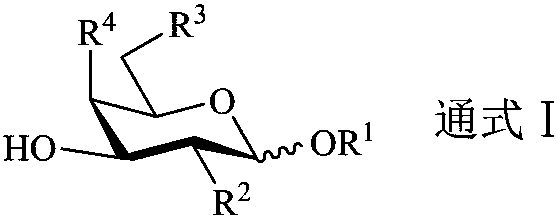
Synthesis method and application of sialylated TF antigen and its fluorination derivatives
A technology of sialylation and synthesis method, applied in the direction of sugar derivatives, sugar derivatives, cancer antigen components, etc., can solve the problem of reducing the electron density of sugar rings
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Synthesis of the compound 2-acetylamino-2-deoxy-D-galactose
[0071] The reaction equation is as follows:
[0072]
[0073] Into a 250mL round bottom flask, add galactosamine hydrochloride 8 (5g, 23mmol), CuSO 4 .5H 2 O (58mg, 0.23mmol), potassium carbonate (1.38g, 10mmol), TfN 3 (5.3g, 27.6mmol) and methanol (100mL), stirred at room temperature for 6h. After the completion of the reaction was detected by thin-layer chromatography, concentrated by rotary evaporation, added dilute hydrochloric acid solution (80 mL) and EtOAc (80 mL) for extraction, and repeated 3 times. The organic phases were combined and concentrated under reduced pressure to obtain an intermediate. Into a 100mL round bottom flask, the intermediate obtained in the previous step and 3-chloro-1-propanol (50mL) were added, acetyl chloride (2mL) was added dropwise, and reacted at room temperature for 3h to obtain compound 9 (4.4g, 67%).
[0074] In the 100mL round bottom flask, add compound 9 (920m...
Embodiment 2
[0076] Synthesis of 2,3,4-tri-acetyl-6-fluoro-α-D-galactosyltrichloroacetimidate 7
[0077] The reaction equation is as follows:
[0078]
[0079] Galactose (1, 10 g, 56 mmol) was dissolved in dry acetone (300 mL), and concentrated sulfuric acid (10 mL) was gradually added under ice-bath conditions. The ice bath was removed and stirred at room temperature for 3 h. After the reaction was complete, water (10 mL) and sodium carbonate (20 g, 189 mmol) were added to quench sulfuric acid to terminate the reaction. Filtrate with celite, concentrate by rotary evaporation, and flash column separation and purification (PE:EtOAc=1:5~1:10) to obtain compound 2 (13.0 g, 90%) as syrup.
[0080] Compound 2 (1.0g, 3.85mmol), diethylaminosulfur trifluoride (DAST, 0.81g, 5.04mmol), 2,4,6-collidine (1.12g, 9.24mmol) and dry 1, 2-Dichloroethane (5 mL) was added into a 10 mL microwave reaction tube, and reacted at 80° C. and 100 W for 1 hour. After cooling to room temperature, methanol was ...
Embodiment 3
[0086] Compound 3-Azidopropyl β-D-6-deoxy-6-fluoro-galactopyranosyl-(1–3)-2-acetamido-2-deoxy-α-D-galactopyranoside (Gal6Fβ1-3GalNAcProN 3 ,13) Synthesis of
[0087] The reaction equation is as follows:
[0088]
[0089] Compound 7 (386 mg, 0.85 mmol) and compound 10 (210 mg, 0.57 mmol) were dissolved in CH 2 Cl 2 (5mL), add Molecular sieves, under ice bath conditions, stirred for 15min, added TMSOTf (11uL, 0.114mmol). After reacting for 20 minutes, triethylamine was added dropwise to stop the reaction, filtered with diatomaceous earth, concentrated by rotary evaporation, and purified by rapid column separation (PE:EtOAc= 5:1) to obtain white solid compound 11 (290 mg, 77%).
[0090] Compound 11 (1.4g 2.0mmol) was dissolved in THF (35mL), acetic acid (16mL), acetic anhydride (5.6mL), zinc powder (11.2g) and reacted at room temperature for 1 hour. Heat to reflux for 24 hours. After the reaction was detected by thin-layer chromatography, it was concentrated by rotary ev...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com