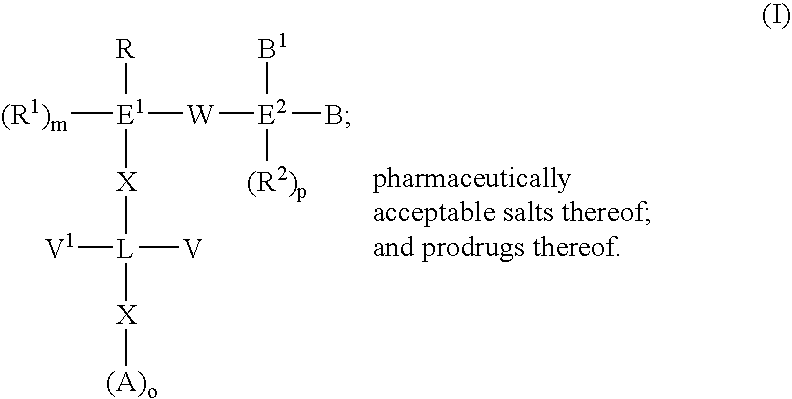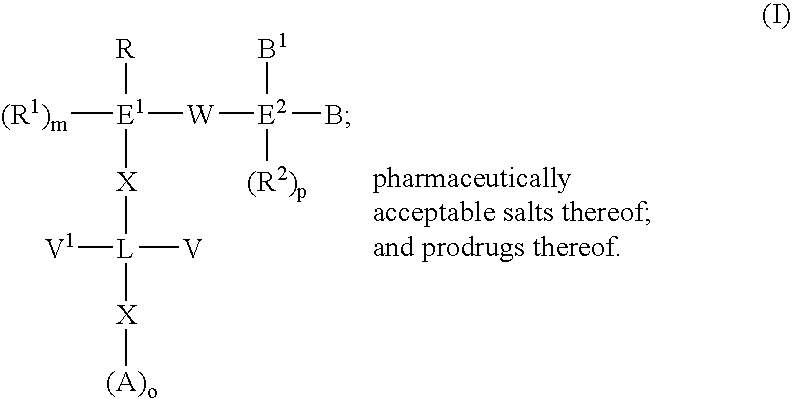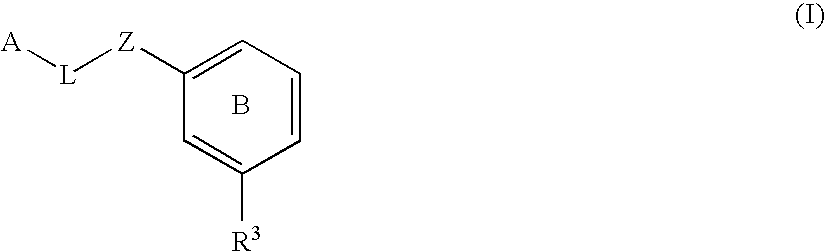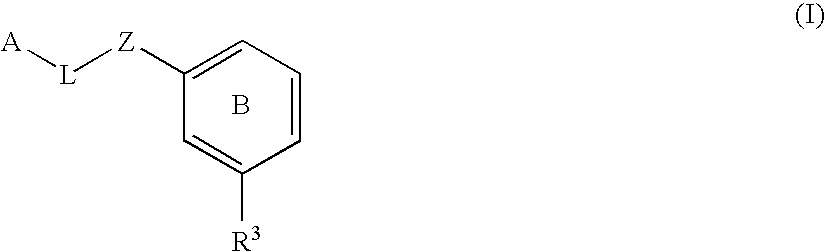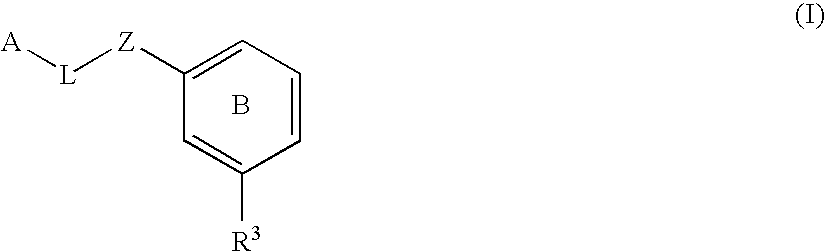Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
720 results about "Thrombin activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
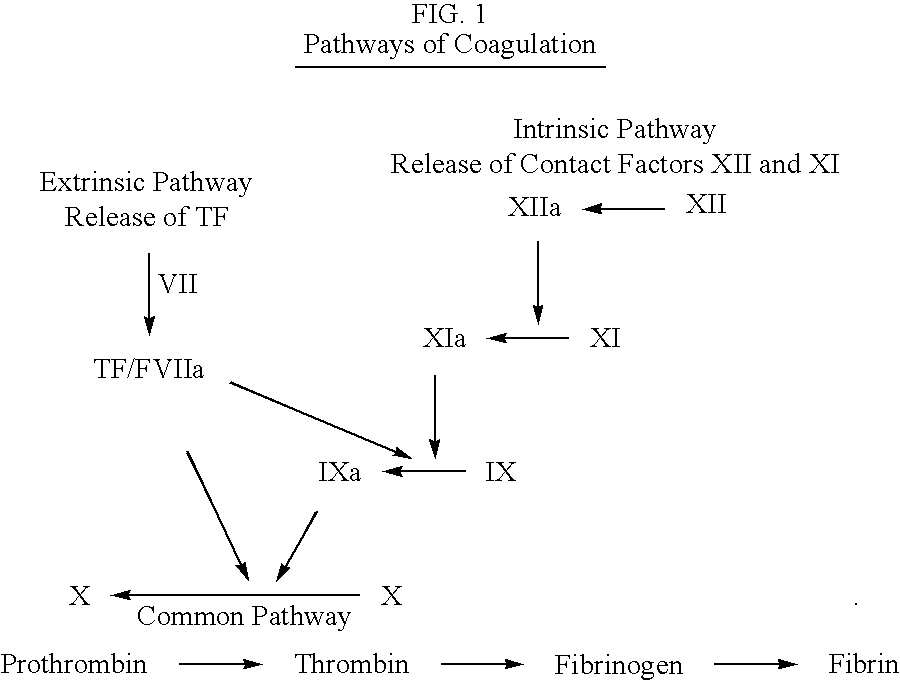
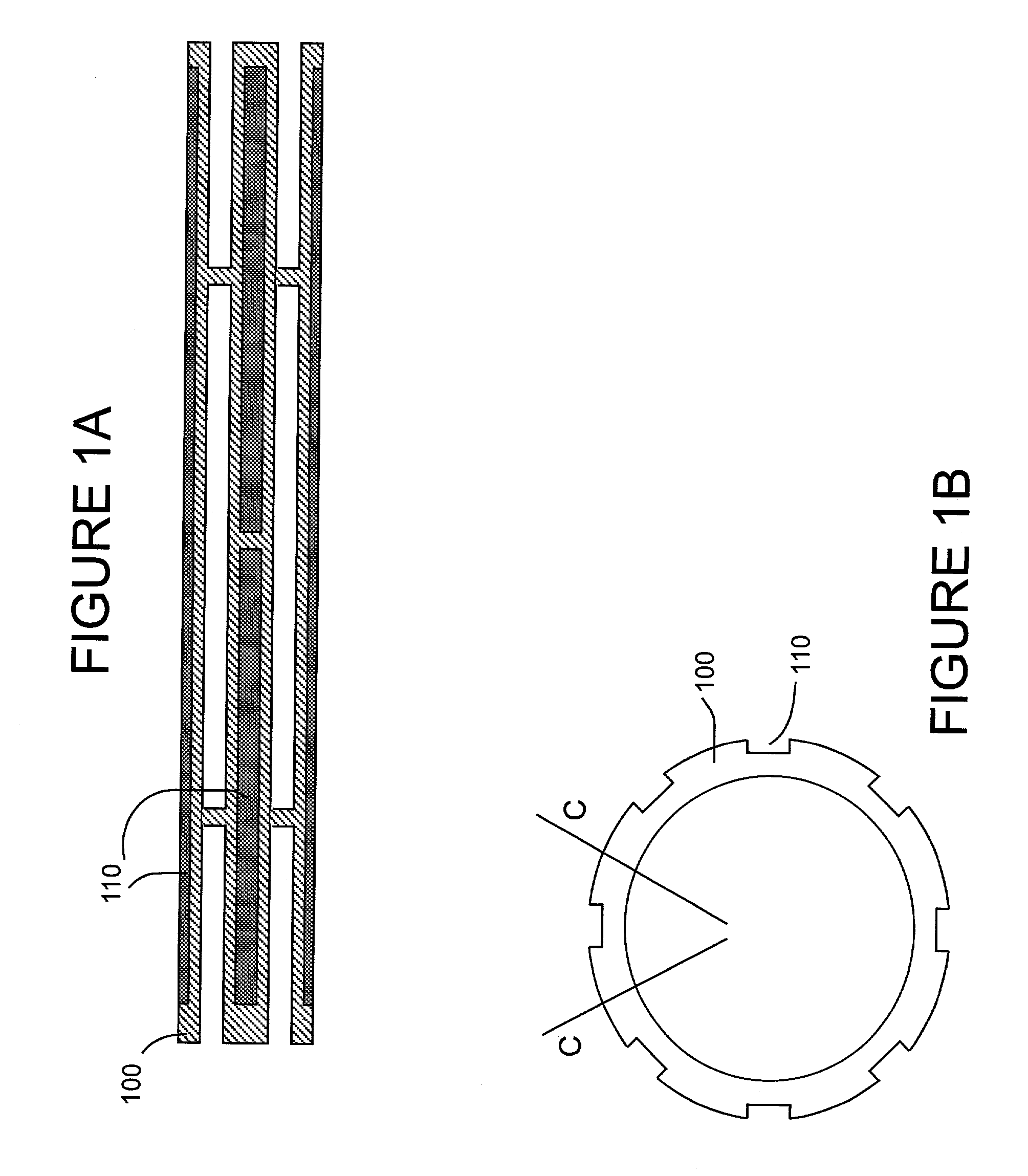
Thrombin is produced by the enzymatic cleavage of two sites on prothrombin by activated Factor X (Xa). The activity of factor Xa is greatly enhanced by binding to activated Factor V (Va), termed the prothrombinase complex.
Method for preparing thrombin for use in a biological glue
InactiveUS6472162B1Derive fast acting, stable autologous thrombinSimple preparatory procedureBioreactor/fermenter combinationsBiological substance pretreatmentsTissue sealantDonors plasma
A sterile method for preparing stable thrombin component from a single donor's plasma in which the thrombin component and the clotting and adhesive proteins component are harvested simultaneously from the same donor plasma in less than one hour. The combined components provide an improved biological hemostatic agent and tissue sealant by virtue of its freedom from the risk of contaminating viruses or bacteria from allogenic human or bovine blood sources. The thrombin provides polymerization of the clotting and adhesive proteins in less than five seconds, and is sufficiently stable to provide that fast clotting over a six hour period. Further, the clotting times can be predictably lengthened by diluting the thrombin with saline.
Owner:ASAHI KASEI MEDICAL CO LTD
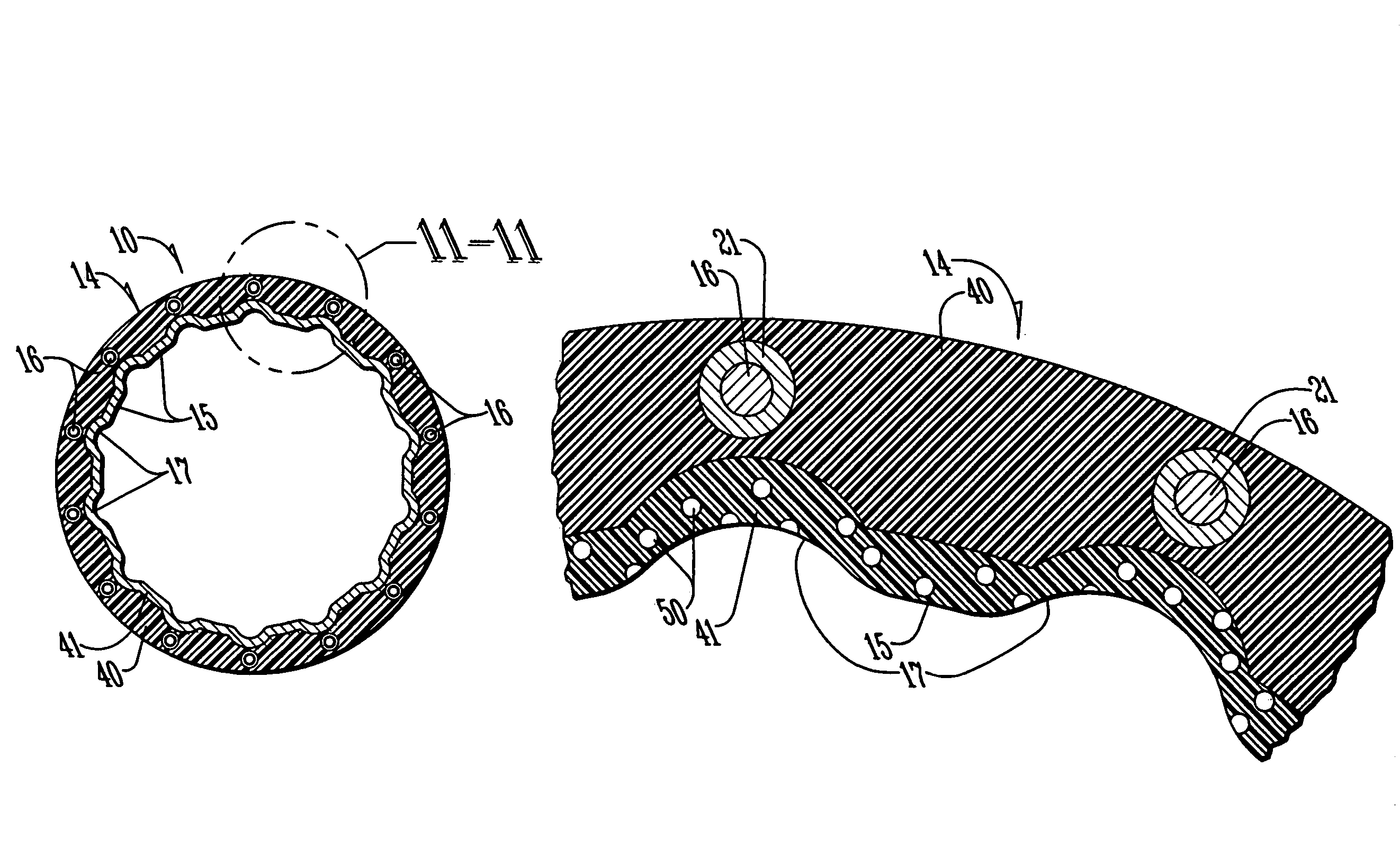
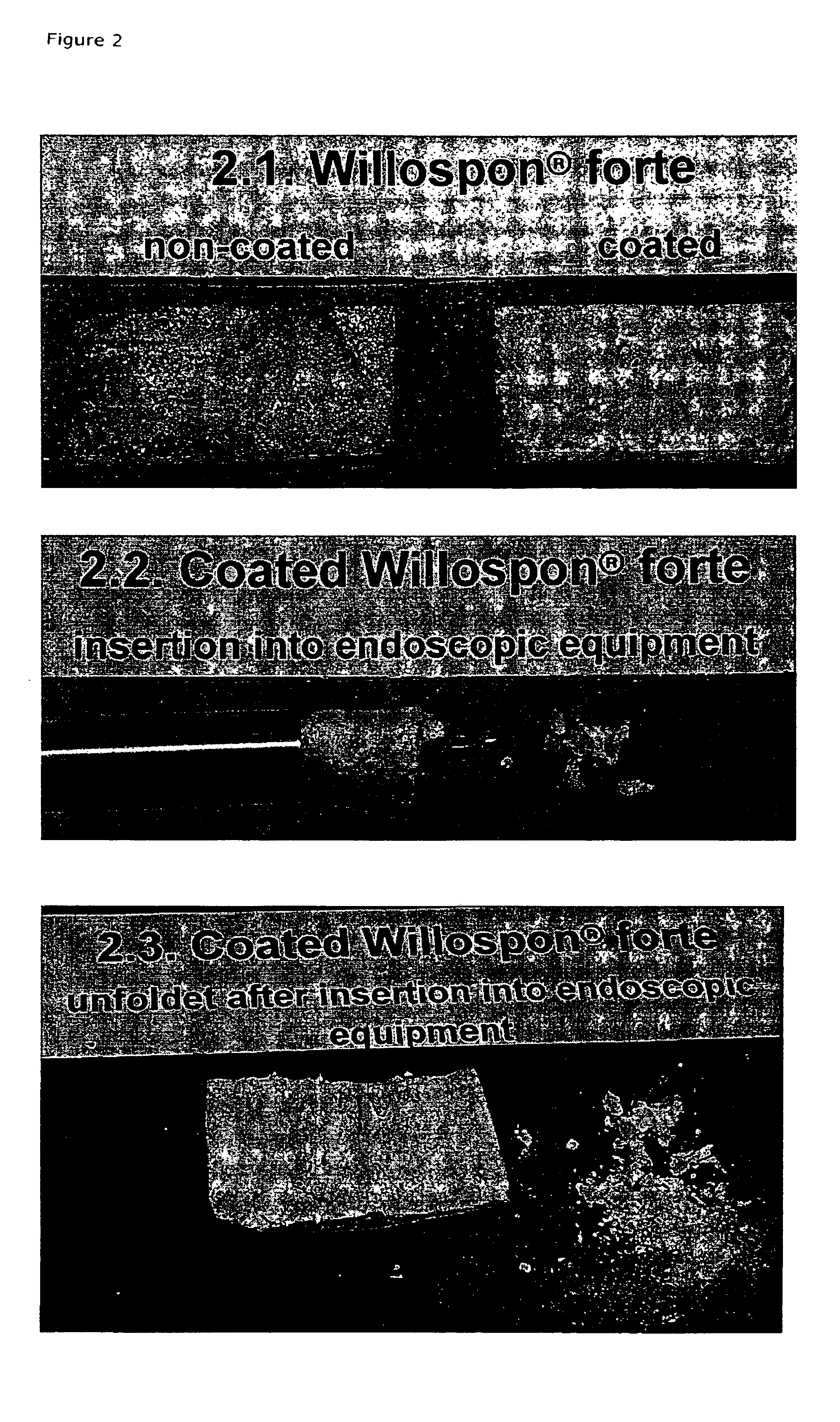
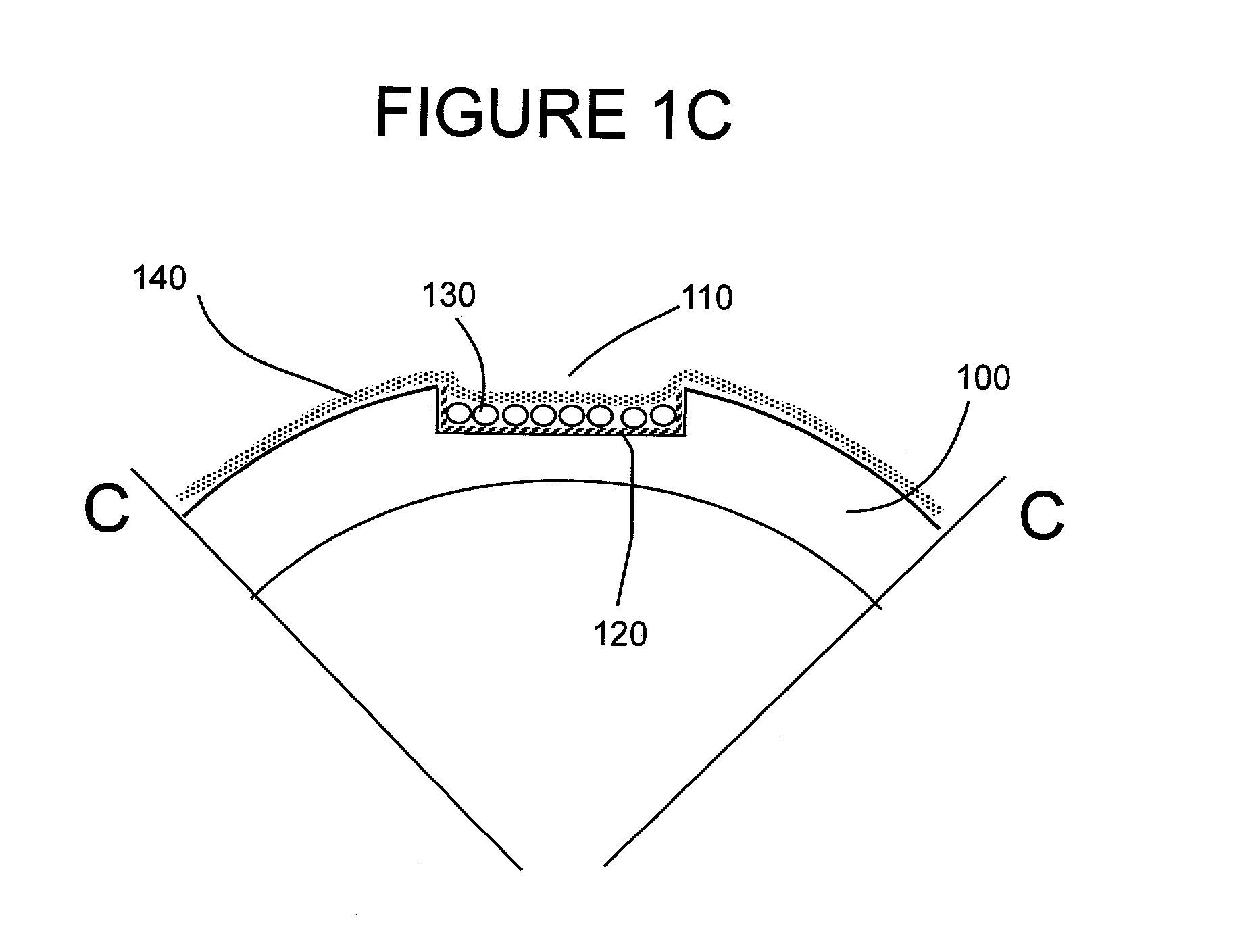
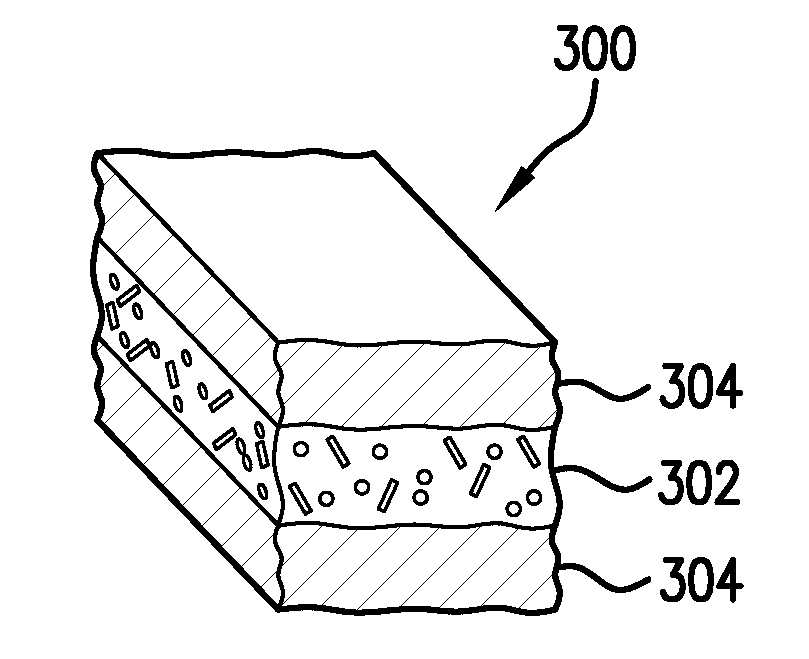
Hemostatic sandwich bandage
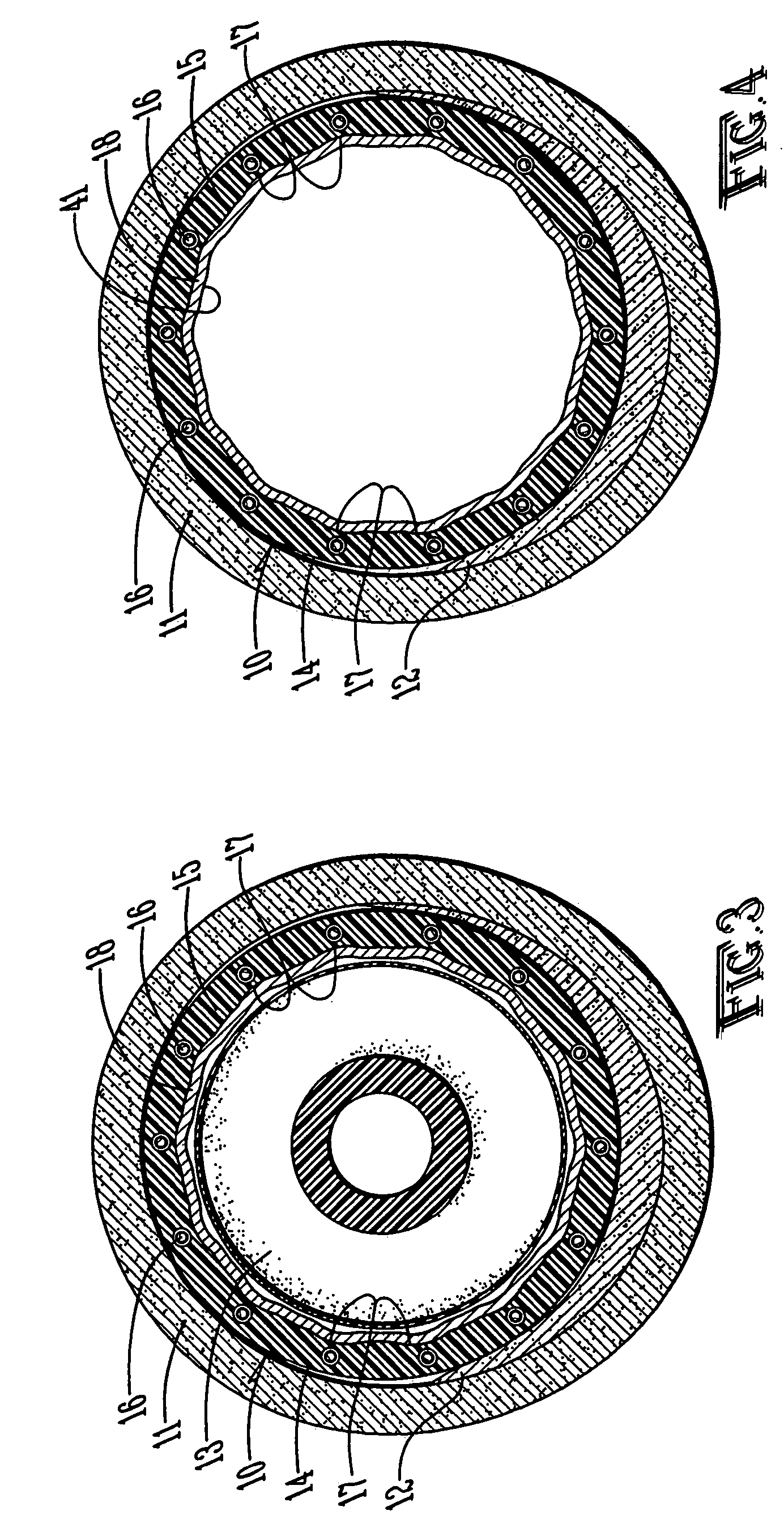
The present invention relates to a haemostatic multilayer bandage that comprises preferably a thrombin layer between two fibrinogen layers. The dressing may contain other resorbable materials such as glycolic acid or lactic acid based polymers or copolymers. The inventive haemostatic bandage is useful for the treatment of wounded tissue.
Owner:AMERICAN NAT RED CROSS
Factor VIII polypeptide
ActiveUS7041635B2Stable and efficiently expressed formFull coagulation activityFactor VIIPeptide/protein ingredientsThrombin activityAmino acid
Owner:SK BIOSCI CO LTD
Composition and method for the repair and regeneration of cartilage and other tissues
InactiveUS7148209B2Add supportImprove coagulation/solidificationBiocidePeptide/protein ingredientsAbnormal tissue growthRepair tissue
Owner:SMITH & NEPHEW ORTHOPAEDICS
Apparatus and method of preparation of stable, long term thrombin from plasma and thrombin formed thereby
InactiveUS6274090B1Derive fast acting, stable autologous thrombinSimple preparatory procedureImmobilised enzymesBioreactor/fermenter combinationsTissue sealantDonors plasma
A sterile method for preparing stable thrombin component from a single donor's plasma in which the thrombin component is harvested simultaneously from the clotting and adhesive proteins component from the same donor plasma in less than one hour. The combined components provide an improved biological hemostatic agent and tissue sealant by virtue of its freedom from the risk of contaminating viruses or bacteria from allogenic human or bovine blood sources. The thrombin provides polymerization of the clotting and adhesive proteins in less than five seconds, and is sufficiently stable to provide that fast clotting over a six hour period. Further, the clotting times can be predictably lengthened by diluting the thrombin with saline.
Owner:ASAHI KASEI MEDICAL CO LTD
Compounds useful in the complement, coagulat and kallikrein pathways and method for their preparation
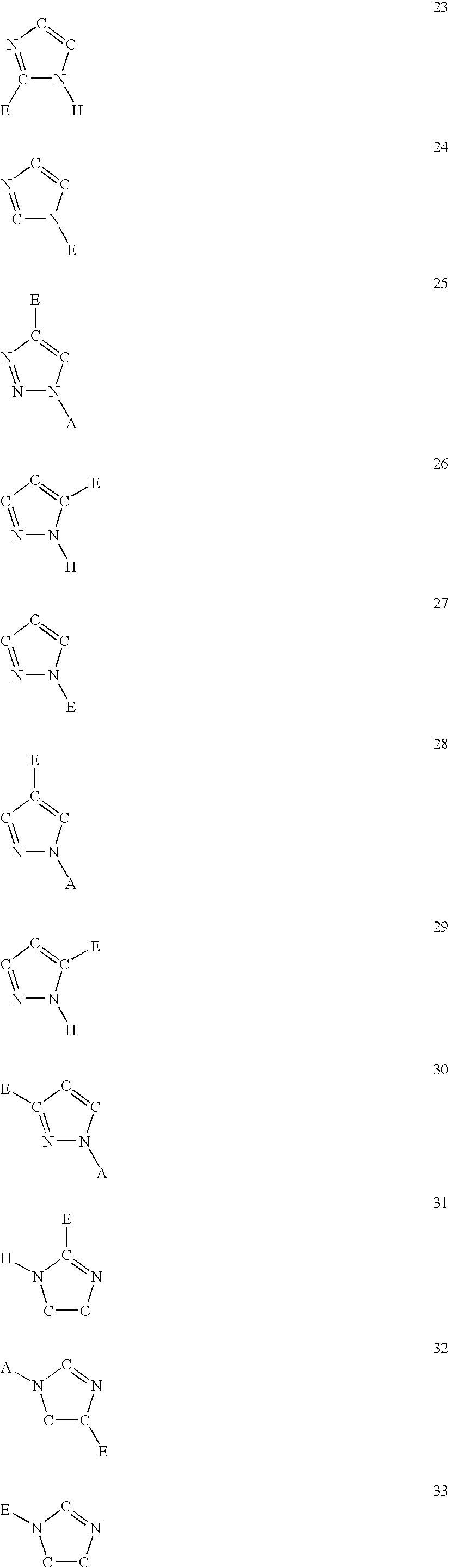
The present invention is concerned with new compounds, and particularly those having a fused bicyclic ring substituted with an amidine moiety. These compounds are each potent inhibitors of Factor D of the alternate pathway of complement, C1s of the classical pathway of complement, Factors Xa, XIIa, VIIa and thrombin of the coagulation pathway, plasmin in the fibrinolytic pathway, and kallikrein and high molecular weight kininogen in the inflammatory pathways. These proteases, which have serine in their active site, are called serine proteases and they are pivotal to most of the processes of inflammation and coagulation. In fact, these various systems are interactive with one another and it is difficult to activate one pathway without it influencing the others.
Owner:BIOCRYST PHARM INC
Composition and method for the repair and regeneration of cartilage and other tissues
InactiveUS20060029578A1Add supportImprove coagulation/solidificationBiocideOrganic active ingredientsAbnormal tissue growthRepair tissue
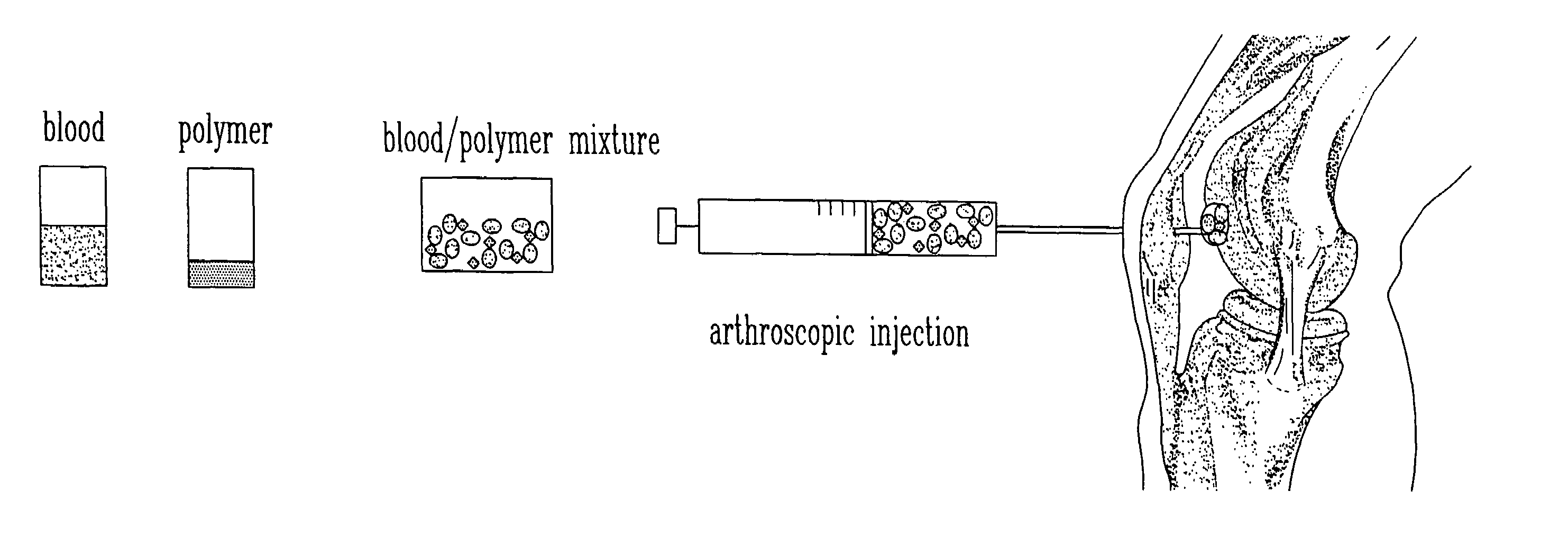
The present invention relates to a new method for repairing human or animal tissues such as cartilage, meniscus, ligament, tendon, bone, skin, cornea, periodontal tissues, abscesses, resected tumors, and ulcers. The method comprises the step of introducing into the tissue a temperature-dependent polymer gel composition such that the composition adhere to the tissue and promote support for cell proliferation for repairing the tissue. Other than a polymer, the composition preferably comprises a blood component such as whole blood, processed blood, venous blood, arterial blood, blood from bone, blood from bone-marrow, bone marrow, umbilical cord blood, placenta blood, erythrocytes, leukocytes, monocytes, platelets, fibrinogen, thrombin and platelet rich plasma. The present invention also relates to a new composition to be used with the method of the present invention.
Owner:SMITH & NEPHEW ORTHOPAEDICS
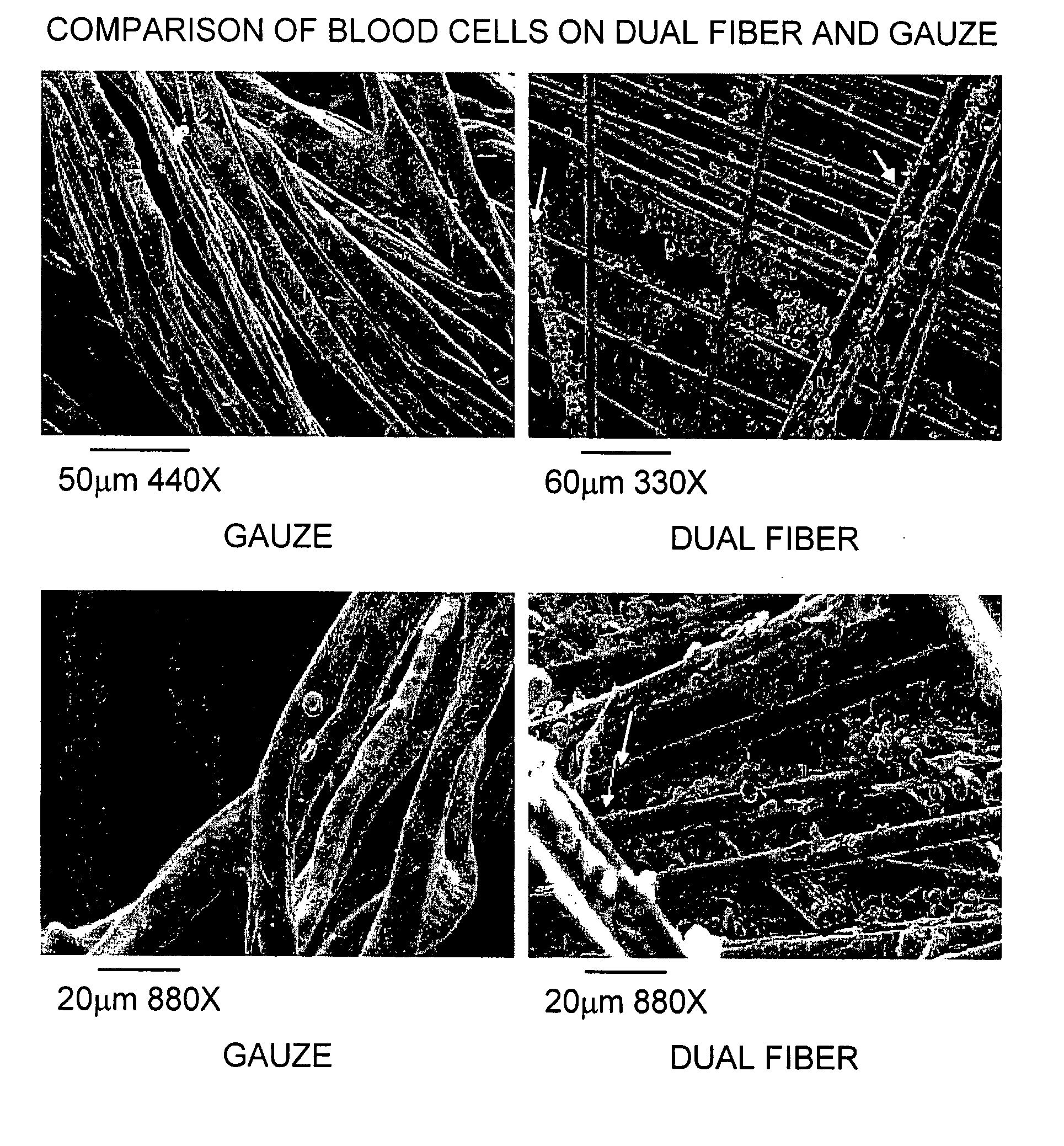
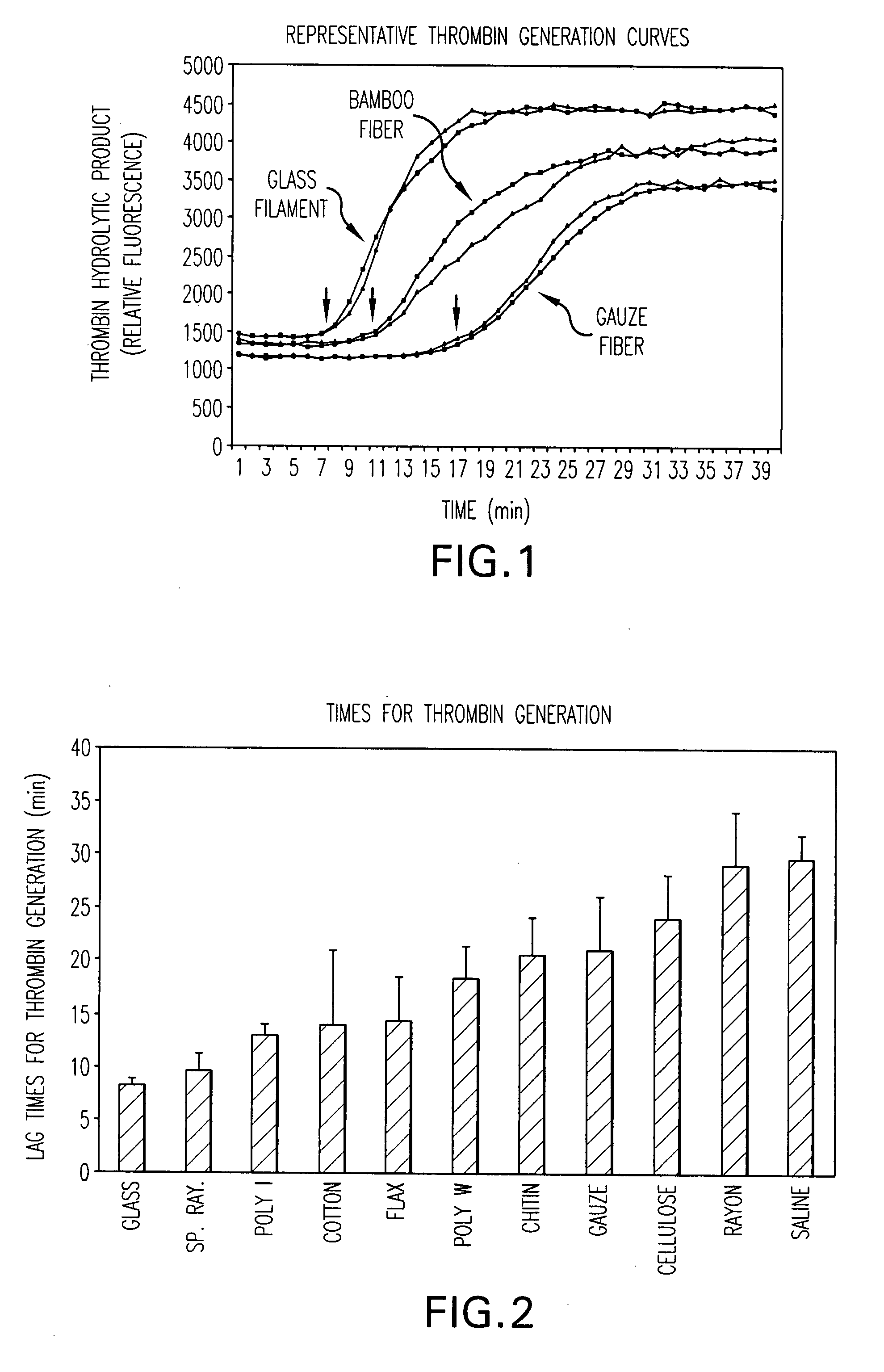
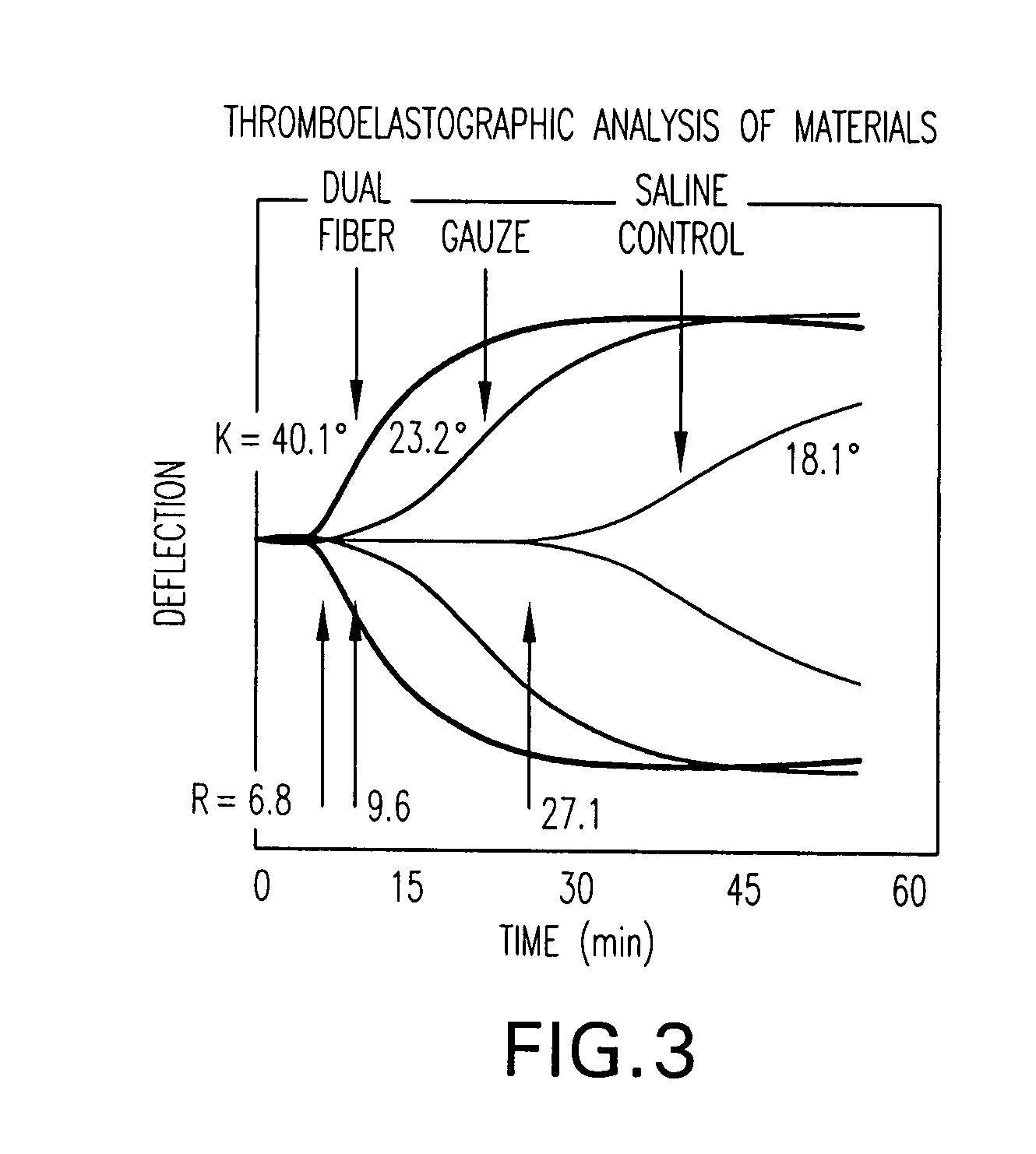
Hemostatic textile
ActiveUS20070160653A1Quick activationNon-adhesive dressingsPeptide/protein ingredientsLactideSisal fiber
The present invention is directed to a hemostatic textile, comprising: a material comprising a combination of glass fibers and one or more secondary fibers selected from the group consisting of silk fibers; ceramic fibers; raw or regenerated bamboo fibers; cotton fibers; rayon fibers; linen fibers; ramie fibers; jute fibers; sisal fibers; flax fibers; soybean fibers; corn fibers; hemp fibers; lyocel fibers; wool; lactide and / or glycolide polymers; lactide / glycolide copolymers; silicate fibers; polyamide fibers; feldspar fibers; zeolite fibers, zeolite-containing fibers, acetate fibers; and combinations thereof; the hemostatic textile capable of activating hemostatic systems in the body when applied to a wound. Additional cofactors such as thrombin and hemostatic agents such as RL platelets, RL blood cells; fibrin, fibrinogen, and combinations thereof may also be incorporated into the textile. The invention is also directed to methods of producing the textile, and methods of using the textile to stop bleeding.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL +1
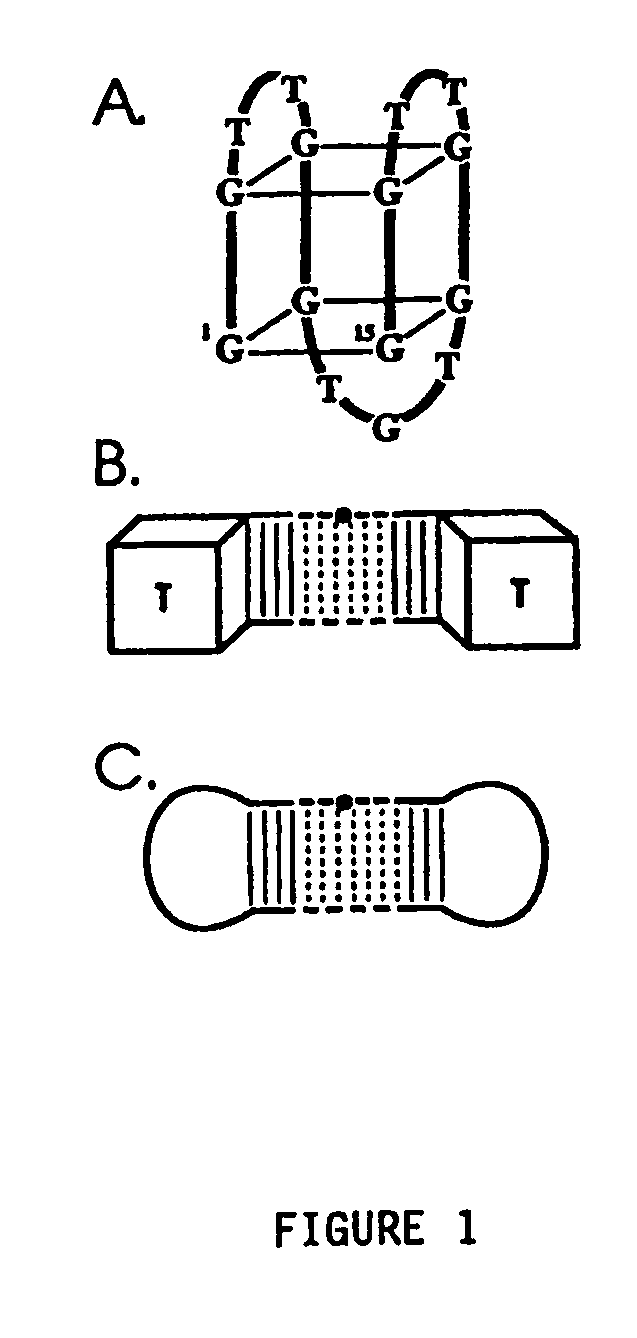
Aptamers and antiaptamers
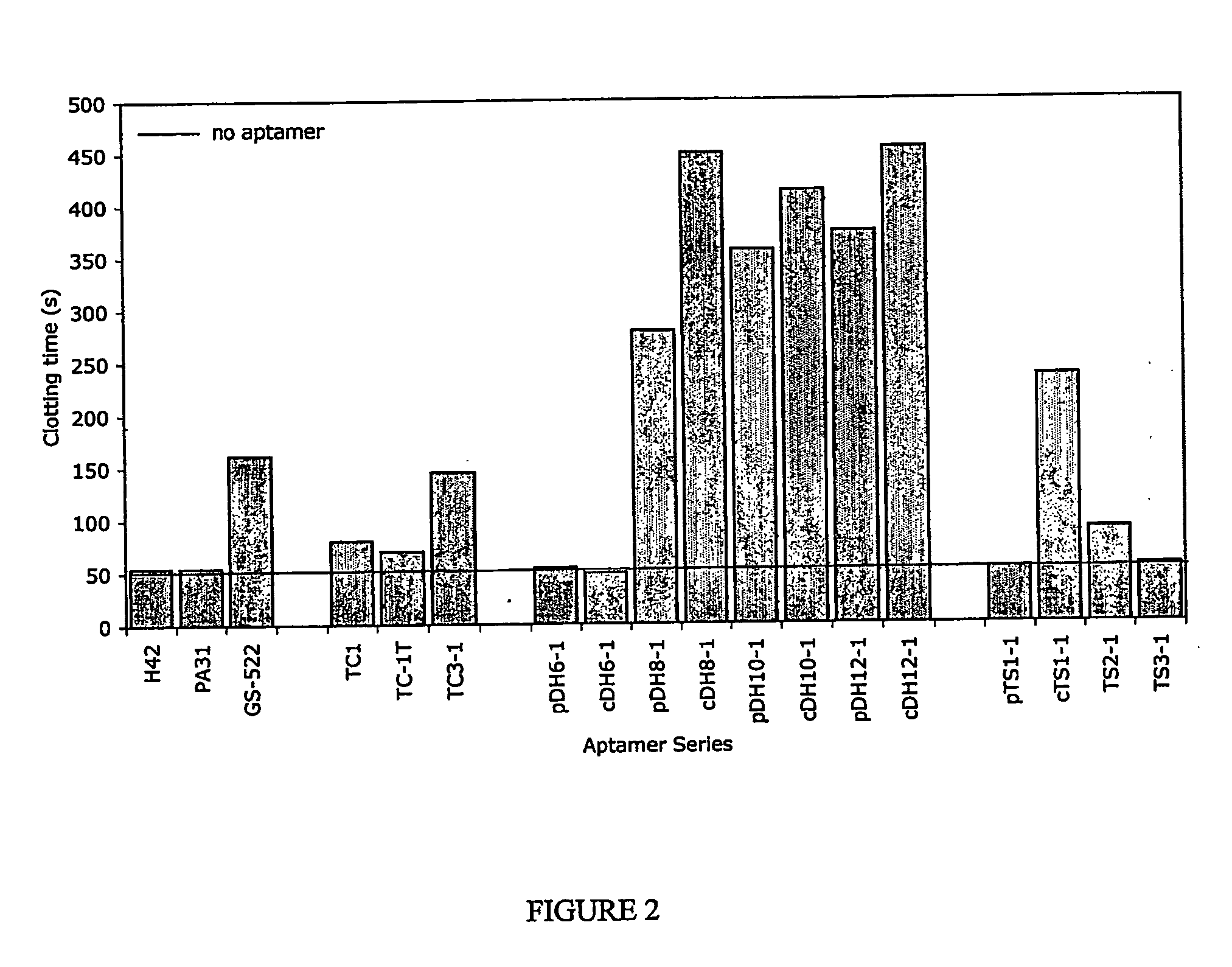
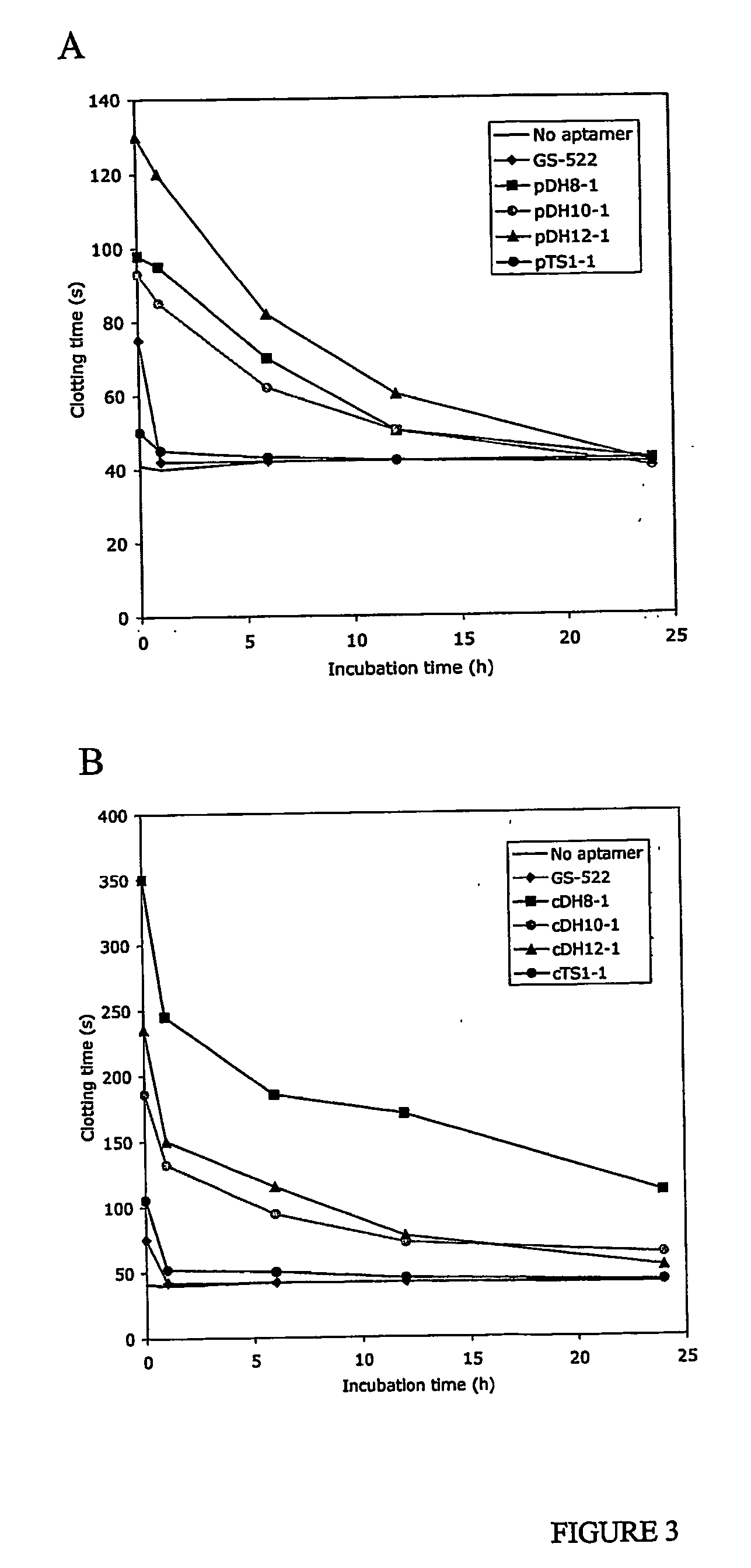
InactiveUS20050176940A1Preventing and reducing coagulation of bloodOrganic active ingredientsSugar derivativesNucleotideThrombin activity
The present invention relates to: An aptamer comprising a circular oligonucleotide defining one to four target binding regions; An aptamer comprising an oligonucleotide defining two, three or four thrombin binding quadruplex regions separated by at least partially duplex regions, wherein the quadruplex regions comprise a GGTMGGXGGTTGG sequence wherein M represents A or T and X represents a sequence of two to five nucleotides and / or nucleotide analogues; An aptamer represented by formula (I): 5′D1, wQxD1D2yQzD2,3′—the variables are as defined in the specification; and Aptamers selected from specific sequences.
Owner:UNISEARCH LTD
Fibrin glue without fibrinogen and biosealant compositions and methods
InactiveUS6168788B1Stop blood flowPrevents unwantedSurgical adhesivesPeptide/protein ingredientsFibrin glueClot formation
The invention is a fibrin glue that avoids the use of fibrinogen and thus eliminates the need for premixing and premature clot formation. The fibrin glue of the invention comprises thrombin, thromboplastin and calcium and may have clotting Factors, VII, IX and X, and the like. The invention also comprises a biosealant for use with the fibrin glue without fibrinogen or for use alone. The biosealant is a two component mixture of gelatin / resorcinol and glyoxal / glutaraldehyde / 4-(p-maleimidophenyl) butyric acid. The two components are mixed on use.
Owner:WORTHAM LEON
Encased stent
InactiveUS7311727B2Prevent restenosisImprove scalabilityStentsSurgeryCell Cycle InhibitionPolyethylene glycol
An encased stent that discourages restenosis by having a homogenous endothelial cell lining along the inner wall of the stent. The endothelial cell lining may be coated on the stent before the stent is placed in the artery, or the endothelial cell lining may be grown after placement by several factors that encourage such growth and discourage restenosis. The endothelial cells to coat the stent may be genetically modified to enhance the growth of the endothelial cells into a homogeneous lining. The stent has a continuous lining in the form of a multi-layer polymer coating, including a conducting biocorrosion inhibiting layer and a continuous film of polyurethane coupled by a coupling agent to polyethylene glycol. Various drugs and cell factors may be incorporated into the lining, such as anti-thrombin, anti-inflammatory and anti-coagulant drugs, cell cycle inhibitors, and vascular endothelial growth factors.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
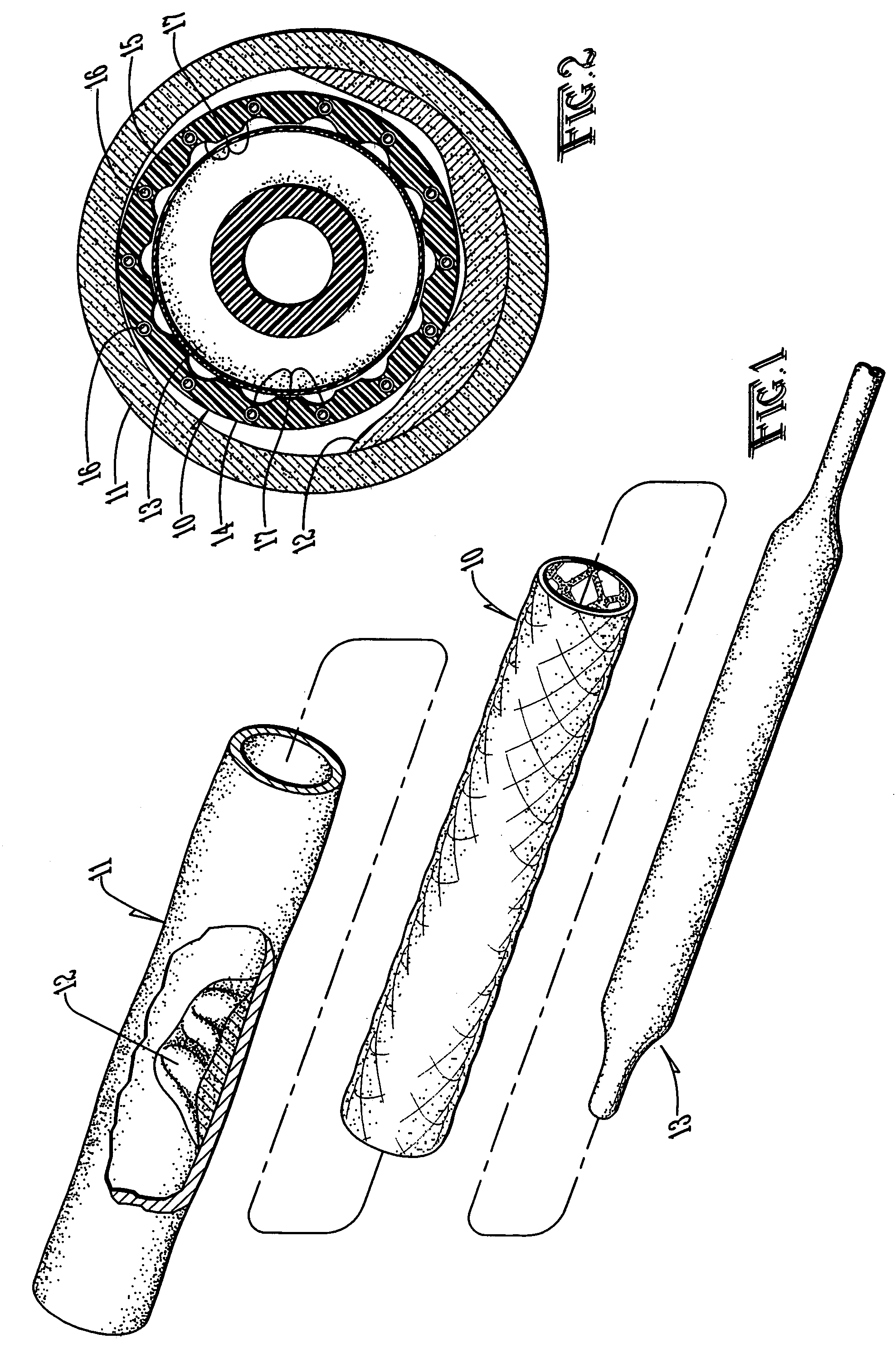
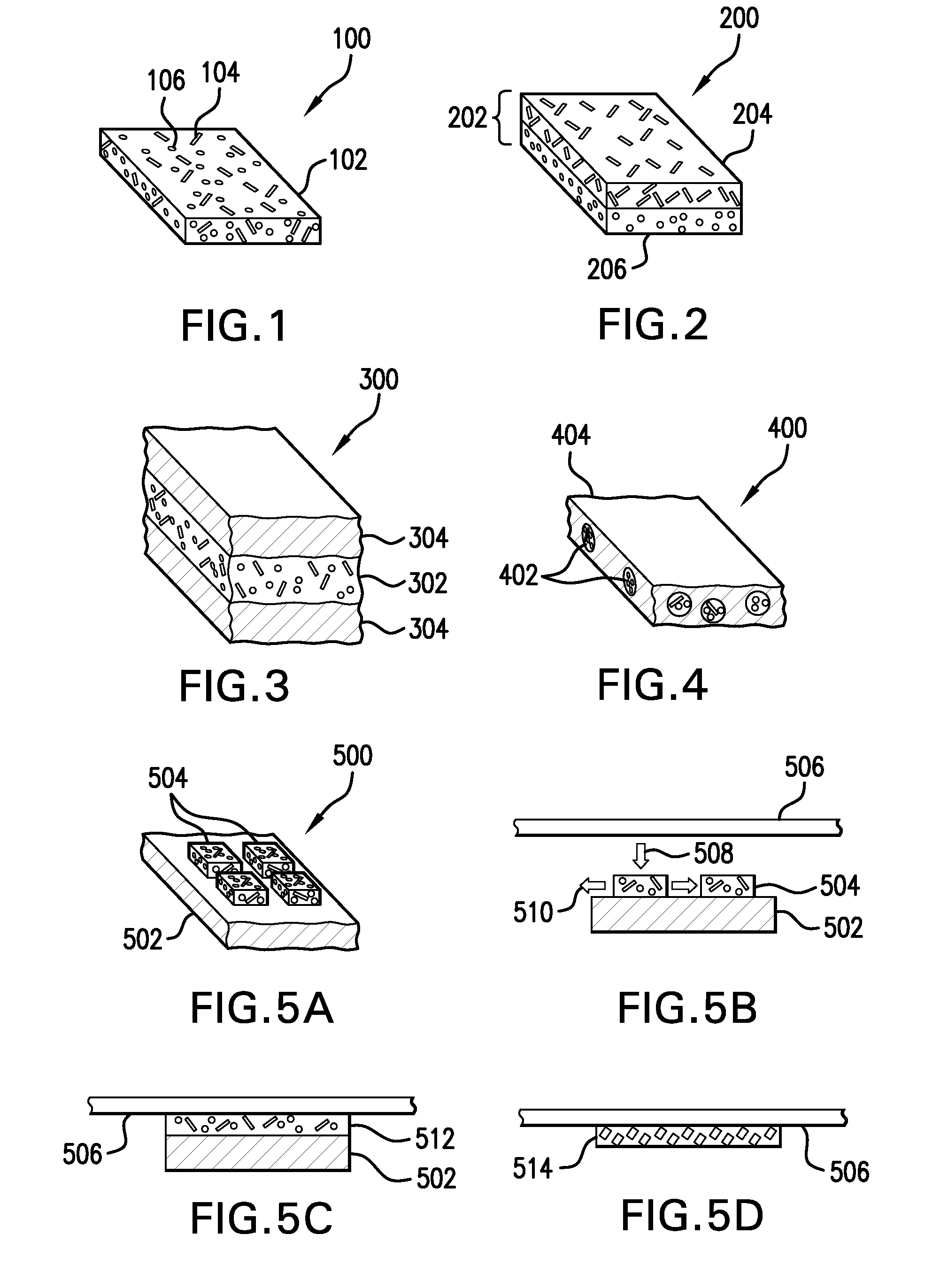
Carrier with solid fibrinogen and solid thrombin
The present invention relates to a solid composition useful for tissue gluing, tissue sealing and haemostasis consisting essentially of a) a carrier which has at least one of the following physical properties: elasticity module in the range of 5-100 N / cm, density of 1-10 mg / cm<3>, chamber diameter of more than 0.75 mm and less than 4 mm and / or having a chamber diameter average below 3 mm and evenly distributed and fixed upon said carrier, b) solid fibrinogen, and c) solid thrombin.The carrier is a biodegradable polymer such as a polyhyaluronic acid, polyhydroxy acid, e.g. lactic acid, glucolic acid, hydroxybutanoic acid, a cellulose, gelatine or collagen, such as a collagen sponge, e.g. a collagen sponge consisting essentially of collagen type I fibres. The fibrinogen and thrombin are preferably human, purified from a natural source, or transgenic or recombinant human fibrinogen and / or thrombin. In a preferred embodiment the composition does not comprise any antifibronolytic agent such as aprotinin, epsilon-aminocaproic acid or alpha2-antiplasmin,
Owner:TOPAZ INVESTMENT AS
Biarylmethyl indolines and indoles as antithromboembolic agents
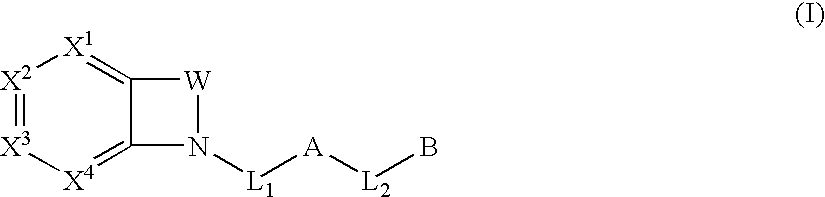
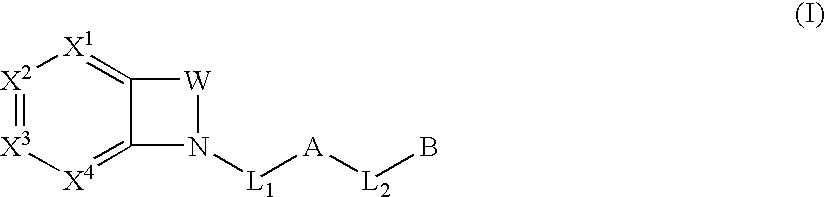
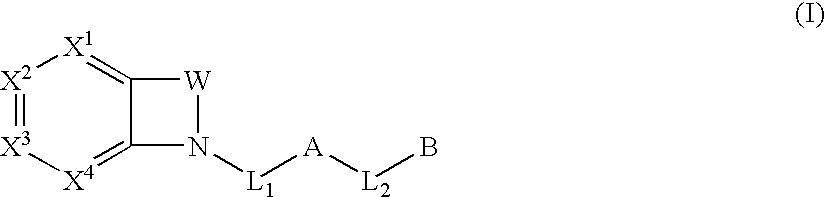
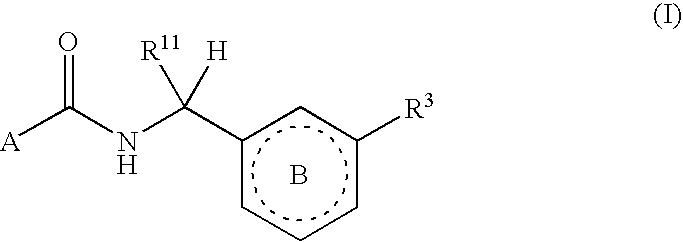
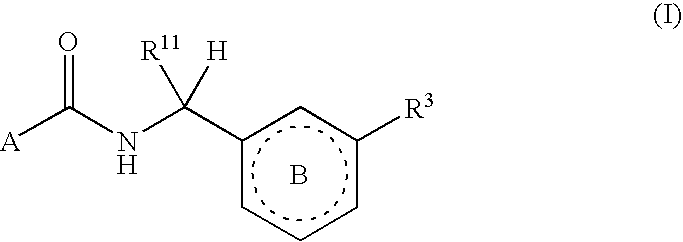
The present invention provides compounds of Formula (I):or a stereoisomer or pharmaceutically acceptable salt or hydrate form thereof, wherein the variables A, B, L1, L2, X1, X2, X3, X4 and W are as defined herein. The compounds of Formula (I) are useful as selective inhibitors of serine protease enzymes of the coagulation cascade and / or contact activation system; for example thrombin, factor Xa, factor XIa, factor IXa, factor VIIa and / or plasma kallikrein. In particular, it relates to compounds that are selective factor XIa inhibitors. This invention also relates to pharmaceutical compositions comprising these compounds and methods of treating thromboembolic and / or inflammatory disorders using the same.
Owner:BRISTOL MYERS SQUIBB CO
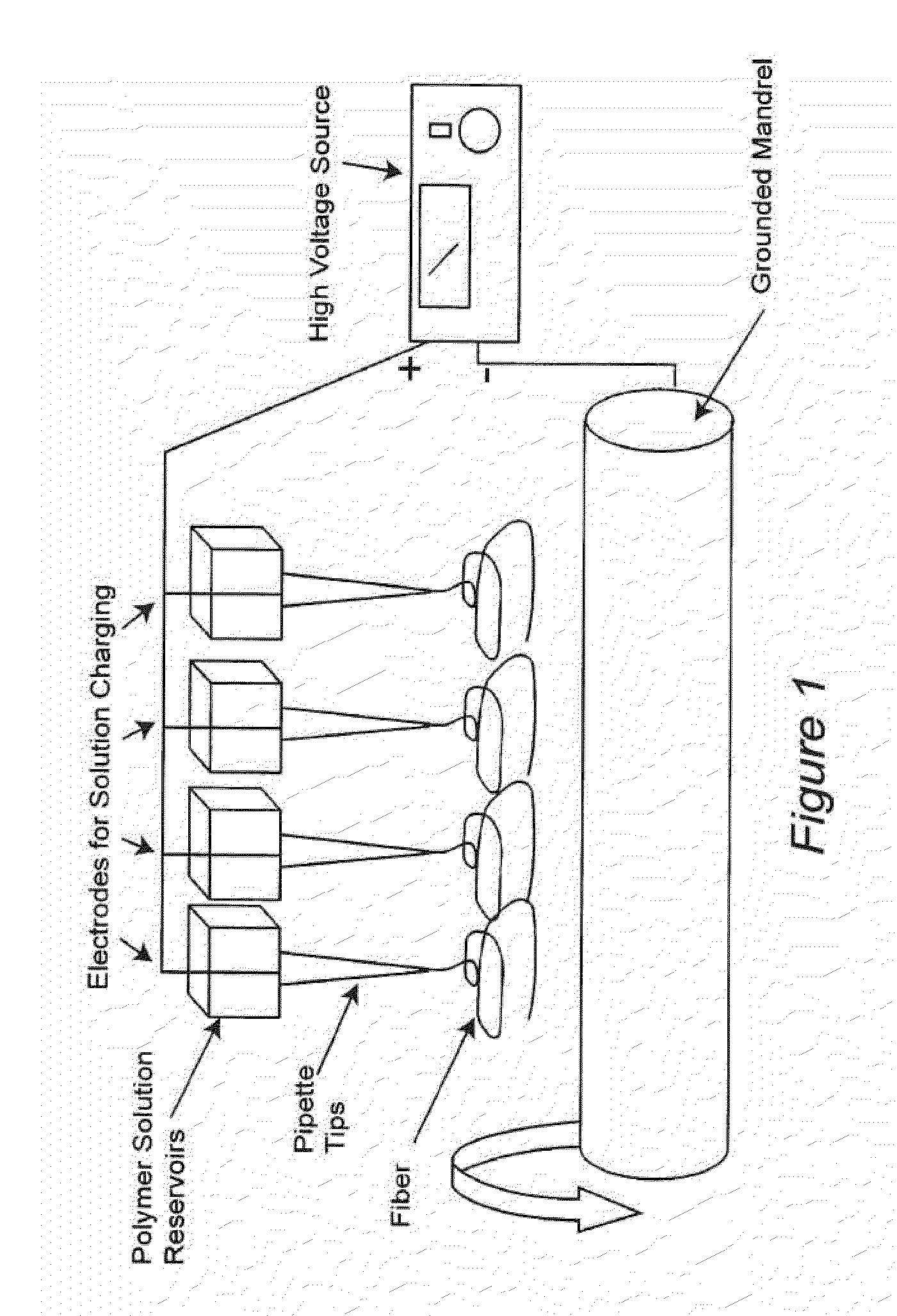
Electrospun dextran fibers and devices formed therefrom
The invention generally relates to dextran fibers which are preferably electrospun and devices formed from such fibers. In particular, such devices may include substances of interest (such as therapeutic substances) associated with the electrospun fibers. Upon exposure to a liquid the electrospun fibers dissolve immediately and the substances of interest are released into the liquid. Exemplary devices include bandages formed from electrospun dextran fibers and associated agents that promote hemostasis, such as thrombin and fibrinogen.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC +1
Chitosan-based styptic sponge and preparation method thereof
InactiveCN102526795AImprove stabilityMaintain coagulation activityAbsorbent padsBandagesWound healingThrombin activity
The invention relates to chitosan-based styptic sponge with a thrombin immobilization effect, and the chitosan-based styptic sponge is a porous sponge made from chitosan with the thrombin immobilization effect and hemostatic. The preparation method of the chitosan-based styptic sponge provided by the invention comprises the following steps: immobilizing thrombin with chitosan or carboxymethyl chitosan, and adding other styptics, cryoprotectants and crosslinking agents to prepare the porous styptic sponge, wherein the weight ratio of the chitosan or carboxymethyl chitosan to the thrombin is 100:(0.1-20); pre-freezing, lyophilizing in vacuum, casting, cutting, encapsulating and sterilizing to prepare the chitosan-based styptic sponge. According to the chitosan-based styptic sponge, the thrombin is immobilized through the chitosan or carboxymethyl chitosan, so that the stability and procoagulant activity of the thrombin can be improved, and the property of the prepared chitosan-based styptic sponge is more stable, the procoagulant and wound healing effects are remarkably improved, and the chitosan-based styptic sponge can be widely applied to wound or surgery hemostasis.
Owner:WUHAN GENERAL HOSPITAL OF GUANGZHOU MILITARY
Biaryl compounds as serine protease inhibitors
InactiveUS6699994B1Organic chemistryPeptide/protein ingredientsSerine Protease InhibitorsFactor VIIa
Owner:BIOCRYST PHARM INC
Methods and compositions for the treatment and diagnosis of vascular inflammatory disorders or endothelial cell disorders
InactiveUS20080199426A1Inhibition effectLow release rateOrganic active ingredientsPeptide/protein ingredientsVasculitisMedicine
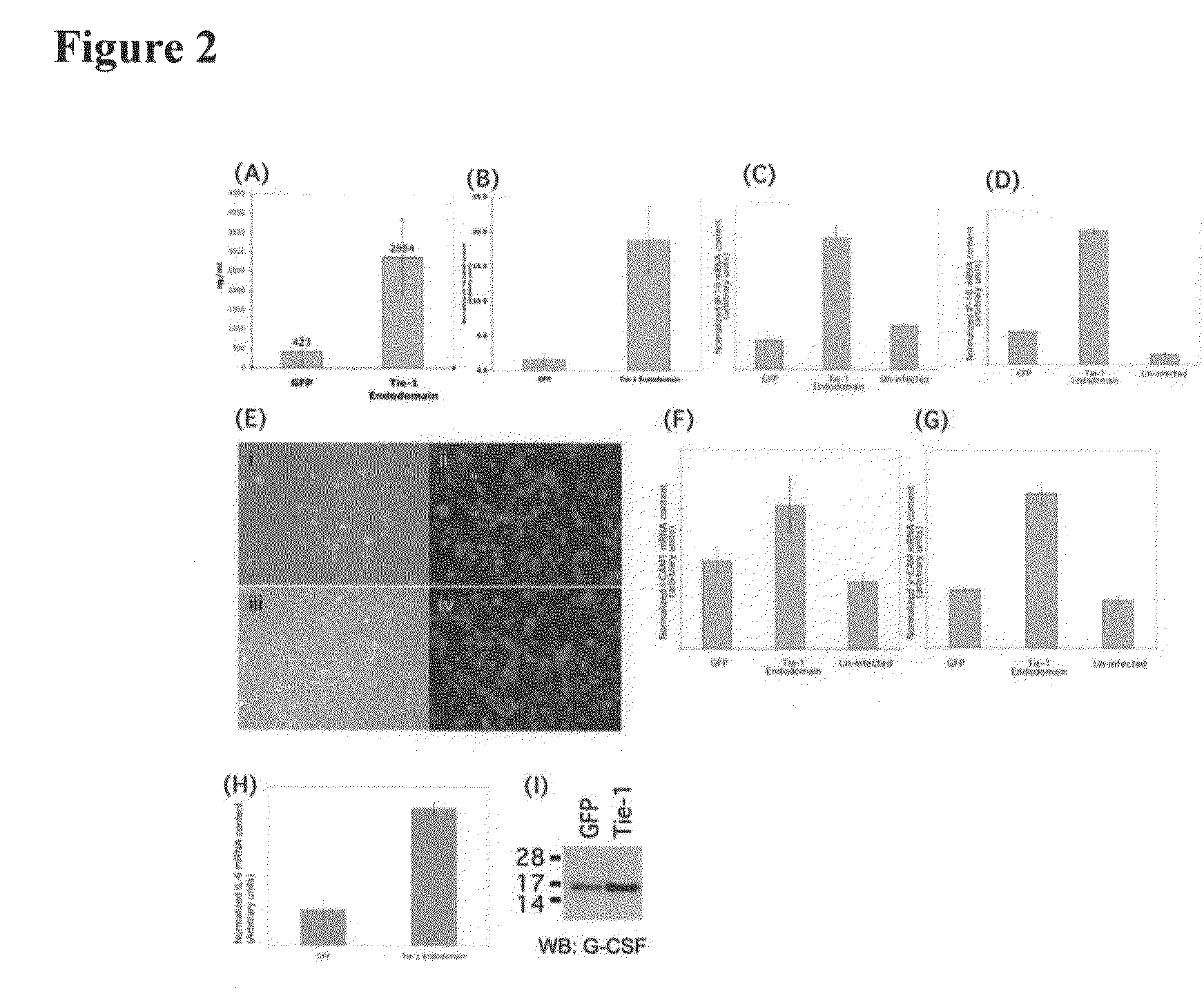
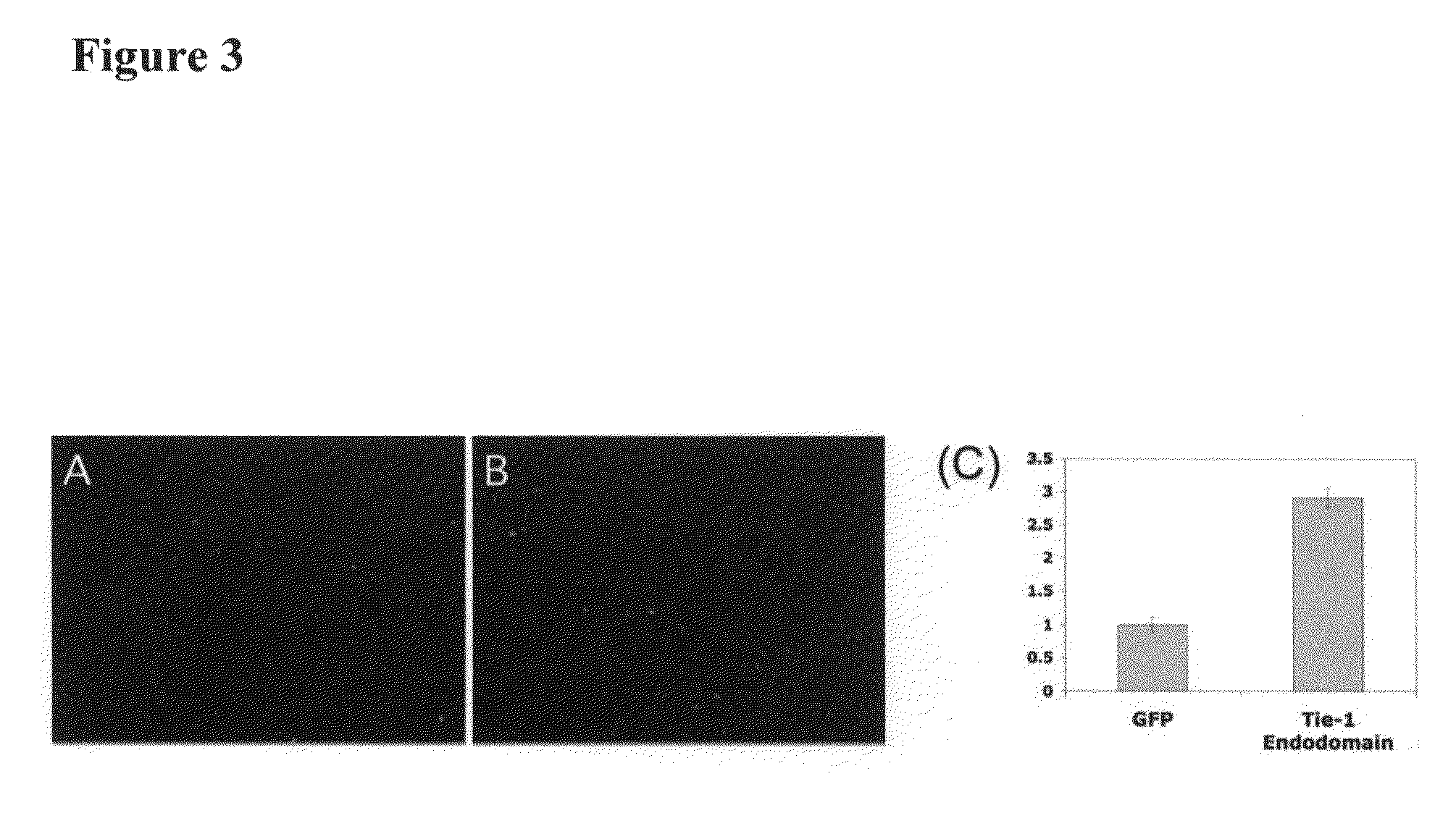
Disclosed herein are methods for treating a vascular inflammatory disorder or endothelial cell disorder using inhibitor compounds that inhibit the expression or biological activity of Tie-1, Tie-1 endodomain, thrombin, VEGFR2, VEGFR2 endodomain, EphA2, and any of the cytokines or kinases that are upregulated by activation of Tie-1 or thrombin, as provided herein. Also disclosed are the use of combinations of inhibitor compounds or the use of an eNOS activator compound in combination with any one or more of the inhibitor compounds. Also disclosed are methods for inhibiting the pro-coagulant activity of thrombin using a Tie-1 or Tie-1 endodomain inhibitor compound or an EphA2 inhibitor compound. Methods for diagnosing and monitoring vascular inflammatory disorders or endothelial cell disorders that include the measurement of any of the polypeptides or nucleic acid molecules of the invention are also disclosed.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Fibrin material and method for producing and using the same
This invention describes a bioerodible fibrin material which is obtained by mixing fibrinogen and thrombin reconstituted or diluted with a particular high tonic strength medium, free of calcium. Such a fibrin-based biomaterial develops a tight structure with thin fibers and small pore size suitable for use as an anti-adhesion barrier. In this invention, thrombin is no longer the variable which governs the tightness and the porosity of the fibrin material obtained, but still controls the clotting time. The mechanical behavior, high-water capacity, and releasable retention properties for therapeutic agents of this fibrin structure causes the fibrin material to be ideally suited for use as a drug delivery device, capable of delivering proteins, hormones, enzymes, antibiotics, antineoplastic agents and even cells for local and systemic treatment of human and non-human patients.
Owner:BAXTER INT INC
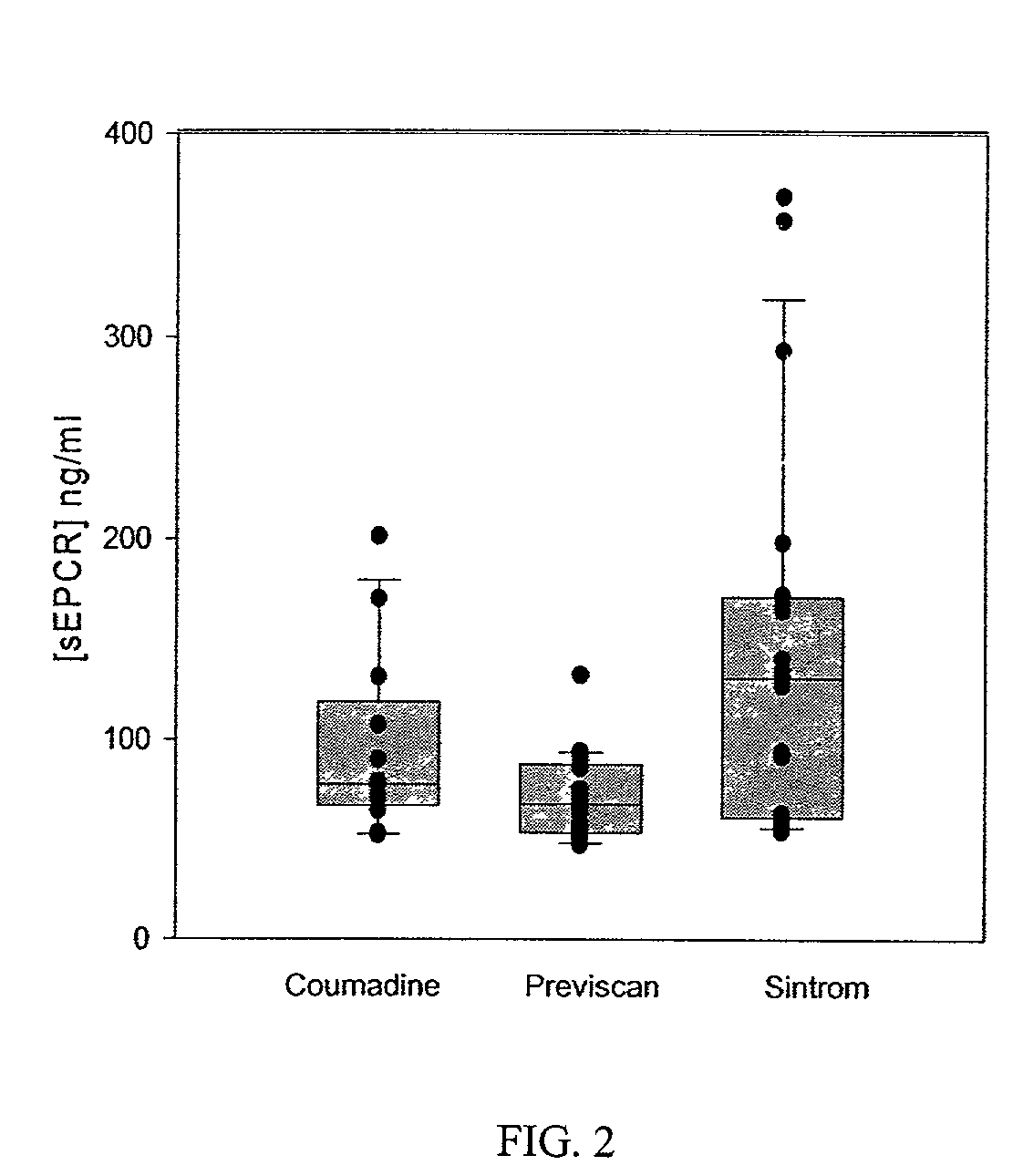
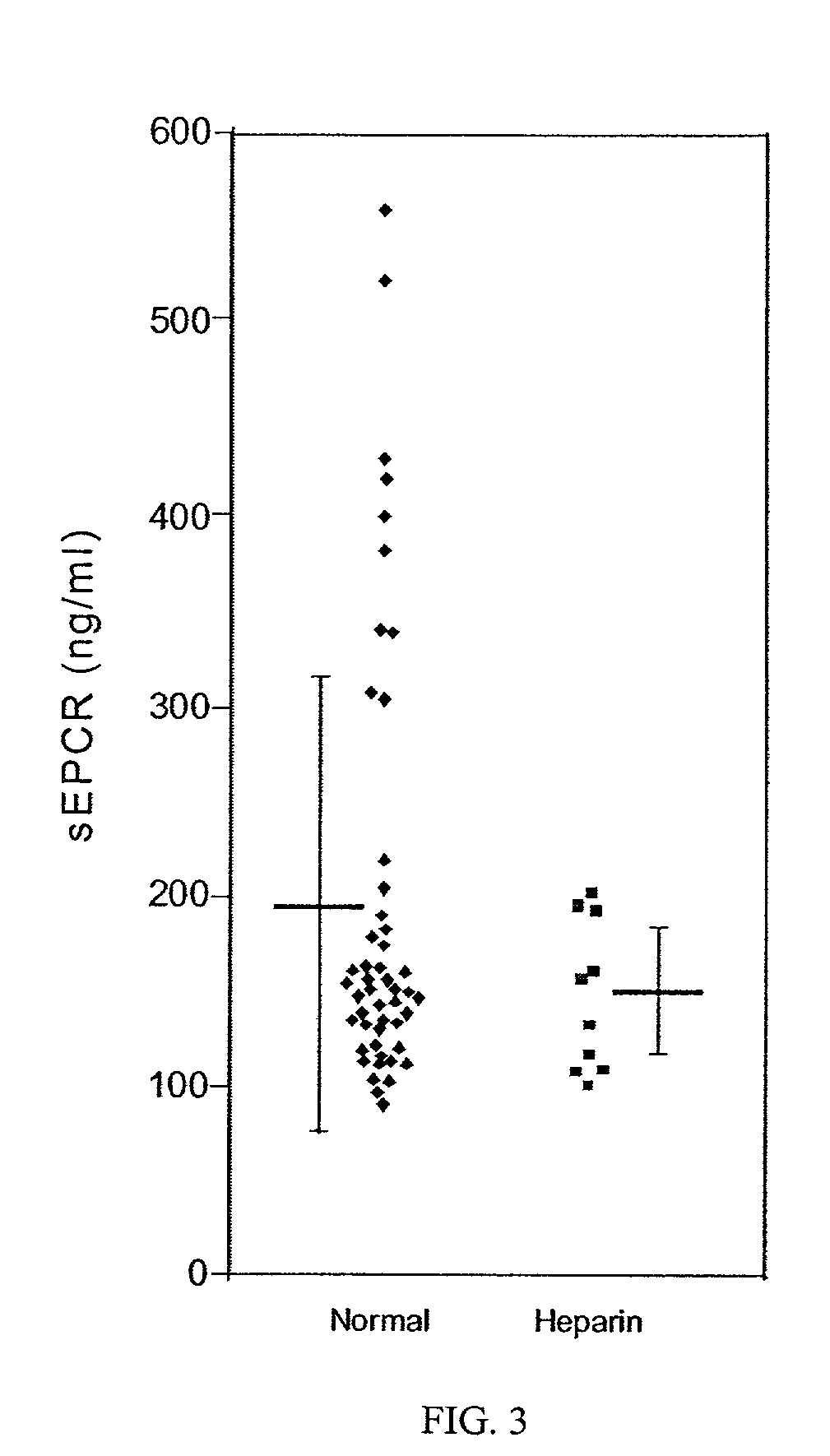
Method for monitoring coagulability and hypercoagulable states
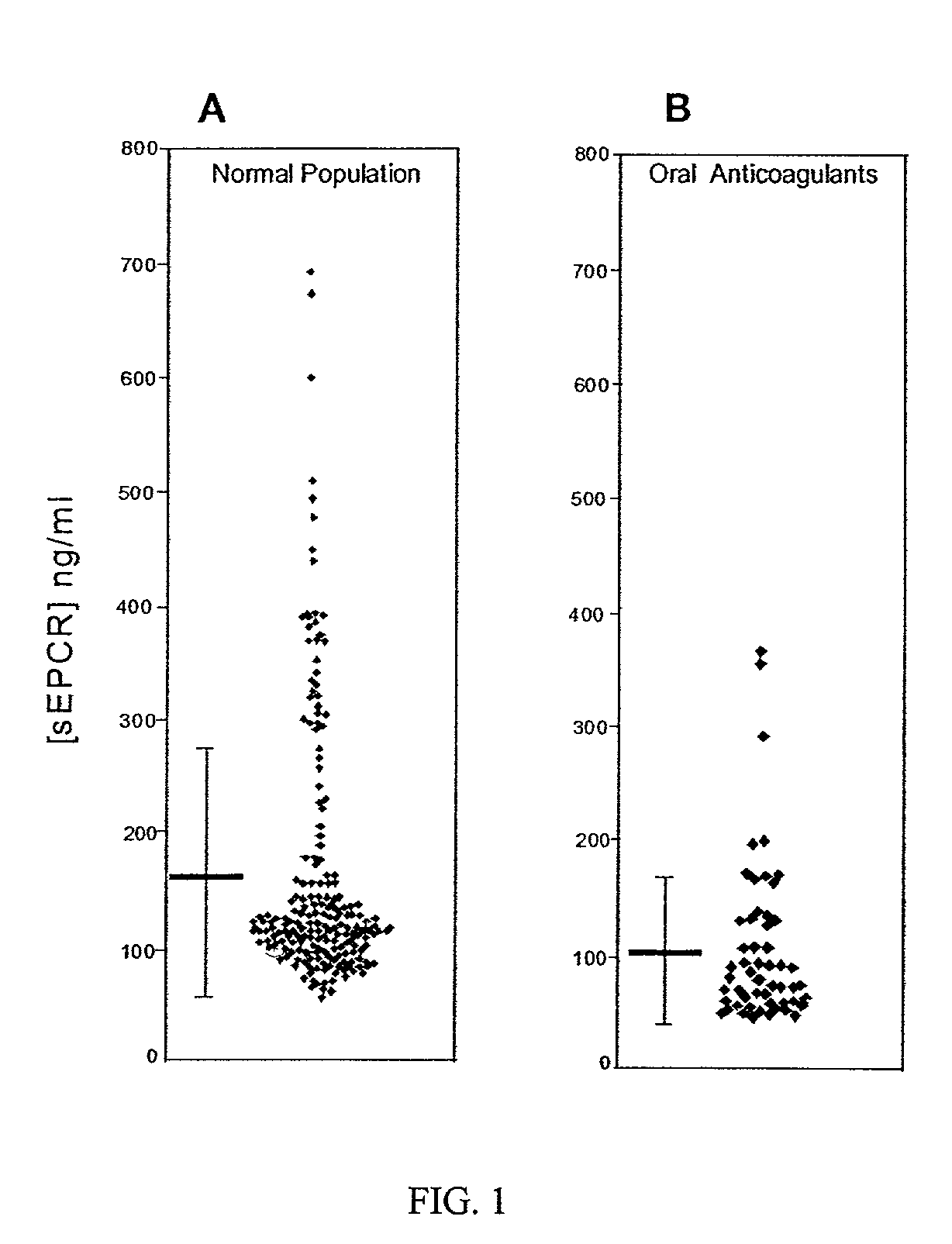
The assay of soluble endothelial protein C receptor (sEPCR) is useful to monitor effective thrombin levels and a hypercoagulable state. An assay for sEPCR is therefore useful to monitor ongoing effectiveness of anticoagulant therapy. A sEPCR ELISA assay is particularly useful for this purpose. A state of hypercoagulability in patients or normal individuals can also be identified by such an assay.
Owner:OKLAHOMA MEDICAL RES FOUND
Carrier with solid fibrinogen and solid thrombin
InactiveUS7052713B2Safety and efficacyShorten hemostasis timePowder deliverySurgical adhesivesNatural sourceFiber
The present invention relates to a solid composition useful for tissue gluing, tissue sealing and hemostasis consisting essentially of a) a carrier which has at least one of the following physical properties: elasticity module in the range of 5-100 N / cm, density of 1-10 mg / cm3, chamber diameter of more than 0.75 mm and less than 4 mm and / or having a chamber diameter average below 3 mm and evenly distributed and fixed upon said carrier, b) solid fibrinogen, and c) solid thrombin.The carrier is a biodegradable polymer such as a polyhyaluronic acid, polyhydroxy acid, e.g. lactic acid, glucolic acid, hydroxybutanoic acid, a cellulose, gelatine or collagen, such as a collagen sponge, e.g. a collagen sponge consisting essentially of collagen type I fibers. The fibrinogen and thrombin are preferably human, purified from a natural source, or transgenic or recombinant human fibrinogen and / or thrombin. In a preferred embodiment the composition does not comprise any antifibronolytic agent such as aprotinin, ε-aminocaproic acid or α2-antiplasmin,
Owner:TOPAZ INVESTMENT AS
Free-standing biodegradable patch
InactiveUS20110071498A1Increase flexibilityImprove mechanical propertiesHydrolasesPeptide/protein ingredientsThrombin activityFibrinogen
Methods and apparatus for a free-standing biodegradable patch suitable for medical applications, especially intravascular, minimally-invasive and intraoperative surgical applications are provided, wherein the patch comprises a free-standing film or device having a mixture of a solid fibrinogen component and a solid thrombin component that, when exposed to an aqueous environment, undergoes polymerization to form fibrin. In alternative embodiments the patch may comprise a solid fibrinogen component, with or without an inorganic calcium salt component. The patch may take a non-adherent form during delivery to a target location within a vessel or tissue, and thereafter may be activated to adhere to vessel wall or tissue, and may include a number of additives, including materials to improve the mechanical properties of the patch, or one or more therapeutic or contrast agents.
Owner:BIOINSPIRE TECH
Methods for the inhibition of neointima formation
Restenosis is inhibited through local delivery of anti-restenotic agents including angiotensin converting enzyme inhibitors; nicotine receptor agonists, agents that increase concentrations of nitric oxide, anti-angiogenic agents, agonists of the TGF-beta receptor; death domain receptor ligands; and thrombin inhibitors. In one embodiment of the invention, the localized delivery is effected through the use of a stent modified for delivery of the agent at the site of injury from balloon angioplasty.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Apparatus and method of preparation of stable, long term thrombin from plasma and thrombin formed thereby
InactiveUS7056722B1Precise repeatable fast slow polymerizationVarious disease riskBioreactor/fermenter combinationsBiological substance pretreatmentsTissue sealantDonors plasma
A sterile method for preparing stable thrombin component from a single donor's plasma in which the thrombin component is harvested simultaneously from the clotting and adhesive proteins component from the same donor plasma in less than one hour. The combined components provide an improved biological hemostatic agent and tissue sealant by virtue of its freedom from the risk of contaminating viruses or bacteria from allogenic human or bovine blood sources. The thrombin provides polymerization of the clotting and adhesive proteins in less than five seconds, and is sufficiently stable to provide that fast clotting over a six hour period. Further, the clotting times can be predictably lengthened by diluting the thrombin with saline.
Owner:ASAHI KASEI MEDICAL CO LTD
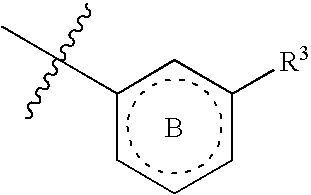
Substituted biaryl compounds as factor XIa inhibitors
The present invention provides compounds of Formula (I): or a stereoisomer, tautomer, pharmaceutically acceptable salt or solvate form thereof, wherein the variables A, L, Z, R3, and ring B are as defined herein. The compounds of Formula (I) are useful as selective inhibitors of serine protease enzymes of the coagulation cascade and / or contact activation system; for example thrombin, factor Xa, factor XIa, factor IXa, factor VIIa and / or plasma kallikrein. In particular, it relates to compounds that are selective factor XIa inhibitors. This invention also relates to pharmaceutical compositions comprising these compounds and methods of treating thromboembolic and / or inflammatory disorders using the same.
Owner:BRISTOL MYERS SQUIBB CO
Biodegradable Metal Barrier Layer for a Drug-Eluting Stent
InactiveUS20080243240A1Reduce and prevent cell proliferationReduce and prevent and inflammationStentsSurgeryAnti mitoticMedical treatment
An implantable medical device includes a substrate, a drug-impregnated layer deposited over the substrate, and a barrier layer at least partially covering the drug-impregnated layer. The barrier layer may be a biodegradable metal, biodegradable metal oxide, or biodegradable metal alloy, such as, magnesium, a magnesium oxide or a magnesium alloy. The drug-impregnated layer includes a therapeutic substance, such as, antineoplastic, anti-inflammatory, antiplatelet, anticoagulant, fibrinolytic, thrombin inhibitor, antimitotic, antiallergic, and antiproliferative substances.
Owner:MEDTRONIC VASCULAR INC
Hemostatic dressing
InactiveUS20060155234A1Easy to handleMore amenable to large-scale manufacturingNon-adhesive dressingsPeptide/protein ingredientsCoagulation proteinThrombin activity
The present invention relates to a hemostatic dressing which comprises a plurality of layers that contain resorbable materials and / or coagulation proteins. In particular, the invention includes dressings in which a layer of thrombin is sandwiched between a first and second layer of fibrinogen and wherein the layer of thrombin is not coextensive with the first and / or second layer of fibrinogen. The hemostatic dressings are useful for the treatment of wounded tissue.
Owner:AMERICAN NAT RED CROSS
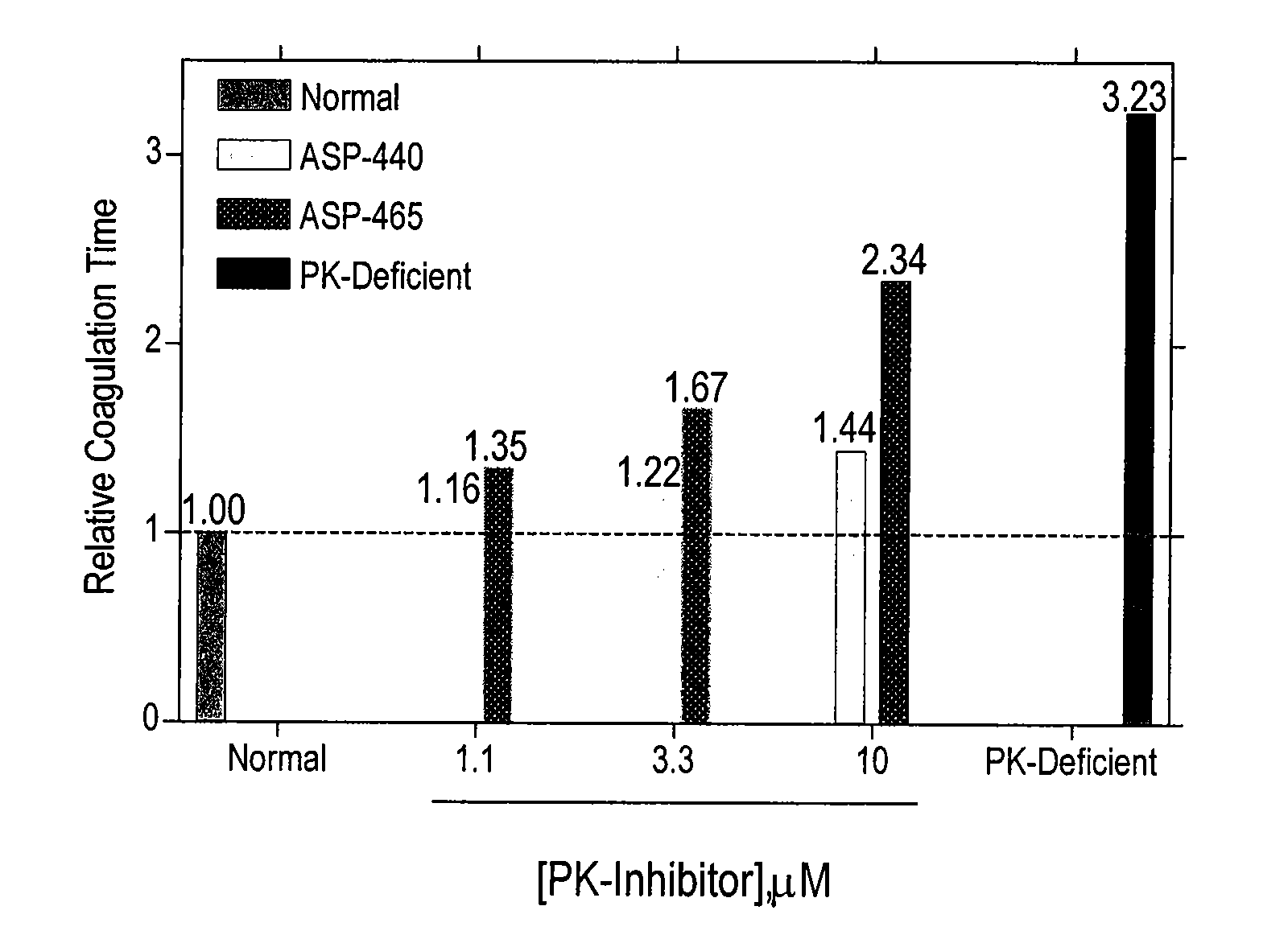
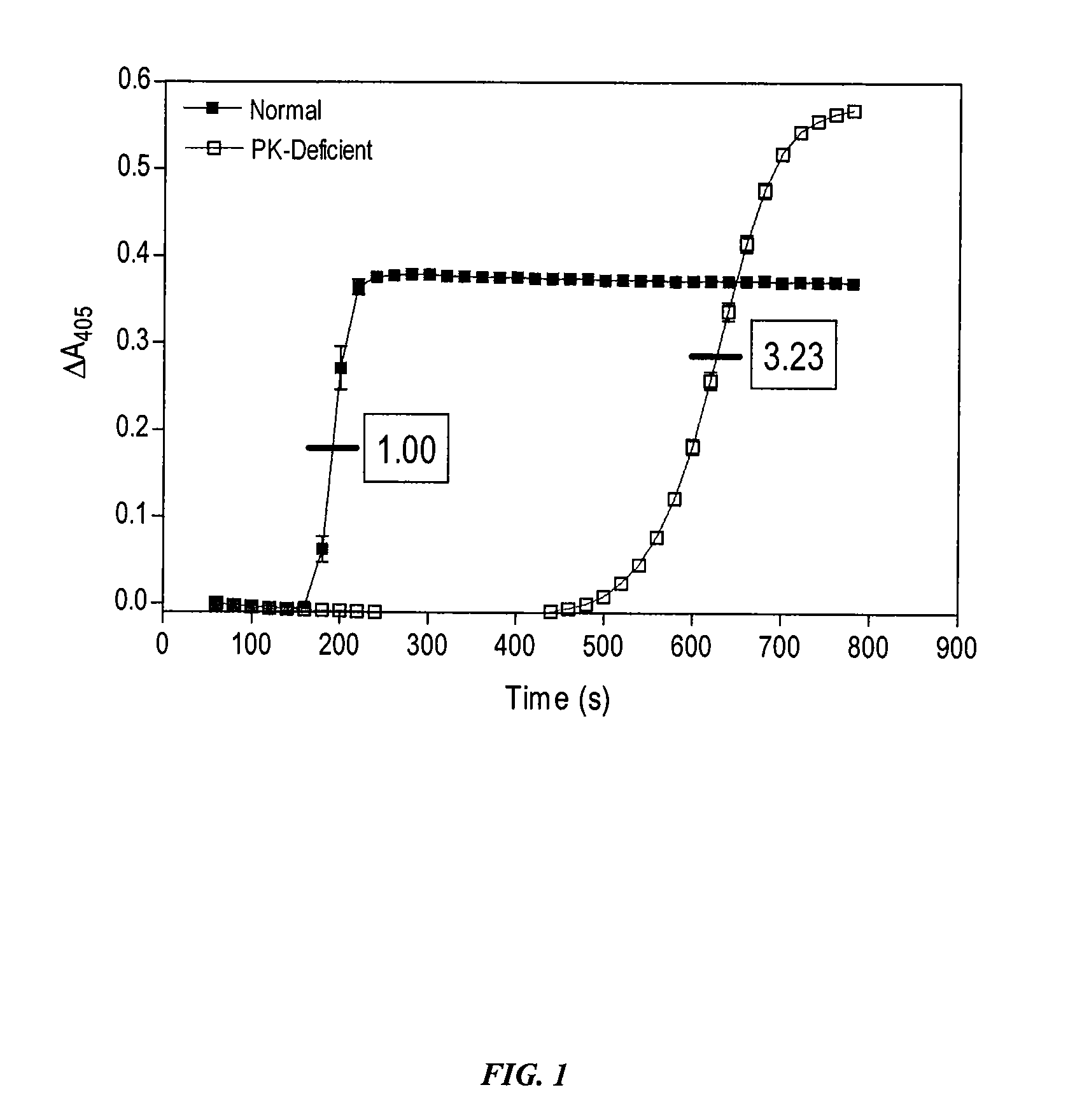
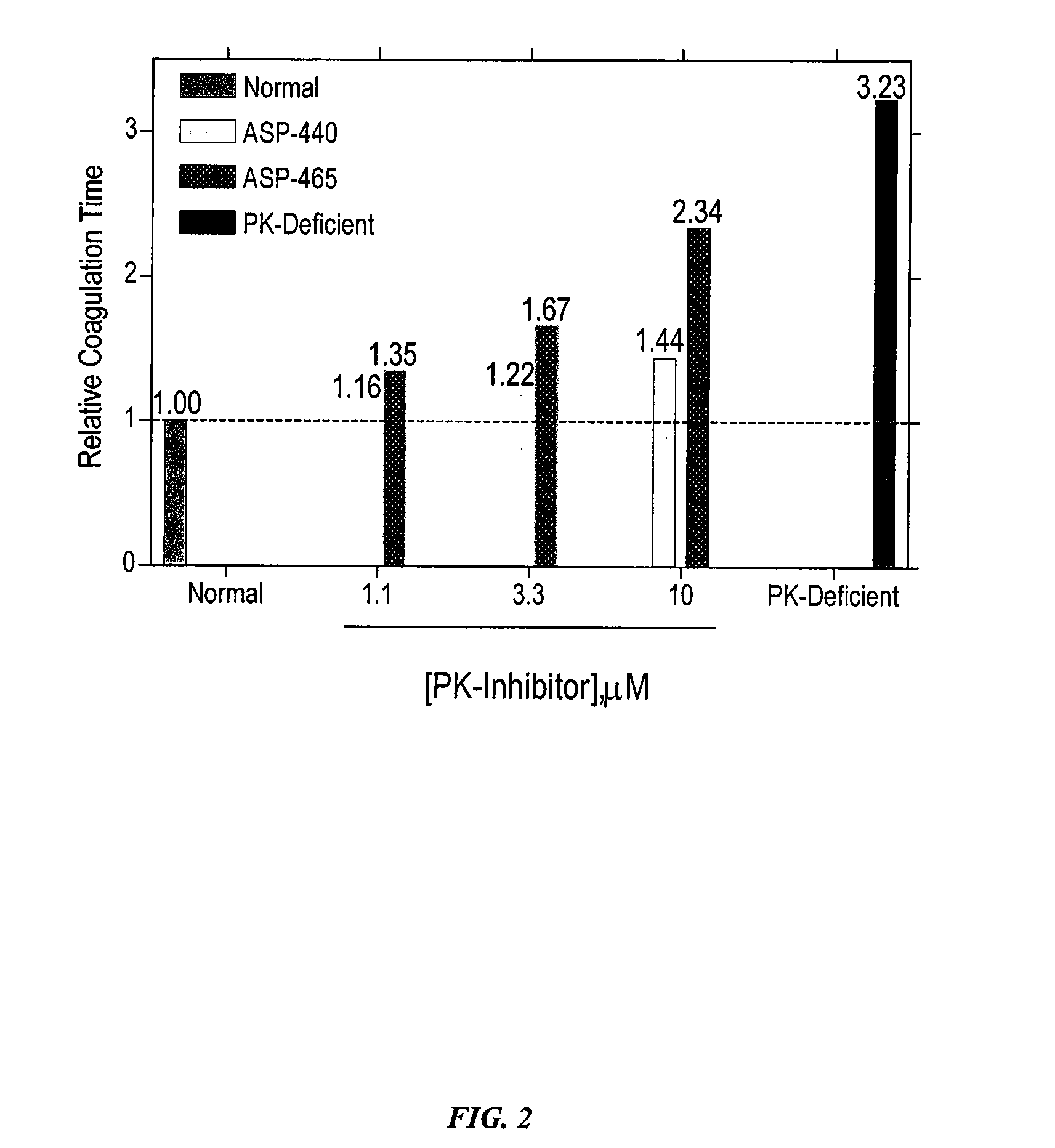
Inhibitors of plasma kallikrein
The present invention provides compounds that inhibit the activity of plasma kallikrein (PK) and methods of preventing and treating the formation of thrombin during or after a PK dependent disease or condition, for example, after fibrinolysis treatment.
Owner:ACTIVESITE PHARMA INC
Free-standing biodegradable patch
InactiveUS20110071499A1Increase flexibilityImprove propertiesBiocidePeptide/protein ingredientsThrombin activityFibrinogen
Methods and apparatus for a free-standing biodegradable patch suitable for medical applications, especially intravascular, minimally-invasive and intraoperative surgical applications are provided, wherein the patch comprises a free-standing film or device having a mixture of a solid fibrinogen component and a solid thrombin component that, when exposed to an aqueous environment, undergoes polymerization to form fibrin. In alternative embodiments the patch may comprise a solid fibrinogen component, with or without an inorganic calcium salt component. The patch may take a non-adherent form during delivery to a target location within a vessel or tissue, and thereafter may be activated to adhere to vessel wall or tissue, and may include a number of additives, including materials to improve the mechanical properties of the patch, or one or more therapeutic or contrast agents.
Owner:BIOINSPIRE TECH
Six-membered heterocycles useful as serine protease inhibitors
The present invention provides compounds of Formula (I):or a stereoisomer, tautomer, pharmaceutically acceptable salt or solvate form thereof, wherein the variables A, B, R3 and R11 are as defined herein. The compounds of Formula (I) are useful as selective inhibitors of serine protease enzymes of the coagulation cascade and / or contact activation system; for example thrombin, factor Xa, factor XIa, factor IXa, factor VIIa and / or plasma kallikrein. In particular, it relates to compounds that are selective factor XIa inhibitors or dual inhibitors of fXIa and plasma kallikrein. This invention also relates to pharmaceutical compositions comprising these compounds and methods of treating thromboembolic and / or inflammatory disorders using the same.
Owner:BRISTOL MYERS SQUIBB CO
Controlled release endoprosthetic device
InactiveUS20050192664A1Overcome problemsVascular smooth cell proliferation caused by stents can be reducedOrganic active ingredientsStentsBULK ACTIVE INGREDIENTPerfusion
The invention relates to improved drug-delivery endoprosthetic device for insertion at a vascular site via catheter placement at the site, comprising: (a) a structural member into the upper and / or lower surface of which one or more micro-deepenings are engraved and / or on which a polymer member is carried, for co-expansion with the polymer member from a contracted state to an expanded state when the device is exposed to said stimulus, (b) optionally a polymer member capable of expanding from a contracted to a stable, expanded state when the polymer member is exposed to a selected stimulus, wherein the device can be delivered from a catheter, with the structural and the optional polymer members in their contracted states, and is adapted to be held in a vessel at the vascular target site by radial pressure against the wall of the vessel, with the structural and the optional polymer members in their expanded states; and wherein the micro-deepenings of said structural member and / or said polymer member comprise a pharmaceutical composition containing one or more active ingredients selected from the group consisting of agents to inhibit or at least reduce excessive proliferation of vessel wall cells, agents to enhance the downstream perfusion of tissue, agents to promote and / or to enhance the neo-formation of capillaries, agents designed to modulate the amount or activity of coagulation factors, agents to reduce the amount of Thrombin- and / or Fibrin-formation, embedded therein for release from the member, with such in its expanded state.
Owner:BOEHRINGER INGELHEIM PHARM KG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com