Monoclonal antibody resistant to SasA (staphylococcus aureus surface protein A) antigen and application of monoclonal antibody
A technology of monoclonal antibody and staphylococcus, applied in the fields of application, antibody, antibacterial drug, etc., can solve the problems of limited protection, unsatisfactory protection of monoclonal antibody, and no surface protein antigen can be found, and achieve highly specific binding activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of Monoclonal Antibody to SasA Protein
[0036] 1. Division of different structural domains of SasA protein
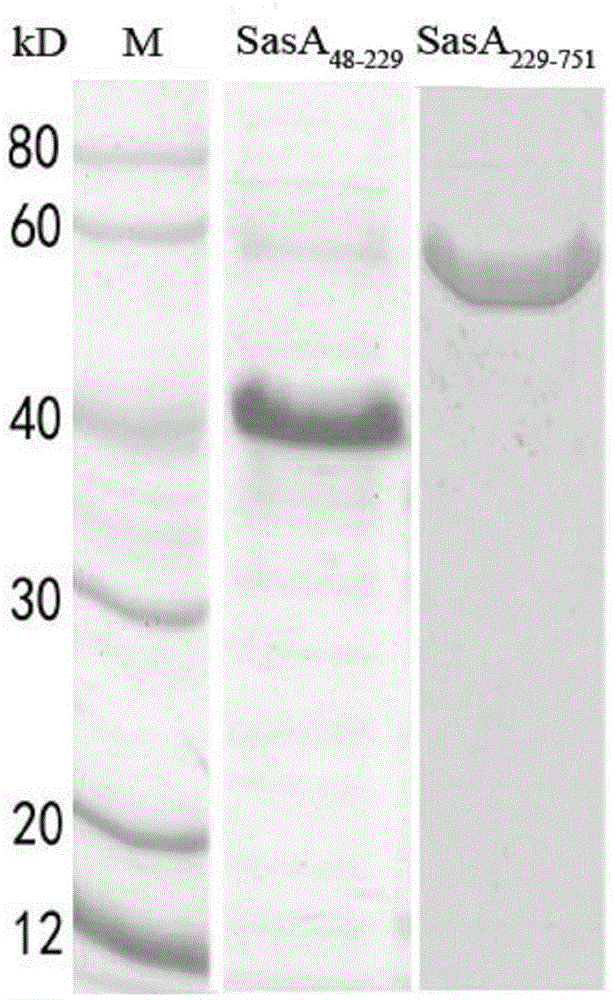
[0037] The SasA protein has a full length of 2271 amino acids and belongs to the SRRP protein (Serine-rich repeat protein) family of Gram-positive bacteria. According to bioinformatics analysis, the SasA protein can be divided into five main structural domains, which are N-terminal signal peptide, serine-rich repeat region 1 (amino acid sequence 48-229), non-repeat region (amino acid sequence 229-751 amino acid sequence), serine-rich repeat region 2 (amino acid sequence 752-2213) and the cell wall anchor sequence at the C-terminus. where SasA 752-2213 Mainly localized in the capsular polysaccharide of Staphylococcus aureus, while SasA 48-229 and SasA 229-751 localizes on the bacterial surface, thus predicting SasA 48-229 and SasA 229-751 It is the main functional domain of SasA protein.
[0038] 2. SasA 48-229 and SasA 229-751 gene...
Embodiment 2
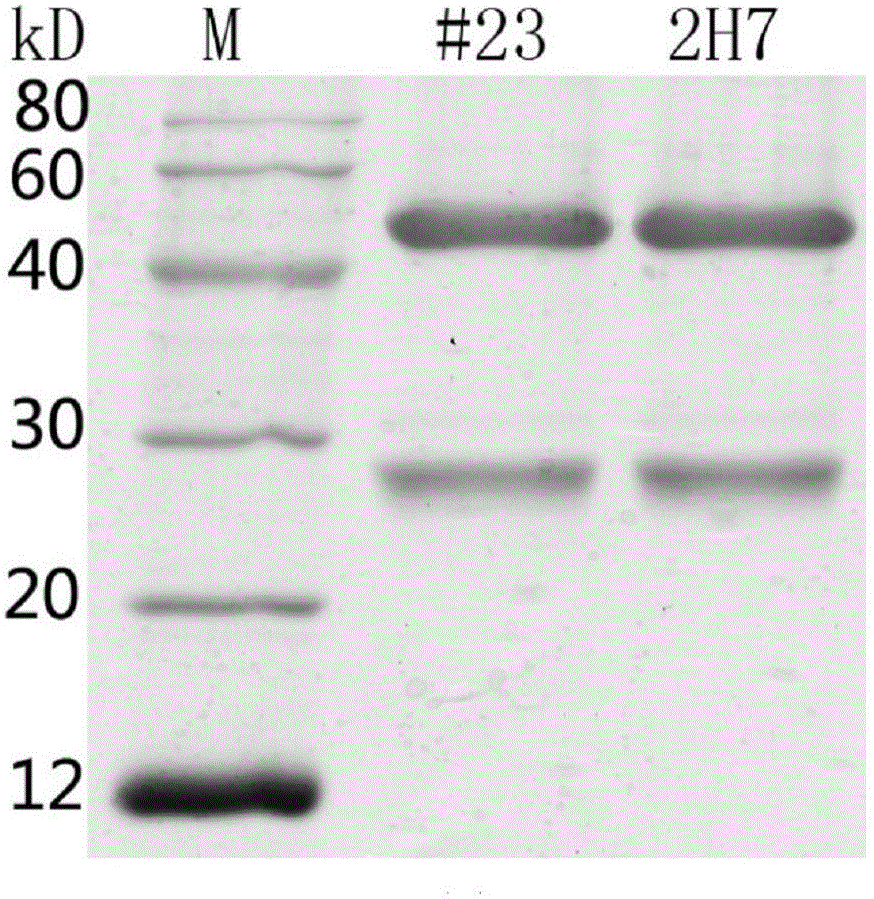
[0055] Example 2. ELISA detection of binding activity of monoclonal antibody #23 and 2H7 to SasA protein
[0056] The SasA protein fragment was coated with 100 μl per well at a concentration of 2 μg / ml, and left overnight at 4°C. After washing 3 times with ELISA washing solution PBST (PBS+0.1% Tween-20), 100 μl of fixative solution (PBST+2% BSA) was added to each well, and blocked at 37° C. for 1 hour. Wash 3 times with PBST, dilute the monoclonal antibody sample with diluent (PBST+0.2% BSA) and add 100 μl to each well, and incubate at 37° C. for 1 h. Wash 4 times with PBST, add 100 μL of horse anti-mouse HRP secondary antibody (CST, #7076) diluted 1:10,000 times to each well, and incubate at 37°C for 1 hour. Wash 4 times with PBST, add TMB to carry out color reaction, stop reaction with 2mol / L sulfuric acid after color development for 5 minutes. Finally, the absorbance value at 450nm is detected on a microplate reader, and the results are shown in Figure 7 . The monoclon...
Embodiment 3
[0057] Example 3. The protective effect of monoclonal antibodies #23 and 2H7 on the challenge of Staphylococcus aureus USA300 strain in mice
[0058] Use 6-week-old BALB / c female mice and divide them into 5 groups (#23 monoclonal antibody group, 2H7 monoclonal antibody group, #23+2H7 monoclonal antibody group, isotype monoclonal antibody control group, PBS control group), 8 mice in each group . #23 monoclonal antibody group intraperitoneally injected monoclonal antibody #23400ug / body, 2H7 monoclonal antibody group intraperitoneally injected monoclonal antibody 2H7400ug / mouse, #23+2H7 monoclonal antibody group intraperitoneally injected #23 and 2H7 monoclonal antibody 200ug / mouse, same type The monoclonal antibody control group was intraperitoneally injected with IgG1 and IgG2b isotype control monoclonal antibodies at 200ug / mouse, and the PBS control group was injected with the same volume of PBS (200ul). After 24 hours of monoclonal antibody injection, the cultured Staphyloco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com