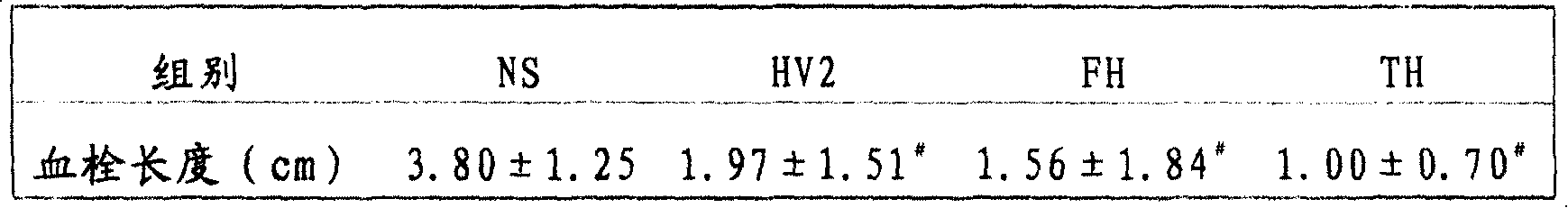Preparation for specificity anticoagulant substance and application thereof
A technology of anticoagulation and thrombin, applied in the prevention and treatment of thrombosis-related diseases, the protein structure and preparation of anticoagulant substances, can solve the problem of no anticoagulant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
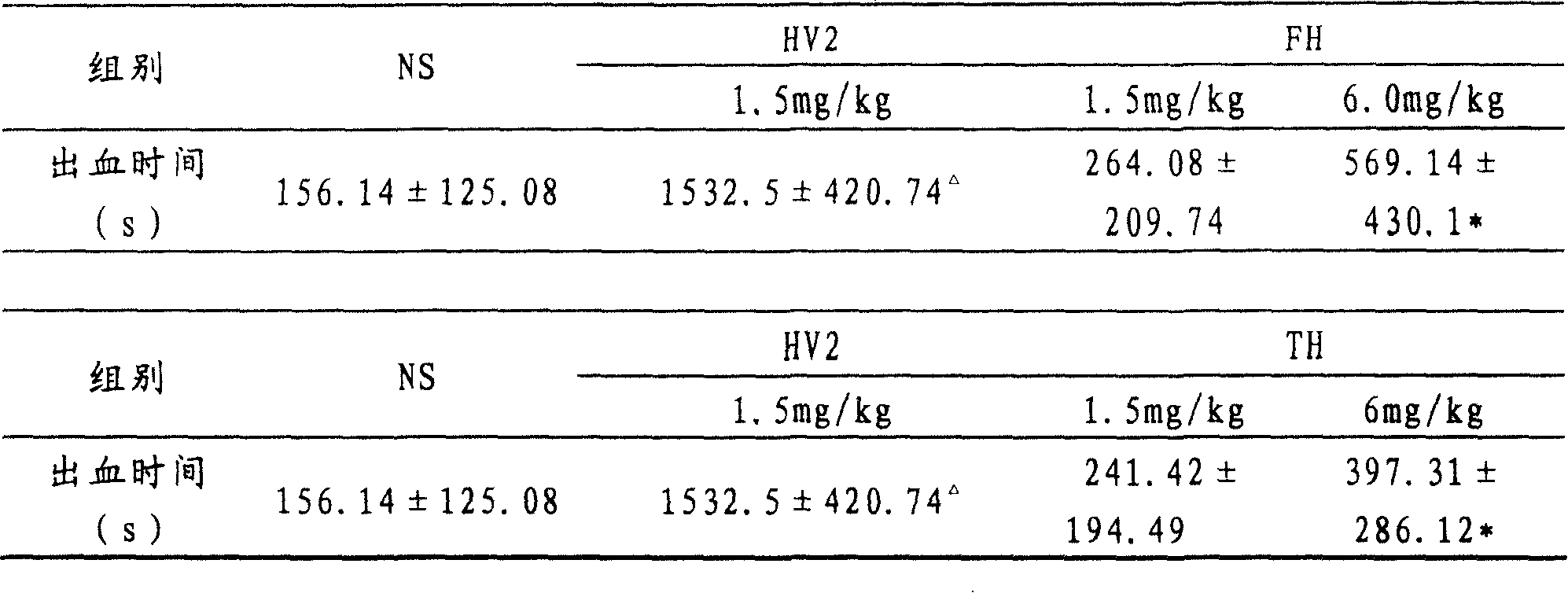
[0019] Example 1 Preparation of IEGR-HV2(FH) and LGPR-HV2(TH) and their function of preventing and treating thrombosis
[0020] 1. Acquisition of FH and TH proteins
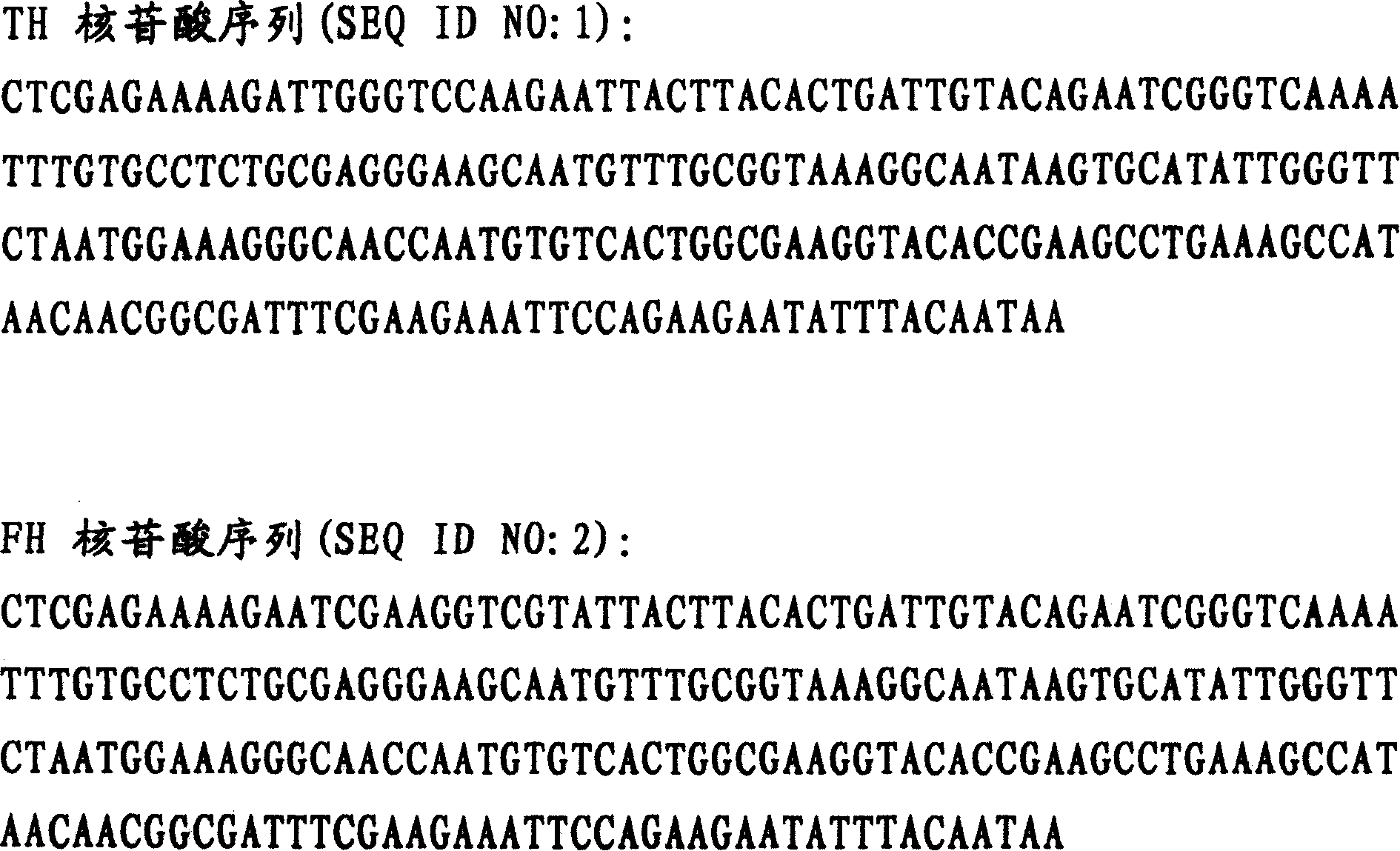
[0021] By PCR, the restriction site Xho I and the base sequence corresponding to the coagulation factor Xa recognition sequence IEGR or the thrombin recognition sequence LGPR are introduced into the upstream of the HV2 gene, and the EcoR I restriction site is introduced downstream of the HV2 gene, and the gene is Ligated to pPIC9 plasmids digested with the same restriction enzymes to obtain recombinant plasmids pPIC9-FH and pPIC9-TH. pPIC9-FH, pPIC9-TH and pPIC9K were digested with BamH I and SalI and ligated to obtain pPIC9K-FH and pPIC9K-TH. The two recombinant plasmids were electroporated and recombined into the yeast genome, and the expression was induced by methanol. The proteins FH and TH were obtained by separation and purification. Note: The nucleotide sequences of TH and FH used in this method are SEQ...
Embodiment 2
[0034] Preparation of Example 2HSA-EFLGPR-HV2 fusion protein (ATH) and HSA-EFIEGR-HV2 fusion protein (AFH) and its antithrombotic and anticoagulant activity
[0035] Add Xho I and EcoR I restriction sites to the upstream and downstream of the human albumin HSA gene, respectively, to construct the HSA gene without a stop codon into the pPIC9 vector, and use BamH I and EcoR I to double-digest the above vector and the pPIC9K vector. HSA is connected to the pPIC9K carrier; the encoding base of EcoR I restriction site and thrombin / coagulation factor Xa recognition sequence is added at the upstream of hirudin gene by PCR method, and the Not I restriction site is introduced at the downstream of hirudin; EcoR I and Not I double-digest the hirudin gene with thrombin / coagulation factor Xa recognition sequence and connect it to the downstream of HSA of the pPIC9K vector with HSA gene constructed above to form the fusion gene ATH / AFH to obtain the plasmid pPIC9K-ATH / AFH. Linearize the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com