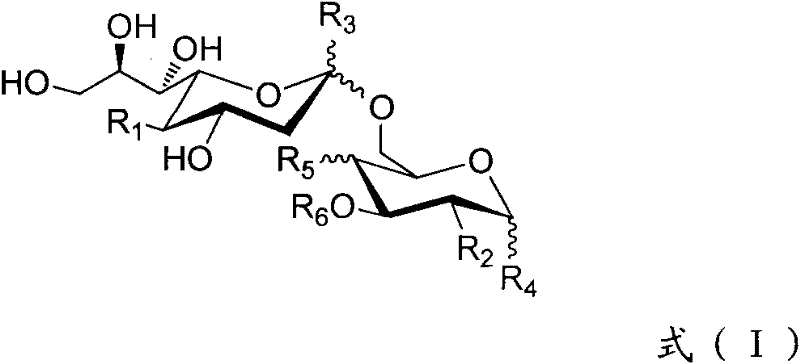
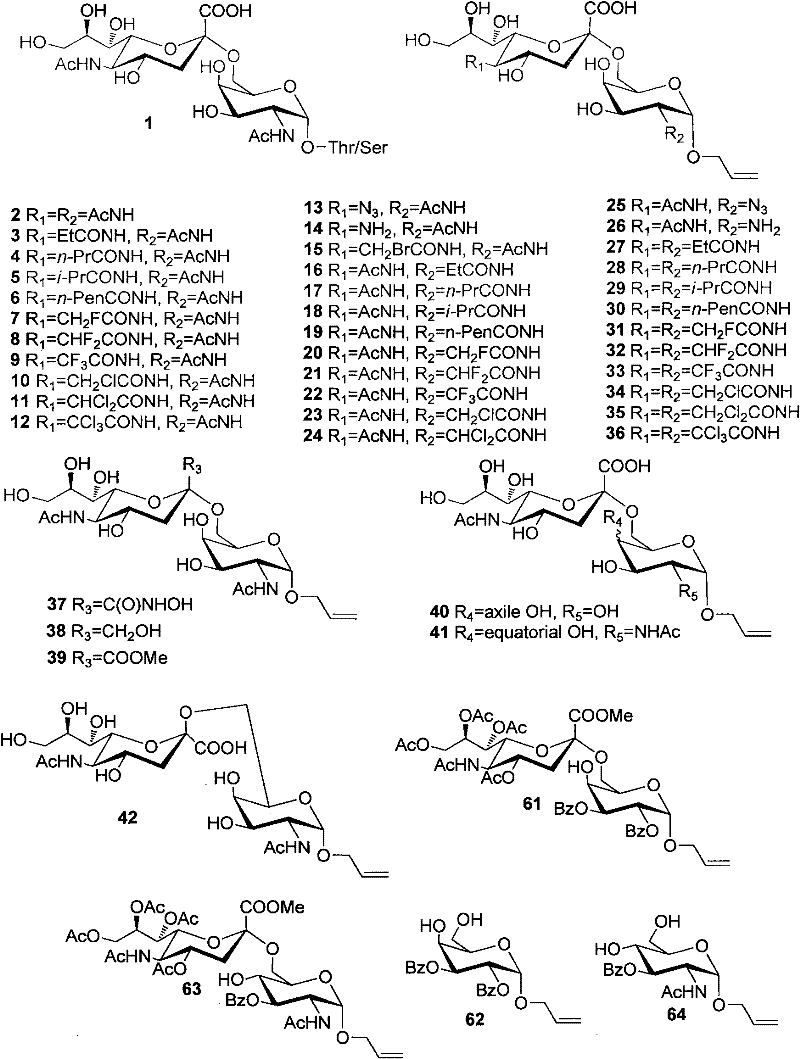
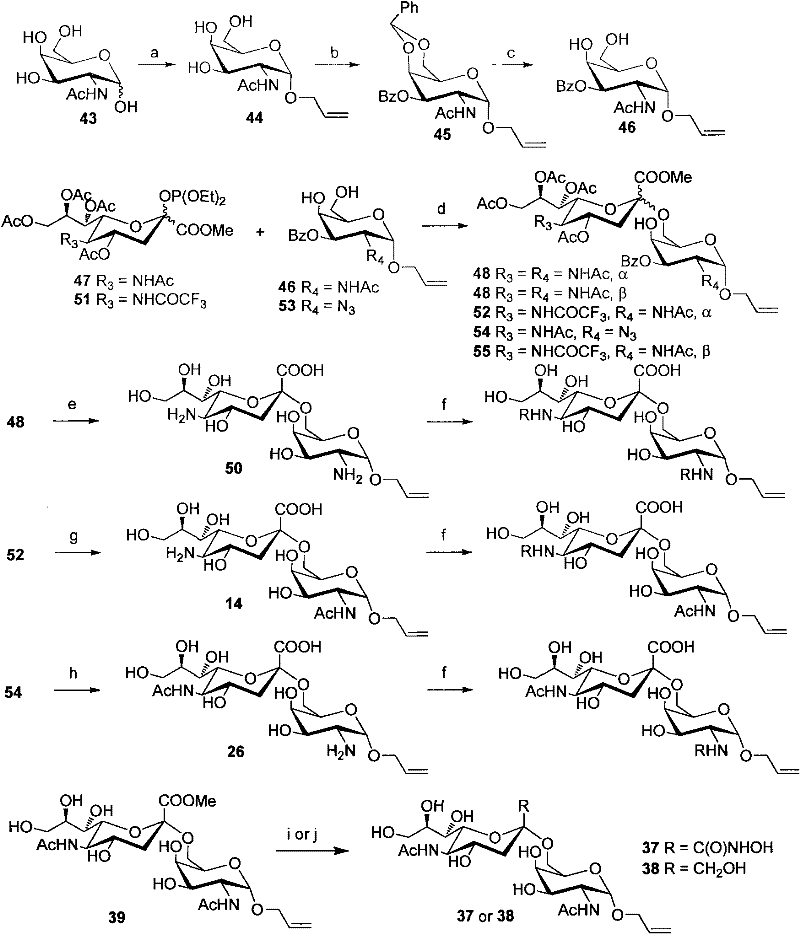
Sialic acid (α-(2→6))-d-pyranose derivative and its synthesis method and application
A technology of sialic acid and derivatives, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problem of ineffective tumor antigens, and achieve effective immune response, IgG/IgM improvement, and good activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The synthesis of embodiment 1 compound 2
[0040] Compound 48 (see Example 42 for its synthesis) (100 mg, 0.119 mmol) was dissolved in 10 mL of methanol, NaOMe / MeOH solution (30%, 0.02 g, 0.11 mmol) was added, and stirred at room temperature for 0.5 hours. After adjusting the pH of the reaction system to 3 with 1N HCl / MeOH, the reaction solution was concentrated in vacuo. The primary product was passed through a P-2 column and eluted with water. Get pure. Yield 95%.
[0041] 1 H-NMR (400MHz, D 2 O) δ5.90-5.77 (m, 1H), 5.22 (dd, 1H, J 1 = 1.6Hz,J 2 = 17.6Hz), 5.14 (d, 1H, J = 10.4Hz), 4.75 (d, 1H, J = 3.6Hz, anomeric H of GalNAc), 4.15 (dd, 1H, J 1 = 4.0Hz,J 2 = 13.6Hz), 4.05(dd, 1H, J 1 = 4.0Hz,J 2 =10.8Hz), 3.96-3.87(m, 3H), 3.82-3.74(m, 5H), 3.60-3.48(m, 4H), 3.46(dd, 1H, J 1 = 1.6Hz,J 2 =8.8Hz), 2.59(dd,1H,J 1 = 4.4Hz,J 2 = 12.4Hz, siaH-3eq), 1.91(s, 6H), 1.56(t, 1H, J=12.4Hz, siaH-3ax); 13 C-NMR (75MHz, D 2 O) 175.59, 175.16, 173.99, 134.221, 118.64,...
Embodiment 2
[0042] The synthesis of embodiment 2 compound 3
[0043] Compound 14 (allyl 4-O-(5-amino-3,5-dideoxy-α-D-pyranoneuraminyl)-2-acetylamino-2-deoxy-α-D- Galactopyranoside, its synthesis see Example 13) as reaction raw material 10mg, be dissolved in 1mL methanol, add 2-3mg NaHCO 3 , add a drop of the corresponding acid anhydride (propionic anhydride, about 5 μl) under ice-cooling, after stirring for one hour, add another drop of acid anhydride, TLC shows that most of the raw materials are converted. The reaction was overnight and gradually warmed to room temperature. TLC showed the reaction was complete. Strengthen the acidic resin to neutralize NaOH, filter with suction, concentrate the solvent, pass through a Biogel-P2 column, and elute with water. Then pass through a C18 column and elute with water-water / methanol. 8-9 mg of product was obtained. (Yield 75-85%).
[0044] 1 H-NMR (500MHz, D 2 O) δ6.02-5.96 (m, 1H), 5.35 (dq, 1H, J = 1.5Hz, 16.5Hz), 5.29-5.24 (m, 1H), 4.93...
Embodiment 3
[0045] The synthesis of embodiment 3 compound 4
[0046] It is prepared from compound 14 and n-butyric anhydride, and the specific operation steps are the same as those of compound 3. (Yield 75-85%).
[0047] 1 H-NMR (500MHz, D 2 O) δ6.02-5.96 (m, 1H), 5.35 (ddd, 1H, J 1 = 1.5Hz,J 2 = 3.0Hz,J 3 =17.0Hz), 5.26(dd,1H,J 1 = 1.0Hz,J 2 = 3.0Hz,J 3 = 10.5Hz), 4.92 (d, 1H, J = 3.5Hz, anomeric H of GalNAc), 4.22 (ddt, 1H, J 1 =J 2 = 1.5Hz,J 3 =5.5Hz,J 4 =13.0Hz), 4.15(dd,1H,J 1 = 3.5Hz,J 2 =11.0Hz), 4.06(dd,1H,J 1 = 4.0Hz,J 2 =8.0Hz), 4.04-4.00(m, 2H), 3.93-3.80(m, 5H), 3.71-3.61(m, 4H), 3.56(dd, 1H, J 1 =1.5,J 2 =9.0Hz), 2.73(dd,1H,J 1 = 4.5Hz,J 2 =12.5Hz, siaH-3eq), 2.26(t, 2H, J=7.5Hz), 2.03(s, 3H), 1.68(t, 1H, J 1 =J 2 =12.5Hz, siaH-3ax), 1.60(hexad, 2H, J=7.5Hz), 0.91(t, 3H, J=7.5Hz); 13 C-NMR (75MHz, D 2 O) δ178.88, 175.31, 174.08, 134.40, 118.81, 101.08, 96.98, 73.36, 72.47, 70.26, 69.45, 69.22, 69.10, 68.80, 68.29, 64.46, 63.35, 51.50, 50.56, 39.5 13.56...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com