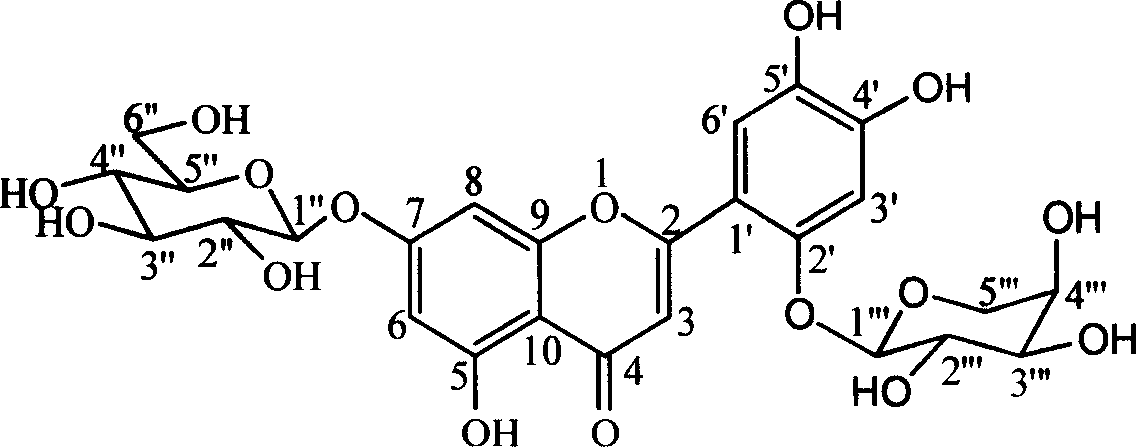
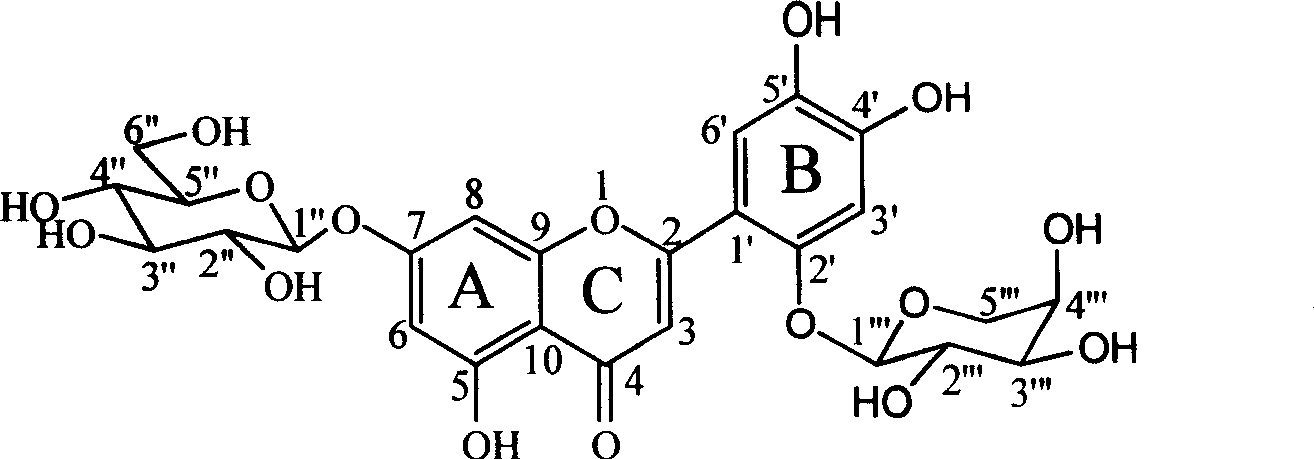
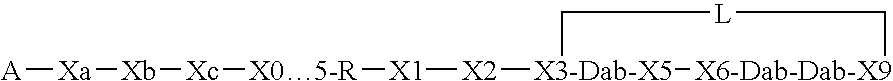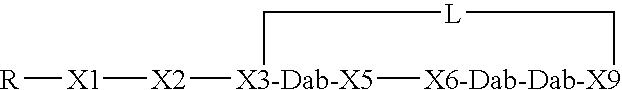Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
89 results about "Bacteremia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bacteremia (also bacteraemia) is the presence of bacteria in the blood. Blood is normally a sterile environment, so the detection of bacteria in the blood (most commonly accomplished by blood cultures) is always abnormal. It is distinct from sepsis, which is the host response to the bacteria.
Chemotherapy treatment
InactiveUS20030040478A1Improve welfareImprove survivalBiocideOrganic active ingredientsRegimenApoptosis
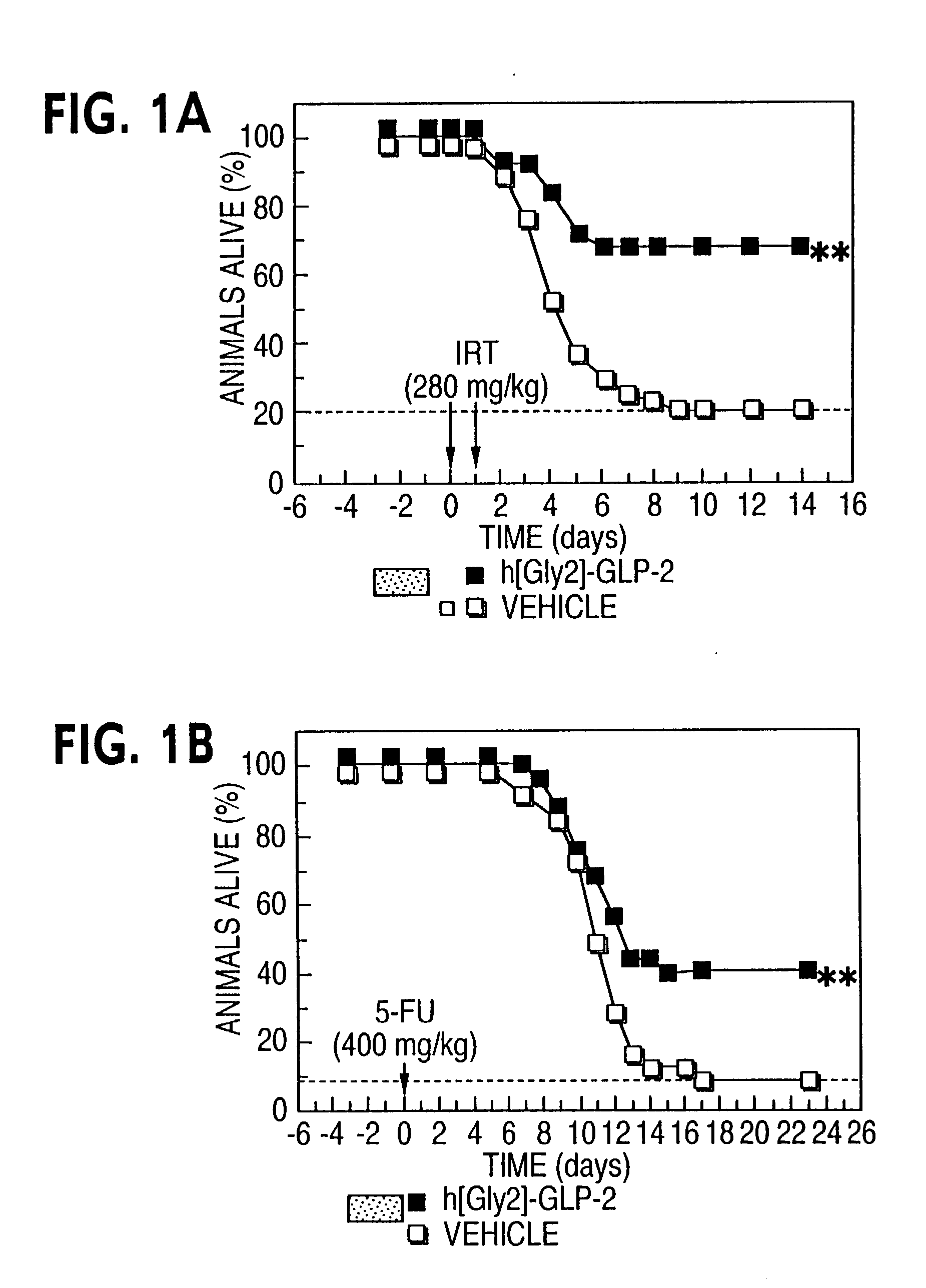
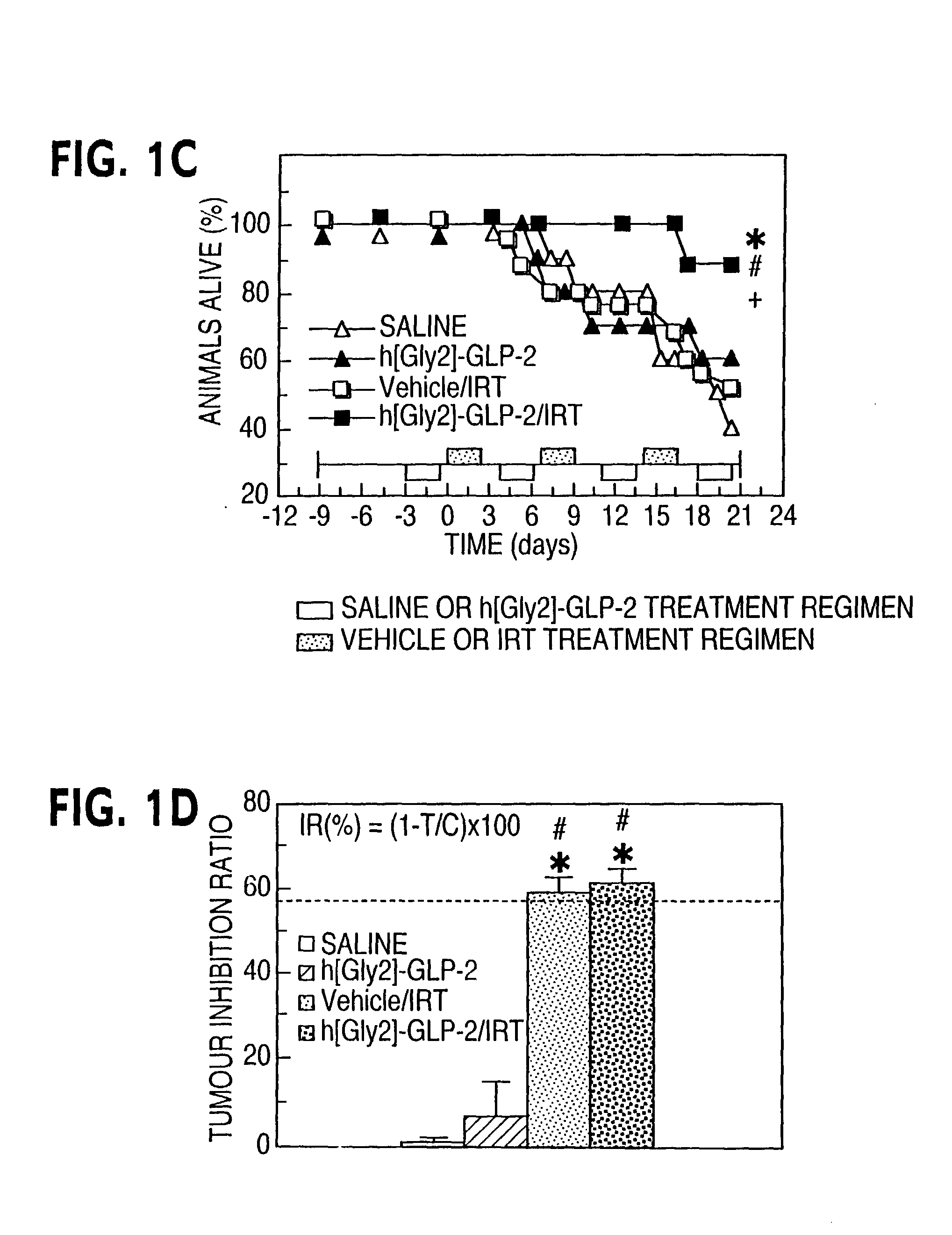
This invention provides a treatment regimen that is effective in inhibiting chemotherapy-induced apoptosis and promoting cell survival. The invention also relates to a treatment regimen that confers resistance to caspase activation, thereby inhibiting caspase-mediated, proteolytic cleavage of functional cellular enzymes. Specifically, subjects undergoing chemotherapy are first exposed to a pretreatment regimen. Under this regimen, a GLP-2 receptor activator, such as h[GLY2]-GLP2, is administered each day for a predetermined beneficial period, e.g., three consecutive days. Approximately about 1 week following pretreatment, the subjects are exposed to an appropriate chemotherapy treatment regimen. Pretreatment with a GLP-2 receptor activator followed by administration of chemotherapeutic agents improves cell survival, reduces bacteremia, attenuates epithelial injury, and inhibits cellular apoptosis. Moreover, it does not impair the effectiveness of chemotherapy nor result in weight loss. The anti-apoptotic effects of GLP-2 may be useful in the reduction of cytoxicity and bacterial infection induced by chemotherapeutic agents.
Owner:1149336 ONTARIO
Compounds pharmaceutical compositions and methods for treatment of bacteremia and/or septicemia
Novel conjugates of bacterial outer membrane binding peptides, preferably having bacterial sensitization activity, and immune cells chemotactic peptides, and pharmaceutical compositions containing same useful in the treatment of bacteremia and / or septicemia following infection by gram negative bacteria administered alone or in combination with conventional antibiotics.
Owner:RAMOT AT TEL AVIV UNIV LTD +1
Antibacterial oral rinse formulation for preventing coronary artery disease
Owner:BONFIGLIO RICHARD PAUL
Streptococcus pneumoniae vaccines
Streptococcus pneumoniae is a major cause of pneumoniae, meningitis, and major cause of morbidity and mortality throughout the world by bacterial otitis media, pneumoniae, meningitis, and bacteraemia. It is an important agent of disease in man especially among infants, the elderly and immunocompromised persons. The present invention provides a solution to this problem by providing a substantially pure or isolated disease related antigen selected from the group consisting of the isolated, recombinant or synthetic S. pneumoniae human immunogenic antigens of SP_0562, SP_0965, SP_0082 (in particular the fragments SP4 and SP 17 of said SP_0082), and a Periplasmic Binding Protein (PBP) (in particular SP_1683 or SP_1386), or a fragments thereof or substantially identical antigen for use in a treatment to induce a immunological memory in a human against S. pneumoniae cells for use in a vaccination treatment of S. pneumoniae disorder in human or against S. pneumoniae in a human. In a particular embodiment, the present invention provides an isolated, recombinant or synthetic S. pneumoniae Periplasmic Binding Protein (PBP) (in particular SP_1683 or SP_1386) as a disease related antigen for use in a treatment to induce a immunological memory in a human against S. pneumoniae cells for use in a vaccination treatment of S. pneumoniae disorder in human or for use in the treatment of an S. pnewnoniae infection in a human. It further provides antibodies that specifically bind to the S. pneumoniae disease related antigens identified herein for use in the treatment of a an S. pneumoniae infection in a human, such as for example in a treatment to induce immunological memory in a human against S. pneumoniae, i.e. in a vaccination treatment of S. pneumoniae. It is also an aspect of the present invention to provide the use of any one of the S. pneumoniae disease related antigens as identified herein, or of the antibodies specific for said antigens in methods to diagnose for a S. pneumoniae disorder in a human.
Owner:KATHOLIEKE UNIV LEUVEN
Methods and Compositions for Treating Bacterial Infection
ActiveUS20100136027A1Enhance immune responseStabilizes and reduces symptomAntibacterial agentsBacteriaEscherichia coliBacteremia
The present invention relates to methods and compositions for the treatment of bacterial infection, for example extraintestinal E. coli infection such as E. coli bacteremia, meningitis and sepsis. The invention relates also to methods of diagnosis and prevention.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method of treating staphylococcus aureus infection
InactiveUS20060153857A1Antibacterial agentsOrganic active ingredientsMonoclonal antibodyPolyclonal antibodies
The present invention provides a method of preventing or treating bacteremia caused by Staphylococcus aureus, comprising administering a monoclonal or polyclonal antibody composition comprising antibodies specific for one or more S. aureus antigens. In one specific embodiment, the composition is a hyperimmune specific IGIV composition. In another specific embodiment, the composition comprise antibodies to a capsular polysaccharide S. aureus antigen, such as the Type 5 and / or Type 8 antigens. In another embodiment, the composition comprises monoclonal antibodies to a capsular polysaccharide S. aureus antigen. This method provides an effective tool for preventing or treating S. aureus bacteremia, and can be used alone or in combination with other therapies.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Method for identifying 28 frequent phthogenic bacteria for clinical bacteremia
InactiveCN1814797AImprove identification efficiencyImprove throughputMicrobiological testing/measurementOrganismBiology
This invention relates to a method for identifying 28 kinds of common pathogens of clinical bacteremias, which crosslinks 30 types of specific probes against the 28 kinds of common pathogens on 30 kinds of different fluorescent microspheres to be reacted with the being tested specimens then to be reacted with the report molecules labeled by fluorescent elements to test the specific 23SrDNA of them on a fluorescent test device.
Owner:ZHEJIANG UNIV
Lignin in dandelion, its bacteria-resisting activity and use for medicine
The invention relates to a lignin compound that could prevent yellow staphylococcal bacteria and B type hemolytic streptococcus, and the medicine combination of the compound. The invention of lignin Morgan dandehon herb acid A and Rufescidride has obviously effect restraining yellow staphylococcal and B type hemolytic streptococcus. It is also respected to prevent the diseases caused by them, like boil, blain, toxicity epidermis necrolysis, pneumonia, etc.
Owner:WENZHOU MEDICAL UNIV
Combination antibiotic and antibody therapy for the treatment of pseudomonas aeruginosa infection
InactiveUS20100272736A1Effective treatmentHigh sensitivityAntibacterial agentsTetracycline active ingredientsMedicineAntibiotic Y
The present invention provides improved pharmaceutical compositions and methods of treating or preventing development of bacteremia associated with Pseudomonas aeruginosa infections, where the method comprises administering an antibiotic and an anti-PcrV antibody.
Owner:KALOBIOS PHARMA +1
Attractive ureteroscope
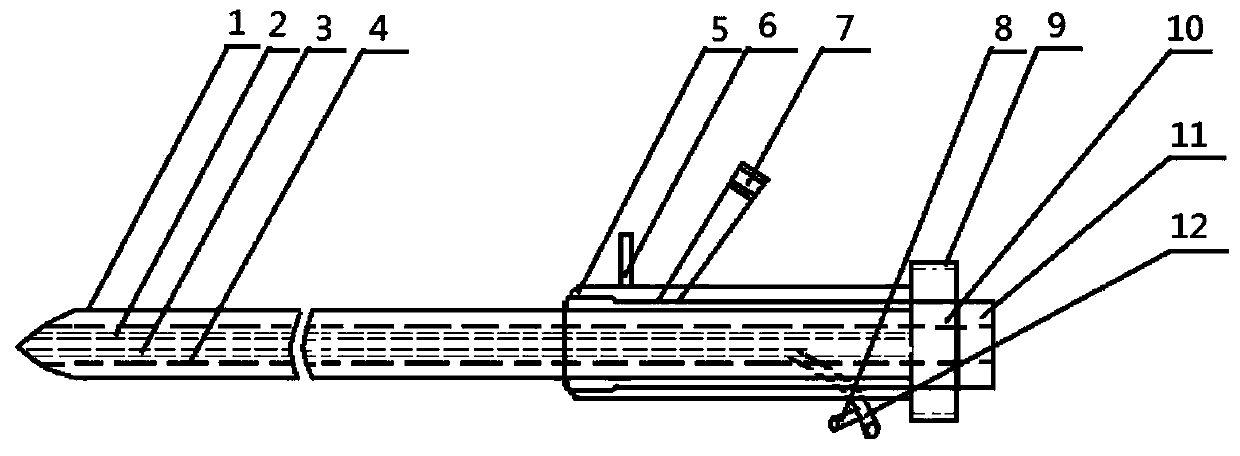
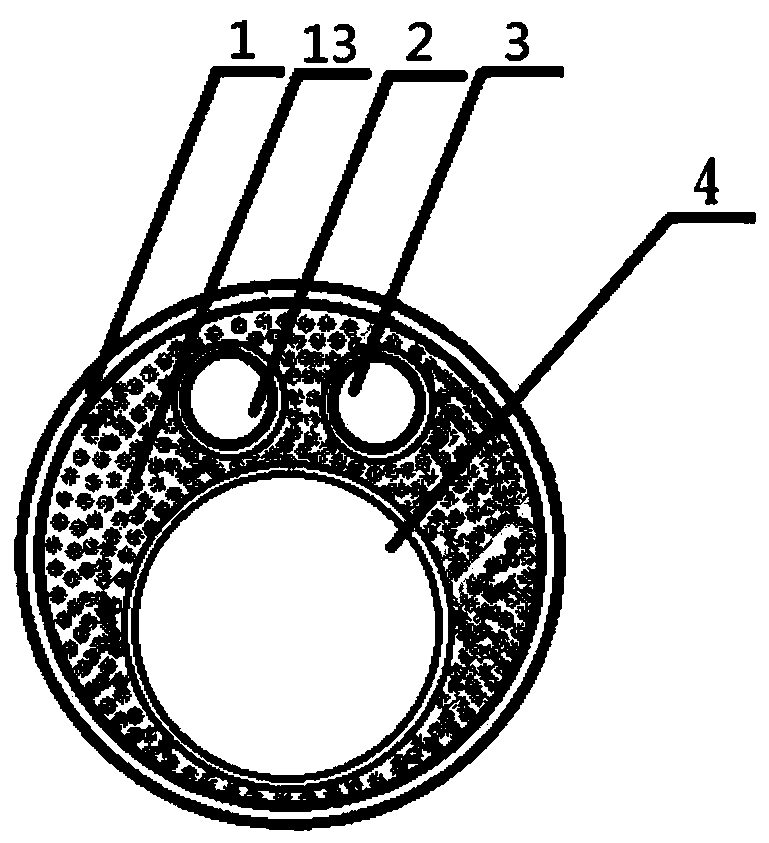
The invention relates to a urological surgical medical device, in particular to an attractive ureteroscope. An influent and holmium laser fiber channel is a cavity shared by the influent and holmium laser fibers during a surgery. An effluent and instrument channel penetrates the entire novel ureteroscope and is a cavity shared by the negative pressure effluent and instruments. An effluent and instrument channel joint is arranged on the rear portion in the ureteroscope, and the outer end of the effluent and instrument channel joint is provided with effluent and instrument channel joint threads.A sleeve of the effluent and instrument channel is arranged behind the effluent and instrument channel. The ureteroscope can accelerate the intraoperative drainage speed without affecting the surgical operation to form continuous water circulation in the surgical field, which can not only maintain the good definition of the visual field during the surgery, but also significantly improve the surgical lithotripsy efficiency. Further, the renal pelvis and ureteral pressure can be reduced to prevent postoperative bacteremia and urinary septicopyemia.
Owner:赣州市人民医院
Rapid antimicrobial susceptibility testing using high-sensitivity direct detection methods
PendingUS20190032104A1Bioreactor/fermenter combinationsBiological substance pretreatmentsBiologyBloodstream infection
The invention features methods, panels, cartridges, kits, and systems for rapid and sensitive detection and identification of pathogens and determination of the pathogen's susceptibility to antimicrobial agents for diagnosis and treatment of disease, including bloodstream infection (e.g., bacteremia and fungemia), and sepsis.
Owner:T2 BIOSYST
Group ABC meningococcus combined vaccine and preparing method thereof
PendingCN106215183AEase the pain of vaccinationEffective preventive effectAntibacterial agentsBacterial antigen ingredientsPericarditisMENINGOCOCCAL POLYSACCHARIDE
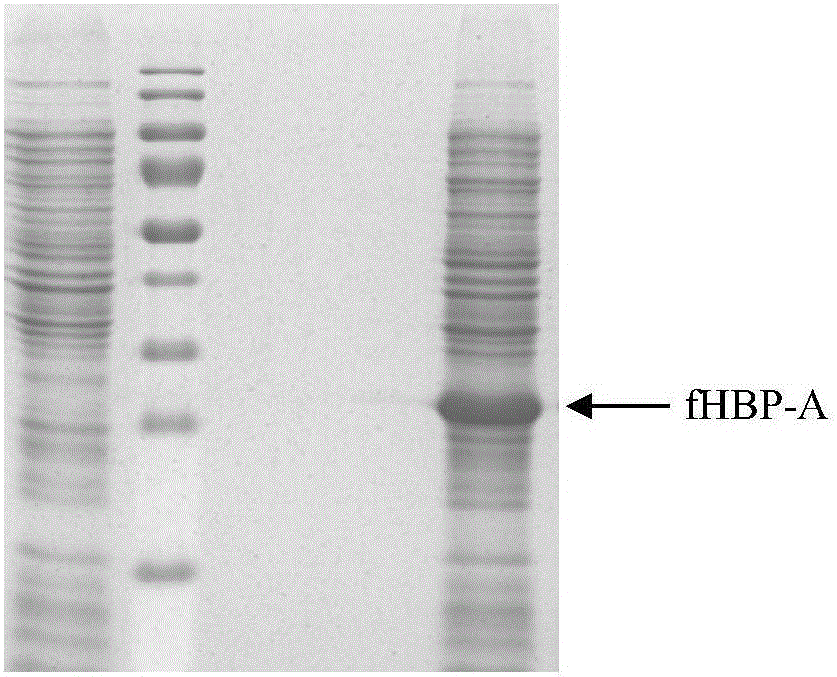
The invention provides a group ABC meningococcus combined vaccine and a preparing method thereof. The group ABC meningococcus combined vaccine is prepared from a group A and group C meningococcus polysaccharide-protein conjugate, and recombinant protein of a human H factor binding protein (fHBP) subgroup A and a human H factor binding protein (fHBP) subgroup B of group B meningococcus. The invention further provides a preparing method of the recombinant fHBP-A protein and fHBP-B protein. The combined vaccine is used for immunity of children 2 or more years old, and used for preventing invasive diseases such as cerebrospinal meningitis, bacteremia, pneumonia and pericarditis caused by group A or group B or group C meningococcus, and providing a better and wider protection effect on meningococcus.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +2
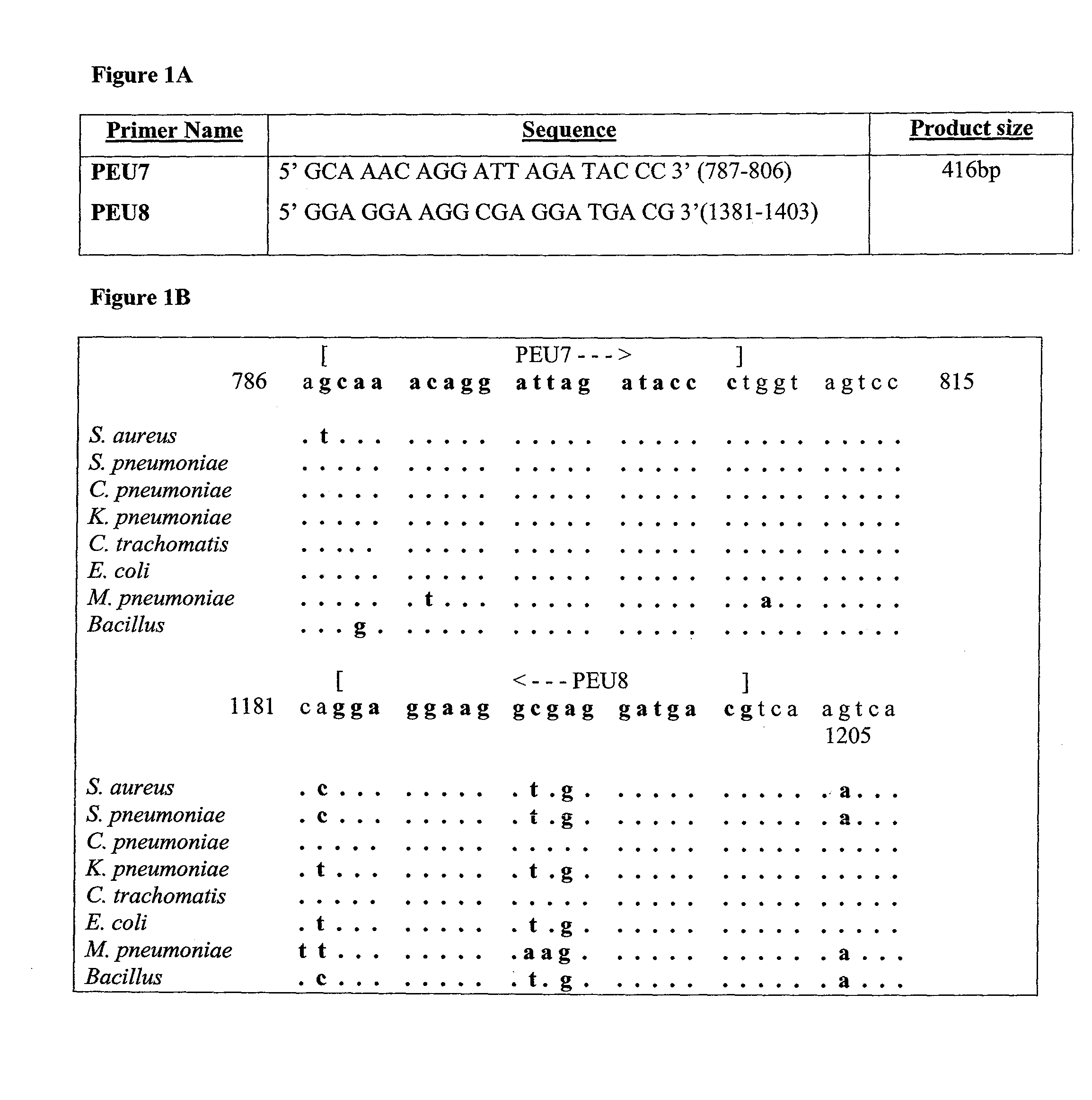
Molecular Diagnosis of Bacteremia
InactiveUS20110008791A1Sugar derivativesMicrobiological testing/measurementClinical settingsDigestion
A highly specific assay can be used for the detection of bacteremia in the clinical setting. The ubiquitous background endogenous DNA present in all PCR reagents is eliminated using a restriction endonuclease digestion. Universal primers for eubacteria are used for detection, and specific primers or probes for bacterial species can be used for identification of species.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Ureteroscope
InactiveCN101879056BReduce water pressureIncrease water pressureEndoscopesUrethroscopesURETEROSCOPEPore water pressure
The invention discloses a ureteroscope, comprising at least two parts of lenses and a lens body, wherein each part of the lenses are connected in sequence; the tail end of the rear part lens is connected with the lens body; communicated tube cavities are arranged in each part of the lenses and the lens body; the external wall of the front part lens is provided with a groove; a tube wall of the rear part lens is provided with a drainage channel; and the groove and the drainage channel are communicated with each other. By additionally arranged a water drainage channel, the invention can accelerate the water drainage speed during an operation, forms continuous water cycle, increases the water drainage amount during the operation on the premise of not influencing the execution in the operation, thus not only can keeping clear view during the operation process and improving operation efficiency, but also reducing water pressure in a kidney and an ureter, preventing occurrence of hyperpyrexia bacteremia after the operation and avoiding rise of the water pressure in a bladder.
Owner:SHANGHAI LINCHAO MEDICAL INSTR
Black purple Tuowusu and medical application in restraining grampostive bacteria
This invention relates to Ligulatrovine A separated from Ligularia actroviolacea that can prevent and treat diseases related to Staphylococcus aureus and beta hemolytic streptococcus, its pharmaceutical salts and drug composition. Ligulatrovine A has significant inhibitive effect on Gram-positive bacteria, and can prevent and treat diseases such as boil, pustule, pneumonia, osteomyelitis, acute myocarditis, endocarditis, meningitis, mastitis, cystitis, pelvic inflammation, urinary inflammation, prostatitis, bacteremia, or abscess in muscle, skin, urogential region or central nervous system caused by Gram-positive bacteria infection.
Owner:WENZHOU MEDICAL UNIV
Graft-port hemodialysis systems, devices, and methods
ActiveUS20160361529A1Small sizeSimple locking mechanismOther blood circulation devicesMedical devicesHemodialysisBlood treatments
The present invention relates to subcutaneously implanted graft-port systems, devices and methods for establishing access to the vascular system of a patient requiring multiple blood treatments over an extended period of time. The systems, devices and methods disclosed herein reduce miscannulation, promote intra-session hemostasis, and decrease the incidence of bacteremia and sepsis among other improvements and advantages. The devices include a port with a flattened plateau-like surface for receiving an access tube. The flat surface may include a tactile or visual guide to assist with placement of the access tube into the tapered seat. Optional valve mechanisms reduce the size and form factor of the implantable graft-port device and seals the conduit of the port closed to physiologic pressures until the valve is opened upon percutaneous insertion of the access tube. The access tube does not pass into the conduit. A mismatch fit between the access tube and tapered seat causes a decrease in the cross-sectional sealing area, a reduction in the overall device size, and an increase in blood flow during treatment. Lock solutions to prevent fowling and infection are also disclosed.
Owner:PROVIFLO A MISSISSIPPI CORP
Molecular Diagnosis of Bacteremia
InactiveUS20090215059A1Sensitively and accurately detectingSugar derivativesMicrobiological testing/measurementBacteroidesClinical settings
A highly specific assay can be used for the detection of bacteremia in the clinical setting. The ubiquitous background endogenous DNA present in all PCR reagents is eliminated using a restriction endonuclease digestion. Universal primers for eubacteria are used for detection, and specific primers or probes for bacterial species can be used for identification of species.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
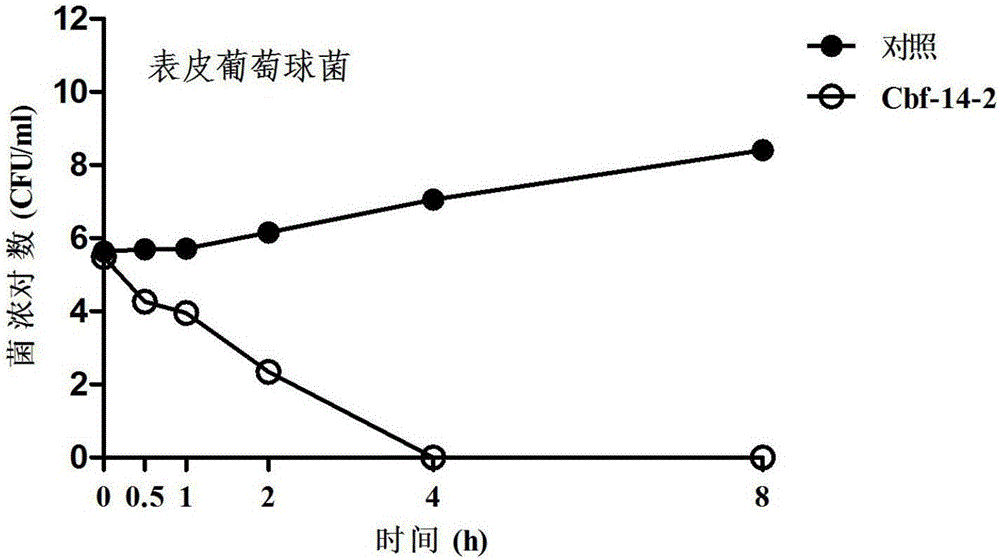
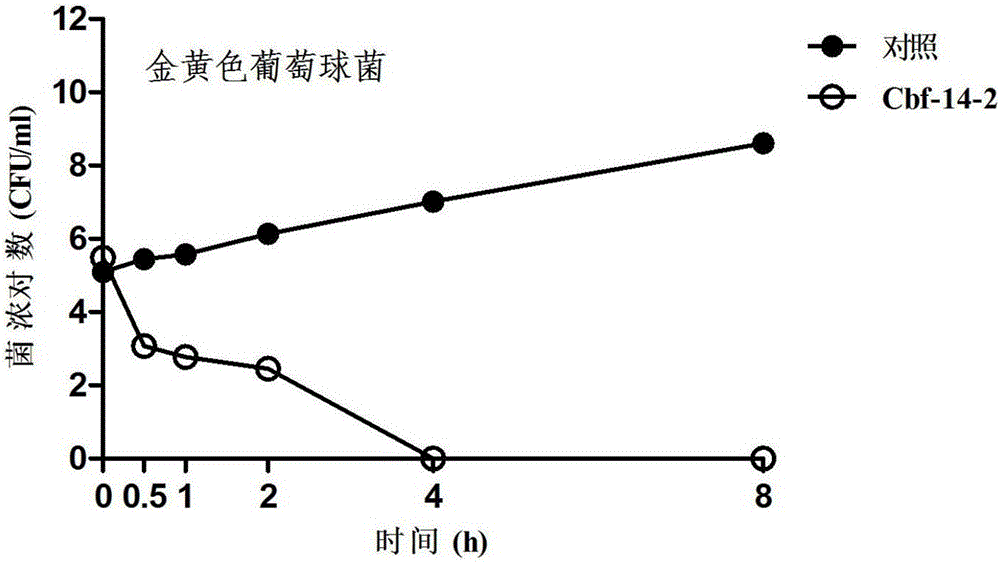
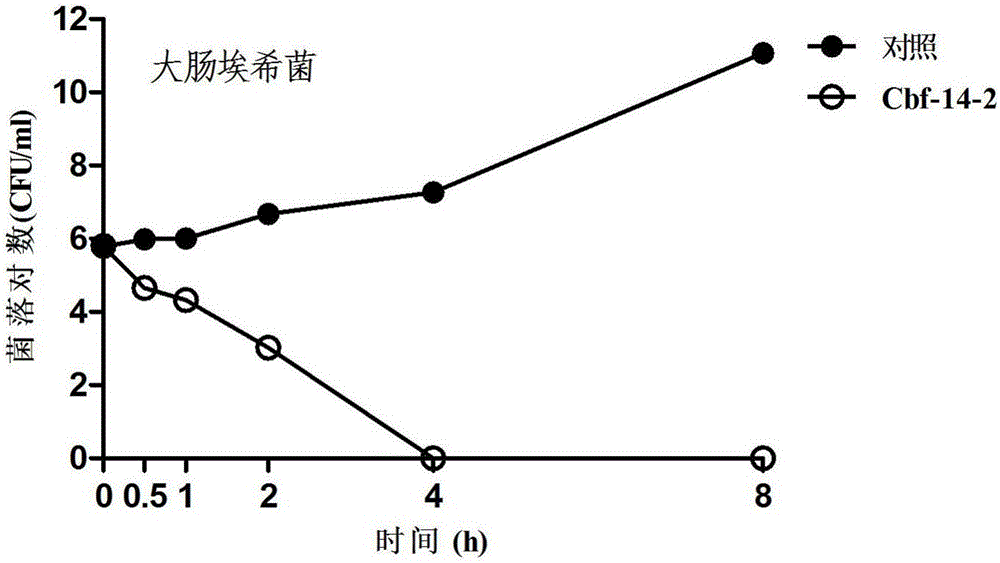
Anti-drug resistant infection polypeptide Cbf-14-2 and application thereof
ActiveCN106543271ALow hemolytic activityLow toxicityAntibacterial agentsPeptide/protein ingredientsAntimicrobial peptidesAntibacterial activity
The invention relates to the technical field of polypeptide drugs in biochemistry, in particular to a polypeptide and application thereof. An unnatural amino acid is introduced according to an antimicrobial peptide Cbf-14 to obtain the polypeptide Cbf-14-2 having the high inhibiting activity on drug-resistant bacteria. An amino acid sequence of the antibacterial polypeptide is shown as SEQ ID: NO:1. An in-vitro antibacterial activity research shows that the polypeptide Cbf-14-2 has a more remarkable killing effect on clinic drug-resistant bacteria compared with a parent peptide Cbf-14. An in-vivo bacteremia model research shows that the polypeptide Cbf-14-2 can remarkably improve the survival rate of a rat suffering from bacteremia, the weight of the rat suffering from the bacteremia is protected, the bacterium concentration of the polypeptide in blood is reduced, and meanwhile the polypeptide has the very good treating effect on lung tissue infection inflammation caused by drug-resistant strains. The polypeptide Cbf-14-2 has very small toxicity to mammalian cells.
Owner:CHINA PHARM UNIV +1
Antibacterial and anti-inflammatory mouthwash and preparation method thereof
InactiveCN106309325ACleans up food residuePlay an antibacterial and anti-inflammatory effectCosmetic preparationsAnthropod material medical ingredientsDiseaseOral disease
The invention discloses antibacterial and anti-inflammatory mouthwash and a preparation method thereof. A main effective component of the mouthwash is a traditional Chinese medicinal extract. The Chinese medicinal extract is prepared from the following raw materials in parts by weight: 25 to 30 parts of rose flower, 4 to 8 parts of flower and root of rosa multiflora, 15 to 20 parts of honeysuckle flower, 6 to 12 parts of herba houttuyniae, 8 to 13 parts of fructus forsythia, 3 to 6 parts of all grass of tagetes patula L, 2 to 4 parts of fructus toosendan, 6 to 8 parts of cortex magnoliae officinalis, 10 to 15 parts of Chinese gall, 4 to 6 parts of roughhaired holly root, 6 to 8 parts of rhizoma polygoni cuspidati, 8 to 12 parts of radix bupleuri, 28 to 35 parts of fresh green lemon, 7 to 10 parts of radix paeoniae alba, 8 to 12 parts of eucalyptus globules and 6 to 9 parts of prepared liquorice root. The mouthwash can realize antibacterial and anti-inflammatory effects while teeth are cleaned in a mouth rinsing process; the mouthwash has the efficacy of effectively inhibiting pathogenic bacteria in an oral cavity from breeding, preventing and treating diseases such as pericoronitis of the wisdom tooth, and preventing serious systemic complications caused by oral diseases such as bacteremia and septicemia.
Owner:QINGDAO HAIZHIYUAN INTELLIGENT TECH
Two flavone glycosides in dandelion and medical use for against Gram-positive bacterium thereof
The invention discloses a flavonoid glycoside and medical salt and application to prevent Micrococcus pyogenes and beta hemolytic streptococcus, which is characterized by the following: preventing pustule, toxic epidermal decay, acute myocarditis, cystitis, cervicitis and tumour of neutral nerve system; fitting for treating prostatitis, pneumococcus, medullitis and knuckle disease.
Owner:DALI PHARMA
Method of performing electropolymerized electrochemically active poly-films as current signal to detect bacteremia
InactiveUS20170199188A1Reduce testing costsEasy to operateBiological material analysisMaterial analysis by electric/magnetic meansElectricityNanoparticle
The present invention is to provide a method of performing electropolymerized electrochemically active poly-films as a current signal to detect bacteremia. The method comprises conjugating an electrochemical redox-active molecular monomer and a specific antibody with a gold nanoparticle to form a modified gold nanoparticle, and the modified gold nanoparticles are conjugated to the surface of bacteria via a specific antibody to form an electrochemically active poly-film by electropolymerization. When applying a voltage, a redox-active current signal of the electropolymerized electrochemically active poly-films can be detected by a usual electrochemical detection system typically in the range between nA and mA.
Owner:NATIONAL TSING HUA UNIVERSITY
Multi-component group B meningococcus vaccine and preparation method thereof
PendingCN108939061AAdequate and widespread preventionAdequate and widespread infection preventionAntibacterial agentsApolipeptidesPericarditisInvasive disease
The invention provides a multi-component group B meningococcus vaccine and a preparation method thereof. The multi-component group B meningococcus vaccine disclosed by the invention comprises a groupB meningococcus low-endotoxin mutant outer membrane vesicle (OMV), recombinant lipoproteins (fHbp-A, fHbp-B) of group B meningococcus subfamily A and subfamily B human factor H binding proteins (fHbp), a group B meningococcus neisserial adhesion A (NadA) recombinant protein, and a group B meningococcus neisseria heparin binding antigen (NHBA) recombinant protein. The vaccine disclosed by the invention is used for preventing cerebrospinal meningitis, bacteremia, pneumonia, pericarditis and other invasive diseases caused by the group B meningococcus, and provides wide effective protection effects to the group B meningococcus.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +2
Antibody against alpha-hemolysin and application thereof
ActiveCN112538112AImprove securityShort course of treatmentAntibacterial agentsAntibody mimetics/scaffoldsPulmonary infectionHemolysis
The invention provides an antibody or a fragment thereof combined with staphylococcus aureus alpha-hemolysin, and application of the antibody or the fragment thereof in preventing or treating staphylococcus aureus infection. The antibody is obtained by screening through a strategy of attenuated immunity and virulent screening of the alpha-hemolysin, has high affinity to the alpha-hemolysin, can effectively block the hemolysis of the alpha-hemolysin, proves a significant protective or therapeutic effect in an alpha-hemolysin sepsis model, an MRSA bacteremia model and an MRSA pulmonary infectionmodel, has a synergistic effect with antibiotics, and is a beneficial supplement to the existing antibiotic therapy of staphylococcus aureus.
Owner:MABWELL (SHANGHAI) BIOSCIENCE CO LTD
Genetically Modified Bacteriophage (Bio-Phage)
InactiveUS20170333500A1Improve abilitiesViral/bacteriophage medical ingredientsBacteriophagesBacteroidesMethicillin sensitive
The present invention describes a genetically modified Staphylococcus aureus bacteriophage VDX-10 comprising the DNA of the bacteriophage VDX-10 being altered by inserting a gene sequence that increases the ability of the bacteriophage to replicate faster as compared to unmodified VDX-10 bacteriophage; a method of producing the genetically modified Staphylococcus aureus bacteriophage VDX-10; and a method of treating infection in a patient by administering an amount of the genetically modified Staphylococcus aureus bacteriophage effective to eliminating the Staphylococcus bacteria cells, where the infection can be Ventilator-Associated Pneumonia (VAP) or bacteremia as incited by methicillin-resistant Staphylococcal aureus (MRSA) or methicillin-sensitive Staphylococcal aureus (MSSA).
Owner:HATFIELD ROC
Bacteriaemia aspartame assay kit and assay method thereof
InactiveCN101864490AShorten identification timeSave rescue timeMicrobiological testing/measurementMicroorganismAssay
The invention relates to the field of clinical microorganism identification, and provides a kit for rapidly identifying a bacterium in a blood source and a method thereof. The kit comprises: two universal primers amplify the V1 variable region of a bacterium 16S r RNA, one sequencing primer measures the sequence of a V1 forward sector, HRP-avidin labels a magnetic bead, and genus is judged by comparing sequencing results and a database. The kit can sequence and distinguish the bacterium in the blood source at a time, can be used for clinical diagnosis of bacteriaemia and ichorrhemia, not only saves the valuable rescuing time for clinical diagnosis and treatment, and has low cost, convenient operation and strong specificity.
Owner:HANGZHOU D A GENETIC ENG
Compounds pharmaceutical compositions and methods for treatment of bacteremia and/or septicemia
Novel conjugates of bacterial outer membrane binding peptides, preferably having bacterial sensitization activity, and immune cells chemotactic peptides, and pharmaceutical compositions containing same useful in the treatment of bacteremia and / or septicemia following infection by gram negative bacteria administered alone or in combination with conventional antibiotics.
Owner:RAMOT AT TEL AVIV UNIV LTD +1
Application of Qingkailing active component radix isatidis extract in preparation of anti-multidrug-resistant bacterium medicine
ActiveCN103040897BDoes not inhibit growthAntibacterial agentsPeptide/protein ingredientsBacteroidesEscherichia coli
The invention discloses a new medical application of a radix isatidis extract and provides a medicine capable of effectively antagonizing a multidrug-resistant bacterium. The radix isatidis preparation can be used for treating diseases caused by the multidrug-resistant bacterium containing an NDM-1 drug-resistant gene, such as intestinal infection, respiratory infection, wound and skin infections, urogenital infection, bacteremia, meningitis and the like, and is particularly applicable to treatment of various infectious diseases caused by calcium acetate acinetobacter, acinetobacter baumannii, stenotrophomonas maltophilia, or escherichia coli containing the NDM-1 drug-resistant gene.
Owner:HEBEI SHINEWAY PHARMA
Methods and compositions for treating bacterial infection
ActiveUS9333250B2Stabilizes and reduces symptomEnhance immune responseAntibacterial agentsBacteriaEscherichia coliBacteroides
The present invention relates to methods and compositions for the treatment of bacterial infection, for example extraintestinal E. coli infection such as E. coli bacteremia, meningitis and sepsis. The invention relates also to methods of diagnosis and prevention.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Serogroup B meningococcus recombinant chimeric protein vaccine and preparation method thereof
ActiveCN107823638AImprove the effect of preventionEffective protectionAntibacterial agentsNervous disorderPericarditisSalmonella serotype typhi
The invention provides a serogroup B meningococcus recombinant chimeric protein vaccine and a preparation method thereof. The vaccine contains three recombinant chimeric proteins of P2-fHBP V1, P2-fHBP V2 and P2-fHBP V3, wherein amino acid sequences of the recombinant chimeric proteins are SEQ ID NO.1, SEQ ID NO.2 and SEQ ID NO.3 respectively; the contents of all the proteins are 30 to 50mu g / dose; optimally, a vaccine preparation also contains a cryoprotectant or vaccine adjuvant. The invention also provides the preparation method of the three recombinant chimeric proteins. By using the recombinant chimeric proteins formed by linking P2 with serogroup B meningococcus fHBP protein, humoral immune response can be effectively induced, and immunogenicity of fHBP is obviously improved. The vaccine provided by the invention can effectively cover all serogroup B meningococcus strains, thereby providing a broad-spectrum preventing effect on invasive diseases such as cerebrospinal meningitis,bacteremia, pneumonia and pericarditis caused by the serogroup B meningococcus.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +2
Application of typhoid fever and paratyphoid fever salmonella in the aspect of fast anti-tumor
InactiveCN101112398AEnhance the ability to phagocytose cancer cellsImprove bindingBacteria material medical ingredientsCancer antigen ingredientsSide effectCancer cell
The present invention discloses an application of typhoid and paratyphoid salmonella in the anti-tumor aspect, which is invented to solve the problems of great side effects and poor effects of the prior art in the treatment of tumors. The present invention provides the application of the typhoid and paratyphoid salmonella in the rapid anti-tumor aspect. Wherein, the tumors are one or more of gastric cancer, lung cancer, liver cancer, tongue squamous cancer, breast cancer, colon cancer, leukemia, brain tumor and sarcoma. The invention makes use of the rapid combination of the typhoid and paratyphoid salmonella with the mononuclear cells after entering into the human body, the strong endotoxin and bacteremia are released and the temperature reaches highly around 40 DEG C to 42 DEG C, so as to affect and inhibit the growth and the life cycle of the tumor cells, furthermore, the large mononuclear cells-macrophages in the reticuloendothelial system are proliferated greatly, thus enhancing the ability of the macrophages in vivo to swallow the cancer cells, so the invention has very significant inhibiting and killing effects of a variety of tumors.
Owner:罗舒仓
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com