Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54 results about "Thrombin generation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
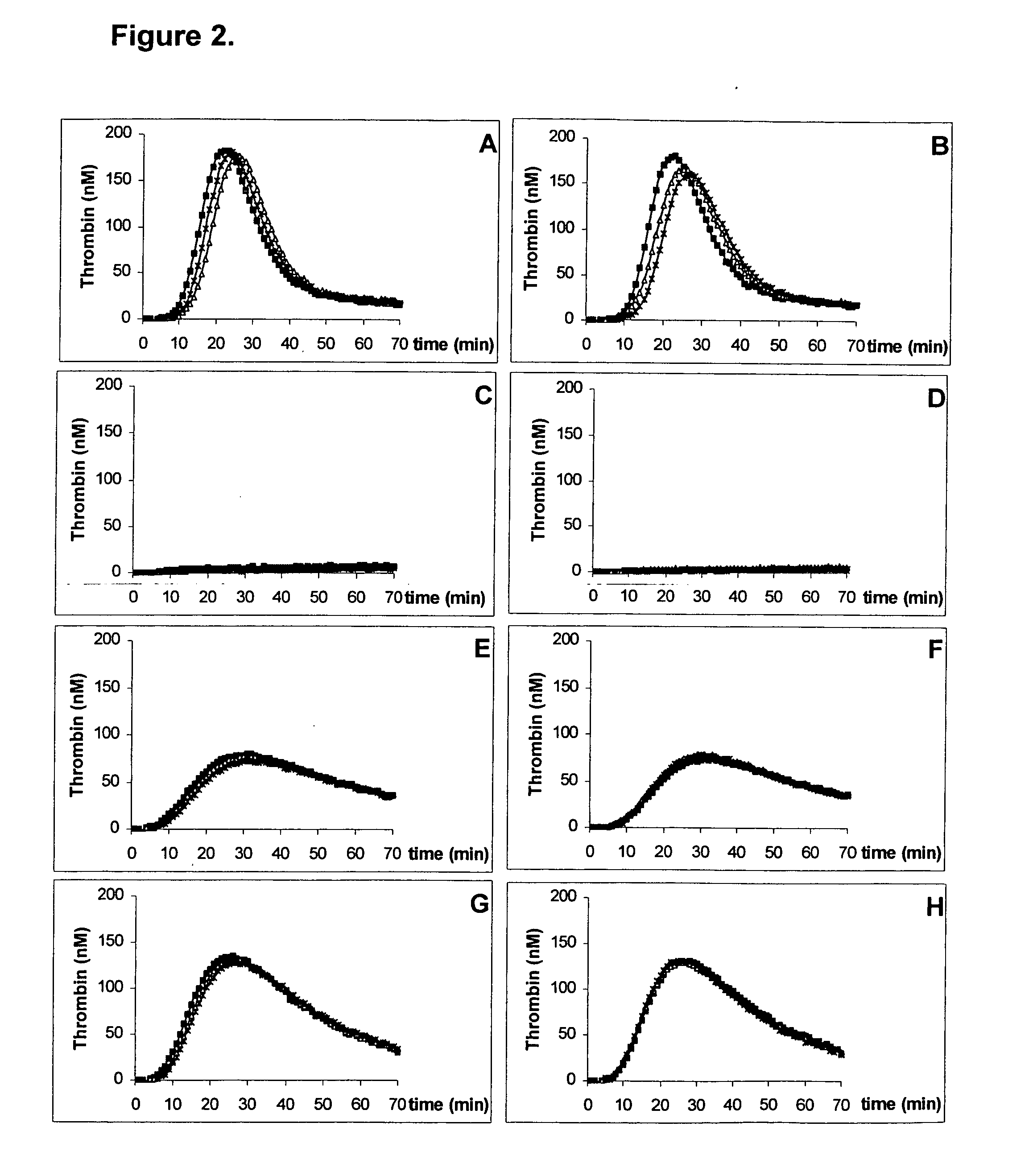
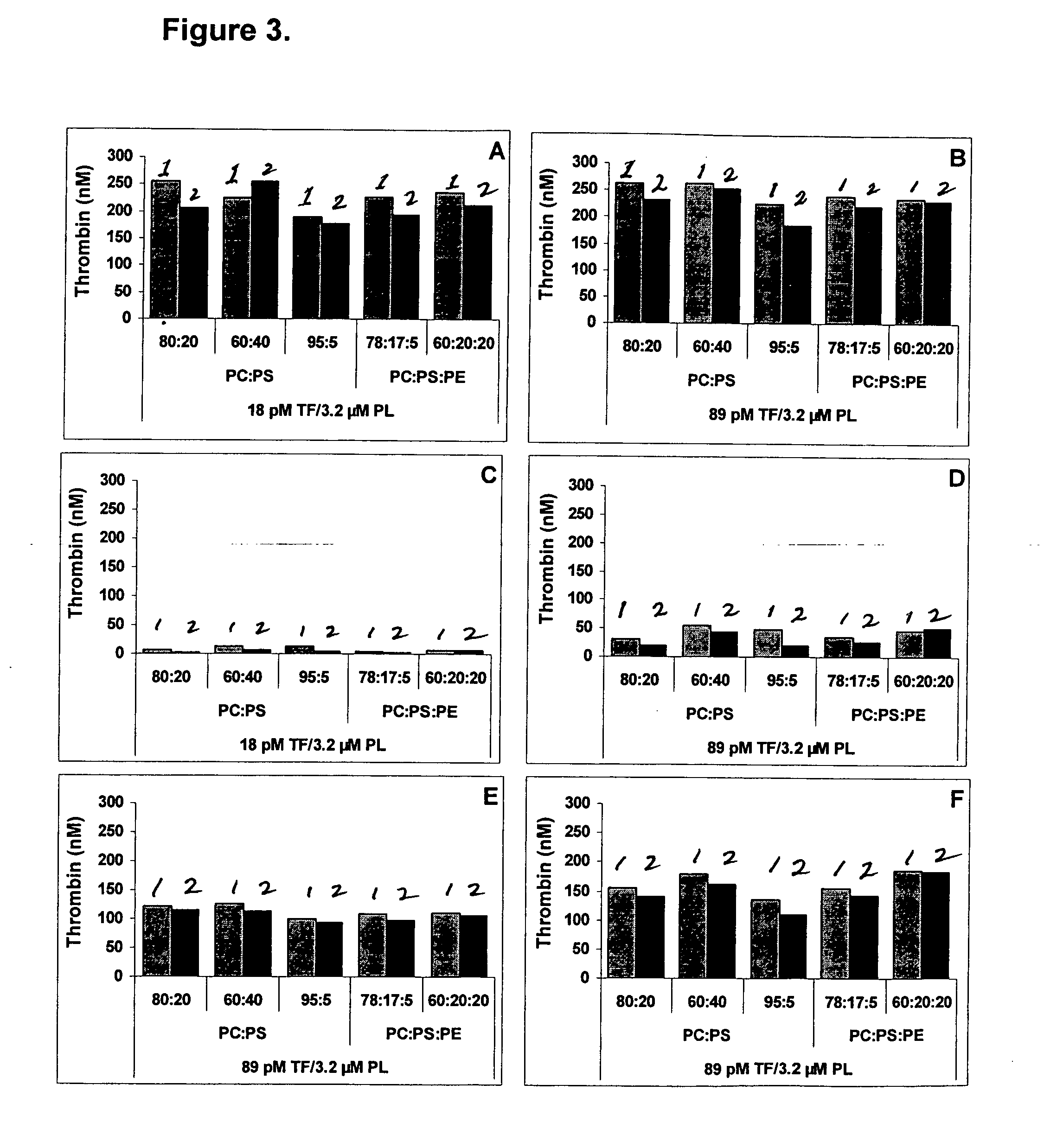
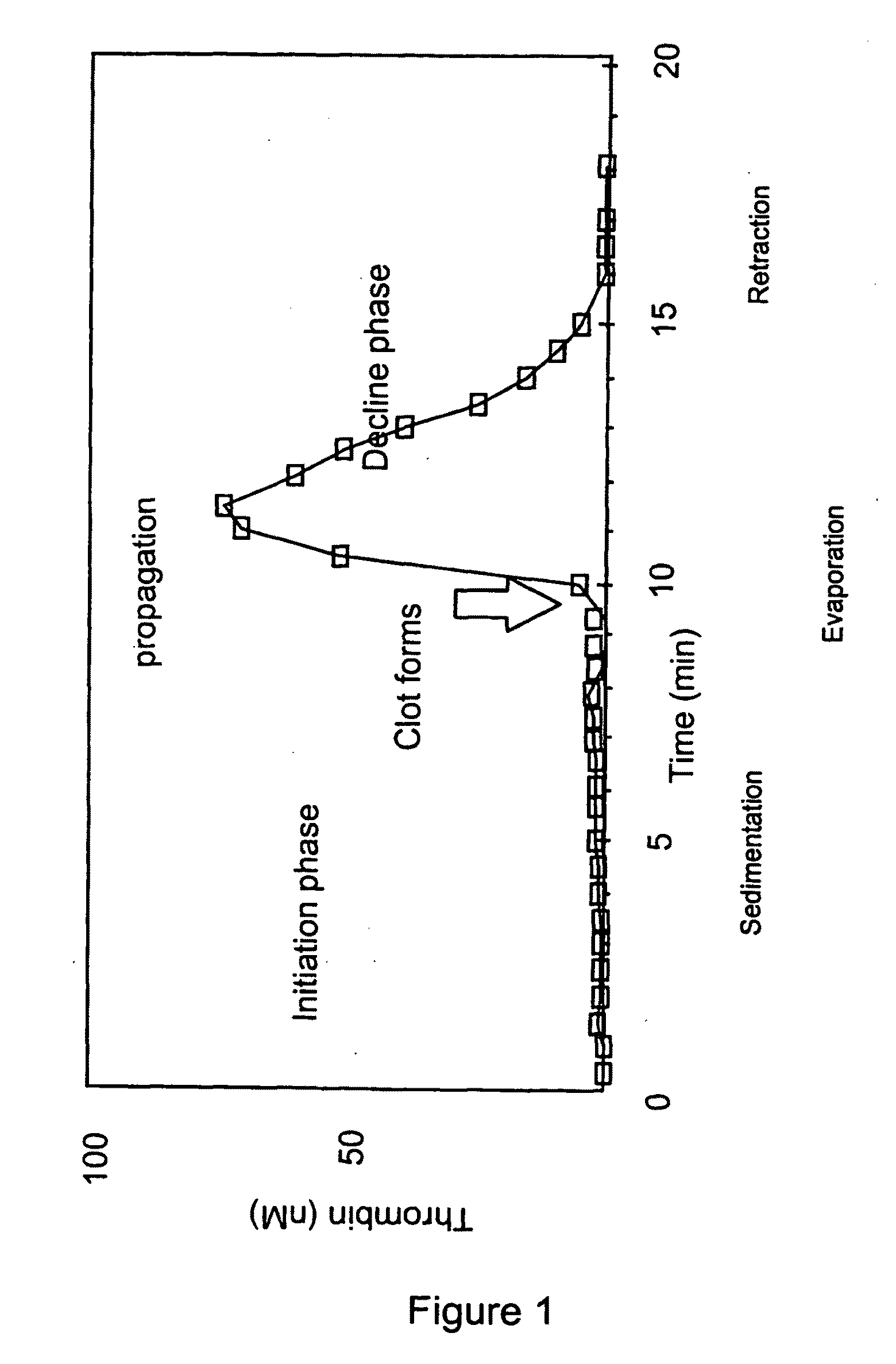
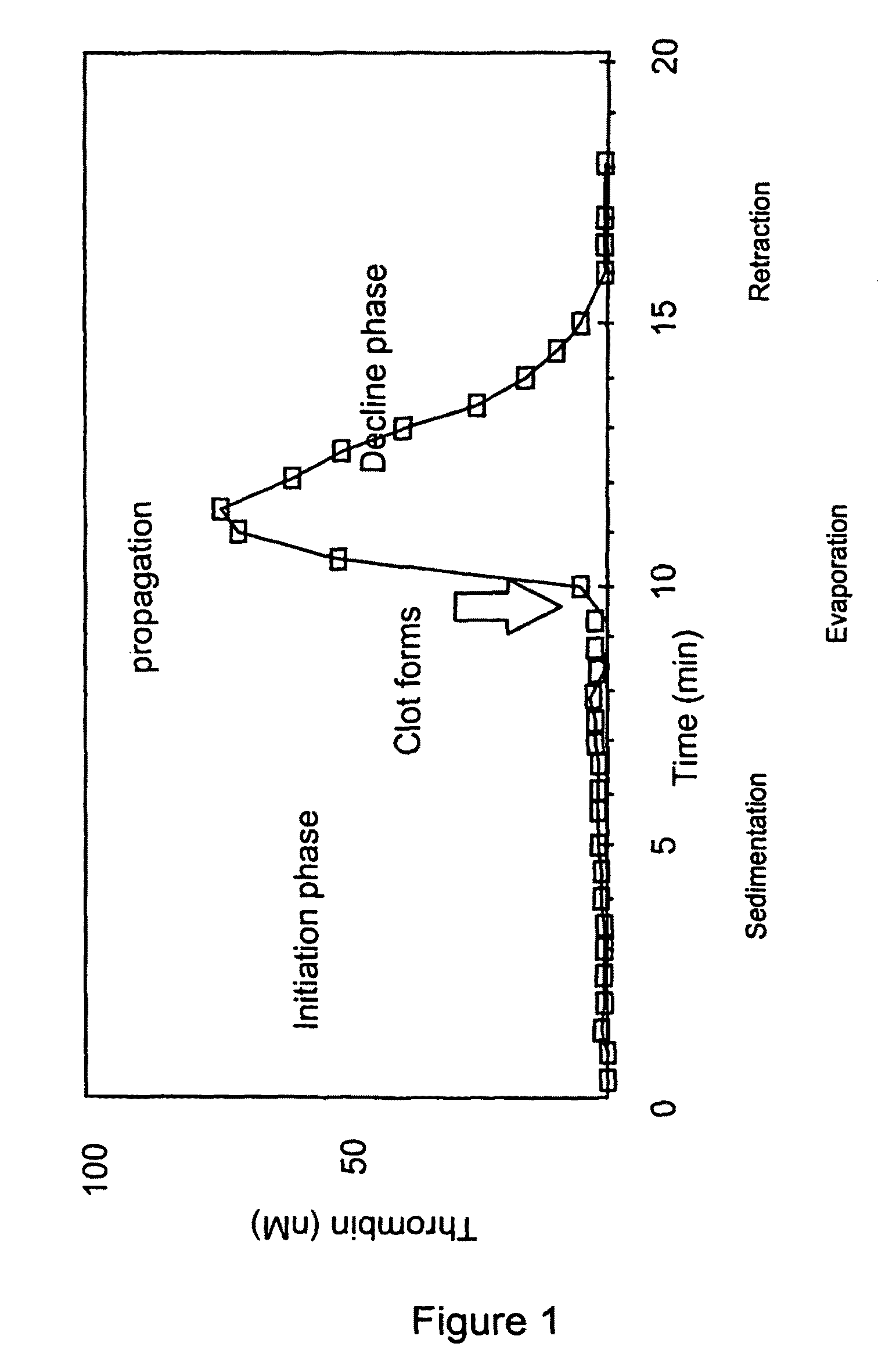
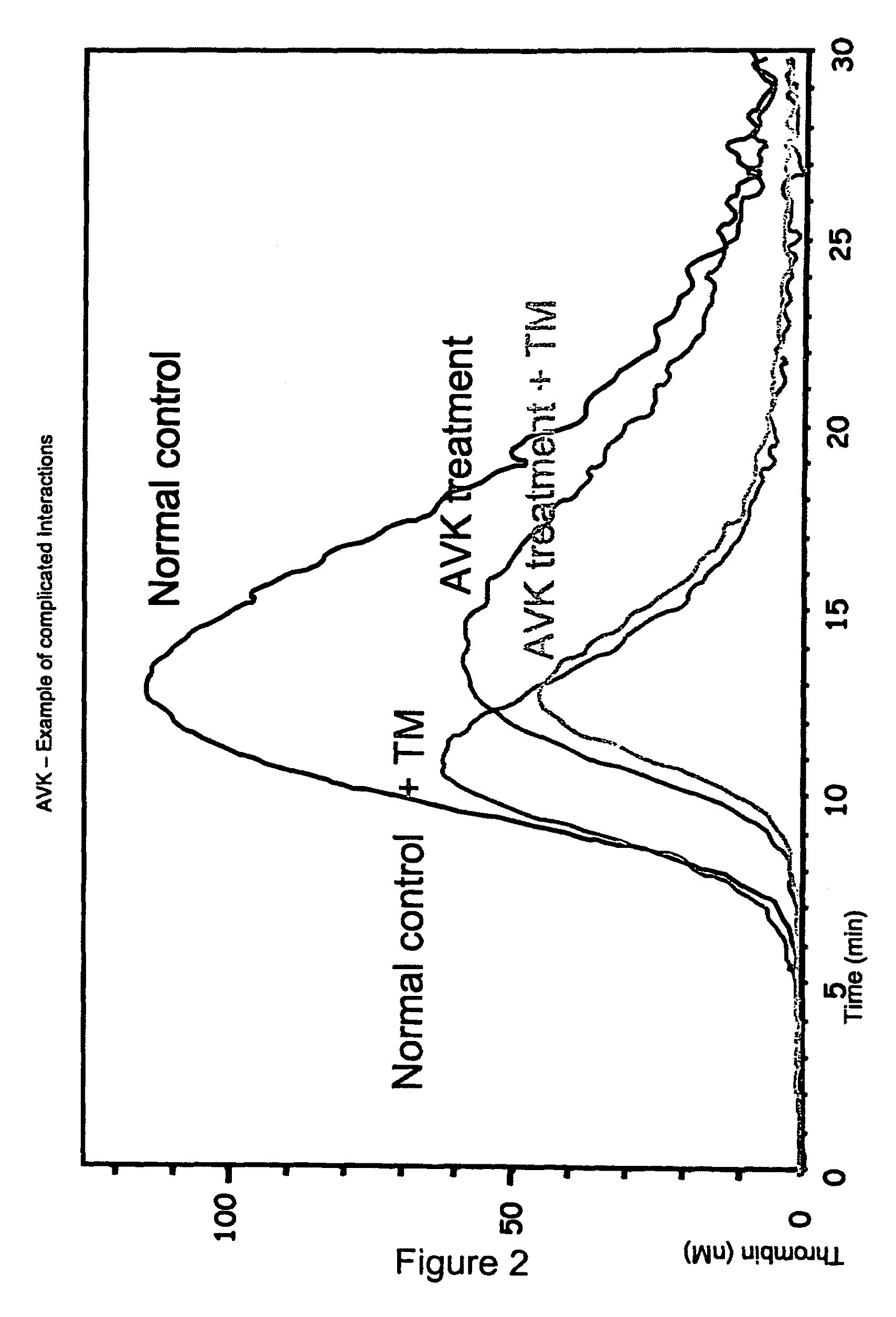
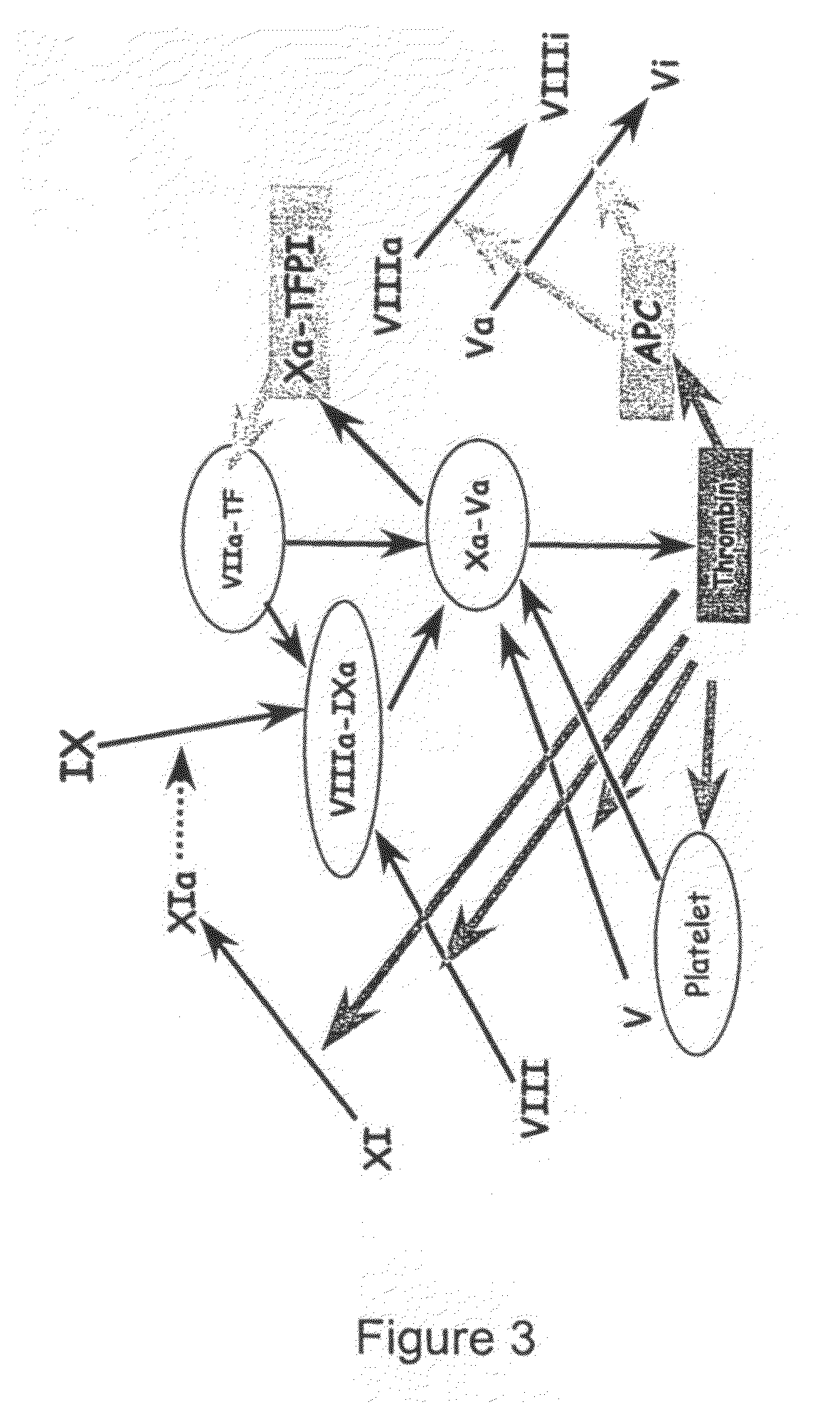
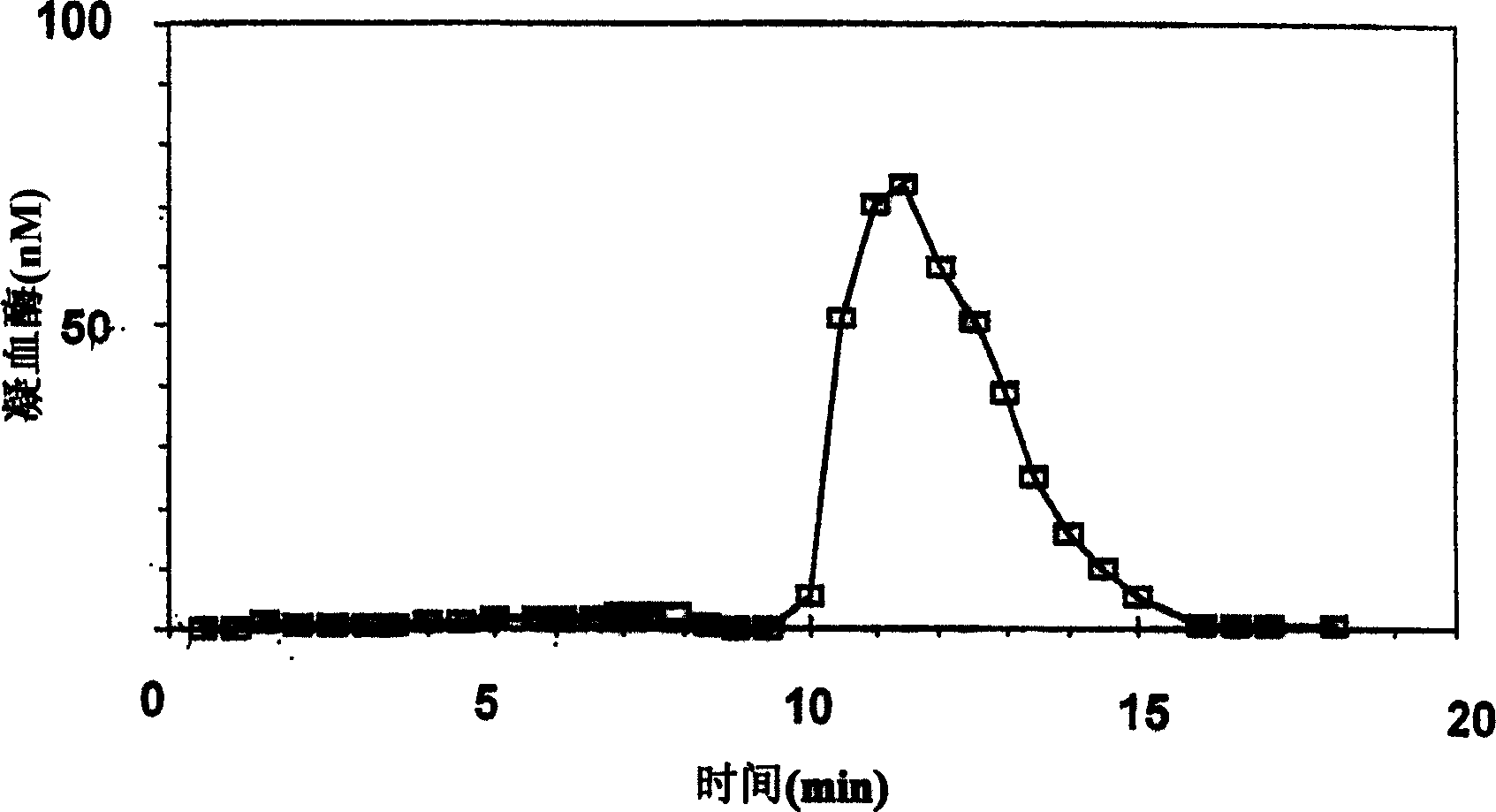
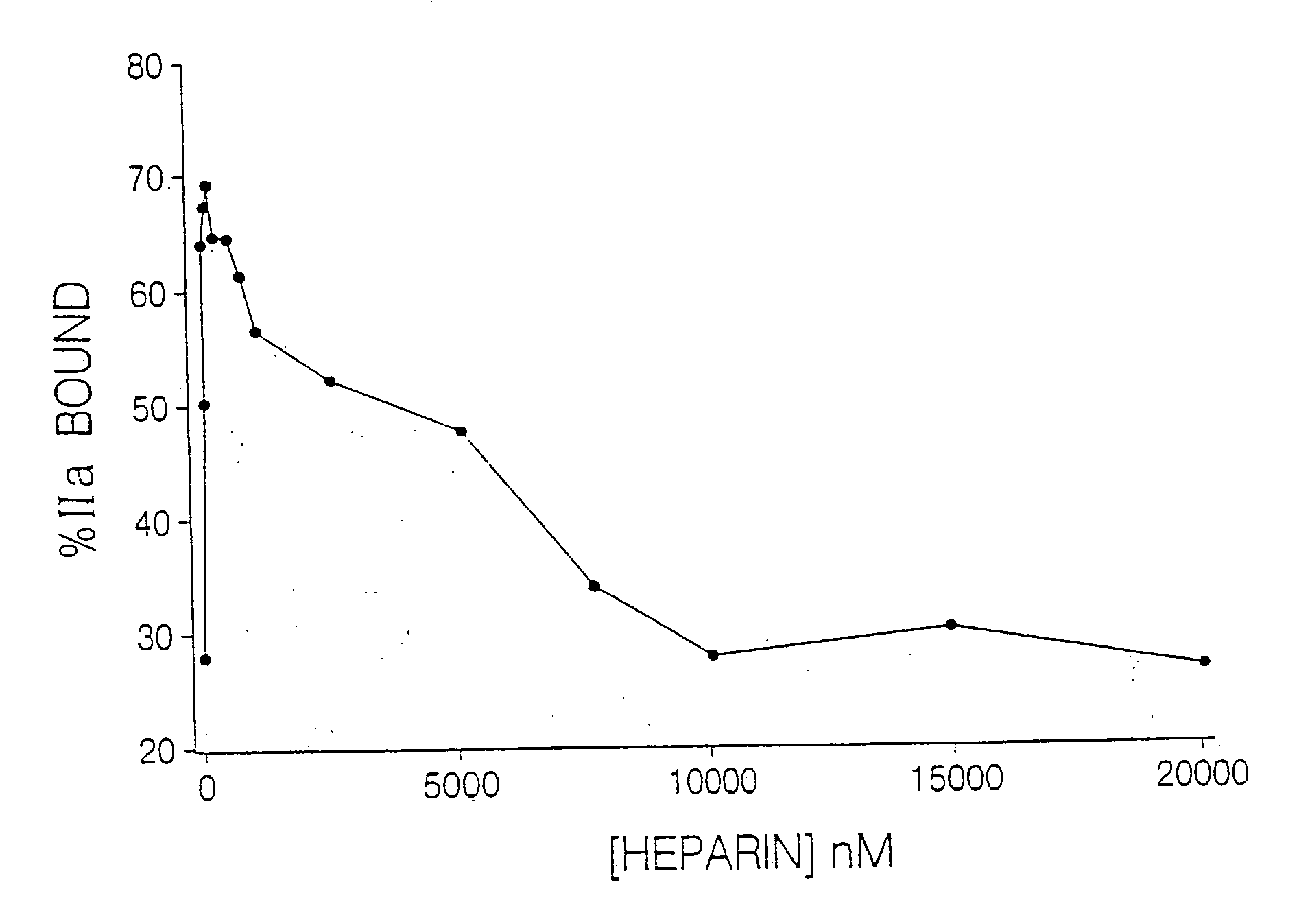
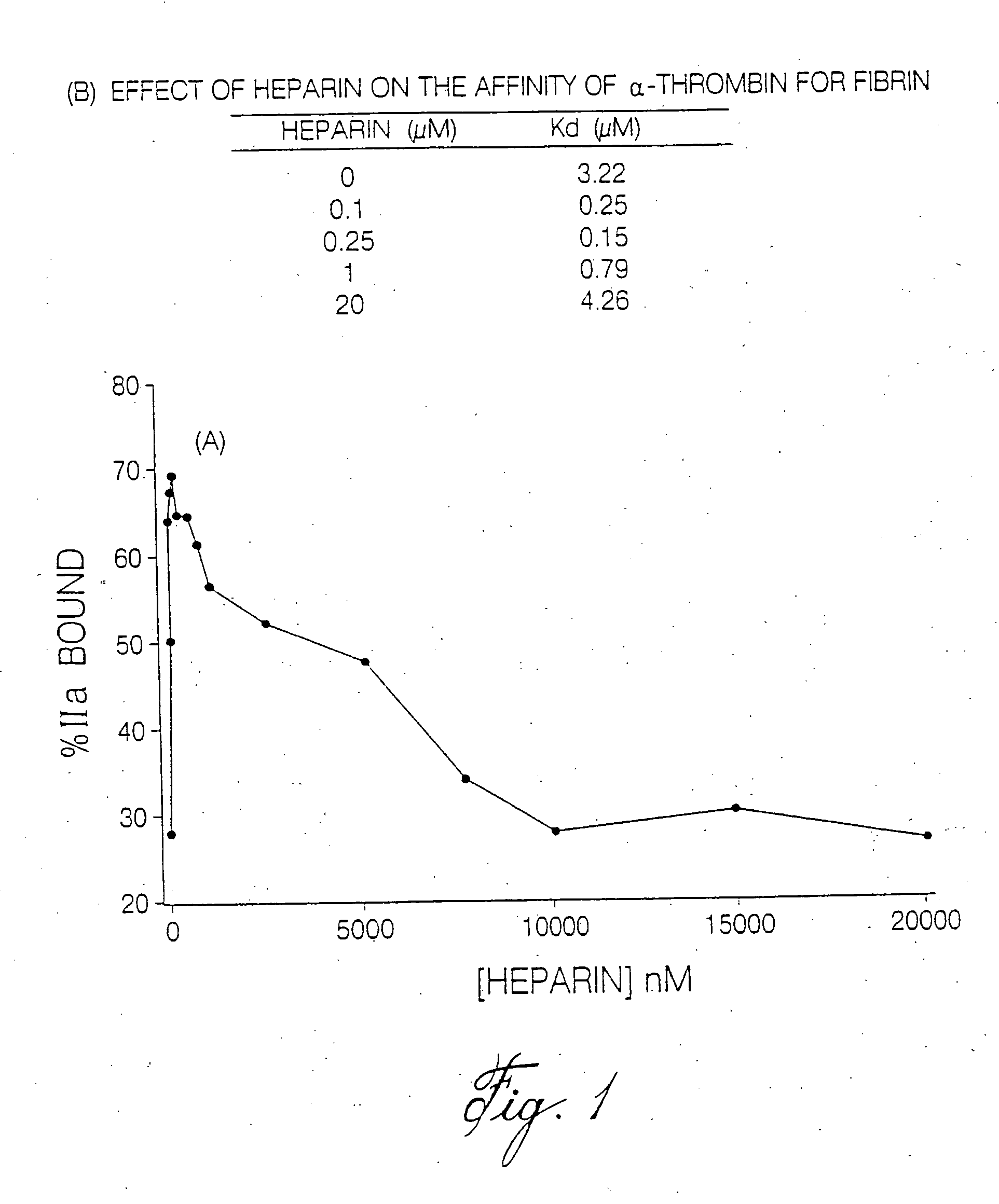
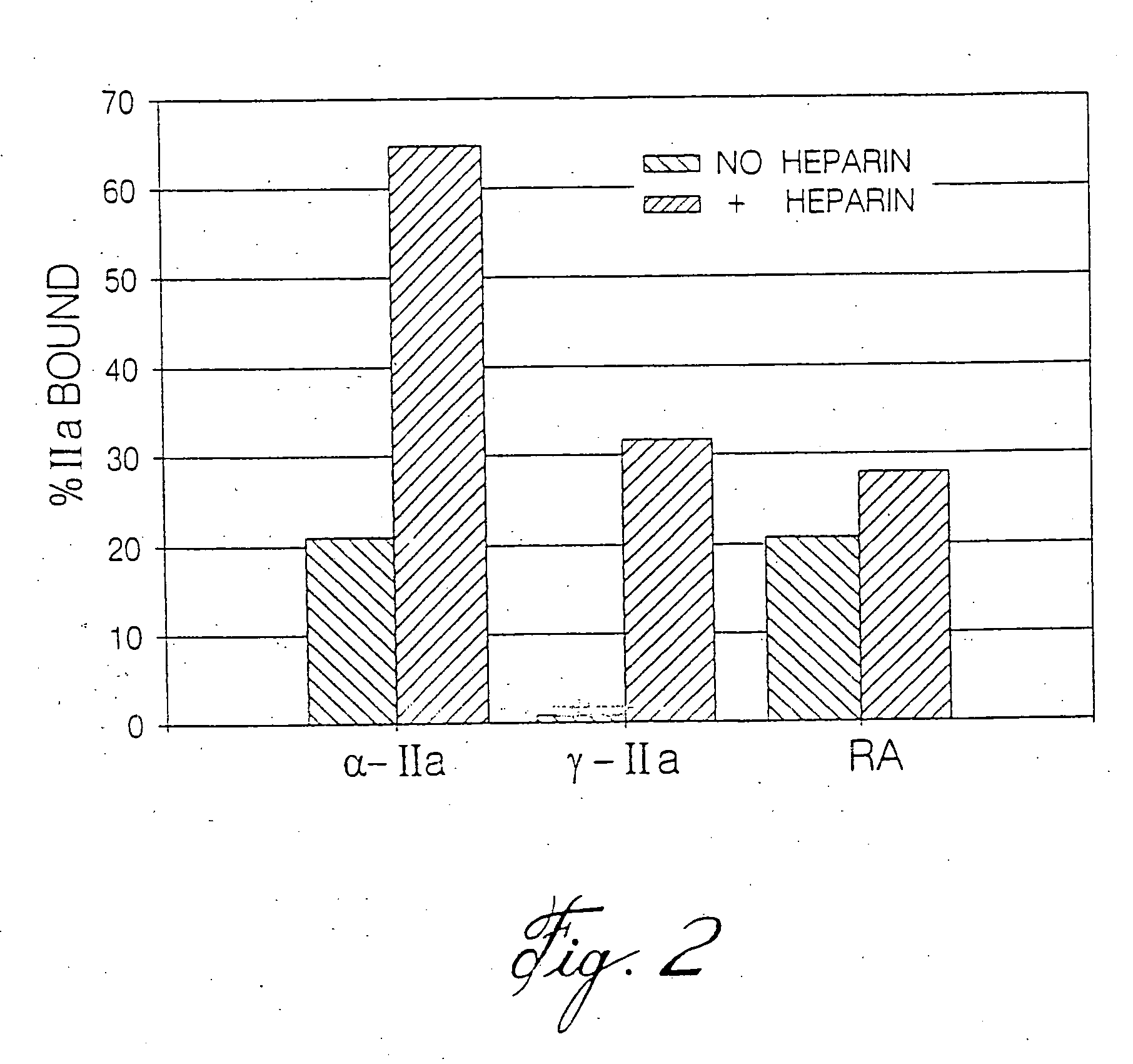
Thrombin Generation (normalized-ETP) Thrombin generation is considered the primary physiological event that initiates clotting. Reduced thrombin generation has been clinically correlated to poor outcomes in hemophilia, liver disease and other coagulopathies.
Inhibition of thrombin generation
The present invention is a method for inhibiting undesirable thrombin generation in a mammal in need thereof. The method comprises administering to the mammal an effective amount of a non-antibacterial tetracycline formulation.
Owner:GALDERMA LAB LP
Onset of force development as a marker of thrombin generation
InactiveUS7202048B2Bioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseClotting factor deficiency
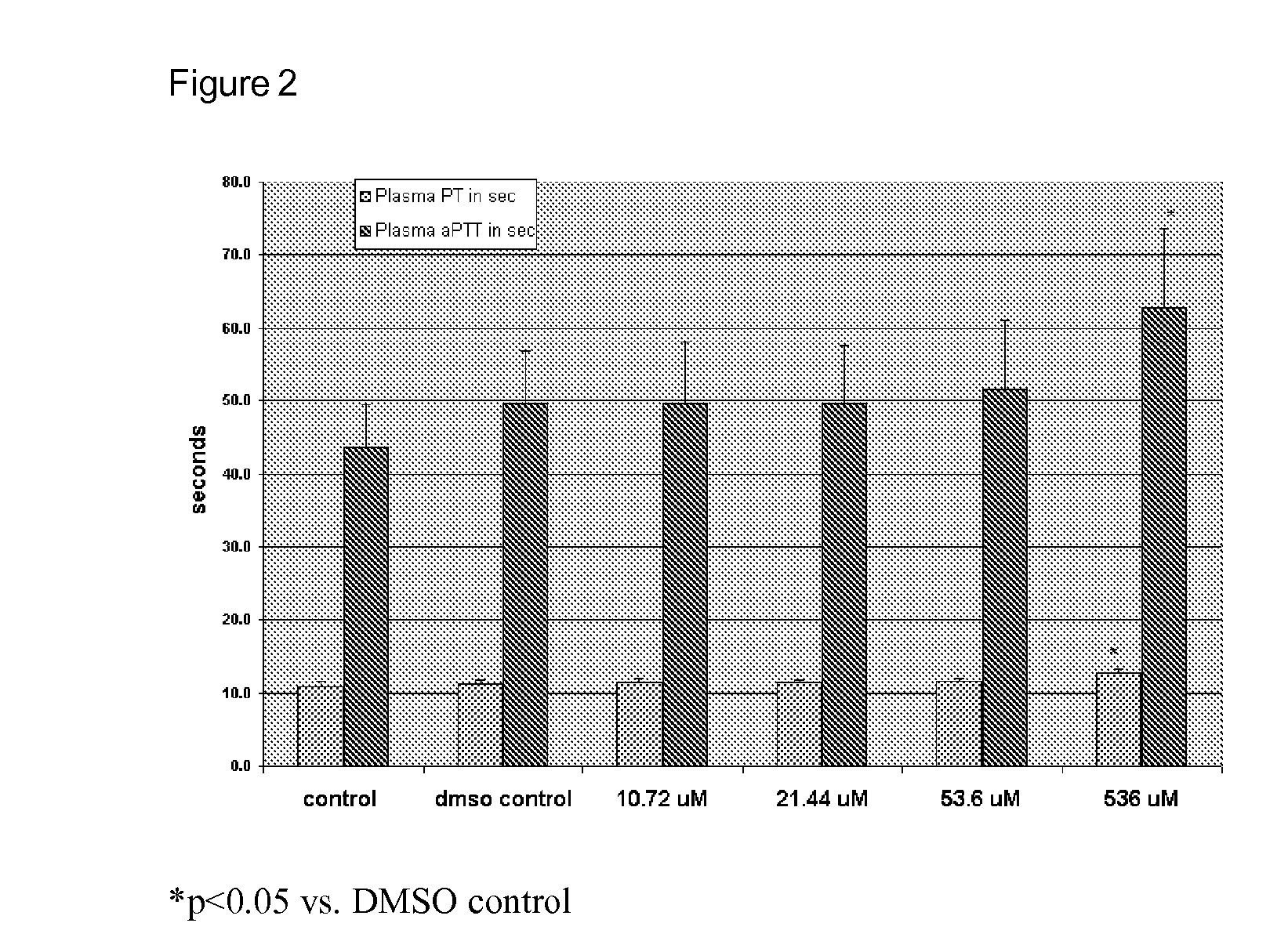
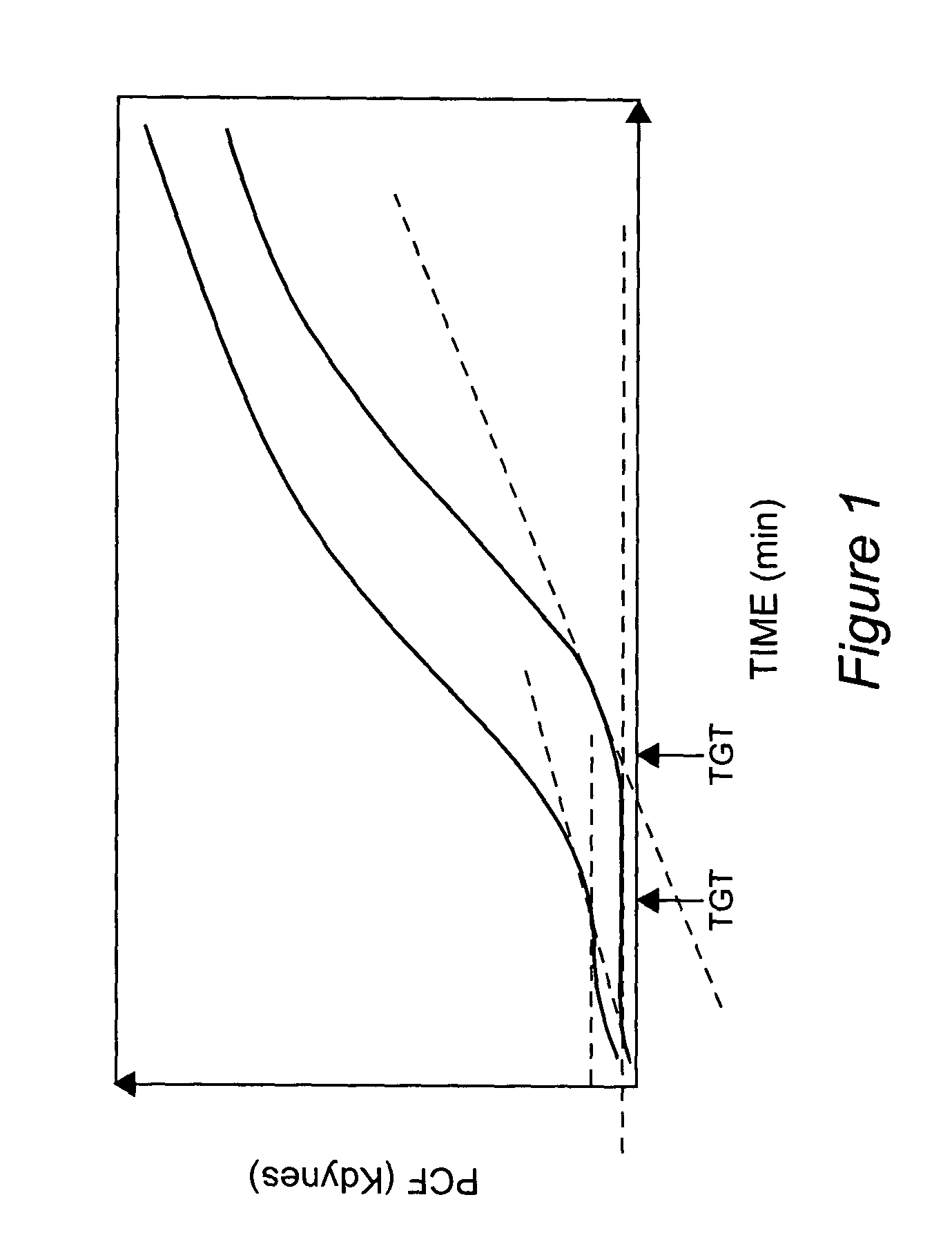
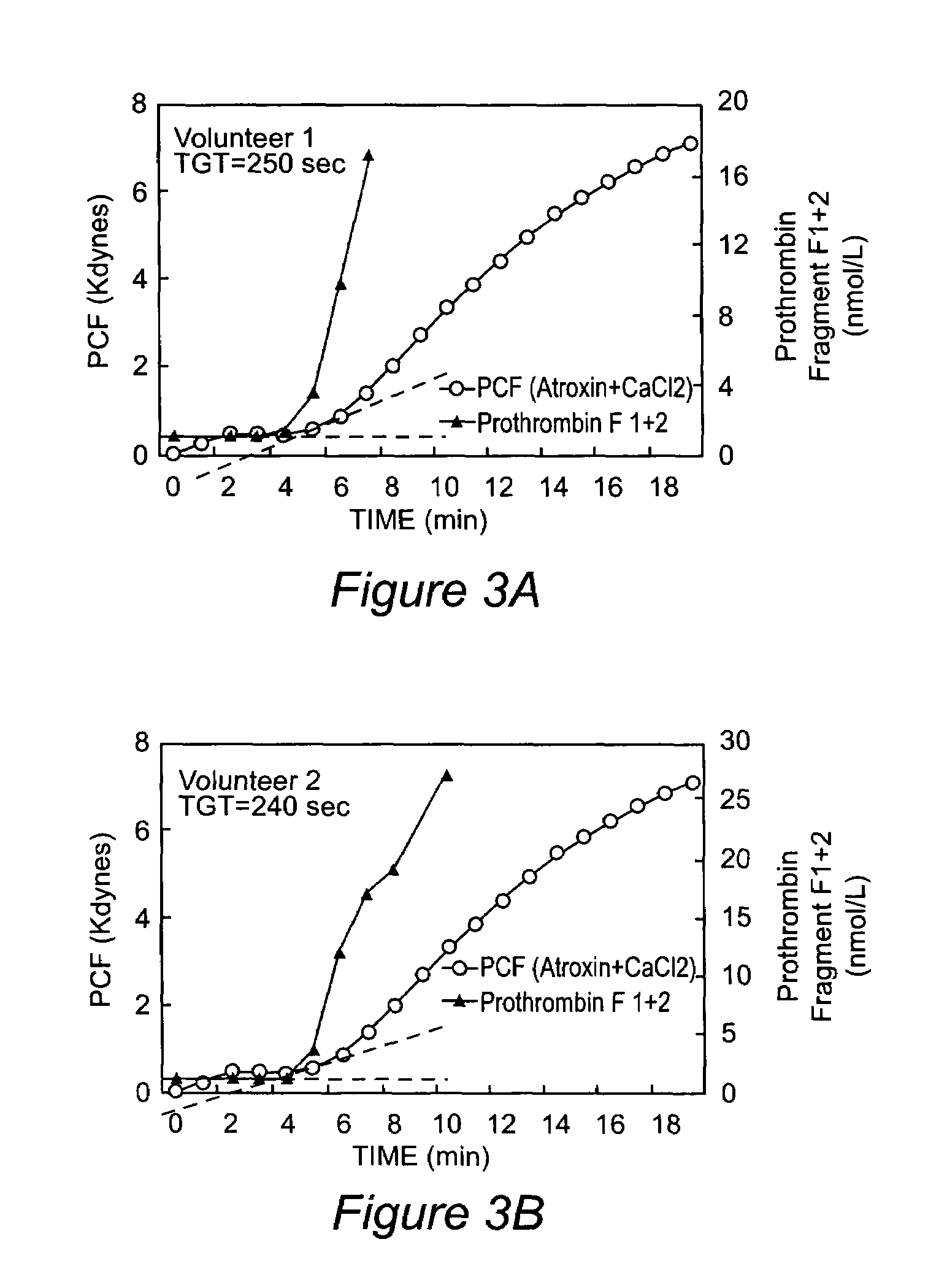
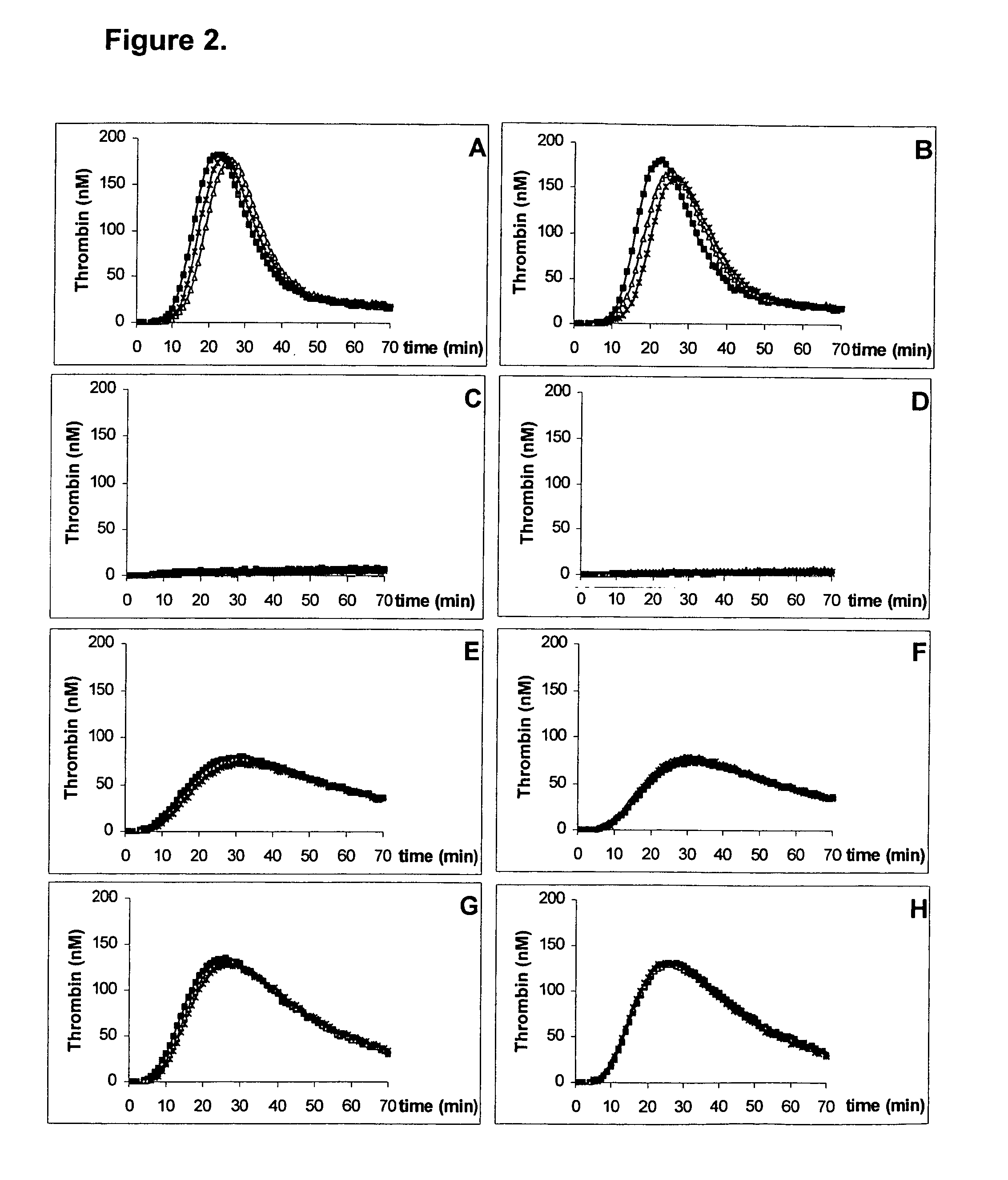
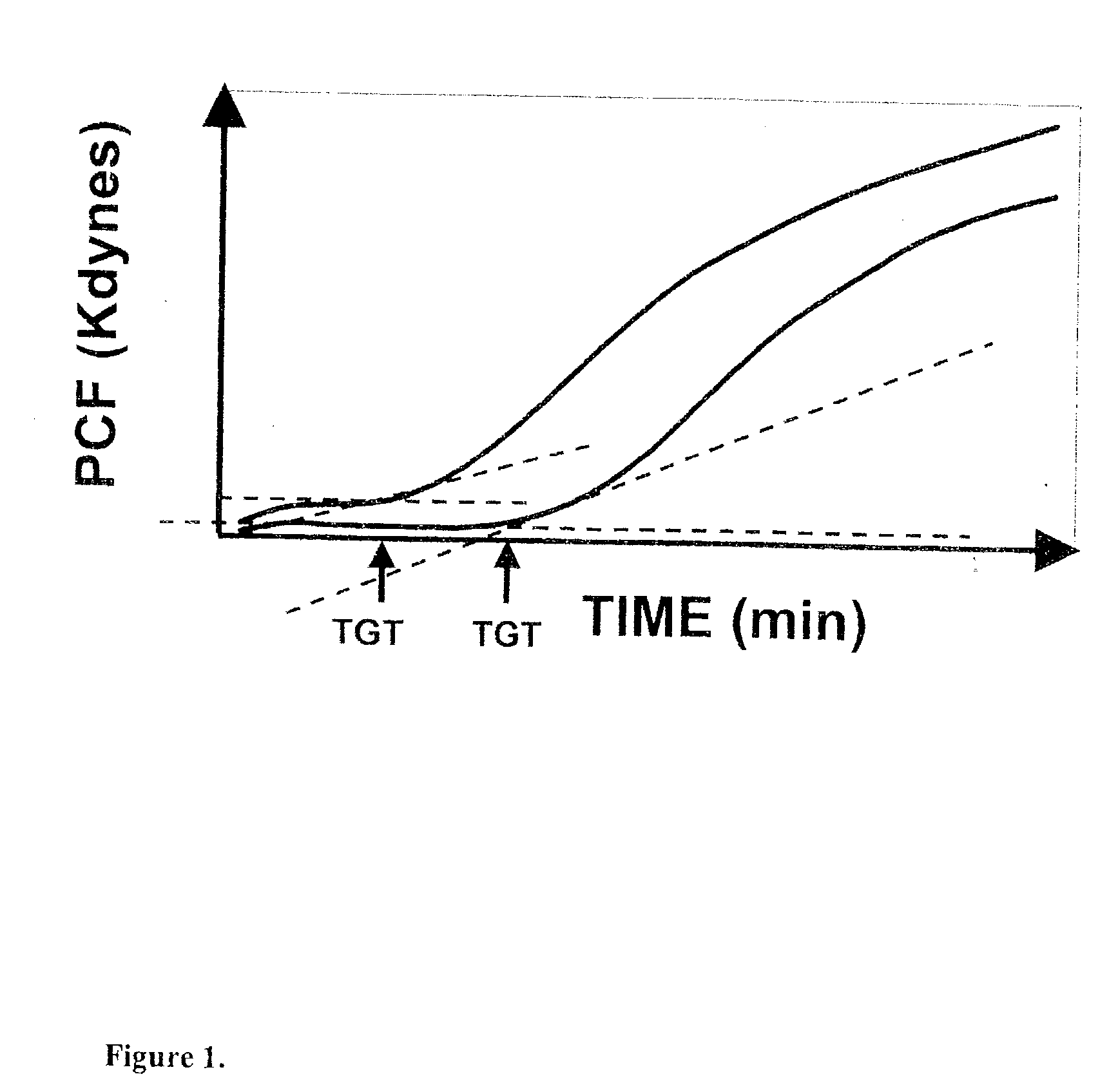
Platelet contractile force (PCF) is used as a surrogate marker of thrombin generation. PCF generation occurs concomitant with the burst of prothrombin fragment F 1+2 release. The time between assay start and PCF onset is identified as the thrombin generation time (TGT), and is used in assessing risk of bleeding, in diagnosing various disorders, and in monitoring the effects of pharmaceutical and other treatments. TGT is prolonged in clotting factor deficiencies and in the presence of direct and indirect thrombin inhibitors. TGT shortens to normal with clotting factor replacement and shortens with administration of rVIIa. TGT is short in thrombophilic states such as coronary artery disease, diabetes and thromboangiitis obliterans and prolongs toward normal with oral and intravenous anticoagulants.
Owner:HEMODYNE
Kit for measuring the thrombin generation in a sample of a patient's blood or plasma
InactiveUS20050221414A1Simple and efficient and fast and reproducible assayConvenient typeMicrobiological testing/measurementBiological material analysisTissue factorThrombin activity
The invention provides a kit for measuring the thrombin generation in a sample of a patient's blood or plasma, or in a sample of clotting factors. The kit contains lyophilized tissue factor / phospholipid-complex and a lyophilized mixture containing a thrombin-substrate and CaCl2. The invention also provides processes for preparing the reagents for the kit. The kit can be used in a method for measuring the thrombin generation in a sample, wherein it is possible to detect changes in thrombin generation kinetics, for example after administration of inhibitor bypassing agents to a patient who has developed inhibitors to an exogenous clotting factor such as Factor VIII.
Owner:BAXTER INT INC +1
Methods for Treating Bleeding Disorders
A method of factor XI-dependent blood coagulation enhancement in a subject in need of enhanced blood coagulation comprising administering a therapeutically effective amount of a composition comprising a non-anticoagulant sulfated polysaccharide (NASP) to the subject. A method of factor XI-dependent blood coagulation enhancement in a subject in need of enhanced blood coagulation comprising: (i) selecting a subject that is not deficient for factor XI; and (ii) administering a therapeutically effective amount of a composition comprising a non-anticoagulant sulfated polysaccharide (NASP) to the subject, wherein the NASP enhances blood coagulation in a factor XI-dependent manner. A method of identifying a non-anticoagulant sulfated polysaccharide (NASP) which is capable of enhancing blood coagulation in dependence on FXI, the method comprising: a) combining a blood or plasma sample comprising activation competent FXI with a composition comprising a sulfated polysaccharide and measuring the clotting or thrombin generation parameters of the blood or plasma sample; b) combining a corresponding blood or plasma sample deficient in activation competent FXI with a composition comprising the sulfated polysaccharide and measuring the clotting or thrombin generation parameters of the blood or plasma sample; and c) comparing the clotting or thrombin generation parameters of the blood or plasma samples as determined in steps (a) and (b) with each other, wherein a decrease in the clotting time of the blood sample or an increase in peak thrombin or decrease in peak time of the plasma sample comprising activation competent FXI compared to the clotting time of the blood sample or peak thrombin or peak time of the plasma sample deficient in activation competent FXI is indicative of a NASP which is capable of enhancing blood coagulation in dependence on FXI.
Owner:TAKEDA PHARMA CO LTD
Chitosan hemostatic sponge made from maggot shells as well as preparation method and application thereof
ActiveCN101974170APromote generationGrowth inhibitionAbsorbent padsBandagesFreeze-dryingPharmacology
The invention discloses a chitosan hemostatic sponge made from maggot shells as well as a preparation method and application thereof. The chitosan hemostatic sponge is obtained by carrying out the following steps of: pulverizing cleaned and dried maggot shells; removing metal ions with a hydrochloric acid solution; removing protein with a sodium hydroxide solution; decoloring with a hydrogen peroxide solution; carrying out gradient deacetylation with the sodium hydroxide solution; drying to obtain powder; then dissolving the powder into a dilute acetic acid solution; freeze drying; and sterilizing. The product can effectively activate partial blood platelets, adsorb erythrocytes, promote the generation of partial thrombase, exert quick and stable hemostatic activity when the product is adhered to a partial tissue wound, have the effects of selective antibiosis and cell proliferation promotion and favorable effects of controlling moderate traumatic hemorrhage and tissue organ diffuse surface hemorrhage, can be suitable for external emergency treatment of traumatic hemorrhage, particularly suitable for implantation hemostasis in a surgery, and also has the functions of preventing wound infection of a wound surface and promoting healing of the wound surface.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
Measuring thrombin activity in whole blood
ActiveUS20090311730A1Increase the areaMicrobiological testing/measurementBiological material analysisThrombin activityThrombin generation
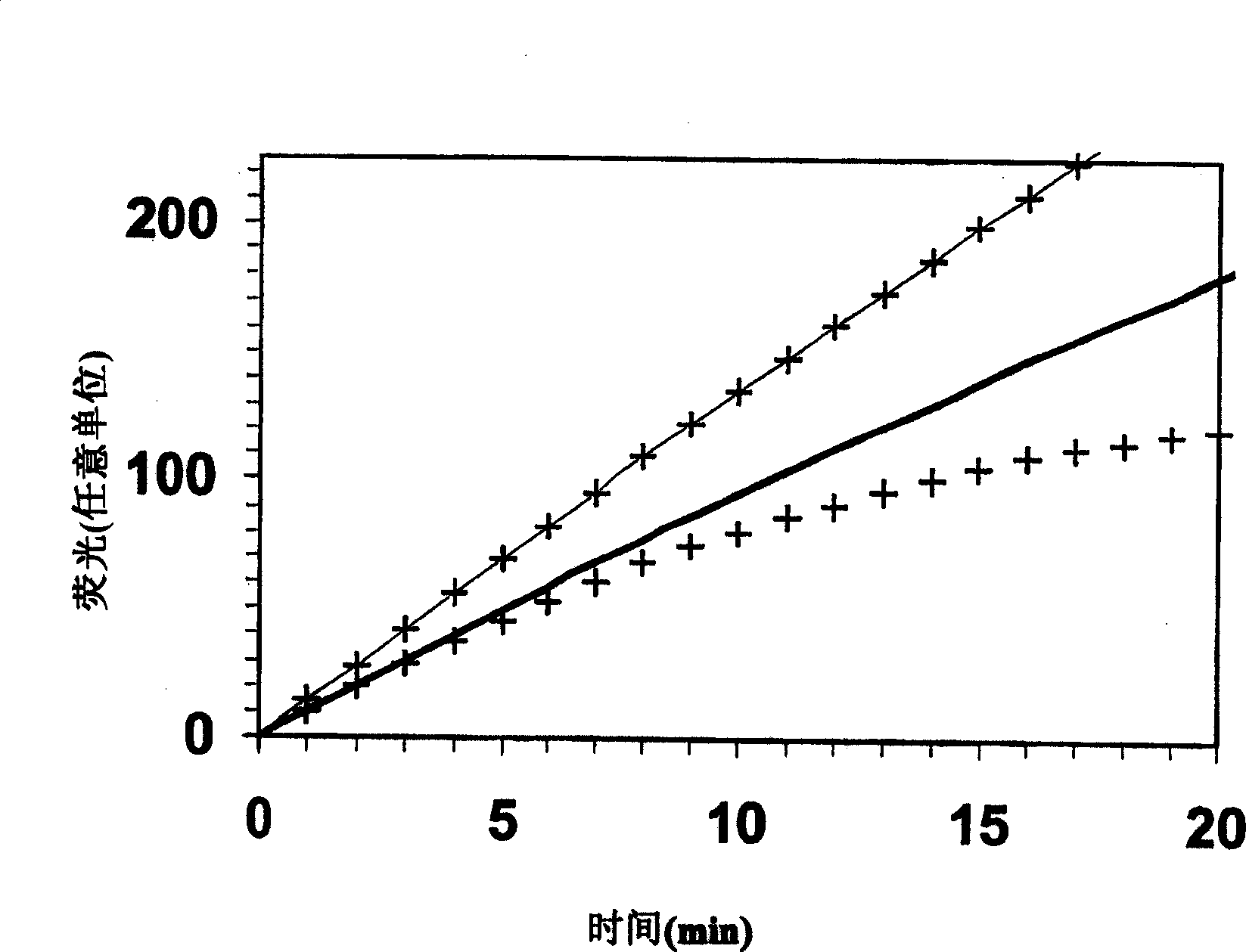
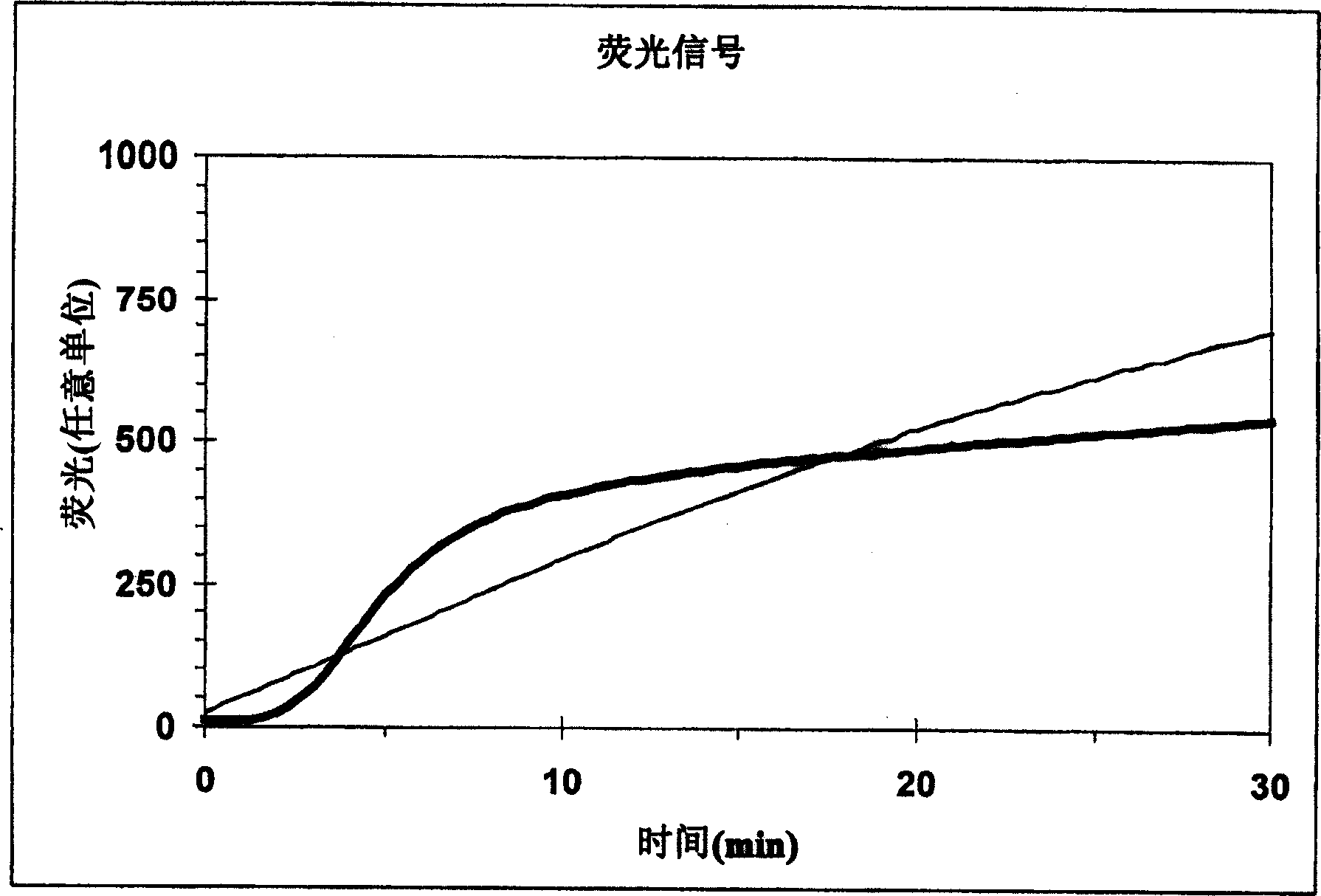
The invention relates to a method for in vitro determining thrombin activity in a sample wherein the sample is a blood sample and thrombin generation is measured by the steps of: —contacting a layer of said sample with a fluorogenic substrate of thrombin, wherein said layer has a thickness within a range of 0.05 to 5 mm and a surface within a range of 10 to 500 mm2; —allowing thrombin to generate in said sample; —measuring the fluorescence emitted from the surface of the layer, by the fluorescent group released from the fluorogenic substrate as a result of enzymatic action of generated thrombin on said fluorogenic substrate.
Owner:SYNAPSE INC
Measuring thrombin activity in whole blood
ActiveUS8916356B2Microbiological testing/measurementBiological material analysisThrombin activityThrombin generation
Owner:SYNAPSE INC
Methods for Treating Bleeding Disorders
A method of factor X1-dependent blood coagulation enhancement in a subject in need of enhanced blood coagulation comprising administering a therapeutically effective amount of a composition comprising a non-anticoagulant sulfated polysaccharide (NASP) to the subject. A method of factor X1-dependent blood coagulation enhancement in a subject in need of enhanced blood coagulation comprising: (i) selecting a subject that is not deficient for factor X1; and (ii) administering a therapeutically effective amount of a composition comprising a non-anticoagulant sulfated polysaccharide (NASP) to the subject, wherein the NASP enhances blood coagulation in a factor X1-dependent manner. A method of identifying a non-anticoagulant sulfated polysaccharide (NASP) which is capable of enhancing blood coagulation in dependence on FXI, the method comprising: a) combining a blood or plasma sample comprising activation competent FXI with a composition comprising a sulfated polysaccharide and measuring the clotting or thrombin generation parameters of the blood or plasma sample; b) combining a corresponding blood or plasma sample deficient in activation competent FXI with a composition comprising the sulfated polysaccharide and measuring the clotting or thrombin generation parameters of the blood or plasma sample; and c) comparing the clotting or thrombin generation parameters of the blood or plasma samples as determined in steps (a) and (b) with each other, wherein a decrease in the clotting time of the blood sample or an increase in peak thrombin or decrease in peak time of the plasma sample comprising activation competent FXI compared to the clotting time of the blood sample or peak thrombin or peak time of the plasma sample deficient in activation competent FXI is indicative of a NASP which is capable of enhancing blood coagulation in dependence on FXI.
Owner:BAXALTA GMBH
Simultaneous measurement of thrombin generation and clot strength in plasma and whole blood
ActiveUS20150118691A1Simultaneous measurementHigh viscosityBioreactor/fermenter combinationsBiological substance pretreatmentsThrombin generationBlood plasma
A method for the simultaneous measurement of proteolylic enzyme generation and clot strength in plasma or whole blood or any appropriate biological sample derived from blood. The measurement method encompasses the use of a detectable substrate which includes a moiety that can be released upon reaction with the targeted proteolytic enzyme, and elements for measurement of an increase in viscosity of clot strength.
Owner:SYNAPSE INC
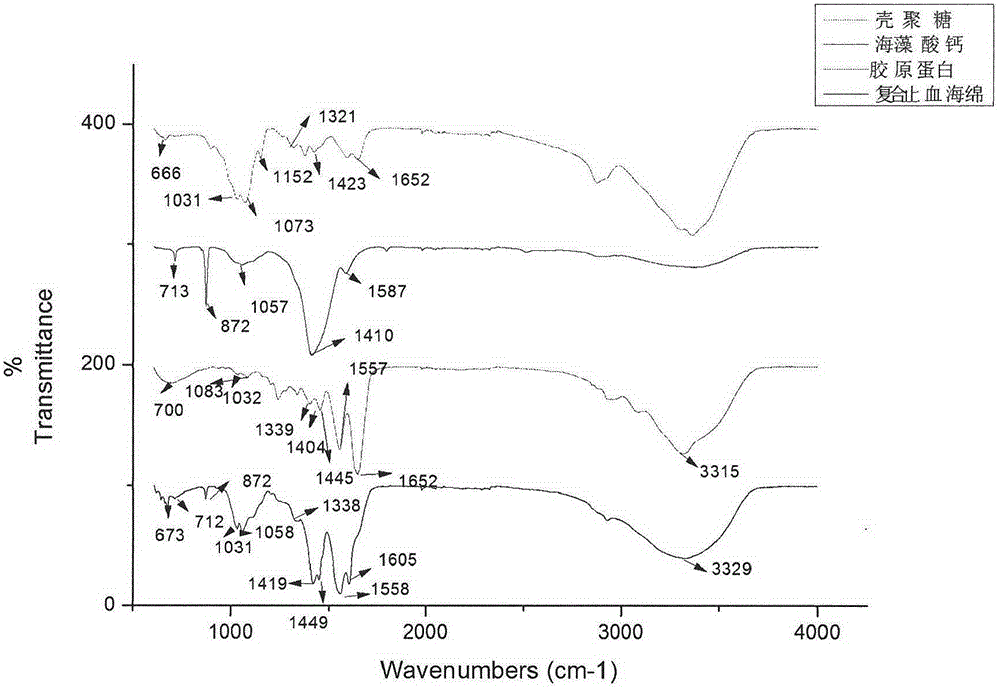
Collagen/calcium alginate/chitosan composite hemostatic sponge and making method
The invention relates to the technical field of local trauma hemostatic dressing, in particular to a collagen / calcium alginate / chitosan composite hemostatic sponge and a making method. The composite hemostatic sponge is characterized by being prepared from dissolving collagen, calcium alginate and chitosan according to a certain proportion and adopting a freeze-drying method. After the composite hemostatic sponge contacts with the surface of a wound, blood platelets can be quickly gathered to form blood scab; the composite hemostatic sponge absorbs a lot of exudate due to good liquid absorbing performance to apply certain pressure on the wound so as to play a certain role in pressing hemostasis; a lot of Ca+ in the composite hemostatic sponge can quickly exchange with Na+ in blood after the composite hemostatic sponge contacts with the surface of the wound, so that thrombin generation is promoted, and blood coagulation of the surface of the wound is accelerated. The composite hemostatic sponge can be used for body wound hemostasis.
Owner:NAVY MEDICINE RES INST OF PLA
Diagnostic test for determining the concentration of transient proteolytic activity in composite biological media
InactiveCN1650170AMagnetic measurementsMicrobiological testing/measurementDiagnostic testThrombin activity
A method is provided for determining in real time the course of thrombin activity in a sample of blood or plasma as it appears in and disappears from the simple which comprises adding a thrombin substrate to the sample that, per unit time, produces a detectable signal in a quantity that bears relation to the amount of thrombin present. Simultaneously, in a control sample of the same blood or plasma in which thrombin generation is not triggered, the activity of a standard preparation with invariable thrombin activity is measured. The exact molar amount of thrombin present at any moment is obtained by comparison of the activity measured in clotting blood and the simultaneously measured calibrator. The method is useful inter alia for diagnosing hyper- and hypo-coaguable states, either congenital, acquired or drug-induced in humans and animals. Also provided is a kit for use in this method.
Owner:SYNAPSE INC
Kit for measuring the thrombin generation in a sample of a sample of a patient's blood or plasma
ActiveUS20130052672A1Rapid diagnosisSimple and efficient and fast and reproducible assayMicrobiological testing/measurementBiological material analysisTissue factorPlasma samples
The invention provides a kit for measuring the thrombin generation in a sample of a patient's blood or plasma, or in a sample of clotting factors. The kit contains lyophilized tissue factor / phospholipid-complex and a lyophilized mixture containing a thrombin-substrate and CaCl2. The invention also provides processes for preparing the reagents for the kit. The kit can be used in a method for measuring the thrombin generation in a sample, wherein it is possible to detect changes in thrombin generation kinetics, for example after administration of inhibitor bypassing agents to a patient who has developed inhibitors to an exogenous clotting factor such as Factor VIII.
Owner:TAKEDA PHARMA CO LTD
Blood collection devices containing contact pathway inhibition additives
InactiveUS20140220552A1Prolong clotting timeStabilize thrombin generationBioreactor/fermenter combinationsBiological substance pretreatmentsBlood collectionMedicine
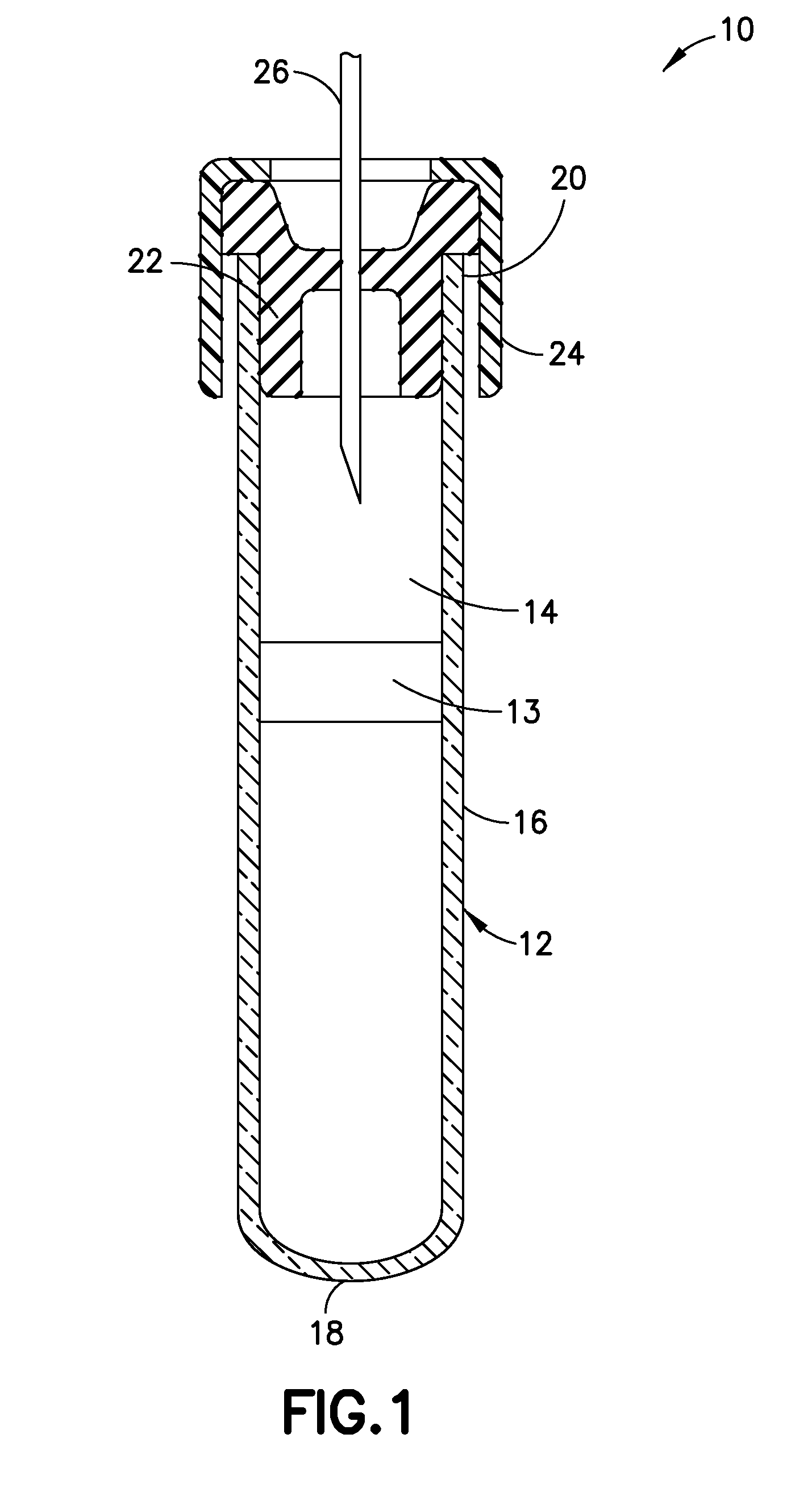
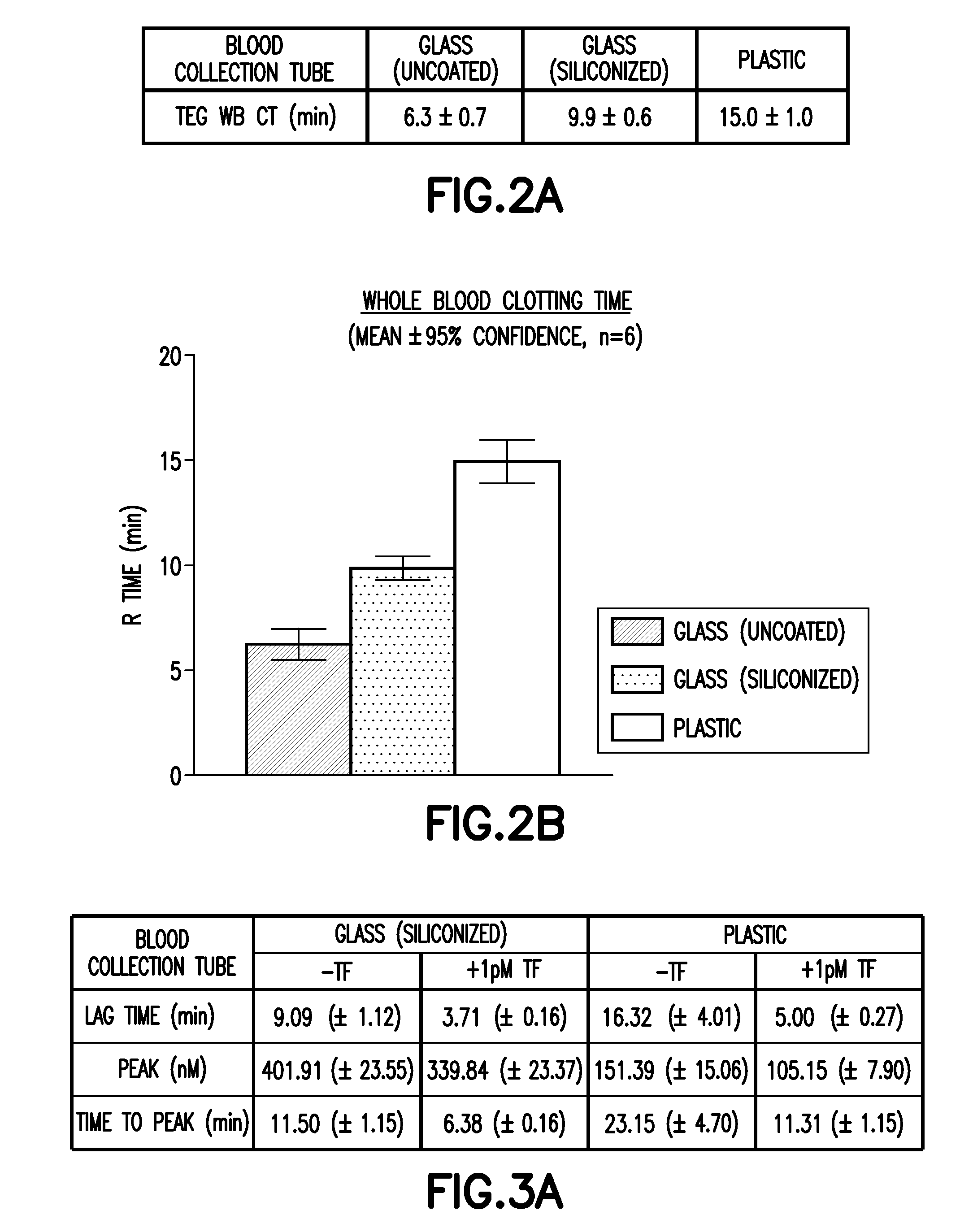
Disclosed are devices for collecting blood that contain an anti-coagulant and an additive that delays clotting by inhibiting the contact pathway for thrombin generation. The additive is a coagulation contact pathway inhibitor additive that is at least one of a Factor XI inhibitor, a Factor XII inhibitor, a kallikrein inhibitor and combinations thereof, each in an amount effective to mediate or suppress the contact pathway for thrombin generation. Methods of making and using the devices, and kits containing the devices, are also provided.
Owner:BECTON DICKINSON & CO
Pharmaceutical preparations and medicines capable of generating, and/or containing, thrombin
Owner:BIO PRODS & BIO ENG AKTIENGES
Heparin compositions that inhibit clot associated coagulation factors
InactiveUS20080119438A1Prevent reactivationAvoid generatingOrganic active ingredientsBlood disorderVenous bloodAngina
The present invention provides compositions and methods for the treatment of cardiovascular diseases. More particularly, the present invention relates to modifying thrombus formation by administering an agent which, inter alia, is capable of (1) inactivating fluid-phase thrombin and thrombin which is bound either to fibrin in a clot or to some other surface by catalyzing antithrombin; and (2) inhibiting thrombin generation by catalyzing factor Xa inactivation by antithrombin III (ATIII). The compositions and methods of the present invention are particularly useful for preventing thrombosis in the circuit of cardiac bypass apparatus and in patients undergoing renal dialysis, and for treating patients suffering from or at risk of suffering from thrombus-related cardiovascular conditions, such as unstable angina, acute myocardial infraction (heart attack), cerebrovascular accidents (stroke), pulmonary embolism, deep vein thrombosis, arterial thrombosis, etc.
Owner:WEITZ JEFFREY I +1
Hemostatic dressing and production method thereof
The invention discloses a hemostatic dressing, which comprises cuttlebone made into nanoscale powder and can also comprise bletilla made into nanoscale powder or pollen typhae made into nanoscale powder or pseudo-ginseng made into nanoscale powder. The invention also discloses a production method of the hemostatic dressing, which comprises the following steps of: cleaning cuttlebone with water and then drying the cuttlebone; crushed the dried cuttlebone into nanoscale powder; and sterilizing the cuttlebone made into the nanoscale powder. The hemostatic dressing has the advantages that the time for thrombin generation can be reduced, the activity of plasmin can be inhibited, the contraction of blood vessels can be realized, bleeding can be stopped rapidly and reliably with no blood stasis, traditional Chinese herbal medicine can be modified into nanoscale powder to prepare hemostatic materials with novel functions, and the blood vessels and tissues on bleeding parts can be recovered.
Owner:ZHUHAI HEFAN MEDICINE
Onset of force development as a marker of thrombin generation
InactiveUS20030199428A1Bioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseCoronary artery disease
Platelet contractile force (PCF) is used as a surrogate marker of thrombin generation. PCF generation occurs concomitant with the burst of prothrombin fragment F 1+2 release. The time between assay start and PCF onset is identified as the thrombin generation time (TGT), and is used in assessing risk of bleeding, in diagnosing various disorders, and in monitoring the effects of pharmaceutical and other treatments. TGT is prolonged in clotting factor deficiencies and in the presence of direct and indirect thrombin inhibitors. TGT shortens to normal with clotting factor replacement and shortens with administration of rVIIa. TGT is short in thrombophilic states such as coronary artery disease, diabetes and thromboangiitis obliterans and prolongs toward normal with oral and intravenous anticoagulants.
Owner:HEMODYNE
Method for identifying a non-anticoagulant sulfated polysaccharide which enhances blood coagulation dependence on FXI
A method of identifying a non-anticoagulant sulfated polysaccharide (NASP) which is capable of enhancing blood coagulation in dependence of FXI, the method comprising: a) combining a blood or plasma sample having activation competent FXI with a sulfated polysaccharide and measuring the clotting or thrombin generation parameters of the blood or plasma sample; b) combining a corresponding blood or plasma sample deficient In activation competent FXI with a sulfated polysaccharide and measuring the clotting or thrombin generation parameters of the blood or plasma sample and c) comparing the clotting or thrombin generation parameters of the blood or plasma samples as determined in steps (a) and (b) with each other.
Owner:TAKEDA PHARMA CO LTD
Hemostasis assay
InactiveUS20080026365A1Determine also hypercoagulabilityMicrobiological testing/measurementWhole blood productPlasmin
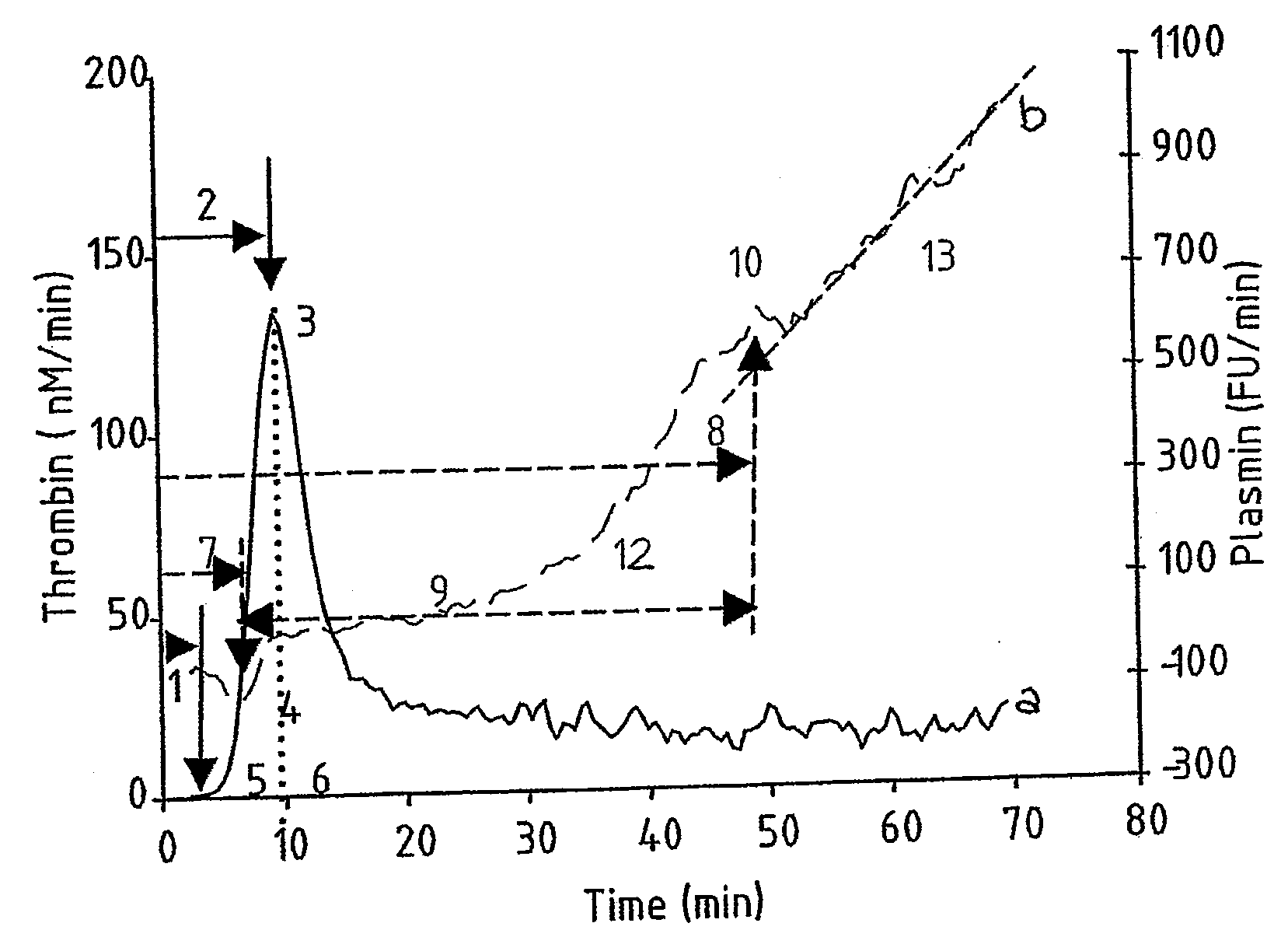
Provided is a hemostasis assay comprising a reaction mixture comprising a blood product to be tested, a trigger molecule for inducing thrombin generation, a thrombin-specific substrate which, upon cleavage by thrombin, produces a measurable thrombin-specific signal, a trigger molecule for inducing plasmin generation, a plasmin-specific substrate which, upon cleavage by plasmin, produces a measurable plasmin-specific signal, a phospholipid-containing surface, and calcium ions. The assay allows determination of the amount of thrombin and the amount of plasmin generated in the reaction mixture in time, starting at t=0, by measuring the thrombin-specific and plasmin-specific signals.
Owner:STICHTING RADBOUD UNIVERSITAIR MEDISCH CENT
Detection of restenosis risk in patients receiving a stent by measuring the characteristics of blood clotting including the measurement of maximum thrombin-induced clot strength
ActiveUS20090198120A1Avoid complicationsReadily availableDisease diagnosisSensorsBare-metal stentPercent Diameter Stenosis
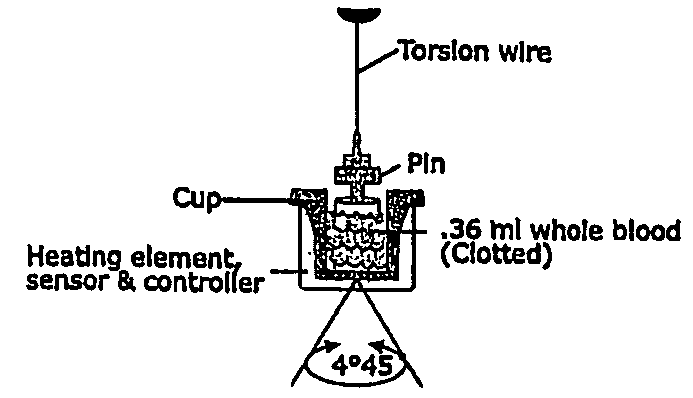
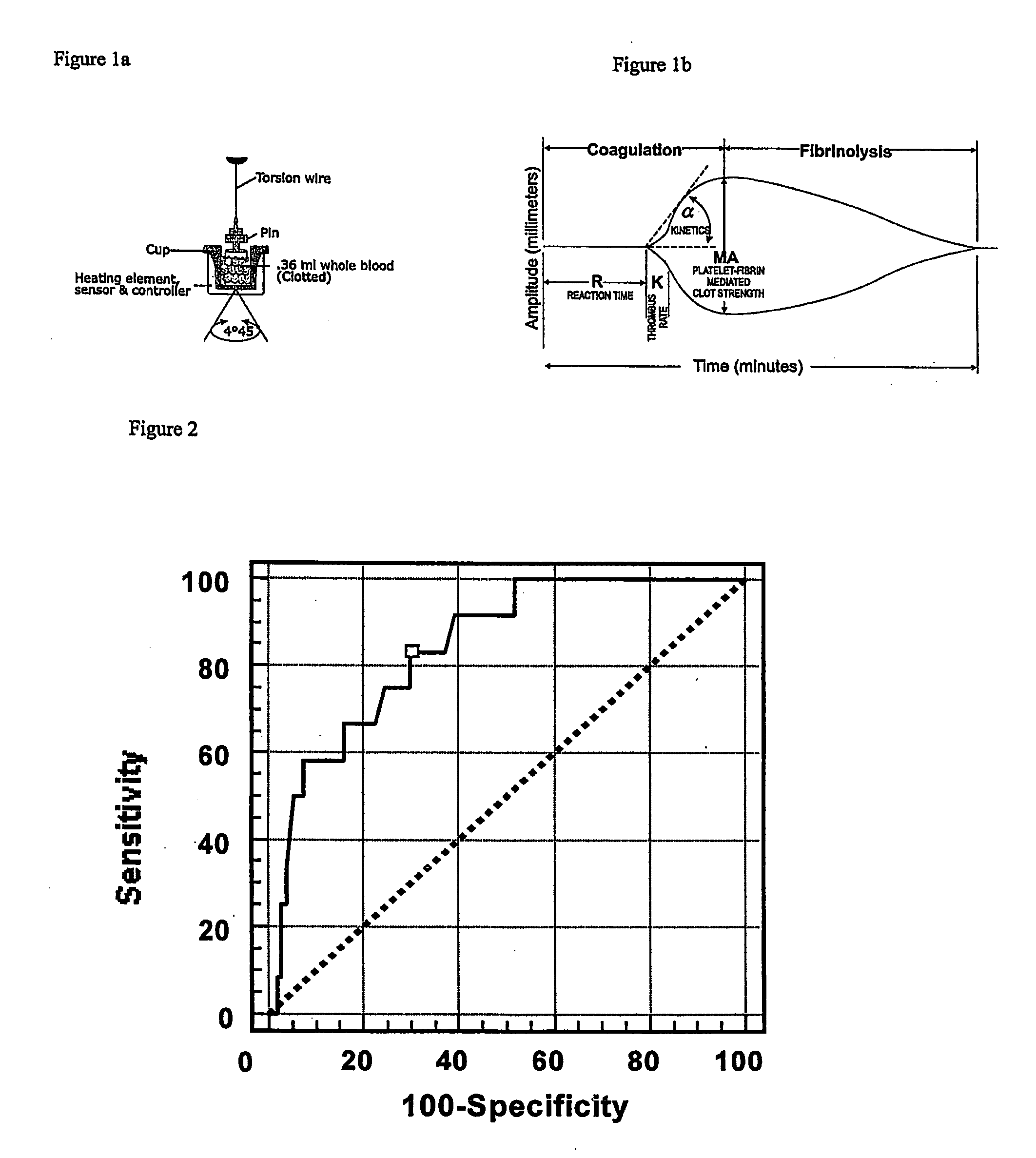
A method of selecting a stent for implantation in the circulatory system of a human being includes the steps of determining a threshold level of platelet hyper-coaguability (PHC). One marker of PHC is platelet-fibrin mediated clot strength (MA) of blood. MA above a threshold demonstrates a risk of restenosis that is relatively high. In practicing the method, a sample of blood is obtained from a patient who requires implantation of a stent, the blood sample is tested for its platelet-fibrin mediated clot strength, the clot strength of the blood sample is compared with the threshold level; if the blood sample has a clot strength below the threshold level, selecting a bare metal stent, and if the blood sample has a clot strength at or above the threshold level, selecting a drug-eluting stent. The method includes, in a preferred embodiment, use of a viscoelastic monitor to perform the testing step. Other markers of PHC may also be employed such as measurement of thrombin generation and / or platelet reactivity.
Owner:GURBEL PAUL A
Thrombin Generation Determination Method
InactiveUS20110183365A1Reliable measurement methodMinimally dilutedMicrobiological testing/measurementBiological testingTemporal changeThrombin generation
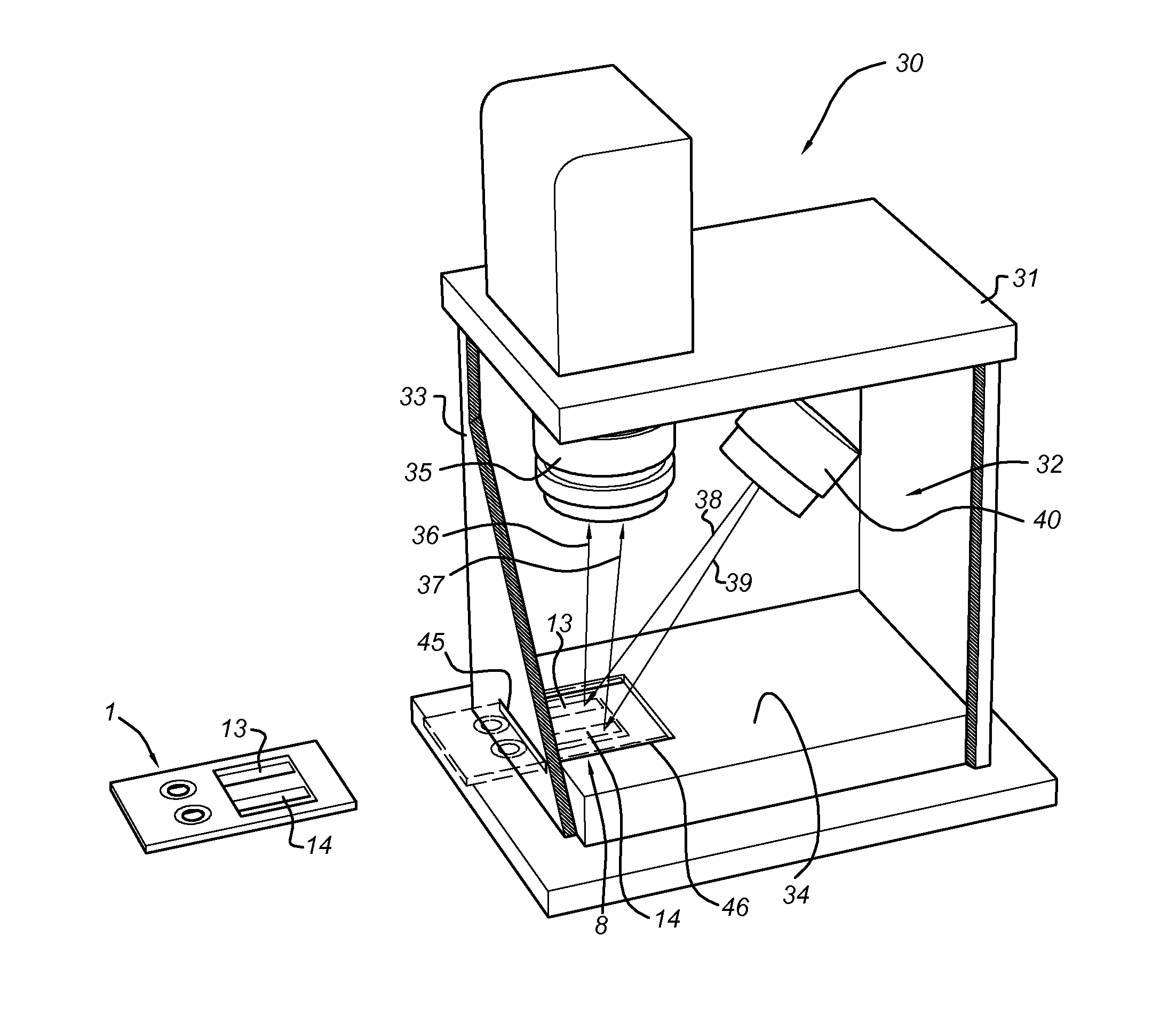
A method for measuring a generation of thrombin in a sample of whole blood as a function of time includes adding to a sample of whole blood a fluorogenic substrate and a thrombin activator to form an activated sample. A conversion product is permitted to form in the activated sample. Fluorescence is measured as a function of time from a fluorescent group that is released during the formation of the conversion product with the use of a fluorescence detector. The fluorescence detector operates in an extended range mode and has an increased sensitivity. Thrombin generation as a function of time can then be calculated from the measured fluorescence as a function of time.
Owner:ADVENTIST HEALTH SYSTSUNBELT
Antibodies capable of specifically binding two epitopes on tissue factor pathway inhibitor
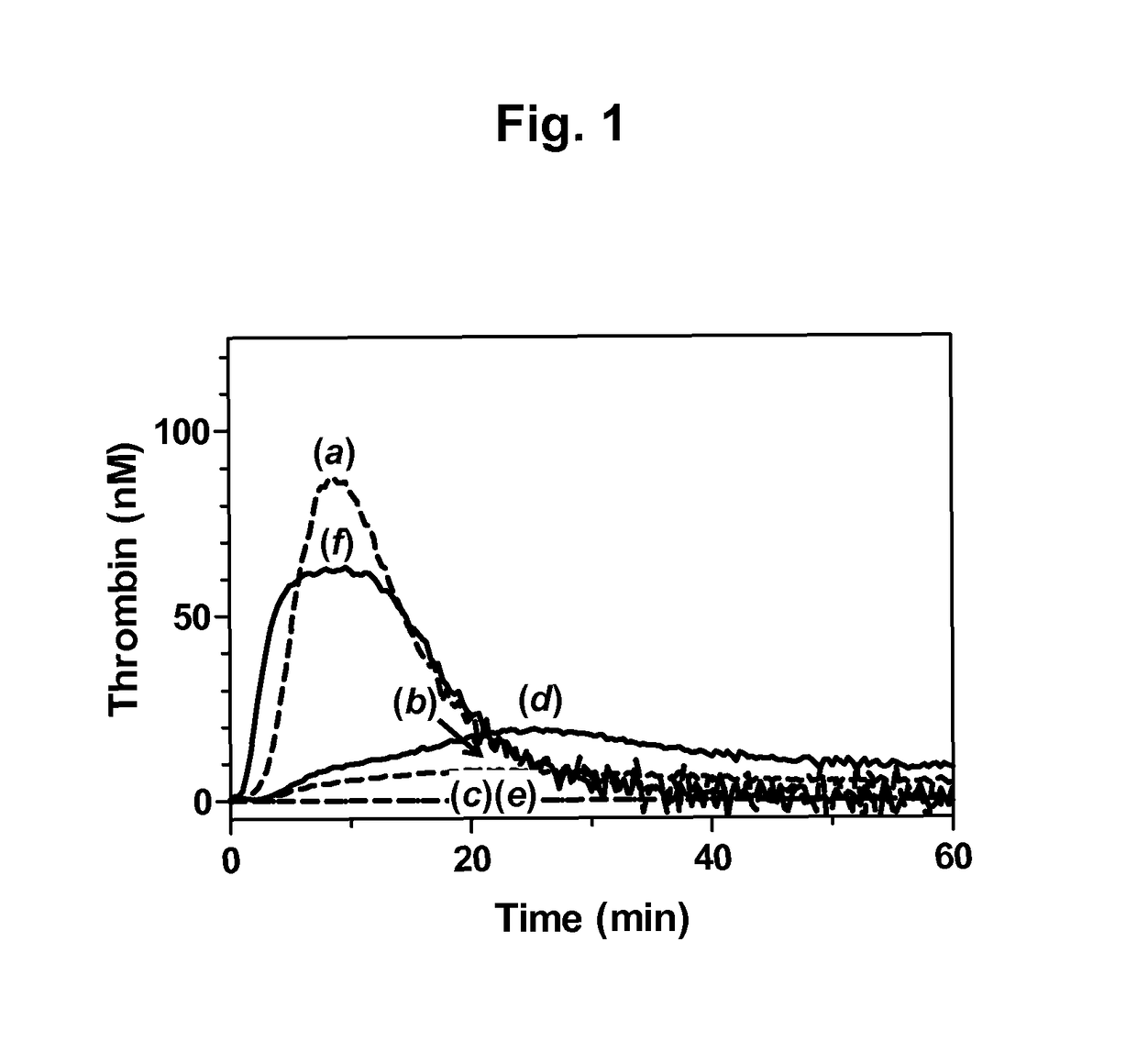
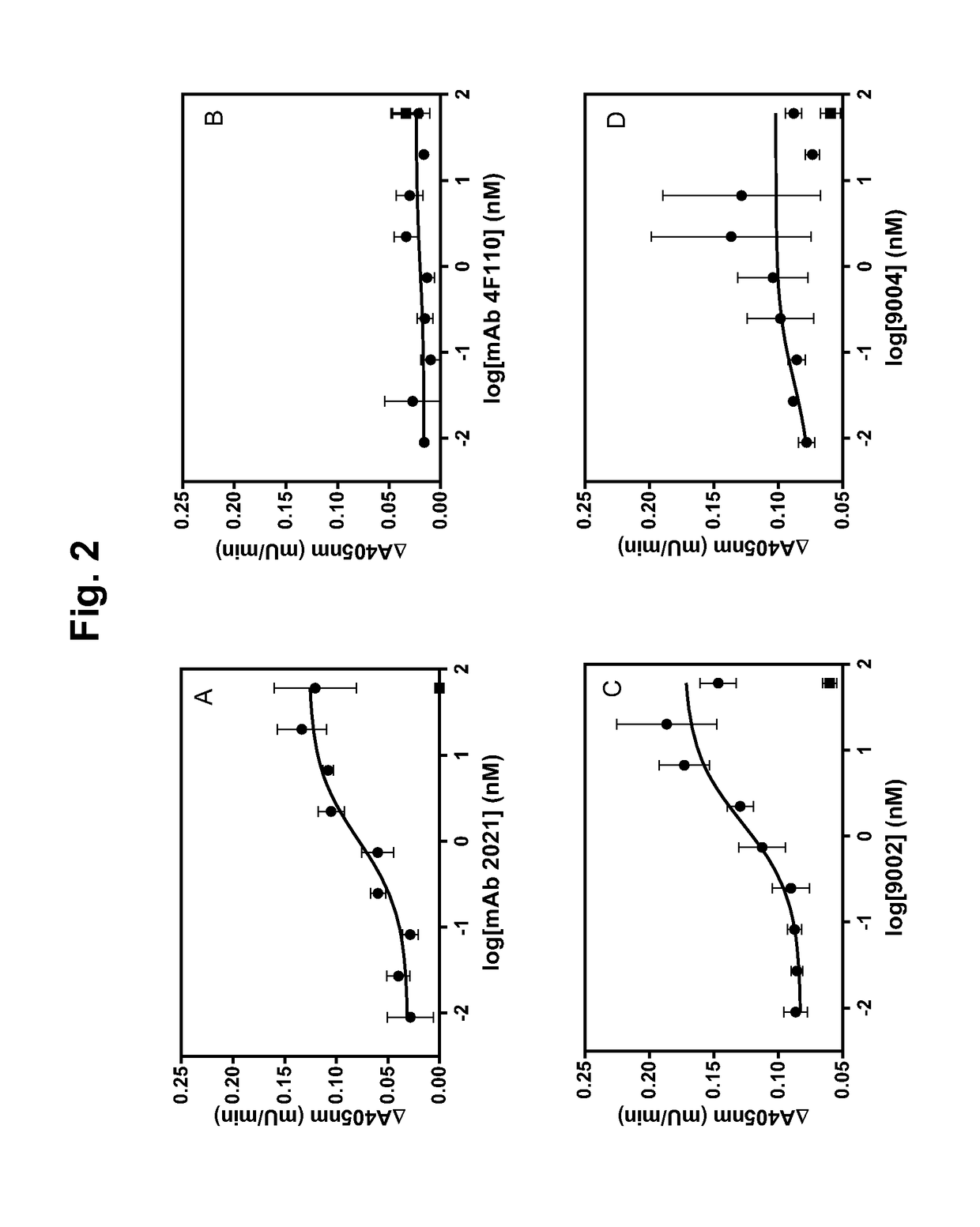
The application discloses a combination of two monospecific TFPI antibodies, wherein one antibody is capable of specifically binding TFPI (1-181) and the other antibody is capable of specifically binding TFPI (182-276), as well as bispecific anti-TFPI antibodies derived from two such monospecific antibodies. Both the combination of the two monospecific antibodies and the bispecific antibody strongly enhance thrombin generation by neutralising full length TFPIα, even where the concentration of TFPI is abnormally elevated.
Owner:NOVO NORDISK AS
Method and assembly for measuring thrombin generation in plasma
InactiveUS20110195441A1Avoid the needBioreactor/fermenter combinationsBiological substance pretreatmentsThrombin generationBlood plasma
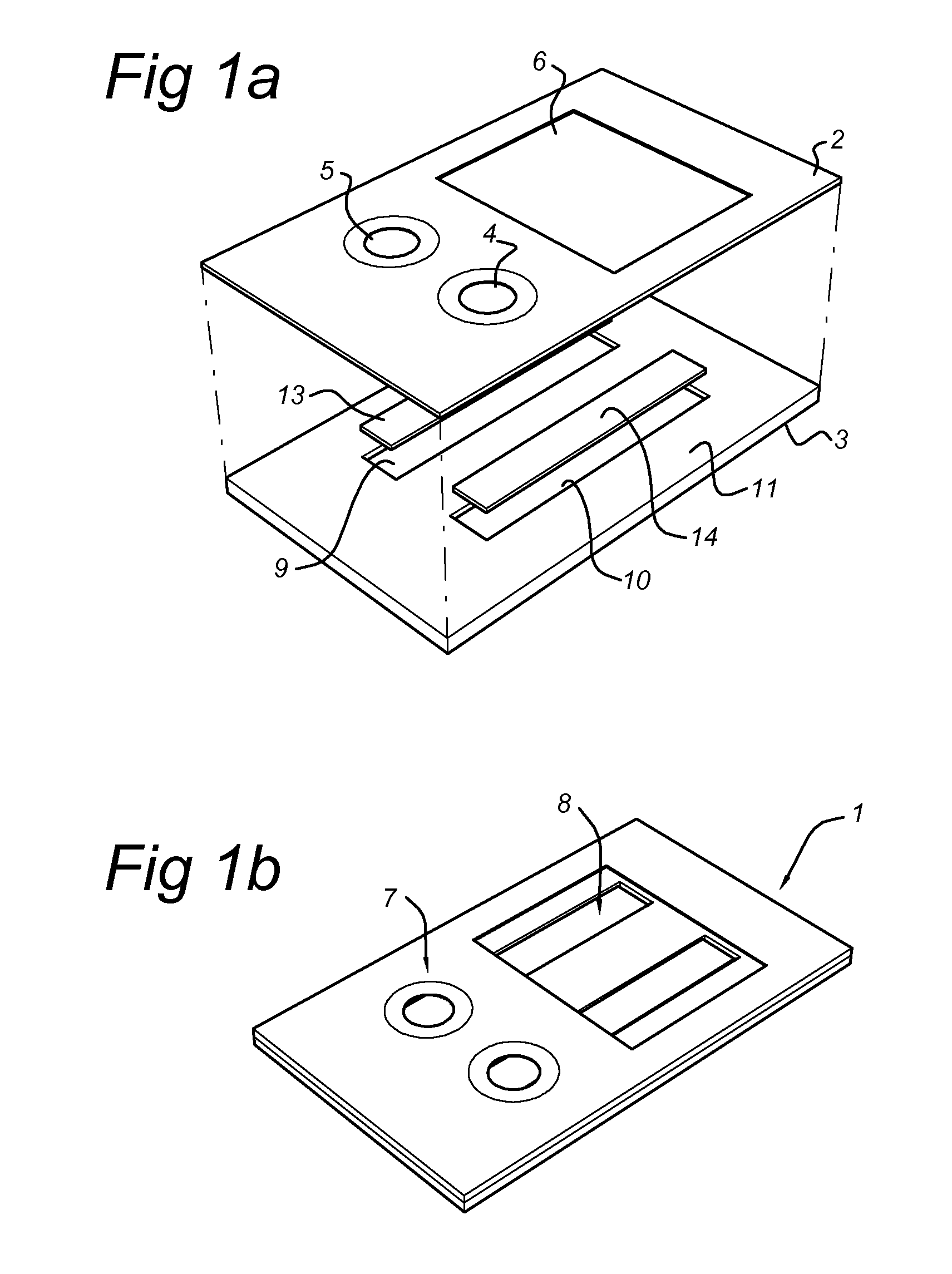
Disclosed is a method for measuring thrombin generation in a whole blood sample. The whole blood sample may be applied forthwith, without prior processing. The blood cells and blood plasma in the whole blood sample are separated by (lateral) flow migration. Also disclosed is an assembly of a sample support and a device dedicated to measure thrombin generation in a whole blood sample. Advantageously, the sample support comprises a separator medium allowing separation of whole blood into blood cells and blood plasma by means of (lateral) flow migration.
Owner:SYNAPSE INC
Modified low molecular weight heparin that inhibits clot associated coagulation factors
InactiveUS20050032745A1Prevent reactivationPacify thrombusOrganic active ingredientsVenous bloodAngina
The present invention provides compositions and methods for the treatment of cardiovascular diseases. More particularly, the present invention relates to modifying thrombus formation by administering an agent which, inter alia, is capable of (1) inactivating fluid-phase thrombin and thrombin which is bound either to fibrin in a clot or to some other surface by catalyzing antithrombin; and (2) inhibiting thrombin generation by catalyzing factor Xa inactivation by antithrombin III (ATIII). The compositions and methods of the present invention are particularly useful for preventing thrombosis in the circuit of cardiac bypass apparatus and in patients undergoing renal dialysis, and for treating patients suffering from or at risk of suffering from thrombus-related cardiovascular conditions, such as unstable angina, acute myocardial infarction (heart attack), cerebrovascular accidents (stroke), pulmonary embolism, deep vein thrombosis, arterial thrombosis, etc.
Owner:HAMILTON CIVIC HOSPITALS RESARCH DEV
Immunoassay for thrombin detection
InactiveUS20120149030A1Immunoglobulins against blood coagulation factorsBiological material analysisBinding siteReactive site
The invention relates to an in vitro immunoassay for quantifying thrombin in a sample comprising anti-thrombin III (AT-III) and thrombin. The method comprises the following steps: contacting the sample with a small molecule that recognizes the substrate binding site of thrombin; contacting the thrombin with a thrombin specific antibody raised against a thrombin blocked in the active site; and measuring the level of bound antibody.
Owner:OMRIX BIOPHARM
Procoagulant assay
InactiveUS6936430B1The testing process is simpleReduce probabilityMicrobiological testing/measurementEnzymesPlasma samplesThrombin activity
Method of detecting coagulation abnormalities in a plasma sample by (a) determining a test rate of thrombin generation over a given time interval by reacting an activator of thrombin with defibrinated normal plasma in the presence of a defibrinated test plasma sample; (b) determining a control rate of thrombin generation over substantially the same time interval in step (a) by reacting the same activator of thrombin with defibrinated normal plasma in the absence of any defibrinated test plasma; and (c) comparing the rates of thrombin generation between test step (a) and control step (b) such that any significant difference between the two thrombin generation rates being indicative of a coagulation abnormality in the test plasma.
Owner:SOUTH EASTERN SYDNEY AREA HEALTH SERVICE
Simultaneous measurement of thrombin generation and clot strength in plasma and whole blood
ActiveUS9428790B2Simultaneous measurementHigh viscosityMicrobiological testing/measurementFlow propertiesThrombin generationBlood plasma
A method for the simultaneous measurement of proteolylic enzyme generation and clot strength in plasma or whole blood or any appropriate biological sample derived from blood. The measurement method encompasses the use of a detectable substrate which includes a moiety that can be released upon reaction with the targeted proteolytic enzyme, and elements for measurement of an increase in viscosity of clot strength.
Owner:SYNAPSE INC
Exosite-Directed Thrombin Inhibitors
Disclosed are an amino acid sequence of the human blood clotting factor Va, peptides containing such sequence, and additional peptides of interest that significantly inhibit thrombin generation. Also disclosed are pharmaceutical compositions containing these peptides and related therapeutic methods for inhibiting thrombin generation and treating blood coagulation disorders.
Owner:KALAFATIS MICHAEL
Antibodies capable of specifically binding two epitopes on tissue factor pathway inhibitor
The application discloses a combination of two monospecific TFPI antibodies, wherein one antibody is capable of specifically binding TFPI (1-181) and the other antibody is capable of specifically binding TFPI (182-276), as well as bispecific anti-TFPI antibodies derived from two such monospecific antibodies. Both the combination of the two monospecific antibodies and the bispecific antibody strongly enhance thrombin generation by neutralising full length TFPI[alpha], even where the concentration of TFPI is abnormally elevated.
Owner:NOVO NORDISK AS
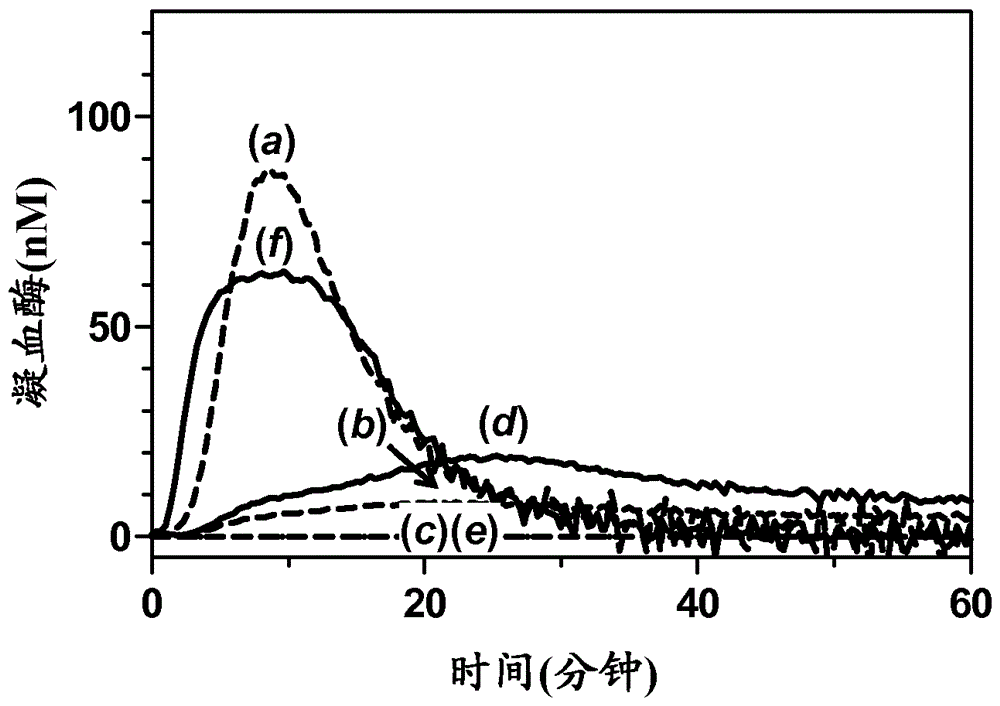
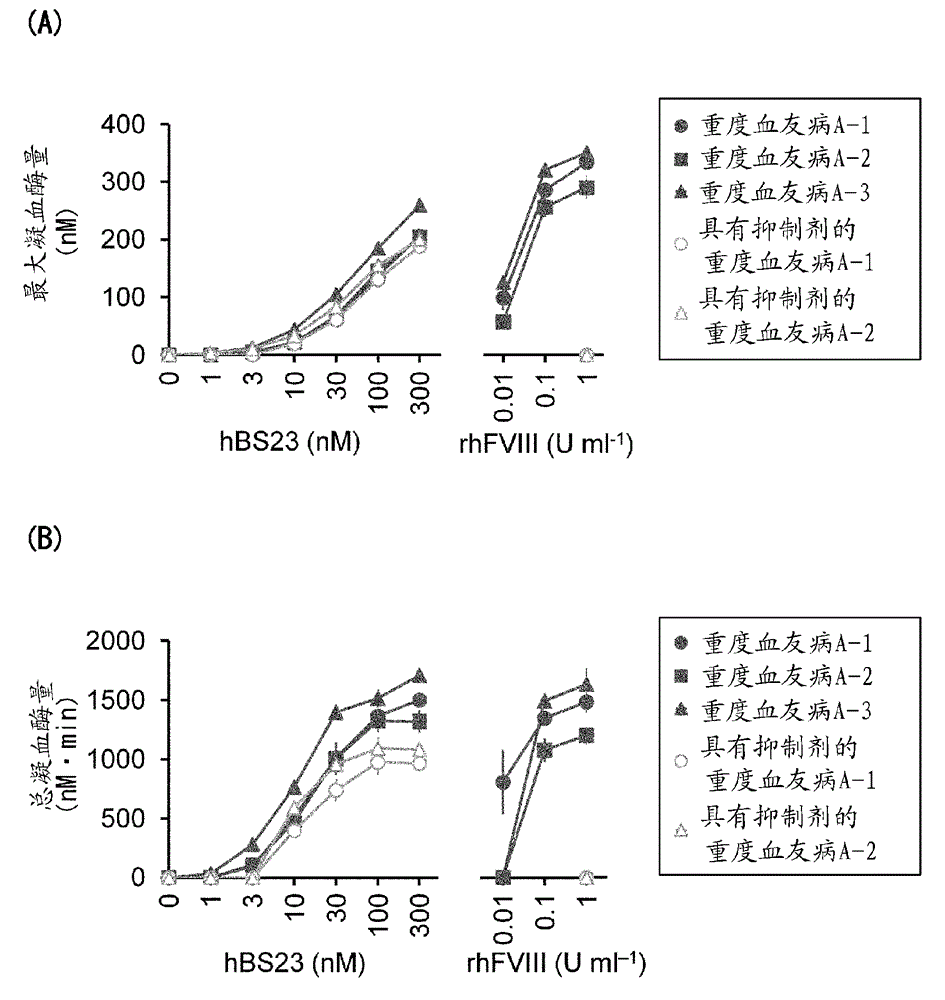
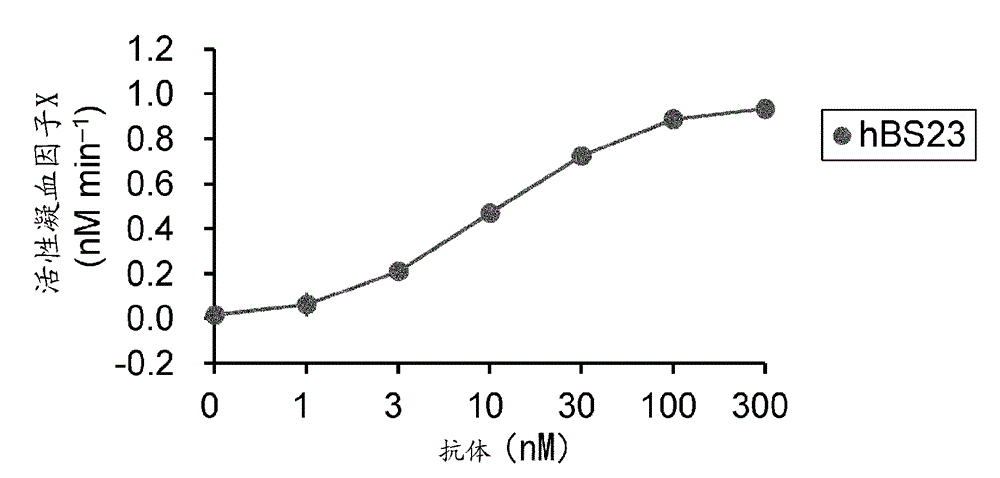
Method for evaluating blood coagulation reaction
ActiveCN104937423AThe effect is accurateAccurate evaluationMicrobiological testing/measurementDisease diagnosisHematological testPhospholipid
Owner:CHUGAI PHARMA CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com