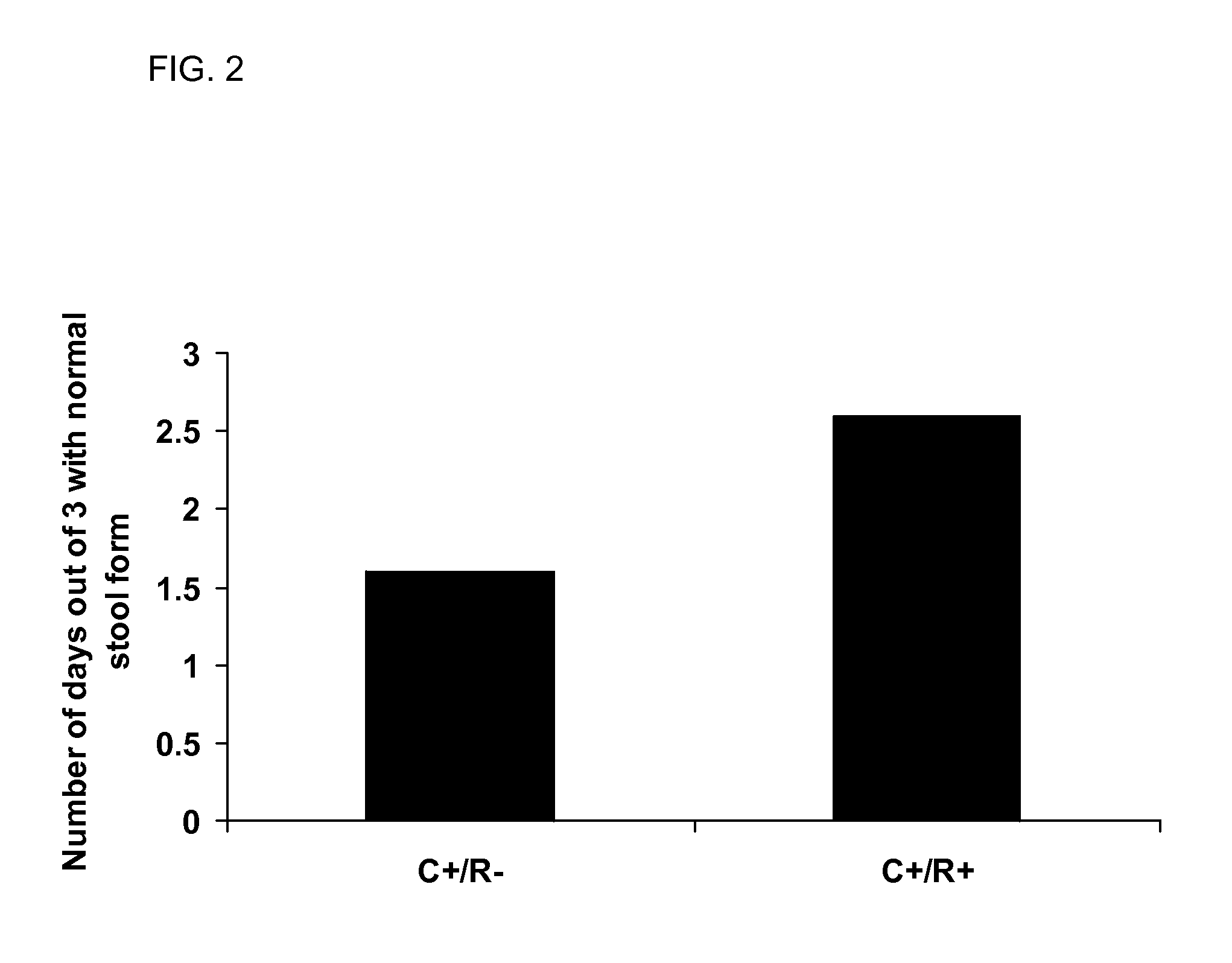
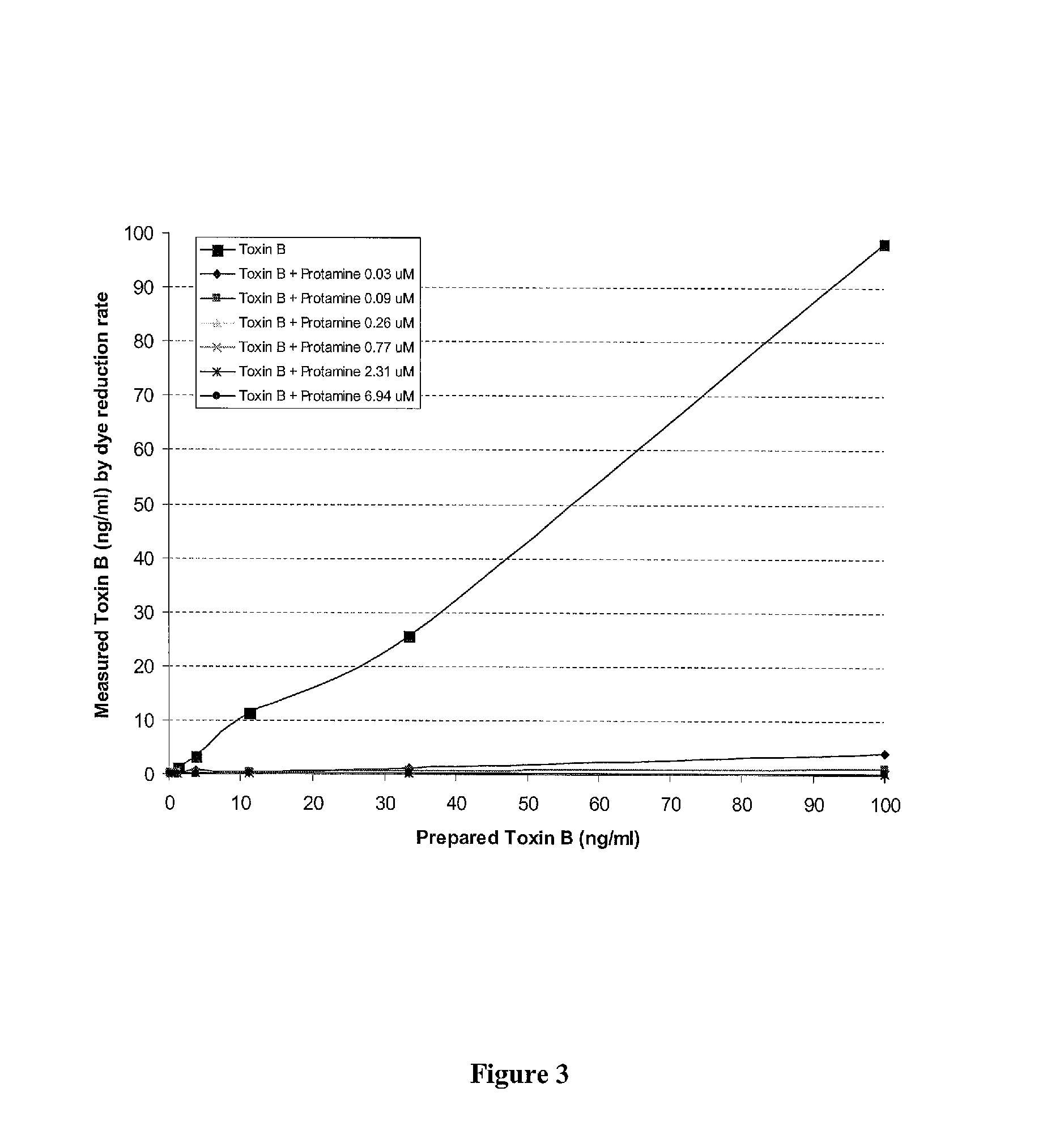
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
102 results about "Antibiotic therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Use of beta-lactamase
InactiveUS20090311234A1Eliminate side effectsAntibacterial agentsHydrolasesSide effectAntibiotic therapy
Class A beta-lactamase may be used for reducing side-effects in the intestine associated with antibiotic therapy with a combination of beta-lactam antibiotic and beta-lactamase inhibitor.
Owner:IPSAT THERAPIES
Convertible multi-lumen catheter
InactiveUS20050055012A1Minimize infection riskRigid enoughMulti-lumen catheterMedical devicesVeinHaemodialysis machine
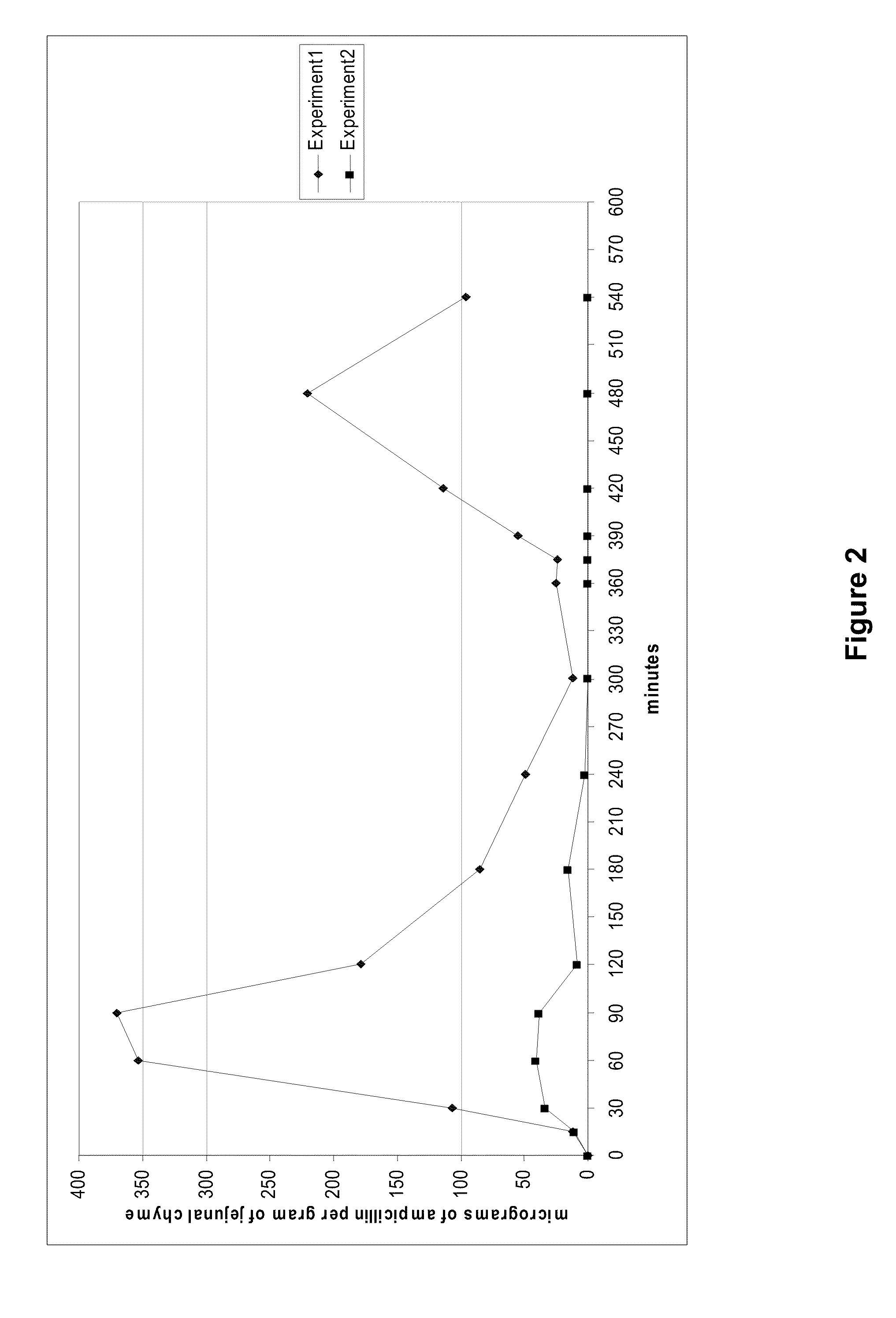
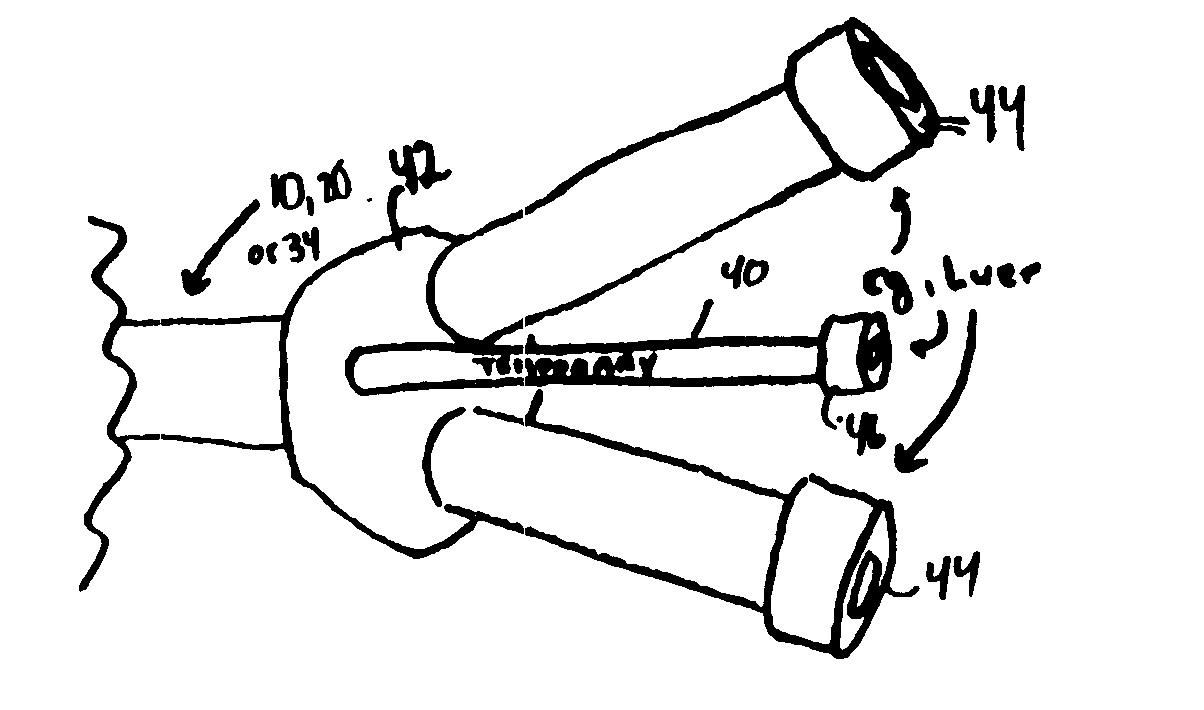
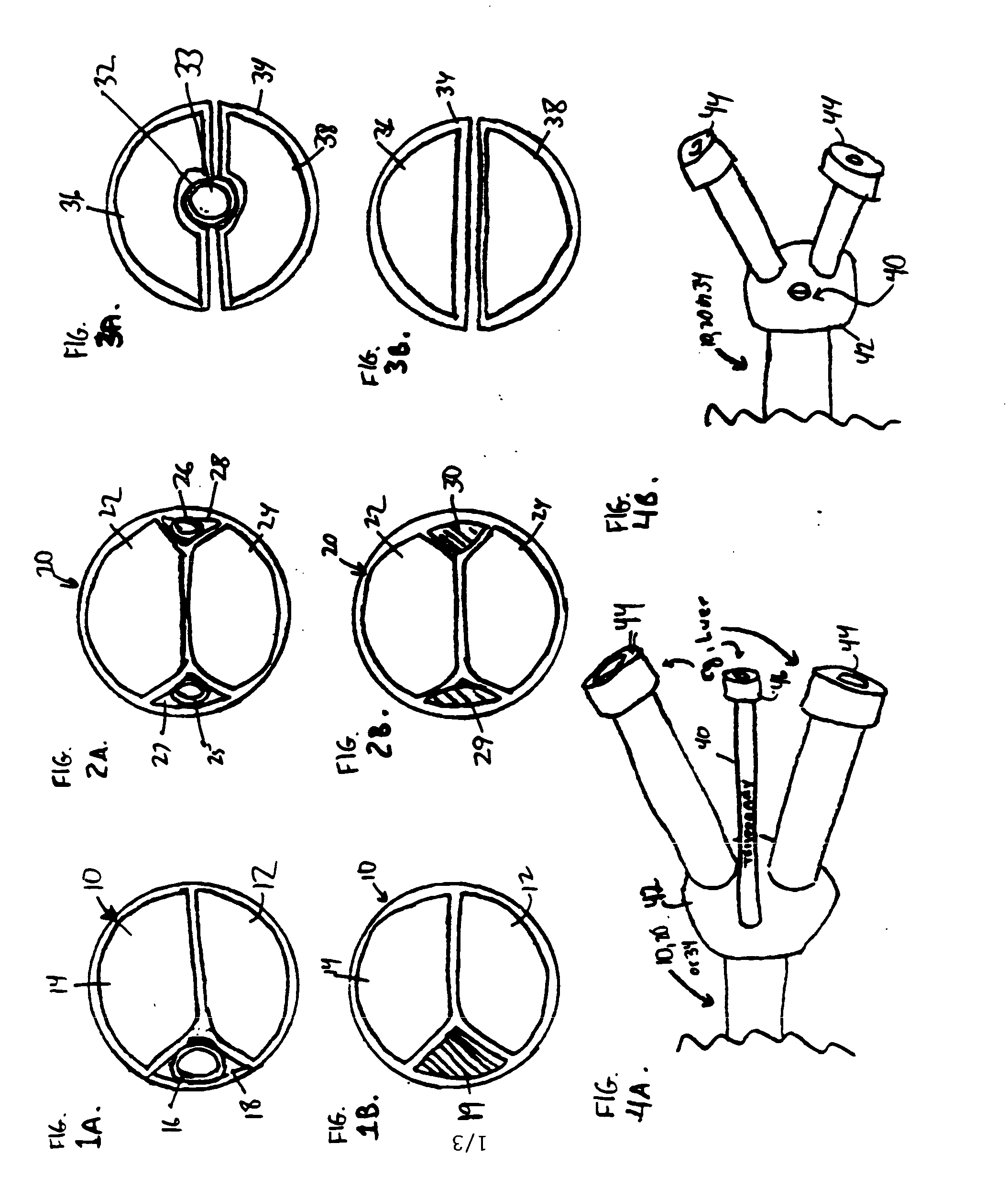
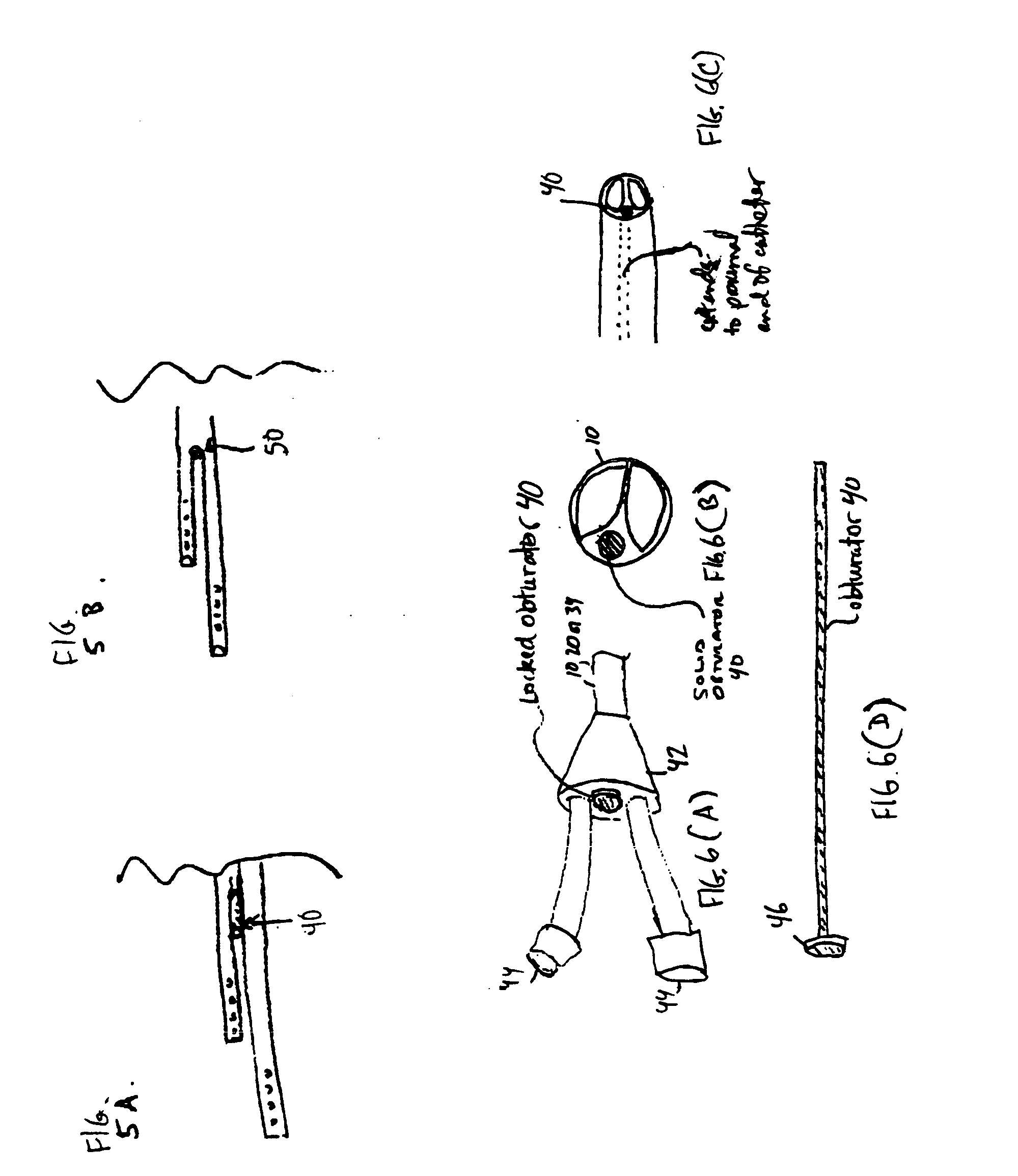
A convertible multi-lumen catheter that may be used for hemodialysis or other indications involving infusion and / or withdrawal of fluids from the body. Unlike existing catheters having a set number of lumens which may limit their utility as both short and long-term venous vascular devices, the catheter of the invention allows one or more additional lumens during the acute phase of catheter use with removal of these lumens (i.e. conversion) for more permanent use. A typical example of this would be a triple lumen device for hemodialysis and antibiotic therapy during an acute infection with conversion to a chronic dual lumen hemodialysis catheter after successful treatment of the infection. The lumen is permanently or semi-permanently blocked using a biocompatible plastic obturator that is inserted into the unused lumen and locked and / or glued into place.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Antibiotic therapy to reduce the likelihood of developing post-infectious irritable bowel syndrome
InactiveUS20110294726A1Reduce the possibilityReduce decreaseAntibacterial agentsBiocideAntibiotic therapyNon ulcer dyspepsia
Owner:CEDARS SINAI MEDICAL CENT
Integrated Device for Diagnostic Analyses, and Relative Method
InactiveUS20070269853A1Promotes and accelerates bacterial growthBioreactor/fermenter combinationsBiological substance pretreatmentsAntibiotic therapyAntibiotic Y
An integrated device for diagnostic analyses used to verify the presence of bacteria in at least a biological sample mixed with a eugonic culture broth, in order to identify the type of bacteria, and to test a series of antibiotics, identifying those effective to determine the antibiotic therapy. The device comprises first examination means to verify the presence of bacteria so as to define corresponding positive biological samples and identify the type of bacteria present in the positive biological samples to define said group of antibiotics, and second examination means to verify the sensitive or resistant response of each positive biological sample to a series of antibiotics of the group of antibiotics defined by the first examination means.
Owner:ALIFAX TECH
Antibiotic Formulations Providing Reduced Gastrointestinal Side Effects and Clostridium difficile Infection Relapse, and Related Methods
InactiveUS20110240512A1Antibacterial agentsSmall article dispensingClostridial infectionAntibiotic-associated diarrhoea
The invention includes a formulation comprising: (i) a therapeutically effective amount of at least one antibiotic; and (ii) a therapeutically effective amount of at least one probiotic material. The formulation is prepared in a dosage form for delivery to the gastrointestinal tract. The invention further includes a method of preventing or minimizing the proliferation of Clostridium difficile in the gastrointestinal tract of a mammal undergoing an antibiotic therapy comprising by administration of the formulation of the invention for the duration of the antibiotic therapy; methods of preventing or minimizing the occurrence of antibiotic-associated diarrhea in a mammal undergoing an antibiotic therapy that includes administering the formulation; and methods of preventing or minimizing the occurrence of antibiotic-associated colitis or pseudomembranous colitis in a patient undergoing an antibiotic therapy that includes administering the formulation.Also included are methods of reducing or preventing a failure of treatment for an antibiotic-treatable infection in a patient comprising orally administering the formulation.Described by the invention are formulations for the treatment of a C. difficile infection comprising: (i) a therapeutically effective amount of at least one antibiotic; and (ii) a therapeutically effective amount of at least one probiotic material. The antibiotic includes a non-systemic gram negative antibiotic (such as, for example, fodaximicin) and formulation is prepared in a dosage form for delivery to the gastrointestinal tract.Also included are methods of treatment of a C. difficile infection in mammal that include administering to the digestive tract of the mammal the above described formulation. Such methods provide that the likelihood that the mammal shall experience a reoccurrence of the C. difficile infection is 70% or less.
Owner:TECOPPA BIOPHARMA
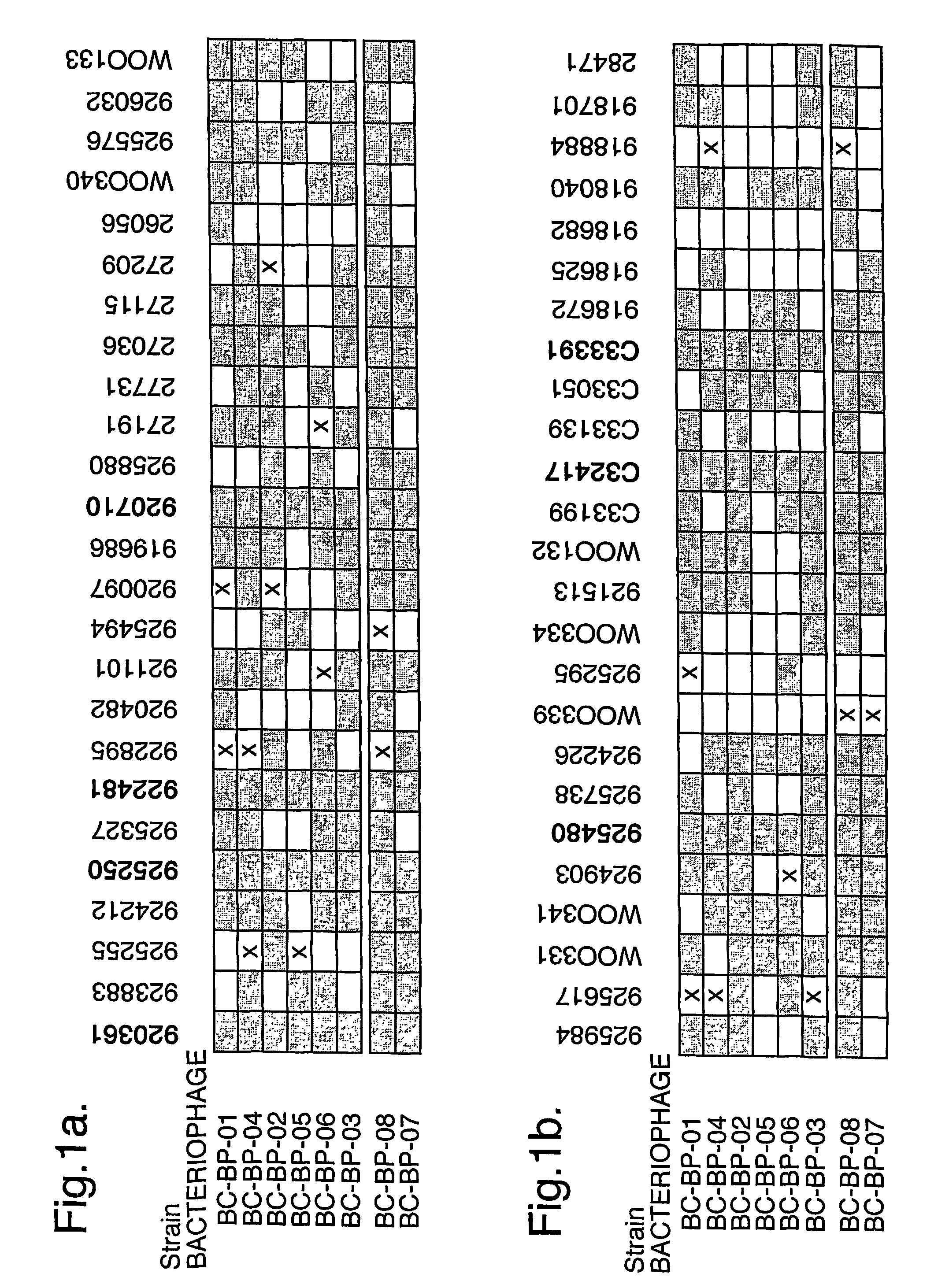
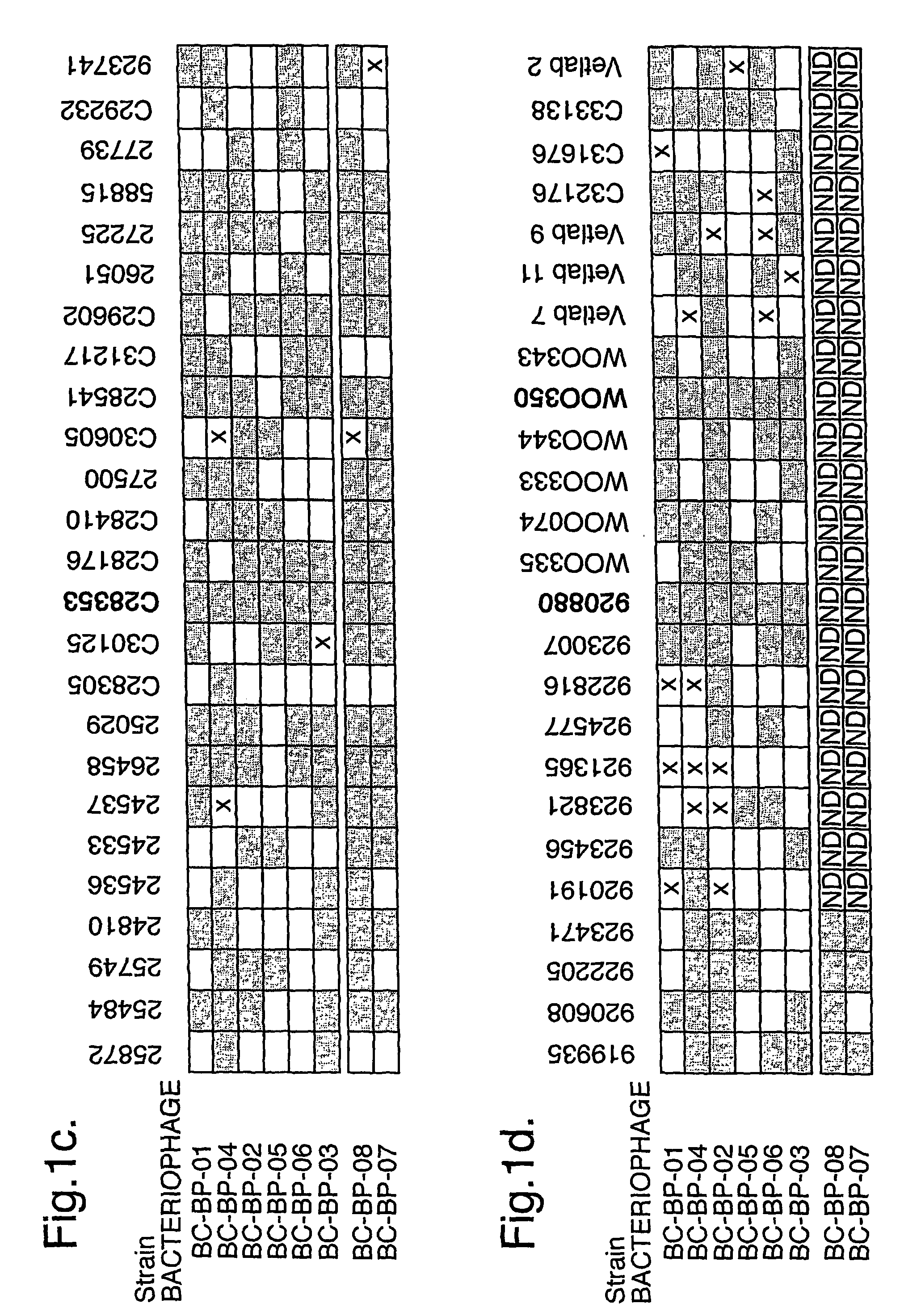
Bacteriophage-containing therapeutic agents
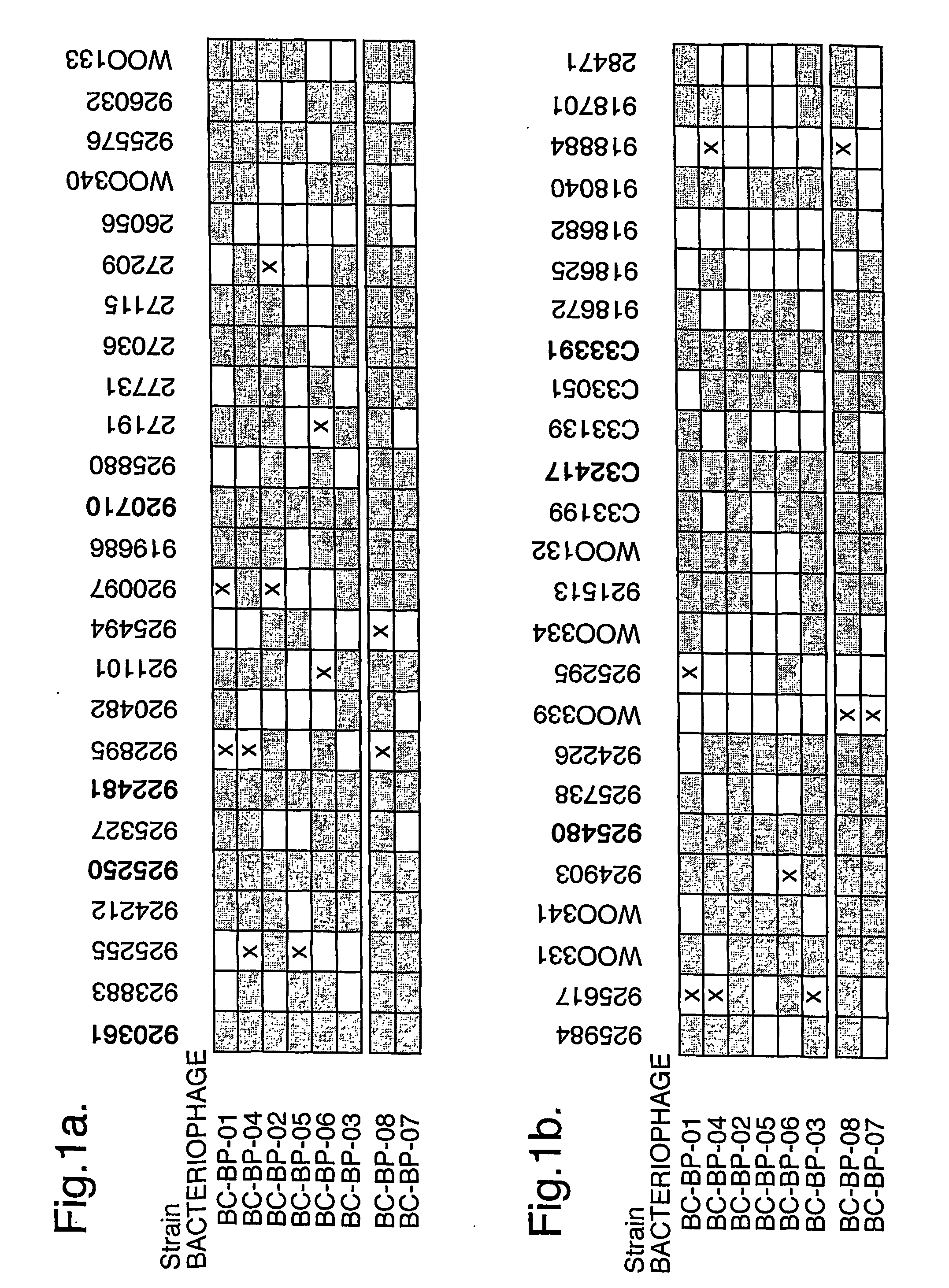
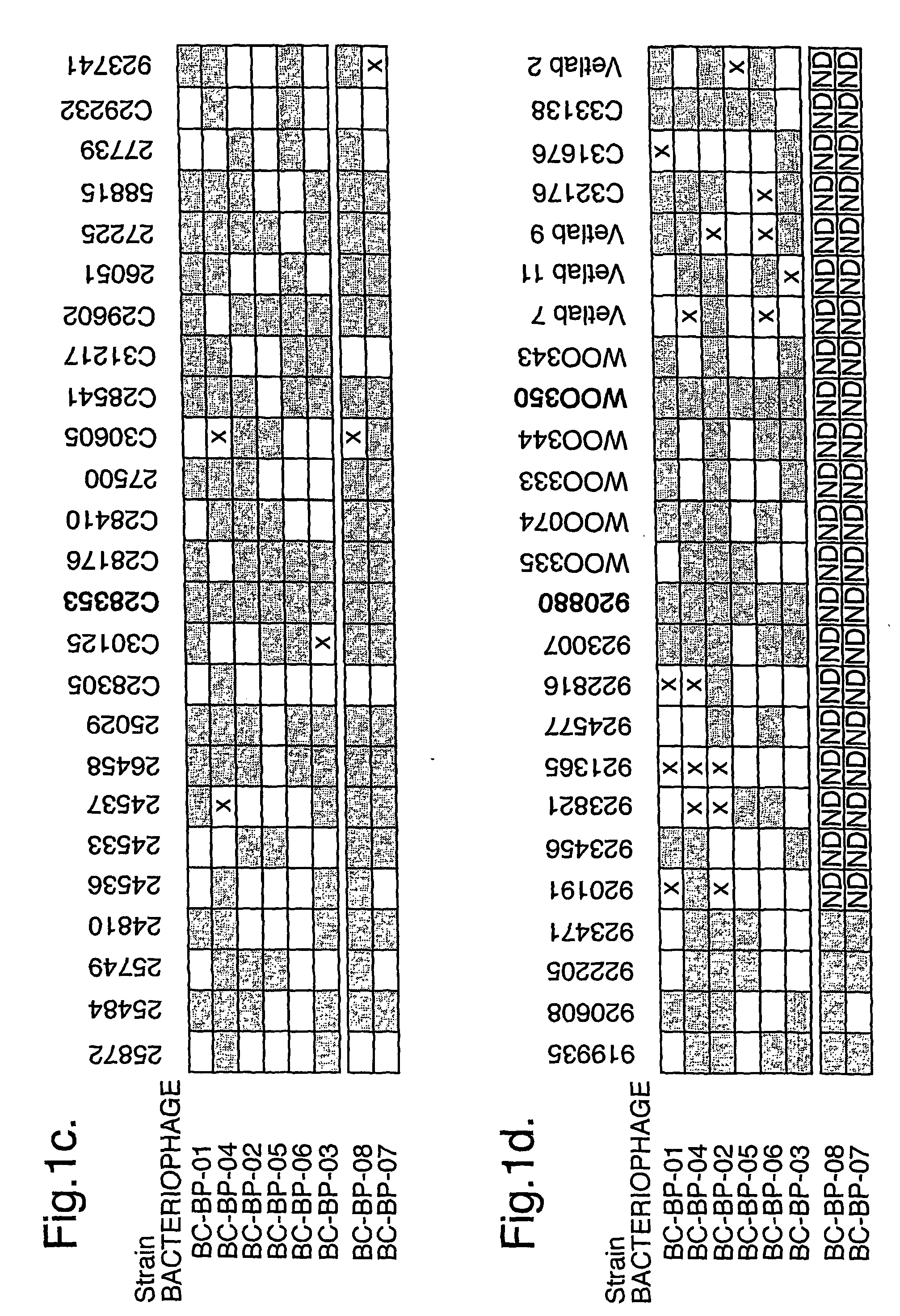
The present invention relates in its broadest aspect to combined phage / antibiotic therapy. More particularly, it relates to use of (i) one or more bacteriophages and (ii) one or more antibiotics in the manufacture of a combined product for simultaneous, separate or sequential administration of (i) and (ii) to treat a bacterial infection characterized by biofilm formation, for example an infection comprising or consisting of P. aeruginosa. Treatment in this context may be either therapeutic or prophylactic treatment. Also provided are deposited bacteriophages each exhibiting different strain specificity against P. aeruginosa and combinations of such bacteriophages, e.g. a panel of six deposited bacteriophages which was found to be effective against a high percentage of clinical isolates of P. aeruginosa from canine ear infections.
Owner:BIOCONTROL
Method for treatment of bowel disorders
InactiveUS20090110663A1Efficiently relievedEasy and quick to administerBiocideBacteria material medical ingredientsSide effectUlcerative colitis
A composition and method for treatment for bowel disorders is described. The composition consists of two parts. First, highly purified, insoluble, chemically unmodified fiber from plants is mixed with an aqueous solution such as water. The second component of the composition is a commercially available probiotic preparation. These commercially probiotic preparations contain large numbers of viable microorganisms of either Lactobacillus sp., Bifidobacter sp. Streptococcus sp. or yeast. Consumption of the fiber preparation or probiotic by patients with constipation secondary to surgery or chronic antibiotic therapy, ulcerative colitis or Crohn's disease results in either a resolution of the symptoms or markedly improvement in symptoms without significant side effects.
Owner:HALOW GEORGE M
A pharmaceutical composition for targeted tumor therapy and its preparation method
The patent of the present invention relates to a pharmaceutical composition containing antitumor antibiotic-dipeptide derivatives that can be used for targeted tumor therapy and a preparation method thereof, belonging to the technical field of pharmaceutical preparations. The pharmaceutical composition is an injection composed of antitumor antibiotic-dipeptide derivatives, phospholipids, stabilizers, organic solvents, antioxidants, surfactants, and pH regulators. Dry powder needle. The pharmaceutical composition greatly increases the solubility and stability of the antitumor antibiotic-dipeptide derivatives with targeted antitumor effects in aqueous solution, and can be effectively used for malignant epithelial tumors (such as breast cancer, ovarian cancer, Targeted therapy for lung cancer, colon cancer, pancreatic cancer, skin melanoma) and other solid tumors.
Owner:重庆寰瑞生物技术有限公司
Primer pair, probe and kit for detecting bacterium MCR-1 gene
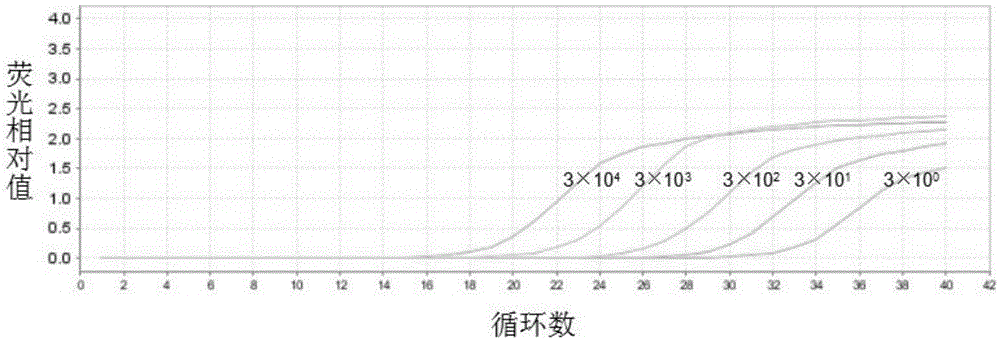
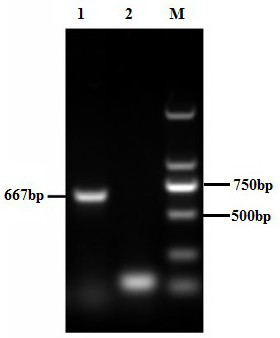
InactiveCN105420394AEasy to detectQuick checkMicrobiological testing/measurementMicroorganism based processesResistant genesFluorescence
The invention relates to a primer pair, a probe and a kit for detecting the bacterium MCR-1 drug-resistant gene. The primer pair comprises an MCR-1 gene detecting upstream primer including the sequence shown by SEQ ID NO.1 and an MCR-1 gene detecting downstream primer including the sequence shown by SEQ ID NO.2. The probe for the MCR-1 gene comprises a sequence shown by SEQ ID NO.3, a fluorescence group is integrated at the 5' end, and a quenching group is integrated at the 3' end of the probe for the MCR-1 gene. The kit for detecting the bacterium MCR-1 drug-resistant gene comprises the primer pair and the probe. The primer pair, the probe and the kit for detecting the bacterium MCR-1 drug-resistant gene have the advantages of being capable of detecting the bacterium MCR-1 drug-resistant gene, good in sensitivity, high in specificity, accurate in rationing and the like. The primer pair, the probe and the kit are good for clinical detection and monitoring of the bacterium MCR-1 drug-resistant gene and guiding antibiotic therapy of patients.
Owner:WUHAN AIMISEN LIFE TECH CO LTD
Paste-like bone cement
ActiveUS20140024739A1Well formedEasy to shapeImpression capsSurgical adhesivesParticulatesFiller Excipient
Paste containing at least one monomer for radical polymerization, at least one polymer that is soluble in said at least one monomer for radical polymerization, and at least one filling agent that is poorly soluble or insoluble in said at least one monomer for radical polymerization, wherein the filling agent is a particulate inorganic filling agent possessing a BET surface of at least 40 m2 / g; kit comprising pastes A and B which, when mixed, form paste C, which pastes are useful for mechanical fixation of articular endoprostheses, for covering skull defects, for filling bone cavities, for femuroplasty, for vertebroplasty, for kyphoplasty, for the manufacture of spacers or for the production of carrier materials for local antibiotics therapy, as well as a form body produced from the pastes.
Owner:HERAEUS MEDICAL
Device and Methods
ActiveUS20160153878A1Quick collectionIncrease capillary forceBioreactor/fermenter combinationsBiological substance pretreatmentsMammalAntibiotic therapy
The present invention relates generally to methods and materials pertaining to assays, for example immunoassays, for biomarkers in body fluids e.g. blood. The invention also relates to diagnostic or screening methods for infections, and methods of differentiating between infectious and non-infectious conditions in mammals, particularly equines, for monitoring response to anti-infective / antibiotic therapy. The invention further relates to a test fluid collection system adapted to permit dilution and analysis of the collected test fluid. The invention further relates to monitoring exertional rhabdomyolysis in equines, and assay devices for all these things.
Owner:EPONA BIOTECH
Bacteria-specific labeled substrtates as imaging biomarkers to diagnose, locate, and monitor infections
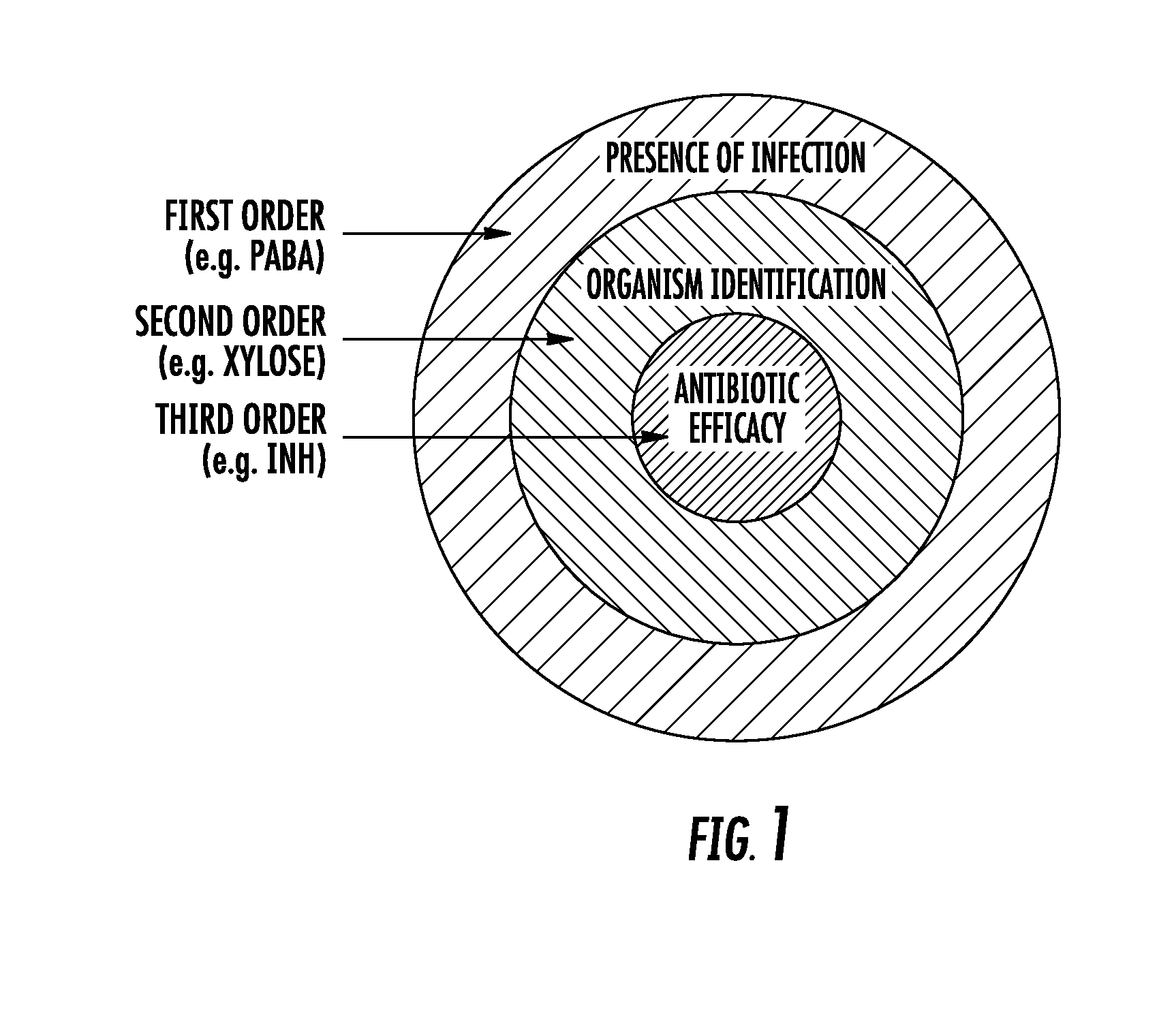
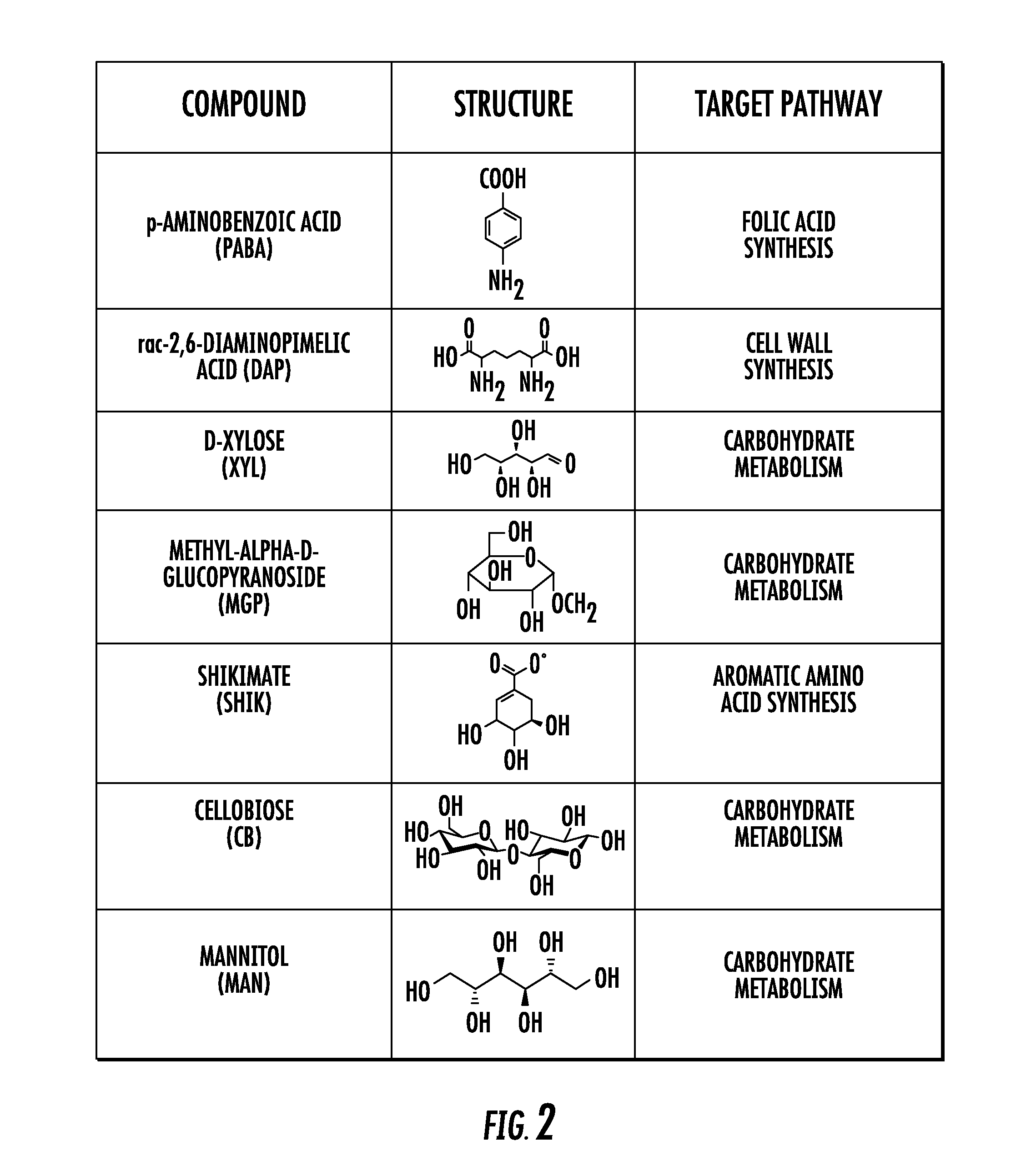
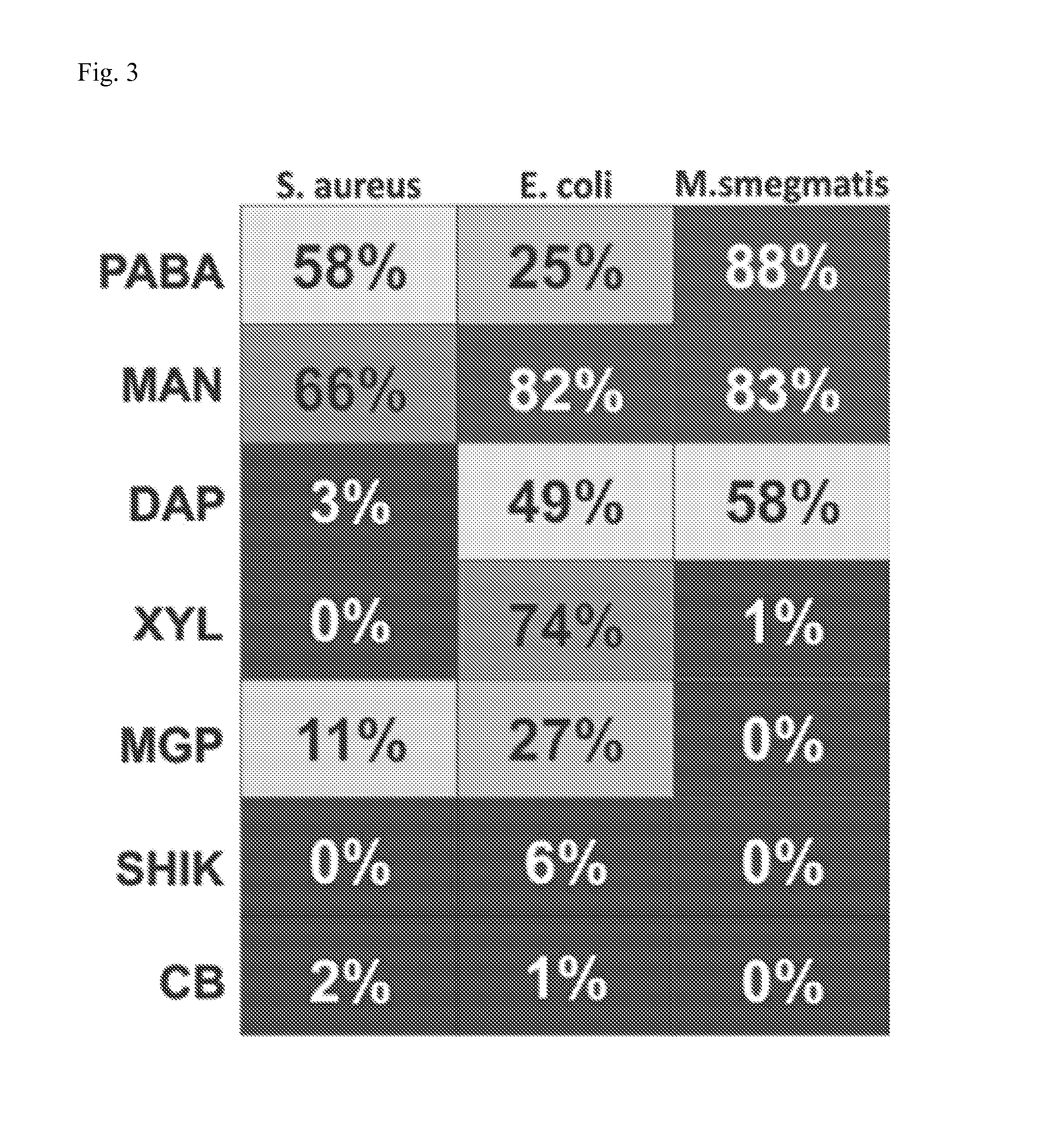
The methods of the present invention exploit unique biochemical pathways present within infectious organisms to develop small molecule metabolic tracers. Labeled substrates created using these inventive methods were created. The labeled substrates can be used to determine whether a subject is infected with an infectious organism by imaging means, and with use of two or more such labeled substrates, methods of differentiating gram negative infection from gram positive infection, and methods of localizing and quantifying infectious disease burden are provided. The methods of the present invention can assist in the clinical decision to begin empiric antibiotic therapy, determine its efficacy, as well as the choice of antibacterial agents.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Colonic delivery using Zn/pectin beads with a Eudragit coating
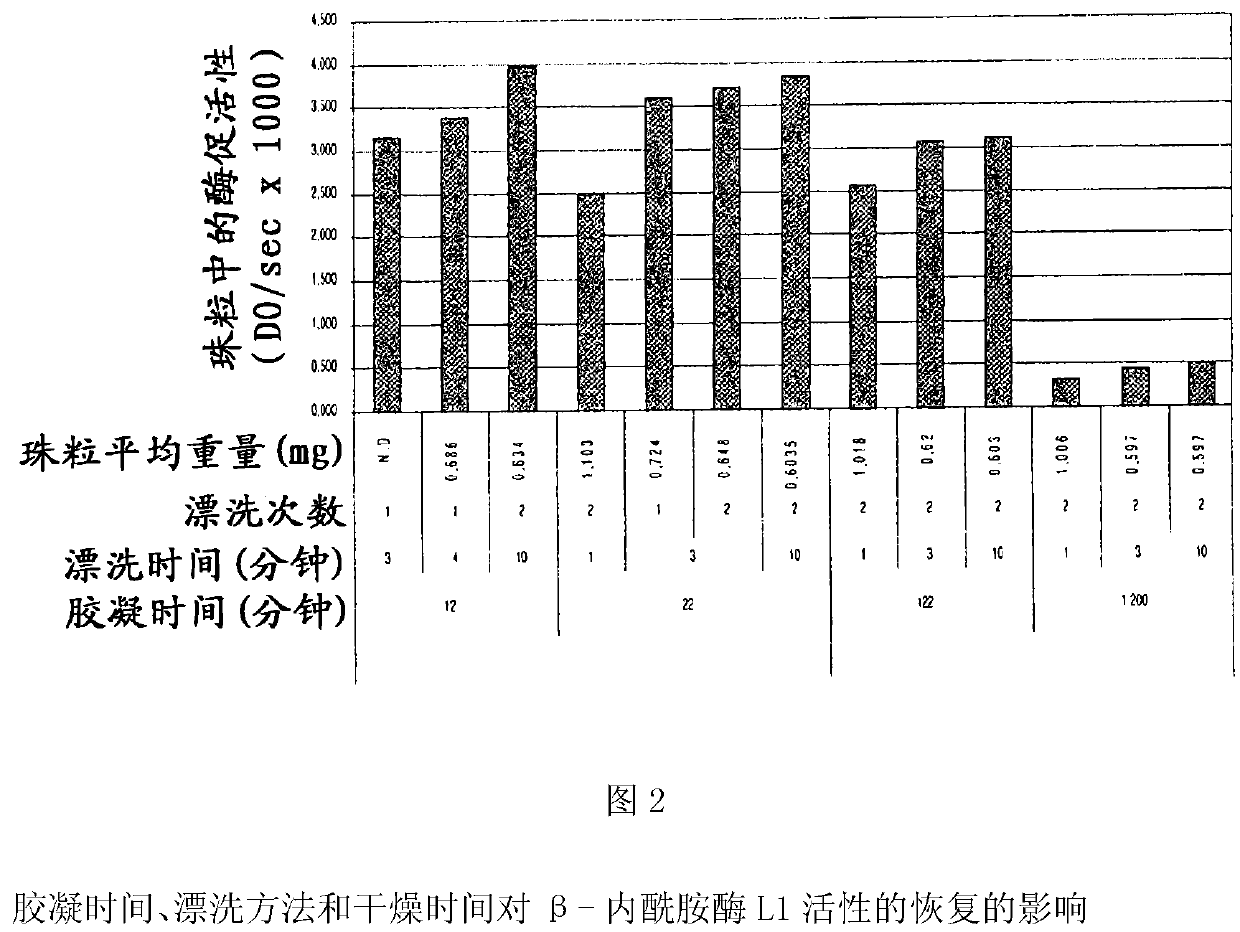
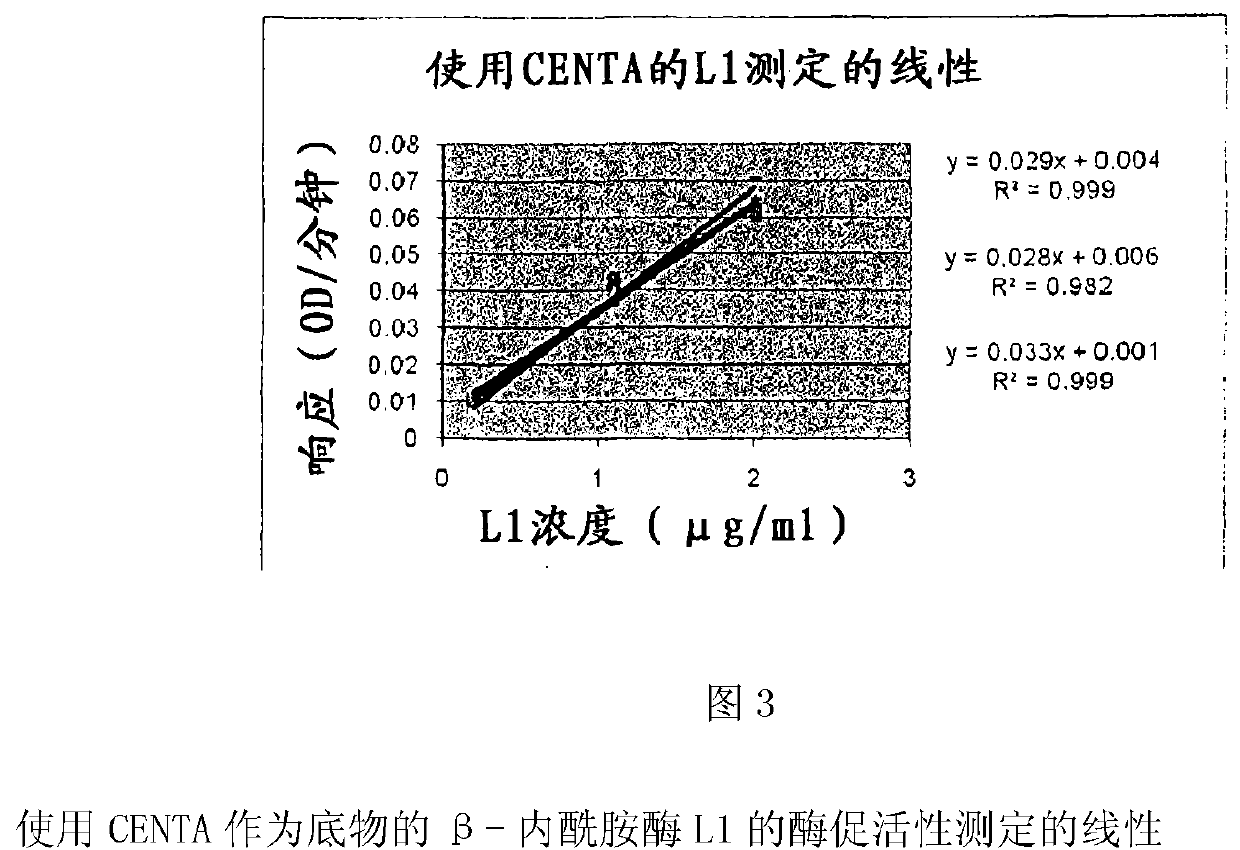
Drug delivery systems that can deliver therapeutic and / or diagnostic agents to the colon are disclosed. The systems include pectin beads crosslinked with zinc or any divalent cation of interest, which beads are then coated with Eudragit TM -type polymers. The drug delivery systems are orally administrable, but can deliver the active agents to the colon, or, in some embodiments, to various other positions in the gastro-intestinal tract. The agents can be used to diagnose, treat, prevent, or investigate a variety of conditions, including infectious diseases, inflammatory diseases, cancers and the like. Certain agents, such as metallo-dependent enzymes, for example, ss-lactamase Ll from Stenotrophomonas maltophilia, as well as agents that inactivate macrolide, quinolone, fluoroquinolone or glycopeptide antibiotics, can reduce the quantity of residual antibiotics reaching the colon following antibiotic therapy.
Owner:DA VOLTERRA
Bacteriophage-containing therapeutic agents
The present invention relates in its broadest aspect to combined phage / antibiotic therapy. More particularly, it relates to use of (i) one or more bacteriophages and (ii) one or more antibiotics in the manufacture of a combined product for simultaneous, separate or sequential administration of (i) and (ii) to treat a bacterial infection characterized by biofilm formation, for example an infection comprising or consisting of P. aeruginosa. Treatment in this context may be either therapeutic or prophylactic treatment. Also provided are deposited bacteriophages each exhibiting different strain specificity against P. aeruginosa and combinations of such bacteriophages, e.g. a panel of six deposited bacteriophages which was found to be effective against a high percentage of clinical isolates of P. aeruginosa from canine ear infections.
Owner:BIOCONTROL
Photosensitizer formula for photodynamically treating periodontitis, as well as preparation method and application thereof
InactiveCN106822894ANo side effectsNo immune responseAntibacterial agentsPhotodynamic therapyOral diseaseGel preparation
The invention discloses a photosensitizer formula for photodynamically treating periodontitis and an application thereof. A hydrogel preparation for treating periodontitis takes toluidine blue as a photoactive substance and carbopol as a gel. A formula for optimizing the gel preparation comprises the following components by mass concentration: 0.05%-3% of carbopol, 0.001%-1% of toluidine blue, 0.01%-0.4% of sodium hydroxide or 0.07%-2% of triethanolamine and the balance of water. A method for photodynamically treating periodontitis, provided by the invention, can be used for replacing an antibiotic therapy, so that the problem of antibiotics abuse can be prevented; when the preparation is used for treating the oral diseases caused by infections, such as, periodontitis, the antibacterial rate of once irradiation treatment can reach up to 99.99%; serious patients can be repeatedly treated at intervals; and the photosensitizer formula is free from toxic or side effect to normal organ tissues and has wide market prospects and high application values.
Owner:ZHEJIANG UNIV OF TECH
Pharmaceutical composition of enterosorbent and prebiotics, dosage forms, and the method for prevention and treatment of gastrointestinal disorders
InactiveCN101460183AEnhanced potencyGood curative effectOrganic active ingredientsDigestive systemAllergic dermatitisIntestinal microorganisms
The pharmaceutical composition is a combination of hydrolytic lignin with moisture of 55% to 65% consisting of the particles measuring 0.15 mm to 0.55 mm, a 45% to 55% aqueous lactulose solution, and a 50% to 55% aqueous oligosaccharide solution at the following ingredient ratio (weight percent): 10-60 of an aqueous lactulose solution; 10-60 of oligosaccharides; sufficient quantity of hydrolytic lignin. Hydrolytic lignin, lactulose and fructose oligosaccharides are sequentially added and mixed using a rotor blender. The composition is administered orally for no less than 14 days and no more than 30 days, two to four times a day, depending on the patient's weight and age. The composition is used as a medicine for treatment of the gastrointestinal disorders, including bacterial, viral, protozoal enteric infections, food poisoning, acute and chronic hepatitis and cirrhosis, diarrhea, peptic ulcer, Crohn's disease, ulcerative colitis, irritable bowel syndrome, mineral disorders with Ca / Mg deficiency, including osteoporosis and other alterations of the bone formation, as an immunomodulator in atopic dermatitis and immunodeficiency conditions, for protection and recovery of intestinal flora after antibiotic therapy, chemotherapy, and radiotherapy. The result is accelerated achievement of the effect and the enhanced action on the state of intestinal microbiocenosis as well as increased effectiveness of treatment of hepatitis and liver cirrhosis, elimination of undesired adverse effects in clinical usage, and extension of indications, i.e. the extended spectrum of usage in prevention and treatment.
Owner:亚历山大·弗拉基米罗维奇·迪科夫斯基
Composite biological material for eliminating bacterial biofilm
InactiveCN105770999ARemove biofilmBiofilm removalCosmetic preparationsToilet preparationsBiological materialsSodium sulfate
The invention discloses a composite biological material for eliminating a bacterial biofilm.Ethylenediaminetetraacetic acid, lactic acid, lauryl sodium sulfate, n-nonanoic acid and glycerol monolaurate are compounded into a biofilm eliminating matrix capable of effectively eliminating the biofilm, the biofilm eliminating matrix and aloperine form the composite biological material, and the composite biological material is applied to eliminating the bacterial biofilm.The novel biofilm eliminating matrix capable of effectively eliminating the biofilm and aloperine form the composite biological material for eliminating the bacterial biofilm, the composite biological material is applied to eliminating the bacterial biofilm and has the advantages of being safe, free of resistance and environmentally friendly, through combined use, the inhibiting capacity of aloperine for the gram-positive bacterium biofilm can be enhanced, the defects of antibiotic therapy can be overcome, and the composite biological material is a novel biofilm eliminating biological material which is effective and free of resistance.
Owner:NINGXIA UNIVERSITY
Human antibiotic proteins
The invention relates to proteins, notably SAP-2 and SAP-3, having an antibiotic action. The invention also relates to a method for purifying certain antimicrobial proteins, as well as to a use of said antimicrobial proteins for antibiotic therapy or to a use of cells which were transfected with a DNA which codes for the proteins provided for in the invention.
Owner:PLANTON
Pharmaceutical composition for prevention and treatment of chicken gland gastritis
InactiveCN104338071AMake up for the shortcomingsAbundant resourcesDigestive systemPlant ingredientsTherapeutic effectSemen
The invention discloses a pharmaceutical composition for prevention and treatment of chicken gland gastritis. The pharmaceutical composition comprises the following components in parts by weight: 25-35 g of scutellaria baicalensis, 25-35 g of phellodendron amurense, 25-35 g of rhizoma sparganii, 25-35 g of rhizoma curcumae, 25-35 g of semen raphani, 25-35 g of radix aucklandiae, 25-35 g of coptis chinensis, 45-55 g of radix codonopsis, 90-110 g of radix isatidis, 25-35 g of liquorice, 25-35 g of semen armeniacae amarae, 25-35 g of rhizoma rehmanniae, 25-35 g of dried orange peel, and 25-35 g of hawthorn. Through full consideration of poultry diet characteristics, optimization design is carried out, the greatest degree of utilization of the poultry self digestive ability is beneficial for treatment effect, and thus the pharmaceutical composition not only can make up for the shortcomings of an antibiotic therapy, but also has the characteristics of rich resources, low cost and convenient use.
Owner:CHENGDU DALIANG POULTRY
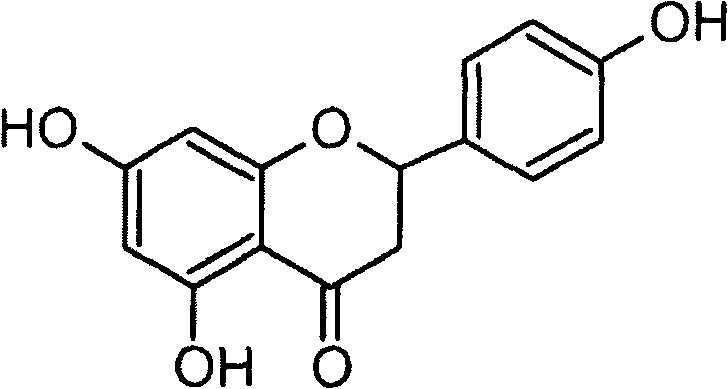
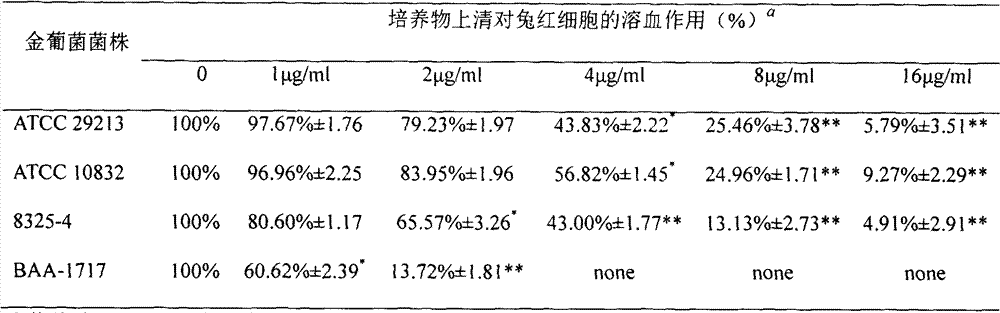
Application of naringenin in preparation of medicine for curing pneumonia
The invention relates to the application of naringenin in the preparation of medicine for curing pneumonia. The effect in staphylococcus aureus infection curing is proved through a rabbit erythrocyte hemolytic test, a pulmonary epithelial cell (A549) damage protection test and a mouse staphylococcus aureus pulmonary inflammation model. Compared with antibiotic therapy, naringenin therapy has the characteristics of no drug tolerance and high cure rate.
Owner:HUBEI WUDANG ANIMAL PHARMA
Compositions and methods to inactivate and/or reduce production of microbial toxins
The present invention is related to compositions and methods to treat, ameliorate and / or prevent morbidity and / or mortality from microbial infections. In particular, bacterial infections that are associated with the production and release of bacterial toxins. For example, many Clostridia bacteria, such as Clostridium difficile, release toxins resulting in tissue and organ damage and death, even after antibiotic therapy that either reduces or eliminates the bacteria. In particular, various peptides, polypeptides, and proteins are disclosed herein that either inactivate Clostridium difficile toxin and / or reduce Clostridium difficile toxin production.
Owner:BIOLOG
Pharmaceutical Composition of Enterosorbent and Prebiotics, Dosage Forms, and the Method for Prevention and Treatment of Gastrointestinal Disorders
InactiveUS20090163427A1Avoid consumptionReduce in quantityDigestive systemCarbohydrate active ingredientsAdditive ingredientIntestinal microorganisms
The pharmaceutical composition is a combination of hydrolytic lignin with moisture of 55% to 65% consisting of the particles measuring 0.15 mm to 0.55 mm, a 45% to 55% aqueous lactulose solution, and a 50% to 55% aqueous oligosaccharide solution at the following ingredient ratio (weight percent): an aqueous lactulose solution: 10÷60; oligosaccharides: 10÷50; hydrolytic lignin: quantity sufficient. Hydrolytic lignin, lactulose and fructose oligosaccharides are sequentially added and mixed using a rotor blender. The composition is administered orally for no less than 14 days and no more than 30 days, two to four times a day, depending on the patient's weight and age. The composition is used as a medicine for treatment of the gastrointestinal disorders, including bacterial, viral, protozoal enteric infections, food poisoning, acute and chronic hepatitis and cirrhosis, diarrhea, peptic ulcer, Crohn's disease, ulcerative colitis, irritable bowel syndrome, mineral disorders with Ca / Mg deficiency, including osteoporosis and other alterations of the bone formation, as an immunomodulator in atopic dermatitis and immunodeficiency conditions, for protection and recovery of intestinal flora after antibiotic therapy, chemotherapy, and radiotherapy. The result is accelerated achievement of the effect and the enhanced action on the state of intestinal microbiocenosis as well as increased effectiveness of treatment of hepatitis and liver cirrhosis, elimination of undesired adverse effects in clinical usage, and extension of indications, i.e. the extended spectrum of usage in prevention and treatment.
Owner:DIKOVSKIY ALEKSANDER VLADIMIROVICH
Application of bacillus subtilis in antagonism helicobacter pylori
InactiveCN102133238AStrong antagonistic effectOvercome side effectsAntibacterial agentsBacteriaNutrient brothSide effect
The invention relates to an application of bacillus subtilis in antagonism helicobacter pylori, wherein substances capable of restraining the growth of helicobacter pylori can be generated when the shake cultivation is performed on the bacillus subtilis for 9 hours at 37 DEG C and at a speed of 180 rpm (revolutions per minute) in a nutrient broth liquid culture medium, thereby efficiently restraining the growth of the helicobacter pylori; when the bacillus subtilis is cultivated for 18 hours, the antibiotic ability is maximum; and antagonistic proteins are acquired by purifying the substances, thereby preparing a preparation for treating gastrointestinal tract diseases. The invention has the following technical effects: the bacillus subtilis from probiotics has a function of maintaining the micro ecological balance of an intestinal tract; the bacillus subtilis culture has stronger antagonism to the helicobacter pylori; and compared with the antibiotic treatment method used for conventionally treating the helicobacter pylori, the bacillus subtilis can be used for overcoming the defects that the side effect by using antibiotic treatment method is strong and the symptoms are easily reappeared after the disease is treated, and the like.
Owner:NANCHANG UNIV
Bovine enterovirus egg yolk antibody and preparation
PendingCN114539396ARich treatment methodsAvoid passingEgg immunoglobulinsDigestive systemAntiendomysial antibodiesRumen
Aiming at the physiological characteristics of ruminants, in order to solve the problems of digestion and inactivation of the egg yolk antibody in the rumen, the invention creatively provides two preparation modes, one is an intestinal preparation which can prevent the egg yolk antibody from passing through the rumen, and research shows that after the intestinal preparation is applied to calves, the intestinal preparation has a treatment effect on calf diarrhea caused by BEV infection, and the intestinal preparation can be used for treating the diarrhea of the calves caused by BEV infection. The effect is close to that of a traditional drug therapy, but compared with an antibiotic therapy, the method is more environment-friendly and safer; the other one is a rumen bypass preparation which can be added as a part of daily ration concentrate and is convenient to use, after the egg yolk antibody is prepared into the rumen bypass preparation, the egg yolk antibody can be prevented from being degraded in the rumen and released in the intestinal tract at a fixed point, and the treatment effect on diarrhea caused by BEV infection is achieved.
Owner:NANYANG NORMAL UNIV
Repurposing compounds for the treatment of infections and for modulating the composition of the gut microbiome
PendingUS20200368218A1Growth inhibitionEliminate side effectsOrganic active ingredientsAgainst vector-borne diseasesClostridium difficile (bacteria)Bacterosira
The present invention relates to agents and compositions for the modification of the growth of bacterial cells. Thus, the compounds of the present invention are useful for the prevention and / or treatment of a disease in a subject. In particular, the present invention relates to the field of repurposing pharmaceutical compounds for treatment strategies of infectious diseases, gastrointestinal disorders, inflammatory diseases, proliferative diseases, metabolic disorders, cardiovascular diseases, and immunological diseases. Some of the compounds of the present invention demonstrate high specificity in inhibiting the growth of single bacterial species. Such compounds enable narrow-spectrum antibacterial therapies, constituting a major effort of current and future drug development strategies in order to reduce side effects of antibacterial treatment plans. Particularly interesting compounds of this invention are effective against pathobiological species such as Clostridium difficile, Clostridium perfingens, Fusobacterium nucleatum, and an enterotoxigenic strain of Bacteroides fragilis. Other compounds of the present invention reveal a strong inhibitory effect on a broad spectrum of bacterial species. Such compounds are useful for broad-spectrum antibiotic therapies of infections with unknown causative infecting bacterial species. Both types of compounds, especially the ones with narrow-spectrum antibacterialactivity, can further be used for modulating the microbiome composition and targeting species associated with dysbiosis and disease.
Owner:EURO LAB FUER MOLEKULARBIOLOGIE EMBL
Antibody against alpha-hemolysin and application thereof
ActiveCN112538112AImprove securityShort course of treatmentAntibacterial agentsAntibody mimetics/scaffoldsPulmonary infectionHemolysis
The invention provides an antibody or a fragment thereof combined with staphylococcus aureus alpha-hemolysin, and application of the antibody or the fragment thereof in preventing or treating staphylococcus aureus infection. The antibody is obtained by screening through a strategy of attenuated immunity and virulent screening of the alpha-hemolysin, has high affinity to the alpha-hemolysin, can effectively block the hemolysis of the alpha-hemolysin, proves a significant protective or therapeutic effect in an alpha-hemolysin sepsis model, an MRSA bacteremia model and an MRSA pulmonary infectionmodel, has a synergistic effect with antibiotics, and is a beneficial supplement to the existing antibiotic therapy of staphylococcus aureus.
Owner:MABWELL (SHANGHAI) BIOSCIENCE CO LTD
Device and method for diagnostic analyses
ActiveUS8927258B2Promotes and accelerates bacterial growthAvoid loadBioreactor/fermenter combinationsBiological substance pretreatmentsAntibiotic therapyAntibiotic Y
An integrated device for diagnostic analyzes used to verify the presence of bacteria in at least a biological sample mixed with a eugonic culture medium in liquid form, to classify at least the type of bacteria, and to test a series of antibiotics, selected from a group of characteristic antibiotics at least for the type of bacteria identified, identifying those effective to determine the antibiotic therapy. The device comprises, inside an integrated structure, first containing means provided with containing elements in which the biological samples to be analyzed are distributed, second containing means comprising recipients or micro-plates thermostated with wells containing a eugonic culture medium in liquid form in which a first fraction of the biological samples to be analyzed is dispensed, and a first recipient and second recipients or plates with a relative first well and second wells in which a further fraction of the biological samples which resulted positive to the analysis is dispensed.
Owner:ALIFAX
Anti-microbial defensin-related peptides and methods of use
An antimicrobial peptide and its analogs that are insensitive to physiological salt and divalent cation concentrations is provided, as are methods for their use to treat and prevent bacterial infections. The peptides are especially useful to treat infections caused by bacteria that are resistant to traditional antibiotic therapy.
Owner:BOARD OF REGENTS FOR OKLAHOMA STATE UNIVERSITY
Medicament for treating enteritis
InactiveCN103372190AAbdominal pain symptoms disappearedReduced symptoms of diarrhea and abdominal painDigestive systemPlant ingredientsSide effectGLYCYRRHIZA EXTRACT
The invention relates to a traditional Chinese medicine and especially provides a medicament for treating enteritis. The medicament is to solve a problem of side effects caused by antibiotic therapy of enteritis. The medicament is prepared from the following medicinal materials in proportion: 7-15 parts of radix bupleuri, 15-21 parts of Scutellaria baicalensis, 14-19 parts of immature bitter orange, 4-6 parts of red peony root, 3-6 parts of root of kudzu vine, 10-14 parts of carbonized sanguisorba root, 11-14 parts of rhizoma cyperi, 8-10 parts of rhizoma zingiberis, 4-6 parts of liquorice, 10-15 parts of dried orange peel, 10-13 parts of rhizoma atractylodis, 1-4 parts of coptis chinensis, 5-8 parts of scorched hawthorn fruit and 8-10 parts of schisandra chinensis. Through 2 courses of treatment, 38 cases are cured, 17 cases get better, 27 cases begin to get better and 8 cases do not get better; therefore, the total effective rate is higher than 90%..
Owner:王振海
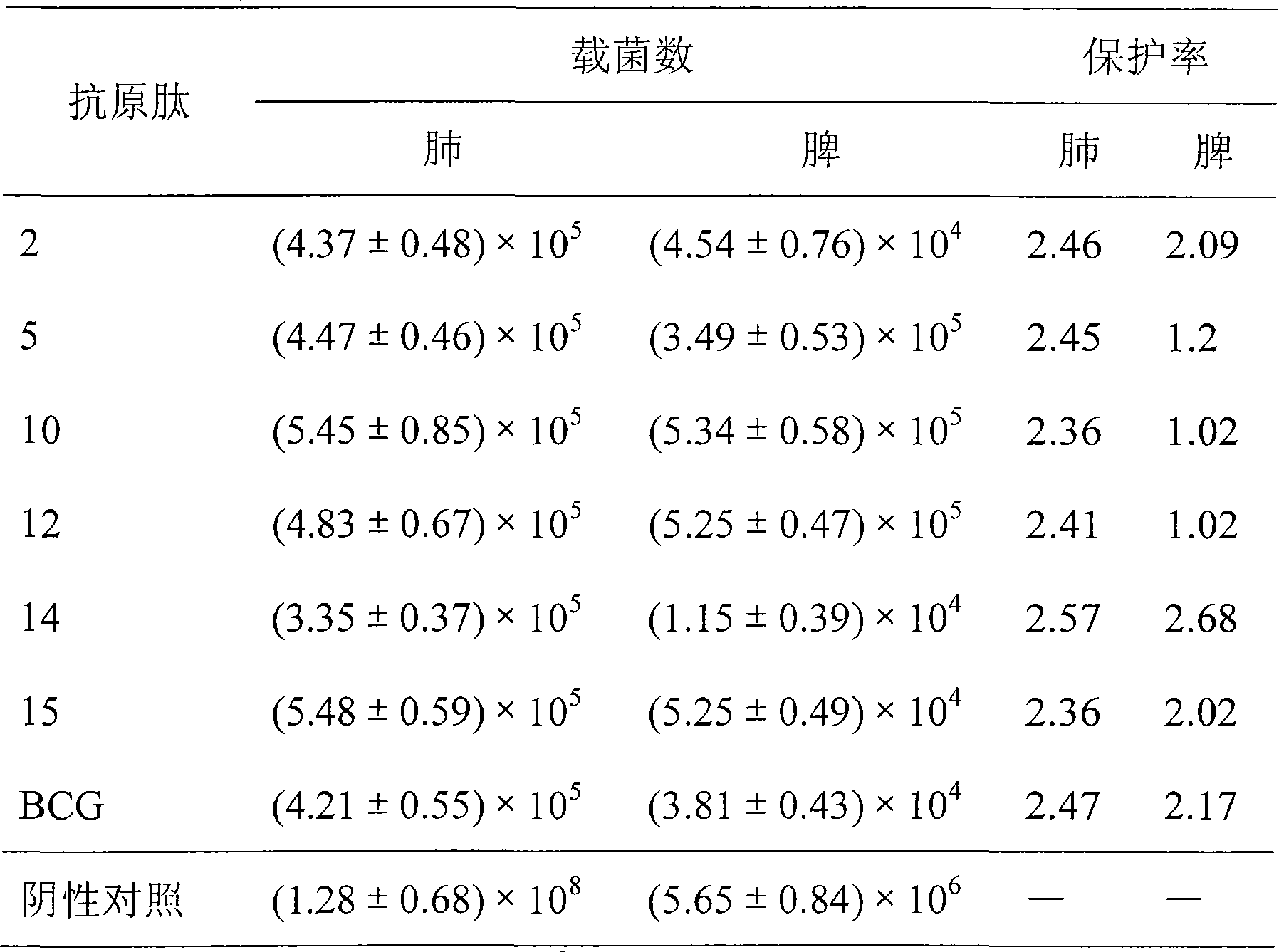
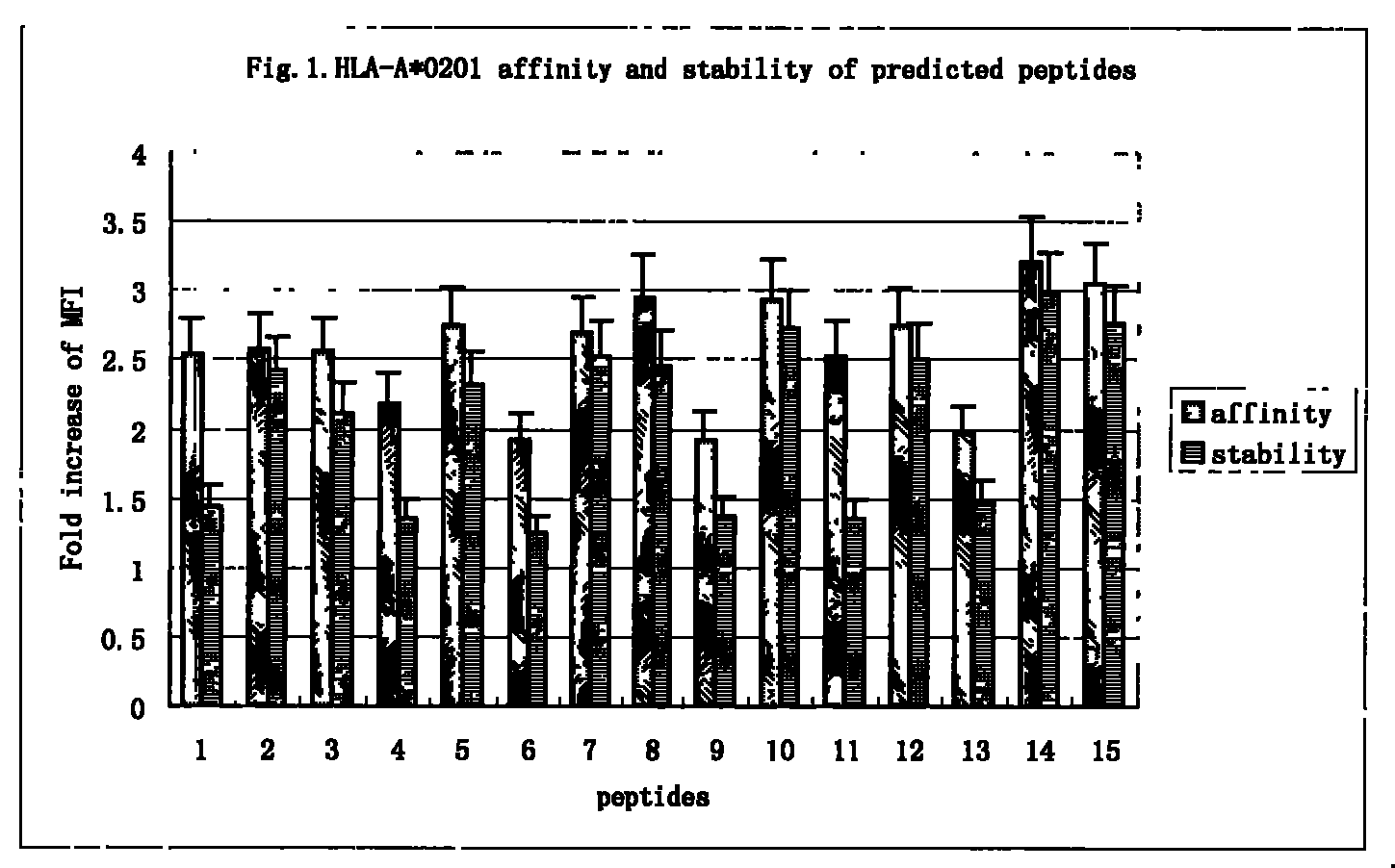
Antigen epitope for exciting protective immunity against tubercle bacillus of human body and uses thereof
InactiveCN101311189BHelp controlMulti-drug resistance problem solvedAntibacterial agentsBacterial antigen ingredientsMolecular ImmunologyScreening method
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com