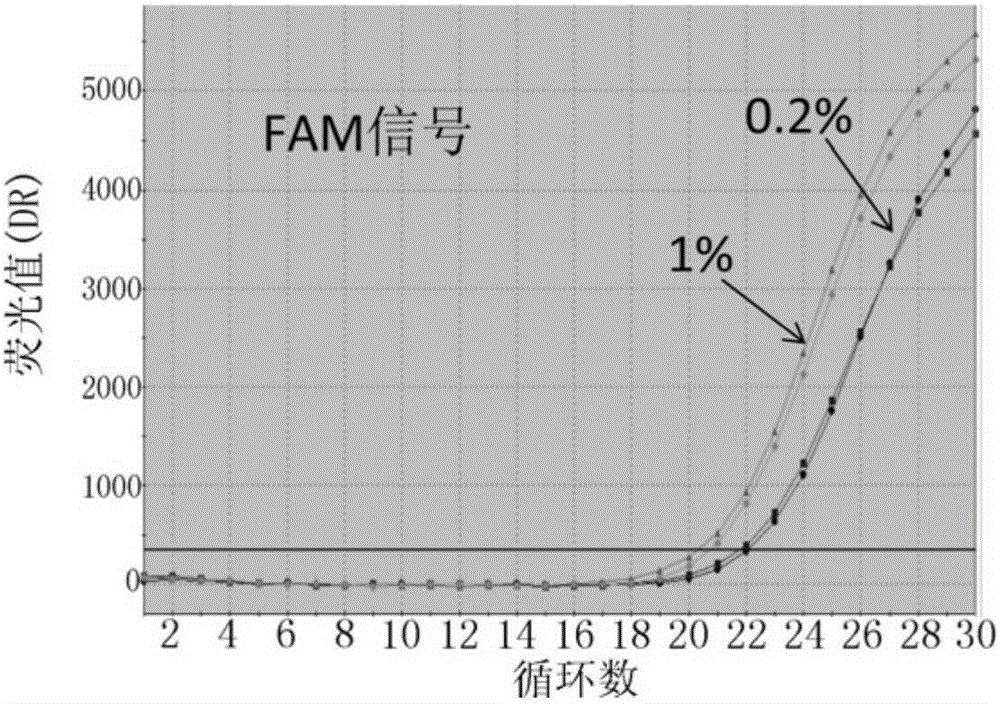
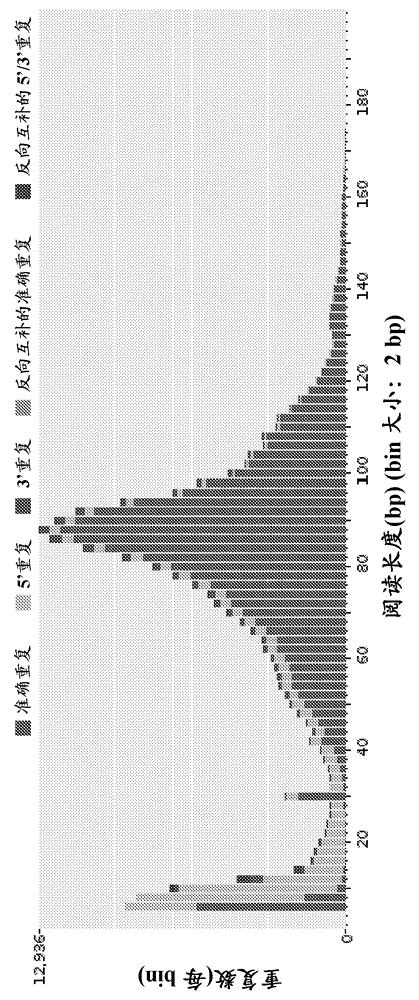
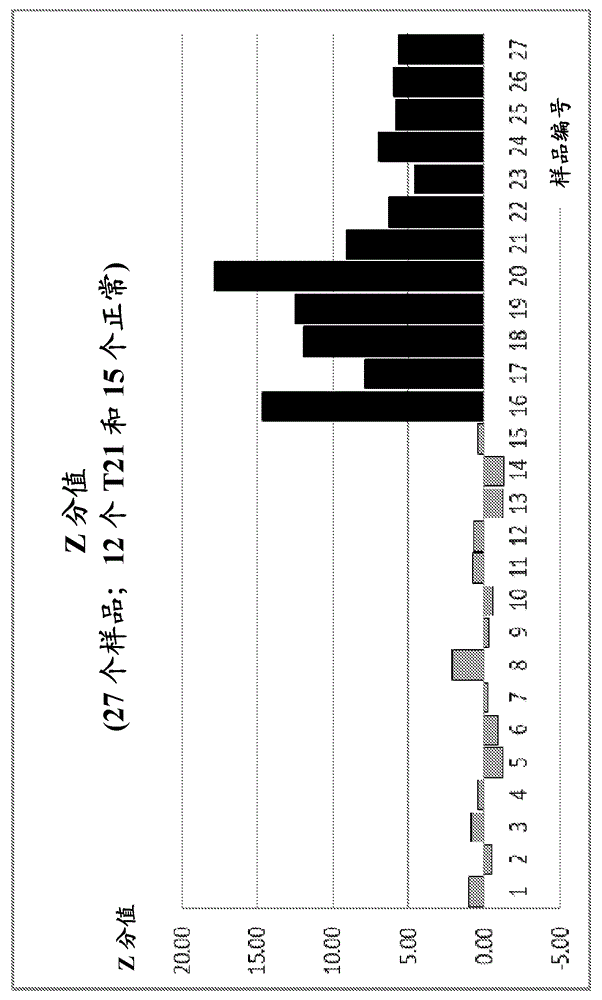
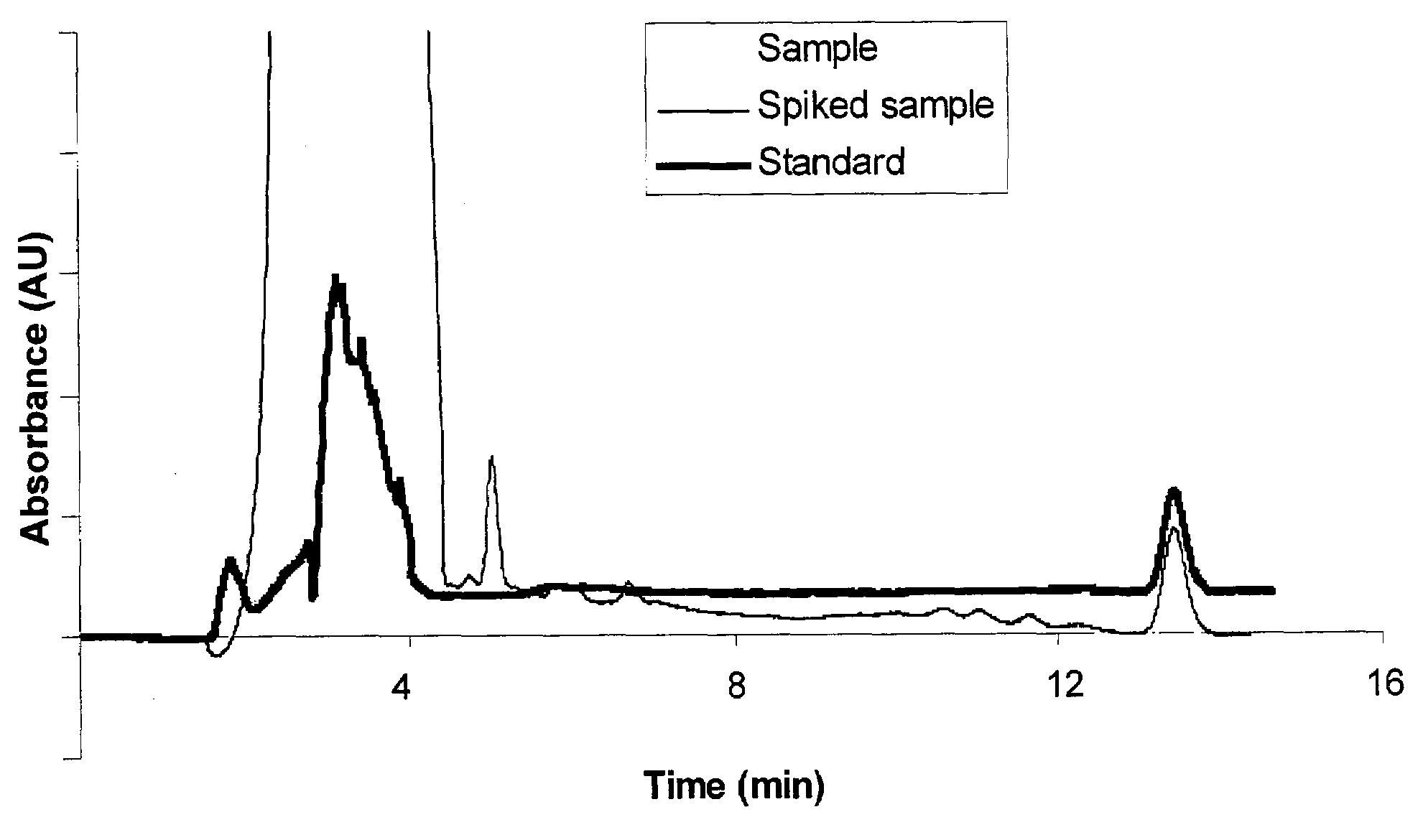
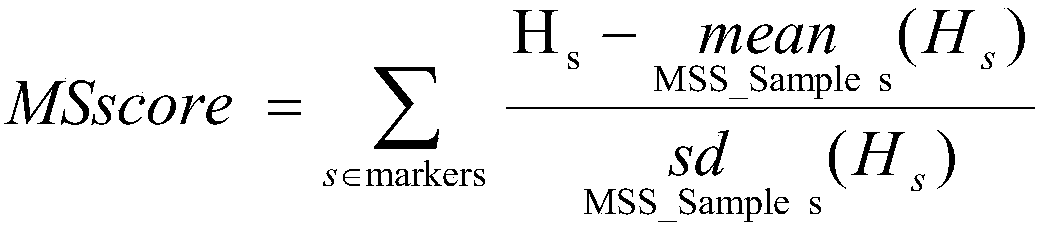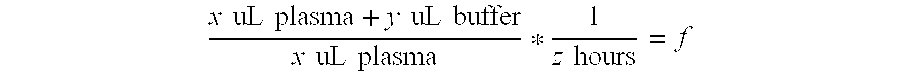Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
408 results about "Plasma sample" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Non-invasive prenatal diagnosis
InactiveUS6258540B1% accurate detection rateIncrease the amount of foetal nucleic acid materialMicrobiological testing/measurementRecombinant DNA-technologyPrenatal diagnosisBlood typing
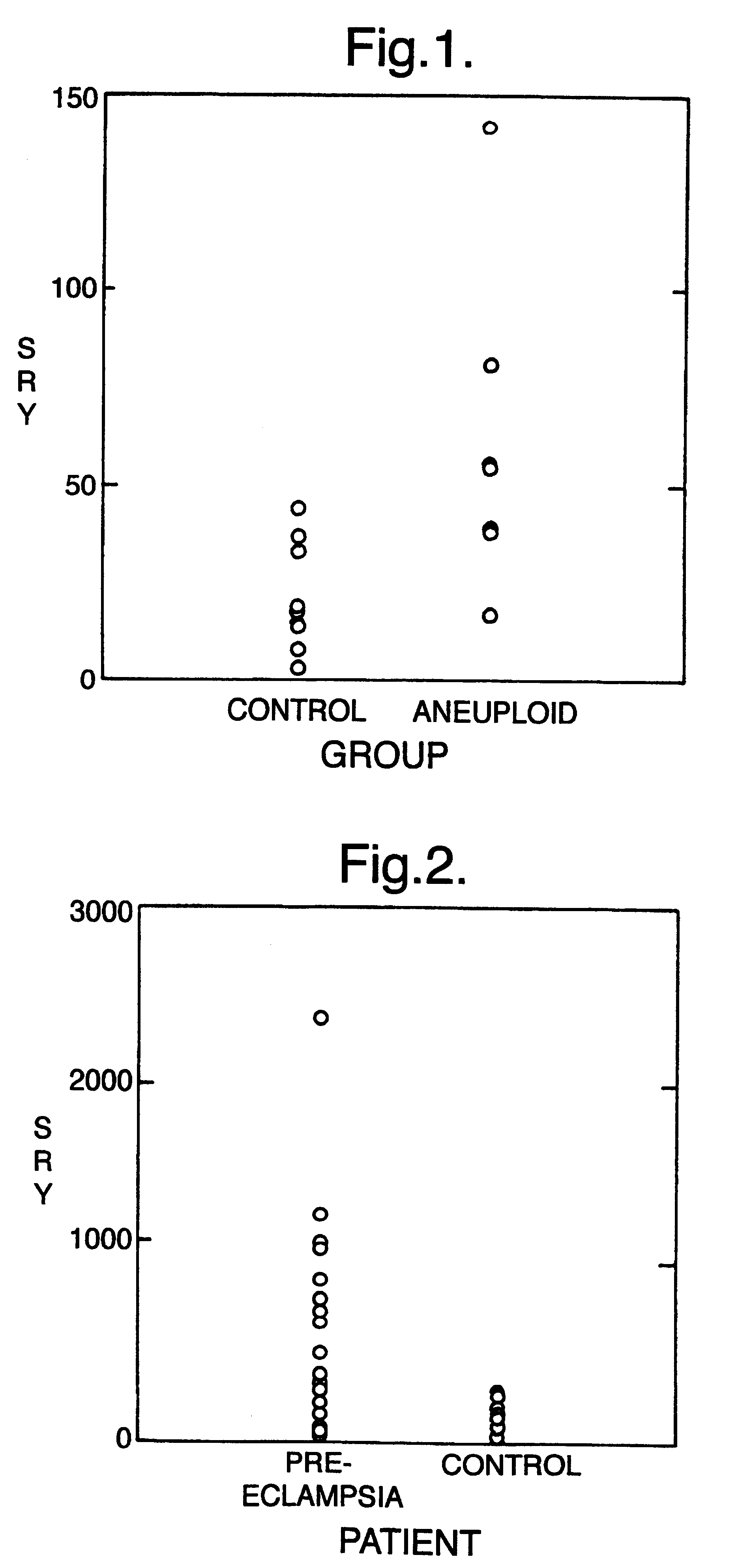
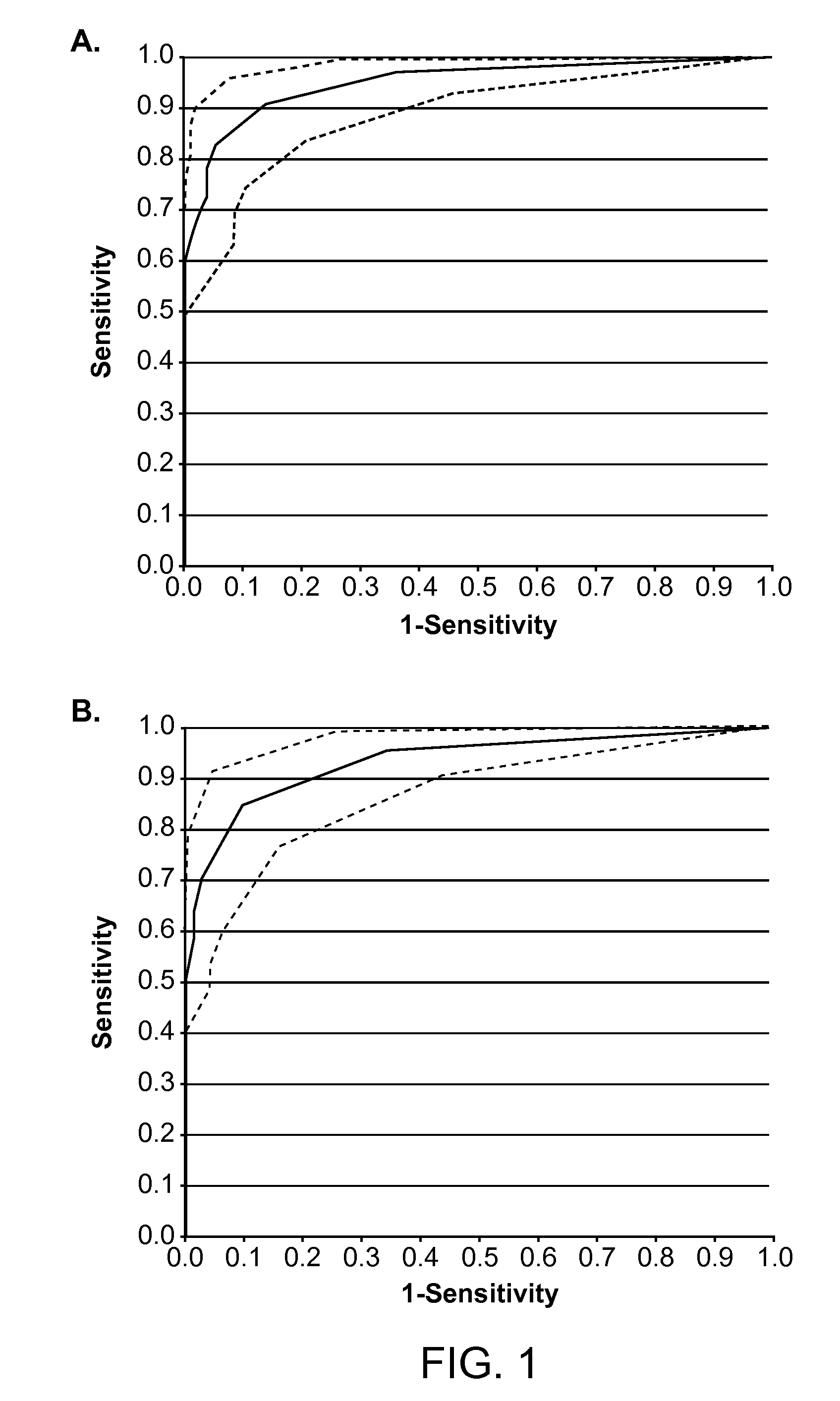
The invention relates to a detection method performed on a maternal serum or plasma sample from a pregnant female, which method comprises detecting the presence of a nucleic acid of foetal origin in the sample. The invention enables non-invasive prenatal diagnosis including for example sex determination, blood typing and other genotyping, and detection of pre-eclampsia in the mother.
Owner:SEQUENOM INC
Personalized Tumor Biomarkers
ActiveUS20150344970A1Microbiological testing/measurementLibrary member identificationBlood plasmaWilms' tumor
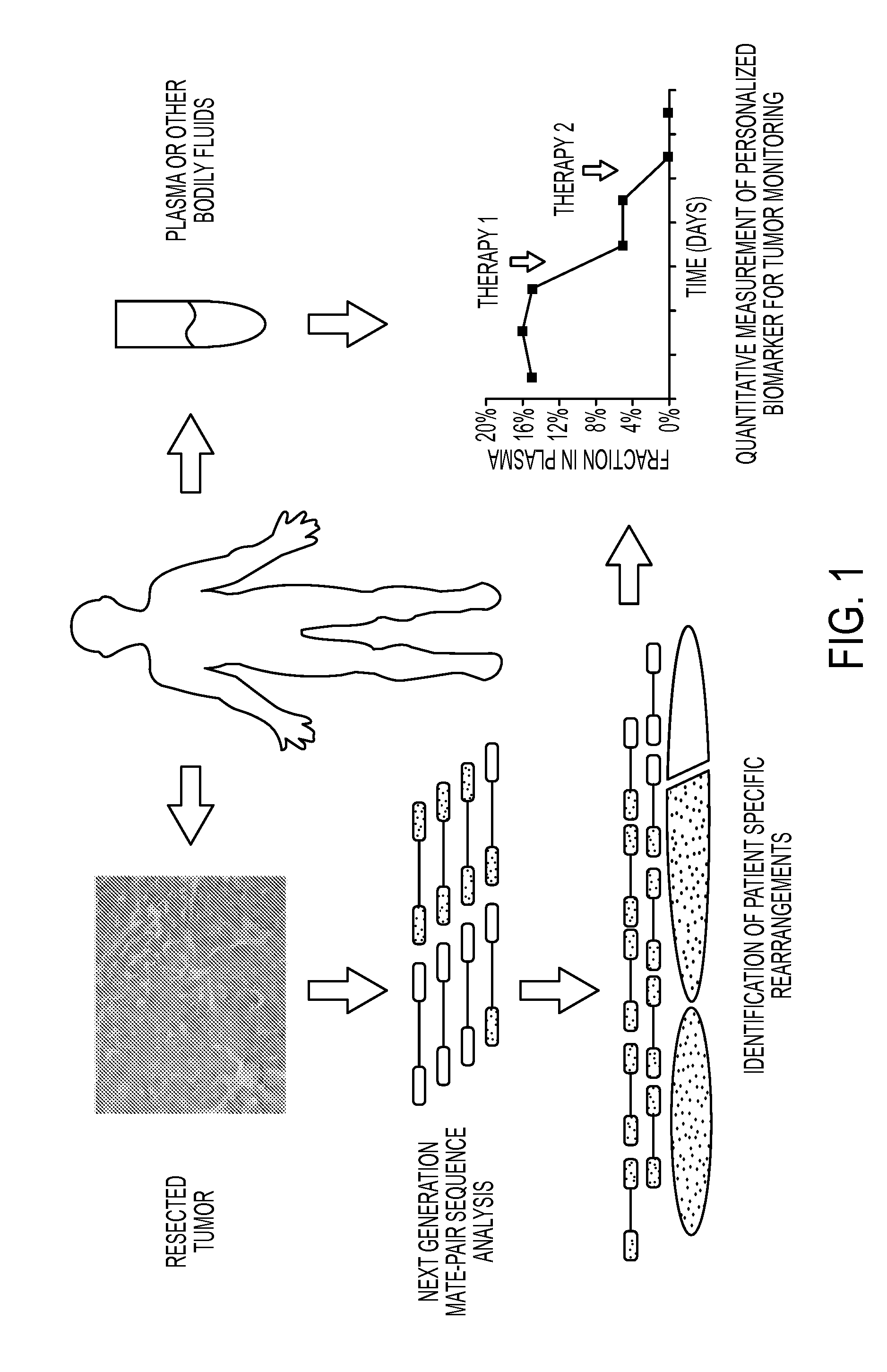
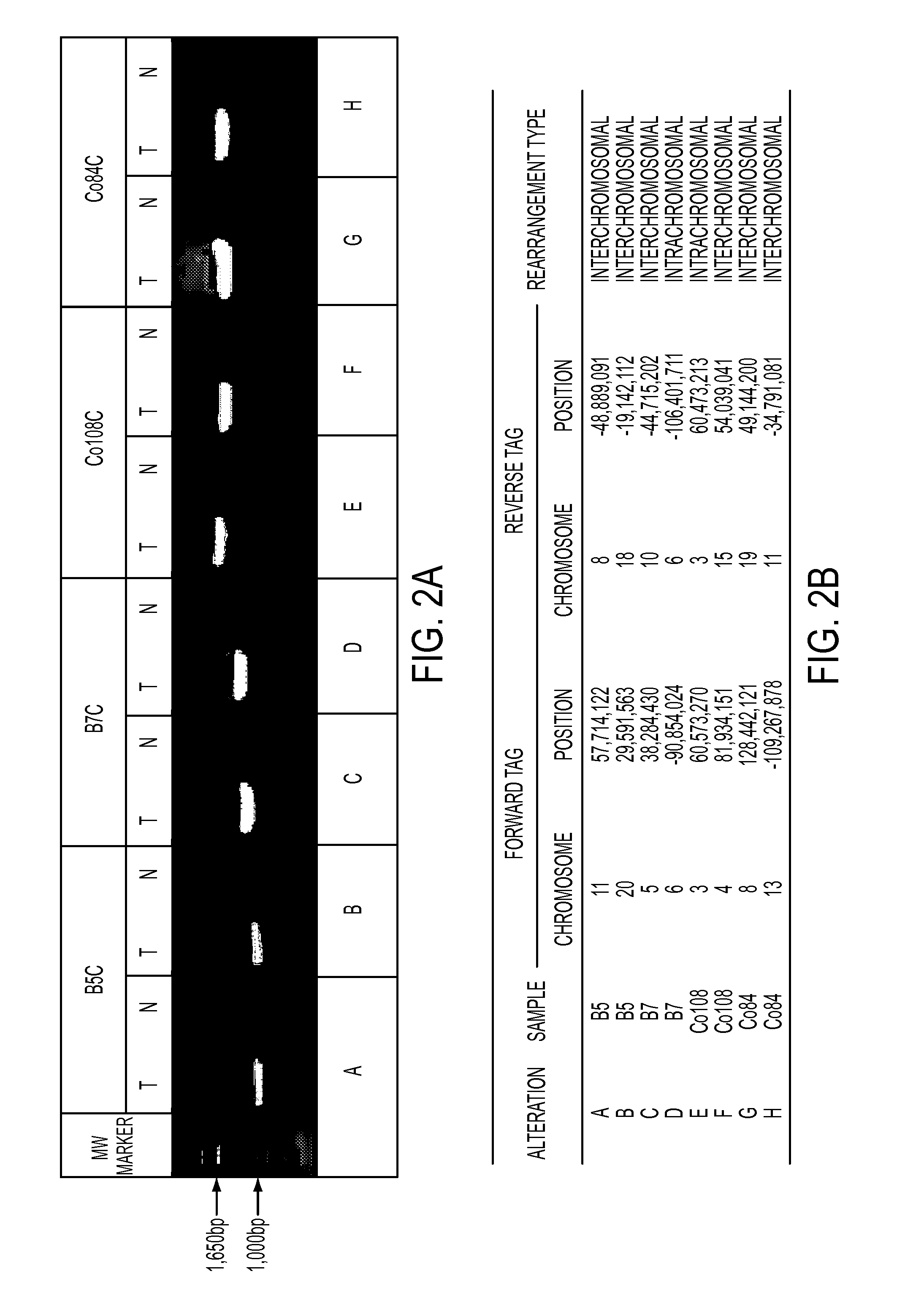
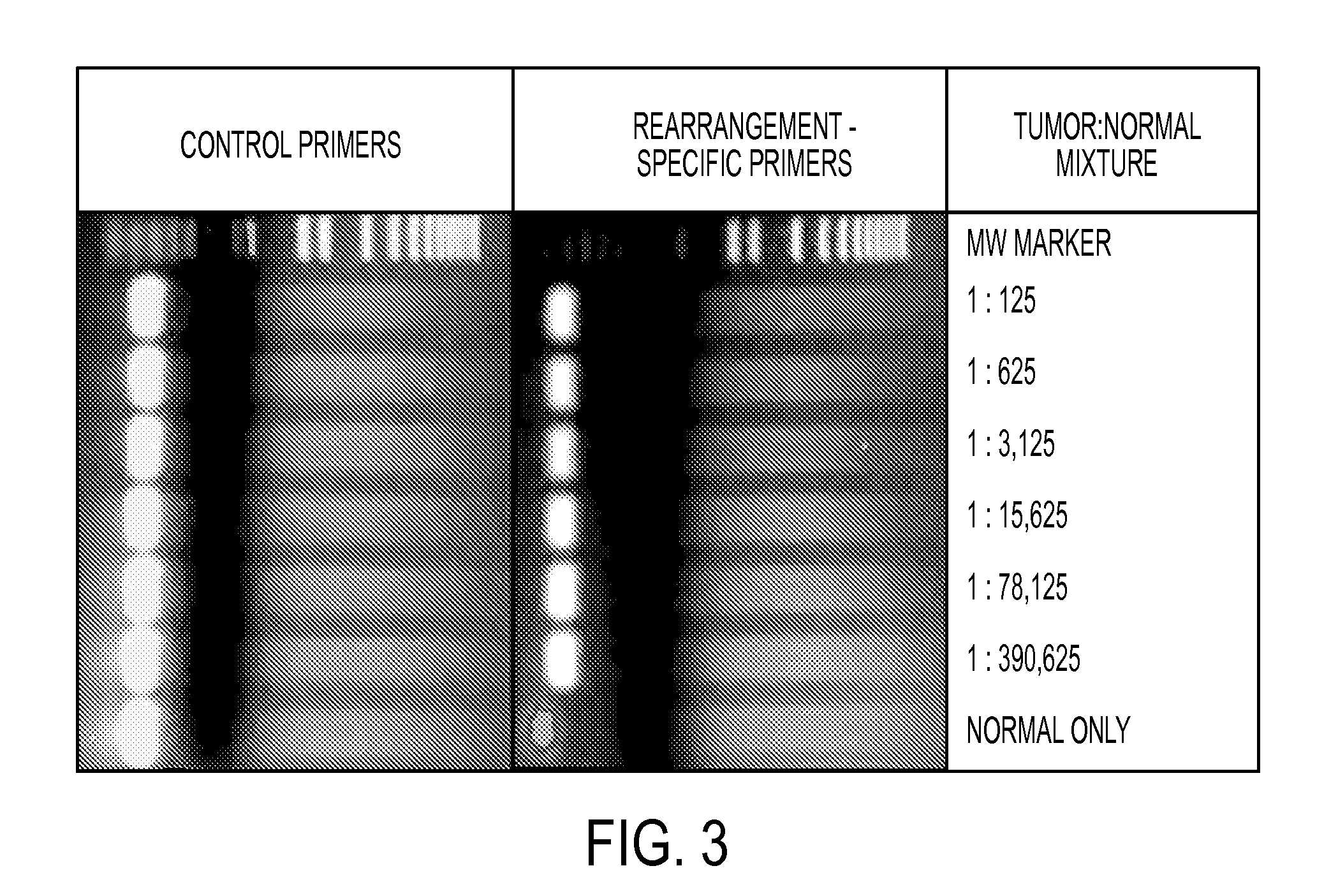
Clinical management of human cancer is dependent on the accurate monitoring of residual and recurrent tumors. We have developed a method, called personalized analysis of rearranged ends (PARE), which can identify translocations in solid tumors. Analysis of four colorectal and two breast cancers revealed an average of nine rearranged sequences (range 4 to 15) per tumor. Polymerase chain reaction with primers spanning the breakpoints were able to detect mutant DNA molecules present at levels lower than 0.001% and readily identified mutated circulating DNA in patient plasma samples. This approach provides an exquisitely sensitive and broadly applicable approach for the development of personalized biomarkers to enhance the clinical management of cancer patients.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
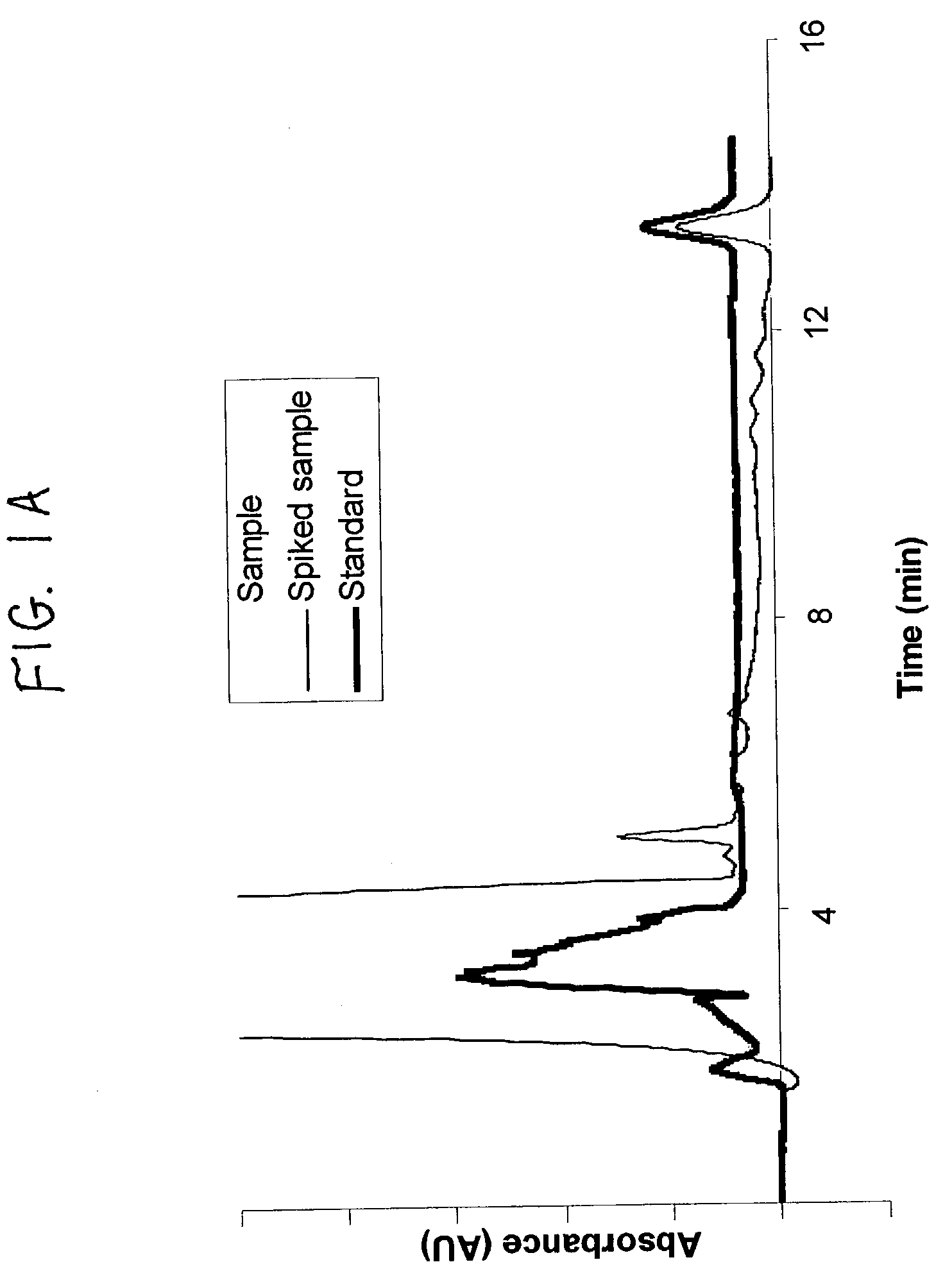
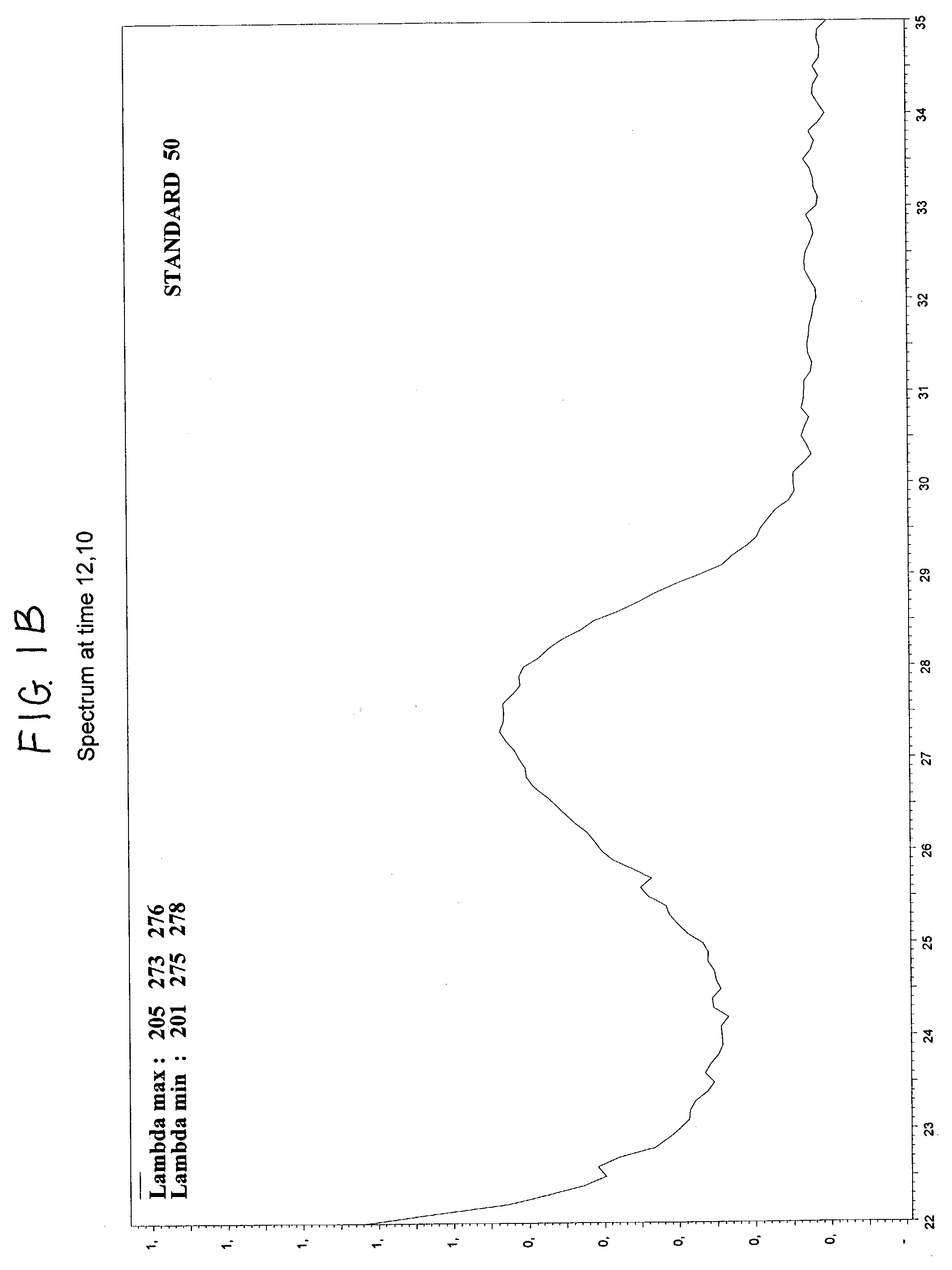
Method for calibrating spectrophotometric apparatus with synthetic fluids to measure plasma and serum analytes
InactiveUS6470279B1Testing/calibration apparatusScattering properties measurementsSample MeasureAnalyte
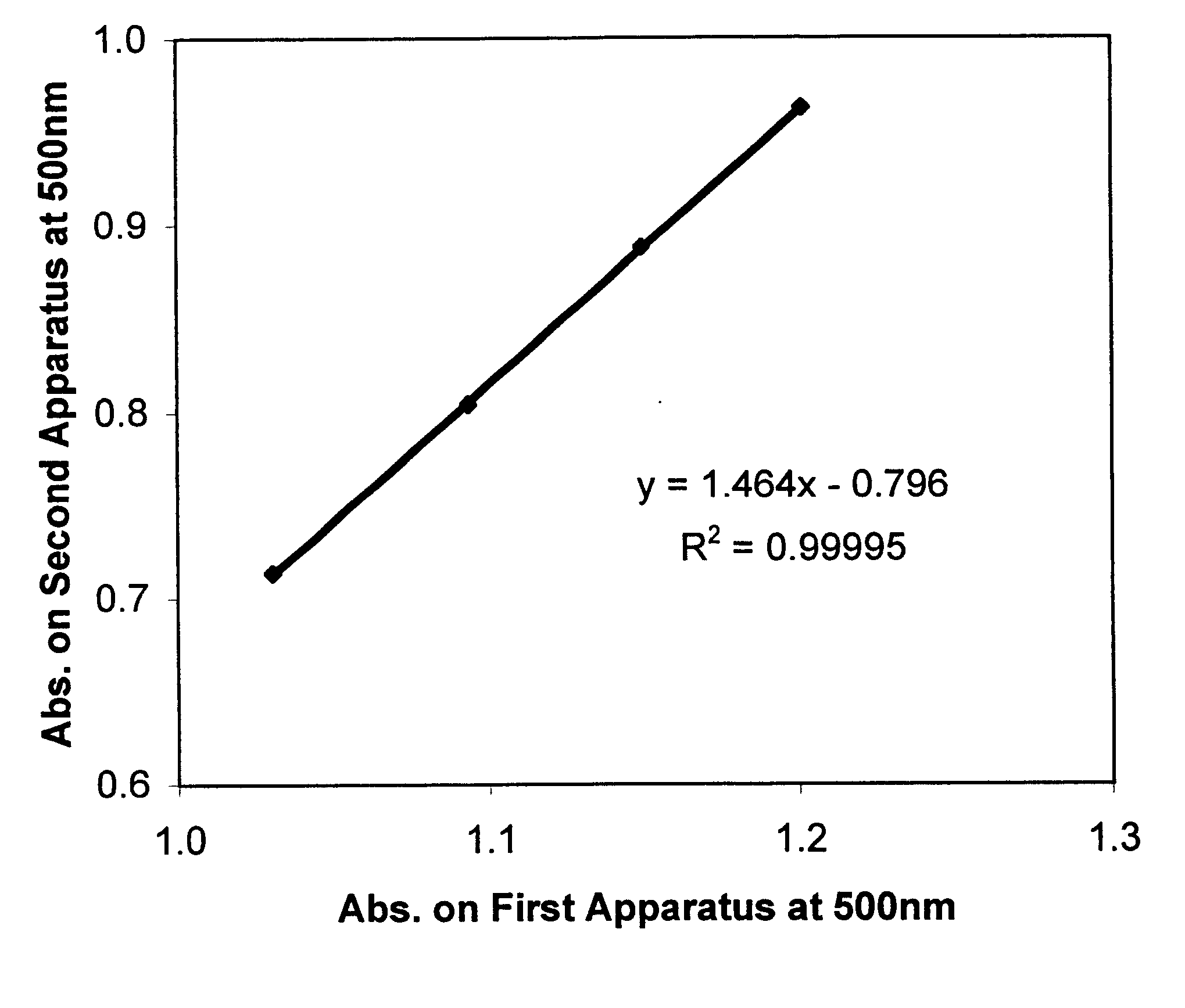
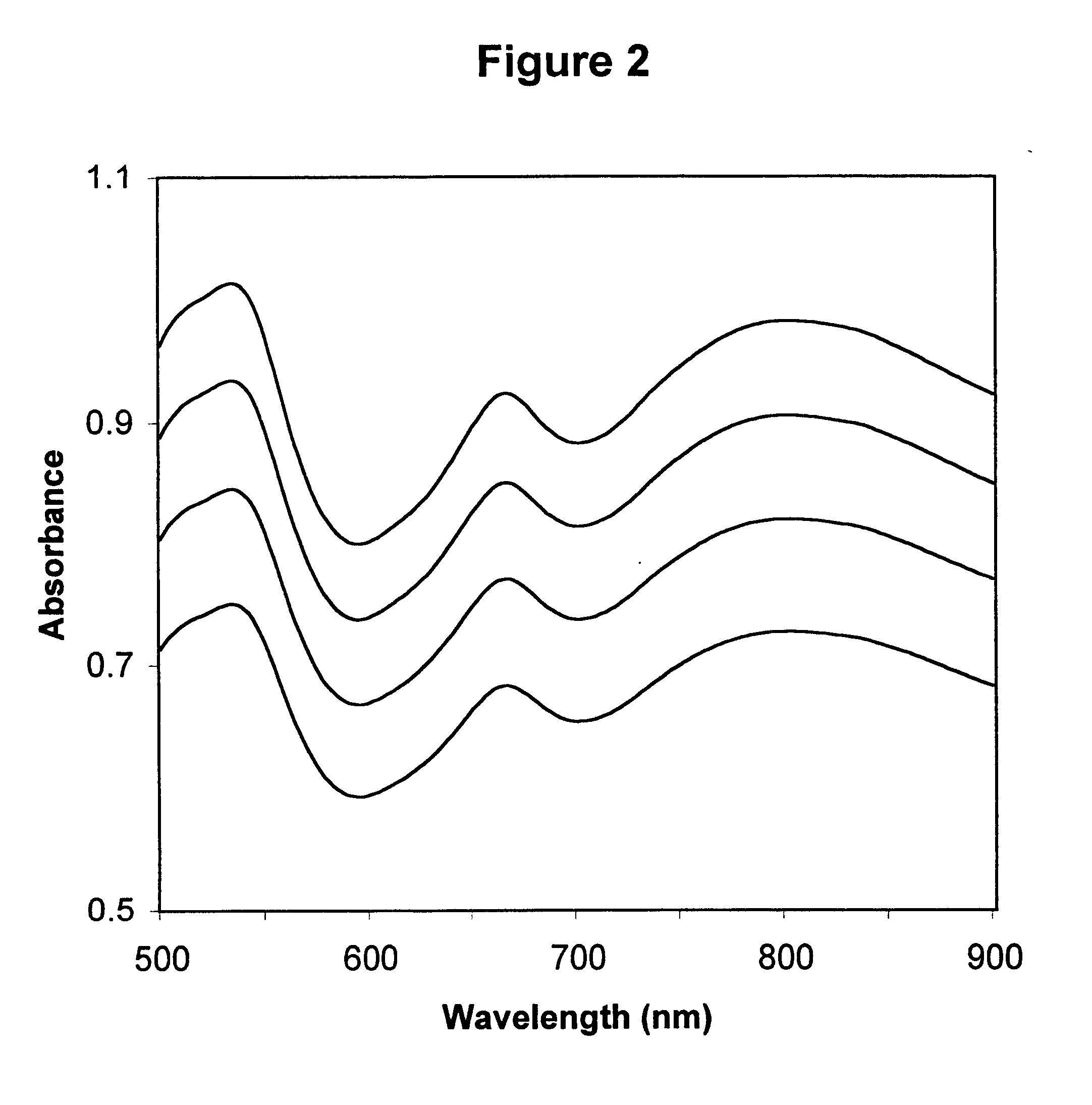
Described is a method for calibrating a spectrophotometric apparatus which is used to measure analytes in plasma and / or serum samples based on the calibration of a First Apparatus, and for recalibrating such apparatus, including recalibration of the First Apparatus all using synthetic calibrators. These apparatus use absorption of radiation to measure analytes in serum or plasma samples. The method described includes using synthetic calibrators which are submitted to the First Apparatus for measurement and compared with measurements of similar calibrators in a Second Apparatus and using the comparison to derive concentrations of analytes in samples measured on the Second Apparatus. As an alternative to making all apparatus identical, in terms of wavelength calibration, the absorbances of all apparatus should be mapped onto a standard set of wavelengths.
Owner:TYCO HEALTHCARE GRP LP
Colorectal cancer associated circulating nucleic acid biomarkers
InactiveUS20140303008A1Raise the possibilityNucleotide librariesMicrobiological testing/measurementBiologic markerBlood plasma
The invention provides methods and reagents for diagnosing colorectal cancer that are based on the detection of biomarkers in the circulating nucleic acids from a patient to be evaluated. In some embodiments, the CNA biomarkers are polynucleotide fragments, e.g., DNA fragments, that are present at an elevated level in blood, e.g., in a serum or plasma sample, of a colorectal cancer patient in comparison to the level in blood, e.g., a serum or plasma sample, obtained from a normal individual who does not have colorectal cancer.
Owner:CHRONIX BIOMEDICAL
Method to measure and characterize microvesicles in the human body fluids
InactiveUS20090220944A1Low efficiencySimple yet reliableMicrobiological testing/measurementDisease diagnosisHuman bodyNon invasive
This disclosure provides a method to capture, detect, characterize and quantify human exosomes in small volumes of human body fluids by using a sandwich ELISA test. This method allows a full characterization of an exosome preparation, thus providing a tool to distinguish a disease-related condition from a healthy state, by the use of a non-invasive assay. In fact, this method may be useful in screening, diagnosis and prognosis of tumors, with a simple plasma sample. At the same time measurement of circulating exosomes may provide information on the level of tumor mass present in a patient. The method provided here is suitable to evaluate presence of some infectious and / or transmissible agents, such as viral proteins or prion proteins, within circulating exosomes.
Owner:EXOSOMICS SPA
Nucleic acid archiving
InactiveUS6872527B2Tightly boundBioreactor/fermenter combinationsBiological substance pretreatmentsSingle strandSingle strand dna
This invention is directed to a process for tightly binding nucleic acid to solid phase and corresponding processes for the utilization thereof. Nucleic acid is bound to solid phase matrices exhibiting sufficient hydrophilicity and electropositivity to tightly bind the nucleic acids from a sample. These processes include nucleic acid (double or single stranded DNA and RNA) capture from high volume and / or low concentration specimens, buffer changes, washes, and volume reductions, and enable the interface of solid phase bound nucleic acid with enzyme, hybridization or amplification strategies. The tightly bound nucleic acid may be used, for example, in repeated analyses to confirm results or test additional genes in both research and commercial applications. Further, a method is described for virus extraction, purification, and solid phase amplification from large volume plasma specimens.
Owner:APPL BIOSYSTEMS INC
Kit for measuring the thrombin generation in a sample of a patient's blood or plasma
InactiveUS20050221414A1Simple and efficient and fast and reproducible assayConvenient typeMicrobiological testing/measurementBiological material analysisTissue factorThrombin activity
The invention provides a kit for measuring the thrombin generation in a sample of a patient's blood or plasma, or in a sample of clotting factors. The kit contains lyophilized tissue factor / phospholipid-complex and a lyophilized mixture containing a thrombin-substrate and CaCl2. The invention also provides processes for preparing the reagents for the kit. The kit can be used in a method for measuring the thrombin generation in a sample, wherein it is possible to detect changes in thrombin generation kinetics, for example after administration of inhibitor bypassing agents to a patient who has developed inhibitors to an exogenous clotting factor such as Factor VIII.
Owner:BAXTER INT INC +1
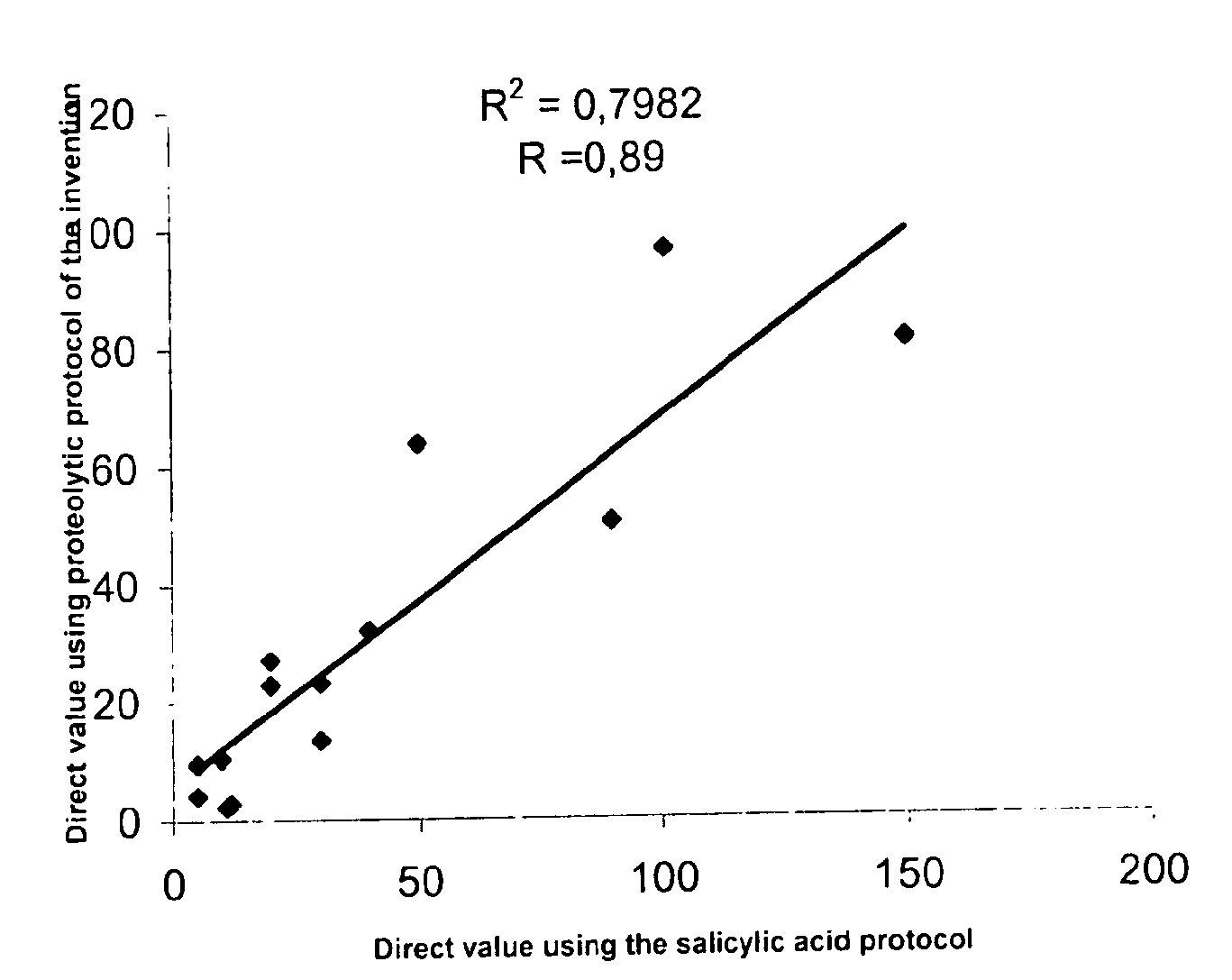
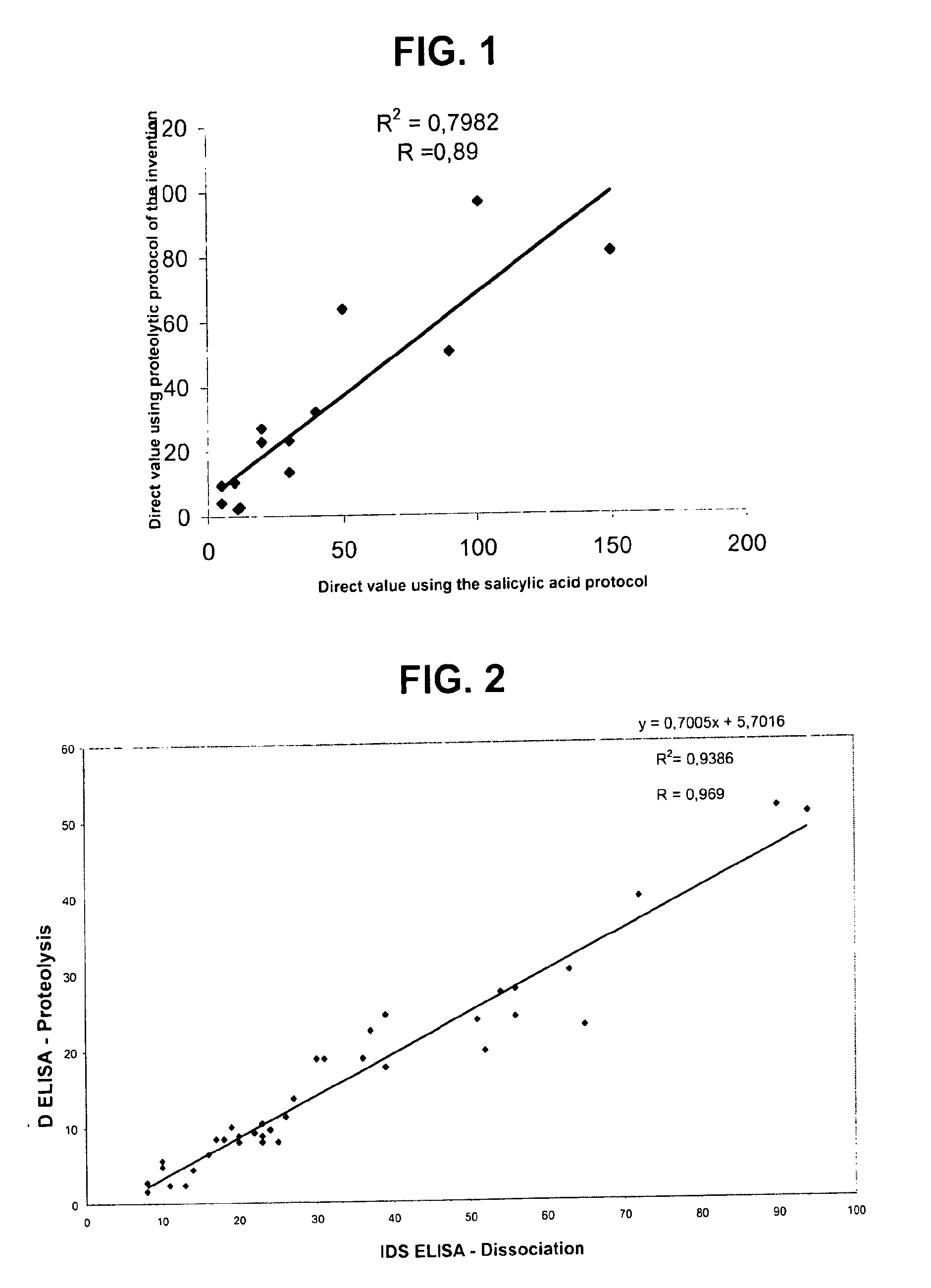
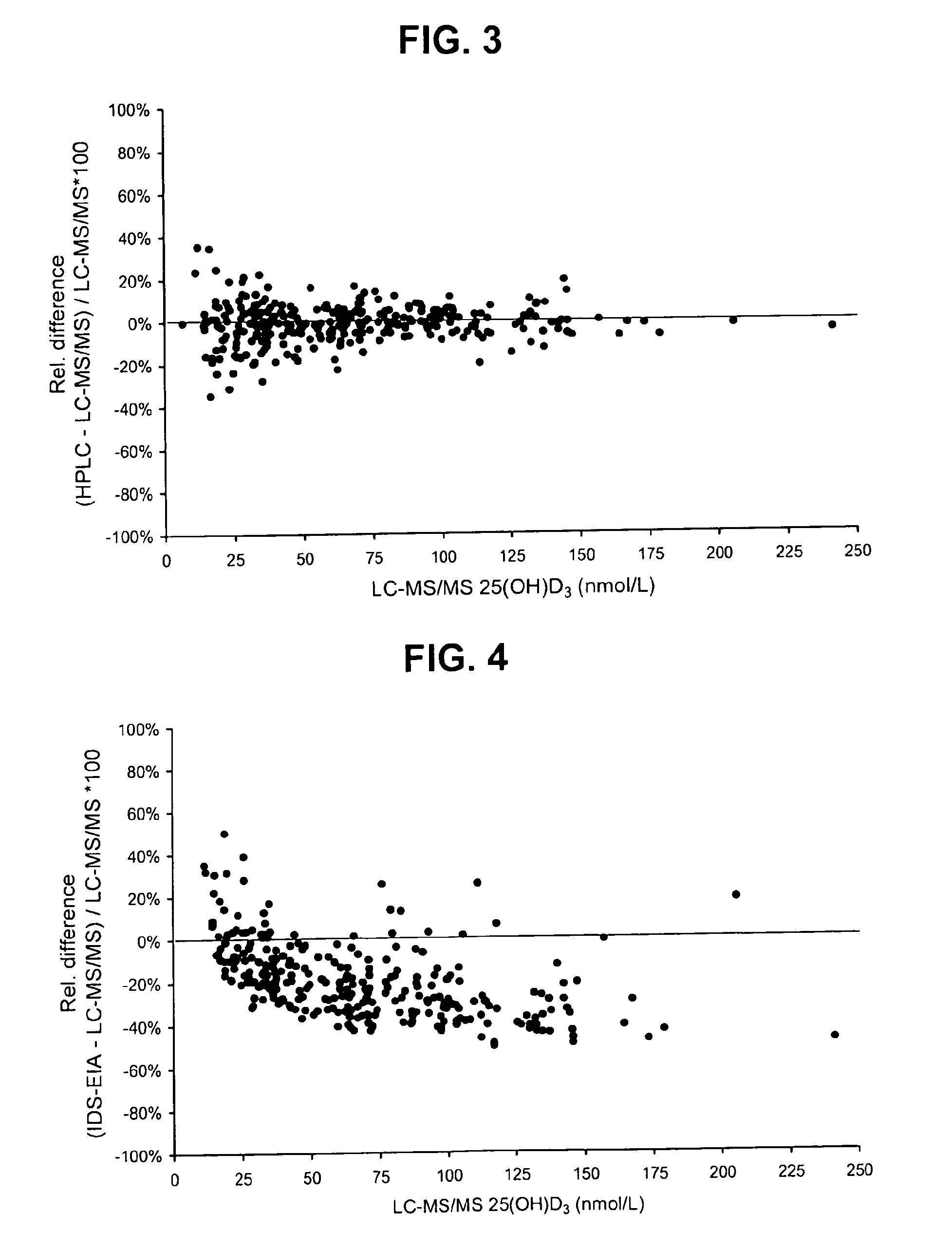
Direct determination of vitamin D in serum or plasma
A method for quantitating vitamin D metabolites directly in blood plasma or serum, without the need for prior purification of the vitamin D metabolites, comprising a digestion of the serum proteins with a serine protease such as proteinase K and sequence of steps for inhibiting the proteinase K activity in the competitive binding analysis. The advantages of this method are its high accuracy over the whole range of physiologically relevant values and that it can be easily adapted for a fully automated analysis of serum and plasma samples.
Owner:IMMUNDIAGNOSTIK AG
Methods for Treating Bleeding Disorders
A method of factor XI-dependent blood coagulation enhancement in a subject in need of enhanced blood coagulation comprising administering a therapeutically effective amount of a composition comprising a non-anticoagulant sulfated polysaccharide (NASP) to the subject. A method of factor XI-dependent blood coagulation enhancement in a subject in need of enhanced blood coagulation comprising: (i) selecting a subject that is not deficient for factor XI; and (ii) administering a therapeutically effective amount of a composition comprising a non-anticoagulant sulfated polysaccharide (NASP) to the subject, wherein the NASP enhances blood coagulation in a factor XI-dependent manner. A method of identifying a non-anticoagulant sulfated polysaccharide (NASP) which is capable of enhancing blood coagulation in dependence on FXI, the method comprising: a) combining a blood or plasma sample comprising activation competent FXI with a composition comprising a sulfated polysaccharide and measuring the clotting or thrombin generation parameters of the blood or plasma sample; b) combining a corresponding blood or plasma sample deficient in activation competent FXI with a composition comprising the sulfated polysaccharide and measuring the clotting or thrombin generation parameters of the blood or plasma sample; and c) comparing the clotting or thrombin generation parameters of the blood or plasma samples as determined in steps (a) and (b) with each other, wherein a decrease in the clotting time of the blood sample or an increase in peak thrombin or decrease in peak time of the plasma sample comprising activation competent FXI compared to the clotting time of the blood sample or peak thrombin or peak time of the plasma sample deficient in activation competent FXI is indicative of a NASP which is capable of enhancing blood coagulation in dependence on FXI.
Owner:TAKEDA PHARMA CO LTD
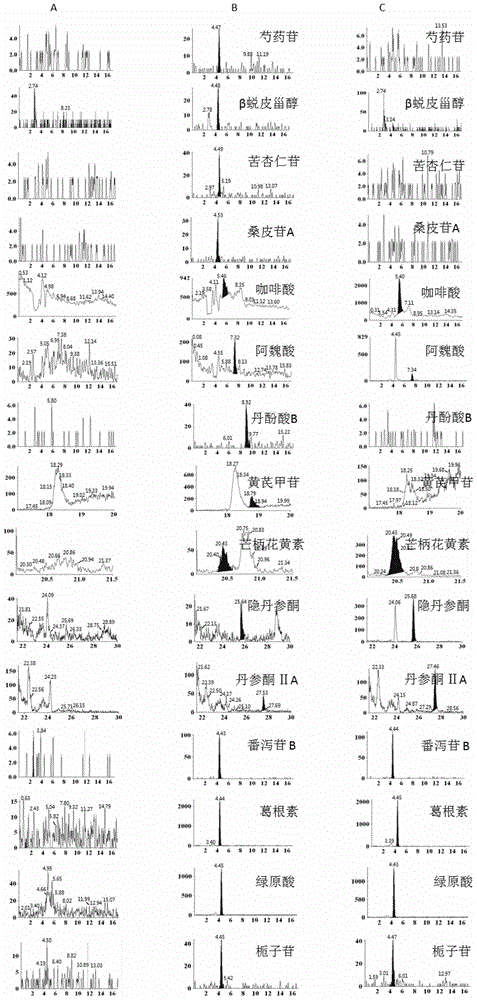
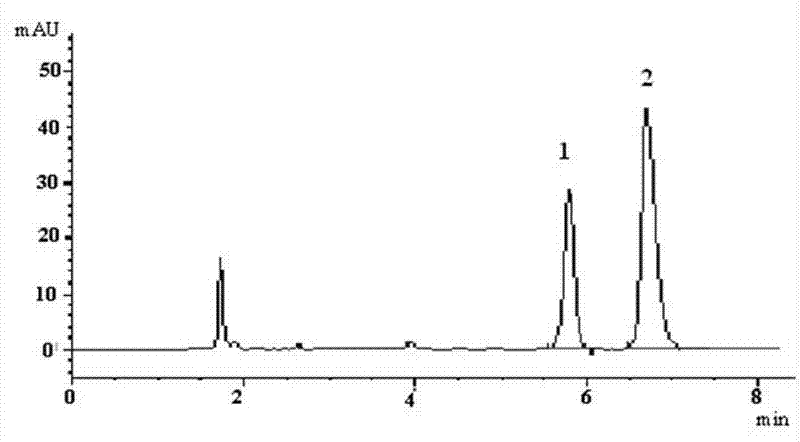
Method for simultaneously detecting main components of Naoxintong capsule in plasma
ActiveCN104614456AInhibit aggregationImprove neurological deficitsComponent separationAstragalosideSalvianolic acid B
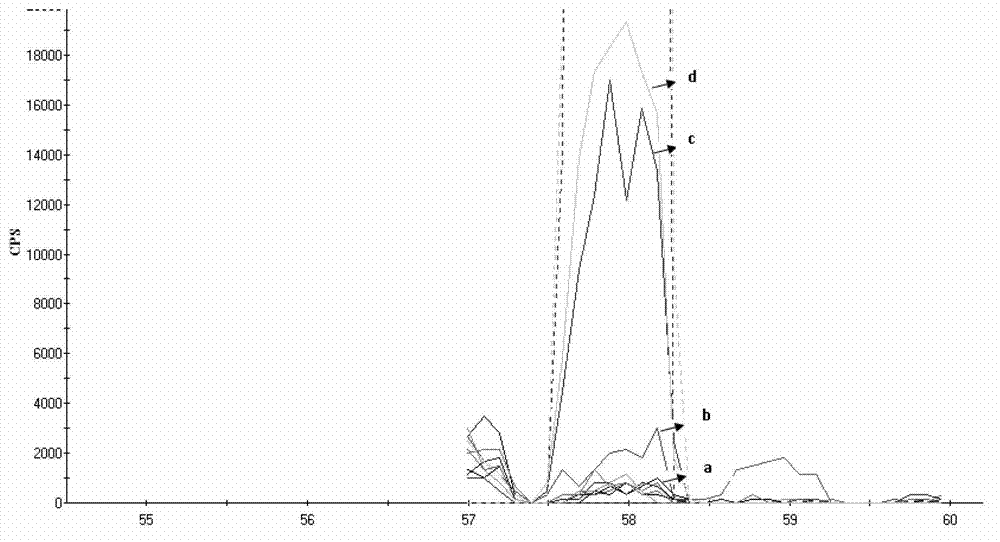
The invention provides a method for simultaneously detecting main components of paeoniflorin, beta ecdysterone, laetrile, mulberroside A, caffeic acid, ferulic acid, salvianolic acid B, astragaloside, formononetin, cryptotanshinone and tanshinone IIA of a Naoxintong capsule in a plasma sample by a liquid chromatography-tandem mass spectrometry (HPLC-MS / MS). In a liquid chromatogram, a mobile phase consists of acetonitrile and a formic acid aqueous solution of which the volume fraction is 0.1%, and gradient elution is used. A mass spectrum uses a quick positive and negative ions switching and analyzing mode and an MRM (Multiple Reaction Monitoring) scanning manner. After the Naoxintong capsule is taken, the situations of the changes of the blood-medicine concentration of several kinds of main components in the plasma of a rat are detected at the same time. The methodological survey results indicate that the established method conforms to determination requirements on biological samples in a body; the method is good in sensitivity, high in specificity, stable, reliable, and suitable for detecting substances with lower contents.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE +1
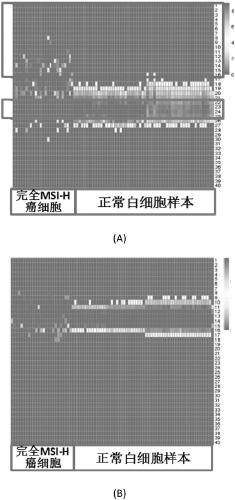
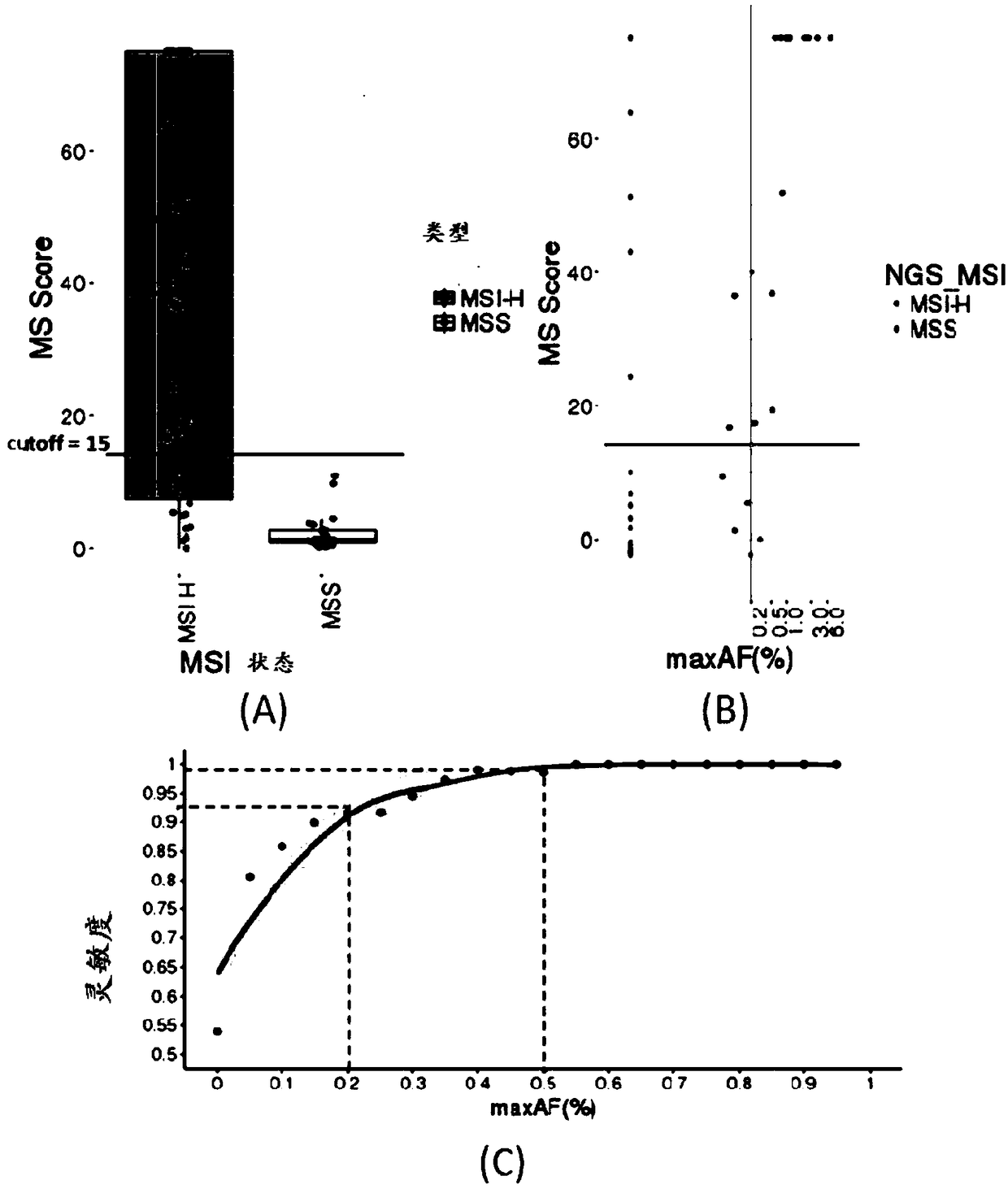
Microsatellite biomarker combination, detection kit and use thereof
ActiveCN109182525AMicrobiological testing/measurementDNA/RNA fragmentationStomach cancerBiomarker (petroleum)
The present invention relates to biomarker combinations, kits for detecting them, and their use in microsatellite instability (MSI) detection and cancer, preferably colorectal cancer (e.g., bowel cancer), gastric cancer or endometrial cancer, prognostic assessment, treatment regimen selection or genetic screening in plasma samples. A method for detecting plasma MSI is provided for the first time,which enables a microsatellite (MS) state of a sample to be judged with high accuracy and sensitivity.
Owner:GUANGZHOU BURNING ROCK DX CO LTD
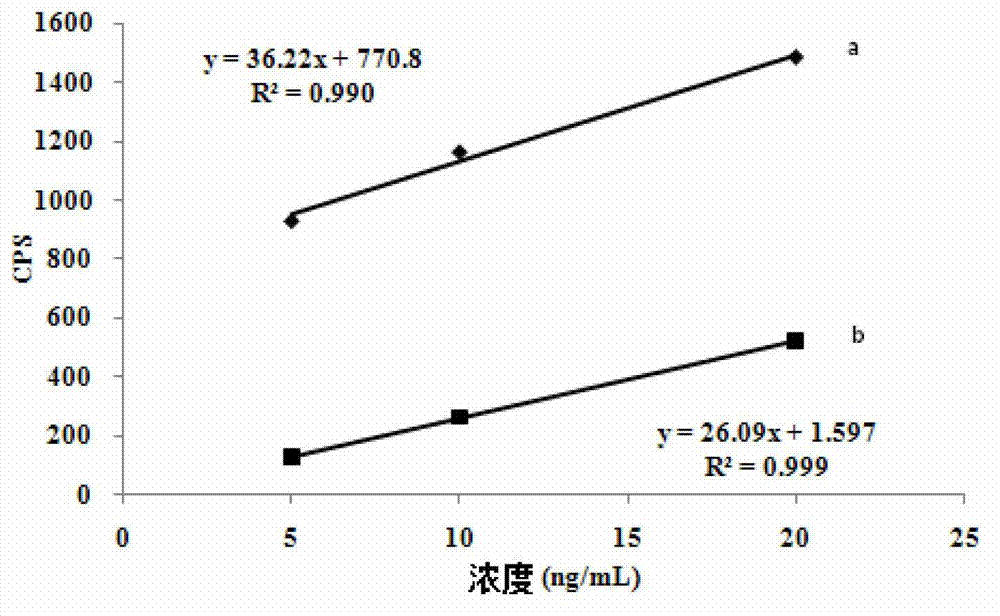
Application of inductively coupled plasma mass spectrometry in drug testing of hemin
The invention relates to an application of inductively coupled plasma mass spectrometry in drug testing of hemin. A method for measuring concentration of stable isotope iron in animal plasma is carried out by an ICP-MS (Inductively Coupled Plasma Mass Spectrometry) method and comprises the following steps of: providing an inductively coupled plasma source mass spectrometer; determining operating conditions of the ICP-MS; providing stable isotope iron powder, marked hemin, internal standard elements and the like; preparing standard solution and internal standard solution; preparing ICP-MS diluent; establishing a standard curve; measuring the stable isotope iron concentration in the plasma; and adding the ICP-MS diluent into a sample tube containing animal plasma sample containing iron, mixing uniformly and putting the sample tube into a refrigerator to be refrigerated, and determining the concentration of the iron in the plasma by the obtained standard curve. The application of the inductively coupled plasma mass spectrometry in the drug testing of the hemin has well performances, e.g., the method has well quantification lower limit, accuracy, absolute recovery test, sample stability, medium effect and quality control requirement, and can be taken as a content measurement method for analyzing the content of the stable isotope iron in a biological sample such as the plasma.
Owner:新疆科丽生物技术有限公司
Fluorescent PCR (Polymerase Chain Reaction) detection kit for IDH1/IDH2 (isocitrate dehydrogenase 1/isocitrate dehydrogenase 2) gene mutation and application thereof
InactiveCN103436613AStrong specificityEnrichmentMicrobiological testing/measurementDNA/RNA fragmentationNucleotideBlood plasma
The invention relates to a fluorescent PCR detection kit for the IDH1 / IDH2 (isocitrate dehydrogenase 1 / isocitrate dehydrogenase 2) gene mutation and application thereof, and particularly provides a nucleotide sequence used for detecting the IDH1 / IDH2 gene mutation, a kit with the nucleotide sequence and the application of the kit to detection of the IDH1 / IDH2 gene mutation. The nucleotide sequence comprises a specific ARMS (Amplification Refractory Mutation System) primer, a general mutation detection TaqMan probe and a nucleic acid amplification retardation primer. The kit can be used for quickly detecting the IDH1 / IDH2 gene mutation with high throughput and at a low cost, is high in sensitivity, good in specificity, low in pollution and quick and safe to operate, can be suitable for high-sensitivity detection of trace mutation in general clinic samples such as fresh frozen tissues and paraffin tissues, especially non-traumatic serums or plasma samples in addition to pathological tissues.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
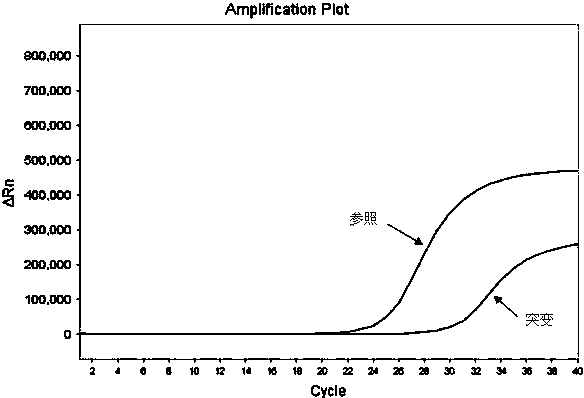
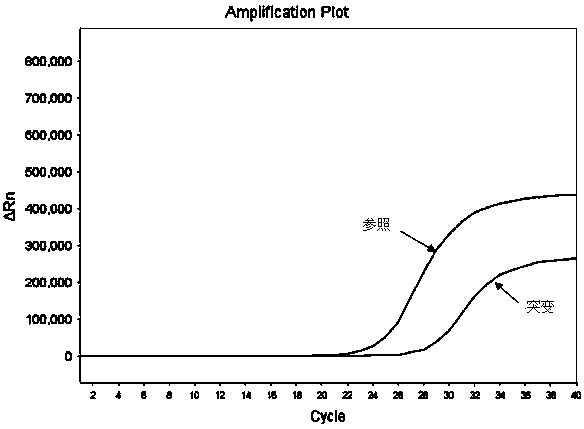
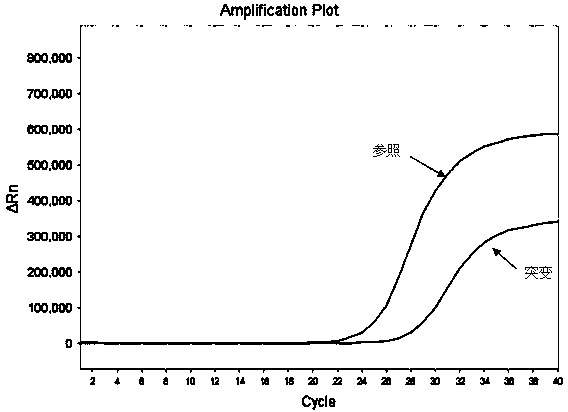
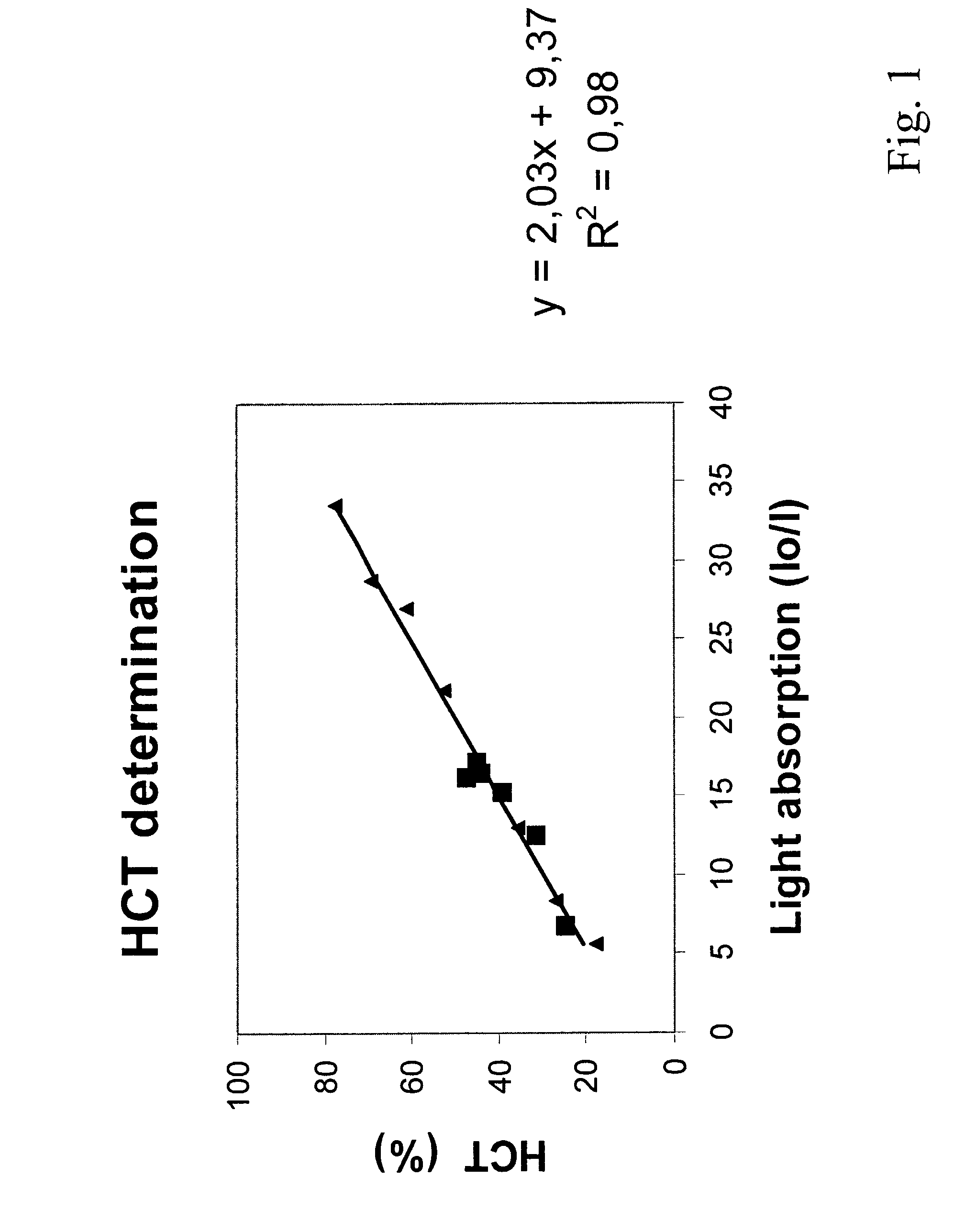
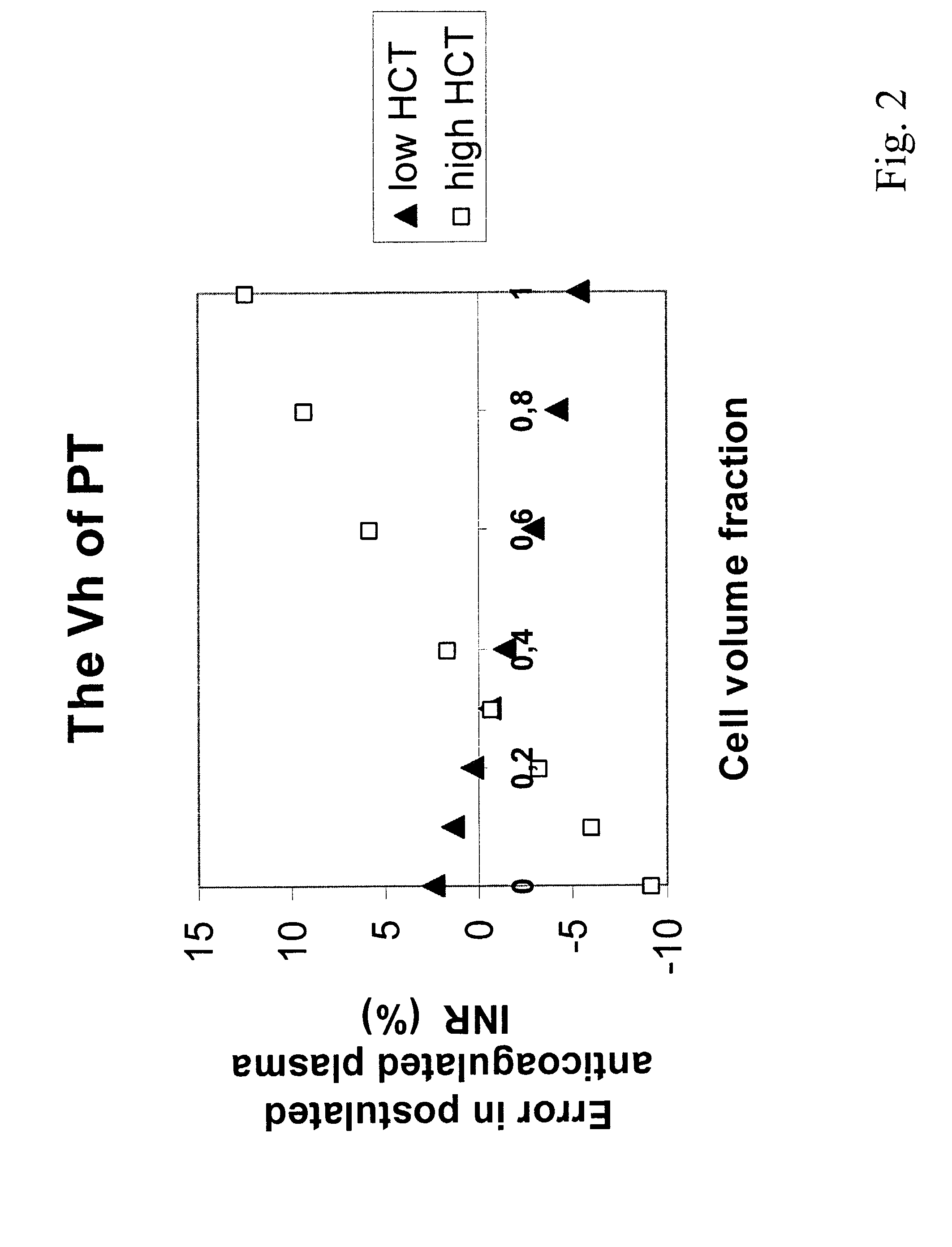
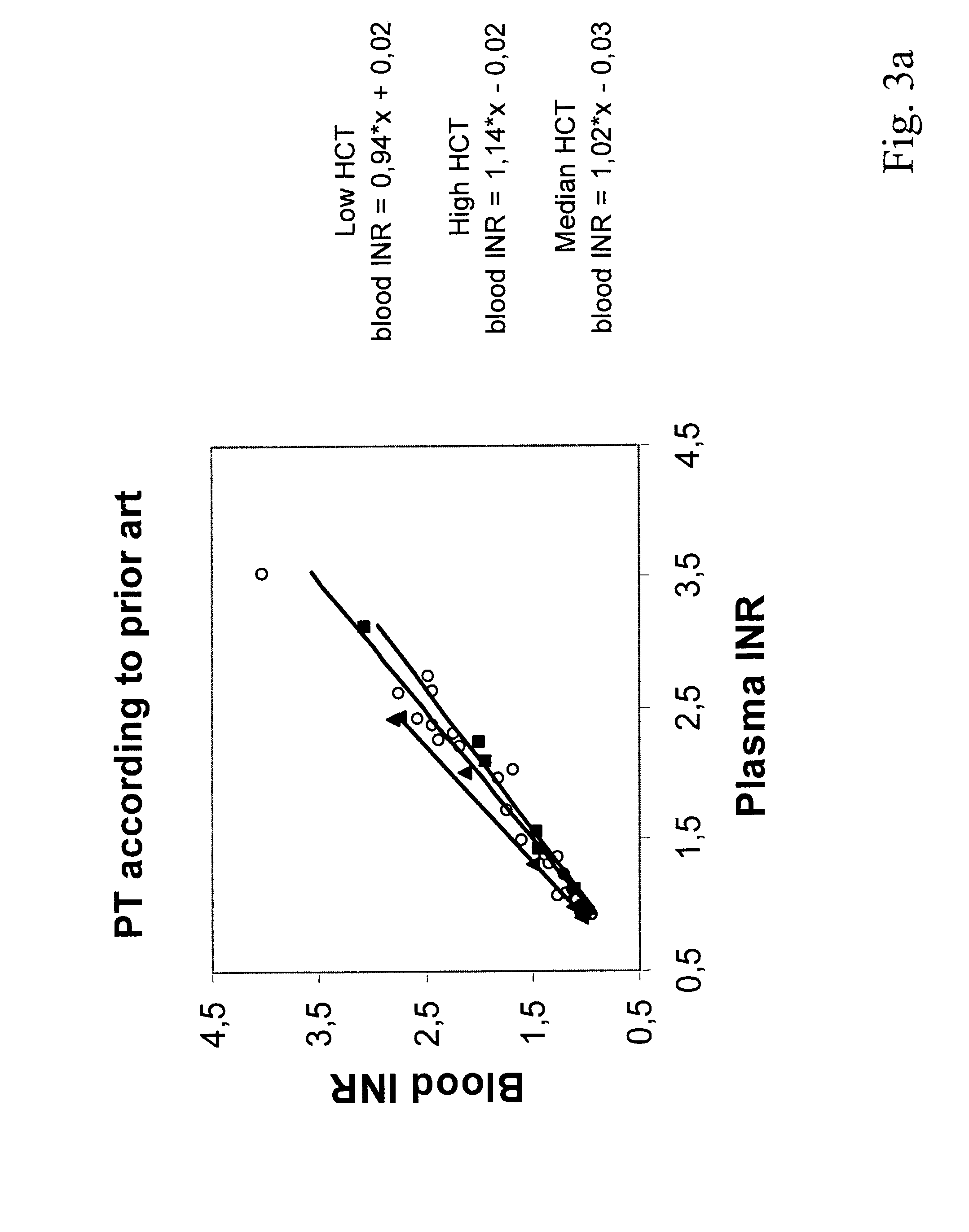
Hematocrit and analyte concentration determination
InactiveUS8759094B2Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteBlood plasma
Owner:ZAFENA
Biomarkers for lymphoma
A biomarker, method, test kit, and diagnostic system for detecting the presence of lymphoma in a person are disclosed. The lymphoma may be Hodgkin's lymphoma or non-Hodgkin's lymphoma. The person may be a high-risk subject. In one embodiment, a plasma sample from a person is obtained. The level of at least one protein listed in Table S3 in the plasma sample is measured. The level of at least one protein in the plasma sample is compared with the level in a normal or healthy subject. The lymphoma is diagnosed based upon the level of the at least one protein in the plasma sample in comparison to the normal or healthy level.
Owner:BATTELLE MEMORIAL INST
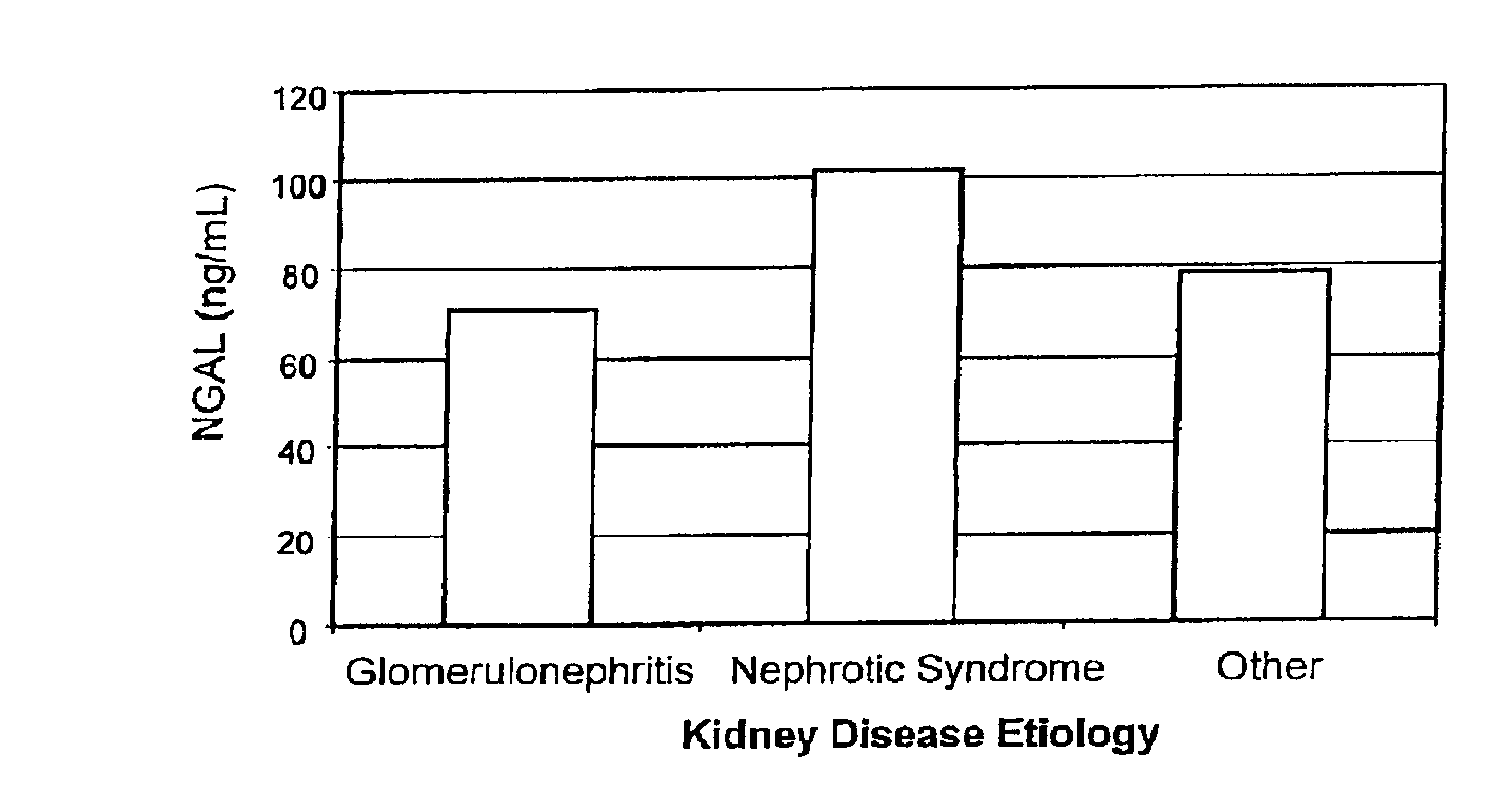
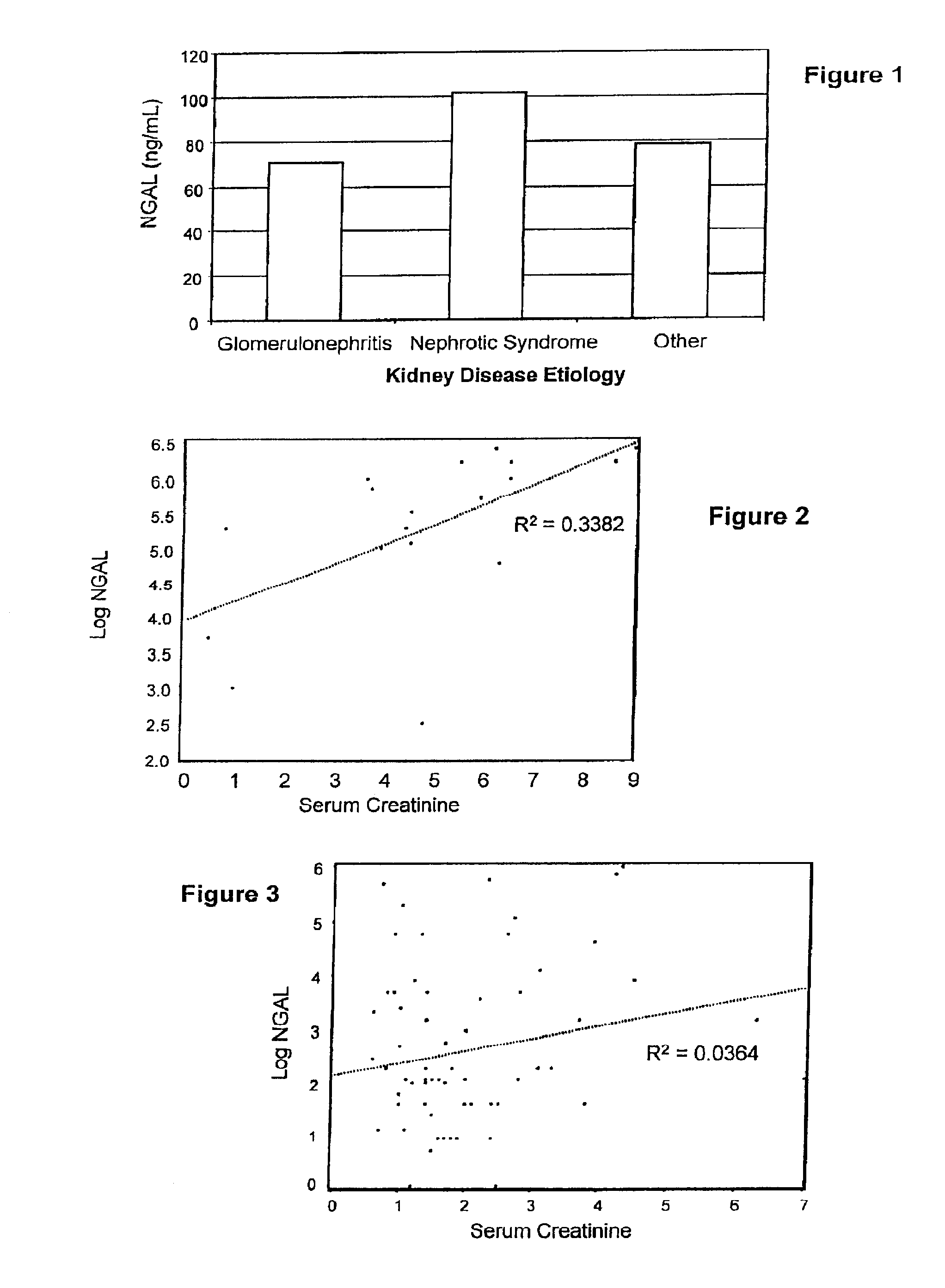
Diagnosis and monitoring of chronic renal disease using ngal
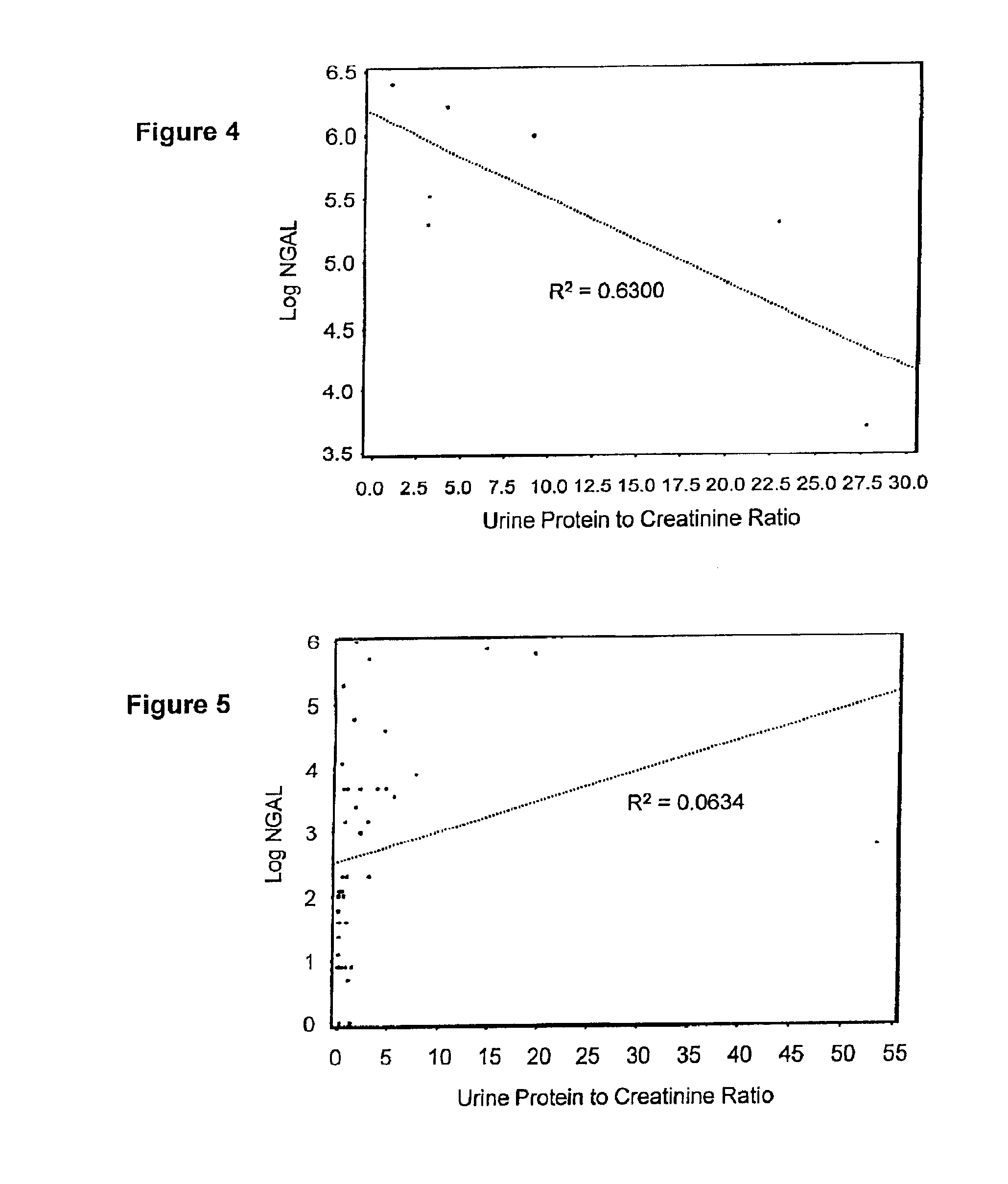
InactiveUS20100234765A1Difficult to levelImprove the level ofDisease diagnosisDiagnostic recording/measuringRegimenProper treatment
A method of assessing the ongoing kidney status of a mammal afflicted with or at risk of developing chronic renal injury or disease, including chronic renal failure (CRF) by detecting the quantity of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in urine, serum or plasma samples at discrete time periods, as well as over time. Incremental increases in NGAL levels in CRF patients over a prolonged period of time are diagnostic of worsening kidney disease. This increase in NGAL precedes and correlates with other indicators of worsening chronic renal disease or CRF, such as increased serum creatinine, increased urine protein secretion, and lower glomerular filtration rate (GFR). Proper detection of worsening (or improving, if treatment has been instituted) renal status over time, confirmed by pre- and post-treatment NGAL levels in the patient, can aid the clinical practitioner in designing and / or maintaining a proper treatment regimen to slow or stop the progression of CRF.
Owner:BARASCH JONATHAN MATTHEW +3
Experimental method for animal model depression degree evaluation based on metabonomics
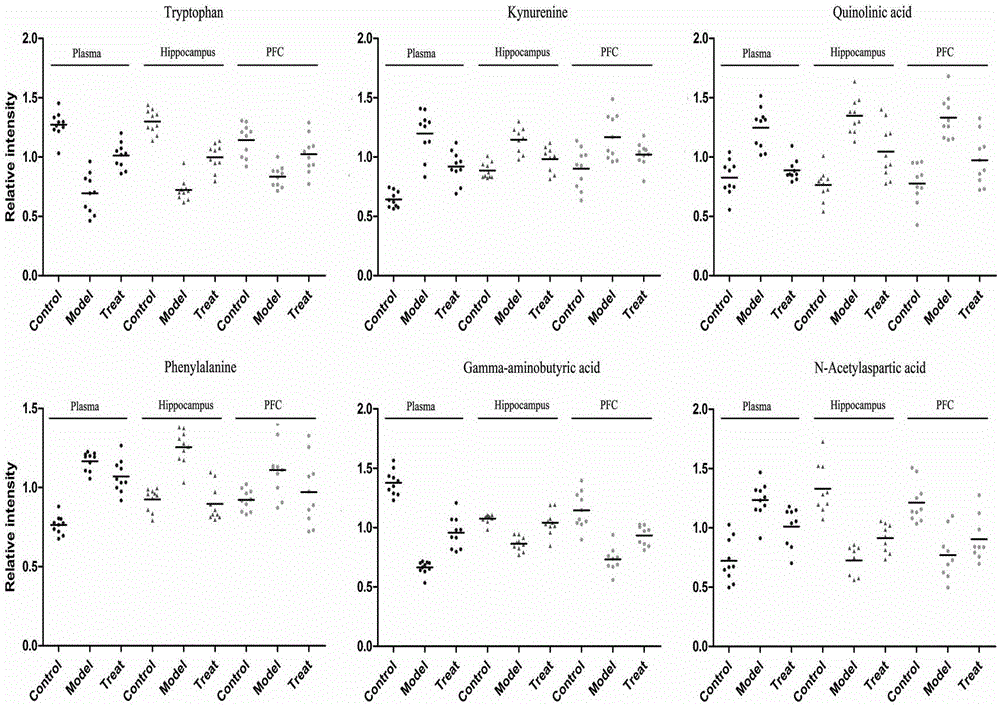
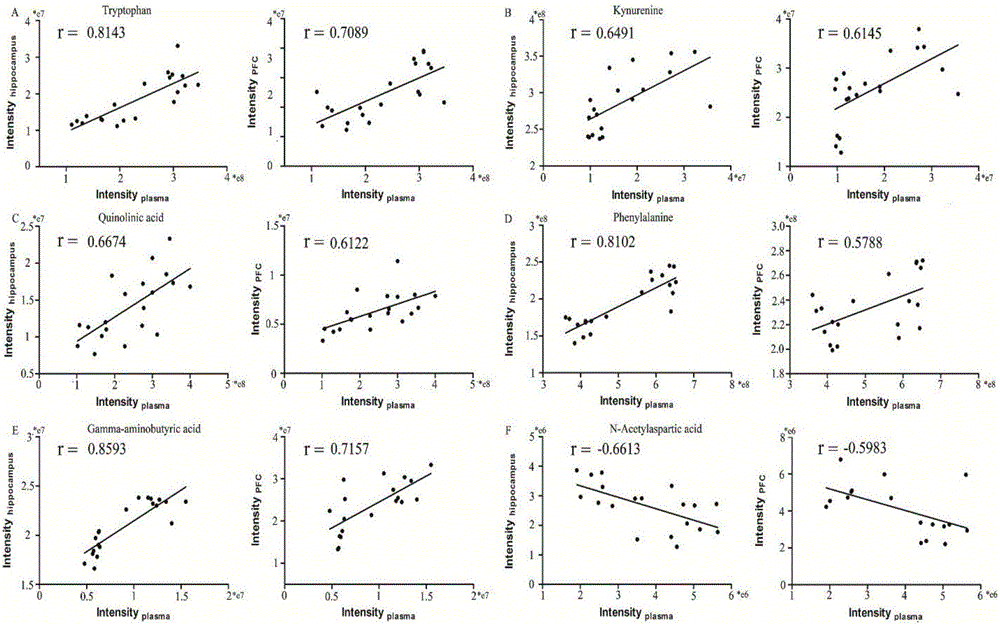
InactiveCN106770857AAccurate measurementComprehensive reflection of metabolic changesComponent separationMass spectrum analysisBehavioral experiment
The invention discloses an experimental method for animal model depression degree evaluation based on metabonomics. The experimental method comprises the following steps: comprehensively and accurately determining endogenous small molecule metabolites in a biological sample by utilizing a metabonomics platform based on high-resolution mass spectrum, screening different metabolites among different samples and different groups according to the obtained metabolic spectrum data by combining a single-variable multi-variable statistical method, utilizing a common different metabolite among three samples, comparing correlation of compounds in a blood plasma sample and a brain tissue sample, and taking a comparison result as a basis of reflecting change of metabolome in brain tissues by utilizing a blood plasma marker; meanwhile, taking a model group and a control group as a training set to construct a model and an administration group as predication capability of a validation set test model, which is an index for evaluating the depression degree by utilizing the change of the blood plasma metabolome. The experimental method disclosed by the invention can be used for solving the problems that the traditional behavioral experiment has many uncontrollable factors, preparation is relatively complex and animal subjectivity is stronger.
Owner:NANJING MEDICAL UNIV
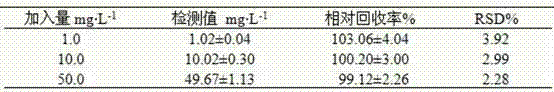
A kind of method for measuring paraquat blood concentration
InactiveCN102288696AEasy pretreatmentThe pre-processing process is simpleComponent separationParaquatBlood plasma
The invention discloses a method for measuring blood concentration of paraquat. The method comprises the following steps of: (1) pretreating a sample, namely adding aqueous solution of an internal standard substance and a protein precipitation agent acetonitrile into a plasma sample, performing whirl mixing, centrifuging and sampling supernatant; (2) separating the sample, namely adopting a universal C18 liquid chromatographic column, and using an ion pairing agent in an acid mobile phase, wherein the acid mobile phase is mixed solution of 3mmol.L<-1> aqueous solution of sodium dodecyl sulfate, 0.2 percent of aqueous solution of trifluoroacetic acid, acetonitrile and water; and (3) detecting by using a diode array detector, namely detecting by using the diode array detector at a detectionwavelength of 250 to 260nm, measuring peak areas of the internal standard substance and the paraquat, and calculating the blood concentration of the paraquat through least square method linear regression. The plasma sample is easy and convenient to pretreat, the detection process is sensitive and rapid, toxic substances and the blood concentration thereof can be rapidly determined in actual application, and the method is high in clinical application value.
Owner:HENAN UNIV OF SCI & TECH
High-flux detection method of circulating DNA liver cancer driver gene
PendingCN107723352AGuaranteed catchHigh sensitivityMicrobiological testing/measurementBlood plasmaExon
The invention discloses a high-flux detection method of a circulating DNA liver cancer driver gene. According to the method, a mutant gene which is the most common of the existing liver cancer is selected and combined with a second-generation high-flux sequencing technology to more comprehensively detect a gene mutant condition of the liver cancer. Since a blood plasma sample of a patient is adopted, the method can greatly reduce the detection injury of a patient; secondly, the whole-exome detection is performed on 75 liver cancer genes, the method is more comprehensive compared to the detection method based on real-time PCR and first-generation Sanger sequencing technology; and since only 75 genes are detected, the sample can be detected in a greater sequencing depth, so that the detection precision can reach 0.1 percent. Therefore, a reference can be provided for more and better individual precision therapeutic schemes of the patient.
Owner:JIAXING YUNYING MEDICAL INSPECTION CO LTD
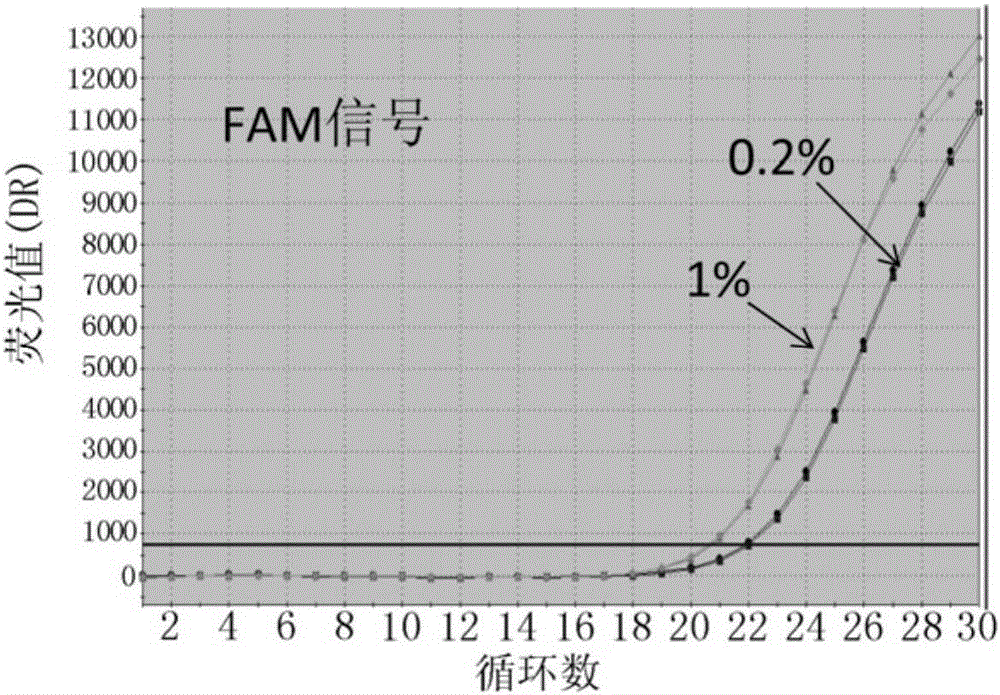
Probes, primers, detection system and kit for detecting mutations of EGFR gene
ActiveCN105349654AHigh sensitivityMeet the actual needs of rapid detectionMicrobiological testing/measurementDNA/RNA fragmentationEGFR Gene MutationBlood plasma
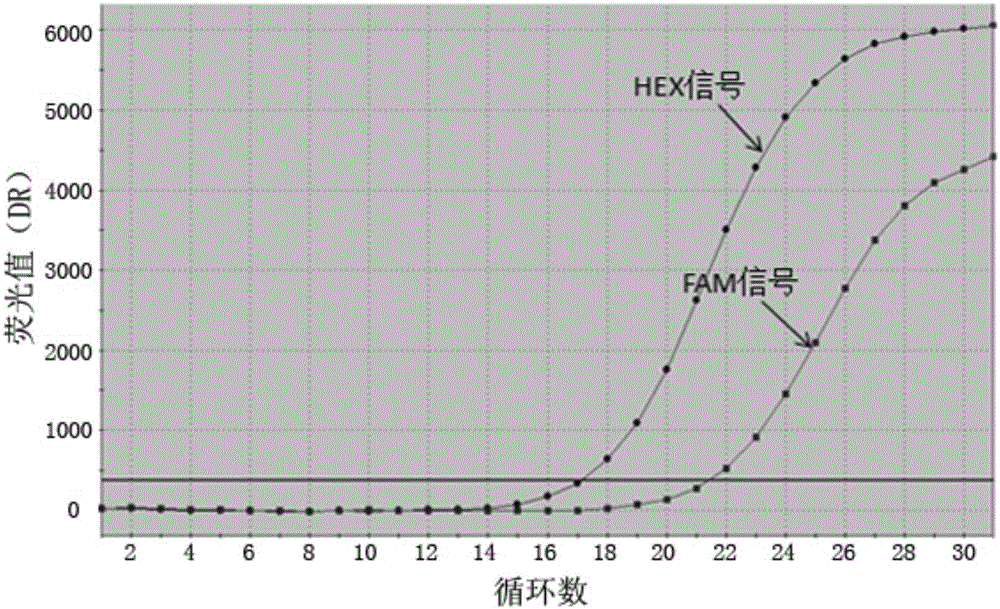
The invention discloses probes, primers, a detection system and a kit for detecting mutations of an EGFR gene, and the probes and the primers have the sequences of SEQ ID NO.1 to SEQ ID NO.24. The invention is characterized in that (1), the amplification efficiency is greatly improved to a largest extent; (2) the sensitivity is high, and the detection sensitivity can reach 0.2%; (3) compared with a digital PCR method, operations are simple, the cost is saved, and the clinical application scope is wide; (4) plasma samples with large reaction volume are detected, the DNA sampling quantity of the plasma samples becomes larger, the system is more stable, and the detection rate of the plasma samples is increased; (5) 25 mutations of the EGFR gene can be detected simultaneously in three reaction tubes, and results are intuitive and clear; (6) the detection speed is fast, and consumed time is only 1 / 2 that of the digital PCR; and (7) a detection method can detect peripheral blood samples, has convenient sampling, and can dynamically monitor curative effect of EGFR-TKI drugs on patients.
Owner:AMOY DIAGNOSTICS CO LTD
Method of detecting chromosomal abnormalities
The invention relates to a method of detecting chromosomal abnormalities, in particular, the invention relates to the diagnosis of fetal chromosomal abnormalities such as trisomy 21 (Down's syndrome) which comprises sequence analysis of cell-free DNA molecules in plasma samples obtained from maternal blood during gestation of the fetus.
Owner:PREMAITHA LTD
Method to assay coenzyme Q10 in blood plasma or blood serum
InactiveUS7303921B2Component separationMicrobiological testing/measurementPara-BenzoquinoneCoenzyme M
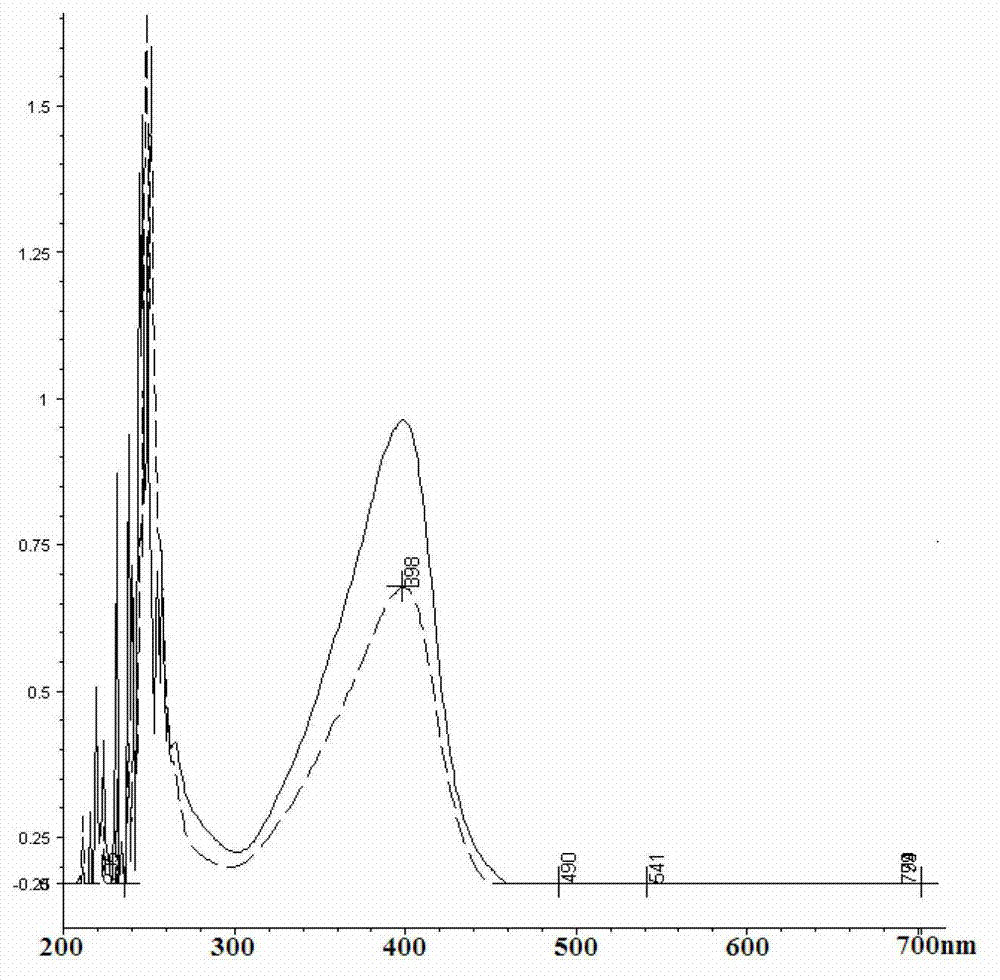
A method is described for determining CoQ10 concentrations in plasma samples. CoQ10 in the plasma sample is oxidized by treating the sample with an oxidizing agent having a redox potential higher than the redox potential of CoQ10, such as, for example, para-benzoquinone. Following oxidation of the CoQ10, the CoQ10 in the plasma sample is extracted with an alcohol, such as, for example, 1-propanol. The alcohol extract is analyzed using direct injection into the HPLC apparatus. This method achieves a rapid, accurate analysis of plasma CoQ10 levels, which can be used for monitoring the bioavailability of orally administered CoQ10 used as a food supplement or as an adjunctive therapy.
Owner:LITTARRU GIAN PAOLO +3
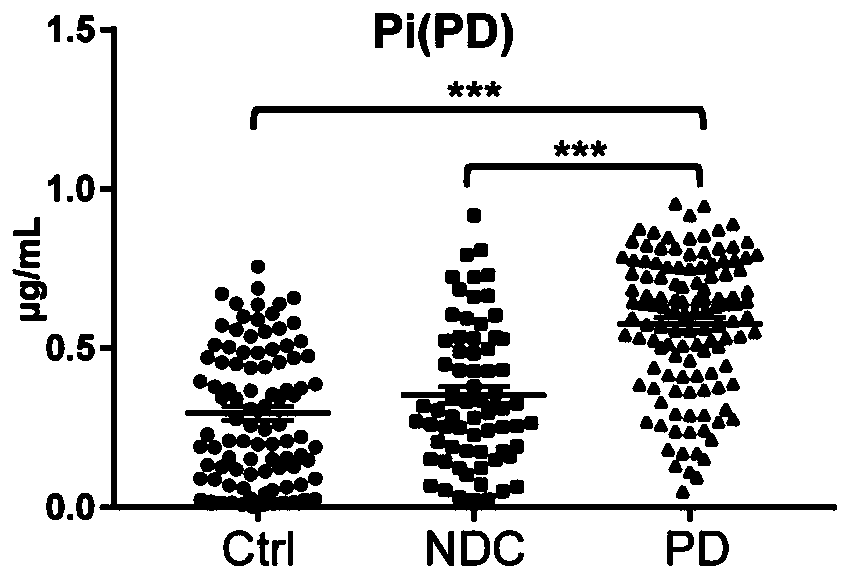
Combined marker and detection kit for diagnosing Parkinson's disease
The invention relates to new application of small molecular metabolites caffeine, creatinine, eicosamide, phenylacetylglutamine, decanoic acid and indolelactic acid in a plasma sample as combined markers in preparation of a kit for diagnosing Parkinson's disease patients in subjects. The invention also relates to the kit for detecting the Parkinson's disease patients in the subjects, and the kit is used for calculating the variables of the combined markers based on a binary logistic regression equation by detecting the respective relative concentrations of the combined markers in the plasma from the subjects, and judging whether the subjects suffer from Parkinson's disease or not based on determined intercept values. The kit can realize high-sensitivity detection of several metabolites involved in the invention, and has the characteristics of low detection cost and good repeatability. The combined use of the small molecule metabolites can be used for assisting the clinical diagnosis ofthe Parkinson's disease, and has higher development and application values.
Owner:FIRST AFFILIATED HOSPITAL OF DALIAN MEDICAL UNIV
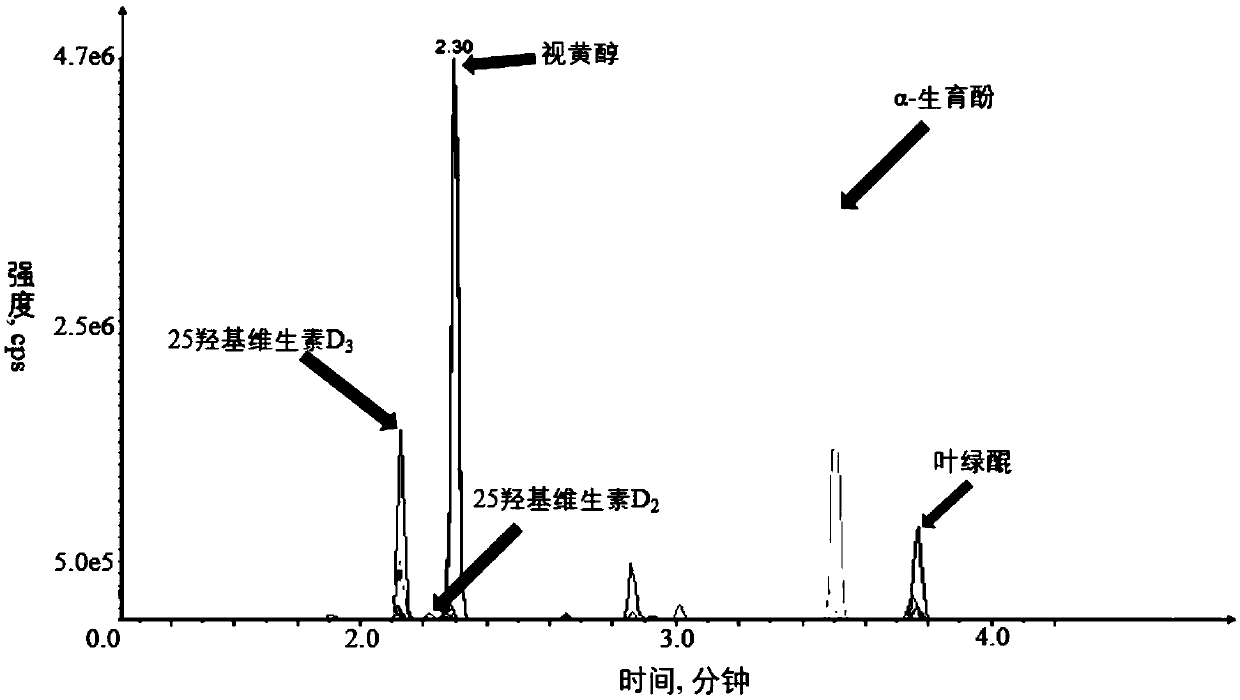
Kit and method for quantitatively detecting five fat-soluble vitamins in blood plasma
PendingCN110763788AFast contentRapid quantitationComponent separationFluid phaseMass Spectrometry-Mass Spectrometry
The invention discloses a kit and method for quantitatively detecting five fat-soluble vitamins in blood plasma. The kit comprises a fat-soluble vitamin calibrator, an isotope mixed standard substance, a fat-soluble vitamin quality control substance, an instrument quality control substance and a mobile phase additive. According to the invention, a solid-phase support liquid-liquid extraction method is adopted and is matched with an automatic pipetting station, so a sample pretreatment method is greatly simplified, and the aim of simultaneously and accurately detecting the five fat-soluble vitamins at high flux and high speed is fulfilled by combining a liquid chromatography-tandem mass spectrometry method. According to the invention, during the detection of a peripheral blood plasma sample, five fat-soluble vitamins can be quantified at a time only by about 250 microliters of plasma sample; moreover, due to a fact that the pretreatment method is simple and quick, the operation time issaved, the content of the fat-soluble vitamins in a human body can be effectively diagnosed and detected according to the indexes, and diseases caused by lack or excess of the fat-soluble vitamins canbe prevented and diagnosed in time. Therefore, the kit and method have extremely high application values in clinical, health examination and scientific research.
Owner:SHENZHEN HUADA GENE INST
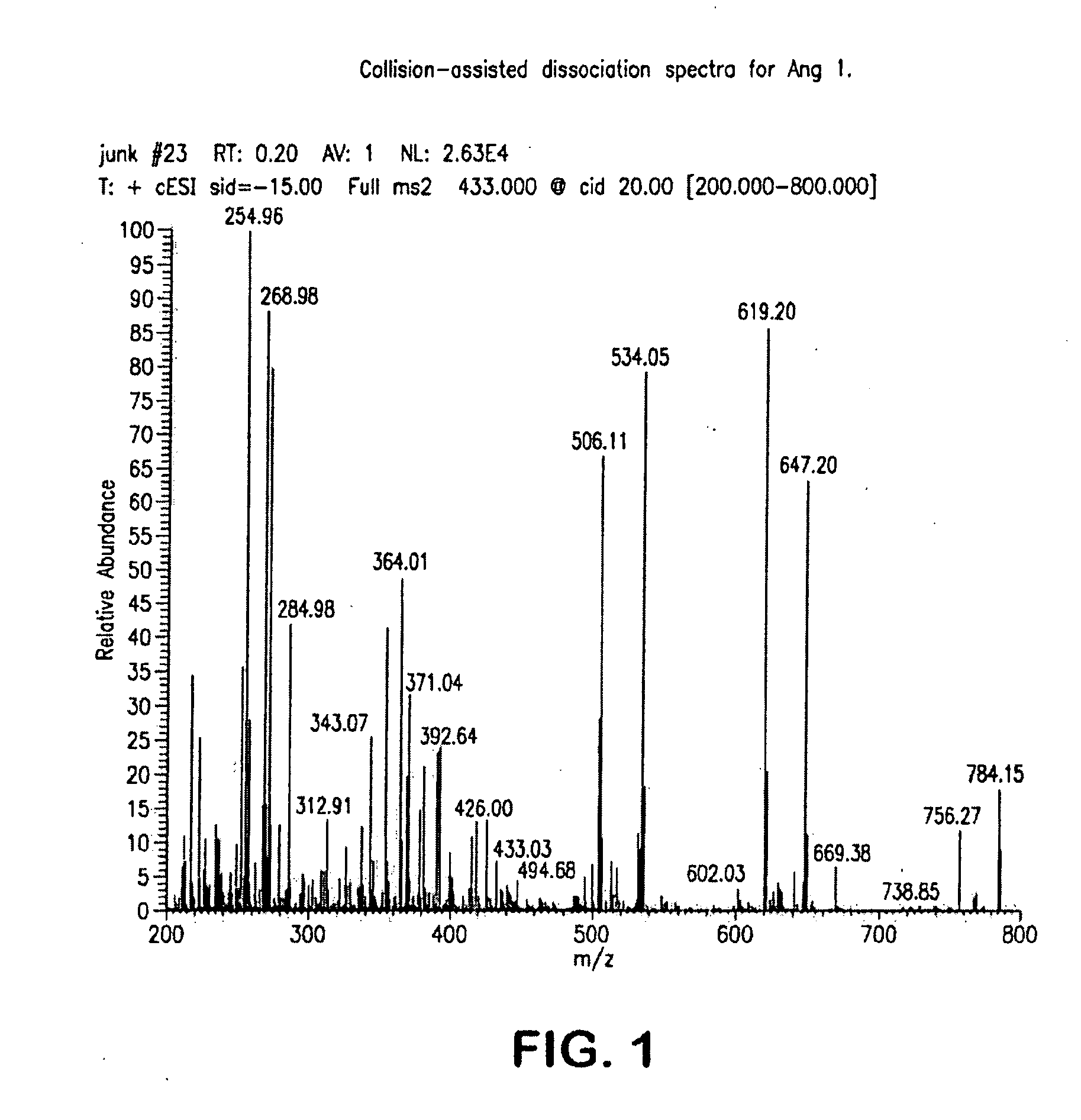
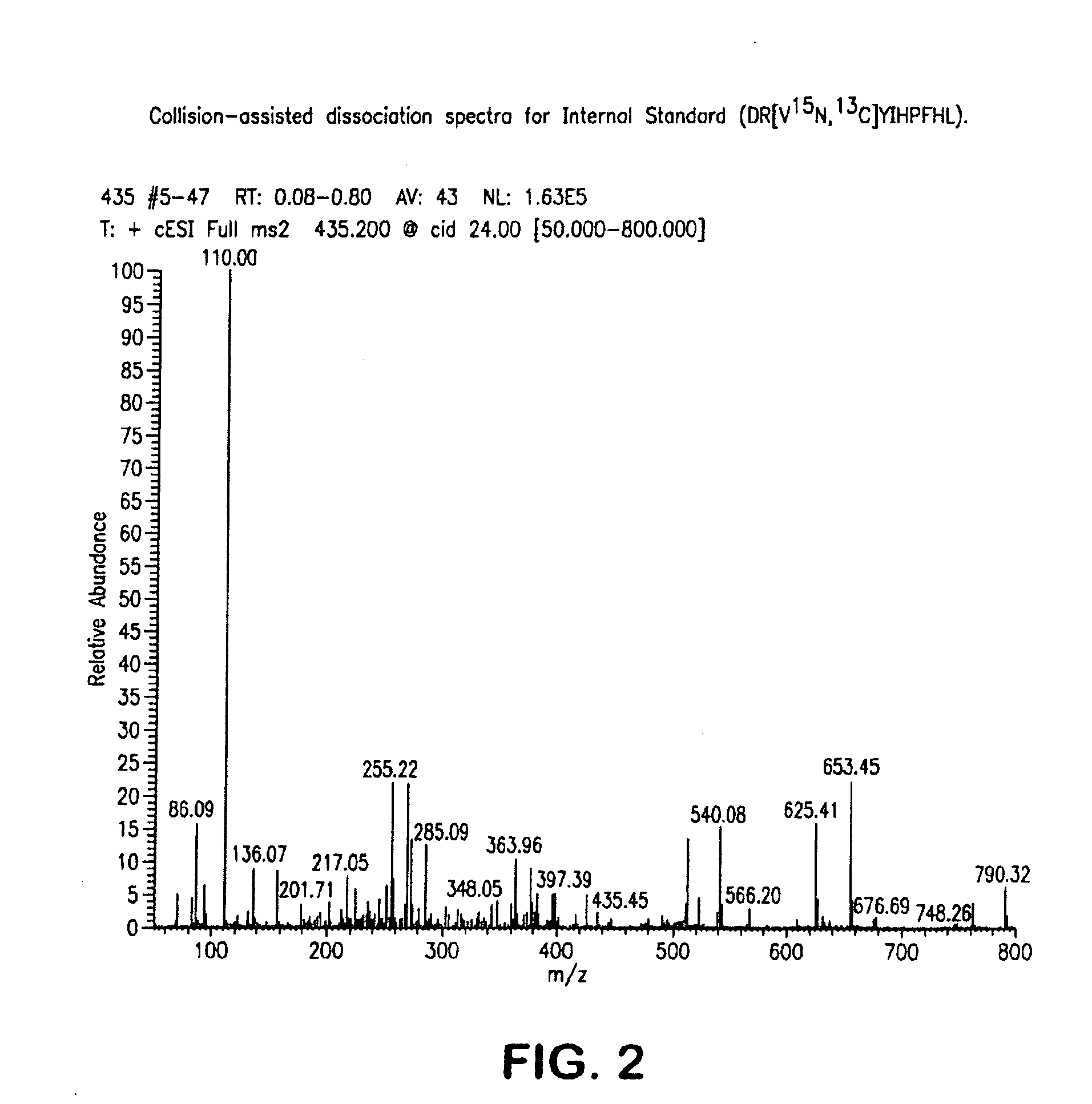
Mass Spectrometry Assay for Plasma-Renin
ActiveUS20100032558A1Avoid condensationSolvent is evaporatedParticle separator tubesBiological material analysisPlasma samplesMass Spectrometry-Mass Spectrometry
Provided are methods for measuring renin activity in a plasma sample using mass spectrometry. The methods generally involve ionizing purified angiotensin 1 from the sample and detecting the amount of angiotensin 1 ions generated. The amount of detected angiotensin 1 ions are then related to the amount of angiotensin 1 generated in the sample, which in turn is related to renin activity in the sample.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
Method for detecting liquid quality of antipsychotic drug in serum or plasma
The invention relates to a method for detecting the liquid quality of an antipsychotic drug in serum or plasma and pretreatment of a specific purification material of the antipsychotic drug. The method comprises the following steps: a serum or plasma sample is subjected to protein precipitation by a certain proportion of organic reagents; a supernatant obtained through centrifugal precipitation ispretreated with a specific purification material to obtain a purified sample solution, the purified sample solution is subjected to liquid chromatography-tandem mass spectrometry detection and analysis, and eight antipsychotic drugs including olanzapine, clozapine, quetiapine, aripiprazole, flupiperidinol, risperidone, pariperidone and ziprasidone in serum or plasma are detected at the same time.The pretreatment of the specific purification material is a mixed solid-phase extraction column taking silica gel as a matrix, and the blood is purified by the specific purification material, so thatthe interference of the matrix in the blood can be removed, the matrix effect of eight antipsychotic drugs can be improved, and the recovery rate of the antipsychotic drugs can be better ensured.
Owner:大连润生康泰医学检验实验室有限公司
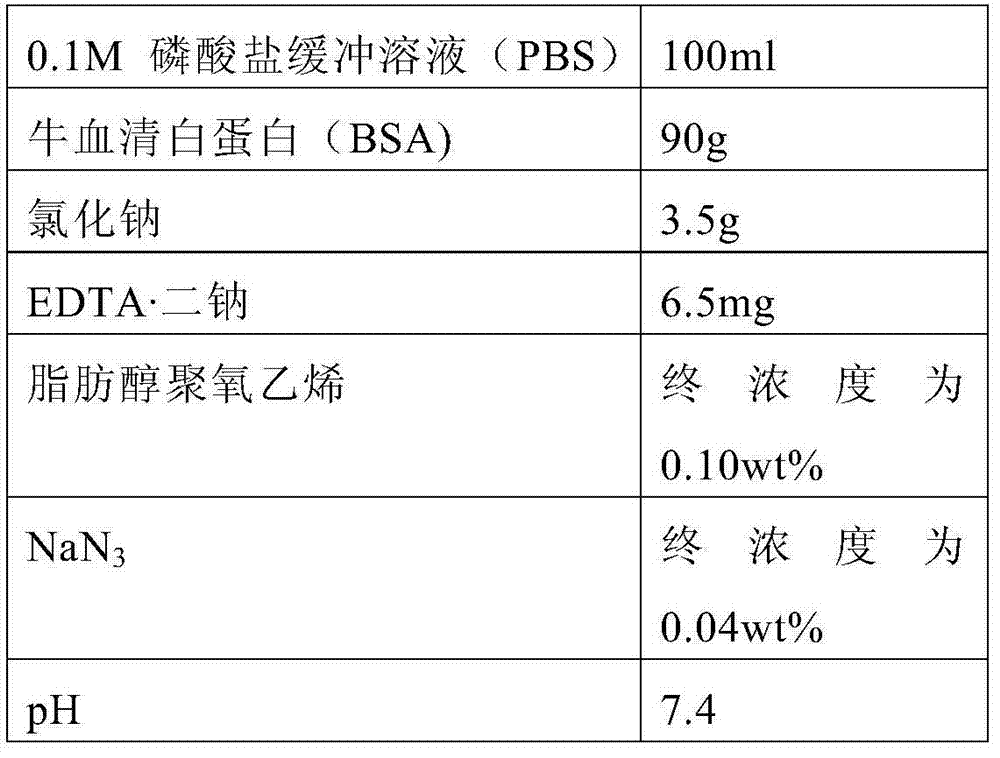
Serum or plasma sample diluent and application thereof
The invention relates to a serum or plasma sample diluent and application thereof. Particularly, the serum or plasma sample diluent comprises a buffer solution (a), albumin (b) with content of 10-100g / L, alkaline chloride (c) and an optional emulsifier (d), wherein the pH value of the diluent is 6.0-8.0. Compared with the existing human negative mixed serum or plasma and calf serum diluent, the diluent provided by the invention has the characteristics of precise and accurate detection result and long-time stable preservation. The invention also discloses a detection kit containing the diluent and application of the diluent in immunoassay.
Owner:AILEX TECH GRP CO LTD +1
Probes, primers and kit for detecting T790M mutation of EGFR gene
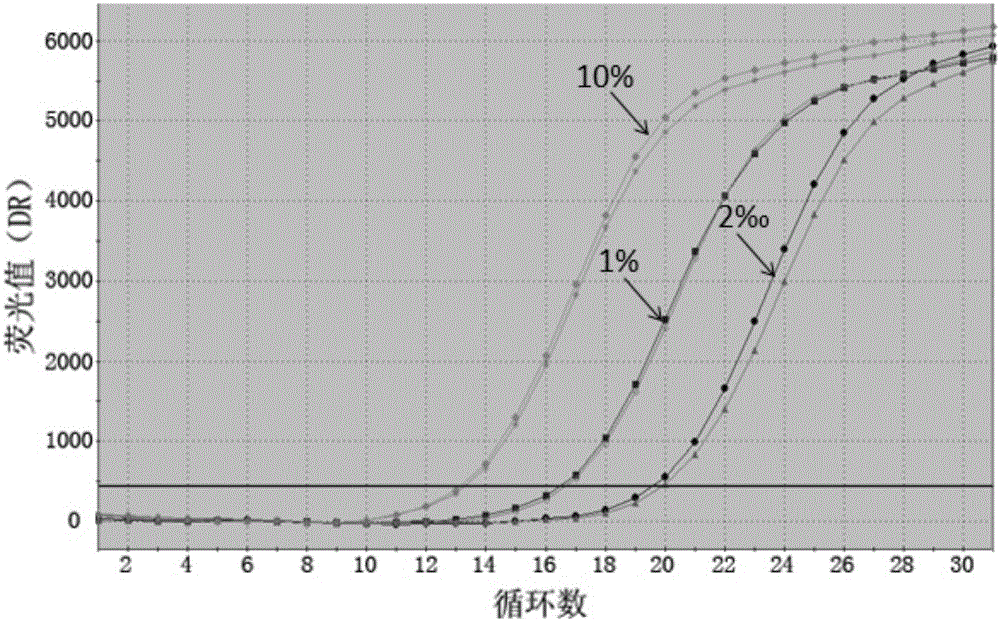
InactiveCN105112544AMeet the actual needs of rapid detectionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationPcr methodBlood plasma
The invention discloses probes, primers and a kit for detecting a T790M mutation of an EGFR gene. The probes and the primers have the following sequence: SEQ ID NO: 01 to SEQ ID NO. 07. The probes, the primers and the kit have the following benefits: (1) SNP sites on the primers are designed as G / A merged basic groups, so that all efficiencies are compatible, and the amplification efficiency is improved; (2) the sensitivity is high, that is, the detection sensitivity can reach 2 permillage; (3) compared with those adopting a digital PCR method, the operation is simple, the cost is reduced, and the clinical application range is wide; (4) through blood plasma sample detection with a large reaction volume, the DNA loading quantity of blood plasma samples is increased, the detection system is more stable, and the detection rate of the blood plasma samples is improved; (5) the detection speed is high, that is, the detection process can be completed within only 120 minutes, and the time consumed in the detection process is only a half of that consumed according to the digital PCR method; (6) the probes, the primers and the kit, provided by the invention, can be utilized for detecting peripheral blood samples, so that convenient sampling and dynamic detection can be realized.
Owner:AMOY DIAGNOSTICS CO LTD
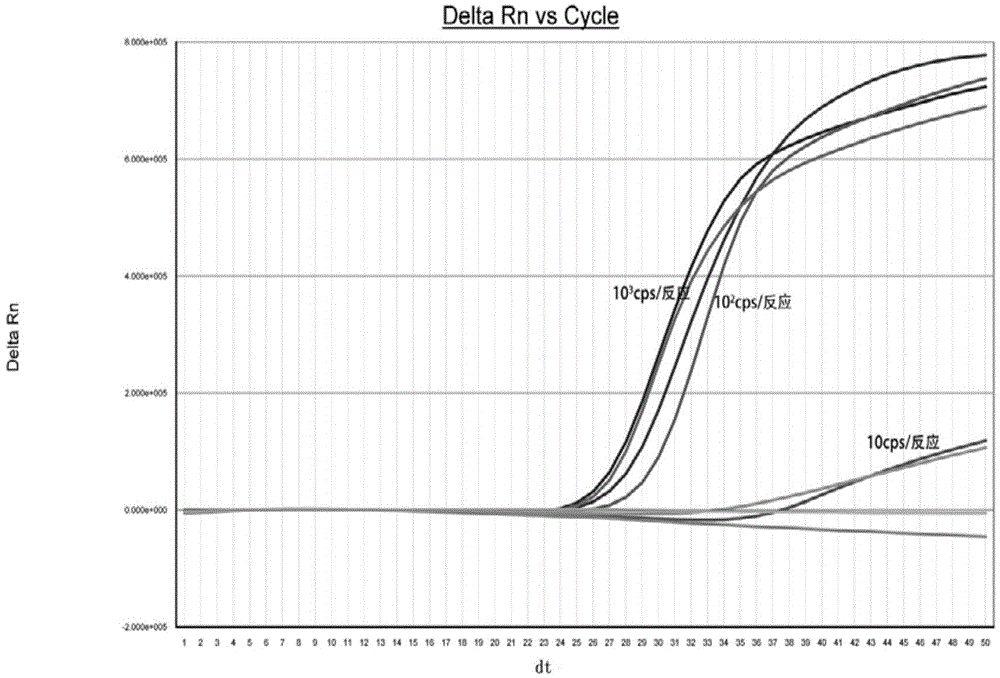
Real-time fluorescence nucleic acid isothermal amplification detection kit for HCV (hepatitis C virus)
ActiveCN105483283AAccurate measurementRapid determinationMicrobiological testing/measurementMicroorganism based processesReverse transcriptaseGenotype
The invention discloses a real-time fluorescence nucleic acid isothermal amplification detection kit for HCV (hepatitis C virus). The detection kit specifically comprises a capture probe, HCV primers, an HCV detecting probe, M-MLV reverse transcriptase, T7RNA polymerase and the like, wherein the HCV primers include a T7 primer and an nT7 primer. By the detection kit which is high in detection efficiency, high in accuracy and promising in application prospect, nucleic acid amplification detection can be performed on serum or plasma samples containing hepatitis C viruses in a high-specificity, high-sensitivity, low-pollution and fast manner, and 6 genotypes of HCV can be detected.
Owner:SHANGHAI RENDU BIOTECH
Screening method of early-stage liver cancer diagnosis markers for people with liver cirrhosis and hepatitis
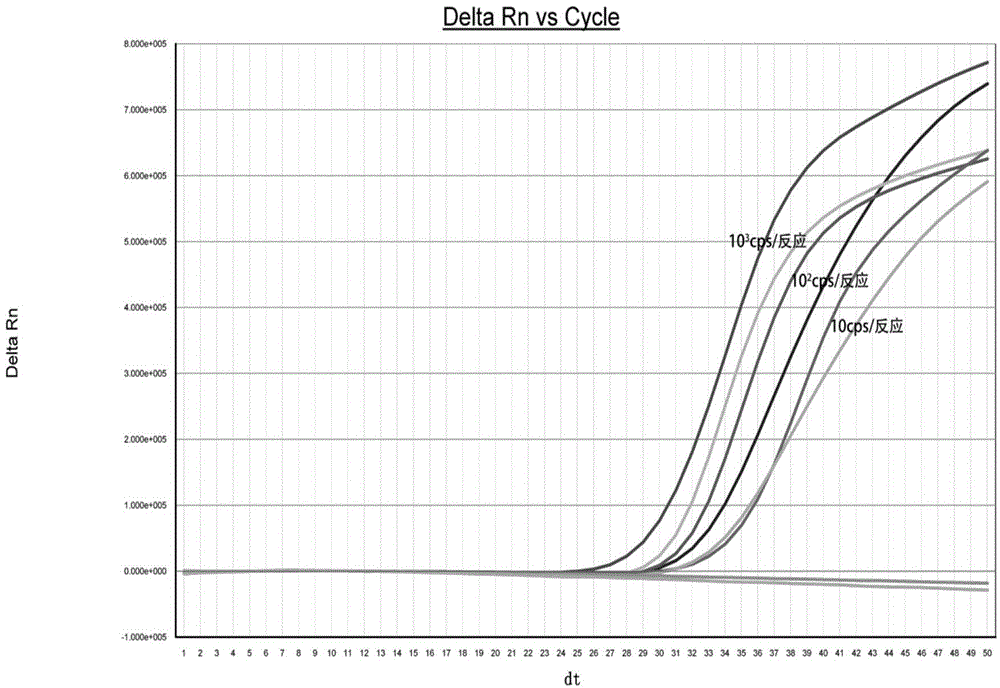
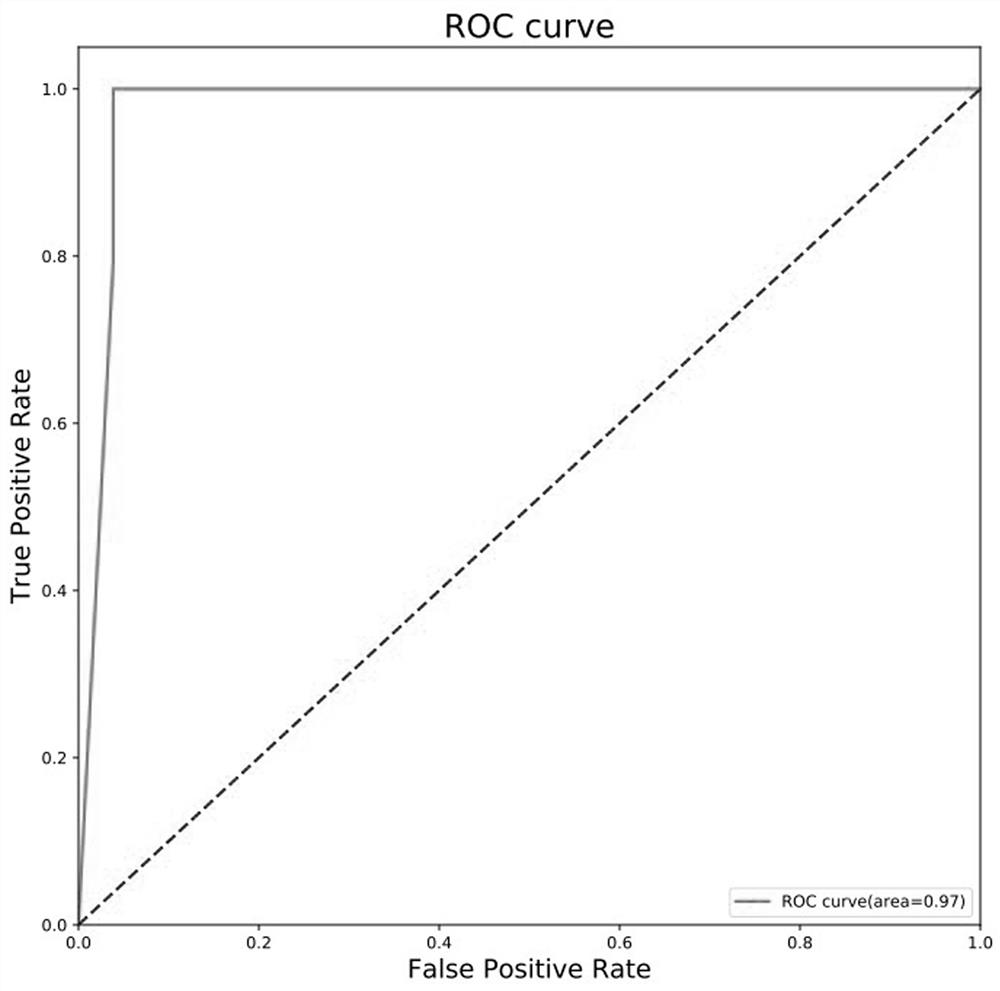
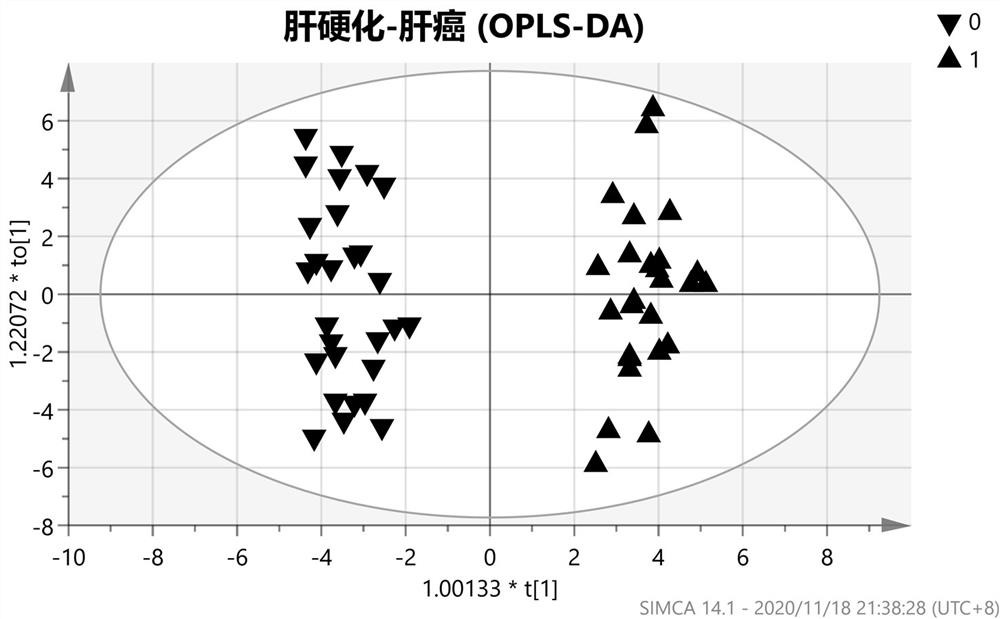
The invention belongs to the technical field of clinical medical diagnosis and relates to a screening method of early-stage liver cancer diagnosis markers for people with liver cirrhosis and hepatitis. The screening method of the early-stage liver cancer diagnosis markers for people with liver cirrhosis and hepatitis liver cirrhosis and hepatitis comprises the following steps of: S1, acquiring data, specifically, collecting a target plasma sample and detecting metabolites in the plasma sample; S2, constructing a discrimination model used for distinguishing people with liver cirrhosis from patients with the primary liver cancer and distinguishing people with the hepatitis from patients with the primary liver cancer; S3, screening markers; and S4, verifying diagnosis capability. According to the screening method of early-stage liver cancer diagnosis markers for people with liver cirrhosis and hepatitis, a high performance liquid chromatography-mass spectrometry system is utilized to obtain the metabolic profiles of blood plasma of patients with liver cirrhosis, hepatitis and hepatocellular carcinoma, a potential tumor metabolic marker group is found, and a diagnosis model is constructed and is used for assisting early diagnosis of tumors of groups with high risk of liver cancer.
Owner:MEI HOSPITAL UNIV OF CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com