Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43results about How to "Specific binding" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
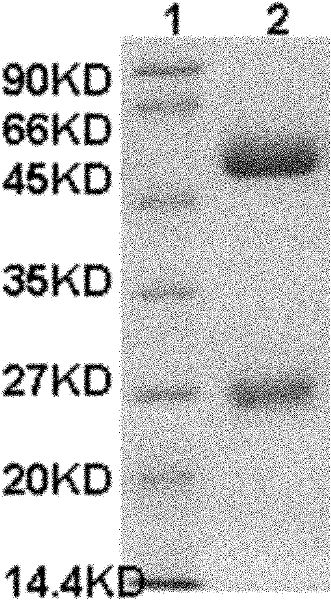
Carbendazim monoclonal antibody, preparation method and application thereof
InactiveCN102675463ASpecific bindingMeet dose dependenceMicroorganism based processesTissue cultureAntigenAscitic fluid
The invention discloses a carbendazim monoclonal antibody, a preparation method and an application thereof. The carbendazim monoclonal antibody is prepared by a mouse hybridoma cell strain MC-3 with a preservation number of CGMCC N0.4575 and the preparation method is as follows: (1) introducing ninth amino acid residue of carbendazim into one amino group to obtain the carbendazim modified by the amino and coupling the carbendazim modified by the amino with a carrier protein to obtain a complete antigen A; (2) immunizing a mouse by the complete antigen A obtained in step (1), taking splenocyteof the immune mice to fuse with a mouse myeloma cell to screen out a positive hybridoma cell strain; extensively culturing the positive hybridoma cell strain and injecting the positive cell strain into a homologous mouse abdominal cavity to induce to generate ascitic fluid and to obtain more carbendazim monoclonal antibodies. The Carbendazim monoclonal antibody can be used for detecting the carbendazim.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
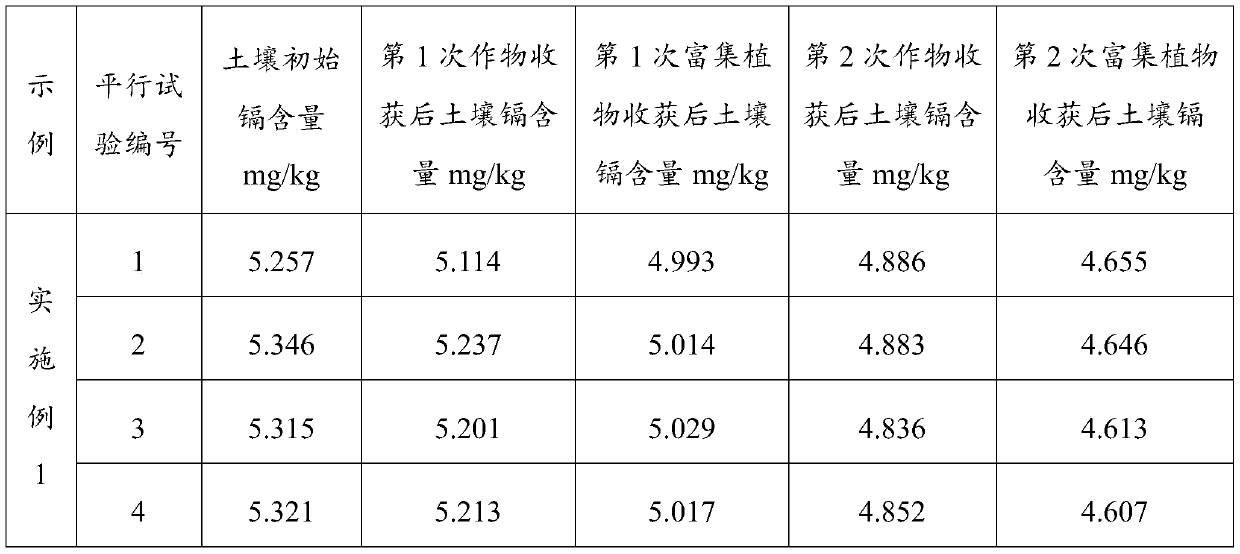
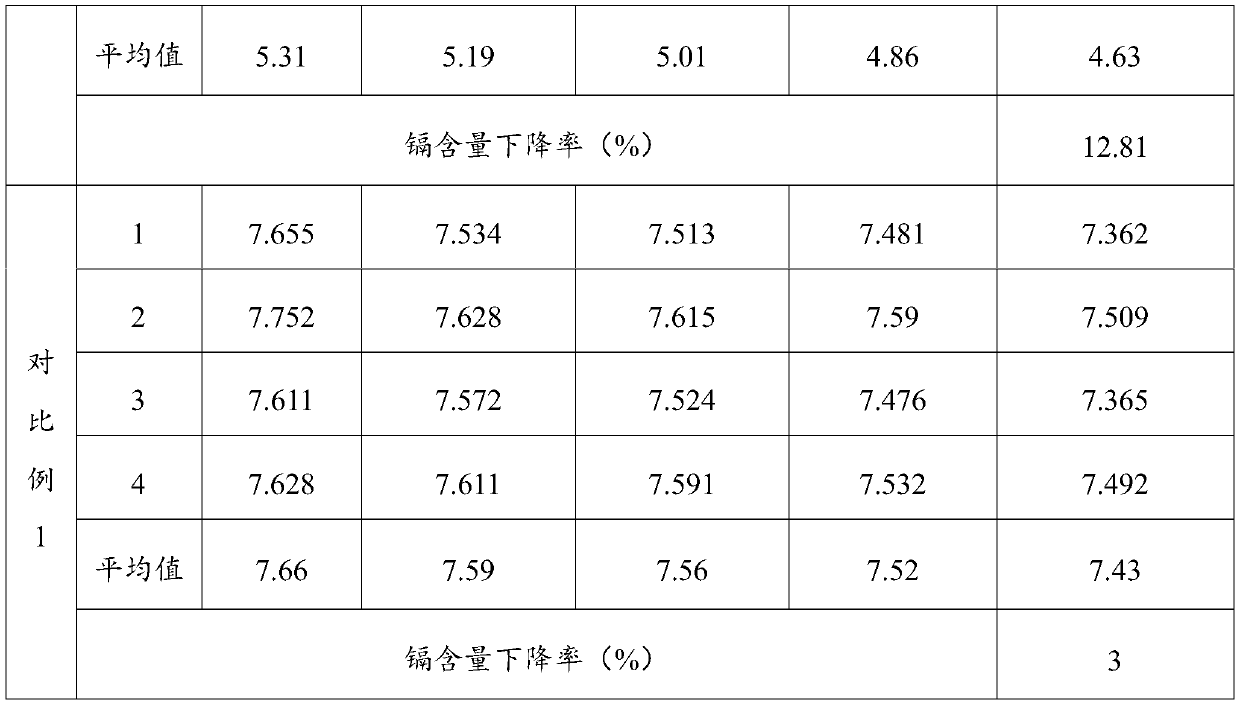
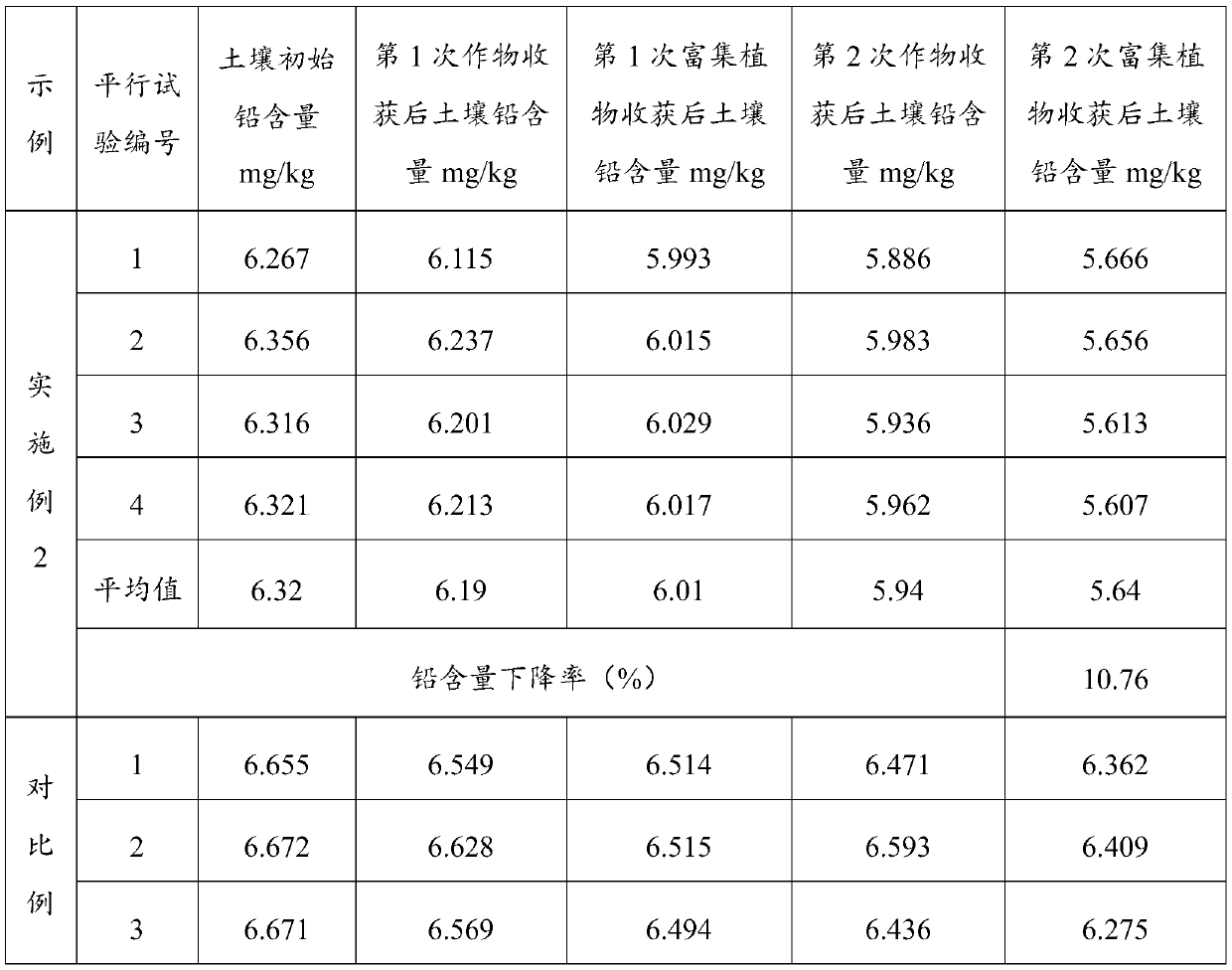
Remediation method for heavy metal pollution of farmland soil
ActiveCN110014032AImprove efficiencyReduce risk of leachingContaminated soil reclamationHyperaccumulatorMaterials science
The invention belongs to the technical field of soil protection, in particular to a remediation method for heavy metal pollution of farmland soil. A heavy metal passivator is uniformly mixed with soil, after conservation, the first crop is planted, and then hyperaccumulators are planted, at that same time, a heavy metal activator is applied and the passivator is applied, after the second crop is planted, the heavy metal passivator is applied at first, so as to reduce the risk of heavy metal leaching, at that same time, heavy metals are avoided to be enriched in crops, and then the heavy metalactivator is applied to plant hyperaccumulators to absorb heavy metals and reduce the content of heavy metals in the soil, and the heavy metals in the soil not covered by the plant roots are kept at alow leaching risk due to the existence of heavy metal passivators. The invention adopts a plant enrichment technology and a method of applying a heavy metal passivator, which can not only fundamentally remove the problem of heavy metals, but also passivate the heavy metals when planting crops, thereby improving the use efficiency of the soil.
Owner:BCEG ENVIRONMENTAL REMEDIATION CO LTD
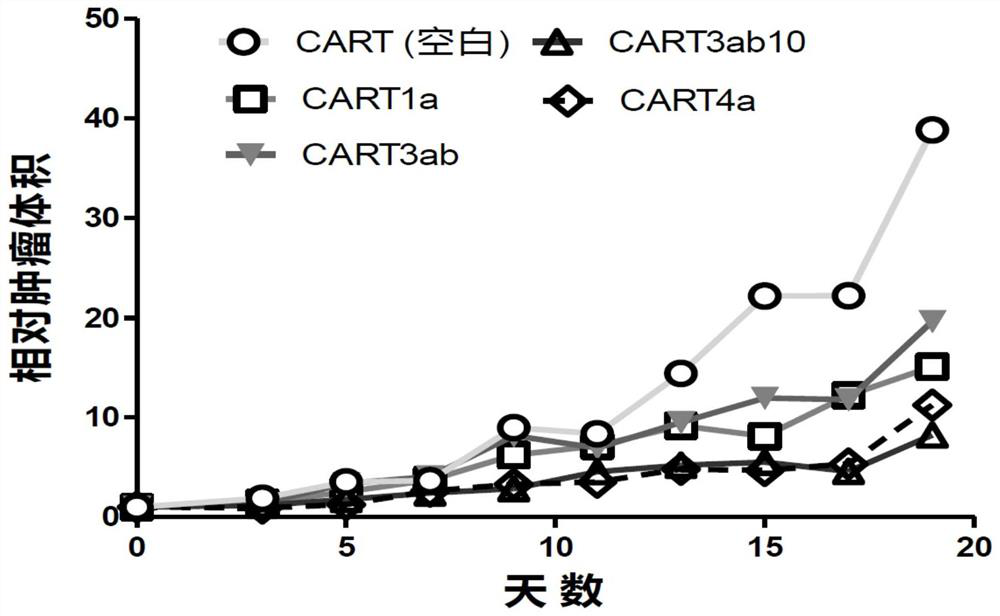
Claudin 18.2-targeting CAR molecule, immune cell modified by Claudin 18.2-targeting CAR molecule and application of Claudin 18.2-targeting CAR molecule
PendingCN111848809AHigh activityHigh affinityImmunoglobulin superfamilyImmunoglobulins against cell receptors/antigens/surface-determinantsTransmembrane domainIntracellular domain
The present invention discloses a nucleic acid construct. The nucleic acid construct is characterized in that the nucleic acid construct has a structure as shown in the formula car-[(IRES)-f]<m>, wherein the IRES is a sequence of an internal ribosome entry site, f encodes a functional protein F, m is 0 or a non-0 natural number, car encodes a CAR, the CAR comprises (a) an extracellular binding domain that specifically recognizes CLDN18.2, (b) a hinge domain, (c) a transmembrane domain, (d) a costimulating intracellular domain, and (e) a signal transduction domain, the extracellular binding domain comprises scFv, and the amino acid sequences and functions of the extracellular binding structural domain are described in the specification. The CAR molecule provided by the invention has excellent safety and effectiveness and shows a very good tumor inhibition effect.
Owner:L&L BIOPHARMA CO LTD
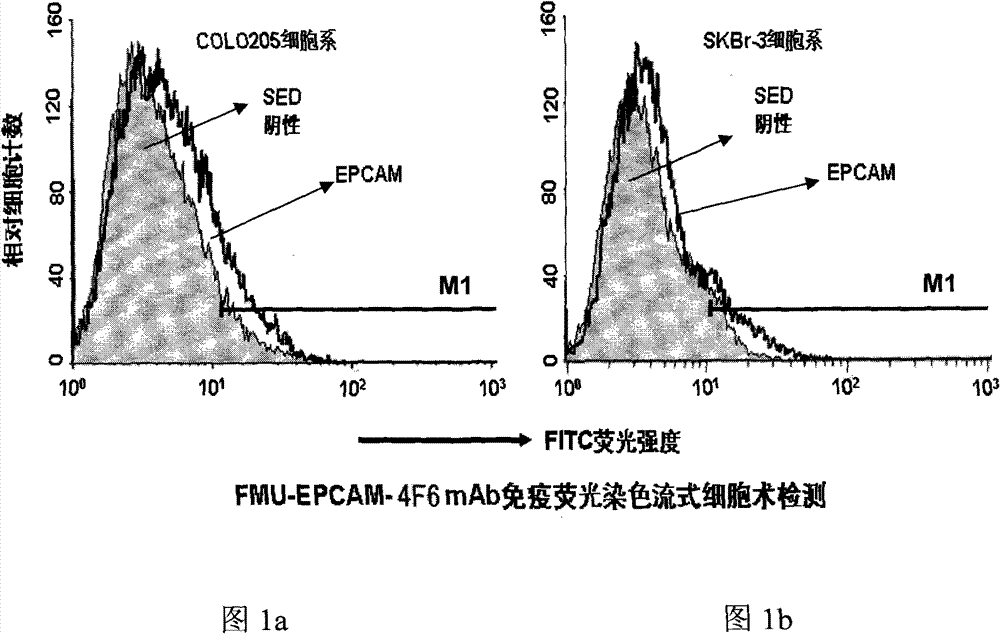
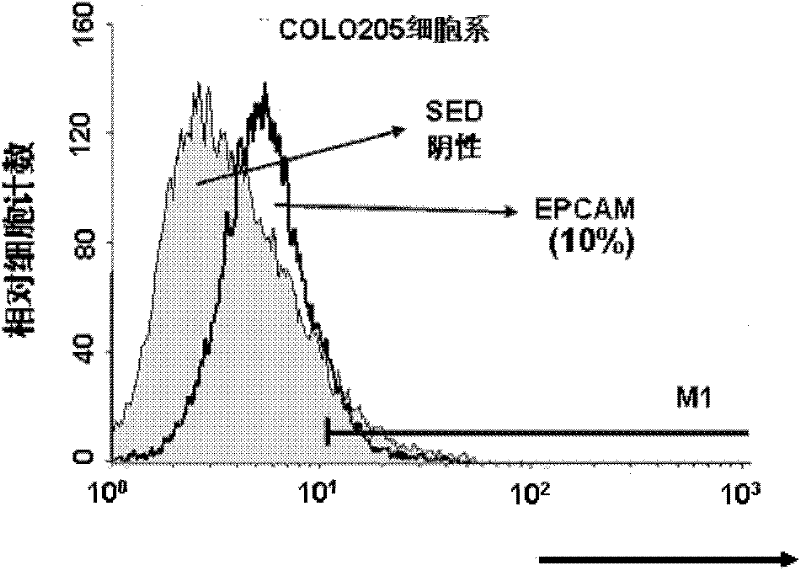
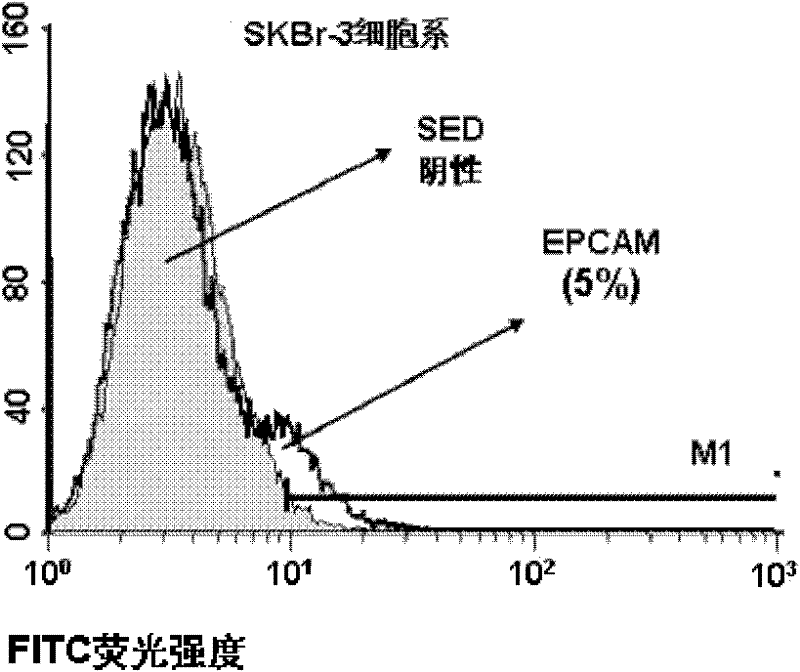
Variable regions of light chains and heavy chains of FMU-EPCAM-2A9 monoclonal antibodies
ActiveCN101701039ASpecific bindingProving uniquenessImmunoglobulins against cell receptors/antigens/surface-determinantsGenetic engineeringBALB/cHeavy chain
The invention discloses variable regions of light chains and heavy chains of FMU-EPCAM-2A9 monoclonal antibodies. The amino acid sequence of a monoclonal antibody light chain variable region is shown as SEQ ID NO.1, and the amino acid sequence of a monoclonal antibody heavy chain variable region is shown as SEQ ID NO.2. A set of mouse anti-human EPCAM monoclonal antibodies are prepared through recombining human EPCAM immune BALB / c mice, and hybridoma cell lines capable of stably secreting high-affinity anti-human EPCAM monoclonal antibodies are screened to prepare ascitic fluid so as to obtain the high-affinity anti-human EPCAM monoclonal antibodies. The uniqueness of the gene sequence and a corresponding protein sequence and a CDR sequence of the gene sequence are confirmed so as to provide supports for anti-human EPCAM chimeric or humanized gene engineering antibodies.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
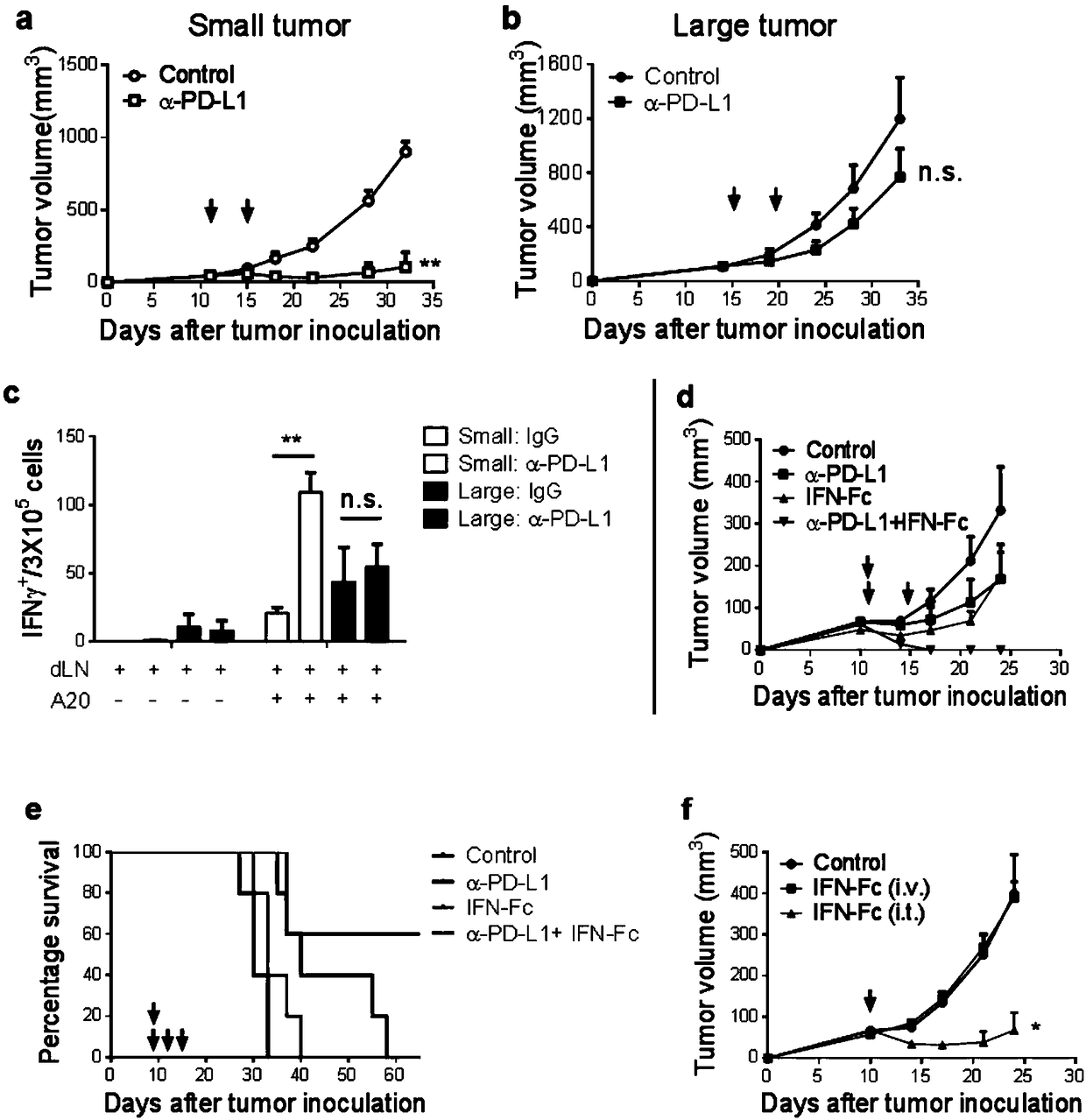
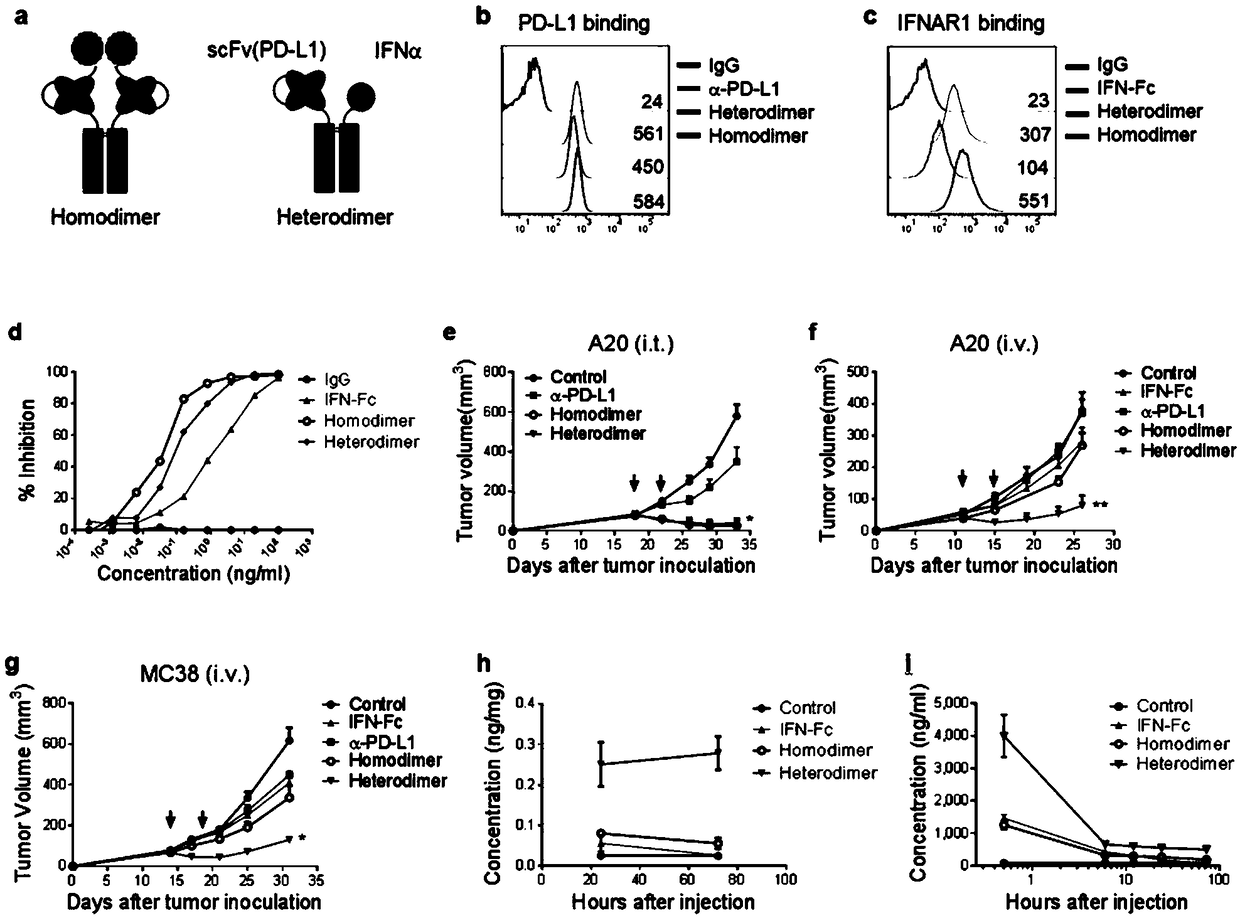
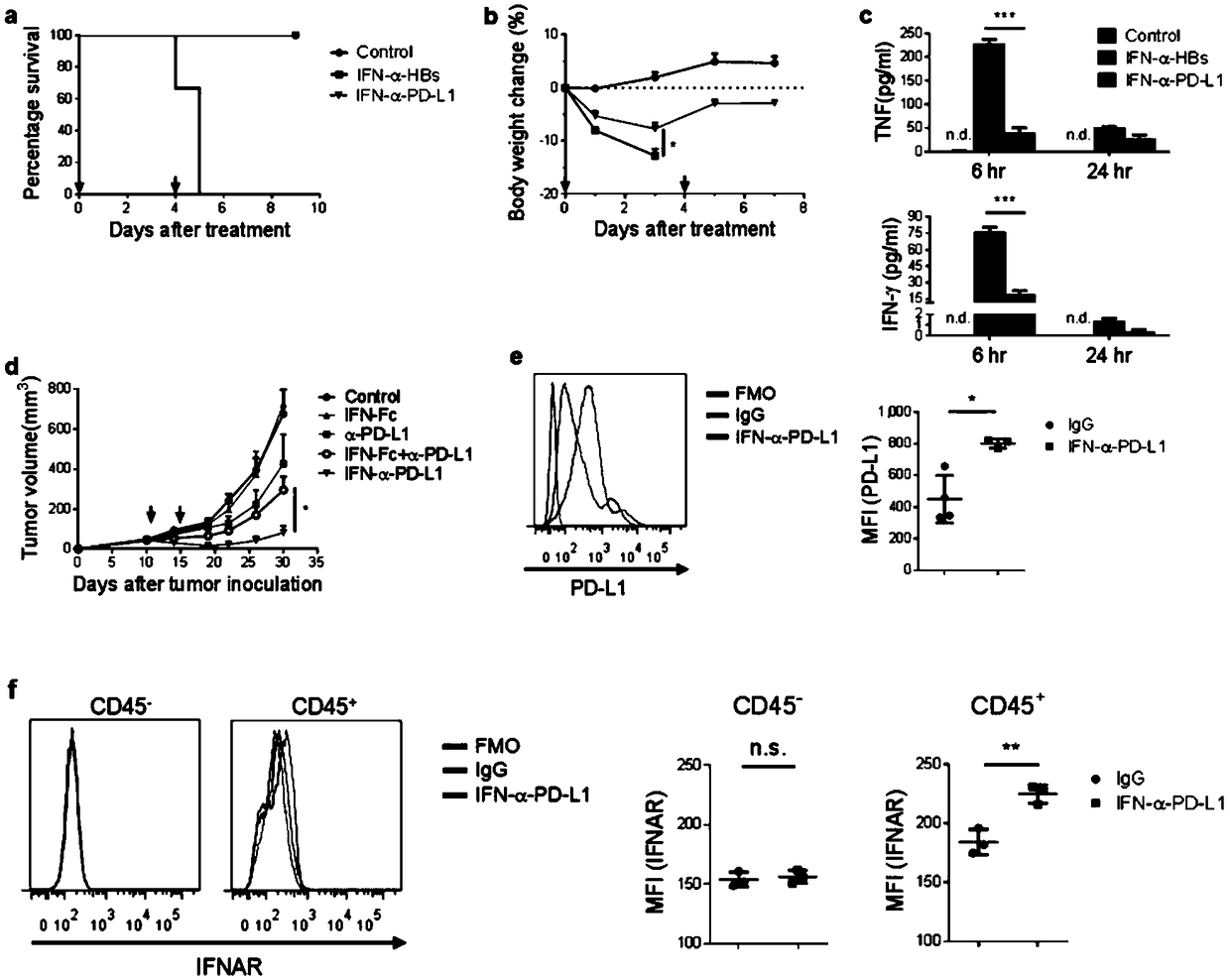
Fusion protein of IFN and anti-PD-L1 antibody and application thereof
ActiveCN108727504AImprove treatmentLow toxicityPeptide/protein ingredientsAntibody mimetics/scaffoldsActivation cellsTumor microenvironment
The invention relates to a fusion protein of an IFN and an anti-PD-L1 antibody, a pharmaceutical composition containing the fusion protein and a kit as well as an application of the fusion protein intumor treatment. The fusion protein can be targeted to PD-L1 and IFN receptors at the same time. An IFN signal in a tumor microenvironment is activated by inducing more powerful T cell activation to enhance PD-1 / PD-L1 treatment on tumors; meanwhile, the anti-PD-L1 antibody can be used for specifically transferring immunoregulation molecules to tumor tissues. The fusion protein acts to generate multiple feedforward responses, so that the targeting effect is increased and the toxicity is reduced, and response to IFN treatment is enhanced, so that the anti-tumor effect is maximized.
Owner:泉州向日葵生物科技有限公司
CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) nanoantibody and preparation method and application thereof
InactiveCN110256563AStable structureDifficult to identifyMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsCompetent cellTotal rna
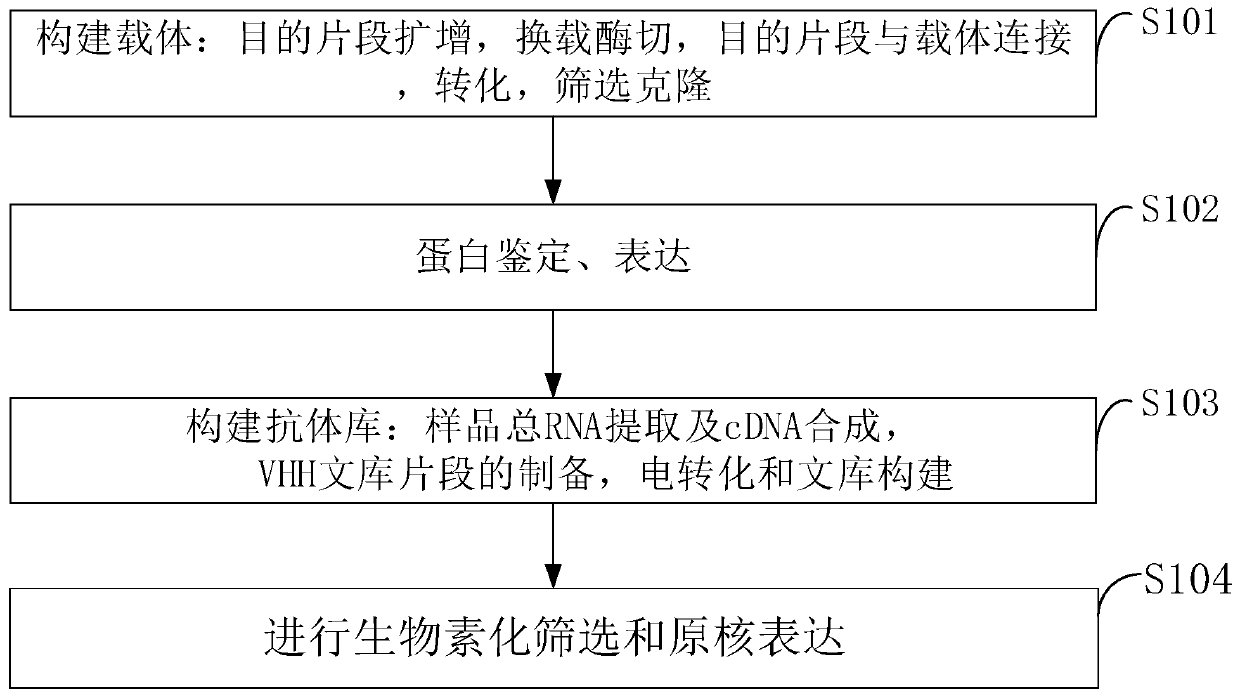
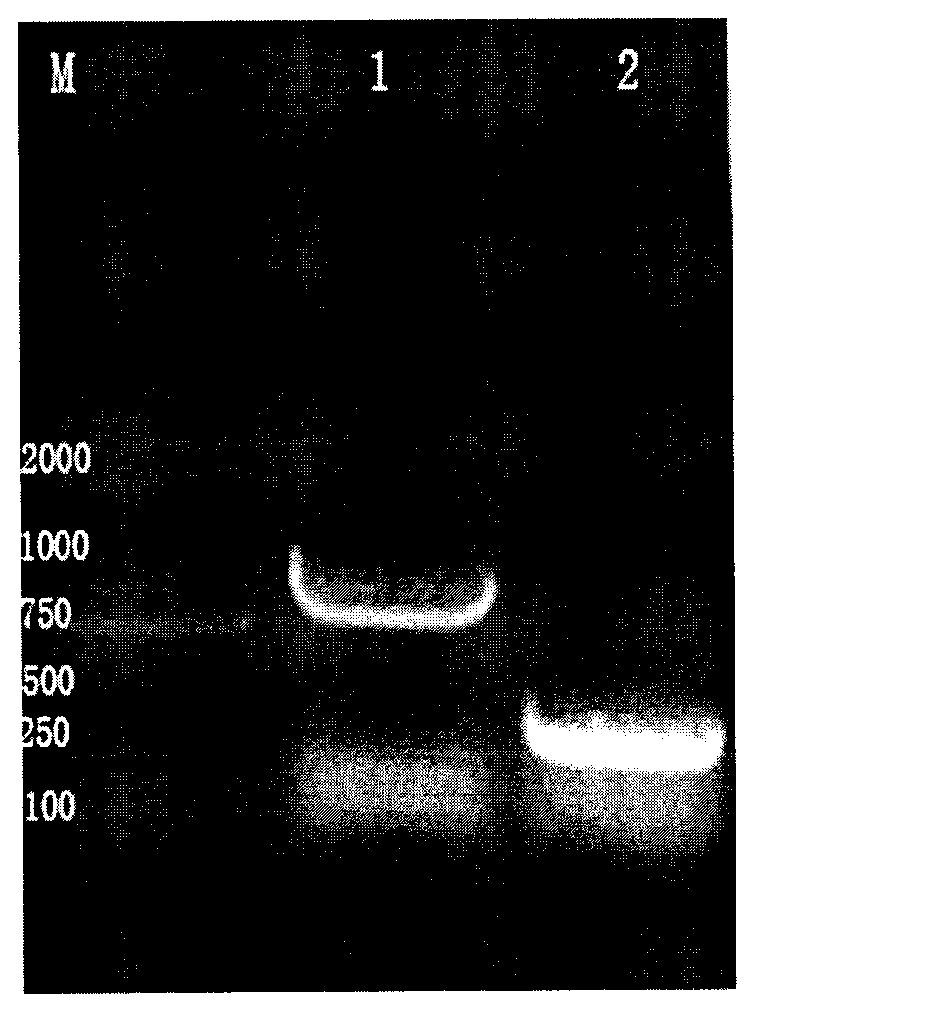
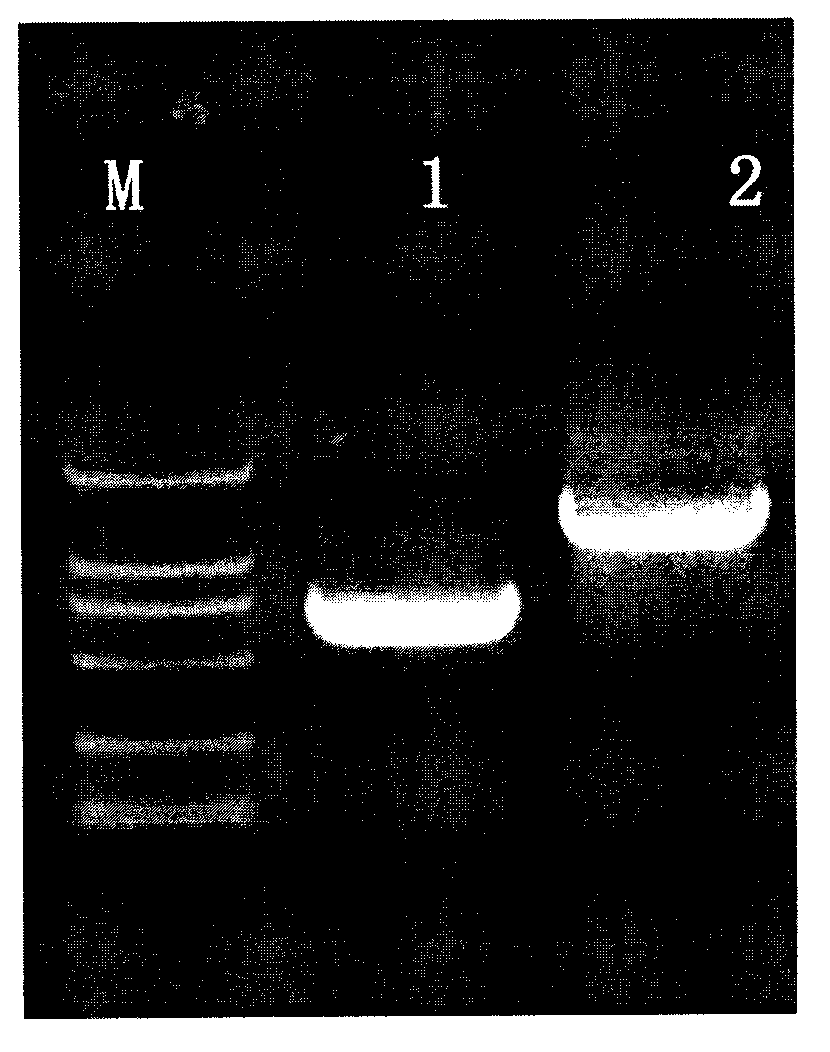
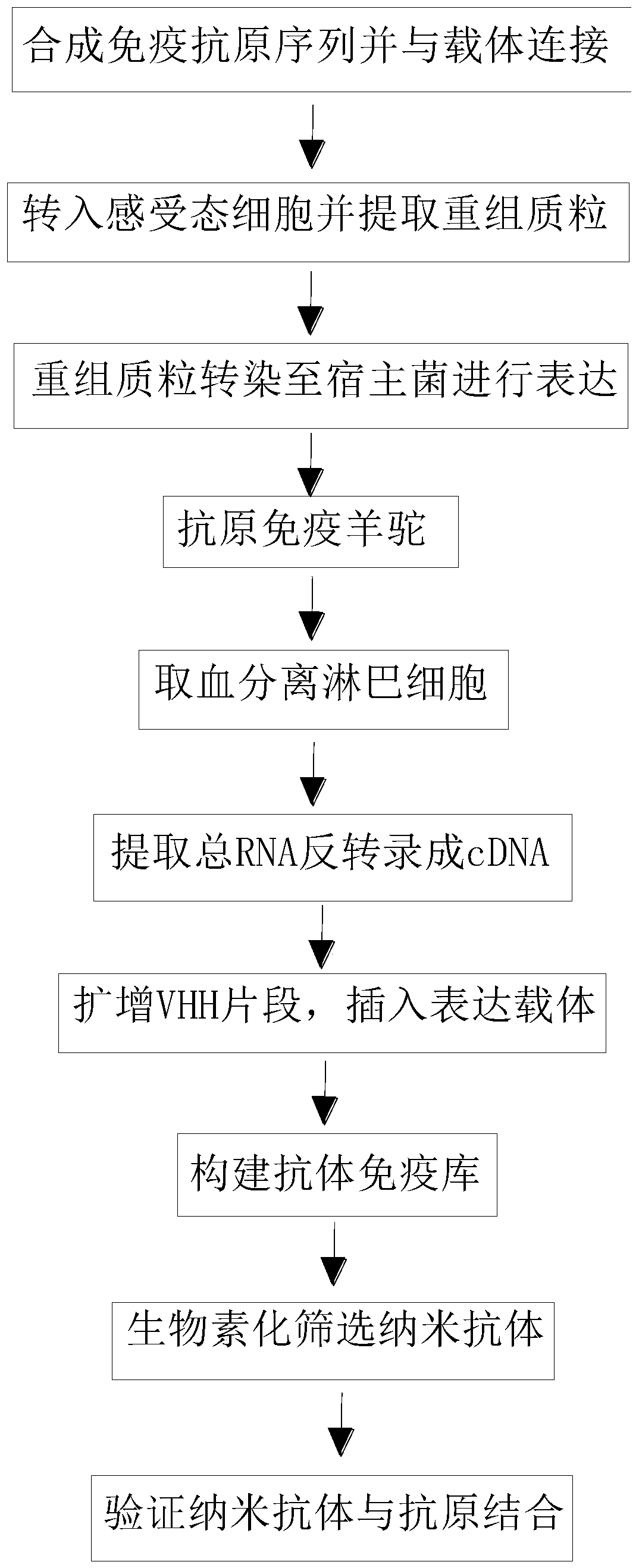
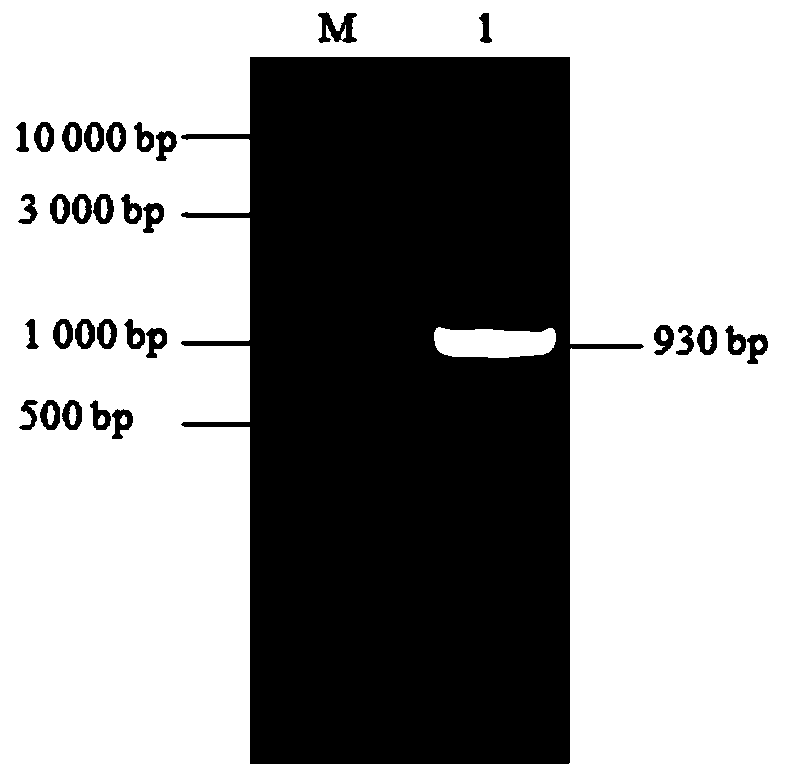
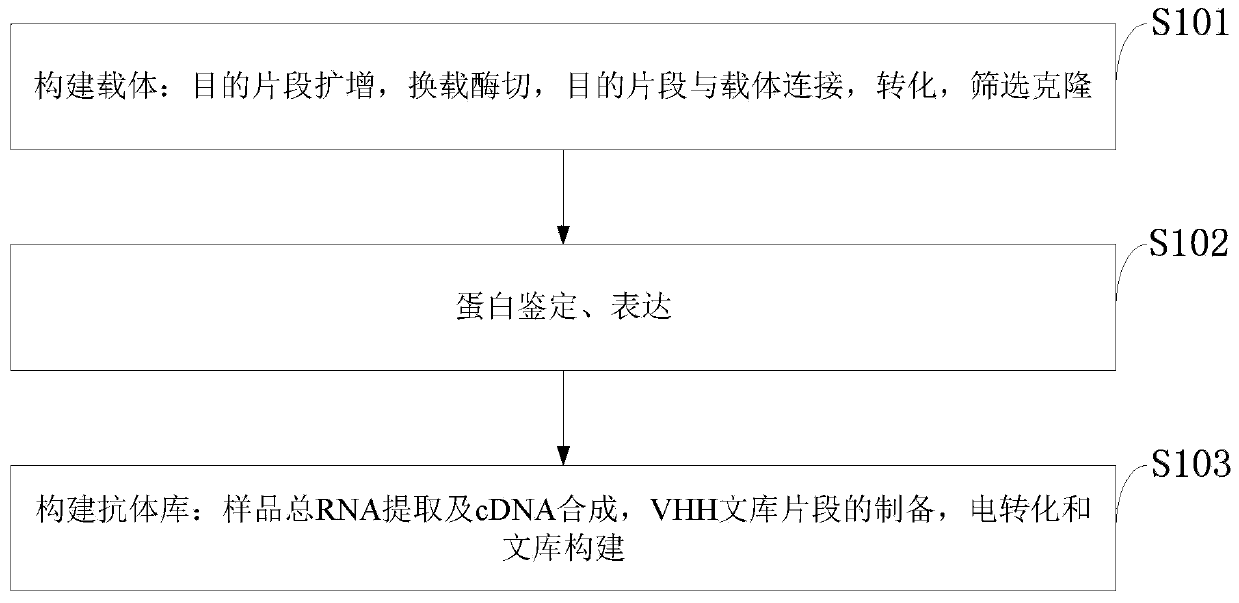
The invention belongs to the technical field of biomedicine and particularly relates to a CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) nanoantibody and a preparation method and application thereof. The ability to bind with antigens can be improved or collaboratively enhanced through the CTLA-4 antibody. The CTLA-4 antibody has no complete antibody structure and lacks Fc end and Y-shaped structure; the antigens bound to the CTLA-4 antibody are not easily recognizable and can escape easily from being caught by the immune system. The technical means includes mainly: carrying out antigen-vector connecting; transferring to competent cells, and extracting recombinant plasmids; transfecting to host bacteria for expressing; immunizing camels with an antigen protein; collecting peripheral blood, extracting total RNA of a lymphocyte sample, inversely transcribing the total RNA into cDNA, performing amplifying to obtain a VHH library fragment, inserting the VHH library fragment into an expression vector, and transferring to the competent cells to construct an antibody immunization library; screening nanoantibodies in the antibody immunization library; verifying binding of the screened nanoantibodies with a CTLA-4 antigen.
Owner:SHIHEZI UNIVERSITY
Preparation and application of hepatitis C virus recombinant protein
InactiveCN105330730AEfficient combinationSpecific bindingGenetic material ingredientsVirus peptidesAntigenPeptide
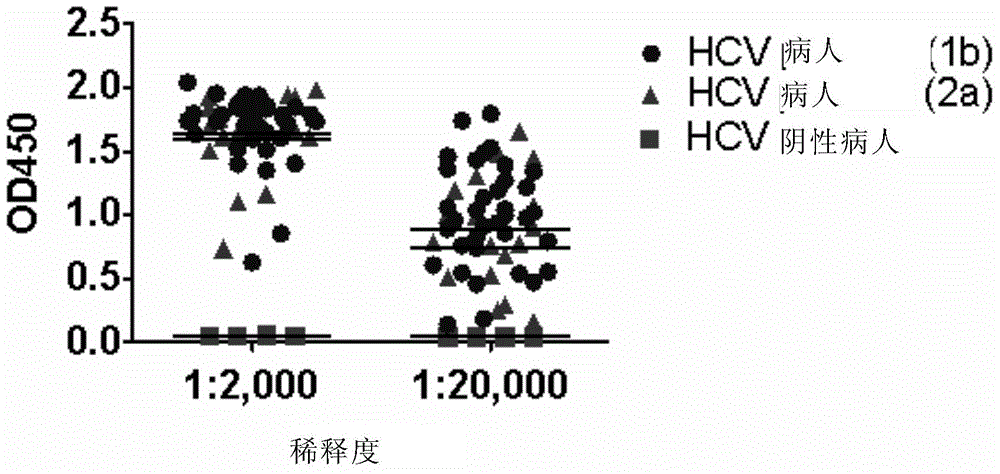
The present invention discloses preparation and application of a hepatitis C virus (HCV) recombinant protein, specifically discloses a separated HCV antigen peptide, which is derived from an E2 protein of HCV virus; the antigenic peptide can bind to an anti-HCV antibody; and the HCV is a Con1 strain of HCV 1b genotype. The invention provides a composition containing the above antigen peptide and a preparation method thereof. The HCV antigen peptide of the invention can effectively and specifically combine with anti-E2 antibody in serum of an infected patient in a broad-spectrum way, and can be used to develop a diagnostic kit for the detection of HCV infection.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Anti-CD38 nano-antibody, encoding gene and application
ActiveCN109232739AHigh affinityEfficient combinationImmunoglobulins against cell receptors/antigens/surface-determinantsBiological testingEscherichia coliGene
The invention relates to an anti-CD38 nano-antibody, an encoding gene and an application. The anti-CD38 nano-antibody is following protein a) or b): a), protein consisting of an amino acid sequence shown as a sequence 2 in a sequence table; b), protein which is obtained by the amino acid sequence shown as the sequence 2 in the sequence table through substitution and / or deletion and / or addition ofone or more amino acids, related with specifically recognized CD38 and derived from a). The anti-CD38 nano-antibody can be efficiently and specifically bound with CD38, affinity is as high as 4 nM, large-scale production with low cost can be realized in an Escherichia coli expression system, and the anti-CD38 nano-antibody has significant application prospect in the fields of detection and pharmaceuticals.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
Zinc finger protein and its antibody preparation and use
InactiveCN1422864ASpecific bindingPeptide/protein ingredientsImmunoglobulins against animals/humansDiseaseAdjuvant
The invention refers to a zinc protein and the antibody, relative to human hematogenic system growth. The ZNF 268 protein is a typical krIIppel-type C2H2 zinc protein; the preparation mainly structures ZNF268 expression carrier, transfers into host cells to express and separate to purify ZNF268 protein; the protein can diagnose and treat the disease because of protein abnormity. The antibody of ZNF268 protein can exceptionally combine with ZNF268 protein. The antibody preparation; mix the ZNF268 protein and assist agent to immunize animal, then separate the immune antimal blood to purify the antibody; the antibody; the antibody can diagnose, treat or prevent the disease relative to the protein abnormity of ZNF268 protein.
Owner:WUHAN UNIV
Method for preparing PD-L1 (programmed death-ligand 1) nanoantibody by immunizing alpacas with PD-L1 antigen
InactiveCN110256564ADifficult to identifyEvade captureMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsSequence signalAntigen
The invention belongs to the technical field of antibodies and discloses a method for preparing PD-L1 (programmed death-ligand 1) nanoantibody by immunizing alpacas with a PD-L1 antigen. The method comprises: designing two synthetic primers, amplifying by PCR (polymerase chain reaction) to obtain sufficient target products for enzyme digestion, linking target fragments to a vector, converting, and carrying out screening and cloning; carrying out protein verifying and expressing; constructing an antibody library; displaying a VHH antibody library through an M13 bacteriophage display system which is composed of a pMECS phagemid vector, E. coli TG1 and an M13KO7 helper phage. In the phasmid vector pMECS, a sequence before a Pst I enzyme digestion site is a coding sequence for part of amino acids in first frame regions of pelB secretory signal peptides and antibodies; the pelB signal peptides can guide subsequent polypeptides secreted to periplasmic cavities; a sequence after a Not I enzyme digestion site is a coding sequence for HA and 6*His tags; the method is suitable for purification or detection of fusion proteins.
Owner:SHIHEZI UNIVERSITY
Drug-loaded polymer vesicle with asymmetric membrane structure, preparation method and application in preparation of drug for treating acute myeloid leukemia
ActiveCN112076159AGuaranteed long cycleGood biocompatibilityOrganic active ingredientsPharmaceutical non-active ingredientsTumor cellsBiophysics
The invention discloses a drug-loaded polymer vesicle with an asymmetric membrane structure, a preparation method and application in preparation of a drug for treating acute myeloid leukemia. An amphiphilic triblock polymer with polyaspartic acid PAsp, a targeted amphiphilic block polymer and a small molecule drug are assembled together to prepare the targeted small molecule drug-loaded polymer vesicle with the asymmetric membrane structure. The drug-loaded polymer vesicle disclosed by the invention has many unique advantages, including small size, simple and controllable preparation, reversible crosslinking, in-vivo stability, targeted delivery, high intracellular drug enrichment concentration, sensitive reduction, efficient killing of tumor cells, significant tumor growth inhibition effect and the like, and especially the drug-loaded vesicle disclosed by the invention has effective inhibition on both acute myeloid leukemia cell lines and patient cells. Therefore, the polymer vesicleis expected to become a simple and multifunctional nano platform for efficient and specific targeted delivery of drugs to tumor cells.
Owner:SUZHOU UNIV
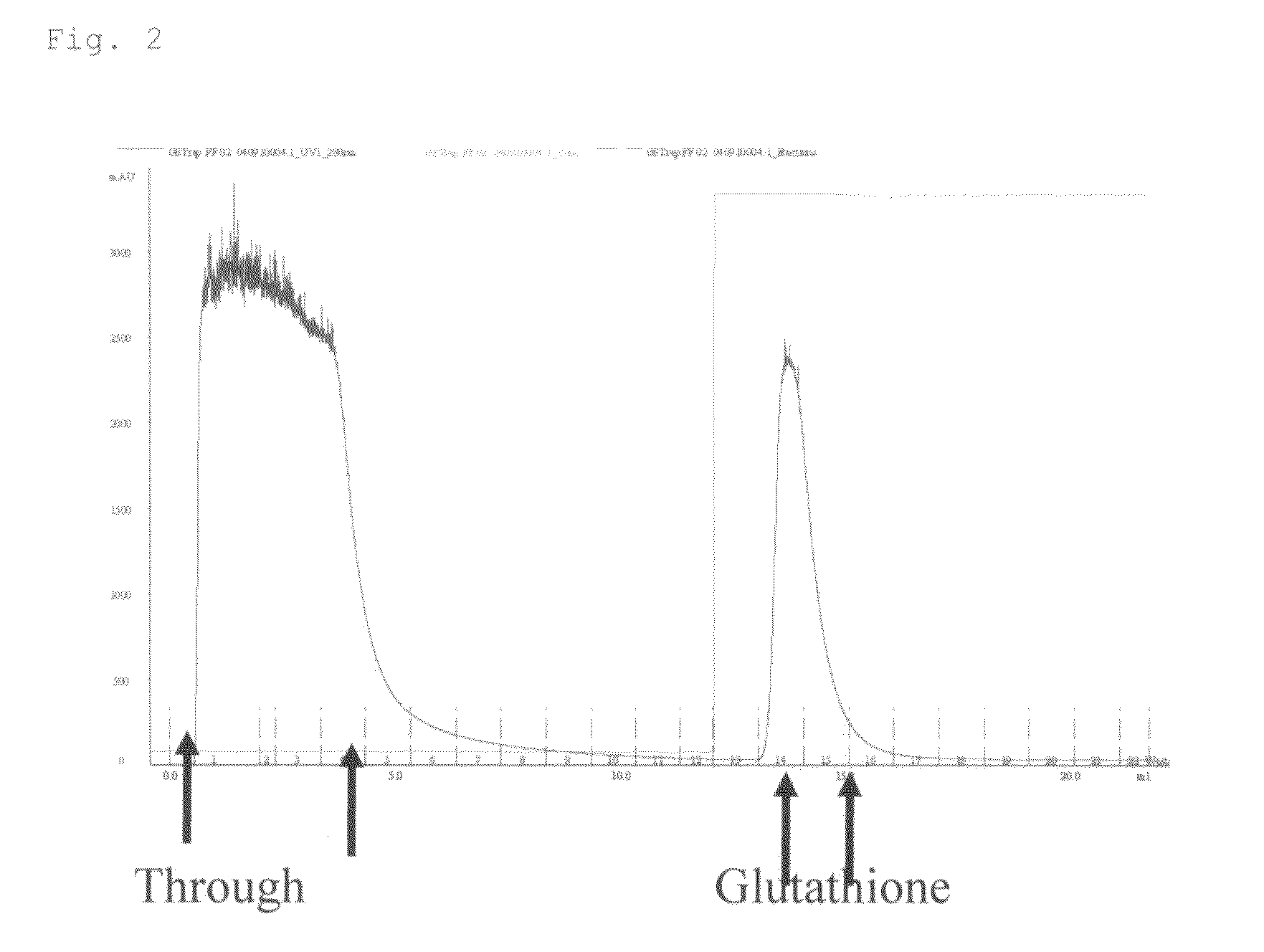
High-Affinity RNA Aptamer Molecule Against Glutathione-S-Transferase Protein
The present invention provides a “nucleic acid adaptor molecule” having specific binding affinity to a GST protein portion serving as an N-terminal fusion partner in a fusion protein consisting of the GST protein and a protein of interest. A “nucleic acid adaptor molecule against a GST protein” according to the present invention is an RNA aptamer molecule having any of the following nucleotide sequences I to III:nucleotide sequence I (SEQ ID NO: 1):GGUAGAUACGAUGGAUGGUUGUGUAAAGGUGGUCGUAUCCGCCGA CAUGACGCGCAGCCAA 61;nucleotide sequence II (SEQ ID NO: 2):GGUAGAUACGAUGGACUAACUGCGCAAAUUACUCGUAUUAGCCGA CAUGACGCGCAGCCAA 61;ornucleotide sequence III (SEQ ID NO: 3):GGUAGAUACGAUGGAUACCGAAAAAUUAGUGUCGUUGACUGCAA CAUGACGCGCAGCCAA 60.
Owner:NEC SOLUTION INNOVATORS LTD +1
Anti-procalcitonin nano antibody and application thereof
ActiveCN110903390ASpecific recognition abilitySpecific bindingBacteriaHydrolasesAntigenAntiendomysial antibodies
The invention discloses an anti-procalcitonin nano antibody. The anti-procalcitonin nano antibody has three unique complementary determining regions CDR1, CDR2, and CDR3. The invention also provides an expression vector containing the variable region coding sequence of the nano antibody, host cells containing the expression vector, a fusion protein of the nano antibody and alkaline phosphatase, and an application of the nano antibody in preparation of a procalcitonin detection kit. The anti-procalcitonin nano antibody provided by the invention has specific recognition and binding capacity on procalcitonin, the affinity with antigen can reach 7.429 E-9, and an excellent detection effect is obtained in procalcitonin detection, especially in application of a double-antibody sandwich method.
Owner:深圳市国创纳米抗体技术有限公司
N-phosphopeptide and protein enrichment material and preparation and application thereof
ActiveCN108079957AMass transfer speedRapid enrichmentOther chemical processesPreparing sample for investigationTert-Butyloxycarbonyl protecting groupMicrosphere
The present invention relates to a novel silica gel material capable of recognizing N-phosphopeptides and proteins under neutral conditions and application thereof in N-phosphopeptide and protein enrichment. Non-porous silica microspheres are prepared by a seed growth method, a core-shell microsphere initial layer is formed by a template-guided dissolution and re-deposition method, and finally sub-2-micron core-shell silica gel with vertical pores can be obtained by acid reflux. N-tert-butyloxycarbonyl-L-tyrosine and N, N'-dipicolylamine are reacted to form a support molecule, and a phosphaterecognition functional molecule is formed by complexation of a metal ion on the support molecule. Finally, the novel phosphoric acid recognition functional silica gel material is obtained by covalently bonding the phosphoric acid recognition functional molecule with the sub-2-micron core-shell silica gel having the vertical pores through an amide bond. The novel phosphoric acid recognition functional silica gel material can rapidly and specifically enrich the N-phosphopeptides and proteins in a neutral buffer system.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Single domain antibody for resisting CEACAM-5 and application of single domain antibody
ActiveCN108659131ASpecific recognition abilitySpecific bindingHydrolasesImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenExpression vector
The invention discloses a single domain antibody for resisting CEACAM-5. The single domain antibody has three unique complementary determining regions CDR1, CDR2 and CDR3. The invention further provides an expression vector containing a variable region coding sequence of the single domain antibody, a host cell containing the expression vector, fusion protein of a variable region of the single domain antibody and human alkaline phosphatase, application of the single domain antibody in preparing a CEACAM-5 detection kit, a method for CEACAM-5 immunological detection by applying the single domainantibody as well as a corresponding detection kit. The single domain antibody for resisting the CEACAM-5, provided by the invention, has specific recognizing and binding capability for the CEACAM-5;the affinity of the single domain antibody can reach 4.51E-09; the single domain antibody has unique antigenic determinant recognition site and can obtain an excellent detection result in CEACAM-5 immunological detection, in particular to a double antibody sandwich method.
Owner:长春力太生物技术有限公司
Anti-anthrax PA antigen monoclonal antibody and applications thereof
ActiveCN102936286AHighly specific PA binding activitySpecific recognition abilityAntibacterial agentsImmunoglobulins against bacteriaDrugs preparationsMonoclonal antibody
The present invention discloses an anti-anthrax PA antigen monoclonal antibody having a humanize form, a chimeric form and a murine form, and applications of the monoclonal antibody in anthrax treatment drug preparation. The anti-anthrax PA antigen monoclonal antibody has characteristics of specific antigen binding, excellent toxin neutralization activity and good animal protection function.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
PD-1 (programmed death 1) nanoantibody and preparation method and application thereof
ActiveCN110256562AStable structureDifficult to identifyImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCompetent cellTotal rna
The invention belongs to the technical field of biomedicine and particularly relates to a PD-1 (programmed death 1) nanoantibody and a preparation method and application thereof. The ability to bind with antigens can be improved or collaboratively enhanced through the PD-1 nanoantibody. The PD-1 nanoantibody has no complete antibody structure and lacks Fc end and Y-shaped structure; the antigens bound to the PD-1 nanoantibody are not easily recognizable and can escape easily from being caught by the immune system. The technical means includes mainly: performing antigen-vector connecting; transferring to competent cells, and extracting recombinant plasmids; transfecting to host bacteria for expressing; immunizing camels with antigen proteins; collecting peripheral blood, extracting total RNA of a lymphocyte sample, reversely transcribing the total RNA into cDNA, performing amplifying to obtain a VHH library fragment, inserting the VHH library fragment into an expression vector, and transferring to the competent cells to construct an antibody immunization library; screening nanoantibodies from the antibody immunization library; verifying the binding of the screened nanoantibodies with a PD-1 antigen.
Owner:SHIHEZI UNIVERSITY
Monoclonal antibody resistant to SasA (staphylococcus aureus surface protein A) antigen and application of monoclonal antibody
ActiveCN104098693AHighly specific binding activitySpecific recognition abilityAntibacterial agentsImmunoglobulins against bacteriaStaphylococcus cohniiMonoclonal antibody
The invention discloses a monoclonal antibody resistant to different structural domains of a SasA (staphylococcus aureus surface protein A) antigen as well as an application of the monoclonal antibody in preparation of a drug for resisting staphylococcus aureus infection. The specific antibody has good protection performance in a mouse model when in combined utilization.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
SgRNA, CREBRF point mutation-type Bama miniature pig constructed by sgRNA and application
ActiveCN110862988ASpecific bindingPromote homologous recombination repairMetabolism disorderPeptidesBiotechnologySingle strand
The invention provides sgRNA, a CREBRF point mutation-type Bama miniature pig constructed by the sgRNA, and application. The sgRNA comprises a nucleic acid sequence as shown in SEQ ID NO: 1 or SEQ IDNO: 2. According to the invention, a scheme of combining CRISPR / Cas9 with homologous recombination is adopted, point mutation of CREBRF gene is carried out, the designed gRNA has specific binding property on a target site near the 1370th site of the CEREBRF gene, the CRISPR / Cas9 can be guided to carry out specific recognition cutting near the target site, double-stranded DNA breakage is formed, homologous recombination repair with exogenous single-stranded DNA as a template is promoted, and therefore the point mutation is introduced. The mutant Bama miniature pig prepared by the method has a typical obesity phenotype and can be used as a model organism to be applied to the field of research and development of medicines for treating obesity and diabetes.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
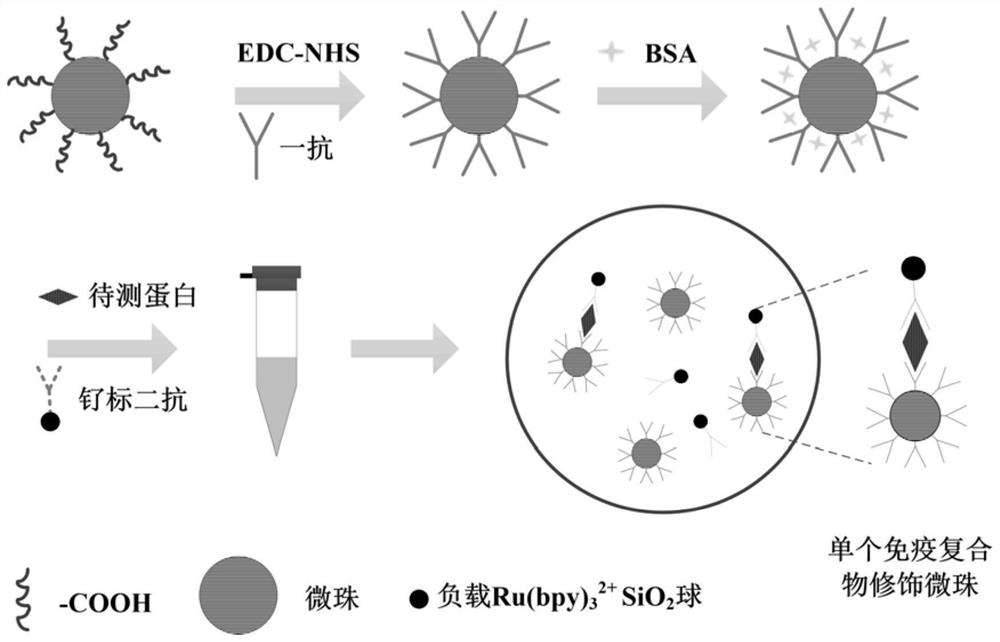
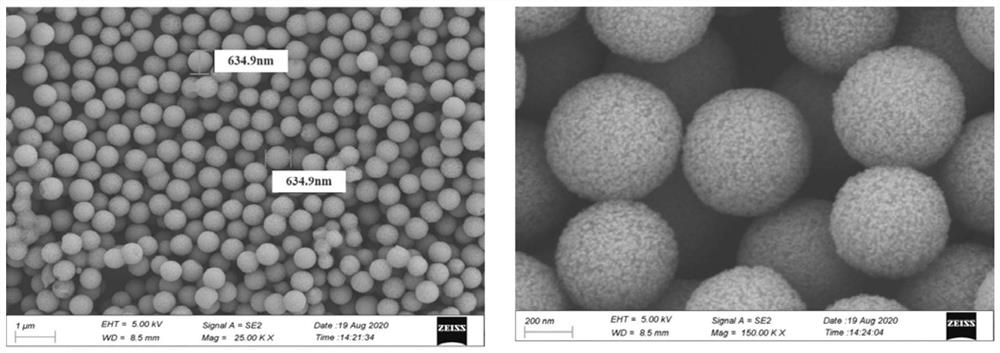
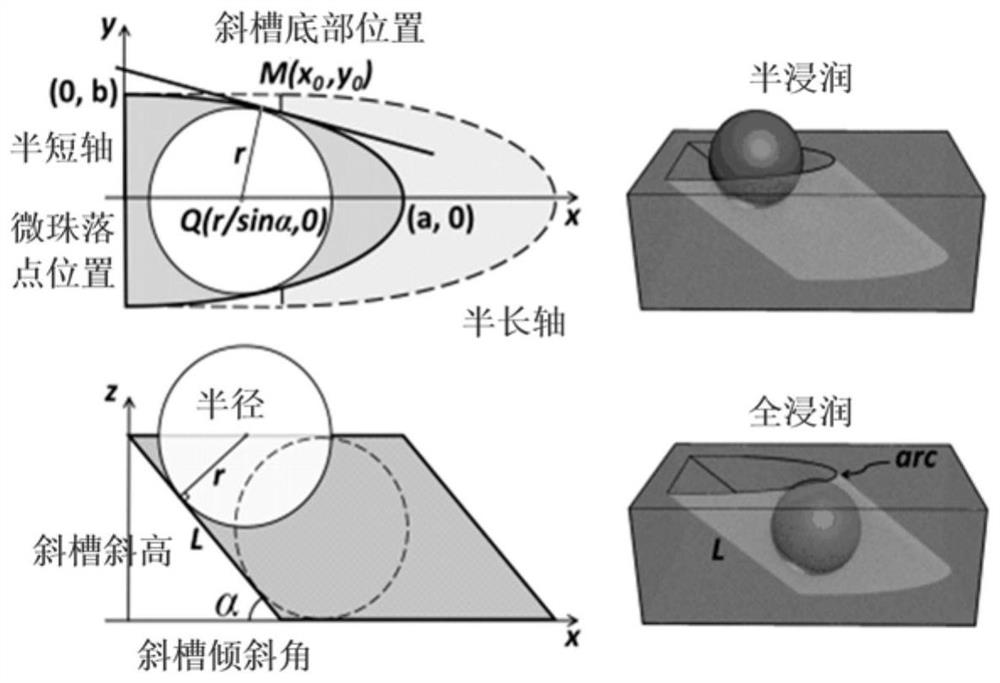
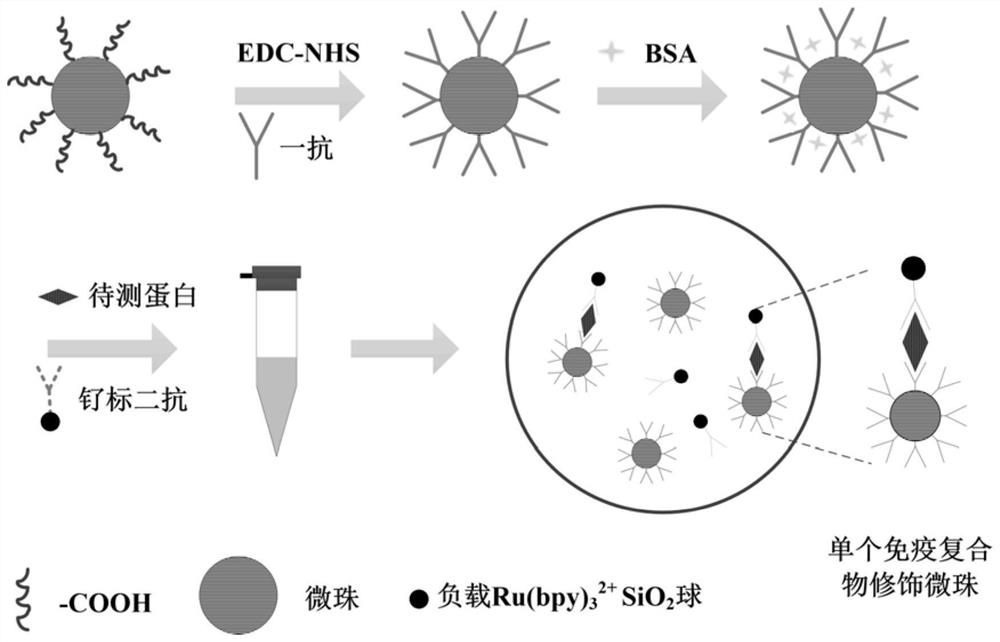
Monomolecular protein detection chip and method based on ultramicro electrode array electrochemiluminescence
ActiveCN112666240ASpecific bindingRealize the combinationMaterial analysis by electric/magnetic meansBiological testingProtein detectionIndium tin oxide
The invention relates to a monomolecular protein detection chip and a monomolecular protein detection method based on ultramicro electrode array electrochemiluminescence. The monomolecular protein detection chip is provided with a substrate of an ITO (indium tin oxide) electrode and an inclined micro trap array with a three-dimensional concave part on the ITO electrode, the two ends of the inclined micro trap array in the transverse direction are respectively provided with wires connected with the positive electrode and the negative electrode of a power supply. The single-molecule protein detection chip is simple to prepare, an electrochemical luminescence detection system further amplifies a signal, and high-sensitivity single-molecule detection of trace protein can be realized.
Owner:SHENZHEN INST OF ADVANCED TECH
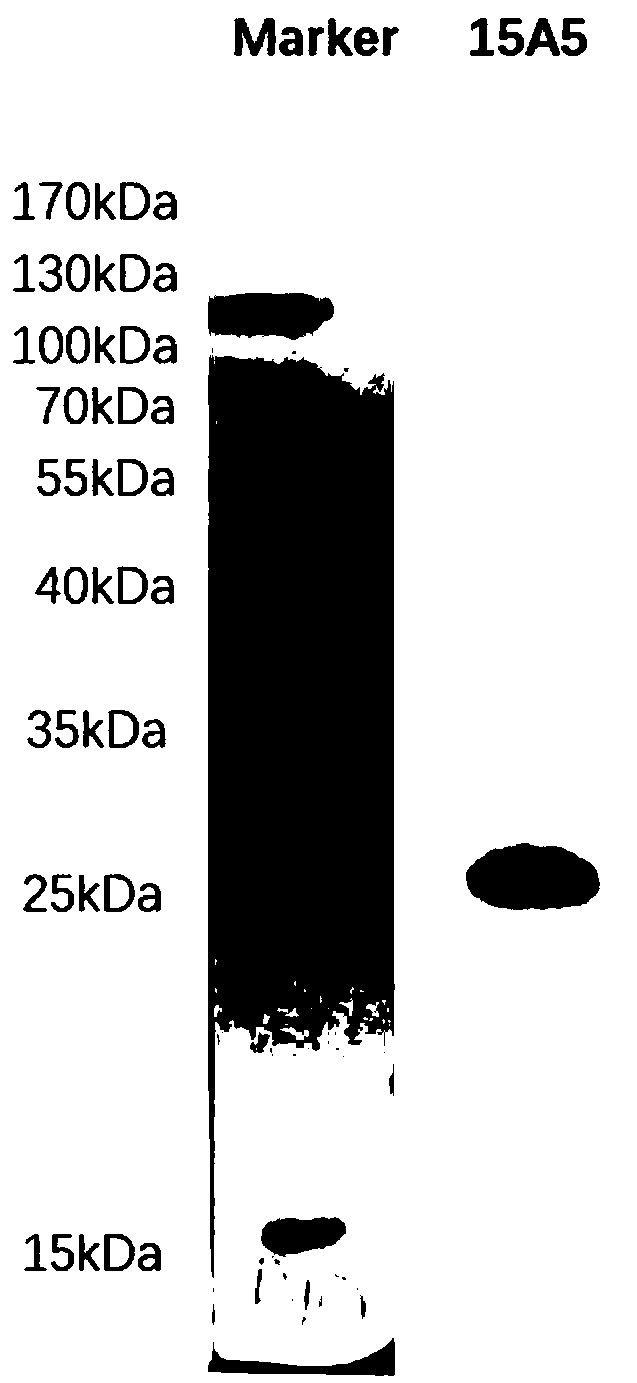
Monoclonal antibody capable of resisting helicobacter pylori protein, and cell line, preparation method and application thereof
ActiveCN108659125ASpecific bindingIncreased sensitivityImmunoglobulins against bacteriaTissue cultureSerum igeHelicobacter
The invention provides a monoclonal antibody capable of resisting helicobacter pylori protein, and a cell line, a preparation method and application thereof. The ultrasound supernatant part of cultured helicobacter pylori mycoprotein is taken as immunogen to immunize a mice, an antibody15A5 capable of specifically combining with the helicobacter pylori protein is obtained after screening, and theantibody is an IgG2a subtype. The antibody can be used for detecting the infected helicobacter pylori in tissues or serum.
Owner:FUZHOU MAIXIN BIOTECH CO LTD
TIM-3 nanobody as well as preparation method and application thereof
ActiveCN111253489AReduce dosageEfficient combinationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenTransient transfection
The invention belongs to the technical field of resistant substances from animals or humans, and discloses a TIM-3 nanobody as well as a preparation method and application thereof. The nanobody is TIM-3 nanobody, and the sequence is shown in SEQ ID NO:1; and a TIM-3 antigen is expressed by mammalian cell transient transfection, a nanobody library is screened for multiple times by using the TIM-3 antigen to obtain specific nanobody phage, and sequencing is performed to obtain a target fragment. An HEK293 cell line is used to express the antigen, a mammalian expression system is used to expresshuman protein to guarantee the original structure of the protein to the maximum extent to ensure that the protein has post-translational modification and specific modification, such as glycosylation of eukaryotic protein, so that the obtained protein has higher activity; the method guarantees the original structure and activity of the protein to the greatest extent; and the screened nanobody can efficiently and specifically bind to a target.
Owner:SHIHEZI UNIVERSITY
Light-chain variable region and heavy-chain variable region of FMU-EPCAM-4F6 monoclonal antibody
ActiveCN101817881ASpecific bindingProving uniquenessImmunoglobulins against animals/humansGenetic engineeringBALB/cHeavy chain
The invention discloses a light-chain variable region and a heavy-chain variable region of an FMU-EPCAM-4F6 monoclonal antibody, wherein an amino acid sequence of the light-chain variable region of the FMU-EPCAM-4F6 monoclonal antibody is shown as SEQ ID NO.1, and an amino acid sequence of the heavy-chain variable region of the FMU-EPCAM-4F6 monoclonal antibody is shown as SEQ ID NO.2. In the invention, a group of mouse anti-human EPCAM monoclonal antibodies is prepared by a recombinant human EPCAM immune BALB / c mouse, hybridoma cell strains which can stably secrete high-affinity anti-human EPCAM monoclonal antibodies are screened, and the high-affinity anti-human EPCAM monoclonal antibodies are obtained by preparing ascitic fluid. The invention confirms the uniqueness of a gene sequence and a corresponding protein sequence as well as a CDR sequence and provides support for anti-body EPCAM chimeric or humanized gene engineering antibodies.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Anti-helicobacter pylori protein monoclonal antibody and its cell line, preparation method and application
ActiveCN108659125BSpecific bindingIncreased sensitivityImmunoglobulins against bacteriaTissue cultureAntiendomysial antibodiesProtein.monoclonal
The invention provides a Helicobacter pylori antibody and its secreting cell line, preparation method and application. In the present invention, the ultrasonic supernatant of the cultured Helicobacter pylori protein is used as an immunogen to immunize mice, and the antibody 15A5 that can specifically bind to the Helicobacter pylori protein is obtained after screening, and the antibody is IgG2a subtype. This antibody detects infected H. pylori in tissue or serum.
Owner:FUZHOU MAIXIN BIOTECH CO LTD
A method for repairing heavy metal pollution in farmland soil
ActiveCN110014032BImprove efficiencyReduce risk of leachingContaminated soil reclamationPlant rootsSoil science
Owner:BCEG ENVIRONMENTAL REMEDIATION CO LTD
Light-chain variable region and heavy-chain variable region of FMU-EPCAM-4F6 monoclonal antibody
ActiveCN101817881BSpecific bindingProving uniquenessImmunoglobulins against animals/humansGenetic engineeringBALB/cHeavy chain
The invention discloses a light-chain variable region and a heavy-chain variable region of an FMU-EPCAM-4F6 monoclonal antibody, wherein an amino acid sequence of the light-chain variable region of the FMU-EPCAM-4F6 monoclonal antibody is shown as SEQ ID NO.1, and an amino acid sequence of the heavy-chain variable region of the FMU-EPCAM-4F6 monoclonal antibody is shown as SEQ ID NO.2. In the invention, a group of mouse anti-human EPCAM monoclonal antibodies is prepared by a recombinant human EPCAM immune BALB / c mouse, hybridoma cell strains which can stably secrete high-affinity anti-human EPCAM monoclonal antibodies are screened, and the high-affinity anti-human EPCAM monoclonal antibodies are obtained by preparing ascitic fluid. The invention confirms the uniqueness of a gene sequence and a corresponding protein sequence as well as a CDR sequence and provides support for anti-body EPCAM chimeric or humanized gene engineering antibodies.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
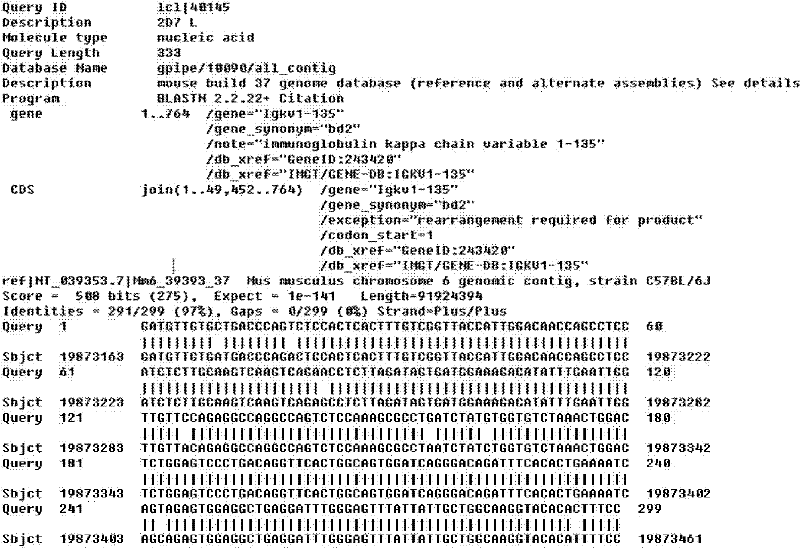
Light chain and heavy chain variable region of FMU-EPCAM-2D7 monoclonal antibody
InactiveCN101899112BSpecific bindingProving uniquenessImmunoglobulins against animals/humansFermentationBALB/cFhit gene
The present invention discloses light chain and heavy chain variable regions of an FMU-EPCAM(Epithelial Cell Adhesion Molecule)-2D7 monoclonal antibody, wherein an amino acid sequence of the light-chain variable region of the FMU-EPCAM-2D7 monoclonal antibody is disclosed as SEQ ID NO.1; and an amino acid sequence of the heavy-chain variable region is disclosed as SEQ ID NO.2. A group of mouse antihuman EPCAM monoclonal antibodies are prepared by recombinating human EPCAM immune BALB / c mousses, ascitic fluid is prepared by screening hybridoma cell lines which can stably secrete high-affinity antihuman EPCAM monoclonal antibodies, and the high-affinity antihuman EPCAM monoclonal antibodies can be obtained. The present invention ensures the uniqueness and a CDR sequence thereof of a gene sequence and a corresponding protein sequence and provides a support for antihuman EPCAM chimerisms or humanized genetic engineering antibodies.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Carbendazim monoclonal antibody, preparation method and application thereof
InactiveCN102675463BSpecific bindingMeet dose dependenceMicroorganism based processesTissue cultureAntigenAscitic fluid
The invention discloses a carbendazim monoclonal antibody, a preparation method and an application thereof. The carbendazim monoclonal antibody is prepared by a mouse hybridoma cell strain MC-3 with a preservation number of CGMCC N0.4575 and the preparation method is as follows: (1) introducing ninth amino acid residue of carbendazim into one amino group to obtain the carbendazim modified by the amino and coupling the carbendazim modified by the amino with a carrier protein to obtain a complete antigen A; (2) immunizing a mouse by the complete antigen A obtained in step (1), taking splenocyte of the immune mice to fuse with a mouse myeloma cell to screen out a positive hybridoma cell strain; extensively culturing the positive hybridoma cell strain and injecting the positive cell strain into a homologous mouse abdominal cavity to induce to generate ascitic fluid and to obtain more carbendazim monoclonal antibodies. The Carbendazim monoclonal antibody can be used for detecting the carbendazim.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
A single-molecule protein detection chip and method based on ultramicroelectrode array electrochemiluminescence
ActiveCN112666240BSpecific bindingRealize the combinationMaterial analysis by electric/magnetic meansBiological testingProtein detectionEngineering
The invention relates to a single-molecule protein detection chip and method based on electrochemiluminescence of an ultra-micro electrode array, and specifically discloses that the single-molecule protein detection chip has a substrate with an ITO electrode and a slanted microstructure with a three-dimensional concave on the ITO electrode. Well array; two ends in the lateral direction of the inclined micro well array are respectively provided with wires connected to the positive and negative electrodes of the power supply. The single-molecule protein detection chip of the invention is simple to prepare, the electrochemiluminescence detection system further amplifies the signal, and can realize the highly sensitive single-molecule detection of trace proteins.
Owner:SHENZHEN INST OF ADVANCED TECH
Application of CAF22 detection reagent in preparation of composition for evaluating renal tubular injury
ActiveCN114578064ASpecific bindingHigh sensitivityDisease diagnosisBiological testingBiologic markerRenal Tubule Epithelium
The invention discloses application of a compact protein C-terminal fragment (CAF22) detection reagent in preparation of a renal tubule injury diagnostic kit and the renal tubule injury diagnostic kit, and provides a new biomarker for diagnosis of renal tubule injury by using the CAF22 as a diagnostic marker of the renal tubule injury. According to the invention, CAF22 detection is combined with other renal injury biomarkers, so that the accuracy and sensitivity of early diagnosis of renal tubule injury can be improved, and the kit has high application value in early diagnosis and treatment of renal injury.
Owner:BEIJING WATSON-CESAR BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com