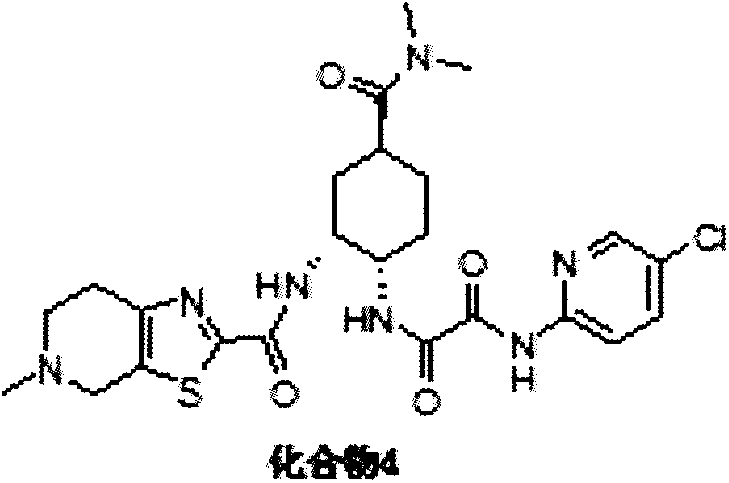
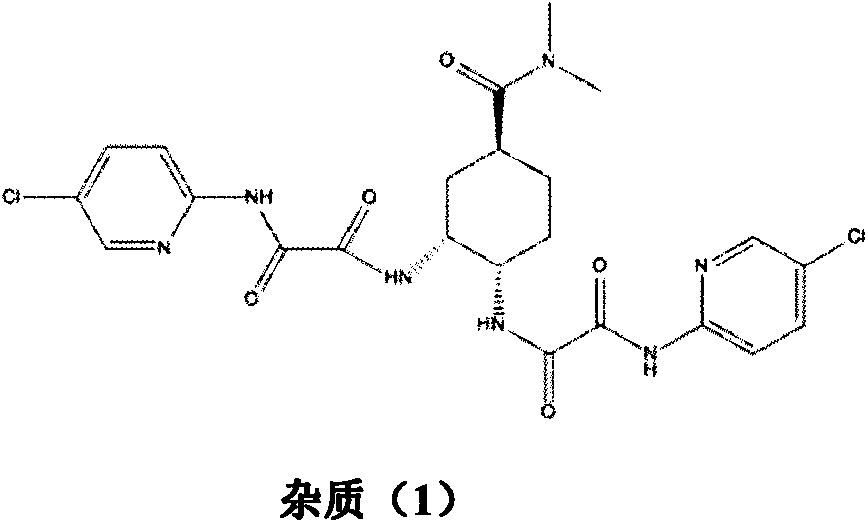
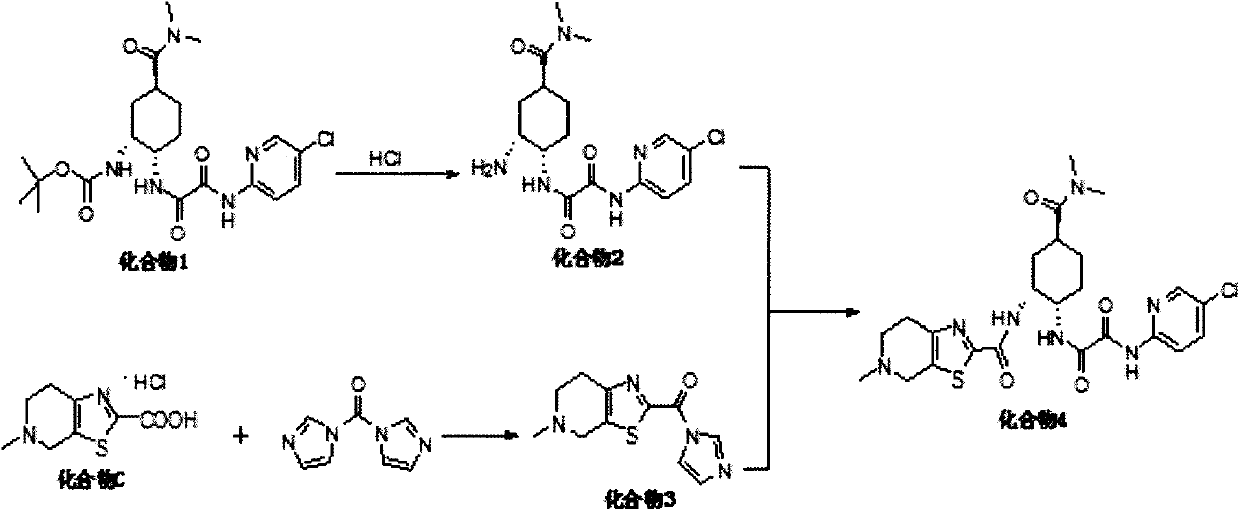
A kind of preparation method of antithrombotic drug
A compound and hydrate technology, which is applied in drug combination, blood diseases, organic chemistry, etc., can solve the problems affecting the purity of compounds, impurity is not easy to remove, and affects the synthesis of compounds, so as to reduce the phenomenon of reaction fuming, curing phenomenon disappears, Conducive to the effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] Steps:
[0032] Add 279.40g of compound C and 1.4L of dichloromethane I into kettle 1, and add 120.42g of triethylamine after stirring; g compound 1 and 5L dichloromethane, stir well and add 514.67g methanesulfonic acid; kettle 2 is heated to 25±5°C and stirred for reaction; kettle 1 is reacted for 7 hours, kettle 2 is reacted for 2 hours; kettle 2 is soaked in triethylamine And, add the solution in kettle 2 to kettle 1, the heat release is not obvious; stir and react at 25±5°C for 20 hours; add 7L water to the system, stir for 1 hour, and let stand to separate the liquid; wash the organic phase with anhydrous magnesium sulfate After drying, filter with suction, and concentrate the filtrate under reduced pressure at 50°C until there is no fraction; add 11L of ethanol to the residue, and reflux for beating for 1 hour; after cooling down, filter with suction, and dry the filter cake to obtain compound 4, 471.6g, with a yield of 80.4%. 99.7% purity. [HPLC met...
Embodiment 2
[0034]
[0035] Add 22.0g of compound C and 220mL of dichloromethane into the reaction flask and stir, add 9.5g of triethylamine, and stir at 25±5°C for 10 minutes. Add 16.7g of CDI, continue to stir at 25±5°C for 7 hours, this system is System 1. Add 39.4g of compound 1 and 800mL of dichloromethane into another reaction flask, add 40.5g of methanesulfonic acid dropwise, and stir at 10±5°C for 5 hours. This system is System 2. To System 2, 42.7 g of triethylamine was added dropwise. After the dropwise addition, system 2 was added dropwise into system 1, kept at 25±5°C and stirred for 24 hours. Add 1L of water to the system, stir for 15 minutes to separate the liquids. The organic phase was dried over anhydrous magnesium sulfate, concentrated under reduced pressure at 50°C until no distillate was present, and the residue was added to 1L of ethanol, and stirred under reflux for 1 hour. After cooling down, it was filtered and the filter cake was dried to obtain compound 4, ...
Embodiment 3
[0037]
[0038] Add 22.0 g of compound C and 220 mL of dichloromethane into the reaction flask and stir, then add 9.5 g of triethylamine and stir for 10 minutes. Add 21.5g EDCI and 15.1g HOBT and stir, this system is System 1. Add 39.4g of compound 1 and 800mL of dichloromethane into another reaction flask, add 40.5g of methanesulfonic acid dropwise, and stir at 10±5°C for 5 hours. This system is System 2. To System 2, 42.7 g of triethylamine was added dropwise. After the dropwise addition, system 2 was added dropwise into system 1, and kept stirring for 24 hours. Add 1L of water to the system, stir for 15 minutes to separate the liquids. The organic phase was dried over anhydrous magnesium sulfate, concentrated under reduced pressure at 50°C until no distillate was present, and the residue was added to 1L of ethanol, and stirred under reflux for 1 hour. After cooling down, it was filtered, and the filter cake was dried to obtain compound 4, 28.5 g, with a yield of 61.7%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com