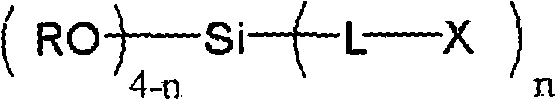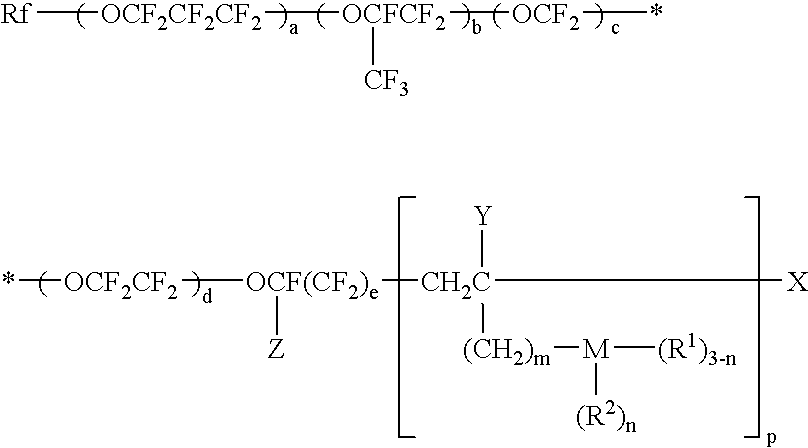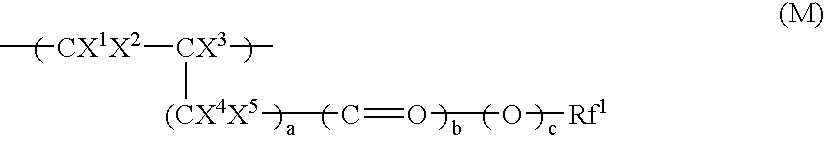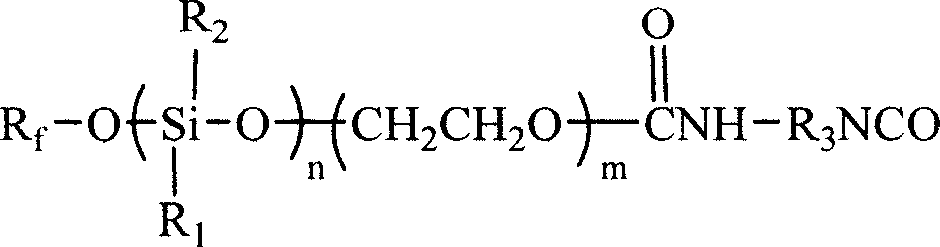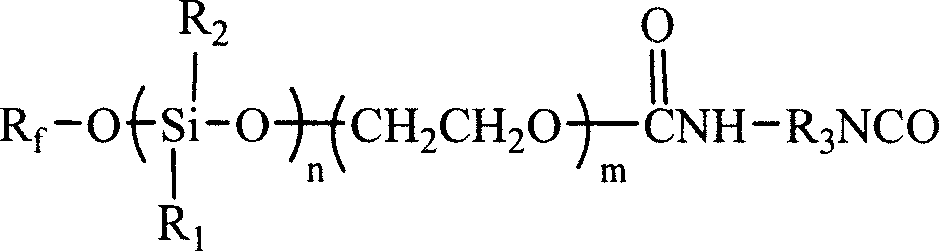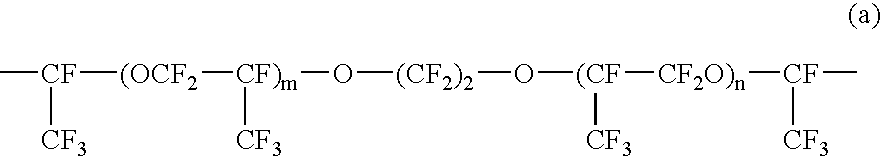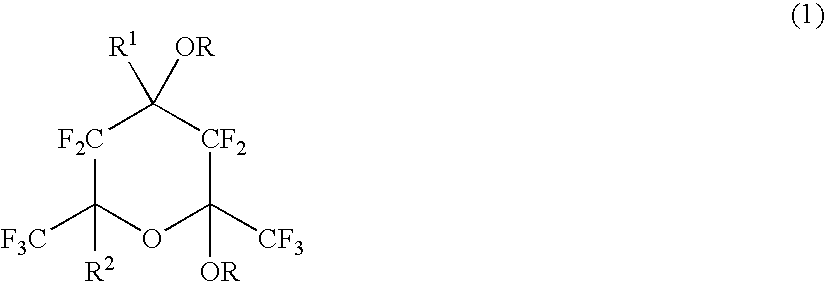Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
849 results about "Compounds of fluorine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding (a weaker bridging link to certain nonmetals). Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements (but not all) the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others (elements in certain groups) the highest oxidation states of oxides and fluorides are always equal.
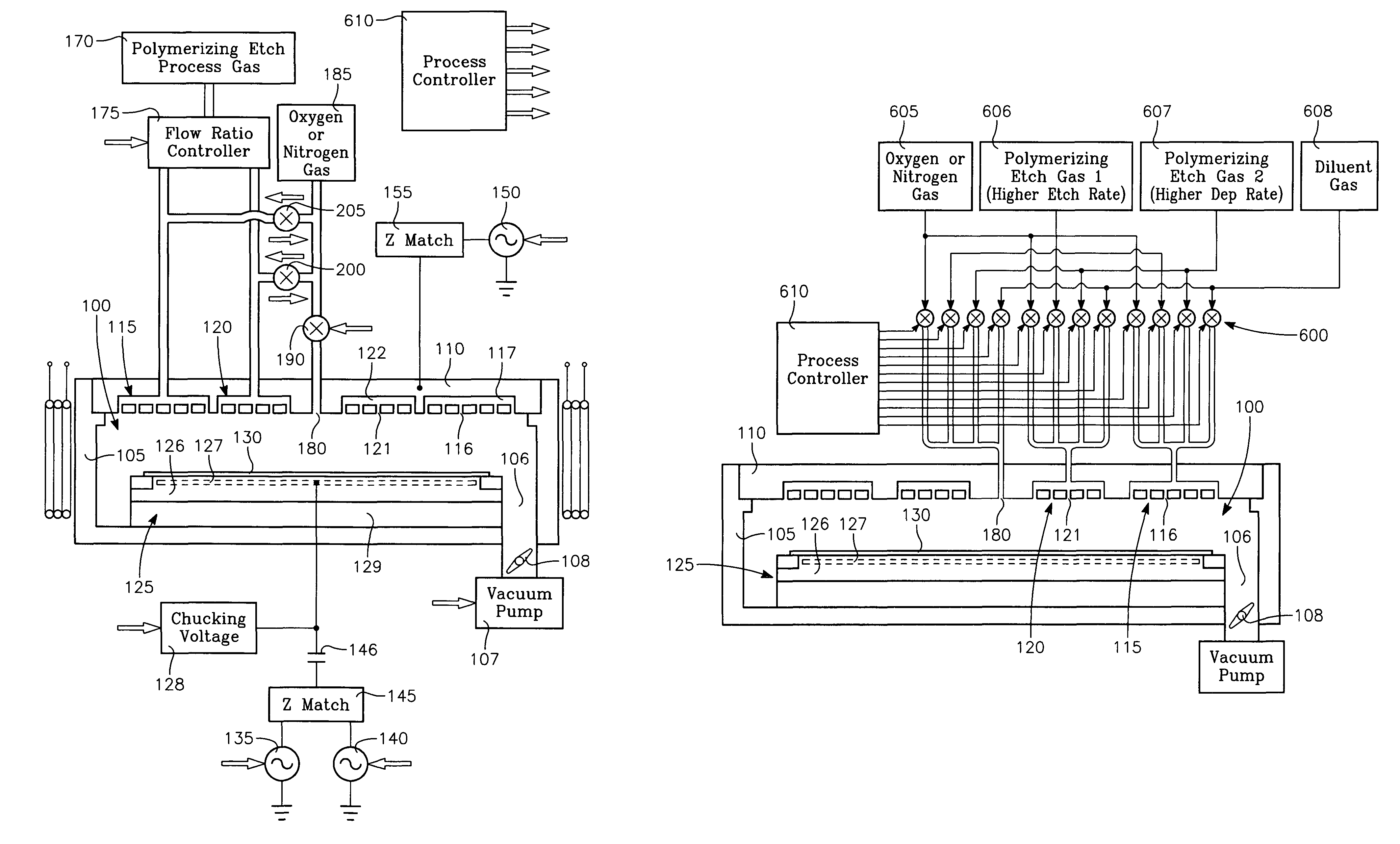
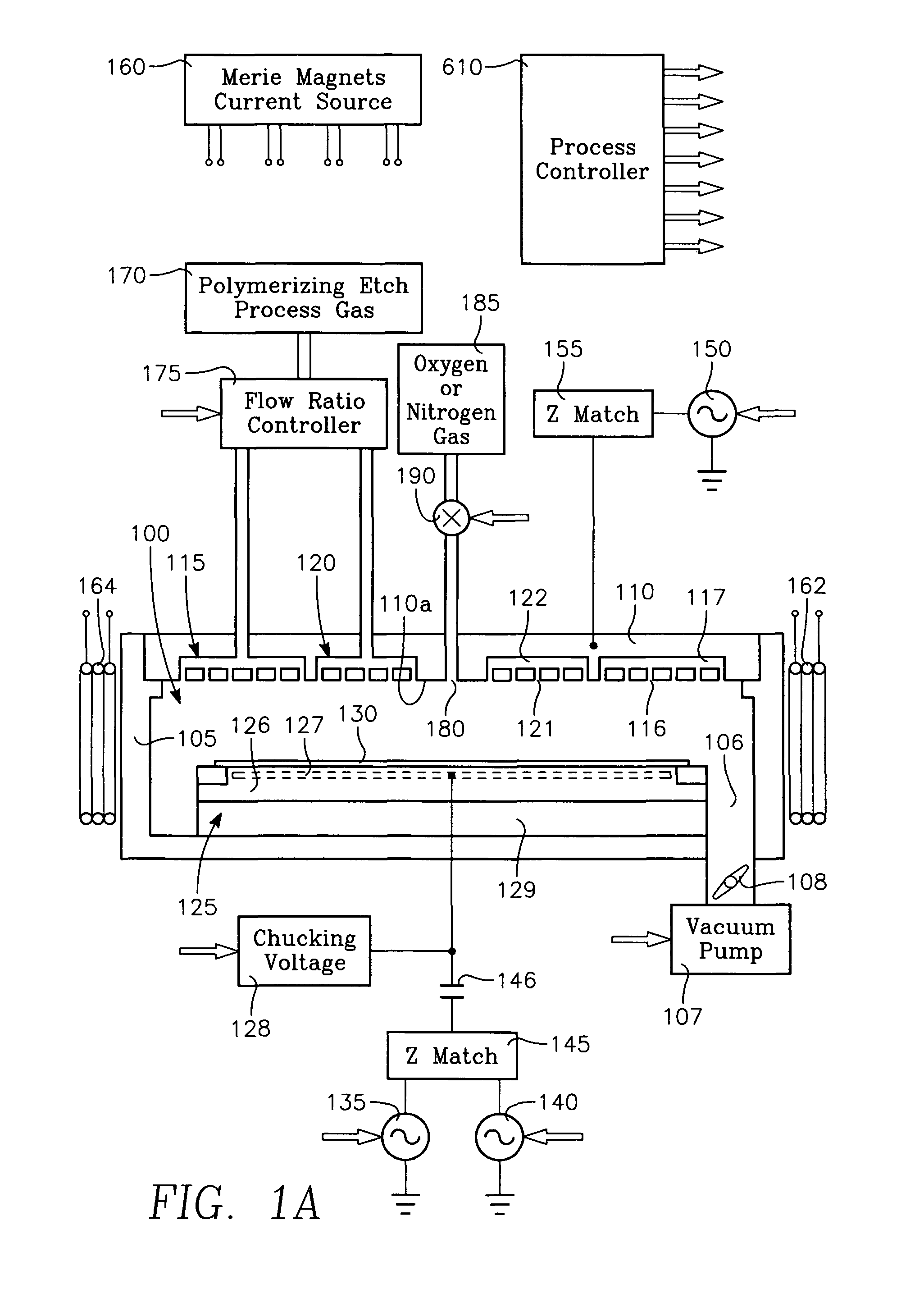
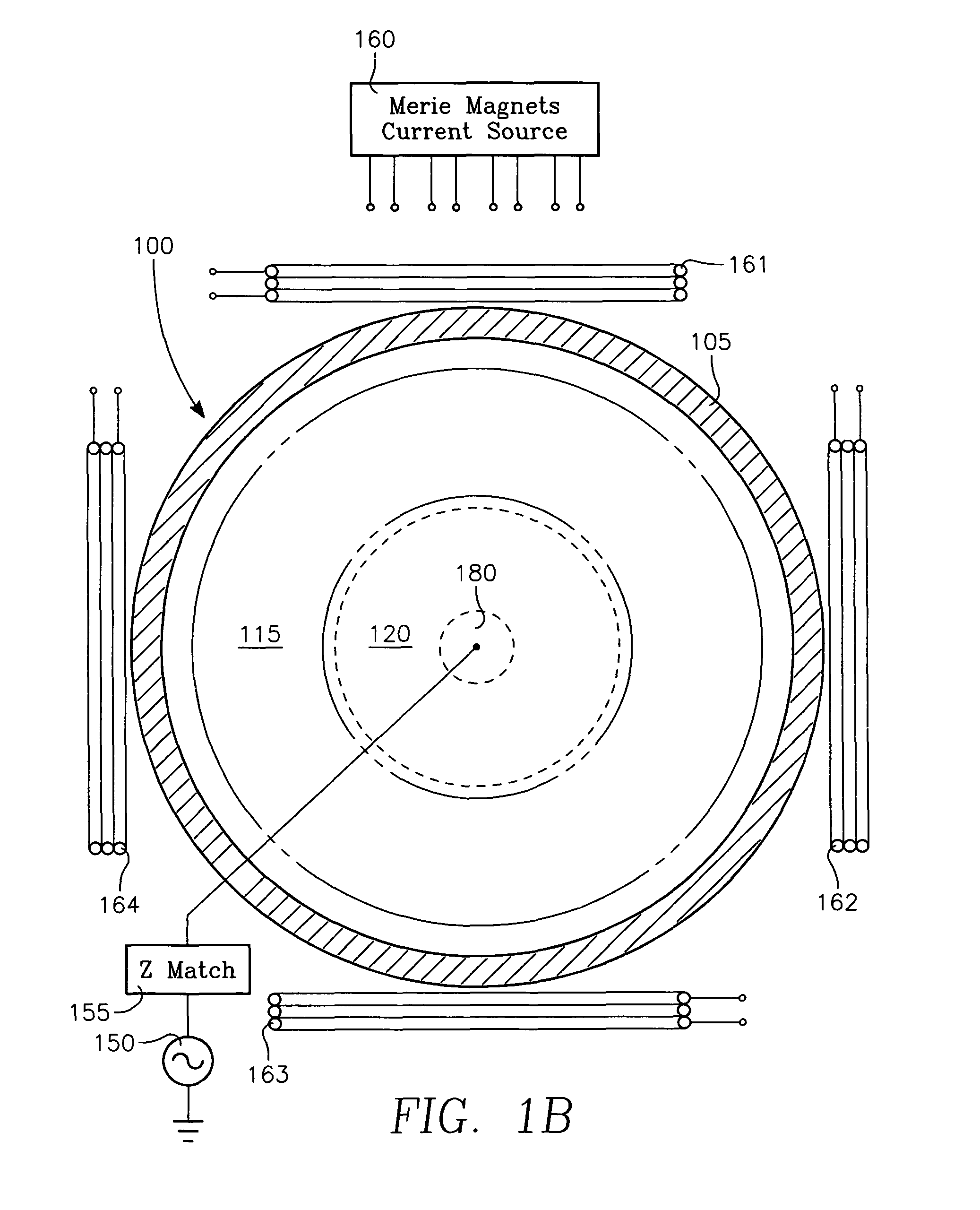
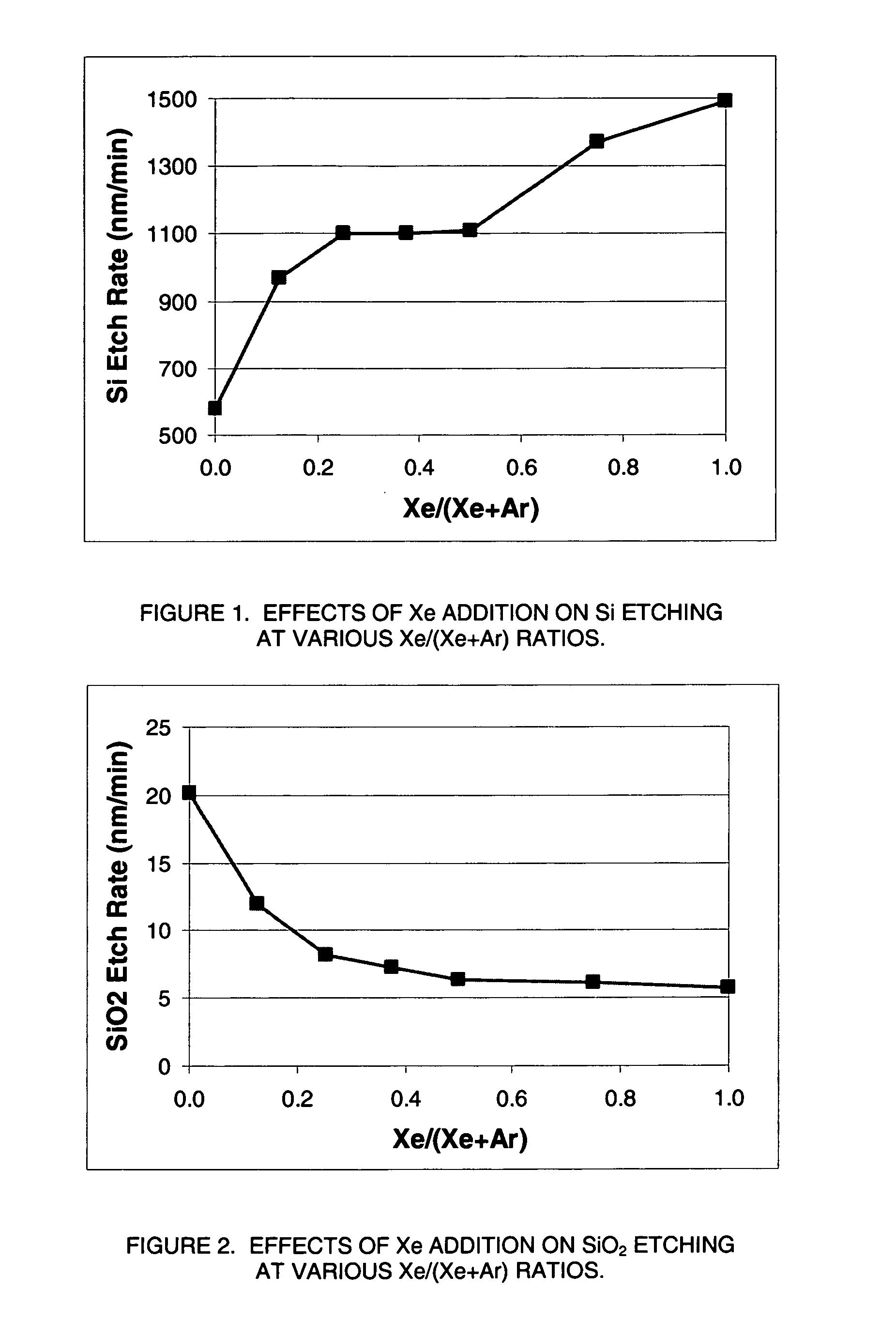
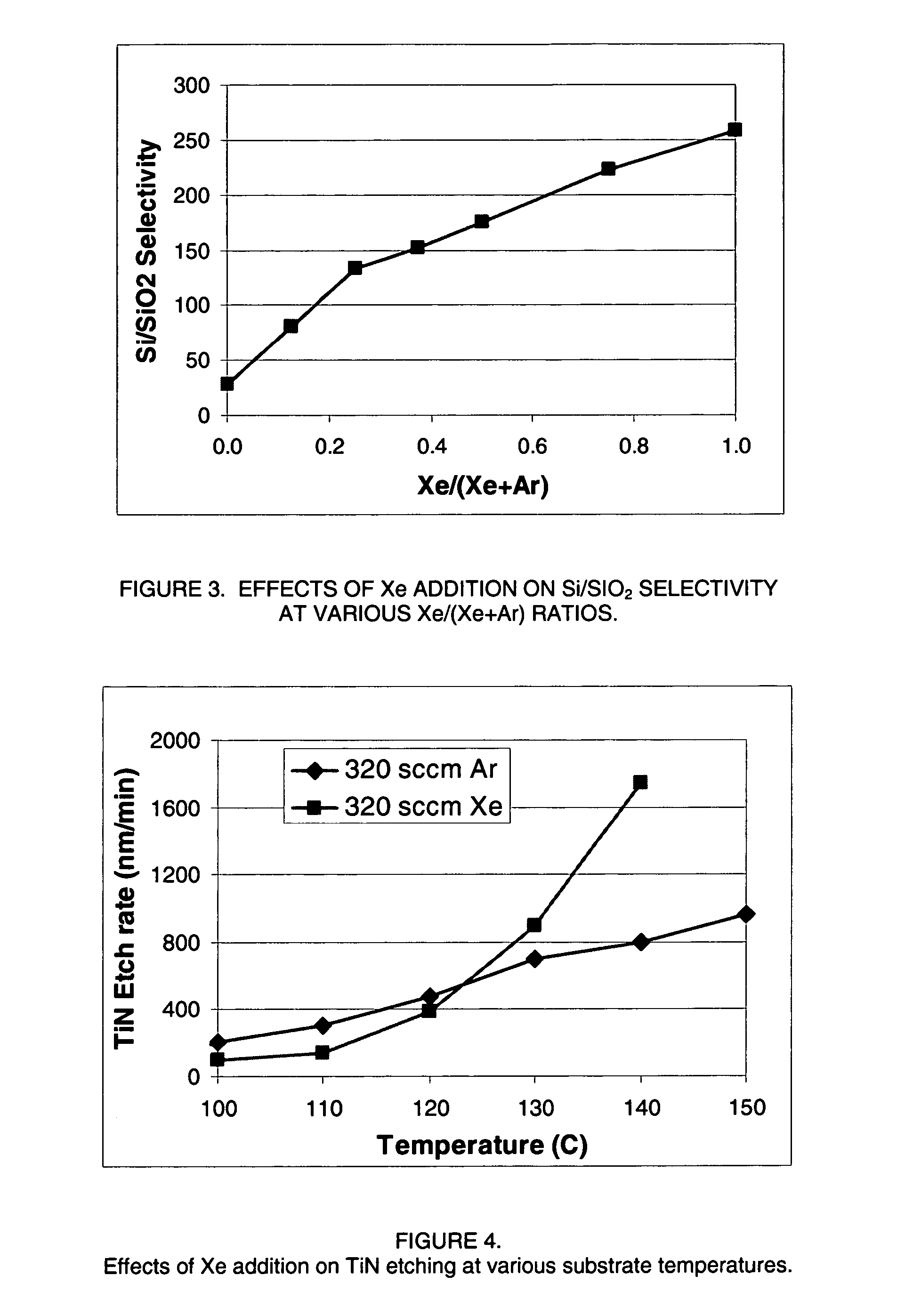
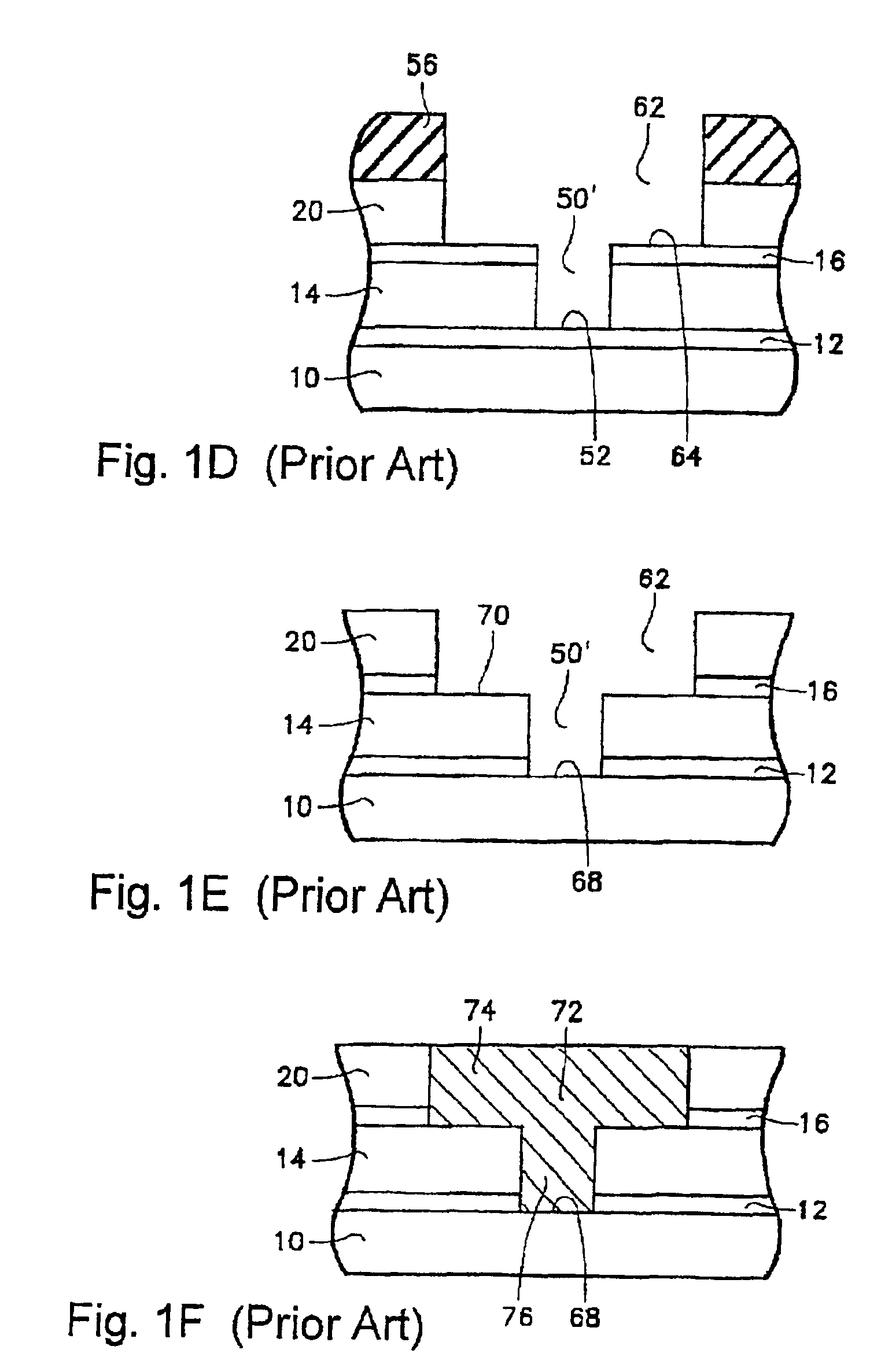
Plasma reactor apparatus with multiple gas injection zones having time-changing separate configurable gas compositions for each zone
ActiveUS8231799B2Electric discharge tubesVacuum gauge using ionisation effectsGas compositionEngineering
Owner:APPLIED MATERIALS INC
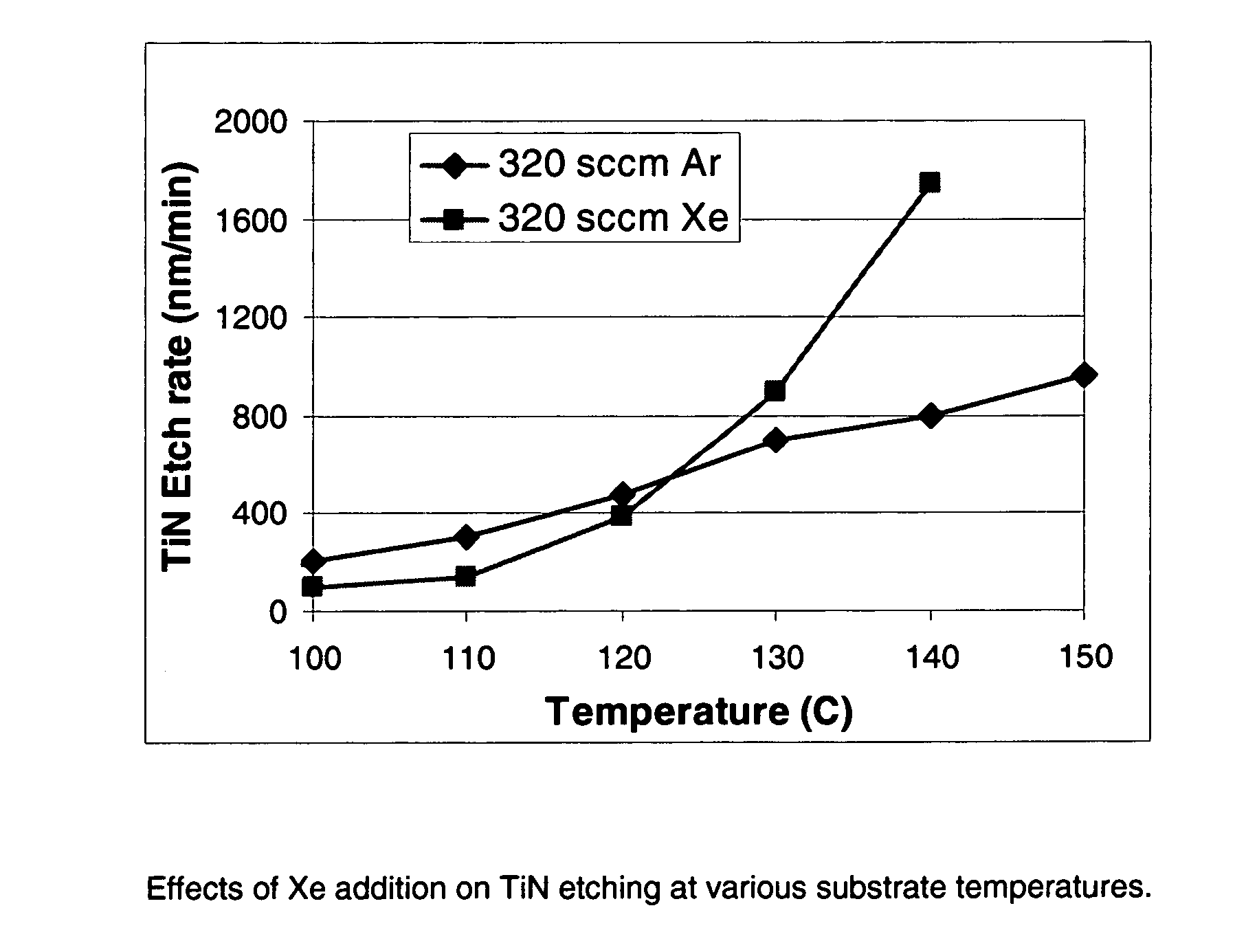
Selective etching of titanium nitride with xenon difluoride
InactiveUS20070117396A1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingEtchingTitanium nitride
This invention relates to an improved process for the selective etching of TiN from silicon dioxide (quartz) and SiN surfaces commonly found in semiconductor deposition chambers equipment and tools. In the process, an SiO2 or SiN surface having TiN thereon is contacted with XeF2 in a contact zone to selectively convert the TiN to a volatile species and then the volatile species is removed from the contact zone. XeF2 can be preformed or formed in situ by reaction between Xe and a fluorine compound.
Owner:VERSUM MATERIALS US LLC
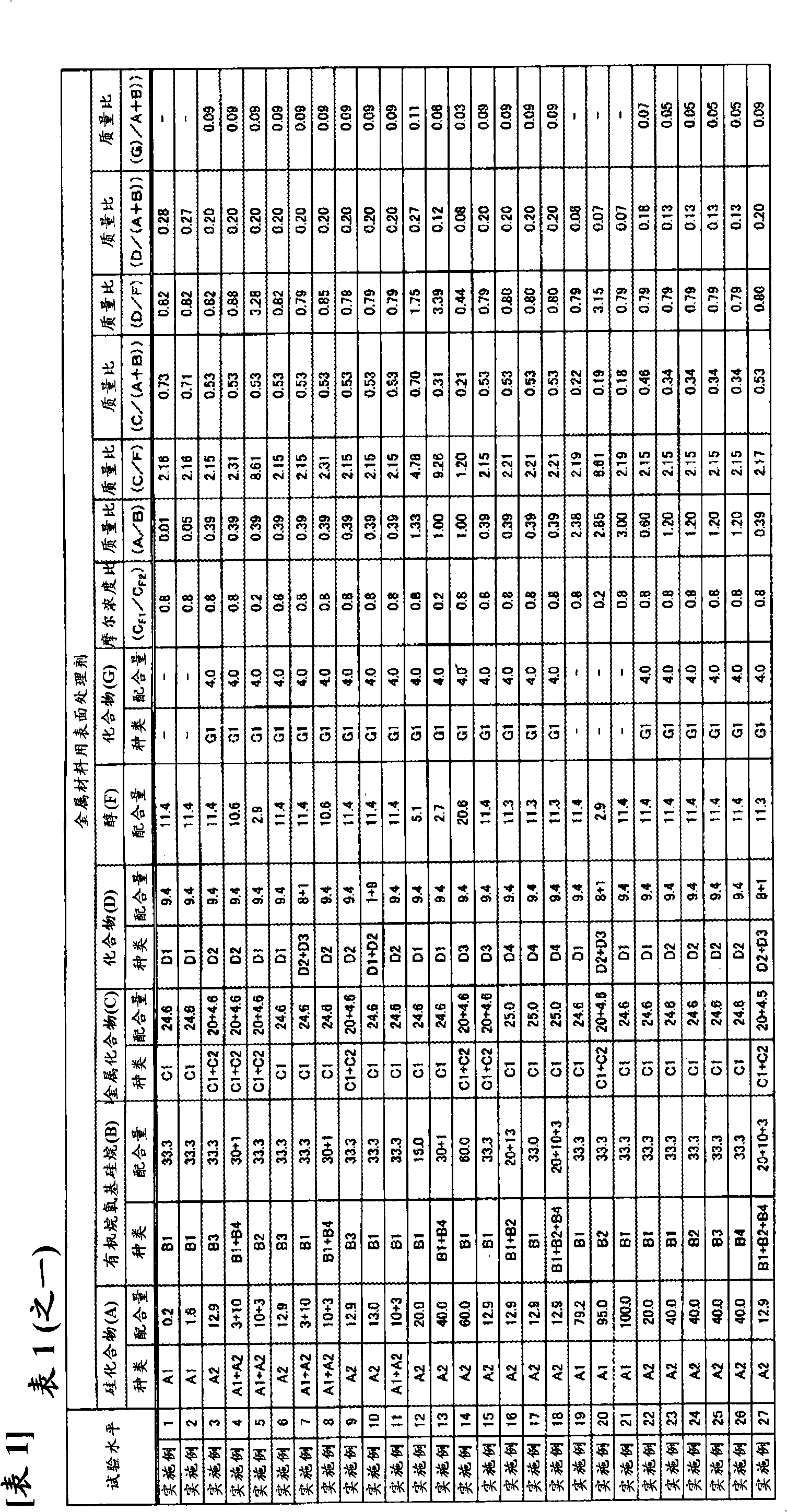
Surface treating agent for metallic materials
ActiveCN102257178AImprove adhesionImprove corrosion resistanceAnti-corrosive paintsMetallic material coating processesAlcoholSilicic acid
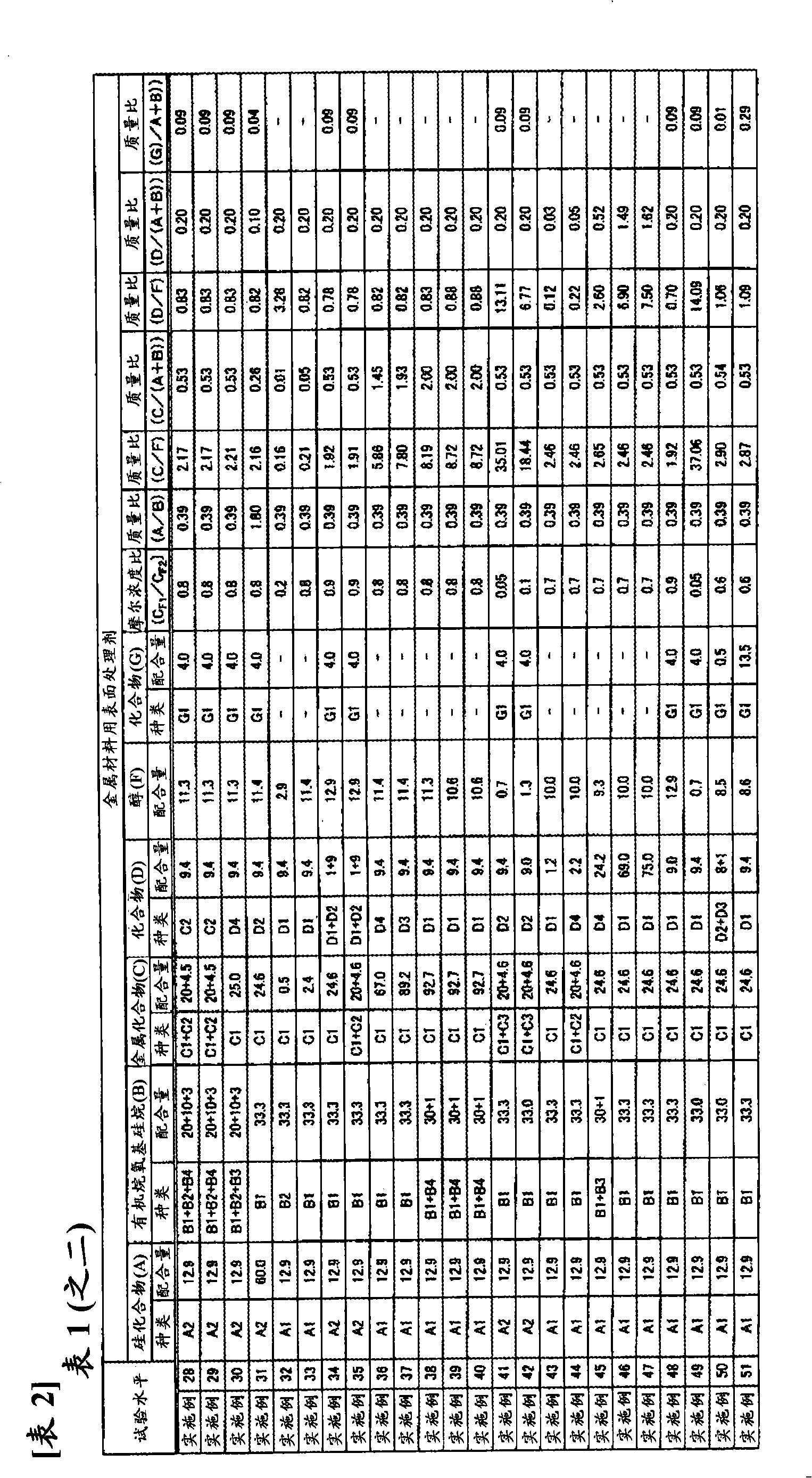
Disclosed is a surface treating agent for metallic materials that has various properties such as corrosion resistance and overcoating properties and, when used in coating-type surface treatment, particularly can form a film , which has excellent adhesion to the surface of metallic materials, and can fix, within the film, a component capable of functioning as a corrosion inhibitor for metallic materials. The surface treating agent for metallic materials comprises a silicic acid compound (A), an organoalkoxysilane (B), a metallic compound (C) containing at least one metal element selected from the group consisting of Zr, Ti, Co, Fe, V, Ce, Mo, Mn, Mg, Al, Ni, Ca, W, Nb, Cr, and Zn, at least one compound (D) selected from the group consisting of phosphoric acid compounds and fluoro compounds, water (E), and an alcohol (F) produced upon hydrolysis of the organoalkoxysilan (B). The molar concentration (mol / L) of the alcohol (F) in the treating agent has been regulated in a predetermined range.
Owner:NIHON PARKERIZING
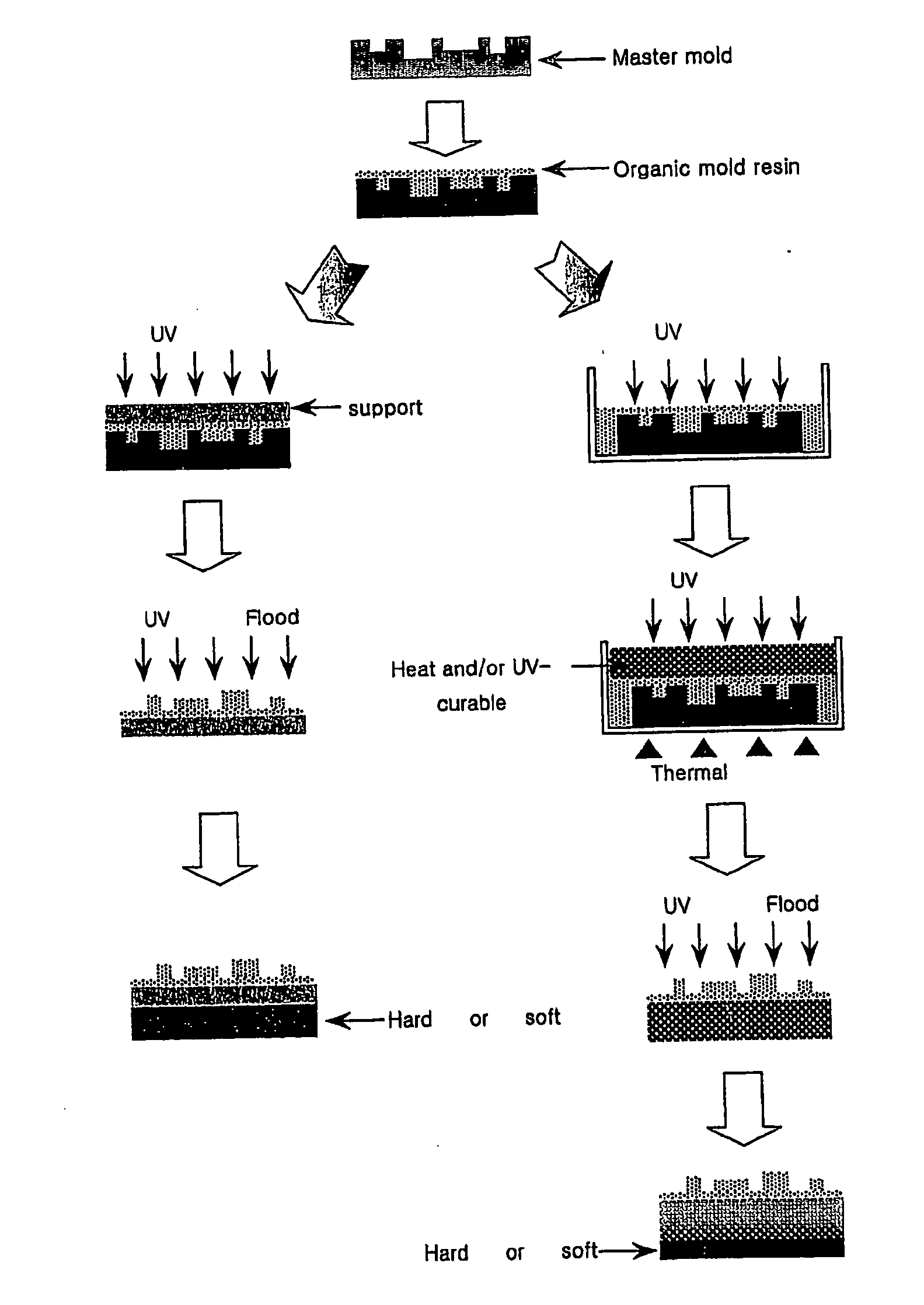
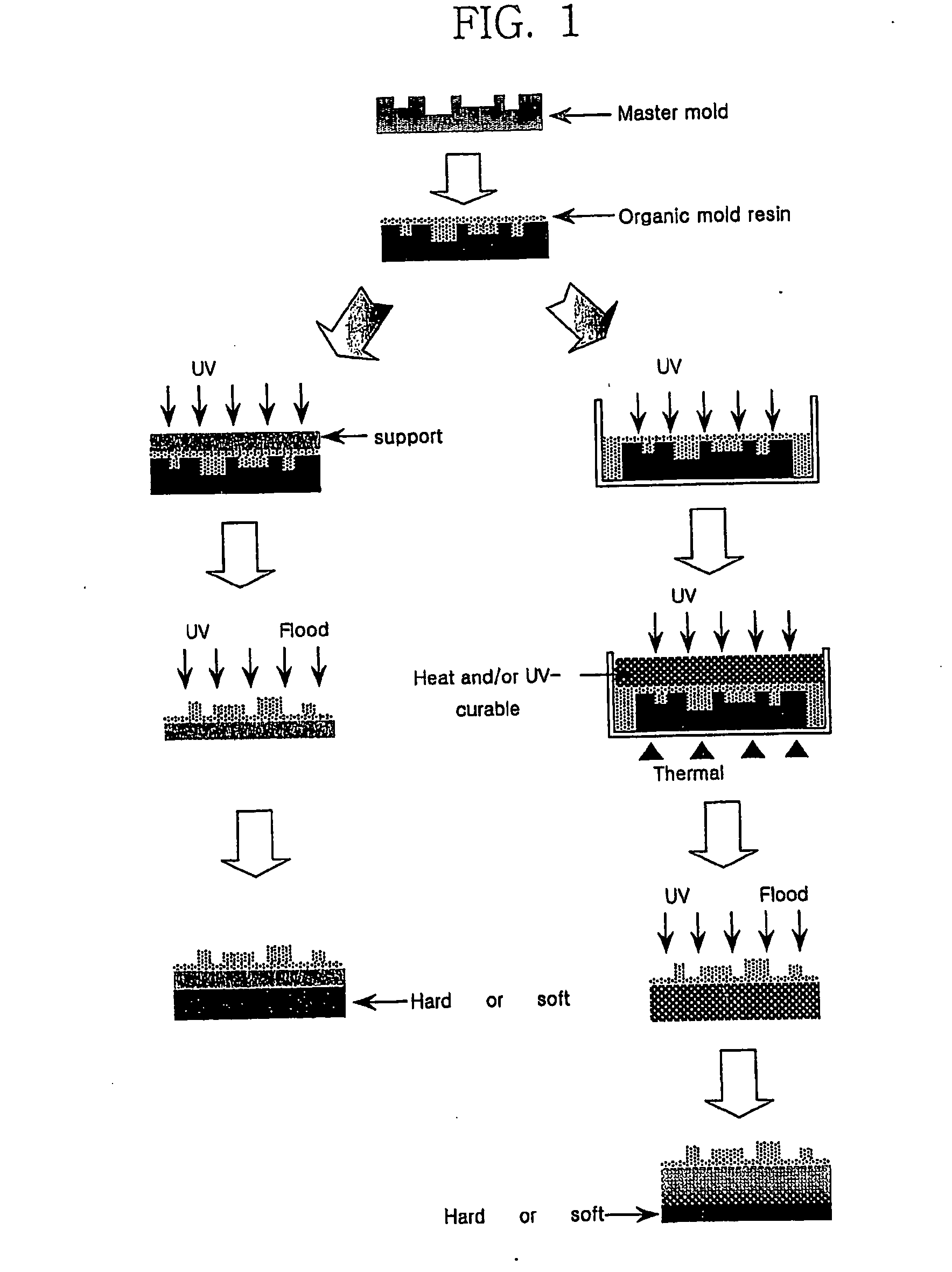
Resin composition for mold used in forming micropattern, and method for fabricating organic mold therefrom
ActiveUS20060214326A1Good chemical and dimensional stabilityHigh modulusNanostructure manufactureNanoinformaticsChemistryPhotoinitiator
A resin composition for a mold used in forming micropatterns comprises (A) 40 to 90 parts by weight of an active energy curable urethane-based oligomer having a reactive group; (B) 10 to 60 parts by weight of a monomer reactive with the urethane-based oligomer, (C) 0.01 to 200 parts by weight of a silicone or fluorine containing compound, based on 100 parts of the sum of the components (A) and (B); and (D) 0.1 to 10 parts by weight of a photoinitiator, based on 100 parts of the sum of the components (A), (B) and (C). The inventive resin composition can be easily cured by the action of an active energy ray, and the organic mold fabricated therefrom is easily lifted off from a master without irreversible adhesion or generation of defects and have excellent dimensional and chemical stabilities.
Owner:MINUTA TECH CO LTD +1
Removal of dielectric oxides
InactiveUS6200891B1Simple methodSemiconductor/solid-state device manufacturingDielectricOrganic solvent
Oxides such as those commonly used in interlevel dielectrics may be removed employing a liquid composition containing a fluoride-containing compound and an organic solvent. Preferred compositions are substantially nonaqueous and include an anhydride. Improved methods for selective removal of oxides, especially for removal of silicon oxides where pre-exposed (or conductive metal - containing) features are present, where metal (conductive metal - contaimg) features are to be exposed by the desired oxide removal, or where the silcon oxide otherwise contacts metal (or conductive metal - containing) features are provided.
Owner:IBM CORP
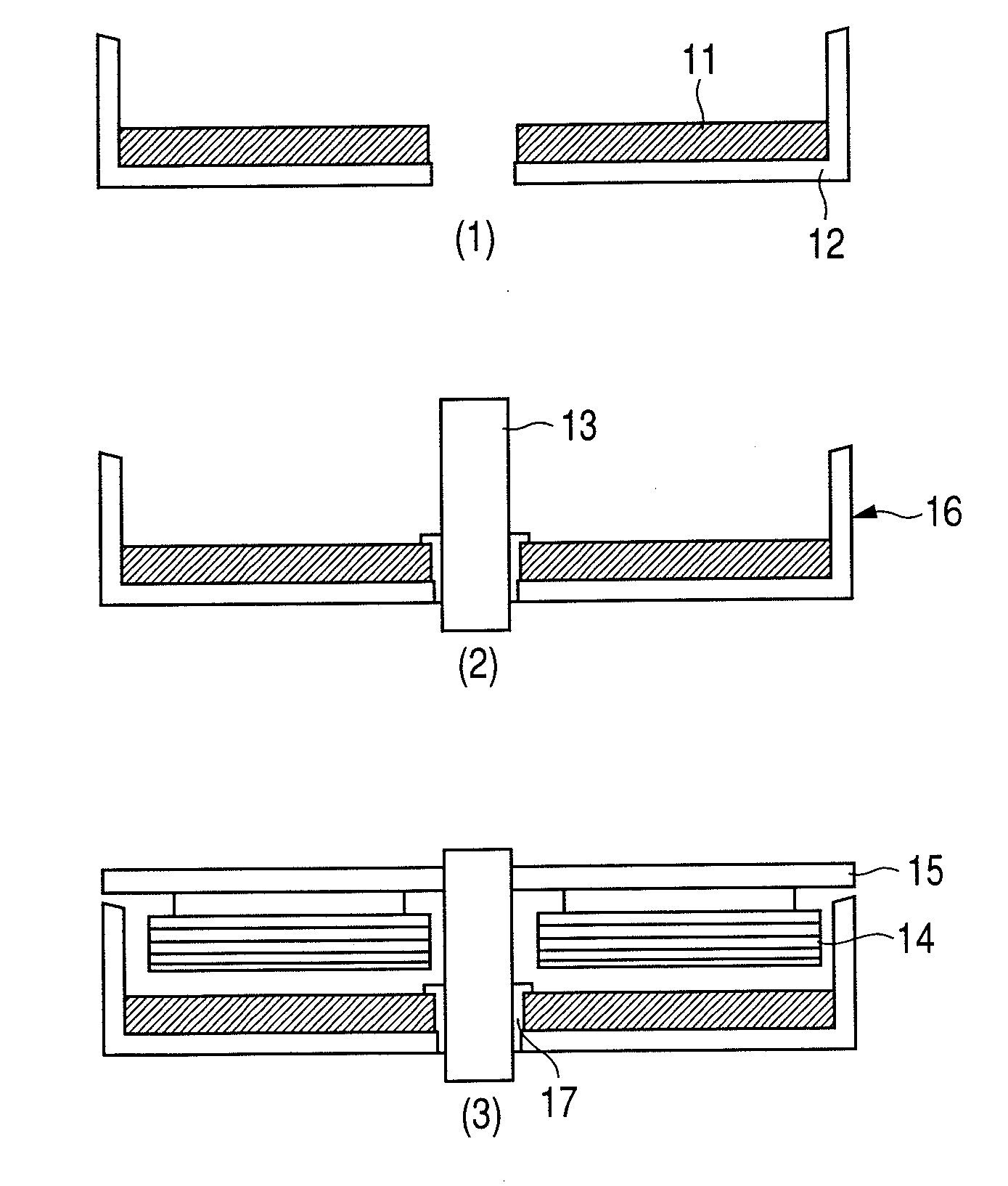
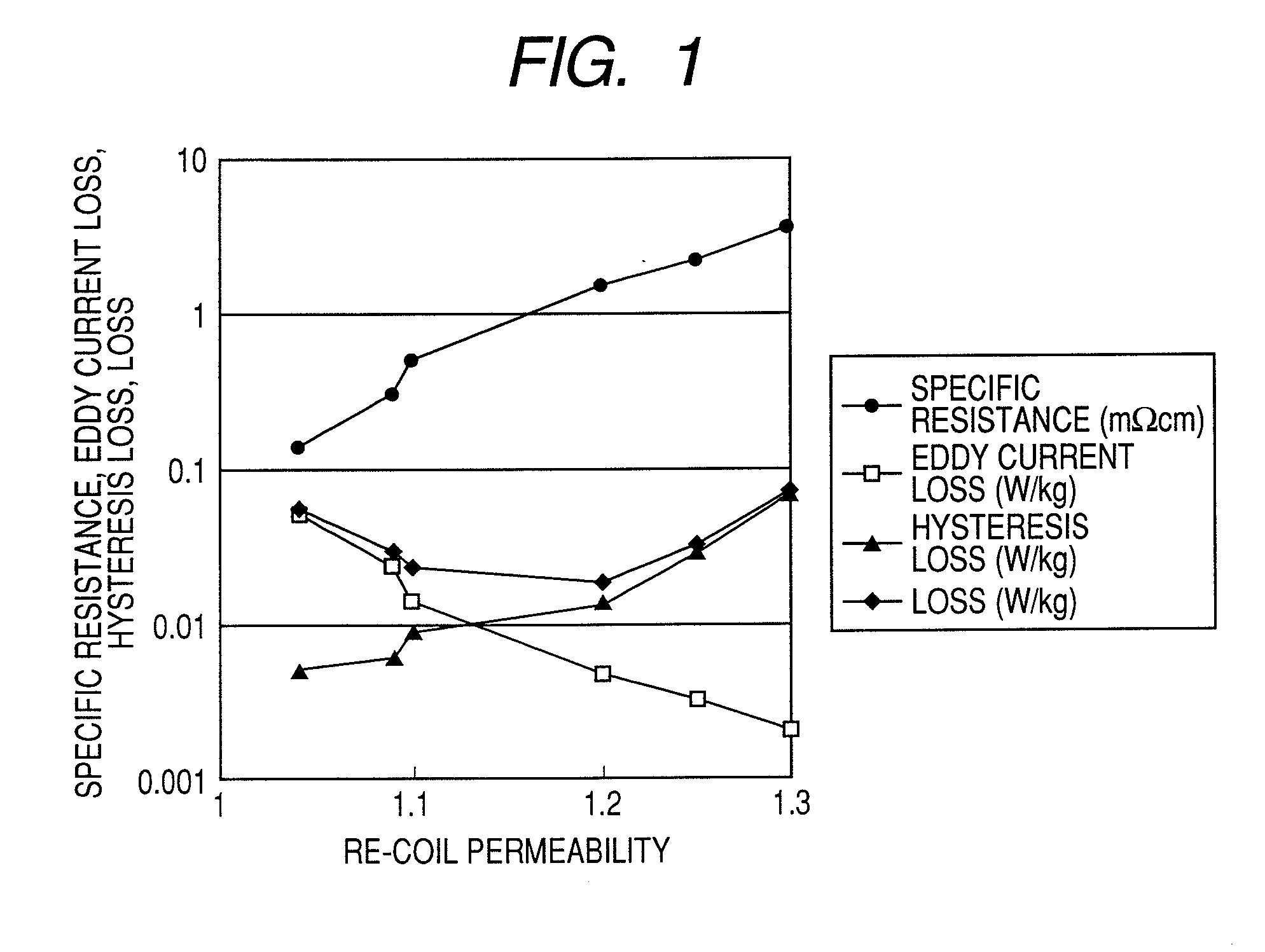
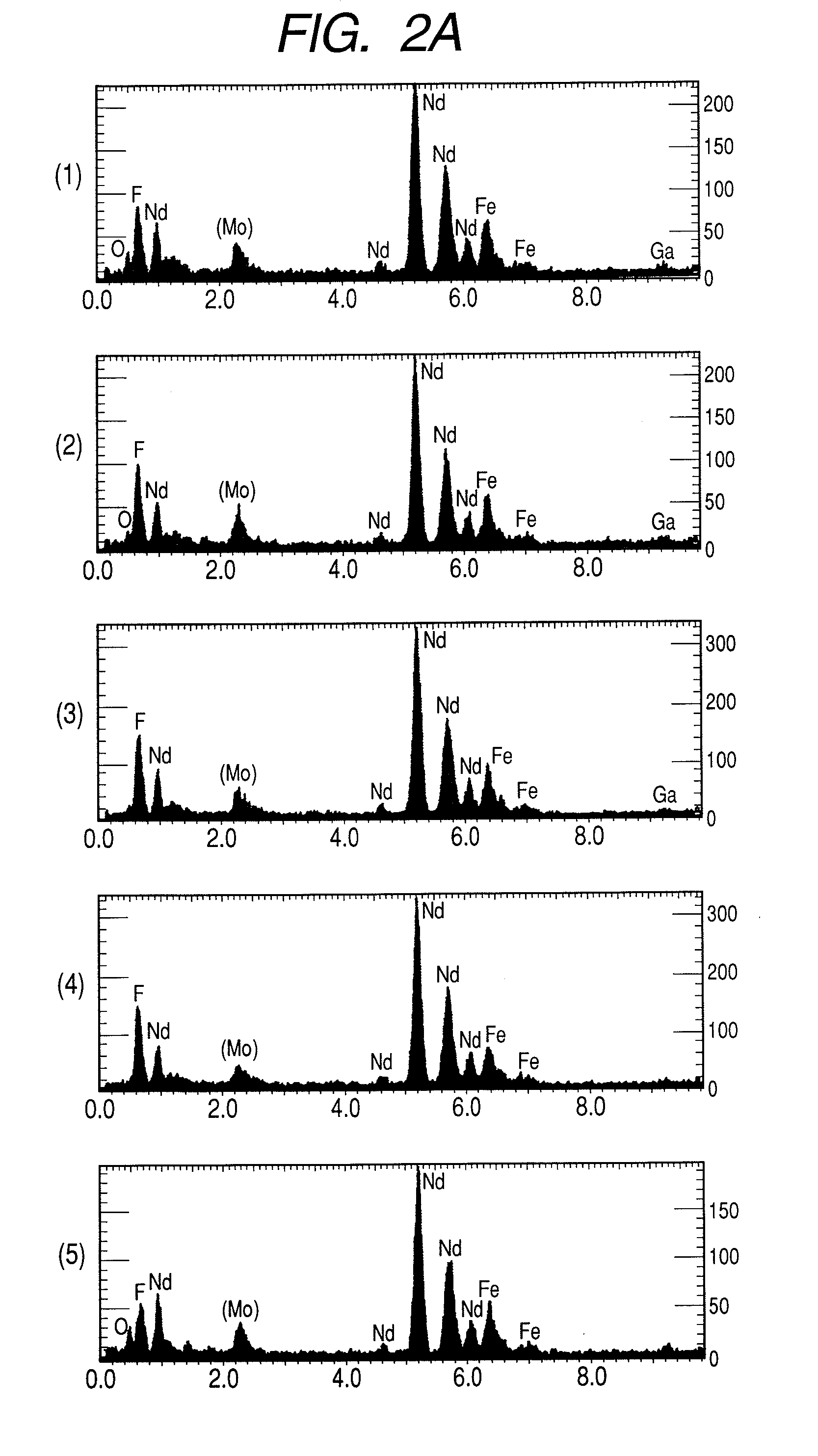
High resistance magnet and motor using the same
InactiveUS20080054738A1Improve magnetic propertiesImprove propertiesLayered productsNanoinformaticsRare-earth elementHigh resistance
A magnet comprising grains of a ferromagnetic material whose main component is iron and a fluorine compound layer or an oxy-fluorine compound layer of fluoride compound particles of alkali metals, alkaline earth metals and rare earth elements, present on the surface of the ferromagnetic material grains, wherein an amount of iron atoms in the fluorine compound particles is 1 to 50 atomic %.
Owner:HITACHI LTD
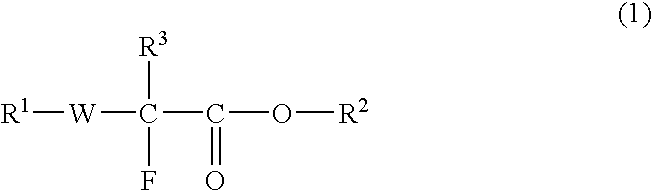
Fluorine-Containing Compound, Fluorine-Containing Polymer, Postive-Type Resist Composition, And Patterning Process Using Same
InactiveUS20080311507A1High rectangularityIncrease acidityOrganic chemistryPhotosensitive materialsPolymer scienceOrganic group
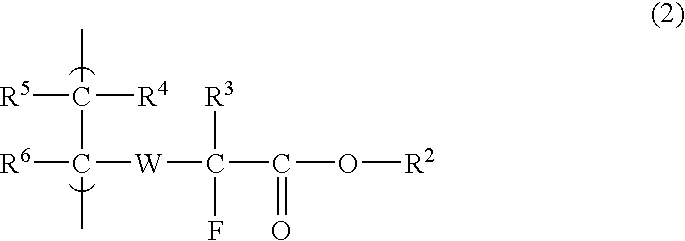
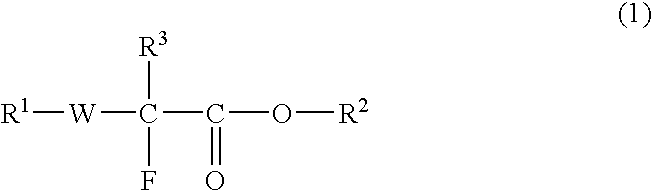
Disclosed is a fluorine-containing compound represented by formula (1),wherein R1 represents a polymerizable double-bond containing group, R2 represents an acid-labile protecting group, R3 represents a fluorine atom or fluorine-containing alkyl group, and W represents a bivalent linking group. This compound can provide a fluorine-containing polymer compound that has a weight-average molecular weight of 1,000-1,000,000 and contains a repeating unit represented by formula (2),wherein R2, R3 and W are defined as above, each of R4, R5 and R6 independently represents a hydrogen atom, fluorine atom or monovalent organic group, at least two of R4, R5 and R6 may be combined to form a ring. This polymer compound can provide a resist composition capable of forming a pattern that is transparent to exposure light and superior in rectangularity.
Owner:CENT GLASS CO LTD
Copolymer for cosmetic preparation
The polymer for cosmetics produced by polymerizing (A) a fluorine-containing (meth)acrylate, and (B) at least one silicon-containing polymerizable compound selected from the group consisting of a mercapto-modified silicone, an azo group-containing silicone and a polymerizable silane can be blended easily in cosmetic preparations and can form a film excellent in a water proofing property, a water- and oil-repellency, feelings in use and safety. This copolymer for cosmetics can improve the drawbacks of fluorine compound-treated powders.
Owner:DAIKIN IND LTD
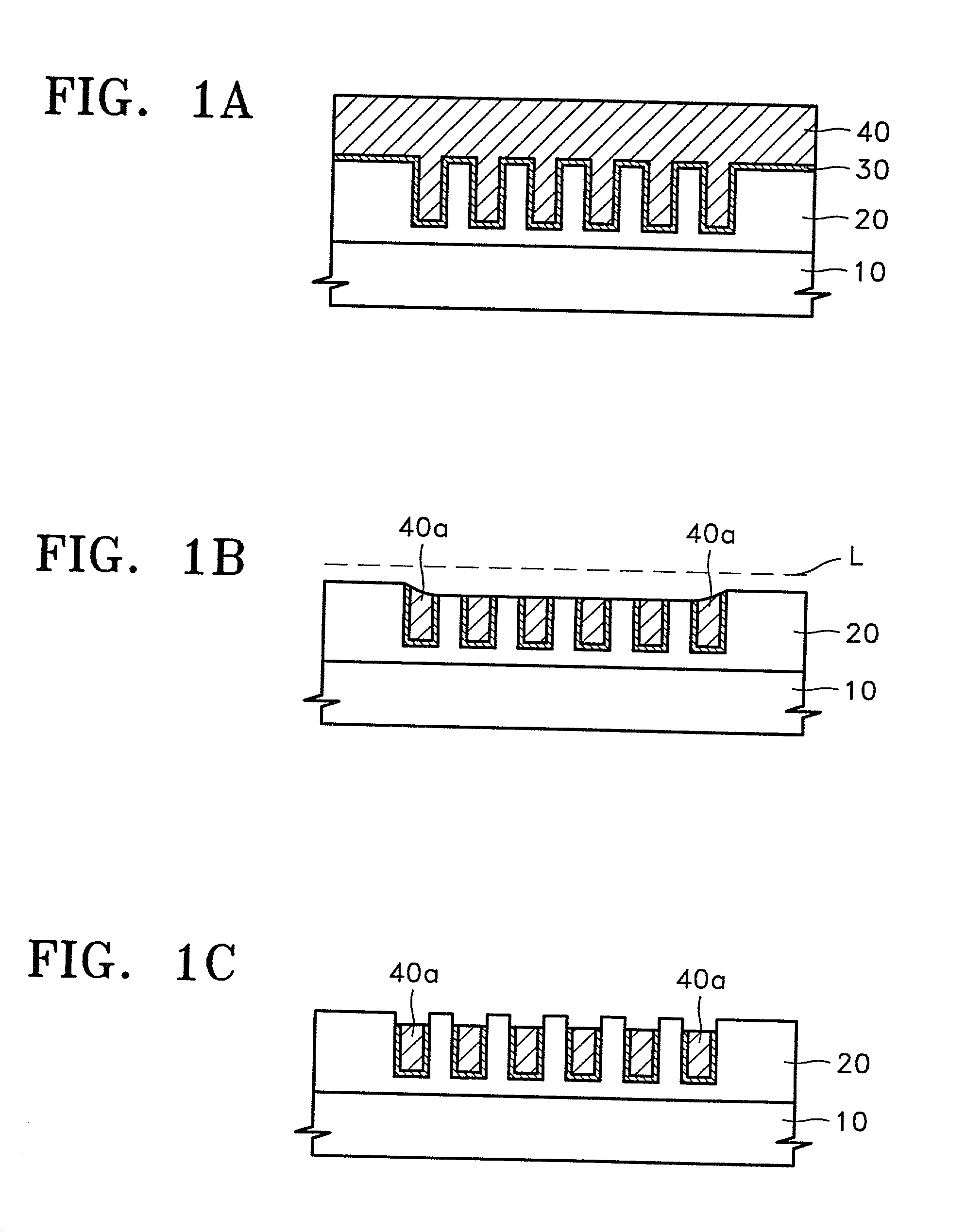
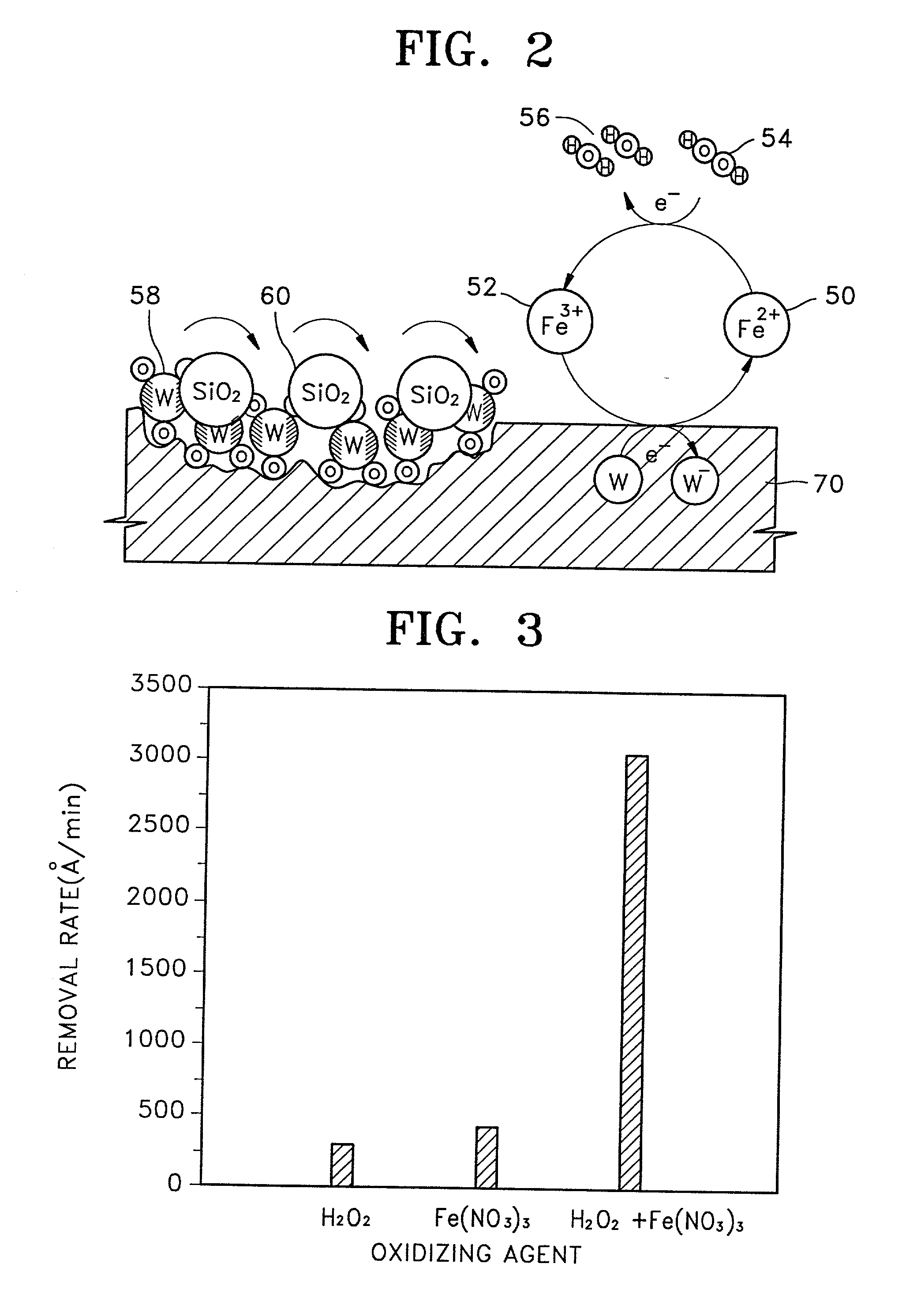
Slurry for chemical mechanical polishing of metal layer, method of preparing the slurry, and metallization method using the slurry
InactiveUS20020019128A1Strong oxidation abilityGood reproducibilitySemiconductor/solid-state device manufacturingPolishing compositions with abrasivesOrganic acidPhysical chemistry
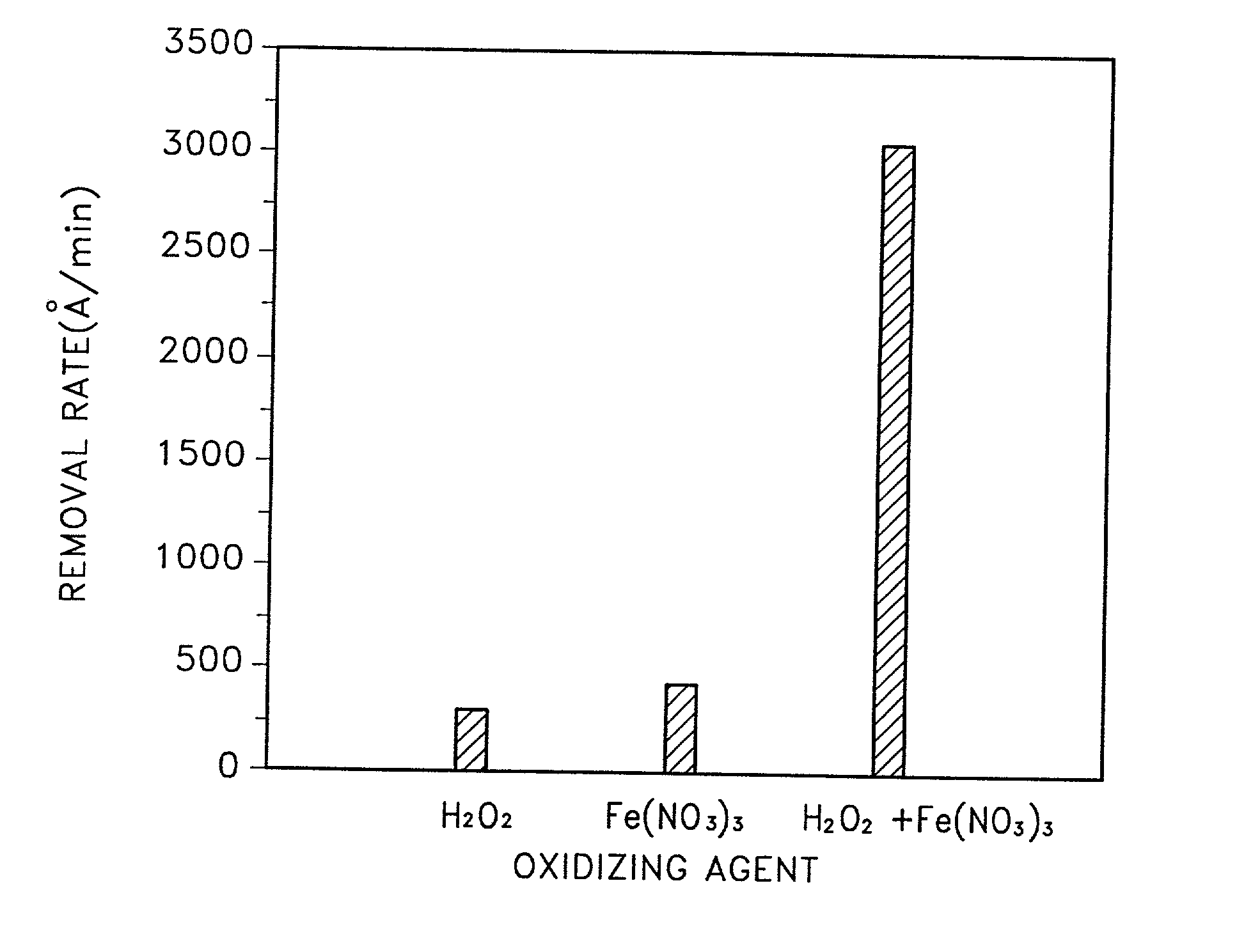
A slurry for use in chemical mechanical polishing (CMP) of a metal layer. The CMP slurry includes an abrasive, a plurality of oxidizing agents, a stabilizer including an organic acid having a carboxyl group, a corrosion inhibitor which suppresses corrosion of a metal, a fluorine compound which reduces a difference in removal rates of a metal layer and a barrier layer, and deionized water. The plurality of oxidizing agents include a second oxidizing agent which oxidizes the metal and a first oxidizing agent which restores an oxidizing ability of the second oxidizing agent.
Owner:SAMSUNG ELECTRONICS CO LTD
Treated textiles and compositions for treating textiles
InactiveUS20050062010A1Good liquid repellencyPhysical treatmentDecorative surface effectsChemical compositionCompound (substance)
Certain chemical compositions provide superior repellency, durability, and soil (stain) release properties when applied to a textile or fabric. Compositions may contain a fluorochemical-containing soil release component or a crosslinking component, or both, and also may contain an antimicrobial agent. In some applications, the crosslinking component may be hydrophobic, so as to be generally not compatible with aqueous environments. Compositions having less than about 6 weight percent of a fluorochemical-containing soil release component, based upon the weight of the treating composition, may be employed in some applications.
Owner:SAGE AUTOMOTIVE INTERIORS INC
Water- and oil-repellent compositions
InactiveUS6960642B2Improve thermal stabilityOther chemical processesLiquid repellent fibresFiberOligomer
Fiber, fabric, film, molded or blown article comprising a water- and oil-repellent composition comprising a melt blend of components (a) and (b) in which:(a) comprises a repellency-imparting, fluorochemical composition comprising at least one fluorine-containing aromatic ester oligomer comprising(1) at least two repeat units derived or derivable from the reaction of at least one dicarboxylic acid (or a derivative thereof) and at least one polyol, with the proviso that either the dicarboxylic acid (or derivative) or the polyol (or both) is aromatic or heteroaromatic, and(2) fluorochemical endgroups derived or derivable from the reaction of(i) the dicarboxylic acid (or derivative) and at least one fluorine-containing monoalcohol, or(ii) the polyol and at least one fluorine-containing monocarboxylic acid; and(b) comprises a treatable substrate material; with the proviso that, when the treatable substrate material comprises a mixture of at least two polymers, the mixture is non-stratifying.
Owner:3M INNOVATIVE PROPERTIES CO
Methods and compositions for improving light-fade resistance and soil repellency of textiles and leathers
ActiveUS20050022313A1Easy to useImpart propertyDispersed particle filtrationPhysical treatmentAdditive ingredientChemical compound
One method includes applying to a post-manufactured textile material a liquid composition resulting from a combination of ingredients. The ingredients include one or more anti-fading compounds, one or more anti-soiling compounds, one or more silicon-based compounds, and one or more carrying media. One composition is a liquid composition resulting from a combination of ingredients, with the ingredients including a benzotriazole, a fluorocarbon, an organosiloxane, and odorless mineral spirits.
Owner:SCHEIDLER KARL J
Surface-treating agent comprising inorganic/organic composite material
InactiveUS20040186216A1Excellent releasabilityHigh transparencySynthetic resin layered productsOrganic dyesRefractive indexOrganic chemistry
A surface treatment agent, which contains: (A) a hydrolyzable metal alkoxide or a hydrolyzate thereof, (B) a fluorocompound containing a perfluoroalkyl group and a functional group reactive with the hydrolyzable metal alkoxide (A), and (C) an adhesion improvement agent, can provide a film having transparency and durability while maintaining excellent soil releasability and low refractive index.
Owner:DAIKIN IND LTD
Flux cored electrode with fluorine
InactiveUS20060219685A1Quality improvementReduce the amount requiredPropellersToothed gearingsSlagCompounds of fluorine
Owner:LINCOLN GLOBAL INC
Fluoroalcohol preparation method, fluorinated monomer, polymer, resist composition and patterning process
ActiveUS20070179309A1Organic compound preparationPhotosensitive materialsPolymer scienceChemical compound
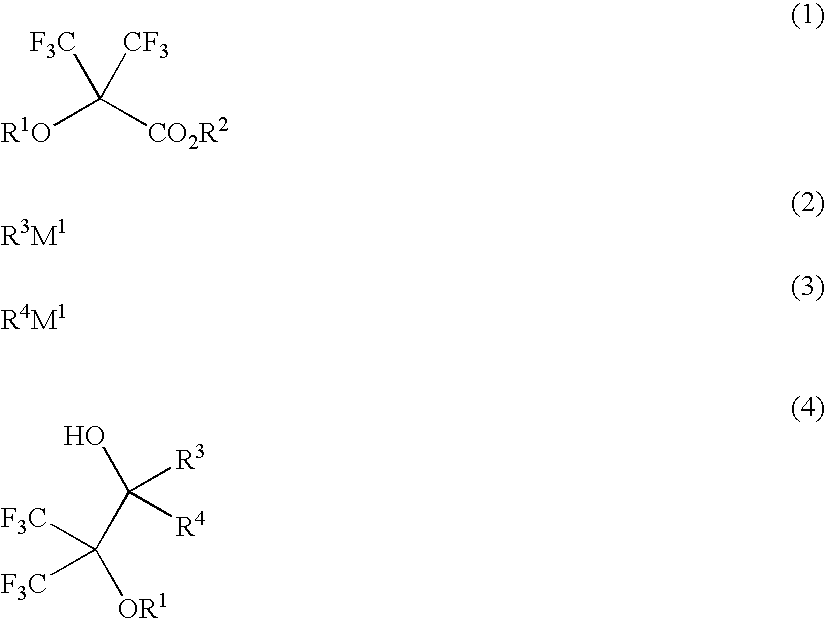
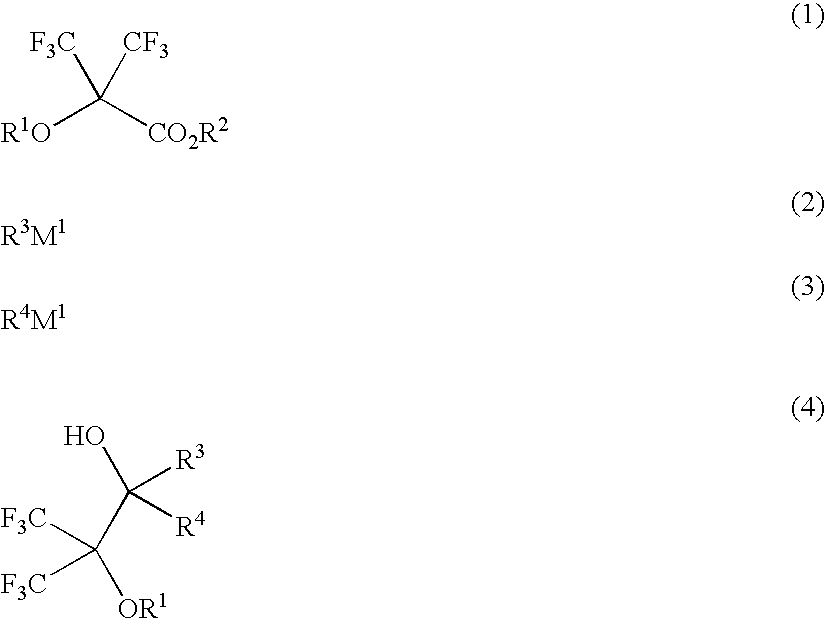
Fluoroalcohol compounds of formula (4) are prepared by reacting a fluorine compound of formula (1) with reducing agents or organometallic reagents of formulas (2) and (3) wherein R1 is H or a monovalent C1-C20 hydrocarbon group in which any —CH2— moiety may be replaced by —O— or —C(═O)—, R2 is H or a monovalent C1-C6 hydrocarbon group, R3 and R4 are H or a monovalent C1-C8 hydrocarbon group, and M1 is Li, Na, K, Mg, Zn, Al, B, or Si. From the fluoroalcohol compounds, fluorinated monomers can be produced in a simple and economic way, which are useful in producing polymers for the formulation of radiation-sensitive resist compositions.
Owner:SHIN ETSU CHEM IND CO LTD
Metal finishing agent using silane coupling agent as main component for metal surface pretreatment
The invention relates to a metal surface treatment agent for metal surface pretreatment, in which silane coupling agent is the main composition. The treatment agent comprises the following raw materials: 1, 2- diethoxy estersil ethane (BTSE), gamma- aminopropyl silane (gamma-APS), water dispersible silicon dioxide, fluorine compound, acetic acid and water. On the basis of silane coupling agent film forming mechanism, by selecting and remixing the main composites silane: coupling agent (SA), surface conditioner and auxiliary film forming agent, a metal surface parkerizing substitute with silicon coupling agent as the main film forming agent is researched. The invention has the characteristics of innocuity, harmlessness, non-pollution, low-temperatrue fast processing, operation simplicity, wide material resource, low price, production clearness, etc.
Owner:合肥华清金属表面处理有限责任公司
Surface treatment agent comprising inorganic-organic hybrid material
InactiveUS7125926B2Excellent releasabilityHigh transparencySynthetic resin layered productsOrganic dyesRefractive indexHybrid material
A surface treatment agent, which contains:(A) a hydrolyzable metal alkoxide or a hydrolyzate thereof,(B) a fluorocompound containing a perfluoroalkyl group and a functional group reactive with the hydrolyzable metal alkoxide (A), and(C) an adhesion improvement agent,can provide a film having transparency and durability while maintaining excellent soil releasability and low refractive index.
Owner:DAIKIN IND LTD
Fluorinated compound, water repellent composition and thin film
InactiveUS20060222865A1Easy to degradeImprove waterproof performanceSilicon organic compoundsOther chemical processesHydrogen atomHalogen
To provide a fluorinated compound, which can readily form a thin film having a hydrophobic / hydrophilic pattern, by employing an ultraviolet light having a relatively low energy. A thin film having a hydrophobic / hydrophilic pattern is formed by irradiating a thin film formed by employing a fluorinated compound represented by the following formula 1 (in the formula 1, each of R1, R2, R3 and R4 which are independent of one another, is a hydrogen atom, a halogen atom or a monovalent organic group, provided that at least one of them is a monovalent organic group having fluorine atoms, and R5 is a hydrogen atom or a monovalent organic group, R6 is a monovalent organic group, and A is a hetero atom) with ultraviolet light.
Owner:ASAHI GLASS CO LTD
Fluorine-containing compound, resist composition for immersion exposure, and method of forming resist pattern
ActiveUS20090047602A1Substance reductionImproving water tacking abilityOrganic chemistryPhotosensitive materialsResistSolubility
A resist composition for immersion exposure including a base component (A) which exhibits changed solubility in an alkali developing solution under action of acid, an acid-generator component (B) which generates acid upon irradiation, and a fluorine-containing compound (C) having a group represented by general formula (c) shown below and containing at least one fluorine atom:wherein Q represents a group in which one hydrogen atom has been removed from a monovalent hydrophilic group; and R1 represents a hydrocarbon group of 2 or more carbon atoms which may have a fluorine atom.
Owner:TOKYO OHKA KOGYO CO LTD
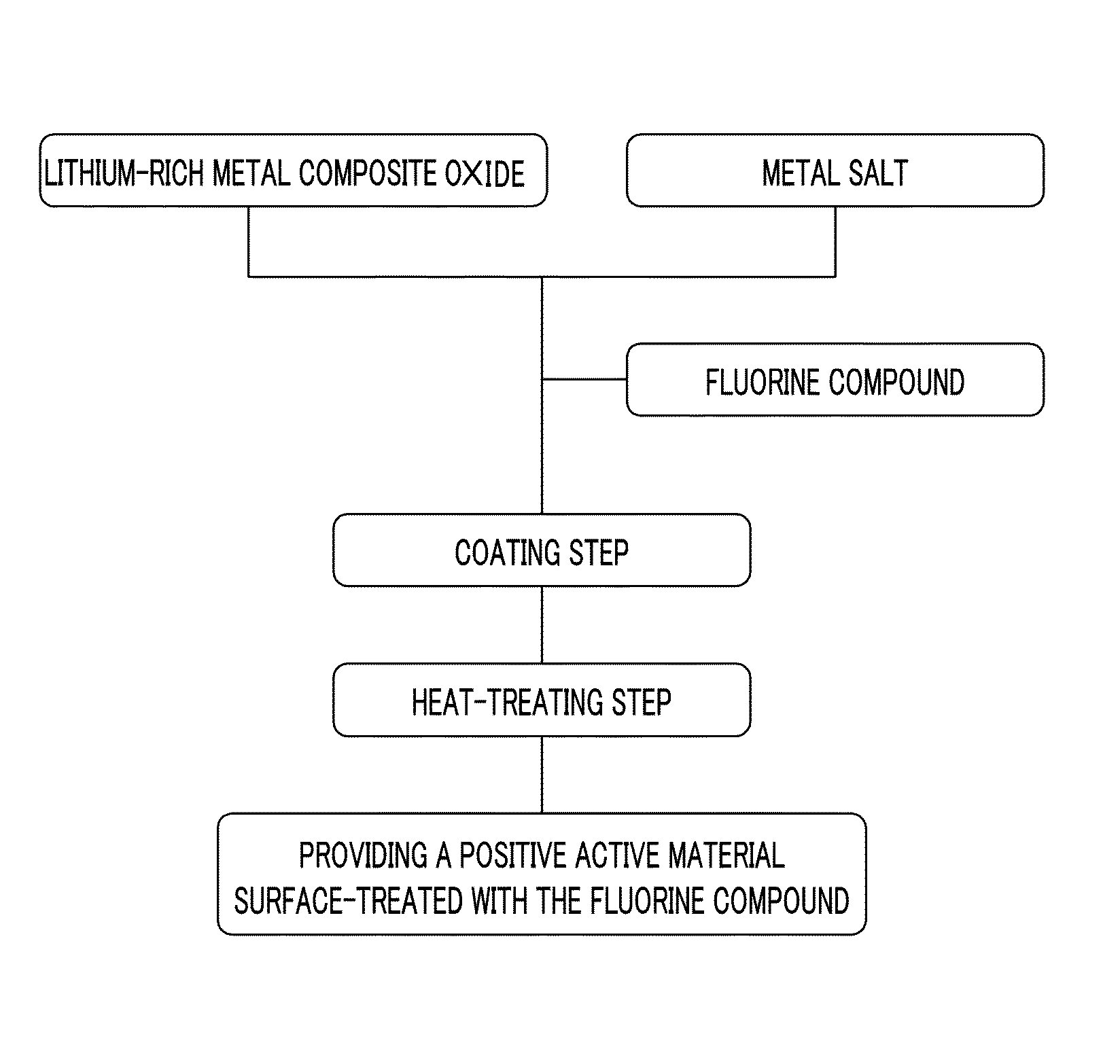
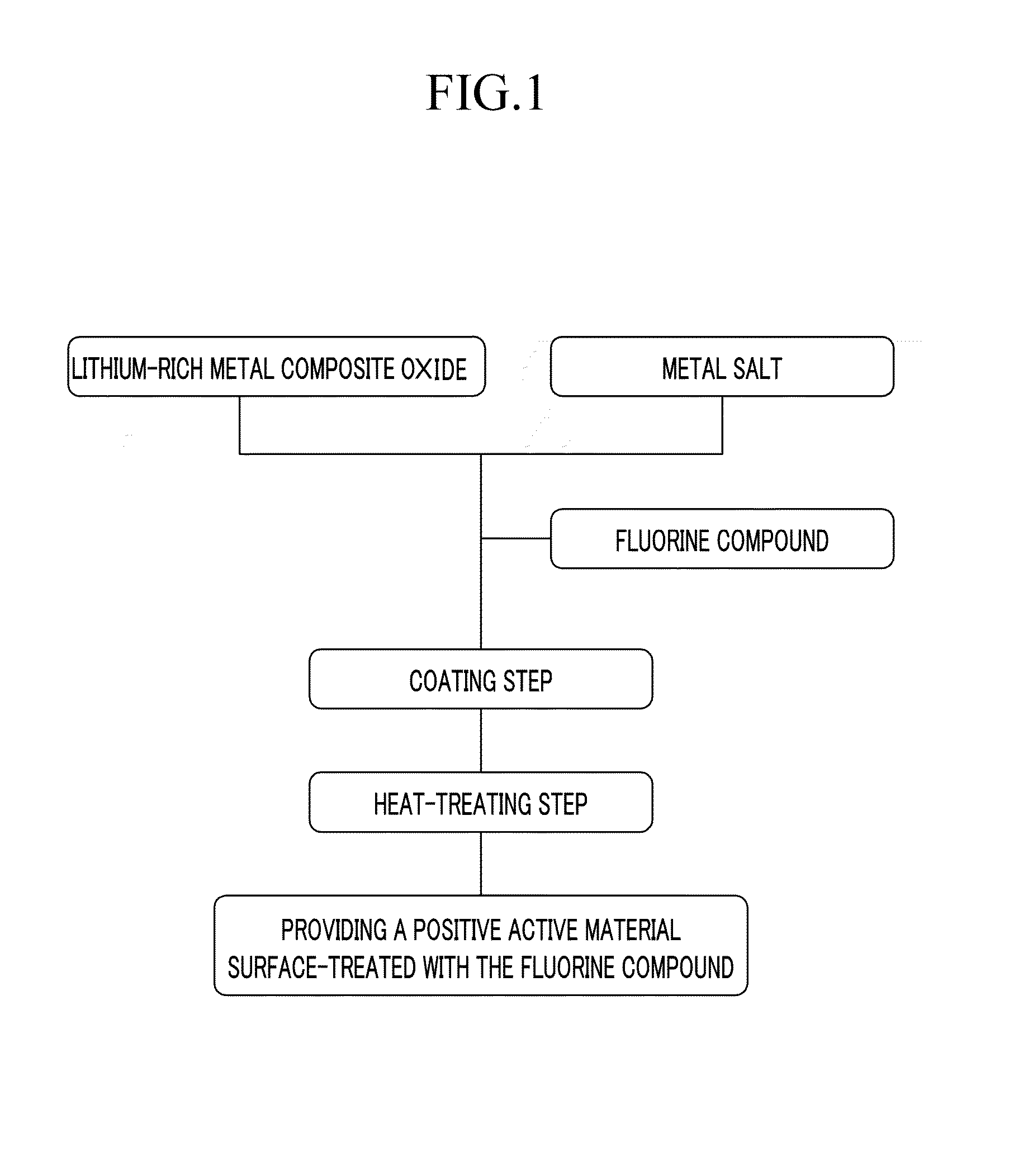
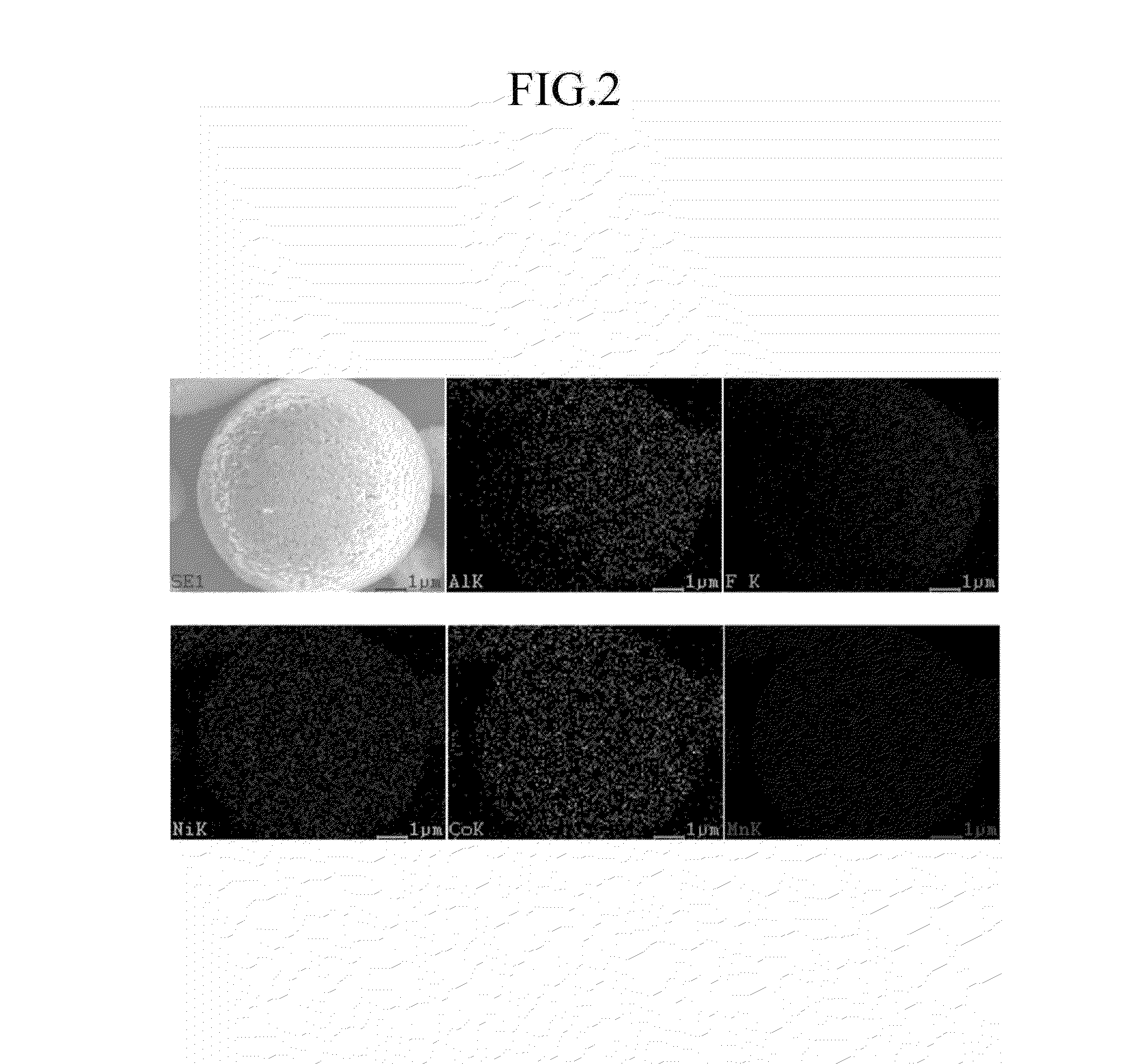
Cathode active material for a lithium secondary battery, method for manufacturing same, and lithium secondary battery including same
InactiveUS20140242463A1Improved cycle life characteristicsSuppressing deterioration and manganese elutionSilver accumulatorsElectrode thermal treatmentElectrical batteryLithium metal
The present invention provides a positive active material for a secondary lithium battery, a method of preparing the positive active material, and a secondary lithium battery including the positive active material, wherein the positive active material includes a lithium metal composite oxide core represented by the following Chemical Formula 1, and a coating layer including a fluorine compound and positioned at a shell of the lithium metal composite oxide core.LiwNixCoyMn1-x-y-zMzO2 [Chemical Formula 1](1.2≦w≦1.5, 0<x<1, 0≦y<1, 0.5≦1-x-y-z, and M is at least one metal selected from the group consisting of Al, Mg, Fe, Cu, Zn, Cr, Ag, Ca, Na, K, In, Ga, Ge, V, Mo, Nb, Si, Ti, and Zr).
Owner:KOREA ELECTRONICS TECH INST
Environment-friendly separation and recovery method of fluorine in fluorine-containing waste liquid
InactiveCN105948083AAchieve separationAchieve recyclingMagnesium fluoridesHydrogen fluorideRecovery methodEnvironmental resistance
The invention discloses an environment-friendly separation and recovery method of fluorine in a fluorine-containing waste liquid. According to the invention, a magnesium-containing compound is added into the fluorine-containing waste liquid as a precipitation agent, such that fluorine in the waste liquid is selectively precipitated; filtering is carried out, and fluorine-removed liquid and magnesium fluoride precipitate are obtained; the fluorine-removed liquid is used in waste water recycling; the magnesium fluoride precipitate is decomposed with sulfuric acid, such that a series of compounds of fluorine are obtained; decomposition residue is subjected to a dissolution-crystallization treatment, such that magnesium sulfate crystals are obtained; the obtained magnesium sulfate crystals are returned and recycled in the fluorine selective precipitation process; the crystallization mother liquor of magnesium sulfate is returned to the dissolution-crystallization process or the magnesium fluoride precipitation decomposition process. The method has the advantages of simple process, simple operation, low production cost, and good fluorine-removing effect. With the method, fluorine resource utilization is realized. The method also has the advantages of no fluorine-containing waste production and no three-waste emission.
Owner:CENT SOUTH UNIV
Process for producing bis(fluorosulfonyl)imide anion compound, and ion-pair compound
InactiveCN102046523AHigh purityLow costNitrosyl chloridePhysical/chemical process catalystsIonic liquidIon pairs
A process for producing a bis(fluorosulfonyl)imide anion compound by substituting a bis(chlorosulfonyl)imide anion compound, obtained from sulfamic acid, chlorosulfonic acid, and a halogenating agent, with fluorine is used as a process for producing a fluorine compound for use, for example, in the synthesis of battery electrolytes and ionic liquids. According to the above process, the inclusion of impurities can be reduced, and a high-purity fluorine compound of a bis(fluorosulfonyl)imide compound can be efficiently produced at a high yield. A base catalyst can be used in the reaction for substituting the bis(chlorosulfonyl)imide anion with fluorine. The base catalyst is preferably a nitrogen-containing compound.
Owner:DAI ICHI KOGYO SEIYAKU CO LTD
Waterproof, grease proof treating compound of fluorine silicon modified polyurethane, and preparation method
ActiveCN101003946AImprove mechanical stabilityAcid-base stability without limitationFibre typesLeather surface finishingFiberChemical structure
The present invention relates to a fluorosilicone modified polyurethane water-proofing oil-proofing finishing agent and its preparation method. Said invention also provides its chemical structure formula. It is characterized by that it uses perfluor-olefin, hydroxyl silicon oil, epoxyethane and diisocyanate as raw material and makes them undergo the process of synthesis treatment so as to obtain the invented emulsion-fluorosilicone modified polyurethane water-proofing oil-proofing finishing agent.
Owner:SHANDONG DONGYUE POLYMER MATERIAL
Adhesive composition
An adhesive composition comprises (A) a linear polyfluoro compound containing alkenyl groups and having a perfluoropolyether structure backbone, (B) a fluorine-bearing organohydrogensiloxane, (C) a platinum compound, (D) a hydrophobic silica powder, (E) an isocyanurate having an epoxy and / or trialkoxysilyl group, (F) an organosiloxane containing an SiH group and an epoxy and / or trialkoxysilyl group, and (G) a carboxylic anhydride. The composition cures into a fluoroelastomer having excellent characteristics and achieves a firm adhesion to a broad range of metal and plastic substrates by brief heating at relatively low temperatures.
Owner:SHIN ETSU CHEM IND CO LTD
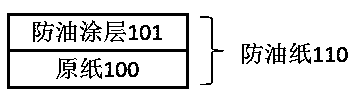
Oil-proof paper and production method thereof
ActiveCN103669105AGood oil resistancePromote degradationNon-fibrous pulp additionPaper coatingPlasticizerProcess engineering
The invention discloses oil-proof paper and a production method thereof, and belongs to the technical field of oil-proof paper. The production method comprises the following steps: coating or sizing the base paper with composite oil-proof paint; and drying to obtain the finished paper, wherein the composite oil-proof paint comprises the following components in parts by weight: 100 parts of starch, 10-40 parts of a plasticizer, 5-100 parts of a coalescing agent and 1-30 parts of a fluorine-containing compound; the solid content of the paint is 5-15%. The composite oil-proof paint comprises the starch, the plasticizer, the coalescing agent and the fluorine-containing compound. The oil-proof paper disclosed by the invention has the advantages of relatively low cost and excellent oil-proof property.
Owner:广东悦声纸业科技有限公司
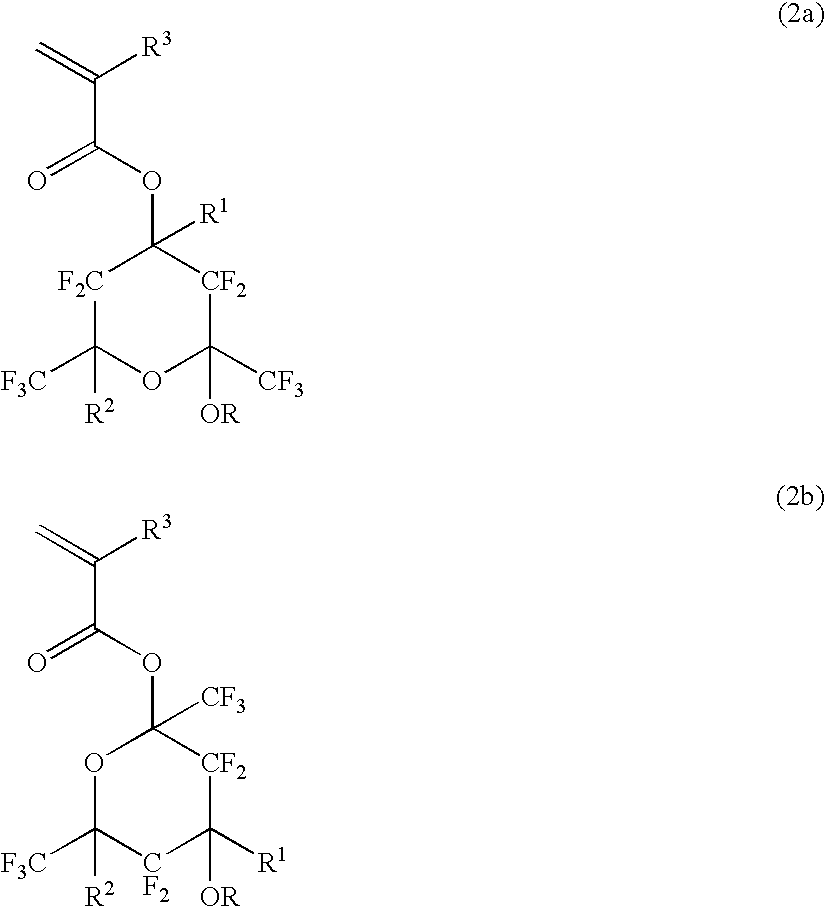
Polymerizable fluorinated compound, making method, polymer, resist composition and patterning process
ActiveUS20060094817A1High resolutionMinimized etch resistanceOrganic compound preparationPhotosensitive materialsResistPolymer science
A polymerizable fluorinated compound having formula (2a) or (2b) wherein R1 and R2 are H or C1-C20 alkyl or fluoroalkyl, R3 is H, F or C1-C4 alkyl or fluoroalkyl, and R is H or a protective group is polymerized into a fluorinated polymer which is used as a base polymer to formulate a resist composition having transparency to laser light of wavelength≦300 nm, alkali development amenability, and dry etch resistance.
Owner:SHIN ETSU CHEM IND CO LTD
Process for selectively etching dielectric layers
InactiveUS6905968B2High dielectric constantReduce capacitanceSemiconductor/solid-state device manufacturingNitrogenSilicon oxide
A method is provided for etching a dielectric structure. The dielectric structure comprises: (a) a layer of undoped silicon oxide or F-doped silicon oxide; and (b) a layer of C,H-doped silicon oxide. The dielectric structure is etched in a plasma-etching step, which plasma-etching step is conducted using a plasma source gas that comprises nitrogen atoms and fluorine atoms. As one example, the plasma source gas can comprise a gaseous species that comprises one or more nitrogen atoms and one or more fluorine atoms (e.g., NF3). As another example, the plasma source gas can comprise (a) a gaseous species that comprises one or more nitrogen atoms (e.g., N2) and (b) a gaseous species that comprises one or more fluorine atoms (e.g., a fluorocarbon gas such as CF4). In this etching step, the layer of C,H-doped silicon oxide is preferentially etched relative to the layer of undoped silicon oxide or F-doped silicon oxide. The method of the present invention is applicable, for example, to dual damascene structures.
Owner:APPLIED MATERIALS INC
Etching composition for metal material and method for manufacturing semiconductor device by using same
ActiveUS20100216315A1Total current dropEasy to driveSemiconductor/solid-state device manufacturingSurface treatment compositionsDielectricMetallic materials
The invention provides an etchant composition employed for selectively etching a metallic material in production of a semiconductor device from an insulating material having high dielectric constant, an insulating material of silicon oxide film or silicon nitride film, and a metallic material, characterized in that the etchant composition is an aqueous solution containing a fluorine compound, and a chelating agent having, in the molecular structure thereof, a phosphorus oxo-acid as a functional group; or is an aqueous solution containing a fluorine compound, a chelating agent having, in the molecular structure thereof, a phosphorus oxo-acid as a functional group, and an inorganic acid and / or an organic acid. The invention also provides a method for producing a semiconductor device employing the etchant composition. According to the invention, a metallic material can be etched selectively and efficiently.
Owner:MITSUBISHI GAS CHEM CO INC
Method for preparing array substrate for liquid crystal display device
ActiveCN103052907AEnvironmental friendlyPrevent poor wiringSolid-state devicesNon-linear opticsCrystallographyOrganic acid
The present invention relates to preparation method for an array substrate for the use in a liquid crystal display device, using an etchant composition comprising: a) 5-25 wt% of hydrogen peroxide (H2O2); b) 0.1-5 wt% of an organic acid; c) 0.1-5 wt% of a phosphate compound; d) 0.1-5 wt% of a water-soluble cyclic amine compound; e) 0.1-5 wt% of a water-soluble compound having a nitrogen atom and a carboxyl group in a molecule; f) 0.01-1.0 wt% of a fluorine-containing compound; g) 0.001-5 wt% of a polyhydric alcohol-based surfactant; and h) the balance of water based on the total weight of the composition.
Owner:DONGWOO FINE CHEM CO LTD
Semiconductor device having improved insulation film and manufacturing method thereof
A semiconductor device which has an interlayer insulating film comprised of molecules with silicon-oxygen bonds and silicon-fluorine bonds and contains a rare gas in concentration higher than 1011 atoms per cm2. The interlayer insulating film is preferably a fluorine-containing silicon oxide film which contains a rare gas. In a manufacturing process, an interlayer insulating is formed by a chemical vapor deposition from a material gas including a silicon-containing gas, a fluorine compound gas, a rare gas, and oxygen. The silicon-containing gas is preferably SiH4 gas, and the fluorine compound gas is preferably SiF4 gas. The flow rate of the rare gas is greater than three times the total flow rate of the SiH4 gas and SiF4 gas. The rare gas is at least one type of gas selected from neon (Ne), argon (Ar), krypton (Kr), and xenon (Xe).
Owner:MITSUBISHI ELECTRIC CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com