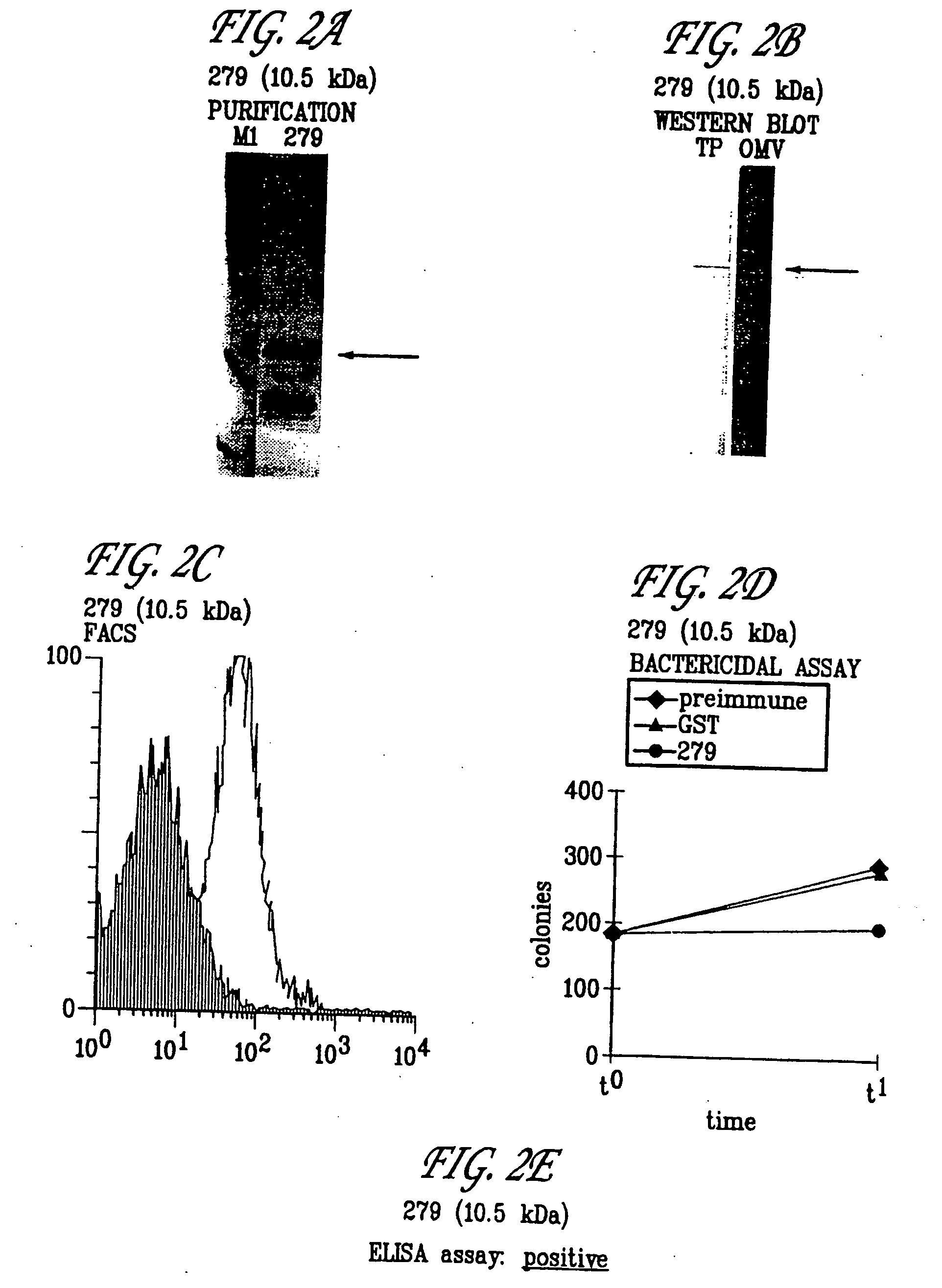
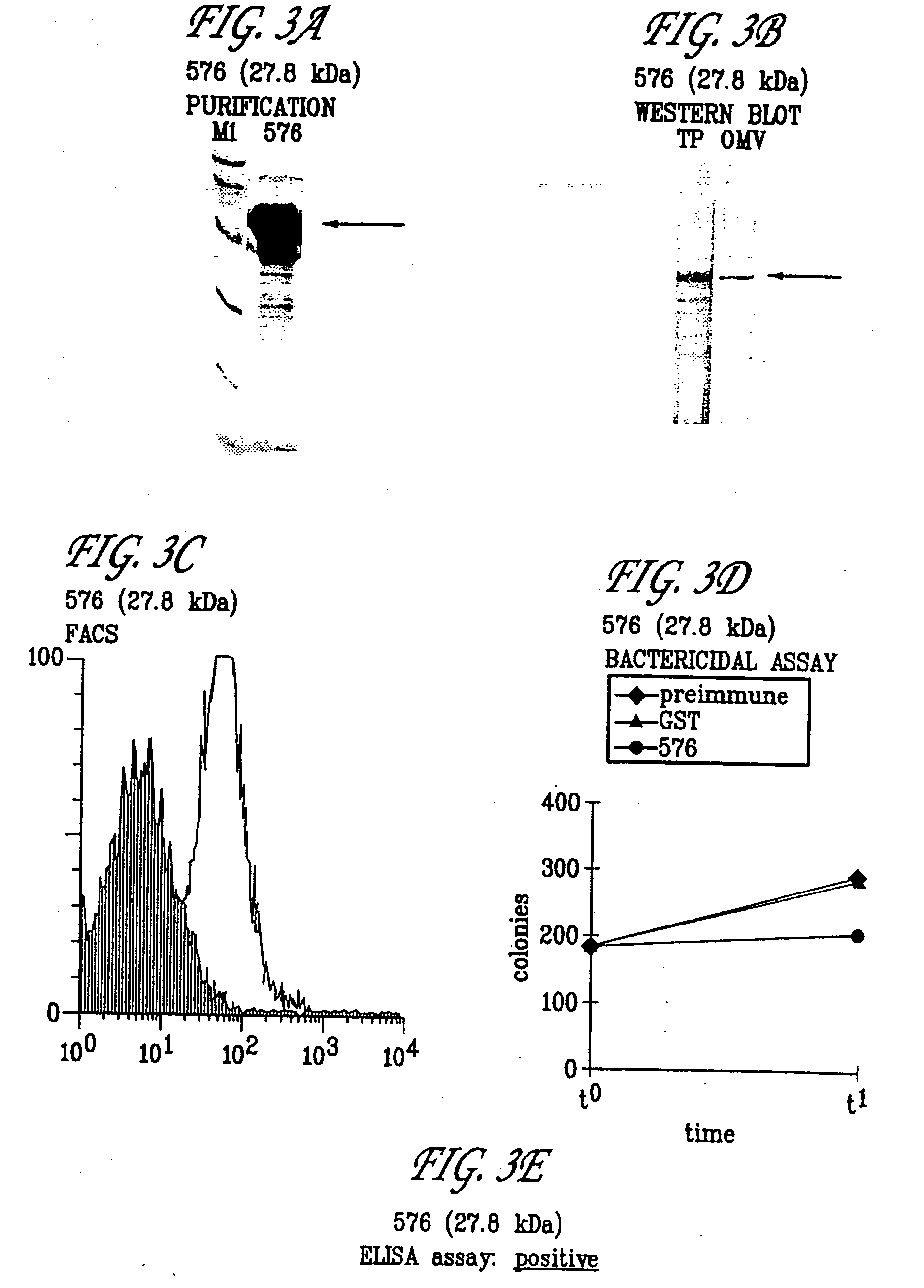
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
227 results about "Meningitides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Brain infection
Vaccines comprising aluminium adjuvants and histidine
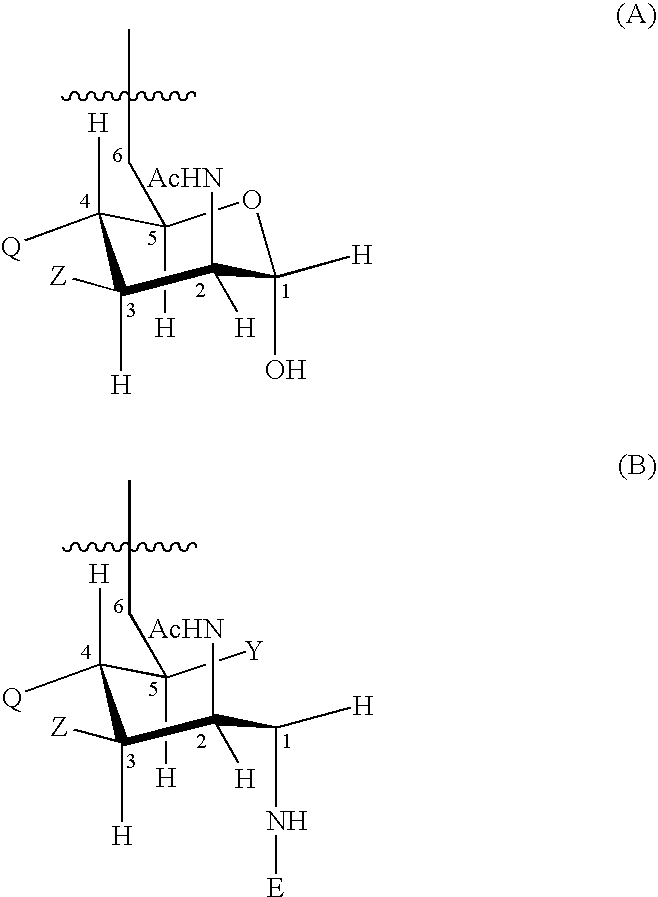
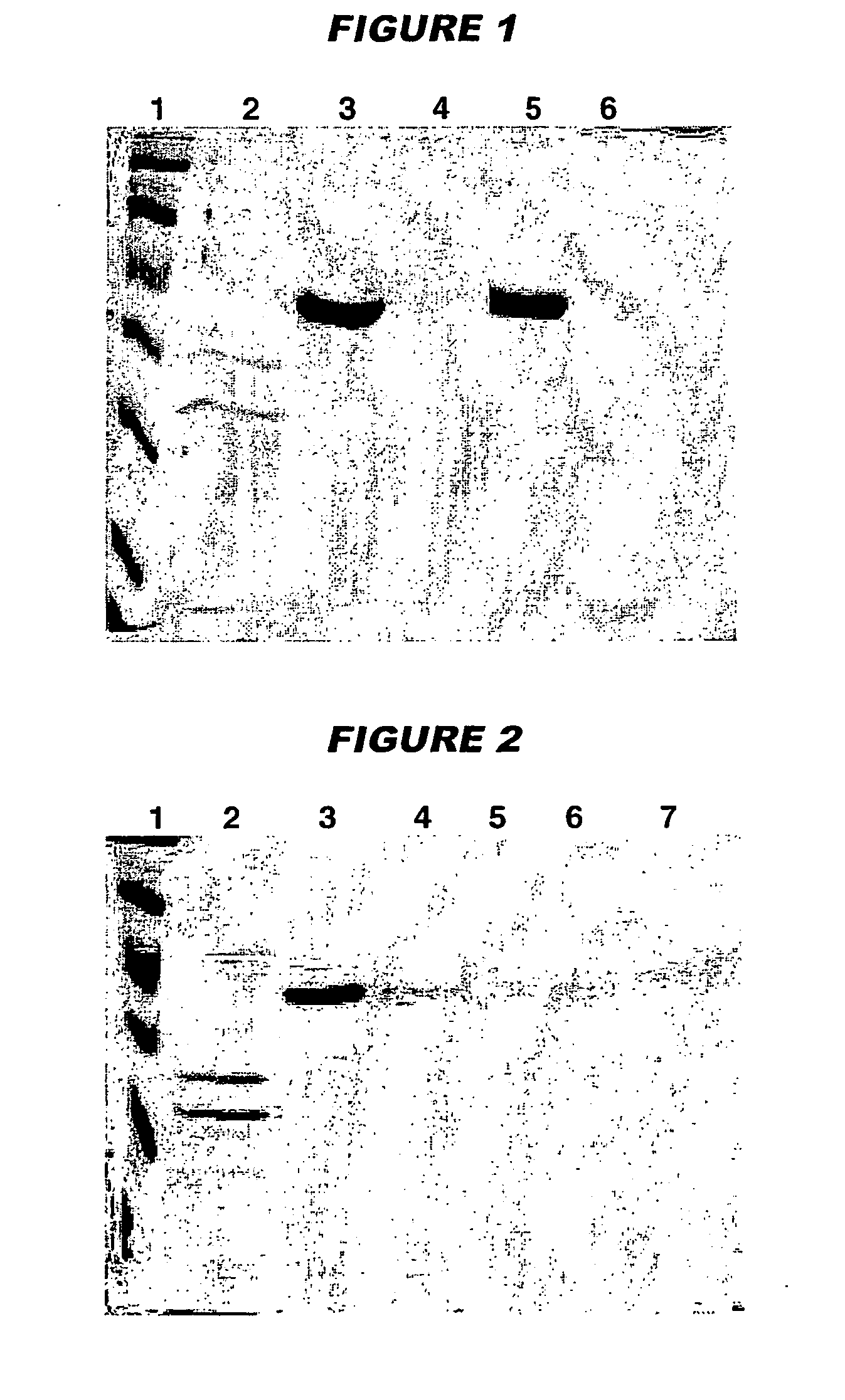
To improve the stability of vaccines comprising aluminum salt(s), the invention uses the amino acid histidine. This can improve pH stability and adjuvant adsorption and can be reduce antigen hydrolysis. Histidine is preferably presen during adsorption to the aluminium salt(s). The antigen in the vaccine may be a protein or a saccharide and is preferably from N. meningitidis.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Polypeptide-vaccines for broad protetion against hypervirulent meningococcal lineages
ActiveUS20060171957A1Improve hydrolysis resistanceAntibacterial agentsBacterial antigen ingredientsSalmonella serotype typhiMeningococcal carriage
A small number of defined antigens can provide broad protection against meningococcal infection, and the invention provides a composition which, after administration to a subject, is able to induce an antibody response in that subject, wherein the antibody response is bactericidal against two or three of hypervirulent lineages A4, ET 5 and lineage 3 of N. meningitidis serogroup B. Rather than consisting of a single antigen, the composition comprises a mixture of 10 or fewer purified antigens, and should not include complex or undefined mixtures of antigens such as outer membrane vesicles. Five protein antigens are used in particular: (1) a ‘NadA’ protein; (2) a ‘741’ protein; (3) a ‘936’ protein; (4) a ‘953’ protein; and (5) a ‘287’ protein.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Proteins and nucleic acids from meningitis/sepsis-associated Escherichia coli
Disclosed herein are various open reading frames from a strain of E. coli responsible for neonatal meningitis (MNEC), and a subset of these that is of particular interest for preparing compositions for immunising against MNEC infections.
Owner:GLAXOSMITHKLINE VACCINES SRL +1
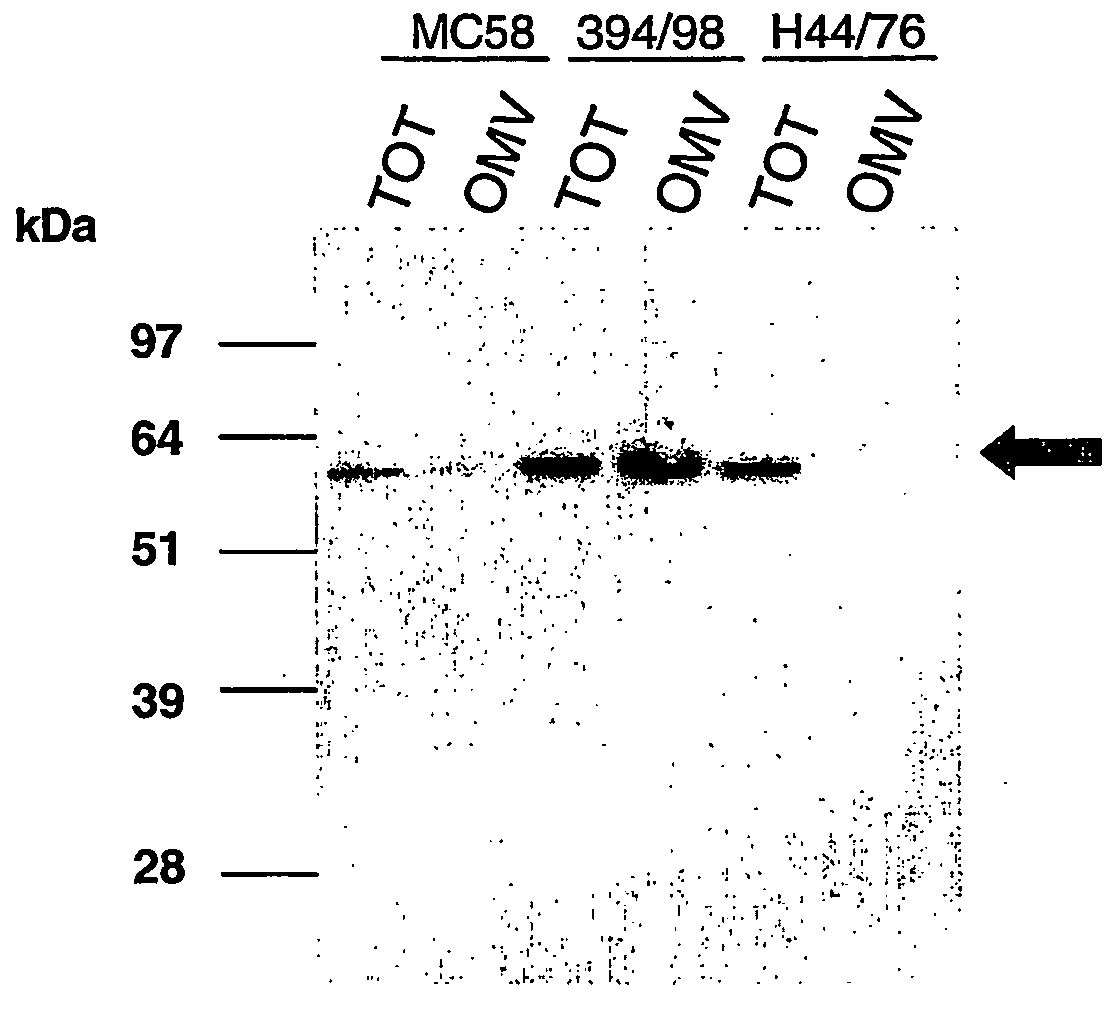
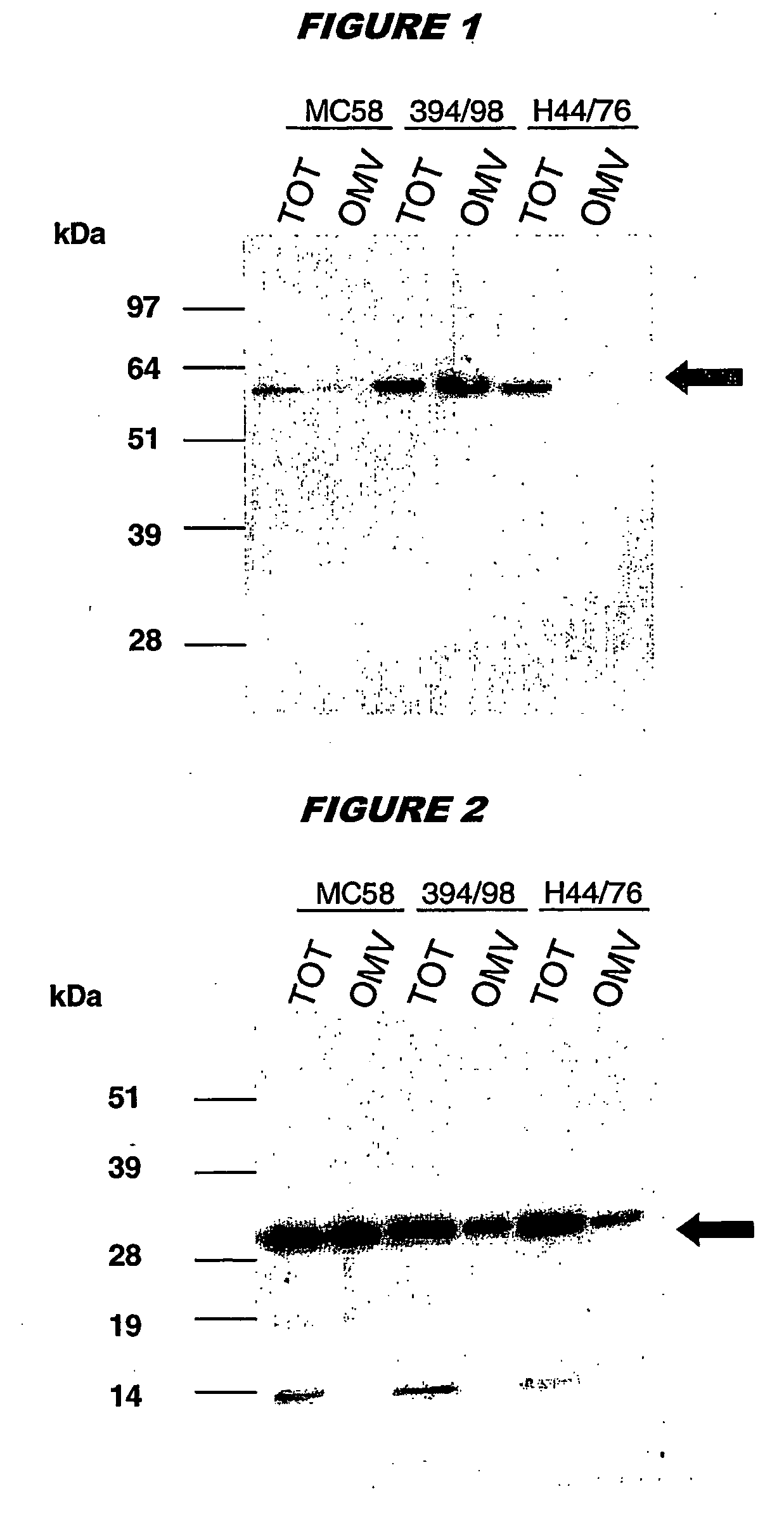
Outer membrane vesicle (OMV) vaccine comprising N. meningitidis serogroup B outer membrane proteins
ActiveUS8273360B2Broadens their efficacyAntibacterial agentsNervous disorderProtective antigenNeisseria weaveri
A composition comprising (a) Neisseria meningitidis serogroup B outer membrane vesicles (OMVs), and (b) an immunogenic component selected from other Neisseria proteins, or immunogenic fragments thereof. Component (b) preferably includes a protein from a different NmB strain from that from which the OMV of component (a) is derived. The OMVs are preferably obtained by deoxycholate extraction. Optionally, the composition may also comprise a protective antigen against other pathogens.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Liquid Vaccines For Multiple Meningococcal Serogroups
Conjugated capsular saccharides from meningococcal serogroups C, W135 and Y are safe and immunogenic in humans when combined in a single dose. This effect is retained when a conjugated capsular saccharide from serogroup A is added. These conjugated antigens can be stably combined in a single aqueous dose without the need for lyophilisation. Broad protection against serogroup B infection can be achieved by using a small number of defined polypeptide antigens. These polypeptide antigens can be combined with the saccharide antigens without loss of protective efficacy for any of the five serogroups. Efficacy if retained even if a Hib conjugate is added. The efficacy of a serogroup W135 conjugate is enhanced by addition of protein antigens derived from a serogroup B strain. Addition of a Hib conjugate to meningococcal conjugates enhances the overall activity against meningococcus serogroup W135.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
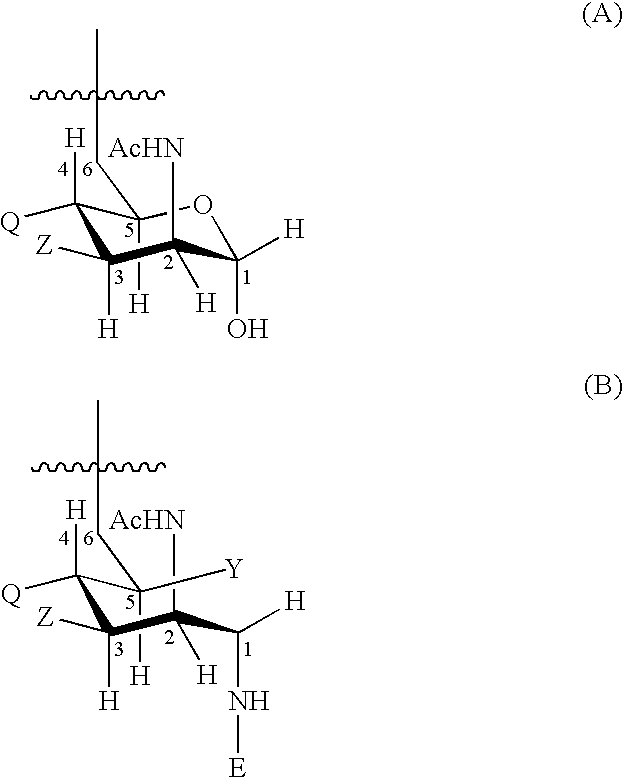
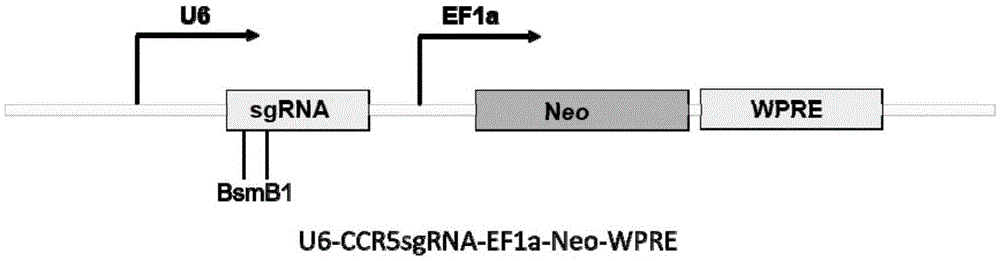
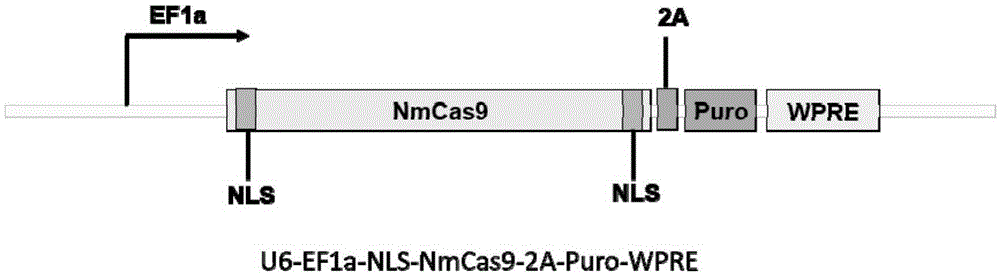
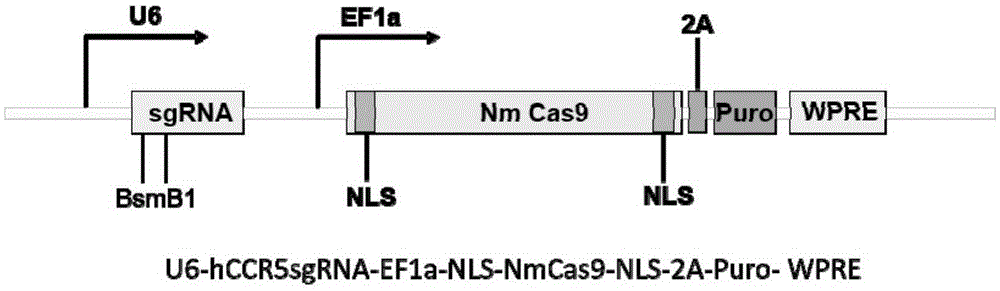
Human CCR5 gene target sequence identified by neisseria meningitidis CRISPR-Cas9 system, sgRNA and application of target sequence and sgRNA
InactiveCN105331609AAchieve preventionAchieve therapeutic effectOrganic active ingredientsHydrolasesGene targetsHIV receptor
The invention belongs to the field of gene engineering and discloses a target sequence identified by a CRISPR-Cas9 system from neisseria meningitidis. The target sequence is as shown in the nth-24th sites of optional one of SEQ ID No. 1-20, and n=1-9. The invention further discloses sgRNA and coding DNA molecules thereof, the sequence of the sgRNA is 5'-identificiation sequence-sequence recruiting Cas9 protein-3', and the DNA sequence corresponding to the identification sequence is identical with the target sequence. The invention also discloses the CRISPR-Cas9 system which comprises the Cas9 protein, the sgRNA and / or a coding sequence carrying the Cas9 protein and a vector of the coding sequence of the sgRNA. The invention also discloses application of the CRISPR-Cas9 system to editing of CCR5 genes and preparation of medicine for HIV infection. By the CRISPR-Cas9 system, the CCR5 genes can be edited, and cells cannot be infected by HIV.
Owner:张竞方
Neisseria meningitidis compositions and methods thereof
ActiveUS20130243807A1Antibacterial agentsBacterial antigen ingredientsSalmonella serotype typhiMeningococcal carriage
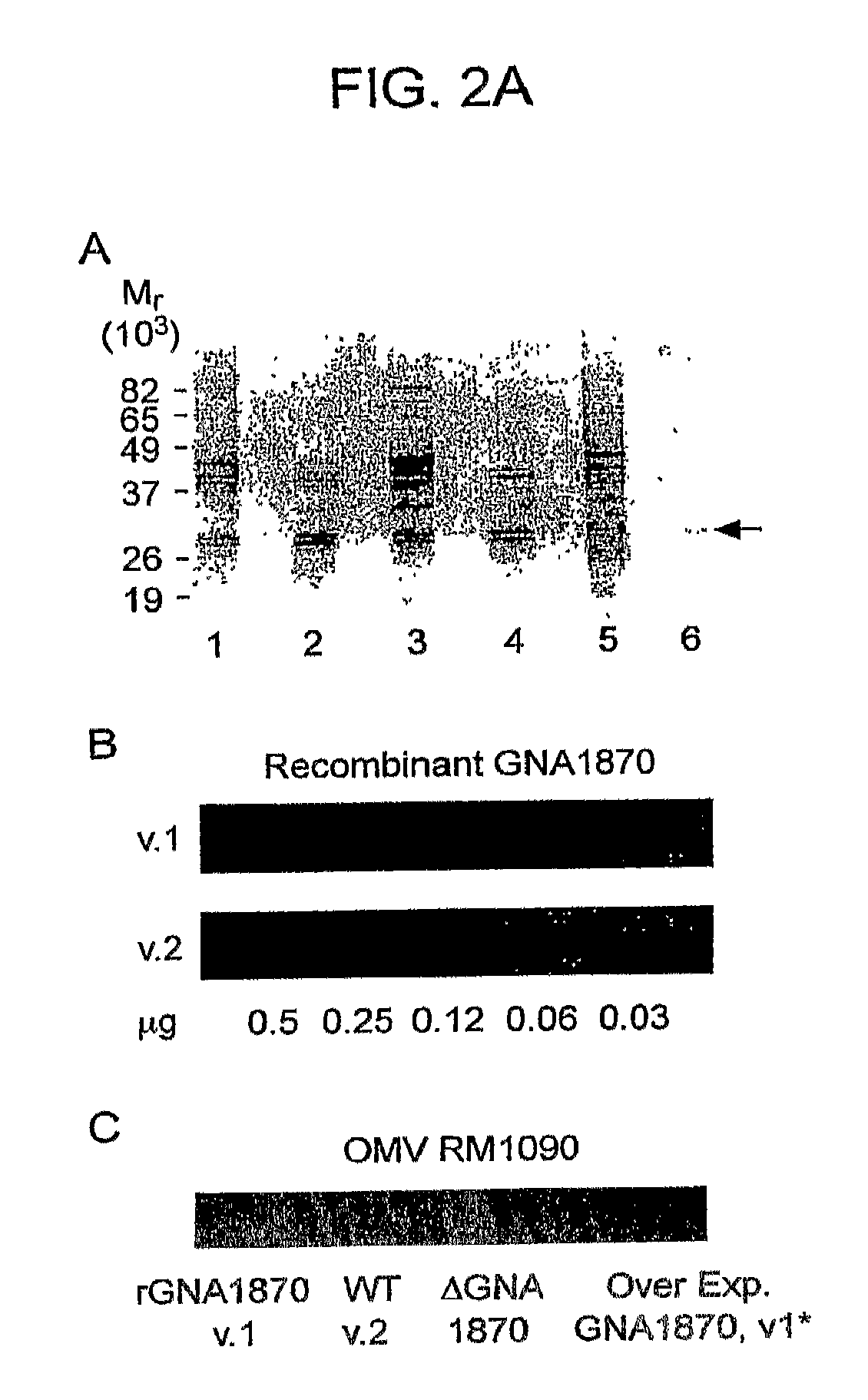
In one aspect, the invention relates to an isolated polypeptide comprising an amino acid sequence that is at least 95% identical to SEQ ID NO: 71. In another aspect, the invention relates to an immunogenic composition including an isolated non-lipidated, non-pyruvylated ORF2086 polypeptide from Neisseria meningitidis serogroup B, and at least one conjugated capsular saccharide from a meningococcal serogroup.
Owner:PFIZER INC
Vaccines comprising aluminium adjuvants and histidine
InactiveUS20050158334A1Easy to controlMinimise adsorptionAntibacterial agentsOrganic active ingredientsAntigenAdjuvant
To improve the stability of vaccines comprising aluminum salt(s), the invention uses the amino acid histidine. This can improve pH stability and adjuvant adsorption and can be reduce antigen hydrolysis. Histidine is preferably presen during adsorption to the aluminium salt(s). The antigen in the vaccine may be a protein or a saccharide and is preferably from N. meningitidis.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
GNA1870-based vesicle vaccines for broad spectrum protection against diseases caused by Neisseria meningitidis
ActiveUS8968748B2Broad protectionReduce expressionAntibacterial agentsBiocideTGE VACCINENeisseria meningitidis
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
Conjugate Vaccines
InactiveUS20080305127A1Improving immunogenicityReduce chain lengthAntibacterial agentsBacterial antigen ingredientsImmunologyHuman patient
The invention provides vaccines against Neisseria meningitidis, pneumococcus and DTPa / w. In particular, it provides vaccines based on conjugated capsular saccharides from multiple meningococcal and / or pneumococcal serogroups. It further provides vaccine administration schemes for the immunisation of human patients with two or more of these vaccines.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Novel immunogenic compositions for the prevention and treatment of meningococcal disease
InactiveUS20110076299A1Antibacterial agentsOrganic active ingredientsDiseaseSalmonella serotype typhi
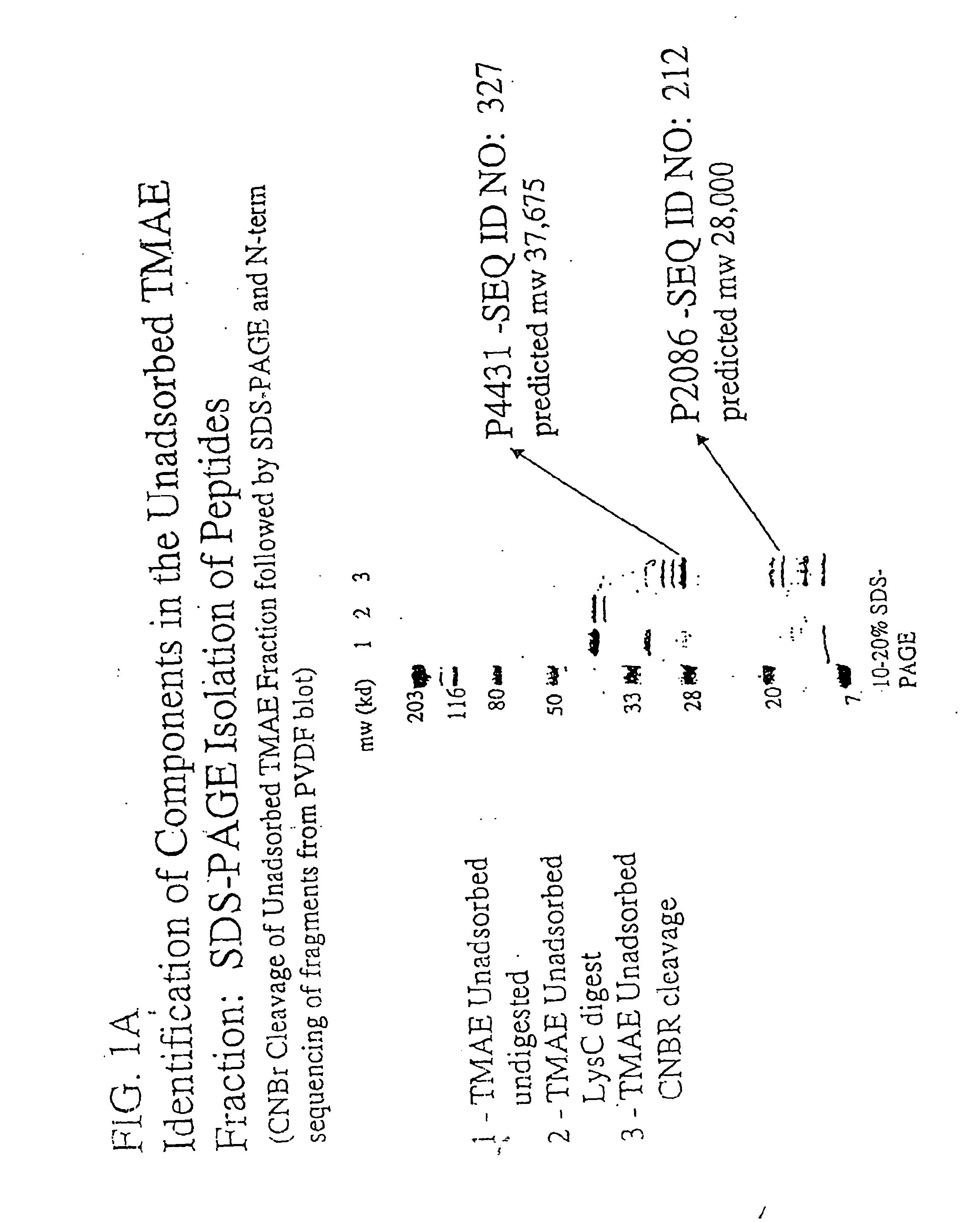
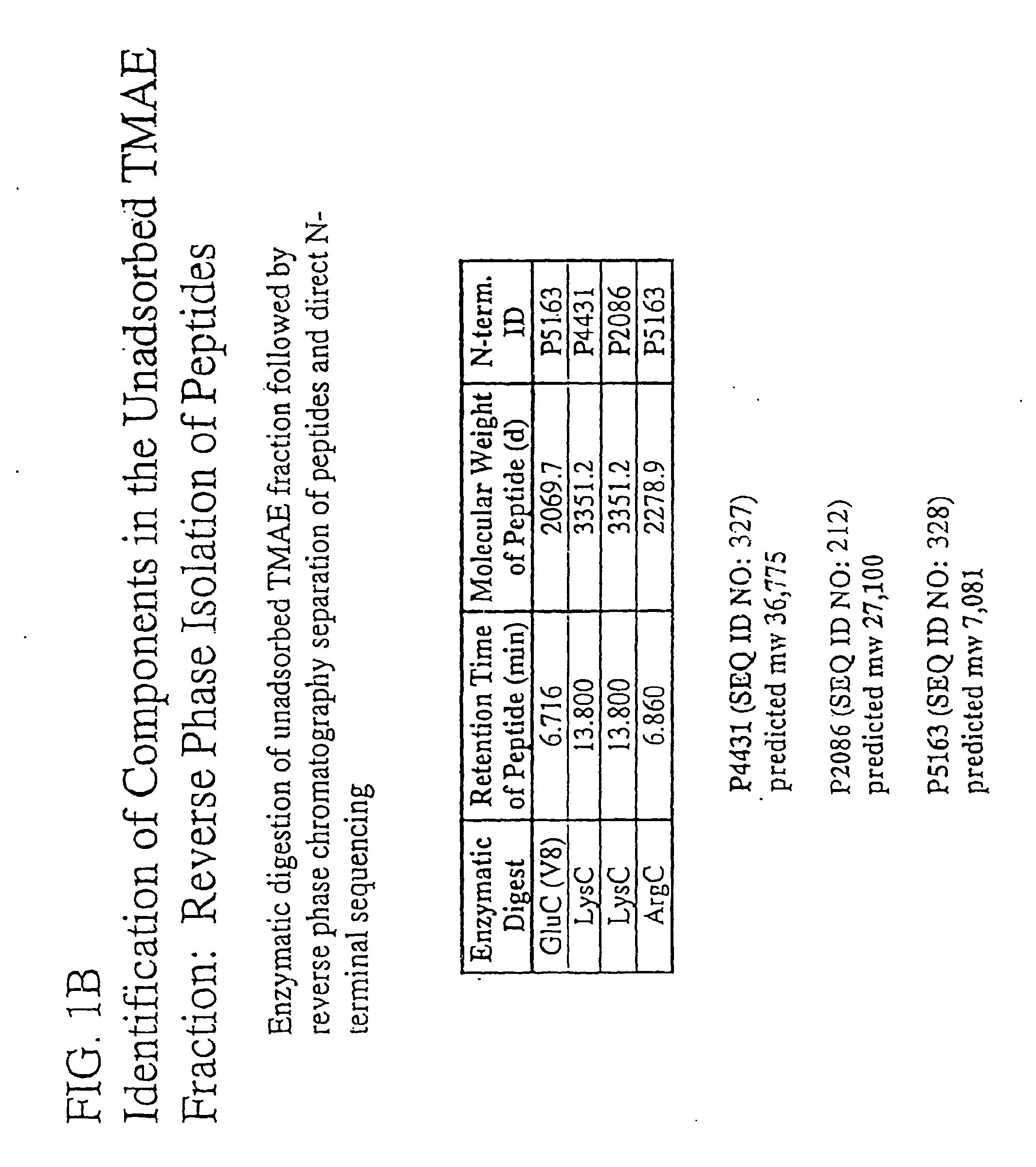
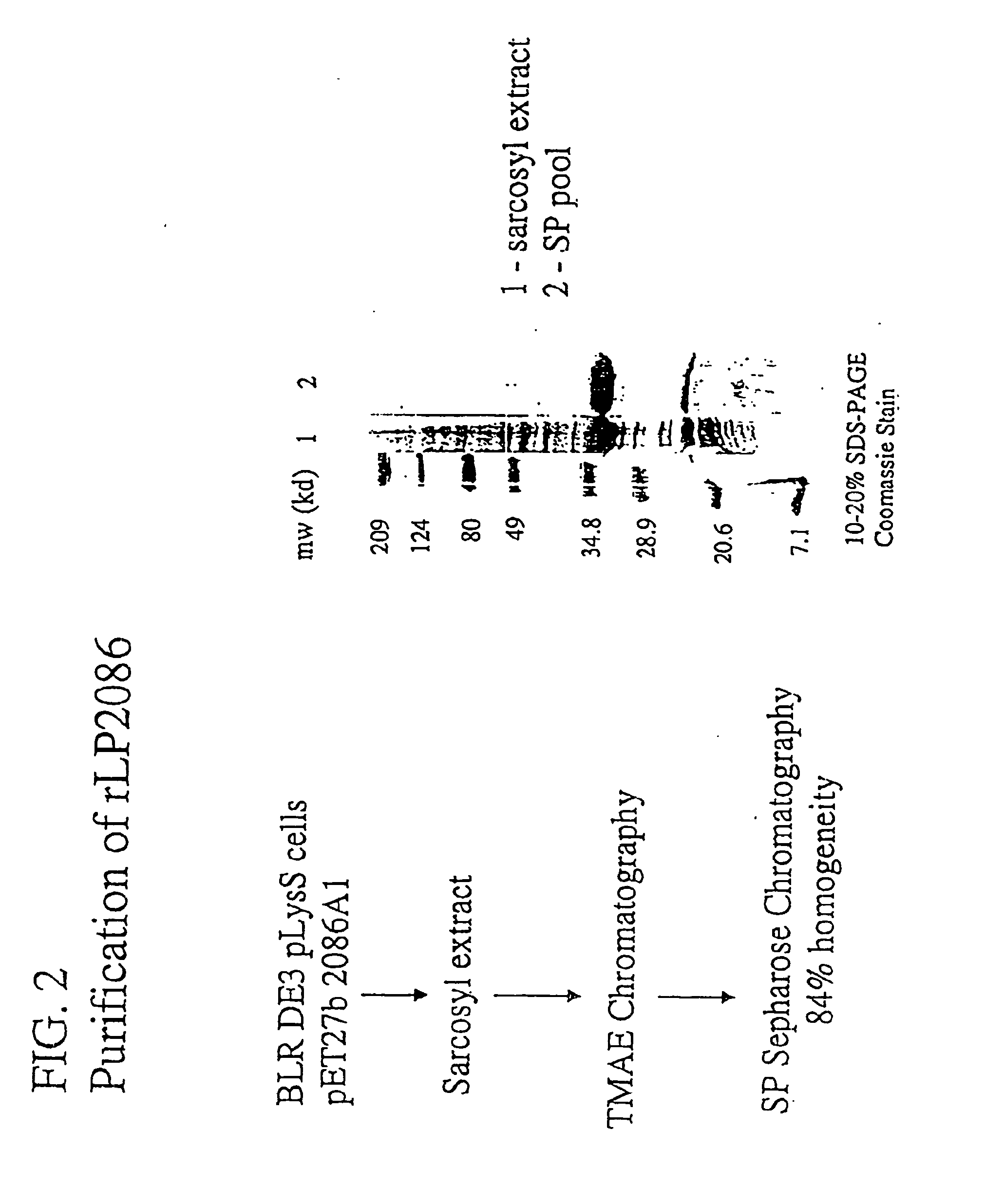
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH HOLDINGS LLC
Bacterial outer memberane vesicles
InactiveUS20060166344A1Good curative effectAvoid problemsAntibacterial agentsBacteriaImmunogenicityTreated cell
Existing methods of meningococcal OMV preparation involve the use of detergent during disruption of the bacterial membrane. According to the invention, membrane disruption is performed substantially in the absence of detergent. The resulting OMVs which retain important bacterial immunogenic components, particularly (i) the protective NspA surface protein, (ii) protein NMB2132 and (iii) protein NMB 1870. A Typical process involves the following steps: (a) treating bacterial cells in the substantial absence of detergent; (b) centrifuging the composition from step (a) to separate the outer membrane vesicles from treated cells and cell debris, and collecting the supernatant; (c) performing a high speed centrifugation of the supernatant from step (b) and collecting the outer membrane vesicles in a pellet; (d) re-dispersing the pellet from step (c) in a buffer; (e) performing a second high speed centrifugation in accordance with step (c), collecting the outer membrane vesicles in a pellet; (f) re-dispersing the pellet from step (e) in an aqueous medium.
Owner:NOVARTIS AG
Multivalent meningococcal derivatized polysaccharide-protein conjugates and vaccine
The present invention describes derivatized polysaccharide-protein conjugates, a composition comprising one or more of such derivatized polysaccharide-protein conjugates and methods of immunizing human patients with the same. The derivatized polysaccharide-protein conjugates are purified capsular polysaccharides from Neisseria meningitidis serogroups A, C, W-135, and Y, derivatized chemically activated and selectively attached to a carrier protein by means of a covalent chemical bond, forming polysaccharide-protein conjugates capable of eliciting long-lasting immunity to a variety of N. meningitidis strains.
Owner:AVENTIS PASTEUR INC
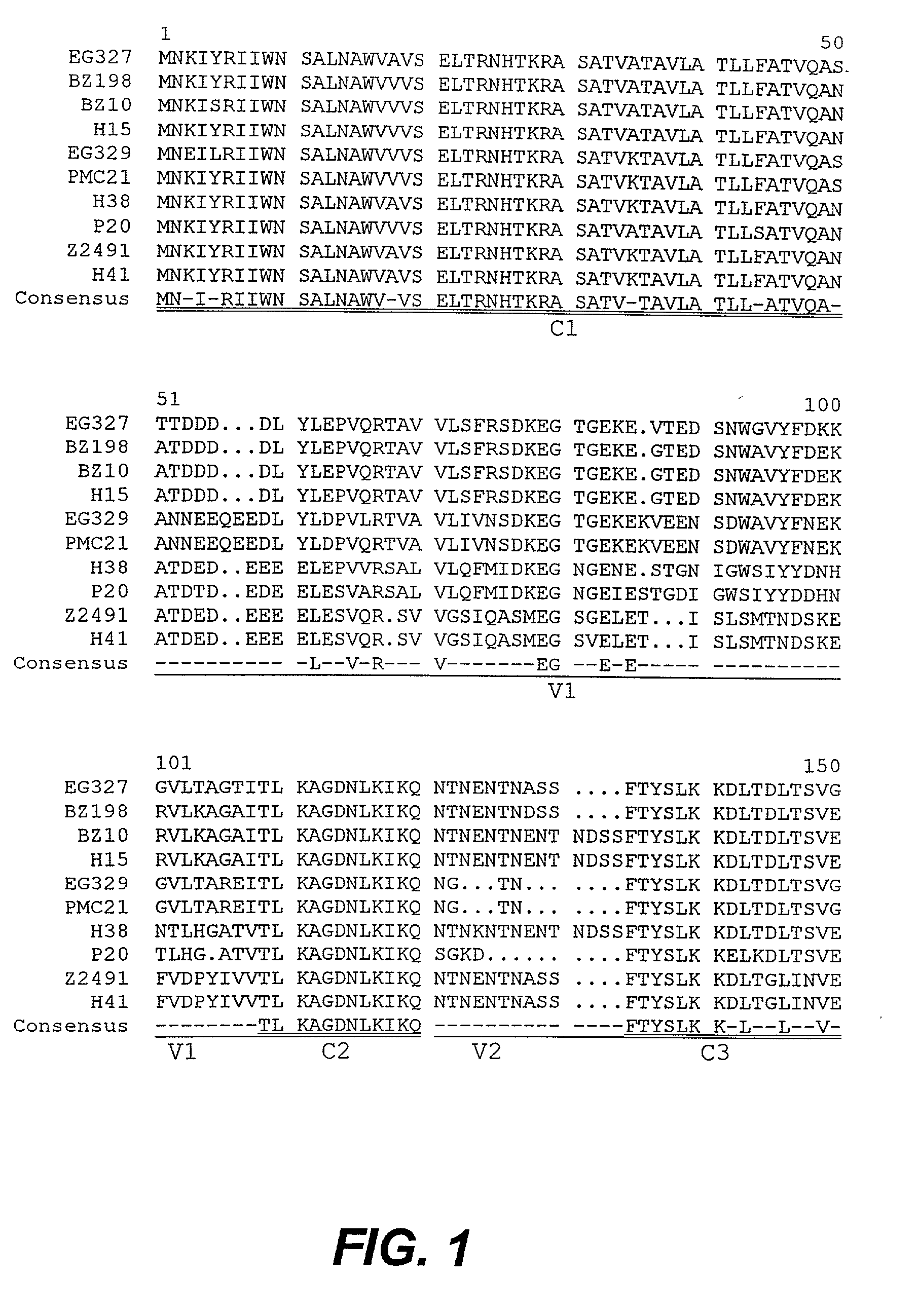
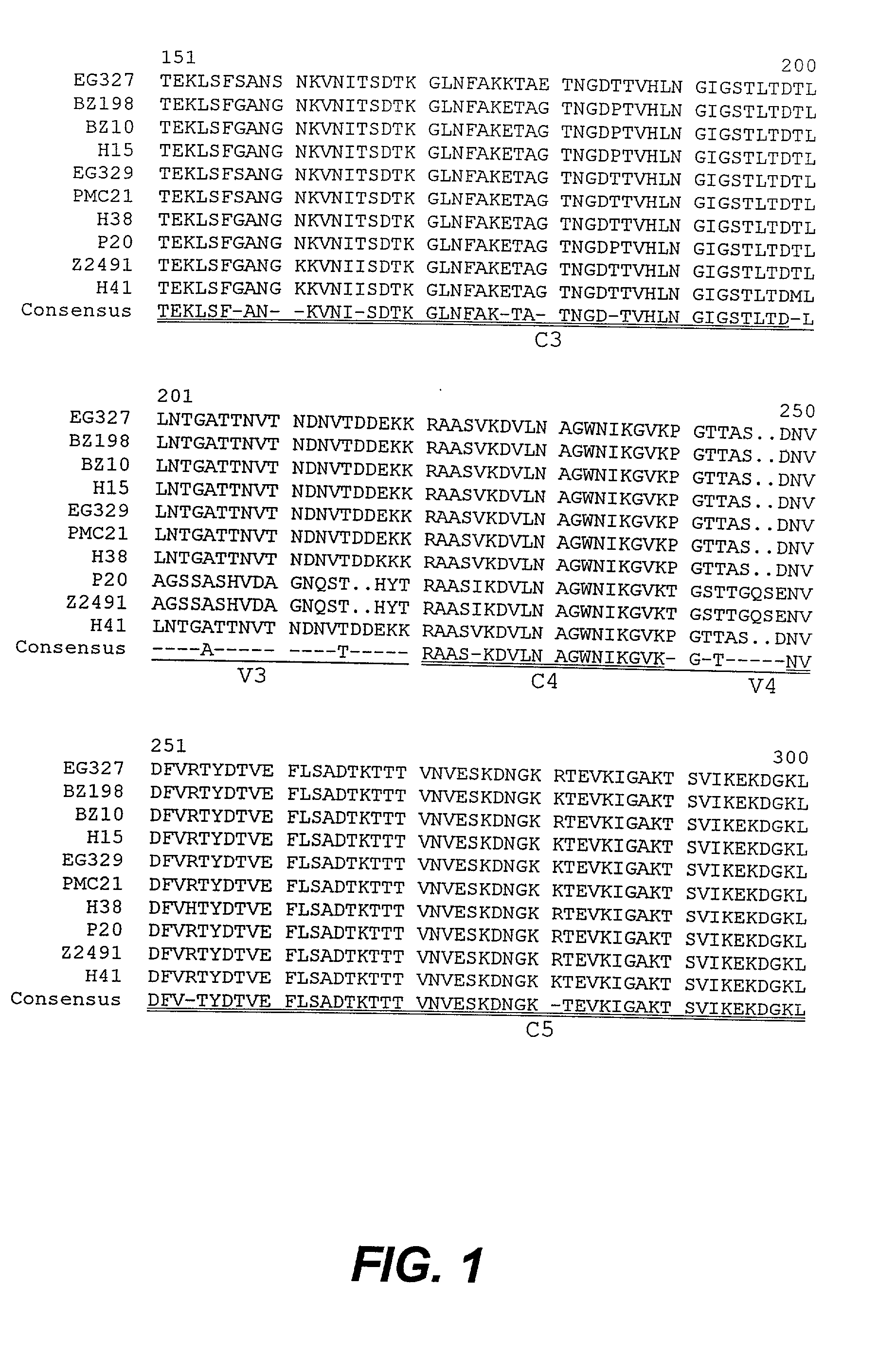
Modified surface antigen
Novel proteins that constitute modified forms of a Neisseria meningitidis surface antigen and encoding nucleic acids are provided. The modified surface proteins are characterized by having deletions of non-conserved amino acids, and thereby being capable of eliciting cross-protective immune responses against Neisseria meningitidis. The invention extends to the use of the modified surface antigens in diagnostics, in therapeutic and prophylactic vaccines and in the design and / or screening of medicaments. The modified surface antigens are particularly useful in vaccines which effectively immunize against a broader spectrum of N. meningitidis strains than would be expected from a corresponding wild-type surface antigen.
Owner:QUEENSLAND THE UNIV OF
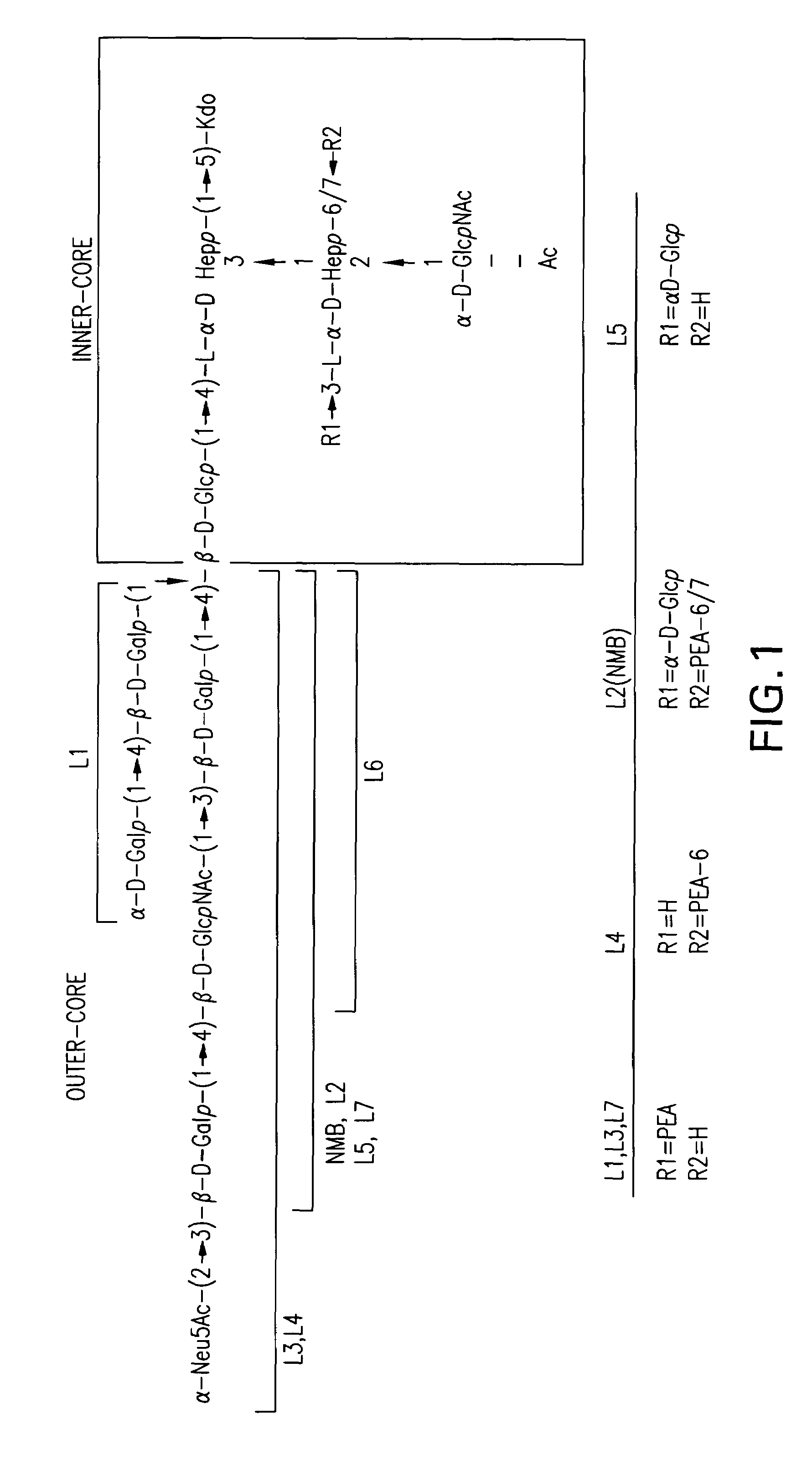
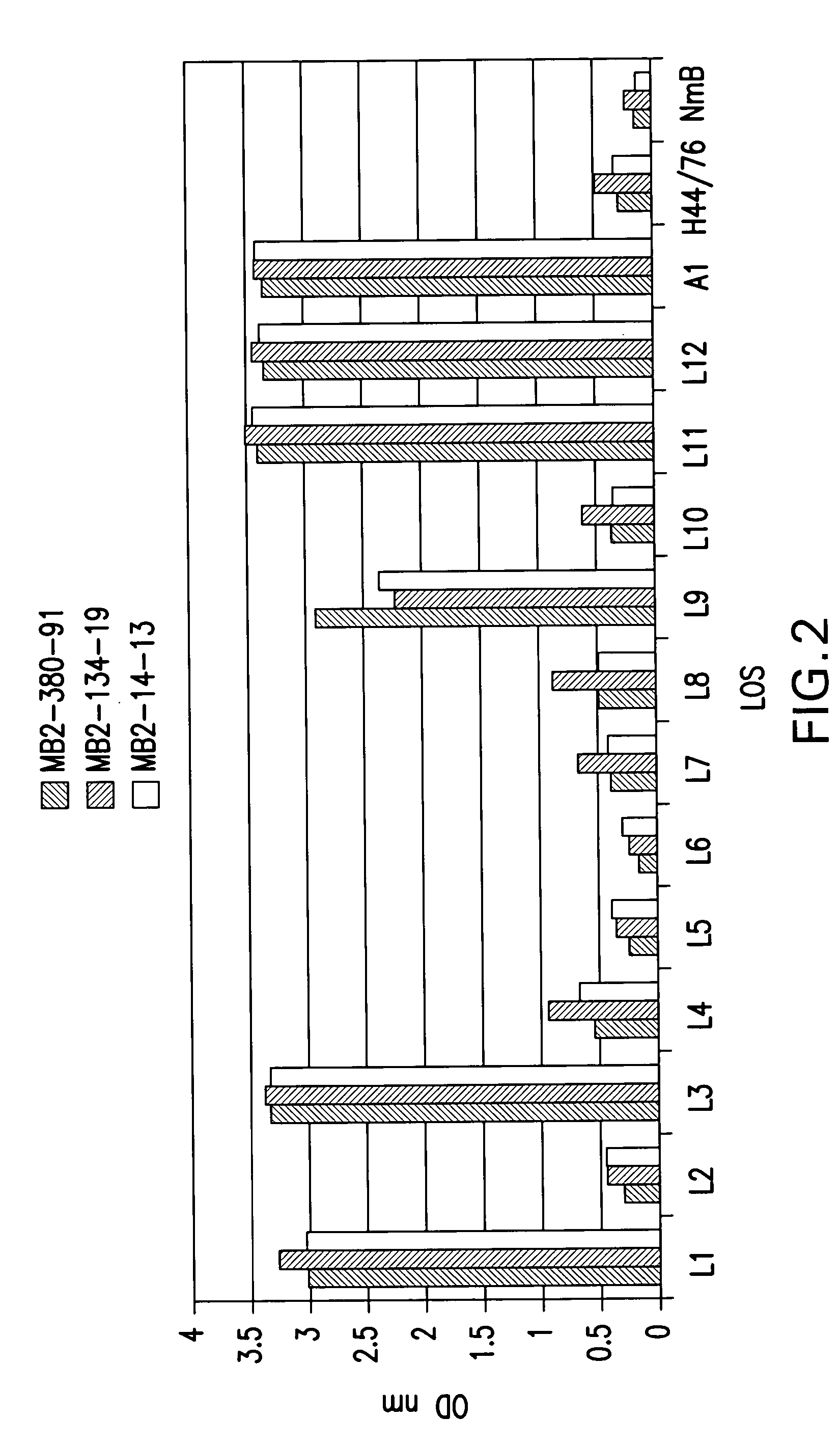
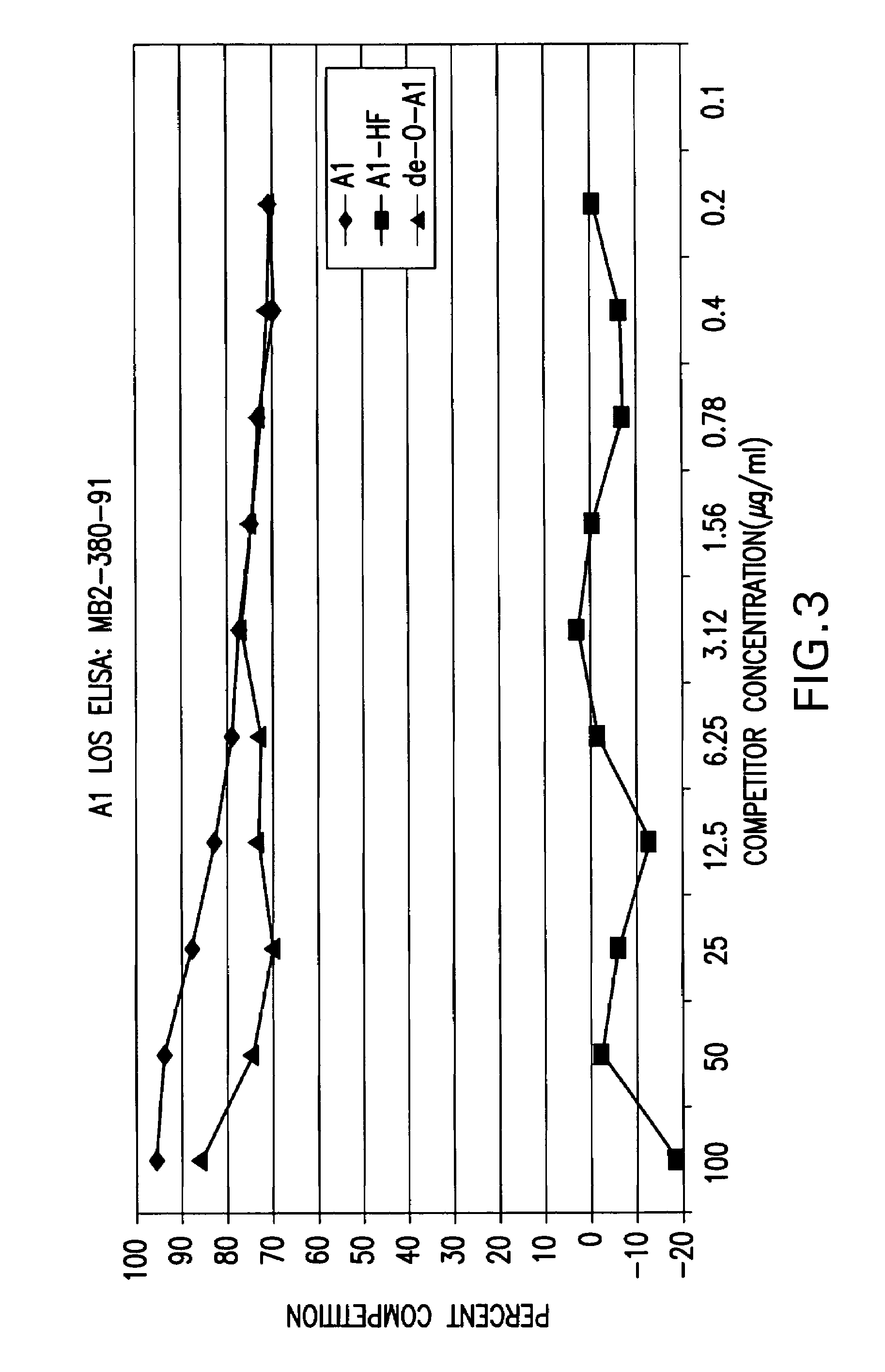
Neisseria meningitdis inner core lipo-oligosaccharide epitopes, multivalent conjugates thereof and immunogenic compositions thereof
The present invention is directed to novel Neisseria meningitidis lipo-oligosaccharide inner core molecules, conjugates thereof and immunogenic compositions thereof. In particular embodiments, the invention relates to multivalent immunogenic compositions comprising five distinct Neisseria meningitidis inner core lipo-oligosaccharide epitope groups, wherein the multivalent compositions induce cross-reactive immune responses against Neisseria meningitidis lipo-oligosaccharide immunotypes.
Owner:WYETH LLC
Novel Immunogenic Compositions for the Prevention and Treatment of Meningococcal Disease
InactiveUS20070253964A1Antibacterial agentsPeptide/protein ingredientsNucleic acid sequencingImmunogenicity
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from neisserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH LLC
Neisseria meningitidis serogroup a capsular polysaccharide acetyltransferase, methods and compositions
InactiveUS20060073168A1Easy to optimizeComplete acetylationAntibacterial agentsBacteriaSalmonella serotype typhiAcetyltransferase
Provided are recombinant DNA molecules that do not occur in nature encoding an O-acetyltransferase, vectors that direct expression of an O-acetyltransferase, recombinant host cells which express an O-acetyltransferase, methods for recombinant production of an O-acetyltransferase, methods for acetylating capsular polysaccharides, especially those of a Serogroup A Neisseria meningitidis using a recombinant O-acetyltransferase, and immunogenic compositions comprising the acetylated capsular polysaccharide.
Owner:EMORY UNIVERSITY +1
Neisseria meningitidis compositions and methods thereof
In one aspect, the invention relates to a composition including a first polypeptide having the sequence set forth in SEQ ID NO: 1 and a second polypeptide having the sequence set forth in SEQ ID NO: 2. In one embodiment, the composition includes about 120 μg / ml of a first polypeptide including the amino acid sequence set forth in SEQ ID NO: 1, 120 μg / ml of a second polypeptide including the amino acid sequence set forth in SEQ ID NO: 2, about 2.8 molar ratio polysorbate-80 to the first polypeptide, about 2.8 molar ratio polysorbate-80 to the second polypeptide, about 0.5 mg / ml aluminum, about 10 mM histidine, and about 150 mM sodium chloride. In one embodiment, a dose of the composition is about 0.5 ml in total volume. In one embodiment, two-doses of the composition induce a bactericidal titer against diverse heterologous subfamily A and subfamily B strains in a human.
Owner:PFIZER INC
Meningococcal fhbp polypeptides
ActiveUS20110020390A1Stable maintenanceSugar derivativesBacteriaAntiendomysial antibodiesMeningococcal carriage
fHBP is a protein in Neisseria meningitidis. Three families of fHBP are known. To increase the ability of a fHBP protein to elicit antibodies that are cross-reactive between the families, fHBP is selected or engineered to have a sequence which can elicit broad-spectrum bactericidal anti-meningococcal antibodies after administration to a host animal.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Polysaccharide derivatives and uses in induction of an immune response
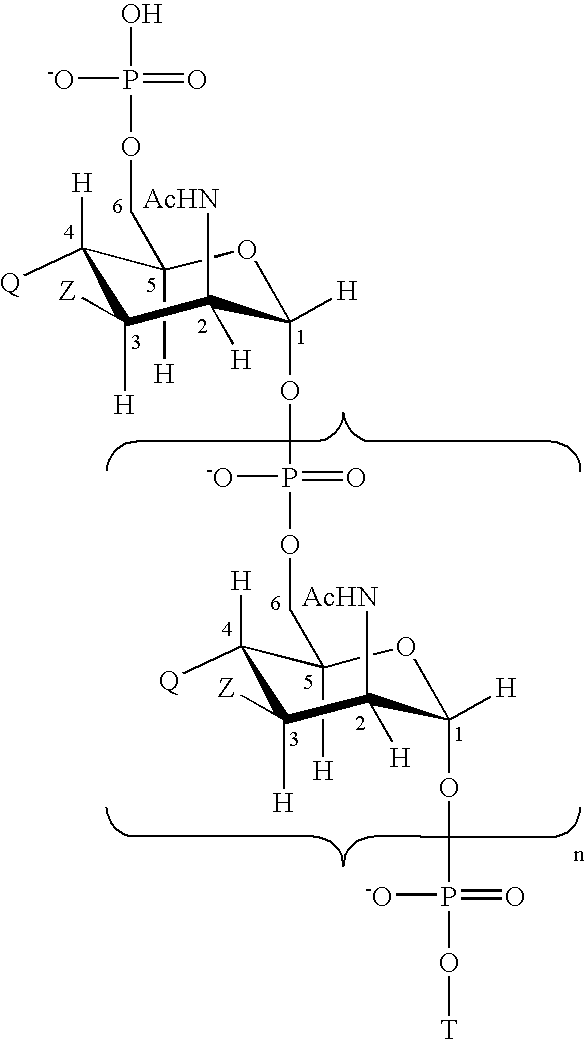
The present invention generally provides compositions comprising a polysaccharide derivative, and methods of their preparation and use for the prevention or treatment of diseases caused by Neisseria meningitidis bacteria, particularly group B (NmB) strains, and by E. coli K1. The invention provides a de-N-acetylated PS derivative in which one or more residues of the PS has been modified by de-N-acetylation. The invention also includes derivatives in which one or more of the N-acetyl groups of PS containing de-N-acetylated PS are replaced with other N-acyl groups, usually a lower acyl group of C2-C3. Further, the invention includes de-N-acetylated PS derivatives containing long chain hydrocarbons, as well as conjugates in which the de-N-acetylated PS derivative is linked to a carrier, e.g., a carrier protein.
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
Molecular mimetics of meningococcal B epitopes which elicit functionally active antibodies
Molecular mimetics of a surface-exposed epitope on loop 4 of PorA of Neisseria meningitidis serogroup B (MenB) P1.2 serosubtype and antibodies produced against the same are disclosed. Compositions containing such molecular mimetics or the antibodies thereto can be used to prevent MenB disease, as well as for diagnosis of MenB infection.
Owner:CHIRON CORP
Microparticles with adsorbed polypeptide-containing molecules
ActiveUS7501134B2Easy to produceSsRNA viruses negative-senseAntibacterial agentsHemagglutininLactide
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Vaccine composition
InactiveUS20060216307A1UpregulationReducing lipid A toxicityAntibacterial agentsSenses disorderProtective antigenBacteroides
The present invention relates to an immuno-protective and non-toxic Gram-negative bleb vaccine suitable for paediatric use. Examples of the Gram-negative strains from which the blebs are made are N. meningitidis, M. catarrhalis and H. influenzae. The blebs of the invention are improved by one or more genetic changes to the chromosome of the bacterium, including up-regulation of protective antigens, down-regulation of immunodominant non-protective antigens, and detoxification of the Lipid A moiety of LPS.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Neisseria genomic sequences and methods of their use
InactiveUS20050191316A1Easy to insertEfficient HarvestingAntibacterial agentsBacteriaSAA proteinNucleotide
The invention provides methods of obtaining immunogenic proteins from genomic sequences including Neisseria, including the amino acid sequences and the corresponding nucleotide sequences, as well as the genomic sequence of Neisseria meningitidis B. The proteins so obtained are useful antigens for vaccines, immunogenic compositions, and / or diagnostics.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Neisseria meningitidis capsular polysacchride conjugates
InactiveUS6080589AEnhance host immune responseImproving immunogenicityBiocideOrganic active ingredientsChemical reactionSialic acid
Capsular polysaccharides containing multiple sialic acid residues, particularly the Group B polysaccharide of Neisseria meningitidis, are modified by chemical reaction to randomly introduce pendant reactive residues of heterobifunctional linker molecules to the polysaccharide backbone. The capsular polysaccharide is deacetylated and the heterobifunctional linker molecule is reacted with the deacetylated material and any residual amino groups are blocked by reaction with alkyl acid anhydride. The introduction of the linker molecules to the polysaccharide chain between the termini enables the polysaccharide to be linked to a carrier molecule, such as a protein, to enhance the immunogenicity of the polysaccharide. The conjugate molecule may be formulated as an immunogenic composition for raising antibodies in a host to the polysaccharide.
Owner:CONNAUGHT LAB
MENINGOCOCCAL fHBP POLYPEPTIDES
InactiveUS20130022633A1Improved antigenHigh affinity bindingAntibacterial agentsBacteriaMeningococcal carriageAmino acid
The factor H binding activity of meningococcal fHBP can be uncoupled from its bactericidal sensitivity. NMR studies have identified various amino acid residues involved in the fHBP / fH interaction and one or more of these residues is modified in a fHBP to reduce or eliminate its ability to bind to fH.
Owner:UNIVERSITY OF FLORENCE +1
A preparing method of a recombinant fusion protein modified with bacterial polysaccharides and applications thereof
ActiveCN105695497AUniform polysaccharide binding siteQuality controllableAntibacterial agentsBacterial antigen ingredientsConjugate vaccineBacteroides
A preparing method of a recombinant fusion protein modified with bacterial polysaccharides and applications thereof are disclosed. The method includes co-expressing a recombinant fusion protein and neisseria meningitide O-oligosaccharyltransferase PglL in a bacterium with O-antigen ligase gene defect, and allowing polysaccharides of the bacterium or exogenous polysaccharides to be connected to the recombinant fusion protein through the neisseria meningitide O-oligosaccharyltransferase PglL to obtain the recombinant fusion protein modified with the bacterial polysaccharides. A specific antibody resisting bacterial polysaccharides can be prepared through immunizing mice with the prepared recombinant fusion protein modified with the bacterial polysaccharides. Through applying the recombinant fusion protein modified with the bacterial polysaccharides to preparation of bacterial polysaccharide protein conjugate vaccines, a plurality of problems of culturing of pathogenic bacteria can be avoided, homogeneity and a production efficiency of the vaccines can be increased and a vaccine preparing cost can be reduced.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Neisseria meningitidis compositions and methods thereof
ActiveUS20180214532A1Elicit immune responseAntibacterial agentsOrganic active ingredientsNeisseria meningitidisPolysaccharide
In one aspect, the invention relates to a composition including a factor H binding protein (fHBP) and a Neisseria meningitidis non-serogroup B capsular polysaccharide. The invention further relates to uses of a composition that includes fHBP, such as, for example, uses to elicit an immune response against N. meningitidis serogroup B strains and non-serogroup B strains. The compositions and methods described herein are directed to administration in humans, including adults, adolescents, toddlers, and infants.
Owner:PFIZER INC
Multiple vaccination including Serogroup C Meningococcus
ActiveUS20100034847A1Accurate measurementAvoid potential suppression effectAntibacterial agentsBacterial antigen ingredientsVaccinationAdjuvant
Various improvements to vaccines that include a serogroup C meningococcal conjugate antigen, including: (a) co-administration with acellular B. pertussis antigen; (b) co-administration with an inactivated poliovirus antigen; (c) supply in a kit together with a separate pneumococcal conjugate component, which may be in a liquid form; and (d) use in combination with a pneumococcal conjugate antigen but without an aluminium phosphate adjuvant. A kit may have: (a) a first immunogenic component that comprises an aqueous formulation of a conjugated capsular saccharide from Streptococcus pneumoniae; (b) a second immunogenic component that comprises a conjugated capsular saccharide from Neisseria meningitidis serogroup C.
Owner:GSK VACCINES GMBH
Neisseria meningitidis inner core lipo-oligosaccharide epitopes, multivalent conjugates thereof and immunogenic compositions thereof
The present invention is directed to novel Neisseria meningitidis lipo-oligosaccharide inner core molecules, conjugates thereof and immunogenic compositions thereof. In particular embodiments, the invention relates to multivalent immunogenic compositions comprising five distinct Neisseria meningitidis inner core lipo-oligosaccharide epitope groups, wherein the multivalent compositions induce cross-reactive immune responses against Neisseria meningitidis lipo-oligosaccharide immunotypes.
Owner:WYETH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com