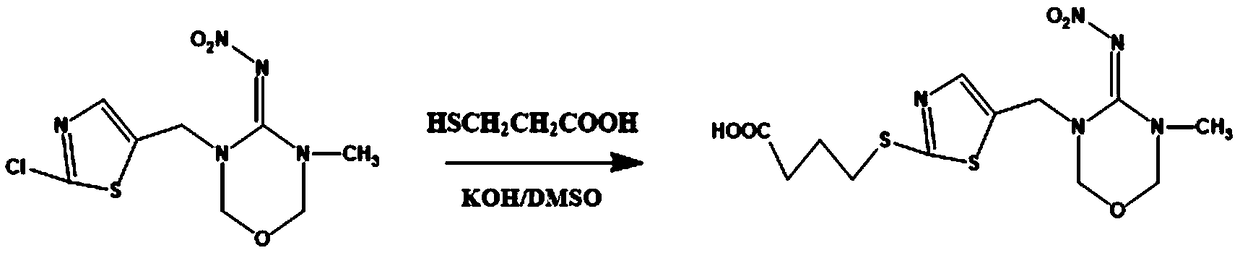
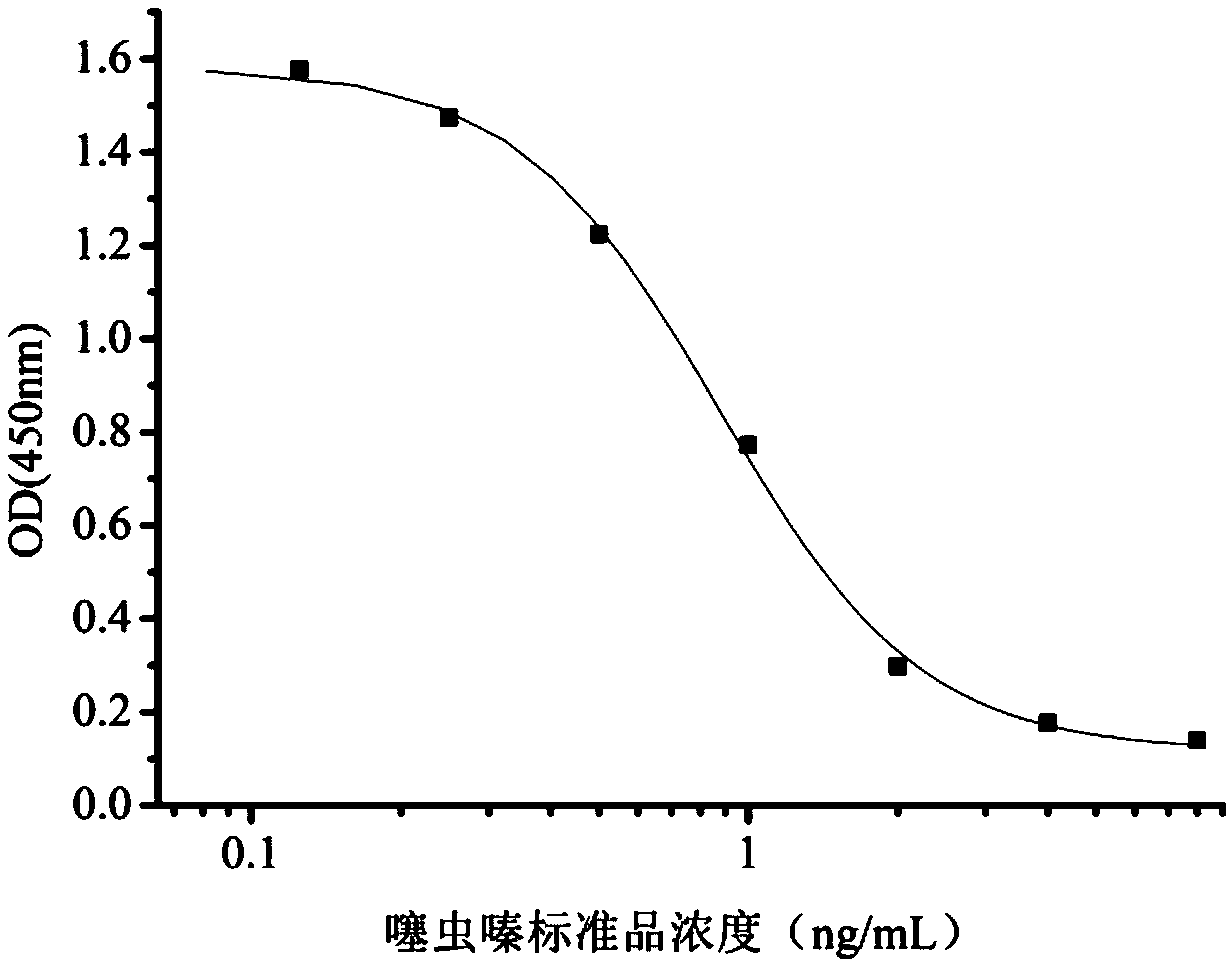
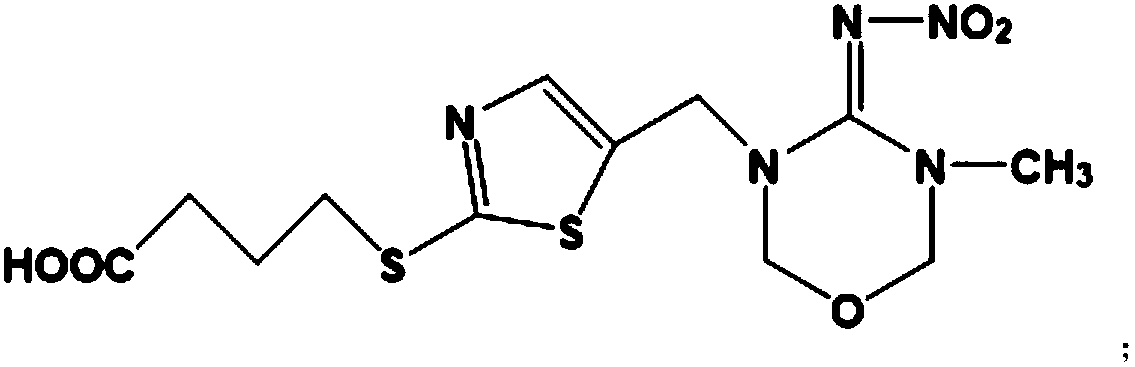
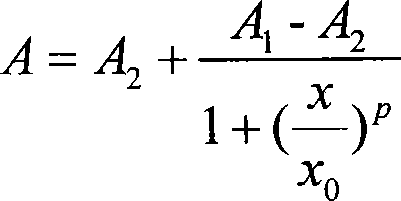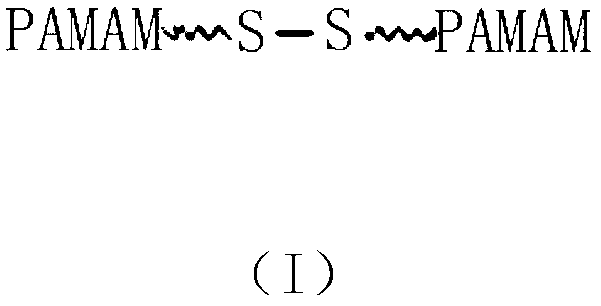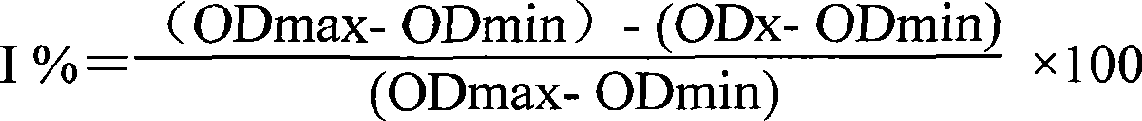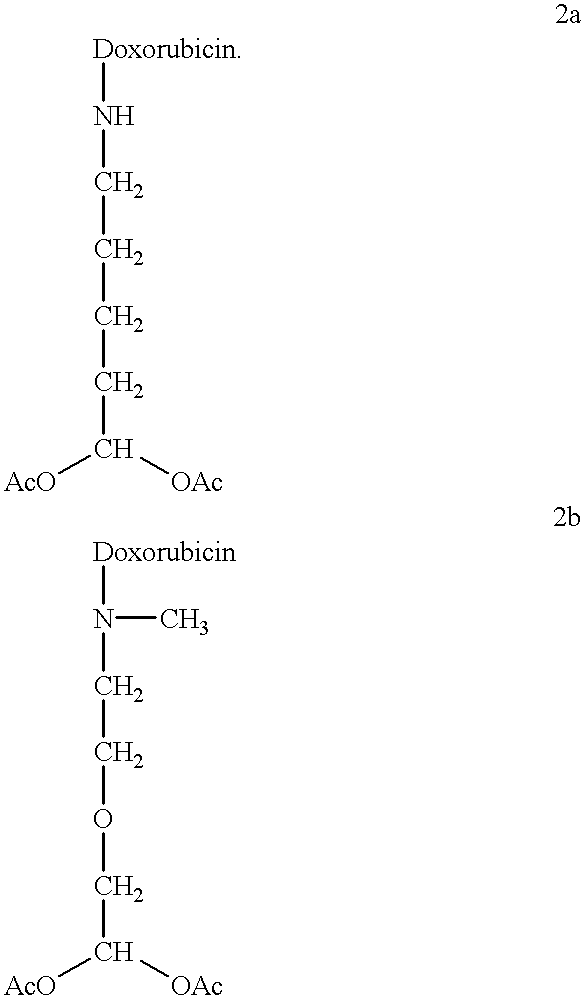Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
541 results about "Myeloma cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Myeloma Myeloma is cancer of the plasma cells. ... Additionally, the myeloma cells commonly produce substances that cause bone destruction, leading to bone pain and/or fractures. Myeloma cells are produced in the bone marrow, the soft tissue inside your bones.
Human SARS-CoV-2 monoclonal antibody and preparation method and application thereof
PendingCN111153991AHigh affinityStrong specificitySsRNA viruses positive-senseVirus peptidesBALB/cMonoclonal antibody agent
The invention discloses a human SARS-CoV-2 monoclonal antibody. The preparation method of the human SARS-CoV-2 monoclonal antibody comprises the steps: adopting SARS-CoV Nucleocapsid recombinant protein as immunogen, immunizing BALB / c mice, performing fusion and subcloning on spleen cells and myeloma cells of mice, then performing a large amount of repeated screening and domestication of cell lines through commercialized products SARS-CoV-2 Nucleocapsid and MERS Nucleocapsid so as to obtain a hybridoma cell line capable of secreting the SARS-CoV-2-resistant N monoclonal antibody with high affinity and high specificity finally and successfully, and finally performing ascites preparation and purification so as to obtain the monoclonal antibody, wherein the amino acid sequence of the SARS-CoVNucleocapsid recombinant protein is shown in SEQ ID No. 1. The invention also discloses application of the monoclonal antibody in preparation of SARS-CoV-2 virus detection products and preparation ofdrugs for inhibiting the SARS-CoV-2 viruses. The monoclonal antibody can be used for detecting the SARS-CoV-2 in human throat swabs / pulmonary secretions and other samples by using a double-antibody sandwich method, and can be applied to diagnosis and prevention and control of SARS-CoV-2 virus infection and scientific researches of viruses and other study.
Owner:BEIJING BIOSYNTHESIS BIOTECH
Chimeric antigen receptors targeting B-cell maturation antigen
ActiveUS9765342B2Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenAntigen receptors
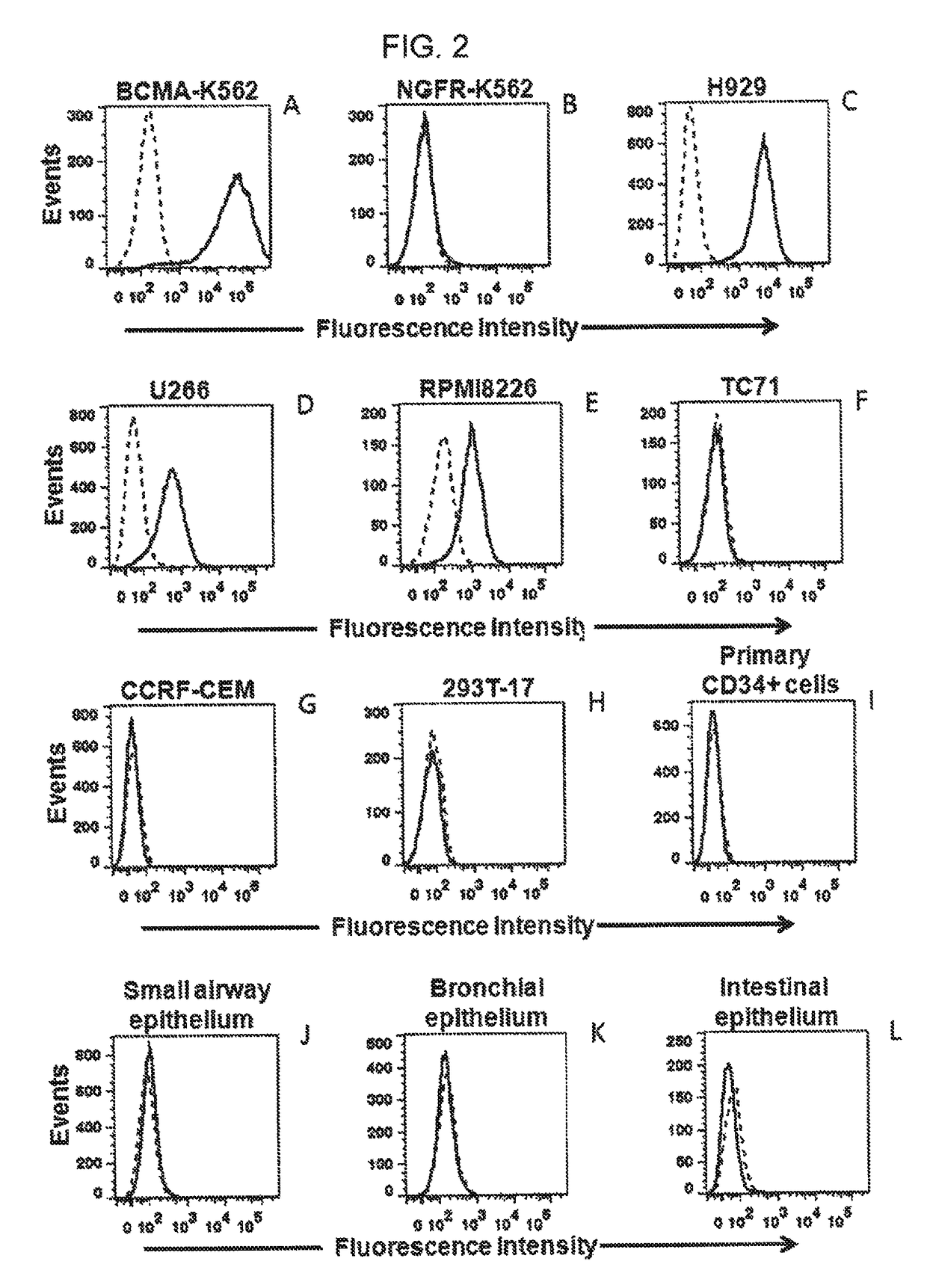
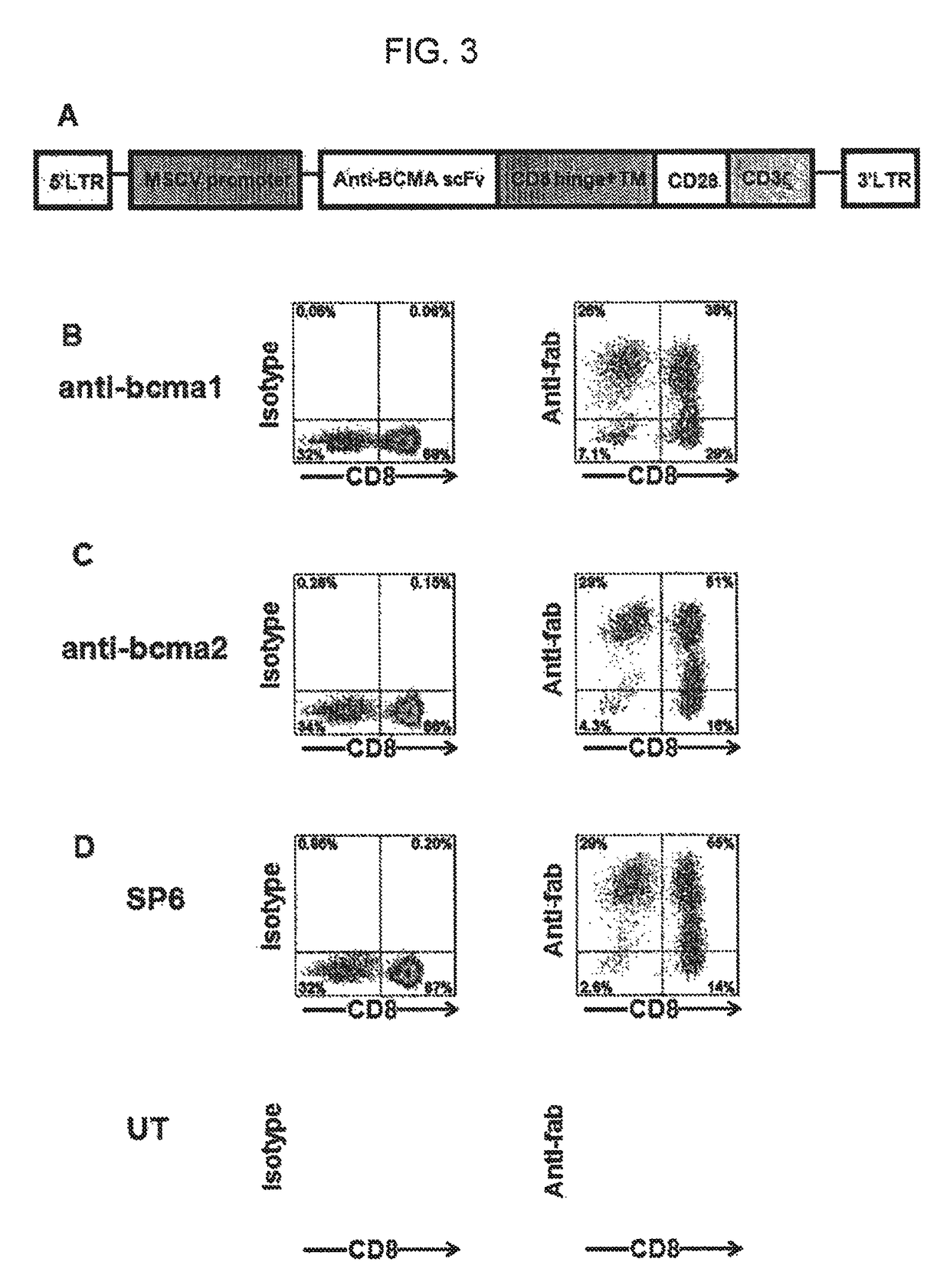
The invention provides an isolated and purified nucleic acid sequence encoding a chimeric antigen receptor (CAR) directed against B-cell Maturation Antigen (BCMA). The invention also provides host cells, such as T-cells or natural killer (NK) cells, expressing the CAR and methods for destroying multiple myeloma cells.
Owner:UNITED STATES OF AMERICA
Combinations for the treatment of diseases involving cell proliferation, migration or apoptosis of myeloma cells or angiogenesis
ActiveUS20080254040A1Improve efficacyRestore sensitivityOrganic active ingredientsBiocideDiseaseCo administration
Owner:BOEHRINGER INGELHEIM INT GMBH
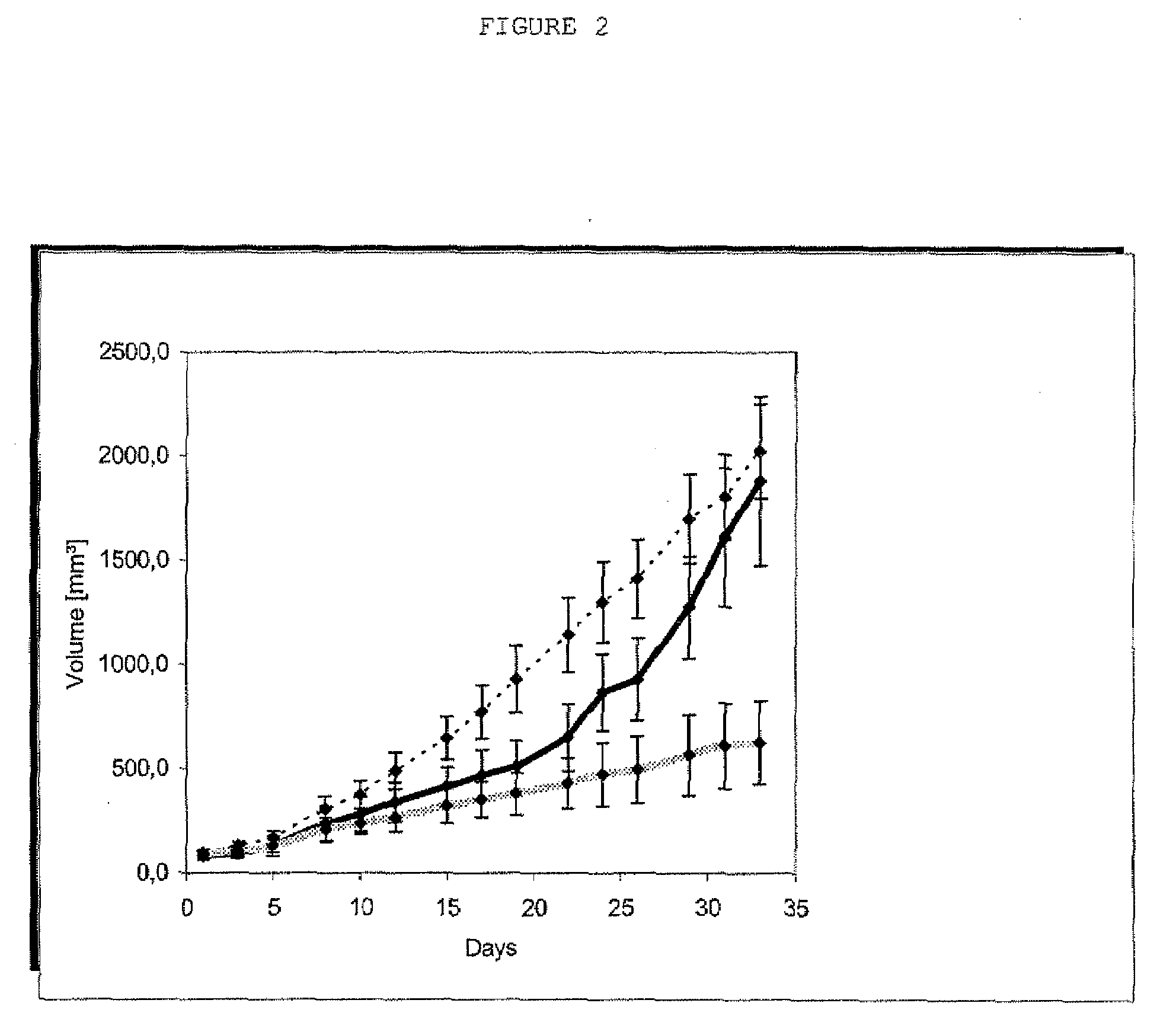
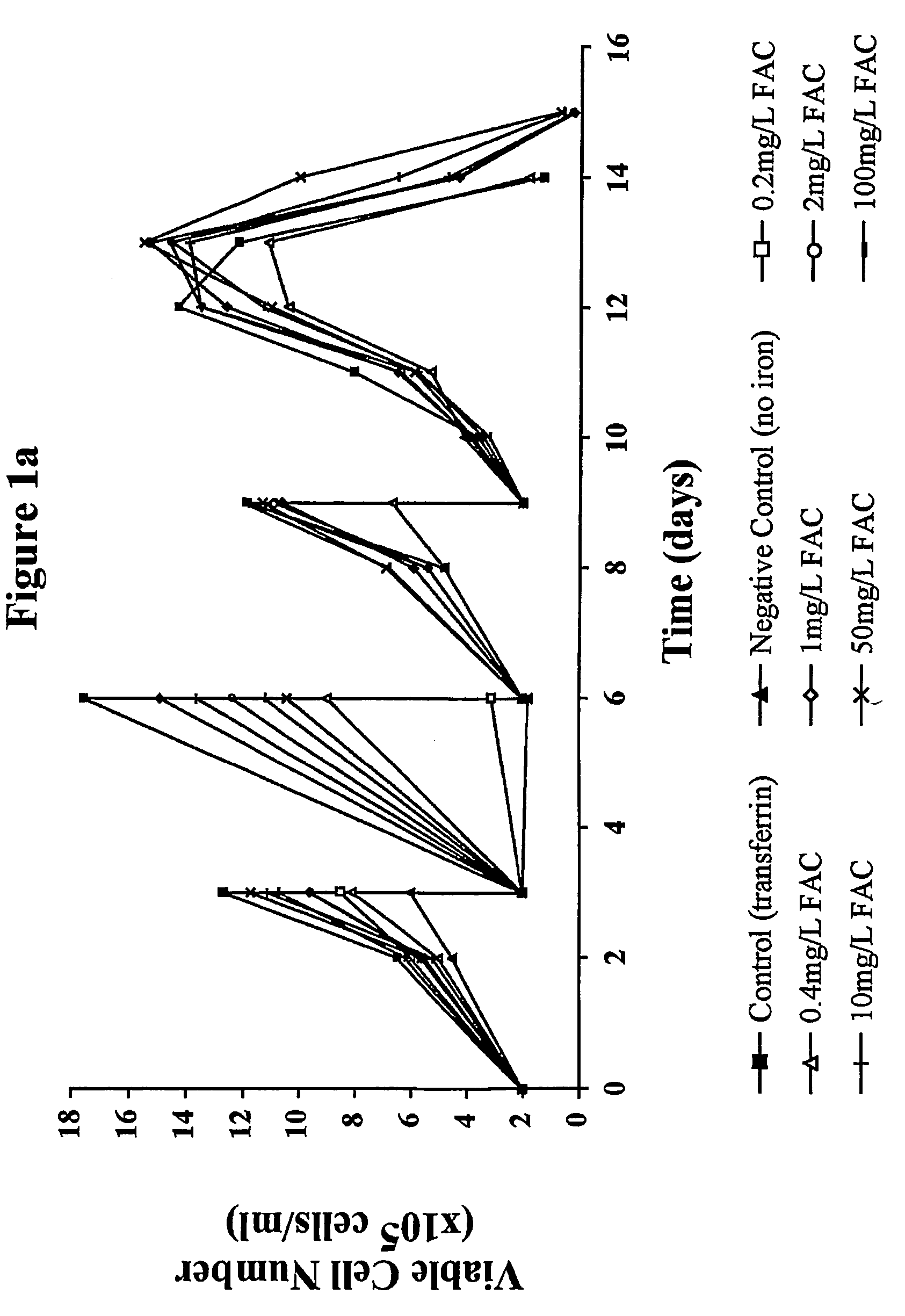
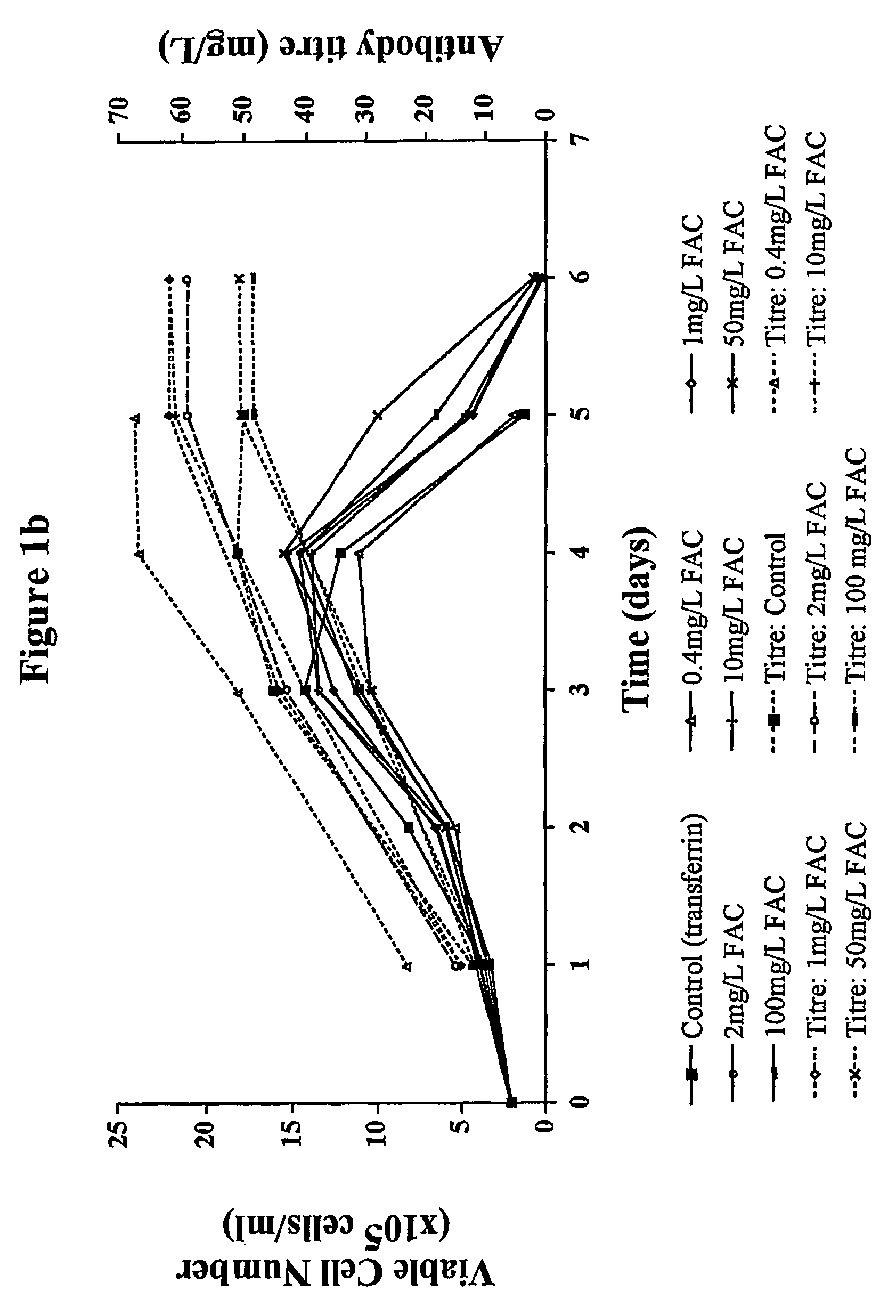
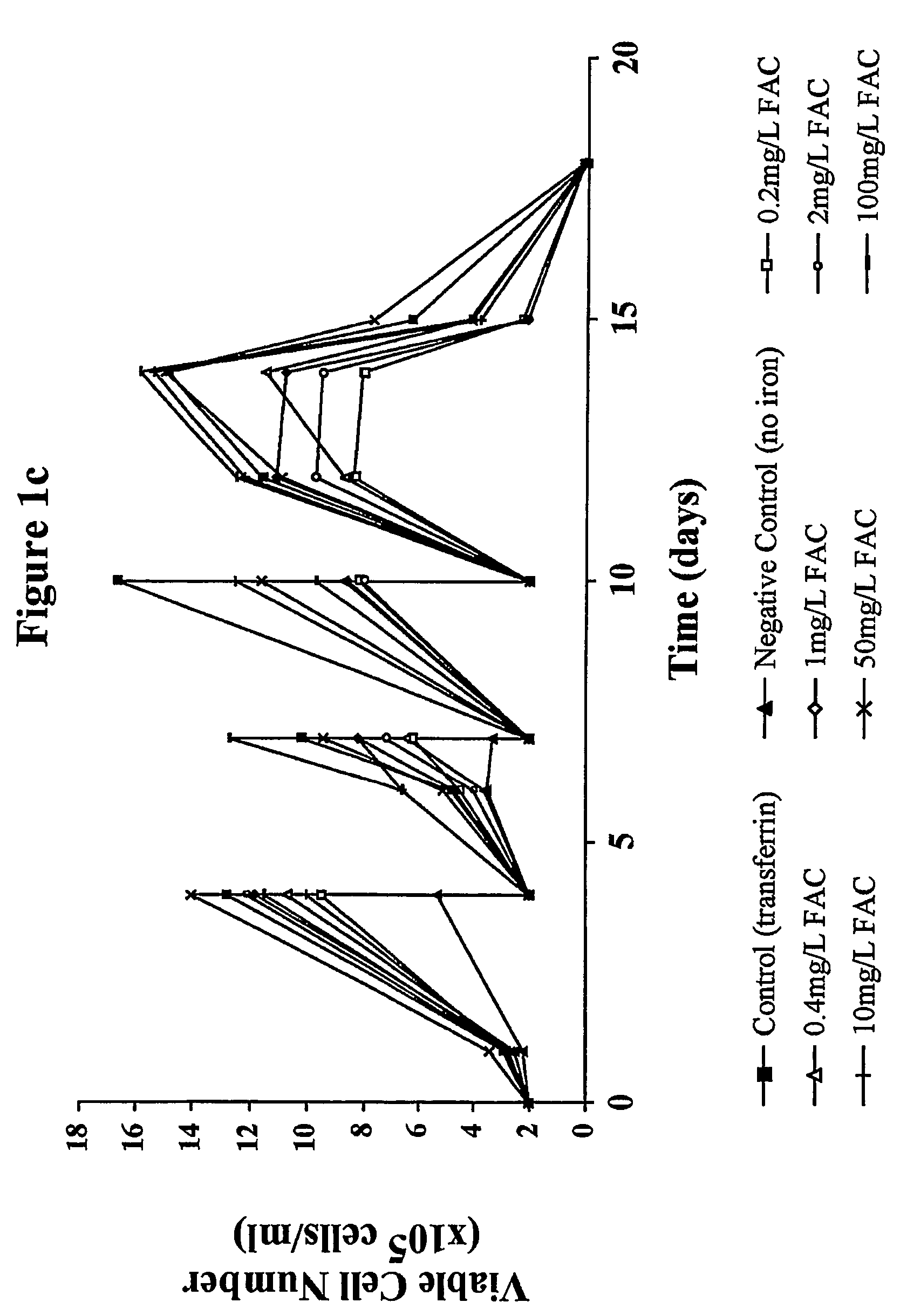
Myeloma cell culture in transferrin-free low iron medium
ActiveUS8361797B2High titerSupport growthGenetically modified cellsCulture processCulture cellMicrobiology
The present invention relates to a method for culturing mammalian cells in a culture medium which is transferrin free and which contains no lipophilic or synthetic nitrogen-containing chelators. Also provided is the use of the medium and a process for providing a mammalian product by culturing cells capable of producing the product in the medium.
Owner:MEDIMMUNE LTD
Monoclonal antibody against platelet membrane glycoprotein VI
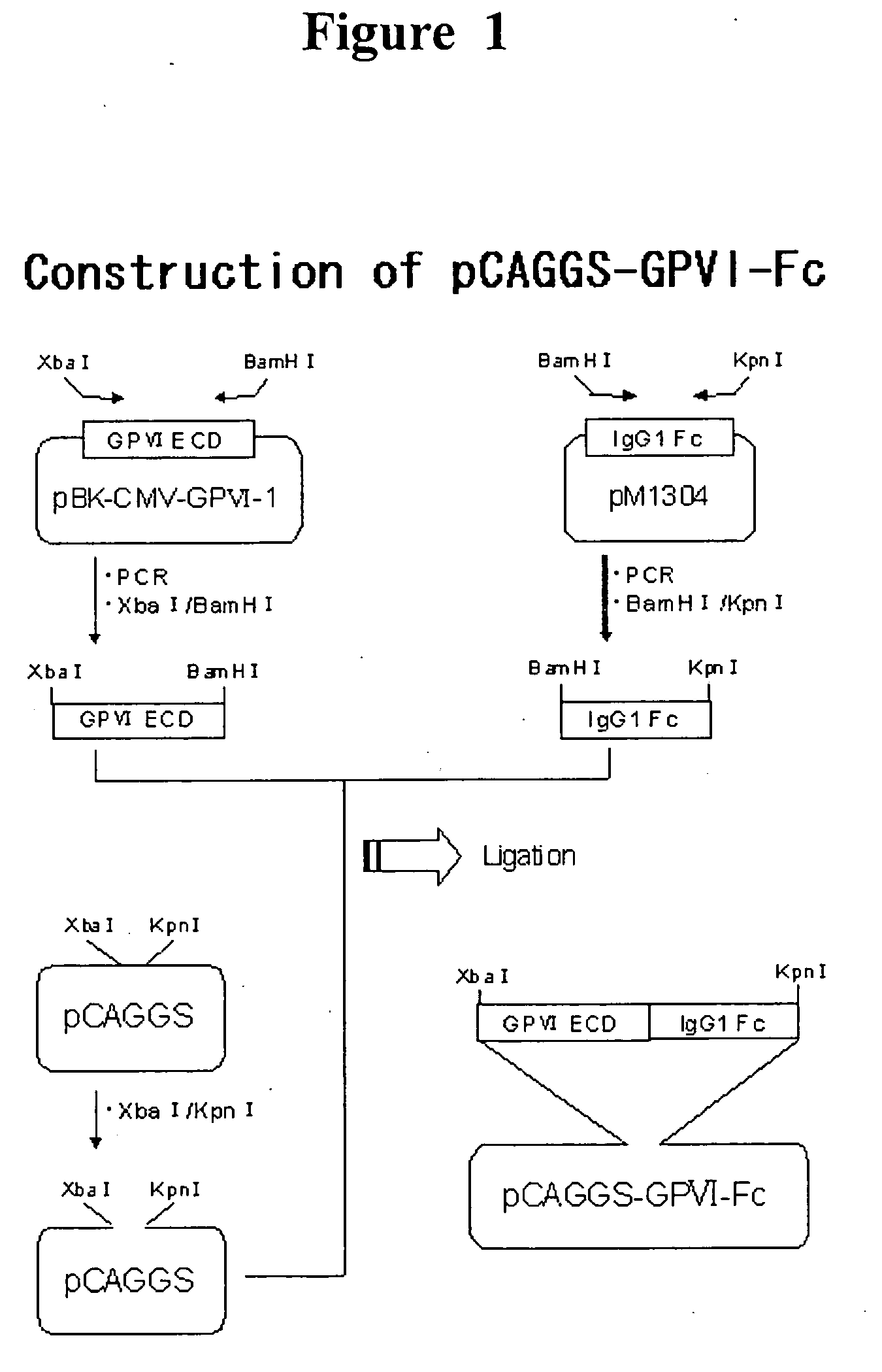
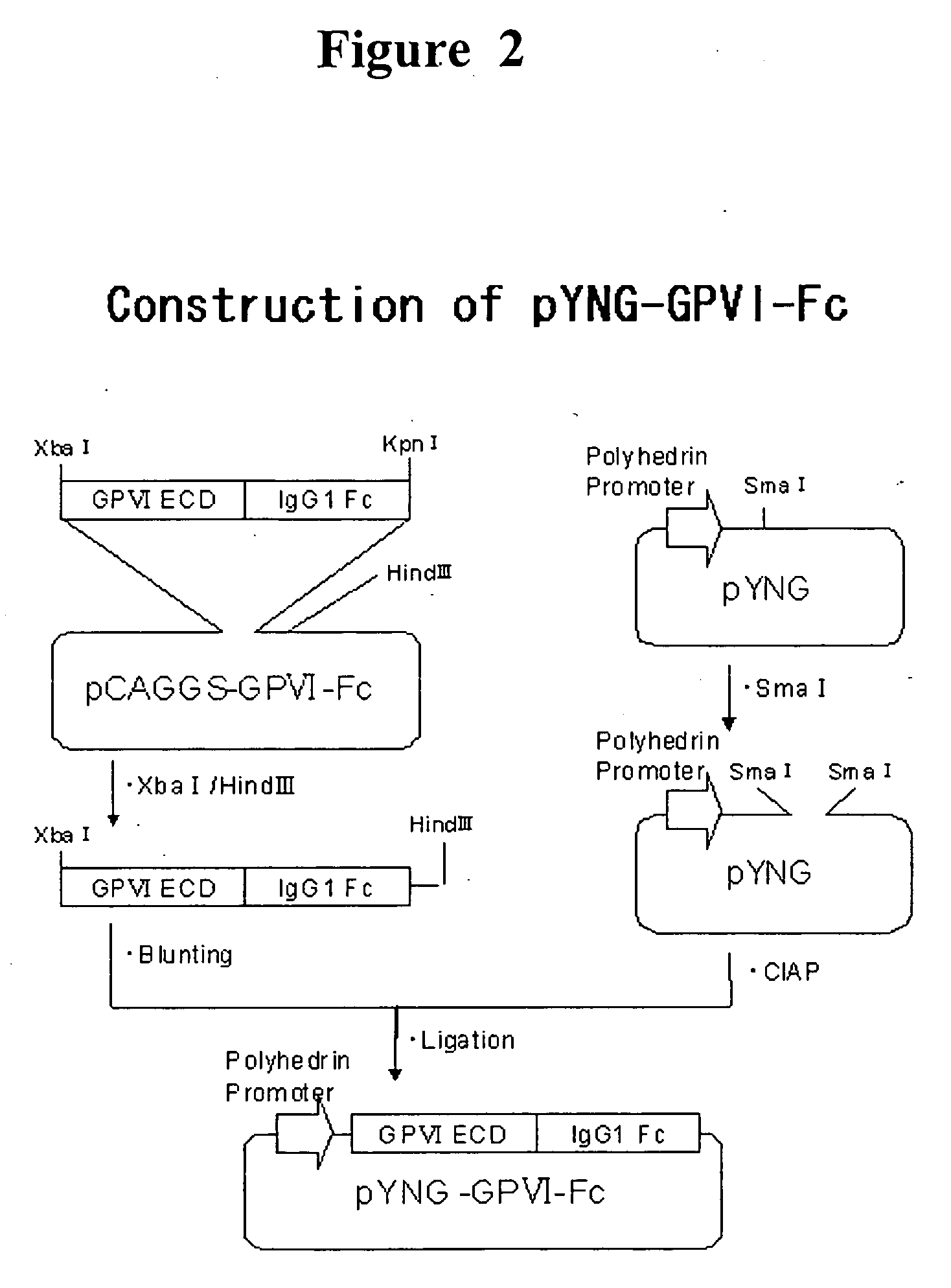
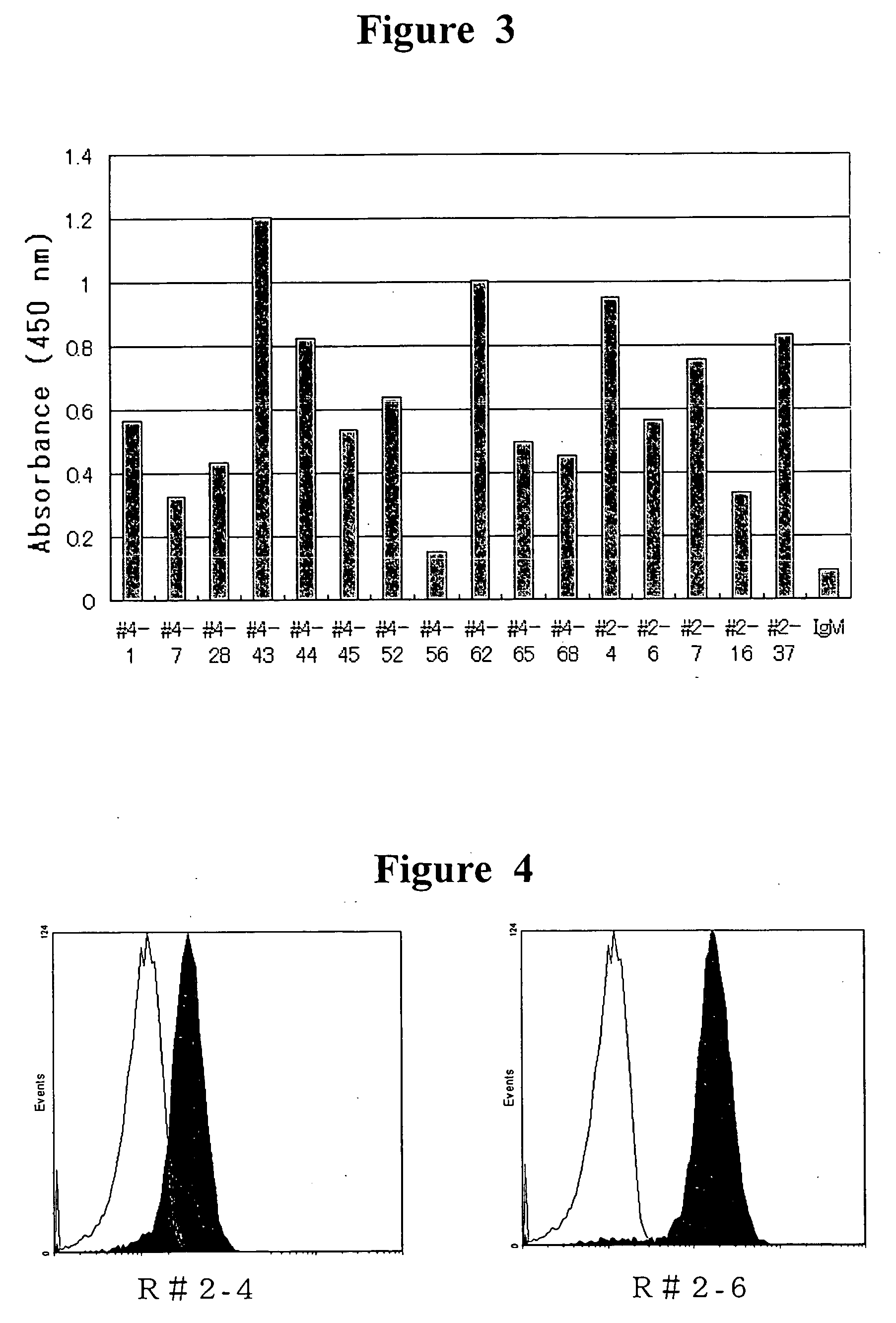
InactiveUS20070025992A1Easy to manufactureSenses disorderAntibody mimetics/scaffoldsHuman plateletBULK ACTIVE INGREDIENT
The present invention provides a human antibody or an active-fragment thereof that specifically binds to human platelet membrane glycoprotein VI and does not induce a human platelet aggregation independently; a cell that produces the antibody or its active-fragment; a pharmaceutical composition that comprises the antibody or its active-fragment as an active ingredient, and so on. The above-mentioned cell can be obtained for example, as follows: a peripheral-blood-lymphocyte of the human that produces an autologous antibody to GPVI is activated by in vitro immunization under specific conditions; a hybridoma with mouse myeloma cell is prepared; and then the hybridoma that produces a monoclonal antibody, which has a binding capacity to GPVI and has an activity that suppresses collagen-mediated agglutinability of the human platelet is selected.
Owner:MOCHIDA PHARM CO LTD
Global nav1.7 knockout mice and uses
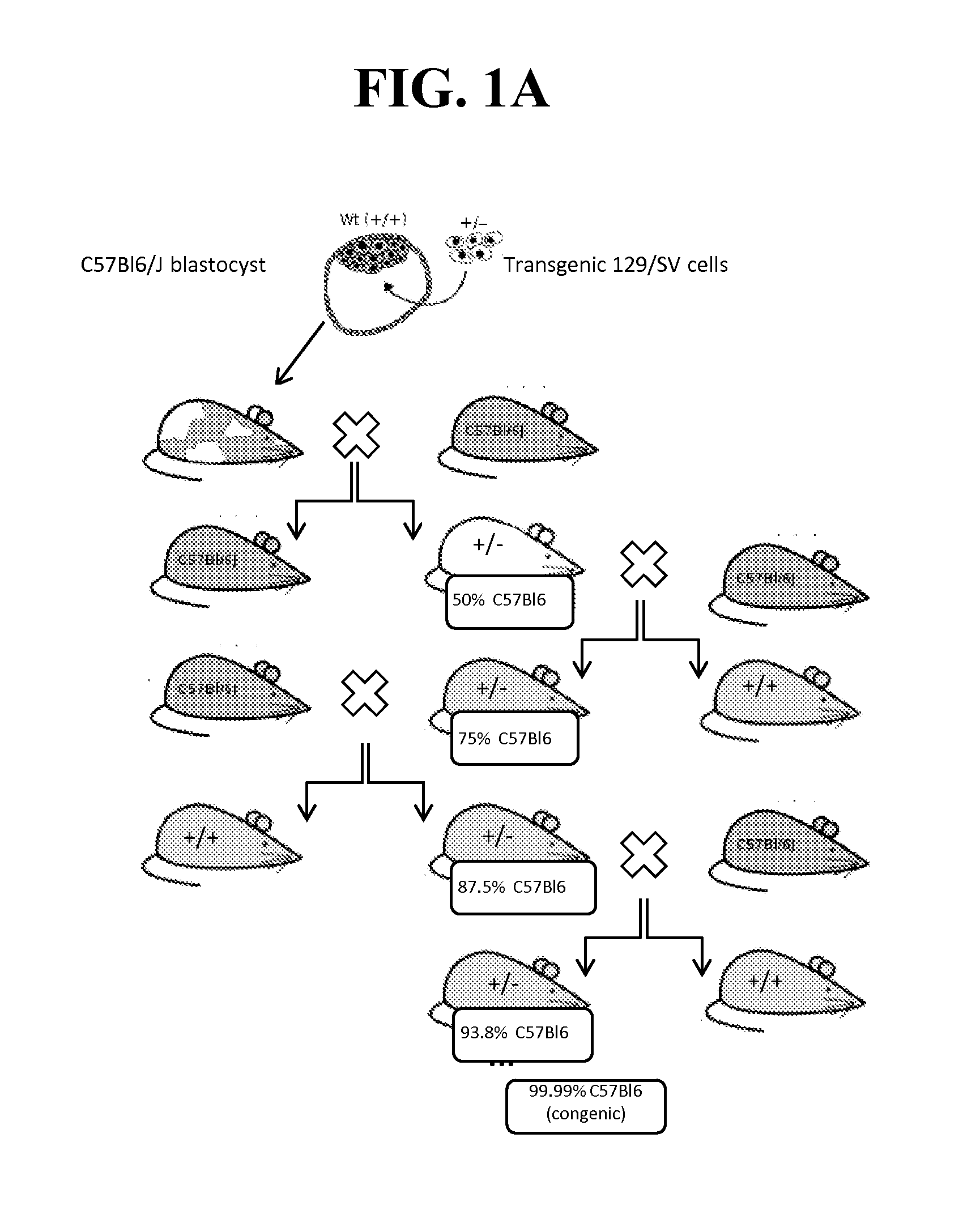
InactiveUS20120185956A1Robust behaviorPrevents painful responseCompounds screening/testingAnimal feeding stuffKnockout animalMyeloma cell
A viable global NaV1.7− / − knockout mouse is disclosed, and a breeding colony of global NaV1.7− / − knockout mice. Also disclosed are an isolated mouse gamete that does not encode a functional NaV1.7− / −, produced by the NaV1.7− / − knockout mouse; an isolated NaV1.7− / − mouse cell, or a progeny cell thereof, isolated from the NaV1.7− / − knockout mouse; and a primary cell culture or a secondary cell line and a tissue or organ explant or culture thereof derived from the NaV1.7− / − knockout mouse. Disclosed also are a hybridoma, wherein the hybridoma was originally formed from the fusion of the isolated NaV1.7− / − mouse cell mouse cell and a myeloma cell, and a method of making an antibody. Also disclosed are assays useful for screening prospective NaV1.7 inhibitors and dose ranging a test NaV17 inhibitor compound, which were validated using the NaV1.7− / − knockout mouse.
Owner:AMGEN INC
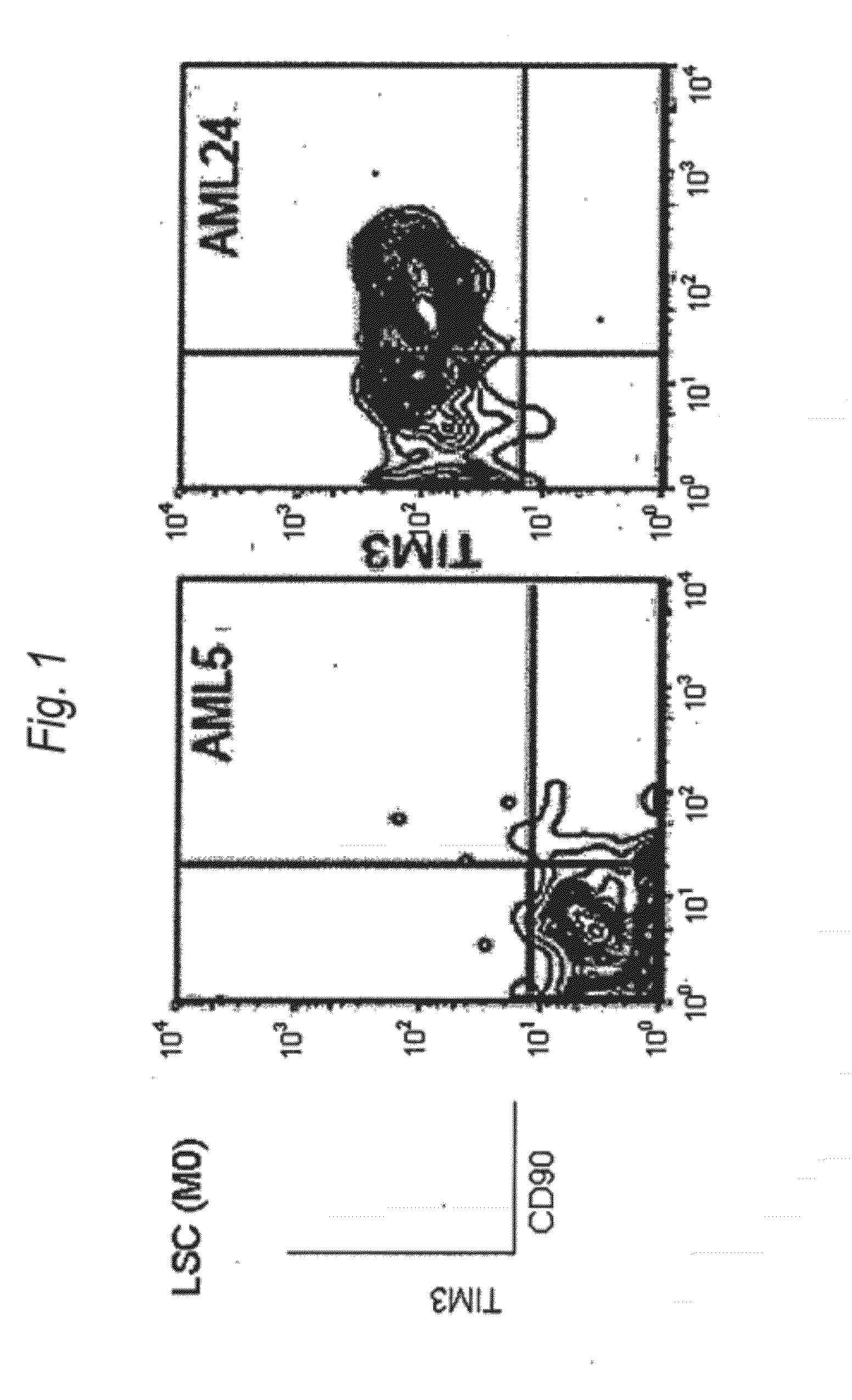
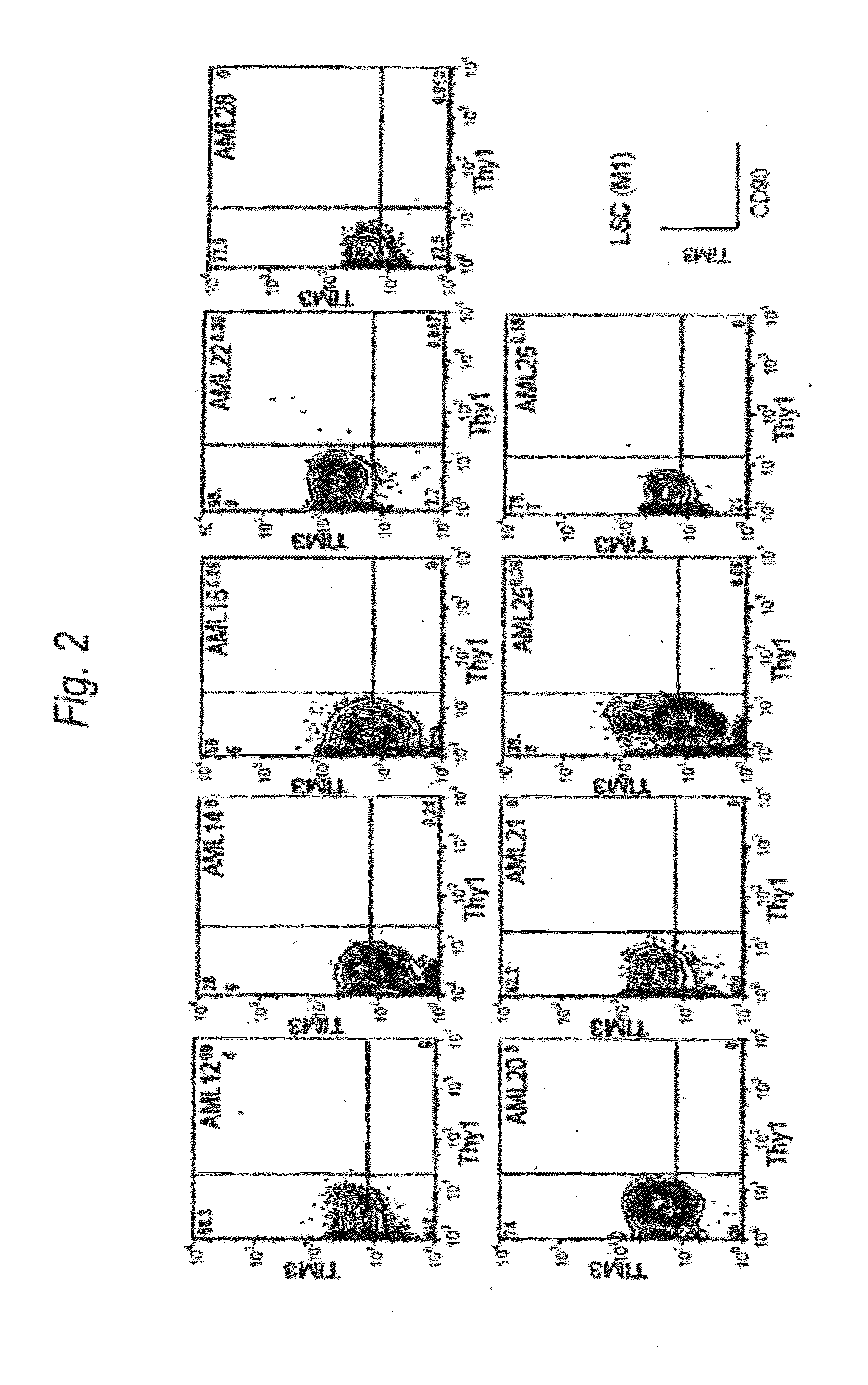
Method for treatment of blood tumor using Anti-tim-3 antibody
ActiveUS20120100131A1Use to establishBiological material analysisAntibody ingredientsDiseaseCell Fraction
Disclosed is a therapeutic method comprising administering a TIM-3 antibody to a subject who is suspected to be suffering from blood tumor and in whom TIM-3 has been expressed in a Lin(−)CD34(+)CD38(−) cell fraction of bone marrow or peripheral blood or a subject who has been received any treatment for blood tumor. Also disclosed is a composition for preventing or treating blood tumor, which comprises a TIM-3 antibody as an active ingredient. Conceived diseases include those diseases which can be treated through the binding or targeting of the TIM-3 antibody to blood tumor cells (AML cells, CML cells, MDS cells, ALL cells, CLL cells, multiple myeloma cells, etc.), helper T cell (e.g., Th1 cells, Th17 cells), and antigen-presenting cells (e.g., dendritic cells, monocytes, macrophages, and cells resembling to the aforementioned cells (hepatic stellate cells, osteoclasts, microglial cells, intraepidermal macrophages, dust cells (alveolar macrophages), etc)), all of which are capable of expressing TIM-3. The diseases for which the therapeutic use is to be examined include blood diseases in which the expression of TIM-3 is observed in bone marrow or peripheral blood, particularly blood tumor.
Owner:KYOWA HAKKO KIRIN CO LTD +1
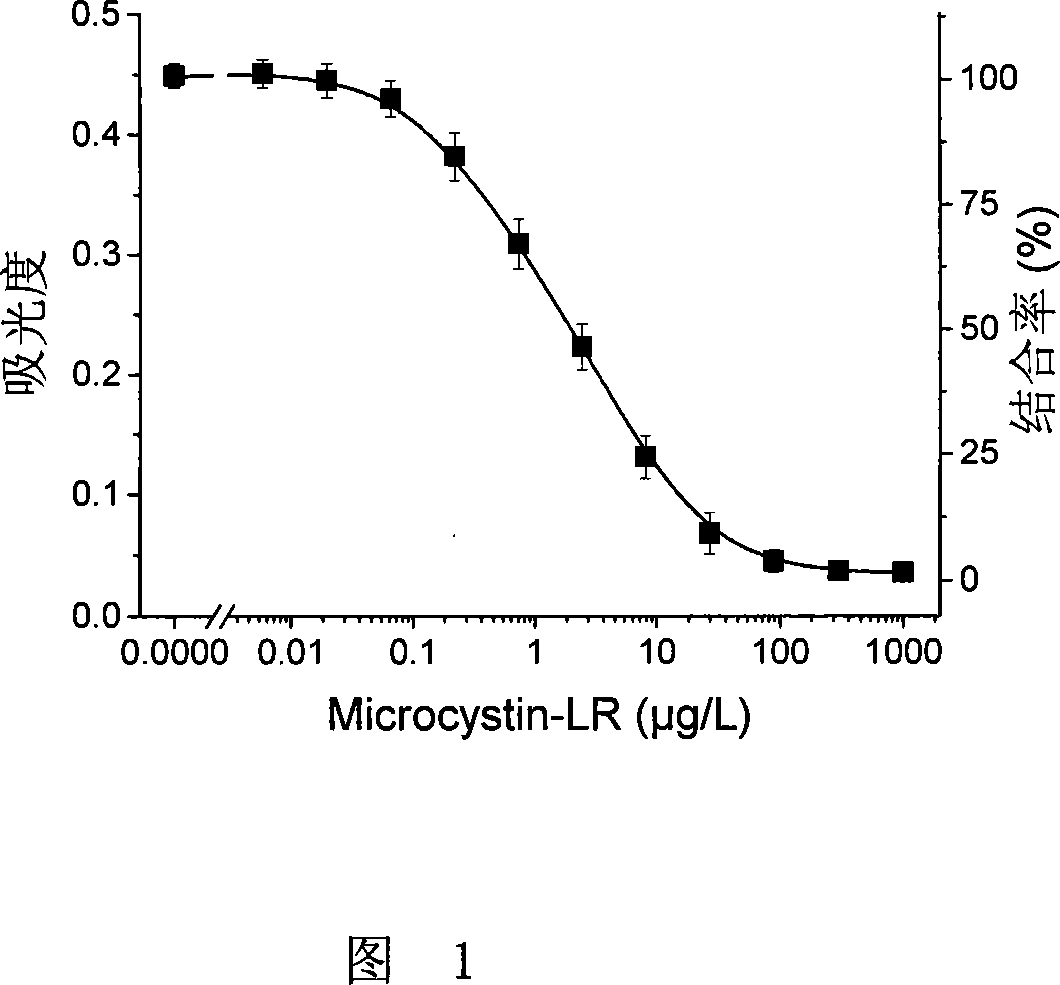
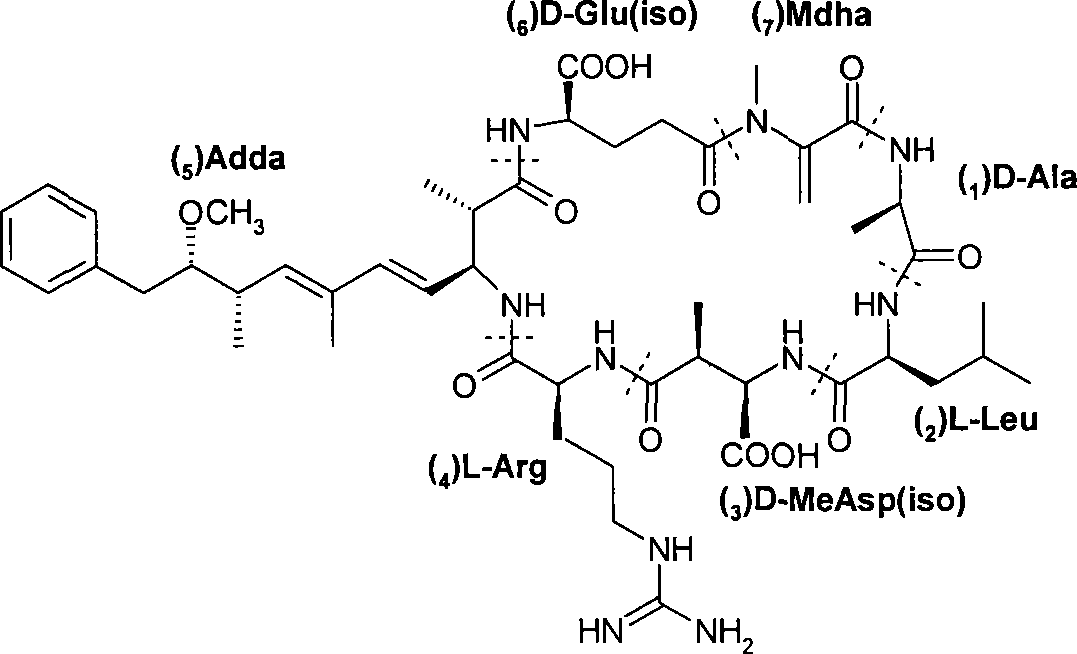
Microcystin monoclonal antibody and its preparation method and application
ActiveCN101125889AUniform textureImprove stabilityImmunoglobulins against animals/humansTissue cultureMicrocystinSpleen cell
The invention discloses a microcystlin monoclonal antibody and the preparation method and the application thereof. The microcystlin monoclonal antibody is a microcystlin-LR monoclonal antibody, and the preparation method thereof is that: 1) an amino group is introduced to a seventh amino acid residue N-methyl dehydroalanine on microcystlin-LR, thus obtaining amino modified microcystlin-LR polypeptide; the amino modified microcystlin-LR polypeptide is coupled with carrier protein to obtain complete A antigen; 2) the immunity mouse of complete A antigen obtained in step 1) to get spleen cell of immunity mouse, which then is fused with myeloma cell of mouse to screen out positive hybrid cancer cell strain; the positive hybrid cancer cell strain is cultured or is injected in sygeneous mouse abdominal cavity to induce ascites to get the microcystlin-LR monoclonal antibody. The monoclonal antibody can be used for examining microcystlin-LR and immunity affinity purification thereof.
Owner:TSINGHUA UNIV
Preparation method of anti-bisphenol A monoclonal antibody
InactiveCN101863981AEffective monitoringRapid Field MonitoringSerum albuminTissue culturePolyethylene glycolCarrier protein
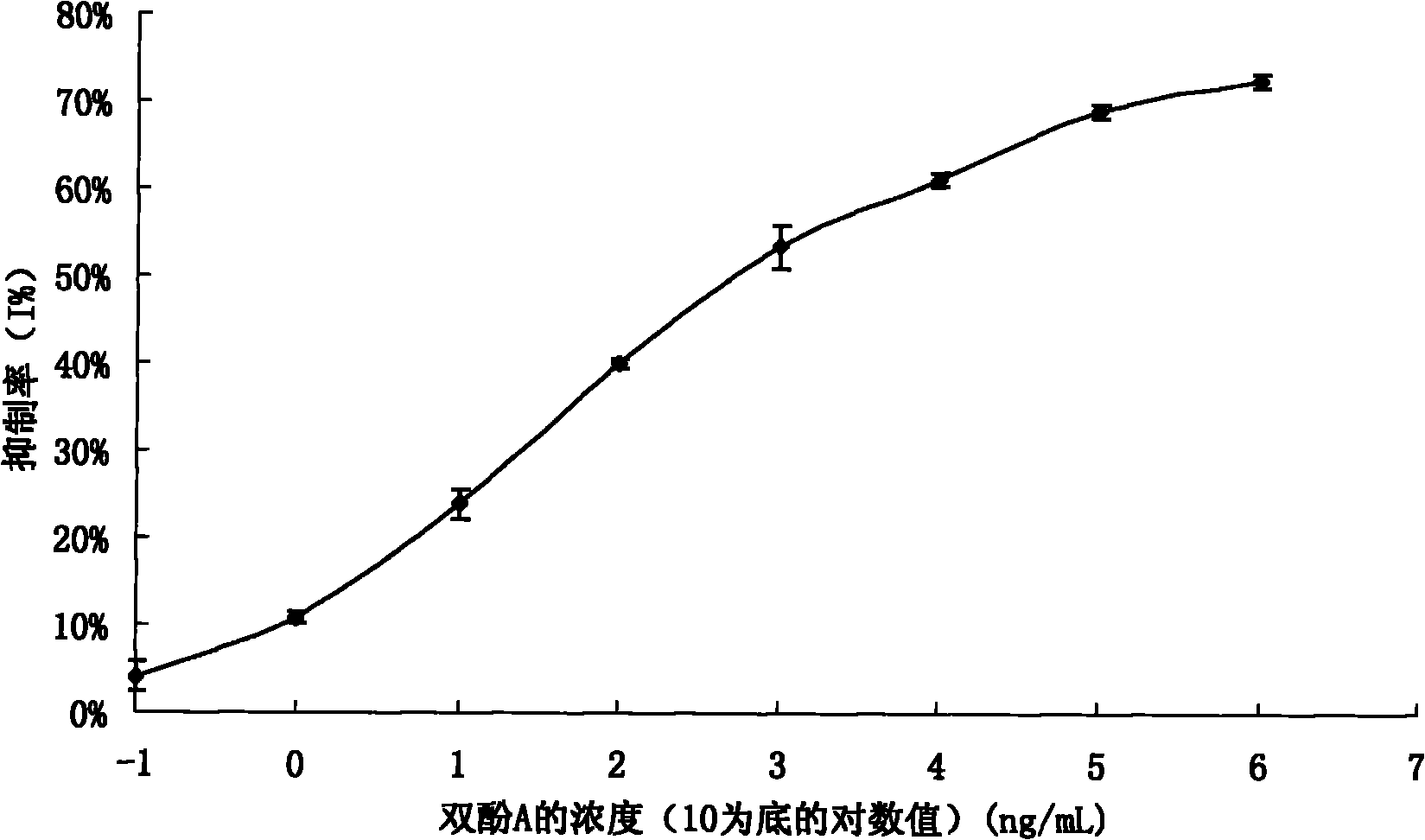
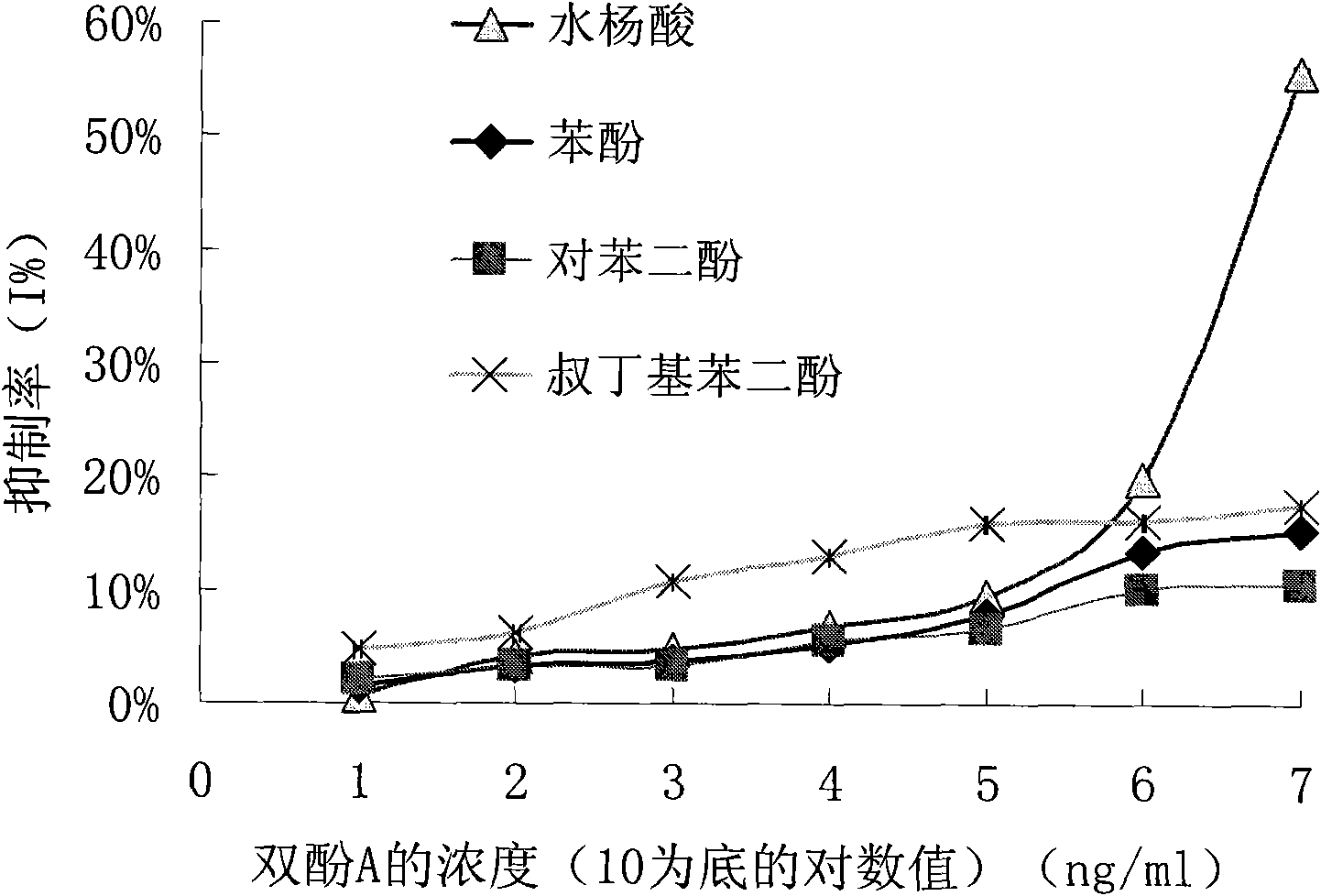
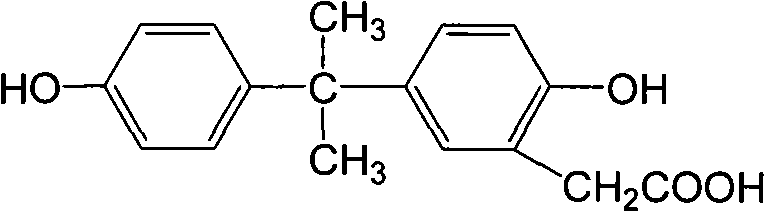
The invention discloses a preparation method of an anti-bisphenol A monoclonal antibody. The preparation method comprises the following steps of: combining bisphenol A and a macromolecule carrier protein to prepare an artificial immunity antigen and a coating antigen; preparing splenocyte suspension from a bisphenol A artificial immunity antigen immunity mouse; fusing the mouse splenocyte and mouse myeloma cells SP2 / 0 in the presence of polyethylene glycol; detecting by hybridoma technology and subcloning for many times to obtain positive hybridoma cells which can stably secrete bisphenol A antibody, wherein the secreted antibody belongs to the IgG1 subclass; and establishing a bisphenol A indirect competition ELISA (Enzyme-Linked Immunosorbent Assay) test by utilizing the monoclonal antibody secreted by cell lines with high antibodytiter, wherein the minimum limit of detection can reach 0.324ng / mL. The antibody has no cross reaction with compounds with benzene ring structures, such as benzene, phenol, tert-butylphenol, p-hydroxylphengl, o-hydroxybenzoic acid and the like, has strong specificity and can be used for immunity detection of bisphenol A.
Owner:GUANGDONG UNIV OF TECH
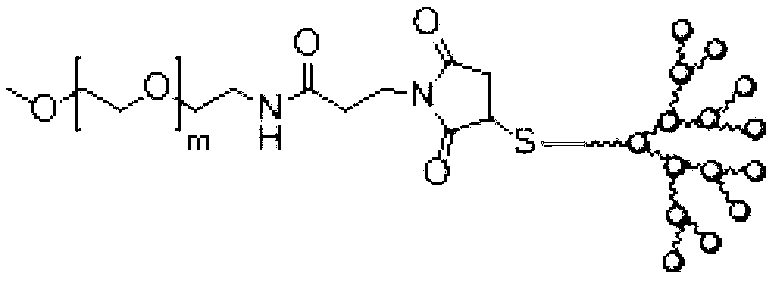
Tree-like polymer targeting nanometer drug delivery carrier and its preparation method
InactiveCN103127526AApplicable treatmentTargeted drug deliveryOrganic active ingredientsPharmaceutical non-active ingredientsTreatment effectBiological activation
The invention relates to a tree-like polymer targeting nanometer drug delivery carrier. The structural general formula of the nanometer drug delivery carrier is PEG-b-PAMAM-(Cys)n. According to the invention, the carrier is sensitive to pH, and a hydrogen bond between the carrier and a drug will break when the pH of the environment is low, so the drug is released, and targeting drug delivery is realized. The carrier has an effective treatment effect on myeloma. A large amount of lactic acid generated in the myeloma cell propagation process because of glycolysis causes a case that the pH of focus positions is lower than the normal pH of a body fluid, and the osteoclast formation and activation and the enzyme catalysis bone break further reduce the pH, so complication syndromes, such as osteolytic damages and the like, are caused. The carrier has the characteristics of specificity, slow release and high drug load, and can substantially improve the treatment effects on liquid tumors, such as myeloma and the like.
Owner:万礼 +2
GPC3 (glypican-3) monoclonal antibody hybridoma cell strain 7D11 and preparation method and application thereof
ActiveCN102634486AReduce manufacturing costConducive to clinical target validationImmunoglobulins against animals/humansMicroorganism based processesMonoclonalSpleen cell
The invention discloses a GPC3 (glypican-3) monoclonal antibody hybridoma cell strain 7D11 and a preparation method and application thereof, which belong to the technical field of bioengineering. The GPC3 monoclonal antibody hybridoma cell strain 7D11 has a preservation code of CGMCC No.5426. The preparation method of the hybridoma cell strain 7D11 includes: using GPC3 protein as an immunogen to immunize a mouse, subjecting spleen cells of the mouse with serum titer more than 1:104 and myeloma cells SP2 / 0 to fusion, using a HATRPMI-1640 medium to screen fused cells, screening by ELISA (enzyme-linked immuno sorbent assay) and multiple limiting dilution process, and finally obtaining the hybridoma cell strain 7D11. The GPC3 monoclonal antibody hybridoma cell strain 7D11 is high in yield of secreted antibodies and easy to survive, the secreted monoclonal antibodies are high in titer, flexible in reaction, easy to detect, low in production cost and widely applicable to detection of GPC3 protein expression.
Owner:GUANGZHOU DARUI BIOTECH
Melamine and carrier protein couplet product, preparation method and uses of melamine antibody
InactiveCN101429243AEfficient detectionEasy to operateSerum immunoglobulinsOvalbuminBALB/cCarrier protein
The invention discloses a preparation method and application of a product obtained by coupling melamine with carrier protein, as well as a melamine antibody. The product obtained by coupling the carrier protein with the melamine is used as artificial antigen and applied to an immunological method for melamine detection. The preparation method comprises the following steps of immunizing animals with the coupled product so as to prepare an antibody used for melamine detection, fusing BALB / C mouse spleen cells immunized with the coupled product and SP2 / 0 mouse myeloma cells, obtaining hybridoma capable of stably transferring culture and secreting anti-melamine specific monoclonal antibodies by screening positive hybridoma and cloning cells, and preparing an ascites monoclonal antibody. The prepared monoclonal antibody is utilized to establish a direct competitive ELISA method having high specificity, sensitivity and accuracy to the melamine, as well as an immune colloidal gold test strip. The preparation method for the product obtained by coupling melamine with carrier protein, as well as the melamine antibody provides service for the rapid detection of melamine-type residue in foods.
Owner:ZHEJIANG UNIV
miRNAs expression profile model related to human multiple myeloma, construction method and application thereof
PendingCN110564852AMicrobiological testing/measurementDNA/RNA fragmentationDifferentially expressed mirnasWilms' tumor
The invention discloses a miRNA expression profile model related to human multiple myeloma, a construction method and an application thereof. The invention discloses a miRNAs expression profile of tumor cells and circulating body fluid of a patient suffering from multiple myeloma, determines a molecular map of differentially expressed miRNAs between the patient suffering from multiple myeloma andhealthy people, and screens a biological marker for early diagnosis, prognosis evaluation and curative effect prediction of myeloma diseases, analyzes the myeloma cell characteristic miRNAs molecularexpression profile, correspondingly regulates mRNA in a targeted manner, and explores the correlation between differential miRNA and mRNA molecules and myeloma disease progression and the molecular mechanism of the correlation. The miRNAs expression profile disclosed by the invention provides a theoretical basis and an experimental basis for the treatment of multiple myeloma.
Owner:INST OF HEMATOLOGY & BLOOD DISEASES HOSPITAL CHINESE ACADEMY OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
Monoclonal antibody and gene encoding the same, hybridoma, pharmaceutical composition, and diagnostic reagent
InactiveUS7396915B2Eliminate side effectsBacteriaImmunoglobulins against cell receptors/antigens/surface-determinantsLiposomeAmino acid
A novel human monoclonal antibody specifically recognizing cancer cells such as non-small cell lung cancer, pancreatic cancer and gastric cancer cells is produced by hybridomas which are obtained by fusing lymphocytes derived from a cancer tissue of a cancer patient with mouse myeloma cells. An anti-cancer drug is obtained by using the antibody alone or anchoring the antibody on the surface of a liposome containing a toxin or an anti-cancer drug encapsulated therein. More specifically, an anti-cancer drug is obtained by using an antibody, in which variable region of its heavy chain comprises the amino acid sequence of SEQ ID NO: 115 and variable region of the light chain comprises the amino acid sequence of SEQ ID NO: 117, alone or anchoring the antibody on the surface of a liposome containing a toxin or an anti-cancer drug encapsulated therein.
Owner:MITSUBISHI TANABE PHARMA CORP +1
Preparation method of heavy metal mercury monoclonal antibody
InactiveCN101139398AFast pushStrong antigenicityImmunoglobulins against animals/humansBiological testingBALB/cSpleen cell
The present invention relates to a preparation method of heavy metal mercury monoclonal antibody, which belongs to the field of biotechnology. The present invention is particularly used in the preparation of specificity-recognition heavy metal mercury monoclonal antibody and in the fast mercury high-sensitivity measurement remained in the agricultural production and the agricultural production environment. The heavy metal mercury ion and the carrier albumen are coupled into full antigens through bifunctional metal chelate 1-(4-separate-cyano phenyl)-EDTA; the full antigens are used on Balb / C mouse and the spleen cells and the Sp2 / 0 myeloma cells are used to prepare hybridoma cells through the hybridoma technology. And monoclonal antibody which can stably excrete anti-Hg-EDTA is generated. The preparation technology in the present invention is easy and practical: the whole preparation process of the antigen requires no special instrument. The present invention is suitable for factory-scale production.
Owner:NANJING AGRICULTURAL UNIVERSITY
Monoclonal antibody of hepatitis B virus X protein and use thereof
The invention relates to a monoclonal antibody of hepatitis B virus X protein (HBx), which is characterized by having specificity reaction to N-end epitope or C-end epitope of HBx, but having no reaction to keyhole limpet hemocyanin (KLH) of carrier protein and other expressed proteins of hepatitis B virus (HBV). The preparation method of the monoclonal antibody comprises the steps of: fusing N-end and C-end antigen epitope polypeptides of HBx respectively with mice immunized with KLH in crosslinking way, and myeloma cells and screening to obtain the monoclonal antibody. The monoclonal antibody can be used for various immunodetection reagents and directed therapeutic drugs of HBx protein or HBx antibody and applied to diagnosis and treatment of diseases such as hepatitis b virus (HBV) infection, hepatocellular carcinoma (HCC) and the like.
Owner:王虹
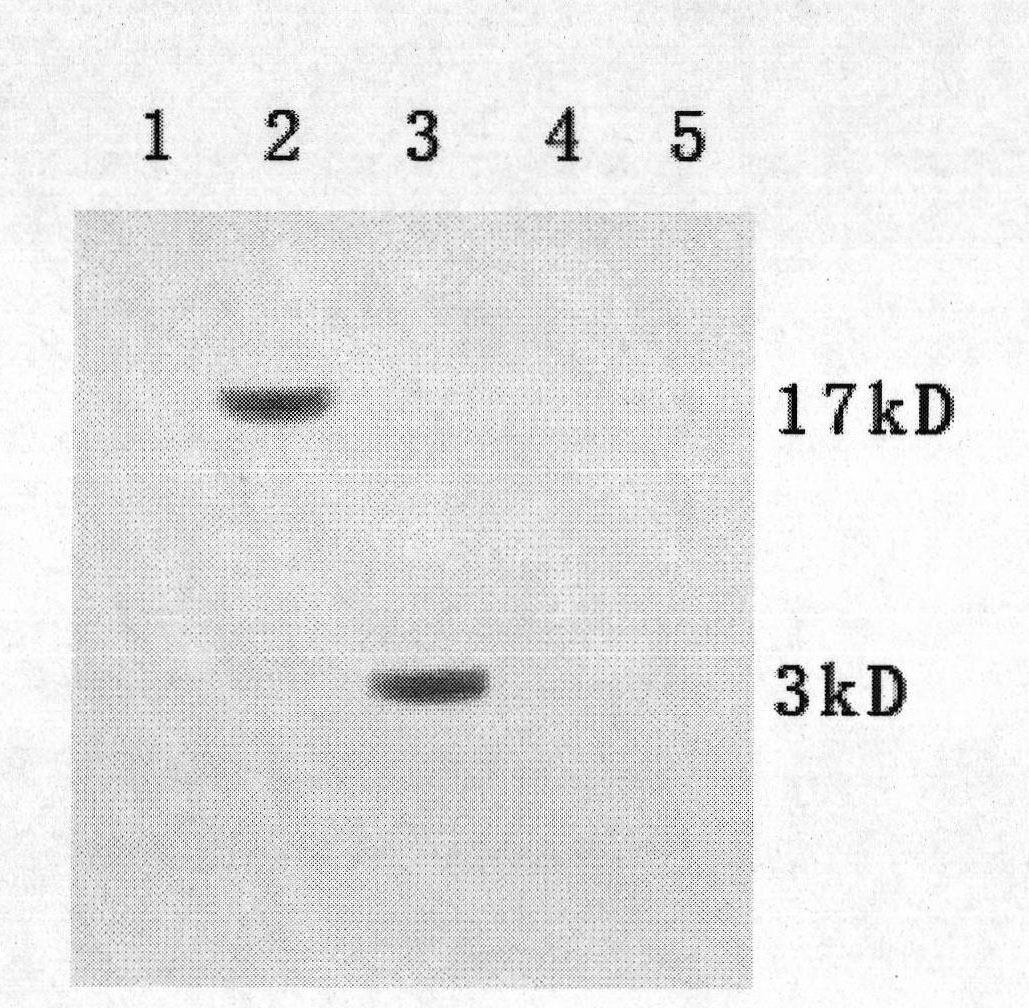
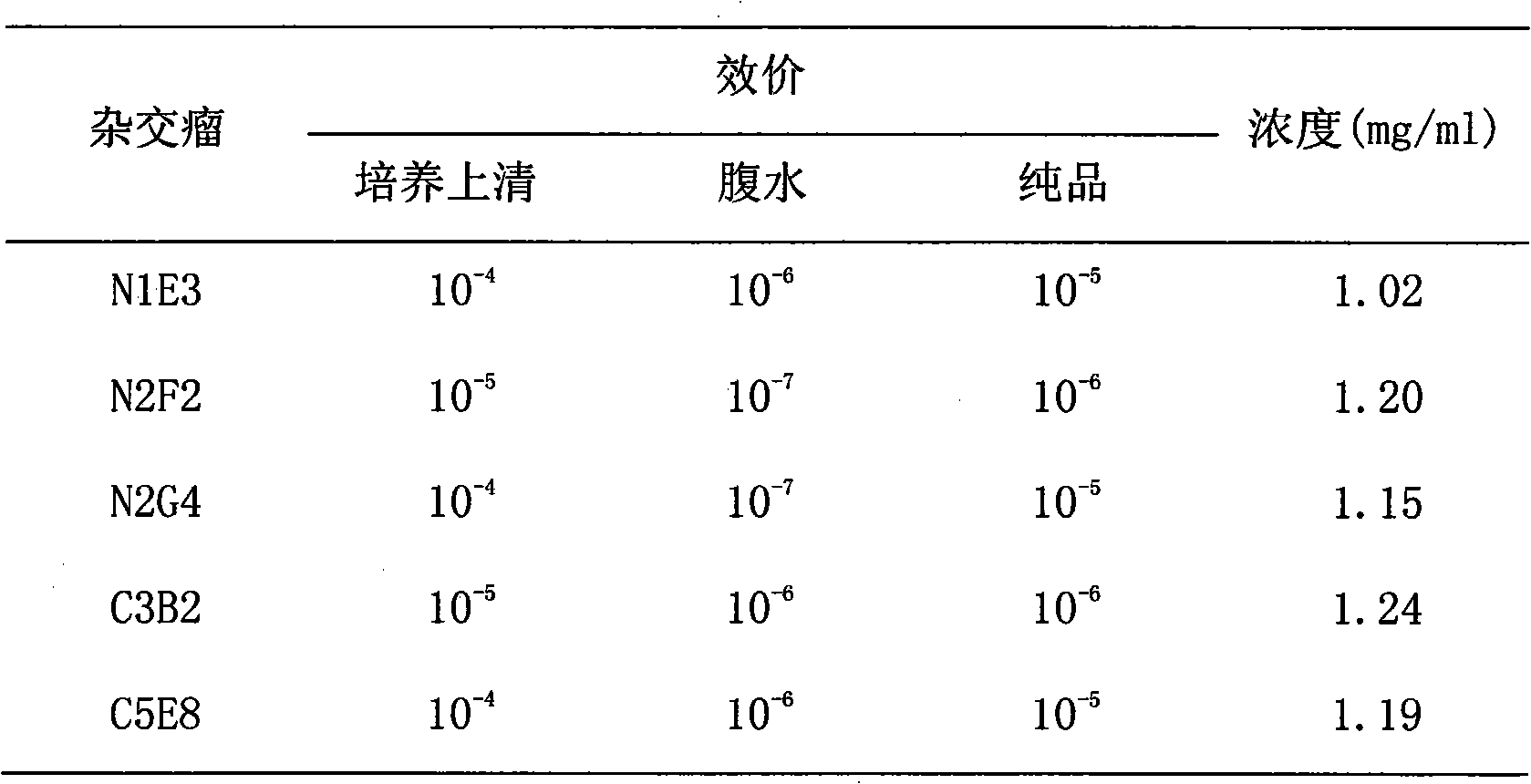
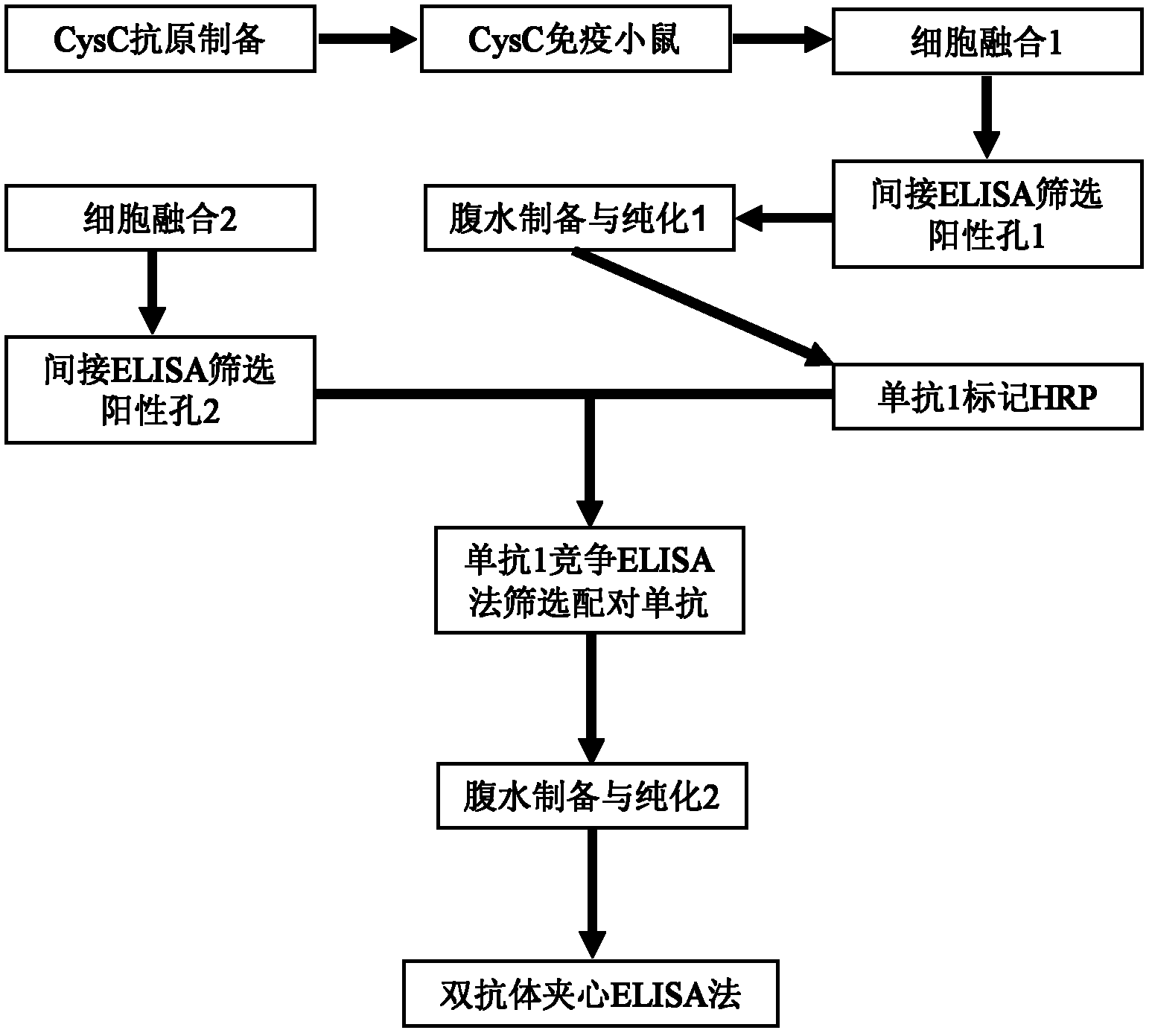
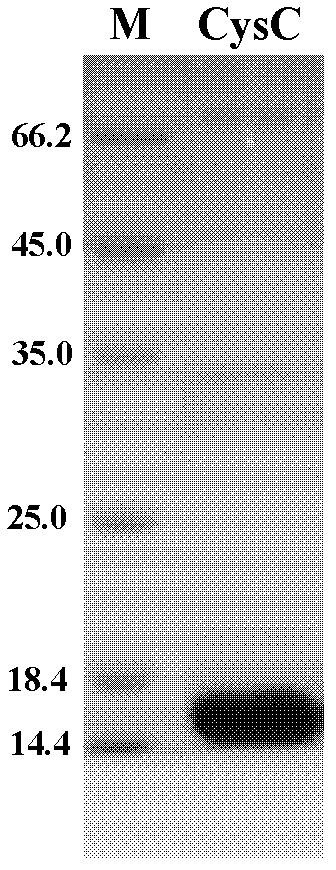
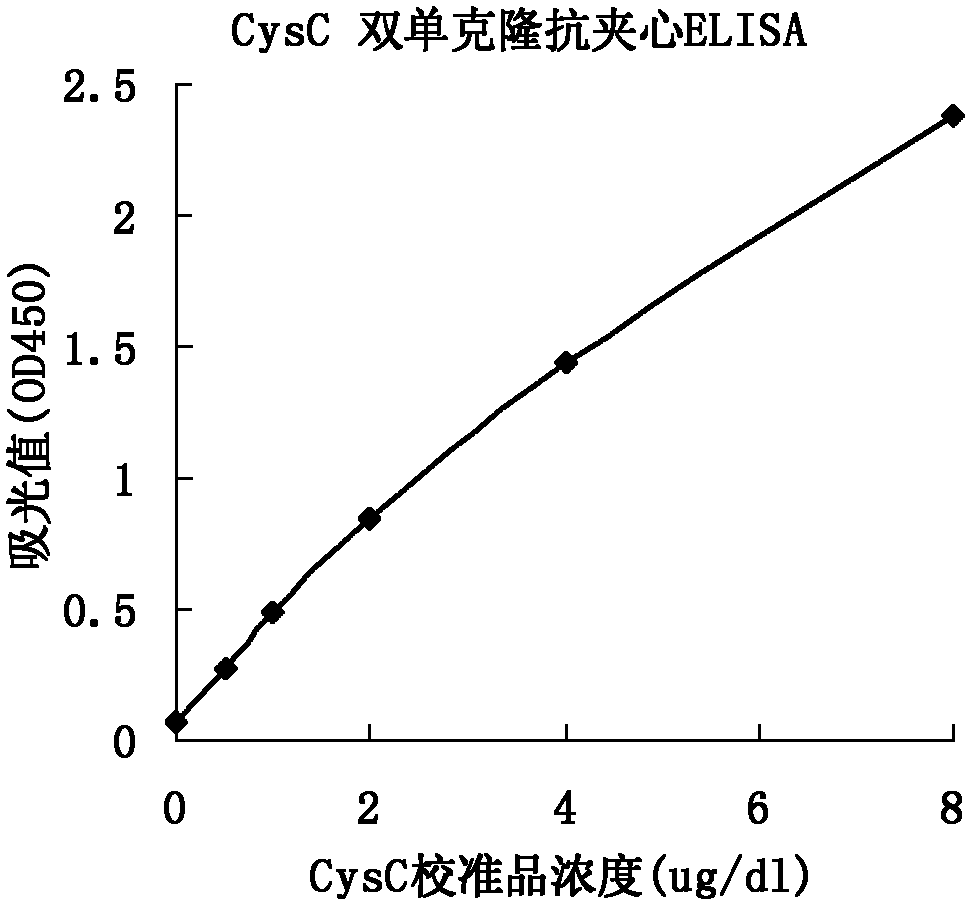
Method for preparing CysC-paired monoclonal antibody
ActiveCN102321176AReduce workloadExpand the scope of the filterImmunoglobulins against protease inhibitorsAntigenPeroxidase
The invention relates to the field of protein engineering, in particular to a method for preparing CysC-paired monoclonal antibodies, which comprises the following steps of: screening hybridoma cells having specific binding with CysC after fusing mouse splenic cells and myeloma cells capable of generating CysC antibodies, preparing cells for generating the monoclonal antibodies, finishing first-time cell fusion, further purifying out the monoclonal antibodies, and selecting one monoclonal antibody to be marked with horse radish peroxidase; then implementing the second-time fusion of the mouse splenic cells and the myeloma cells; finishing CysC-antigen coating on an elisa plate, adding a hybridoma-cell cultural supernatant obtained through the second-time cell fusion to elisa-plate holes, arranging a blank control hole, subsequently adding the monoclonal antibody marked with the horse radish peroxidase to the elisa-plate holes, incubating at the temperature of 37 DEG C for 30 minutes, adding a substrate to each hole for color rendering, and measuring a light absorption value under 450nm after stopping reaction through sulfuric acid; and selecting the uninhibited holes as the paired monoclonal antibodies.
Owner:BEIJING LEADMAN BIOCHEM
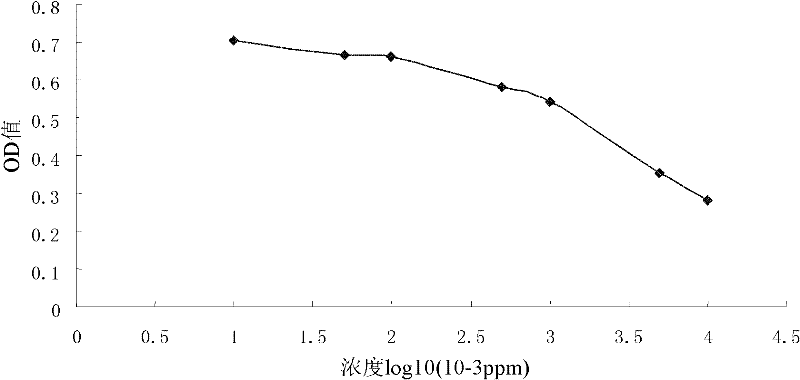
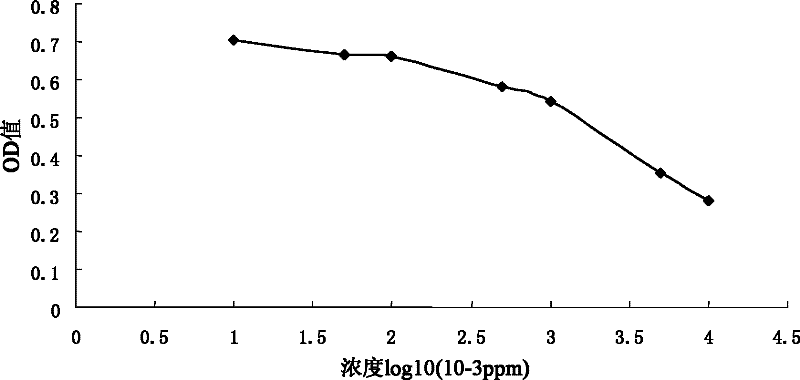
Carbendazim monoclonal antibody, preparation method and application thereof
InactiveCN102675463ASpecific bindingMeet dose dependenceMicroorganism based processesTissue cultureAntigenAscitic fluid
The invention discloses a carbendazim monoclonal antibody, a preparation method and an application thereof. The carbendazim monoclonal antibody is prepared by a mouse hybridoma cell strain MC-3 with a preservation number of CGMCC N0.4575 and the preparation method is as follows: (1) introducing ninth amino acid residue of carbendazim into one amino group to obtain the carbendazim modified by the amino and coupling the carbendazim modified by the amino with a carrier protein to obtain a complete antigen A; (2) immunizing a mouse by the complete antigen A obtained in step (1), taking splenocyteof the immune mice to fuse with a mouse myeloma cell to screen out a positive hybridoma cell strain; extensively culturing the positive hybridoma cell strain and injecting the positive cell strain into a homologous mouse abdominal cavity to induce to generate ascitic fluid and to obtain more carbendazim monoclonal antibodies. The Carbendazim monoclonal antibody can be used for detecting the carbendazim.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Process for preparing high-flux monoclonal antibody
The invention discloses a monoclonal antibody preparing method of high-flux, which comprises the following steps: (a) proceeding immune inoculation for non-human mammal animal through multiple immunogen; (b) compounding splenocyte of immune inoculation animal and bone marrow oncocyte to fuse into cross oncocyte; (c) collecting the cross oncocyte; culturing; preparing cross oncocyte suspension; (d) screening cross oncocyte through biological chip to produce cross oncocyte system of immunogen monoclonal antibody.
Owner:SHANGHAI BIOCHIP
Penicillin and carrier protein couplet product, method for producing beta-lactam penicillin antibody, and uses thereof
The invention provides a preparation method and application of a product obtained by coupling penicillin with carrier protein, as well as a beta-lactam type penicillin antibody. Animals are immunized with penicillin artificial antigen coupled in the invention so as to prepare the antibody which can be used for detecting beta-lactam type penicillin in foods. The preparation method comprises the following steps: immune BALB / C mouse spleen cells and SP2 / 0 mouse myeloma cells are fused; beta-lactam type antibiotics coupled with the carrier protein are used as coating antigen to screen positive hybridoma; hybridoma capable of stably transferring culture and secreting anti-beta-lactam type antibiotic antibodies through cell clones is obtained; and an ascites monoclonal antibody is prepared. The prepared monoclonal antibody is utilized to establish a direct competitive ELISA method having high specificity, sensitivity and accuracy to the beta-lactam type antibiotics, as well as an immune colloidal gold test strip. The preparation method for the product obtained by coupling penicillin with carrier protein, as well as the beta-lactam type antibiotic antibodies can serve the rapid detection of beta-lactam type antibiotic residue in foods.
Owner:ZHEJIANG UNIV
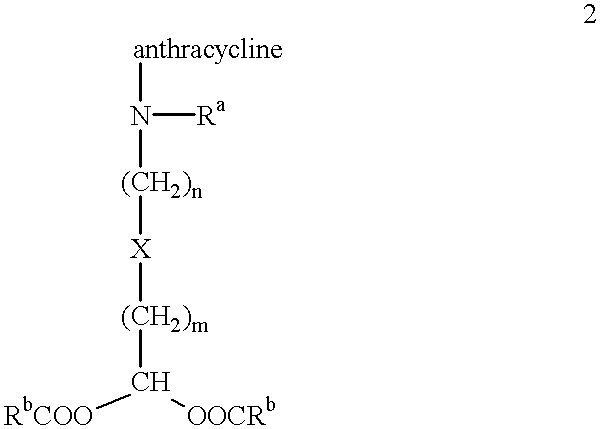
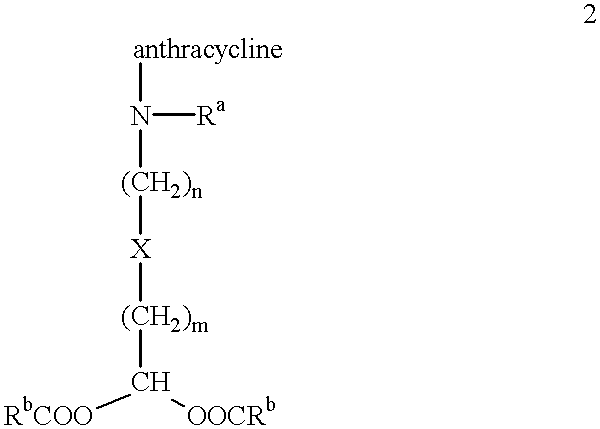
Methods of treatment with anthracycline derivatives
The present invention is a compound having the structurewhere:anthracycline is doxorubicin, daunorubicin or a derivative thereof;N is the 3' nitrogen of daunosamine;Ra is H or alkyl;X is, O, S, CRc2 or NRc where Rc is H or alkyl;Rb is alkyl or aryl;n is 1 to 6; andm is 0 to 6.Ra and Rc are preferably H, methyl, ethyl, propyl or butyl, although other alkyl substituents are usable. Rb is alkyl or aryl. The compound of the present invention as described above is activatable in vivo by esterases and spontaneous dehydration to form an aldehyde. The aldehyde may couple to nucleophiles of intracellular macromolecules. The compounds of the present invention are cytotoxically effective in the inhibition of human myeloma cells.
Owner:PRO NEURON INC
HCMVPP65 antigenemia indirect immunofluorescence method detection reagent kit
InactiveCN101261272AImprove featuresIncreased sensitivityFluorescence/phosphorescenceBALB/cFluorescence
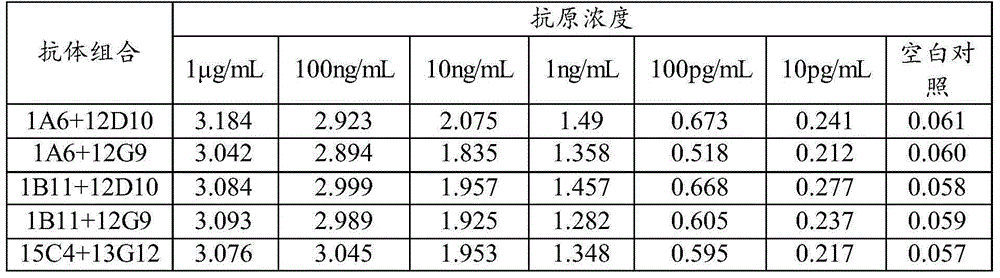
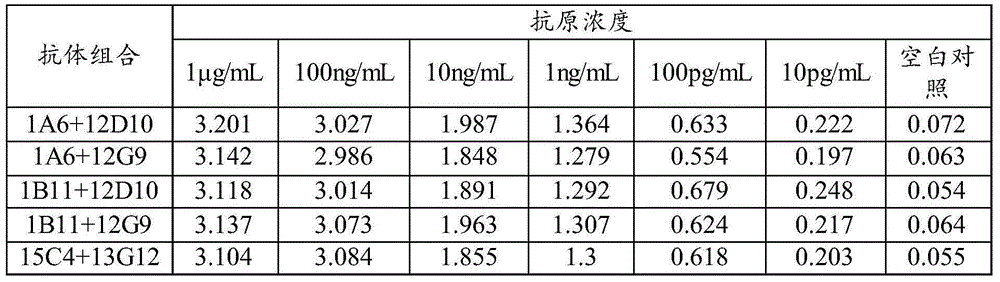
The invention discloses a HCMVPP65 antigenemia indirect immune fluorescence method detection kit, which uses cytomegalovirus-AD169 virus strain pp65 protein as the immunogen to immune a Balb / c mouse. The spleen cells of the immunized mouse and the myeloma cells of the mouse which belongs to the same type with the immune mouse are conventionally integrated, by indirect ELISA screening and finite dilution cloning, the hybridoma cell lines of the mouse cytomegalovirus pp65 protein cloning antibody are obtained, and the characteristics of the hybridoma cell lines are identified by ELISA, immune fluorescence experiment and other methods; two monoclonal antibodies that stably secrete the pp65 protein are established successfully and named respectively as 1A6 and 4A8. A monoclonal antibody which differs from the former report and aims at the pp65 protein of the cytomegalovirus (CMV) is prepared and a method used for preparing erythrocyte fast pyrolysis is established; compared with other detection kits which belongs to the same kind, the detection kit of the invention is faster, simpler and more convenient and has higher specificity and sensitivity.
Owner:天津市秀鹏生物技术开发有限公司
Chloramphenicol universal monoclonal antibody hybridoma cell strain and application thereof
ActiveCN104263701AHigh affinityHigh detection sensitivityMicroorganism based processesTissue cultureBALB/c1,3-Propanediol
Owner:JIANGNAN UNIV
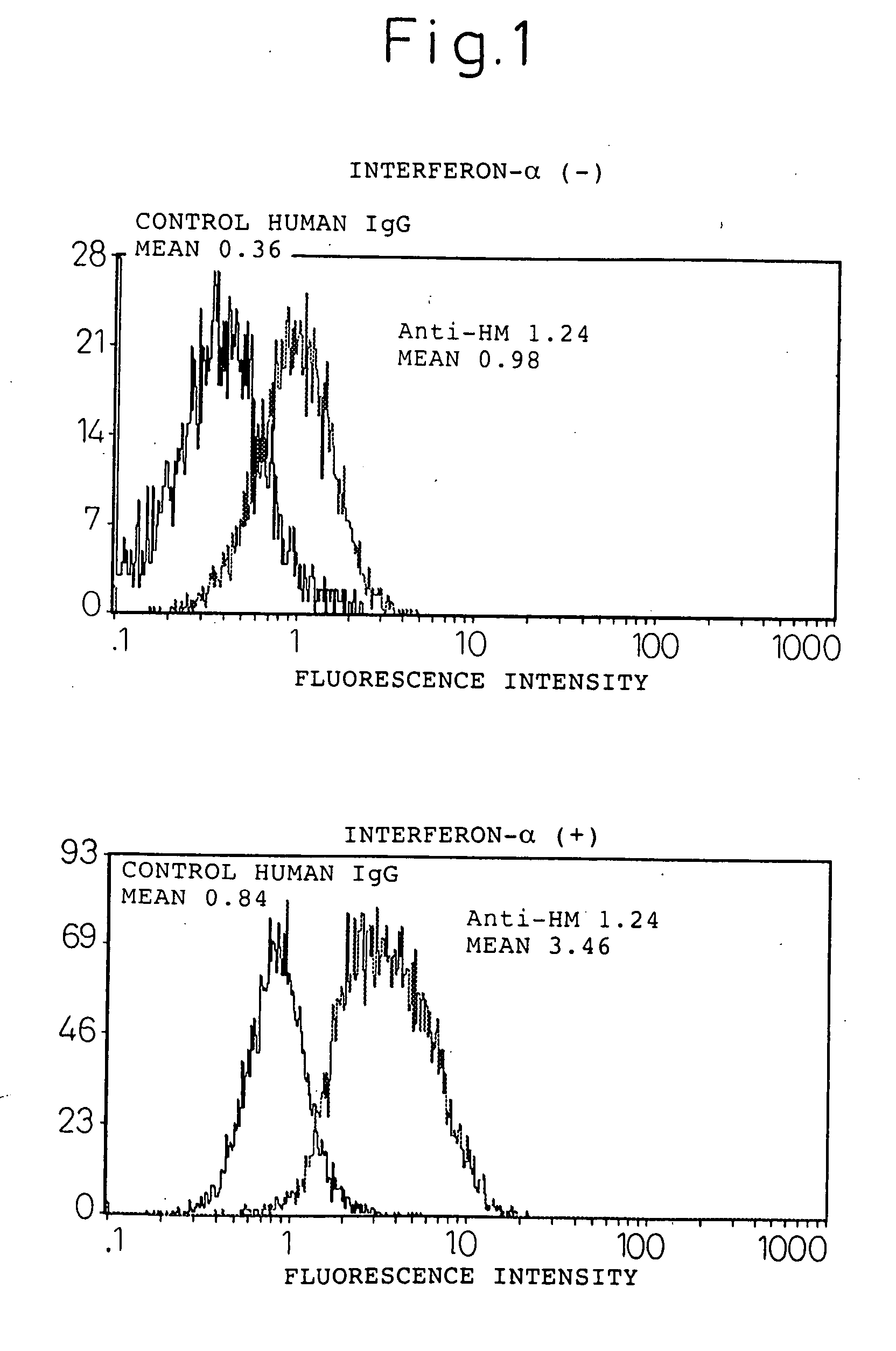
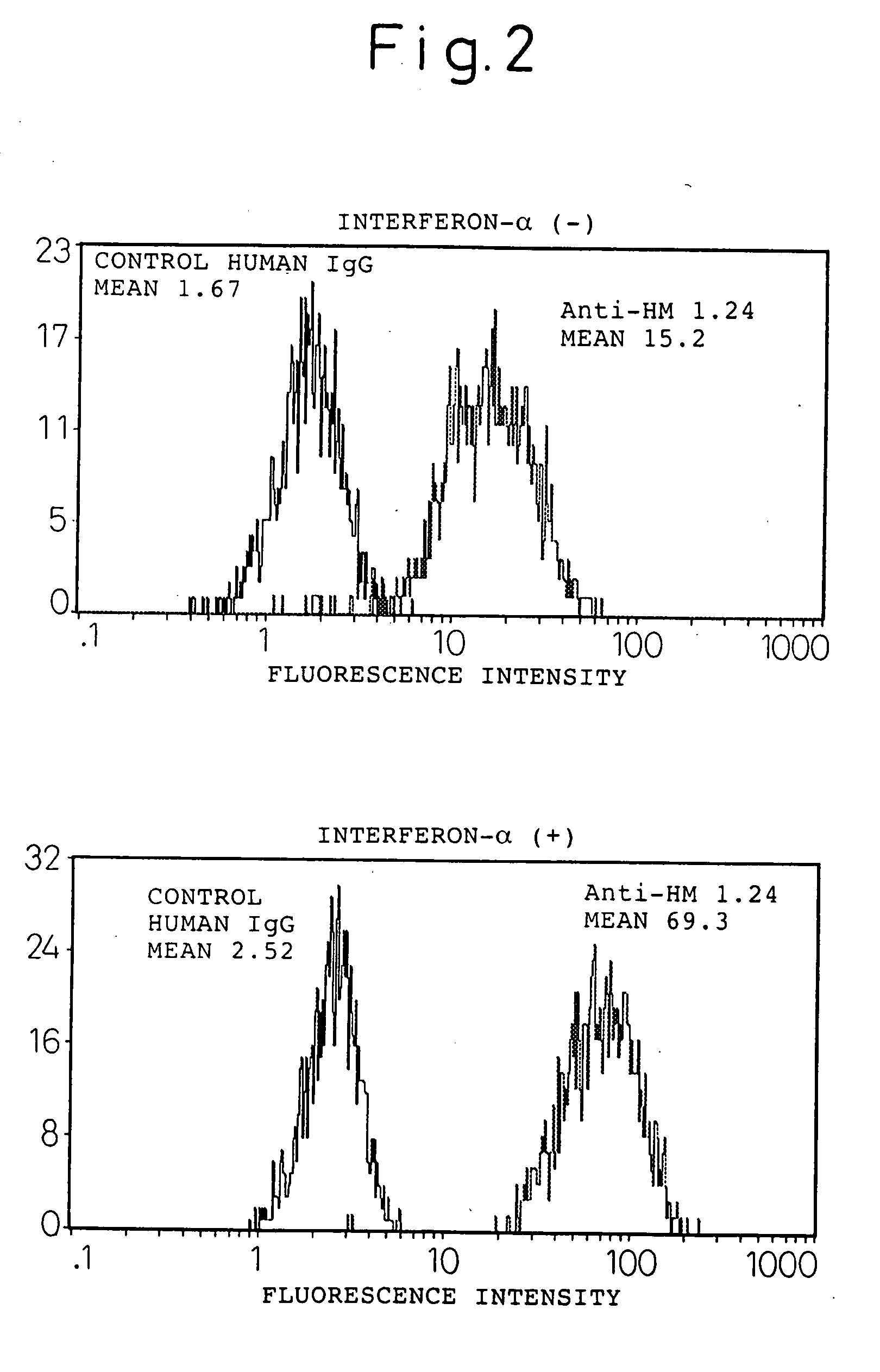
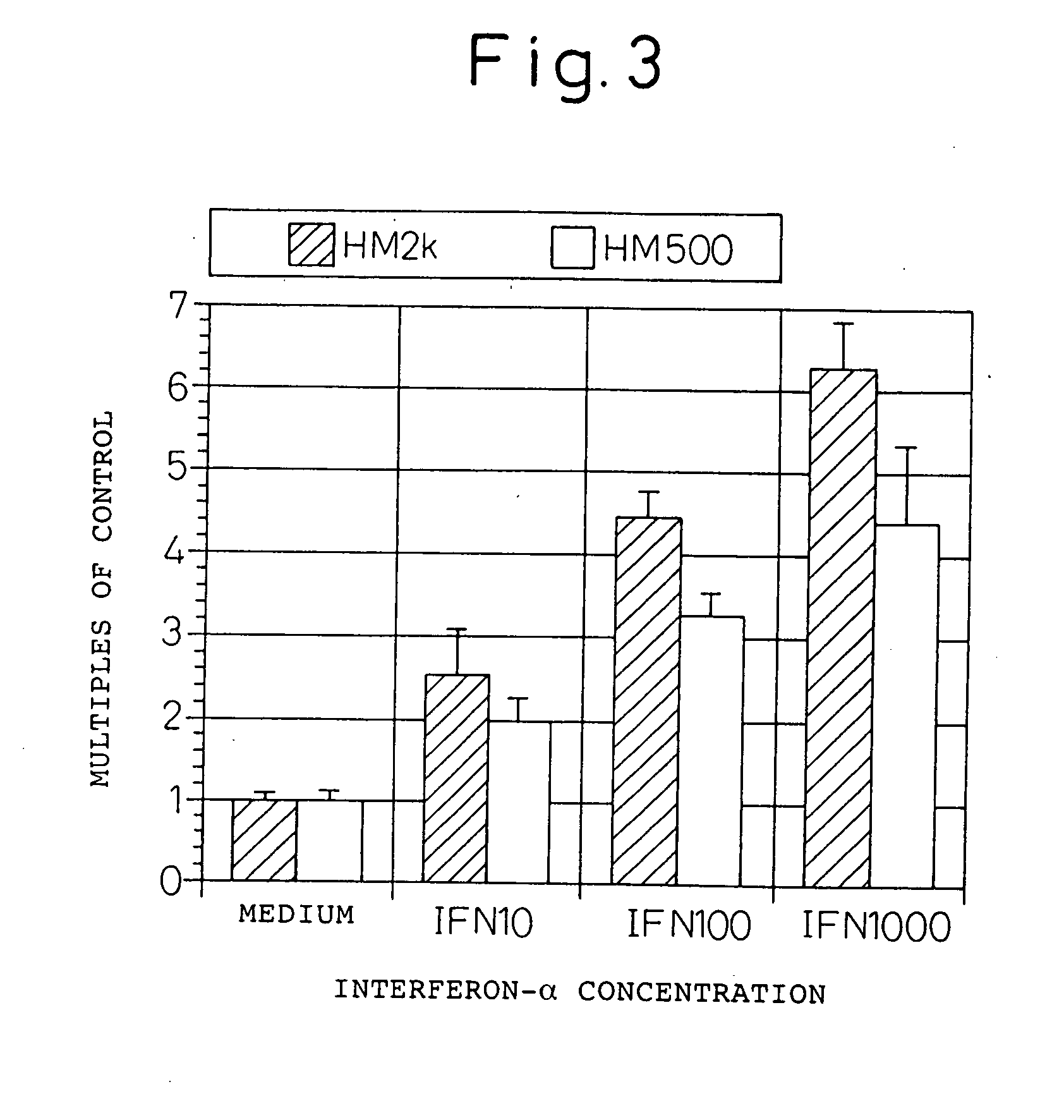
Expression enhancer for HM1.24 antigen
InactiveUS20060193828A1Improve survivalPeptide/protein ingredientsSkeletal disorderAntigenInterferon α
An enhancer of expression, in the myeloma cell, of HM1.24 antigen, said enhancer comprising interferon-α or interferon-γ, or IRF-2 protein as an active ingredient. Interferon-α or interferon-γ is expected to enhance the expression of HM1.24 antigen by activating the promoter of a gene encoding HM1.24 antigen.
Owner:CHUGAI PHARMA CO LTD
Anti-rabbit hemorrhagic disease virus VP60 albumen monoclonal antibody
InactiveCN101519447AThe preparation method is simple and feasibleStrong specificityImmunoglobulins against virusesTissue cultureBALB/cCell culture supernatant
The invention relates to an anti-rabbit hemorrhagic disease virus (RHDV)VP60 albumen monoclonal antibody, and belongs to the technical field of biology. An SP2 / 0 myeloma cell and a BALB / c mouse splenic cell immunized by utilizing RHDV to recombine VP60 albumen undergo cell fusion, are selectively cultured by an HAT culture medium and undergo double ELISA screening by utilizing the recombined VP60 albumen and RHDV; the obtained cell culture supernatant is checked up and screened respectively to obtain a hybrid tumor cell strain A3C which can stably excrete the anti-RHDV VP60 albumen monoclonal antibody; the ascitic fluid ELISA titer of the A3C is detected to be 1:327,600; and according to identification, the monoclonal antibody can specifically combine the expressed recombined VP60 albumen as well as the RHDV, and one single reaction strip appears in both specific combinations, thereby proving that the anti-RHDV VP60 albumen monoclonal antibody is a VP60 specific antibody of the RHDV capsid albumen.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Hybridoma cell strain secreting thiamethoxam monoclonal antibody and application thereof
InactiveCN108998422AHigh detection sensitivityImprove featuresMicroorganism based processesDepsipeptidesBALB/cIc50 values
The invention relates to a hybridoma cell strain secreting thiamethoxacin monoclonal antibody and application thereof, belonging to the field of food safety immunodetection. The accession number of the hybridoma cell strain is CGMCC No. 14699. According to the invention, a complete Freund's adjuvant is used for primary immunization of a BALB / c mouse, then an incomplete Freund's adjuvant is used for booster immunization three times, and a thiamethoxam complete antigen containing no adjuvant is used for impact immunization once, so the BALB / c mouse is immunized; and then the high-titer low-IC50spleen cells of the immunized mouse are fused with mouse myeloma cells by using a PEG method, and then the cell strain is obtained through indirect competitive ELISA screening and subcloning three times. The monoclonal antibody secreted by the cell strain has good specificity and detection sensitivity (with an IC50 value of 0.81 ng / mL) to thiamethoxam and can be used for detection of thiamethoxamresidues in food.
Owner:JIANGNAN UNIV +1
Preparation method of monoclonal antibody and kit thereof
InactiveCN105198996AStrong specificitySame natureImmunoglobulins against hormonesFermentationSpleen cellElisa method
The invention relates to the technical field of preparation of monoclonal antibodies, and in particular relates to a preparation method of a monoclonal antibody and a kit thereof. The preparation method comprises the following steps: performing immunization on an animal by adopting an antigen, and taking immune spleen cells; fusing the immune spleen cells with myeloma cells to obtain fusion cells; detecting the fusion cells by adopting an indirect ELISA (enzyme-linked immunosorbent assay) method to obtain positive hybridoma cells; detecting the positive hybridoma cells by adopting a competitive inhibition ELISA method to obtain hybridoma cells with competitive reactions; sub-cloning the hybridoma cells with competitive reactions to obtain monoclonal antibody hybridoma cells; and excreting the monoclonal antibody hybridoma cells to obtain the monoclonal antibody. According to the preparation method provided by the invention, the monoclonal antibody is screened by adopting a method combining the indirect ELISA method and the competitive inhibition ELISA method, the operation is simple and convenient, the purposiveness is strong, the workload is small, and a high-quality hybridoma cell strain can be locked in a short time.
Owner:SINOCARE
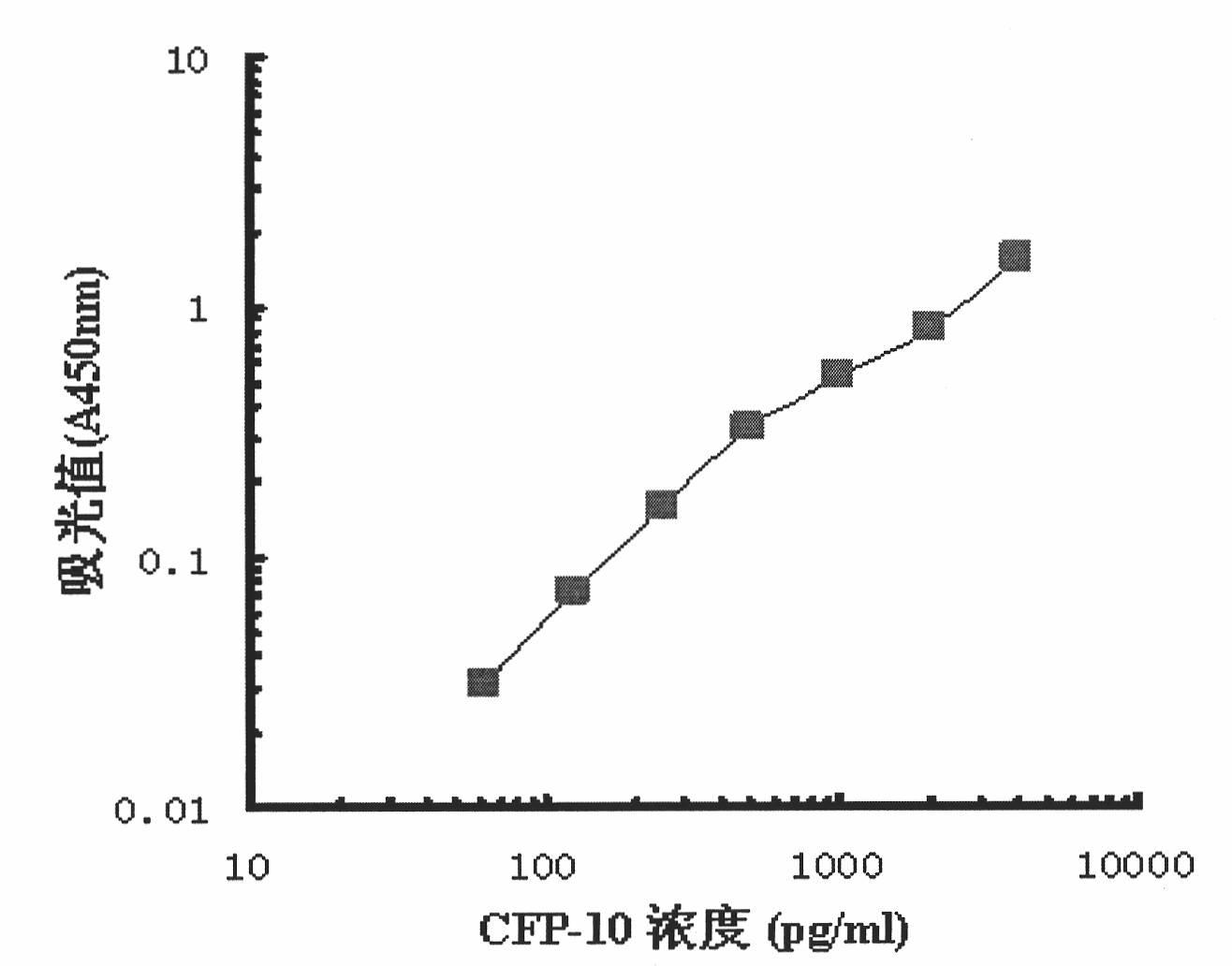
Monoclonal antibody TBCD6 resistant to mycobacterium tuberculosis CFP-10 and application
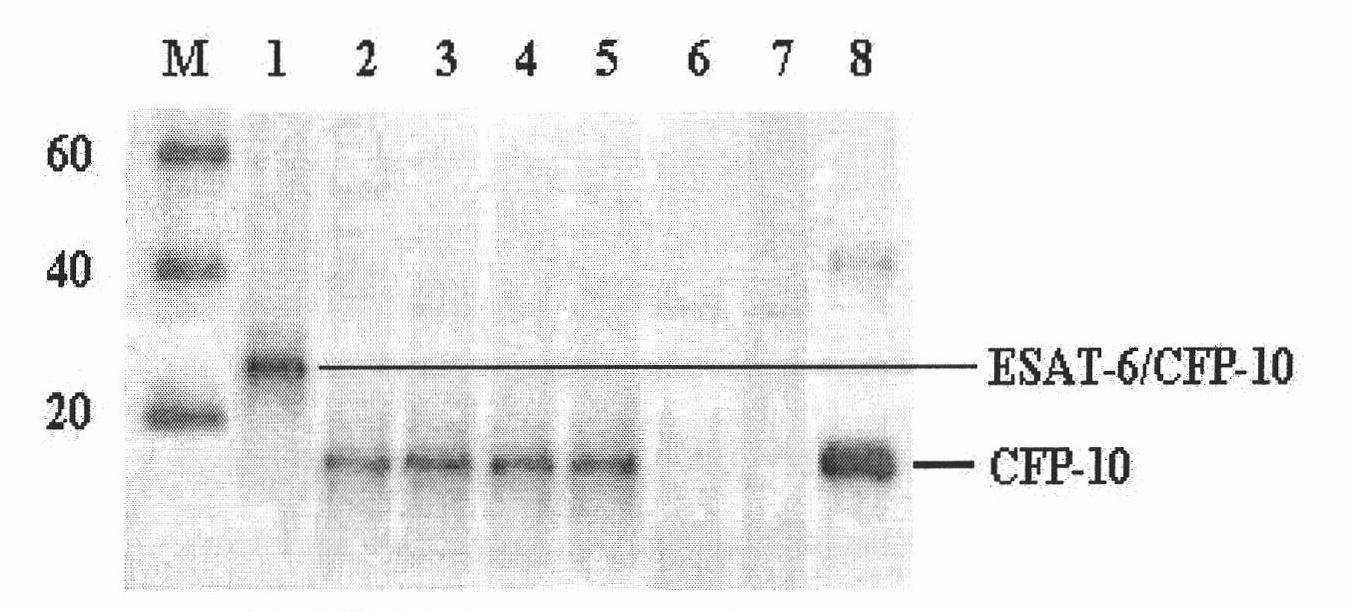
InactiveCN101985472AStable secretionNo decline in secretionImmunoglobulins against bacteriaMicroorganism based processesMycobacterium tuberculosis cultureHIV Western Blot
The invention provides a monoclonal antibody TBCD6 resistant to mycobacterium tuberculosis culture filter protein (CFP) 10. A preparation method of the monoclonal antibody is characterized by immunizing the mice with the specific antigenic peptide, collecting sensitized B lymphocyte of the mice to undergo fusion with the myeloma cells of the mice, judging positive clones by utilizing enzyme-linked immunosorbent assay (ELISA) and western blotting, building a hybridoma cell system TBCD6 which secretes the monoclonal antibody, amplifying, culturing and injecting the positive cells to the peritoneal cavities of the mice of the same strain, using the mice to induce ascites containing the antibody and obtaining the monoclonal antibody through collection and affinity purification of the antibody. The invention provides application of the monoclonal antibody to detection and quantitative analysis of the mycobacterium tuberculosis CFP 10.
Owner:ZHEJIANG UNIV +1
Enrofloxacin monoclonal antibody and application
InactiveCN101565690AStrong specificityRapid and Sensitive DetectionTissue cultureImmunoglobulinsBALB/cIon exchange
The invention relates to an enrofloxacin monoclonal antibody and application, relates to hybridoma strains thereof, and belongs to the technical field of immunochemistry. The enrofloxacin monoclonal antibody is generated by mouse hybridoma strains 6A4 and 8E6. The preparation method comprises the following steps that: enrofloxacin and carrier proteins BSA, HAS and OVA are coupled by a carbodiimide method to synthesize artificial immunogens EnR-BSA, EnR-HSA and coatingen EnR-OVA; a Balb / c mouse is immunized by the synthesized artificial immunogens EnR-BSA and EnR-HSA; a spleen cell of the immunized mouse is extracted to be fused with a SP2 / O myeloma cell and coated by the coatingen EnR-OVA; indirect ELISA method and indirect competition ELISA method are established to screen the hybridoma strains which can stably secrete specific antibody; the obtained cell strain immunized Balb / c mouse is used to prepare ascites; the ascites is purified by a caprylic acid-ammonium method and an ion exchange method; and valences of antibodies of two purified cell strains reach over 1.024*10 and 1.28*10. The monoclonal antibody has strong specificity, can be applied to preparation of enrofloxacin residue inspection kit and aerosol test strip, and can sensibly and quickly inspect the enrofloxacin residue.
Owner:泰州市蛋白质工程研究院
Antibody capable of recognizing HLA class i
InactiveUS20120244142A1Inhibit cell growthProlong lifeImmunoglobulins against animals/humansAntibody ingredientsSpleen cellCell Aggregations
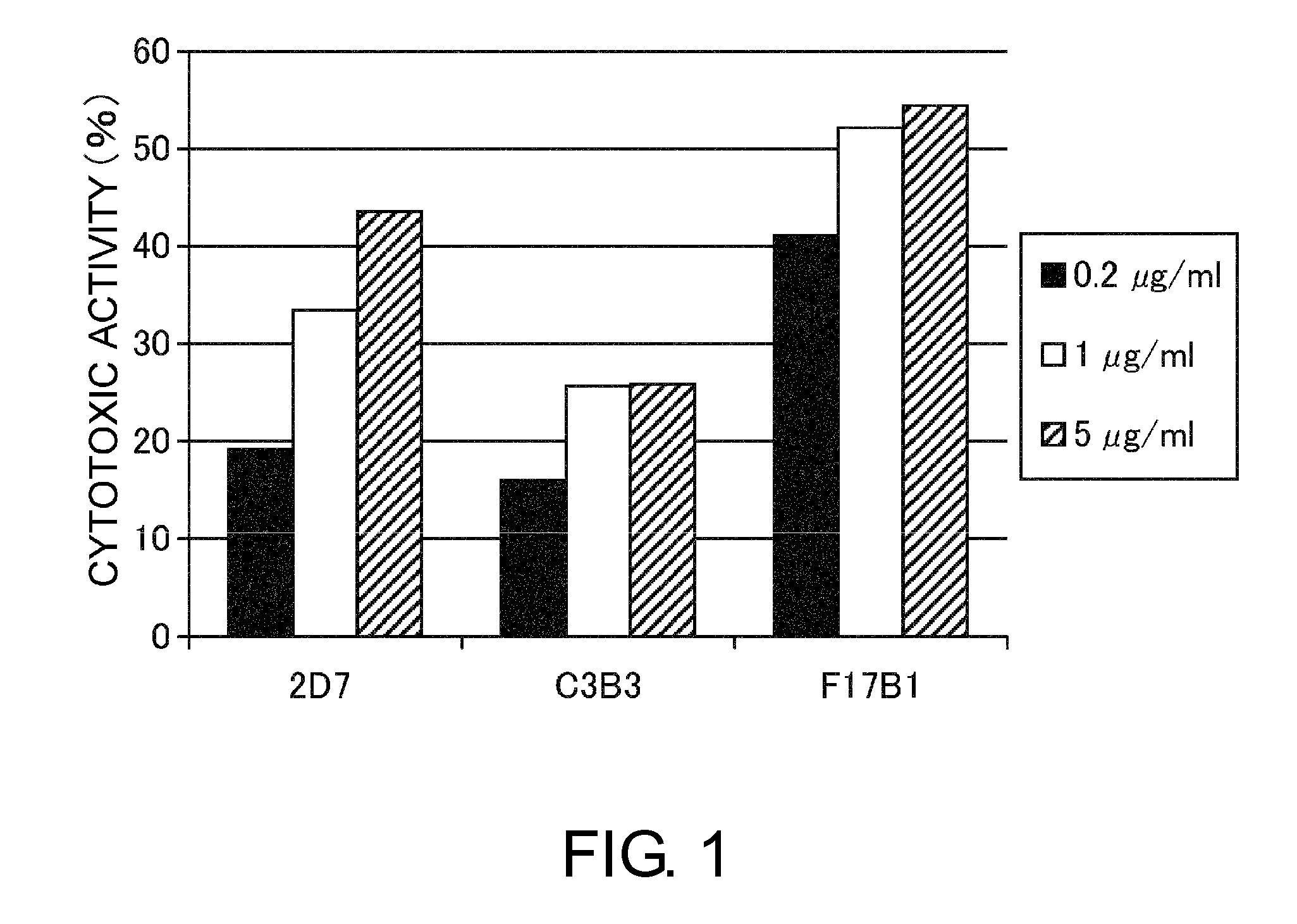
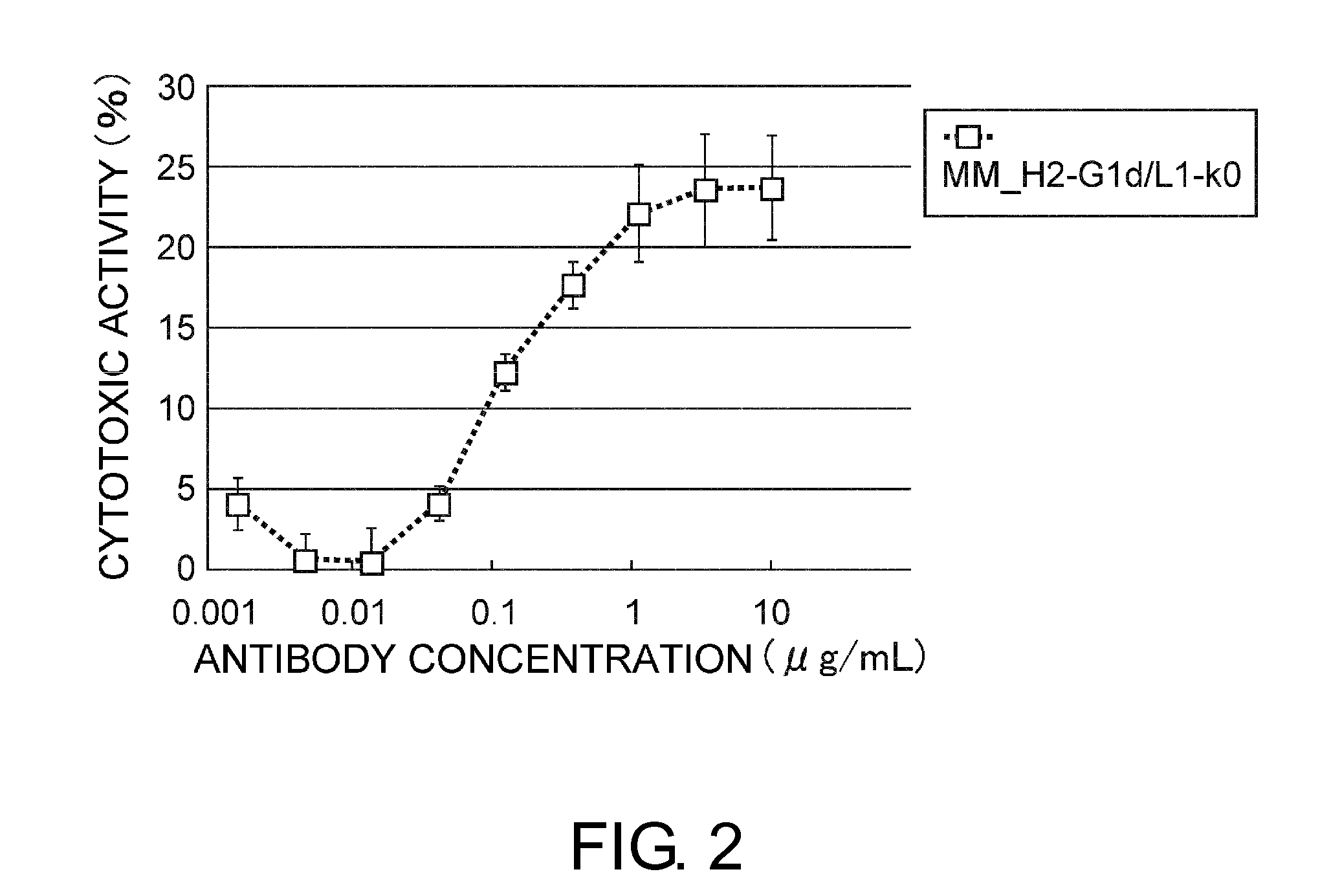
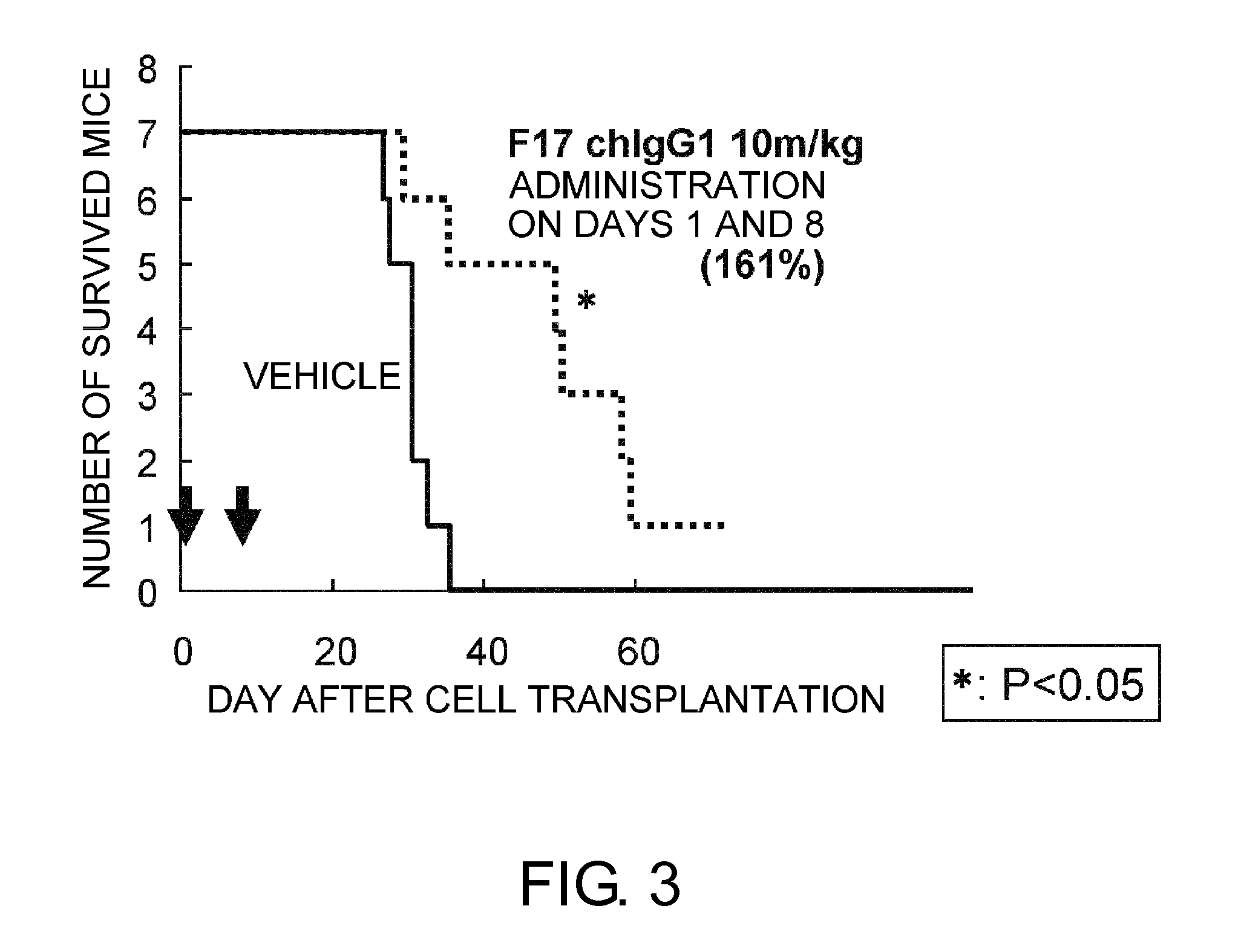
An objective of the present invention is to provide antibodies that recognize HLA class I and have significant cell death-inducing activity. To achieve the above objective, first, the present inventors immunized mice with soluble HLA-A to which a FLAG tag is attached. Four days after the final immunization, spleen cells from the mice were fused with mouse myeloma cells. Then, hybridoma screening was carried out using the cell aggregation-inducing activity and cell death-inducing activity as an index. Thus, the monoclonal antibody F17B1 that has strong cell death-inducing activity was selected. The sequences of the H chain and L chain variable regions of the mouse monoclonal antibody F17B1 were determined to humanize this antibody. Then, the humanized anti-HLA class I antibody H2-G1d_L1-k0 was assessed for cell death induction. The result showed that H2-G1d_L1-k0 suppressed the growth of the IM9 cell line in a concentration dependent manner.
Owner:CHUGAI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com