Monoclonal antibody of antiplague bacillus F1 antigen and application of monoclonal antibody
A monoclonal antibody, F1 antigen technology, applied in applications, antibodies, antibacterial drugs, etc., can solve the problems of poor affinity and specificity of monoclonal antibodies, no mention of protective effect, low immunogenicity, etc., and achieve high specific binding activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
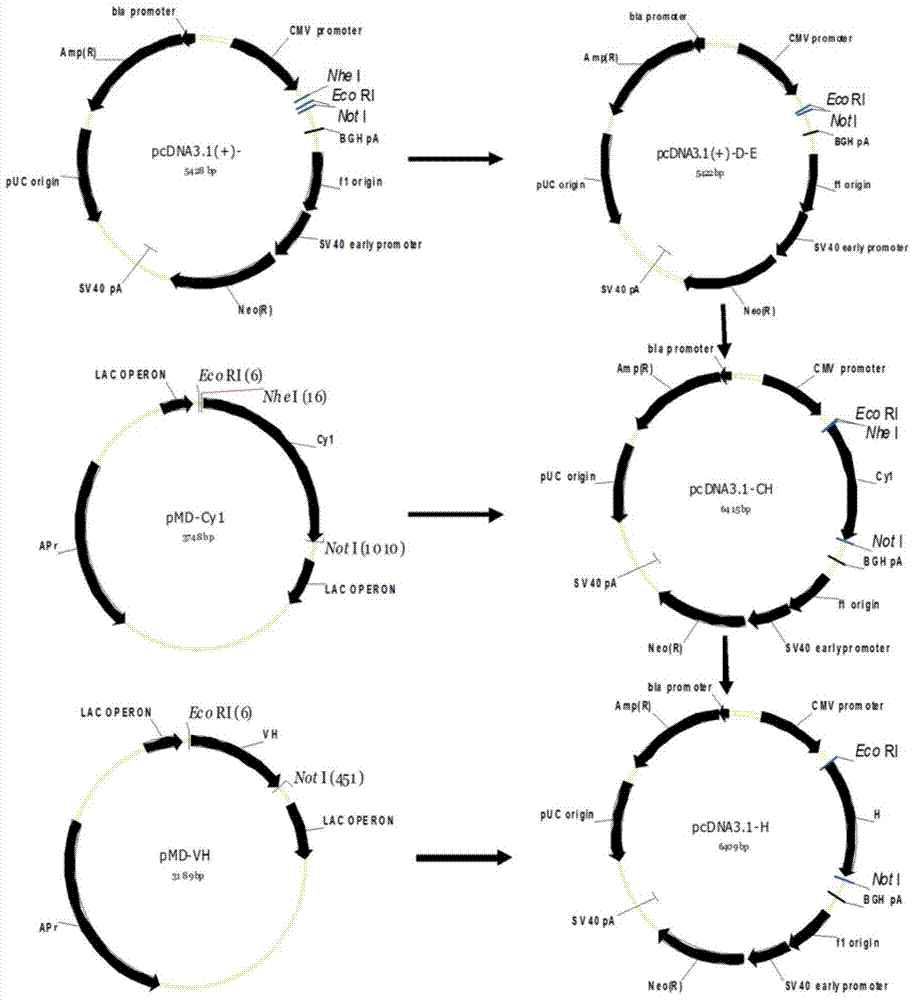
[0029] The preparation of embodiment 1 chimeric antibody
[0030] 1. Cloning of mouse monoclonal antibody gene
[0031] 1.1 Preparation of mouse monoclonal antibody
[0032] Using recombinant plague capsular antigen F1 as the immunogen, a high-affinity and high-specificity mouse-derived anti-F1 monoclonal antibody F2H5 hybridoma cell line was obtained by conventional monoclonal antibody preparation methods, and the subtype of the secreted antibody molecule is IgG1 Subclass, the light chain is the Kappa subtype.
[0033]1.2 Cloning of the variable region gene of the monoclonal antibody F2H5 light and heavy chain
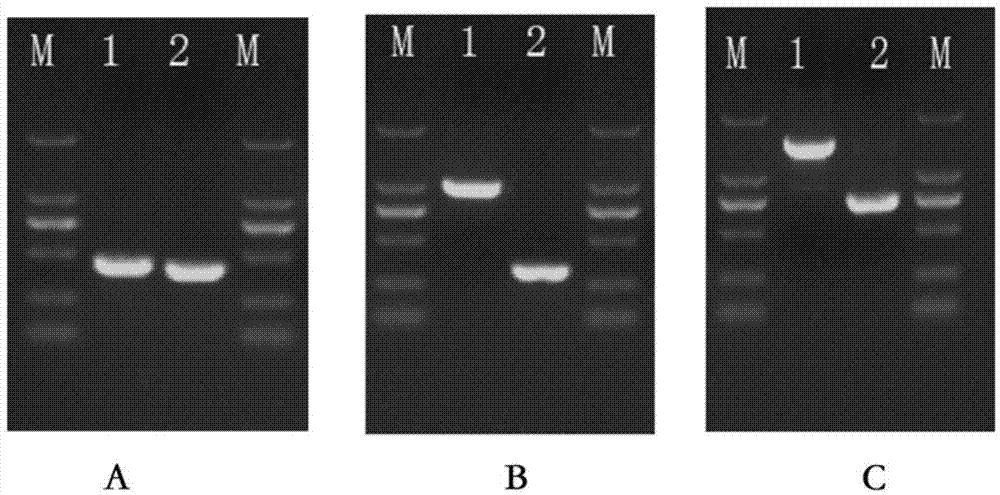
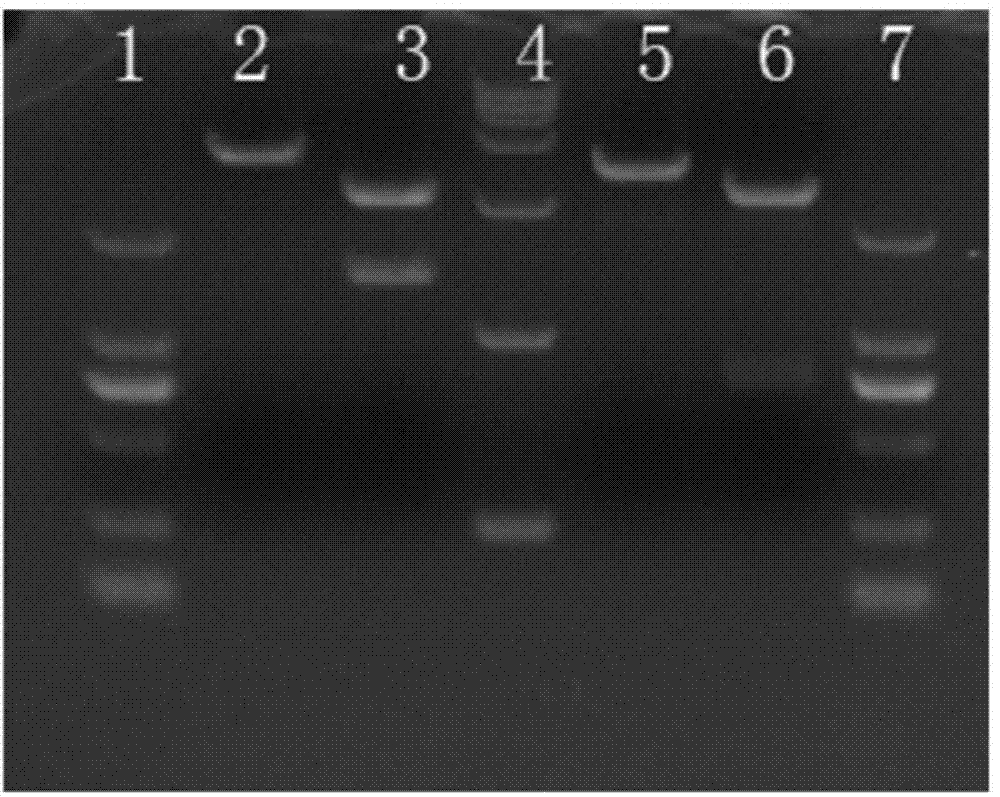
[0034] The monoclonal antibody is composed of a variable region and a constant region. Because the gene variation of the variable region of the mouse monoclonal antibody is large, and the gene sequence of the constant region is very conservative, the 5'-RACE method is used for cloning. A primer sequence was added to the 5' end, and PCR amplification was performed w...
Embodiment 2
[0095] Example 2 Preparation of mouse-derived monoclonal antibody from ascites and analysis of its binding activity with antigen
[0096] 7-10 days before the inoculation of the tumor strain, each mouse was intraperitoneally injected with 0.5 ml of paraffin oil, and the abdomen of the mouse was gently rubbed with a cotton ball to fully diffuse the paraffin oil in the mouse abdominal cavity. Prepare hybridoma cells, recover and observe their growth status, select those cultures with good cell shape and activity, when they are in the logarithmic growth phase, gently blow down with RPMI l640, centrifuge and remove bovine serum in the culture medium , suspended in saline, and injected into the peritoneal cavity of human mice. The amount of cells injected into each mouse l0 5 –l0 6 . 8-10 days after the inoculation of the tumor strain, there may be accumulation of ascites. When the abdomen is obviously swollen, the ascites should be collected. Centrifuge 4mL of ascitic fluid at...
Embodiment 3
[0097] Embodiment 3.ELISA detects the binding activity of chimeric antibody and F1
[0098] Dilute the recombinant F1 to 2μg / ml, coat the enzyme-linked plate with 100ul / well, overnight at 4°C, block with blocking solution (20mM PB, 0.15M NaCl, 5% milk powder) the next day at 37°C for 1h, wash the plate, and add serial dilutions Wash the plate after incubating at 37°C for 1 hour, add 1:1 000 diluted HRP-labeled goat anti-human Fc (Sigma, A0170) and incubate at 37°C for 1 hour, wash the plate, develop color with OPD, and measure OD with a microplate reader 450 . See Figure 9 . Serially diluted monoclonal antibodies 256, 128, 64, 32, 16, 8, 4, 2ng / ml react with coated recombinant F1, showing a dose-effect response, with a sensitivity of 4-8ng / ml, and recombinant anthrax protective antigen PA, Recombinant plague V antigen, recombinant tetanus antigen, etc. did not react (not shown in the figure). It shows that the monoclonal antibody against plague F1 antigen provided by the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com