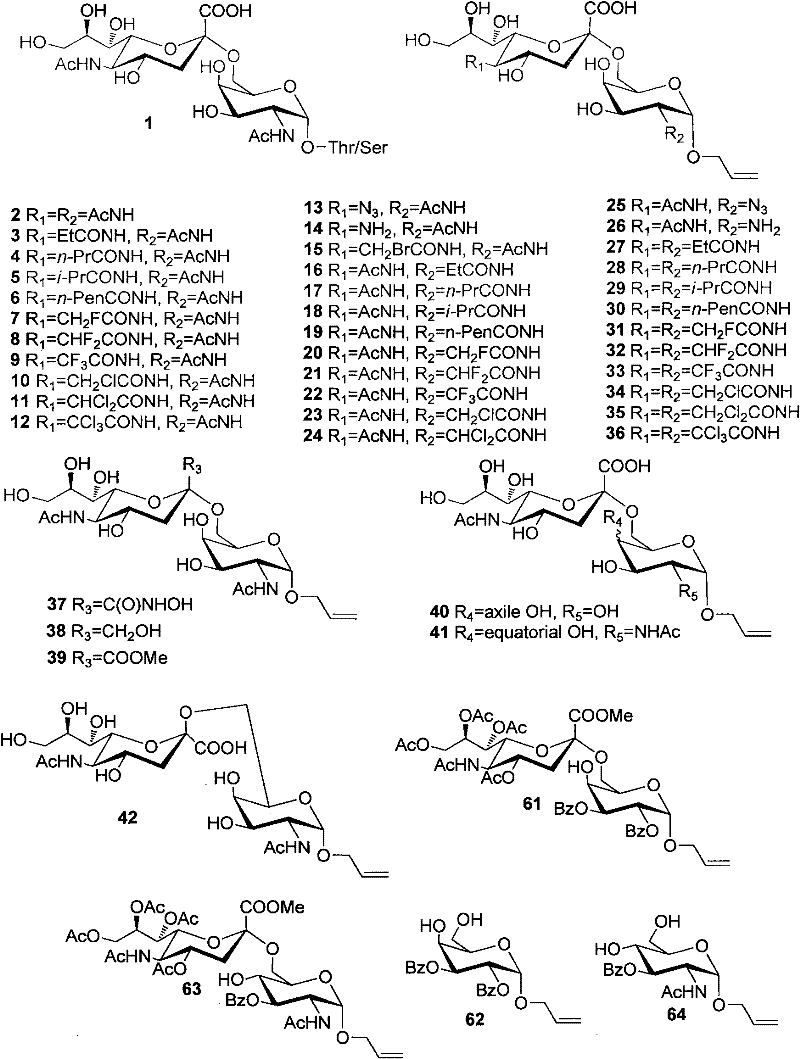
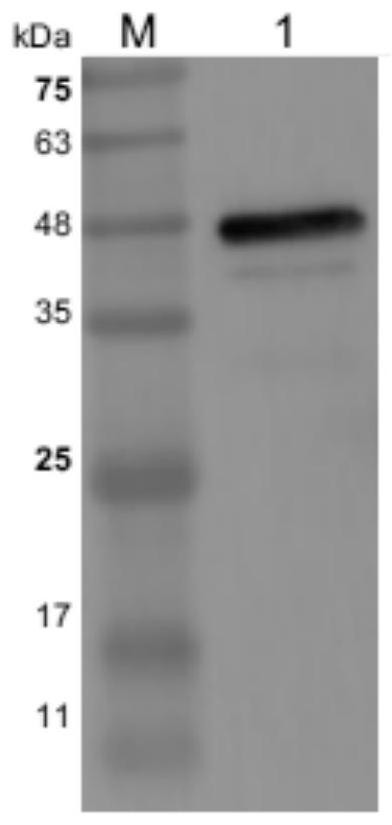
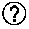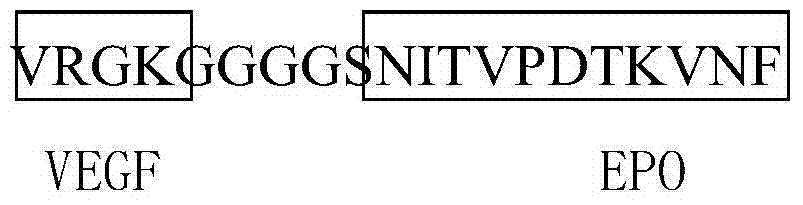Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
73results about How to "Effective immune response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chimeric viruses presenting non-native surface proteins and uses thereof
ActiveUS20120122185A1Readily immunizeEffective immune responseSsRNA viruses negative-sensePolypeptide with localisation/targeting motifEctodomainVirosome
The present invention provides chimeric negative-stand RNA viruses that allow a subject, e.g., an avian, to be immunized against two infectious agents by using a single chimeric virus of the invention. In particular, the present invention provides chimeric influenza viruses engineered to express and incorporate into their virions a fusion protein comprising an ectodomain of a protein of an infectious agent and the transmembrane and cytoplasmic domain of an influenza virus protein. Such chimeric viruses induce an immune response against influenza virus and the infectious agent. The present invention also provides chimeric Newcastle Disease viruses (NDV) engineered to express and incorporate into their virions a fusion protein comprising the ectodomain of a protein of an infectious agent and the transmembrane and cytoplasmic domain of an NDV protein. Such chimeric viruses induce an immune response against NDV and the infectious agent.
Owner:MT SINAI SCHOOL OF MEDICINE
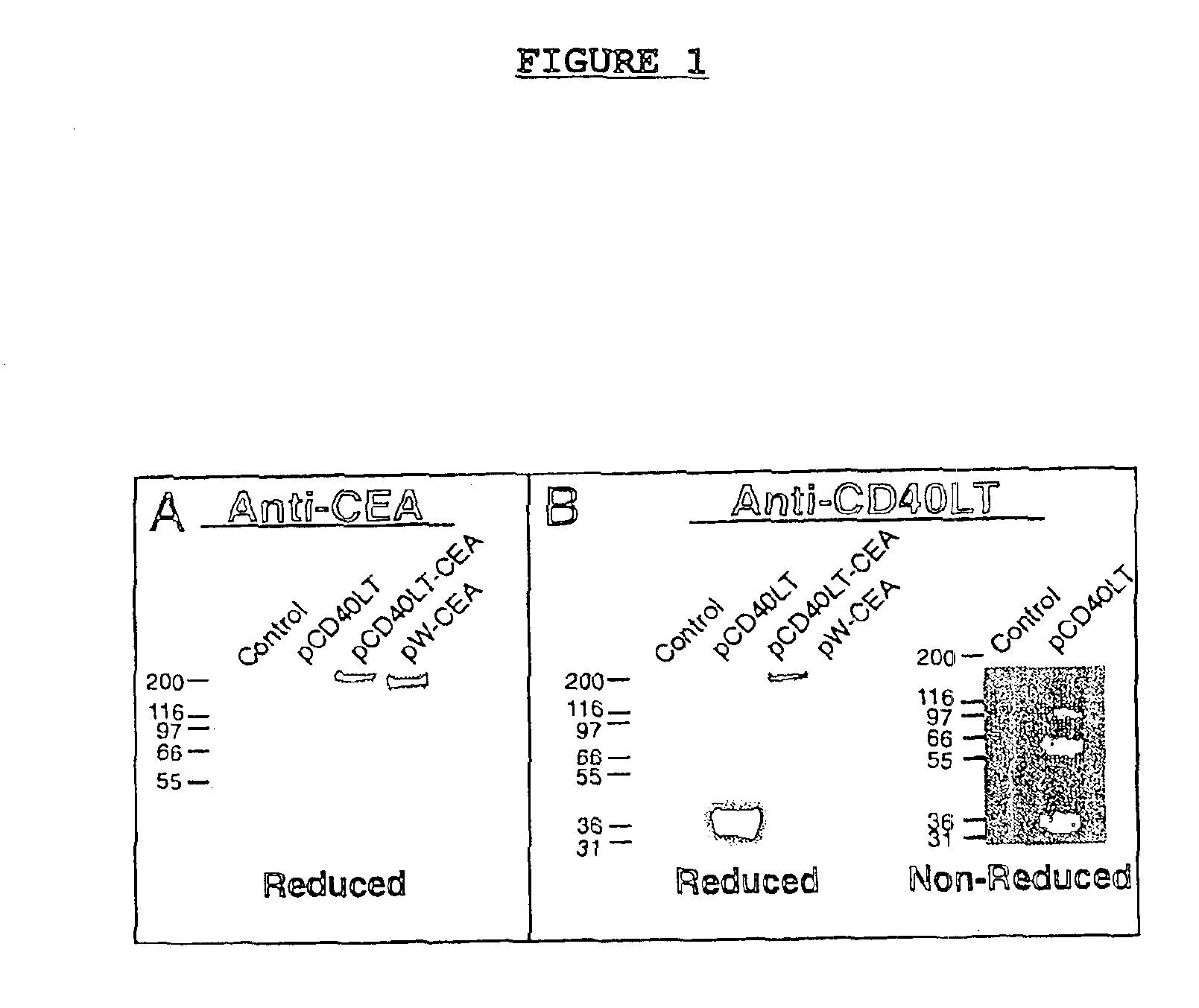
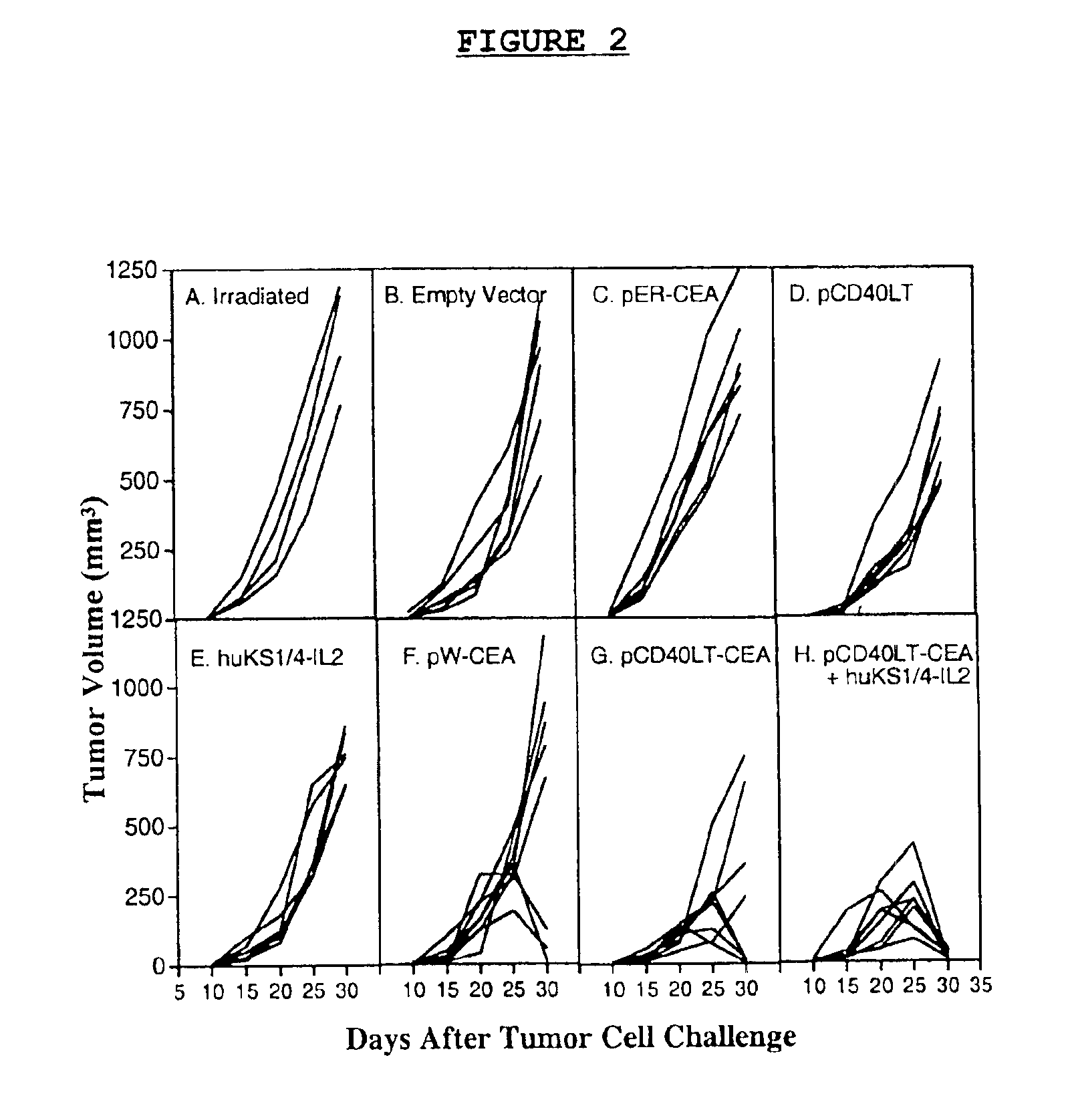
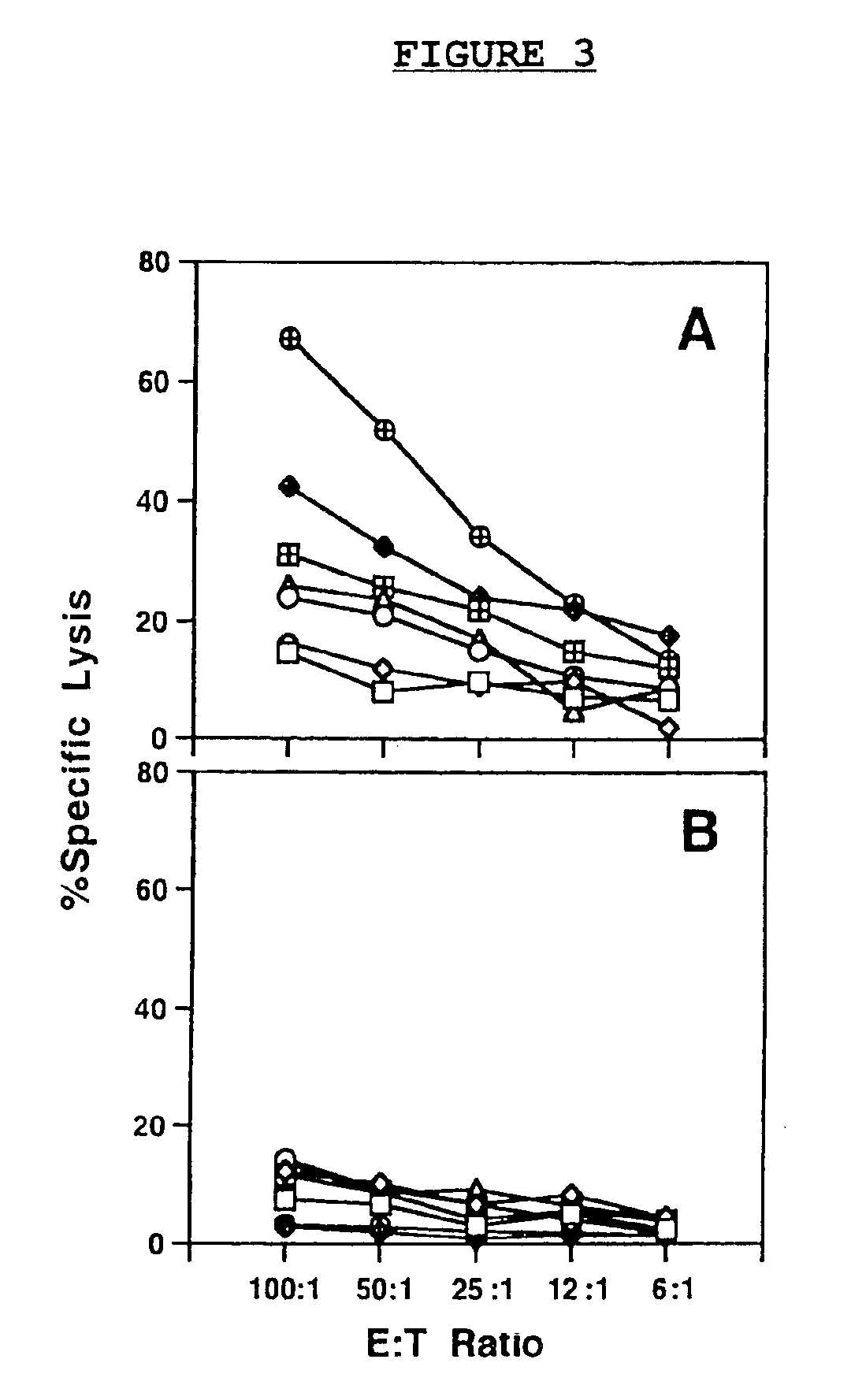
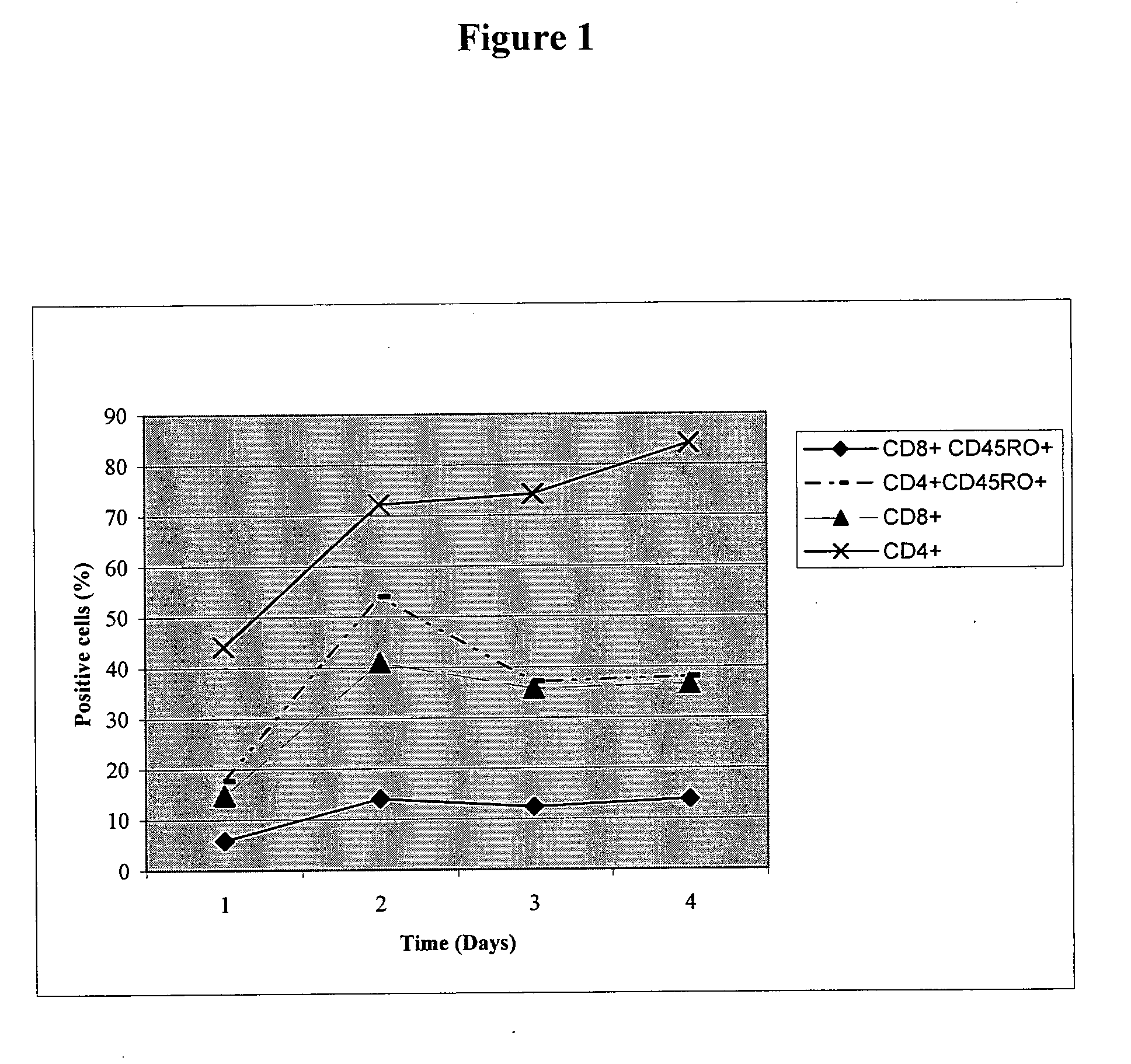
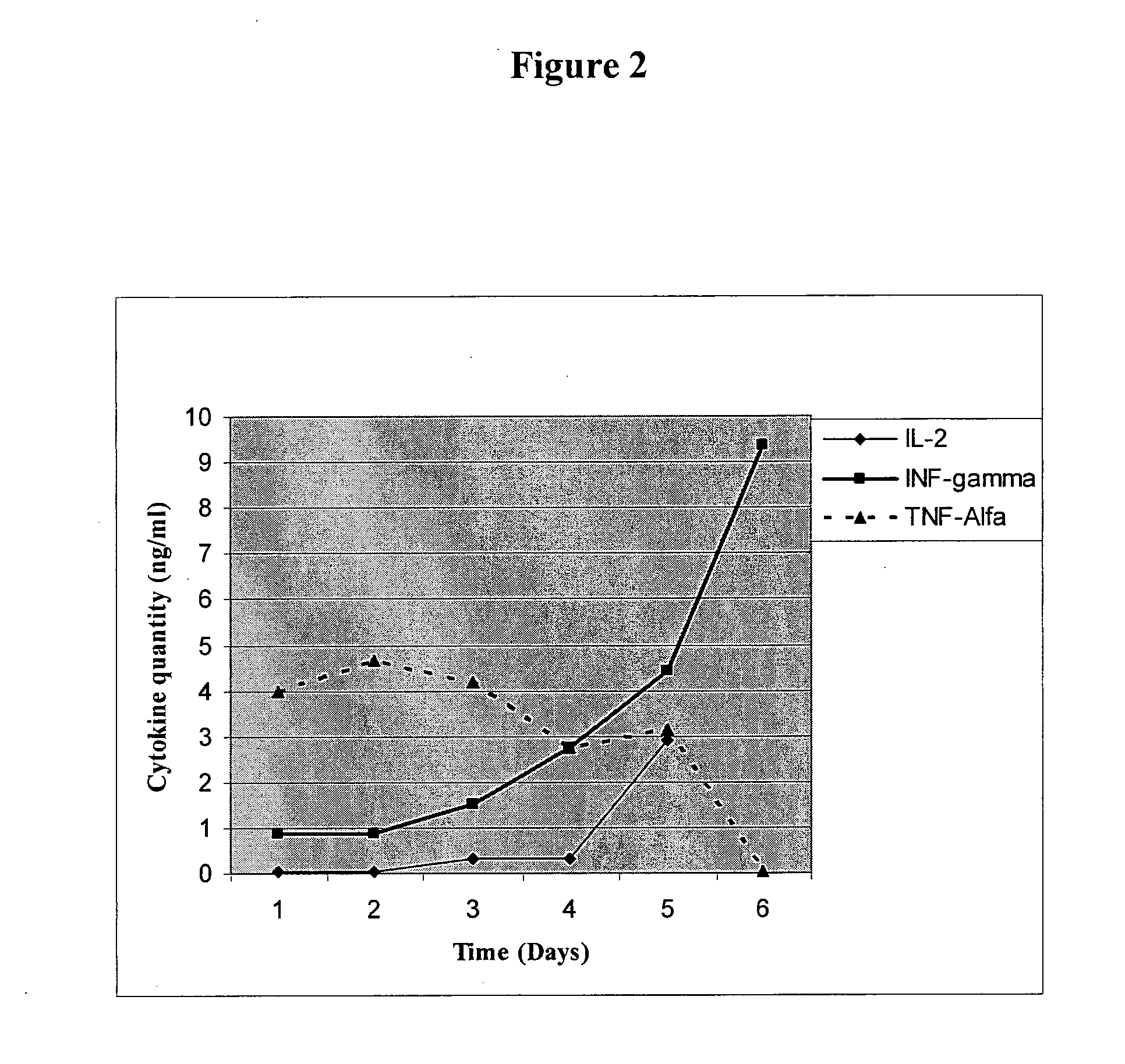
DNA vaccines encoding CEA and a CD40 ligand and methods of use thereof
InactiveUS6923958B2Enhance immune responseEffective immune responseOrganic active ingredientsPeptide/protein ingredientsLipofectamineMammal
A DNA vaccine effective for eliciting an immune response against cells that present a carcinoembryonic antigen (CEA) comprises a DNA operably encoding a CEA and a DNA operably encoding a CD40 ligand, SEQ ID NO:1 and SEQ ID NO: 2, respectively, or its homotrimer, CD40LT. The DNA vaccine can be incorporated in a delivery vector such as an attenuated live bacterium or virus, or a liposome carrier. In a method embodiment, the DNA vaccine is administered orally to a mammal, such as a human, to elicit an immune response against CEA presenting cells such as colon cancer cells. A preferred method embodiment includes the additional step of treating the mammal with recombinant antibody fusion protein huKS1 / 4-IL2 to enhance the immune response effectiveness of the vaccine.
Owner:THE SCRIPPS RES INST
Antigen specific multi epitope vaccines
ActiveUS20100074925A1Strong and comprehensive responseEffective immune responseTumor rejection antigen precursorsSugar derivativesMHC class IProtein target
The present invention relates to cancer vaccines composed of the signal peptide domain of tumor associated antigens or proteins. The peptide vaccines of the invention are characterized by having multiple MHC class I and class II epitopes which are highly abundant in the population. Therefore, these vaccines are likely to induce a strong, comprehensive immune response against the target proteins in the majority of the vaccinated population, and thereby induce an immune reaction against tumors expressing such target proteins. Specifically, the invention relates to peptide vaccines composed of the signal peptide domain of Mucin (MUC1), BAGE-1 or ARMET, and their use for the treatment of cancers which express Mucin (MUC1), BAGE-1 or ARMET.
Owner:VAXIL BIOTHERAPEUTICS
Method and apparatus for treating tumors using low strength electric fields
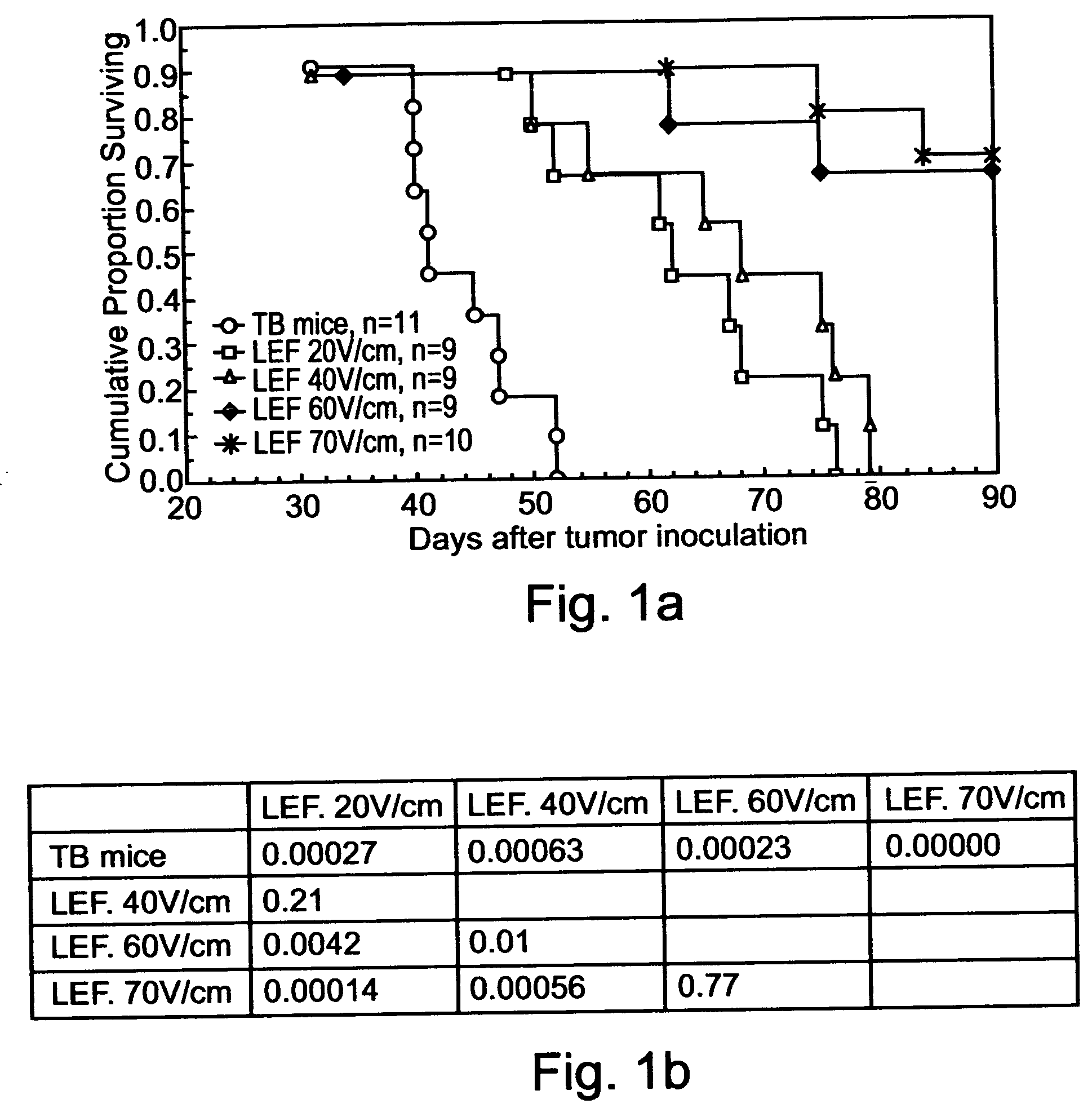
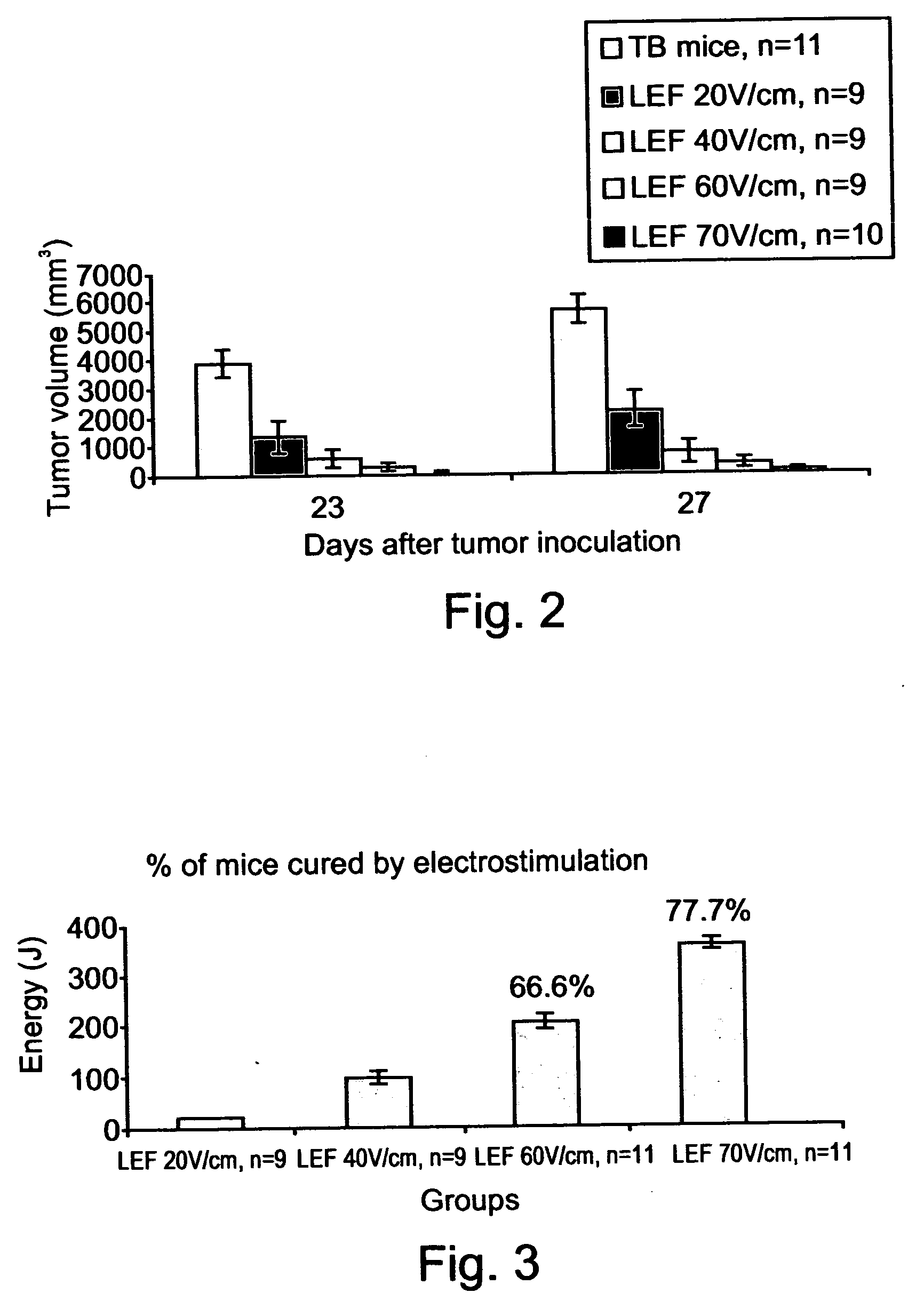
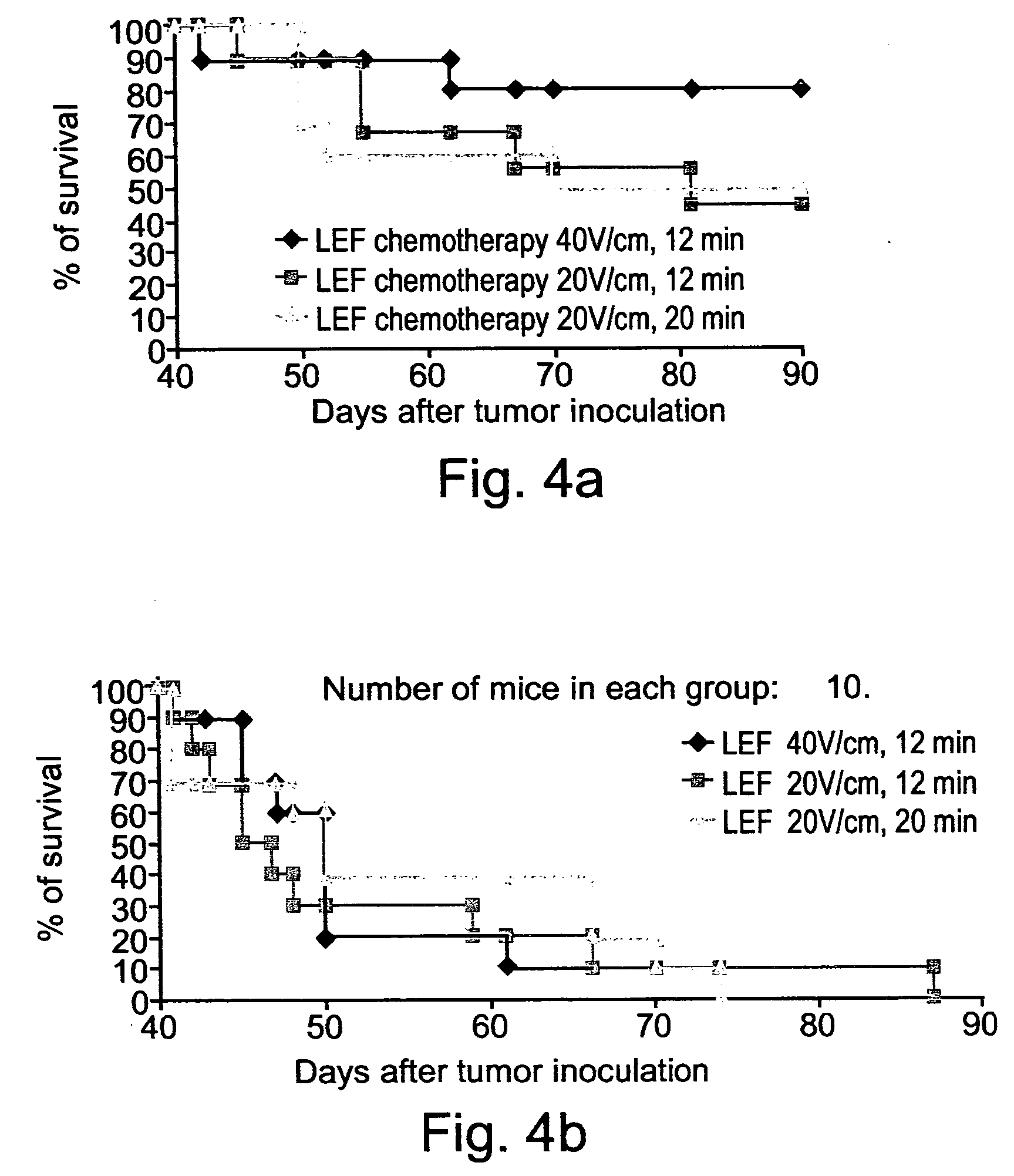
InactiveUS20040158288A1Effective treatment of cancerEffective immune responseHeavy metal active ingredientsEnergy modified materialsElectric field pulseOncology
A method of treating tumor tissue of an individual is provided. The method is effected by applying to cells of the tumor tissue electrical field pulses having a strength, a repetition frequency and a pulse width selected capable of inducing endocytosis mediated cell death thereby treating the tumor tissue.
Owner:RAMOT AT TEL AVIV UNIV LTD
PCV 2-Based Methods and Compositions for the Treatment of Pigs
InactiveUS20100150959A1Effective immune responseSugar derivativesViral antigen ingredientsBiologyDisease
The present invention relates to methods and compositions for vaccinating pigs against porcine circovirus associated diseases.
Owner:VECTOGEN
Microneedle compositions and methods of using same
ActiveUS20170196966A1Enhance immune responseEffective immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseViral replicationVirology
Described herein, are microneedle devices comprising a recombinant alphavirus replicon encoding an exogenous polypeptide, wherein the recombinant alphavirus replicon is coated onto or embedded into a plurality of microneedles. Also described herein are methods of preparing a microneedle device comprising a recombinant alphavirus replicon encoding an exogenous polypeptide. Also disclosed herein are methods of inducing an immune response in an individual comprising contacting the individual with a microneedle device comprising a recombinant alphavirus replicon encoding an exogenous polypeptide.
Owner:VERNDARI INC
Novel feedstuff matched with safe aquatic product
InactiveCN1836542AImprove immunityImprove survival rateAnimal feeding stuffAccessory food factorsDiseaseAquatic animal
The safe mixed feed for aquatic animal includes base feed and added compound microecological preparation in the amount of 0.02-0.05 wt% of the base feed. The compound microecological preparation is mixture of fissiparous vibrio and bacillus in the weight ration of 1 to 1-2. The safe mixed feed can raise the immunity of aquatic animal, reduce diseases and raise survival rate obviously.
Owner:缪淑华
Application of chitosan oligosaccharide and vaccine containing chitosan oligosaccharide
InactiveCN104623654AExcellent immune responseSmall molecular weightAntiviralsImmunological disordersAntigenResponse effect
The invention provides anapplication of chitosan oligosaccharide and a vaccine containing the chitosan oligosaccharide. The chitosan oligosaccharide is applied to the vaccine as an adjuvant, namely each single vaccine contains 2.5-10mg of chitosan oligosaccharide. The chitosan oligosaccharide provided by the invention is a chitosan oligosaccharidemixturewhich is formed by 2-10 glucosamines and of which the molecular weight is smaller than 3000, and is small in toxic or side effect, and is safe and reliable when being used in an immunizing dose range; antigen-specifichumoral immune responsecan be effectively induced; the induced humoral immune response effect is superior to that of a group free of an adjuvant; and the chitosan oligosaccharide is available in raw materialwhich is a commercially available product, is simple in preparation technology, low in cost, stable in performance, relatively high in biological potency, and free of a toxic or side effect, and can be add to a plurality of traditional vaccines or genetic engineering vaccines as the adjuvant.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Apolipoprotein E as an adjuvant for lipid antigens
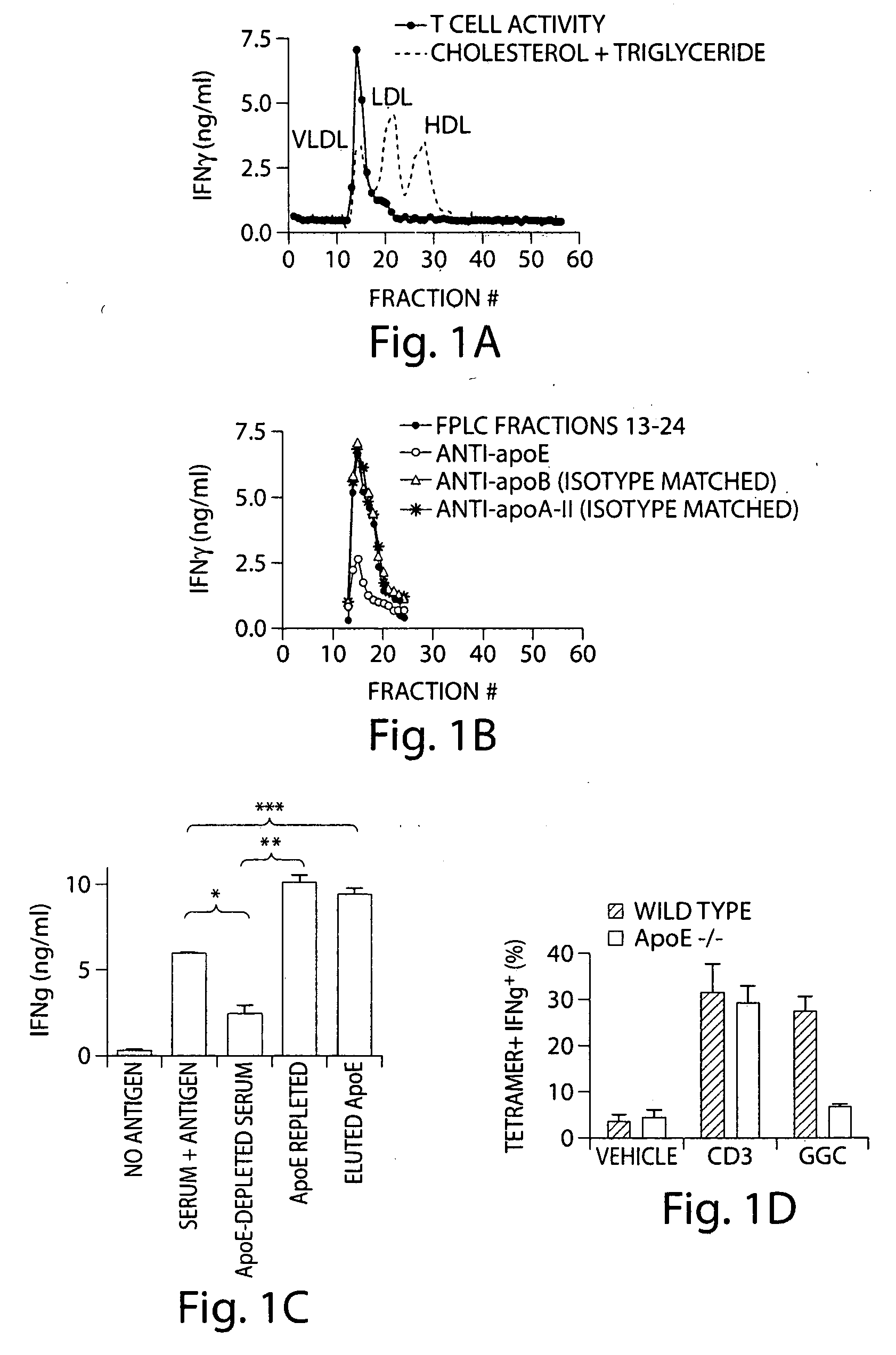
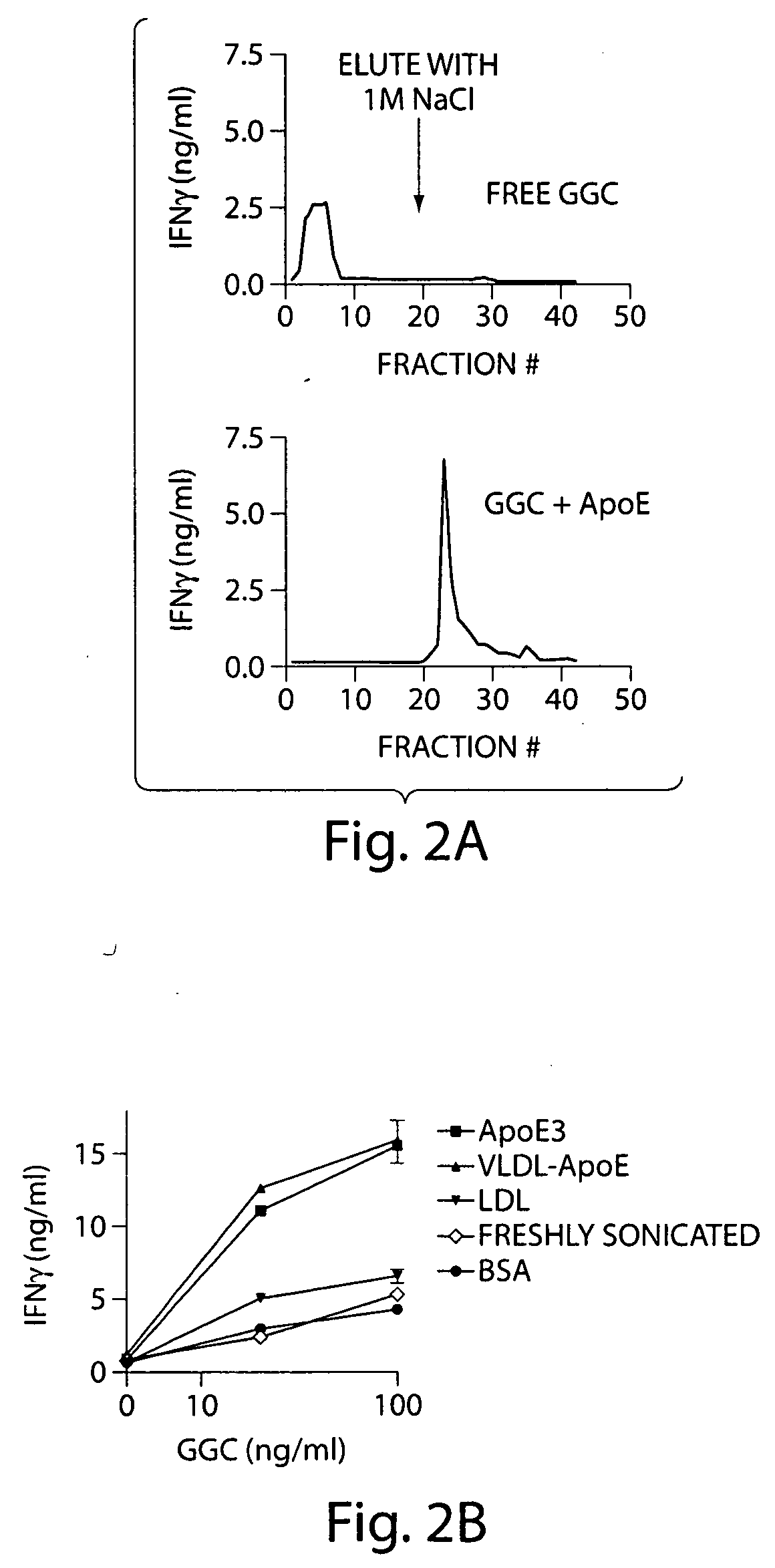
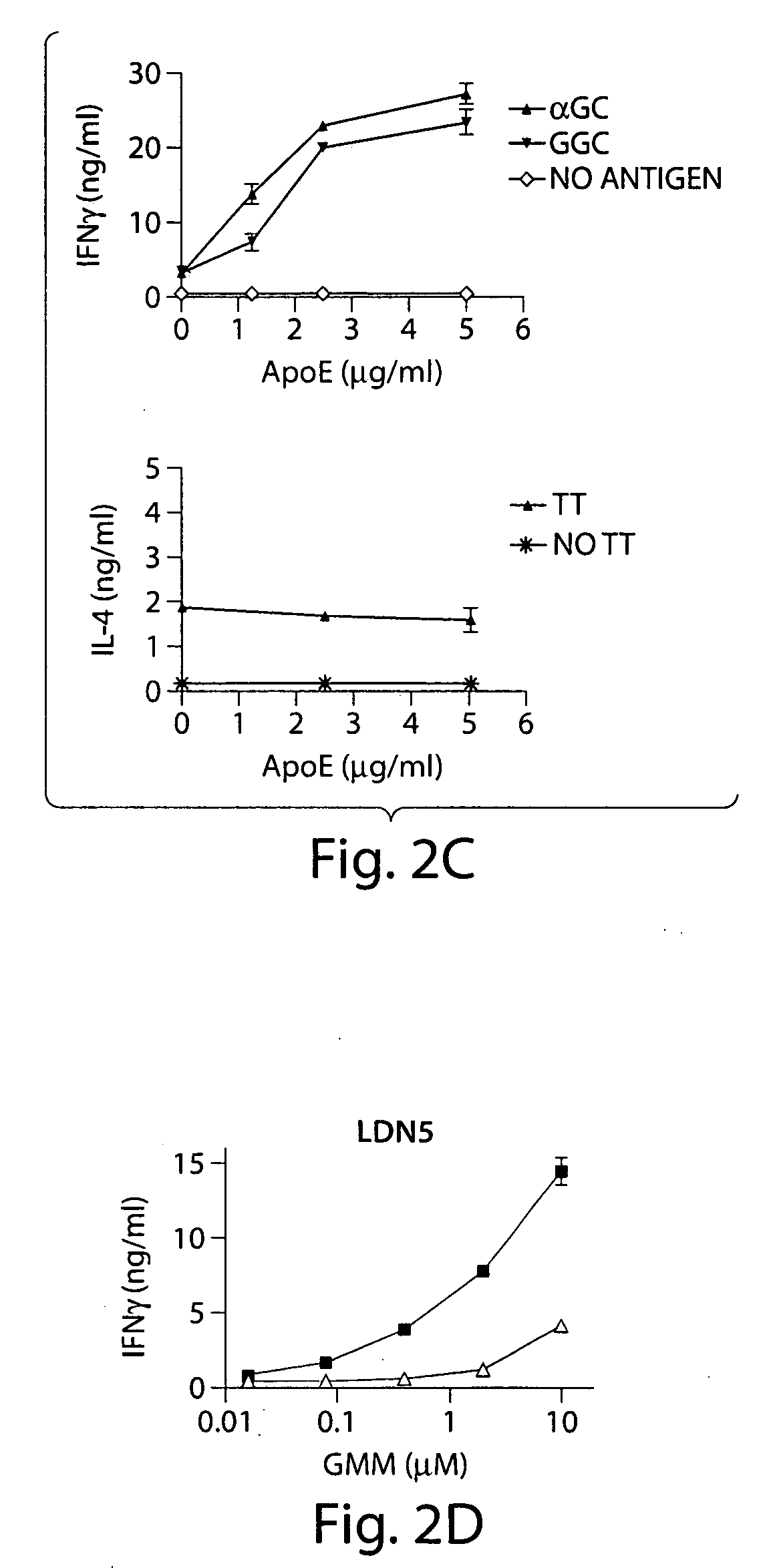
InactiveUS20070110770A1Stimulate immune responseEffective immune responseAntibacterial agentsOrganic active ingredientsDrugAdjuvant
Owner:THE BRIGHAM & WOMENS HOSPITAL INC
Sialic acid (α-(2→6))-d-pyranose derivative and its synthesis method and application
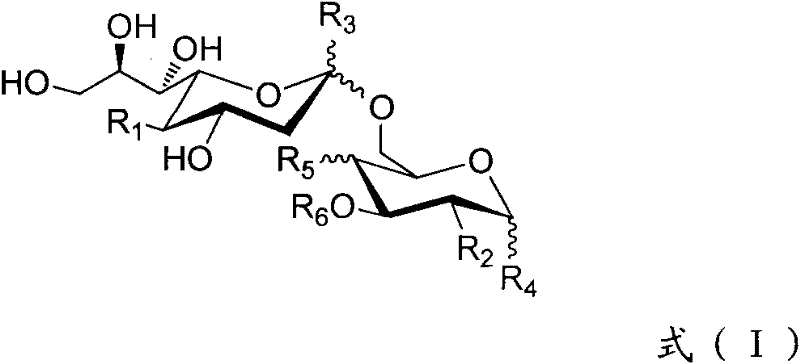
ActiveCN102276662ANovel structureHigh activityEsterified saccharide compoundsOrganic active ingredientsPyranoseAntiendomysial antibodies
The invention discloses an N-acyl group modified sialic acid (alpha-(2-6))-D-amino pyranose derivative and its synthetic method and use. The sialic acid (alpha-(2-6))-D-amino pyranose derivative shown in the formula (I) is synthesized from raw materials of D-galactosamine (glucose) and sialic acid and is coupled with a carrier protein or a polypeptide to form a glycoprotein (glycopeptide) conjugate. In a structure of the N-acyl group modified sialic acid (alpha-(2-6))-D-amino pyranose derivative, a derivative acyl group replaces an acetyl group and thus the structure is novel. The N-acyl group modified sialic acid (alpha-(2-6))-D-amino pyranose derivative has good activity in anti-tumor vaccines. A result of an experiment on mice shows that through structural derivatization, a carbohydrate antigen based vaccine can produce an effective immune response and a mass of antibodies and IgG / IgM is improved obviously. The antibodies can identify specifically tumor cells expressing STn and thus anti-tumor effects are realized. STn antigens can be expressed on multiple tumors and thus the N-acyl group modified sialic acid (alpha-(2-6))-D-amino pyranose derivative has a wide application scope.
Owner:PEKING UNIV
Carbohydrate specific cellular immunity inducing microorganisms and fractions thereof
InactiveCN101600454AEnhance specific immune responseEffective immune responseBacterial antigen ingredientsBacteriaMolecular biologyAbnormal tissue growth
The present invention relates to the field of prevention and treatment of disorders associated with the occurrence of certain carbohydrate epitopes. More particularly, the present invention relates to the prevention and treatment of carbohydrate epitope positive tumors. It relates to formulations and methods for the induction of an effective carbohydrate specific cellular immune response.
Owner:GLYCOTOPE GMBH
The pIL-6 gene adjuvant for pig vaccine and its prepn process
InactiveCN1772298AActivity is not affectedEasy to makeProtozoa antigen ingredientsGenetic material ingredientsBiologyFoot-and-mouth disease virus
The present invention discloses one kind of adjuvant for pig vaccine and its preparation process and constituted pig vaccine. The gene adjuvant for pig vaccine is adjuvant including pig interleukin-6 gene (pIL-6), or recombinant plasmid pcDNA-pIL-6 of animal cell expression plasmid pcDNA3.1 and pig interleukin-6 gene (pIL-6). The pig vaccine is composition of pigí»s cysticercus-resisting vaccine composition; pigí»s deactivated foot-and-mouth disease virus vaccine and the gene adjuvant. The gene adjuvant of the present invention is prepared through gene cloning, recombination, recombinant plasmid proliferation, extraction and purification and other processes.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
PCV3 Cap protein epitope peptide, anti-PCV3 Cap protein monoclonal antibody, and preparation method and application thereof
ActiveCN112812178AStrong specificityImprove featuresImmunoglobulins against virusesGenetic engineeringProtein engineeringAntibodies monoclonal
The invention relates to a PCV3Cap protein epitope peptide, an anti-PCV3Cap protein monoclonal antibody, and a preparation method and application thereof. The invention provides the anti-PCV3Cap protein monoclonal antibody, and further provides a heavy chain variable region sequence and a light chain variable region nucleotide and amino acid sequence of the monoclonal antibody. On the basis, the antibody can be prepared by a genetic engineering method; and meanwhile, modification such as addition, deletion and replacement of one or more amino acids can be performed by genetic engineering and protein engineering methods to obtain an active fragment or a conservative variant thereof, thereby laying a foundation for further improving the specificity and affinity of the antibody. The antibody has relatively high specificity, has no cross reaction with PCV1, PCV2 and other porcine viruses such as CSFV, PRRSV and PRV, and has wide research and application values and commercial use values in immunological detection such as antigen / antibody detection kits, antigen / antibody immunochromatographic test paper, IFA, IPMA and Western Blotting.
Owner:河南中泽生物工程有限公司
Hydrophilic antigen and/or hydrophobic antigen vaccine delivery system and preparation method thereof
ActiveCN111658767AParticle size adjustableEasy to packAntibacterial agentsPowder deliveryVaccine deliveryAntigen delivery
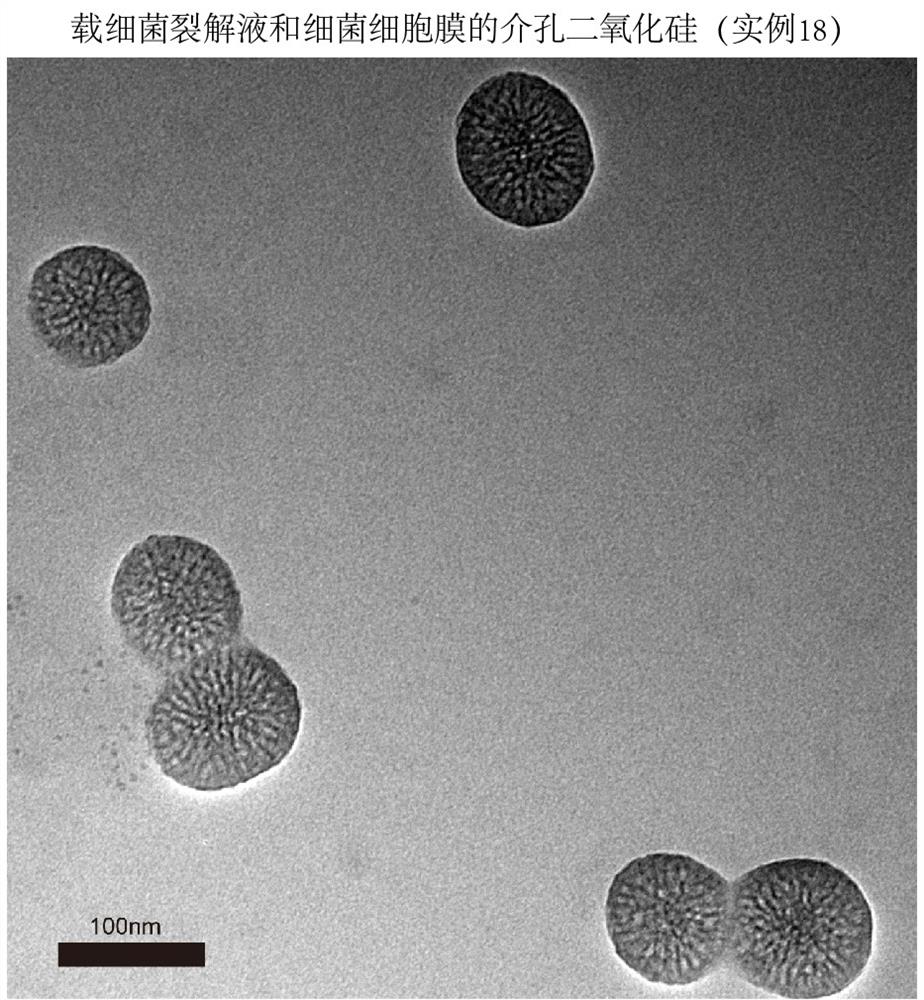
The invention provides a hydrophilic antigen and / or hydrophobic antigen vaccine delivery system and a preparation method thereof. According to the invention, mesoporous silica with good dispersibilityand controllable particle size and pore diameter is synthesized by adopting a two-phase method. A hydrophilic antigen is entrapped by the mesoporous silica through mesopores, a hydrophobic antigen isentrapped by a lipid membrane, and the vaccine delivery system is a multivalent antigen delivery system. The prepared vaccine can be transferred to lymph nodes, is efficiently taken by antigen presenting cells, induces antigen-specific immune response, and has a wide application prospect.
Owner:SICHUAN UNIV
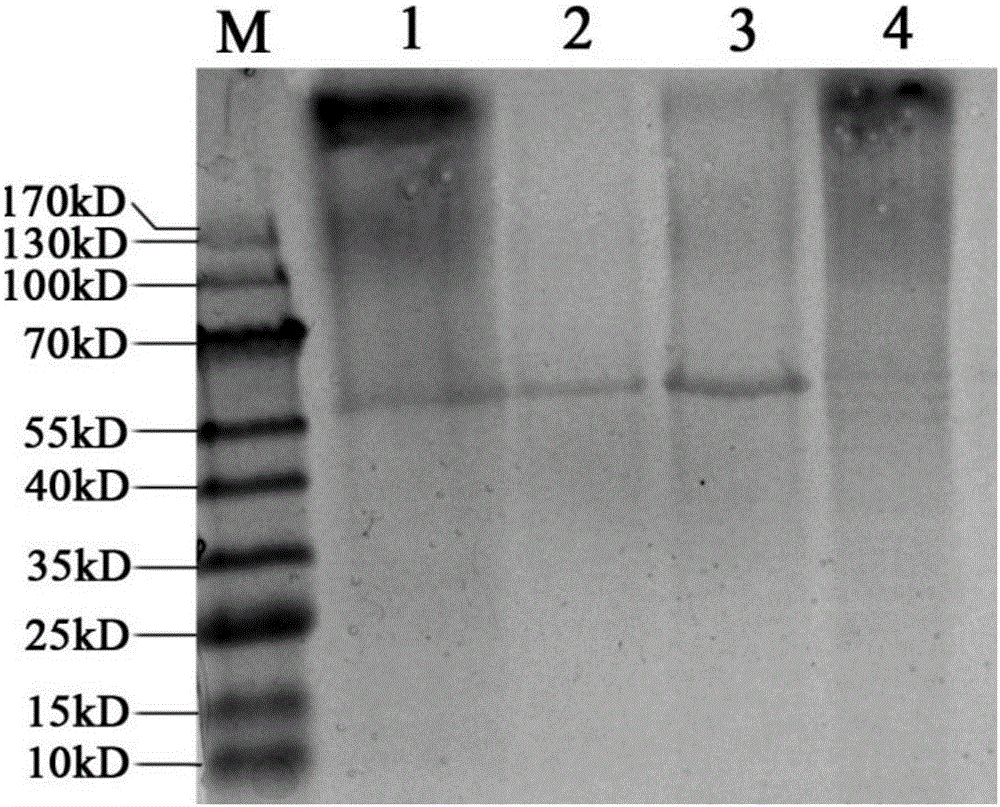
rBCG for expression of Br. Melitensis P39 and L7/L12 fusion gene and construction method thereof
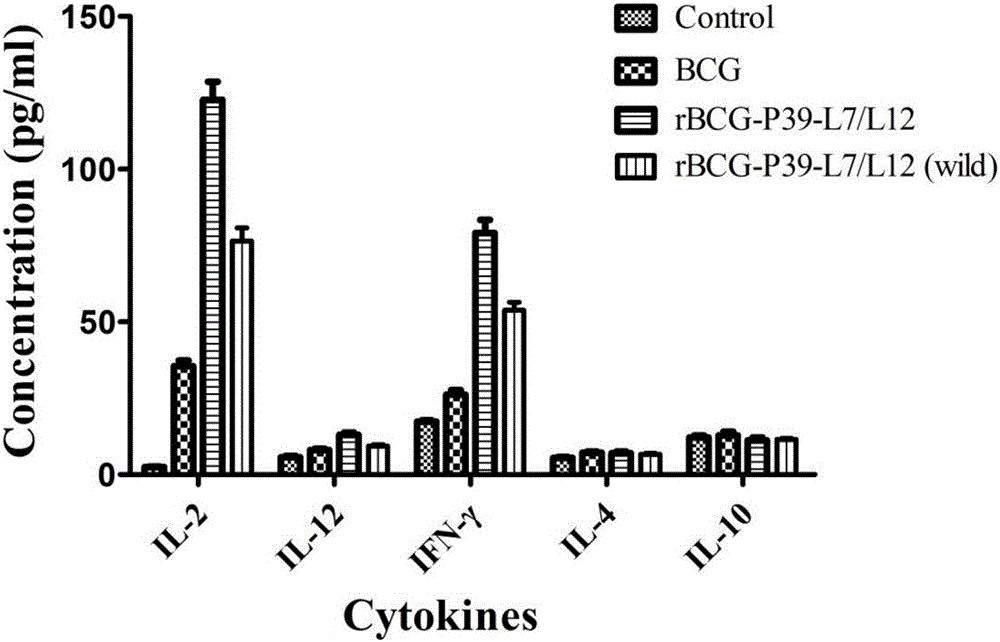
ActiveCN106834331ASignificant immune adjuvant effectLow costAntibacterial agentsBacterial antigen ingredientsRibosomal proteinBCG vaccine
The invention provides rBCG for expression of Br. Melitensis P39 and L7 / L12 fusion gene. The rBCG is constructed by transferring an expression vector carrying codon-optimized Br. Melitensis P39 and L7 / L12 fusion gene into BCG. Brucellosis-generated cytoplasm binding protein PBP39 (coding gene is P39) and Brucellosis ribosomal protein L7 / L12 are both T-cell antigen. Bacillus Calmette-Guerin (BCG) vaccine is the only one commercial vaccine for preventing tuberculosis so far. The BCG vaccine has a remarkable immunologic adjuvant effect and is an exogenous gene expression host with good performance and high safety. By BCG expression of the codon-optimized Brucellosis P39 and L7 / L12 fusion gene, expression quantity of the target gene can be increased. The rBCG vaccine can simulate intracellur infection and parasitic characteristics of Brucellosis to more effectively induce body to generate immune response, can perform advantages of high safety, simple preparation, low cost, etc. of BCG as the expression host as well as the immunologic adjuvant effect of the BCG itself, and is expected to become a novel Brucellosis vaccine.
Owner:INNER MONGOLIA MEDICAL UNIV
Swine fever antigen epitope peptide and use thereof
ActiveCN112679584AIncrease abundanceGood immune effectViral antigen ingredientsVirus peptidesAfrican swine feverAntigenic protein
The invention belongs to the field of biomedicines and particularly relates to a swine fever antigen epitope peptide and use thereof. The swine fever antigen epitope peptide is selected from 100th-550th amino acids of a N-terminal of an African swine fever viral protein P72 and has the length of 20-210 amino acids, and an independent swine fever antigen epitope peptide maybe comprise a recombinant antigen of the swine fever antigen epitope peptide and can induce an immune reaction of a mammal to the African swine fever viral protein P72. Inventors disclose epitope information on a major antigen protein P72 of an African swine fever virus according to a resolved full virus structure of a natural African swine fever virus; and compared with epitope information predicted by means of bioinformatics and the like, the swine fever antigen epitope peptide disclosed by the invention is more real, more reliable and more effective.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
Microemulsion-based vaccine delivery system, preparation method and application thereof
ActiveCN111658611AStability is not affectedGood dispersionInorganic non-active ingredientsImmunological disordersVaccine deliveryMicroemulsion
The invention provides a microemulsion-based vaccine delivery system, and also provides a preparation method and application thereof. According to microemulsion in the invention, a series of metal ioncompounds are adsorbed; and antigens are added in a preparation process, so that antigen entrapment can be realized and a stable vaccine preparation is obtained. The vaccine prepared by the inventioncan be efficiently taken in by antigen presenting cells, effectively transferred to lymph nodes and induce antigen-specific immune response, and has a wide application prospect.
Owner:SICHUAN UNIV
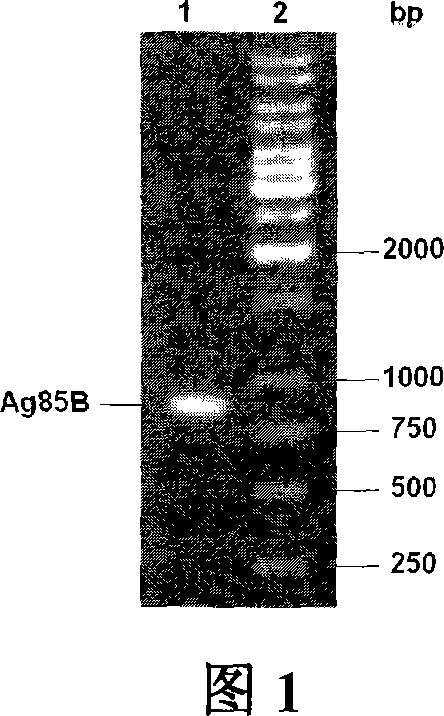
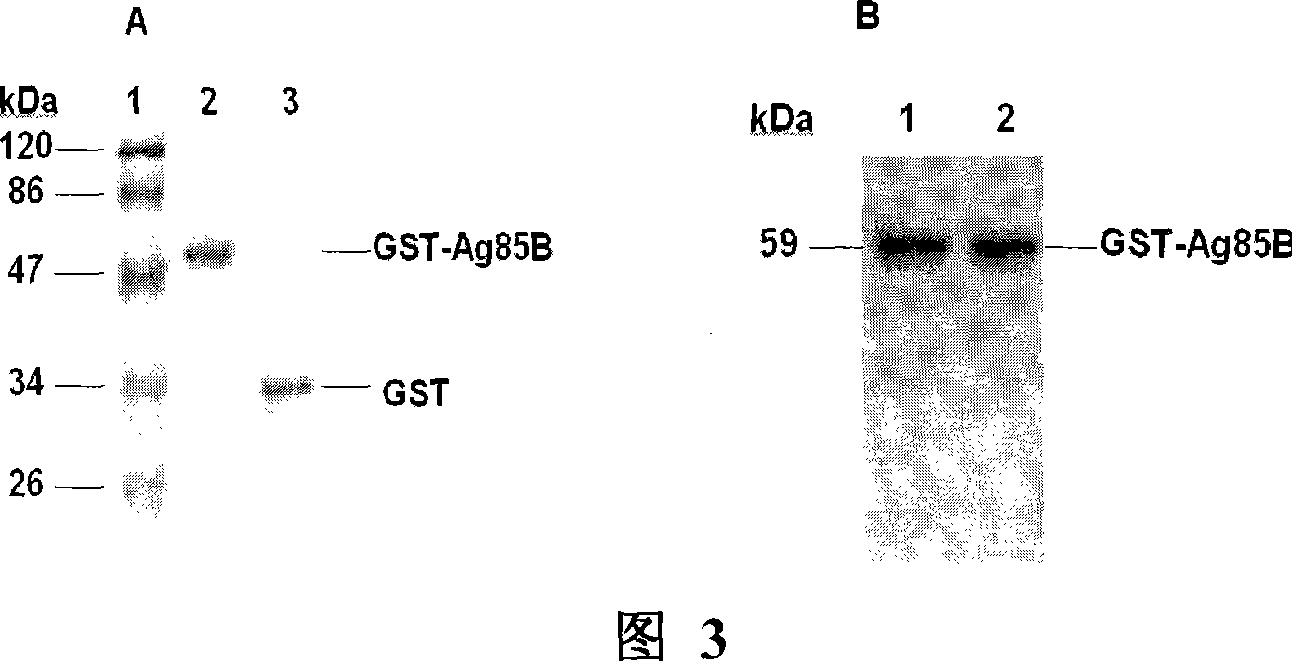
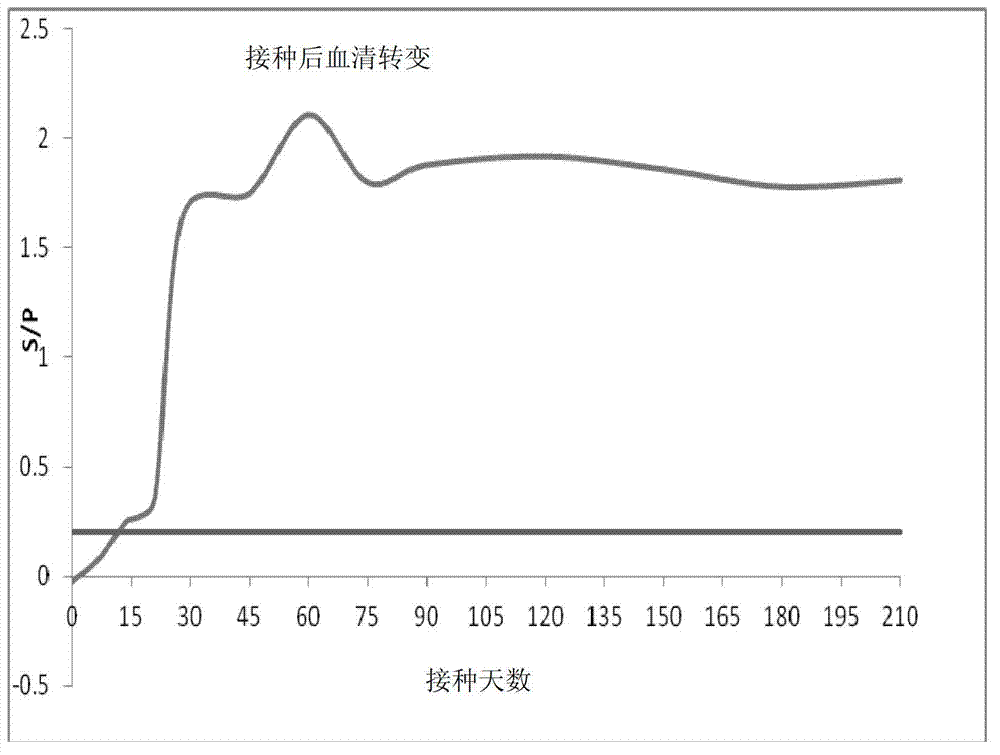
Carrier bacterin of attenuated typhoid bacterium of carrying tubercle branch bacillus Ag85B
InactiveCN101049508AImprove the ability to synthesize IgAEffective cellular immunityBacterial antigen ingredientsBacteriaMicrobiologyMycobacterium
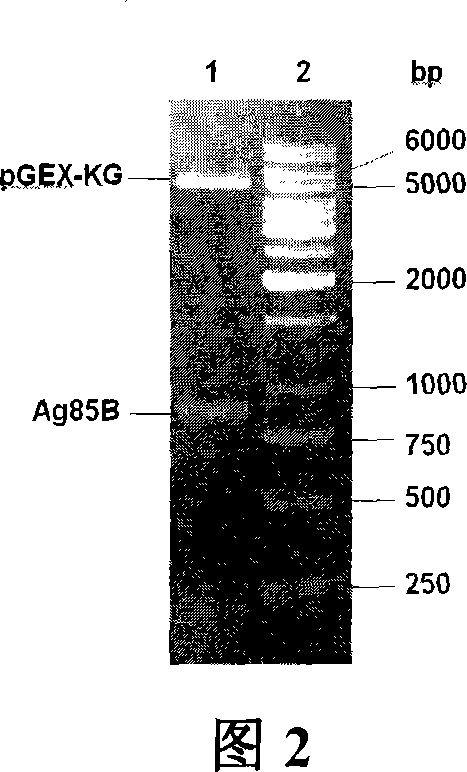
An attenuated Salmonella typhi carrier vaccine carrying tubercular mycobacterium Ag85B is prepared through preparing prokaryotic recombinant expression plasmid pGEX-Ag85B, configuring eukaryotic recombinant expression plasmid pVAX1-Ag85B, and preparing said antitubercular vaccine. It can be orally taken.
Owner:WUHAN UNIV
Multi-epitope fusion antigen for detecting virus serum antibody of porcine reproductive and respiratory syndrome and kit prepared with multi-epitope fusion antigen
The invention relates to a multi-epitope fusion antigen for detecting virus serum antibody of porcine reproductive and respiratory syndrome, wherein an amino acid sequence of the multi-epitope fusion antigen is shown in SEQ ID No:1. The invention also relates to a preparation method of the multi-epitope fusion antigen, and a kit for detecting the virus antibody of porcine reproductive and respiratory syndrome. The multi-epitope fusion antigen prepared by the preparation method has the advantages of high purity, good stability, simple preparation process, and low production cost. The kit has the characteristics of high sensitivity and strong safety.
Owner:重庆业为基生物科技集团有限公司
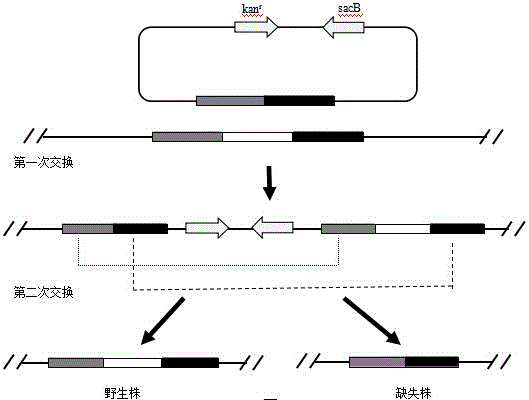
Double-gene-deficient Salmonella enteritidis strain and establishment method thereof, and vaccine containing double-gene-deficient Salmonella enteritidis strain
ActiveCN106190943AReduced net proliferationEnhance humoral and cellular immunityAntibacterial agentsBacteriaImmune effectsSalmonella bongori
The invention discloses a double-gene-deficient Salmonella enteritidis strain and an establishment method thereof, and a vaccine containing the double-gene-deficient Salmonella enteritidis strain. The double-gene-deficient Salmonella enteritidis strain is a Salmonella enteritidis strain of which rfaH gene and sopB gene are knocked out. According to the establishment method, a homologous recombination process and a sucrose-sensitive gene negative screening process are adopted to knock out the rfaH gene and sopB gene in the Salmonella enteritidis strain. The vaccine comprises the double-gene-deficient Salmonella enteritidis strain prepared by the method. The cellular immunity and mucosa immunity of the double-gene-deficient Salmonella enteritidis strain are enhanced; and the double-gene-deficient Salmonella enteritidis strain has favorable protective effects and favorable immune effects, has favorable removing effects in the host, can be colonized and removed in the body within 3-4 weeks, and thus, has excellent safety performance.
Owner:CHONGQING UNIV OF TECH
Nasal influenza vaccine composition
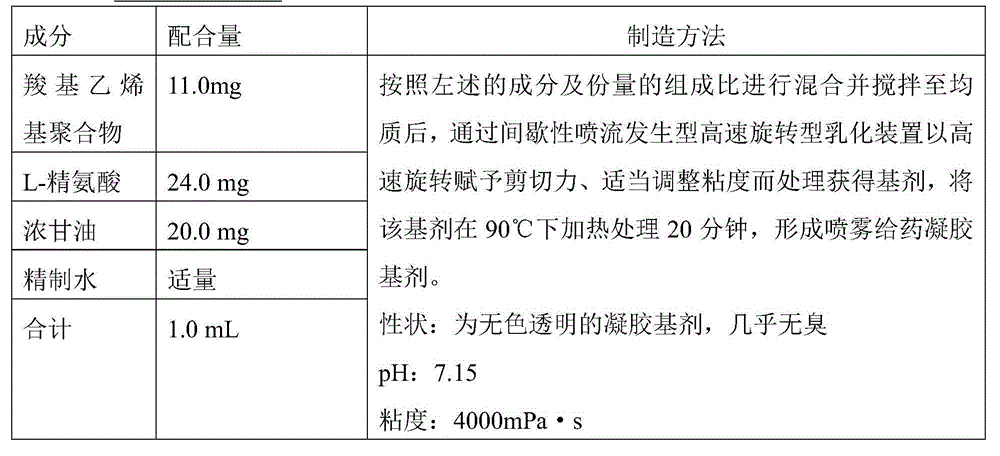
InactiveCN104884086ATo deal with the epidemicEffective immune responseSsRNA viruses negative-senseViral antigen ingredientsAntigenAdjuvant
The present invention relates to a nasal mucosal spray delivery type influenza vaccine composition which is characterized in that the composition comprises a gel base including an influenza virus inactivated whole particle antigen and a carboxyvinyl polymer but does not comprise an adjuvant.
Owner:JAPAN AS REPRESENTED BY DIRECTOR GENERAL OF NAT INST OF INFECT IOUS DISEASES +1
Vibrio anguillarum (listonella anguillarum) virulent strain and application thereof
InactiveCN103275890AHigh immune protectionReduce lossesAntibacterial agentsBacteriaLaboratory cultureVirulence
The invention relates to a Vibrio anguillarum strain and an application method thereof and in particular relates to a Vibrio anguillarum MN strain. The Vibrio anguillarum MN strain is separated from a Scophthamus maximus body, is a wild strain with stronger toxicity and has a preservation number of CGMCC (China General Microbiological Culture Collection Center) No.7198. Vaccine antigens of one or more expression products of inactivated thallus, bacterial ghost components, attenuated strains, protective antigens, antigen subunits, antigenic determinants or antigenic gene expression vectors, which are prepared from the Vibrio anguillarum MN strain can be applied to an immunological technology of the Vibrio anguillarum MN strain; and the vaccine antigens in the immune application can be inoculated by adopting an injection immunization, trauma immunization, immersion bath immunization or oral immunization manner, and can be independently used, or used together with other adjuvants, or prepared into single preparations which can be used together with the adjuvants or not together with the adjuvants.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Anti-tumor angiogenesis immune composite peptide, and preparation method and application thereof
InactiveCN104744593AGrowth inhibitionSuppress generationPeptide/protein ingredientsPeptide preparation methodsVascular endotheliumActive immunization
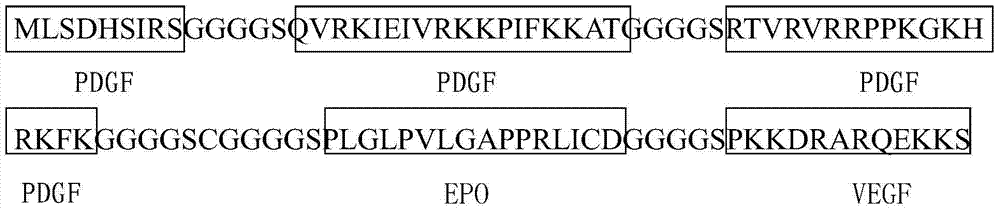
The invention provides an anti-tumor angiogenesis immune composite peptide. The anti-tumor angiogenesis immune composite peptide includes an immunogenicity epitope of a vascular endothelial growth factor (VEGF), an immunogenicity epitope of platelet-derived growth factor (PDGF) and an immunogenicity epitope of erythropoietin EPO. The anti-tumor angiogenesis immune composite peptide has the capability of targeting three target sites, VEGF, PDGF and EPO, thus effectively inhibiting tumor growth; in addition, the anti-tumor angiogenesis immune composite peptide can be used as an active immunization vaccine, and effectively reduce the antibody drug immunogenic response. The invention also provides a preparation method and application of the anti-tumor angiogenesis immune composite peptide.
Owner:SHENZHEN INST OF ADVANCED TECH
Construction method of multivalent epitope and subunit vaccine
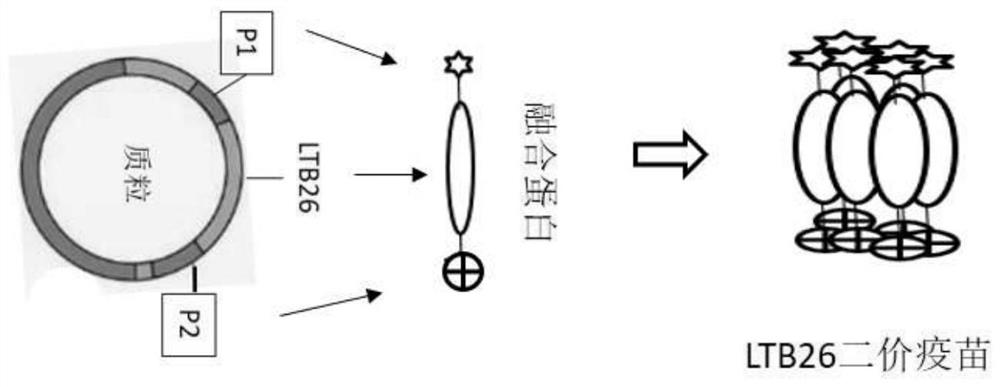
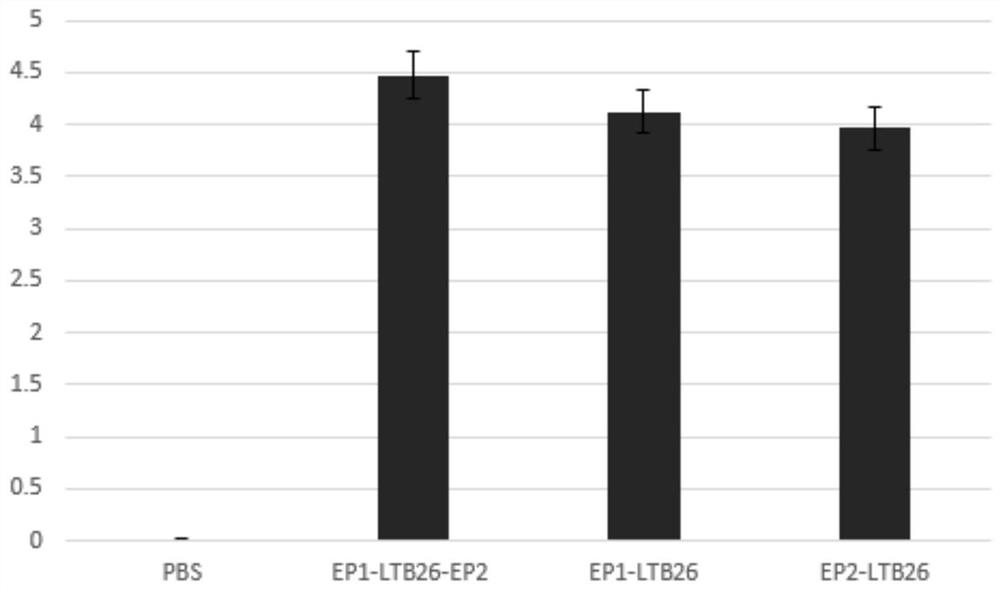
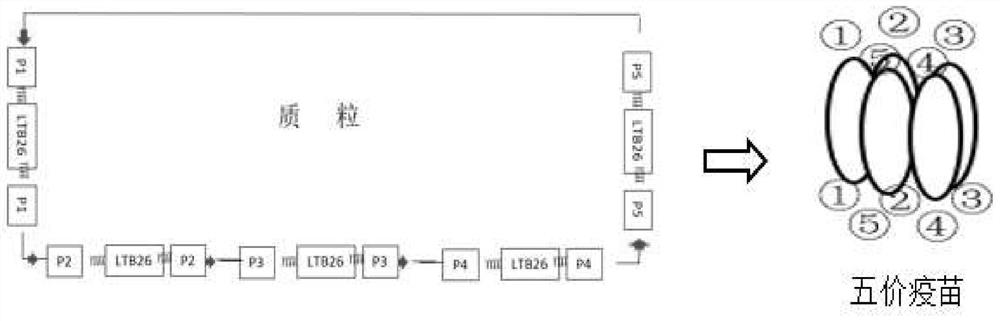
PendingCN111607605AEnlarge the quantityBoost antigen doseSsRNA viruses positive-senseViral antigen ingredientsPentamerBULK ACTIVE INGREDIENT
The invention discloses a construction method of a multivalent epitope and subunit vaccine, and belongs to the field of vaccines. An antigen protein is connected to two ends of LTB26 through fusion expression to obtain a fusion protein, and a pentamer of the fusion protein is obtained by means of the characteristic that LTB26 can be self-assembled to form a pentamer. The active ingredient of the vaccine is the pentamer of the fusion protein. The vaccine is rich in immunogen quantity and variety, and the activity of a LTB26 immunologic adjuvant and the immunogen activity of an antigen peptide are combined together, so that the vaccine can stimulate an organism to generate a large number of specific antibodies, effective immune response is stimulated, and a procedure of adding an immunologicadjuvant is omitted. The protein fused to two ends of LTB26 can also be other proteins other than an antigen protein, and can be applied to the fields other than vaccine preparation, such as large-scale preparation of antibodies, research and development of medical detection kits, and the like.
Owner:CHONGQING MEDICAL UNIVERSITY
Method of inactivating and preserving respiratory syncytial virus (RSV)
PendingCN110684747AEffective immune responseSsRNA viruses negative-senseViral antigen ingredientsDiseaseF protein
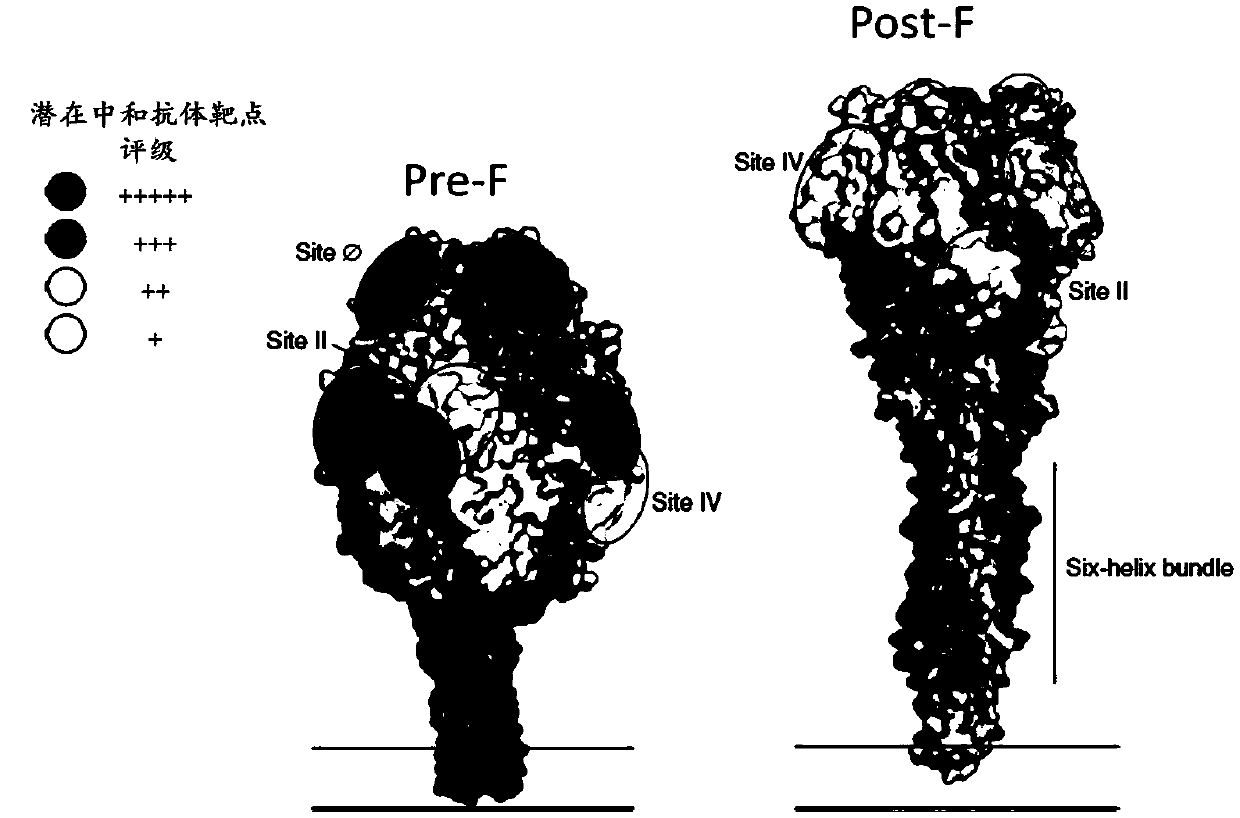
The invention relates to the field of virology and immunology, in particular to an inactivated and isolated respiratory syncytial virus (RSV) and a method of stabilizing pre-F proteins in the RSV, further relates to a method of preserving the RSV and stabilizing the pre-F proteins in the RSV and further relates to a vaccine containing the inactivated RSV, the inactivated RSV prepared and / or preserved by the method of the present invention and a use of the vaccine for the preventing or treating RSV infection or diseases associated with RSV infection.
Owner:XIAMEN UNIV +1
Preparation method of nano vaccine with pH and reduction dual sensitivity and obtained product
InactiveCN112315941ANot easy to dissociateImprove efficiencyCancer antigen ingredientsPharmaceutical non-active ingredientsDisulfide bondingType antigen
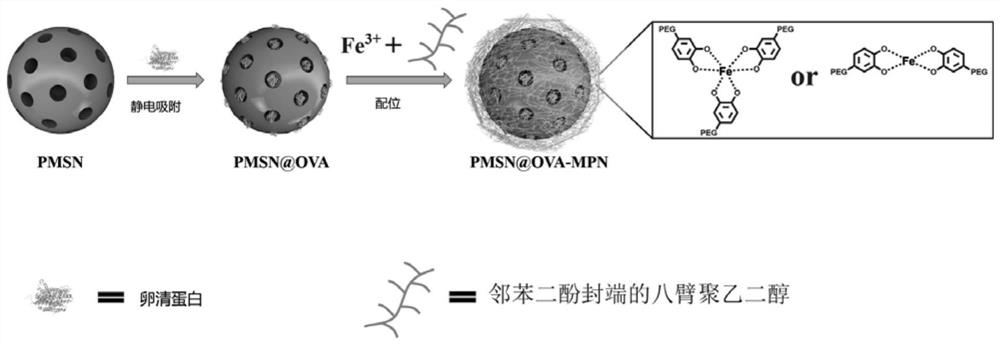
The invention discloses a preparation method of a nano vaccine with pH and reduction double sensitivity and an obtained product. The nano vaccine is obtained by taking PEI-modified mesoporous silica nanospheres as a raw material, adsorbing a model antigen through electrostatic interaction, and then wrapping an outer layer with a metal phenolic network with reduction sensitive disulfide bonds as aprotection network. The preparation method is simple and convenient to operate, low in energy consumption, environment-friendly and easy to expand production, and the obtained nano vaccine has very good in-vivo long-term stability and biocompatibility and has remarkable treatment and prevention effects on tumors.
Owner:HAINAN UNIVERSITY
Live attenuated RTX-producing bacteria of family pasteurellaceae
InactiveCN1117862CEffective immune responseBacteriaViral antigen ingredientsBacteroidesUltrasound attenuation
The present invention relates to live attenuated RTX-toxin producing bacteria of the family Pasteurellaceae, of which the attenuation is due to the fact that they produce RTX toxin in a non-activated form. The invention also relates to vaccines for the protection of mammals against infection with RTX-toxin producing bacteria of the family Pasteurellaceae, and to methods for the preparation of said live attenuated bacteria and vaccines.
Owner:INTERVET INT BV
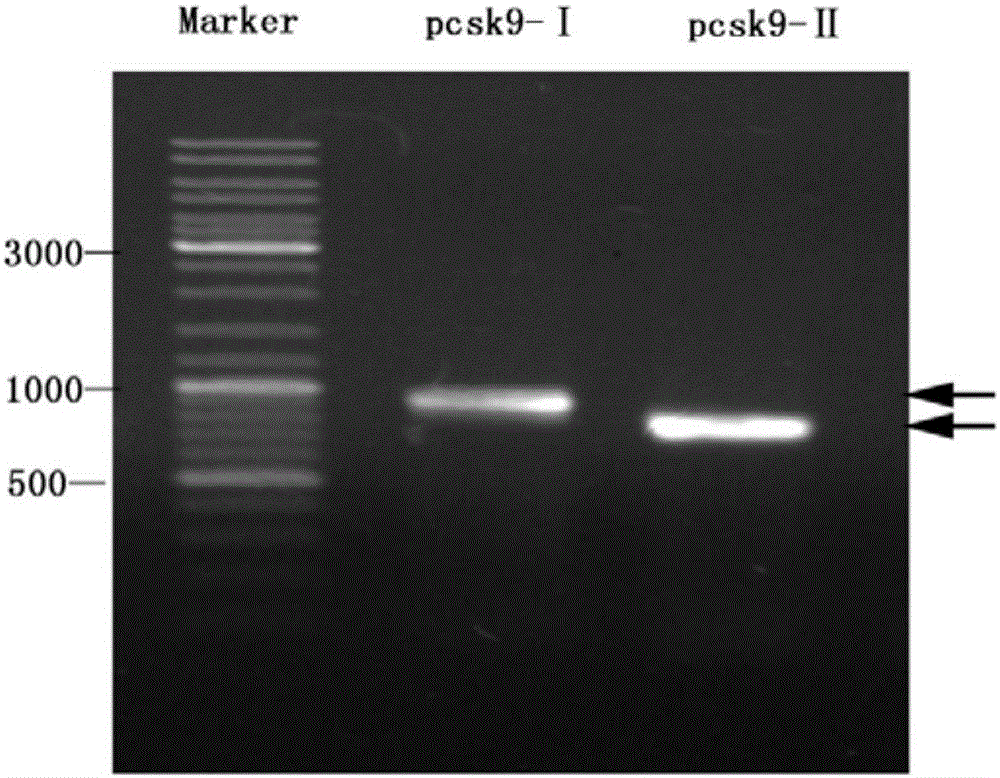
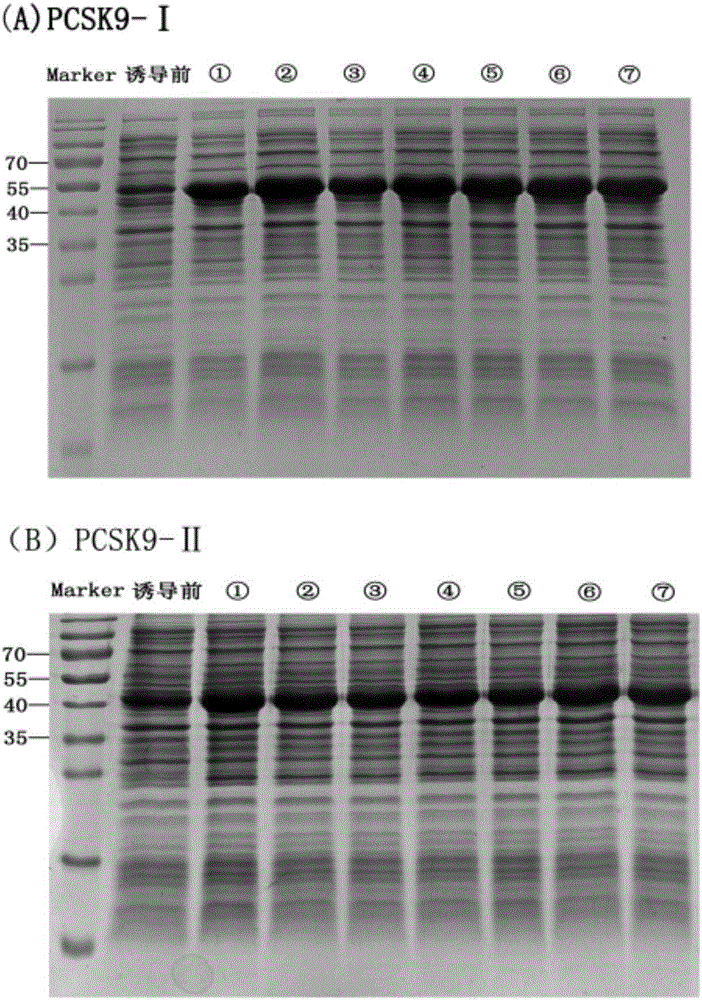
Anti-hyperlipidemia protein vaccine aiming at PCSK9 (Proprotein convertase subtilisin/kexin type 9)
InactiveCN106822881AAvoid degradationAvoid toleranceMetabolism disorderVertebrate antigen ingredientsKexinSubtilisin
The invention belongs to the field of biomedicines and relates to an anti-hyperlipidemia protein vaccine aiming at PCSK9 (Proprotein convertase subtilisin / kexin type 9). Aiming at solving the technical problems, the anti-hyperlipidemia protein vaccine which has good performances and takes the PCSK9 as a target is researched and developed. The protein vaccine aiming at the PCSK9, which is prepared by the invention, can be used for remarkably lowering the level of blood lipid of serum and has remarkable immunogenicity and good safety; a preparation method is simple and feasible and is low in cost. The protein vaccine aiming at the PCSK9 can be used for preventing and treating hyperlipidemia diseases and can realize treatment effect without the need of completely blocking the PCSK9; a condition that individuals is intolerant to complete blocking of the PCSK9 is avoided and the anti-hyperlipidemia protein vaccine has a better application prospect.
Owner:SICHUAN UNIV
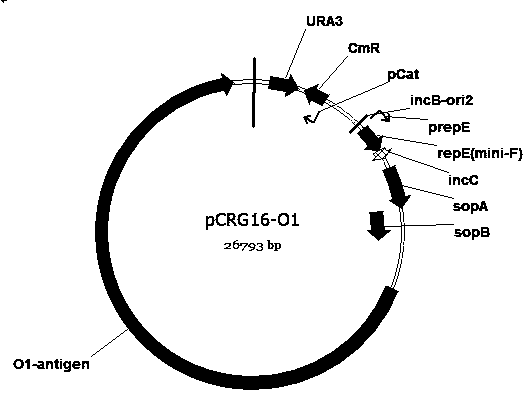
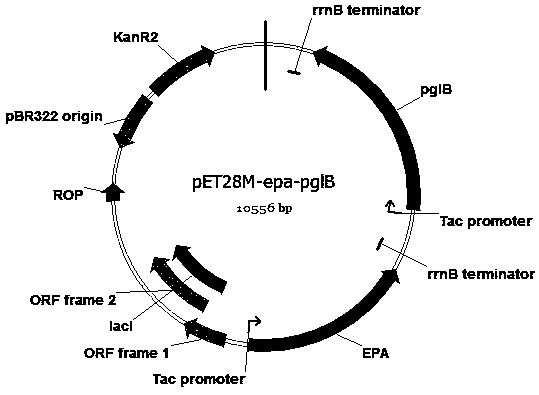
Escherichia coli engineering bacterium for synthesizing glycoprotein conjugate vaccines for neonatal meningitis escherichia coli and application
ActiveCN108774628AImproving immunogenicityStrong specificityAntibacterial agentsBacteriaEscherichia coliAntigen
The invention discloses an escherichia coli engineering bacterium for synthesizing glycoprotein conjugate vaccines for neonatal meningitis escherichia coli and application, and relates to a method forconstructing a cell factory for synthesizing O1 serum type glycoprotein conjugate vaccines for neonatal meningitis escherichia coli. O1 antigens are constructed based on a DNA Assembler method by utilizing efficient homologous recombination efficiency of saccharomyces cerevisiae so as to synthesize gene cluster plasmids; the O1 antigens are converted in escherichia coli JM109 to synthesize gene cluster shuttle plasmids, and the plasmids are identified by virtue of lipopolysaccharide extraction, gel electrophoresis and silver staining; waaL and wecA in the JM109 are deleted with the aid of FLP-FRT so as to eliminate the interference of original incomplete O antigens; and pET28a(+) plasmids are reconstructed and induced to synthesize the glycoprotein conjugate vaccines, the glycoprotein ispurified by virtue of an AKTA Primeplus protein purification workstation, and the glycoprotein is identified by virtue of western-blotting. The constructed recombinant escherichia coli provides a novel idea for synthesizing the glycoprotein conjugate vaccines by a biological method.
Owner:NANKAI UNIV
Subunit vaccine against feline infectious peritonitis virus and application
InactiveCN112691189AThe characteristics of overcoming the lack of immune antigenicityImprove the level ofSsRNA viruses positive-senseViral antigen ingredientsReceptorImmunogenicity
The invention discloses a subunit vaccine against feline infectious peritonitis virus and an application thereof. The vaccine is a fusion protein RBD-FTH formed by connecting a surface protein epitope sequence containing FIPV particles and ferritin through a flexible Linker. According to the fusion protein RBD-FTH1, RBD-FTH1 monomers can be assembled into spherical 24-mer nanoparticles through the self-assembly effect of ferritin, and antigens in a RBD region are displayed on the surfaces of the nanoparticles, thereby causing stronger immune response of a receptor. According to the invention, the RBD of the FIPV is used as antigen fragments to achieve multimerization of the antigens based on the ferritin, thereby overcoming insufficient immunogenicity of RBD monomers, effectively causing stronger immune response and significantly improving antibody neutralization level of a host against FIPV.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com