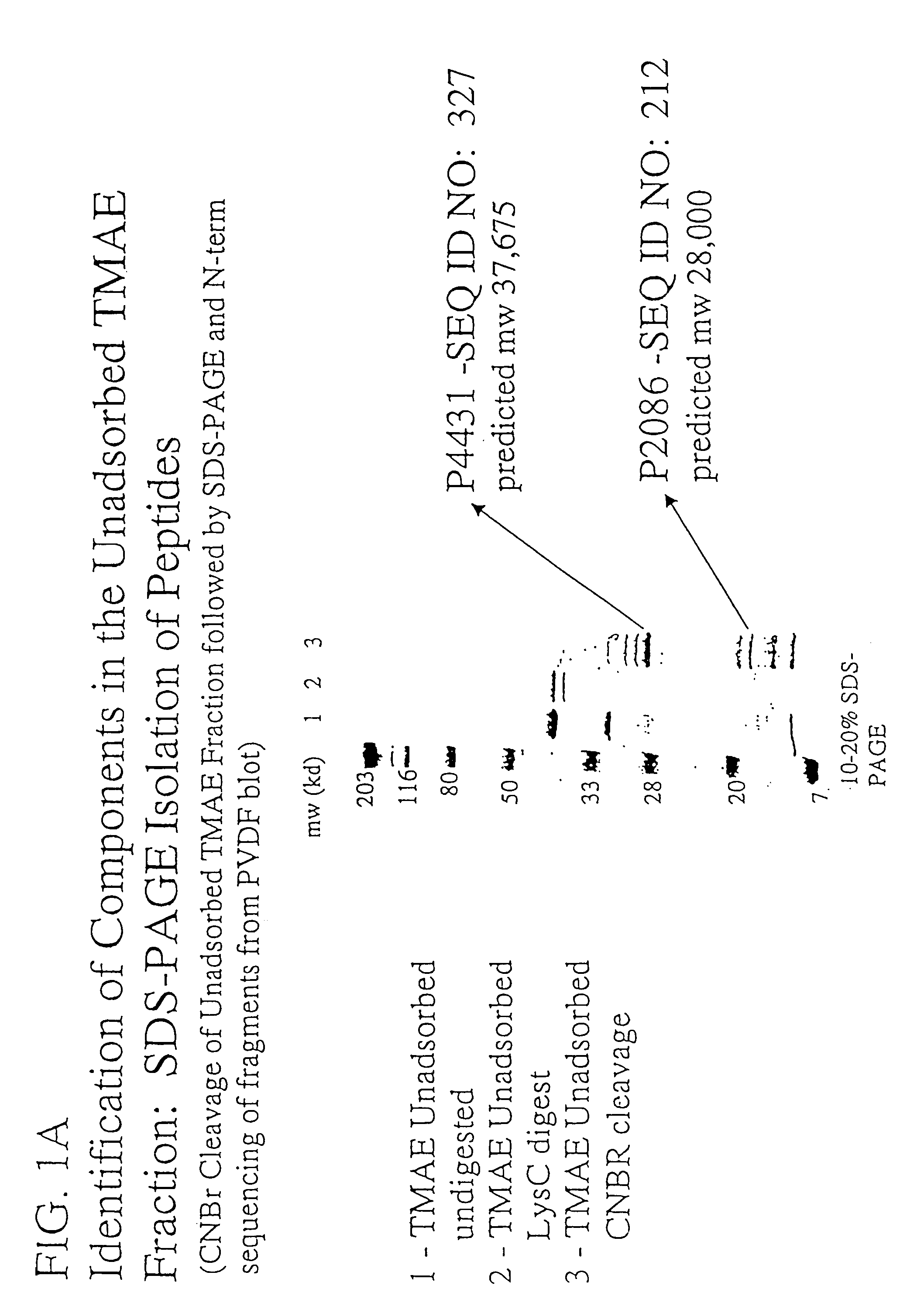
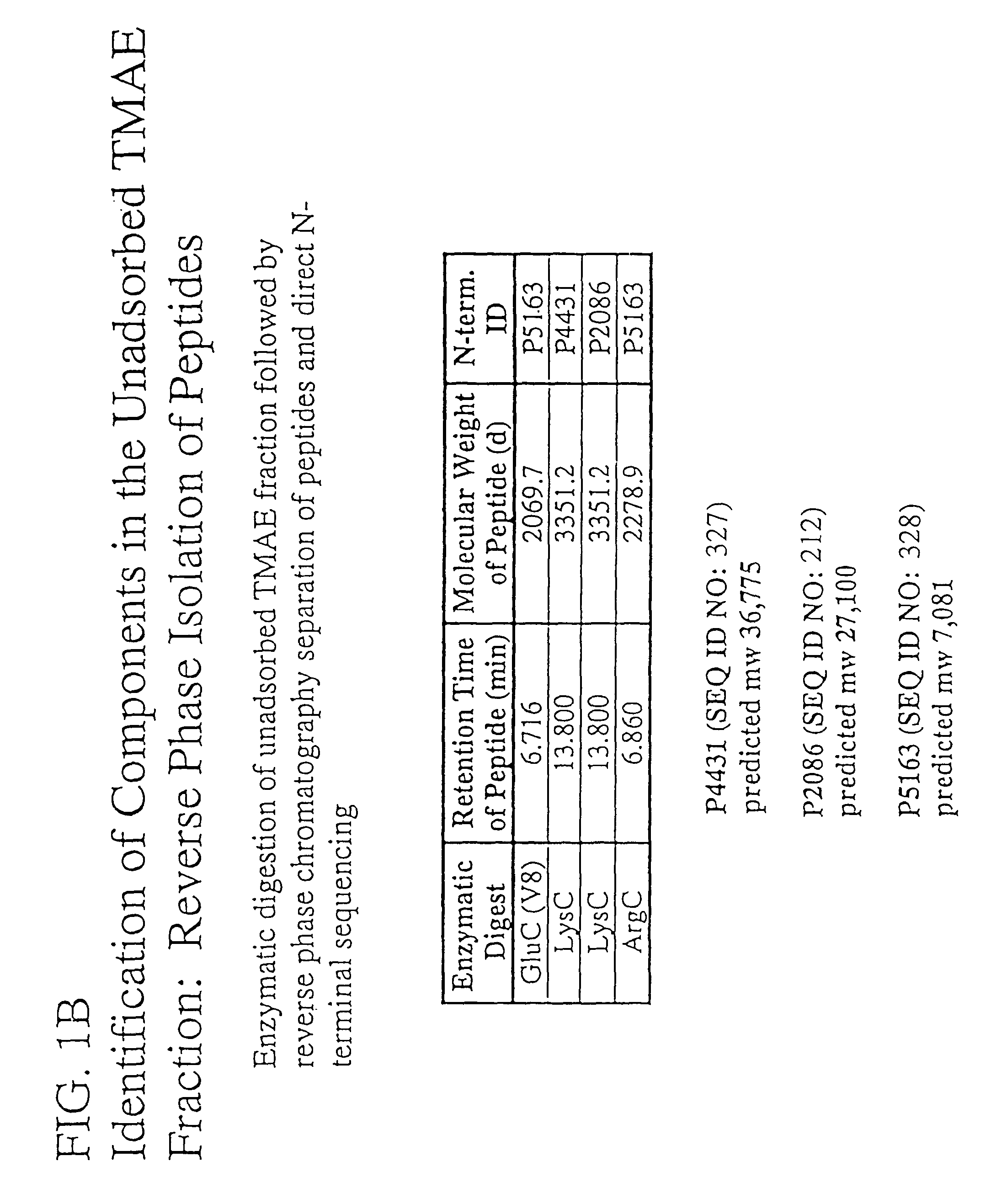
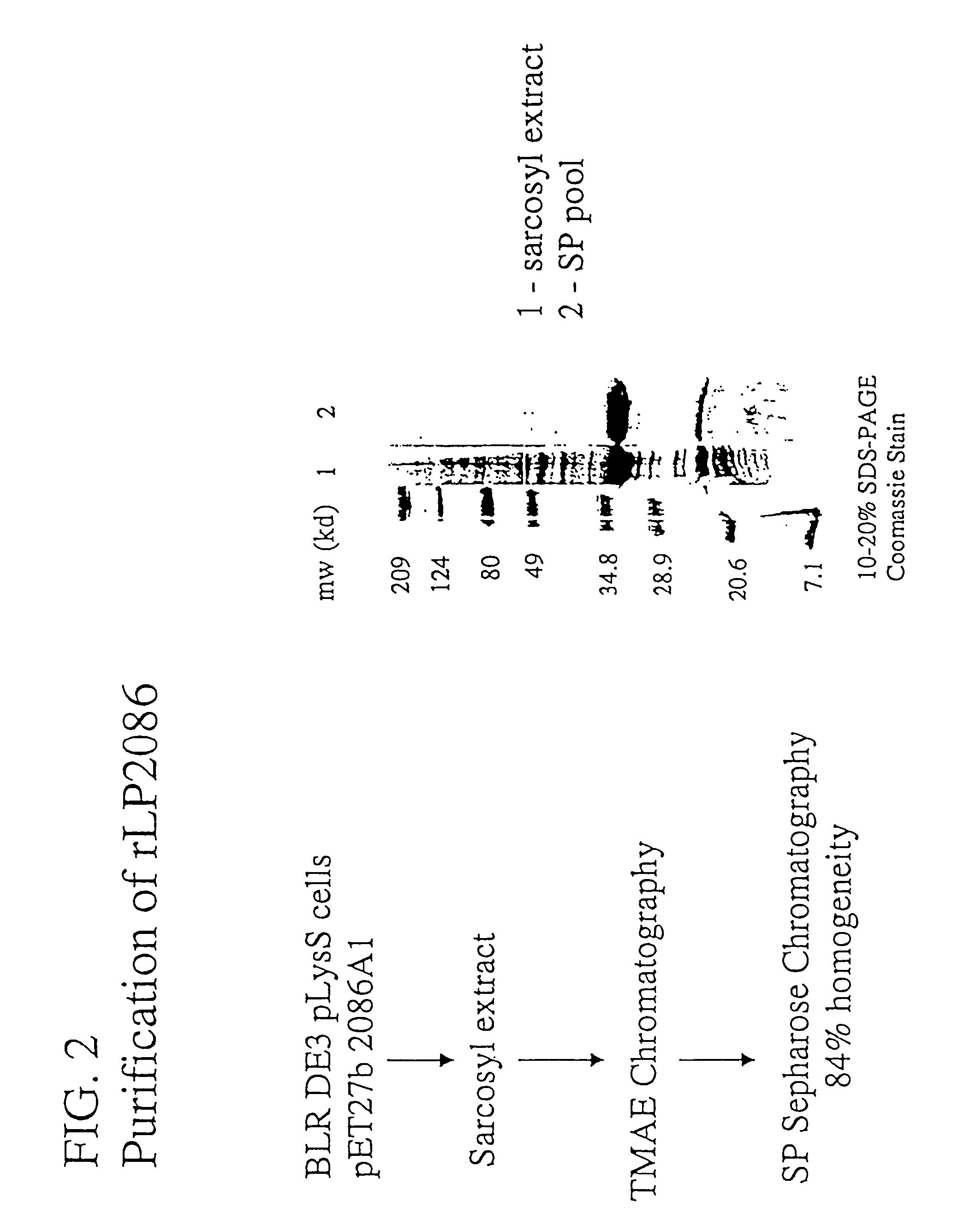
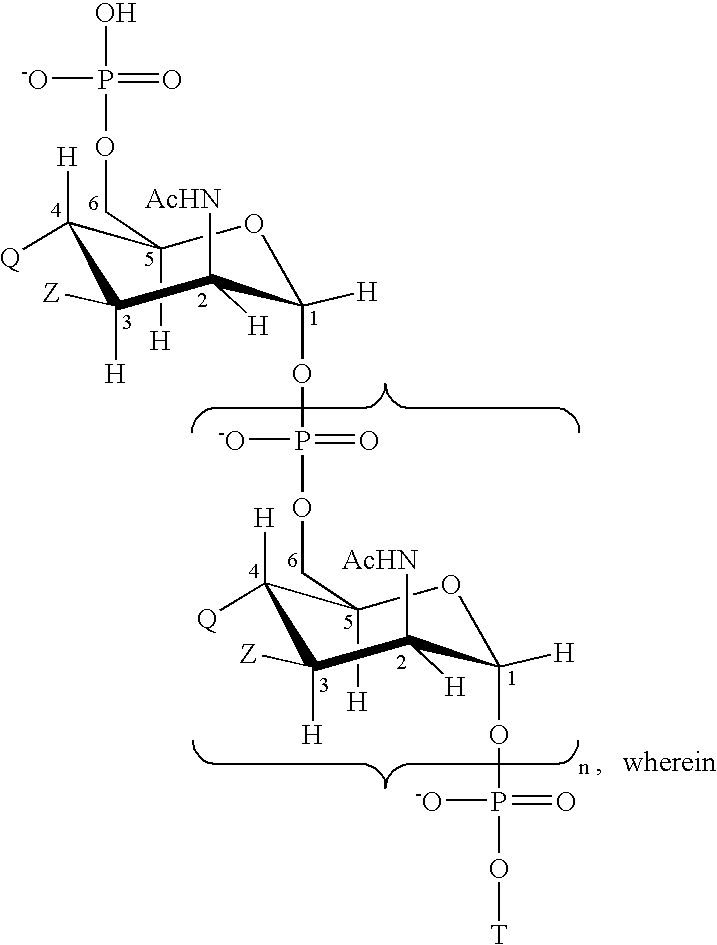
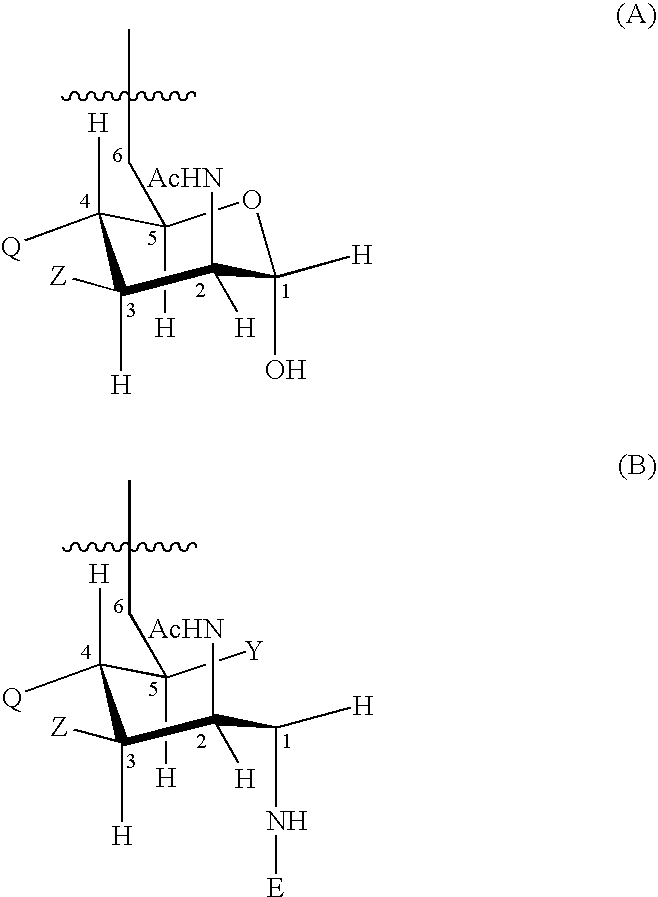
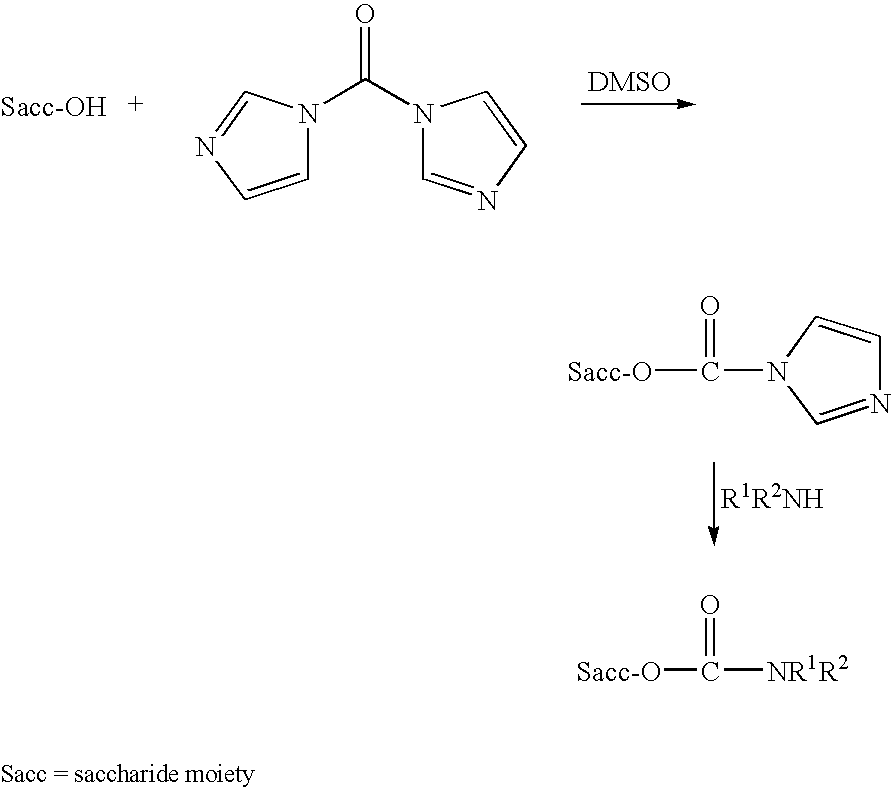
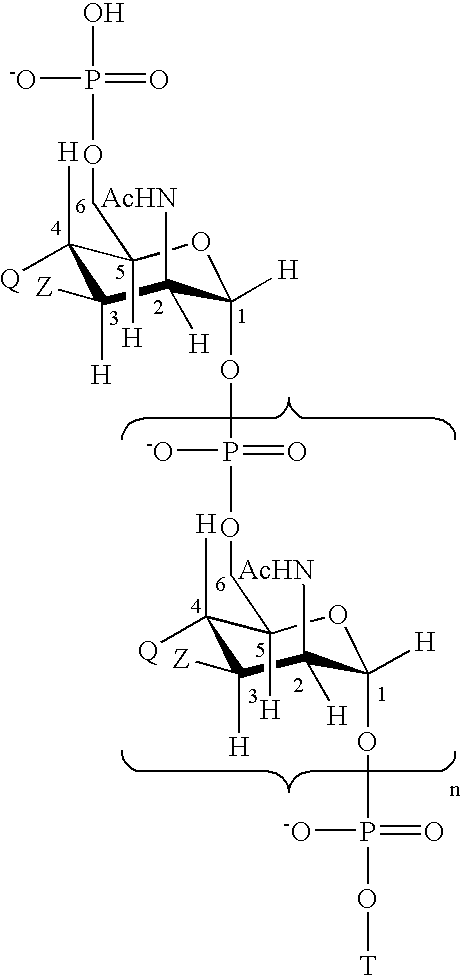
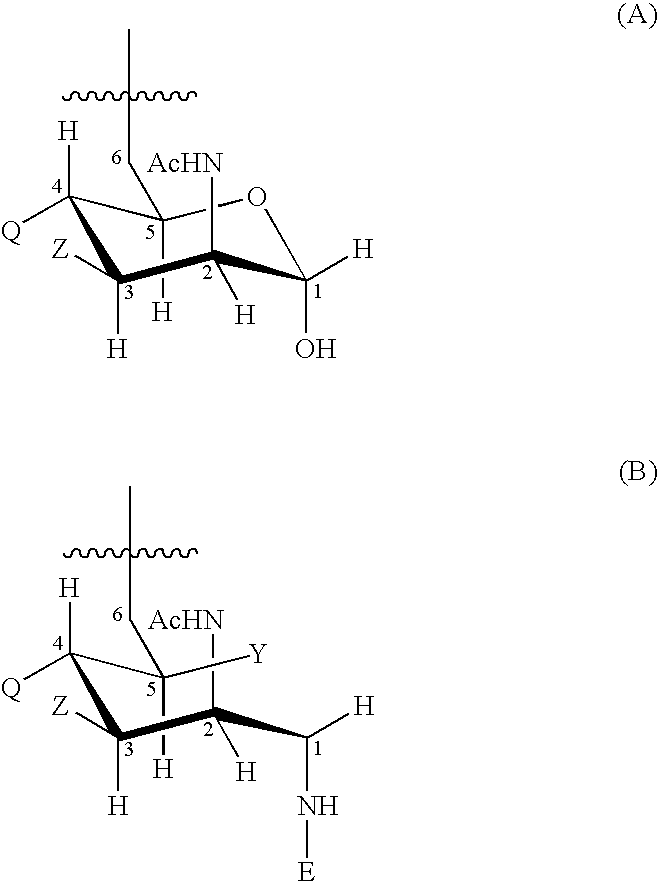
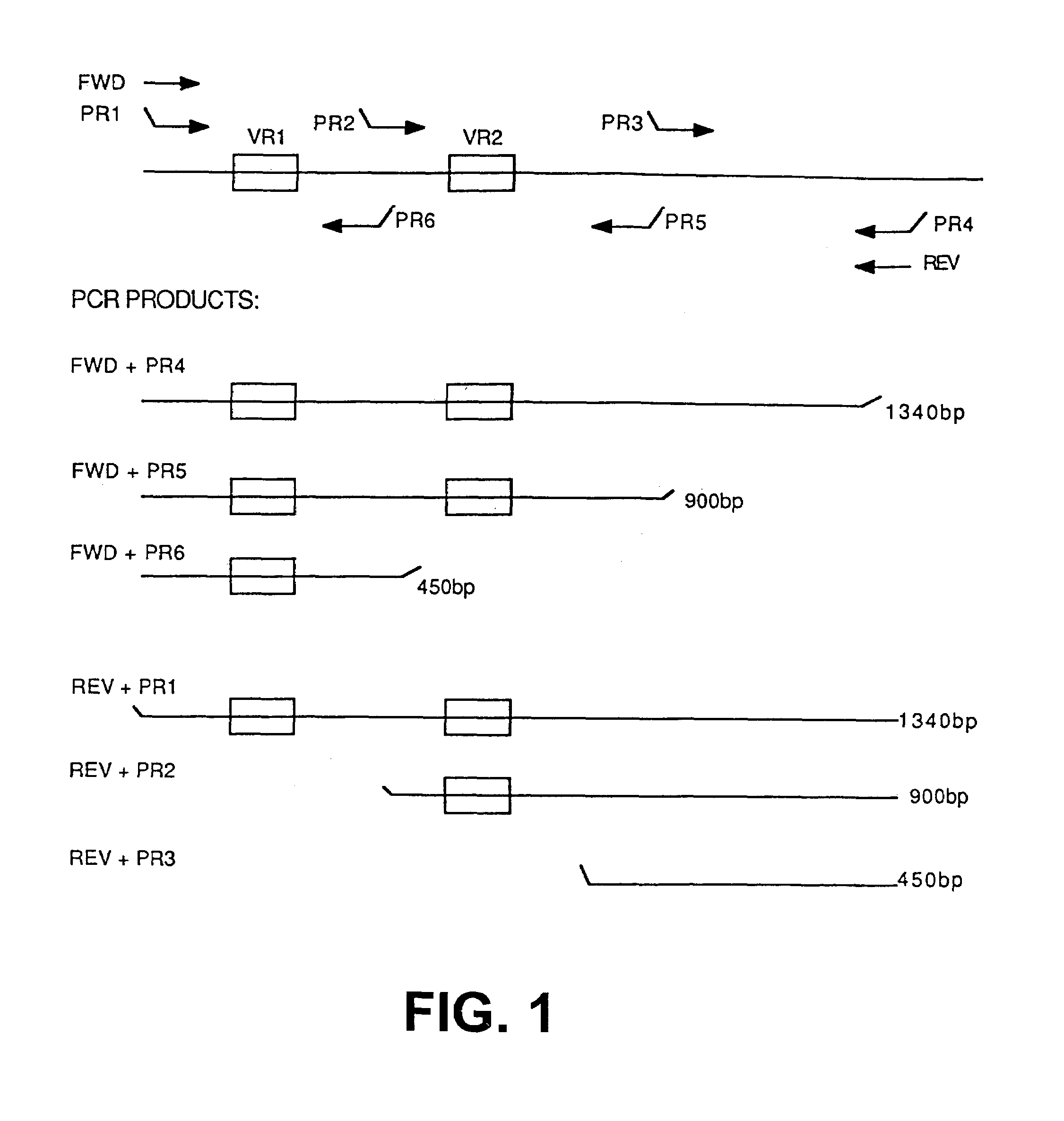
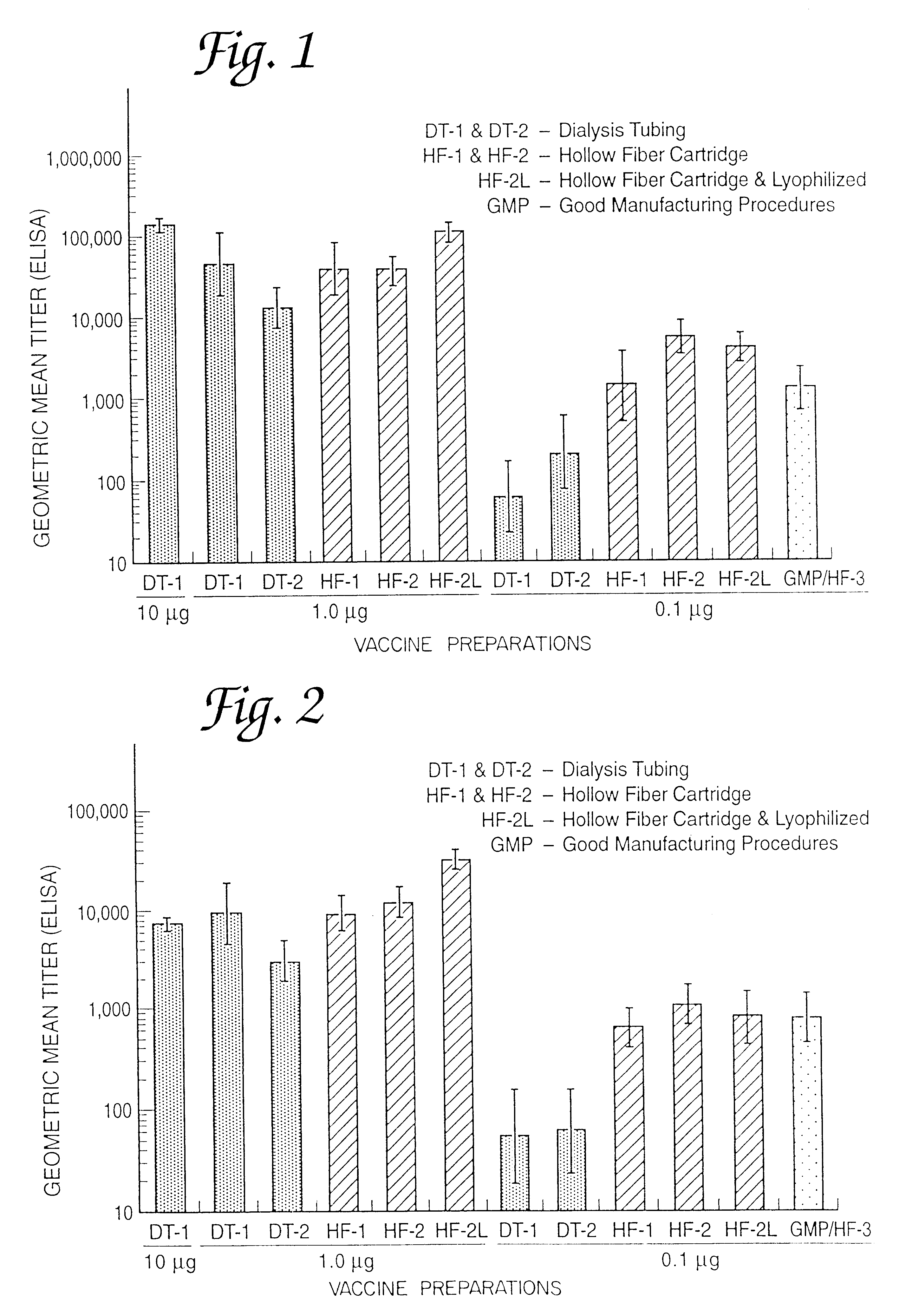
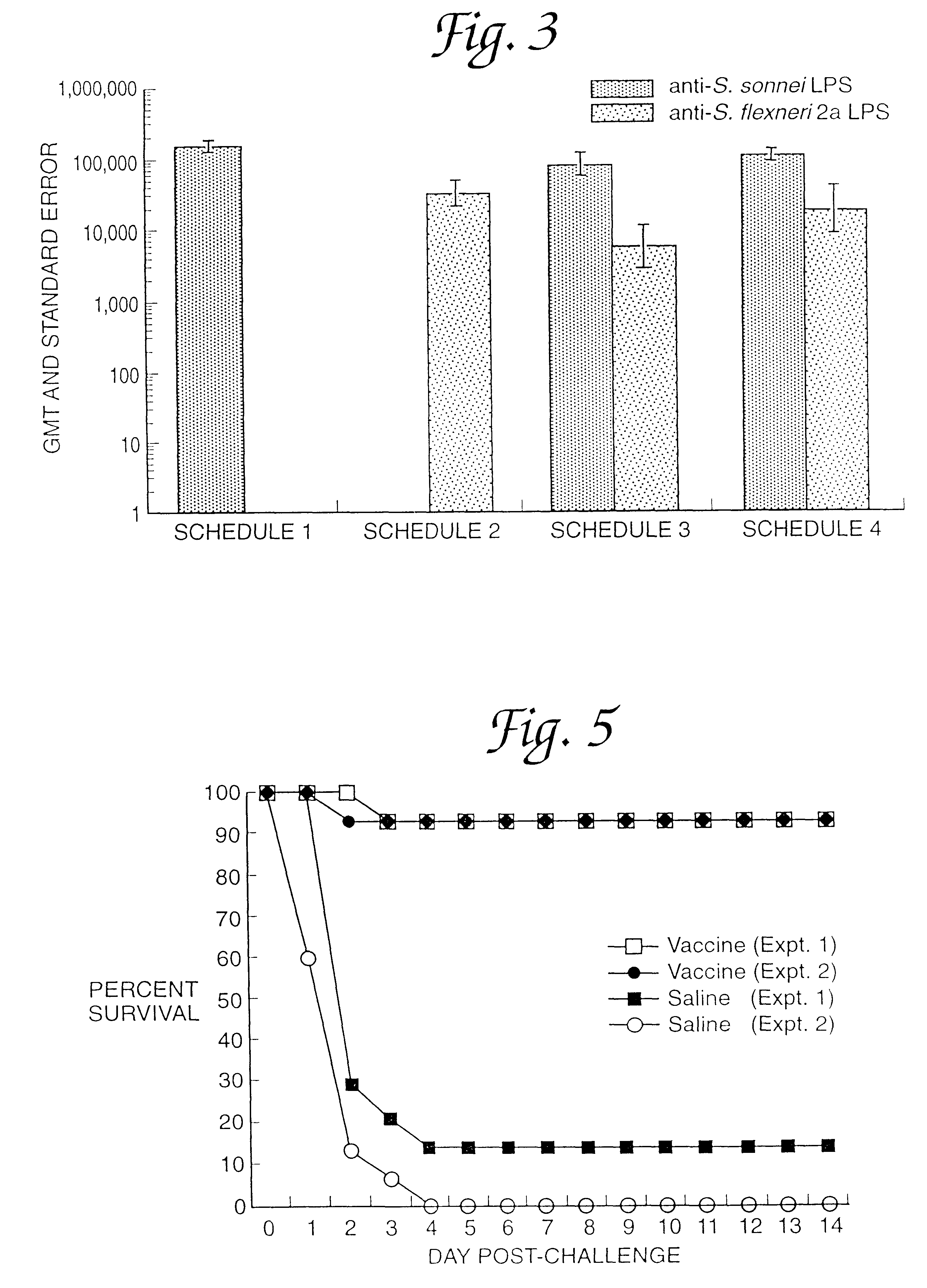
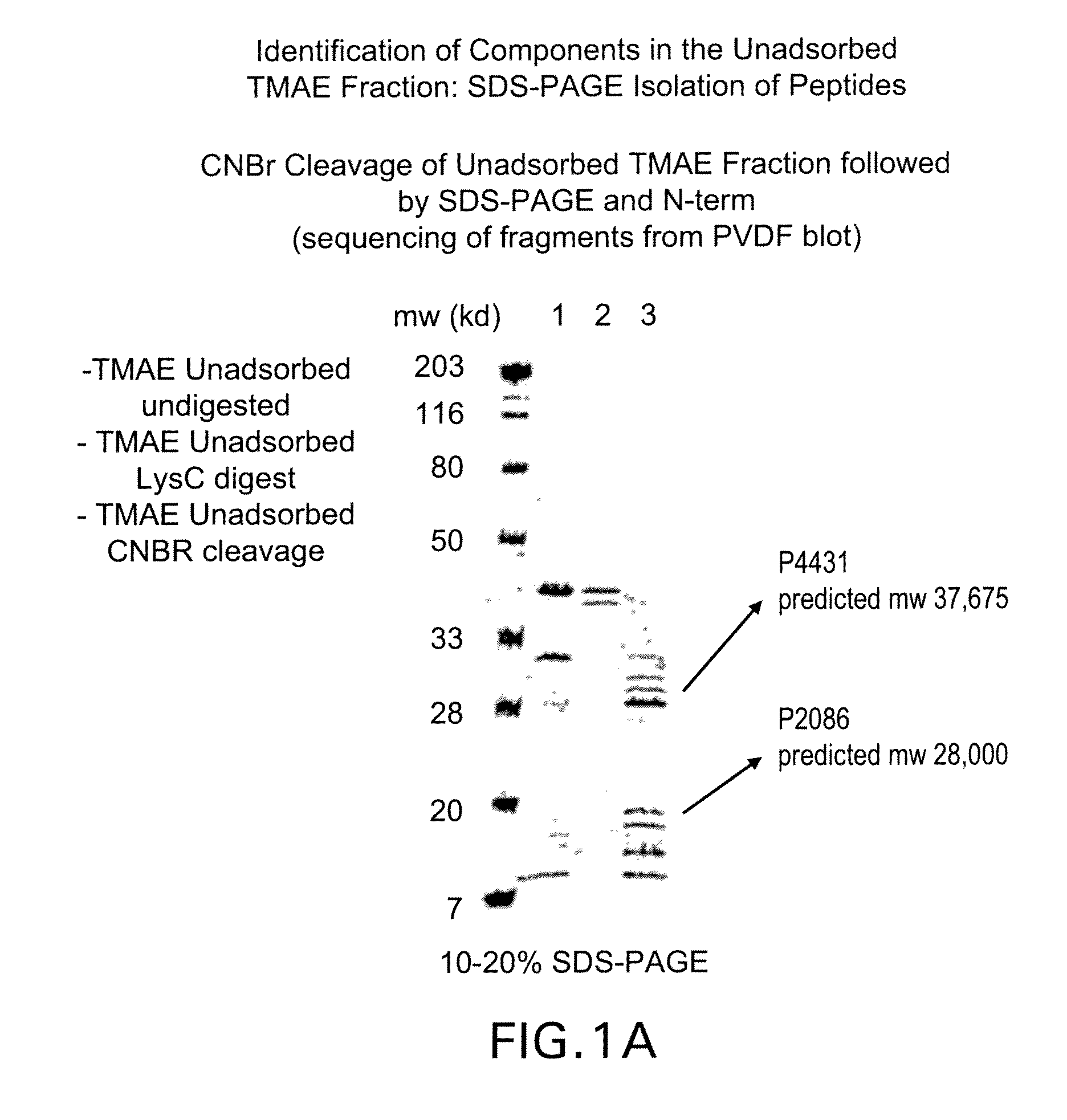
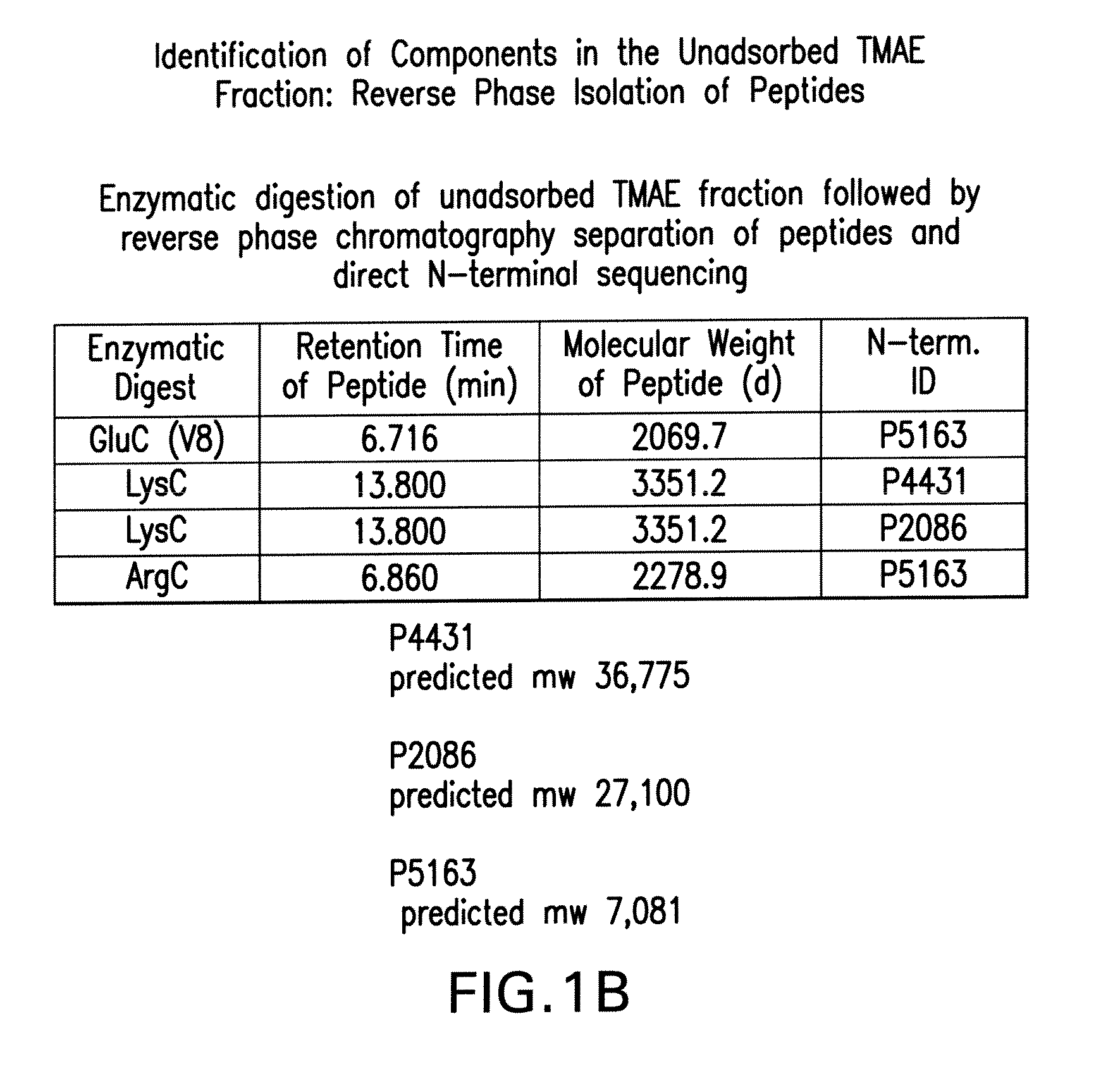
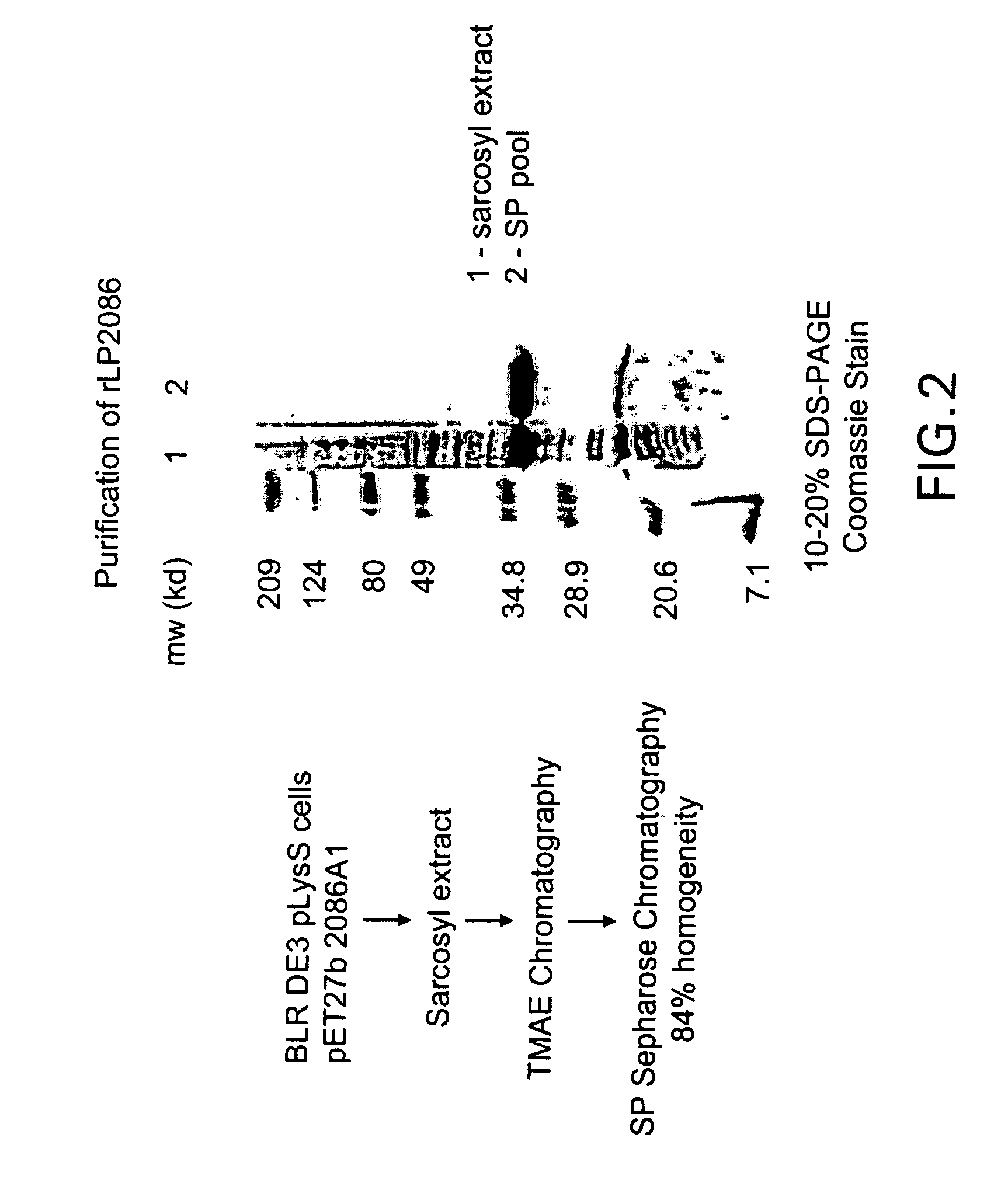
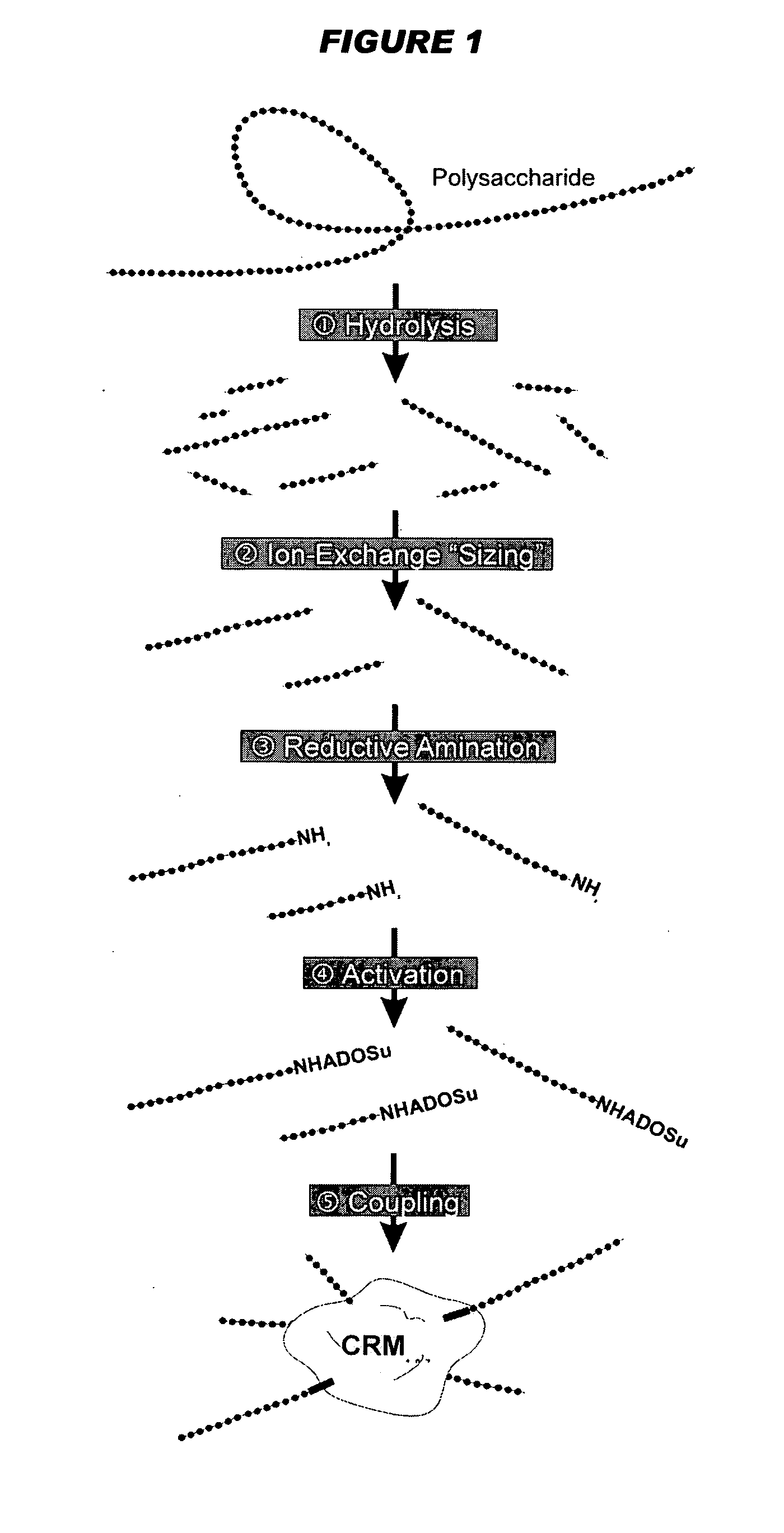
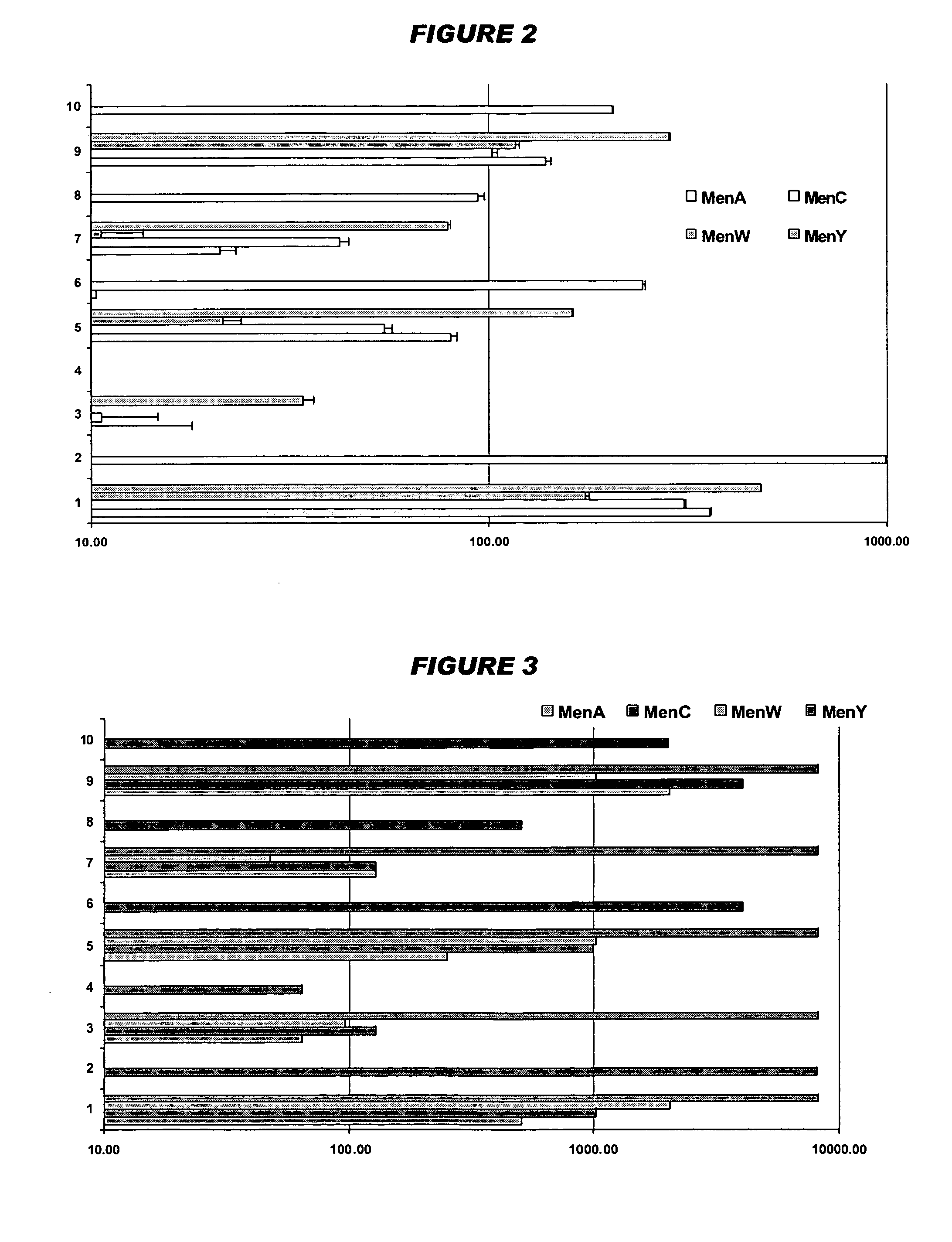
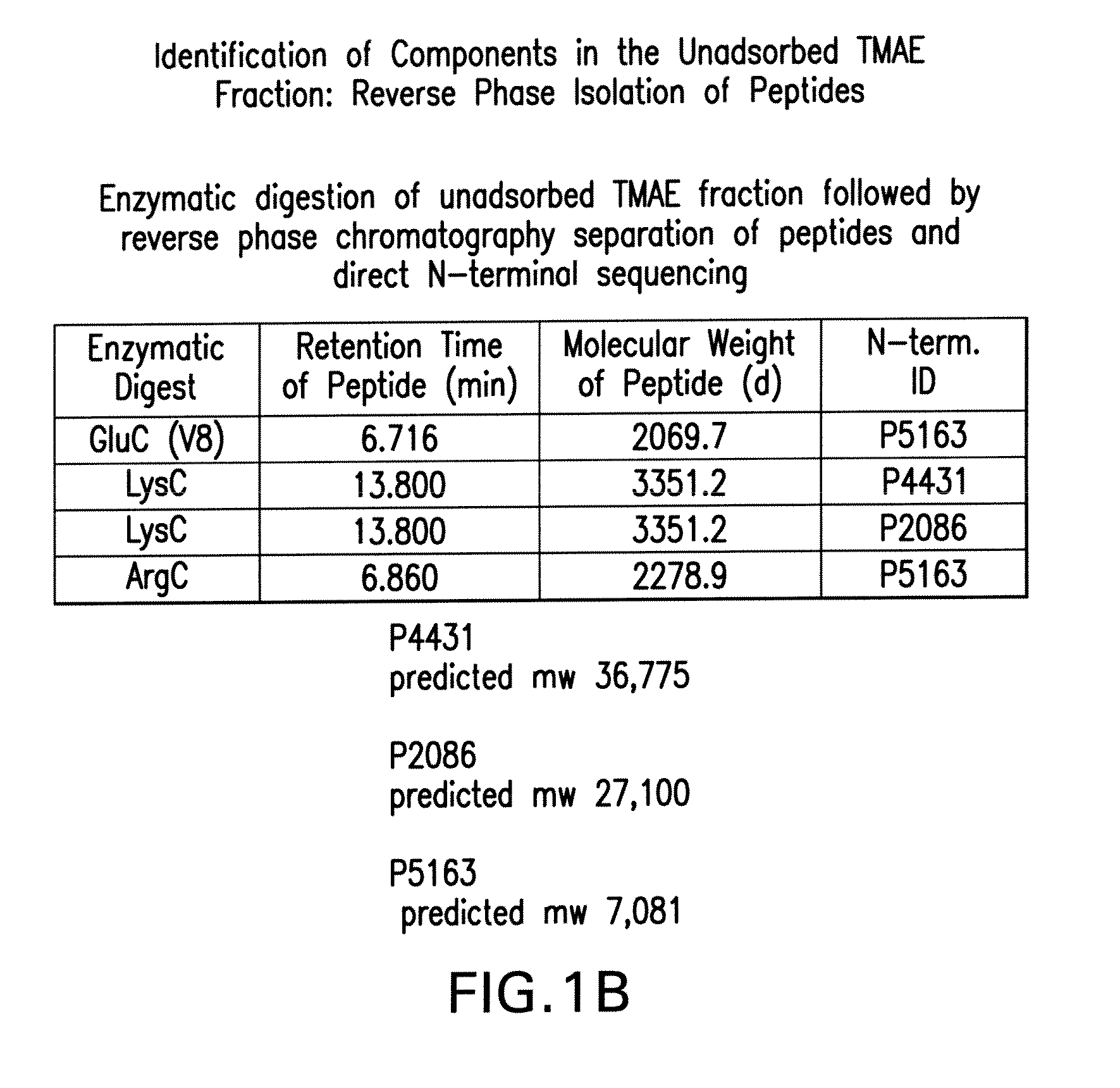
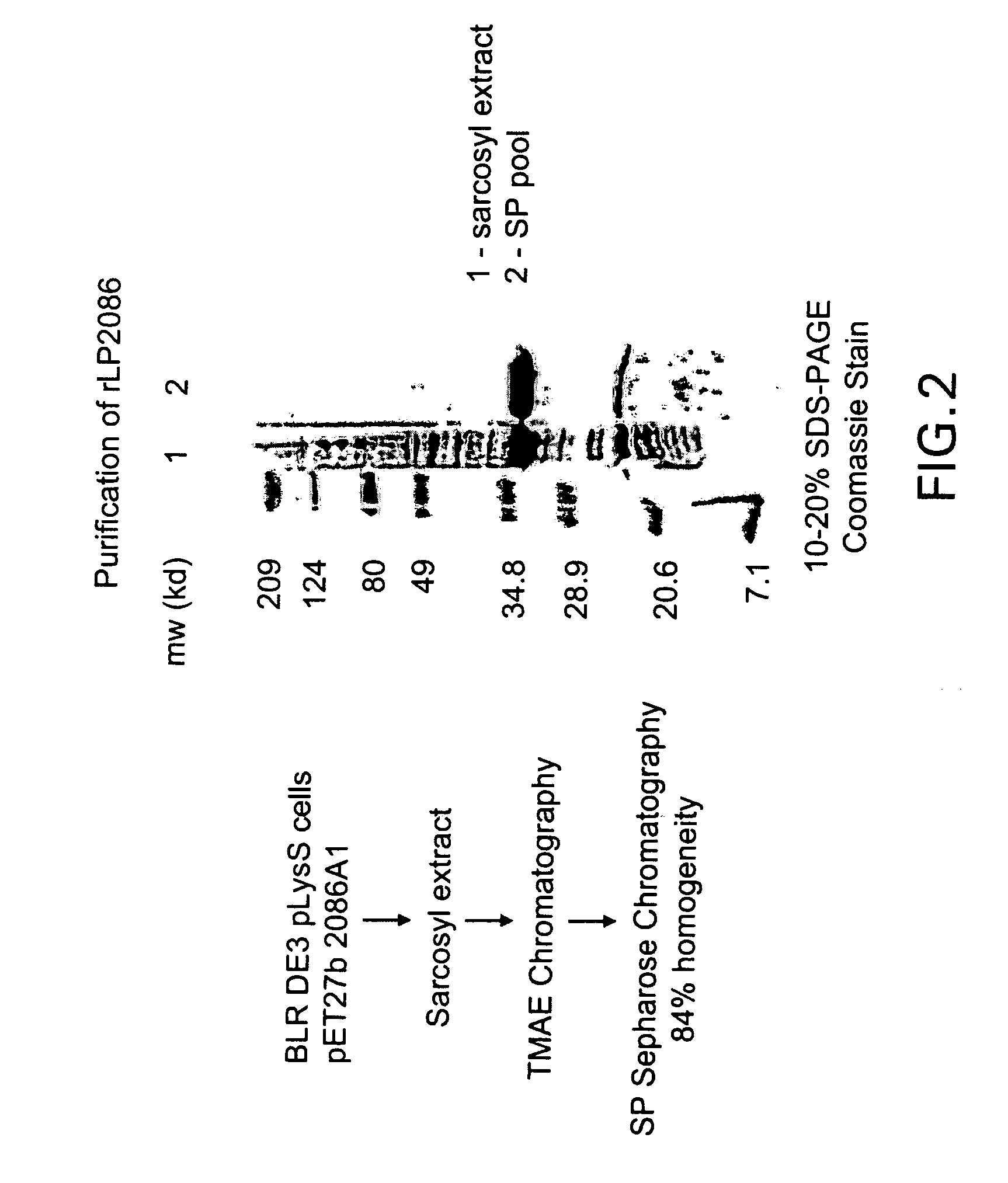
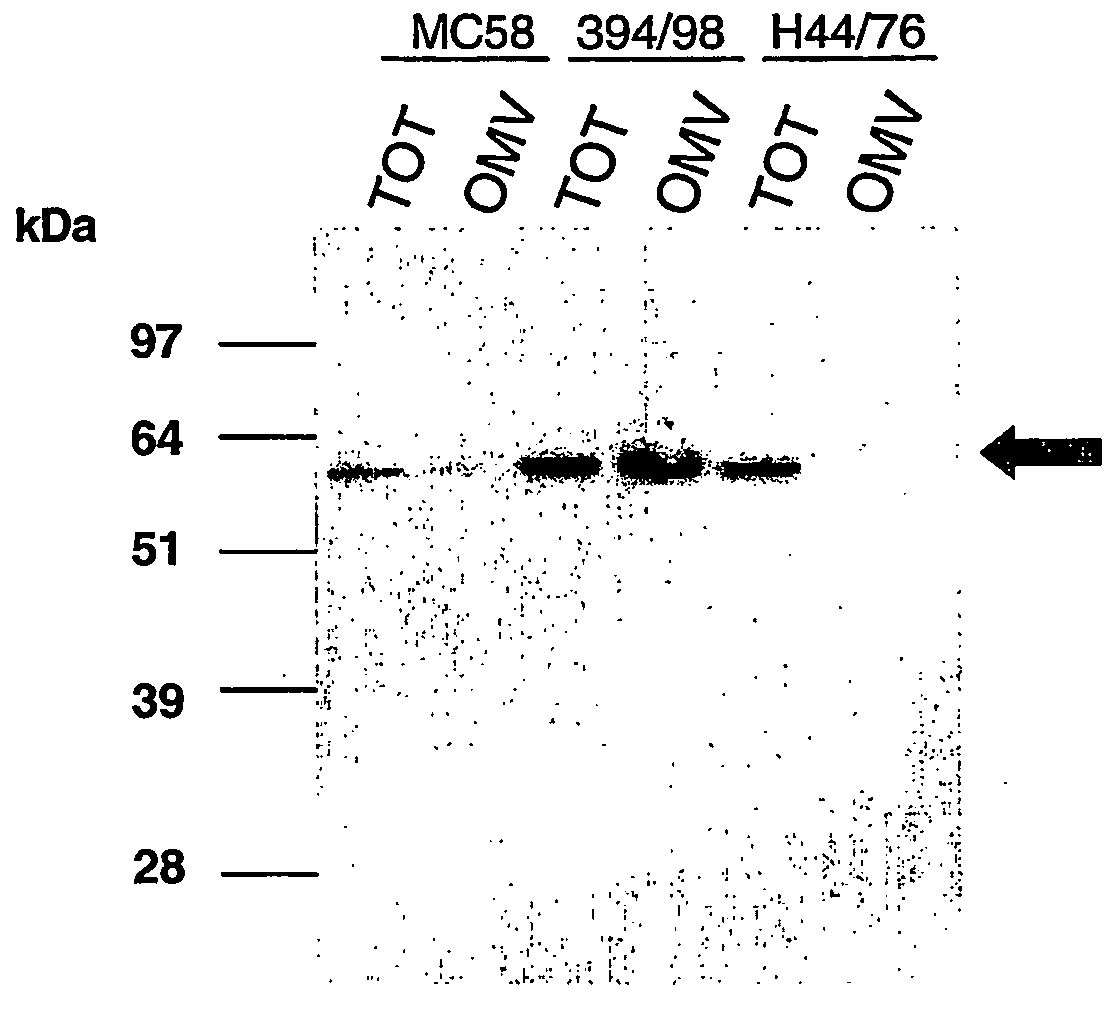
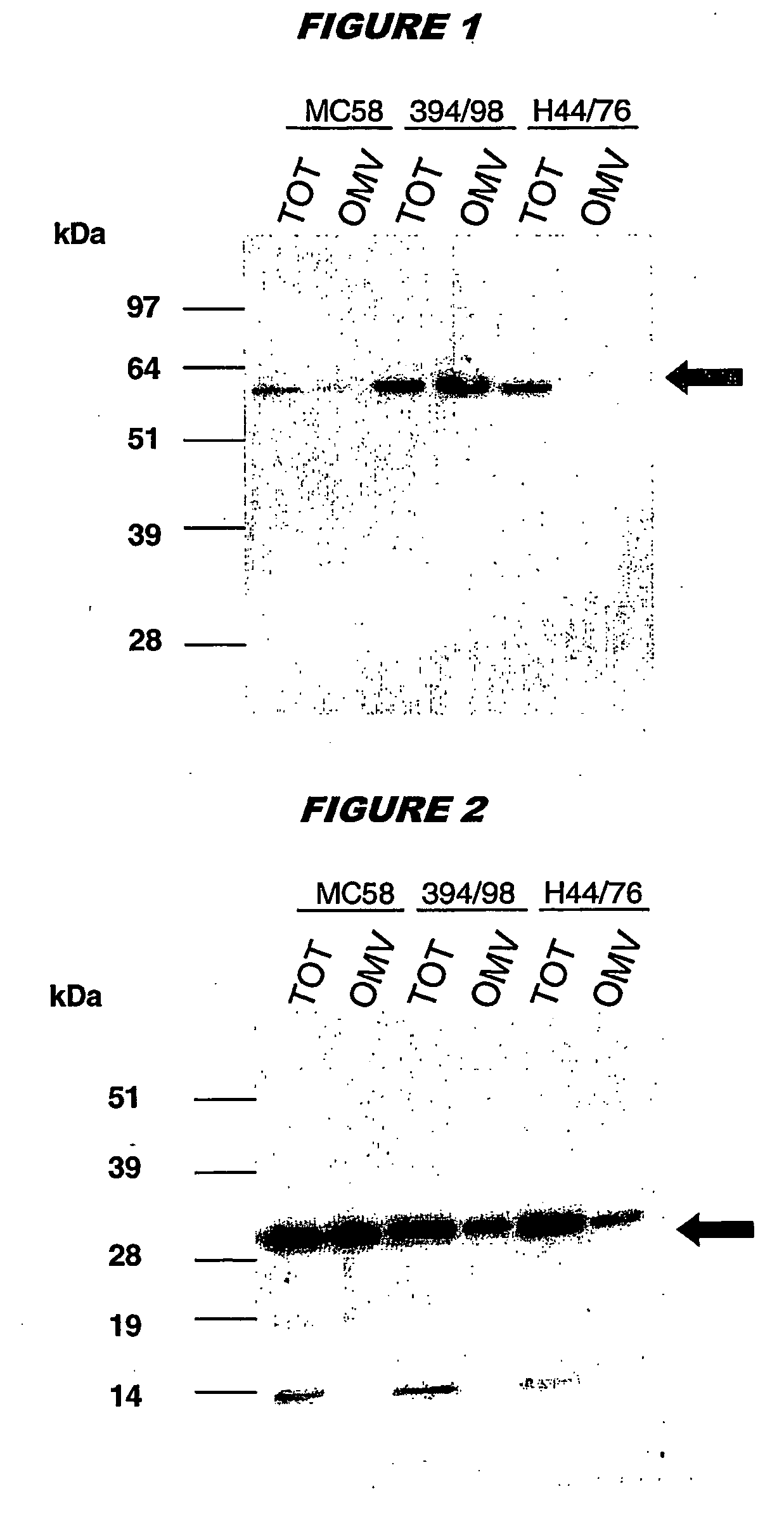
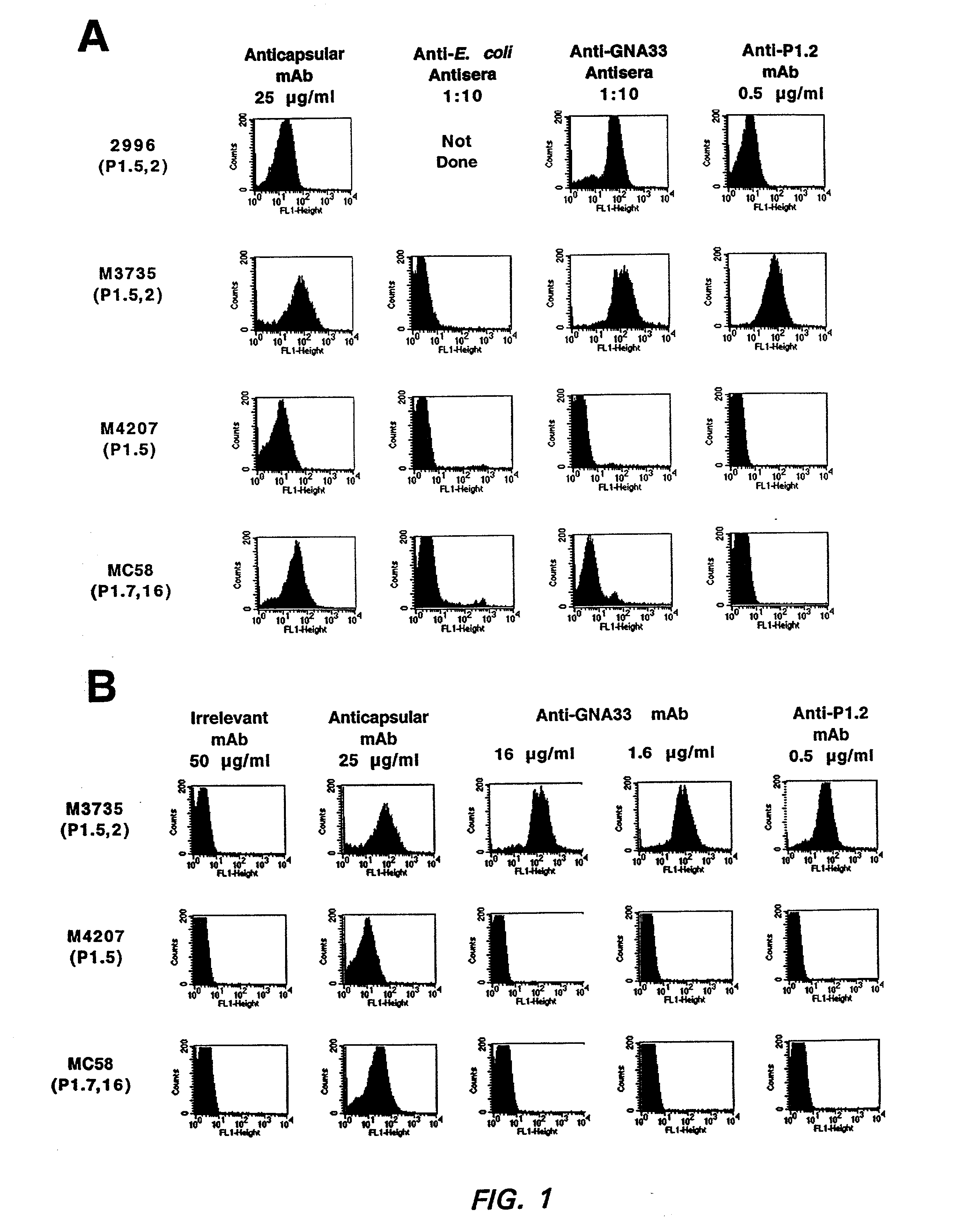
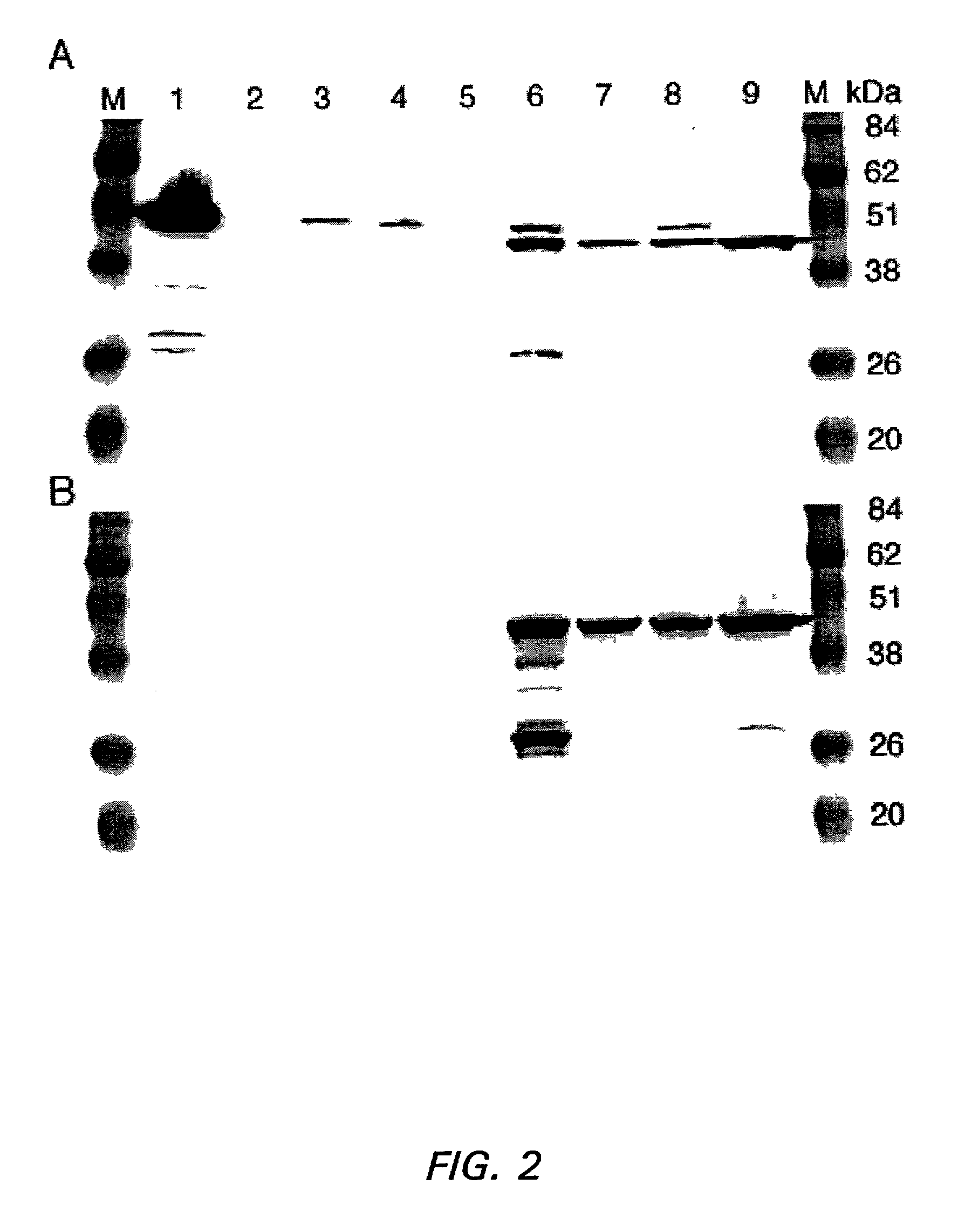
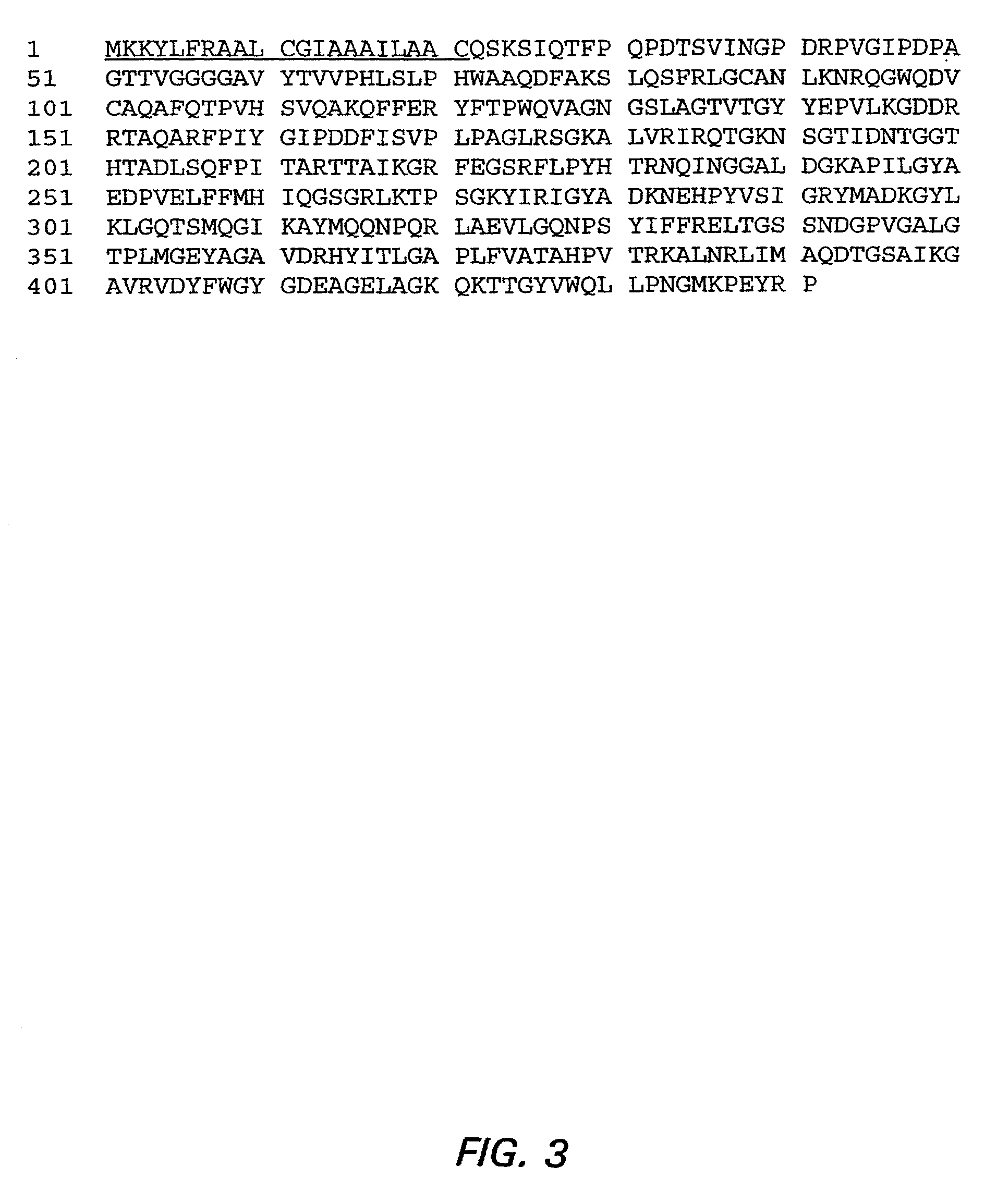
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
234 results about "Meningococcal carriage" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
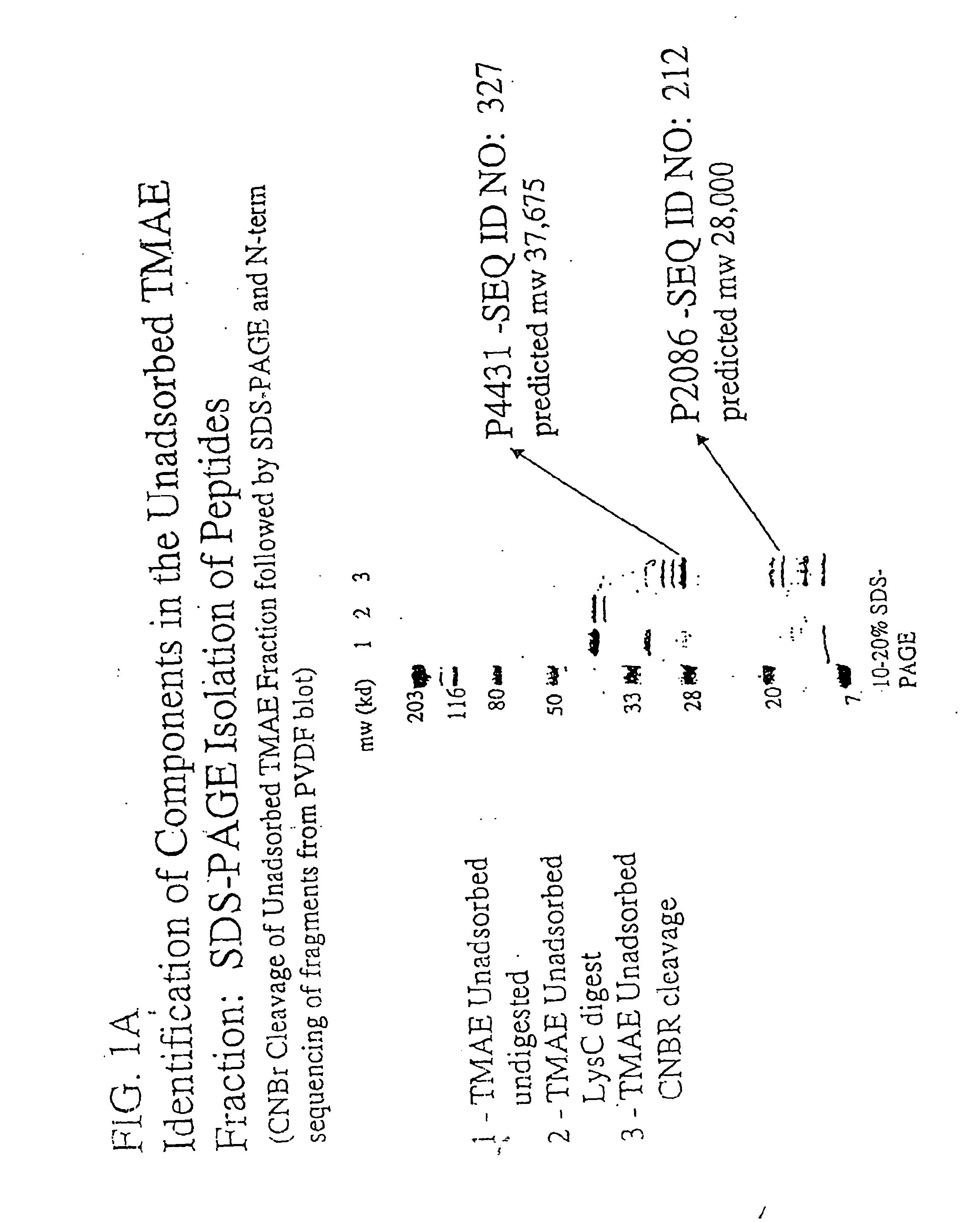
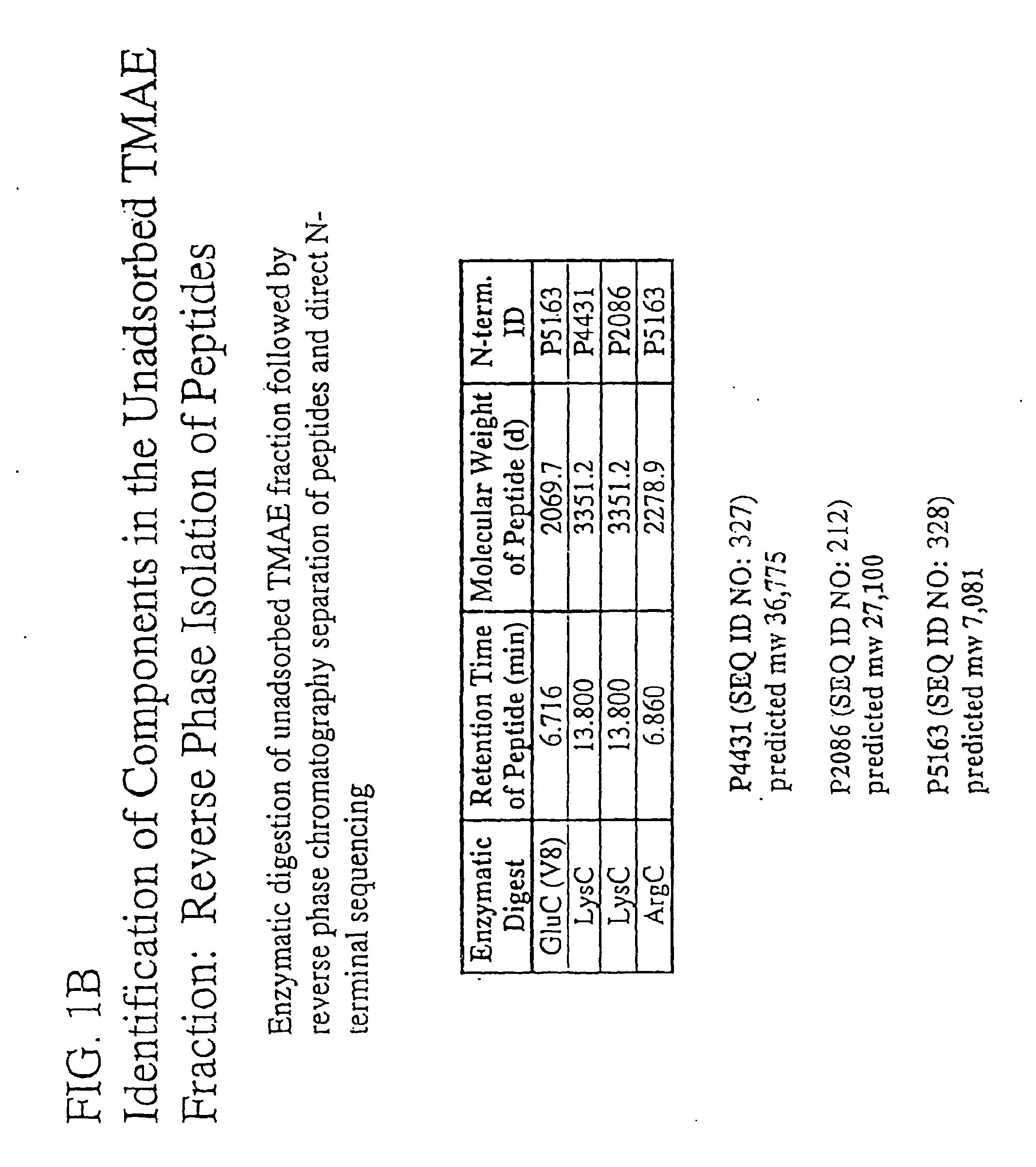
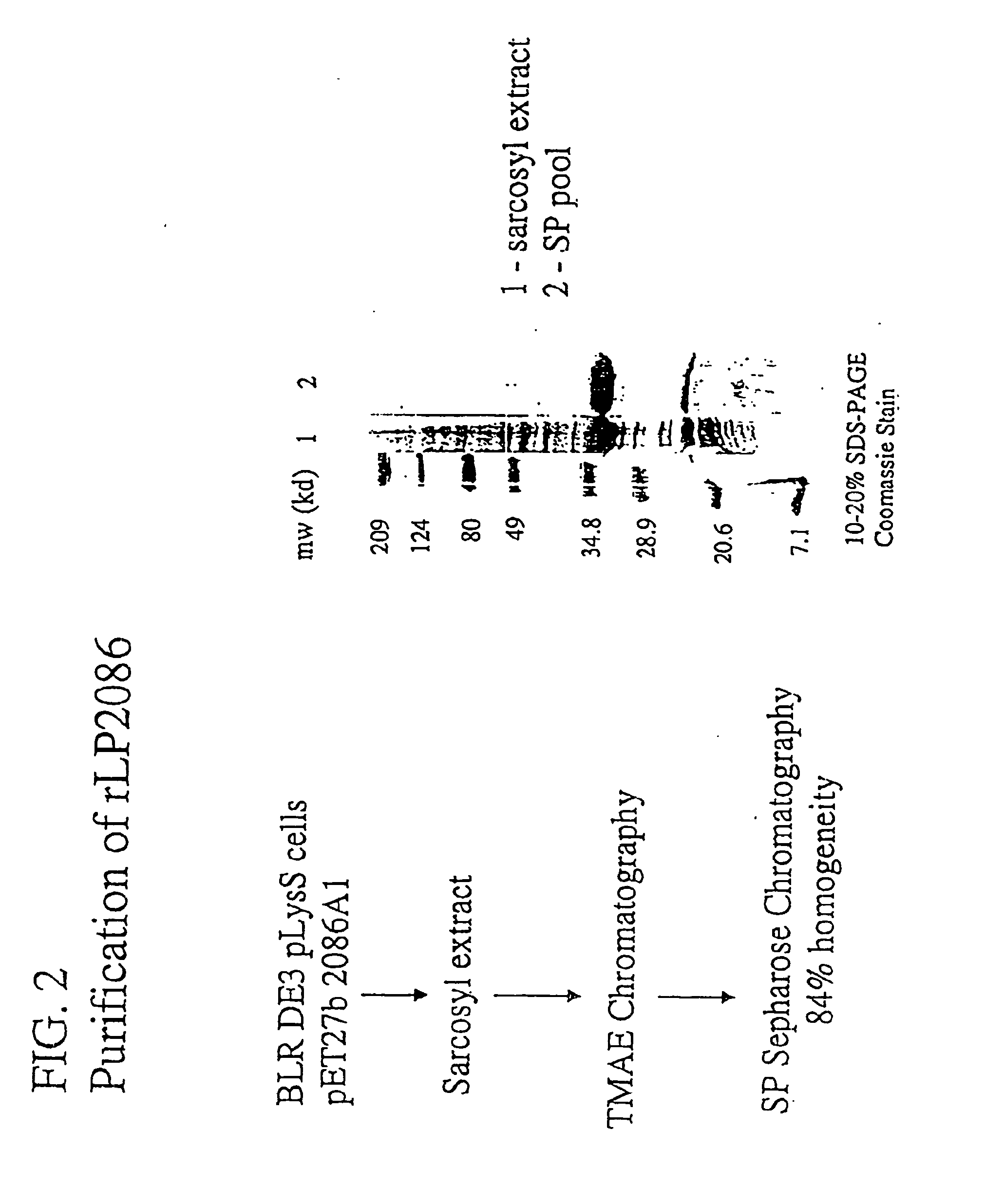
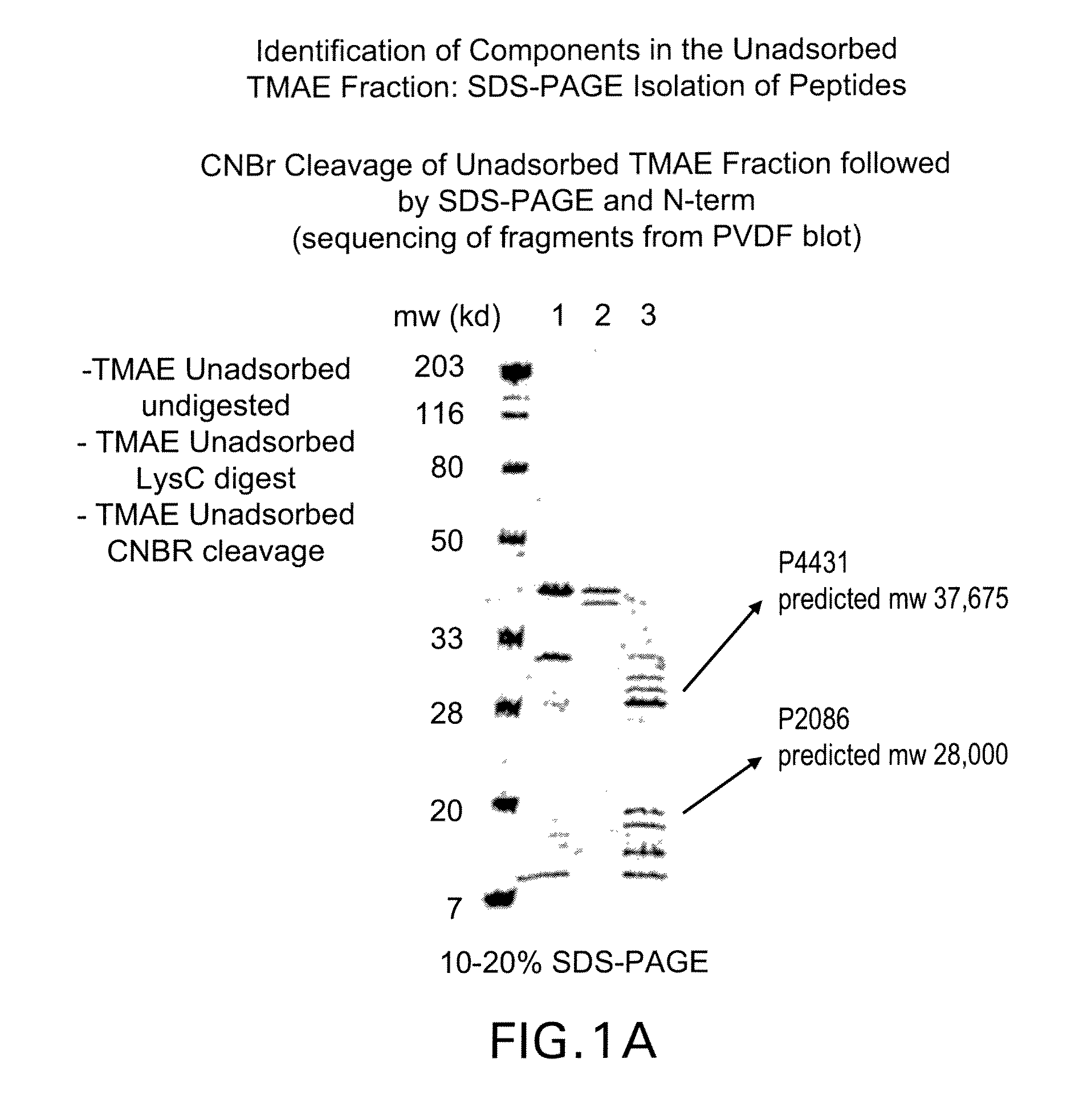
Immunogenic compositions for the prevention and treatment of meningococcal disease
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH HOLDINGS LLC
Polypeptide-vaccines for broad protetion against hypervirulent meningococcal lineages
ActiveUS20060171957A1Improve hydrolysis resistanceAntibacterial agentsBacterial antigen ingredientsSalmonella serotype typhiMeningococcal carriage
A small number of defined antigens can provide broad protection against meningococcal infection, and the invention provides a composition which, after administration to a subject, is able to induce an antibody response in that subject, wherein the antibody response is bactericidal against two or three of hypervirulent lineages A4, ET 5 and lineage 3 of N. meningitidis serogroup B. Rather than consisting of a single antigen, the composition comprises a mixture of 10 or fewer purified antigens, and should not include complex or undefined mixtures of antigens such as outer membrane vesicles. Five protein antigens are used in particular: (1) a ‘NadA’ protein; (2) a ‘741’ protein; (3) a ‘936’ protein; (4) a ‘953’ protein; and (5) a ‘287’ protein.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Injectable vaccines against multiple meningococcal serogroups
ActiveUS20070082014A1Pharmaceutical delivery mechanismDepsipeptidesStreptococcus pneumoniaeSalmonella serotype typhi
An injectable immunogenic composition comprising capsular saccharides from at least two of serogroups A, C, W135 and Y of Neisseria meningitidis, wherein said capsular saccharides are conjugated to carrier protein(s) and / or are oligosaccharides, and wherein (i) the composition comprises <50 mug meningococcal saccharide per dose, and / or (ii) the composition further comprises an antigen from one or more of: (a) serogroup B N. meningitidis; (b) Haemophilus influenzae type B; and / or (c) Streptococcus pneumoniae. Saccharide antigens in the compositions are generally conjugated to a carrier.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Novel immunogenic compositions for the prevention and treatment of meningococcal disease
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH HOLDINGS LLC
Combinations of meningococcal factor h binding proteins
ActiveUS20130189295A1Reduce chain lengthOptimum chain lengthAntibacterial agentsBacterial antigen ingredientsMeningococcal carriageBioinformatics
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Meningococcal class 1 outer-membrane protein vaccine
InactiveUS7118757B1Enhance immune responseBacterial antigen ingredientsPeptide/protein ingredientsEpitopeMeningococcal carriage
Owner:WYETH HOLDINGS CORP
Methods for the production of non-covalently complexed and multivalent proteosome sub-unit vaccines
InactiveUS6476201B1Shorten the timeIncrease temperatureAntibacterial agentsOrganic active ingredientsContinuous monitoringContamination
A continuous method for preparing proteosome-amphiphilic determinant vaccines for parenteral or mucosal administration using diafiltration or ultrafiltration technology. The amphiphilic determinants include lipopolysaccharides from gram negative bacteria, e.g. S. flexneri, P. shigelloides and S. sonnei. Proteosomes are obtained from group B type 2b meningococci. The active proteosome-amphiphilic determinant complexes (non-covalent complexes) of the vaccine are formed using diafiltration or ultrafiltration to remove the detergent under non-static conditions. The use of diafiltration or ultrafiltration decreases processing time and the opportunity for contamination and further permits the use of ambient temperature and efficient scale-up. In addition, the process permits the reliable and continuous monitoring of the dializate which enhances the efficiency of the entire process. The time of dialysis for the production of a lot of vaccine is reduced from 7-10 days to less than 72 hours and usually less than 48 or 24 hours. The use of the process optimizes the presence of each antigenic component in the preparation of multivalent vaccines.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY +1
Immunogenic compositions for the prevention and treatment of meningococcal disease
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH LLC
Mucosal meningococcal vaccines
InactiveUS20070207090A1Improve securityReduced dosAntibacterial agentsBacterial antigen ingredientsMucosal Immune ResponsesAdjuvant
The invention provides immunogenic compositions for mucosal delivery comprising capsular saccharides from at least two of serogroups A, C, W135 and Y of N. meningitidis. It is preferred that the capsular saccharides in the compositions of the invention are conjugated to carrier protein(s) and / or are oligosaccharides. Conjugated oligosaccharide antigens are particularly preferred. The invention also provides immunogenic compositions comprising (a) a capsular saccharide antigen from serogroup C of N. meningitidis, and (b) a chitosan adjuvant. The composition preferably comprises (c) one or more further antigens and / or (d) one or more further adjuvants. The compositions are particularly suitable for mucosal delivery, including intranasal delivery. The use of chitosan and / or detoxified ADP-ribosylating toxin adjuvants enhances anti-meningococcal mucosal immune responses and can shift the Th1 / Th2 bias of the responses.
Owner:NOVARTIS AG
Neisseria meningitidis compositions and methods thereof
ActiveUS20130243807A1Antibacterial agentsBacterial antigen ingredientsSalmonella serotype typhiMeningococcal carriage
In one aspect, the invention relates to an isolated polypeptide comprising an amino acid sequence that is at least 95% identical to SEQ ID NO: 71. In another aspect, the invention relates to an immunogenic composition including an isolated non-lipidated, non-pyruvylated ORF2086 polypeptide from Neisseria meningitidis serogroup B, and at least one conjugated capsular saccharide from a meningococcal serogroup.
Owner:PFIZER INC
Gonococcal proteins and nucleic acids
InactiveUS7504111B2Stable maintenanceAntibacterial agentsOrganic active ingredientsAntigenSalmonella serotype typhi
The invention provides proteins from gonococcus (Neisseria gonorrhoeae), including amino acid sequences, the corresponding nucleotide sequences, expression data, and serological data. The proteins are useful antigens for vaccines, immunogenic compositions, and / or diagnostics. They are also useful for distinguishing between gonococcus and meningococcus and, in particular, between gonococcus and serogroup B meningococcus.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Novel immunogenic compositions for the prevention and treatment of meningococcal disease
InactiveUS20070082007A1Avoid infectionInhibition of colonizationAntibacterial agentsNervous disorderDiseaseSalmonella serotype typhi
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:ZLOTNICK GARY W +5
Novel immunogenic compositions for the prevention and treatment of meningococcal disease
InactiveUS20110076299A1Antibacterial agentsOrganic active ingredientsDiseaseSalmonella serotype typhi
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH HOLDINGS LLC
Novel immunogenic compositions for the prevention and treatment of meningococcal disease
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH LLC
Bacterial outer memberane vesicles
InactiveUS20060166344A1Good curative effectAvoid problemsAntibacterial agentsBacteriaImmunogenicityTreated cell
Existing methods of meningococcal OMV preparation involve the use of detergent during disruption of the bacterial membrane. According to the invention, membrane disruption is performed substantially in the absence of detergent. The resulting OMVs which retain important bacterial immunogenic components, particularly (i) the protective NspA surface protein, (ii) protein NMB2132 and (iii) protein NMB 1870. A Typical process involves the following steps: (a) treating bacterial cells in the substantial absence of detergent; (b) centrifuging the composition from step (a) to separate the outer membrane vesicles from treated cells and cell debris, and collecting the supernatant; (c) performing a high speed centrifugation of the supernatant from step (b) and collecting the outer membrane vesicles in a pellet; (d) re-dispersing the pellet from step (c) in a buffer; (e) performing a second high speed centrifugation in accordance with step (c), collecting the outer membrane vesicles in a pellet; (f) re-dispersing the pellet from step (e) in an aqueous medium.
Owner:NOVARTIS AG
Polypeptide-vaccines for broad protection against hypervirulent meningococcal lineages
ActiveUS8663656B2Antibacterial agentsBacterial antigen ingredientsSalmonella serotype typhiMeningococcal carriage
A small number of defined antigens can provide broad protection against meningococcal infection, and the invention provides a composition which, after administration to a subject, is able to induce an antibody response in that subject, wherein the antibody response is bactericidal against two or three of hypervirulent lineages A4, ET 5 and lineage 3 of N. meningitidis serogroup B. Rather than consisting of a single antigen, the composition comprises a mixture of 10 or fewer purified antigens, and should not include complex or undefined mixtures of antigens such as outer membrane vesicles. Five protein antigens are used in particular: (1) a ‘NadA’ protein; (2) a ‘741’ protein; (3) a ‘936’ protein; (4) a ‘953’ protein; and (5) a ‘287’ protein.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
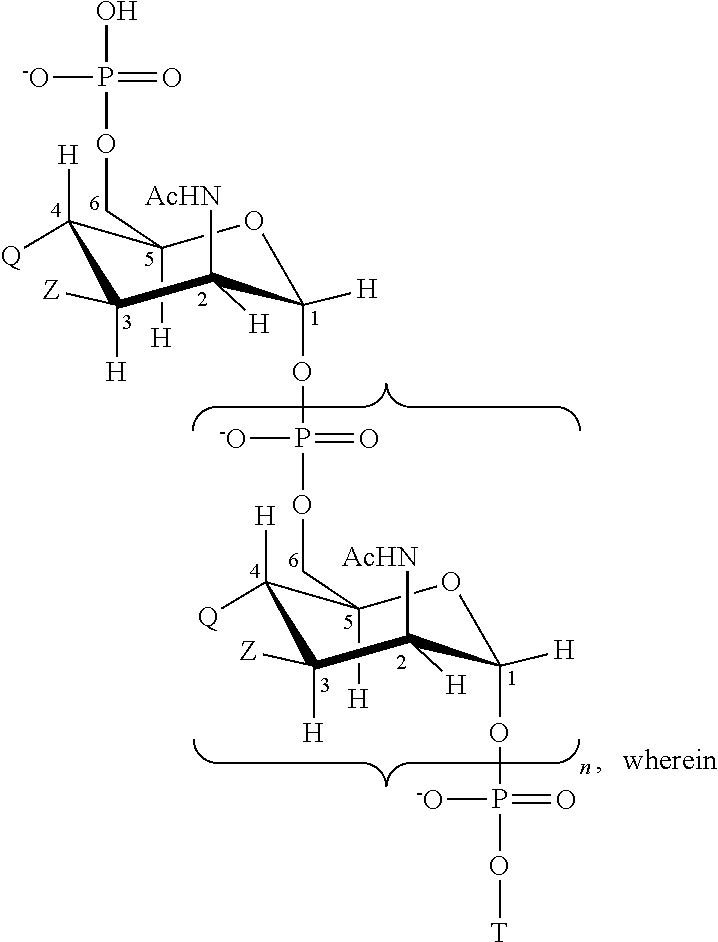
Multivalent meningococcal derivatized polysaccharide-protein conjugates and vaccine
The present invention describes derivatized polysaccharide-protein conjugates, a composition comprising one or more of such derivatized polysaccharide-protein conjugates and methods of immunizing human patients with the same. The derivatized polysaccharide-protein conjugates are purified capsular polysaccharides from Neisseria meningitidis serogroups A, C, W-135, and Y, derivatized chemically activated and selectively attached to a carrier protein by means of a covalent chemical bond, forming polysaccharide-protein conjugates capable of eliciting long-lasting immunity to a variety of N. meningitidis strains.
Owner:AVENTIS PASTEUR INC
Neisseria meningitidis compositions and methods thereof
ActiveUS8986710B2Antibacterial agentsBacterial antigen ingredientsMeningococcal carriageImmunogenicity
In one aspect, the invention relates to an isolated polypeptide comprising an amino acid sequence that is at least 95% identical to SEQ ID NO: 71. In another aspect, the invention relates to an immunogenic composition including an isolated non-lipidated, non-pyruvylated ORF2086 polypeptide from Neisseria meningitidis serogroup B, and at least one conjugated capsular saccharide from a meningococcal serogroup.
Owner:PFIZER INC
Meningococcal fhbp polypeptides
ActiveUS20110020390A1Stable maintenanceSugar derivativesBacteriaAntiendomysial antibodiesMeningococcal carriage
fHBP is a protein in Neisseria meningitidis. Three families of fHBP are known. To increase the ability of a fHBP protein to elicit antibodies that are cross-reactive between the families, fHBP is selected or engineered to have a sequence which can elicit broad-spectrum bactericidal anti-meningococcal antibodies after administration to a host animal.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Formulation of meningitis vaccines
InactiveUS20100203137A1Improving immunogenicityWithout riskAntibacterial agentsPowder deliveryCoccidiaMeningococcal carriage
A liquid Hib component is used to reconstitute a lyophilised meningococcal component, thereby producing a combined meningitis vaccine. A lyophilised meningococcal component can also be reconstituted with an oil-in-water emulsion.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Separated and purified acellular pertussis-diphtheria-tetanus, b-type haemophilus influenzae and A-group and C-group meningococcus combined vaccine and preparation method thereof
InactiveCN104689309ARelieve painReduce the burden onAntibacterial agentsBacterial antigen ingredientsHemagglutininAluminium hydroxide
The invention discloses a combined vaccine and a preparation method thereof. The combined vaccine is formed by a component A and a component B, wherein the component A is a liquid preparation and composed of pertussis toxin, filamentous hemagglutinin, pertussis adhesion, diphtheria toxoid, tetanus toxoid, aluminium hydroxide and sodium chloride; the component B is a freeze-drying preparation and composed of A-group meningitis polysaccharide conjugate, C-group meningitis polysaccharide conjugate, b-type haemophilus influenzae polysaccharide conjugate and lactose. The preparation method of the vaccine includes the steps of preparing of acellular pertussis-diphtheria-tetanus vaccine semi-finished products, A-group and C-group meningococcocci and b-type haemophilus influenzae combined vaccine semi-finished products, split charging and packaging. The vaccine has the characteristics of being safe, effective, controllable and capable of preventing diseases through an injection, the preparation method is easy to operate, preparation is facilitated, cost is low, and the combined vaccine is suitable for industrialized mass production.
Owner:CHENGDU OLYMVAX BIOPHARM
Gonococcal proteins and nucleic acids
InactiveUS20050260581A1Easy to insertEfficient HarvestingAntibacterial agentsOrganic active ingredientsSalmonella serotype typhiVaccine Immunogenicity
The invention provides proteins from gonococcus (Neisseria gonorrhoeae), including amino acid sequences, the corresponding nucleotide sequences, expression data, and serological data. The proteins are useful antigens for vaccines, immunogenic compositions, and / or diagnostics. They are also useful for distinguishing between gonococcus and meningococcus and, in particular, between gonococcus and serogroup B meningococcus.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Molecular mimetics of meningococcal B epitopes which elicit functionally active antibodies
Molecular mimetics of a surface-exposed epitope on loop 4 of PorA of Neisseria meningitidis serogroup B (MenB) P1.2 serosubtype and antibodies produced against the same are disclosed. Compositions containing such molecular mimetics or the antibodies thereto can be used to prevent MenB disease, as well as for diagnosis of MenB infection.
Owner:CHIRON CORP
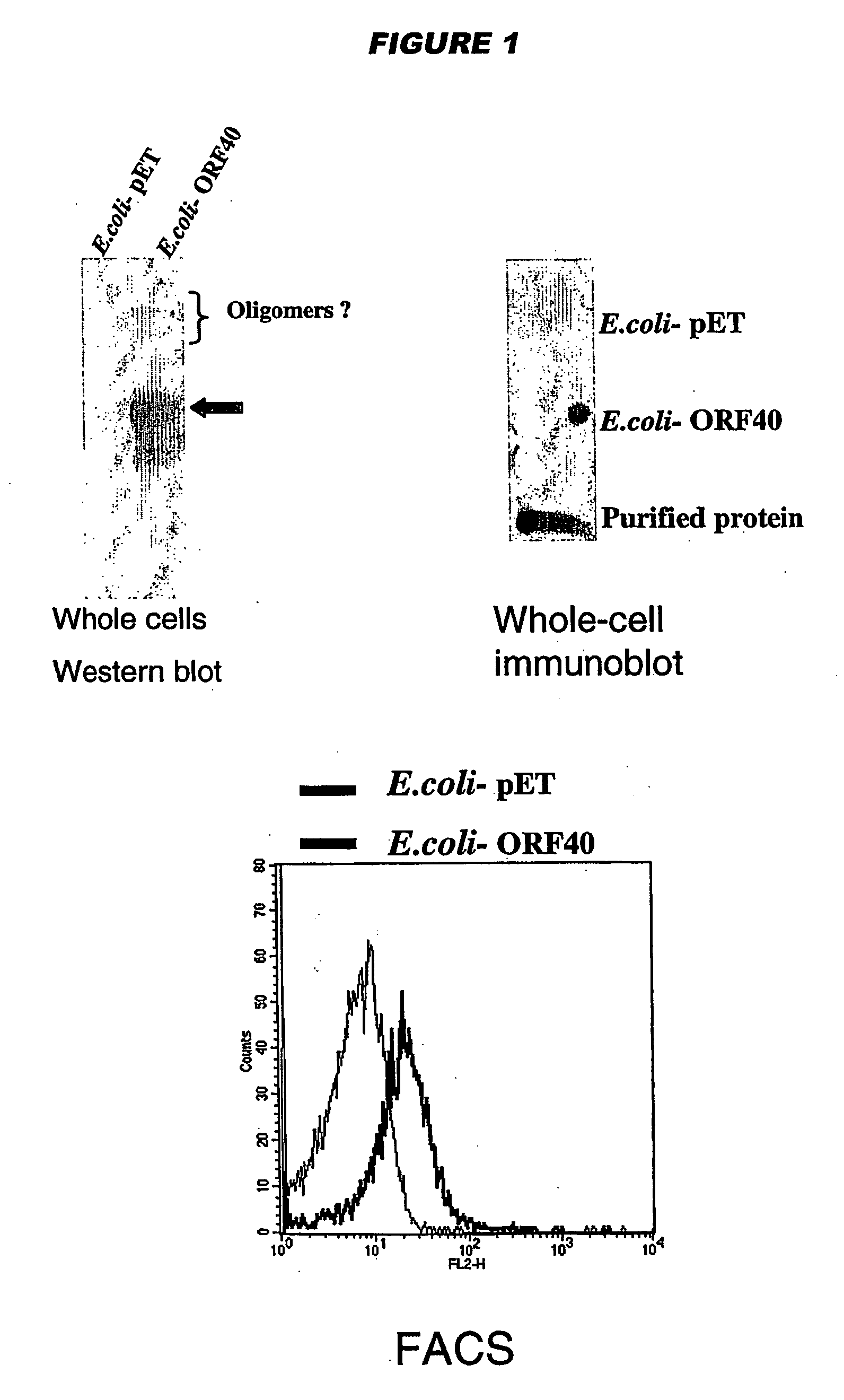
Meningococcus adhesins nada, app and orf 40
InactiveUS20050232936A1Inhibition of attachmentLower Level RequirementsAntibacterial agentsBacteriaEscherichia coliBacterial Adhesins
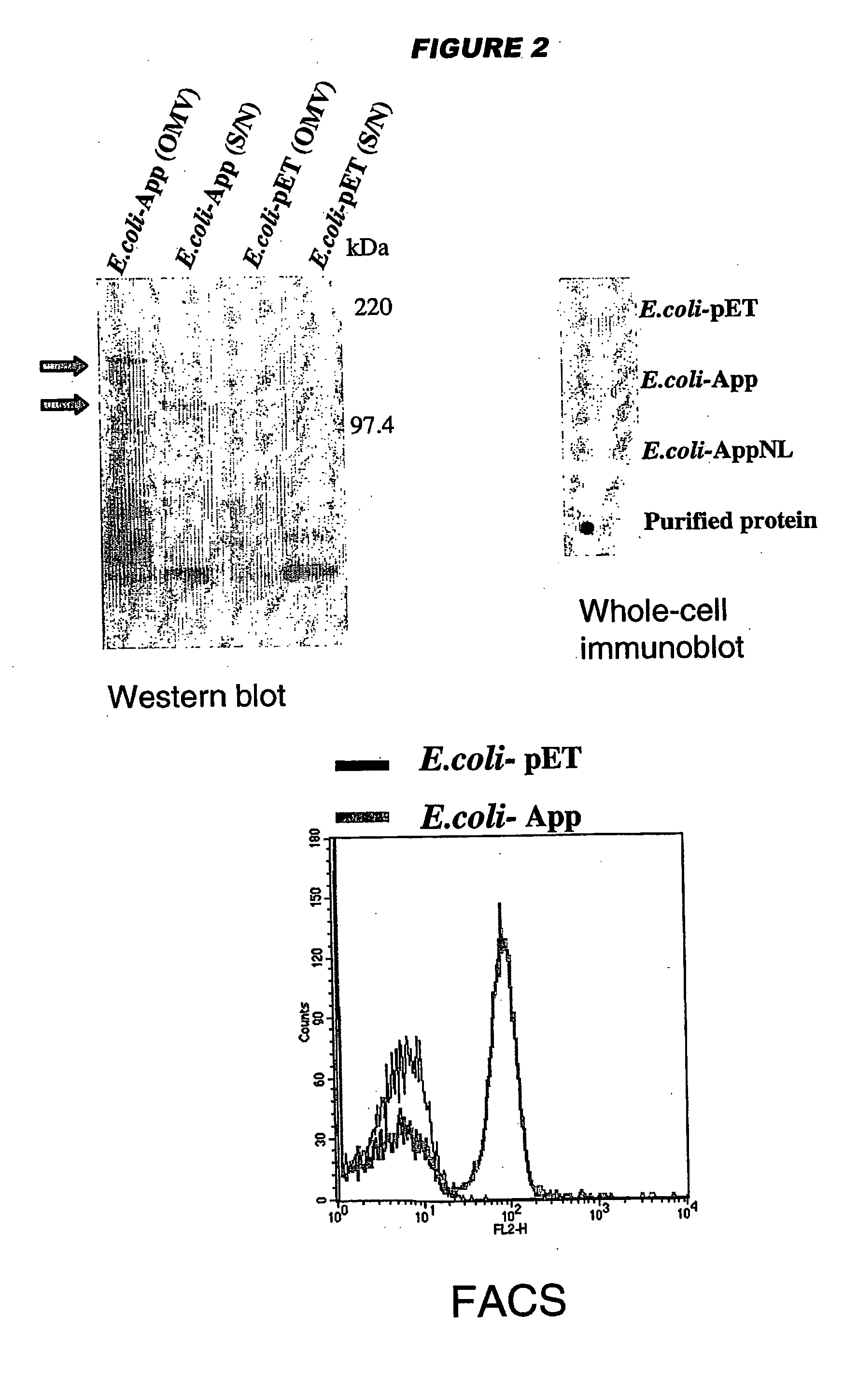
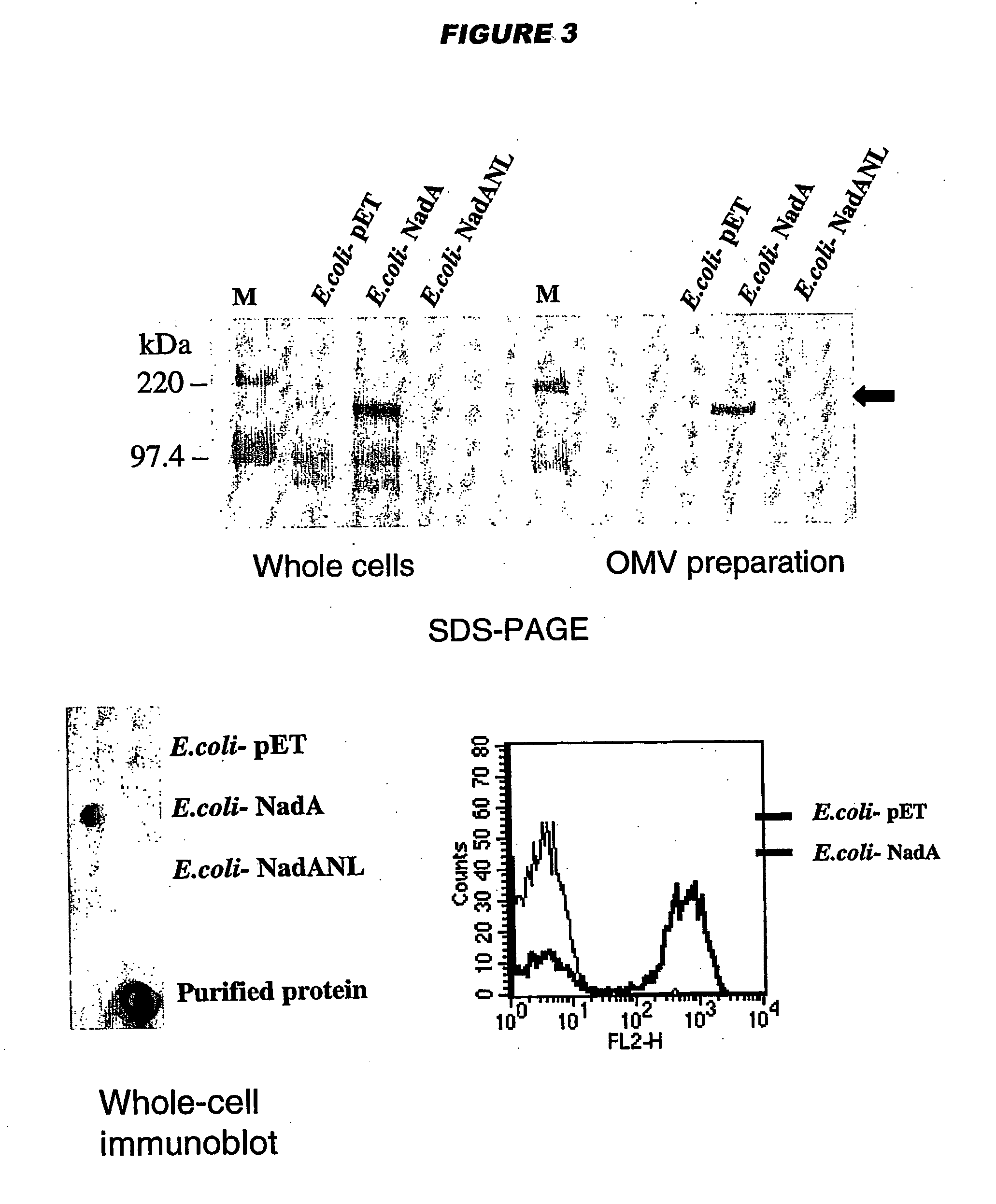
NadA, App and ORF40 function as adhesins in N. meningitidis. Adhesion can be modulated by targeting these three proteins. NadA allelic variants are disclosed. Autoproteolytic cleavage of App is disclosed, as is removal of the activity by mutagenesis. App is processed and secreted into culture medium when expressed in E. coli. Mature App proteins are disclosed. Knockout mutants are disclosed. Vesicles from non-Neisserial hosts with heterologous adhesin expression are disclosed.
Owner:NOVARTIS AG
Slow-release preparation containing beta-lactamase inhibitor and cephalosporin and its use
The present invention relates to a slow-released preparation containing beta-lactamase inhibitor and cephalosporin. Said slow-released preparation can be made into antibiotic slow-released injection or slow-released implant preparation. Said injection is formed from slow-released microsphere and solvent, the slow-released microsphere contains slow-released auxiliary material and beta-lactamase inhibitor with antibacterial effective dose and cephalosporin, the solvent is special one containing suspension adjuvant of carboxymethyl cellulose sodium, etc. and its viscosity is 100 cp-3000 cp (20 deg.C-30 deg.C). The slow-released auxiliary material is selected from EVAc, polylactic acid copolymer, sebacic acid copolymer, albumin glue and gelatin, etc. The slow-released implant preparation is prepared by using slow-released microsphere or adopting melting process. Said invention also provides its application method. and can obtain obvious therapeutic effect for curing various infective diseases.
Owner:JINAN SHUAIHUA PHARMA TECH
Multiple variants of meningococcal protein NBM1870
ActiveUS8980286B2Improve purification effectImprove stabilityAntibacterial agentsBacterial antigen ingredientsSerum igeAntigen
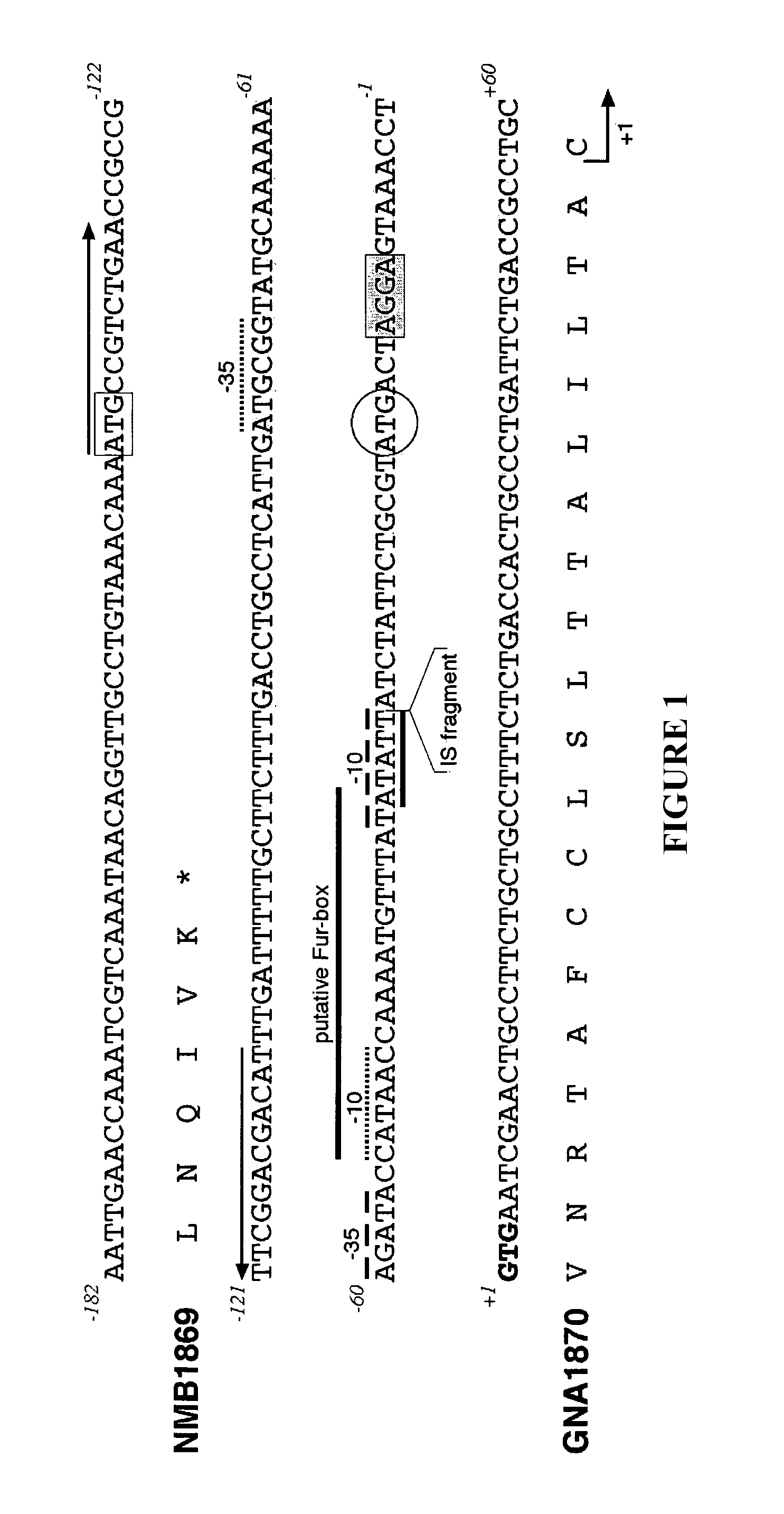
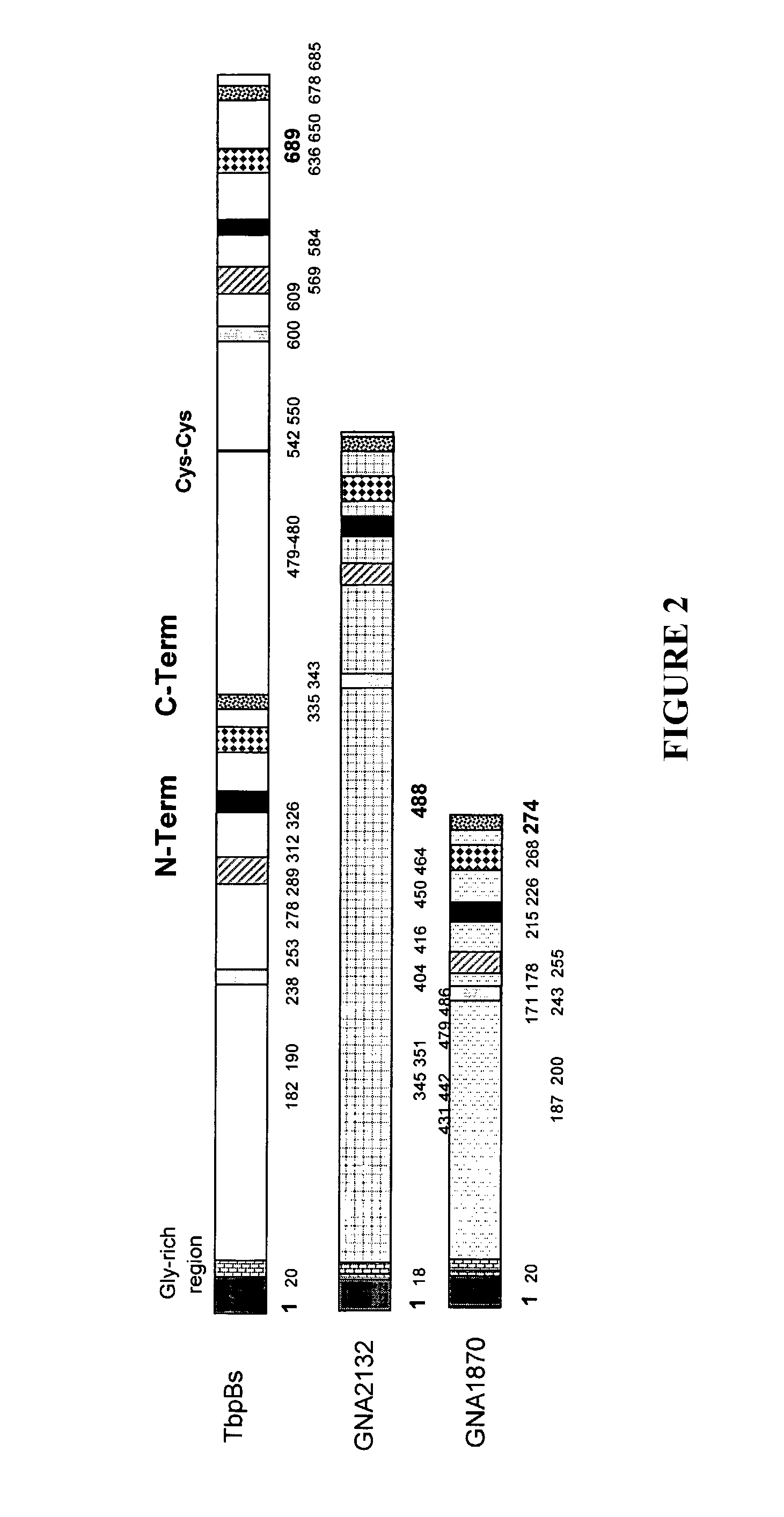
Meningococcal protein NMB 1870 has been described in the prior art. The inventors have found that NMB 1870 is an effective antigen for eliciting anti-meningococcal antibody responses, and that it is expressed across all meningococcal serogroups. Forty-two different NMB 1870 sequences have been identified, and these group into three variants. Serum raised against a given variant is bactericidal within the same variant group, but is not active against strains which express one of the other two variants i.e. there is intra-variant cross-protection, but not inter-variant cross-protection. For maximum cross-strain efficacy, therefore, the invention uses mixture comprising different variants of NMB 1870.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Liquid vaccines for multiple meningococcal serogroups
ActiveUS8765135B2Antibacterial agentsBacterial antigen ingredientsMeningococcal carriageProtein antigen
Conjugated capsular saccharides from meningococcal serogroups C, W135 and Y are safe and immunogenic in humans when combined in a single dose. This effect is retained when a conjugated capsular saccharide from serogroup A is added. These conjugated antigens can be stably combined in a single aqueous dose without the need for lyophilisation. Broad protection against serogroup B infection can be achieved by using a small number of defined polypeptide antigens. These polypeptide antigens can be combined with the saccharide antigens without loss of protective efficacy for any of the five serogroups. Efficacy if retained even if a Hib conjugate is added. The efficacy of a serogroup W135 conjugate is enhanced by addition of protein antigens derived from a serogroup B strain. Addition of a Hib conjugate to meningococcal conjugates enhances the overall activity against meningococcus serogroup W135.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process
InactiveCN105031634AKeep healthyAvoid pollutionAntibacterial agentsCarrier-bound antigen/hapten ingredientsConjugate vaccineVaccine manufacturing
The invention discloses an A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process. The problem that highly-toxic chemical reagents, such as cyanogen bromide, are used in the vaccine manufacturing process in the prior art and accordingly are harmful to protection of human health and environment is solved. The A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process comprises the following steps of 1 cultivating Neisseria meningitidis, 2 utilizing the cultivated Neisseria meningitidis to extract crude polysaccharide, 3 purifying the crude polysaccharide to prepare refined Neisseria meningitidis polysaccharide and 4 activating and deriving the refined polysaccharide. The A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process has the advantages that the extraction rate of the polysaccharide from a capsule is higher, the precipitation rate of the polysaccharide is higher, the recovery rate and purity of the refined polysaccharide are high, the derivation effect is better and the like.
Owner:CHENGDU OLYMVAX BIOPHARM
Meningococcal conjugate vaccination
ActiveUS20100104593A1Improving immunogenicityReduce chain lengthAntibacterial agentsBacterial antigen ingredientsCoccidiaMedicine
Conjugated meningococcal capsular saccharides will be introduced into immunisation schedules in the near future, but the phenomenon of “carrier suppression” must first be addressed, particularly where multiple conjugates are to be used. In the invention, tetanus toxoid is used as the carrier protein, even where multiple meningococcal conjugates are administered at the same time and where a patient has previously been exposed to the carrier protein, either in the form of a previous immunogen (e.g. in a DTP vaccine) or as a previous carrier protein (e.g. in a Hib or pneumococcal conjugate vaccine). The invention provides a method for immunising a patient, comprising administering multiple conjugates of meningococcal capsular saccharides, wherein each conjugate comprises a tetanus toxoid carrier protein, and the capsular saccharide, and wherein the patient has been pre-immunised with a tetanus toxoid.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
MENINGOCOCCAL fHBP POLYPEPTIDES
InactiveUS20130022633A1Improved antigenHigh affinity bindingAntibacterial agentsBacteriaMeningococcal carriageAmino acid
The factor H binding activity of meningococcal fHBP can be uncoupled from its bactericidal sensitivity. NMR studies have identified various amino acid residues involved in the fHBP / fH interaction and one or more of these residues is modified in a fHBP to reduce or eliminate its ability to bind to fH.
Owner:UNIVERSITY OF FLORENCE +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com