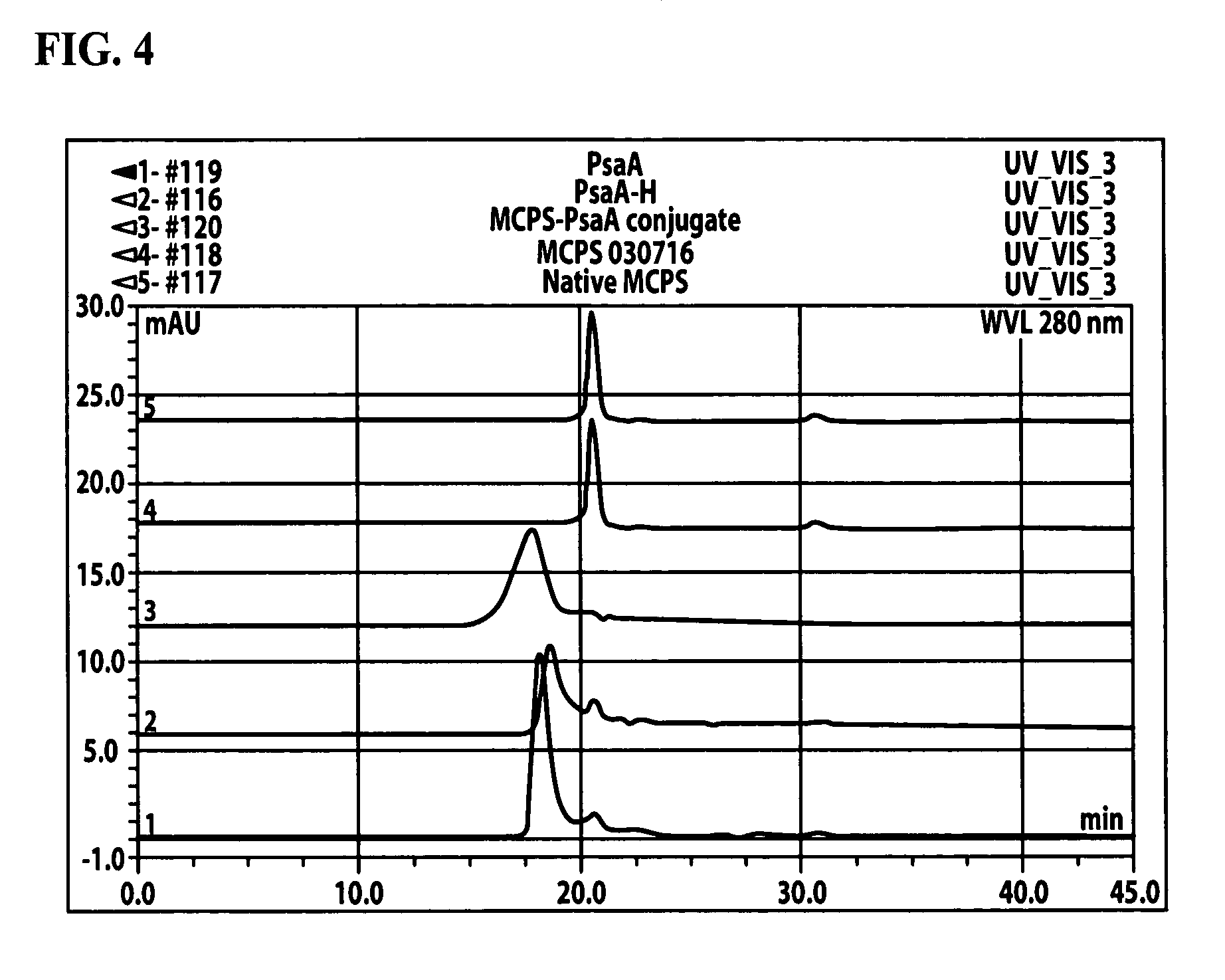
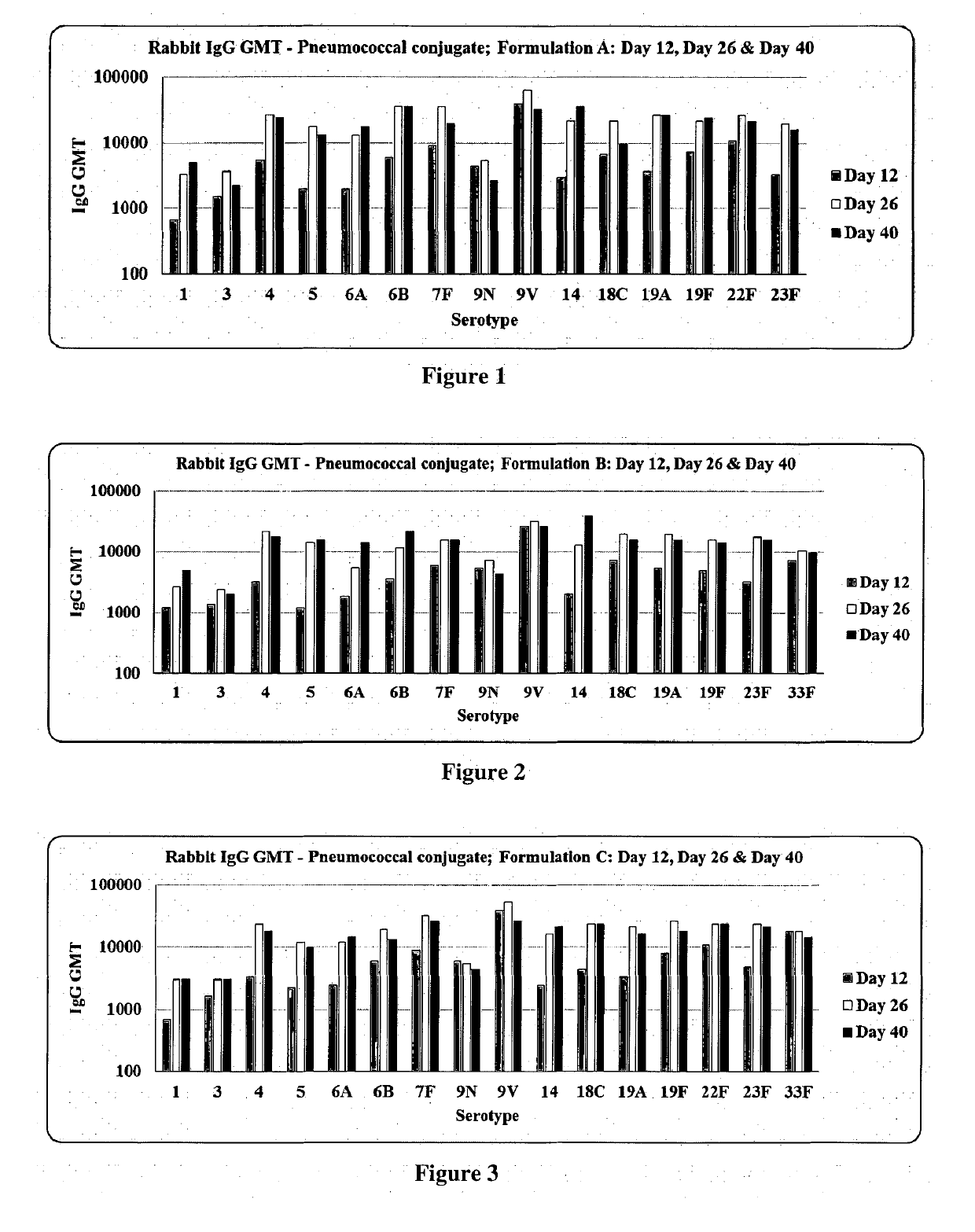
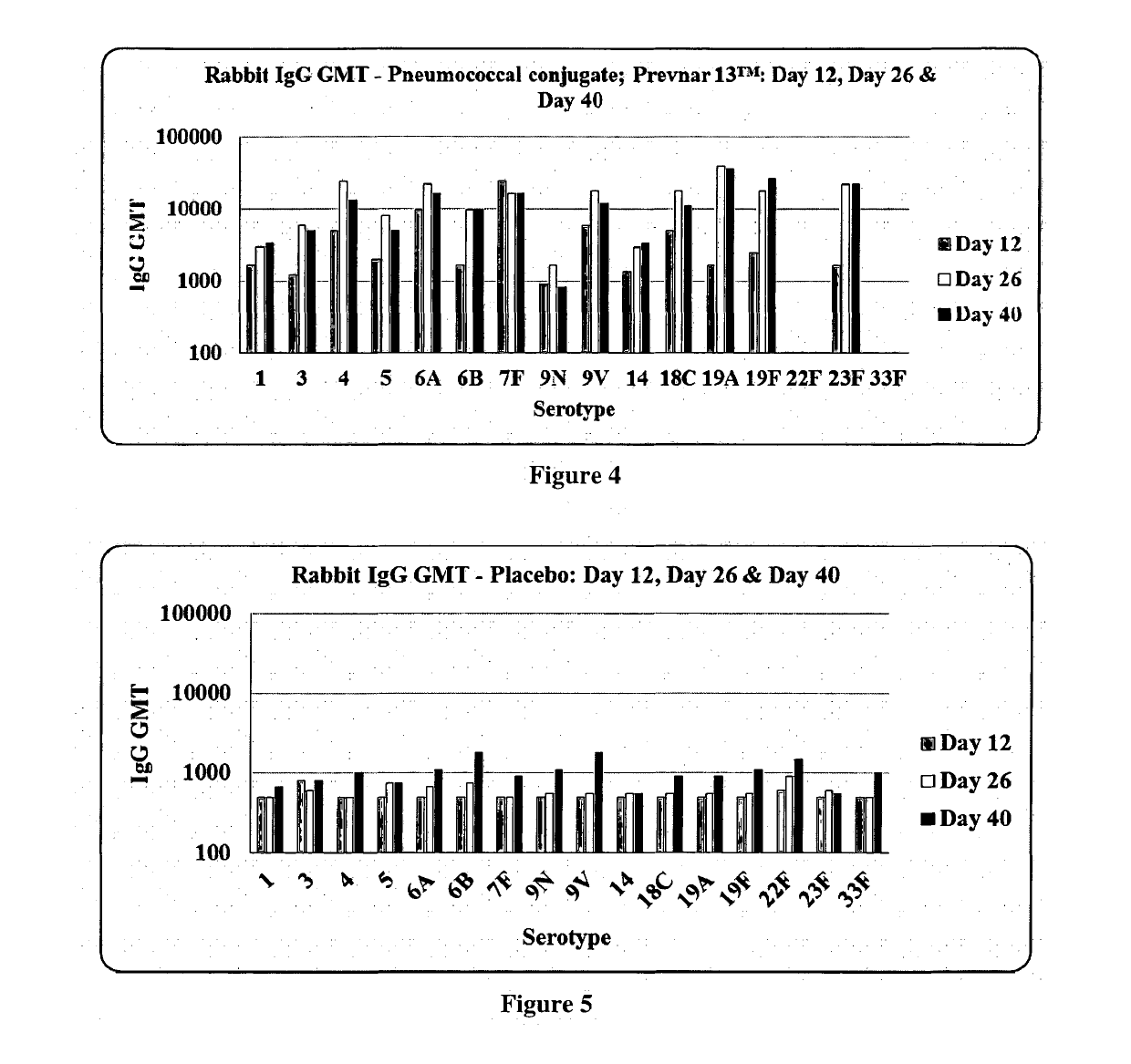
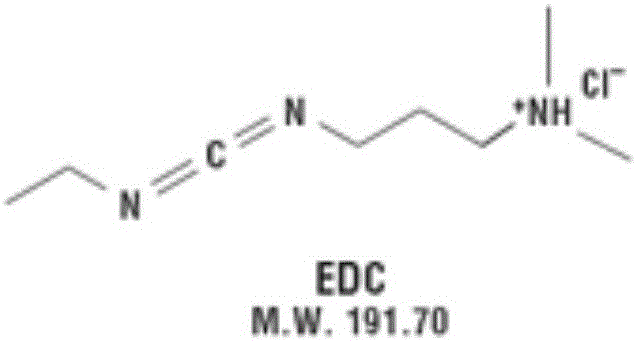
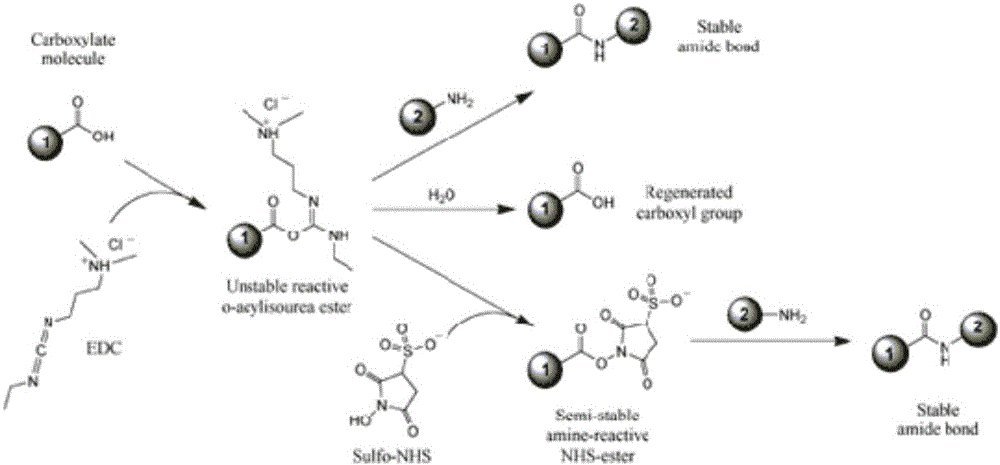
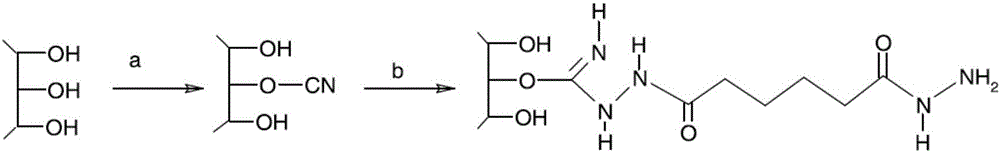
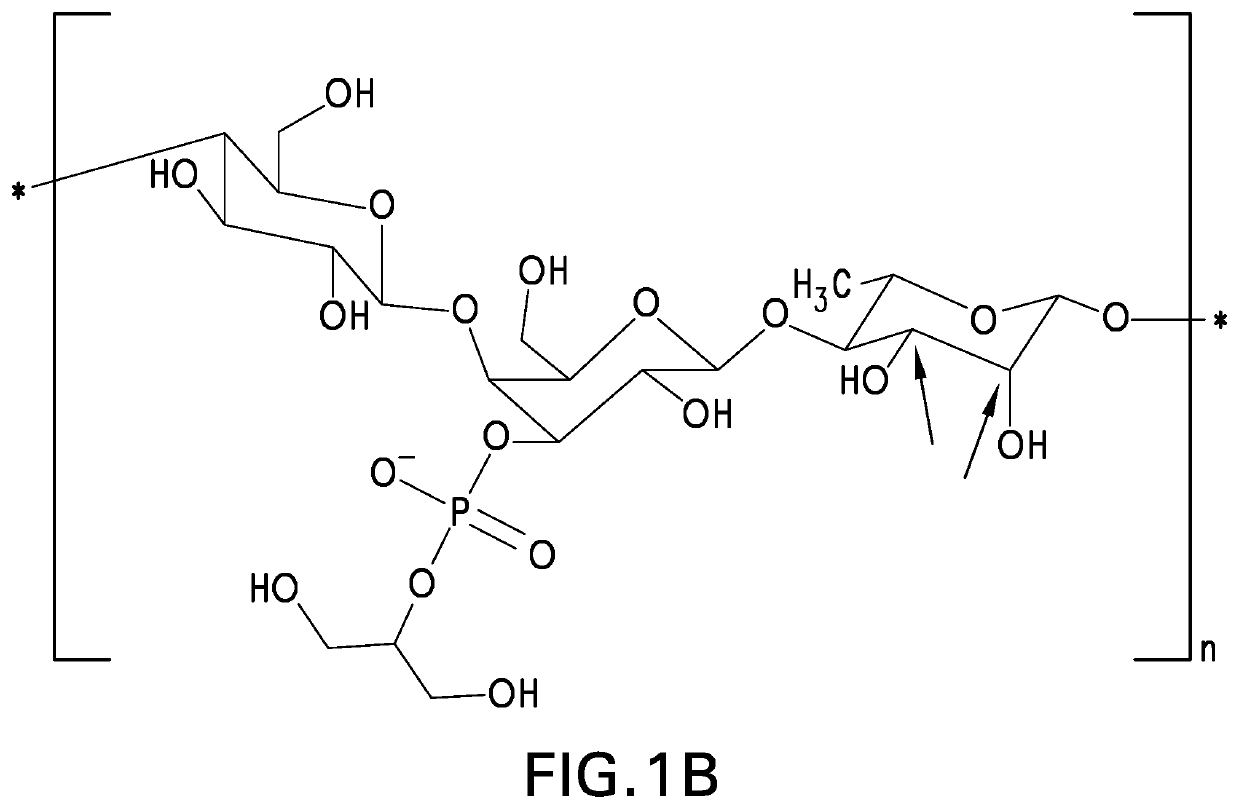
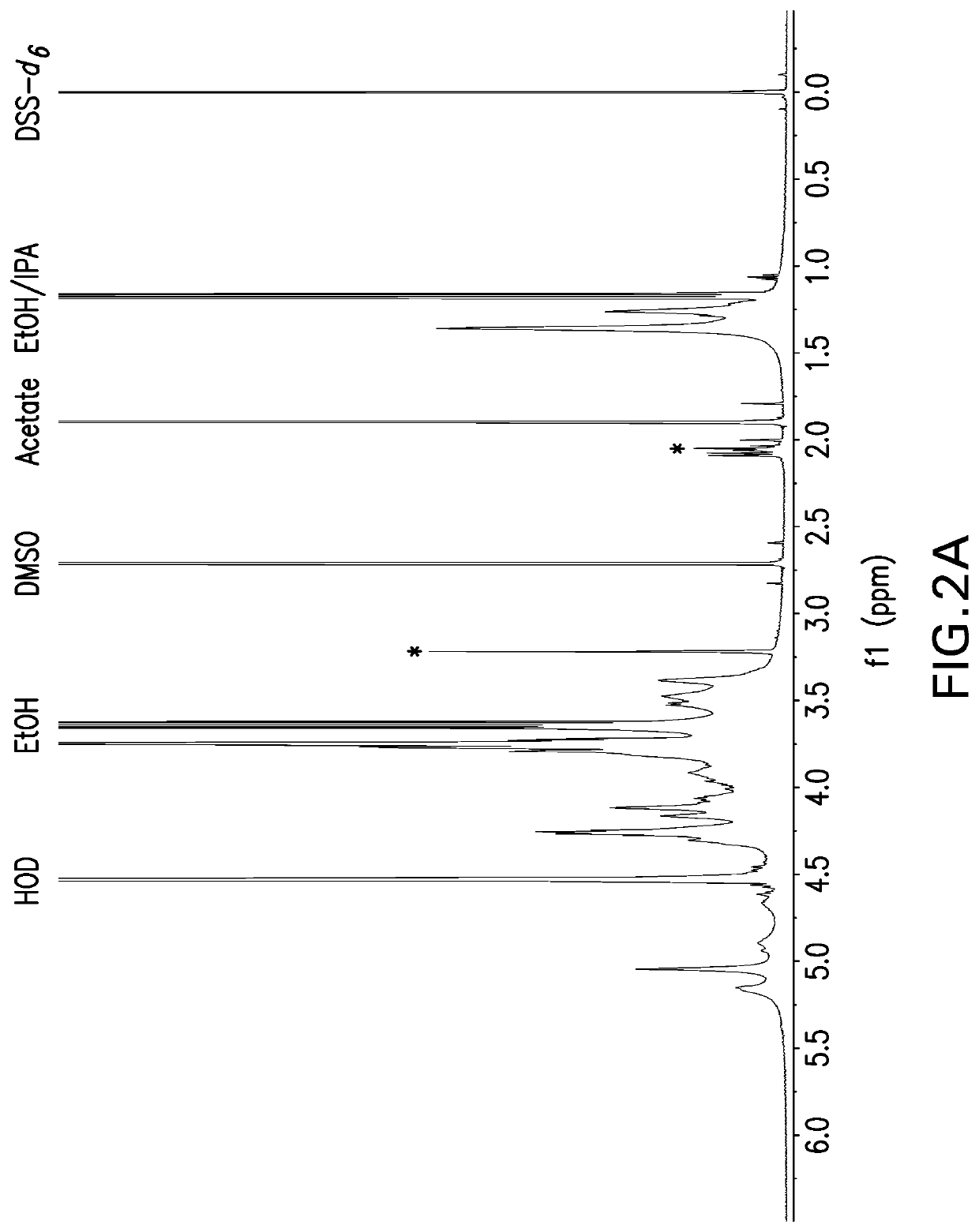
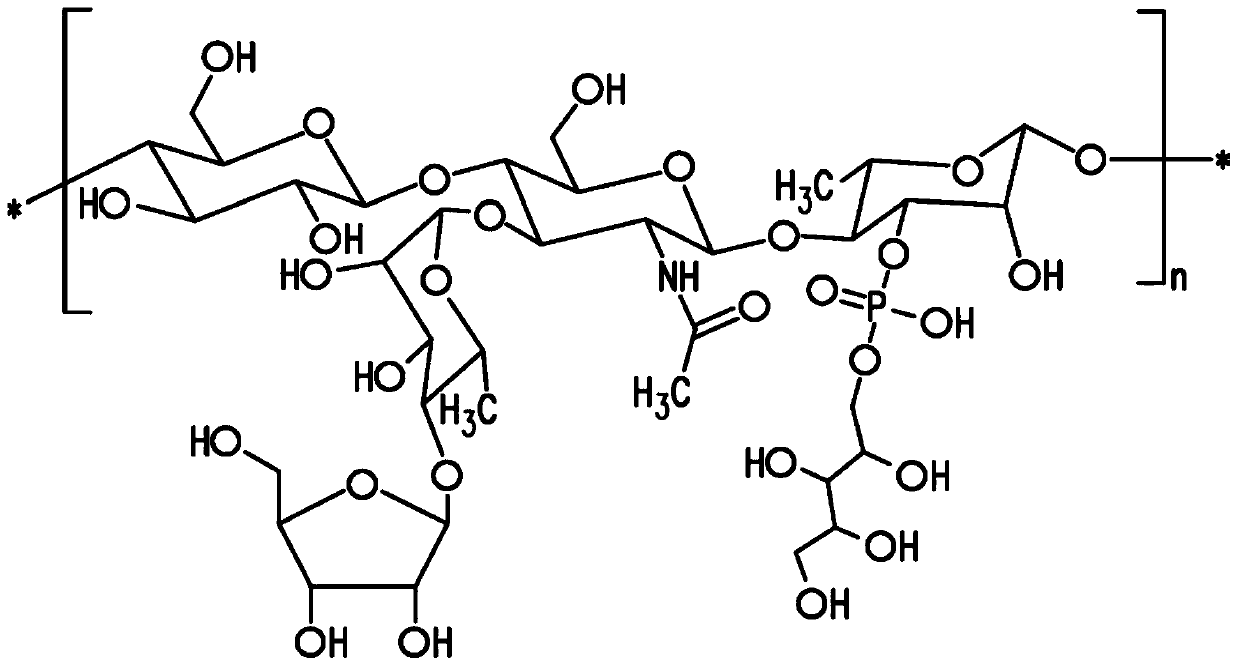
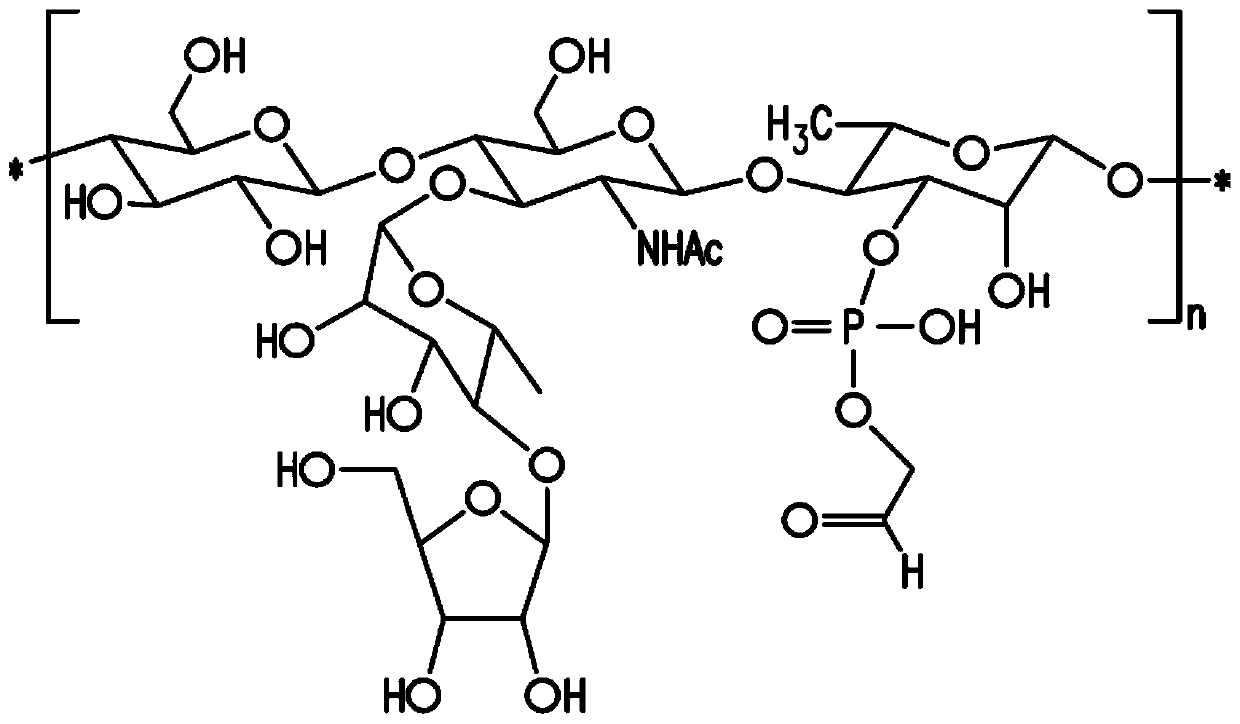
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43 results about "Pneumococcal conjugate vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pneumococcal conjugate vaccine (PCV) is a pneumococcal vaccine and a conjugate vaccine used to protect infants, young children, and adults against disease caused by the bacterium Streptococcus pneumoniae (the pneumococcus). There are currently three types of PCV available on the global market, which go by the brand names: Prevnar (called Prevenar in some countries), Synflorix and Prevnar 13.
High-efficiency 14-valent pneumococcal conjugate vaccine
InactiveCN101590224AEffective protectionAntibacterial agentsBacterial antigen ingredientsCoccidiaHaemophilus
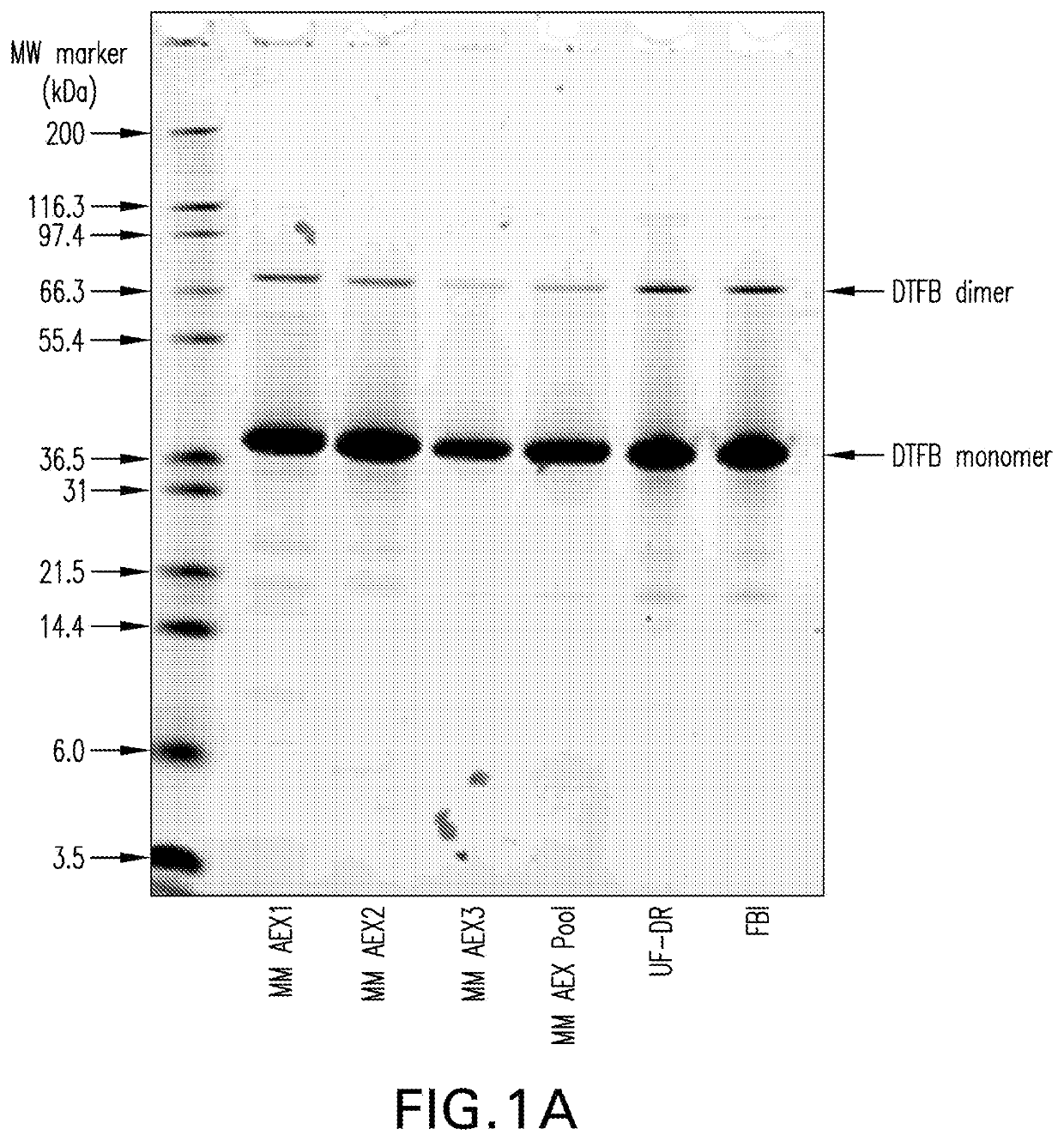
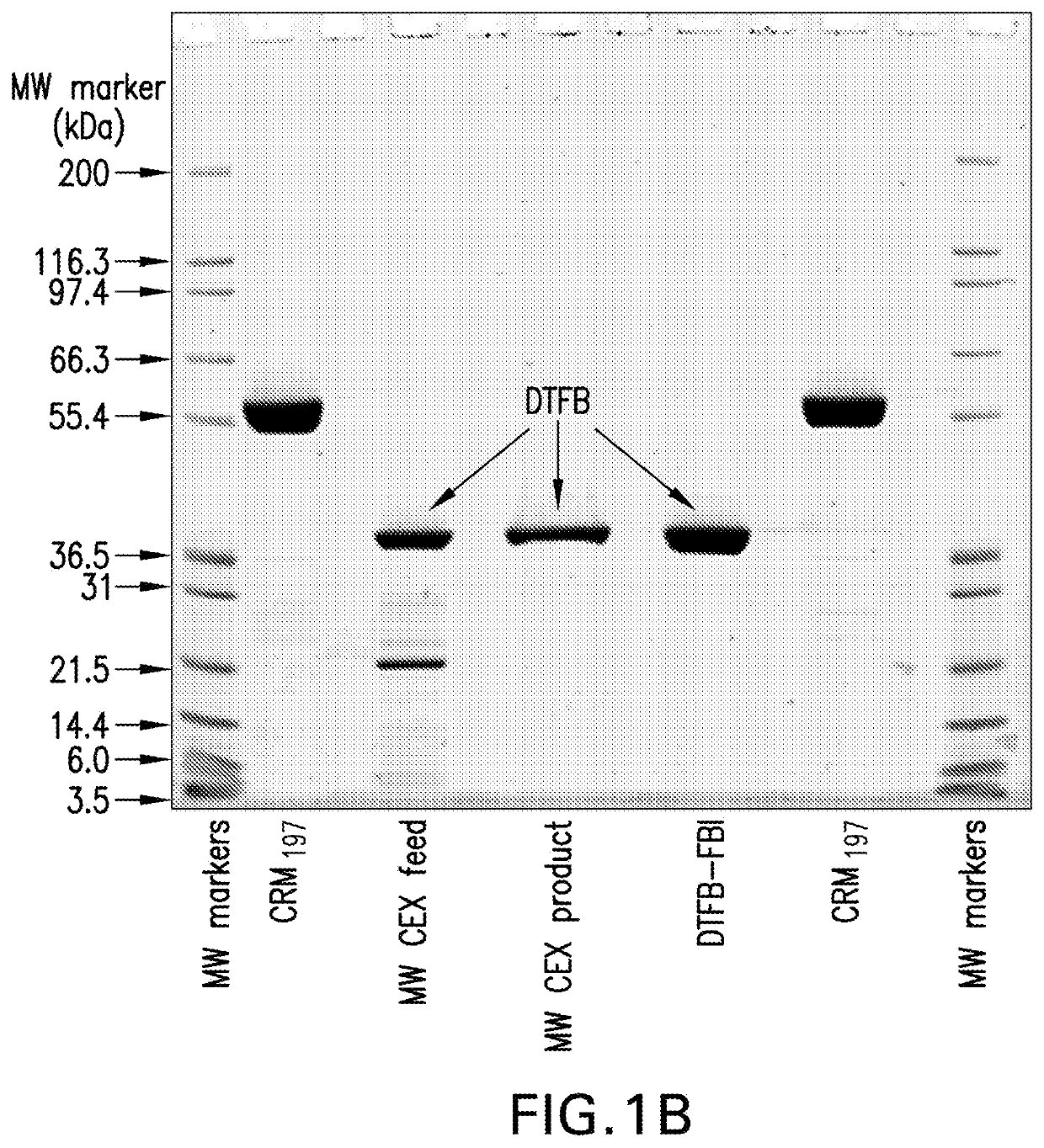
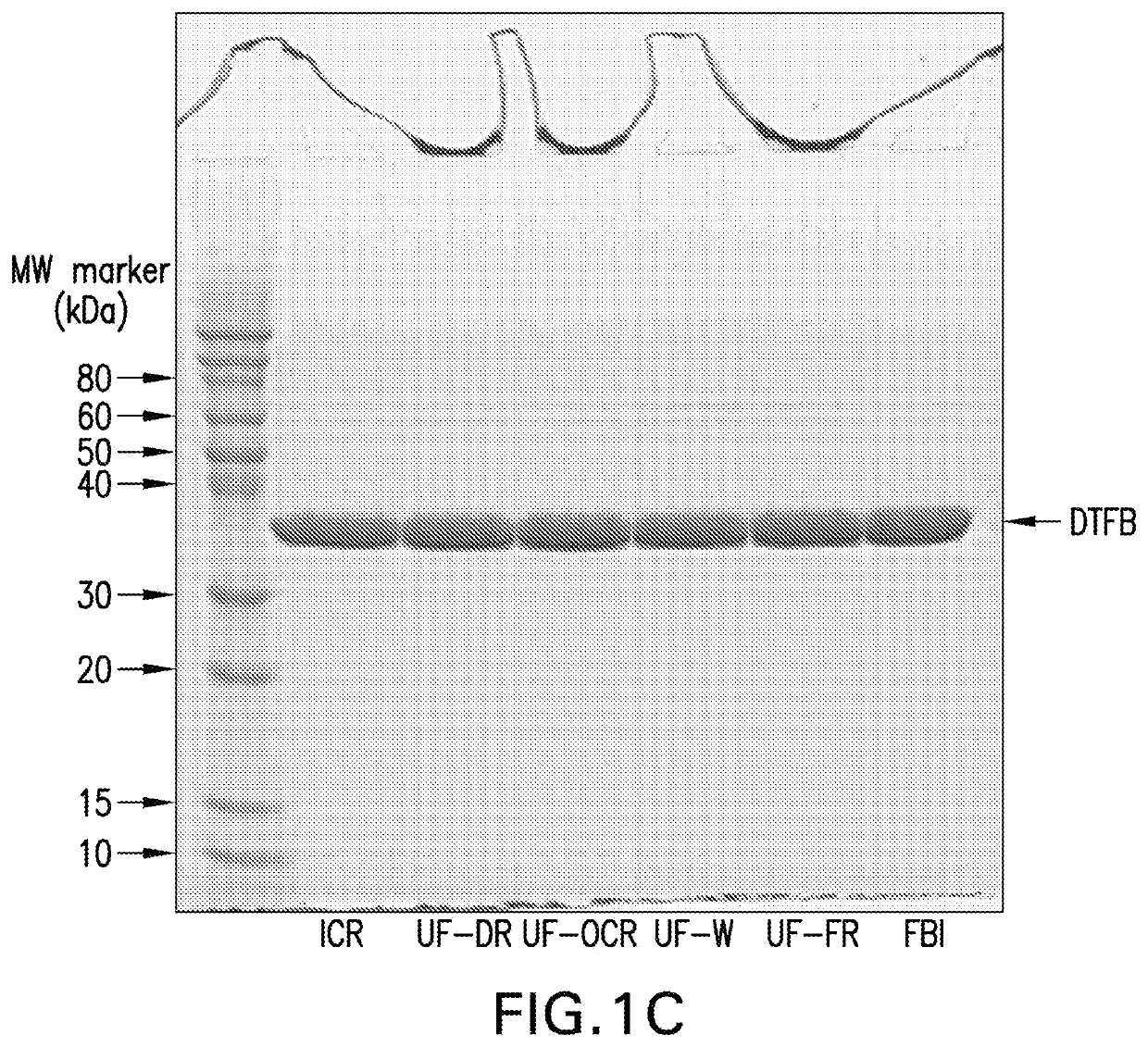
The invention relates to a high-efficiency 14-valent pneumococcal conjugate vaccine, which is formed by coupling and combining capsular polysaccharide extracted from fourteen types of serotype pneumococcuses and carrier protein; the fourteen types of the serotype pneumococcuses are 1, 2, 4, 5, 6A, 6B, 7F, 9N, 9V, 14, 18C, 19A, 19F and 23F; and the carrier protein is diphtherin non-toxic mutated protein CRM197, tetanus toxin protein and influenza haemophilus protein D. Compared with the prior pneumococcal conjugate vaccine, the high-efficiency 14-valent pneumococcal conjugate vaccine has the advantages that: (1) the cross-protection rate of the pneumococcuses of fourteen serotypes (1, 2, 4, 5, 6A, 6B, 7F, 9N, 9V, 14, 18C, 19A, 19F and 23F) reaches more than 90 percent; and (2) the high-efficiency 14-valent pneumococcal conjugate vaccine can generate effective protection on senior citizens which are susceptible to pneumonia and is suitable for children under two years old.
Owner:广州精达医学科技有限公司
Novel pneumococcal conjugate vaccine and preparation method thereof
ActiveCN101785857AImproving immunogenicityReduce immune competitionAntibacterial agentsBacterial antigen ingredientsConjugate vaccineSugar
The invention relates to a novel pneumococcal conjugate vaccine and a preparation method thereof. The novel pneumococcal conjugate vaccine comprises a dual-valent serum type pneumococcal capsular sugar-protein combination and / or a multi-valent serum type pneumococcal capsular sugar-protein combination. The dual-valent serum type capsular sugar-protein combination is a combination formed by combining two different serum types of capsular sugar with a protein carrier through chemical bonds, and the two different serum types of capsular sugar form links in the structure according to the combination with the protein carrier through the chemical bonds. The multi-valent serum type capsular sugar-protein combination is a combination formed by combining two different serum types of capsular sugar with a protein carrier through chemical bonds, and the two different serum types of capsular sugar form links in the structure according to the combination with the protein carrier through the chemical bonds. In addition, the invention also provides a method of preparing the bacterial capsular sugar-protein conjugate vaccine.
Owner:复星安特金(成都)生物制药有限公司
Multiple vaccination including serogroup C meningococcus
Various improvements to vaccines that include a serogroup C meningococcal conjugate antigen, including: (a) co-administration with acellular B. pertussis antigen; (b) co-administration with an inactivated poliovirus antigen; (c) supply in a kit together with a separate pneumococcal conjugate component, which may be in a liquid form; and (d) use in combination with a pneumococcal conjugate antigen but without an aluminum phosphate adjuvant. A kit may have: (a) a first immunogenic component that comprises an aqueous formulation of a conjugated capsular saccharide from Streptococcus pneumoniae; (b) a second immunogenic component that comprises a conjugated capsular saccharide from Neisseria meningitidis serogroup C.
Owner:GSK VACCINES GMBH
Meningococcal conjugate vaccination
ActiveUS20100104593A1Improving immunogenicityReduce chain lengthAntibacterial agentsBacterial antigen ingredientsCoccidiaMedicine
Conjugated meningococcal capsular saccharides will be introduced into immunisation schedules in the near future, but the phenomenon of “carrier suppression” must first be addressed, particularly where multiple conjugates are to be used. In the invention, tetanus toxoid is used as the carrier protein, even where multiple meningococcal conjugates are administered at the same time and where a patient has previously been exposed to the carrier protein, either in the form of a previous immunogen (e.g. in a DTP vaccine) or as a previous carrier protein (e.g. in a Hib or pneumococcal conjugate vaccine). The invention provides a method for immunising a patient, comprising administering multiple conjugates of meningococcal capsular saccharides, wherein each conjugate comprises a tetanus toxoid carrier protein, and the capsular saccharide, and wherein the patient has been pre-immunised with a tetanus toxoid.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Integration of meningococcal conjugate vaccination
ActiveUS20090060945A1Easy to useAvoid carrier suppressionAntibacterial agentsCarrier-bound antigen/hapten ingredientsDiphtheria vaccinationCarrier protein
Conjugated meningococcal capsular saccharides will be introduced into immunisation schedules in the near future, but the phenomenon of “carrier suppression” must first be addressed, particularly where multiple conjugates are to be used. It has been found that diphtheria toxoid and its derivatives (such as CRM197) can safely be used as the carrier protein, even where multiple meningococcal conjugates are administered at the same time and where a patient has previously been exposed to the carrier protein, either in the form of a previous immunogen (e.g. in a DTP vaccine) or as a previous carrier protein (e.g. in a Hib or pneumococcal conjugate vaccine). The invention provides a method for immunising a patient, comprising administering multiple conjugates of meningococcal capsular saccharides, wherein each conjugate comprises a diphtheria toxoid (or derivative thereof) carrier protein, and the capsular saccharide, and wherein the patient has been pre-immunised with a diphtheria toxoid (or derivative thereof).
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Meningococcal and Pneumococcal Conjugate Vaccine and Method of Using Same
ActiveUS20100266625A1Low costReduce the burden onAntibacterial agentsBacterial antigen ingredientsCoccidiaMeningococcal carriage
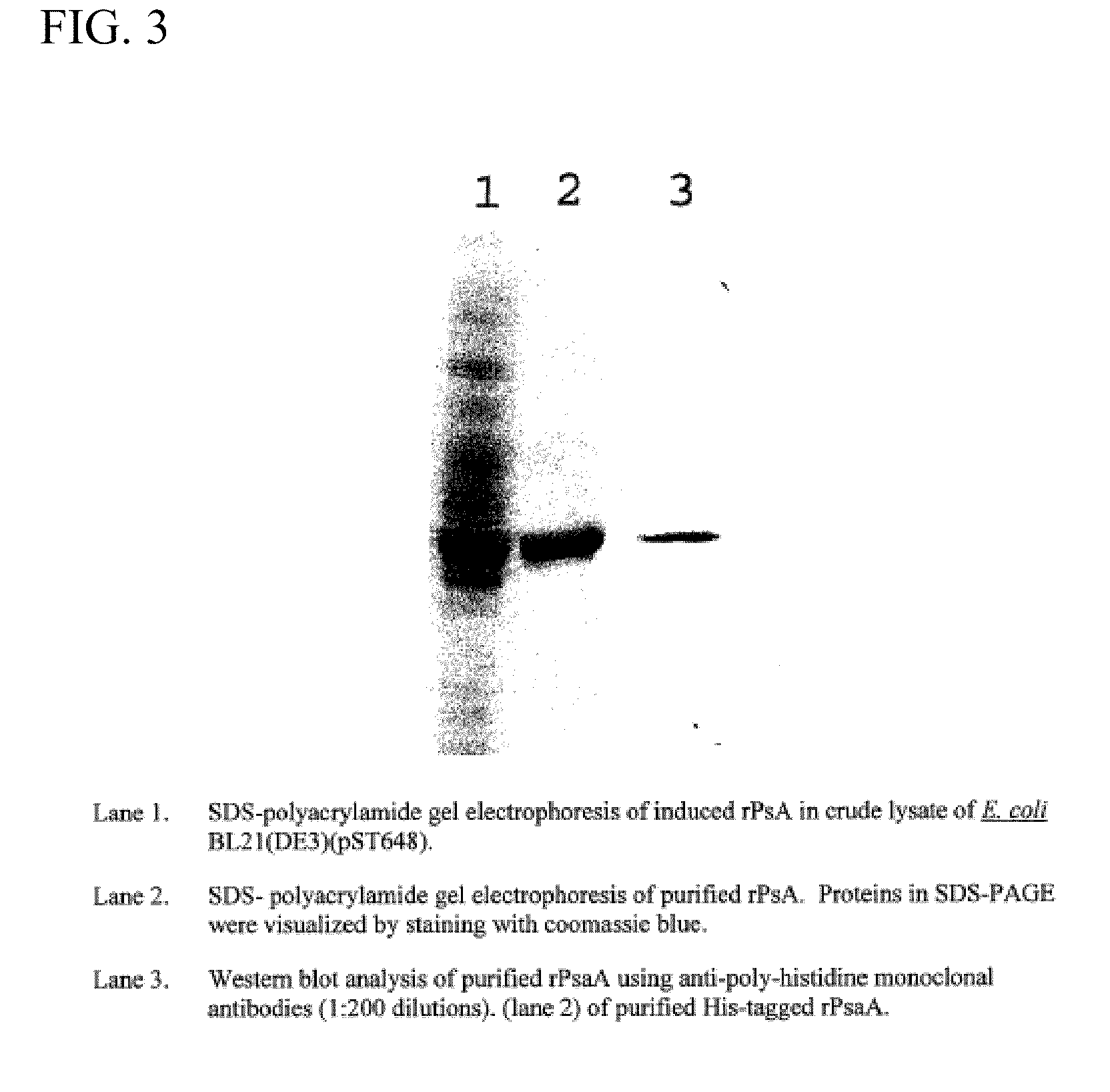
This disclosure relates to vaccine formulations comprising an immunogenic composition for inducing antibodies to both S. pneumoniae and N. meningitides in a subject. In a preferred aspect, the immunogenic composition comprises covalently conjugated recombinant PsaA (“rPsaA”) from S. pneumoniae and capsular polysaccharide from N. meningitidis serogroup C. This disclosure further relates to methods for producing the immunogenic composition as well as methods for their use.
Owner:HOWARD UNIVERSITY +1
Multivalent pneumococcal conjugate vaccine
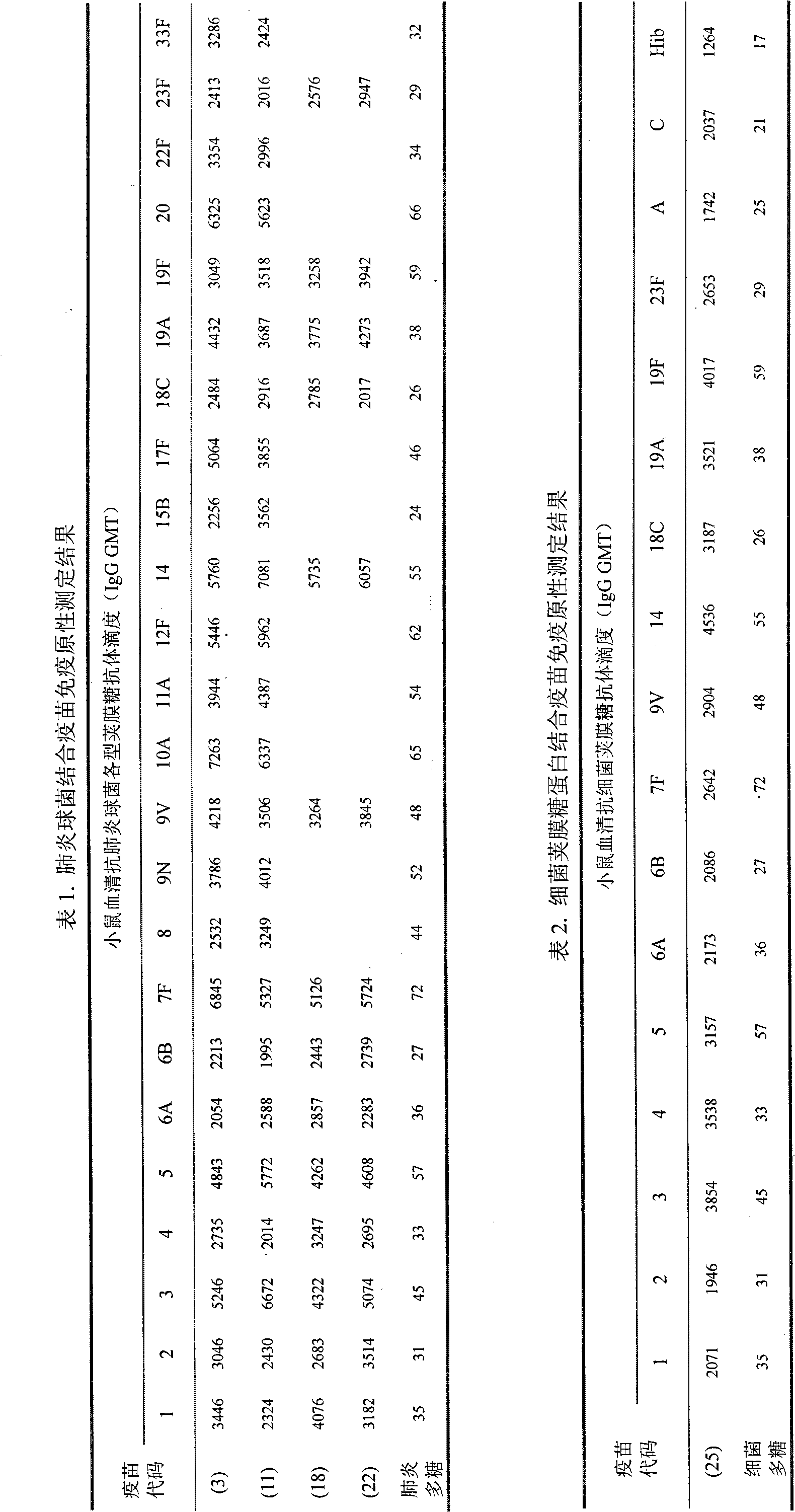
The present invention relates to a multivalent Pneumococcal conjugate vaccine (PCV) composition comprising: 1) at least 12 capsular polysaccharides selected from serotypes 1, 3, 4, 5, 6B, 7F, 9N, 9V, 15B, 14, 18C, 19A, 19F, 22F, 23F and 33F of S. pneumoniae activated with CDAP and conjugated to carrier protein selected from CRM197, pneumococcal surface protein A (PspA), pneumococcal adhesin protein (PsaA) or combination thereof and 2) a pharmaceutically acceptable carrier, wherein the composition does not contain capsular polysaccharide from serotype 6A.
Owner:BIOLOGICAL E LTD
Preparation method of pneumococcal conjugate combination vaccine
PendingCN106110316AImmunogenicity ComparisonExcellent titerAntibacterial agentsBacterial antigen ingredientsImmunogenicityInfective disorder
The invention relates to pneumococcal conjugate combinations. High-immune conjugates are prepared with selected combination methods suitable for different polysaccharide types and constitute one combination which is the pneumococcal conjugate combination with high immunogenicity, and the combination is used for preventing infectious diseases caused by pneumococcus. The combination vaccine is prepared from pneumococcal conjugate combinations selected from pneumococcus serotypes 1, 2, 3, 4, 5, 6A, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33 F.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +2
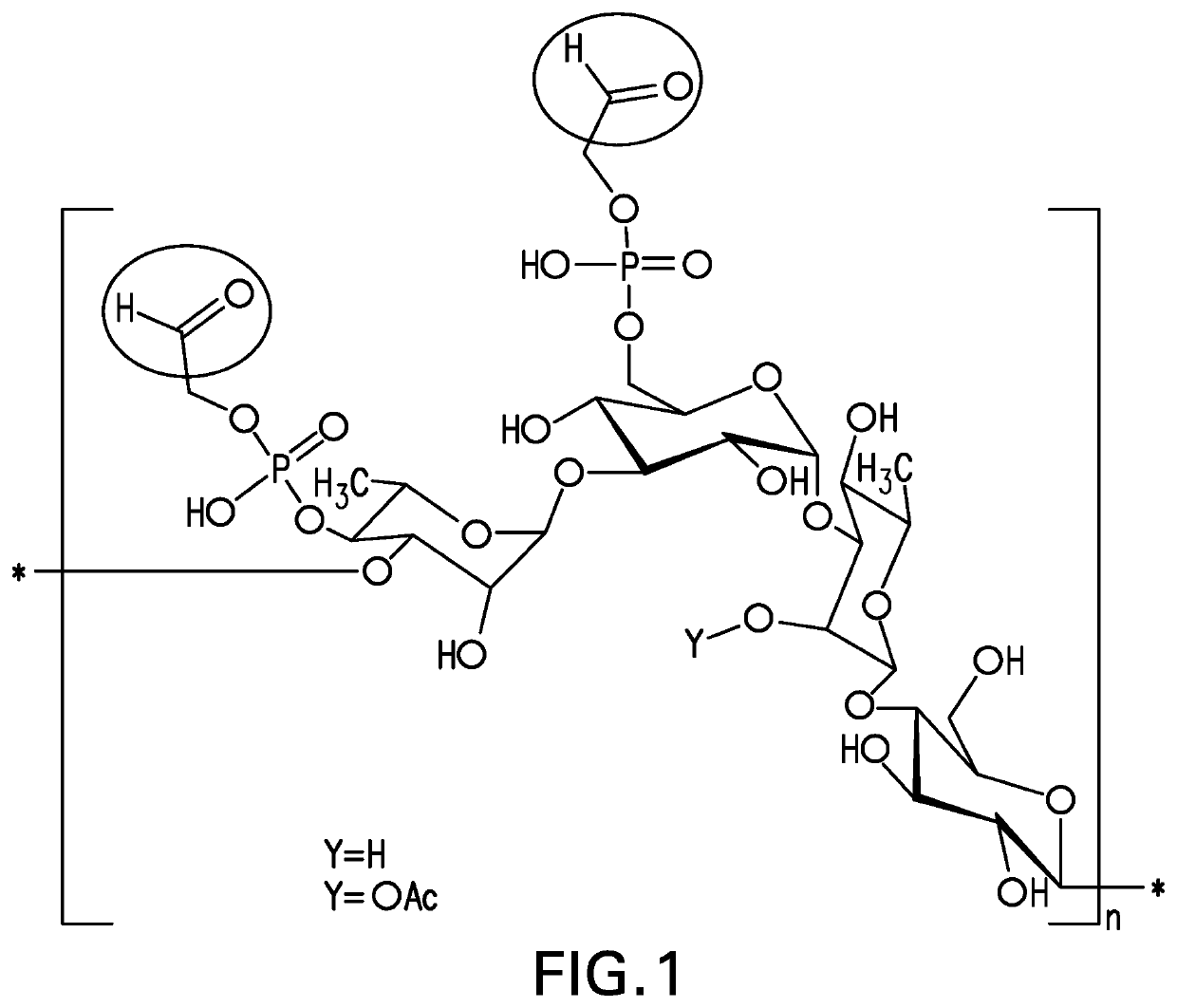
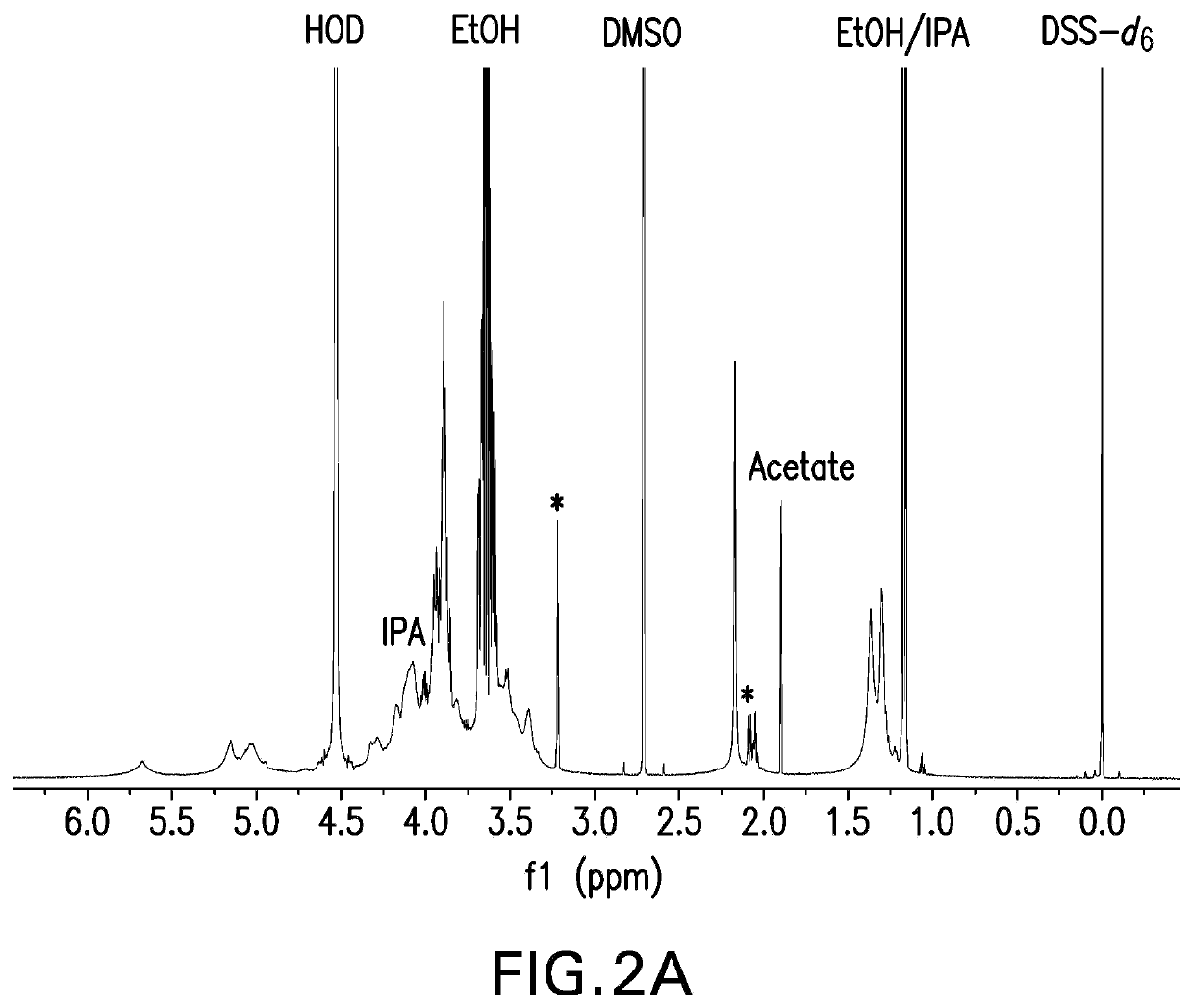
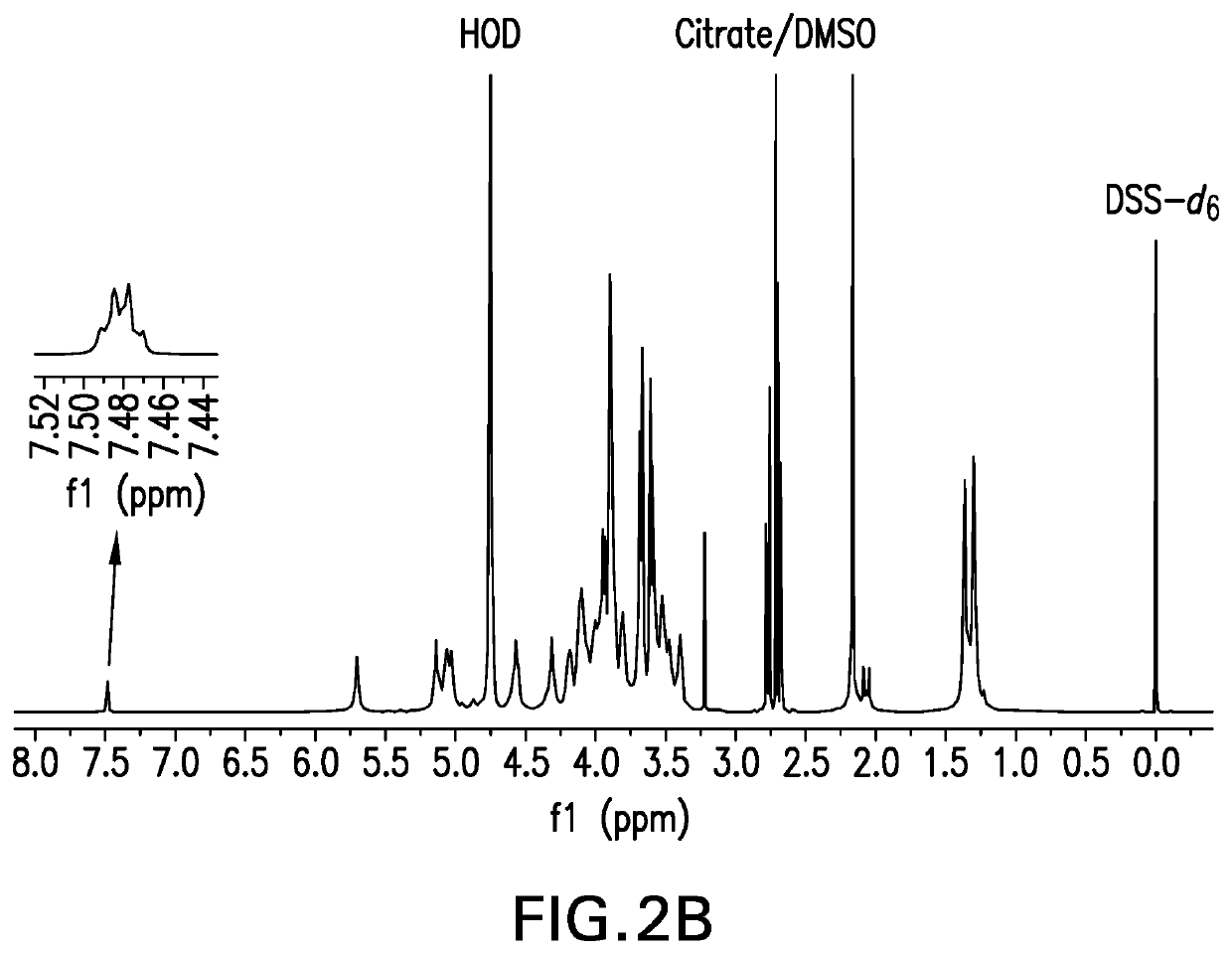
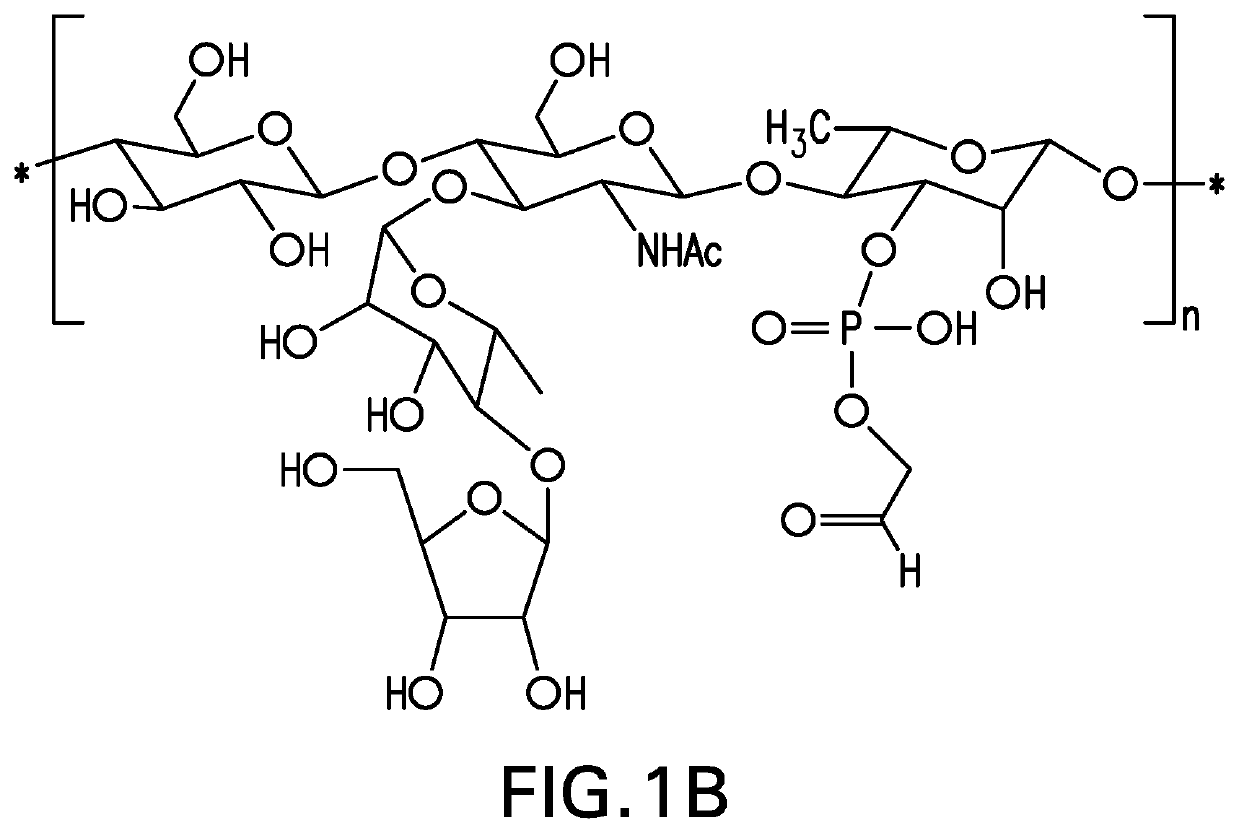
Pneumococcal polysaccharides and their use in immunogenic polysaccharide-carrier protein conjugates
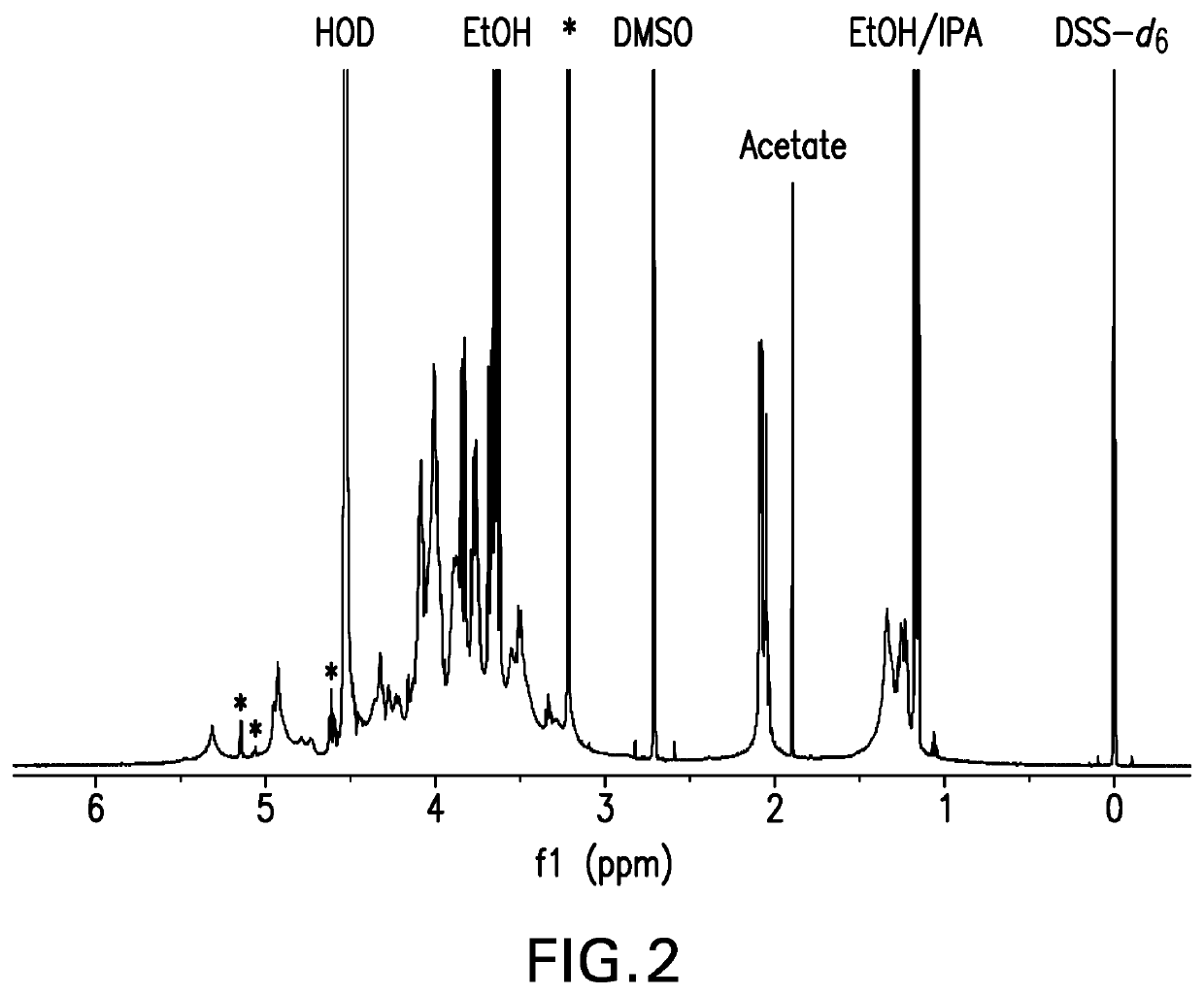
The present invention provides capsular polysaccharides from Streptococcus pneumoniae serotypes identified using NMR. The present invention further provides polysaccharide-protein conjugates in which capsular polysaccharides from one or more of these serotypes are conjugated to a carrier protein such as CRM197. Polysaccharide-protein conjugates from one or more of these serotypes may be included in multivalent pneumococcal conjugate vaccines having polysaccharides from multiple additional Streptococcus pneumoniae serotypes.
Owner:MERCK SHARP & DOHME LLC
Pneumococcal polysaccharides and their use in immunogenic polysaccharide-carrier protein conjugates
The present invention provides capsular polysaccharides from Streptococcus pneumoniae serotypes identified using NMR. The present invention further provides polysaccharide-protein conjugates in which capsular polysaccharides from one or more of these serotypes are conjugated to a carrier protein such as CRM197. Polysaccharide-protein conjugates from one or more of these serotypes may be included in multivalent pneumococcal conjugate vaccines having polysaccharides from multiple additional Streptococcus pneumoniae serotypes.
Owner:MERCK SHARP & DOHME LLC
Pneumococcal polysaccharides and their use in immunogenic polysaccharide-carrier protein conjugates
The present invention provides capsular polysaccharides from Streptococcus pneumoniae serotypes identified using NMR. The present invention further provides polysaccharide-protein conjugates in which capsular polysaccharides from one or more of these serotypes are conjugated to a carrier protein such as CRM197. Polysaccharide-protein conjugates from one or more of these N serotypes may be included in multivalent pneumococcal conjugate vaccines having polysaccharides from multiple additional Steptococcus pneumoniae serotypes.
Owner:MERCK SHARP & DOHME LLC
Pneumococcal polysaccharides and their use in immunogenic polysaccharide-carrier protein conjugates
ActiveUS20210038723A1Antibacterial agentsPharmaceutical delivery mechanismCarrier proteinStreptococcus halichoeri
The present invention provides capsular polysaccharides from Streptococcus pneumoniae serotypes identified using NMR. The present invention further provides polysaccharide-protein conjugates in which capsular polysaccharides from one or more of these serotypes are conjugated to a carrier protein such as CRM197. Polysaccharide-protein conjugates from one or more of these serotypes may be included in multivalent pneumococcal conjugate vaccines having polysaccharides from multiple additional Steptococcus pneumoniae serotypes.
Owner:MERCK SHARP & DOHME LLC
Pneumococcal vaccine containing pneumococcal surface protein a
ActiveUS20150320851A1Induce immune responseInduce protective immunityAntibacterial agentsBacterial antigen ingredientsCoccidiaProtein s antigen
A pneumococcal vaccine comprising a fusion protein at least comprising a full-length family 1 pneumococcal surface protein A (PspA) or a fragment thereof, and a full-length family 2 PspA or a fragment thereof, in particular any one of the following fusion proteins (1) to (3):(1) a fusion protein at least comprising a family 1, clade 2 PspA and a family 2, clade 3 PspA,(2) a fusion protein at least comprising a family 1, clade 2 PspA and a family 2, clade 4 PspA, and(3) a fusion protein at least comprising a family 1, clade 2 PspA and a family 2, clade 5 PspA,is useful as a pneumococcal vaccine comprising a single protein antigen that has broadly cross-reactive immunogenicity and can induce immune response against a wide range of pneumococcal clinical isolates.
Owner:OSAKA UNIV
Pneumococcal conjugate vaccine formulations
The present invention provides pneumococcal conjugate vaccine formulations comprising surfactant systems incorporating polysorbate 20 or a combination of a poloxamer and a polyol.
Owner:MERCK SHARP & DOHME CORP
Integration of meningococcal conjugate vaccination
ActiveUS9402915B2Antibacterial agentsCarrier-bound antigen/hapten ingredientsCarrier proteinDiphtheria vaccination
Conjugated meningococcal capsular saccharides will be introduced into immunization schedules in the near future, but the phenomenon of “carrier suppression” must first be addressed, particularly where multiple conjugates are to be used. It has been found that diphtheria toxoid and its derivatives (such as CRM197) can safely be used as the carrier protein, even where multiple meningococcal conjugates are administered at the same time and where a patient has previously been exposed to the carrier protein, either in the form of a previous immunogen (e.g. in a DTP vaccine) or as a previous carrier protein (e.g. in a Hib or pneumococcal conjugate vaccine). The invention provides a method for immunizing a patient, comprising administering multiple conjugates of meningococcal capsular saccharides, wherein each conjugate comprises a diphtheria toxoid (or derivative thereof) carrier protein, and the capsular saccharide, and wherein the patient has been pre-immunized with a diphtheria toxoid (or derivative thereof).
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Meningococcal conjugate vaccination
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Pneumococcal conjugate vaccine formulations
The present invention provides polysaccharide-protein conjugate vaccine formulations comprising a buffer, surfactant, sugar, alkali or alkaline salt, aluminum adjuvant, optionally a bulking agent, and optionally a polymer.
Owner:MERCK SHARP & DOHME CORP
Meningococcal and pneumococcal conjugate vaccine and method of using same
ActiveUS8003112B2Efficient infectionLow costAntibacterial agentsBacterial antigen ingredientsCoccidiaMeningococcal carriage
This disclosure relates to vaccine formulations comprising an immunogenic composition for inducing antibodies to both S. pneumoniae and N. meningitides in a subject. In a preferred aspect, the immunogenic composition comprises covalently conjugated recombinant PsaA (“rPsaA”) from S. pneumoniae and capsular polysaccharide from N. meningitidis serogroup C. This disclosure further relates to methods for producing the immunogenic composition as well as methods for their use.
Owner:HOWARD UNIVERSITY +1
Method for detecting contents of various types of specific sugar in polyvalent pneumococcal conjugate vaccine
ActiveCN106018832AImprove signal-to-noise ratioLower background requirementsScattering properties measurementsBiological testingGlycoprotein bindingDesorption
The invention relates to a method for detecting the contents of various types of specific sugar in polyvalent pneumococcal conjugate vaccine. The method comprises the following steps: step I: preparing solutions of certain concentrations from various types of monovalent capsular polysaccharide-protein conjugates according to sugar concentrations; step II: taking several parts of pneumococcal conjugate vaccine, adding the conjugate solutions obtained in the first step into the parts of pneumococcal conjugate vaccine except the first part, and diluting all the samples with a solvent to the same volume, wherein the volumes of the conjugate solutions added into the parts of pneumococcal conjugate vaccine increase from the second art to the last part in sequence; step III: adding alkali into each sample for desorption, and then adding acid for neutralization to obtain standards; step IV: carrying out reactions between the standards and a detection reagent, and drawing a standard curve by taking the sugar contents of the conjugate solutions as horizontal coordinates and taking reacting values as vertical coordinates; step V: pushing the standard curve outwards to the content axis, and taking the intercepts on the content axis as the sugar contents of monovalent capsular polysaccharide-protein conjugates in the polyvalent pneumococcal conjugate vaccine.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +2
Processes for the formulation of pneumococcal polysaccharides for conjugation to a carrier protein
ActiveUS20200276316A1Desire propertyPromote dissolutionAntibacterial agentsSugar derivativesSucroseStreptococcus pneumoniae conjugated
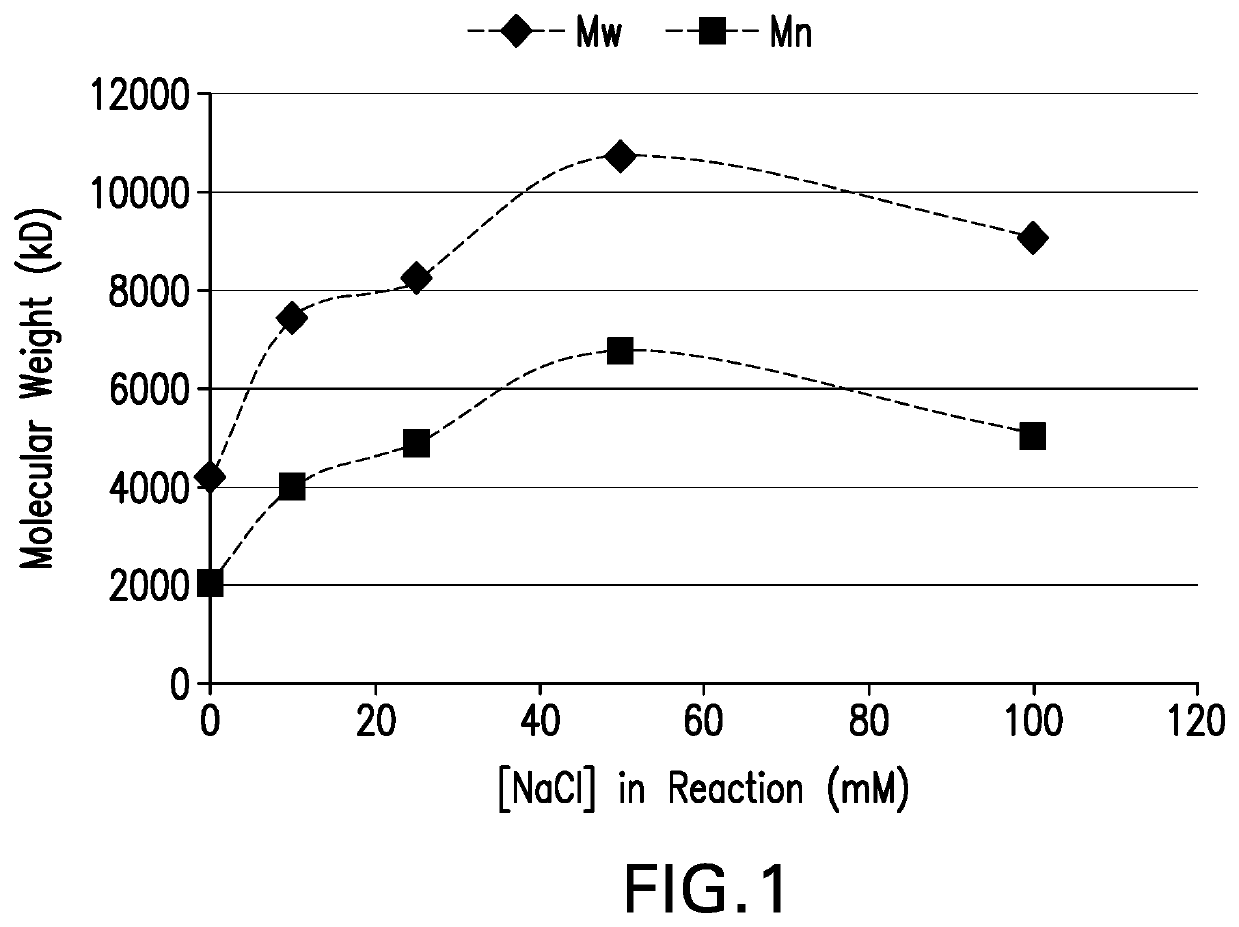
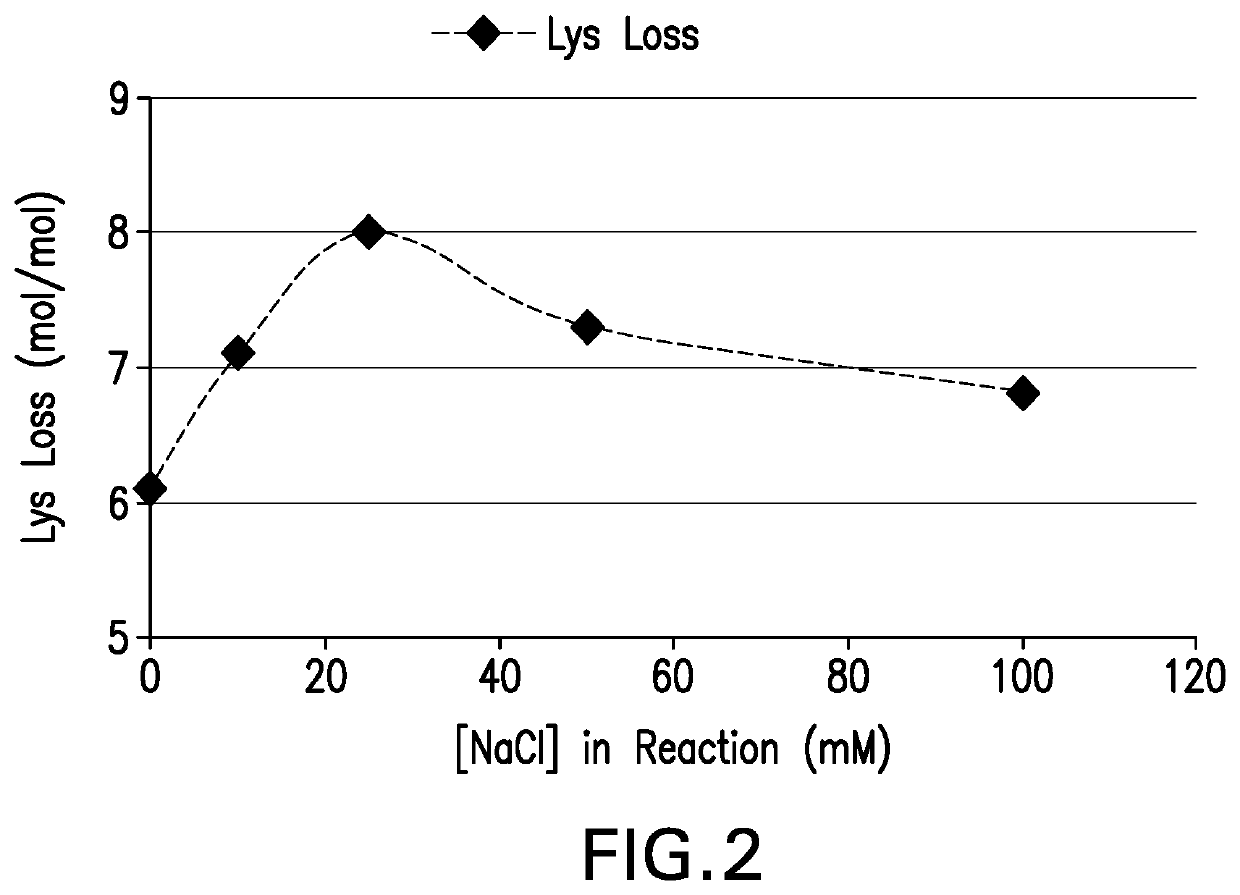
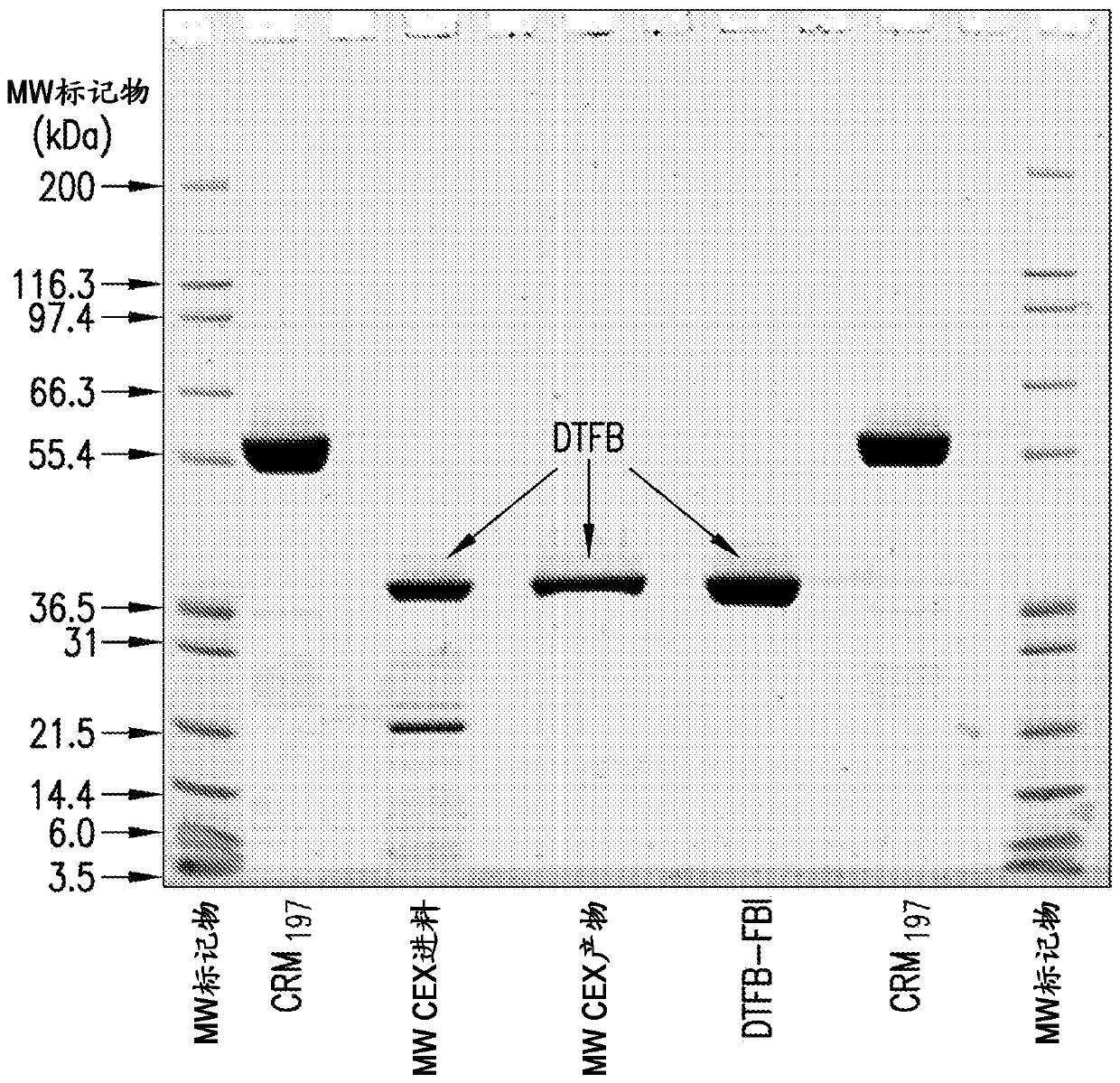
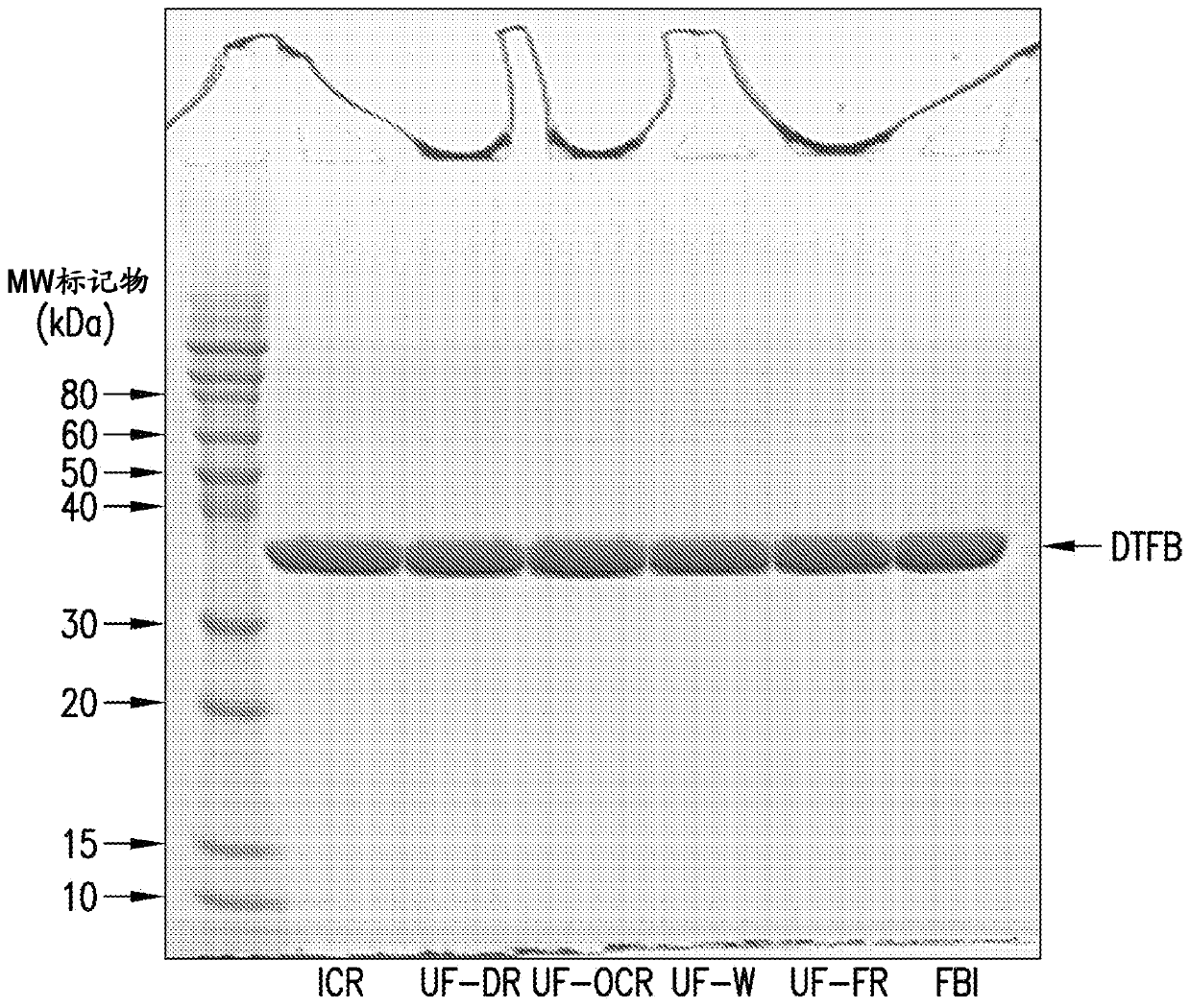
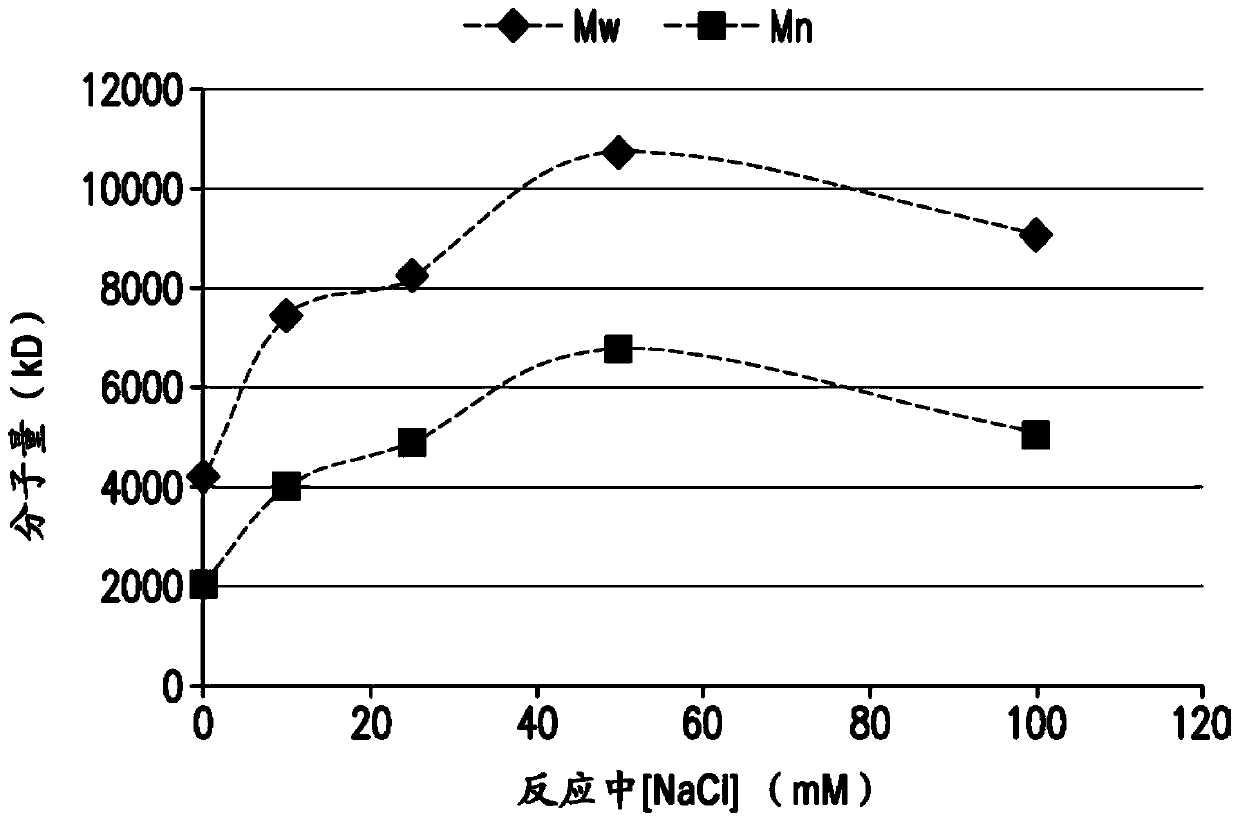
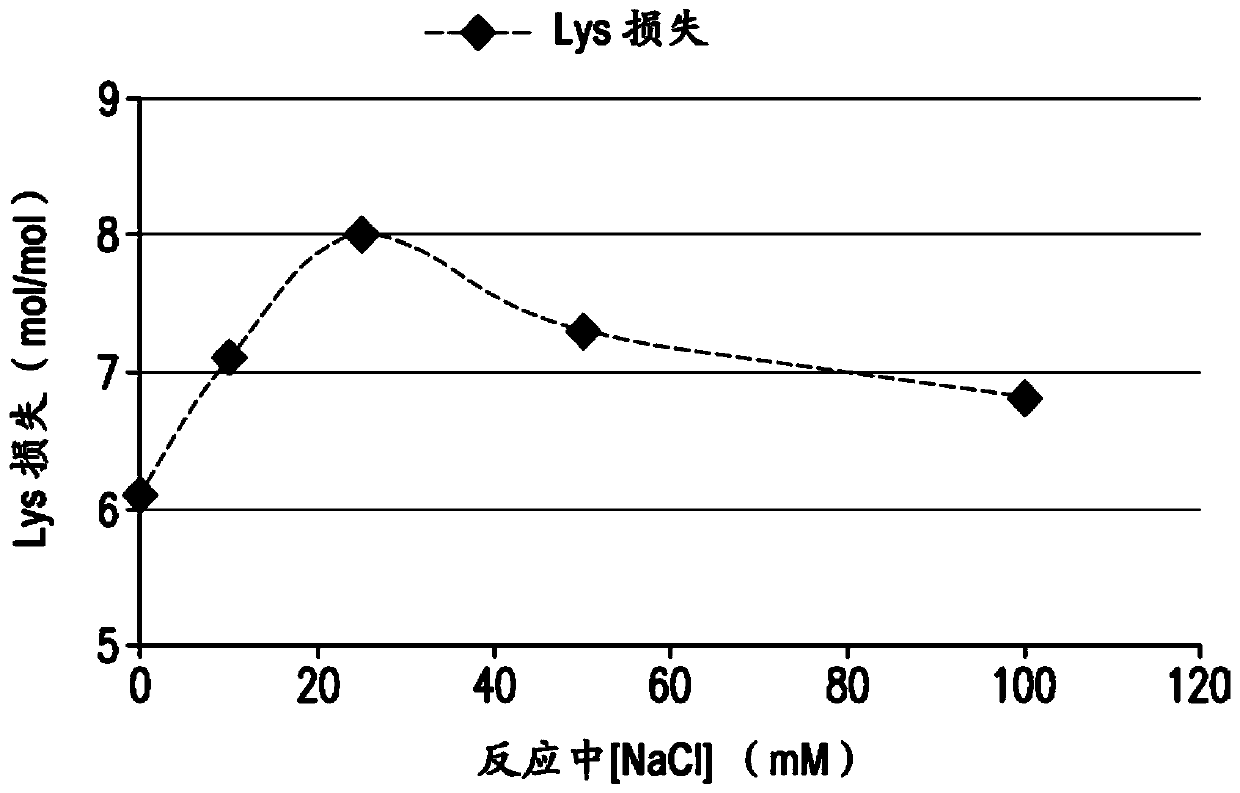
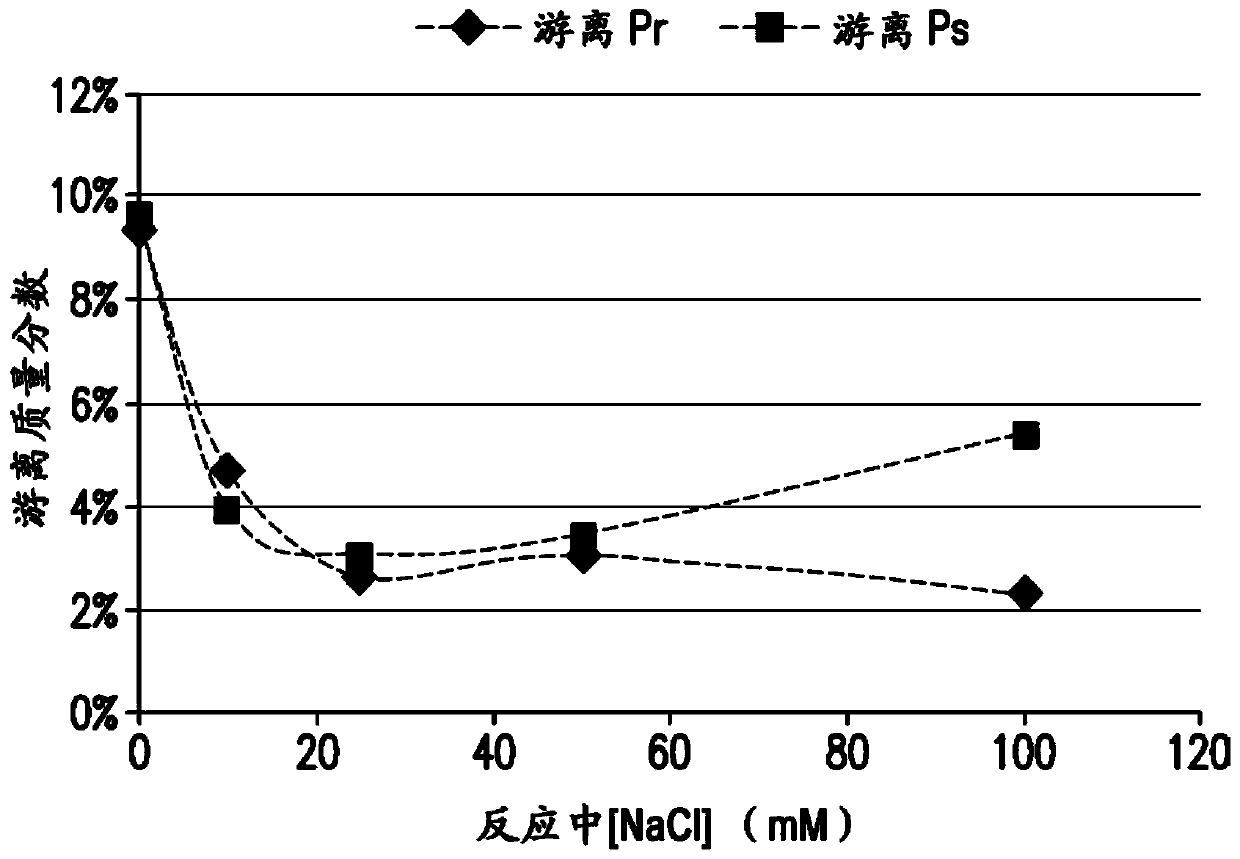
The present invention provides a number of process improvements related to the conjugation of capsular polysaccharides from Streptococcus pneumoniae to a carrier protein. These process are serotype specific and include acid hydrolysis, addition of sodium chloride to the reductive amination reaction, and addition of sucrose to dissolve polysaccharides. Polysaccharide-protein conjugates prepared using the processes of the invention can be included in multivalent pneumococcal conjugate vaccines.
Owner:MERCK SHARP & DOHME LLC
Preparation combination of multivalence pneumococcal conjugate vaccine and application thereof
ActiveCN108524926AResolving Serum InhibitionEffective control of molecular weightAntibacterial agentsBacterial antigen ingredientsSerum igeDisease
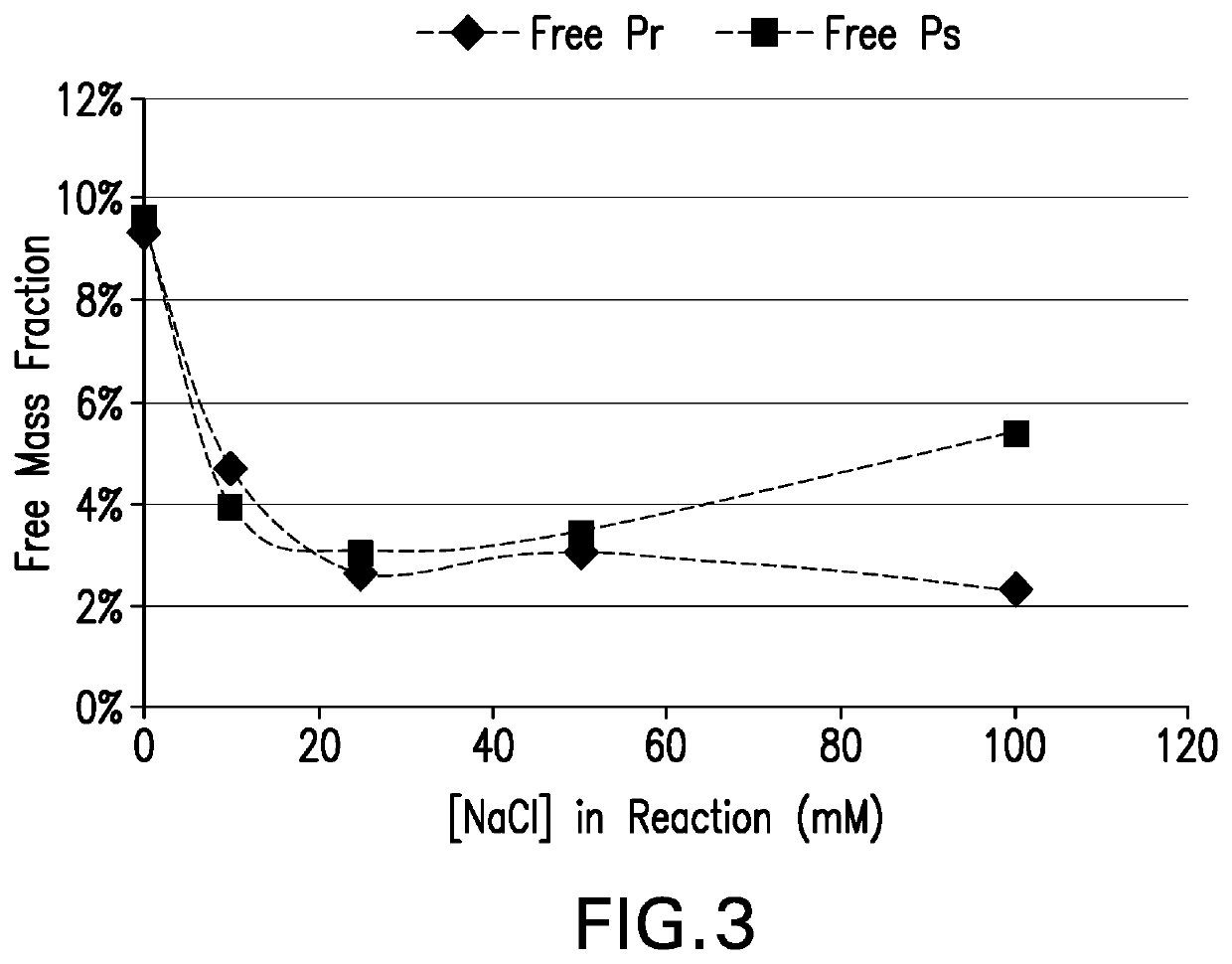
The invention provides a preparation combination of multivalence pneumococcal conjugate vaccine, a preparation method thereof and application in medicine preparation for preventing and treating diseases caused by pneumococcus. The invention further discloses a control method for conjugate molecular weight in the preparation combination of the multivalence pneumococcal conjugate vaccine. The seruminhibition problem in the multivalence pneumococcal conjugate vaccine is solved by the provided pneumococcal conjugate vaccine, and the immunogenicity in the preparation combination can be effectivelyimproved. The preparation method for the preparation combination of the multivalence pneumococcal conjugate vaccine can effectively control the molecular weight in the conjugate, and free protein andfree polysaccharide which do not join in conjugation can be easily removed.
Owner:CANSINO BIOLOGICS INC
Novel pneumococcal conjugate vaccine and preparation method thereof
ActiveCN101785857BImproving immunogenicityReduce immune competitionAntibacterial agentsBacterial antigen ingredientsSugarTGE VACCINE
The invention relates to a novel pneumococcal conjugate vaccine and a preparation method thereof. The novel pneumococcal conjugate vaccine comprises a dual-valent serum type pneumococcal capsular sugar-protein combination and / or a multi-valent serum type pneumococcal capsular sugar-protein combination. The dual-valent serum type capsular sugar-protein combination is a combination formed by combining two different serum types of capsular sugar with a protein carrier through chemical bonds, and the two different serum types of capsular sugar form links in the structure according to the combination with the protein carrier through the chemical bonds. The multi-valent serum type capsular sugar-protein combination is a combination formed by combining two different serum types of capsular sugarwith a protein carrier through chemical bonds, and the two different serum types of capsular sugar form links in the structure according to the combination with the protein carrier through the chemical bonds. In addition, the invention also provides a method of preparing the bacterial capsular sugar-protein conjugate vaccine.
Owner:复星安特金(成都)生物制药有限公司
Pneumococcal conjugate vaccine formulations
The present invention provides pneumococcal conjugate vaccine formulations comprising surfactant systems incorporating polysorbate 20 or a combination of a poloxamer and a polyol.
Owner:MERCK SHARP & DOHME BV
Processes for the formulation of pneumococcal polysaccharides for conjugation to a carrier protein
The present invention provides a number of process improvements related to the conjugation of capsular polysaccharides from Streptococcus pneumoniae to a carrier protein. These processes are serotypespecific and include acid hydrolysis, addition of sodium chloride to the reductive amination reaction, and addition of sucrose to dissolve polysaccharides. Polysaccharide-protein conjugates prepared using the processes of the invention can be included in multivalent pneumococcal conjugate vaccines.
Owner:MERCK SHARP & DOHME BV
Multivalent pneumococcal conjugate vaccine
ActiveUS11147863B2Antibacterial agentsBacterial antigen ingredientsCarrier proteinPharmaceutical medicine
The present invention relates to a multivalent Pneumococcal conjugate vaccine (PCV) composition comprising: 1) at least 12 capsular polysaccharides selected from serotypes 1, 3, 4, 5, 6B, 7F, 9N, 9V, 15B, 14, 18C, 19A, 19F, 22F, 23F and 33F of S. pneumoniae activated with CDAP and conjugated to carrier protein selected from CRM197, pneumococcal surface protein A (PspA), pneumococcal adhesin protein (PsaA) or combination thereof and 2) a pharmaceutically acceptable carrier, wherein the composition does not contain capsular polysaccharide from serotype 6A.
Owner:BIOLOGICAL E LTD
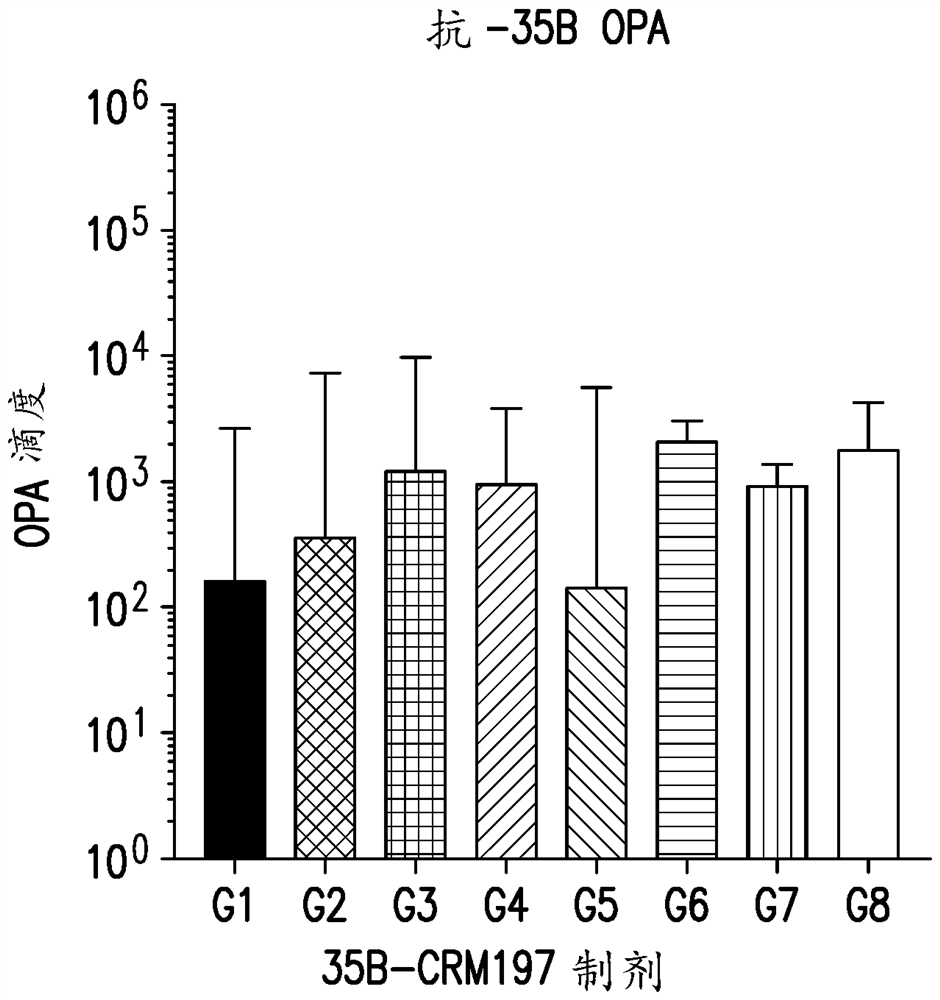
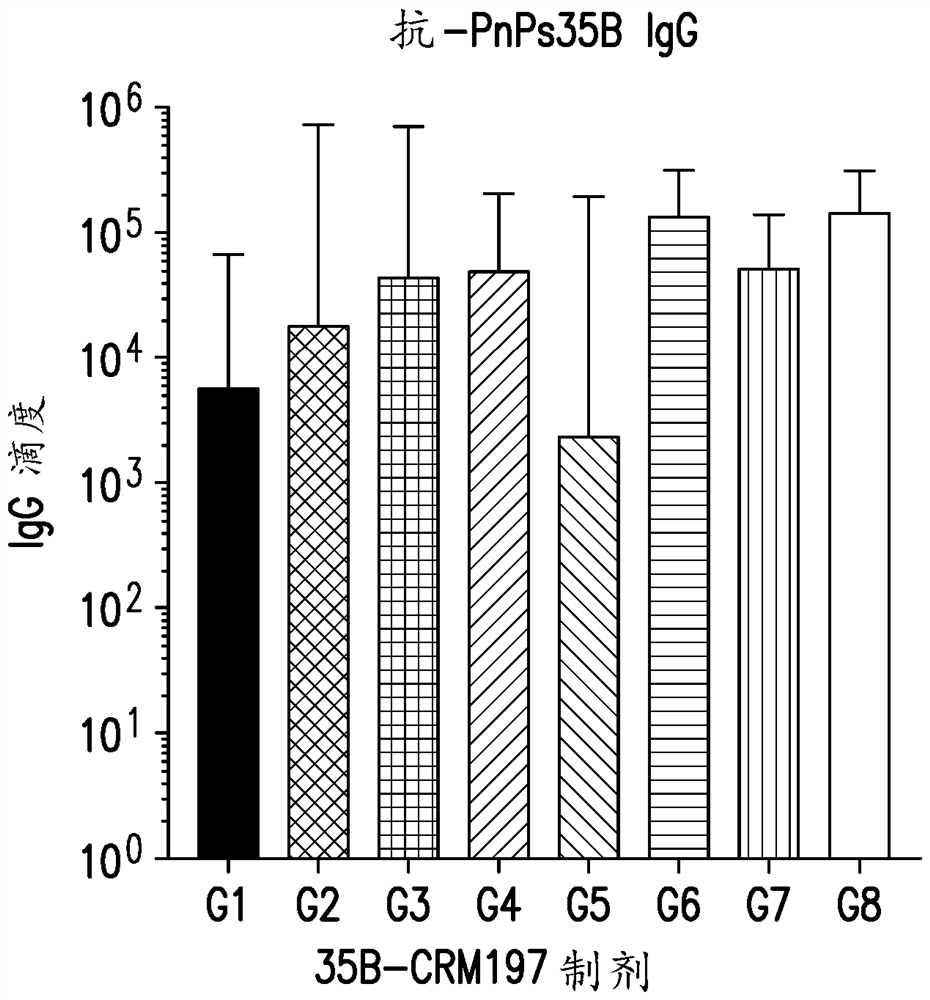
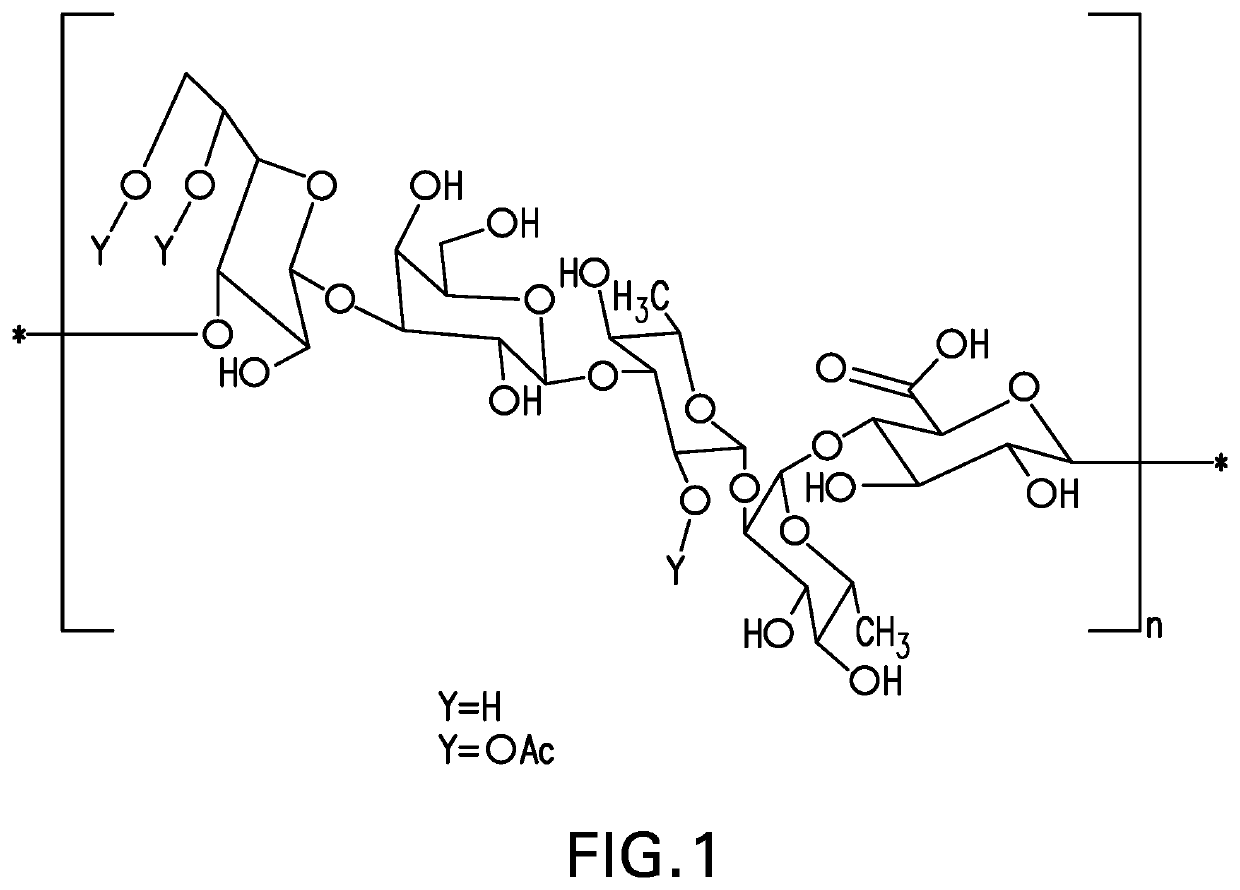
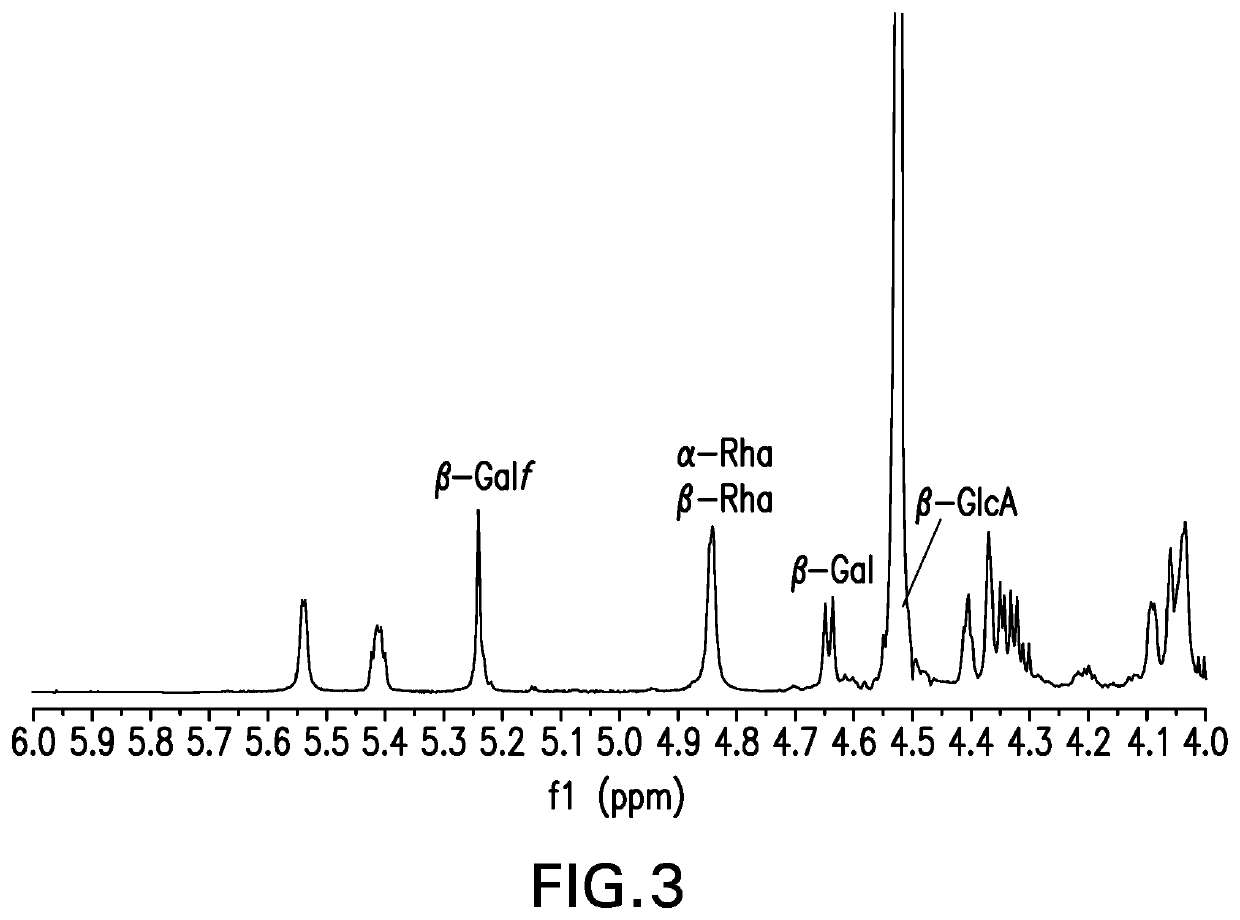
An immunogenic serotype 35b pneumococcal polysaccharide-protein conjugate and conjugation process for making the same
PendingUS20220233674A1Antibacterial agentsBacterial antigen ingredientsCarrier proteinStreptococcus halichoeri
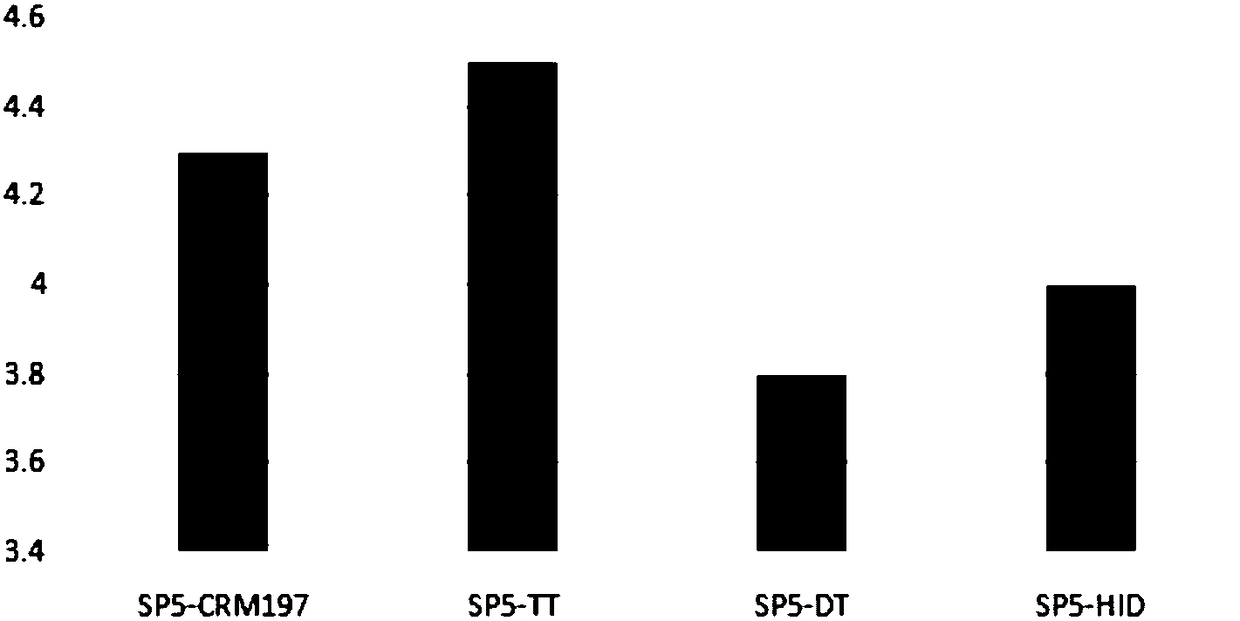
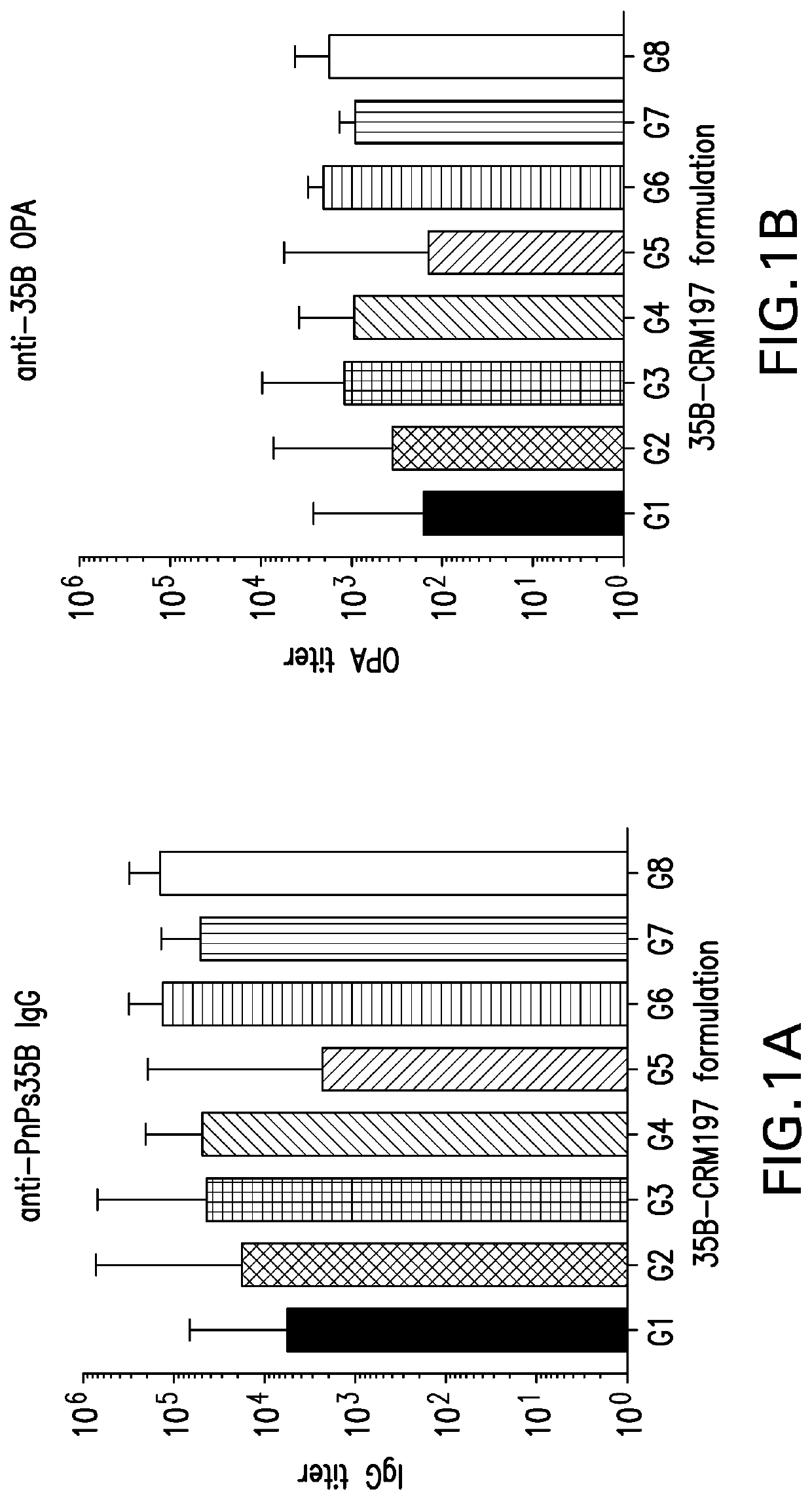
The present invention provides a process improvement related to the conjugation of capsular polysaccharides from Streptococcus pneumoniae (S. pneumoniae) serotype 35B to a carrier protein. The serotype 35B polysaccharide-protein conjugate, prepared by the disclosed process, is, among other things, more immunogenic than similar conjugates made by prior art methods. S. pneumoniae serotype 35B polysaccharide-protein conjugates prepared using the processes of the invention can be included in multivalent pneumococcal conjugate vaccine compositions.
Owner:MERCK SHARP & DOHME LLC
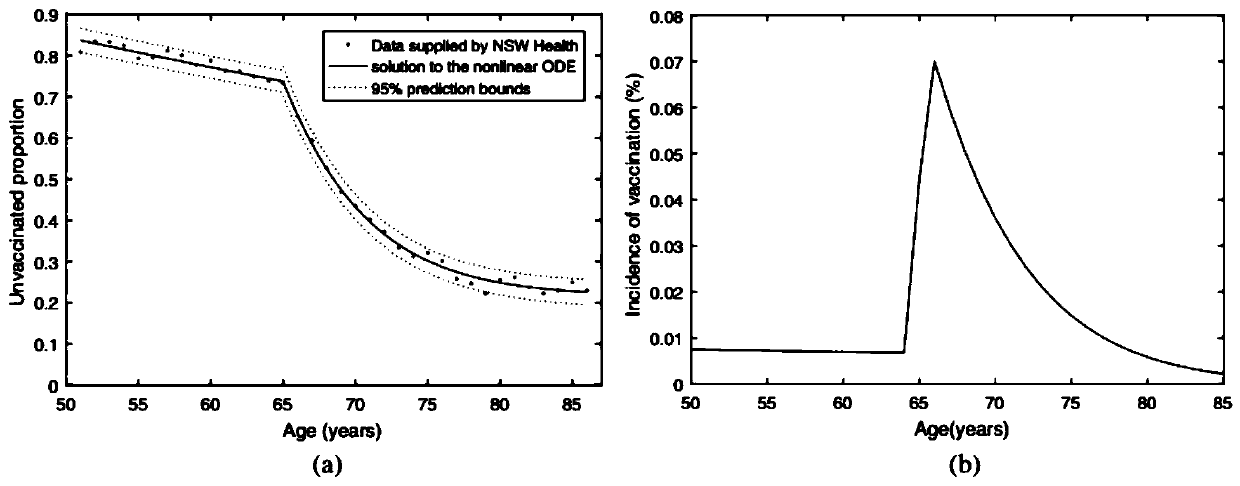
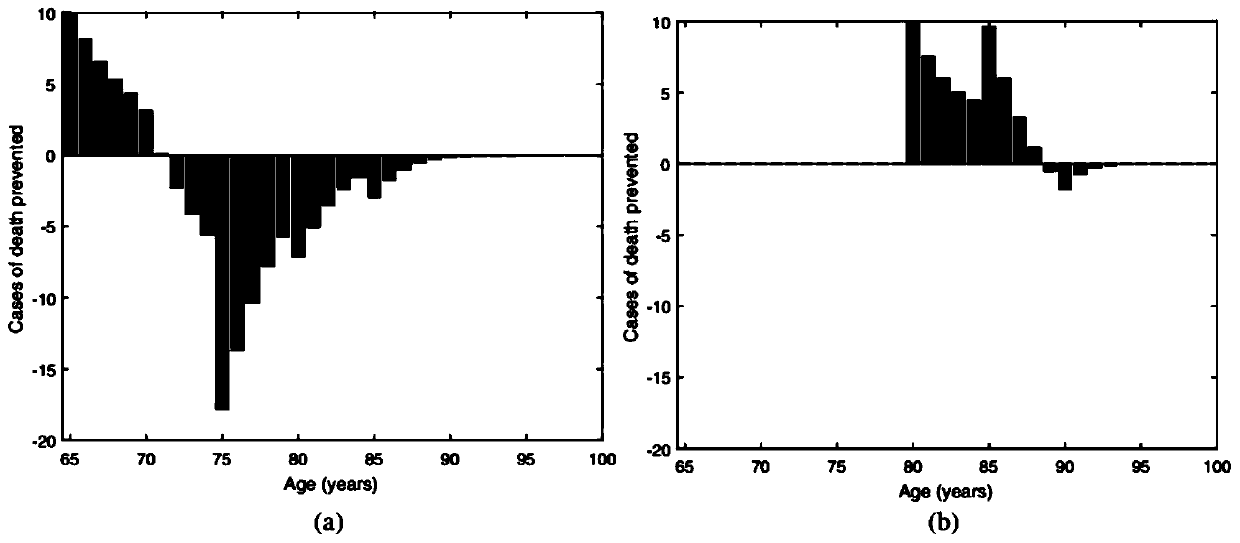
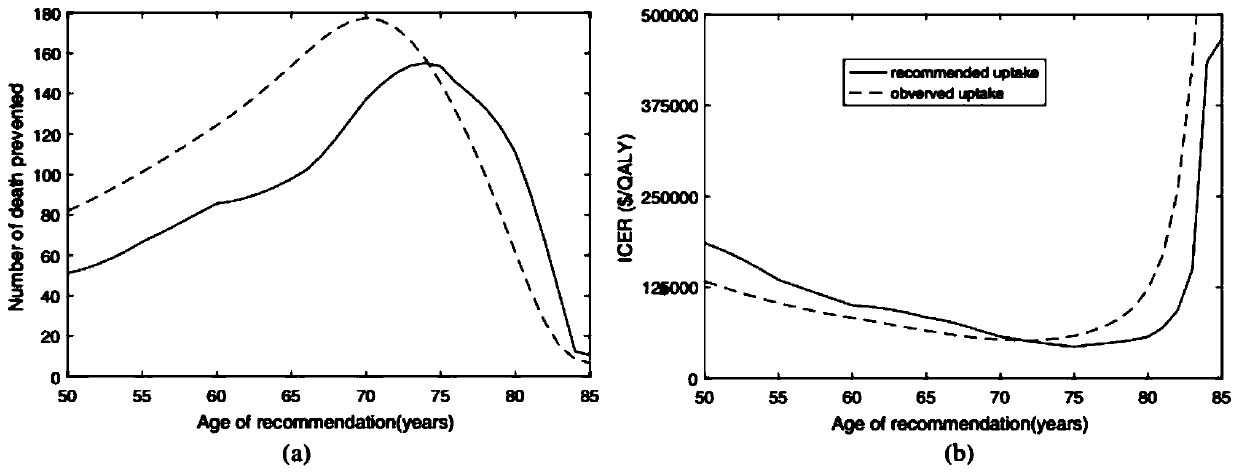
Numerical and statistical modeling method for medical intervention
The invention discloses a numerical and statistical modeling method for medical intervention. The method discusses the use of more realistic observation of the impact of vaccination distribution to formulate more effective vaccine policy suggestions. With the 13-valent pneumococcal conjugate vaccine (PCV13) as an example, the method discusses the timeliness and outcome of vaccination. One dose ofPCV13 is recommended to adults more than 65 years old recently so as to replace the existing 23-valence pneumococcal polysaccharide vaccine (PPV23) plan. The suggestion to the 65 age is consistent with that of most other developed countries.
Owner:陈晨
An immunogenic serotype 35b pneumococcal polysaccharide-protein conjugate and conjugation process for making the same
The present invention provides a process improvement related to the conjugation of capsular polysaccharides from Streptococcus pneumoniae (S. pneumoniae) serotype 35B to a carrier protein. The serotype 35B polysaccharide-protein conjugate, prepared by the disclosed process, is, among other things, more immunogenic than similar conjugates made by prior art methods. S. pneumoniae serotype 35B polysaccharide-protein conjugates prepared using the processes of the invention can be included in multivalent pneumococcal conjugate vaccine compositions.
Owner:MERCK SHARP & DOHME BV
Pneumococcal polysaccharides and their use in immunogenic polysaccharide-carrier protein conjugates
The present invention provides capsular polysaccharides from Streptococcus pneumoniae serotypes identified using NMR. The present invention further provides polysaccharide-protein conjugates in which capsular polysaccharides from one or more of these serotypes are conjugated to a carrier protein such as CRM197. Polysaccharide-protein conjugates from one or more of these serotypes may be included in multivalent pneumococcal conjugate vaccines having polysaccharides from multiple additional Streptococcus pneumoniae serotypes.
Owner:MERCK SHARP & DOHME LLC
Pneumococcal polysaccharides and their use in immunogenic polysaccharide-carrier protein conjugates
PendingCN111093650AInorganic non-active ingredientsMultivalent vaccineCarrier proteinStreptococcus halichoeri
The present invention provides capsular polysaccharides from Streptococcus pneumoniae serotypes identified using NMR. The present invention further provides polysaccharide-protein conjugates in whichcapsular polysaccharides from one or more of these serotypes are conjugated to a carrier protein such as CRM197. Polysaccharide-protein conjugates from one or more of these serotypes may be included in multivalent pneumococcal conjugate vaccines having polysaccharides from multiple additional Steptococcus pneumoniae serotypes.
Owner:MERCK SHARP & DOHME BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com