Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Circumsporozoite protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
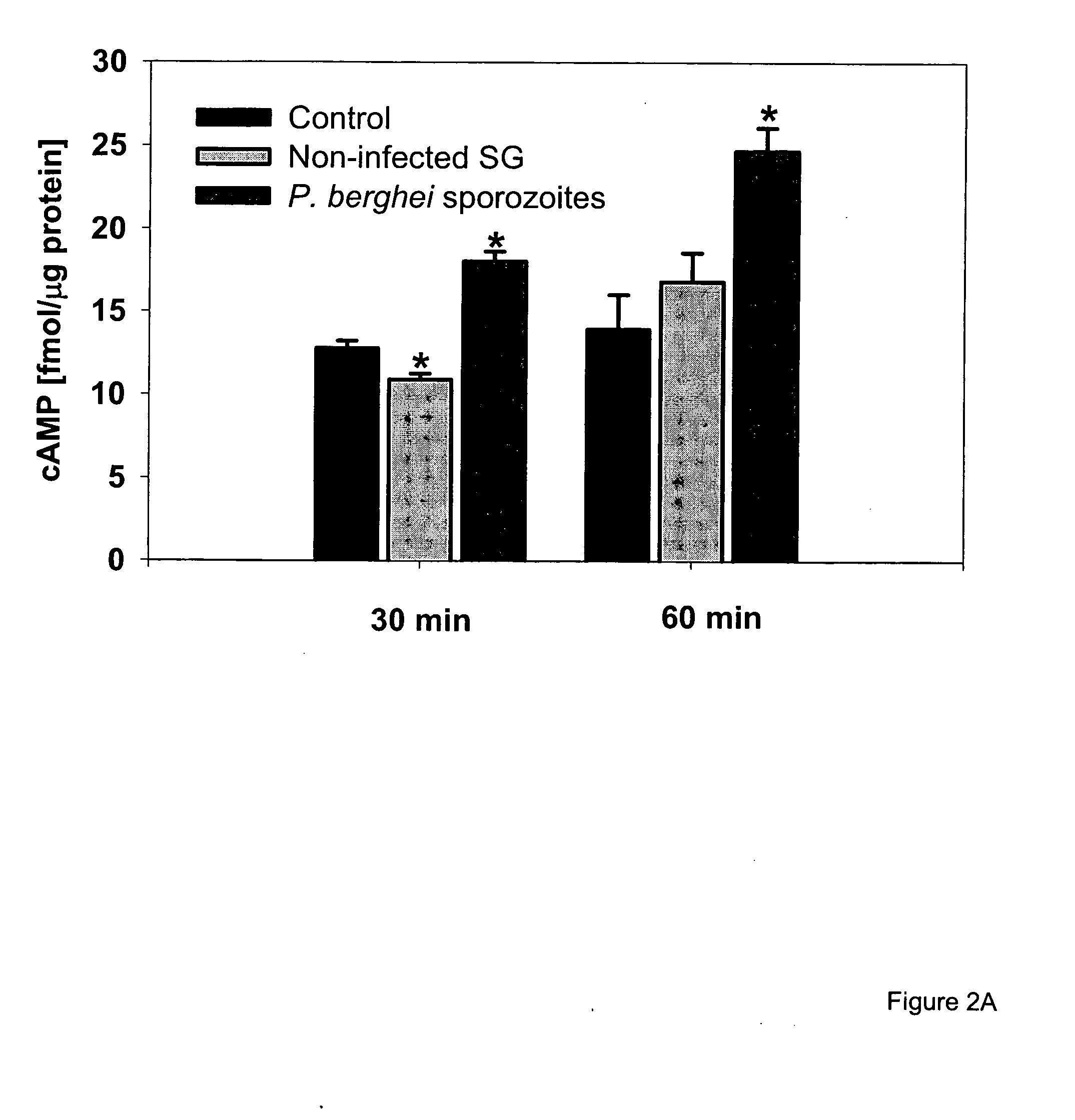
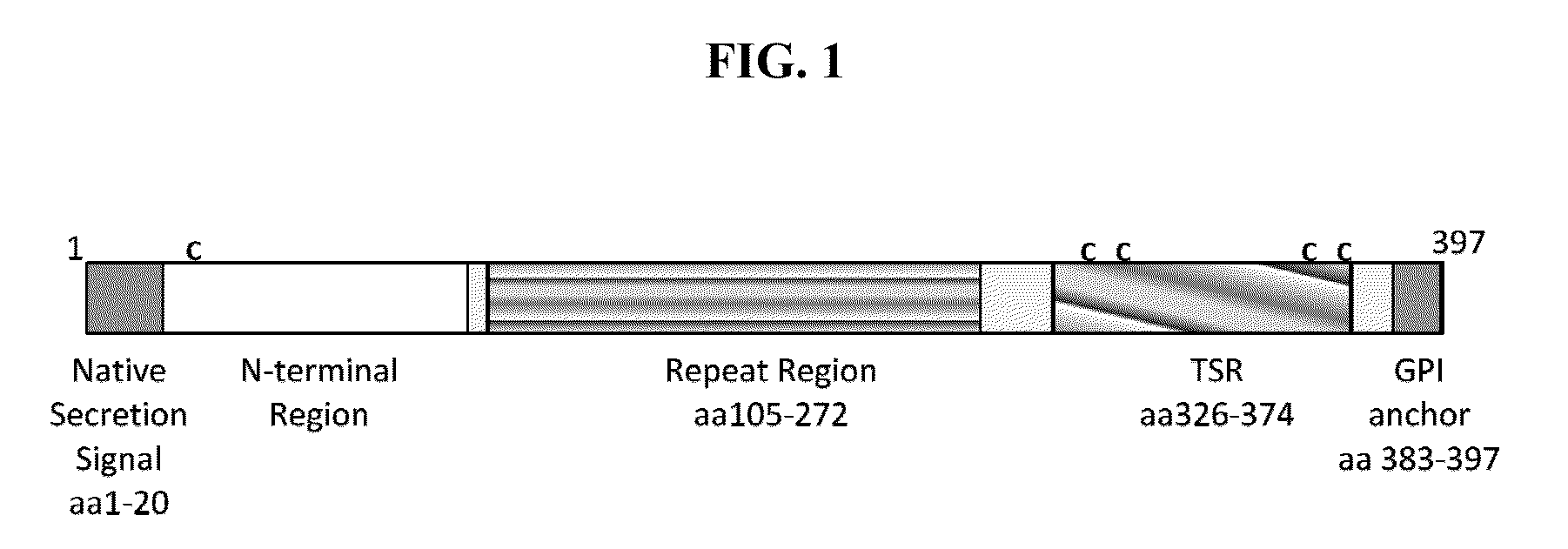
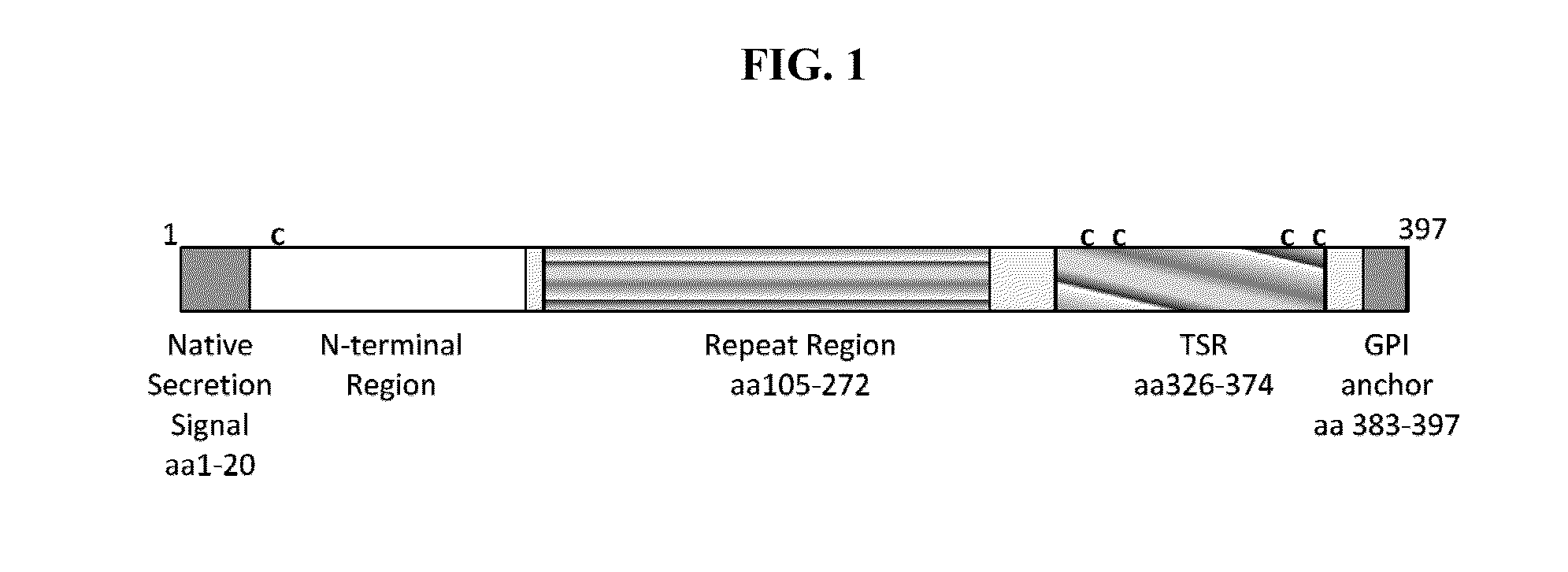
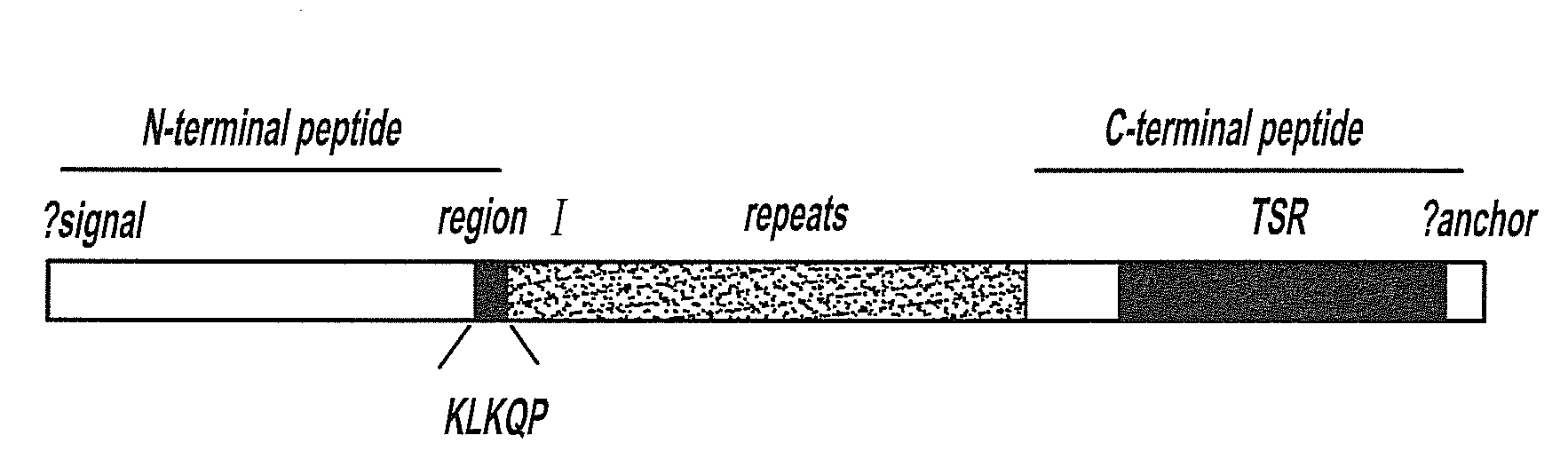
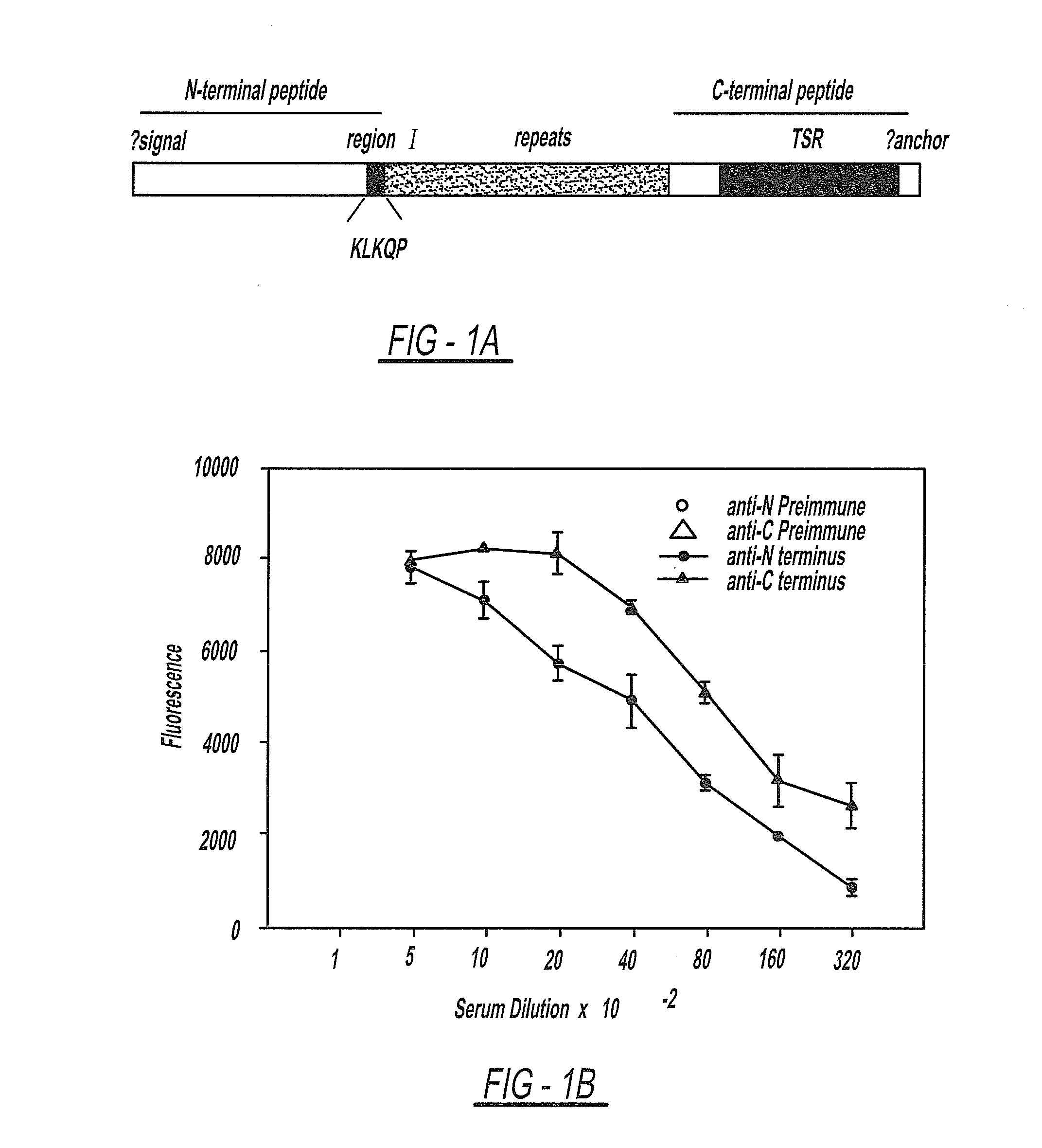
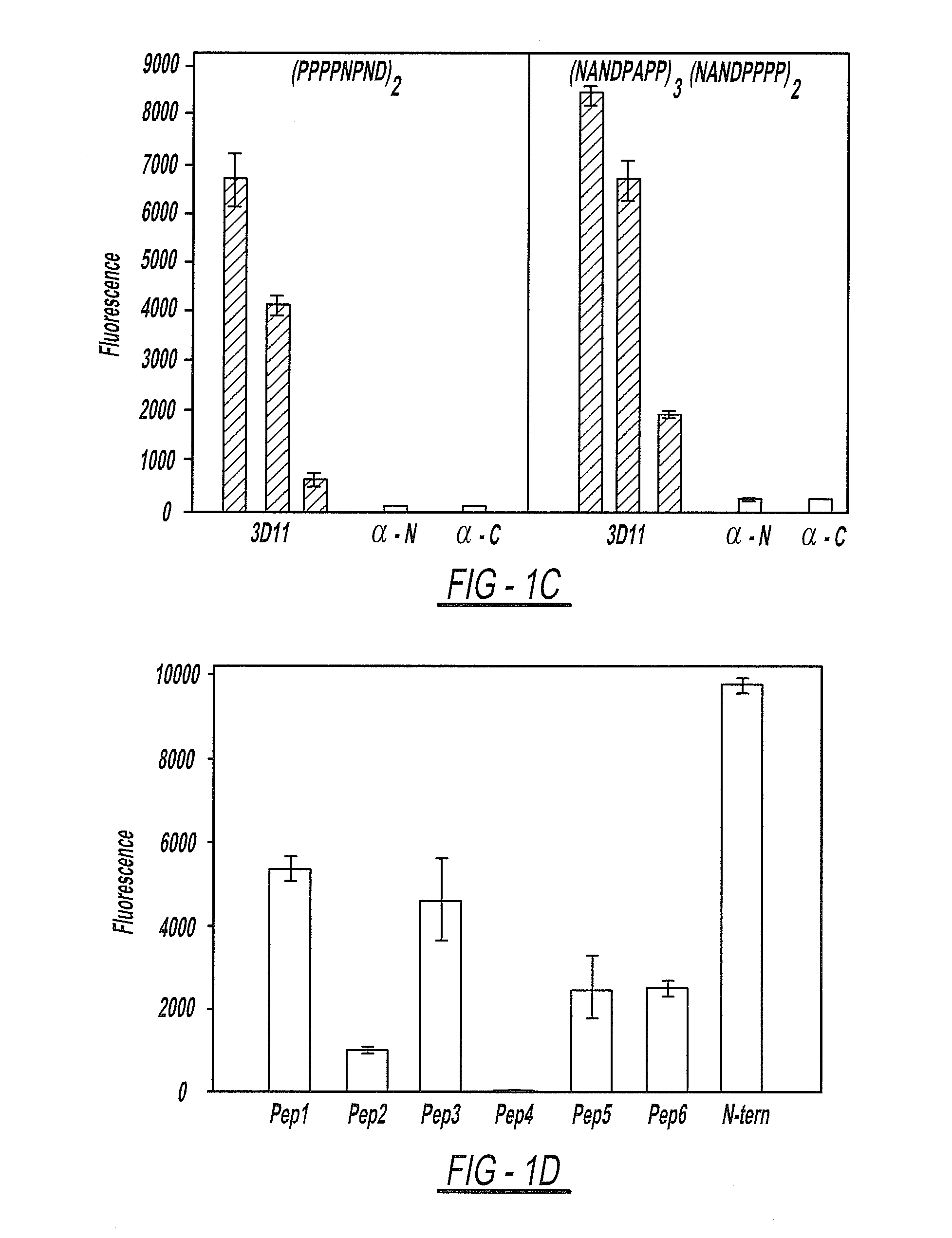
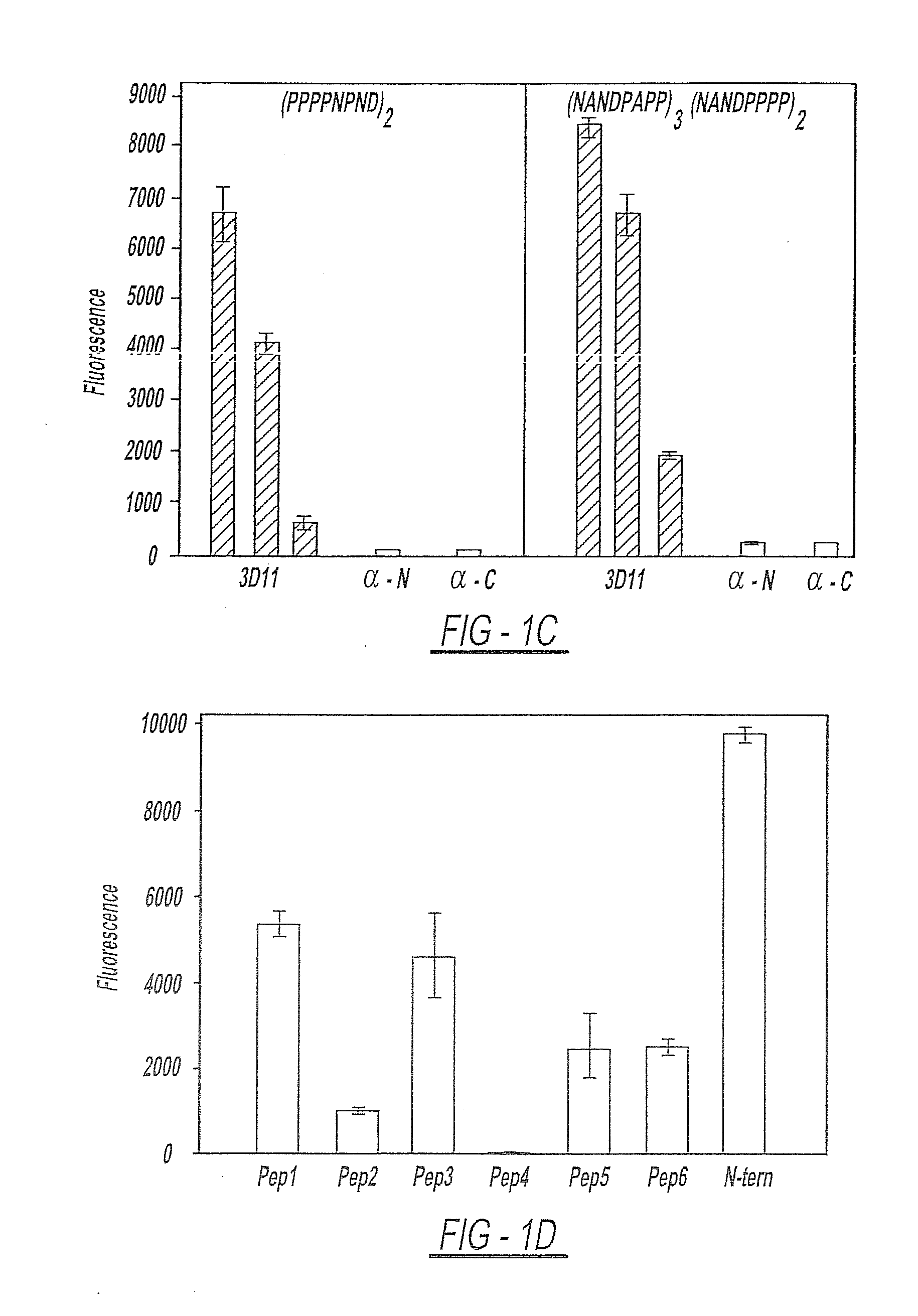
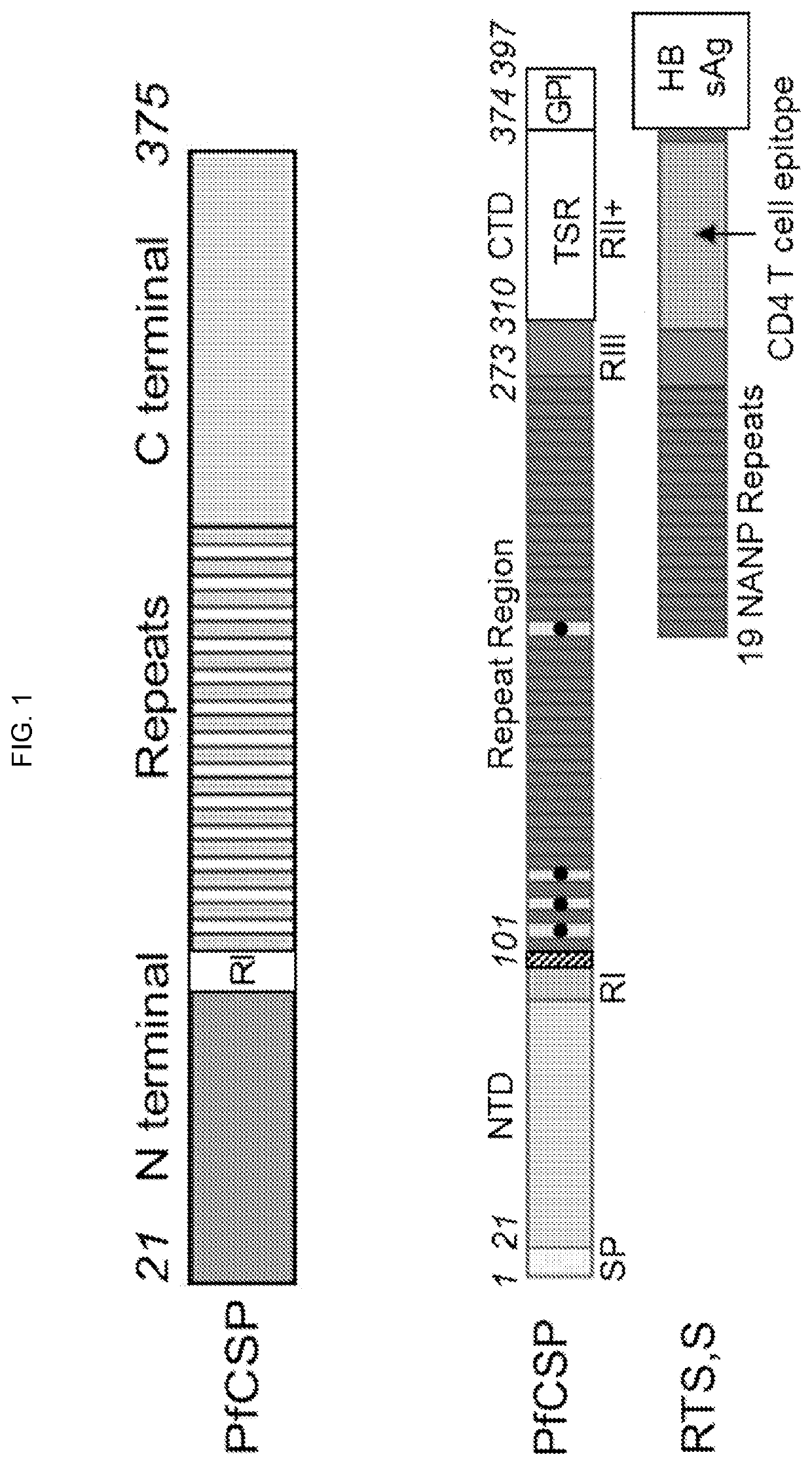
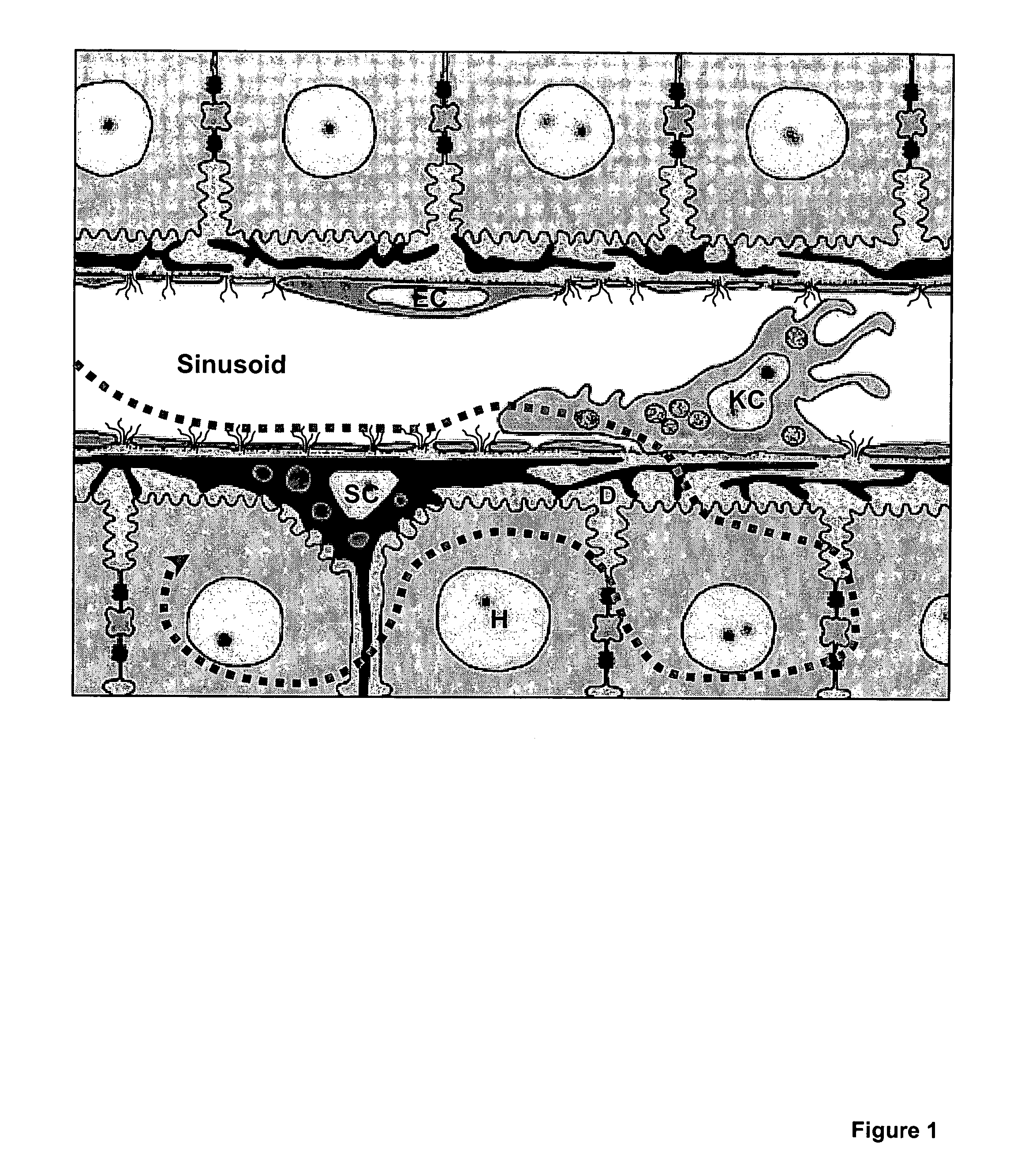
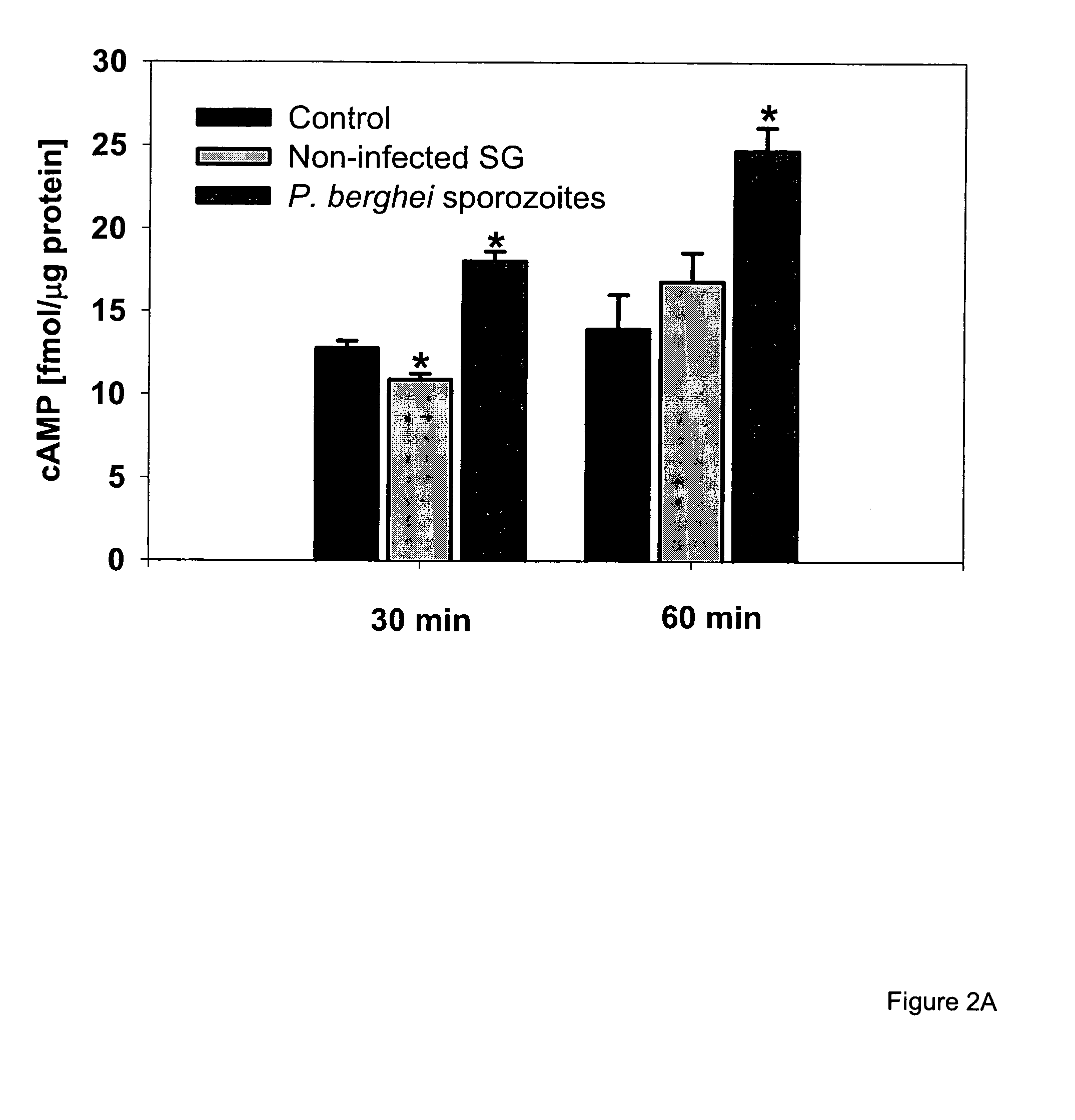
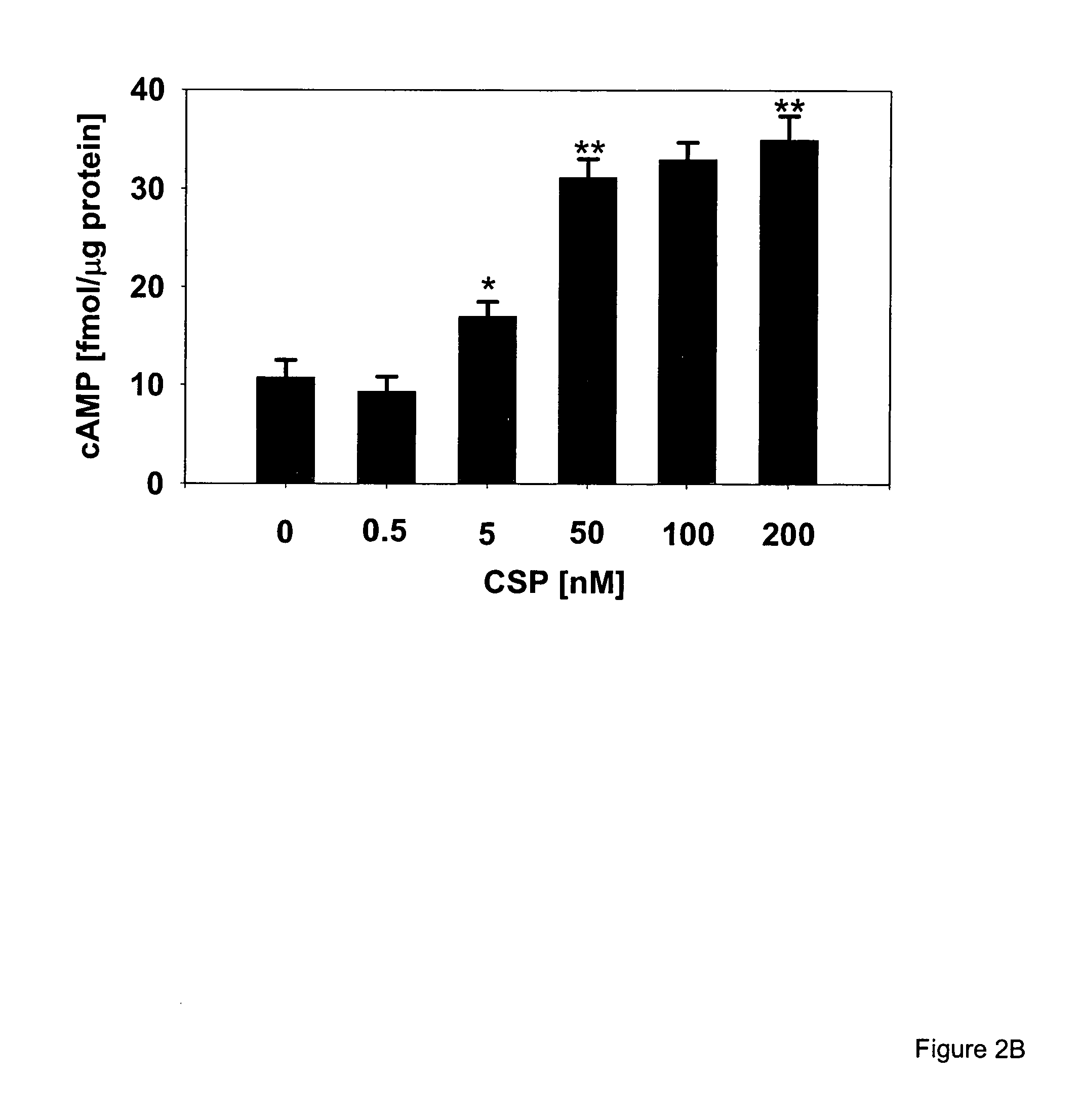
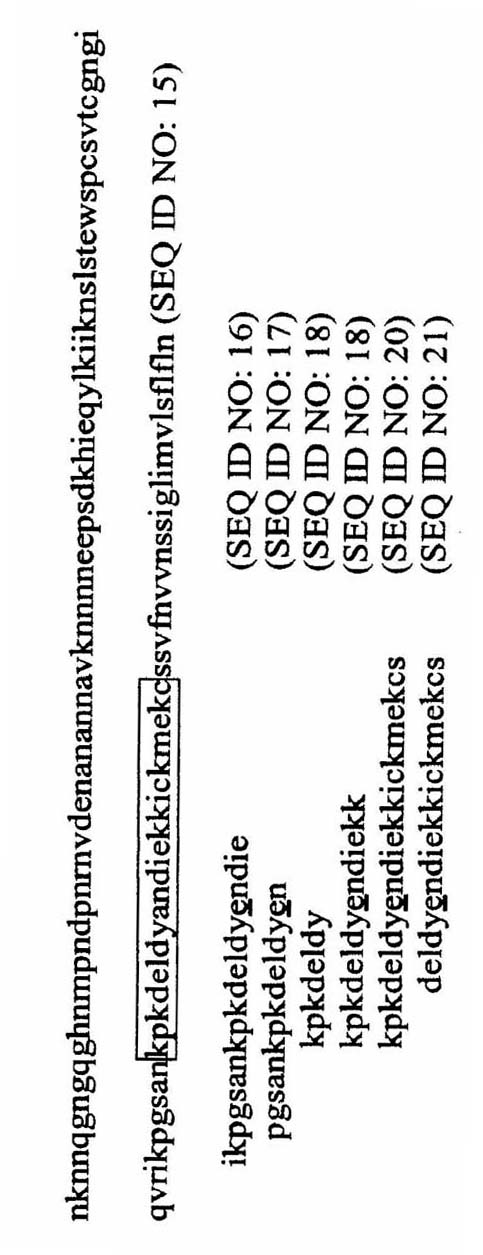
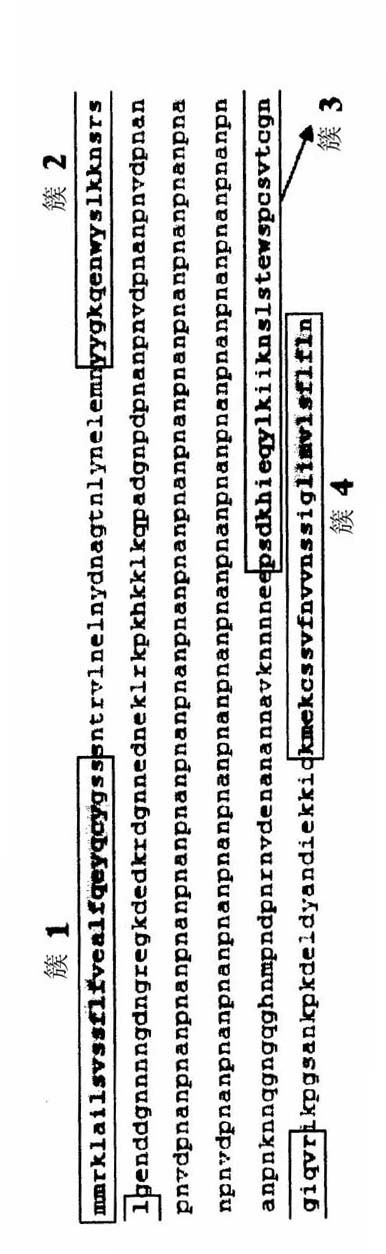
Circumsporozoite protein (CSP) is a secreted protein of the sporozoite stage of the malaria parasite (Plasmodium sp.) and is the antigenic target of RTS,S, a pre-erythrocytic malaria vaccine currently undergoing clinical trials. The amino-acid sequence of CSP consists of an immunodominant central repeat region flanked by conserved motifs at the N- and C- termini that are implicated in protein processing as the parasite travels from the mosquito to the mammalian vector.
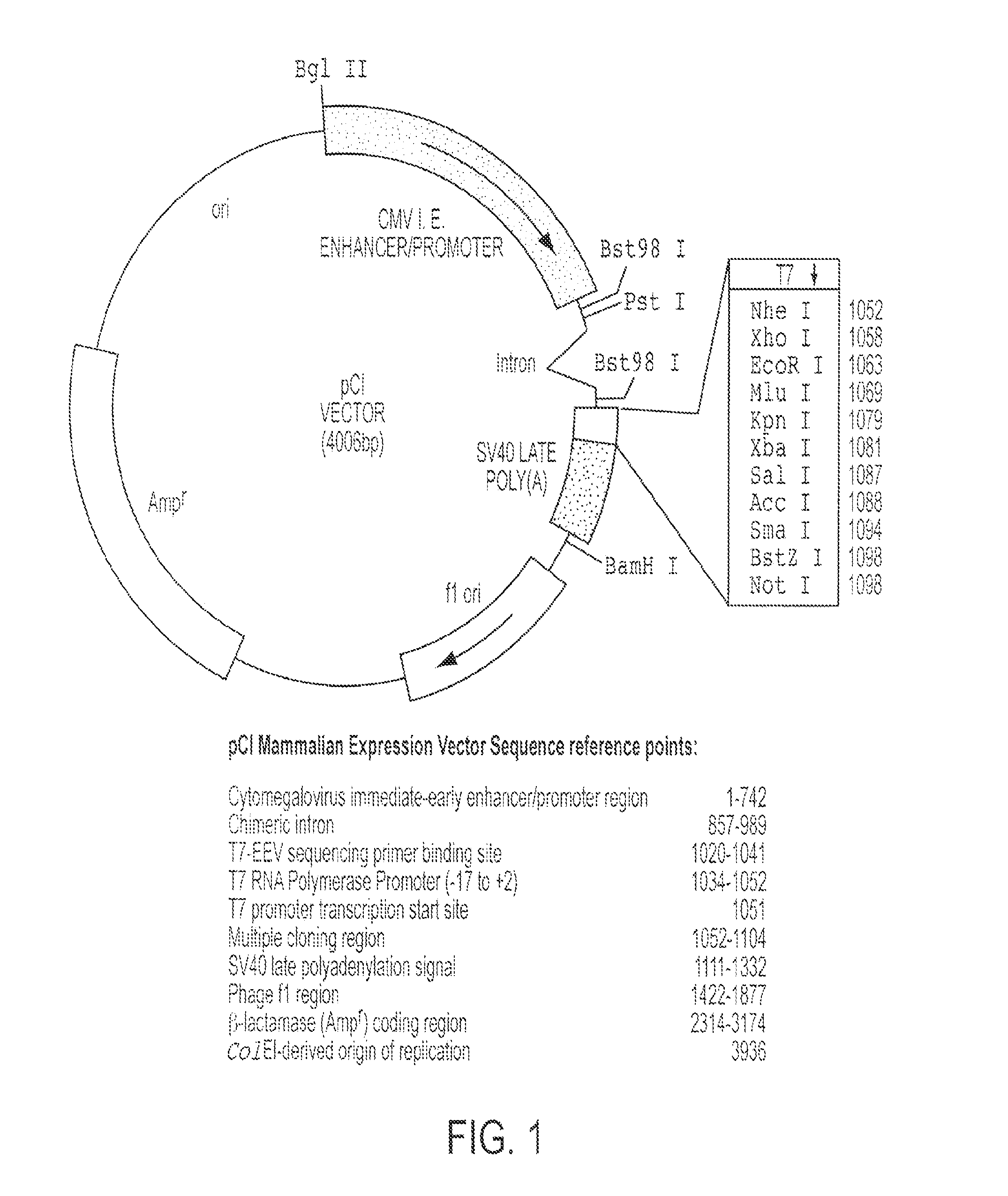
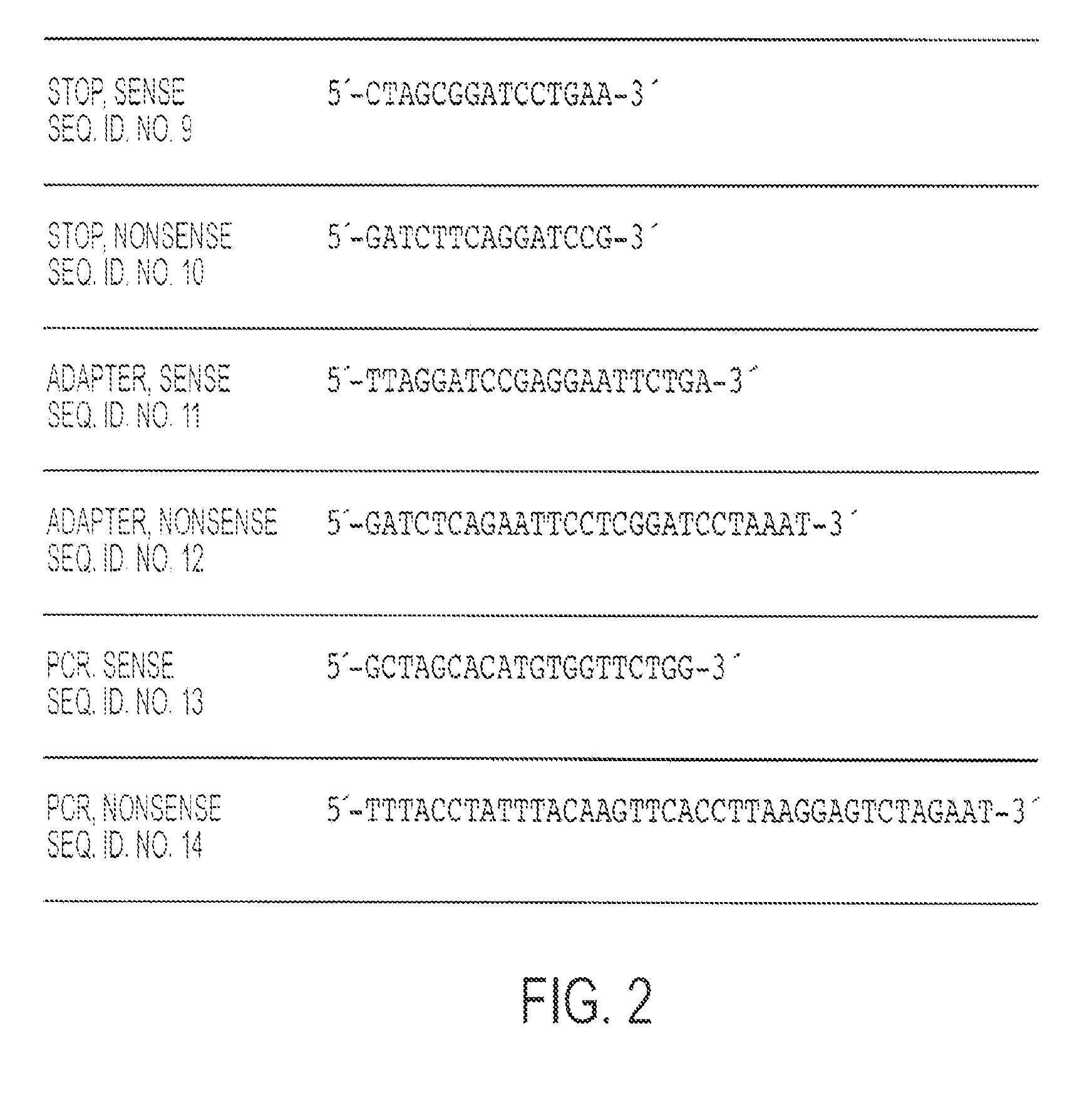
CR-2 binding peptide P28 as molecular adjuvant for DNA vaccines
InactiveUS8470560B2Improve responseRobust productionBiocidePeptide/protein ingredientsAdjuvantBinding peptide
The invention is an DNA vaccine and method of use thereof for modulating the immune response against the circumsporozoite protein (CSP) of malaria parasites, using the CR2 binding motifs of C3d, especially p28.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Plasmodium vivax hybrid circumsporozoite protein and vaccine
InactiveUS7790186B2Decreased number of repeatMaximize possibility of generatingPeptide/protein ingredientsAntibody mimetics/scaffoldsCircumsporozoite proteinAntibody production
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Methods and compositions for malaria prophylaxis
InactiveUS20060122266A1Malaria prophylaxisPreventing cell invasionOrganic active ingredientsBiocideCell invasionProteinase activity
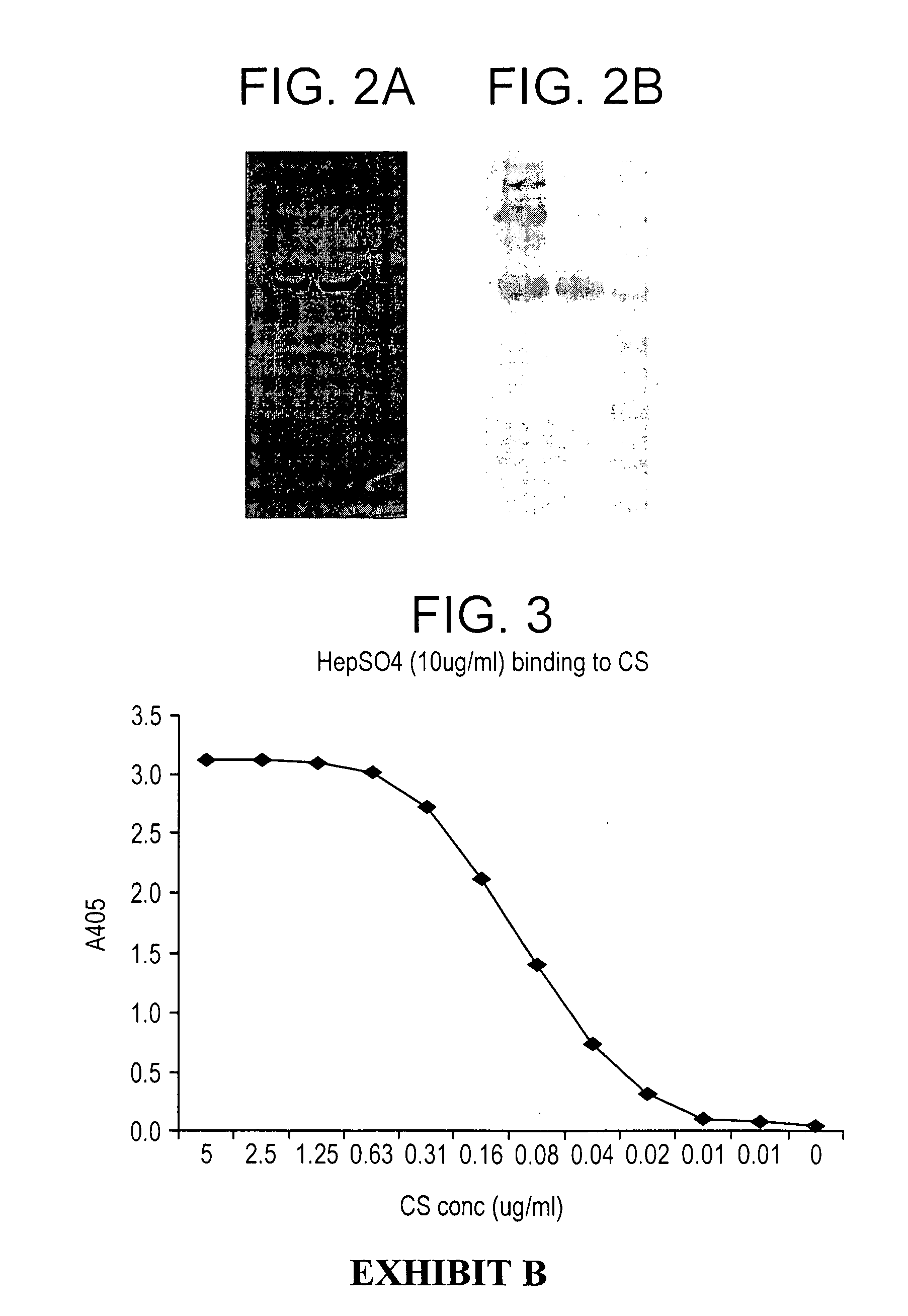
A composition for preventing malaria infection including a protease inhibitor. A pharmaceutical composition for preventing malaria infection including a protease inhibitor and a pharmaceutical carrier. A method of malaria infection prophylaxis including the step of administering an effective amount of the composition of the present invention. A method of malaria prophylaxis by inhibiting circumsporozoite protein processing or by inhibiting a protease of a sporozoite. Methods of preventing sporozoite cell invasion or preventing circumsporozoite processing.
Owner:SINNIS PHOTINI +1
Plasmodium vivax hybrid circumsporozoite protein and vaccine
InactiveUS20090196883A1Not painful to administerIntrinsically safePeptide/protein ingredientsAntibody mimetics/scaffoldsCircumsporozoite proteinAntibody production
Described in this application is a synthetic P. vivax circumsporozoite protein useful as a diagnostic reagent, for antibody production, and as a vaccine protective against infection with any strain of P. vivax.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Adenoviral vector-based malaria vaccines
InactiveUS20080248060A1Induce immune responseViral antigen ingredientsPeptidesAntigenNucleic acid sequencing
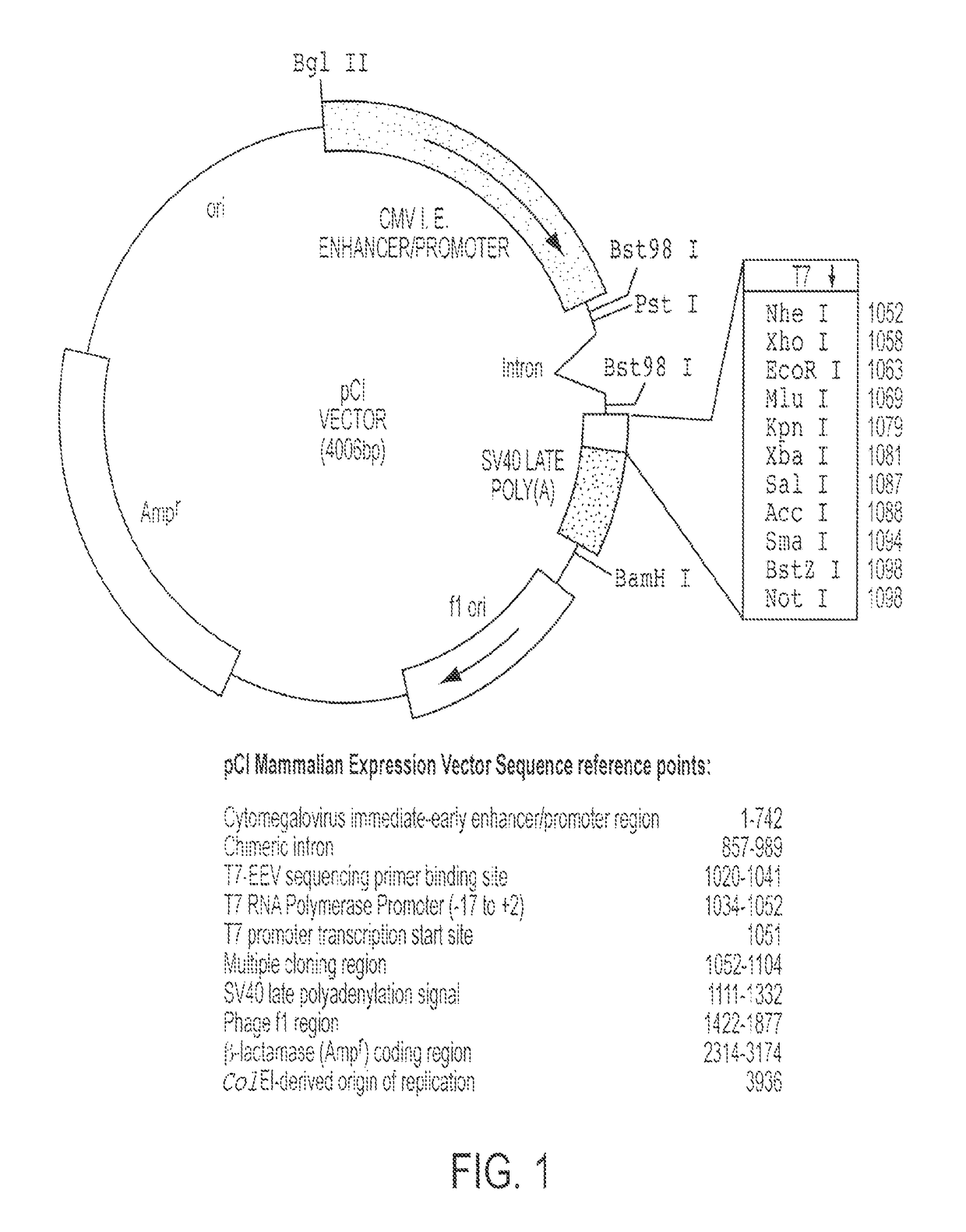
The invention provides a method of inducing an immune response against malaria in a mammal. The method comprises intramuscularly administering to a mammal a composition comprising a pharmaceutically acceptable carrier and either or both of (a) a first adenoviral vector comprising a nucleic acid sequence encoding a P. falciparum circumsporozoite protein (CSP) operably linked to a human CMV promoter, and / or (b) a second adenoviral vector comprising a nucleic acid sequence encoding a P. falciparum apical membrane antigen 1 (AMA-1) antigen operably linked to a human CMV promoter.
Owner:GEN VEC INC +1
Compositions and methods for inactivating or suppressing inflammatory cells
InactiveUS20070041992A1Reduce probabilitySuppress respiratory burstProtozoa antigen ingredientsPeptide/protein ingredientsAutoimmune conditionAutoimmune disease
Methods are provided for reducing the activity or function of an inflammatory cell by contacting a cell with, or administering to a subject in need thereof, an effective amount of a circumsporozoite protein or homolog thereof, or a fragment thereof. Methods of treating an inflammatory disease, or an autoimmune disease or for inducing tolerance are also disclosed as are pharmaceutical compositions comprising a therapeutically effective amount of a circumsporozoite protein or homolog thereof, or a fragment thereof and a pharmaceutically acceptable carrier.
Owner:NEW YORK UNIV
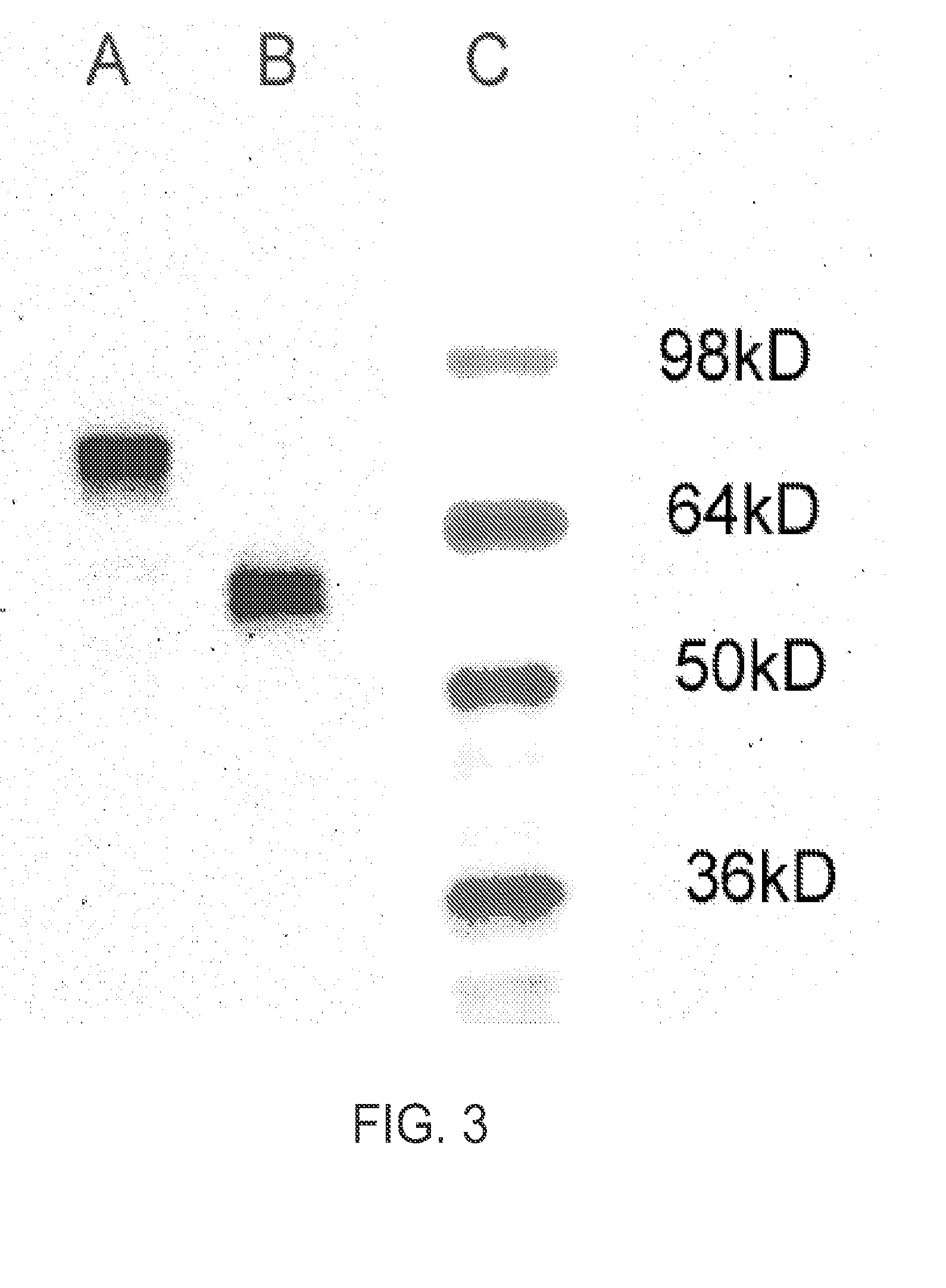
Process for purifying recombinant Plasmodium falciparum circumsporozoite protein
ActiveUS9169304B2High yieldOvercome obstaclesPeptide/protein ingredientsInorganic non-active ingredientsBiochemistryCircumsporozoite protein
The present invention relates to processes for purifying high-quality recombinant Plasmodium falciparum circumsporozoite protein at high yields.
Owner:PELICAN TECH HLDG INC
Hepatic targeting peptide and angiogenesis inhibitor fusion protein as well as preparation method and application thereof
ActiveCN103319605AStrong specificityImprove the dose-effect ratioPeptide/protein ingredientsPharmaceutical non-active ingredientsSide effectWhole body
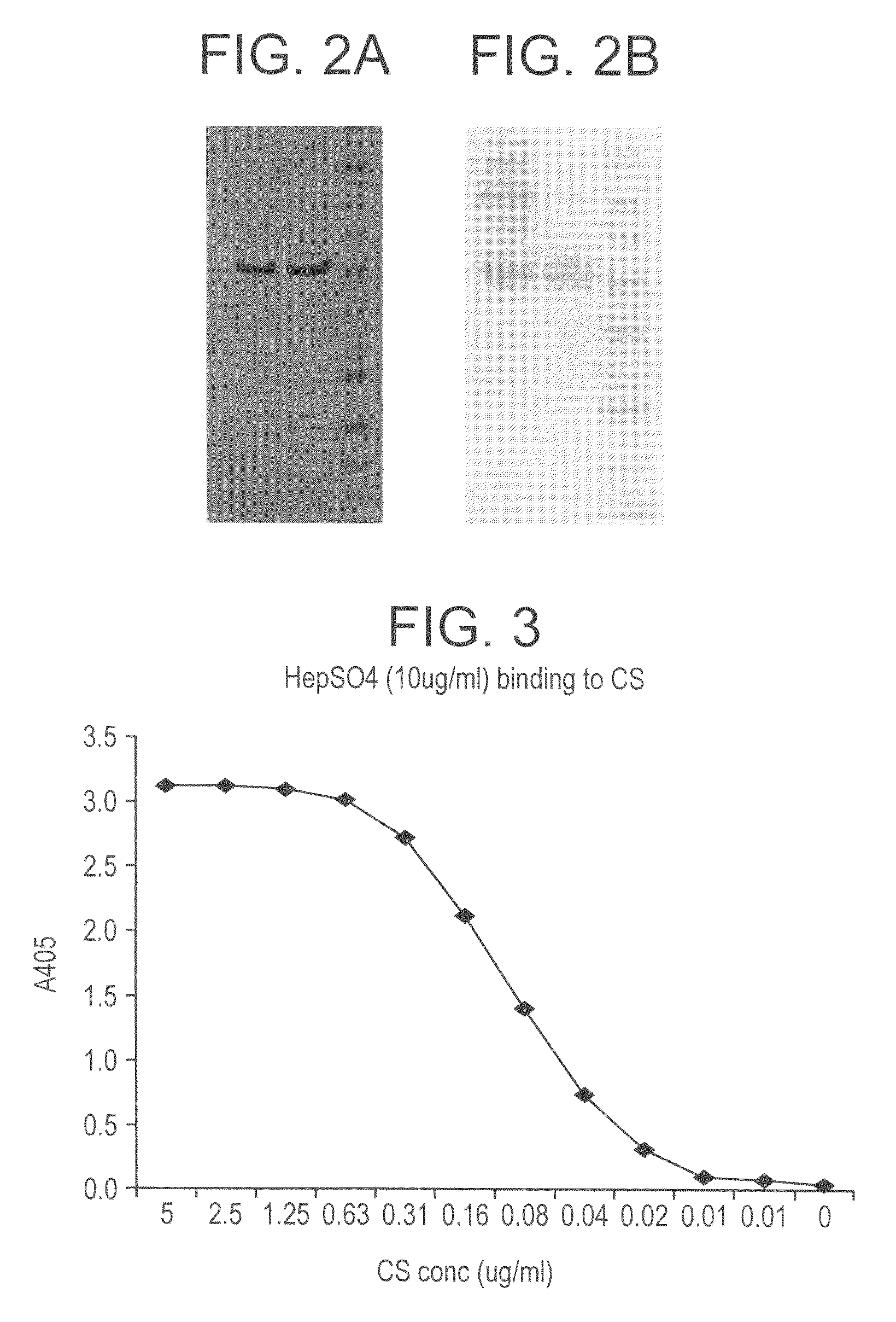
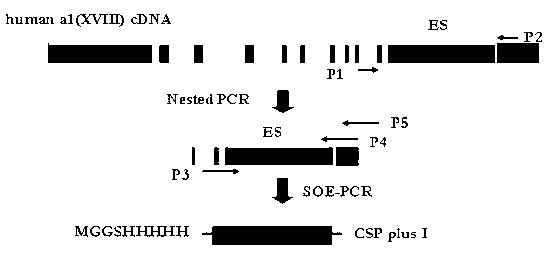
The invention discloses a hepatic targeting peptide and angiogenesis inhibitor fusion protein as well as a preparation method and an application thereof. According to the invention, 19 amino acids, which can be specifically combined with an acceptor on the hepatocyte surface (namely heparin sulfate proteoglycan), in a circumsporozoite protein (CSP) N-terminal conserved block I (CSPI-plus) are adopted, and are fused on the amino or carboxyl terminal of the angiogenesis inhibitor by a genetic engineering process to prepare the hepatic targeting peptide and angiogenesis inhibitor fusion protein. The fusion protein can specifically target the liver to inhibit the neovascularization, improve the local concentration of focus part, reduce the dosage of a whole body and reduce the toxic and side effects.
Owner:GUANGDONG PHARMA UNIV
Compositions and methods for inhibiting hepatocyte invasion by malarial sporozoites
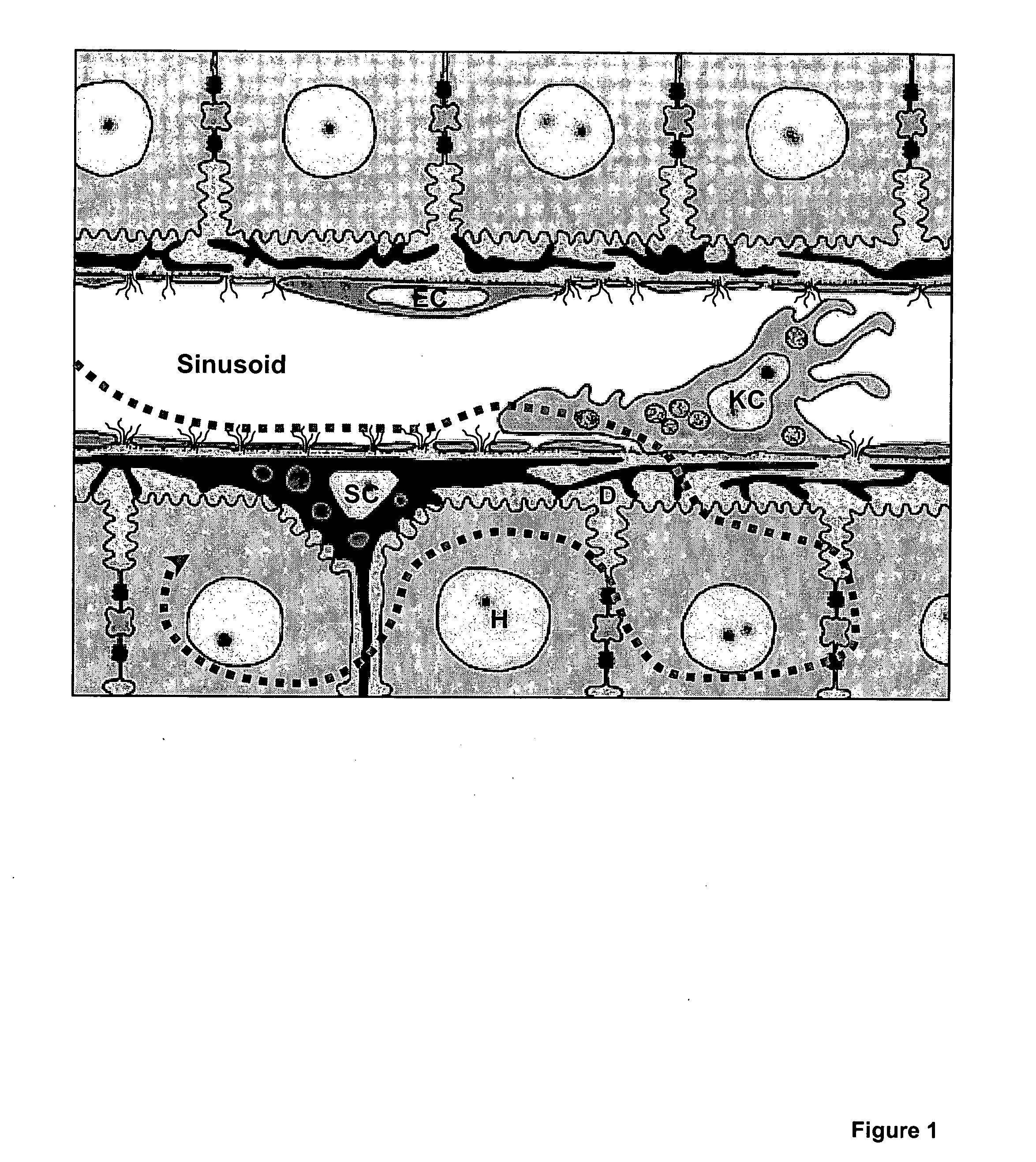
There is provided peptide and mimetic inhibitors for the binding of a circumsporozoite polypeptide to receptors of hepatocytes from malaria-susceptible mammals. Also contemplated is a method of inhibiting the binding of a malaria sporozoites to hepatocytes susceptible to sporozoite invasion. A peptide of Region II+ of the circumsporozoite protein is also provided, as is a method of targeting the delivery of substances to hepatocytes.
Owner:NEW YORK UNIVERSITY
Geographically-specific Plasmodium vivax molecule marker, and its application in strain tracing
PendingCN103865979AGuaranteed to be scientificTo achieve the purpose of traceabilityMicrobiological testing/measurementBiological material analysisMicrosatelliteMosquito infection
The invention relates to a geographically-specific Plasmodium vivax molecule marker, and its application in strain tracing. The molecule marker includes a Plasmodium vivax circumsporozoite protein center replication region reflecting mosquito infection differences of different media, a height polymorphism microsatellite reflecting mutation, migration and genetic drift, and a drug resistance related gene mutation reflecting the population positivity selection of drug control. Results show that each of a Plasmodium vivax circumsporozoite protein center replication region sequence, dihydrofolate reductase SNP, dihydrobiopterin synthetase SNP, a neutral microsatellite and the like has a geographic specificity, and can be used as a classification index in tracing detection.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Methods and compositions for malaria prophylaxis
InactiveUS20100113609A1BiocideSulfur/selenium/tellurium active ingredientsCell invasionProteinase activity
Owner:NEW YORK UNIV
Process for purifying recombinant plasmodium falciparum circumsporozoite protein
ActiveUS20140051841A1High yieldOvercome obstaclesPeptide/protein ingredientsInorganic non-active ingredientsBiochemistryCircumsporozoite protein
The present invention relates to processes for purifying high-quality recombinant Plasmodium falciparum circumsporozoite protein at high yields.
Owner:PFENEX
Heterologous prime boost vaccination regimen against malaria
ActiveUS20130216580A1High protection levelHigh levelProtozoa antigen ingredientsDsDNA virusesRegimenSerotype
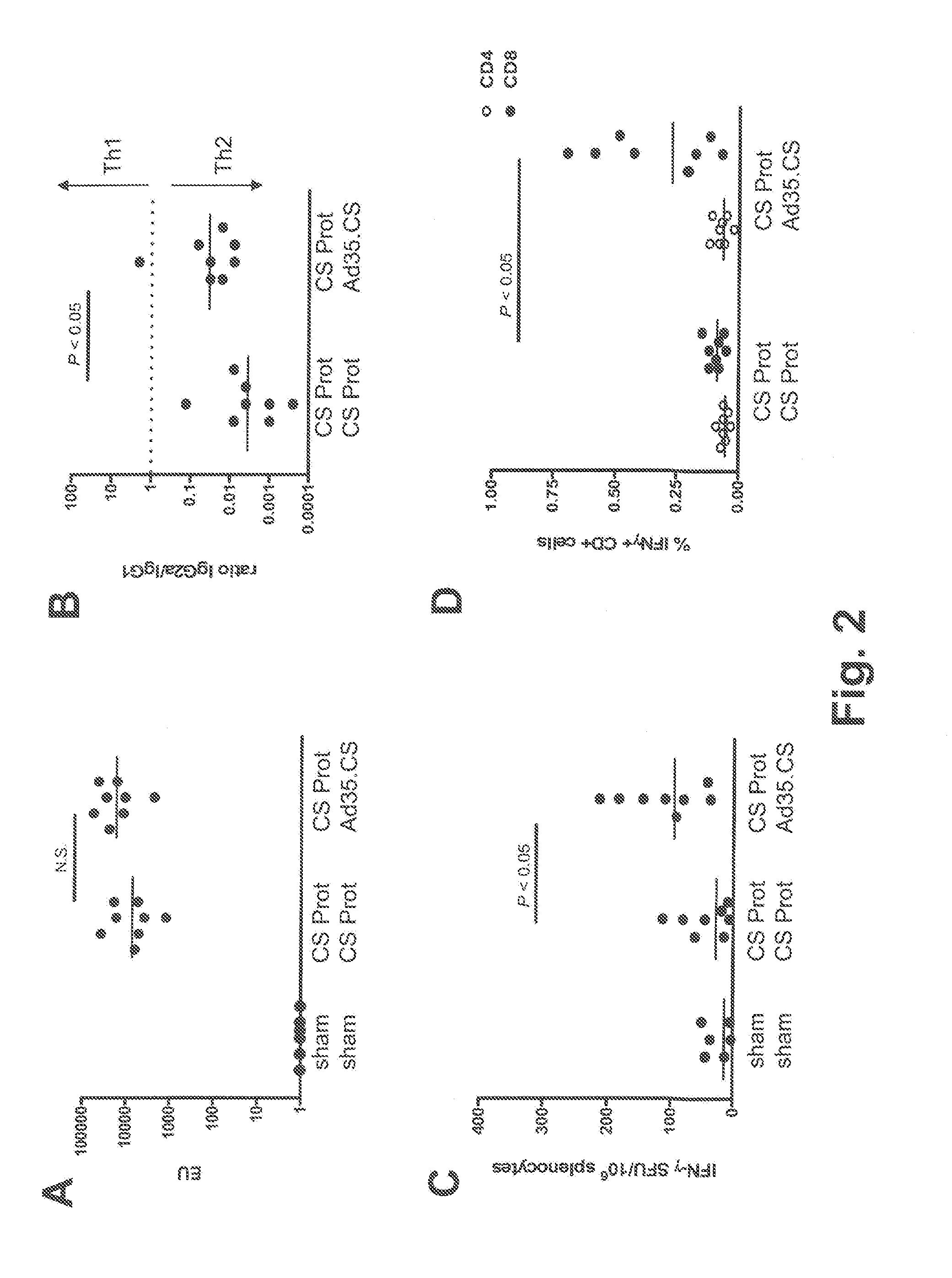
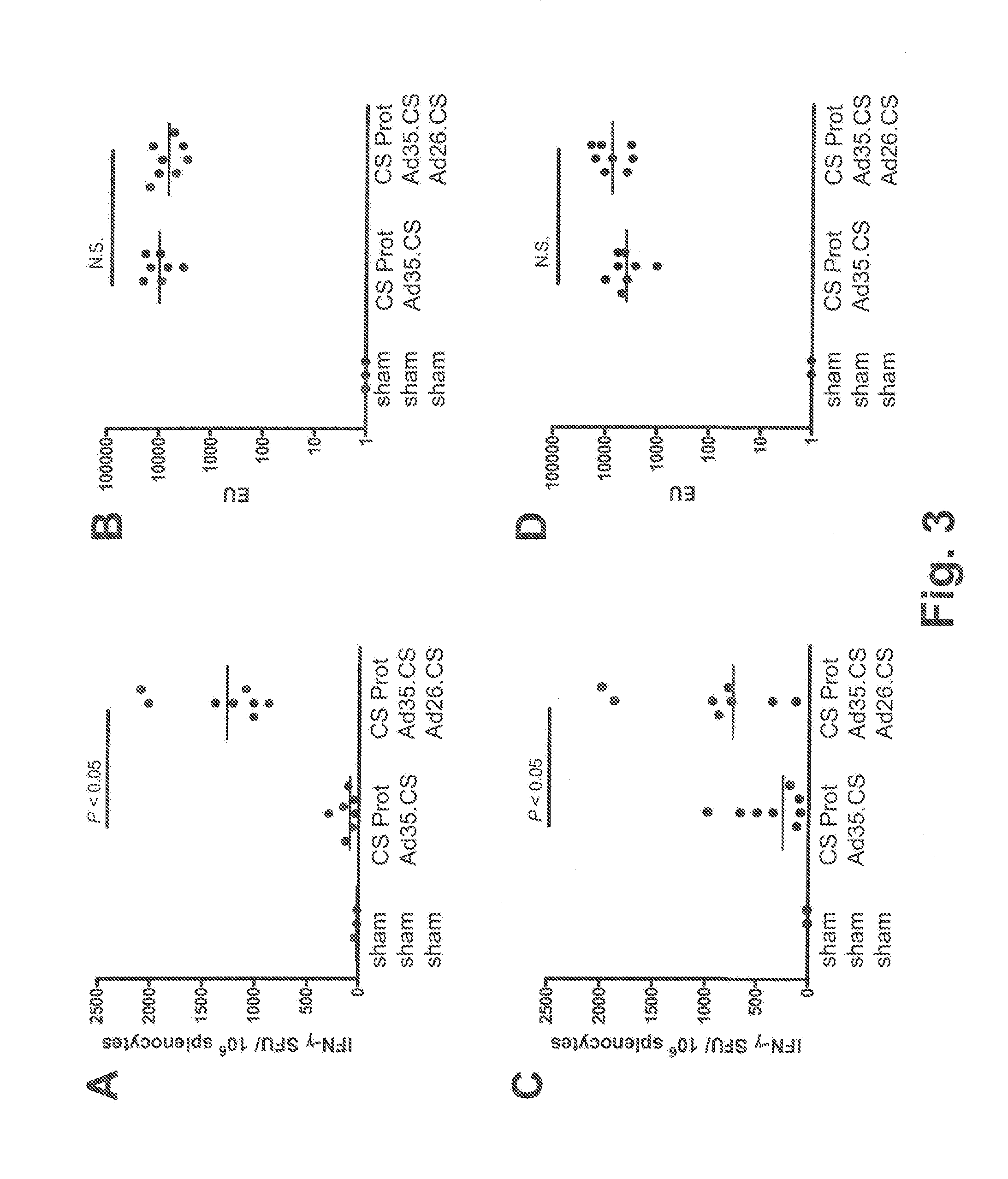
Described are methods for inducing an immune response in a subject against an antigen from a malaria-causing parasite, preferably P. falciparum, the method comprising: (i) administering to a subject a priming composition comprising adjuvanted proteinaceous antigen-comprising circumsporozoite (CS) protein or an immunogenic part thereof from a malaria-causing parasite; (ii) administering to the subject a first boosting composition comprising a recombinant adenovirus vector that comprises nucleic acid encoding CS protein or immunogenic part thereof from a malaria-causing parasite; and (iii) administering to the subject a second boosting composition comprising a recombinant adenovirus vector that comprises nucleic acid encoding CS protein or an immunogenic part thereof from a malaria-causing parasite, wherein either the first boosting composition comprises a recombinant adenovirus vector of serotype 35 (Ad35) and the second boosting composition comprises a recombinant adenovirus of Ad26, or wherein the first boosting composition comprises a recombinant adenovirus vector of Ad26 and the second boosting composition comprises a recombinant adenovirus of Ad35.
Owner:JANSSEN VACCINES & PREVENTION BV
Novel uses of plasmodium circumsporozoite protein in resisting proliferation and migration of tumor
InactiveCN101480489APrevent proliferationPeptide/protein ingredientsGenetic material ingredientsWilms' tumorFhit gene
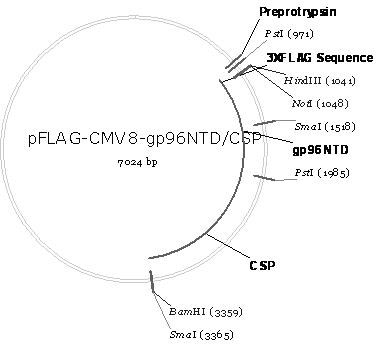
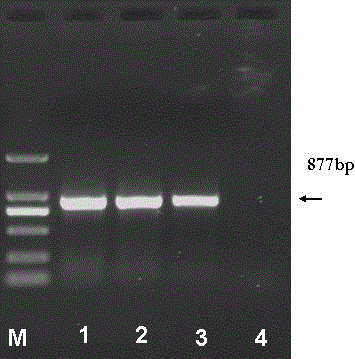
The invention discloses a new application of plasmodium circumsporozoite protein CSP for preparing medicaments for anti-tumor multiplication and migration. The invention successfully constructs a recombinant expression carrier pFLAG-CMV8-CSP of the CSP and transfects the recombinant expression carrier into a human liver cancer cell strain HepG2 and a colorectal cancer cell strain SW480 in a manner of liposome transfection. The research result shows that the CSP is mainly expressed in the cytoplasm of HepG2 cells, can restrain TNF-Alpha and LPS, stimulate the HepG2 cells and SW480 cells to activate NF-Kappa B and restrain the multiplication of the SW480 cells, and is further developed into a novel protein medicament for the anti-tumor multiplication and migration hopefully; and the recombinant expression carrier containing the gene of the CSP is hopefully further developed into the novel protein medicament for the anti-tumor multiplication and migration, therefore, the invention has favorable clinical application prospect.
Owner:ARMY MEDICAL UNIV
Methods and compositions for malaria prophylaxis
InactiveUS20090092619A1Malaria prophylaxisPreventing cell invasionOrganic active ingredientsAntibody ingredientsCell invasionProteinase activity
A composition for preventing malaria infection including a steric inhibitor of circumsporozoite protein cleavage. A pharmaceutical composition for preventing malaria infection including a steric inhibitor and a pharmaceutical carrier. A method of malaria infection prophylaxis including the step of administering an effective amount of the composition of the present invention. A method of malaria prophylaxis by sterically inhibiting circumsporozoite protein processing or by directly inhibiting a protease of a sporozoite from binding to its target. Methods of preventing sporozoite cell invasion or preventing circumsporozoite processing through steric or direct inhibition.
Owner:NEW YORK UNIV
Methods and compositions for malaria prophylaxis
InactiveUS20110223179A1Low quantity requiredAvoid splittingOrganic active ingredientsAntibody ingredientsCell invasionProteinase activity
A composition for preventing malaria infection including a steric inhibitor of circumsporozoite protein cleavage. A pharmaceutical composition for preventing malaria infection including a steric inhibitor and a pharmaceutical carrier. A method of malaria infection prophylaxis including the step of administering an effective amount of the composition of the present invention. A method of malaria prophylaxis by sterically inhibiting circumsporozoite protein processing or by directly inhibiting a protease of a sporozoite from binding to its target. Methods of preventing sporozoite cell invasion or preventing circumsporozoite processing through steric or direct inhibition.
Owner:NEW YORK UNIV
CR-2 Binding Peptide P28 as Molecular Adjuvant for DNA Vaccines
InactiveUS20100255075A1Increased cellular responseImprove protectionBiocideOrganic active ingredientsAdjuvantBinding peptide
The invention is an DNA vaccine and method of use thereof for modulating the immune response against the circumsporozoite protein (CSP) of malaria parasites, using the CR2 binding motifs of C3d, especially p28.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Malaria vaccine compositions and constituents which elicit cell mediated immunity
Malaria vaccines based on polyepitope constructs that elicit cell-mediated immunity against a broad spectrum of malaria parasites and which cover the majority of HLA alleles are provided. Epitopes in the polyepitope constructs are from regions of the Plasmodium falciparum circumsporozoite protein (CSP) known to contain CD4 and CD8 T cell epitopes, and include both epitopes from highly variable and highly conserved regions of CSP.
Owner:INT AIDS VACCINE INITIATIVE INC
Application of circumsporozoite protein polypeptide CSP I-plus of plasmodium in preparing anti-malarial medicine
InactiveCN103611151AReduced number of infecting parasitesPeptide/protein ingredientsGenetic material ingredientsMalarial parasiteCell invasion
The invention discloses novel application of the peptide fragment CSP I-plus of circumsporozoite protein CSP I-plus of plasmodium in treating plasmodium infection. Researches discover that the circumsporozoite protein CSP plays an important role in adhesion and hepatic cell invasion of plasmodium sporozoite, and the key point of malaria infection is that sporozoite of plasmodium invades liver cells, so that malaria infection can be prevented by stopping invasion of sporozoite. CSP I-plus is an important functional domain of CSP, can be specifically combined with a liver cell surface molecule, namely heparan sulfate proteoglycan (HSPG), and can stop combination of CSP and the liver cells. Therefore, the CSP I-plus is expected to be developed into a novel micro-molecule polypeptide medicine for treating plasmodium falciparum infection by stopping adhesion and liver cell invasion of CSP mediated plasmodium sporozoite, and the recombinant expression plasmid containing CSP I-plus coding genes is expected to be further developed into a novel anti-malarial genetic medicine, so that the circumsporozoite protein polypeptide CSP I-plus has an excellent clinical application prospect.
Owner:GUANGDONG PHARMA UNIV
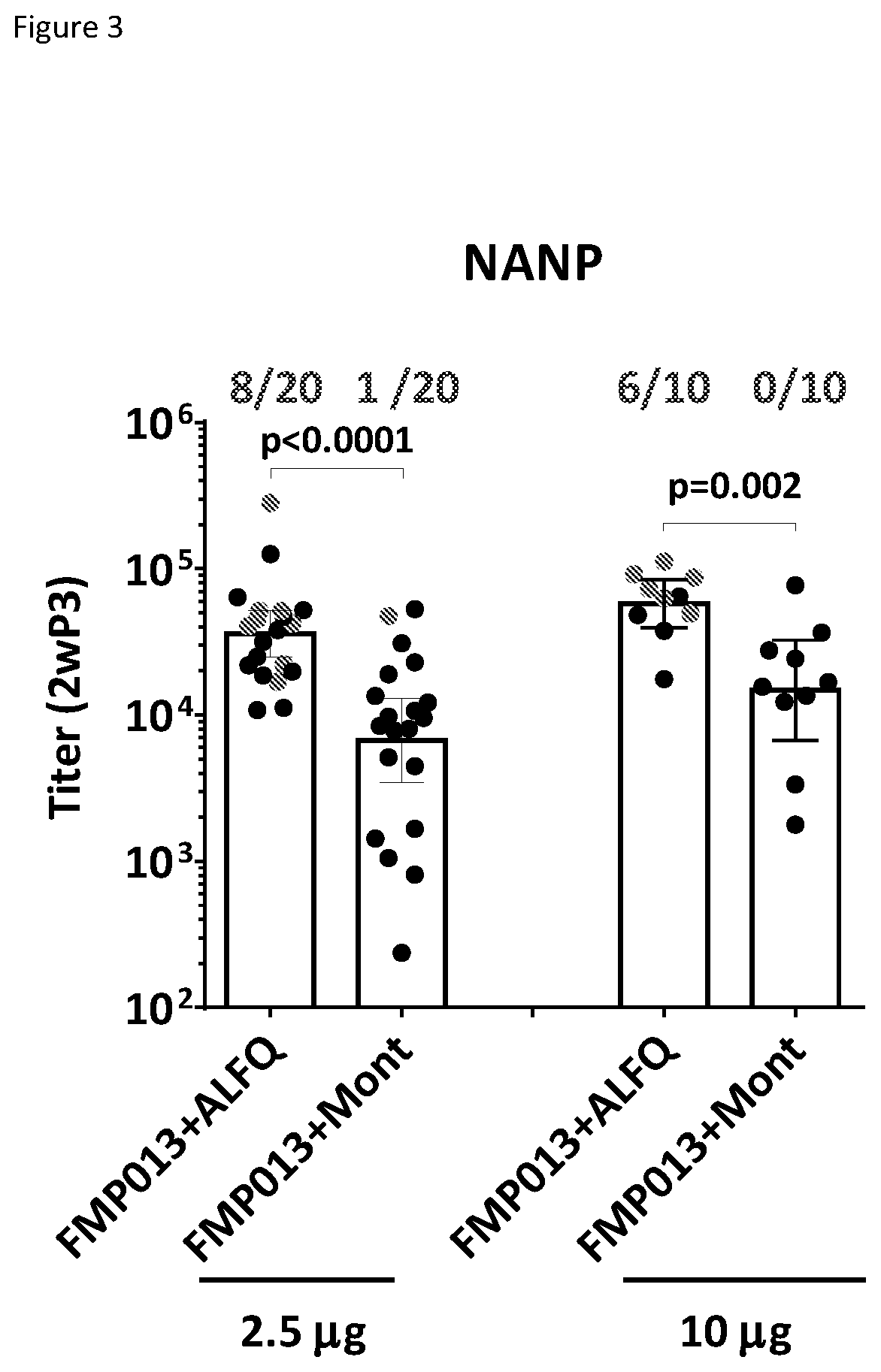
Compositions and Methods for Vaccine Delivery
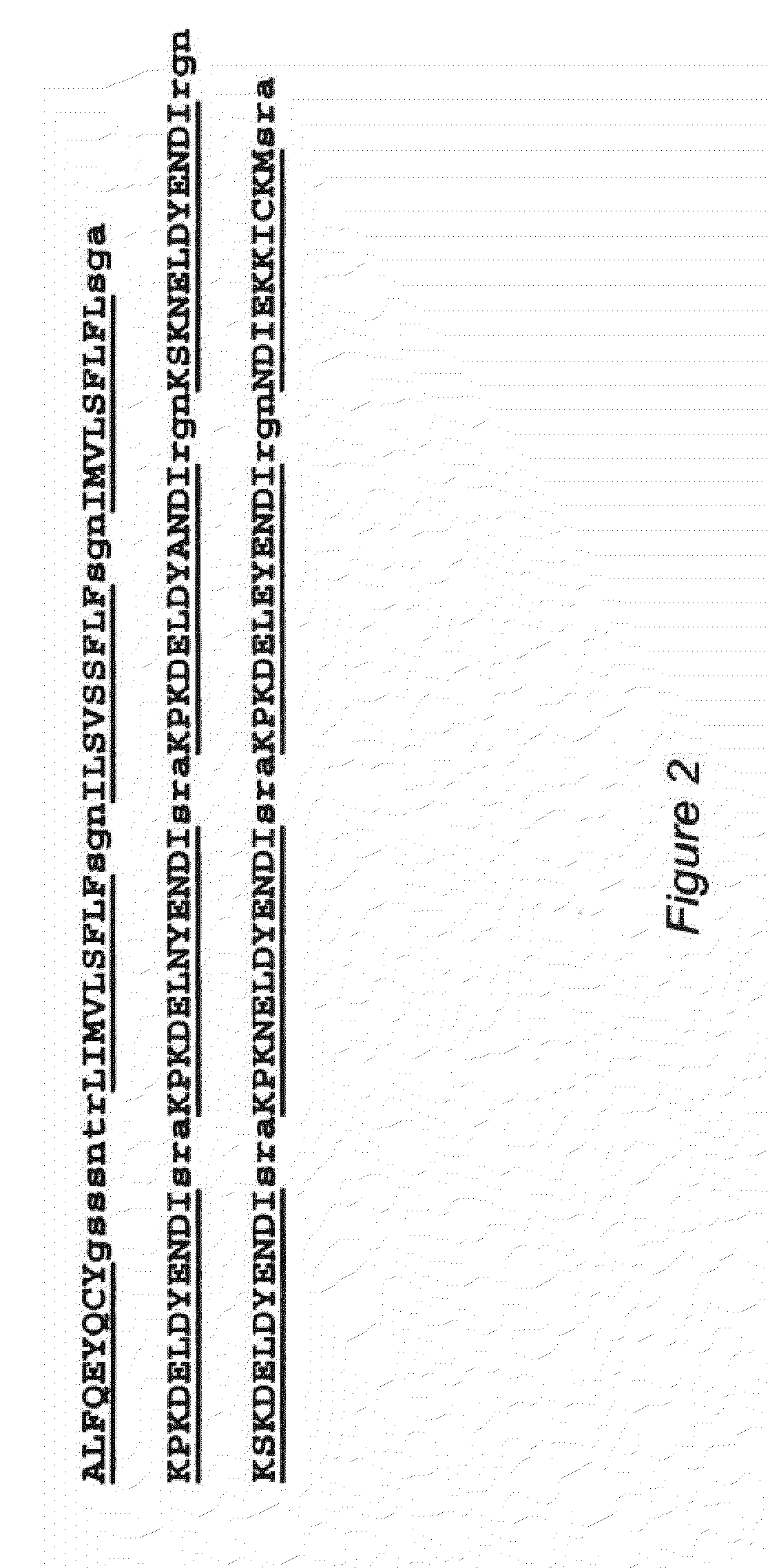
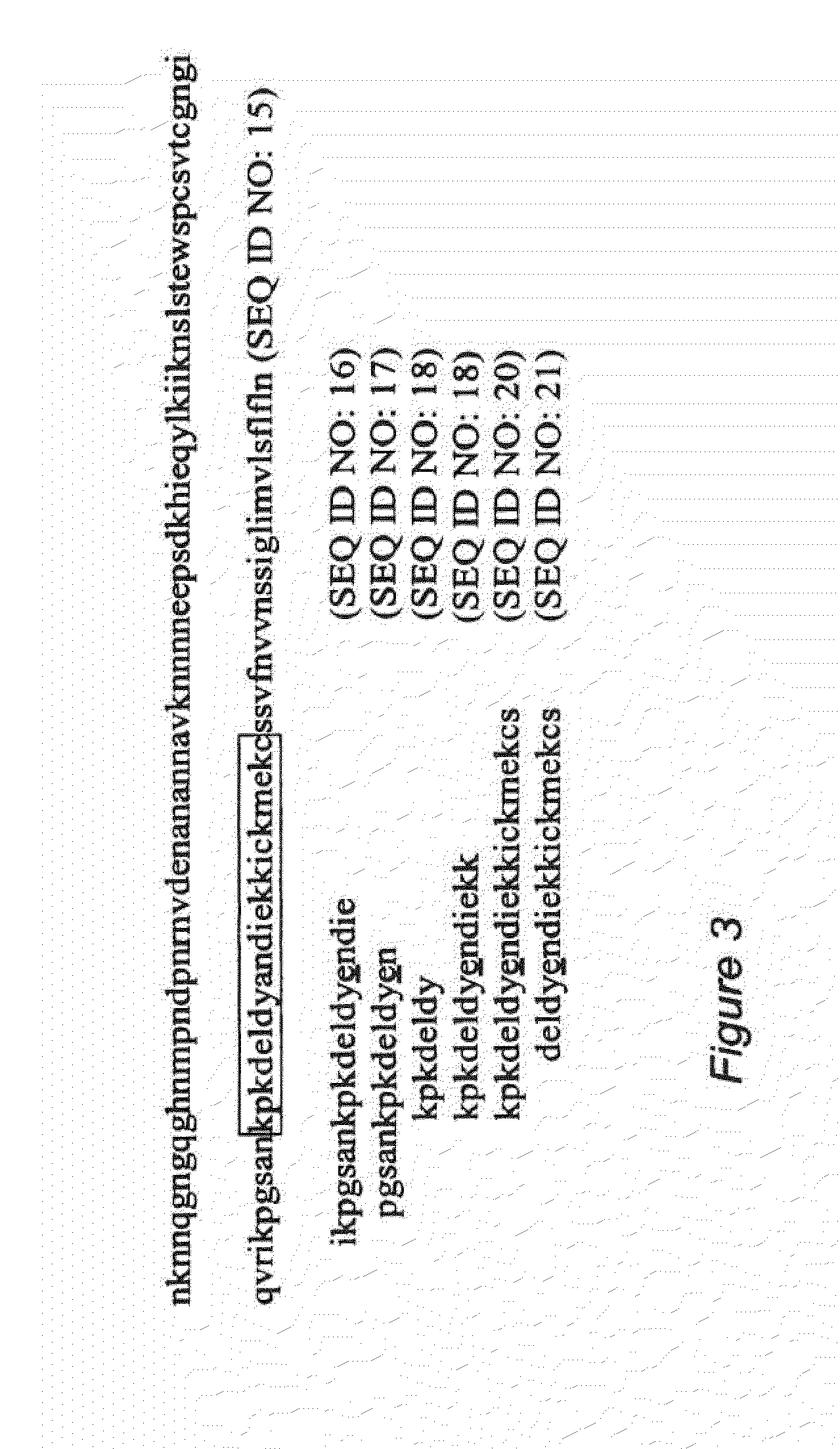
The invention relates to pharmaceutical compositions comprising at least one antigen and an adjuvant composition, where the adjuvant composition comprises a saponin and a liposome. The liposome of the composition comprises monophosphoryl lipid A (MPLA), cholesterol and a phospholipid that is in a liquid crystalline state at greater than or equal to 23° C., and the concentration of cholesterol to lipid in the liposome is greater than 50% (mol / mol). The antigen in the composition is a soluble Plasmodium falciparum recombinant circumsporozoite protein (rCSP) comprising the amino acid sequence of SEQ ID NO:1, or a P. falciparum rCSP peptide that is at least 95% identical to the amino acid sequence of SEQ ID NO:1.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC +1
Novel uses of plasmodium circumsporozoite protein in resisting proliferation and migration of tumor
InactiveCN101480489BPrevent proliferationPeptide/protein ingredientsGenetic material ingredientsLiposomeWilms' tumor
The invention discloses a new application of plasmodium circumsporozoite protein CSP for preparing medicaments for anti-tumor multiplication and migration. The invention successfully constructs a recombinant expression carrier pFLAG-CMV8-CSP of the CSP and transfects the recombinant expression carrier into a human liver cancer cell strain HepG2 and a colorectal cancer cell strain SW480 in a manner of liposome transfection. The research result shows that the CSP is mainly expressed in the cytoplasm of HepG2 cells, can restrain TNF-Alpha and LPS, stimulate the HepG2 cells and SW480 cells to activate NF-Kappa B and restrain the multiplication of the SW480 cells, and is further developed into a novel protein medicament for the anti-tumor multiplication and migration hopefully; and the recombinant expression carrier containing the gene of the CSP is hopefully further developed into the novel protein medicament for the anti-tumor multiplication and migration, therefore, the invention has favorable clinical application prospect.
Owner:ARMY MEDICAL UNIV
Malaria infrared stage DNA vaccine and preparing method thereof
InactiveCN102166349ASpecific complete protective immunityAvoid infectionAntibody medical ingredientsVector-based foreign material introductionCD8T cell
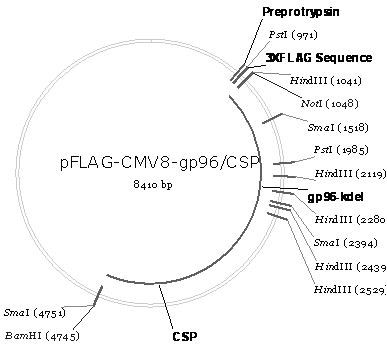
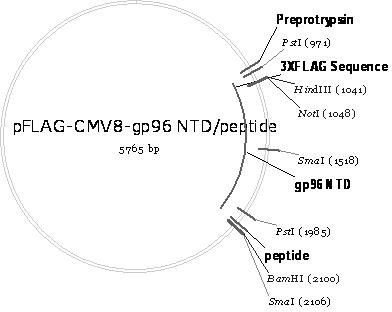
The invention belongs to the biomedicine field, relating to a malaria infrared stage DNA vaccine and a preparing method thereof. The vaccine is obtained by connecting the coding gene of N end segment (amino acid sequence shown as SEQ ID No. 1) of heat shock protein (gp96) with the coding gene of the circumsporozoite protein (amino acid sequence shown as SEQ ID No. 3) in series and inserting into eukaryotic expression plasmid. The preparing method of the invention comprises three steps: the construction of recombinant plasmid pMD19 simple T / CSP, the construction of recombinant plasmid pFLAG-CMV8-gp96NTD, and the construction of recombinant plasmid pFLAG-CMV8-gp96NTD / CSP. The vaccine is able to simultaneously induce anti-CSP antibody generation and CD8+T cell reaction, thereby obtaining the plasmodium-specified complete protective immunization, and has great application prospect in preventing malaria inflection.
Owner:ARMY MEDICAL UNIV
Fusion protein of liver-targeting peptide and recombinant human endostatin and its preparation method and application
ActiveCN103319606BStrong specificityImprove the dose-effect ratioBacteriaPeptide/protein ingredientsSide effectWhole body
The invention discloses a fusion protein of liver-targeting peptide and recombinant human endostatin, its preparation method and application. The present invention selects 19 amino acids that can be specifically combined with the receptor--heparan sulfate proteoglycan HSPG on the surface of liver cells in the conserved I region of the N-terminal of the Plasmodium circumsporozoite protein CSP, and uses genetic engineering to fuse it in The carboxy-terminus of the novel human endostatin nhES with 9 amino acid sequences (MGGSHHHHH) added to the amino-terminus, and the liver-targeting peptide and recombinant human endostatin fusion protein (ES‑CSP) were prepared. The fusion protein is easy to purify, can specifically target the liver to inhibit the formation of new blood vessels, increase the local concentration of the drug at the lesion site, reduce the systemic dosage, and reduce its toxic and side effects. It provides ideas and scientific basis for the clinical development of drugs that target angiogenesis to treat liver cancer, and has great scientific significance and practical value for further improving the treatment level of liver cancer in my country.
Owner:GUANGDONG PHARMA UNIV
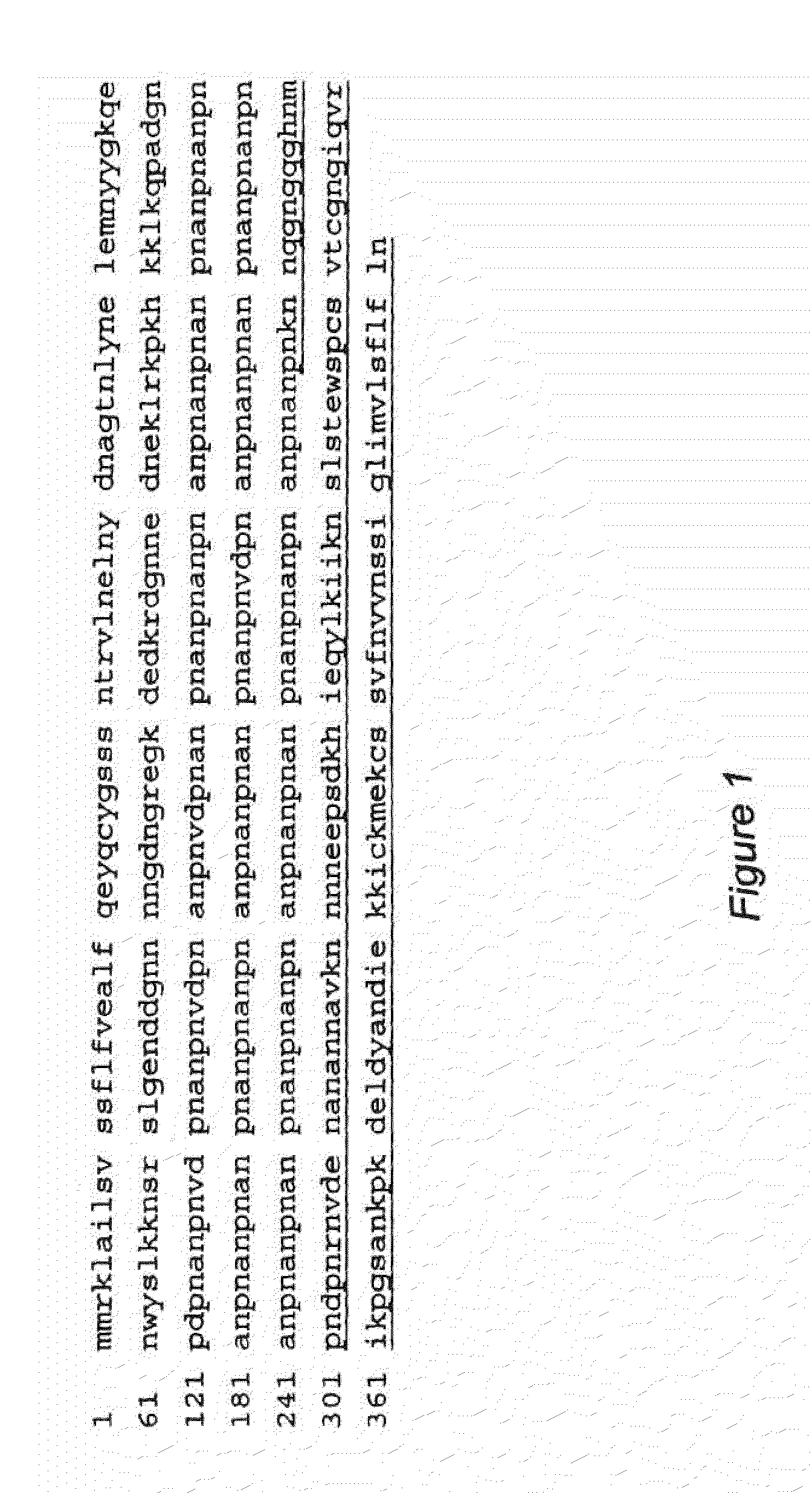
Circumsporozoite proteins with increased expression in mammalian cells
PendingUS20220280626A1Improve stabilityHigh yieldOrganic active ingredientsAntibody mimetics/scaffoldsAntigenMalaria
Mutated and / or truncated malarial circumsporozoite proteins (CSP) and associated nucleic acids that are more stable and highly expressed in mammalian cells are described. The mutated and / or truncated CSP and associated nucleic acids can be expressed to produce malaria vaccine antigens.
Owner:FRED HUTCHINSON CANCER CENT
Adenoviral vector-based malaria vaccines
The invention provides a method of inducing an immune response against malaria in a mammal. The method comprises intramuscularly administering to a mammal a composition comprising a pharmaceutically acceptable carrier and either or both of (a) a first adenoviral vector comprising a nucleic acid sequence encoding a P. falciparum circumsporozoite protein (CSP) operably linked to a human CMV promoter, and / or (b) a second adenoviral vector comprising a nucleic acid sequence encoding a P. falciparum apical membrane antigen 1 (AMA-1) antigen operably linked to a human CMV promoter.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Compositions and methods for inactivating or suppressing inflammatory cells
InactiveUS8865657B2Suppress respiratory burstInhibition releaseProtozoa antigen ingredientsPeptide/protein ingredientsDiseaseAutoimmune disease
Methods are provided for reducing the activity or function of an inflammatory cell by contacting a cell with, or administering to a subject in need thereof, an effective amount of a circumsporozoite protein or homolog thereof, or a fragment thereof. Methods of treating an inflammatory disease, or an autoimmune disease or for inducing tolerance are also disclosed as are pharmaceutical compositions comprising a therapeutically effective amount of a circumsporozoite protein or homolog thereof, or a fragment thereof and a pharmaceutically acceptable carrier.
Owner:NEW YORK UNIV
Malaria vaccine compositions and constituents which elicit cell mediated immunity
Malaria vaccines based on polyepitope constructs that elicit cell-mediated immunity against a broad spectrum of malaria parasites and which cover the majority of HLA alleles are provided. Epitopes in the polyepitope constructs are from regions of the Plasmodium falciparum circumsporozoite protein (CSP) known to contain CD4 and CD8 T cell epitopes, and include both epitopes from highly variable and highly conserved regions of CSP.
Owner:AERAS GLOBAL TB VACCINE FOUND
Multiple malaria pre-erythrocytic antigens and their use in the elicitation of a protective immune response in a host
The invention relates to Plasmodium antigenic polypeptides identified through the use of a specifically devised functional immunization screening assay. In particular, the invention relates to antigenic polypeptides of malaria parasites wherein said antigenic polypeptides that exhibit a protective effect, especially that of eliciting a protective immune response in a host against challenge by Plasmodium sporozoites. The invention relates to a combination of compounds, comprising at least 2 distinct active ingredients wherein each active ingredient consists of an antigenic polypeptide of a Plasmodium parasite, a polynucleotide encoding the antigenic polypeptide, or a vector, in particular a viral vector, especially a lentiviral vector, expressing such antigenic polypeptide of a Plasmodium parasite, wherein one antigenic polypeptide is the circumsporozoite protein (CSP) or a polypeptidic derivative thereof and another antigenic polypeptide is either protein Ag40 (11-09) or protein Ag45 (11-10).
Owner:INST PASTEUR +1
Recombinant polypeptide construct comprising Plasmodium falciparum circumsporozoite protein HLA class I restricted T-cell epitopes
The invention relates to a recombinant polypeptide construct comprising epitopes from Plasmodium falciparum protein circumsporozoite protein (CSP). The epitopes contain HLA class I binding motifs and stimulate an anti-malaria CD8+T-cell response. The polypeptides can be incorporated into immunogenic formulations against malaria. Additionally, the antigens are useful for facilitating evaluation of immunogenicity of candidate malaria vaccines.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Adenoviral vector-based malaria vaccines
ActiveUS20140335128A1Induce immune responseWhole-cell/virus/DNA/RNA ingredientsDsDNA virusesMalarial parasiteAntigen
The invention provides a method of inducing an immune response against malaria in a mammal. The method comprises intramuscularly administering to a mammal a composition comprising a pharmaceutically acceptable carrier and either or both of (a) a first adenoviral vector comprising a nucleic acid sequence encoding a P. falciparum circumsporozoite protein (CSP) operably linked to a human CMV promoter, and / or (b) a second adenoviral vector comprising a nucleic acid sequence encoding a P. falciparum apical membrane antigen 1 (AMA-1) antigen operably linked to a human CMV promoter.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com