Colonic delivery using Zn/pectin beads with a Eudragit coating
A colon and pectin technology, applied in the directions of capsule delivery, microcapsules, peptide/protein components, etc., can solve problems such as aggravating the discomfort of the original disease and the bad feeling of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
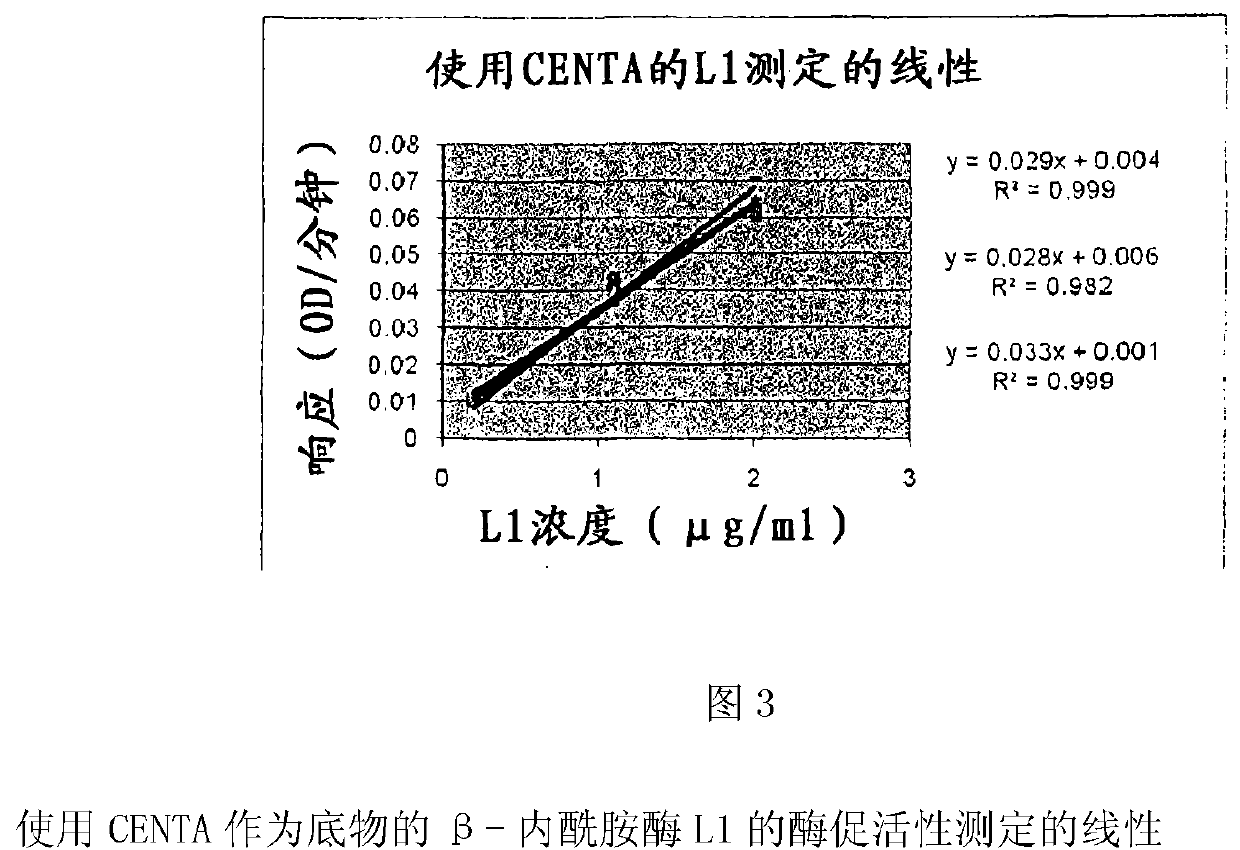
[0268] Example 1: Development of a sensitive, quantitative and specific assay for β-lactamase L1
[0269] Hydrolysis of nitroceftin is a well-known technique for quantification of penicillinase activity. However, the usual format is in a single tube and is not suitable for the analysis of large numbers of samples. This example describes the development and fit for purpose qualification of this assay performed in a 96-well microplate format.
[0270] A stock solution of nitroceftin was obtained by dissolving dry powder of nitroceftin in dimethyl sulfoxide (DMSO) at a concentration of 10 mM. Stock solutions were stored at -20°C and diluted 100-fold immediately before use in 50 mM sodium phosphate buffer (Hepes buffer) pH 7.0 containing 0.1 mg / ml bovine serum albumin (BSA). The choice of buffer is described in Table 1.
[0271] Add 20 μl of the solution to be analyzed to 180 μl of the diluted nitrocefamin. The kinetics of nitrocefaxifen hydrolysis was measured by absorbance m...
Embodiment 2
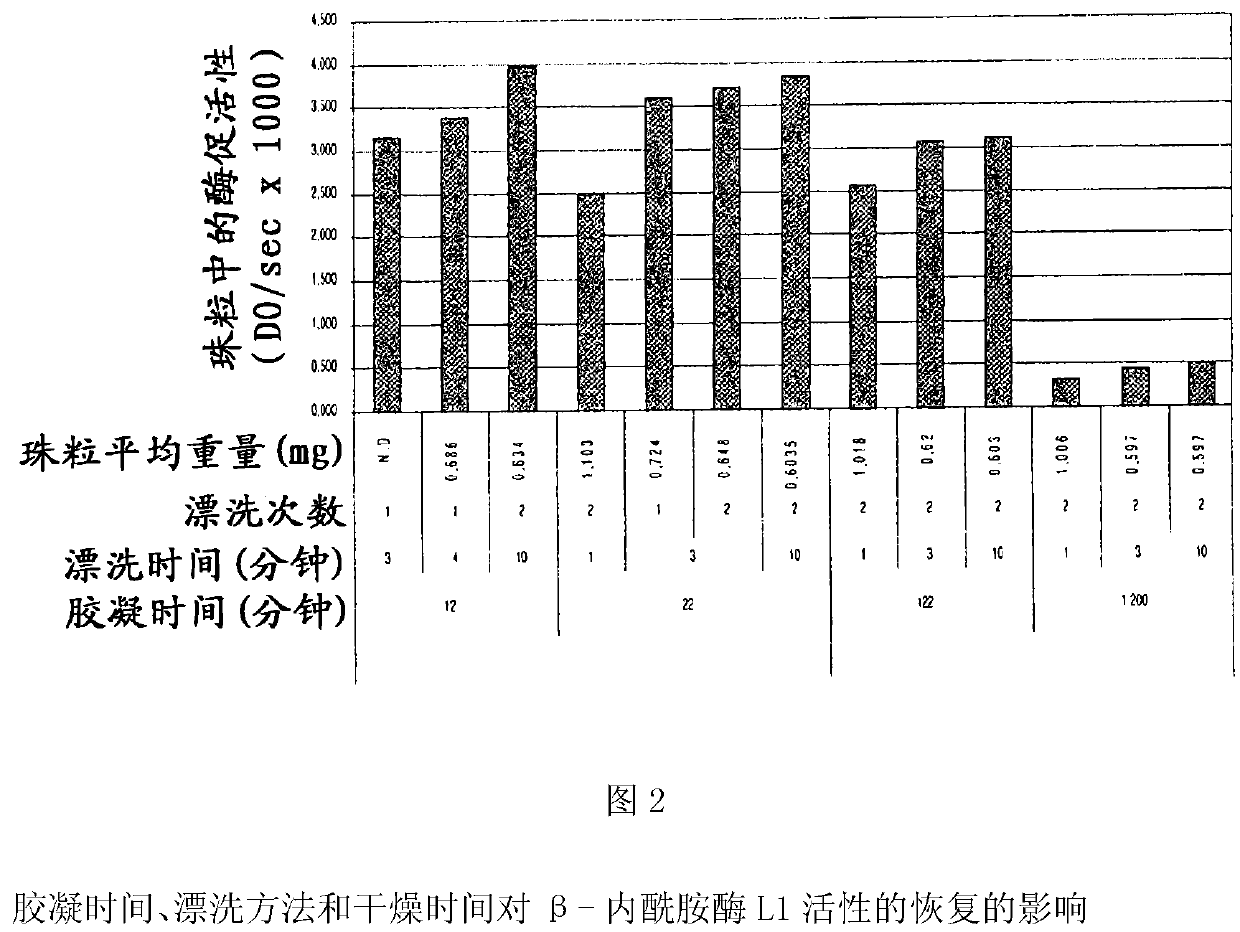
[0278] Example 2: Instability of β-lactamase L1 in raw pectin mixtures and the role of metal counterions
[0279] 0.3 ml of β-lactamase L1 (Eurogentec, Belgium, approximately 10 mg / mL by μBCA assay) was mixed with 10 g of a 6% pectin solution (low methoxylated amidated pectin (Unipectine ), Texturant Systems, cat#OG175C) mixed; the pH of the pectin solution was not adjusted.
[0280] Under the condition of stirring (200rpm) at room temperature, the pectin / β-lactamase L1 mixture was added dropwise to 40ml of calcium chloride (6 %) of the beaker.
[0281]After further incubation to equilibrate free and bound calcium ions, the beads were recovered by filtration and washed 3 times in 200 ml of purified water to remove excess free calcium. At this stage, the beads are referred to as "gelled beads".
[0282] The beads were dried in an oven at 37°C for 2 hours, resulting in dried beads.
[0283] 2x5 drop and 2x15 drop samples were taken at the outlet of the needle to measure init...
Embodiment 3
[0288] Example 3: Optimization of metal ions for gelling pectin, and effect of pH of pectin solution
[0289] To determine the effect of pectin solution parameters and zinc ions, an experiment comparing 4 formulations was carried out. The design scheme was established according to the factorial design Design Expert 6.0.10, Stat-Ease, Minneapolis. Check two parameters:
[0290] (a) pH of pectin solution: 4.0 and 7.0.
[0291] (b) Metal cations in the gelling water bath: Ca 2+ (CaCl 2 ) or Zn 2+ (Zinc acetate is abbreviated as ZnAc)
[0292] Beads were prepared as described in Example 2. However, since the stability of pectin decreases with increasing pH, the concentration of pectin solution was reduced from 6% to 4%.
[0293] Encapsulation yield was measured by assaying the enzymatic activity of β-lactamase L1, as described in Example 1.
[0294] Five beads were dissolved in 20 ml at 4°C in the presence or absence of 1% pectinase (Pectinase from Aspergillus aculeatus, P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com