Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53results about "Uranium fluorides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
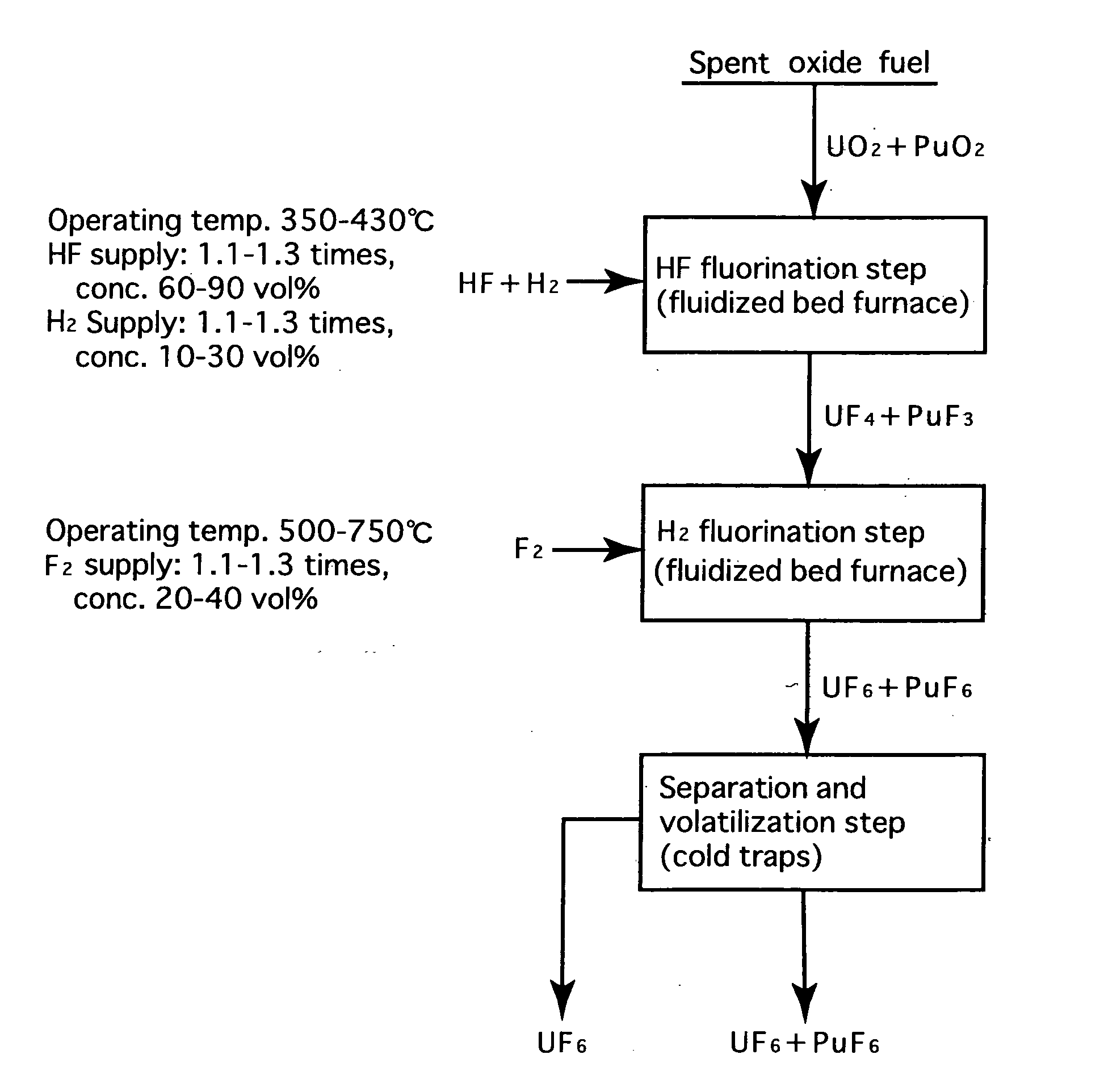
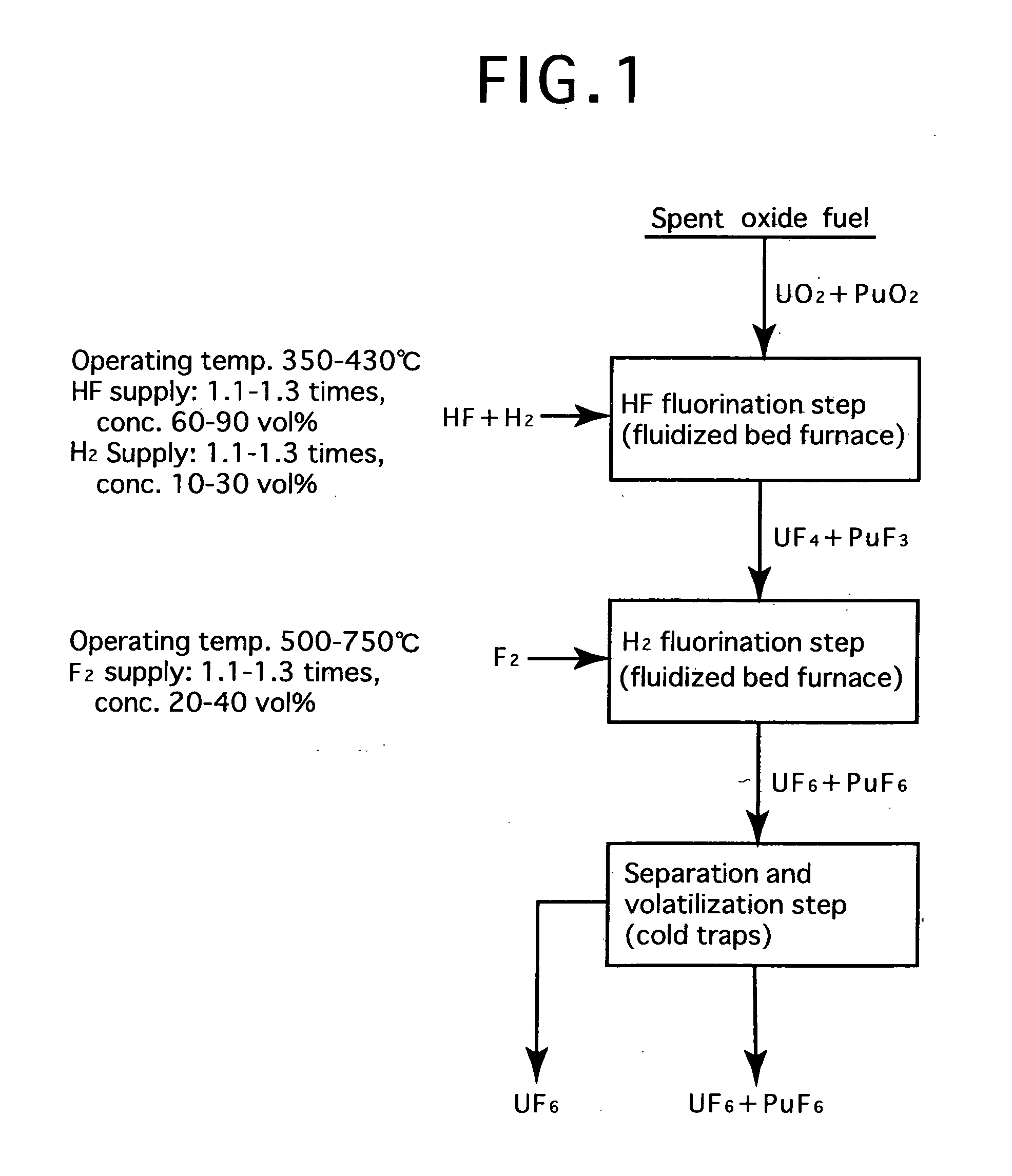
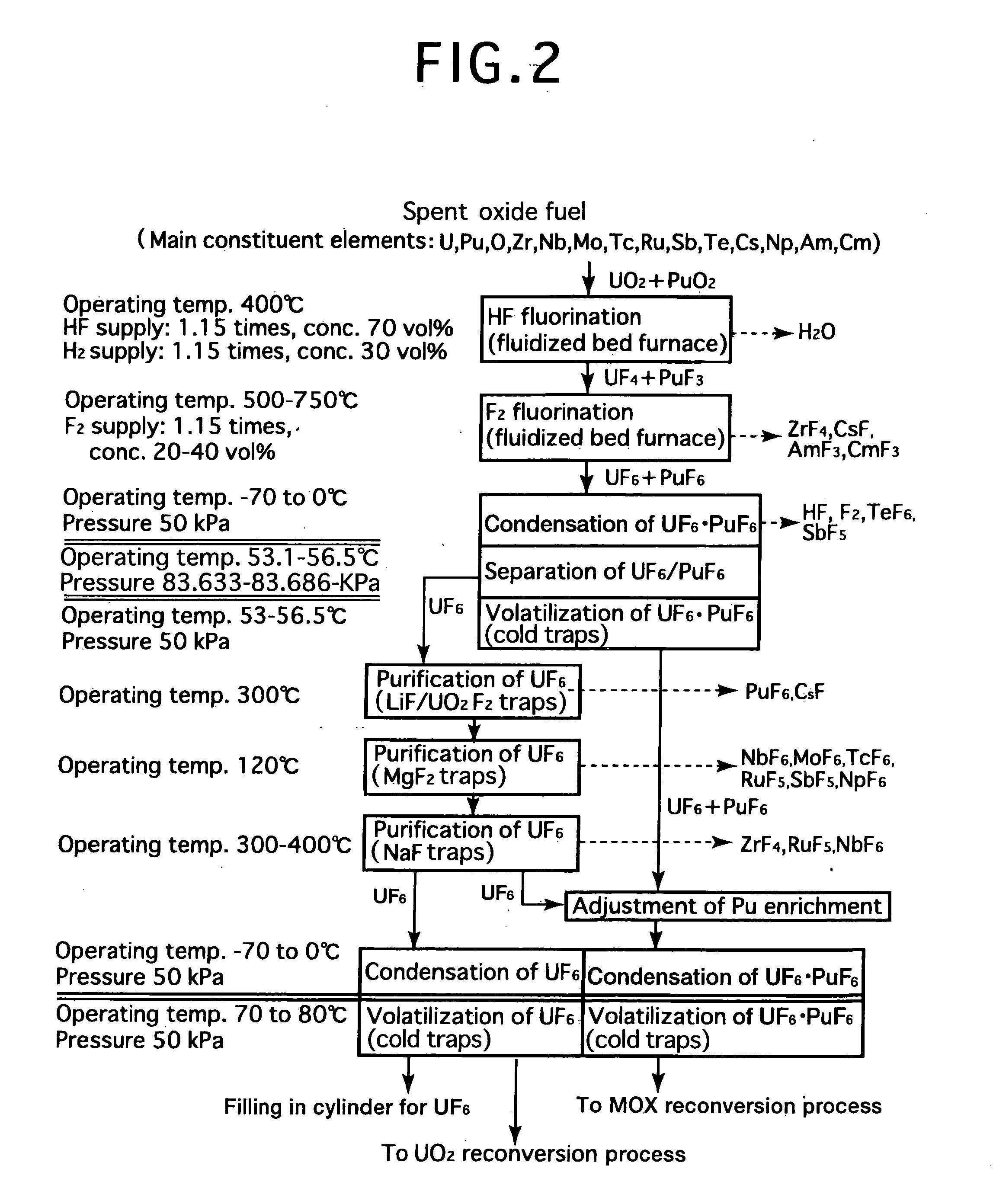
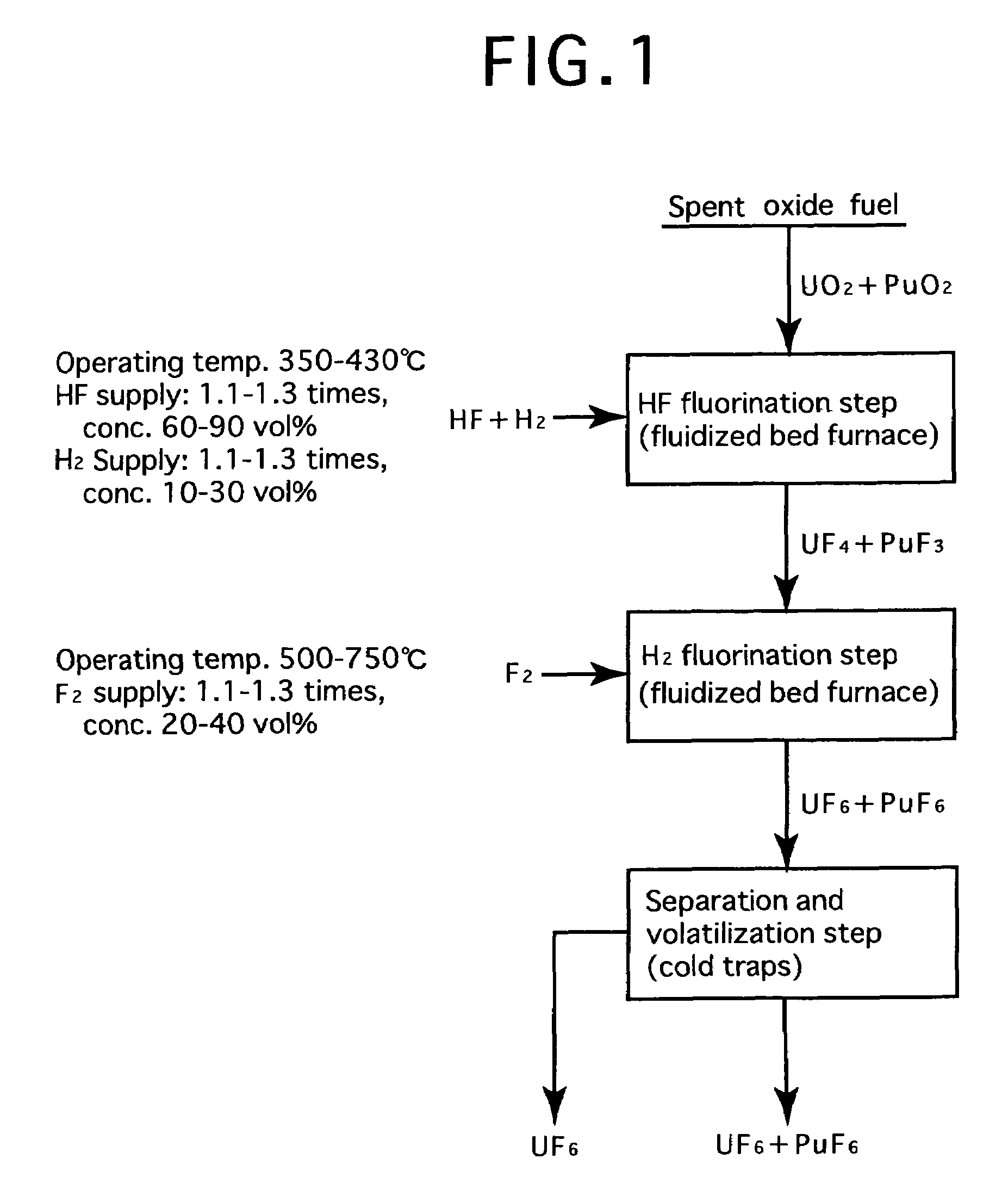
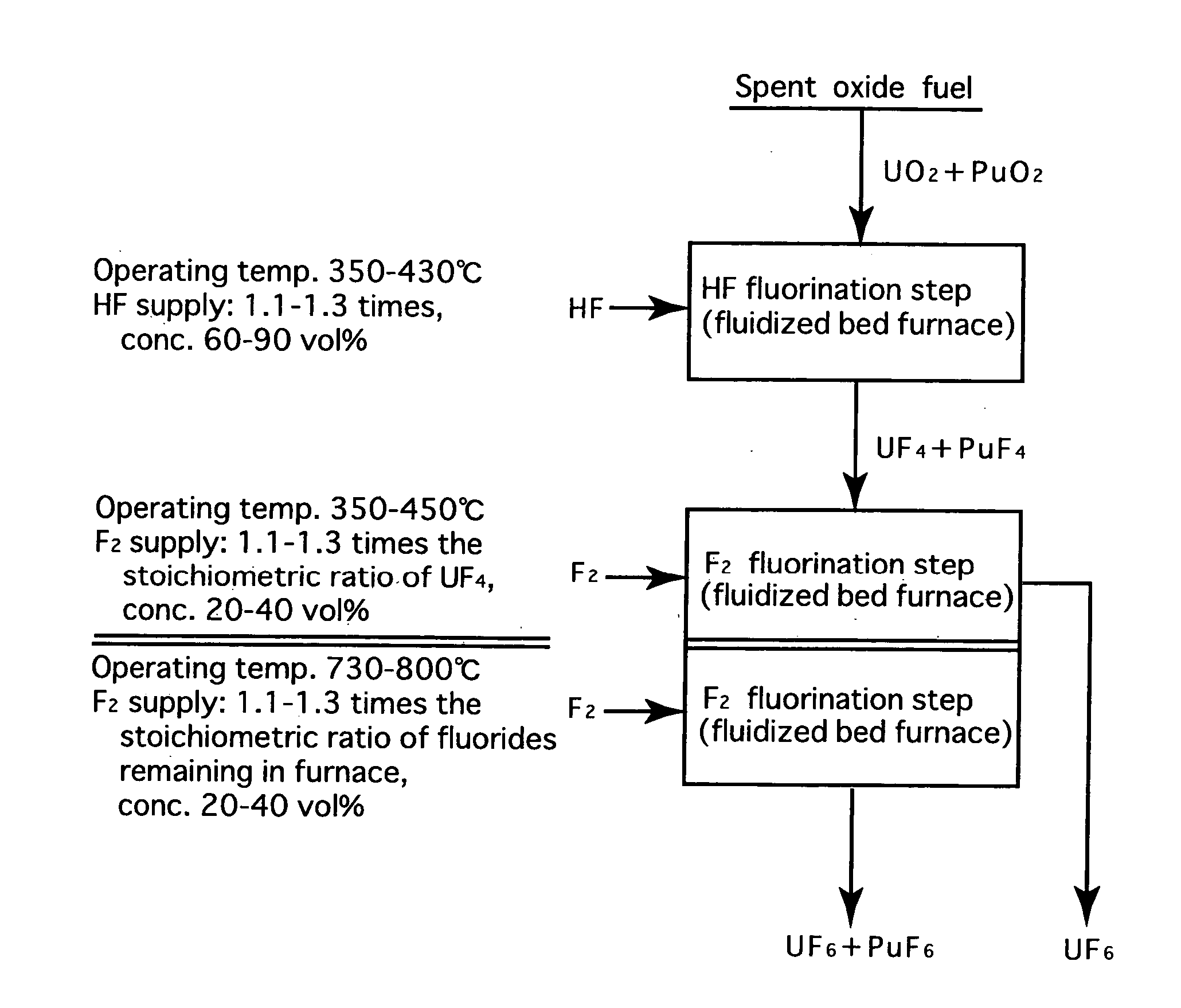
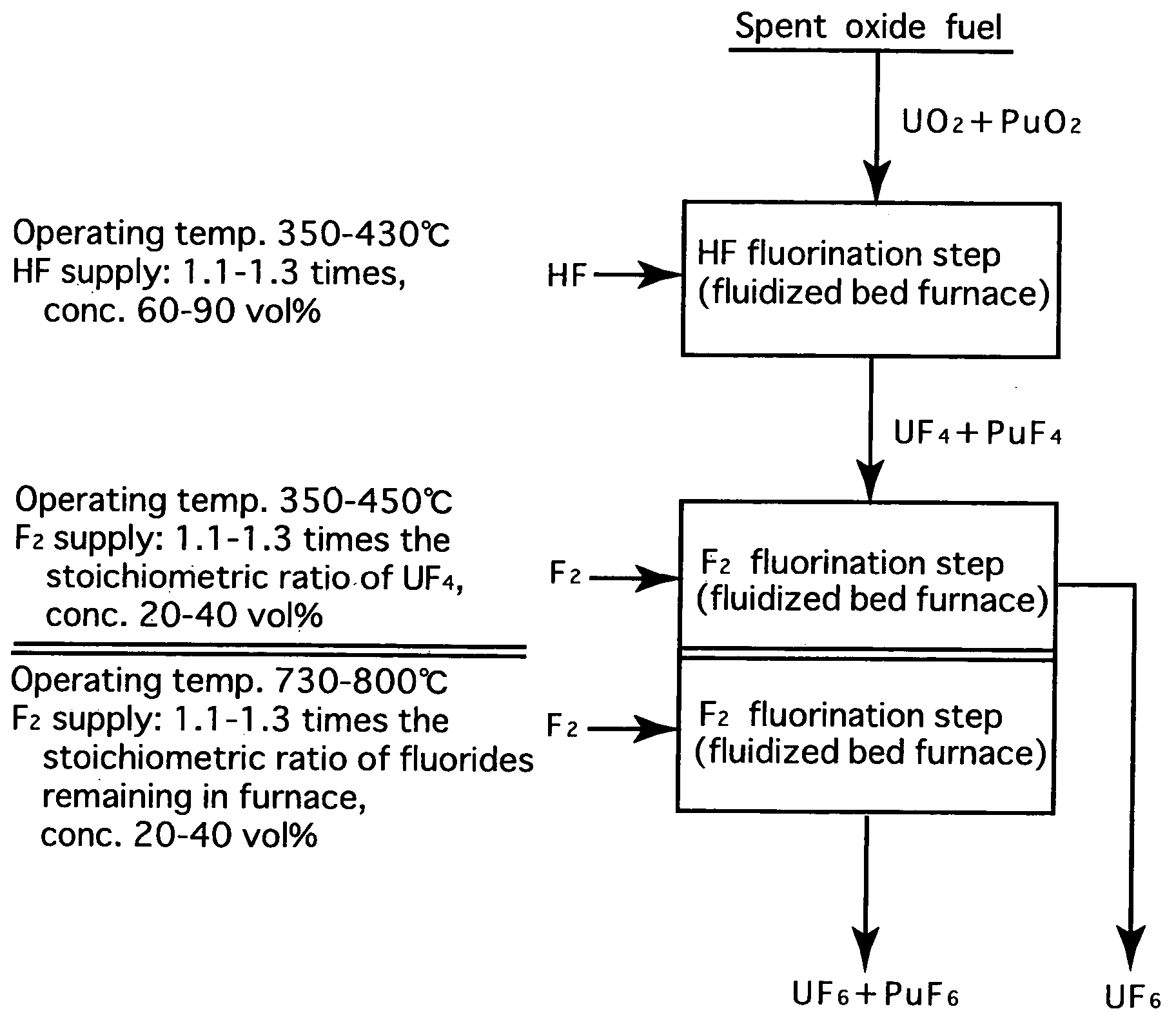
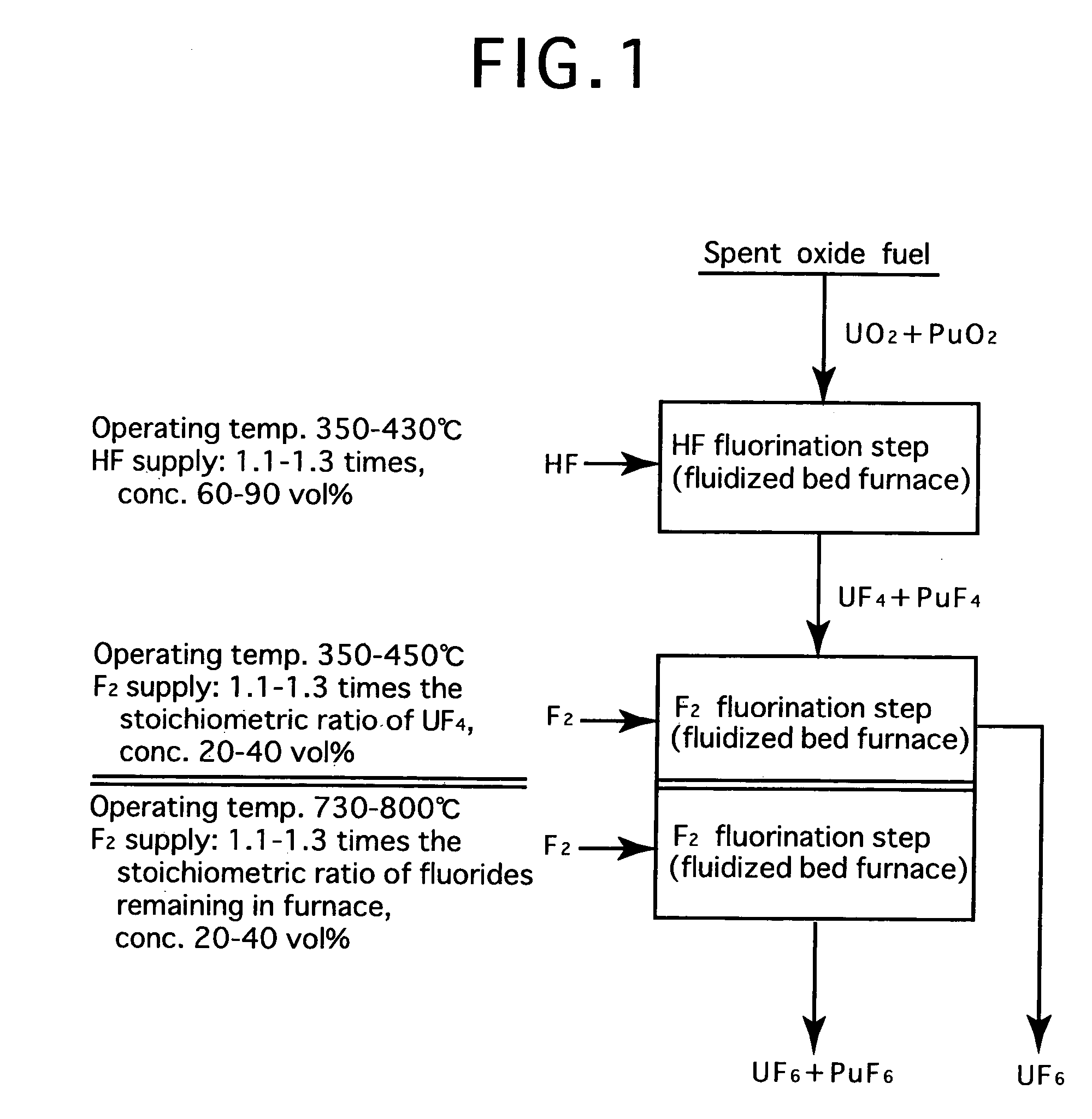
Reprocessing method by fluoride volatility process using fractional distillation
InactiveUS20060057042A1Increase ratingsRaise the ratioPlutonium compoundsUranium fluoridesHydrogen fluorideHydrogen
Fluorine or a fluorine compound is subjected to a reaction with a spent oxide fuel to produce fluorides of uranium and plutonium, and recovering the fluorides using a difference in volatility behavior. The method includes steps of: subjecting a mixture of UO2 and PuO2 with hydrogen fluoride mixed with hydrogen to HF-fluorinate uranium and plutonium into UF4 and PuF3; subjecting UF4 and PuF3 with a fluorine gas to F2-fluorinate uranium and plutonium into UF6 and PuF6; and fractionating UF6 and PuF6 using a difference in phase change of obtained UF6and PuF6, removing a part of UF6, and volatilizing the remaining UF6 and PuF6 at the same time. By such a reprocessing method, PuF4 hard to undergo a reaction is prevented from being formed as an intermediate fluoride, the material of a reactor is hard to be corroded, and a consumption of expensive fluorine gas is reduced.
Owner:JAPAN ATOMIC ENERGY AGENCY INDEPENDANT ADMINISTRATIVE CORP
Method of separating uranium from irradiated nuclear fuel
InactiveUS6960326B1Simplified cutting stepsReduce stepsTransuranic element compoundsNuclear energy generationHydrogen fluorideFluoride
The invention provides a method of separating uranium from at least fission products in irradiated nuclear fuel, said method comprising reacting said irradiated nuclear fuel with a solution of ammonium fluoride in hydrogen fluoride fluorinating said reacted irradiated nuclear fuel to form a volatile uranium fluoride compound and separating said volatile uranium fluoride compound from involatile fission products. The invention thus provides a reprocessing scheme for irradiated nuclear fuel. The method is also capable of reacting, and breaking down Zircaloy cladding and stainless steel assembly components. Thus, whole fuel elements may be dissolved as one thereby simplifying procedures over conventional Purex processes.
Owner:NEXIA SOLUTIONS
Reprocessing method by fluoride volatility process using fractional distillation
InactiveUS7323153B2Reduce consumptionNot easy to corrodePlutonium compoundsUranium fluoridesHydrogen fluorideFluoride volatility
Fluorine or a fluorine compound is subjected to a reaction with a spent oxide fuel to produce fluorides of uranium and plutonium, and recovering the fluorides using a difference in volatility behavior. The method includes steps of: subjecting a mixture of UO2 and PuO2 with hydrogen fluoride mixed with hydrogen to HF-fluorinate uranium and plutonium into UF4 and PuF3; subjecting UF4 and PuF3 with a fluorine gas to F2-fluorinate uranium and plutonium into UF6 and PuF6; and fractionating UF6 and PuF6 using a difference in phase change of obtained UF6 and PuF6, removing a part of UF6, and volatilizing the remaining UF6 and PuF6 at the same time. By such a reprocessing method, PuF4 hard to undergo a reaction is prevented from being formed as an intermediate fluoride, the material of a reactor is hard to be corroded, and a consumption of expensive fluorine gas is reduced.
Owner:JAPAN ATOMIC ENERGY AGENCY INDEPENDANT ADMINISTRATIVE CORP
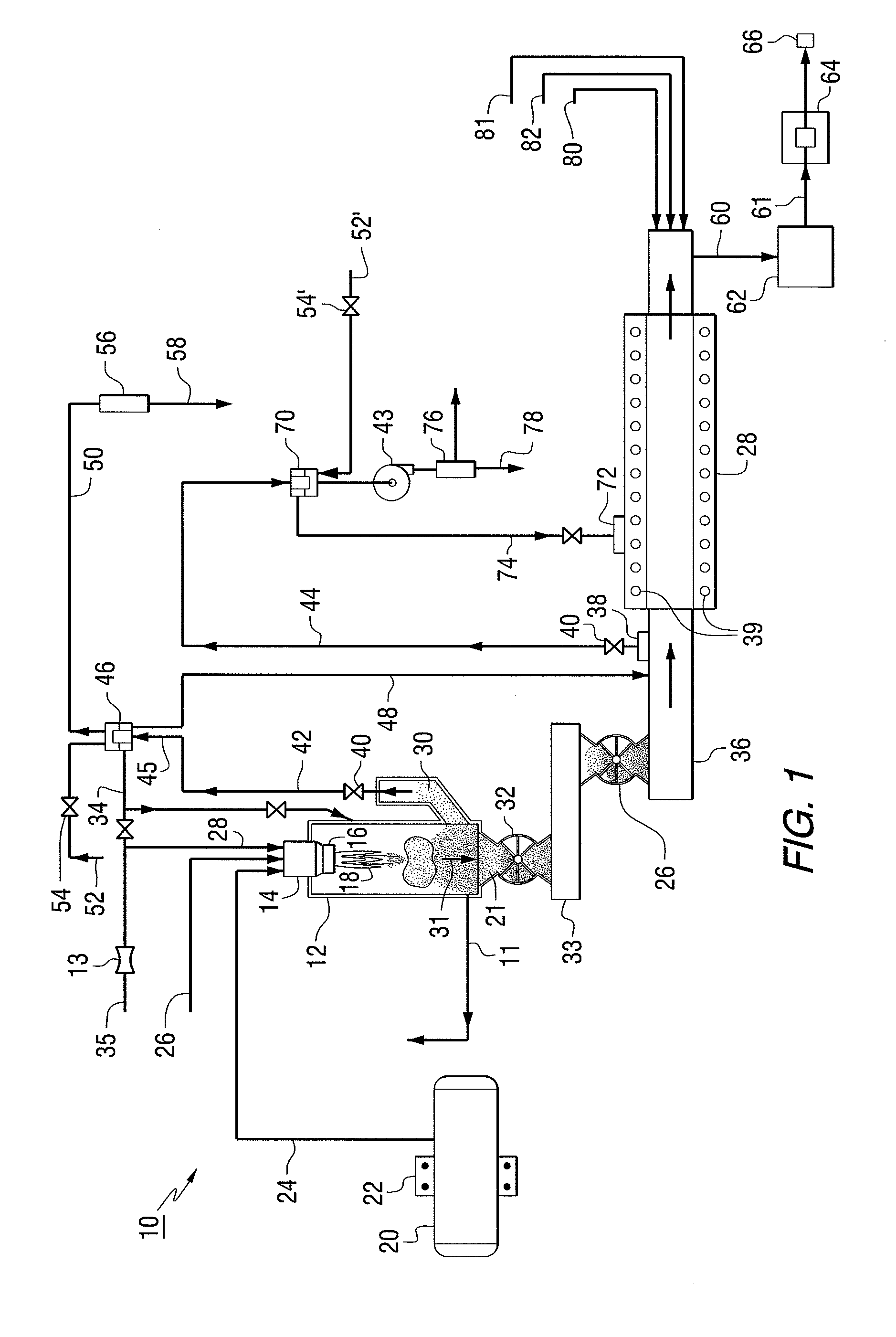
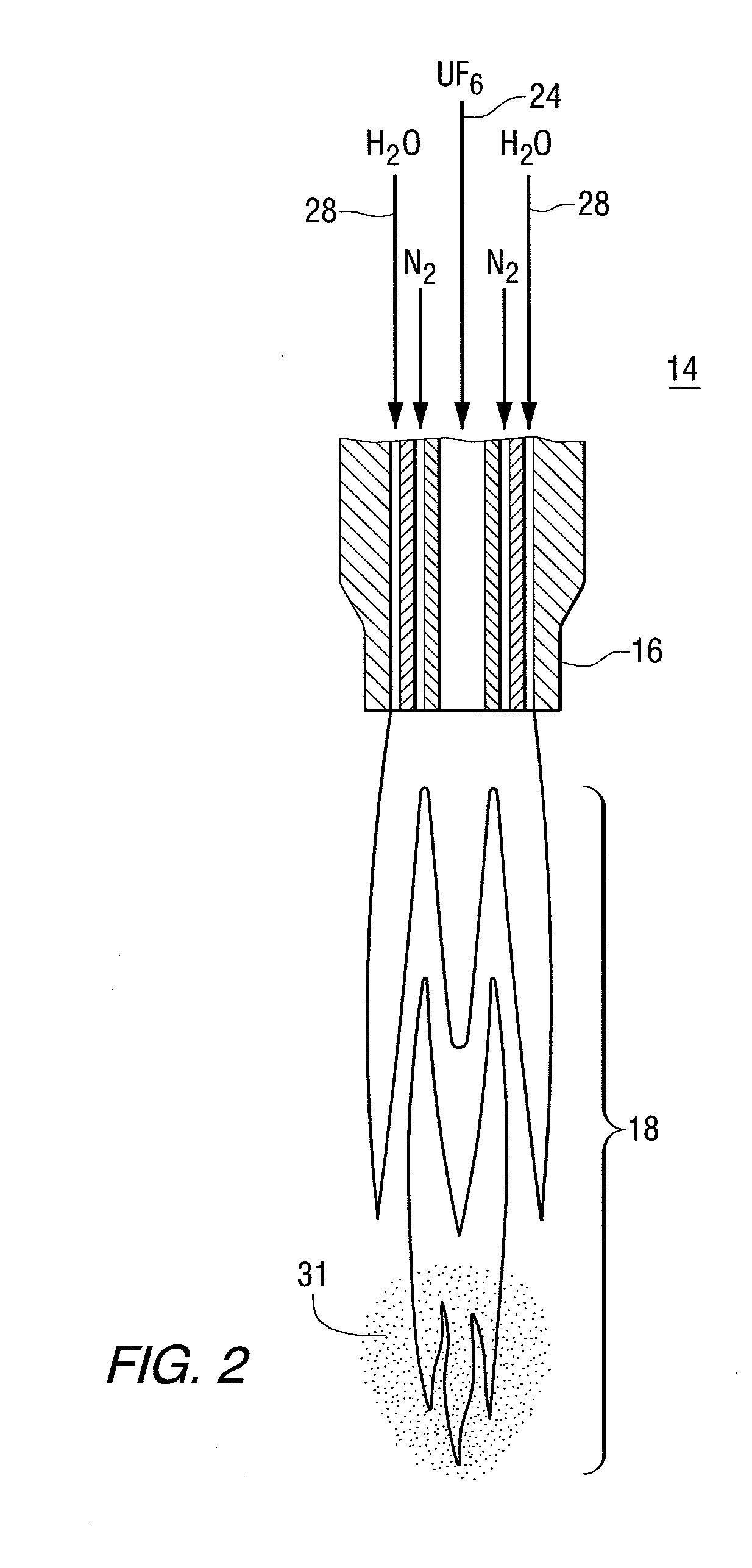
Method and device for declogging filter
A method of declogging at least one filter of a plant for manufacturing uranium oxide from uranium hexafluoride, including separating, from the wall of the filter, uranium oxyfluoride particles deposited, by a stream of inert gas such as nitrogen, injected into the filter, in a counter-currentwise direction to the flow of hydrofluoric acid.
Owner:AREVA NP SAS
Two step dry UO2 production process utilizing a positive sealing valve means between steps
ActiveUS7824640B1Inhibition amountHigh densityTransuranic element compoundsNuclear energy generationTemperature controlNuclear grade
The present invention provides a two-step process for producing nuclear grade, active uranium dioxide (UO2) powder in which the first step comprises reacting uranium hexafluoride (UF6) with steam in a flame reactor to yield uranyl fluoride (UO2F2); and the second step comprises removing fluoride and reducing UO2F2 to uranium dioxide (UO2) in a kiln under a steam / hydrogen atmosphere. The two-step process, each step separated by a positive sealed valve means to prevent gas, particularly H2 flow back, tightly controls the exothermicity of the reaction, which allows for a very tight temperature control which controls the growth of the particles and results in UO2 powder that is active and of consistent morphology.
Owner:WESTINGHOUSE ELECTRIC CORP
Low temperature route to uranium nitride
A method of preparing an actinide nitride fuel for nuclear reactors is provided. The method comprises the steps of a) providing at least one actinide oxide and optionally zirconium oxide; b) mixing the oxide with a source of hydrogen fluoride for a period of time and at a temperature sufficient to convert the oxide to a fluoride salt; c) heating the fluoride salt to remove water; d) heating the fluoride salt in a nitrogen atmosphere for a period of time and at a temperature sufficient to convert the fluorides to nitrides; and e) heating the nitrides under vacuum and / or inert atmosphere for a period of time sufficient to convert the nitrides to mononitrides.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
Method for producing uranium oxide from uranium oxyfluoride
InactiveUS6096281AReduced thermodynamic stabilityAvoid vaporizationPhosphorus halides/oxyhalidesFluoride preparationUranium oxideOxidizing agent
A method for producing uranium oxide includes combining uranium oxyfluoride and a solid oxidizing agent having a lower thermodynamic stability than the uranium oxide after "oxide"; heating the combination below the vapor point of the uranium oxyfluoride to sufficiently react the uranium oxyfluoride and the oxidizing agent to produce uranium oxide and a non-radioactive fluorine compound; and removing the fluorine compound after "compound".
Owner:INT ISOTOPES
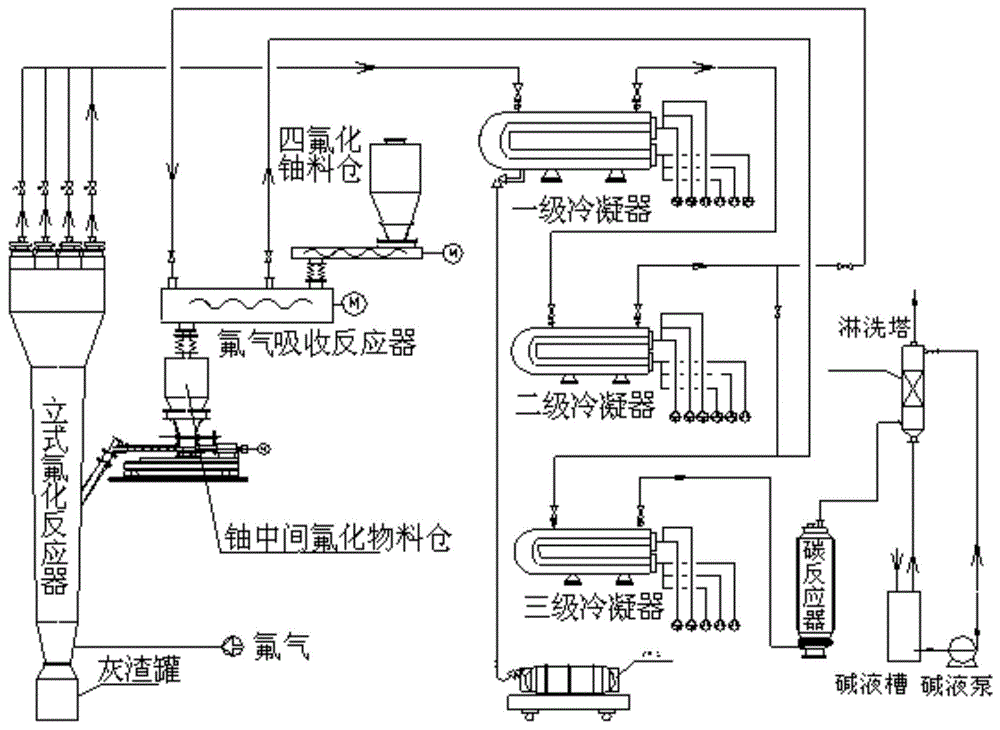
Fluorination technology for recovering fluorine gas
ActiveCN104628038AIncrease profitImprove absorption efficiencyUranium fluoridesUranium hexafluorideMaterial transfer
The invention discloses a fluorination technology for recovering fluorine gas. The method comprises the steps of fluorination and fluorine gas absorption. In the invention, a fluorine gas absorption reactor is abutted to a fluorination reactor, the solid material feeding end of absorption reactor is directly used as a system material inlet end, and the solid material inlet end of the fluorination reactor is substituted by the material outlet end of the fluorine gas absorption reactor, so a material transfer process is omitted. Compared with foreign fluorination technologies, the method has the advantages of short process flow and simple operation control. The technical scheme of the method adapts to the self characteristic of the technical route of uranium hexafluoride production technologies in China, and can be realized on the basis of original mature process flow without complex change.
Owner:THE 404 CO LTD CHINA NAT NUCLEAR
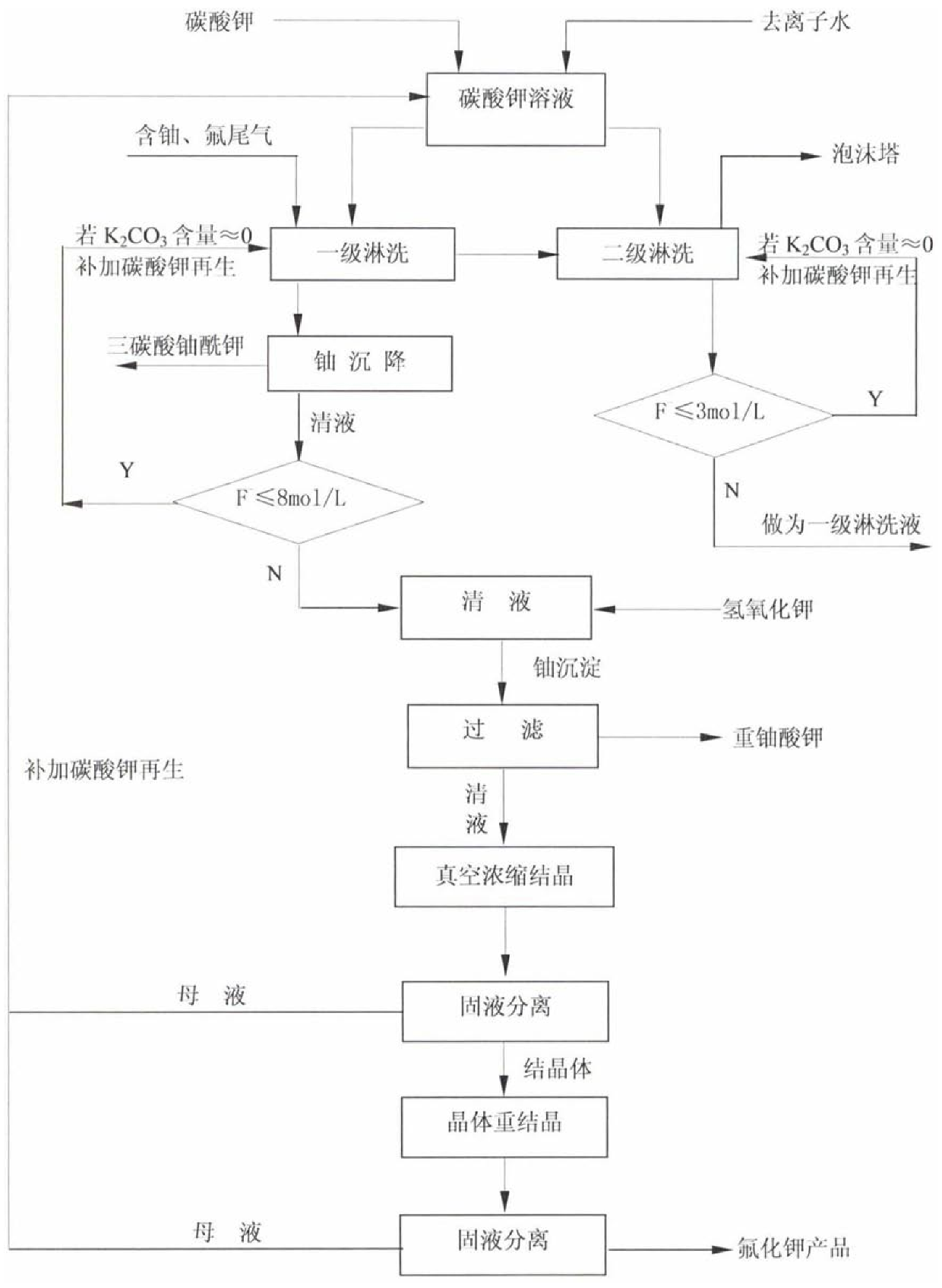
Uranium and fluorine-containing tail gas leaching and eluent regeneration process
ActiveCN106508071BSave resourcesTo achieve the goal of "turning waste into treasure"Uranium fluoridesDispersed particle separationChemical industryFiltration
The invention relates to a process for treating uranium and fluorine-containing tail gas and waste liquid in the field of uranium chemical industry, in particular to a uranium- and fluorine-containing tail gas leaching and eluent regeneration process, which is carried out in sequence as follows: (1) K2CO3 solution is used for leaching Tail gas containing uranium and fluorine; (2) Potassium uranyl tricarbonate is separated by sedimentation. If the mass percentage concentration of K2CO3 in the resulting clear liquid is ≈0 and the concentration of KF is >8mol / l, add KOH to the clear liquid to generate diuranic acid Potassium precipitation; (3) Potassium diuranate precipitate is separated by filtration, and the obtained clear liquid is crystallized, and the crystal is KF crystal. The process of the invention can reduce the impact of radioactive waste on the environment, and has good social benefits; the closed loop of the eluent avoids the loss of uranium elements, saves resources, and recycles fluorine ions in the form of KF by-products to achieve It achieves the purpose of "turning waste into treasure" and has certain economic benefits.
Owner:CNNC LANZHOU URANIUM ENRICHMENT
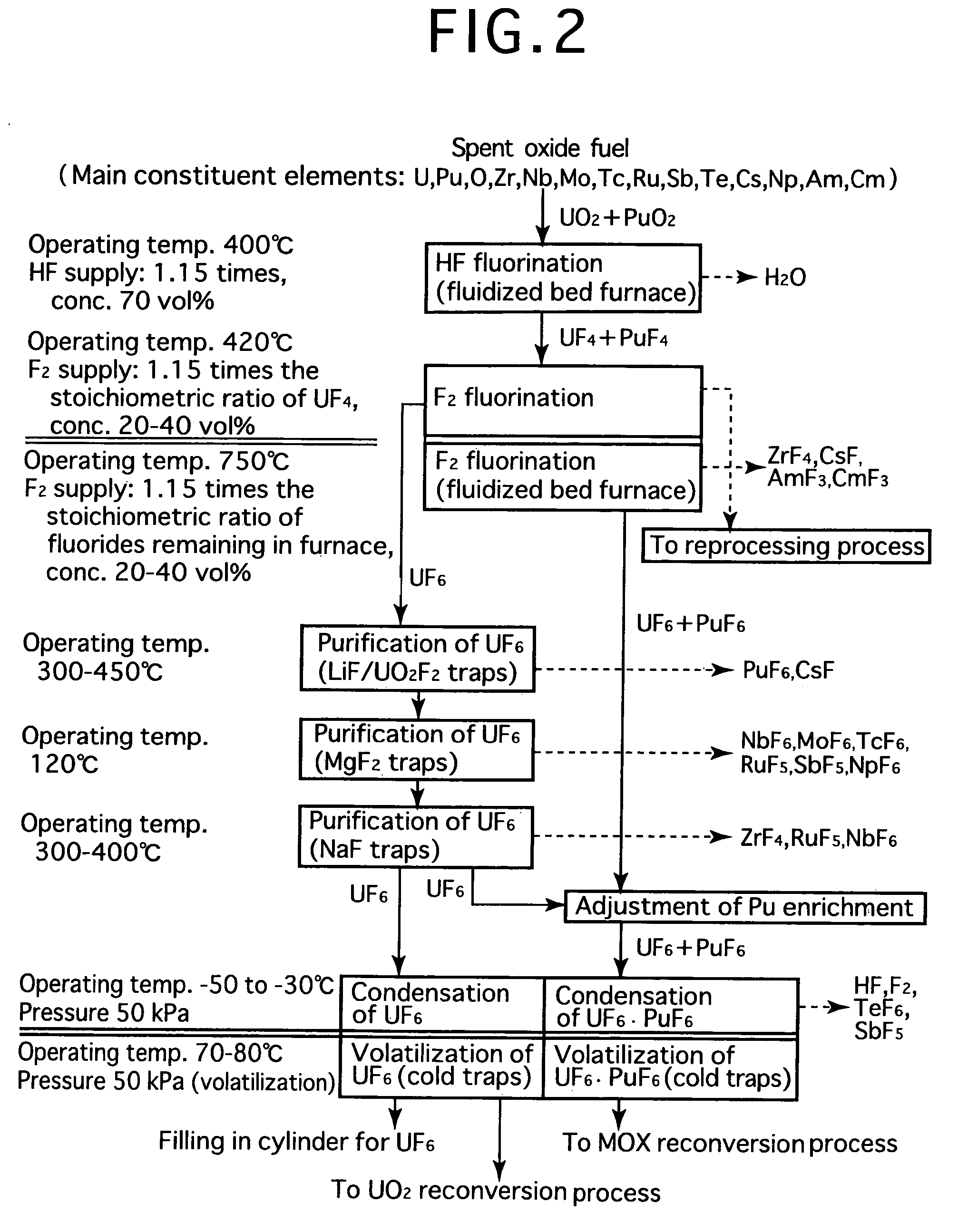
Reprocessing method by fluoride volatility process using solid-gas separation
InactiveUS20060057043A1Simple stepsEasy to separatePlutonium compoundsFluoride preparationFluoride volatilityHexafluoride
Fluorine or a fluorine compound is subjected to a reaction with a spent oxide fuel to produce fluorides of uranium and plutonium, and the fluorides are recovered using a difference in volatility behavior. The spent oxide fuel is subjected to a reaction with an HF gas, whereby uranium, plutonium and most impurities are converted into solid fluorides having low valences or remained as oxides to inhibit volatilization thereof, and then in an F2 fluorination step, the HF fluorination product is subjected to a reaction with a fluorine gas in two stages: one at a low temperature and the other at a high temperature, whereby a certain amount of gaseous uranium and volatile impurities are separated with plutonium kept in a solid form in the first stage, and mixed fluorides of remaining uranium and plutonium are fluorinated into hexafluorides at the same time in the second stage. By such a reprocessing method, plutonium enrichment can be adjusted, uranium and plutonium can be purified, and steps are simplified as well. In addition, reactors are hard to be corroded or deteriorated.
Owner:JAPAN ATOMIC ENERGY AGENCY INDEPENDANT ADMINISTRATIVE CORP
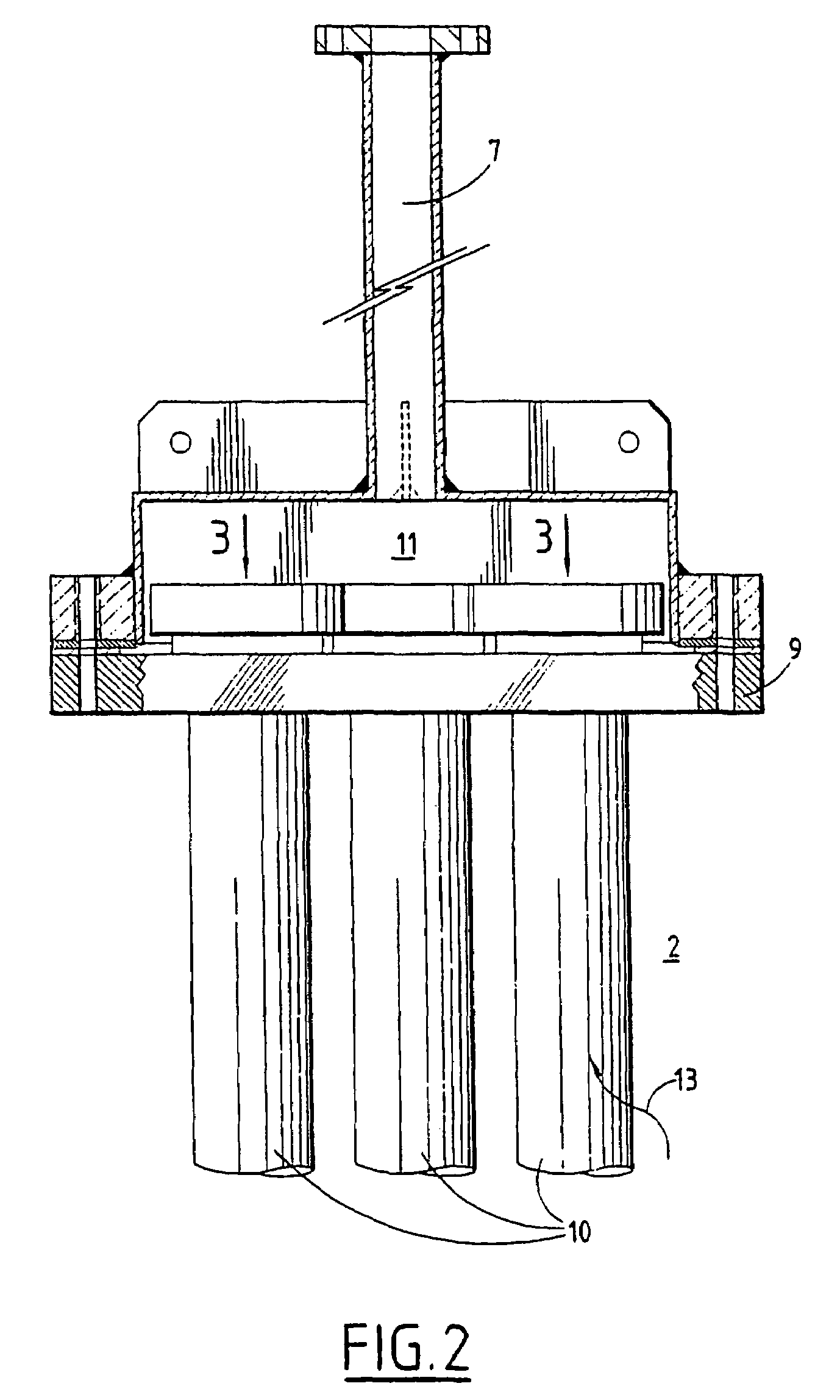
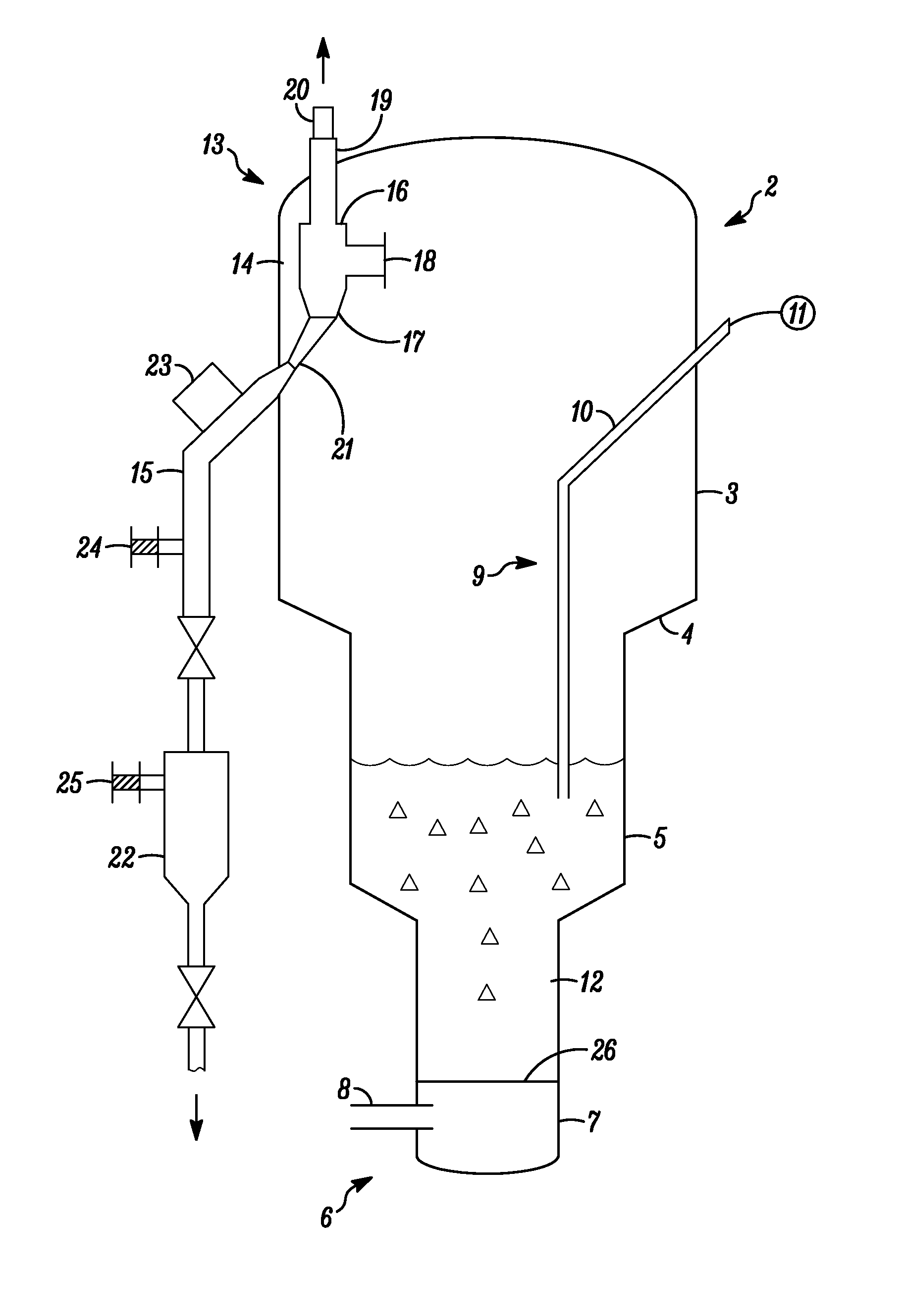
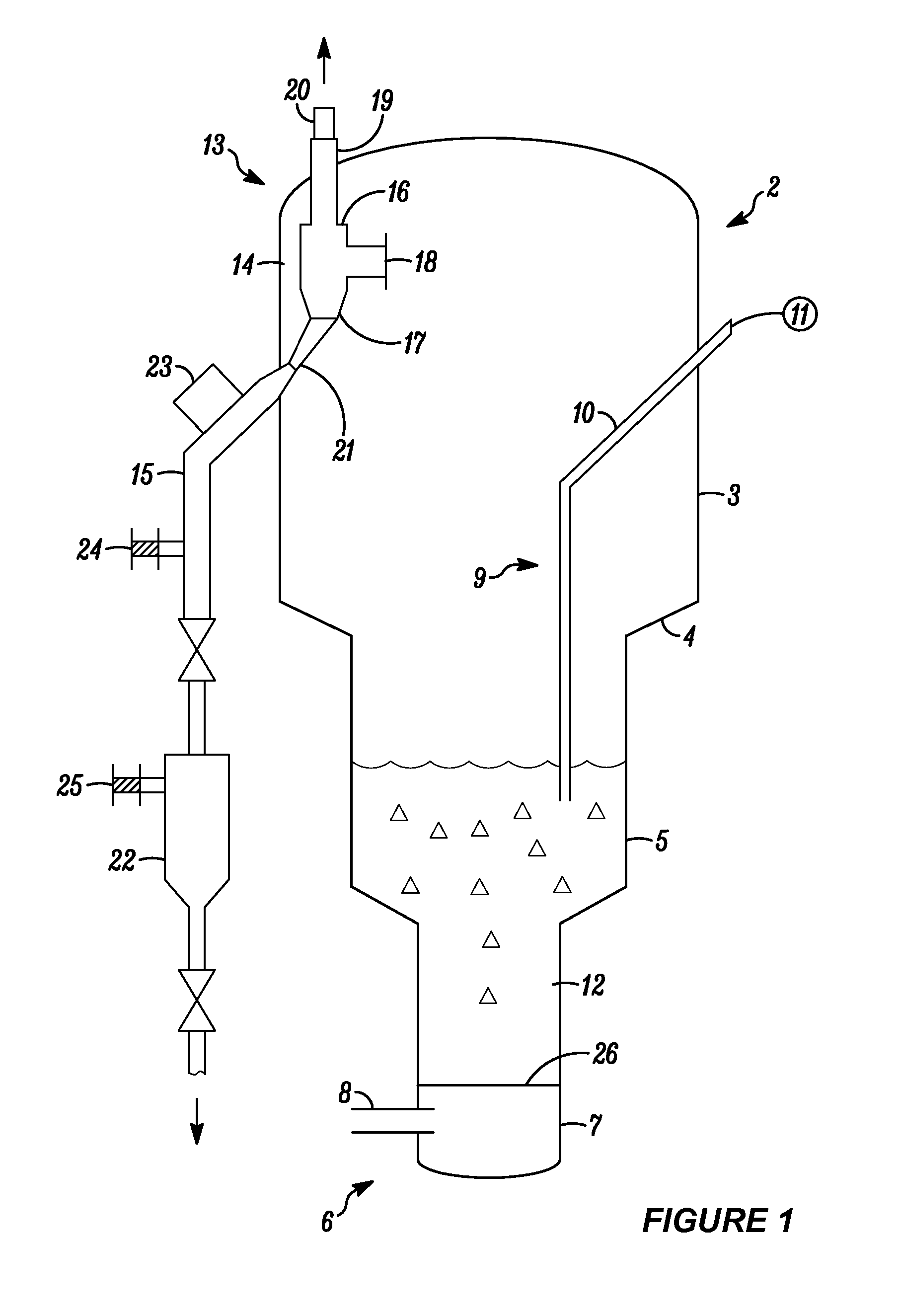
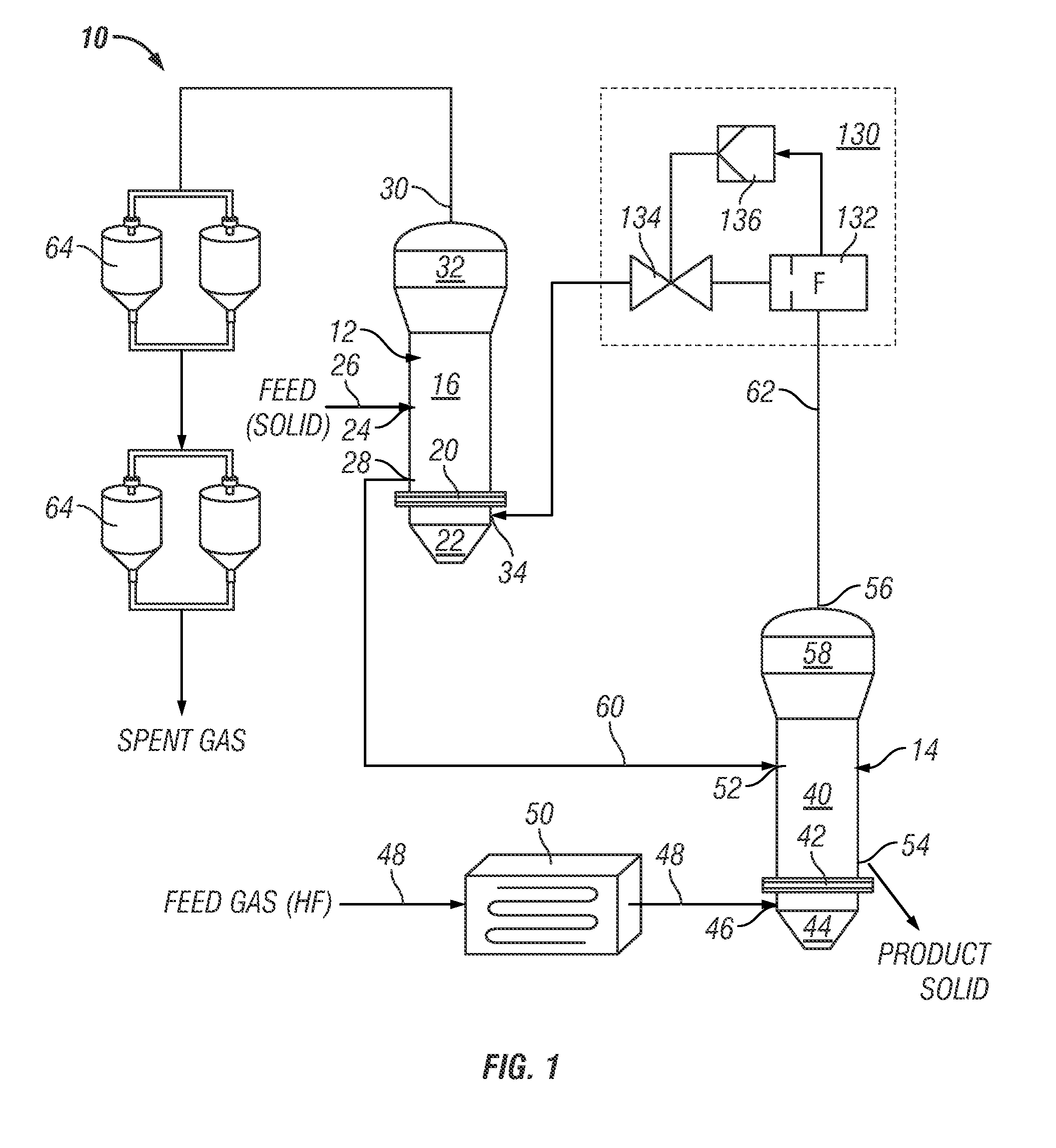
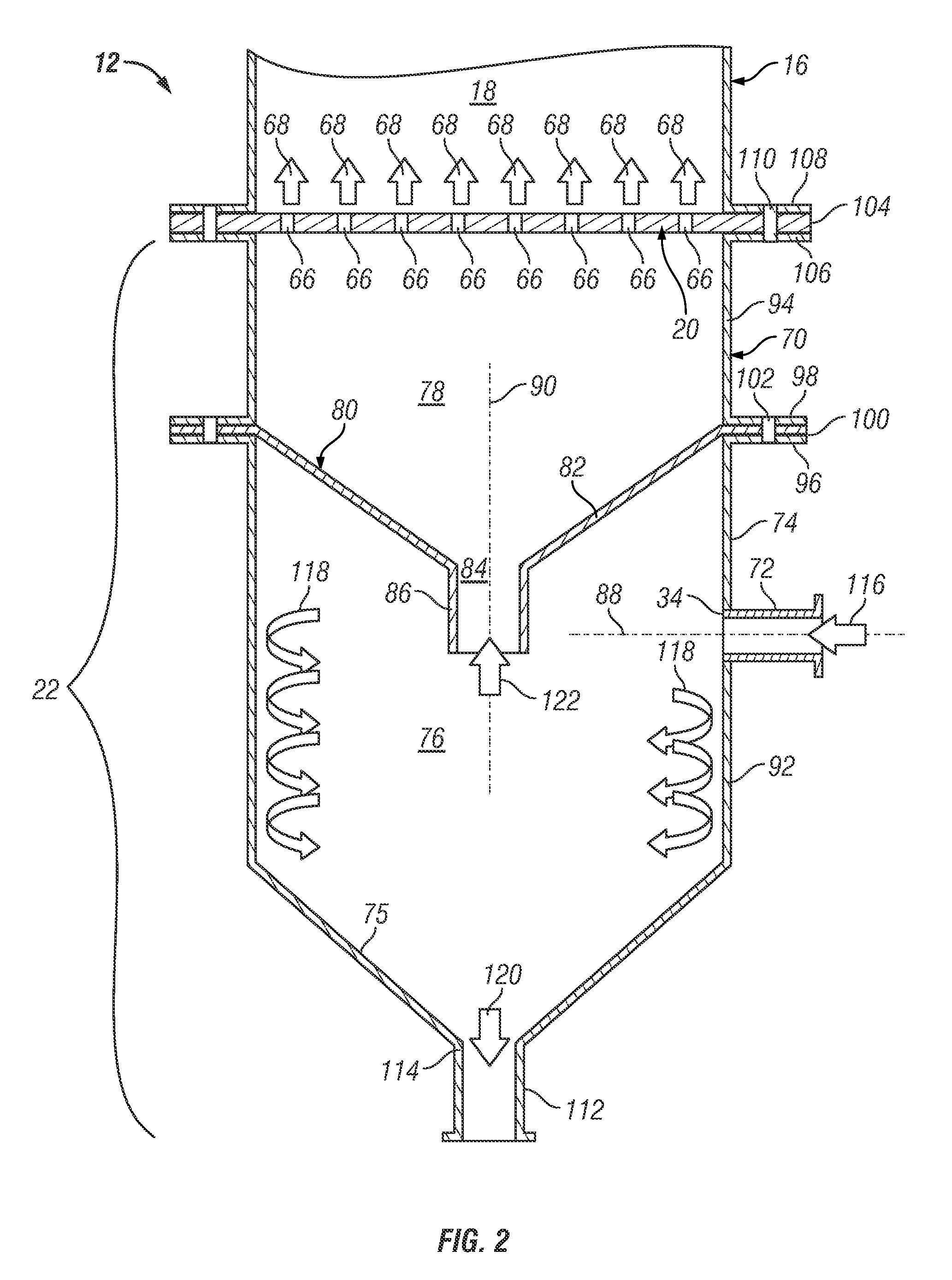
Series-coupled fluidized bed reactor units including cyclonic plenum assemblies and related methods of hydrofluorination
ActiveUS20150064090A1Easy to separatePromote cyclonic separationNuclear energy generationUranium fluoridesCycloneHydrogen
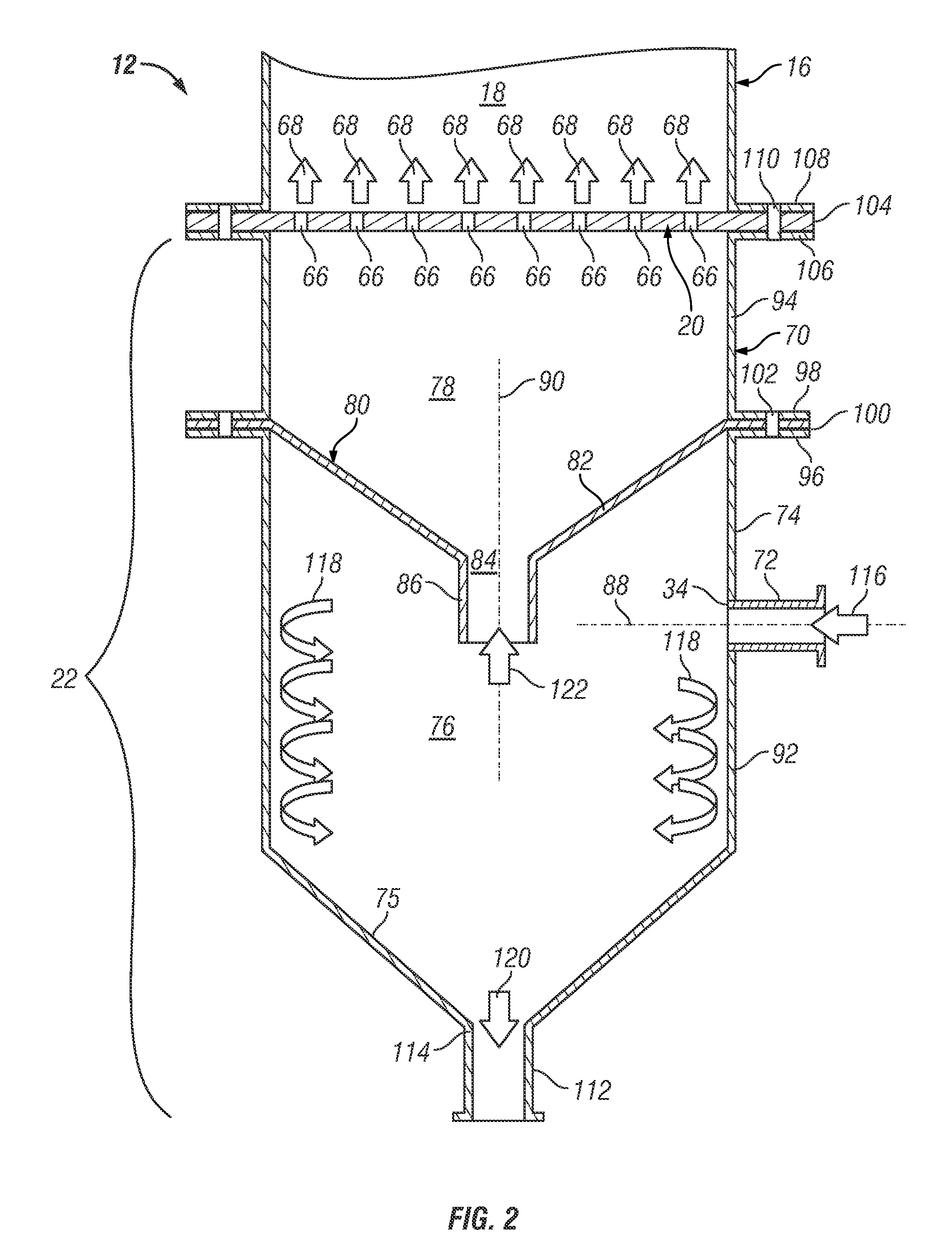
Embodiments of a series-coupled fluidized bed reactor unit are provided. In one embodiment, the reactor unit includes primary and secondary reactors. The primary reactor includes a reaction vessel, a gas distributor fluidly coupled to the reaction vessel, and a cyclonic plenum assembly. The cyclonic plenum assembly includes a plenum assembly housing, which is fluidly coupled to the gas distributor and which has an annular sidewall; and a gas / solids inlet pipe, which fluidly couples a partially-reacted gas outlet of the secondary reactor to the plenum assembly housing. The gas / solids inlet pipe is tangentially positioned with respect to the annular sidewall of the plenum assembly housing to induce vortex flow within the plenum assembly housing of the partially-reacted gas received from the secondary fluidized bed reactor through the gas / solids inlet pipe to promote the cyclonic separation of entrained solids from the partially-reacted gas prior to entry into the gas distributor.
Owner:HONEYWELL INT INC
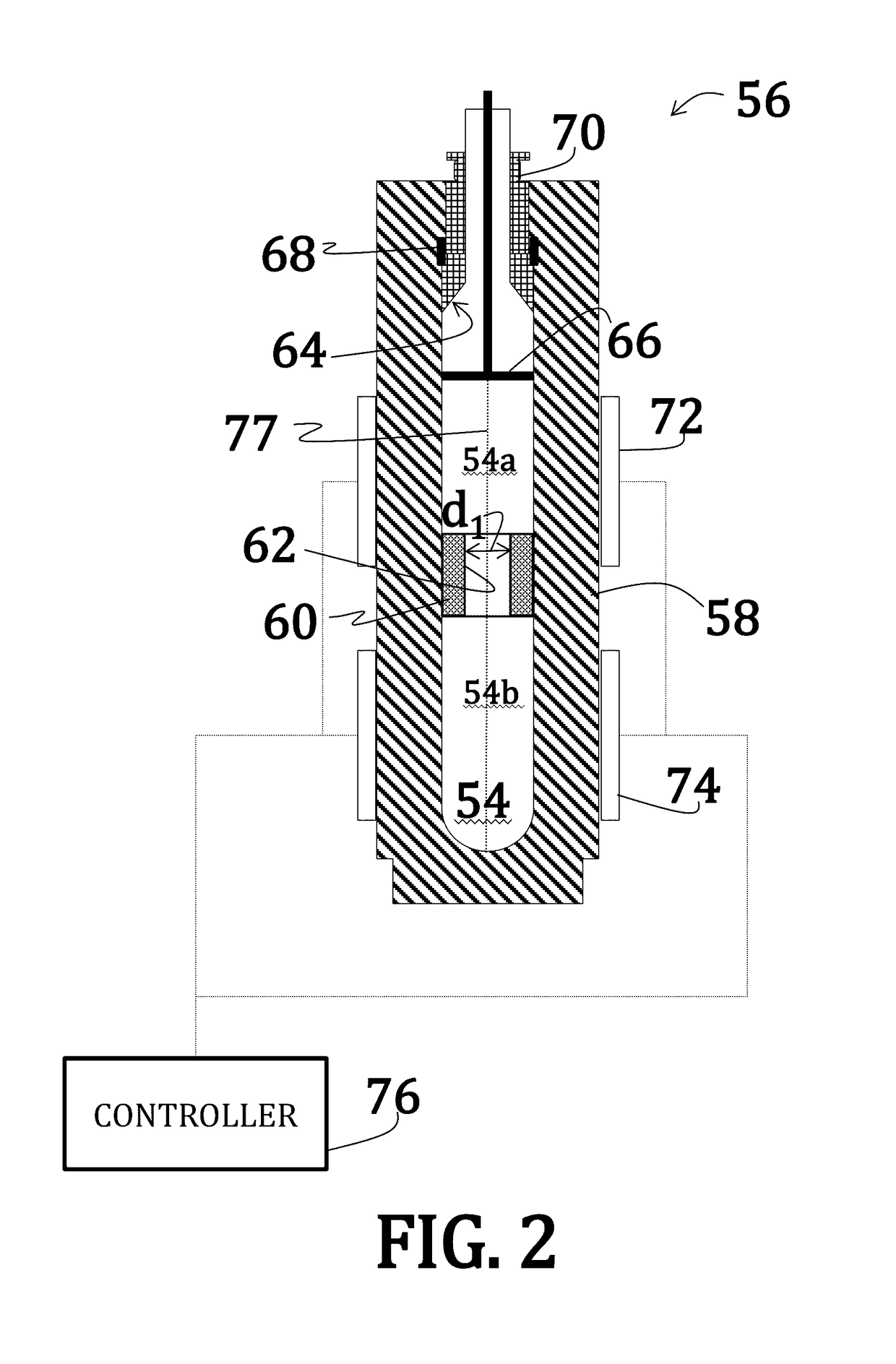
A method of condensing and collecting uf6
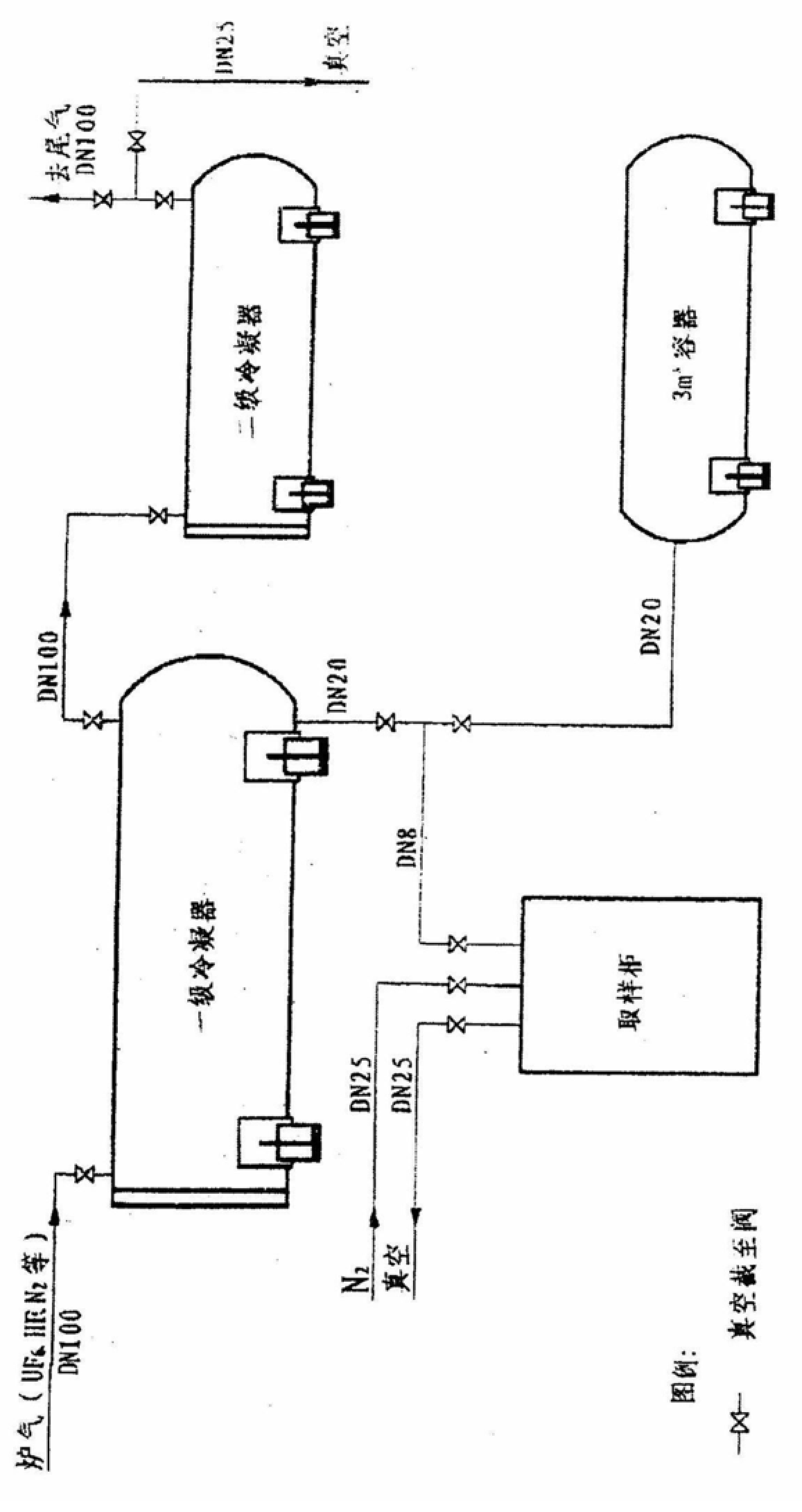
The invention belongs to a nuclear fuel preparation method, and specifically discloses a method for collecting UF6, which includes: inputting the UF6 gas mixture obtained by the gas-solid reaction of UF4 and F2 in a vertical fluorination furnace into a first-stage internal cooling and internal heating UF6 condenser, and passing through After condensing and separating most of the UF6, it is then input into the secondary internal cooling and internal heating UF6 condenser to condense and collect the remaining UF6, and the residual gas enters the exhaust gas treatment system to be purified and then emptied; among them, the refrigerant in the internal circulation pipe of the primary condenser is demineralized water , The temperature for receiving UF6 gas is 5-10°C. The present invention adopts demineralized water with low corrosion and no scaling as the secondary refrigerant of the first-stage internal cooling and internal heating type UF6 condenser, which reduces the corrosion of the internal circulation pipe of the internal cooling and internal heating type condenser; the temperature of the receiving gas is changed to 5-10 ℃, reduce the working temperature difference of the internal circulation pipe and reduce the stress corrosion of the internal circulation pipe. The working life of the condenser is extended to more than 1238tU, and the service life can reach more than 10 years; and the UF6 collection rate can still reach more than 99.6%.
Owner:CNNC LANZHOU URANIUM ENRICHMENT
Method for converting UO3 or u3o8 into hydrated UO4
ActiveUS20130280157A1Improve responseQuick conversionPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesUranium fluoridesHydration reactionWater of crystallization
A method for converting UO3 and / or U3O8 into hydrated UO4 of formula UO4.nH2O wherein n is 2 or 4, comprising the following successive steps:a) preparing an aqueous suspension of a UO3 powder and / or a U3O8 powder;b) adding hydrogen peroxide H2O2 to the aqueous suspension of a UO3 and / or U3O8 powder, converting the UO3 and / or U3O8 into hydrated UO4 and precipitating, crystallizing the hydrated UO4 in the suspension;g) recovering the precipitate, crystals of UO4 hydrate;h) optionally, washing the recovered UO4 hydrate precipitate, crystal(s);i) optionally, repeating step d);j) optionally, drying the precipitate, the crystals;wherein the addition of H2O2 to the aqueous suspension is carried out so that the suspension contains a stoichiometric excess of H2O2 relatively to the stoichiometry of the reaction from UO3:UO3+nH2O+H2O2→UO4.nH2O+H2O (1)or of the reaction from U3O8 UO2.67+1.33H2O2+nH2O→UO4.nH2O+H2O (2)and the pH of the suspension is maintained in steps a) and b) at a value comprised between 2 and 3.
Owner:ORANO CYCLE
Particle blend for synthesizing a porous structure of the aluminum titanate type
The invention relates to a particle blend comprising mainly or consisting of an oxide phase of the pseudo-brookite type comprising at least titanium and aluminum, said blend being obtained from at least two particle size fractions, namely a coarse particle size fraction, the median diameter d50 of which is greater than 12 microns, and a fine particle size fraction, the median diameter d50 of which is between 0.5 and 3 microns, the mass ratio of said coarse fraction to said fine fraction being between 1.5 and 20, limits inclusive, and the ratio of the median diameter of the coarse fraction to that of the fine fraction being greater than 12.
Owner:SAINT GOBAIN CENT DE RES & DEVS & DETUD EUROEN
Method for cleaning filtering tube of hydrofluorination fluidized bed in uranium conversion
ActiveCN109925795AGuaranteed anti-corrosion performanceEfficient removalUranium fluoridesChemical/physical processesFluidized bedEconomic benefits
A method for cleaning the filtering tube of a hydrofluorination fluidized bed in uranium conversion comprises the following steps: (1) removal of surface dirt; (2) removal of internal dirt; and (3) passivation and drying. A chemical medicament is prepared for cleaning of the blocked filtering tube of the hydrofluorination fluidized bed. the dirt inside the filtering tube can be effectively removed. The dirt in the filtering tube is further removed through chemical medicament immersion, bubbling and other processes after the dirt on the surface of the filtering tube is washed out. The passivating and drying process is used after the removal of the dirt in the filtering tube in order to ensure the anticorrosion performance of the filtering tube. The filtering tube cleaned with the cleaning method can meet the process requirements of the hydrofluorination fluidized bed to achieve the purpose of repeated use, has certain economic benefits, and reduces the production cost.
Owner:THE 404 COMPANY LIMITED CHINA NAT NUCLEAR
Use of a kmgf3 compound for trapping metals in the form of fluorides and/or oxyfluorides in a gaseous or a liquid phase
InactiveUS20140112846A1Retain these metals very effectivelyEfficient retentionMagnesium fluoridesTransuranic element compoundsGas phaseMetal impurities
The invention relates to the use of a compound of formula KMgF3 to trap metals present in the form of fluorides and / or of oxyfluorides in a gaseous or liquid phase.It also relates to a compound of formula KMgF3 which has a surface specific area at least equal to 30 m2 / g and at most equal to 150 m2 / g and also to its methods of preparation.The invention notably finds application in the nuclear industry, in which it can advantageously be used to purify uranium hexafluoride (UF6) present in a gaseous or liquid stream, with regard to metal impurities which are also present in this stream.
Owner:COMURHEX
Two step dry uo2 production process utilizing a positive sealing valve means between steps
ActiveUS20100278704A1Inhibition amountHigh densityTransuranic element compoundsNuclear energy generationTemperature controlNuclear grade
The present invention provides a two-step process for producing nuclear grade, active uranium dioxide (UO2) powder in which the first step comprises reacting uranium hexafluoride (UF6) with steam in a flame reactor to yield uranyl fluoride (UO2F2); and the second step comprises removing fluoride and reducing UO2F2 to uranium dioxide (UO2) in a kiln under a steam / hydrogen atmosphere. The two-step process, each step separated by a positive sealed valve means to prevent gas, particularly H2 flow back, tightly controls the exothermicity of the reaction, which allows for a very tight temperature control which controls the growth of the particles and results in UO2 powder that is active and of consistent morphology.
Owner:WESTINGHOUSE ELECTRIC CORP
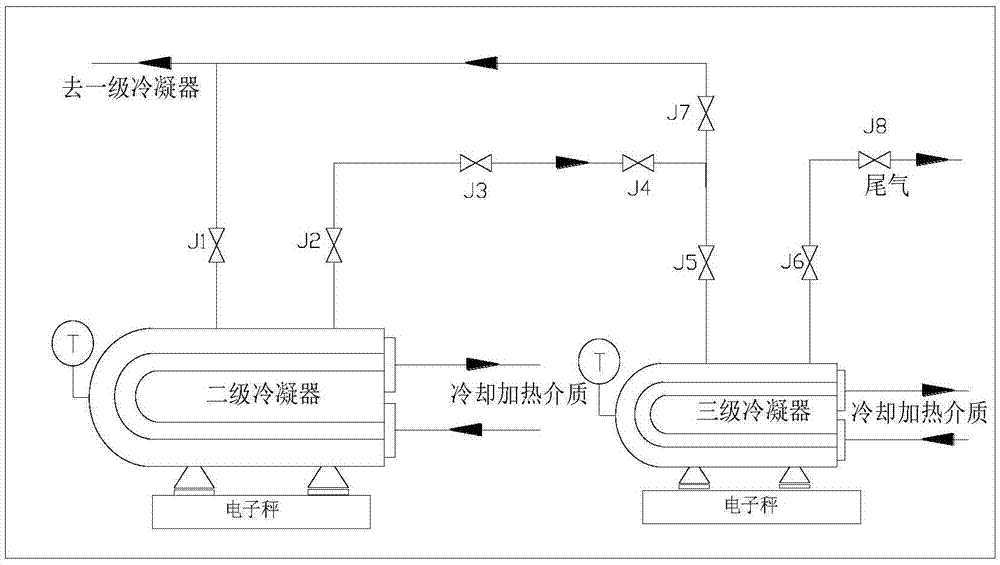
Natural uranium hexafluoride gas-phase transfer system and natural uranium hexafluoride gas-phase transfer method
ActiveCN106924989AImprove direct yieldAchieve purificationSolidificationLiquefactionNatural uraniumTransfer system
The invention belongs to the technical field of natural uranium hexafluoride gas-phase transfer, in particular to a natural uranium hexafluoride gas-phase transfer system and a natural uranium hexafluoride gas-phase transfer method. A secondary condenser and a tertiary condenser are arranged above two electronic scales respectively, and ethylene glycol and steam serve as a cold medium and a heat medium respectively. The secondary condenser is provided with two outlets, one of the outlets is connected to an inlet of a primary condenser through a valve J1, and the other outlet is connected to an inlet of the tertiary condenser through a valve J2, a valve J3, a valve J4 and a valve J5. The tertiary condenser is provided with two outlets, one of the outlets is connected to the inlet of the primary condenser through the valve J5 and a valve J7, and the other outlet is connected to an external tail gas treatment process system through a valve J6 and a valve J8. By the system and the method, assistance in pressure-relieved purification and gas-phase transfer of UF6 products in a cold trap can be realized, purity of the natural UF6 products is improved, the metal direct recovery rate is increased, and safety risks in a transfer process are reduced.
Owner:THE 404 CO LTD CHINA NAT NUCLEAR
Method for preparing uranium tetrafluoride through dry fluorination
PendingCN112939085AAvoid it happening againShort manufacturing processUranium fluoridesHydrogen fluorideUranium tetrafluoride
The invention belongs to a treatment method, and particularly relates to a method for preparing uranium tetrafluoride through dry fluorination. The method comprises the following steps of 1, calcining metal uranium, a uranium compound, a uranium-silicon mixture and the like at high temperature to form oxides, 2, uniformly mixing the oxide obtained by calcining with fluorinating agents such as ammonium fluoride and ammonium bifluoride according to a certain proportion, and 3, putting the mixed materials into a muffle furnace or a converter with a heating function, introducing inert gases such as nitrogen, hydrogen and ammonia gas or reducing gases, and electrifying and heating until the oxides are completely converted into uranium tetrafluoride powder. The method has the remarkable effects that uranium-containing wastewater is prevented from being generated, and the cost is reduced. The method can be applied to preparation of uranium tetrafluoride by taking metal uranium, uranium compounds, uranium-silicon mixtures and the like as raw materials in the fields of nuclear chemical engineering and fuel element manufacturing and recovery treatment of unqualified uranium-silicon mixtures in the fuel element manufacturing process.
Owner:CHINA NUCLEAR BAOTOU GUANGHUA CHEM IND
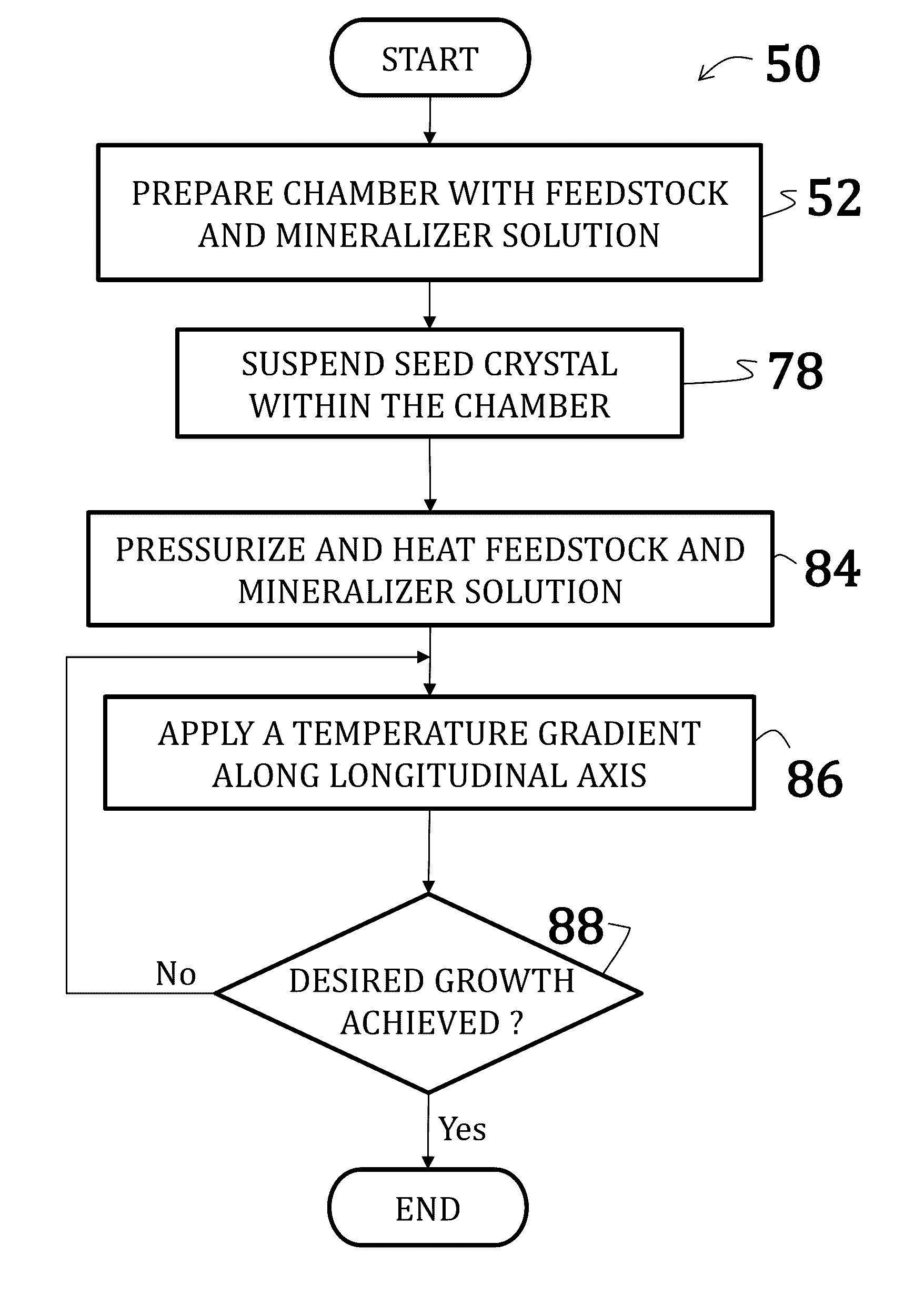
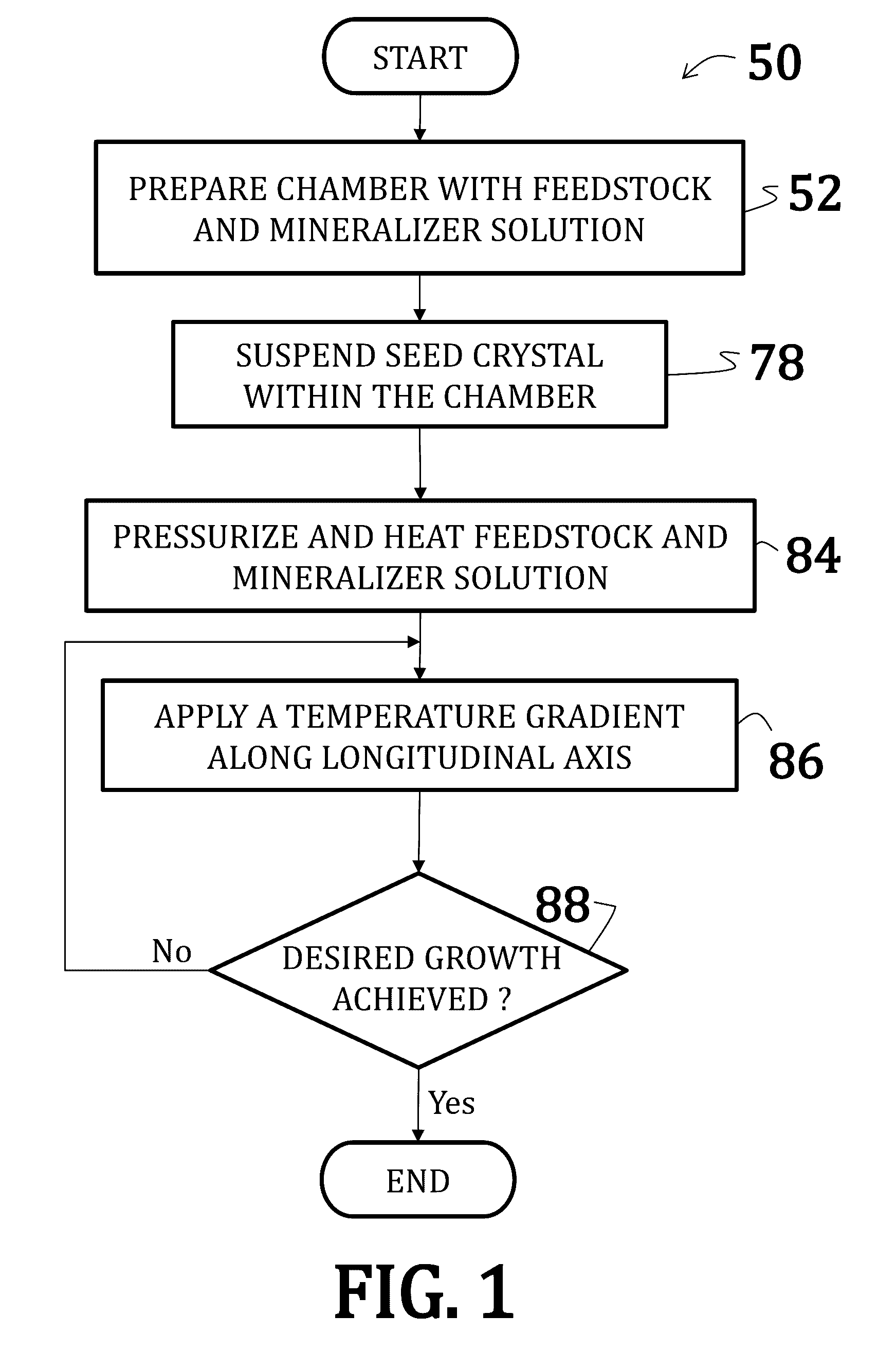
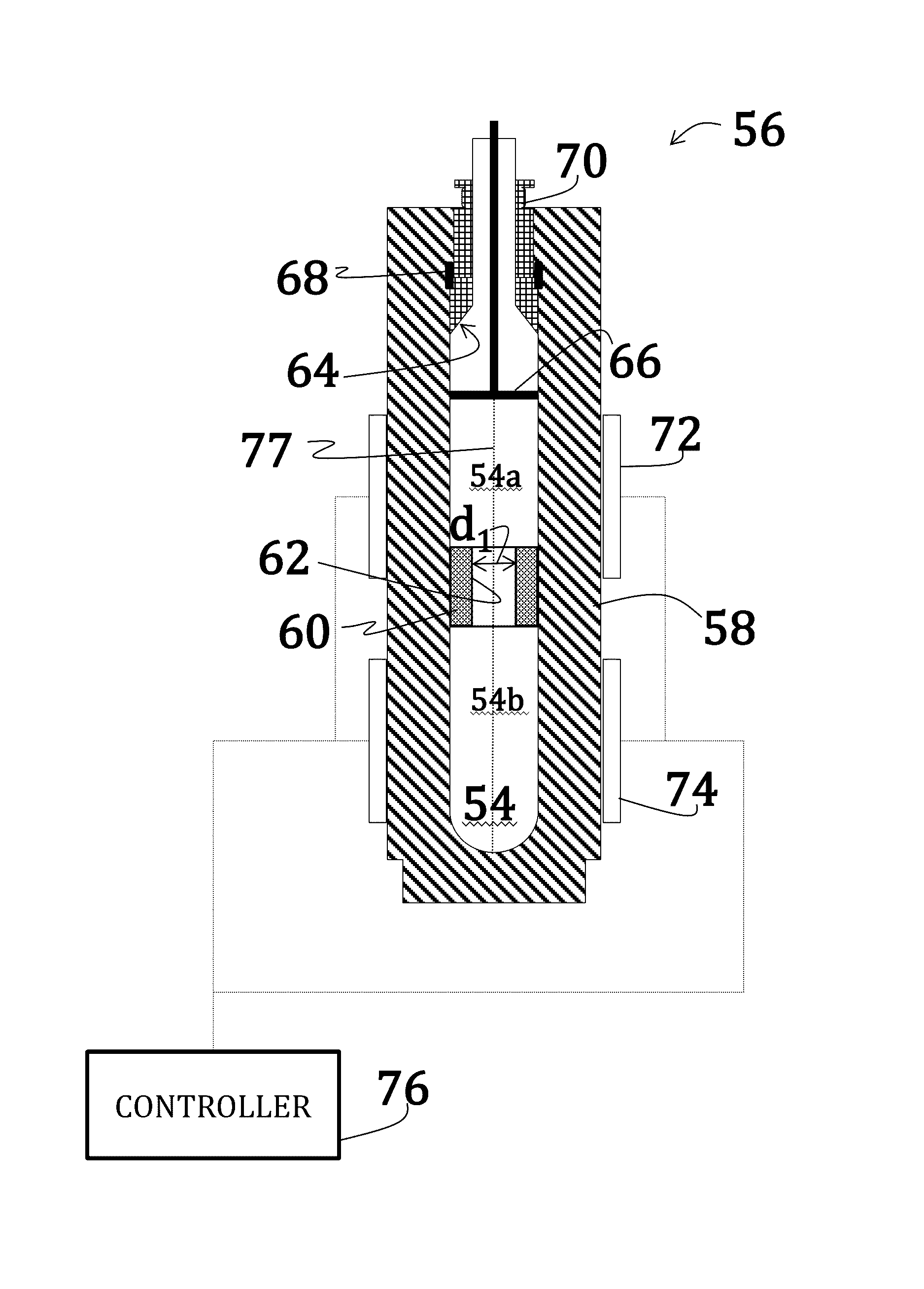
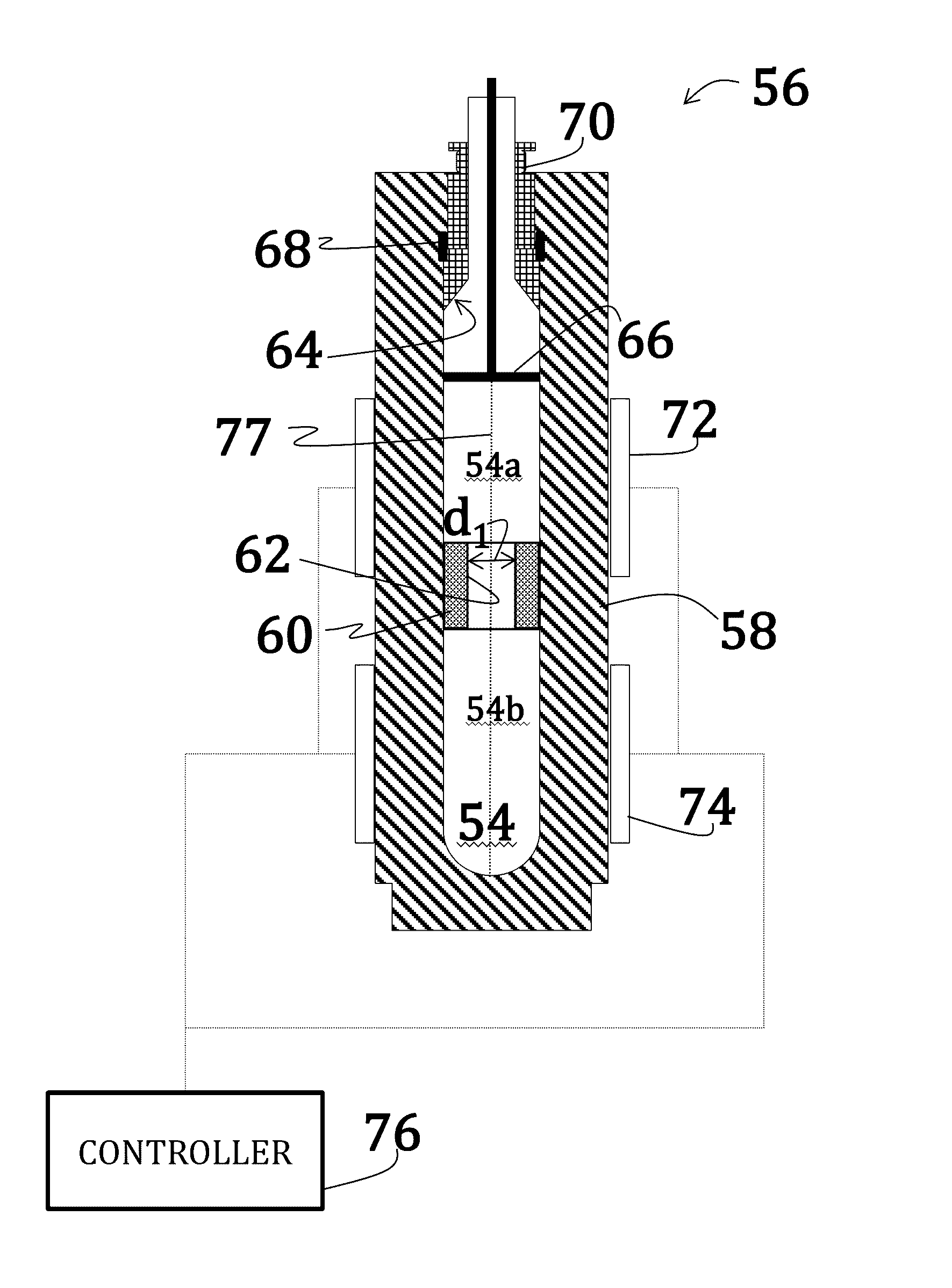
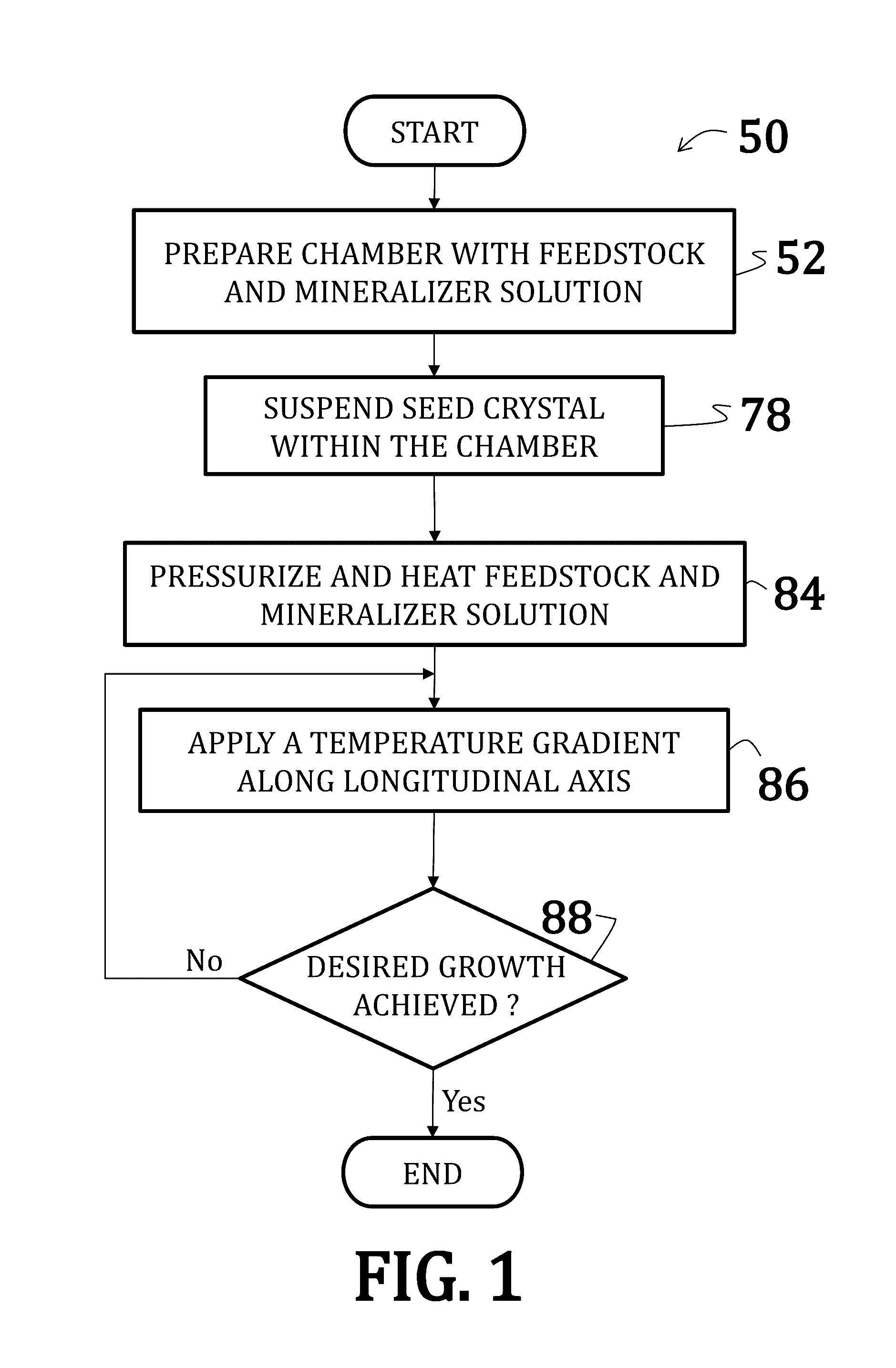
Alkali uranium fluorophospahte-based crystals and methods of fabrication
ActiveUS20160289860A1Problem and drawbackPolycrystalline material growthFrom normal temperature solutionsPhosphateFluoride
A method of synthesizing alkali uranium fluorophosphate crystals. The method includes combining a uranium-based feedstock with a mineralizer solution. The mineralizer solution includes an alkali nutrient, a phosphate, and a fluoride. The feedstock and mineralizer solution are pressurized and a thermal gradient applied thereto such that a first portion of the feedstock and the mineralizer solution is heated to a temperature that is greater than a temperature of a second portion of the feedstock and the mineralizer solution. Uranium nutrient enters the mineralizer solution from the feedstock in the first portion and uranium nutrient precipitates to spontaneously form crystals in the second portion.
Owner:GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE AIR FORCE
Internal cyclone for fluidized bed reactor
InactiveUS20140065049A1Removing heavy cloggageFluidized bed combustionUranium fluoridesCycloneFluidized bed
A fluidized bed reactor comprising a reaction column having a fluid portion; a gas inflow means for flowing a gas upwardly from the fluid portion of the reaction column; a particle feed means for feeding particles to the fluid portion of the reaction column; a cyclone capable of separating particles from the gas flowing upwardly from the fluid portion of the reaction column, the cyclone being located within the reaction column and being in communication with the gas flowing upwardly, wherein the cyclone comprises a cyclone body having an inlet, a gas outlet, and a particle drop port; and a particle discharge pipe having an upper part connected to the particle drop port of the cyclone body, and a lower part, wherein the particle discharge pipe is located substantially outside of the reaction column.
Owner:HONEYWELL INT INC
Rubidium uranium fluoride-based crystals and methods of fabrication
ActiveUS20160348268A1Polycrystalline material growthFrom normal temperature solutionsFluoride crystalsRubidium
A method of synthesizing rubidium uranium fluoride crystals. The method includes combining uranium-based feedstock with a mineralizer solution that includes a rubidium fluoride. The feedstock and mineralizer solution are pressurized and a thermal gradient applied thereto such that a first portion of the feedstock and the mineralizer solution is heated to a temperature that is greater than a temperature of a second portion of the feedstock and the mineralizer solution. Uranium nutrient enters the mineralizer solution from the feedstock in the first portion and uranium nutrient precipitates to spontaneously form crystals in the second portion.
Owner:THE UNITED STATES OF AMERICA AS REPRESETNED BY THE SEC OF THE AIR FORCE
Separation and recovery of molybdenum values from uranium process distillate
A method for treating process distillate heavies produced during uranium fluoride purification is described. The heavies contain primarily uranium hexafluoride, UF6, and molybdenum oxytetrafluoride, MoOF4. The uranium hexafluoride is removed via distillation at reduced pressure leaving essentially MoOF4 containing <0.1% of residual uranium hexafluoride. This mixture is hydrolyzed in water, then treated with a solution of sodium hydroxide until a pH of at least 7.5 is reached. The precipitated sodium diuranate and sodium fluoride are removed by filtration. The filtrate is reacted with calcium chloride to precipitate the molybdenum values as calcium molybdate containing trace quantities of calcium fluoride.
Owner:HONEYWELL INT INC
Series coupled fluidized bed reactor units including cyclonic plenum assemblies and related methods of hydrofluorination
ActiveUS9511339B2Promote cyclonic separationFluoride preparationNuclear energy generationCycloneHydrogen
Owner:HONEYWELL INT INC
Alkali uranium fluorophosphate-based crystals and methods of fabrication
ActiveUS9670589B2Polycrystalline material growthFrom normal temperature solutionsPhosphate crystalsFluoride
A method of synthesizing alkali uranium fluorophosphate crystals. The method includes combining a uranium-based feedstock with a mineralizer solution. The mineralizer solution includes an alkali nutrient, a phosphate, and a fluoride. The feedstock and mineralizer solution are pressurized and a thermal gradient applied thereto such that a first portion of the feedstock and the mineralizer solution is heated to a temperature that is greater than a temperature of a second portion of the feedstock and the mineralizer solution. Uranium nutrient enters the mineralizer solution from the feedstock in the first portion and uranium nutrient precipitates to spontaneously form crystals in the second portion.
Owner:THE UNITED STATES OF AMERICA AS REPRESETNED BY THE SEC OF THE AIR FORCE
Separation and recovery of molybdenum values from uranium process distillate
InactiveUS20140140905A1Inhibition formationTransuranic element compoundsMolybdeum compoundsMolybdateFiltration
A method for treating process distillate heavies produced during uranium fluoride purification is described. The heavies contain primarily uranium hexafluoride, UF6, and molybdenum oxytetrafluoride, MoOF4. The uranium hexafluoride is removed via distillation at reduced pressure leaving essentially MoOF4 containing <0.1% of residual uranium hexafluoride. This mixture is hydrolyzed in water, then treated with a solution of sodium hydroxide until a pH of at least 7.5 is reached. The precipitated sodium diuranate and sodium fluoride are removed by filtration. The filtrate is reacted with calcium chloride to precipitate the molybdenum values as calcium molybdate containing trace quantities of calcium fluoride.
Owner:HONEYWELL INT INC
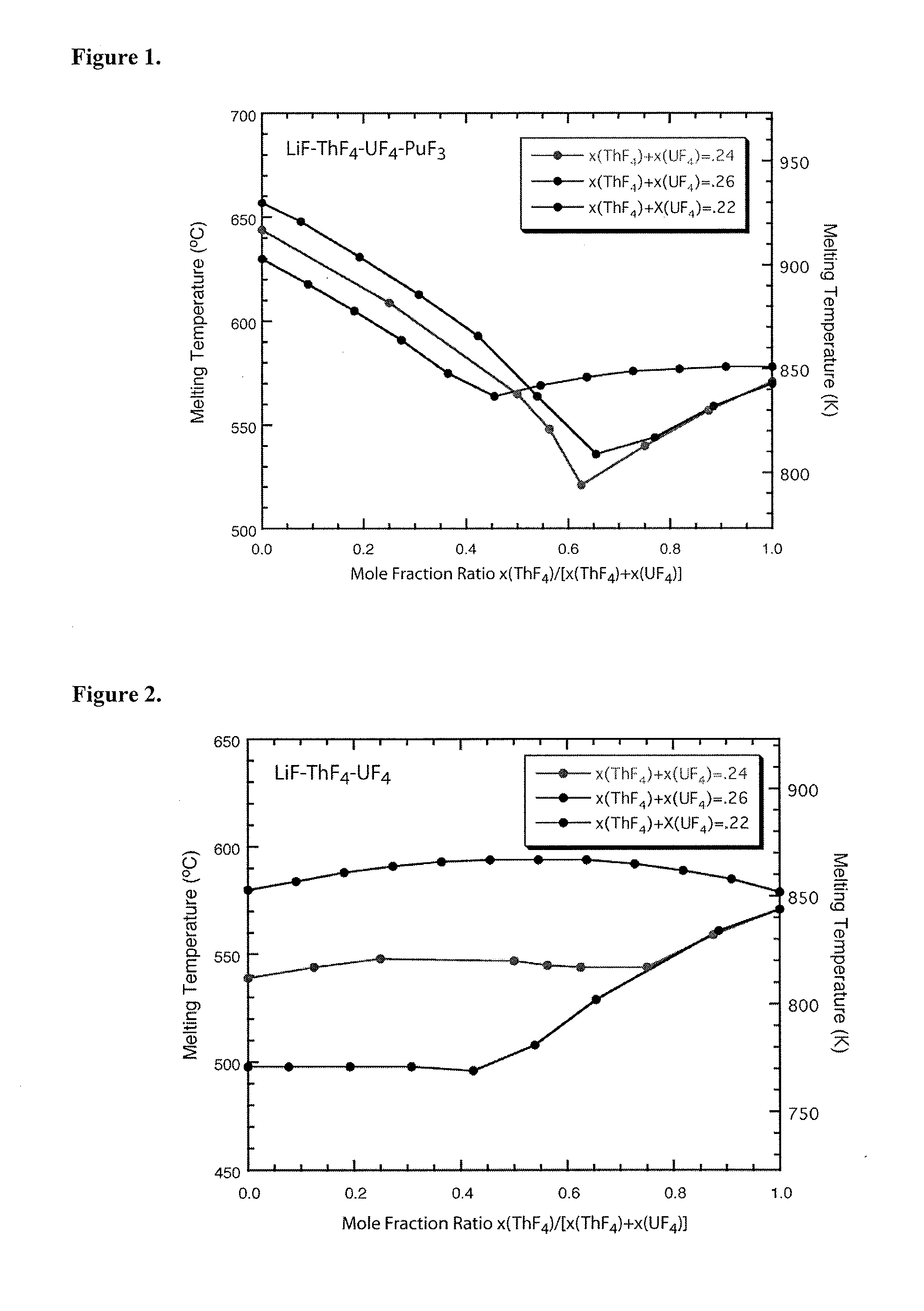
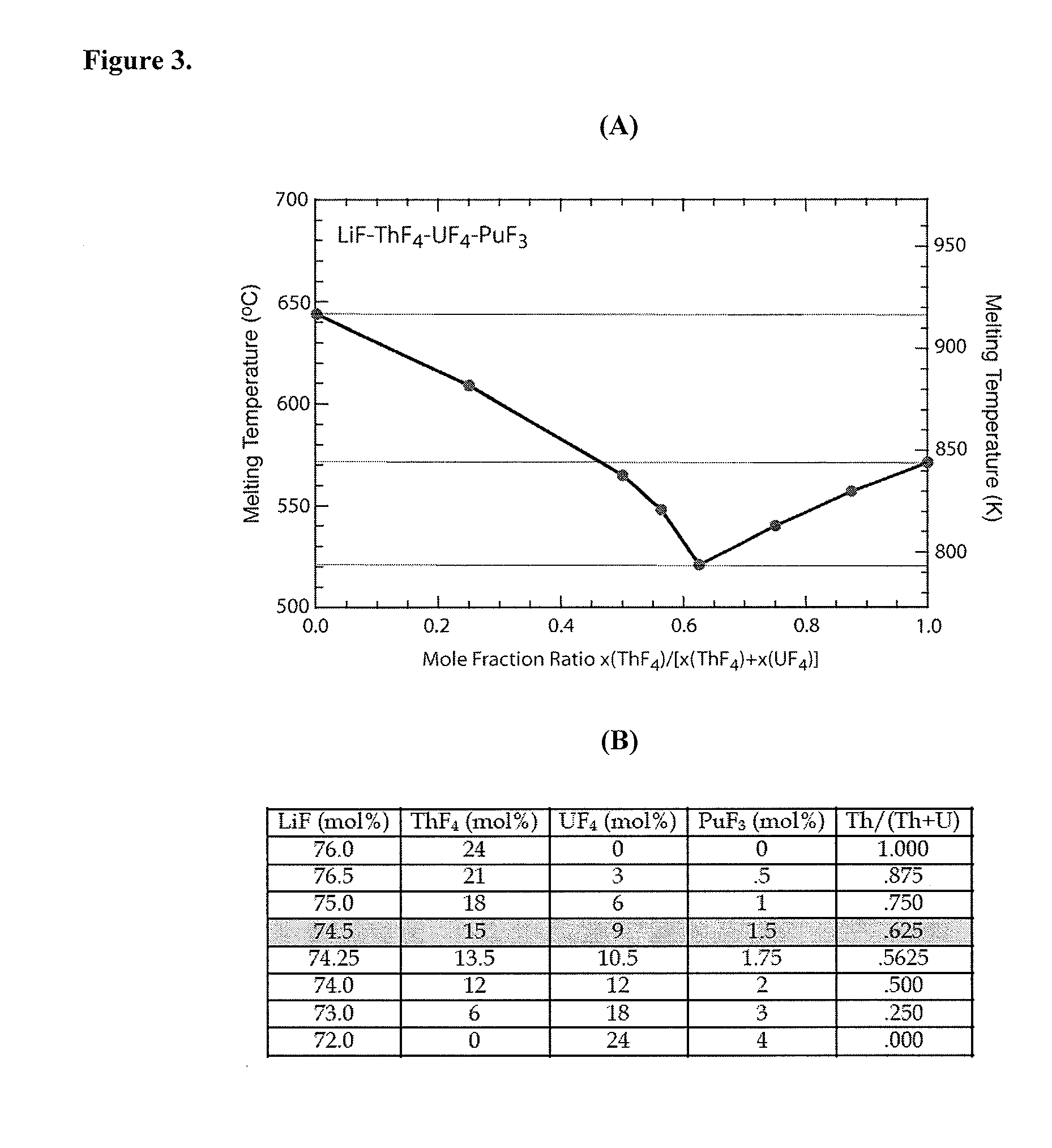
Molten salt fuels with high plutonium solubility
Owner:LAWRENCE LIVERMORE NAT SECURITY LLC
Use of a KMgF.sub.3 compound for trapping metals in the form of fluorides and/or oxyfluorides in a gaseous or a liquid phase
InactiveUS8858901B2Retain these metals very effectivelyEfficient retentionMagnesium fluoridesNuclear fuel reprocessingGas phaseMetal impurities
The invention relates to the use of a compound of the formula KmgF3 to trap metals in the form of fluorides and / or of oxyfluorides in a gaseous or liquid phase. It also relates to a compound of the formula KMgF3 which has a surface area at least equal to 30 m2 / g and at most equal to 150 m2 / g and also to its methods of preparation. The invention notably finds application in the nuclear industry, in which it can advantageously be used to purify uranium hexafluoride (UF6) present in a gaseous or liquid stream, with regard to metal impurities which are also present in this stream.
Owner:COMURHEX
Uranium dioxide-based crystals and methods of fabrication
ActiveUS20160348267A1Polycrystalline material growthFrom normal temperature solutionsUranium tetrafluorideUranium trioxide
Owner:THE UNITED STATES OF AMERICA AS REPRESETNED BY THE SEC OF THE AIR FORCE
Reprocessing method by fluoride volatility process using solid-gas separation
InactiveUS7208129B2Easy to separateReduce consumptionFluoride preparationPlutonium compoundsFluoride volatilityHexafluoride
Fluorine or a fluorine compound is subjected to a reaction with a spent oxide fuel to produce fluorides of uranium and plutonium, and the fluorides are recovered using a difference in volatility behavior. The spent oxide fuel is subjected to a reaction with an HF gas, whereby uranium, plutonium and most impurities are converted into solid fluorides having low valences or remained as oxides to inhibit volatilization thereof, and then in an F2 fluorination step, the HF fluorination product is subjected to a reaction with a fluorine gas in two stages: one at a low temperature and the other at a high temperature, whereby a certain amount of gaseous uranium and volatile impurities are separated with plutonium kept in a solid form in the first stage, and mixed fluorides of remaining uranium and plutonium are fluorinated into hexafluorides at the same time in the second stage. By such a reprocessing method, plutonium enrichment can be adjusted, uranium and plutonium can be purified, and steps are simplified as well. In addition, reactors are hard to be corroded or deteriorated.
Owner:JAPAN ATOMIC ENERGY AGENCY INDEPENDANT ADMINISTRATIVE CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com