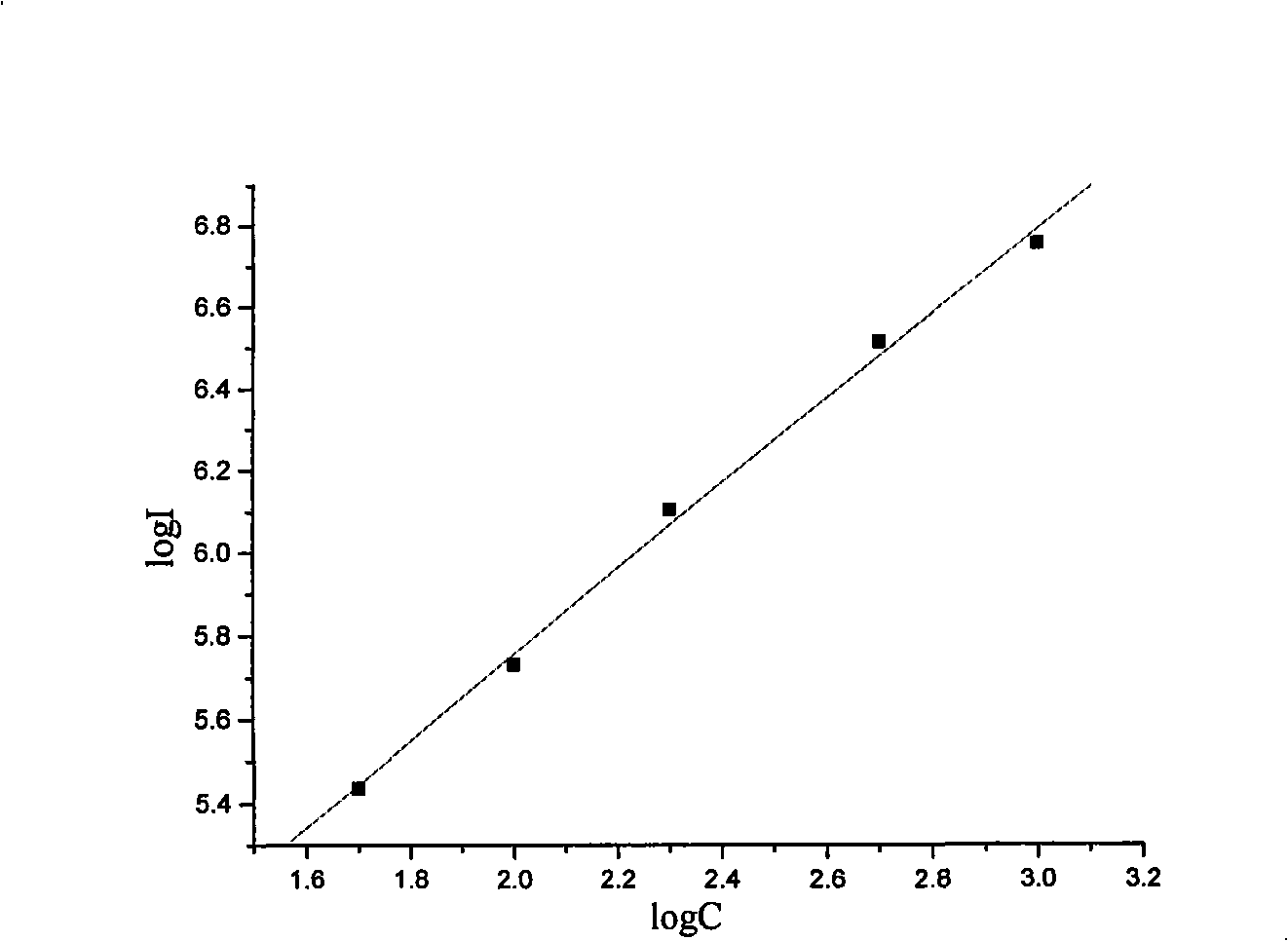
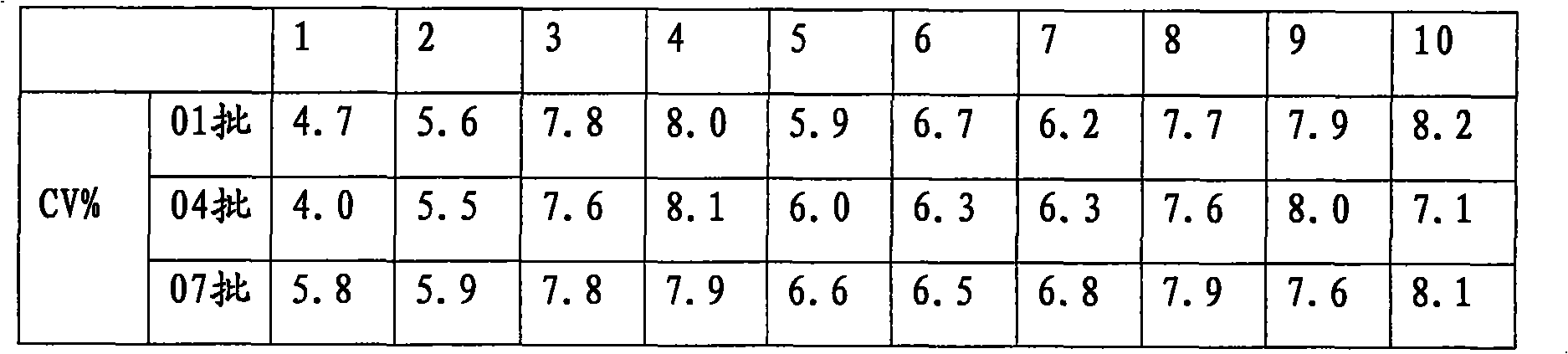
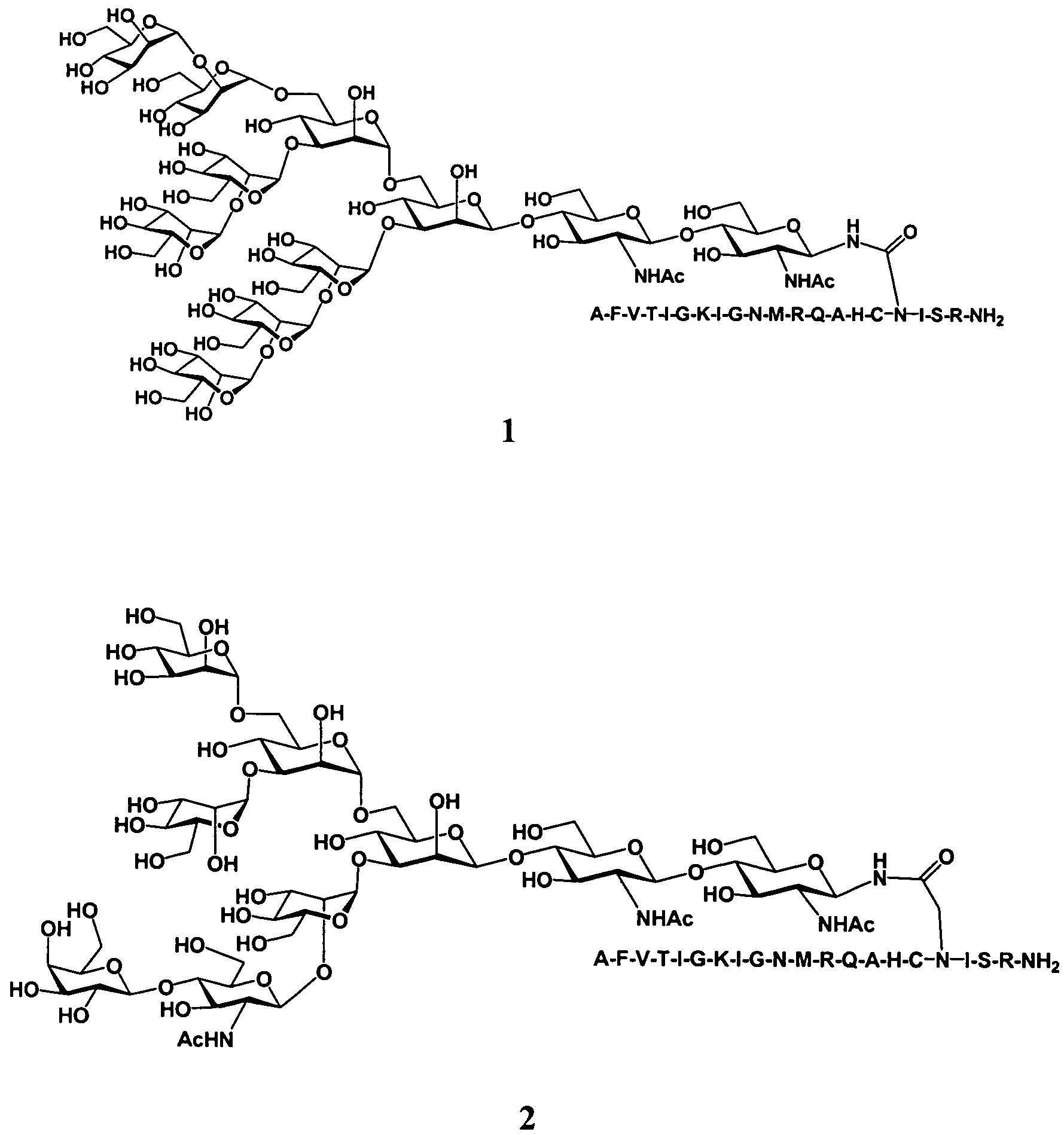
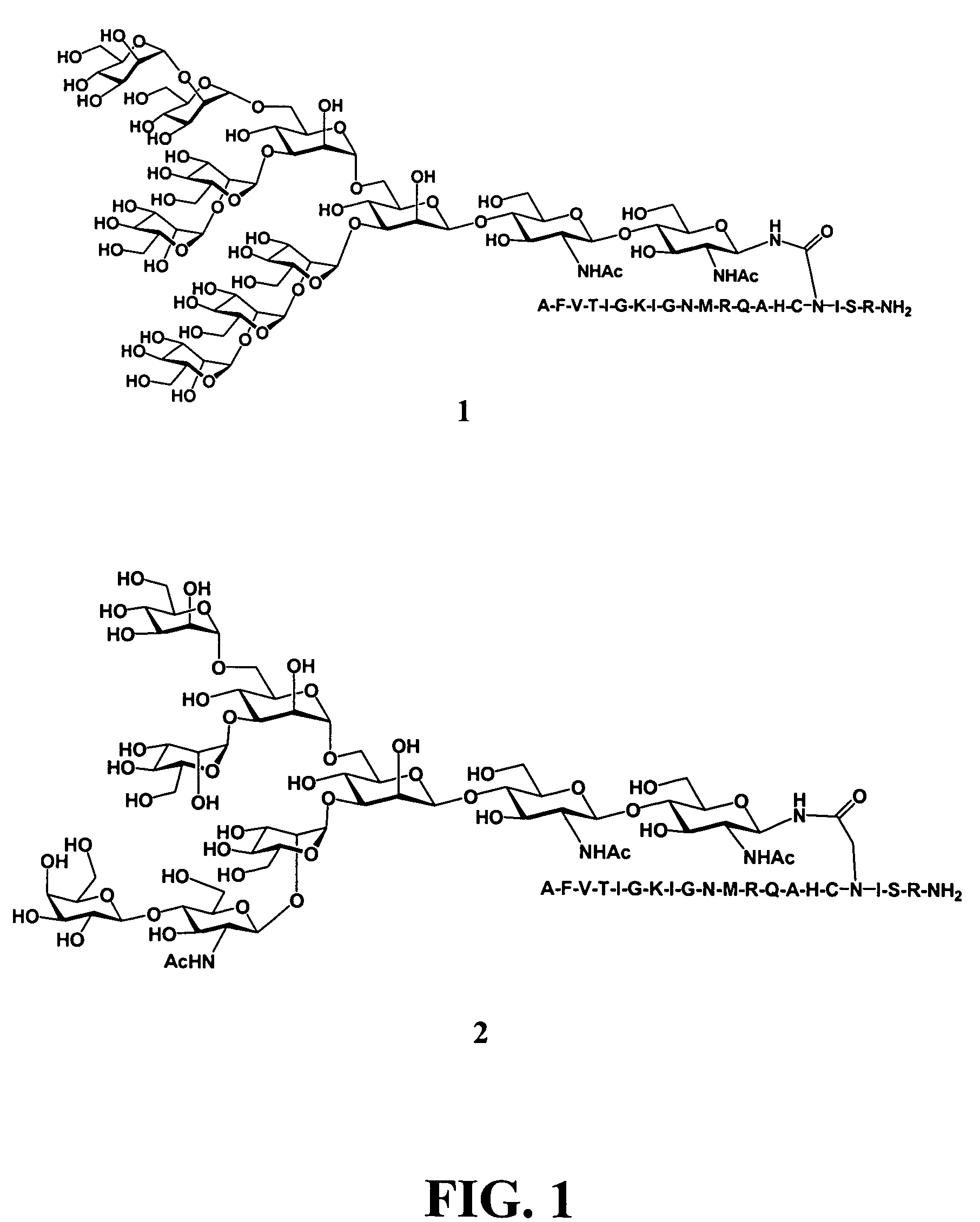
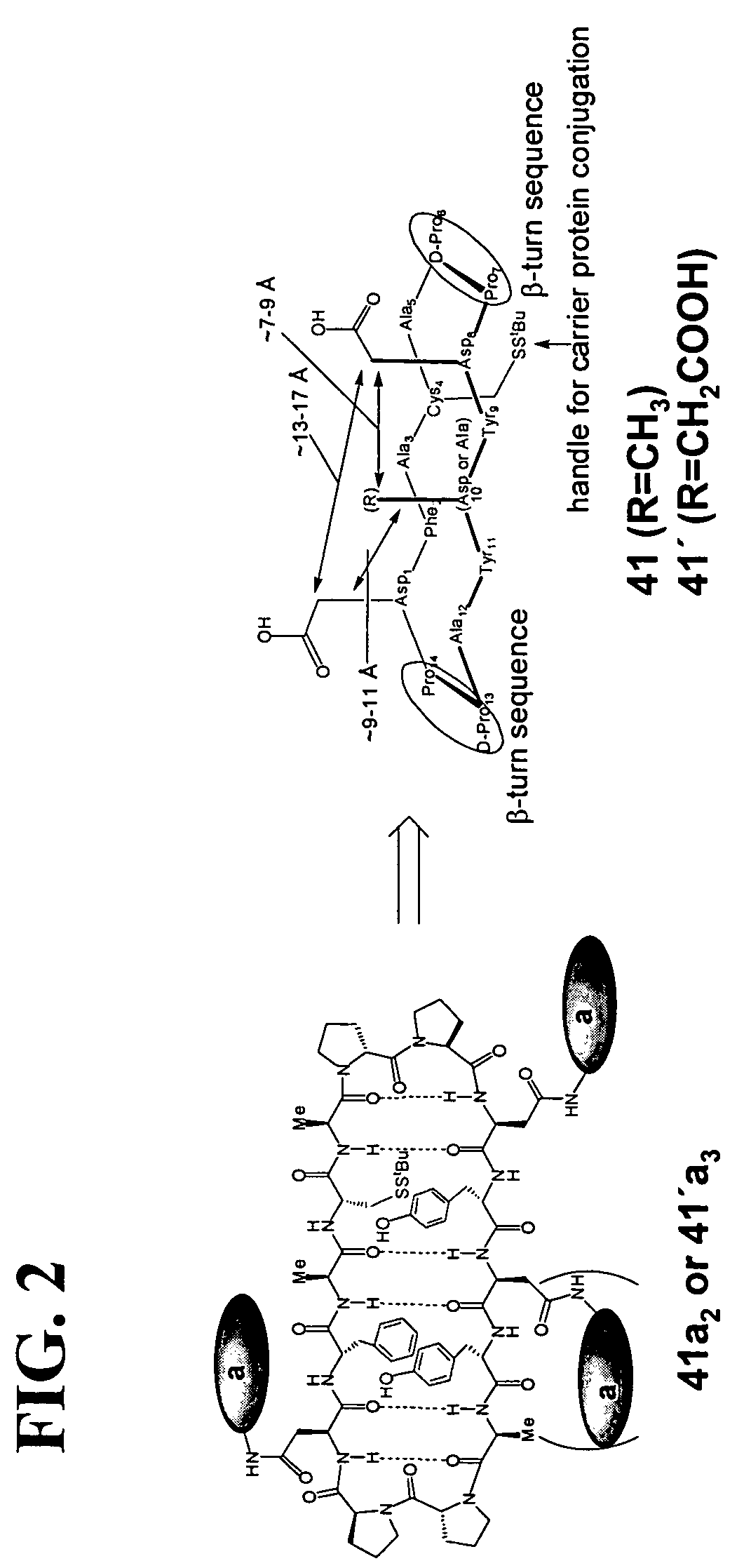
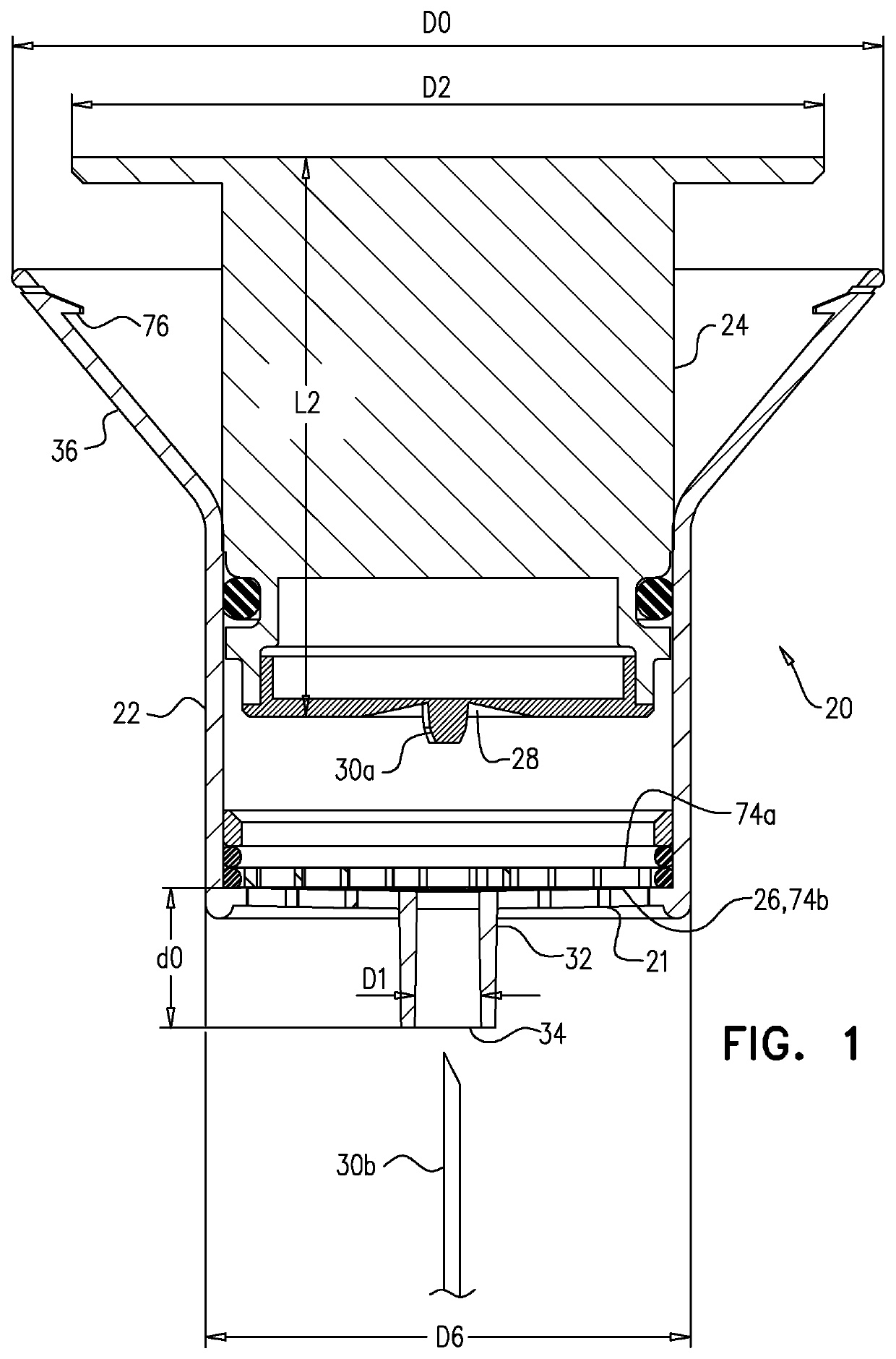
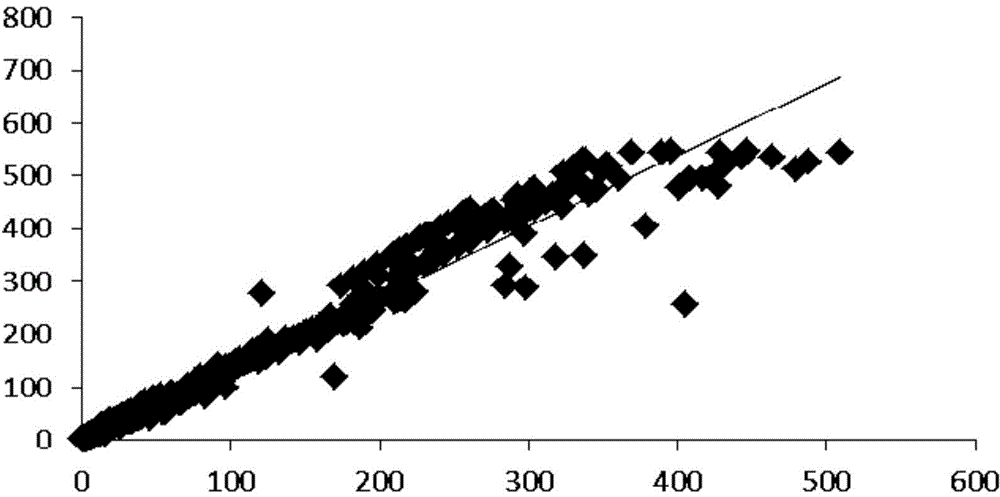
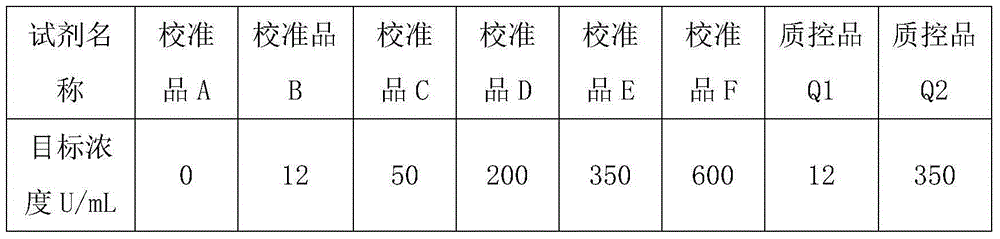
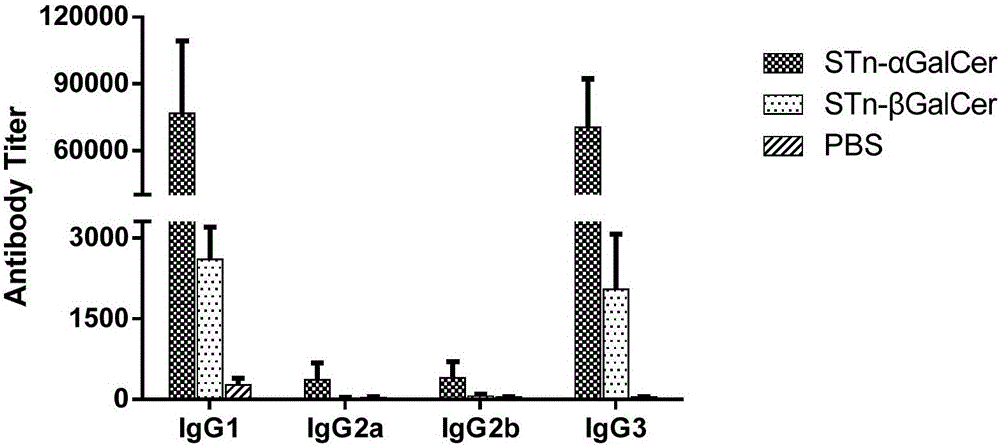
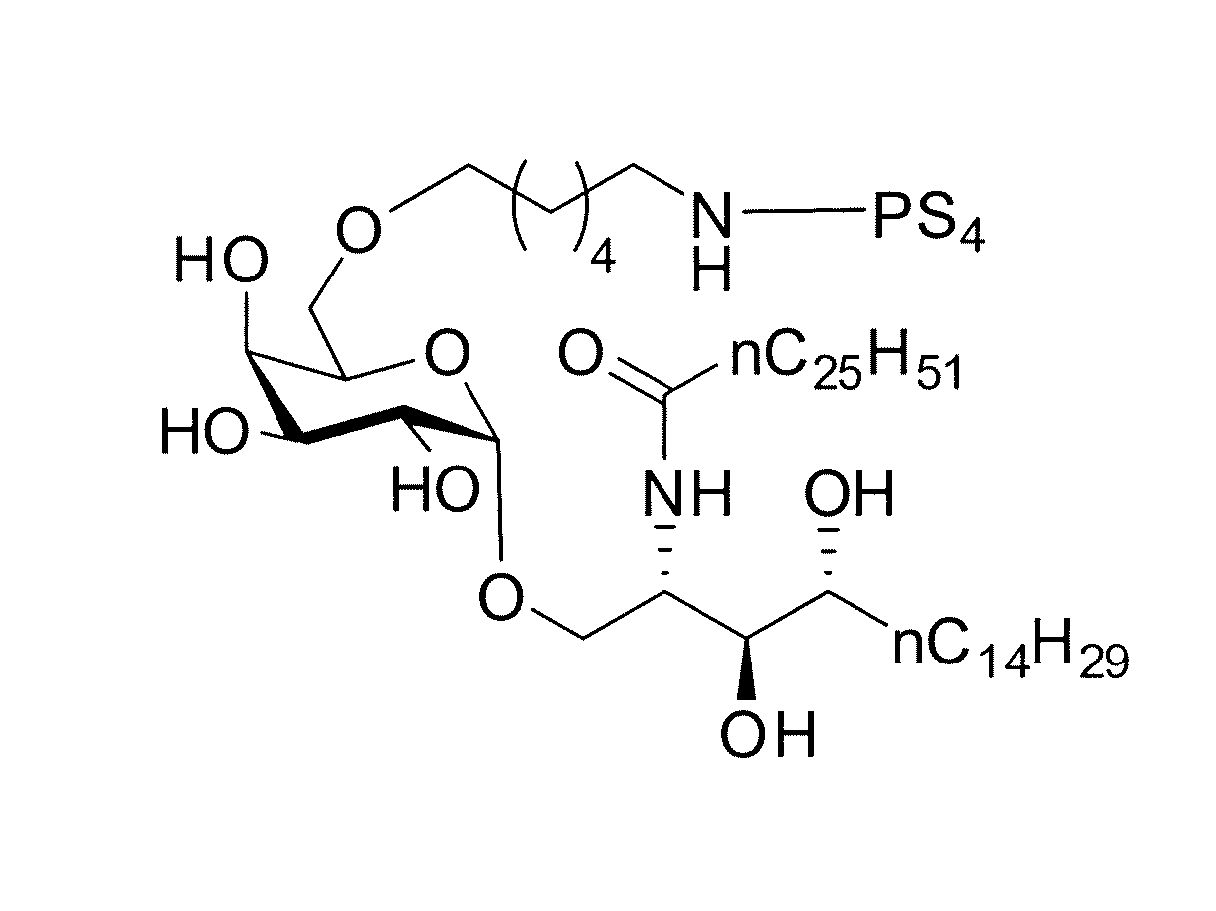
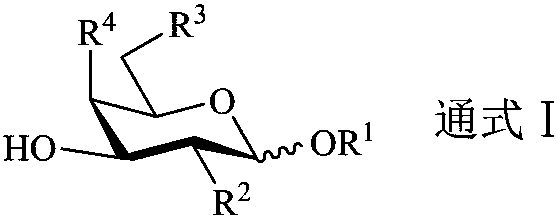
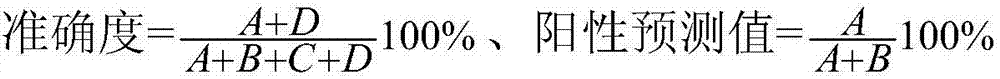Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
119 results about "Carbohydrate antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
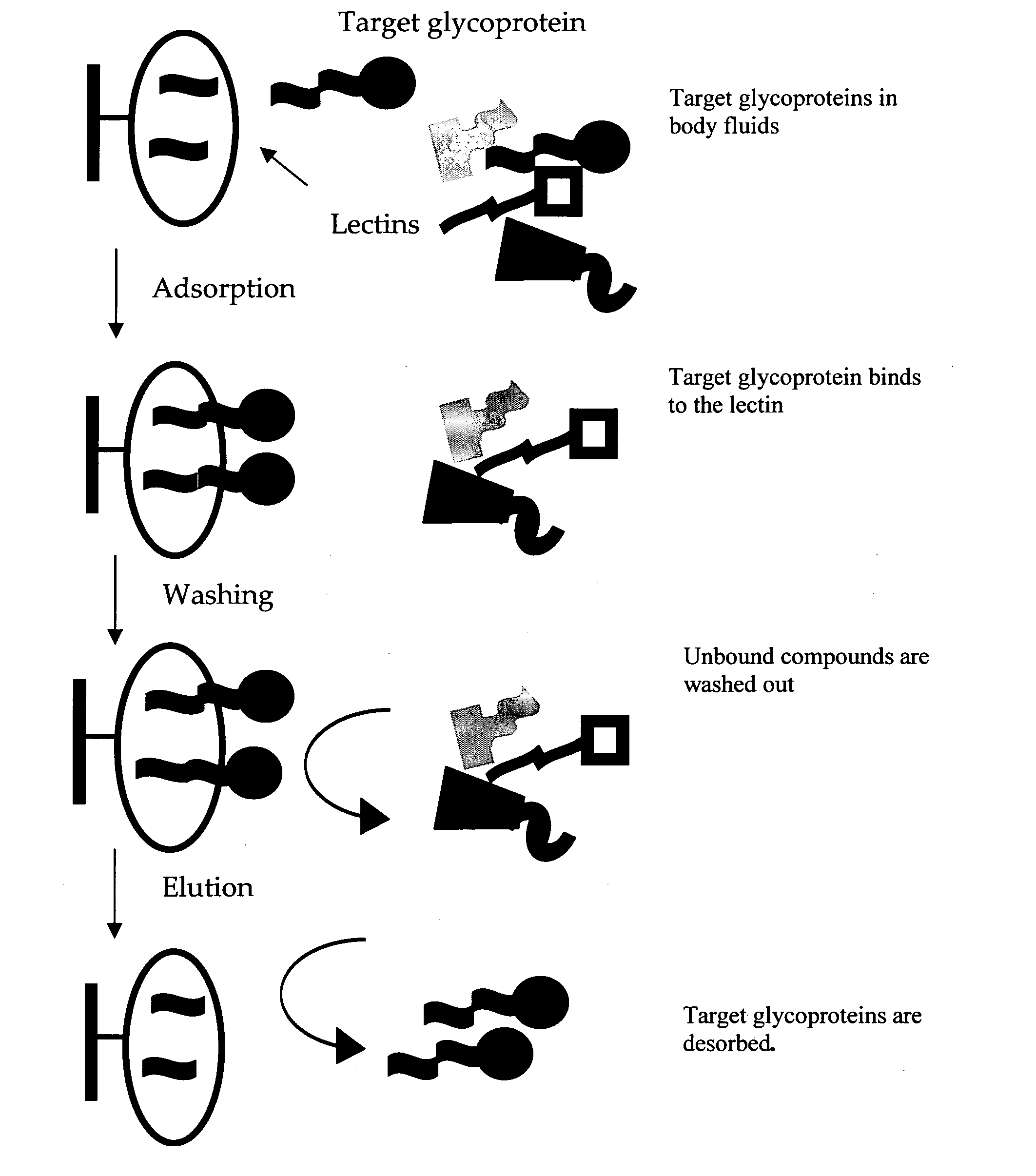
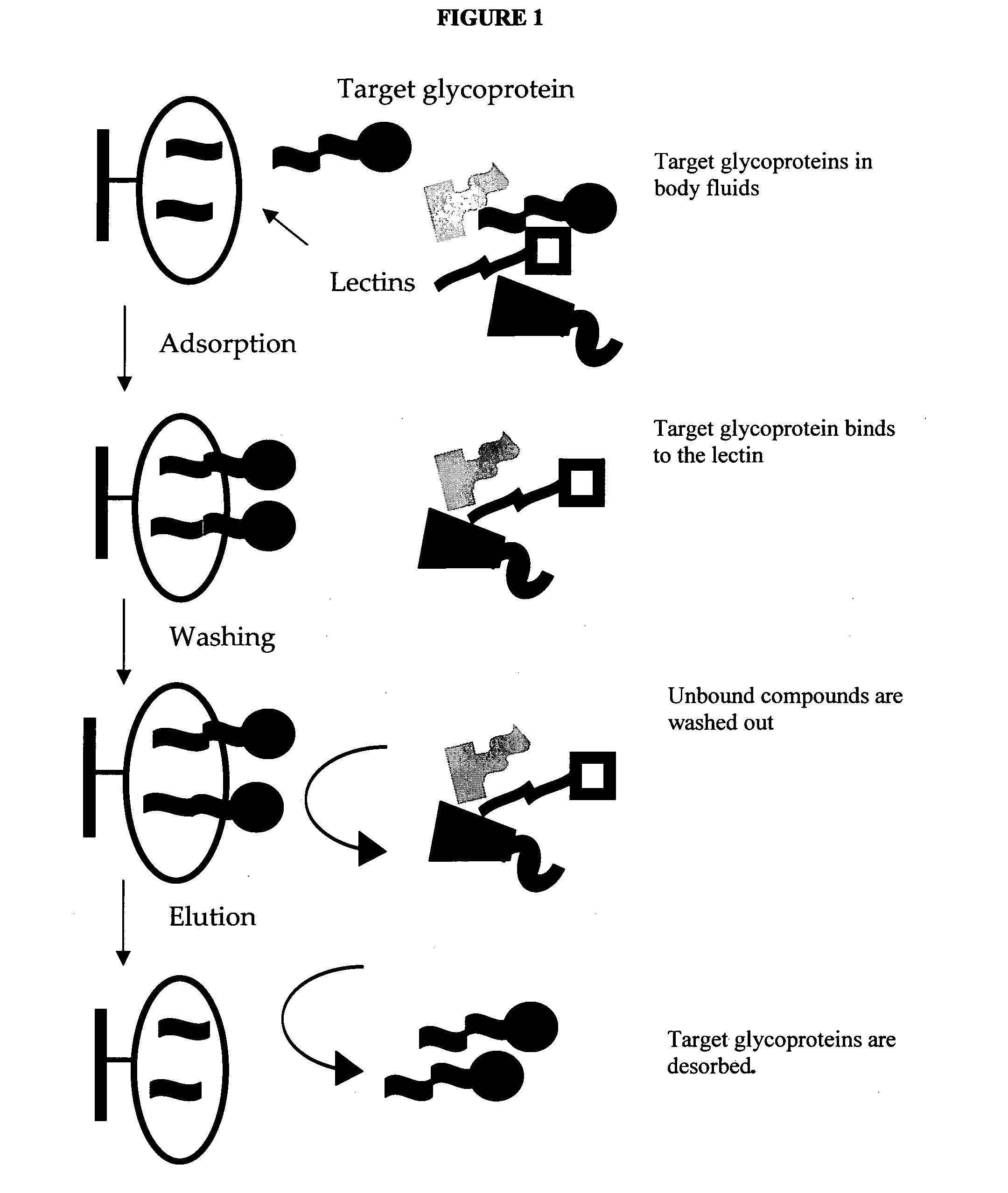
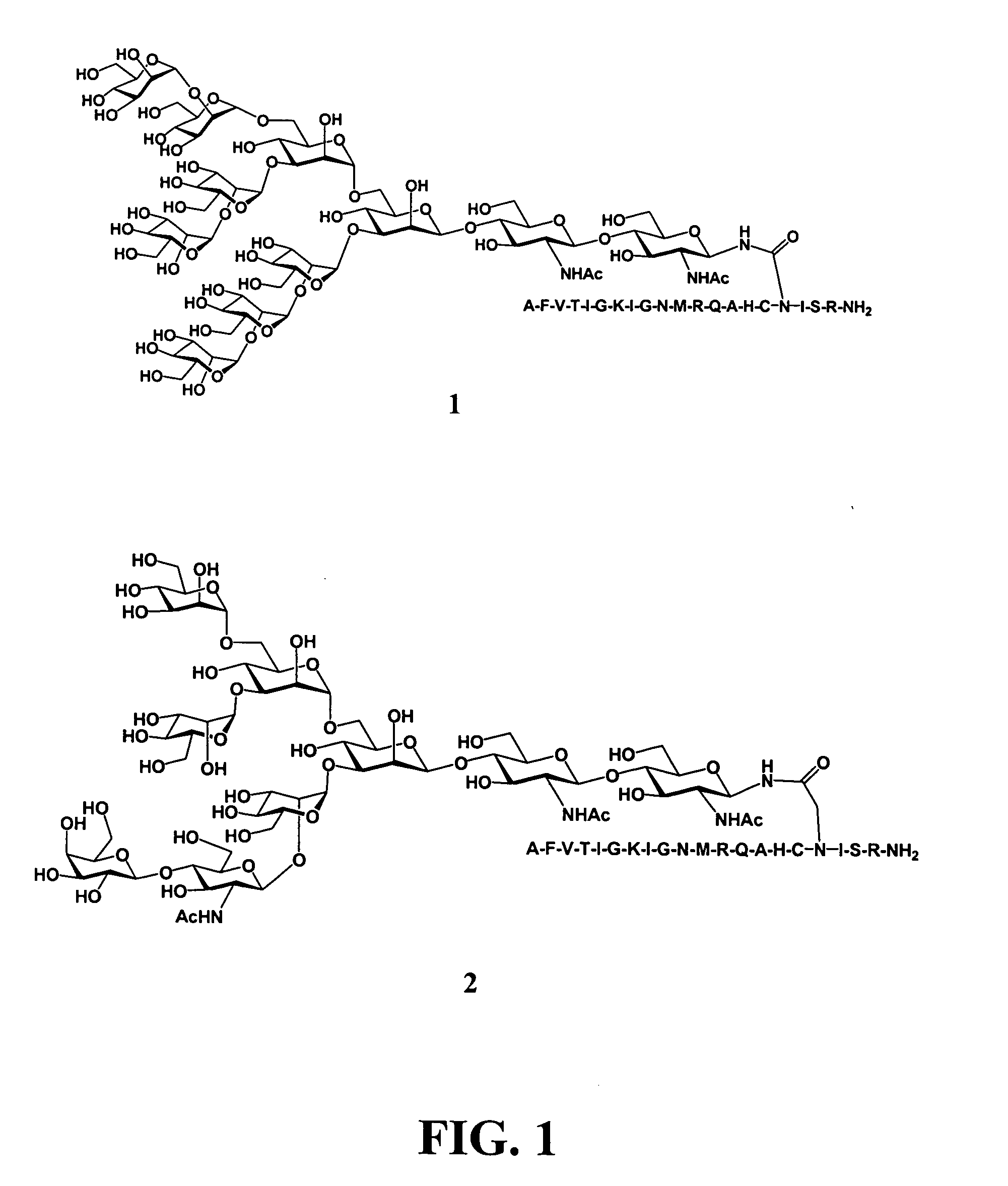
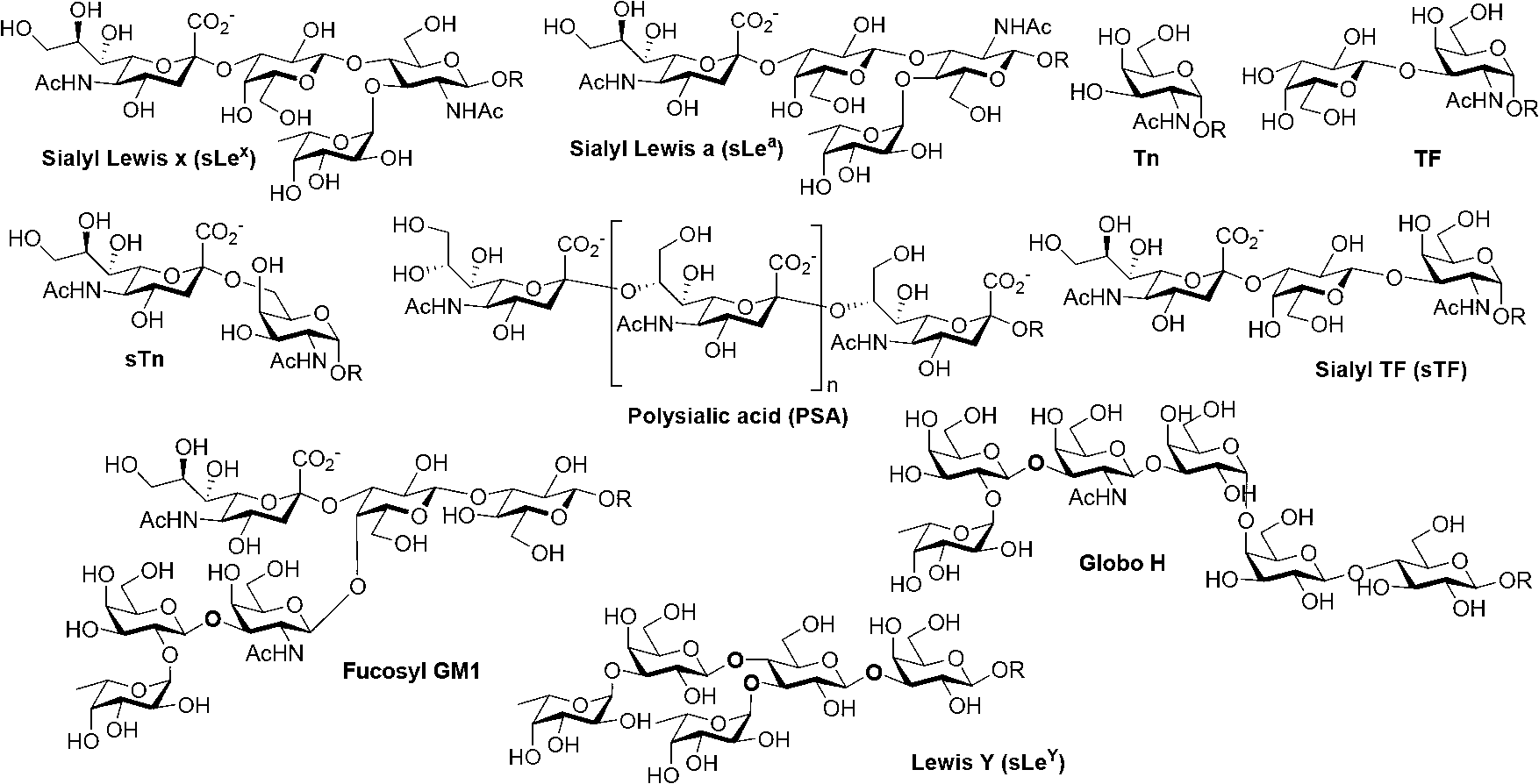
Carbohydrate antigens are polymeric chains of diverse monomeric sugar molecules. Carbohydrate antigens can induce antibody production without the help of T lymphocytes and are also recognized by cell‐surface lectins.
Reaction plate and protein chip kit for integrated detection of multiple gynecologic tumor markers
InactiveCN1880960AChemiluminescene/bioluminescenceColor/spectral properties measurementsCarbohydrate antigenGynecologic Tumor
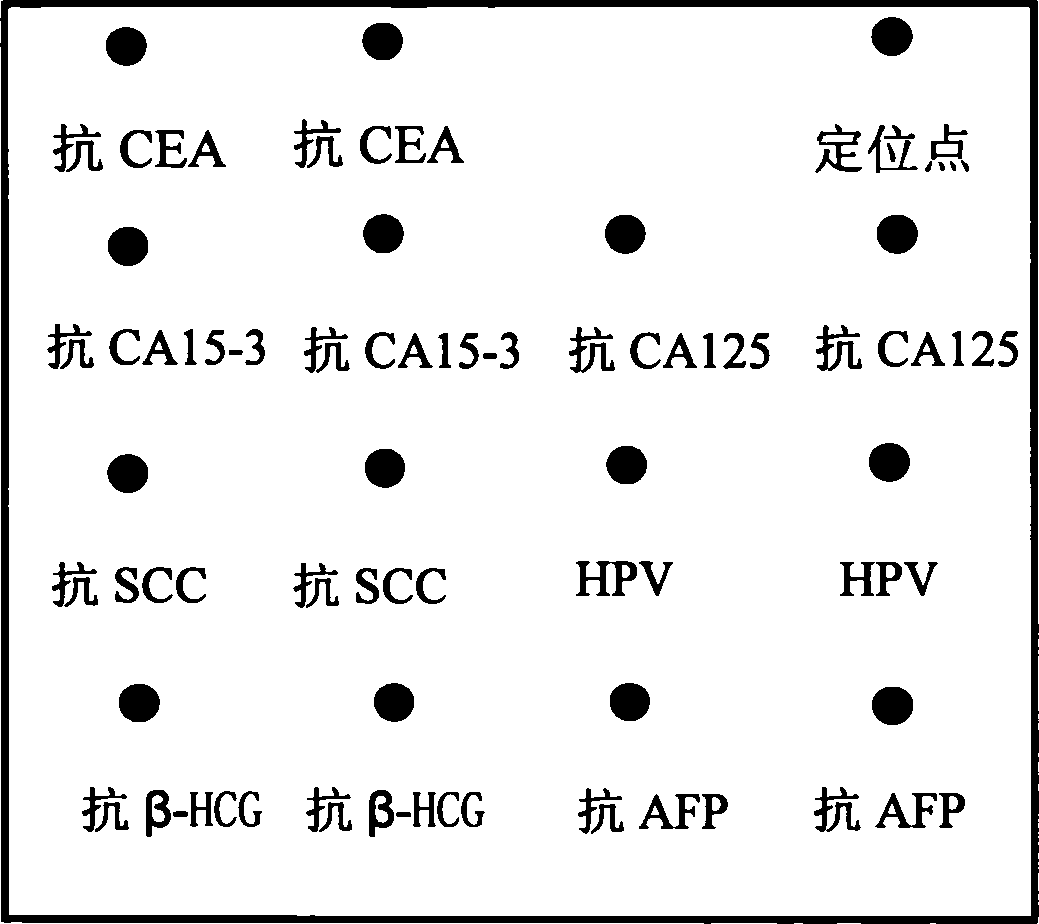
The invention discloses an integral detecting reacting board and protein chip agent box of Carcinoembryonic antigen,CEA, 15-3 CA15-3(Carbohydrate Antigen 15-3,CA15-3), CA 125 (Cancer antigen 125,CA125), Squamous cell carcinoma antigen,SCC, Human papillomavirus antibody,HPVAb, beta-human chorionic gonadotropin, beta-HCG and alpha-fetoprotein,AFP, wherein the reacting hole board contains base and reacting hole on the base; the reacting hole contains 2-384 sample holes, 2-200 standard sample holes; the fixing phase carrier is set on the bottom of each reacting hole, which is covered by seven antigen or antibody molecule arrays with anti-CEA, anti-CA15-3,anti-CA125,anti-SCC,HPV,anti- beta-HCG,anti-AFP.
Owner:汪宁梅
Cancer stem cell markers and uses thereof
InactiveUS20120294859A1Delay is slowLarge indexBiological material analysisAntibody ingredientsDiseaseCell marker
The present invention inter alia pertains to therapeutic methods which are based on the use of an agent specifically binding a tumor-associated carbohydrate antigen for the treatment of cancer stem cells and related diseases. Also provided are diagnostic and prognostic methods using a tumor-associated carbohydrate antigen as marker for cancer stem cells.
Owner:GLYCOTOPE GMBH
Magnetic microparticle chemiluminescence enzyme immune analytic reagent kit for detecting saccharide antigen and its use method
InactiveCN101324579AQuantitative detection of carbohydrate antigen contentLow pre-processing requirementsMaterial analysisCarbohydrate antigenMicroparticle
The invention relates to a magnetic corpuscule chemiluminescent enzyme immunoassay kit for detecting carbohydrate antigen and the application method thereof. The kit comprises FITC antibody-coated magnetic corpuscules; a marker solution prepared by mixing the FITC-marked carbohydrate antigen monoclonal antibody and the enzyme-marked carbohydrate antigen monoclonal antibody; a carbohydrate antigen standard sample solution; a concentrated washing solution; and a luminescent substrate solution, wherein carbohydrate antigen optionally adopts one of CA72-4, CA50, CA19-9, CA242, CA15-3, CA27-29 and CA125. The enzyme-marked antibody and the FITC-marked antibody are the monoclonal antibodies corresponding to the antigens. The FITC antibody coating the magnetic corpuscules adopts a polyclonal antibody or a monoclonal antibody. The marker solution is prepared by mixing an FITC-marked capture antibody working solution and an enzyme-marked antibody pair working solution by the volume ratio of 1:(1-3). Compared with the known kit for mensurating the carbohydrate antigen, the kit has the advantages of high flux, high sensitivity, wide linear range, rapidness, etc., and has a wide application prospect for the clinical inspection, etc.
Owner:TSINGHUA UNIV
RM2 antigen (beta1,4-GalNAc-disialyl-Lc4) as prostate cancer-associated antigen
InactiveUS20050221397A1Useful predictionBiological material analysisBiological testingAntigenSerum ige
A novel carbohydrate antigen, β1,4-GalNAc-disialyl-Lc4, defined by monoclonal antibody RM2, is expressed in human prostate cancer, but not in benign prostate hypertrophy (BPH) or normal prostate gland. Monoclonal antibody RM2 or other antibodies with similar specificity are useful for diagnosis of prostate cancer by immunohistology of biopsy samples, specifications from a total prostatectomy, and quantitative determination of RM2 antigen in sera of patients.
Owner:NORTHERN ADVANCEMENT CENT FOR SCI & TECH
Surface plasmon resonance biosensor system for detection of antigens and method for determining the presence of antigens
The present invention provides an SPR system and corresponding methods of use, for determining the presence or concentration of tumor-associated antigens in cancer patient samples. The SPR system may have multiple channels, with each channel having operably affixed thereto an antibody specific for a tumor-associated antigen, so as to allow detection of multiple tumor-associated antigens simultaneously. When a biological sample from a patient is applied to the SPR system, the presence of two or more tumor-associated antigens can be determined by measuring an SPR signal shift from each channel. The SPR system may detect the presence or concentration of a tumor-associated carbohydrate antigen, where the sensor surface contains affixed thereto an antibody specific for the glycosyl epitope, as well as an antibody specific for the polypeptide to which the carbohydrate antigen is naturally associated in cancer patients.
Owner:TAYA MINORU +5
Methods for screening for therapeutic molecules and use of the molecules therefrom
Owner:PROCOGNIA ISRAEL
Gp120 specific antigens and uses thereof
InactiveUS20060229432A1Improving immunogenicitySlow and sustained releasePeptide/protein ingredientsVirus peptidesCarbohydrate antigenAntibody fragments
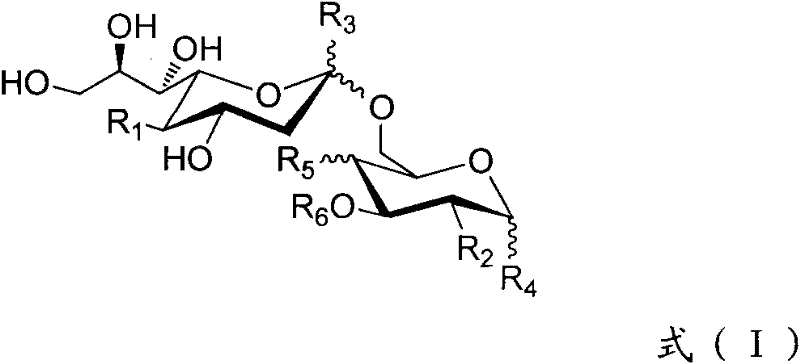
The present invention provides multi-antigenic constructs comprising one or more carbohydrate antigens having the formula: wherein R1, R2A, R2B, R3, R4, W1, W2 and W3 are as defined herein; and additionally provides compositions thereof, and methods for their use in the treatment and / or prevention of HIV infection, and methods for inducing HIV-specific antibodies in a subject, comprising administering to a subject in need thereof, an effective amount of any of the inventive compounds as disclosed herein, either in conjugated form or unconjugated and in combination with a suitable immunogenic carrier. In another aspect, the invention provides an antibody or antibody fragment which binds specifically to a gp120 glycan or glycopeptide of the invention.
Owner:SLOAN KETTERING INST FOR CANCER RES
Gp120 specific antigens and uses thereof
InactiveUS7531181B2Improving immunogenicitySlow and sustained releasePeptide/protein ingredientsVirus peptidesCarbohydrate antigenAntibody fragments
The present invention provides multi-antigenic constructs comprising one or more carbohydrate antigens having the formula:wherein R1, R2A, R2B, R3, R4, W1, W2 and W3 are as defined herein; and additionally provides compositions thereof, and methods for their use in the treatment and / or prevention of HIV infection, and methods for inducing HIV-specific antibodies in a subject, comprising administering to a subject in need thereof, an effective amount of any of the inventive compounds as disclosed herein, either in conjugated form or unconjugated and in combination with a suitable immunogenic carrier. In another aspect, the invention provides an antibody or antibody fragment which binds specifically to a gp120 glycan or glycopeptide of the invention.
Owner:SLOAN KETTERING INST FOR CANCER RES
Testing for particulates
ActiveUS20190381498A1Heating or cooling apparatusMicrobiological testing/measurementParticulatesAntigen
A method is provided for testing for presence of a particulate selected from the group consisting of: a microorganism, a fungus, a bacteria, a spore, a virus, a mite, a biological cell, a biological antigen, a protein, a protein antigen, and a carbohydrate antigen. The method includes (a) collecting, in a tube (22), fluid that potentially contains the particulate, (b) using a plunger (24) to push the fluid through a filter (26) disposed at a distal portion of the tube or at a distal end of the plunger, and subsequently, (c) while the filter is inside the tube, ascertaining if any of the particulate was trapped by the filter by applying a particulate-presence-testing-facilitation solution to the filter. Other embodiments are also described.
Owner:HERO SCI LTD
Carbohydrate antigen 19-9 (CA 19-9) quantitative assay kit, preparation method and detection method thereof
The invention discloses a carbohydrate antigen 19-9 (CA 19-9) quantitative assay kit, which comprises a CA 19-9 calibration material, a CA 9-19 quality control material, an anti-reagent, a magnetic micro particle reagent, and a luminescent substrate. The invention also discloses a preparation method of the kit and a method using the kit to detect the carbohydrate antigen 19-9 (CA 19-9). Fluorescein isothiocyanate labeled CA 19-9 coated antibody and alkaline phosphatase labeled CA 19-9 labeled antibody are used to prepare the anti-reagent; anti-fluorescein isothiocyanate antibody is coupled to carboxyl magnetic beads to prepare the magnetic micro particle reagent, thus the materials can be more easily mixed and separated in immunity reactions, moreover, the reaction speed is greatly increased; a novel chemical luminescent substrate APLS is taken as the substrate, and the sensitivity and specificity of the kit are both improved. The provided kit has the advantages of high detection sensitivity, good specificity, and little variation, and the shelf life is one year or more.
Owner:JIANGSU ZECEN BIOTECH CO LTD
Carbohydrate antigen CA125 quantitative determination kit, and making method and detection method thereof
InactiveCN105445470AHigh sensitivityQuick responseChemiluminescene/bioluminescenceBiological testingAntigenCarbohydrate antigen
The invention discloses a carbohydrate antigen CA125 quantitative determination kit. The kit comprises a calibration substance, a quality control substance, a resisting reagent, a magnetic particle reagent and a luminescence substrate. The invention also discloses a making method of the kit, and a method for detecting a carbohydrate antigen CA125 by using the kit. The resisting reagent is prepared by adopting a fluorescein isothiocyanate labeled CA125 coated antibody and an alkaline phosphatase labeled CA125 labeled antibody, and the magnetic particle reagent is prepared through coupling an anti-fluorescein isothiocyanate antibody with a carboxyl magnetic sphere, so uniform mixing and separation are easy in an immunization reaction, and the reaction speed is greatly improved; and a novel chemiluminescence substrate APLS is adopted as the substrate, so the sensitivity and the specificity of the kit are improved. The determination kit has the advantages of reliable performances, high sensitivity and wide linear range, and can be used together with a semi-automatic and fully-automatic apparatus.
Owner:JIANGSU ZECEN BIOTECH CO LTD
Construction method for light-induced electrochemical immunosensor for detecting double tumour markers
InactiveCN107860923AEasy to separateInhibitory complexAlkaline earth titanatesMaterial analysis by electric/magnetic meansAntigenStrontium titanate
The invention discloses a construction method for a double-component light-induced electrochemical immunosensor based on a dendritic titanium dioxide nano-rod-strontium titanate (B-TiO2 NRs-SrTiO3) hetero-junction, and through the method, detecting alpha-fetoprotein and carbohydrate antigen 153 simultaneously an be achieved. The B-TiO2 NRs-SrTiO3 hetero-junction is synthesized by utilizing a hydrothermal method, so that carrier separation and hole transportation are promoted, and the photocurrent density produced by the B-TiO2 NRs-SrTiO3 is remarkably increased by compared with that produced by B-TiO2 NRs. On the basis of the B-TiO2 NRs-SrTiO3 hetero-junction, beta-galactosidase and acetylcholin esterase are taken as a signal marker for specifically catalyzing hydrolysis of p-aminobenzypylgalactoside and acetylcholine to produce p-aminophenol and choline in situ to serve as sacrificial electron donors respectively for distinguishing photocurrent signals. Because of proper signal difference, the sensor can realize high-sensitivity detection of the alpha-fetoprotein and the carbohydrate antigen 153.
Owner:UNIV OF JINAN
Conjugate of monophosphate A (MPLA) and carbohydrate antigen Globo H and preparation method and application thereof
InactiveCN109432415AReach killHigh antibody titerCancer antigen ingredientsCarrier-bound antigen/hapten ingredientsAntigenAdjuvant
The invention relates to a conjugate of monophosphate A (MPLA) and carbohydrate antigen Globo H and capable of serving as an antitumor vaccine and a preparation method and application thereof. The conjugate can be prepared through a chemical method and has the advantages of definite structure, clear composition and adjuvant needlessness. In addition, the invention further provides the applicationof the conjugate in preparing drug for treating or preventing cancer.
Owner:广州粤美医药科技有限公司
Thomsen-Friedenreich (TF) antigen and TF antigen analogue and their chemoenzymatic synthesis method and use
InactiveCN102796144AImprove pharmacokinetic propertiesSugar derivativesCarrier-bound antigen/hapten ingredientsAntigenChemical synthesis
The invention discloses a Thomsen-Friedenreich (TF) antigen and a TF antigen analogue and their chemoenzymatic synthesis method and use. The chemoenzymatic synthesis method comprises the following steps of 1, carrying out chemosynthesis of fluorogalactose and a fluoro-galactosamine analogue, 2, carrying out enzymatic synthesis of a fluoro-TF antigen, and 3, carrying out enzymatic synthesis of a sialyl TF antigen. The preparation method has flexibility of a chemosynthesis method, and high regioselectivity and high efficiency of an enzymatic synthesis method, creatively realizes enzymatic synthesis of the fluoro-TF antigen, and solves the problem that the existing fluoro-TF antigen chemosynthesis method has low substrate reactivity, complex synthesis steps and a low yield. A tumor-associated fluoro-carbohydrate antigen is superior to a natural carbohydrate antigen in pharmacokinetic properties and thus the preparation method has wide application prospects in development of a novel anti-tumor vaccine.
Owner:SHANDONG UNIV
Stomach cancer serological detection and identification kit and method
ActiveCN106755377AExclude false positive resultsAvoid pressure lossMicrobiological testing/measurementAntigenCarbohydrate antigen
The invention discloses a stomach cancer serological detection and identification kit and a method. The stomach cancer serological detection and identification kit is prepared from a cel-miR-39-5p fragment, a primer, an exosome isolating reagent, an exosome miRNA (Micro Ribonucleic Acid) extracting reagent, a reverse transcription reagent and a real-time fluorescent quantitative PCR (Polymerase Chain Reaction) reagent, wherein the primer comprises a cel-miR-39-5p internal reference primer, an hsa-miR-375 primer, an hsa-miR-590-5p primer, an hsa-miR-19b-3p primer, an hsa-miR-100-5p primer and an hsa-miR-16 primer. When any results of detecting stomach cancer CA (Carbohydrate Antigen)19-9, CA24-2 and CEA (Carcino Embryonie Antigen) of a patient by using serum are positive, and the stomach cancer serological detection and identification kit provided by the invention is then used for carrying out aided detection, false positive results of serological detection can be effectively removed, so that huge mental stress and property loss brought to the patient can be avoided; meanwhile, aiming at the situation that when the results of detecting the stomach cancer CA19-9, CA24-2 and CEA of the patient by using the sera are negative, and the stomach cancer serological detection and identification kit provided by the invention is then used for carrying out the aided detection,, the false negative results of the serological detection can be effectively found, so that the life of the patient can be rescued in time.
Owner:ZHEJIANG PROVINCIAL HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Immunological detection method and kit for detecting mycobacterium tuberculosis
InactiveCN102243233AGuaranteed SensitivityStrong specificityMaterial analysisAntigenCarbohydrate antigen
The invention discloses an immunological detection method and a kit for detecting mycobacterium tuberculosis. According to the immunological detection method, mycobacterium tuberculosis specific antigens are adopted. The mycobacterium tuberculosis specific antigens comprise one or two components selected from protein 38kDa, protein CFP10, carbohydrate antigen LAM, and carbohydrate antigen TBGL. According to the invention, a multiple antigen composition is adopted as a mixed antigen for detecting tuberculosis antibodies. The method and the kit have advantages of high sensitivity, high specificity, and low false positive rate. During a real operation process, a multiple point combined determination result is improved into a single point determination result. Therefore the operation is more convenient. With the method and the kit, antibodies in tuberculosis patient body fluid samples such as serum and hydrothorax can be detected specifically in half a day. The kit can be produced in large scale with low cost. Therefore, the invention has an important significance in the controlling of tuberculosis.
Owner:张舒林 +2
Liposomal vaccine preparation and use thereof, as well as method for producing IgG antibody
InactiveCN106620682ASimple structureHigh titerCancer antigen ingredientsPharmaceutical non-active ingredientsAdjuvantCarbohydrate antigen
The invention relates to the field of liposomal vaccines, and discloses a liposomal vaccine preparation and an application thereof, as well as a method for producing IgG antibodies. The liposomal vaccine preparation comprises: (a) a tumor-associated carbohydrate antigen or glycopeptide antigen; (b) a linking unit; and (c) an adjuvant represented by formulas (1) to (3). According to the technical scheme disclosed by the invention, a high-titer and high-affinity IgG antibody can be guaranteed to be produced while the molecular structure of the vaccine is simplified. In addition, the vaccine molecules are good at thermal stability, thereby being relatively easy to store and transport.
Owner:HUAZHONG NORMAL UNIV
Modification of bioassays for detection of antigens characteristic of bacteria that are causative of ear and respiratory infections to eliminate false positive results caused by nasopharyngeal colonization of children
The present invention relates to modifying rapid immunochromatographic (“ICT”) tests for the detection of characteristic carbohydrate antigens of bacteria that are known to be causative of otitis media and respiratory diseases in children under the age of approximately 12 years. The test modifications involve either (1) reducing the total amount of antibodies to the carbohydrate antigen employed in each test, (2) adding at least one fixed “scrub” line located just prior to the capture line in the sample flow path to the prepared ICT test strip to “scrub” out an identical amount of target antigen from all bodily fluid test samples obtained from both colonized but otherwise healthy children and diseased children, or (3) combinations of (1) and (2).
Owner:ALERE SCARBOROUGH
Cancer stem cell markers and uses thereof
InactiveCN102753199AImmunoglobulins against blood group antigensBiological material analysisAntigenCarbohydrate antigen
The present invention pertains to therapeutic methods which are based on the use of an agent specifically binding a tumor-associated carbohydrate antigen for the treatment of cancer stem cells and related diseases. Also provided are diagnostic and prognostic methods using a tumor- associated carbohydrate antigen as marker for cancer stem cells.
Owner:GLYCOTOPE GMBH
Modification of bioassays for detection of antigens characteristic of bacteria that are causative of ear and respiratory infections to eliminate false positive results caused by nasopharyngeal colonization of children
InactiveUS20050260694A1Reduce eliminateReduce morbidityBiological testingCarbohydrate antigenRespiratory disease
The present invention relates to modifying rapid immunochromatographic (“ICT”) tests for the detection of characteristic carbohydrate antigens of bacteria that are known to be causative of otitis media and respiratory diseases in children under the age of approximately 12 years. Children of this age group are also prone to nasopharyngeal colonization with the same bacteria, and urine samples taken from colonized, but otherwise healthy, children were shown to exhibit an unduly high incidence of test results that were false positive for the presence of disease. The test modifications, which maintain the test sensitivity unchanged and the test specificity at a value above 90% were developed to insure that healthy, albeit colonized, children were not medicated for disease the bacteria are known to cause. The modifications involve either (1) reducing the total amount of antibodies to the carbohydrate antigen employed in each test, (2) adding at least one fixed “scrub” line located just prior to the capture line in the sample flow path to the preprepared ICT test strip to “scrub” out an identical amount of target antigen from all bodily fluid test samples obtained from both colonized but otherwise healthy children and diseased children, or (3) combinations of (1) and (2).
Owner:ALERE SCARBOROUGH
Carbohydrate antigen-125 quantitative determination kit and application thereof
InactiveCN103675278ANo radioactivityEliminate hazardsMaterial analysisAntigenAntiendomysial antibodies
The invention provides a carbohydrate antigen-125 quantitative determination kit and a detection method thereof. According to the scheme, a principle of a double-antibody sandwich enzyme immunoassay chemiluminescence method is adopted to prepare a carbohydrate antigen-125 quantitative determination kit. The kit comprises a CA125 (carbohydrate antigen-125) antibody-coated micro-plate / strip, six calibration material bottles, two quality control bottle, an enzyme conjugate, a washing concentrate bottle, a luminous substrate A1 bottle and a luminous substrate B1 bottle. The kit is applied to large-scale detection and manual detection one by one or group by group and has the beneficial effects of high sensitivity, high accuracy and high reproducibility.
Owner:上海裕隆医学检验所股份有限公司
Sialic acid (α-(2→6))-d-pyranose derivative and its synthesis method and application
ActiveCN102276662ANovel structureHigh activityEsterified saccharide compoundsOrganic active ingredientsPyranoseAntiendomysial antibodies
The invention discloses an N-acyl group modified sialic acid (alpha-(2-6))-D-amino pyranose derivative and its synthetic method and use. The sialic acid (alpha-(2-6))-D-amino pyranose derivative shown in the formula (I) is synthesized from raw materials of D-galactosamine (glucose) and sialic acid and is coupled with a carrier protein or a polypeptide to form a glycoprotein (glycopeptide) conjugate. In a structure of the N-acyl group modified sialic acid (alpha-(2-6))-D-amino pyranose derivative, a derivative acyl group replaces an acetyl group and thus the structure is novel. The N-acyl group modified sialic acid (alpha-(2-6))-D-amino pyranose derivative has good activity in anti-tumor vaccines. A result of an experiment on mice shows that through structural derivatization, a carbohydrate antigen based vaccine can produce an effective immune response and a mass of antibodies and IgG / IgM is improved obviously. The antibodies can identify specifically tumor cells expressing STn and thus anti-tumor effects are realized. STn antigens can be expressed on multiple tumors and thus the N-acyl group modified sialic acid (alpha-(2-6))-D-amino pyranose derivative has a wide application scope.
Owner:PEKING UNIV
Method for auxiliary diagnosis of liver cancer by means of combined detection of tumor markers based on artificial neural network
InactiveCN107024586AImprove accuracyIncrease positive rateMaterial analysis by observing effect on chemical indicatorDisease diagnosisStatistical analysisCarbohydrate antigen
The invention discloses a method for auxiliary diagnosis of liver cancer by means of combined detection of tumor markers based on an artificial neural network. The method comprises the following steps: collecting a blood sample of a patient; adopting a chemiluminesent immunoassay kit to respectively measure the contents of alpha fetoprotein (AFP), carcino-embryonic antigen (CEA) and carbohydrate antigen 125 (CA125) in serum; measuring the level of sialic acid (SA) in the serum by applying a visible spectrophotometry; measuring the level of Ca in the serum by using an azo arsenic III end-point method; carrying out descriptive statistical analysis on all data by using statistics, and taking diagnostic sensitivity, specificity, accuracy, a positive predictive value and a negative predictive value as evaluation indexes; adopting a back-propagation neural network algorithm to obtain a trained model; using the trained model to predict a corresponding test set. The method provided by the invention is high in accuracy rate and can be used for well distinguishing the liver cancer, benign and normal, thus having good popularization and application prospects.
Owner:中国人民解放军第一五九医院
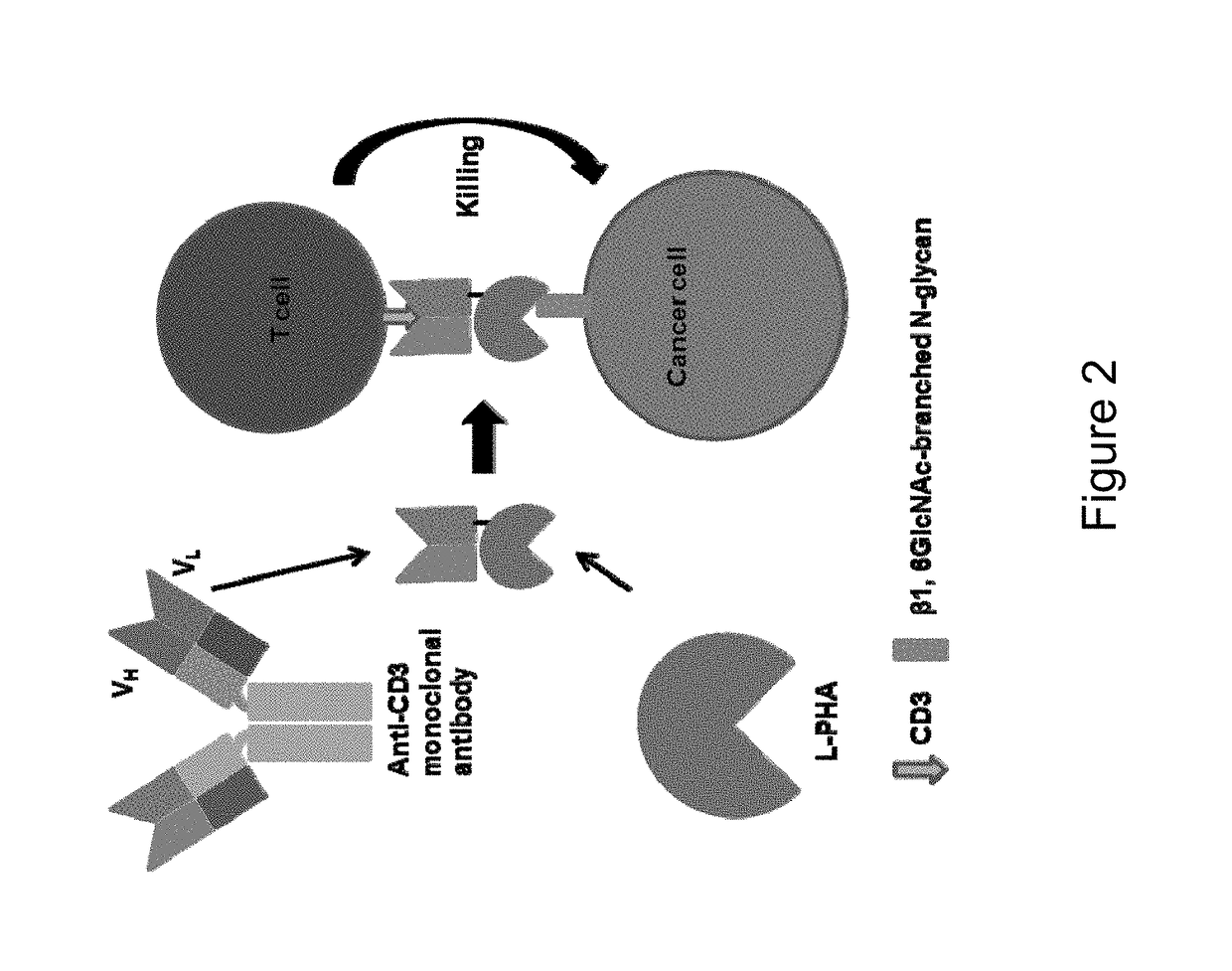
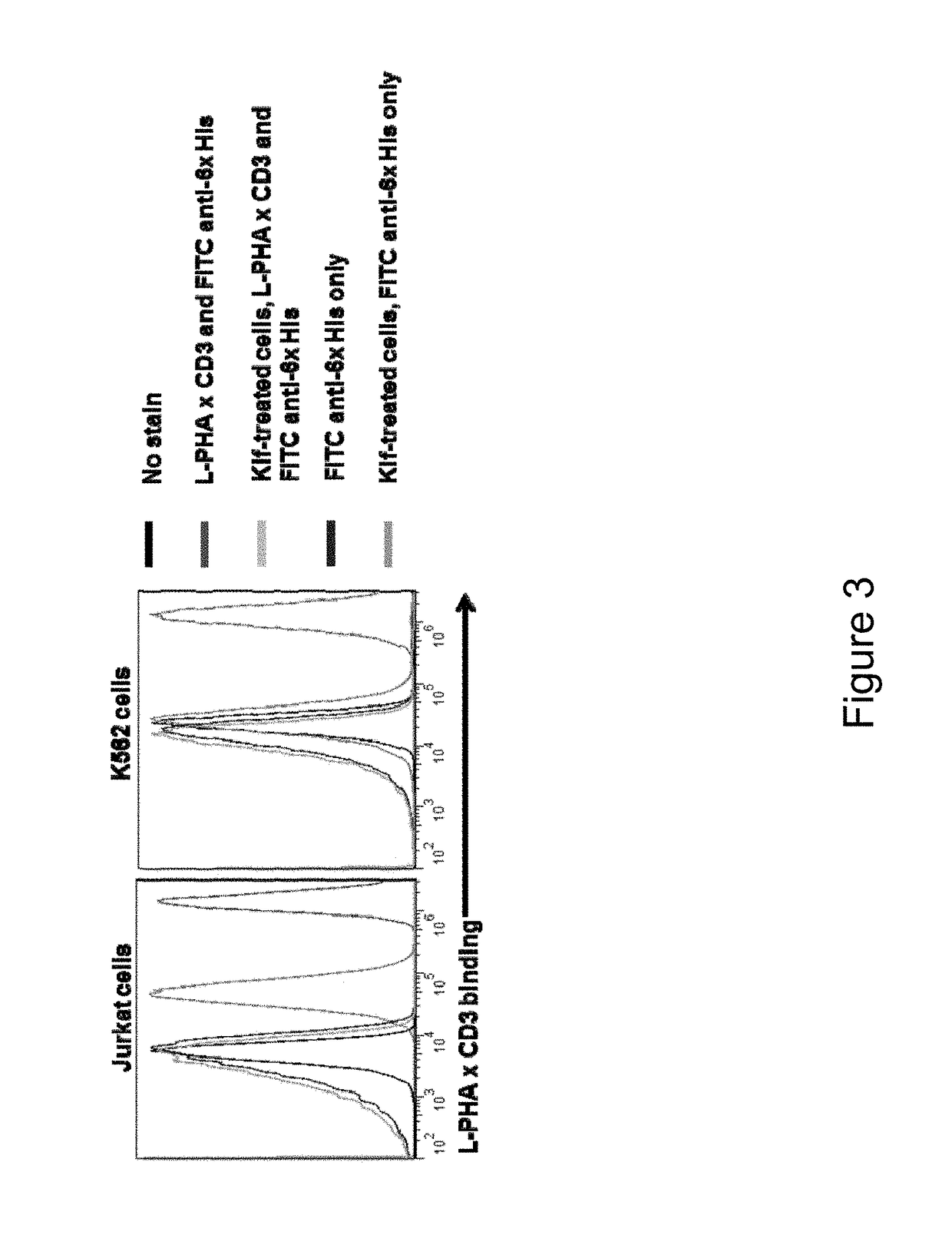
Glycan-dependent Immunotherapeutic Molecules
ActiveUS20180169260A1Polypeptide with localisation/targeting motifPeptide/protein ingredientsCarbohydrate antigenBinding domain
The present invention relates to compositions and methods for treating cancer. The invention makes use of peptides, nucleic acids encoding such peptides, and cells expressing such peptides, where the peptide comprises a tumor-associated carbohydrate antigen (TACA)-binding domain.
Owner:RGT UNIV OF CALIFORNIA
Quantitative determination kit for carbohydrate antigen 125 (CA125), and detection method thereof
InactiveCN102323252AHigh detection sensitivityImprove featuresChemiluminescene/bioluminescenceAntigenCarbohydrate antigen
The present invention discloses a quantitative determination kit for carbohydrate antigen 125 (CA125). The quantitative determination kit is characterized in that: the kit comprises a CA125 magnetic separation reagent, an enzyme reactant, a reaction reinforcing agent, a diluent, a CA125 standard sample, a CA125 quality control sample, a cleaning solution, a concentrated solution and a substrate solution. The invention further discloses a preparation method for the kit. According to the kit provided by the present invention, a chemiluminescence technology is combined with immunomagnetic nanoparticles; a reaction system approaching to the homogeneous phase is provided; compared to the prior art, the kit provided by the present invention has higher detection sensitivity and higher specificity, and reaches better performance parameters, and the product cost is substantially reduced.
Owner:INNER MONGOLIA KEHUI BIOLOGICAL TECH
Anti-CA125 carbohydrate antigen nano-antibody, and application thereof
ActiveCN108178799ASignificant ADCC effectAchieve precise imagingIn-vivo radioactive preparationsBacteriaAntigenComplementarity determining region
The invention discloses an anti-CA125 carbohydrate antigen nano-antibody. The nano-antibody has three unique complementarity determining regions CDR1, CDR2 and CDR3. The invention also discloses an application of the nano-antibody in the preparation of tumor treatment drug and tumor diagnosis reagents. The anti-CA125 nano-body has highly specific recognizing and binding ability to the CA125, has aremarkable ADCC effect on ovarian cancer cells, and can accurately image tumors in mouse bodies.
Owner:深圳市国创纳米抗体技术有限公司
Carbohydrate antigen CA19-9 assay kit and detection method using the same
InactiveCN107389946ALong validity periodHigh Luminescence Quantum YieldBiological testingCarbohydrate antigenMagnetic bead
The invention discloses a carbohydrate antigen CA19-9 assay kit. The kit comprises a magnetic separation reagent R1, a labeling reagent R2, a CA19-9 calibration product and a CA19-9 quality control product. The invention also provides a preparation method of the kit. The magnetic separation reagent R1 in the kit is prepared through coupling a CA19-9 monoclonal antibody to the surface of a carboxyl magnetic bead. The labeling reagent R2 is prepared through coupling a luminescent substance acridinium ester to a paired CA19-9 monoclonal antibody. The reactions occur under nearly homogeneous conditions. Through use of an excitation solution, the reaction product antibody-antigen-antibody sandwich complex can immediately release photons at 430 nm and can release strong photons without use of a luminescent enhancer and a catalyst so that luminescence detection can be directly carried out. Through optimizing the experimental processes of magnetic bead coating with an antibody and antibody labeling with acridinium ester, the stability and the detection sensitivity of the kit are greatly improved. The kit has a wide linear detection range, is easy to operate and has a fast detection rate.
Owner:北京健安生物科技有限公司
Carbohydrate-glycolipid conjugate vaccines
ActiveUS20150238597A1Antibacterial agentsBacterial antigen ingredientsConjugate vaccineCarbohydrate antigen
The present invention relates to the field of synthesizing and biologically evaluating of a novel class of carbohydrate-based vaccines. The new vaccines consist of a multi-modular structure which allows applying the vaccine to a whole variety of pathogenes. This method allows preparing vaccines against all pathogens expressing immunogenic carbohydrate antigens. As conjugation of antigenic carbohydrates to proteins is not required the conjugate vaccine is particularly heat stable. No refrigeration is required, a major drawback of protein-based vaccines.
Owner:MAX PLANCK GESELLSCHAFT ZUR FOERDERUNG DER WISSENSCHAFTEN EV
Chemiluminescence quantitative detection kit for carbohydrate antigen 242
InactiveCN102095846AQuick checkEasy to detectChemiluminescene/bioluminescenceCarbohydrate antigenBiology
The invention discloses a chemiluminescence quantitative detection kit for a carbohydrate antigen 242 (CA242). The kit comprises a CA242 detection reaction plate, an enzyme conjugate, a luminescent substrate, a calibrator, a quality control material and washing concentrate, wherein the reaction plate is coated with a CA242 antibody. The kit can specifically and quantitatively detect the content of the CA242 in patient serum, and is used for the auxiliary diagnosis and prognosis of malignant tumors of a digestive tract such as pancreatic cancer and the like, and monitoring of curative effects. Compared with the conventional enzyme linked immunosorbent assay (ELISA) technology, the chemiluminescence immunoassay keeps high specificity of the ELISA technology, stability and reliability of a detection result, and convenience of operation, and can improve detection sensitivity simultaneously.
Owner:上海裕隆生物科技有限公司
Synthesis method and application of sialylated TF antigen and its fluorination derivatives
ActiveCN108164573AGood metabolic stabilitySugar derivativesCancer antigen ingredientsEnzymatic synthesisChemical synthesis
The invention discloses a synthesis method and an application of a sialylated TF antigen and its fluorination derivatives. The method includes the following steps: (1) chemically synthesizing fluorogalactose and fluorogalactosamine analogues; (2) chemically synthesizing a fluorinated TF antigen; and (3) synthesizing the sialylated TF antigen and its fluorination derivatives through an enzyme technology. The flexibility of a chemical synthesis technology is combined with the high regioselectivity and the high efficiency of the enzyme synthesis technology, so the enzymatic synthesis of the fluorosialylated TF antigen is achieved for the first time, and the disadvantages of many synthesis steps, poor stereoselectivity, low yield and use of a heavy metal salt in existing chemical synthesis ofthe fluorosialylated TF antigen are overcome. A fluorotumor-associated carbohydrate antigen has a higher stability than natural carbohydrate antigen, so the sialylated TF antigen and its fluorinationderivatives have a broad application prospect in the development of novel antitumor vaccines.
Owner:TIANJIN UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com