Preparation method of nano vaccine with pH and reduction dual sensitivity and obtained product
A nano-vaccine, a sensitive technology, is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc. It can solve the problems of antigen dissociation, toxic and harmful tissue cells, etc. , the effect of improving efficacy and biosafety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
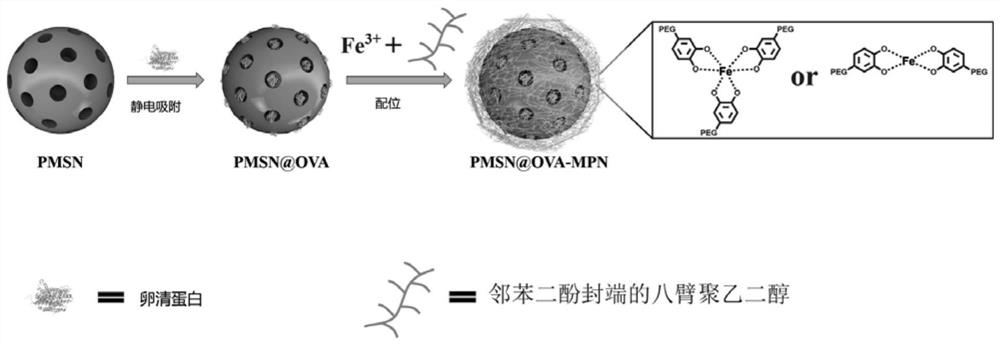
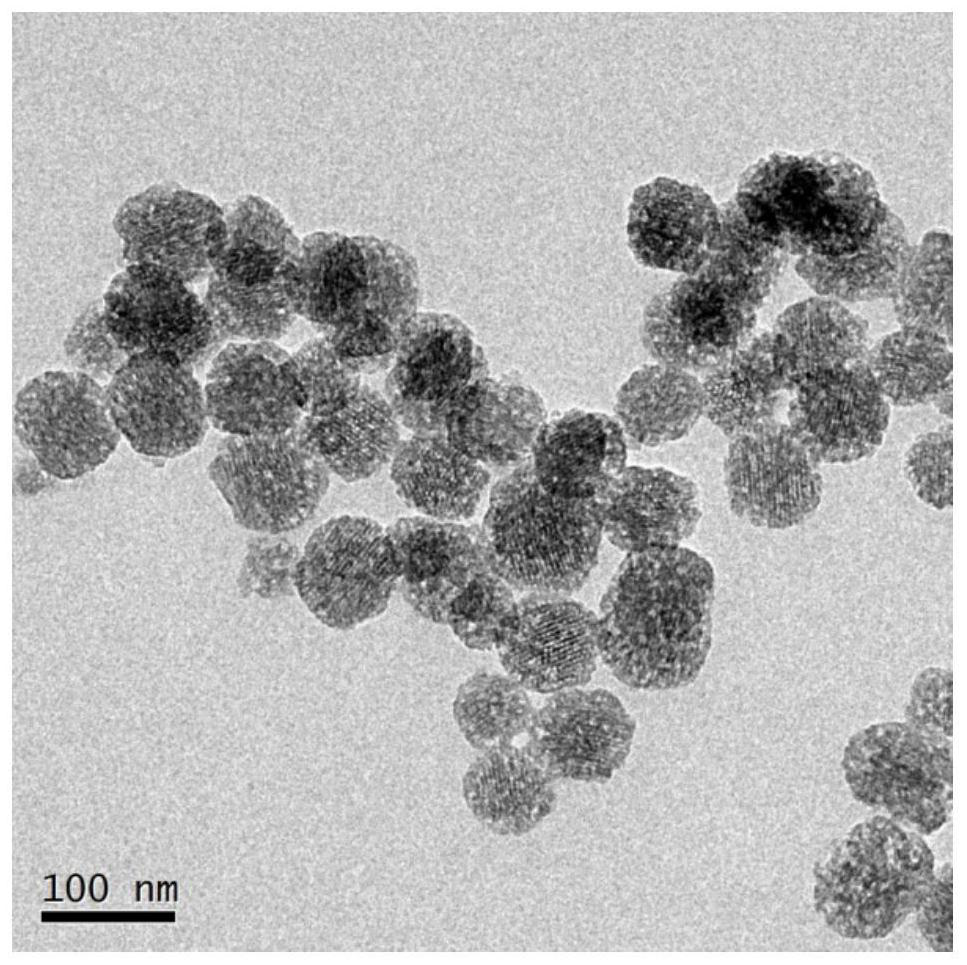
[0040] The preparation process of nano-vaccine with pH and reduction sensitivity is as follows: figure 1 As shown, the specific steps are as follows:
[0041]Synthesis of PEI-modified mesoporous silica nanospheres (PMSNs): 20 mg of lyophilized MSNs were dispersed in 10 mL of ultrapure water by sonication. Add PEI solution (2 mL, 20 mg / mL) to the above MSN suspension. After magnetic stirring for 2 hours, the PEI-modified mesoporous silica nanospheres were collected by centrifugation (12000 rpm, 15 min), and washed 3 times with pure water to remove free PEI. Finally, pure PEI-modified mesoporous silica nanospheres were obtained by lyophilization, denoted as PMSN.
[0042] Loading of ovalbumin on PMSN: Suspend PMSN (1 mg) in 0.5 mL of pure water, and then suspend 0.5 mL of 2.0 mg / mL ovalbumin (OVA) solution in the suspension. The mixture was shaken rapidly at room temperature for 1 hour and then centrifuged (12000 rpm, 15 min). The solid at the bottom was washed three times w...
Embodiment 2
[0047] Synthesis of PEI-modified mesoporous silica nanospheres (PMSNs): 18 mg of lyophilized MSNs were dispersed in 10 mL of ultrapure water by sonication. PEI solution (2 mL, 20 mg / mL) was added to the above MSN suspension. After magnetic stirring for 2.5 h, the PEI-modified nanoparticles were collected by centrifugation (12000 rpm, 15 min), and washed 3 times with pure water to remove free PEI. Finally, pure PEI-modified mesoporous silica nanospheres were obtained by lyophilization, denoted as PMSN.
[0048] Loading of ovalbumin on PMSN: Suspend PMSN (1 mg) in 0.5 mL of pure water, and then suspend 0.5 mL of 1.6 mg / mL ovalbumin (OVA) solution in the suspension. The mixture was shaken rapidly at room temperature for 1 h, and then centrifuged (12000 rpm, 15 min). The bottom solid after centrifugation was washed three times with pure water to remove free OVA to obtain PMSN@OVA.
[0049] Synthesis of catechol-terminated eight-armed PEG (CPEG): 4-(2-aminoethyl)-1,2-benzenediol...
Embodiment 3
[0053] Study on OVA release behavior of PMSN@OVA-MPN in vitro
[0054] In order to study the OVA release behavior of PMSN@OVA-MPN in different media, take an appropriate amount of PMSN@OVA-MPN prepared in Example 1 (in which OVA is marked with FITC), divide it into three parts on average, and disperse them in A : 4 mL PBS (pH=7.4), B: 4 mL PBS (pH=7.4), and C: 4 mL PBS (pH=5) in three portions of PBS and stirred at 37°C. At the 7th hour of stirring, reduced glutathione was added to one neutral PBS suspension at pH = 7.4 to reach a concentration of 10 mM, but another neutral PBS and weakly acidic PBS suspension was maintained throughout No glutathione was added in the process. Centrifuge at different time points (12000 rpm, 5 min), then extract 800 μL supernatant from these suspensions for detection, and supplement the remaining suspension with corresponding 800 μL glutathione solution (10 mM), neutral PBS or weakly acidic PBS, and then continue to stir. Among them, add glut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com