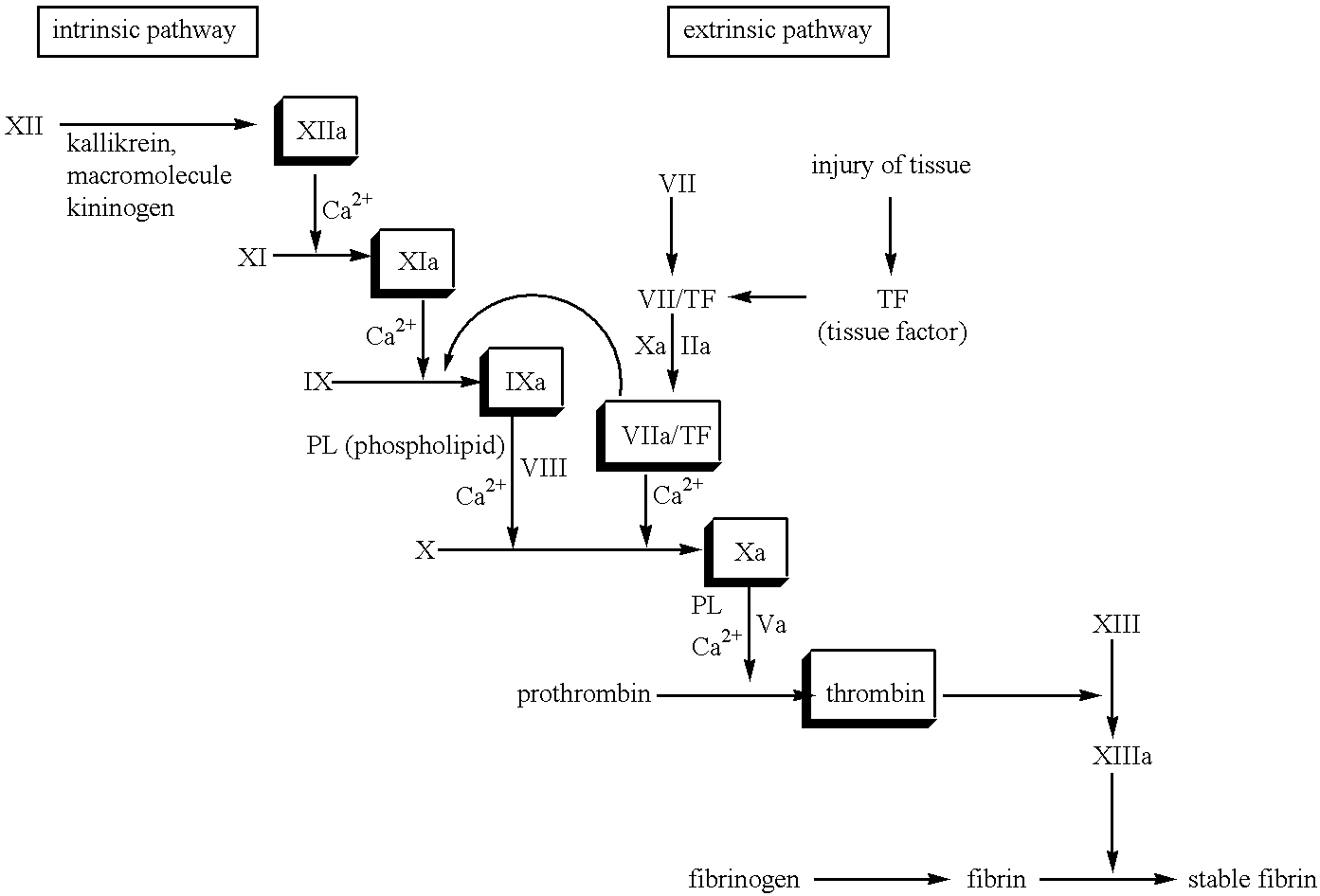
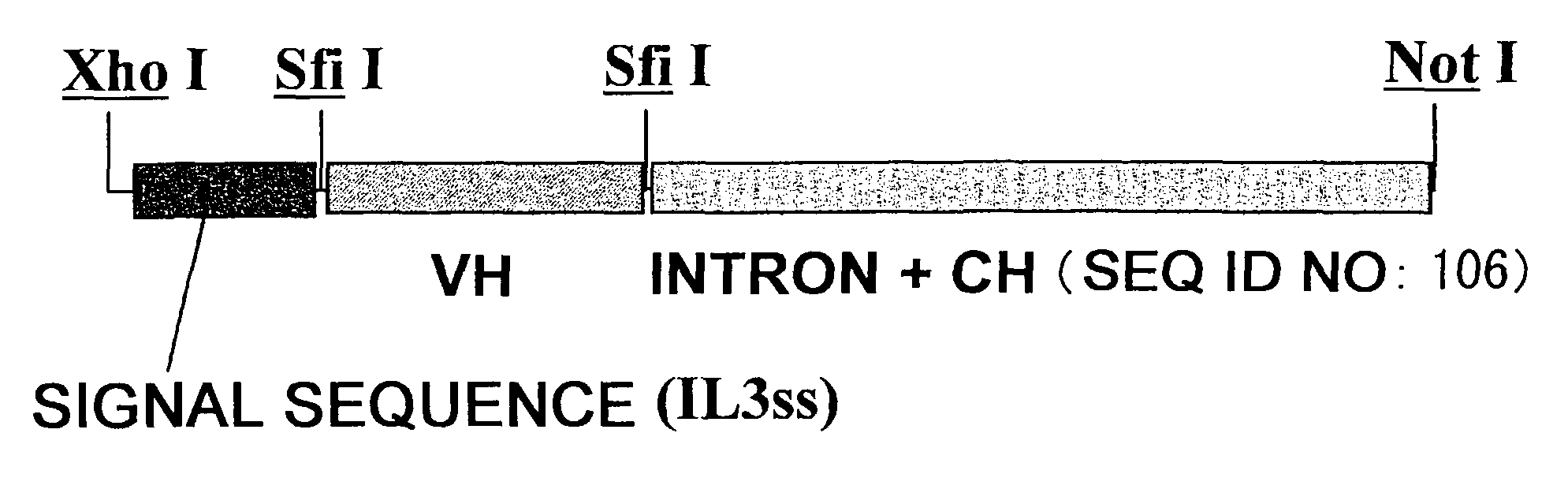
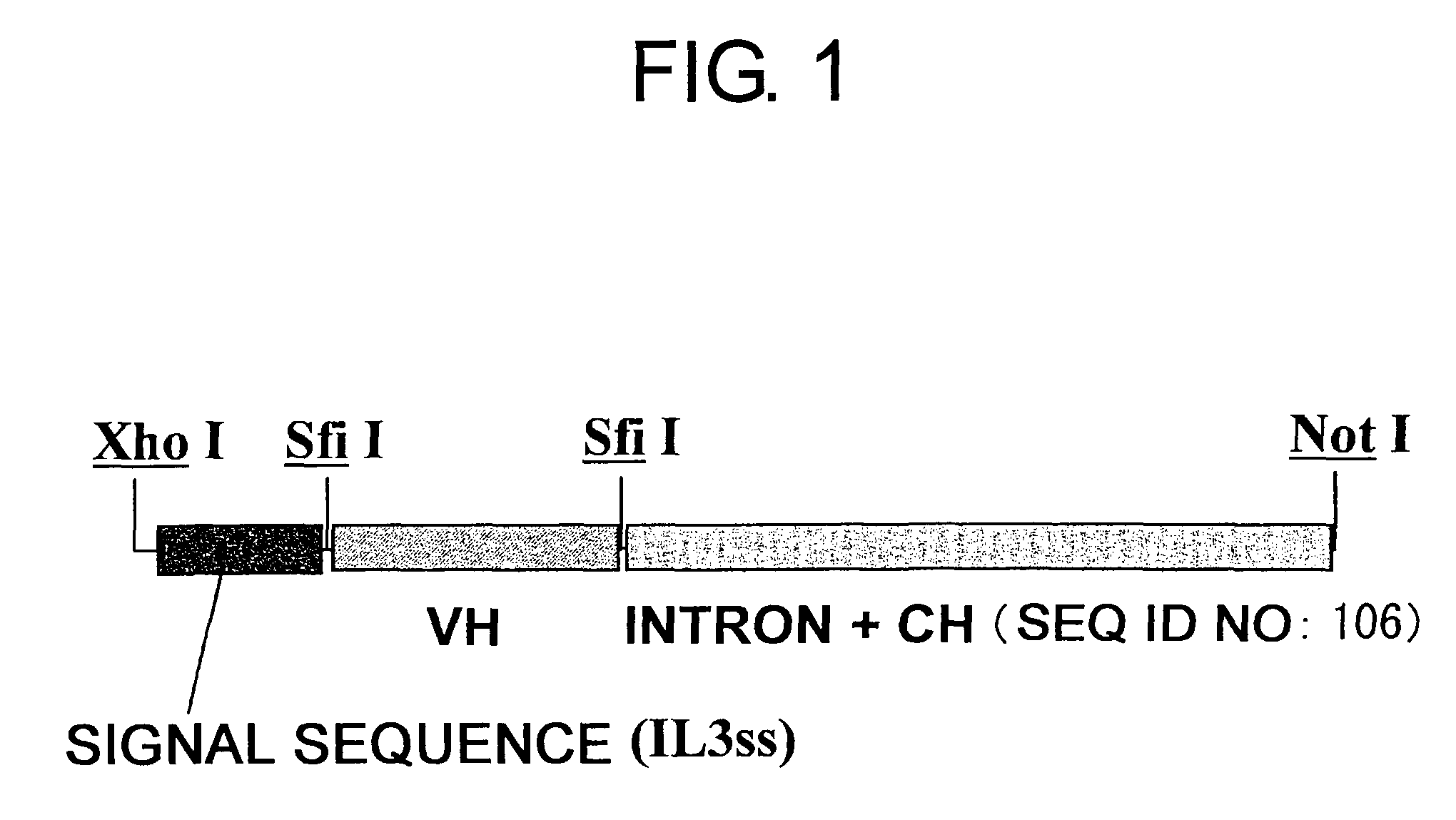
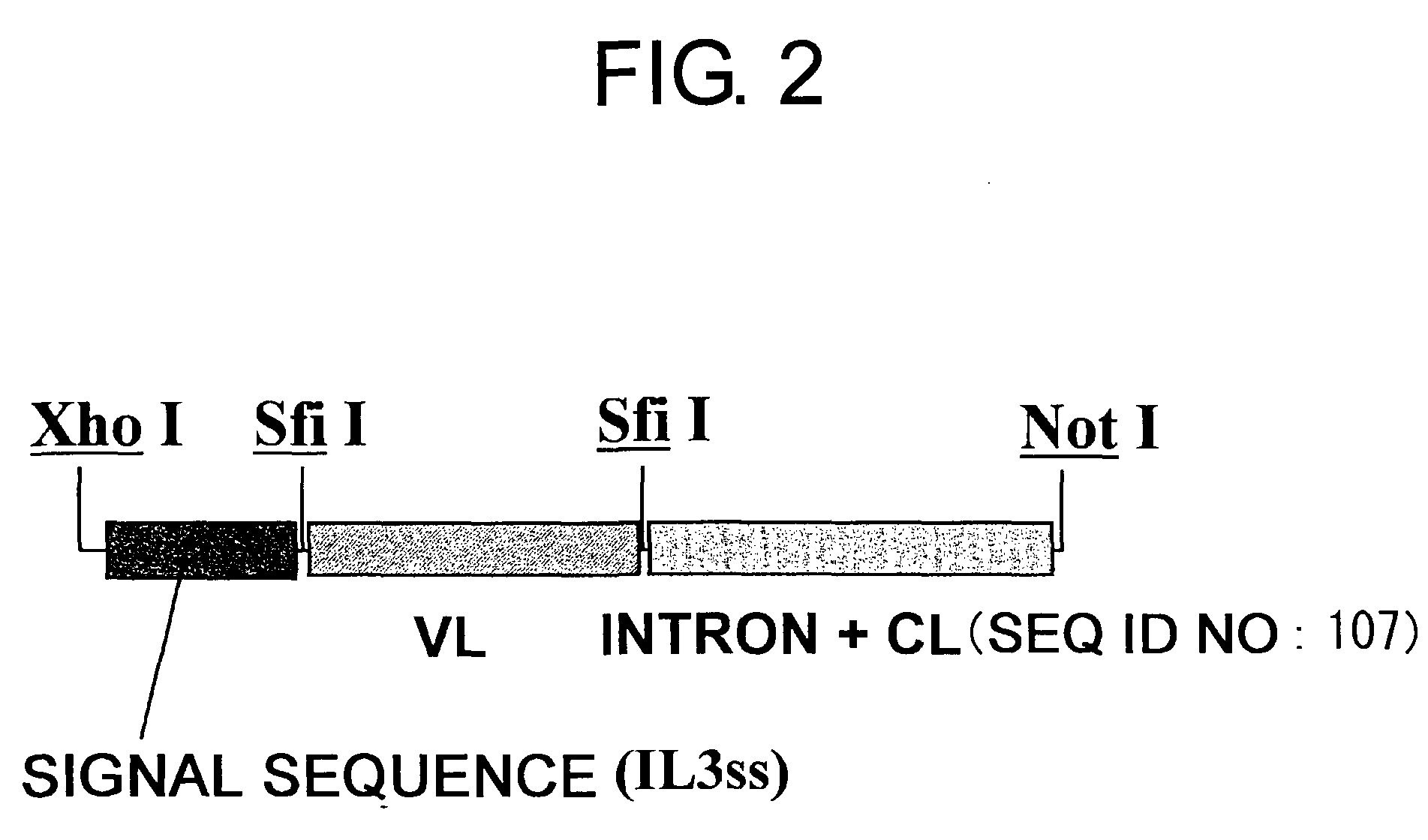
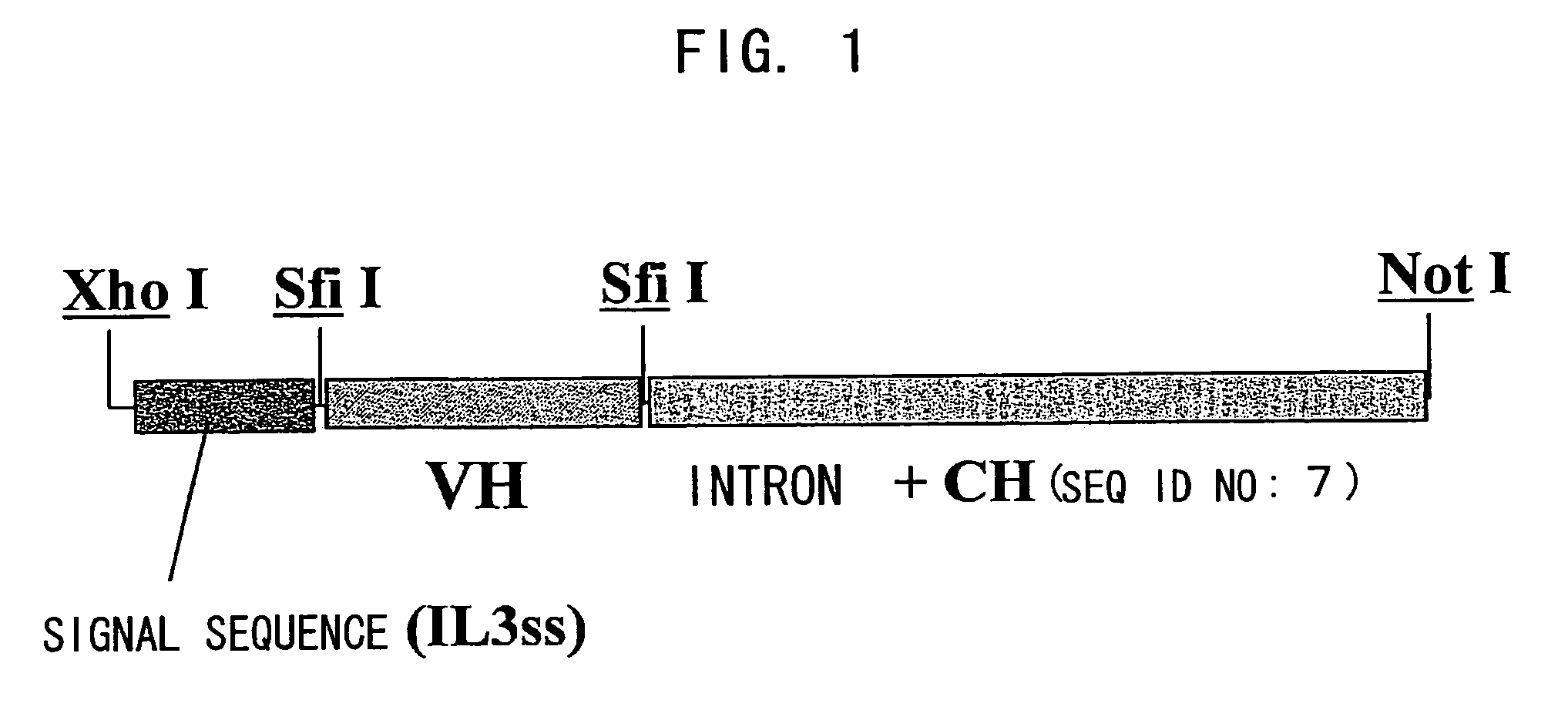
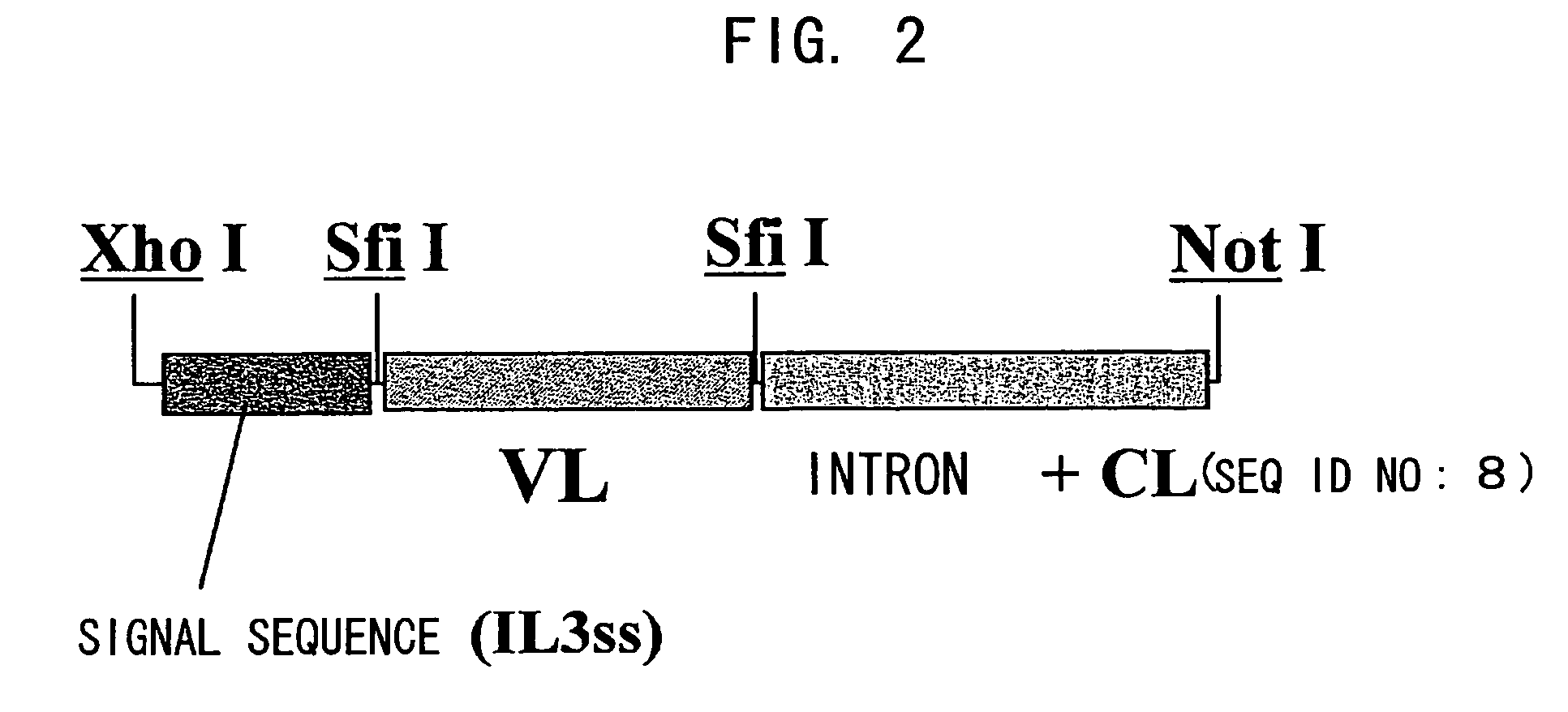
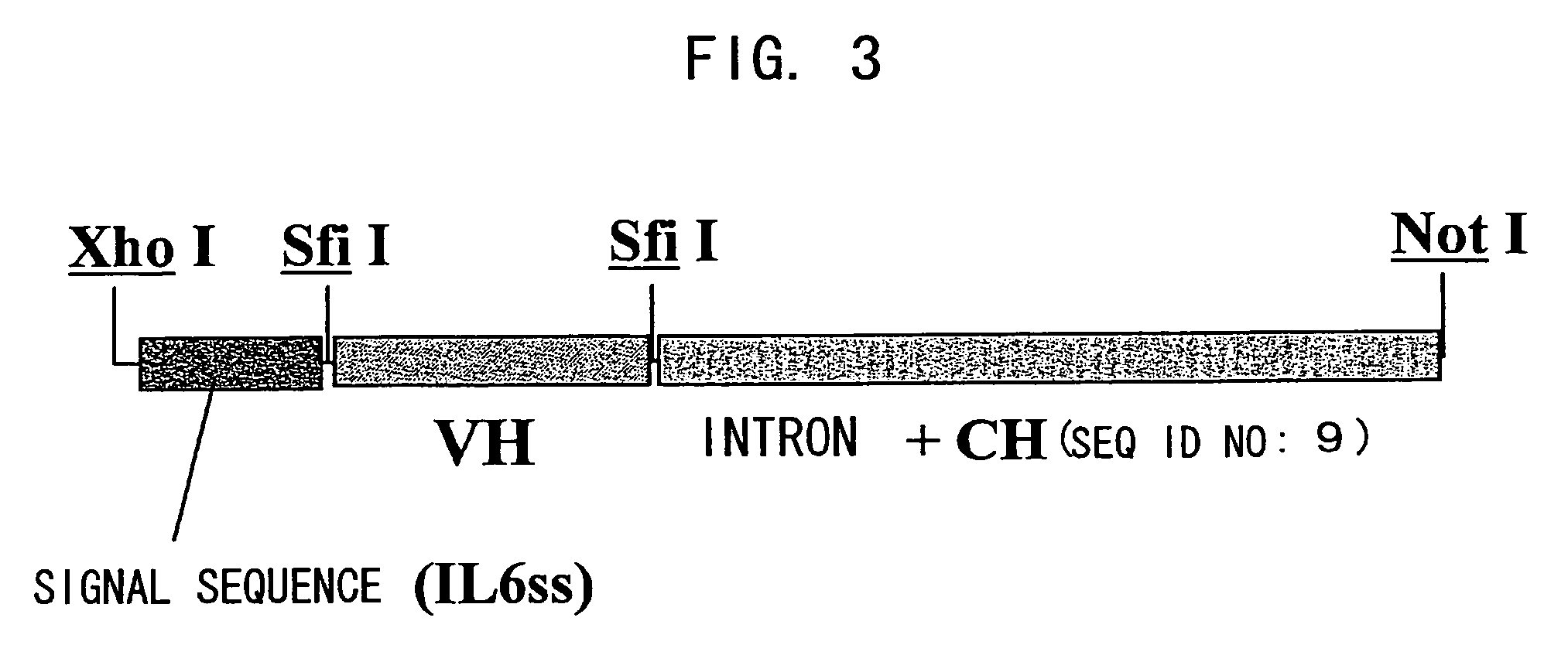
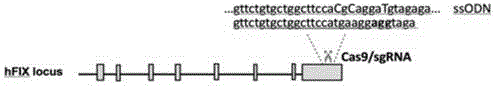
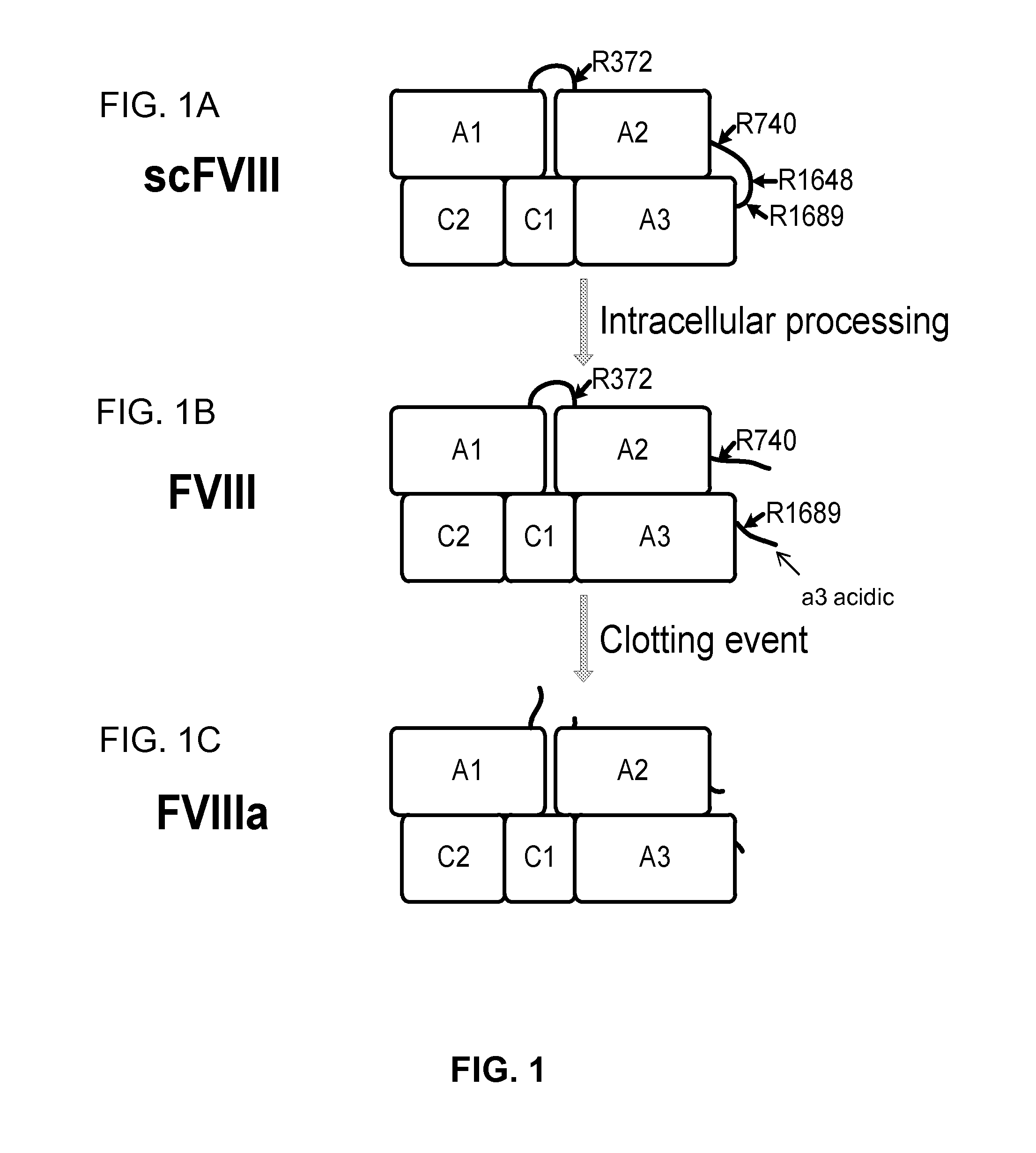
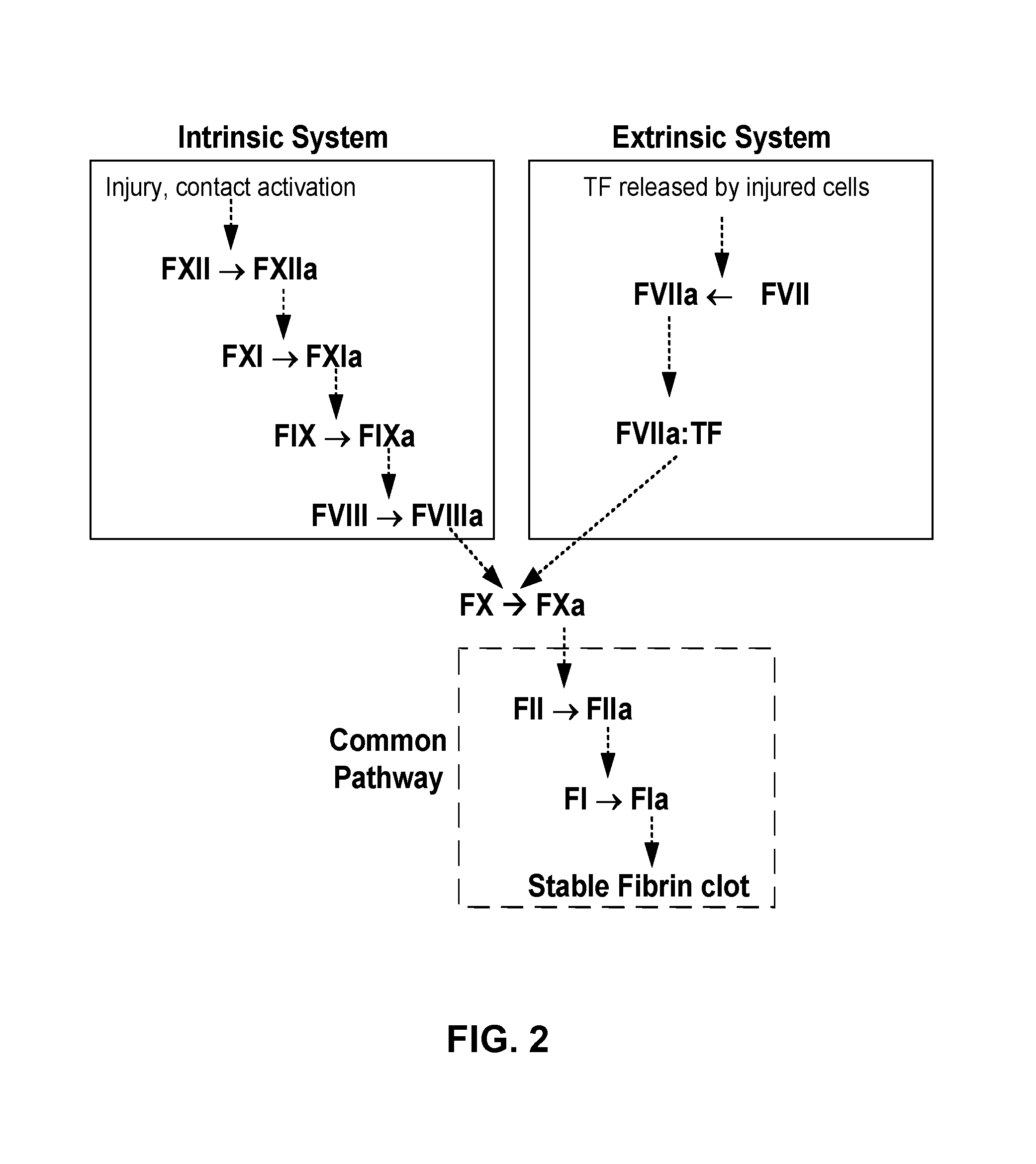
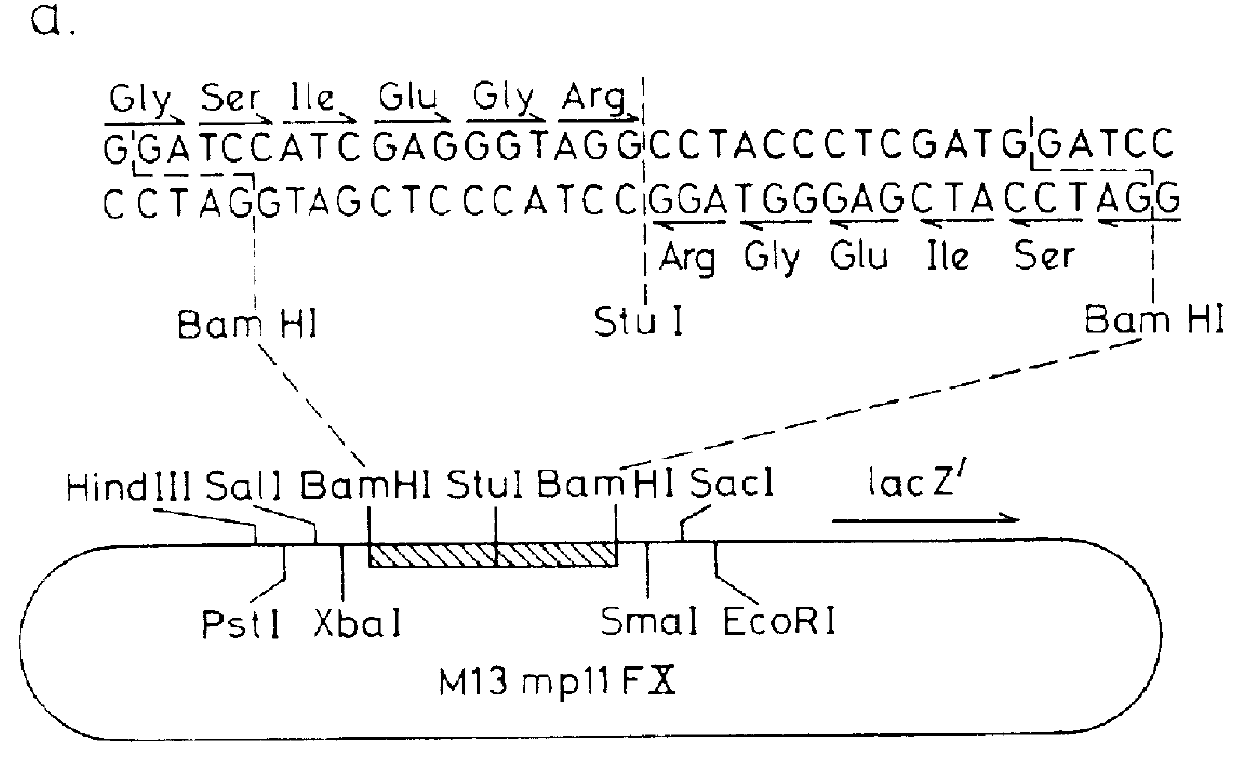
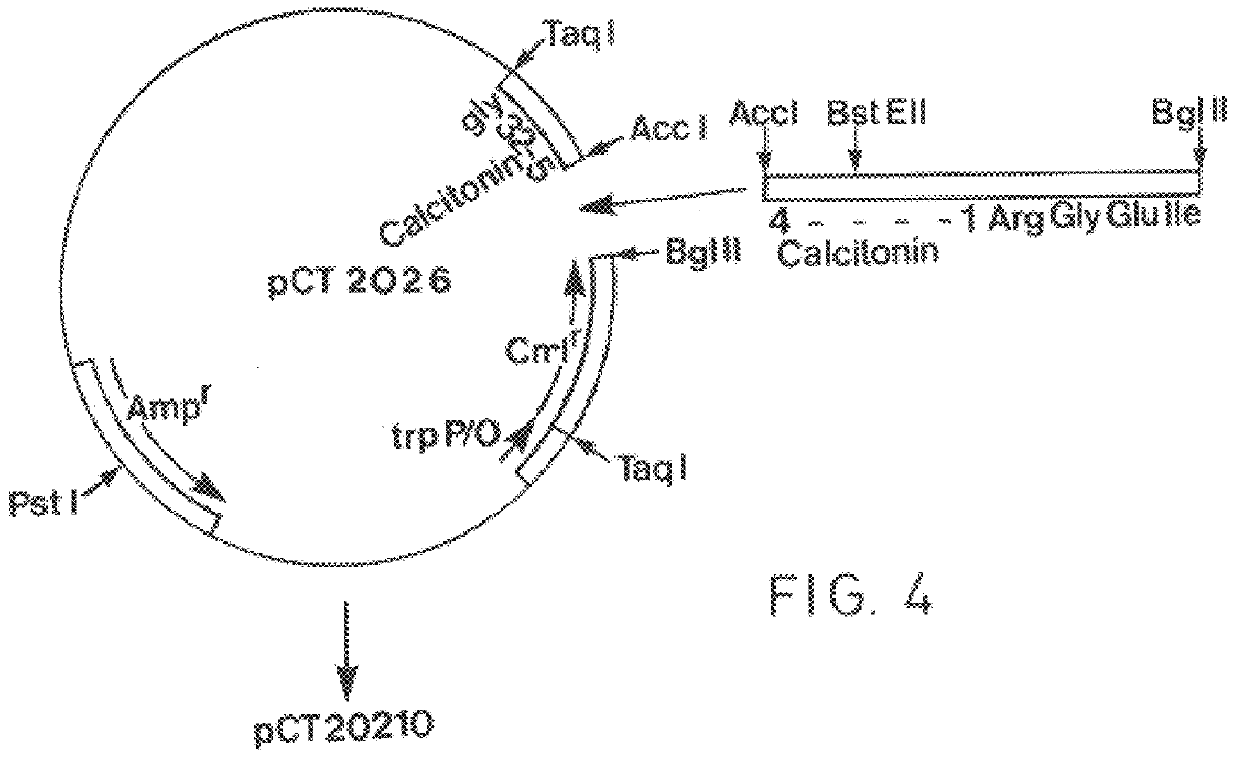
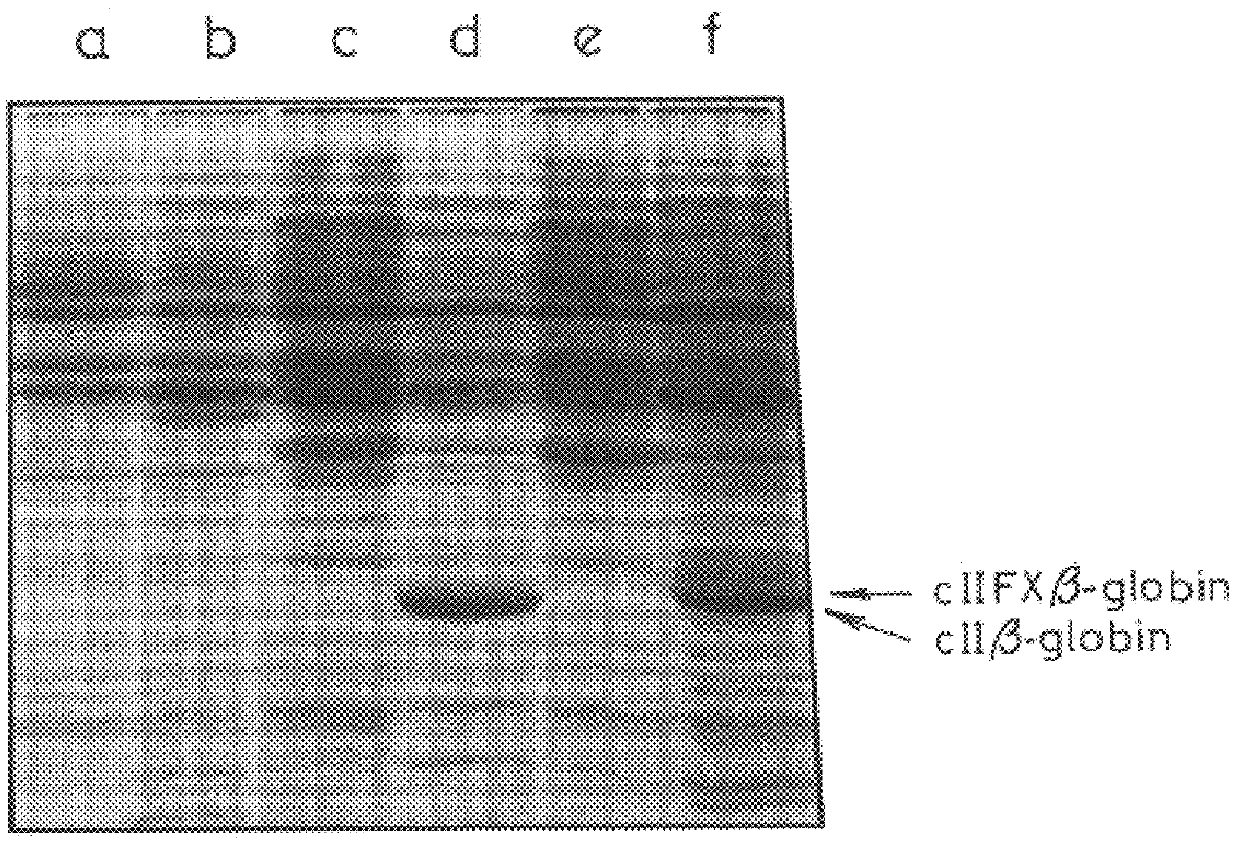
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
388 results about "Blood coagulation factors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bispecific antibody substituting for functional proteins
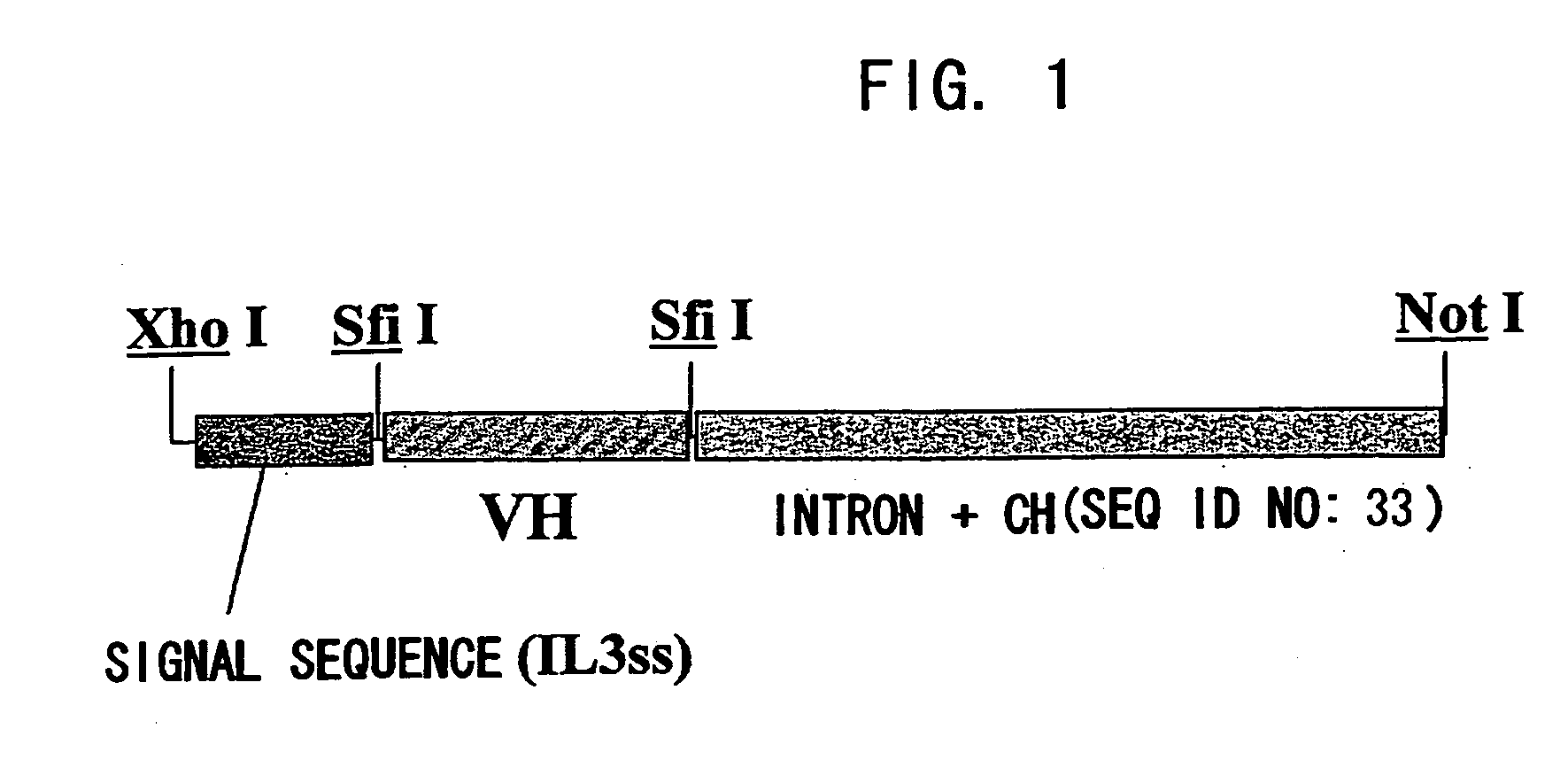
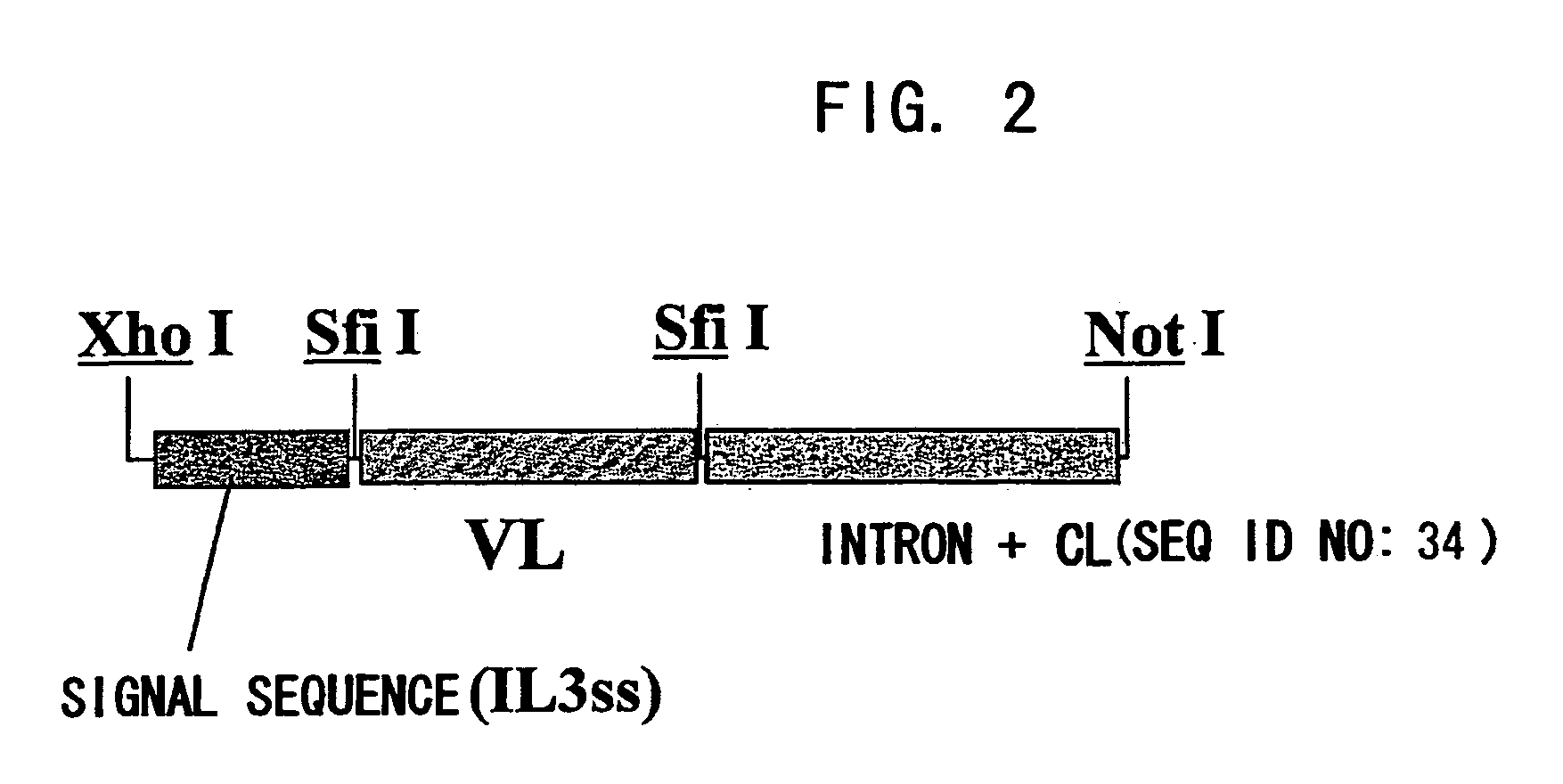
ActiveUS20070041978A1Enhances enzymatic reactionImmunoglobulins against blood coagulation factorsFactor VIIBlood coagulation factor VIIIBlood Coagulation Factor X
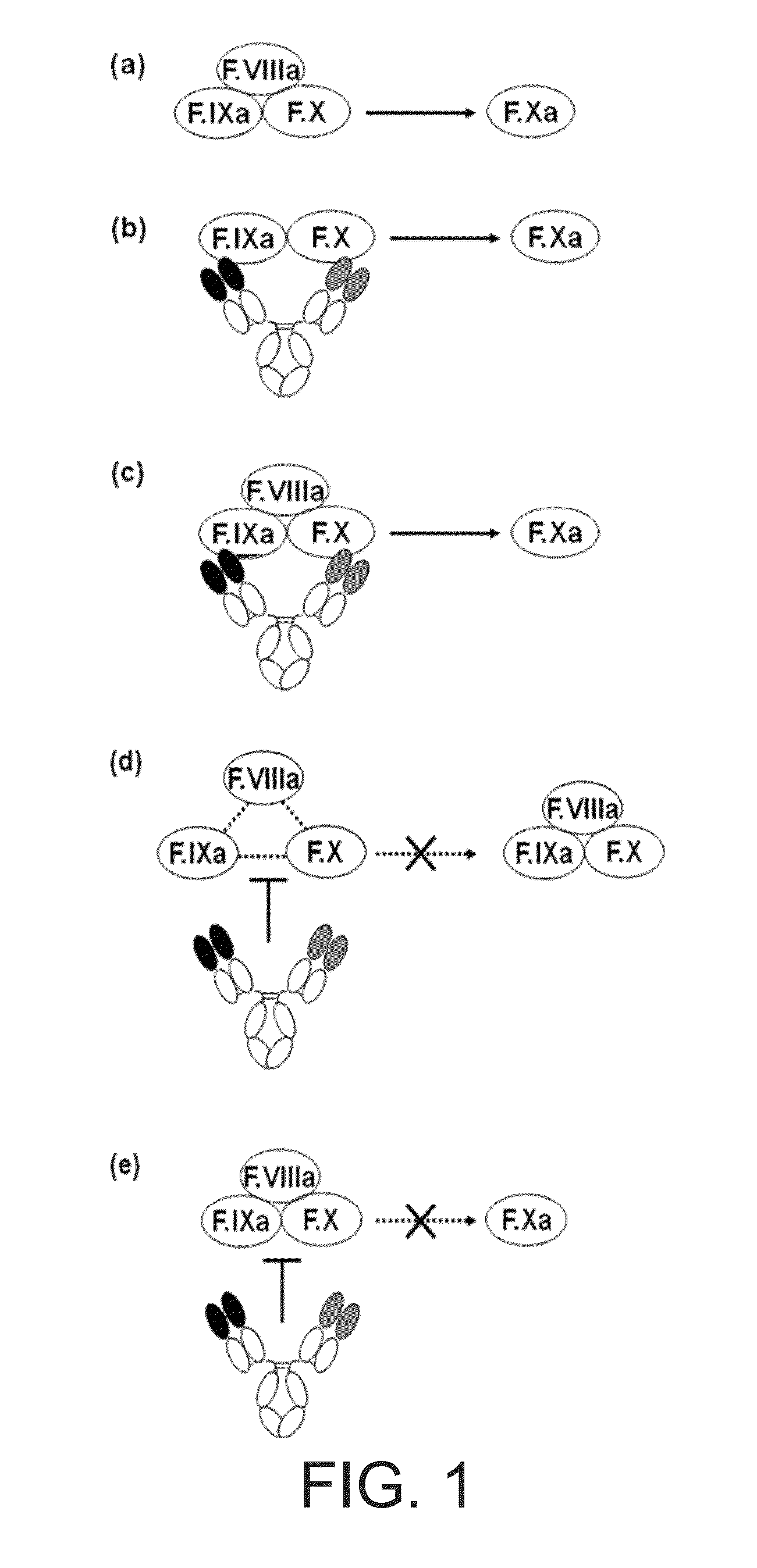
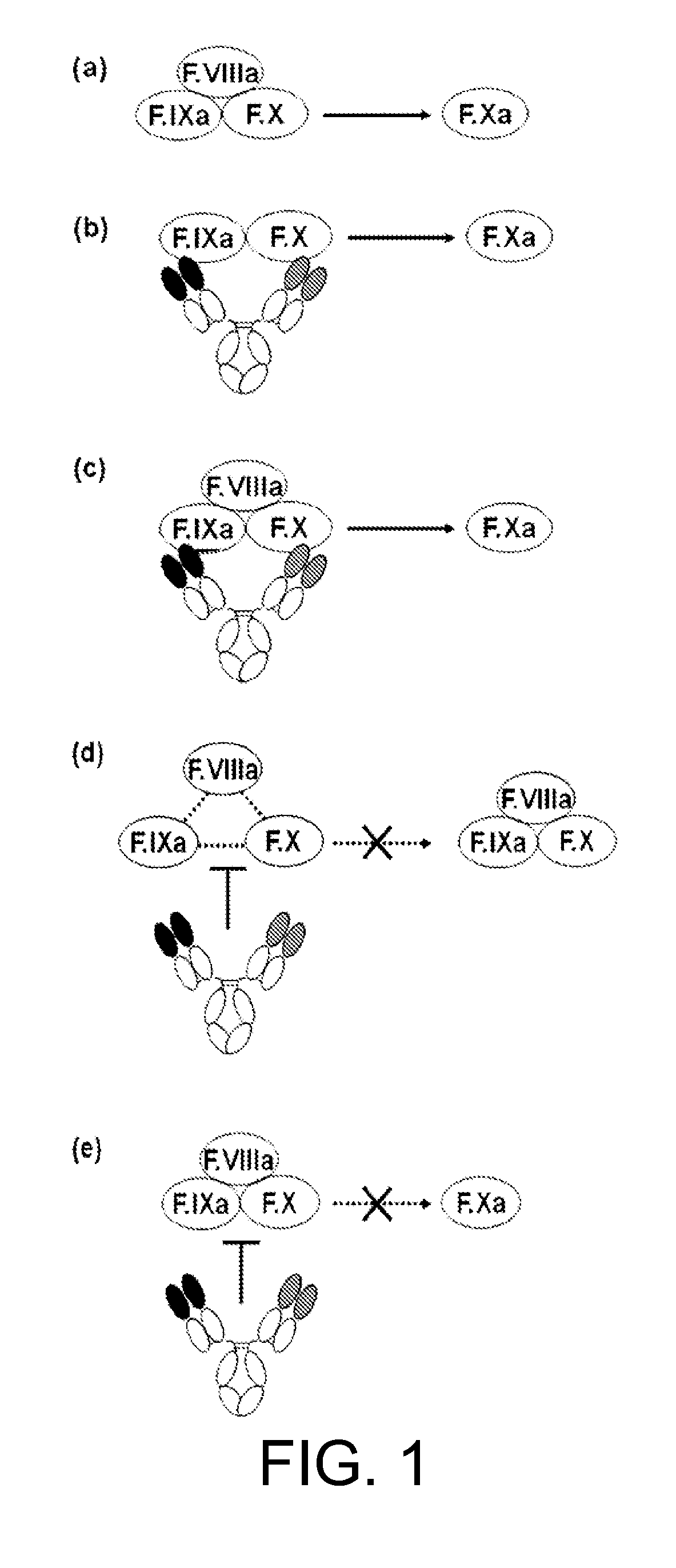
The present inventors succeeded in constructing bispecific antibodies, which bind to both the blood coagulation factor IX / activated blood coagulation factor IX and blood coagulation factor X, and functionally substitute for blood coagulation factor VIII / activated blood coagulation factor VIII which enhances the enzymatic reaction.
Owner:CHUGAI PHARMA CO LTD
Double Specific Antibodies Substituting For Functional Proteins
InactiveUS20080075712A1Reduced activityOutstanding sustainabilityImmunoglobulins against blood coagulation factorsNervous disorderBlood coagulation factor VIIIBlood Coagulation Factor X
The present inventors succeeded in separating bispecific antibodies that functionally substitute for ligands of type I interferon receptors comprising two types of molecules: AR1 chain and AR2 chain. Furthermore, the present inventors succeeded in producing bispecific antibodies that substitute for the enzyme reaction-accelerating function of blood coagulation factor VIII / activated blood coagulation factor VIII, which bind to both blood coagulation factor IX / activated blood coagulation factor IX and blood coagulation factor X.
Owner:CHUGAI PHARMA CO LTD
Amidino derivatives and drugs containing the same as the active ingredient
InactiveUS6358960B1BiocideGroup 5/15 element organic compoundsExtracorporeal circulationDisseminated coagulopathy
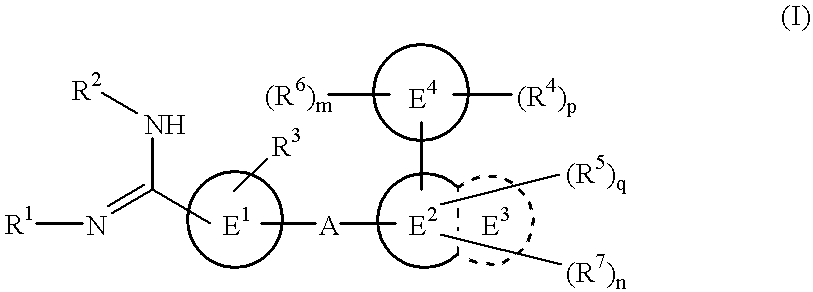
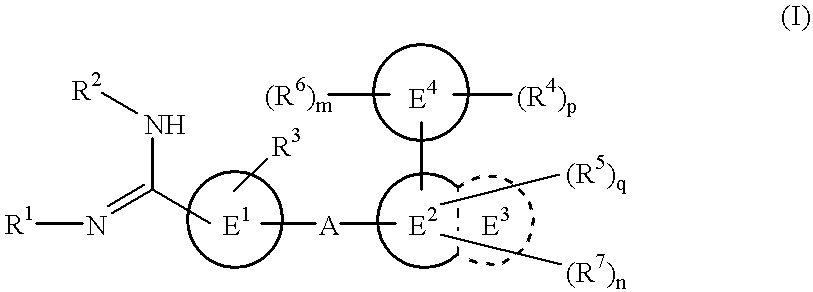
The novel amidino derivatives of the formula (I):wherein all the symbols are as in specification defined;have an inhibitory activity of a blood coagulation factor VIIa and are useful for treatment and / or prevention of several angiopathy caused by enhancing a coagulation activity, such as disseminated intravascular coagulation, coronary thrombosis, cerebral infarction, cerebral embolism, transient ischemic attack, cerebrovascular disorders, pulmonary vascular diseases, deep venous thrombosis, peripheral arterial obstruction, thrombosis after artificial vascular transplantation and artificial valve transplantation, post-operative thrombosis, reobstruction and restenosis after coronary artery bypass operation, reobstruction and restenosis after PTCA or PTCR, thrombosis by extracorporeal circulation and procoagulative diseases such as glomerlonephriitis.
Owner:ONO PHARMA CO LTD
Antibody Substituting for Function of Blood Coagulation Factor VIII
InactiveUS20100003254A1Enhances enzymatic reactionShorten clotting timeImmunoglobulins against blood coagulation factorsAntibody ingredientsBlood coagulation factor VIIIAntiendomysial antibodies
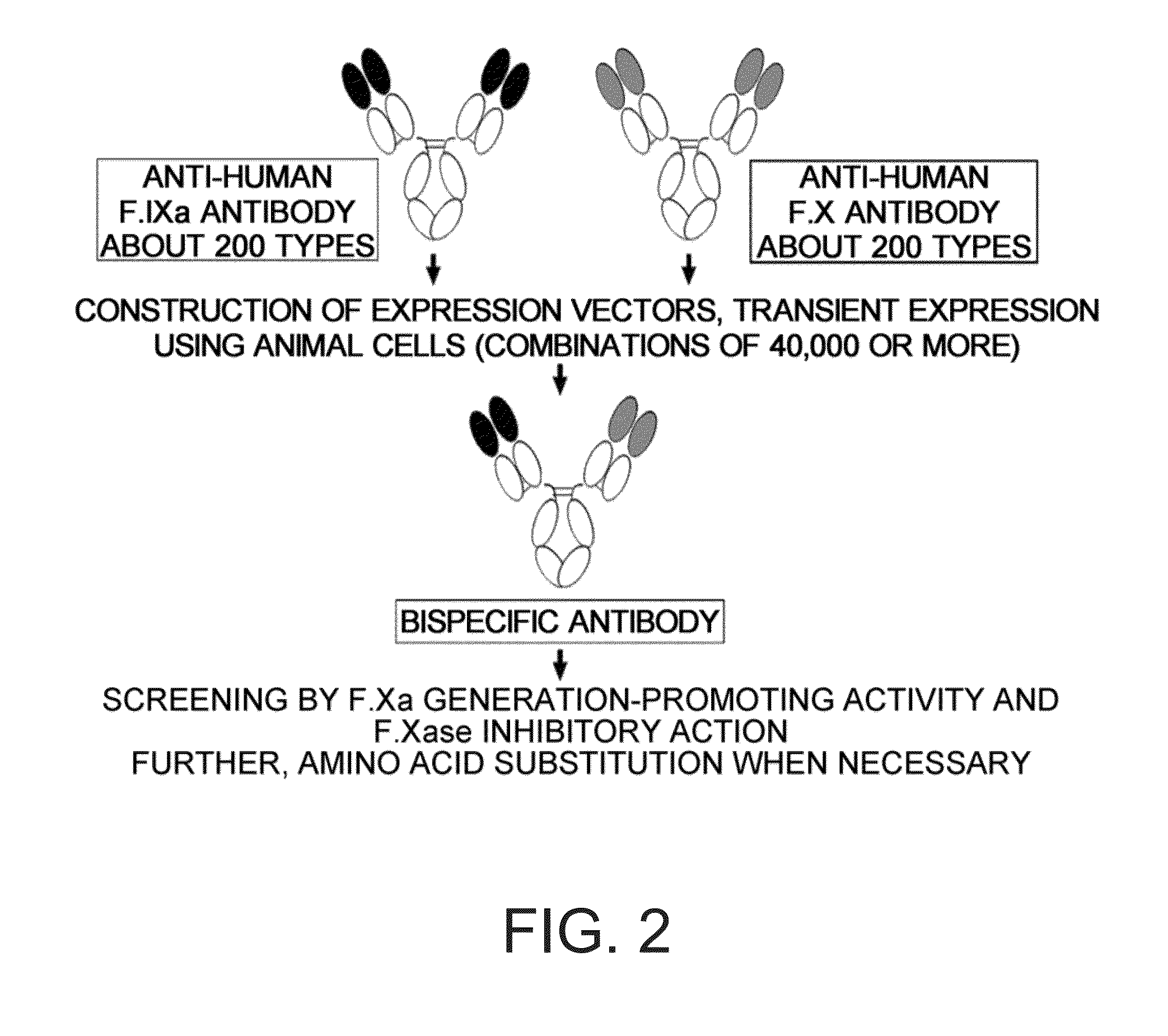
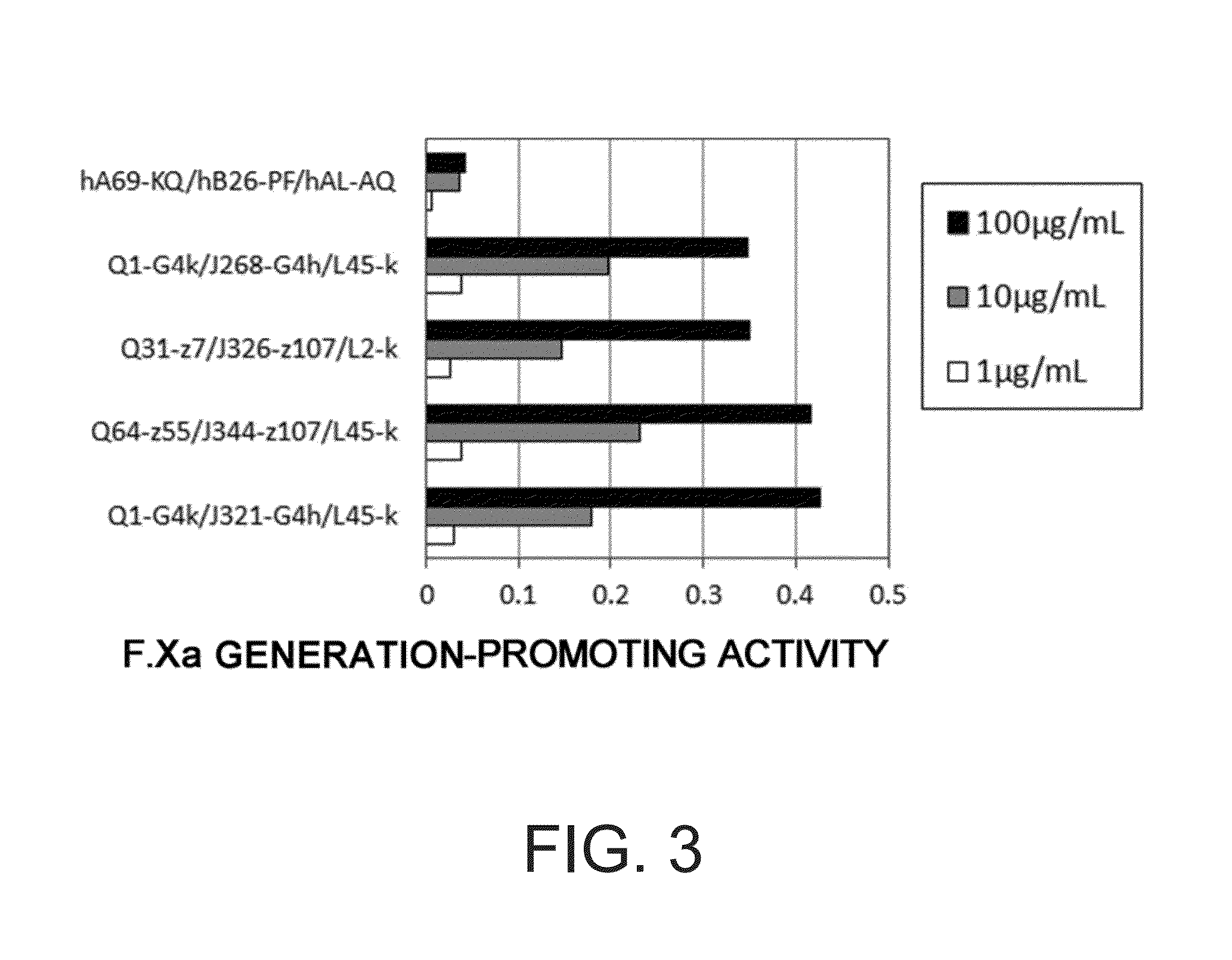
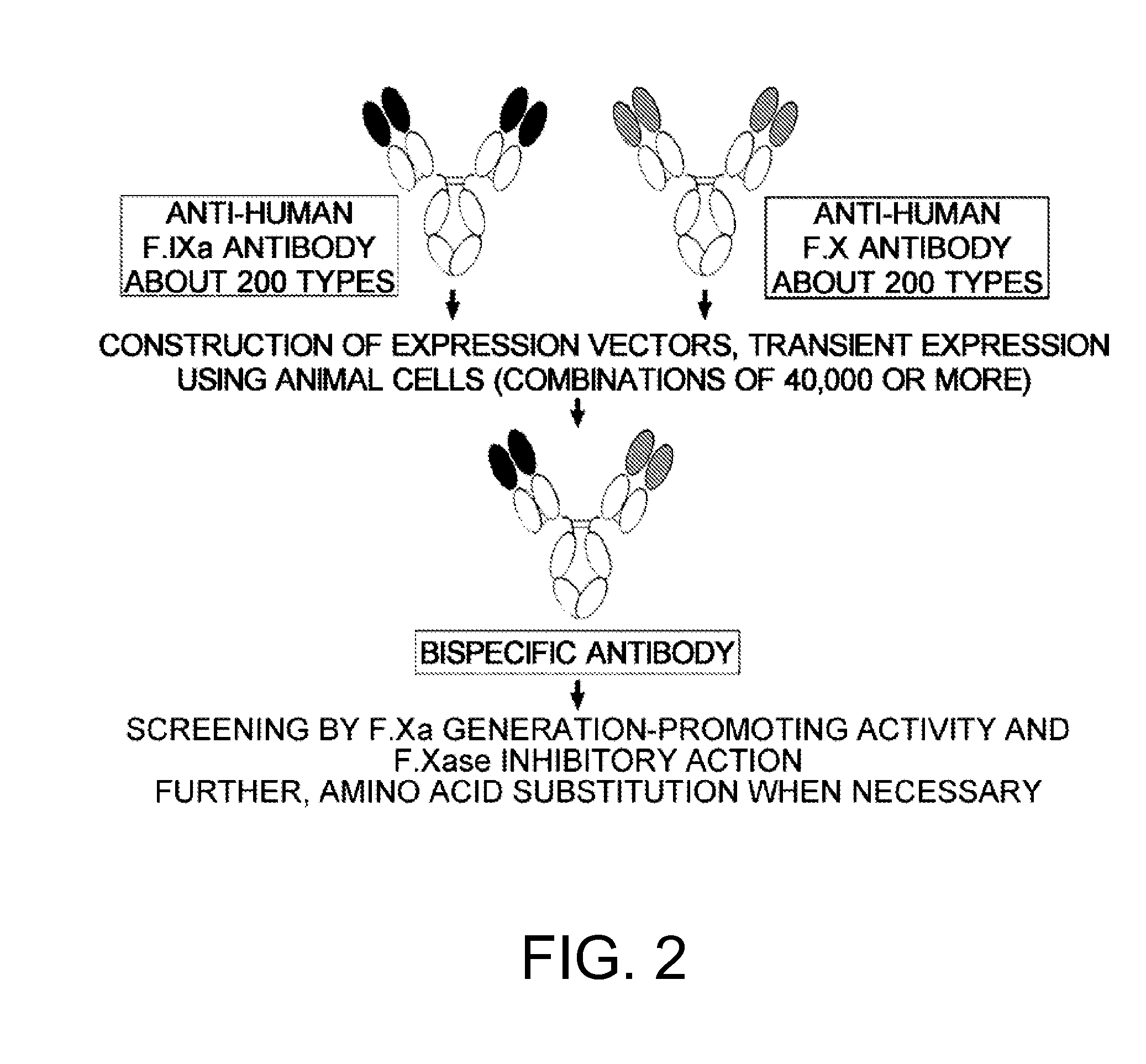
The present inventors produced a variety of bispecific antibodies that specifically bind to both F. IX / F. IXa and F. X, and functionally substitute for F. VIIIa, i.e., have a cofactor function to promote F. X activation via F. IXa. Among these antibodies, the antibody A44 / B26 reduced coagulation time by 50 seconds or more as compared to that observed when the antibody was not added. The present inventors produced a commonly shared L chain antibody from this antibody using L chains of A44, and showed that A44L can be used as commonly shared L chains, although the activity of the resulting antibody is reduced compared to the original antibody (A44HL-B26HL). Further, with appropriate CDR shuffling, the present inventors successfully produced highly active multispecific antibodies that functionally substitute for coagulation factor VIII.
Owner:CHUGAI PHARMA CO LTD
Bispecific antibody substituting for functional proteins
ActiveUS8062635B2Enhances enzymatic reactionImmunoglobulins against blood coagulation factorsFactor VIIBlood coagulation factor VIIIBlood Coagulation Factor X
The present inventors succeeded in constructing bispecific antibodies, which bind to both the blood coagulation factor IX / activated blood coagulation factor IX and blood coagulation factor X, and functionally substitute for blood coagulation factor VIII / activated blood coagulation factor VIII which enhances the enzymatic reaction.
Owner:CHUGAI PHARMA CO LTD
Site specific repairing carrier system and method of blood coagulation factor genetic mutation
The invention discloses a method for carrying out in-situ repairing on blood coagulation factor F8 / F9. The method comprises the following steps: in a target genome sequence, selecting the mutation sites of blood coagulation factor as the gene sites for in-situ repairing; designing the binding sites of nuclease of sgRNA sequence of a CRISPR / Cas system; designing a homologous recombinant repairing donor sequence for in-situ repairing; delivering nuclease protein and / or sgRNA and the nucleotide sequence of the homologous recombinant repairing donor to the gene sites of in-situ repairing by a delivering carrier; generating damages to the genome DNA by the nuclease on the gene in-situ repairing sites; and inserting the homologous recombinant repairing donor sequence into the gene in-situ repairing sites so as to repair the gene or supplement the expression of gene. The in-situ repairing of mutation sites of blood coagulation factor can be applied to the clinic, and the method has the advantages of precise induction, safer and controllable process, and definite target.
Owner:EAST CHINA NORMAL UNIV
Multi-specific antigen-binding molecule having alternative function to function of blood coagulation factor viii
ActiveUS20130330345A1High activityLow F.Xase inhibitory actionImmunoglobulins against blood coagulation factorsAnimal cellsBlood coagulation factor VIIIAntigen
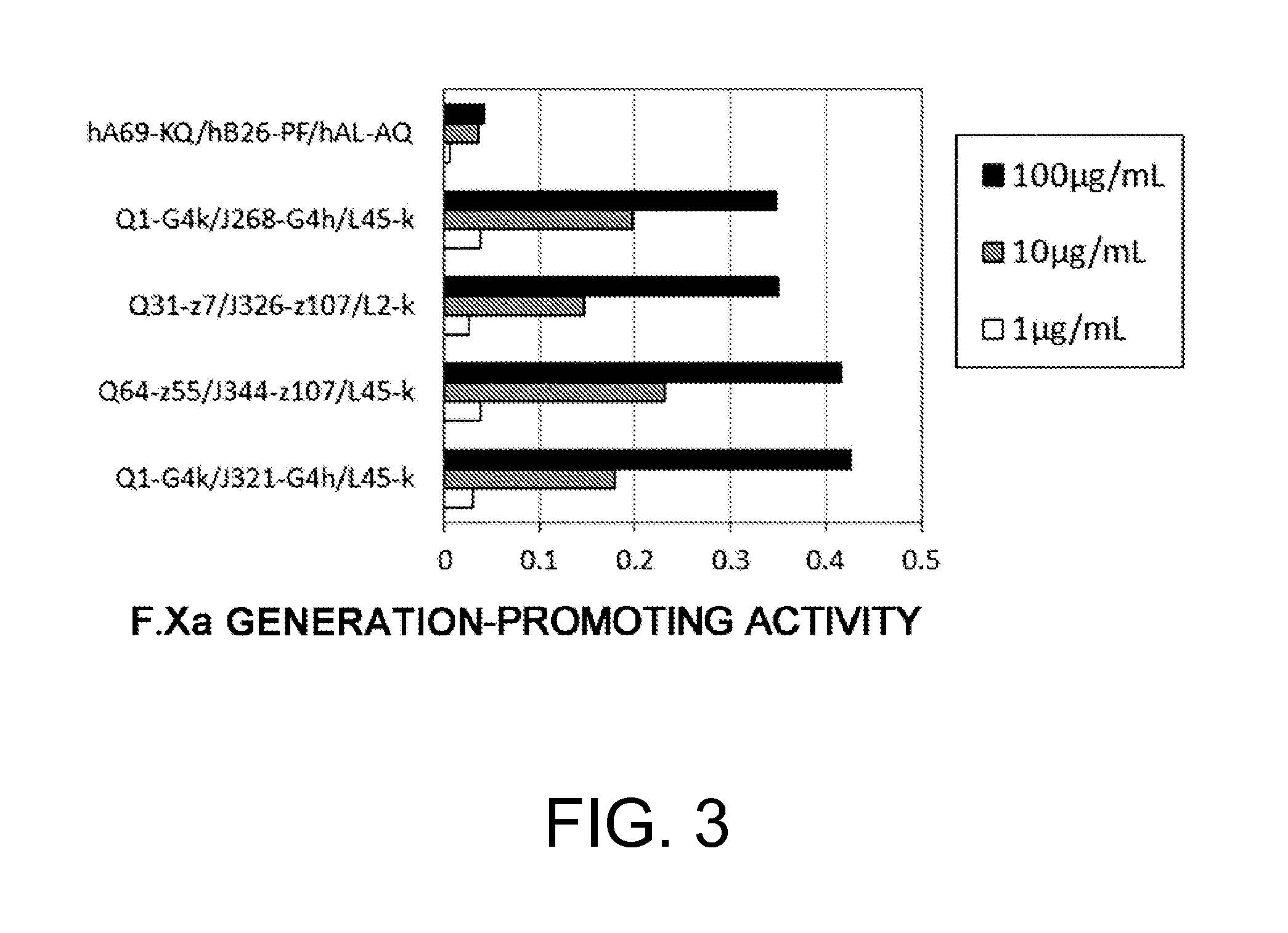
Various bispecific antibodies that specifically bind to both blood coagulation factor IX / activated blood coagulation factor IX and blood coagulation factor X and functionally substitute for the cofactor function of blood coagulation factor VIII, that is, the function to promote activation of blood coagulation factor X by activated blood coagulation factor IX, were produced. From these antibodies, multispecific antigen-binding molecules having a high activity of functionally substituting for blood coagulation factor VIII were successfully discovered.
Owner:CHUGAI PHARMA CO LTD
Factor viii compositions and methods of making and using same
ActiveUS20150158929A1Dosing is convenientGood curative effectFactor VIIPeptide/protein ingredientsDiseaseNucleic acid
Owner:BIOVERATIV THERAPEUTICS INC
Recombinant fusion proteins
This invention provides a DNA sequence coding for a cleavage site which is specifically cleaved by blood coagulation Factor Xa, a vector containing such a sequence, and a host organism transformed with such a vector. Preferably, in the vector, the Factor Xa cleavage site coding sequence is fused at one end to a product and at its other end to an ATG codon or a sequence coding for at least part of a host protein. This invention also provides a process, for the production of a desired protein or peptide product in native form, comprising: transforming a host organism with a vector as described above; expressing the desired protein or peptide product as a fusion protein comprising the desired protein or peptide product fused to a Factor Xa cleavage site; and a cleaving the fusion protein with Factor Xa to yield the foreign gene product in native form.
Owner:CELLTECH R & D LTD
Preparation method of hemostatic material
ActiveCN104721878AGood hemostatic effectSave rescue timeBiochemical fibre treatmentAbsorbent padsMedicineElectrospinning
The invention discloses a preparation method for a hemostatic material, an absorbable carrier material prepared by an electrospinning technology or a combination of the absorbable carrier material and a blood coagulation factor film-shaped material prepared by the electrospinning technology, wherein blood coagulation factors are fibrinogen and thrombin from human or animal blood. The superfine fibre prepared by the electrospinning technology has the advantages that the specific surface area of the reticulate structure of the hemostatic material is increased, and the mutual fusion action between the reticulate structure and tissues is promoted. The hemostatic material is good in biocompatibility, more remarkable in hemostatic effect and convenient to use, and can be used for hemostasis in trauma treatment and clinical operations.
Owner:GUANGZHOU DIANFANG BIOTECH CO LTD
Human coagulation factor VII polypeptides
InactiveUS6960657B2High activityPromote formationPeptide/protein ingredientsMammal material medical ingredientsPharmaceutical drugPolynucleotide
The present invention relates to novel human coagulation Factor VIIa variants having coagulant activity as well as polynucleotide constructs encoding such variants, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
Process for preparing human immunoglobulin for intravenous injection
ActiveCN102584934AReduce adverse effectsImprove biological activityPeptide preparation methodsIon exchangeTwo step
The invention relates to a process for preparing human immunoglobulin for intravenous injection, and belongs to the field of biological pharmacy. The precipitate of components II and III in the process for preparing the human immunoglobulin for intravenous injection is treated by a caprylic acid and calcium chloride precipitation method, miscellaneous proteins are removed from the precipitate of the components II and III, and two-step chromatography is performed. Compared with the general caprylic acid precipitation method, the process has the advantages that various blood coagulation factors are effectively removed from a precipitate solution of the components II and III by adding calcium chloride, the stability of the immunoglobulin is improved, and the yield of the product is obviously improved, namely more than 7g of immunoglobulin can be prepared from each liter of blood plasma; upper column chromatographic purification is performed by two ion exchange columns and a chromatographic technology, so that the miscellaneous proteins can be effectively removed, and the purity of the product is over 99.5 percent; and in addition, caprylic acid is added, and viruses are removed and filtered by a DV20 filter element, so that a virus removing effect can be obviously improved, and the safety of clinical medication is improved.
Owner:华润博雅生物制药集团股份有限公司
Multi-specific antigen-binding molecules and uses thereof
InactiveUS20140037632A1High activityHigh stability in bloodImmunoglobulins against blood coagulation factorsAntibody ingredientsBlood coagulation factor VIIIAntigen
Various bispecific antibodies that specifically bind to both blood coagulation factor IX / activated blood coagulation factor IX and blood coagulation factor X and functionally substitute for the cofactor function of blood coagulation factor VIII, that is, the function to promote activation of blood coagulation factor X by activated blood coagulation factor IX, were produced. From these antibodies, multispecific antigen-binding molecules having a high activity of functionally substituting for blood coagulation factor VIII were successfully discovered.
Owner:CHUGAI PHARMA CO LTD
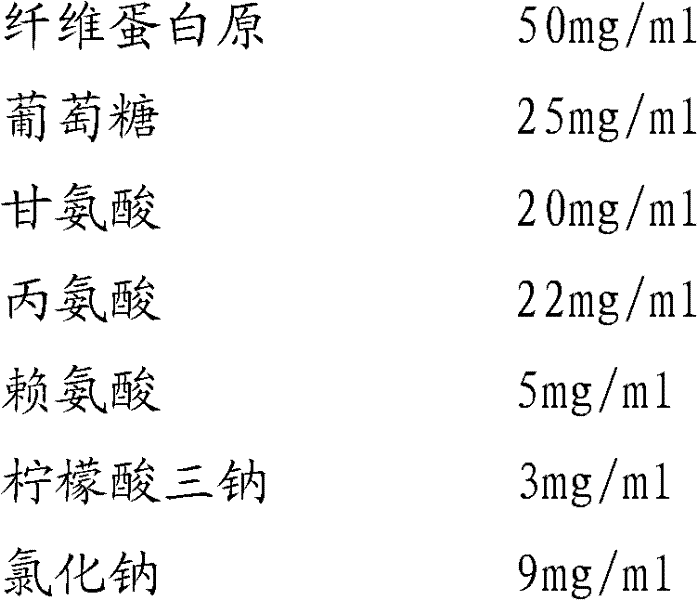
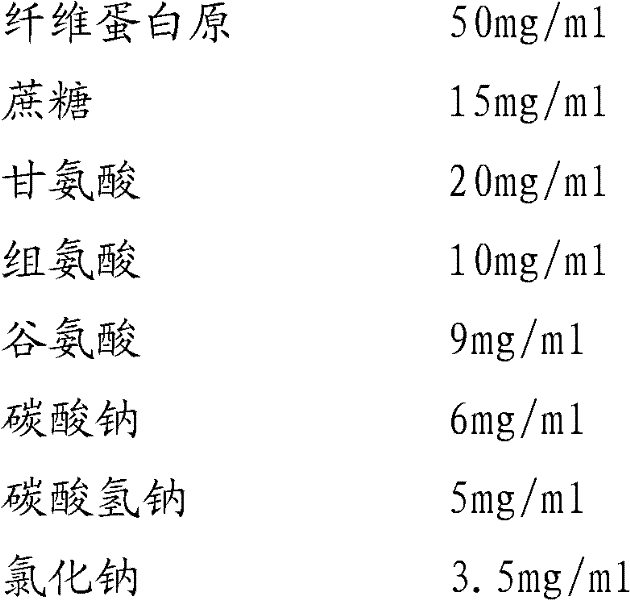
Preparation method of fibrinogen
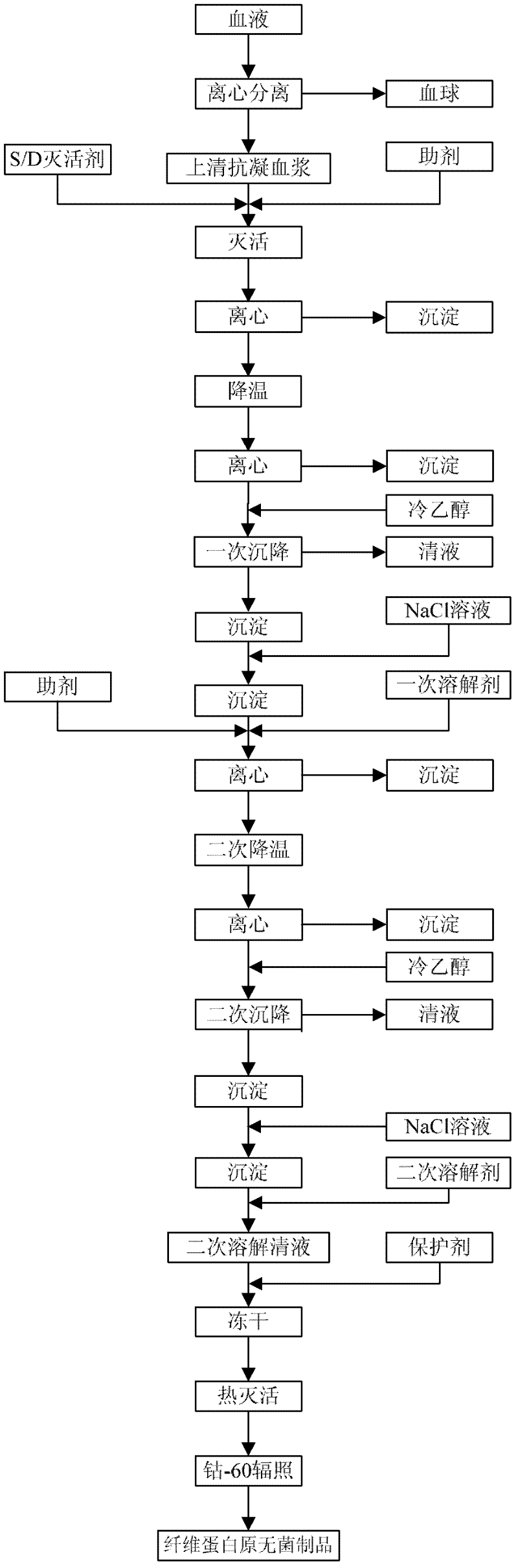
ActiveCN102286095AHigh purityImprove extraction efficiencyFibrinogenPeptide preparation methodsSolubilityFreeze-drying
The invention provides a preparation method for fibrinogen with the advantages of high dissolution rate in the production process, short production period, fast solubility in clinical use, good biological activity, high safety in clinical use and high yield. An aid is added into supernate anticoagulant plasma. Compared with the prior art, the preparation method has the advantages that: 1, the preparation method provided by the invention is high in extraction efficiency; 2, the aid adopted in the method can improve the quality of the fibrinogen product, and plays a role in protecting the biological activities of the fibrinogen and blood coagulation factors XIII in virus inactivation and cold ethanol settlement processes; 3, in the process, the process solubility time of the fibrinogen is shortened to about 10 minutes; and 4, the solubility time of the freeze dried product of the fibrinogen is within 30 seconds, so precious time is won for rescuing patients in time in clinic.
Owner:DATIAN HUACAN BIO TECH
Blood pump and ventricular assist device
InactiveUS6769871B2Improve hydrophilicityEasy to eluteSpecific fluid pumpsPump componentsHemocompatible MaterialsImpeller
The blood pump according to the present invention comprises a casing having a blood inlet and a blood outlet, and an impeller for circulating blood by rotating inside the casing, wherein both the casing's and the impeller's surfaces in contact with blood are formed of a biocompatible metal, and onto the surfaces in contact with blood a coating film of a hemocompatible material comprising a phospholipid polymer is formed. According to the present invention, the blood pump effectively suppresses the development of thrombi without activating blood coagulation factors such as thrombocites (blood platelets) in the blood, thanks to a coating film of a hemocompatible material made of a phospholipid polymer being formed onto the surfaces in contact with blood of the casing and the impeller composing the main part of the blood pump.
Owner:SUN MEDICAL TECH RES
Serum protein marker group for diagnosing MODY (maturity-onset-diabetes of the young) and application thereof
InactiveCN106645757AAccurate diagnosisReliable resultsApolipeptidesHydrolasesFactor iiMass spectrometry
The invention relates to a serum protein marker group for diagnosing MODY (maturity-onset-diabetes of the young) and application thereof. The serum protein marker group comprises five proteins: apolipoprotein C-IV (APOC4), apolipoprotein a (LPA), a complement component C6 (C6), a blood coagulation factor v (F5) and thyroid binding globulin (SERPINA7). ITRAQ (isobaric tags for relative and absolute quantitation) are used to combine with an MALDI-TOF / MS technology to detect; mass spectrum detection shows that expression levels of the serum apolipoprotein C-IV, the apolipoprotein a, the complement component C6 and the blood coagulation factor v protein in serum of a MODY patient are obviously improved, and an expression level of the thyroid binding globulin in the serum of the MODY patient is obviously reduced. Mass spectrum MRM (multiple-reaction-monitoring) also verifies that the five proteins are abnormal in expression in serums of the MODY patient, type-1 and type-2 diabetics and a healthy control group, and are specific proteins.
Owner:XINJIANG MEDICAL UNIV
Factor VIII:Ca chromogenic assay
InactiveUS6100050AGood reproducibilityHigh degree of sensitivityHydrolasesMicrobiological testing/measurementBlood coagulation factor VIIIFactor ii
A chromogenic assay for determination of blood coagulation Factor VIII:Ca, using an indicator of Factor Xa simultaneously as a measure of Factor VIII:Ca concentration and as an inhibitor of Factor Xa. This technique can be applied to measure the concentration of an activating enzyme using the rate of conversion of the indicator molecule of the product of that enzyme as an indirect indicator of enzyme concentration.
Owner:DADE BEHRING
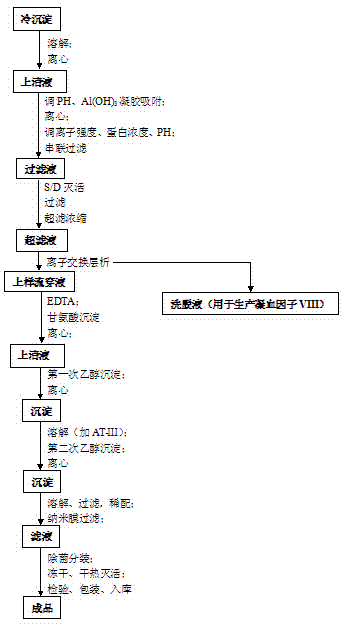
Preparation process for extracting human fibrinogens from waste for extracting cryoprecipitated blood coagulation factor VIII
ActiveCN104231072AHigh purityShort reconstitution timeFactor VIIFibrinogenBlood coagulation factor VIIIEthanol precipitation
The invention discloses a preparation process for extracting human fibrinogens from waste for extracting cryoprecipitated blood coagulation factor VIII. The preparation process is characterized by comprising the steps of cryoprecipitation dissolution, 2% aluminium hydroxide gel absorption, ion strength adjustment, series connection filtering, S / D viral inactivation, ion-exchange chromatography, EDTA Ca2+ removal, glycine precipitation, primary low-temperature ethanol precipitation, AT-III thrombin inhibition, secondary low-temperature ethanol precipitation, nanofilm filtering and dry-heat inactivation. For ensuring the safety, a nanofilm ia added to filter virus except S / D and dry-heat inactivation. By means of an added AT-III inactivated thrombin and EDTA Ca2+ removal process, fibrinogen in the production process is effectively prevented from being activated into fibrous protein. The glycine precipitation is utilized to remove fibrous protein monomers and polymers in products so as obtain high-purity human fibrinogen. The preparation products are safe and reliable, redissolution time is short, the clinic first-aid demand is met, and meanwhile the preparation process has important significance on indirect saving of scarce plasma resources.
Owner:华润博雅生物制药集团股份有限公司
Stabilized factor viii variants
InactiveUS20130183280A1Increase in vitro stabilityIncreased in vivo circulatory half lifeFactor VIIPeptide/protein ingredientsHalf-lifeOrganic chemistry
Owner:NOVO NORDISK AS
Methods for delivering recombinant adeno-associated virus virions to the liver of a mammal
Methods for introducing recombinant adeno-associated virus (rAAV) virions into the liver of a mammal are provided. In these methods, the liver is partially or completely isolated from its blood supply, a catheter is introduced into the liver via a peripheral blood vessel, and rAAV virions are then infused through the catheter to the liver. The methods described herein may be used, for example, to deliver heterologous genes encoding therapeutic proteins to the hepatocytes of humans. This can be accomplished, for example, by introducing the catheter into a femoral artery, threading the catheter into the hepatic artery, and infusing rAAV virions through the catheter and into the liver. Exemplary examples of heterologous genes include those coding for blood coagulation factors.
Owner:GENZYME CORP
Hybrid molecules having Factor VII/VIIa activity
InactiveUS7432352B2Same and increased activityIncreased serum half-lifePeptide/protein ingredientsMammal material medical ingredientsSynthetic analogueFactor ii
Owner:NOVO NORDISK HEALTH CARE AG
Water gel fast forming process based on bionic process
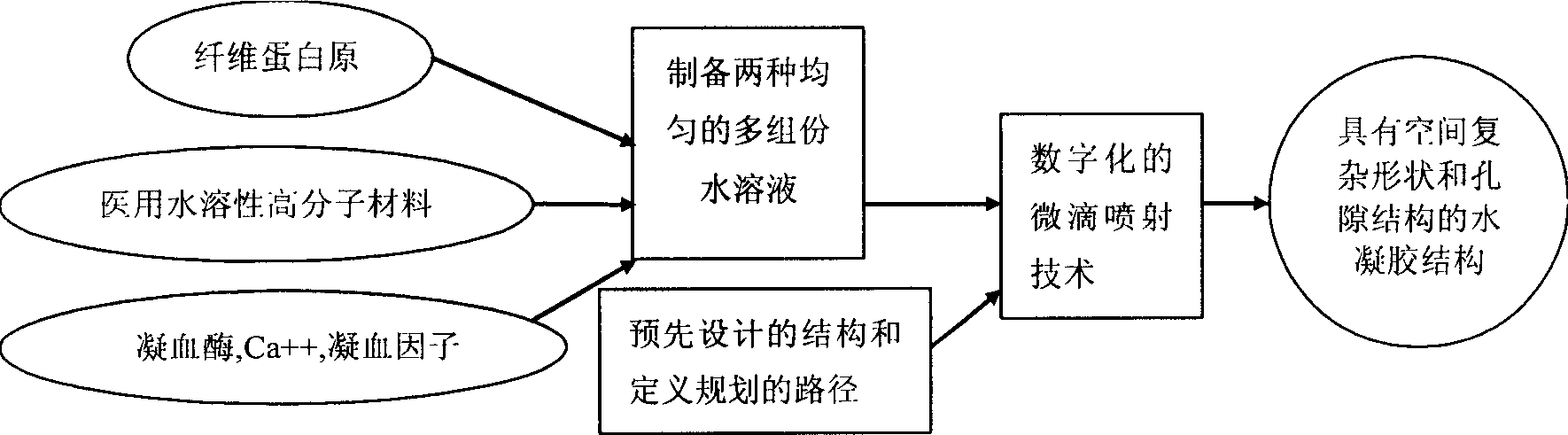
The fast bionic water gel forming process simulates human blood coagulation process to produce fibrin gel. Fibrinogen and medical water soluble polymer material, as well as thrombin, Ca ion and blood coagulation factor are prepared into two multiple-component water solutions; and the two water solutions are made to mix and accumulate in certain spatial position by means of digital micro dropping and jetting technology while producing enzyme reaction to form water gel with fibrin as matrix, so as to make 3D water gel structure with complicated spatial shape and porous structure. The coagulation process has complicated and effective regulating and controlling mechanism, and the water gel structure has excellent mechanical and biological performance, stable structure and effectively regulated degradation speed. The present invention is suitable for forming tissue engineering sample, medicine slow releasing carrier, etc.
Owner:TSINGHUA UNIV
FIX-Mutant Proteins for Hemophilia B Treatment
InactiveUS20080214462A1Improved clot activityHigh activityPeptide/protein ingredientsMammal material medical ingredientsHEK 293 cellsDisease
The present invention relates to recombinant blood coagulation factor IX (rFIX) mutants having improved FIX clotting activity. Three full length FIX proteins with combinations of mutations of amino acids important for functional activity of FIX and FIX wild type were cloned and expressed in HEK 293 cells. The proteins were tested by an activated partial thromboplastin time (aPTT) assays in FIX-depleted plasma. Two mutant proteins had increased specific FIX activity. Furthermore, a pre-activated FIX protein had an increased activity in FIX-depleted plasma. Therefore these FIX mutants can be used for the treatment of FIX associated bleeding disorders.
Owner:BAXTER INT INC +1
Therapeutic ultrasonic microvesicle for tumour ultrasonic therapy and its preparing method
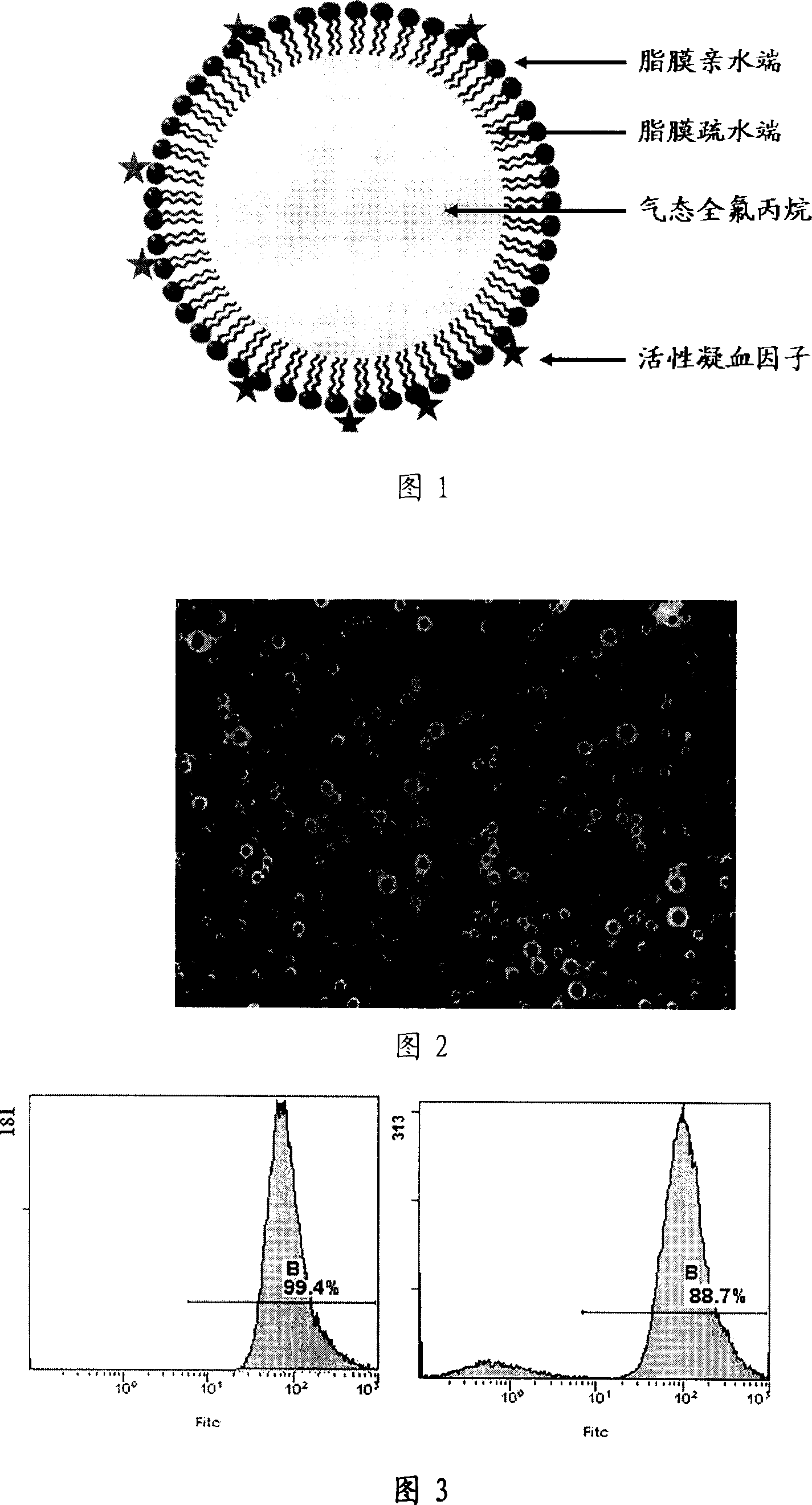
The present invention relates to a therapeutic type ultrasonic microvesicle for tumor ultrasonic therapy, it includes ultrasonic contrast medium microvesicle containing gas endonucleus and blood coagulation factor, the blood coagulation factor is absorbed on the ultrasonic contrast medium microvesicle surface or the blood coagulation factor is adsorbed on the microvesicle surface and covered in its interior so as to form ultrasonic microvesicle in which the blood coagulation factor is combined. Under the proper therapeutic ultrasonic action said therapeutic type ultrasonic microvesicle can promote local tissue microcirculation thrombosis, and can be used for curing the diseases of tumor, etc.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
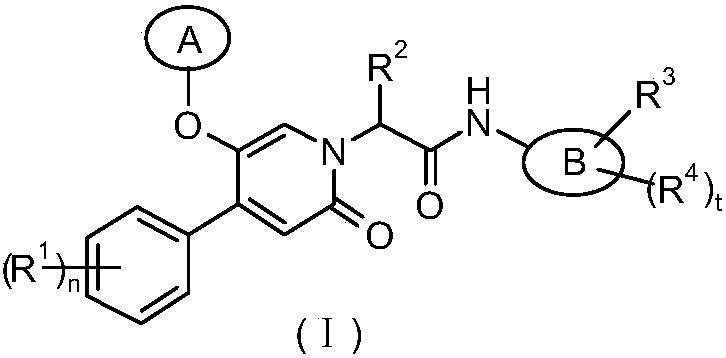
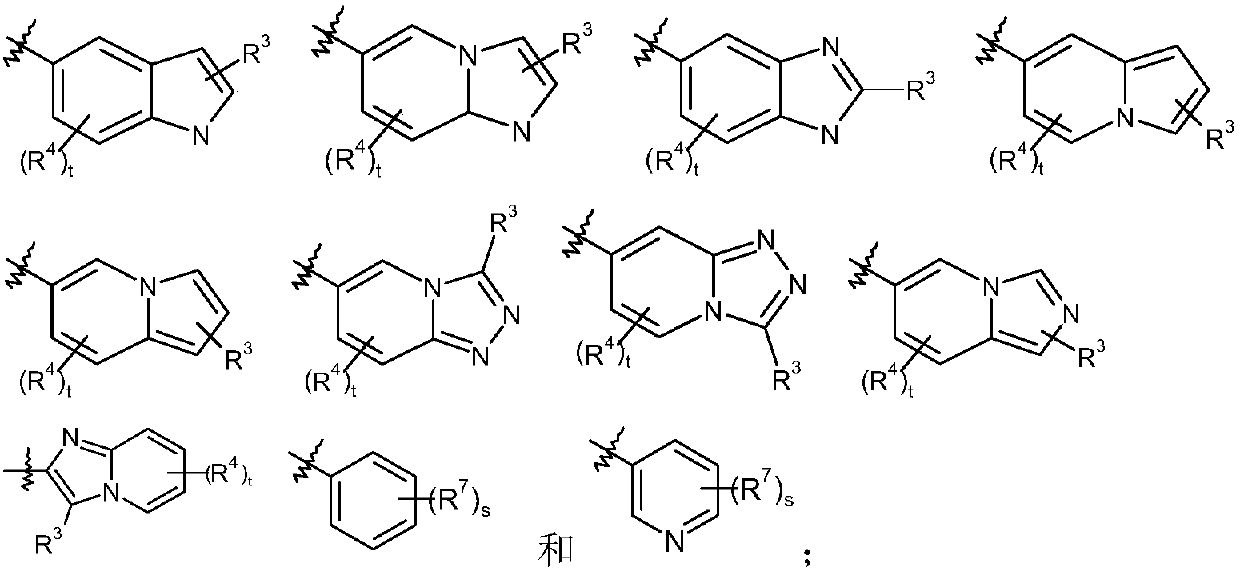
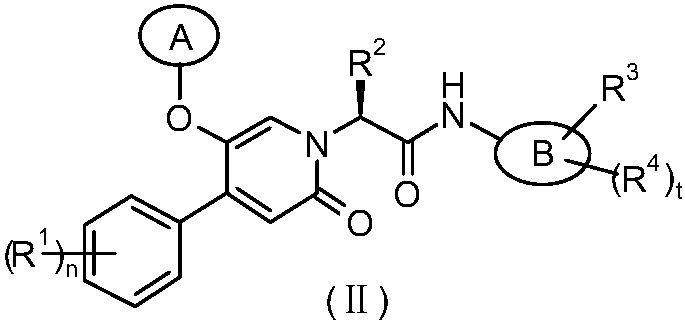
Epoxy-substituted oxopyridine derivatives and their preparation method and application in medicine
The invention relates to epoxy-substituted oxopyridine derivatives and their preparation method and application in medicine and concretely, relates to epoxy-substituted oxopyridine derivatives shown in the general formula (I), their preparation method, a pharmaceutical composition containing the derivatives and a use of the derivatives as therapeutic agents and especially as blood coagulation factor XIa (called as FXIa for short) inhibitors and in preparation of drugs for treating diseases such as thromboembolism. The definition of each substituent in the general formula (I) is the same as thedefinition in the specification.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
In-vitro non-implantable maglev heart chamber assisting centrifugal blood pump
InactiveCN105641762AAvoid damageReduce usageIntravenous devicesProsthesisHeparin coatingExtracorporeal circulation
The invention belongs to the field of medical apparatuses, and relates to an artificial heart assisting device for treating heart failure caused by acute cardiogenic shock, in particular to an in-vitro non-implantable maglev heart chamber assisting centrifugal blood pump. The in-vitro non-implantable maglev heart chamber assisting centrifugal blood pump adopts a non-bearing magnetic fluid levitation technology to substitute mechanical bearing driving of the conventional centrifugal blood pump, an impeller rotor is suspended in a magnetic field without any mechanical contact, and the position and the speed of the impeller rotor are precisely regulated by a digital signal processor system, so that damage to blood cells and visible components (platelets, blood coagulation factors and the like) of blood is reduced to the most extent; meanwhile, by a heparin coating technology applicable to the inner wall of the blood pump and the surface of the impeller rotor, coagulation and thrombosis as well as use of an anticoagulant medicine in the assisting process are further reduced, so that complications of hemorrhage of a patient during a surgery are reduced. The in-vitro non-implantable maglev heart chamber assisting centrifugal blood pump has the characteristics of being simple in structure, reliable in operation, convenient to maintain, stepless in speed regulation and the like, can be used for treating the heart failure caused by acute cardiogenic shock triggered by a variety of reasons, and in combination with an oxygenator, can also be used for assisting circulation and blood oxygenation during extracorporeal membrane oxygenation (ECMO) treatment.
Owner:ZHENGREN BEIJING MEDICAL INSTR CO LTD
Preparation method of human coagulation factor VIII
ActiveCN104231073ASuitable for a wide range of peopleHigh potencyFactor VIIPeptide/protein ingredientsAlcohol sugarsDiabetic patient
The invention discloses a preparation method of a human coagulation factor VIII. The human coagulation factor VIII prepared by the method does not contain human serum albumin or other animal-derived protein, does not contain sugar or sugar alcohol, does not have the risk for transmitting other viruses or pathogene, and is wide in applicable crowd scope, and can be used by diabetic patients; the human coagulation factor VIII prepared by the method is fast to redissolve and good in redissolving effect, and still keeps high titer and high specific activity which are respectively larger than 80 percent and 40 IU / mg; in addition, the preparation method is simple, the cost is low, the human coagulation factor VIII is safe and effective, and has a good industrial application prospect.
Owner:广东双林生物制药有限公司
Long-acting coagulation factors and methods of producing same
Polypeptides comprising at least one carboxy-terminal peptide (CTP) of chorionic gonadotrophin attached to the carboxy terminus but not to the amino terminus of a coagulation factor and polynucleotides encoding the same are disclosed. Pharmaceutical compositions comprising the polypeptides and polynucleotides of the invention and methods of using and producing same are also disclosed.
Owner:OPKO BIOLOGICS
Preparation method and application of marine organism active hemostasis dressing
ActiveCN103467771AGood biocompatibilityEnhance anti-inflammatoryAbsorbent padsBandagesCross-linkMicrosphere
The invention discloses a preparation method and application of a marine organism active hemostasis dressing. The preparation method comprises the steps of adding equal sodium alga acid solution while stirring a water solution comprising hydroxypropyl trimethyl ammonium chloride chitosan and / or an acetum of chitosan so as to obtain a liquor 1, taking an emulsion comprising paraffin as a liquor 2, mixing the liquor 1 and the liquor 2 according to the proportion of (2-3):5 and stirring uniformly so as to obtain a stable emulsification system, adding a cross-linking agent calcium chloride solution for cross-linking and solidifying the emulsification system, then washing, dewatering and drying so as to obtain the marine organism active hemostasis dressing. The materials of the prepared microballoon product have good biocompatibility and biodegradability as well as special effects of resisting bacterium, stopping bleeding, and promoting healing, when being contacted with a wound, the cross-linking and solidification product can rapidly absorb moisture in blood and concentrate blood platelets and blood coagulation factors into sticky gel, thus effectively sealing the bleeding wound and enhancing the hemostasis performance of the microballoon material, and therefore, the marine organism active hemostasis dressing is stable, efficient and environment-friendly.
Owner:山东贝诺医药生物科技有限公司
FVIII-Independent FIX-Mutant Proteins for Hemophilia A Treatment
ActiveUS20080214461A1Peptide/protein ingredientsMammal material medical ingredientsHEK 293 cellsMutated protein
The present invention relates to recombinant blood coagulation factor IX (rFIX) mutants having factor VIII (FVIII) independent factor X (FX) activation potential. Five full length FIX proteins with combinations of mutations of amino acids important for functional activity of FIX and FIX wild type were cloned and expressed in HEK 293 cells. The proteins were tested by an activated partial thromboplastin time (aPTT) assay in FVIII-depleted plasma as well as in FVIII-inhibited patient plasma. In FVIII-depleted plasma functional activity of the FIX mutants was calculated as increased FVIII equivalent activity. The mutant proteins had increased FVIII equivalent activity. In FVIII-inhibited patient plasma the FEIBA equivalent activity was calculated for analysis of FVIII independent FX activation potential. The proteins had also increased FEIBA equivalent activity. Furthermore, the pre-activated FIX proteins had an increased activity in FIX-depleted plasma containing FVIII inhibitors. Therefore these FIX mutants are alternatives as bypassing agents for treatment of FVIII inhibitor patients.
Owner:TAKEDA PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com