Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
596 results about "Malarial parasites" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Malaria parasites belong to the genus Plasmodium (phylum Apicomplexa). In humans, malaria is caused by P. falciparum, P. malariae, P. ovale, P. vivax and P. knowlesi.
Therapy via targeted delivery of nanoscale particles
InactiveUS20050090732A1Destroying inhibiting vascularityAntibacterial agentsNervous disorderDiseaseProstate cancer
Disclosed are compositions, systems and methods for treating a subject's body, body part, tissue, body fluid cells, pathogens, or other undesirable matter involving the administration of a targeted thermotherapy that comprises a bioprobe (energy susceptive materials that are attached to a target-specific ligand). Such targeted therapy methods can be combined with at least one other therapy technique. Other therapies include hyperthermia, direct antibody therapy, radiation, chemo- or pharmaceutical therapy, photodynamic therapy, surgical or interventional therapy, bone marrow or stem cell transplantation, and medical imaging, such as MRI, PET, SPECT, and bioimpedance. The disclosed therapies may be useful in the treatment of a variety of indications, including but not limited to, cancer of any type, such as bone marrow, lung, vascular, neuro, colon, ovarian, breast and prostate cancer, epitheleoid sarcomas, AIDS, adverse angiogenesis, restenosis, amyloidosis, tuberculosis, cardiovascular plaque, vascular plaque, obesity, malaria, and illnesses due to viruses, such as HIV.
Owner:NANOTX INC
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
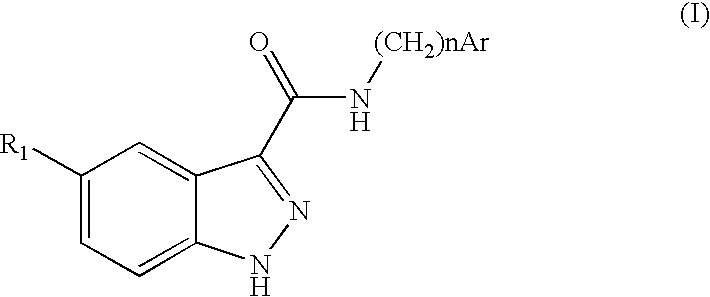
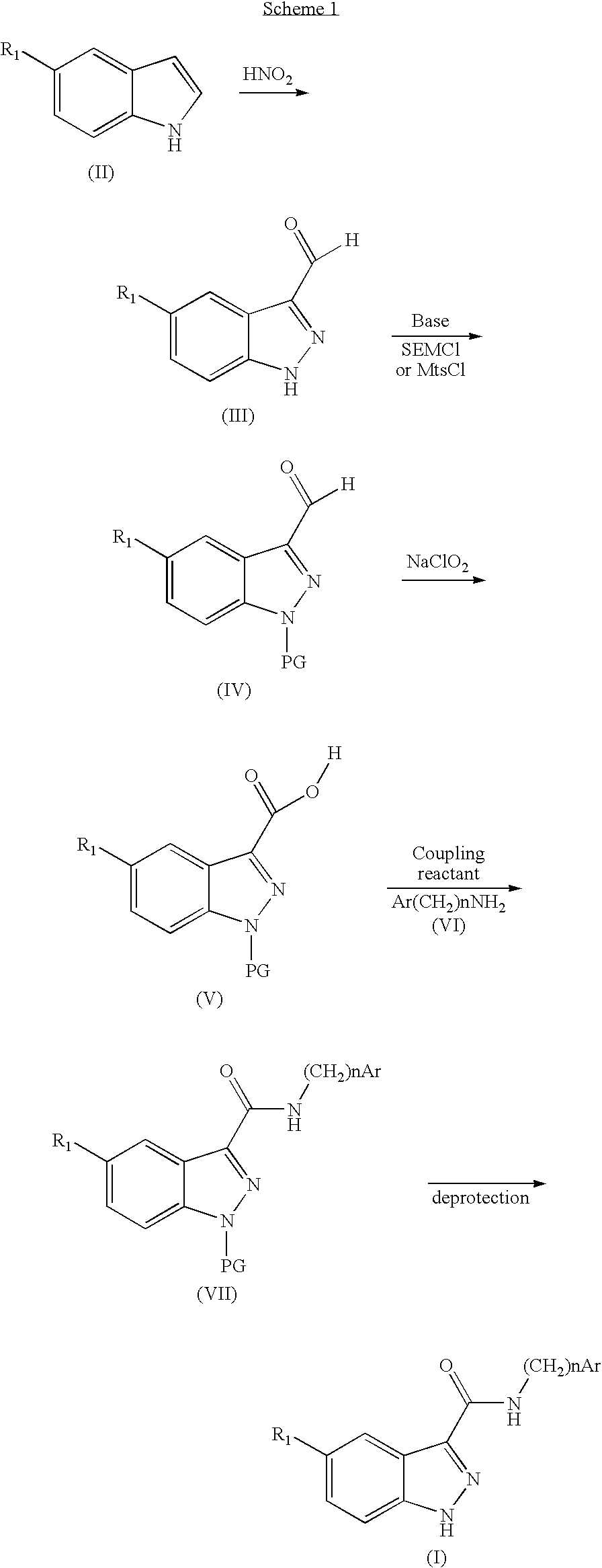
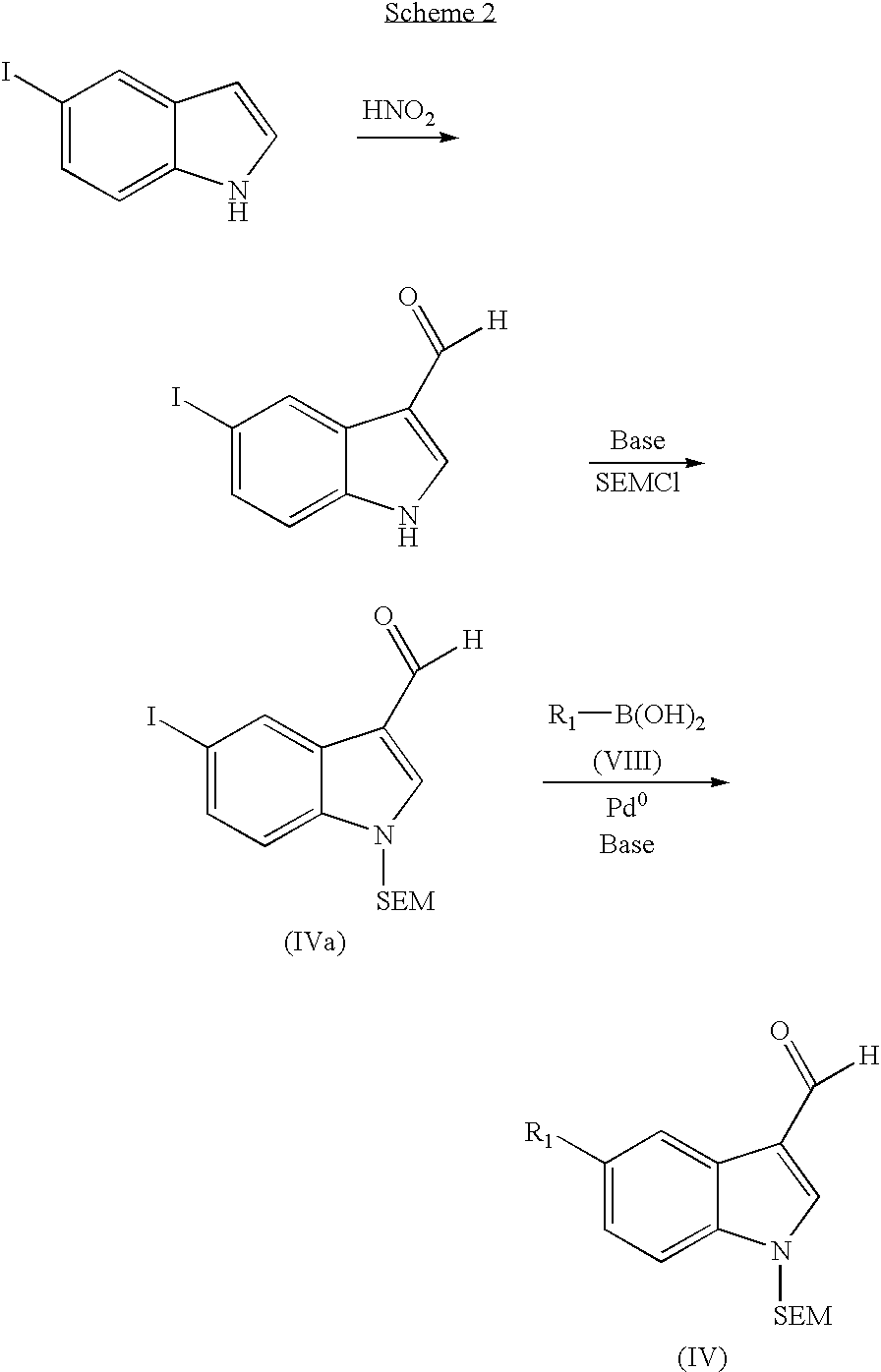
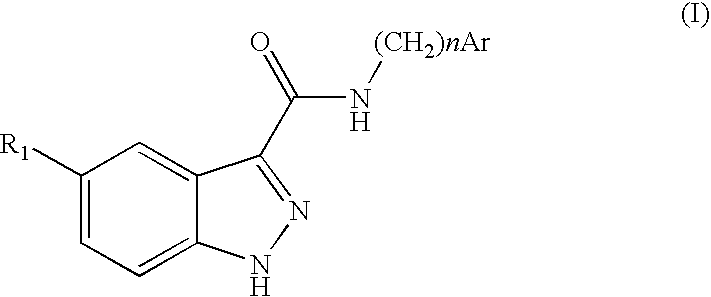
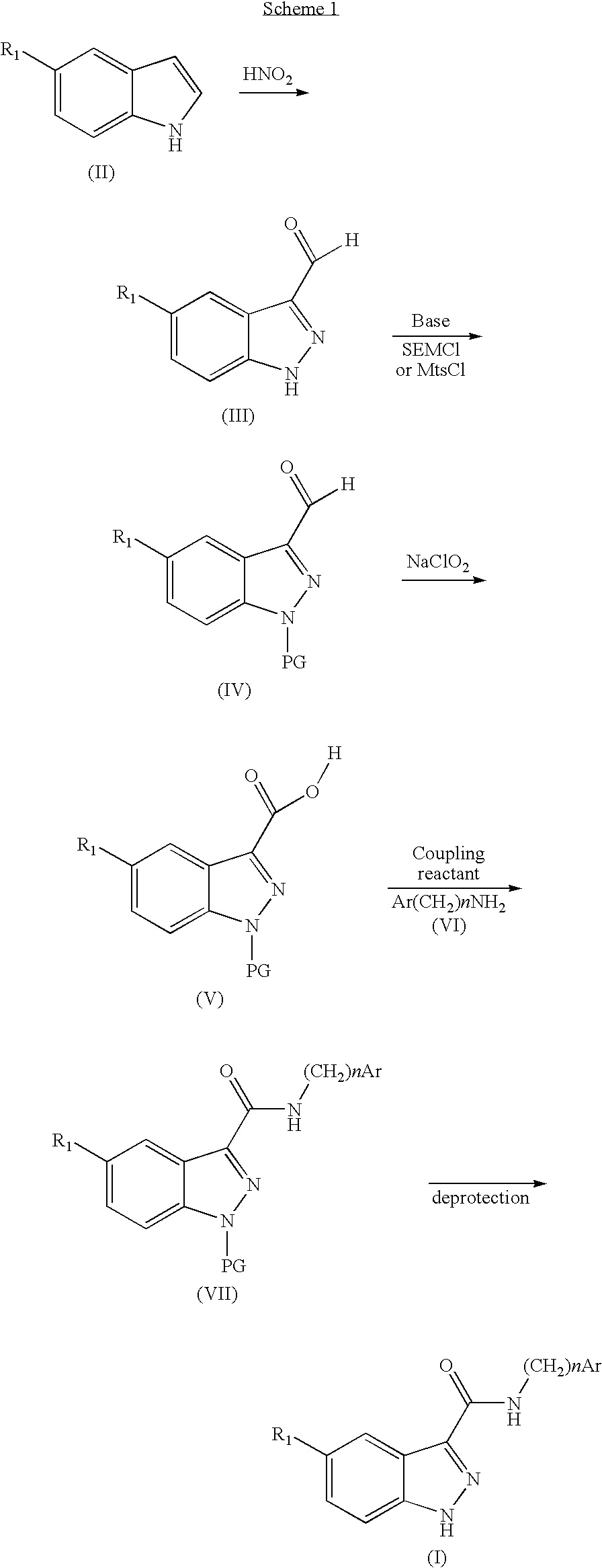
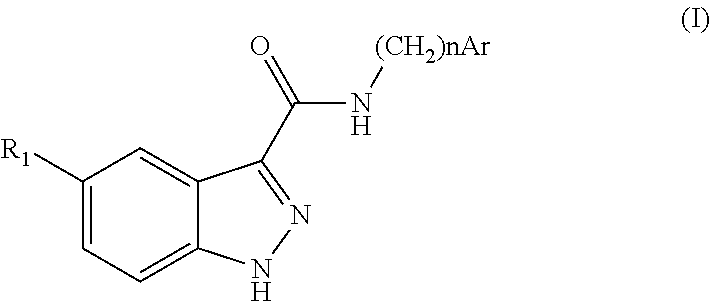
Indazolecarboxamide derivatives for the treatment and prevention of malaria
The invention relates to methods of treating or preventing malaria which comprises administering to a patient in need thereof, an effective amount of a 1H-indazole-3-carboxamide derivative of general formula (I), in the form of a base or of an addition salt with an acid, or in the form of a hydrate or of a solvate of said base or acid addition salt.
Owner:SANOFI AVENTIS SA
Indazolecarboxamide derivatives for the treatment and prevention of malaria
The invention relates to methods of treating or preventing malaria which comprises administering to a patient in need thereof, an effective amount of a 1H-indazole-3-carboxamide derivative of general formula (I), in the form of a base or of an addition salt with an acid, or in the form of a hydrate or of a solvate of said base or acid addition salt.
Owner:SANOFI AVENTIS SA
Indazolecarboxamide derivatives for the treatment and prevention of malaria
The invention relates to methods of treating or preventing malaria which comprises administering to a patient in need thereof, an effective amount of a 1H-indazole-3-carboxamide derivative of general formula (I), in the form of a base or of an addition salt with an acid, or in the form of a hydrate or of a solvate of said base or acid addition salt.
Owner:SANOFI AVENTIS SA
Methods of treatment of disease using adsorbent carriers
InactiveUS6498007B1Increase productionReduce in quantityAntibacterial agentsSolvent extractionDiseaseAcetic acid
The invention relates to a method for the removal of leucocytes from blood which comprises bringing blood that comprises infected leucocytes into contact with an adsorbent carrier that has a greater affinity for infected, activated and / or defective leucocytes than for uninfected leucocytes especially cellulose acetate. The method can be used in the apheresis treatment of diseases caused by pathogenic organisms, for example, HIV, HCV or malaria. It is especially useful for treatment of HIV.
Owner:JIMRO
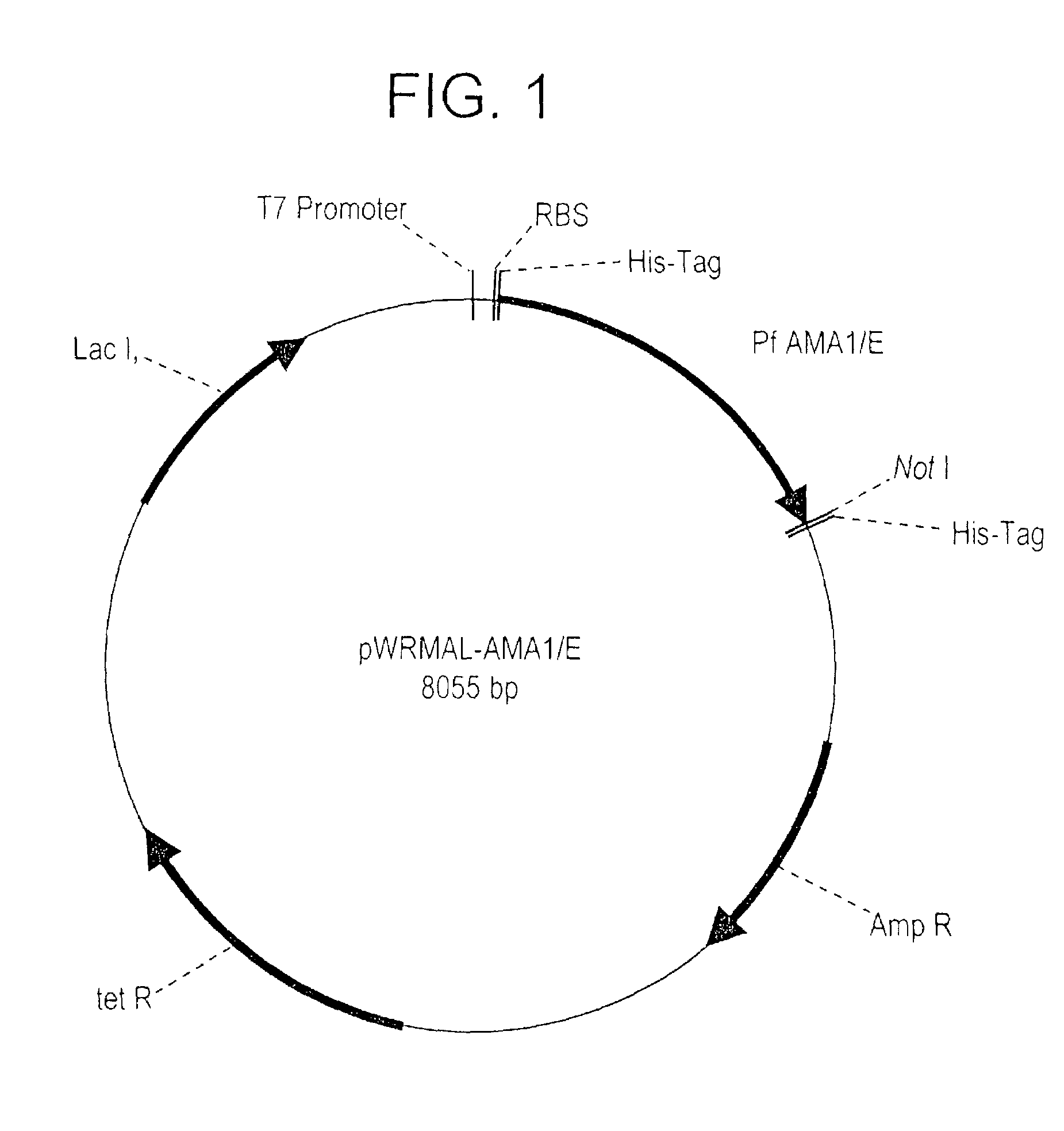
Plasmodium falciparum AMA-1 protein and uses thereof
InactiveUS7029685B2Eliminate the problemImprove responseProtozoaFermentationADAMTS ProteinsMalarial parasites
In this application is described the expression and purification of a recombinant Plasmodium falciparum (3D7) AMA-1 ectodomain. The method of the present invention produces a highly purified protein which retains folding and disulfide bridging of the native molecule. The recombinant AMA-1 is useful as a diagnostic reagent, for use in antibody production, and as a protein for use alone, or as part of, a vaccine to prevent malaria.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Peptide sequences specific for the hepatic stages of P. falciparum bearing epitopes capable of stimulating the T lymphocytes
The present invention relates to an in vitro diagnostic method for malaria in an individual comprising placing a tissue or a biological fluid taken from an individual in contact with a molecule or polypeptide composition, wherein said molecule or polypeptide composition comprises one or more peptide sequences bearing all or part of one or more T epitopes of the proteins resulting from the infectious activity of P. falciparum, under conditions allowing an in vitro immunological reaction to occur between said composition and the antibodies that may be present in the tissue or biological fluid, and in vitro detection of the antigen-antibody complexes formed. The invention further relates to a polypeptide comprising at least one T epitope from a liver-stage specific protein produced by P. falciparum and a vaccine composition directed against malaria comprising a molecule having one or more peptide sequences bearing all or part of one or more T epitopes resulting from the infectious activity of P. falciparum in the hepatic cells.
Owner:INST PASTEUR
Methods for the prevention of malaria
InactiveUS20050208078A1Avoid dangerAvoids impracticalityAntiparasitic agentsWhole-cell/virus/DNA/RNA ingredientsMalaria preventionMalarial parasites
The invention comprises a novel method for protecting subjects against malaria. The method of the invention involves inoculation with attenuated sporozoites, and in particular, but not limited to subcutaneous, intramuscular, intradermal, mucosal, submucosal, and cutaneous administration.
Owner:SANARIA INC
Substituted quinoline and quinazoline inhibitors of quinone reductase 2
The present invention provides composition and methods of inhibiting quinone reductase 2 (QR2). The methods are useful in the treatment of malaria and autoimmune diseases. The compositions of the invention comprise quinoline and quinazoline derivatives. The invention also provides methods for inhibiting the activity of QR2 by contacting the enzyme with one or more compositions of the invention.
Owner:SERENEX INC
Composition and methods for site-specific drug delivery to treat malaria and other liver diseases
A system for selectively delivering drugs to target tissues is provided. The system includes a drug-linker-saccharide-drug conjugate (D-L-A-D1). The linker includes a functional group that is recognized and cleaved by enzyme in the target phases. The recognition segment is preferably a malaria drugs. The carrier is preferably hydrophilic, biodegradable and biocompatible particle. Any drug may be delivered using a conjugate prepared according to the invention.
Owner:ANSARI ASLAM +1
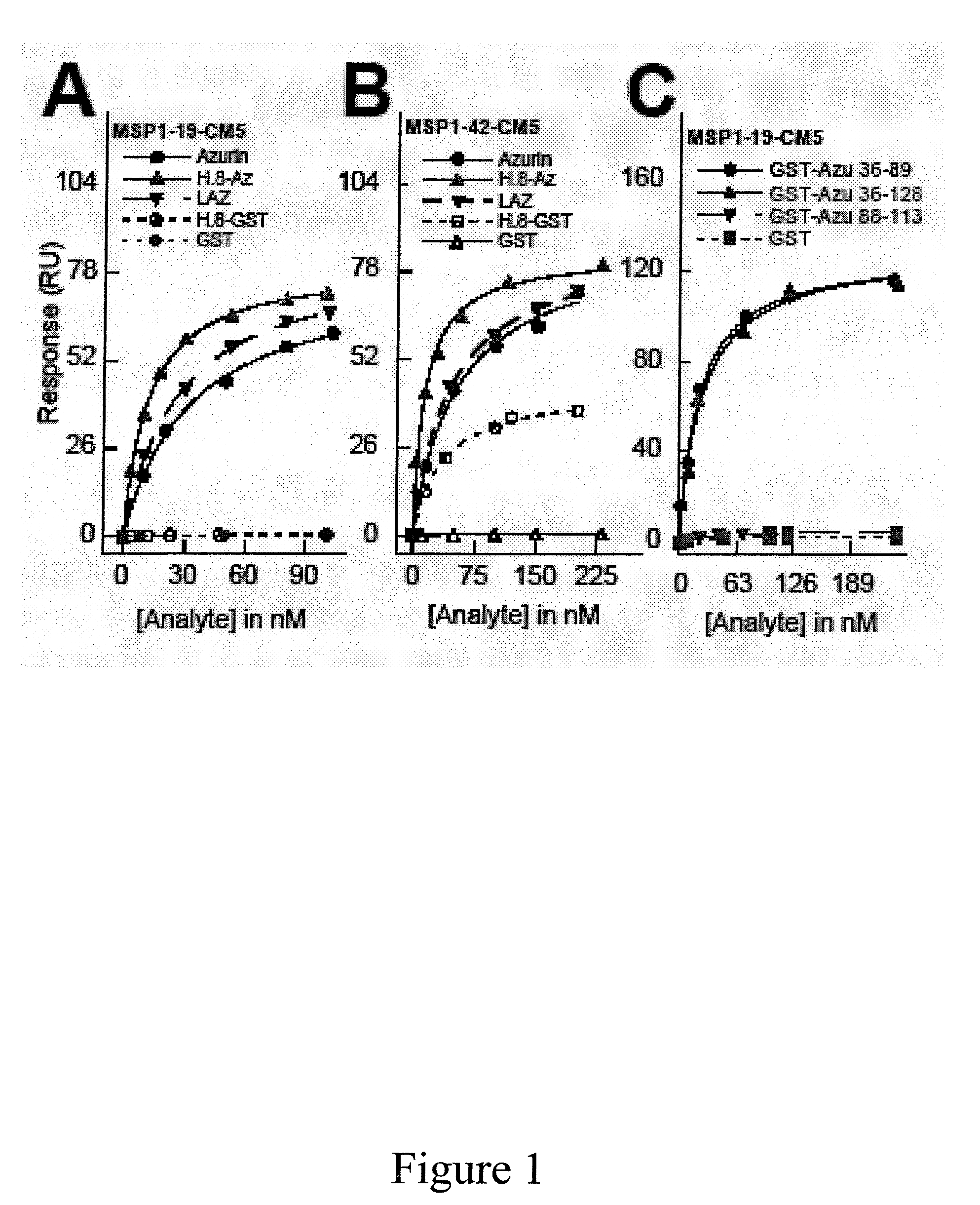
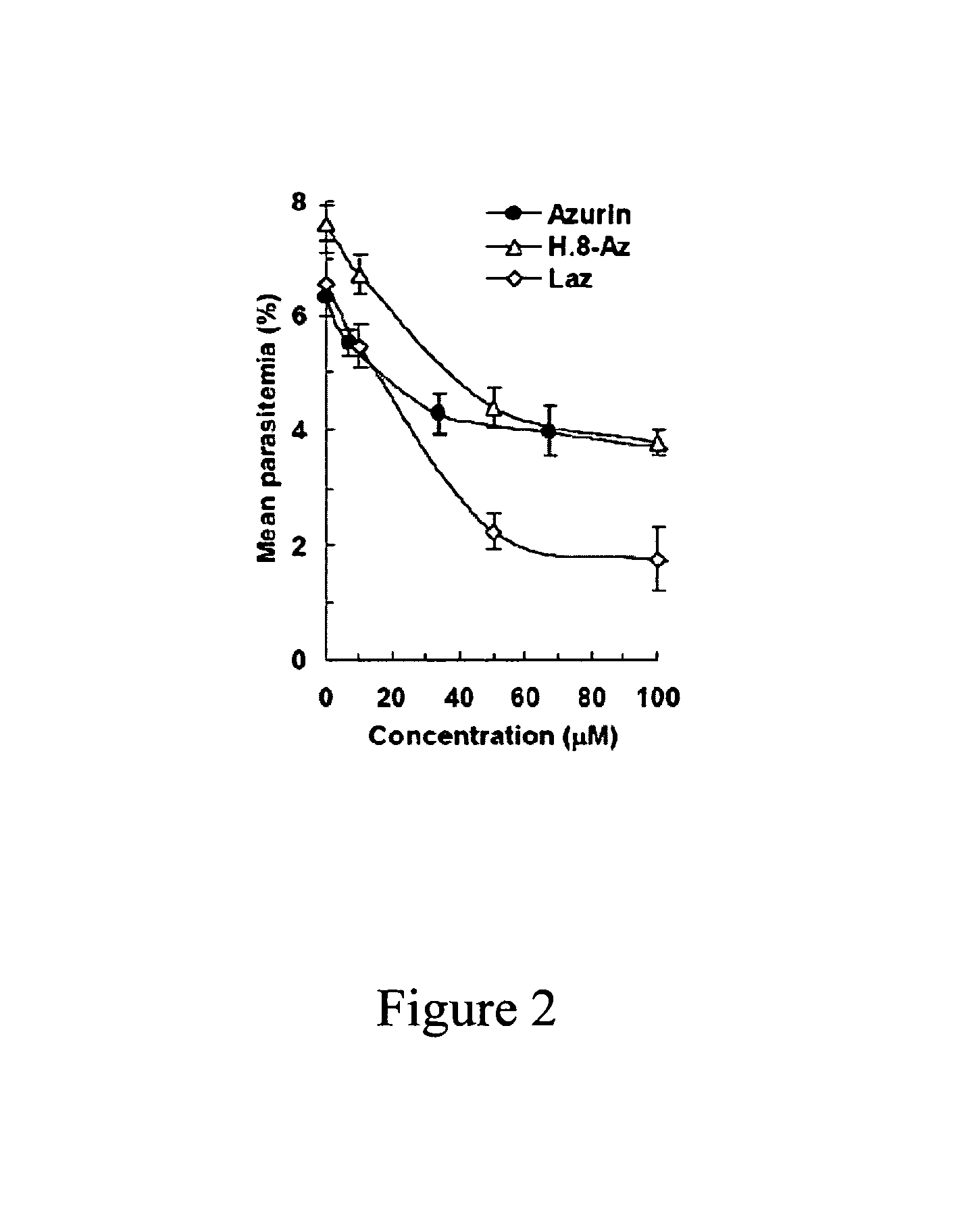
Compositions and methods for treating malaria with cupredoxin and cytochrome
The present invention relates to cupredoxin and cytochrome and their use, separately or together, to inhibit the spread of parasitemia in mammalian red blood cells and other tissues infected by the malaria parasite, and in particular the parasitemia of human red blood cells by P. falciparum. The invention provides isolated peptides that are variants, derivatives or structural equivalents of cupredoxins or cytochrome c, and compositions comprising cupredoxins and / or cytochrome c, or variants, derivatives or structural equivalents thereof, that are useful for treating or preventing malaria infection in mammals. Further, the invention provides methods to treat mammalian patients to prevent or inhibit the growth of malarial infection in mammals. The invention also provides methods to prevent the growth of malaria infection in insect vectors.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
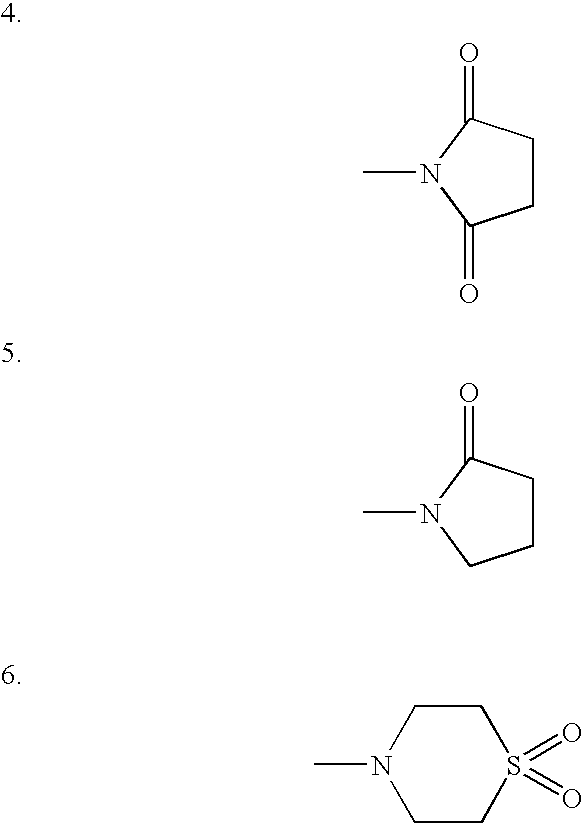
Compositions comprising trimetrexate and methods of their synthesis and use
InactiveUS6258821B1Improve stabilityEnsure long-term stabilityOrganic active ingredientsBiocideDiseaseOxygen
This invention is directed to the novel composition of matter trimetrexate ascorbate, to compositions comprising trimetrexate ascorbate, and to compositions comprising trimetrexate and ascorbic acid. These compositions are useful in the treatment of diseases in mammals such as, but not limited to, cancer, bacterial and protozoal infections, malaria, psoriasis, and rheumatoid arthritis. The invention is further related to methods of stabilizing trimetrexate to degradation caused by heat, light, oxygen, or water.
Owner:MEDIMMUNE ONCOLOGY
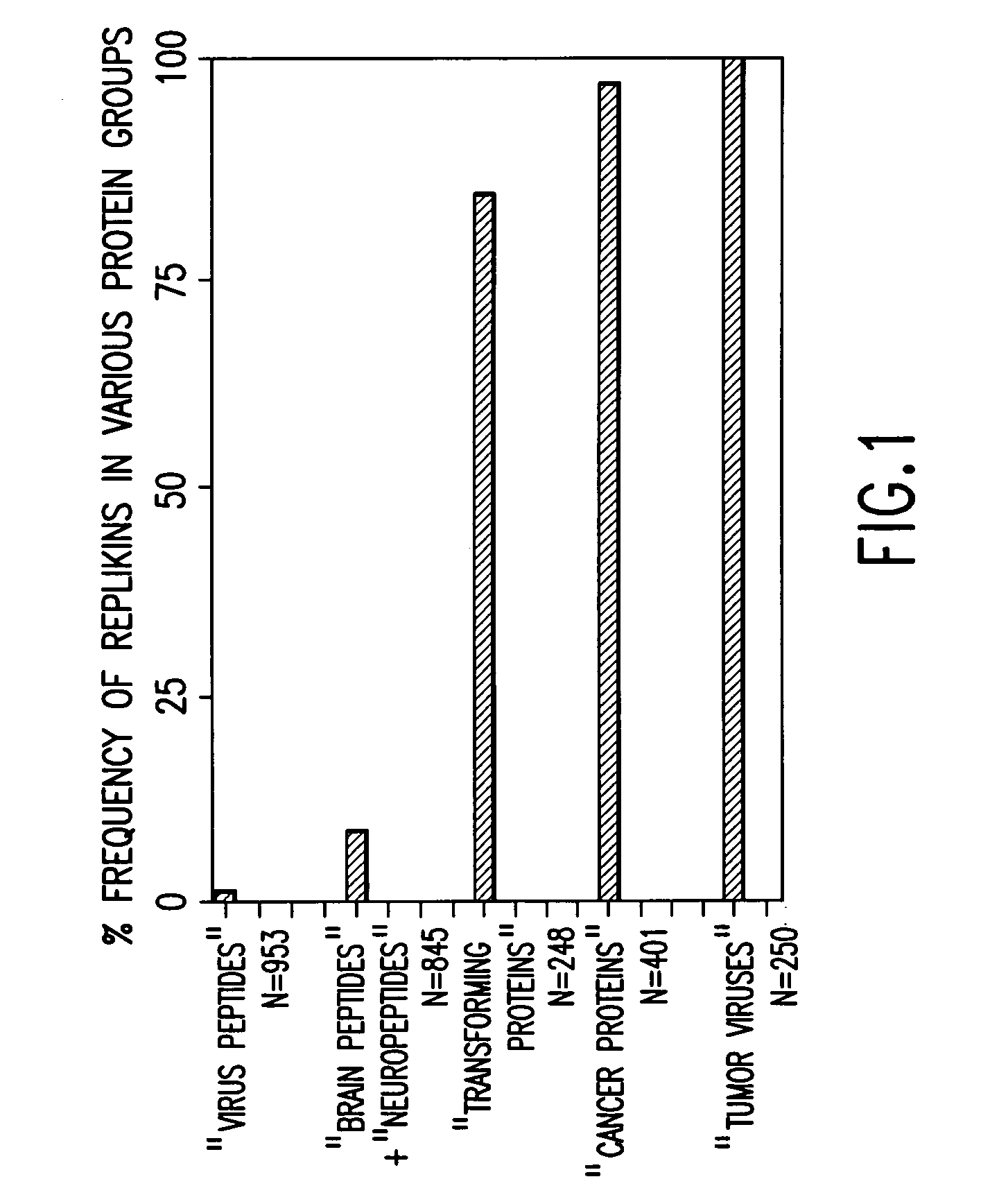
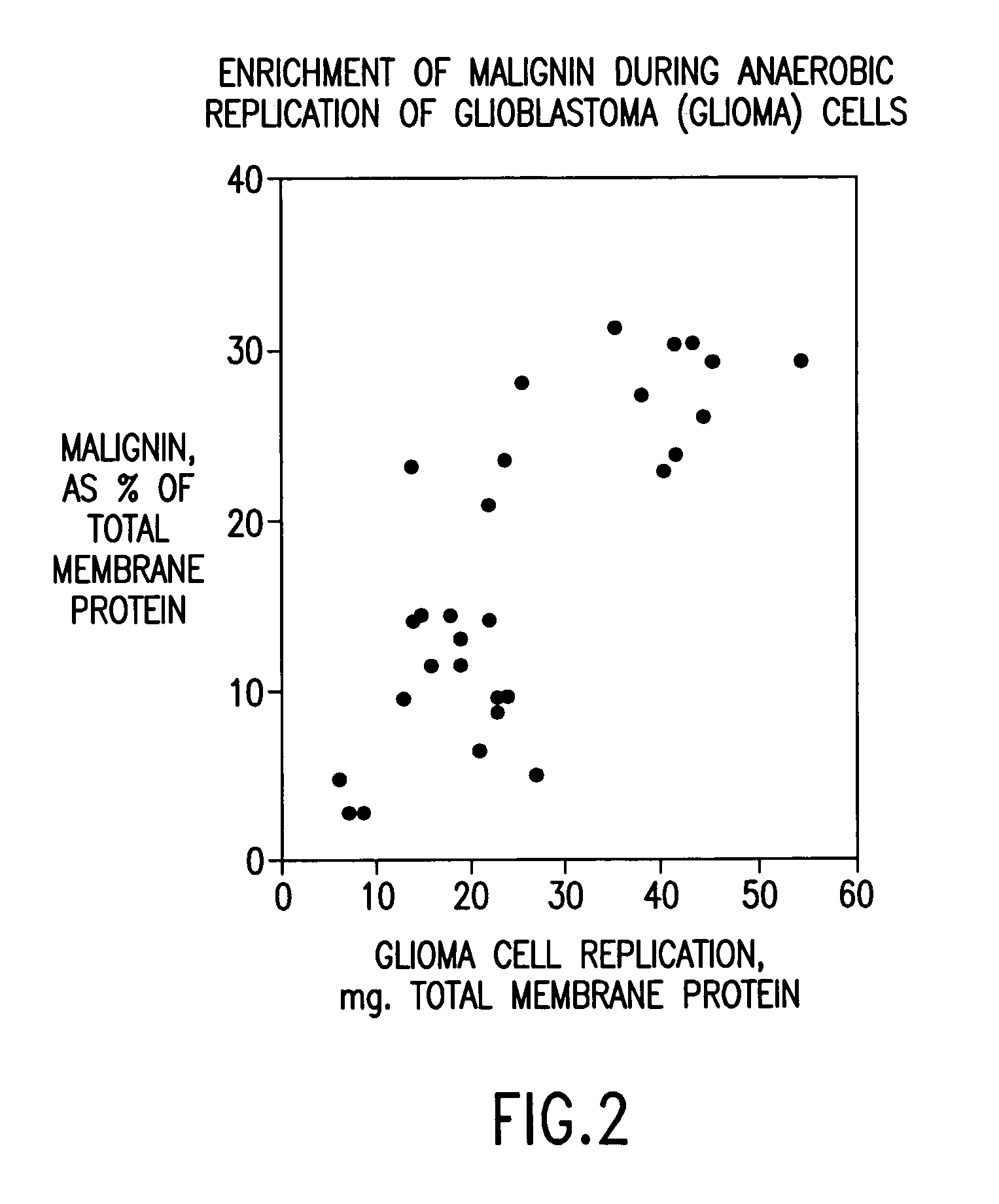
Replikin peptides in rapid replication of glioma cells and in influenza epidemics
Peptides of influenza virus hemagglutinin protein and Plasmodium falciparum malaria antigen, antibodies specific for the peptides, influenza vaccines, malaria vaccines and methods of stimulating the immune response of a subject to produce antibodies to influenza virus or malaria are disclosed. Also disclosed are methods for formulating vaccines for influenza virus.
Owner:BOGOCH SAMUEL +1
Methods for the prevention of malaria
InactiveUS20050220822A1Provide protectionMitigates or prevents malaria pathology and/or symptomsWhole-cell/virus/DNA/RNA ingredientsAgainst vector-borne diseasesMalaria preventionMalarial parasites
The invention comprises novel compositions and methods for protecting subjects against malaria. The compositions of the invention include aseptic, live attenuated Plasmodium sporozoites, and the methods include the inoculation of subjects with these compositions by means of parenteral, non-intravenous inoculation, in particular, but not limited to subcutaneous, intramuscular, intradermal, mucosal, submucosal, and cutaneous administration.
Owner:SANARIA INC
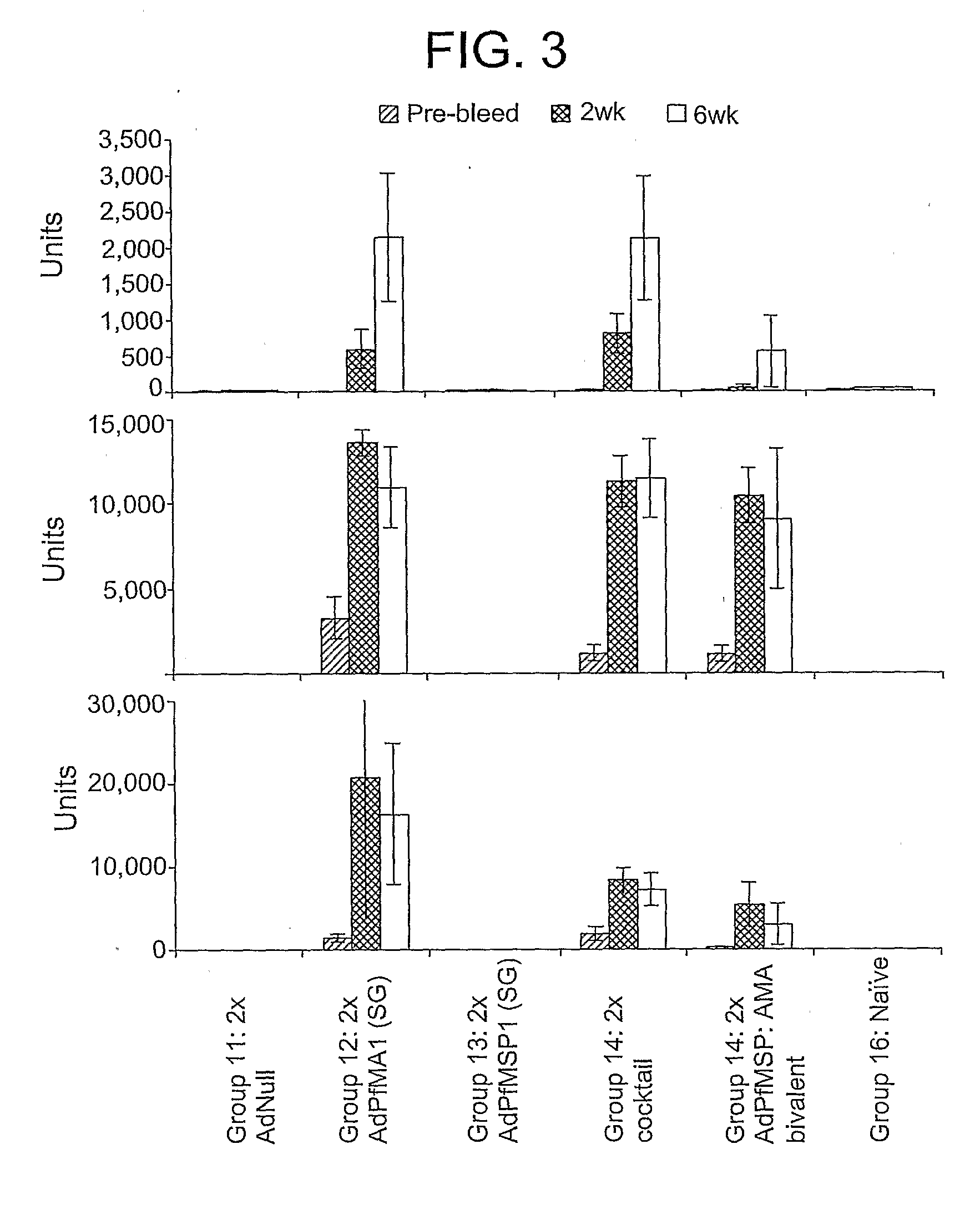
Adenoviral vector-based malaria vaccines
The invention provides adenoviral vectors comprising an adenoviral genome comprising heterologous antigen-encoding nucleic acid sequences, such as Plasmodium nucleic acid sequences, operably linked to promoters. The invention further provides a method of inducing an immune response against malaria in a mammal comprising administering the adenoviral vectors to the mammal.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY +2
HDAC inhibitors and therapeutic methods using the same
ActiveUS9249087B2High sensitivityDegree of isoform selectivity for an HDACIOrganic active ingredientsOrganic chemistryAutoimmune diseaseMalaria
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA +1
Methods and arrangements for identifying dermatological diagnoses with clinically negligible probabilties
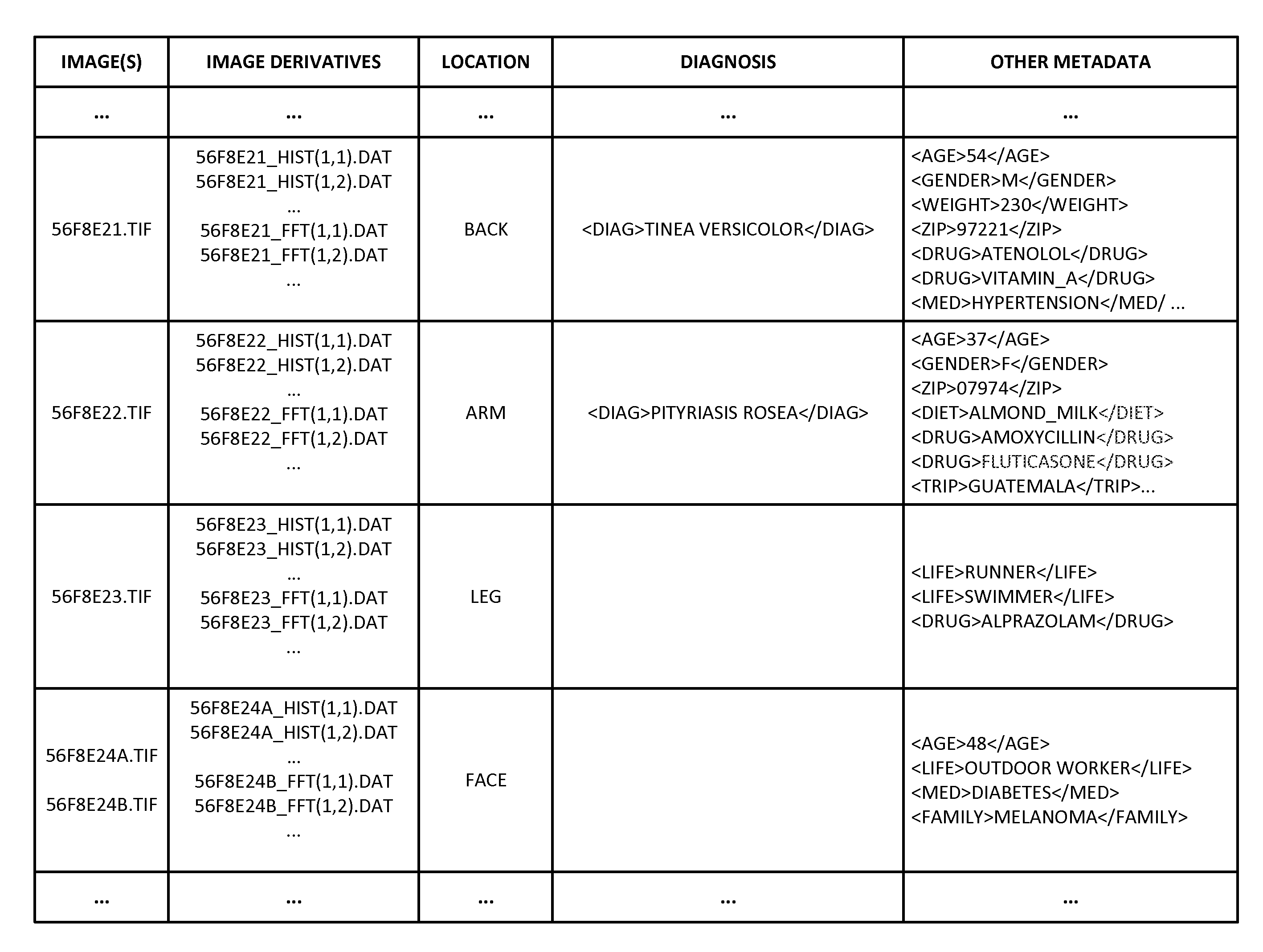
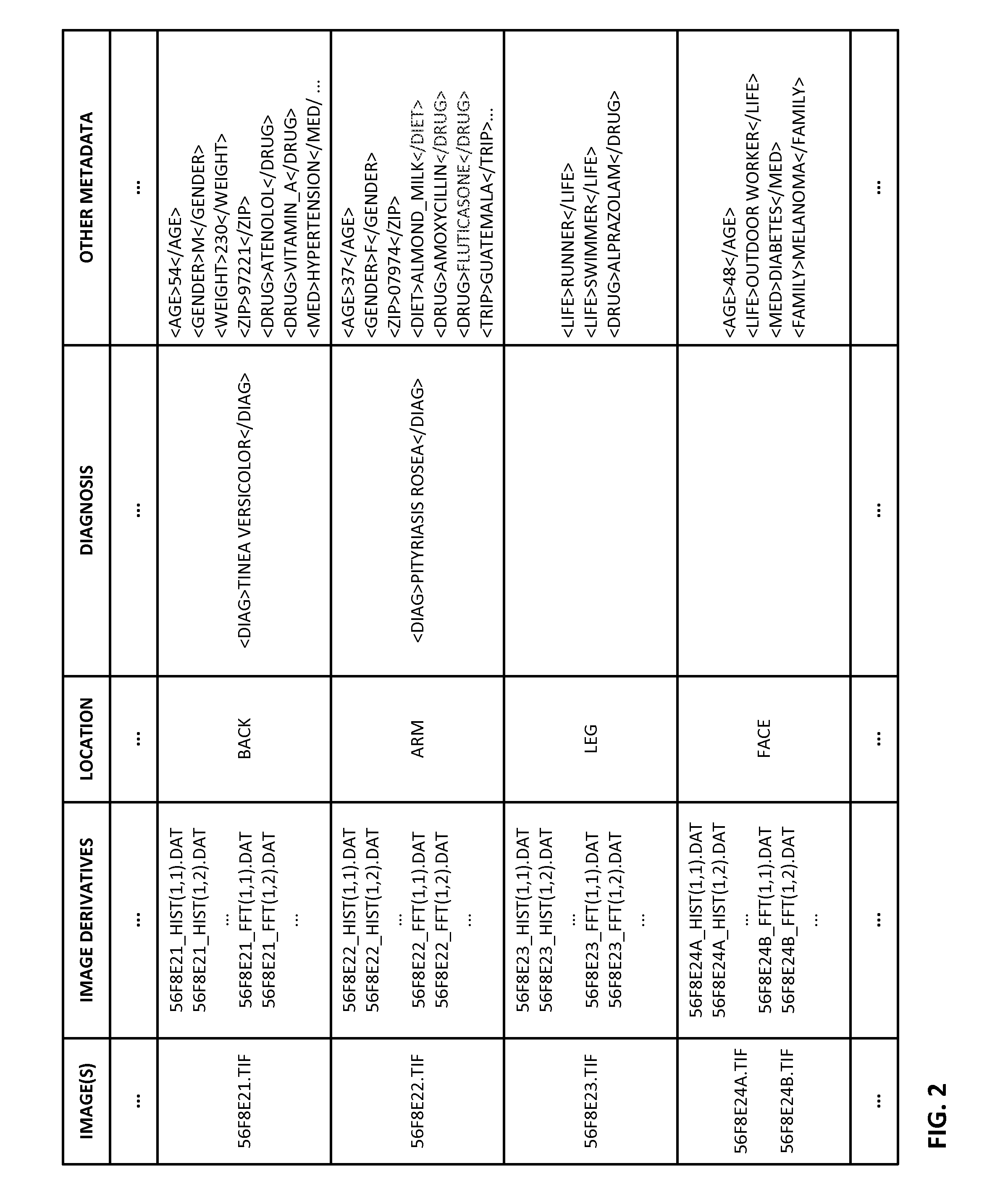
ActiveUS20150003699A1Lower cost of careImage enhancementMedical imagingPattern recognitionBiopsy procedure
Reference imagery of dermatological conditions is compiled in a crowd-sourced database (contributed by clinicians and / or the lay public), together with associated diagnosis information. A user later submits a query image to the system (e.g., captured with a smartphone). Image-based derivatives for the query image are determined (e.g., color histograms, FFT-based metrics, etc.), and are compared against similar derivatives computed from the reference imagery. This comparison identifies diseases that are not consistent with the query image, and such information is reported to the user. Depending on the size of the database, and the specificity of the data, 90% or more of candidate conditions may be effectively ruled-out, possibly sparing the user from expensive and painful biopsy procedures, and granting some peace of mind (e.g., knowledge that an emerging pattern of small lesions on a forearm is probably not caused by shingles, bedbugs, malaria or AIDS). A great number of other features and arrangements are also detailed.
Owner:DIGIMARC CORP
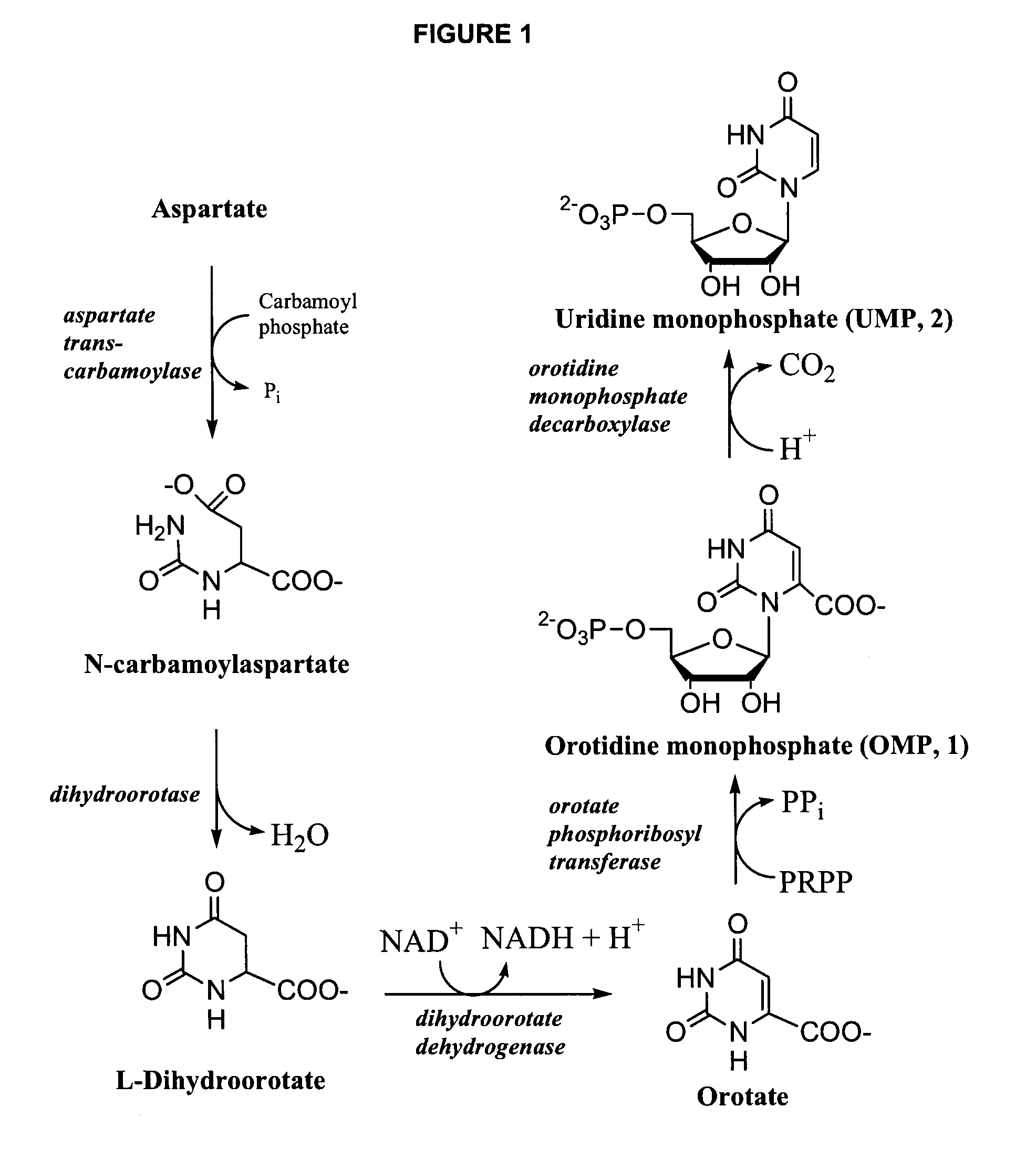
Odcase inhibitors for the treatment of malaria
The present invention includes methods of treating or preventing malaria by administering an anti-malarial effective amount of 6-substituted uridine derivatives to a subject need thereof. The invention also includes new 6-substituted uridine derivatives for use as therapeutics, in particular to treat malaria.
Owner:UNIV HEALTH NETWORK
Methods for vaccinating against malaria
ActiveUS20060188527A1Reduce chanceReduce severityBiocideGenetic material ingredientsVaccinationA-DNA
The invention pertains to methods for protecting against malaria infection by vaccination. The method of the invention involves priming an anti-malaria immune response with a DNA-based vaccine and boosting that response with a protein-based a vaccine. The method of the invention also relates to broadening the resulting immune response by boosting with a protein-based vaccine.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Insecticide-impregnated fabric and method of production
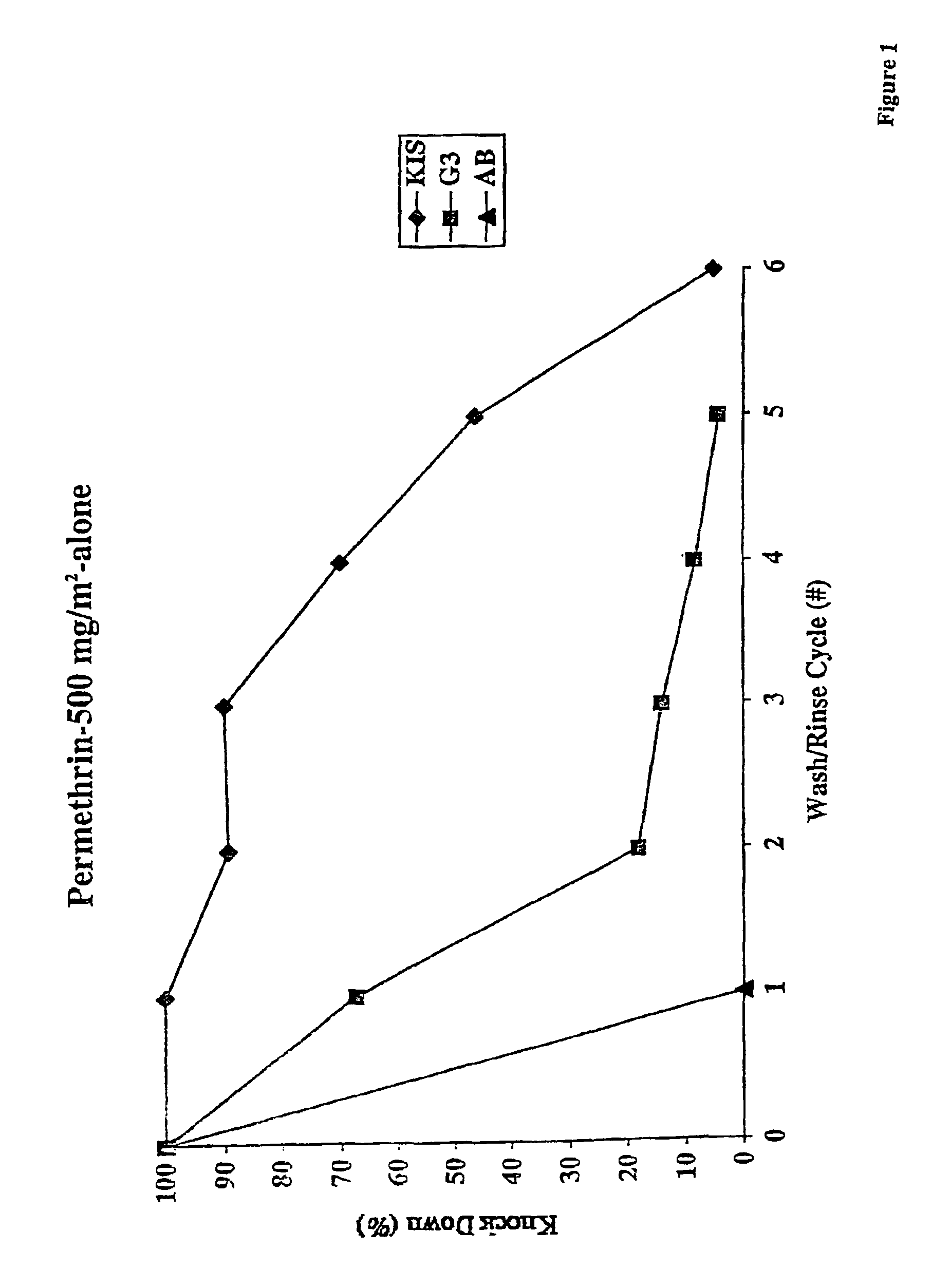
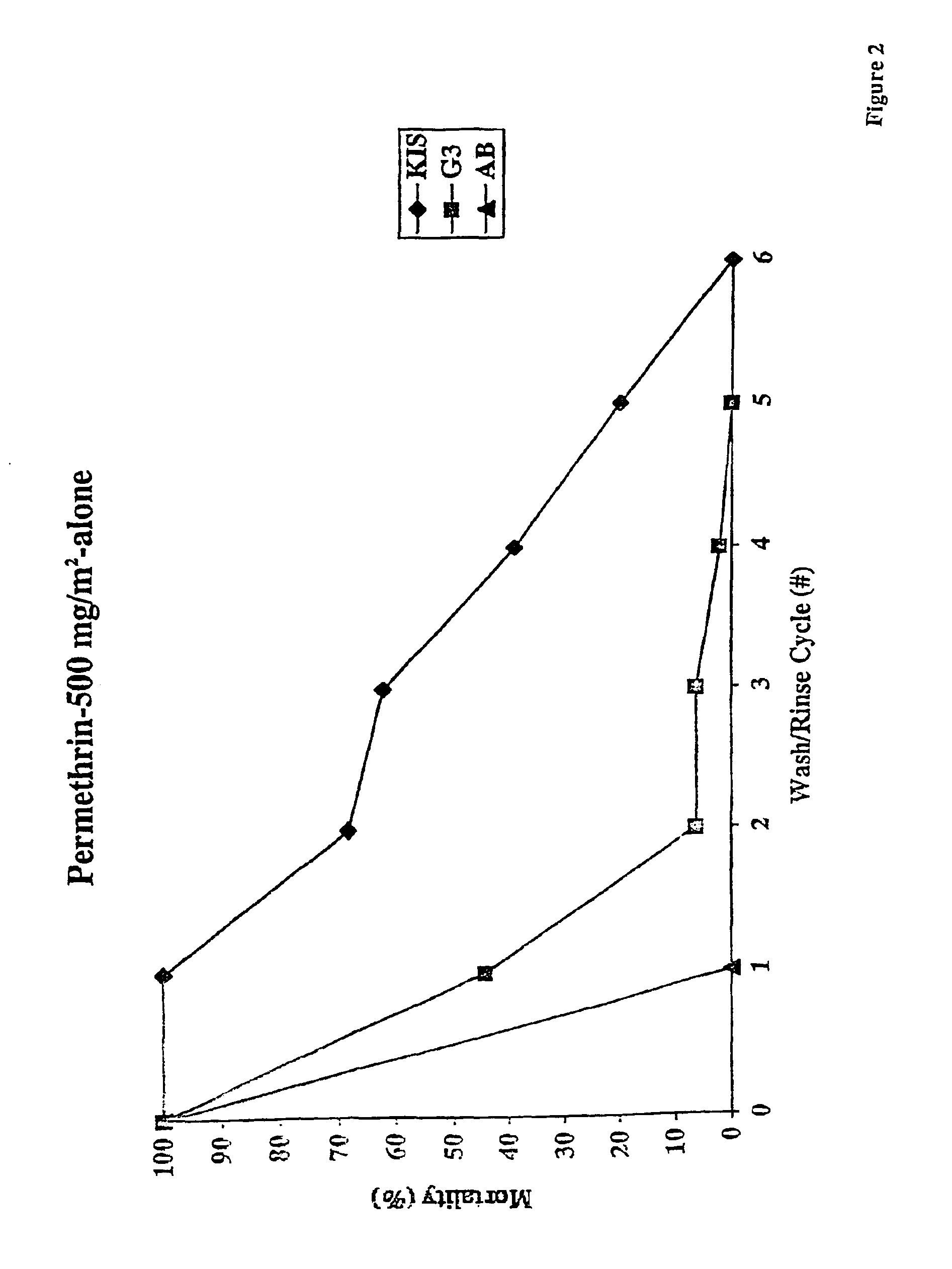
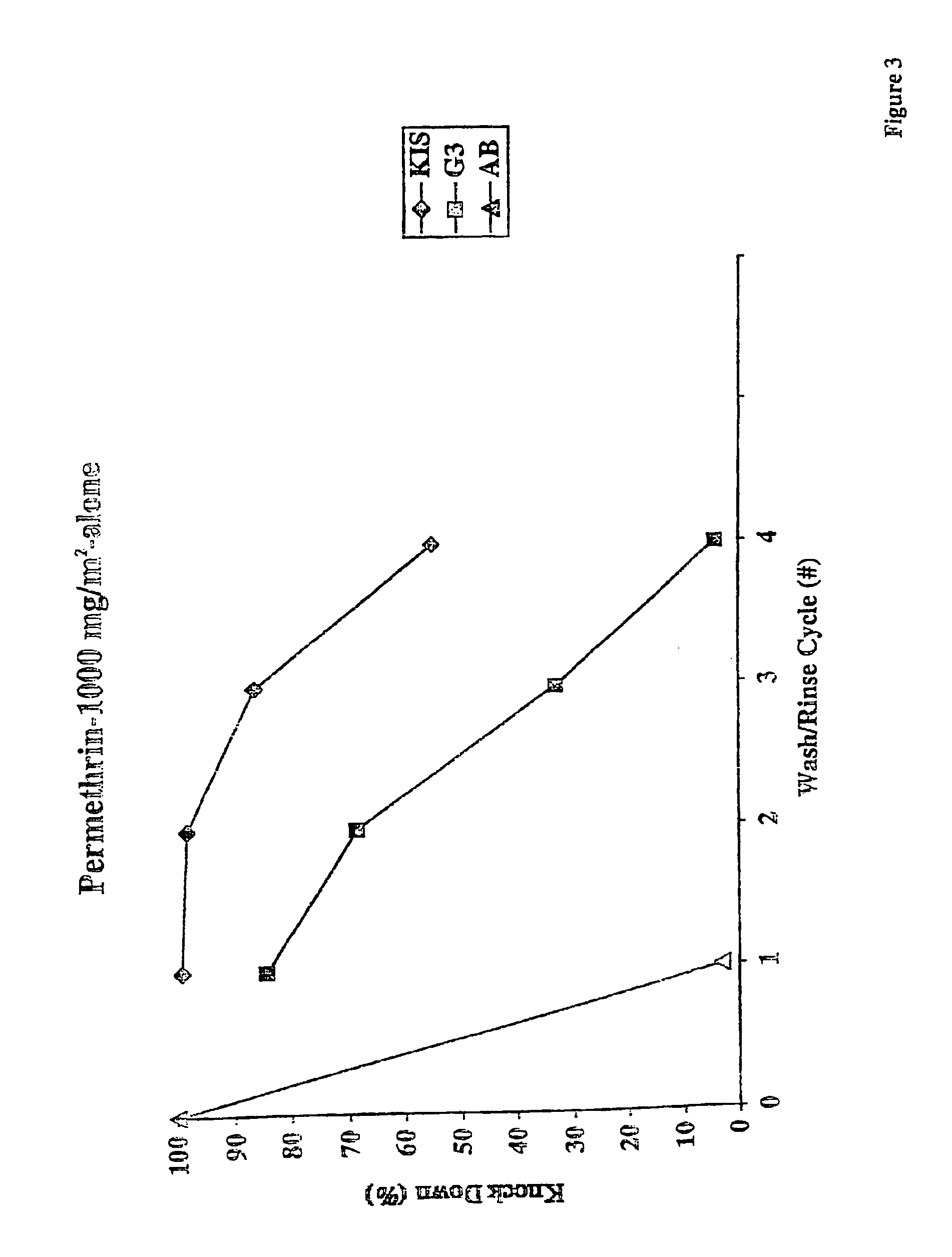
InactiveUS6896892B2Reduce removalHigh insect mortalityBiocideBiochemical fibre treatmentCyclodextrinMalarial parasites
An insecticide-impregnated fabric that remains sufficiently effective at killing and repelling disease vector insects after repeated washings with detergent and water is described. The fabric is impregnated with an insecticide composition containing an insecticide, a cyclodextrin, and a binding agent. The resulting fabric is useful for providing personal protection against disease-carrying insect vectors, particularly when assembled as a bednet in regions of the world where malaria is prevalent, and will remain effective for a longer period of time before re-impregnation is necessary.
Owner:DEPT OF HEALTH & HUMAN SERVICES US SEC THE CENTS OF DISEASE CONTROL & PREVENTION TECH TRANSFER OFFICE
Methods using proton pump inhibitors
The invention provides methods of treating and preventing asthma, laryngitis, symptomatic gastroesophageal reflux disease, pregnancy-induced gastroesophageal reflux disease, noncardiac chest pains, coughing, apnea, dyspepsia, inflammatory bowel disease, irritable bowel syndrome, gastritis, stress ulcers, bleeding peptic ulcers, acute gastrointestinal bleeding, infectious enteritis, collagenous colitis, lymphocytic colitis, chronic diarrhea in immunocompromised patients, esophageal ulcers in immunocompromised patients, idiopathic gastric acid hypersecretion, gastroparesis, gastrointestinal motility disorders, Zollinger-Ellison syndrome, short bowel syndrome, emesis, regurgitation, early satiety, chronic sore throat, abdominal pain, abdominal bloating, nausea, sour stomach, diarrhea, constipation, bacterial infections, refractory ulcers, gastrointestinal disorders induced by NSAIDs, Barrett's esophagus, gastrointestinal disorders caused by steroids, gastrointestinal disorders induced by cholinergic compounds, and fungal or viral-induced ulcers in the gastrointestinal tract by administering a therapeutically effective amount of at least one proton pump to a patient in need thereof. The invention also provides on demand relief of symptoms associated with gastroesophageal reflux disease (GERD), and provides relief from symptoms caused by the consumption of excessive amounts of food and / or alcohol by administering a therapeutically effective amount of at least one proton pump inhibitor to a patient in need thereof. The invention also provides methods for treating parasitic infections, such as malaria, by administering a therapeutically effective amount of at least one proton pump inhibitor to a patient in need thereof.
Owner:EISAI CO LTD
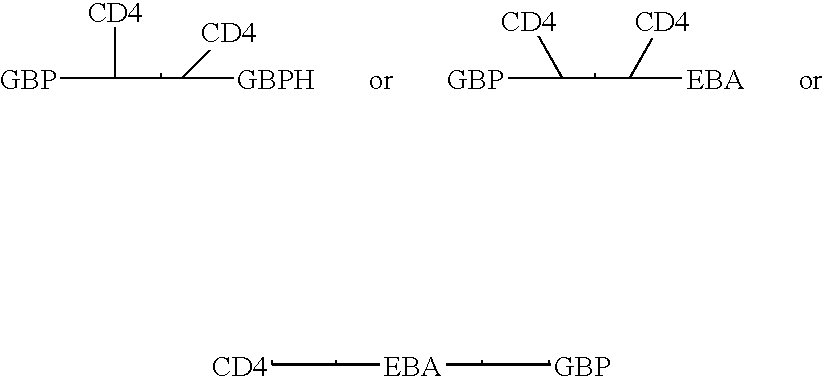
Fusion proteins comprising CD4 and the malaria parasite merozoite glycophorin binding protein 130 (GBP-130)
InactiveUS7585508B1Reduce infectivityRelieve symptomsFusions with soluble cell surface receptorAntiviralsGlycophorinBinding peptide
Novel hybrid fusion peptides are disclosed. The novel peptides are formed by the fusion of two or more components. One component is a peptide sequence or variant of a peptide sequence derived from a malaria parasite merozoite peptide which has affinity for and binding capability to red blood cells.In particular segments of the glycophorin binding peptide 130 (GBP130), are preferred for the first component. Also disclosed are alternative first components, the glycophorin binding peptide homologues (GBPH), or the erythrocyte binding antigen 175 (EBA175), or the plasmodium vivax Duffy receptor or the pre major merozoite surface antigen PMMSA or the (P200) peptide.The first component peptide is fused to all or part of a peptide segment derived from the CD4 molecule or part thereof or variant thereof which shows binding affinity for the HIV virus.The resulting fusion peptide being exemplified asNH2-CD4-GBP130-COOH1-371 201-774Also disclosed are the methods of manufacture and means to use the novel hybrid peptides as clinical agents to treat, prevent or test for HIV infection.
Owner:PRENDERGAST KENNETH F
Resonance driven changes in chain molecule structure
InactiveUS6060293AEfficient inductionPeptide preparation methodsElectrical/wave energy microorganism treatmentChemical industryDisease
PCT No. PCT / DK96 / 00158 Sec. 371 Date Nov. 26, 1997 Sec. 102(e) Date Nov. 26, 1997 PCT Filed Apr. 1, 1996 PCT Pub. No. WO96 / 30394 PCT Pub. Date Oct. 3, 1996The invention relates to the technical application of electromagnetic radiation such as microwaves and radiowaves and application of ultra sound to chain molecules. In particular, the present invention relates to the utilization of topological excitations such as wring, twist and torsional modes, e.g., for generating structure, such as in folding, refolding or renaturation, and denaturation or unfolding of peptides, polypeptides, proteins, and enzymes; for generating changes in molecular affinity; for stimulating drug receptor interactions; and for changing molecular communication, is described. The technique is based on a new understanding of the underlying physical phenomenon and can also be applied to other chain molecules and biologically active biomolecules and tailored polymers such as glucoproteins, antibodies, genomic chain molecules such as DNA and RNA as well as PNA, carbohydrates, and synthetic and natural organic polymers. The invention is especially applicable for solving problems related to inclusion bodies and aggregation when using recombinant DNA and protein engineering techniques. Furthermore, the invention can be utilized in therapeutic treatment and in development and production of pharmaceuticals. The area of applicability ranges from biotechnological industry, food industry, drug industry, pharmacological industry, chemical industry, and concerns, e.g., the treatment of conditions and diseases related to influenza, hepatitis, polio, malaria, borrelia, diabetes, Alzheimer's disease, Creutzfeldt Jakob disease, other prion related diseases, multiple sclerosis, cataract, heart diseases, cancer, and aging.
Owner:PROKYON
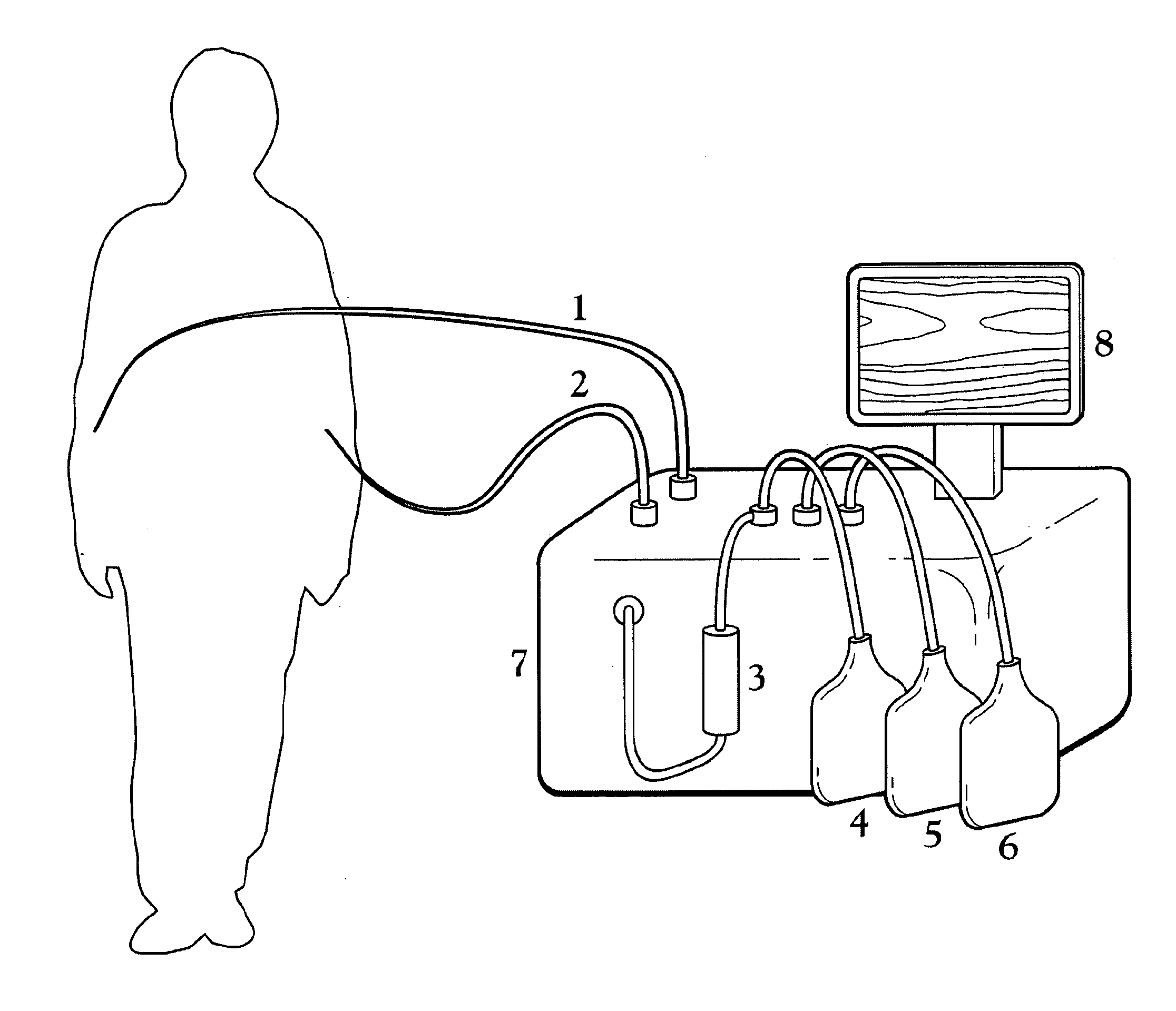
A Blood Purification Method and Apparatus for the Treatment of Malaria
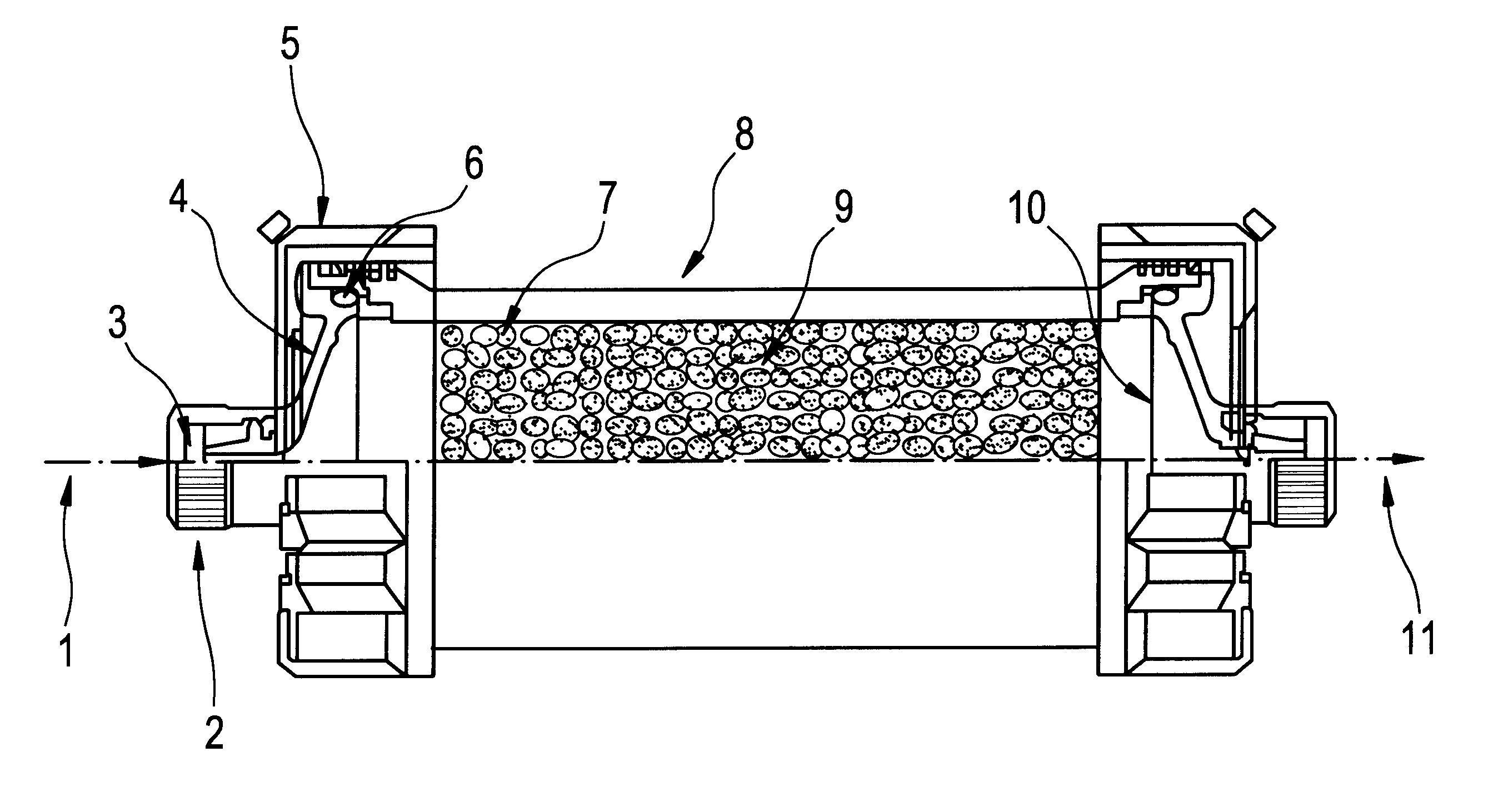
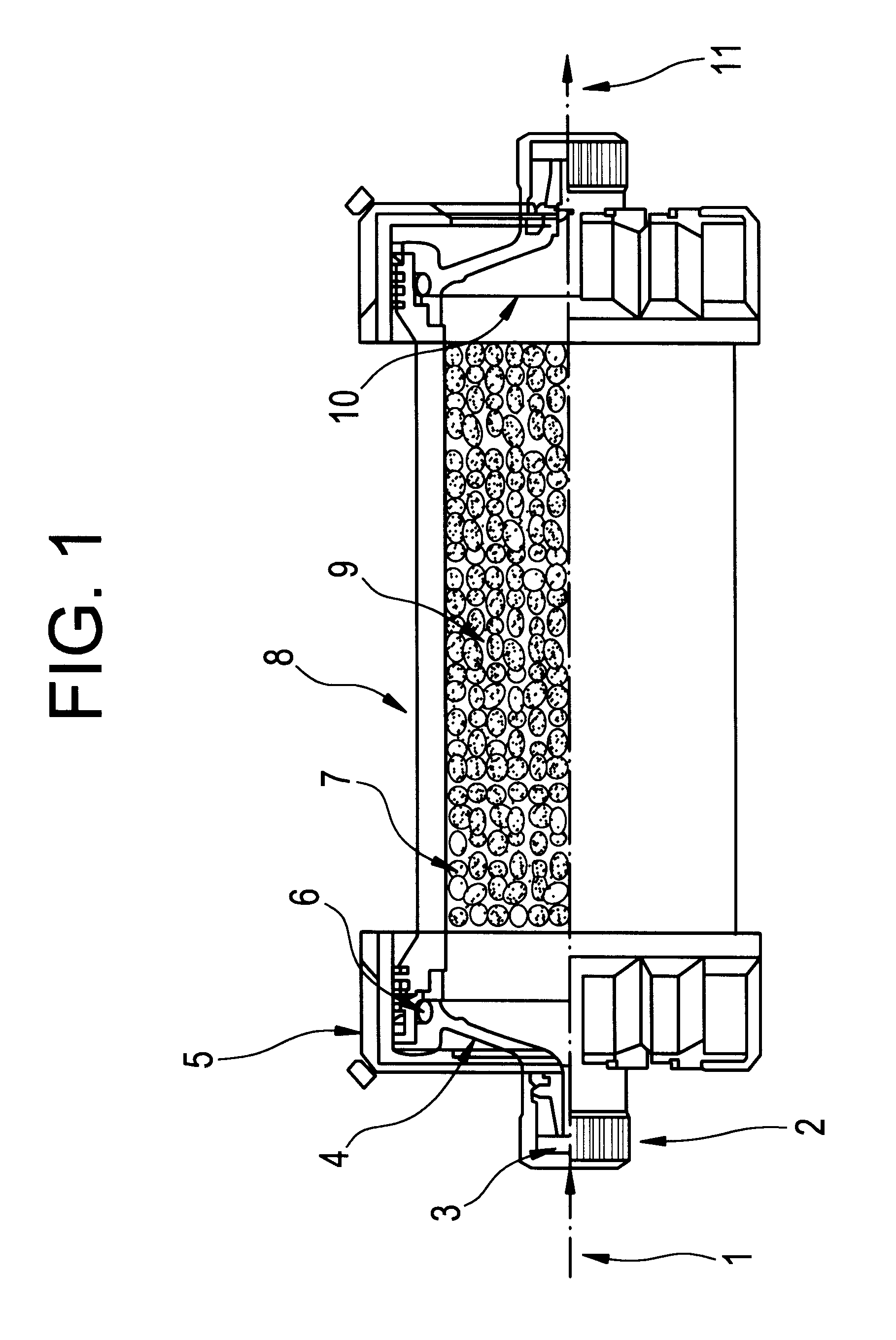
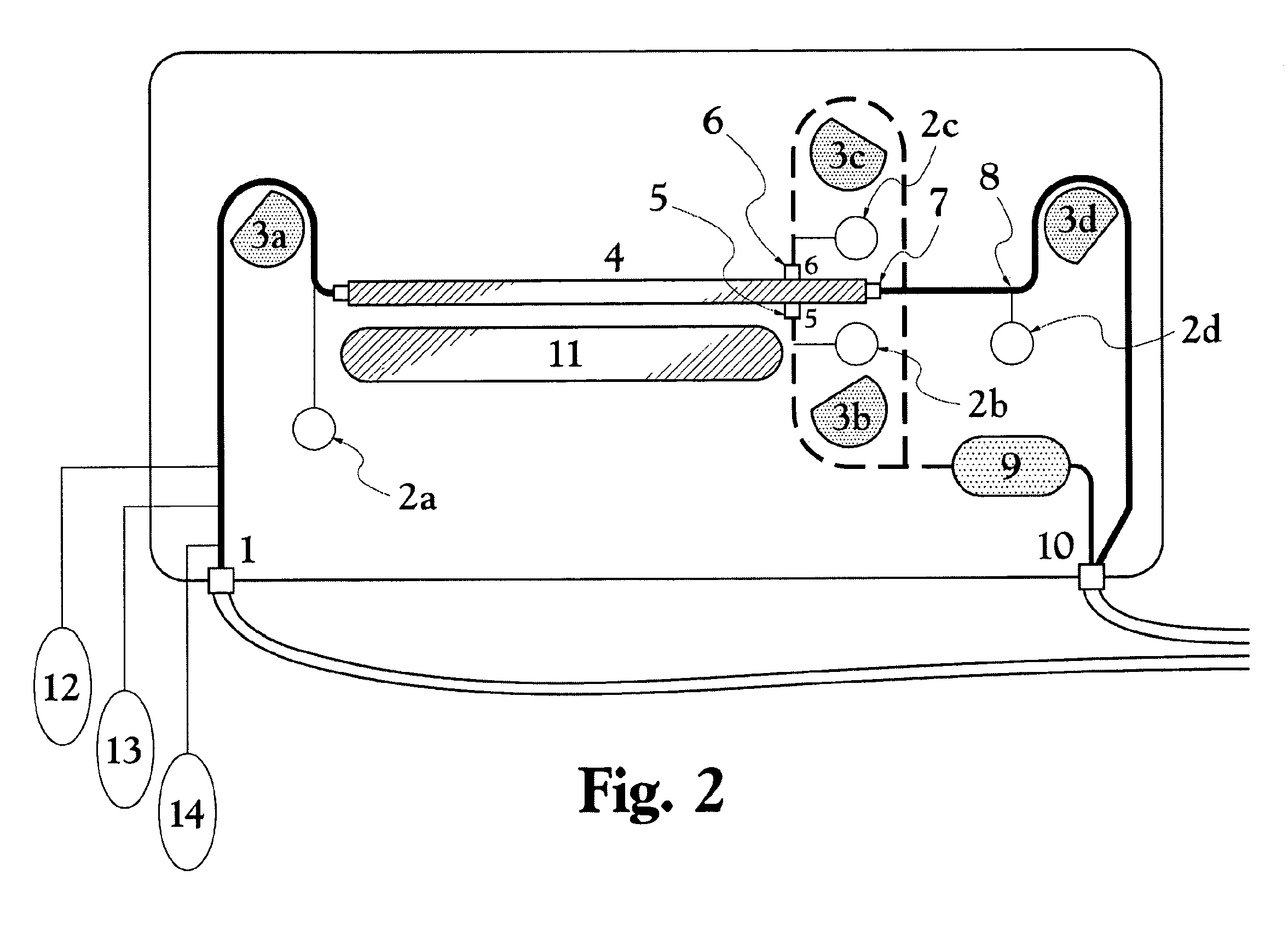
ActiveUS20100331753A1Promote recoveryQuick effectElectrostatic separationMedical devicesDiseaseMagnetic field gradient
Methods for treating malaria are provided, the treatment comprising the step of removing malaria-infected red blood cells from the patient s blood. Blood is drawn from the patient s circulatory system and circulated through a blood purification device that selectively eliminates the infected red blood cells from all other blood s components and replaces the cleansed blood back into the patient s circulatory system. A blood purification device, which is useful to perform the therapeutic methods of the invention, is also provided. The device leverages the magnetic properties of the hemozoin contained within the infected red blood cells and comprises one or more separation chambers (4) though which blood flows through a high-gradient magnetic field generated by an array of wires (5) separated from the chambers and not in contact with the patient blood. The magnetic field gradient acting on the cells magnetic properties displaces the infected and non-infected red blood cells on different layers of the blood flow across the chamber height. The blood flow is split into separated streams and blood streams containing infected cells are filtrated thereby trapping infected cells. Blood containing non-infected red blood cells is circulated back to the patient. The device application is not limited to the treatment of malaria and includes other blood related diseases that affect the magnetic properties of a patient's red blood cells.
Owner:TROPICAL HEALTH SYST
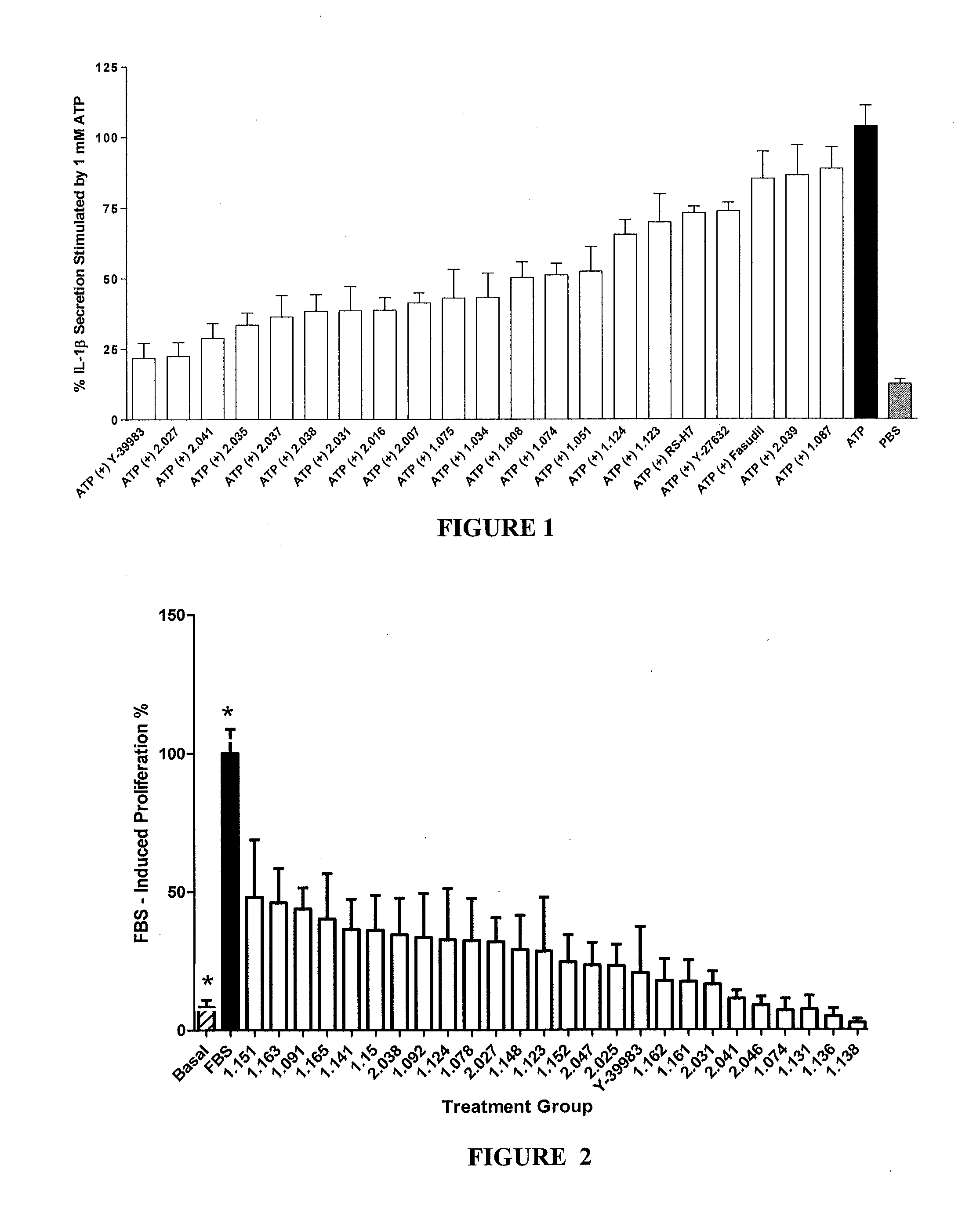
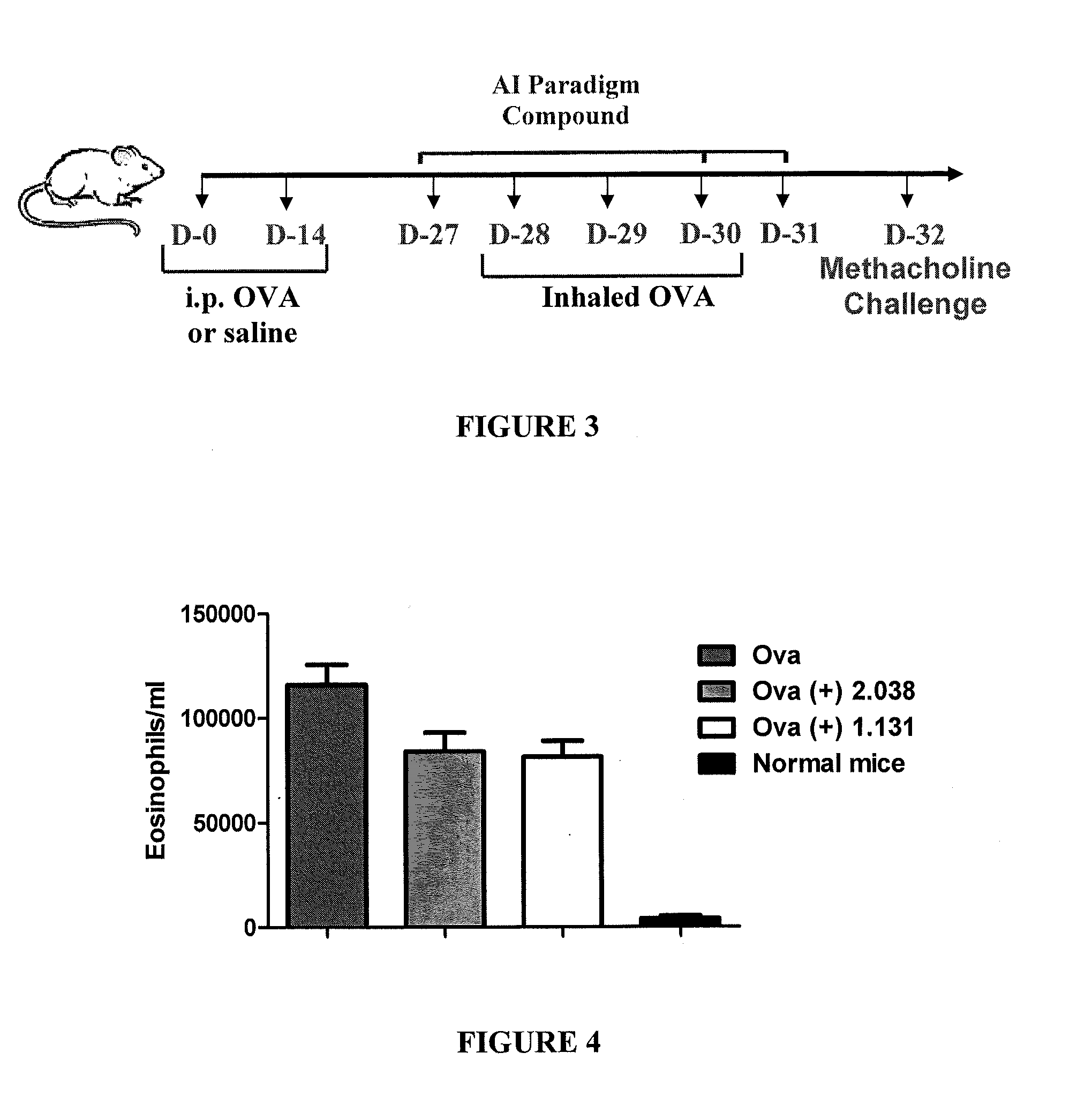
Method for treating diseases associated with alterations in cellular integrity using Rho kinase inhibitor compounds
This invention is directed to methods of preventing or treating diseases or conditions associated with alterations in cellular integrity including alterations in endothelial permeability, excessive cell proliferation or tissue remodeling. Particularly, this invention is directed to methods of treating diabetic nephropathy, malaria, or cancer. The method comprises identifying a subject in need of the treatment, and administering to the subject an effective amount of a novel rho kinase inhibitor compound to treat the disease.
Owner:INSPIRE PHARMA
Loop-mediated isothermal amplification technology-based plasmodium genus and species nucleic acid screening method
InactiveCN102010910ASimple methodMicrobiological testing/measurementAgainst vector-borne diseasesScreening methodMolecular level
The invention discloses a set of loop-mediated isothermal amplification technology-based plasmodium genus and species nucleic acid screening method and belongs to the field of biological detection. The method is performed by loop-mediated isothermal amplification (LAMP) technology, specific positions of target genes are amplified by using an LAMP technology platform through specific primers of plasmodium genera and specificity primers of plasmodium species, the plasmodium genera are screened or detected at a molecular level under assistance of positive and negative quality control and an internal control detection system, and plasmodium species screening or detection is performed on four kinds of plasmodia, namely Plasmodiumfalciparum, Plasmodiummalariae, Plasmodiumovale and Plasmodiumvivax which can make humans infected with malaria in plasmodium genus organisms. The invention has the characteristics that: the method is simple, economic and rapid, and has high sensitivity, high specificity and wide application prospect.
Owner:中华人民共和国徐州出入境检验检疫局
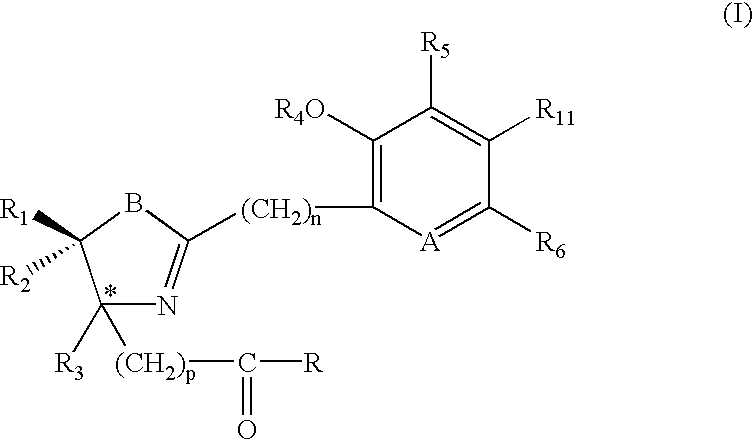
Iron binding agents
Composition, article of manufacture for and method of treating malaria in a human having an infestation of Plasmodium protozoans are described. The method comprises administering a therapeutically-effective amount of a compound of formula (I) or (IV), i.e. sufficient quantity to reduce the population of Plasmodium. The composition of the invention is a compound of formula (I) or (IV) with a pharmaceutical excipient. The article of manufacture is the composition in combination with labeling for treating malaria. The substituents are detailed in the specification.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Mosquito-repelling bactericide
InactiveCN104585255AInhibition of abnormal fermentationPromote peristalsisBiocideAntisepticsDiseaseAdditive ingredient
The invention relates to a mosquito-repelling bactericide. The raw material formula comprises main ingredients, wherein the main ingredients comprise the following components: clove, folium artemisiae argyi, honeysuckle, elsholtzia, radix angelicae, purple perilla, calamus, mint and agastache rugosus. The mosquito-repelling bactericide disclosed by the invention has the beneficial effects that according to the common sense and principle that mosquitoes bite to suck blood and spread diseases, the bactericide has the main effect of repelling mosquitoes, and harmful pathogenic bacteria and bacteria in air can be killed by virtue of the flavor volatilized by the traditional Chinese medicines, so that the family members are prevented from being troubled by the mosquitoes and are even prevented from suffering from various diseases due to mosquito bites, and people are kept away from diseases such as malaria, filariasis, epidemic encephalitis B and dengue fever.
Owner:刘向上
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com