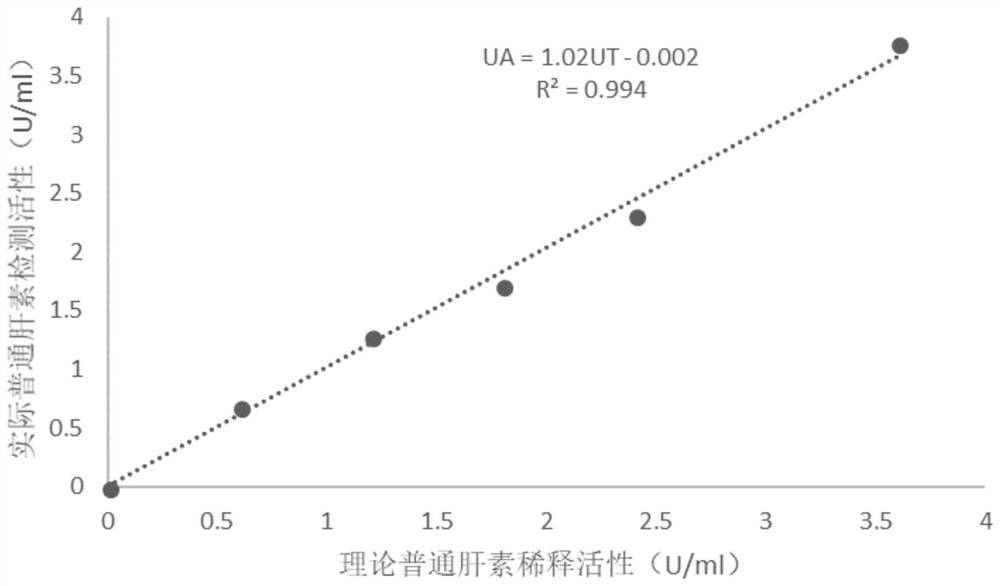
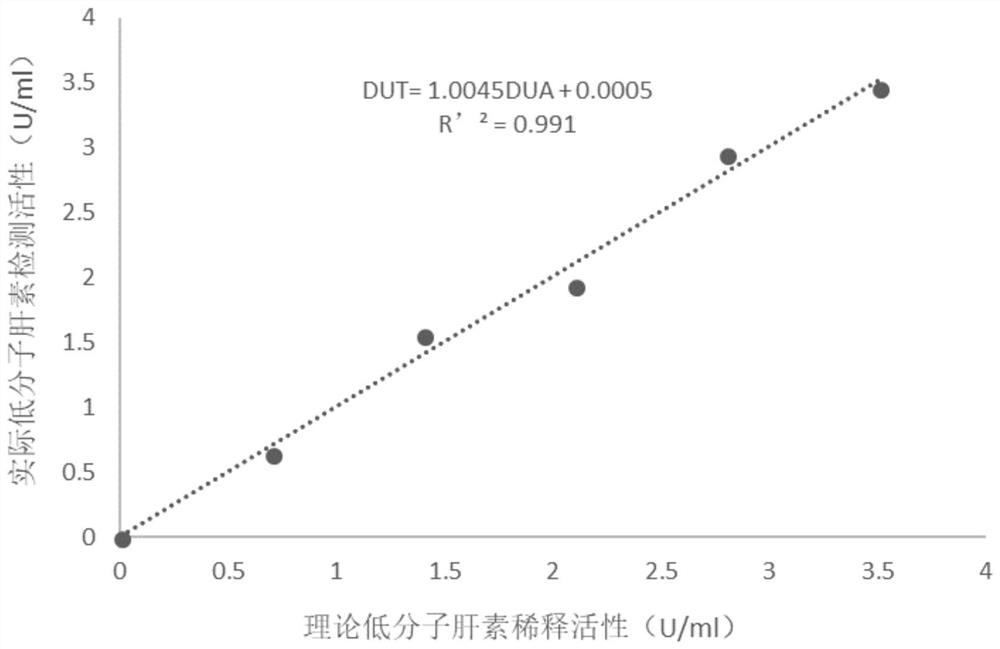
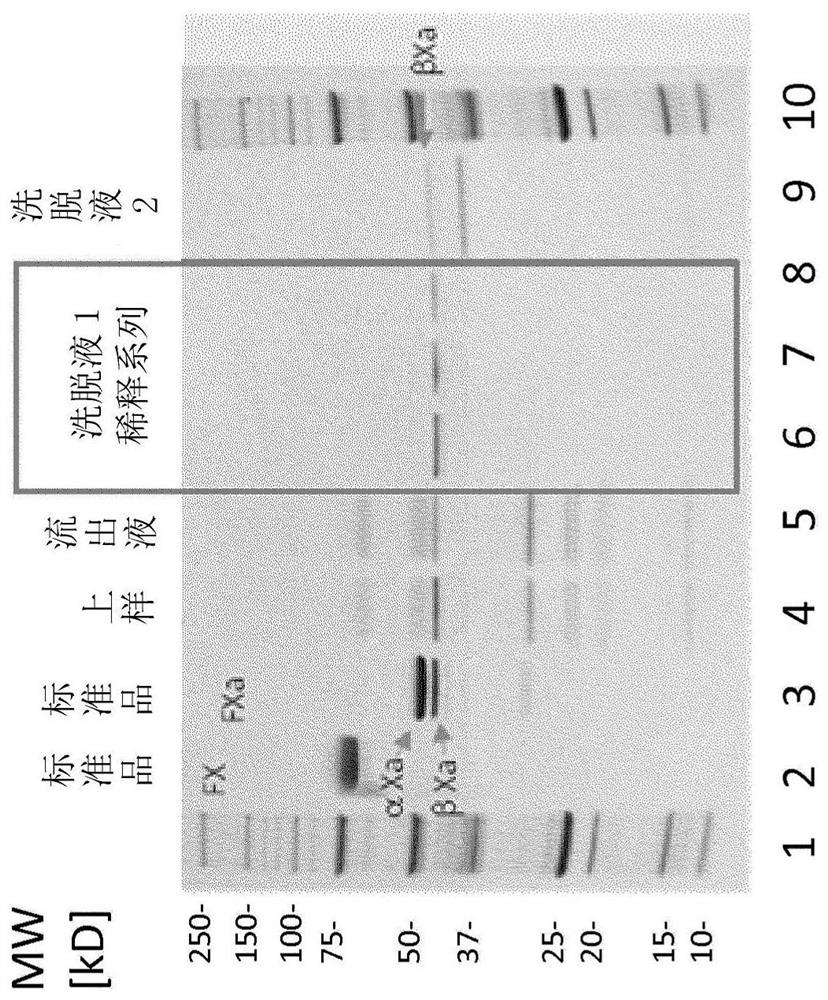
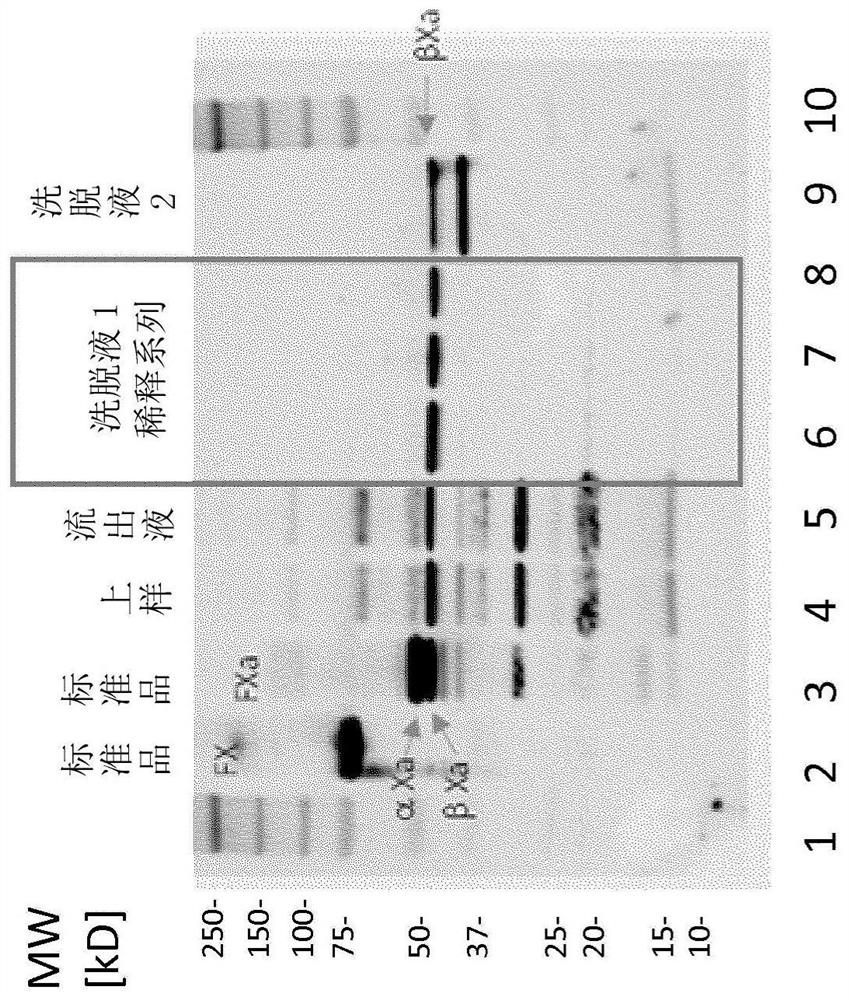
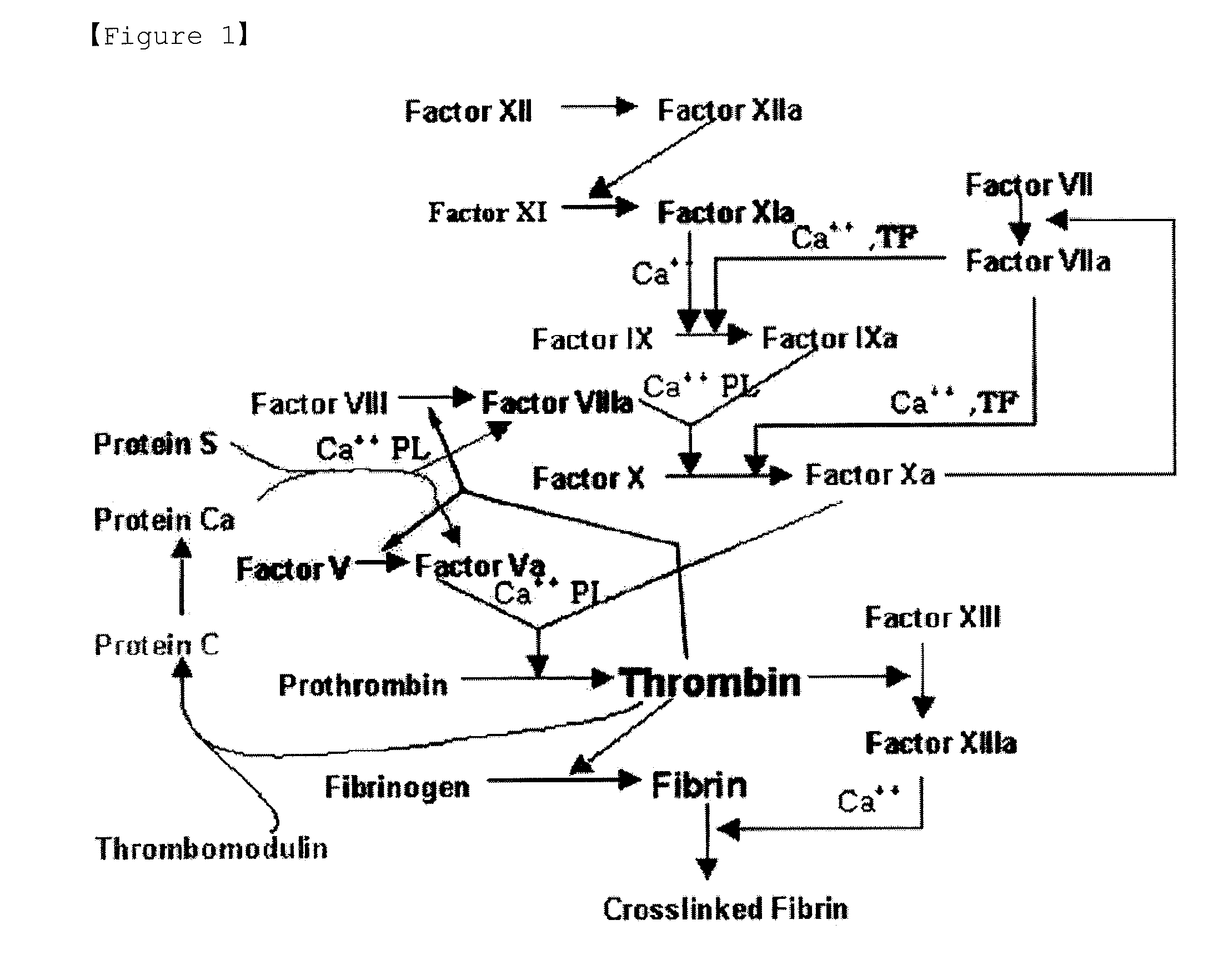
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Coagulation Factor Xa" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation for specificity anticoagulant substance and application thereof
InactiveCN101190945APeptide/protein ingredientsHybrid peptidesThrombin activityCoagulation Factor Xa
The invention relates to an anticoagulant protein containing an oligopeptide which is identified and cracked by thrombin or blood coagulation factor Xa; more particularly, the invention relates to a new anticoagulant substance connected by an anticoagulant substance and an amino acid sequence which can be identified and cracked by thrombin or blood coagulation factor Xa, or a new substance connected by an anticoagulant substance and other substances through taking an amino acid sequence which can be identified and cracked by thrombin or blood coagulation factor Xa as oligopeptide, as well as medicine science application of these new anticoagulant substances.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA +1
Sustained release preparation
InactiveUS20110045028A1Inhibit appropriatelyLess side effectsOrganic active ingredientsBiocideHydrophilic polymersMedicine
Disclosed is a sustained-release preparation which is prepared by shaping a granule comprising a blood coagulation factor Xa inhibitor and a mixture of at least two hydrophilic polymers. Also disclosed is a pharmaceutical composition comprising a combination of the sustained-release preparation and an immediate release preparation comprising a blood coagulation factor Xa inhibitor. It becomes possible to provide a controlled release preparation comprising a blood coagulation factor Xa inhibitor for the prevention or treatment of thrombosis, which can control the activity of blood coagulation factor Xa for a long term and is excellent in convenience and compliance. It is also becomes possible to provide a method for producing the controlled release preparation.
Owner:TAKEDA PHARMA CO LTD
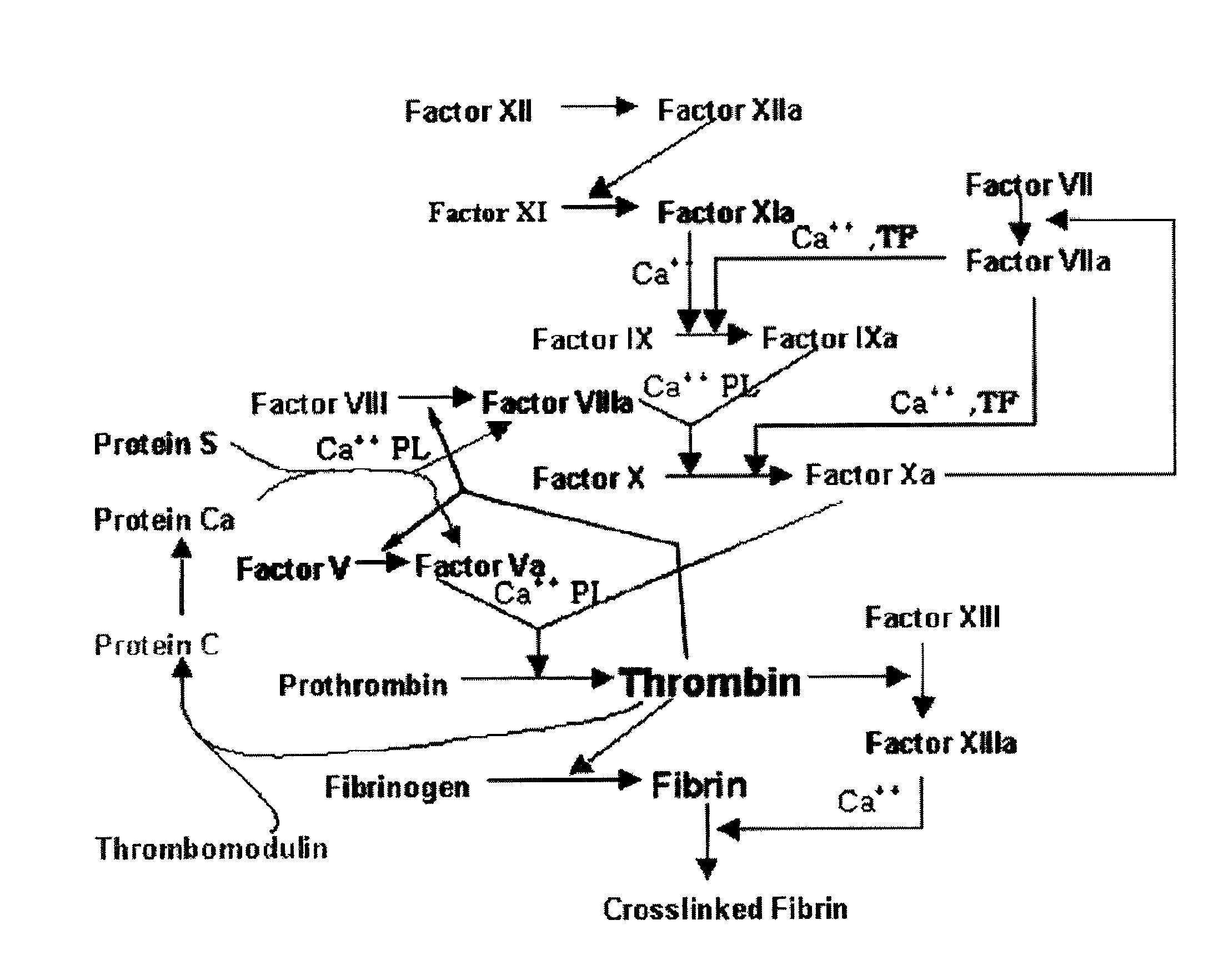
Chlorothiophene-amides as inhibitors of coagulation factors xa and thrombin
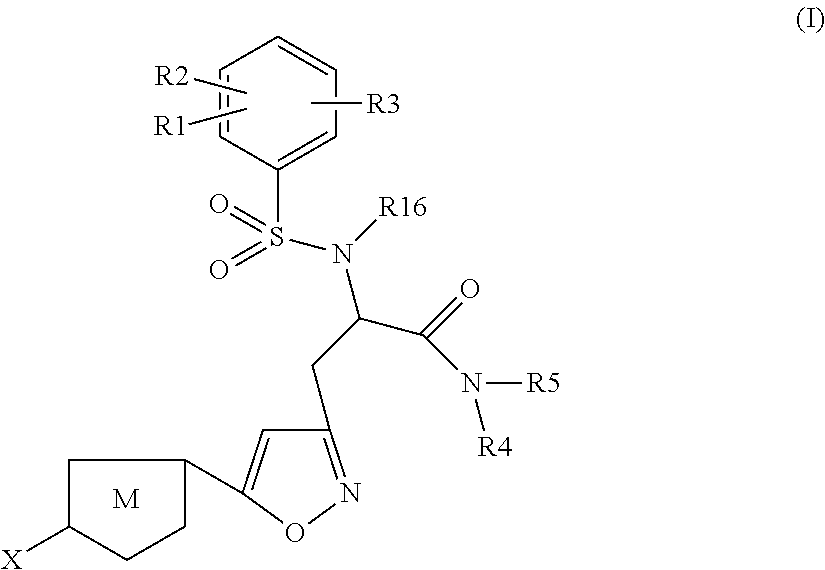
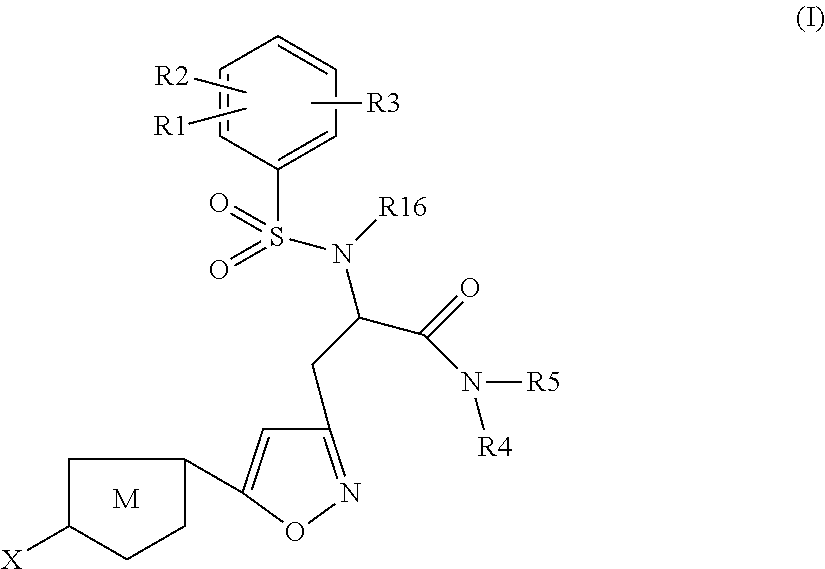
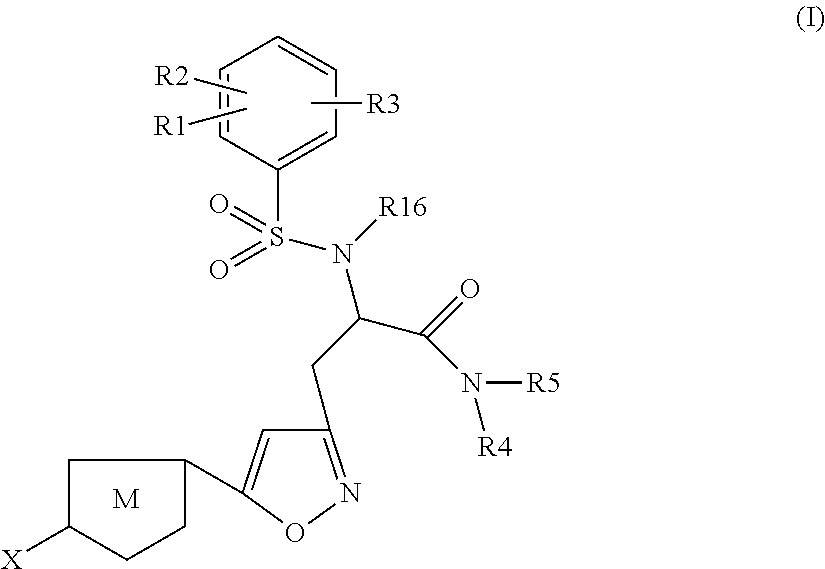
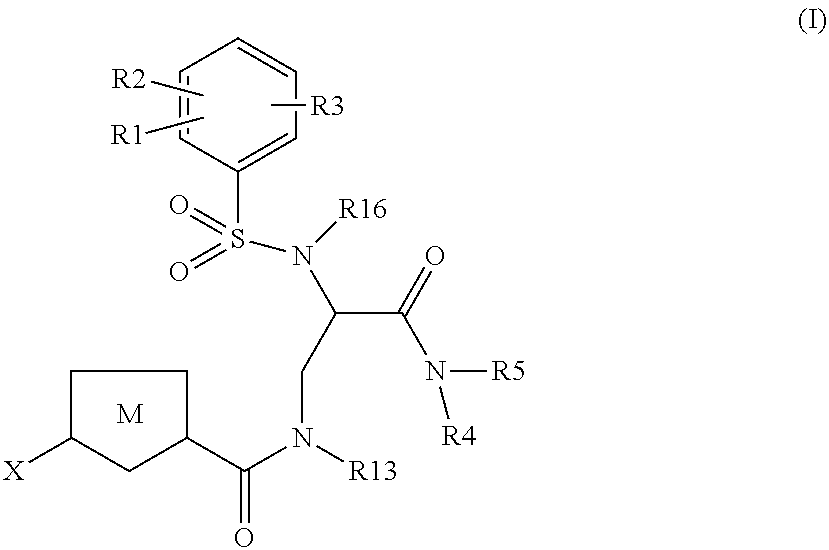
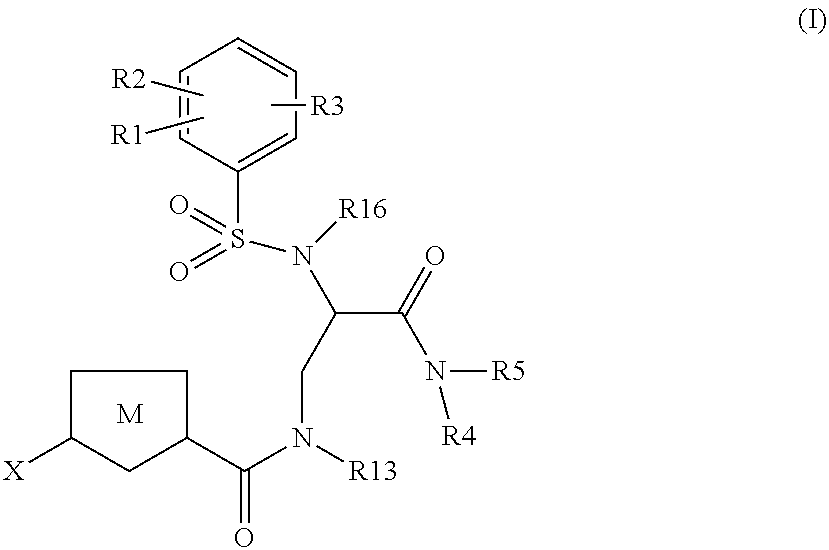
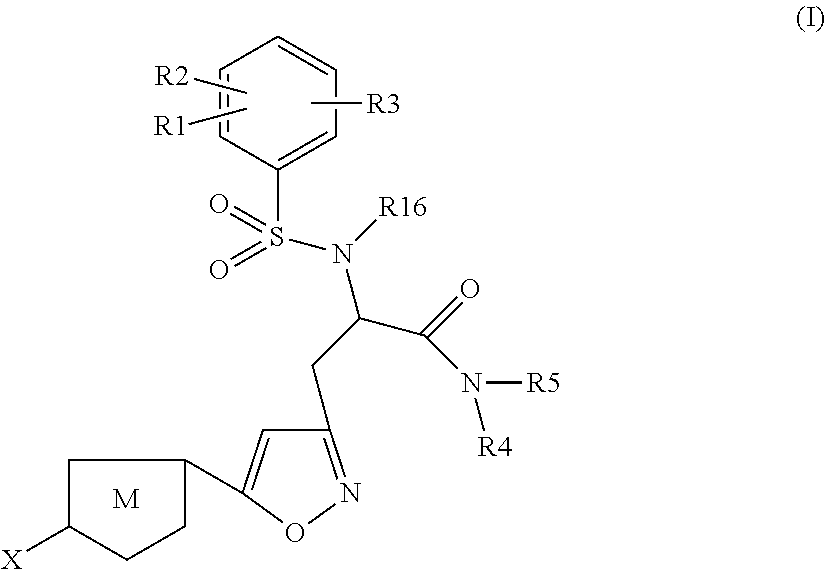
The present invention relates to compounds of the formula I,wherein R1; R2; R3; R4; R5, R13, R16, X and M have the meanings indicated in the claims. The compounds of formula I are valuable pharmacologically active compounds. They exhibit a strong anti-thrombotic effect and are suitable, for example, for the therapy and prophylaxis of cardio-vascular disorders like thromboembolic diseases or restenoses. They are reversible inhibitors of the blood clotting enzymes factor Xa (FXa) and thrombin and can in general be applied in conditions in which an undesired activity of factor Xa and / or thrombin are present or for the cure or prevention of which an inhibition of factor Xa and thrombin are intended. The invention furthermore relates to processes for the preparation of compounds of the formula I, their use, in particular as active ingredients in pharmaceuticals, and pharmaceutical preparations comprising them.
Owner:BEIJING LIANXIN PHARMA CO LTD
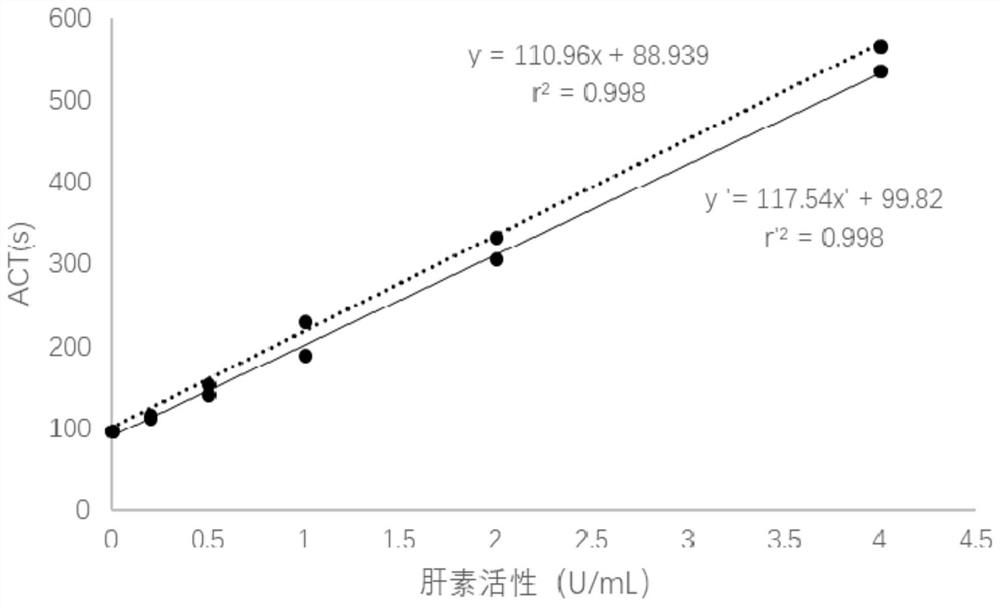
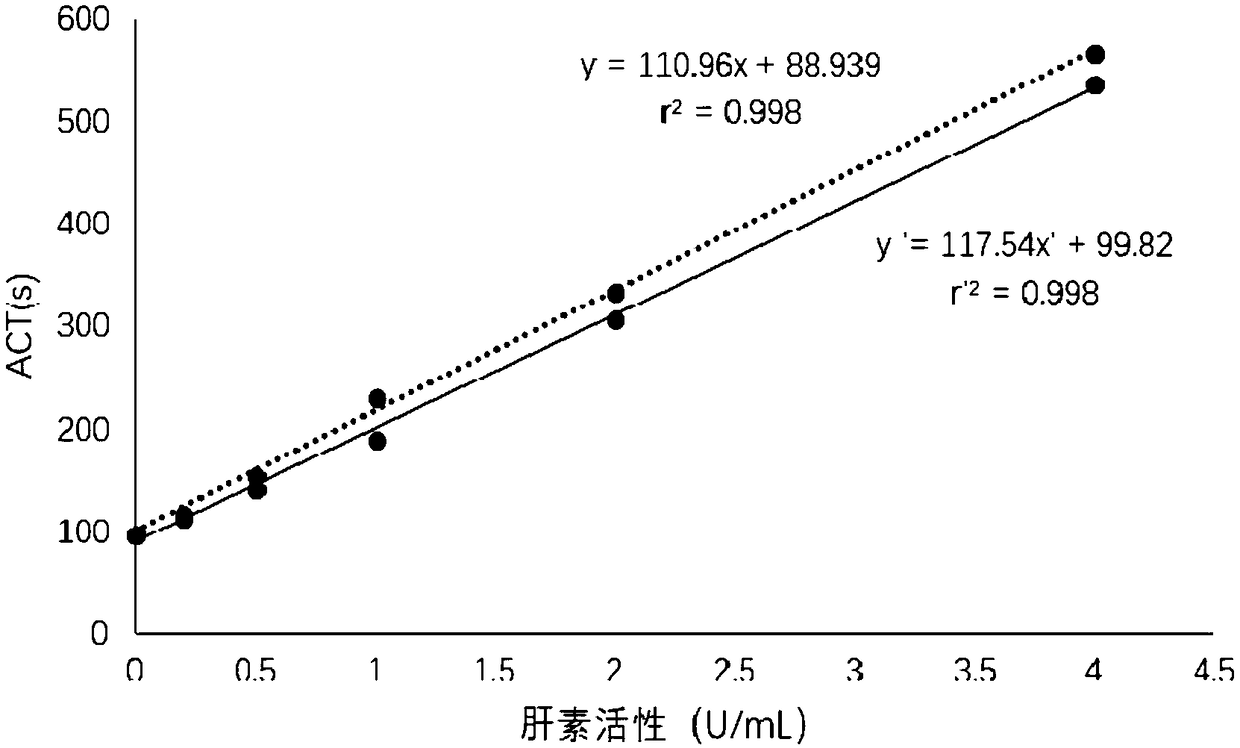
Thromboelastography heparin quantitative detection kit and preparation method thereof
ActiveCN108982865AQuantitative detection is convenient and accurateSimplify the inspection processBiological material analysisBiological testingCoagulation Factor XaCoefficient of variation
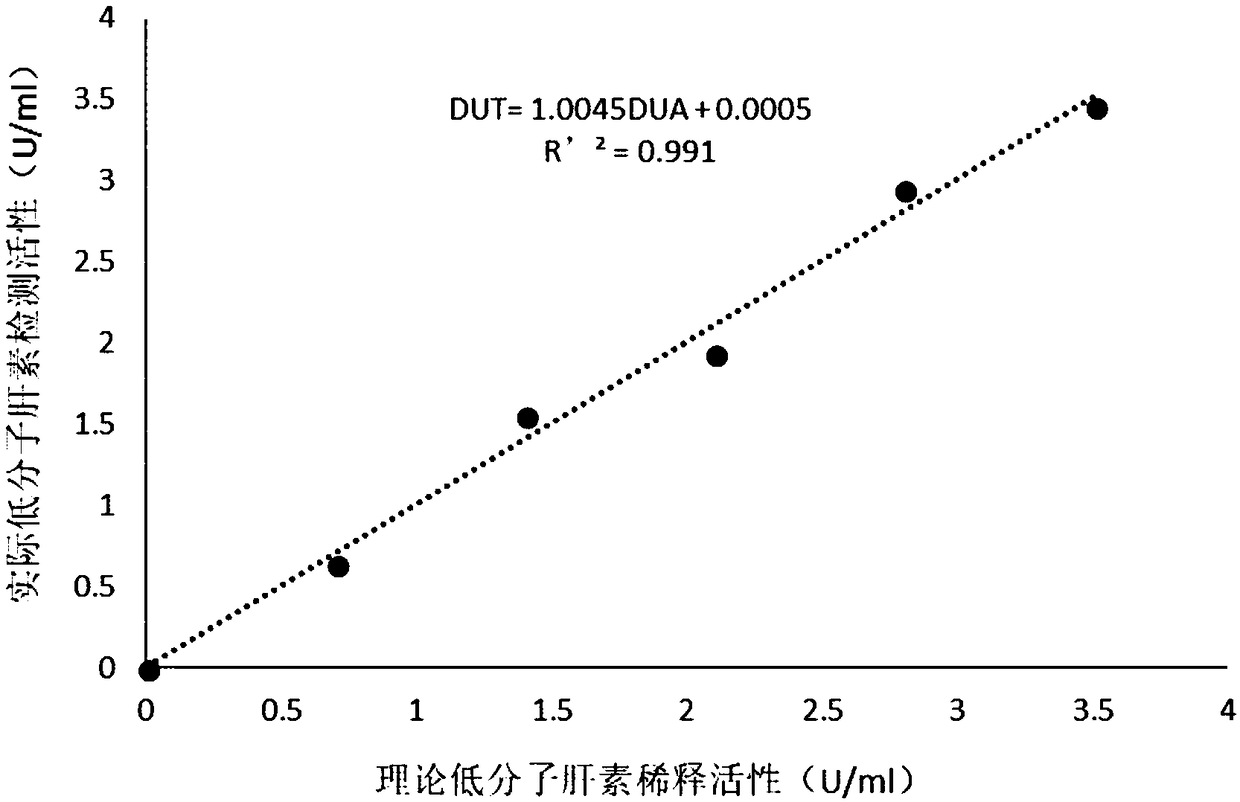
The invention relates to a thromboelastography heparin quantitative detection kit, which comprises a buffer agent, a blood coagulation factor Xa, rabbit brain congealed fat, a coagulation factor activator, a supporting agent, and a biological preservative. The preparation method comprises the following steps: preparing a buffer solution, a blood coagulation factor Xa stock solution, a blood coagulation factor activator stock solution, a biological preservative solution, and a rabbit brain congealed fat stock solution; taking the support agent and adding the buffer solution to prepare a supportsolution; adding the blood coagulation factor Xa, the blood coagulation factor activator, and the rabbit brain congealed fat stock solution to the supporting solution according to the requirements ofkit specification, adding the biological preservative solution after uniformly mixing, then adding the buffer solution to the specified amount, subpackaging and lyophilizing after uniformly mixing. The thromboelastography heparin quantitative detection kit provided by the invention can quantitatively detect the heparin in a sample by a thromboelastography, has good correlation in a linear range,meets the heparin detection limit standard and has a coefficient of variation of not more than 10%, and the sample adopts whole blood without extra treatment; the integrity of protein in a clotting cascade is also not required, and the inspection operating process is simplified, thereby facilitating doctors and patients.
Owner:上海原科实业发展有限公司
Target multifunctional anti-embolism fusion protein as well as preparation method and application thereof
InactiveCN102180973AInhibition formationInhibition of developmentFungiPeptide/protein ingredientsArginineCoagulation Factor Xa
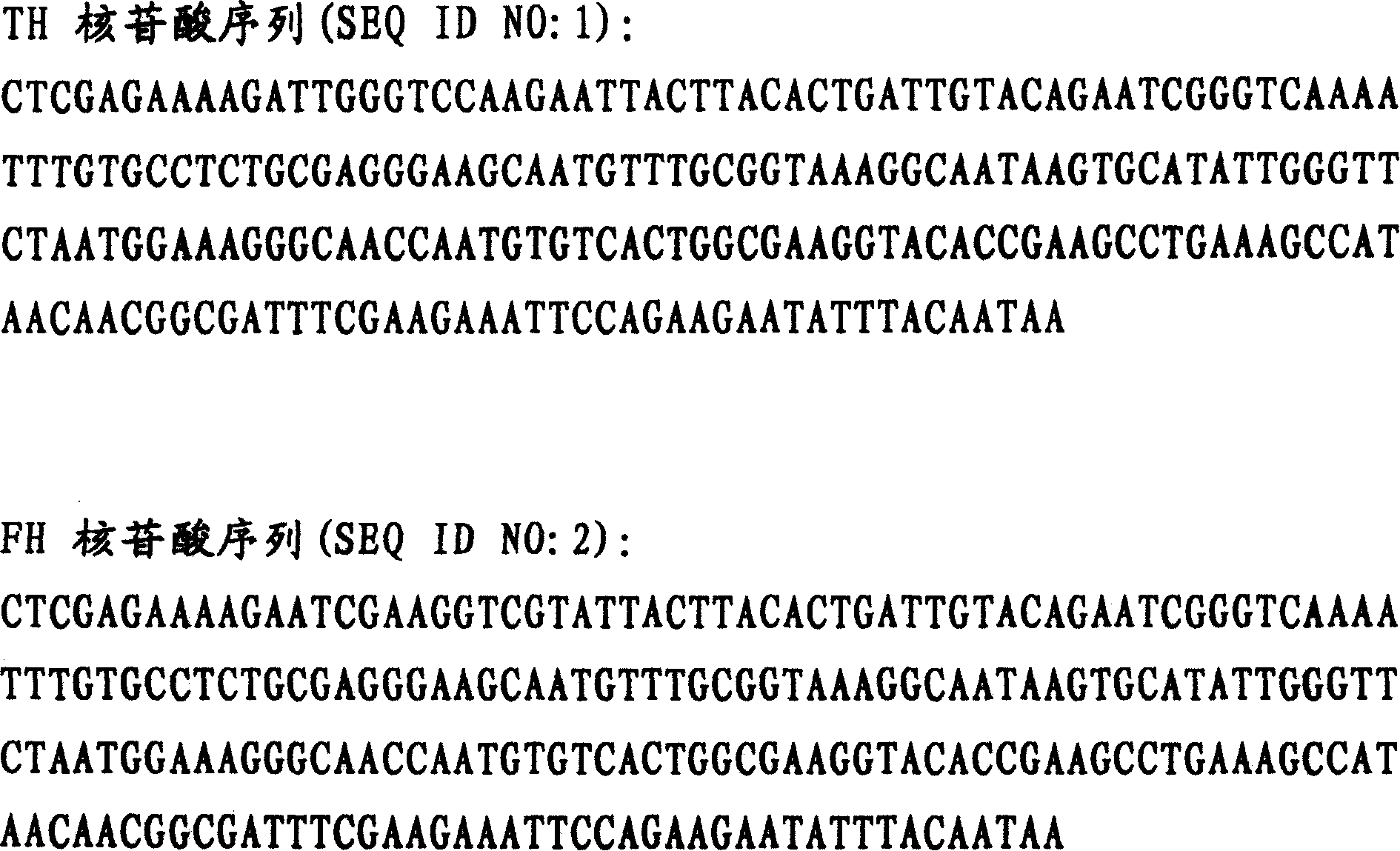
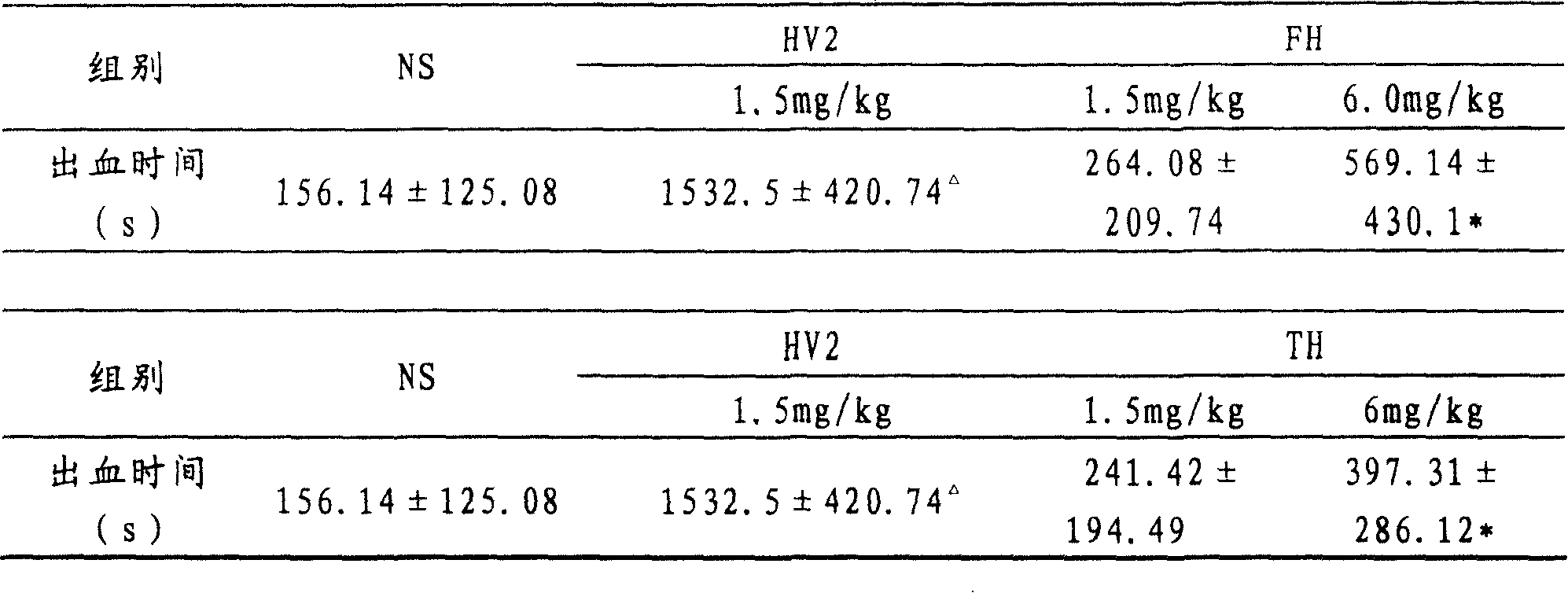
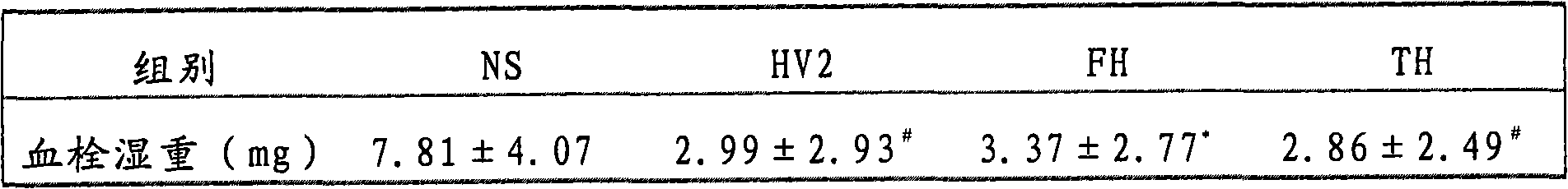
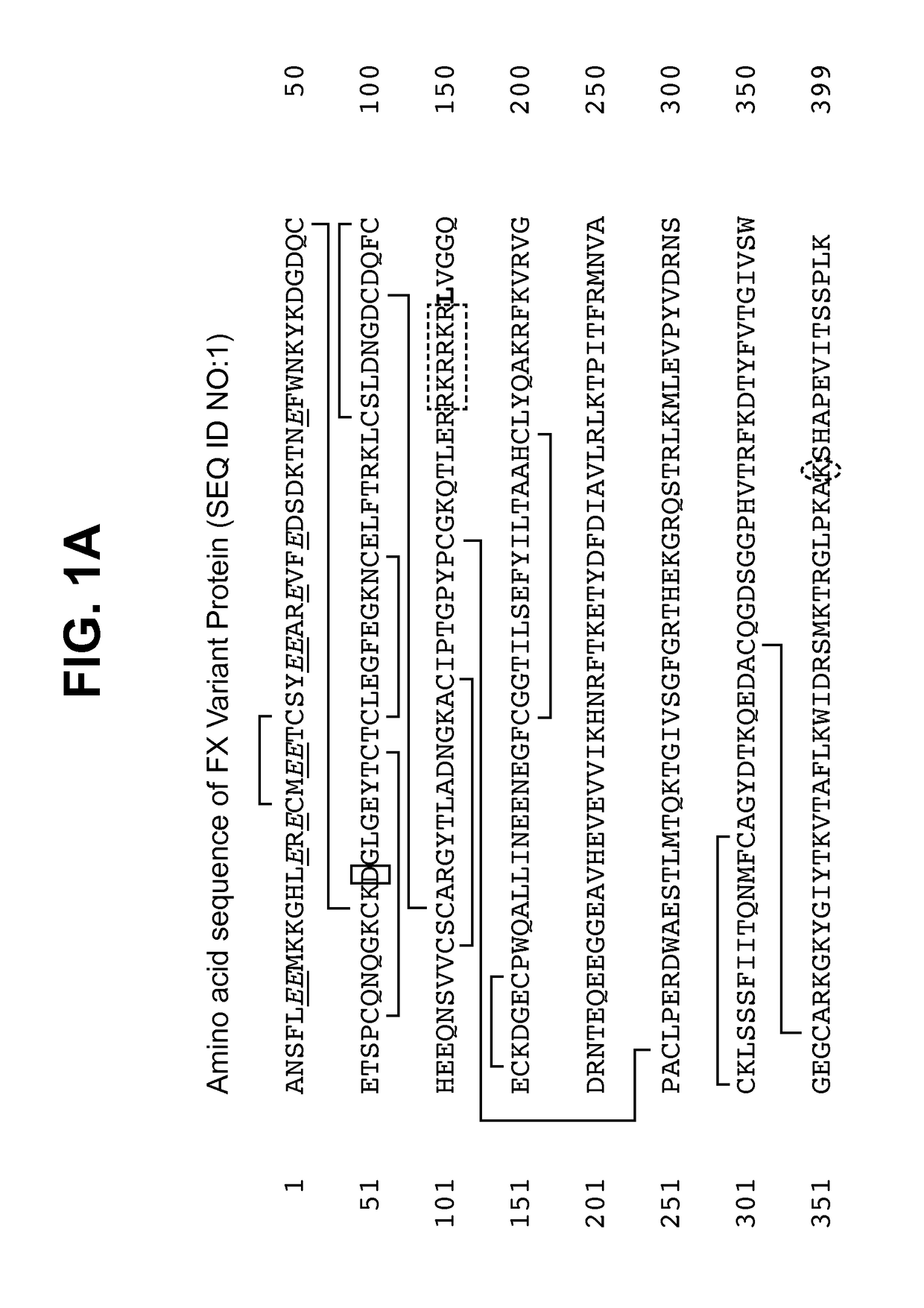
The invention belongs to the technical field of genetic engineering, relating to a target multifunctional anti-embolism fusion protein an amino acid sequence of which is as shown in SEQ ID NO.1. The invention also relates to a gene for encoding the fusion protein, a recombinant expression vector containing the gene, a transformant containing the recombinant expression vector and a method for preparing the fusion protein. The fusion protein can reasonably splice human antibacterial peptide LL-37, leech peptide Hirudin-12, Agkistrodon acutus peptide (AAP), arginine-glycin-aspartate (RGD) and human blood coagulation factor Xa identification sites, so that the functions among a target component, an antibacterial component, a thrombin resisting component and a platelet aggregation resisting component are complementary and in synergistic effect; and the fusion protein can well exert target anti-inflammatory and anticoagulation activities at a thrombus position, and simultaneously can repair a vessel endothelial cell, can commonly inhibit the formation and development of thrombus by virtue of multiple ways, and can be used for preparing a medicament for preventing and treating thrombotic diseases.
Owner:CHONGQING UNIV
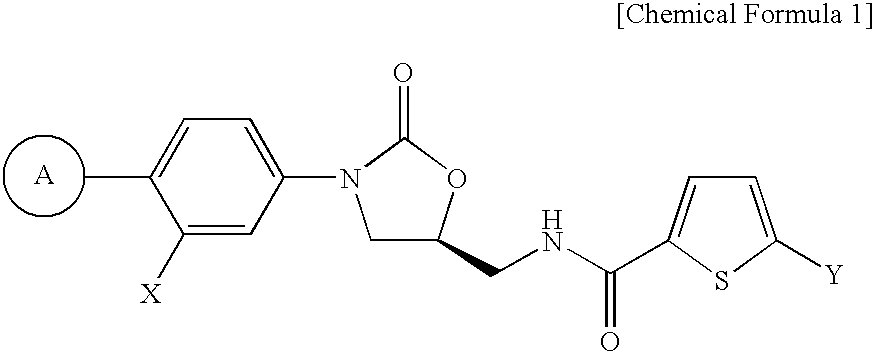
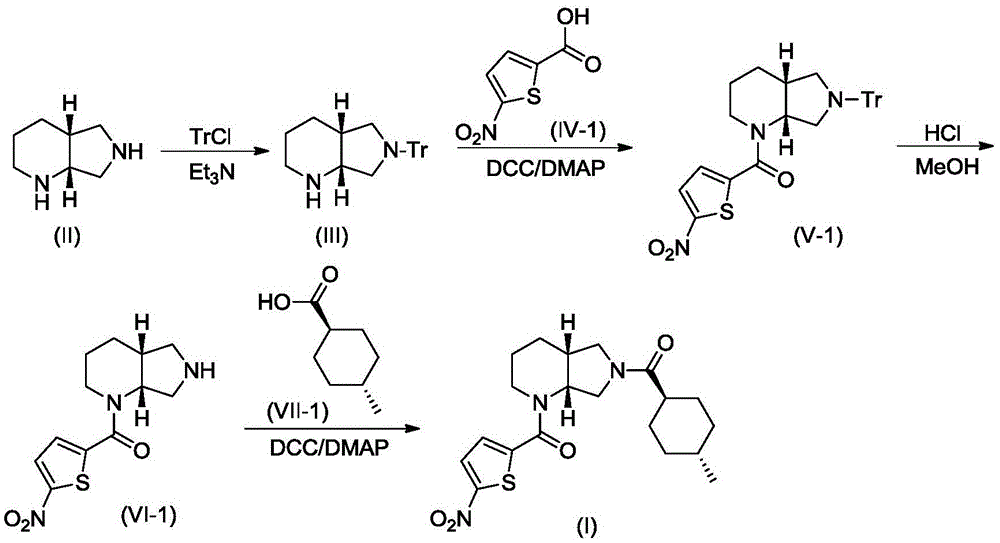
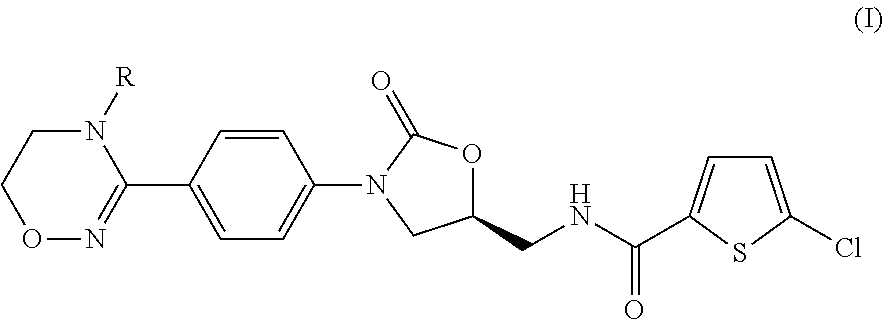
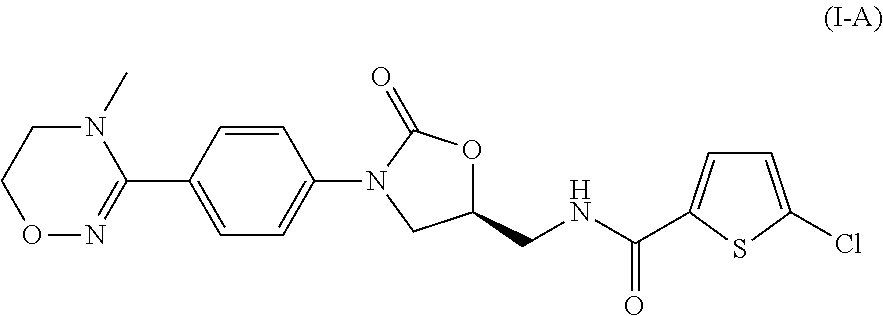
Method for preparing 5-chloro-n-(methyl)thiophen-2-carboxamide derivative and intermediate used therein
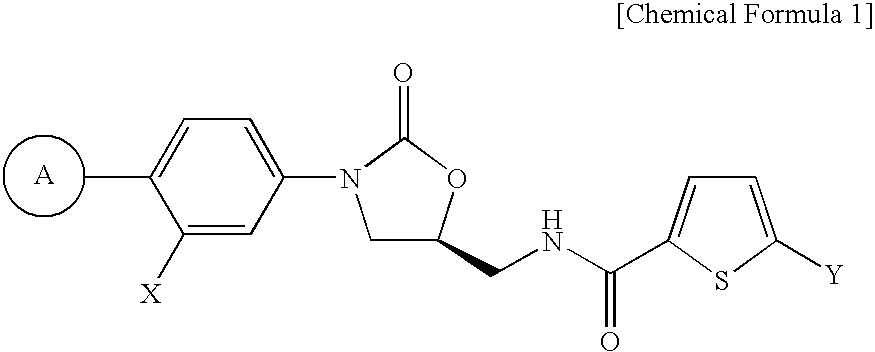
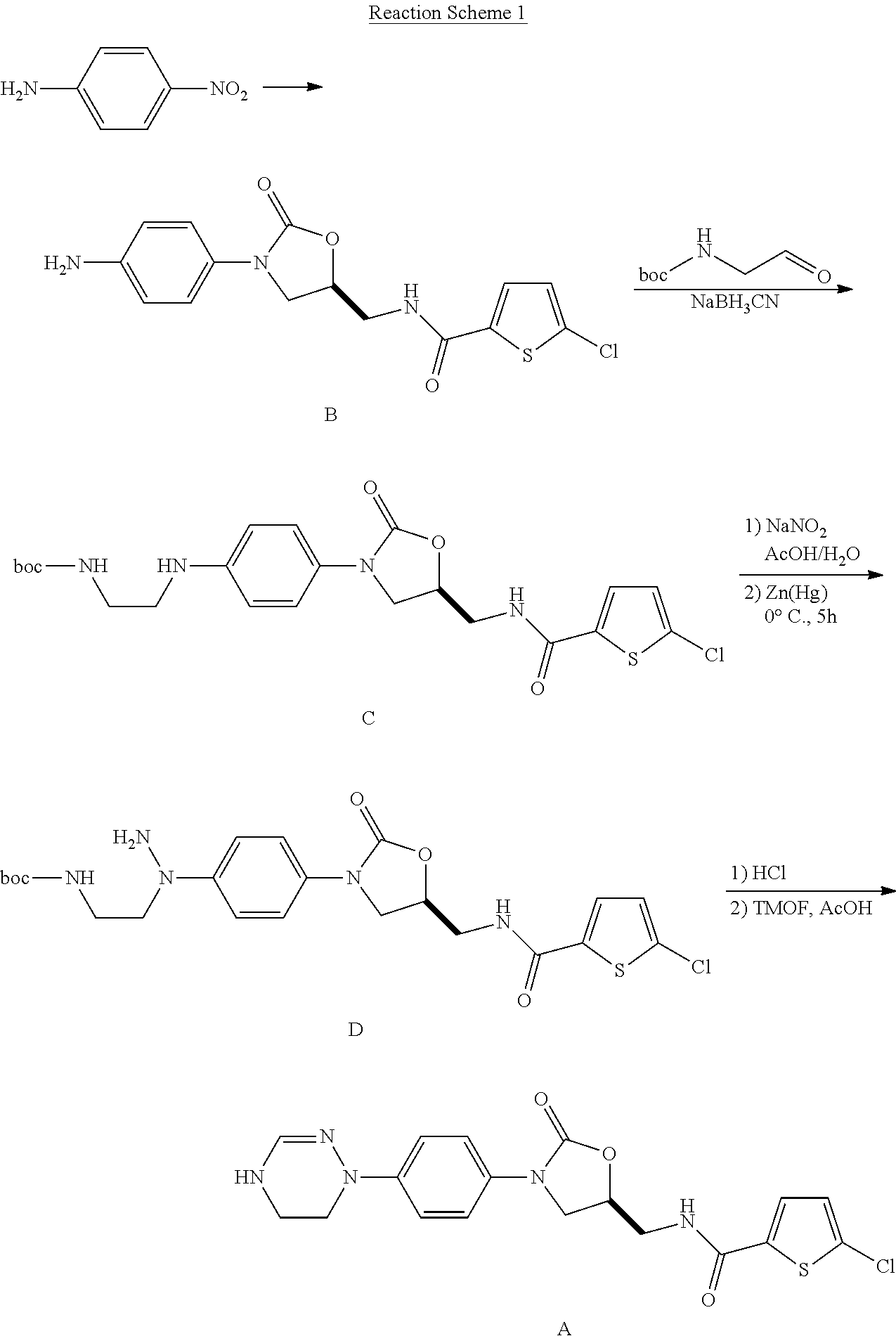
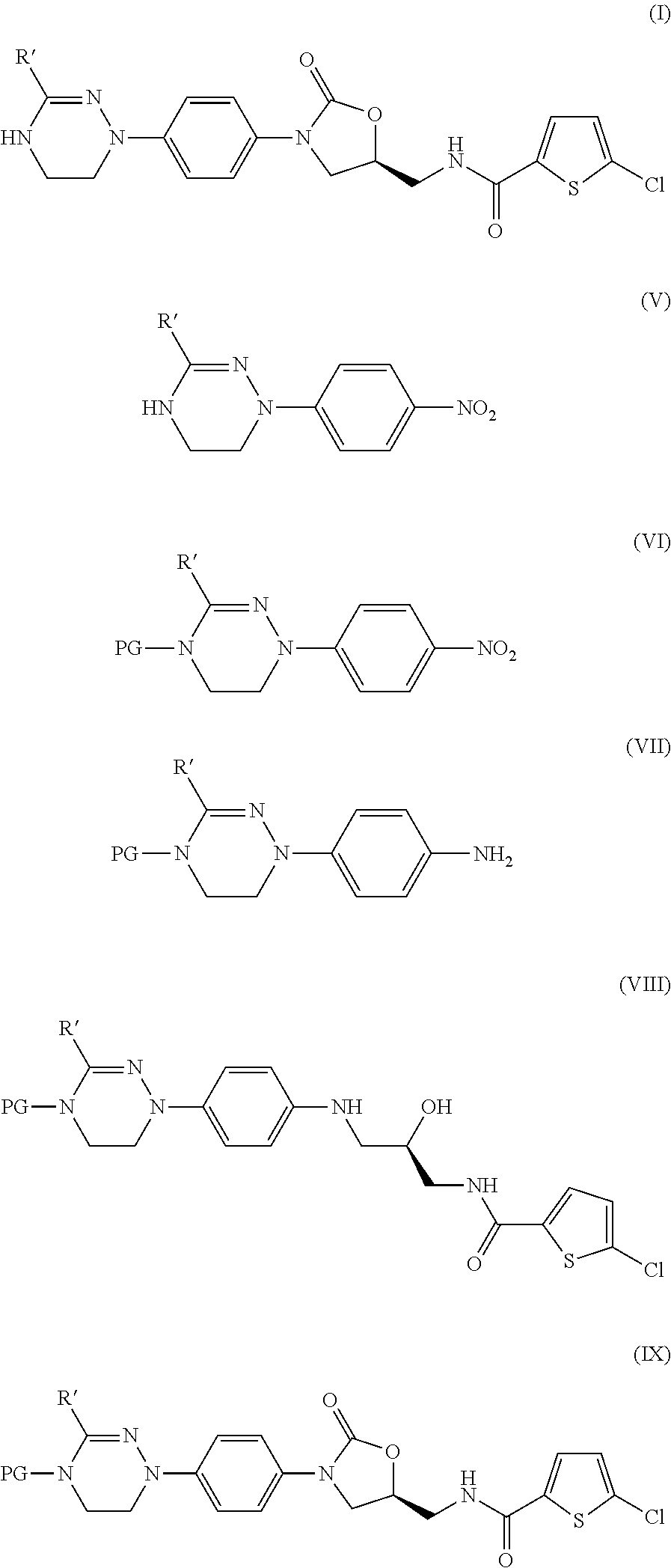
Disclosed are: a method for preparing a 5-chloro-N-({(5S)-2-oxo-3-[4-(5,6-dihydro-4H-[1,2,4]triazin-1-yl)phenyl]-1,3-oxazolidin-5-yl}-methyl)thiophene-2-carboxamide derivative, which is a inhibitor of blood coagulation factor Xa, in a high purity and yield; and a novel intermedicate used therein.
Owner:THE GREEN CROSS CORP +1
Chlorothiophene-amides as inhibitors of coagulation factors Xa and thrombin
The present invention relates to compounds of the formula I,wherein R1; R2; R3; R4; R5, R13, R16, X and M have the meanings indicated in the claims. The compounds of formula I are valuable pharmacologically active compounds. They exhibit a strong anti-thrombotic effect and are suitable, for example, for the therapy and prophylaxis of cardio-vascular disorders like thromboembolic diseases or restenoses. They are reversible inhibitors of the blood clotting enzymes factor Xa (FXa) and thrombin and can in general be applied in conditions in which an undesired activity of factor Xa and / or thrombin are present or for the cure or prevention of which an inhibition of factor Xa and thrombin are intended. The invention furthermore relates to processes for the preparation of compounds of the formula I, their use, in particular as active ingredients in pharmaceuticals, and pharmaceutical preparations comprising them.
Owner:BEIJING LIANXIN PHARMA CO LTD
Preparation of specific anticoagulation matter and its use
Production of specific anticoagulant substance and its use disclosed. The process is carried out by connecting amino acid sequence into anticoagulant substance, which is recognized and cracked by zymoplasm or blood coagulation factor Xa, or connecting anticoagulant substance with amino acid sequence as oligopeptide to obtain final product.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Chlorothiophene-isoxazoles as inhibitors of coagulation factors xa and thrombin
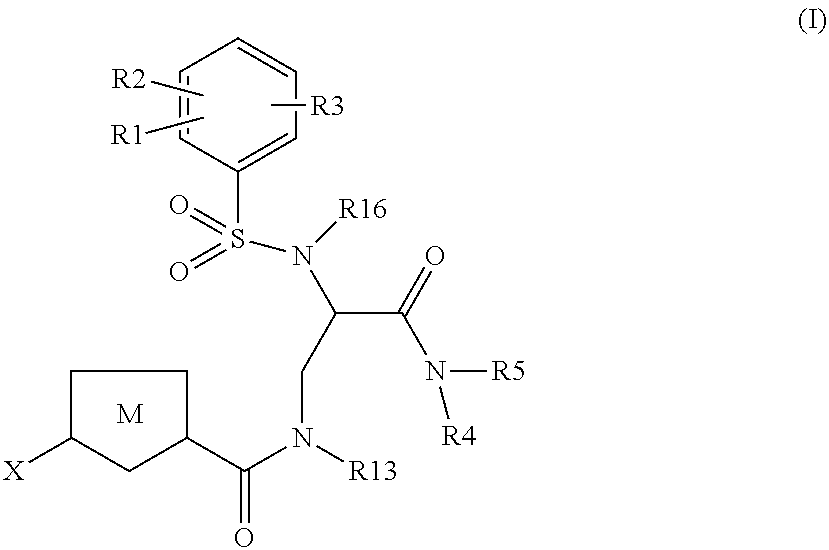
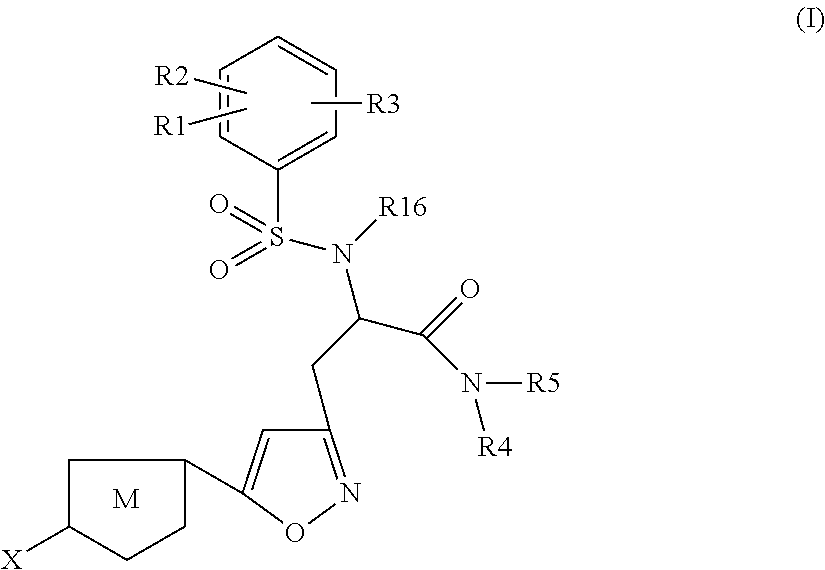
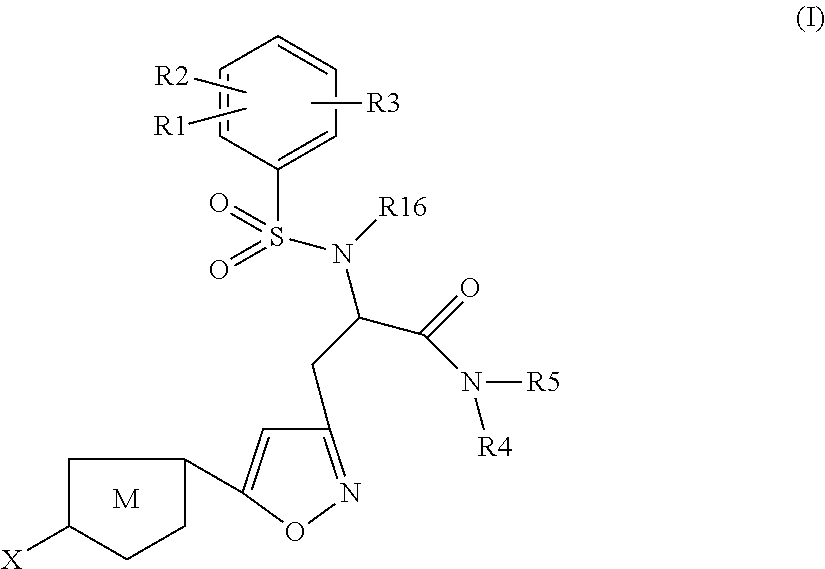
The present invention relates to compounds of the formula I,wherein R1; R2; R3; R4; R5, R16, X and M have the meanings indicated in the claims. The compounds of formula I are valuable pharmacologically active compounds. They exhibit a strong anti-thrombotic effect and are suitable, for example, for the therapy and prophylaxis of cardio-vascular disorders like thromboembolic diseases or restenoses. They are reversible inhibitors of the blood clotting enzymes factor Xa and thrombin and can in general be applied in conditions in which an undesired activity of factor Xa and / or thrombin are present or for the cure or prevention of which an inhibition of factor Xa and thrombin are intended. The invention furthermore relates to processes for the preparation of compounds of the formula I, their use, in particular as active ingredients in pharmaceuticals, and pharmaceutical preparations comprising them.
Owner:SANOFI SA
Preparation for specificity anticoagulant substance and application thereof
The invention relates to an anticoagulant protein containing an oligopeptide which is identified and cracked by thrombin or blood coagulation factor Xa; more particularly, the invention relates to a new anticoagulant substance connected by an anticoagulant substance and an amino acid sequence which can be identified and cracked by thrombin or blood coagulation factor Xa, or a new substance connected by an anticoagulant substance and other substances through taking an amino acid sequence which can be identified and cracked by thrombin or blood coagulation factor Xa as oligopeptide, as well as medicine science applications of these new anticoagulant substances.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
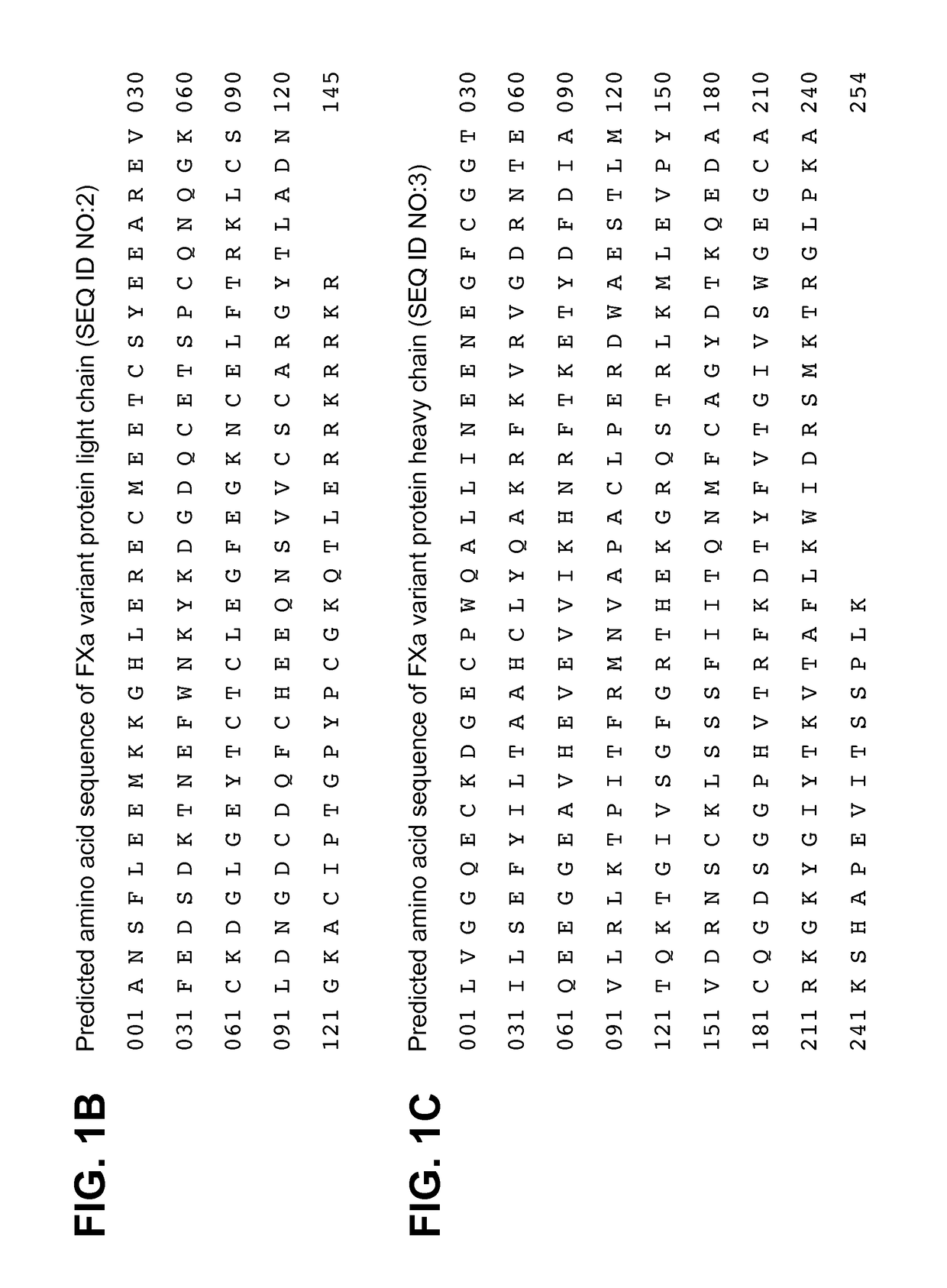
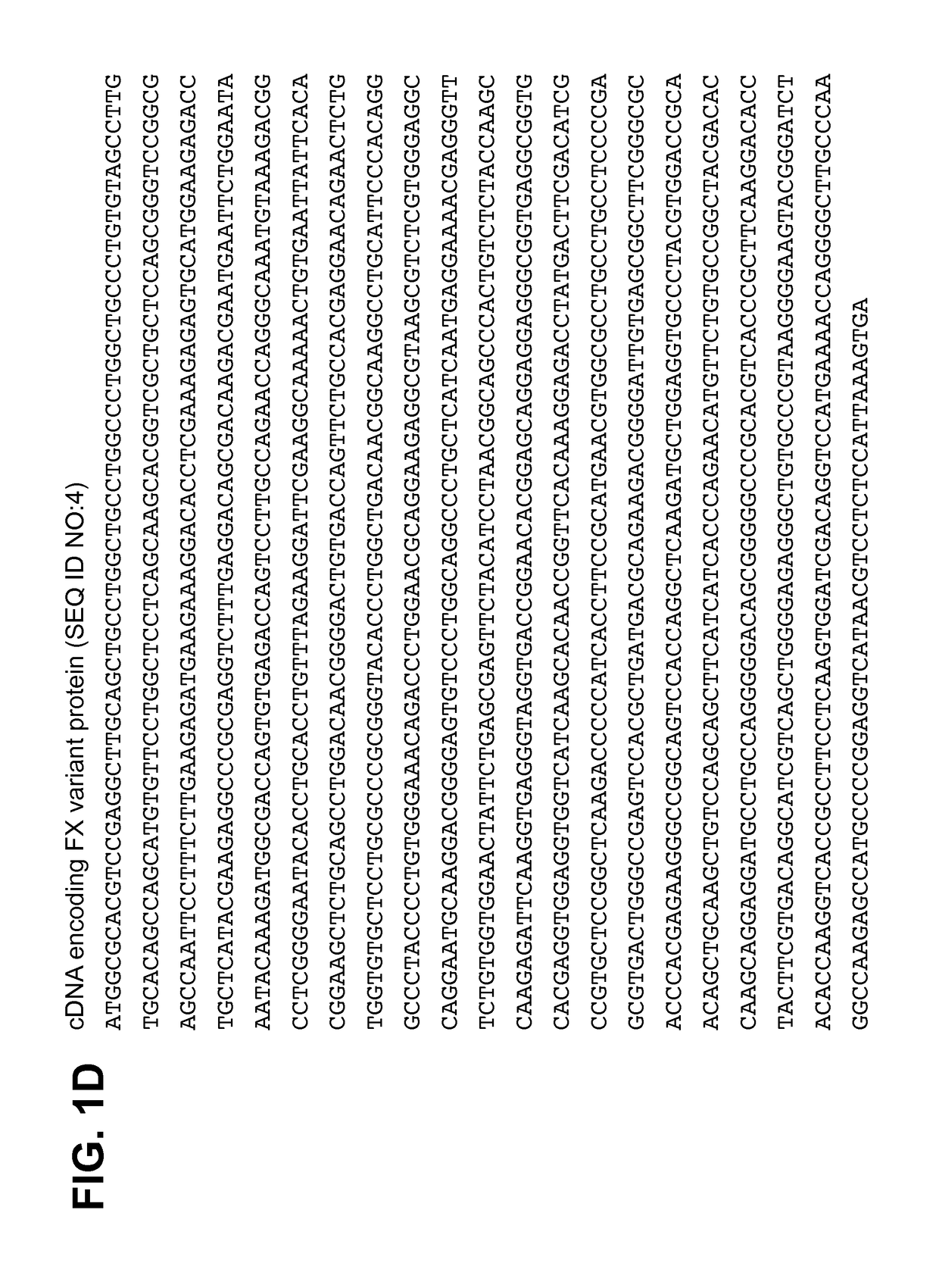
FXa variant compositions
ActiveUS9757434B2Prevent uncontrolled bleedingBioreactor/fermenter combinationsBiological substance pretreatmentsPost translationalCoagulation Factor Xa
Compositions are provided comprising recombinant variants of the human clotting Factor Xa. Such compositions include a wide variety of isoforms and post-translational modifications of FXa and are useful for treating subjects in need of hemostasis.
Owner:PFIZER INC
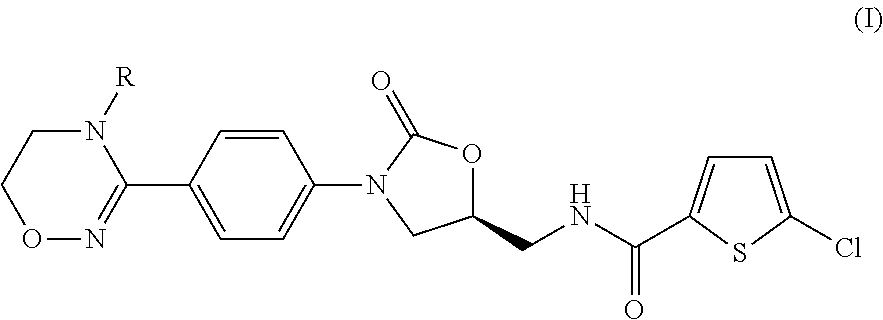
Method for preparing (S)-5-chloro-N-((3-(4-(5,6-dihydro-4H-1,2,4-oxadiazin-3-yl)phenyl)-2-oxooxazolidin-5-yl)methyl)thiophene-2-carboxamide derivatives
ActiveUS8637667B2Organic active ingredientsOrganic chemistryCoagulation Factor XaCombinatorial chemistry
Provided is a method for preparing (S)-5-chloro-N-((3-(4-(5,6-dihydro-4H-1,2,4-oxadiazin-3-yl)phenyl)-2-oxooxazolidin-5-yl)methyl)thiophene-2-carboxamide derivatives of Formula (I) which are useful as blood coagulation factor Xa inhibitors, and said method using 1-fluoro-4-nitrobenzen as a starting material. According to the method of the present invention, (S)-5-chloro-N-((3-(4-(5,6-dihydro-4H-1,2,4-oxadiazin-3-yl)phenyl)-2-oxooxazolidin-5-yl)methyl)thiophene-2-carboxamide derivatives of Formula (I) which are useful as blood coagulation factor Xa inhibitors can be prepared in a high purity and a high yield.
Owner:LEGOCHEM BIOSCIENCES LTD
Compositions comprising heterogeneous populations of recombinant human clotting factor xa proteins
ActiveUS20150182604A1Prevent uncontrolled bleedingPeptide/protein ingredientsHydrolasesPost translationalProtein composition
Compositions are provided comprising recombinant variants of the human clotting Factor Xa. Such compositions include a wide variety of isoforms and post-translational modifications of FXa and are useful for treating subjects in need of hemostasis.
Owner:PFIZER INC
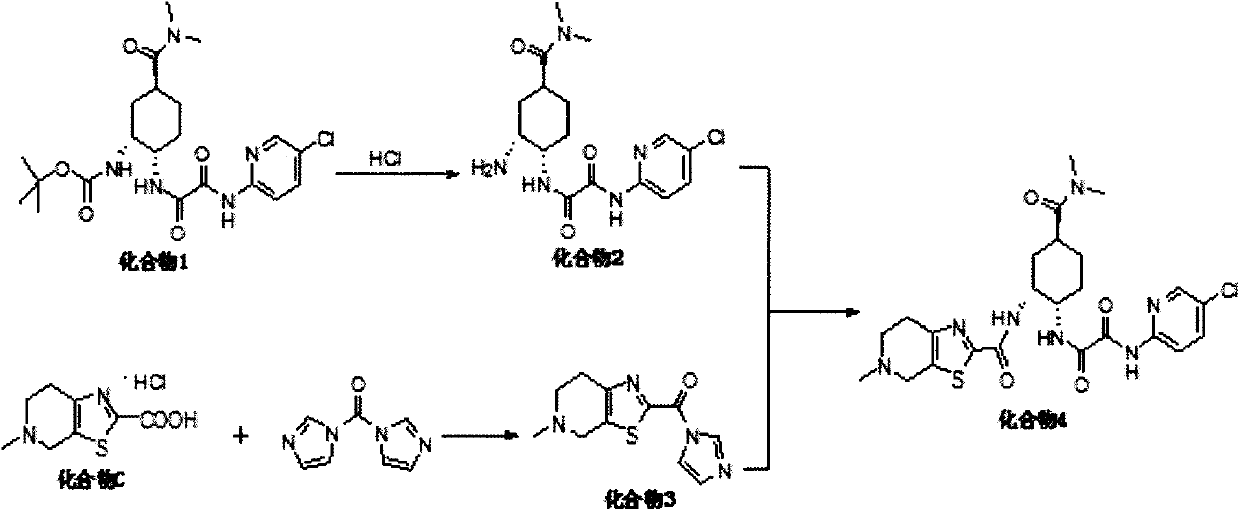
A kind of preparation method of antithrombotic drug
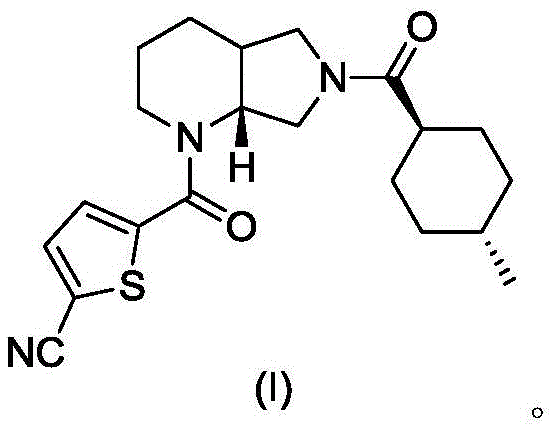
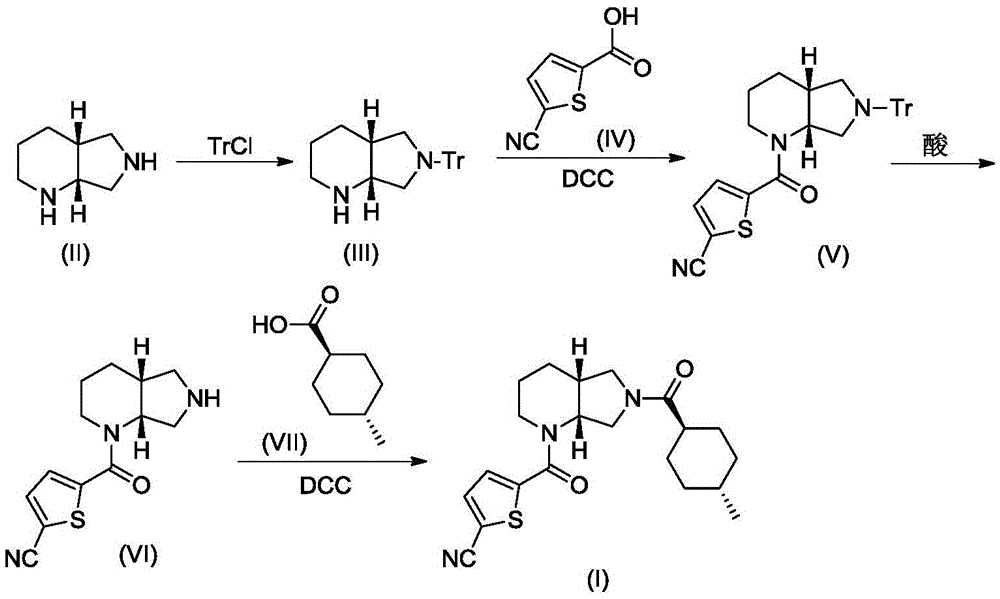
ActiveCN107641131BReduce generationEase of industrial productionOrganic chemistryBlood disorderCoagulation Factor XaCombinatorial chemistry
The invention relates to a preparation method of an antithrombotic drug, belongs to the technical field of medicinal chemistry and in particular relates to a preparation method of a direct coagulationfactor Xa inhibitor, which is shown as the following reaction. Compared with the existing process, the preparation method has the advantages of mild reaction condition in each step, high yield, simplicity and convenience operation, stable process and suitability for large-scale industrial production.
Owner:JIANGSU VCARE PHARMATECH
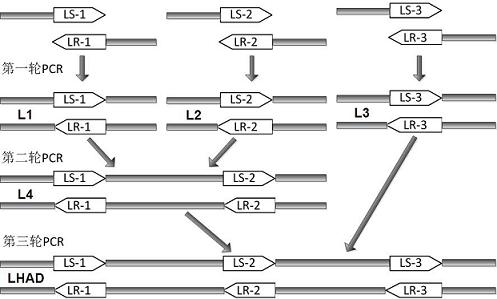
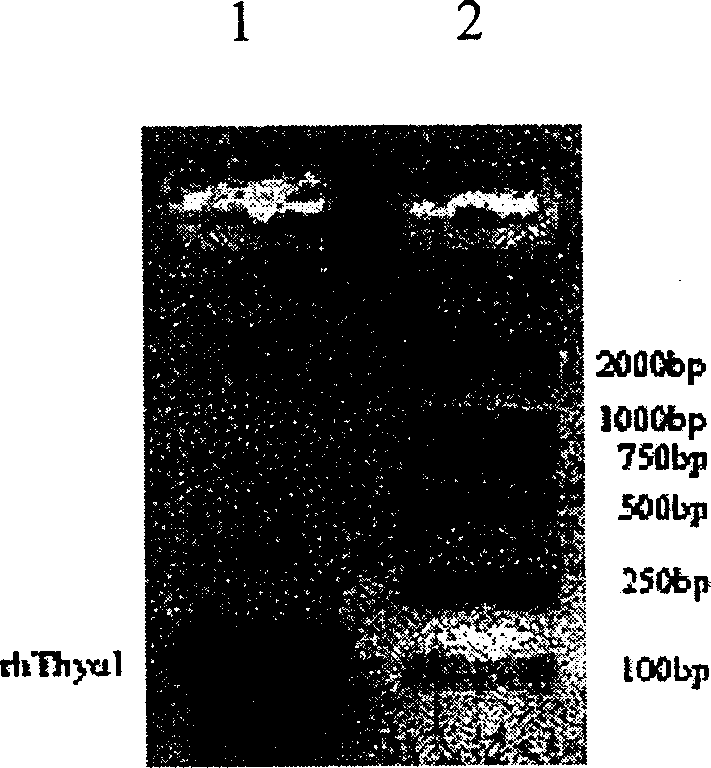
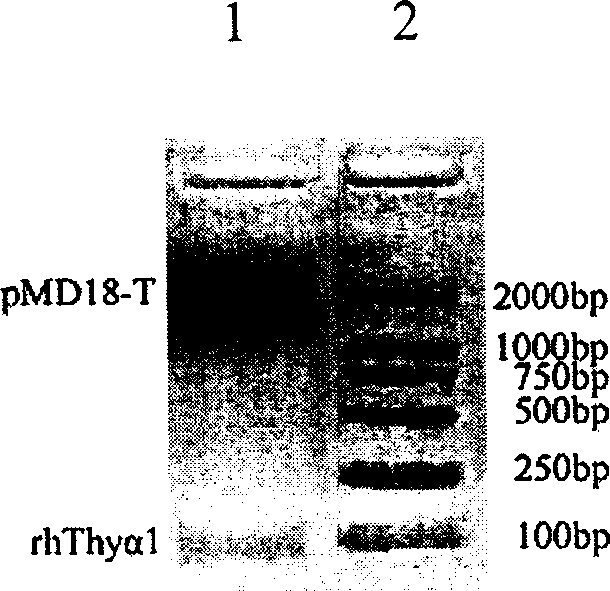
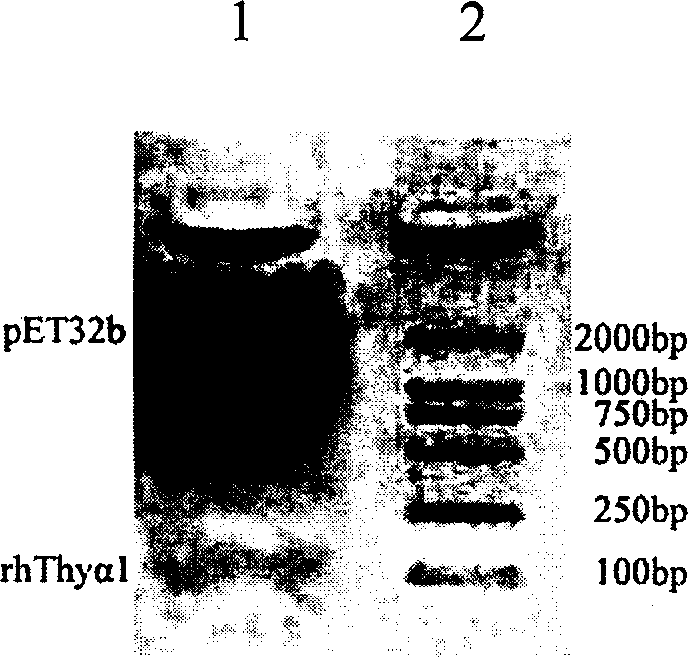
Expression purification in colibacillus and activity identification method of recombinant thymulin alpha-1
InactiveCN1706946AReduce manufacturing costShort cycleGenetic engineeringFermentationEscherichia coliChemical synthesis
The present invention relates to medicine biological engineering technology, and is expression and purification in colibacillus and activity identification method of recombinant human thymulin alpha-1. Through PCR, chemically synthesized recombinant human thymulin alpha-1 gene is amplified and cloned to pMD18-T vector, and through KpnI / SacI restriction and connection, the thymulin gene is inserted to the downstream of thioredoxin in expression vector pET32b. Recombinant plasmid is converted to expression host BL21(DE3), and through IPTG induction, Zn-Sepharose affinity chromatographic purification and renaturation, thioredoxin-thymulin alpha-1 fusion protein in the amount of 35 mg / L may be obtained from the bacteria liquid. Through further blood coagulation factor Xa restriction, Zn-Sepharose affinity chromatography and reverse efficient liquid chromatographic purification, recombinant human thymulin is obtained.
Owner:NANJING UNIV
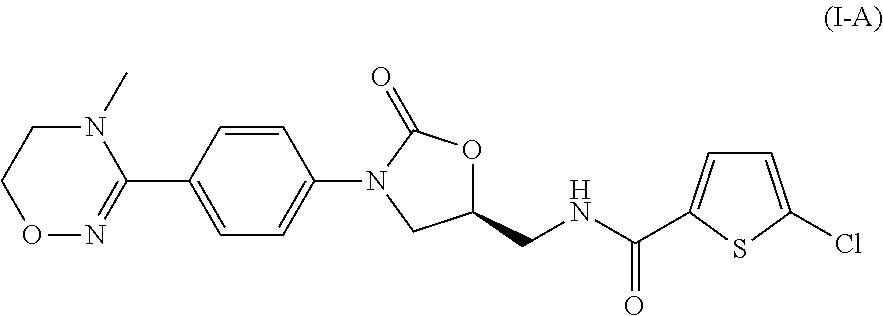
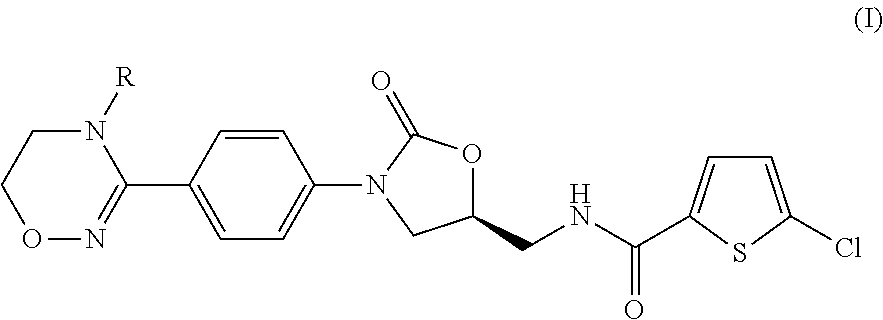
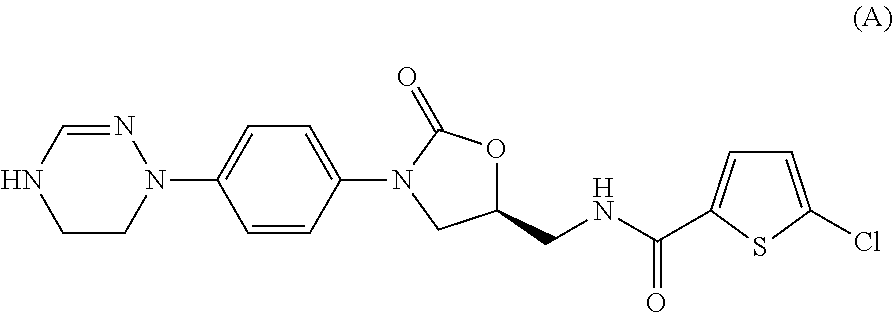
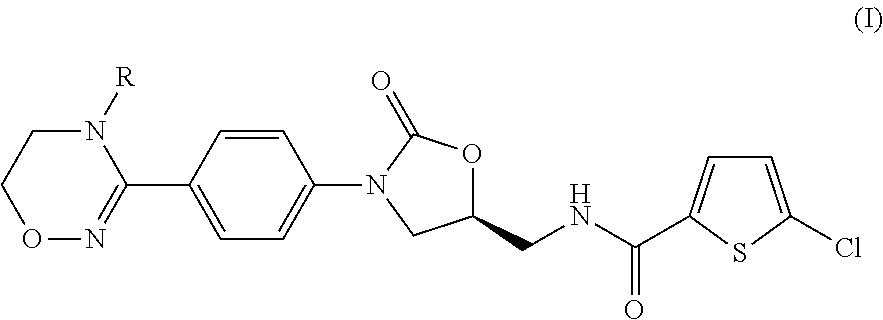
Method for preparing 5-chloro-N-({(5S)-2-oxo-3-[4-(5,6-dihydro-4H-[1,2,4]triazin-1-yl)phenyl]-1,3-oxazolidin-5-yl}methyl) derivative and intermediate used therein
Disclosed are: a method for preparing a 5-chloro-N-({(5S)-2-oxo-3-[4-(5,6-dihydro-4H-[1,2,4]triazin-1-yl)phenyl]-1,3-oxazolidin-5-yl}-methyl)thiophene-2-carboxamide derivative, which is a inhibitor of blood coagulation factor Xa, in a high purity and yield; and a novel intermedicate used therein.
Owner:THE GREEN CROSS CORP +1
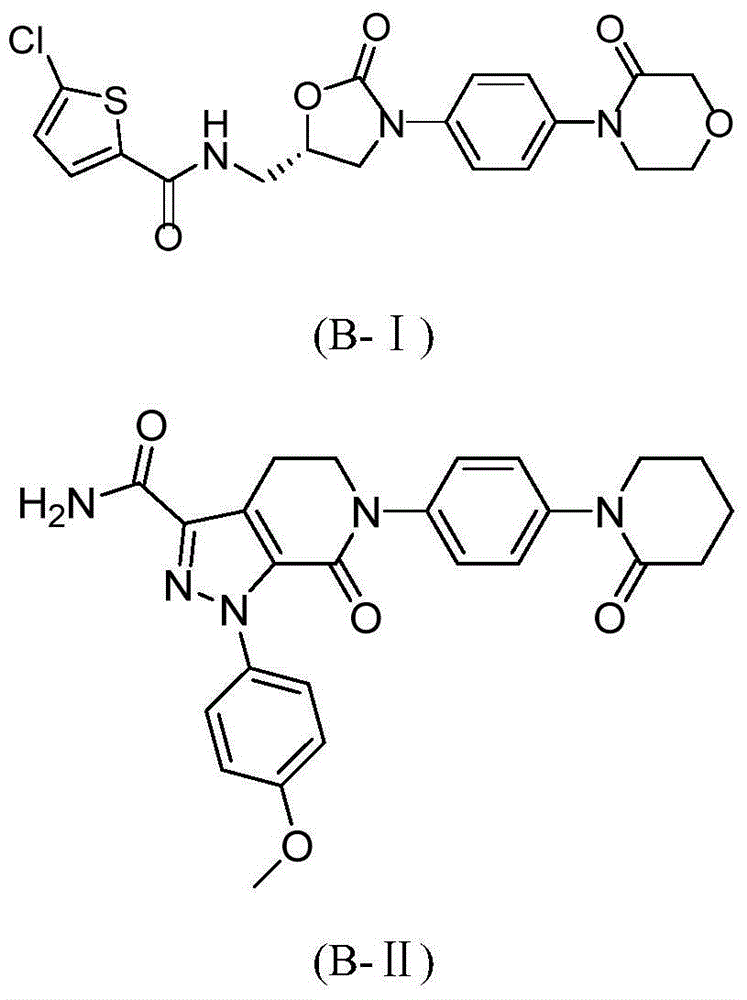
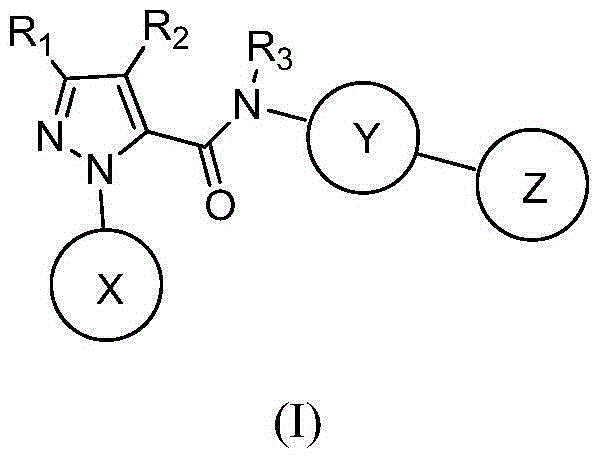
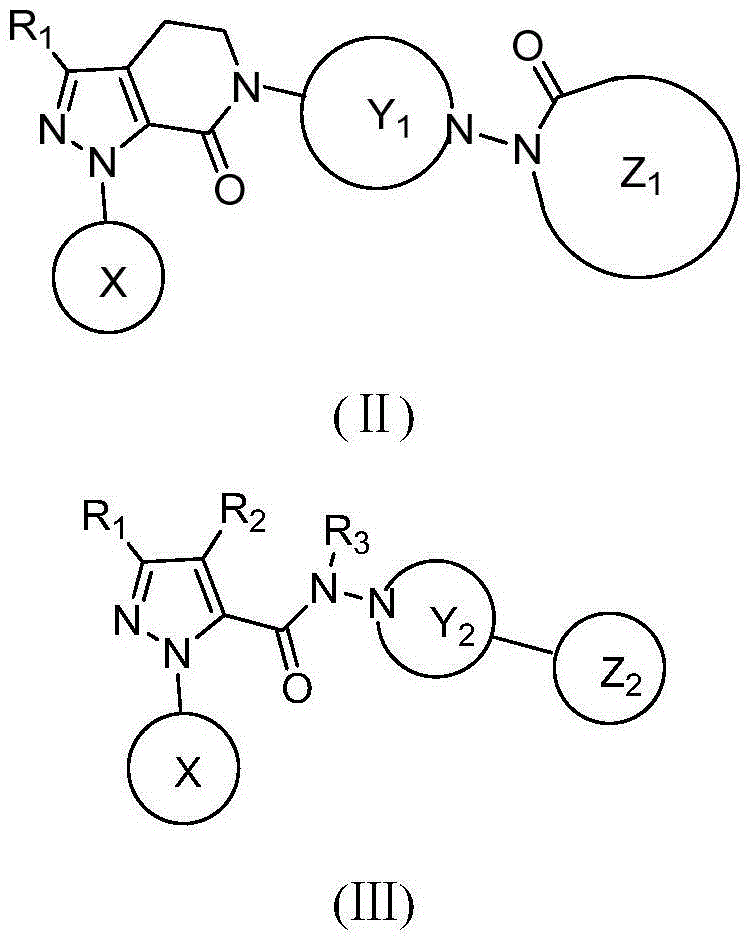
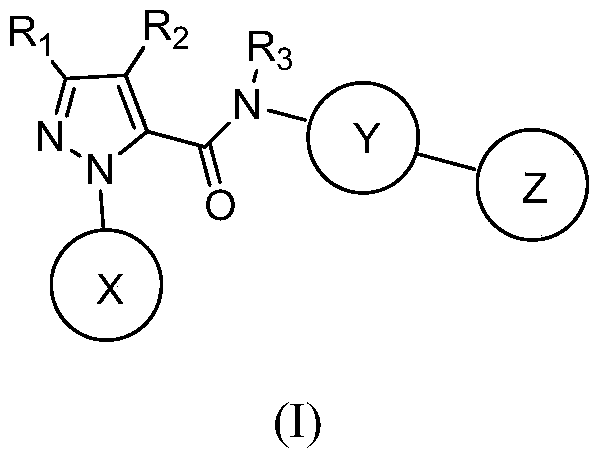
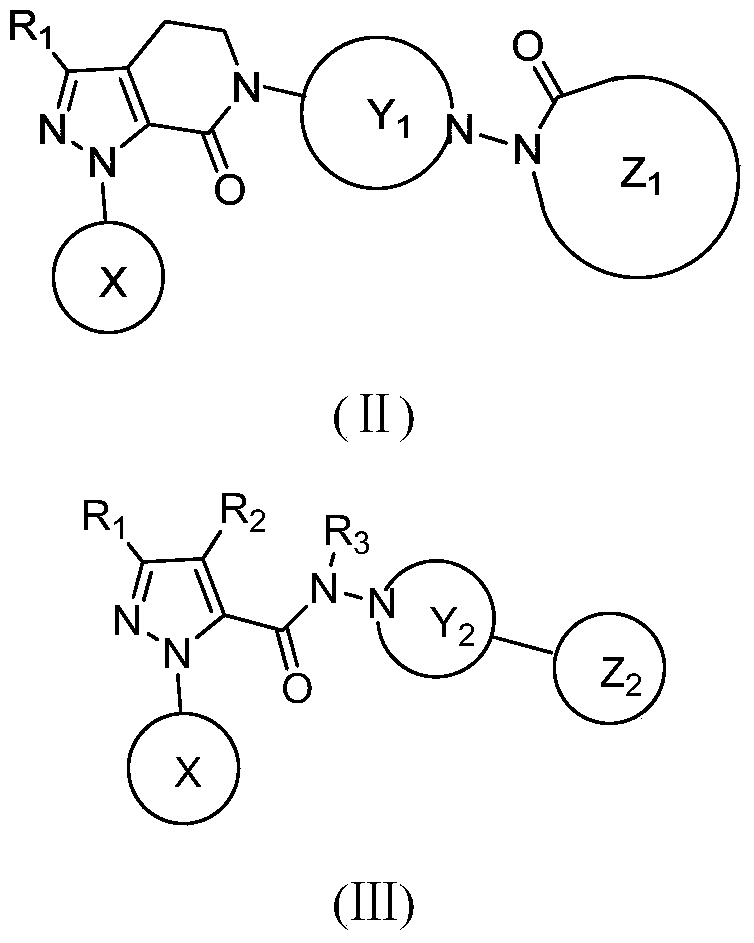
Hydrazide compound as blood coagulation factor Xa inhibitor
ActiveCN105085515AGood water solubilityGood curative effectOrganic active ingredientsOrganic chemistryDiseaseBenzene
The invention relates to a new compound represented by formula (I) or a pharmaceutically acceptable salt thereof. In the formula (I), X is selected from a 3-9-membered carbon ring or a benzo ring thereof and a 4-10-membered heterocyclic ring or a benzo ring thereof; Y and Z are respectively independently selected from 4-9-membered saturated heterocyclic rings; and R1-3 are respectively independently selected from H, F, Cl, Br, I, CN, OH, SH, NH2, CHO and COOH, or are selected from R01 substituted C1-10 alkyl or heteroalkyl groups, C3-C10 cycloalkyl or heterocycloalkyl groups, and C3-10 cycloalkyl or heterocycloalkyl group substituted C1-10 alkyl or heteroalkyl groups. The compound can be used as an anticoagulant for treating and preventing thrombus abnormity diseases. The strong blood coagulation factor Xa inhibitor is provided to meet practical demands of selectivity and the strong inhibitor of the blood coagulation factor Xa.
Owner:NORTH CHINA PHARMA COMPANY
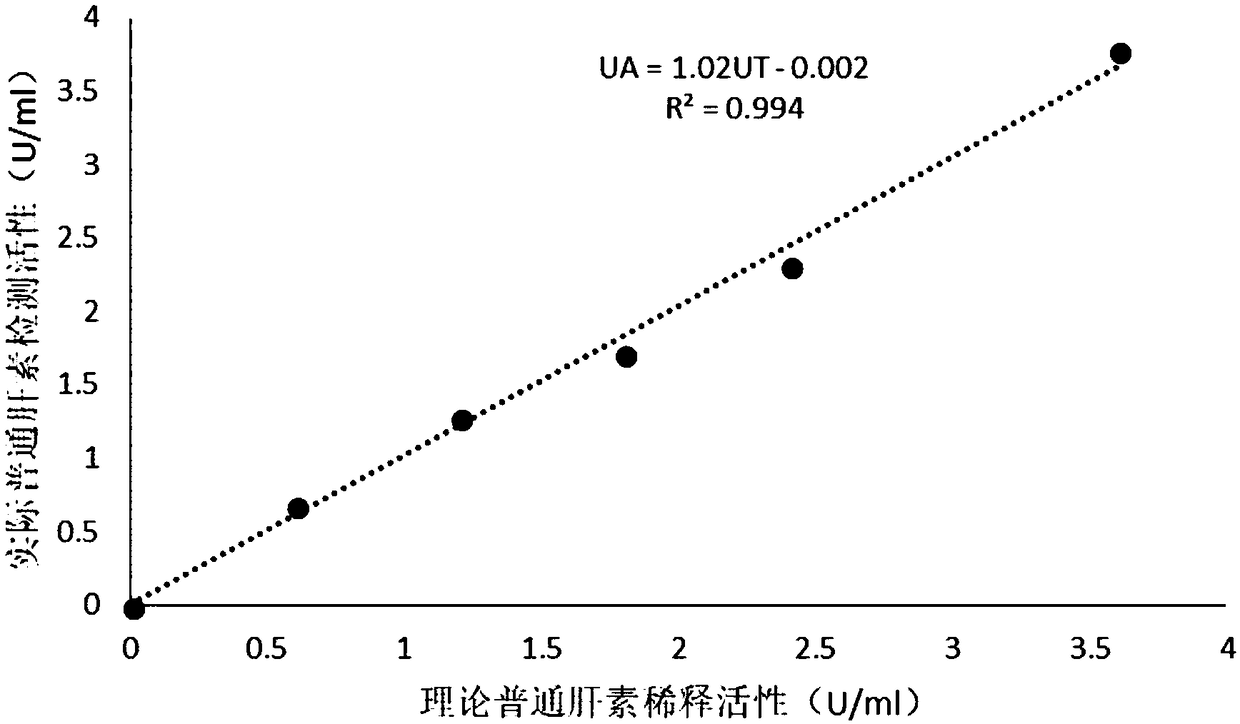
Thromboelastometry heparin quantitative detection kit and preparation method thereof
ActiveCN108982865BQuantitative detection is convenient and accurateEasy to detectBiological material analysisBiological testingCoagulation Factor XaThrombus
A kit for quantitative detection of heparin by thromboelastography, comprising a buffer, blood coagulation factor Xa, rabbit brain fat coagulation, blood coagulation factor activator, support agent, biological preservative; the preparation method includes: preparing buffer solution, blood coagulation factor Xa stock solution, Blood coagulation factor activator stock solution, biological antiseptic solution, rabbit brain lipid coagulation stock solution; take support agent and add buffer solution to prepare support solution; add coagulation factor Xa, coagulation factor activator, rabbit brain fat coagulation stock solution according to kit specifications In the support solution, add biological preservative solution after mixing, and then add buffer solution to the specified amount, mix and freeze-dry. The invention can quantitatively detect the heparin in the sample by the thromboelastography detection method, the correlation is good in the linear range, meets the detection limit standard of heparin, and the coefficient of variation is not more than 10%. There is also no requirement for the integrity of the protein in the waterfall, which simplifies the inspection process and is convenient for doctors and patients.
Owner:上海原科实业发展有限公司
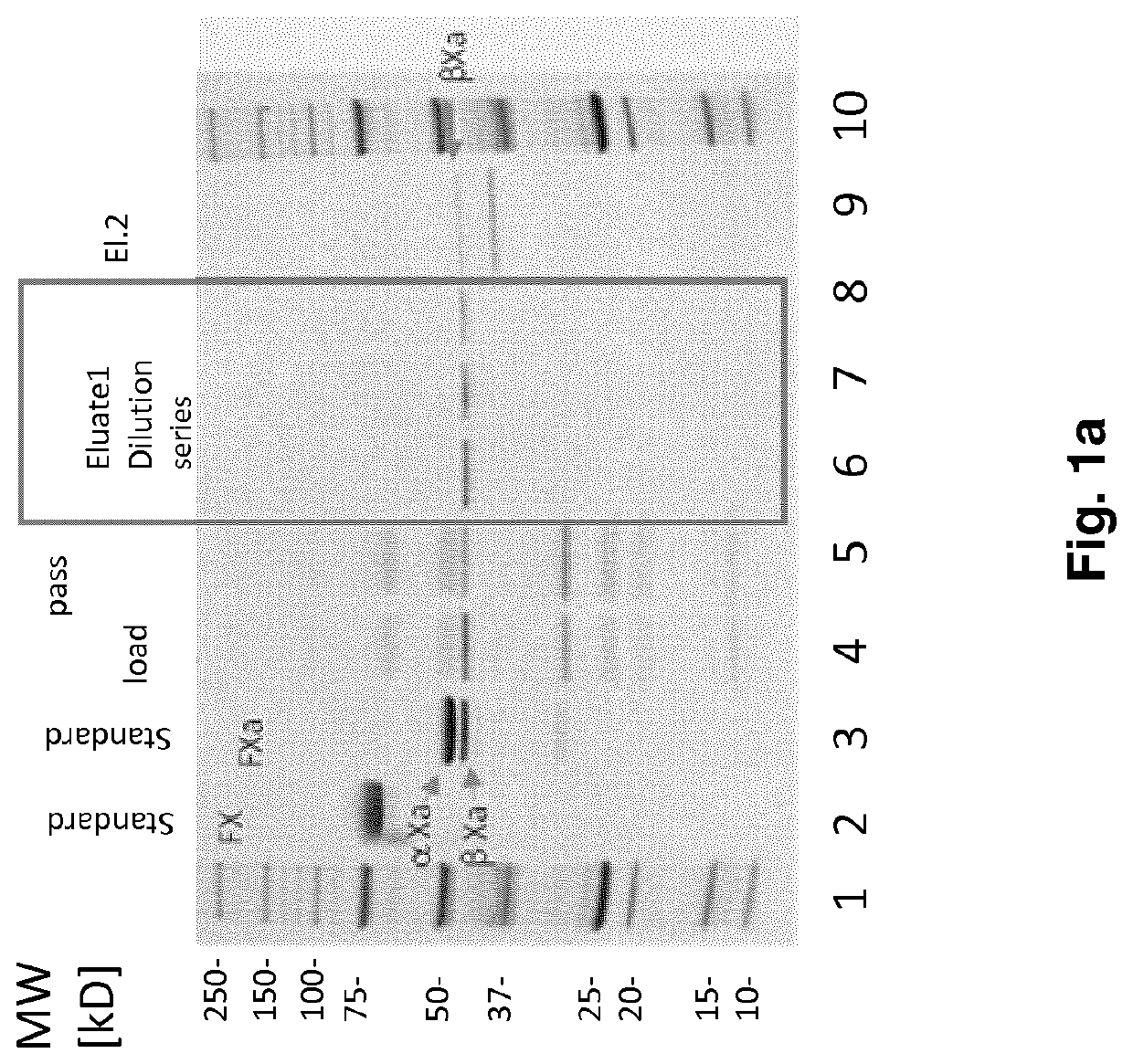
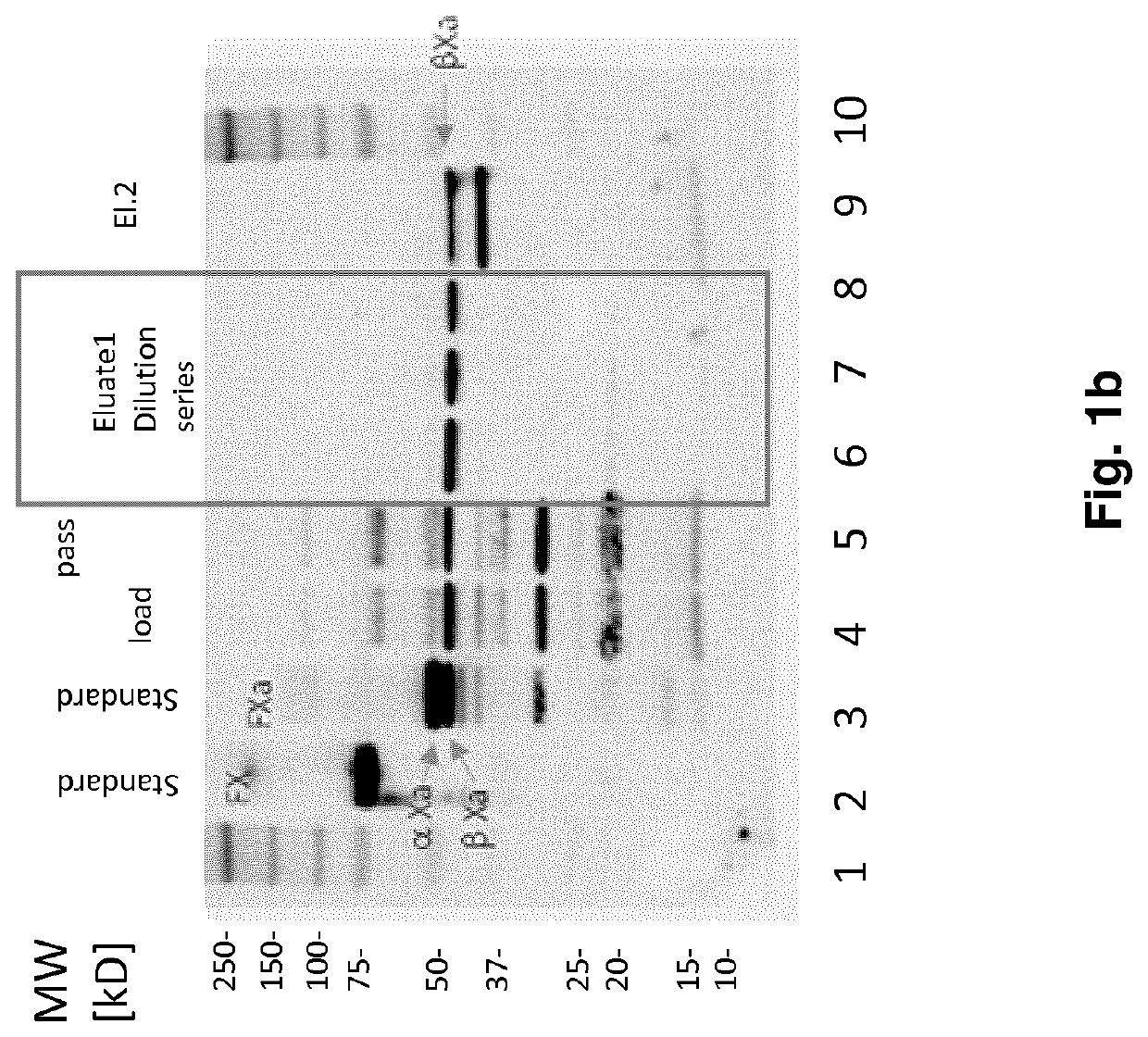
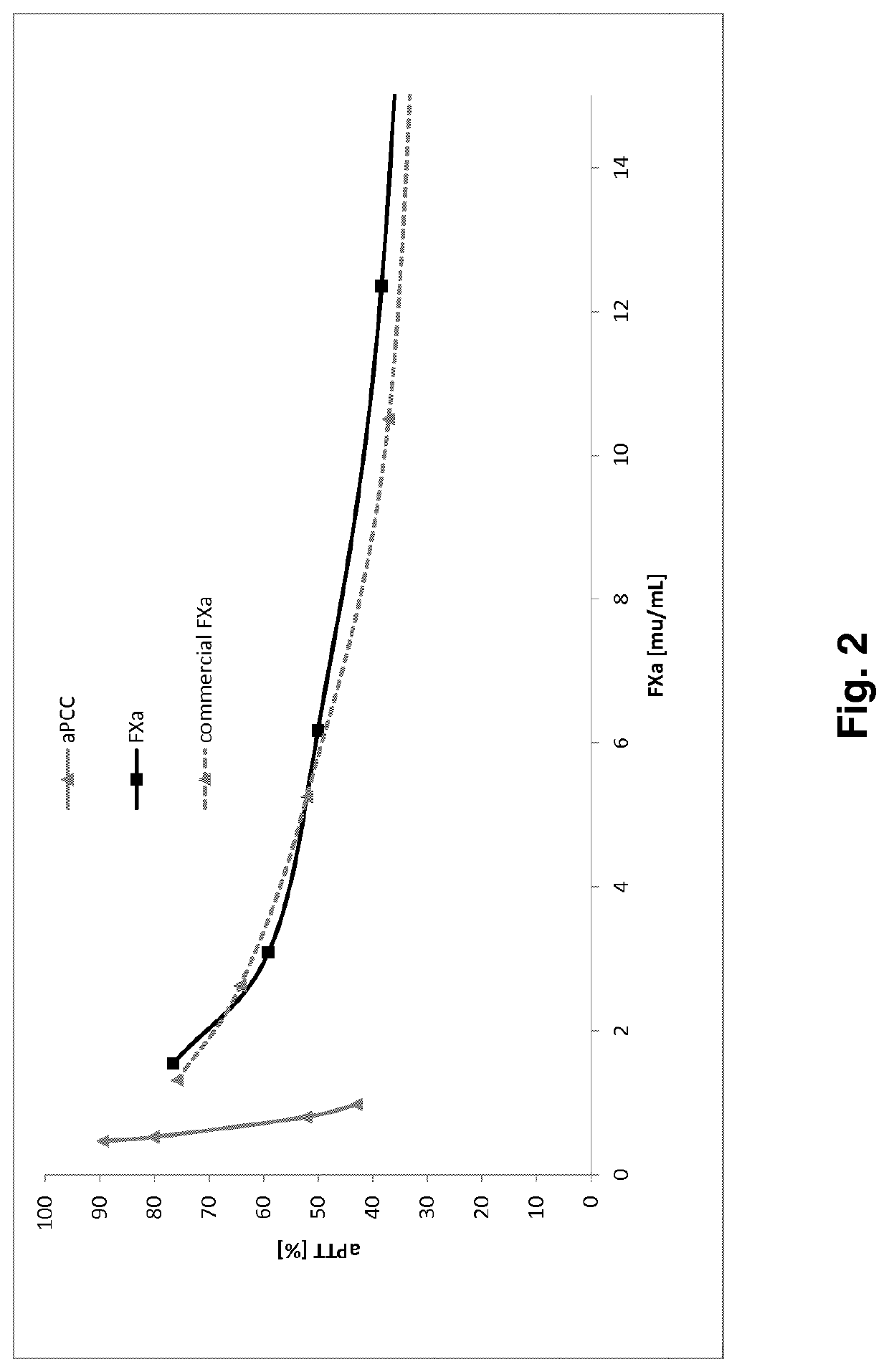
FX ACTIVATION PROCESS AND USE THEREOF IN PREPARATION OF FXa COMPOSITION
PendingCN112423782APeptide/protein ingredientsPeptide preparation methodsProtein activationCoagulation Factor Xa
The invention relates to a high purity coagulation factor Xa (FXa or activated coagulation factor X) preparation and an activation and purification process to obtain the FXa of high purity and high degree of activation without the addition of proteinaceous activators during manufacturing.
Owner:OCTAPHARMA
Polypeptide for inhibiting activity of bacillus subtilis transglutaminase (BTG) and its screening method and use
ActiveCN103060283AInhibitory activityRestore activityTransferasesMicroorganism based processesCoagulation Factor XaCytotoxicity
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
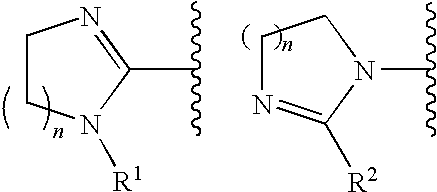
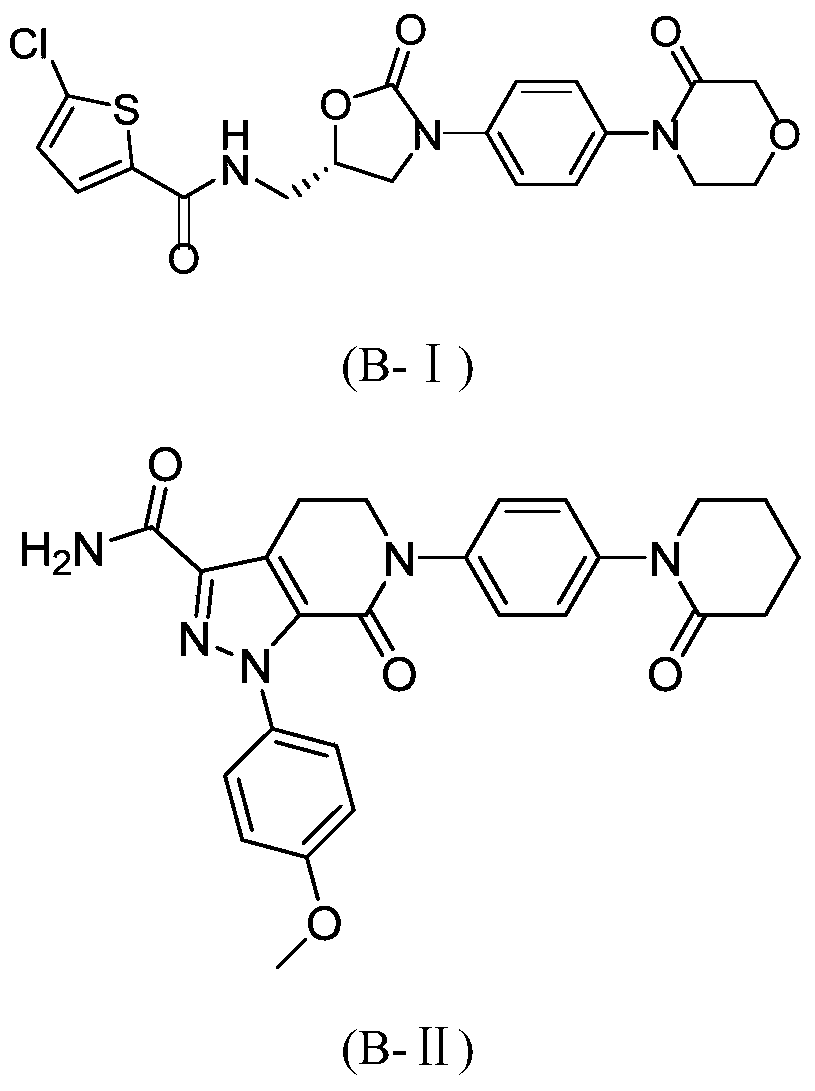
Fxa inhibitors with cyclic amidines as p4 subunit, processes for their preparations, and pharmaceutical compositions and derivatives thereof
The present invention relates to novel oxazolidinone derivatives with cyclic amidines, and prodrugs, hydrates, solvates, isomers and pharmaceutically acceptable salts thereof, and processes for preparing the same, and pharmaceutical compositions comprising the same. The oxazolidinone derivatives with cyclic amidines, and prodrugs, hydrates, solvates, isomers and pharmaceutically acceptable salts thereof can be usefully employed as an anticoagulant for treating thromboembolism and tumors via inhibition of coagulation factor Xa.
Owner:LEGOCHEM BIOSCIENCES LTD
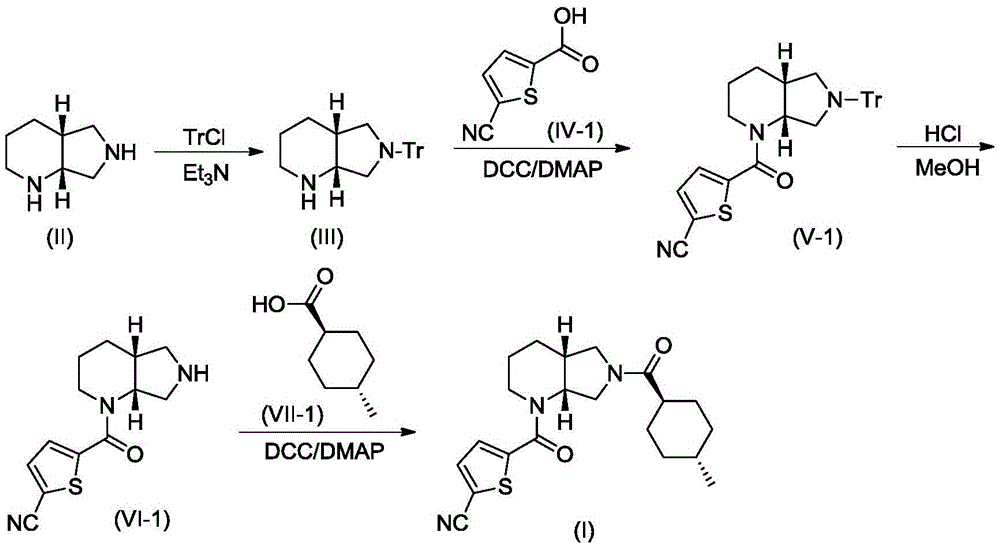
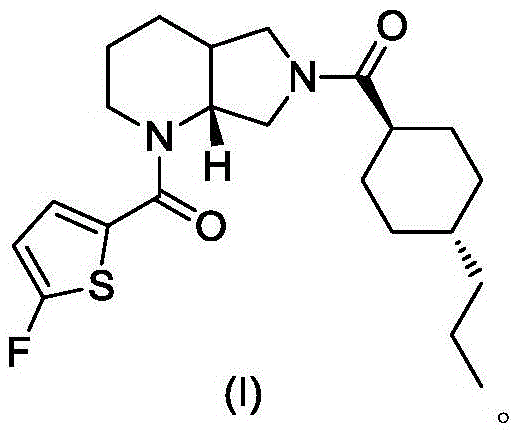
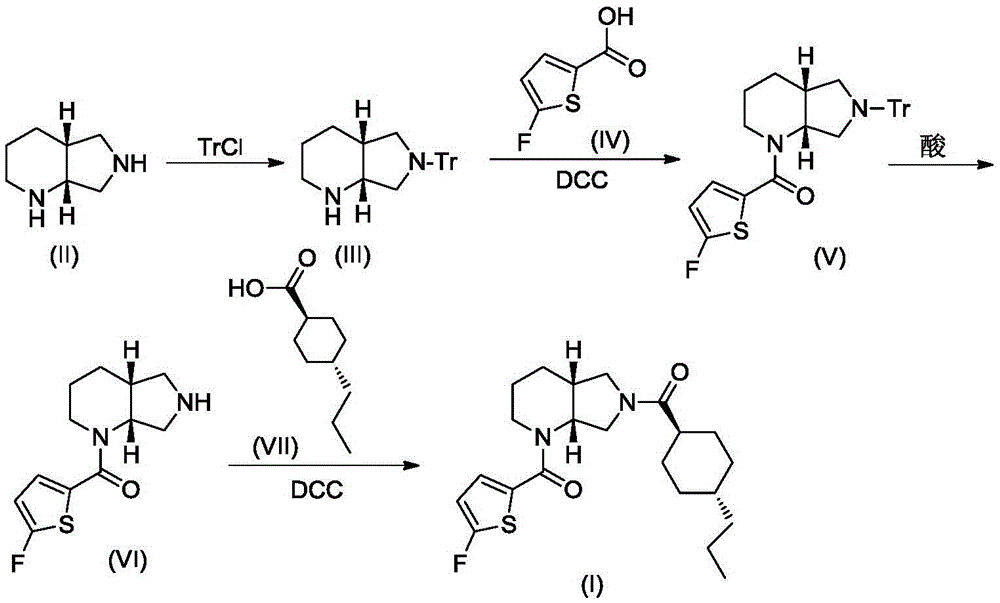
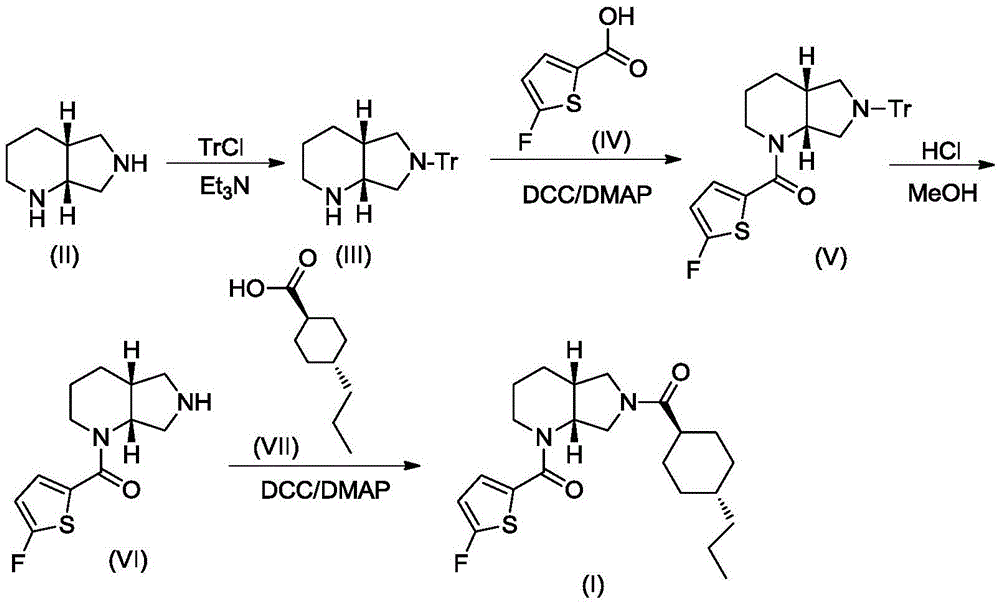
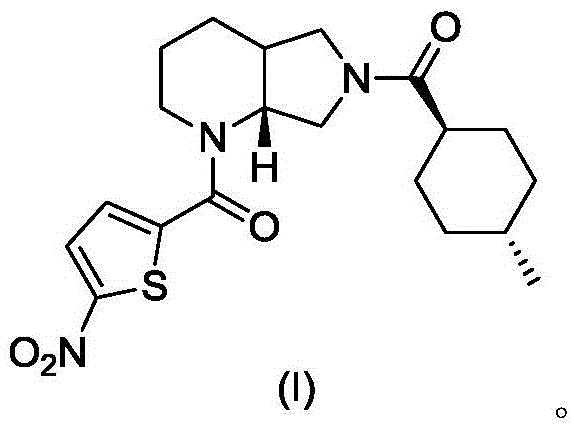
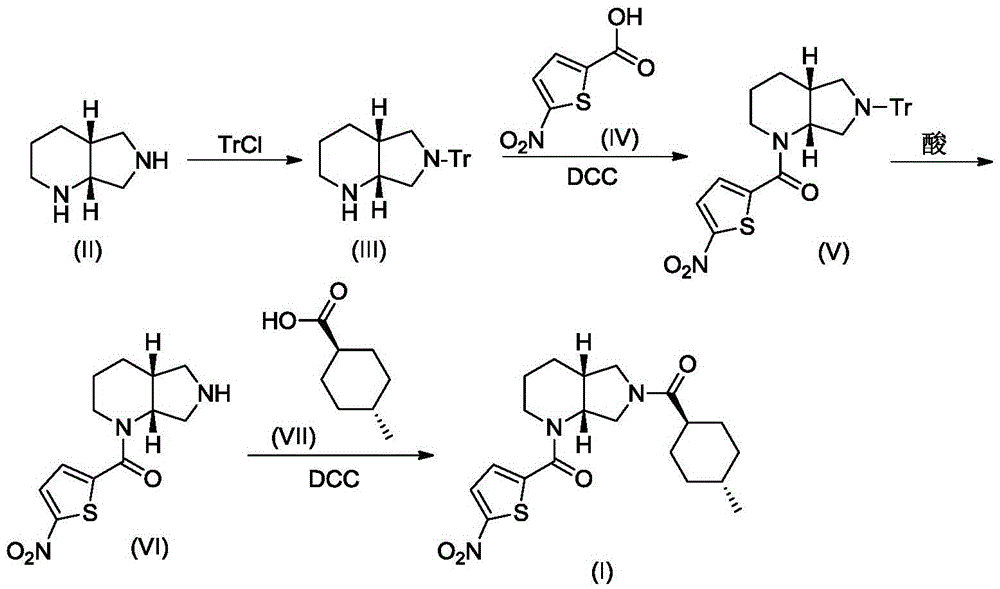
A blood coagulation factor Xa inhibitor containing bicyclic amide structure, its preparation method and use
The invention relates to the field of medicines related to venous thromboembolic diseases, and particularly relates to an FXa inhibitor containing a bicycle-amide structure, a preparation method of the FXa inhibitor and application of the FXa inhibitor in preparation of medicines for treating venous thromboembolic diseases. The inhibitor structure is as shown in the specification.
Owner:上海启锚生物科技有限公司
A kind of blood coagulation factor Xa inhibitor containing bicyclic amide structure and its application
The invention relates to the field of medicines related to venous thromboembolic diseases, and particularly relates to an FXa inhibitor containing a bicycle-amide structure, a preparation method of the FXa inhibitor and application of the FXa inhibitor in preparation of medicines for treating venous thromboembolic diseases. The inhibitor structure is as shown in the specification.
Owner:上海鑫合医药有限公司
FXa inhibitors with cyclic amidines as P4 subunit, processes for their preparations, and pharmaceutical compositions and derivatives thereof
Owner:LEGOCHEM BIOSCIENCES LTD
Method for preparing (s)-5-chloro-n-((3-(4-(5,6-dihydro-4h-1,2,4-oxadiazin-3-yl)phenyl)-2-oxooxazolidin-5-yl)methyl)thiophene-2-carboxamide derivatives
Provided is a method for preparing (S)-5-chloro-N-((3-(4-(5,6-dihydro-4H-1,2,4-oxadiazin-3-yl)phenyl)-2-oxooxazolidin-5-yl)methyl)thiophene-2-carboxamide derivatives of Formula (I) which are useful as blood coagulation factor Xa inhibitors, and said method using 1-fluoro-4-nitrobenzen as a starting material. According to the method of the present invention, (S)-5-chloro-N-((3-(4-(5,6-dihydro-4H-1,2,4-oxadiazin-3-yl)phenyl)-2-oxooxazolidin-5-yl)methyl)thiophene-2-carboxamide derivatives of Formula (I) which are useful as blood coagulation factor Xa inhibitors can be prepared in a high purity and a high yield.
Owner:LEGOCHEM BIOSCIENCES LTD
Polypeptide for inhibiting activity of bacillus subtilis transglutaminase (BTG) and its screening method and use
ActiveCN103060283BInhibitory activityRestore activityTransferasesMicroorganism based processesCoagulation Factor XaScreening method
The invention relates to a polypeptide for inhibiting an activity of bacillus subtilis transglutaminase (BTG) and its screening method and use. The polypeptide has an amino acid sequence shown in the formula of SEQ ID No: 1. The polypeptide provided by the invention can inhibit the activity of BTG by a fusion protein and has an inhibition rate more than 60%. Through the polypeptide, the activity of expressed BTG having strong cytotoxicity is inhibited in a host cell and then is recovered after the inhibitor is removed under the action of a blood coagulation factor Xa.
Owner:TIANJIN UNIV OF SCI & TECH
Chlorothiophene-isoxazoles as inhibitors of coagulation factors Xa and thrombin
The present invention relates to compounds of the formula I,wherein R1; R2; R3; R4; R5, R16, X and M have the meanings indicated in the claims. The compounds of formula I are valuable pharmacologically active compounds. They exhibit a strong anti-thrombotic effect and are suitable, for example, for the therapy and prophylaxis of cardio-vascular disorders like thromboembolic diseases or restenoses. They are reversible inhibitors of the blood clotting enzymes factor Xa and thrombin and can in general be applied in conditions in which an undesired activity of factor Xa and / or thrombin are present or for the cure or prevention of which an inhibition of factor Xa and thrombin are intended. The invention furthermore relates to processes for the preparation of compounds of the formula I, their use, in particular as active ingredients in pharmaceuticals, and pharmaceutical preparations comprising them.
Owner:SANOFI SA
Fx activation process and its use in the preparation of a fxa composition
PendingUS20210290738A1Eliminate riskPeptide/protein ingredientsPeptide preparation methodsCoagulation Factor XaBiology
The invention relates to a high purity Coagulation Factor Xa (FXa or activated Coagulation Factor X) preparation and an activation and purification process to obtain said FXa of high purity and high degree of activation without addition of proteinaceous activators during manufacturing.
Owner:OCTAPHARMA
Hydrazides as Inhibitors of Coagulation Factor Xa
ActiveCN105085515BGood water solubilityGood curative effectOrganic active ingredientsOrganic chemistryBenzeneCoagulation Factor Xa
The invention relates to a new compound represented by formula (I) or a pharmaceutically acceptable salt thereof. In the formula (I), X is selected from a 3-9-membered carbon ring or a benzo ring thereof and a 4-10-membered heterocyclic ring or a benzo ring thereof; Y and Z are respectively independently selected from 4-9-membered saturated heterocyclic rings; and R1-3 are respectively independently selected from H, F, Cl, Br, I, CN, OH, SH, NH2, CHO and COOH, or are selected from R01 substituted C1-10 alkyl or heteroalkyl groups, C3-C10 cycloalkyl or heterocycloalkyl groups, and C3-10 cycloalkyl or heterocycloalkyl group substituted C1-10 alkyl or heteroalkyl groups. The compound can be used as an anticoagulant for treating and preventing thrombus abnormity diseases. The strong blood coagulation factor Xa inhibitor is provided to meet practical demands of selectivity and the strong inhibitor of the blood coagulation factor Xa.
Owner:NORTH CHINA PHARMA COMPANY
A blood coagulation factor Xa inhibitor containing bicyclic amide structure and its application
The invention relates to the field of medicines related to venous thromboembolic diseases, and particularly relates to an FXa inhibitor containing a bicycle-amide structure, a preparation method of the FXa inhibitor and application of the FXa inhibitor in preparation of medicines for treating venous thromboembolic diseases. The inhibitor structure is as shown in the specification.
Owner:浙江西塘实业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
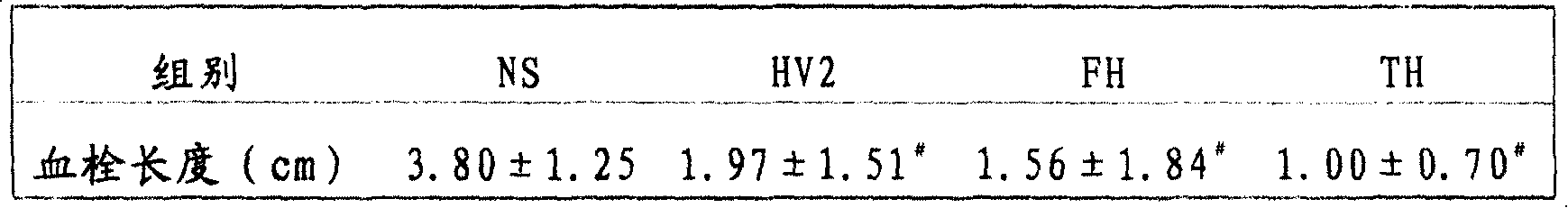
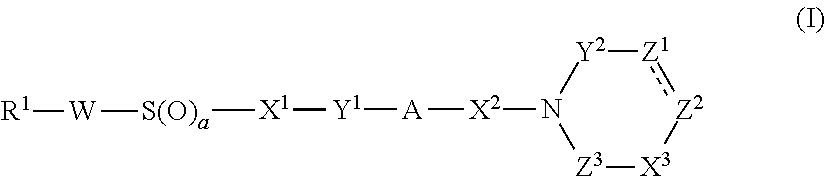







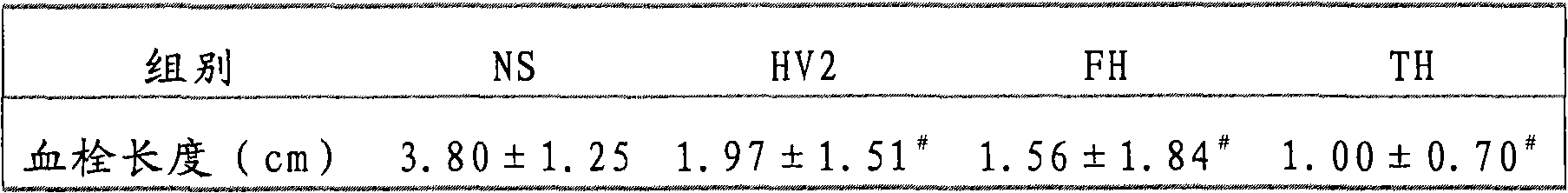


![Method for preparing 5-chloro-N-({(5S)-2-oxo-3-[4-(5,6-dihydro-4H-[1,2,4]triazin-1-yl)phenyl]-1,3-oxazolidin-5-yl}methyl) derivative and intermediate used therein Method for preparing 5-chloro-N-({(5S)-2-oxo-3-[4-(5,6-dihydro-4H-[1,2,4]triazin-1-yl)phenyl]-1,3-oxazolidin-5-yl}methyl) derivative and intermediate used therein](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e63dd9dc-b161-4c7e-be6b-64478d4d8c31/US08754210-20140617-C00001.png)
![Method for preparing 5-chloro-N-({(5S)-2-oxo-3-[4-(5,6-dihydro-4H-[1,2,4]triazin-1-yl)phenyl]-1,3-oxazolidin-5-yl}methyl) derivative and intermediate used therein Method for preparing 5-chloro-N-({(5S)-2-oxo-3-[4-(5,6-dihydro-4H-[1,2,4]triazin-1-yl)phenyl]-1,3-oxazolidin-5-yl}methyl) derivative and intermediate used therein](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e63dd9dc-b161-4c7e-be6b-64478d4d8c31/US08754210-20140617-C00002.png)
![Method for preparing 5-chloro-N-({(5S)-2-oxo-3-[4-(5,6-dihydro-4H-[1,2,4]triazin-1-yl)phenyl]-1,3-oxazolidin-5-yl}methyl) derivative and intermediate used therein Method for preparing 5-chloro-N-({(5S)-2-oxo-3-[4-(5,6-dihydro-4H-[1,2,4]triazin-1-yl)phenyl]-1,3-oxazolidin-5-yl}methyl) derivative and intermediate used therein](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e63dd9dc-b161-4c7e-be6b-64478d4d8c31/US08754210-20140617-C00003.png)