Conjugate of monophosphate A (MPLA) and carbohydrate antigen Globo H and preparation method and application thereof
A technology of monophosphate esters and conjugates, applied in the field of anti-tumor sugar vaccine development, can solve problems such as suppression, difficult immune response, and difficult confirmation of protein coupling site structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Preparation of sugar vaccine GL-01
[0042]
[0043] 1) Preparation of Compound 4
[0044]
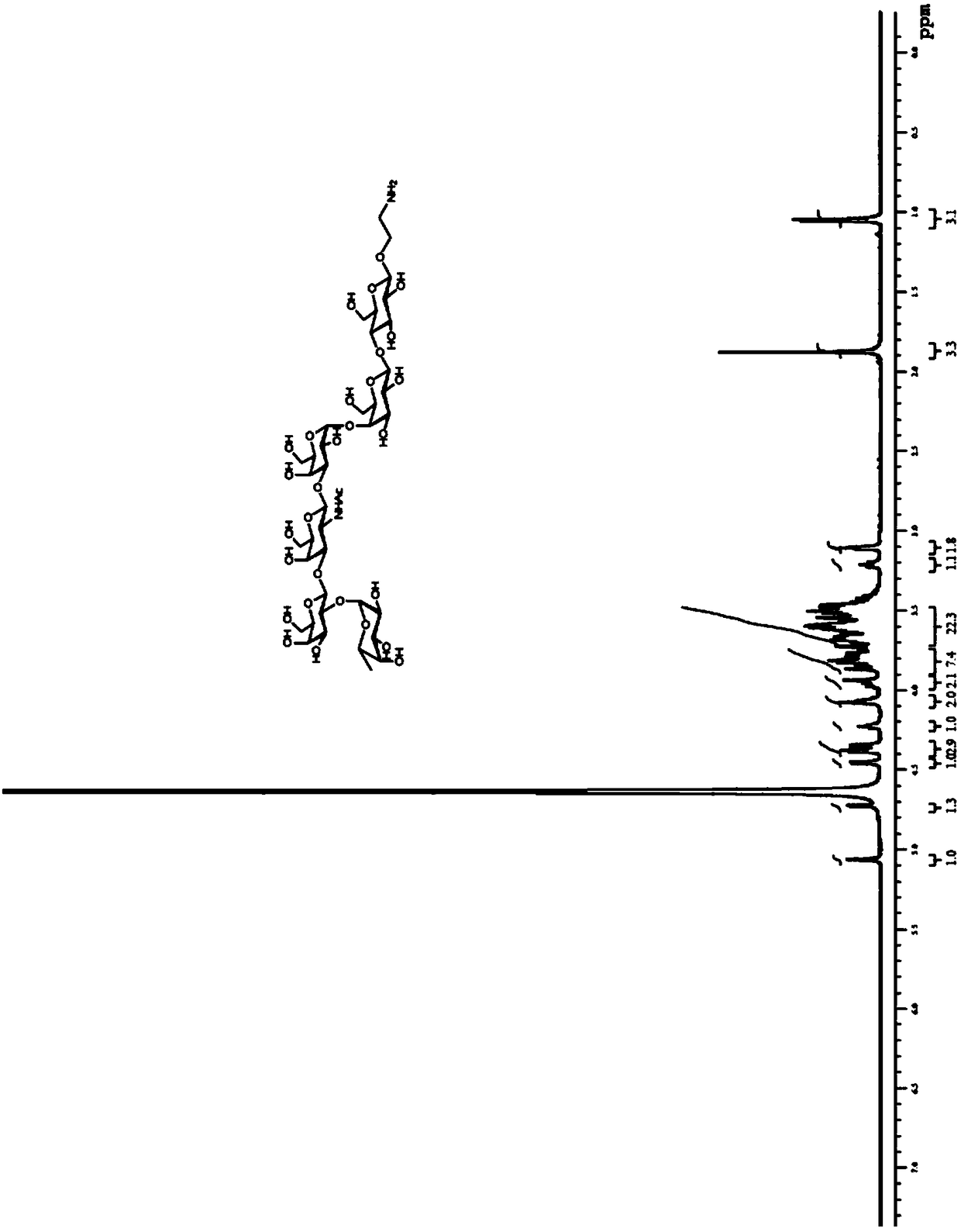
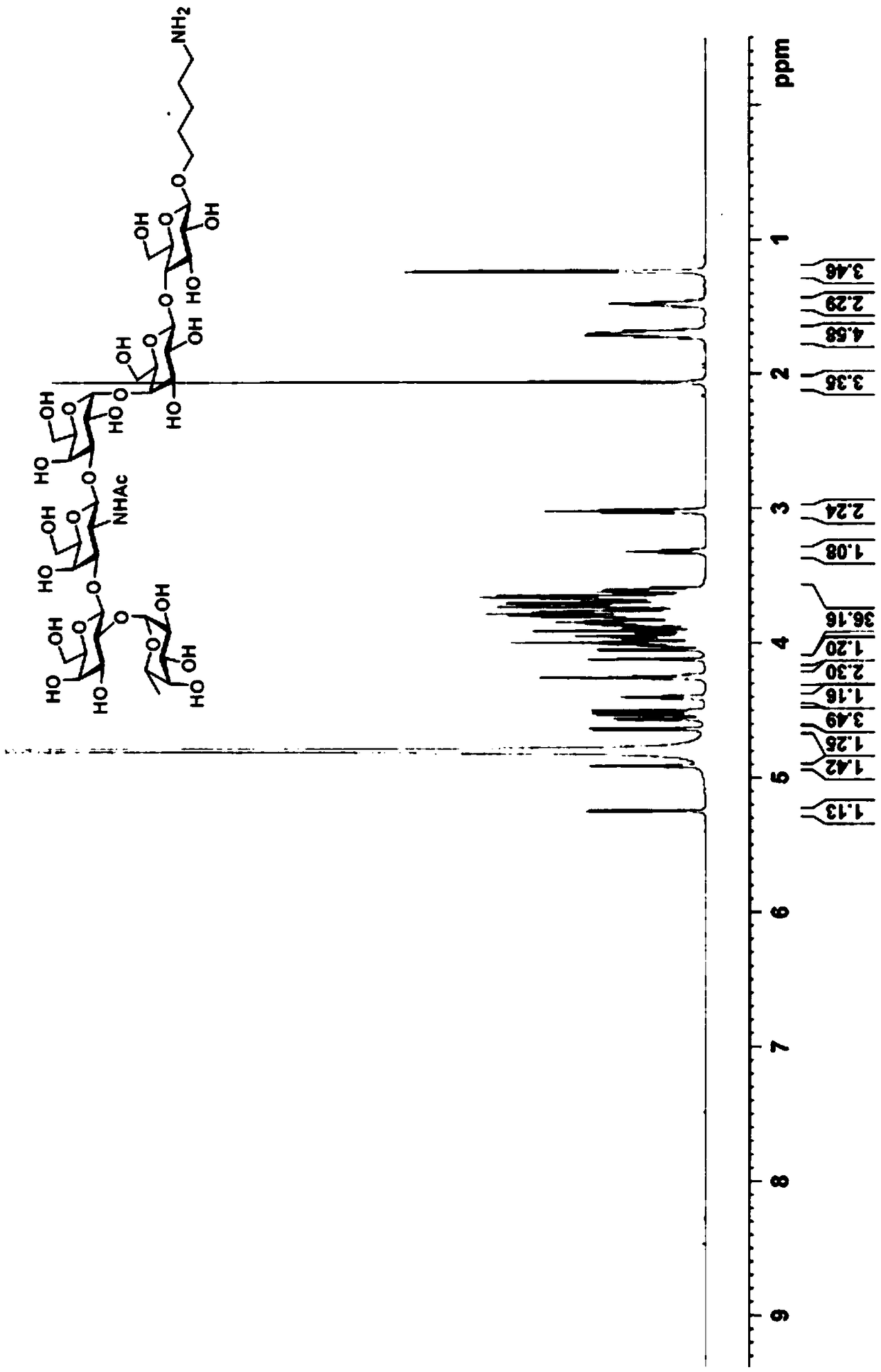
[0045] Dissolve compound 3 (42 mg, 0.02 mmol) in 10 mL of dichloromethane, add appropriate amount of active zinc powder and acetic acid, react at room temperature for 6 h, remove the solid by filtration, remove dichloromethane and acetic acid under reduced pressure, and then extract the obtained oil Dissolve in 10mL dichloromethane containing adipic anhydride (5mg, 0.04mmol), add an appropriate amount of N-methylmorpholine to make the pH of the reaction solution greater than 7, react at room temperature for 12h, dilute with an appropriate amount of dichloromethane, and use saturated NaHCO 3 Washed and extracted 3 times, dried over anhydrous sodium sulfate, filtered, concentrated, and separated by silica gel column chromatography (methanol:dichloromethane=1:4) to obtain compound 4 (36 mg, yield 82%). ESI-MS(m / z):2281.5[M+H] + . 1H-NMR(CDCl3,400MHz):7.35-7....
Embodiment 2
[0055] Embodiment 2: the preparation of sugar vaccine GL-02
[0056] 1) Preparation of Compound 9
[0057] Compound 9 (9 mg, 63%) was prepared by reacting compound 5 (12 mg, 5 μmol) with Globo H derivative 7 (6 mg, 8 μmol) using a method similar to step 3 of Example 1. ESI-MS(m / z):3364.0 [M+H] + . 1 H NMR (600MHz, CDCl 3 :CD 3 OD:D 2 O=3:3:1):δ:7.33-7.12(m,25H, ArH),5.51(m,1H,lipid-H-3'),5.37-5.29(m,1H,lipid-H-3) ,5.28-5.19(m,3H,2H of lipid,,H-1""'),5.17-5.00(m,4H,(Ph CH 2 O) 2 P),1.99(s,3H,NH Ac); 1.63-1.40 (m, 12H, lipid), 1.36-1.09 (br, 98H, 48x CH 2 ,lipid),0.98-0.78(18H,6x CH3,lipid).
[0058]
[0059] 2) Preparation of sugar vaccine GL-02
[0060] Prepared by a method similar to step 4 of Example 1, compound 9 (7 mg, 2.2 μmol) was reacted with hydrogen to prepare Globo H sugar vaccine GL-02 (5 mg, 83%). ESI-MS(m / z):2913.7[M+H] + . 1 H NMR (600MHz, CDCl 3 :CD 3 OD:D 2 O=5:3:1):δ:1.98(s,3H,NH Ac ); 1.82-1.57(m,12H,lipid),1.52-1.12(br,98H,48x CH 2 ...
Embodiment 3
[0062] Embodiment 3: the determination of the immune antibody titer of Globo H sugar vaccine GL-01 and GL-02
[0063] 1) Mouse immunization experiment: 6-8 week old mice were taken and divided into immunization group and control group (PBS), 5 mice in each group. The sugar vaccines GL-01 and GL-02 prepared in Example 1 and Example 2, as well as Compound 1 as a positive control, were prepared into liposomes, and the immune test was carried out by subcutaneous injection in mice, using an initial Immunization and three booster immunization schemes, inject the prepared vaccine on days 0, 14, 21, and 28 respectively, and take 0.1ml to 0.2ml of blood from each mouse on days 0, 27, and 38, and place it at 37 degrees for half an hour , placed at 4 degrees for half an hour, centrifuged at 5000 rpm, and the clear serum in the upper layer was separated for ELISA detection and analysis. (Org.Biomol.Chem.,.2014,12,3238; Chem.Sci.,2015,6,7112)
[0064] 2) ELISA immunoassay: 96-well plate,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com