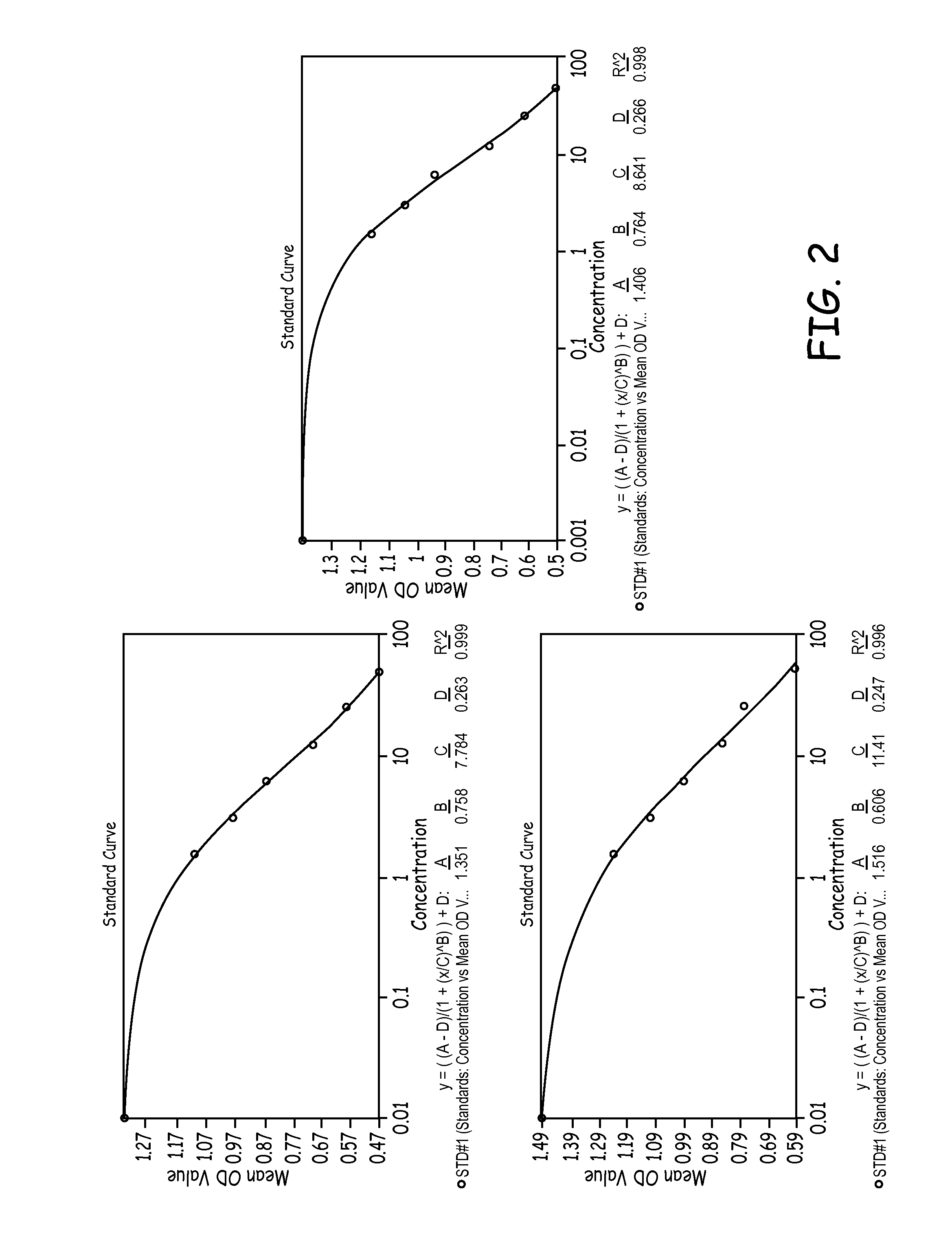
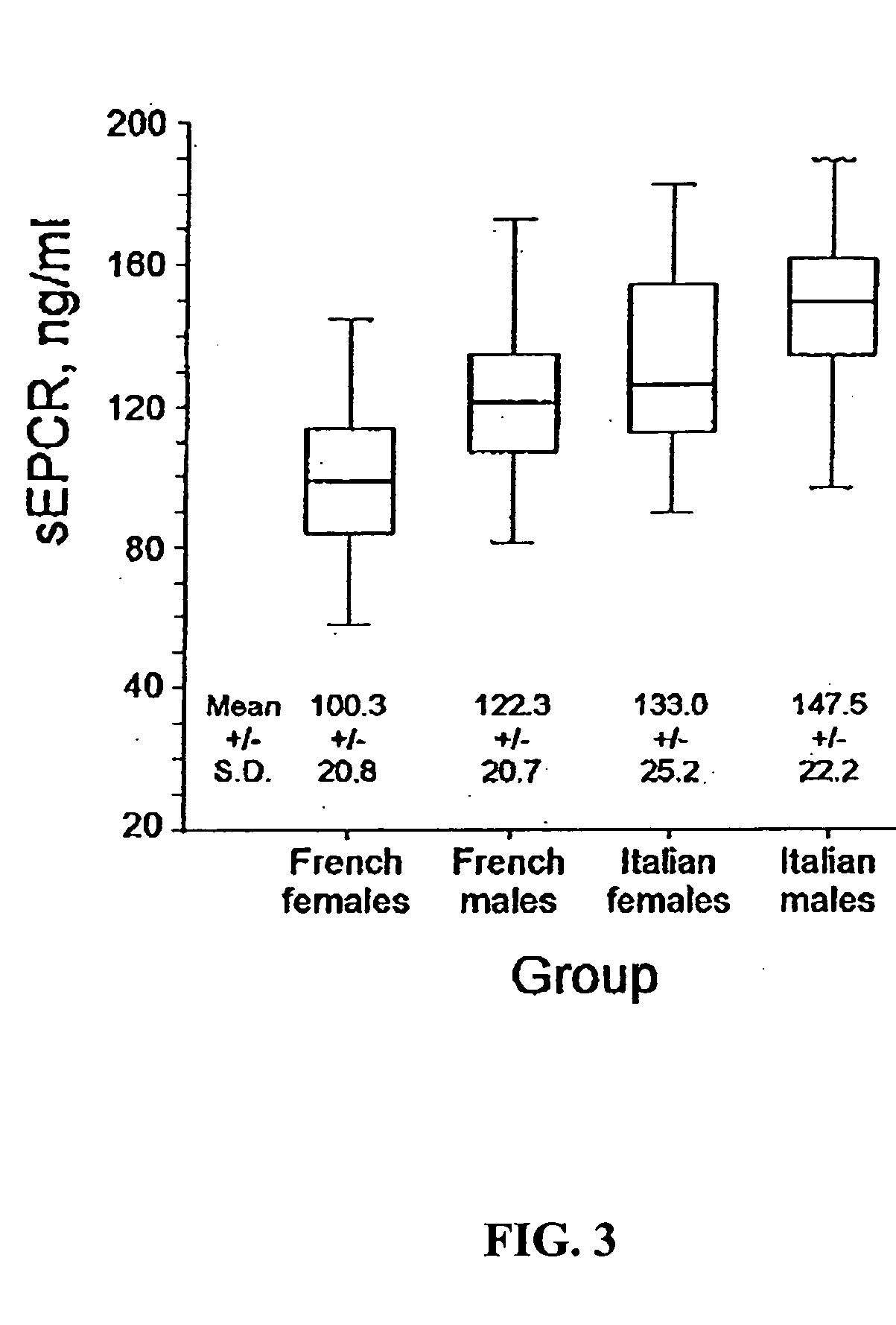Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
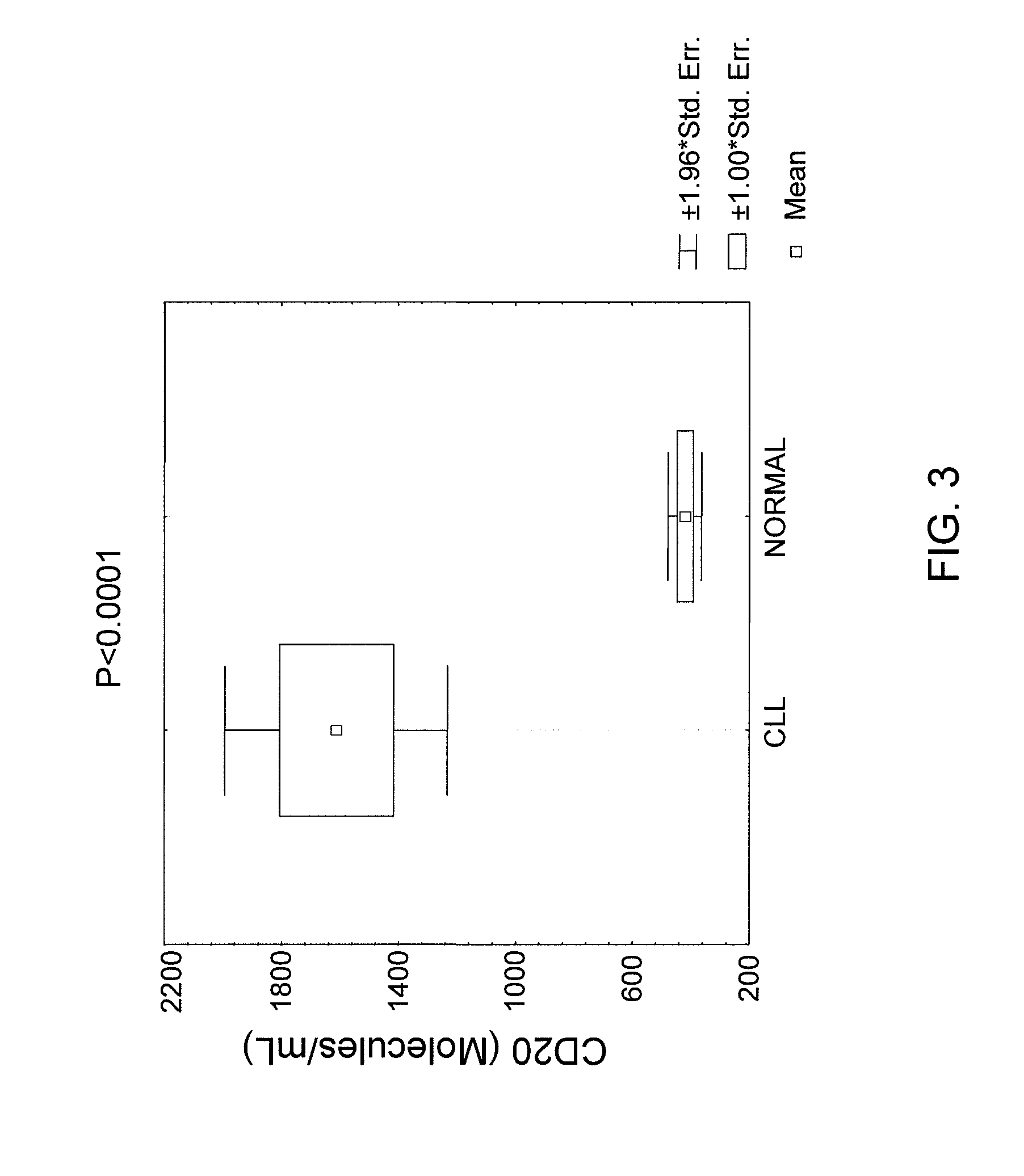
85 results about "Elisa assay" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for monitoring coagulability and hypercoagulable states
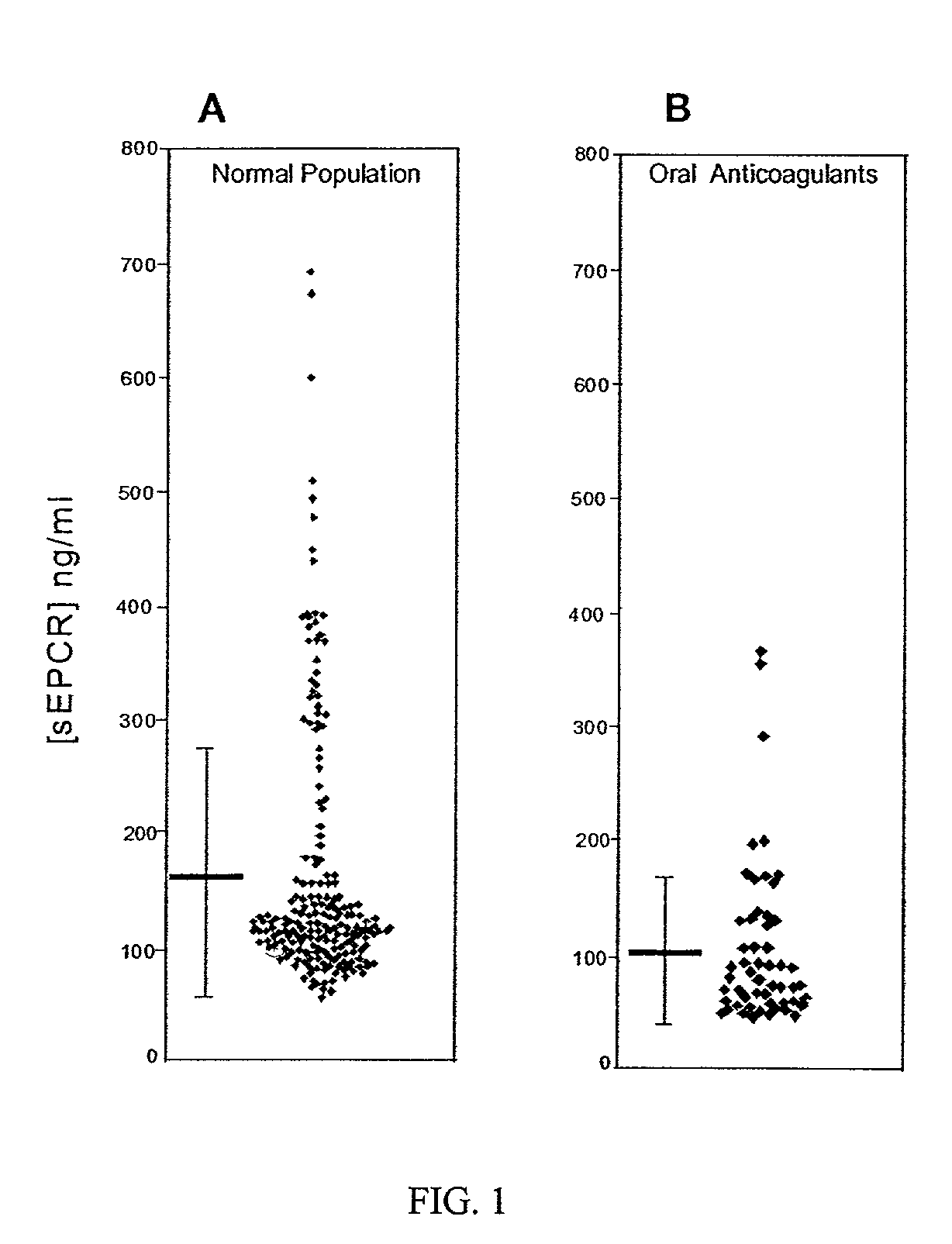
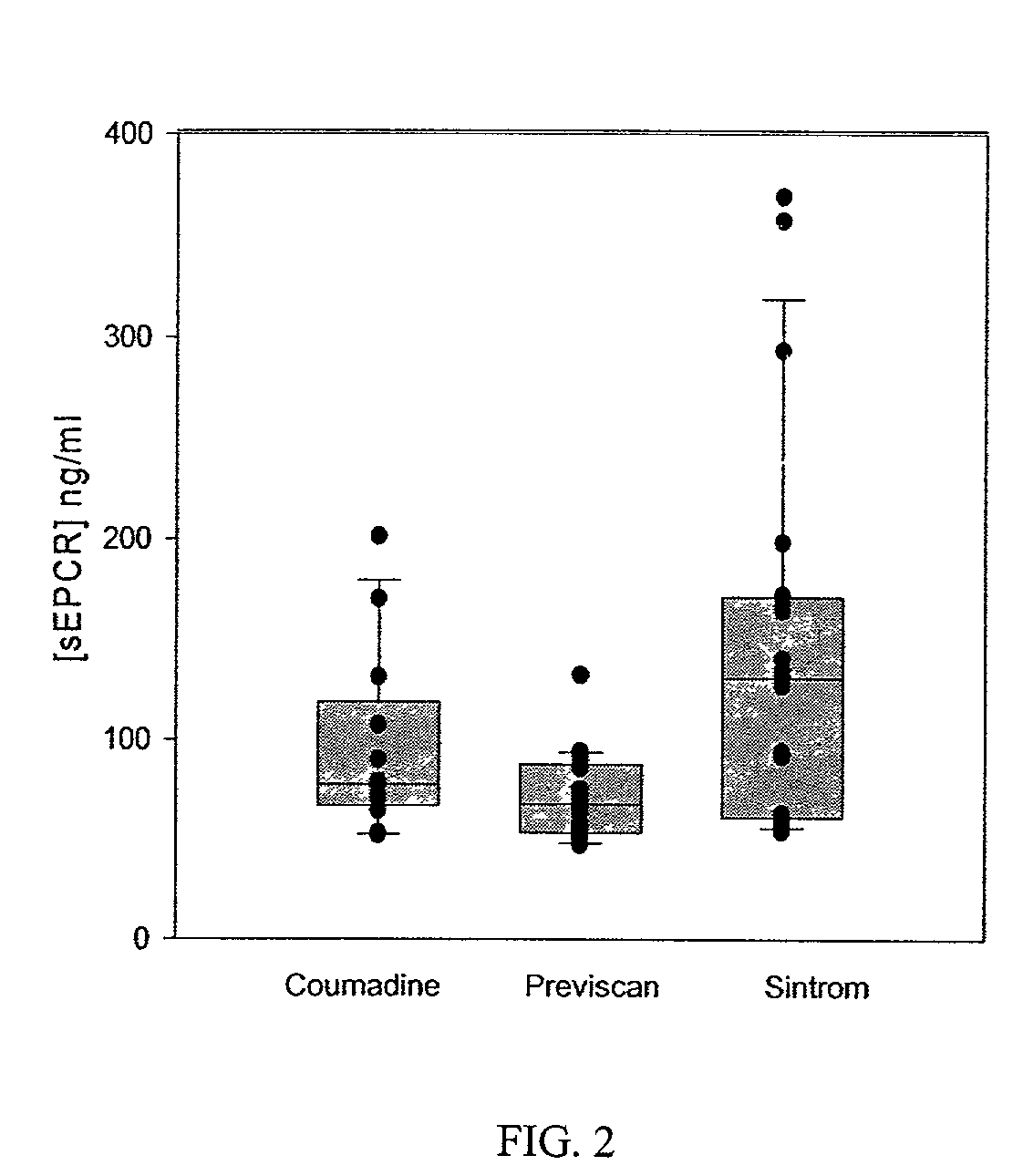
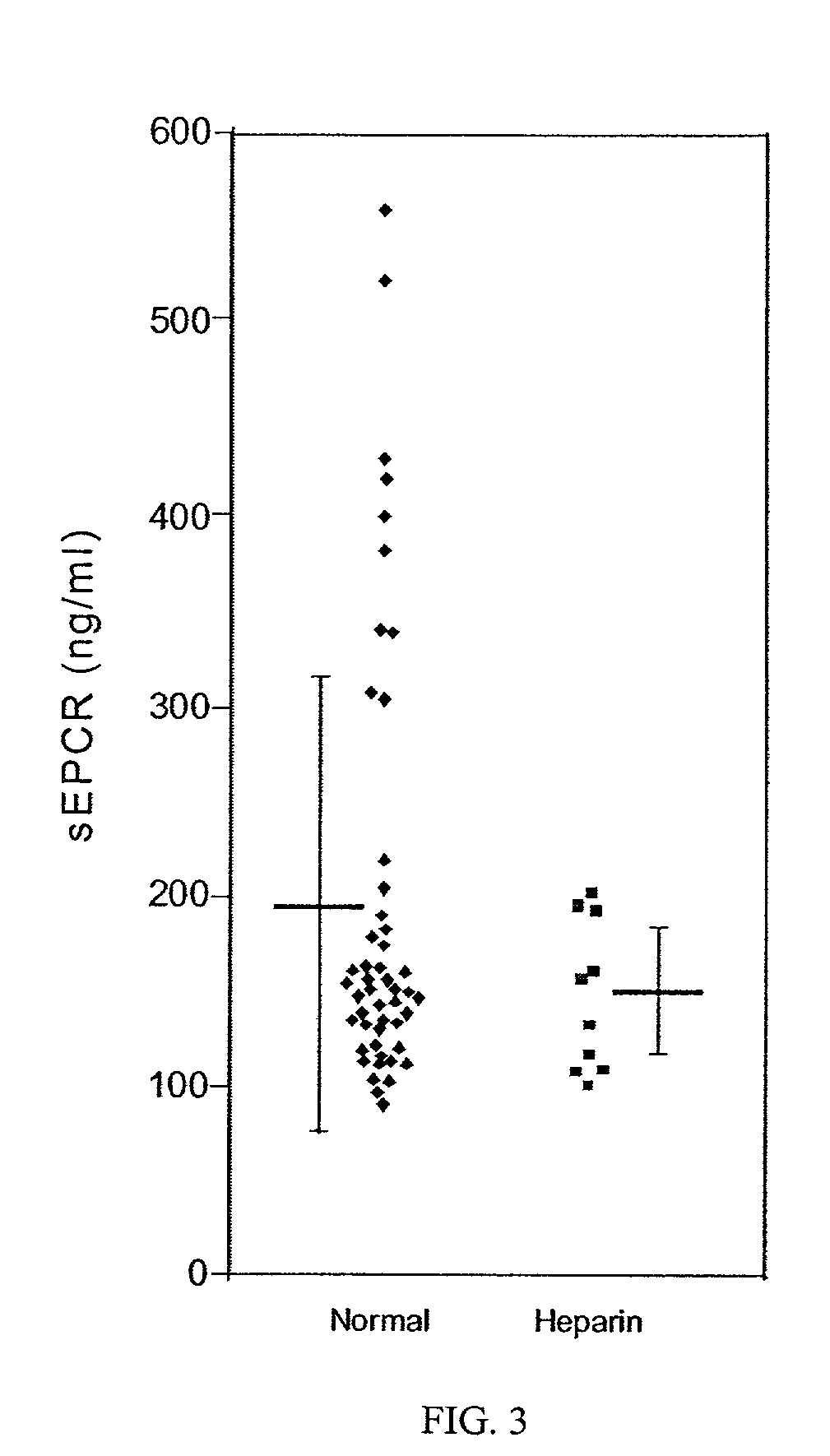
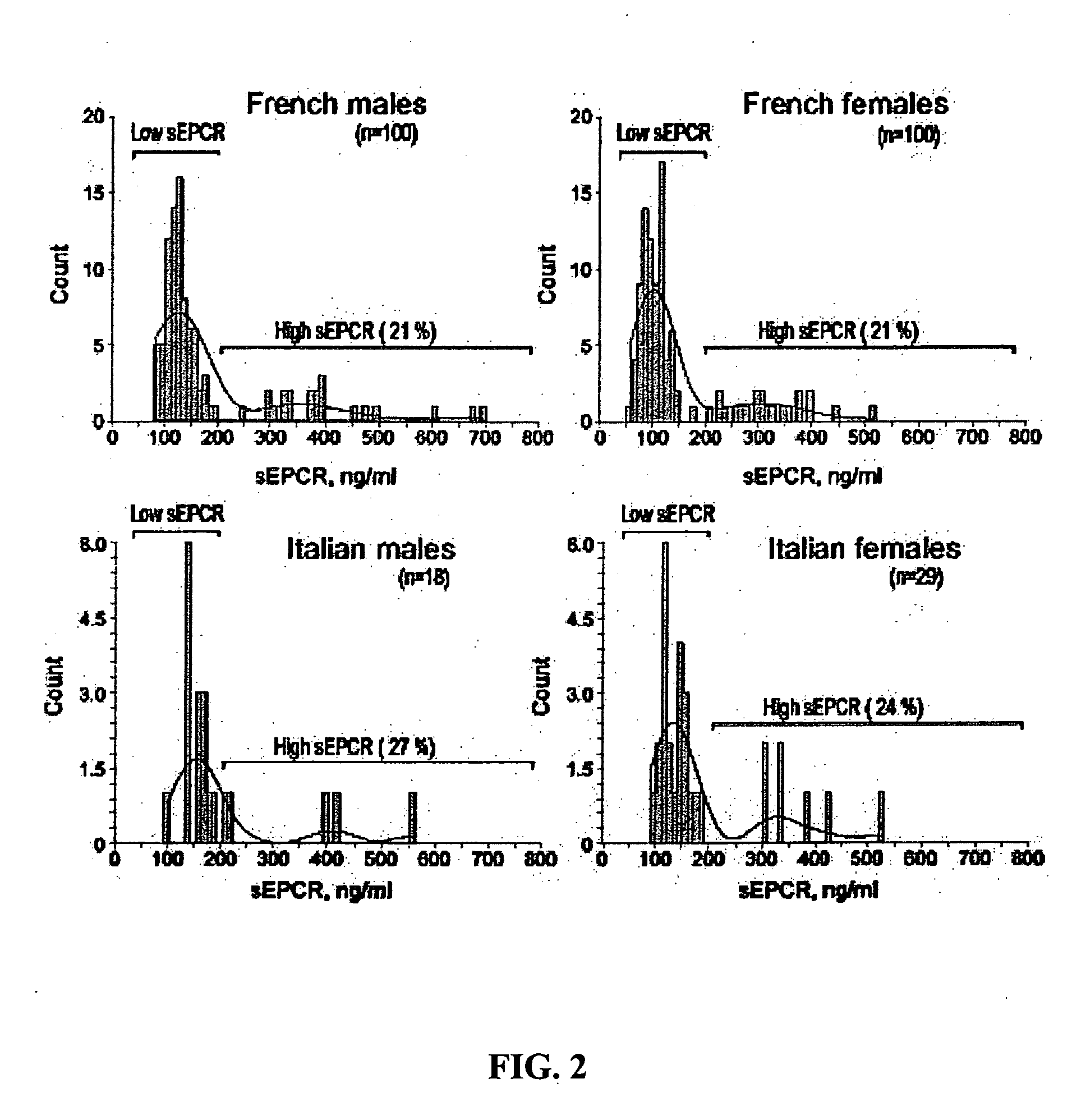
The assay of soluble endothelial protein C receptor (sEPCR) is useful to monitor effective thrombin levels and a hypercoagulable state. An assay for sEPCR is therefore useful to monitor ongoing effectiveness of anticoagulant therapy. A sEPCR ELISA assay is particularly useful for this purpose. A state of hypercoagulability in patients or normal individuals can also be identified by such an assay.
Owner:OKLAHOMA MEDICAL RES FOUND
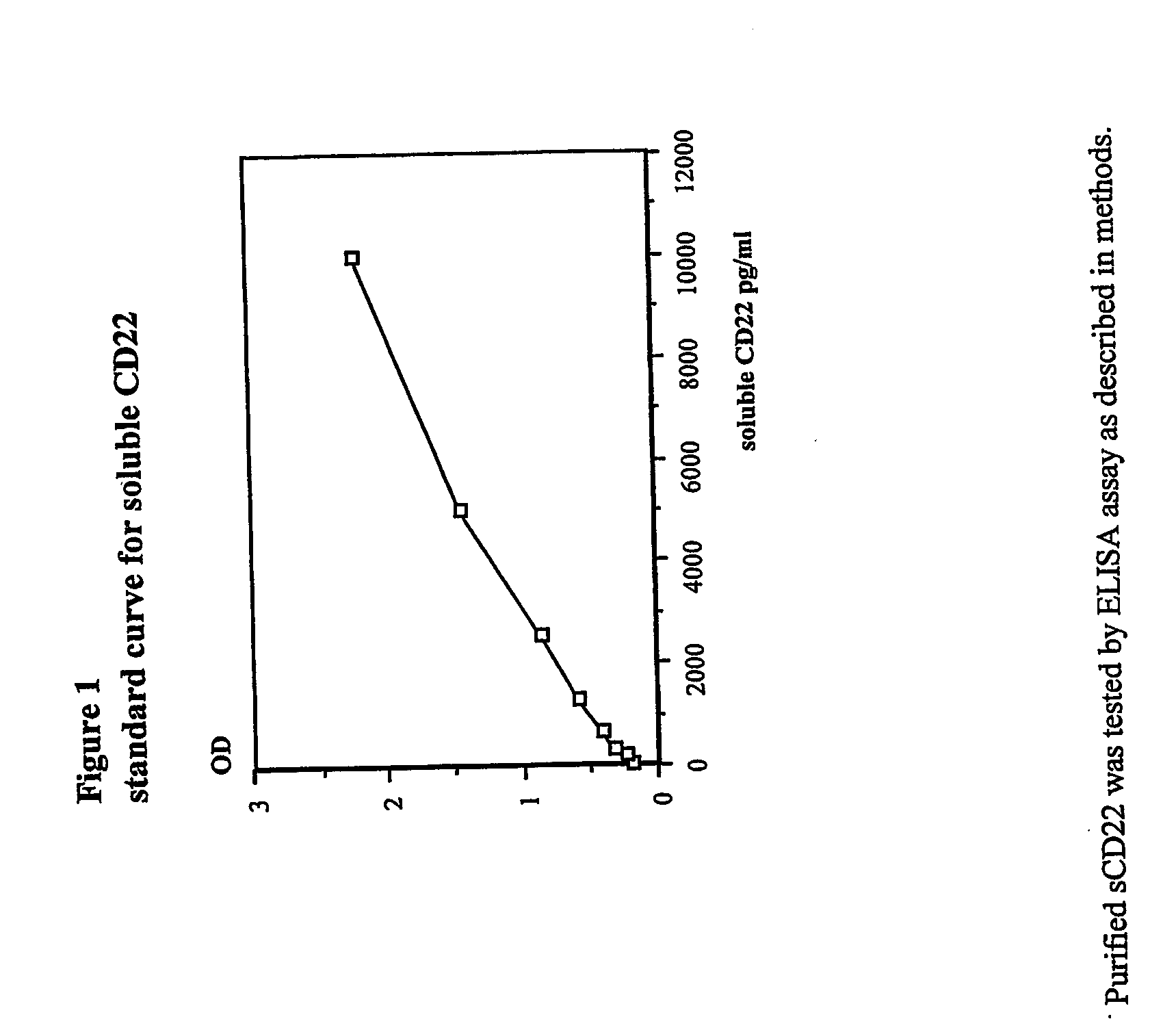
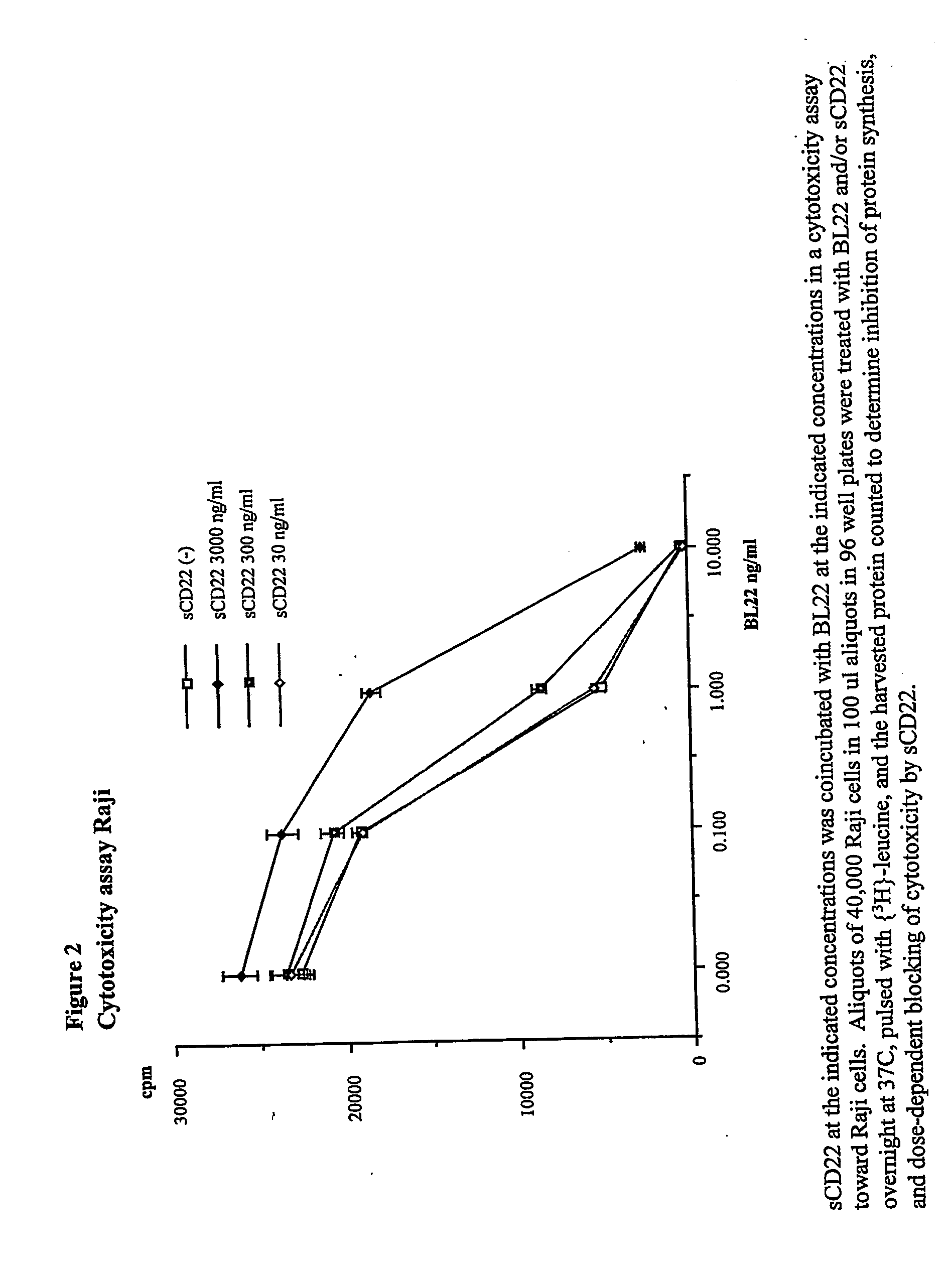
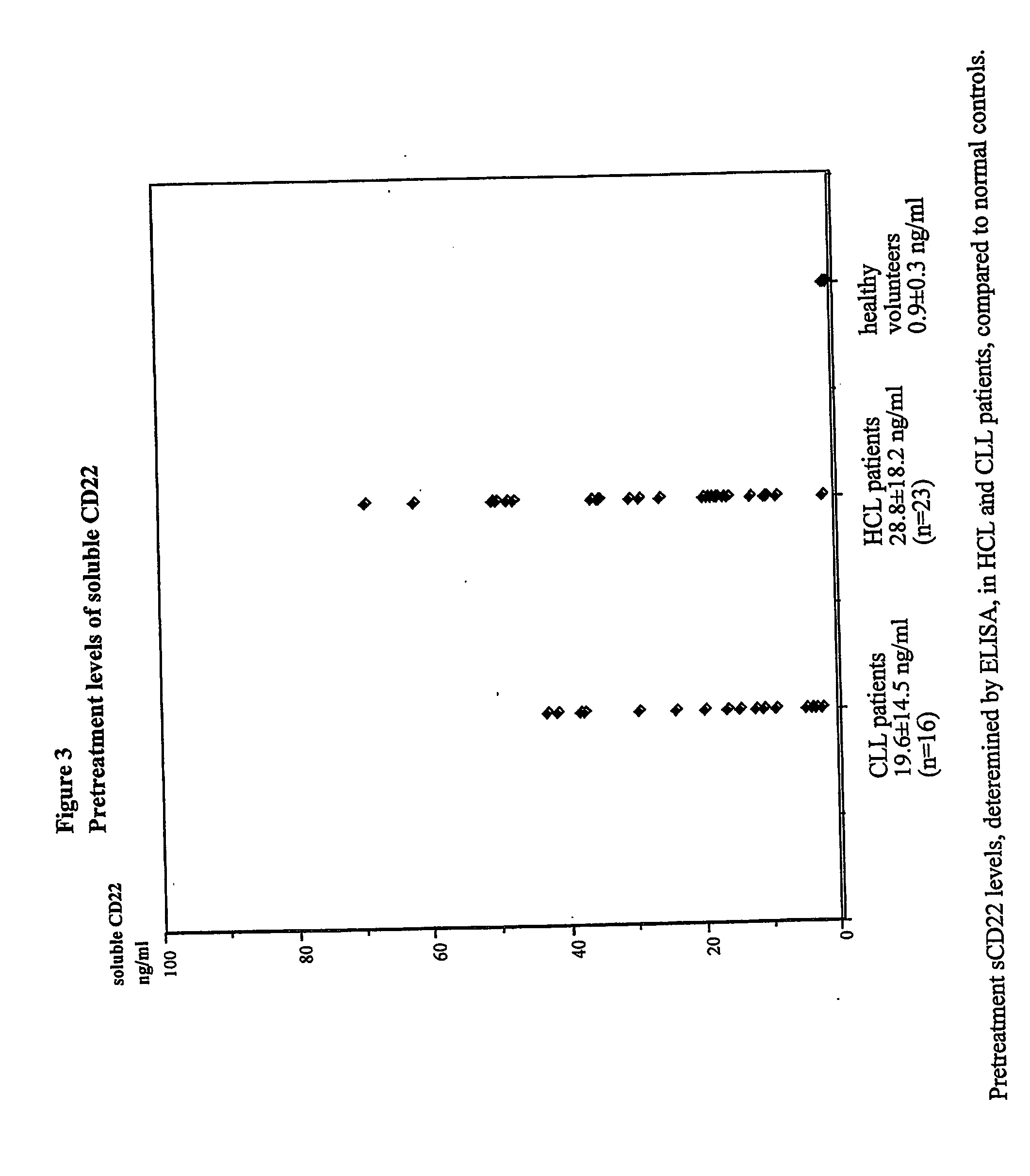
Elisa assay of serum soluble cd22 to assess tumor burnden/relapse in subjects with leukemia and lymphoma
InactiveUS20050244828A1Microbiological testing/measurementBiological material analysisAbnormal tissue growthTumor Load
Disclosed herein are methods of using previously unknown soluble forms of CD22 (sCD22) present in the serum of subjects with B-cell leukemias and lymphomas to assess tumor burden in the subjects. Also disclosed are methods of diagnosing or prognosing development or progression of a B-cell lymphoma or leukemia in a subject, including detecting sCD22 in a body fluid sample taken or derived from the subject, for instance serum.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICAS AS REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES THE
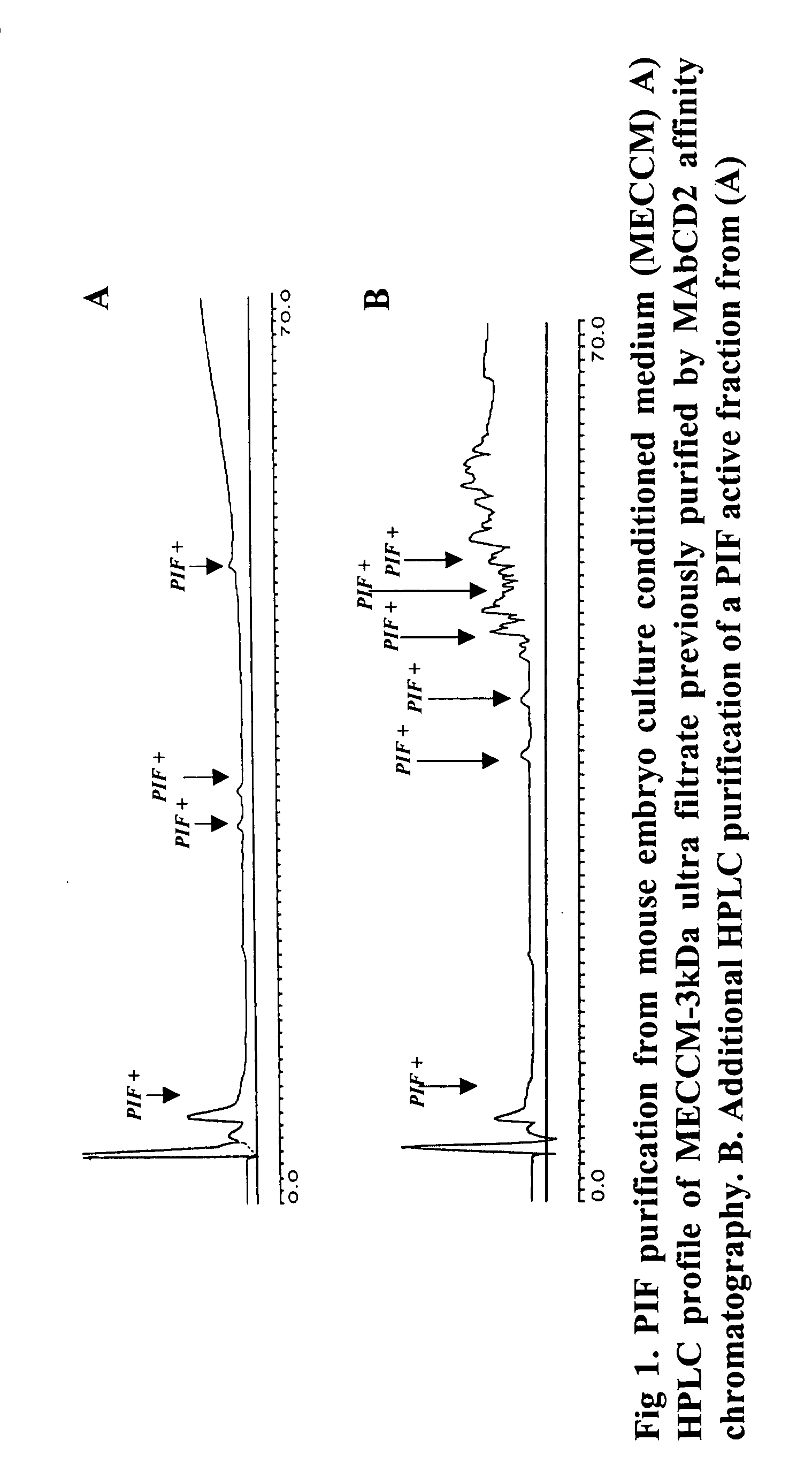
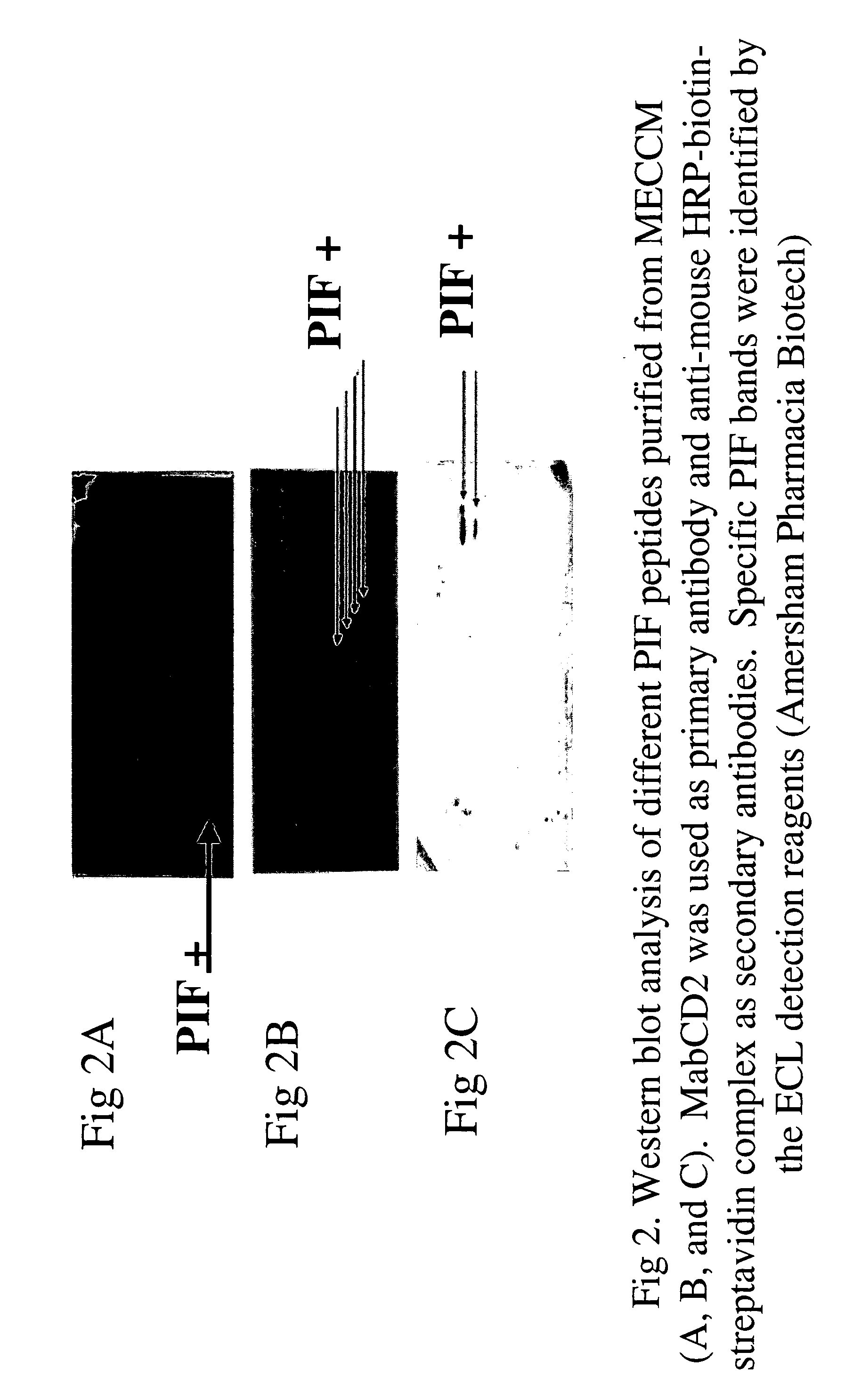
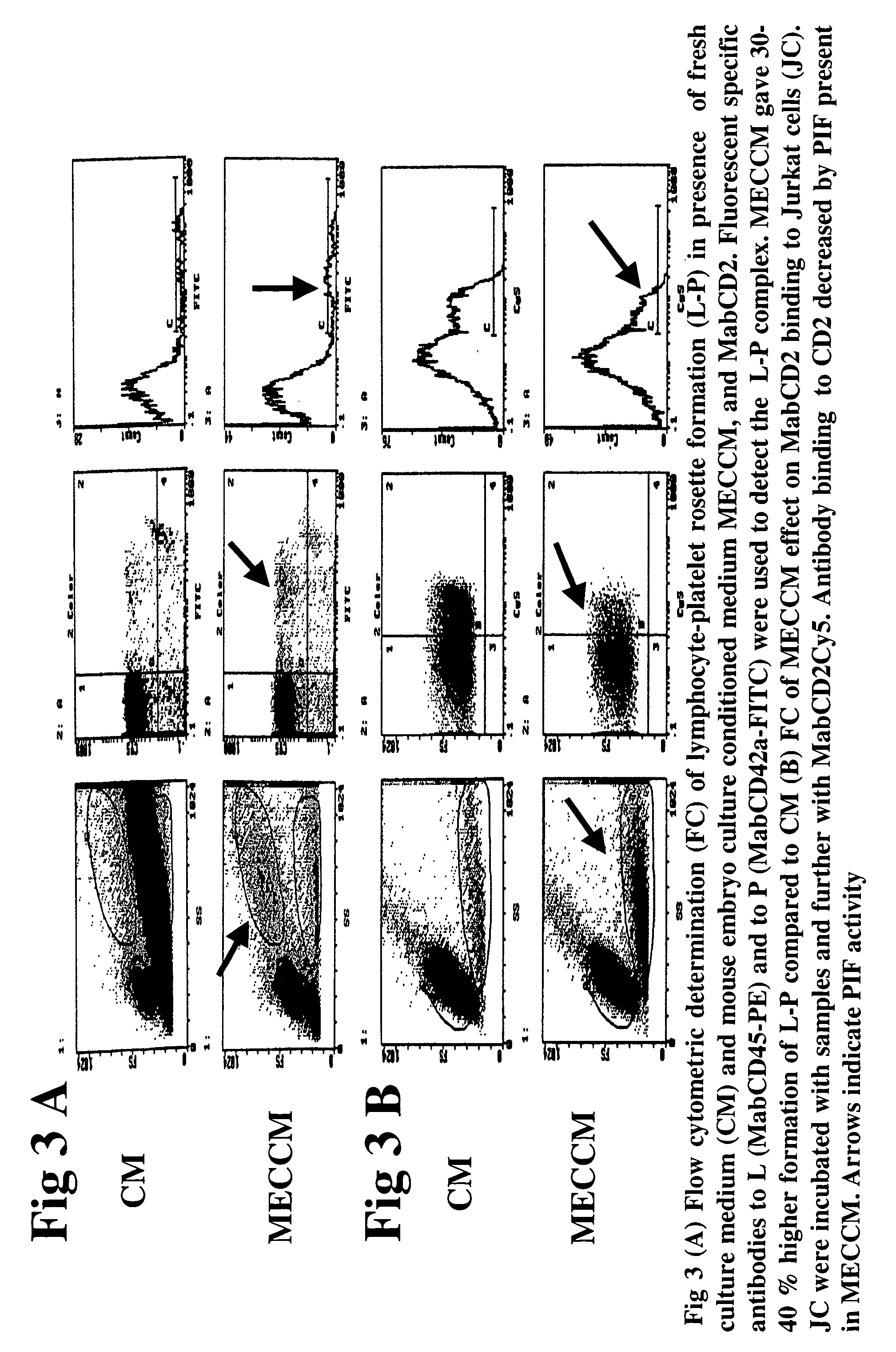
New assays for preimplantation factor and preimplantation factor peptides
InactiveUS20050064520A1High expressionReduce cell viabilityBiological material analysisBiological testingPIF peptideFluorescence
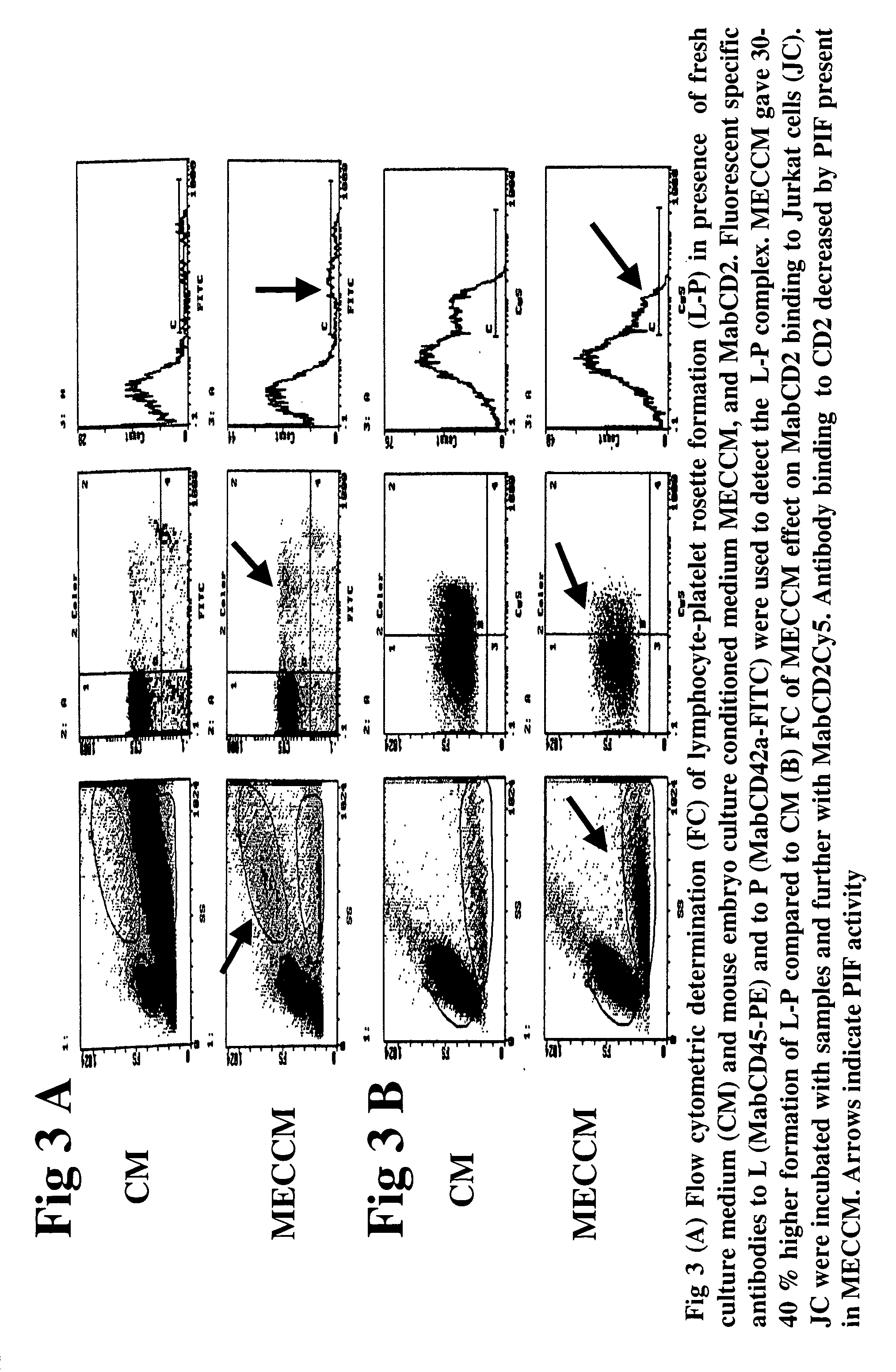
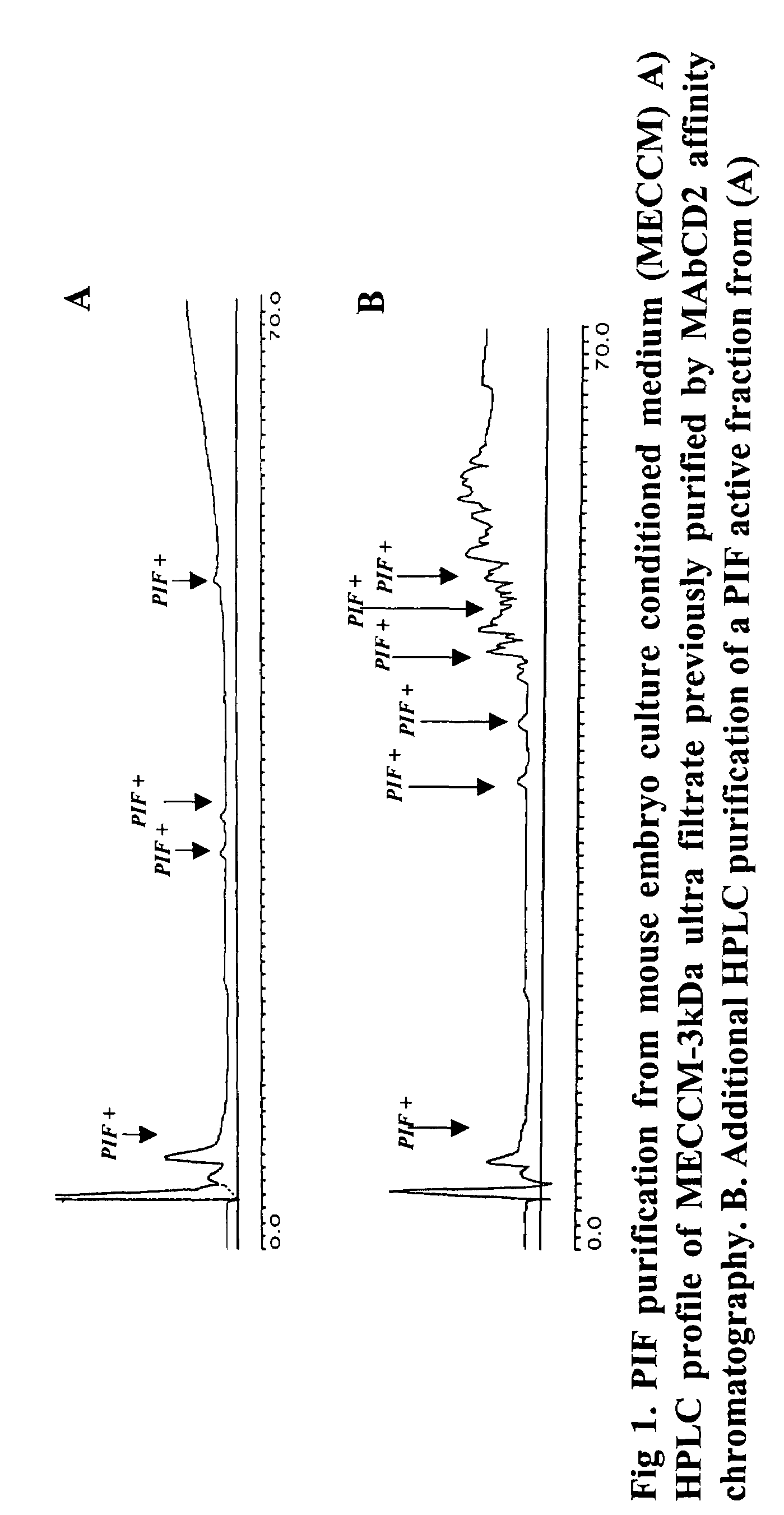
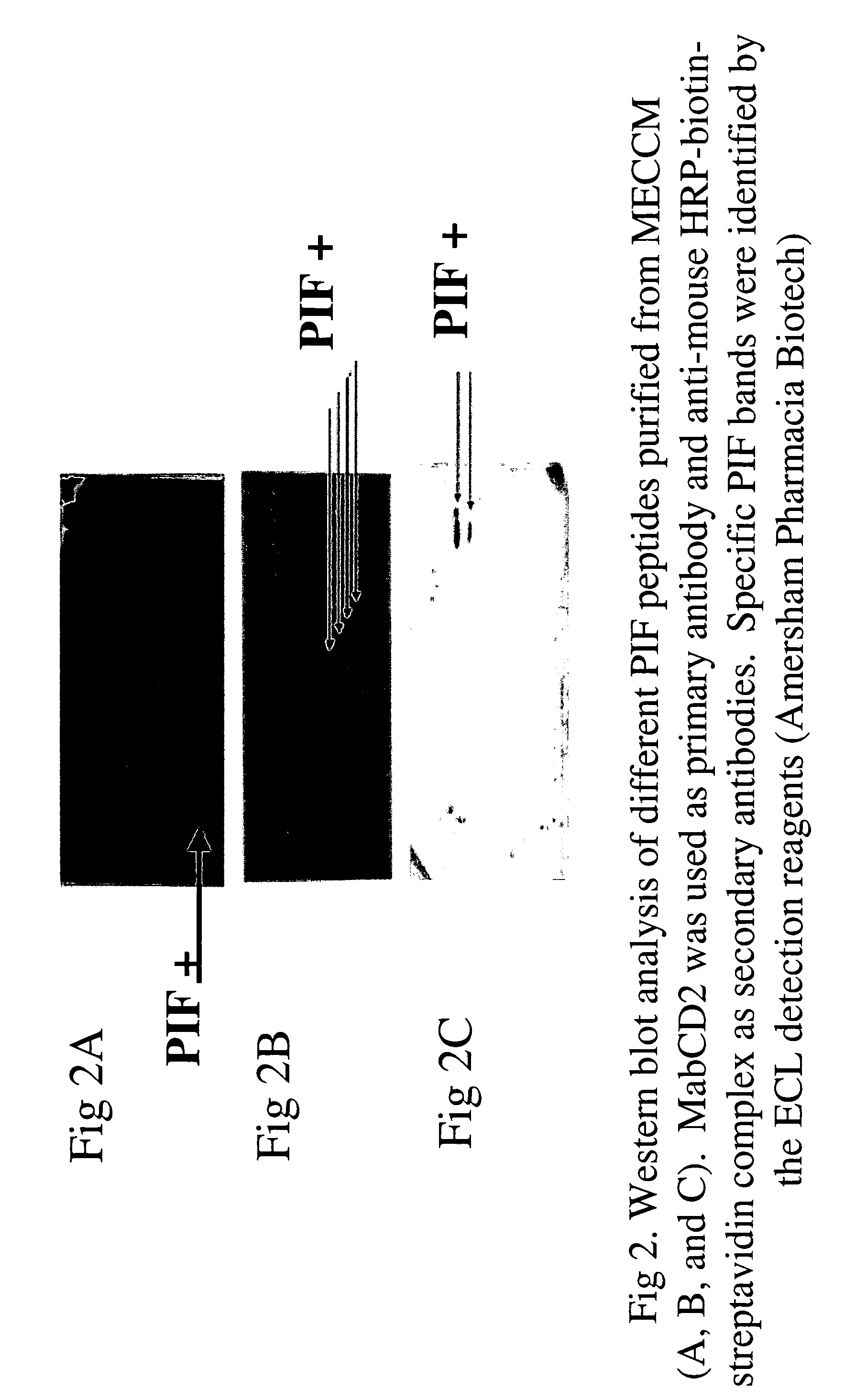
The present invention relates to assay methods used for detecting the presence of PIF, and to PIF peptides identified using this assay. In particular, the present invention relates to flow cytomery assays for detecting PIF. It is based, at least in part, on the observation that flow cytometry using fluorescently labeled antilymphocyte and anti-platelet antibodies demonstrated an increase in rosette formation in the presence of PIF. It is further based on the observation that flow cytometry demonstrated that monoclonal antibody binding to CD2 decreased in the presence of PIF. The present invention further relates to PIF peptides which, when added to Jurkat cell cultures, have been observed to either (I) decrease binding of anti-CD2 antibody to Jurkat cells; (ii) increase expression of CD2 in Jurkat cells; or (iii) decrease Jurkat cell viability. In additional embodiments, the present invention provides for ELISA assays which detect PIF by determining the effect of a test sample on the binding of anti-CD2 antibody to a CD2 substrate.
Owner:BIOLNCEPT INC
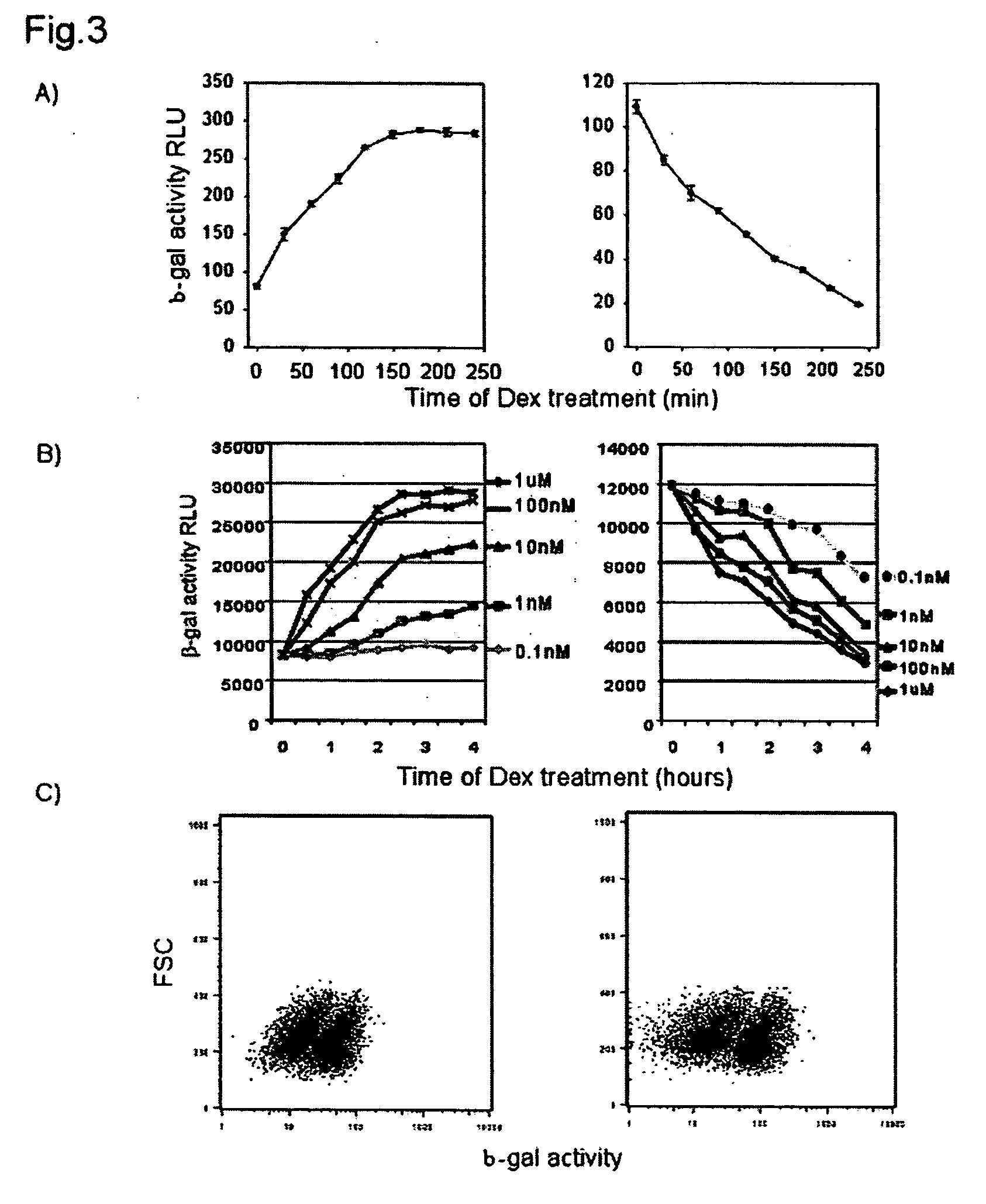
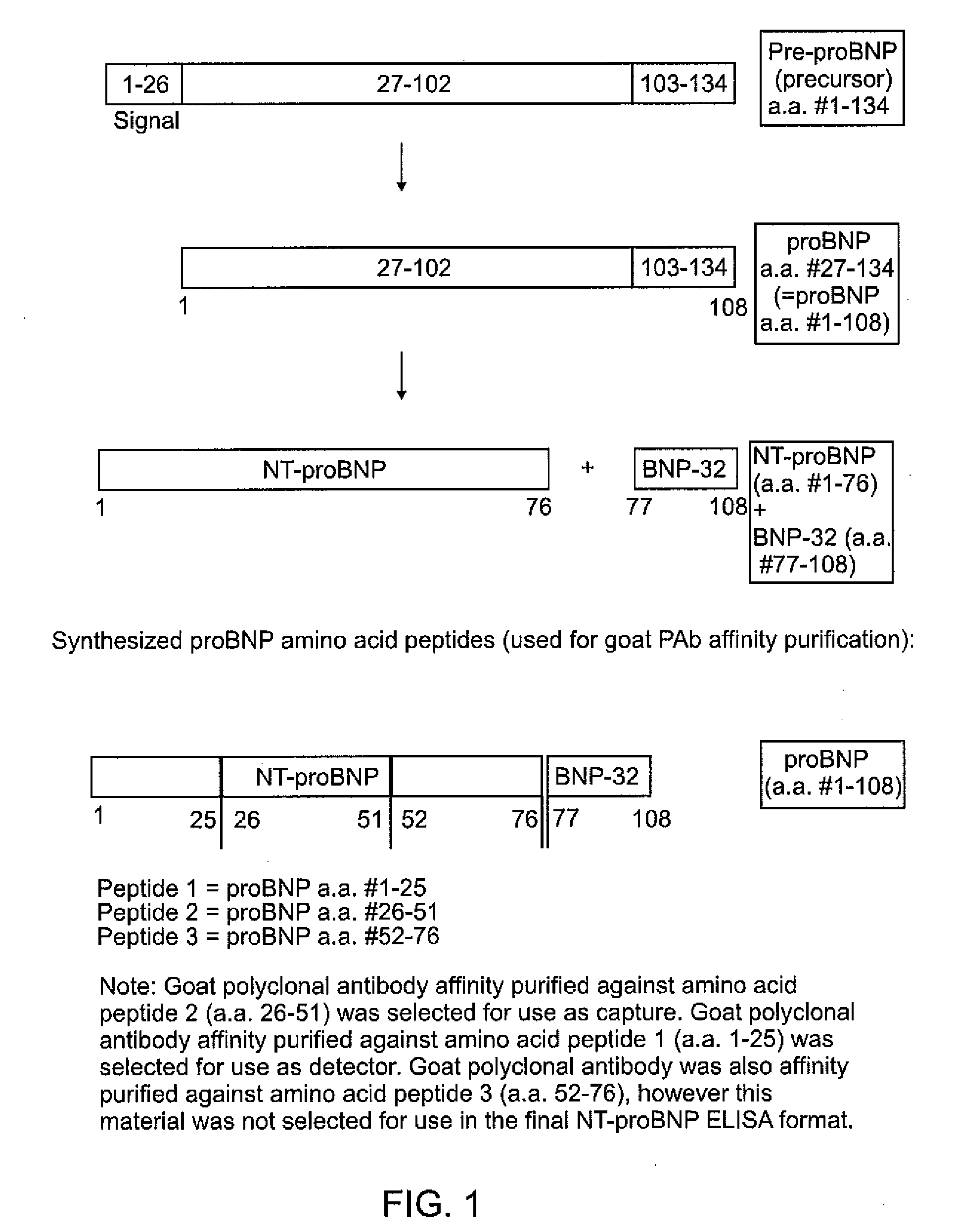
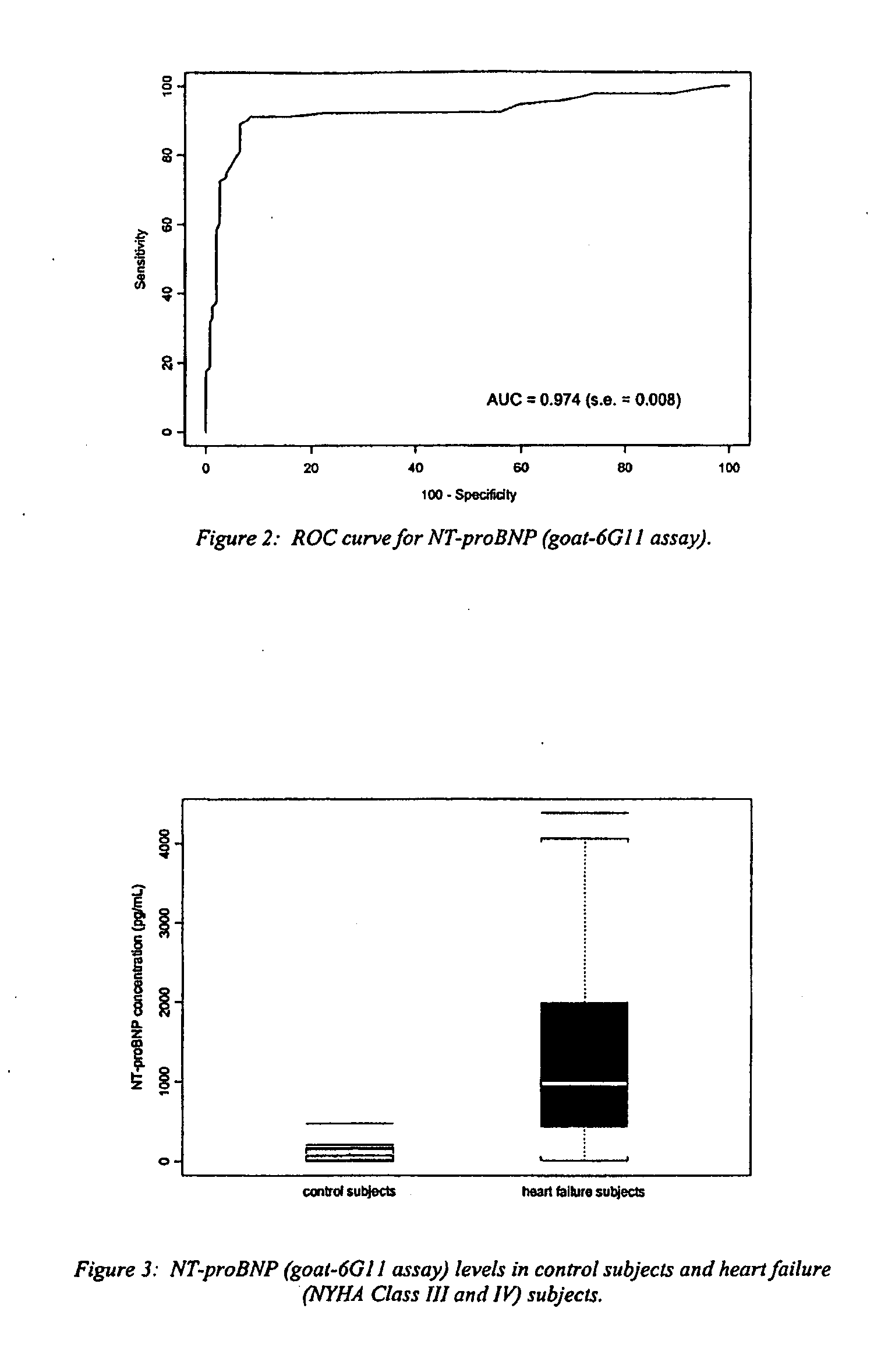
Polyclonal-monoclonal ELISA assay for detecting N-terminus proBNP
InactiveUS7527939B2Accurately predicting mortalityDisease diagnosisImmunoglobulins against hormonesBlood plasmaAmino acid
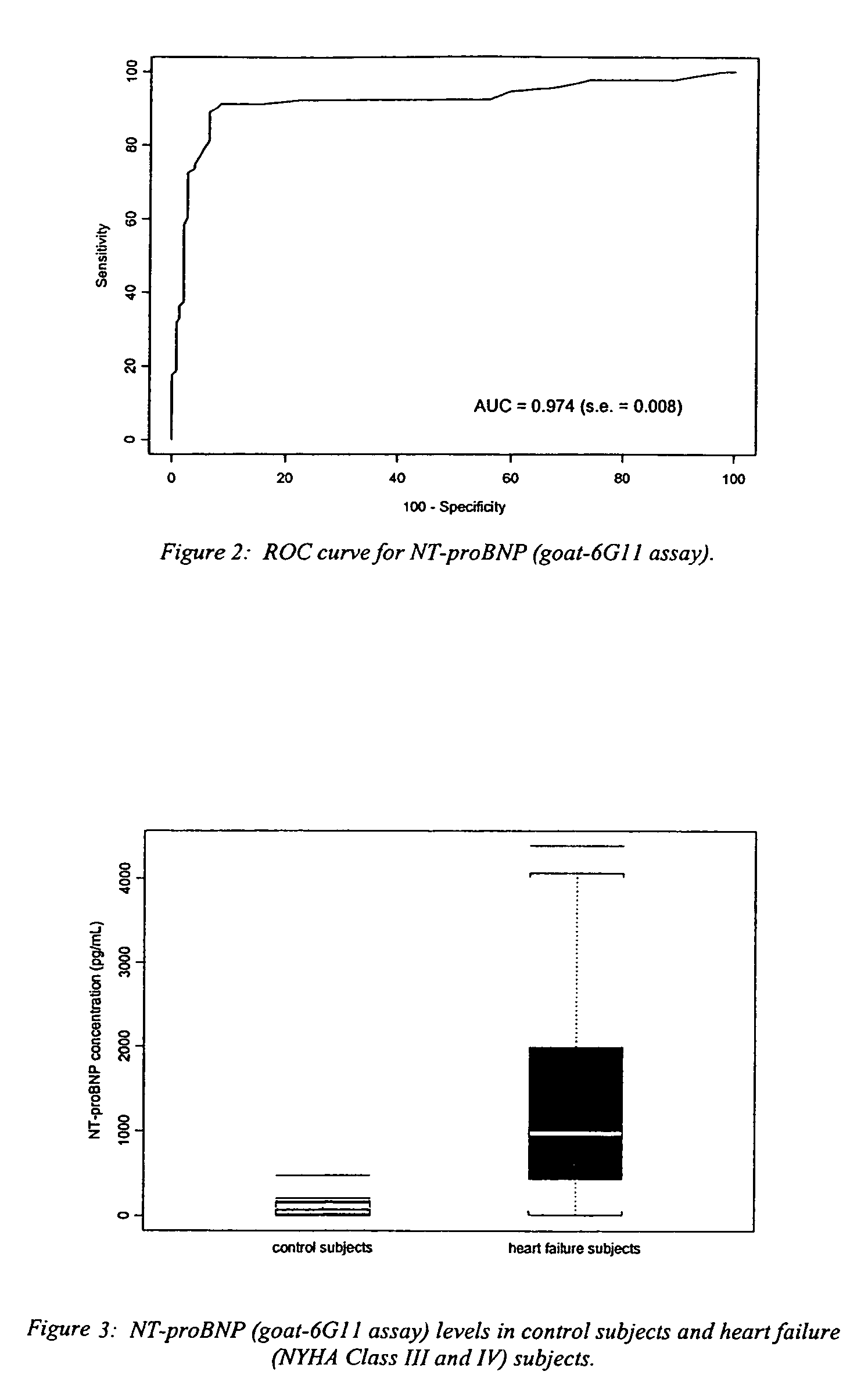
A specific and sensitive in vitro ELISA assay and diagnostic test kit is disclosed for determining levels of NT-proBNP protein in a variety of bodily fluids, non-limiting examples of which are blood, serum, plasma, urine and the like. The NT-proBNP ELISA assay test employs the sandwich ELISA technique to measure circulating NT-proBNP in human plasma. In order to obtain antibodies with specific binding properties for targeted amino acid sequences within human proBNP, recombinant human proBNP (or rhproBNP) was expressed and purified for use as an immunogen. Polyclonal antibodies (PAb) to specific amino acid sequences were subsequently purified from goat serum by sequential affinity purification. Monoclonal antibodies were raised against specific polypeptides. Recombinant human NT-proBNP (or rhNT-proBNP) was expressed and purified in order to obtain material for use in calibration of a quantitative method for measurement of human NT-proBNP.
Owner:NANOGEN POINT OF CARE
Assays for preimplantation factor and preimplantation factor peptides
The present invention relates to assay methods used for detecting the presence of PIF, and to PIF peptides identified using this assay. In particular, the present invention relates to flow cytomery assays for detecting PIF. It is based, at least in part, on the observation that flow cytometry using fluorescently labeled antilymphocyte and anti-platelet antibodies demonstrated an increase in rosette formation in the presence of PIF. It is further based on the observation that flow cytometry demonstrated that monoclonal antibody binding to CD2 decreased in the presence of PIF. The present invention further relates to PIF peptides which, when added to Jurkat cell cultures, have been observed to either (I) decrease binding of anti-CD2 antibody to Jurkat cells; (ii) increase expression of CD2 in Jurkat cells; or (iii) decrease Jurkat cell viability. In additional embodiments, the present invention provides for ELISA assays which detect PIF by determining the effect of a test sample on the binding of anti-CD2 antibody to a CD2 substrate.
Owner:BIOLNCEPT INC
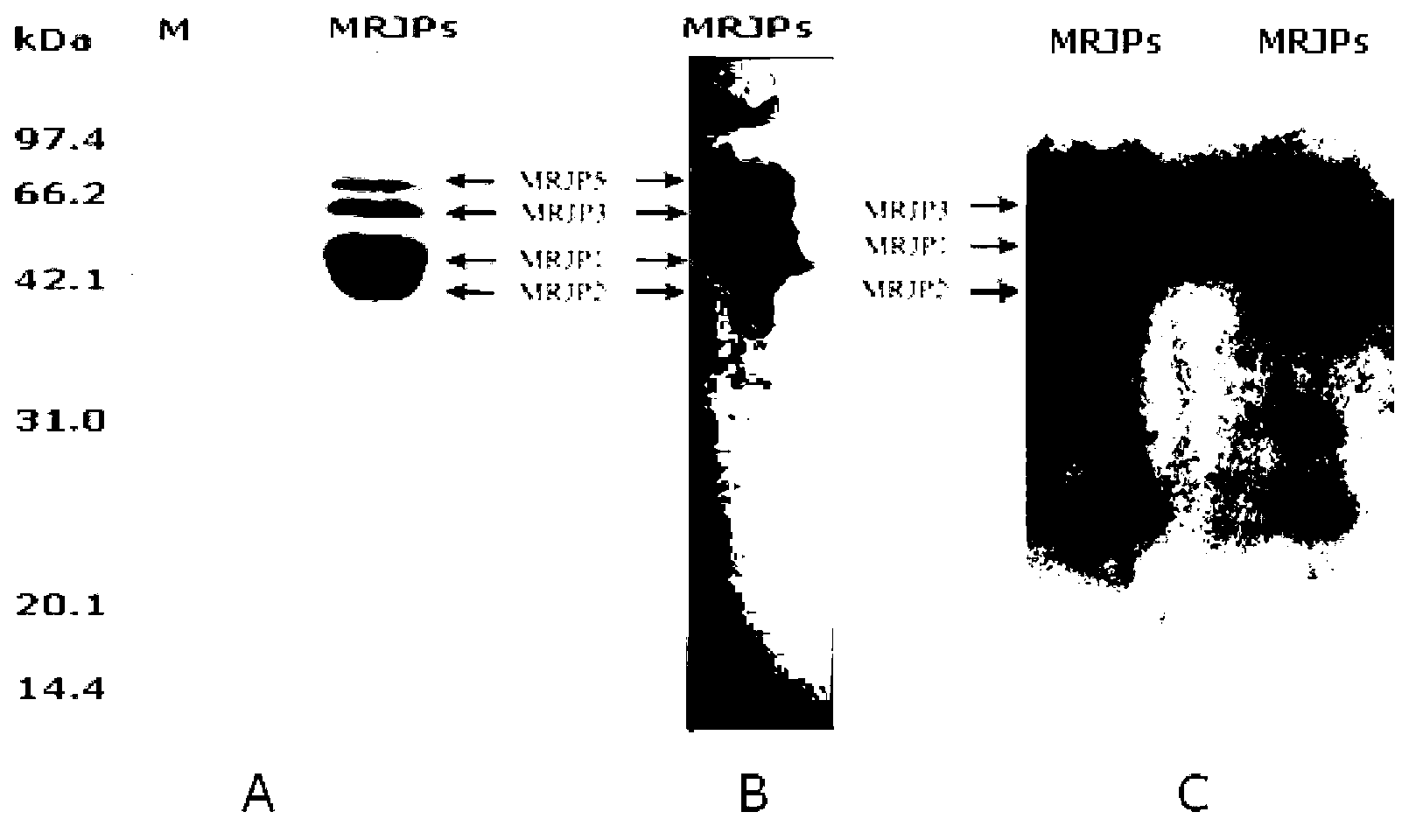
A specific antibody of major royal jelly protein MRJP1 and a preparation method thereof and Elisa quantitative detection thereof
ActiveCN103059135AStrong specificityIndividual bigSerum immunoglobulinsImmunoglobulins against animals/humansNew Zealand white rabbitSpecific antibody
The invention discloses a specific antibody of major royal jelly protein MRJP1 and a preparation method thereof and Elisa quantitative detection thereof. First, homology analysis is performed to amino acid sequences of proteins of all members of the Apismellifera major royal jelly protein MRJPs family (MRJP1-MRJP9) to select a specific polypeptide amino acid sequence unlike other MRJPs family members in MRJP1. The related specific polypeptide is synthesized by using a chemical method, and is used as an antigen to immunize New Zealand white rabbits; taking serum, and performing Elisa assay obtaining polyclonal antibody R2 with a relatively high titer, then purifying the antibody by using an affinity column prepared from the synthesized MRJP1 polypeptide. The titer of antibody R2 is detected via Elisa assay by using MRJP1 as the antigen, and the titer of the antibody is greater than 1:20000. The present invention provides a very reliable new rapid detection method for the qualitative and quantitative detection of MRJP1 in royal jelly, and also provides a very reliable technical means for quality control, freshness detection, and identification of genuine products of royal jelly and honey products for bee product quality supervision departments and processing and trading enterprises.
Owner:ZHEJIANG UNIV
Detection of protein translocation by beta-galactosidase reporter fragment complementation
ActiveUS20050287522A1Precise and accurate monitoringMicrobiological testing/measurementBiological material analysisBeta-GalactosidasesElisa assay
Methods and compositions are provided for detecting molecular translocations, particularly protein translocations within and between subcellular copartments, using at least two components that exhibit a localization-dependent difference in complementation activity. In particular, alpha-complementing β-galactosidase fragments are provided. These β-galactosidase reporter fragments display significantly enhanced enzymatic activity when one fragment is localized in a membrane. Methods for carrying out no-wash ELISA assays based on the reporter component system are also provided.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Polyclonal-monoclonal elisa assay for detecting n-terminus pro-bnp
InactiveUS20090325195A1Accurately predicting mortalityDisease diagnosisFused cellsBound propertyBlood plasma
A specific and sensitive in vitro ELISA assay and diagnostic test kit is disclosed for determining levels of NT-proBNP protein in a variety of bodily fluids, non-limiting examples of which are blood, serum, plasma, urine and the like. The NT-proBNP ELISA assay test employs the sandwich ELISA technique to measure circulating NT-proBNP in human plasma. In order to obtain antibodies with specific binding properties for targeted amino acid sequences within human proBNP, recombinant human proBNP (or rhproBNP) was expressed and purified for use as an immunogen. Polyclonal antibodies (PAb) to specific amino acid sequences were subsequently purified from goat serum by sequential affinity purification. Monoclonal antibodies were raised against specific polypeptides. Recombinant human NT-proBNP (or rhNT-proBNP) was expressed and purified in order to obtain material for use in calibration of a quantitative method for measurement of human NT-proBNP.
Owner:NEXUS DX
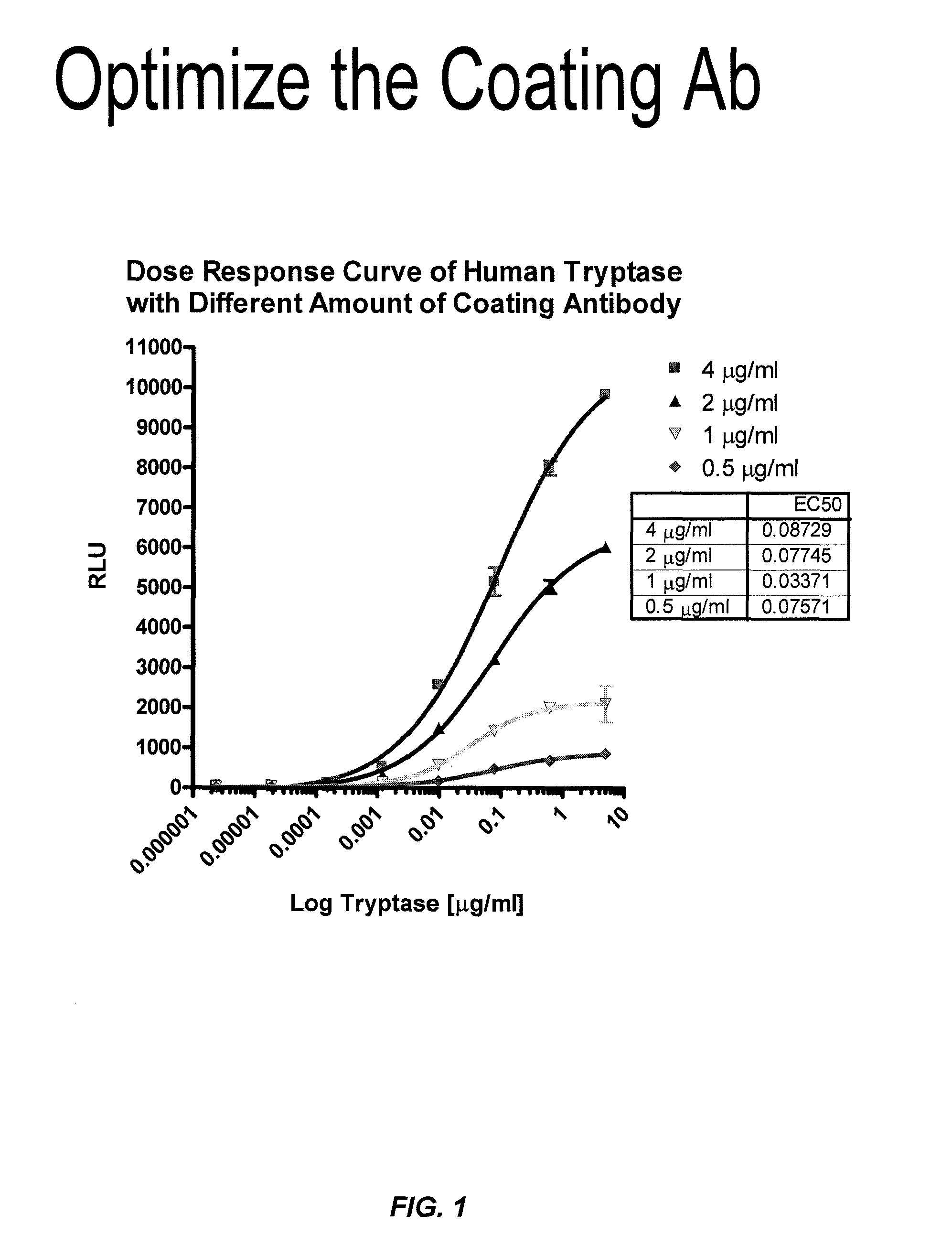
Methods for diagnosing irritable bowel syndrome
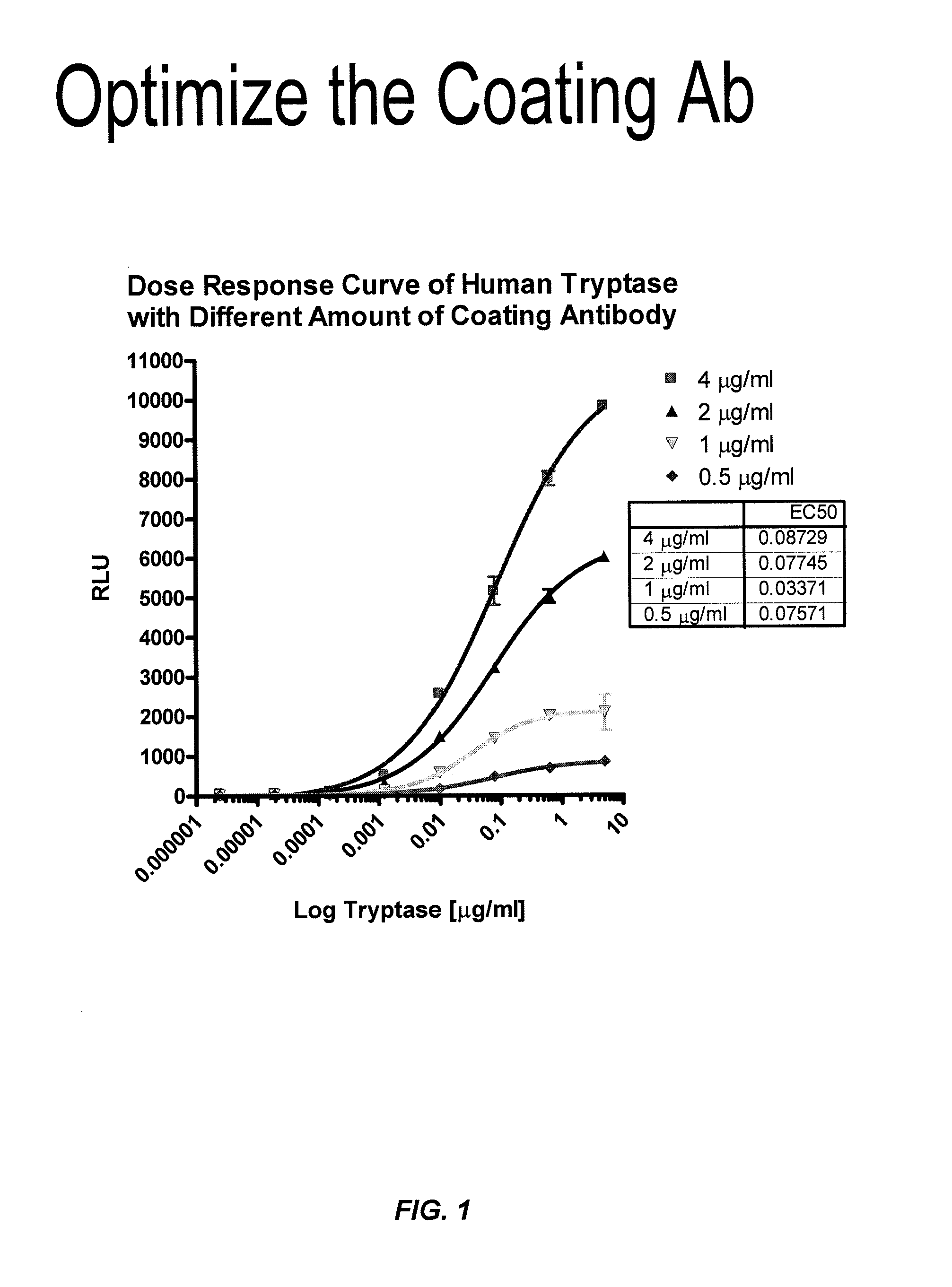
ActiveUS8114616B2Accurate diagnostic predictionBioreactor/fermenter combinationsBiological substance pretreatmentsPhysiologyCapture antibody
The invention provides an ELISA assay for the determination of serum mast cell β-tryptase levels using rabbit anti-tryptase as the capture antibody and alkaline phosphatase conjugated G3 as the detecting antibody. Luminescent substrate CPSD was used to enhance the assay sensitivity. Also provided are methods for aiding in the diagnosis of irritable bowel syndrome by detecting the serum level of β-tryptase, histamine and / or prostaglandin E2.
Owner:PROMETHEUS BIOSCIENCES INC
Methods for diagnosing irritable bowel syndrome
ActiveUS20110159521A1Accurate classificationAccurate predictionBioreactor/fermenter combinationsBiological substance pretreatmentsCapture antibodyProstaglandin E2
The invention provides an ELISA assay for the determination of serum mast cell β-tryptase levels using rabbit anti-tryptase as the capture antibody and alkaline phosphatase conjugated G3 as the detecting antibody. Luminescent substrate CPSD was used to enhance the assay sensitivity. Also provided are methods for aiding in the diagnosis of irritable bowel syndrome by detecting the serum level of β-tryptase, histamine and / or prostaglandin E2.
Owner:PROMETHEUS BIOSCI INC
Ileitis diagnostic assay
Improved immunoassays for the protection of antibodies against Lawsonia intracellularis are provided which permit rapid, easy detection of low concentrations of anti-Lawsonia antibodies in animal-derived specimens. The preferred assay is an ELISA assay employing an antigenic extract of L. intracellularis lipopolysaccharide.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
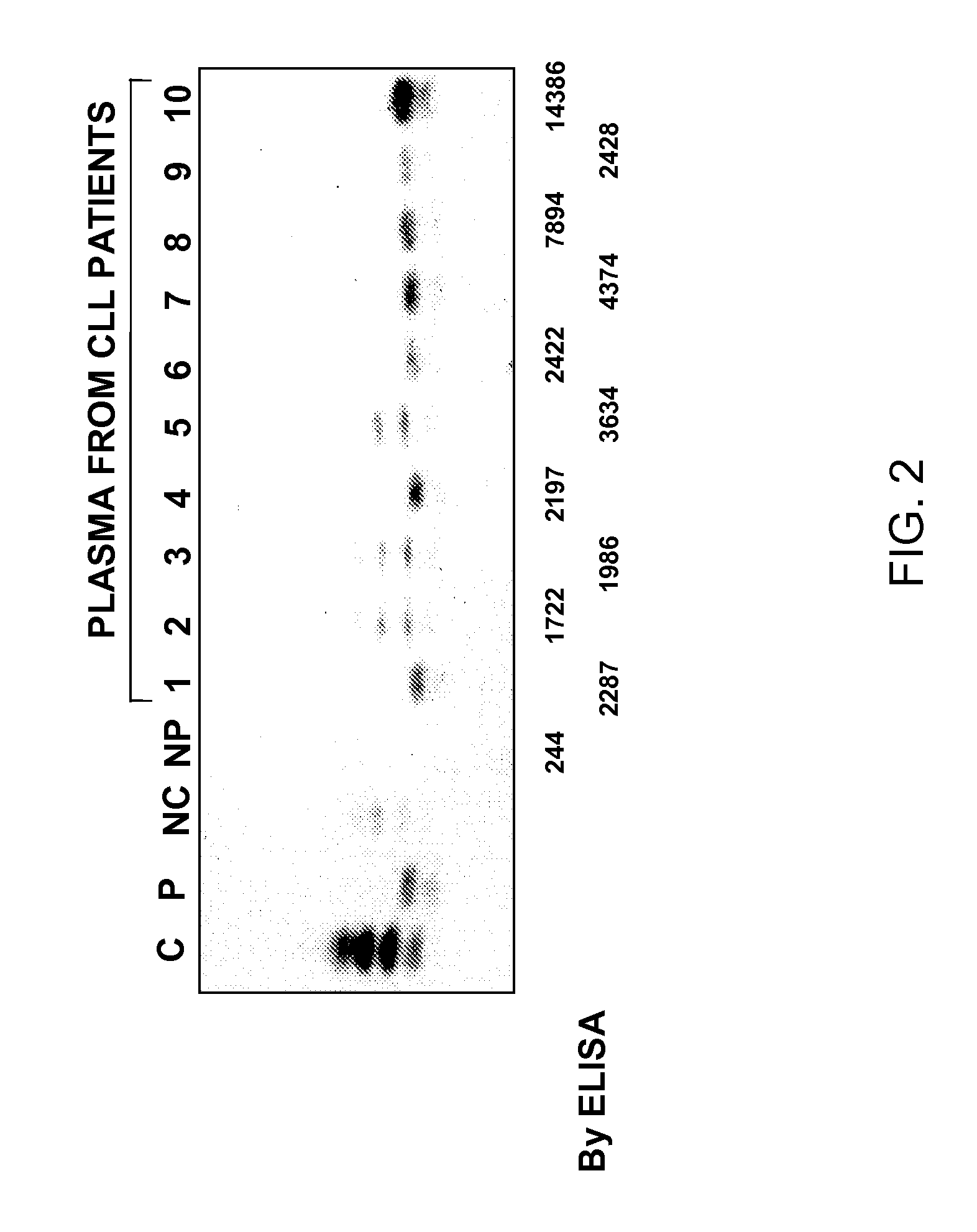
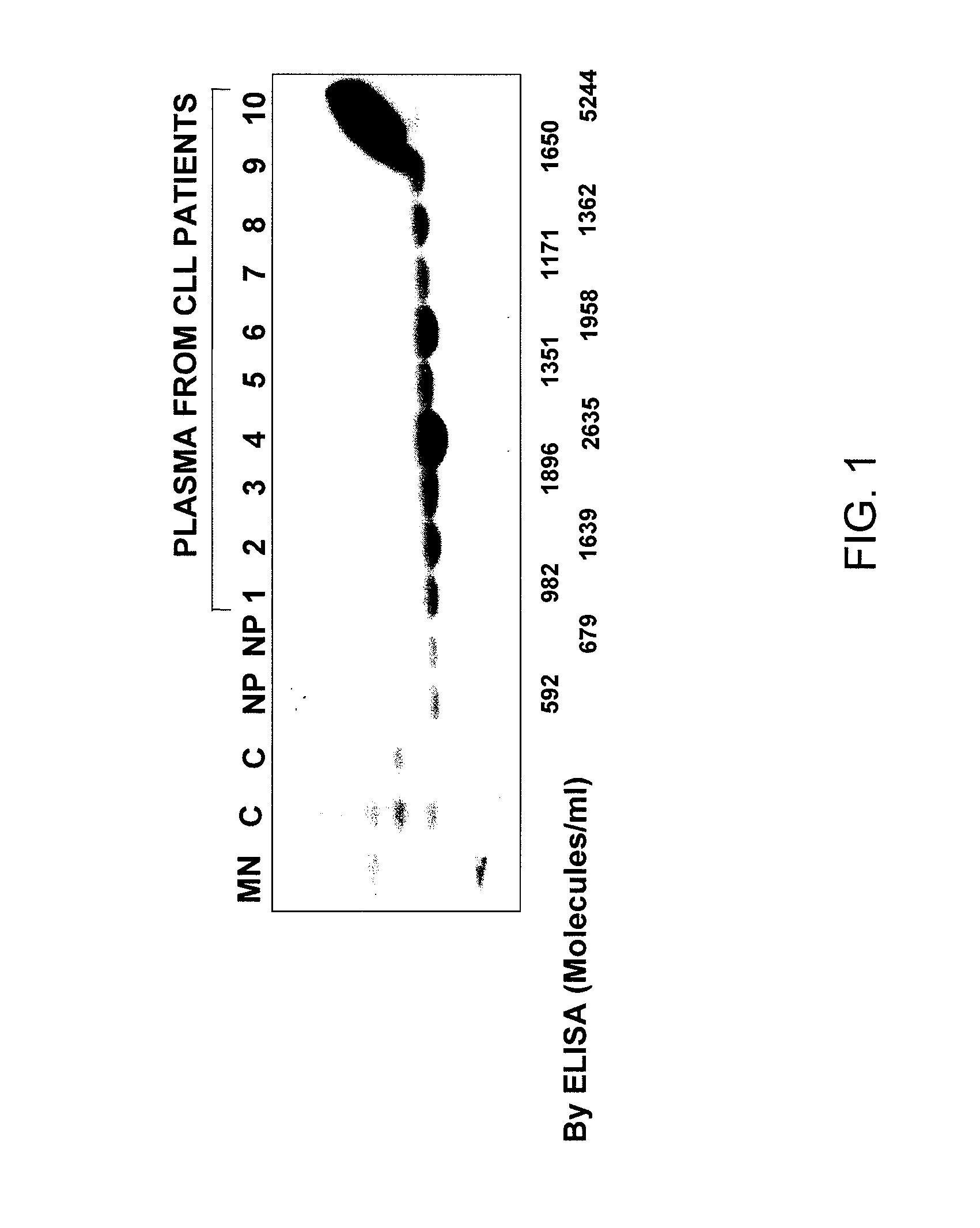
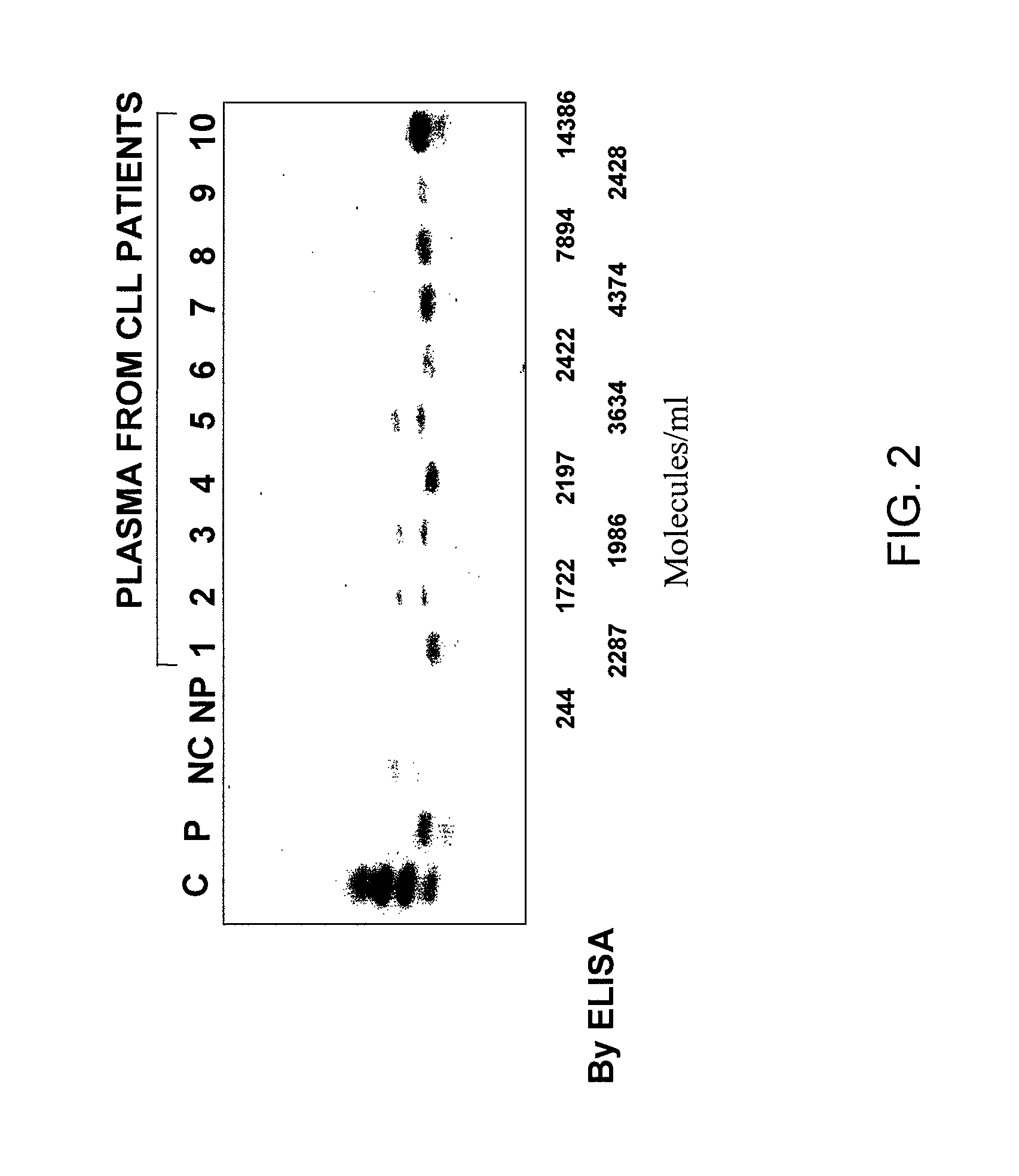
Measuring circulating therapeutic antibody, antigen and antigen/antibody complexes using ELISA assays
The present invention relates to the field of immunology and hyperproliferative diseases. More specifically, the present invention relates to a method of detecting and monitoring therapeutic antibody:antigen complex, soluble antigen and soluble therapeutic antibody, wherein a patient has undergone at least one course of immunotherapy. Yet further, levels of therapeutic antibody:antigen complexes, soluble antigens or soluble therapeutic antibodies may be measured and used to stage or monitor a hyperproliferative disease.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Measuring circulating therapeutic antibody, antigen and antigen/antibody complexes using ELISA assays
The present invention relates to the field of immunology and hyperproliferative diseases. More specifically, the present invention relates to a method of detecting and monitoring therapeutic antibody:antigen complex, soluble antigen and soluble therapeutic antibody, wherein a patient has undergone at least one course of immunotherapy. Yet further, levels of therapeutic antibody:antigen complexes, soluble antigens or soluble therapeutic antibodies may be measured and used to stage or monitor a hyperproliferative disease.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Peripheral Blood Biomarkers for Idiopathic Interstitial Pneumonia and Methods of Use
InactiveUS20120329666A1Increased riskMicrobiological testing/measurementLibrary screeningPcr assayProteomics
Owner:DUKE UNIV +1
Neosporosis recombinant enzyme-linked immunosorbent assay (rELISA) antibody assay kit
InactiveCN102455360ADemonstration effect is goodSignificant technological progressHybrid peptidesVector-based foreign material introductionAntigenEscherichia coli
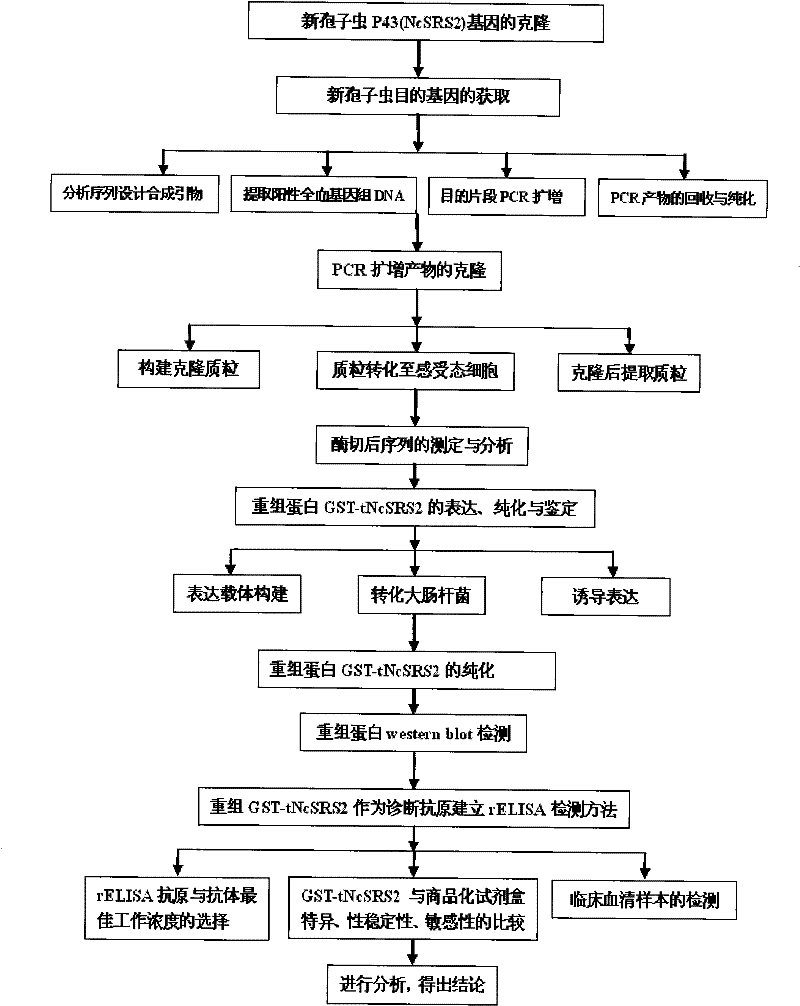
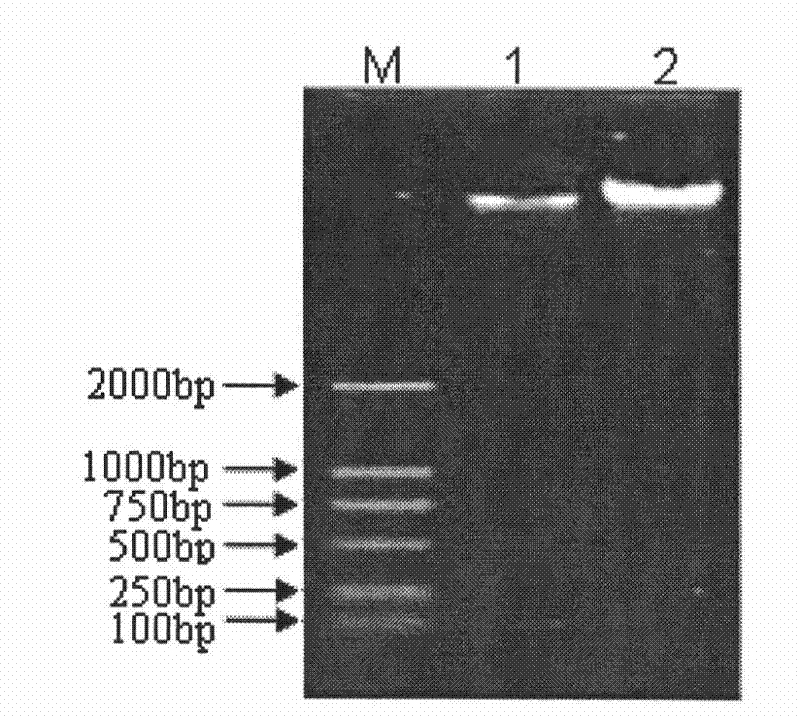
The invention provides a neosporosis rELISA antibody assay kit. The neosporosis recombinant enzyme-linked immunosorbent assay (rELISA) antibody assay kit is characterized in that designing a primer set according to a neospora caninum NcSRS2 gene of the GenBank, introducing the primer set to an enzyme digestion site, removing a signal peptide zone of an NcSRS2 protein, extracting a DNA from Xinjiang cow positive serum, carrying out polymerase chain reaction (PCR) amplification of the DNA to obtain a tNcSRS2 genetic fragment having length of 975bp, cloning the tNcSRS2 genetic fragment into a pMD-18-T carrier, carrying out enzyme digestion and sequencing, further directionally connecting the desired fragment obtained by the previous step to a pGEX-4T-2 expression carrier, transferring the pGEX-4T-2 expression carrier with the desired fragment into escherichia coli, carrying out inducible expression and purification, wherein expressed fusion protein molecular weight is about 62kDa, and carrying out ELISA antigen coating by the expressed fusion protein to obtain the neosporosis rELISA antibody assay kit. A use method of the neosporosis rELISA antibody assay kit comprises the following steps that a coated enzyme label reaction plate is taken out, is subjected to first washing, is enclosed, is subjected to second washing, is added with a sample needing to be detected, is subjected to third washing, is added with a secondary antibody, is subjected to fourth washing, and undergoes a color reaction; and when the color reaction is finished, an OD450 / 630 value of the coated enzyme label reaction plate is read and a result is determined. Compared with a commercial ELISA assay kit, the neosporosis rELISA antibody assay kit has a higher coincidence rate above 95% The neosporosis rELISA antibody assay kit also is suitable for the ELISA of a neospora caninum antibody in ruminant serum and blood plasma.
Owner:XINJIANG AGRI UNIV
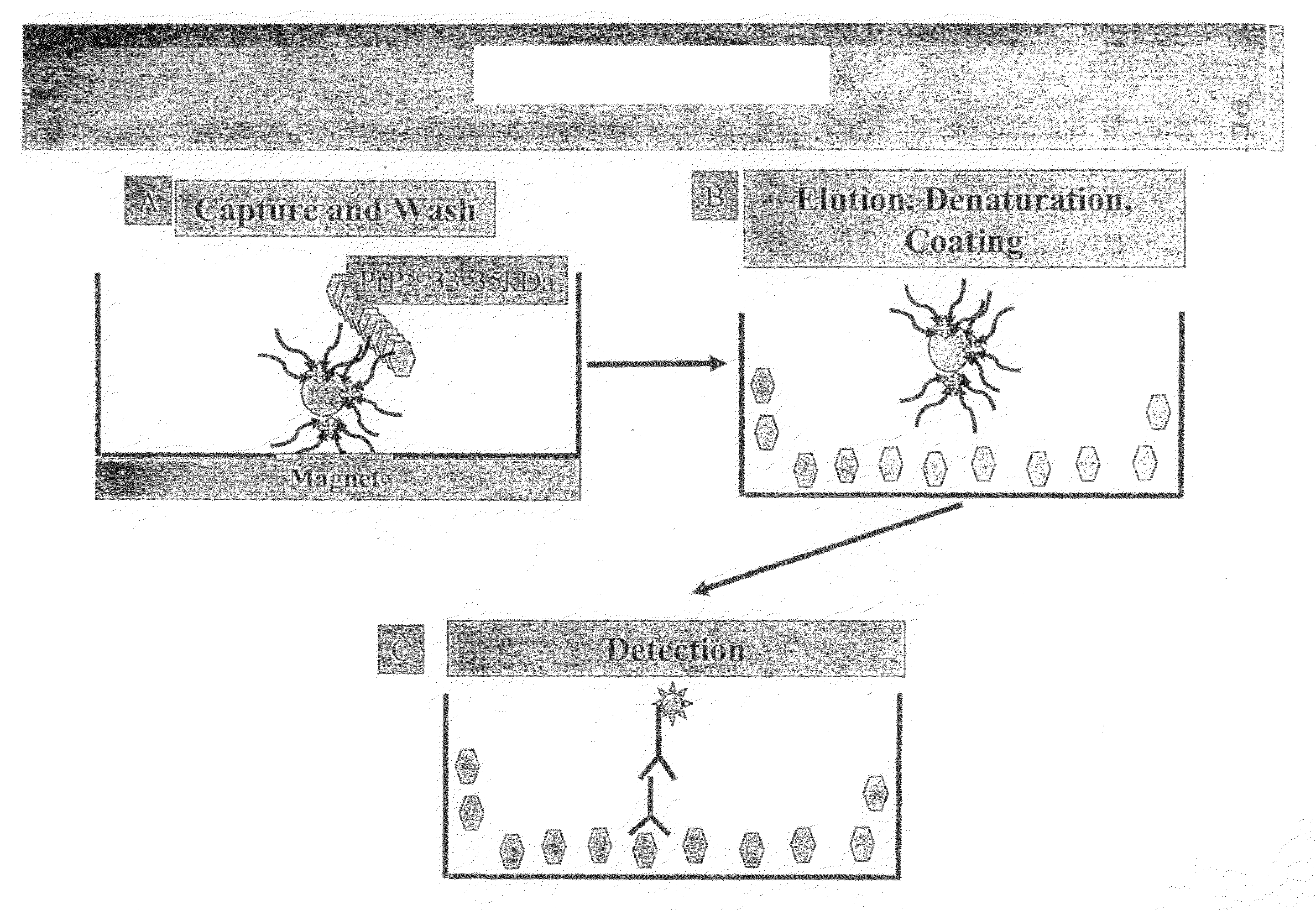
Elisa assays using prion-specific peptide reagents
InactiveUS20090130774A1Easy to produceMaterial nanotechnologyDisease diagnosisElisa assayProteinaceous infectious particle
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
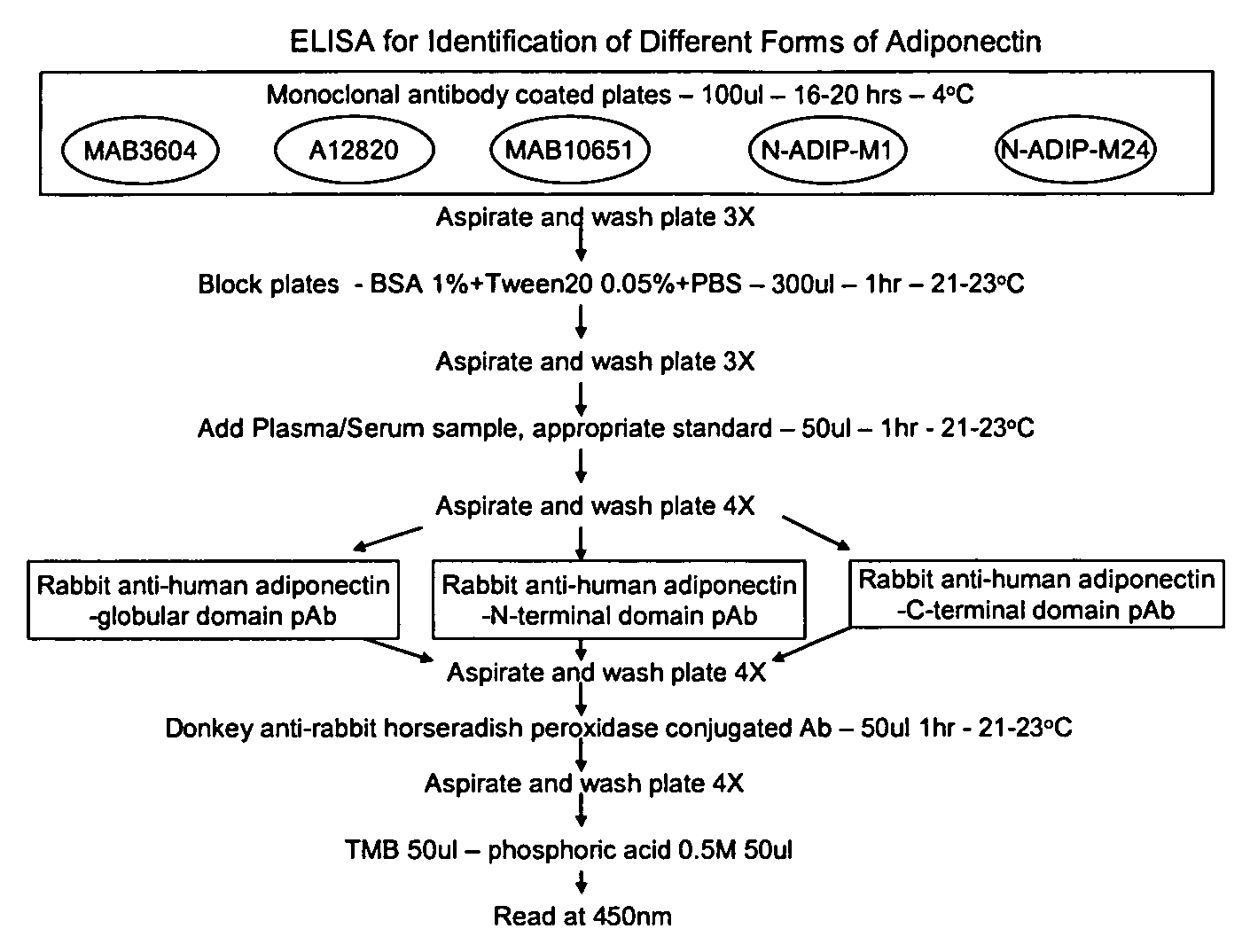
Characterization and identification of unique human adiponectin isoforms and antibodies
The invention pertains to methods for measuring different forms of human adiponectin that are present in human plasma / serum, and more specifically methods are based on an ELISA assay that utilizes different monoclonal antibodies directed against adiponectin, in combination with different polyclonal antibodies directed against different domains of human adiponectin. The invention also provides unique isoforms of adiponectin and antibodies thereto, including polyclonal and monoclonal antibodies.
Owner:F HOFFMANN LA ROCHE INC
Methods for predicting susceptibility to cardiovascular disease
InactiveUS20050032140A1Disease diagnosisBiological testingAtherosclerotic cardiovascular diseaseIncreased risk
The assay of soluble endothelial protein C receptor (sEPCR) is useful to predict cardiovascular disease, particularly atherosclerotic cardiovascular disease (ASCVD). An assay for sEPCR is therefore useful to identify individuals at risk of developing ASCVD. An sEPCR ELISA assay is particularly useful for this purpose. Elevated sEPCR is indicative of an increased risk of developing cardiovascular disease.
Owner:OKLAHOMA MEDICAL RES FOUND
Ileitis diagnostic assay
ActiveUS20060035287A1Bacterial antigen ingredientsSugar derivativesLawsonia intracellularisElisa assay
Improved immunoassays for the protection of antibodies against Lawsonia intracellularis are provided which permit rapid, easy detection of low concentrations of anti-Lawsonia antibodies in animal-derived specimens. The preferred assay is an ELISA assay employing an antigenic extract of L. intracellularis lipopolysaccharide.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Rapid, immunochemical process for measuring thiopurine methyltransferase
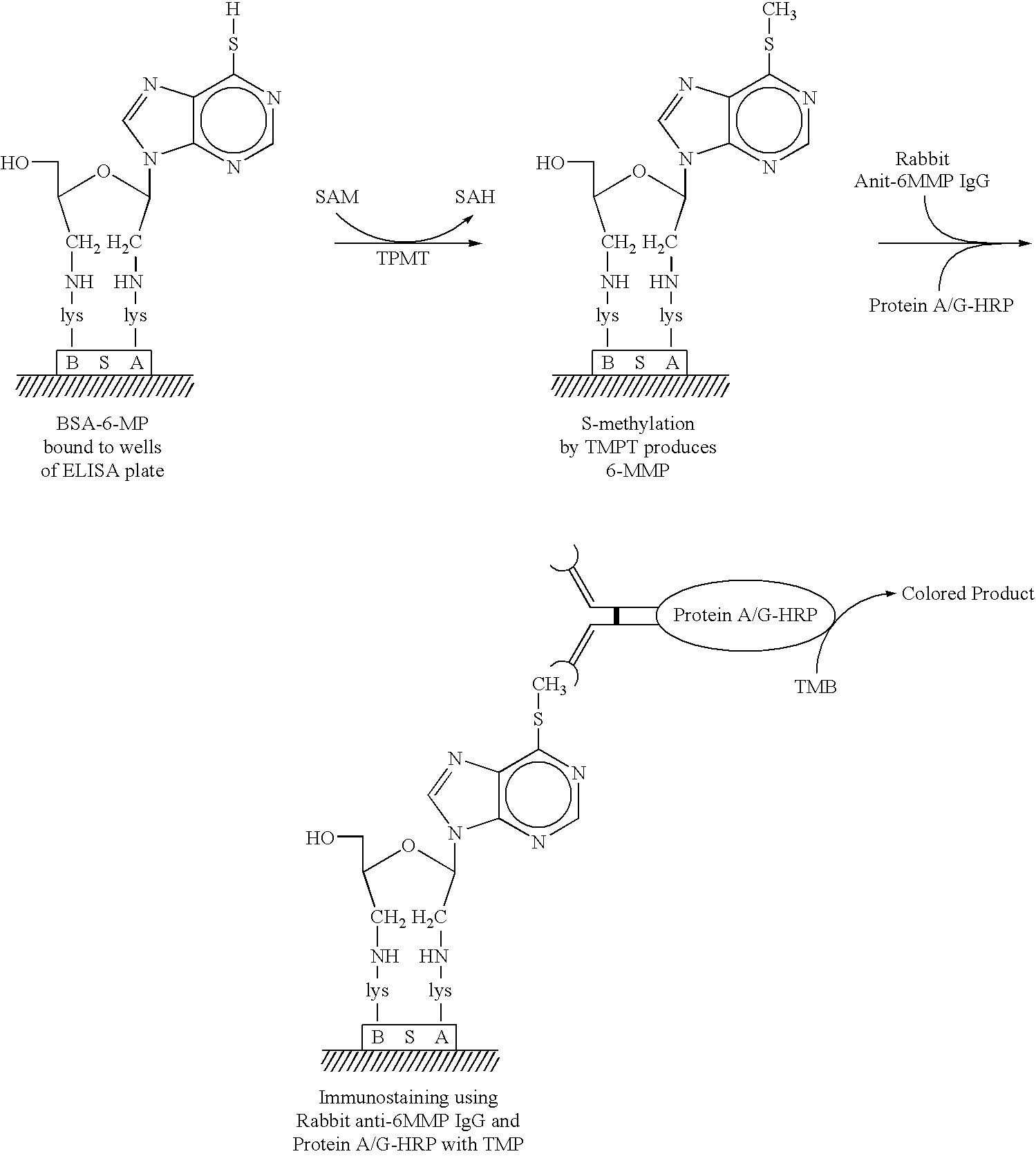
InactiveUS6946258B2Low costMitigate environmental hazardsBiological material analysisBiological testingHeterologousThiopurine methyltransferase
The present invention relates to non-isotopic immunoassays for thiopurine methyltransferase (TPMT). The immunoassays of this invention may be homogenous or heterogenous, in which detection of the TPMT-catalyzed reaction product relies upon specific binding of antibody to 6-MMP or other TPMT-catalyzed reaction products. Preferred embodiments of this invention include a Rapid Immunomigration Cassette and an assay carried out in ELISA assay format.
Owner:BIOLOGIX DIAGNOSTICS +1
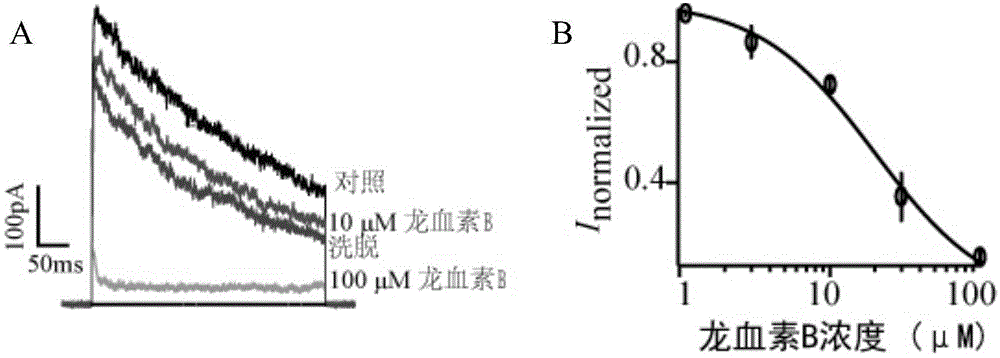
Application of Loureirin B in preparation of Kv1.3 channel blocker
InactiveCN104523663AClear ingredientsQuality is easy to controlMetabolism disorderAntipyreticDiseaseSide effect
The invention discloses an application of Loureirin B in the preparation of a Kv1.3 channel blocker and provides a new drug approach for treating autoimmune diseases. The Loureirin B contains clear components and quality of the Loureirin B is controllable. The Loureirin B can effectively inhibit a Kv1.3 potassium channel, thus effectively treating various autoimmune diseases. Through various verification means of patch clamp experiment, calcium imaging techniques, ELISA assay and manufacturing of animal models of autoimmune encephalitis, rheumatoid arthritis and the like, it is found that the Loureirin B has a good therapeutical effect on autoimmune diseases at the overall level, can effectively overcome side effects of traditional therapeutics such as adrenocortical hormone and the like and can maintain normal protective immune responses of patients.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
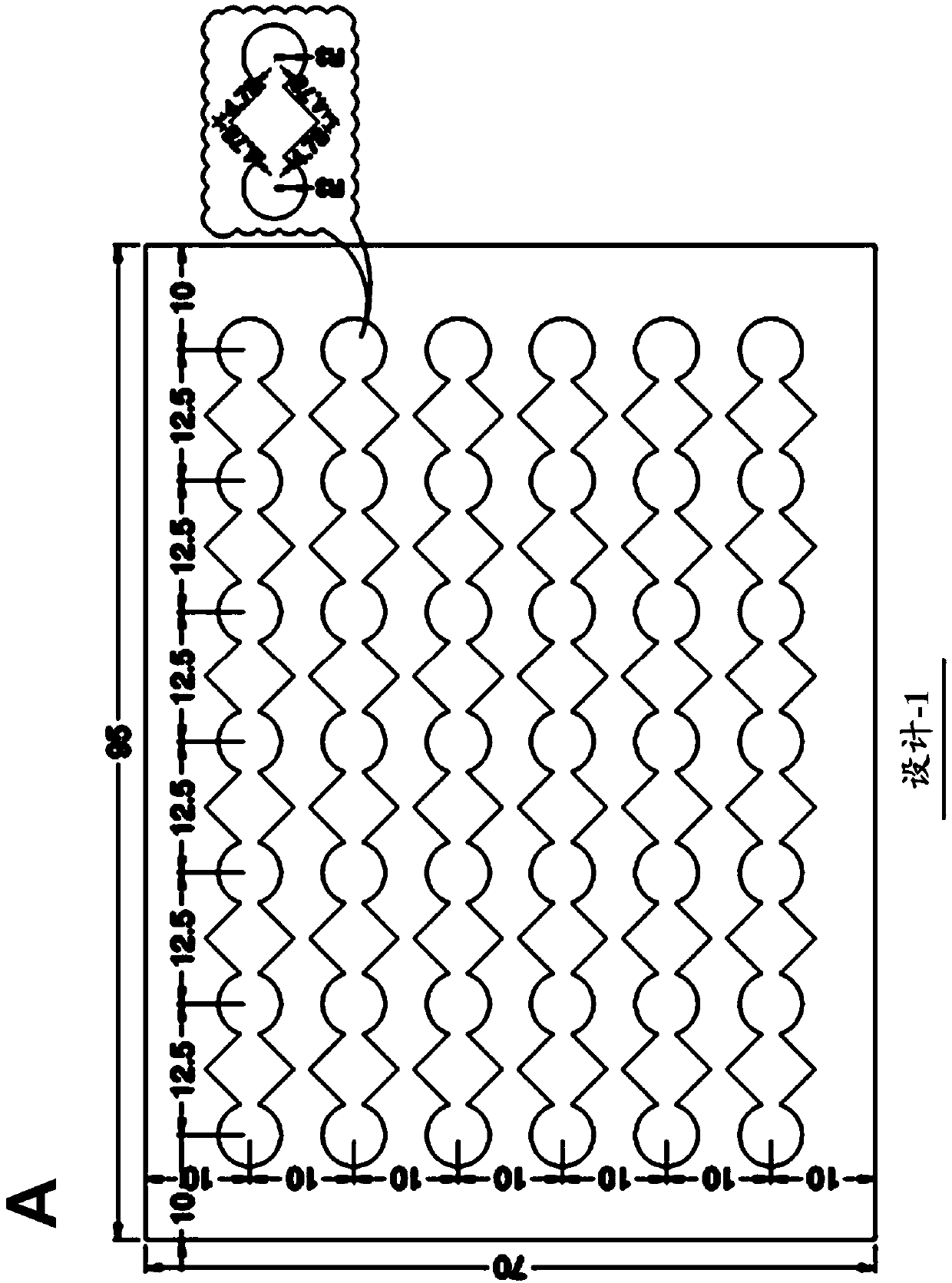
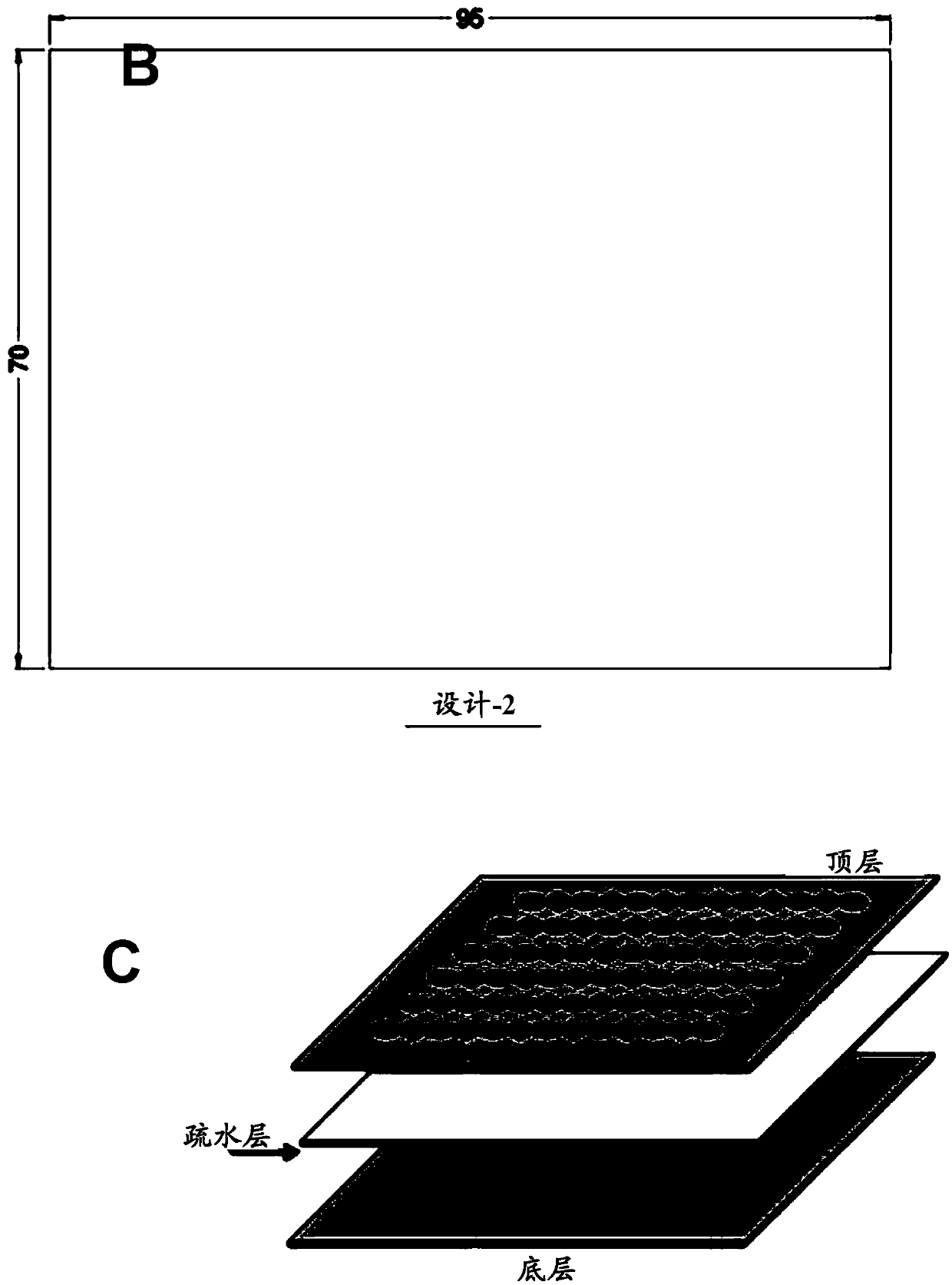
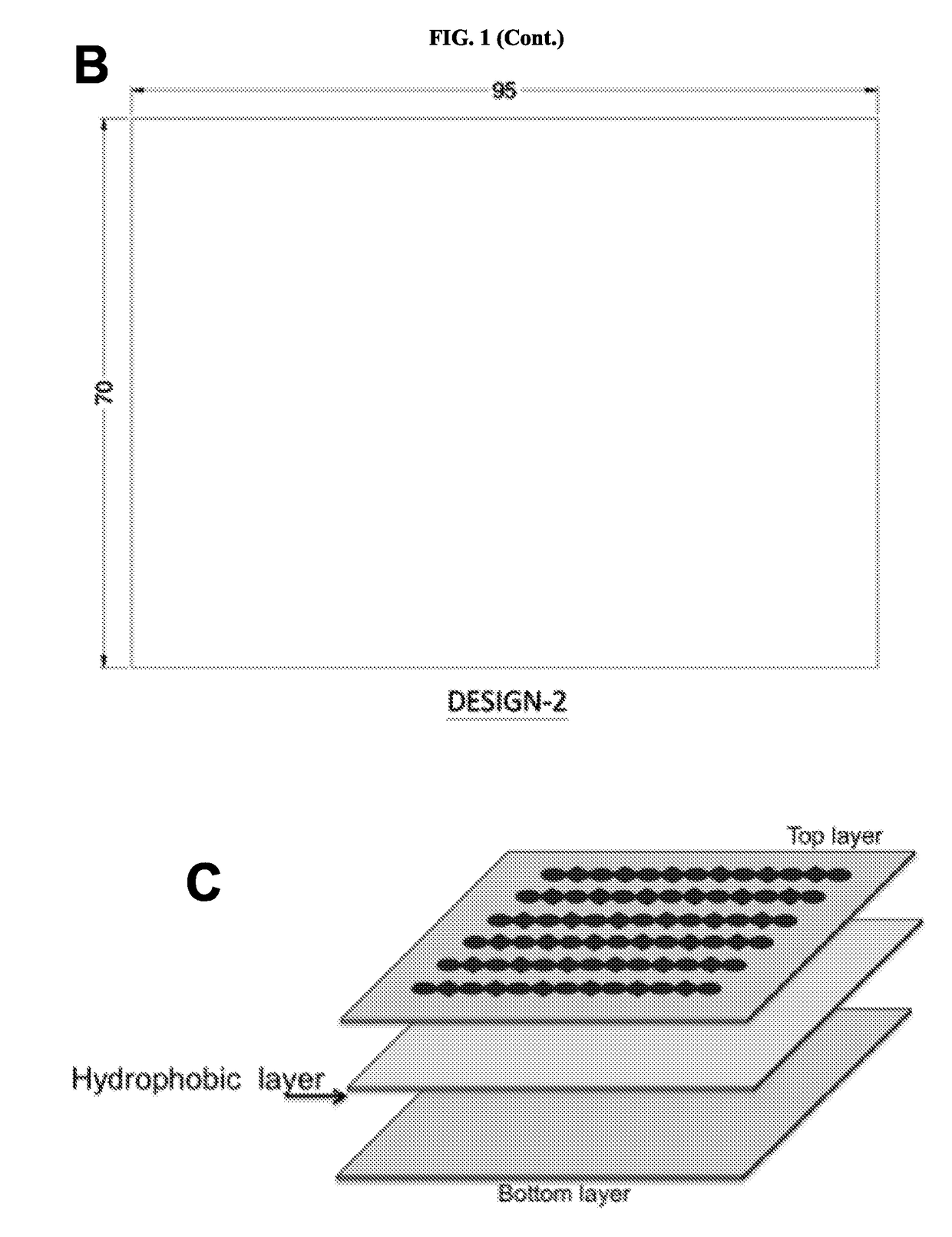
Novel design of enzyme-linked immunsorbent assay plates and systems and methods of use thereof
The present disclosure refers to an enzyme-linked immunosorbent assay (ELISA) plate comprising at least one row of reaction chambers, wherein the reaction chambers in the same row are in fluid communication with each other. Also enclosed is a system for detecting one or more target analytes comprising an ELISA plate as described herein, a plurality of magnetic beads and a magnet configured to cooperate with the magnetic beads. Also encompassed is a method of performing an ELISA assay which comprises of moving magnetic beads through subsequent reaction chambers, wherein the reaction chambers are alternatingly filled with a non-aqueous liquid, such as silicone oil, and aqueous ELISA reagents.
Owner:AGENCY FOR SCI TECH & RES
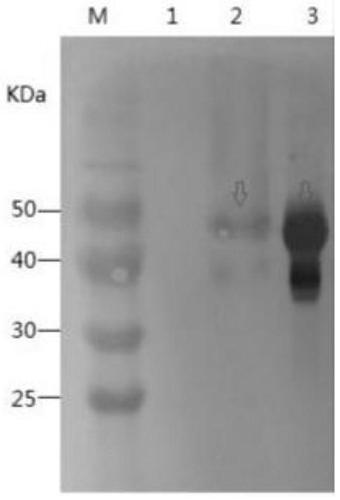
Monoclonal antibody hybridoma cell line 1H6 for resisting infectious bursal disease virus VP2 protein
ActiveCN110373393AStrong specificityHighly competitiveVirus peptidesImmunoglobulins against virusesBALB/cTitin Antibody
The invention relates to a monoclonal antibody hybridoma cell line 1H6 for resisting infectious bursal disease virus VP2 protein, and belongs to the technical field of biology. In the invention, BALB / c mice are immunized with purified IBDV QL strain antigen, splenocytes are prepared and fused to an SP2 / 0 myeloma cell line, double screening is carried out by IBDV recombinant VP2 (rVP2) protein expressed by prokaryote and purified IBDV QL strain to obtain the monoclonal antibody hybridoma cell line 1H6 which not only can react with rVP2 protein expressed by prokaryote, but also can react with IBDV, the antibody subclass is IgG1 kappa, and the antibody titers of induced ascites are 108 respectively. Sandwich ELISA assay shows that 1H6 has no cross-reaction with other four avian viruses; indirect immunofluorescence test proves that 1H6 has good specific reaction; and immunoblot analysis shows that 1H6 can produce specific protein bands with rVP2 protein and IBDV.
Owner:JIANGSU ACAD OF AGRI SCI
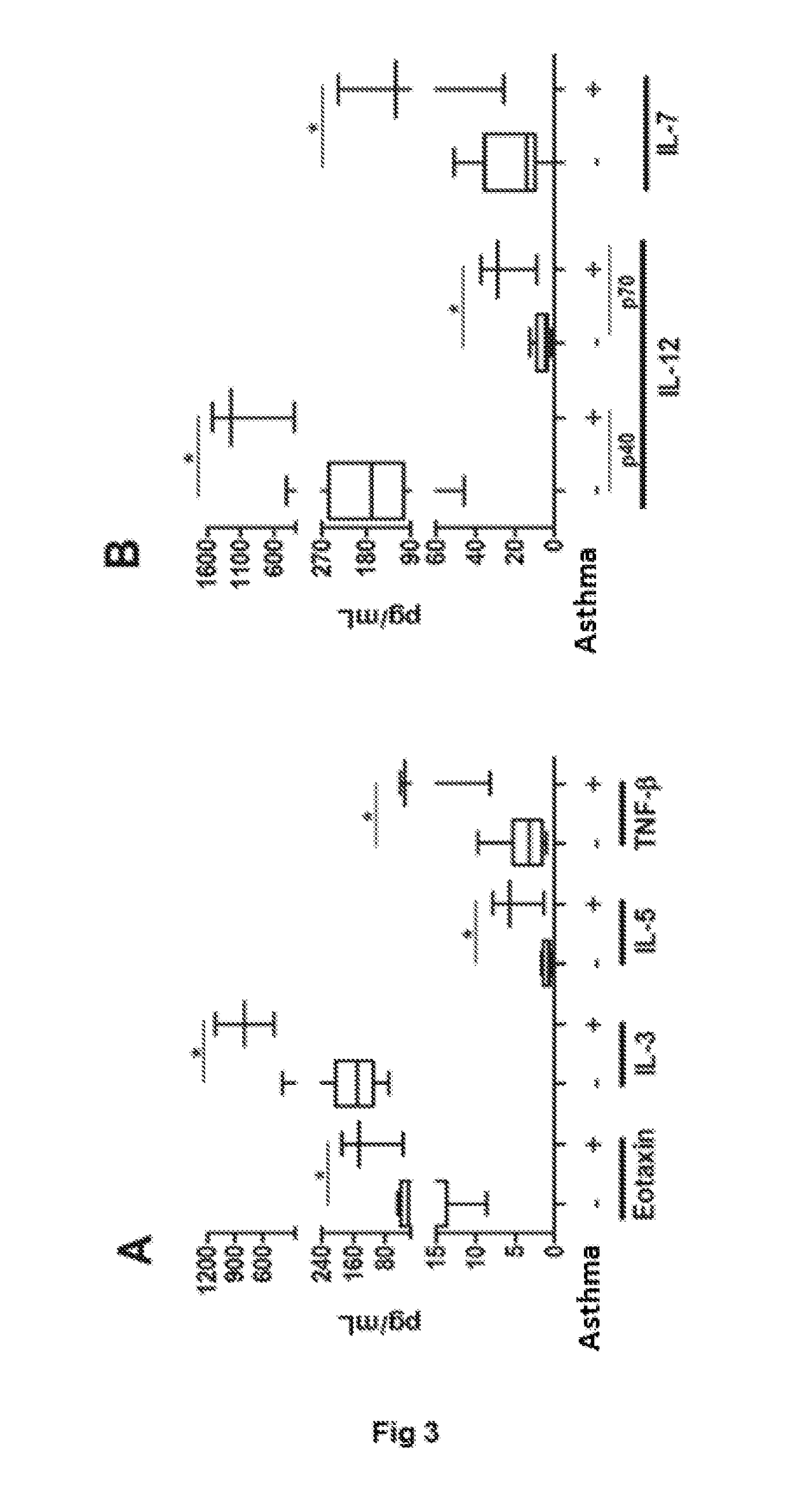
USE OF IL-3, IL-33, AND IL-12p40 FOR CHARACTERIZATION OF THE RESPIRATORY INFECTIONS BY SYNCYTIAL RESPIRATORY VIRUS
ActiveUS20180327843A1Microbiological testing/measurementDisease diagnosisRespiratory diseaseHuman metapneumovirus RNA
The present invention is related to detecting respiratory diseases using molecular markers as prognostic tool of the evolution of respiratory infection cases. Concretely, during the differential diagnostic of respiratory infections caused by the Syncytial Respiratory Virus and human Metapneumovirus, it will be established the expression pattern of the severity markers of IL-3, IL-33 and IL12p40. The expression pattern of the molecular markers can be defined in biological samplers using ELISA assays, flow cytometry or PCR in real time. The confirmation of the etiological agent of the infection in combination to the pattern definition of the molecular markers IL-3, IL-33 and IL12p40 will indicate a prognostic of the disease severity.
Owner:PONTIFISIA UNIVERSIDAD KATOLIKA DE CHILE
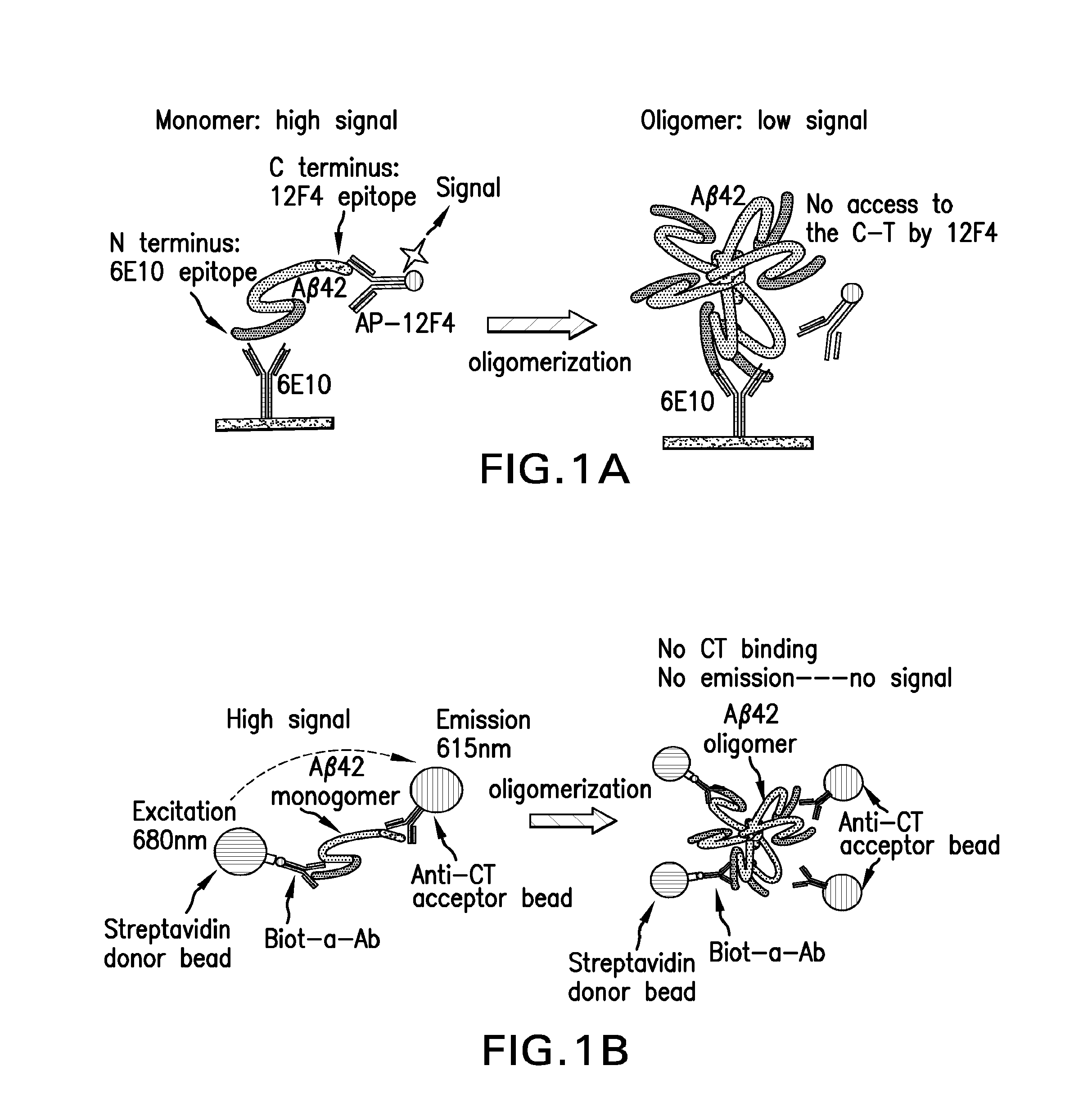
Methods for identifying inhibitors of abeta42 oligomers
The invention herein is directed to immunoassays for the detection of Aβ42 oligomers. The inventive assays are based on the observations herein that the presence of Aβ42 oligomers in a preparation is directly related to a decrease in a C-terminal (CT) immunosignal and a correlated increase in an N-terminal (NT) immunosignal, relative to the immunosignal generated in the absence of Aβ42 oligomers, in an Aβ42 CT and NT ELISA assay and an Aβ42 CT AlphaLISA assay. The invention herein involves the use of these assays alone or in combination to screen for inhibitors of Aβ42 oligomerization.
Owner:MERCK SHARP & DOHME CORP
Elisa assay kit, elisa for determining desmosine levels from urine samples and diagnostic urine assays for aneurysms
InactiveUS20130273580A1Effective isolationChemiluminescene/bioluminescenceBiological material analysisBacteriuriaPolyclonal antibodies
Improved ELISA assay formats are described that provide for effective measurement of desmosine, an elastin degradation product, in urine samples. The urine samples can be effectively introduced into the assay with little or no sample preparation. The competitive ELISA assay based on high titer polyclonal antibodies is suitable for commercial application. Desirable antibodies can be generated using desmosine bound to a protein or the like using a protein crosslinking agent. The desmosine assay are found to be useful for the diagnosis of aortic aneurysms in which desmosine levels of patients with aortic aneurysms have been found to be significantly elevated relative to a control group.
Owner:ADI
Characterization and Identification of Unique Human Adiponectin Isoforms and Antibodies
InactiveUS20090123943A1Easy to measureAntibody ingredientsDisease diagnosisSerum igeMonoclonal antibody
The invention pertains to methods for measuring different forms of human adiponectin that are present in human plasma / serum, and more specifically methods are based on an ELISA assay that utilizes different monoclonal antibodies directed against adiponectin, in combination with different polyclonal antibodies directed against different domains of human adiponectin. The invention also provides unique isoforms of adiponectin and antibodies thereto, including polyclonal and monoclonal antibodies.
Owner:F HOFFMANN LA ROCHE INC
Novel design of enzyme-linked immunosorbent assay plates and systems and methods of use thereof
The present disclosure refers to an enzyme-linked immunosorbent assay (ELISA) plate comprising at least one row of reaction chambers, wherein the reaction chambers in the same row are in fluid communication with each other. Also enclosed is a system for detecting one or more target analytes comprising an ELISA plate as described herein, a plurality of magnetic beads and a magnet configured to cooperate with the magnetic beads. Also encompassed is a method of performing an ELISA assay which comprises of moving magnetic beads through subsequent reaction chambers, wherein the reaction chambers are alternatingly filled with a non-aqueous liquid, such as silicone oil, and aqueous ELISA reagents.
Owner:AGENCY FOR SCI TECH & RES
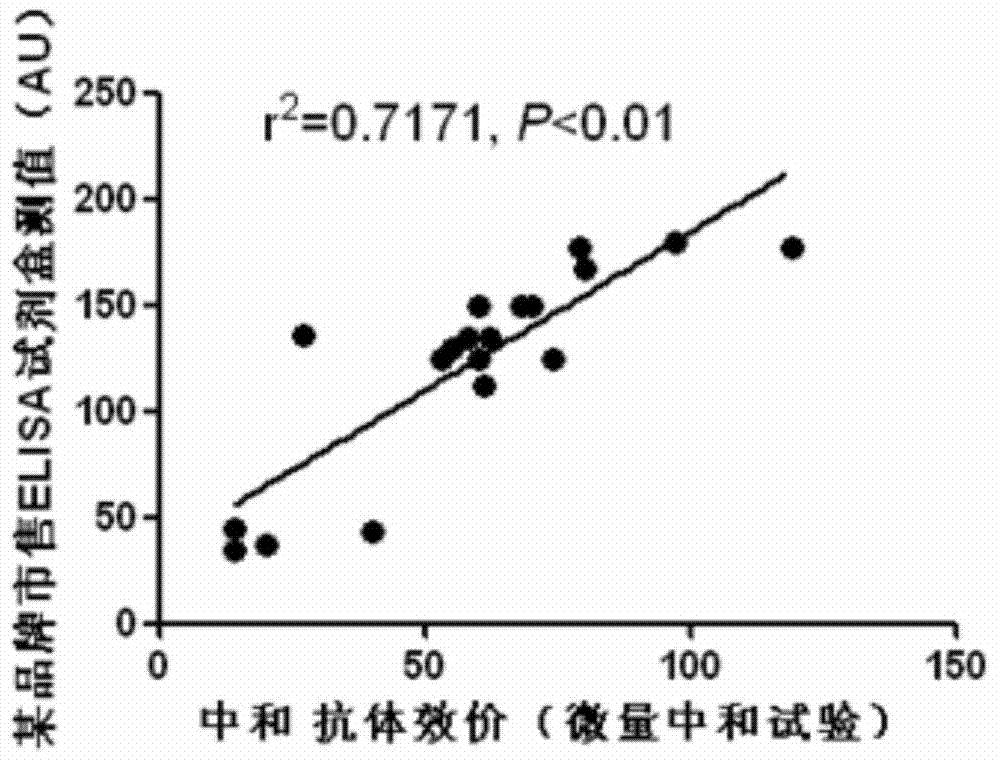
Preparation method of recombinant protein IE1-coated ELISA (Enzyme linked immune sorbent assay) reaction plate and assay kit for quantitatively detecting HCMV (human cytomegalovirus) neutralized antibody in human plasma
The invention discloses a preparation method of a recombinant protein IE1-coated ELISA (Enzyme linked immune sorbent assay) reaction plate and an assay kit for quantitatively detecting HCMV (human cytomegalovirus) neutralized antibody in human plasma and relates to a preparation method of a novel recombinant antigen IE1-coated ELISA reaction plate and a prepared ELISA assay kit for quantitatively detecting HCMV neutralized antibody in human plasma based on the preparation method of the novel recombinant antigen IE1-coated ELISA reaction plate. The preparation method comprises the following steps: preparing specificity recombinant HCMVIE1 antigen, purifying protein, coating the ELISA reaction plate and assembling the ELISA assay kit for quantitatively detecting HCMV neutralized antibody in human plasma. Compared with the other commercial ELISA assay kit, the ELISA assay kit disclosed by the invention can highly specifically, sensitively and rapidly detect IgG antibody titer with neutralized HCMV specificity in plasma and the detection result is highly consistent with that of a micro neutralization experiment. The preparation method of the recombinant protein IE1-coated ELISA (Enzyme linked immune sorbent assay) reaction plate and the assay kit for quantitatively detecting HCMV (human cytomegalovirus) neutralized antibody in human plasma are mainly used for screening high-titer HCMV intravenous injected gamma globulin production raw materials for laboratory research, clinical screening and blood product enterprises screening.
Owner:王明丽 +1
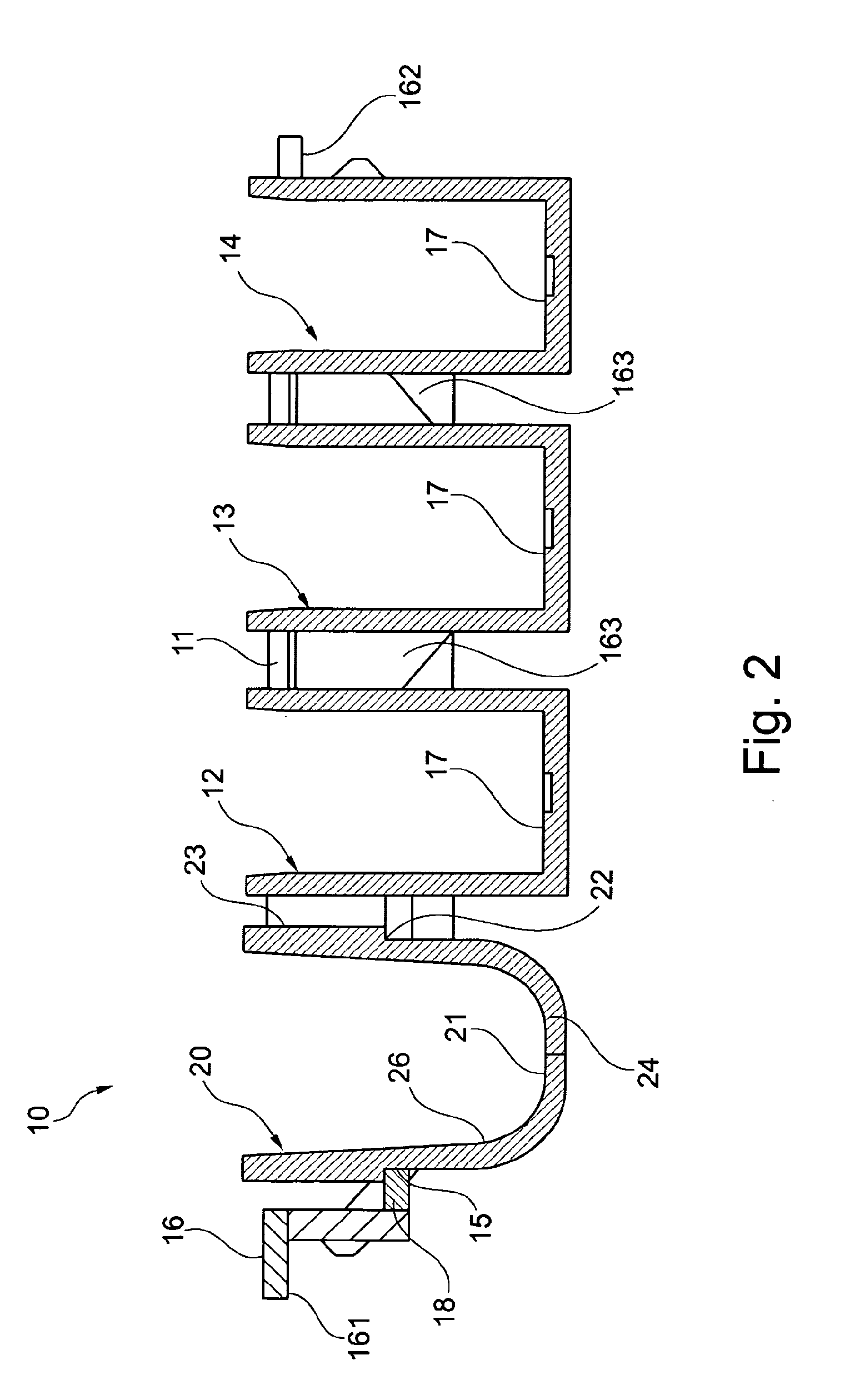
Reagent cartridge for an assembly for selectively performing a clincial chemical test or an elisa test, use of said reagent cartridge and assembly
InactiveUS20120252109A1Improve bindingSuitable for storageBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenChemical test
The invention relates to a reagent cartridge 10 for an assembly for selectively performing a clinical chemical test or an ELISA assay comprising a housing 11 having at least one cavity 12, 13, 14 that contains a reaction or diluting component and having a recess 15 in which a solid phase 20 to which an antigen or antibody can be coupled is inserted into the recess 15 of the housing 11. It is particularly advantageous if the reagent cartridge 10 comprises three cavities 12, 13, 14 and the reagent cartridge 10 can be used for analyzing clinical chemical parameters, for analyzing immunodiagnostic parameters, for providing additional reagents, and as a diluting cartridge.
Owner:DRG INSTR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com