SARS-CoV-2 (severe acute respiratory syndrome-corona virus disease-2) inhibitor and application thereof
A sars-cov-2 and sars-cov-2-s technology, applied in the field of neutralizing antibodies against SARS-CoV-2, can solve problems such as limited sources, mismatch of donor and recipient blood types, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] The cultivation and material preparation of embodiment 1 virus
[0076] The SARS-CoV-2 virus was isolated by the patent applicant from the peripheral blood of a Jiangsu patient in the acute phase in 2020. After the virus was inoculated into Vero cells, at 37°C, 5% CO 2 Cultured for 5 days under certain conditions, collected the supernatant and determined the 50% tissue infection dose (50% tissue culture infectious dose, TCID 50 ). All manipulations are performed in BSL-3 laboratories.
[0077] Recombinant S protein (SARS-CoV-2-S), commercially purchased, the sequence is shown in SEQ ID NO.1.
Embodiment 2
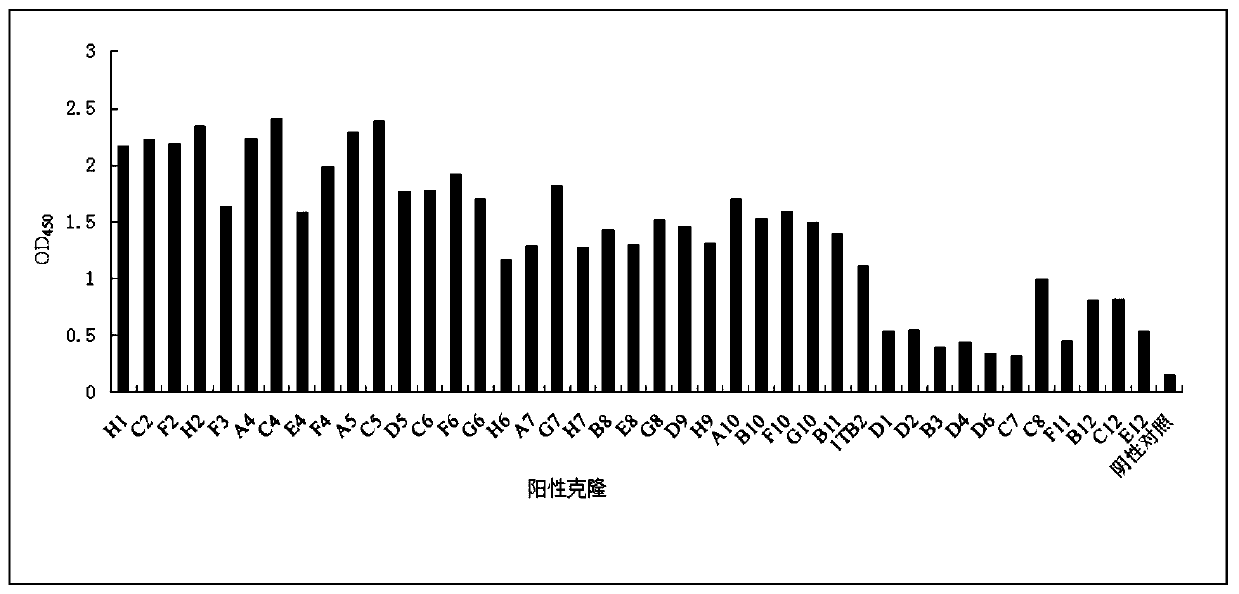
[0078] Example 2 Construction of ScFv human antibody library and screening of anti-SARS-CoV-2-S protein single chain antibody
[0079] 2.1 Materials
[0080] Primers: Family-specific light chain (Vκ and Vλ), IgG heavy chain (VH) and overlap-PCR primers were designed according to the book "Phage Display", including Vκ12 pair, Vλ24 pair, VH 6 pair, and overlap-PCR1 pair.
[0081] Primers for constructing human scFv library:
[0082] Vκforward primers:
[0083] 5'GGGCCCAGGCGGCCGAGCTCCAGATGACCCAGTCTCC 3' (SEQ ID NO.66)
[0084] 5'GGGCCCAGGCGGCCGAGCTCGTGATGACYCAGTCTCC 3' (SEQ ID NO. 67)
[0085] 5'GGGCCCAGGCGGCCGAGCTCGTGWTGACRCAGCTCC 3' (SEQ ID NO. 68)
[0086] Vκreverse primers:
[0087] 5'GGAAGATCTAGAGGAACCACCTTTTGATYTCCACCTTGGTCCC 3' (SEQ ID NO. 69)
[0088] 5'GGAAGATCTAGAGGAACCACCTTTTGATCTCCAGCTTGGTCCC 3' (SEQ ID NO. 70)
[0089] 5'GGAAGATCTAGAGGAACCACCTTTAATCTCCAGTCGTGTCCC 3' (SEQ ID NO. 71)
[0090] 5'GGAAGATCTAGAGGAACCACCTTTGATATCCACTTTGGTCCC 3' (SEQ ID NO. 72)
[0...
Embodiment 3
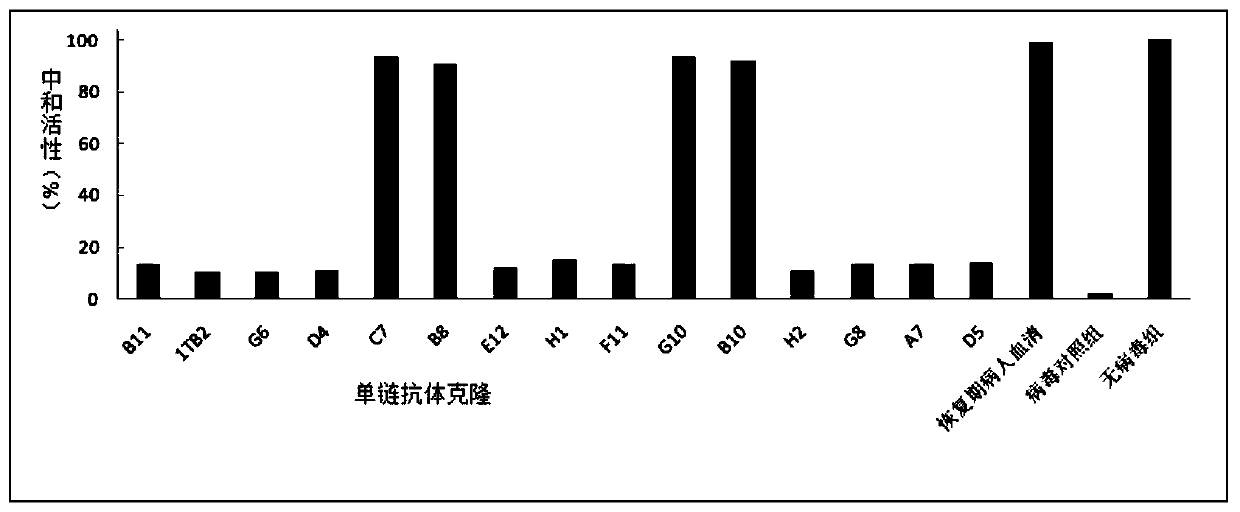
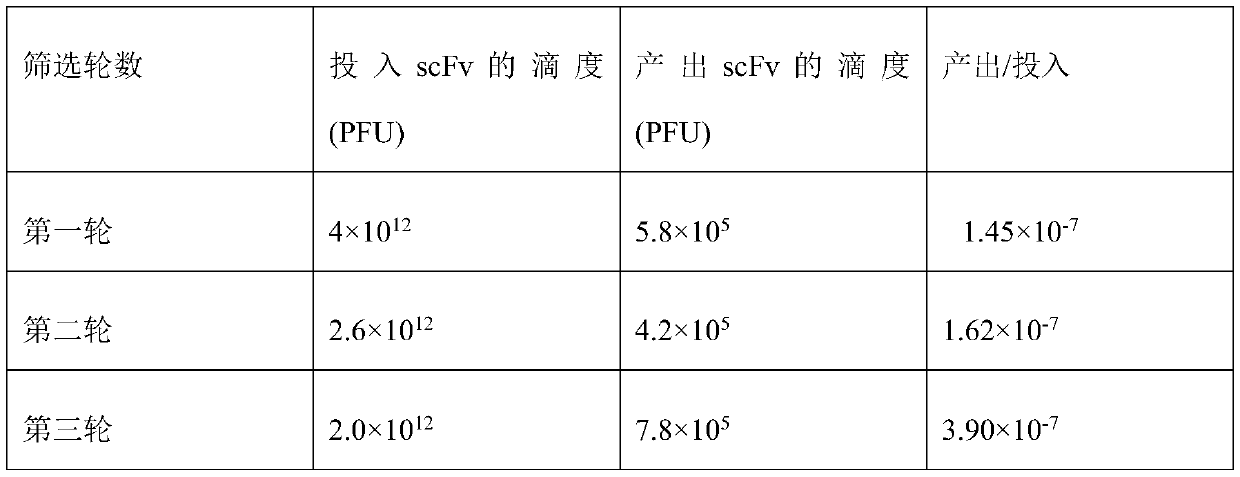
[0134] Example 3 Candidate scFv monoclonal microneutralization experiment (all operations were carried out in BSL-3 laboratory)
[0135] 3.1 Preparation of phage antibody
[0136] Pick 15 single colonies that were positive in the Phage-ELISA test and place them in 2ml 2×YT medium (containing 100 μg / mL ampicillin, 12.5 μg / mL tetracycline, and 1 g / mL glucose), culture them overnight at 37°C with shaking, and the next day 1:10 were inoculated into 10ml of 2×YT medium (containing 100 μg / mL ampicillin and 30 μg / mL tetracycline) and cultured with shaking at 37°C for 6 hours, and the helper phage VCSM13 (final concentration of 1×10 9 PFU / mL), incubate at 37°C for 1 h, add kanamycin (final concentration is 50 μg / mL), shake at 30°C overnight, centrifuge at 900 g for 30 minutes, discard the precipitate, add 5×PEG / NaCl to the supernatant, mix Put it on ice for 6 hours, centrifuge at 900g for 30min, discard the supernatant, resuspend the pellet with 1ml PBS, centrifuge at 900g for 30min,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com