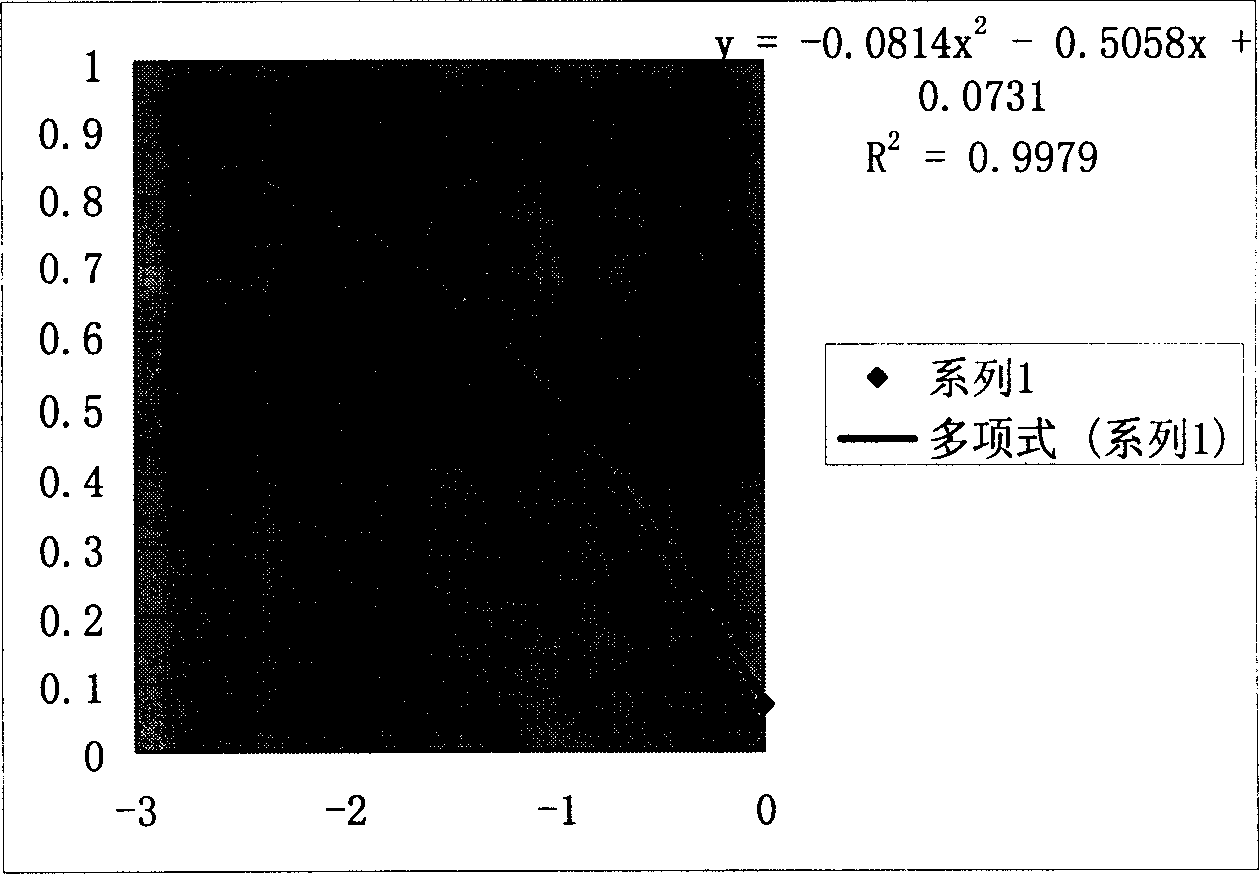
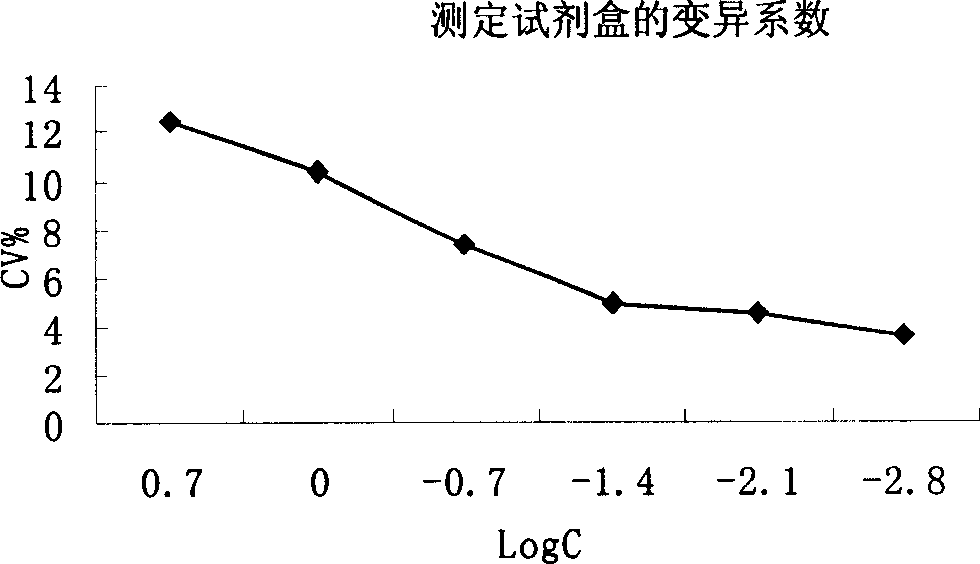
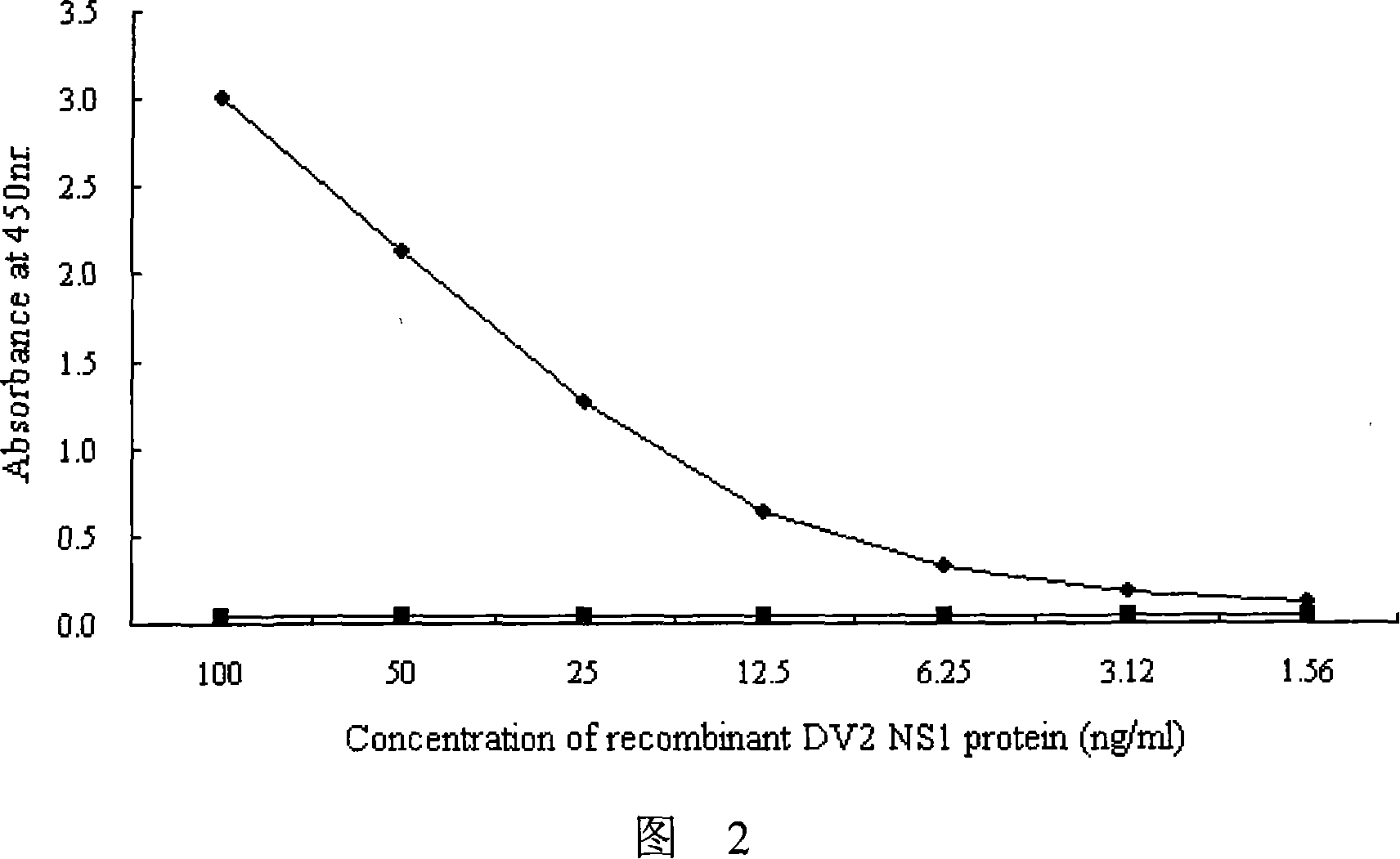
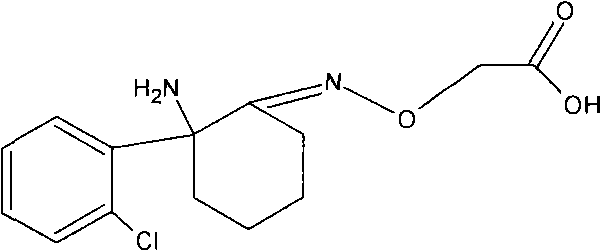
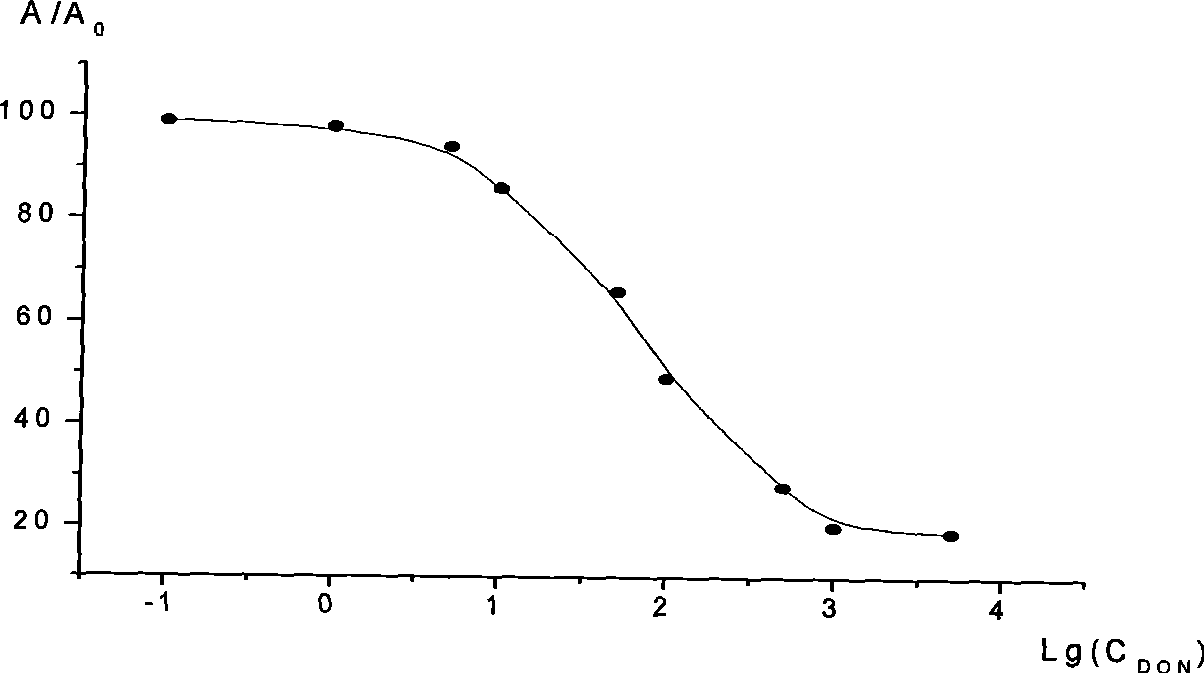Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
108 results about "Elisa test" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
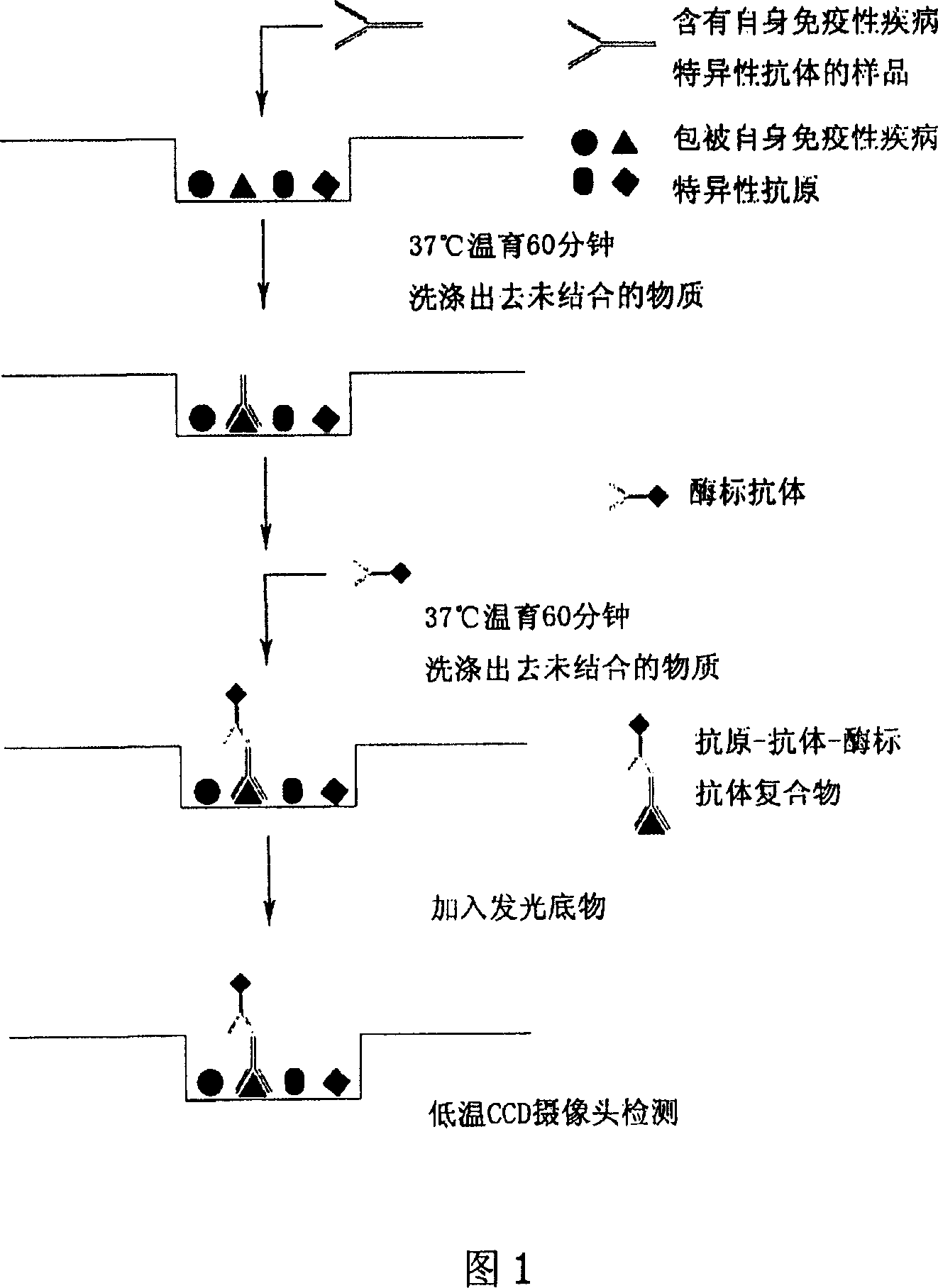
The ELISA HIV test was one of the original diagnostic tools used for HIV and AIDS. An ELISA test is a test used to determine if there are antibodies or antigens in the body. When conducting an ELISA HIV test, a special slide is prepared that contains HIV antigens. Serum from the person receiving the test is applied to the slide.
Method to measure and characterize microvesicles in the human body fluids
InactiveUS20090220944A1Low efficiencySimple yet reliableMicrobiological testing/measurementDisease diagnosisHuman bodyNon invasive
This disclosure provides a method to capture, detect, characterize and quantify human exosomes in small volumes of human body fluids by using a sandwich ELISA test. This method allows a full characterization of an exosome preparation, thus providing a tool to distinguish a disease-related condition from a healthy state, by the use of a non-invasive assay. In fact, this method may be useful in screening, diagnosis and prognosis of tumors, with a simple plasma sample. At the same time measurement of circulating exosomes may provide information on the level of tumor mass present in a patient. The method provided here is suitable to evaluate presence of some infectious and / or transmissible agents, such as viral proteins or prion proteins, within circulating exosomes.
Owner:EXOSOMICS SPA
Indirect competitive ELISA kit for detecting malachite green in aquatic product
InactiveCN1766623AEasy to operateStrong specificityTesting medicinal preparationsMalachite greenElisa kit
The invention relates to a colorless green malachite green indirect compete ELISA test agent box of an aquatic product. The test plate of the agent box is a split 96 holes enzyme mark plate which coats colorless green malachite green and albumin coupling material; the anti-colorless green malachite green antibody is a monoclonal antibody which is prepared by the colorless green malachite green and the albumin coupling material immune mouse; the tested sample uses ethyl hexoate and cyclohexane to extract after uniform and adjusts PH value for detecting. The quoting standard of the sample detecting is that it is a regression curve which uses the logarithm value of the sample density as abscissa and uses the degradation rate as ordinate. We could read the density of the corresponding sample from the degradation rate of each sample. The sensibility of the test method is 0.0023ª–g / ml; the test range is 0.0016-1ª–g / ml.
Owner:SHANGHAI ENTRY EXIT INSPECTION & QUARANTINE BUREAU OF P R C +1
Immunologic diagnosis kit for detecting type II dengue virus NS1 antigen
ActiveCN101226196AAccurate detectionQuick checkMaterial analysisAgainst vector-borne diseasesSerotypeElisa test
The invention provides an immunity diagnosis test kit for detecting II-type dengue virus antigen, which comprises a porous reaction plate covering monoclonal antibody DV2-M6, a sample treatment liquid, a monoclonal antibody DV2-M15 marked with a label, a positive contrast, a negative contrast, a concentration washing liquid, a develop liquid and a termination liquid, wherein the monoclonal antibodies DV2-M6 and DV2-M14 of the test kit can be specifically combined with NS1 protein of II-type dengue virus, without cross reaction with other three kinds of serotype dengue viruses NS1 and respectively combined with different antigen points of NS1, while the check sensitivity of NS1 protein of II-type dengue virus can reach 3ng / ml and the check sensitivity of culture supernatant of II-type dengue virus infection cell is 8 power of Pan-E dengue early elisa test kit, thereby improving the sensitivity of clinical serum sample check.
Owner:SOUTHERN MEDICAL UNIVERSITY
Biologically safe Africa swine fever antigen multifactorial serum for ELISA diagnosis
The invention relates to a biologically safe Africa swine fever antigen multifactorial serum for ELISA (enzyme-linked immuno sorbent assay) diagnosis. A technical scheme adopted in the invention includes: adopting gene-expressed structural protein P72, K205R, P54, and A104R, conducting chemical purification, carrying out coating with a Freund's incomplete adjuvant, performing intramuscular immunization on laboratory swine in three batches, collecting swine blood after one month, separating serum, implementing serological testing, and conducting subpackaging and preservation. The serum is subpackaged into ELISA kits to undergo test according to conventional ELISA test methods.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
ELISA test box for detecting zearalenone and preparing and detecting method thereof
The invention relates to an ELISA kit for detecting zearalenone, the detection is rapid, sensitive, accurate, quantitative, simple in operation, low in requirements on sample purity and strong in specificity, thereby being particularly applicable to the detection of large quantities of samples; and the invention also provides a preparation of the kit and a detection method. The kit comprises washing liquid, color developing liquid A, color developing liquid B and stop solution, and the kit is characterized in that: the kit also comprises a coated plate, a zearalenone standard product, a zearalenone monoclonal antibody freeze-dried product and an enzyme-labeled goat anti-mouse antibody free-dried product; when in detection, the coated plate is taken, 50mu1-100mu1 of the ZEN standard product or a well processed sample is added into the respective micropores, 50mul-100mul of the anti-ZEN antibody is added, the incubation is carried out at 35 DEG C-45 DEG C for about 0.5 hour-1 hour, the washing liquid is used for washing for 3 times-5 times, 50mu1-100mu1 of the horseradish peroxidase (HRP)-goat anti-mouse antibody is added, the incubation is carried out at about 35 DEG C-45 DEG C for about 0.5 hour-1 hour, the washing liquid is used for washing for 3 times-5 times, 50mu1-100mu1 of the color developing liquid A and 50mu1-100mu1 of the color developing liquid B are added, the mixture is placed still in the dark for 10 minutes-20 minutes, then the stop solution is added, the absorbance value is measured at 450nm, and the ZEN content in the sample is calculated from a standard curve.
Owner:BEOSON JIANGSU FOOD SAFETY TECH CO LTD
Micro-plate reader for elisa testing
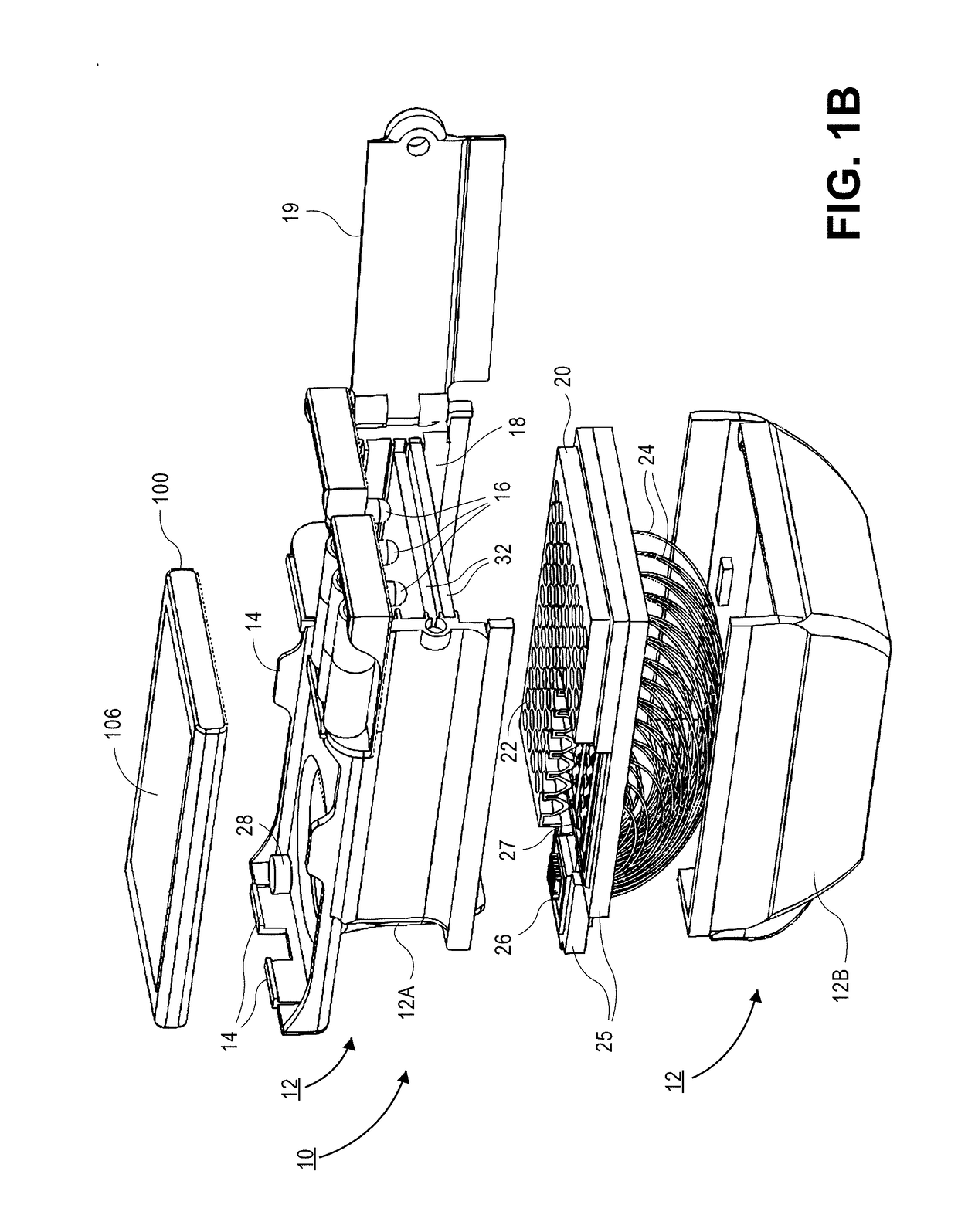
ActiveUS20180196193A1More compactMore denseTelevision system detailsDigital computer detailsFiberReduced size
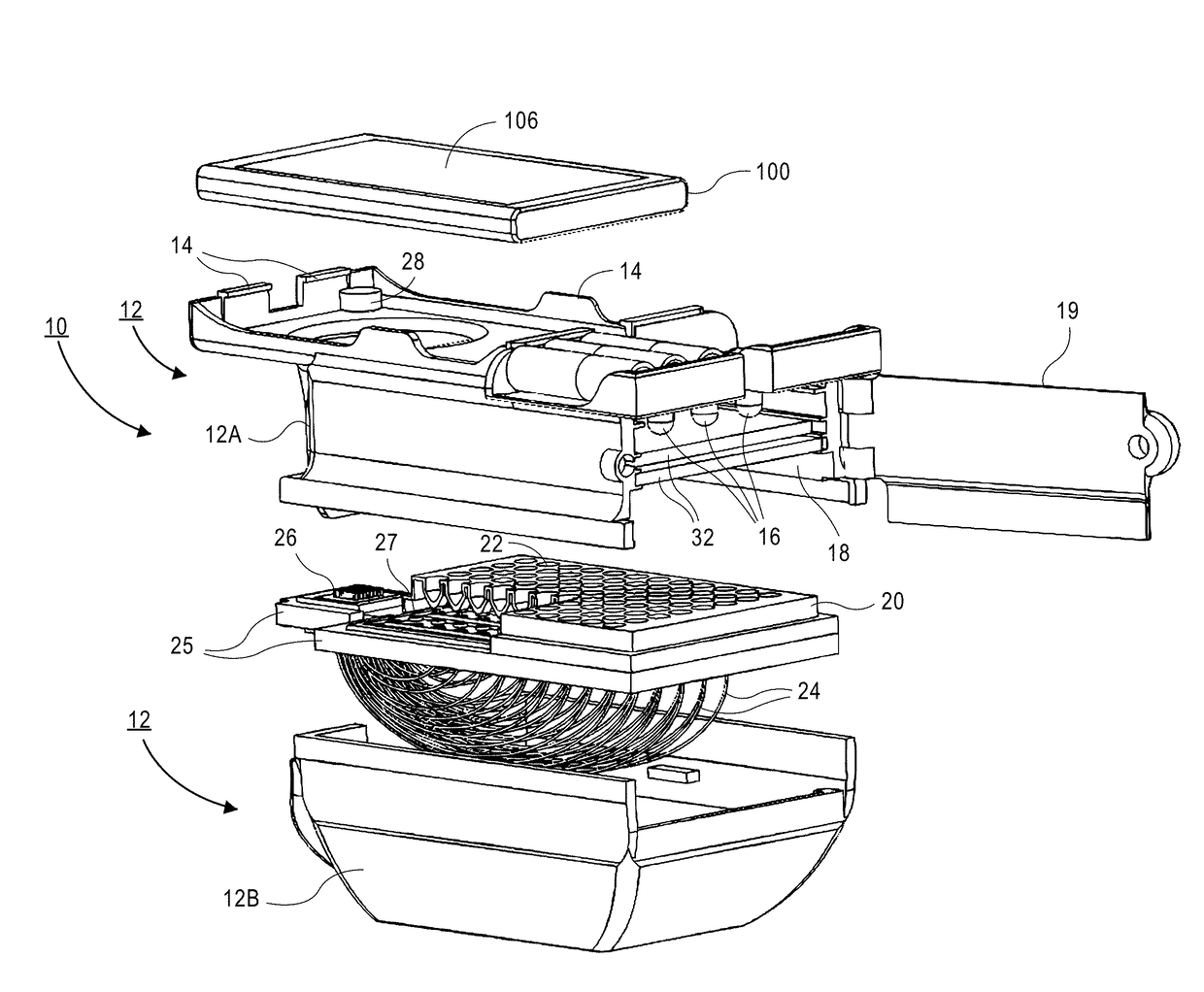
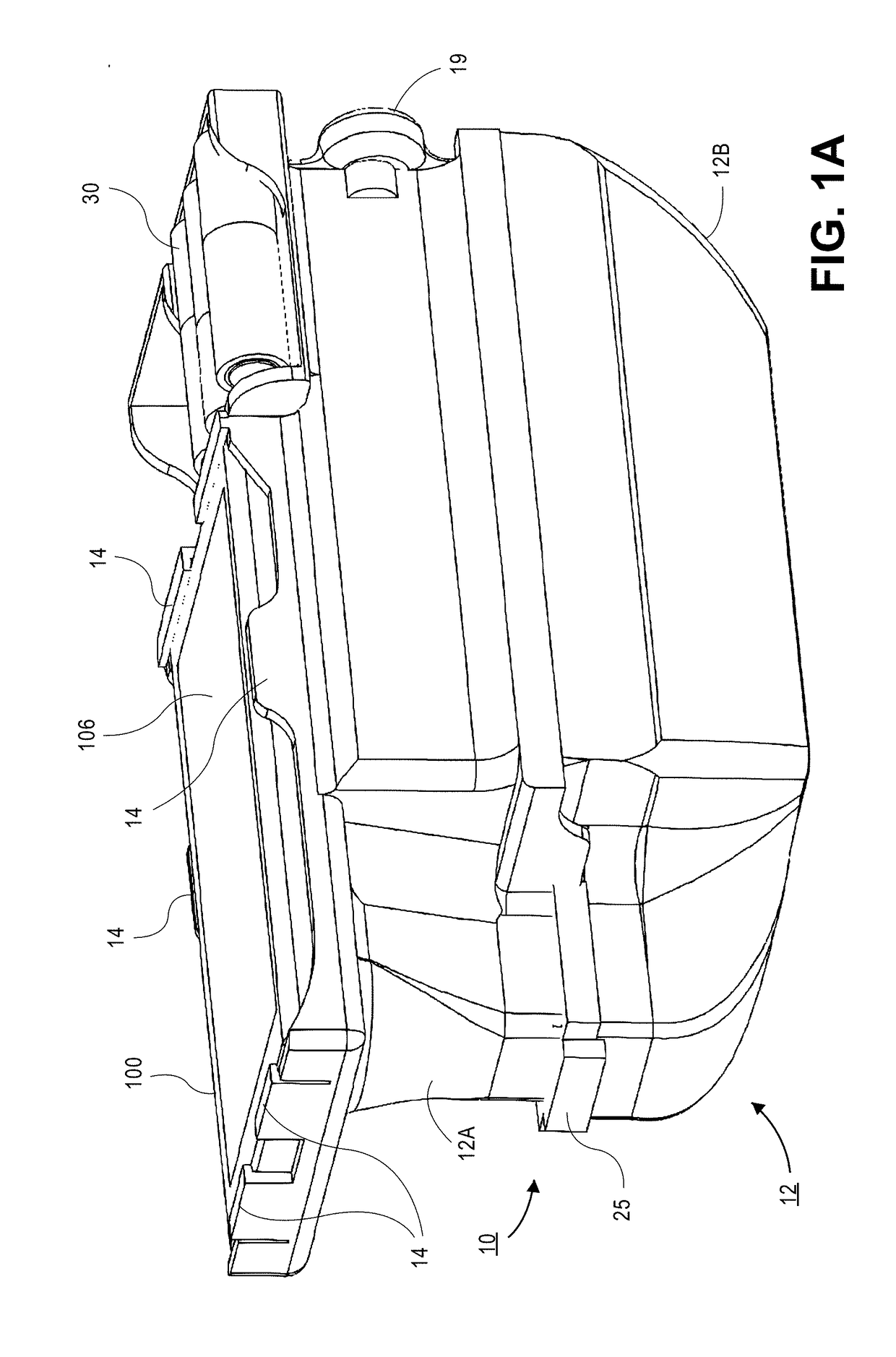
A micro-plate reader for use with a portable electronic device having a camera includes an opto-mechanical attachment configured to attach / detach to the portable electronic device and includes an array of illumination sources. A slot in the opto-mechanical attachment is dimensioned to receive an optically transparent plate containing an array of wells. Optical fibers are located in the opto-mechanical attachment and transmit light from each well to a reduced size header having, wherein the fiber array in the header has a cross-sectional area that is ≤10× the cross-sectional area of the wells in the plate. A lens located in the opto-mechanical attachment transmits light from the header fibers to the camera. Software executed on the portable electronic device or other computer is used to process the images to generate qualitative clinical determinations and / or quantitative index values for the separate wells.
Owner:RGT UNIV OF CALIFORNIA
Microarray-ELISA detecting reagent kit for detecting autoimmunity disease relevant antibody spectrum
InactiveCN101063680AImprove throughputHigh parallelMaterial analysisDiffuse sclerodermaAutoimmune responses
This invention relates to one anti-extracting nuclear antigen spectrum array to ELISA test agent case for selecting for systematic lupus erythematosus, mixed connective tissue disease, Sjogren syndrome, systemic scleroderma, polymyositis and atrophic arthritis system self immune property antigen ENA spectrum micro array to enzyme immune agent case.
Owner:BEIJING BGI GBI BIOTECH +4
Classical swine fever virus recombinant E2 protein and IgM (immune globulin M) antibody ELISA (enzyme-linked immunosorbent assay) test kit thereof
ActiveCN102532281AGood antigenicityGood repeatabilityBacteriaVirus peptidesEscherichia coliClassical swine fever virus CSFV
The invention belongs to the field of molecular biology and relates to a classical swine fever virus recombinant E2 protein and an IgM (immune globulin M) antibody ELISA (enzyme-linked immunosorbent assay) test kit thereof. The classical swine fever virus E2 protein expressed by recombinant Escherichia coli is obtained by cloning the main antigen region of E2 protein into a pronucleus expression vector to obtain a recombinant expression vector, transforming the recombinant expression vector to Escherichia coli BL21 (DE3) and expressing and purifying with the recombinant Escherichia coli. A Westernblot test indicates that the protein has good antigenicity. According to the invention, an elisa plate is coated the protein; an ELISA method is established through optimization of antigen coating concentration, serum dilution and action time, secondary antibody concentration and action time as well as developing time for the purpose of detecting negative serum so as to determine a critical value and a judgment standard. According to the invention, the expression of a recombinant strain constructed by the recombinant E2 protein on a heterologous protein is stable, and the recombinant strain is good in antigenicity; and on the basis, the recombinant E2 protein disclosed by the invention is used for establishing a CSFV (classical swine fever virus) IgM antibody ELISA test kit for the first time.
Owner:JIANGSU ACAD OF AGRI SCI
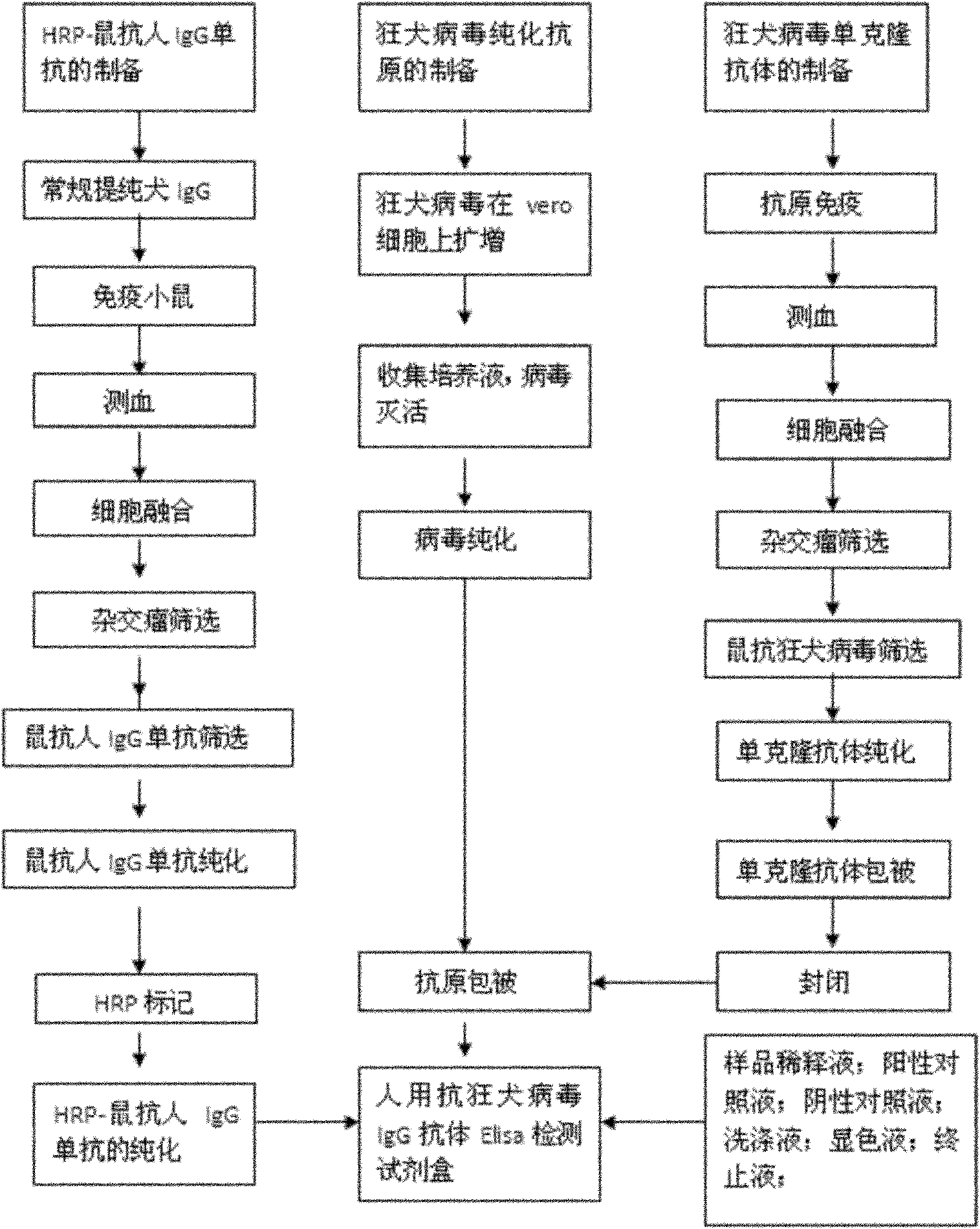
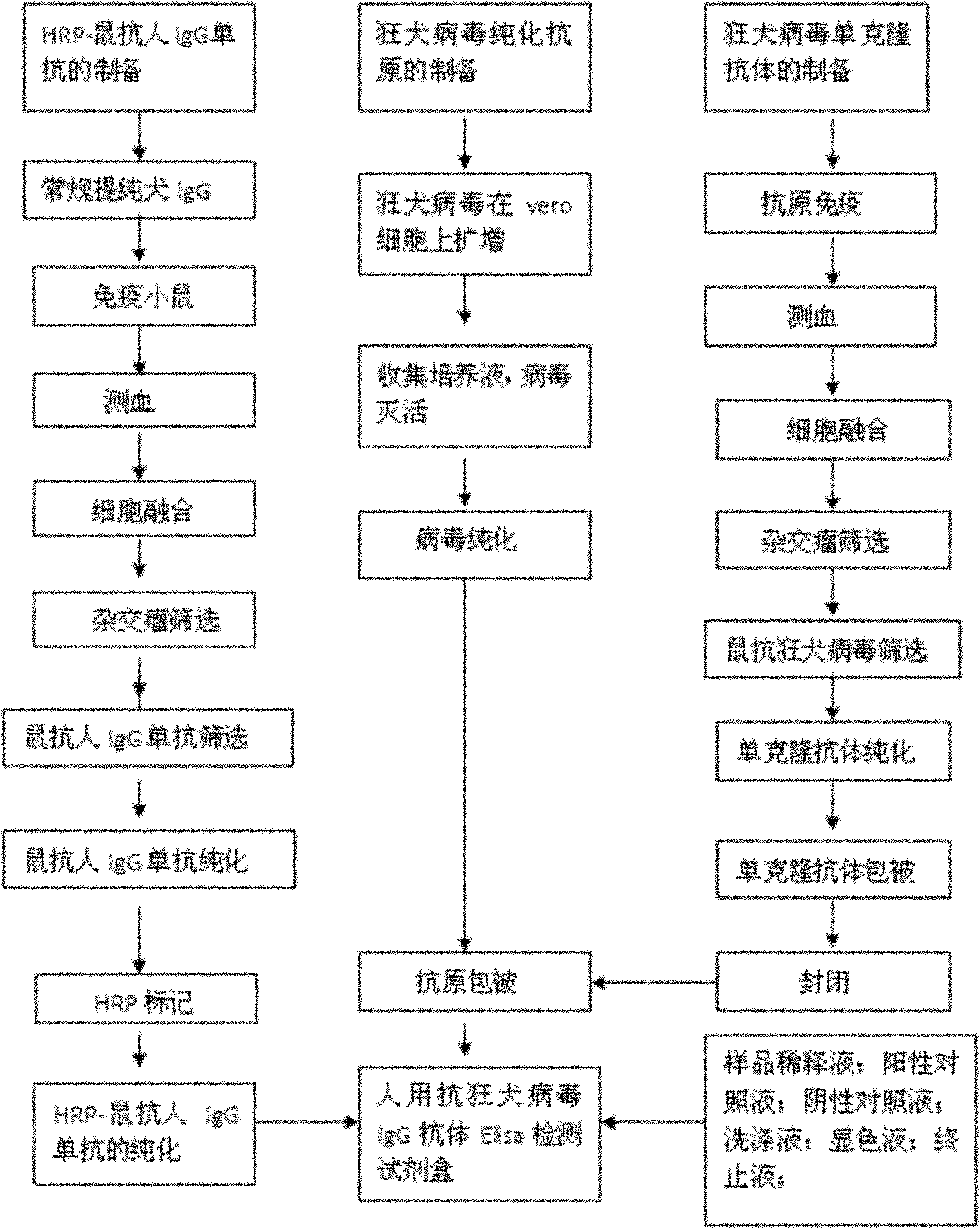
Human anti-rabies virus IgG antibody ELISA test kit
ActiveCN101936997AMake up for the shortcomings of low sensitivityHigh sensitivityDepsipeptidesMaterial analysisAntigenPositive control
The invention relates to a human anti-rabies virus IgG antibody ELISA test kit. An ELISA plate is firstly coated with an anti-rabies virus monoclonal antibody, wherein the coating buffer solution is a 0.05M carbonate buffer solution of which the pH value is 9.6, and the coating amount is 0.1-1ug per hole; a blocking solution is a BSA or skimmed milk of which the mass concentration is 1-10%; the ELISA plate is coated with a rabies virus purified antigen after being blocked, wherein the coating amount is 0.1-1ug per hole; a sample diluent is a 0.01mol / L phosphate buffer solution (PBS) which contains bovine serum albumin (BSA) with a mass concentration of 0.1-10% and NaN3 with a mass concentration of 0.01-0.05 and has a pH value of 7.2-7.4; an enzyme conjugate is a horse radish peroxidase-mouse anti-human IgG enzyme conjugate; a concentrated cleaning solution is a 0.01mol / L PBS which contains tween-20 with a volume concentration of 0.05% and has a pH value of 7.2-7.4; a zymolyte A solution is a 3,3'-5,5'-tetramethyl benzidine solution, and a zymolyte B solution is an oxydol solution; and a stop solution is a 1mol / L H2SO4 solution, and a positive control and a negative control are arranged in the kit. The specificity of the kit is up to 100%, and the sensitivity is 1:640. The kit is used for evaluating the immunity effect of humans inoculated with rabies vaccines.
Owner:WUHAN CHOPPER BIOLOGY
Full-automatic enzyme-linked immunoassay system
InactiveCN1885037AImprove test efficiencyHeating or cooling apparatusMaterial analysisAntigenProduction line
The invention relates to a full-automatic enzyme couple immune analyze system, used in ELISA test of antigen and antibody of medical organizations, which can overcome the defects of low efficiency. The invention uses enzyme couple immune processing method of industrial product line, to distribute the structure based on needed program and condition of enzyme couple immune reaction, to realize the liquid adding unit, the constant-temperature cultivate unit and plate washing unit of said reaction, which can independently complete mission and cooperate too. The invention can improve the efficiency 1-10 times than present instruments.
Owner:YANTAI ADEKANG BIOTECHNOLOGY CO LTD
ELISA test kit of human TFF3
The invention relates to a new ELISA test kit of human TFF3, and the key technique is characterized in that the specific anti-TFF3 antibody is prepared by a gene engineering method, an elisa plate coated by the antibody is used as an important part of the kit, and then is assembled with TFF3 multi-antibody, secondary antibody marked by horse radish peroxidase, TFF3 standard, TFF3 positive control, sample diluent, washing liquor, coloration solution and stopping solution into the ELISA test kit of human TFF3; ELISA is carried out by a bi-anti sandwich method to establish a measurement method which can sensitively and rapidly capture TFF3 concentration in human serum or cell and tissue culture, and has the characteristics of high specificity and high stability. The kit is mainly used for clinical research use such as general laboratory or gastrointestinal tract pathology.
Owner:WUHAN SANYING BIOTECH
Tobacco mosaic virus Yunnan isolate TAS-ELISA test kit and its preparing method
InactiveCN1611945ALow costHigh detection sensitivityPreparing sample for investigationNicotiana tabacumTobacco mosaic virus
This invention discloses a kin of tobacco mosaic virus yunnan outlier three antibody sandwich enzyme immune adsorbing rule detection kit. It includes case, enzyme standard board and liquid set in the case. The liquid comprises positive and negative contrast, enzyme standard antibody, buffer solution and substrate. It also includes multiple and single antibodies of tobacco mosaic virus yunnan outlier. The single and multiple antibodies take tobacco mosaic virus extracting solution with 20-30mg / ml virus concentration as immune antigen. They are prepared by immune injection method and single clone antibody method. The detection sensitivity of the detection kit in this invention can reach 0.01-0.001ng / ml, and it is 100-1000 times than indirect ELISA and DAS-ELISA detection sensitivity.
Owner:YUNNAN ACAD OF AGRI SCI +2
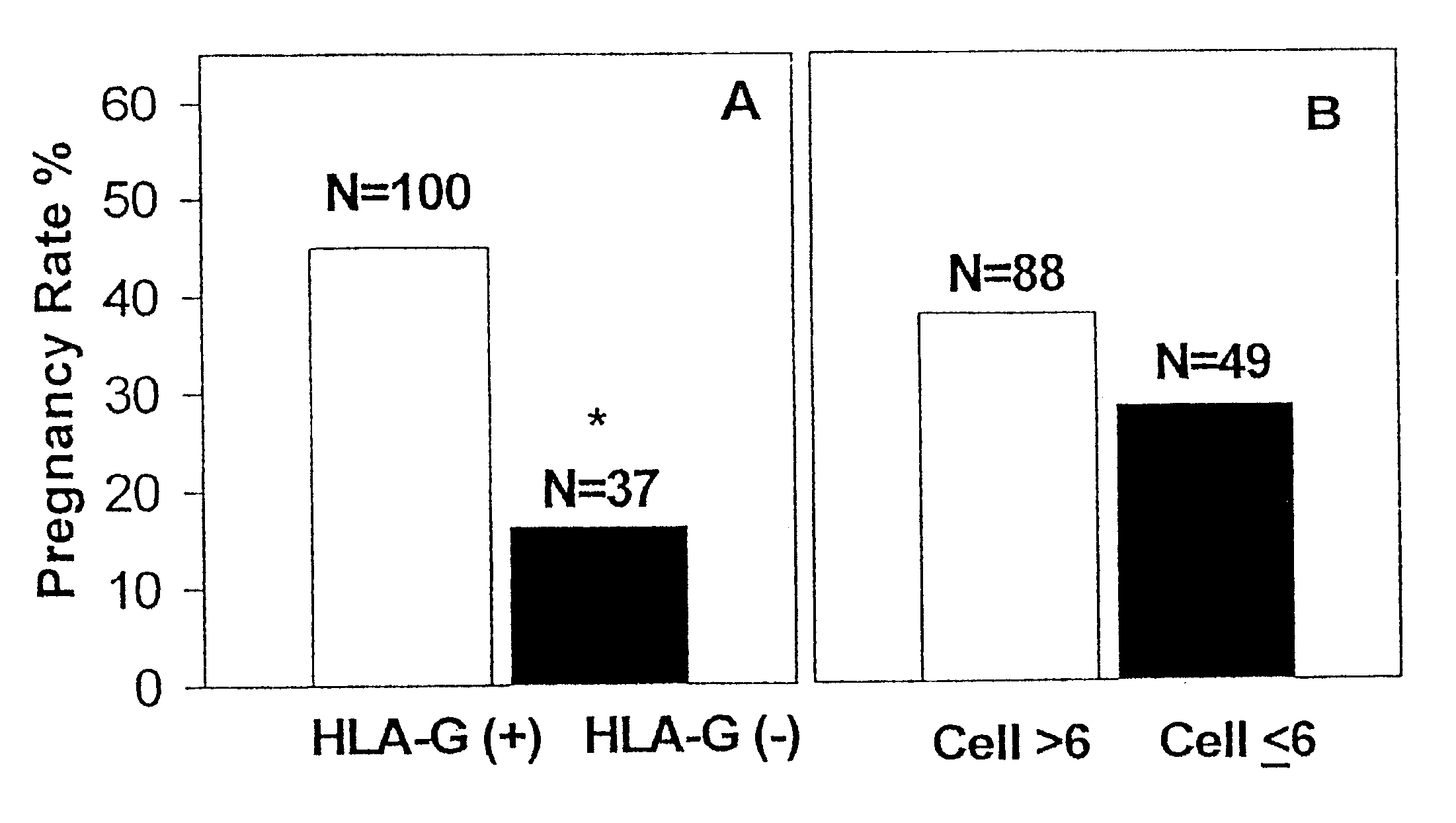
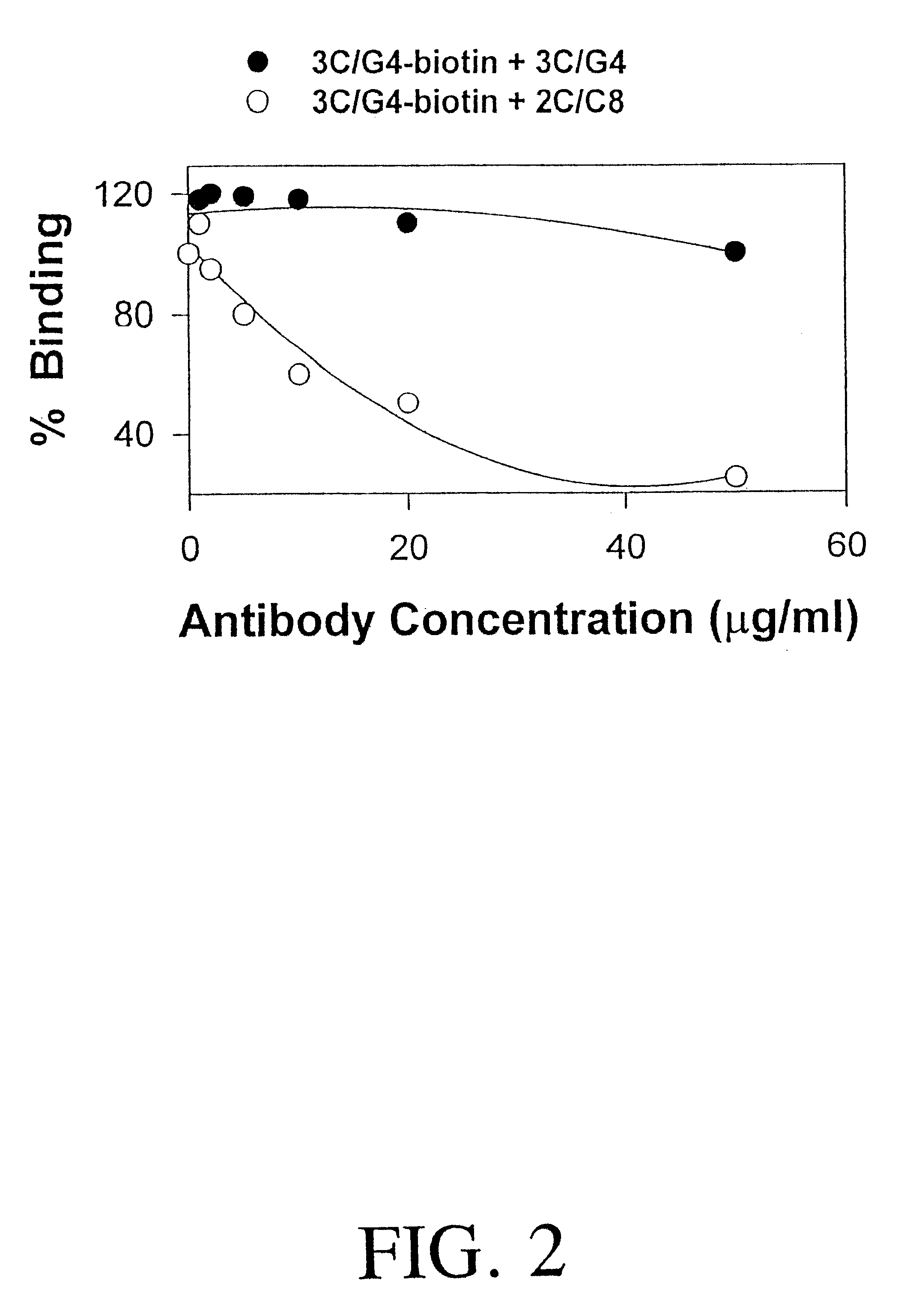
Detection of HLA-G
InactiveUS20020015973A1Raise the possibilityFast quantitative measurementAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsClinical settingsHLA-G
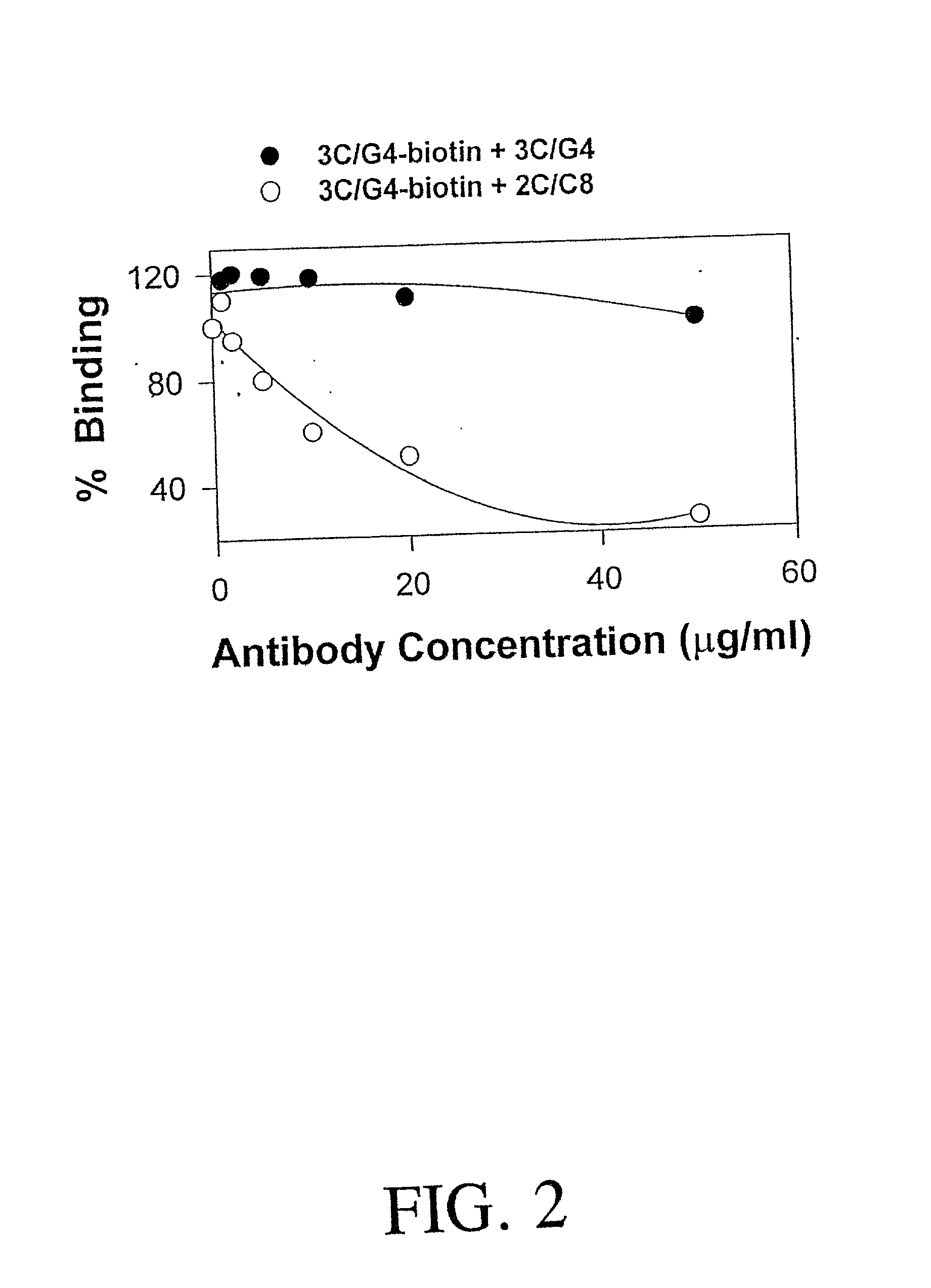
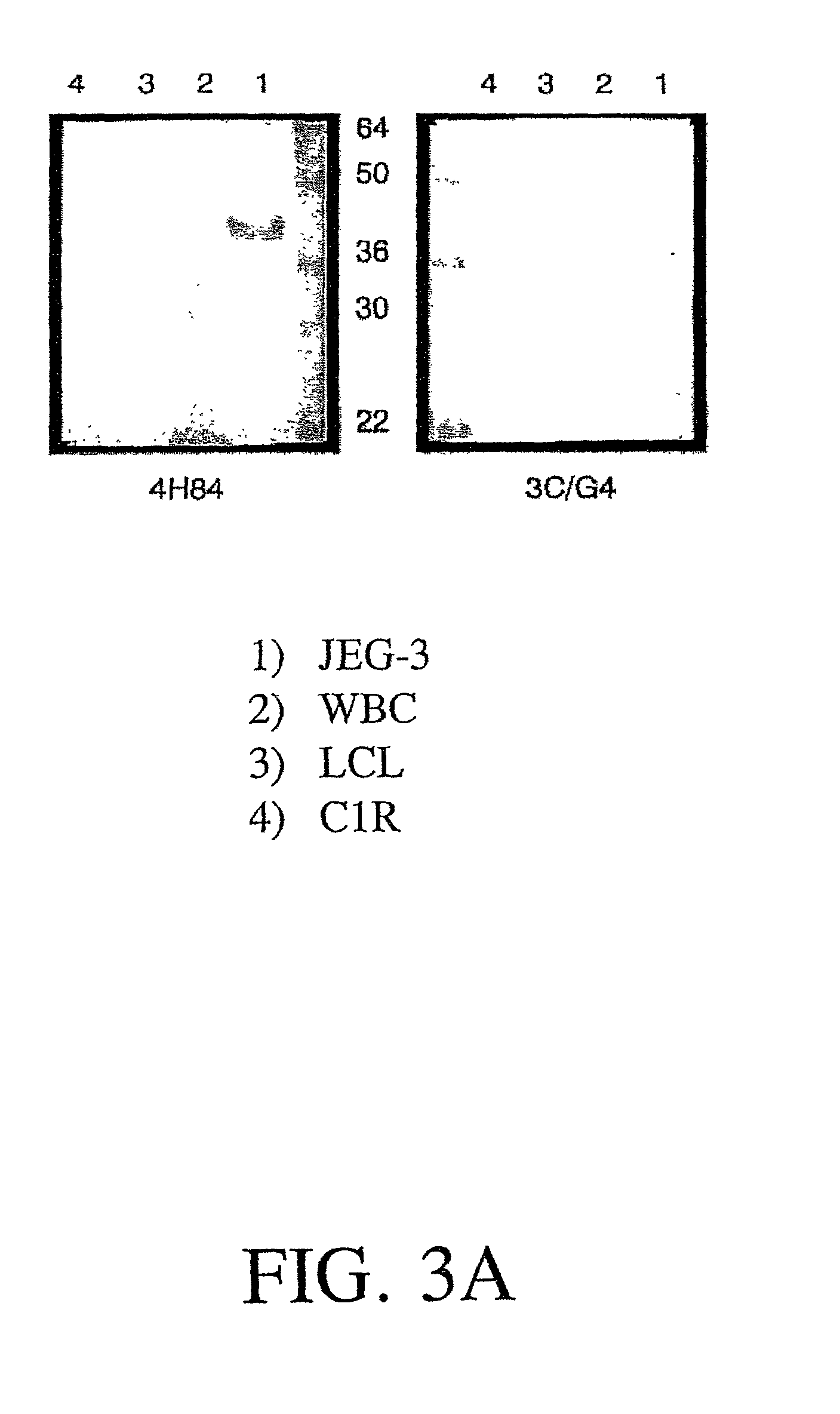
The present invention relates to the detection of HLA-G. Antibodies to both soluble and membrane bound HLA-G are disclosed. Exemplary antibodies include 2C / C8, 3C / G4, and 4H84. 2C / C8 and 4H84 antibodies bind to the same region of HLA-G, which is a different region than that bound by 3C / G4. Methods of detection and diagnosis are disclosed as well as kits, including a miniaturized assay suitable for a clinical setting. Further, a method of selecting an embryos for in vitro fertilization is disclosed. A sandwich ELISA test is provided using two antibodies that bind to HLA-G at different regions. The HLA-G test according to the invention is over 1000 times more selective in binding to HLA-G than to antigens HLA-A2, HLA-B4, HLA-C, or mixed WBC preparations.
Owner:LIBRACH CLIFFORD L +1
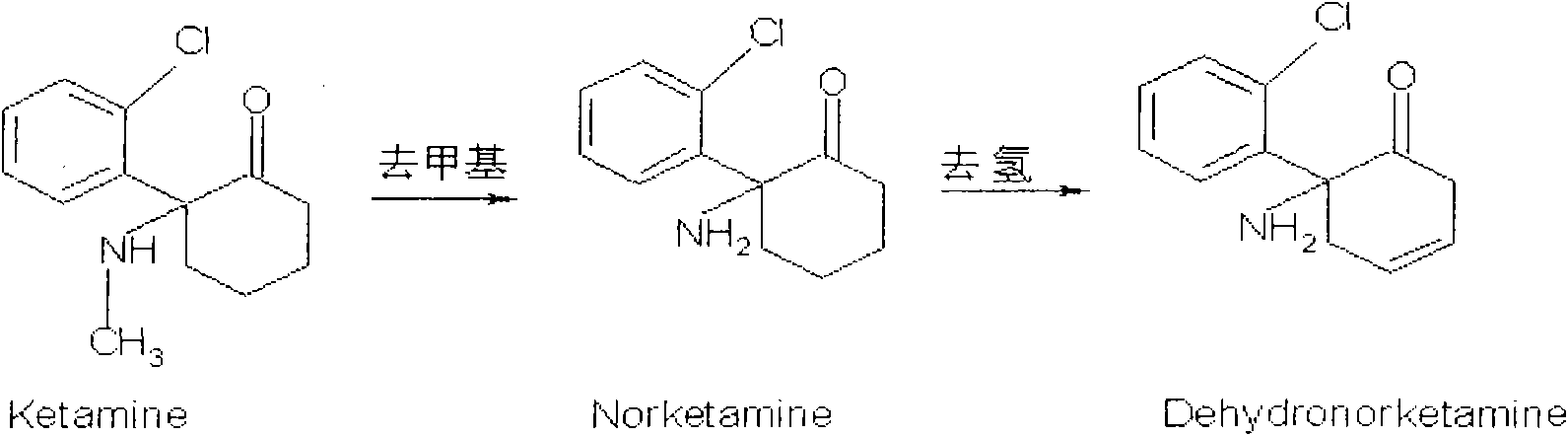
ELISA testing kit for detecting norketamine and preparation method thereof
InactiveCN101620231AQuick checkSensitive detectionBiological testingGenetic engineeringAntigenMonoclonal antibody
The invention provides an ELISA testing kit for detecting norketamine (NKET) and a preparation method thereof. The testing kit comprises cleaning solution, color development solution A, color development solution B, stop solution, a coated microplate, a norketamine (NKET) standard, an anti-NKET monoclonal antibody and enzyme labeling goat anti-mouse antibody cryodesiccate, wherein the coated microplate coats a solid phase antigen, the norketamine (NKET) standard is obtained by diluting pure norketamine, and the anti-NKET monoclonal antibody is prepared through preparing a complete antigen of norketamine firstly by utilizing mice immunity. The ELISA testing kit for detecting norketamine uses an enzyme linked immunosorption detection method to calculate NKET content of a sample from a standard curve by detecting absorbance values of norketamine. The invention has simple and convenient operation, rapid, sensitive and accurate detection as well as convenient use and low price of special testing kits, and is suitable for detecting large batch samples.
Owner:江苏省苏微微生物研究有限公司
Monoclonal antibody of anti-H9 subtype flu virus haemagglutinin protein and application thereof
InactiveCN103059132AStrong specificityHigh sensitivityImmunoglobulins against virusesMaterial analysisPurification methodsInfluenza virus A hemagglutinin
The invention discloses a monoclonal antibody of anti-H9 subtype flu virus haemagglutinin protein, which is secreted by hybridoma cell strain 4D10 with the preserving number of CCTCC No: C2012152. The invention further discloses a double antibody sandwich ELISA test kit of H9 subtype flue virus and a detection method. The monoclonal antibody of anti-H9 subtype flu virus haemagglutinin protein is used as a primary antibody, a monoclonal antibody marked by horseradish peroxidase is used as a second antibody, and a separation, augmentation, inactivation and purification method of H9 subtype flue virus and a preparation and purification method of anti-H9 subtype flu virus haemagglutinin monoclonal antibody are disclosed. According to the test kit and the detection method disclosed by the invention, the H9 subtype flue virus can be detected directly, and the monoclonal antibody has the characteristics of being high in specificity, high in sensitivity, short in detection time, wide in detection sample range and the like.
Owner:HUAZHONG AGRI UNIV
Enzyme-linked immuno sorbent assay (ELISA) enzyme immunoassay color developing agent for increasing developing duration and preparation method and application thereof
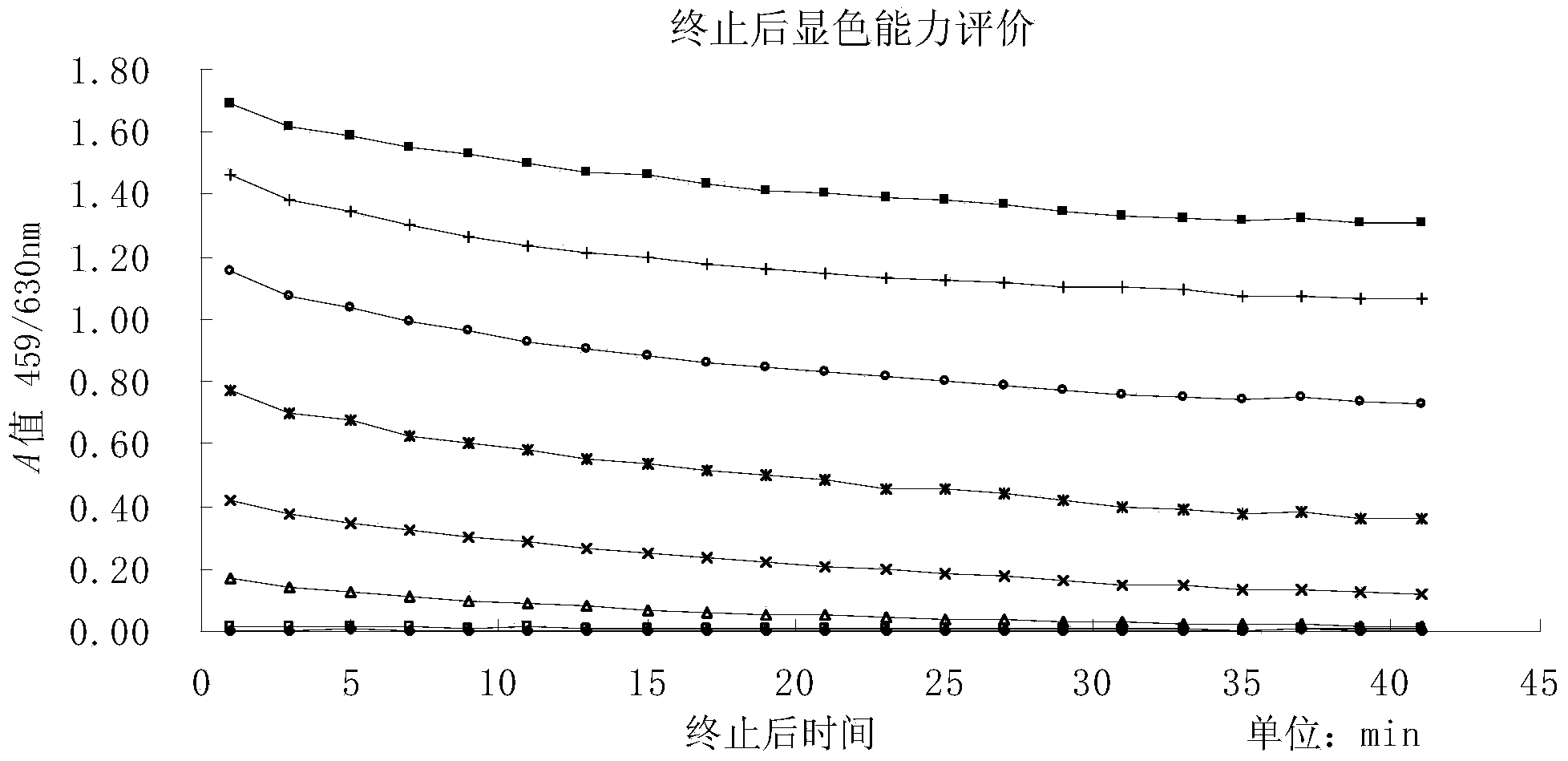
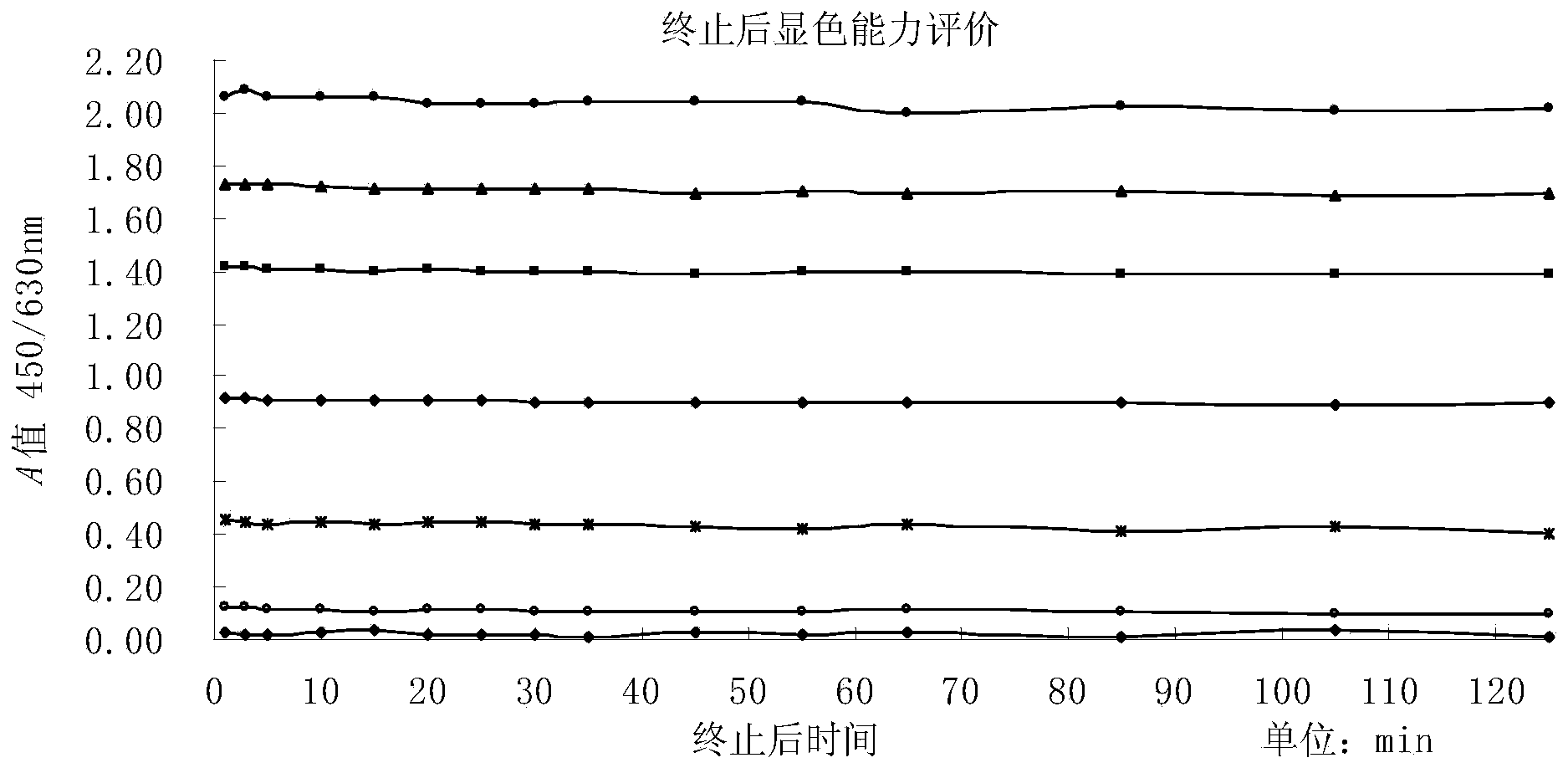
The invention discloses an enzyme-linked immuno sorbent assay (ELISA) enzyme immunoassay color developing agent for increasing developing duration and a preparation method and application thereof. Compared with the prior art, the color developing agent prepared by the method can be maintained to be close to a constant color developing state after termination within 120 minutes, so that the color developing agent maintains a quite steady state after developing is terminated. Therefore, the stability, accuracy and precision of OD value measurement are increased, and an adverse effect of the instable color developing process on the accuracy of the ELISA testing result is effectively reduced.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
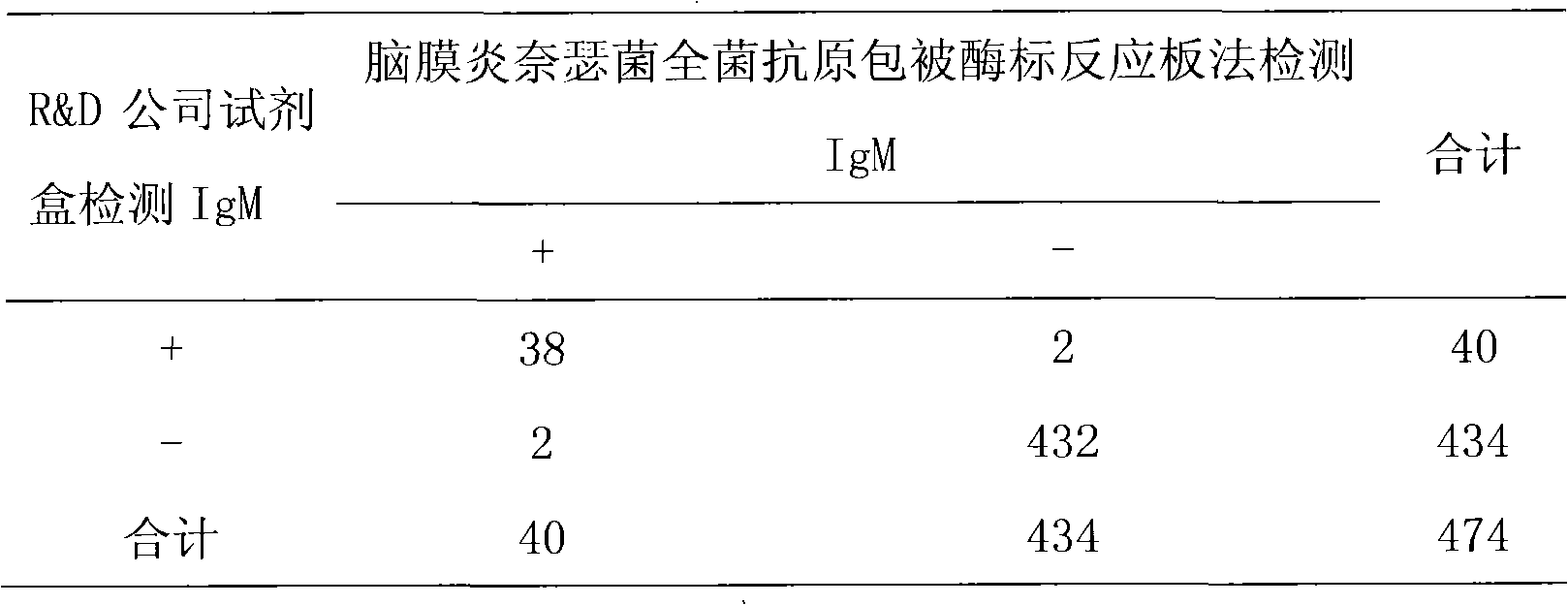
Method for producing whole neisseria meningitidis antigen coated enzyme-labeled reaction plate and enzyme-linked immuno sorbent assay (ELISA) test kit
The invention discloses a method for producing a whole neisseria meningitidis antigen coated enzyme-labeled reaction plate and an enzyme-linked immuno sorbent assay (ELISA) test kit. The whole neisseria meningitidis antigen coated enzyme-labeled reaction plate is produced by mixing an equal amount of A-type neisseria meningitides and C-type neisseria meningitides and coating the mixture on an enzyme-labeled reaction plate and is used for conveniently and rapidly detecting the specific neisseria meningitides IgM antibody in the serum with the indirect ELISA. The ELISA test kit comprises the whole neisseria meningitidis antigen coated enzyme-labeled reaction plate, sample diluent, washing liquid, color rendering solution and stop solution. The invention discloses a method for sensitively and rapidly capturing the specific neisseria meningitides IgM antibody in the serum with the ELISA. The ELISA test kit has high specificity and stability and is mainly used for the laboratory research and the clinical diagnosis.
Owner:赵俊 +1
Detection of HLA-G
InactiveUS6613538B2Easy accessImprove the level ofAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsClinical settingsHLA-G
The present invention relates to the detection of HLA-G. Antibodies to both soluble and membrane bound HLA-G are disclosed. Exemplary antibodies include 2C / C8, 3C / G4, and 4H84. 2C / C8 and 4H84 antibodies bind to the same region of HLA-G, which is a different region than that bound by 3C / G4. Methods of detection and diagnosis are disclosed as well as kits, including a miniaturized assay suitable for a clinical setting. Further, a method of selecting an embryos for in vitro fertilization is disclosed. A sandwich ELISA test is provided using two antibodies that bind to HLA-G at different regions. The HLA-G test according to the invention is over 1000 times more selective in binding to HLA-G than to antigens HLA-A2, HLA-B4, HLA-C, or mixed WBC preparations.
Owner:LIBRACH CLIFFORD L +1
Competitive ELISA detection method for DHAV-1 antibodies
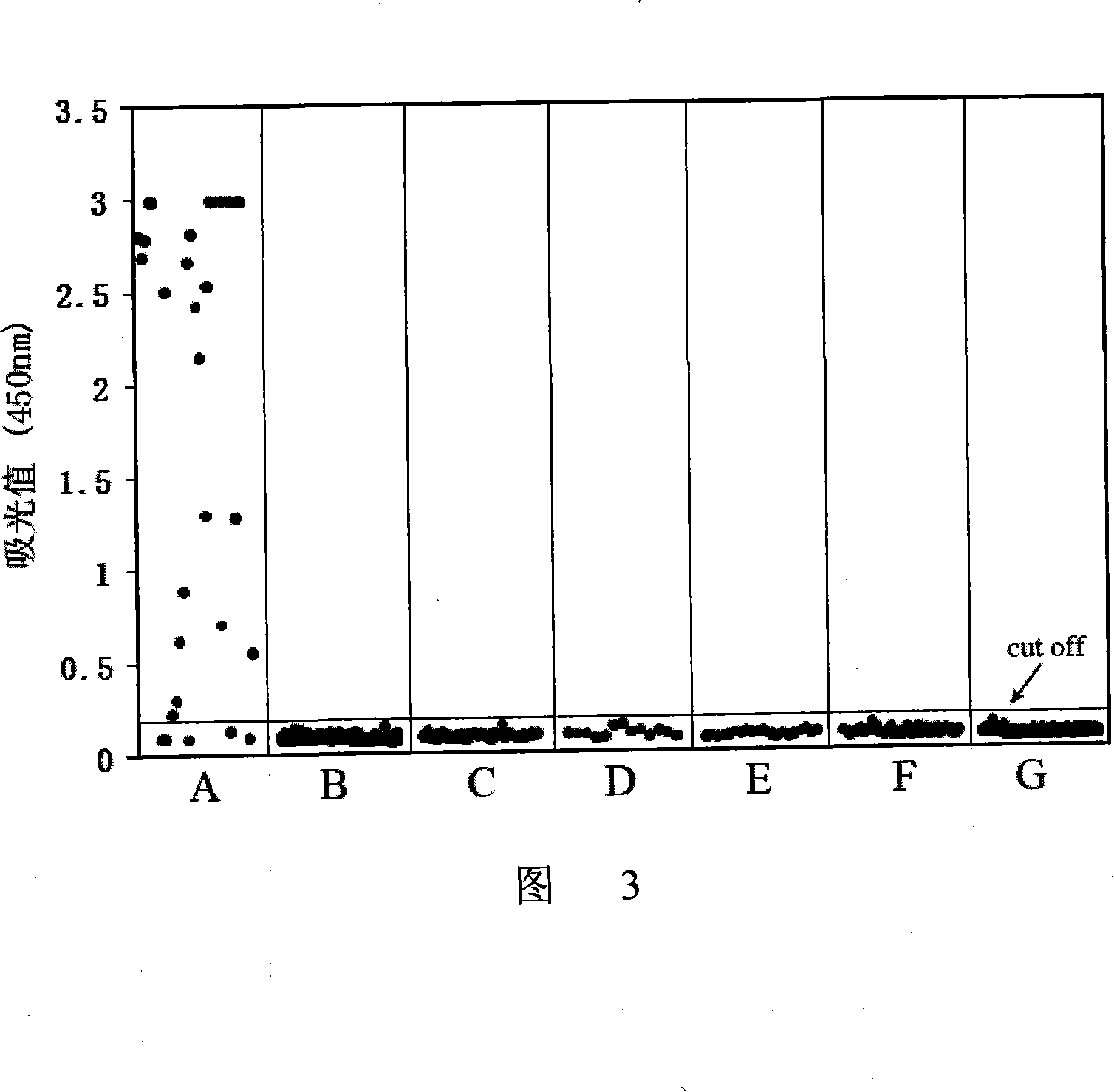
The invention provides a competitive ELISA detection method for DHAV-1 antibodies. The detection method solves the problem that at present, the valence of the DHAV-1 antibodies in the bodies of breed ducks and ducklings and the valence of commercial DHAV-1 egg yolk antibodies are difficult to evaluate. The detection method includes the steps of performing monoclonal antibody making, antigen coating, washing, closing, washing, enzyme-labeled antibody treating, washing and TMB developing; determining the OD450 nm value of each reaction hole; calculating the inhibition ratio of a serum sample, wherein the inhibition ratio (%)=(the OD value of an enzyme-labeled antibody-the OD value of the sample)*100% / the OD value of the enzyme-labeled antibody; if PI>=15.21%, judging that serum is positive; if PI<15.21%, judging that the serum is negative. According to the competitive ELISA detection method for the DHAV-1 antibodies, high specificity of monoclonal antibodies and high sensitivity of ELISA tests are achieved, stability of results is good, commercialization and automation can be achieved, and judgment can be made by naked-eye observation.
Owner:SHANDONG VOCATIONAL ANIMAL SCI & VETERINARY COLLEGE
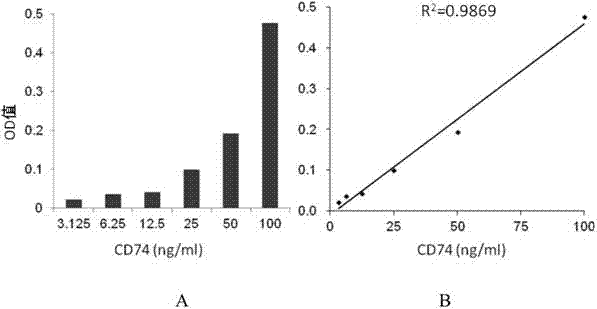
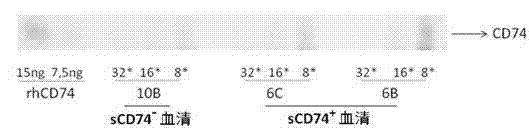
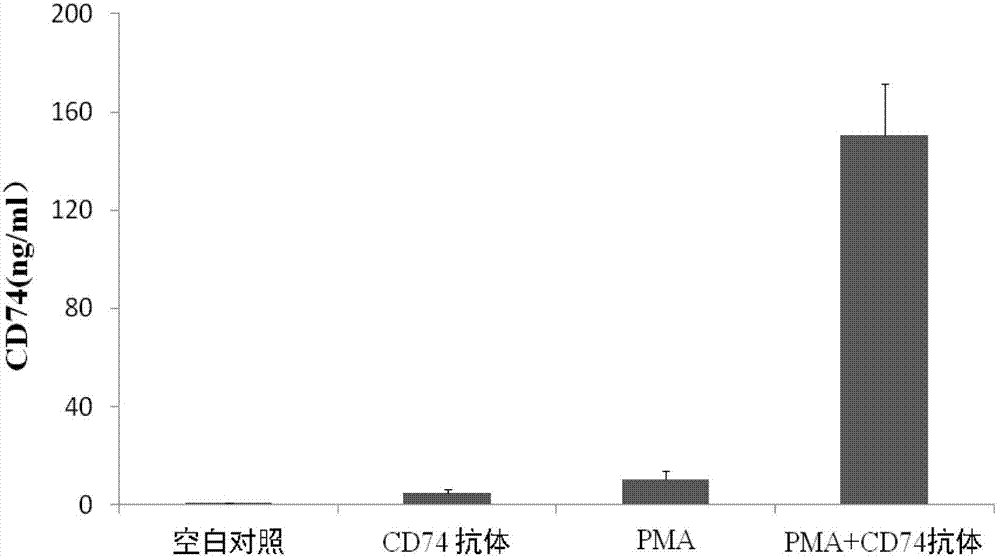
ELISA test kit of human-derived soluble CD74 protein and detection method thereof
ActiveCN103207277AReduce subjectivityHigh sensitivityBiological testingWestern blotSemiquantitative Method
The present invention belongs to the field of immunology and biotechnology, and provides an ELISA test kit of human-derived soluble CD74 protein and a detection method thereof. The kit of the present invention can accurately detect the content of human-derived soluble CD74 protein in body fluid or cell supernatant, and results are quantitatively analyzed by an enzyme-labeling instrument to exclude subjectivity of semi-quantitative methods such as immunohistochemistry, immunofluorescence, Western blot and the like; the kit has high sensitivity, the detected content of CD74 protein is as low as 800 pg / ml; according to the invention, no complicated instrument is necessary in detection; the kit is suitable for popularization and application in scientific research institutions and medical institutions, is suitable for large-scale detection of clinical samples, and can rapidly obtain massive data and information related to human-derived CD74 protein.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Saliva test for early diagnosis of cancers
InactiveUS20050106642A1Reduce concentrationReduce amountDisease diagnosisFermentationCancer antigenOvary cell
Proteonic cancer markers (PCMs) for breast, colon, liver and ovary were isolated, from the respective lysate of transformed cells, by differential centrifugation. Polyclonal antibodies were generated in mice against the (PCMs) for breast, colon, liver and ovary individually and combination thereof. Saliva from normal people was assayed by ELISA for antimixture of PCMs; breast, colon, liver and ovary cells individually. It was revealed that cancer antigen was detectable in saliva from normal people and the ELISA titer / 100 μl ranged from 1:200 to 1:1600. Out of 32 normal salivas tested, ELISA titer was higher than 1:1000 in seven specimens. Those specimens were assayed by ELISA tests for individual PCM using anti-breast, anti-colon, anti-liver and anti-ovary. Each saliva specimen showed highest titer for one type of cancer antigen. Four saliva specimens showed high titers for breast PCM, two for colon one for liver. Only one saliva specimen showed high titer for ovary and colon PCMs. Thus, the invention further relates to the quantitative assessment of specific PCMs for breast, colon, liver and ovary in human saliva, by using antibodies against these markers individually.
Owner:LIPPS BINIE V +1
ELISA test method on basis of nanometer enzyme with peroxidase activity
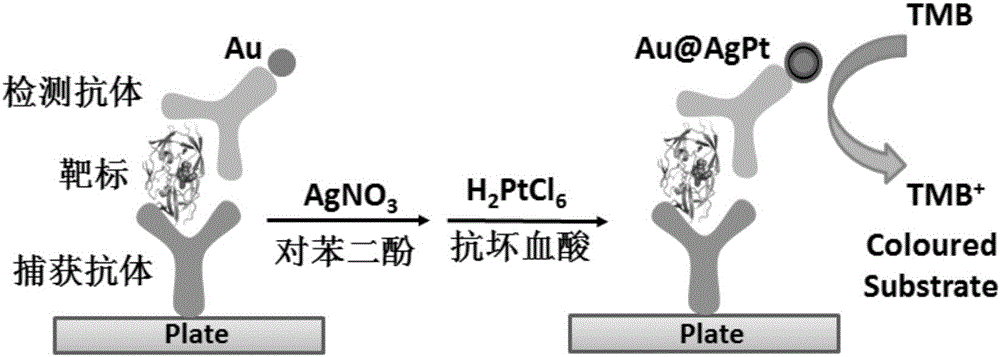
ActiveCN106018819AAvoid non-specific adsorptionAvoid the reduction of catalytic activityOrganic-compounds/hydrides/coordination-complexes catalystsBiological testingAntigenPlatinum
The invention discloses an ELISA test method on the basis of nanometer enzyme with peroxidase activity. The ELISA test method is fit for a double antibody sandwich method or a double antigen sandwich method. The ELISA test method comprises the following steps: modifying gold nanoparticle with detection antibody / detection antigen; performing ELISA detection to form the compound of capture antibody / capture antigen, to-be-detected antigen / to-be-detected antibody, detection antibody / detection antigen and gold nanoparticle; performing silver platinum dyeing and wrapping a silver shell layer and a platinum shell layer on the surface of the gold nanoparticle, thereby acquiring the nanometer enzyme Au@AgPt particle with peroxidase activity; utilizing the nanometer enzyme Au@AgPt particle to catalyze a peroxidase enzyme substrate to acquire the colored product so as to detect the quantity of the to-be-detected antigen / to-be-detected antibody. The method of forming enzyme after modifying is adopted, so that the method has the advantages of simplicity, quickness, convenience and high stability; the reduction of the catalytic activity of nanometer enzyme particle caused by nonspecific adsorption and modification can be effectively avoided; and therefore, a new platform is supplied for environment monitoring and disease diagnosis.
Owner:XIAMEN UNIV
Method for expressing human insulin for reducing blood sugar by transgenic lucid ganoderma
InactiveCN1879891ALower blood sugarLower blood sugar levelsMetabolism disorderGenetic material ingredientsElisa testBlood sugar
The invention relates to an artificial insulinogen analogue gene, while it adds the internal stay sequence of KDEL to accumulate the protein in the internal network to avoid degrading enzyme of cell. And it uses GPD of high level epiphyte to start the target gene and arranged in the carrier transformed with lucid ganoderma; uses electric hitting method or agricillin or PEG medium gene method to transform lucid ganoderma; the ELISA test has proved that the content of artificial insulinogen analogue is 105.0 mug / g-174.8 mug / g of the fresh weight, and at 4.40%-10.40% of soluble protein. And the invention can effectively reduce blood sugar.
Owner:林忠平
ELISA (Enzyme Linked Immunosorbent Assay) test kit for testing difenoconazole residue and test method
InactiveCN106674351ASensitive detectionEasy to detectSerum immunoglobulinsBiological material analysisNew Zealand white rabbitEthyl group
The invention discloses an ELISA (Enzyme Linked Immunosorbent Assay) test kit for testing difenoconazole residue and a test method. Chloroethane is utilized to substitute hydrogen atoms of three sites on a benzene ring in a difenoconazole molecular structure with ethyl groups, which are oxidized into carboxyl groups, afterwards, the mixed anhydride method is utilized to connect a hapten onto a carrier, so that an artificial antibody is formed, the carrier is bovine serum albumin, an artificial difenoconazole antigen is utilized to immunize New Zealand white rabbits to prepare an antibody, and along with related agents, such as standard difenoconazole, washing concentrate, substrate color-developing solution and stop solution, the antibodies compose the ELISA test kit. The main steps of a test include: sample preprocessing, kit testing, and test result analysis. Compared with a variety of current conventional test methods applied widely, the enzyme linked immunosorbent assay method disclosed by the invention has advantages, such as low cost and high testing sensitivity, and an effective method is provided for difenoconazole residue testing.
Owner:SHENYANG JINCHENG TECH CO LTD
Immunogen of cycloproply thaastral and preparation process thereof
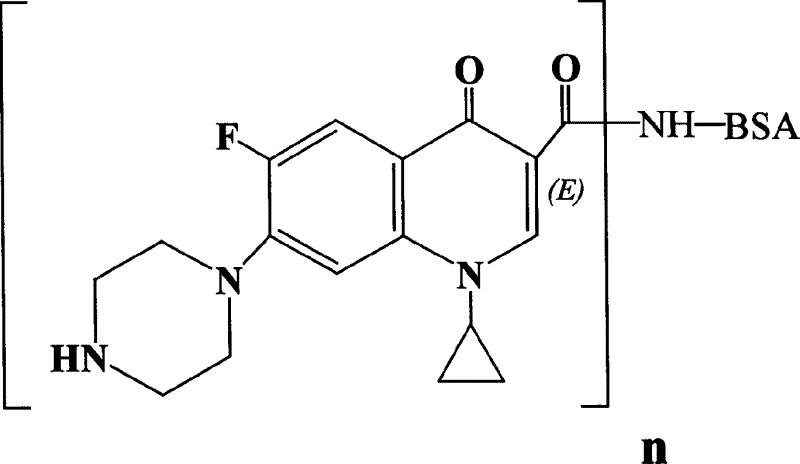
The invention is a Ciprofloxacin immunogen, coupling Ciprofloxacin with bovine serum albumin at room temperature, using PBS buffer liquor to dialyze, freeze-drying supernatant by high-speed centrifugation and obtaining Ciprofloxacin crude product, then using glucose gel chromatography for purifying, freeze-drying to obtain Ciprofloxacin pure product. The research shows it succeeds in synthesizing the immunogen able to be used in preparing Ciprofloxacin specific antibody. It lays the foundation of development of ELISA test method of Ciprofloxacin medicine residues.
Owner:SHANDONG UNIV
ELISA kit for identification of pig hoof-and-mouth disease immunity animal and taint animal
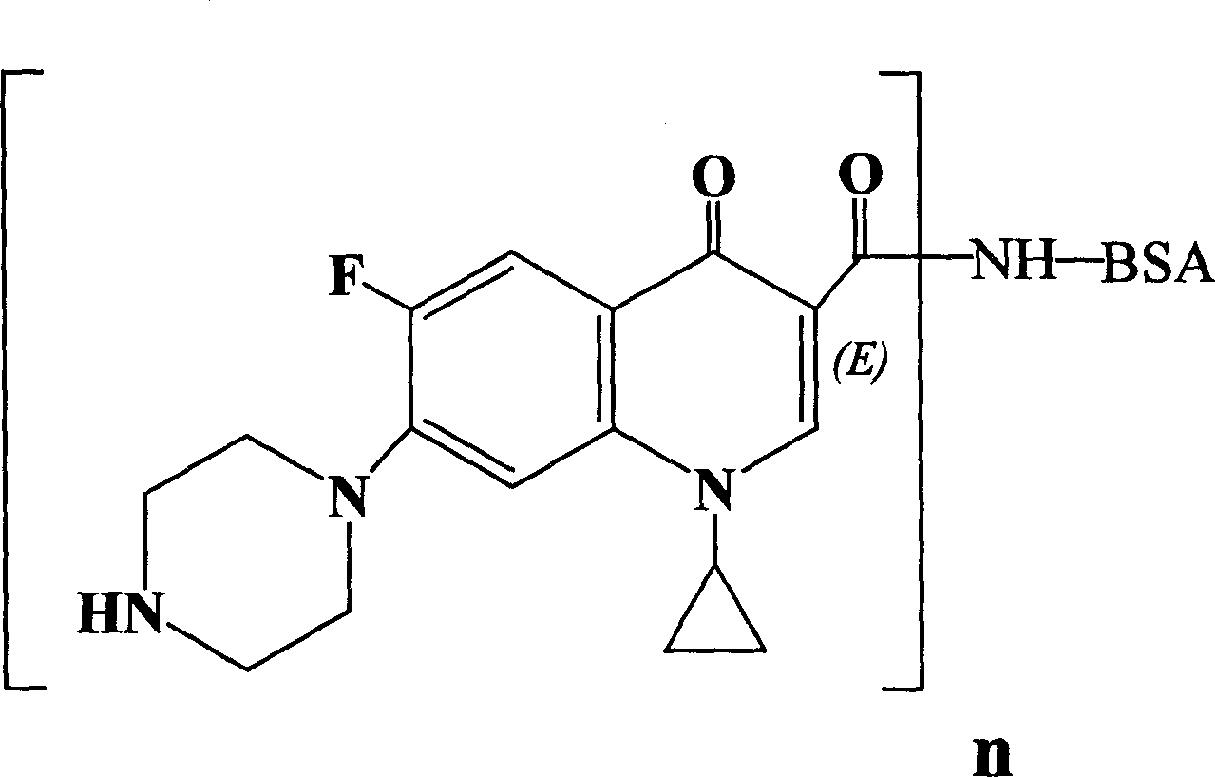
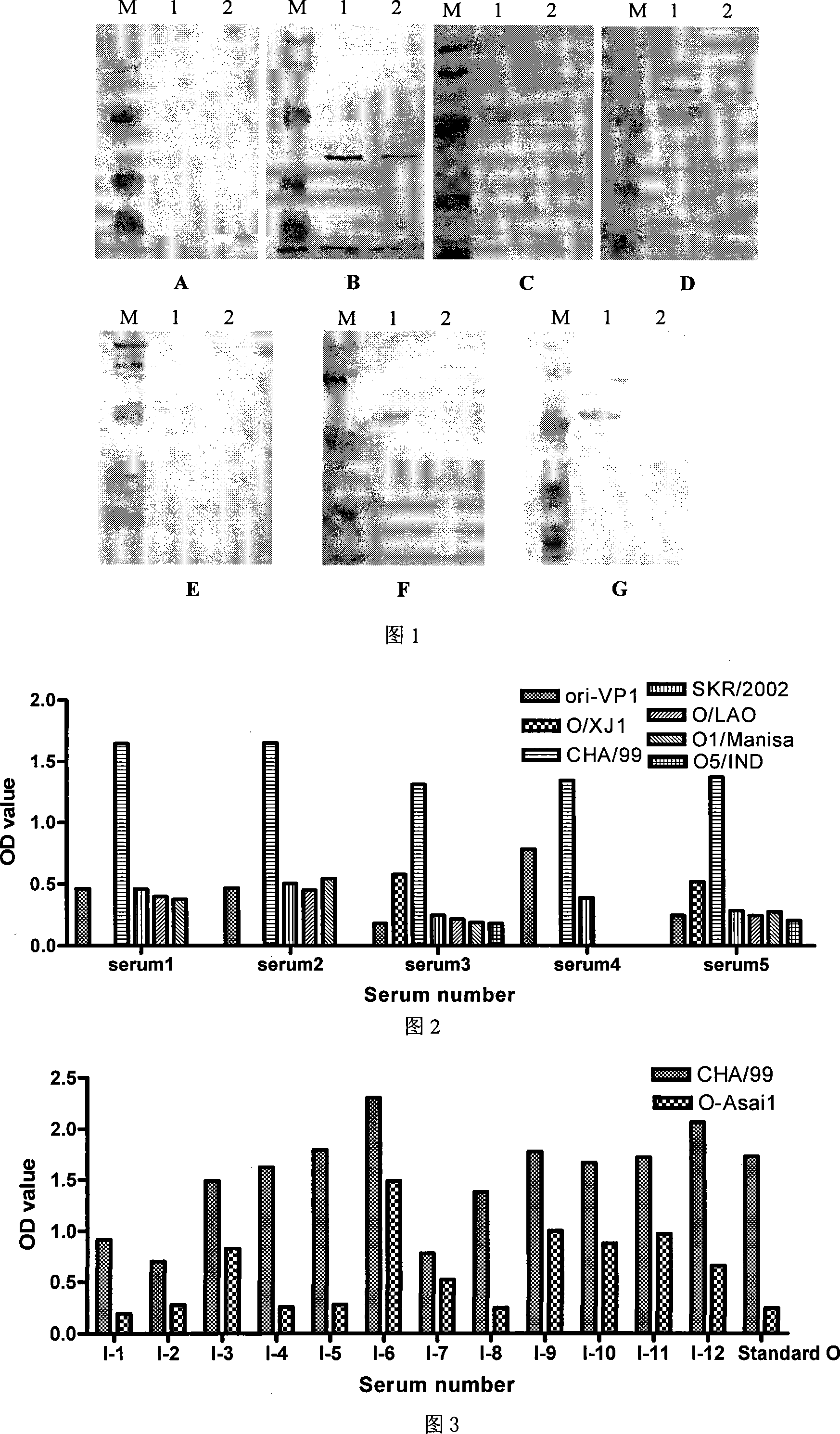
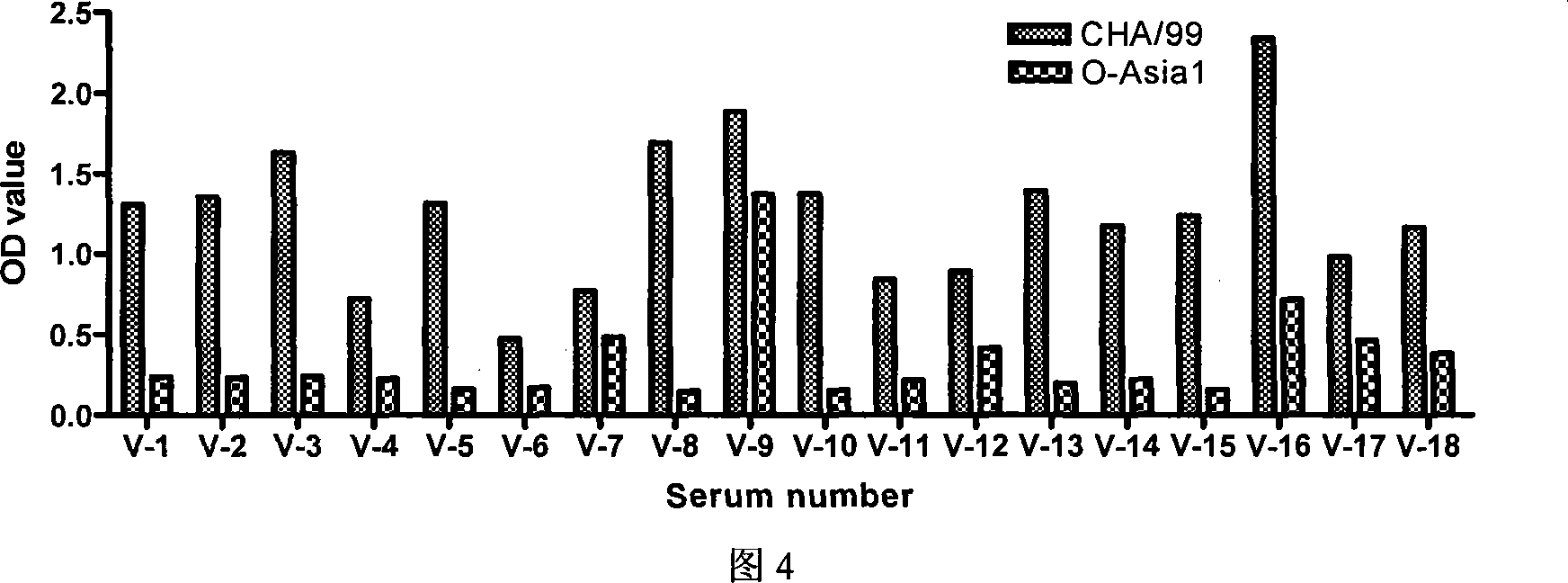
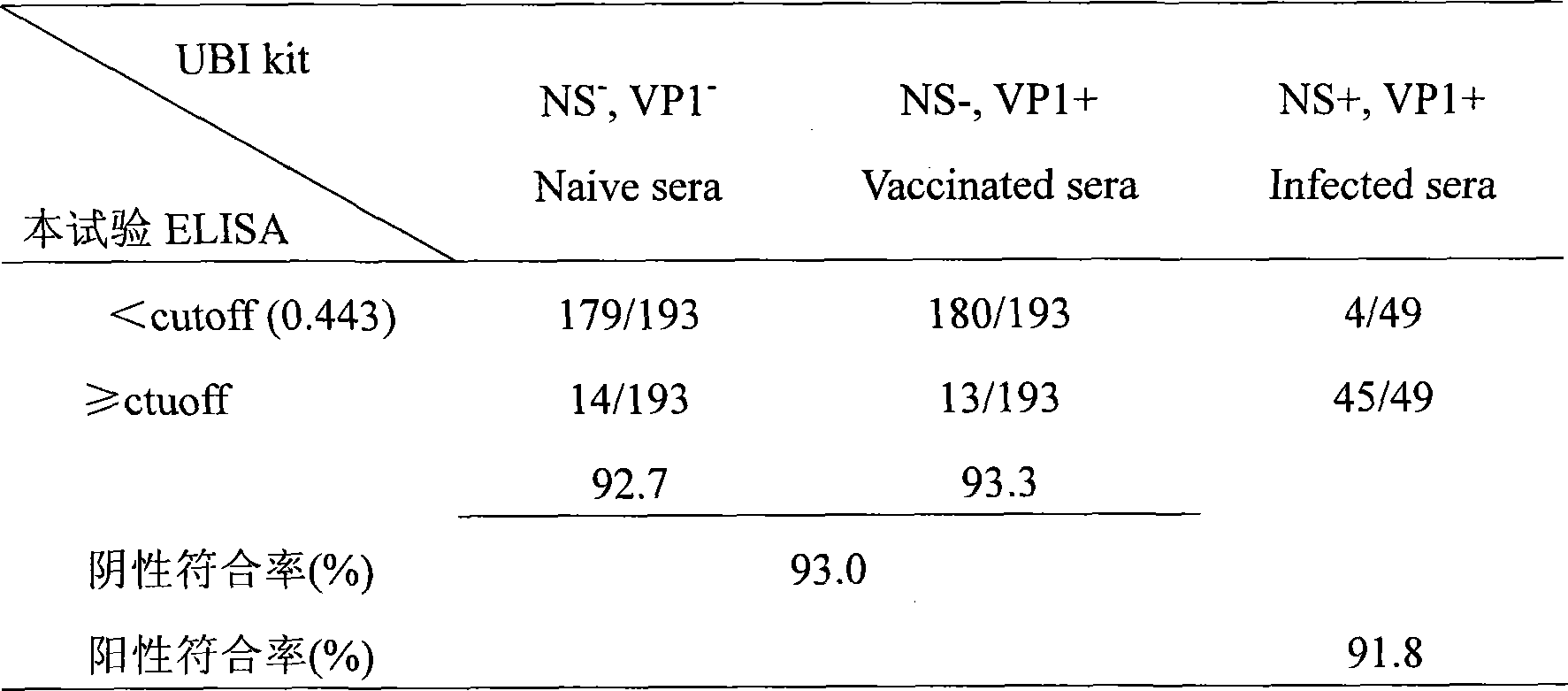
The invention relates to an ELISA test kit for identifying pig foot and mouth disease immunity animal and infected animal, wherein one orifice plate comprises the protein NS-AGD pure product of all linear antigen epitopes of FMDV non-structural protein 3ABC and another one orifice plate comprises the mutant protein CHA / 99 pure product of structural protein VP1 with wide antigen spectrum. The result is judged by cut-off that when in the infected animal non-structural protein test, the object sample OD492>=0.443, judges it positive, or else, judges it negative, when in the immunity animal structural protein test, when the object sample OD492>=0.450, judges it positive, or else, judges it negative. The test kit can quickly and synchronously identify pig foot and mouth disease vaccine antibody and wild virus infection, and can evaluate immunity effect according to the result.
Owner:ZHEJIANG UNIV
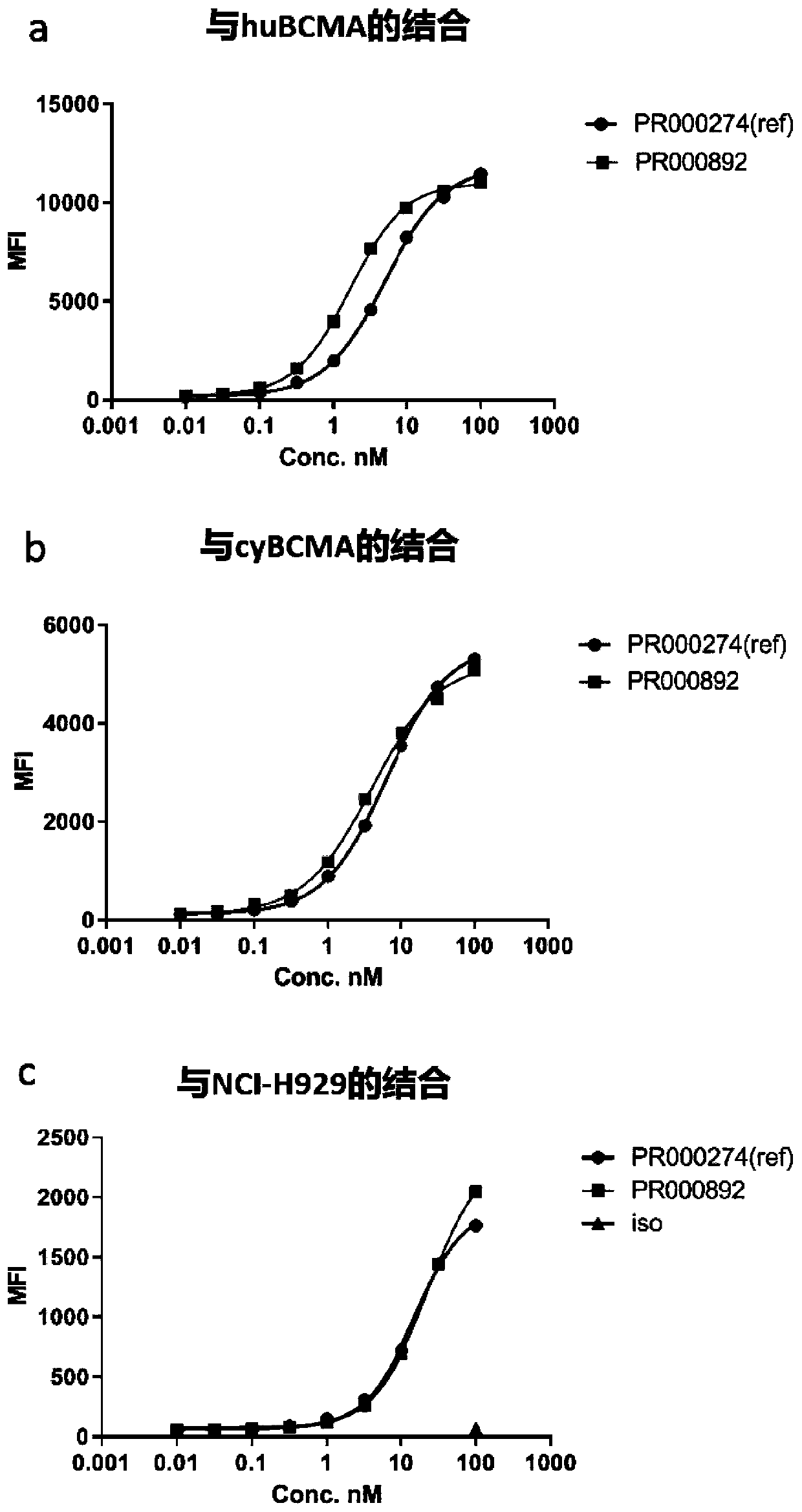
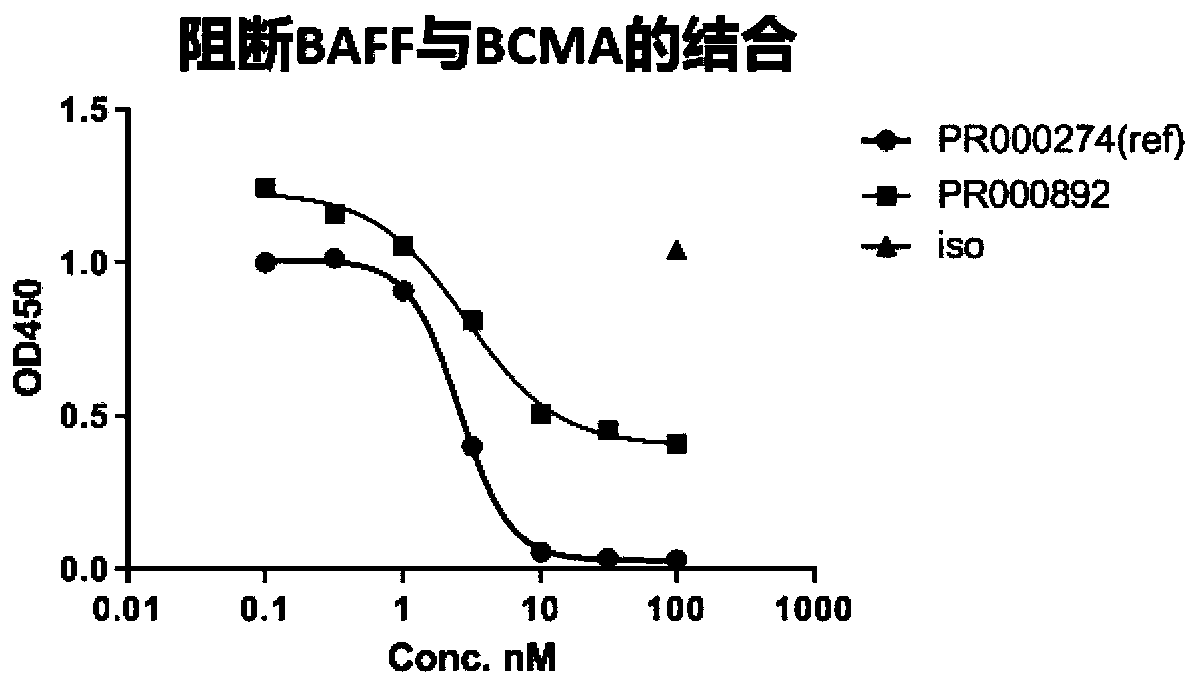
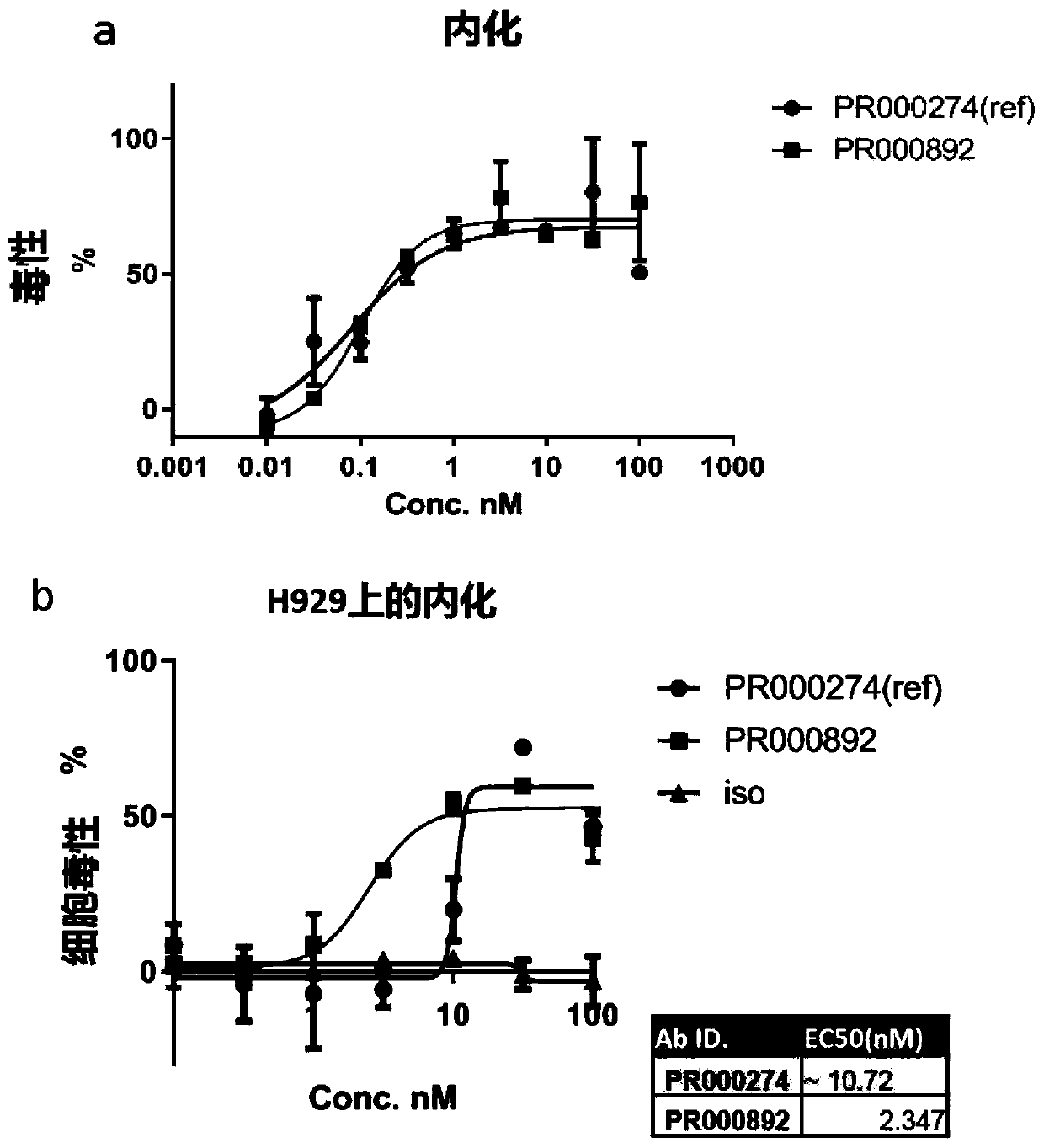
BCMA binding protein and preparation method and application thereof
ActiveCN111234020AHas an internalizing effectGood specific affinityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsNin one binding proteinHeavy chain
The invention discloses BCMA binding protein and a preparation method and application thereof. The BCMA binding protein includes a heavy chain variable region and a light chain variable region, wherein the light chain variable region includes LCDR1, LCDR2 and LCDR3, the heavy chain variable region includes HCDR1, HCDR2 and HCDR3, and the amino acid sequences of the LCDR1, LCDR2 and LCDR3 and aminoacid sequences of the HCDR1, HCDR2 and HCDR3 are shown in detail in the specification. The BCMA binding protein has good specific affinity, and has better binding on a tumor cell line and better internalization effects when compared with a control antibody, and therefore the BCMA binding protein can be further developed as an ADC candidate; and after ELISA tests are performed, the BCMA binding protein antibody has a function of partial blocking, and additional curative effects can be achieved when the BCMA binding protein is adopted as mAbs or CAR-T during treatment.
Owner:NONA BIOSCIENCES CO LTD
Anti-African swine fever virus single-chain antibody as well as preparation method and application thereof
ActiveCN111560069AControl the spread of the diseaseReactivity hasMicroorganism based processesImmunoglobulins against virusesSingle-Chain AntibodiesAfrican swine fever
The invention discloses an anti-African swine fever virus single-chain antibody as well as a preparation method and application thereof. According to the invention, lymphocytes are separated from peripheral blood of naturally infected African swine fever immune tolerant pigs; extracting is carried out to obtain the total mRNA of the separated lymphocytes; the total cDNA fragments are obtained through an RT-PCR method; cDNA is taken as a template, under the action of a corresponding primer with a Linker joint, swine ScFv antibody gene sequence is obtained through an SOE-PCR method; constructingof an ScFv antibody gene sequence to a pET-30a vector is carried out, and BL21 competent cell transformation is carried out; the ScFv antibody (VH-VL kappa 6) aiming at the African swine fever virusis screened from a single colony after transformation, and preliminary activity identification is carried out on the screened ScFv antibody (VH-VL kappa 6) by adopting an ELISA test, it shows that theantibody has African swine fever reaction activity. The invention provides a new material for early diagnosis, prevention and control of African swine fever, and provides a new technical means for controlling epidemic situation propagation of African swine fever as soon as possible.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Enzyme-linked immunosorbent assay (ELISA) test kit for testing lawsonia intracellularis (LI) of pigs
InactiveCN107576791AAchieving high-level expressionIncrease profitMaterial analysisTrue positive rateLawsonia intracellularis
The invention discloses an enzyme-linked immunosorbent assay (ELISA) test kit for testing lawsonia intracellularis (LI) of pigs. The ELISA test kit for testing the LI of the pigs comprises a solid phase carrier and an antigen enveloped in the solid phase carrier, wherein the antigen is LsaA recombinant protein, and the amino acid sequence of the LsaA recombinant protein is as shown in SEQ ID No. 1. The kit has the characteristics of being higher in sensitivity, high in specificity, good in stability, and the like, and has the advantages of convenient use, simple operation, short detection timeand low use cost; the kit can be used for carrying out serological investigation, antibody detection, vaccine immune effect evaluation and the like on the LI infection condition of the pigs, and is suitable for large-scale popularization and use.
Owner:HENAN AGRICULTURAL UNIVERSITY
Method for biopanning affinity ligand of bromelin
InactiveCN102121043AHigh affinityRich experimental dataMicrobiological testing/measurementMaterial analysisEscherichia coliSorbent
The invention relates to a method for biopanning the affinity ligand of bromelin. The method comprises the following steps: (1) biopanning; (2) performing directed amplification and enrichment on bacteriophage; (3) testing the titer; (4) performing monoclonal amplification on bacteriophage; (5) performing an enzyme-linked immuno sorbent assay (ELISA); and (6) performing DNA sequencing, wherein the sequence is Ile-XXX-Ser-Pro-XXX-XXX-XXX (LXSPXXX). The preparation method is simple and has low cost, the biological resources of Escherichia coli and bacteriophage are rich; the selected heptapeptide ligand which has affinity to bromelin has higher affinity to bromelin, and can be used to discover the concensus sequence and provide rich experimental data for the interaction of protein in the proteomics; and the heptapeptide ligand can also be used in the separation and purification researches of pineapple protein.
Owner:DONGHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com