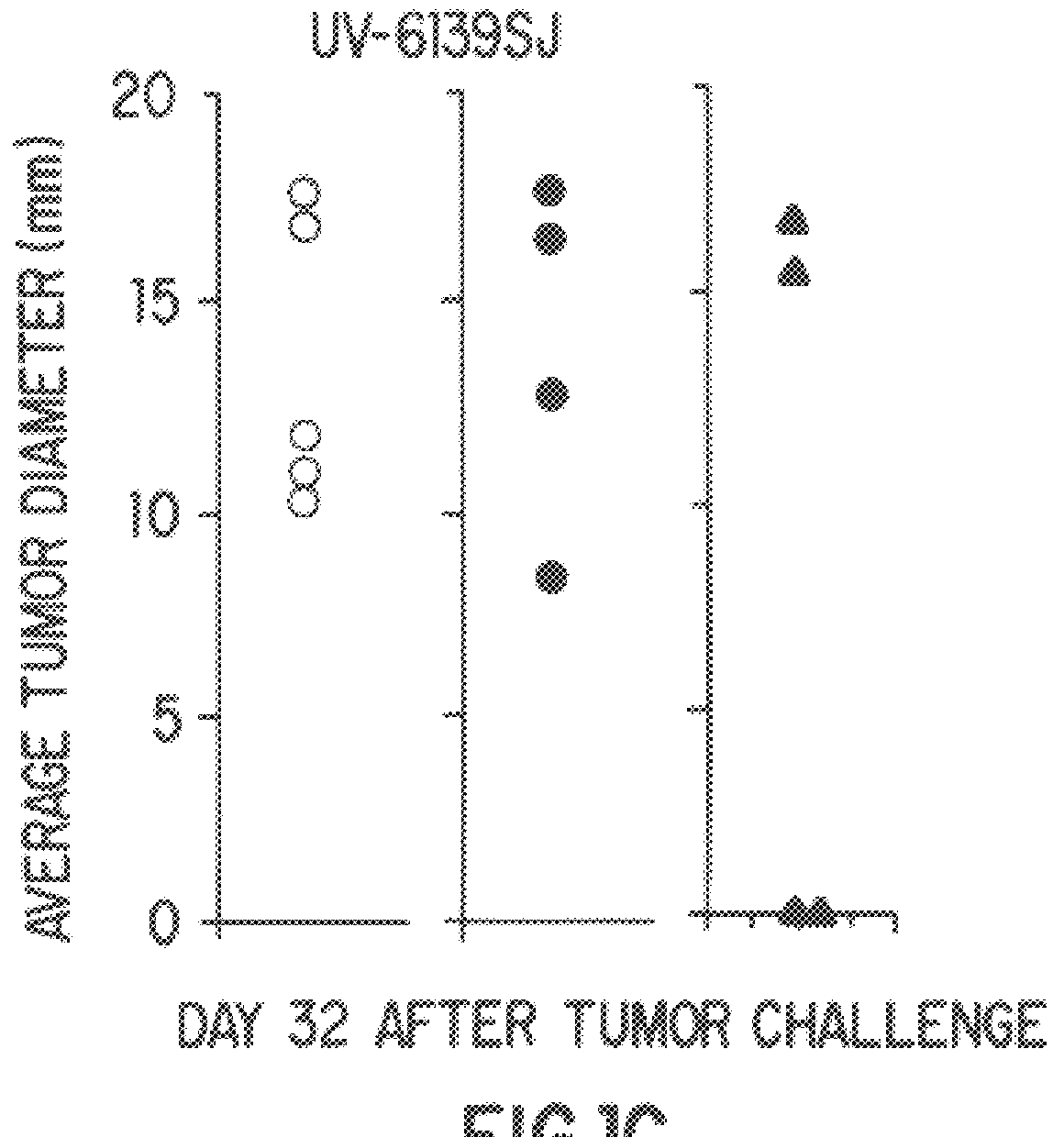
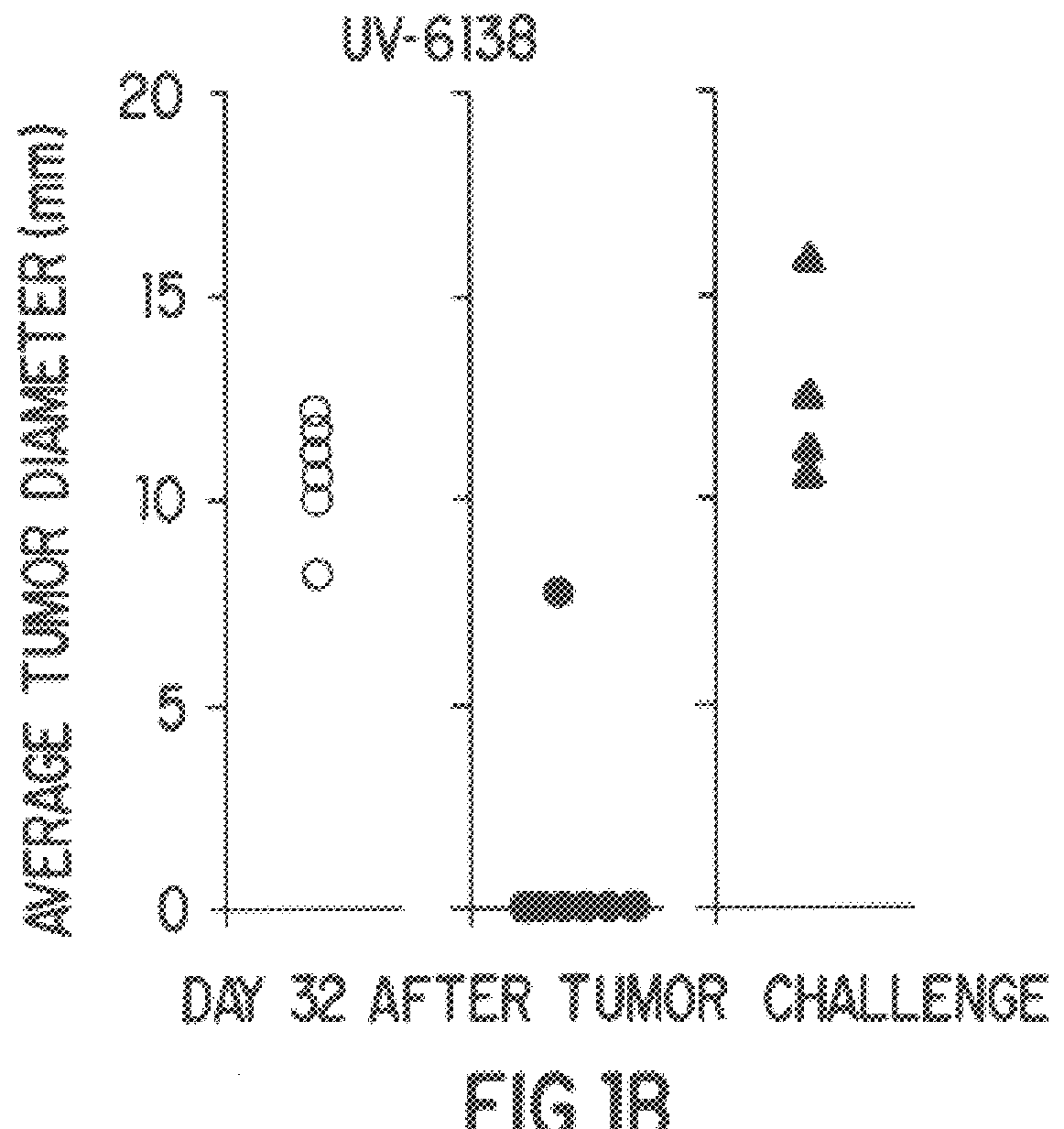
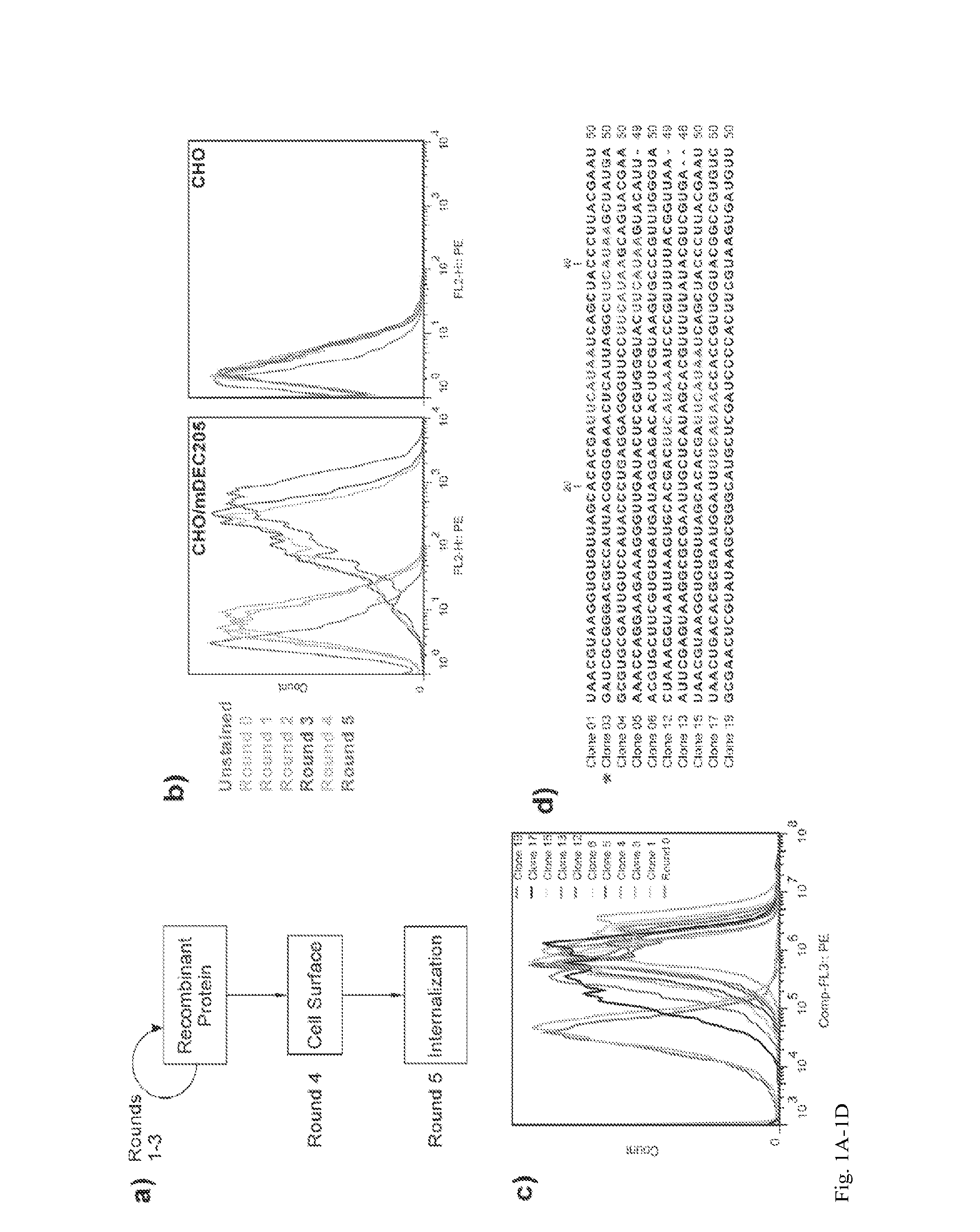
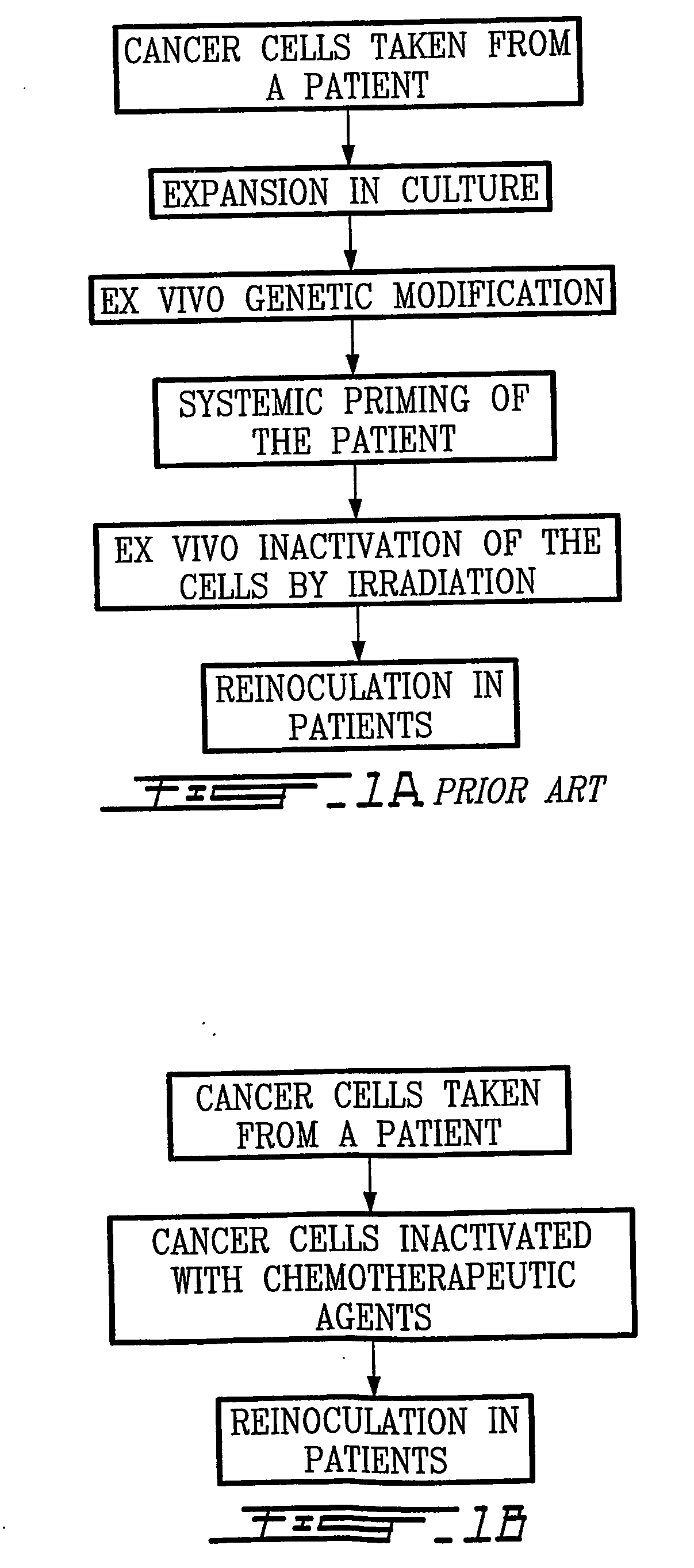
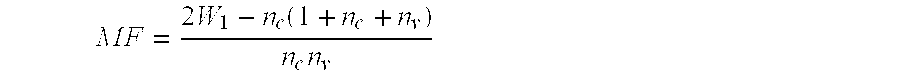Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
76results about How to "Elicit immune response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
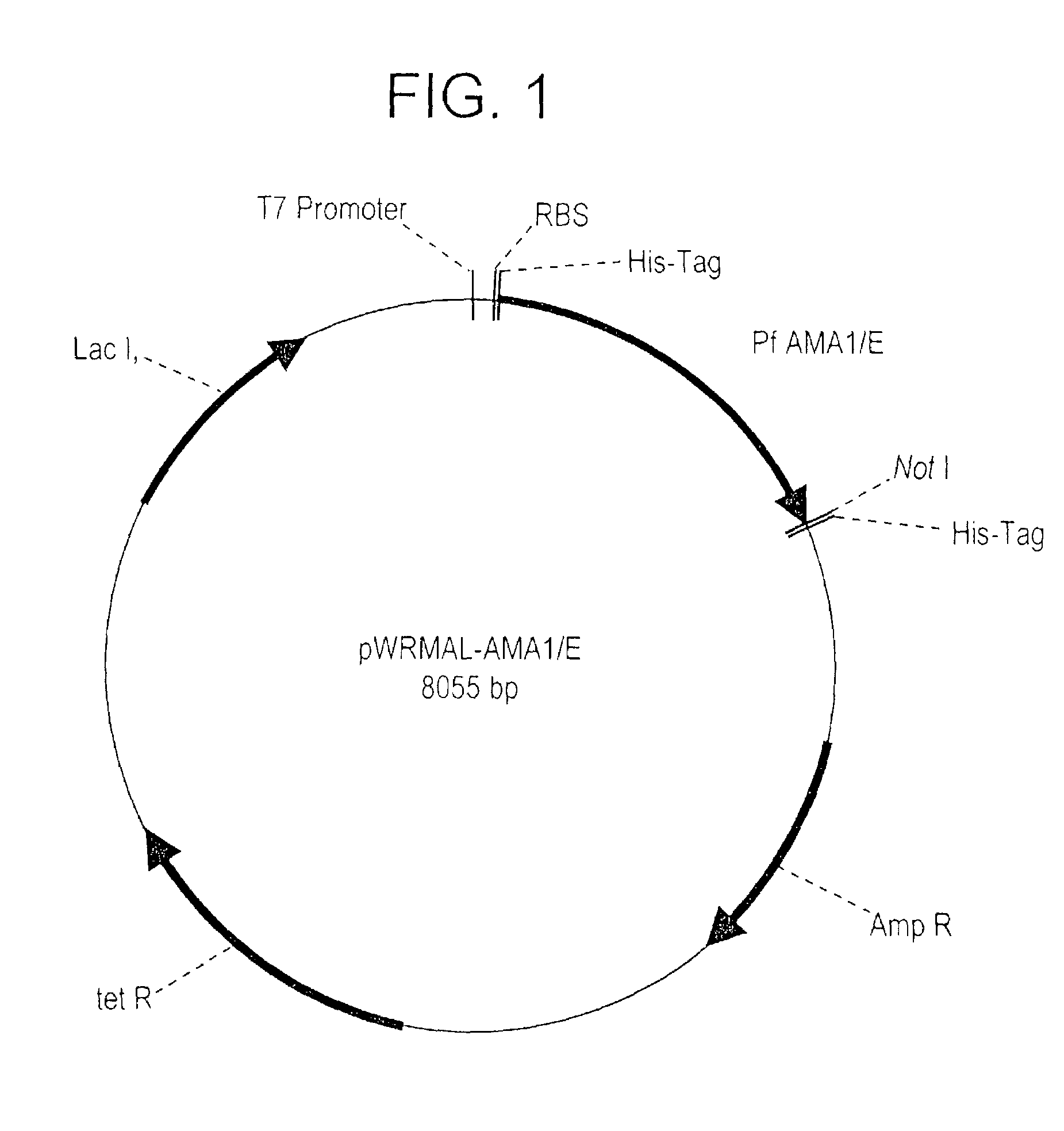
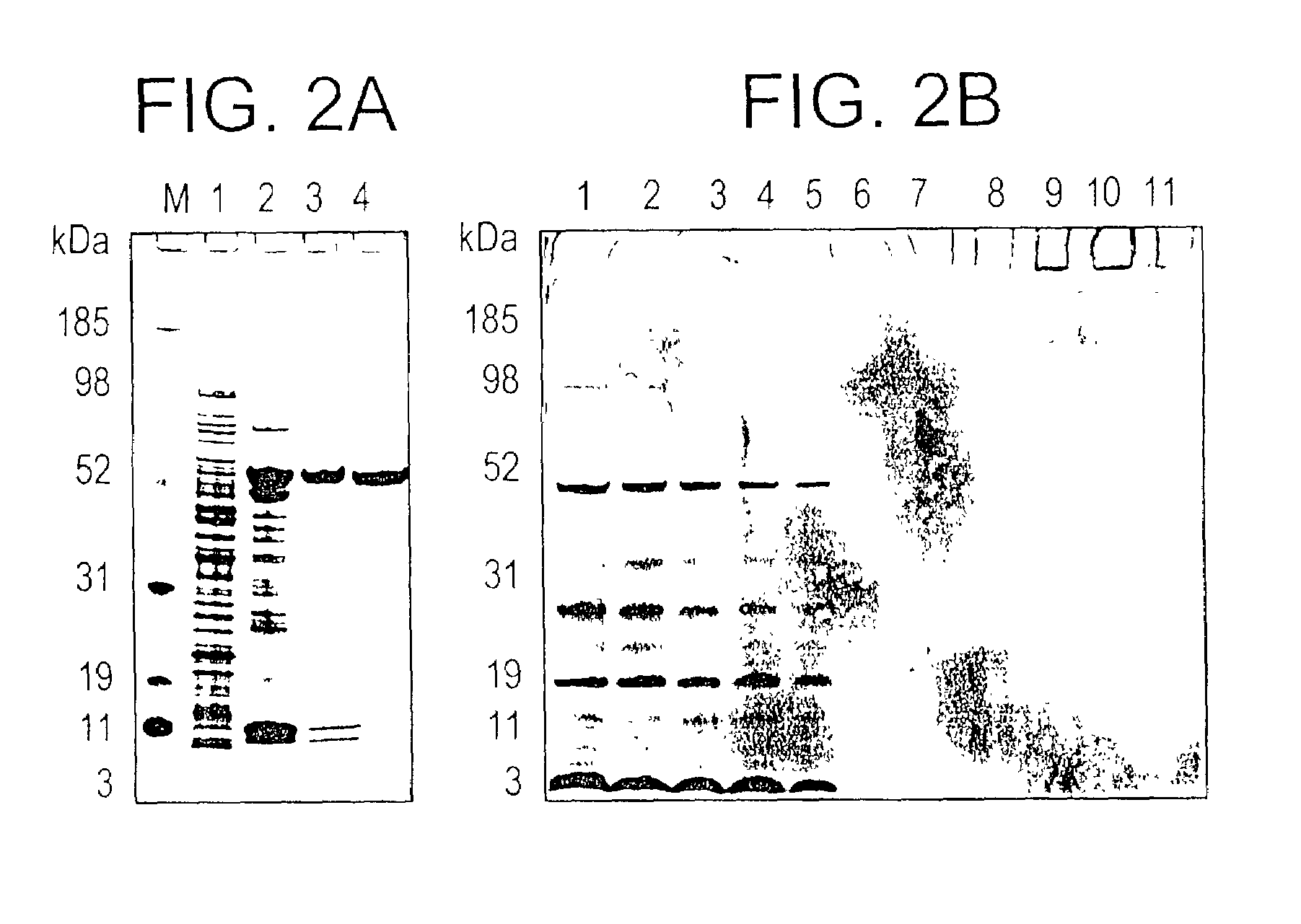
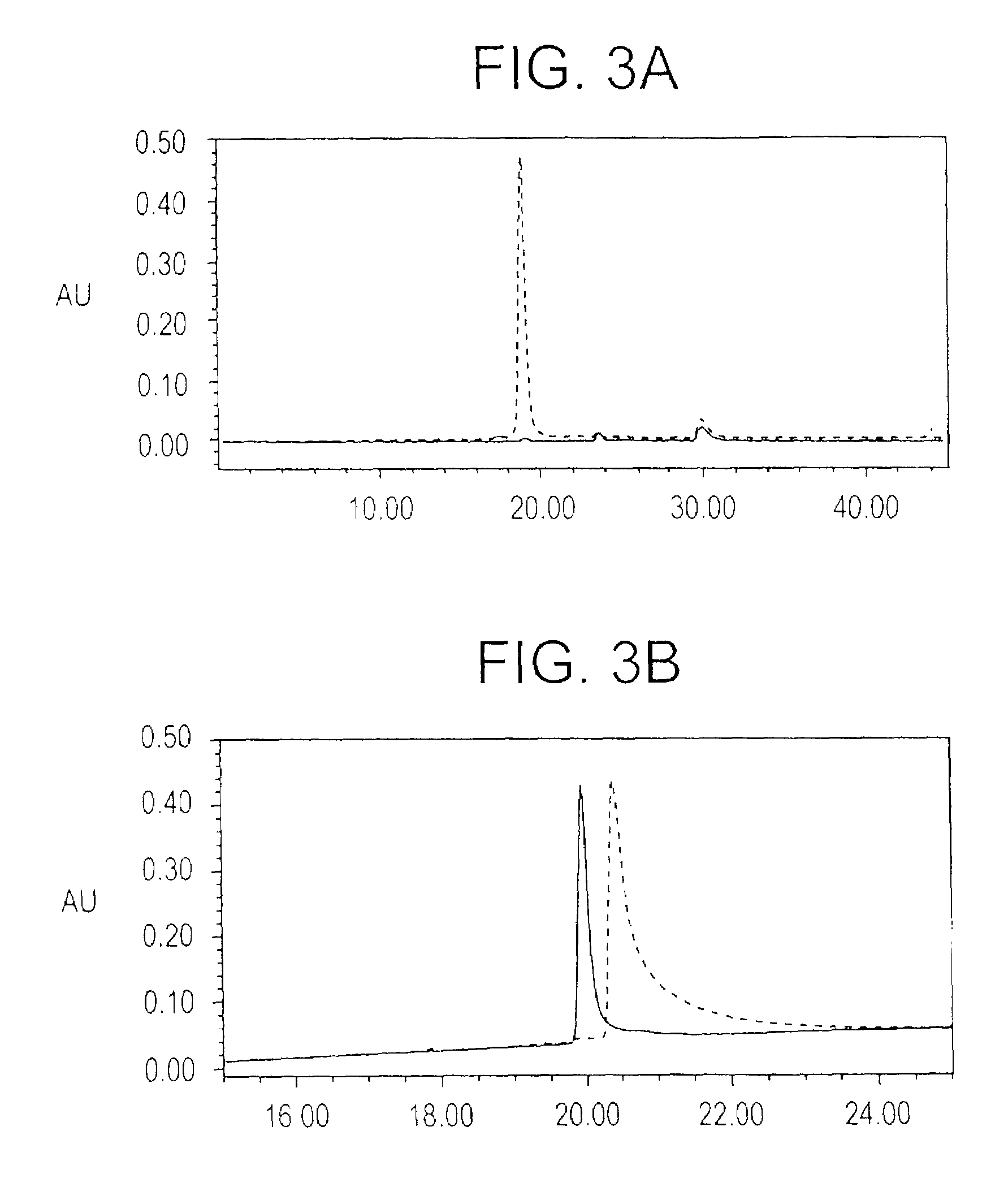
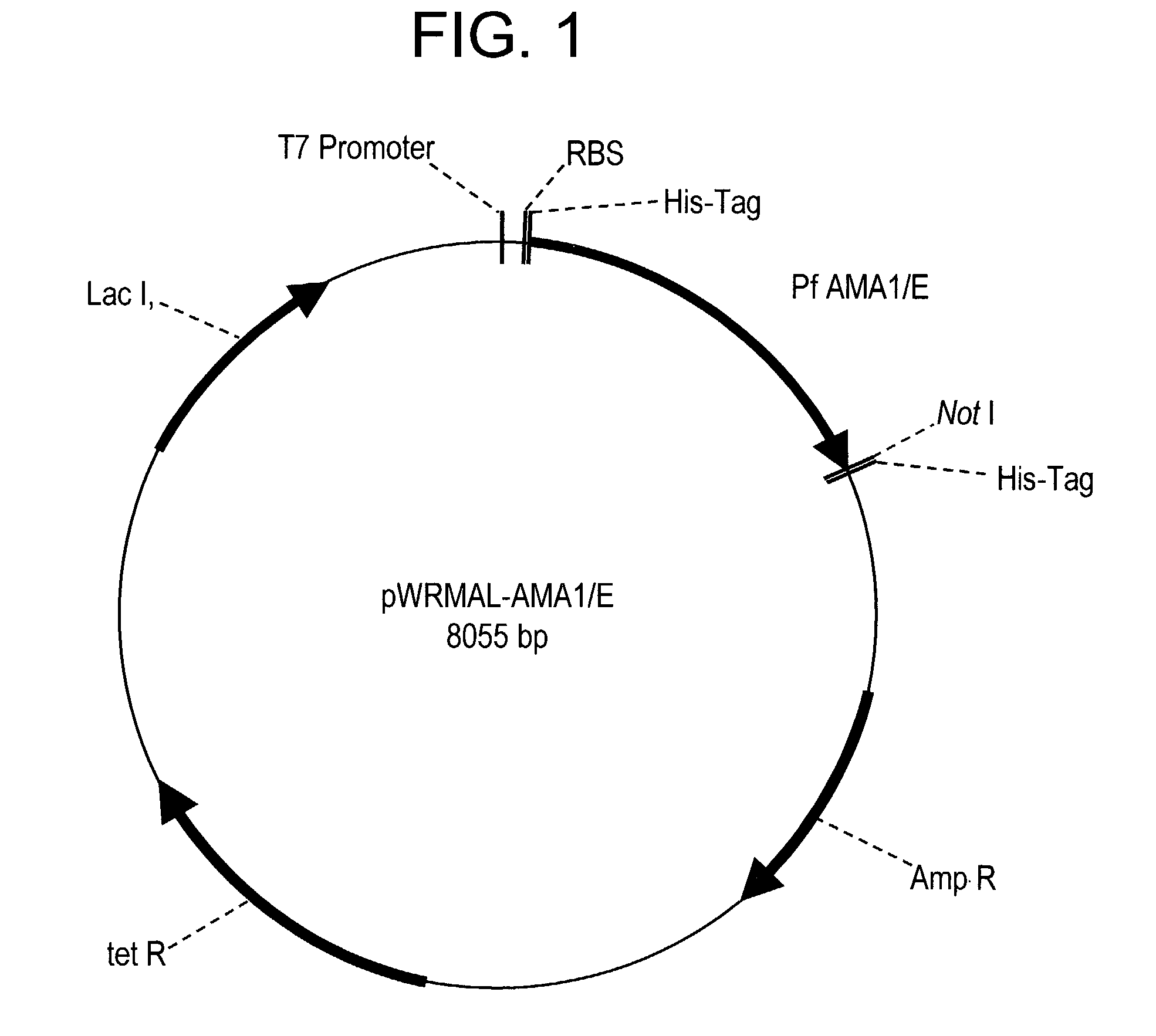
Plasmodium falciparum AMA-1 protein and uses thereof
InactiveUS7029685B2Eliminate the problemImprove responseProtozoaFermentationADAMTS ProteinsMalarial parasites
In this application is described the expression and purification of a recombinant Plasmodium falciparum (3D7) AMA-1 ectodomain. The method of the present invention produces a highly purified protein which retains folding and disulfide bridging of the native molecule. The recombinant AMA-1 is useful as a diagnostic reagent, for use in antibody production, and as a protein for use alone, or as part of, a vaccine to prevent malaria.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Plasmodium falciparum AMA-1 protein and uses thereof
InactiveUS7060276B2Eliminate the problemImprove responseSugar derivativesViral antigen ingredientsADAMTS ProteinsPlasmodium falciparum
In this application is described the expression and purification of a recombinant Plasmodium falciparum (3D7) AMA-1 ectodomain. The method of the present invention produces a highly purified protein which retains folding and disulfide bridging of the native molecule. The recombinant AMA-1 is useful as a diagnostic reagent, for use in antibody production, and as a vaccine.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Complexes of peptide-binding fragments of heat shock proteins and their use as immunotherapeutic agents
InactiveUS20010034042A1Elicit immune responsePrevent and substantially inhibit growthAntibacterial agentsBiocideDrugPeptide
The present invention relates to pharmaceutical compositions comprising peptide-binding fragments of heat shock proteins (HSPs) and noncovalent complexes of peptide-binding fragments of HSPs in noncovalent association with antigenic molecules. The invention further relates to methods for the use of such pharmaceutical compositions as immunotherapeutic agents for the treatment and prevention of infectious diseases and cancer.
Owner:CONNECTICUT HEALTH CENT UNIV OF
Genetically stable recombinant modified vaccinia ankara (RMVA) vaccines and methods of preparation thereof
ActiveUS20100316667A1Elicit immune responseVectorsSugar derivativesHeterologousModified vaccinia Ankara
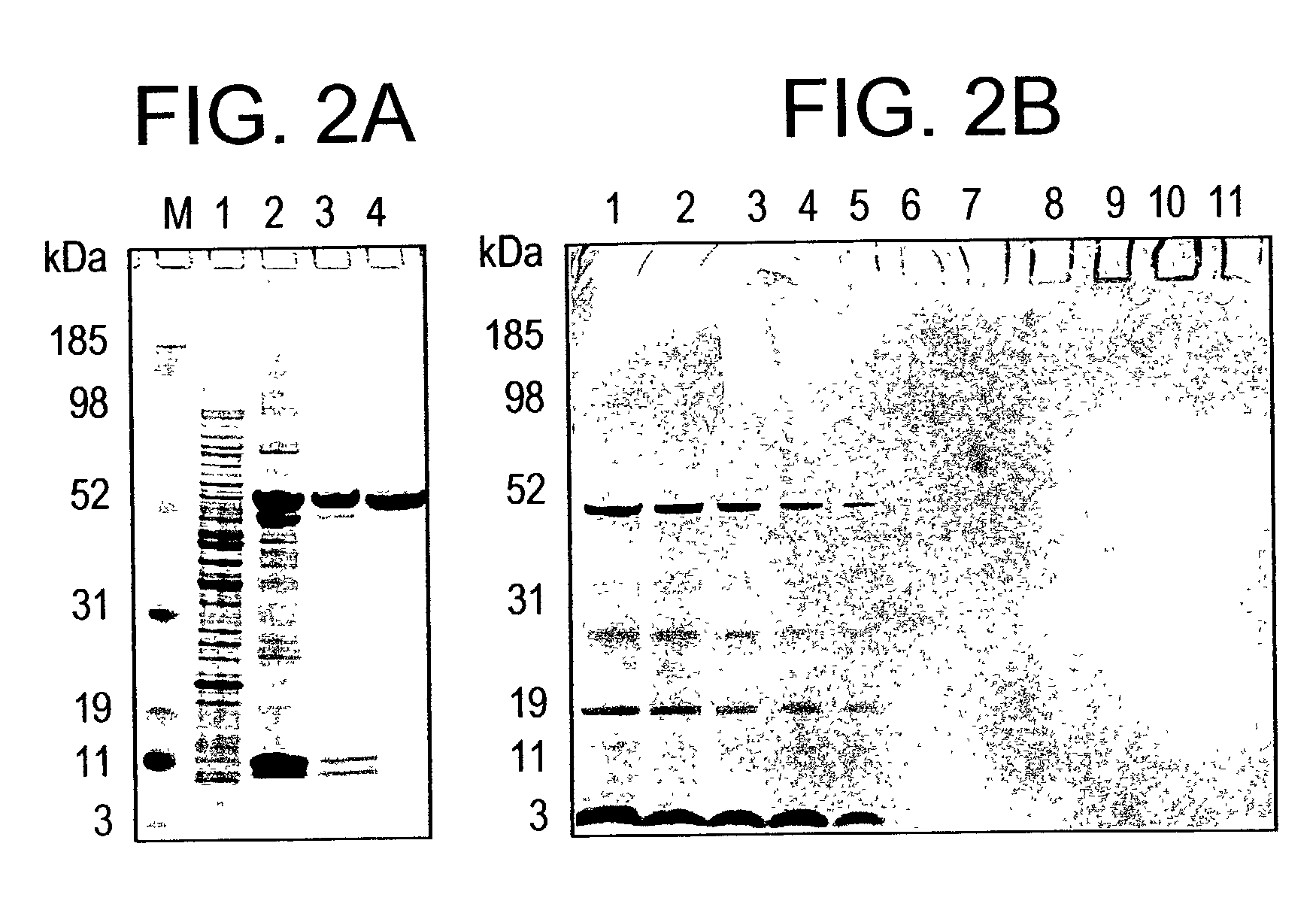
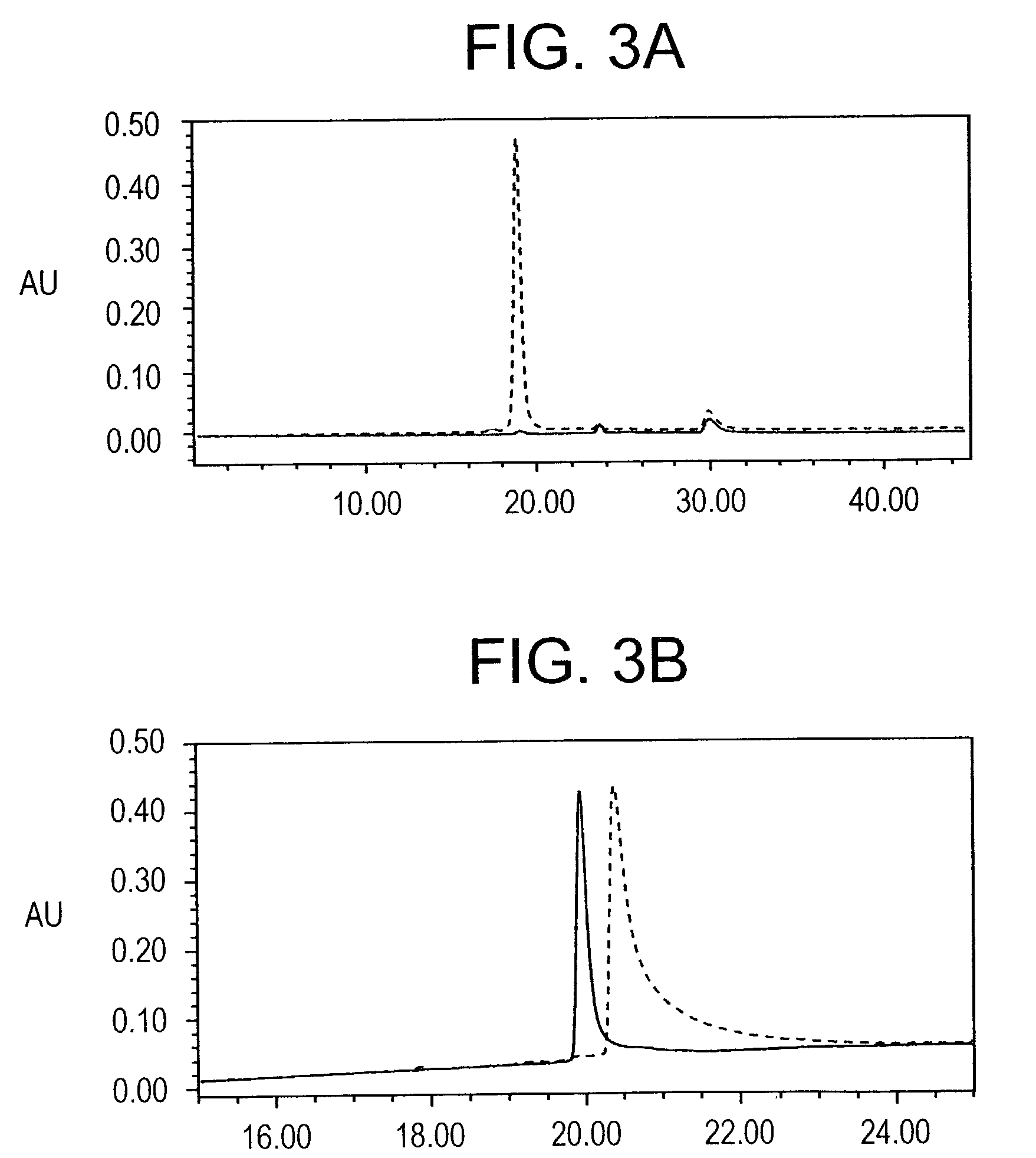
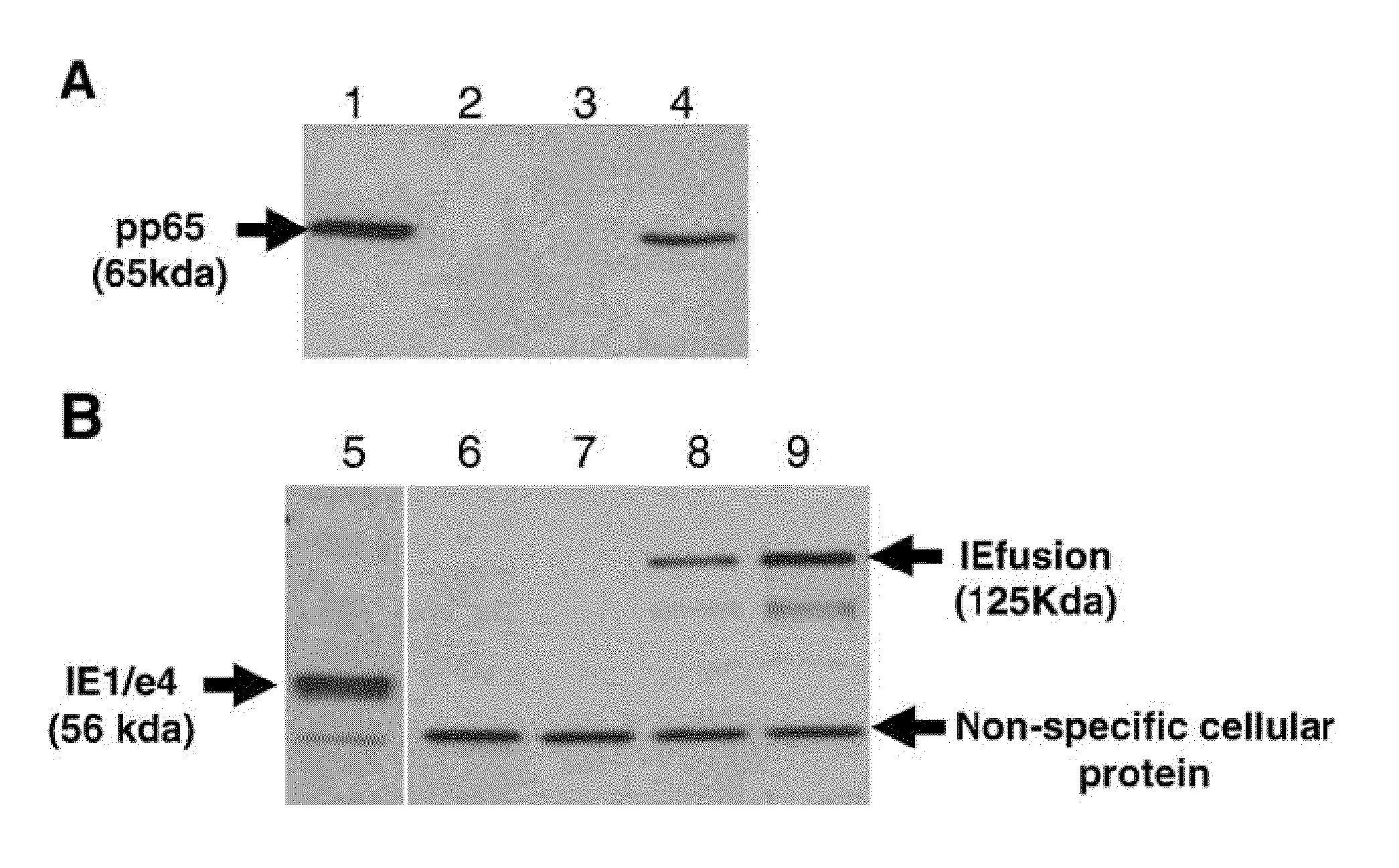
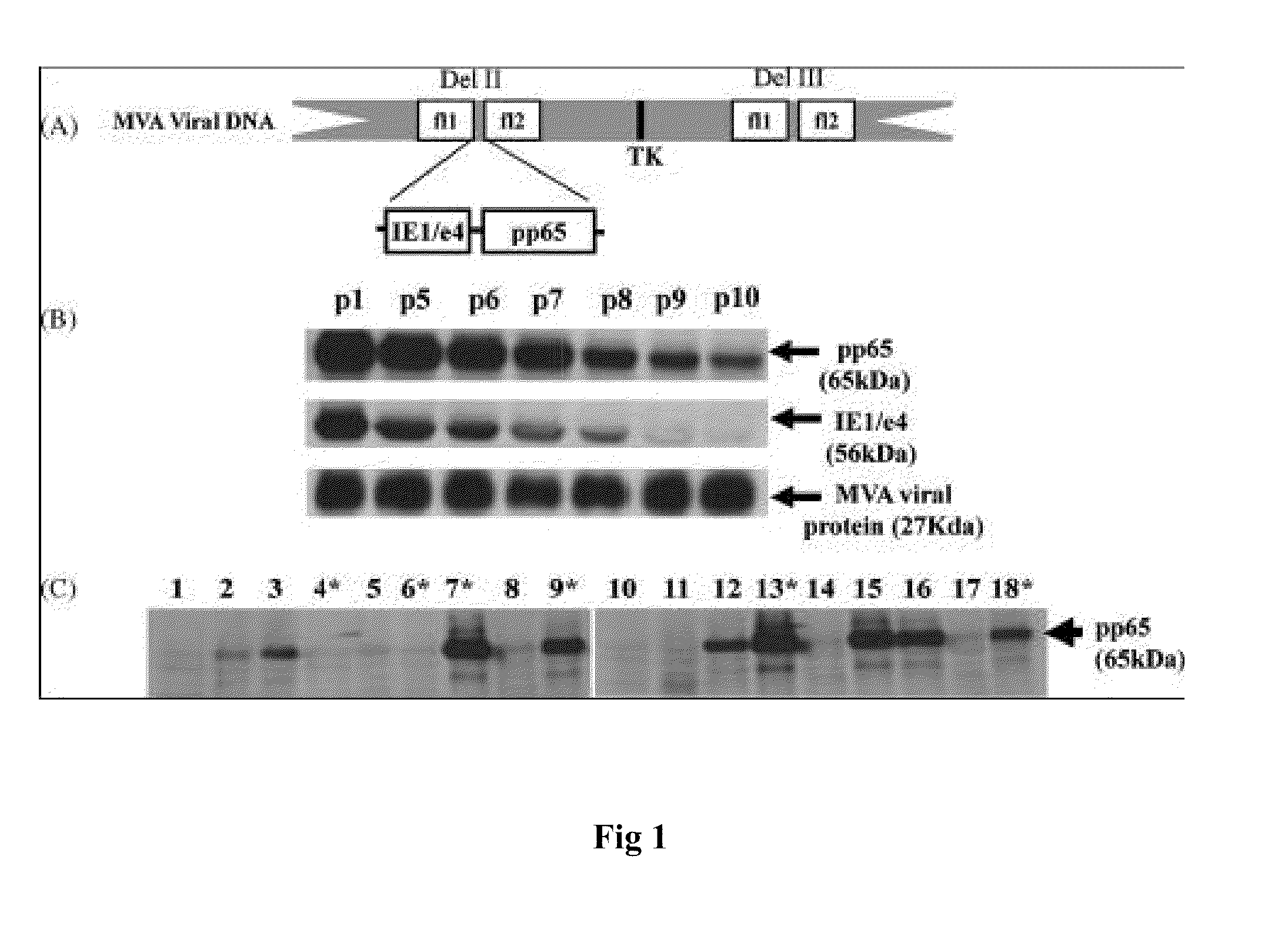
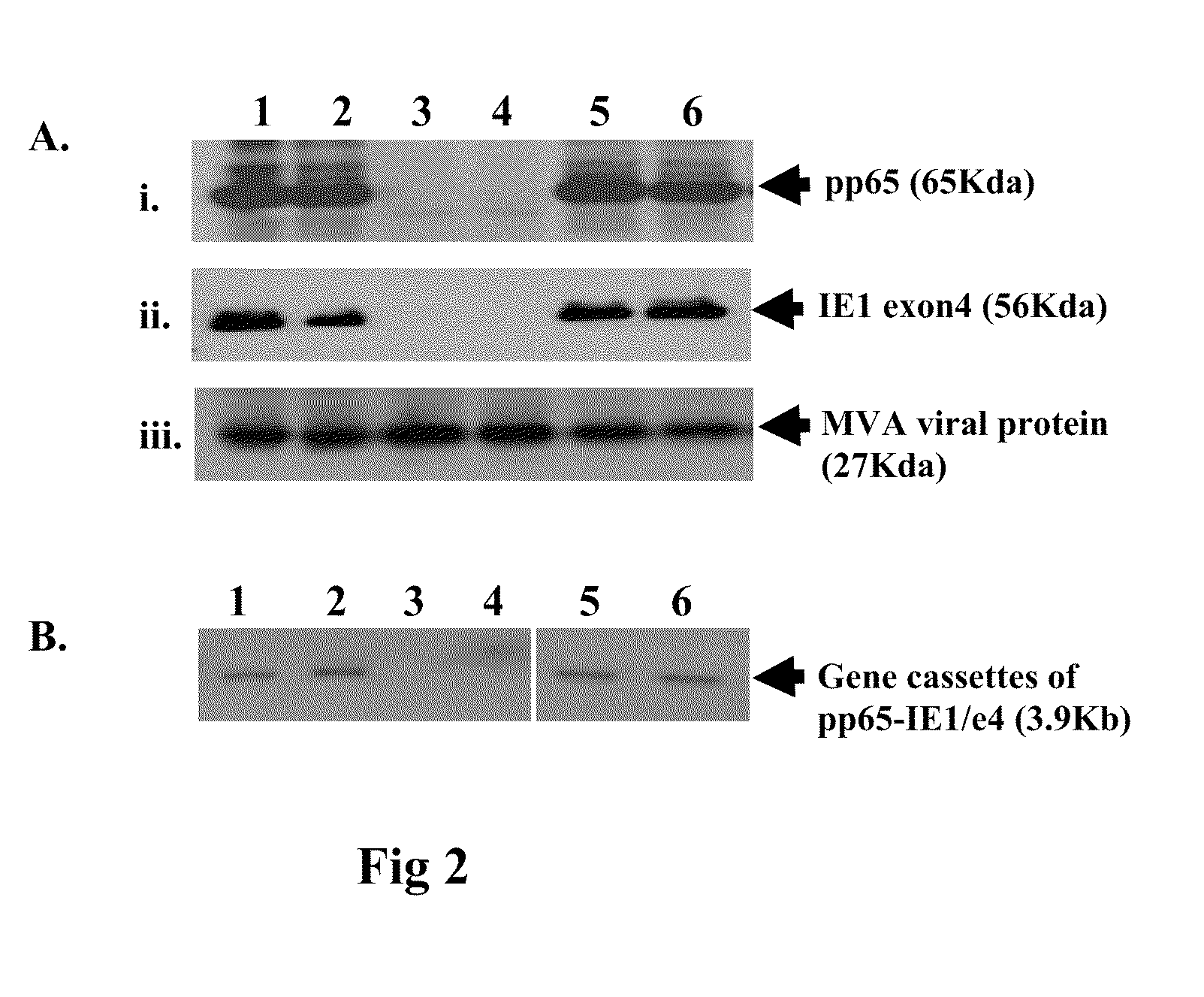
A vaccine comprising an immunologically effective amount of recombinant modified vaccinia Ankara (rMVA) virus which is genetically stable after serial passage and produced by a) constructing a transfer plasmid vector comprising a modified H5 (mH5) promoter operably linked to a DNA sequence encoding a heterologous foreign protein antigen, wherein the expression of said DNA sequence is under the control of the mH5 promoter; b) generating rMVA virus by transfecting one or more plasmid vectors obtained from step a) into wild type MVA virus; c) identifying rMVA virus expressing one or more heterologous foreign protein antigens using one or more selection methods for serial passage; d) conducting serial passage; e) expanding an rMVA virus strain identified by step d); and f) purifying the rMVA viruses from step e) to form the vaccine. One embodiment is directed to a fusion cytomegalovirus (CMV) protein antigen comprising a nucleotide sequence encoding two or more antigenic portions of Immediate-Early Gene-1 or Immediate-Early Gene-2 (IEfusion), wherein the antigenic portions elicit an immune response when expressed by a vaccine.
Owner:CITY OF HOPE
Mycoplasma Hyopneumoniae Avirulent Adjuvanted Live Vaccine
ActiveUS20090117152A1Preventing and minimize severityElicit immune responseAntibacterial agentsBacterial antigen ingredientsViral antigensImmunogenicity
Provided are immunogenic and vaccine compositions and methods for their preparation and use, which compositions are effective in protecting against, minimizing the severity of, preventing, and / or ameliorating M. hyopneumoniae infection. Administration to an animal of one or two doses of an adjuvanted live avirulent M. hyopneumoniae composition disclosed herein is effective in providing immunity to the animal and protection from infection with a virulent strain of M. hyopneumoniae thereby reducing the severity of and / or preventing disease caused by one or more virulent strain of M. hyopneumoniae. Also provided are compositions, which further comprise one or more antigen such as, for example, one or more live bacteria, bacterin, toxoid, and / or virus and / or viral antigen. Exemplified are immunogenic compositions, comprising an adjuvanted live avirulent M. hyopneumoniae and compositions, comprising Porcine Circovirus Type 1-Type 2 chimera modified live vaccine (cPCV1-2) in further combination with an adjuvanted live avirulent M. hyopneumoniae.
Owner:ZOETIS SERVICE LLC
Recombinant cancer cell secreting modified heat shock protein-antigenic peptide complex
InactiveUS20080026012A1Elicit immune responseAntibacterial agentsFungiInfected cellCancer prevention
The present invention relates to methods for purifying immunogenic, prophylactically and therapeutically effective complexes of modified heat shock proteins noncovalently associated with antigenic peptides of cancer or infected cells. The claimed methods comprise the constructing of a nucleotide sequence encoding a secretable modified heat shock protein, expressing the sequence in an appropriate host cell, recovering the immunogenic complexes from the cell culture and the cells, and purifying the immunogenic complexes by affinity chromatography. Large amounts of such immunogenic complexes can be obtained by large-scale culturing of host cells containing the genetic sequence. The complexes can be used as a vaccine to elicit specific immune responses against cancer or infected cells, and to treat or prevent cancer or infectious diseases.
Owner:UNIV OF MIAMI
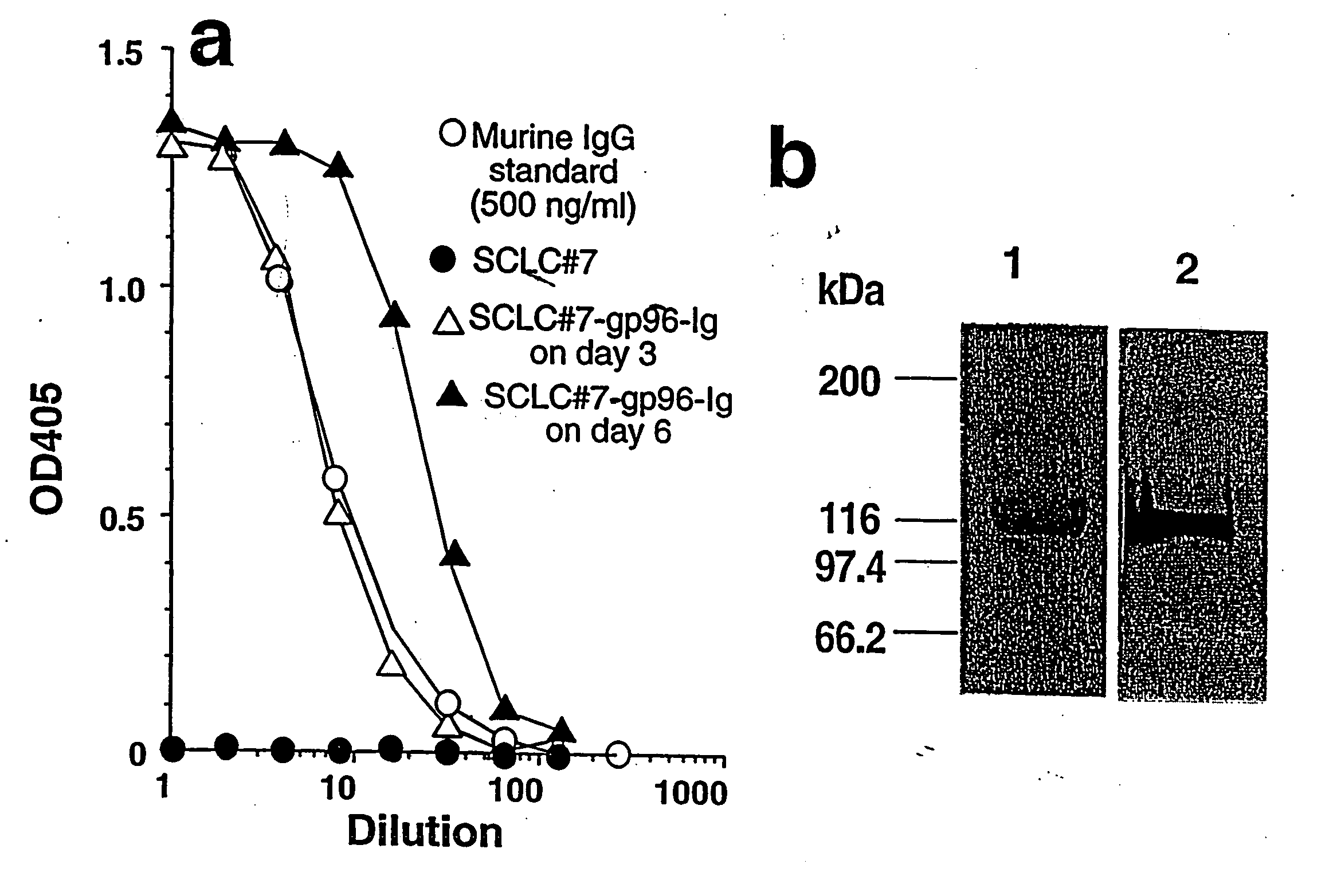
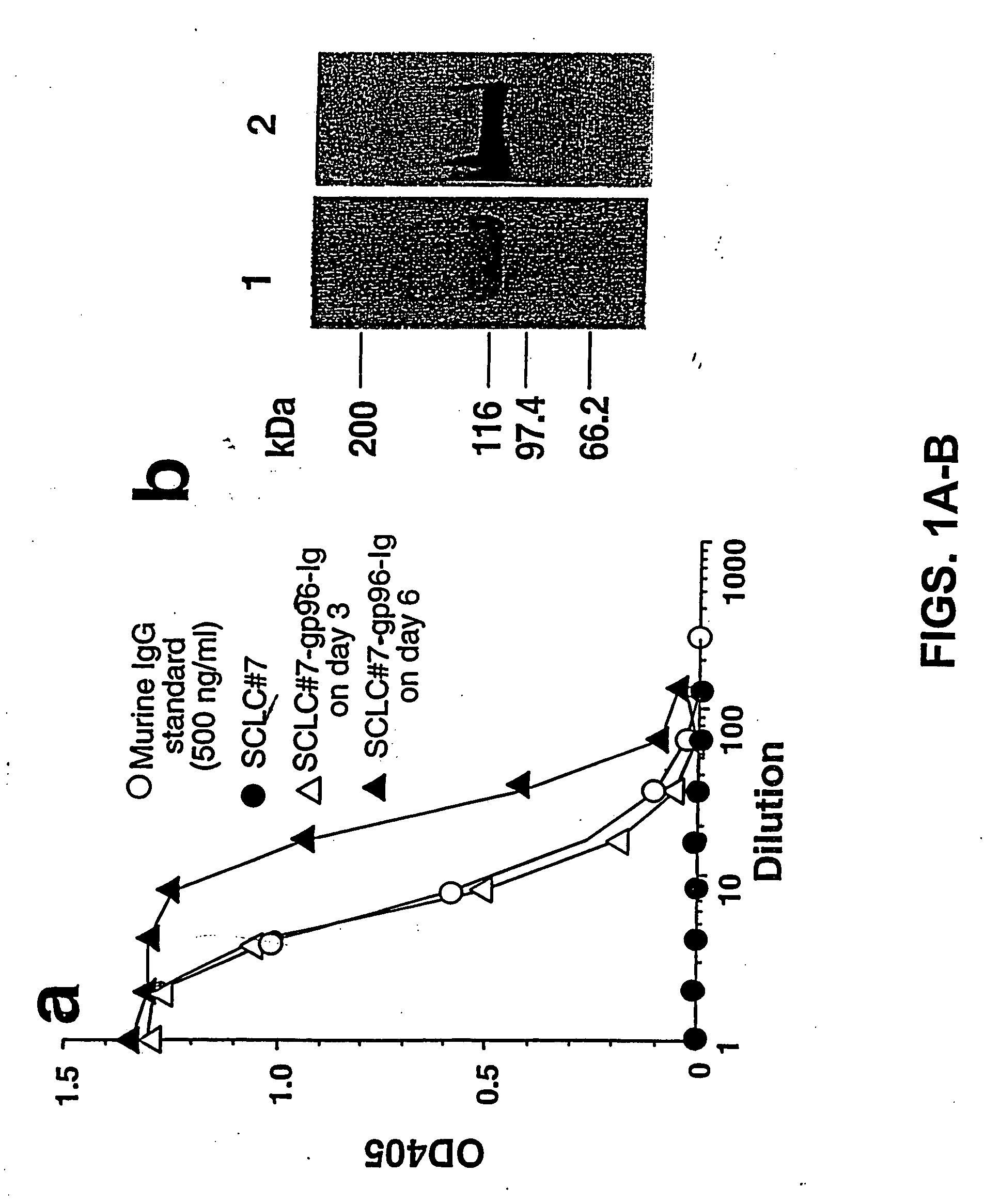
Compositions and methods using complexes of heat shock protein gp96 and antigenic molecules for the treatment and prevention of infectious diseases
InactiveUS6143299AElicit immune responseOrganic active ingredientsPeptide/protein ingredientsMHC class IAntigenic valence
The present invention relates to methods and compositions for eliciting an immune response and the prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases. The methods of the invention comprise administering a composition comprising an effective amount of a complex, in which the complex consists essentially of a heat shock protein (hsp) noncovalently bound to an antigenic molecule. "Antigenic molecule" as used herein refers to the peptides with which the hsps are endogenously associated in vivo as well as exogenous antigens / immunogens (i.e., with which the hsps are not complexed in vivo) or antigenic / immunogenic fragments and derivatives thereof. In a preferred embodiment, the complex is autologous to the individual. The effective amounts of the complex are in the range of 10-600 micrograms for complexes comprising hsp70, 50-1000 micrograms for hsp90, and 10-600 micrograms for gp96. The invention also provides a method for measuring tumor rejection in vivo in an individual, preferably a human, comprising measuring the generation by the individual of MHC Class I-restricted CD8+ cytotoxic T lymphocytes specific to the tumor. Methods of purifying hsp70-peptide complexes are also provided.
Owner:FORDHAM UNIVERSITY
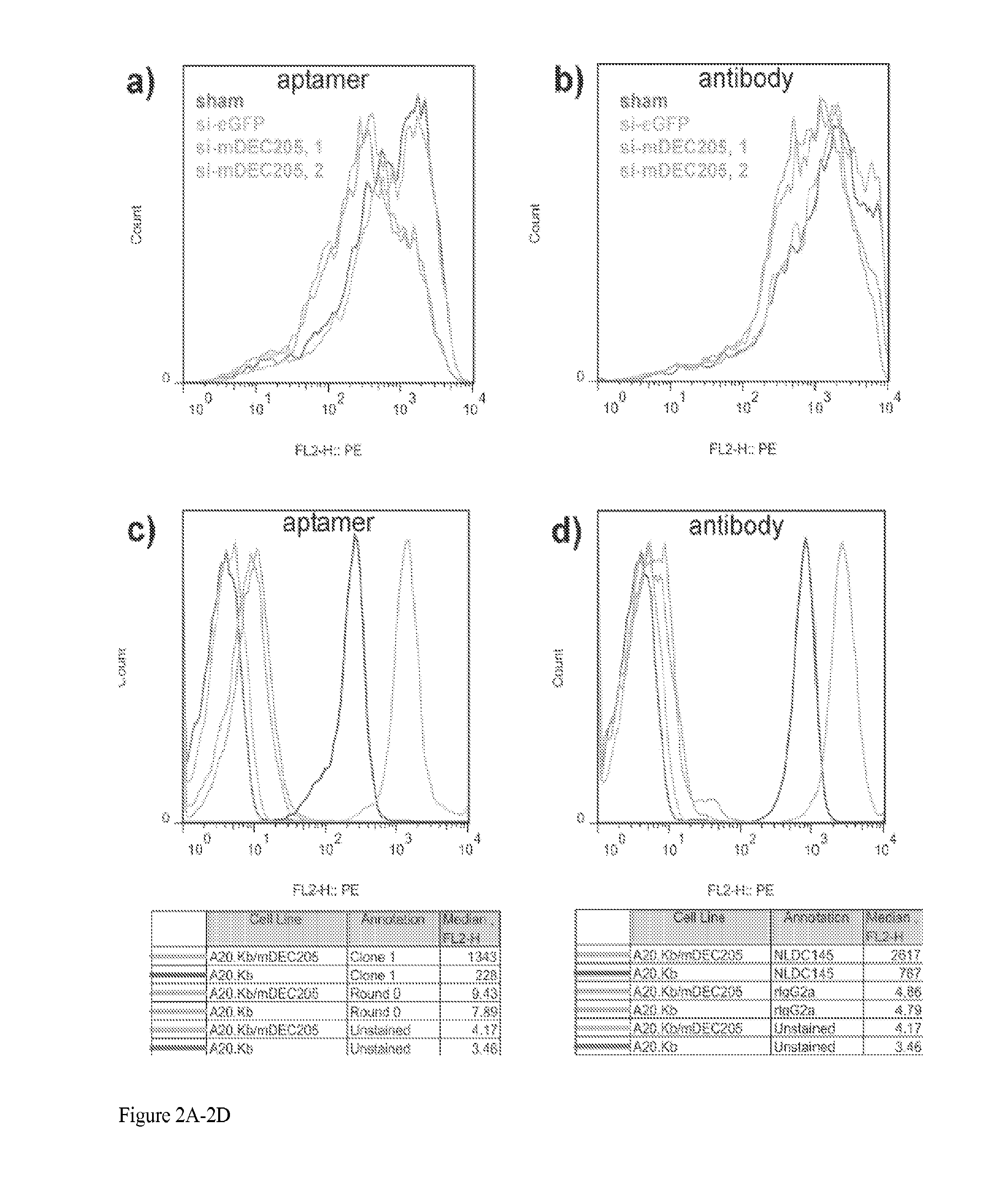
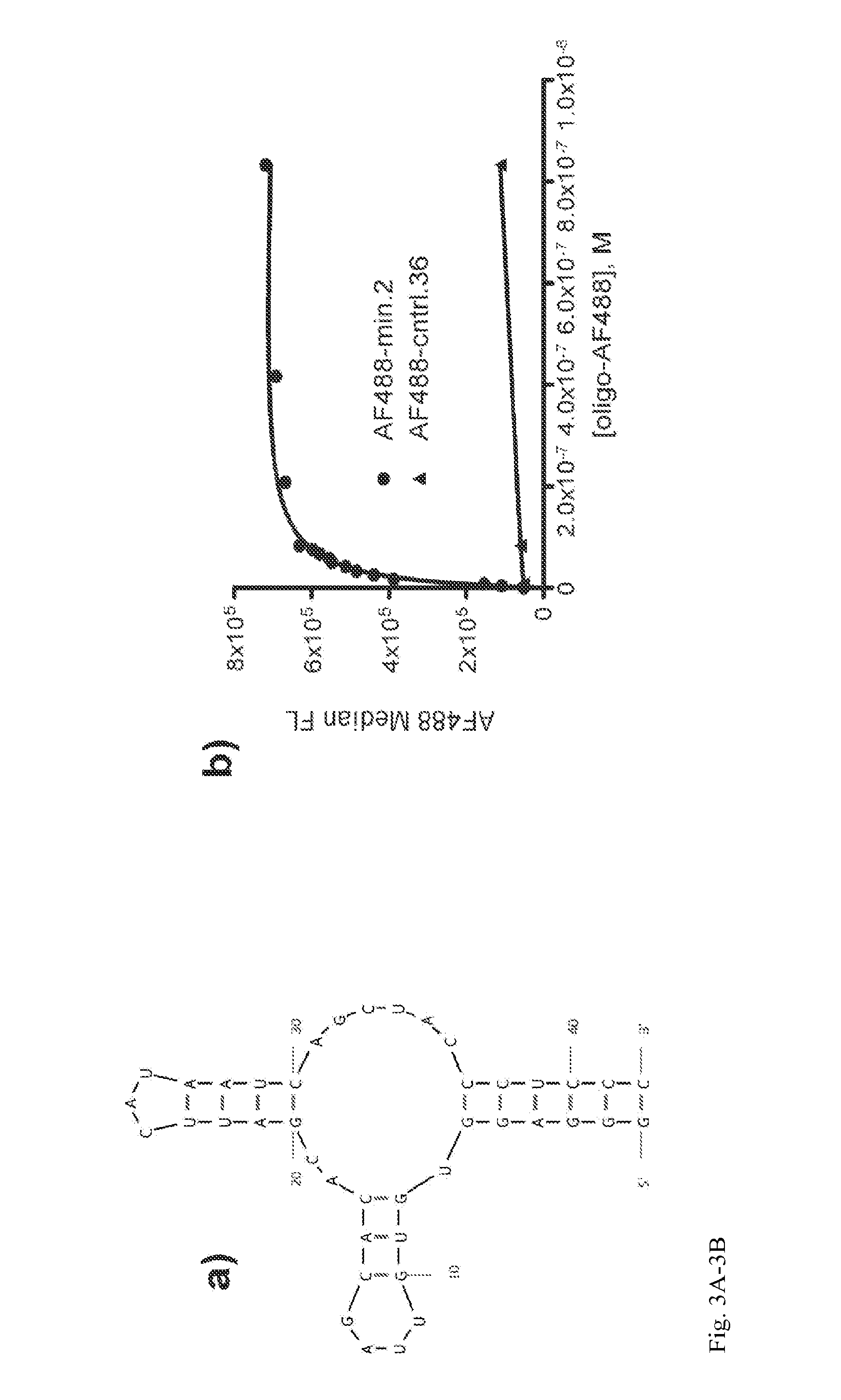
Aptamer-targetted antigen delivery
ActiveUS20150191730A1Elicit immune responseSpecial deliverySugar derivativesGene deliveryAntigen delivery
A composition is provided comprising an oligonucleotide aptamer conjugated to an antigen, wherein the aptamer is directed against a cell-surface target of an antigen-presenting cell. Also provided are methods of delivering an antigen to a dendritic cell and of eliciting an immune response in a subject.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
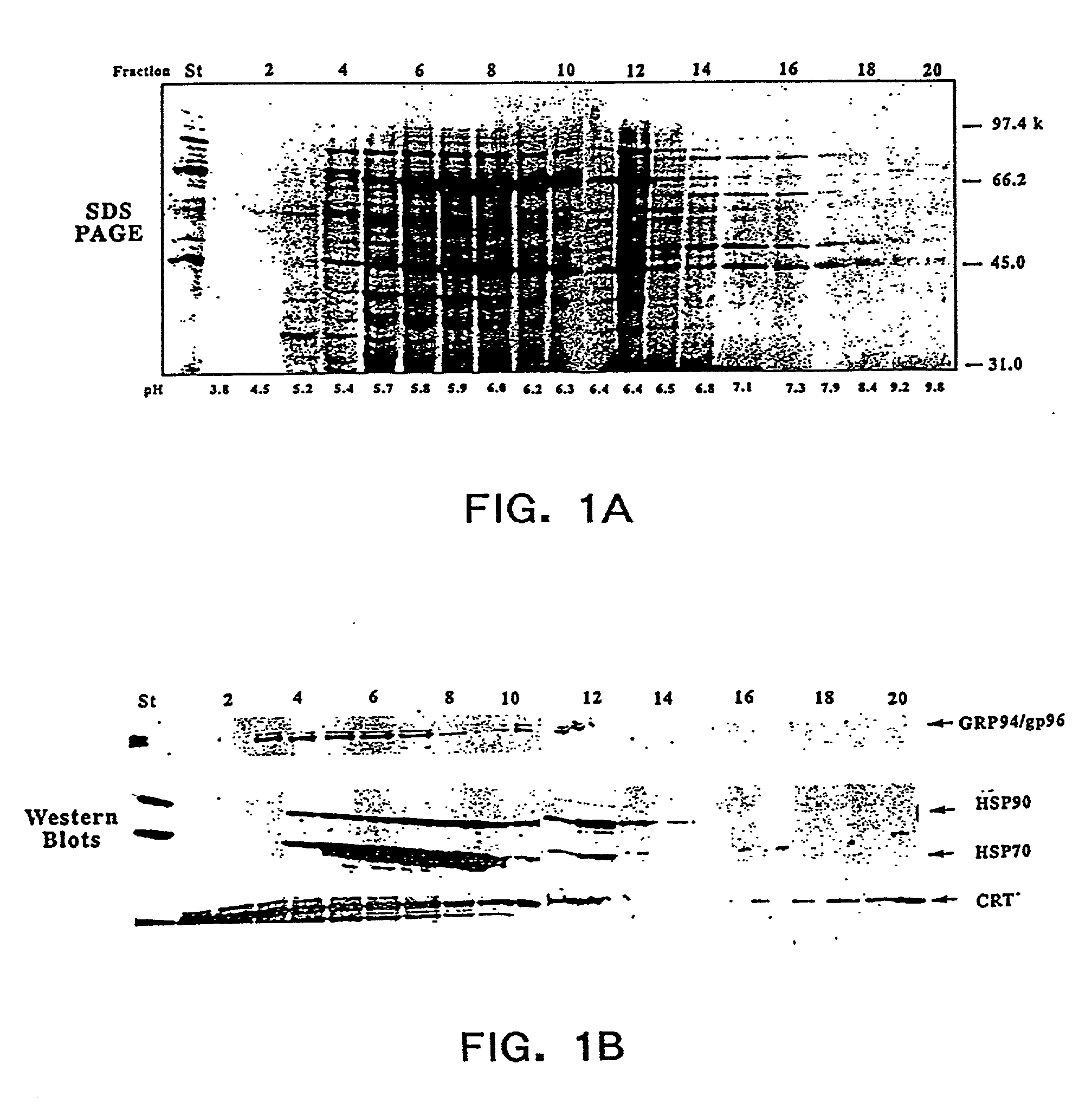
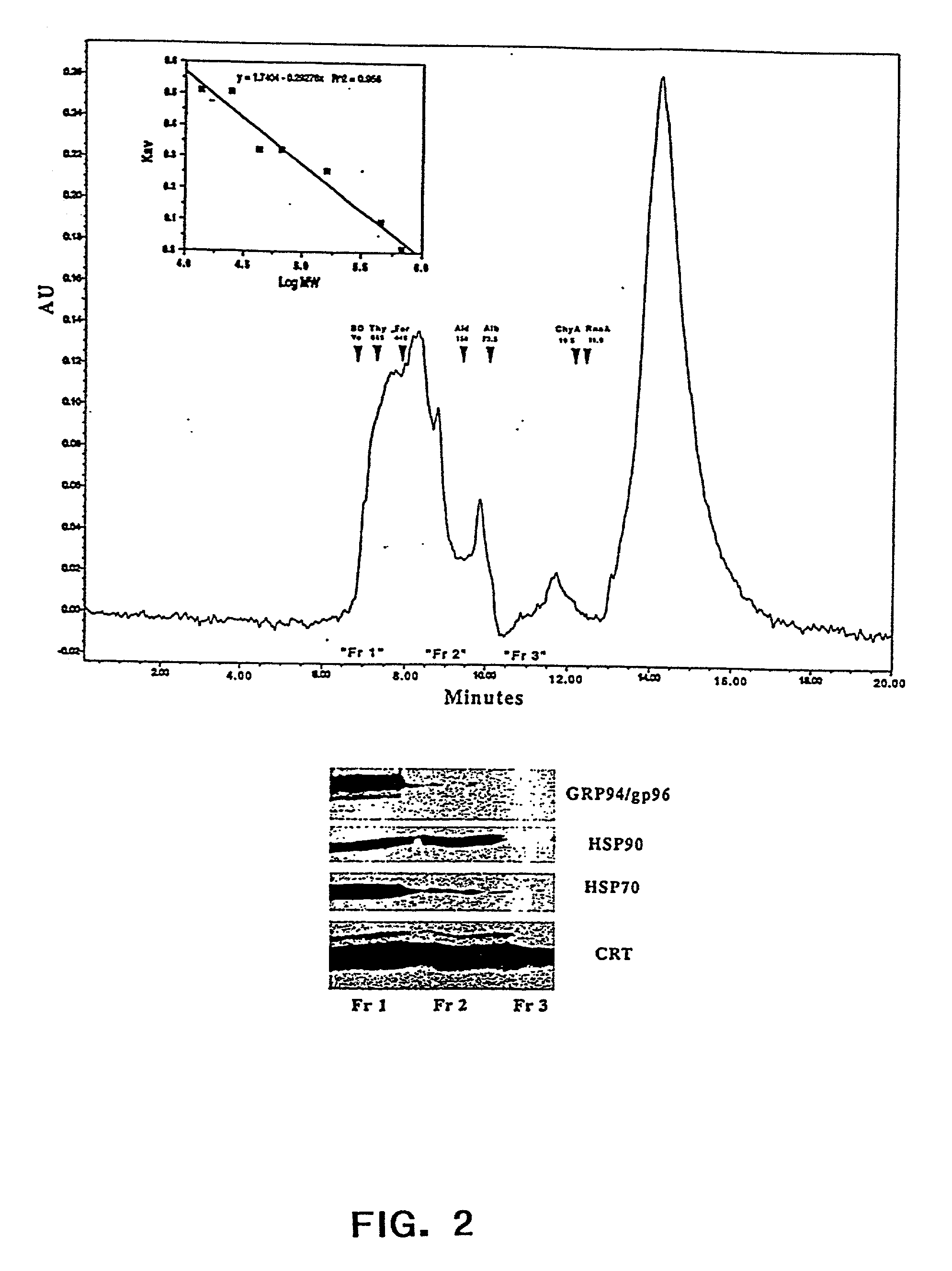
Methods of recovering chaperone proteins and complexes thereof
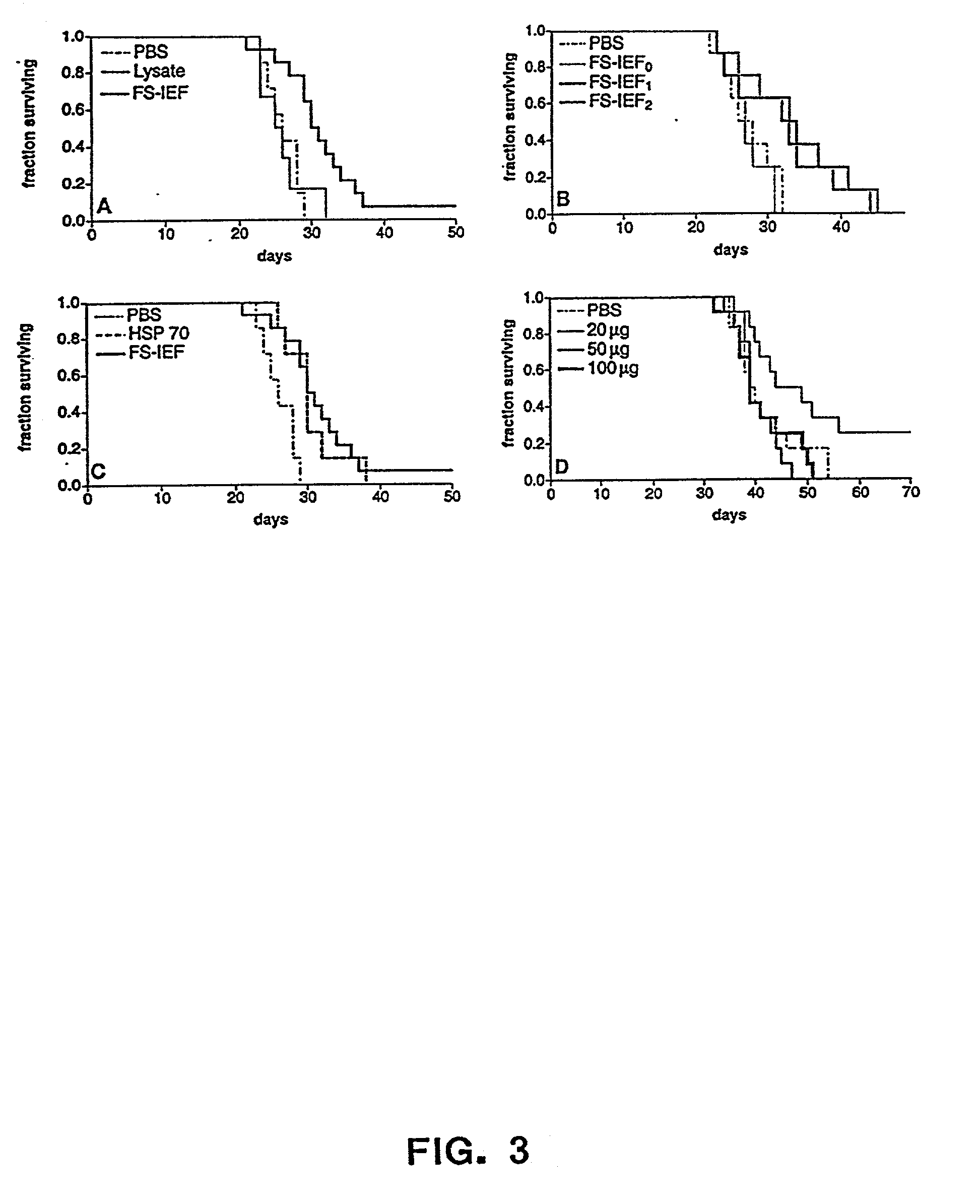
The present invention provides methods for efficient and concomitant recovery of multiple chaperone proteins and / or chaperone protein complexes from a limited sample source. Disclosed are methods involving the use of Free Solution Iso-Electric Focusing (FS-IEF) which can enrich samples containing chaperone proteins and / or chaperone protein complexes from a given sample. The chaperone proteins can be, but are not restricted to calreticulin, gp96, hsp86, hsp84, hsp70, hsp60 and hsp40. The invention also provides methods of recovering chaperone protein complexes for the preparation of vaccines containing chaperone complexes.
Owner:ARIZONA BOARD OF REGRENTS ON BEHALF OF THE UNIV OF ARIZONA THE
Vaccine
InactiveUS20050214329A1Elicit immune responsePrevention and ameliorationAntibacterial agentsBacterial antigen ingredientsTGE VACCINEConjugated vaccines
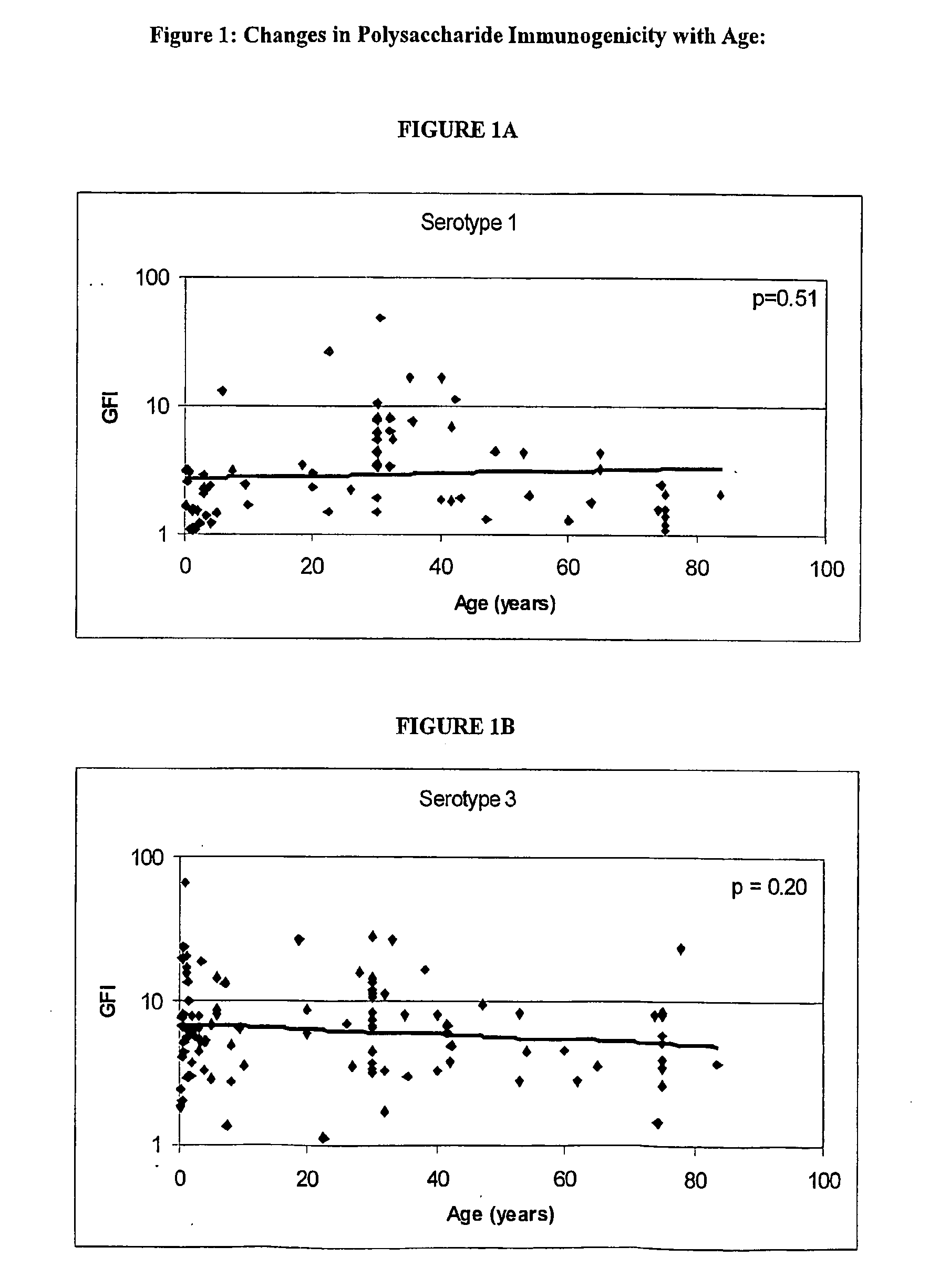
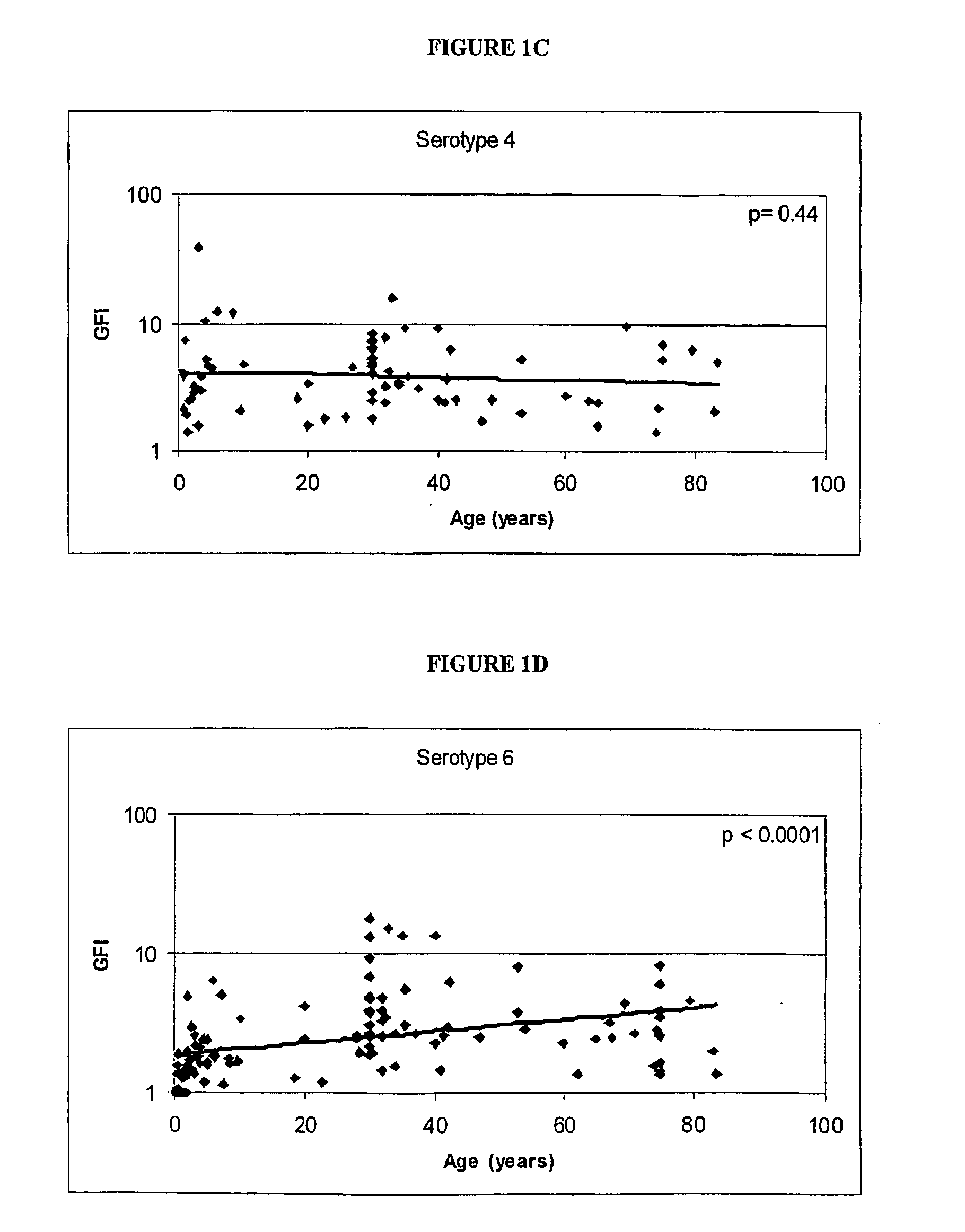
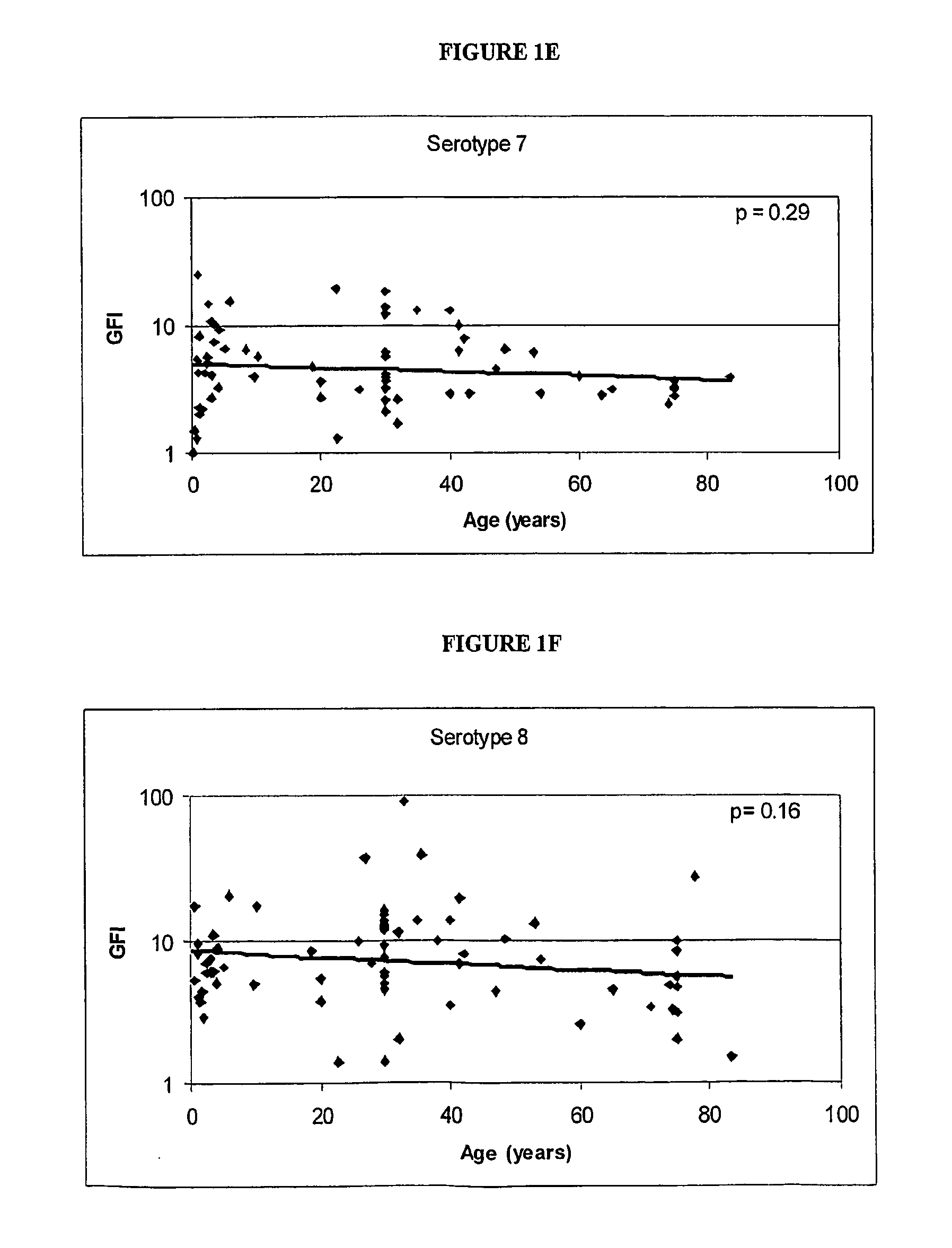
The present invention provides an optimal formulation of multiple serotype Streptococcus pneumoniae conjugate vaccines.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
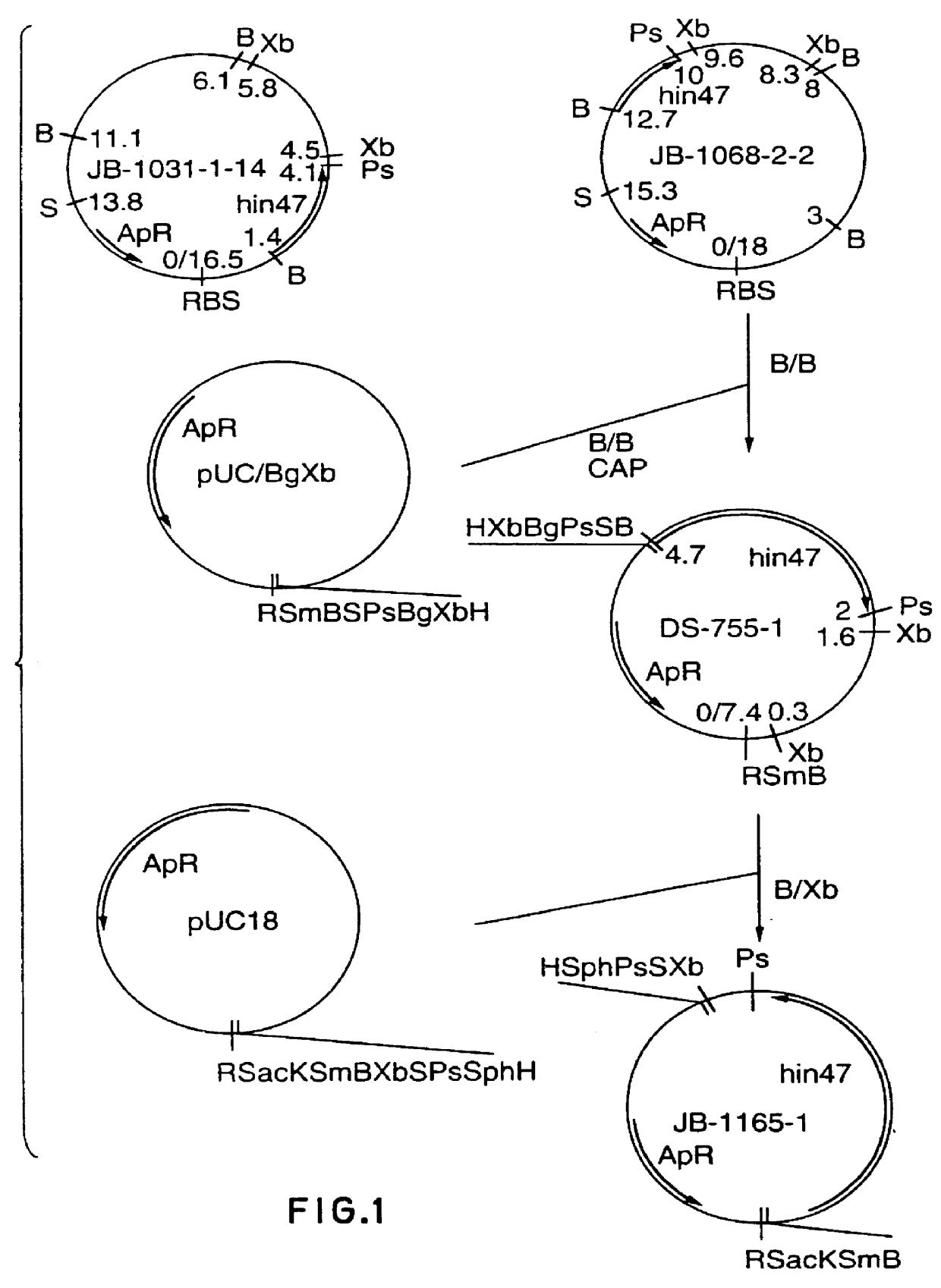
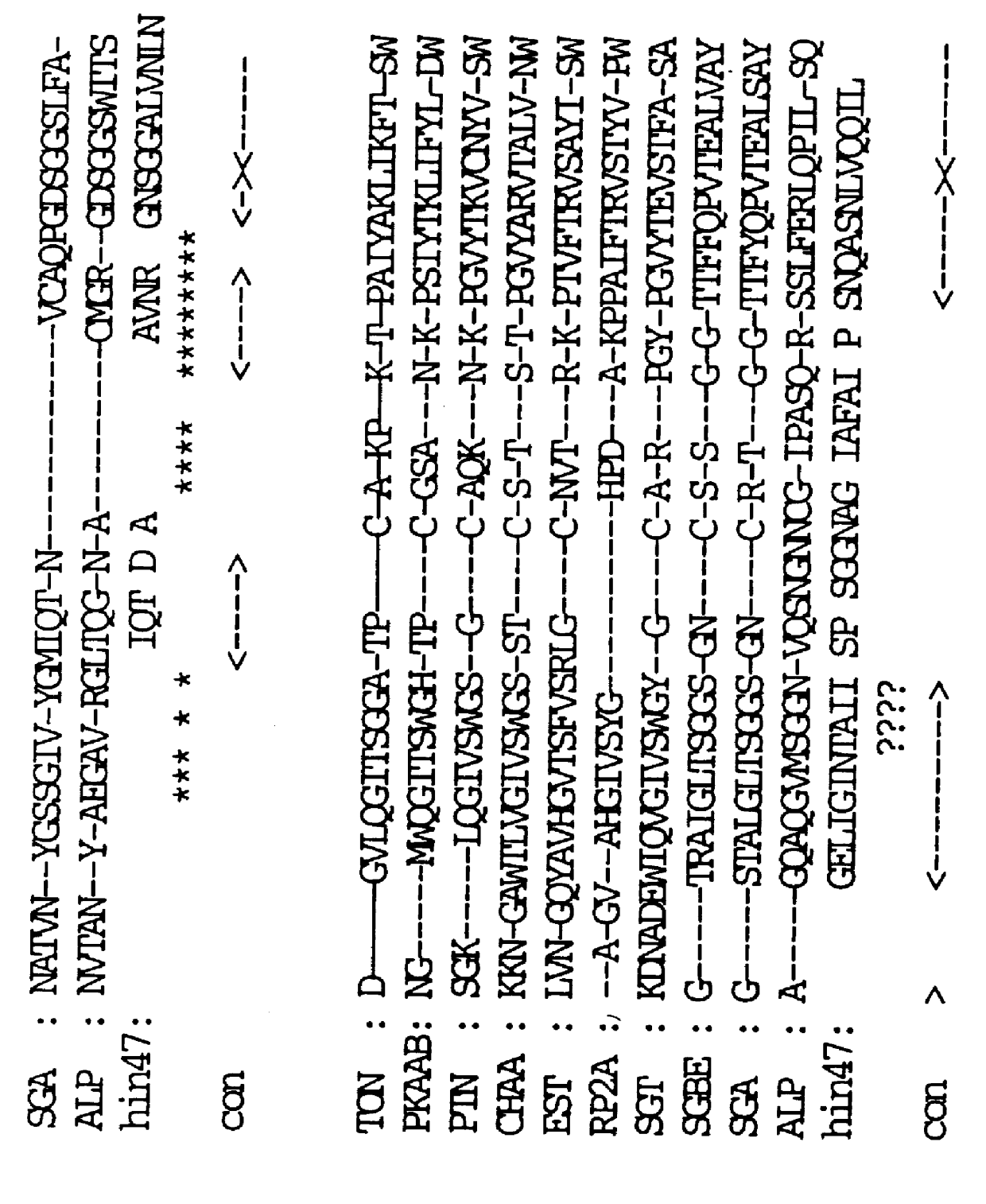
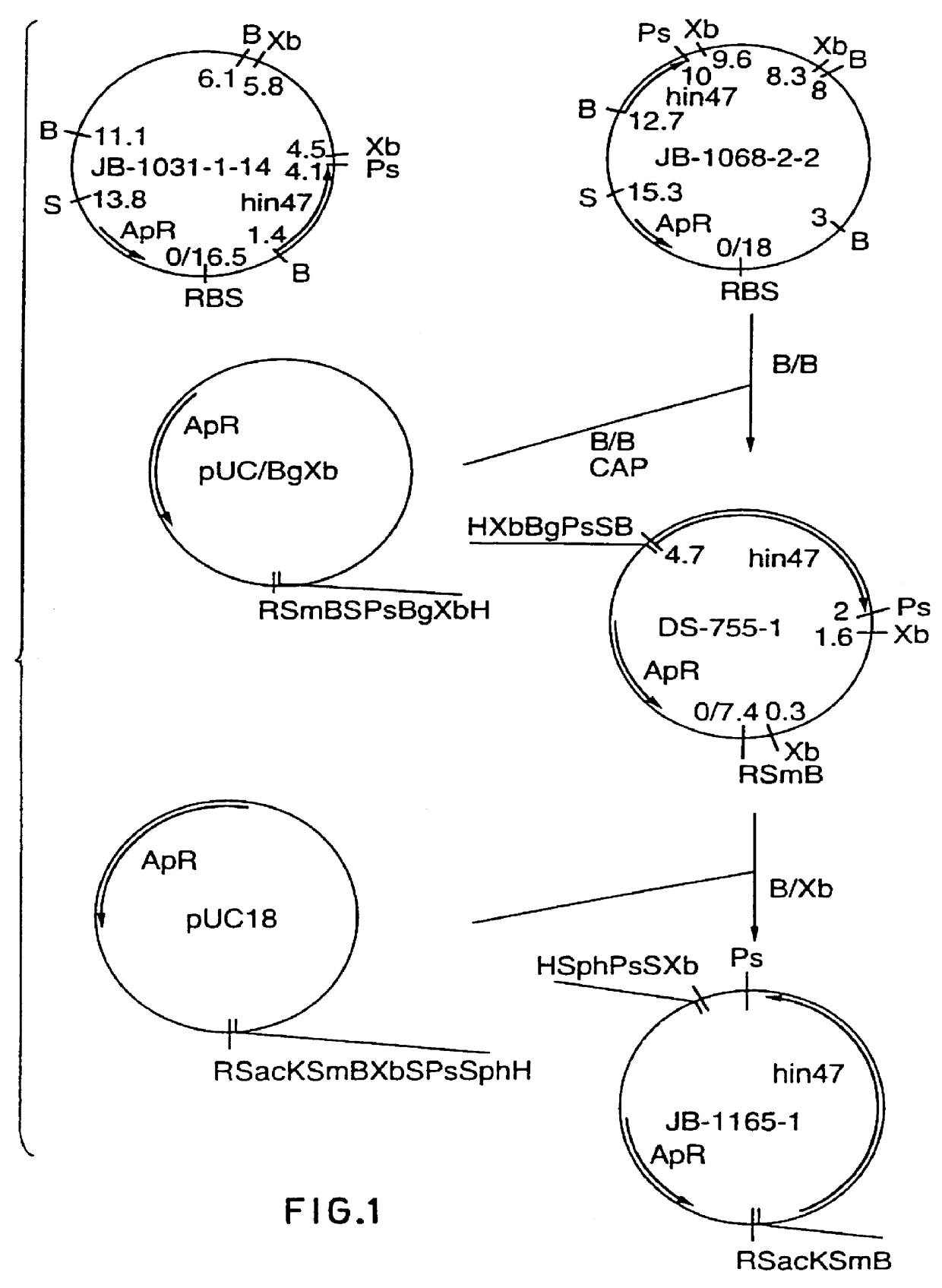
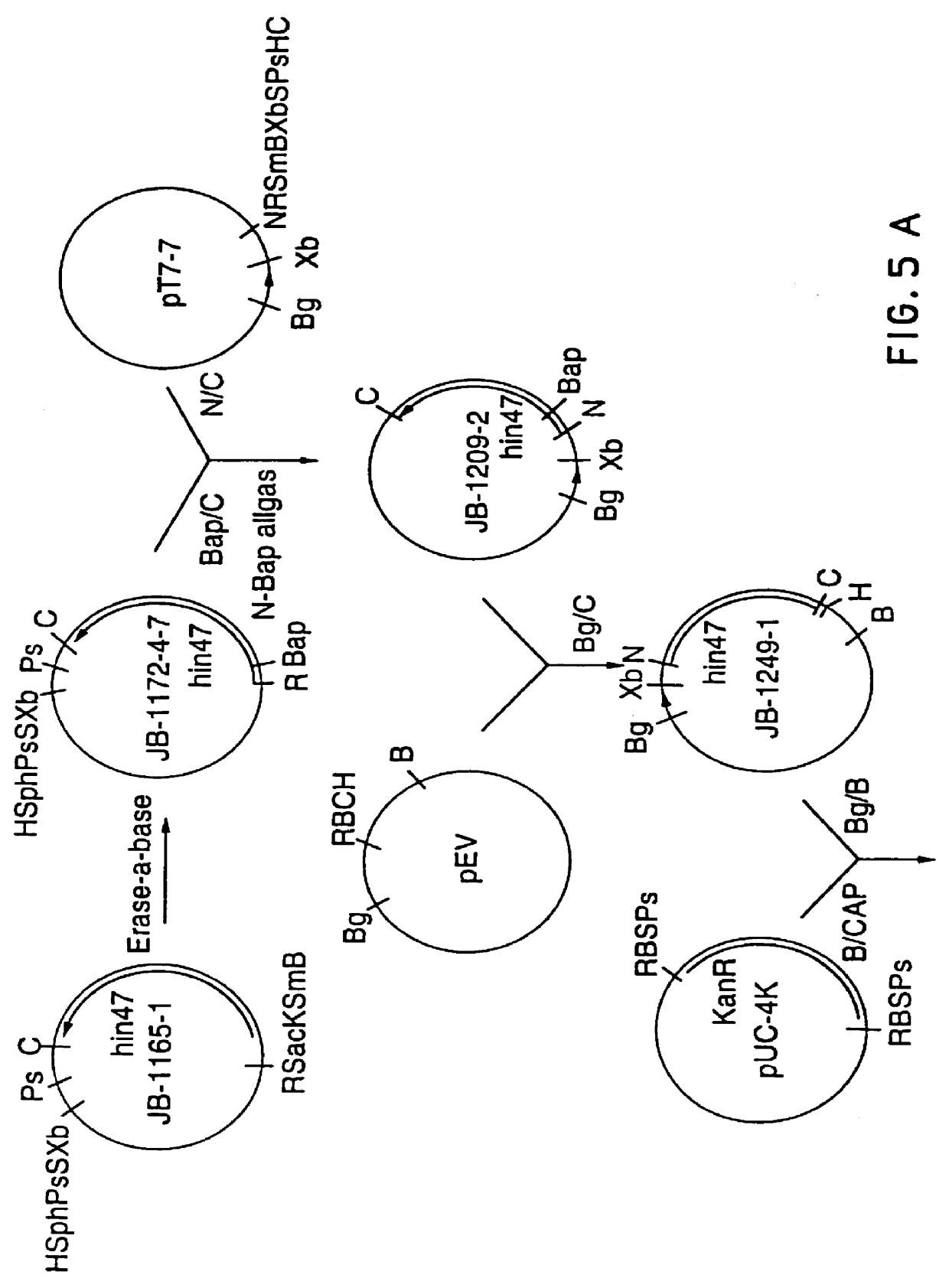
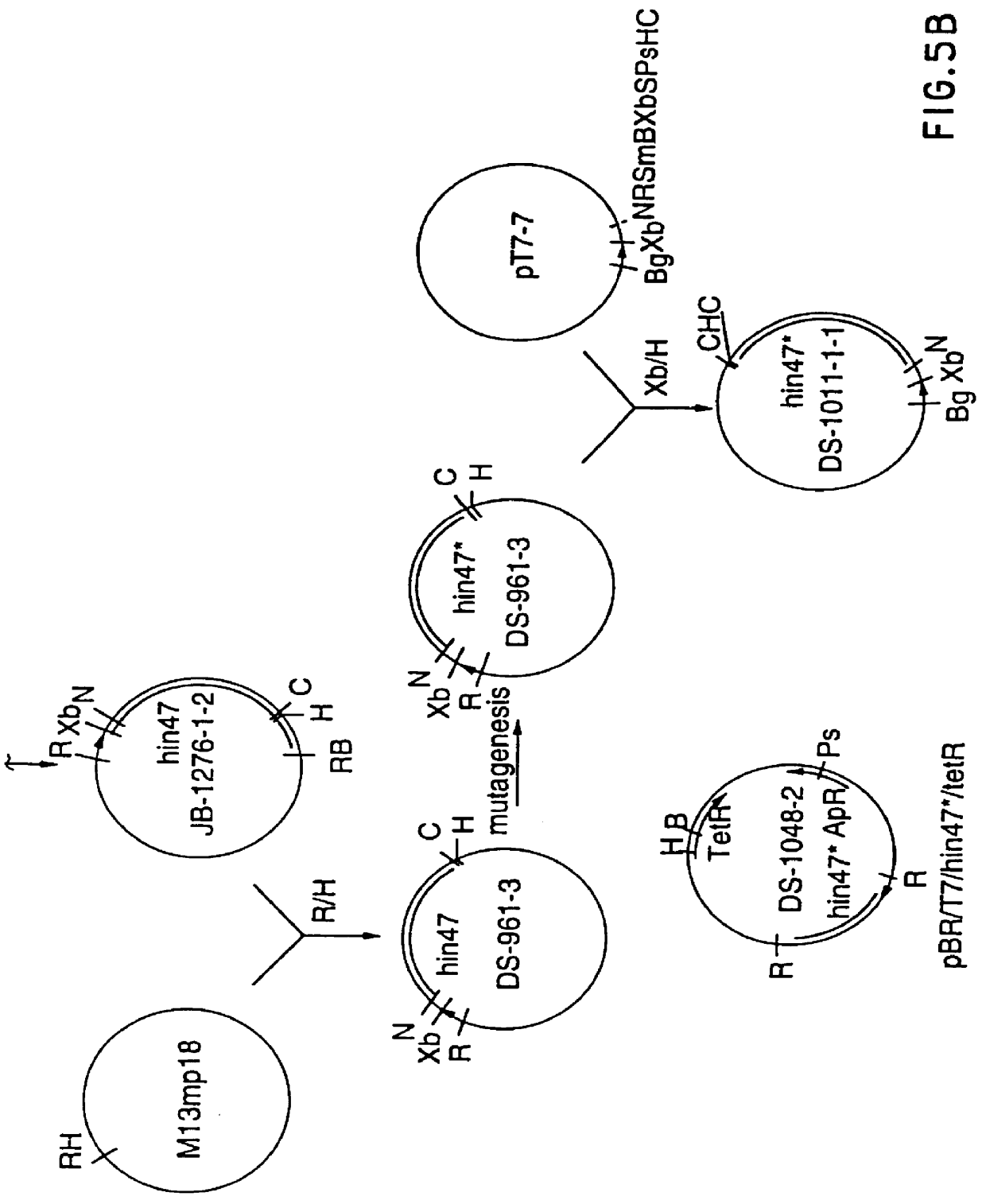
Analog of Haemophilus Hin47 with reduced protease activity
An isolated and purified analog of Haemophilus influenza Hin47 protein has a decreased protease activity which is less than about 10% of that of natural Hin47 protein and preferably substantially the same immunogenic properties as natural Hin47 protein. An isolated and purified nucleic acid molecule encoding the Hin47 analog may be provided in a recombinant plasmid which may be introduced into a cell which is grown to produce the Hin47 analog. Immunogenic compositions comprising the Hin47 analog and the encoding nucleic acid may be formulated as vaccines for in vivo administration to a host, including a human, to confer protection against diseases caused by a bacterial pathogen, including Haemophilus species, such as Haemophilus influenzae, that produces Hin47 protein or a protein capable of inducing antibodies in the host specifically reactive with Hin47 protein. The Hin47 analog and the encoding nucleic acid also may be employed in diagnostic applications.
Owner:CONNAUGHT LAB
Plasmodium vivax hybrid circumsporozoite protein and vaccine
InactiveUS7790186B2Decreased number of repeatMaximize possibility of generatingPeptide/protein ingredientsAntibody mimetics/scaffoldsCircumsporozoite proteinAntibody production
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Expression of lipoproteins
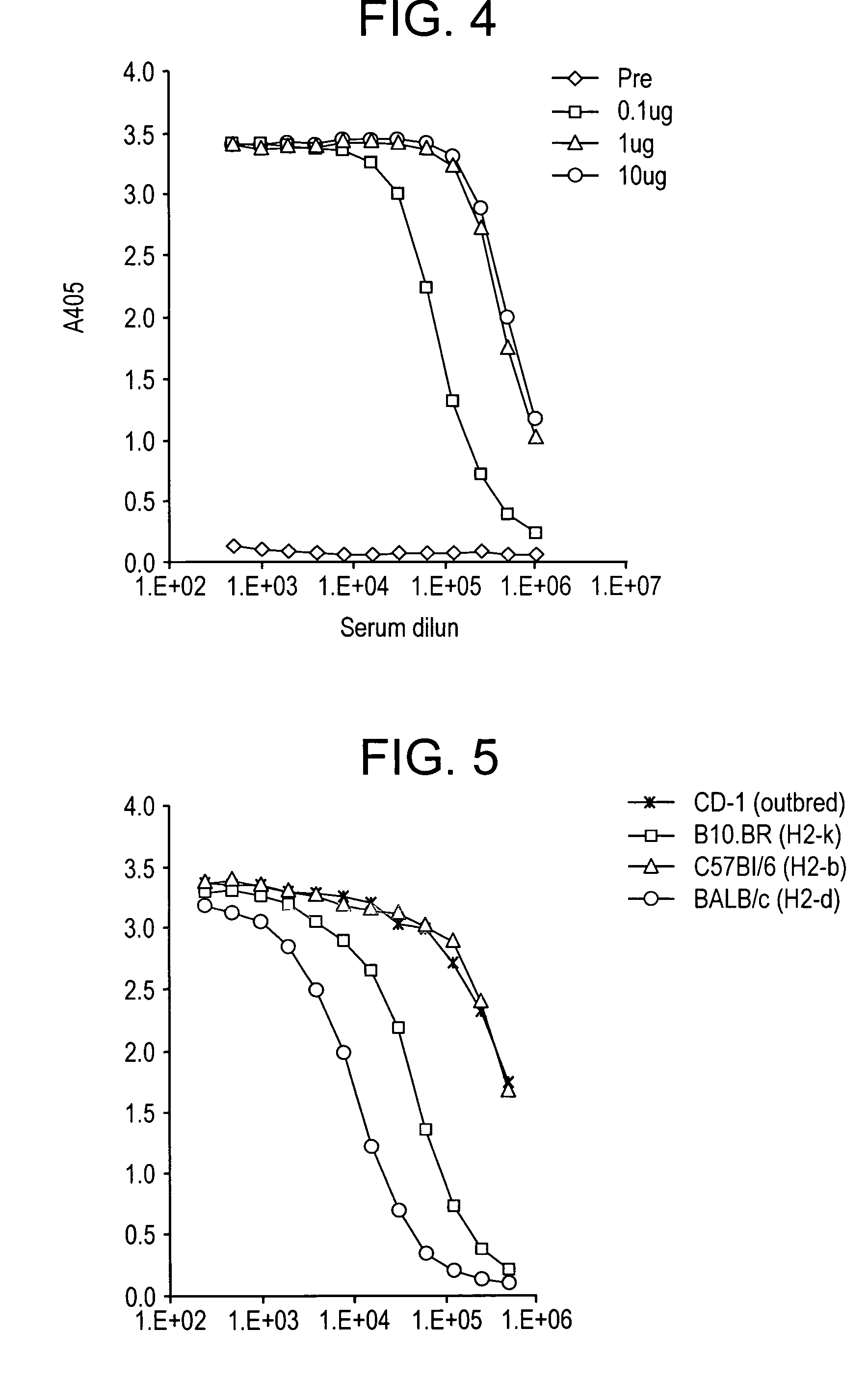
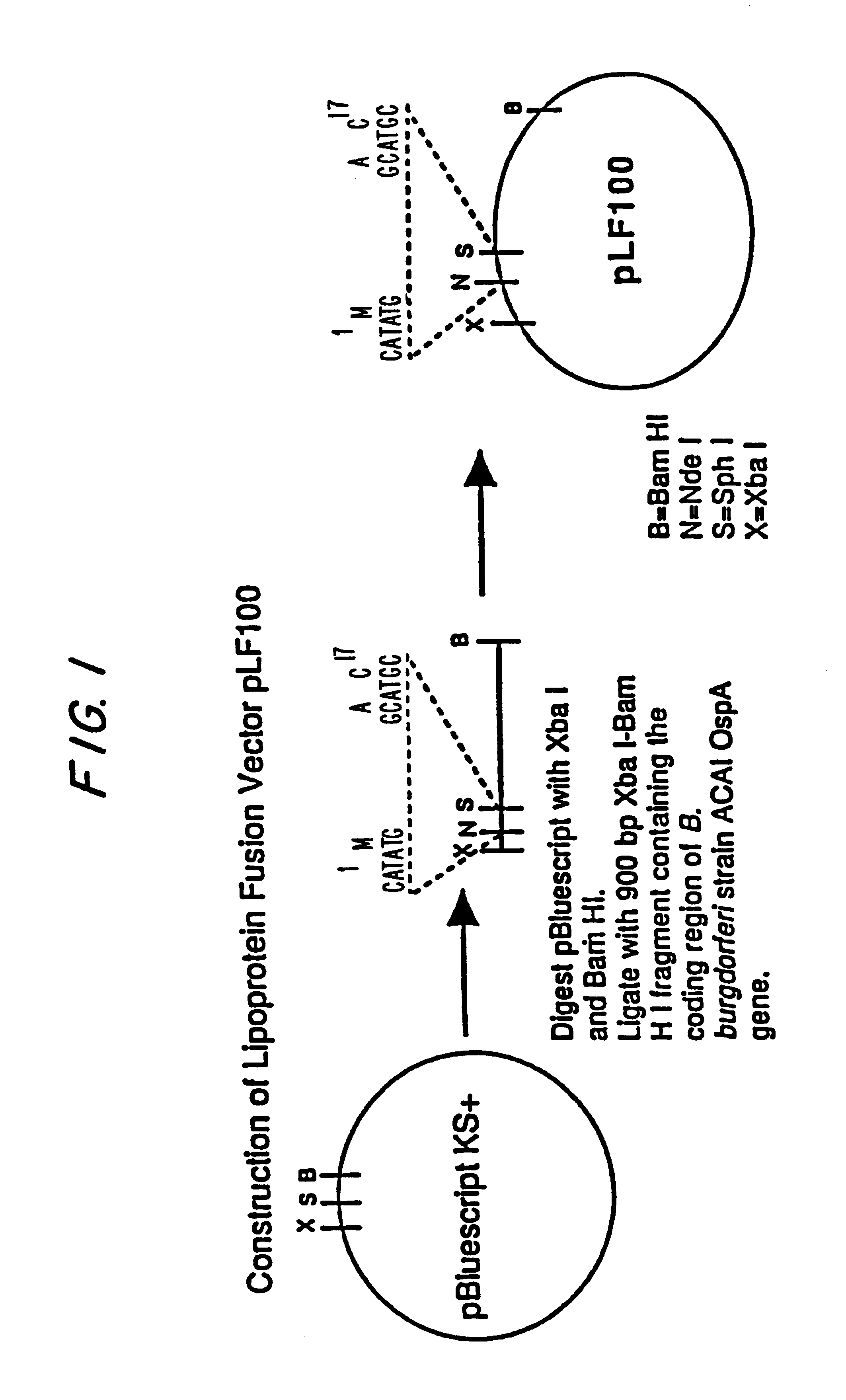
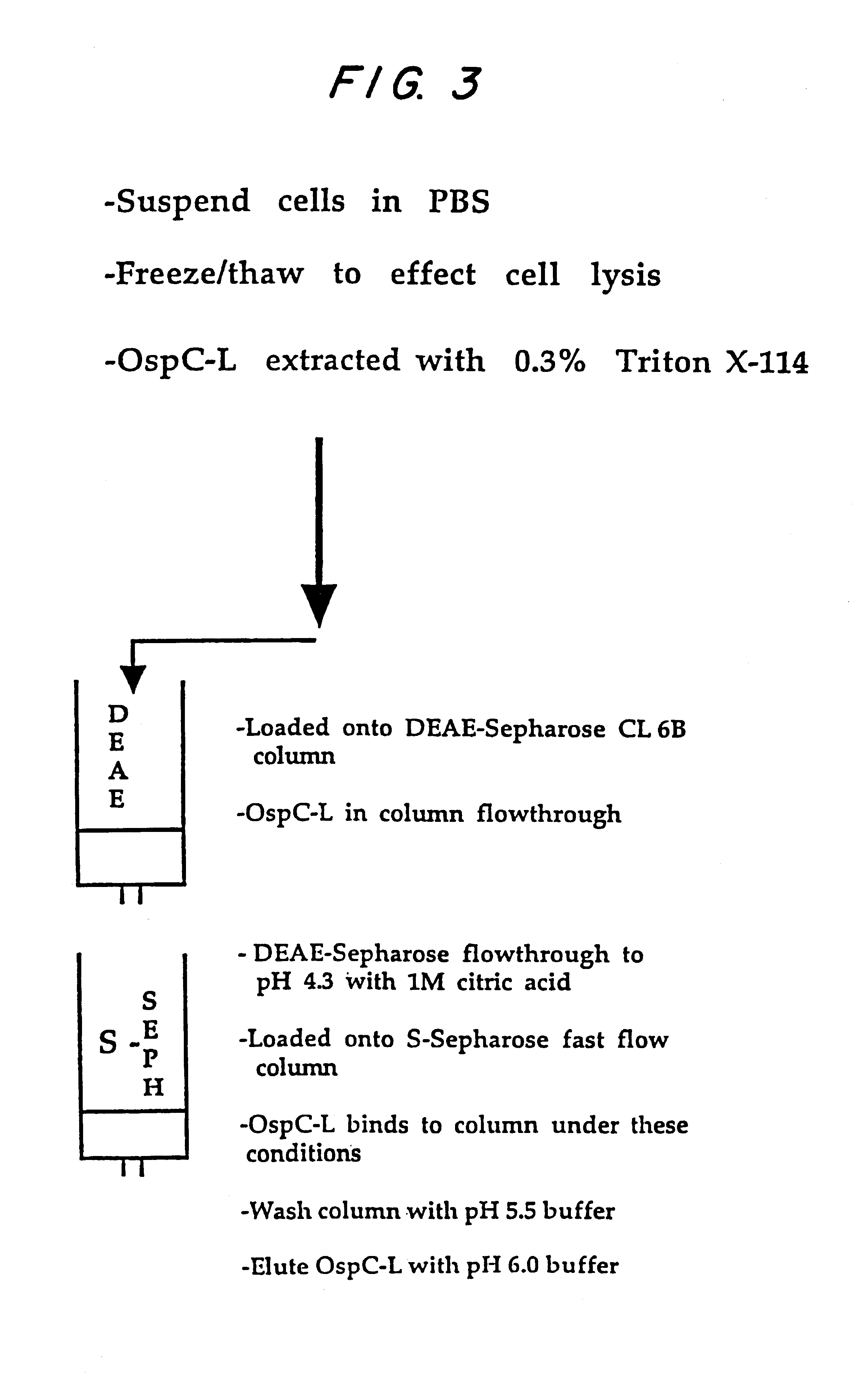
InactiveUS6538118B1Elicit immune responsePolypeptide with localisation/targeting motifSugar derivativesBorreliaEscherichia coli
Heterologous lipidated proteins formed recombinantly are disclosed and claimed. The expression system can be E. coli. The heterologous lipidated protein has a leader sequence which does not naturally occur with the protein portion of the lipidated protein. The lipidated protein can have the Borrelia OspA leader sequence. The protein portion can be OspC, PspA, UreA, Ure B, or a fragment thereof. Methods and compositions for forming and employing the proteins are also disclosed and claimed.
Owner:CONNAUGHT LAB
Chemotherapeutic agents as anti-cancer vaccine adjuvants and therapeutic methods thereof
InactiveUS20060051354A1Elicit immune responseSlow tumor growthVaccinesAntibody ingredientsAntigenTumor growth
The present invention relates to an anti-cancer vaccine composition comprising an antigen in association with an effective amount of at least one immunomodulator chemotherapeutic adjuvant eliciting an immune response in a patient and a pharmaceutically acceptable carrier. It also relates to method of preventing tumor growth and reducing tumor growth using the anti-cancer vaccine composition of the present invention.
Owner:TECH BIOLACTIS
Analog of Haemophilus Hin47 with reduced protease activity
An isolated and purified analog of Haemophilus influenzae Hin47 protein has a decreased protease activity which is less than about 10% of that of natural Hin47 protein and preferably substantially the same immunogenic properties as natural Hin47 protein. An isolated an purified nucleic acid molecule encoding the Hin47 analog may be provided in a recombinant plasmid which may be introduced into a cell which is grown to produce the Hin47 analog. Immunogenic compositions comprising the Hin47 analog and the encoding nucleic acid may be formulated as vaccines for in vivo administration to a host, including a human, to confer protection against diseases caused by a bacterial pathogen, including Haemophilus species, such as Haemophilus influenzae, that produces Hin47 protein or a protein capable of inducing antibodies in the host specifically reactive with Hin47 protein. The Hin47 analog and the encoding nucleic acid also may be employed in diagnostic applications.
Owner:CONNAUGHT LAB
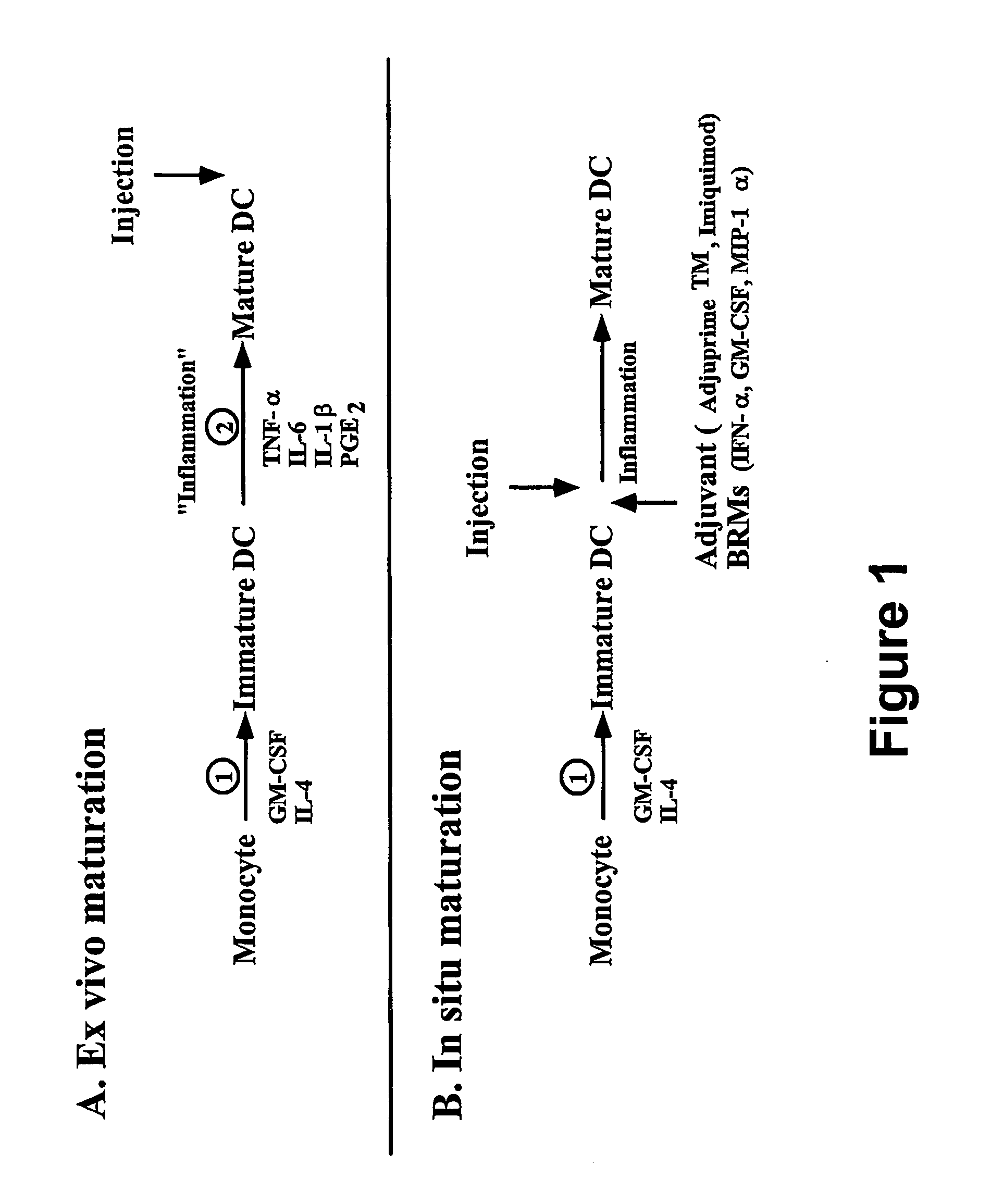
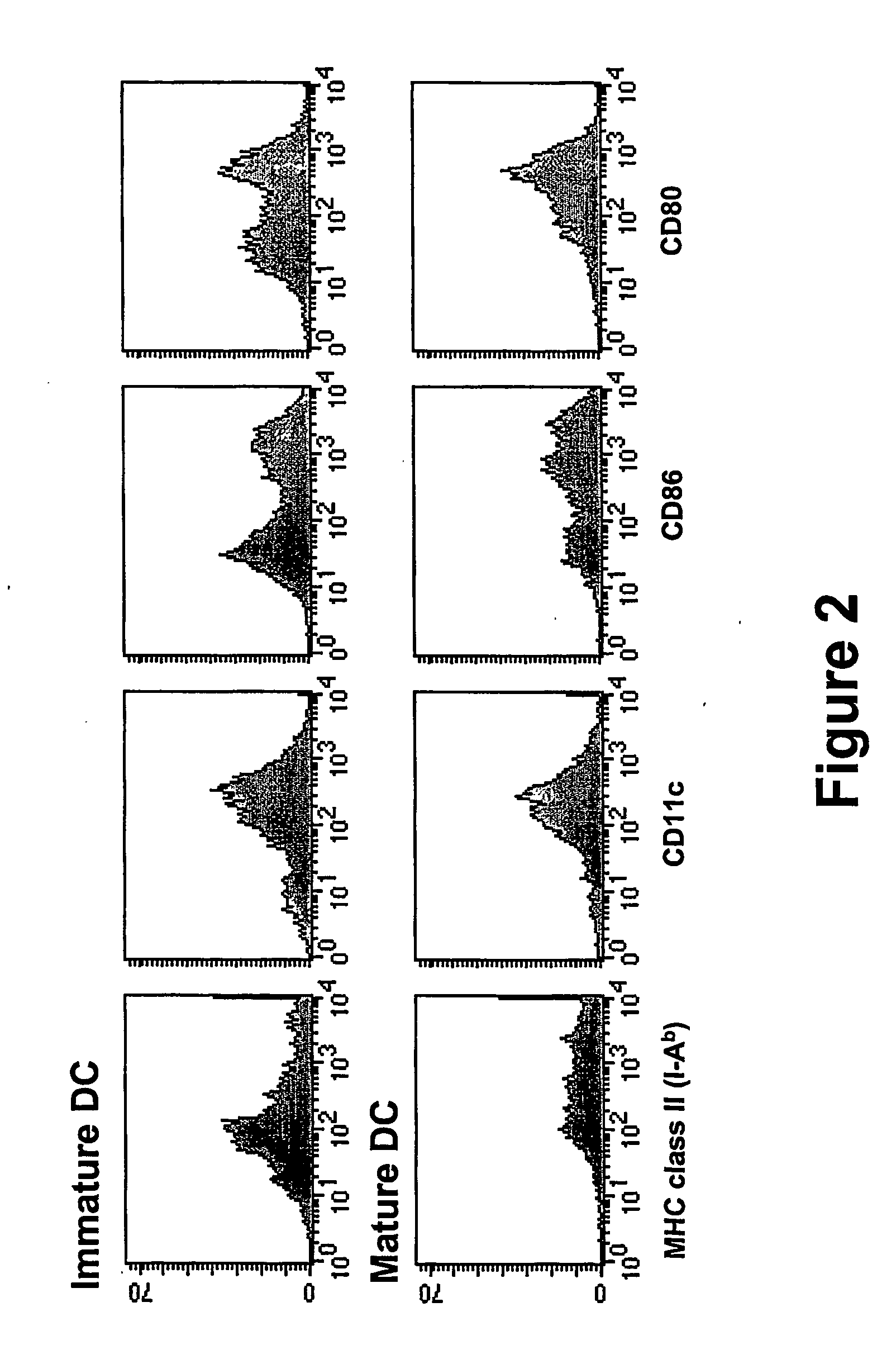
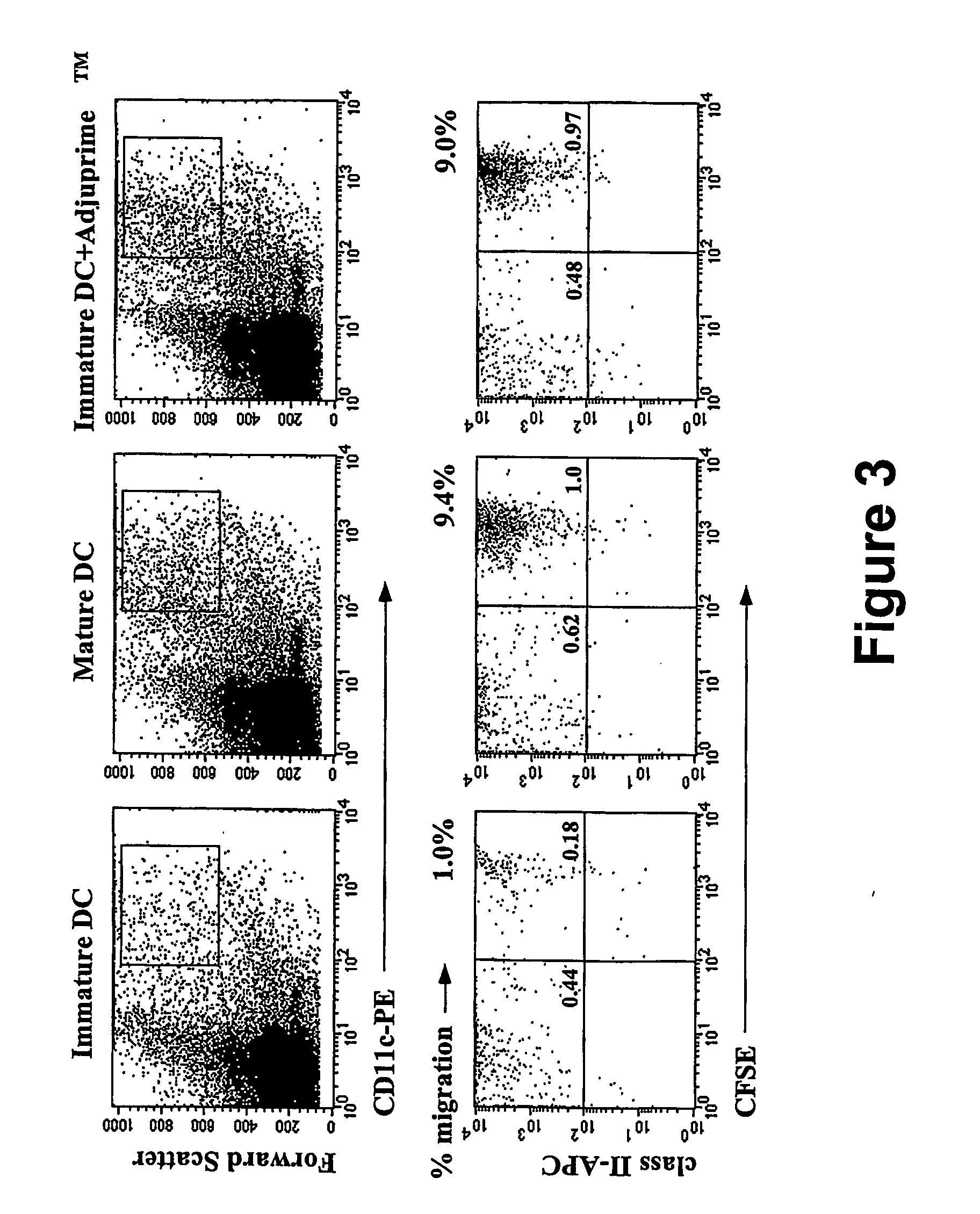
In situ maturation of dendritic cells
ActiveUS20060121003A1Elicit immune responseBiocideOrganic active ingredientsDendritic cellTreating Site
Owner:COIMMUNE INC
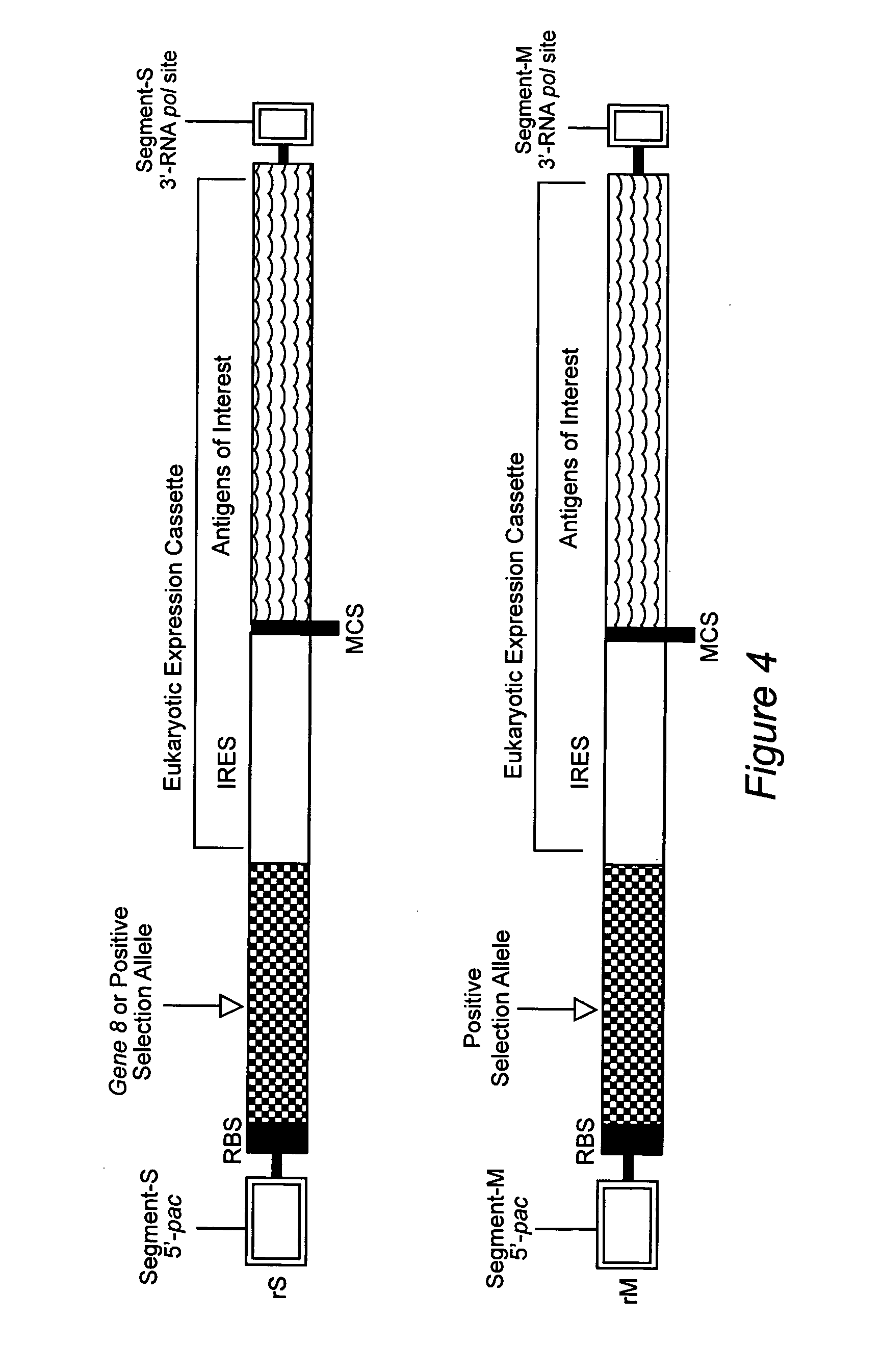
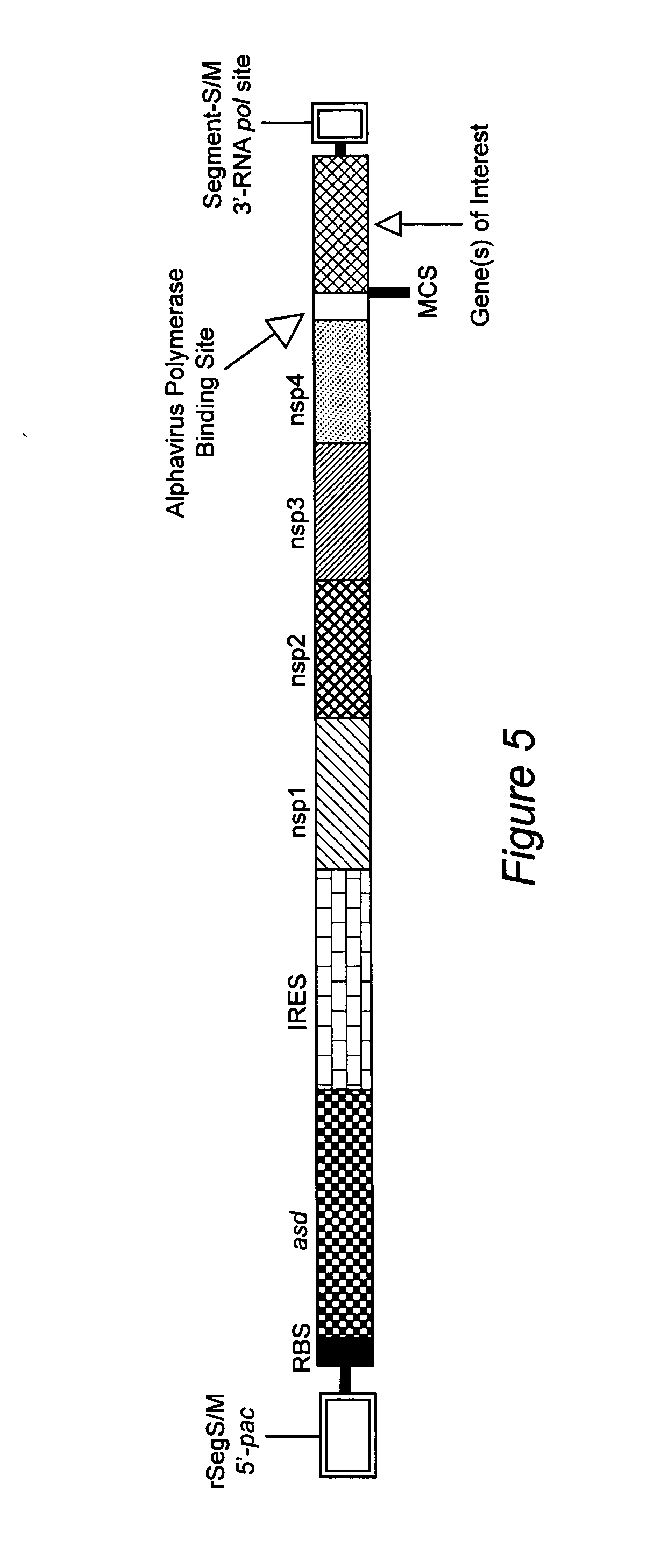
Bacterial packaging strains useful for generation and production of recombinant double-stranded RNA nucleocapsids and uses thereof
ActiveUS20060115493A1Elicit immune responseBacterial antigen ingredientsSugar derivativesEukaryotic cellRNA
Bacterial packaging strains useful for generating recombinant double-stranded RNA nucleocapsids (rdsRNs) are provided. The packaging strains are useful for the production of RNA encoding vaccine antigens, bioactive proteins, immunoregulatory proteins, antisense RNAs, and catalytic RNAs in eukaryotic cells or tissues. Recombinant ssRNA is introduced into the strains and packaged to form rdsRNs de novo.
Owner:INT AIDS VACCINE INITIATIVE INC
Methods for designing and preparing vaccines comprising directed sequence polymer compositions via the directed expansion of epitopes
InactiveUS20110129497A1Elicit immune responseSsRNA viruses negative-senseAntibacterial agentsEpitopeMedicine
The instant invention comprises a process of preparing a composition comprising directed sequence polymer (DSP) mixtures that act as epitopes and useful as vaccines, such DSP synthesized according to a set of rules regarding the identity and the frequency of occurrence of amino acids that substitute a base or native amino acid of a known epitope. The resulting composition is a mixture of related peptides for therapeutic use as a vaccine, preferably for infectious agents that are immune evasive.
Owner:DECLION HLDG
Extraneous DNA sequence that facilitates hantavirus gene expression
InactiveUS7217812B2Not painful to administerAvoid infectionSugar derivativesViral antigen ingredientsDiseaseSerum ige
In this application is described a protective DNA vaccines against infection with HFRS- and HPS-associated hantaviruses. The vaccines were constructed by subcloning cDNA representing the medium (M) (encoding the G1 and G2 glycoproteins) into the DNA expression vector pWRG7077. Animals vaccinated with the M construct developed a neutralizing antibody response. Passive transfer experiments show that serum from vaccinated animals, when injected on days 4 or 5 after challenge, protected animals from lethal disease.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Analog of haemophilus Hin47 with reduced protease activity
InactiveUS6153580AImprove stabilityImprove thermal stabilityAntibacterial agentsBiocideBacteroidesDisease
An isolated and purified analog of Haemophilus influenzae Hin47 protein has a decreased protease activity which is less than about 10% of that of natural Hin47 protein and preferably substantially the same immunogenic properties as natural Hin47 protein. An isolated an purified nucleic acid molecule encoding the Hin47 analog may be provided in a recombinant plasmid which may be introduced into a cell which is grown to produce the Hin47 analog. Immunogenic compositions comprising the Hin47 analog and the encoding nucleic acid may be formulated as vaccines for in vivo administration to a host, including a human, to confer protection against diseases caused by a bacterial pathogen, including Haemophilus species, such as Haemophilus influenzae, that produces Hin47 protein or a protein capable of inducing antibodies in the host specifically reactive with Hin47 protein. The Hin47 analog and the encoding nucleic acid also may be employed in diagnostic applications.
Owner:LOOSMORE SHEENA M +4
Plasmodium vivax hybrid circumsporozoite protein and vaccine
InactiveUS20090196883A1Not painful to administerIntrinsically safePeptide/protein ingredientsAntibody mimetics/scaffoldsCircumsporozoite proteinAntibody production
Described in this application is a synthetic P. vivax circumsporozoite protein useful as a diagnostic reagent, for antibody production, and as a vaccine protective against infection with any strain of P. vivax.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Neisseria meningitidis compositions and methods thereof
ActiveUS10183070B2Elicit immune responseAntibacterial agentsOrganic active ingredientsSalmonella serotype typhiNeisseria meningitidis
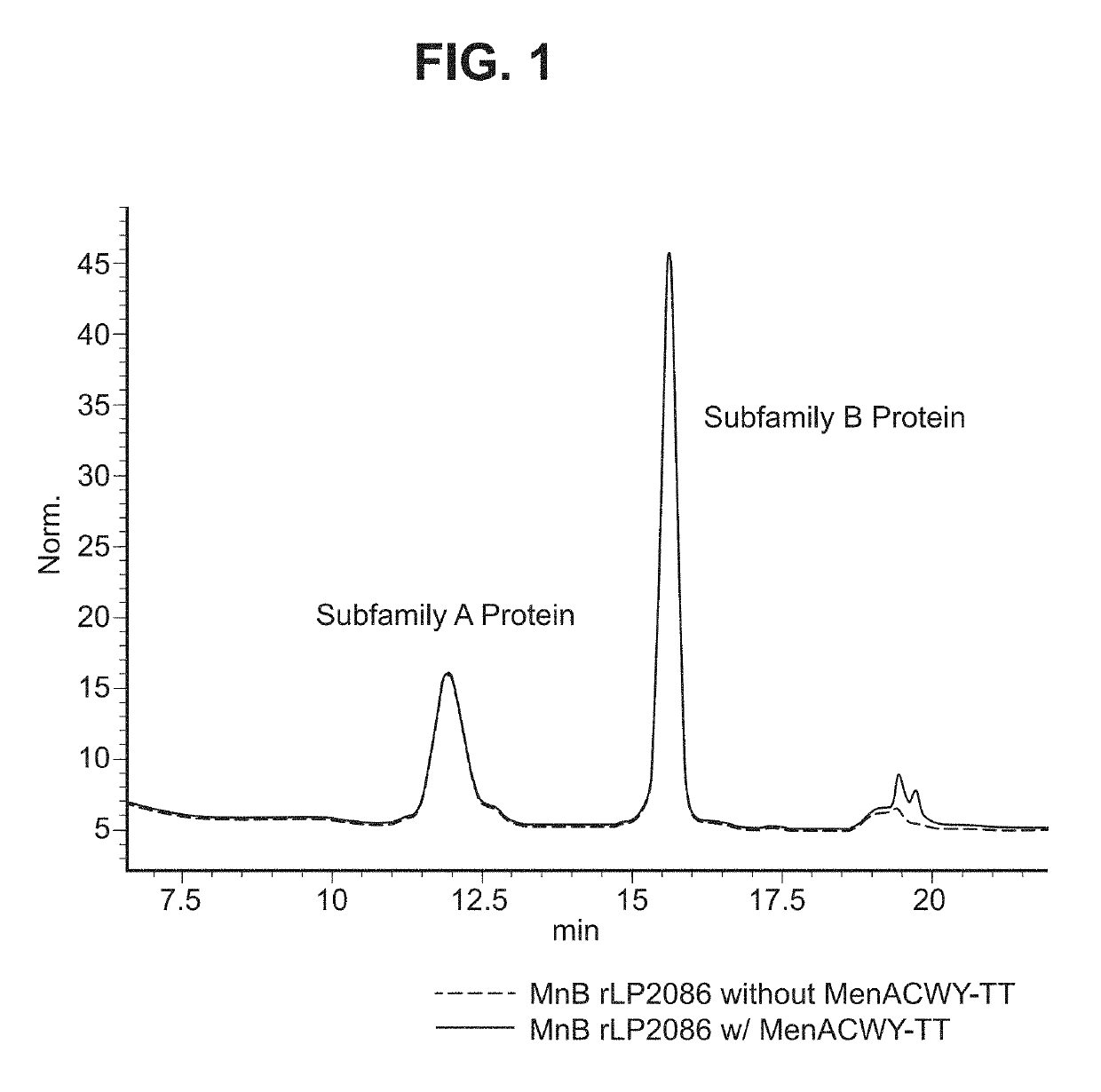
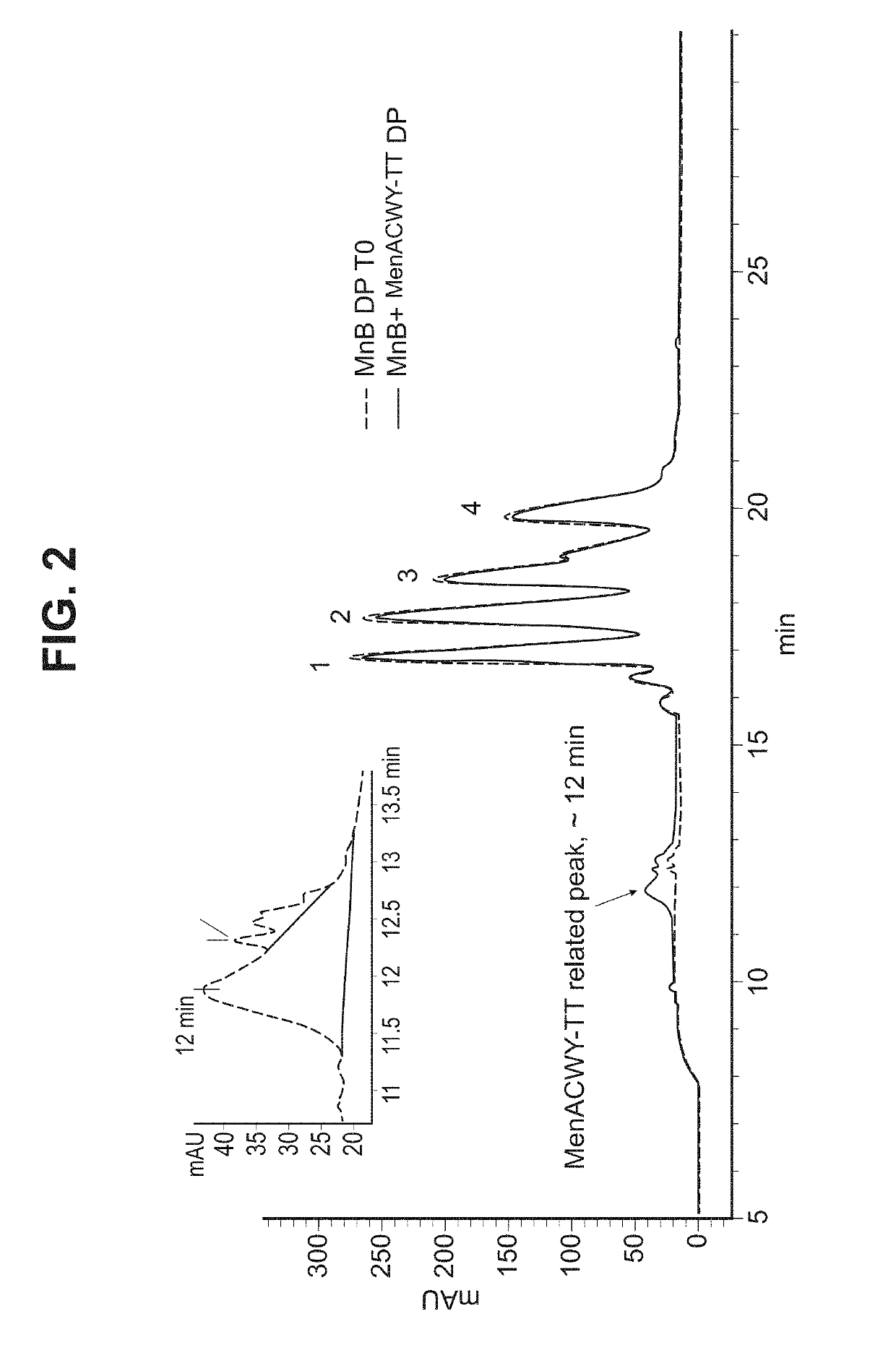
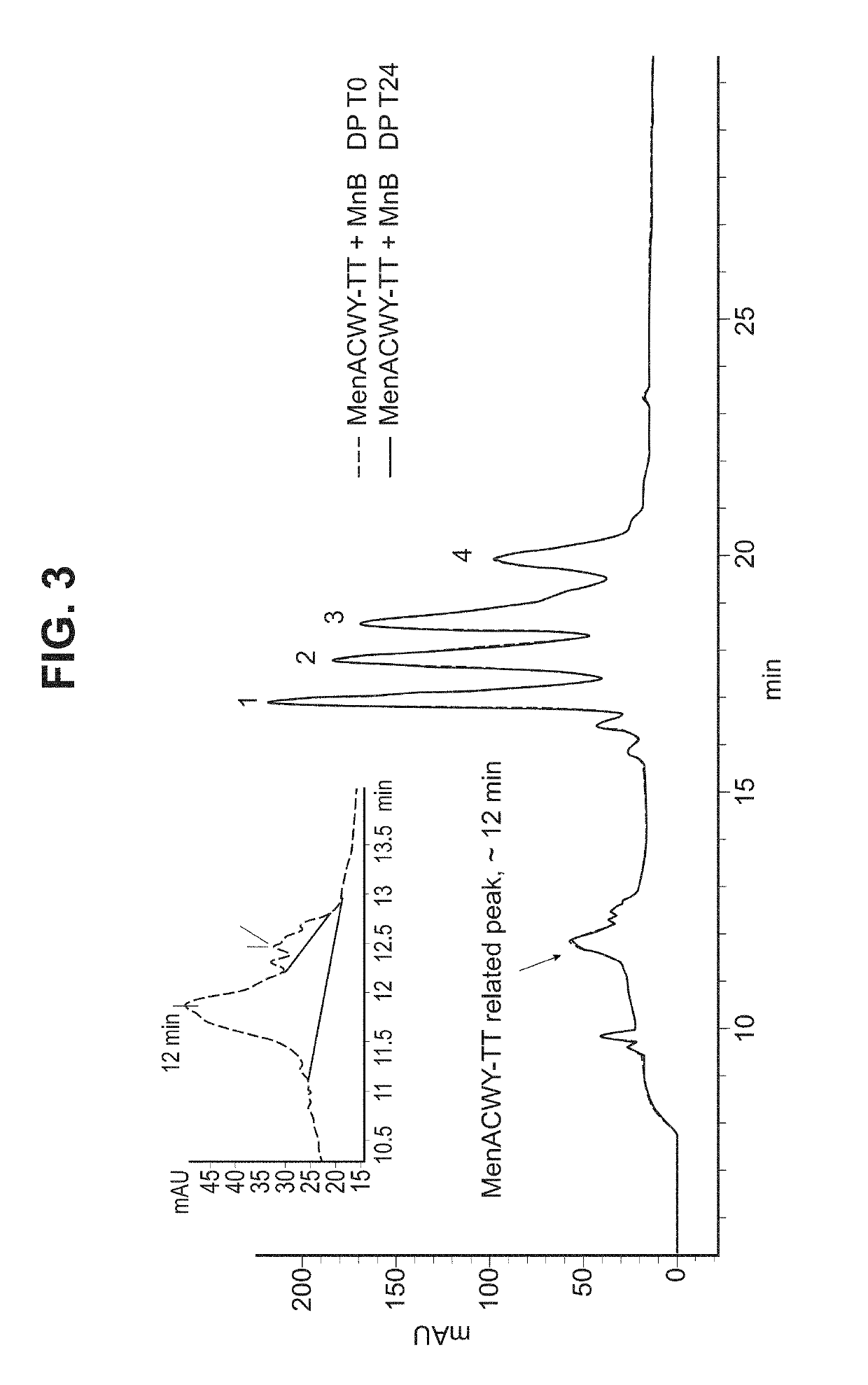
In one aspect, the invention relates to a composition including a factor H binding protein (fHBP) and a Neisseria meningitidis non-serogroup B capsular polysaccharide. The invention further relates to uses of a composition that includes fHBP, such as, for example, uses to elicit an immune response against N. meningitidis serogroup B strains and non-serogroup B strains. The compositions and methods described herein are directed to administration in humans, including adults, adolescents, toddlers, and infants.
Owner:PFIZER INC
Extracellular serine protease
InactiveUS20060205054A1Elicit immune responseCompound screeningTumor rejection antigen precursorsColon carcinomaA-DNA
The present invention provides a DNA encoding a novel extracellular serine protease termed Tumor Antigen Derived Gene-14 (TADG-14) which is overexpressed in ovarian, breast and colon carcinoma samples. Also provided are vector and host cells capable of expressing the DNA of the present invention, as well as the uses of the DNA and protein of the present invention. Also provided is a TADG-14 protein variant that has a potential role for detecting and targeting of ovarian carcinomas.
Owner:UNIV OF ARKANSAS FOR MEDICAL SCI THE +1
Fully human Anti-vap-1 monoclonal antibodies
ActiveUS20110117021A1Elicit immune responseImproved profileSenses disorderNervous disorderMonoclonal antibodyBiology
Novel fully human anti-VAP-1 antibodies and fragments thereof are disclosed. Nucleic acids encoding anti-VAP-1 antibodies or fragments thereof, as well as expression vectors and host cells incorporating these nucleic acids for the recombinant expression of anti-VAP-1 antibodies are also given. Pharmaceutical compositions comprising said antibodies and therapeutic uses thereof are also disclosed.
Owner:BIOTIE THERAPIES CORP
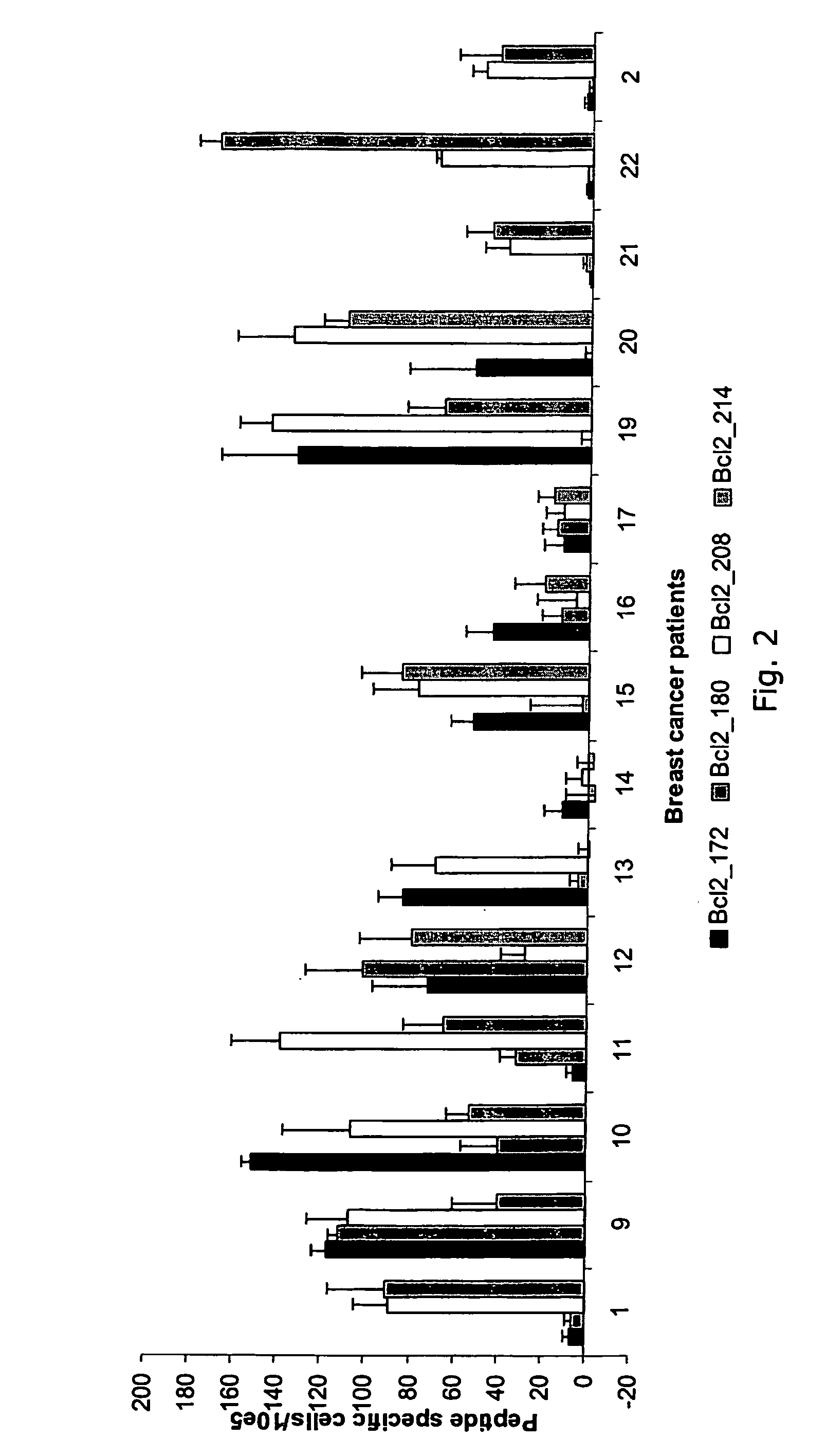
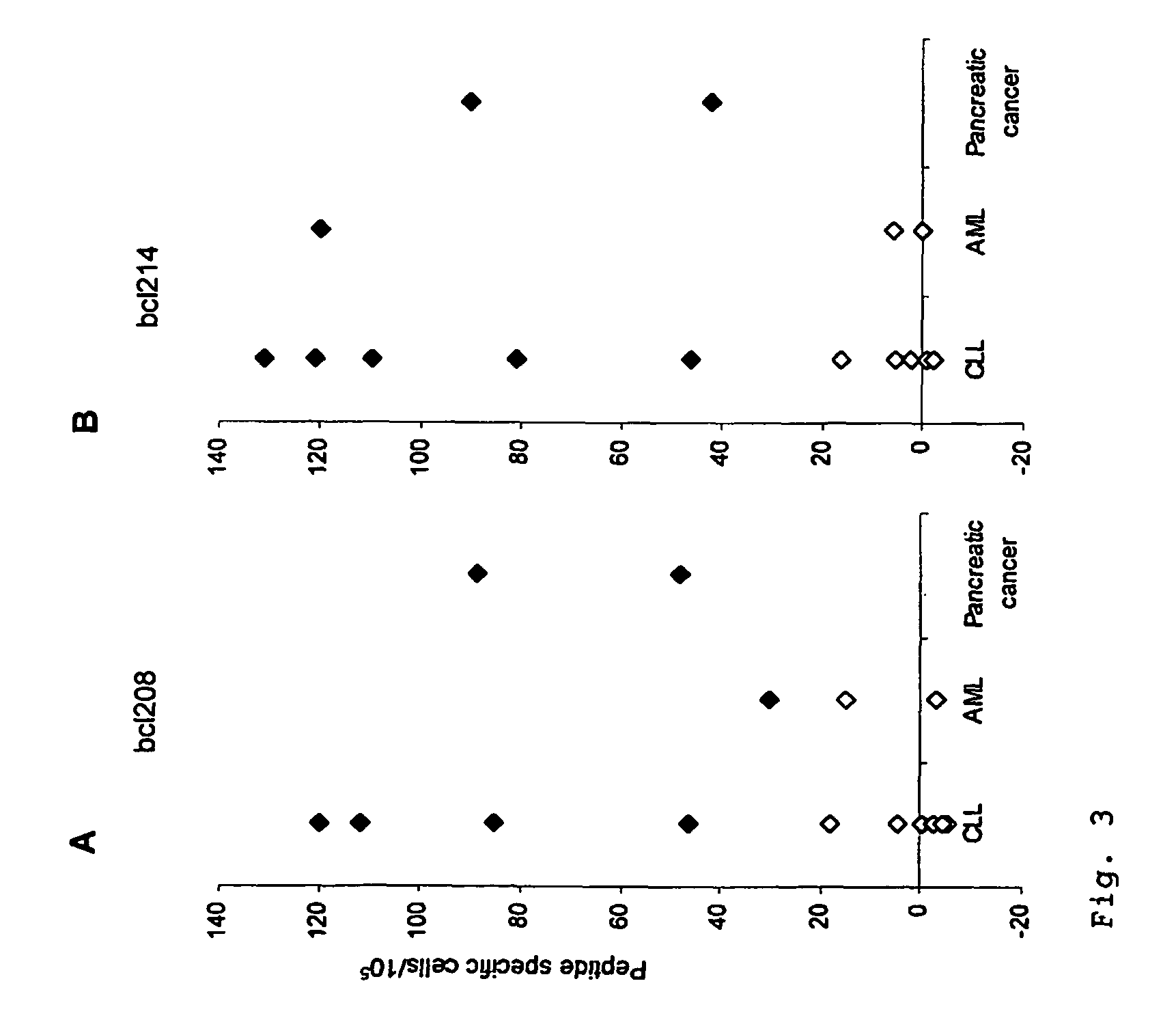
Proteins belonging to the Bcl-2 family and fragments thereof, and their use in cancer patients
ActiveUS7842294B2Elicit immune responseTumor rejection antigen precursorsPeptide/protein ingredientsADAMTS ProteinsTreatment use
The present invention relates to proteins belonging to the Bcl-2 family and peptides fragments thereof for use in pharmaceutical compositions. The disclosed proteins and peptide fragments are in particularly useful in vaccine compositions for treatment of cancer. The invention furthermore relates to methods of treatment using said compositions. It is also an aspect of the invention to provide T-cells and T-cell receptors specifically recognising the disclosed proteins and peptide fragments.
Owner:SURVAC
Allogenic tumor cell vaccine
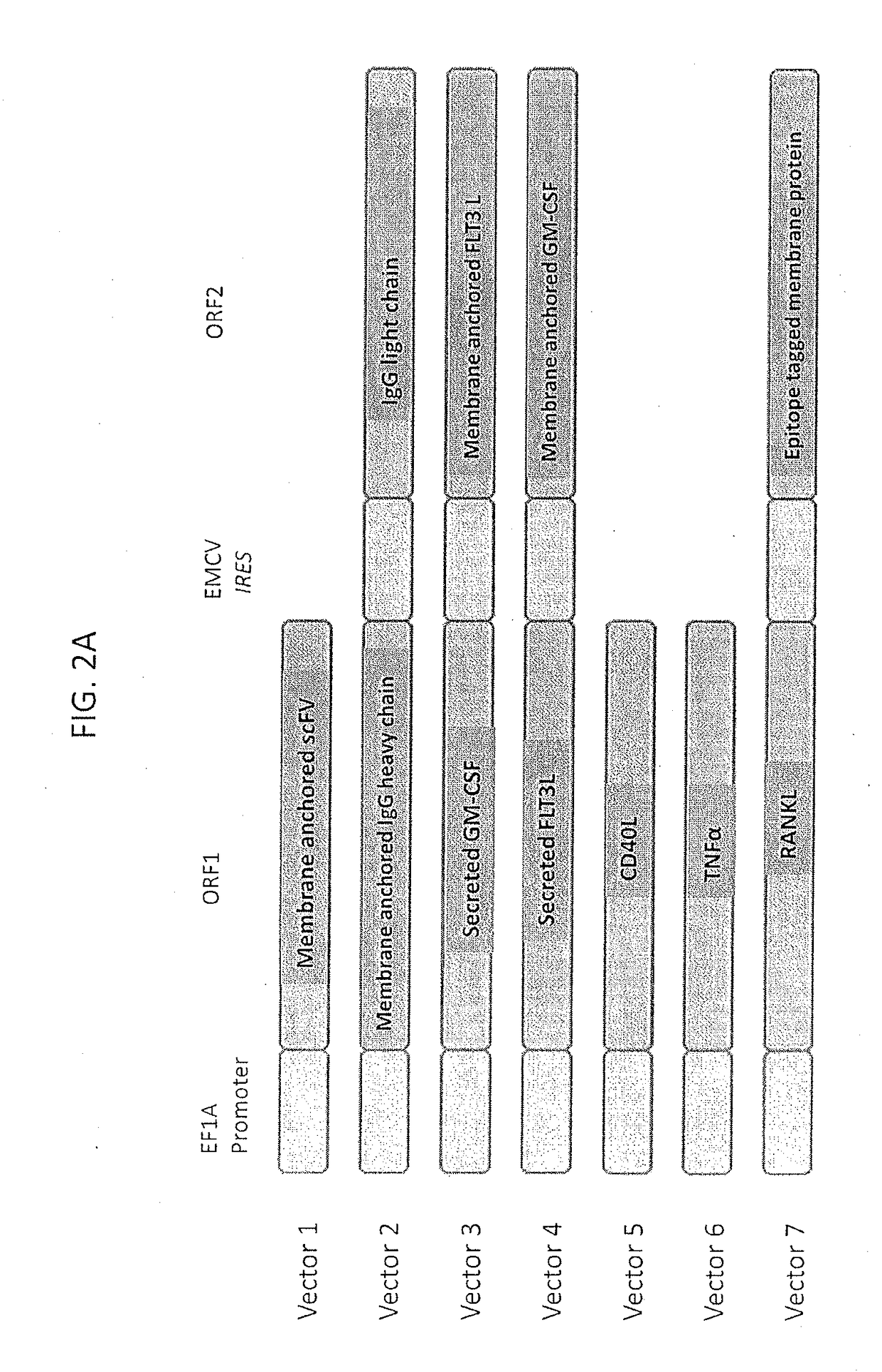
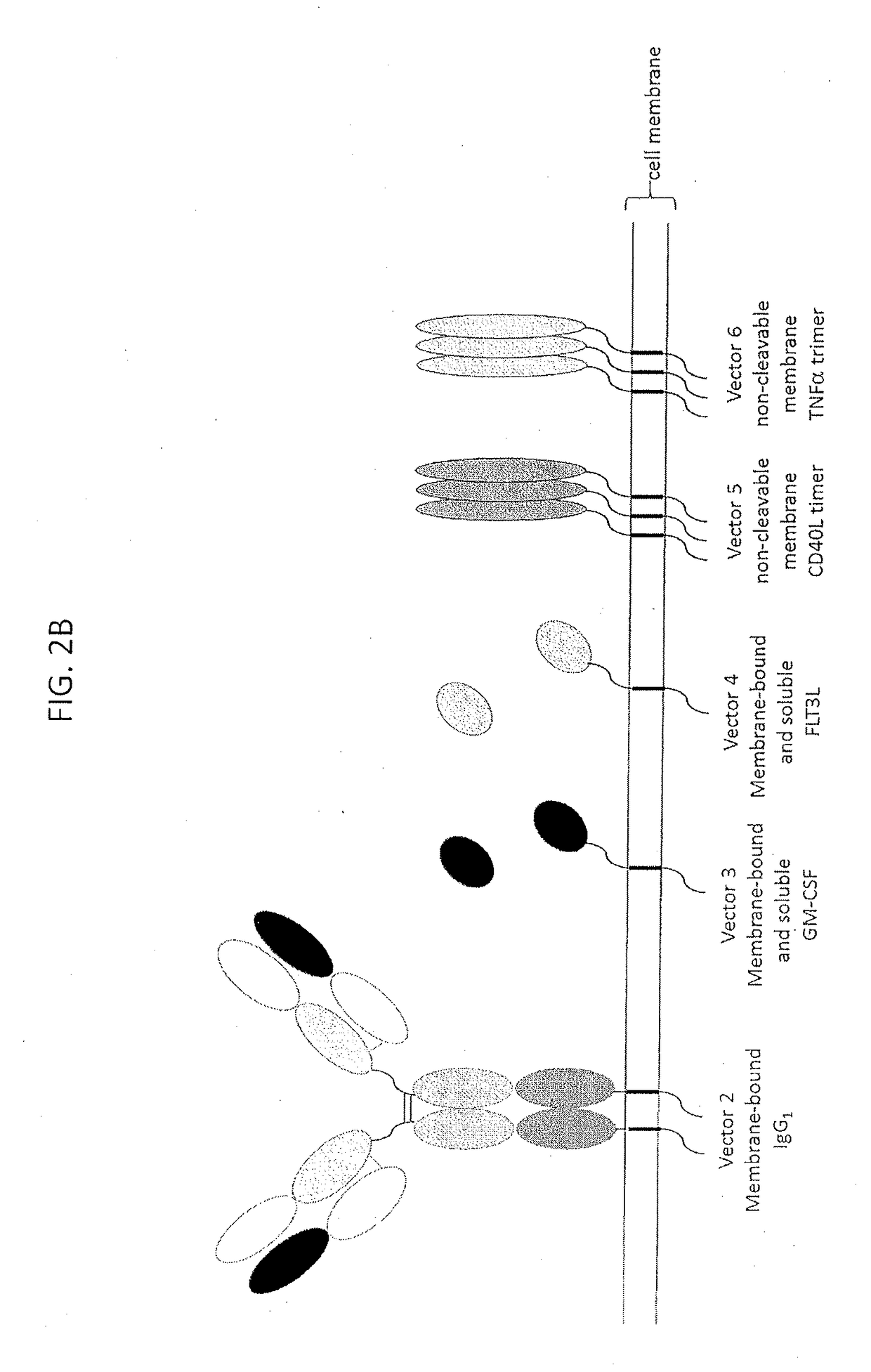
ActiveUS20180185463A1Improve clinical outcomeImprove clinical outcomesTumor necrosis factorSkin cancer vaccineGreek letter alphaImmunogen
The described invention provides a tumor cell vaccine comprising genetically modified tumor cell line of a particular tumor type that stably expresses high levels of two or more immunomodulators. According to some embodiments, an immunogenic amount of the tumor cell line variants may be selected for concomitant expression of two or more of recombinant membrane expressed IgG1, CD40L, TNF-alpha, as well as membrane and soluble forms of GM-CSF, and Flt-3L peptides that are effective to elicit an anti-tumor immune response compared to the parent unmodified tumor cell line as measured in vitro by a one-way mixed lymphocyte tumor reaction assay using human peripheral blood mononuclear cells and the genetically modified allogeneic cell vaccine candidate. According to some embodiments, the tumor cell vaccine candidate will induce an immune response in the recipient cancer patient that cross reacts with the patient's own (autologous) tumor cells, the effects of which will be sufficient to result in enhanced anti-tumor immunity contributing to the increased survival of a vaccinated patient cohort compared to a matched unvaccinated patient cohort.
Owner:ALLOPLEX BIOTHERAPEUTICS
Human cytomegalovirus vaccine
InactiveUS20130164289A1Elicit immune responsePrevent entryPeptide/protein ingredientsVirus peptidesPeptideCytomegalovirus disease
Combination peptides, polypeptides and proteins that elicit high titer neutralizing antibodies against cytomegalovirus (CMV) are provided. The combination peptides, polypeptides and proteins encompass epitopes located within the UL130 and UL131 components of the gH / gL / UL128-131 protein complex, in particular, epitopes located within amino acid residues 27-46 of UL130 and amino acid residues 90-106 of UL131. The combination peptides, polypeptides and proteins, and the nucleic acids encoding them, may be used in vaccines, and as diagnostic and research tools.
Owner:VIRGINIA COMMONWEALTH UNIV
Neisseria meningitidis compositions and methods thereof
ActiveUS20190231861A1Elicit immune responseAntibacterial agentsOrganic active ingredientsNeisseria meningitidisPolysaccharide
In one aspect, the invention relates to a composition including a factor H binding protein (fHBP) and a Neisseria meningitidis non-serogroup B capsular polysaccharide. The invention further relates to uses of a composition that includes fHBP, such as, for example, uses to elicit an immune response against N. meningitidis serogroup B strains and non-serogroup B strains. The compositions and methods described herein are directed to administration in humans, including adults, adolescents, toddlers, and infants.
Owner:PFIZER INC
Antigenic peptide of hsv-2 and methods for using same
InactiveUS20120027789A1Elicit immune responseBacteriaPeptide/protein ingredientsT-Cell SpecificityPeptide
The invention provides HSV antigens that are useful for the prevention and treatment of HSV infection. Disclosed herein are epitopes confirmed to be recognized by T-cells derived from herpetic lesions. T-cells having specificity for antigens of the invention have demonstrated cytotoxic activity against cells loaded with virally-encoded peptide epitopes, and in many cases, against cells infected with HSV, The identification of immunogenic antigens responsible for T-cell specificity provides improved anti-viral therapeutic and prophylactic strategies. Compositions containing antigens or polynucleotides encoding antigens of the invention provide effectively targeted vaccines for prevention and treatment of HSV infection.
Owner:FRED HUTCHINSON CANCER RES CENT +1
Novel therapeutic target for protozoal diseases
ActiveUS20070087012A1Elicit immune responsePeptide/protein ingredientsImmunoglobulinsEpitopeHemozoin
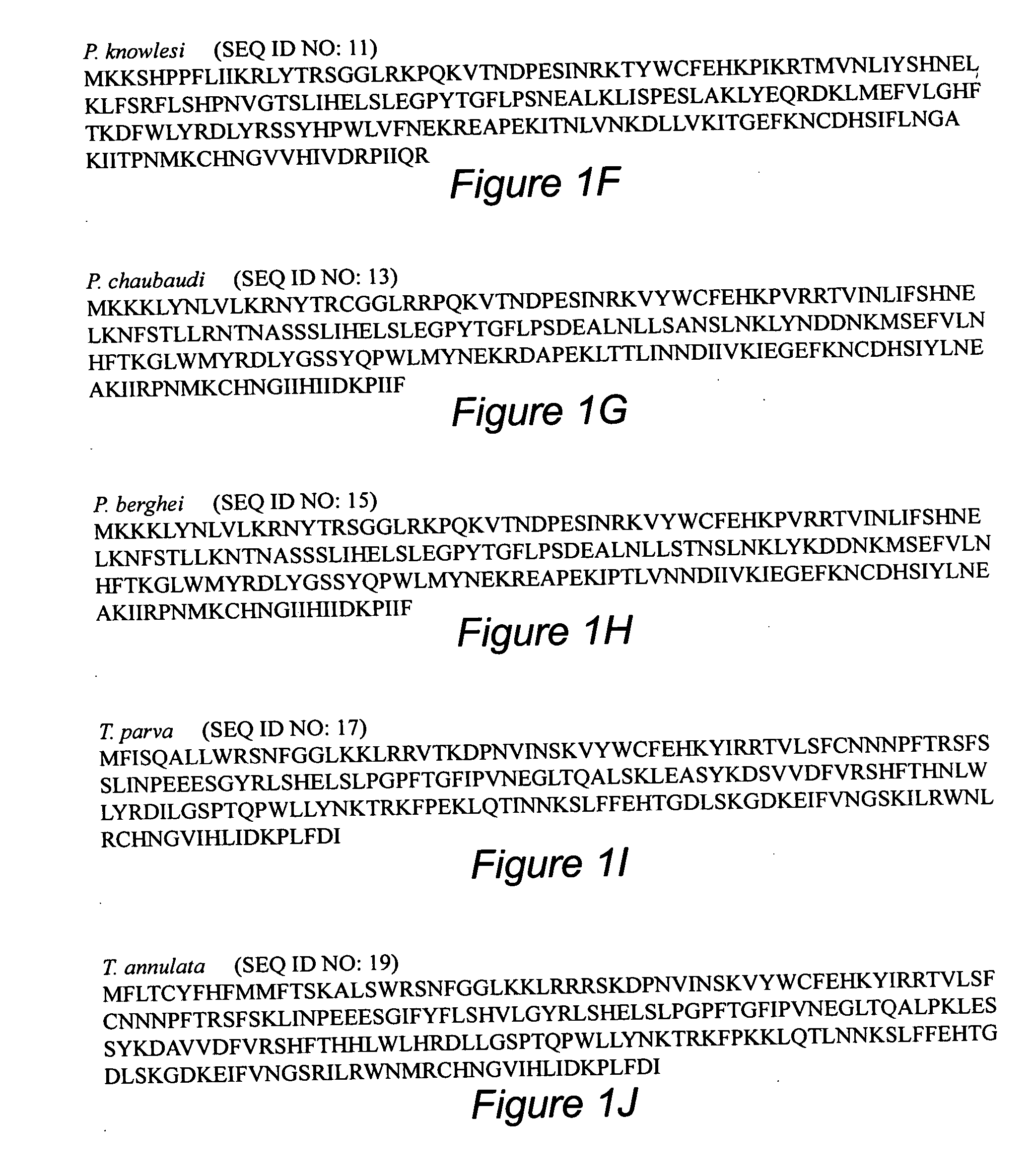
A novel Fasciclin Related Adhesive Protein (FRAP) from Plasmodium and related parasites is provided as a target for therapeutic intervention in diseases caused by the parasites. FRAP has been shown to play a critical role in adhesion to, or invasion into, host cells by the parasite. Furthermore, FRAP catalyzes the neutralization of heme by the parasite, by promoting its polymerization into hemozoin. This invention provides methods and compositions for therapies based on the administration of protein, DNA or cell-based vaccines and / or antibodies based on FRAP, or antigenic epitopes of FRAP, either alone or in combination with other parasite antigens. Methods for the development of compounds that inhibit the catalytic activity of FRAP, and diagnostic and laboratory methods utilizing FRAP are also provided.
Owner:VIRGINIA TECH INTPROP INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com