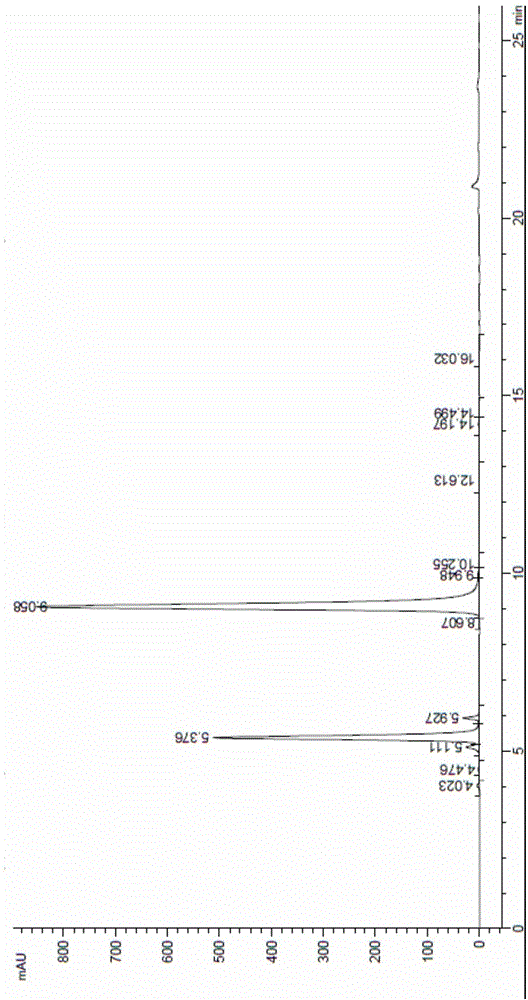
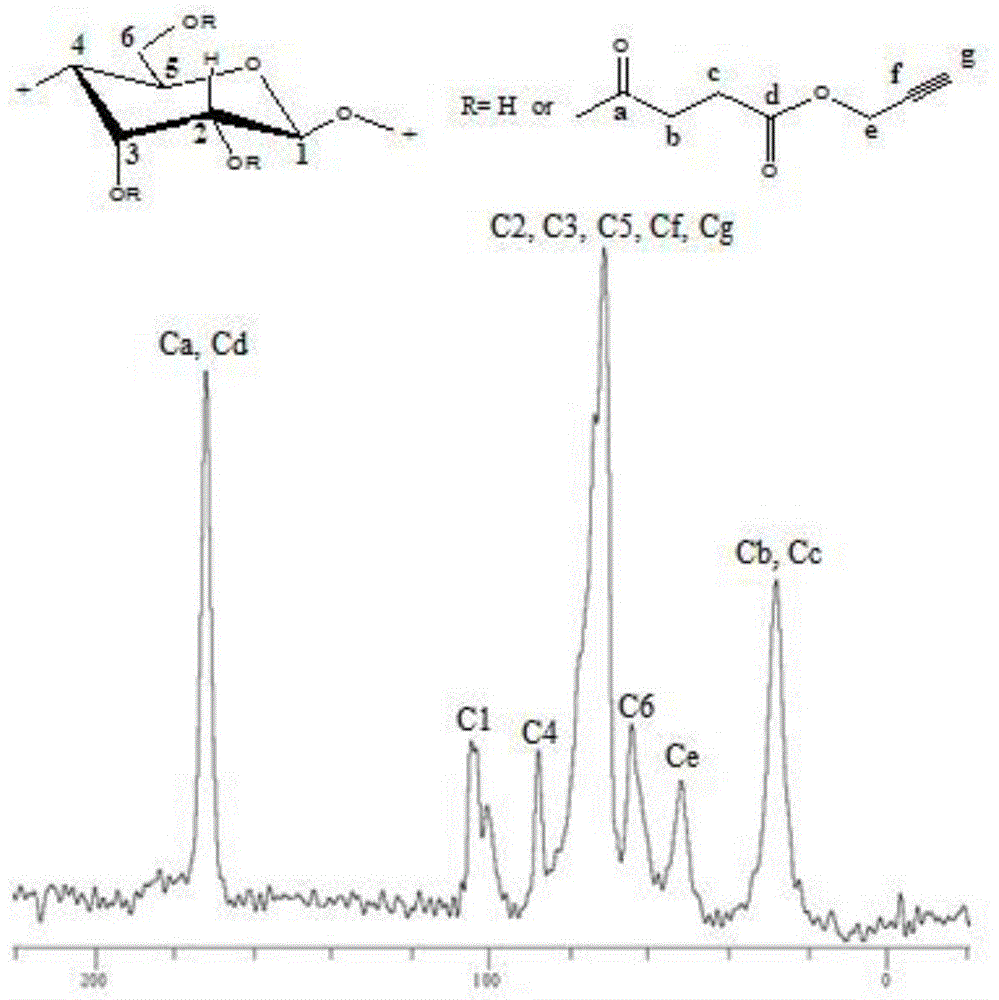
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
63 results about "Butyric anhydride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Butyric anhydride (butanoic anhydride) is a chemical compound with the formula (CH₃CH₂CH₂CO)₂O. It is a clear colorless liquid that smells strongly of butyric acid, formed by its reaction to moisture in the air.
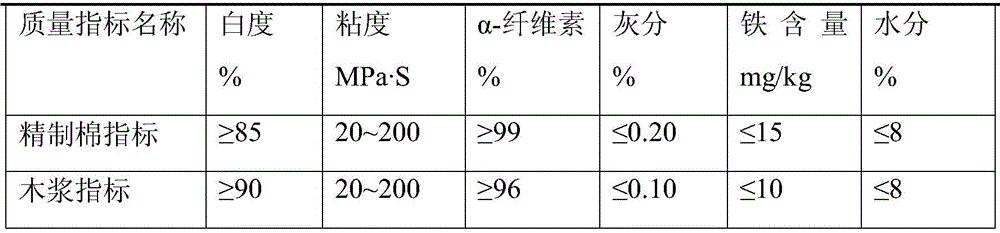
Preparation method of medium-viscosity cellulose acetate butyrate
The invention discloses a preparation method of medium-viscosity cellulose acetate butyrate. The preparation method comprises the following steps of pulverizing wood pulp; uniformly spraying a mixture of acetic acid and butyric acid into the pulverized wood pulp, and activating for 1h to 6h at the normal temperature; cooling a mixture of acetic anhydride, butyric anhydride, butyric acid and a liquid acid catalyst to minus 5 DEG C to minus 20 DEG C for standby use; slowly throwing the activated wood pulp into the well-cooled mixture, and maintaining the temperature to be less than or equal to 65 DEG C; adding magnesium acetate solution into the reacted system, and adding 200 to 500 parts by weight of acetic acid with the mass percentage of 50 to 70 percent for hydrolysis, wherein the system temperature is controlled at 60 to 70 DEG C, and the hydrolysis time is 2h to 5h; continuing adding magnesium acetate to neutralize the liquid acid catalyst, and adding the material into water for chromatographic solid-liquid separation; washing the separated CAB (cellulose acetate butyrate) solids with water until the acid value is less than 300ppm, and drying the CAB solids to obtain the CBA finished product. Through the method, a method for preparing medium / high-viscosity CAB is developed, and the content of butyryl in CAB is 36 to 40 percent; the power viscosity is 400cps to 1000cps.
Owner:JIANGSU RUICHEN CHEM
Cellulose acetate butyrate mixed ester with high butyryl content, and preparation method thereof
The invention discloses cellulose acetate butyrate mixed ester with high butyryl content, and a preparation method thereof. The cellulose ester is prepared from the following components in parts by weight: 600 parts of wood pulp, 800-1200 parts of butyric acid, 2500-3500 parts of butyric anhydride, 20-40 parts of acetic anhydride, and concentrated sulfuric acid catalyst accounting for 5-10% of total weight parts of wood pulp. The preparation method comprises the following steps: activating, performing esterification reaction, mixing, adding magnesium acetate solution, hydrolyzing, performing chromatography, and then sequentially conventionally filtering, washing and drying to obtain a finished product. According to cellulose acetate butyrate mixed ester with high butyryl content, cellulose acetate butyrate with the butyryl content of 48-53% and acetyl content of less than or equal to 4% can be obtained after the combination according to specific formula and proportion, and the product is stable in performances. The preparation method is simple in steps, the preparation period is short, the preparation conditions are not strict and easily achieved, and especially, the activated solid is fed into a cooling liquid within 2-5min, so that the quality of products can be guaranteed while the fast follow-up reaction is guaranteed.
Owner:JIANGSU RUICHEN CHEM
Production method of spherical cellulose acetate butyrate
The invention belongs to the technical field of organic acid ester cellulose preparation, in particular relates to a production method of spherical cellulose acetate butyrate, and solves the technical problem of low product yield. The production method comprises the following steps: a, treating velvet wood pulp or cellulose acetate grade refined cotton by acetic acid; b, mixing butyric anhydride, acetic anhydride and concentrated sulfuric acid, cooling to obtain cooled mixed liquid, adding the treated velvet wood pulp or cellulose acetate grade refined cotton into the cooled mixed liquid, and adding a solvent for acylation; c, adding hydrolysis liquid into acylation reaction liquid for hydrolysis, and neutralizing; d, precipitating, hardening, performing solid-liquid separation, washing, stewing, and drying to obtain a spherical cellulose acetate butyrate product. According to the method provided by the invention, the apparent viscosity of materials in reaction liquid can be effectively reduced, the mixing effect of the materials can be improved, the reaction performance of the materials can be improved, and the reaction yield of the materials can be increased.
Owner:SICHUAN NITROCELLULOSE CORP
Preparation method for biomass-based carbon nanofiber
InactiveCN111197187AImprove plasticityReduce hydrogen bondingMaterial nanotechnologyFibre chemical featuresMeth-Spinning
The invention discloses a preparation method for a biomass-based carbon nanofiber. The method particularly includes the following steps: first, mixing lignin with butyric anhydride and 1-methylimidazole, stirring under a nitrogen atmosphere for reaction, washing with n-hexane, then placing in deionized water, stirring, performing extraction filtration, and drying to obtain esterification modifiedlignin; dissolving polyacrylonitrile and the esterification modified lignin in a N, N-dimethylformamide solution to obtain a spinning solution; performing electrostatic spinning on the spinning solution to prepare a carbon nanofiber precursor, performing pre-oxidation on the carbon nanofiber precursor in a muffle furnace, and then performing carbonization treatment to obtain the biomass-based carbon nanofiber.The biomass-based carbon nanofiber prepared by the method of the invention is used as an electrode material of a super-capacitor, is excellent in electrochemical performance, and can meetapplication requirements in the fields of electric automobiles, high-load industries, new energy power storage, and the like.
Owner:XIAN UNIV OF TECH
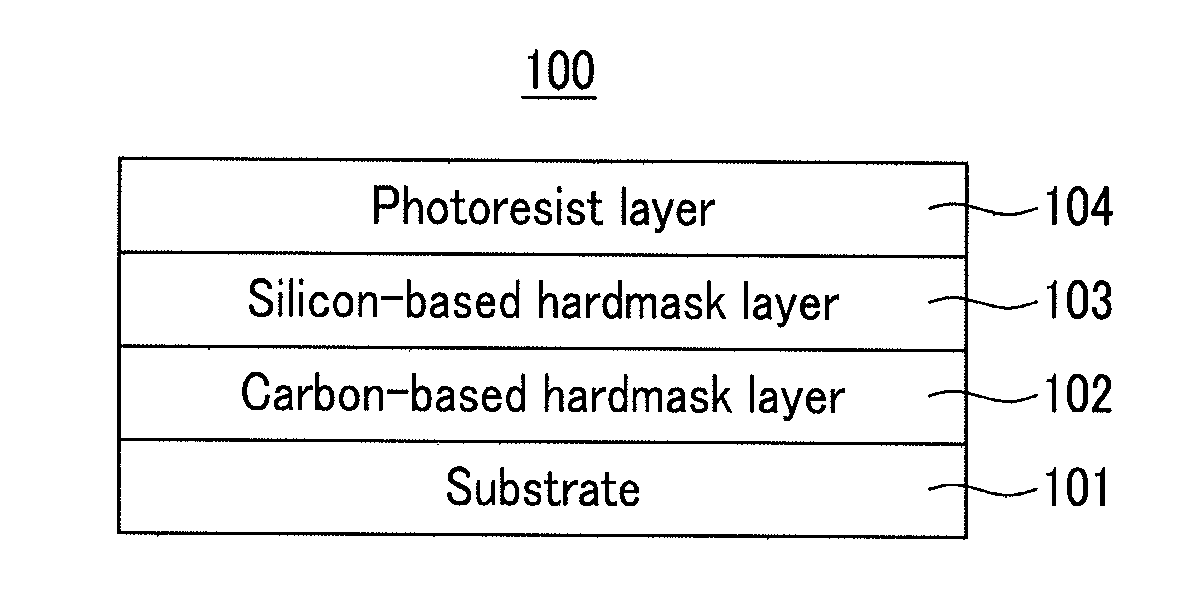
Hardmask composition for forming resist underlayer film, process for producing a semiconductor integrated circuit device, and semiconductor integrated circuit device
InactiveUS20110241175A1Good coating performanceExquisite patternPhotomechanical apparatusSemiconductor/solid-state device manufacturingResistButyric anhydride
A hardmask composition for forming a resist underlayer film, a process for producing a semiconductor integrated circuit device, and a semiconductor integrated circuit device, the hardmask composition including an organosilane polymer, and a stabilizer, the stabilizer including one of acetic anhydride, methyl acetoacetate, propionic anhydride, ethyl-2-ethylacetoacetate, butyric anhydride, ethyl-2-ethylacetoacetate, valeric anhydride, 2-methylbutyric anhydride, nonanol, decanol, undecanol, dodecanol, propylene glycol propyl ether, propylene glycol ethyl ether, propylene glycol methyl ether, propylene glycol, phenyltrimethoxysilane, diphenylhexamethoxydisiloxane, diphenylhexaethoxydisiloxane, dioctyltetramethyldisiloxane, hexamethyltrisiloxane, tetramethyldisiloxane, decamethyltetrasiloxane, dodecamethylpentasiloxane, hexamethyldisiloxane, and mixtures thereof.
Owner:CHEIL IND INC
Preparation method for cellulose acetate-butyrate
InactiveCN102898530AUniform degree of substitutionGood reproducibilityCellulose acetateButyric anhydride
The invention provides a preparation method for cellulose acetate-butyrate. The preparation method comprises the following concrete steps: (1) stirring butyric acid, acetic acid and refined cotton linter in a mixer and carrying out activation at a temperature of 80 to 95 DEG C for 2 to 4 h; (2) adding butyric anhydride into the activated refined cotton linter and carrying out esterification at a temperature of 80 to 95 DEG C for 4 to 6 h under stirring, with sulfuric acid used as a catalyst; (3) after esterification, adding an aqueous solution of butyric acid into esterification liquid and carrying out hydrolysis at a temperature of 80 to 95 DEG C for 4 to 6 h under stirring; and (4) after hydrolysis, maintaining stirring, adding sodium butyrate to neutralize sulfuric acid, adding the aqueous solution of butyric acid for dilution, dumping an obtained diluted solution into water under stirring for precipitation and rinsing and drying an obtained product. The method provided by the invention has the advantages of a simple process, conservation of raw materials, optimized steps and low cost.
Owner:WUXI KAILI PHARMA
Method for preparing cellulose acetate butyrate microsphere adsorption material
InactiveCN103990439AAvoid deformationImprove molding rateOther chemical processesAlkali metal oxides/hydroxidesCelluloseMicrosphere
The invention relates to a method for preparing a cellulose acetate butyrate microsphere adsorption material by an ionic liquid as a solvent. The method comprises that cellulose is dissolved in an ionic liquid, the cellulose solution reacts respectively with butyric anhydride and acetic anhydride to produce cellulose acetate butyrate products having different substitution degrees, the cellulose acetate butyrate solution is condensed to form liquid drops by reversed phase suspension and programmed cooling, a curing agent is added into the cellulose acetate butyrate liquid drops to cure the liquid drops so that microspheres are formed, and the ionic liquid is removed by distilled water washing so that cellulose acetate butyrate microspheres are obtained. The method has simple processes, realizes recycle of the solvent, utilizes the cellulose raw material having a wide source and a low cost, and realizes high-value utilization of natural plant cellulose. The cellulose acetate butyrate microspheres prepared by the method have acetyl content of 3-20% and butyryl group content of 5-43%, have controllable acyl content and have regular spherical shapes. 95% of the cellulose acetate butyrate microspheres have grain diameters of 50-200 microns. The cellulose acetate butyrate microspheres have good mechanical properties, good thermostability and acid and alkali resistance and are good carriers and adsorption materials.
Owner:中国科技开发院广西分院 +1
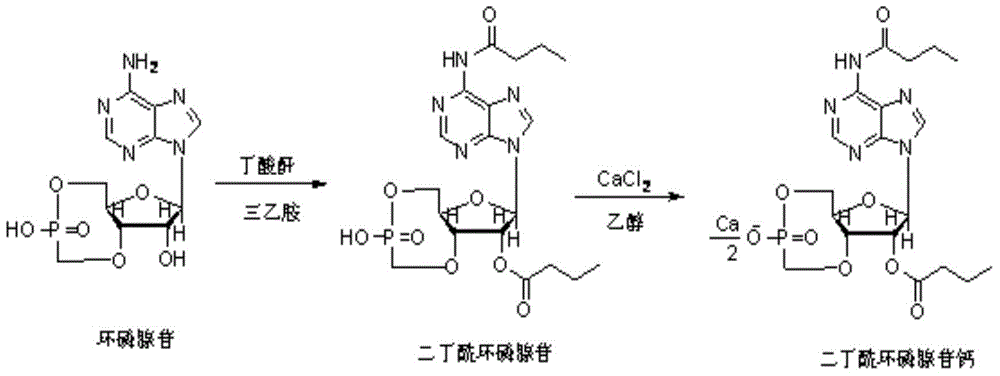
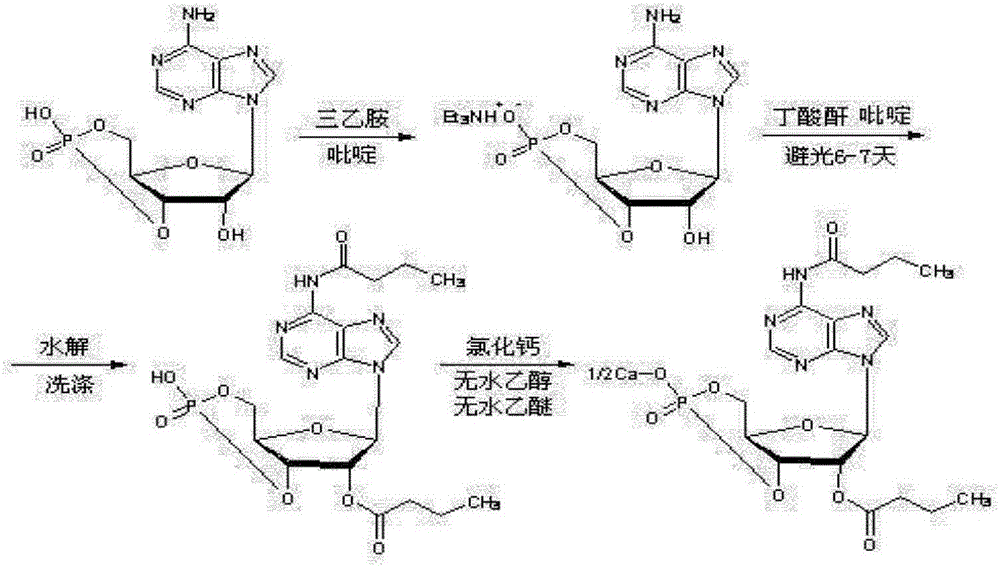
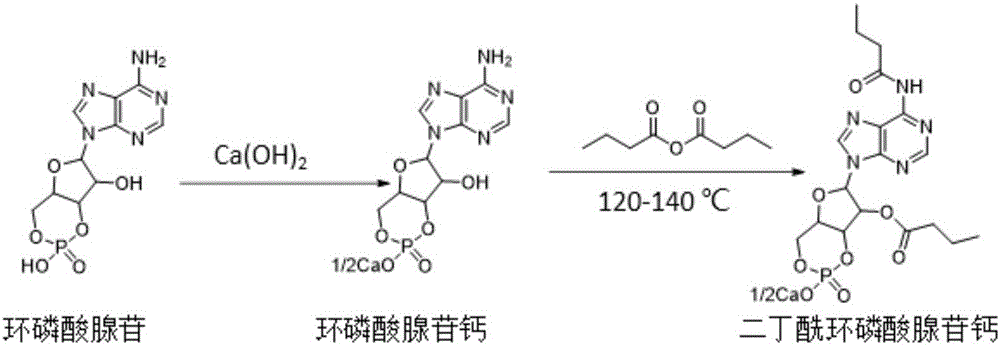
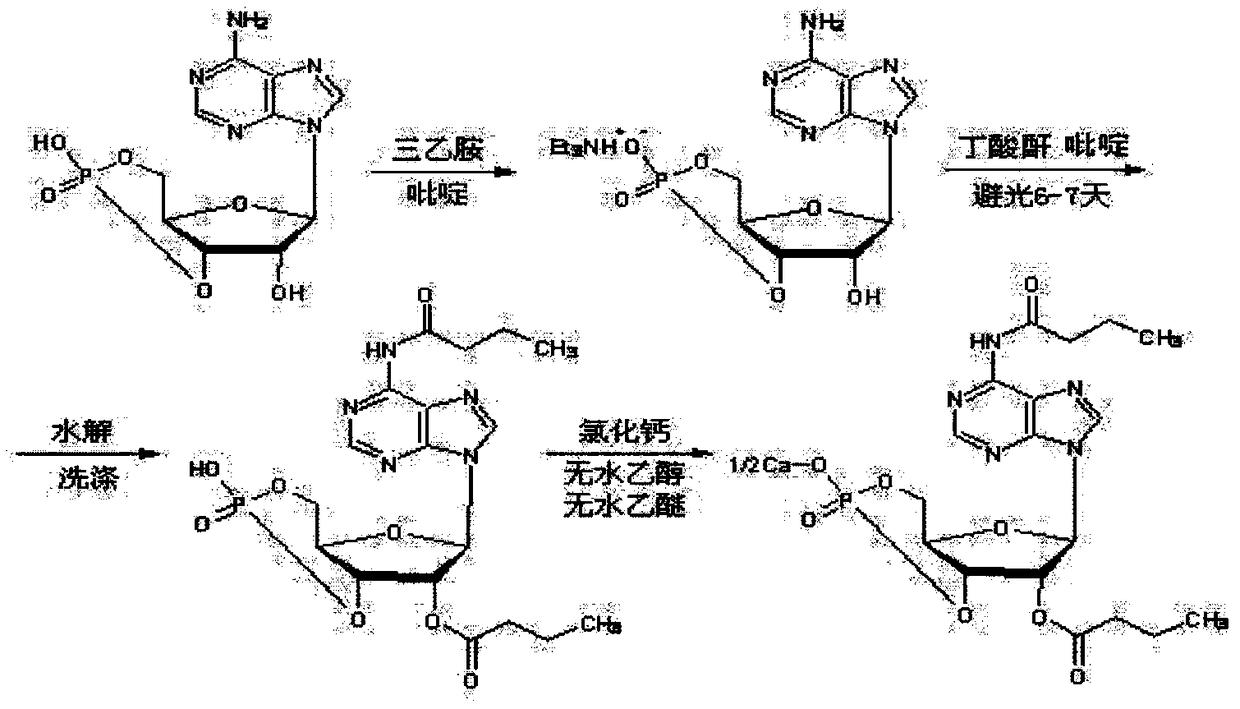
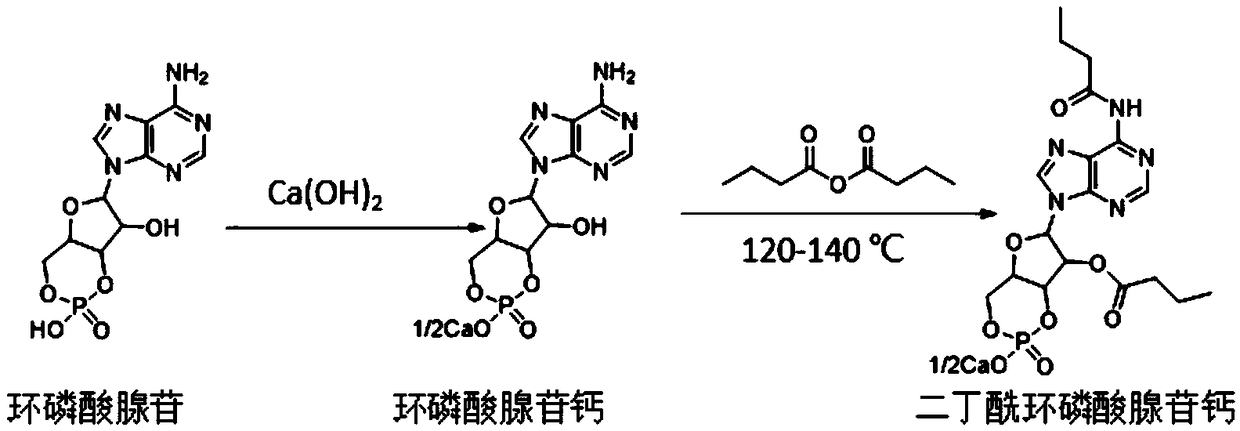
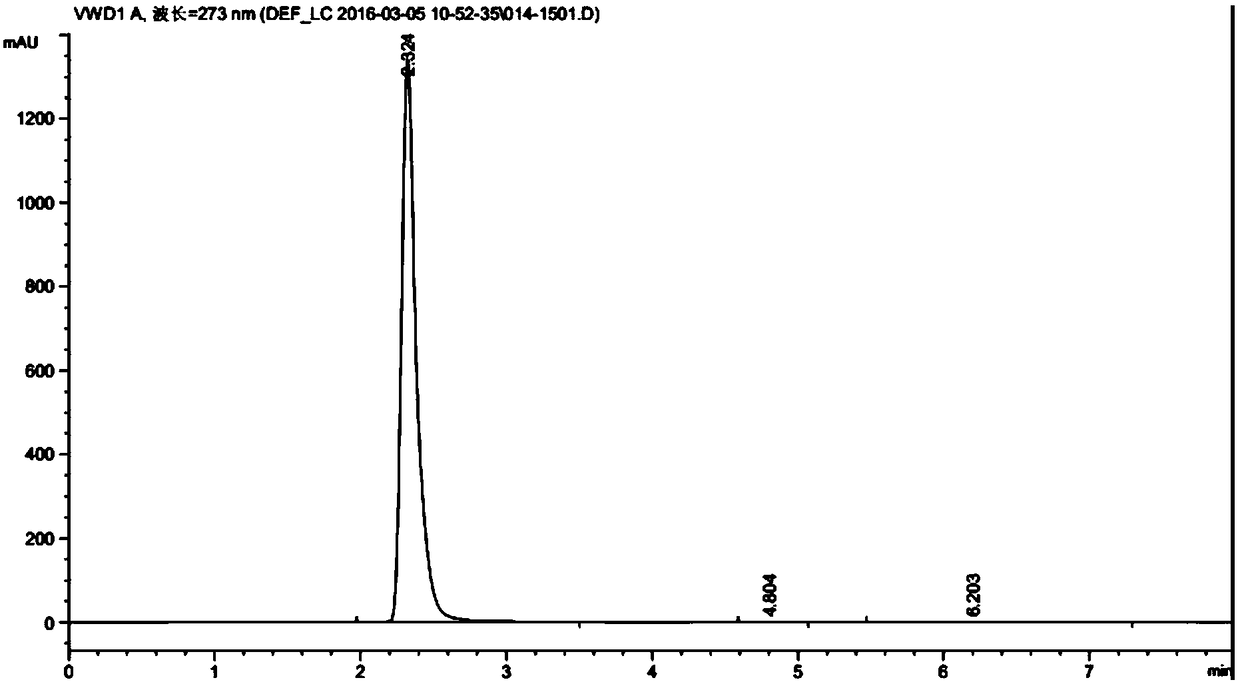
Method for preparing calcium dibutyryladenosine cyclophosphate
ActiveCN105566424AShorten the production cycleHigh yieldSugar derivativesSugar derivatives preparationButyric anhydrideAdenosine
The invention relates to the technical field of drug synthesis, in particular to a method for preparing calcium dibutyryladenosine cyclophosphate. The preparation method comprises the following steps: taking adenosine cyclophosphate as a raw material, and preparing adenosine cyclophosphate triethylamine salt with triethylamine under an aprotic solvent; taking butyric anhydride as an acylating agent, and synthesizing the calcium dibutyryladenosine cyclophosphate in an organic aprotic solvent. Compared with the traditional process, the method has the advantages that the production period is shortened from the original 9-10 days to 3-4 days, the yield of crude products is increased to 78-82 percent, the purification yield is increased to 90-94 percent, and the total reaction yield is increased to 70-75 percent. The method disclosed by the invention adopts a continuous washing method and performs reversed phase extraction, and the liquid chromatogram purity of the obtained dibutyryladenosine cyclophosphate is 98-98.5 percent.
Owner:SHANGHAI ZIYUAN PHARMA
Preparation method of cellulose nanometer crystal with functionalized alkynyl on surface
The present invention relates to a preparation method of a cellulose nanometer crystal with functionalized alkynyl on the surface. The preparation method comprises: 1) preparing 4-oxo-4-(prop-2-yn-1-oxy)butyric anhydride; 2) carrying out ultrasonic and uniform mixing reaction according to a mass ratio of the cellulose nanometer crystal to the 4-oxo-4-(prop-2-yn-1-oxy)butyric anhydride of 1:5-1:20, wherein the mass of a catalyst is 0.1-20% of the mass of the 4-oxo-4-(prop-2-yn-1-oxy)butyric anhydride, the reaction temperature of 60-100 DEG C, and the reaction time is 2-8 h; and 3) after completing the reaction, cooling to a room temperature to obtain a primary product, washing a plurality of times, and drying to obtain the cellulose nanometer crystal with the functionalized alkynyl on the surface, wherein the surface hydroxyl substitution degree is 4.5-13.8%. The preparation method of the present invention has advantages of low-cost, environmental-protection and completely-biodegradable raw materials, simpleness, rapidness, and high efficiency.
Owner:WUHAN UNIV OF TECH
Hydrophobized polysaccharide, and preparation method and application thereof
The invention especially relates to a hydrophobized polysaccharide, and a preparation method and application thereof, belonging to the technical field of organic synthesis. The hydrophobized polysaccharide is prepared by reacting the hydroxyl group of glucan with succinic anhydride to prepare glucan butyrate with a butyric acid group at first and then reacting glucan butyrate with dicyclohexylcarbodiimide (DCC) so as to allow a carboxyl group to be modified by dicyclohexylurea (DCU); so the terminal group of the hydrophobized polysaccharide is modified by DCU, and butyric acid is used as a linking arm for connection with glucan via an ester bond. According to the preparation method, glucan or other polysaccharide is used as a raw material and reacts butyric anhydride and DCC to prepare the hydrophobized polysaccharide. The prepared hydrophobized polysaccharide has a molecular weight in a range of 20 to 500 K, has self-emulsifying performance and is applicable as a proteinic drug, especially as a carrier for amino acid and vaccine drugs.
Owner:BENGBU MEDICAL COLLEGE
Butyrate starch preparing method
ActiveCN109400725ASolve the problem of irritating odor and volatilePrevent volatilizationFood ingredient functionsButyric anhydrideLarge intestine
The invention discloses a butyrate starch preparing method and belongs to the technical fields of preparation and application of modified starch and deep processing of agricultural products. Accordingto the butyrate starch preparing method, starch serve as the principal raw material for esterification with butyric anhydride to achieve stabilization of butyric acid and small releasing of the butyric acid in gastric juice; resistant starch prepared through esterification of the butyric acid and the starch can well avoid being absorbed in small intestines, resist digestion by enzymes secreted bybrush borders, selectively release micromolecular effective load in large intestines; fermented by enteric microorganisms, the resistant starch can produce more short-chain fatty acids and probiotics, thereby improving the bioavailability of the butyric acid and expanding the application range of the starch. Compared with other butyric acid products, the butyrate starch is odorless and can be esterified into the resistant starch to convey the butyric acid to the rear end of the intestines; the butyrate starch can be fermented by the enteric microorganisms and decomposed by bacterial esteraseinto butyric acid and other short-chain fatty acids and increase the content of the probiotics, thereby belonging to probiotic products.
Owner:JIANGNAN UNIV
Lithium-manganese primary battery
InactiveCN109301274AImprove discharge characteristicsImprove working environment temperatureOrganic electrolyte cellsGlutaric anhydrideHigh temperature storage
For solving the problems that a lithium-manganese primary battery in the prior art generates gas and influences the discharge characteristic under a high temperature environment, the invention provides a lithium-manganese primary battery, which includes a cathode, an anode and an electrolyte. The cathode includes MnO2. The electrolyte includes an additive. The additive includes an acid anhydride compound being one or more of: maleic anhydride, methyl maleic anhydride, dimethyl maleic anhydride, succinic anhydride, glutaric anhydride, methyl succinic anhydride, dimethyl succinic anhydride, acetic anhydride, propionic anhydride and butyric anhydride. By means of the specific acid anhydride compound, the high-temperature storage performance of the lithium-manganese primary battery is effectively improved.
Owner:SHENZHEN CAPCHEM TECH CO LTD
Preparation method of high-molecular-weight cellulose acetate butyrate
ActiveCN112521516AHigh molecular weightSave operating timeChemical recyclingPtru catalystButyric anhydride
The invention discloses a preparation method of high-molecular-weight cellulose acetate butyrate, which comprises the steps of 1) conducting activation, specifically, soaking cellulose powder in acetic acid to obtain activated cellulose powder; 2) conducting esterification, specifically, enabling the activated cellulose powder to react with acetic anhydride and butyric anhydride under the condition of an acid catalyst to obtain an esterification reaction solution; 3) conducting neutralization, specifically, adding a neutralizer into the esterification reaction liquid to neutralize the acid catalyst into salt; and 4) conducting reduction, specifically, adding sodium triacetoxyborohydride into the neutralized esterification reaction solution to partially reduce the ester group into hydroxyl,thereby obtaining the high-molecular-weight cellulose acetate butyrate. The method avoids cellulose acetate butyrate glucose unit breakage and molecular degradation caused by hydrolysis by adding water, and also avoids the problems of difficult separation and high metal content caused by adding a heterogeneous catalyst.
Owner:WANHUA CHEM GRP CO LTD
The preparation method of monoglyceride butyrate
ActiveCN104086422BPromote generationImprove responseMolecular sieve catalystsOrganic compound preparationMonoglycerideN-Butyric acid
The invention discloses a preparation method for glyceryl tributyrate. The preparation method comprises: taking glycerin and n-butyric acid as raw materials, adding a catalyst and heating to 100-110 DEG C with stirring, performing heating reflux as well as dropwise adding butyric anhydride, stopping reaction until no water is generated in the reaction solution during dropwise adding, cooling to room temperature, and filtering, so as to obtain glyceryl tributyrate. The catalyst is a mixture of sulfonic-acid mesoporous molecular sieve and ZSM-5 acidic zeolite, and the usage amount of the catalyst is 2-10% by weight of the sum of glycerin and n-butyric acid. By taking sulfonic-acid mesoporous molecular sieve and ZSM-5 acidic zeolite as the catalyst, the selectivity is high, the reaction time is shortened, the generation of by-products is reduced and the catalyst is reusable. By employing the method, the reaction system does not need a water-carrying agent, so that the reaction cost and safe hidden trouble are reduced, the purification step of the product is simplified, the post-processing process is simple, environment is protected and energy is saved. The obtained glyceryl tributyrate is high in purity and high in yield, the reaction speed is fast, and industrial production is easy to realize.
Owner:HUBEI XINZHOU CHEM
Method of synthesizing 2,2,4,4-tetramethyl-1,3-cyclobutadione
InactiveCN111718252AFor subsequent applicationOrganic compound preparationKetenes preparationButyric anhydrideCombinatorial chemistry
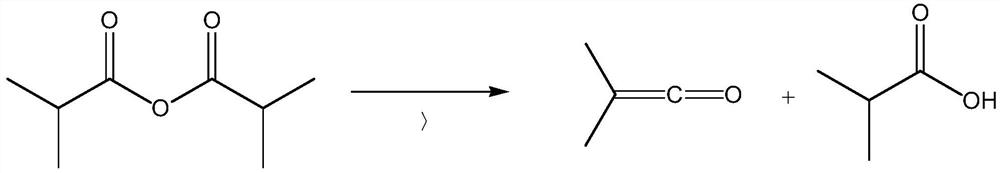
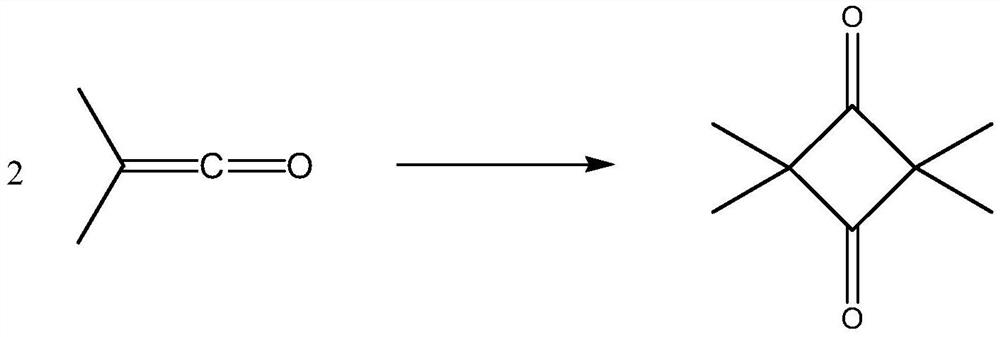
The present disclosure provides a process for synthesizing 2, 2, 4, 4-tetramethyl-1, 3-cyclobutanedione, comprising the steps of (a) cleaving isobutyric anhydride to form a dimethyl ketene-containinggas; (b) introducing gas containing dimethyl ketene into an ester solvent to dissolve the dimethyl ketene in the ester solvent to form an ester solution containing the dimethyl ketene; and (c) standing the ester solution containing the dimethyl ketene under inert gas, so that the dimethyl ketene is dimerized to form the 2, 2, 4, 4-tetramethyl-1, 3-cyclobutanedione. The synthetic method of the present invention is a synthetic method of collecting DMK generated by cracking IBAN in a new manner and dimerizing DMK to form TMCD, thereby being capable of basically overcoming various defects in the prior art.
Owner:IND TECH RES INST
Preparation method of phenolic resin composite material for storage tank
The invention provides a preparation method of a phenolic resin composite material for a storage tank. The method comprises the steps that 1, microcrystalline cellulose is dissolved in ionic liquid, the mixture is placed in water bath to be stirred, a cellulose solution is obtained, butyric anhydride is added into the cellulose solution to conduct the reaction, acetic anhydride is added to conduct the reaction continuously, extracting, sedimentation, washing and drying are conducted, and cellulose acetate butyrate is obtained; 2, a coupling agent is dissolved in ethanol, nano-silica is added into an ethanol solution with the coupling agent and taken out for drying, and modified nano-silica is obtained; 3, phenolic resin is dissolved in dimethyl sulfoxide, curing agents, curing catalysts, release agents, toughening agents, glass fibers, the cellulose acetate butyrate and the modified nano-silica are added into the dimethyl sulfoxide with the phenolic resin, ultrasonic agitation and vacuum defoamation are conducted, and a mixture is obtained; 4, the mixture is poured into a mold, after solidification is completed, the mixture is naturally cooled to a room temperature, and the composite material is obtained by taking the material out of the mold. According to the preparation method of the phenolic resin composite material for the storage tank, the composite material is better in low temperature resistance and better in basic performance at low temperatures.
Owner:青岛澳科仪器有限责任公司 +1
A kind of method that improves caprolactone yield
The present invention discloses a method for improving the yield of caprolactone, and anhydride substances such as acetic anhydride, propionic anhydride, butyric anhydride, valeric anhydride, hexanoic anhydride, succinic anhydride, glutaric anhydride, adipic anhydride, phthalic anhydride and the like are added in the last phase of the reaction for production of caprolactone from cyclohexanone and a peracid to improve the yield of caprolactone. The method is simple and easy to implement and suitable for industrial application, can greatly reduce the production cost of the caprolactone, and brings huge economic benefits.
Owner:CHINA PETROLEUM & CHEM CORP
Calcium dibutyryladenosine cyclophosphate preparation method
ActiveCN106336439ALow costShort reaction timeSugar derivativesSugar derivatives preparationAdenosineButyric anhydride
The invention discloses a calcium dibutyryladenosine cyclophosphate preparation method, which is characterized by comprising: (1) adding adenosine cyclophosphate and calcium hydroxide to water according to a molar ratio of 1:1-1.2, carrying out a reaction for 30-40 min, filtering to remove insoluble matters, adding ethanol having the volume 2-3 times the volume of the filtrate to the filtrate, filtering after a calcium adenosine cyclophosphate salt is precipitated, and carrying out vacuum drying at a temperature of 60-80 DEG C to obtain calcium adenosine cyclophosphate; (2) adding the calcium adenosine cyclophosphate obtained in the step (1) and butyric anhydride to a reaction kettle according to a ratio of 1 g:7-12 ml, protecting with nitrogen, and carrying out a stirring reaction for 1-3 h; and (3) adding 0-4 DEG C water having the volume 3-4 times the volume of the reaction liquid obtained in the step (2) to the reaction liquid, stirring for 30-40 min, carrying out nano-filtration on the reaction liquid, concentrating the trapping liquid, and carrying out freeze drying or spray drying to obtain the calcium dibutyryladenosine cyclophosphate. According to the present invention, the selectivity of the obtained calcium dibutyryladenosine cyclophosphate is more than 90%, the reaction time is short, the reaction process is simple and environmental friendly, and the method is suitable for industrial application.
Owner:NANJING UNIV OF TECH
Brass surface passivation treating agent and application method thereof
InactiveCN104120419AImprove the lubrication effectAvoid the phenomenon of ring cracksMetallic material coating processesButyric anhydrideBottle
The invention discloses a brass surface passivation treating agent and an application method thereof, and relates to the technical field of lubrication of metal parts. The application method comprises the following steps: passivant is prepared; 200-250 g / L of butyric anhydride, 30-50 g / L of nitric acid and 300 g / L of zinc stearate solution are put in a preparation bottle for preparing the passivant; the temperature of preparing the passivant is controlled at 20 DEG C; parts are flushed by hot water at a temperature of 60-120 DEG C, are secondarily cleaned by using cold water after the flushing is finished, and are put in a passivation pool to perform the passivation treatment for 5-10 S after the cleaning is finished; after the parts are flushed for the third time by using the cold water after being taken out, and are flushed for the fourth time by using the hot water; after the flushing is finished, the parts are taken out for airing, and are dried and stacked; and the passivation treatment process is finished. The brass surface passivation treating agent has good lubricating effect, in particular can prevent an inner hole wall from generating cracks when extruding hollow pieces, and facilitates the promotion of product quality.
Owner:ANHUI CHENGYOU AUTO PARTS MFG
Starch-based nanoparticles of double-embedded beta-carotene as well as preparation method and application of starch-based nanoparticles
ActiveCN114868907APreparation raw materials are non-toxicWide variety of sourcesFood shapingFood ingredient functionsBiotechnologyButyric anhydride
The invention discloses double-embedded beta-carotene starch-based nano-particles as well as a preparation method and application of the double-embedded beta-carotene starch-based nano-particles. The preparation method comprises the following steps: carrying out enzymolysis treatment and precipitation grading treatment on starch to prepare dextrin; butyric anhydride is adopted to carry out acylation treatment on dextrin, and butyrylated dextrin is prepared; carrying out embedding treatment on the butyrylated dextrin and beta-carotene by adopting a coprecipitation method, so as to prepare a butyrylated dextrin / beta-carotene inclusion compound; the gliadin is subjected to ultrasonic modification treatment, and ultrasonic modified gliadin is prepared; enabling an alkaline mixed system containing the butyrylated dextrin / beta-carotene inclusion compound, the ultrasonic modified gliadin and the beta-carotene to be subjected to self-assembly, so as to prepare the starch-based nanoparticles with the double embedded beta-carotene. The double-embedded starch-based nanoparticles prepared by the method have relatively high inclusion rate and high environmental stress resistance stability, and have wide application prospects in the field of bioactive substances or emulsions.
Owner:HEFEI UNIV OF TECH
Method of preparing cellulose acetate butyrate by means of dichloromethane solvent method
The invention discloses a method of preparing cellulose acetate butyrate by means of a dichloromethane solvent method. The method comprises the following steps: (1) spraying acetic acid to wood pulp and performing activation at constant temperature; in addition, uniformly mixing acetic anhydride, butyric anhydride, butyric acid and a sulfuric acid catalyst; (2) inputting the material in an esterification kettle with a dichloromethane solvent for esterification reaction, after reaction, adding magnesium acetate into the reaction system, uniformly stirring the mixture, adding acetic acid into the mixture to dilute, and discharging the diluted solution into a hydrolysis kettle; (3) adding acetic acid into the hydrolysis kettle to promote hydrolysis, wherein the temperature of the system is 60-85 DEG C, and the time is controlled within 3-7h; and after hydrolysis, adding magnesium acetate into the system; and (4) performing filter pressing, performing flash evaporation on the filtrate, adding water for chromatography, and performing washing and drying. By this way, the cellulose acetate butyrate, the dynamic viscosity of which is 5000cps-7000cps, is obtained. The method is simple in operating step, high in controllability, good in atom economy, safe in operating environment and small in wastewater quantity.
Owner:JIANGSU RUICHEN CHEM
Beta receptor blocker Acebutolol intermediate synthesized by photochemical Fries rearrangement
PendingCN113527123ASynthetic method is simpleLow pollution and cheapOrganic compound preparationCarboxylic acid amide separation/purificationM-aminophenolButyric anhydride
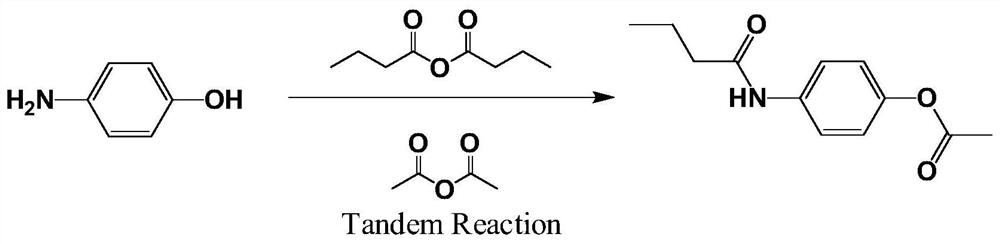
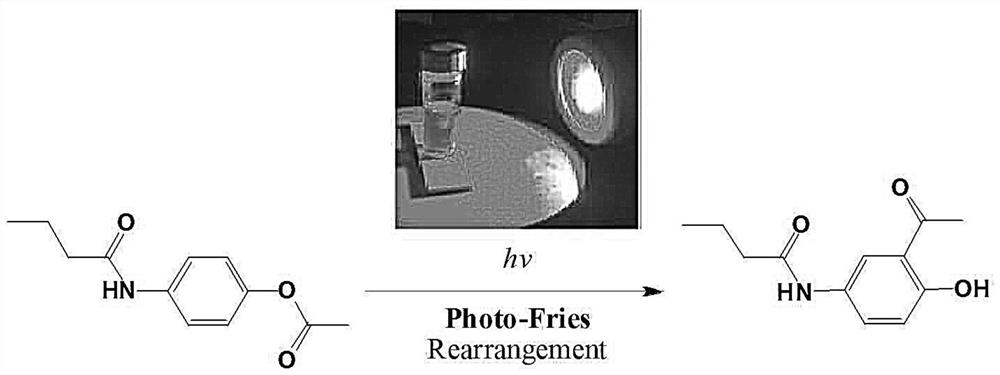
The invention belongs to the field of organic photochemistry and medicinal chemistry, and particularly relates to a beta receptor blocker Acebutolol intermediate synthesized by photochemical Fries rearrangement. An efficient continuous acetylation synthesis method is adopted, raw materials including p-aminophenol and n-butyric anhydride are mixed and subjected to a water diversion reaction with a water-carrying agent, then the water-carrying agent is removed through distillation, reactants are cooled, water is added as a dispersing agent, then liquid caustic soda is added for a reaction, phenate is generated, acetic anhydride is dropwise added, the mixture is stirred for a reaction, ester is generated, recrystallizing is carried out to generate a first-step product N-(4-acetoxyphenyl) butyrylamide; and in the step 2 photochemical Fries liquid phase rearrangement reaction is adopted, the product in the first step is dissolved in an organic solvent, then Fries rearrangement is carried out by irradiation of ultraviolet-visible light with a specific wavelength, the solvent is heated and evaporated after the reaction is completed, and the important intermediate 2-acetyl-4-n-butyryl aminophenol of Acebutolol is obtained after recrystallization. The method is simple and easy to implement, and low-toxicity, efficient and cheap green chemical reagents are used in the synthesis process.
Owner:山东盛安贝新材料有限公司
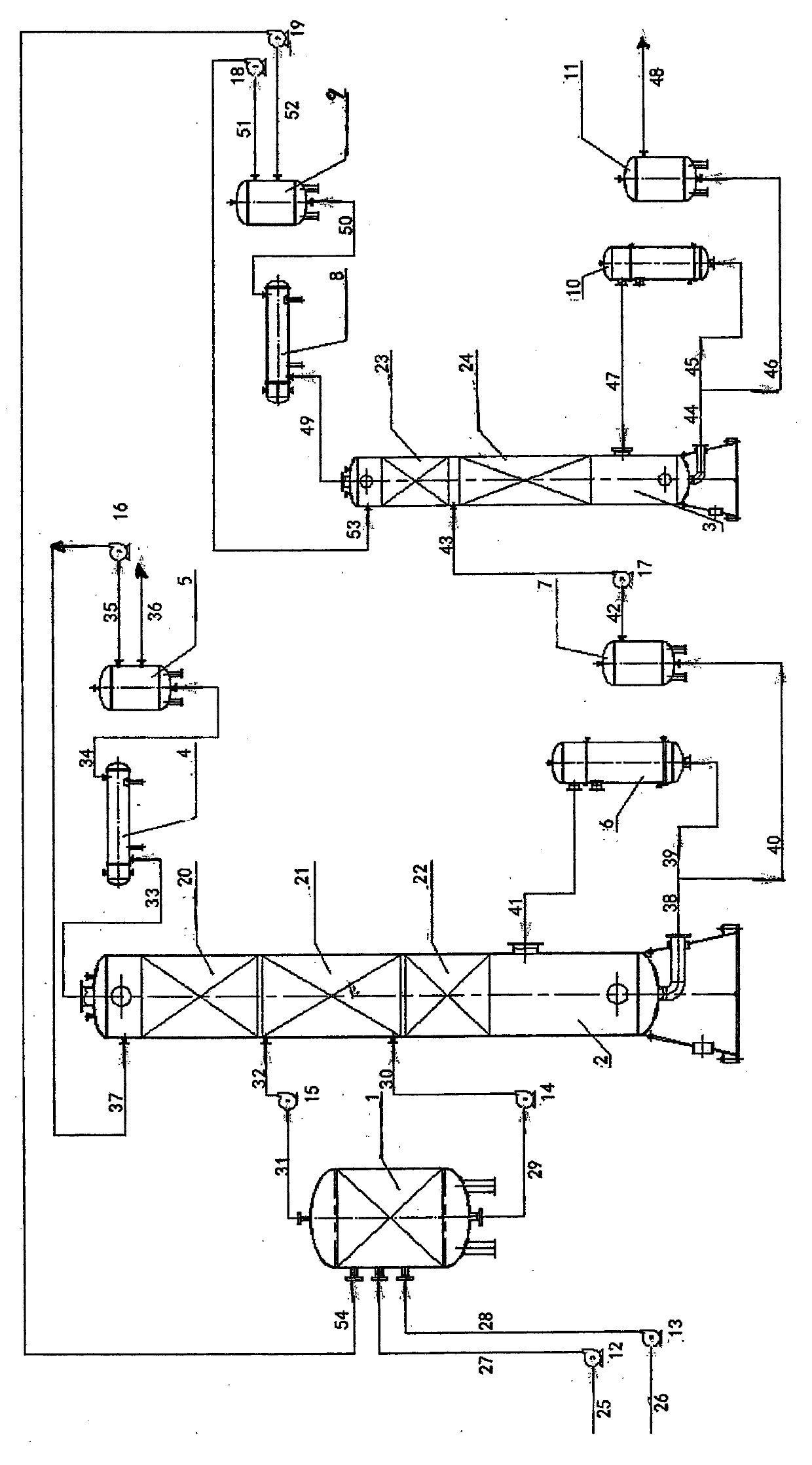
Apparatus for preparing butyric anhydride through reactive distillation of acetic anhydride and butyric acid, and process thereof
InactiveCN104086405AFix stability issuesSettlement yieldChemical industryCarboxylic acid anhydrides preparationAcetic acidButyric anhydride
The invention discloses an apparatus for preparing butyric anhydride through reactive distillation of acetic anhydride and butyric acid, and a process thereof. The apparatus comprises a reactor, a reactive distillation tower, a butyric anhydride refining tower, a condenser, a condenser, a reboiler, a refluxing tank, a kettle tank, a pump and pipelines. The process using the combination of the reactive distillation tower, the rector and the butyric anhydride refining tower comprises the following steps: adding acetic anhydride and butyric acid to the reactor, reacting, and carrying out reaction and rectification on acetic anhydride and butyric acid flowing into the reactive distillation tower on each layer of trays of a reaction section; carrying out stripping section rectification on a material flowing down in the reaction section; carrying out rectifying section rectification on a material rising in the reactions section, and extracting from the tower top to obtain 80-100% acetic acid; pressurizing a material from the bottom of the reactive distillation tower to enter the butyric anhydride refining tower for rectification; and obtaining some of acetic acid, acetic anhydride, butyric acid, acetic butyric anhydride and butyric anhydride at the top of the butyric anhydride refining tower and butyric anhydride with the purity of 80-100% at the bottom of the refining tower. The apparatus and the process solve the problems of unstable quality, low yield and low production efficiency of products intermittently produced by using butyric anhydride.
Owner:TIANJIN PULAI CHEM TECH
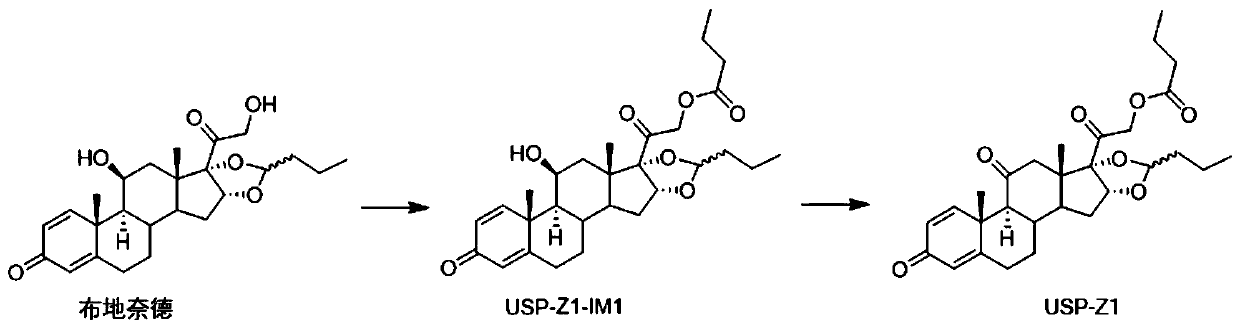
Synthesis method of budesonide impurity USP-Z1
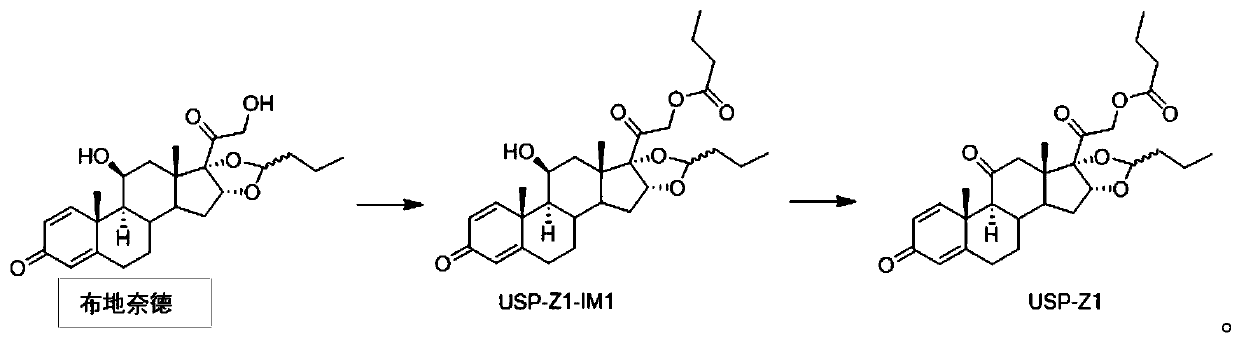
The invention provides a synthesis method of a budesonide impurity USP-Z1. According to the synthesis method, firstly, budesonide is taken as a raw material and subjected to a reaction with butyryl chloride or butyric anhydride under the condition of an organic solvent and an acid-binding agent to produce a compound USP-Z1-IM1; then, the compound USP-Z1-IM1 is dissolved in the organic solvent, anoxidant is added, oxidation is performed, and the impurity USP-Z1 is produced. The synthesis method adopts a simple process route and is convenient to operate, good in selectivity and high in yield; the synthesized budesonide impurity USP-Z1 can serve as a reference substance for detection and study of budesonide and is applied to quality control of budesonide and related preparations to control the purity of raw materials or preparations of budesonide.
Owner:BIONNA (BEIJING) MEDICAL TECH CO LTD
Method for synthesizing 2-chlorobutyric acid
InactiveCN102241583AReduce dosageIncrease profitPreparation from carboxylic acid anhydridesChemical recyclingN-Butyric acidHydrogen
The invention discloses a method for synthesizing 2-chlorobutyric acid, which comprises the following steps that: n-butyric acid and liquid chlorine are reacted in the presence of butyric anhydride serving as a catalyst at 50 to 150 DEG C for 5 to 15 hours, namely chloride ions substitute hydrogen ions at position-2 of the molecules of butyric anhydride to form 2-chlorobutyric anhydride and then the 2-chlorobutyric anhydride reacts with n-butyric acid to form butyric anhydride and 2-chlorobutyric acid; and after reaction, the 2-chlorobutyric acid with a purity of over 99 percent can be obtained by rectification. In the invention, butyric anhydride is used as a catalyst, and by controlling the reaction temperature and chlorine content, the conversion rate can be kept between 85 and 95 percent, the by-products generated can be reduced, and excessive chlorination can be prevented; and after being hydrolyzed, the catalyst can be recycled, and unreacted n-butyric acid can be reclaimed by rectification and can be reused in subsequent production. In the invention, the used amount of chlorine is small, fewer byproducts are produced, the utilization rate of n-butyric acid is high, and the environment-protection effect is improved considerably.
Owner:JIAXING BOYUAN BIO CHEM TECH
Preparation method for glyceryl tributyrate
ActiveCN104086422APromote generationImprove responseMolecular sieve catalystsOrganic compound preparationN-Butyric acidButyric anhydride
The invention discloses a preparation method for glyceryl tributyrate. The preparation method comprises: taking glycerin and n-butyric acid as raw materials, adding a catalyst and heating to 100-110 DEG C with stirring, performing heating reflux as well as dropwise adding butyric anhydride, stopping reaction until no water is generated in the reaction solution during dropwise adding, cooling to room temperature, and filtering, so as to obtain glyceryl tributyrate. The catalyst is a mixture of sulfonic-acid mesoporous molecular sieve and ZSM-5 acidic zeolite, and the usage amount of the catalyst is 2-10% by weight of the sum of glycerin and n-butyric acid. By taking sulfonic-acid mesoporous molecular sieve and ZSM-5 acidic zeolite as the catalyst, the selectivity is high, the reaction time is shortened, the generation of by-products is reduced and the catalyst is reusable. By employing the method, the reaction system does not need a water-carrying agent, so that the reaction cost and safe hidden trouble are reduced, the purification step of the product is simplified, the post-processing process is simple, environment is protected and energy is saved. The obtained glyceryl tributyrate is high in purity and high in yield, the reaction speed is fast, and industrial production is easy to realize.
Owner:HUBEI XINZHOU CHEM
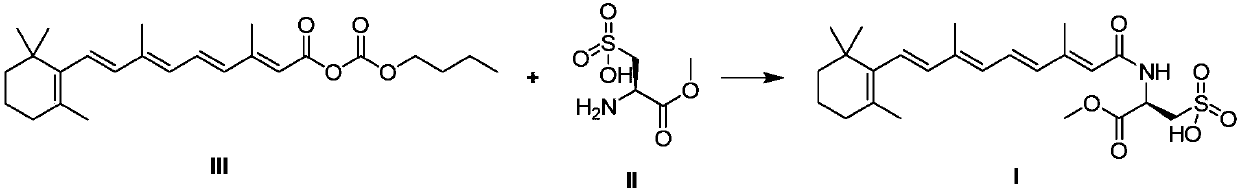
Preparation method of sodium salt of N-(all trans-retinol)-L-cystathionine methyl ester
The invention discloses a preparation method of sodium salt of N-(all trans-retinol)-L-cystathionine methyl ester shown as formula I-Na. The preparation method comprises the following steps: step 1, in a dipolar aprotic solvent, performing acylation reaction on all-trans retinoic acid butyric anhydride shown in formula III and L-cystathionine methyl ester shown in formula II in the presence of organic base to obtain N-(all-trans-retinol)-L-cystathionine methyl ester shown in formula I; and step 2, prepare the n-(all trans-retinol)-l-cystathionine methyl ester shown in formula I obtained in thestep 1 into the sodium salt of n-(all trans-retinol)-l-cystathionine methyl ester shown in the formula I-Na. The preparation method disclosed by the invention has mild reaction conditions, simple operation, high yield and high purity of the obtained product. The production cost is low, the equipment requirement is low, and the method is suitable for industrial production.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT +1
Preparation method of cellulose acetate butyrate
The invention belongs to the technical field of cellulose derivatives, and particularly relates to a preparation method of cellulose acetate butyrate. The method comprises the steps: adding the refined cotton linters and the activated carbon adsorbing the glacial acetic acid into a butyric acid aqueous solution, stirring, activating, and filtering to obtain an activated mixture of the refined cotton linters and the activated carbon; adding the mixture of the activated refined cotton linters and the activated carbon into a solvent, stirring, and filtering to obtain a solution containing activated cellulose; adding concentrated sulfuric acid, acetic anhydride and butyric anhydride into the solution containing the activated cellulose for esterification reaction to obtain esterified feed liquid; adding water into the esterified feed liquid for stirring, pressurizing and hydrolyzing to obtain hydrolysate; and neutralizing, filtering and precipitating the hydrolysate to obtain a precipitate, and washing, cooking and drying the precipitate to obtain the cellulose acetate butyrate. The method has the characteristics of good activation effect, high reaction efficiency, high yield and short hydrolysis time.
Owner:WEIFANG ENG VOCATIONAL COLLEGE
Production method of spherical cellulose acetate butyrate
InactiveCN104193829BGood application effectReduce apparent viscosityButyric anhydrideCellulose acetate
Owner:SICHUAN NITROCELLULOSE CORP
A kind of preparation method of calcium dibutyryl cyclic adenosine phosphate
ActiveCN106336439BLow costShort reaction timeSugar derivativesSugar derivatives preparationFreeze-dryingButyric anhydride
The invention discloses a calcium dibutyryladenosine cyclophosphate preparation method, which is characterized by comprising: (1) adding adenosine cyclophosphate and calcium hydroxide to water according to a molar ratio of 1:1-1.2, carrying out a reaction for 30-40 min, filtering to remove insoluble matters, adding ethanol having the volume 2-3 times the volume of the filtrate to the filtrate, filtering after a calcium adenosine cyclophosphate salt is precipitated, and carrying out vacuum drying at a temperature of 60-80 DEG C to obtain calcium adenosine cyclophosphate; (2) adding the calcium adenosine cyclophosphate obtained in the step (1) and butyric anhydride to a reaction kettle according to a ratio of 1 g:7-12 ml, protecting with nitrogen, and carrying out a stirring reaction for 1-3 h; and (3) adding 0-4 DEG C water having the volume 3-4 times the volume of the reaction liquid obtained in the step (2) to the reaction liquid, stirring for 30-40 min, carrying out nano-filtration on the reaction liquid, concentrating the trapping liquid, and carrying out freeze drying or spray drying to obtain the calcium dibutyryladenosine cyclophosphate. According to the present invention, the selectivity of the obtained calcium dibutyryladenosine cyclophosphate is more than 90%, the reaction time is short, the reaction process is simple and environmental friendly, and the method is suitable for industrial application.
Owner:NANJING TECH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com