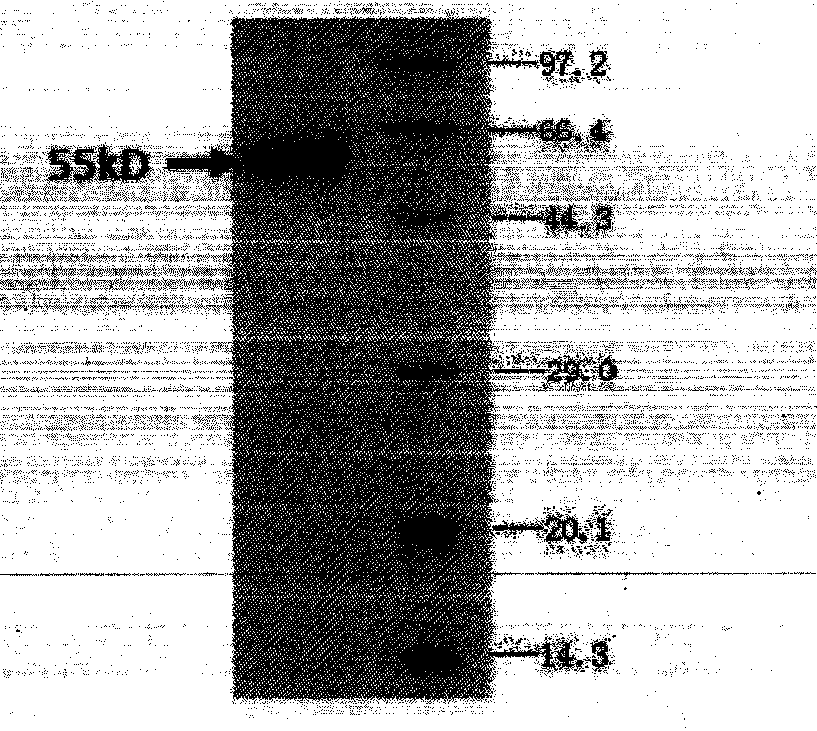Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
215 results about "Cellular antigens" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cell surface antigens of leukocytes are called CD antigens, and important for immune reactions of organisms. As lymphocytes mature, they express different protein receptors on the cell surface, which can aid in determining the type and maturation stage of the cells being examined.
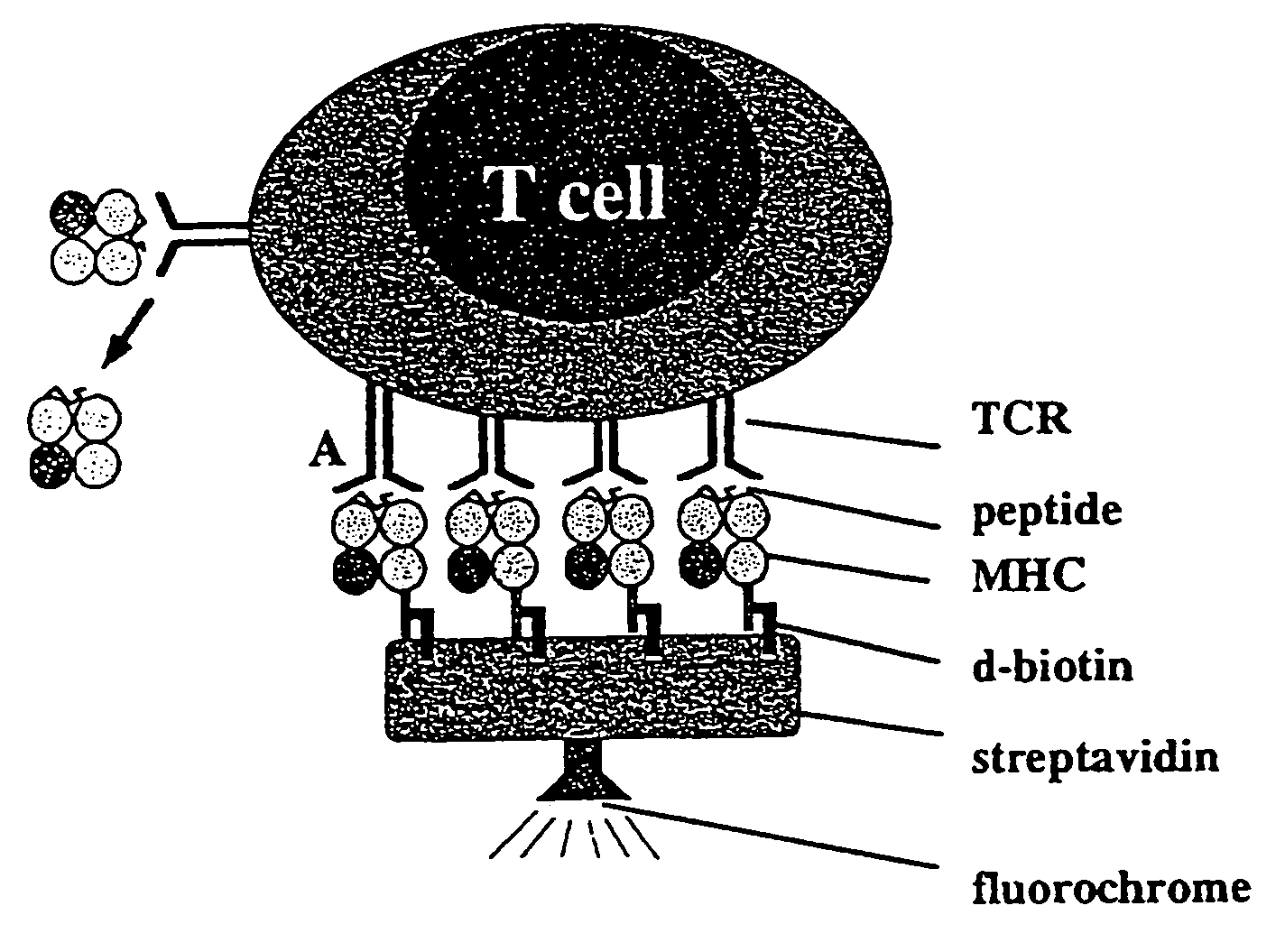
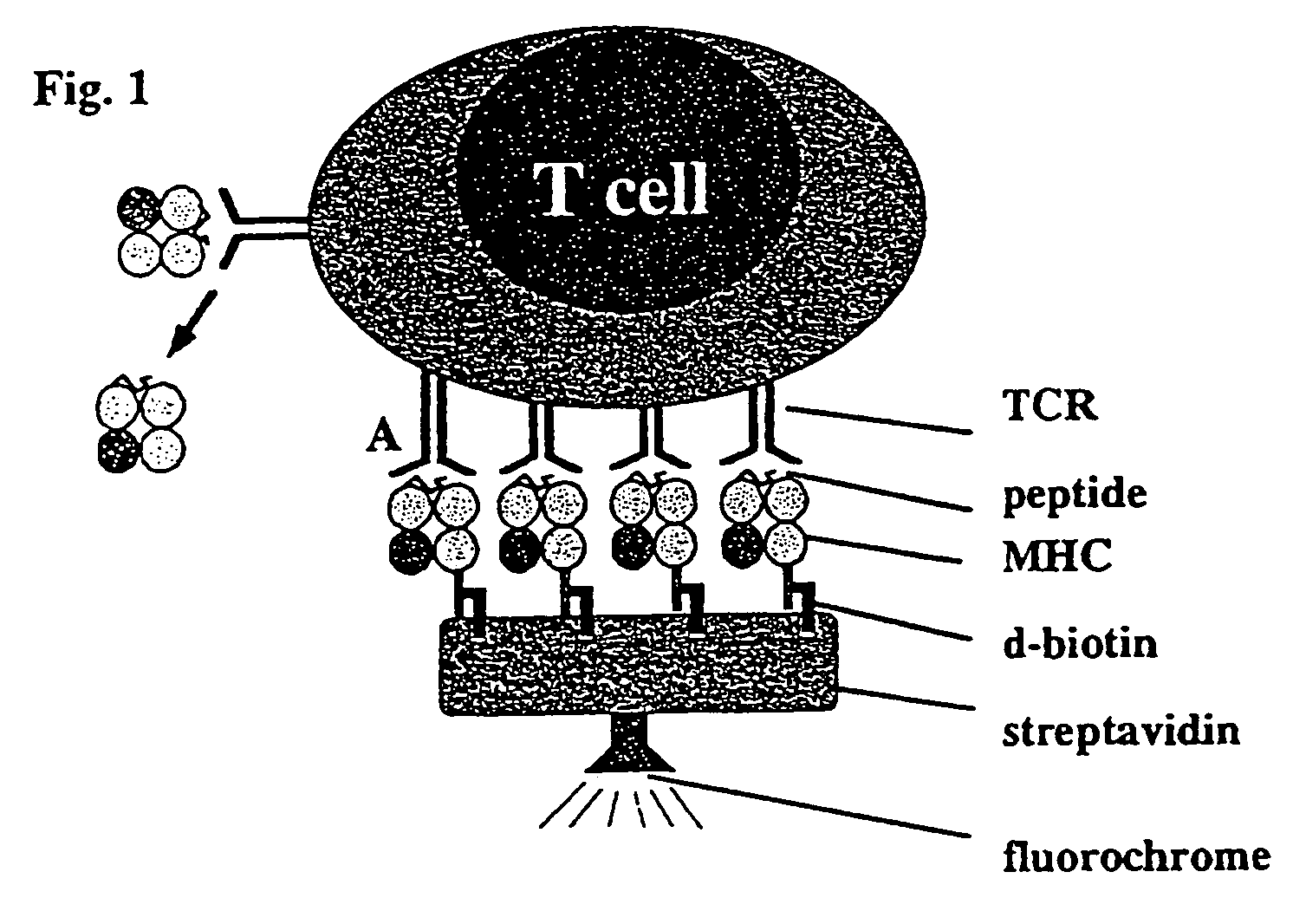
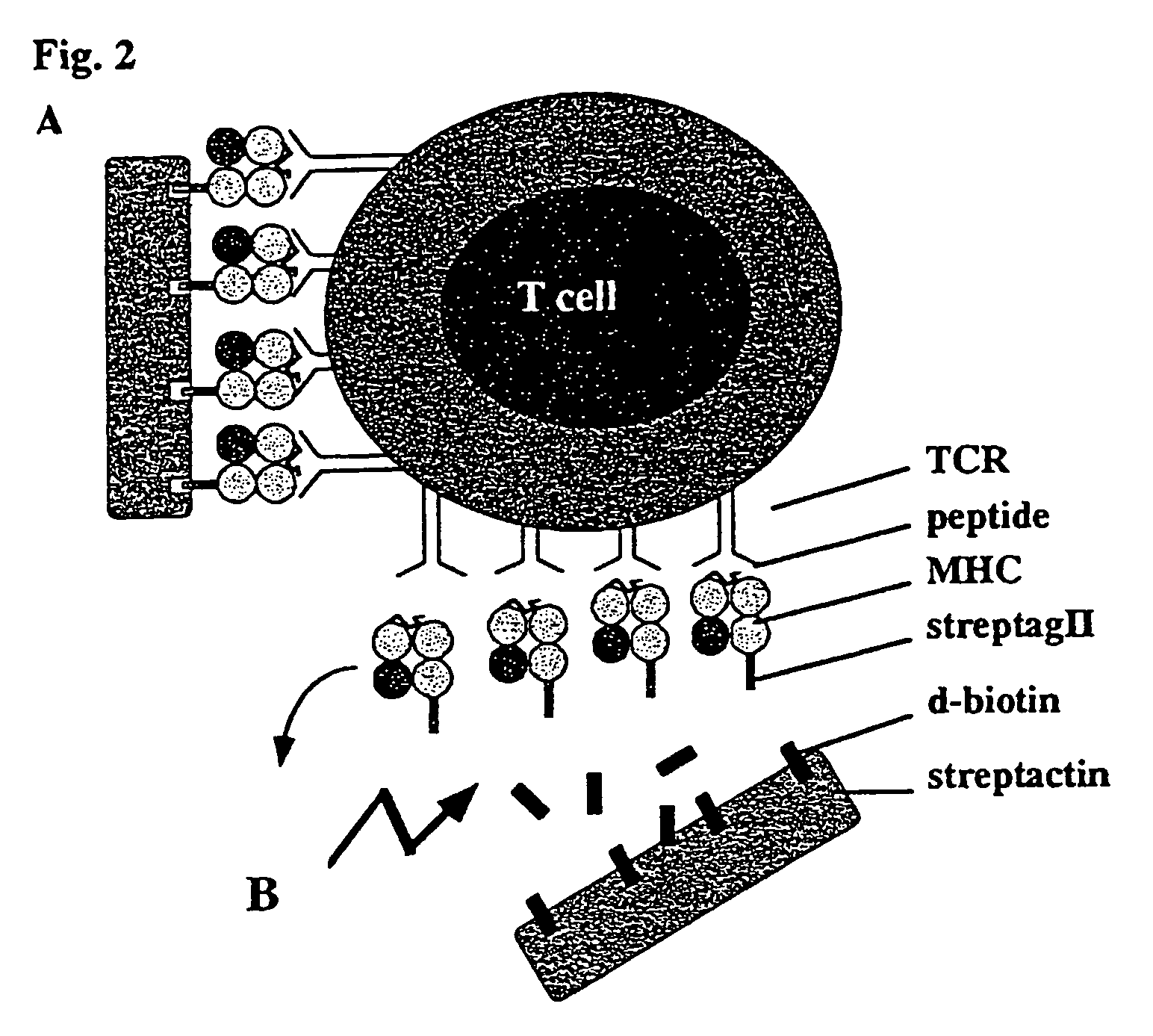
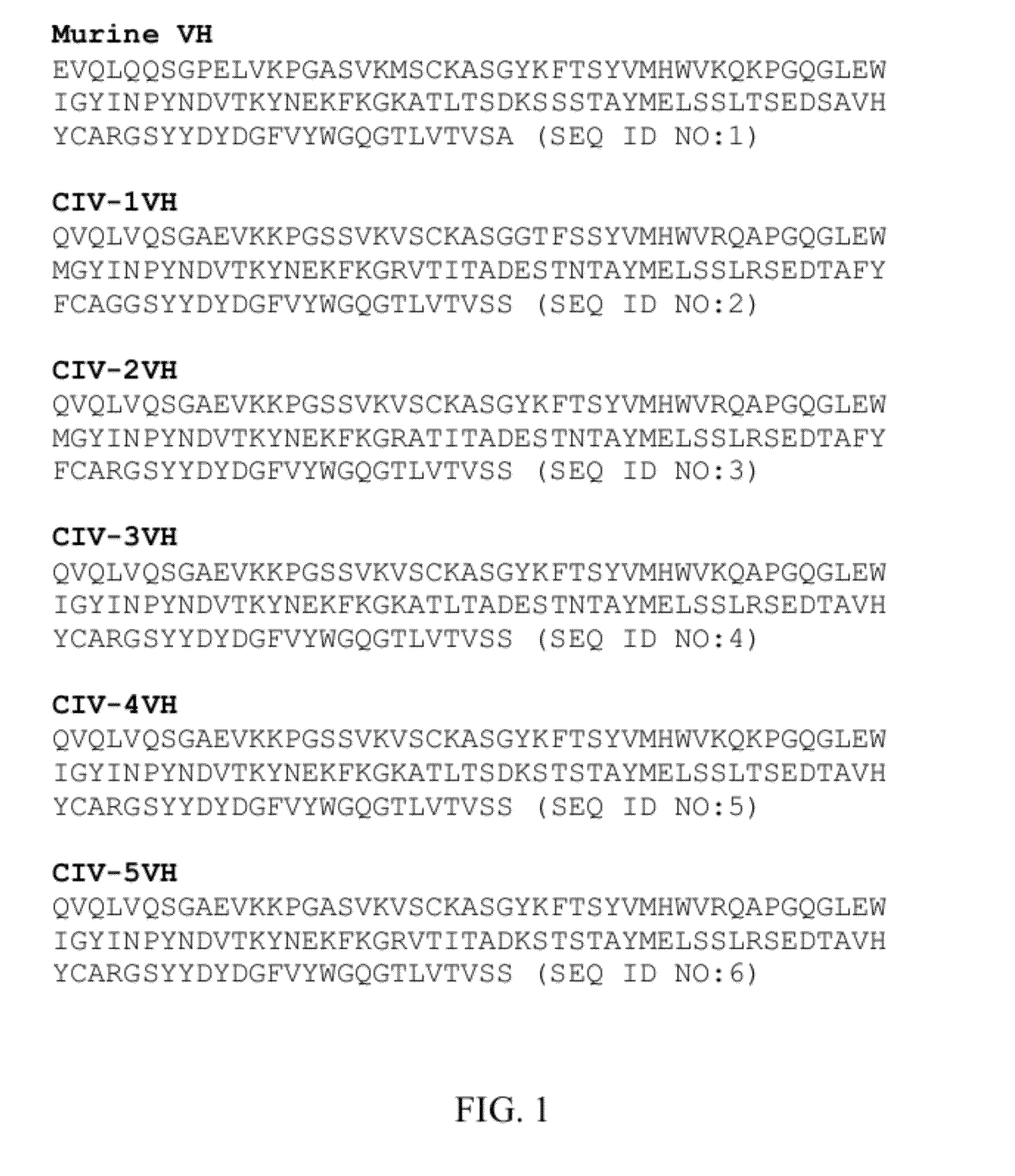
Reversible MHC multimer staining for functional purification of antigen-specific T cells
ActiveUS7776562B2Preserving functional statusHigh affinityMammal material medical ingredientsDepsipeptidesStainingBasic research
The present invention relates to a new method for reversible staining and functional isolation or characterization of cells, e.g. antigen-specific T cells. With this technique, the original functional status of cells can be substantially maintained after their identification and purification. Thus, this new method is of broad benefit for basic research and clinical applications.
Owner:IBA LIFESCIENCES GMBH
Novel methods for therapeutic vaccination
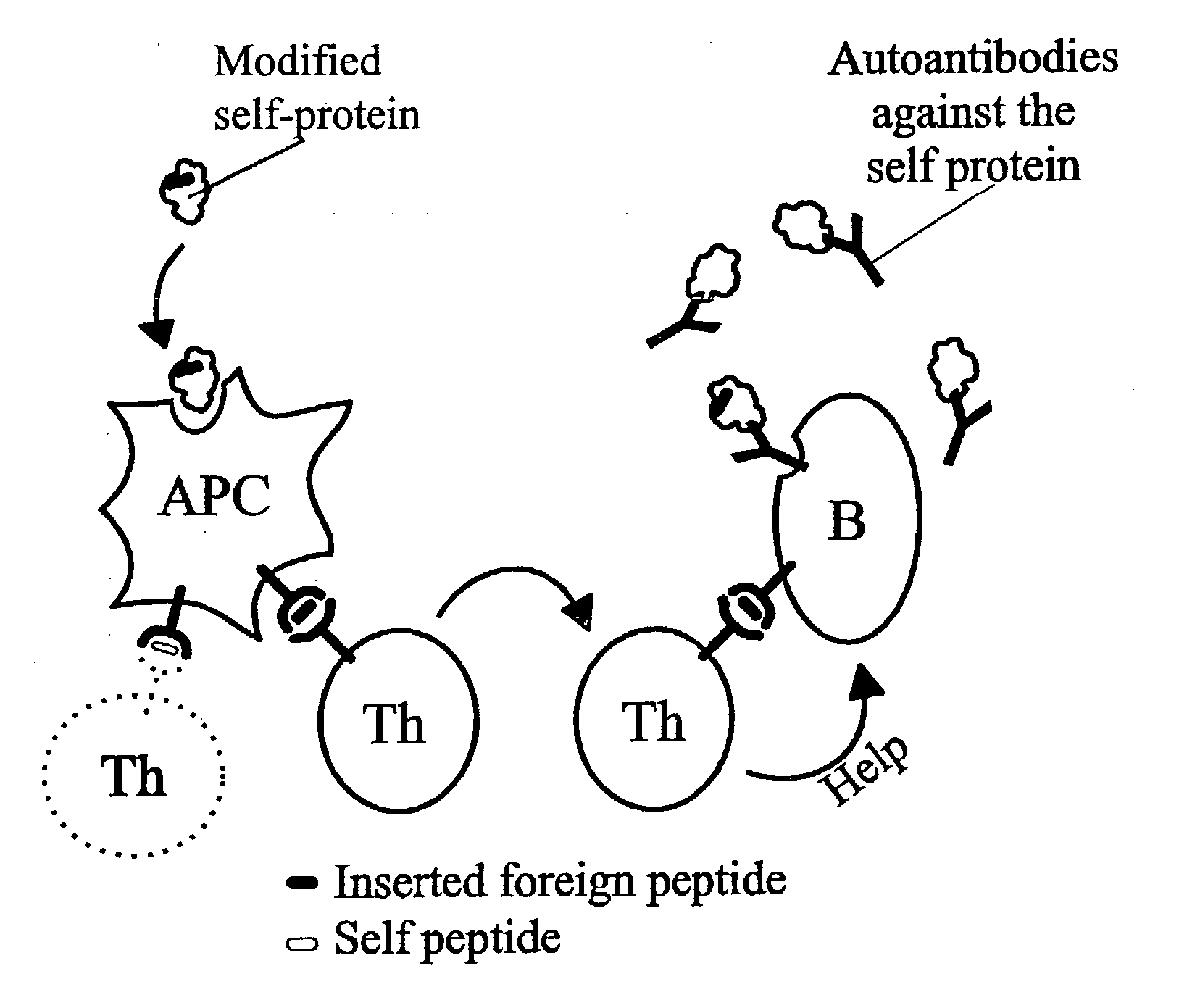
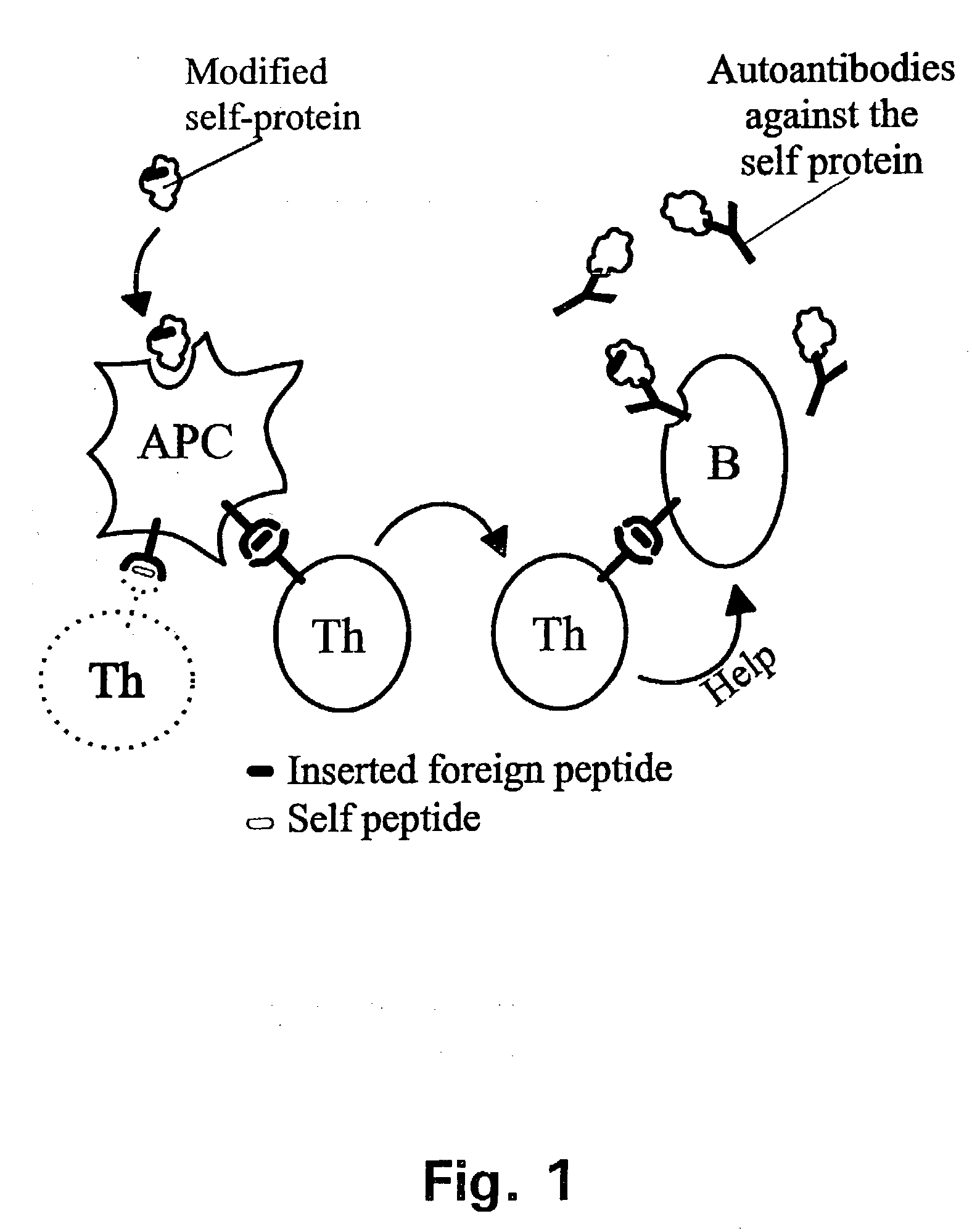
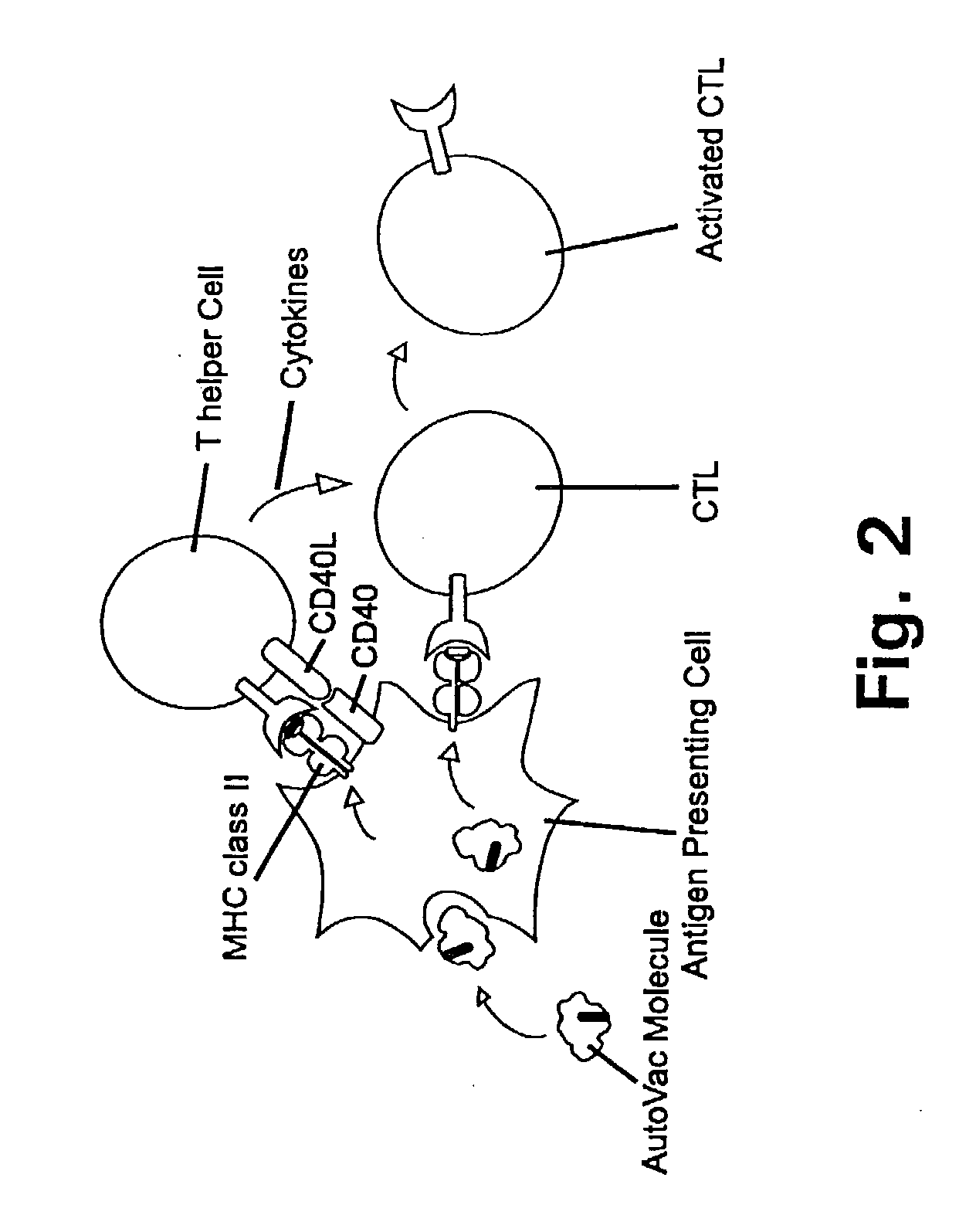
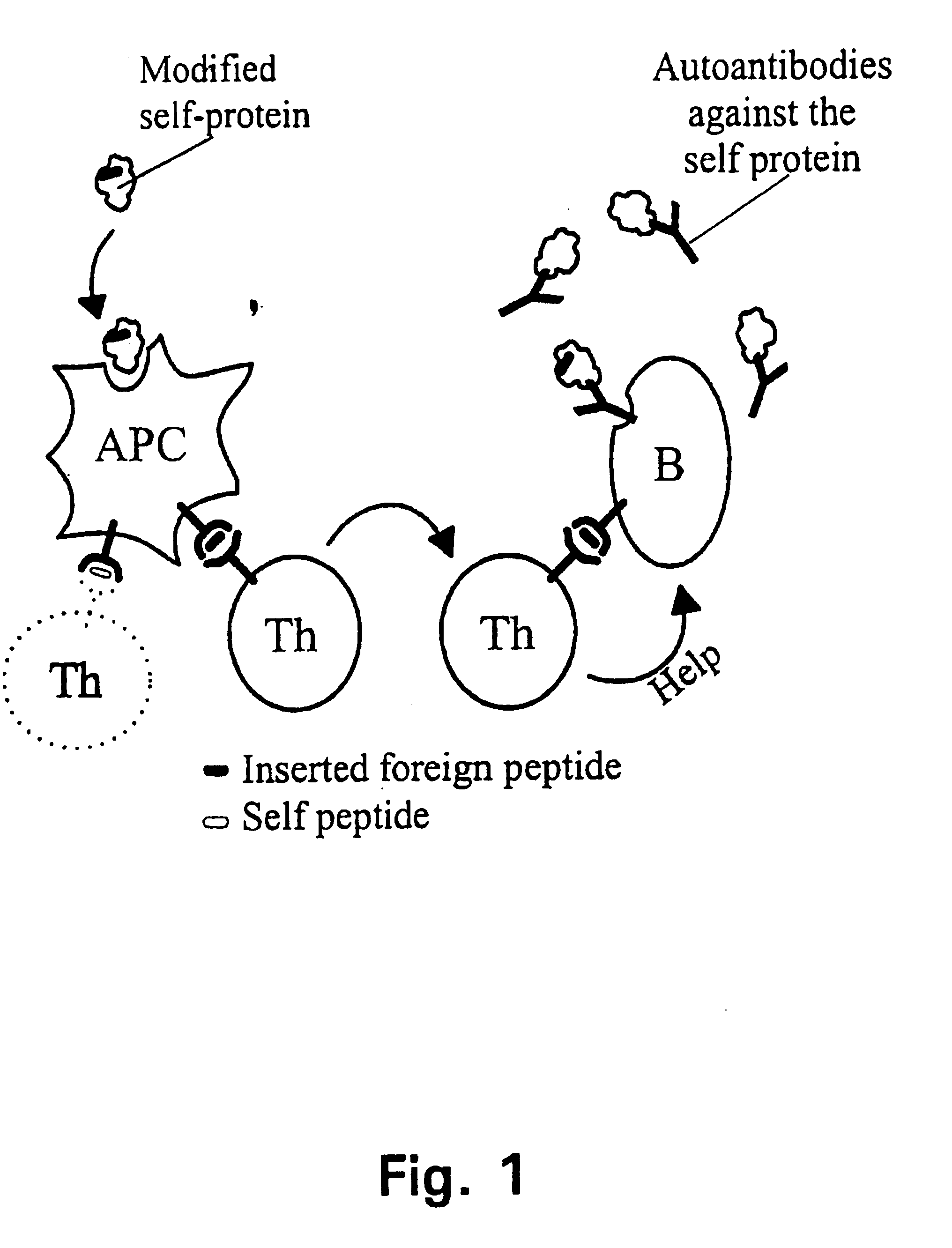
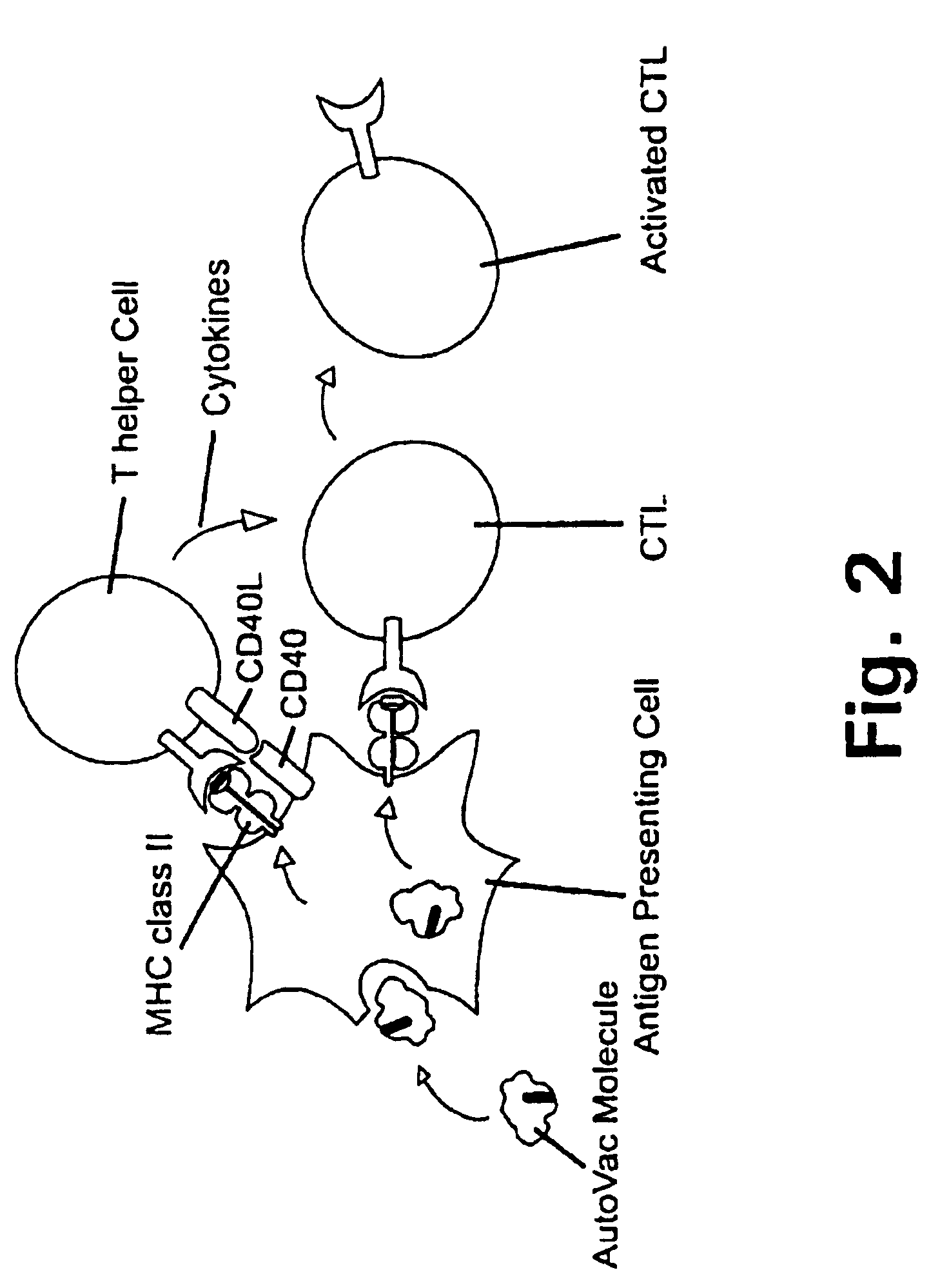
A method is disclosed for inducing cell-mediated immunity against cellular antigens. More specifically, the invention provides for a method for inducing cytotoxic T-lymphocyte immunity against weak antigens, notably self-proteins. The method entails that antigen presenting cells are induced to present at least one CTL epitope of the weak antigen and at the same time presenting at least one foreign T-helper lymphocyte epitope. In a preferred embodiment, the antigen is a cancer specific antigen, e.g. PSM, Her2, or FGF8b. The method can be exercised by using traditional polypeptide vaccination, but also by using live attenuated vaccines or nucleic acid vaccination. The invention furthermore provides immunogenic analogues of PSM, Her2 and FGF8b, as well as nucleic acid molecules encoding these analogues. Also vectors and transformed cells are disclosed. The invention also provides for a method for identification of immunogenic analogues of weak or non-immunogenic antigens.
Owner:BAVARIAN NORDIC AS
Novel methods for therapeutic vaccination
A method is disclosed for inducing cell-mediated immunity against cellular antigens. More specifically, the invention provides for a method for inducing cytotoxic T-lymphocyte immunity against weak antigens, notably self-proteins. The method entails that antigen presenting cells are induced to present at least one CTL epitope of the weak antigen and at the same time presenting at least one foreign T-helper lymphocyte epitope. In a preferred embodiment, the antigen is a cancer specific antigen, e.g. PSM, Her2, or FGF8b. The method can be exercised by using traditional polypeptide vaccination, but also by using live attenuated vaccines or nucleic acid vaccination. The invention furthermore provides immunogenic analogues of PSM, Her2 and FGF8b, as well as nucleic acid molecules encoding these analogues. Also vectors and transformed cells are disclosed. The invention also provides for a method for identification of immunogenic analogues of weak or non-immunogenic antigens.
Owner:BAVARIAN NORDIC AS
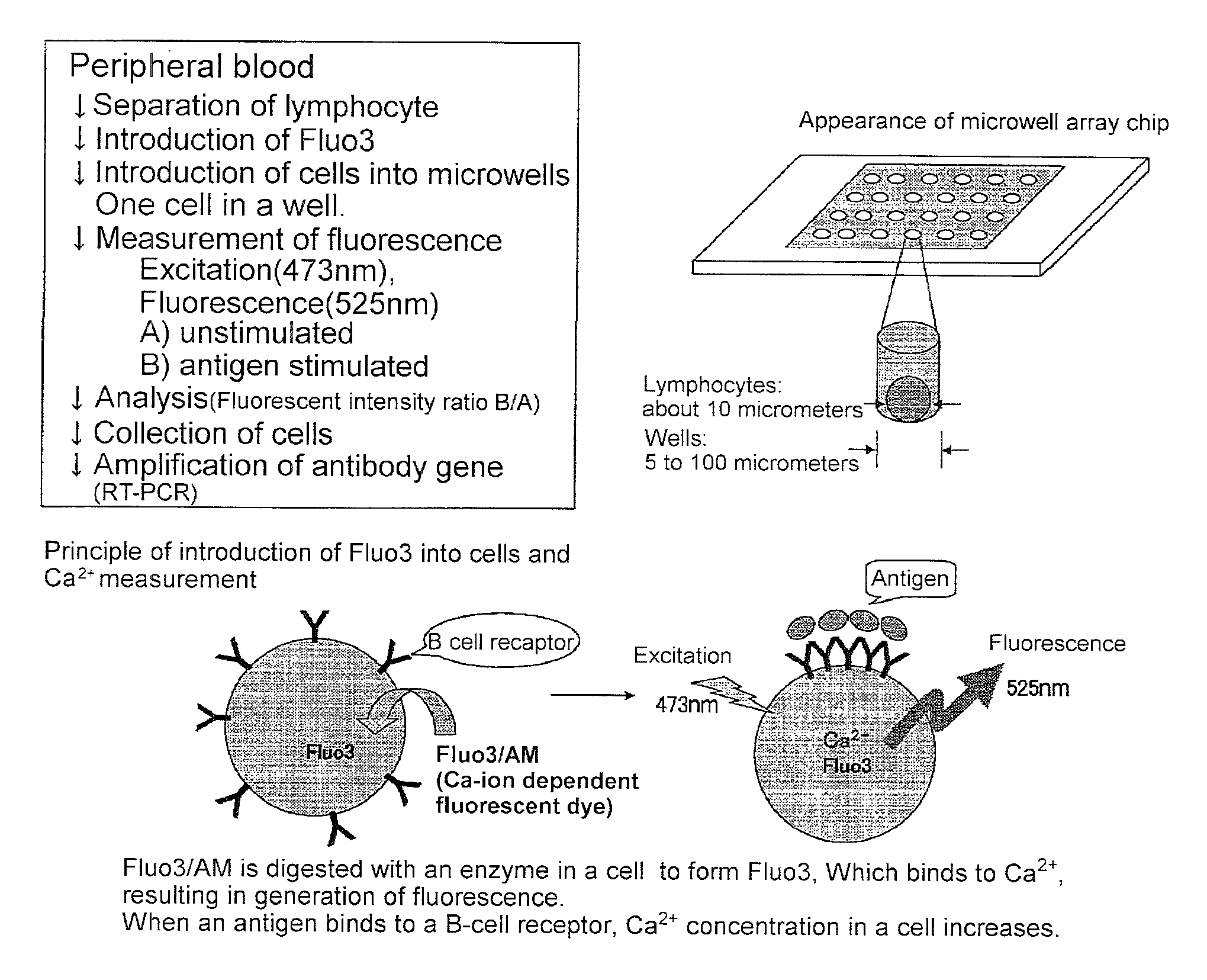
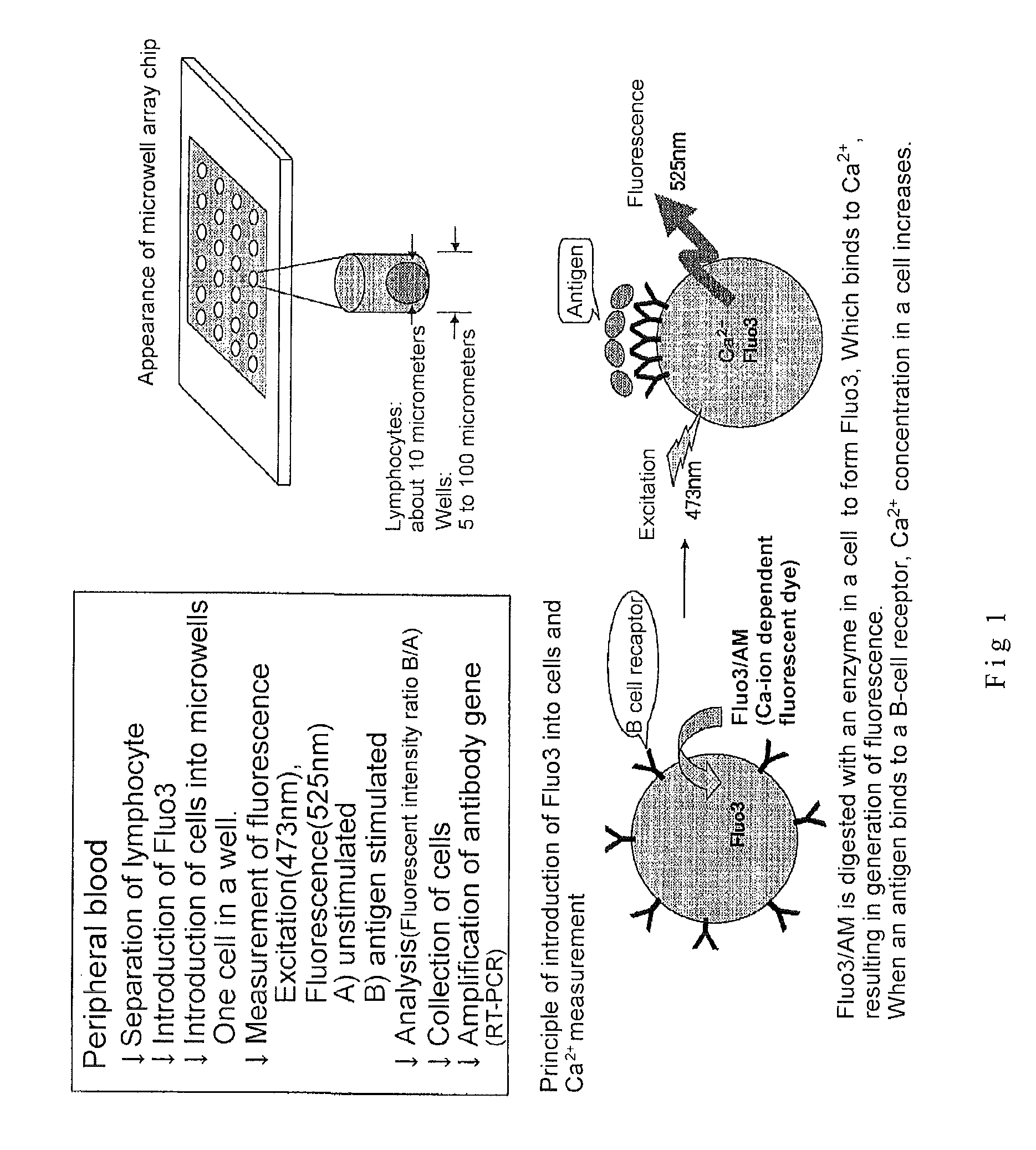
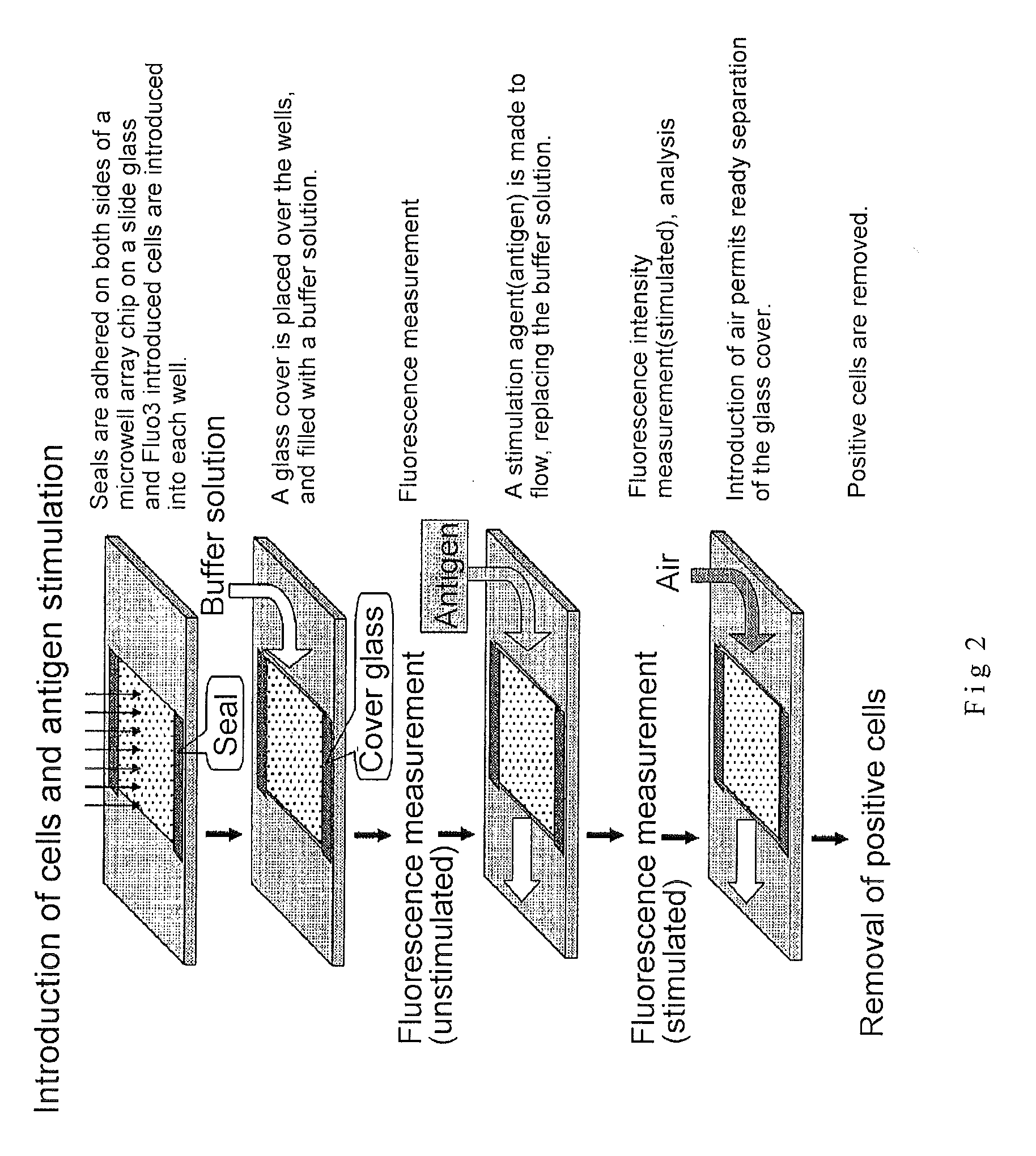
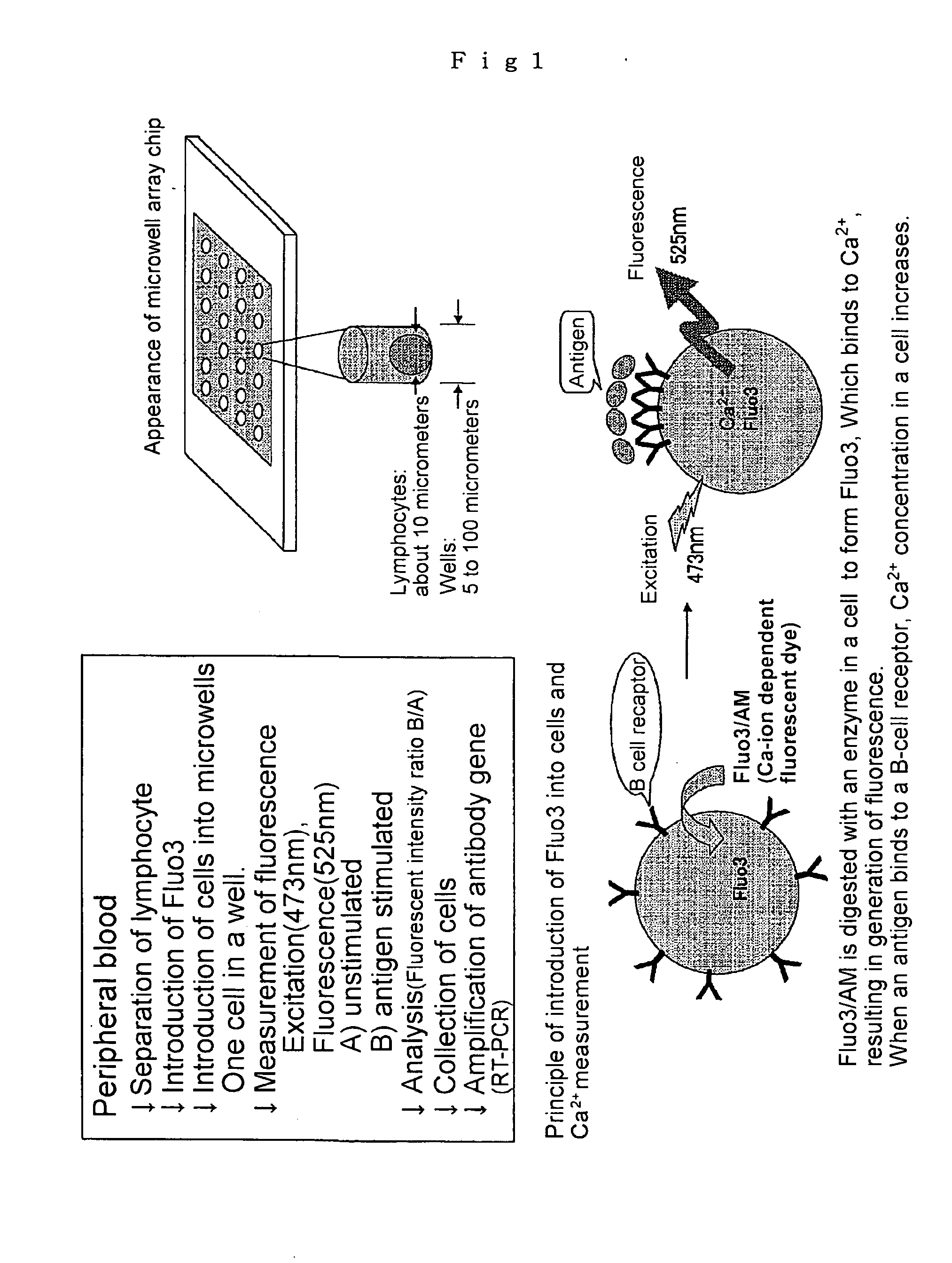
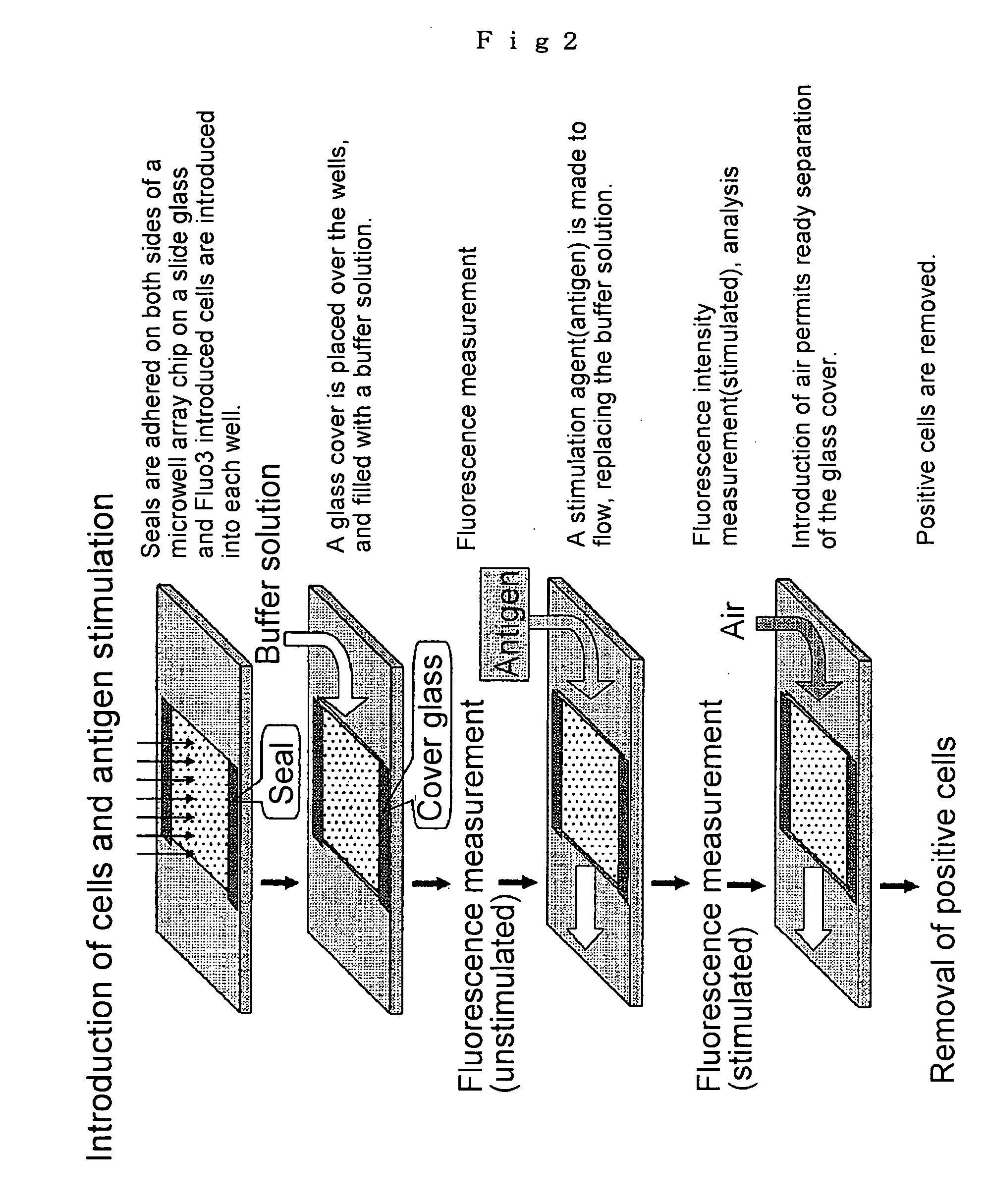
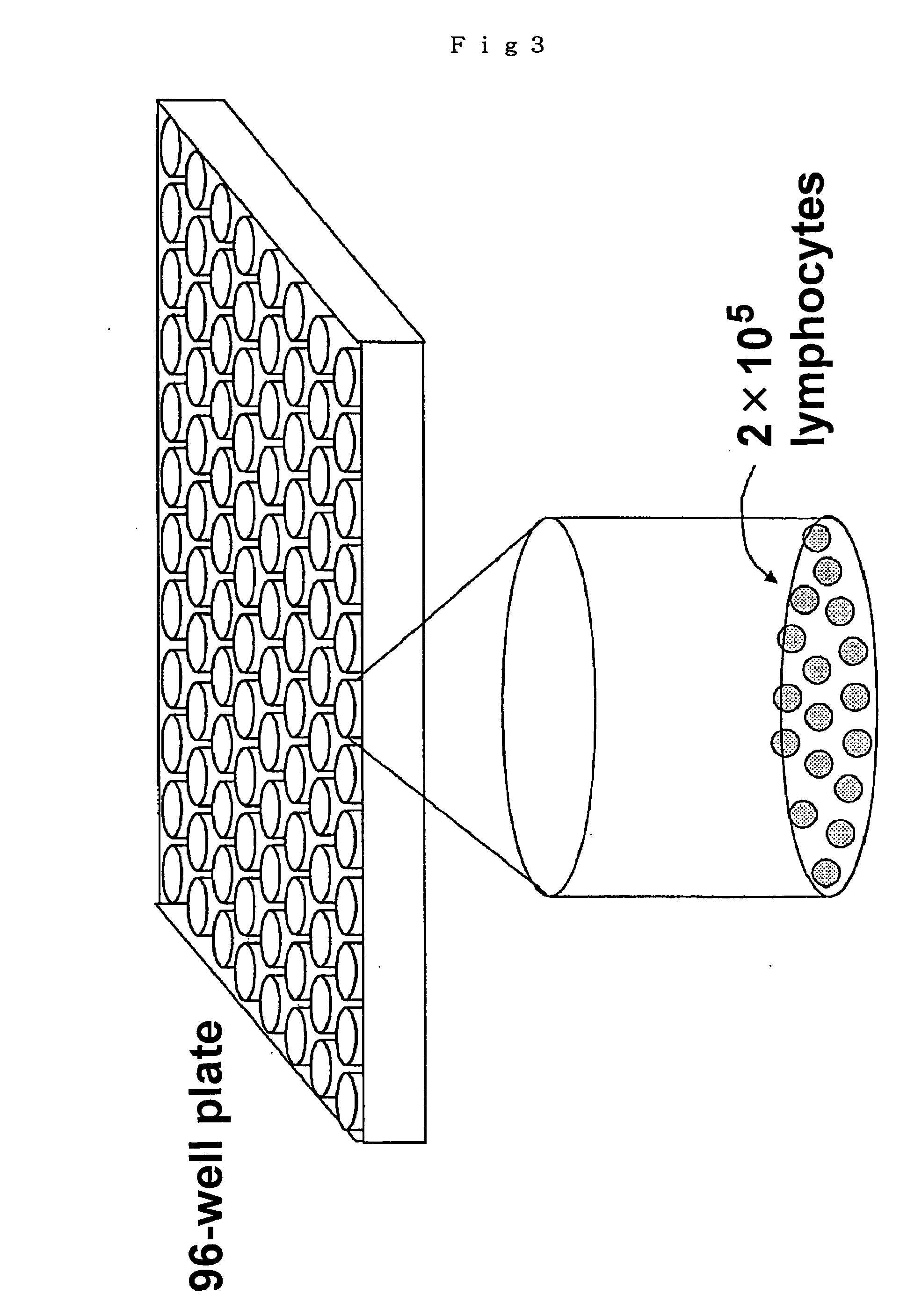
Microwell array chip for detecting antigen-specific lymphocytes, method of detecting and method of manufacturing antigen-specific lymphocytes, and method of cloning antigen-specific lymphocyte antigen receptor genes
ActiveUS20090181859A1Library screeningImmunoglobulins against virusesLymphocyte antigen receptorCellular antigens
A microwell array chip that has multiple microwells and is employed to contain a single lymphocyte specimen in each microwell and detect antigen-specific lymphocytes in single units; wherein the microwell array chip is of a shape and of dimensions where only one lymphocyte is contained in each microwell. A method of detecting antigen-specific lymphocytes comprising the steps of adding antigen to each microwell in the above microwell array chip, stimulating the lymphocyte specimen, and detecting lymphocyte specimens reacting with the antigen.
Owner:BLINK BIOMEDICAL SAS
Inhibiting B cell activation with soluble CD40 or fusion proteins thereof
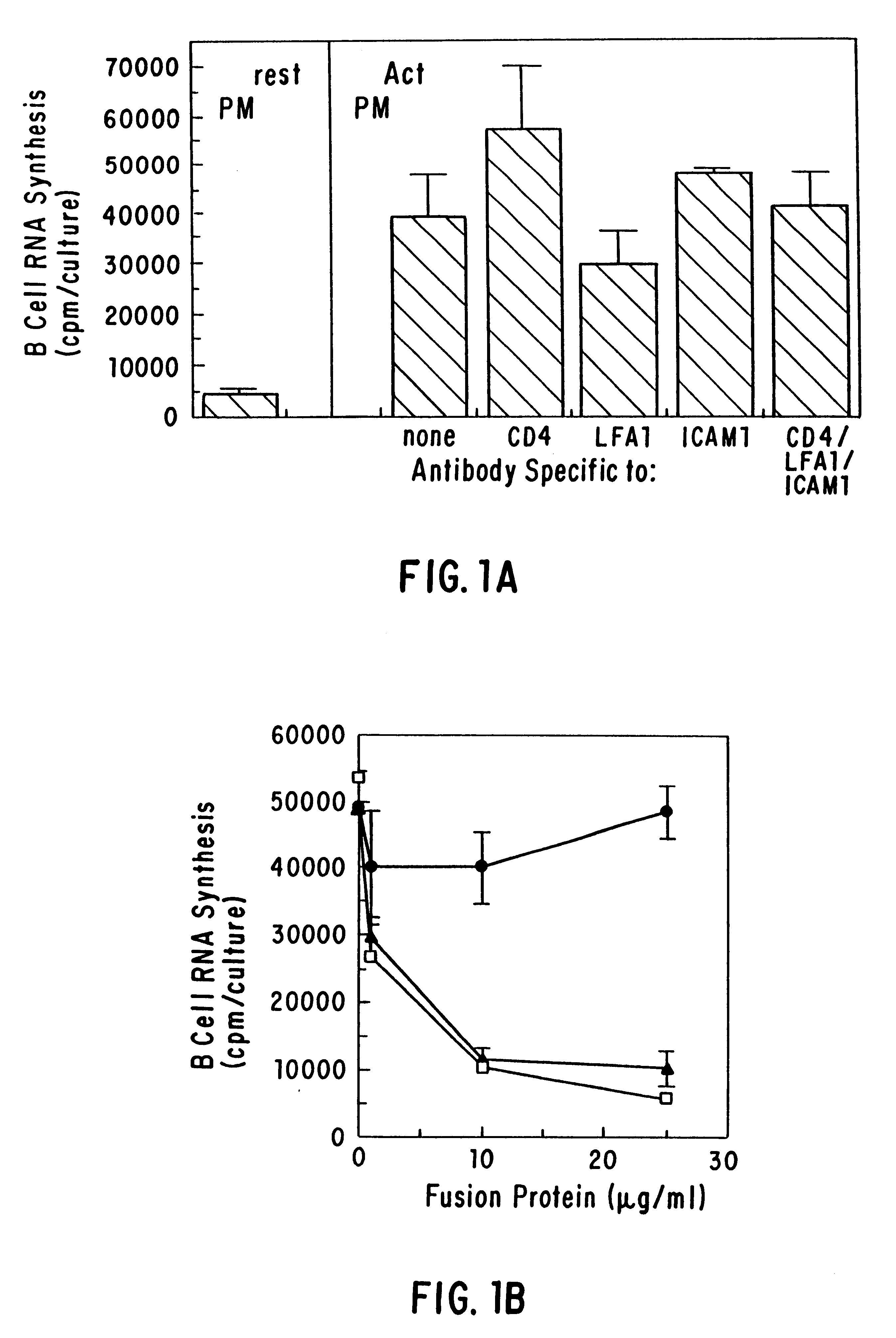
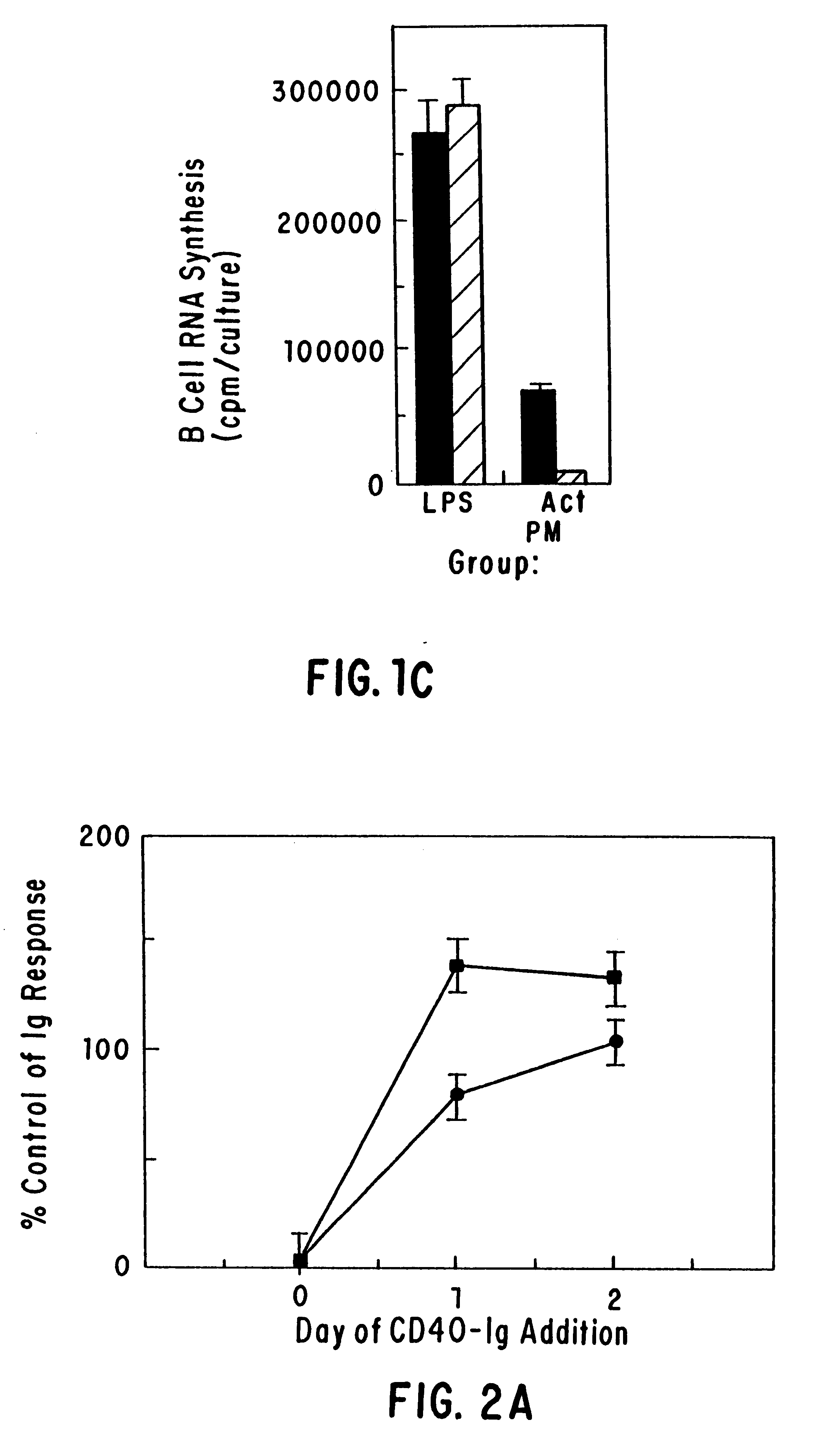
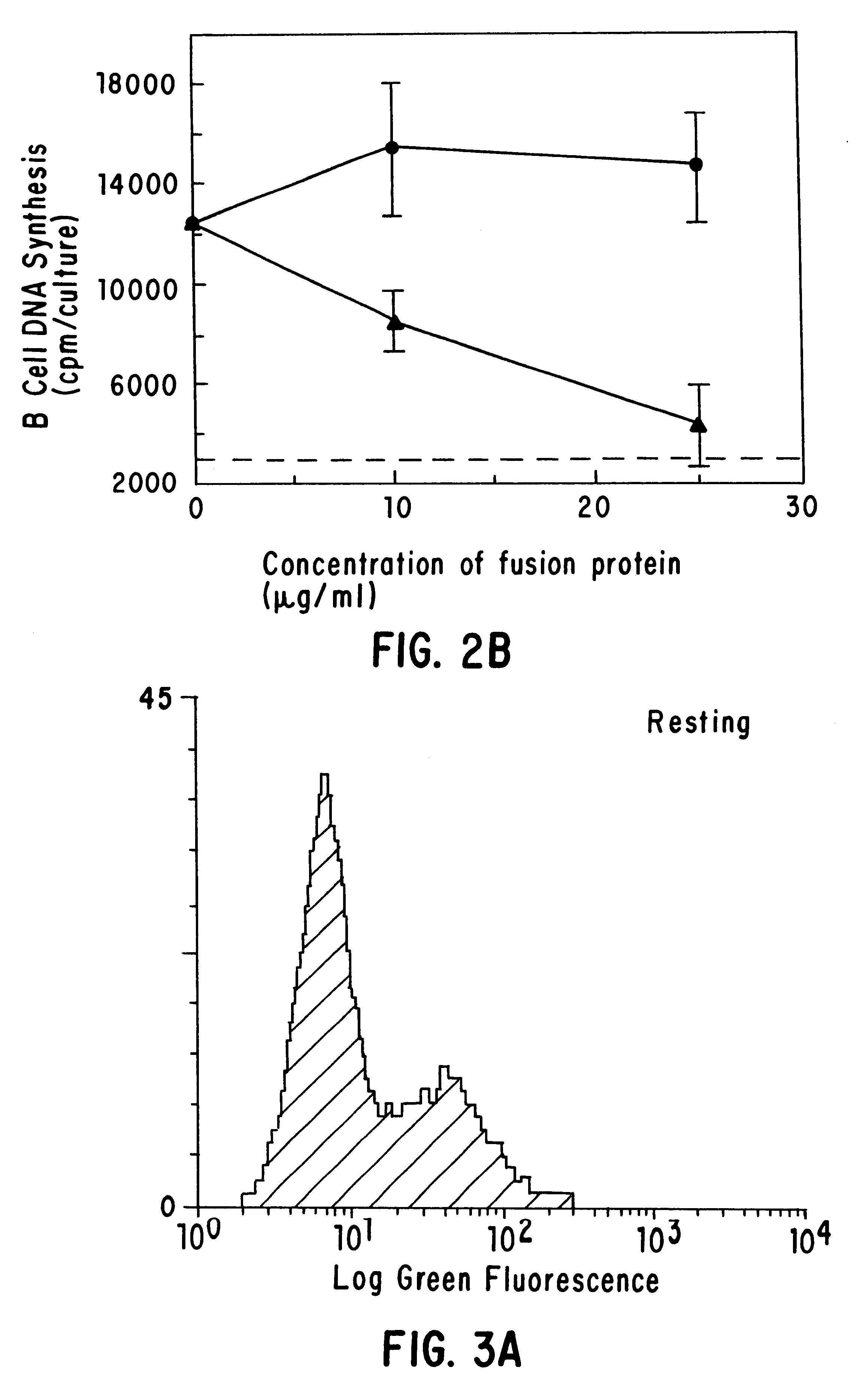
The present invention relates to a counter-receptor, termed CD40CR, for the CD40 B-cell antigen, and to soluble ligands for this receptor, including fusion molecules comprising at least a portion of CD40 protein. It is based, at least in part, on the discovery that a soluble CD40 / immunoglobulin fusion protein or antibody specific for gp39 on T cells was able to inhibit helper T-cell mediated B-cell activation by binding to a novel 39 kD protein receptor on helper T-cell membranes. The present invention provides for a substantially purified CD40CR receptor; for soluble ligands of CD40CR, including antibodies as well as fusion molecules comprising at least a portion of CD40 protein; and for methods of controlling B-cell activation which may be especially useful in the treatment of allergy or autoimmune disease, including graft-versus-host disease and rheumatoid arthritis.
Owner:BRISTOL MYERS SQUIBB CO +1
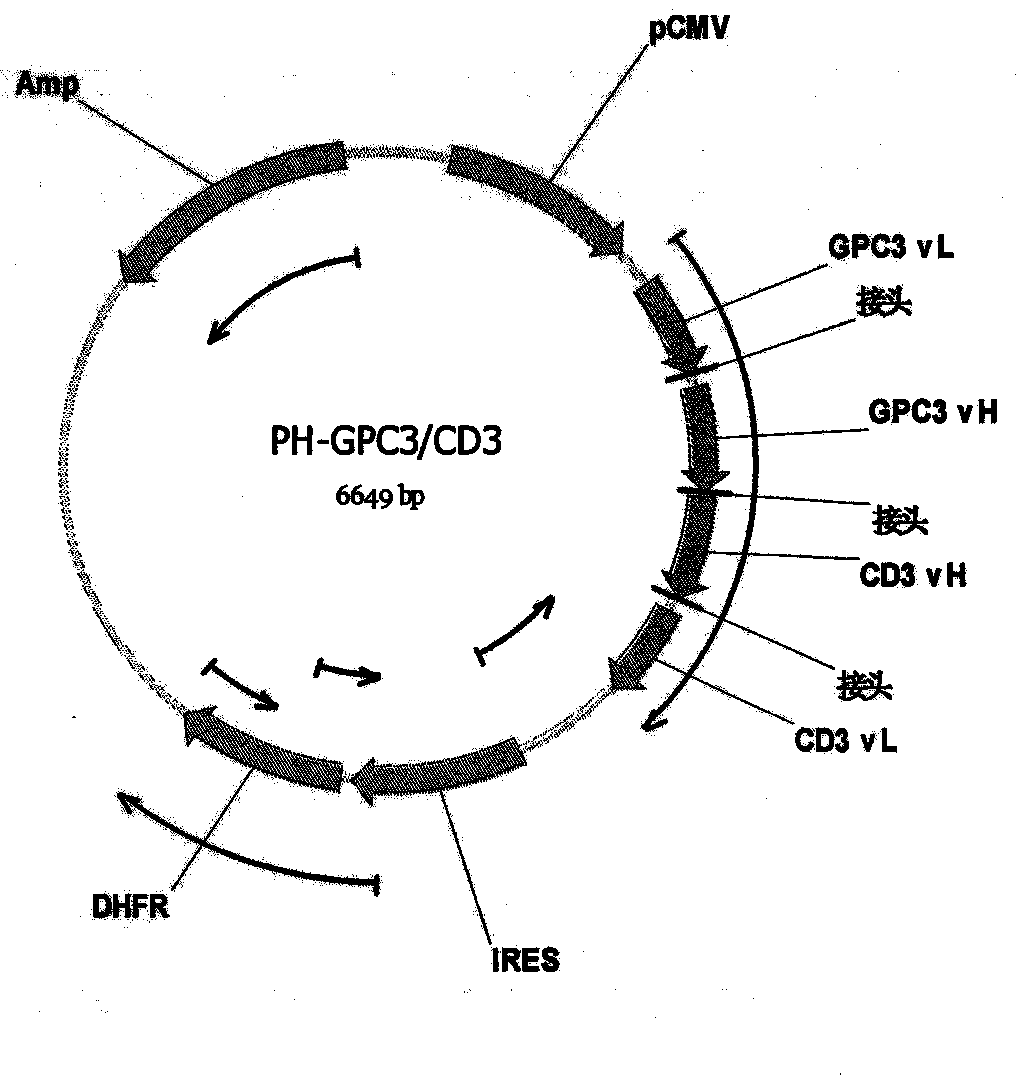
Bispecific antibody aiming at phosphatidylinositols protein polysaccharide-3 and T cell antigen
InactiveCN103833852ABacteriaImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenNucleotide
The first aspect of the invention relates to a bispecific antibody, which comprises a first functional domain for specific identification of phosphatidylinositol protein polysaccharide-3, a second domain for specific identification of human T cell antigen CD3, and a connection for connecting the functional domains. The second aspect of the invention relates to a nucleotide sequence encoding the above antibody. The third aspect of the invention relates to a carrier containing the above nucleotide sequence, and includes an expressive vector. The fourth aspect of the invention relates to a eukaryotic or prokaryotic expression system containing the above carrier. The fifth aspect of the invention relates to application of the above antibody to preparation of medicament for treating or preventing tumor.
Owner:SHANGHAI INST OF ONCOLOGY
Microwell array chip for detecting antigen-specific lymphocytes, method of detecting and method of manufacturing antigen-specific lymphocytes, and method of cloning antigen-specific lymphocyte antigen receptor genes
InactiveUS20060134704A1Bioreactor/fermenter combinationsBiological substance pretreatmentsLymphocyte antigen receptorCellular antigens
A microwell array chip that has multiple microwells and is employed to contain a single lymphocyte specimen in each microwell and detect antigen-specific lymphocytes in single units; wherein the microwell array chip is of a shape and of dimensions where only one lymphocyte is contained in each microwell. A method of detecting antigen-specific lymphocytes comprising the steps of adding antigen to each microwell in the above microwell array chip, stimulating the lymphocyte specimen, and detecting lymphocyte specimens reacting with the antigen.
Owner:VIVALIS SA
Control of antibody responses to synthetic nanocarriers
Disclosed are synthetic nanocarrier compositions that comprise B cell antigen for desired antibody production and an off-target response attenuating polymeric coating as well as related methods.
Owner:SELECTA BIOSCI
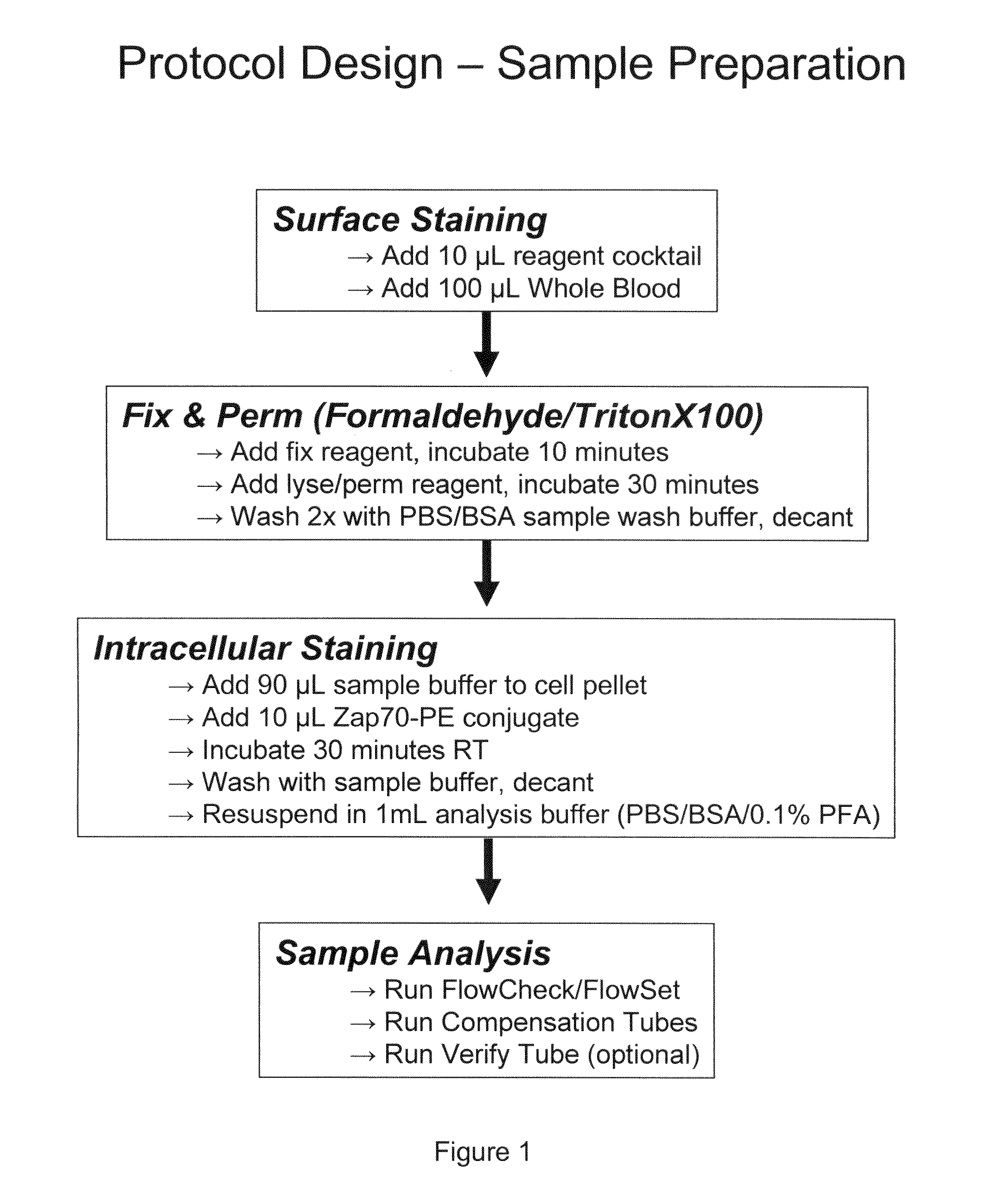
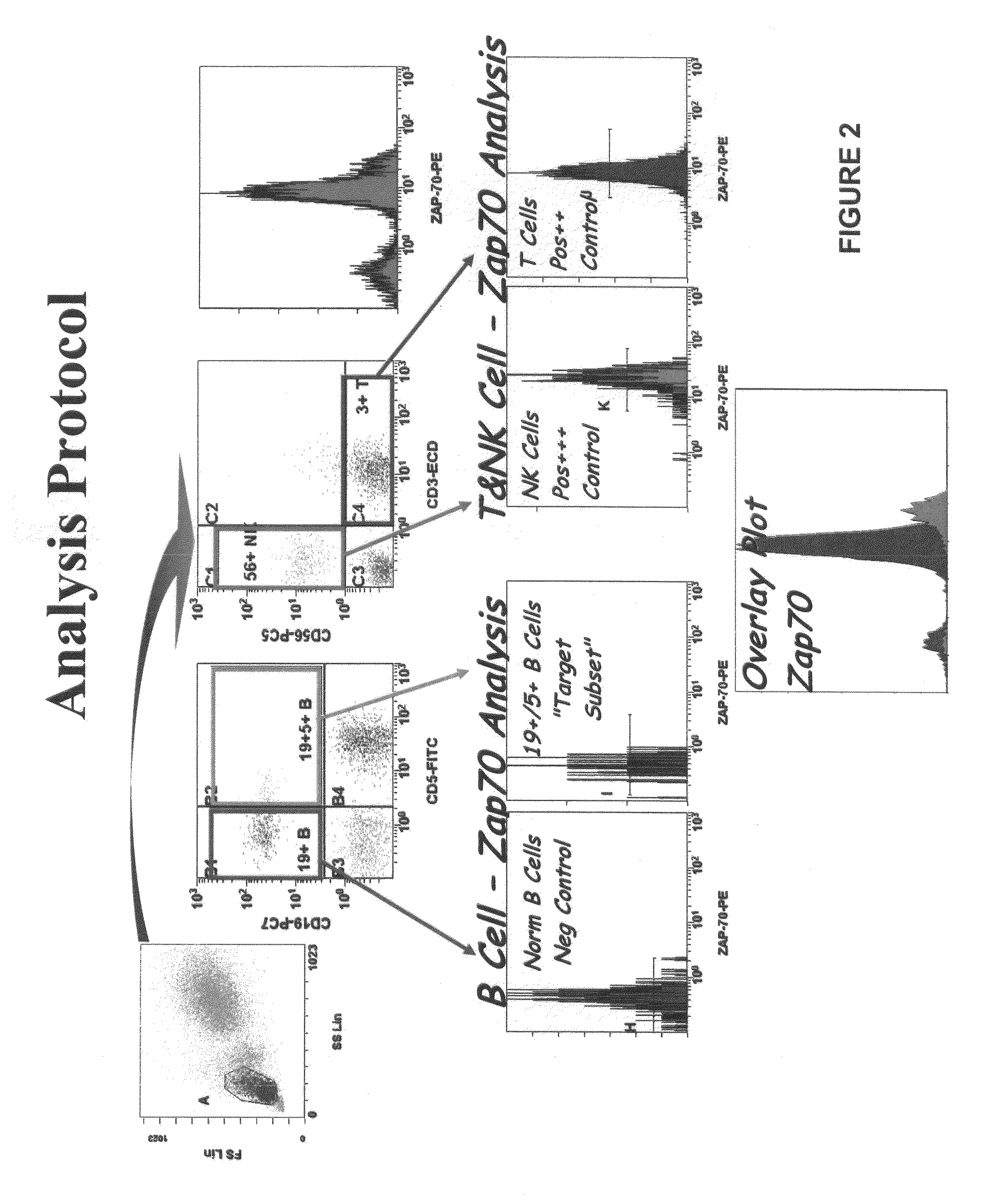
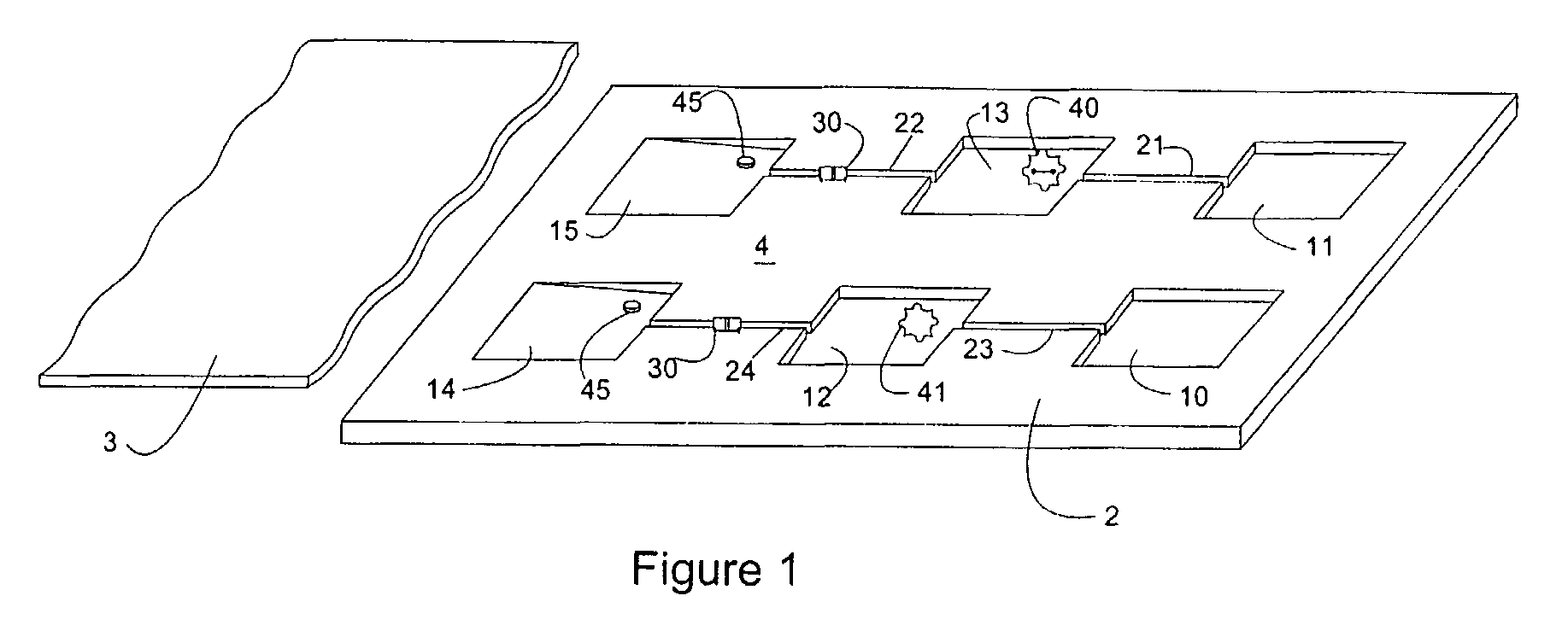
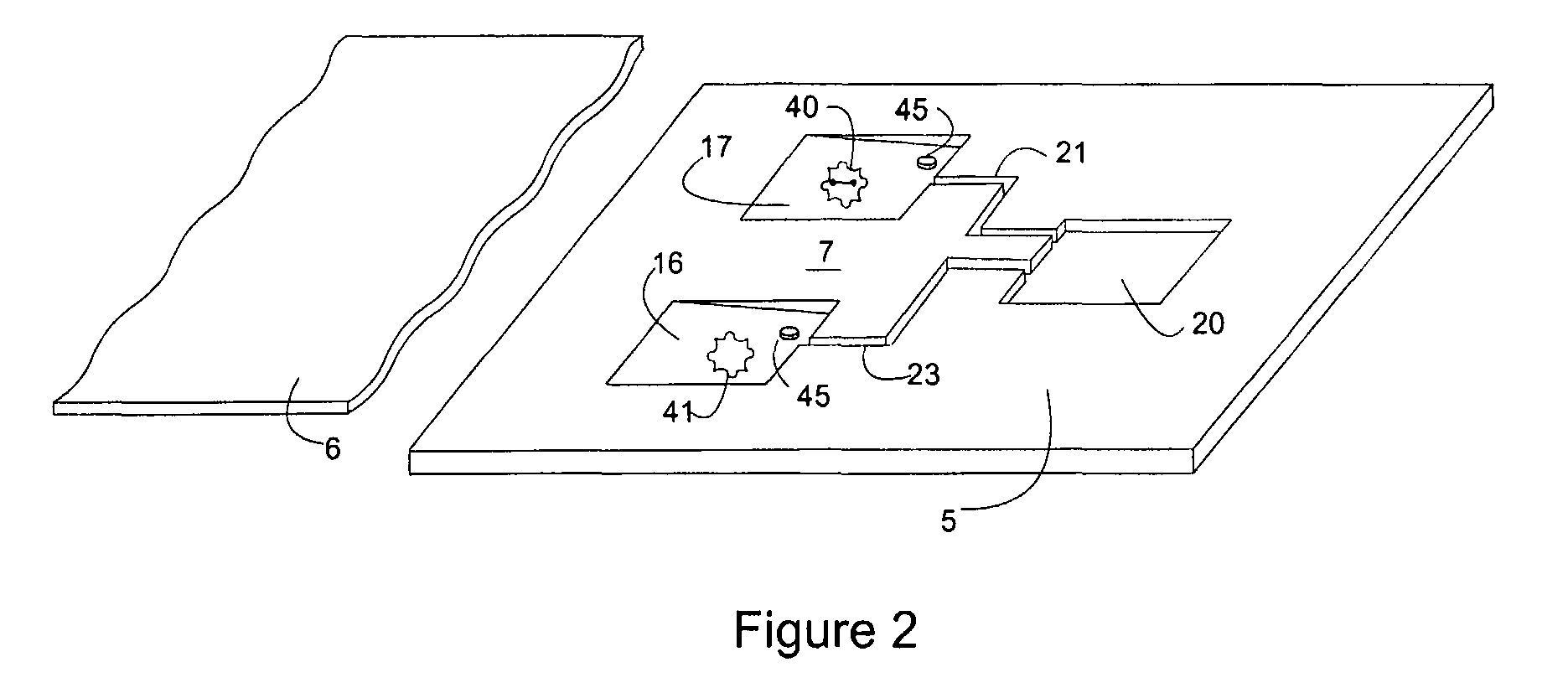
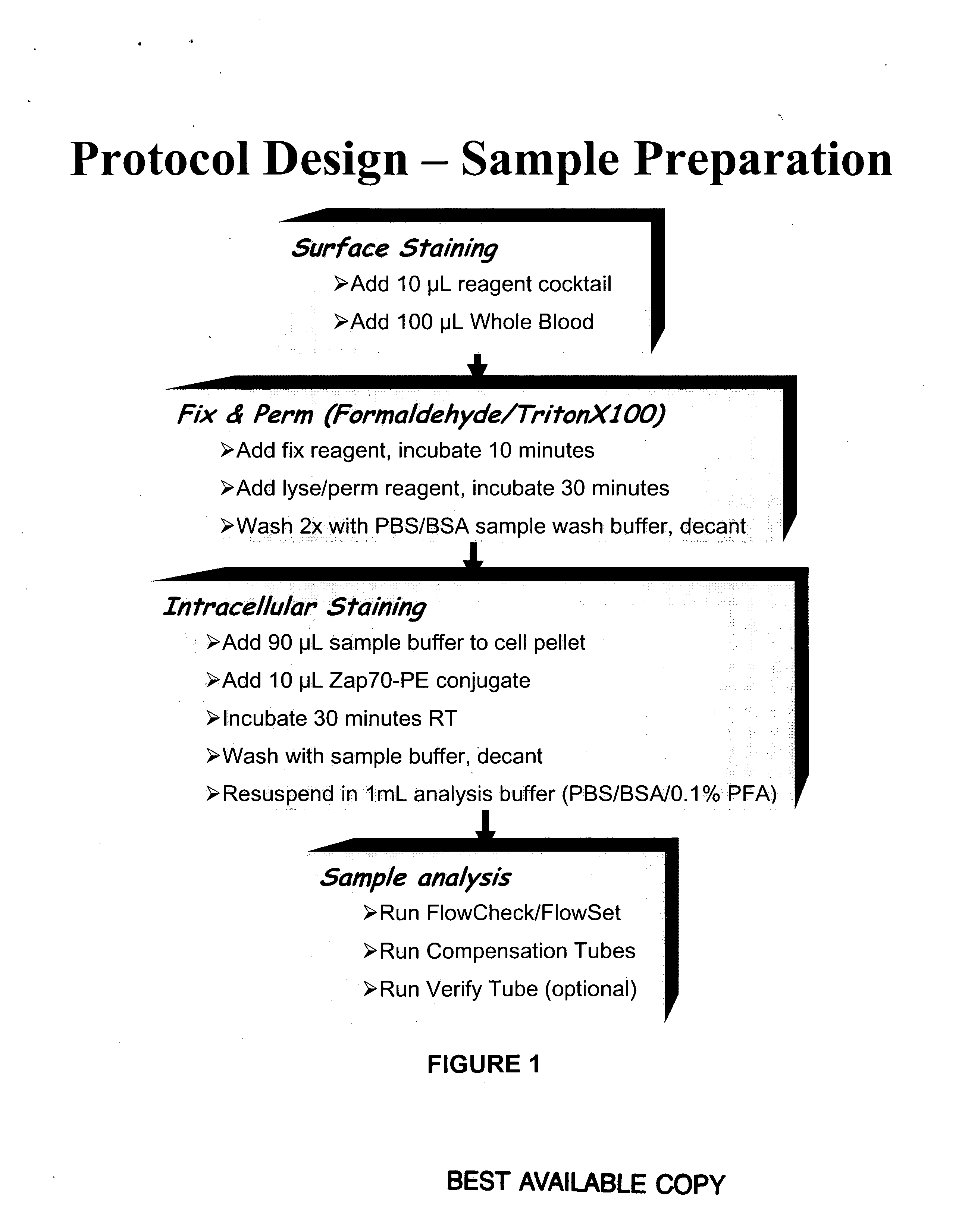
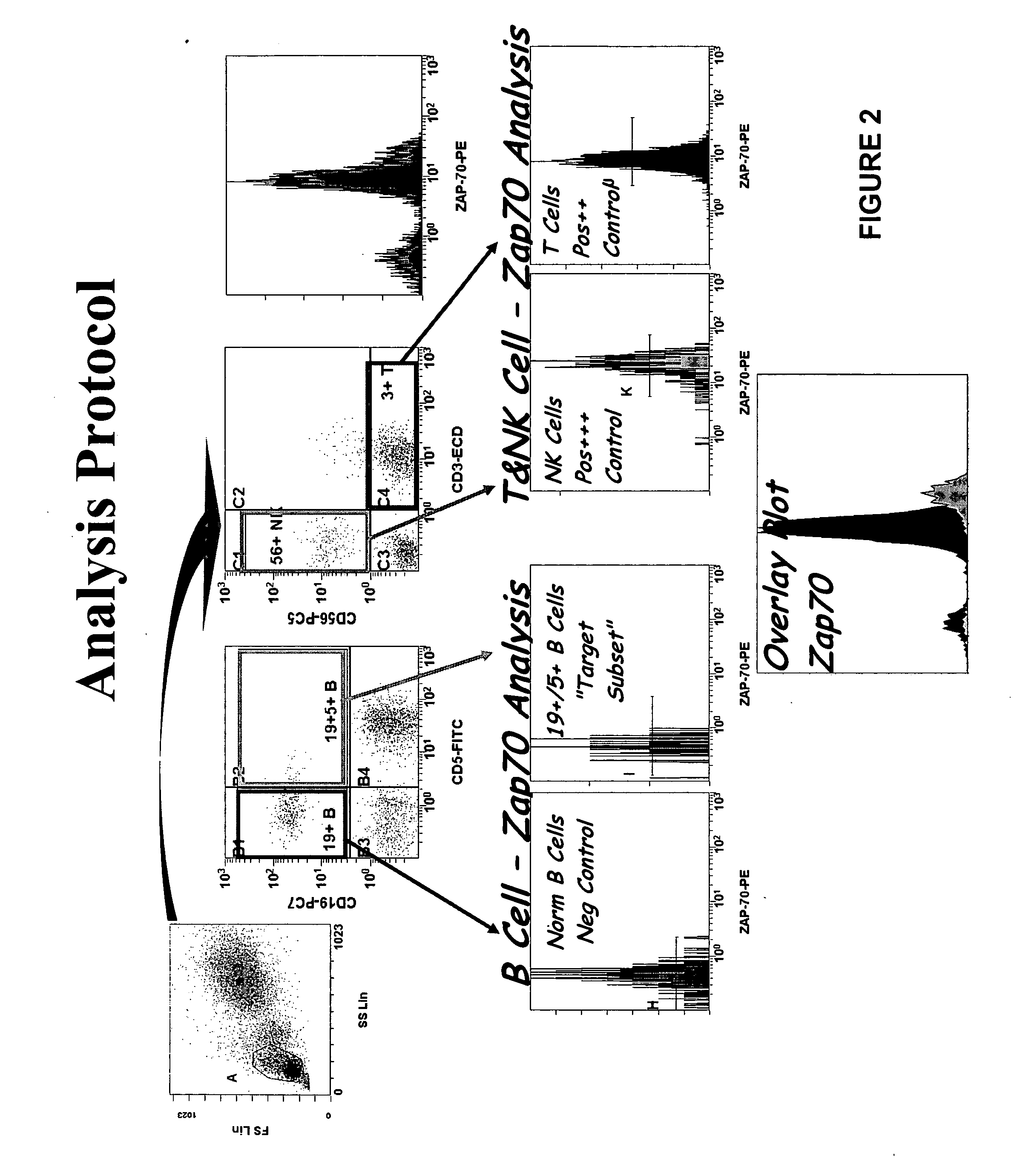
Composite Profiles of Cell Antigens and Target Signal Transduction Proteins for Analysis and Clinical Management of Hematologic Cancers
InactiveUS20100261204A1Increased riskDetermining prognosisDisease diagnosisBlood/immune system cellsCellular antigensTarget signal
Owner:BECKMAN COULTER INC
T-Cell Receptor Antibodies And Methods of Use Thereof
InactiveUS20120034221A1Good curative effectAvoid managementFungiBacteriaDiseaseT-Cell Antigen Receptors
The present invention is directed to the production and use of monoclonal antibodies, or antigen binding fragments thereof, that specifically bind the T cell antigen receptor (TCR) and their use for immunomodulation. In preferred embodiments, the antibody or antigen binding fragment of the invention specifically binds the constant region of the α chain of the TCR, or otherwise specifically binds the α chain regardless of TCR clonal origin (i.e., is pan specific for TCR). The antibodies of the invention may be used, for example, in immunosuppressive therapies for transplant maintenance and the treatment of autoimmune diseases, and / or as targeting molecules for use in the treatment of T-cell malignancies.
Owner:MACROGENICS INC
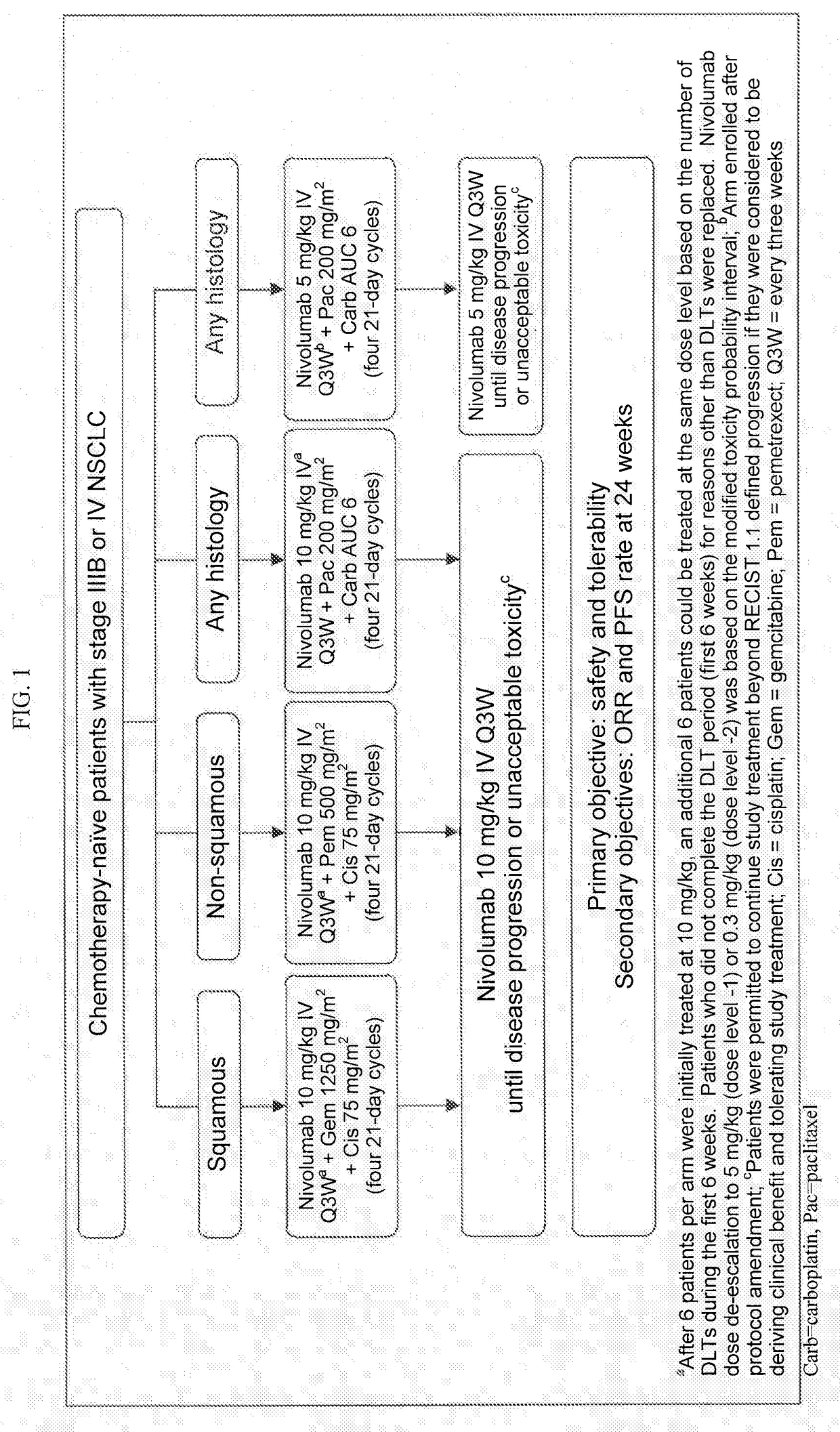
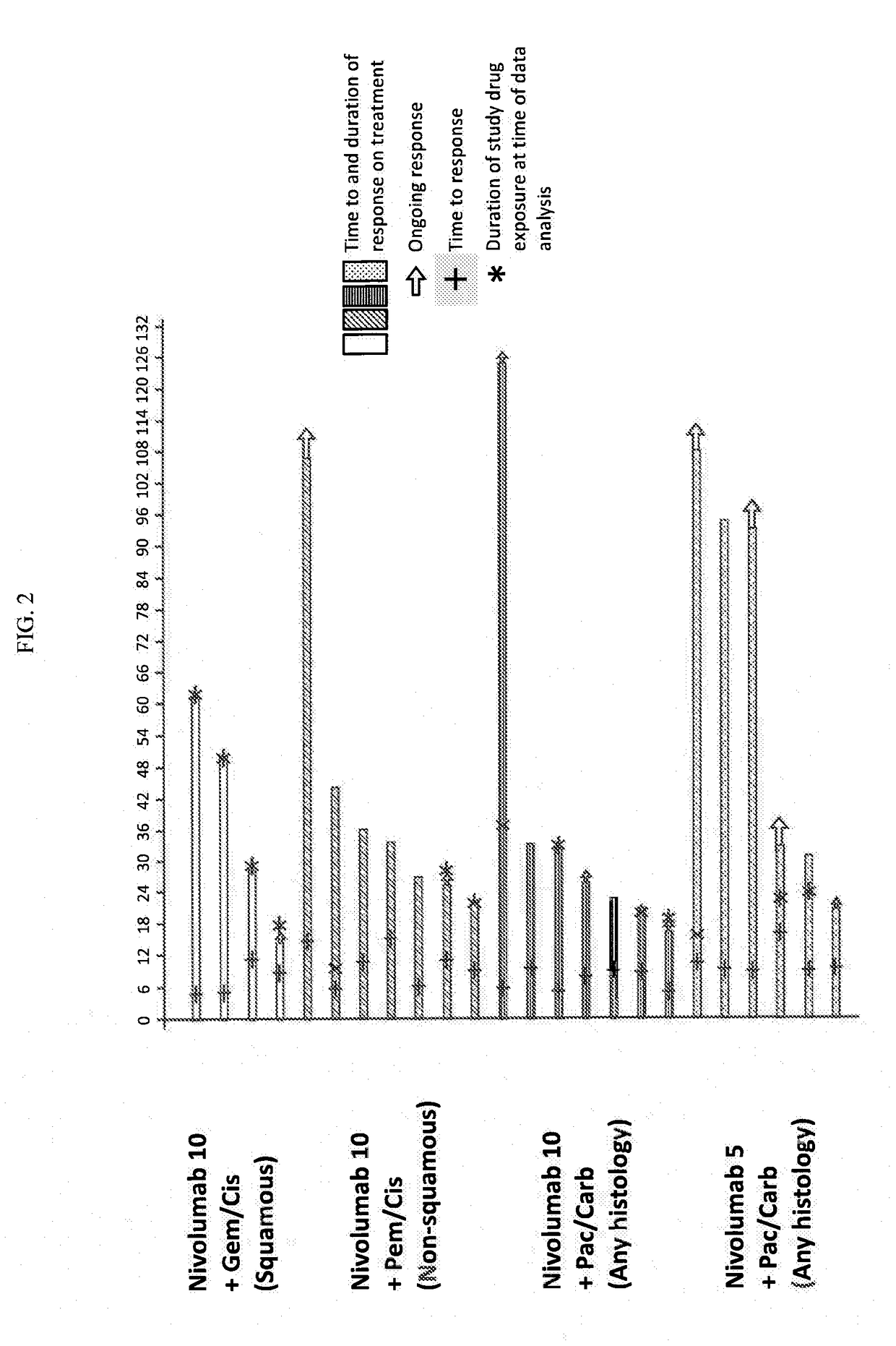
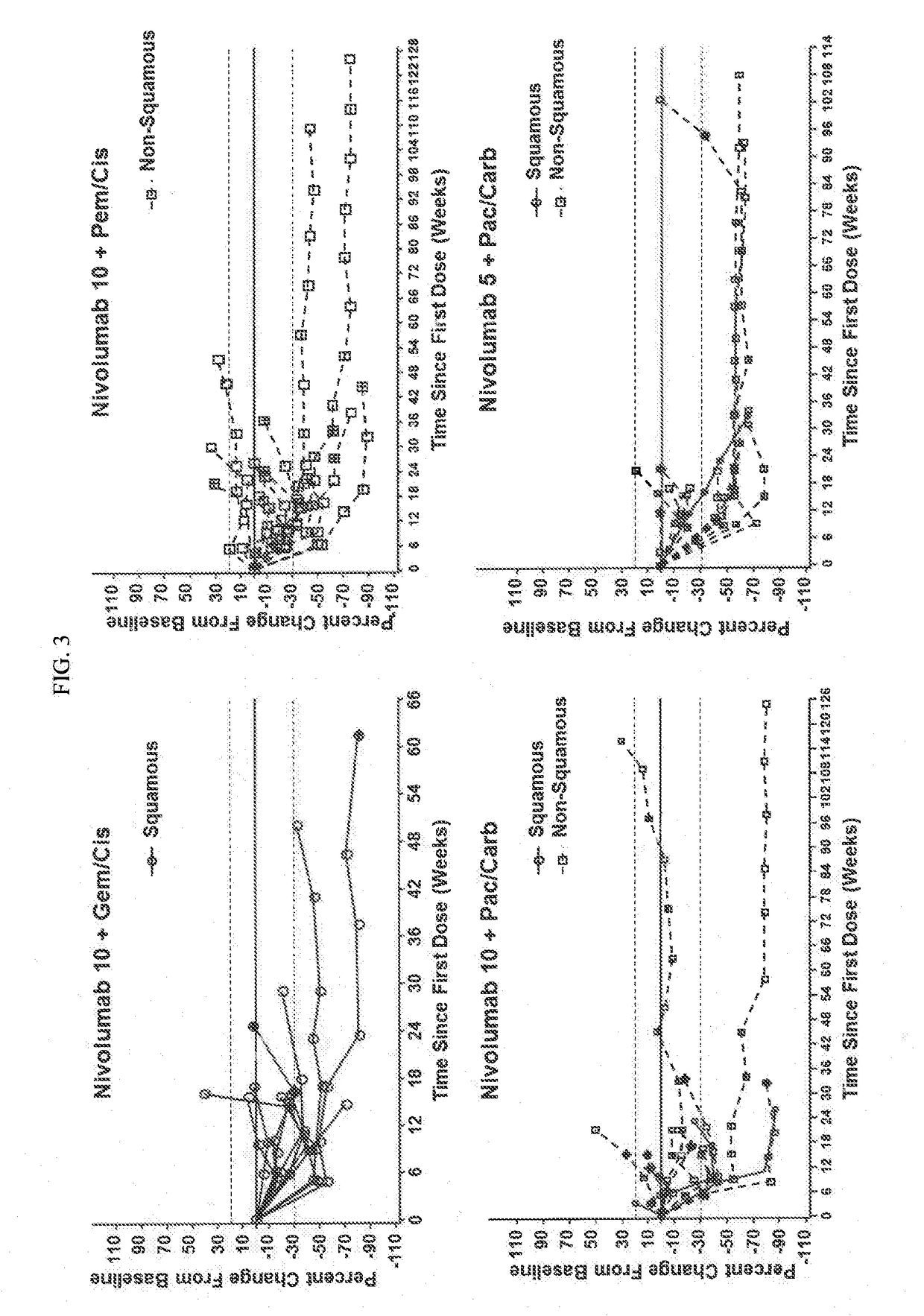
Treatment of lung cancer using a combination of an Anti-pd-1 antibody and another Anti-cancer agent
InactiveUS20170158776A1Durable clinical responseReliable responseHeavy metal active ingredientsImmunoglobulins against growth factorsAnticarcinogenTyrosine-kinase inhibitor
This disclosure provides a method for treating a subject afflicted with a lung cancer, which method comprises administering to the subject therapeutically effective amounts of: (a) an anti-cancer agent which is an antibody or an antigen-binding portion thereof that specifically binds to a Programmed Death-1 (PD-1) receptor and inhibits PD-1 activity; and (b) another anti-cancer agent. The other anti-cancer agent can be a platinum-based doublet chemotherapy, an EGFR-targeted tyrosine kinase inhibitor, bevacizumab, an anti-Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) antibody, or any other therapy used to treat lung cancer in the art or disclosed herein.
Owner:BRISTOL MYERS SQUIBB CO
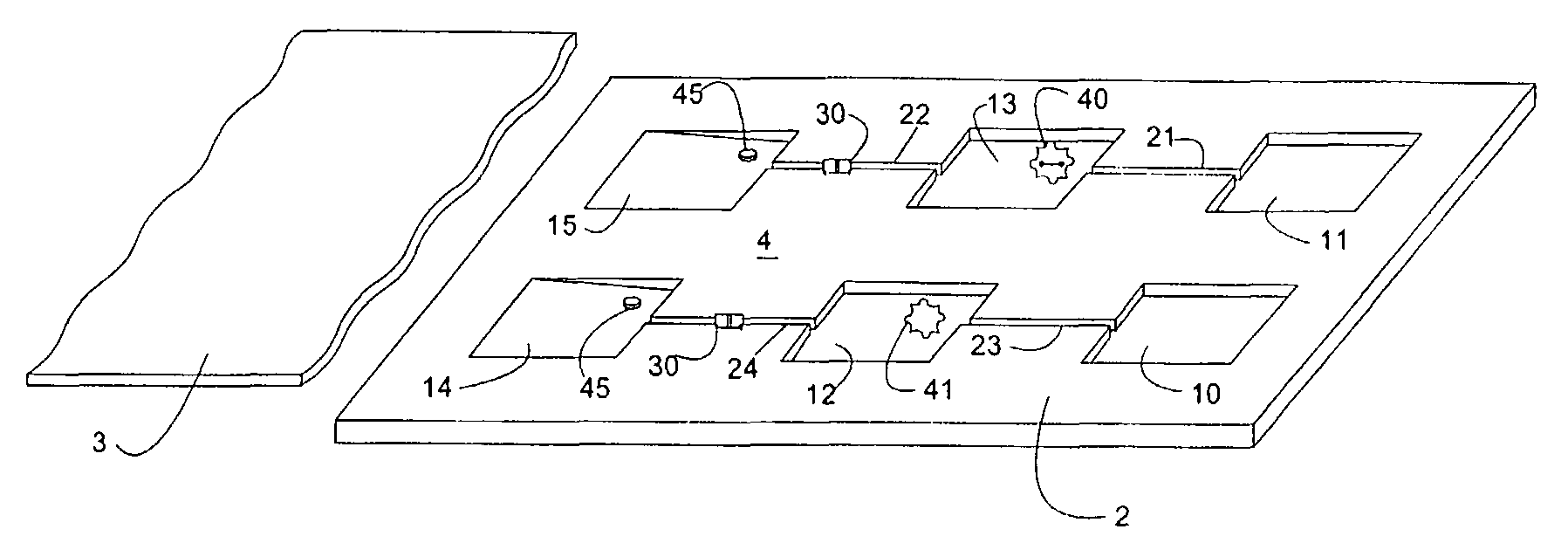
Microfluidic device and leucocyte antigen mediated microfluidic assay
ActiveUS9023641B2Bioreactor/fermenter combinationsBiological substance pretreatmentsCellular antigensWhite blood cell
Owner:ADVANCED ANIMAL DIAGNOSTICS
Composite profiles of cell antigens and target signal transduction proteins for analysis and clinical management of hematologic cancers
InactiveUS20070105165A1Increased relapse riskDetermining prognosisDisease diagnosisBlood/immune system cellsCellular antigensTarget signal
The present invention is directed to methods for establishing a composite marker profile for a sample derived from an individual suspected having a neoplastic condition. A composite marker profile of the invention allows for identification of prognostically and therapeutically relevant subgroups of neoplastic conditions and prediction of the clinical course of an individual. The methods of the invention provide tools useful in choosing a therapy for an individual afflicted with a neoplastic condition, including methods for assigning a risk group, methods of predicting an increased risk of relapse, methods of predicting an increased risk of developing secondary complications, methods of choosing a therapy for an individual, methods of determining the efficacy of a therapy in an individual, and methods of determining the prognosis for an individual. In particular, the method of the present invention discloses a method for establishing a composite marker profile that can serve as a prognostic indicator to predict whether the course of a neoplastic condition in a individual will be aggressive or indolent, thereby aiding the clinician in managing the patient and evaluating the modality of treatment to be used. In particular embodiments disclosed herein, the methods of the invention are directed to establishing a composite marker profile for a leukemia selected from the group consisting of Chronic Lymphocytic Leukemia (CLL), Acute Myelogenous Leukemia (AML), Chronic Myelogenous Leukemia (CML), and Acute Lymphocytic Leukemia (ALL).
Owner:BECKMAN COULTER INC +4
Use of anti-CD45 leukocyte antigen antibodies for immunomodulation
InactiveUS7160987B2Altered expressionInduce toleranceAnimal cellsPeptide/protein ingredientsDiseaseEpitope
A method for the prevention or reversal of transplant rejection, or for therapy for autoimmune diseases, is provided comprising administering compounds such as monoclonal antibodies, that bind specifically to one or more preselected CD45R leukocyte antigens, including the CD45RB epitope.
Owner:RES CORP TECH INC
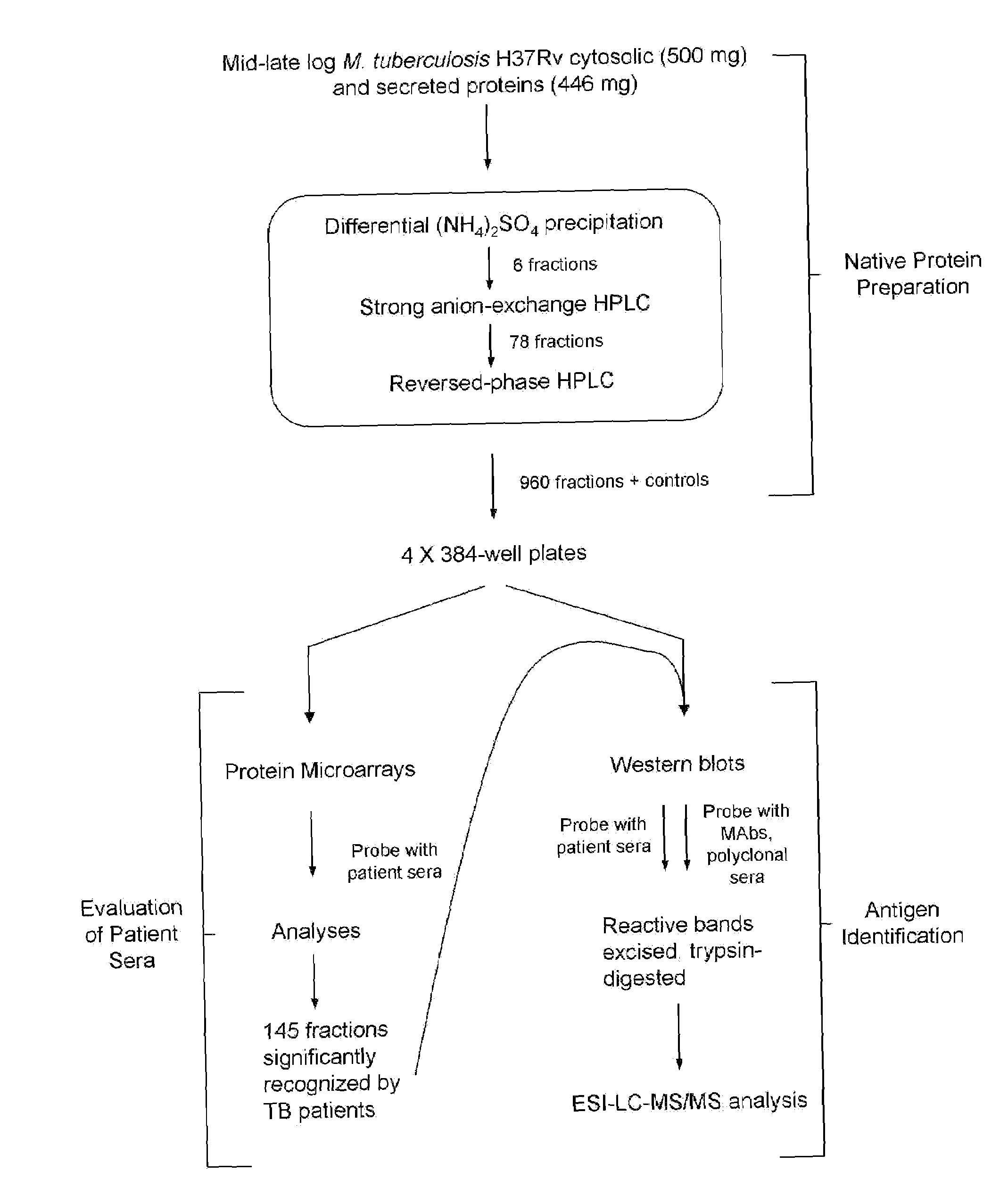
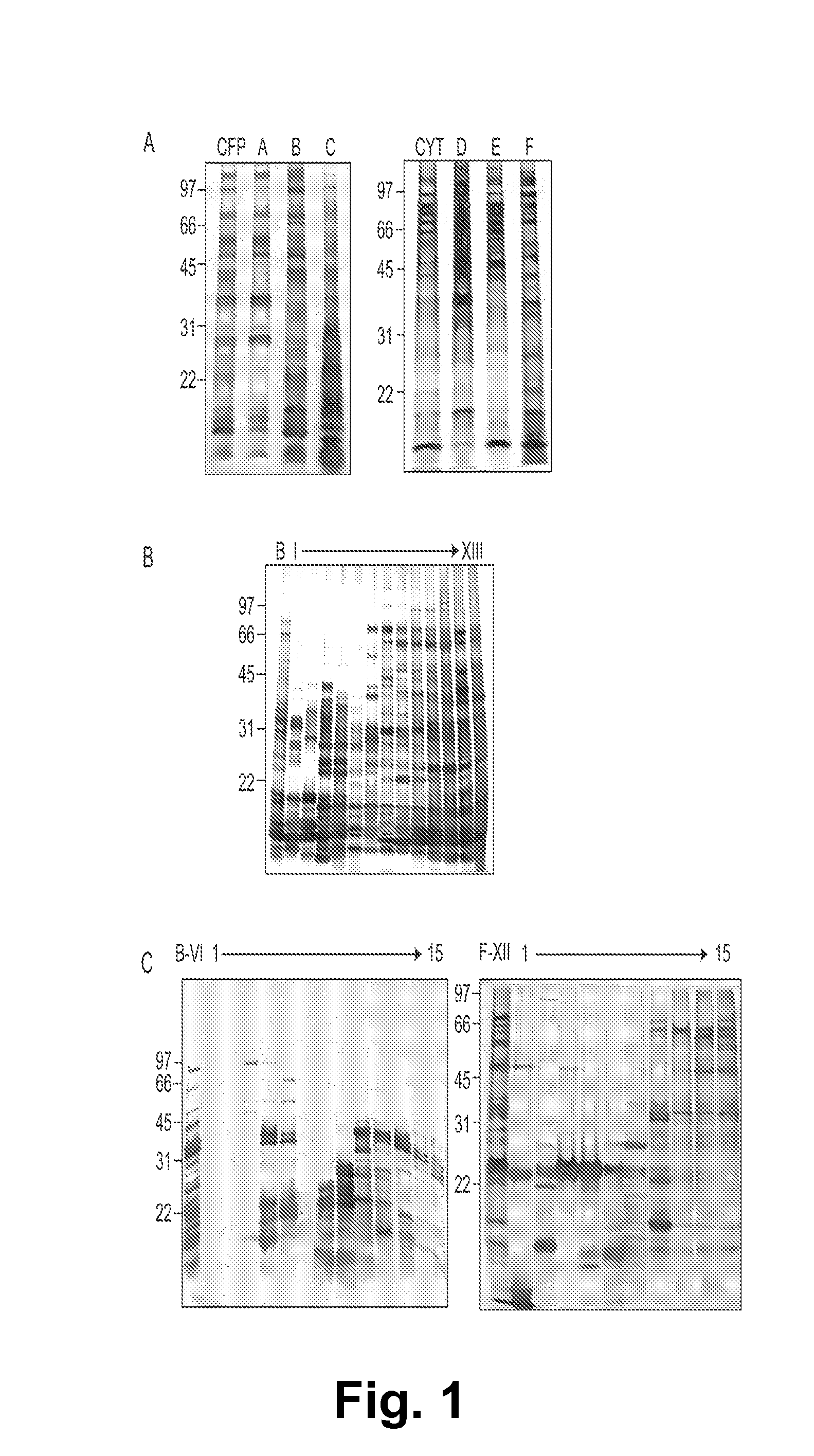
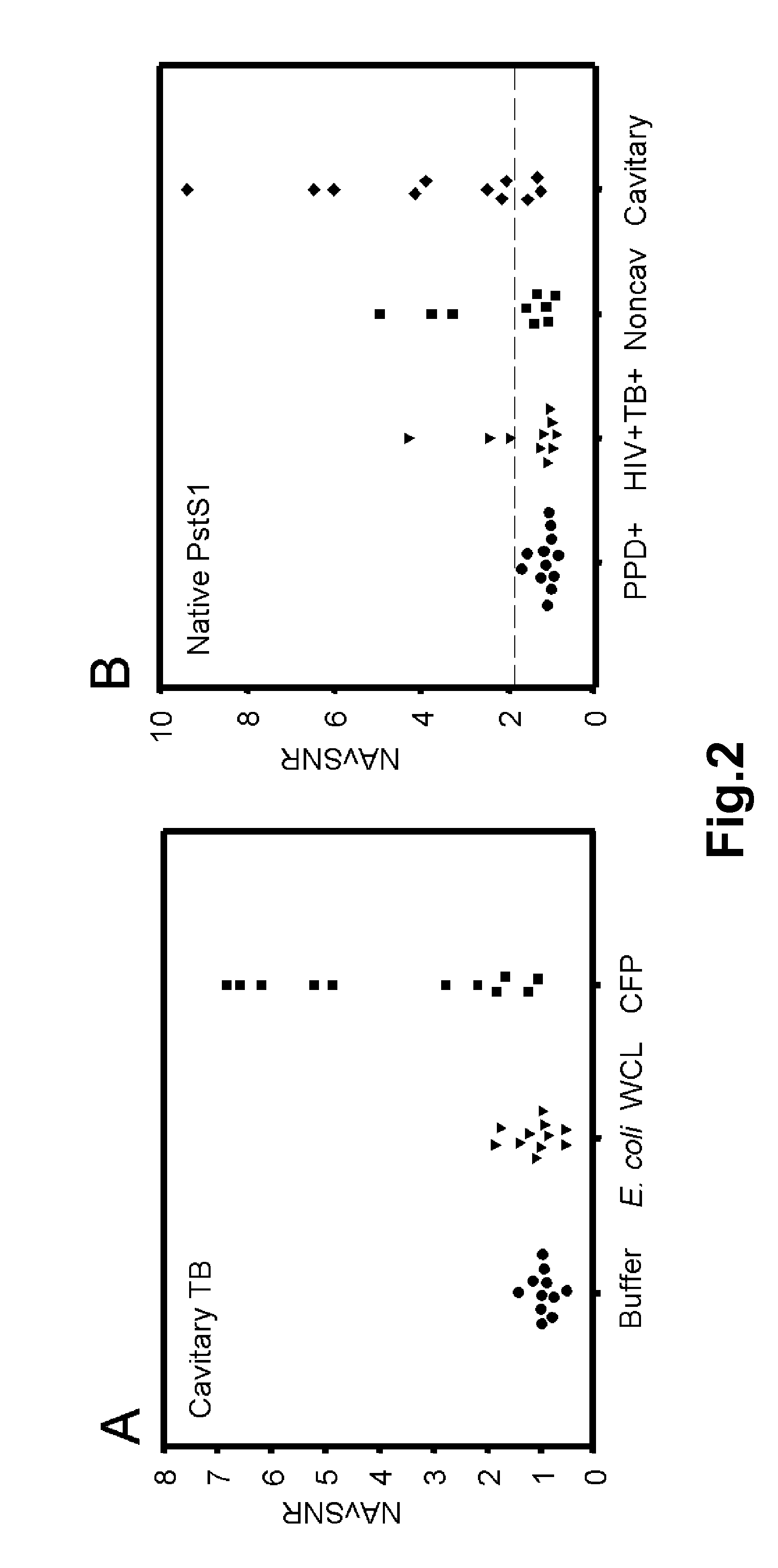
Biomarkers of tuberculosis that distinguish disease categories: use as serodiagnostic antigens
Mycobacterial proteins from culture filtrate or cytosol are disclosed as being useful B cell antigens for early diagnosis of mycobacterial disease, particularly in humans. These proteins include four that had not previously been recognized as B cell antigens (LppZ protein encoded by Mtb gene Rv3006; SodC protein encoded by Mtb gene Rv0432; BfrB protein encoded by Mtb gene Rv3841 and TrxC protein encoded byMtb gene Rv3914). Antigenic compositions include these proteins and / or peptide fragments thereof, in various combinations with each other or with one or more of a set of 10 additional Mtb proteins known to be antigens (in paricular early antigens. Methods and kits for using these antigenic composition for early diagnosis of mycobacterial infection and disease are also disclosed.
Owner:NEW YORK UNIV +1
Multi-index disease combined detection microfluidic device
ActiveCN109647553ARealize joint detectionWell mixedLaboratory glasswaresMaterial analysisCell specificAntigen
The invention discloses a multi-index disease combined detection microfluidic device, which comprises a microfluidic reaction chip, a microfluidic storage chip, a direct current motor driving module,a reaction chip fluid control module, a storage release fluid control module, a temperature control module, and a CCD detection module. Through the cooperation between the reaction chip fluid controlmodule, the storage release fluid control module, the temperature control module, and the microfluidic reaction chip, a sample and a sample diluting solution are mixed; microsphere surface coated antibodies capture target cell specific antigens, non-specific adsorbed cell antigens on the microsphere surface are washed off, target cell antigens specifically absorbed on the microsphere surface capture enzyme labeled antibodies, exciting liquids (A and B) are mixed, enzymes specifically adsorbed on the microsphere surface catalyze the chemiluminescence of the exciting liquids; the CCD detection module detects and collects the chemiluminescence signals of the microsphere surface, and multiple index antigens in a serum sample are captured and detected automatically.
Owner:BEIJING UNIV OF CHEM TECH
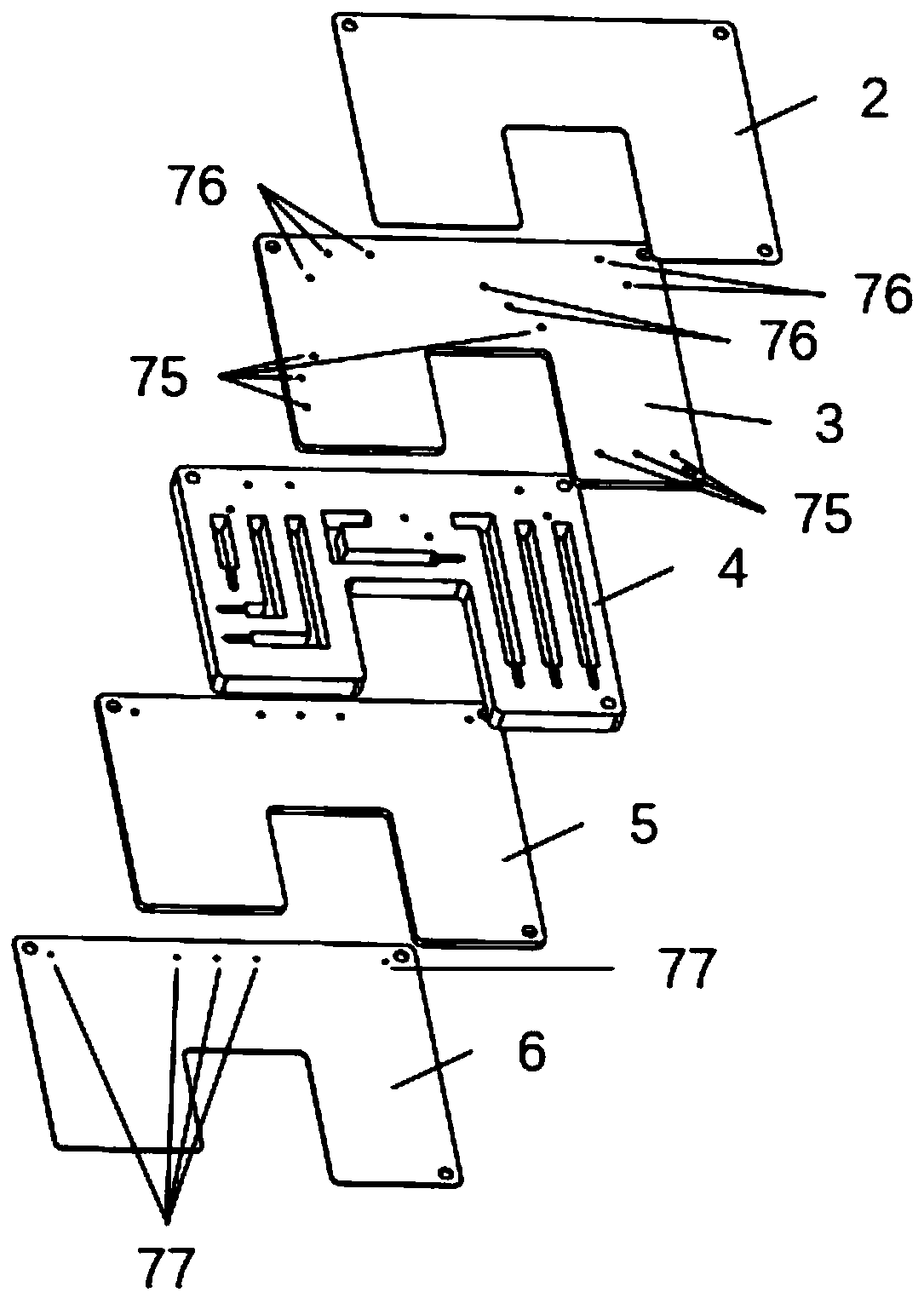
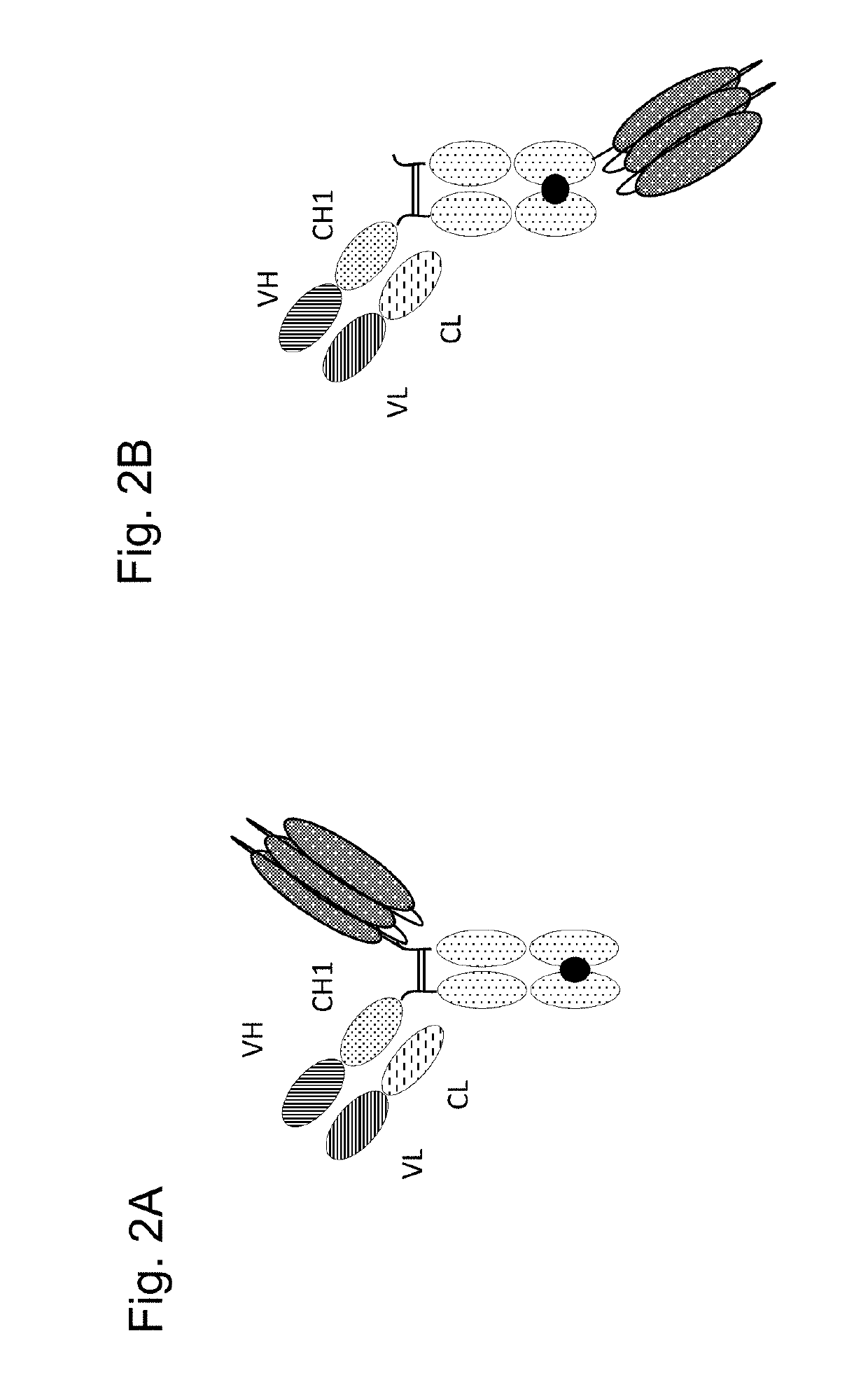
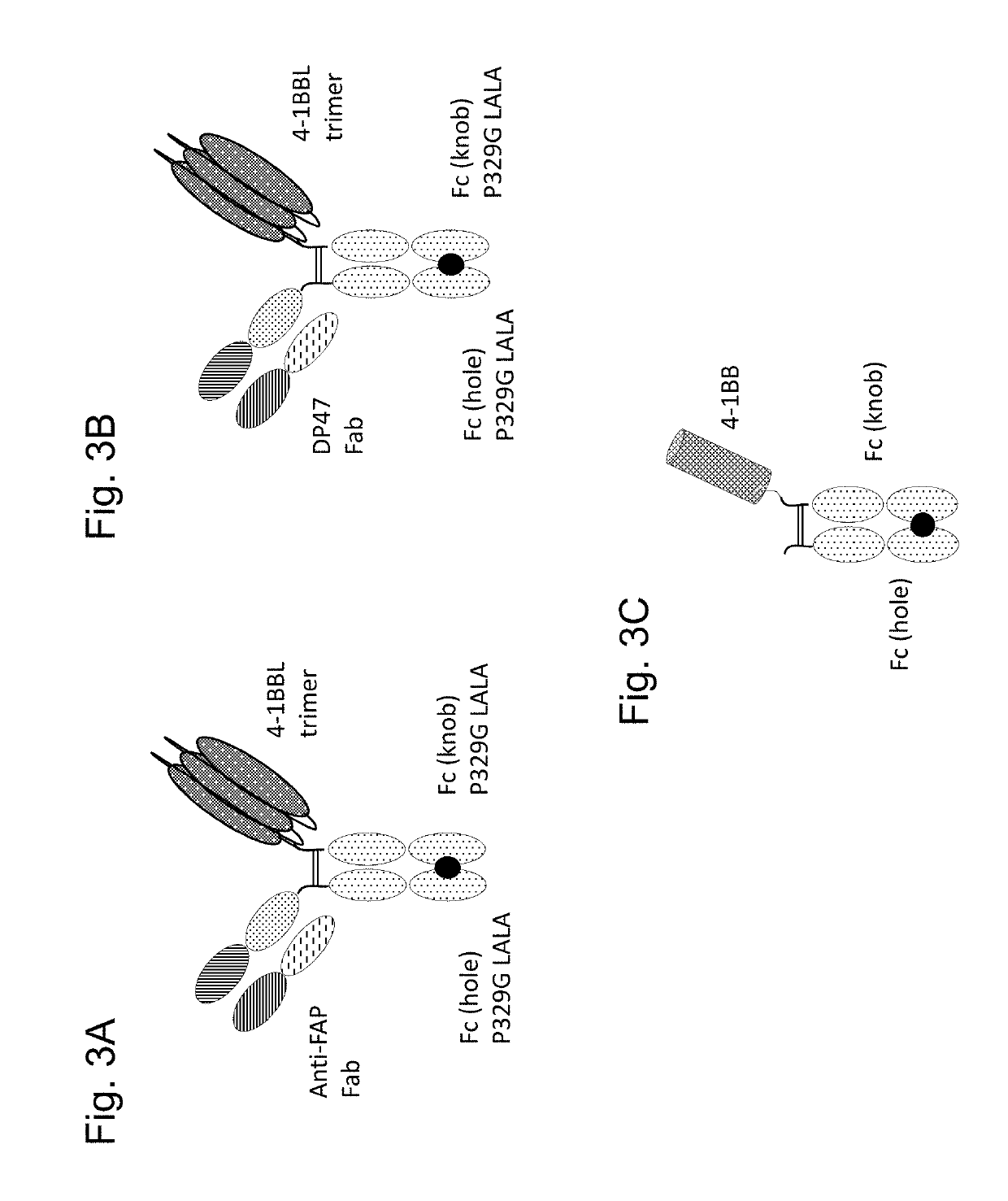
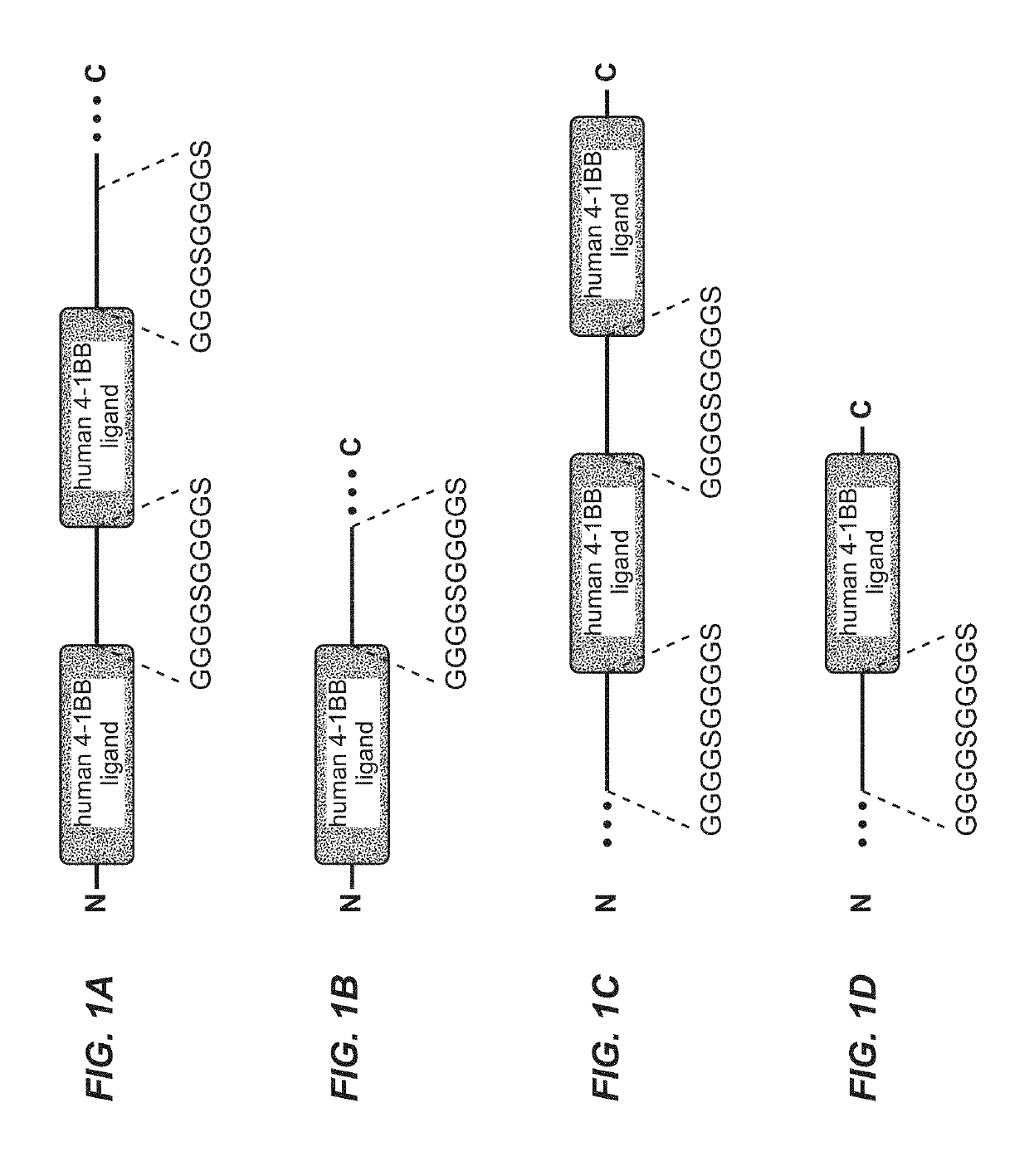
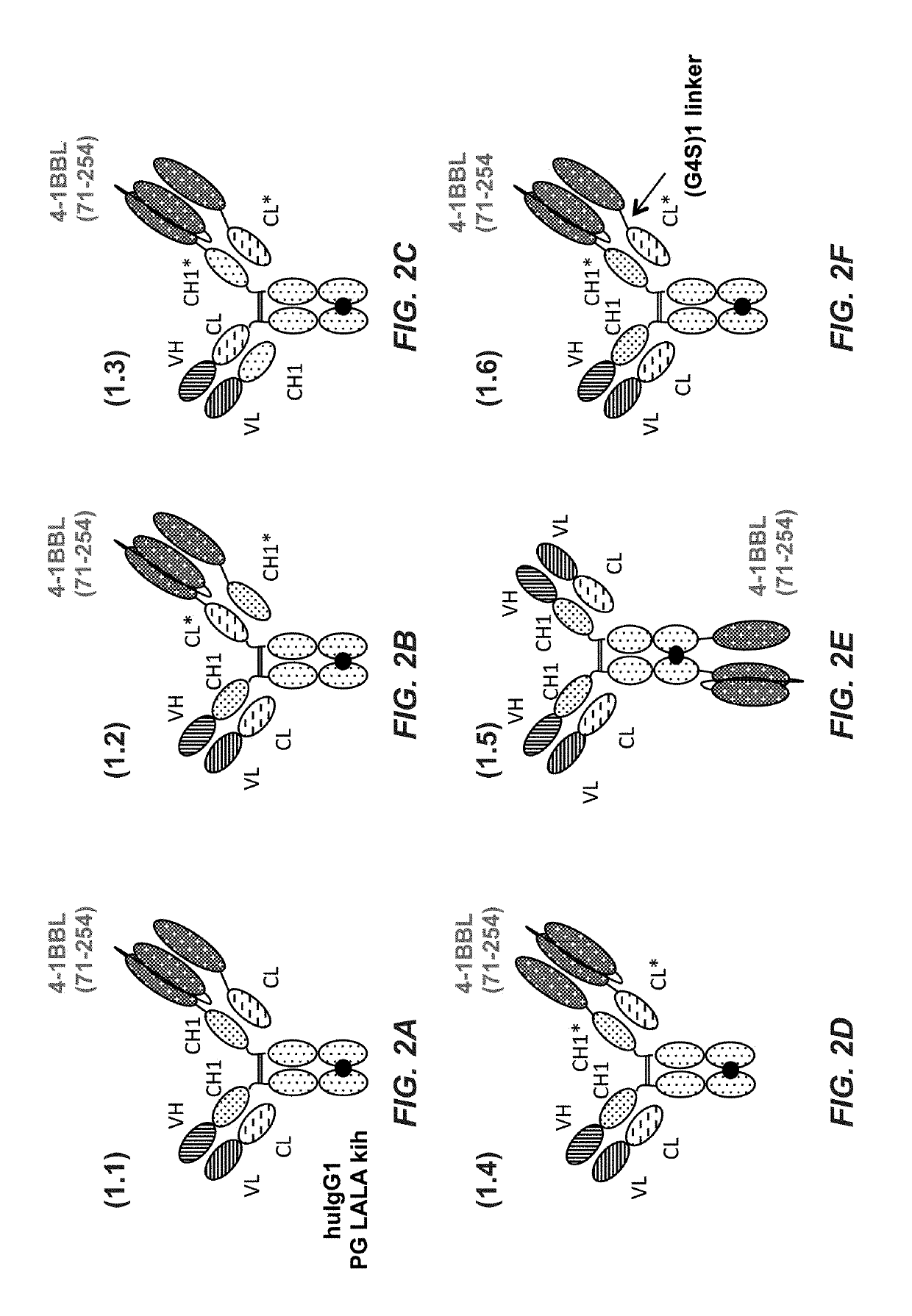
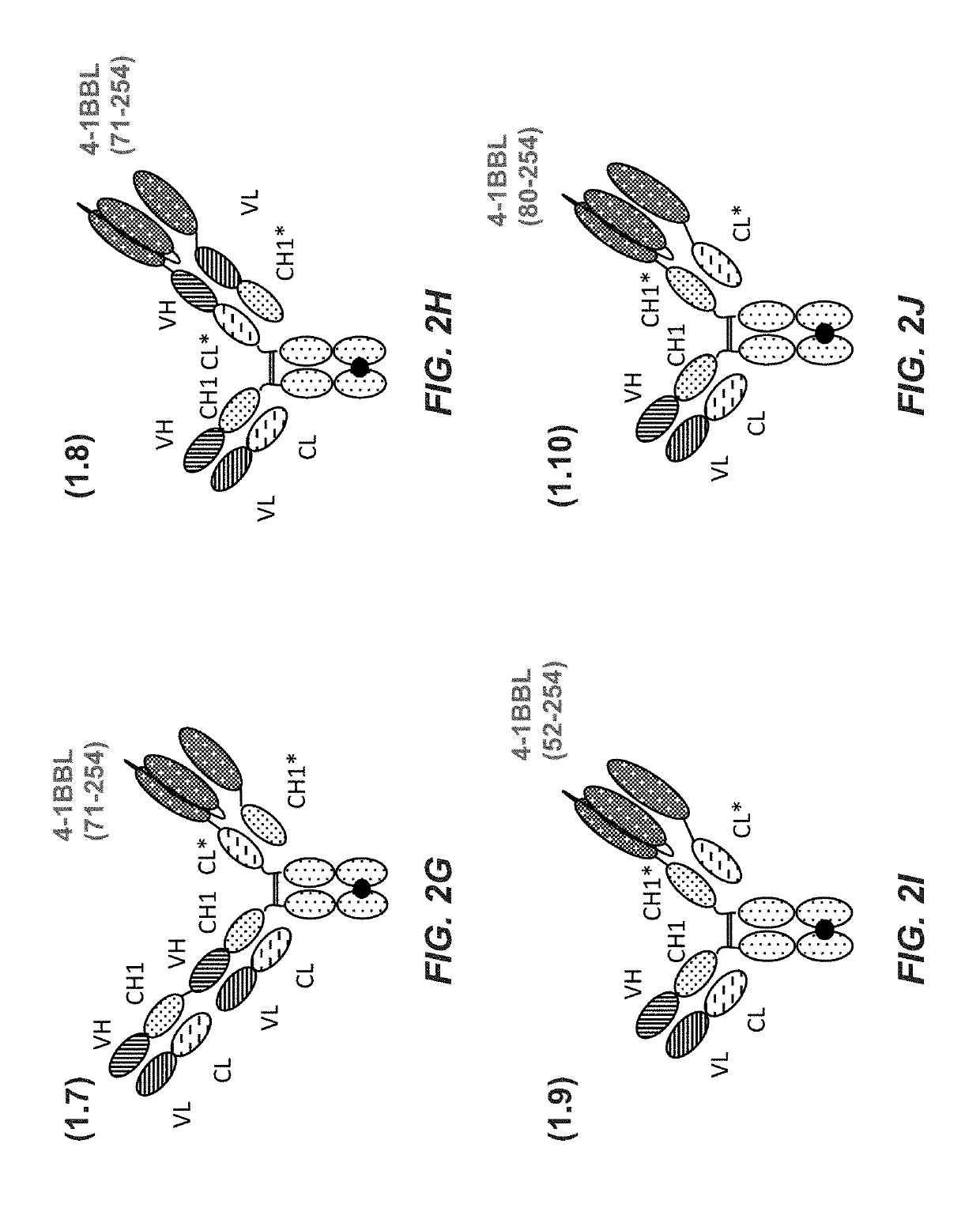
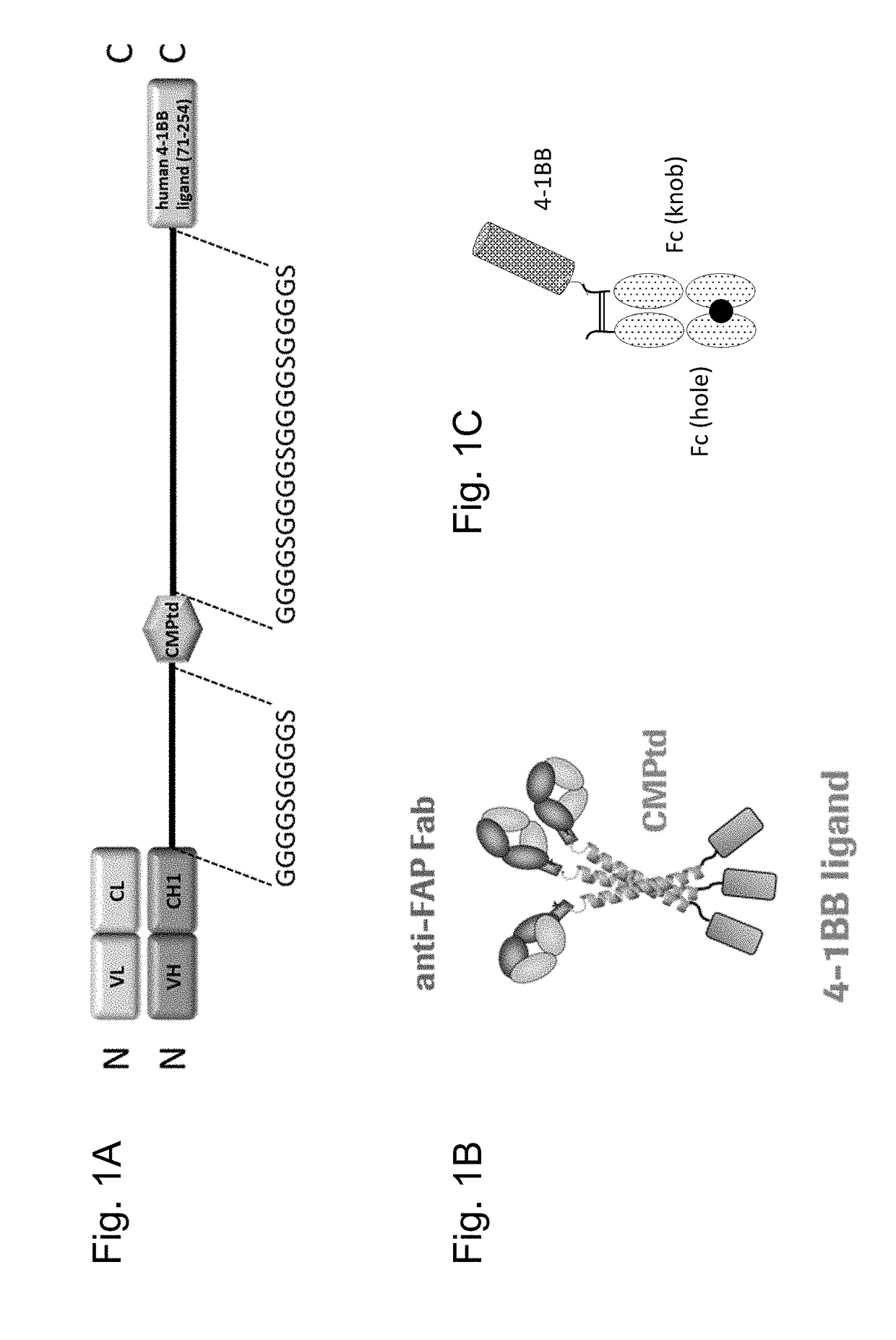
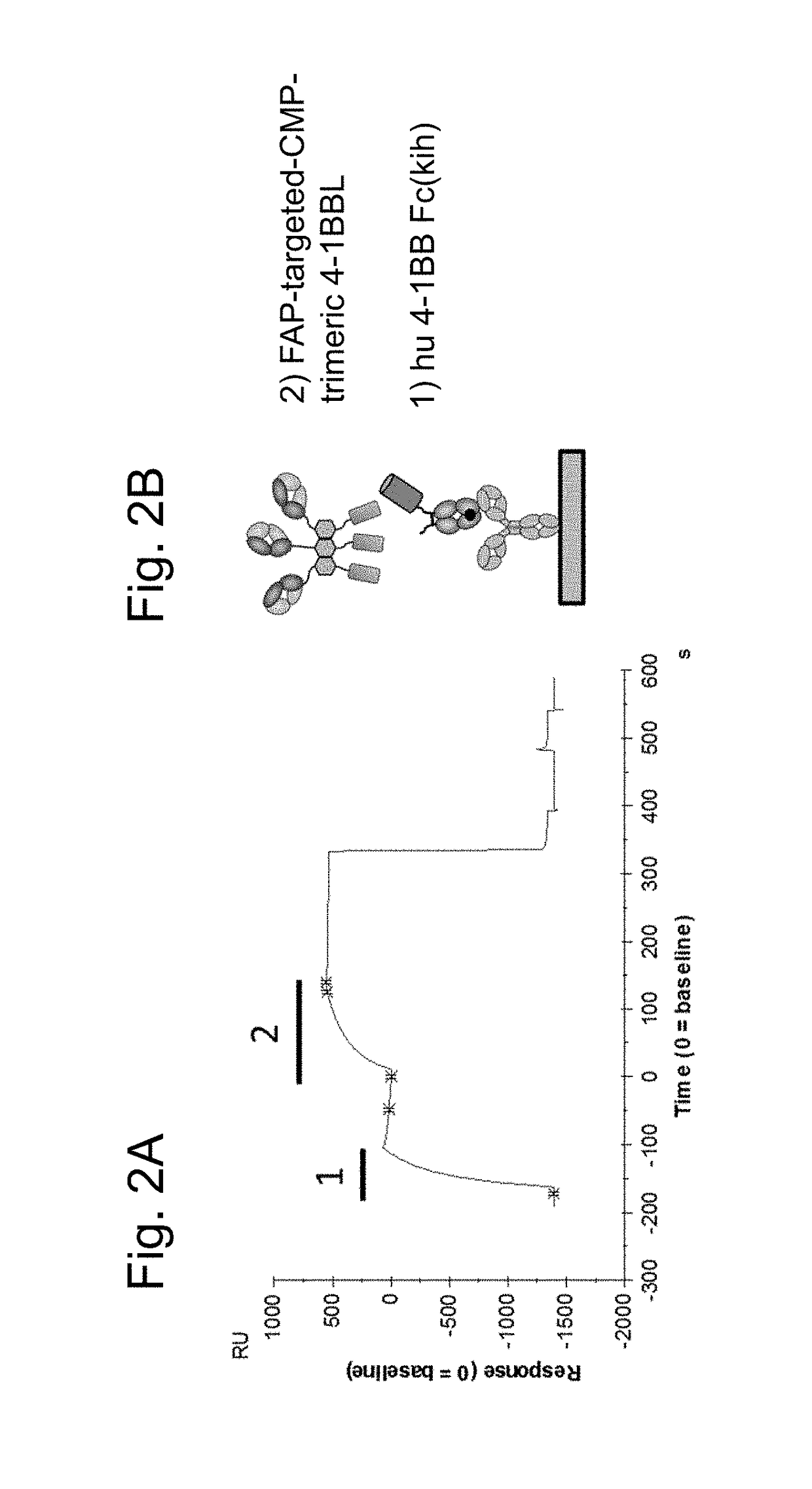
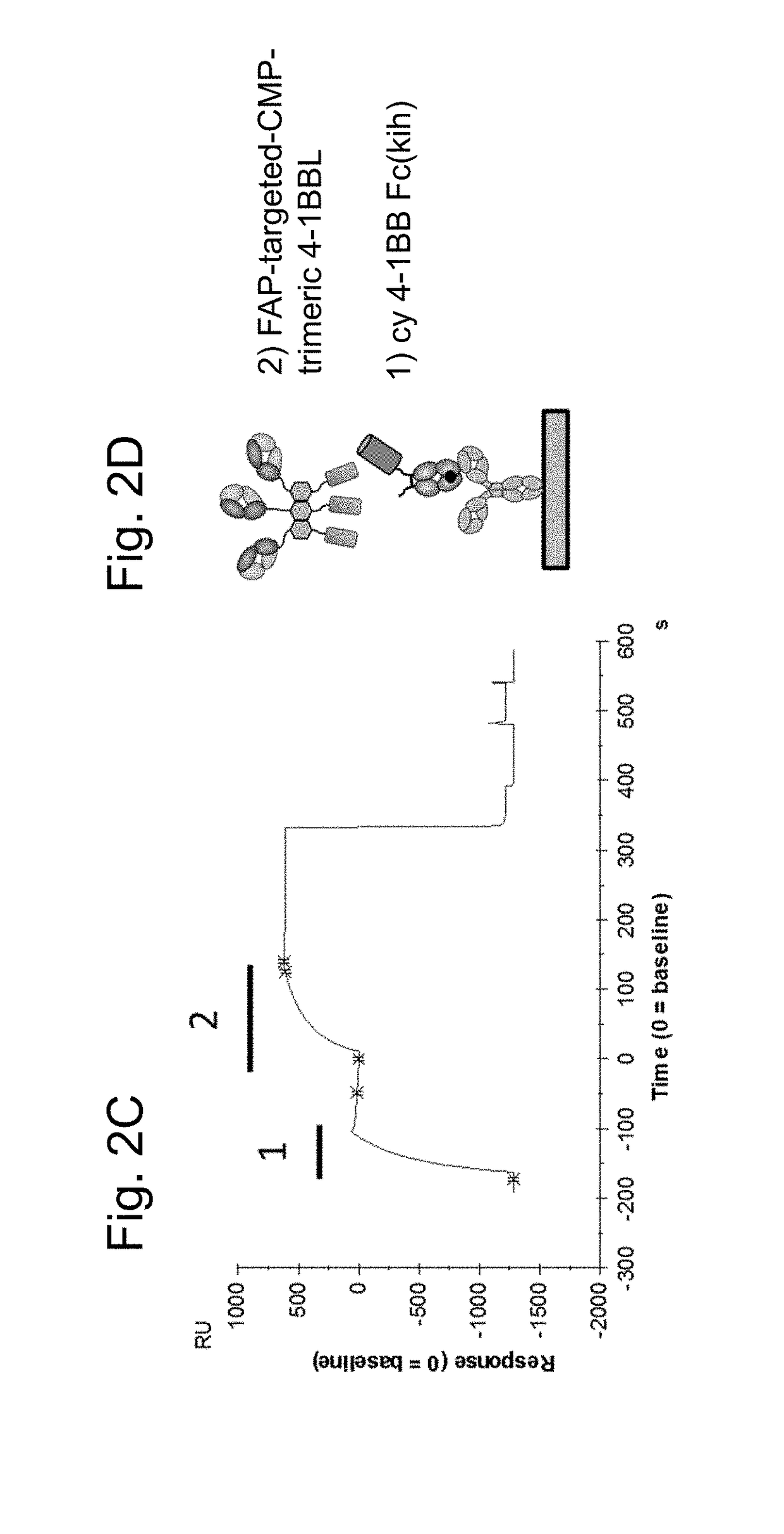
Tumor necrosis factor (TNF) family ligand trimer-containing antigen binding molecules
ActiveUS10464981B2Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsCellular antigensFc domain
The invention relates to novel TNF family ligand trimer-containing antigen binding molecules comprising (a) at least one moiety capable of specific binding to a target cell antigen, (b) a polypeptide comprising three ectodomains of a TNF ligand family member or fragments thereof that are connected to each other by peptide linkers and (c) a Fc domain composed of a first and a second subunit capable of stable association, and to methods of producing these molecules and to methods of using the same.
Owner:F HOFFMANN LA ROCHE INC
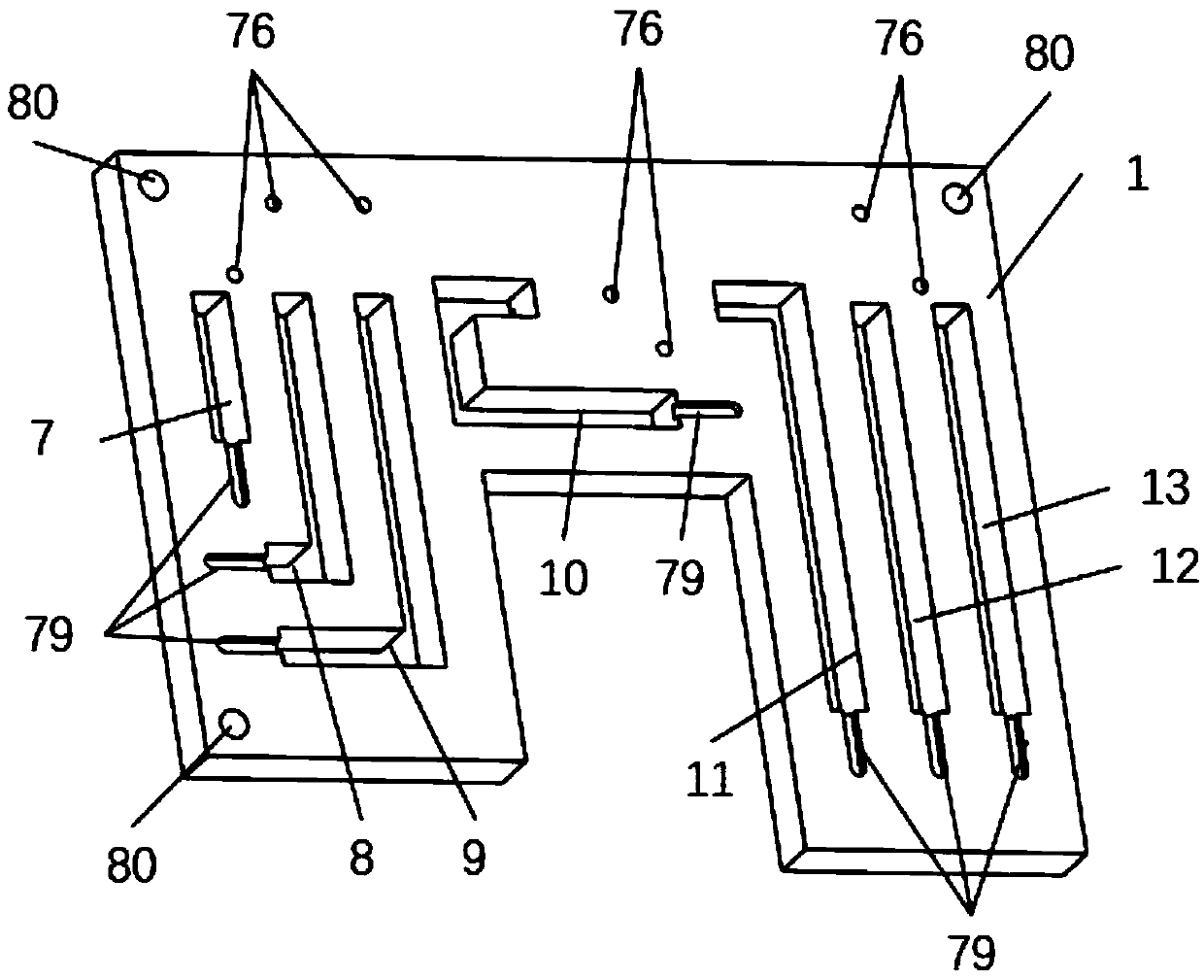
Tumor necrosis factor (TNF) family ligand trimer-containing antigen-binding molecules
The invention relates to novel TNF family ligand trimer-containing antigen binding molecules comprising (a) at least one moiety capable of specific binding to a target cell antigen and (b) a first and a second polypeptide that are linked to each other by a disulfide bond, characterized in that the first polypeptide comprises two ectodomains of a TNF ligand family member or fragments thereof that are connected to each other by a peptide linker and in that the second polypeptide comprises only one ectodomain of said TNF ligand family member or a fragment thereof.
Owner:F HOFFMANN LA ROCHE & CO AG
Microfluidic device and leucocyte antigen mediated microfluidic assay
ActiveUS20090011451A1Easy to doMicrobiological testing/measurementBiological material analysisCellular antigensWhite blood cell
Owner:ADVANCED ANIMAL DIAGNOSTICS
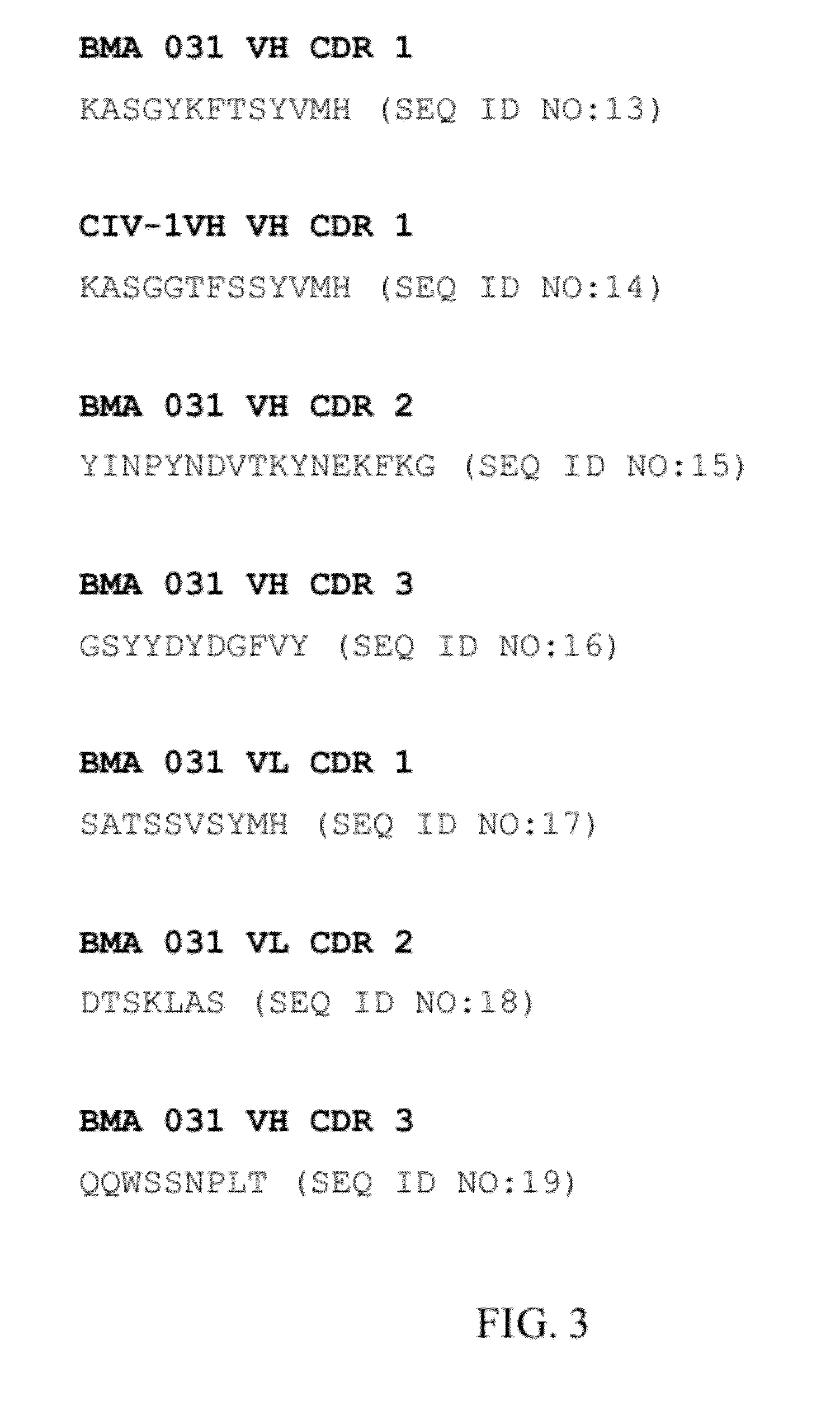
Monoclonal antibodies reactive with defined regions of the T cell antigen receptor
The present invention relates to monoclonal antibodies which recognize defined regions of the T-cell receptor (TCR). In a specific embodiment, the invention provides monoclonal antibodies which are reactive with a constant region of the alpha chain of the TCR. In particular embodiments, the invention relates to two monoclonal antibodies, termed alpha F1 and alpha F2, which react with two different epitopes on the framework region of the alpha monomer of the TCR molecule. In another specific embodiment, the invention is directed to monoclonal antibodies reactive with a variable region of the beta chain of the TCR. In particular, the invention provides two monoclonal antibodies, termed W112 and 2D1, which react with beta chain variable regions V beta 5.3 and V beta 8.1, respectively. In another specific embodiment, the invention is directed to monoclonal antibodies reactive with a variable region of the delta chain of the TCR. In particular, the invention provides monoclonal antibody delta TCS1, isotype IgG2a. The monoclonal antibodies of the invention have value in diagnosis and therapy and are useful tools for study of the immune system.
Owner:ASTRAZENECA AB
A group of antigen epitope polypeptide and uses thereof
InactiveCN101492495AViral antigen ingredientsMicroorganism based processesCarrier proteinB-Cell Epitopes
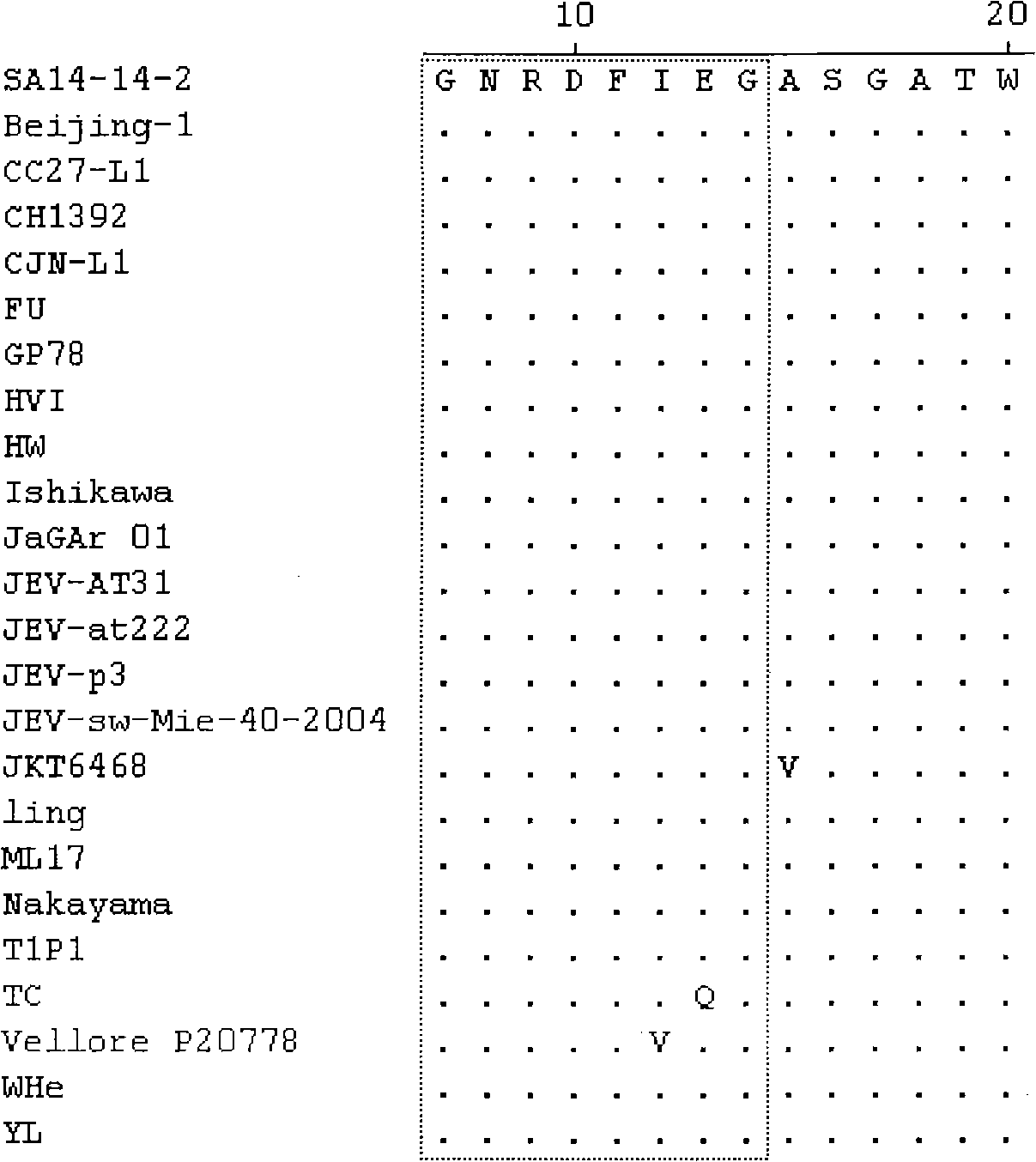
The invention discloses a set of epitope polypeptides, particularly relates to a neutralizing B cell epitope sequence of encephalitis B virus E protein, and further discloses application of controlling and diagnosing encephalitis B virus by these epitopes. The amino acid sequences of the epitope polypeptides in the invention are respectively any amino acid sequence of SEQ ID NO: 64, SEQ ID NO: 79, SEQ ID NO: 95, SEQ ID NO: 108, SEQ ID NO: 124 and SEQ ID NO: 139. After the epitope polypeptides in the invention are coupled or fused with carrier proteins to be expressed as immunogenic or vaccine immuno animal organism, neutralizing antibodies aiming at JEV can be generated, and the JEV can be neutralized in vivo or in vitro so as to prevent virus from infecting animal organism. The epitope polypeptides in the invention or the junctional complex thereof can be used as reagents for detecting encephalitis B virus antibodies or encephalitis B virus polypeptide antibodies.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Identification, quantification, and characterization of t cells and t cell antigens
Methods of quantifying and / or characterizing antigen-specific T cell populations, identifying T cell antigens and epitopes, and preparing targeted pharmaceutical compositions by (i) introducing a peptide into an antigen-presenting cell (APC) expressing a fusion protein comprising a major histocompatibility complex portion and a reporter peptide portion such that a complex forms between the fusion protein and the peptide and the peptide is displayed by the APC and (ii) contacting the APC with a population of cells, such that T cells in the population of cells that react with the peptide detectably internalize the complex; novel APCs useful in such methods; and related therapeutic and diagnostic methods.
Owner:THE GOV OF THE US AS REPRESENTED BY THE SEC
Helicobacter pylori urease B subunit B cell antigen epitope polypeptide, identification method and application
InactiveCN101033468AGood immunogenicityClear Helicobacter pylori infectionBacterial antigen ingredientsMicrobiological testing/measurementAnti-Helicobacter pylori IgGCarrier protein
This invention provides a B cell epitope peptide and its identification method to neutralize helicobacterpylori urease B subunit, determines the antigen epitope corresponding with the monoclonal antibody 6E6 of helicobacterpylori urease B subunit, and confirms this B cell epitope is specific for the 6E6. The invention also provides a chimeric epitope vaccine and its preparation method containing B cell epitope and carrier protein BSA, and the B cell epitope has strong antigenicity.
Owner:ARMY MEDICAL UNIV
SARS-CoV-2 lymphocyte antigen epitope peptides and application thereof
InactiveCN112961223ASsRNA viruses positive-senseViral antigen ingredientsAntigen epitopeLymphocyte antigen
The invention discloses lymphocyte antigen epitope peptides and application thereof, belongs to the fields of medical immunology and infectious diseases, and particularly relates to 164 thymus dependent lymphocyte antigen epitope peptides of five proteins of SARS-CoV-2 and application thereof. The antigen epitope peptides can be presented by HLA-A molecules to stimulate activation, proliferation and differentiation of SARS-CoV-2 specific thymus dependent lymphocytes, so that the immune effect of resisting SARS-CoV-2 infection is exerted. The antigen peptides can be used for preparing mixed polypeptide vaccines of SARS-CoV-2, recombinant protein vaccines connected with a plurality of epitope peptides in series and DNA and RNA vaccines, can be used for preparing a detection kit for detecting SARS-CoV-2 specific thymus dependent lymphocytes, can also be used for preparing effector thymus dependent lymphocytes or drugs for treating SARS-CoV-2 infection, and have a potential application value in the prevention, treatment and diagnosis of SARS-CoV-2 infection and COVID-19.
Owner:SOUTHEAST UNIV +2
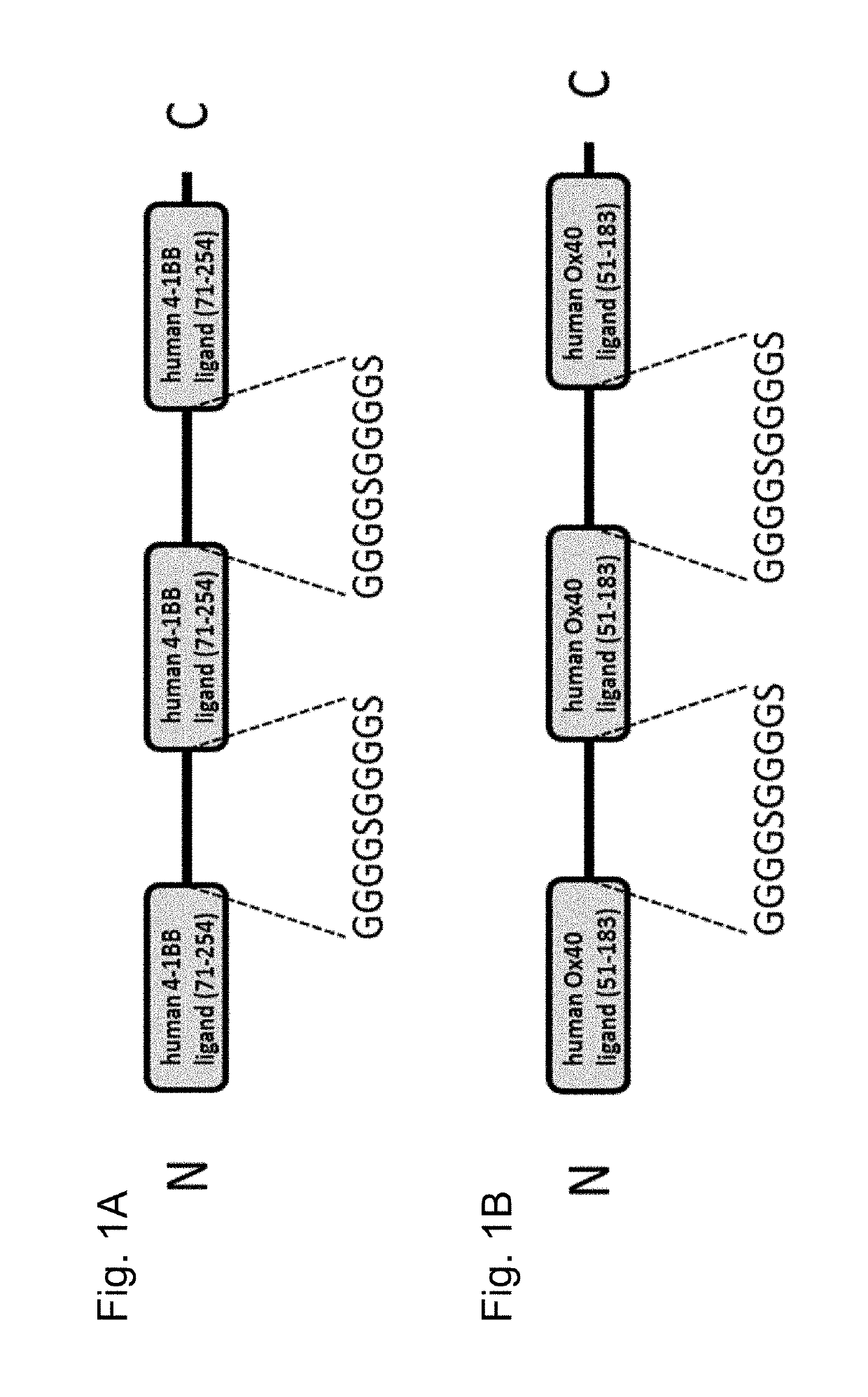
Trimeric costimulatory TNF family ligand-containing antigen binding molecules
InactiveUS20190016771A1Connective tissue peptidesHybrid immunoglobulinsCellular antigensAntigen binding
The invention relates to novel trimeric costimulatory TNF family ligand-containing antigen binding molecules comprising three fusion polypeptides, each of the three fusion polypeptides comprising (a) an ectodomain of a costimulatory TNF family ligand selected from the group consisting of 4-1BBL, OX40L and GITRL or fragments thereof, (b) a trimerization domain, in particular a trimerization domain derived from human cartilage matrix protein (huCMP) of SEQ ID NO:1, and (c) a moiety capable of specific binding to a target cell antigen, and to methods of producing these molecules and to methods of using the same.
Owner:F HOFFMANN LA ROCHE & CO AG
Compositions, methods and kits for detection of an antigen on a cell and in a biological mixture
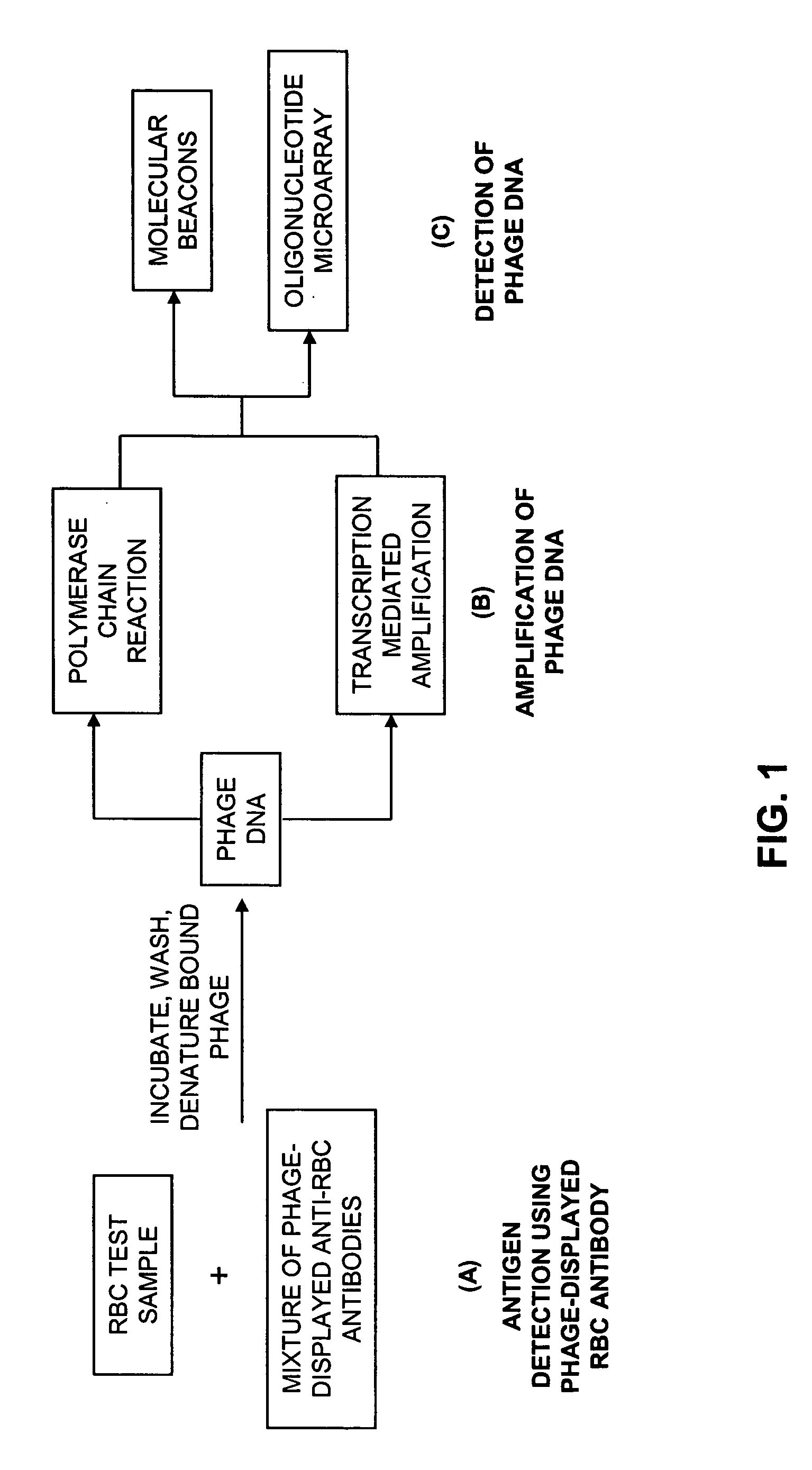
InactiveUS20050191622A1Antibody mimetics/scaffoldsMicrobiological testing/measurementCellular antigensBacteriophage
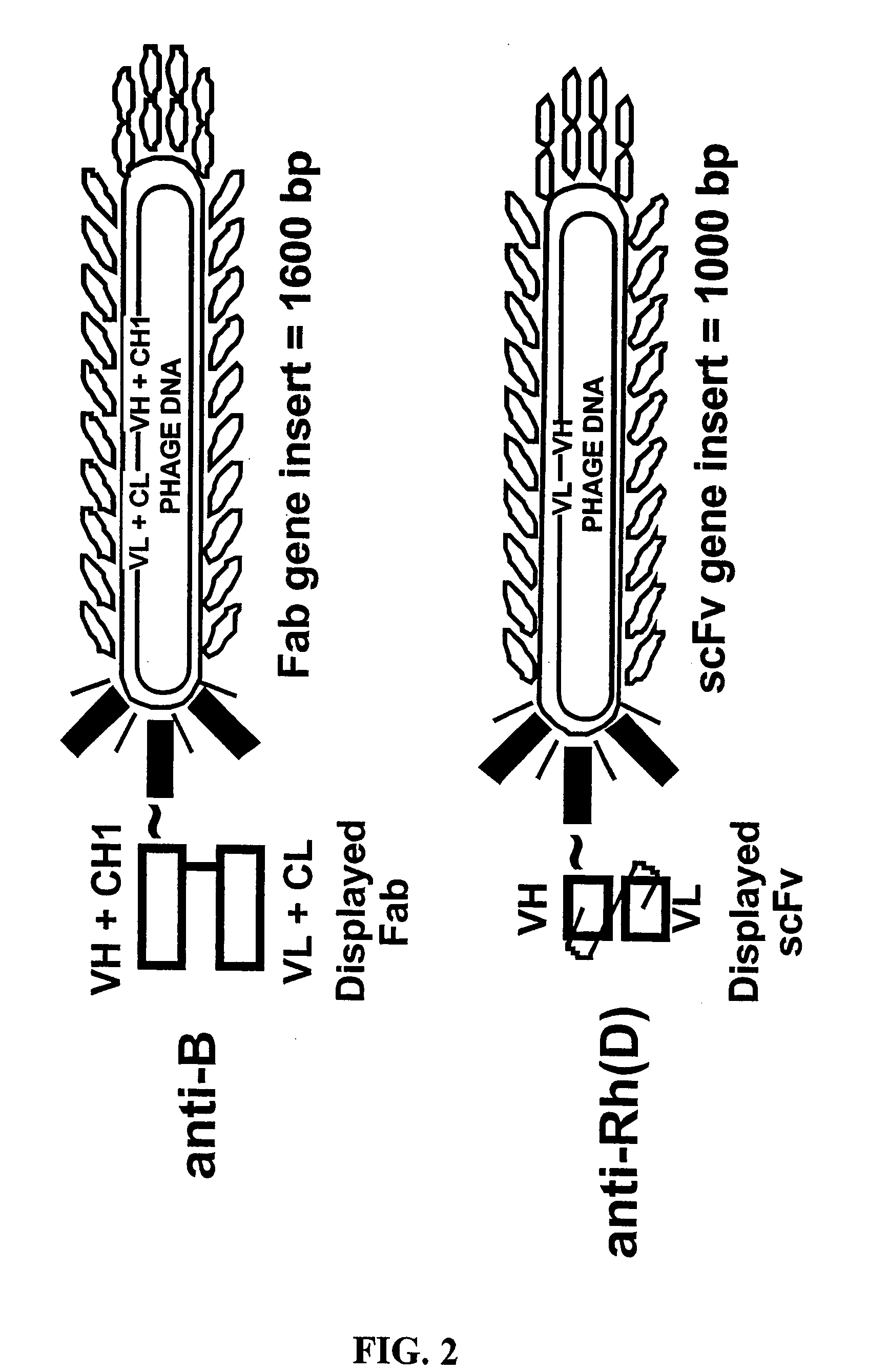
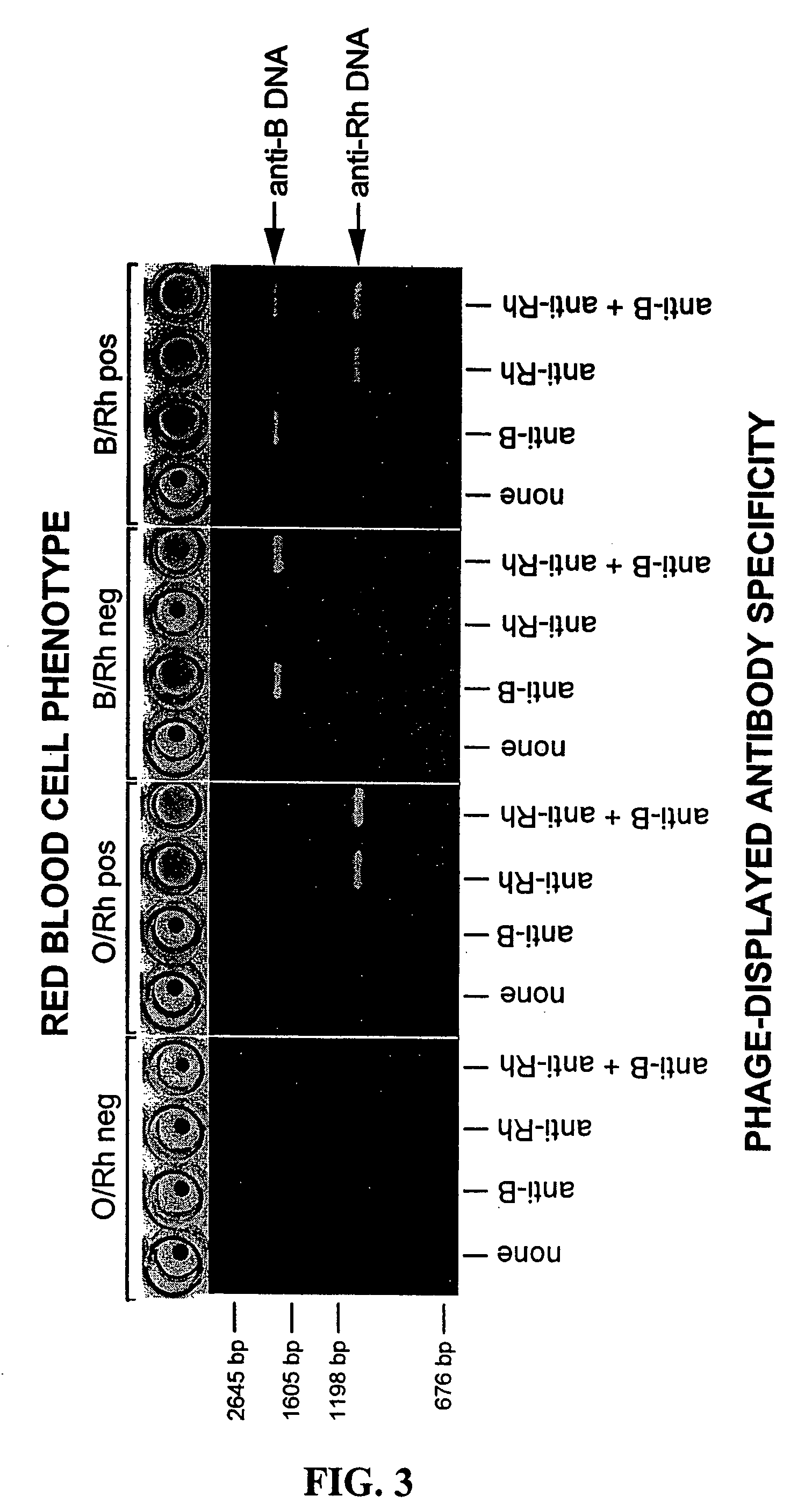
The present invention relates to novel methods for detecting a member of a known binding pair in a sample, including a cell, where one member of the pair (termed the “receptor”) is expressed by a bacteriophage, which phage is then used to detect the presence of the other member of the pair (termed the “ligand” or “target”). Rather than detecting the binding of the phage using antibody-based technology, the present invention relates to detecting the nucleic acid associated with the phage. In one aspect, the invention relates to identifying an antigen-bearing moiety (e.g., a red blood cell antigen) of interest present on a cell, e.g., a red blood cell, using antibody-displaying bacteriophage, as well as detecting anti-red blood cell auto- or alloantibodies and / or complement in a sample, using antiglobulin reagent-displaying bacteriophage and detecting a nucleic acid associated with the phage.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
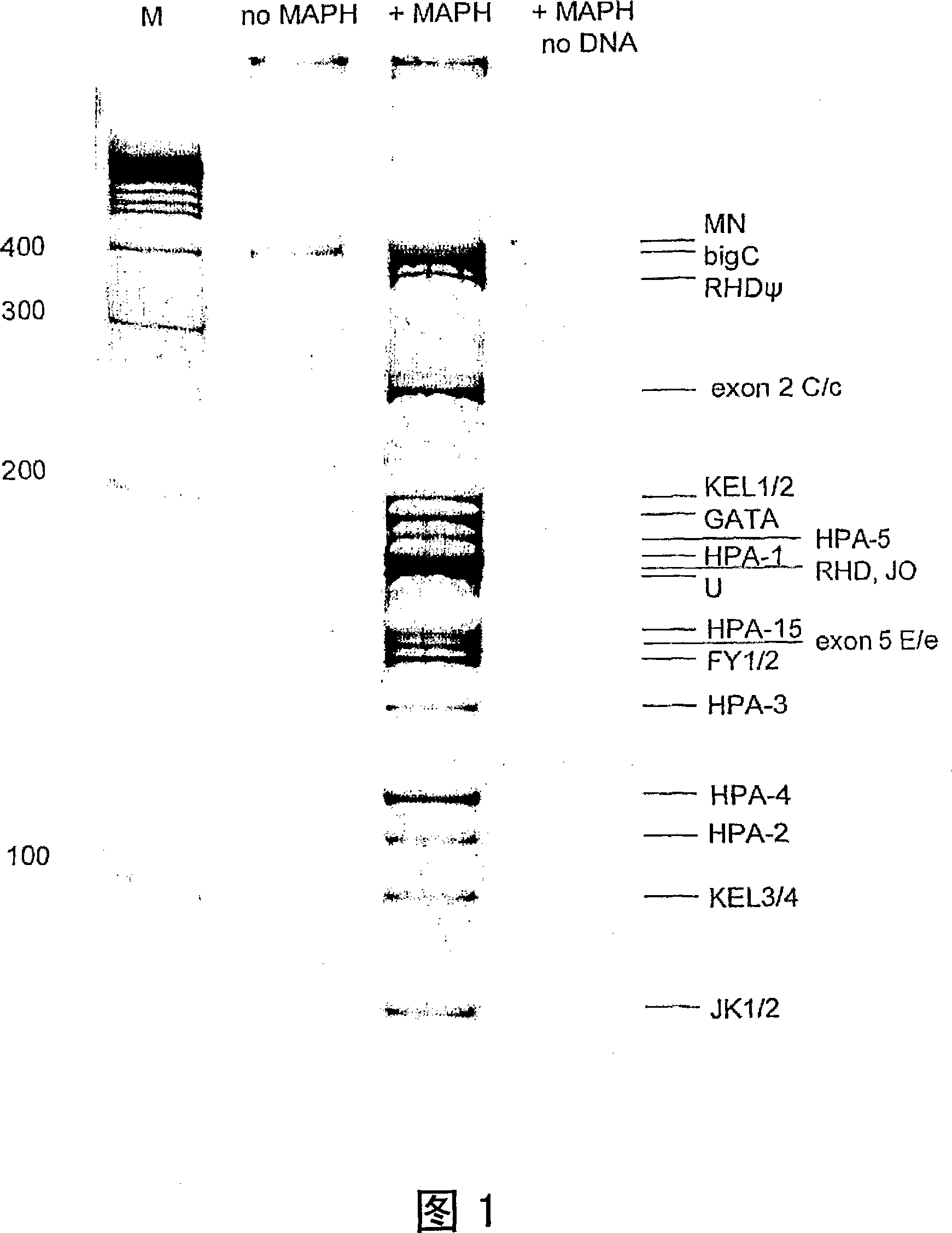
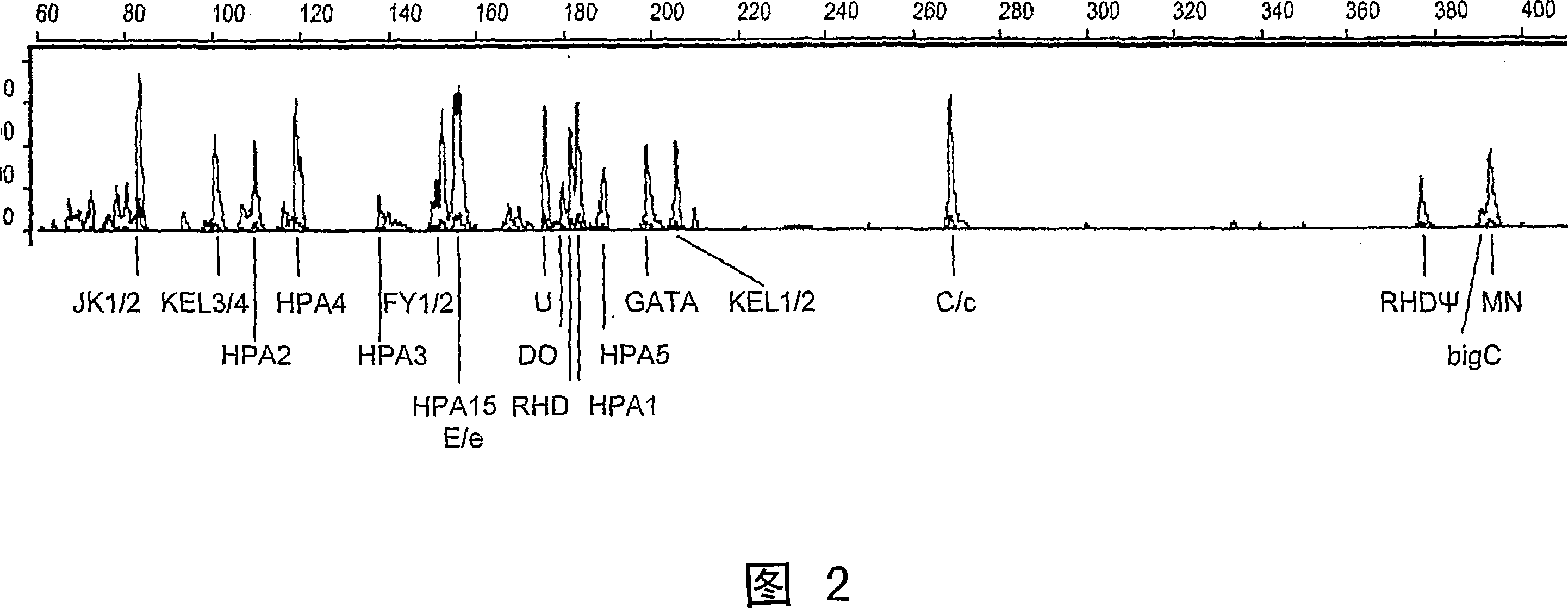
A method of genotyping blood cell antigens and kit suitable for genotyping blood cell antigens
A method of genotyping blood cell antigens comprising subjecting DNA from an individual of a mammalian species to a multiplex Polymerase Chain Reaction (PCR) to amplify and detectably label a region of the locus of at least two different blood cell antigens which contains the site of nucleotide polymorphism of said blood cell antigen and using the thus amplified and labeled DNA fragments to determine the genotype for each of said blood cell antigens. The multiplex PCR comprises the use of at least one pair of blood cell antigen-specific chimeric primers for each blood cell antigen to be genotyped and at least one detectably labeled universal primer, preferably a pair of detectably labeled universal primers. The universal primer(s) have a unique sequence not occurring in the DNA of said mammalian species. Each chimeric primer pair comprises a left chimeric primer and a right chimeric primer, each of them comprising a blood cell antigen-specific part at the 3' end and a universal part at the 5' end. The base sequence of the universal part of the chimeric primers corresponds to the base sequence of said at lea st one universal primer. The blood cell antigen-specific parts of the chimeric primer pair enclose a region of the locus of the blood cell antigen which contains the site of nucleotide polymorphism of said blood cell antigen. A k it for genotyping blood cell antigens by this method. A set of blood cell antig en- specific chimeric primer pairs and a set of blood cell antigen allele-specif ic oligonucleotide probes.
Owner:桑昆血液供给基金会
Antinuclear antibody quantitative detection kit and use method thereof
The present invention provides a kit for quantitative detection of homogeneous or speckled antinuclear antibody, and a use method thereof. The kit comprises: (1) a Hep-2 cell antigen sheet; (2) a fluorescence labeled secondary antibody; (3) fluorescence intensity calibration microspheres; (4) a buffer; (5) a sheet sealing agent; and (6) an experimental operation and quantitative fluorescence intensity analysis instruction. According to the present invention, the (1) Hep-2 cell antigen sheet provided by the kit, the (2) fluorescence labeled secondary antibody provided by the kit, and a serum sample to be detected are subjected to an indirect immunofluorescence reaction, the (3) fluorescence intensity calibration microspheres are added to the (1) Hep-2 cell antigen sheet to be detected, observing and microscopic image shooting are performed through a fluorescence microscope, a microscopic image analysis software is used to carry out fluorescence intensity analysis, and the fluorescence intensity value is converted into the corresponding serum antinuclear antibody titer value according to the (6) experimental operation and quantitative fluorescence intensity analysis instruction provided by the kit, such that the quantitative detection of the homogeneous or speckled antinuclear antibody in the serum sample is achieved.
Owner:NAT INST OF METROLOGY CHINA
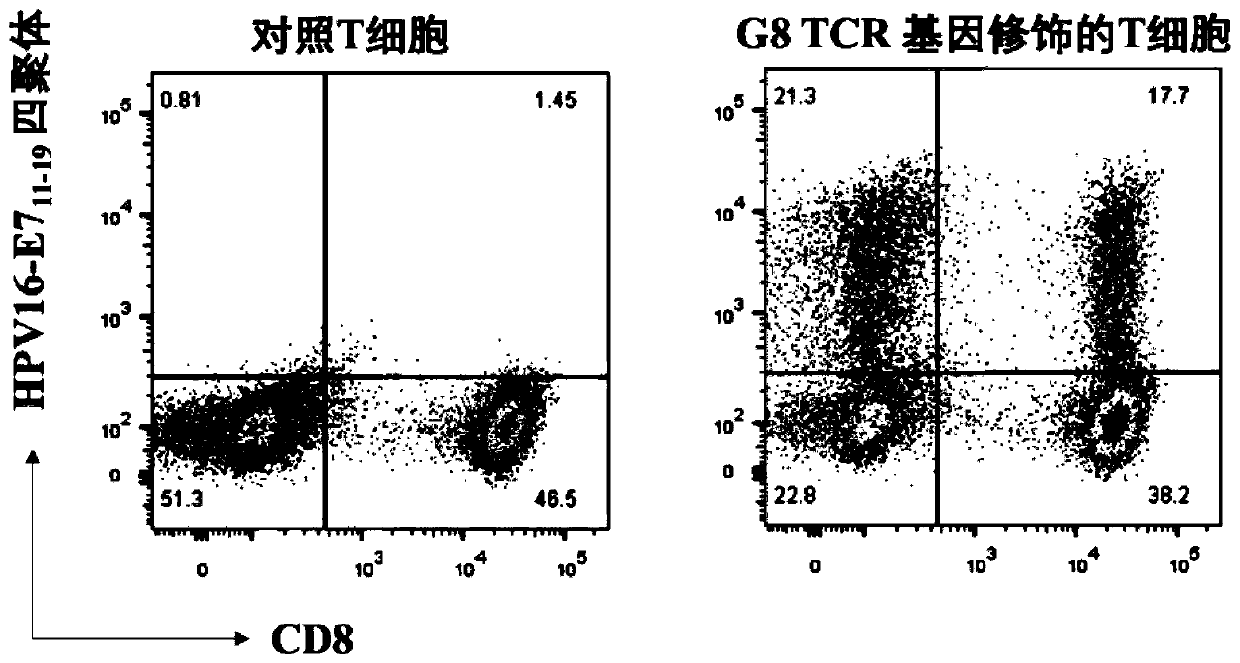
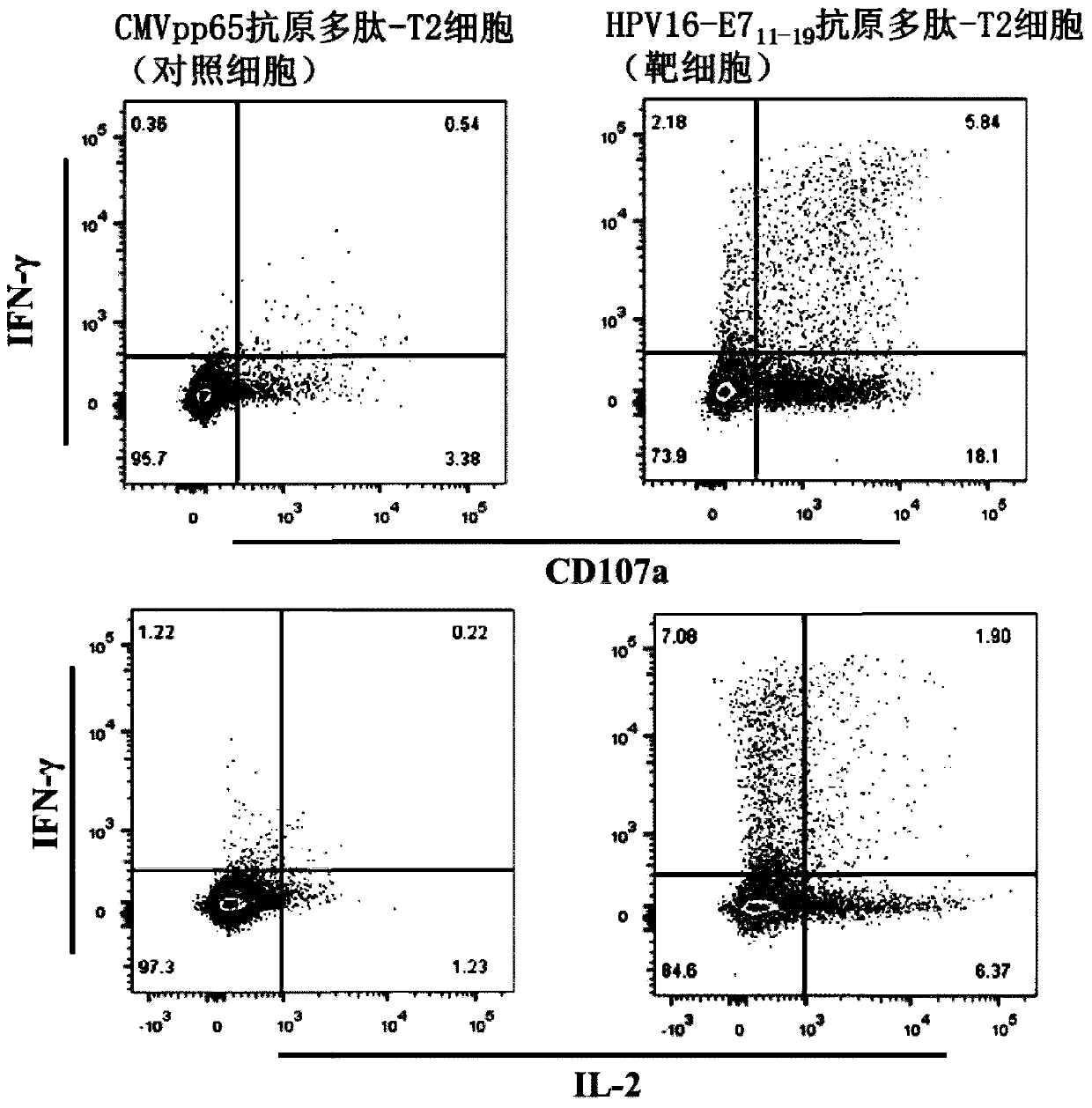
TCR for identifying human papilloma virus HPV16-E7 antigen
ActiveCN110357952AImmunoglobulin superfamilyMammal material medical ingredientsAntigenT-Cell Antigen Receptors
The invention relates to the technical field of T cell antigen receptors, in particular to TCR capable of specifically identifying and combining with a human papilloma virus antigen HPV16-E7, a nucleotide sequence encoding the TCR or nucleic acid molecules of a complementary sequence of the nucleotide sequence, a carrier containing the nucleic acid molecules, cells transducing the nucleic acid molecules or carrier, a drug composition containing the TCR, the nucleic acid molecules, the carrier or the cells which are taken as active ingredients and application of the TCR, nucleic acid molecules,carrier, cells and drug composition which are used for preparing drugs for treating tumors or virus infection respectively.
Owner:深圳市因诺转化医学研究院 +1
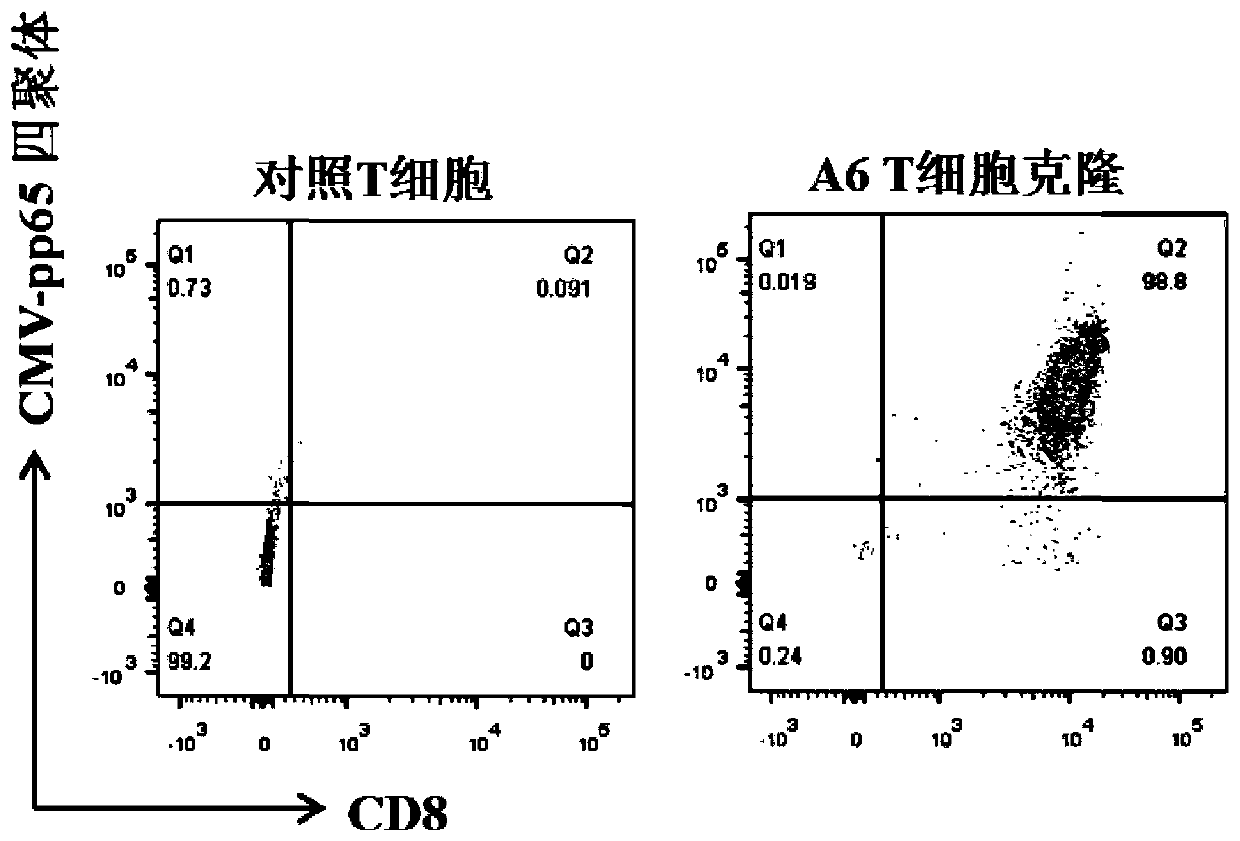
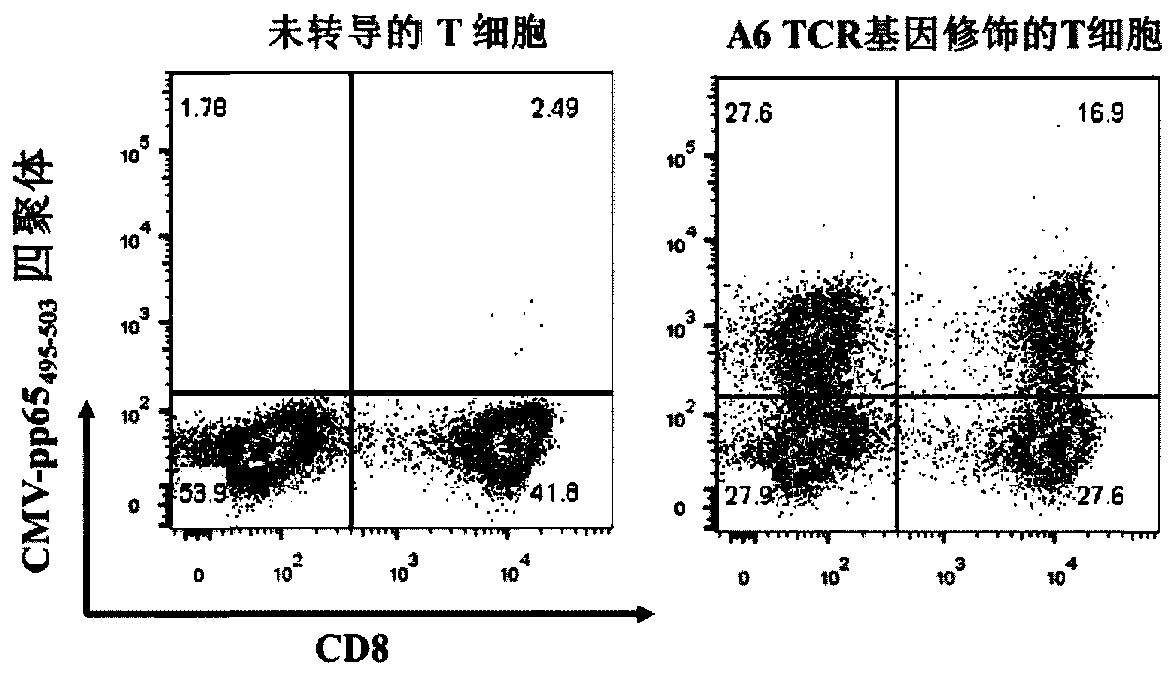
TCR for identifying human cytomegalovirus pp65 antigen
The invention relates to the technical field of T cell antigen receptors, in particular to TCR capable of identifying and combining with an HLA-A2-CMV-pp65495-503 antigen complex, a nucleotide sequence encoding the TCR or nucleic acid molecules of a complementary sequence of the nucleotide sequence, a carrier containing the nucleic acid molecules, cells transducing the nucleic acid molecules or carrier, a drug composition containing the TCR, the nucleic acid molecules, the carrier or the cells which are taken as active ingredients and application of the TCR, nucleic acid molecules, carrier, cells and drug composition which are used for preparing drugs for treating tumors or virus infection respectively.
Owner:深圳市因诺转化医学研究院 +1
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com