Human rotavirus Delta VP8* subunit recombinant protein and application thereof
A rotavirus and recombinant protein technology, applied in the field of animal molecular biology and genetic engineering, can solve the problem of low immunogenicity, achieve strong neutralizing antibody titers, improve immune efficacy, and overcome potential risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Construction of an expression vector containing a fragment of interest.
[0038]The material used was the Wa strain of human rotavirus with genotype G1P[8]. The Wa strain of human rotavirus is a classic strain, which is cultured with primary African green monkey kidney (AGMK) cells. The medium used was EMEM, in which trypsin was added to a final concentration of 0.5 μg / ml, penicillin 100 IU / ml, streptomycin 100 μg / ml, amphotericin B 2.5 μg / ml. The formula of EMEM medium is shown in Table 1:
[0039] Table 1 EMEM medium formula
[0040] serial number
Content (mg / L)
serial number
Content (mg / L)
1
Anhydrous Calcium Chloride.2H2O
265.00
18
L-serine
42.00
2
0.10
19
95.00
[0041] 3
400.00
20
16.00
4
97.67
21 ...
Embodiment 2
[0051] Example 2 Expression of human rotavirus subunit recombinant protein
[0052] The expression plasmid pET28a-P2-P[8]ΔVP8* was transformed into E.coli BL21(DE3)pLysS competent cells by heat shock method. Pick a single clone from the agar plate and inoculate it in LB liquid medium containing 50 μg / ml kanamycin (add 2% w / v glucose, 1% v / v ethanol). When the absorbance value at 600 nm reached 0.5, IPTG was added to a final concentration of 1 mM, and the protein expression was induced overnight at 18°C. SDS-PAGE electrophoresis results such as image 3 shown. Then, the recombinant E.coli cells were collected by centrifugation at 10,000 g at 4°C for 15 minutes, and stored at -80°C for later use. After testing, the yield of soluble protein obtained by this method is 46 mg / L.
[0053] The present invention further adopts the second method to induce recombinant protein expression, the steps of which are the same as the above method, the difference is that glucose and ethanol a...
Embodiment 3
[0055] Embodiment 3Western blot method detects protein
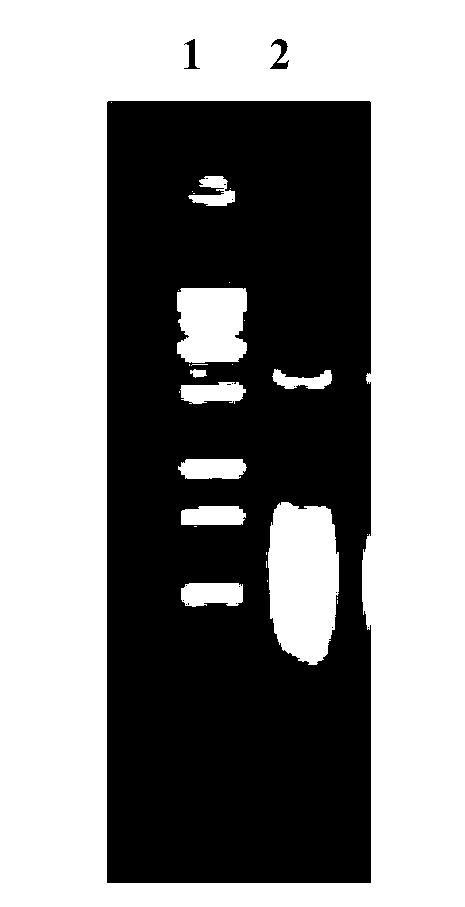
[0056] Proteins were analyzed on 4-12% NuPAGE gels and transferred to nitrocellulose membranes (Whatman). The membrane was incubated overnight at 4°C with hyperimmune guinea pig anti-Wa(P[8]) serum (1:50). After washing three times, the membrane was incubated with peroxidase-labeled goat anti-guinea pig IgG (H+L) (KPL) for 1 h at room temperature. 3,3'Diaminoblue aniline (DAB) (Sigma) was used for color development. The result is as Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com