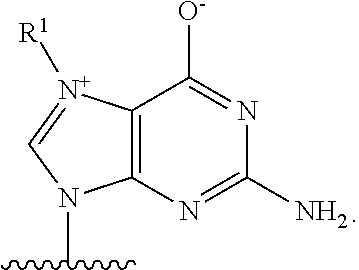
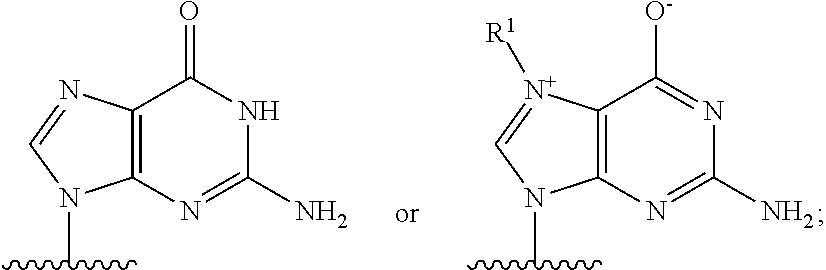Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
162results about How to "Reduce infectivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
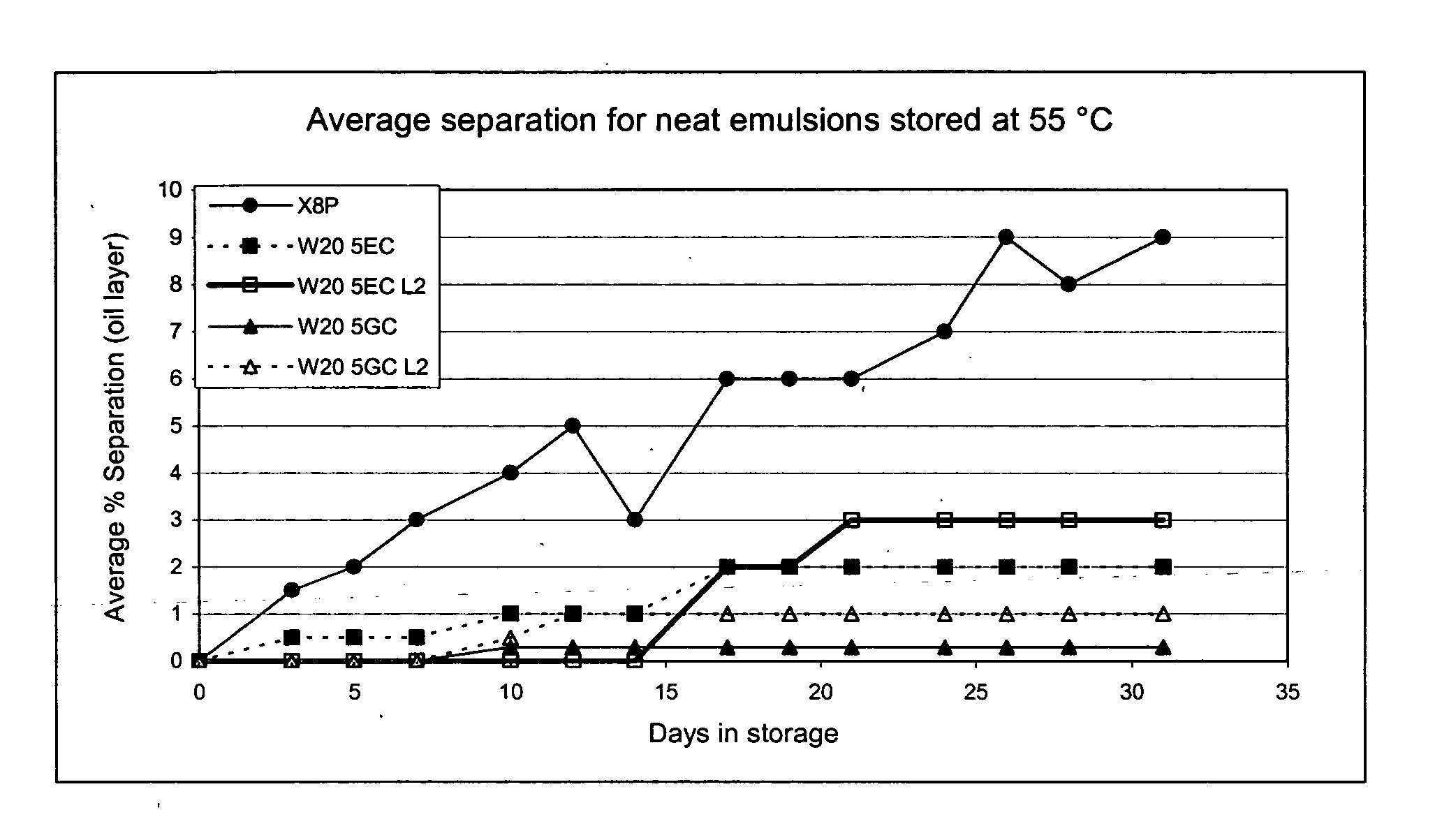
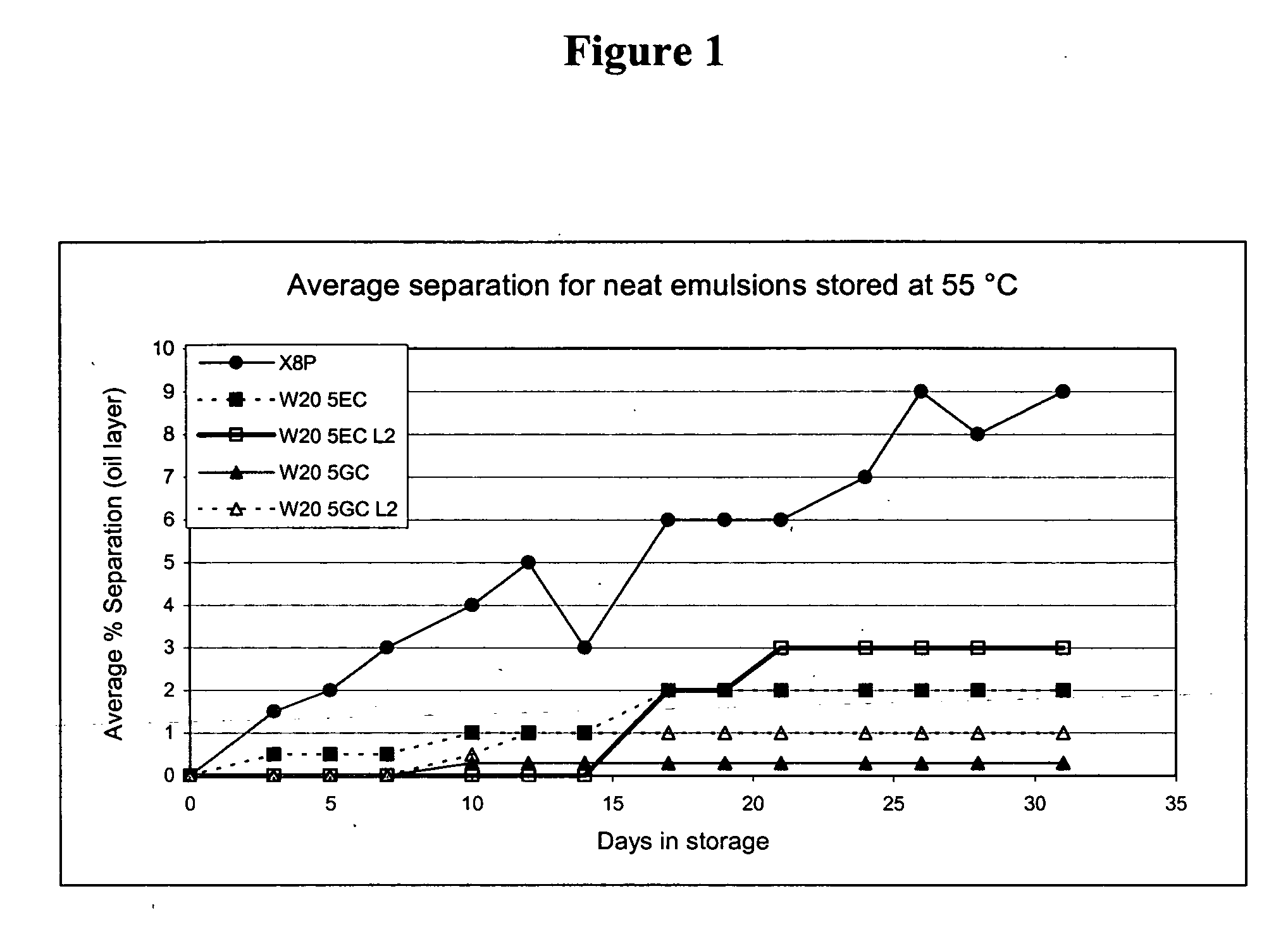
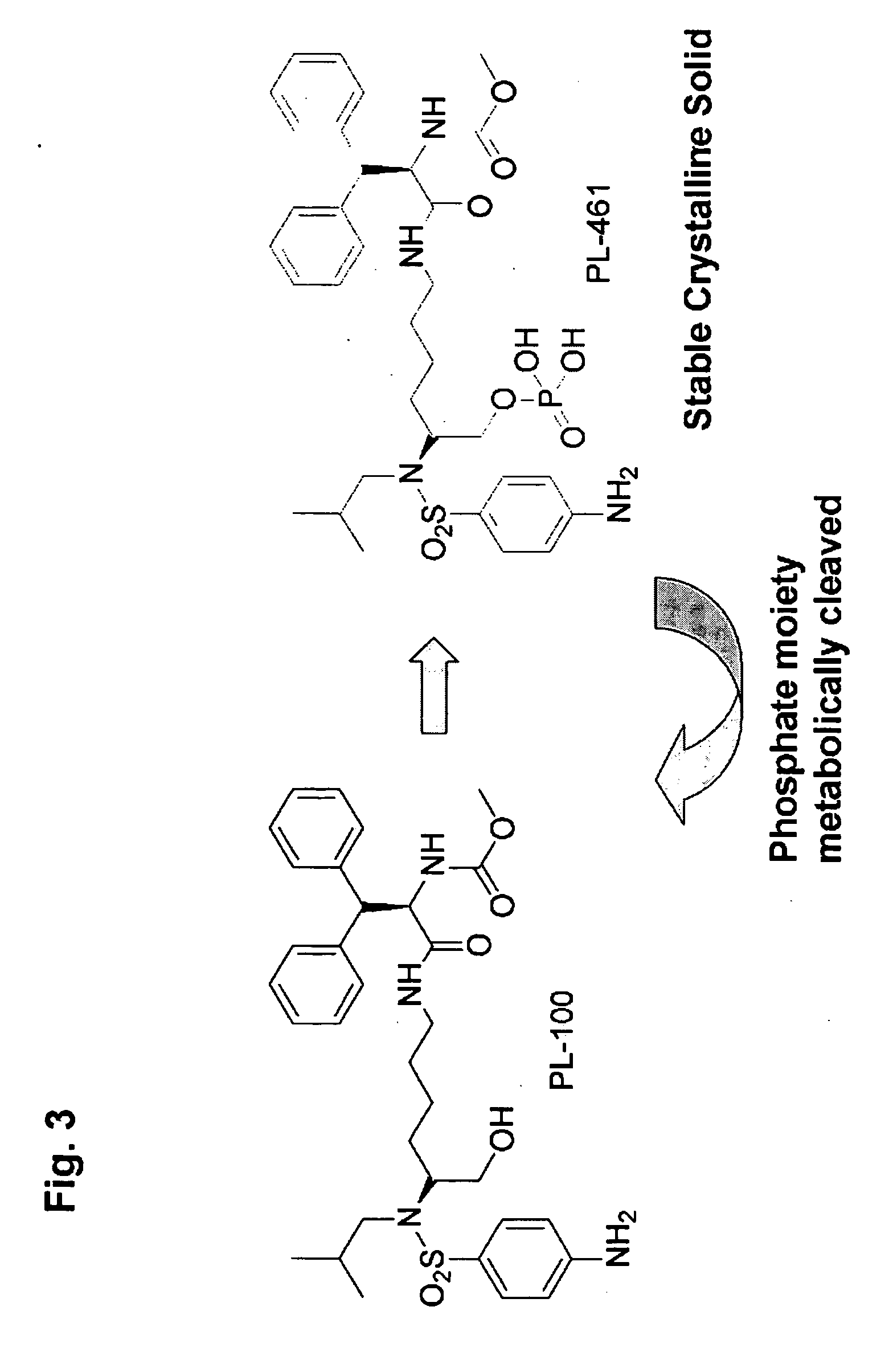
Compositions for inactivating pathogenic microorganisms, methods of making the compositions, and methods of use thereof
InactiveUS20060251684A1Reduce infectivityReduce morbiditySsRNA viruses negative-senseAntibacterial agentsPathogenic microorganismOrganic solvent
Nanoemulsion compositions with low toxicity that demonstrate broad spectrum inactivation of microorganisms or prevention of diseases are described. The nanoemulsions contain an aqueous phase, an oil phase comprising an oil and an organic solvent, and one or more surfactants. Methods of making nanoemulsions and inactivating pathogenic microorganisms are also provided.
Owner:NANOBIO CORP
Methods of production and use of liquid formulations of plasma proteins
InactiveUSRE38431E1Enhanced quality of lifeReduce infectivityPeptide/protein ingredientsInorganic non-active ingredientsMethods of productionFactor XIIIa
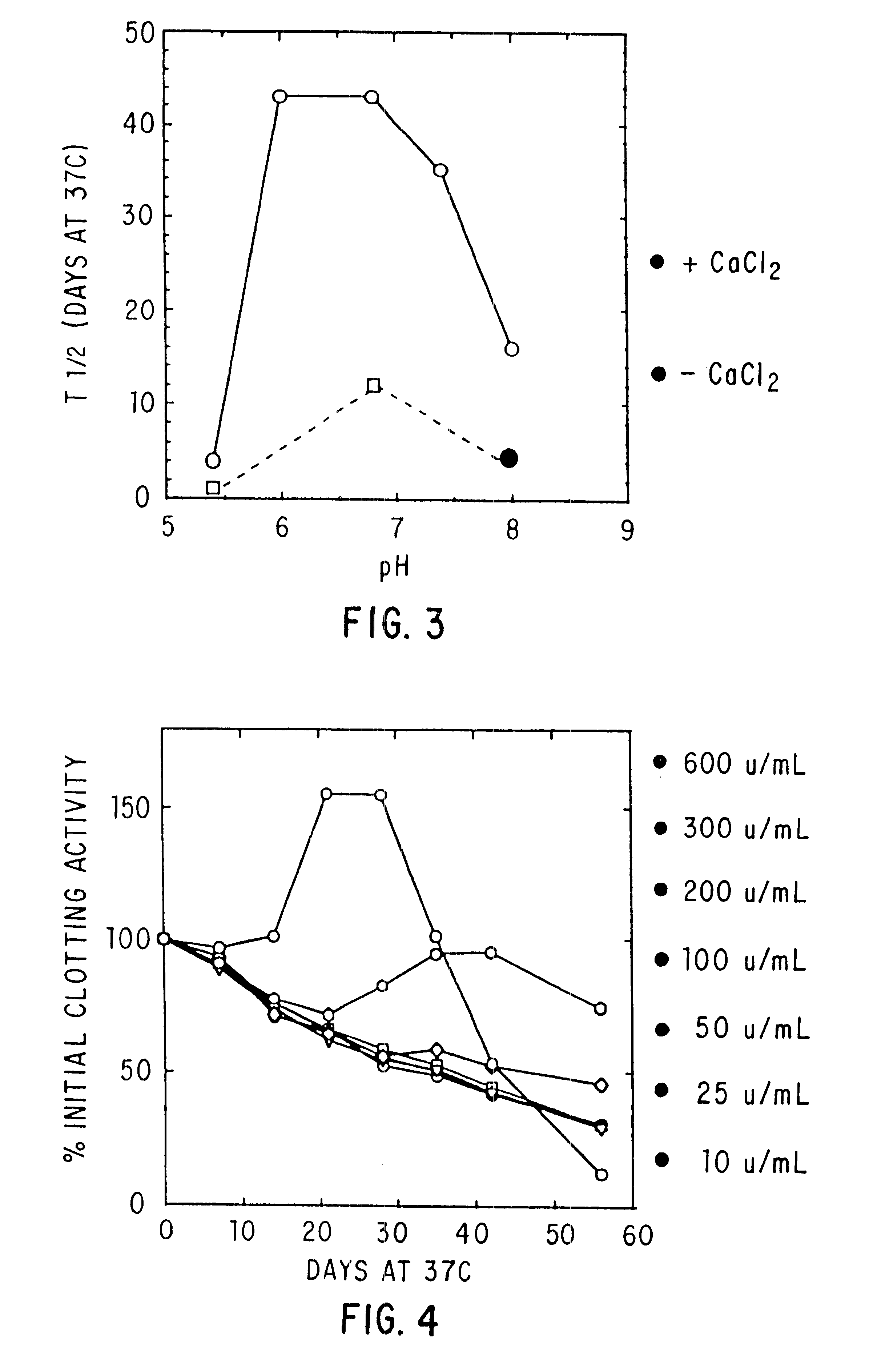
The present invention relates to the preparation and use of liquid formulations of plasma proteins, particularly blood coagulation factors. More specifically, the present invention relates to stable liquid formulations of Factor VIII and Factor IX that can be administered by injection or infusion to provide a constant level of the coagulation factor in the blood.
Owner:THE COALITION FOR HEMOPHILIA B +1
Chimeric Arterivirus-like particles
InactiveUS7122347B2Reducing stability of interactionReduce infectivityFungiSsRNA viruses positive-senseStructural proteinArterivirus
The invention relates to the field for Arteriviruses and vaccines directed against infections caused by these viruses. The invention provides an Arteriviruses-like particle comprising at least a first structural protein derived from a first Arterivirus and a second structural protein wherein the second structural protein is at least partly not derived from said first Arterivirus.
Owner:ID LEYLSTAD INST VOOR DIERHOUDERIJ +1
Systems and methods for contaminant detection within a fluid, ultraviolet treatment and status notification
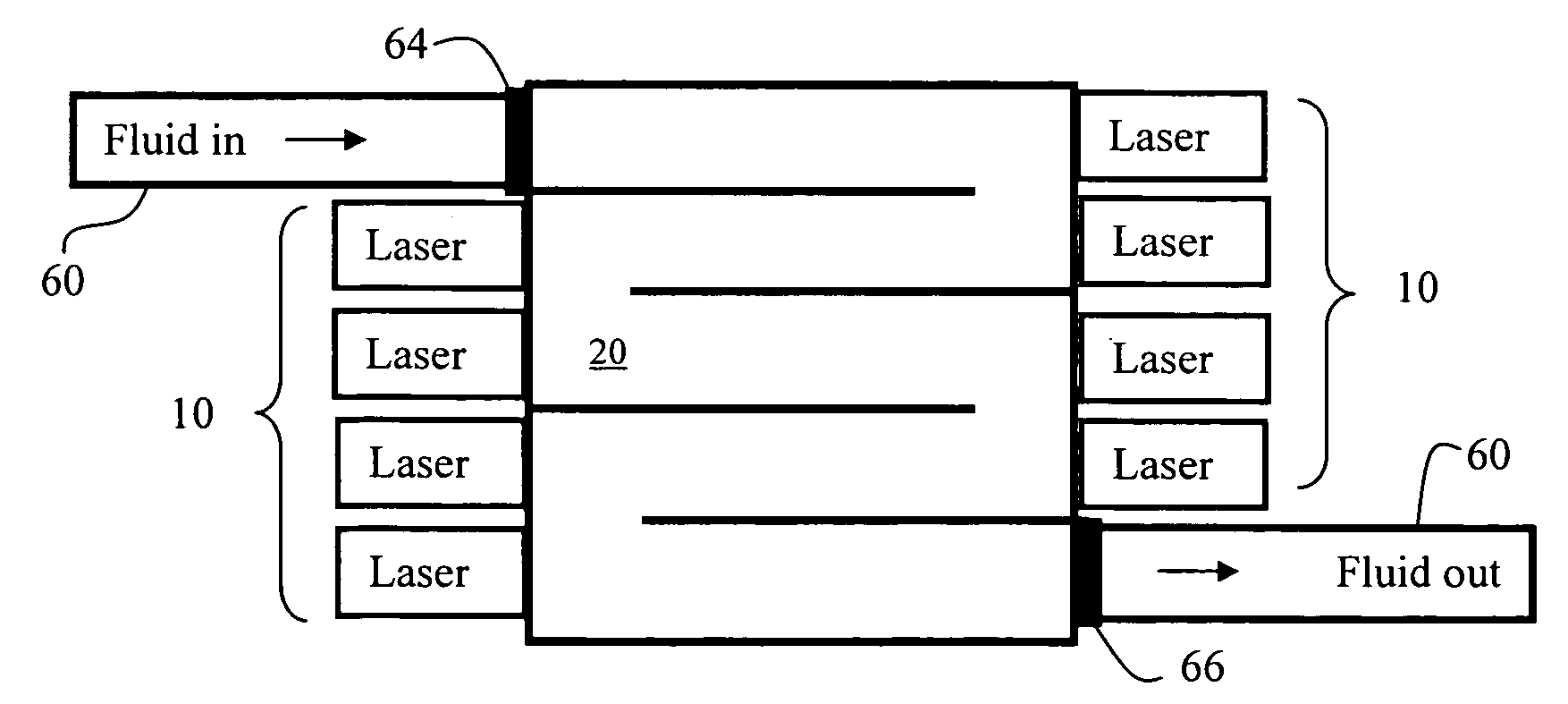
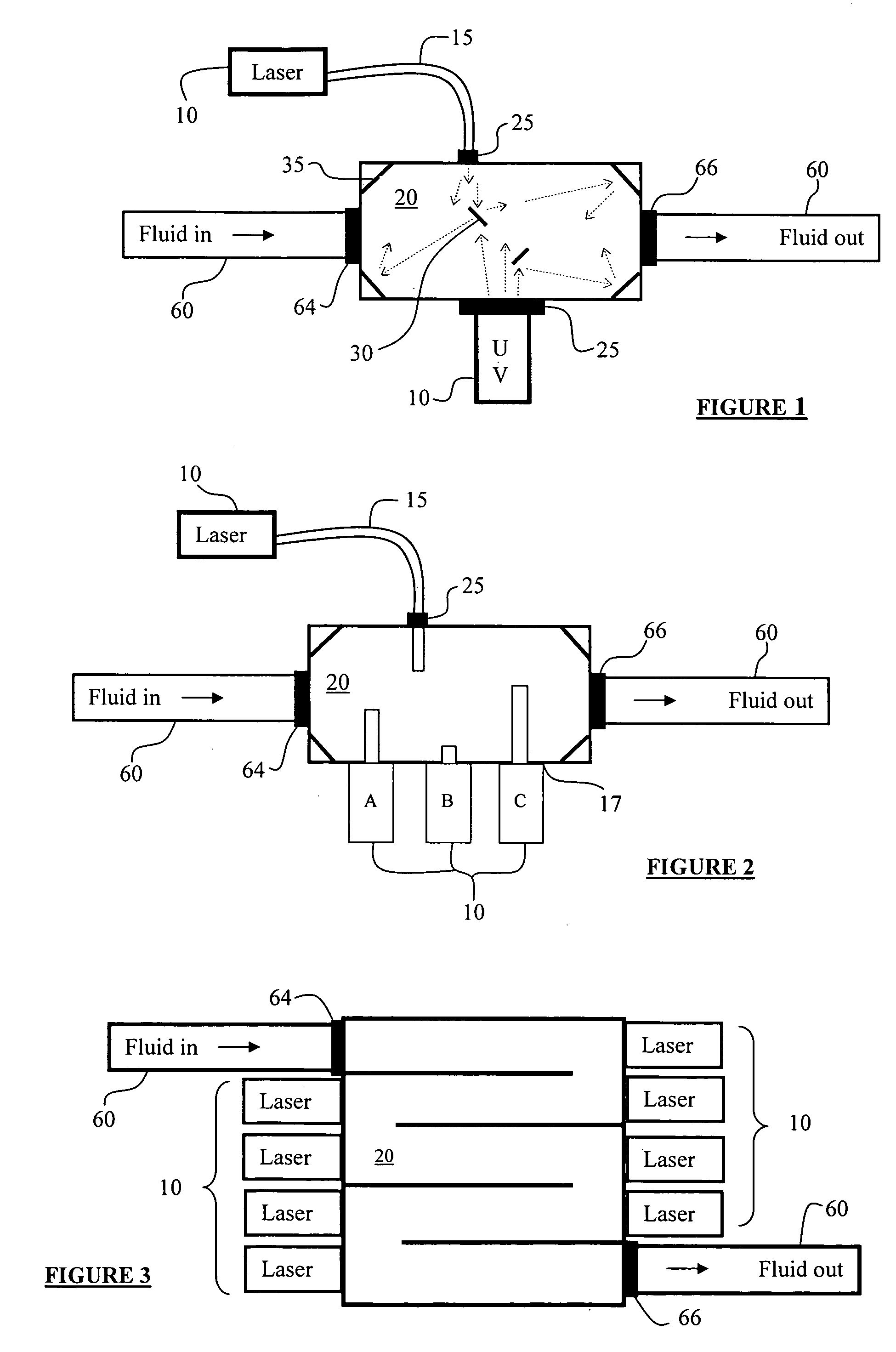
InactiveUS7160370B2Reduce infectivityHarmful effectCombination devicesAuxillary pretreatmentUltravioletDistribution system
A fluid-borne (e.g., water, air) biological and chemical hazard detection and treatment system can include sensors (e.g., flow rate, contaminant detectors), treatment using ultraviolet laser-emitted light, can be microprocessor controlled and can communicate and be controlled over data networks. Treatment and detection systems can be deployed at various stages along a fluid distribution system, allowing for protection coverage and redundancy. During treatment, fluid enters into and / or passes through a “treatment area” wherein the fluid is subjected to light emanating from a laser at wavelengths within the ultraviolet range. DNA for microorganisms contained within fluid (including blood) are reactive to laser light as they pass through treatment areas and are rendered un-infective. Treatment systems can be staged in close proximity, providing more than one treatment area and associated light sources to a fluid stream. Such staging can provide for concentrated redundancy prior to its delivery to the intended point of use.
Owner:SALTECH CORP
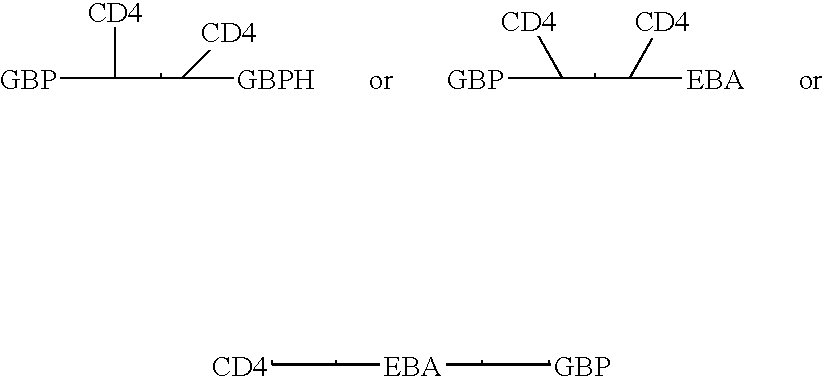
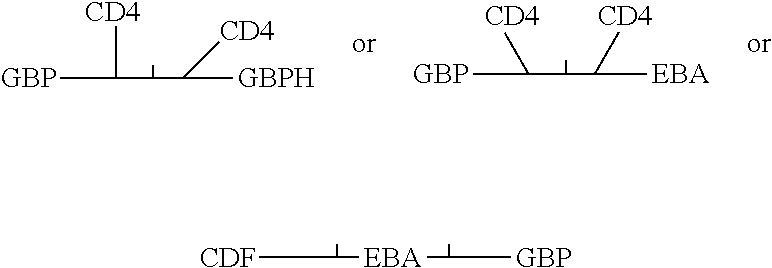
Fusion proteins comprising CD4 and the malaria parasite merozoite glycophorin binding protein 130 (GBP-130)
InactiveUS7585508B1Reduce infectivityRelieve symptomsFusions with soluble cell surface receptorAntiviralsGlycophorinBinding peptide
Novel hybrid fusion peptides are disclosed. The novel peptides are formed by the fusion of two or more components. One component is a peptide sequence or variant of a peptide sequence derived from a malaria parasite merozoite peptide which has affinity for and binding capability to red blood cells.In particular segments of the glycophorin binding peptide 130 (GBP130), are preferred for the first component. Also disclosed are alternative first components, the glycophorin binding peptide homologues (GBPH), or the erythrocyte binding antigen 175 (EBA175), or the plasmodium vivax Duffy receptor or the pre major merozoite surface antigen PMMSA or the (P200) peptide.The first component peptide is fused to all or part of a peptide segment derived from the CD4 molecule or part thereof or variant thereof which shows binding affinity for the HIV virus.The resulting fusion peptide being exemplified asNH2-CD4-GBP130-COOH1-371 201-774Also disclosed are the methods of manufacture and means to use the novel hybrid peptides as clinical agents to treat, prevent or test for HIV infection.
Owner:PRENDERGAST KENNETH F
Unit dosage forms for the treatment of herpes simplex
InactiveUS7351715B2Increase successSafe and effective and inexpensiveBiocidePeptide/protein ingredientsDiseaseCell membrane
The components of this invention are chosen because of their complementarity for the prevention or treatment of diseases caused by the herpes simplex virus. L-Lysine favorably increases the physiologic immunomodulation necessary for defense against this virus. Zinc improves and maintains a normal immune response. 2-Deoxy-2-D-glucose and heparin sodium alter the surface interaction between the herpes virus and the cell, preventing fusion and infectivity. N-Acetyl-L-cysteine increases glutathione levels thereby creating a thiol redox barrier to the virus at the cell membrane. Quercetin reduces intraoellular replication of the herpes virus and viral infectivity. Ascorbate, in concert with copper and D-α-tocopherol, provides an antioxidant defense against the herpes virus, which tends to lose latency during period of oxidative, free radical excess. Selenium and quercetin also participate in reducing various oxidative stresses. Together the components of this invention provide the potential for improved resistance to, improved recovery from, and a decreased frequency of recurrence of herpes simplex virus infection.
Owner:CHRONORX
Compositions and methods for treating viral infections
InactiveUS6043347AImprove hydrophobicityEasy to fixPeptide/protein ingredientsVirus peptidesEpitopeImmunodeficiency virus
Methods and compositions for treatment, diagnosis, and prevention of a virus comprise administering to a patient antibodies which react with regions of viral proteins and result in neutralization of infectivity and inactivation of functionally essential events in the life cycle of the virus. The antibodies recognize viral epitopes which fail to elicit an immune response in man when encountered through infection or naturally through the environment. In a preferred embodiment, the invention provides compositions and methods useful in the treatment and diagnosis of human immunodeficiency virus (HIV) infections.
Owner:TECH HLDG +1
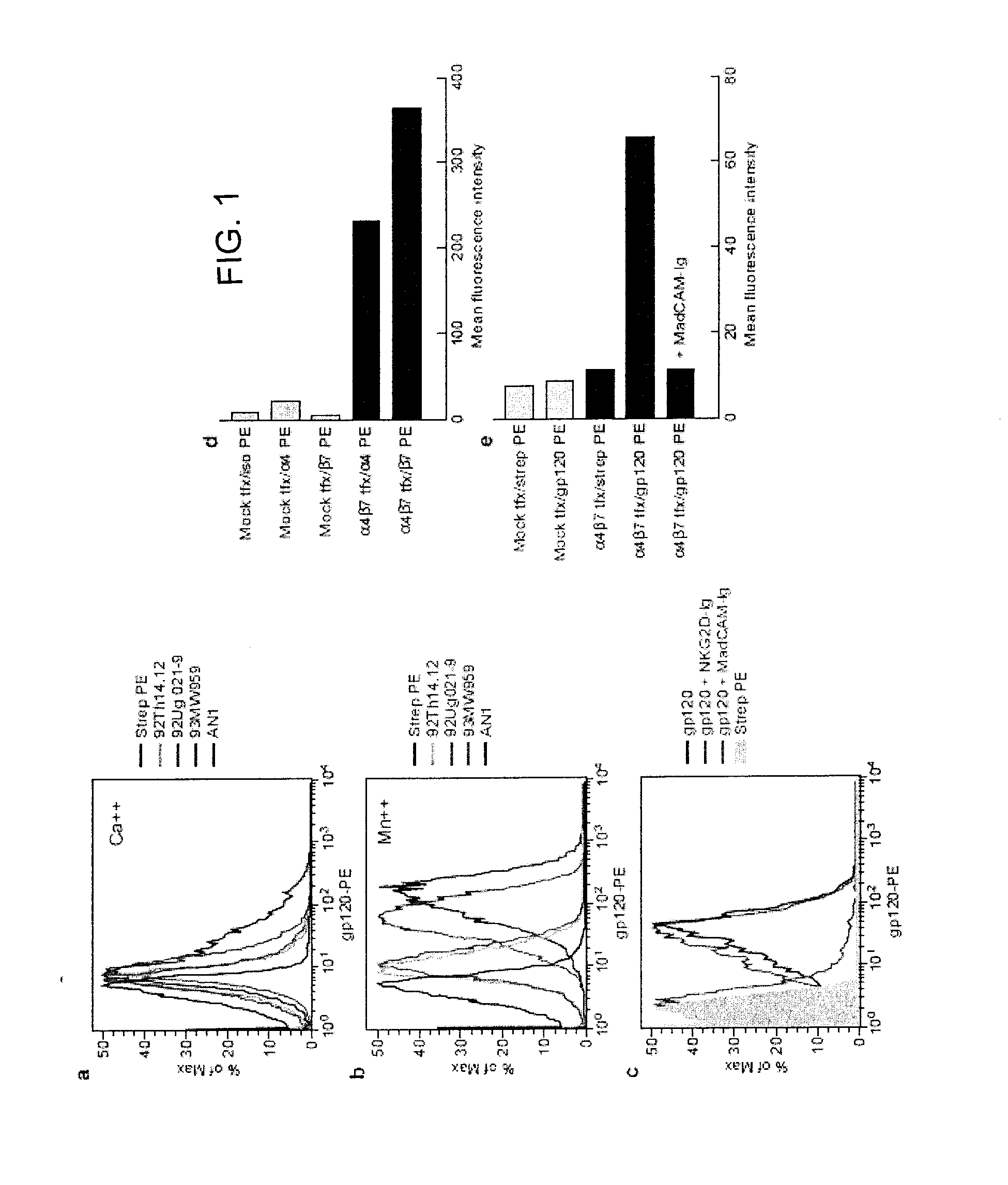
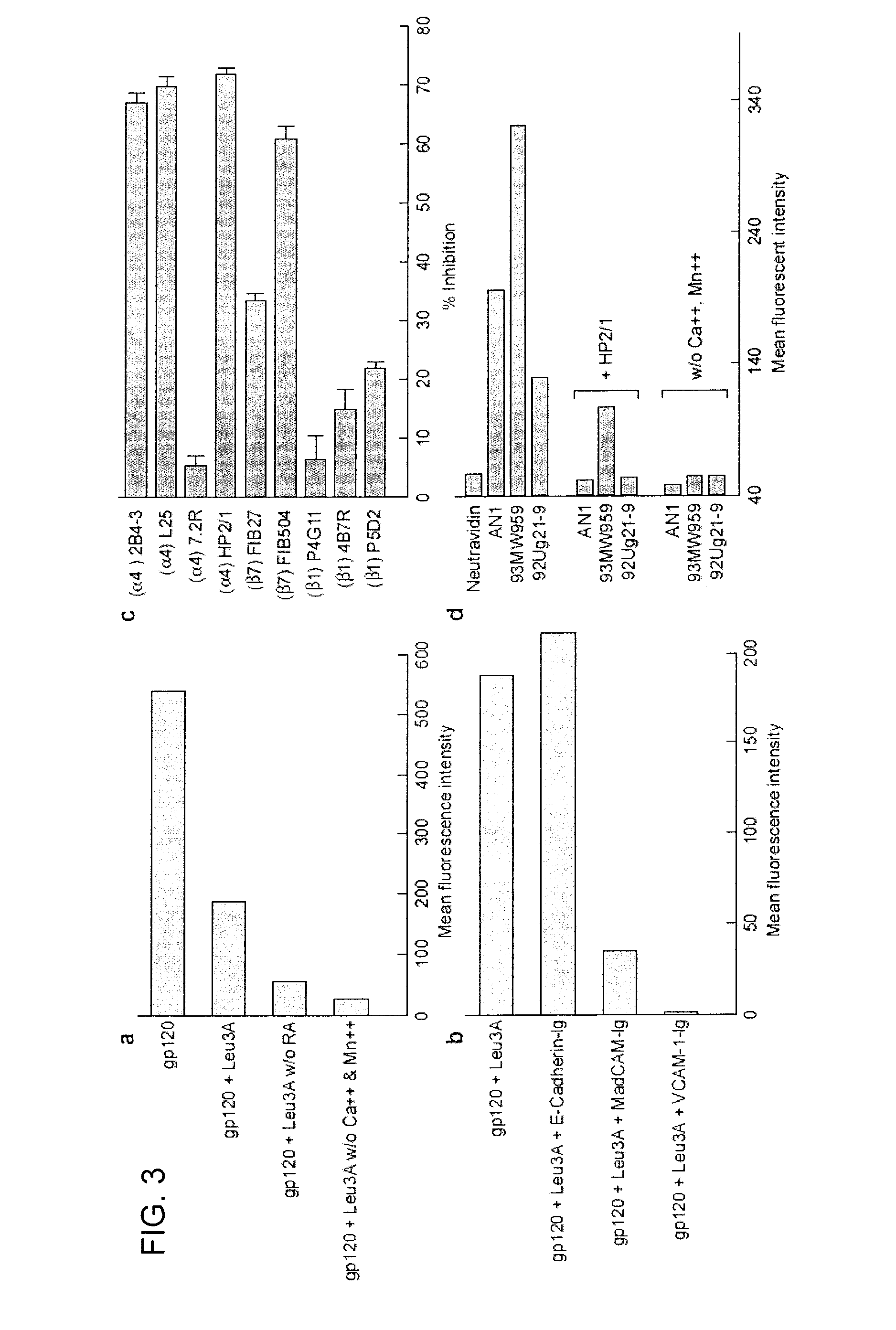
Use of antagonists of the interaction between HIV gp120 and a4b7 integrin
ActiveUS20110086024A1Infection efficiency can be decreasedLow efficiencyCompound screeningApoptosis detectionMonoclonal antibodyΒ7 integrin
Methods are provided for the treatment of a HIV infection. The methods can include administering to a subject with an HIV infection a therapeutically effective amount of an agent that interferes with the interaction of gp120 and α4 integrin, such as a α4β1 or α4β7 integrin antagonist, thereby treating the HIV infection. In several examples, the α4 integrin antagonist is a monoclonal antibody that specifically binds to a α4, β1 or β7 integrin subunit or a cyclic hexapeptide with the amino acid sequence of CWLDVC. Methods are also provided to reduce HIV replication or infection. The methods include contacting a cell with an effective amount of an agent that interferes with the interaction of gp120 and α4 integrin, such as a α4β1 or α4β7 integrin antagonist. Moreover, methods are provided for determining if an agent is useful to treat HIV.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Compositions and methods for treating viral infections
InactiveUS6258599B1Neutralize and inactivate essential stepReduce infectivityPeptide/protein ingredientsVirus peptidesEpitopeImmunodeficiency virus
Owner:PROBE INT
Anti-viral fusion peptides
InactiveUS20110082075A1Reduce circulationReduce infectivityBiocidePeptide/protein ingredientsErythrocyte bindingBinding peptide
Owner:PRENDERGAST KENNETH FRANCIS
SARS Vaccine Compositions and Methods of Making and Using Them
InactiveUS20090017069A1The process is simple and effectiveThe method is simple and efficientSsRNA viruses positive-senseViral antigen ingredientsLipid formationViral Vaccine
Owner:ELI LILLY & CO +1
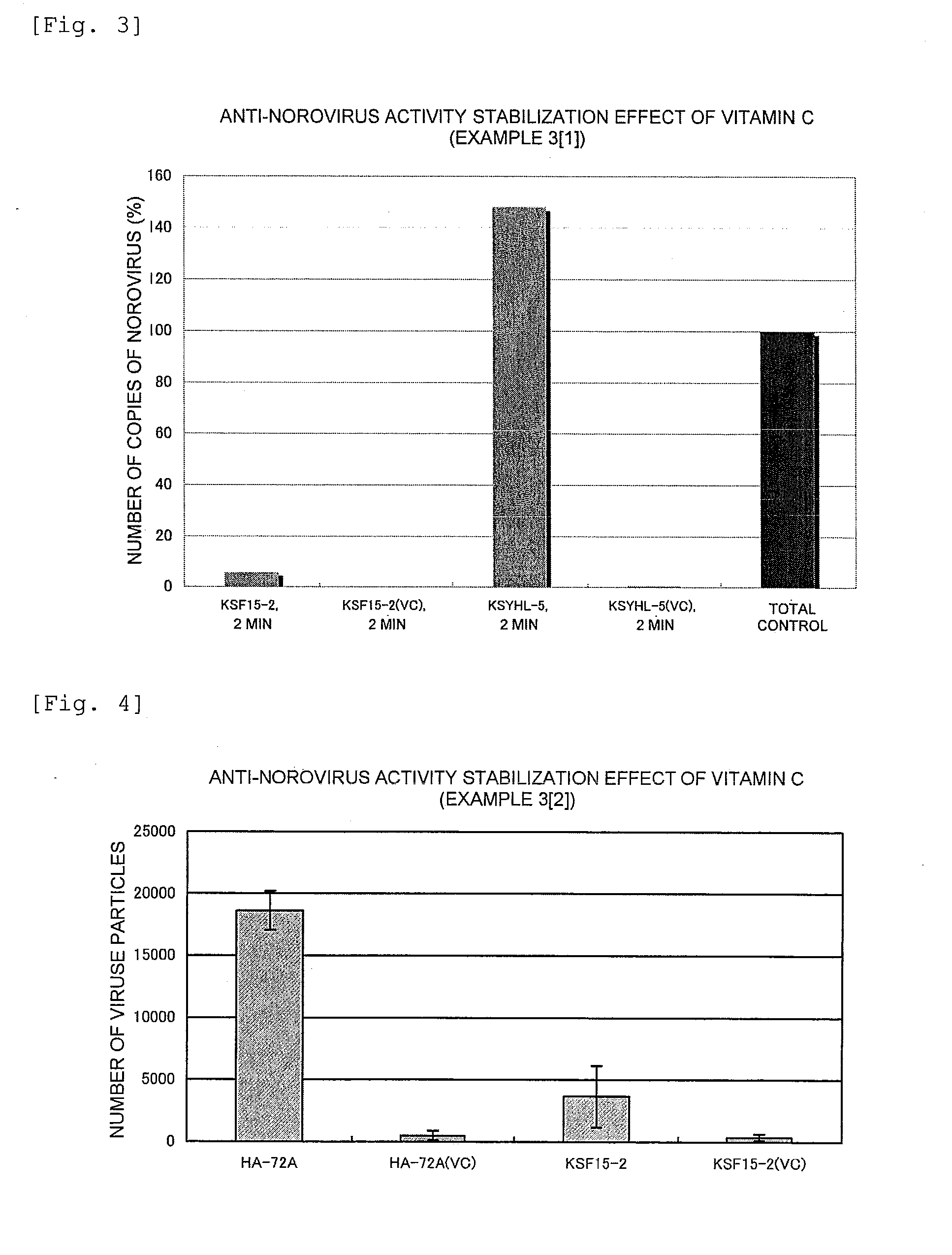
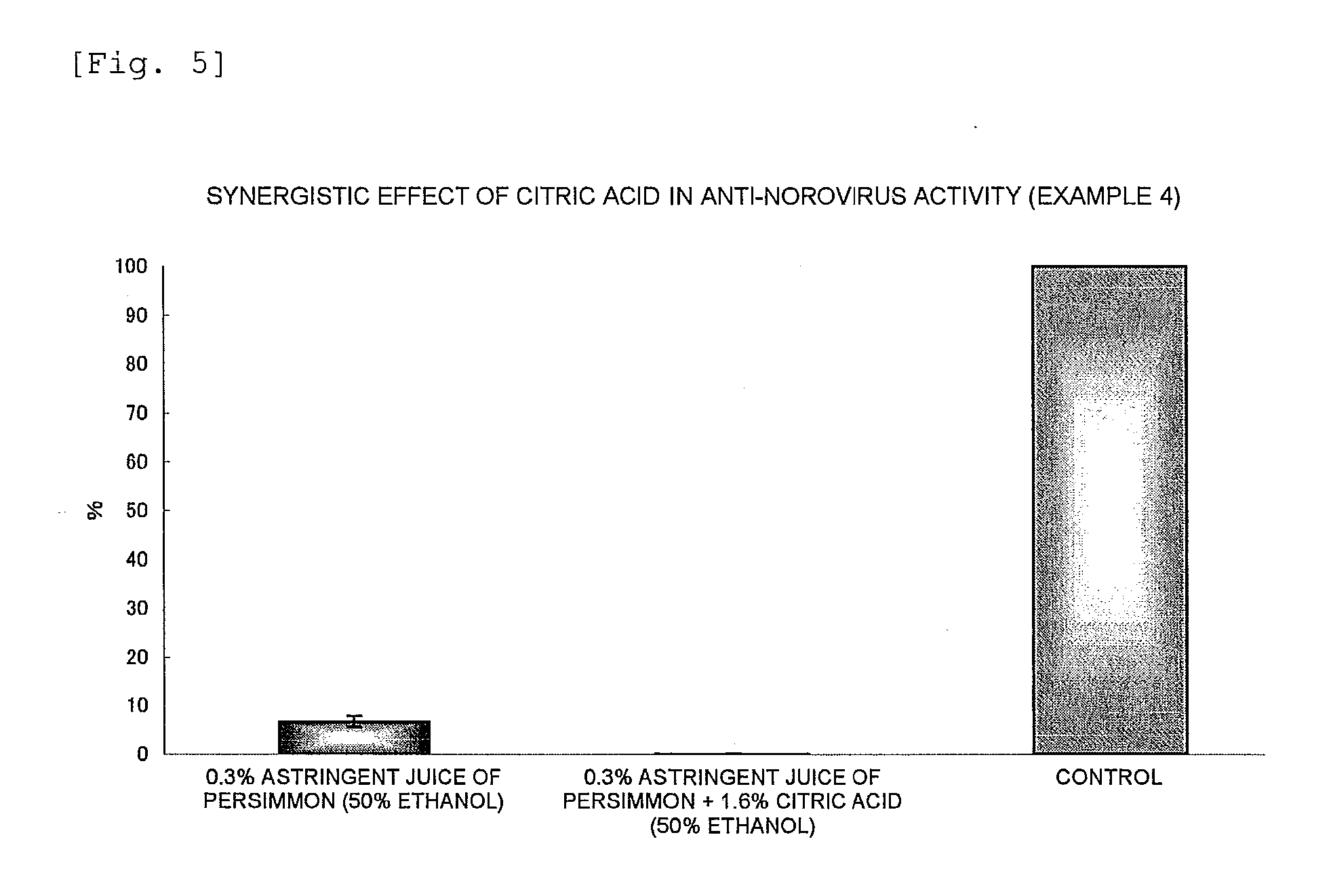
Anti-Norovirus Agent and Composition Containing the Same
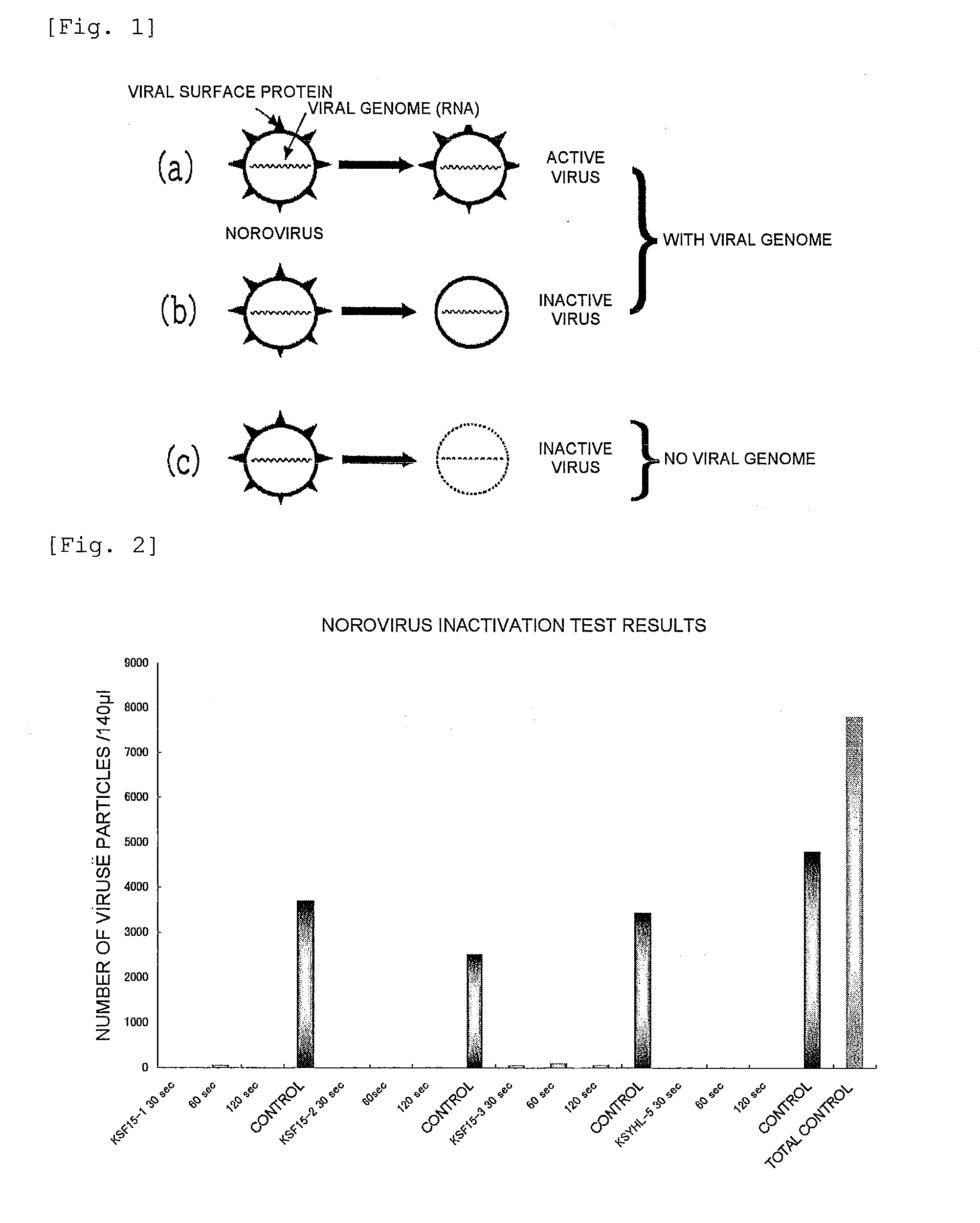
ActiveUS20100240600A1Excellent anti-norovirus characteristicEffective disinfectionAntibacterial agentsBiocideFruit juiceVitamin C
Provided is an anti-norovirus agent that has high norovirus-inactivating activity and is safe for the human body, and an anti-norovirus composition that contains the anti-norovirus agent and is useful for disinfection and infection control against the norovirus. The anti-norovirus agent includes, as an active ingredient, an extract from a plant of the genus Diospyros containing tannin (hereinafter referred to as a “persimmon extract”), preferably a persimmon extract produced by heating squeezed juice or an extract from the fruit of a plant of the genus Diospyros or treating the squeezed juice or the extract with an alcohol. The anti-norovirus composition contains the anti-norovirus agent and at least one selected from the group consisting of alcohols, surfactants, antimicrobial agents, humectants, and cosmetic fats and oils, and preferably further containing an organic acid, such as citric acid, and / or a salt thereof or vitamin C.
Owner:HIROSHIMA UNIVERSITY +1
Method for improving pharmacokinetics of protease inhibitors and protease inhibitor precursors
InactiveUS20060287316A1Preventing and reducing and probabilityPreventing and reducing riskBiocideAntiviralsProteinase activityDepressant
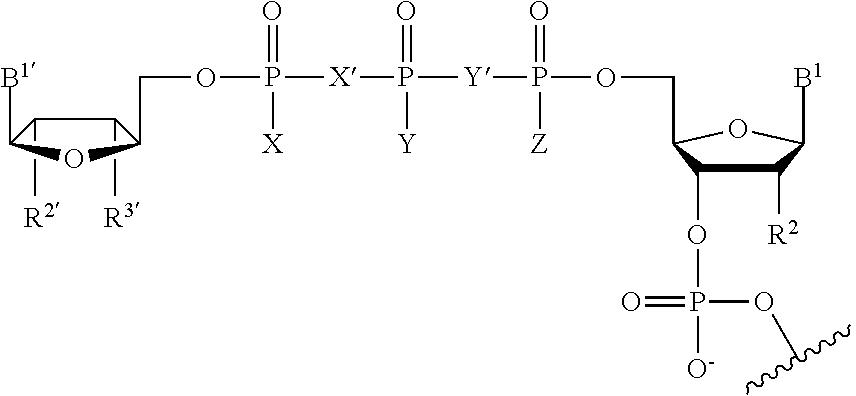
The present invention provides methods for improving the pharmacokinetics of protease inhibitors and protease inhibitor precursors and pharmaceutical composition comprising protease inhibitors or protease inhibitor precursors of formula I and a cytochrome P450 monooxigenase inhibitor; when the compound of formula I comprises an amino group, pharmaceutically acceptable ammonium salts thereof, wherein R1 may be, for example, (HO)2P(O)—, (NaO)2P(O)—, alkyl-CO— or cycloalkyl-CO—, wherein X may be, for example, F, Cl, and Br, and wherein R2 and R3 are as defined herein.
Owner:AMBRILIA BIOPHARMA INC
Compositions for inactivating pathogenic microorganisms, methods of making the compositions, and methods of use thereof
ActiveUS20120171249A1Reduce infectivityReduce morbidityAntibacterial agentsSsRNA viruses negative-sensePathogenic microorganismDisease
Nanoemulsion compositions with low toxicity that demonstrate broad spectrum inactivation of microorganisms or prevention of diseases are described. The nanoemulsions contain an aqueous phase, an oil phase comprising an oil and an organic solvent, and one or more surfactants. Methods of making nanoemulsions and inactivating pathogenic microorganisms are also provided.
Owner:NANOBIO CORP
Method and compostitions for treating coronavirus infection
ActiveUS10729735B1Inhibiting infectivityReducing intercellular transmissionOrganic active ingredientsAntiviralsOleandrinViral infection
A method of treating viral infection, such as viral infection caused by a virus of the Coronaviridae family, is provided. A composition having at least oleandrin is used to treat viral infection.
Owner:PHOENIX BIOTECH INC
Method of sanitizing a biological tissue
ActiveUS20050202137A1Preserve the flavorPreserve colorMilk preservationFruit and vegetables preservationBiotechnologyMicroorganism
A method for treating biological tissue, particularly meats for human consumption, so as to sanitize the tissue, is described. The method inactivates microorganisms and pathogenic prions (proteins) in the tissue.
Owner:URTHTECH
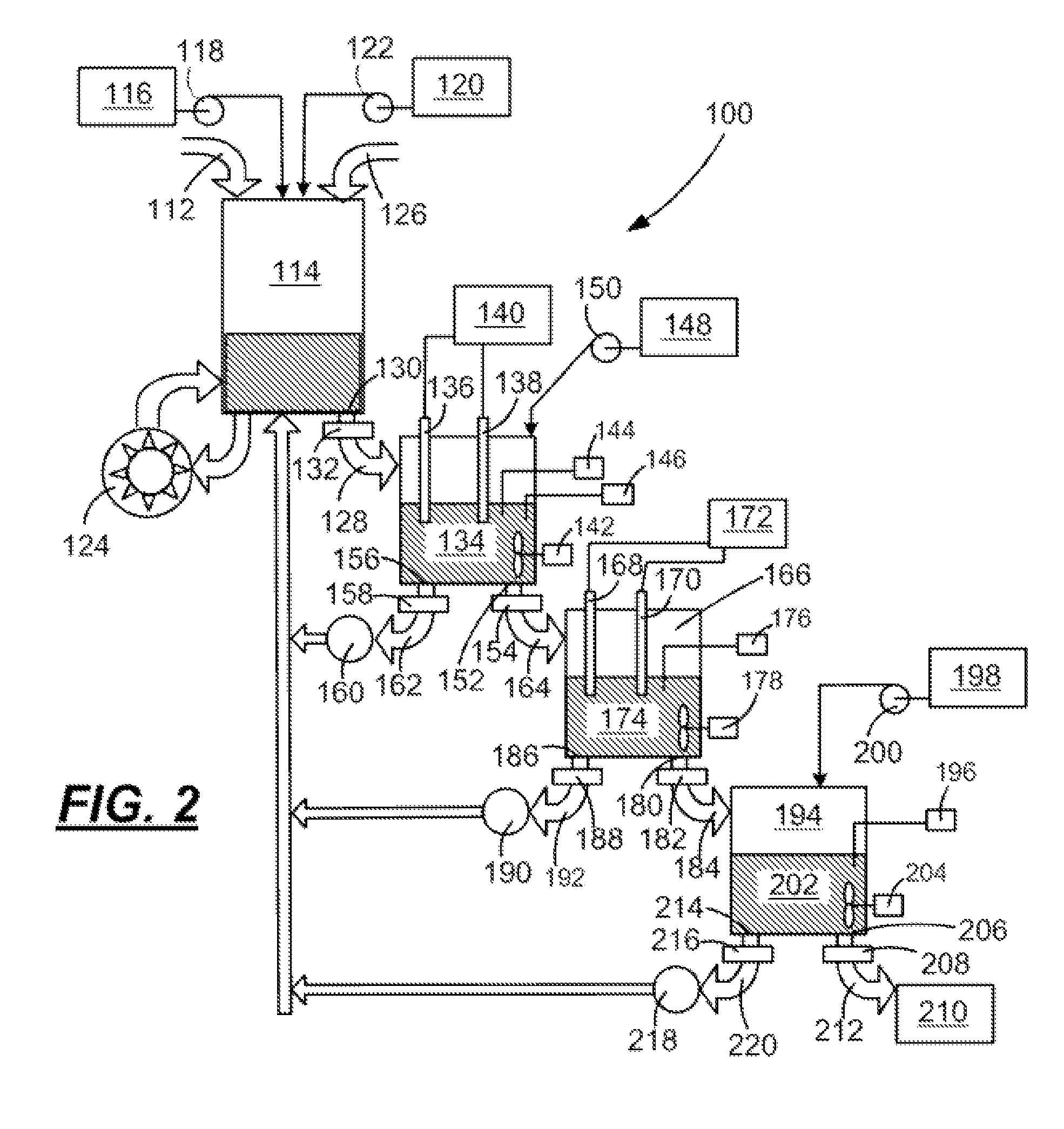
Immunoadhesin comprising a chimeric ICAM-1 molecule produced in a plant
InactiveUS7951378B2Improve efficiencyIncrease in valencySsRNA viruses positive-sensePeptide/protein ingredientsMammalADAMTS Proteins
The immunoadhesions of the present invention are useful in treating rhinovirus infections. The immunoadhesions contain a chimeric ICAM molecule and may optionally also contain J chain and secretory compounds. The chimeric ICAM molecule is a fusion protein that has a rhinovirus receptor protein linked to an immunoglobulin protein. This invention also includes the greatly increased and improved method of producing immunoadhesions in plants. Each of the components of an immunoadhesin is produced in a plant cell and thereby assembles within the plant cell. This method of producing the immunoadhesions of the present invention results in the efficient and economic production of these molecules. The present invention also contemplates the production of immunoadhesions in a variety of eukaryotic cells including plants and mammalian cells. The immunoadhesions of the present invention are useful as a therapeutic against the common cold in humans which is caused by rhinoviruses.
Owner:PLANET BIOTECH
Method for establishing carrier by plant DNA virus infestation
InactiveCN1424400ASmall amount of copyIncreased copy volumeMicrobiological testing/measurementFermentationSatelliteViral genomes
A process for configuring plant DNA virus infections carrier includes such steps as infectious cloning to genom DNA-A and satellite modecule DNA beta by means of the separated yellowing-leaf-curing virus of tomato, overlap-PCR and enzyme severing to obtain two directly repeated genoms, inserting them to high-cloning plant expression carrier, and introducing high-infection Agrobacterium strain by triparental cross.
Owner:ZHEJIANG UNIV
Modified immunodeficiency virus particles
InactiveUS7407663B2The process is simple and effectivePositive immunologic responseAntibacterial agentsSsRNA viruses positive-senseLipid formationImmunodeficiency virus
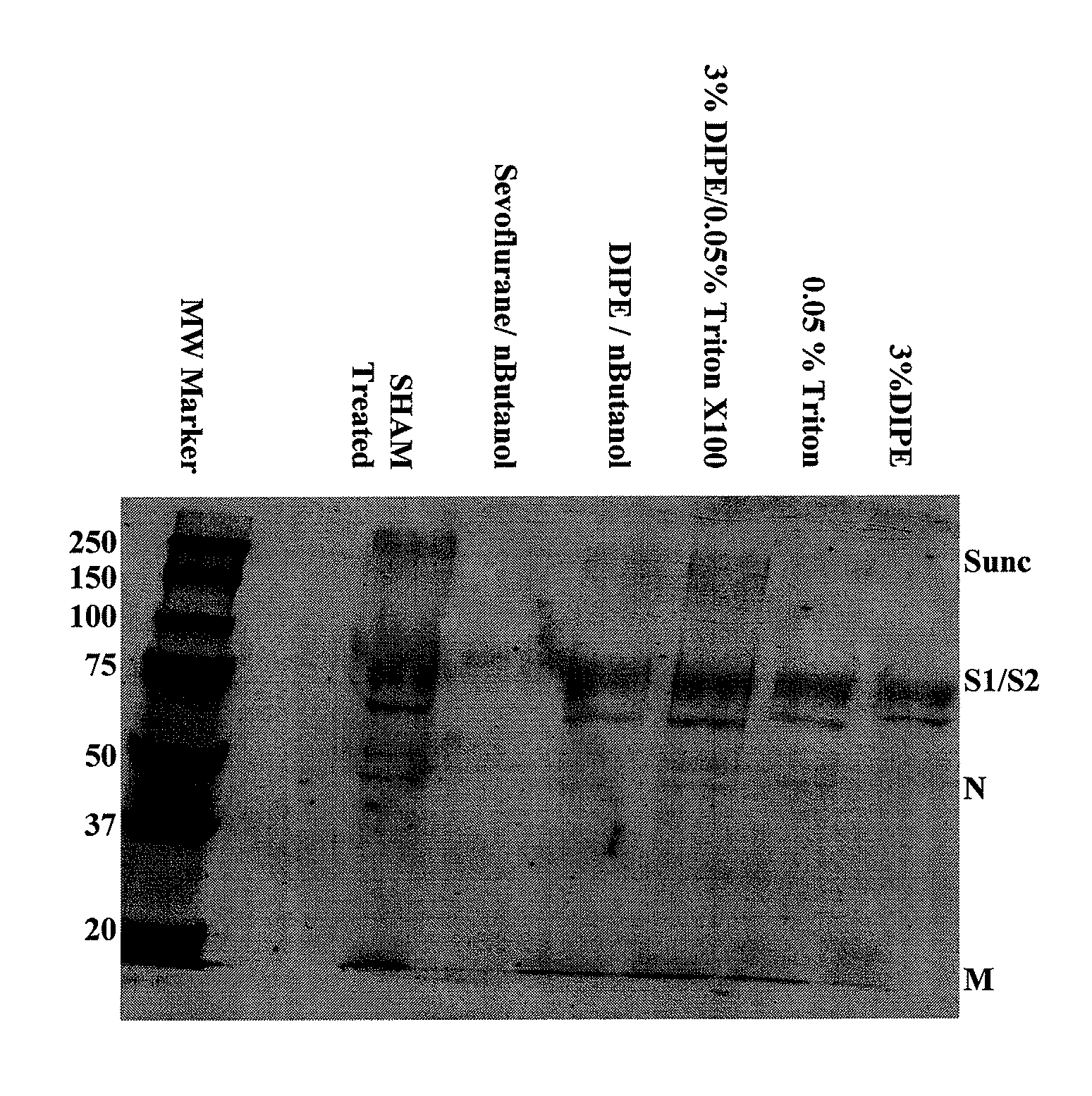
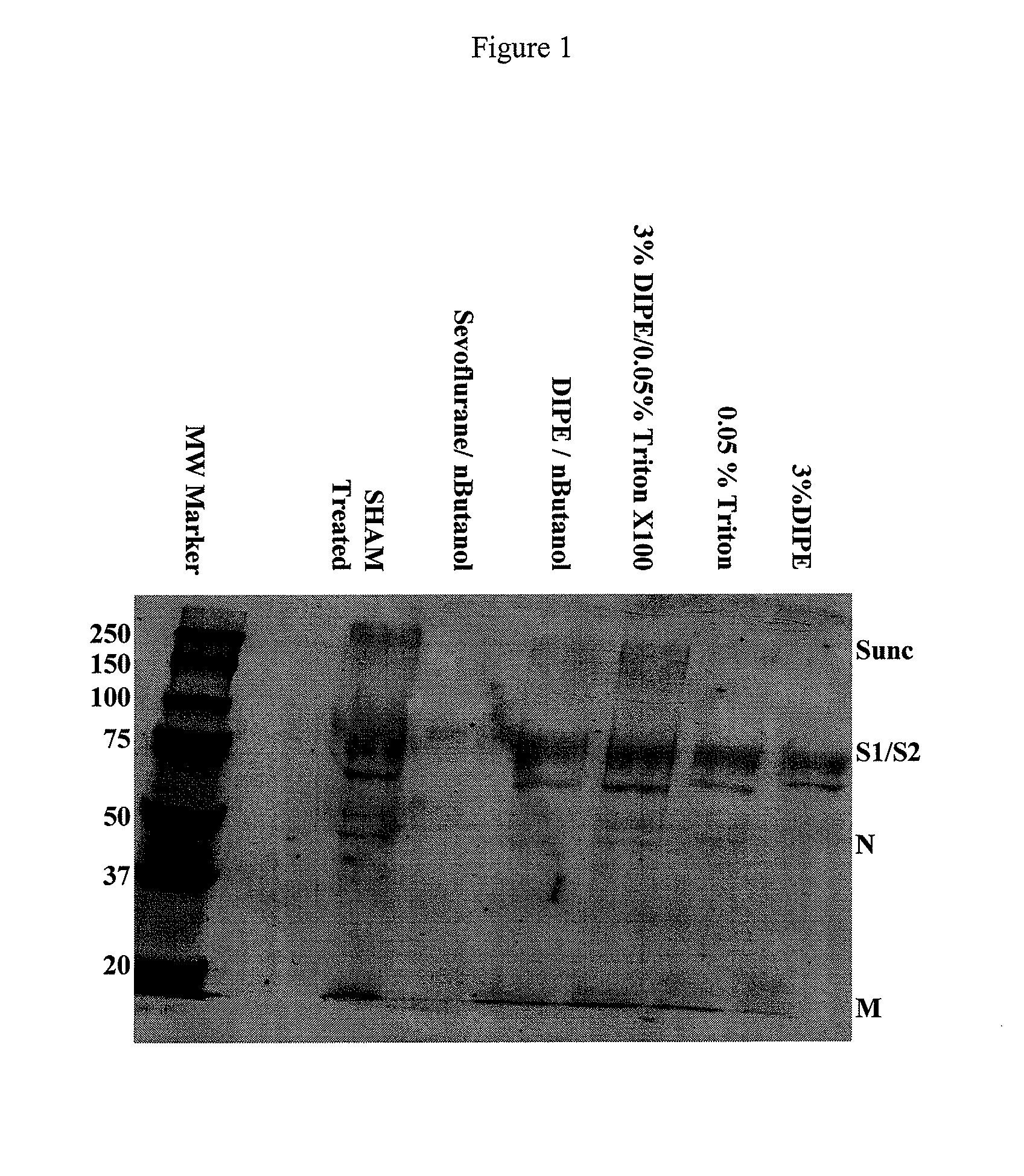
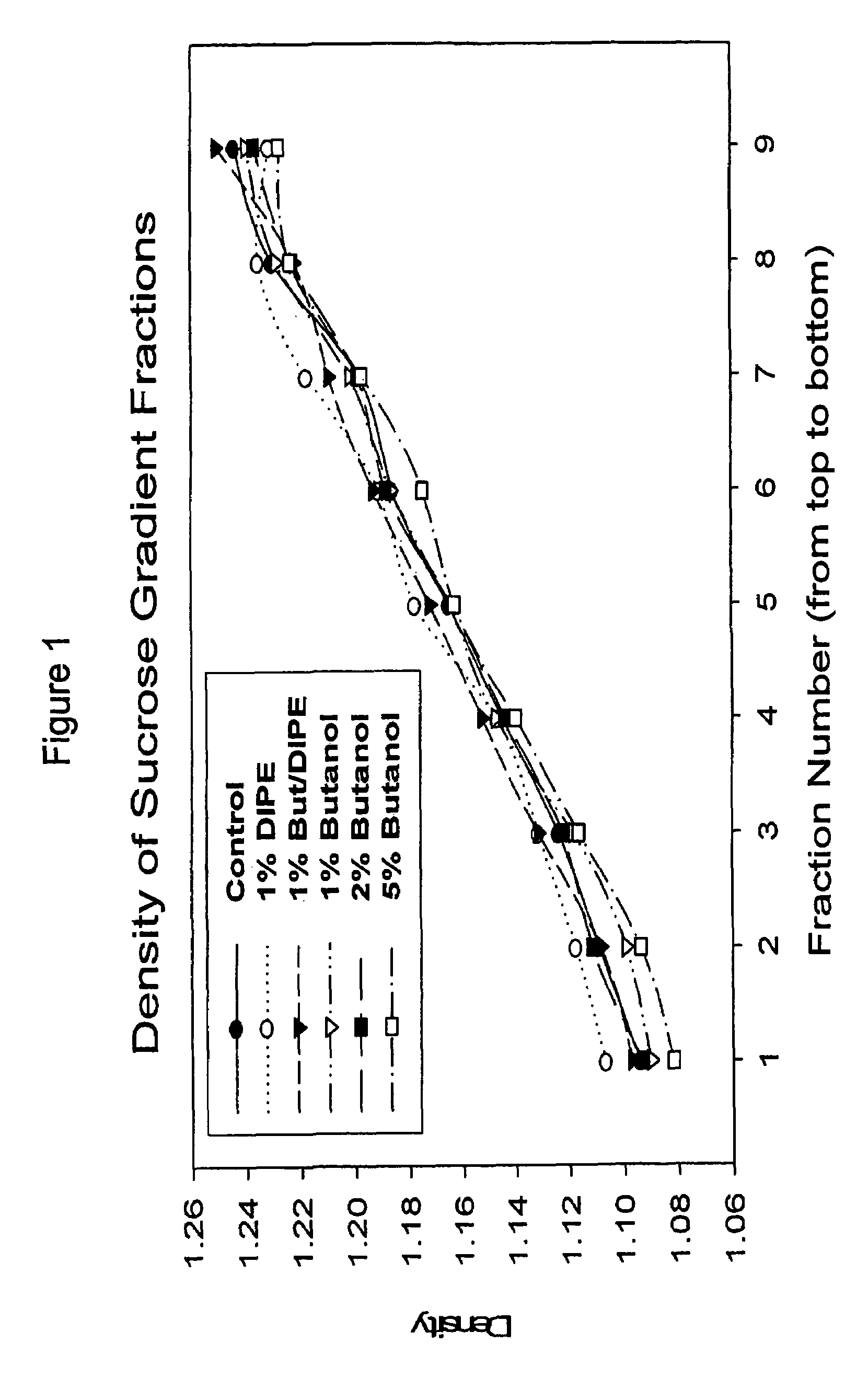
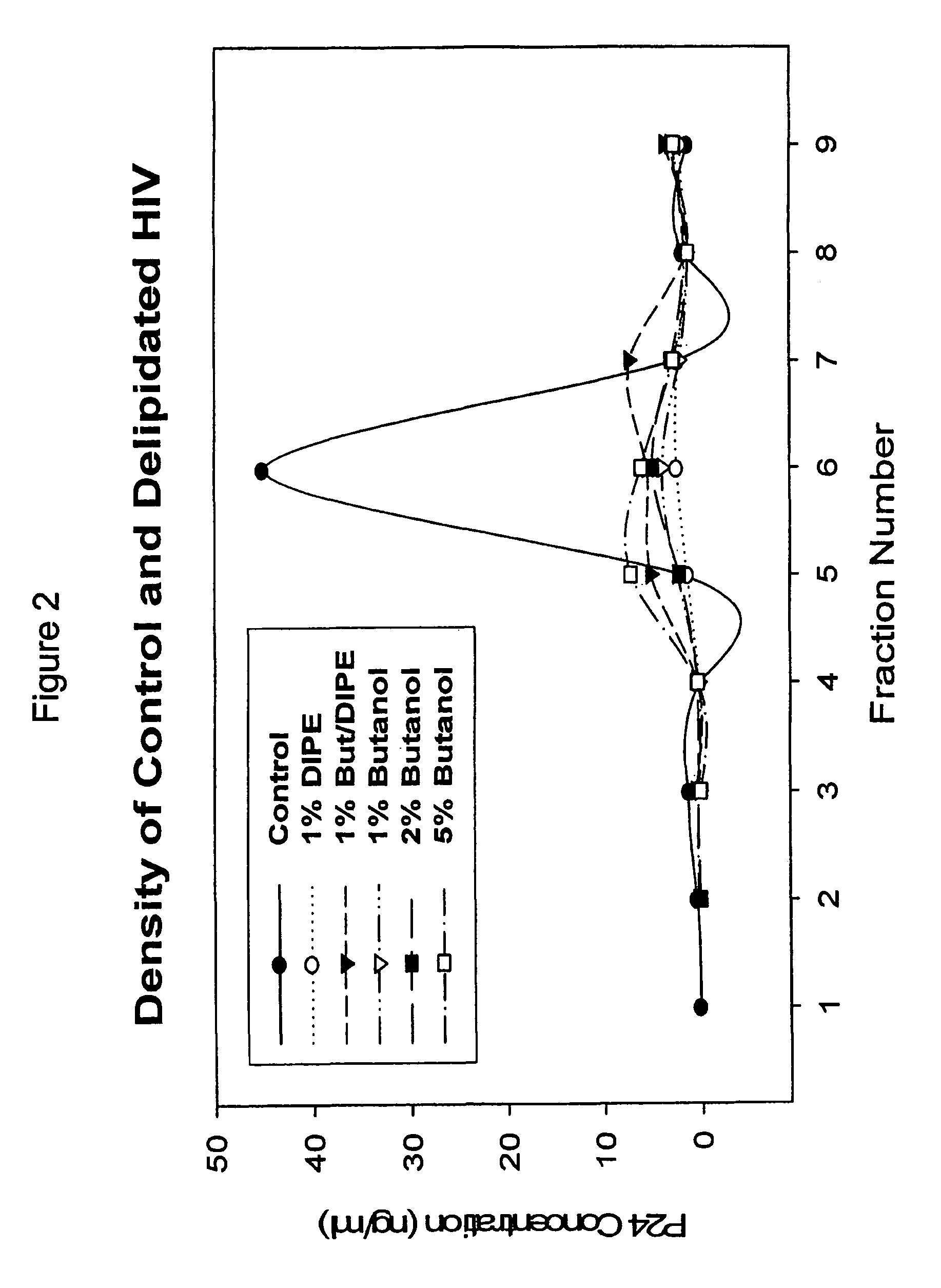
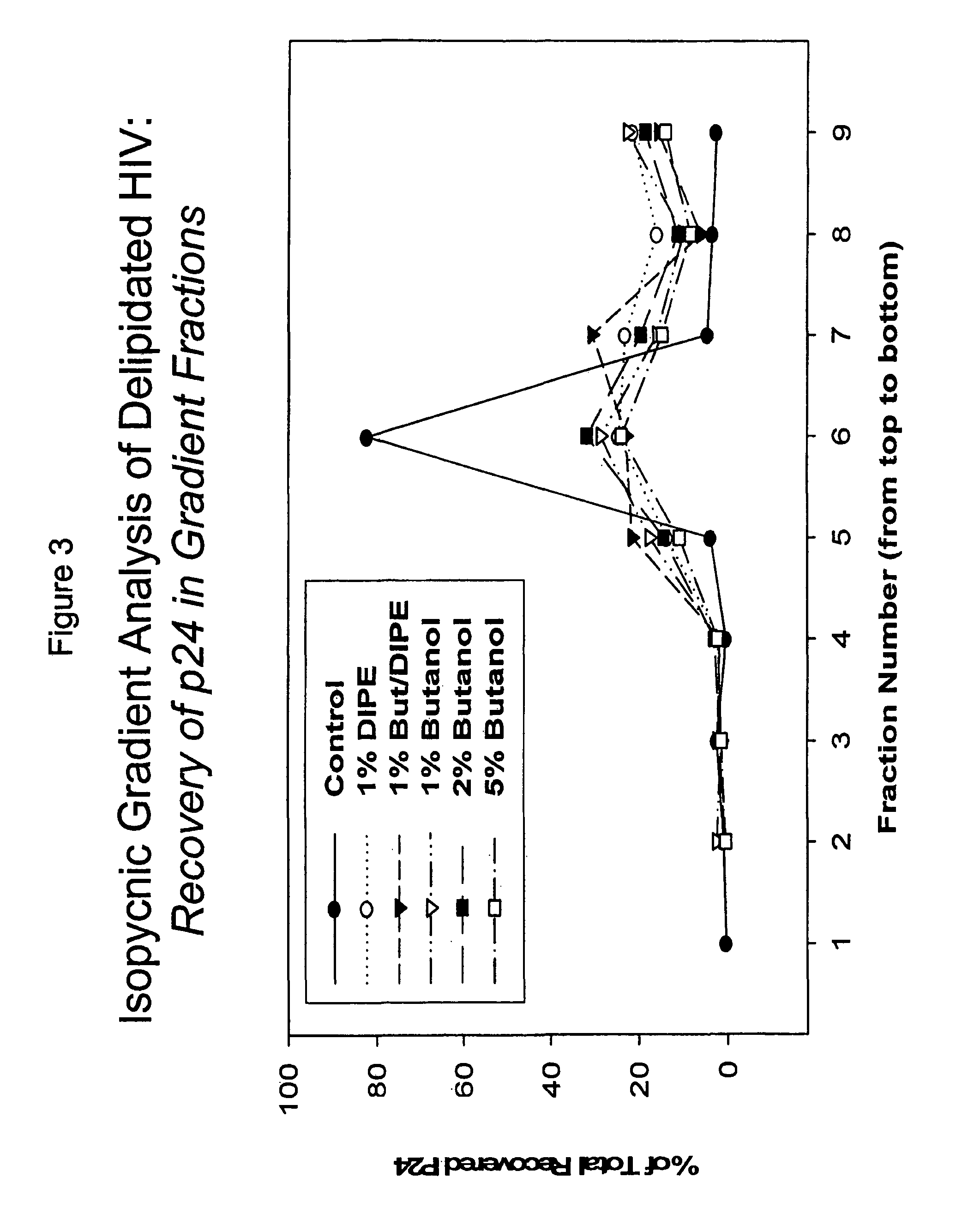
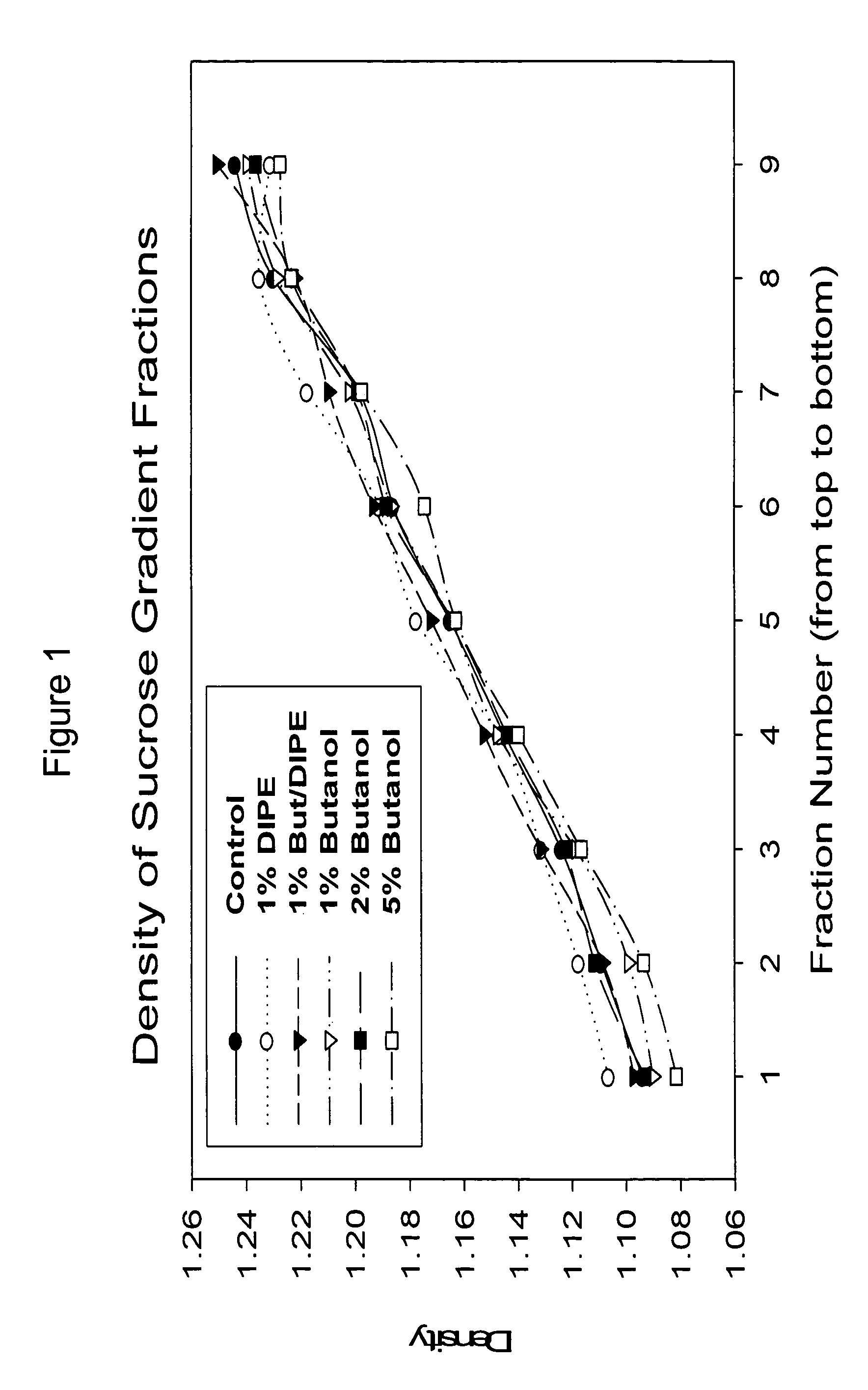
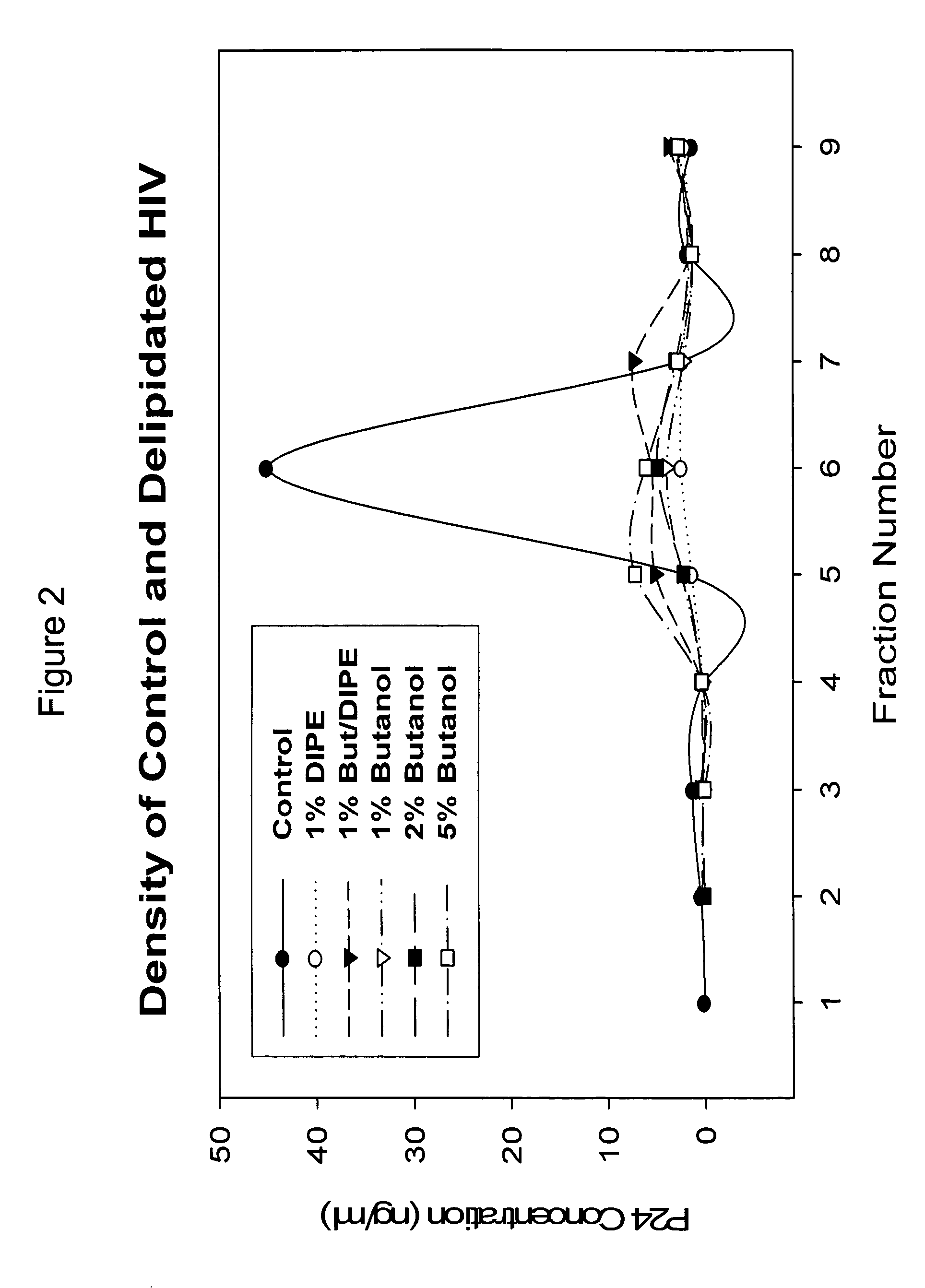
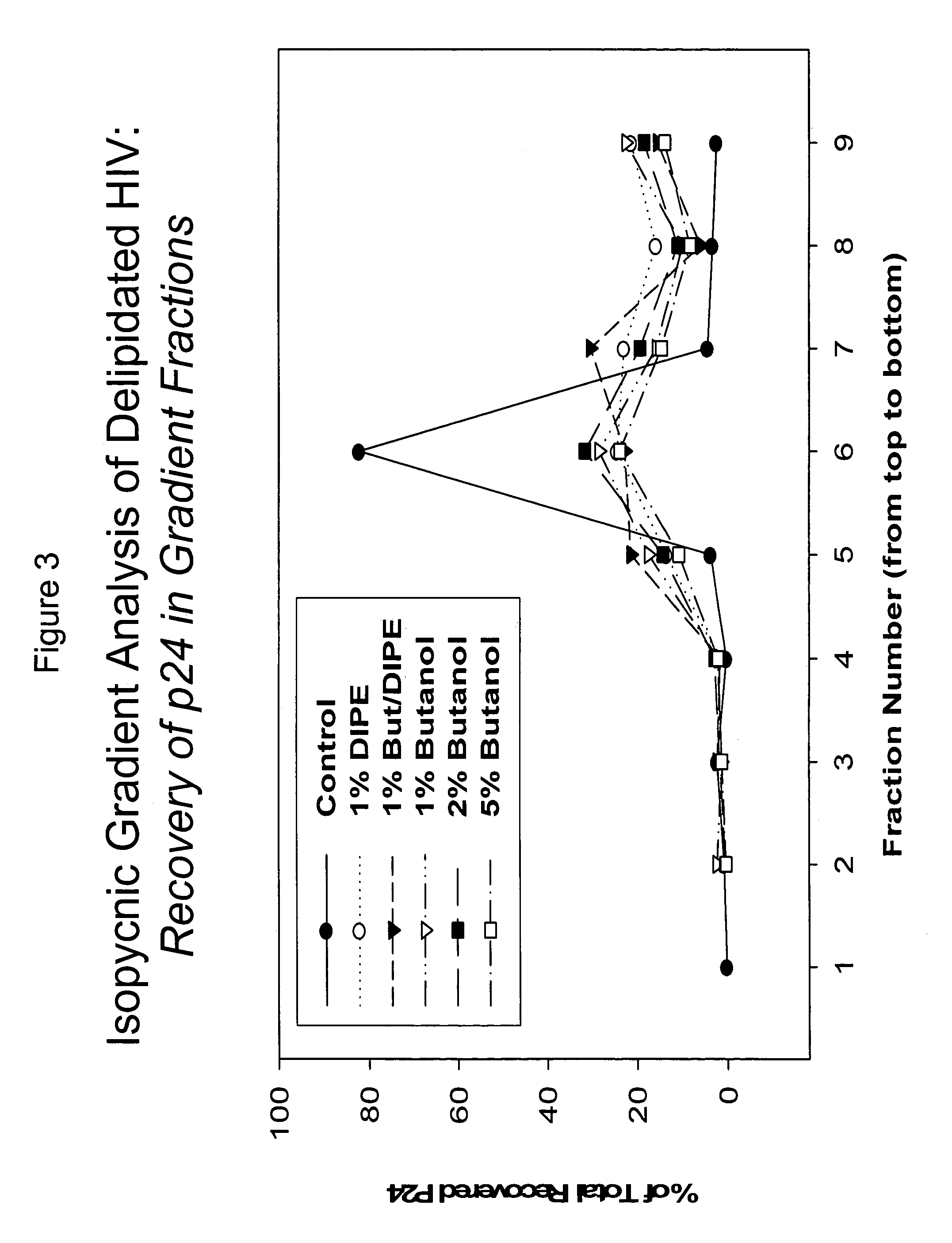
The present invention relates to a method for reducing the occurrence and severity of infectious diseases, especially infectious diseases in which lipid-containing infectious organisms are found in biological fluids, such as blood. The present invention employs solvents useful for extracting lipids from the lipid-containing infectious organism, thereby reducing the infectivity of the infectious organism. The present invention uses optimal solvent systems such that the lipid envelope around the viral particle is dissolved while the viral particle remains intact, resulting in a modified viral particle. The present invention also provides an autologous vaccine composition, comprising a lipid-containing infectious organism, treated with solvents to reduce the lipid content of the infectious organism, combined with a pharmaceutically acceptable carrier. The vaccine composition is administered to an animal or a human to provide protection against the lipid-containing infectious organism. The present invention further provides a simple, inexpensive and easy to use kit for delipidating fluids and for delipidation of lipid-containing organisms in a fluid.
Owner:ELI LILLY & CO +1
Method of making modified immunodeficiency virus particles
InactiveUS7439052B2The process is simple and effectivePositive immunologic responseSsRNA viruses positive-senseViral antigen ingredientsLipid formationImmunodeficiency virus
Described are a composition and method for reducing the occurrence and severity of infectious diseases, especially infectious diseases in which lipid-containing infectious viral organisms are found in biological fluids, such as blood. Solvents useful for extracting lipids from lipid-containing infectious viral organisms are employed thereby creating immunogenic modified, partially delipidated viral particles with reduced infectivity. Provided are delipidated viral vaccine compositions, such as therapeutic vaccine compositions, comprising these modified, partially delipidated viral particles with reduced infectivity, optionally combined with a pharmaceutically acceptable carrier or an immunostimulant. The vaccine composition is administered to a patient to provide protection against a lipid-containing infectious viral organism or, as a therapeutic vaccine, to treat or alleviate infection by the lipid-containing infectious viral organism. The vaccine compositions of the present invention include combination vaccines of modified viral particles obtained from one or more strains of a virus and / or one or more types of virus.
Owner:LIPID SCI +1
Crispr/cas-related methods and compositions for treating herpes simplex virus
ActiveUS20180251770A1Reduce infectivityReduce replicationSenses disorderHydrolasesHuman cellBioinformatics
CRISPR / CAS-related systems, compositions and methods for editing RS1, RL2, and / or LAT genes in human cells are described, as are cells and compositions including cells edited according to the same.
Owner:EDITAS MEDICINE
Modified viral particles with immunogenic properties and reduced lipid content
InactiveUS7407662B2The process is simple and effectivePositive immunologic responseSsRNA viruses positive-senseViral antigen ingredientsLipid formationSolvent
The present invention relates to a method for reducing the occurrence and severity of infectious diseases, especially infectious diseases in which lipid-containing infectious viral organisms are found in biological fluids, such as blood. The present invention employs solvents useful for extracting lipids from the lipid-containing infectious viral organism thereby creating modified viral particles with reduced infectivity and enhanced antigenicity. The present invention provides vaccine compositions, comprising these modified viral particles with reduced infectivity and enhanced antigenicity, optionally combined with a pharmaceutically acceptable carrier or an immunostimulant. The vaccine composition is administered to a patient to provide protection against the lipid-containing infectious viral organism. The vaccine compositions of the present invention include combination vaccines of modified viral particles obtained from one or more strains of a virus and / or one or more types of virus.
Owner:LIPID SCI +1
Crispr/cas-related methods and compositions for treating hepatitis b virus
InactiveUS20180236103A1Avoid sequelaeReduce expressionHydrolasesStable introduction of DNAGenome editingHepatitis B virus
Owner:EDITAS MEDICINE
Novel Anti-Viral Method
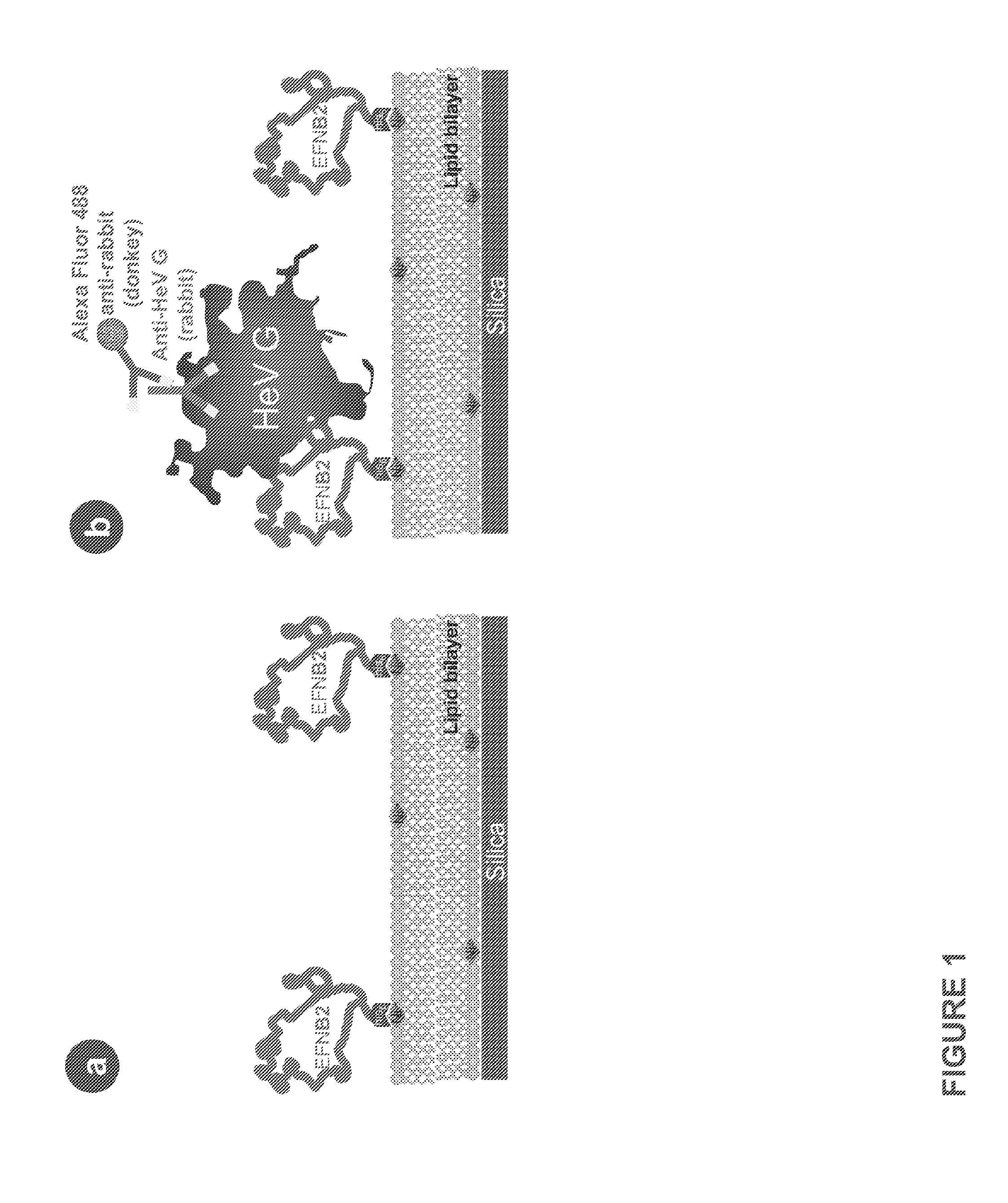
ActiveUS20120039978A1Reduce infectivitySsRNA viruses negative-sensePowder deliveryLipid formationBiological activation
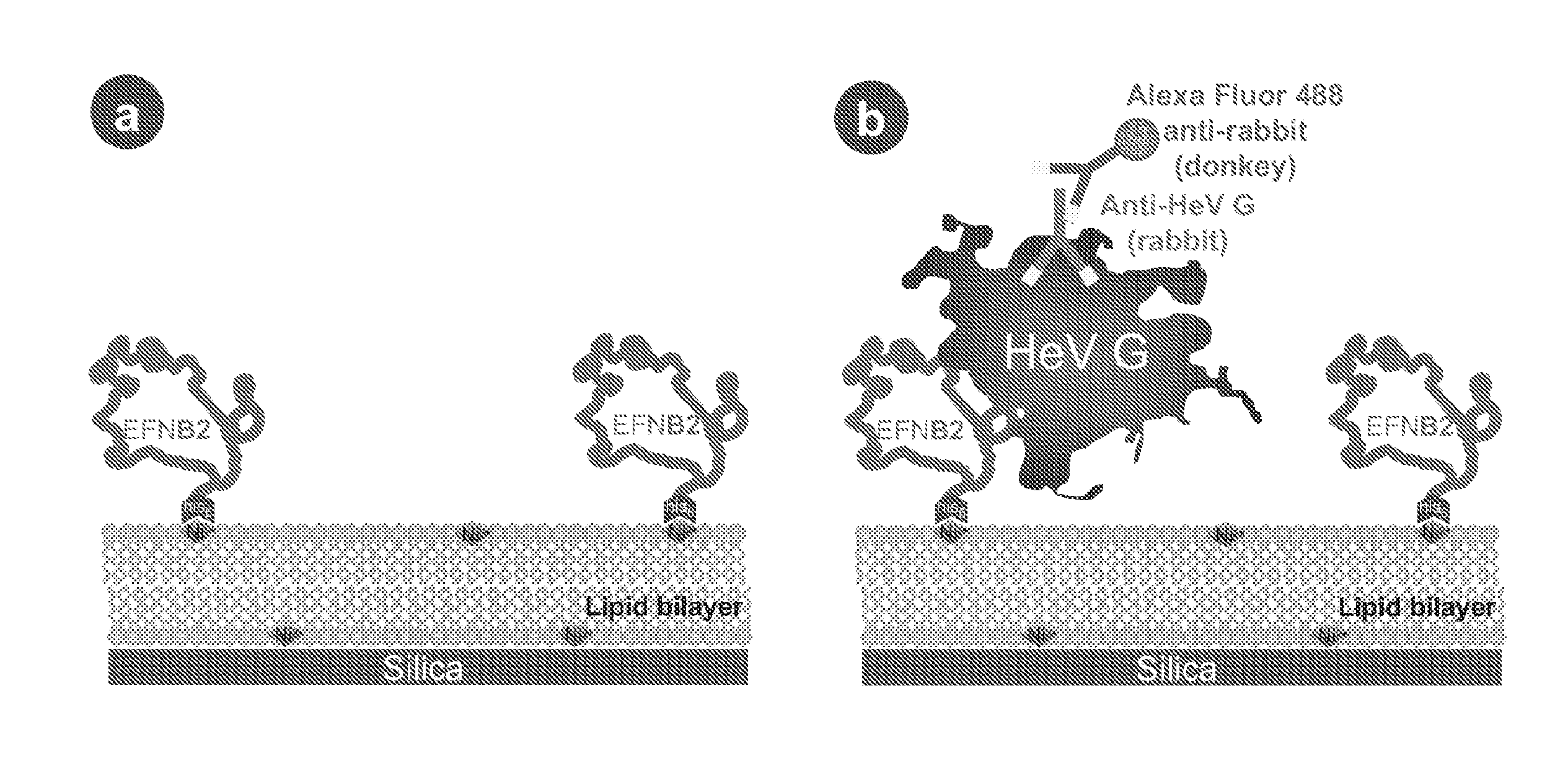
The invention provides a method of reducing viral infectivity in a sample compnsing contacting the sample with a earner matrix wherein lipids are attached to the carrier matrix, and wherein at least one virus-specific agent is attached to the lipids The virus-specific agent is capable of binding to at least one viral component The virus specific agent may be a receptor The earner matrix may be a silica particle, which may be coated with a lipid bilayer The invention further provides a method of inactivating a virus comprising contacting the virus with a earner matrix wherein lipids are attached to the earner matrix, and wherein at least one receptor is attached to the lipids, binding the receptor to at least one viral receptor-binding protein, and activating at least one viral fusion protein, wherein activation inactivates the virus, and releases the virus from the earner matrix
Owner:CORNELL UNIVERSITY +1
Dedicated Hemolysin for flow cytometer and preparing method and application thereof
InactiveCN1632576AProtect integrityProtection from infectionPreparing sample for investigationBiological testingBlood testWhite blood cell number
It is a process method of hemolysin in special cell device and its application, which belongs to medical test agent technique field. The hemolysin is composed of NaCI, Na#-[2] HPO#-[4]íñ12H#-[2] O, KH#-[2] PO#-[4], cavaform and double stilled water. Each component is mixed and is put in the water for four to five hours and is shaken every thirty to forty minutes and to be filtered to get the agent. The hemolysin in this invention is used as surface marks of leucocyte in blood tested by the flow cell device.
Owner:JINAN CENTER HOSPITAL
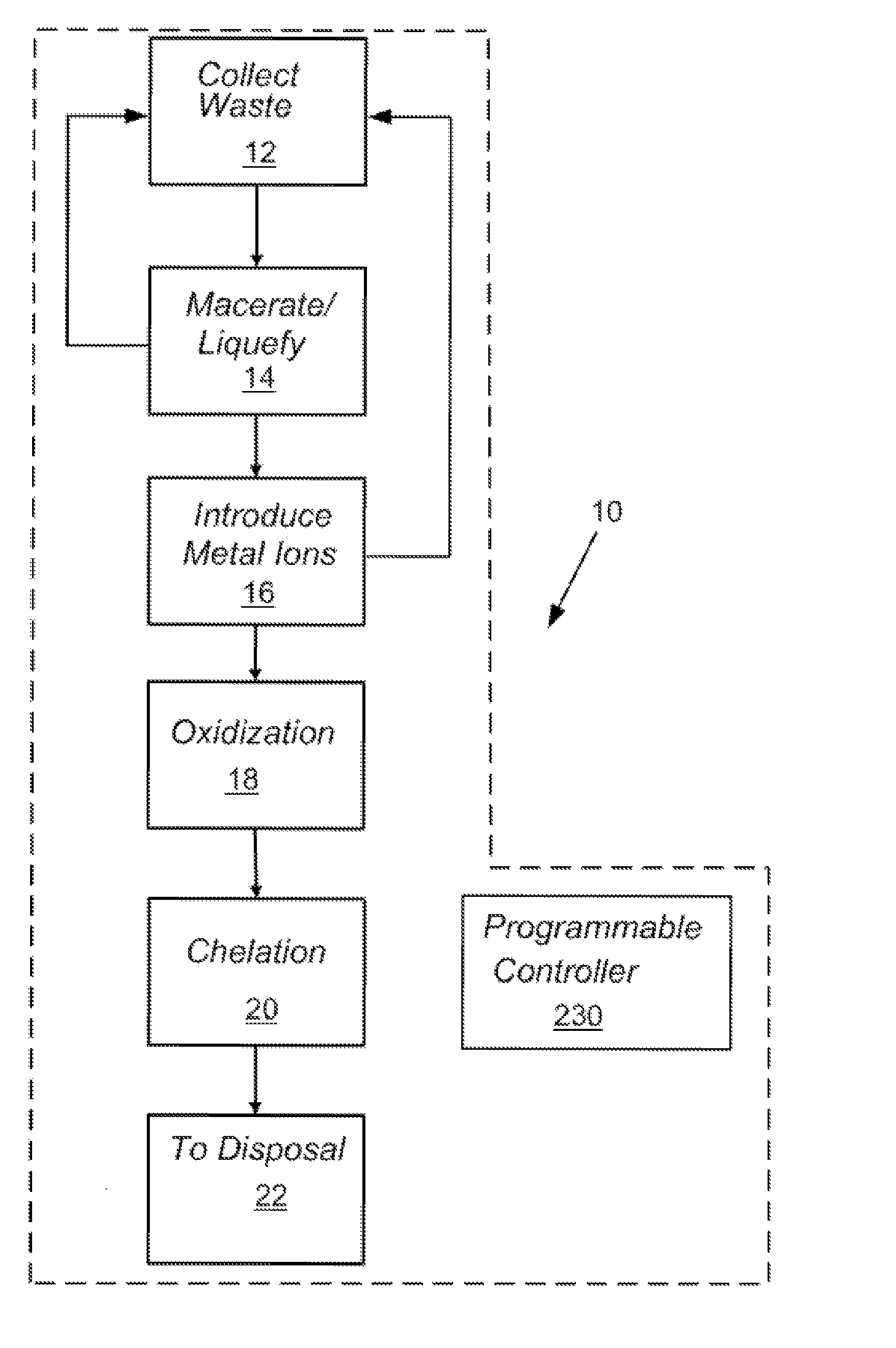
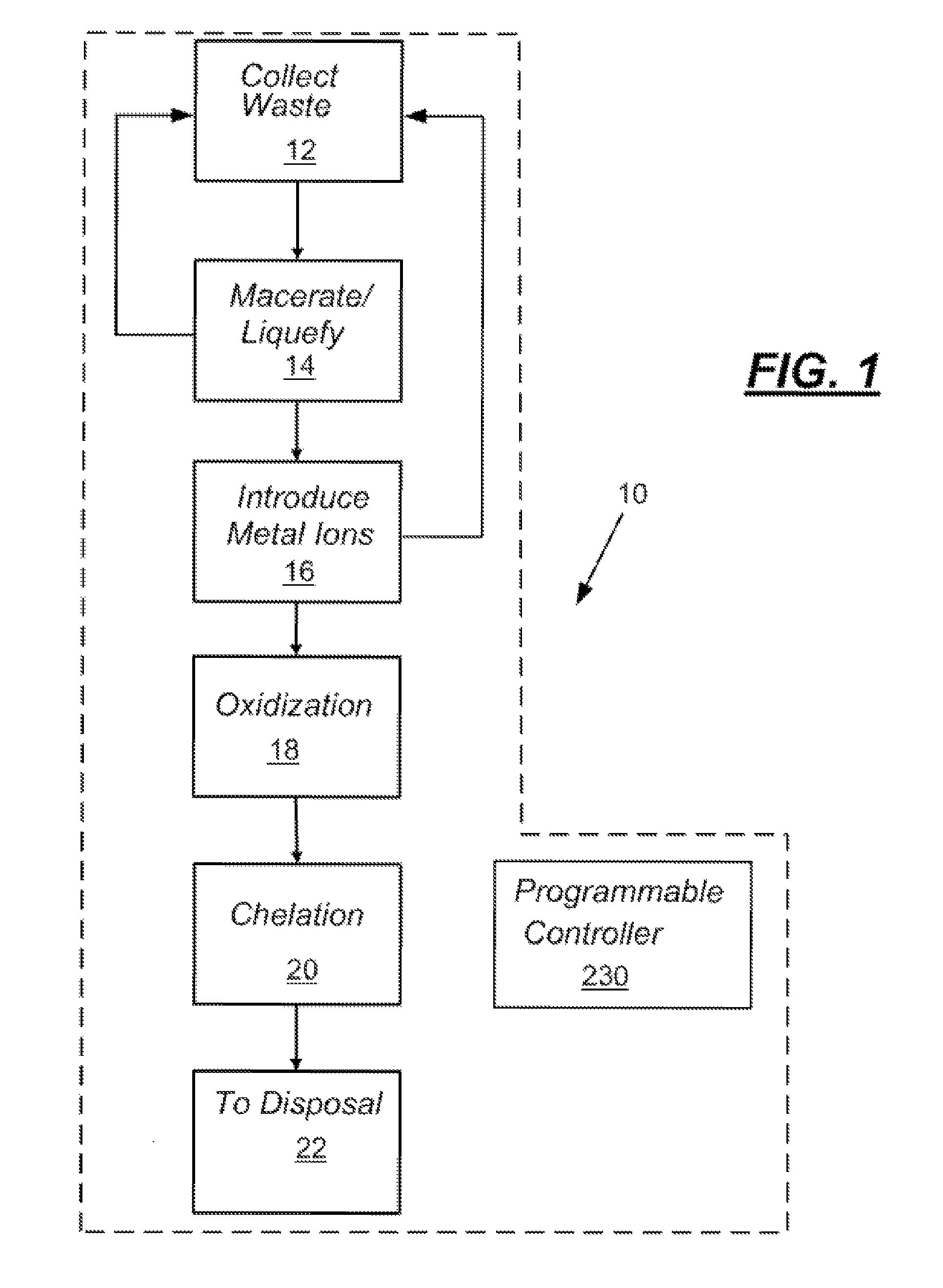
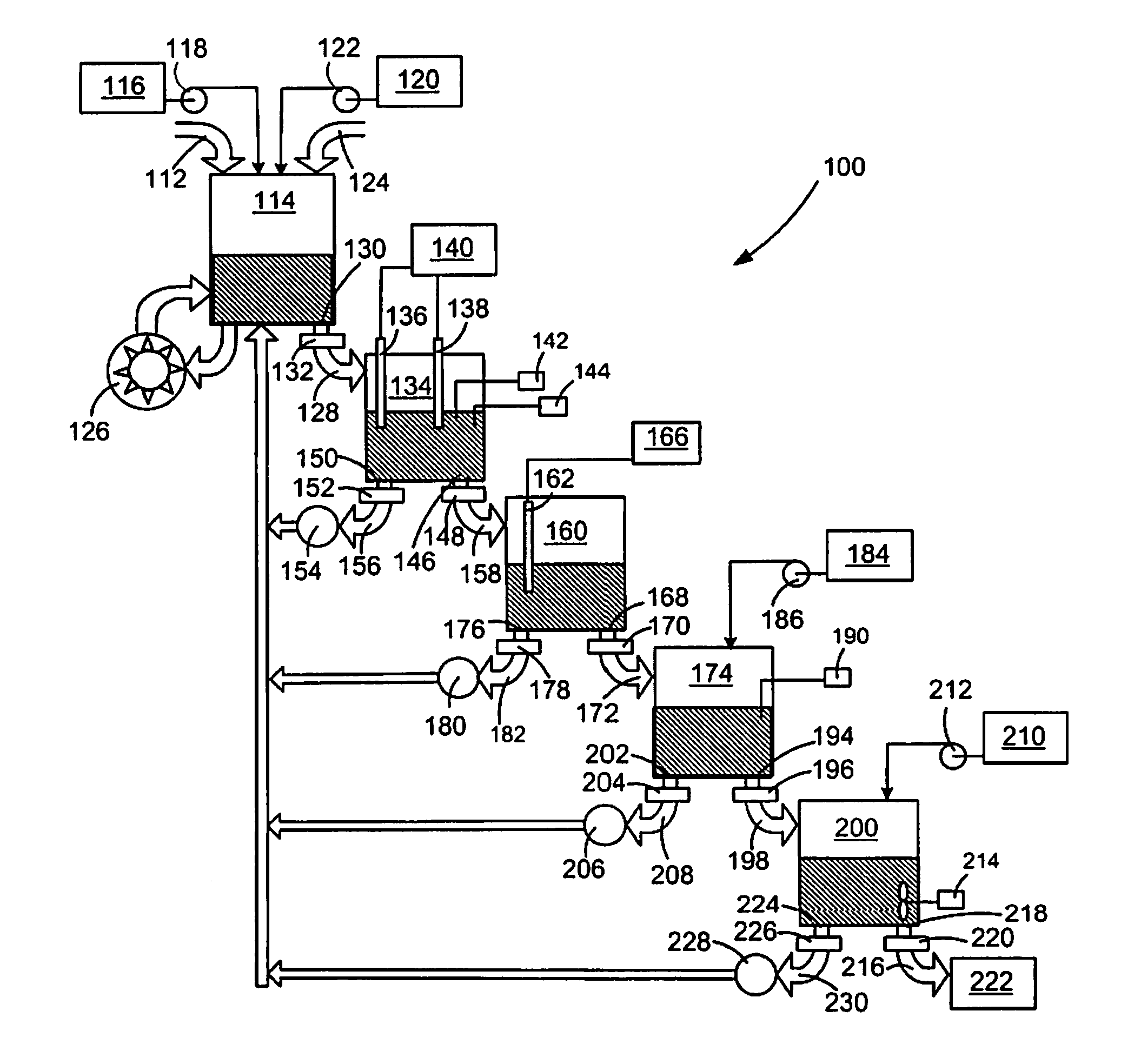
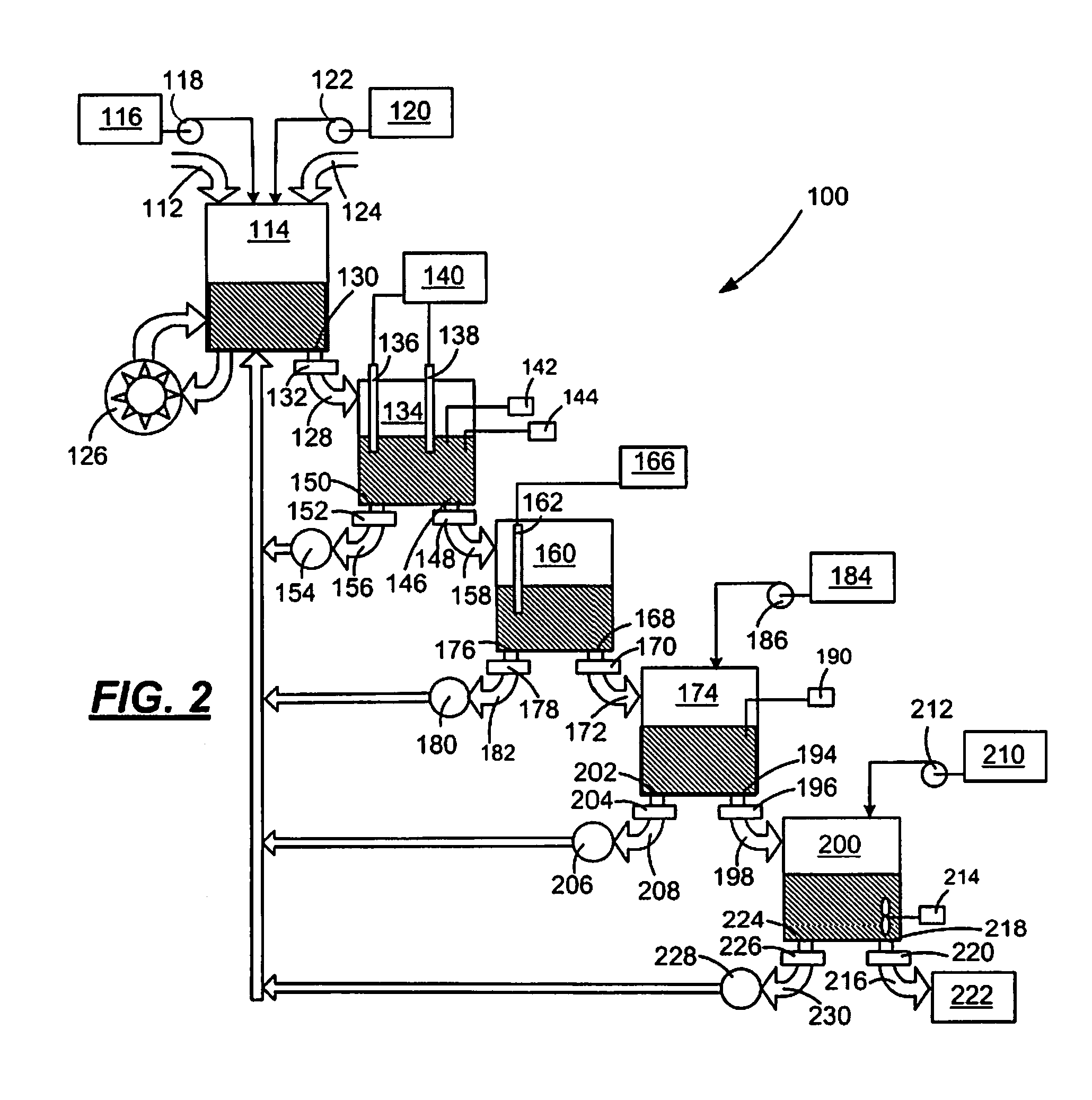
In-line waste disinfection method
InactiveUS7799234B2Easy to deployReduce infectivityWater treatment parameter controlWater/sewage treatment by magnetic/electric fieldsWaste streamDisinfection methods
A modular waste disinfection system for the disinfection of substantially liquid infectious waste streams and methods of treating such waste streams are disclosed. The modular waste disinfection system includes a metal ion generation chamber for introducing metal ions into the waste material; an oxidant generation chamber in fluid flow communication with the metal ion generation chamber for disinfection of the waste material with an oxidizing agent; and a chelation chamber in fluid flow communication with the oxidant generation chamber for deactivation of metal ions in the waste material, wherein the waste is discharged to a sanitary sewer after disinfection.
Owner:INNOVATION SERVICES INC
Novel immunoadhesins for treating and prventing toxicity and pathogen-mediated diseases
InactiveUS20060015969A1Effect be exertImprove efficiencyAntibacterial agentsVirusesCommon coldToxicant
Immunoadhesins active against toxins and pathogens are described, with specific examples directed to immunoadhesins for thwarting pathogens such as anthrax and the common cold. The immunoadhesin-receptor ligand principle can be employed to counter virtually any pathogen, toxicant or toxin, including, e.g., natural and synthetic metabolic poisons.
Owner:PLANET BIOTECH
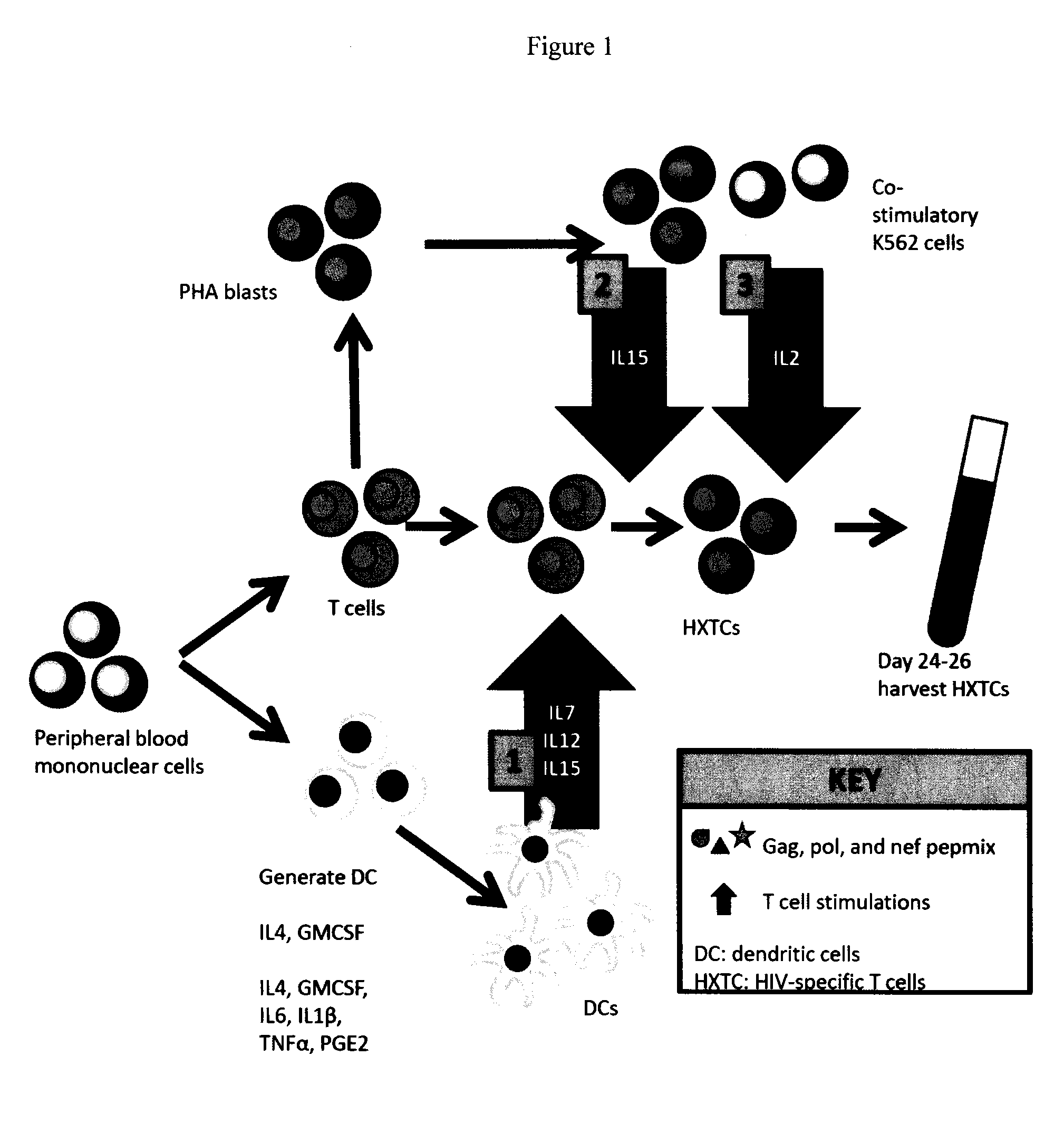
Generation of broadly-specific, virus-immune cells targeting multiple HIV antigens for preventive and therapeutic use
ActiveUS20150359876A1Improve antiviralImprove antitumorArtificial cell constructsBlood/immune system cellsImmunotherapyT cell
Compositions for T cell-based immunotherapy of HIV, HIV-associated malignancies, HIV-associated viral infections, or other HIV-related complications. Modified T cells that are resistant to invasion or infection with HIV, such as T-cells modified to decrease or eliminate expression of mannosyl-oligosacharide glucosidase enzyme (“MOGS”). Methods for producing such compositions by expanding HIV-specific T cells from different sources to recognize multiple HIV antigens.
Owner:CHILDRENS NAT MEDICAL CENT
Waste treatment and disinfection unit
InactiveUS20110290740A1Easy to deployReduce infectivityWaste water treatment from animal husbandryWaste water treatment from animal processingWaste streamCatalytic oxidation
A waste treatment system and method for treating a substantially liquid waste stream. The waste treatment system includes a conditioning stage for conditioning the waste stream for treatment. A metal ion generation stage is provided for generating metal ions for disinfection of the waste stream and for catalytic oxidation. A wet oxidation stage is provided in fluid flow communication with the metal ion generation stage for denaturing the waste stream using an oxygen-containing gas. A chelation stage in fluid flow communication with the oxidation stage is provided for deactivating metal ions in the waste stream.
Owner:INNOVATION SERVICES INC
SARS vaccine compositions and methods of making and using them
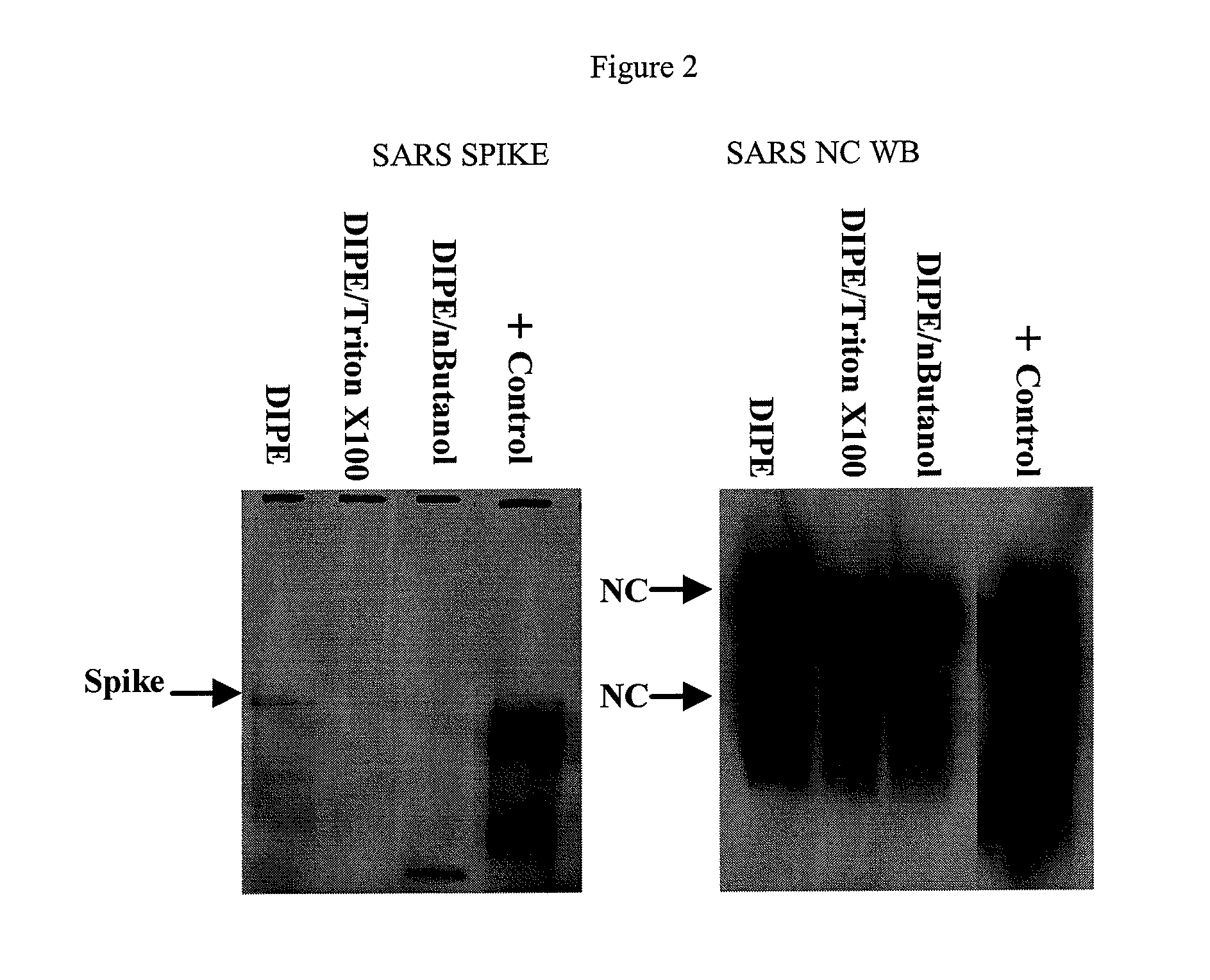
InactiveUS8506968B2The process is simple and effectiveThe method is simple and efficientSsRNA viruses positive-senseViral antigen ingredientsLipid formationViral Vaccine
Described is a composition and method for reducing the occurrence and severity of infectious diseases, especially infectious diseases such as SARS, in which lipid-containing infectious viral organisms are found in biological fluids, such as blood. The present invention employs solvents useful for extracting lipids from the lipid-containing infectious viral organism thereby creating immunogenic modified, partially delipidated viral particles with reduced infectivity. The present invention provides delipidated viral vaccine compositions, such as therapeutic vaccine compositions, comprising these modified, partially delipidated viral particles with reduced infectivity, optionally combined with a pharmaceutically acceptable carrier or an immunostimulant. The vaccine composition is administered to a patient to provide protection against the lipid-containing infectious viral organism or, in case of a therapeutic vaccine, to treat or alleviate infection against the lipid-containing infections viral organism. The vaccine compositions of the present invention include combination vaccines of modified viral particles obtained from one or more strains of a virus and / or one or more types of virus.
Owner:ELI LILLY & CO +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com