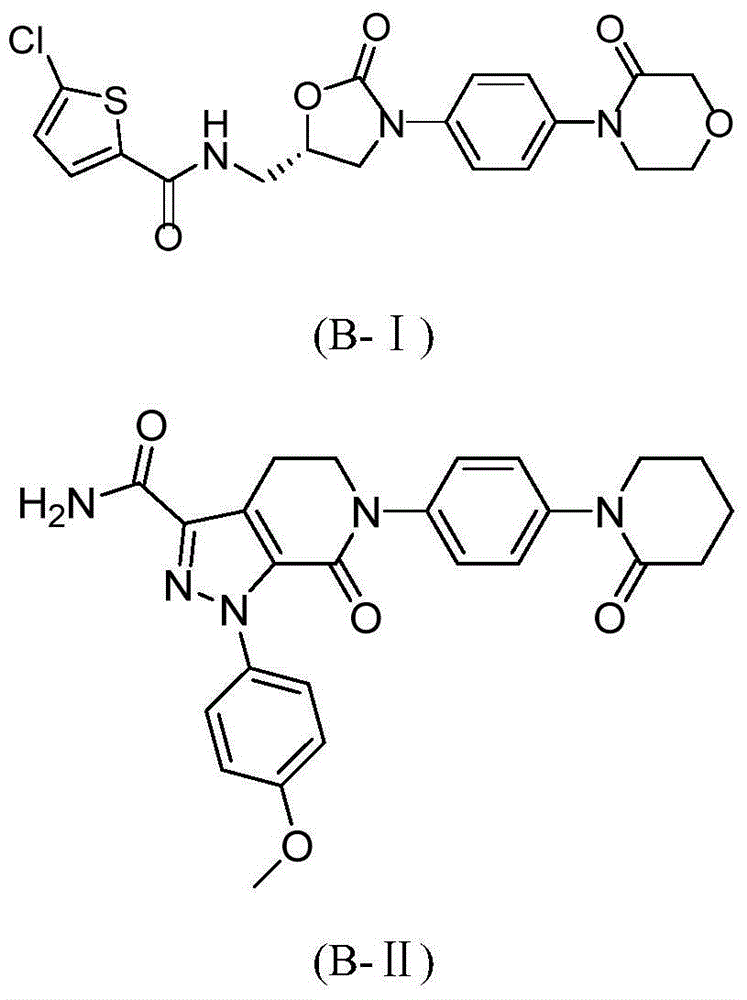
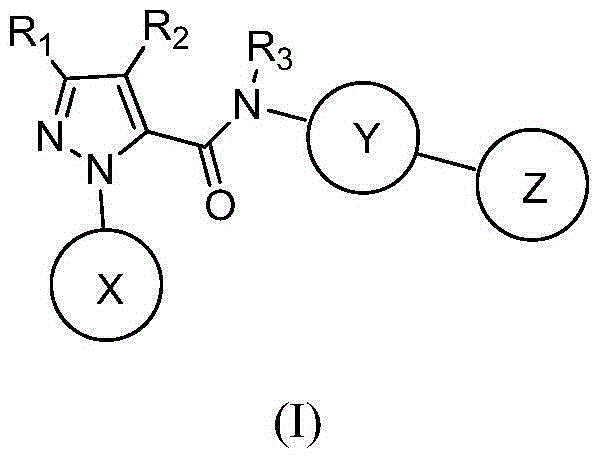
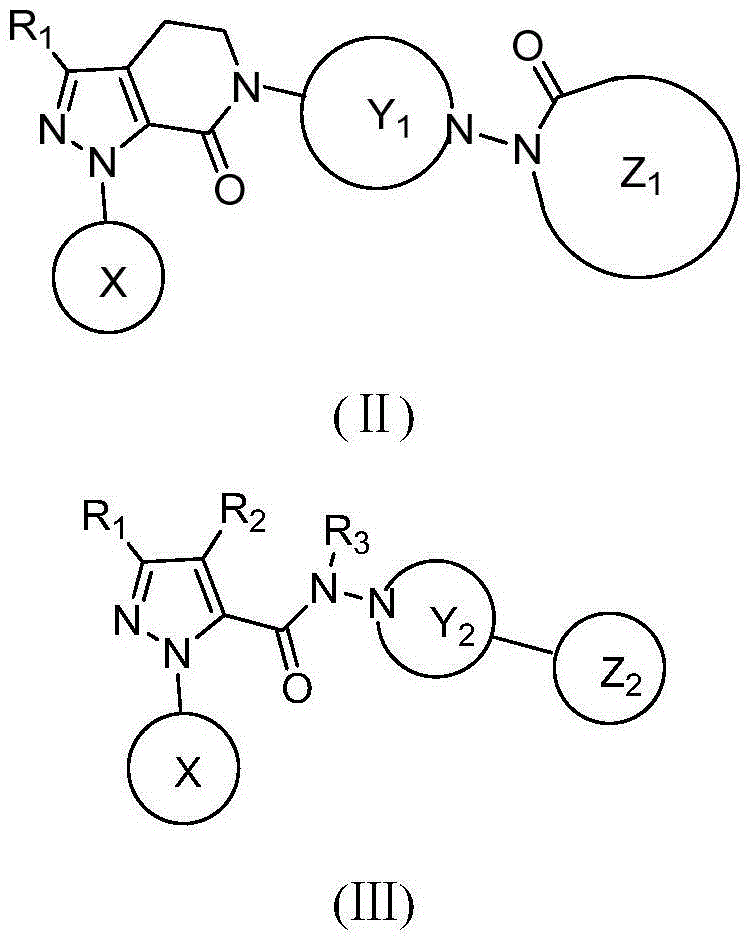
Hydrazide compound as blood coagulation factor Xa inhibitor
A compound, heteroatom technology, applied in the field of new hydrazide compounds, can solve problems such as high risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] 4-(4-(1-(4-methoxyphenyl)-7-oxo-3-(trifluoromethyl)-4,5-dihydro-1H-pyrazolo[3,4-c ]pyridin-6(7H)-yl)piperidine-1- base) morpholin-3-one
[0152]
[0153] Reaction flow:
[0154]
[0155] Step A: Dissolve (4-methoxyphenyl)hydrazine hydrochloride (4.83 g, 27.7 mmol) in ethanol (50 mL) and add 1-ethoxy-2,2,2-trifluoroethanol (4.8 g, 33.3 mmol), then the mixture was refluxed for 16 hours. The reaction was concentrated, then the residue was dissolved in DMF (50 mL) and cooled to 0 °C. NBS (6.1 g, 27.7 mmol) was slowly added to the solution. The reaction mixture was warmed to 25°C and stirred for 16 hours. The reaction mixture was quenched with water and extracted with ethyl acetate. The combined organic phases were washed with brine, dried over anhydrous sodium sulfate, filtered and concentrated. The crude residue was purified by silica gel chromatography (PE to PE:EA=20:1) to give (Z)-2,2,2-trifluoro-N'-(4-methoxyphenyl)bromoacetyl Hydrazine (3.3 g, 39%) as ...
Embodiment 2
[0165] 1-(4-methoxyphenyl)-6-(2'-oxo-[1,1'-dipiperidinyl]-4-yl)-3-(trifluoromethyl)-5,6 -Dihydro-1H-pyrazolo[3,4-c]pyridine pyridin-7(4H)-one
[0166]
[0167] Step A: 6-(1-aminopiperidin-4-yl)-1-(4-methoxyphenyl)-3-(trifluoromethyl)-5,6-dihydro-1H-pyrazole And[3,4-c]pyridin-7(4H)-one (500 mg, 1.22 mmol) was dissolved in DMF (8 mL), and DIEA (315 mg, 2.44 mmol) and 5-bromopentyl Acid chloride (267 mg, 1.34 mmol). The mixture was stirred at 25°C for 3 hours, poured into water (30 mL), extracted with EA (30 mL×2), dried over anhydrous sodium sulfate, filtered and concentrated. The crude residue was purified by thin layer chromatography (EA) to give 5-bromo-N-(4-(1-(4-methoxyphenyl)-7-oxo-3-(trifluoromethyl)-4 , 5-Dihydro-1H-pyrazolo[3,4-c]pyridin-6(7H)-yl)piperidin-1-yl)pentanamide (180 mg, 25%) as a yellow solid. LCMS(ESI)m / z:528.2(M+1),530.2(M+3).
[0168] Step B: Add 5-bromo-N-(4-(1-(4-methoxyphenyl)-7-oxo-3-(trifluoromethyl)-4,5-di Hydrogen-1H-pyrazolo[3,4-c]pyr...
Embodiment 3
[0170] 1-(4-methoxyphenyl)-6-(1-(2-oxopyrrolidin-1-yl)-piperidinyl-4-yl)-3-(trifluoromethyl)-5, 6-Dihydro-1H-pyrazolo [3,4-c]pyridin-7(4H)-one
[0171]
[0172] Step A: To 6-(1-aminopiperidin-4-yl)-1-(4-methoxyphenyl)-3-(trifluoromethyl)-5,6-dihydro-1H-pyrazole To [3,4-c]pyridin-7(4H)-one (250 mg, 0.611 mmol) in DCM (8 mL) was added Et 3 N (62 mg, 0.611 mmol) and 4-chlorobutyryl chloride (86 mg, 0.611 mmol). The mixture was stirred at 25 °C for 16 hours, the resulting mixture was poured into water (30 mL), extracted with DCM (30 mL×2), dried over anhydrous sodium sulfate and concentrated. The crude product was purified by TLC (EA) to give 4- Chloro-N-(4-(1-(4-methoxyphenyl)-7-oxo-3-(trifluoromethyl)-4,5-dihydro-1H-pyrazolo[3,4 -c] pyridin-6(7H)-yl)piperidin-1-yl)butanamide (100 mg, 32%) as a yellow solid. LCMS(ESI)m / z:514.2(M+1),516.2(M+3).
[0173] Step B: At 0°C, to 4-chloro-N-(4-(1-(4-methoxyphenyl)-7-oxo-3-(trifluoromethyl)-4,5-di Hydrogen-1H-pyrazolo[3,4-c]py...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com