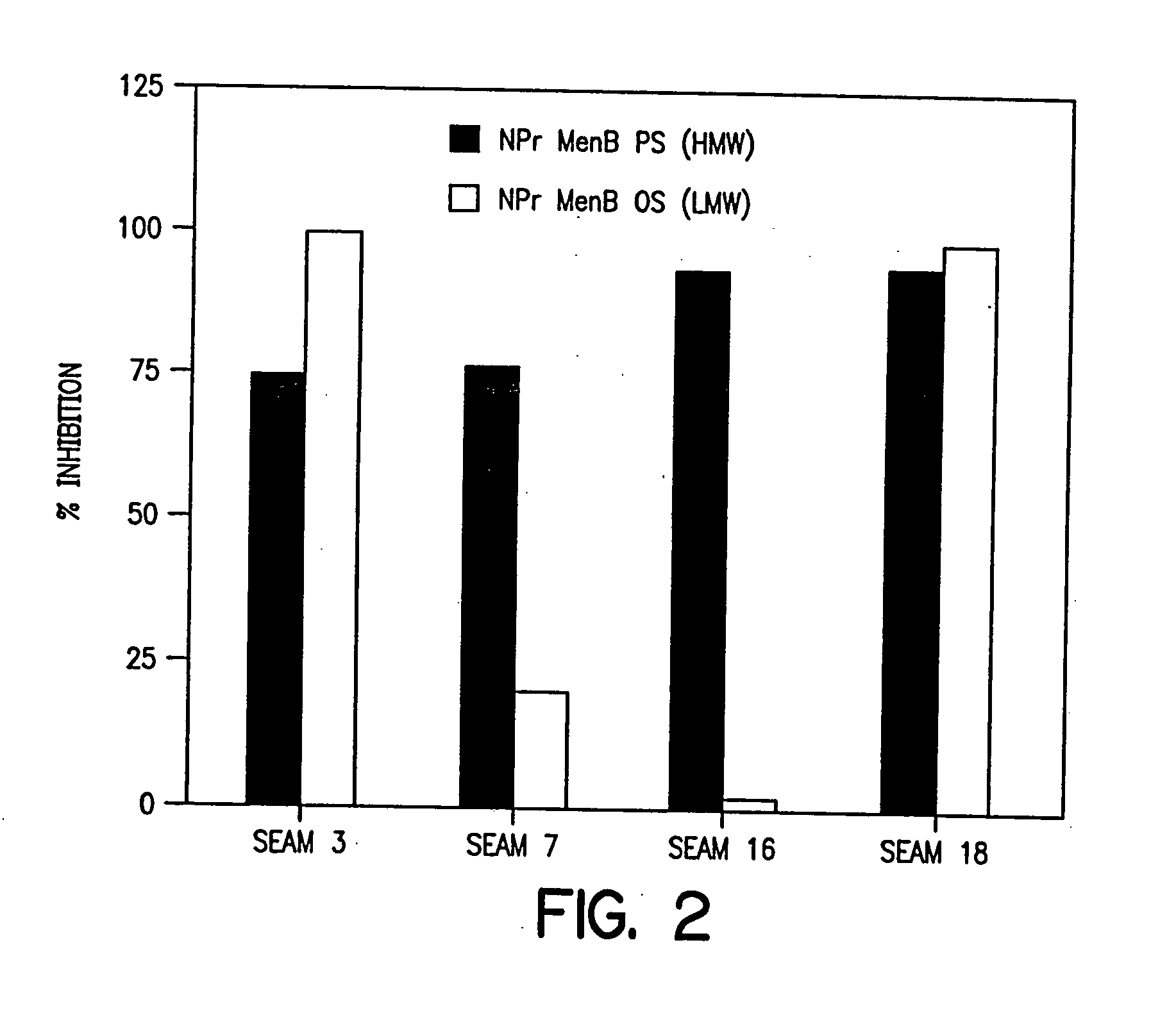
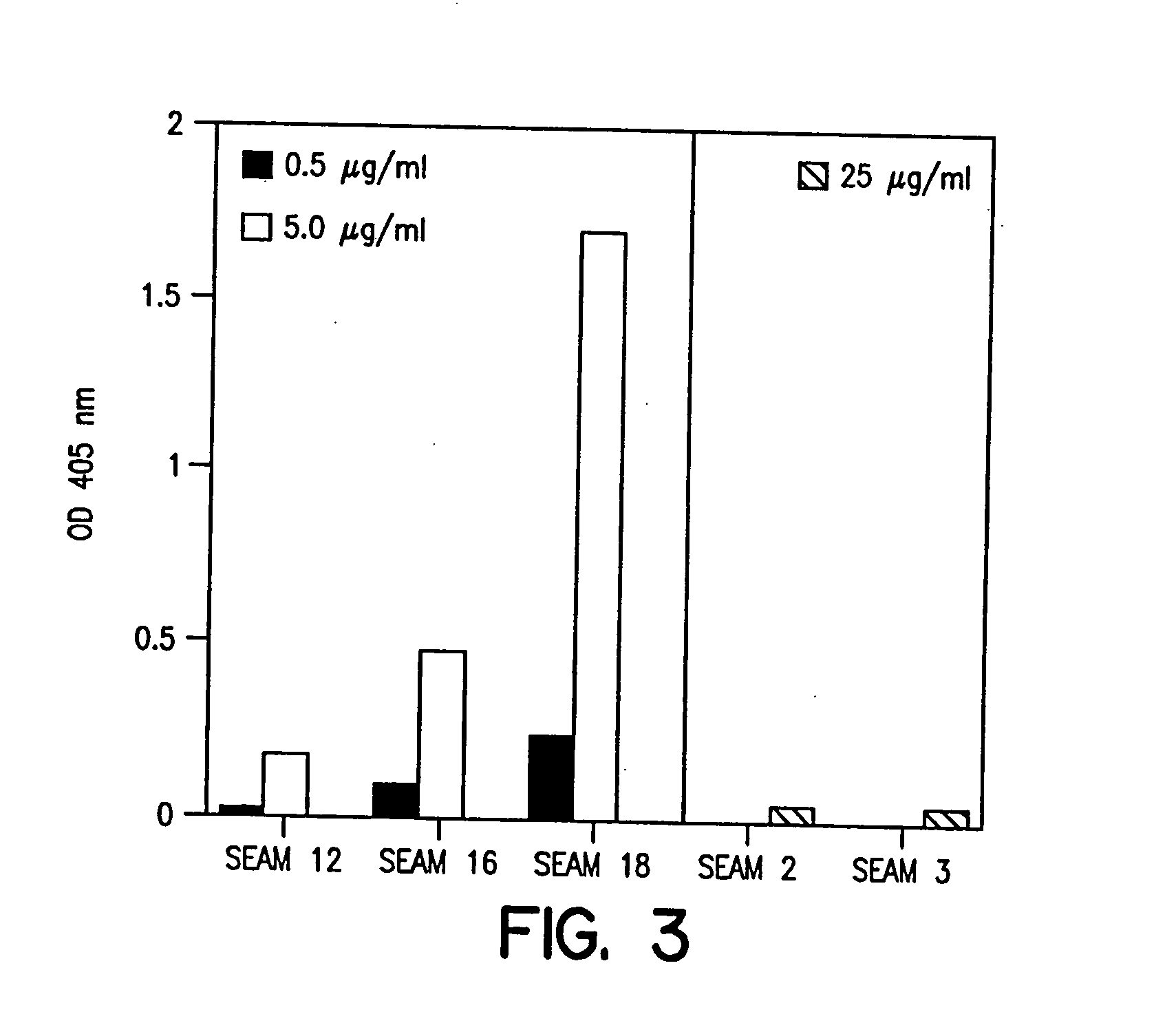
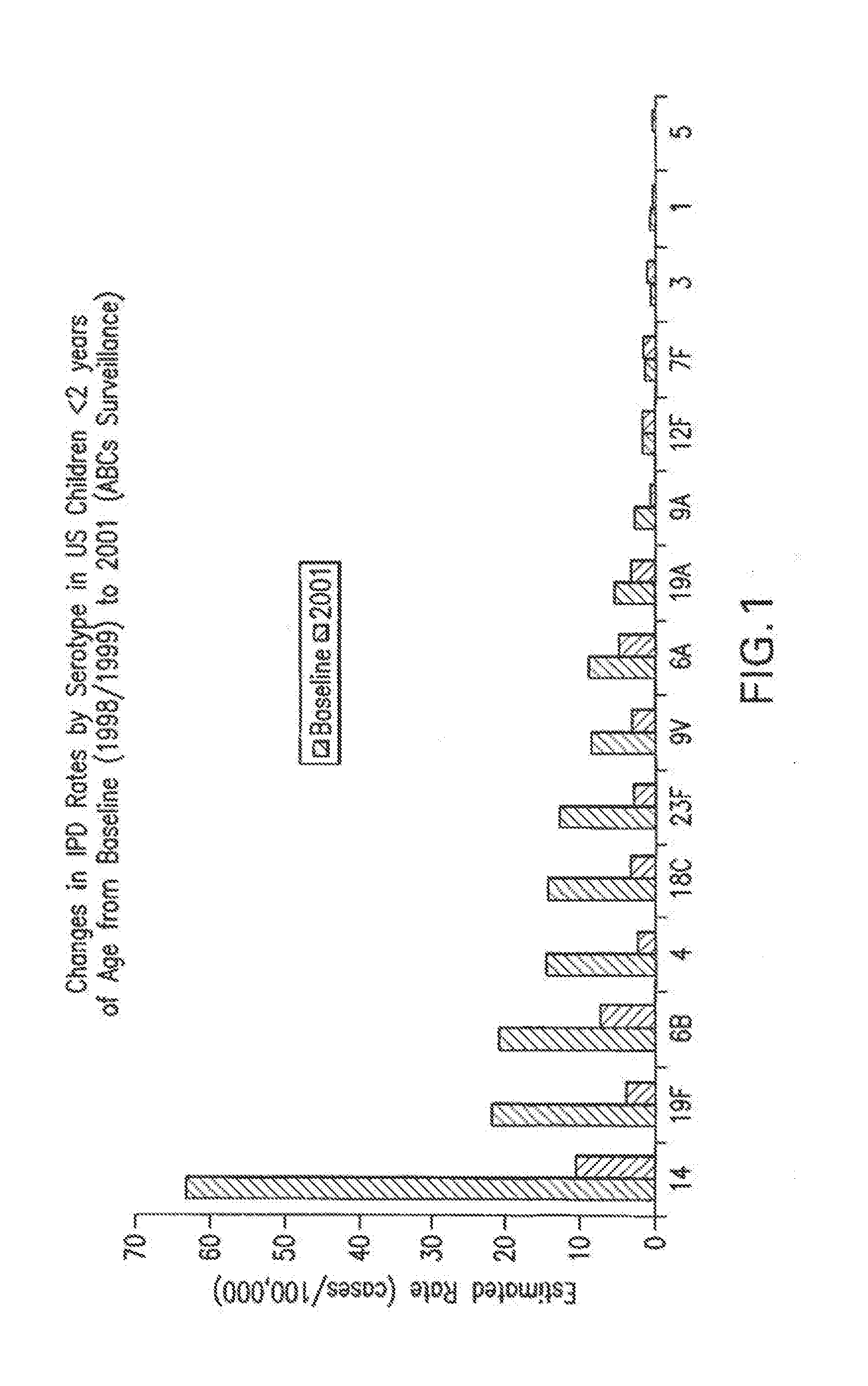
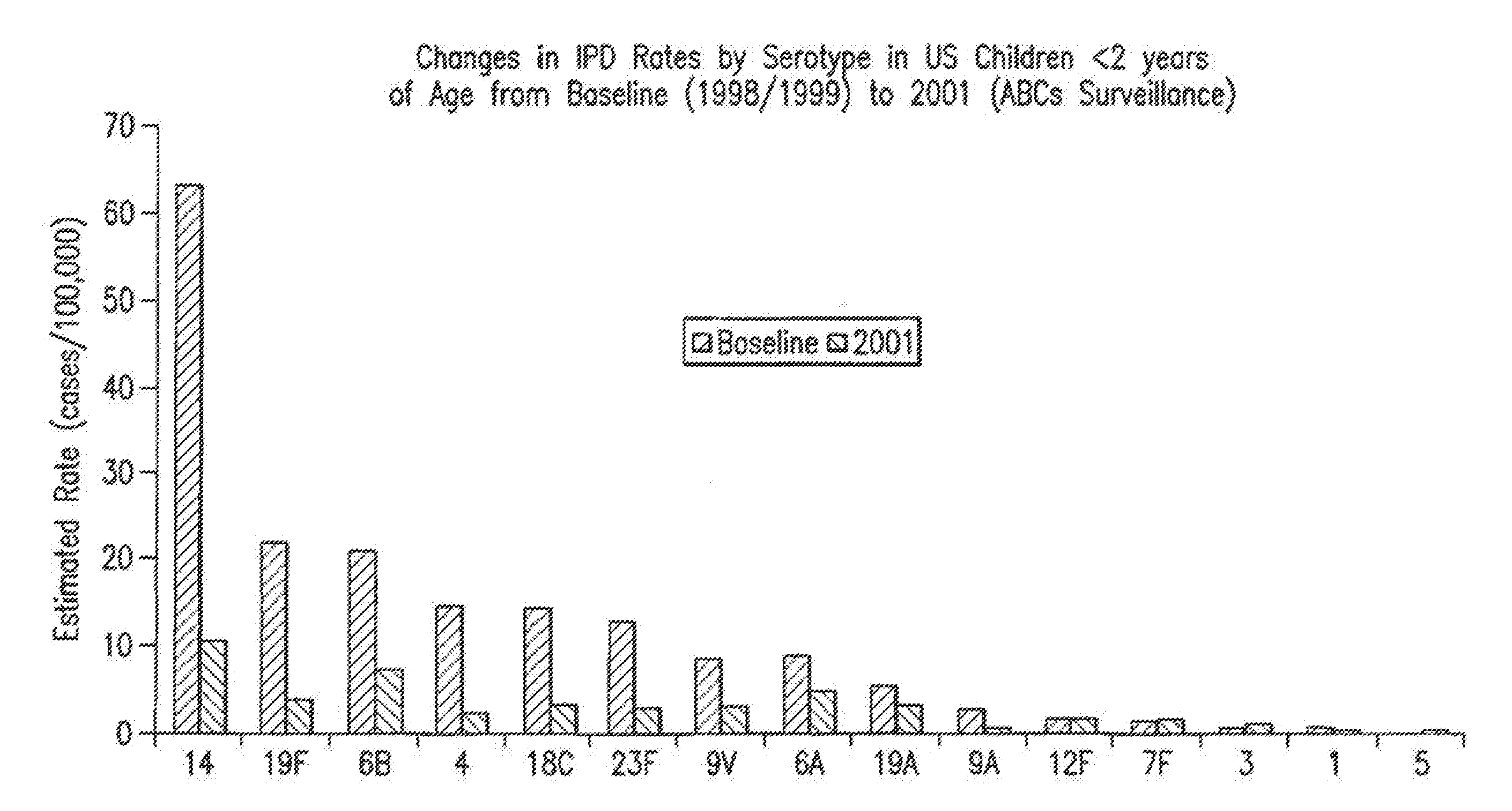Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
103 results about "Salmonella serotype typhi" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
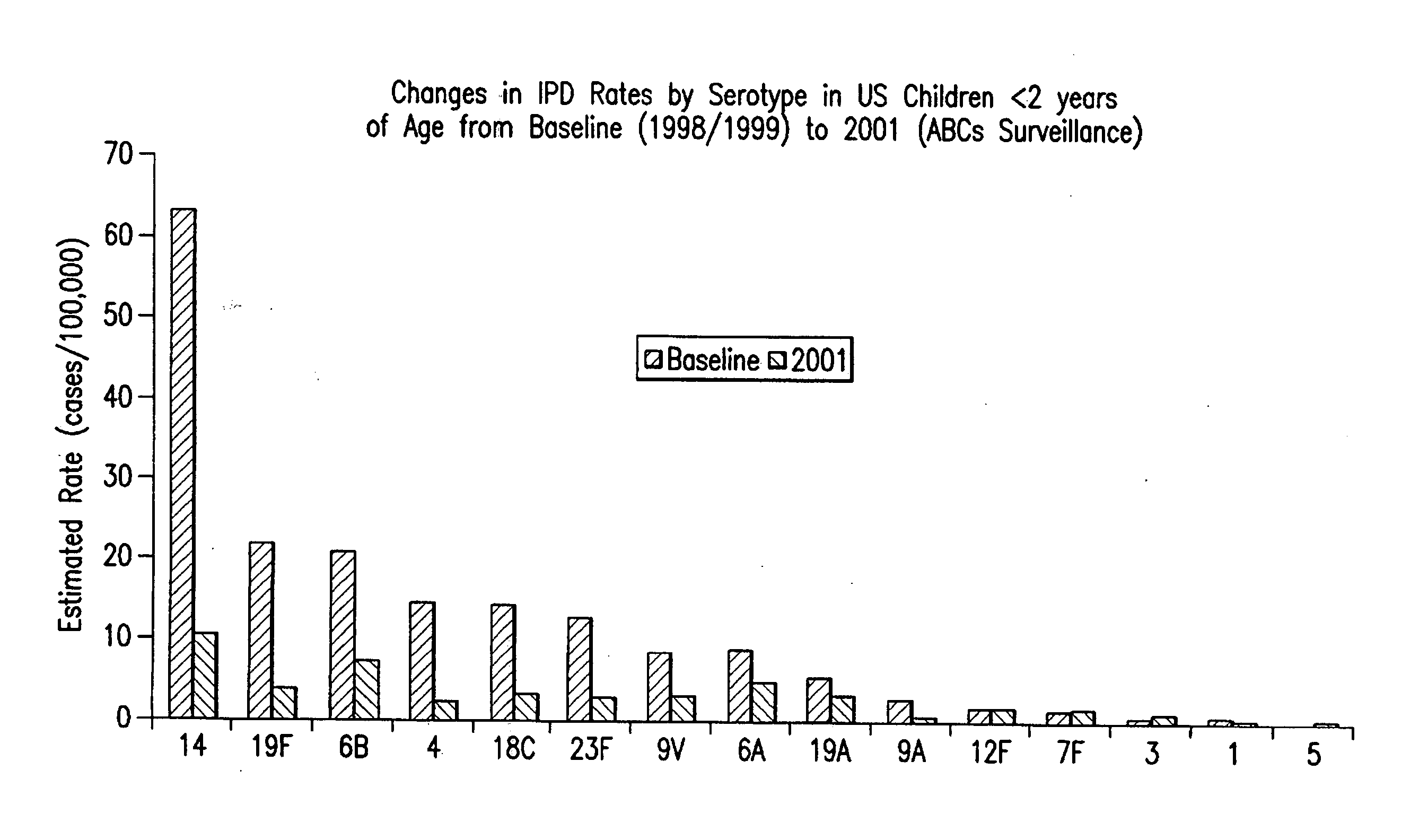
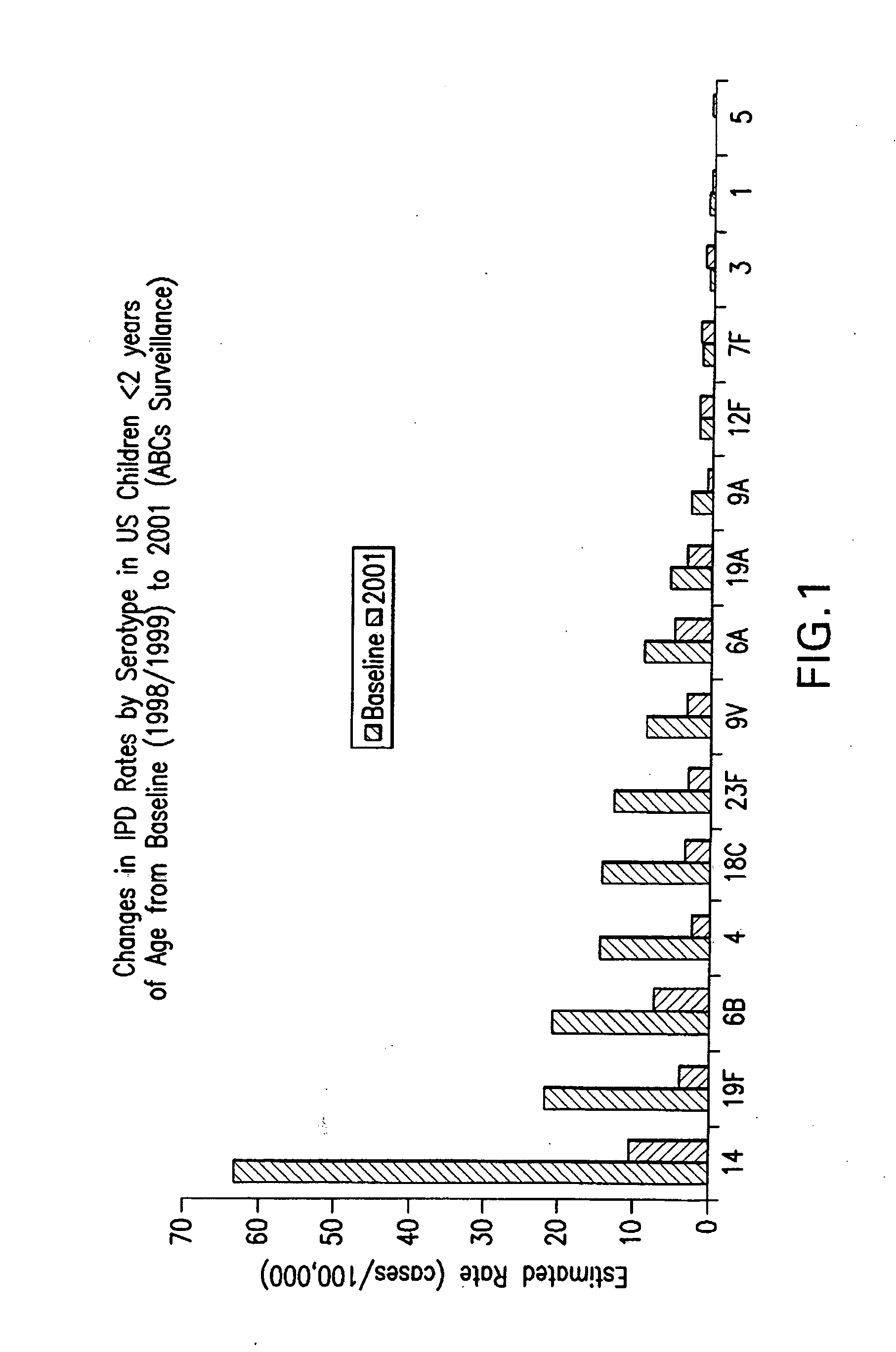
The Salmonella genus of bacteria, for example, has been determined to have over 2600 serotypes, including Salmonella enterica serovar Typhimurium, S. enterica serovar Typhi, and S. enterica serovar Dublin. Vibrio cholerae, the species of bacteria that causes cholera, has over 200 serotypes, based on cell antigens.
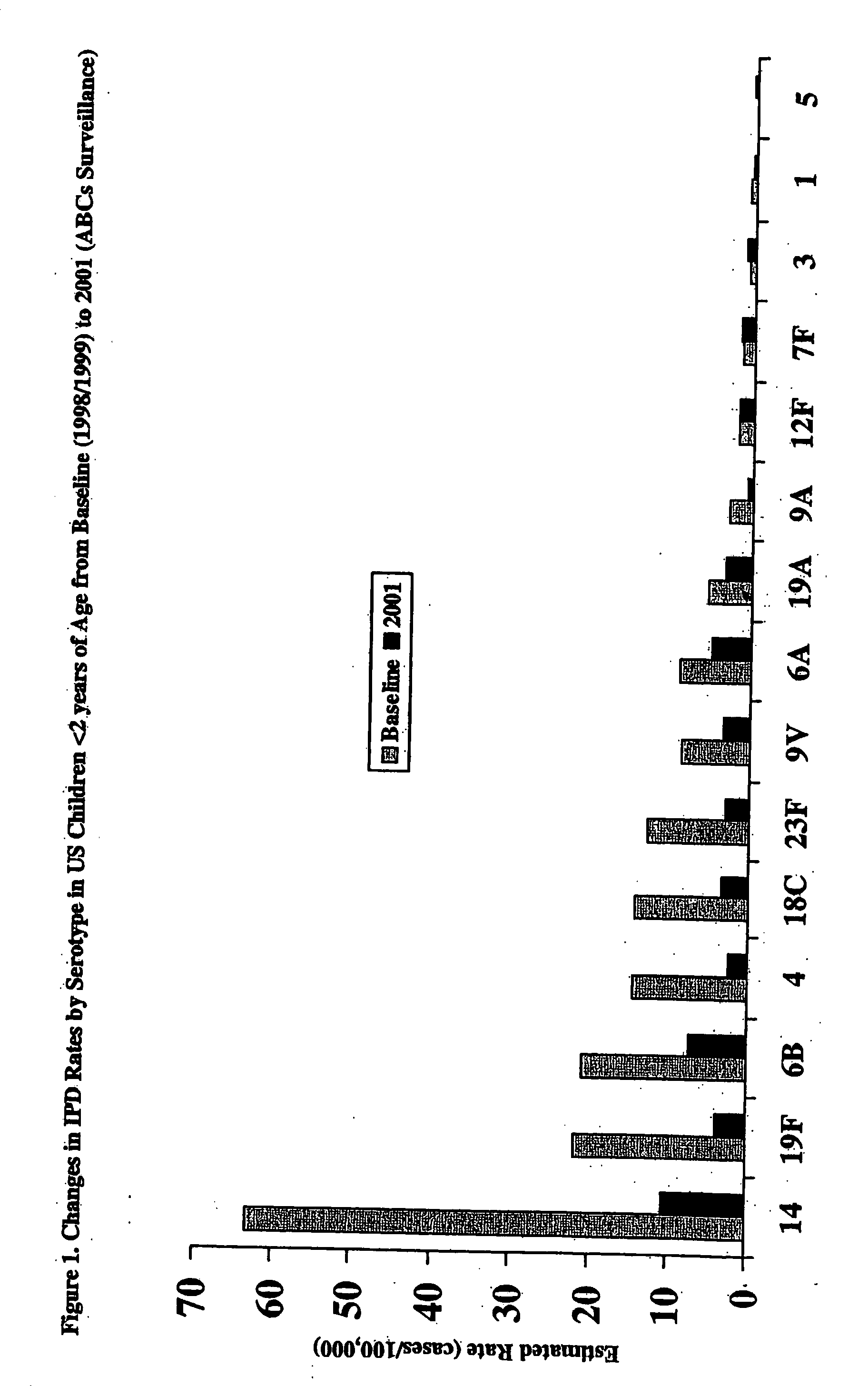
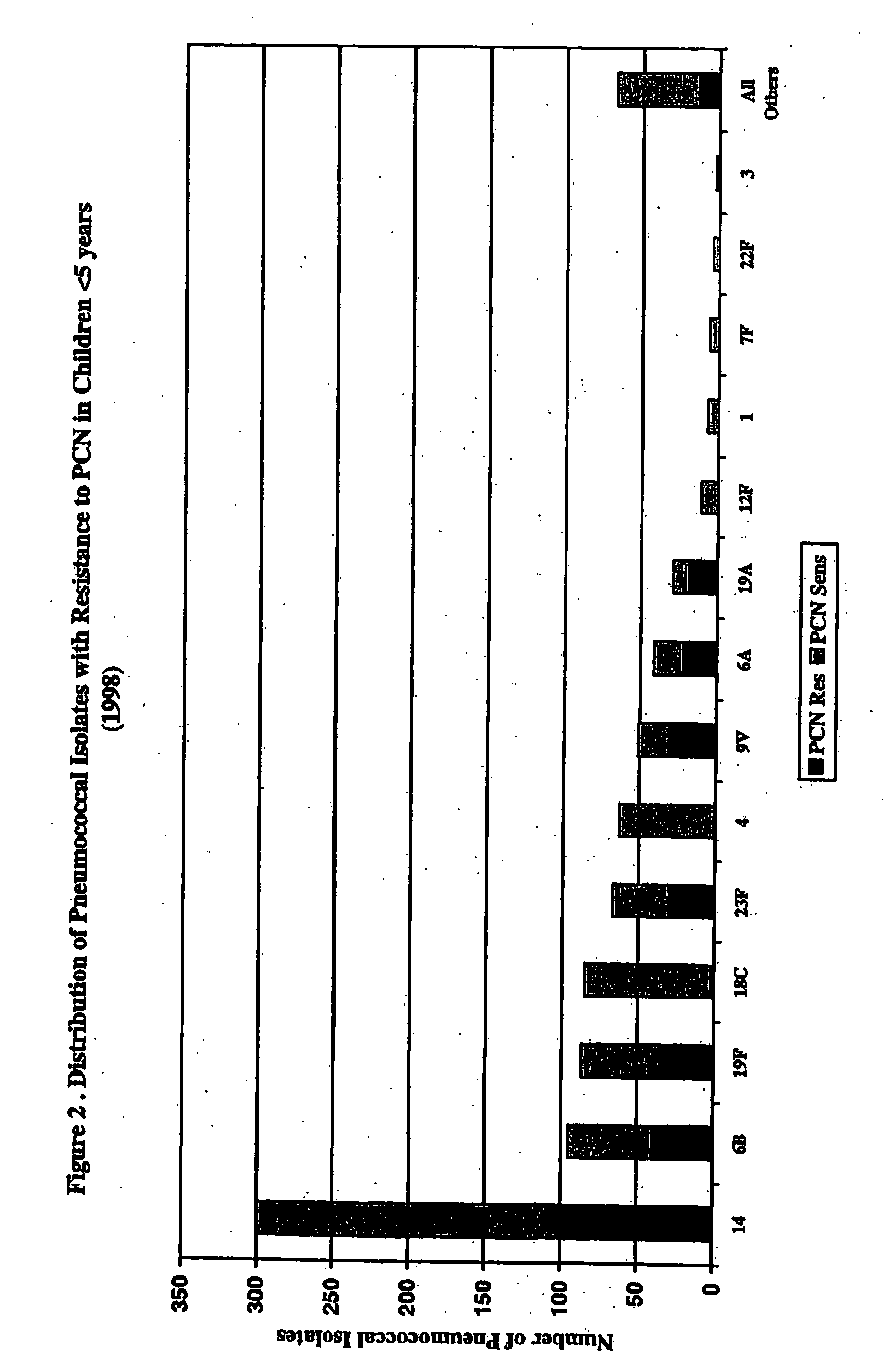
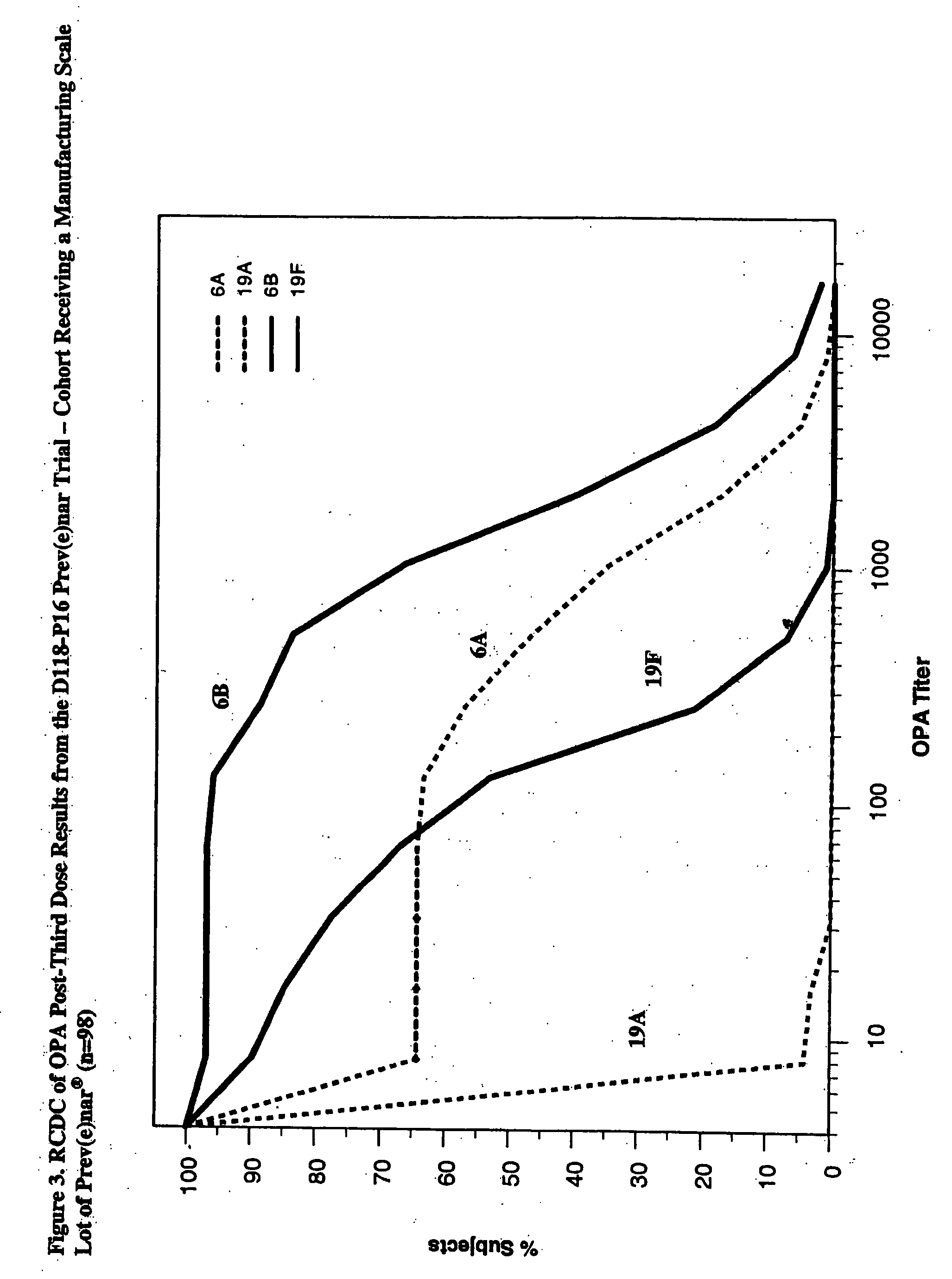
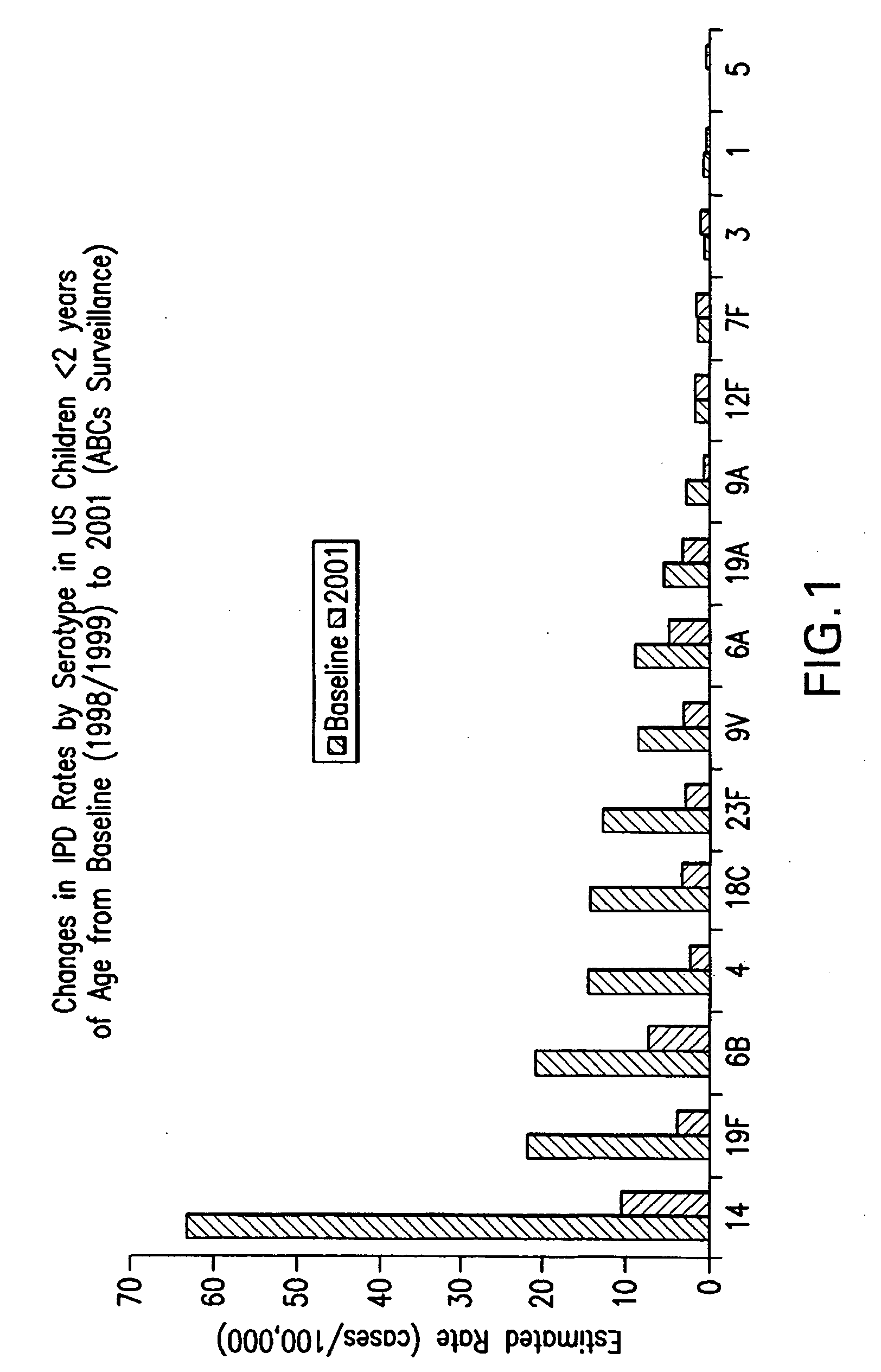
Multivalent pneumococcal polysaccharide-protein conjugate composition
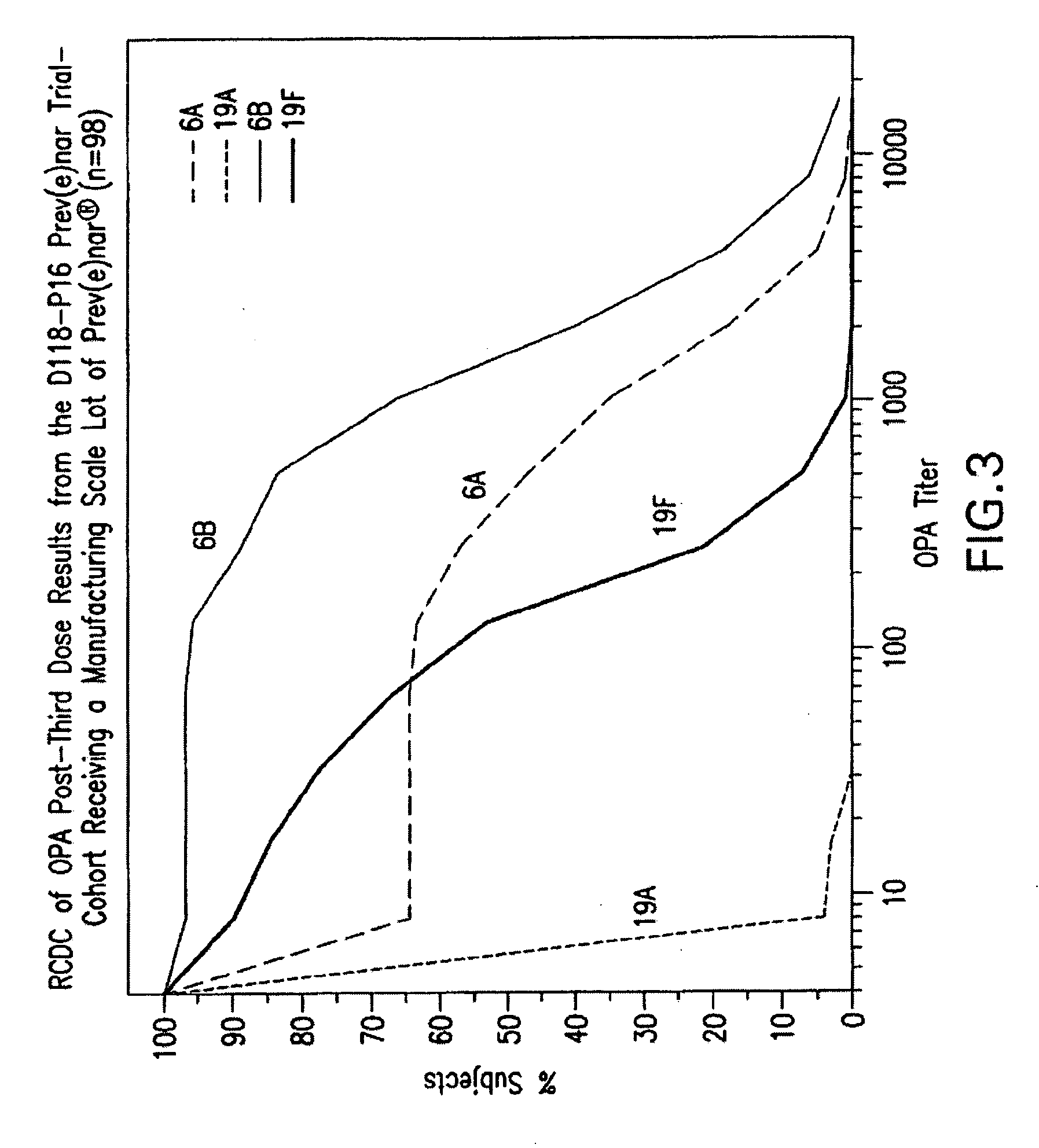
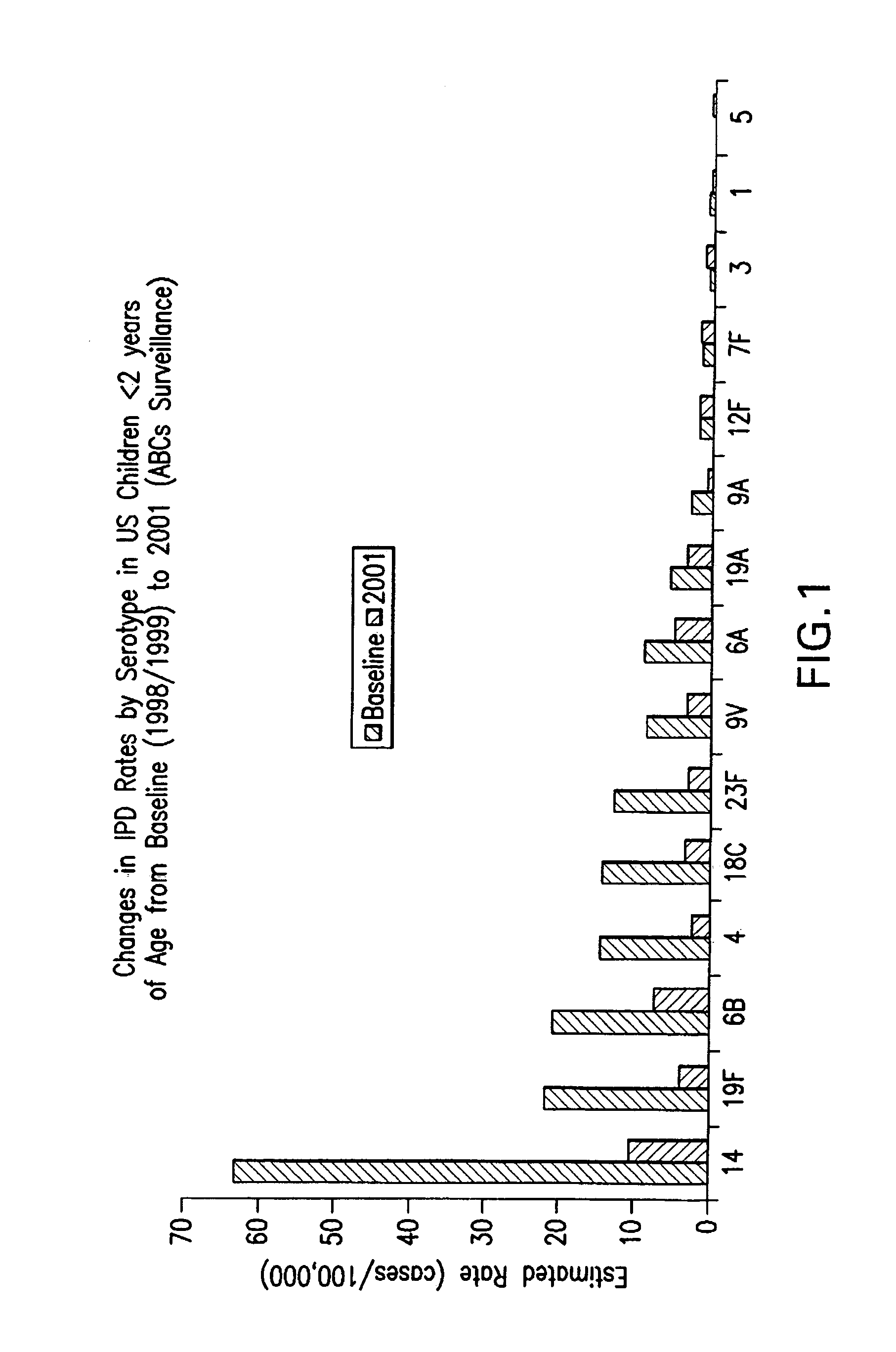
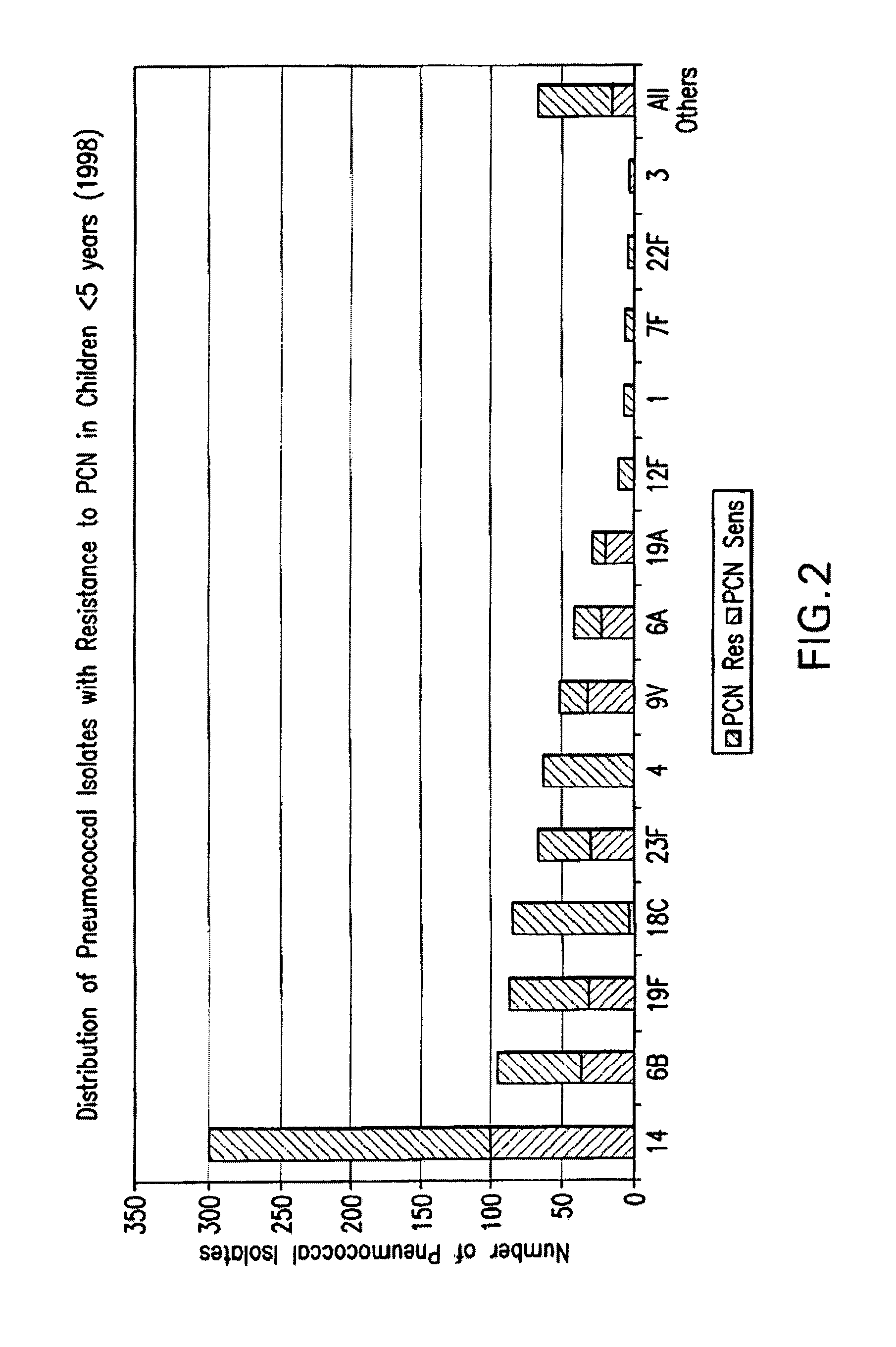
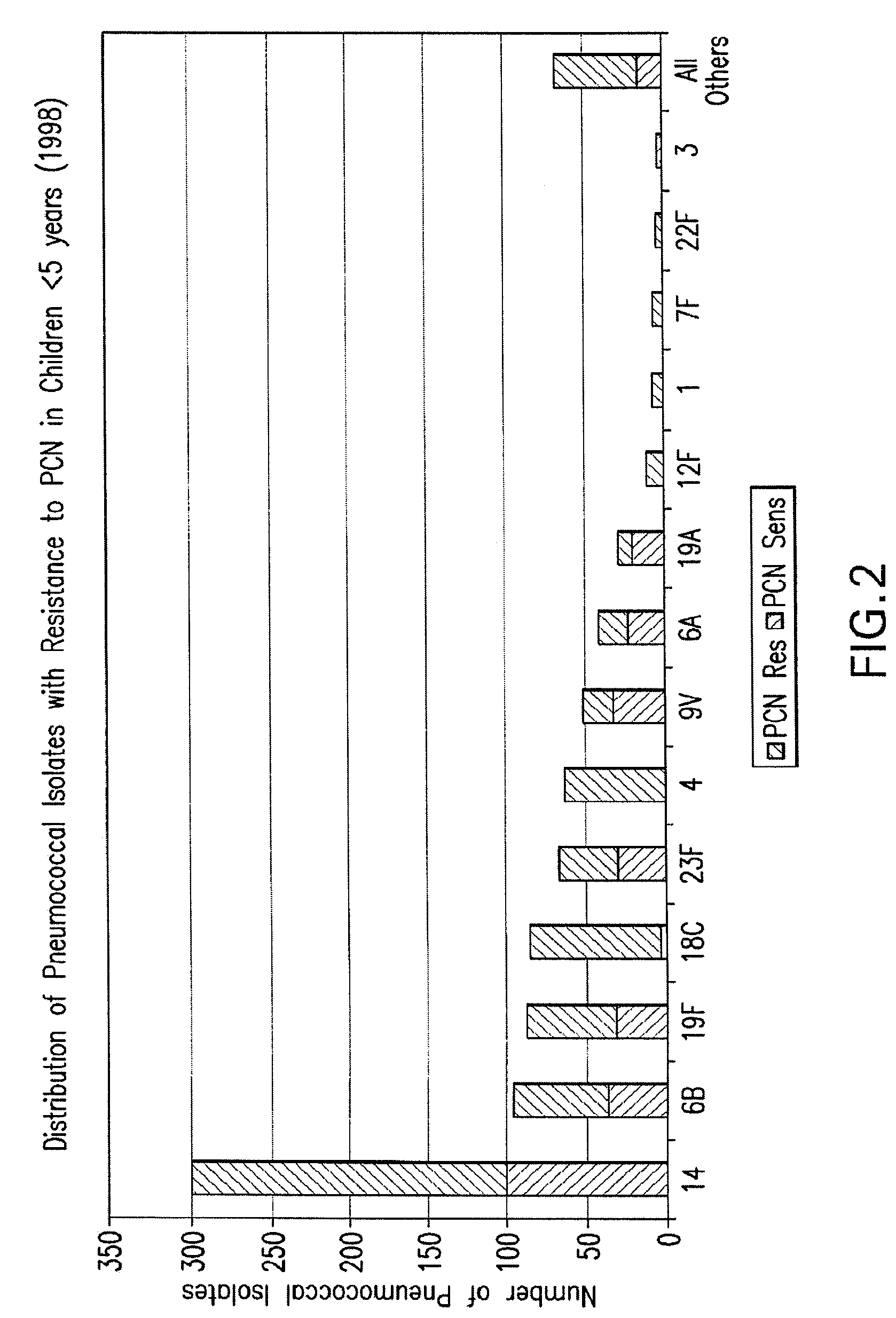
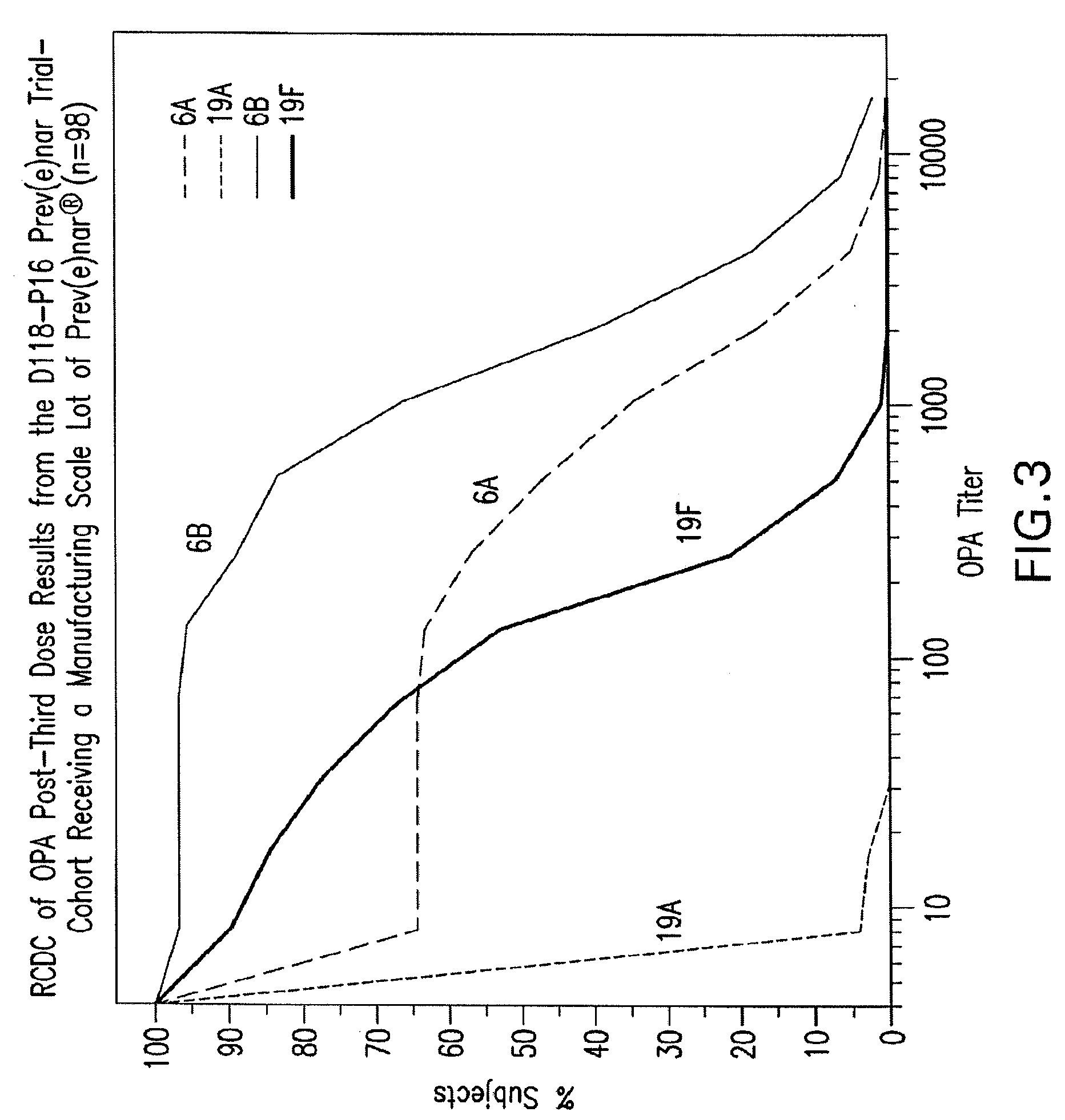
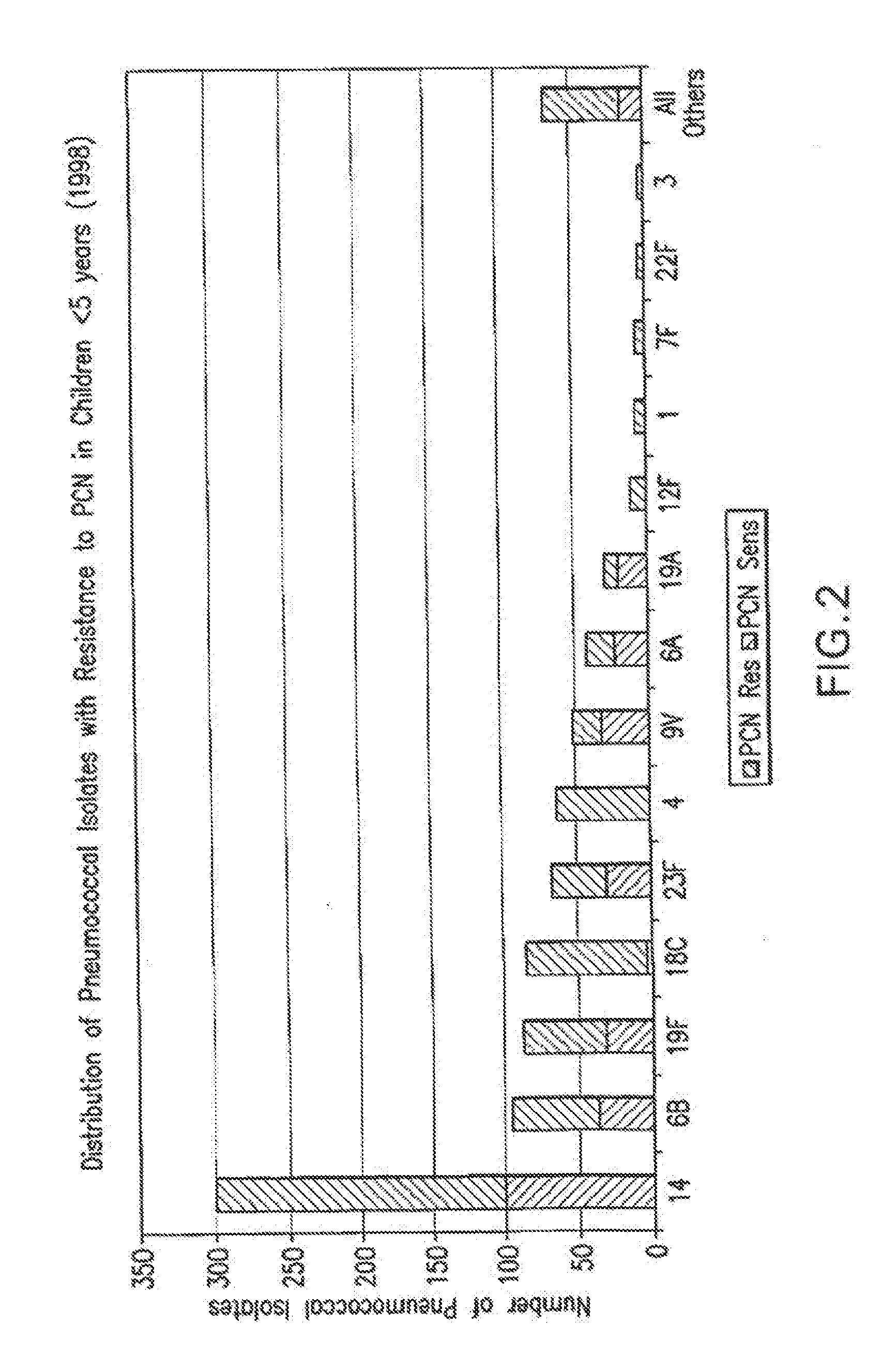
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection.
Owner:WYETH LLC
Multivalent pneumococcal polysaccharide-protein conjugate composition
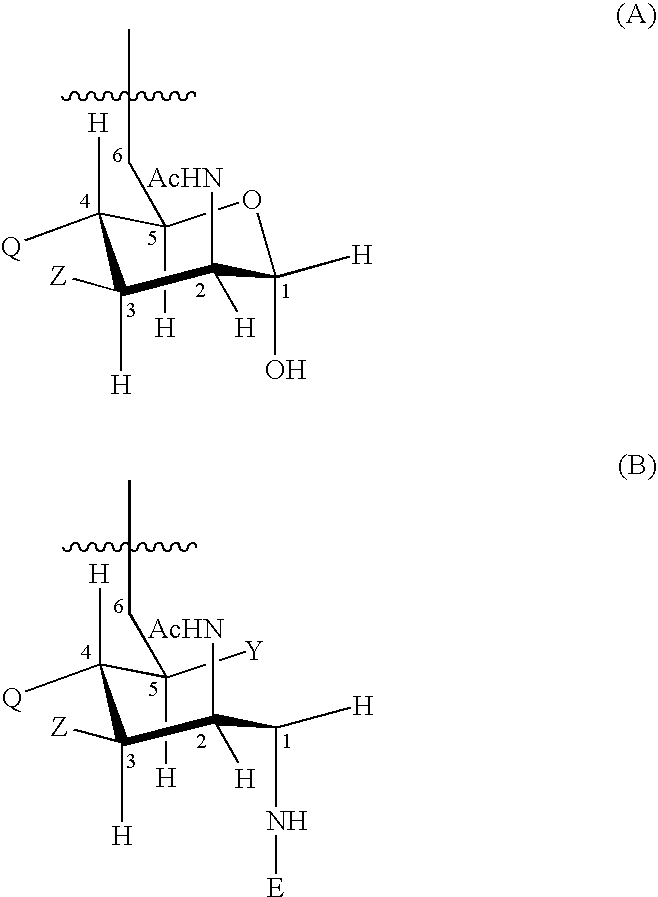
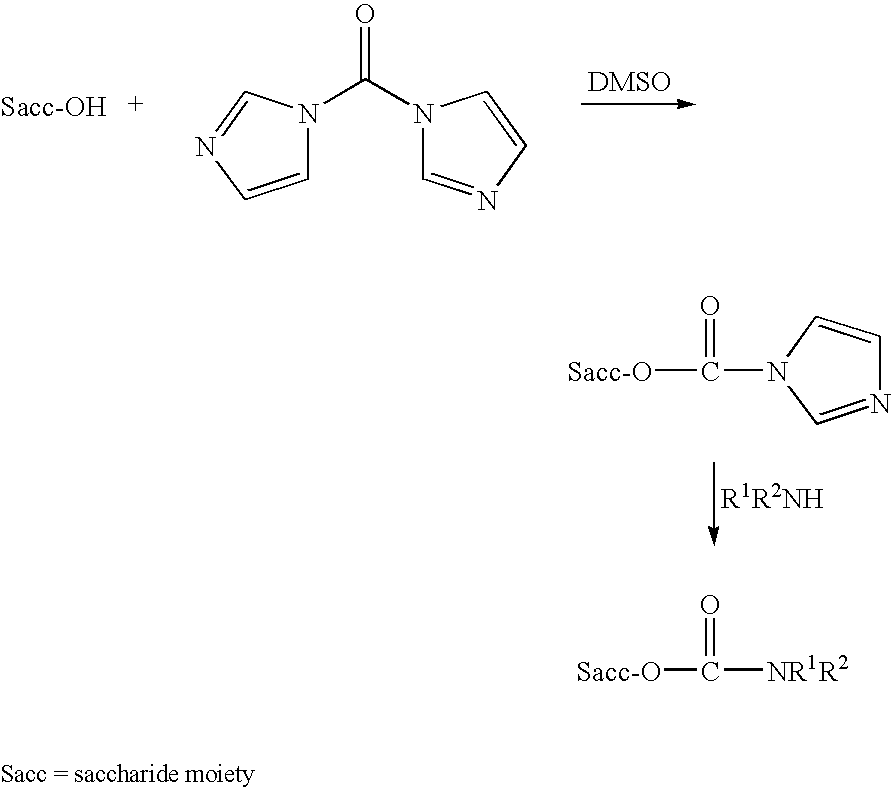
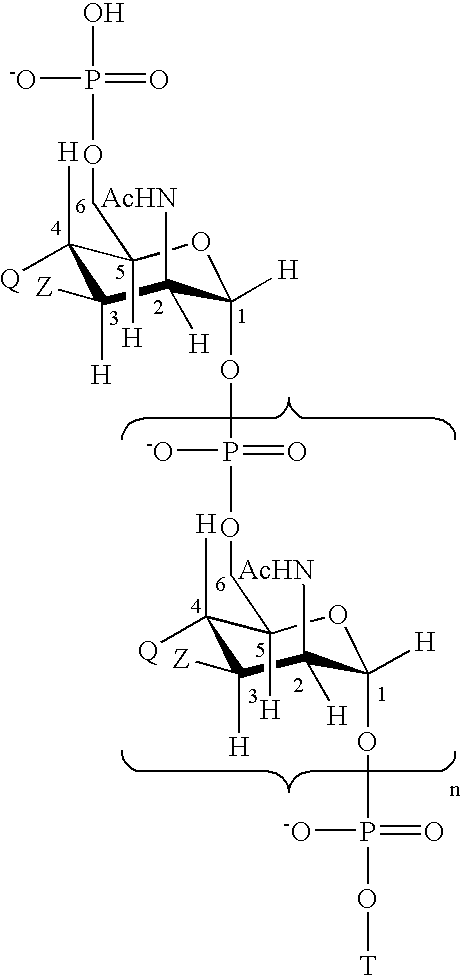
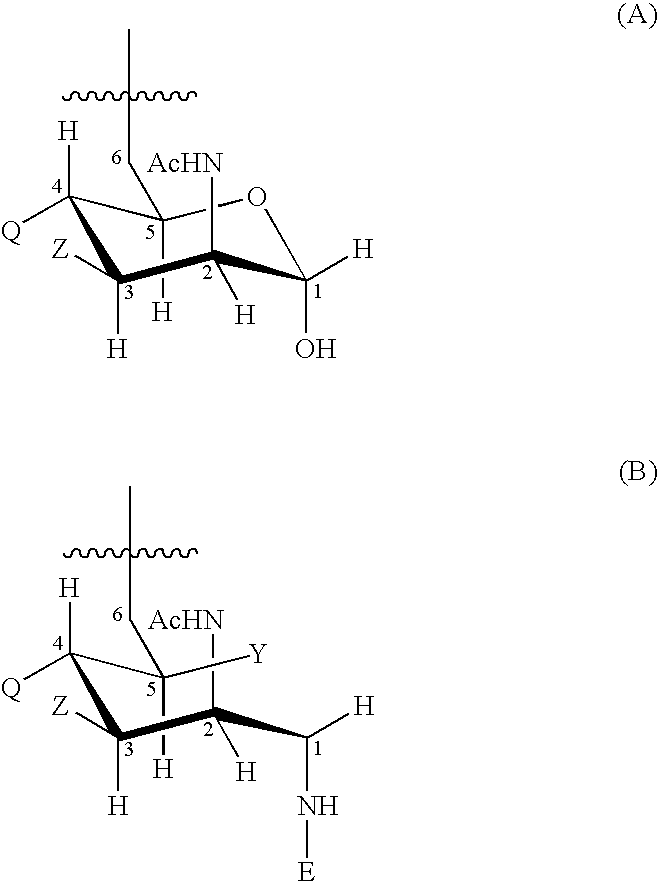
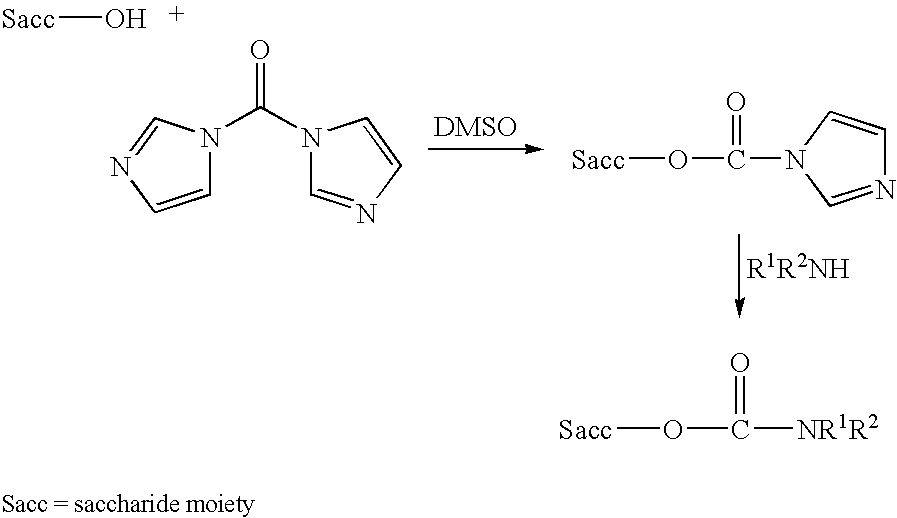
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Methods for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 19A polysaccharide are also provided in which the serotype 19A polysaccharide is co-lyophilized with a carrier protein and conjugation is carried out in dimethyl sulfoxide (DMSO) via a reductive amination mechanism.
Owner:WYETH LLC
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Also described is a method for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 1 polysaccharide covalently linked to a carrier protein, the method including partial de-O-acetylation of the polysaccharide by mild hydrolysis in an alkaline pH buffer.
Owner:WYETH LLC
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Also described is a method for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 3 polysaccharide covalently linked to a carrier protein, the method including periodic acid oxidation of the polysaccharide in the presence of bivalent cations.
Owner:WYETH LLC
Immunogenic compositions for the prevention and treatment of meningococcal disease
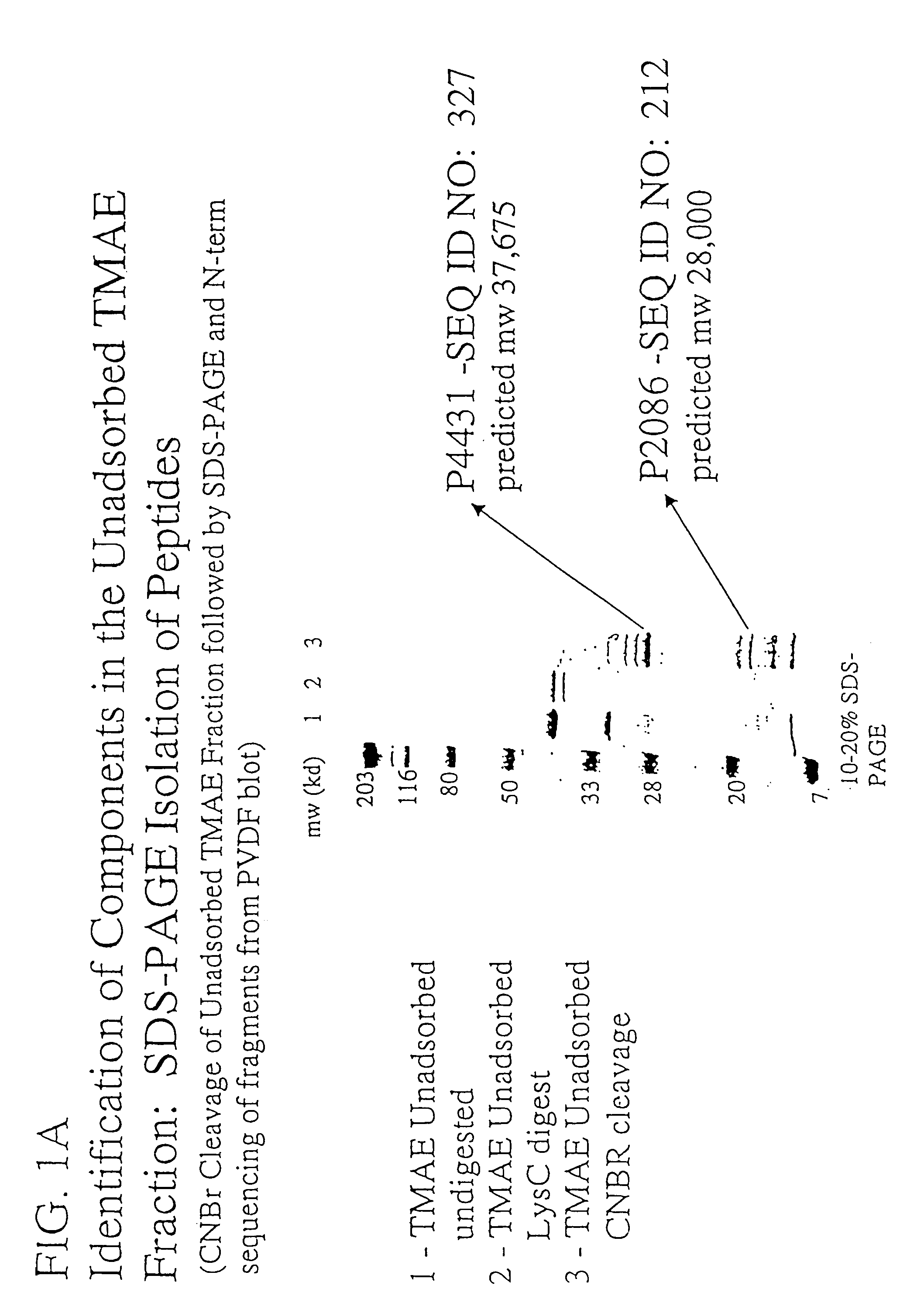
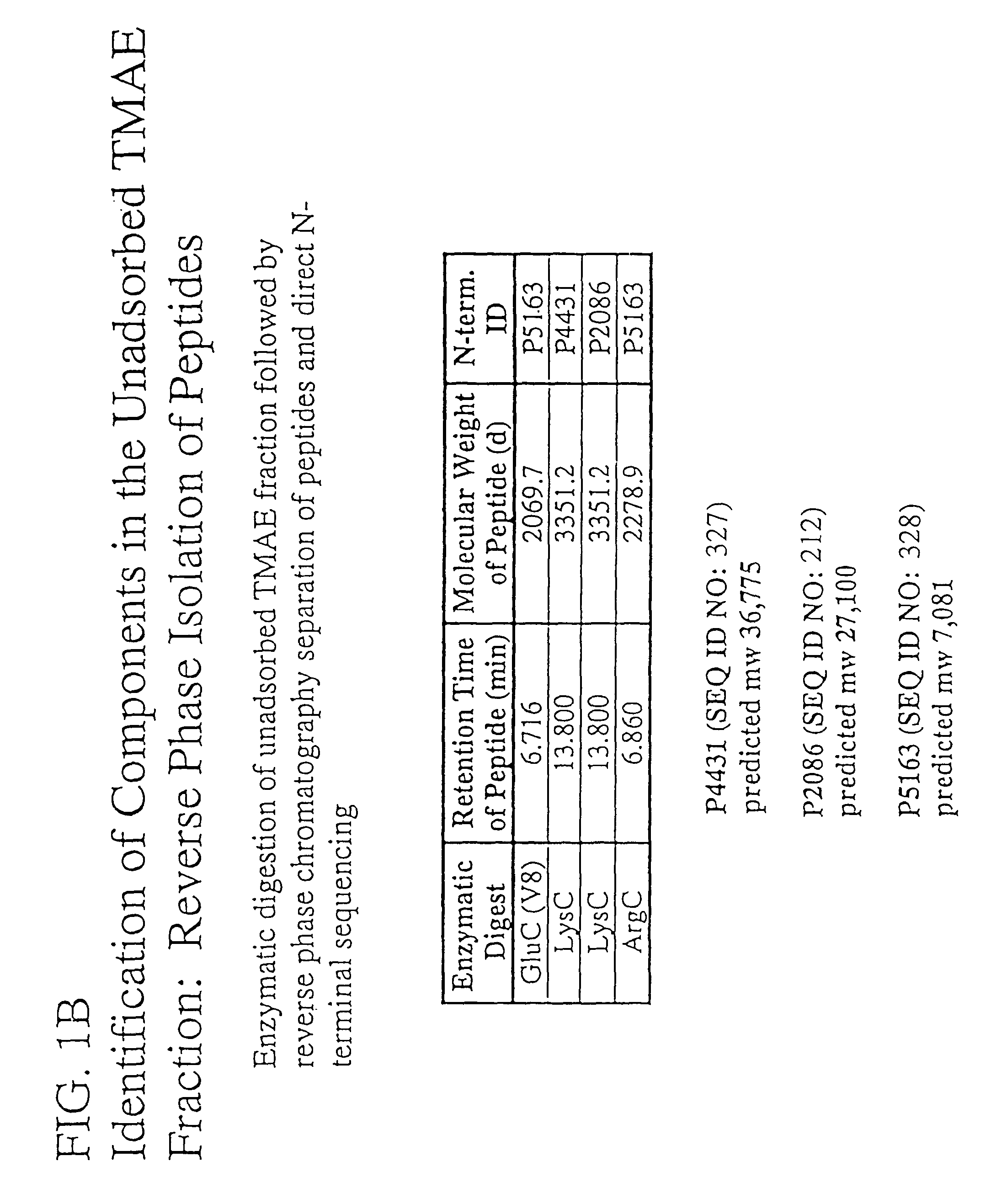
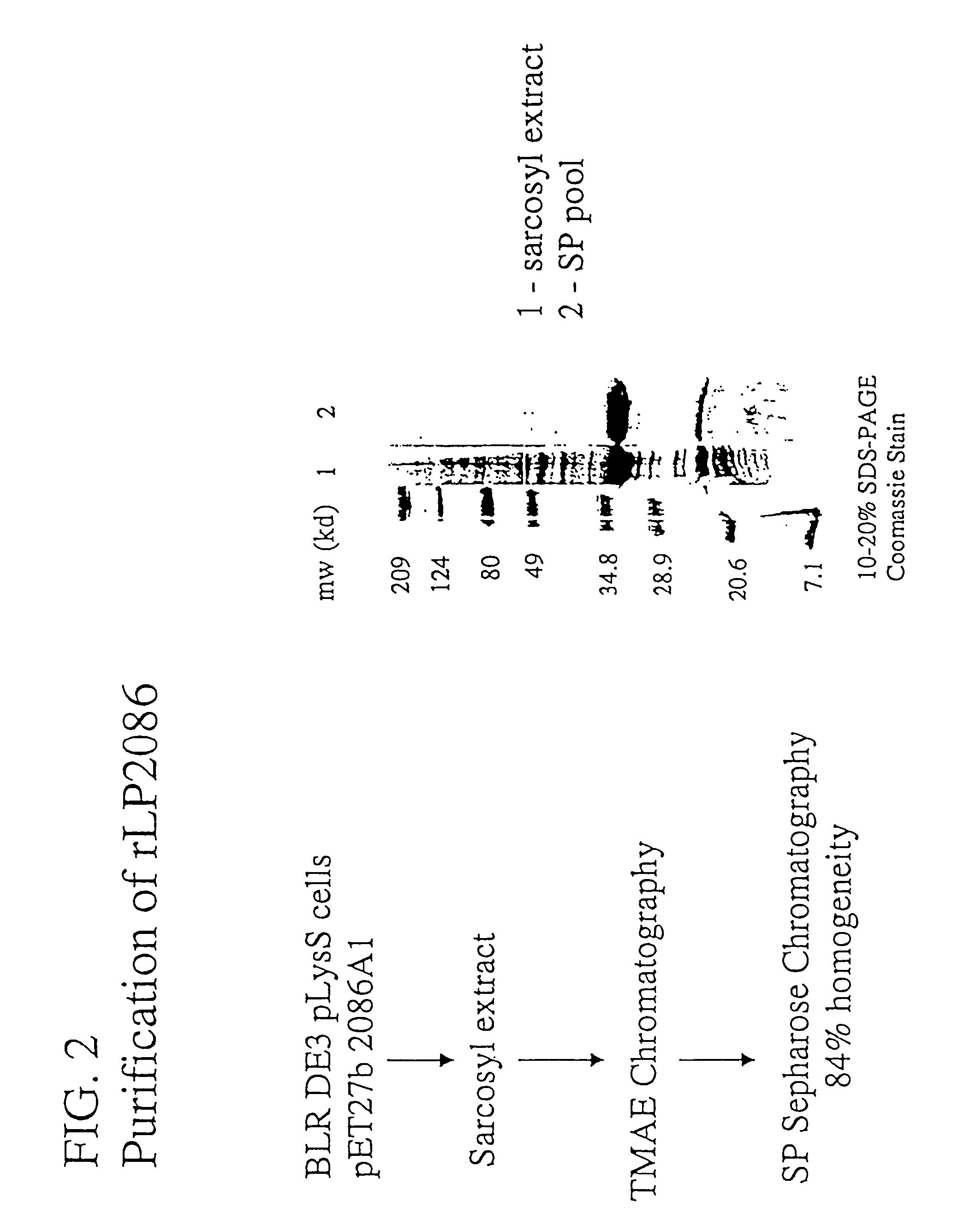
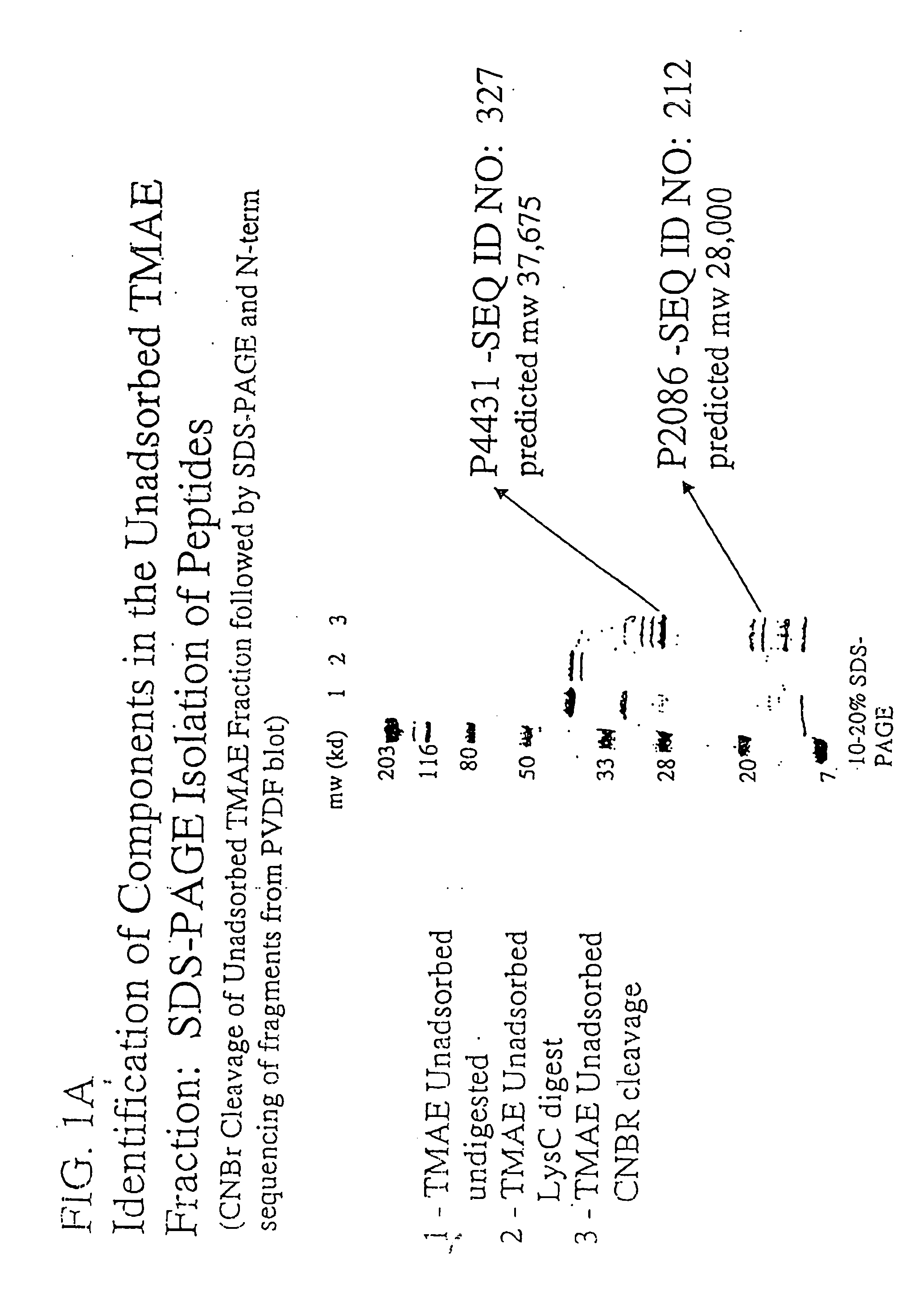
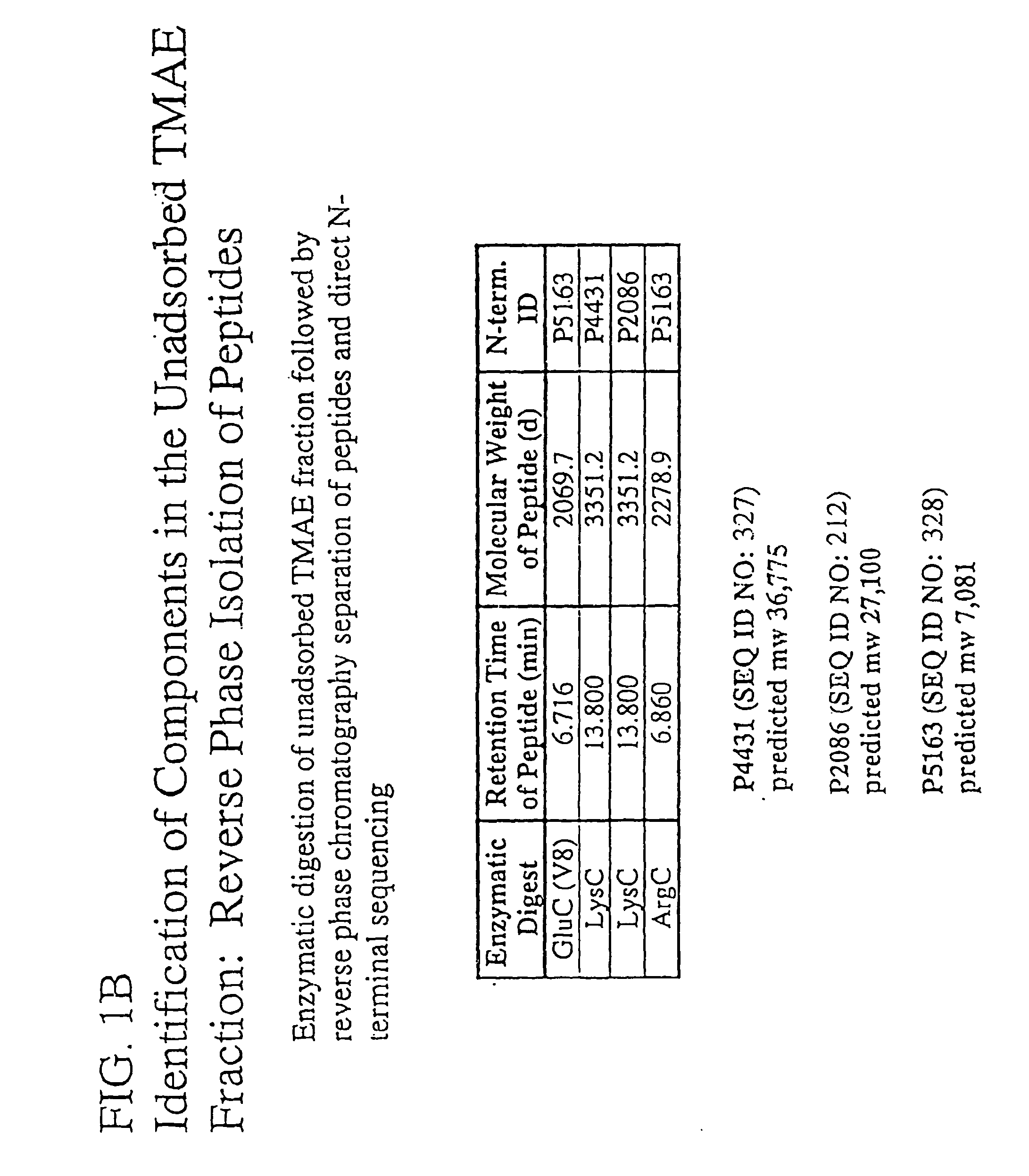
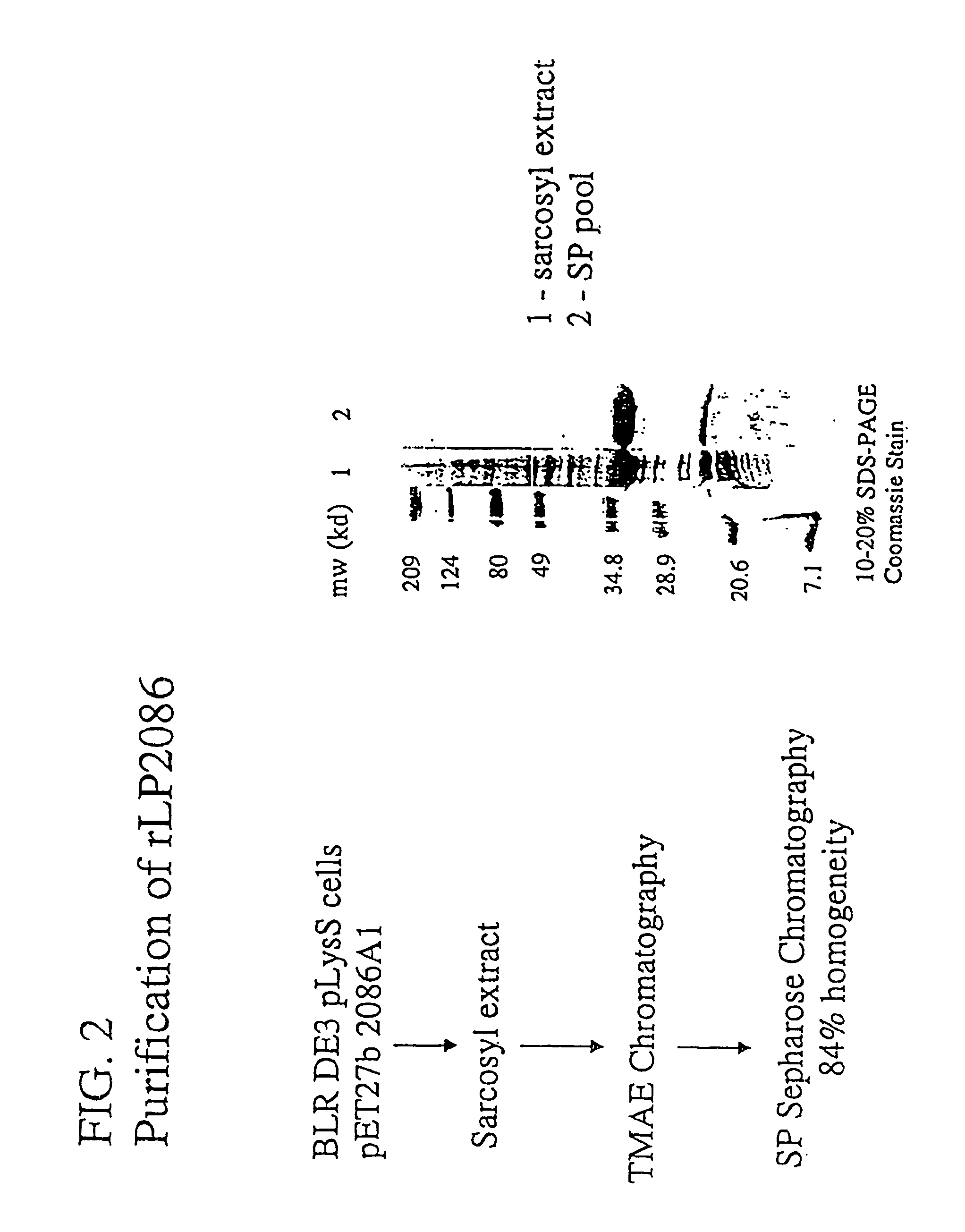
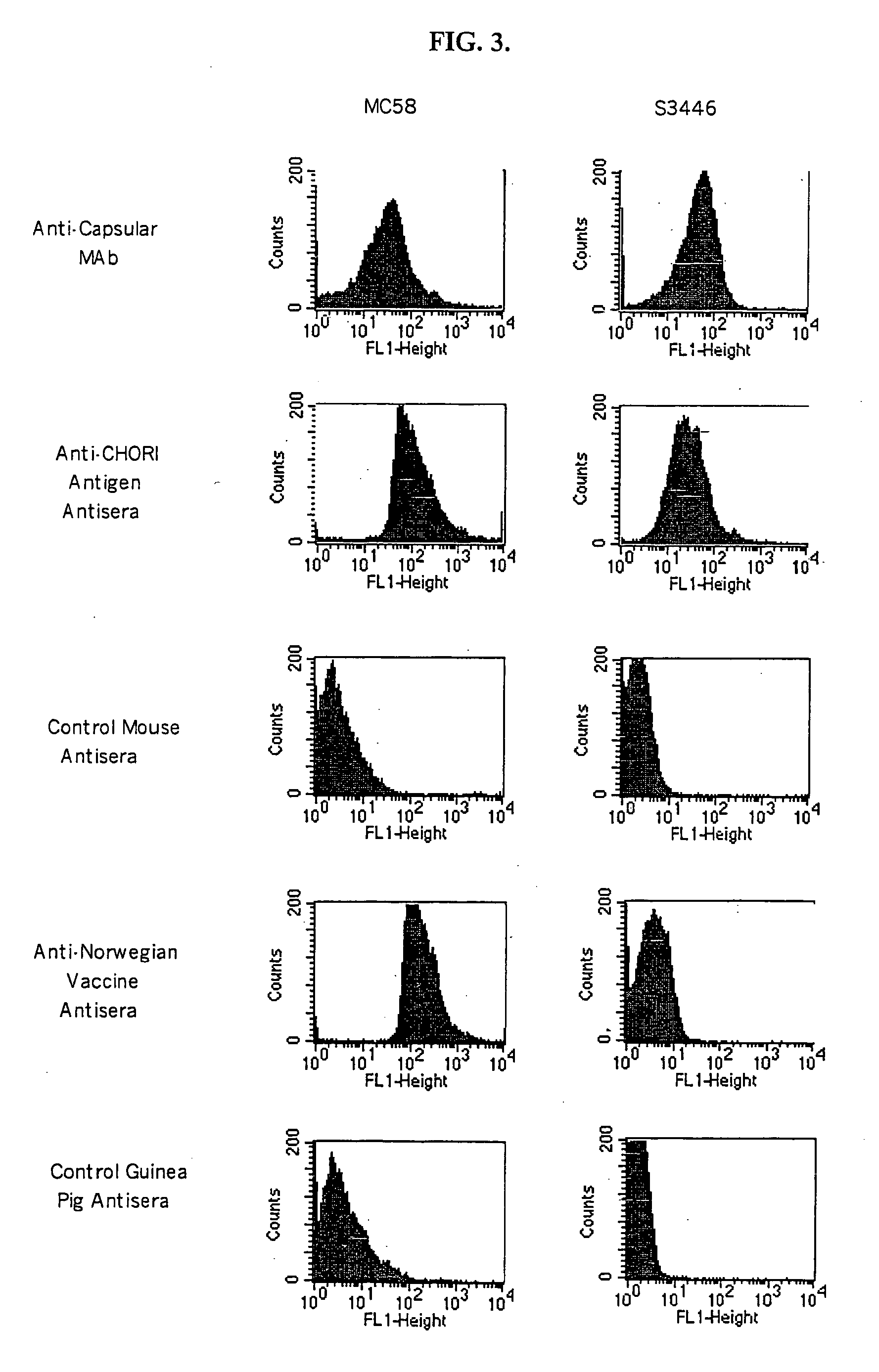
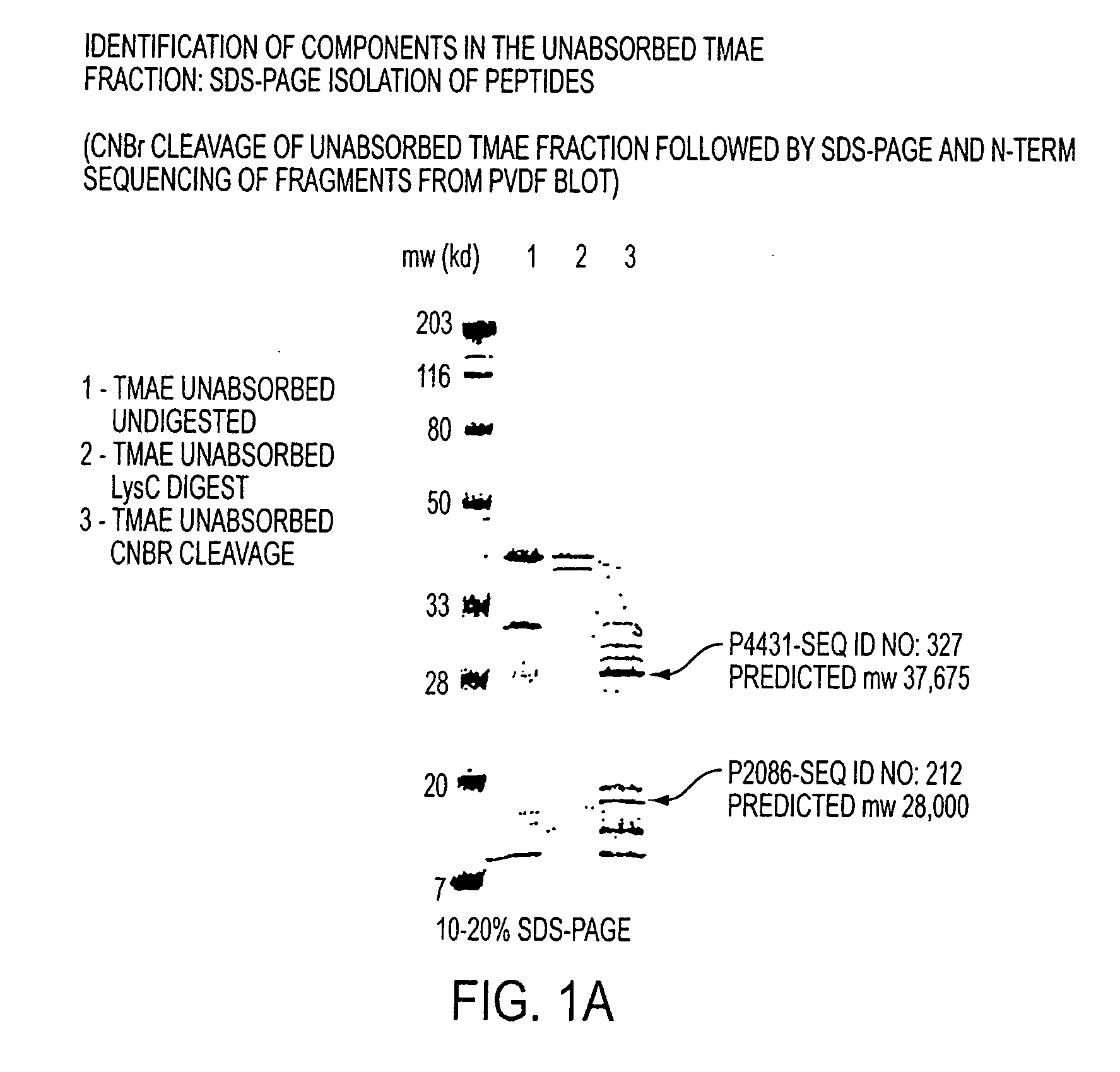
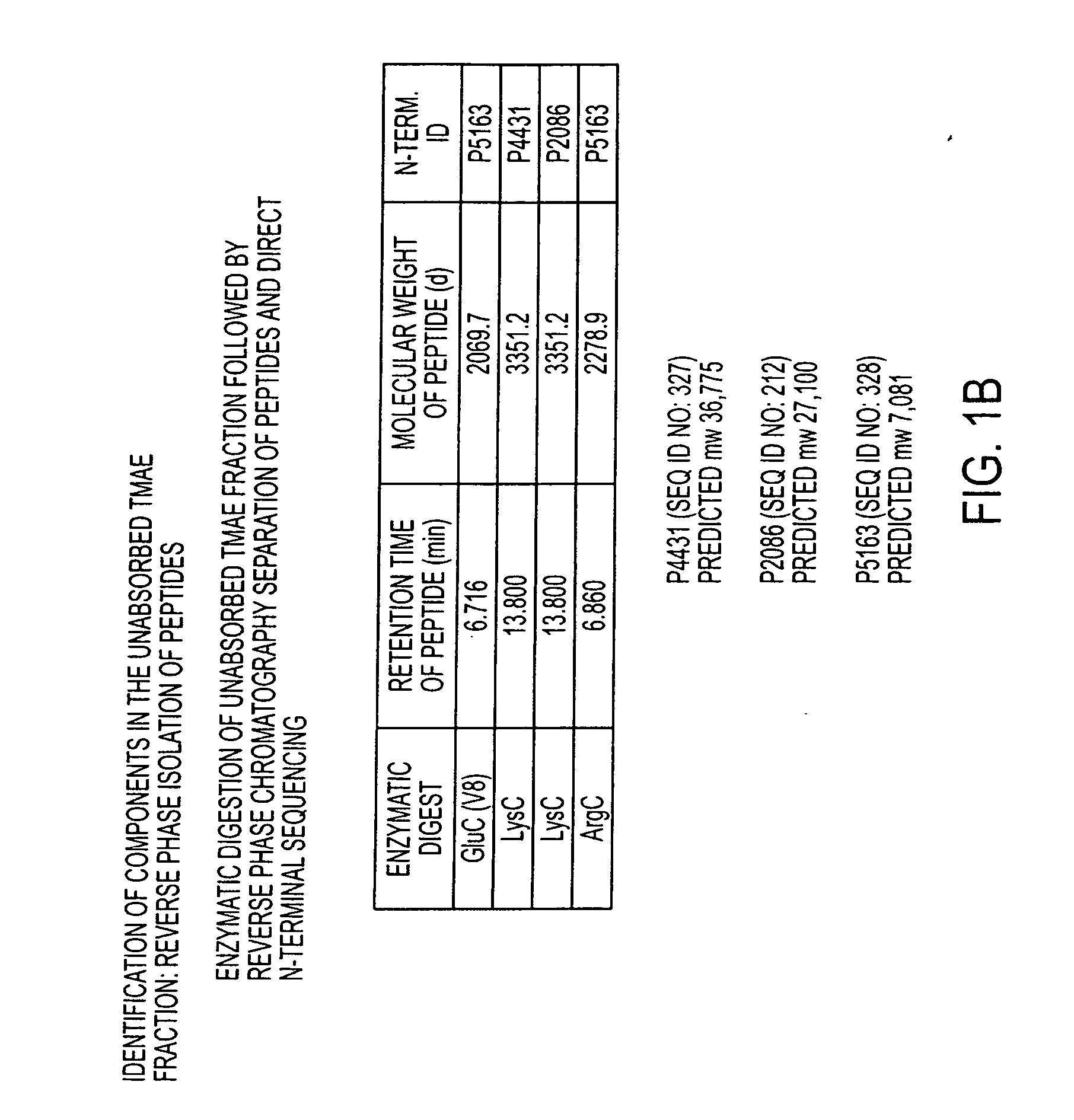
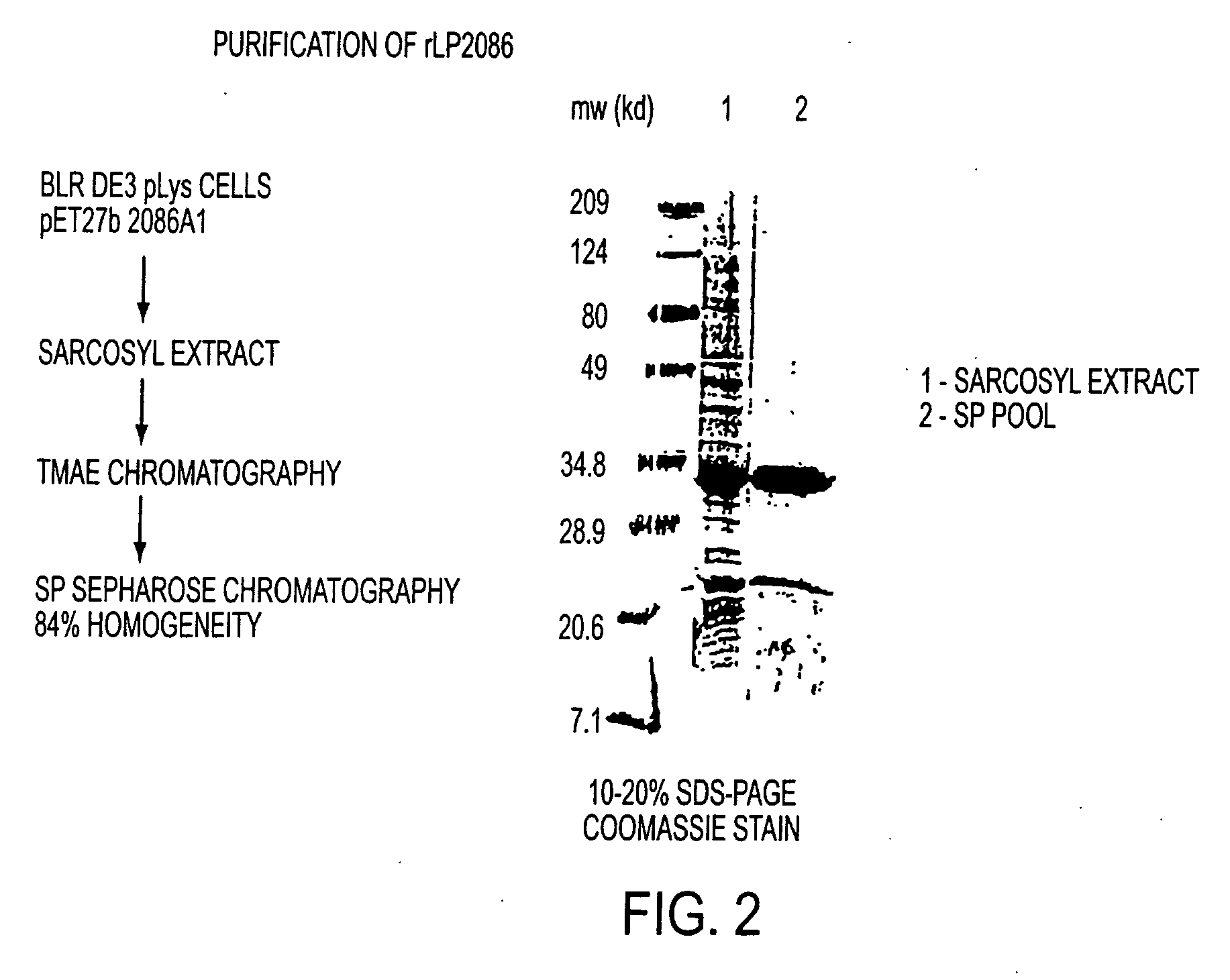
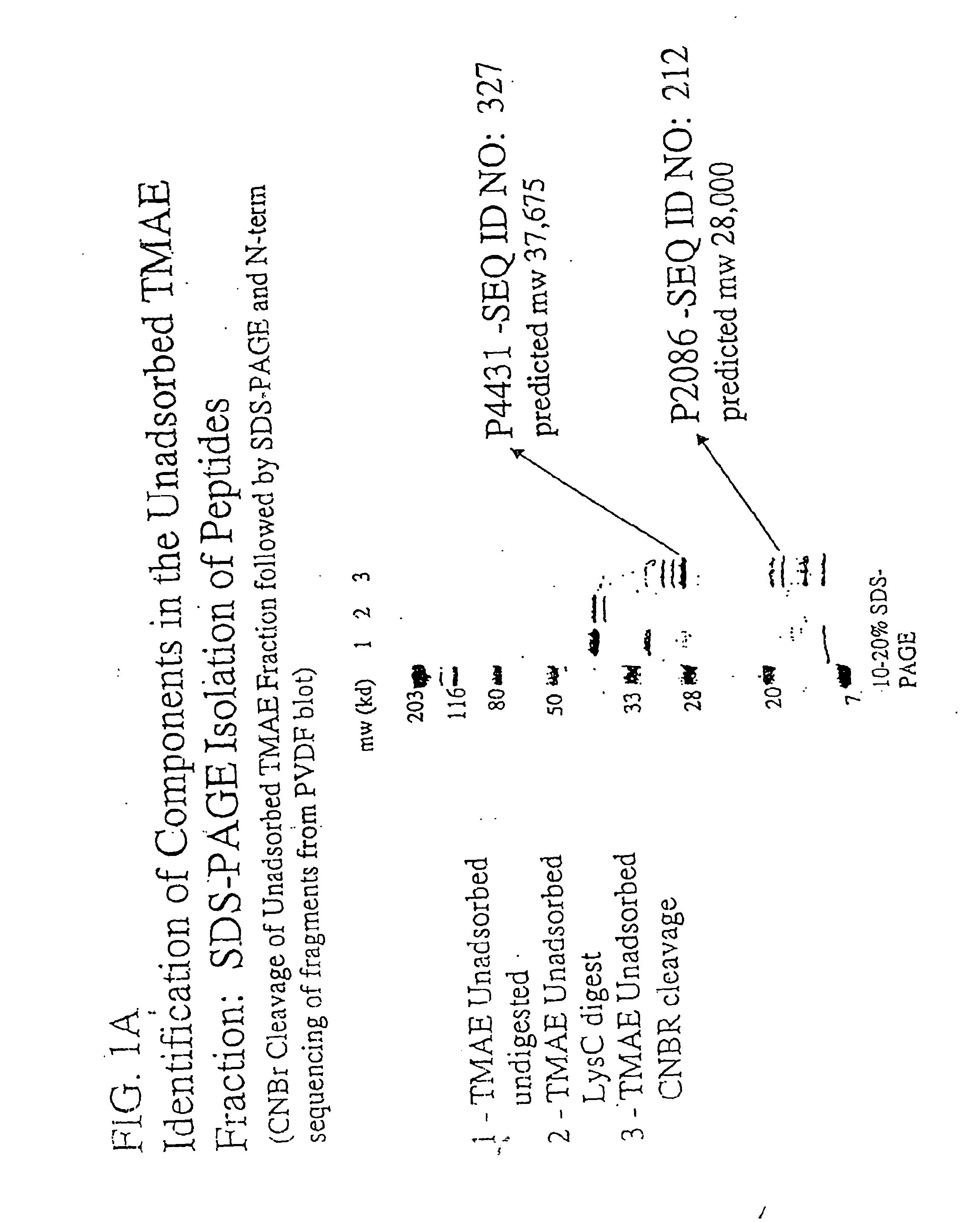
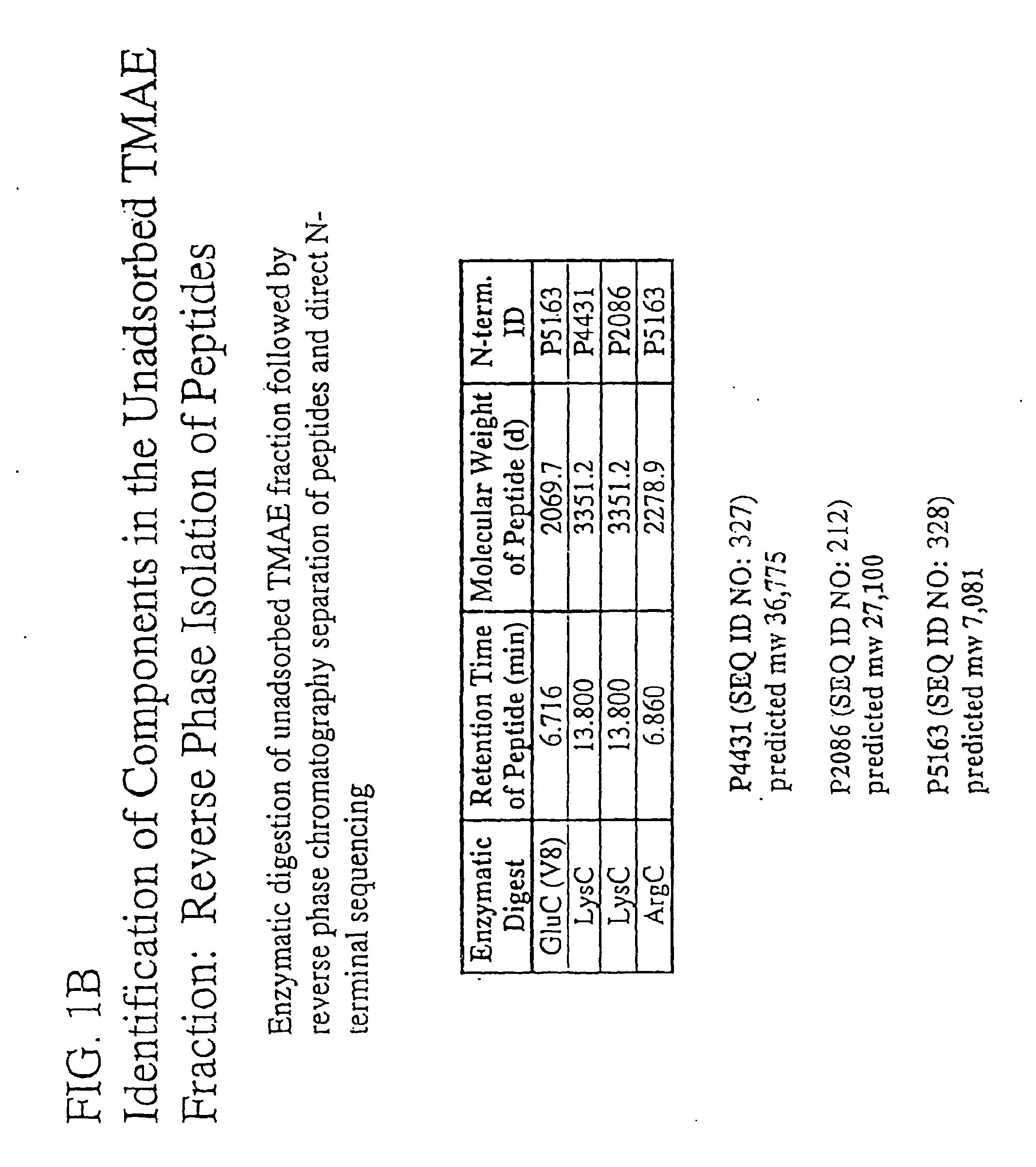
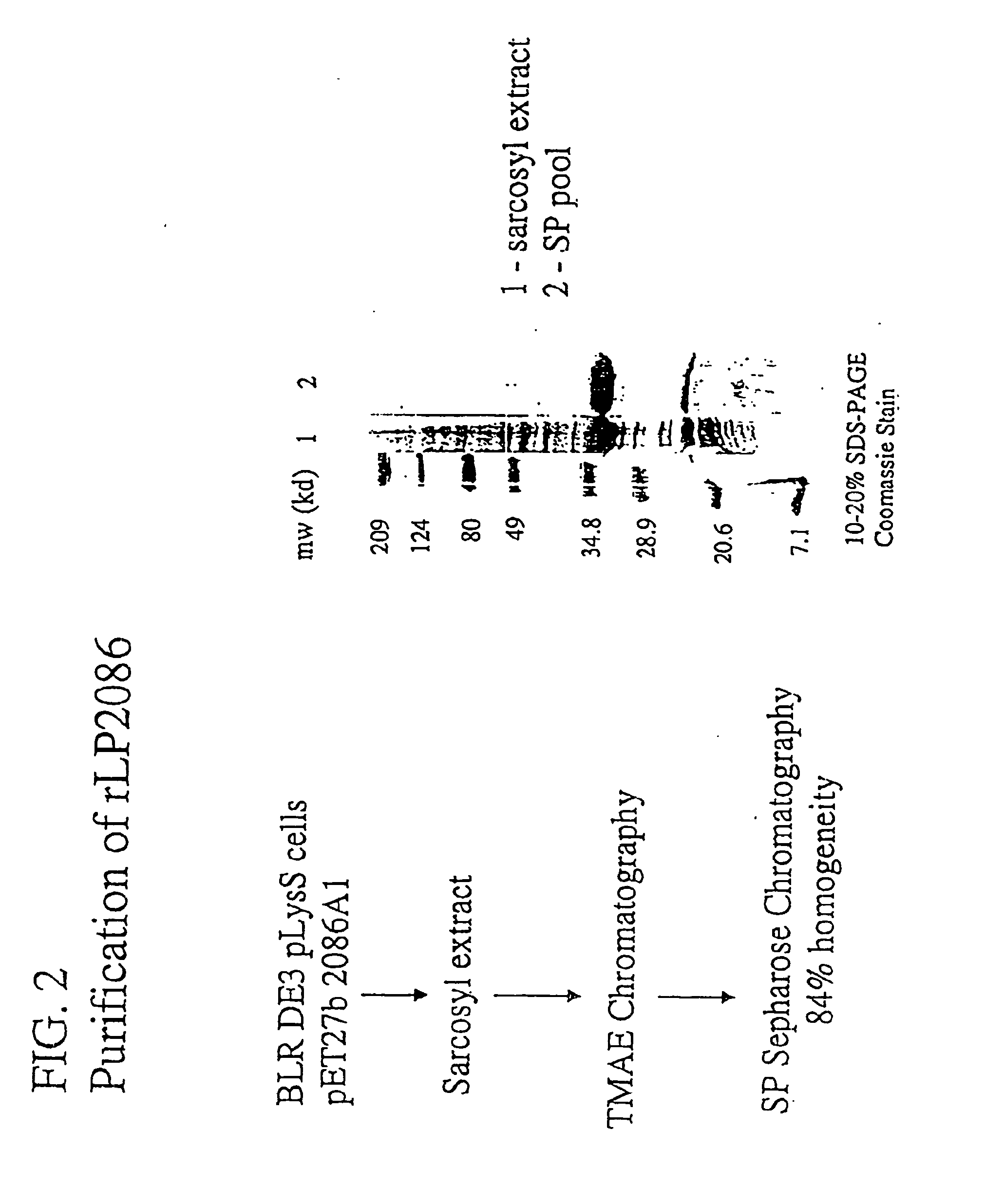
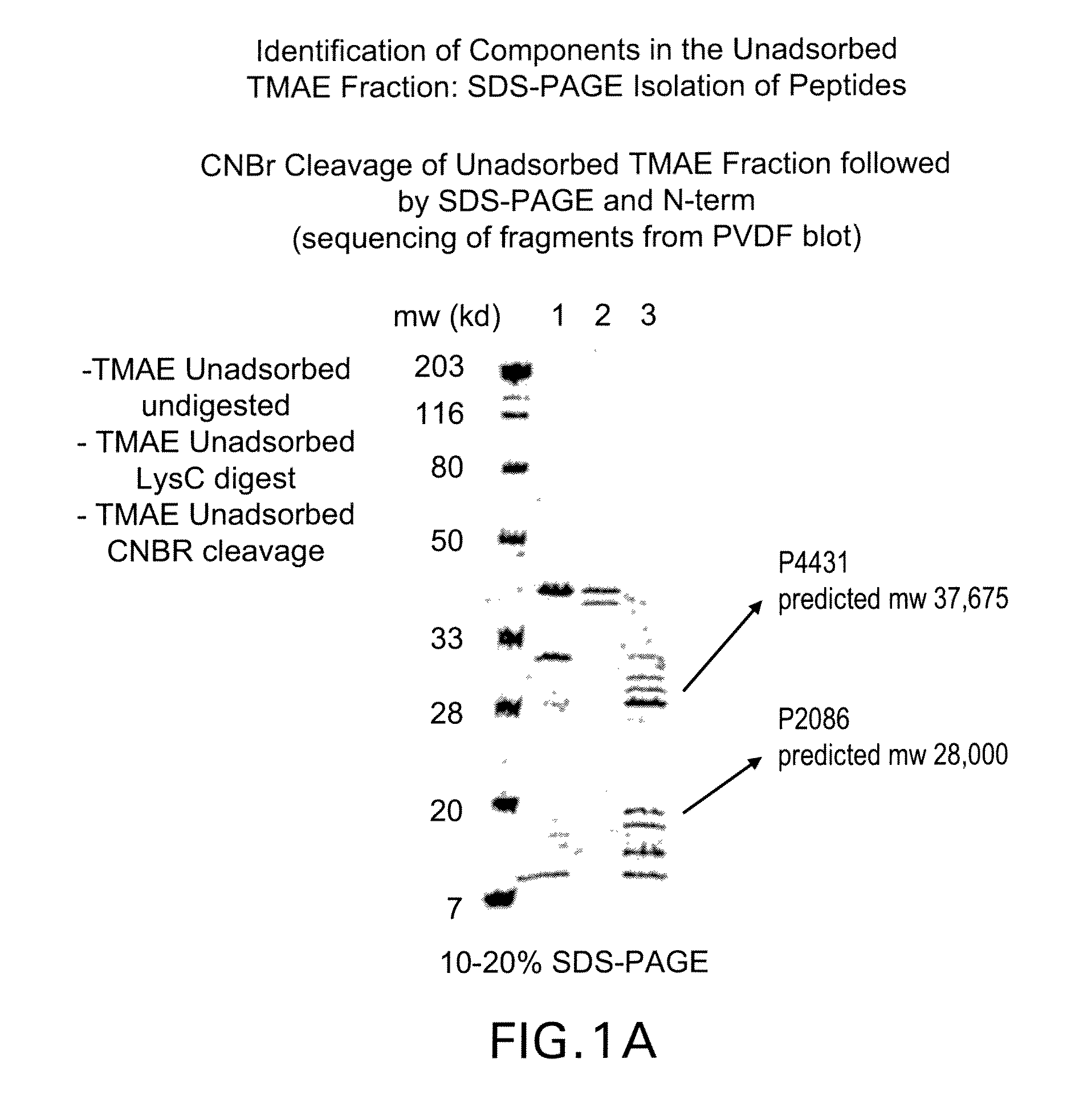
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH HOLDINGS LLC
Polypeptide-vaccines for broad protetion against hypervirulent meningococcal lineages
ActiveUS20060171957A1Improve hydrolysis resistanceAntibacterial agentsBacterial antigen ingredientsSalmonella serotype typhiMeningococcal carriage
A small number of defined antigens can provide broad protection against meningococcal infection, and the invention provides a composition which, after administration to a subject, is able to induce an antibody response in that subject, wherein the antibody response is bactericidal against two or three of hypervirulent lineages A4, ET 5 and lineage 3 of N. meningitidis serogroup B. Rather than consisting of a single antigen, the composition comprises a mixture of 10 or fewer purified antigens, and should not include complex or undefined mixtures of antigens such as outer membrane vesicles. Five protein antigens are used in particular: (1) a ‘NadA’ protein; (2) a ‘741’ protein; (3) a ‘936’ protein; (4) a ‘953’ protein; and (5) a ‘287’ protein.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Injectable vaccines against multiple meningococcal serogroups
ActiveUS20070082014A1Pharmaceutical delivery mechanismDepsipeptidesStreptococcus pneumoniaeSalmonella serotype typhi
An injectable immunogenic composition comprising capsular saccharides from at least two of serogroups A, C, W135 and Y of Neisseria meningitidis, wherein said capsular saccharides are conjugated to carrier protein(s) and / or are oligosaccharides, and wherein (i) the composition comprises <50 mug meningococcal saccharide per dose, and / or (ii) the composition further comprises an antigen from one or more of: (a) serogroup B N. meningitidis; (b) Haemophilus influenzae type B; and / or (c) Streptococcus pneumoniae. Saccharide antigens in the compositions are generally conjugated to a carrier.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Novel immunogenic compositions for the prevention and treatment of meningococcal disease
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH HOLDINGS LLC
Outer membrane vesicle (OMV) vaccine comprising N. meningitidis serogroup B outer membrane proteins
ActiveUS8273360B2Broadens their efficacyAntibacterial agentsNervous disorderProtective antigenNeisseria weaveri
A composition comprising (a) Neisseria meningitidis serogroup B outer membrane vesicles (OMVs), and (b) an immunogenic component selected from other Neisseria proteins, or immunogenic fragments thereof. Component (b) preferably includes a protein from a different NmB strain from that from which the OMV of component (a) is derived. The OMVs are preferably obtained by deoxycholate extraction. Optionally, the composition may also comprise a protective antigen against other pathogens.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Also described is a method for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 3 polysaccharide covalently linked to a carrier protein, the method including periodic acid oxidation of the polysaccharide in the presence of bivalent cations.
Owner:WYETH LLC
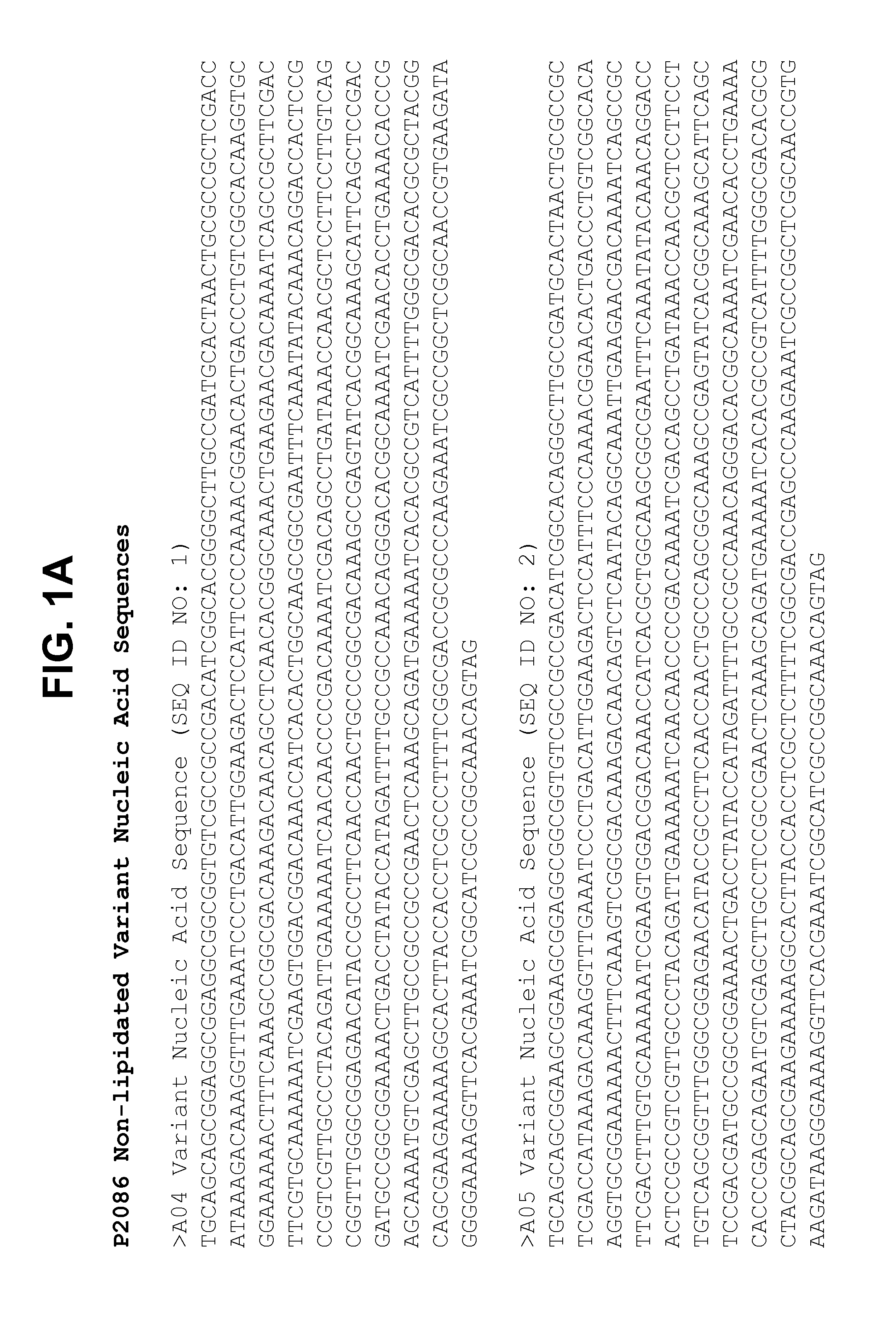
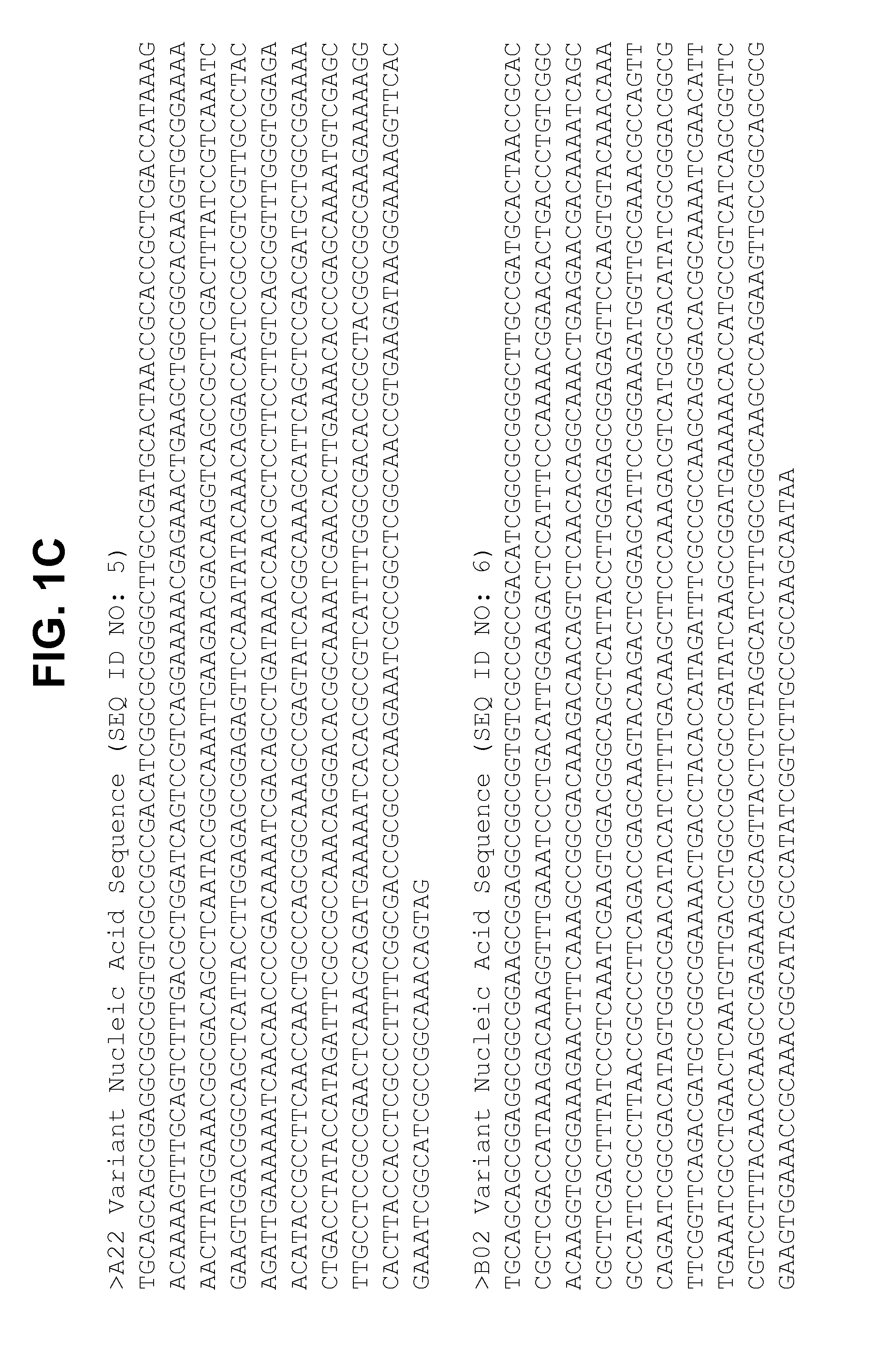
Non-lipidated variants of neisseria meningitidis orf2086 antigens
ActiveUS20120093852A1Raise immunogenic responseImprove responseAntibacterial agentsBacterial antigen ingredientsSalmonella serotype typhiNeisseria meningitidis
The present invention relates to compositions including an isolated non-pyruvylated non-lipidated ORF2086 polypeptide, and methods thereof. In an exemplary embodiment, the compositions described herein are immunogenic. The present invention further relates to compositions that elicit a bactericidal immune response in a mammal against an ORF2086 subfamily B polypeptide from serogroup B Neisseria meningitidis, and methods related thereto.
Owner:WYETH LLC
Gna1870-Based Vesicle Vaccines for Broad Spectrum Protection Against Diseases Caused by Neisseria Meningitidis
ActiveUS20080248065A1Broad protectionDecrease of emergenceAntibacterial agentsBacterial antigen ingredientsBacteroidesDisease
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
Immunogenic compositions for the prevention and treatment of meningococcal disease
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH LLC
Mucosal meningococcal vaccines
InactiveUS20070207090A1Improve securityReduced dosAntibacterial agentsBacterial antigen ingredientsMucosal Immune ResponsesAdjuvant
The invention provides immunogenic compositions for mucosal delivery comprising capsular saccharides from at least two of serogroups A, C, W135 and Y of N. meningitidis. It is preferred that the capsular saccharides in the compositions of the invention are conjugated to carrier protein(s) and / or are oligosaccharides. Conjugated oligosaccharide antigens are particularly preferred. The invention also provides immunogenic compositions comprising (a) a capsular saccharide antigen from serogroup C of N. meningitidis, and (b) a chitosan adjuvant. The composition preferably comprises (c) one or more further antigens and / or (d) one or more further adjuvants. The compositions are particularly suitable for mucosal delivery, including intranasal delivery. The use of chitosan and / or detoxified ADP-ribosylating toxin adjuvants enhances anti-meningococcal mucosal immune responses and can shift the Th1 / Th2 bias of the responses.
Owner:NOVARTIS AG
Neisseria meningitidis compositions and methods thereof
ActiveUS20130243807A1Antibacterial agentsBacterial antigen ingredientsSalmonella serotype typhiMeningococcal carriage
In one aspect, the invention relates to an isolated polypeptide comprising an amino acid sequence that is at least 95% identical to SEQ ID NO: 71. In another aspect, the invention relates to an immunogenic composition including an isolated non-lipidated, non-pyruvylated ORF2086 polypeptide from Neisseria meningitidis serogroup B, and at least one conjugated capsular saccharide from a meningococcal serogroup.
Owner:PFIZER INC
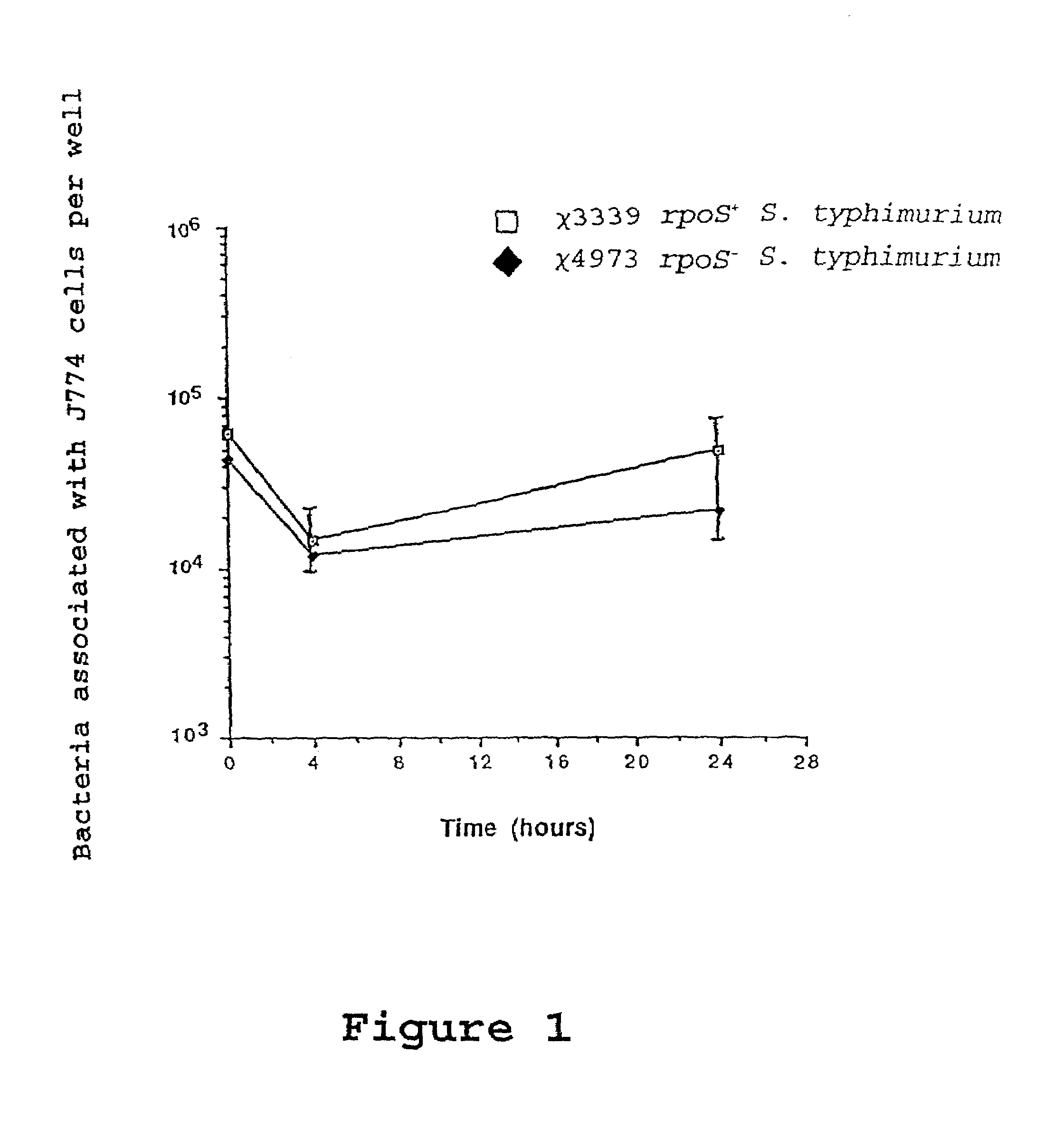
Recombinant vaccines comprising immunogenic attenuated bacteria having RpoS positive phenotype
InactiveUS7083794B2Improve balanceImproving immunogenicityAntibacterial agentsBiocideSalmonella entericaSalmonella serotype typhi
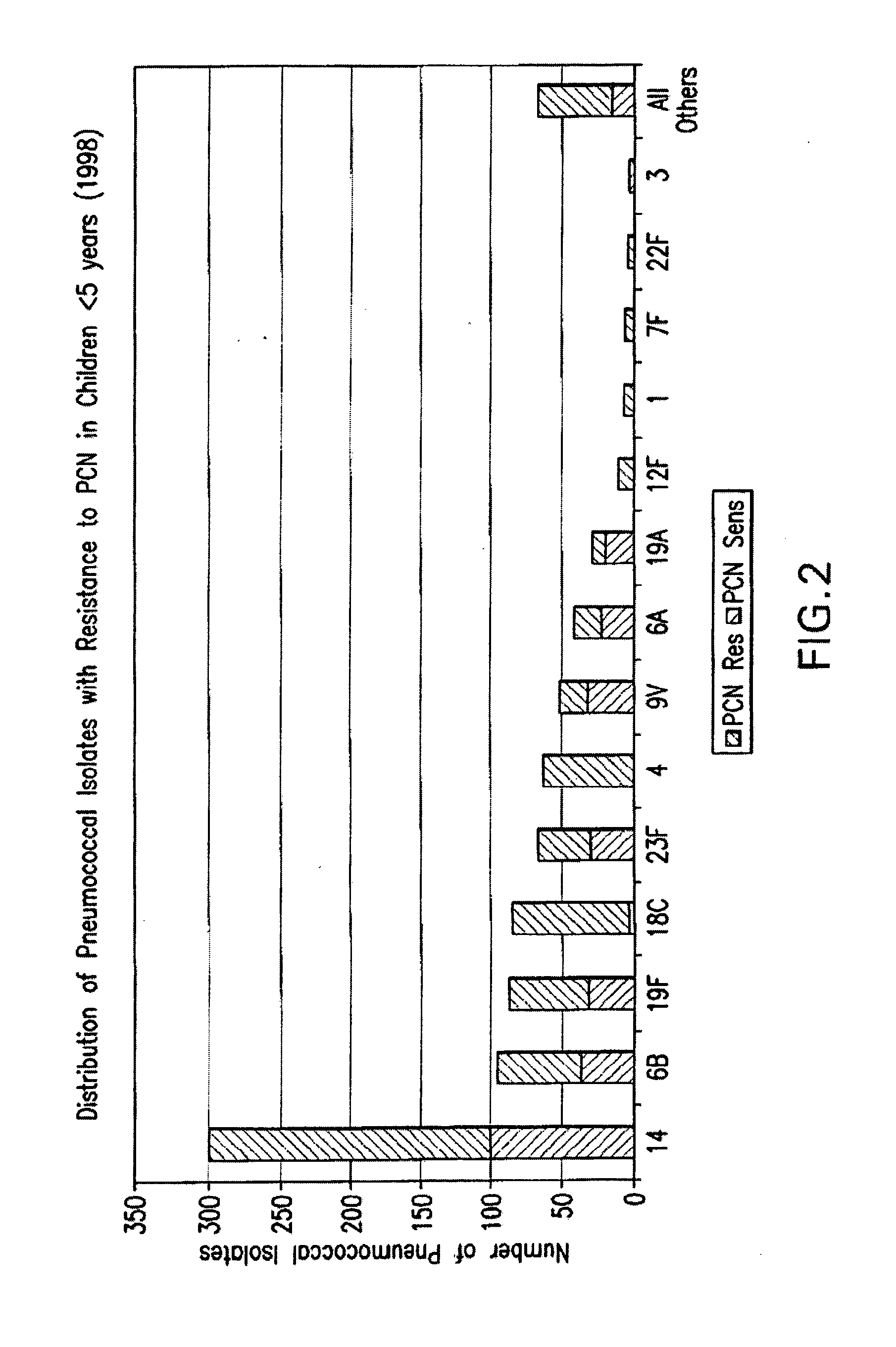
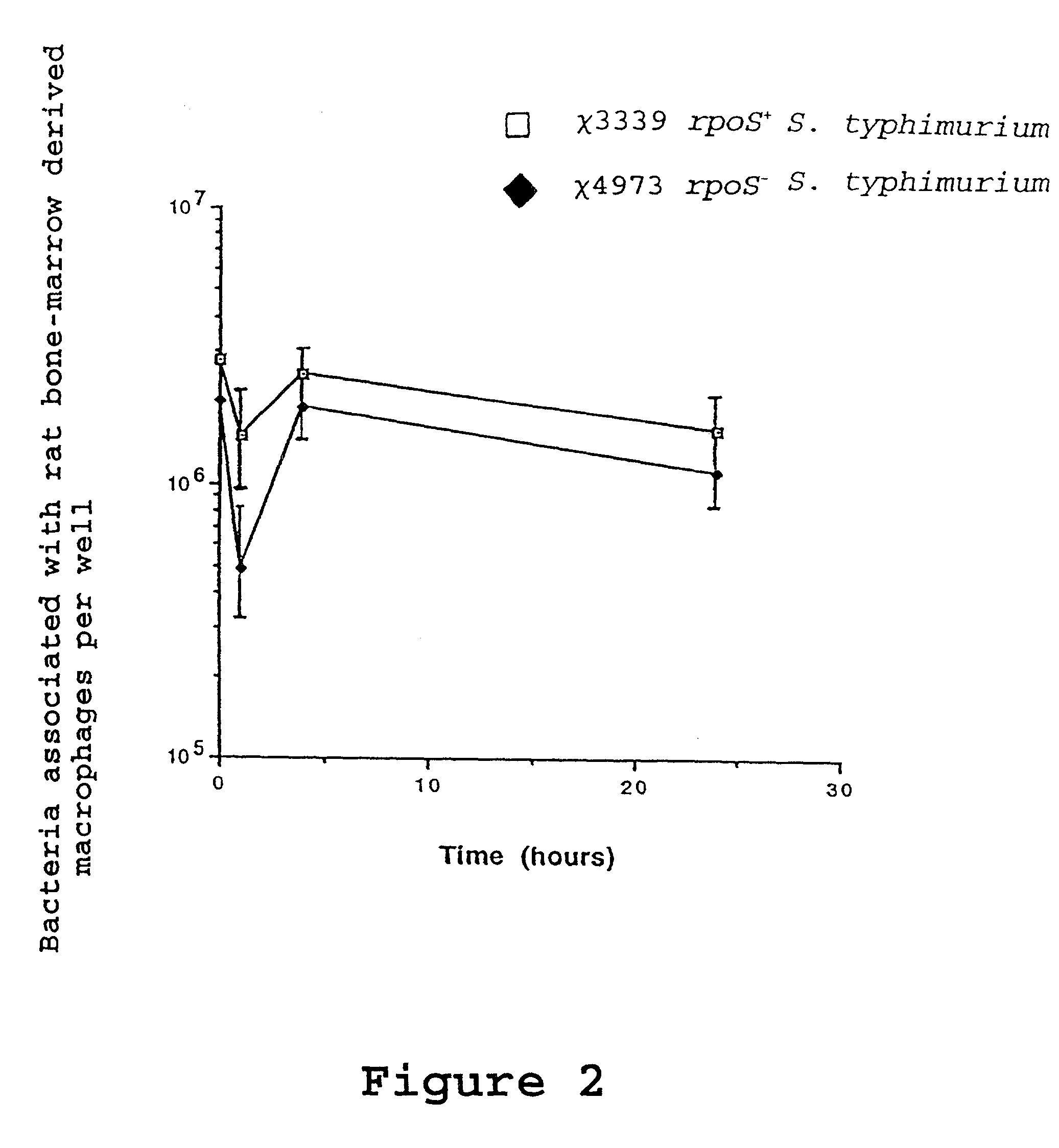
Attenuated immunogenic bacteria having an RpoS+ phenotype, in particular, Salmonella enterica serotype Typhi having an RpoS+ phenotype and methods therefor are disclosed. The Salmonella have in addition to an RpoS+ phenotype, an inactivating mutation in one or more genes which render the microbe attenuated, and a recombinant gene capable of expressing a desired protein. The Salmonella are attenuated and have high immunogenicity so that they can be used in vaccines and as delivery vehicles for genes and gene products. Also disclosed are methods for preparing the vaccine delivery vehicles.
Owner:WASHINGTON UNIV IN SAINT LOUIS
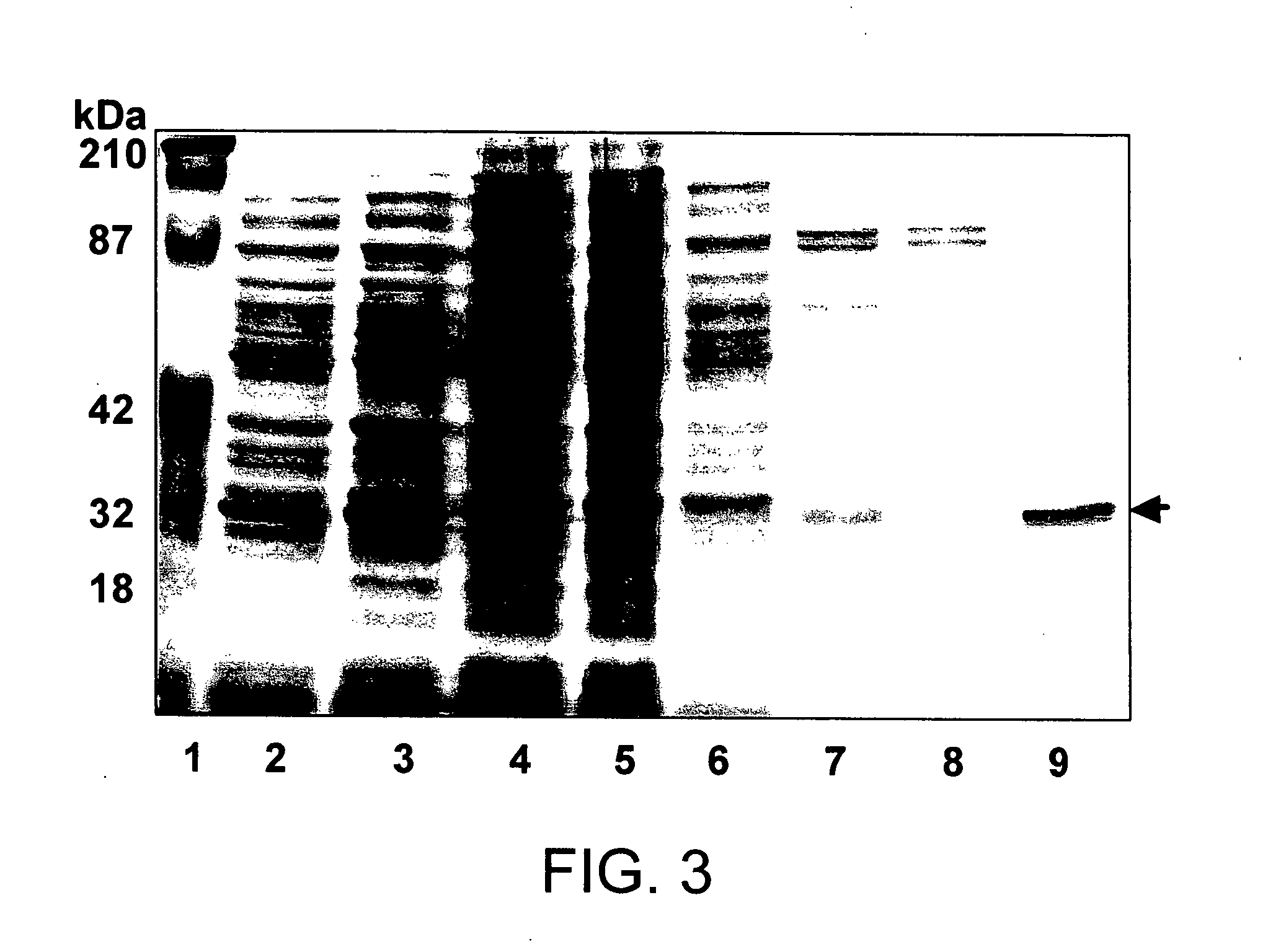
Gonococcal proteins and nucleic acids
InactiveUS7504111B2Stable maintenanceAntibacterial agentsOrganic active ingredientsAntigenSalmonella serotype typhi
The invention provides proteins from gonococcus (Neisseria gonorrhoeae), including amino acid sequences, the corresponding nucleotide sequences, expression data, and serological data. The proteins are useful antigens for vaccines, immunogenic compositions, and / or diagnostics. They are also useful for distinguishing between gonococcus and meningococcus and, in particular, between gonococcus and serogroup B meningococcus.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Vaccines for broad spectrum protection against diseases caused by Neisseria meningitidis
InactiveUS20060029621A1Wide applicabilityAntibacterial agentsOrganic active ingredientsSalmonella serotype typhiSerotype
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
Novel immunogenic compositions for the prevention and treatment of meningococcal disease
InactiveUS20070082007A1Avoid infectionInhibition of colonizationAntibacterial agentsNervous disorderDiseaseSalmonella serotype typhi
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:ZLOTNICK GARY W +5
Novel immunogenic compositions for the prevention and treatment of meningococcal disease
InactiveUS20110076299A1Antibacterial agentsOrganic active ingredientsDiseaseSalmonella serotype typhi
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH HOLDINGS LLC
Novel immunogenic compositions for the prevention and treatment of meningococcal disease
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH LLC
Polypeptide-vaccines for broad protection against hypervirulent meningococcal lineages
ActiveUS8663656B2Antibacterial agentsBacterial antigen ingredientsSalmonella serotype typhiMeningococcal carriage
A small number of defined antigens can provide broad protection against meningococcal infection, and the invention provides a composition which, after administration to a subject, is able to induce an antibody response in that subject, wherein the antibody response is bactericidal against two or three of hypervirulent lineages A4, ET 5 and lineage 3 of N. meningitidis serogroup B. Rather than consisting of a single antigen, the composition comprises a mixture of 10 or fewer purified antigens, and should not include complex or undefined mixtures of antigens such as outer membrane vesicles. Five protein antigens are used in particular: (1) a ‘NadA’ protein; (2) a ‘741’ protein; (3) a ‘936’ protein; (4) a ‘953’ protein; and (5) a ‘287’ protein.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
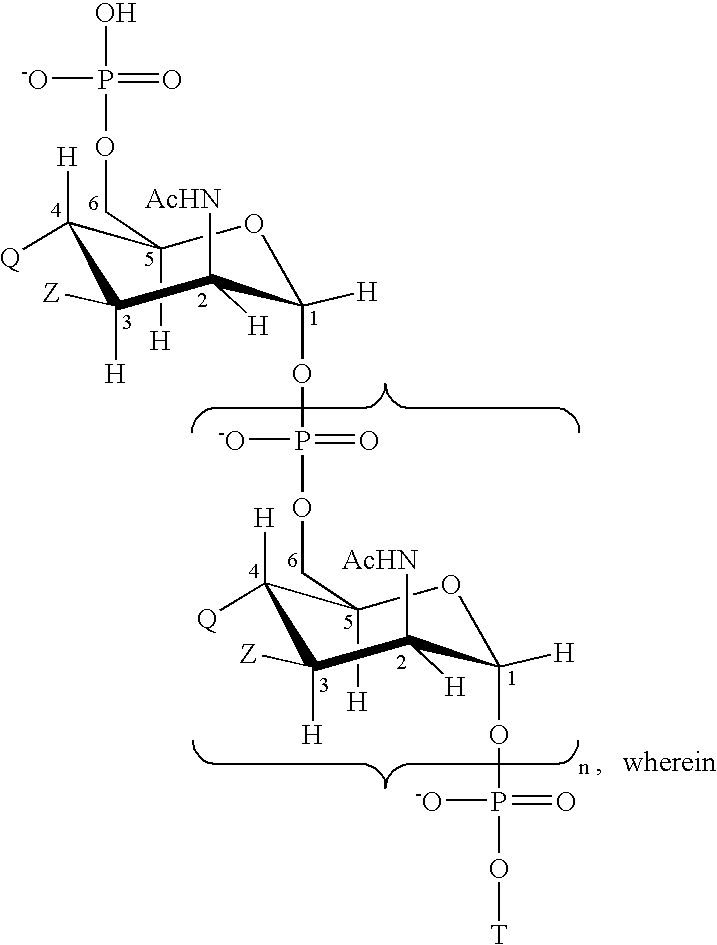
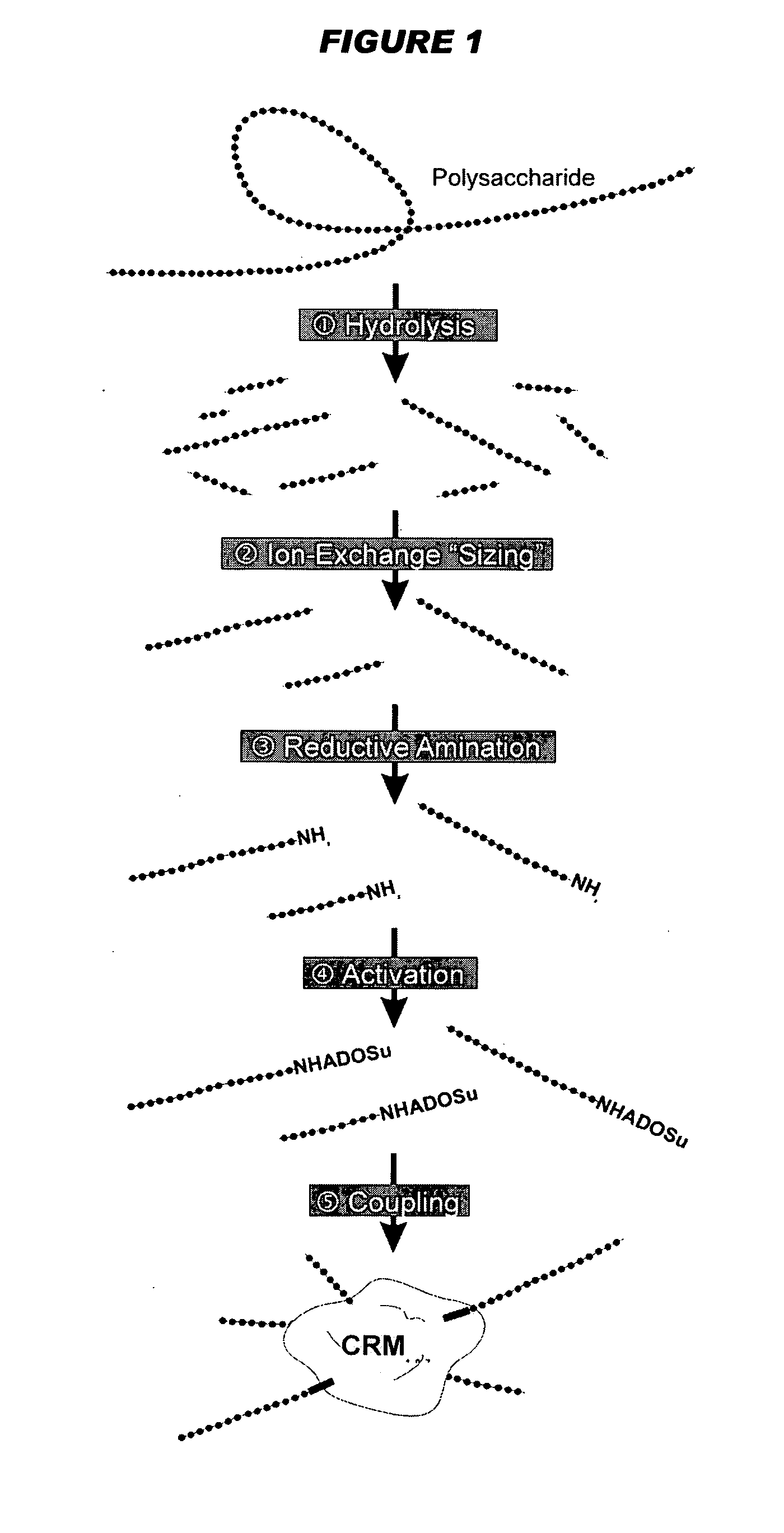
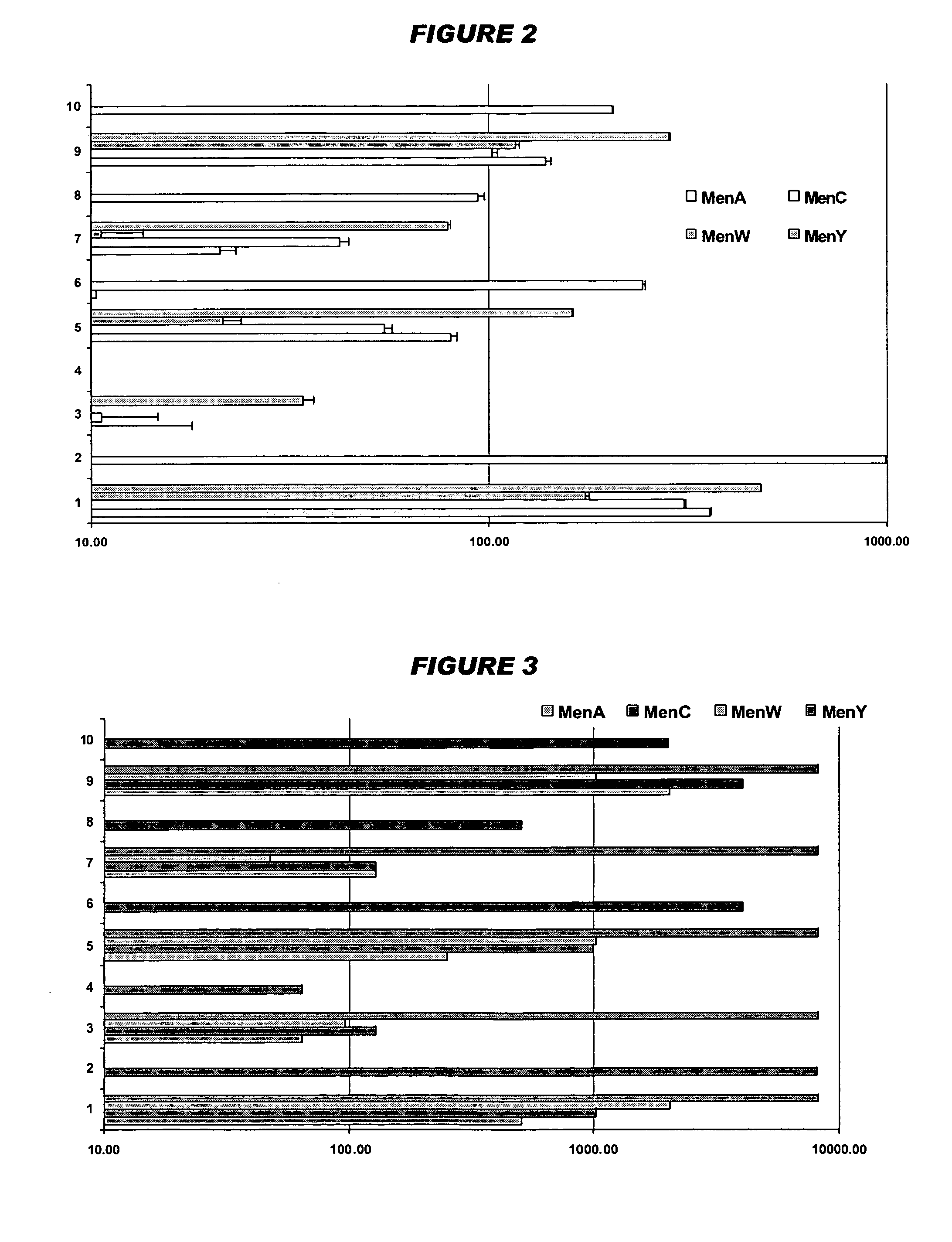
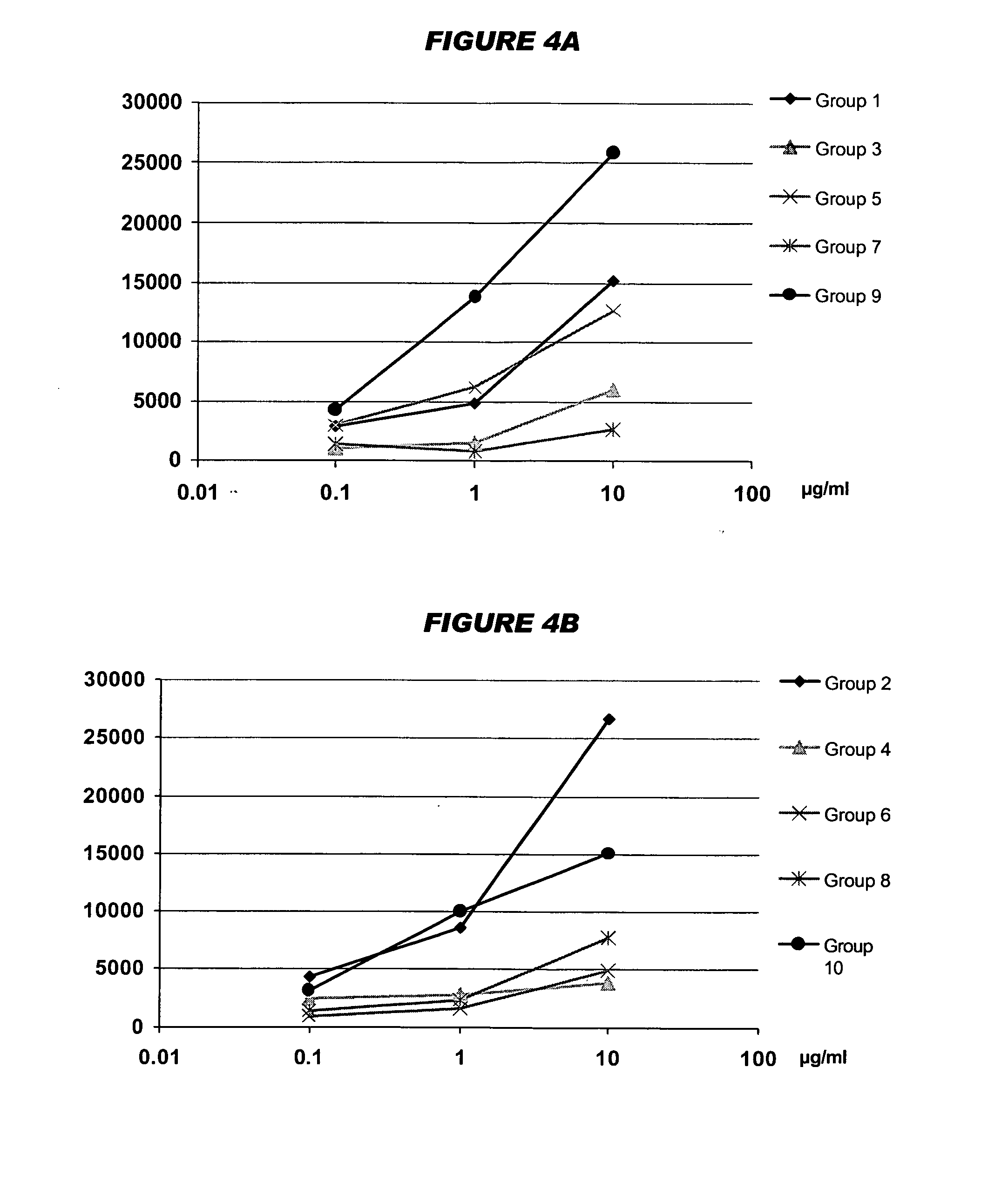
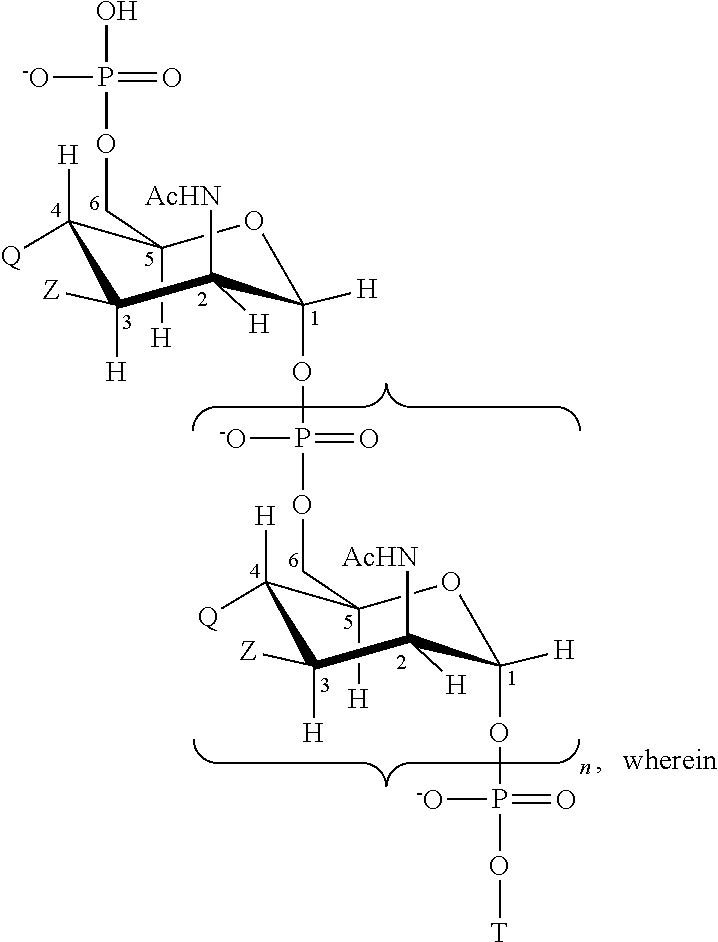
Multivalent meningococcal derivatized polysaccharide-protein conjugates and vaccine
The present invention describes derivatized polysaccharide-protein conjugates, a composition comprising one or more of such derivatized polysaccharide-protein conjugates and methods of immunizing human patients with the same. The derivatized polysaccharide-protein conjugates are purified capsular polysaccharides from Neisseria meningitidis serogroups A, C, W-135, and Y, derivatized chemically activated and selectively attached to a carrier protein by means of a covalent chemical bond, forming polysaccharide-protein conjugates capable of eliciting long-lasting immunity to a variety of N. meningitidis strains.
Owner:AVENTIS PASTEUR INC
Conserved Neisserial antigens
InactiveUS7368261B1Easy to useReduce complexityAntibacterial agentsPeptide/protein ingredientsAntigenSalmonella serotype typhi
To ensure maximum cross-strain recognition and reactivity, regions of proteins that are conserved between different Neisserial species, serogroups and strains can be used. The invention provides proteins which comprise stretches of amino acid sequence that are shared across the majority of Neisseria, particularly N. meningitidis and N. gonorrhoeae.
Owner:NOVARTIS AG
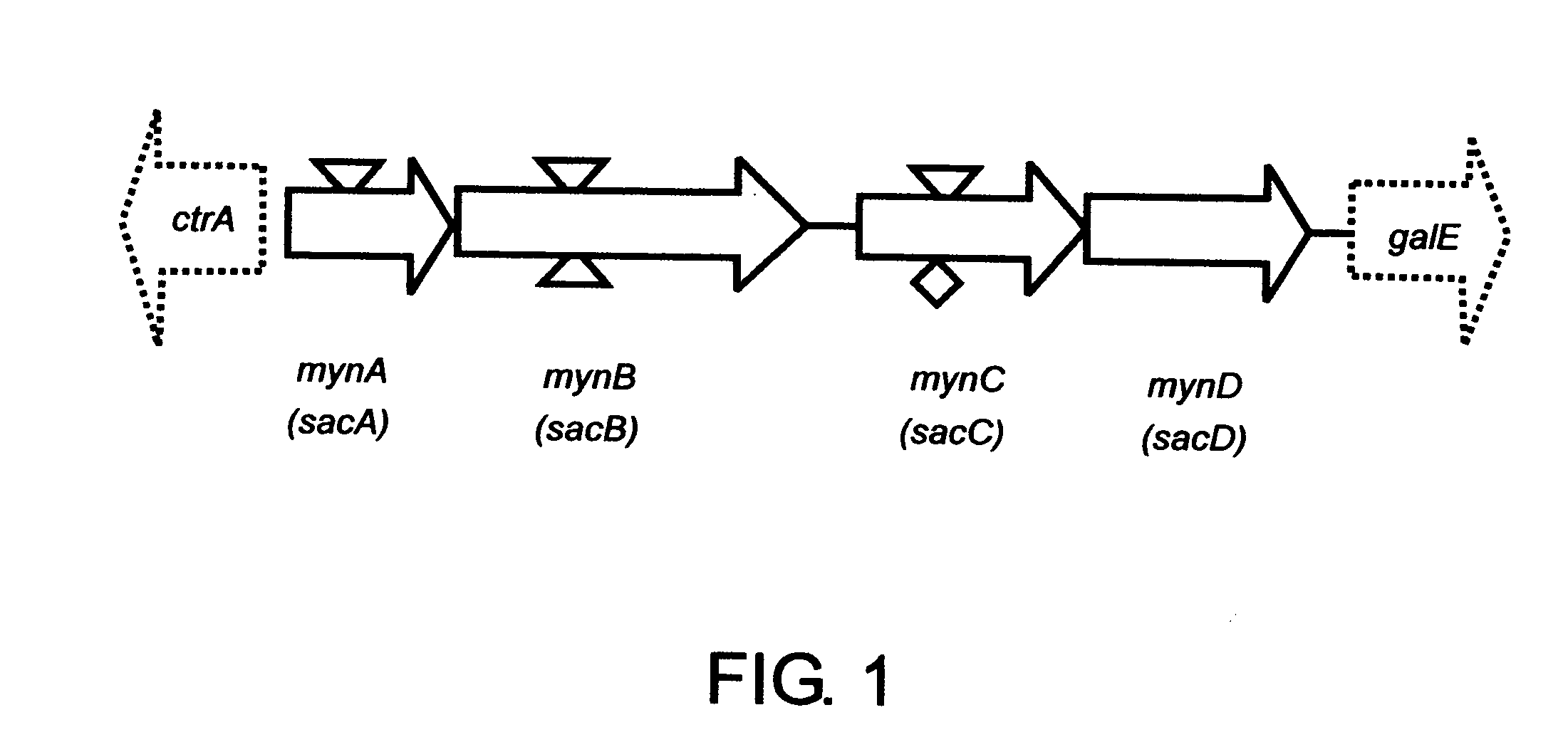
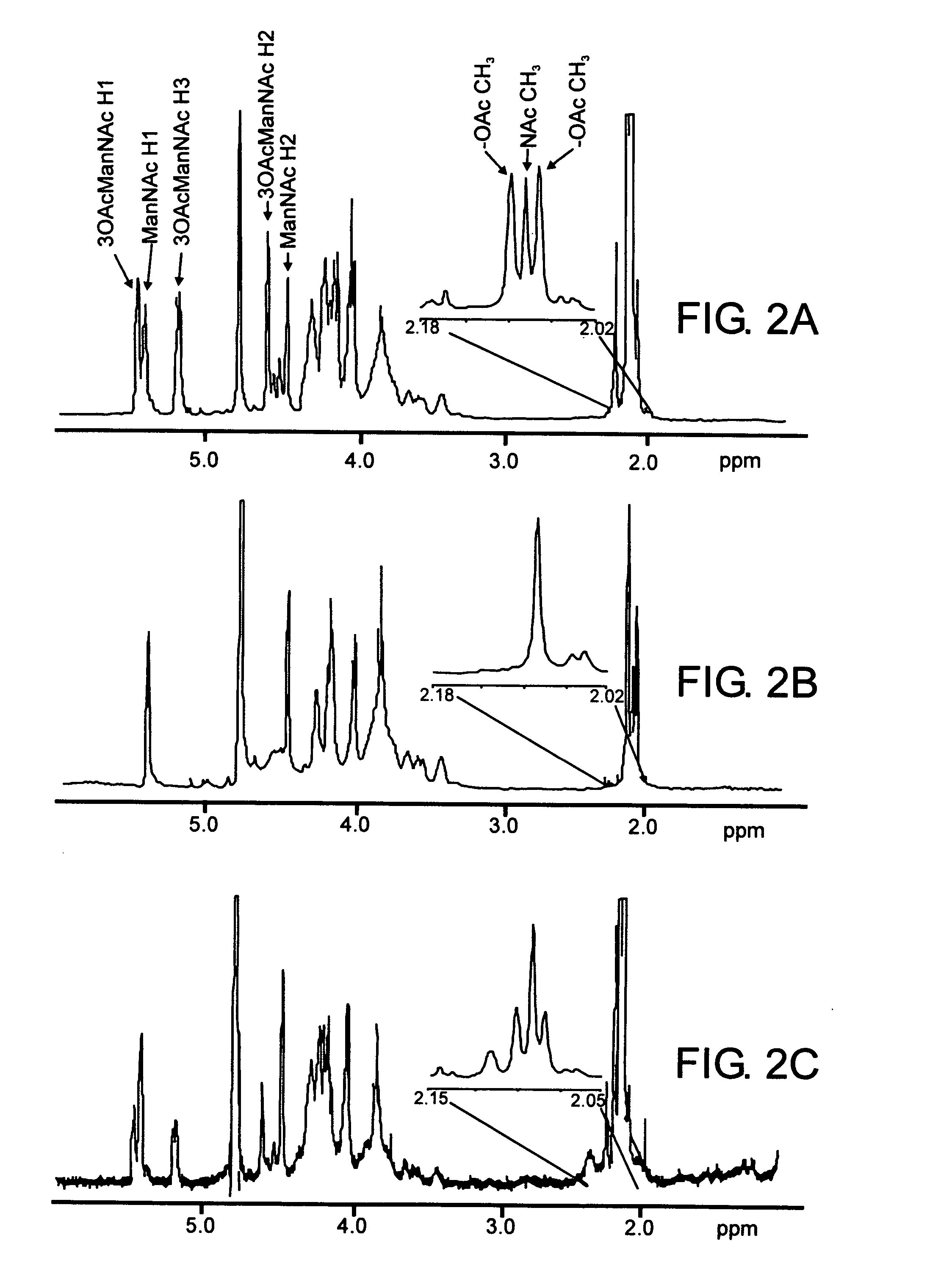
Neisseria meningitidis serogroup a capsular polysaccharide acetyltransferase, methods and compositions
InactiveUS20060073168A1Easy to optimizeComplete acetylationAntibacterial agentsBacteriaSalmonella serotype typhiAcetyltransferase
Provided are recombinant DNA molecules that do not occur in nature encoding an O-acetyltransferase, vectors that direct expression of an O-acetyltransferase, recombinant host cells which express an O-acetyltransferase, methods for recombinant production of an O-acetyltransferase, methods for acetylating capsular polysaccharides, especially those of a Serogroup A Neisseria meningitidis using a recombinant O-acetyltransferase, and immunogenic compositions comprising the acetylated capsular polysaccharide.
Owner:EMORY UNIVERSITY +1
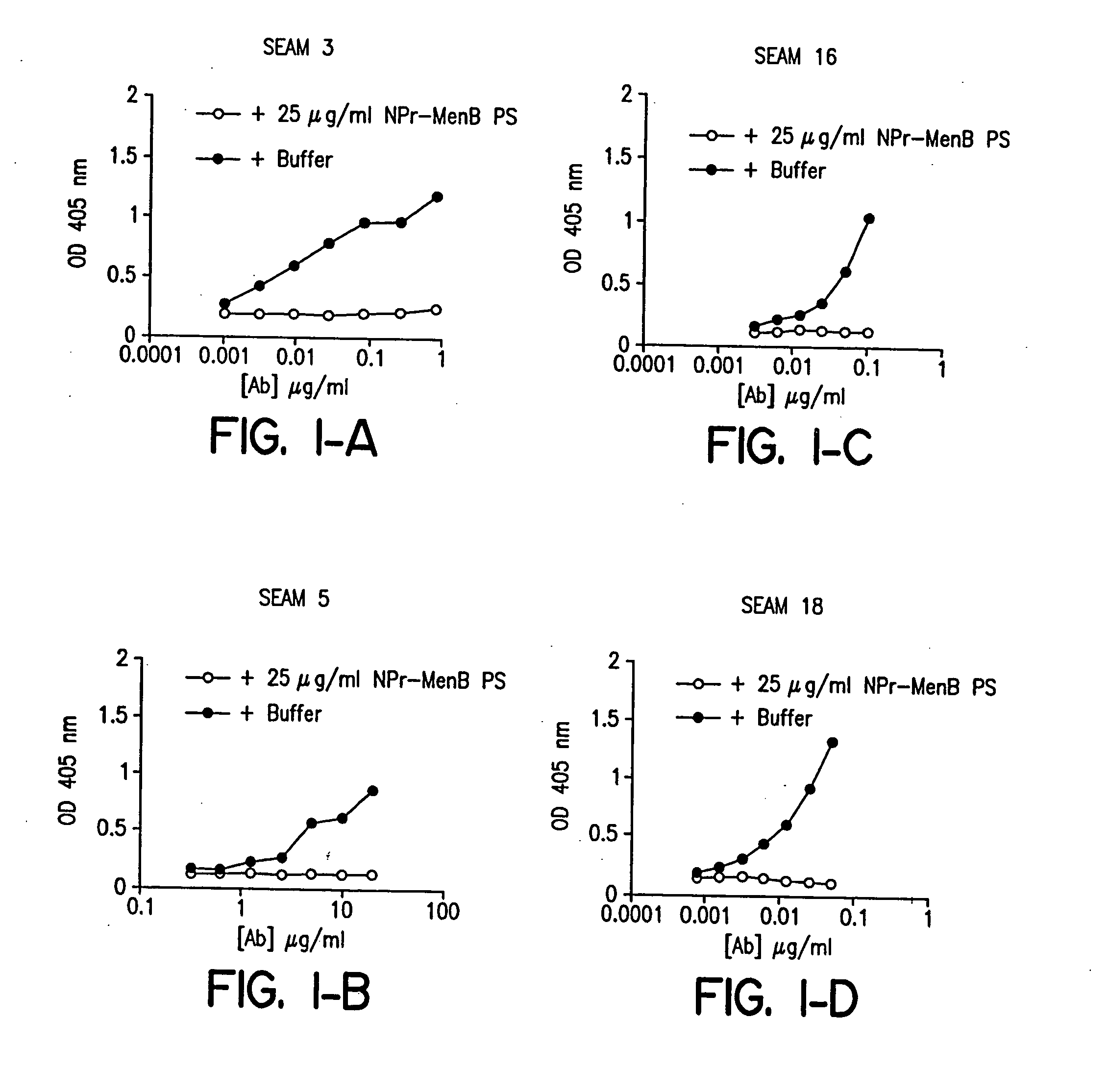
Methods for isolating molecular mimetics of unique Neisseria meningitidis serogroup B epitopes
InactiveUS20060035284A1Determine autoreactivityLeast riskAntibacterial agentsAnimal cellsEscherichia coliSalmonella serotype typhi
Novel bactericidal antibodies against Neisseria meningitidis serogroup B (“MenB”) are disclosed. The antibodies either do not cross-react or minimally cross-react with host tissue polysialic acid and hence pose minimal risk of autoimmune activity. The antibodies are used to identify molecular mimetics of unique epitopes found on MenB or E. coli K1. Examples of such peptide mimetics are described that elicit serum antibody capable of activating complement-mediated bacteriolysis of MenB. Vaccine compositions containing such mimetics can be used to prevent MenB or E. coli K1 disease without the risk of evoking autoantibody.
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Methods for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 19A polysaccharide are also provided in which the serotype 19A polysaccharide is co-lyophilized with a carrier protein and conjugation is carried out in dimethyl sulfoxide (DMSO) via a reductive amination mechanism.
Owner:WYETH LLC
Gonococcal proteins and nucleic acids
InactiveUS20050260581A1Easy to insertEfficient HarvestingAntibacterial agentsOrganic active ingredientsSalmonella serotype typhiVaccine Immunogenicity
The invention provides proteins from gonococcus (Neisseria gonorrhoeae), including amino acid sequences, the corresponding nucleotide sequences, expression data, and serological data. The proteins are useful antigens for vaccines, immunogenic compositions, and / or diagnostics. They are also useful for distinguishing between gonococcus and meningococcus and, in particular, between gonococcus and serogroup B meningococcus.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com