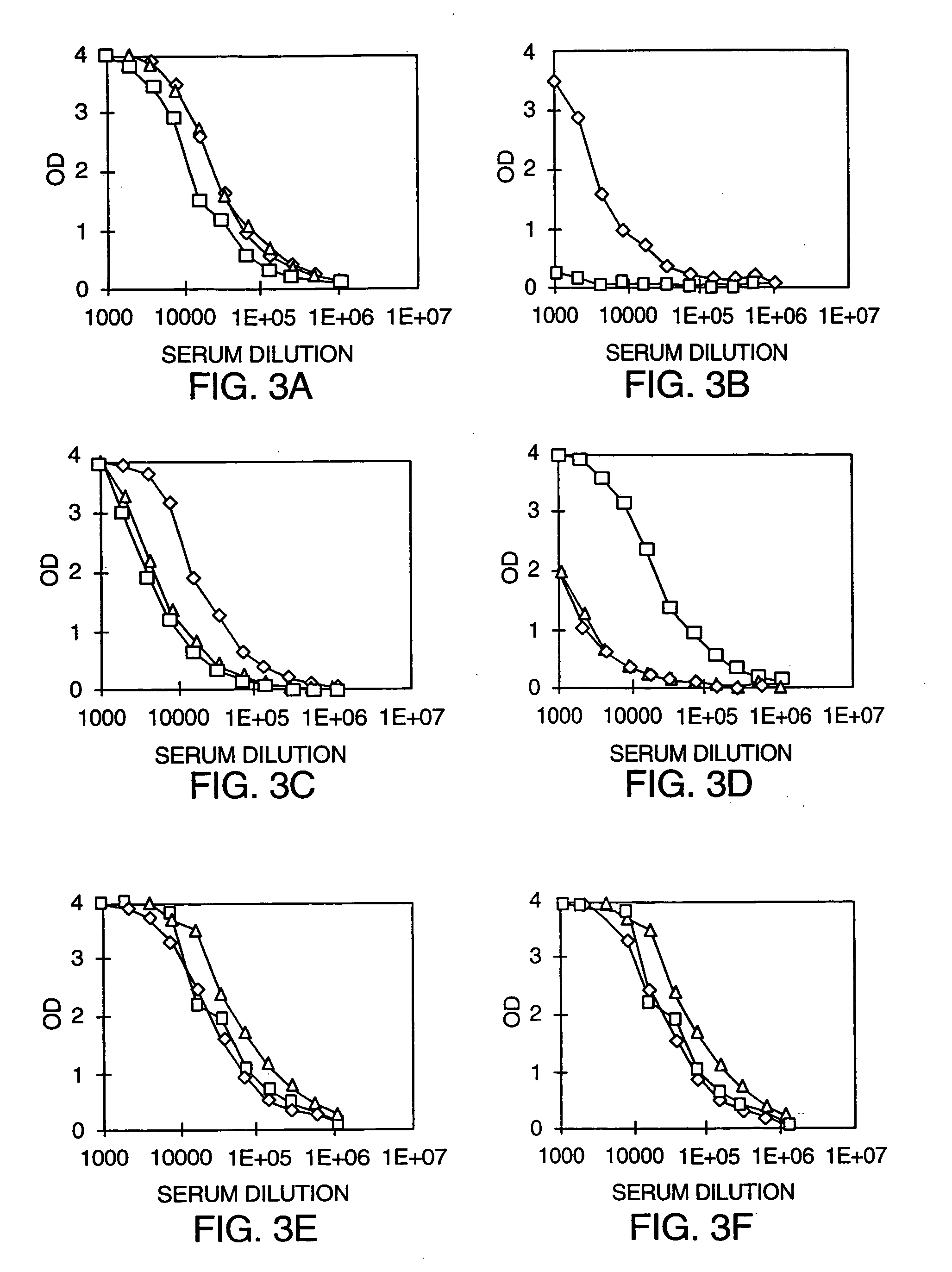
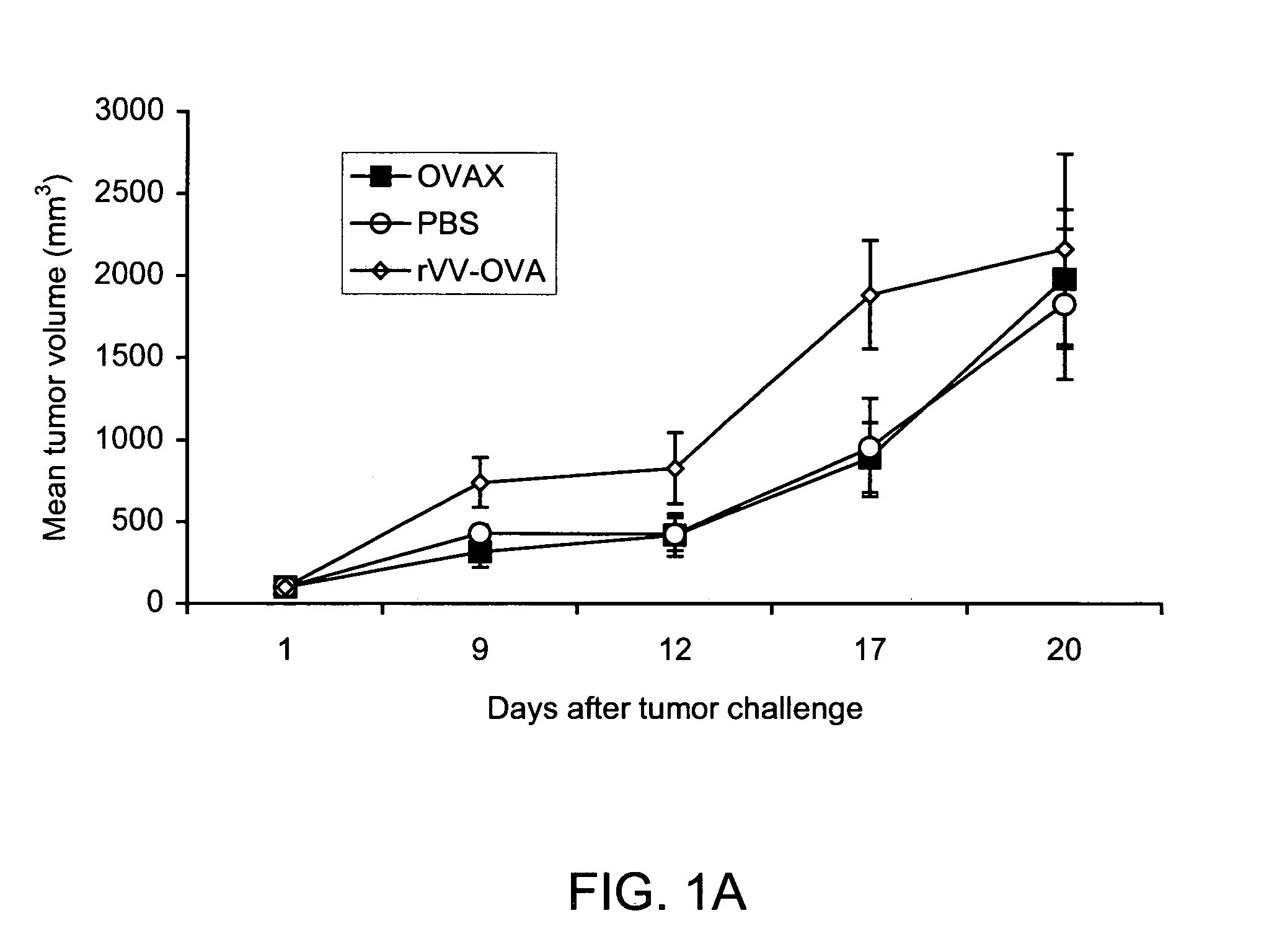
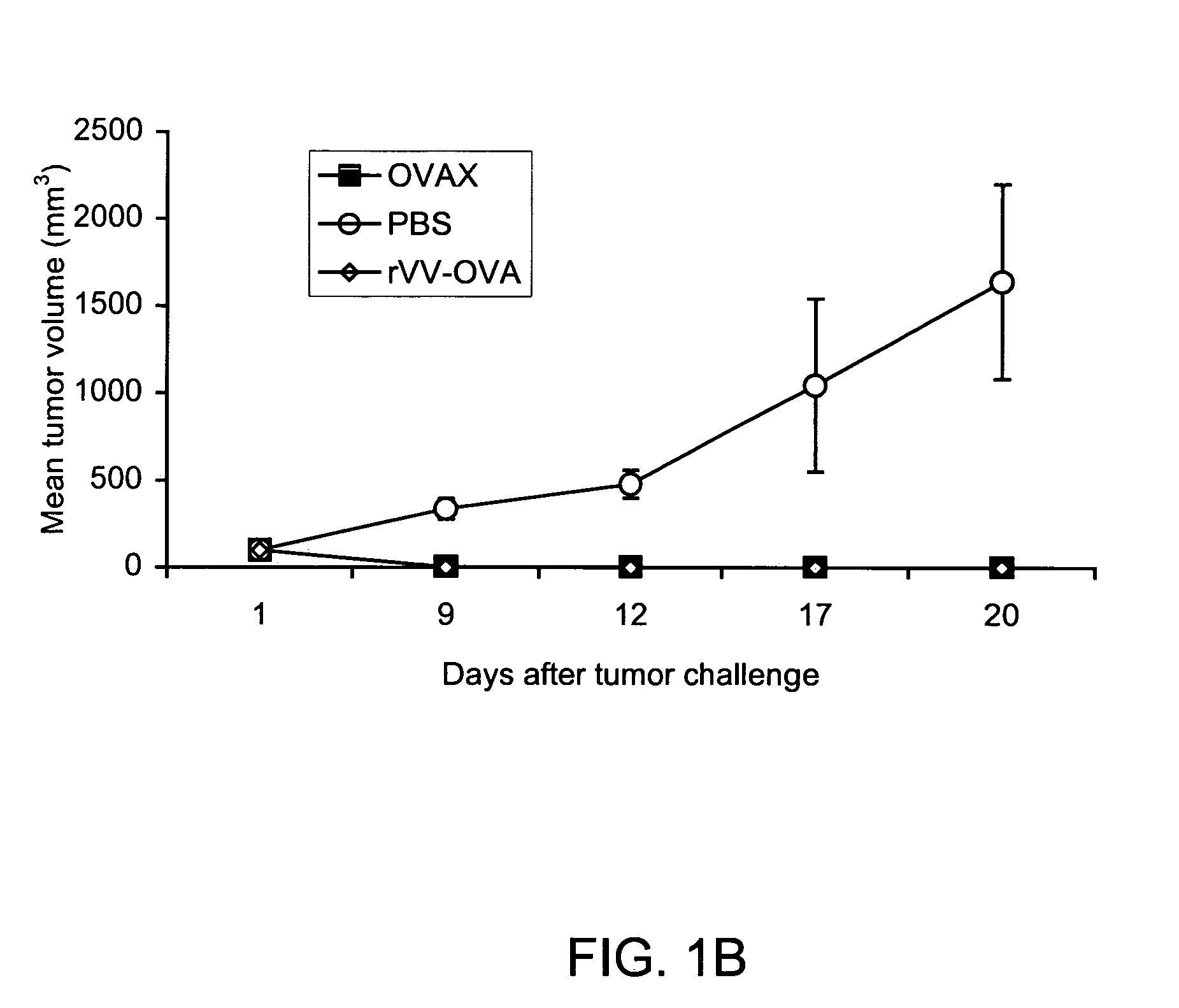
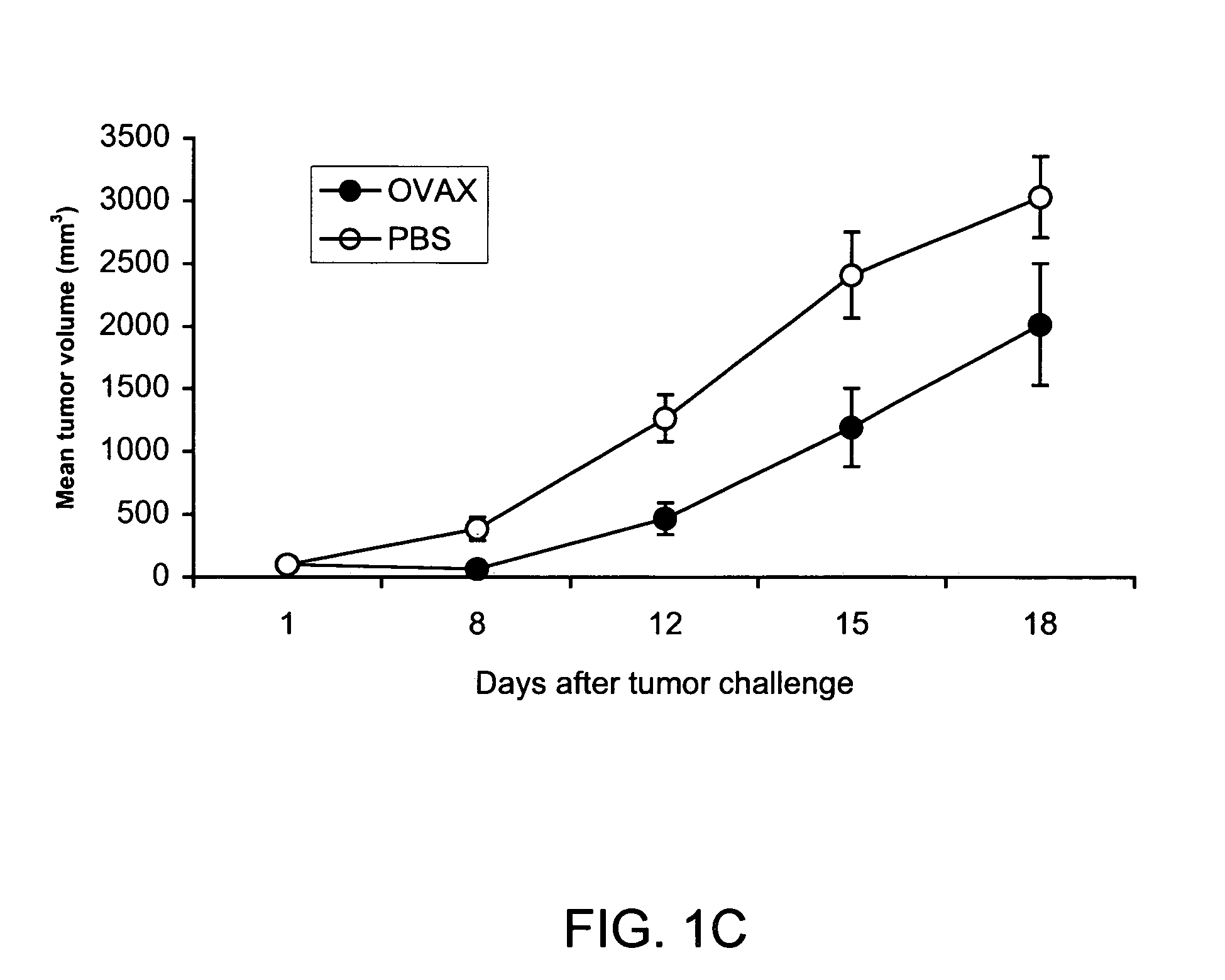
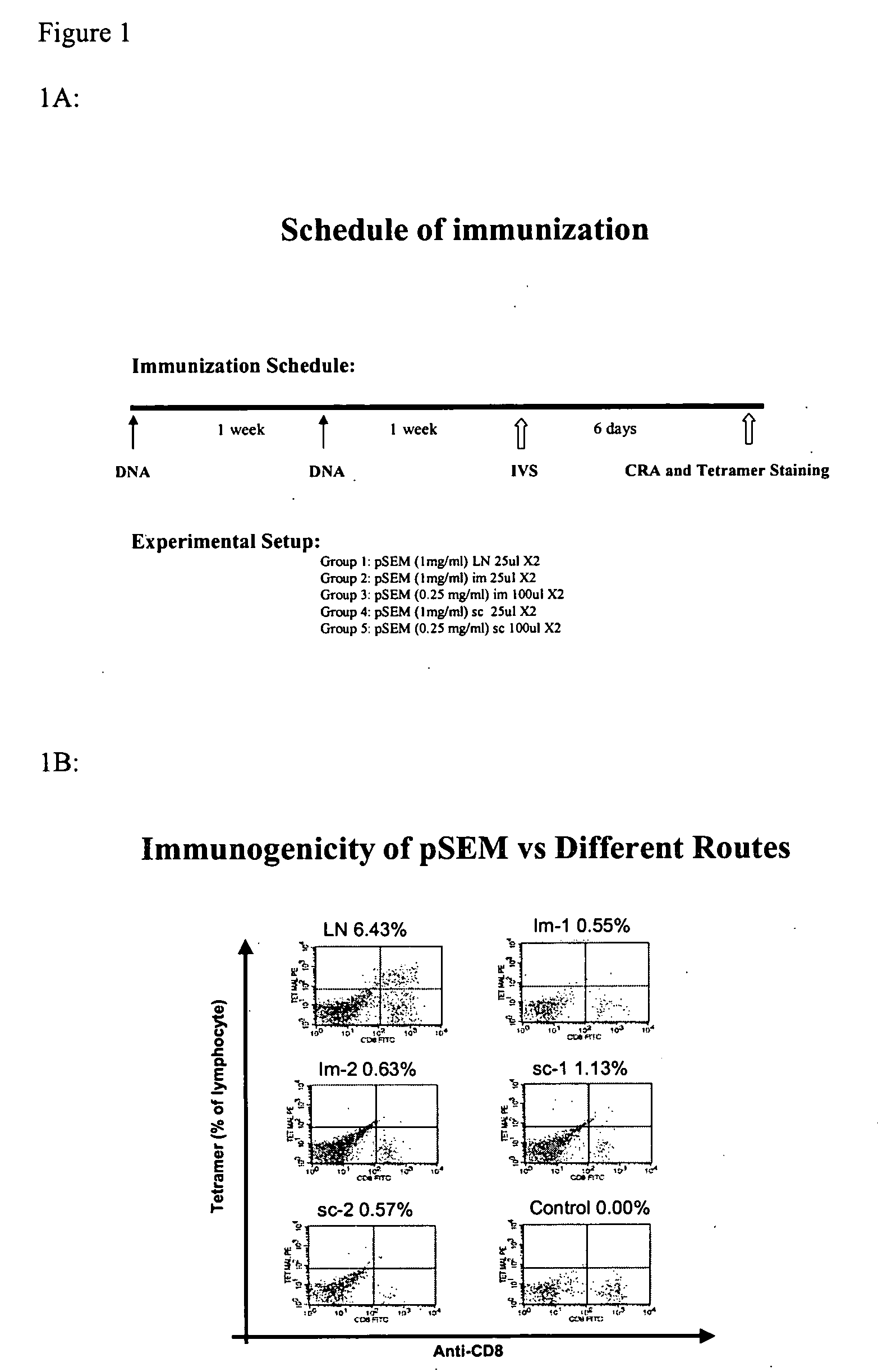
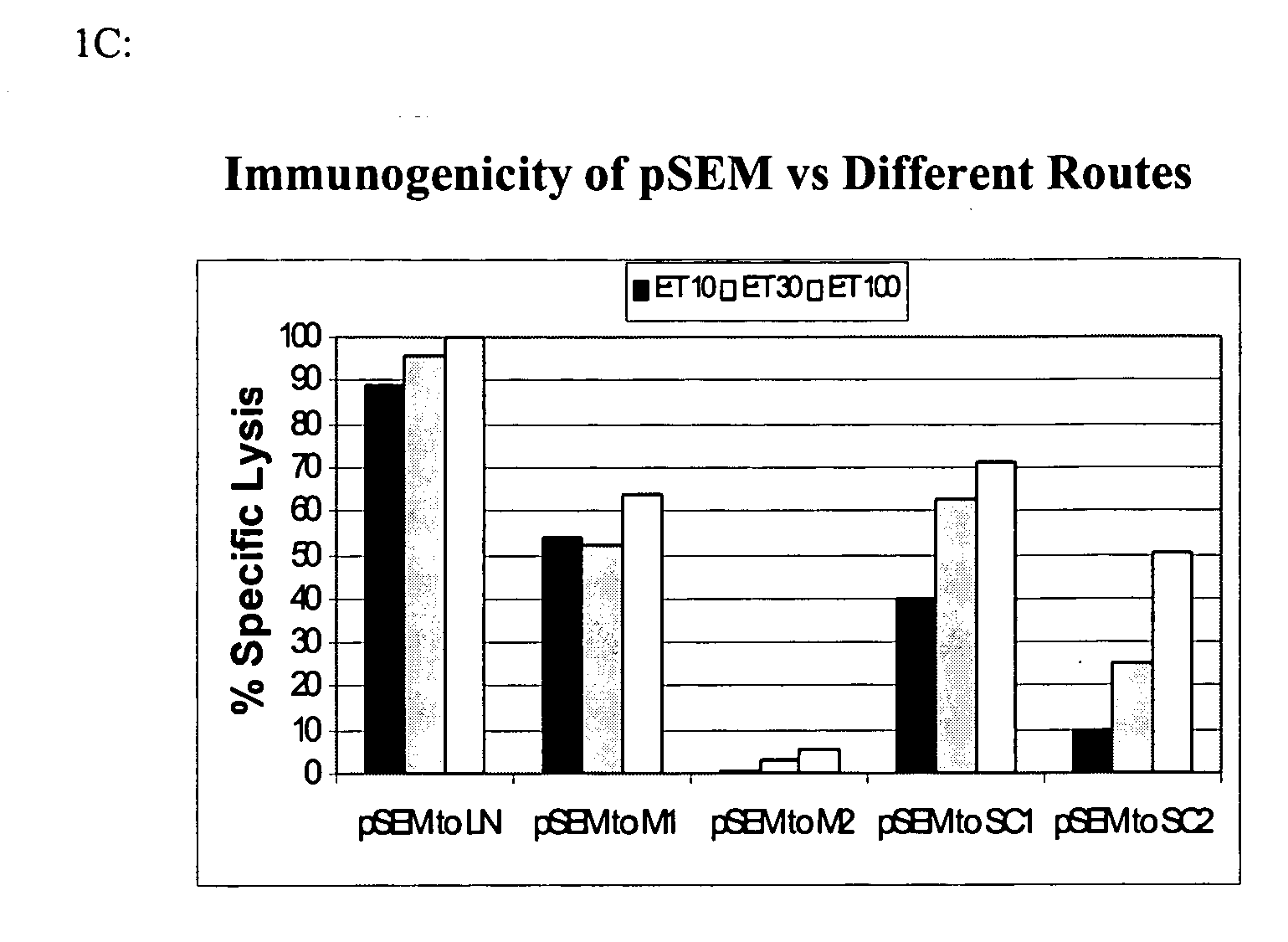
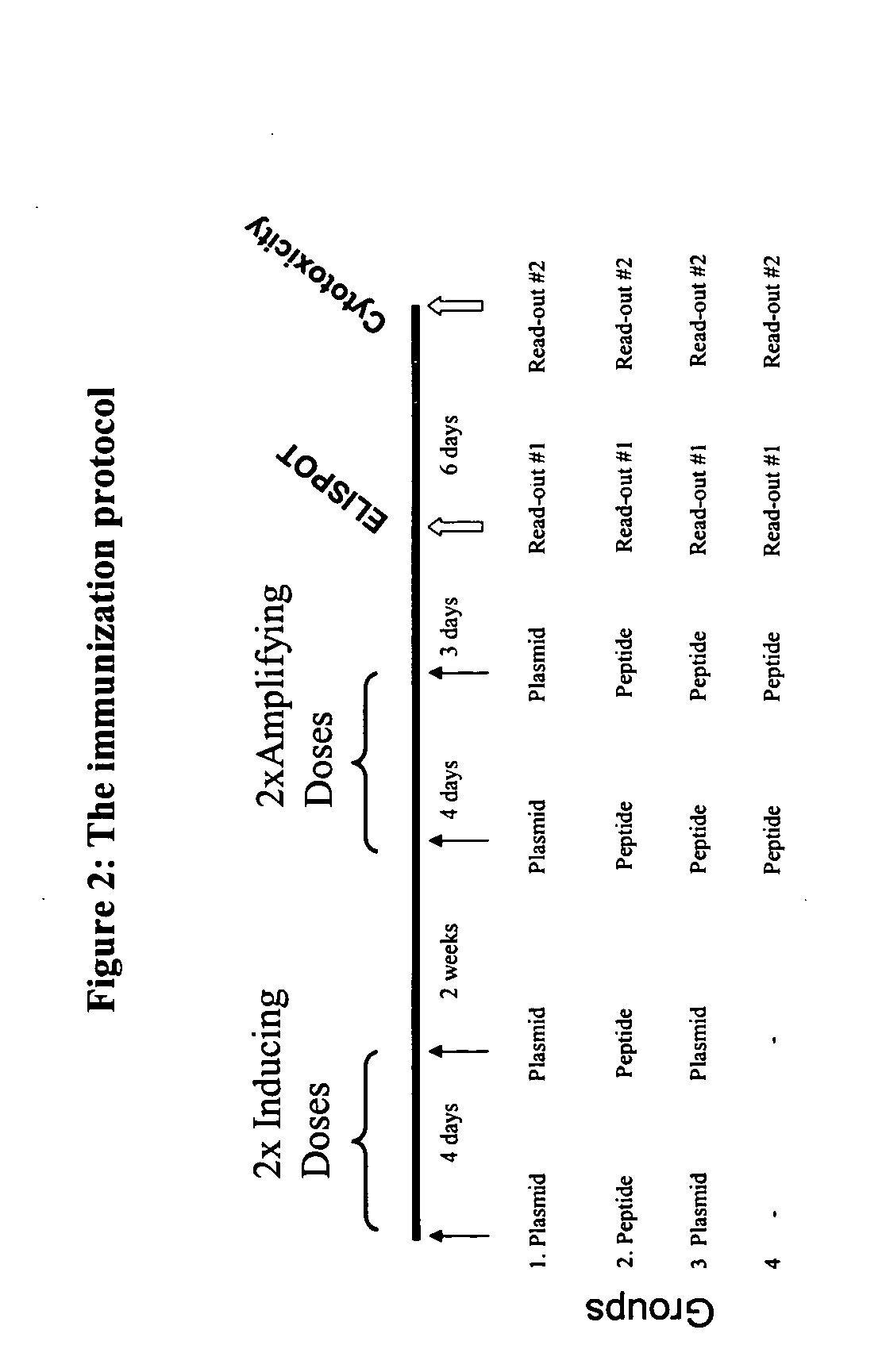
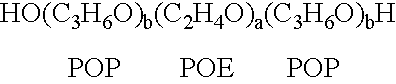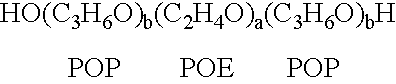Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3534results about "Whole-cell/virus/DNA/RNA ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
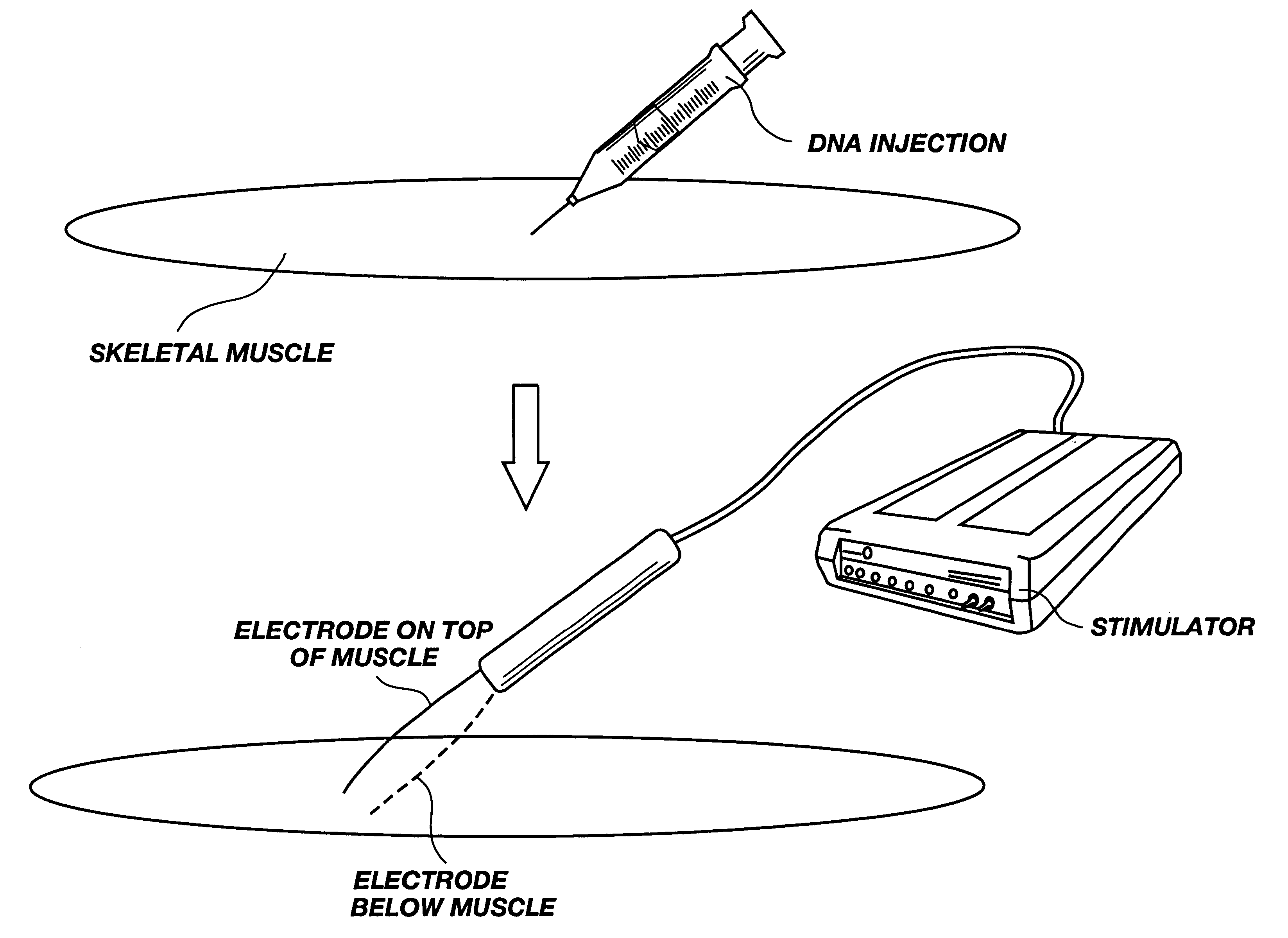
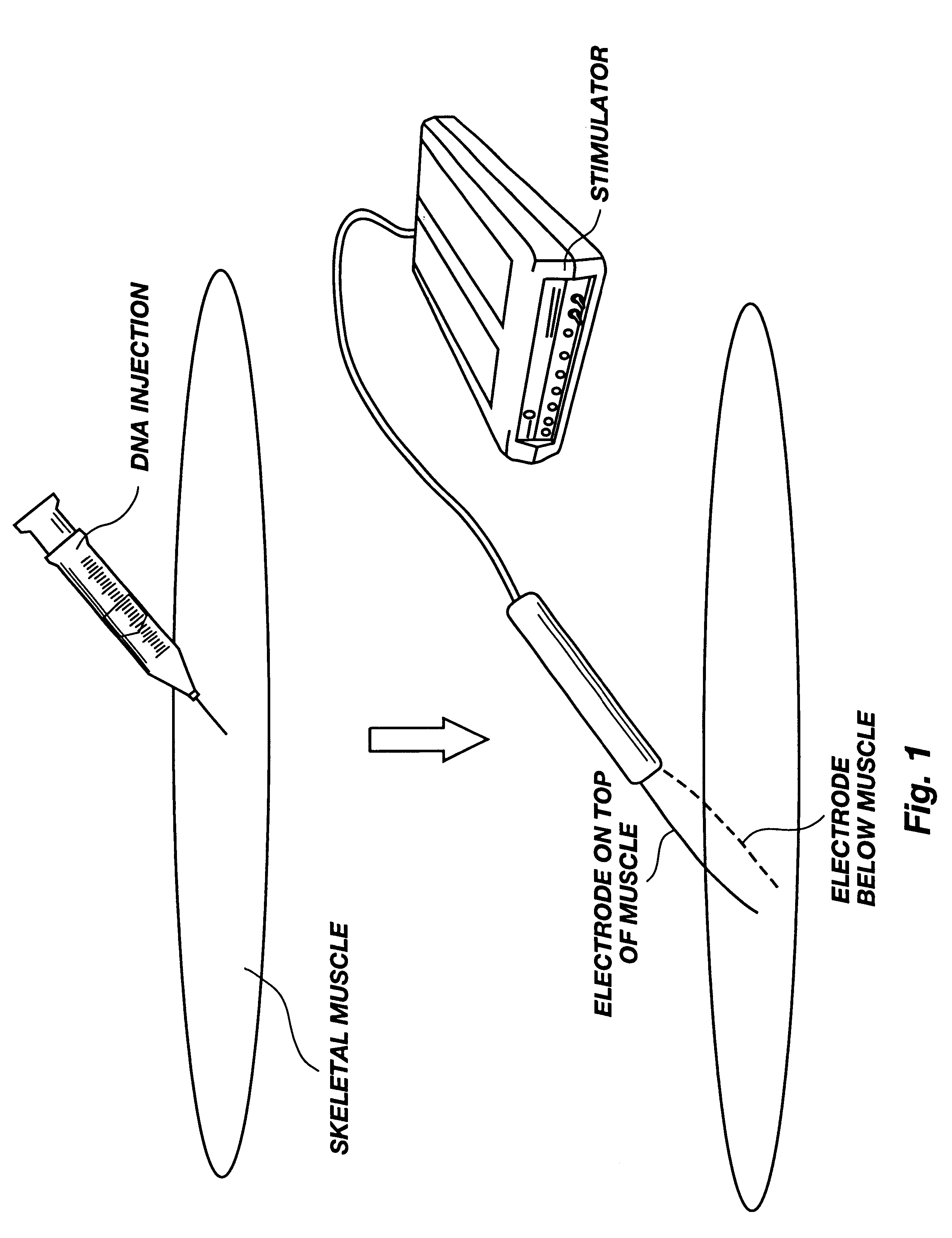
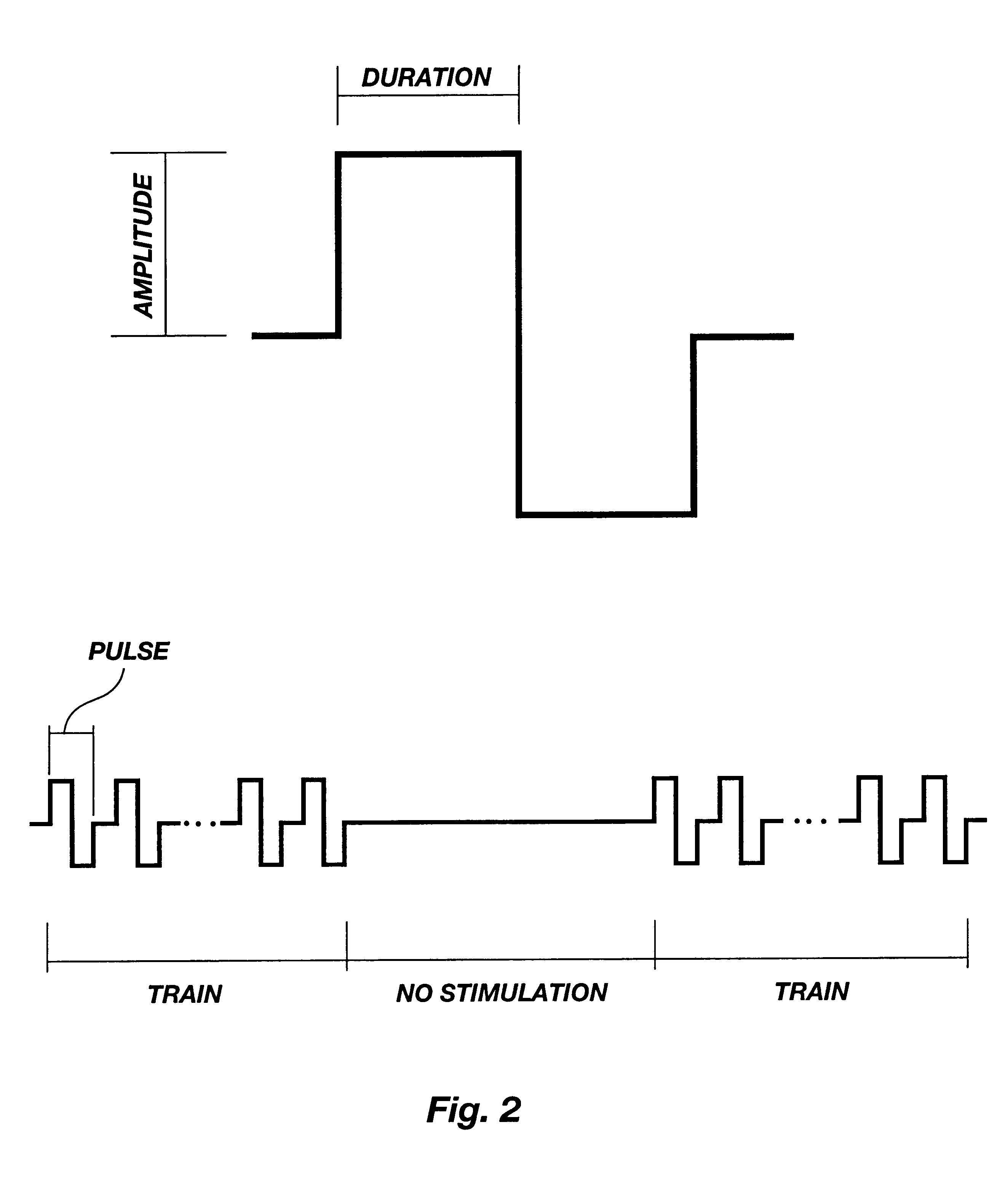
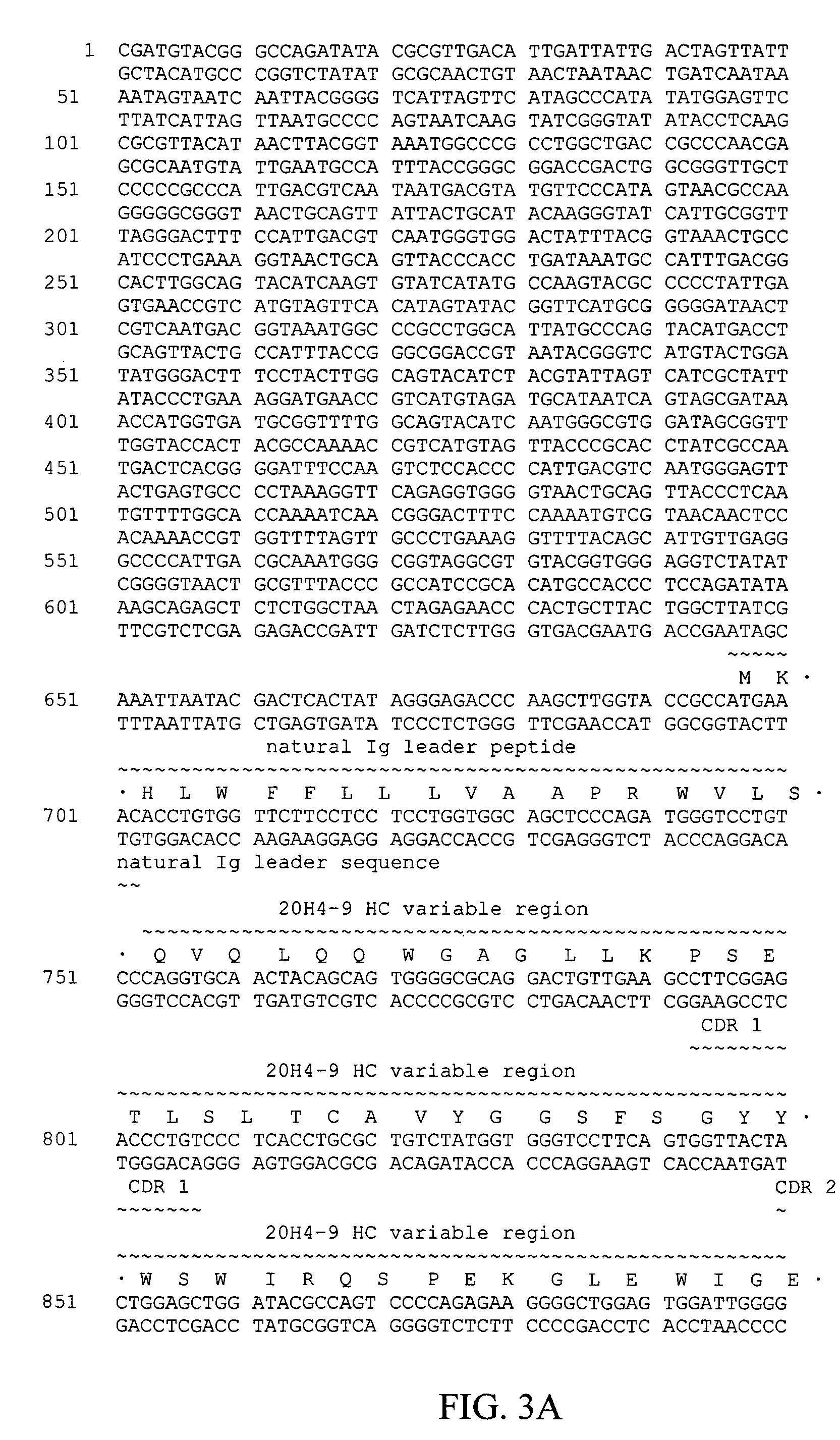
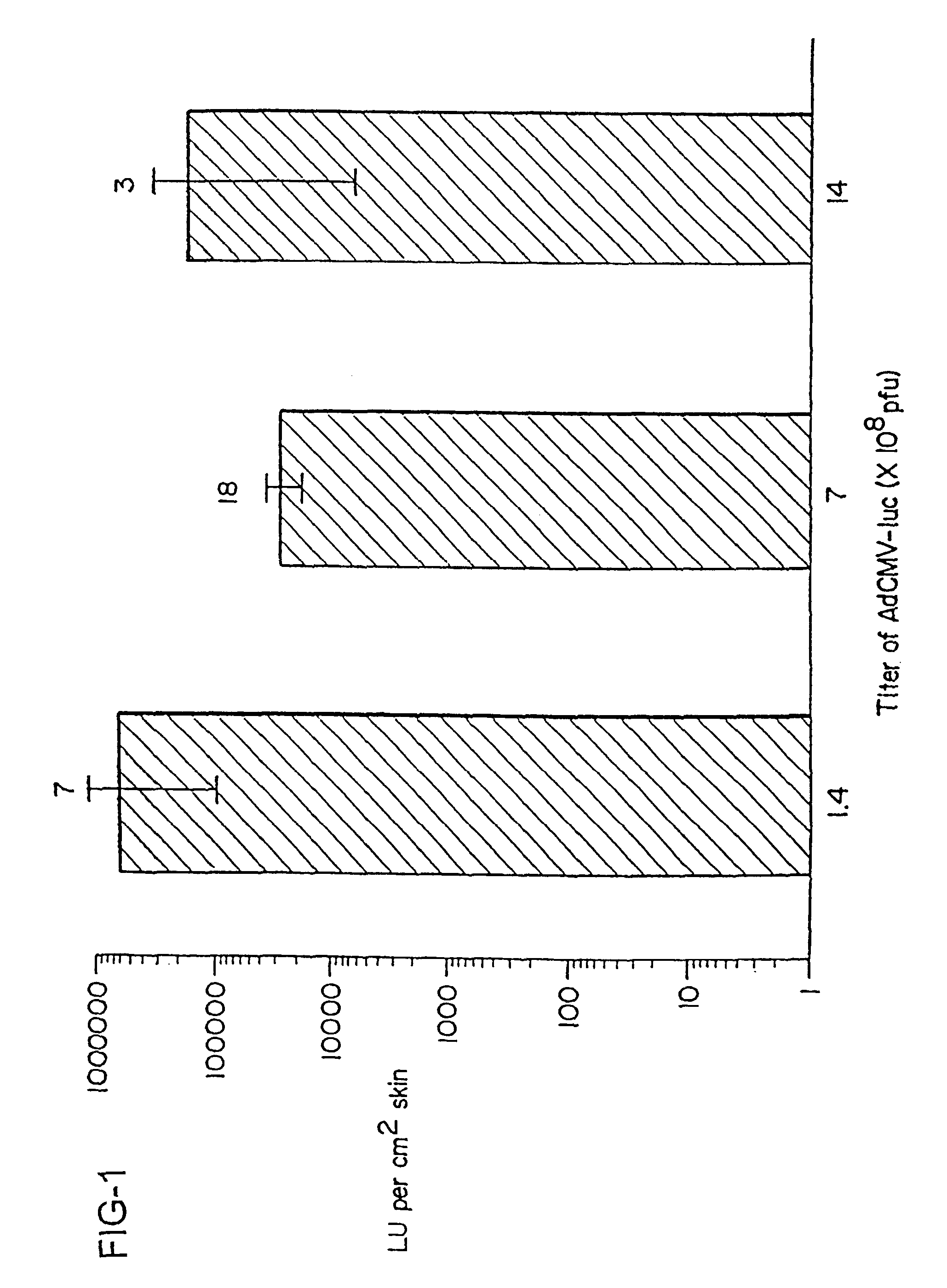
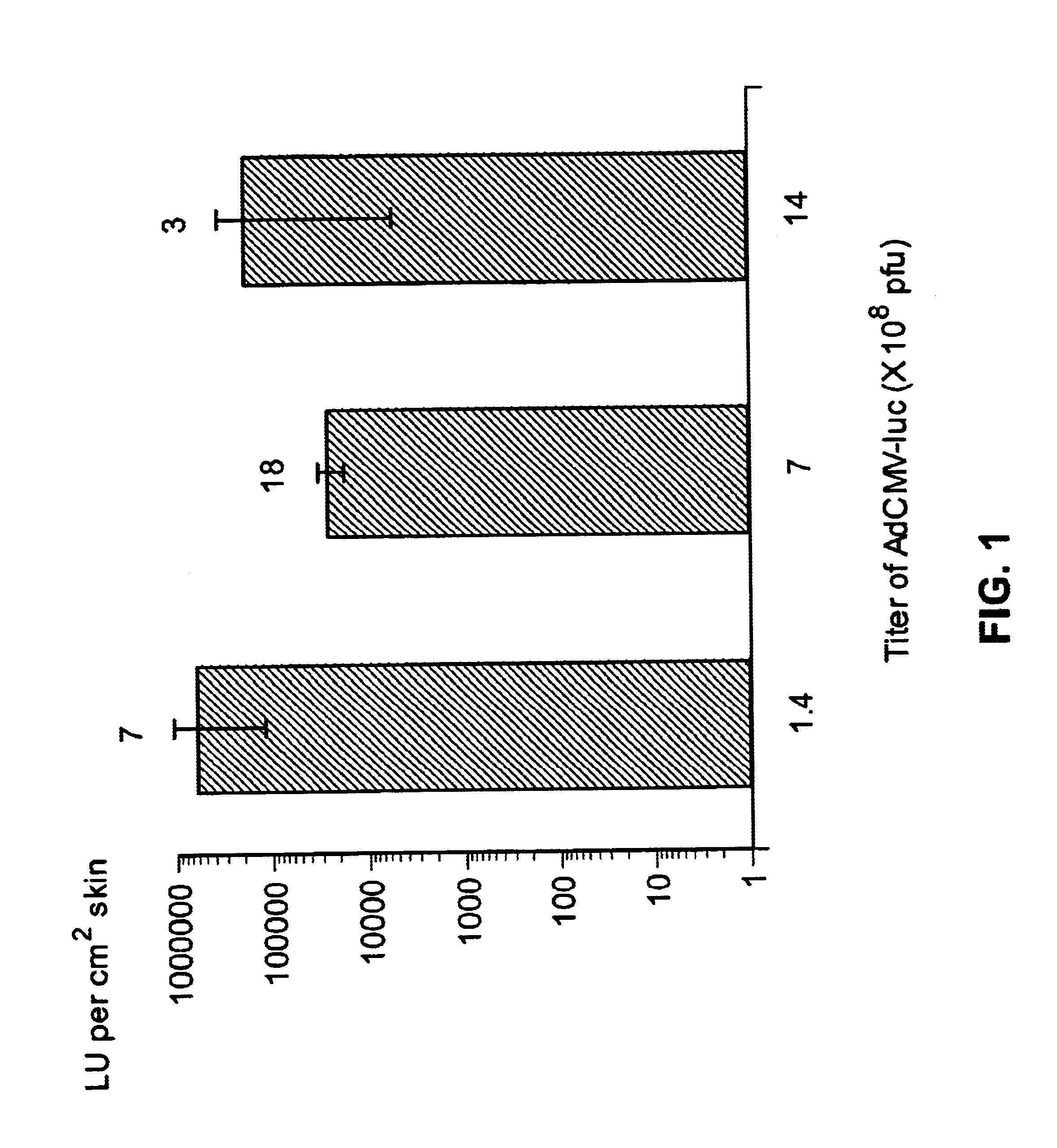
Method for genetic immunization and introduction of molecules into skeletal muscle and immune cells
InactiveUS6261281B1High transfection efficiencyGreat luciferace activityBacterial antigen ingredientsElectrotherapyVaccinationWhole body
A method is disclosed for enhanced vaccination and genetic vaccination of mammals. The vaccination is accomplished by delivering molecules such as proteins and nucleic acids into skeletal muscle and other cells residing in the skeletal muscle in vivo. The protein or nucleic acid is first injected into the muscle at one or multiple sites. Immediately or shortly after injection, electrodes are placed flanking the injection site and a specific amount of electrical current is passed through the muscle. The electrical current makes the muscle permeable, thus allowing the pharmaceutical drug or nucleic acid to enter the cell. The efficiency of transfer permits robust immune responses using DNA vaccines and produces sufficient secreted proteins for systemic biological activity to be observed.
Owner:INOVIO
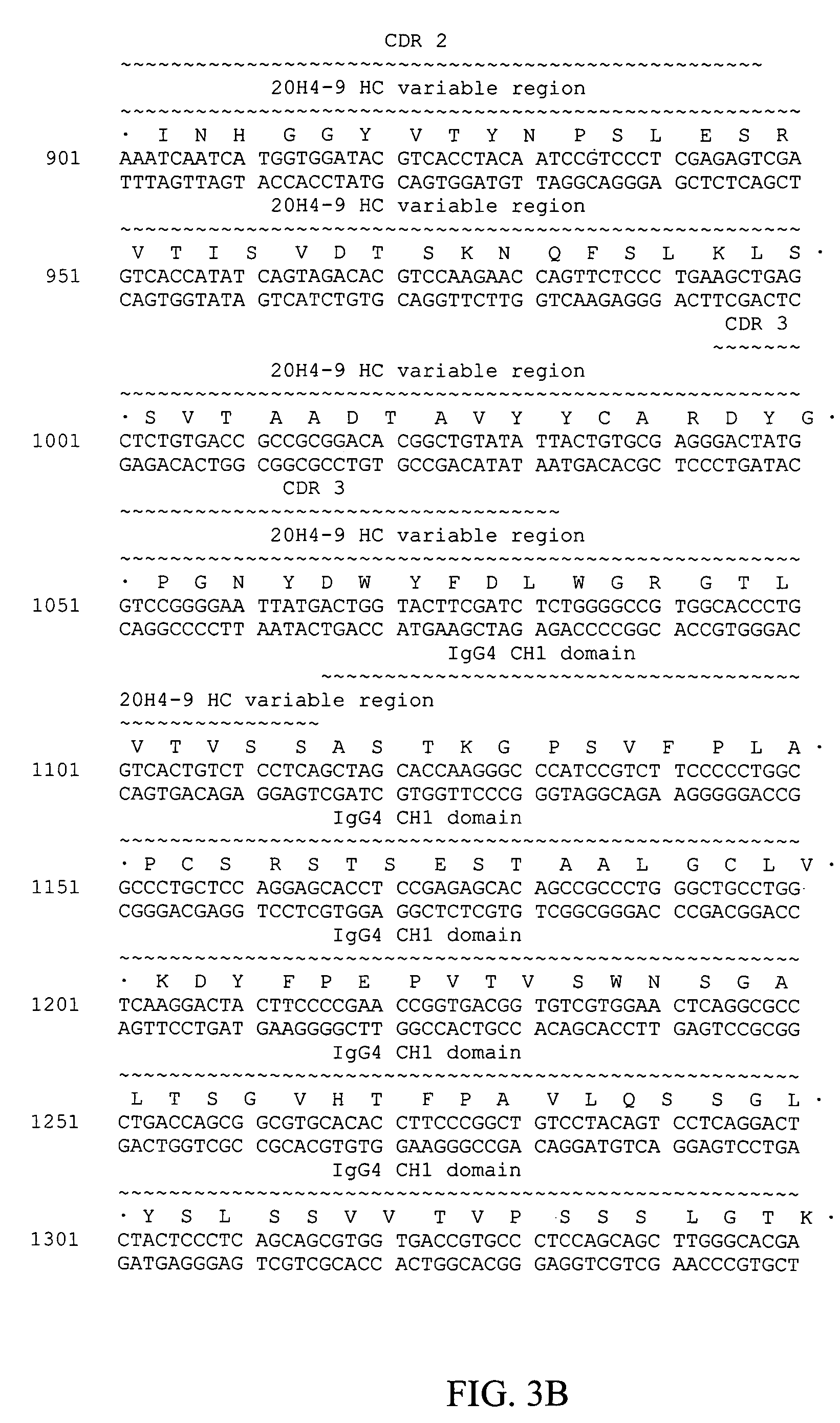
Fully human antibodies against human 4-1BB
Fully human antibodies and antigen-binding portions thereof that bind to human 4-1BB and that allow binding of human 4-1BB to a human 4-1BB ligand. In one aspect, the antibody is an IgG4 antibody. Also provided is a method for treating a disease in a subject comprising administering a therapeutically effective amount of the antibody to said subject.
Owner:BRISTOL MYERS SQUIBB CO
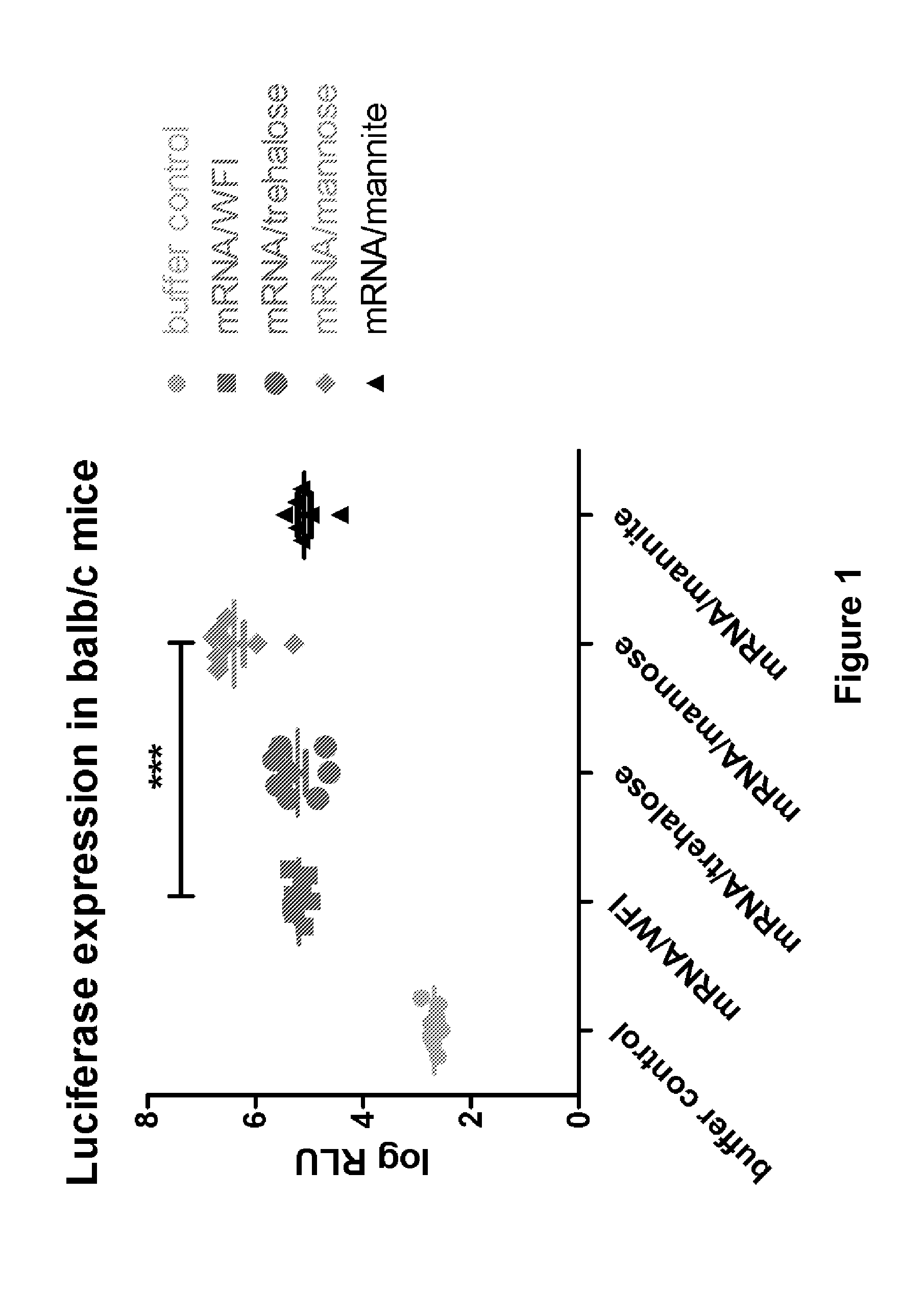
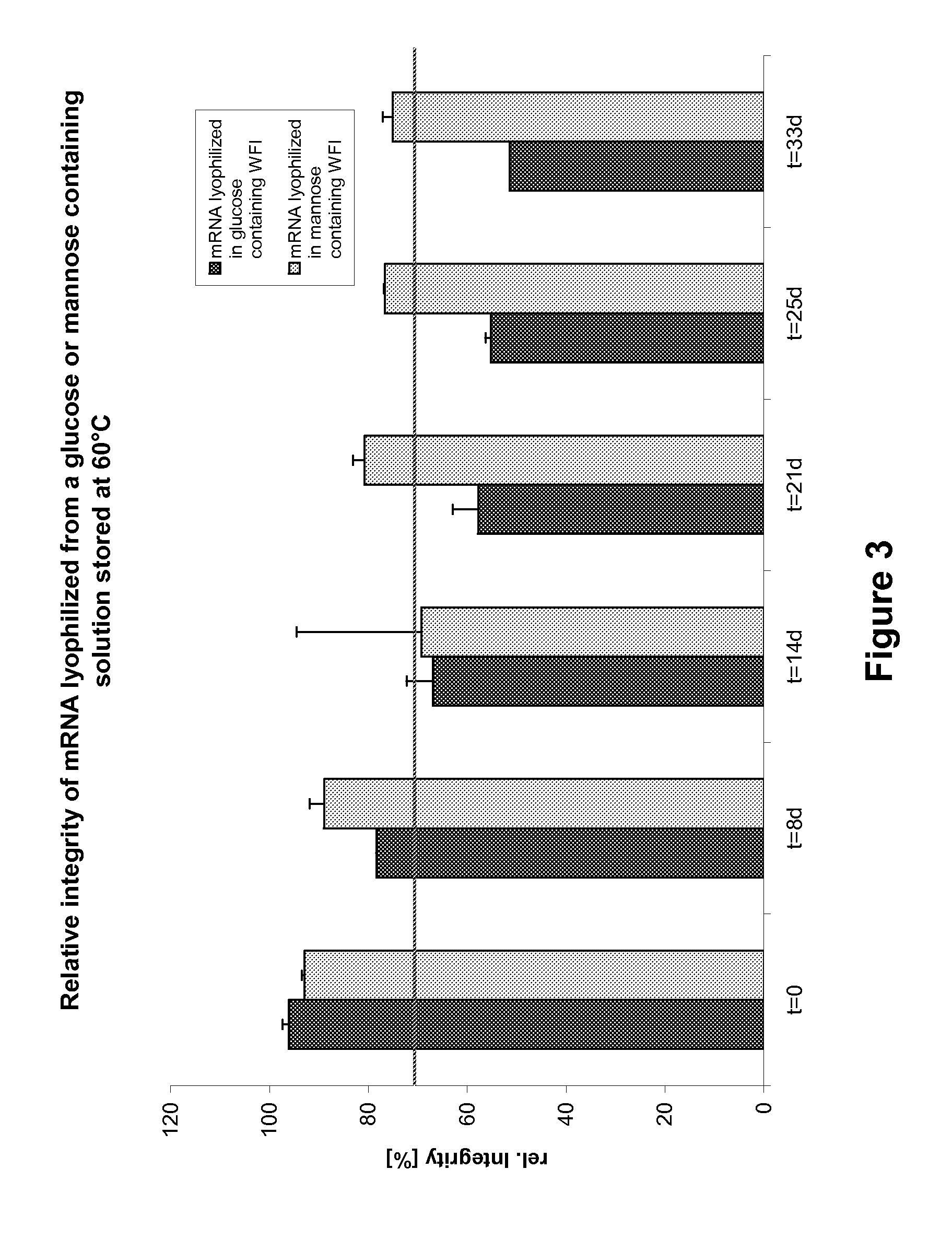
Mannose-containing solution for lyophilization, transfection and/or injection of nucleic acids
InactiveUS20120258046A1High transfection efficiencyEnhance protein expressionOrganic active ingredientsGenetic material ingredientsIn vivoTransfection
The present invention is directed to (the use of) a solution containing at least one nucleic acid (sequence) and free mannose for lyophilization, transfection and / or injection, particularly of RNA and mRNA. The inventive solution exhibits a positive effect on stabilization of the nucleic acid (sequence) during lyophilization and storage but also leads to a considerable increase of the transfection efficiency of a nucleic acid. It thus also increases in vivo expression of a protein encoded by such a nucleic acid upon increased transfection rate. The present invention is furthermore directed to a method of lyophilization using the mannose-containing solution, to pharmaceutical compositions, vaccines, kits, first and second medical uses applying such a mannose-containing solution and / or a nucleic acid (sequence) lyophilized or resuspended with such a solution.
Owner:CUREVAC SE
Noninvasive genetic immunization, expression products therefrom and uses thereof
InactiveUS6348450B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideHemagglutininWhole body
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, tetanus toxin C-fragment, anthrax protective antigen, HIV gp 120, human carcinoembryonic antigen, and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector.
Owner:UAB RES FOUND
Modification of RNA, Producing an Increased Transcript Stability and Translation Efficiency
ActiveUS20100129877A1Improve stability efficiencyImprove translation efficiencySugar derivativesFermentationTranslational efficiencyCell biology
Owner:JOHANNES GUTENBERG UNIV MAINZ VERTRETEN DURCH DEN PRASIDENTEN
Microparticles with adsorbent surfaces, methods of making same, and uses thereof
InactiveUS6884435B1Stimulate immune responseEasy to usePowder deliverySsRNA viruses positive-senseAntigenDisease
The present invention is directed to microparticles, to microparticle compositions containing the same, to methods of forming the same, and to uses for the same, including use for a vaccine, for raising an immune response, for treatment of a disease and for diagnosis of a disease. The microparticles comprise a biodegradable polymer, such as a poly(α-hydroxy acid), a polyhydroxy butyric acid, a polycaprolactone, a polyorthoester, a polyanhydride, or a polycyanoacrylate, and a detergent selected from a cationic detergent and an anionic detergent. The microparticles further comprise an antigen adsorbed on the surface of the microparticle.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Influenza hemagglutinin and neuraminidase variants
InactiveUS20050042229A1Efficient productionSsRNA viruses negative-senseHydrolasesHemagglutininNeuraminidase
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising influenza hemagglutinin and neuraminidase variants are provided.
Owner:MEDIMMUNE LLC
Reduction of porcine circovirus-2 viral load with inactivated PCV-2
InactiveUS6517843B1Improving immunogenicityImprove performanceBiocideGenetic material ingredientsDiseaseStaining
Porcine circovirus-2 (PCV-2) is a recently identified agent wherein the potential spectrum of PCV-2-associated disease has been expanded by evidence of vertical and sexual transmission and associated reproductive failure in swine populations. PCV-2 was isolated from a litter of aborted piglets from a farm experiencing late term abortions and stillbirths. Severe, diffuse myocarditis was present in one piglet associated with extensive immunohistochemical staining for PCV-2 antigen. Variable amounts of PCV-2 antigen were also present in liver, lung and kidney of multiple fetuses. Inoculation of female pigs with a composition including an immunogen from PCV-2 or an epitope of interest from such an immunogen or with a vector expressing such an immunogen or epitope of interest prior to breeding, such as within the first five weeks of life, or prior to the perinatal period, or repeatedly over a lifetime, or during pregnancy, such as between the 6th and 8th and / or the 10th and 13th weeks of gestation, can prevent myocarditis, abortion and intrauterine infection associated with porcine circovirus-2. In addition, innoculation of male and / or female pigs with the aforementioned compositions can be carried out to prevent transmission of PCV-2 from male to female (or vice versa) during mating. Thus, the invention involves methods and compositions for preventing myocarditis, abortion and intrauterine infection associated with porcine circovirus-2.
Owner:QUEENS UNIV OF BELFAST +4
Fc fusion proteins for enhancing the immunogenicity of protein and peptide antigens
InactiveUS20050261229A1Stimulate productionEnhanceVirusesPeptide/protein ingredientsFc receptorAdjuvant
Disclosed herein are methods and compositions for enhancing the immunogenicity of a preselected protein or peptide antigen in a mammal. Immunogenicity is enhanced by fusing the preselected antigen to an immunoglobulin heavy chain constant region to produce an Fc-antigen fusion protein. The Fc-antigen fusion proteins bind Fc receptors on the surface of antigen presenting cells, thereby targeting the antigen to the antigen presenting cells in the mammal. In addition, disclosed is a family of adjuvants, for example, an Fc-adjuvant fusion protein, for use in combination with the Fc-antigen fusion proteins to enhance or modulate a particular immune response against the preselected antigen.
Owner:MERCK PATENT GMBH
Yeast-dendritic cell vaccines and uses thereof
Owner:UNIV OF COLORADO THE REGENTS OF +1
Cytotoxic t-lymphocyte-inducing immunogens for prevention, treatment, and diagnosis of cancer
The present invention relates to compositions and methods for the prevention, treatment, and diagnosis of cancer, especially carcinomas, such as breast carcinoma. The invention discloses peptides, polypeptides, and polynucleotides that can be used to stimulate a CTL response against breast or cancer.
Owner:IMMUNOTOPE
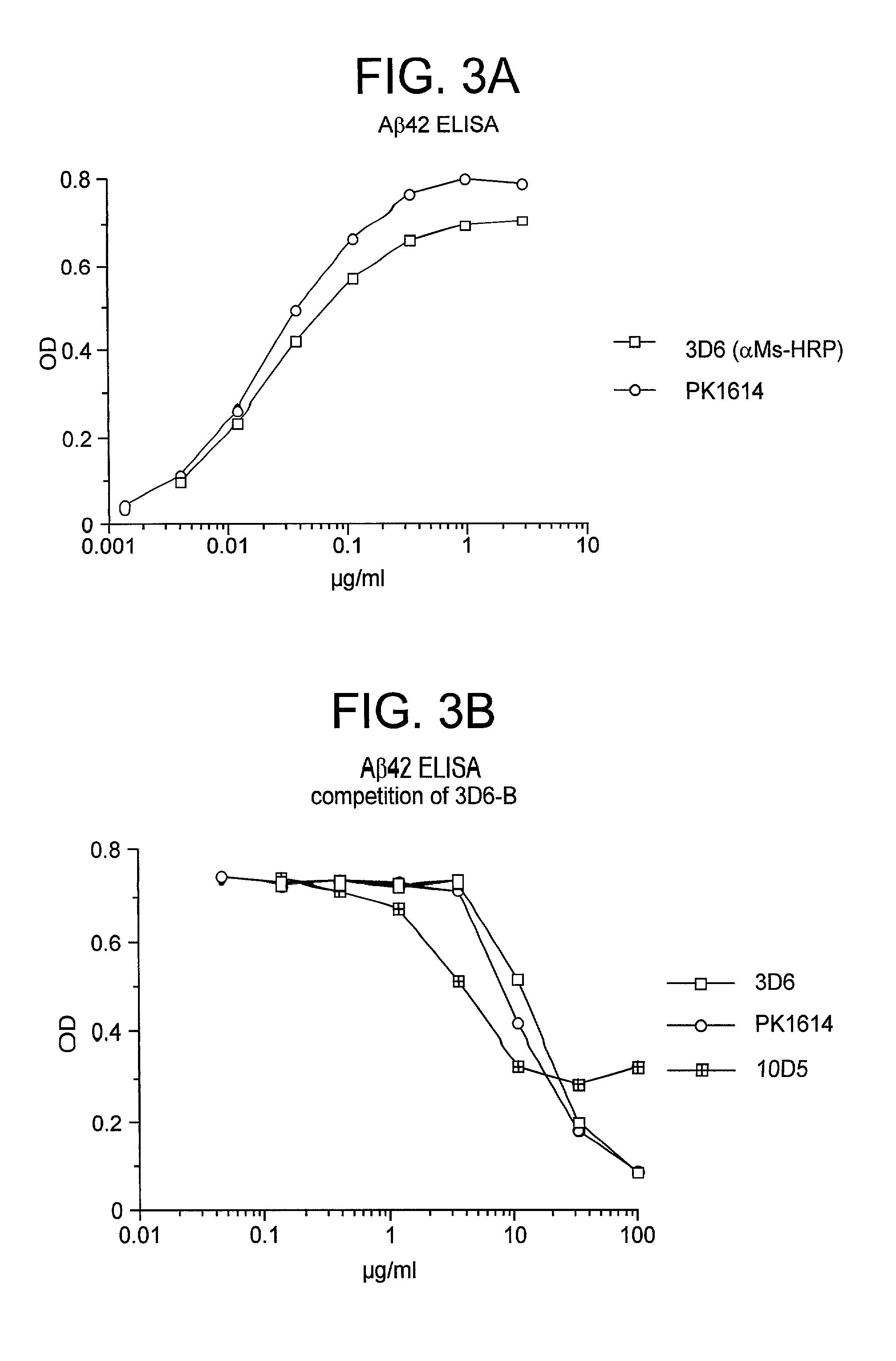
Passive immunization of Alzheimer's disease
InactiveUS6913745B1Peptide/protein ingredientsAntibody mimetics/scaffoldsPassive ImmunizationsAmyloid disease
Disclosed are pharmaceutical compositions and methods for preventing or treating a number of amyloid diseases, including Alzheimer's disease, prion diseases, familial amyloid neuropathies and the like. The pharmaceutical compositions include immunologically reactive amounts of amyloid fibril components, particularly fibril-forming peptides or proteins. Also disclosed are therapeutic compositions and methods which use immune reagents that react with such fibril components.
Owner:JANSSEN SCI IRELAND UC
Fully human antibodies against human 4-1BB
ActiveUS20050095244A1Enhance immune responseSugar derivativesViral antigen ingredientsDisease4-1BB ligand
Fully human antibodies and antigen-binding portions thereof that bind to human 4-1BB and that allow binding of human 4-1BB to a human 4-1BB ligand. In one aspect, the antibody is an IgG4 antibody. Also provided is a method for treating a disease in a subject comprising administering a therapeutically effective amount of the antibody to said subject.
Owner:BRISTOL MYERS SQUIBB CO
Retrovirus and viral vectors
InactiveUS6635472B1Prevent and attenuate diseaseEnhance immune responseGenetic material ingredientsVirus peptidesVirus-RetrovirusDna viral
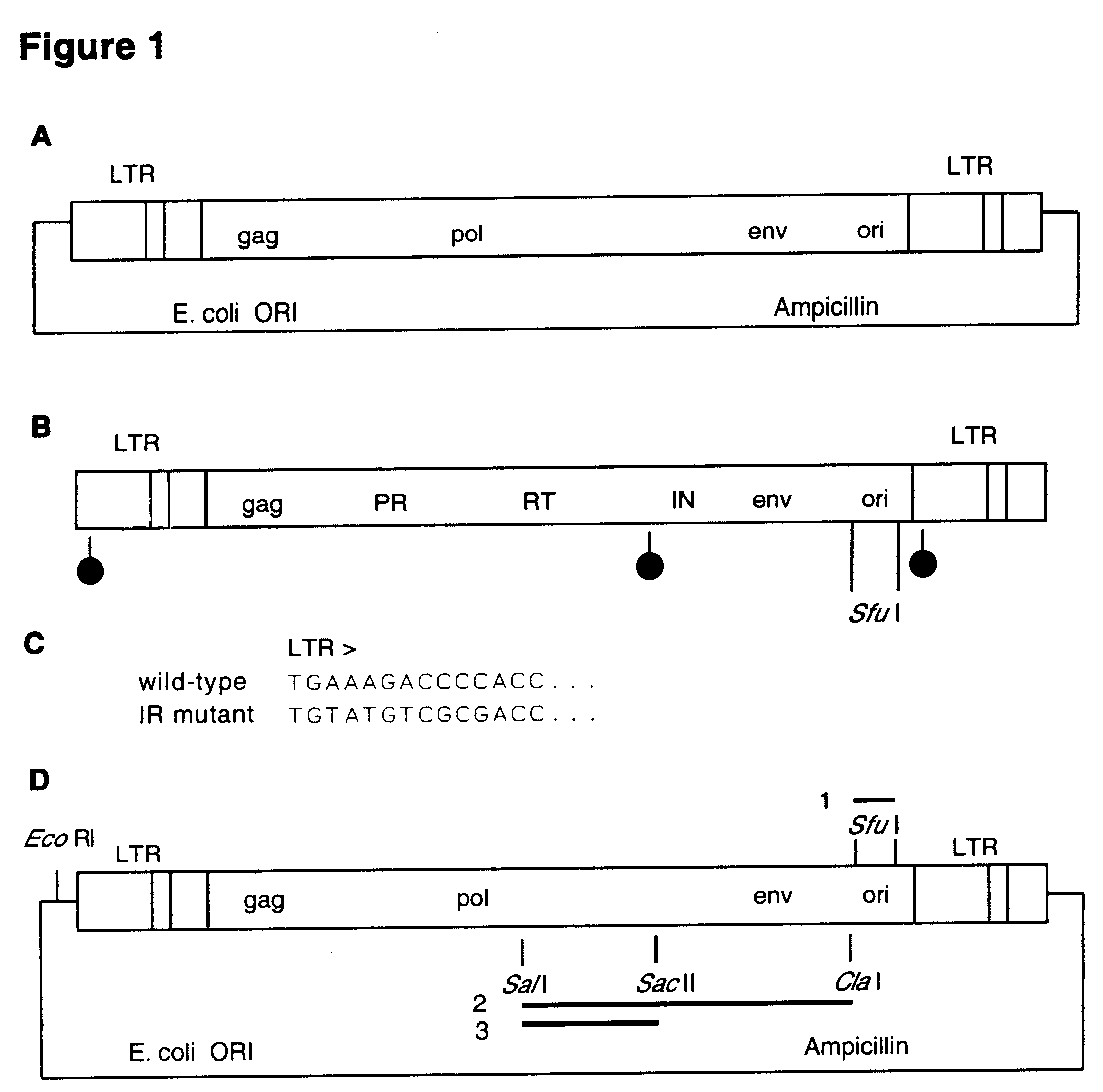
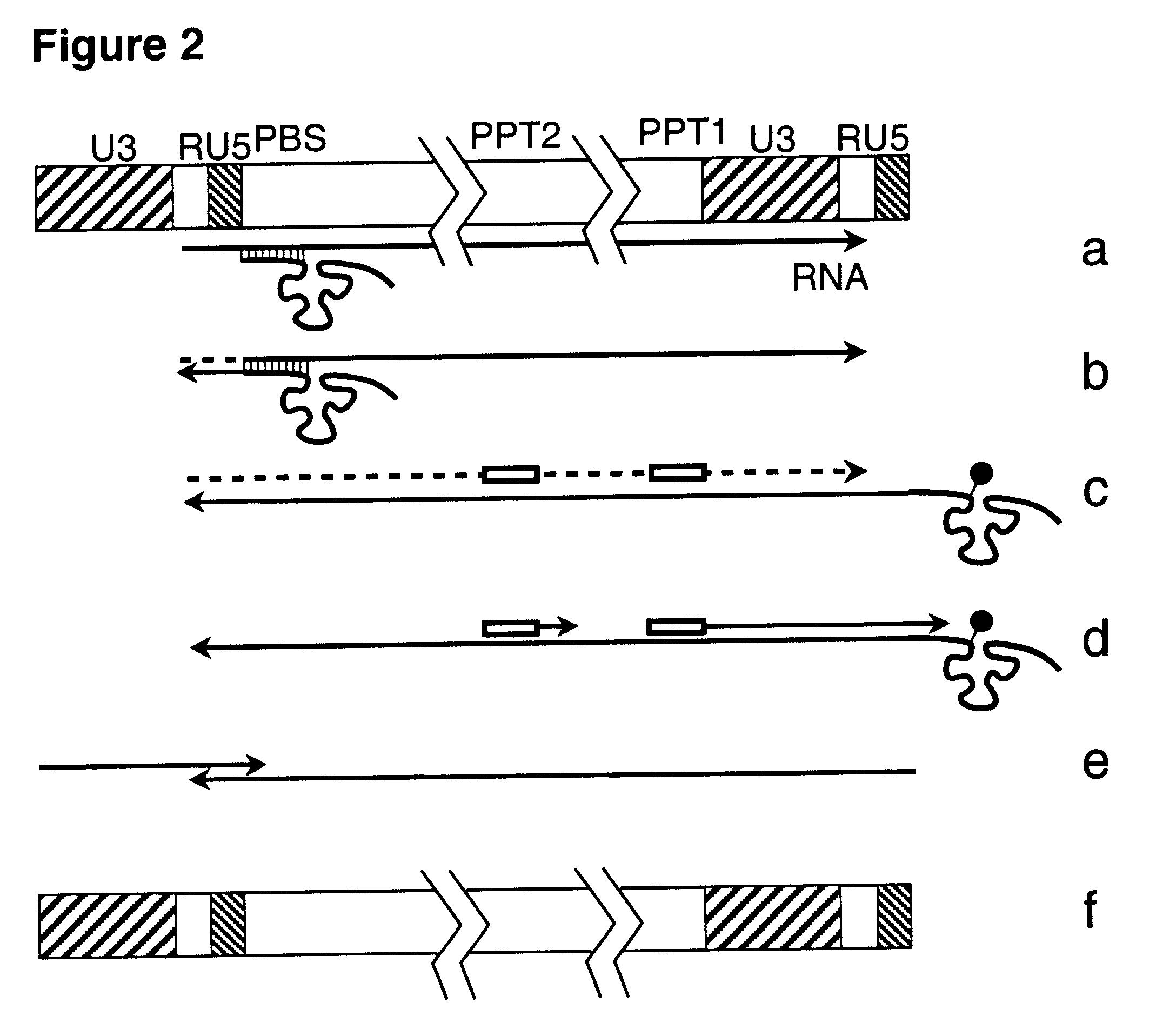
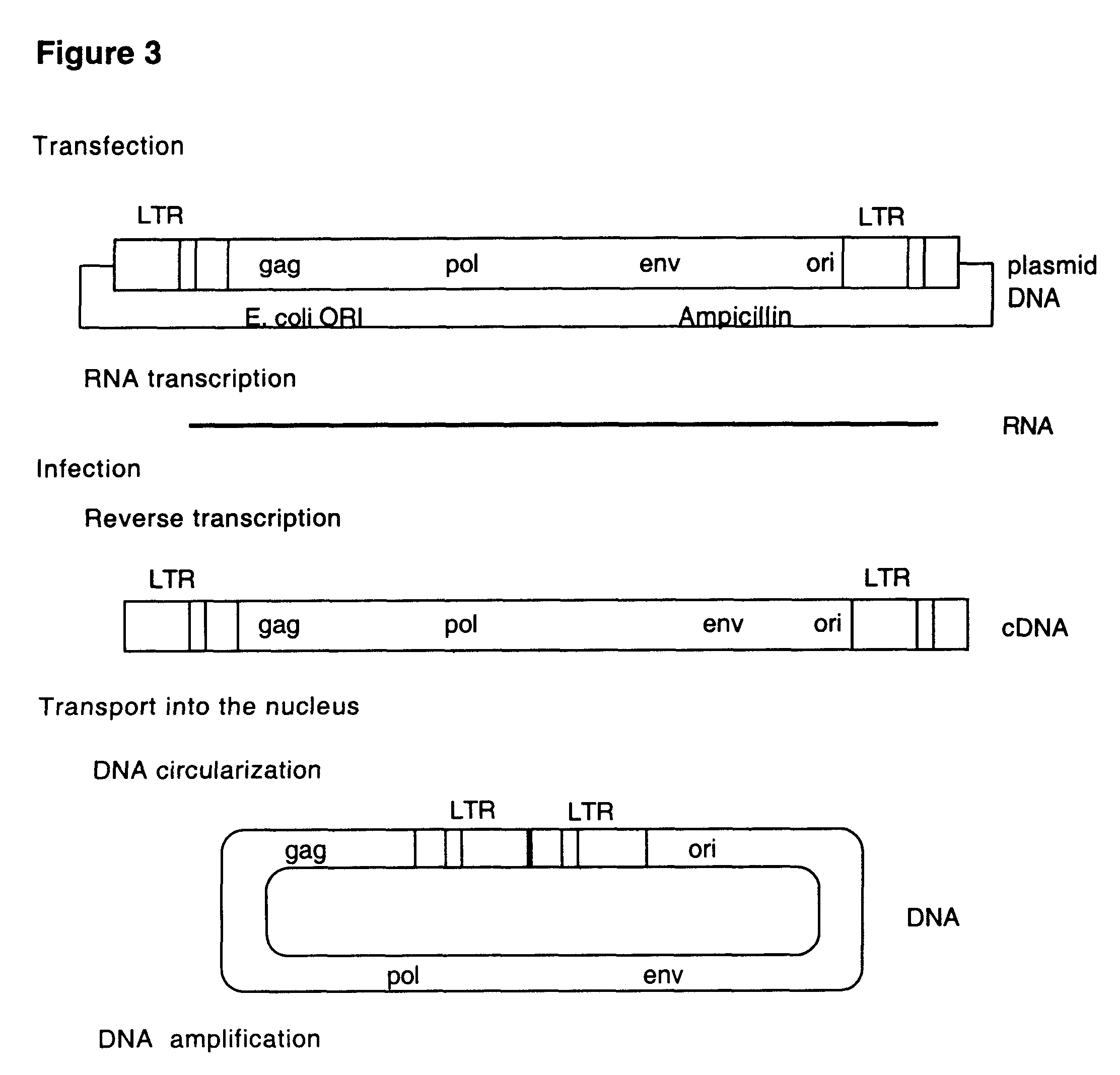
This invention relates to the fields of genetic engineering, virus replication and gene transfer. More specifically, this invention relates to polynucleotide construct, recombinant virus, transposon, and their vectors, wherein an ori derived from a DNA virus capable of replicating in vertebrate cells is inserted into the retrovirus, allowing the retrovirus following the reverse transcription to efficiently replicate as extrachromosomal or episomal DNA without the necessity of integration into the host cell chromosome. Additionally, this invention relates to polynucleotide construct, recombinant virus, transposon, and their vectors replicating episomally without aid of an ori and related elements. Also, this invention encompasses preventive, therapeutic, and diagnostic applications employing said constructs, viruses and vectors.
Owner:RUBICON LABS
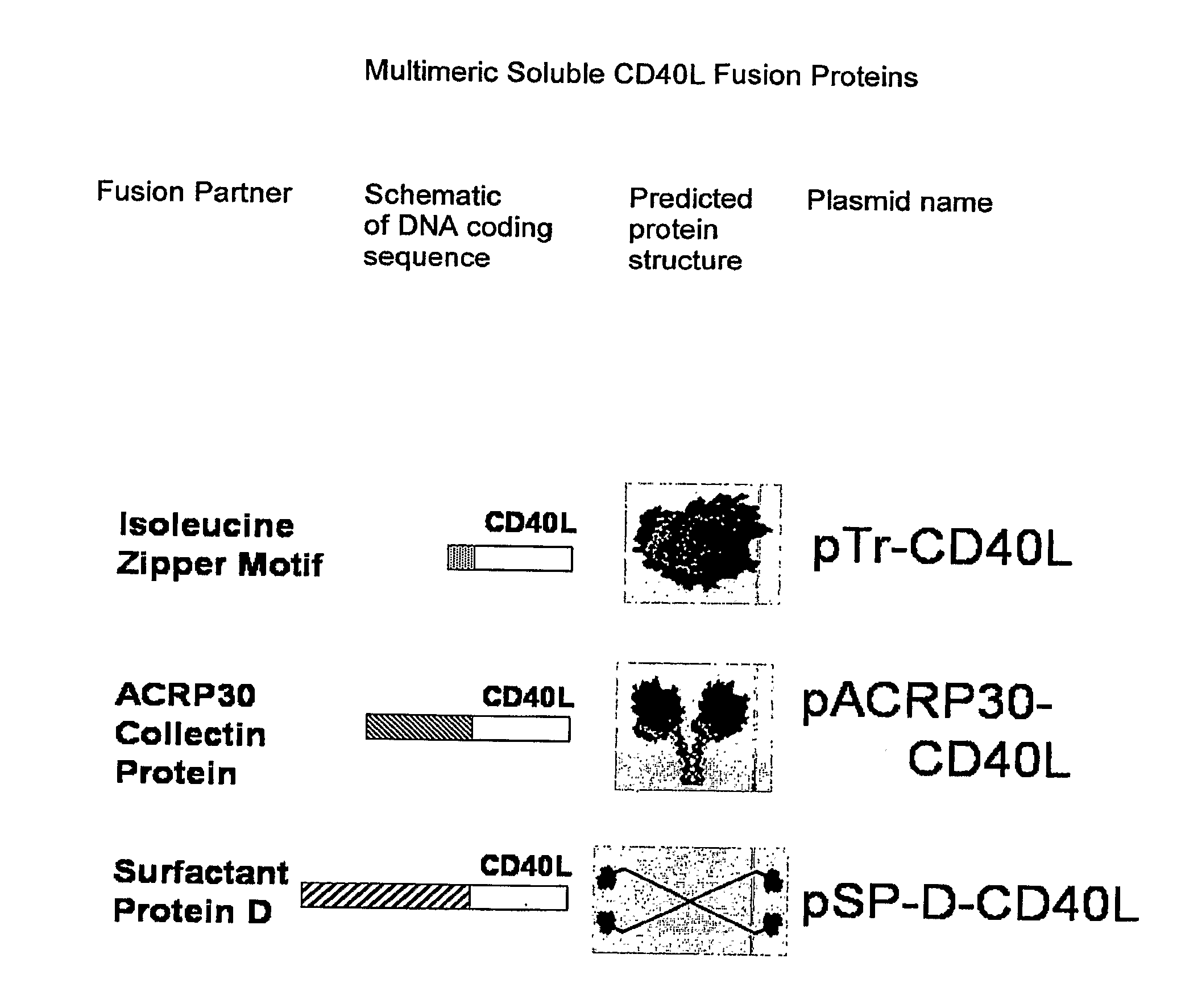
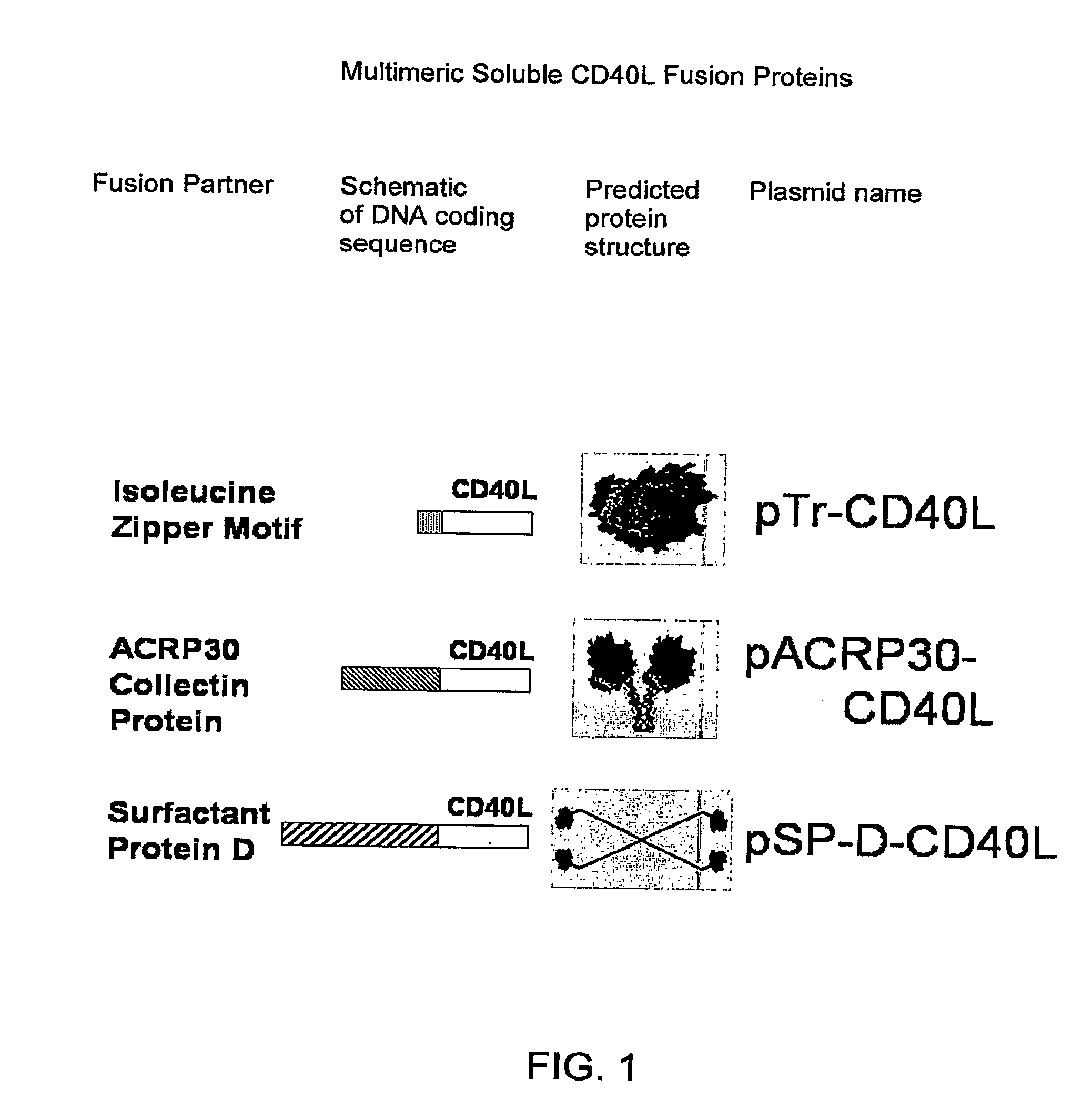
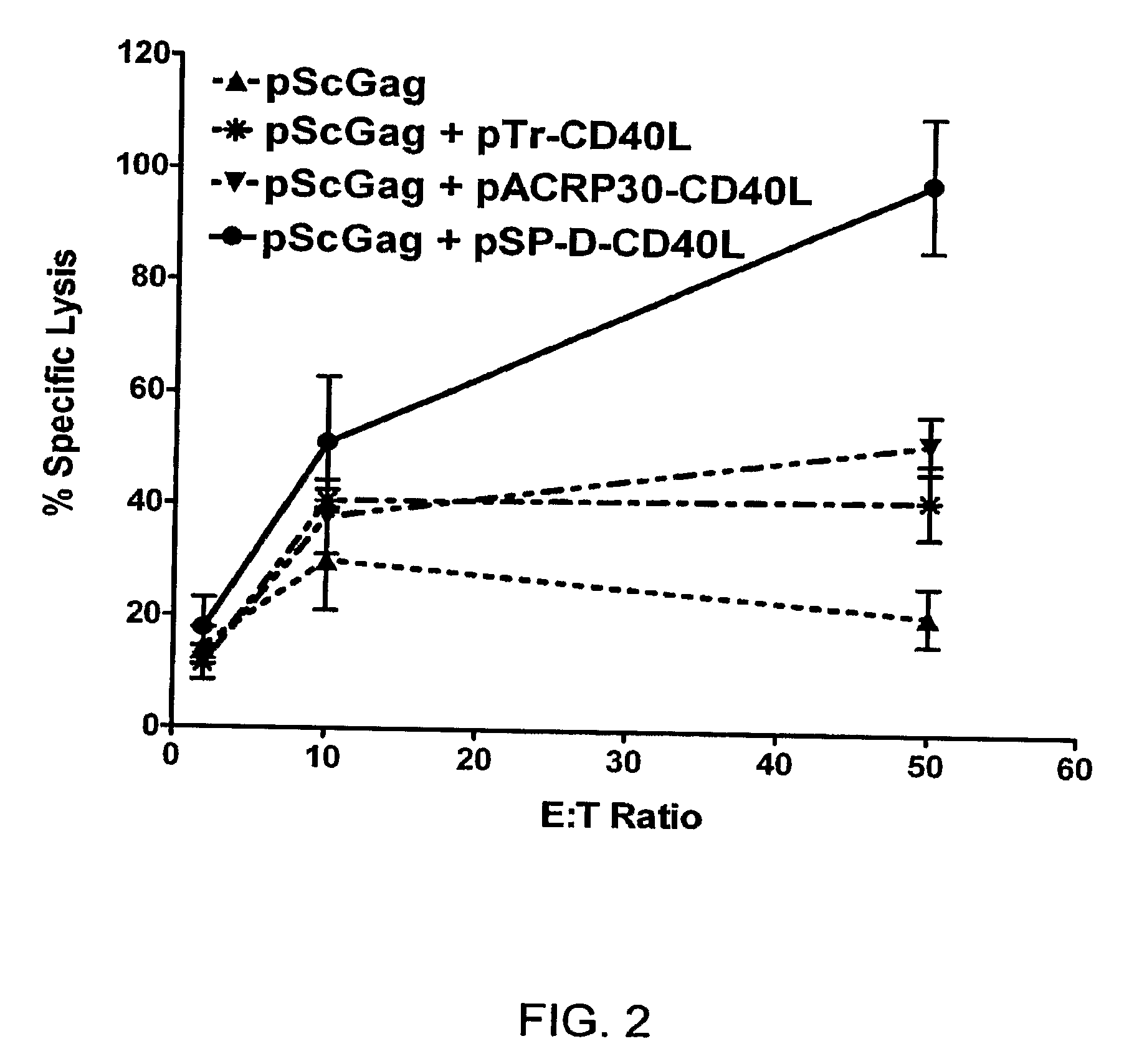
DNA vaccines encoding antigen linked to a domain that binds CD40
InactiveUS7118751B1Improve abilitiesEasy to demonstrateAntibody mimetics/scaffoldsVirus peptidesPeptide antigenEukaryotic plasmids
Vaccines that target one or more antigens to a cell surface receptor improve the antigen-specific humoral and cellular immune response. Antigen(s) linked to a domain that binds to a cell surface receptor are internalized, carrying antigen(s) into an intracellular compartment where the antigen(s) are digested into peptides and loaded onto MHC molecules. T cells specific for the peptide antigens are activated, leading to an enhanced immune response. The vaccine may comprise antigen(s) linked to a domain that binds at least one receptor or a DNA plasmid encoding antigen(s) linked to a domain that binds at least one receptor. A preferred embodiment of the invention targets HIV-1 env antigen to the CD40 receptor, resulting in delivery of antigen to CD40 positive cells, and selective activation of the CD40 receptor on cells presenting HIV-1 env antigens to T cells.
Owner:HAYDEN LEDBETTER MARTHA S +1
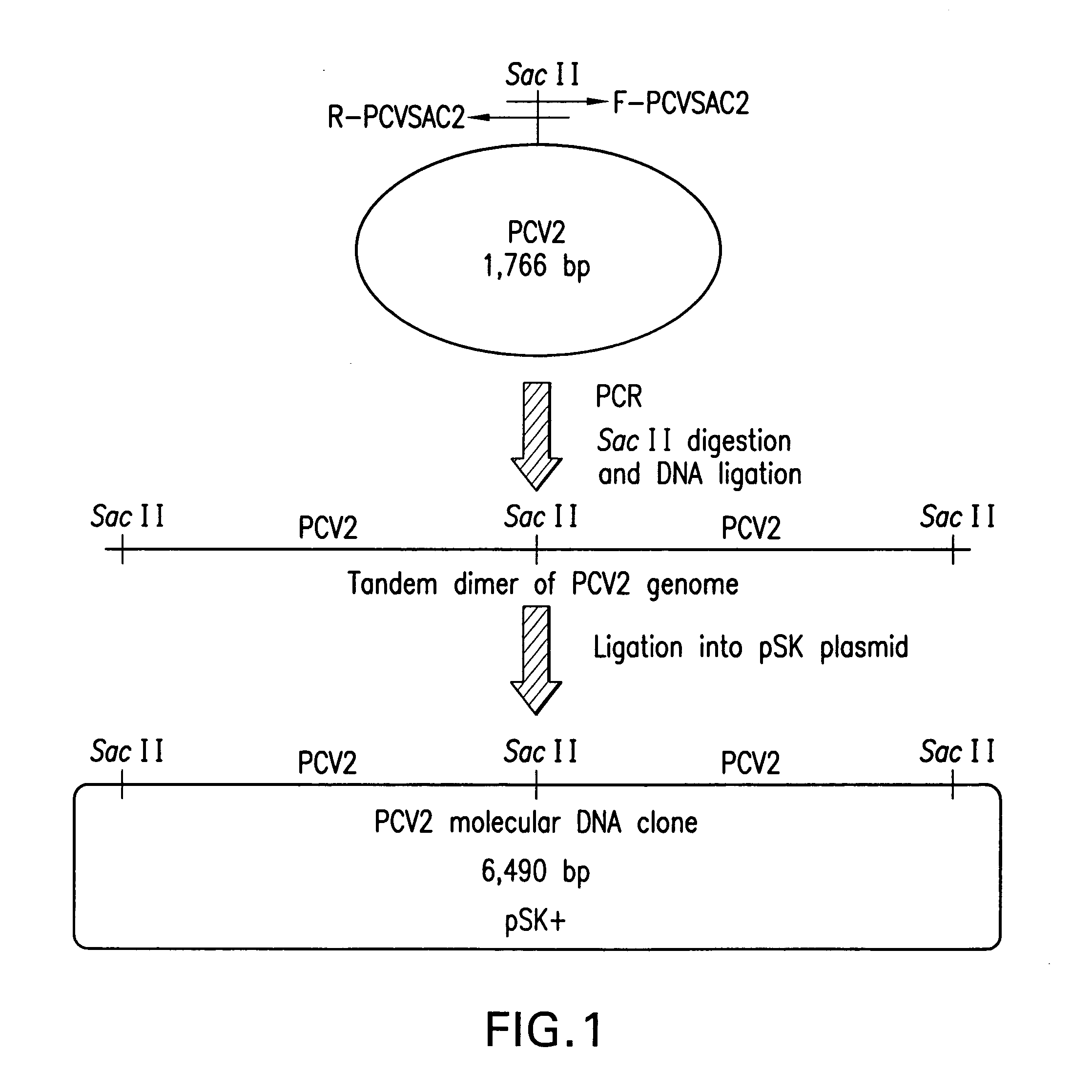
Chimeric infectious DNA clones, chimeric porcine circoviruses and uses thereof
InactiveUS7279166B2Facilitate cell culture growthEnsure vaccine safetyFungiBacteriaSpecific immunityADAMTS Proteins
The present invention relates to infectious DNA clones, infectious chimeric DNA clones of porcine circovirus (PCV), vaccines and means of protecting pigs against viral infection or postweaning multisystemic wasting syndrome (PMWS) caused by PCV2. The new chimeric infectious DNA clone and its derived, avirulent chimeric virus are constructed from the nonpathogenic PCV1 in which the immunogenic ORF gene of the pathogenic PCV2 replaces a gene of the nonpathogenic PCV1, preferably in the same position. The chimeric virus advantageously retains the nonpathogenic phenotype of PCV1 but elicits specific immune responses against the pathogenic PCV2. The invention further embraces the immunogenic polypeptide expression products. In addition, the invention encompasses two mutations in the PCV2 immunogenic capsid gene and protein, and the introduction of the ORF2 mutations in the chimeric clones.
Owner:IOWA STATE UNIV RES FOUND +1
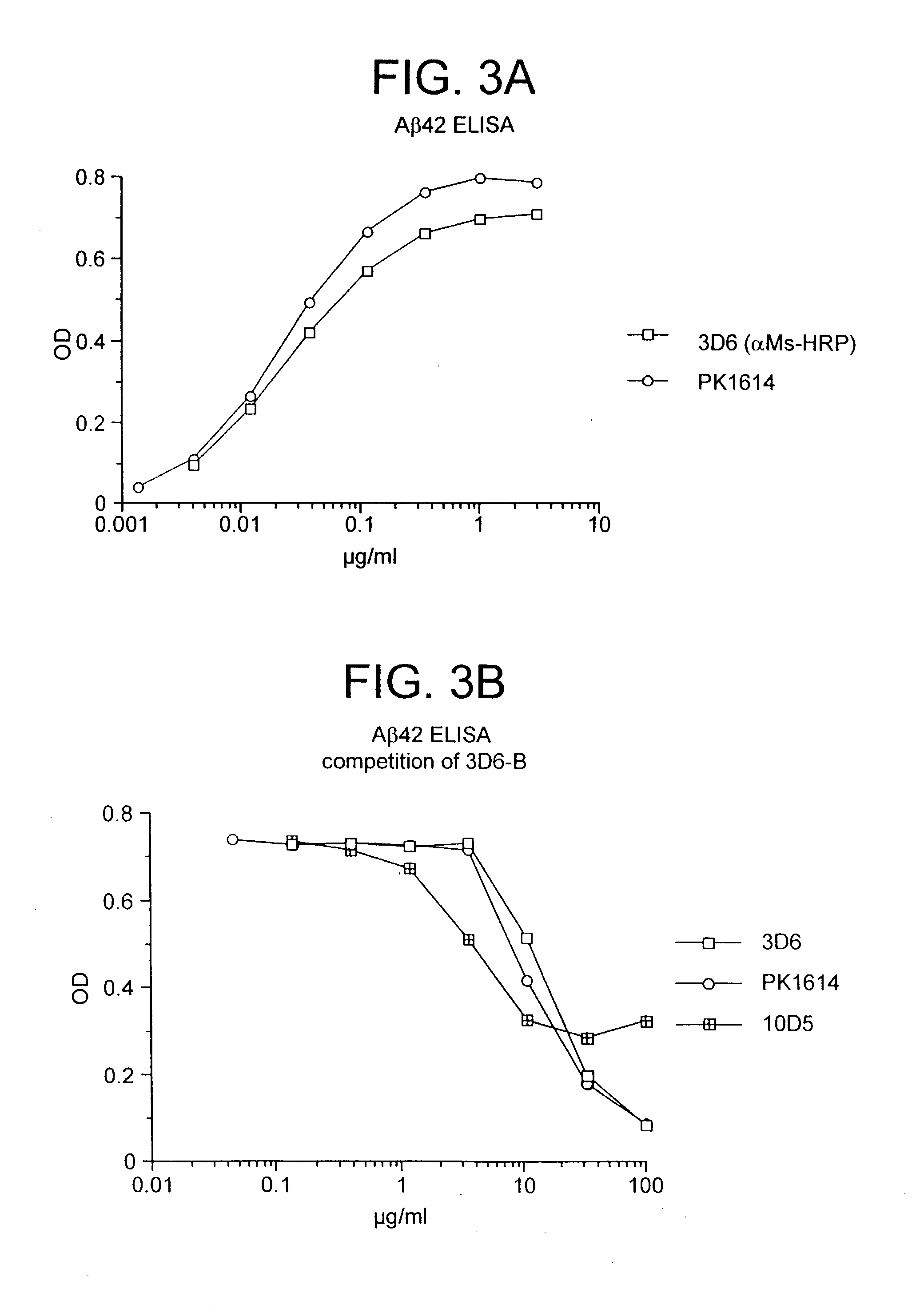
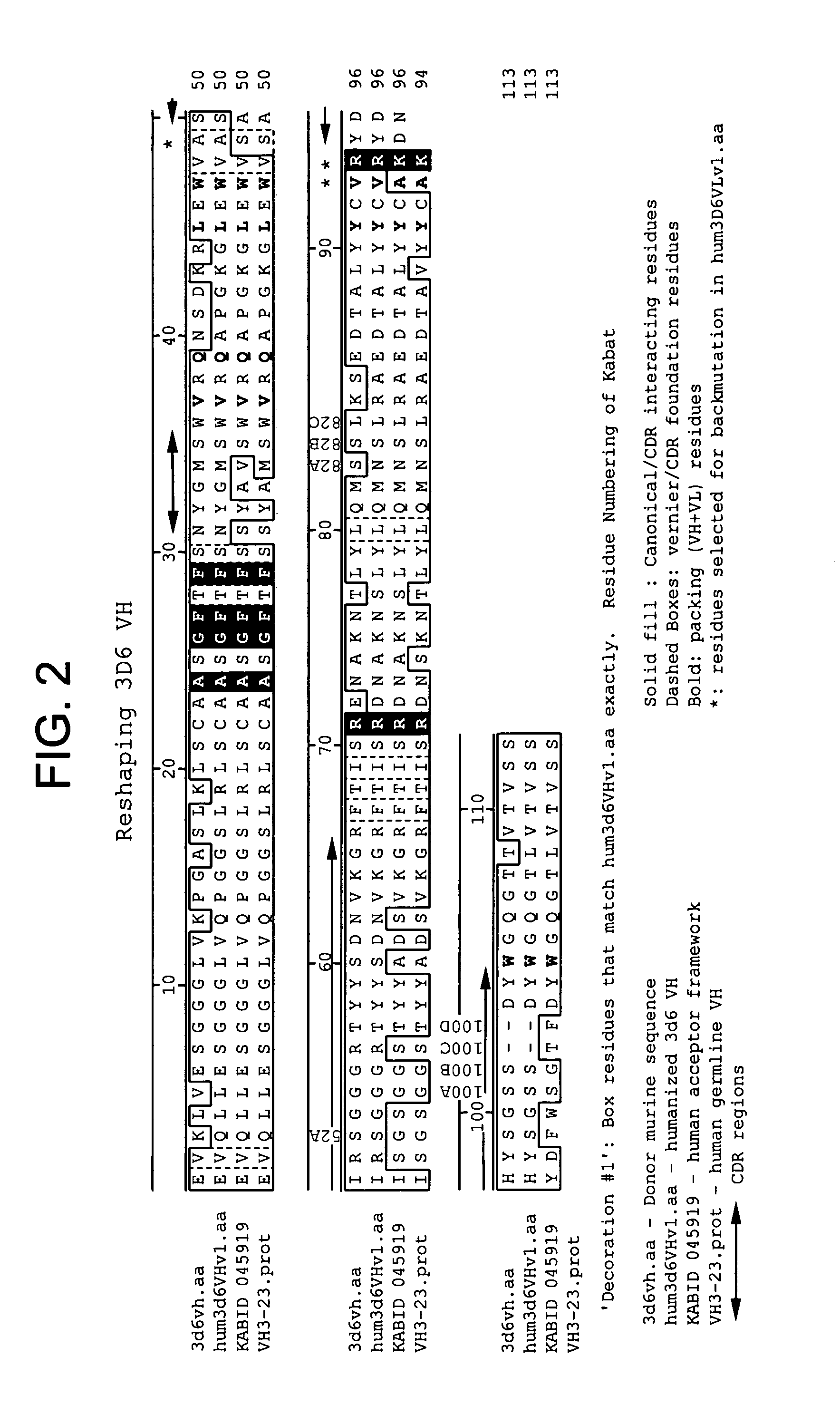
Humanized antibodies that recognize beta amyloid peptide
InactiveUS20050009150A1High binding affinityLow immunogenicityFungiNervous disorderHumanized antibodyΒ amyloid peptide
The invention provides improved agents and methods for treatment of diseases associated with amyloid deposits of Aβ in the brain of a patient. Preferred agents include humanized antibodies.
Owner:JANSSEN ALZHEIMER IMMUNOTHERAPY +1
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
Methods of elicit, enhance and sustain immune responses against MHC class I-restricted epitopes, for prophylactic or therapeutic purposes
InactiveUS20050079152A1Improve responseEfficient amplificationAntibacterial agentsBiocideEpitopeMHC class I
Embodiments relate to methods and compositions for eliciting, enhancing, and sustaining immune responses, preferably against MHC class I-restricted epitopes. The methods and compositions can be used for prophylactic or therapeutic purposes.
Owner:MANNKIND CORP
Therapeutic delivery compositions and methods of use thereof
InactiveUS20020128218A1Reduce deliveryFacilitate transmission and introductionBiocidePeptide/protein ingredientsHydrophileNucleic acid sequencing
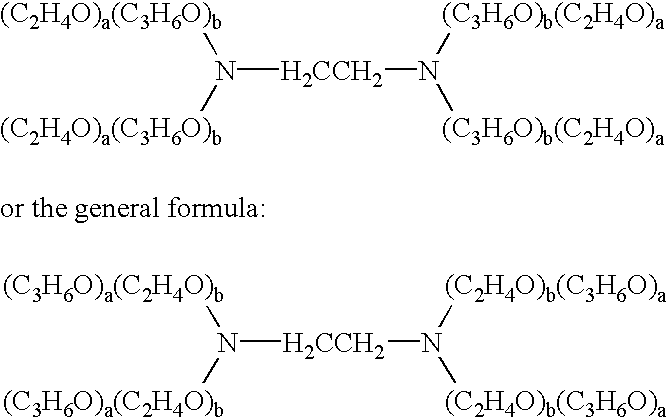
The present invention relates to compositions and methods for treating infectious diseases and genetic disorders through gene therapy and intracellular delivery of antisense oligonucleotides or other nucleic acid sequences. The present invention comprises a therapeutic delivery composition effective for treating a disease state comprising an administerable admixture of an effective amount of a therapeutic compound capable of altering nucleic acid sequence function and an effective amount of a block copolymer having the following general formula: 1 wherein: the mean aggregate molecular weight of the portion of the octablock copolymer represented by polyoxypropylene is between about 5000 and about 7000 Daltons; a is a number such that the portion represented by polyoxyethylene constitutes between about 10% to about 40% of the compound by weight; and b is a number such that the polyoxypropylene portion of the total molecular weight of the octablock copolymer constitutes between about 60% and about 90% of the compound by weight. The present invention also includes compositions and methods using biologically active nonionic reverse block copolymers. The reverse copolymers have an inner core of polyoxypropylene (POP) that is flanked on either end by polyoxyethylene (POE). The reverse block copolymers have the following formula: 2 wherein "b" represents a number such that the molecular weight of the hydrophobe (C.sub.3H.sub.6O).sub.b is between approximately 2,000 and 10,000, and "a" represents a number such that the percentage of hydrophile (C.sub.2H.sub.4O).sub.a is between approximately 5% and 30%.
Owner:EMANUELE R MARTIN +3
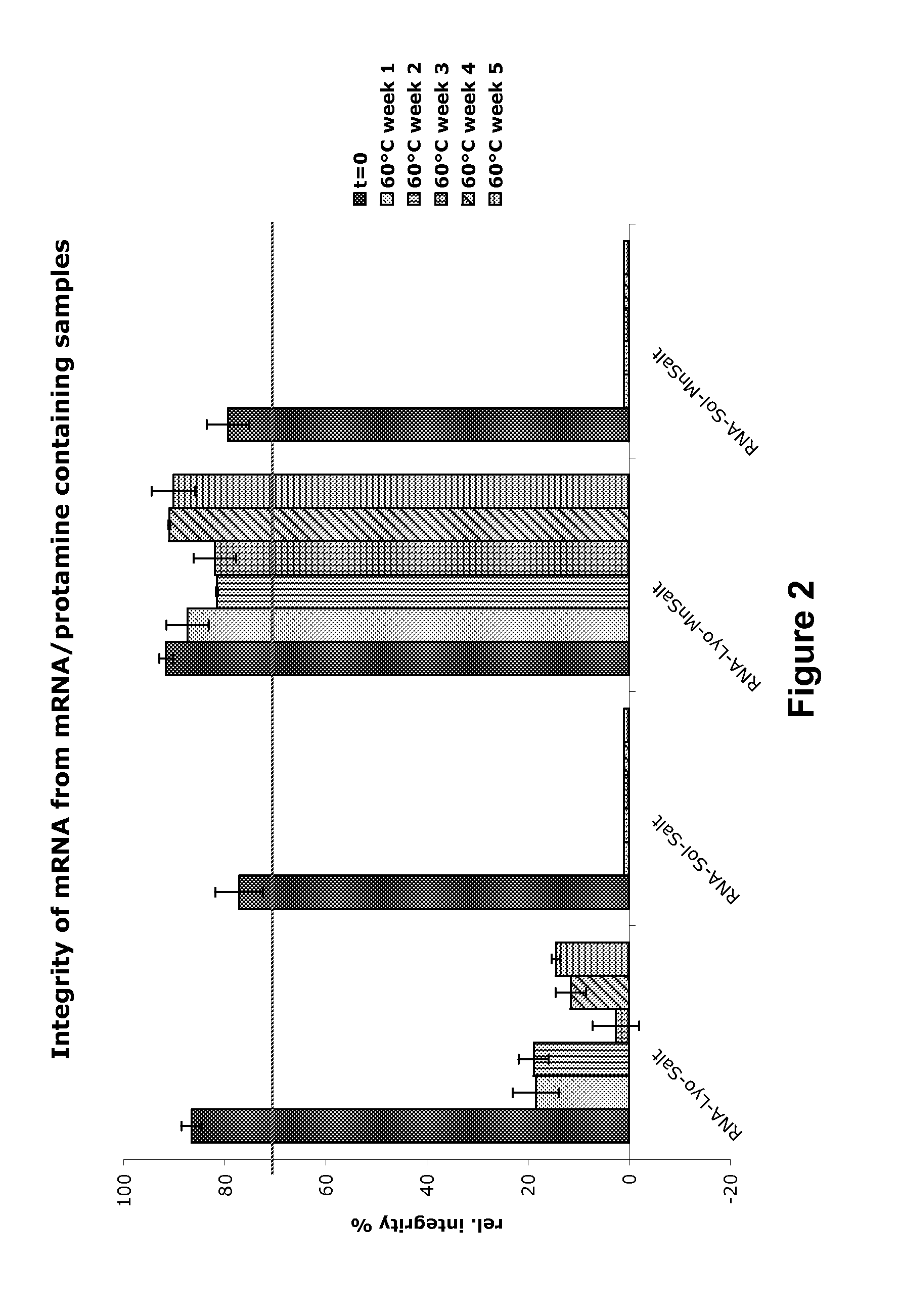
Immunisation of large mammals with low doses of RNA
ActiveUS20130149375A1Conveniently preparedImprove stabilityAntibacterial agentsSsRNA viruses negative-senseMammalImmunity response
RNA encoding an immunogen is delivered to a large mammal at a dose of between 2 μg and 100 μg. Thus the invention provides a method of raising an immune response in a large mammal, comprising administering to the mammal a dose of between 2 μg and 100 μg of immunogen-encoding RNA. Similarly, RNA encoding an immunogen can be delivered to a large mammal at a dose of 3 ng / kg to 150 ng / kg. The delivered RNA can elicit an immune response in the large mammal
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Method of inducing an antigen-specific immune response by administering a synergistic combination of adjuvants comprising unmethylated CpG-containing nucleic acids and a non-nucleic acid adjuvant
The present invention relates generally to adjuvants, and in particular to methods and products utilizing a synergistic combination of immunostimulatory oligonucleotides having at least one unmethylated CpG dinucleotide (CpG ODN) and a non-nucleic acid adjuvant. Such combinations of adjuvants may be used with an antigen or alone. The present invention also relates to methods and products utilizing immunostimulatory oligonucleotides having at least one unmethylated CpG dinucleotide (CpG ODN) for induction of cellular immunity in infants.
Owner:UNIV OF IOWA RES FOUND +2
Inducing cellular immune responses to human papillomavirus using peptide and nucleic acid compositions
InactiveUS20070014810A1Reduce the possibilityImproving immunogenicitySugar derivativesViral antigen ingredientsEpitopeT cell
This invention uses our knowledge of the mechanisms by which antigen is recognized by T cells to identify and prepare human papillomavirus (HPV) epitopes, and to develop epitope-based vaccines directed towards HPV. More specifically, this application communicates our discovery of pharmaceutical compositions and methods of use in the prevention and treatment of HPV infection.
Owner:GENIMMUNE NV +1
Pharmaceutical compositions and methods for treatment of amyloid diseases
Disclosed are pharmaceutical compositions and methods for preventing or treating a number of amyloid diseases, including Alzheimer's disesase, prion diseases, familial amyloid neuropathies and the like. The pharmaceutical compositions include immunologically reactive amounts of amyloid fibril components, particularly fibril-forming peptides or proteins. Also disclosed are therapeutic compositions and methods which use immune reagents that react with such fibril components.
Owner:JANSSEN ALZHEIMER IMMUNOTHERAPY
Ultrasound assisted transdermal vaccine delivery method and system
InactiveUS20050112135A1Adequate buffering capacityHigh viscosityViral antigen ingredientsSurgeryVaccine deliveryBiocompatible coating
An apparatus and method for transdermally delivering a vaccine comprising a delivery system having (i) a microprojection member (or system) that includes a plurality of microprojections (or array thereof) that are adapted to pierce through the stratum corneum into the underlying epidermis layer, or epidermis and dermis layers and (ii) an ultrasonic device. In one embodiment, the vaccine is contained in a biocompatible coating that is applied to the microprojection member. In a further embodiment, the delivery system includes a gel pack having a vaccine-containing hydrogel formulation that is disposed on the microprojection member after application to the skin of a patient. In an alternative embodiment, the vaccine is contained in both the coating and the hydrogel formulation.
Owner:ALZA CORP
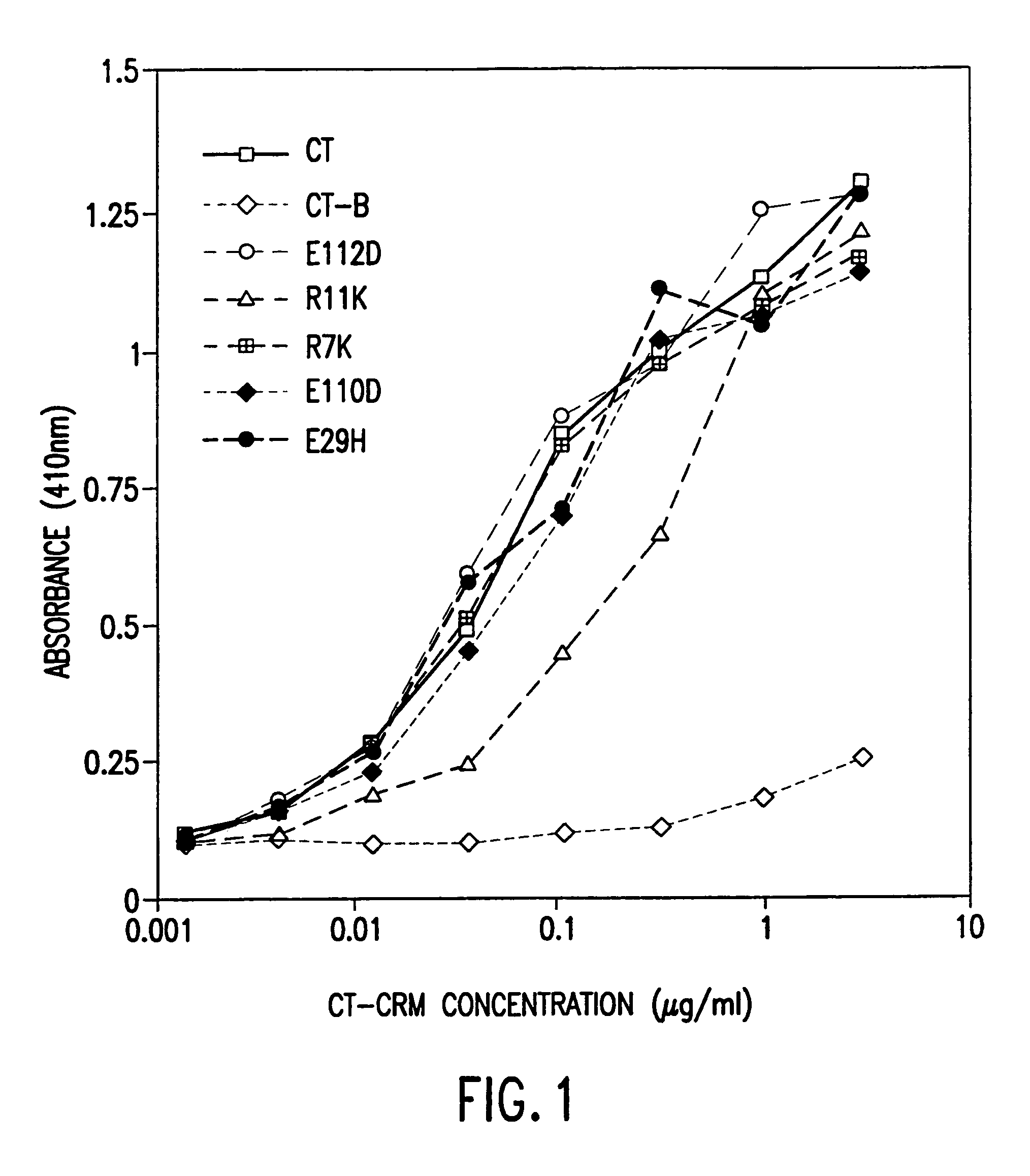
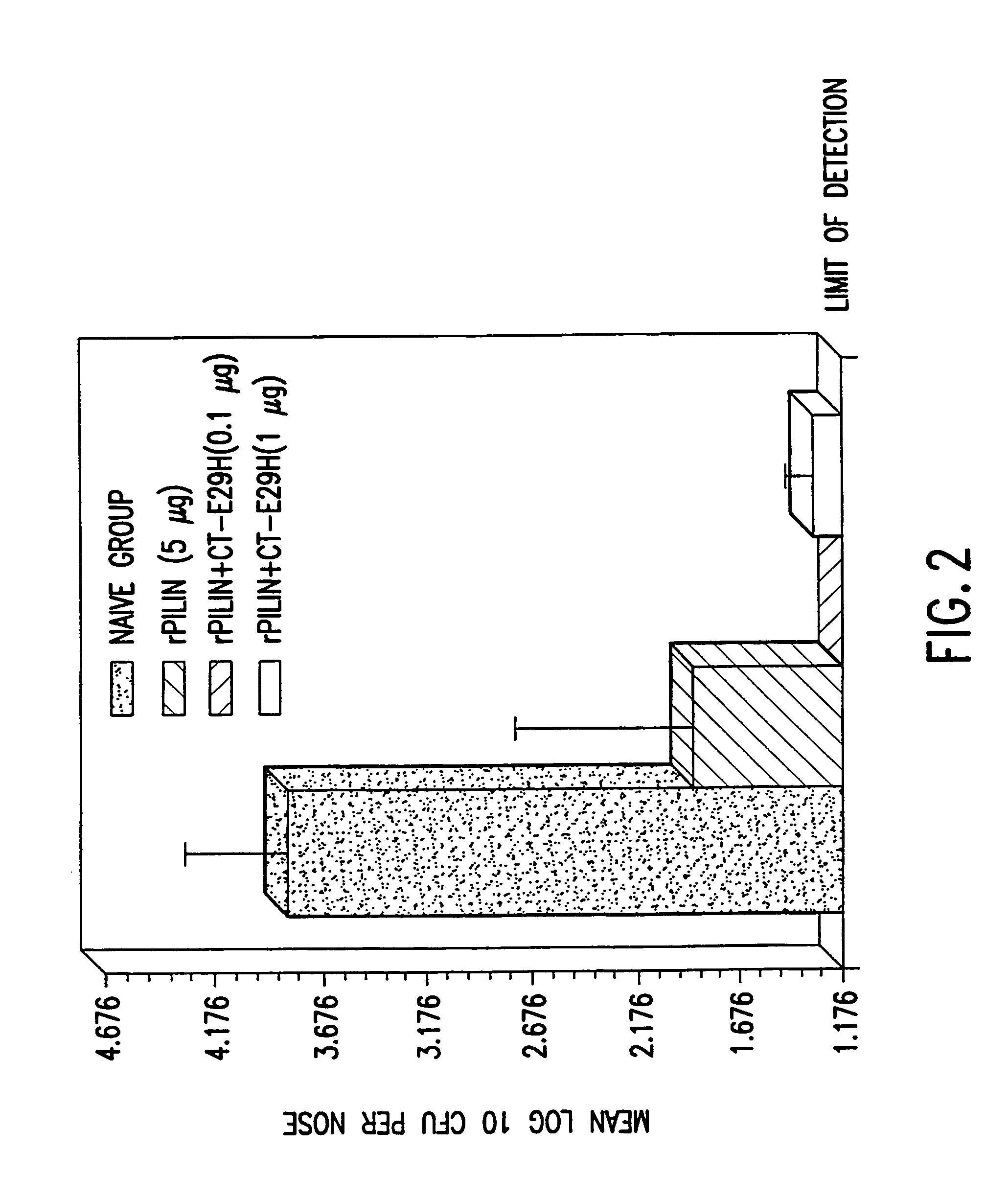
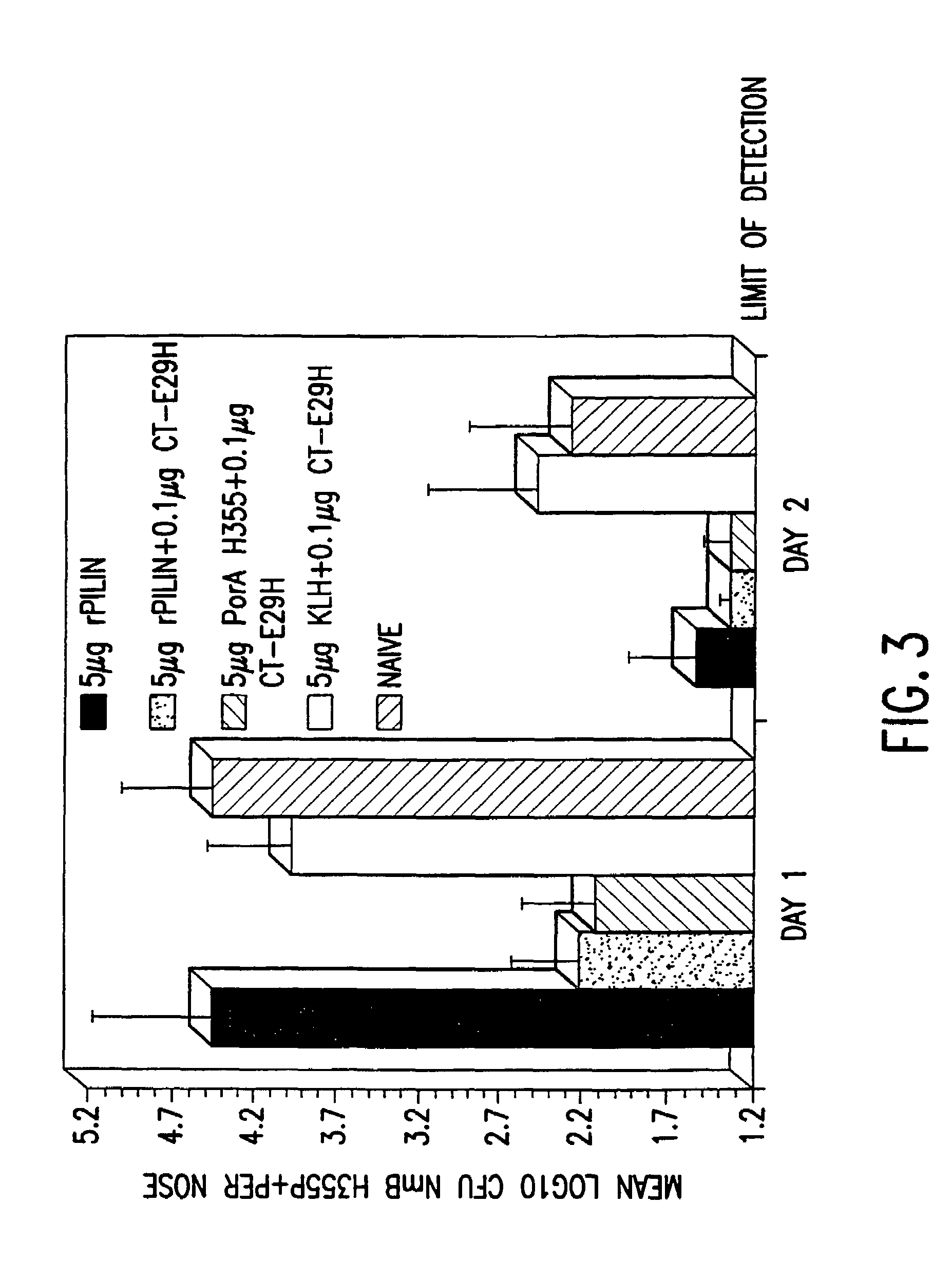
Mutant cholera holotoxin as an adjuvant
InactiveUS7384640B1Low toxicityEnhance immune responseSsRNA viruses negative-senseBacteriaAntigenAdjuvant
A mutant cholera holotoxin featuring a point mutation at amino acid 29 of the A subunit, wherein the glutamic acid residue is replaced by an amino acid other than aspartic acid, is useful as an adjuvant in an antigenic composition to enhance the immune response in a vertebrate host to a selected antigen from a pathogenic bacterium, virus, fungus or parasite. In a particular embodiment, the amino acid 29 is histidine. The mutant cholera holotoxin may contain at least one additional mutation in the A subunit at a position other than amino acid 29. The antigenic composition may include a second adjuvant in addition to the mutant cholera holotoxin.
Owner:UNIFORMED SERVICES UNIV OF HEALTH SCI UNITED STATES OF AMERICA AS REPRESENTED BY THE +1
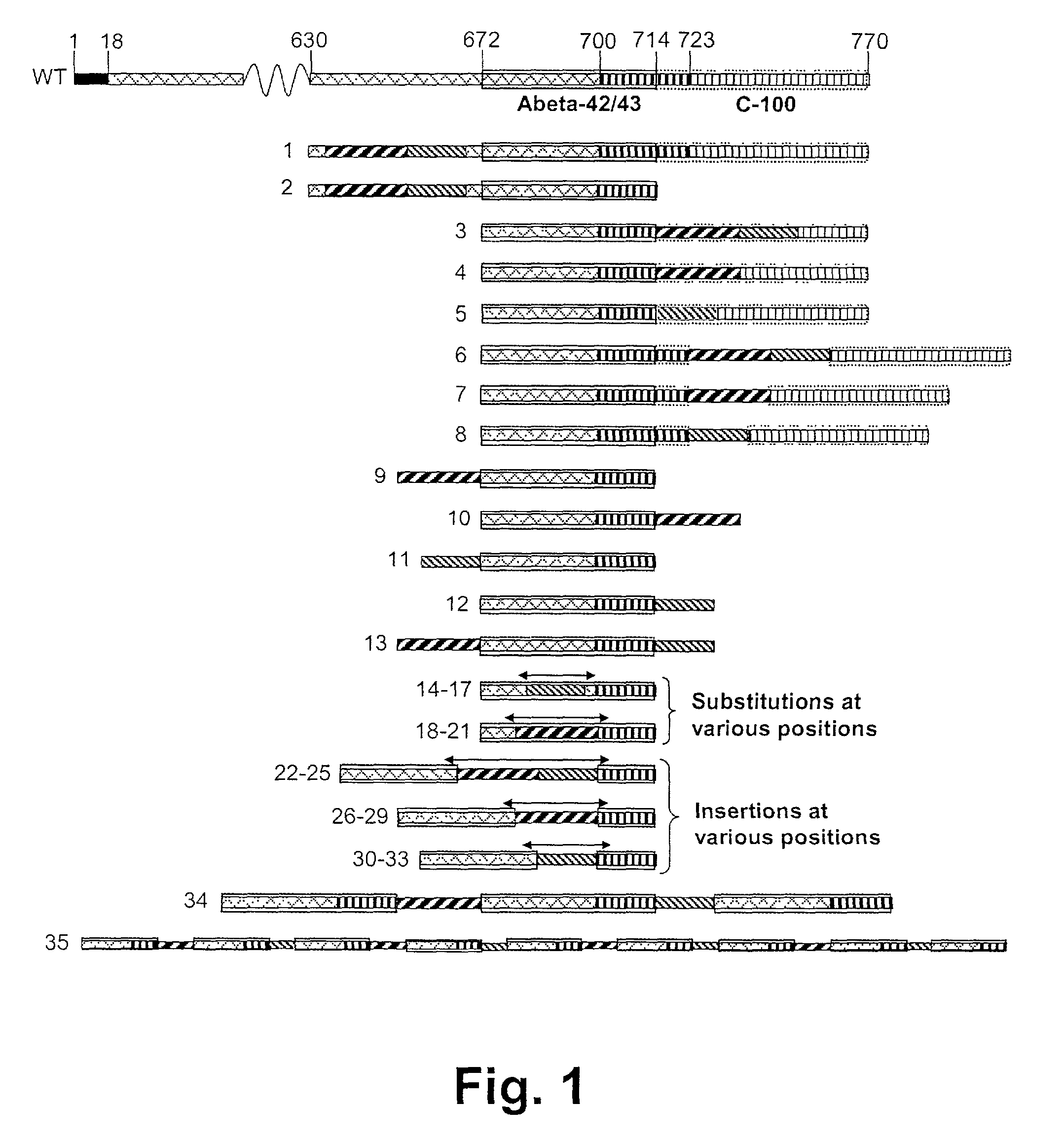
Novel method for down-regulation of amyloid
A method for in vivo down-regulation of amyloid protein in an animal, including a human being, the method comprising effecting presentation to the animal's immune system of an immunogenically effective amount of at least one amyloidogenic polypeptide or subsequence thereof which has been formulated so that immunization of the animal with the amyloidgenic polypeptide or subsequence thereof induces production of antibodies against the amyloidogenic polypeptide, and / or at least one analogue of the amyloidogenic polypeptide wherein is introduced at least one modification in the amino acid sequence of the amyloidogenic polypeptide which has as a result the immunization of the animal with the analogue induces production of antibodies against the amyloidogenic polypeptide.
Owner:H LUNDBECK AS
Fc fusion proteins for enhancing the immunogenicity of protein and peptide antigens
InactiveUS7067110B1Improving immunogenicityStrengthIn-vivo radioactive preparationsAntibody mimetics/scaffoldsPeptide antigenFc(alpha) receptor
Disclosed herein are methods and compositions for enhancing the immunogenicity of a preselected protein or peptide antigen in a mammal. Immunogenicity is enhanced by fusing the preselected antigen to an immunoglobulin heavy chain constant region to produce an Fc-antigen fusion protein. The Fc-antigen fusion proteins bind Fc receptors on the surface of antigen presenting cells, thereby targeting the antigen to the antigen presenting cells in the mammal. In addition, disclosed is a family of adjuvants, for example, an Fc-adjuvant fusion protein, for use in combination with the Fc-antigen fusion proteins to enhance or modulate a particular immune response against the preselected antigen.
Owner:MERCK PATENT GMBH
Immunostimulatory Combinations for Vaccine Adjuvants
This invention discloses immunostimulatory combinations of Tumor Necrosis Factor Receptor Superfamily (TN-FRSF) agonists, Toll-Like Receptor (TLR) agonists, “domain present in NAIP, CIITA, HET-E, TP-I (NACHT)-Leucine Rich Repeat (LRR)” or “NLR” agonists, RIG-I-Like Helicase or “RLH” agonists, purinergic receptor agonists and cytokine / chemokine receptor agonists, together with delivery methods. The combinations, when used alone at the site of pathology, provide immunostimulation that induces host humoral and cellular immunologic responses to eliminate pathogens or neoplasms. Alternatively, when the combinations are used with a defined antigens, these combinations can induce focused humoral and cellular immunologic responses useful as prophylactic and / or ameliorative therapeutic modalities for infections and the treatment of neoplastic disorders.
Owner:RGT UNIV OF CALIFORNIA
Humanized antibodies that recognize beta amyloid peptide
InactiveUS7189819B2Reduce the burden onReducing the neuritic dystrophyFungiNervous disorderHumanized antibodyΒ amyloid peptide
The invention provides improves agents and methods for treatment of diseases associated with amyloid deposits of Aβ in the brain of a patient. Preferred agents include humanized antibodies.
Owner:JANSSEN ALZHEIMER IMMUNOTHERAPY +3
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com