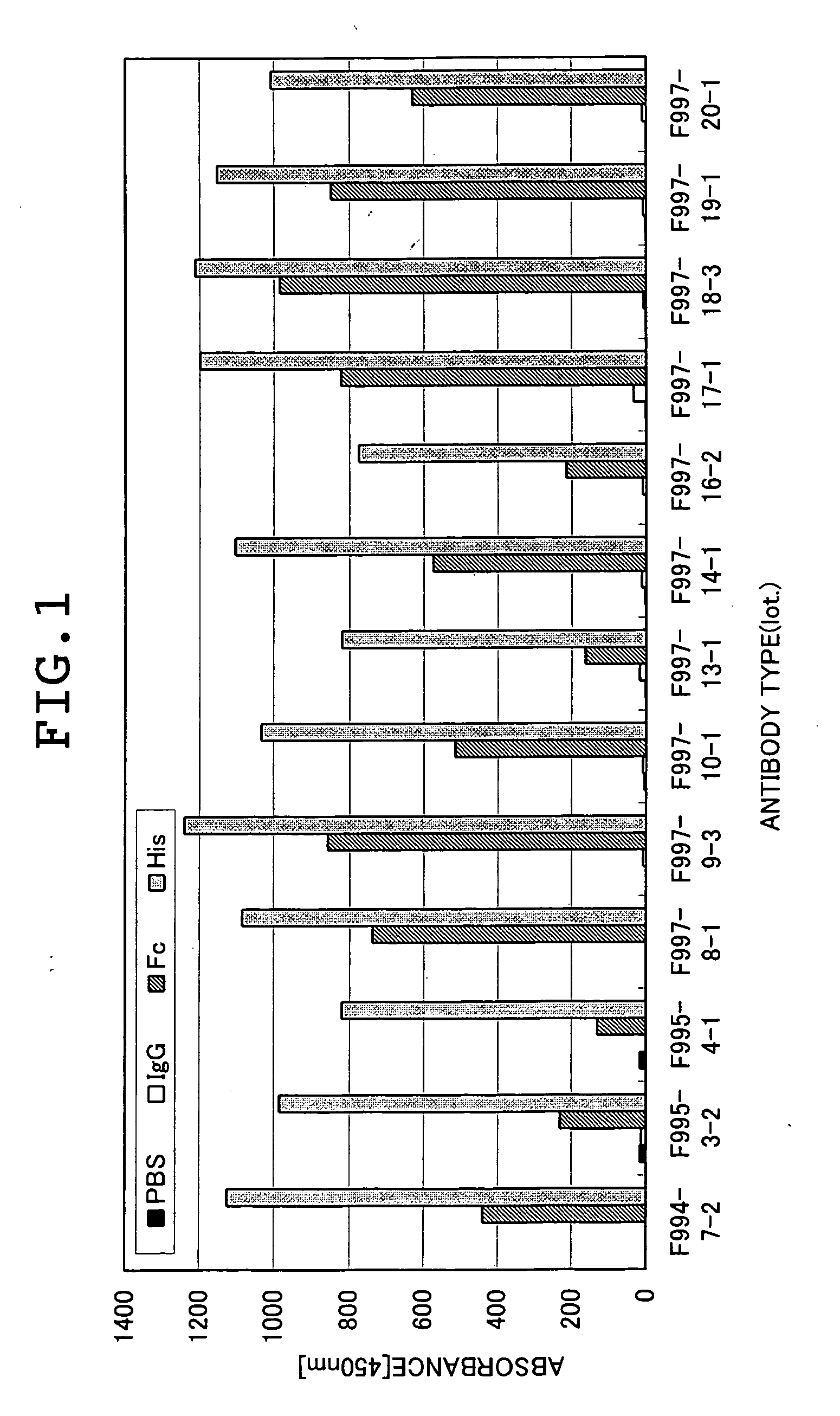
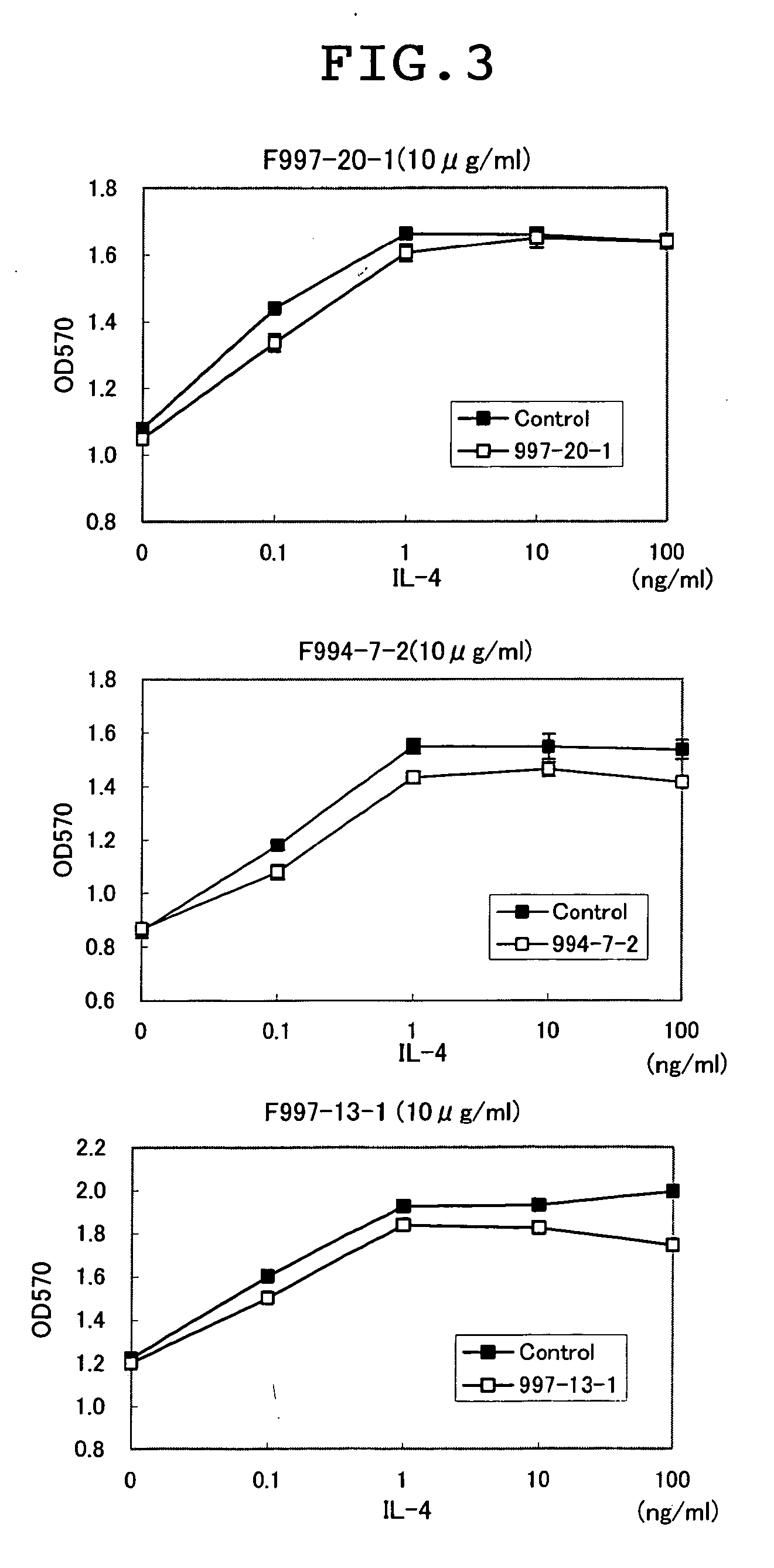
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
715 results about "Neutralising antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A neutralizing antibody (NAb) is a type of antibody that is produced naturally as part of immune system responses. These antibodies inhibit the effects of or destroy foreign agents that invade the body. Neutralizing antibodies can be triggered by infection or vaccination.
Mutant adeno-associated virus virions and methods of use thereof
ActiveUS20050053922A1Reduce the binding forceAltered infectivityAntibacterial agentsVirusesReassortant VirusesNeutralizing antibody
The present invention provides mutant adeno-associated virus (AAV) that exhibit altered capsid properties, e.g., reduced binding to neutralizing antibodies in serum and / or altered heparin binding and / or altered infectivity of particular cell types. The present invention further provides libraries of mutant AAV comprising one or more mutations in a capsid gene. The present invention further provides methods of generating the mutant AAV and mutant AAV libraries, and compositions comprising the mutant AAV. The present invention further provides recombinant AAV (rAAV) virions that comprise a mutant capsid protein. The present invention further provides nucleic acids comprising nucleotide sequences that encode mutant capsid proteins, and host cells comprising the nucleic acids. The present invention further provides methods of delivering a gene product to an individual, the methods generally involving administering an effective amount of a subject rAAV virion to an individual in need thereof.
Owner:INTEGRATIVE GENE THERAPEUTICS +1
Antigen delivery platforms
InactiveUS20140030292A1Improve stabilityAvoid reduce stimulationSsRNA viruses positive-senseFusion with post-translational modification motifAntigen deliveryHerpes simplex virus DNA
This disclosure provides platforms for delivery of herpes virus proteins to cells, particularly proteins that form complexes in vivo. In some embodiments these proteins and the complexes they form elicit potent neutralizing antibodies. Thus, presentation of herpes virus proteins using the disclosed platforms permits the generation of broad and potent immune responses useful for vaccine development.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
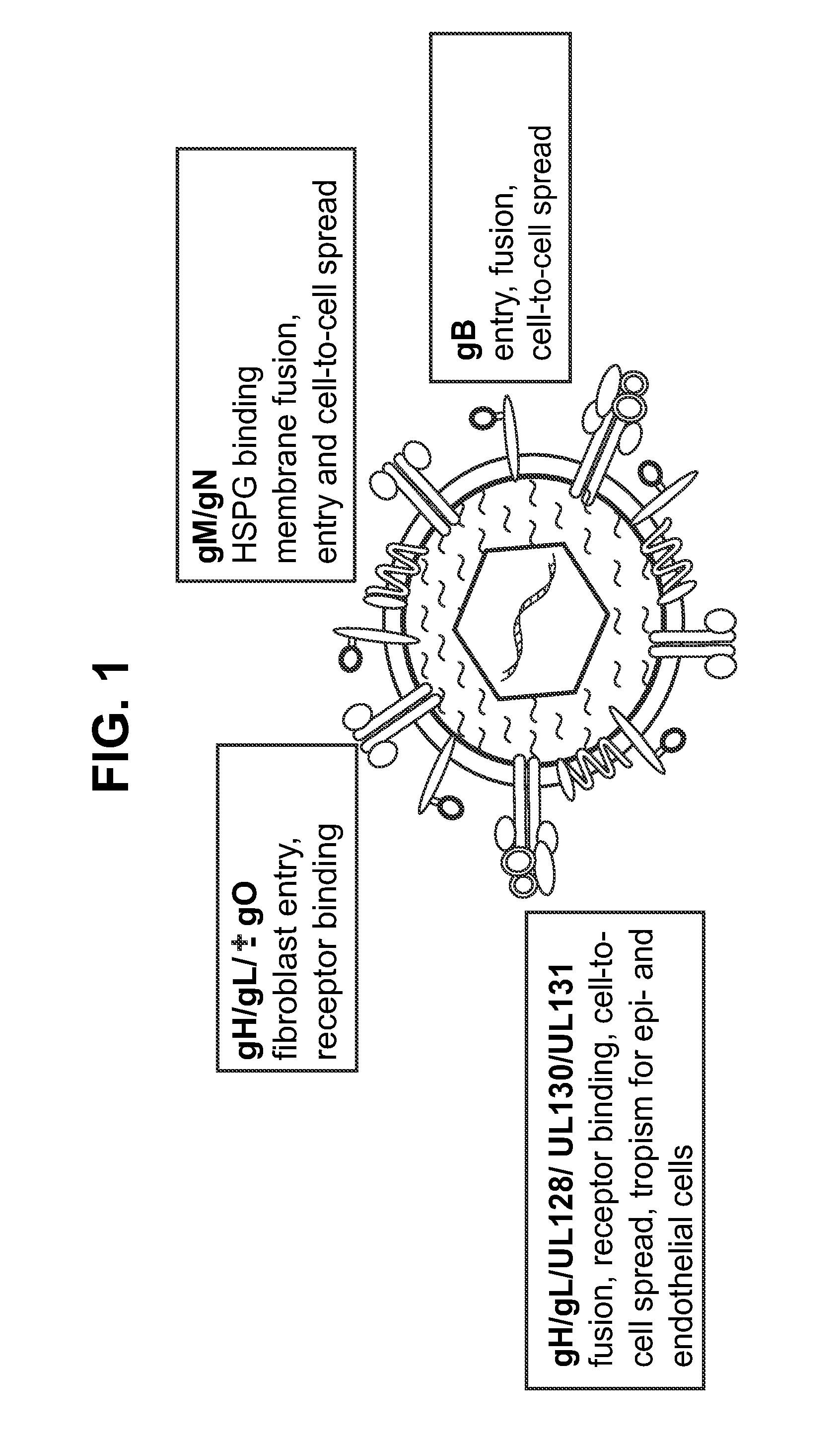
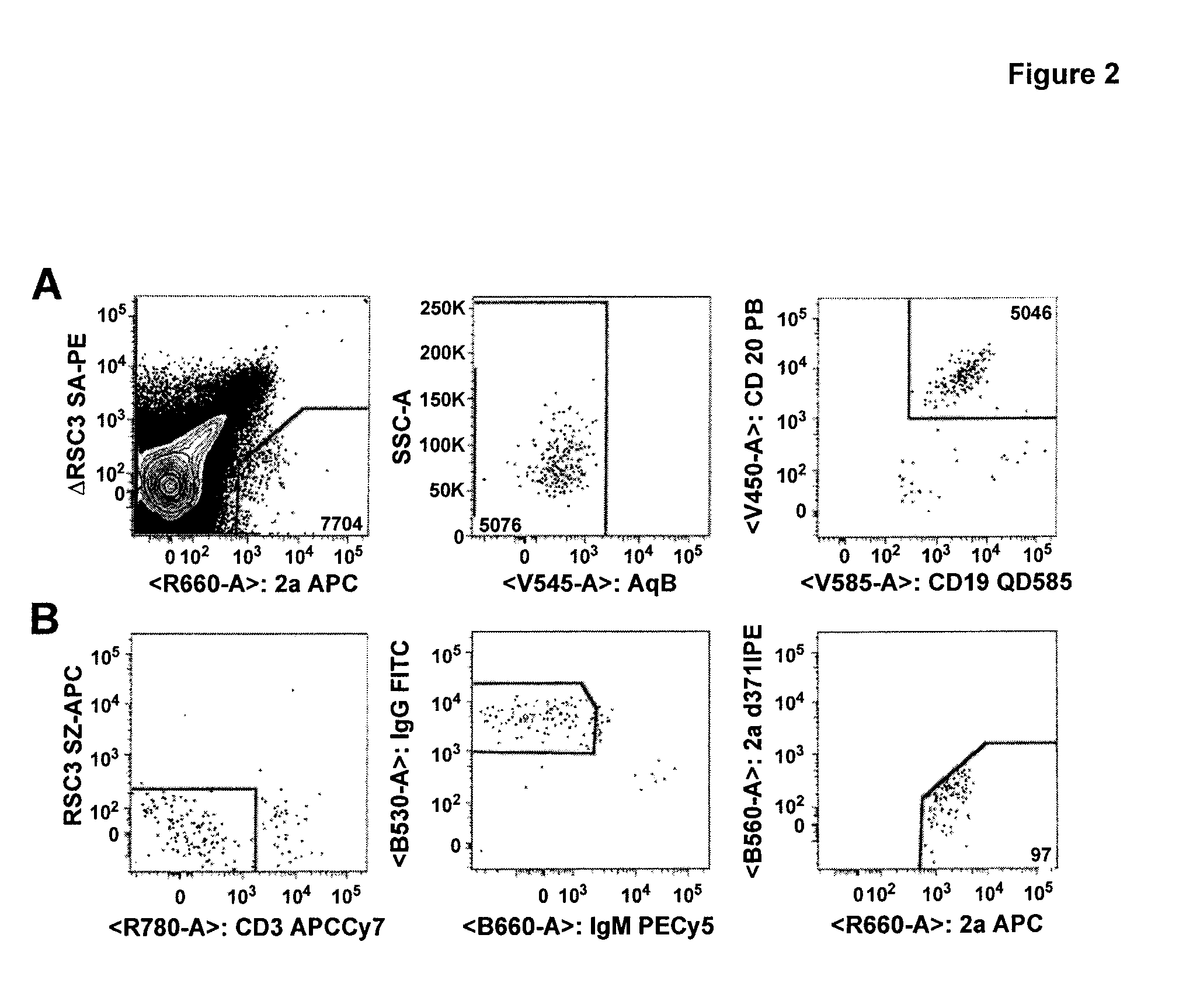
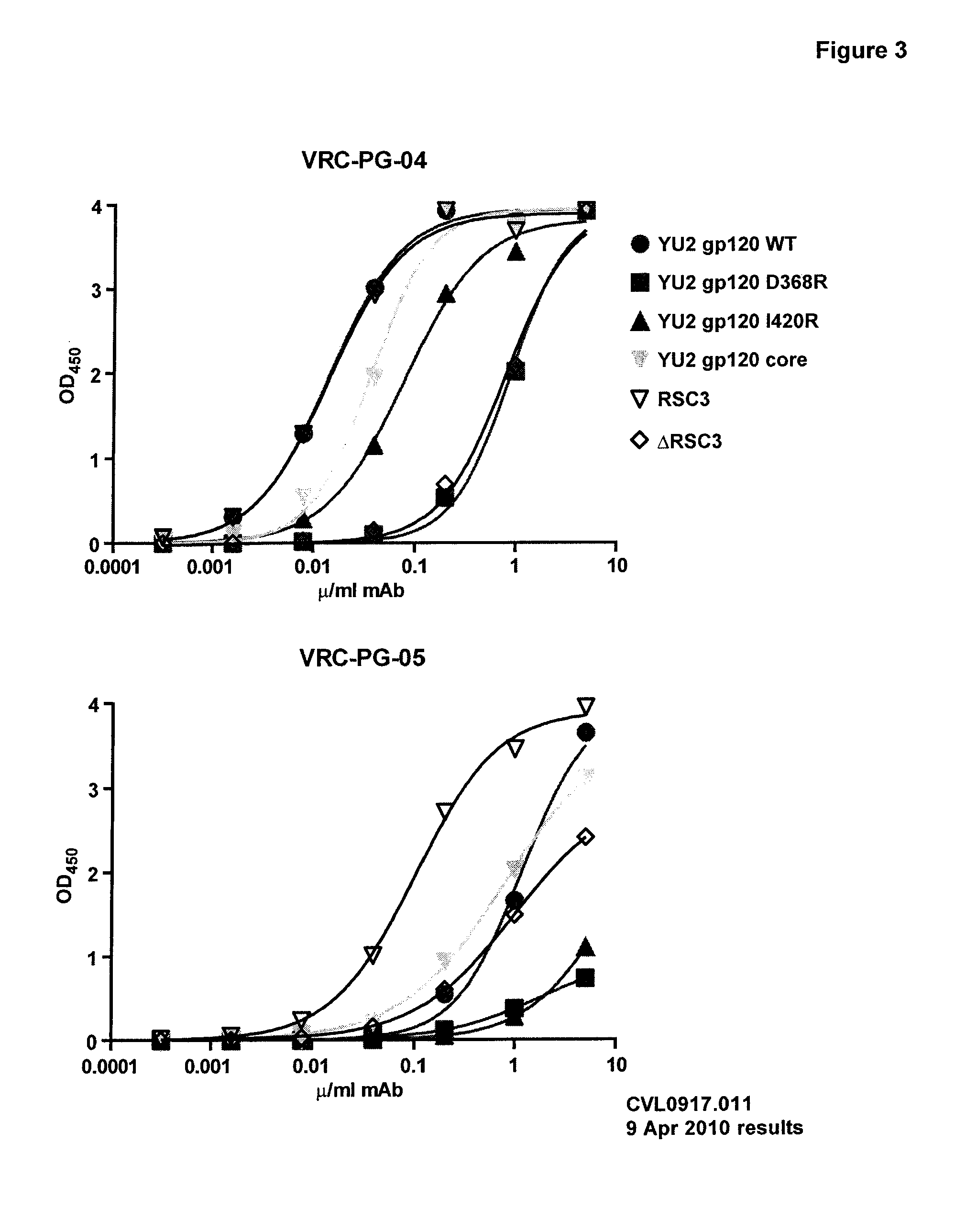
Human cytomegalovirus neutralising antibodies and use thereof
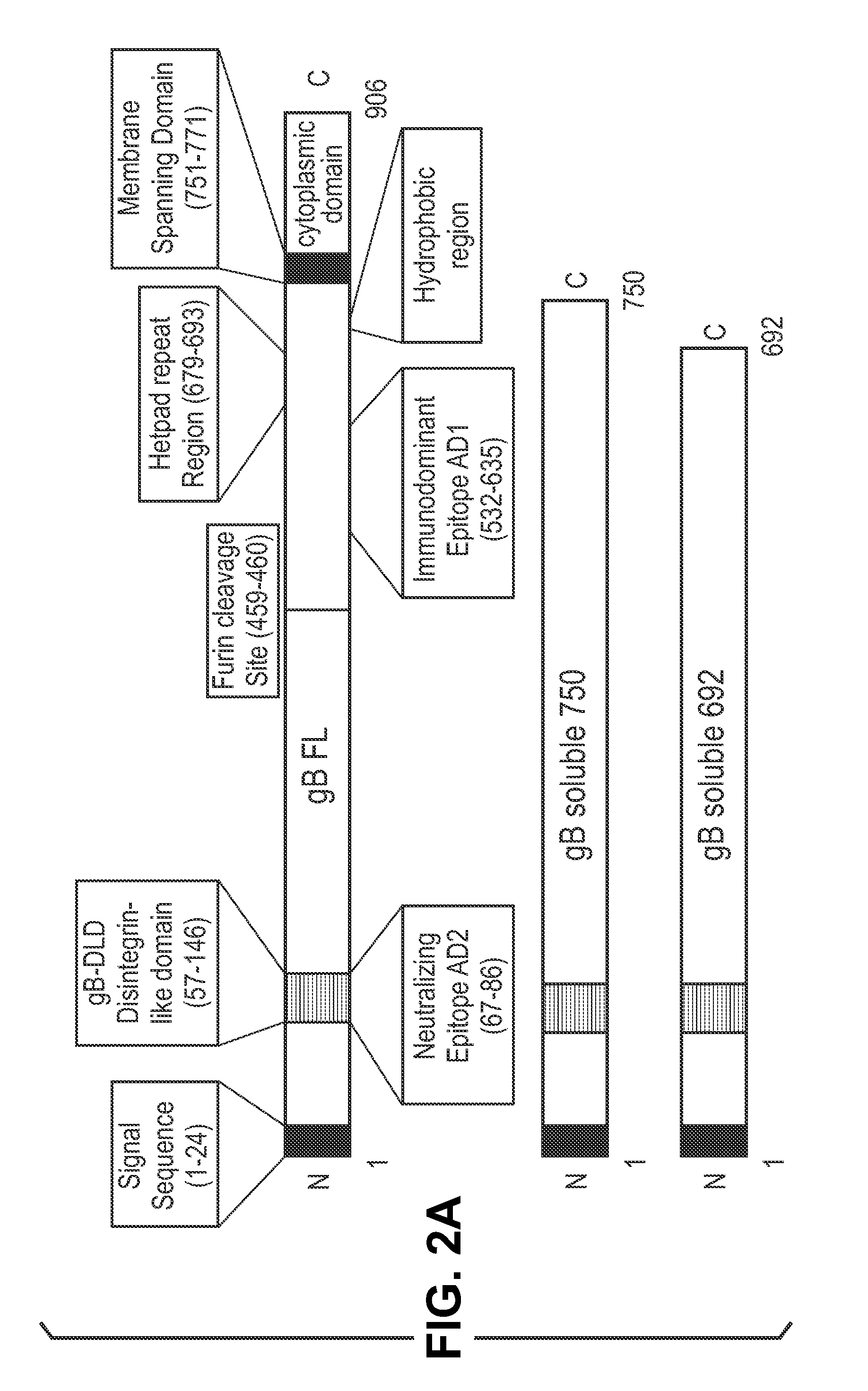
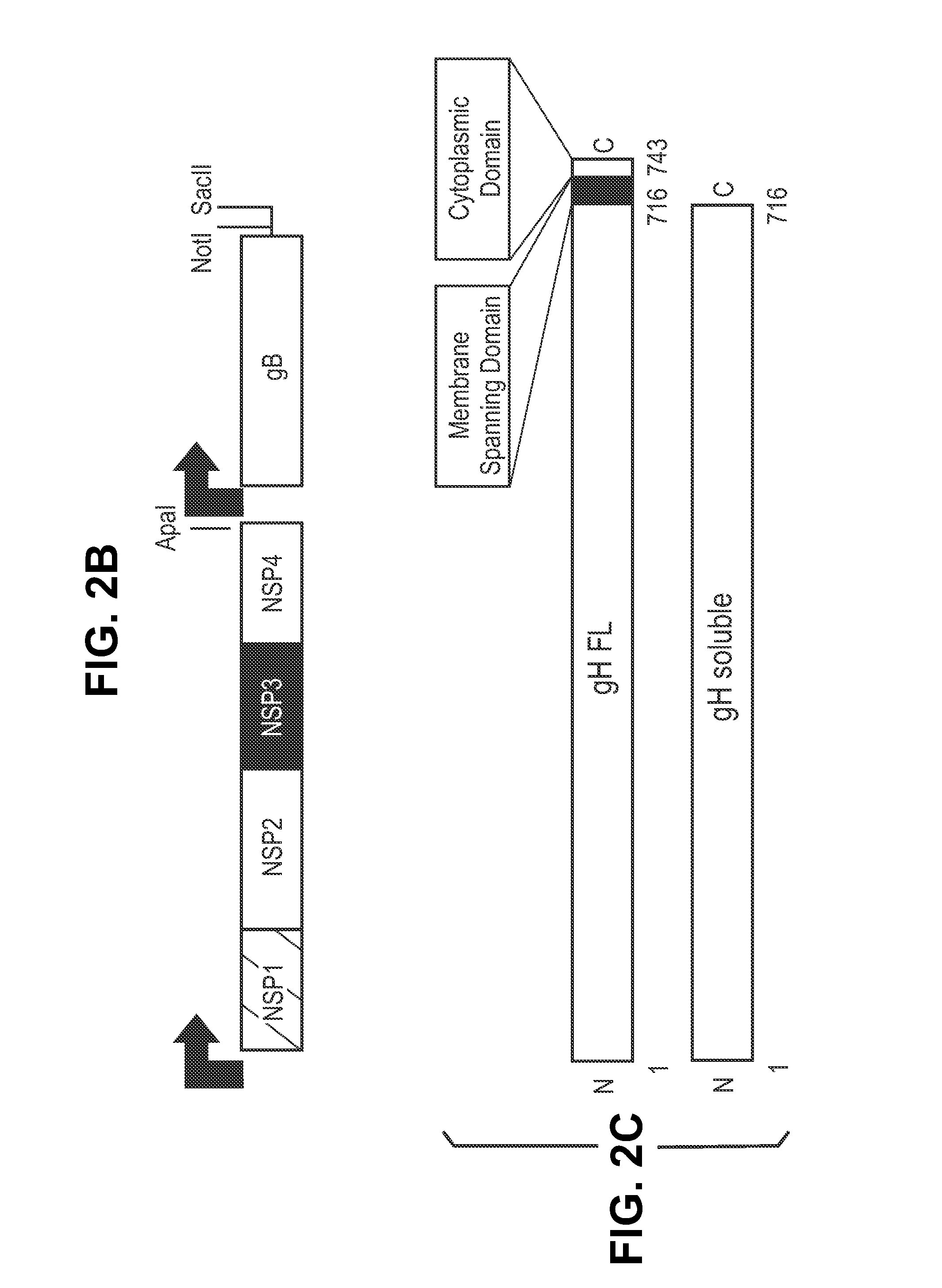
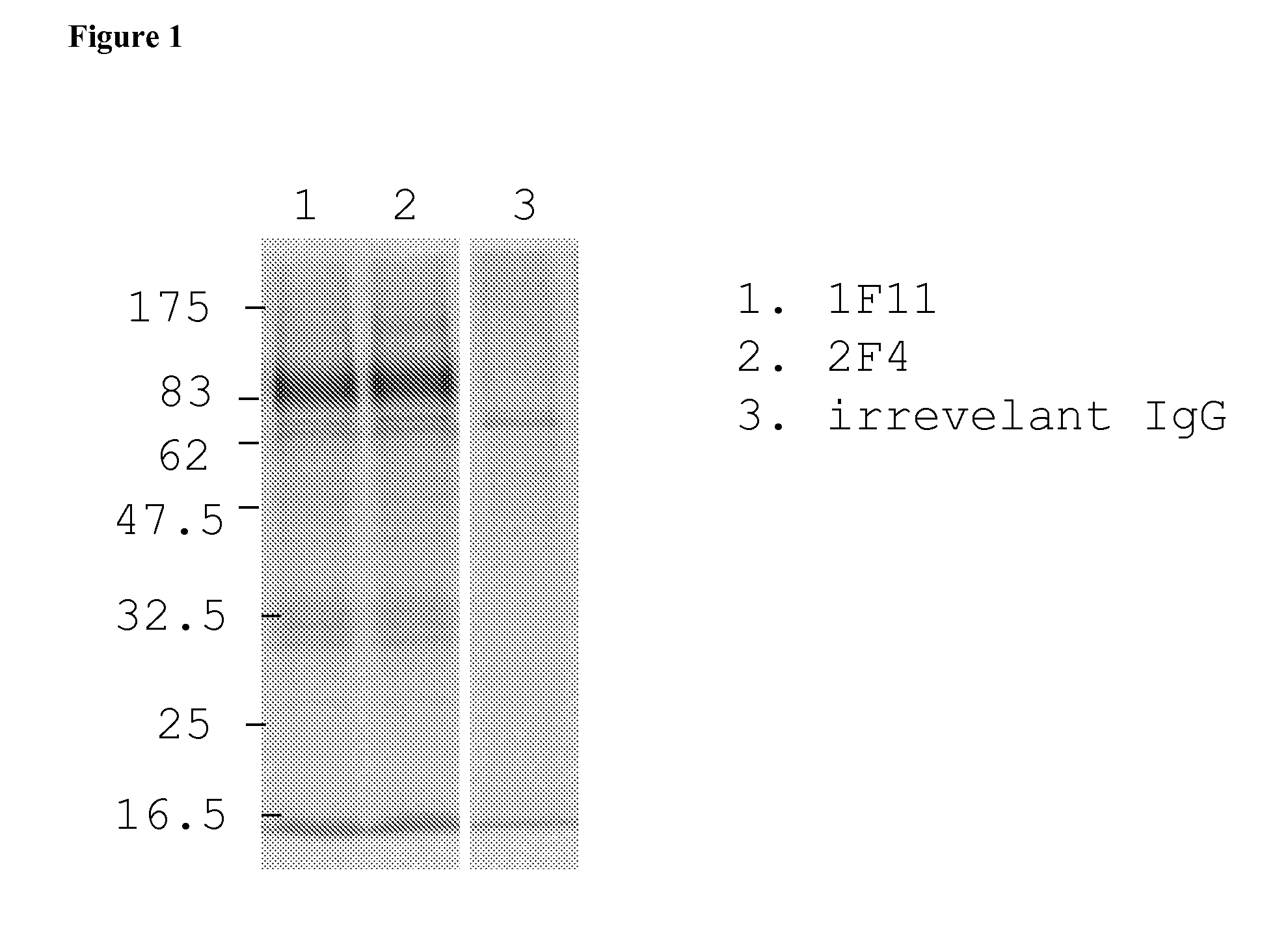
The invention relates to neutralizing antibodies, and antibody fragments thereof, having high potency in neutralizing hCMV, wherein said antibodies and antibody fragments are specific for one, or a combination of two or more, hCMV gene UL products. The invention also relates to immortalized B cells that produce, and to epitopes that bind to, such antibodies and antibody fragments. In addition, the invention relates to the use of the antibodies, antibody fragments, and epitopes in screening methods as well as in the diagnosis, prevention, and therapy of disease.
Owner:INSTITUTE FOR RESEARCH IN BIOMEDECINE
Antibodies That Bind Myostatin, Compositions And Methods
There are disclosed selective myostatin antagonists (including antibodies), nucleic acids encoding them, and methods of making and using them. Neutralizing antibodies recognizing the conformational epitope near position 21 to 31 and position 50 to 60.
Owner:AMGEN INC
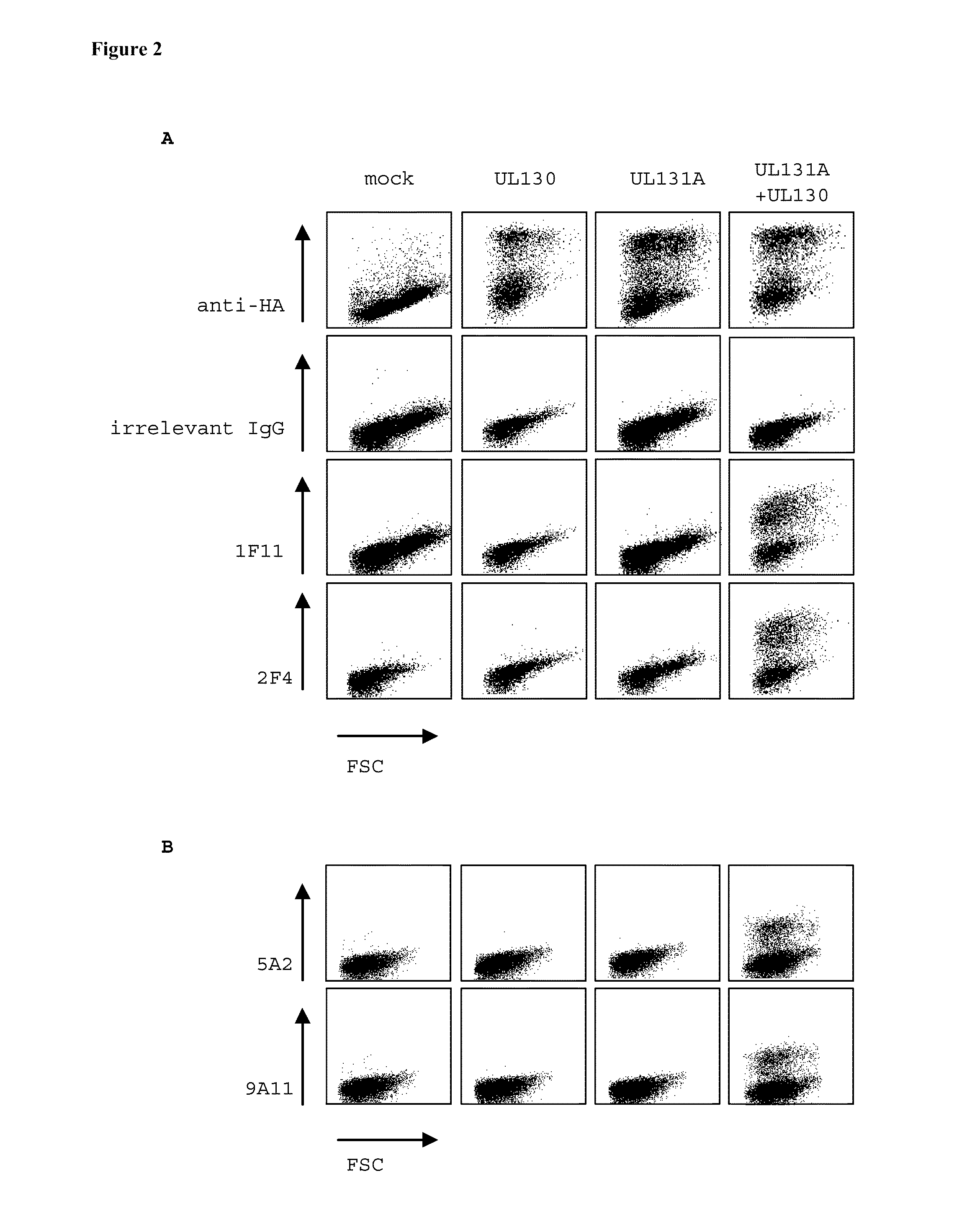
Human cytomegalovirus neutralising antibodies and use thereof
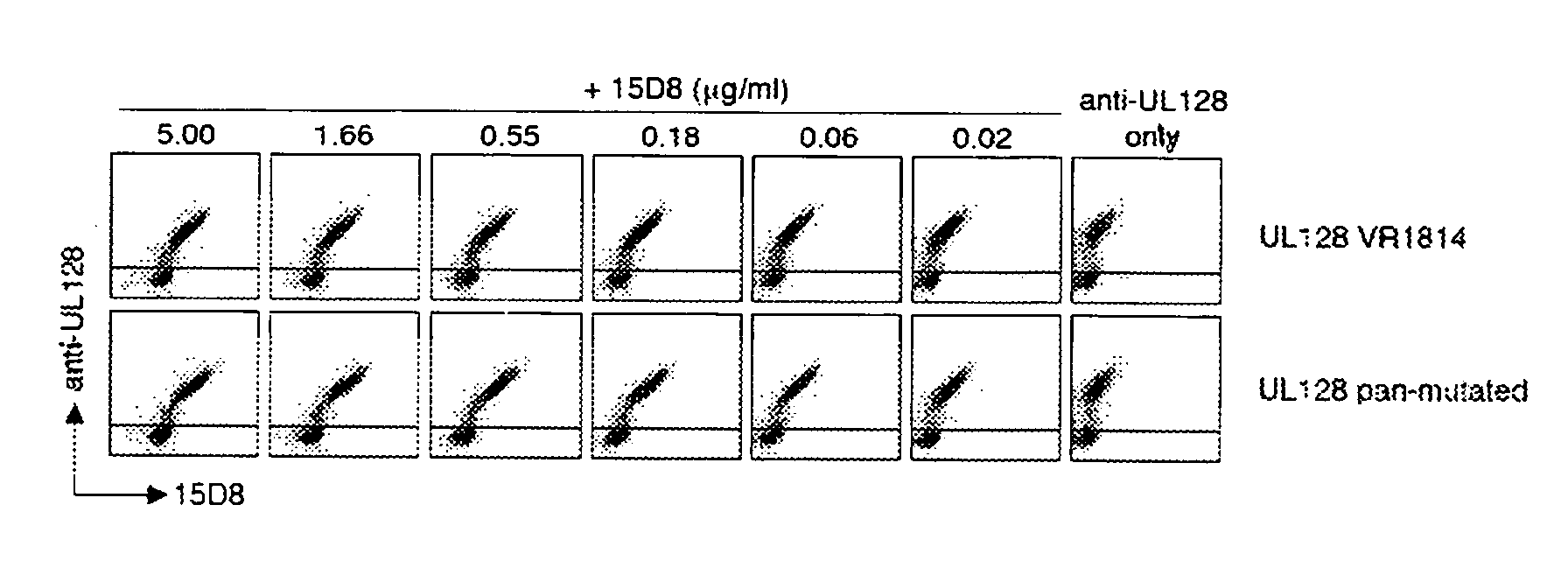
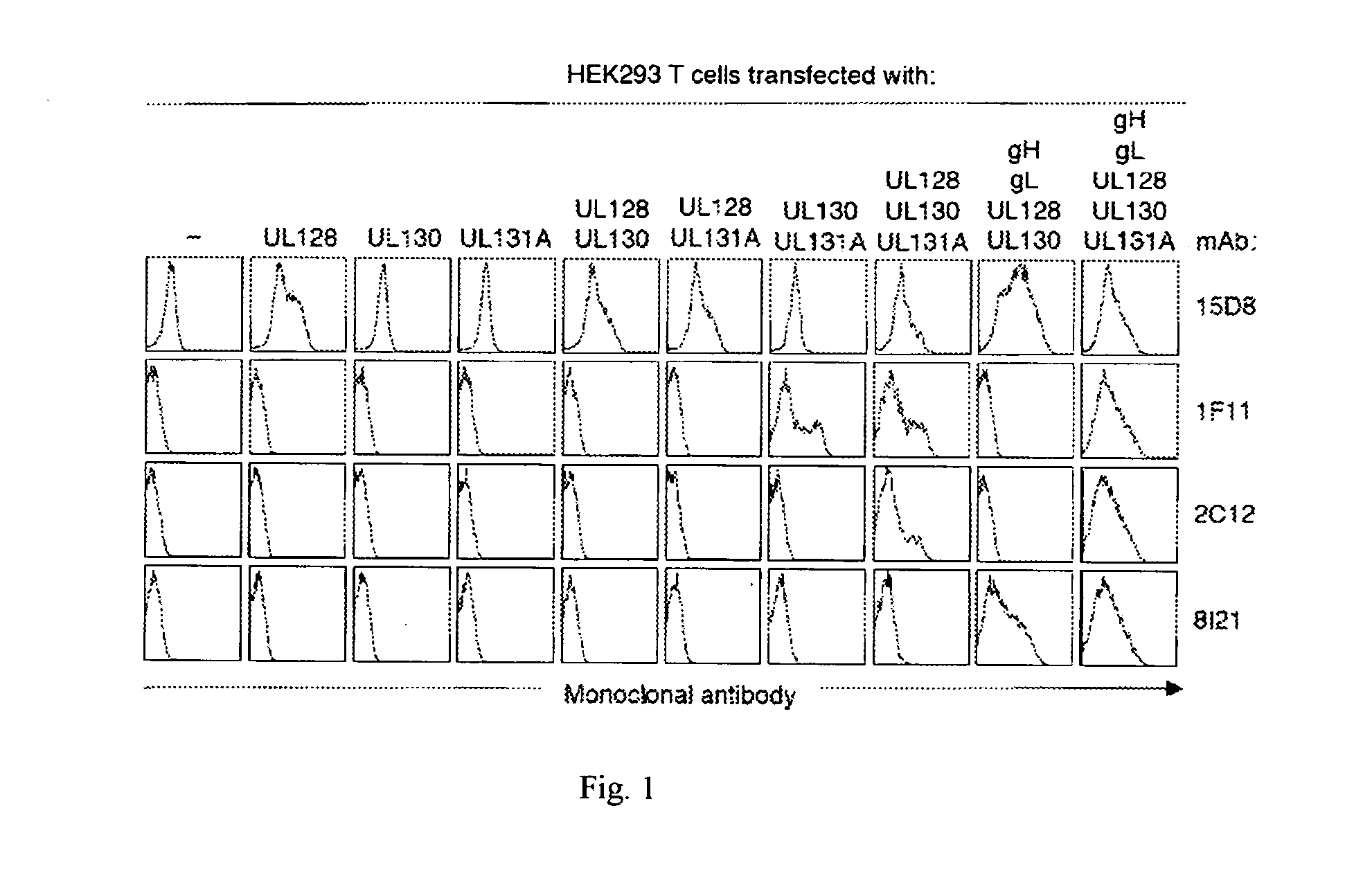
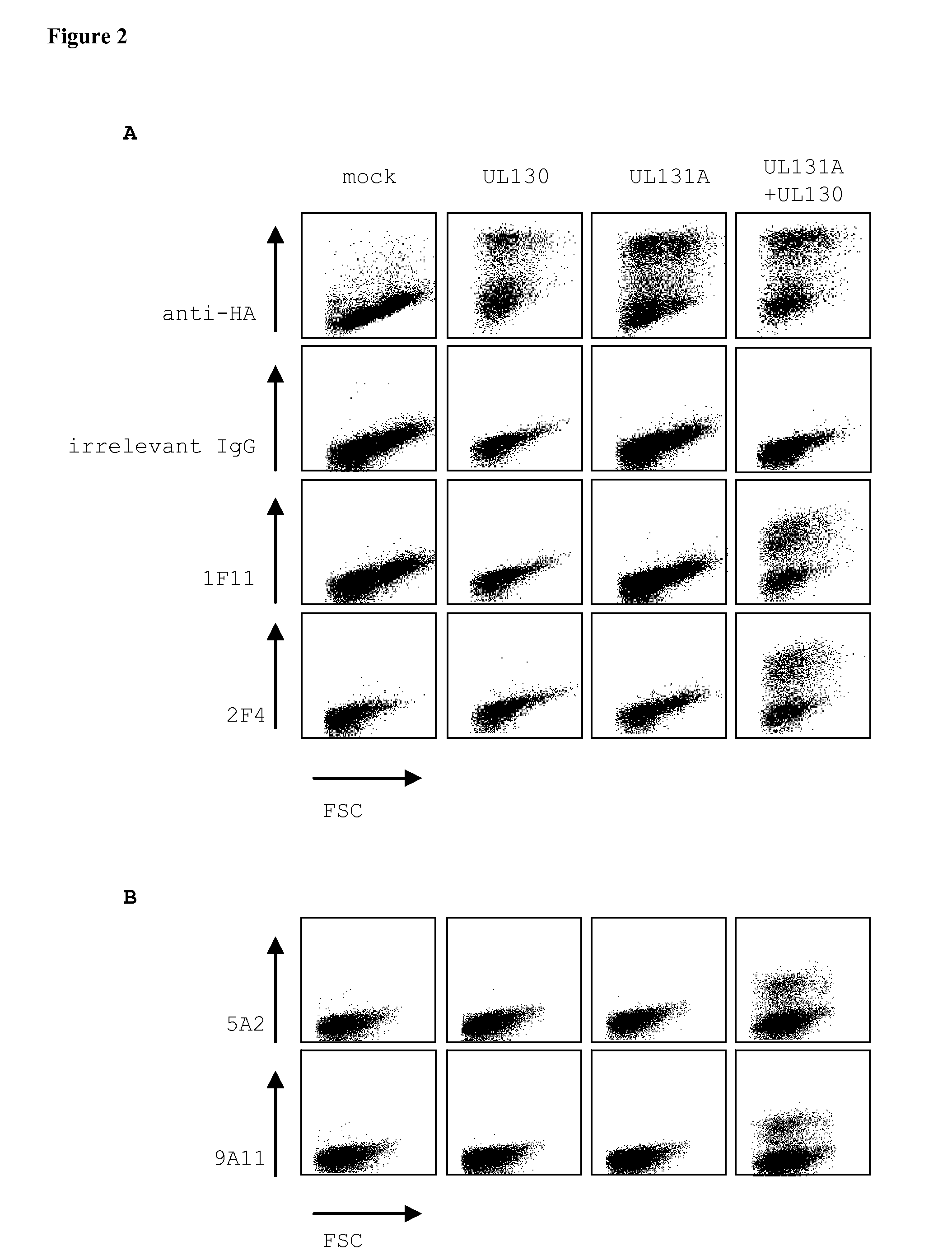
The invention relates to neutralizing antibodies and antibody fragments having high potency in neutralizing hCMV, wherein said antibodies and antibody fragments are specific for a combination of hCMV proteins UL130 and UL131A, or for a combination of hCMV proteins UL128, UL130 and UL131A. The invention relates also to immortalized B cells that produce, and to epitopes that bind to, such antibodies and antibody fragments. In addition, the invention relates to the use of the antibodies, antibody fragments, and epitopes in screening methods as well as in the diagnosis and therapy of disease.
Owner:HUMABS LLC
Optimization of gene sequences of chimeric virus-like particles for expression in insect cells
InactiveUS20050118191A1Minimize the numberSequence minimizedAnimal cellsViral antigen ingredientsDiagnostic testTGE VACCINE
Owner:NOVAVAX
Human anti-OPGL neutralizing antibodies as selective OPGL pathway inhibitors
Monoclonal antibodies and hybridomas producing them that interact with osteoprotegerin ligand (OPGL) are provided. Methods of treating osteopenic disorders by administering a pharmaceutically effective amount of antibodies to OPGL are also provided. Methods of detecting the amount of OPGL in a sample using antibodies to OPGL are further provided.
Owner:ER SQUIBB & SONS INC
SARS-CoV-2 (severe acute respiratory syndrome-corona virus disease-2) inhibitor and application thereof
InactiveCN111333722AHigh affinityRealize standardized productionBiological material analysisImmunoglobulins against virusesPhage antibodiesAntibody fragments
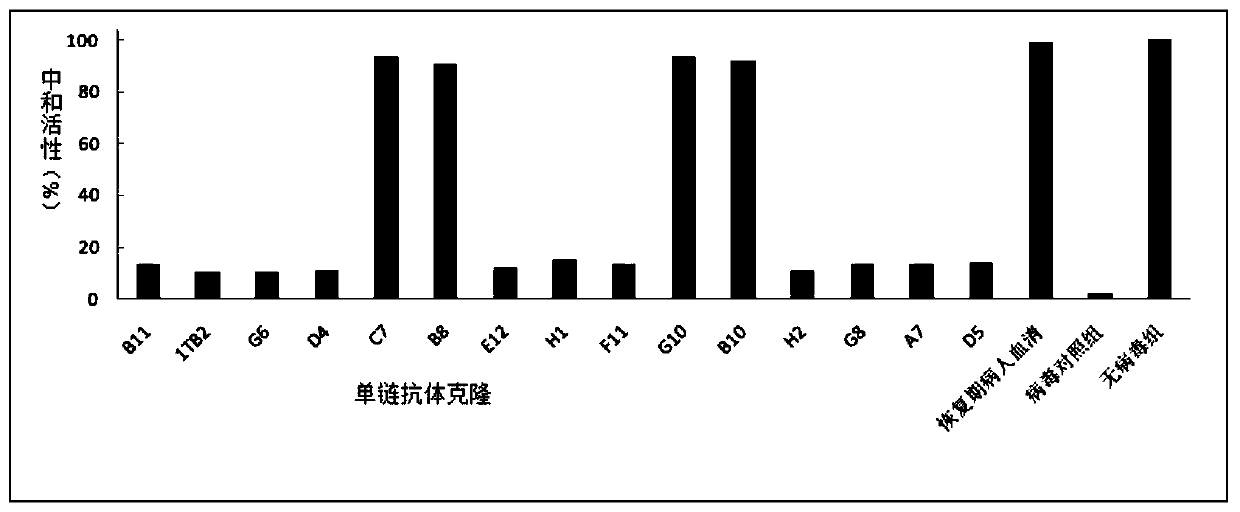
The invention relates to an SARS-CoV-2 (severe acute respiratory syndrome-corona virus disease-2) inhibitor and application thereof and in particularly relates to a neutralizing antibody for SARS-CoV-2 and application of the neutralizing antibody. According to the antibody, a phage display technology is adopted to construct a high-capacity human immunity phage antibody library, and an SARS-CoV-2 protein is adopted a target, human antibody single-chain antibody fragments are screened, and an antibody with a good neutralizing function on SARS-CoV-2 viruses is obtained. The antibody provided by the invention can be used for treating diseases caused by infection of novel corona viruses, and has significant clinical application value.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE CONTROL & PREVENTION PUBLIC HEALTH RES INST OF JIANGSU PROVINCE
SARS-CoV-2 neutralizing antibody detection kit
PendingCN111562369AGood repeatabilityStrong specificityImmunoassaysImmunodiagnosticsProtein s antigen
The invention relates to an SARS-CoV-2 neutralizing antibody detection kit. The SARS-CoV-2 neutralizing antibody detection kit comprises a solid phase carrier, an S protein antigen of SARS-CoV-2 and acompetitive substance. The competitive substance is marked with a signal substance and can be specifically combined with the new coronavirus S protein antigen. Whether a tested person is infected bythe new coronavirus or not and whether infection risks exist or not are judged by detecting a neutralizing antibody through an immunodiagnosis technology, and the method is reliable in theory, practical and feasible and can be completed only in a secondary biosafety laboratory.
Owner:威海威高生物科技有限公司
Subunit vaccine for novel coronavirus and application of subunit vaccine
InactiveCN111533809AImproving immunogenicityImprove stabilitySsRNA viruses positive-senseViral antigen ingredientsAntibody fragmentsTGE VACCINE
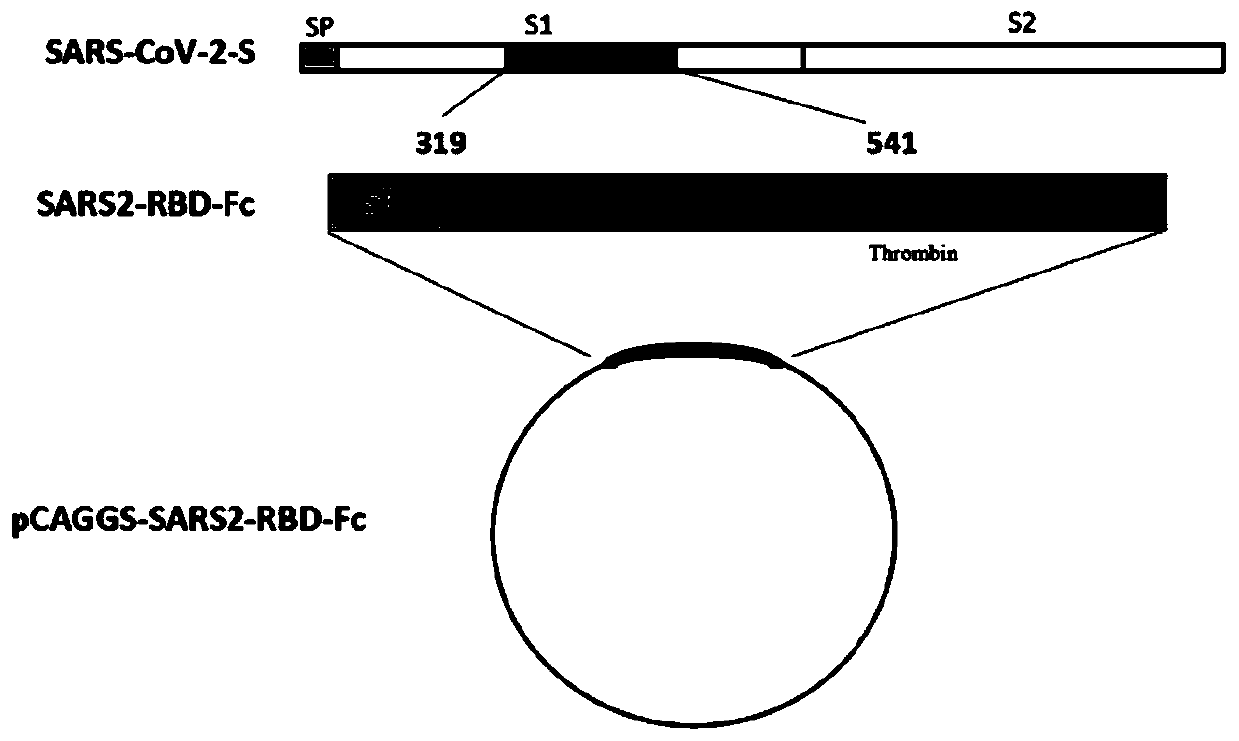
The invention discloses a fusion protein of a novel coronavirus envelope protein and an application of the fusion protein. The fusion protein (SARS2-RBD-Fc) is obtained by fusing an RBD structural domain of a novel coronavirus envelope protein S with an antibody Fc fragment; and as a subunit vaccine, the fusion protein can induce an organism to generate an efficient neutralizing antibody through nasal drip immunization and intramuscular injection. It indicates that the SARS2-RBD-Fc can be used as a candidate vaccine for preventing and treating new coronavirus infection.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Methods and Compositions for Vaccination of Animals with Prrsv Antigens with Improved Immunogenicity
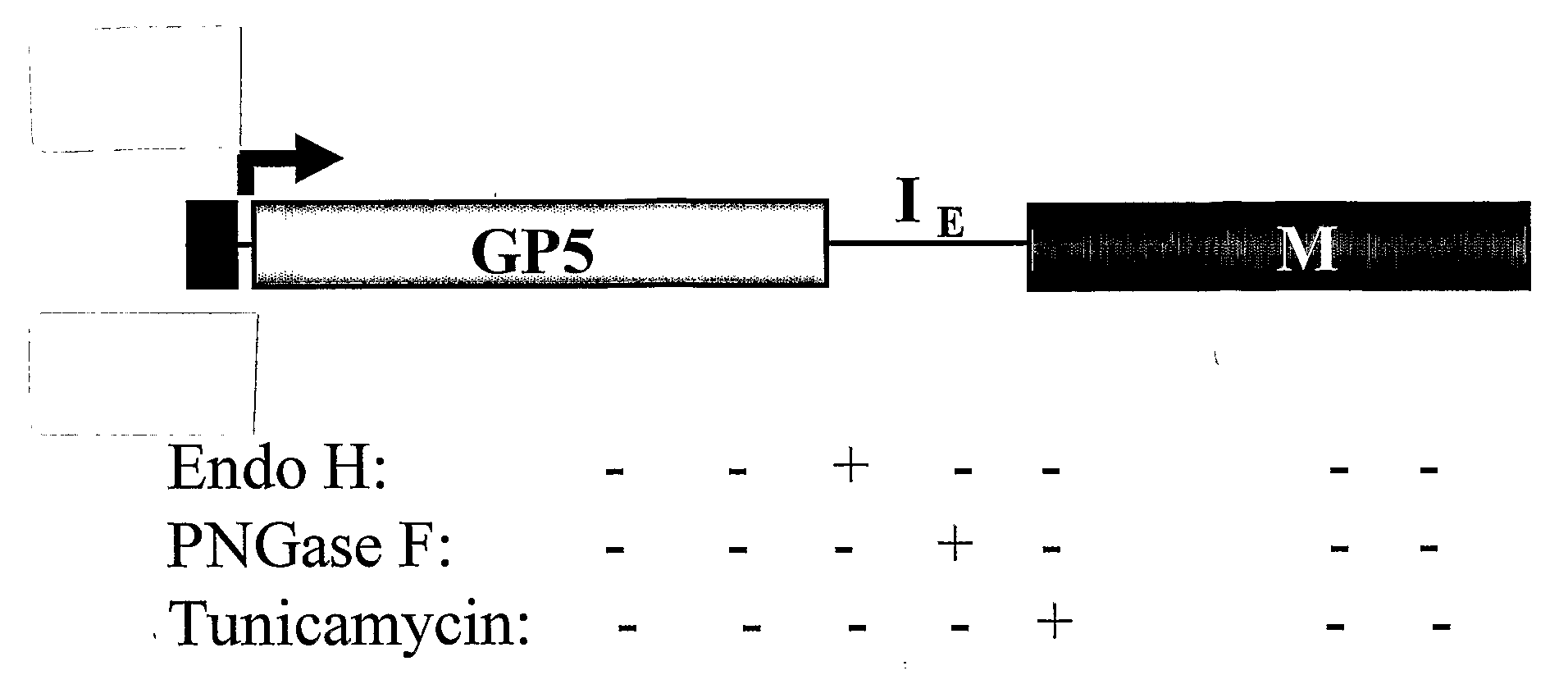
Pigs challenged with hypoglycosylated variants of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) major surface protein GP5 exhibited increased production of PRRSV-neutralizing antibodies relative to the levels of neurtalizing antibodies produced by pigs immunized with wild type (wt) or glycosylated GP5. This invention provides for methods of obtaining improved immune responses in pigs to PRRSV, compositions useful for obtaining the improved immune responses as well as isolated polynucleotides that encode hypoglycosylated variants of PRRSV major surface protein GP5.
Owner:NUTECH VENTURES
Optimization of gene sequences of virus-like particles for expression in insect cells
InactiveUS20040121465A1Improve the level ofMinimize the numberAnimal cellsViral antigen ingredientsPolynucleotideTGE VACCINE
Codon optimized polynucleotides for optimal expression of recombinant proteins in eukaryotic cells are provided. The codon optimized polynucleotides encode a viral capsid protein that self assembles into a virus-like particle. The virus-like particle is expressed extracellularly and exhibits conformational antigenic epitopes capable of raising neutralizing antibodies. Pharmaceutical compositions, vaccines, and diagnostic test kits containing the gene products of the codon-optimized polynucleotides are also provided.
Owner:NOVAVAX
Novel coronavirus S protein two-region subunit nano vaccine based on pyrococcus furiosus ferritin
ActiveCN111217919AMultimerizationOvercoming the disadvantage of insufficient immunogenicitySsRNA viruses positive-senseViral antigen ingredientsEucaryotic cellNeutralising antibody
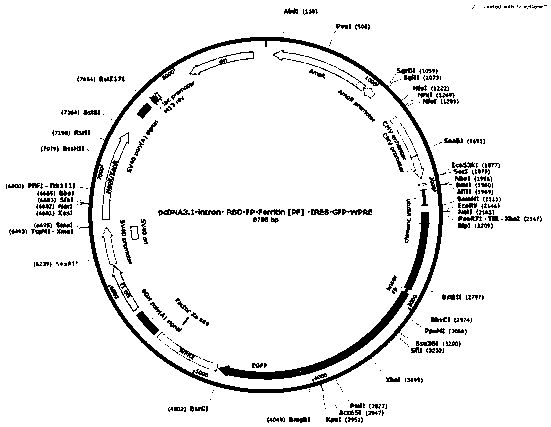
The invention discloses a novel coronavirus S protein double-region subunit nano vaccine based on pyrococcus furiosus ferritin. The virus receptor binding domain (RBD) and fusion peptide (FP) are usedtogether as a double antigen, and are connected with pyrococcus furiosus ferritin (PF_Ferritin) to from a fusion protein RBD-FP-PF_Ferritin to realize antigen multimerization; and then an eukaryoticcell expression system is used for expressing, and a 24-mer nano antigen can be formed through self-assembly of PF_Ferritin. The scheme can overcome the shortcoming of insufficient immunogenicity of RBD monomers, the obtained vaccine can significantly increase the level of a neutralizing antibody against the virus of a host, and the produced antibody has the ability to strongly block the virus from invading target cells. In addition, the vaccine of the invention is simple in preparation method, is easy to purify, and is high in safety, and the vaccine can be relatively quickly applied to clinical trials.
Owner:SUN YAT SEN UNIV
Novel HIV -1 broadly neutralizing antibodies
ActiveUS20130251726A1Reduce the impactMicrobiological testing/measurementImmunoglobulins against virusesHeavy chainNeutralising antibody
The present application relates novel HIV-1 broadly neutralizing antibodies. The antibodies of the present invention are further characterized by their ability to bind epitopes from the Env proteins. The invention also provides light and heavy chain variable region sequences. Compositions for prophylaxis, diagnosis and treatment of HIV infection are provided.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +2
Human cytomegalovirus neutralising antibodies and use thereof
The invention relates to neutralizing antibodies and antibody fragments having high potency in neutralizing hCMV, wherein said antibodies and antibody fragments are specific for a combination of hCMV proteins UL130 and UL131A, or for a combination of hCMV proteins UL128, UL130 and UL131A. The invention relates also to immortalized B cells that produce, and to epitopes that bind to, such antibodies and antibody fragments. In addition, the invention relates to the use of the antibodies, antibody fragments, and epitopes in screening methods as well as in the diagnosis and therapy of disease.
Owner:HUMABS LLC
Subunit coronavirus vaccine for dimerization-based receptor binding domains
ActiveCN106928326AOvercoming the disadvantage of insufficient immunogenicityIncrease neutralizing antibody productionSsRNA viruses positive-senseBacteriaCoronavirus vaccinationMiddle East respiratory syndrome coronavirus
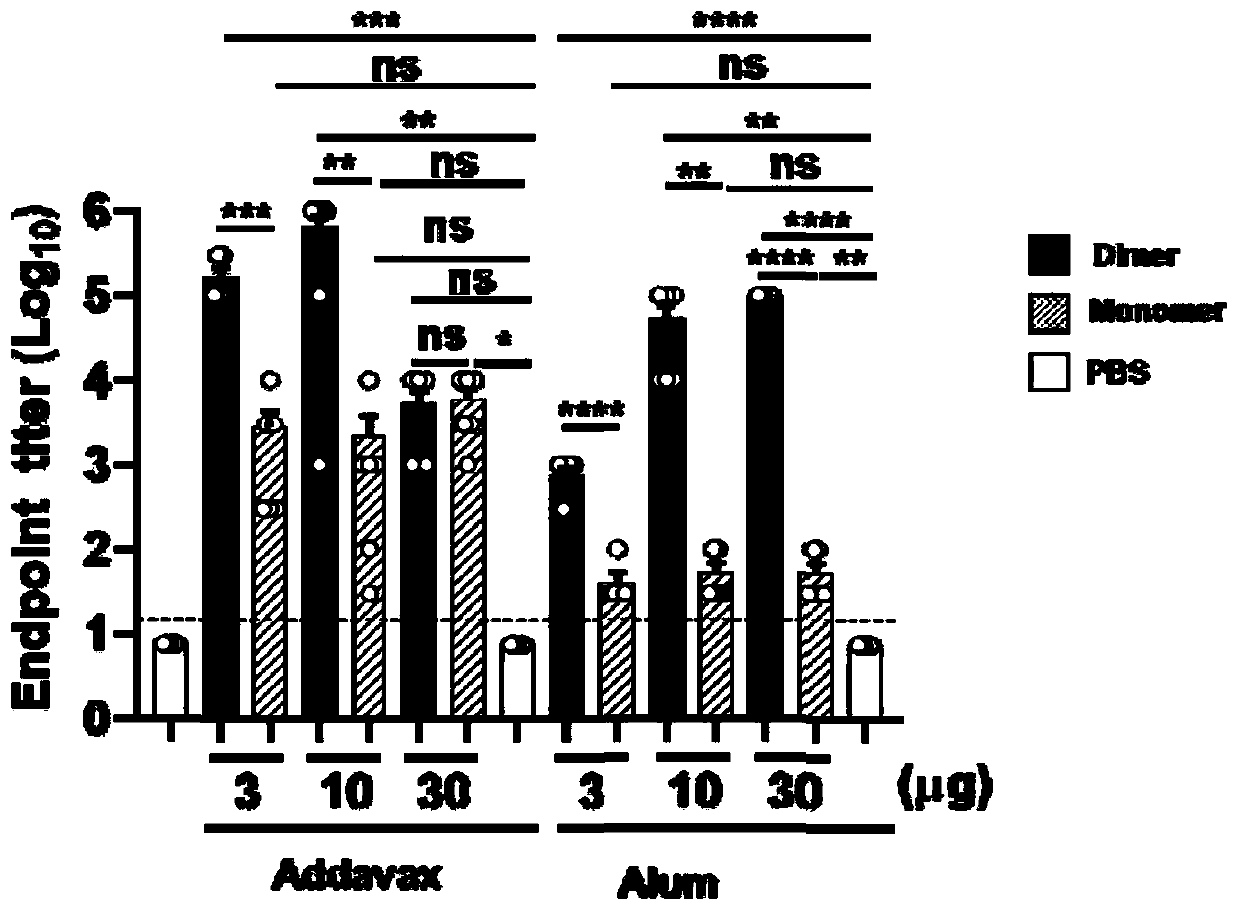
The invention discloses a subunit coronavirus vaccine for dimerization-based receptor binding domains and belongs to the technical field of medicine. A baculovirus expresses RBD (receptor binding domain (E367-Y606) of MERS-CoV (middle east respiratory syndrome coronavirus) protein and RBD (R294-F515) of SARS-CoV (severe acute respiratory syndrome coronavirus) in insect cells, the RBDs may form a dimer through cysteine residue at 603 of S (spike) protein or form a dimer through cysteine residue at 512 of the S protein, and purified RBD protein dimer and monomer are used respectively to immunize Bald / c mice. The dimerized RBDs have the advantages that the defect that RBD monomers have poor immunogenicity is overcome and the generation of neutralizing antibodies in against MERS-CoV is increased greatly.
Owner:ANHUI ZHIFEI LONGCOM BIOPHARM CO LTD
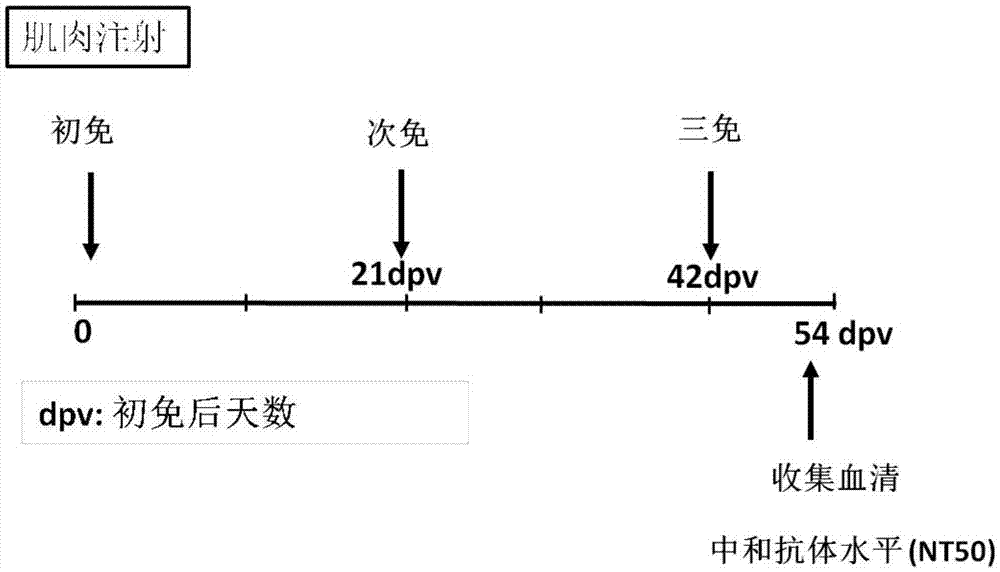
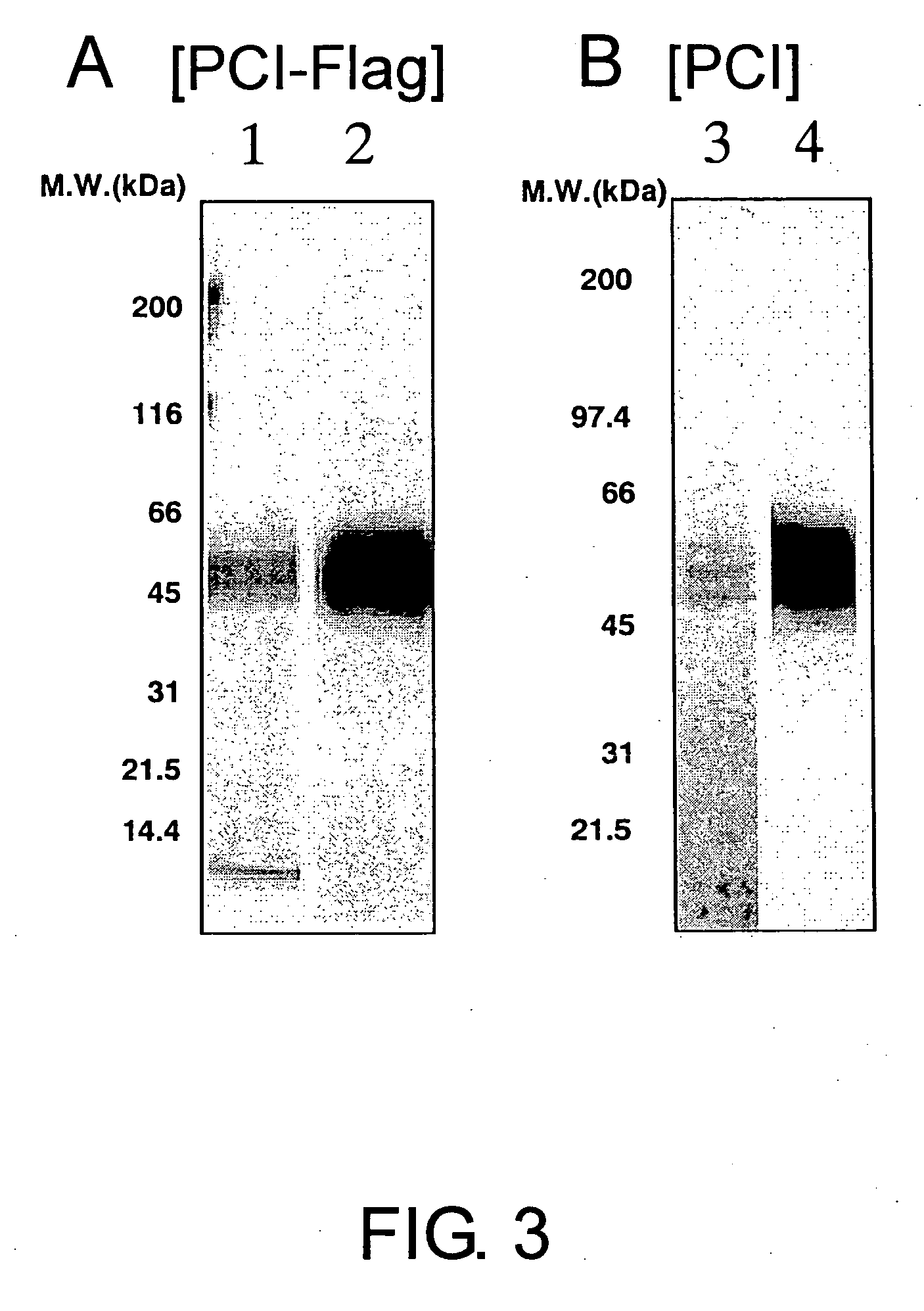
Anti-pci neutralizing antibody
InactiveUS20060167230A1Increase productionHigh activityAntibacterial agentsAntipyreticProtein C inhibitorTherapeutic effect
The present invention provides anti-PCI antibodies having Protein C inhibitor (PCI)-neutralizing activity, and the uses thereof. Through the generation and screening of anti-PCI antibodies, the inventors successfully isolated anti-PCI antibodies which inhibit PCI's inhibitory effect on the production and activity of activated Protein C (aPC). The antibodies of the present invention suppress PCI's inhibitory effect on aPC production and / or the aPC inactivation by PCI, and thus can be used to maintain aPC activity and sustain the effects of aPC physiological activities, such as suppression of the activation of blood coagulation system and anti-inflammatory functions. The present invention also provides uses of the antibodies of the present invention in treating diseases such as thrombosis and sepsis using aPC. In treatments by aPC administration, the therapeutic effect of aPC can be sustained by administering an antibody of the present invention. The antibodies of the present invention can be used in the treatment and prevention of diseases such as thrombosis and sepsis.
Owner:CHUGAI PHARMA CO LTD
Anti-il13 receptor alpha1 neutralizing antibody
InactiveUS20050154192A1High expressionEffective therapyAnimal cellsBiological material analysisNeutralizing antibodyAmino acid
A novel anti-IL13 receptor α1 antibody having an activity of inhibiting a cell response by IL13, in particular, having an activity of inhibiting the cell response by IL13 but not inhibiting the cell response by IL4, a characteristic amino acid sequence in a variable region thereof, and a method of detecting IL13 receptor α1 in a sample using the same are provided.
Owner:MOCHIDA PHARM CO LTD
Human rotavirus Delta VP8* subunit recombinant protein and application thereof
ActiveCN103319604AImprove immune efficiencyFast titerBacteriaViral antigen ingredientsCross neutralizationRotavirus RNA
The invention relates to human rotavirus Delta VP8* subunit recombinant protein and application thereof. The human rotavirus Delta VP8* subunit recombinant protein comprises a T cell epitope P2 in tetanus toxin and a rotavirus Delta VP8* subunit. By the recombinant protein disclosed by the invention, the immune efficacy of a Delta VP8* subunit vaccine can be greatly improved; faster and stronger neutralization antibody titer can be induced; moreover, anti-p[4] genotype specific rotavirus cross neutralization antibody of high titer can be induced; simultaneously, the potential risk of inducing intussusception by taking attenuated rotavirus vaccine orally can be overcome; therefore, the recombinant protein is applicable to preparing a rotavirus vaccine.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
High-Yield Method For The Production Of Human Antibodies Blocking The Biological Activity Of A Human Cytokine
The invention concerns a pharmaceutical composition comprising, as the active ingredient, human natural antibodies of the IgG isotype, that neutralize the activity of a human cytokine selected from VEGF, IFNα, IL-4, TNFα and TGFβ, the said neutralizing antibodies inhibiting at least 50% of the maximum biological activity induced by an amount ranging from 0.006 ng to 0.05 ng of the said cytokine in vitro.
Owner:NEOVACS SA
African swine fever B and T cell tandem epitope fusion vaccine
InactiveCN111018995AGood immune effectAvoid the risk of accelerated viral infectionAntibody mimetics/scaffoldsViral antigen ingredientsAfrican swine feverTGE VACCINE
The invention, which belongs to the technical field of vaccines, particularly relates to an African swine fever B and T cell tandem epitope fusion vaccine. The main component of the African swine fever B and T cell tandem epitope fusion vaccine is African swine fever tandem epitope fusion protein. The African swine fever tandem epitope fusion protein comprises a B cell neutralizing epitope peptidefragment and a T cell epitope; and the B cell neutralizing epitope peptide comprises the following fragments: at least one neutralizing epitope peptide of each of p72, p54, p30 proteins. When the African swine fever tandem epitope fusion protein is used as a vaccine, the immune effect is good; and the antibody level significantly higher than that of a control group can be detected after one immunization. Since the non-neutralizing epitope is reduced as much as possible in the fusion protein, the risk of accelerating virus infection (ADE effect and antibody dependence enhancement effect) by anon-neutralizing antibody after immunization can be avoided.
Owner:河南省生物工程技术研究中心 +1
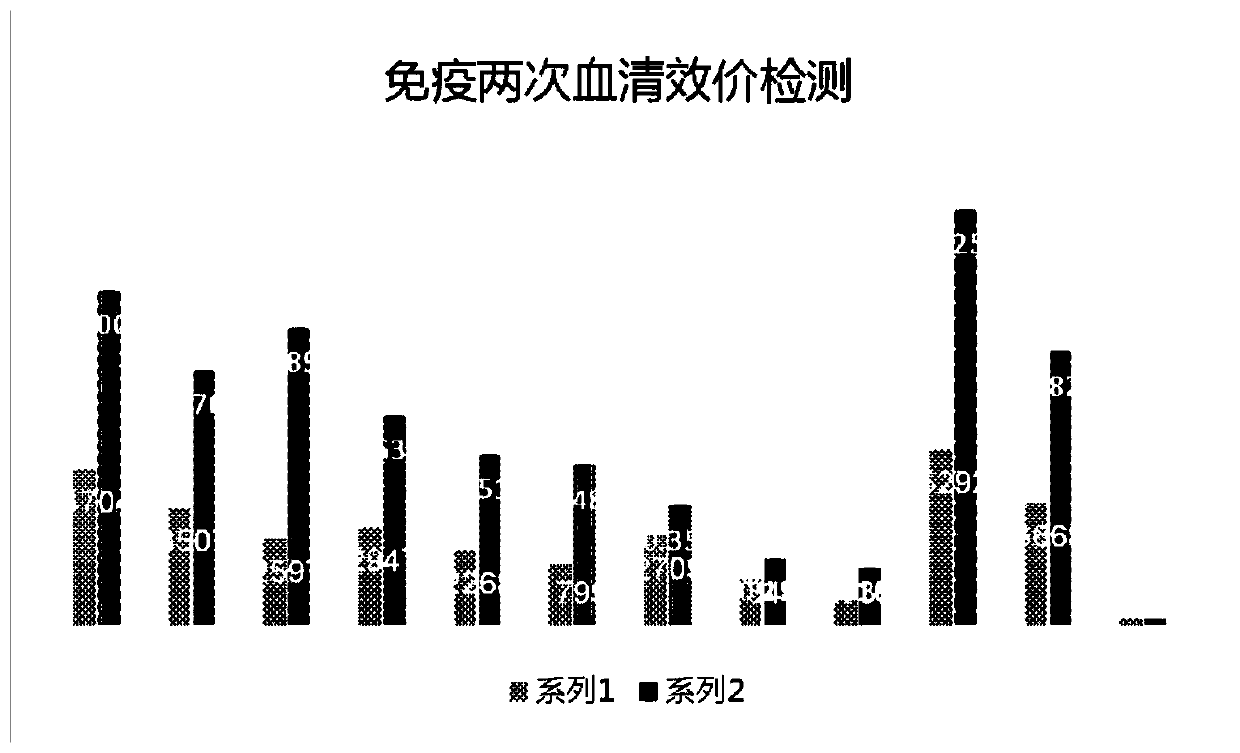
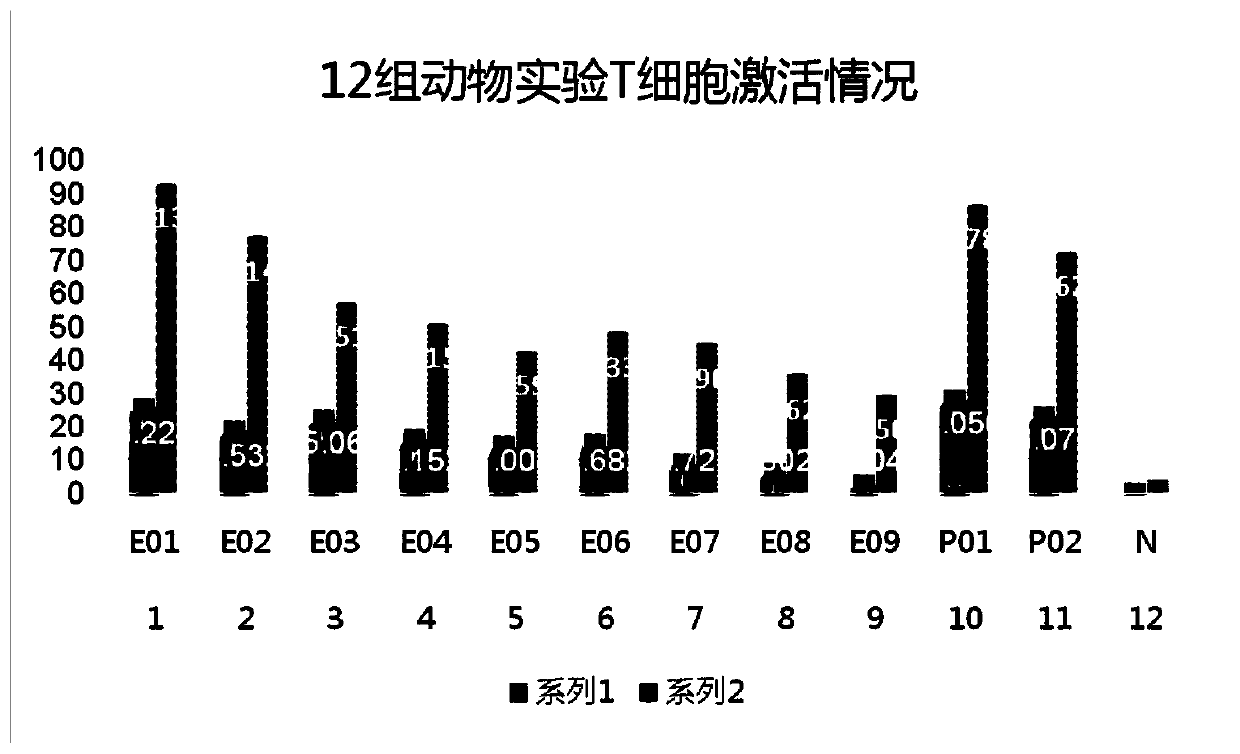
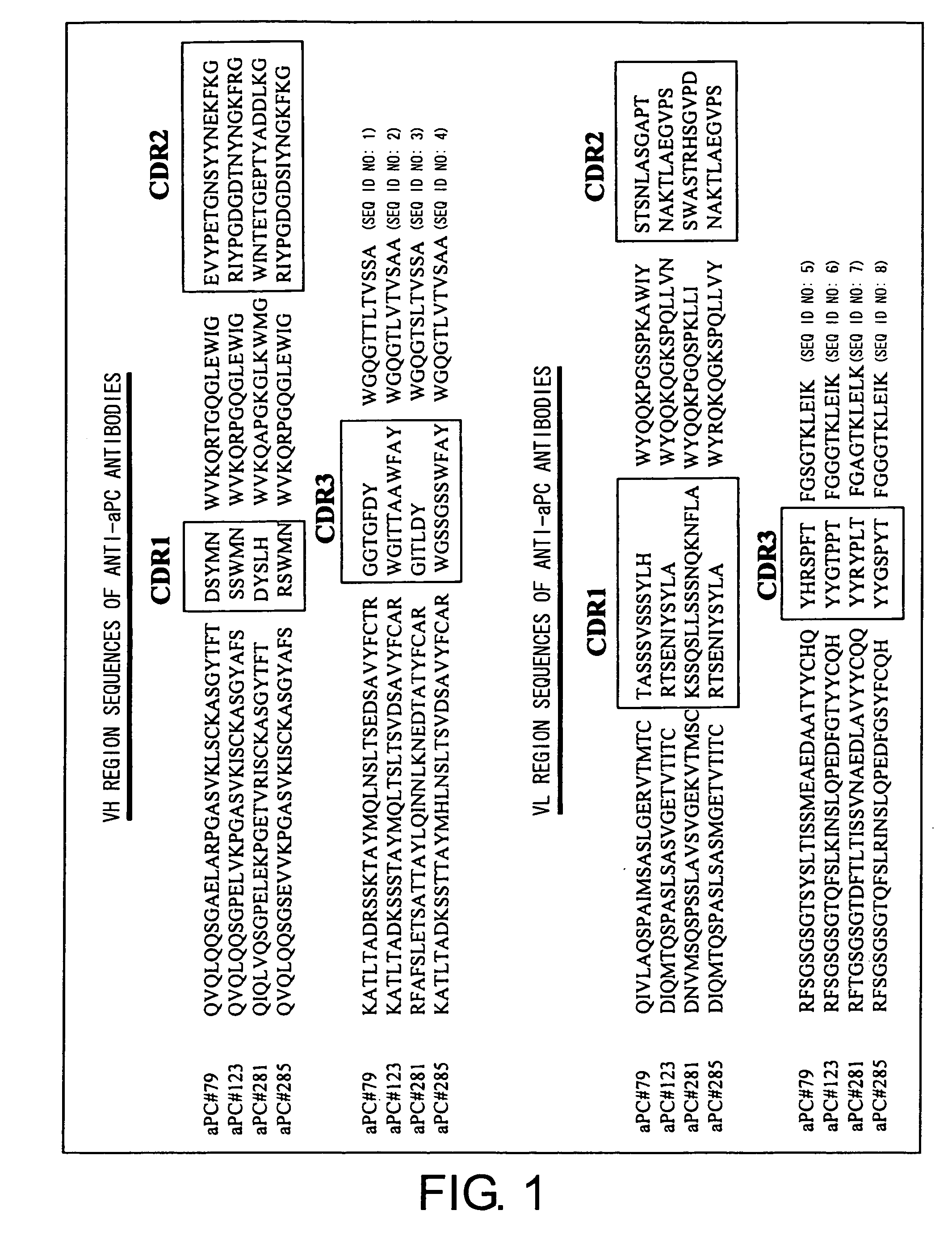
Apc non-neutralizing anti-body
ActiveUS20060121022A1High activityPotentiate actionAntibacterial agentsImmunoglobulins against animals/humansDiseaseAntiendomysial antibodies
The present invention provides anti-aPC antibodies that suppress the inactivation of activated protein C (aPC), and uses thereof. The present inventors screened anti-aPC antibodies, and succeeded in isolating anti-aPC antibodies comprising the activity of suppressing aPC inactivation in blood. The antibodies of the present invention can be used to maintain aPC activity by suppressing aPC inactivation, and can thus be used to sustain aPC bioactivities, such as the activity of suppressing activation of the blood coagulation system, and anti-inflammatory activity. In addition, the present invention provides uses of the antibodies of the present invention in aPC therapy for diseases such as thrombosis and sepsis. The therapeutic effect of aPC can be prolonged in treatment that uses aPC administration by allowing an antibody of the present invention to bind with aPC. The antibodies of the present invention can be used in the treatment and prevention of diseases such as thrombosis and sepsis.
Owner:CHUGAI PHARMA CO LTD
SARS-CoV-2 neutralizing antibody detection kit
PendingCN111562368AHigh sensitivityEasy to operateBiological material analysisImmunodiagnosticsNeutralising antibody
The invention relates to an SARS-CoV-2 neutralizing antibody detection kit. The SARS-CoV-2 neutralizing antibody detection kit comprises a solid phase carrier, a first antigen and a second antigen ofS protein of SARS-CoV-2, and the second antigen is marked with a signal substance. Whether a tested person is infected by the new coronavirus or not and whether infection risks exist or not are judgedby detecting a neutralizing antibody through an immunodiagnosis technology, and the method is reliable in theory, practical and feasible and can be completed only in a secondary biosafety laboratory.
Owner:威海威高生物科技有限公司
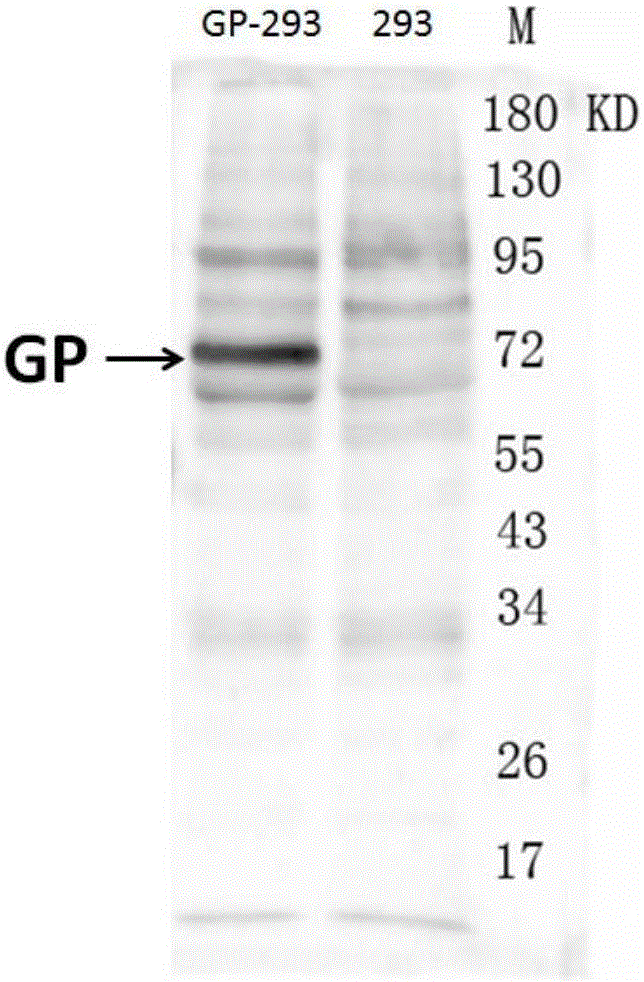
Hybridoma cell strain ZJEB8-01, Ebola-virus GP albumen resistant monoclonal antibody, and preparation and application of Ebola-virus GP albumen resistant monoclonal antibody
ActiveCN105087497AStable secretionNo decline in secretionImmunoglobulins against virusesTissue cultureAntigenEbola virus
The invention relates to a hybridoma cell strain ZJEB8-01, an Ebola-virus GP albumen resistant monoclonal antibody, and preparation and application of the Ebola-virus GP albumen resistant monoclonal antibody. The hybridoma cell strain ZJEB8-01 can be used for secreting the Ebola-virus GP albumen resistant monoclonal antibody. Binding of the monoclonal antibody and the 412th-431st amino acid sequence antigen peptides of Ebola-virus GP albumen has high specificity and sensitiveness, and the monoclonal antibody can be applied to preparation of Ebola-virus GP albumen detection reagents. Sequencing of the monoclonal antibody is completed, and the material basis is laid for humanization transformation of monoclonal antibody sequences, research and development of the neutralizing antibody for neutralizing Ebola viruses and development of the antibody into clinical Ebola virus treatment antibody in later period.
Owner:THE FIRST AFFILIATED HOSPITAL ZHEJIANG UNIV COLLEGE OF MEDICINE
Remedies for arthritis
InactiveUS20050175608A1Increase productionFacilitates dispersion and absorptionAntipyreticAnalgesicsGrowth retardantDiagnostic agent
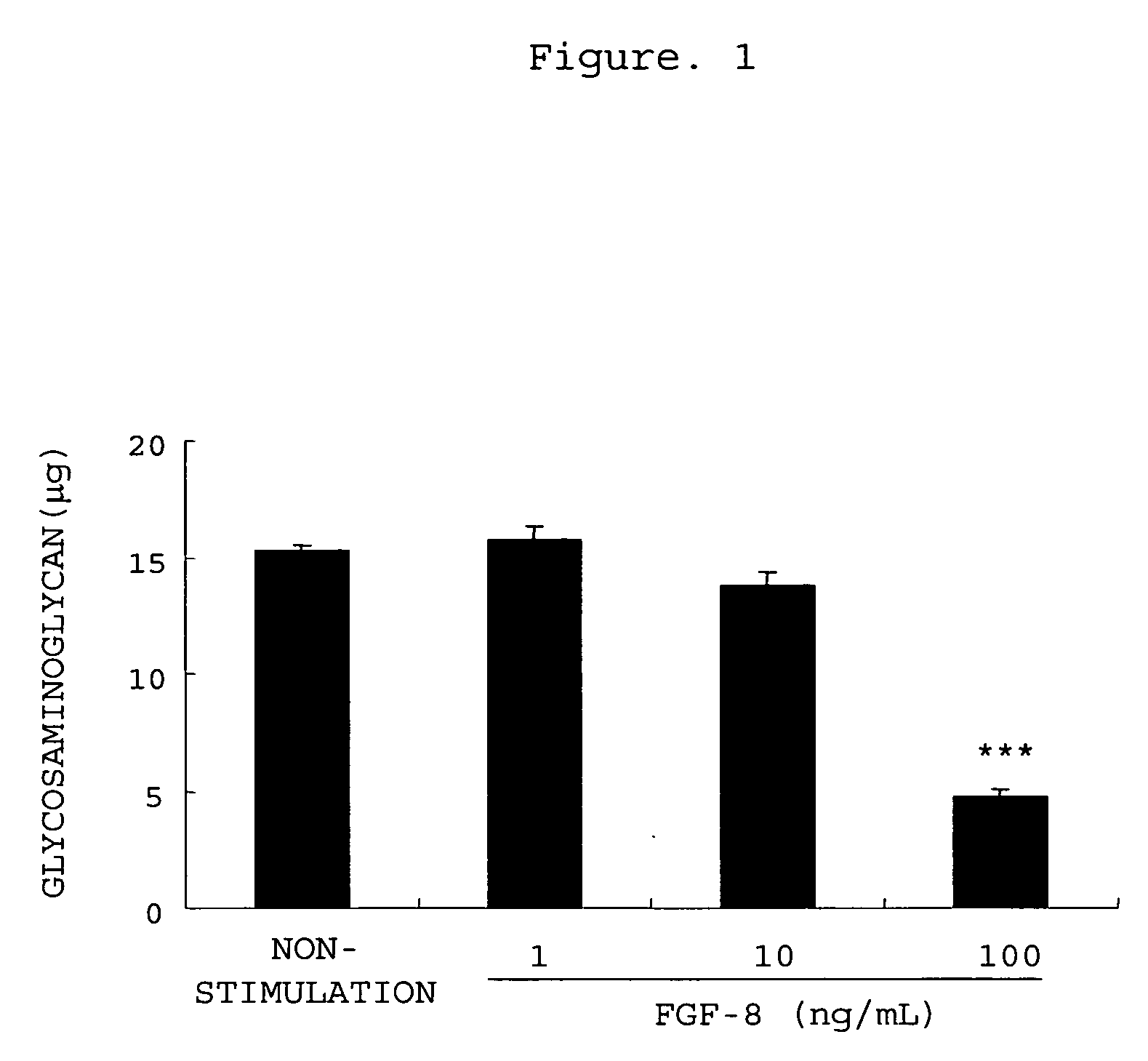
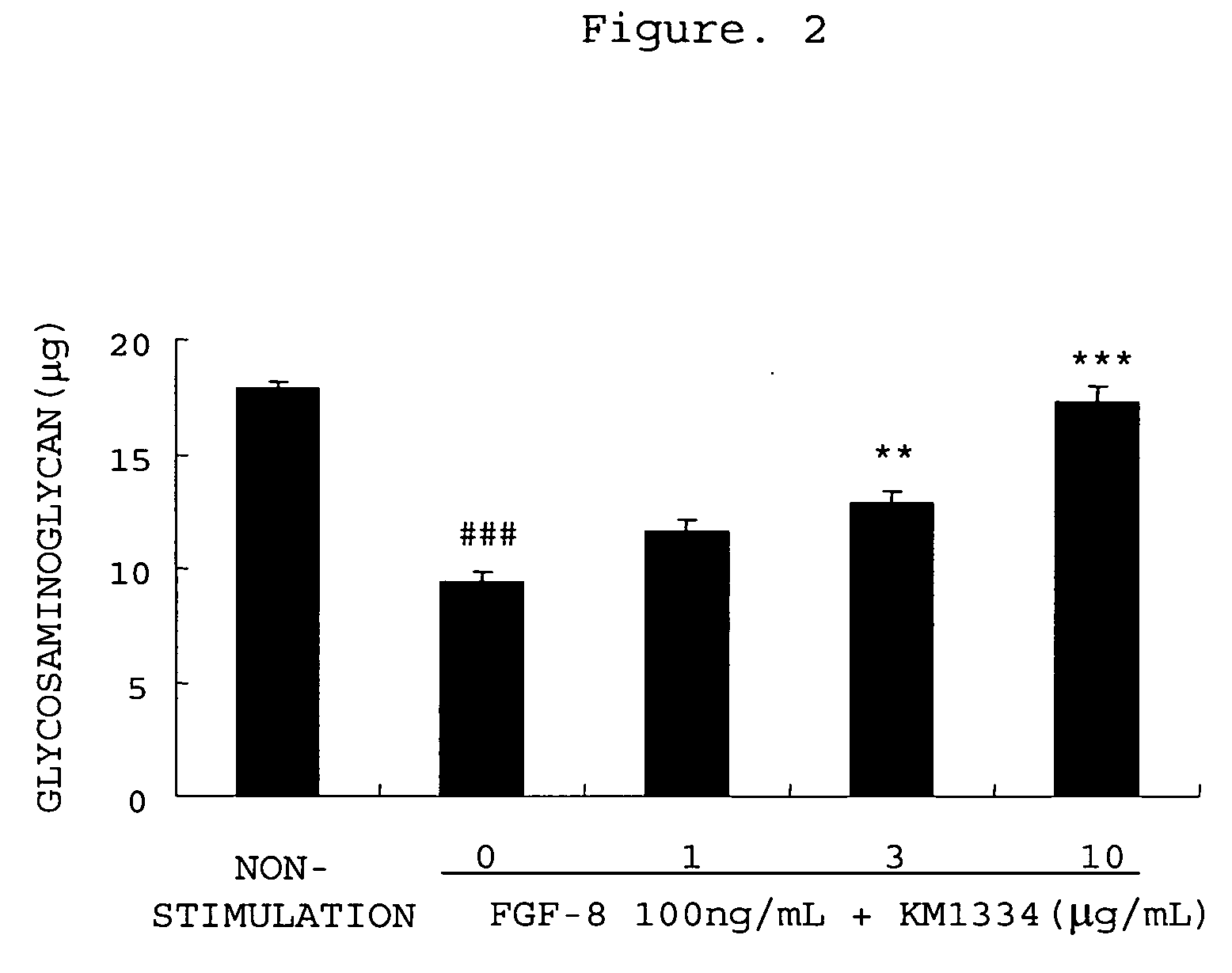
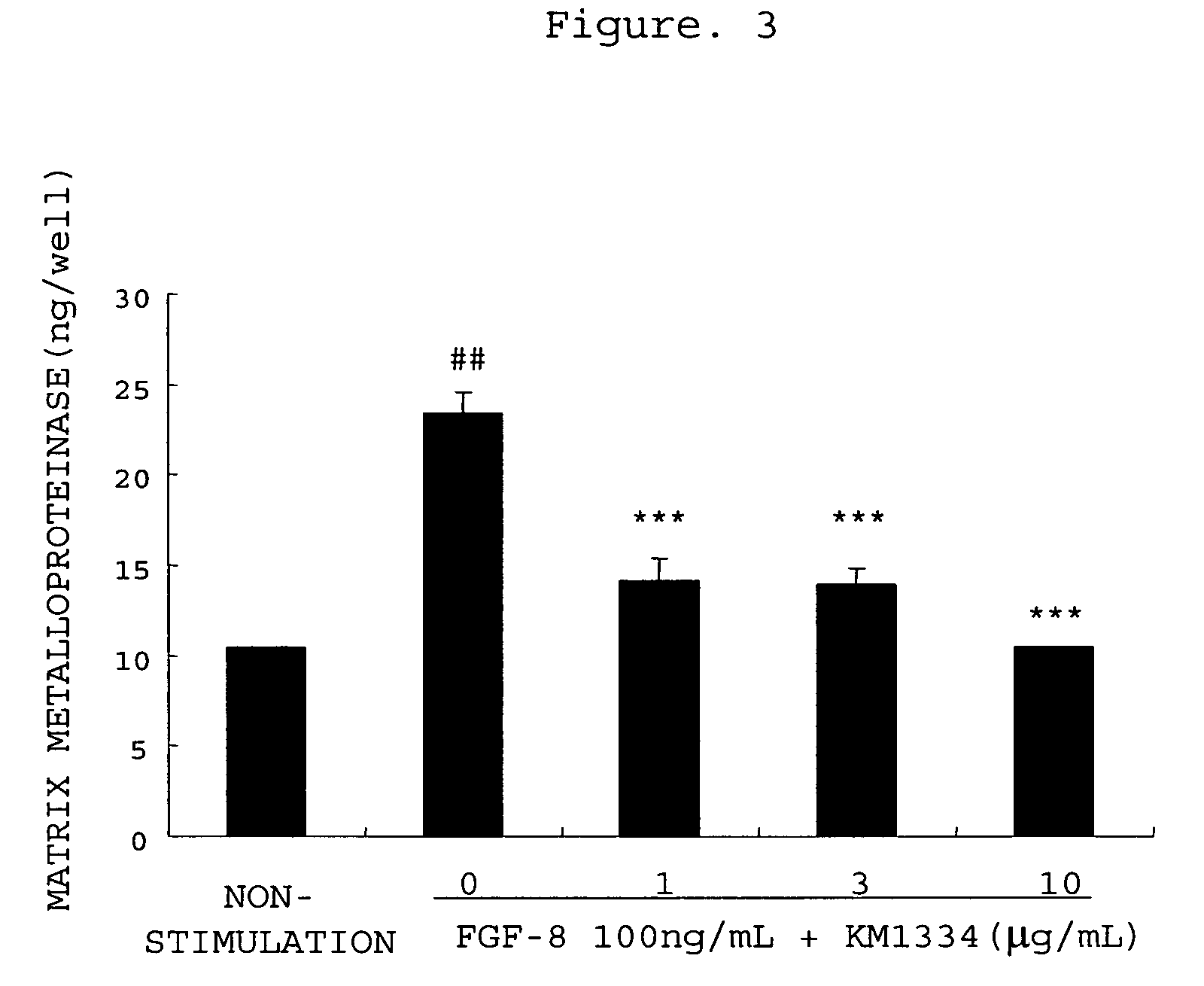
The invention provides an agent for preventing or treating arthritis, a cartilage protecting agent, a joint destruction inhibitor and a synovial membrane growth inhibitor comprising an anti-FGF-8 neutralizing antibody as an active ingredient, as well as a diagnostic agent of arthritis comprising an anti-FGF-8 antibody as an active ingredient and a method for judging arthritis using the antibody.
Owner:KYOWA HAKKO KIRIN CO LTD
Epitope-transplant scaffolds and their use
InactiveUS20100068217A1Sugar derivativesAnalogue computers for chemical processesEpitopeNeutralizing antibody
Owner:UNITED STATES OF AMERICA +1
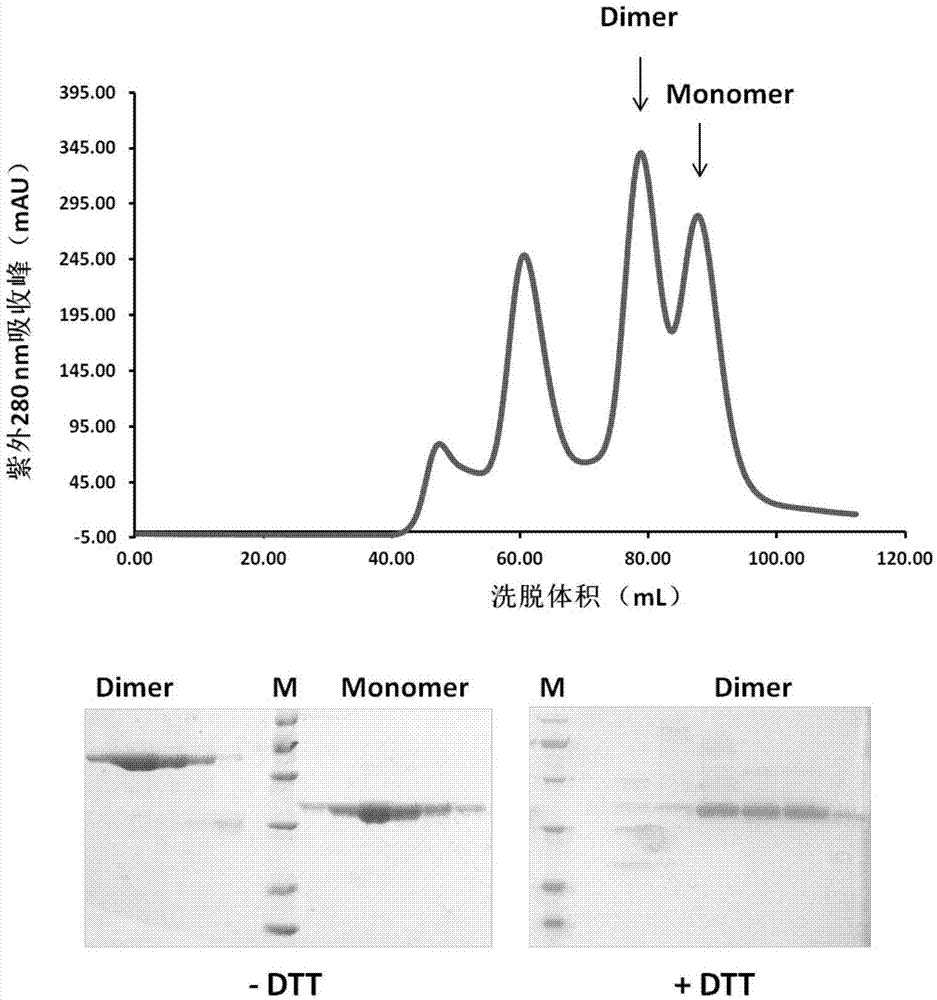
Beta-coronavirus antigen as well as preparation method and application thereof
ActiveCN111592602AGood immune effectHigh expressionPolypeptide with localisation/targeting motifSsRNA viruses positive-senseDimerNeutralising antibody
The embodiment of the invention relates to a beta-coronavirus antigen as well as a preparation method and application thereof. Wherein the amino acid sequence of the beta-coronavirus antigen comprisesan amino acid sequence arranged according to a (A-B)-(A-B) style or an amino acid sequence arranged according to a (A-B)-C-(A-B) style or an amino acid sequence arranged according to a (A-B)-(A-B ')style or an amino acid sequence arranged according to a (A-B)-C-(A-B') style, and the beta-coronavirus antigen has a single-chain dimer structure. The single-chain dimer expressed by the embodiment ofthe invention is stable in content and has good immunogenicity as a beta-coronavirus antigen, and a vaccine prepared by using the single-chain dimer as a beta-coronavirus antigen can stimulate mice to generate a neutralizing antibody with very high titer.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
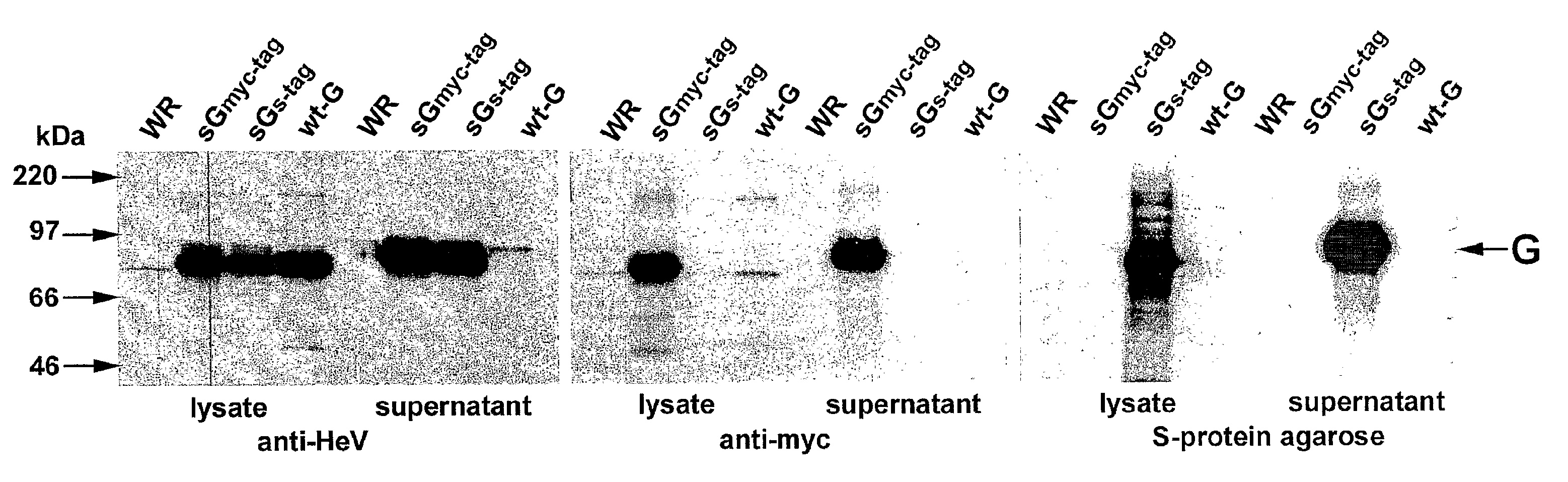
Soluble Forms of Hendra and Nipah Virus G Glycoprotein
ActiveUS20090041772A1Improve stabilityImproving immunogenicitySsRNA viruses negative-sensePeptide/protein ingredientsTherapeutic antibodyNeutralizing antibody
This invention relates to soluble forms of G glycoprotein from Hendra and Nipah virus. In particular, this invention relates to compositions comprising soluble forms of G glycoprotein from Hendra and Nipah virus and also to diagnostic and therapeutic methods using the soluble forms of G glycoprotein from Hendra and Nipah virus. Further, the invention relates to therapeutic antibodies including neutralizing antibodies, and vaccines for the prevention and treatment of infection by Hendra and Nipah viruses.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Novel vaccine for preventing COVID-19 and preparation method thereof
ActiveCN111939250AHighly conservativeAntibody induction ability is weakSsRNA viruses positive-senseAntibody mimetics/scaffoldsNucleotideReceptor
Provided is a novel vaccine for preventing COVID-19, the nucleotide sequence of an antigen of the novel vaccine is SEQ NO: 1, the amino acid sequence of the antigen of the novel vaccine is SEQ NO: 2,and the antigen of the vaccine comprises two functional parts: an S protein receptor binding structural domain capable of inducing a specific neutralizing antibody and a T cell related N protein truncated peptide fragment capable of inducing and activating effector T cells; The vaccine disclosed by the invention has the characteristics that the T cell related N protein truncated peptide fragment has weak capability of inducing the generation of the N protein antibody, so that a vaccine inoculator and a COVID-19 infected patient can be identified by using the N protein antibody, and the vaccineantigen does not induce the generation of the N protein antibody, so that lung injuries can be reduced, and the vaccine is safer. The cell vaccine disclosed by the invention is low in manufacturing cost, and can induce generation of virus-specific neutralizing antibodies and T cell immune response.
Owner:ZHENGZHOU UNIV
Neutralizing molecules to viral antigens
The present invention concerns methods and means for identifying, producing, and engineering neutralizing molecules against influenza A viruses, and to the neutralizing molecules produced. In particular, the invention concerns neutralizing molecules against various influenza A virus subtypes, including neutralizing antibodies against H5 and / or H3 and / or H1, such as, for example all of H1, H3, and H5 subtypes, and methods and means for making such molecules.
Owner:BIOASSETS LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com