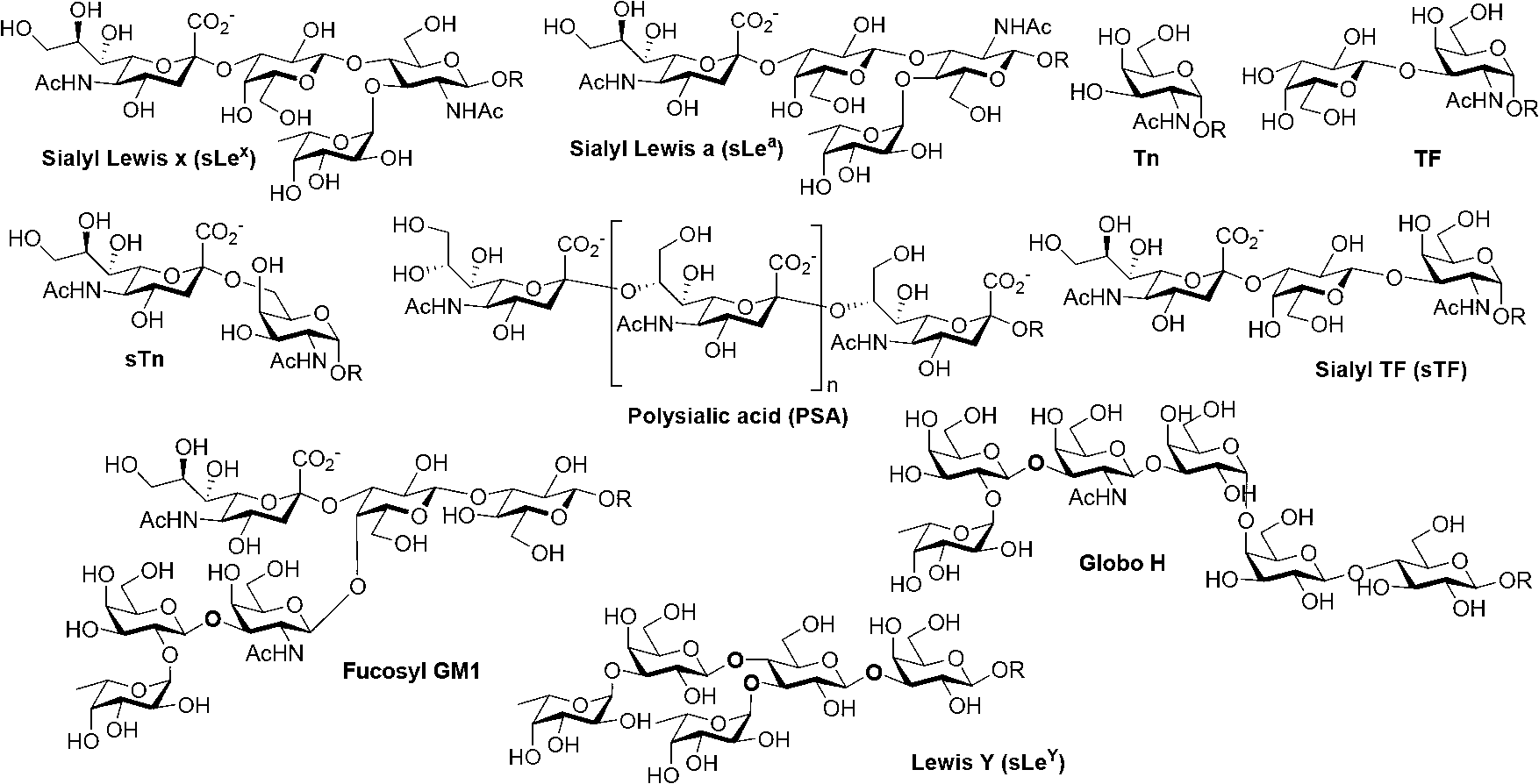
Thomsen-Friedenreich (TF) antigen and TF antigen analogue and their chemoenzymatic synthesis method and use
A synthesis method and analog technology are applied in the field of chemical enzymatic synthesis of tumor-related carbohydrate antigens and their analogs, and can solve the problems of reducing sugar ring electron density and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0070] 1. Chemoenzymatic synthesis of T antigen, sT antigen and their fluorinated derivatives
[0071] Synthesis of the compound 2-acetylamino-2-deoxy-D-galactose
[0072] The reaction equation is as follows:
[0073]
[0074] Into a 500mL round bottom flask, add galactosylamino hydrochloride (7.01g, 32.51mmol), acetic anhydride (60mL), pyridine (Pyr 120mL), dimethylaminopyridine (DMAP 1.06g, 8.68mmol), and stir at room temperature for 12h . Thin-layer chromatography detection (petroleum ether: ethyl acetate = 3:2), after the reaction is complete, add 20mL toluene to the reaction solution, rotary evaporation and concentration, repeat 3 times. The obtained solid was redissolved in 30 mL of methanol, placed in ultrasonic for 0.25 h, and then left to stand at 4°C for 12 h. Filtrate, discard the filtrate, collect the resulting solid, and dry to obtain white solid compound 18 (10.75 g, yield 85%).
[0075] Into a 500 mL round bottom flask, add solid compound 18 (5.69 g, 14.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com