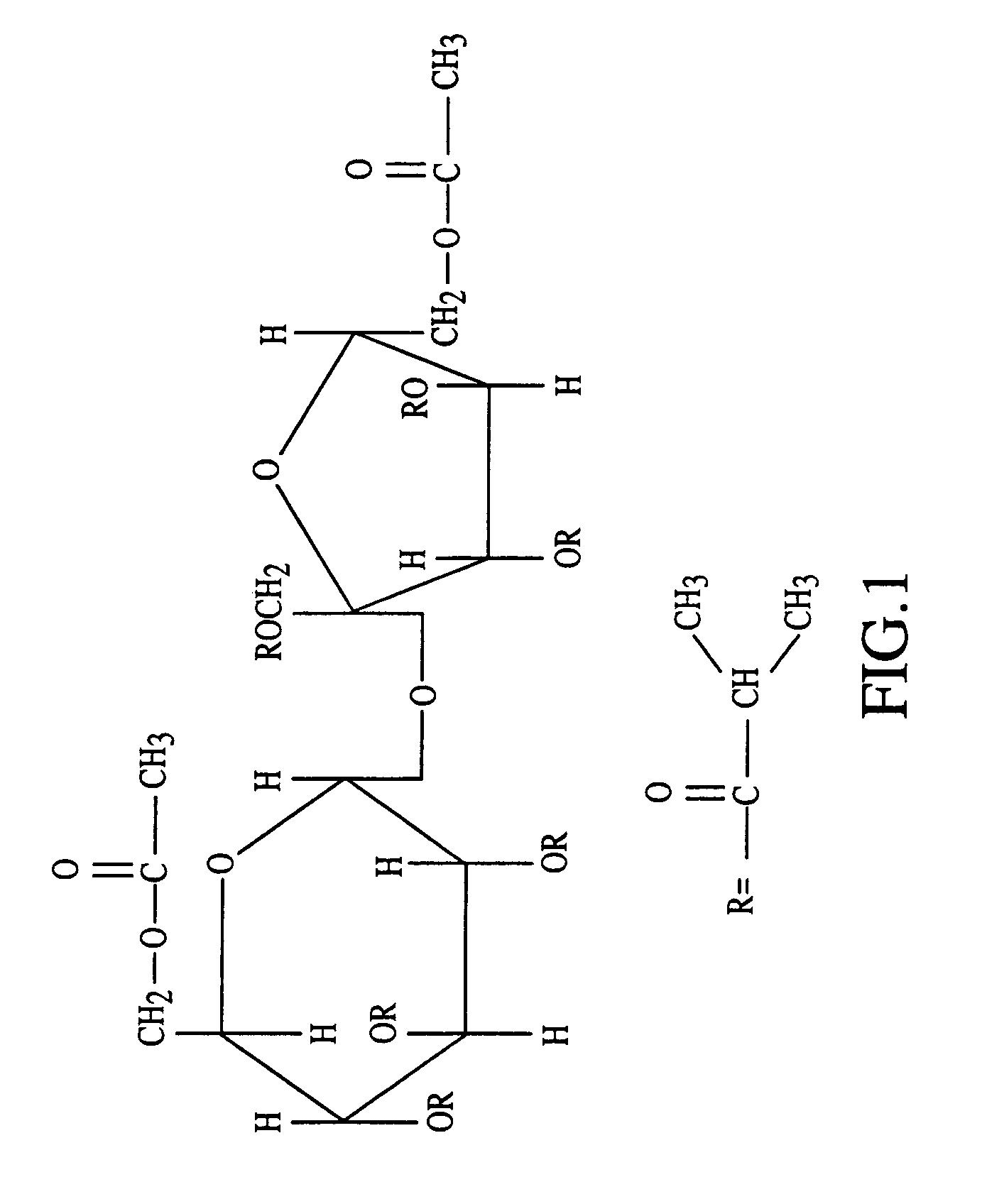
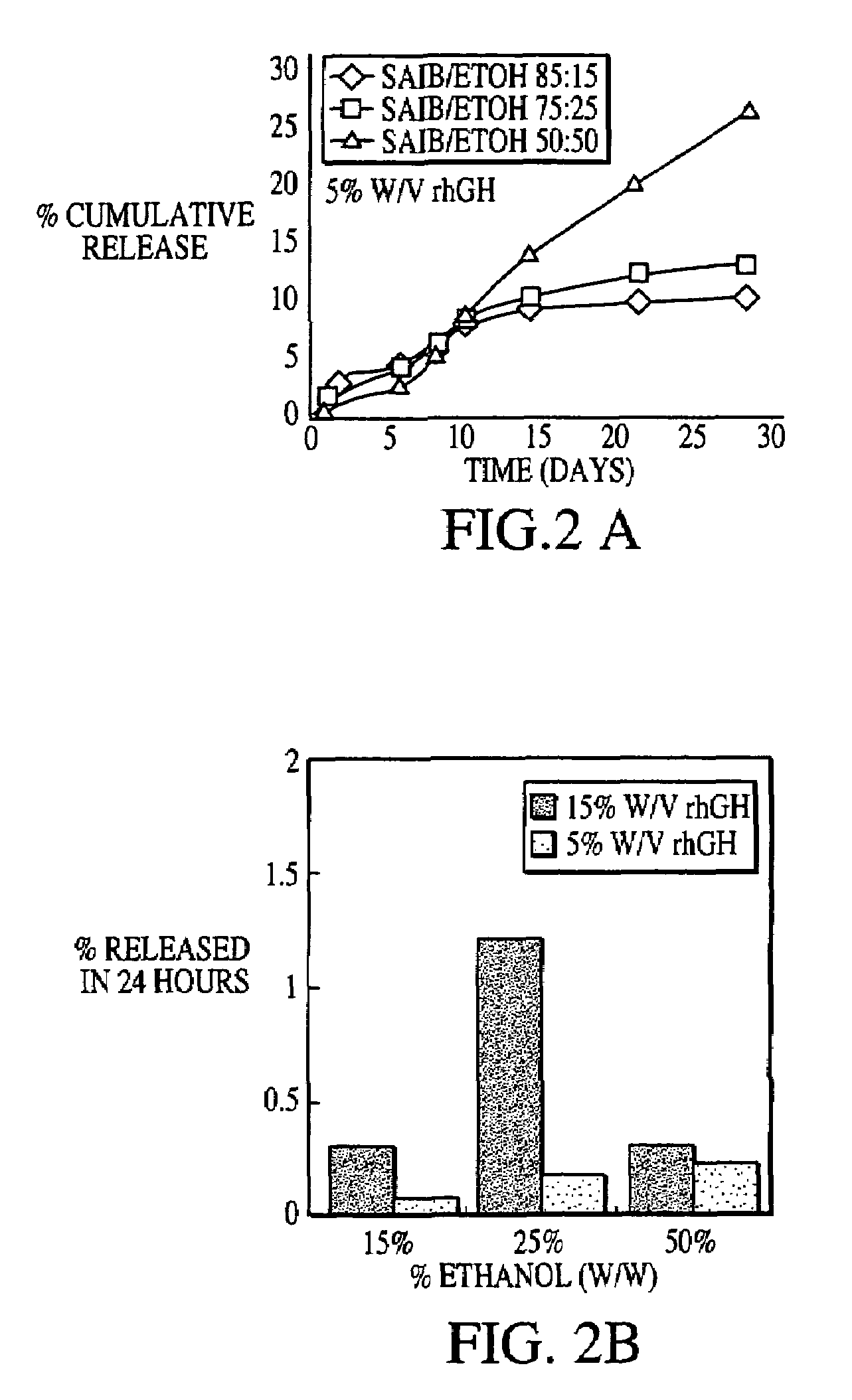
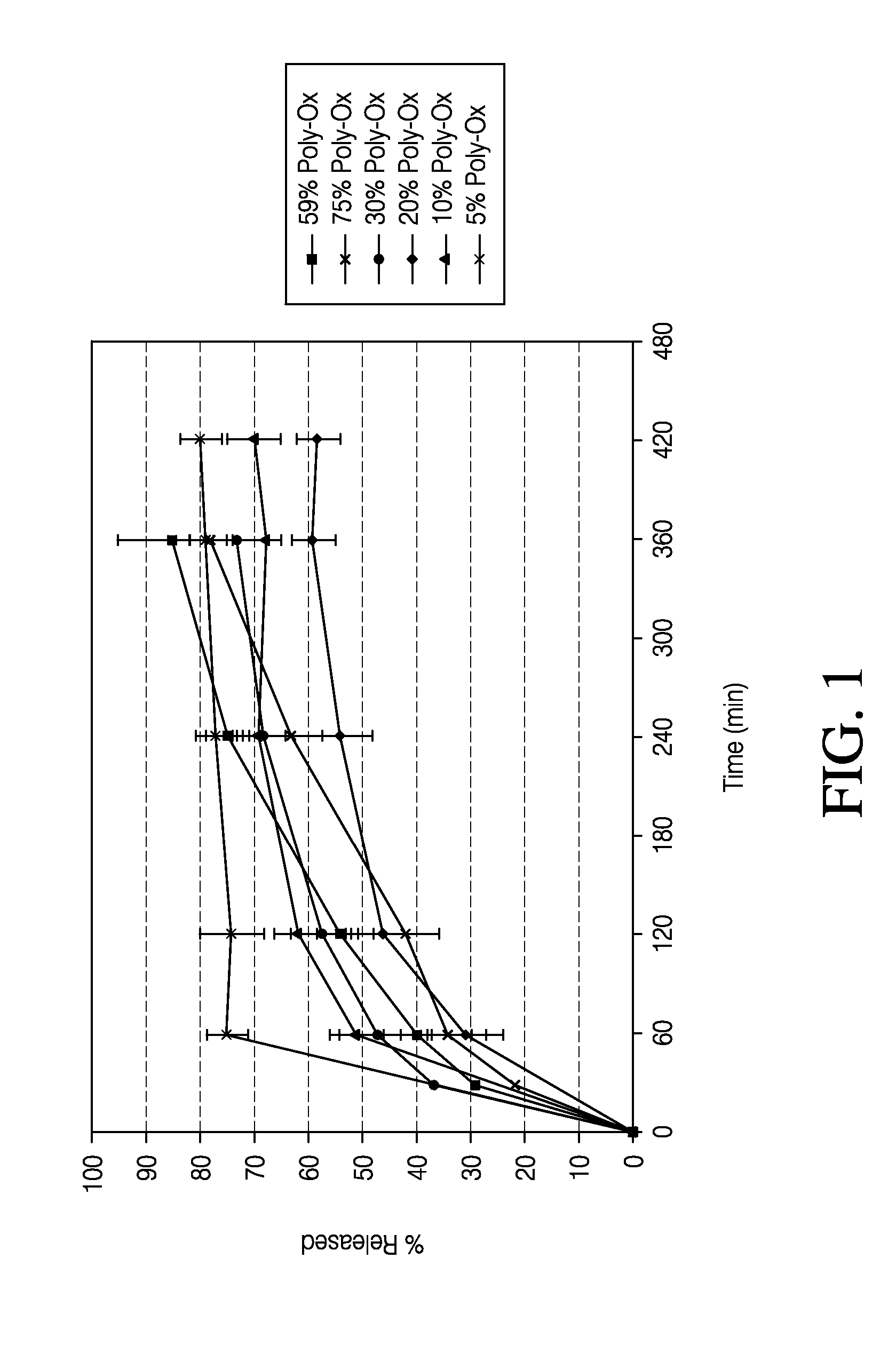
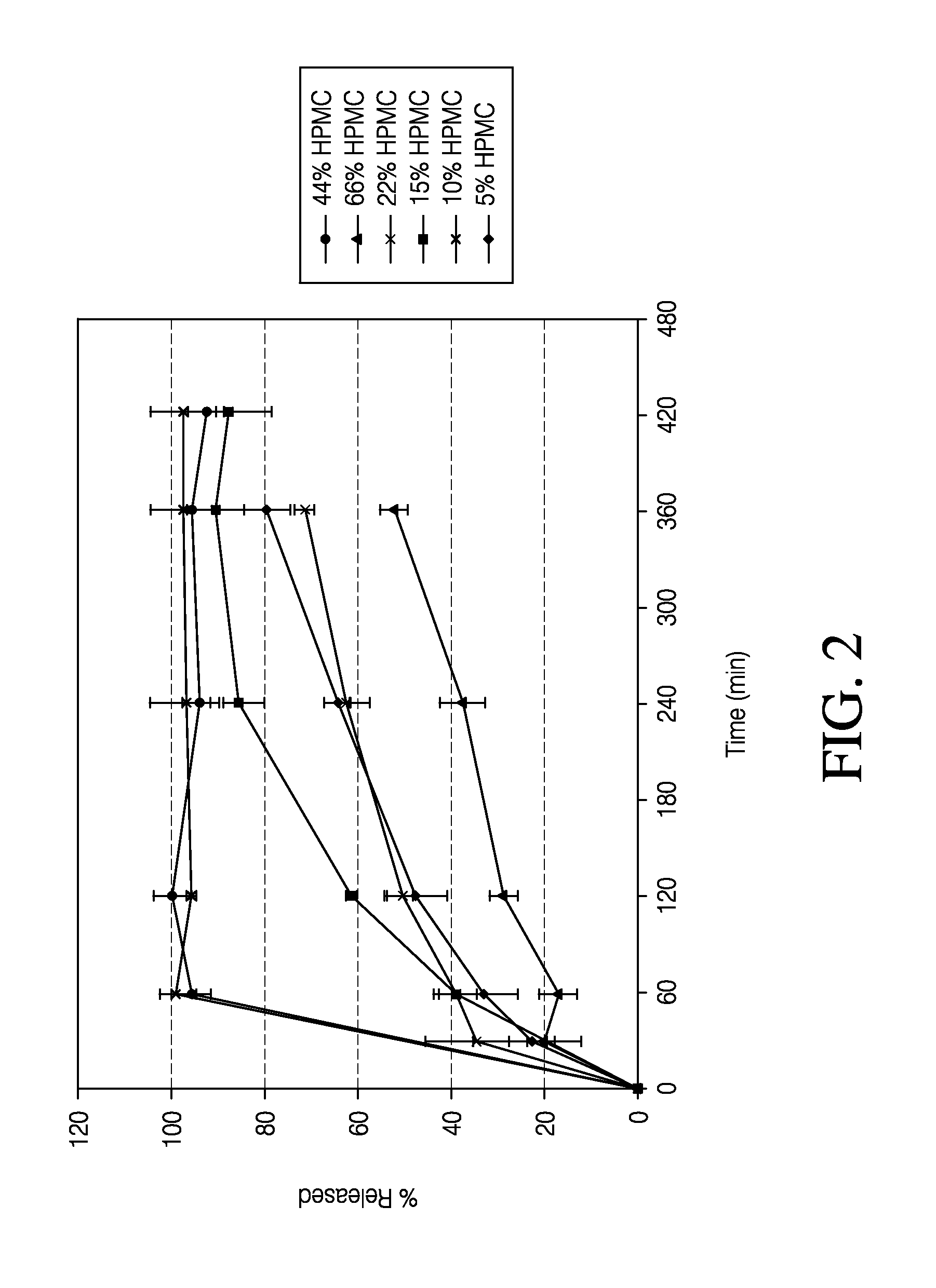
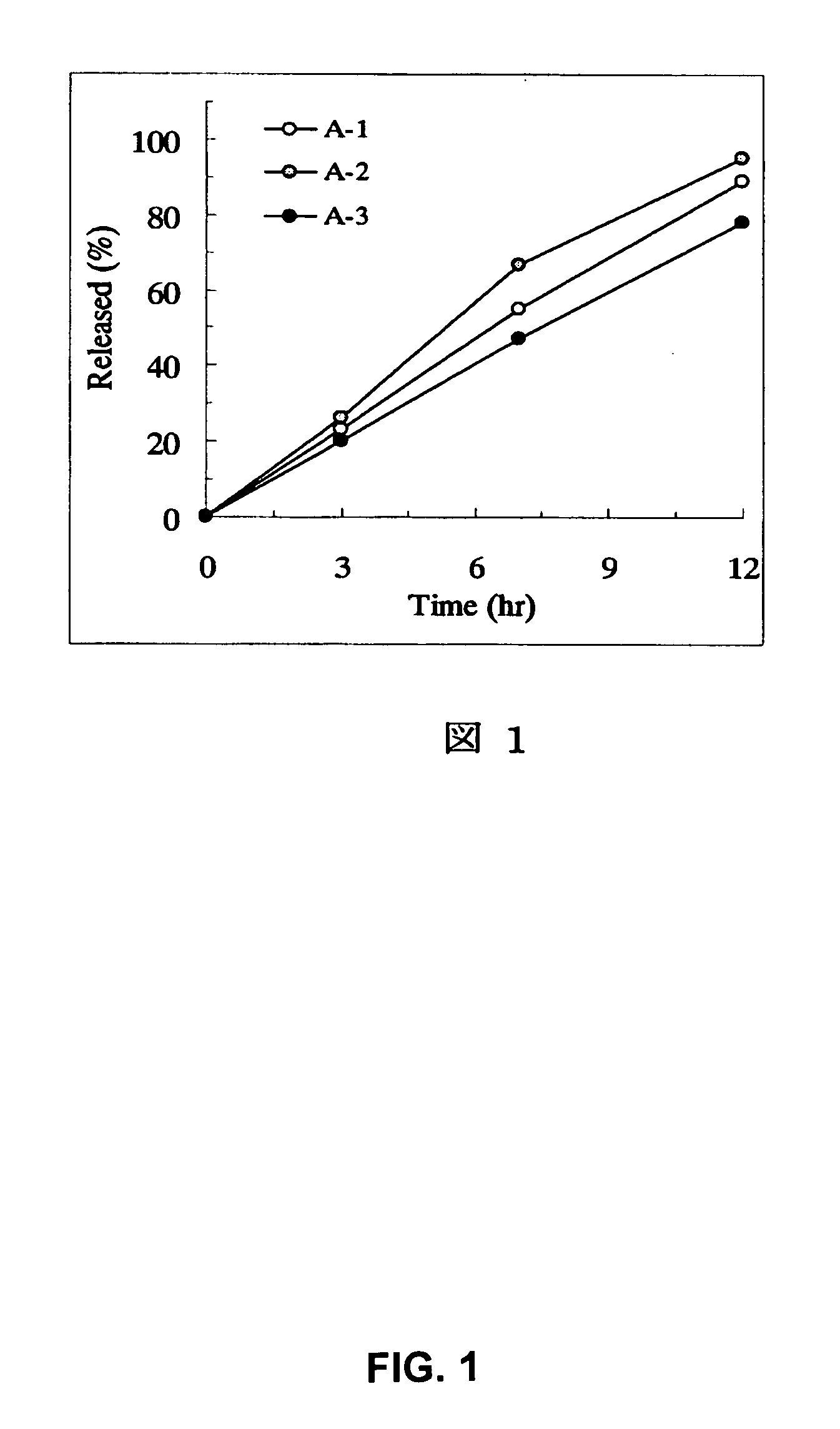
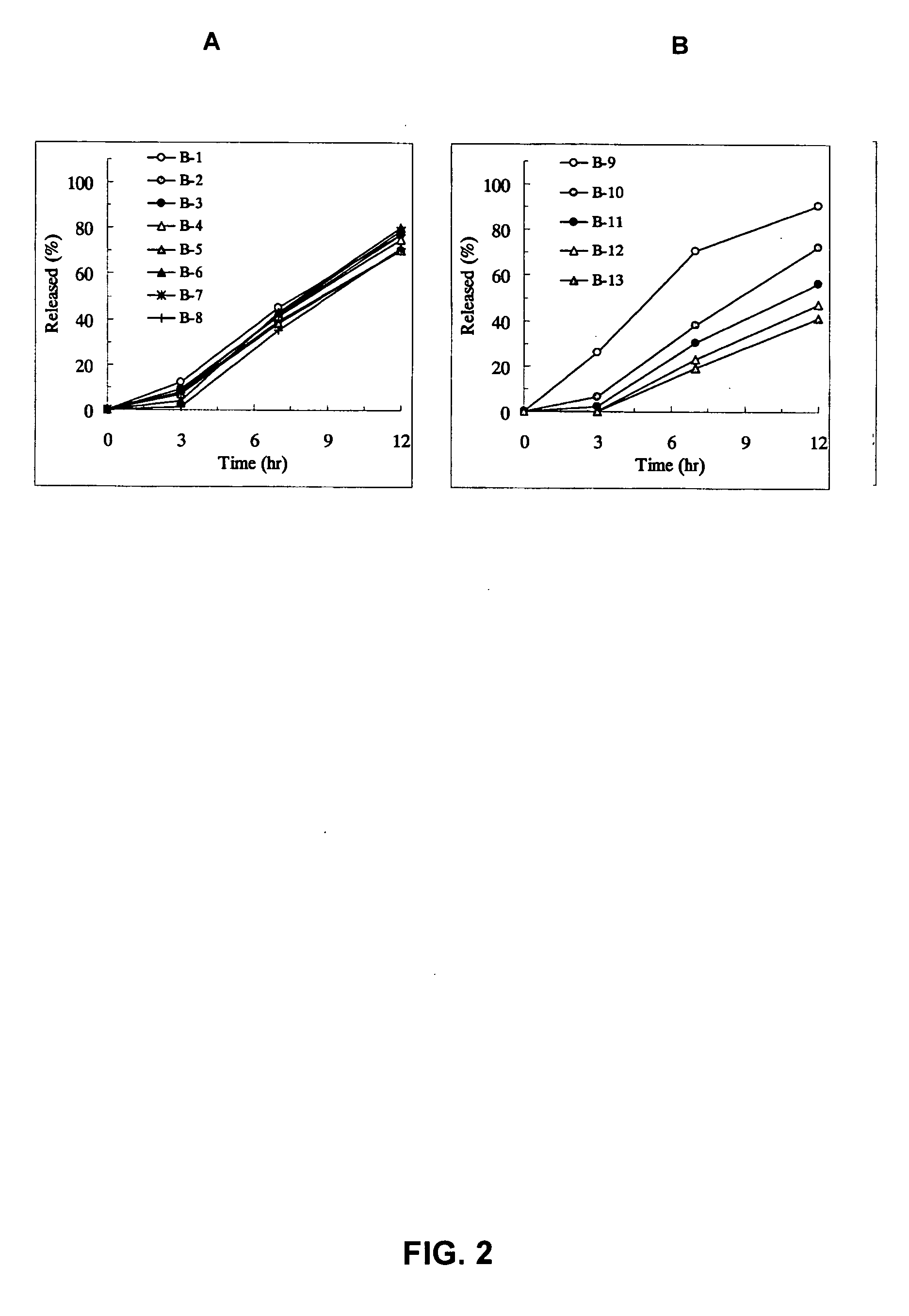
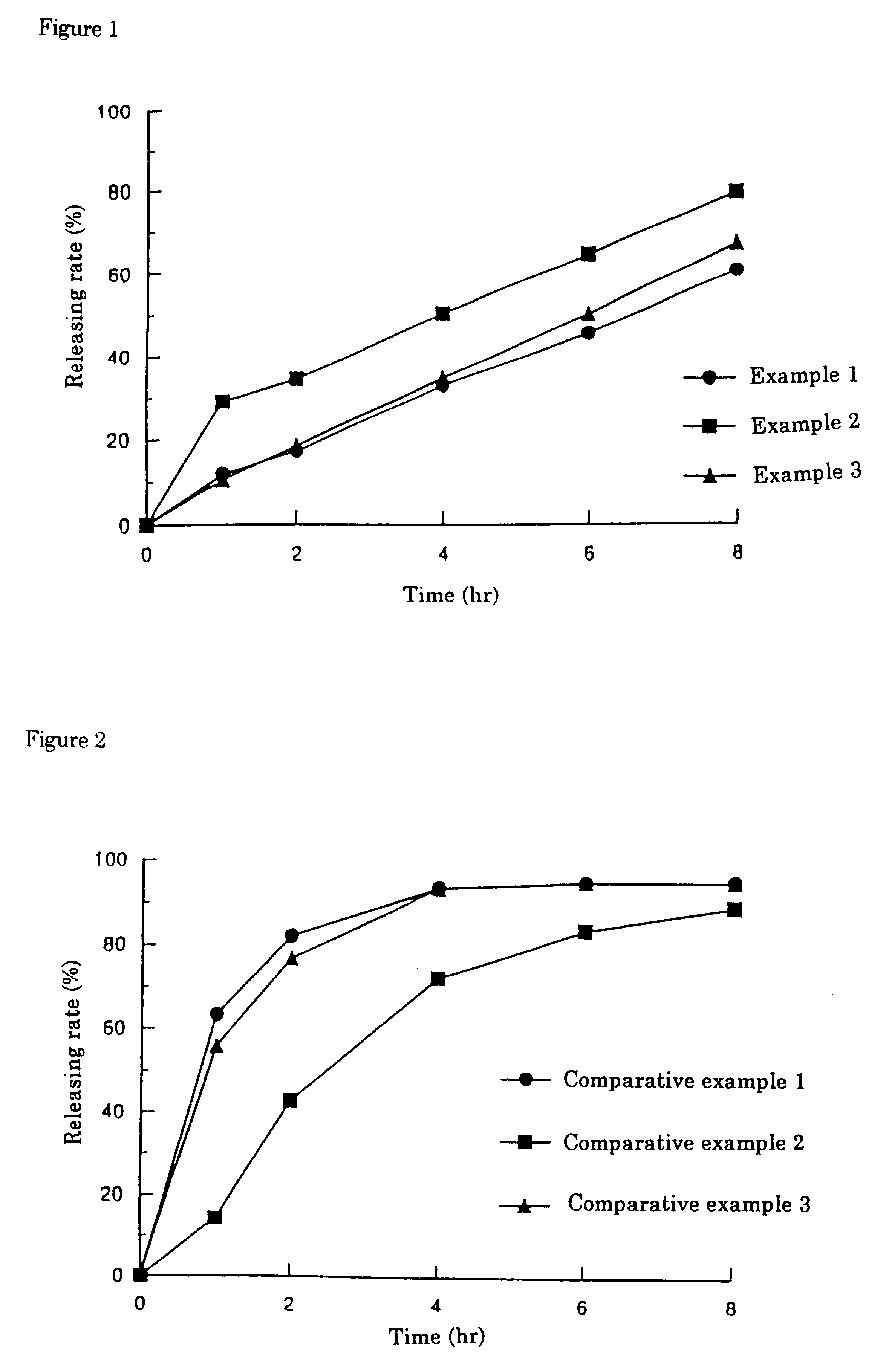
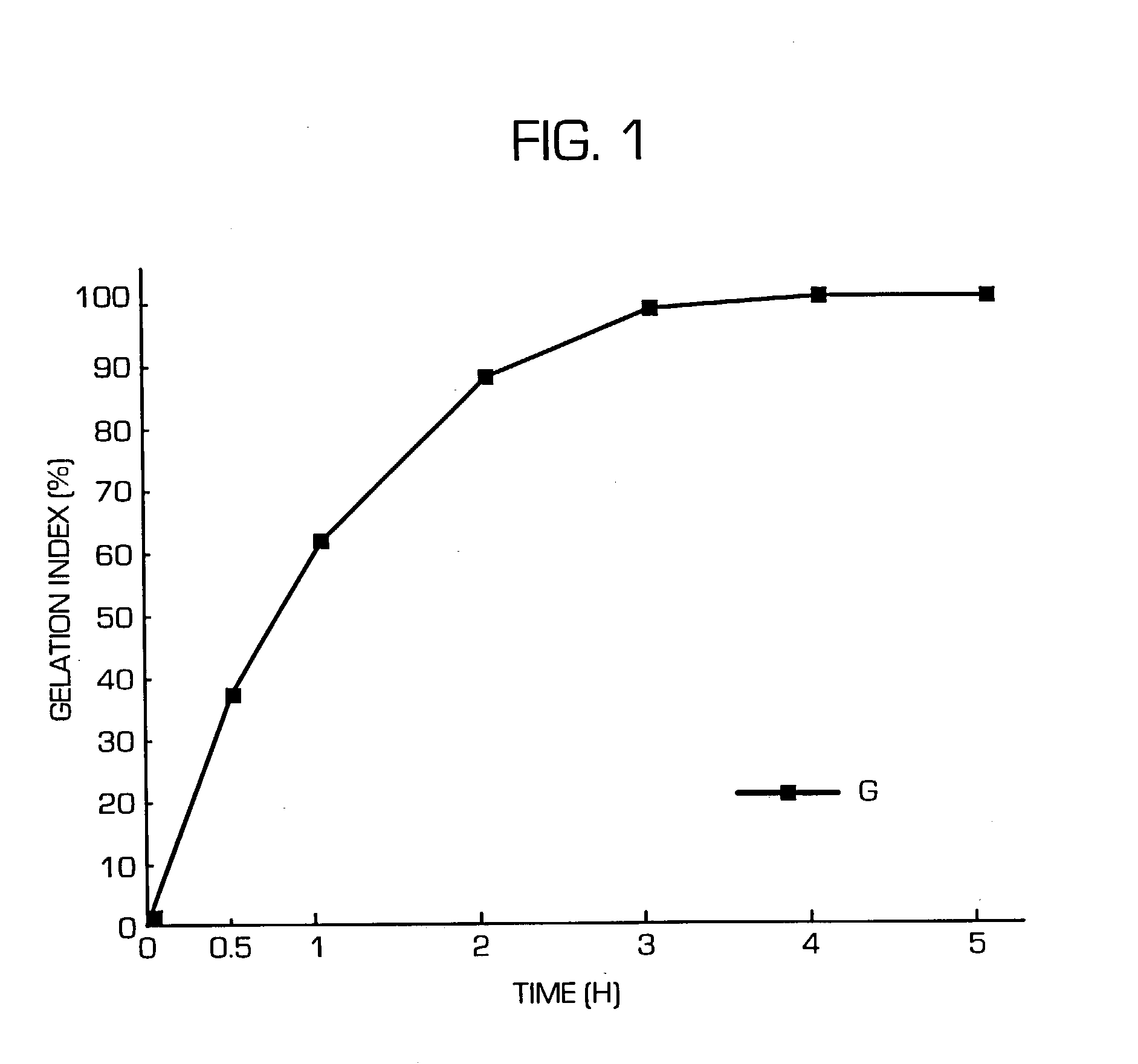
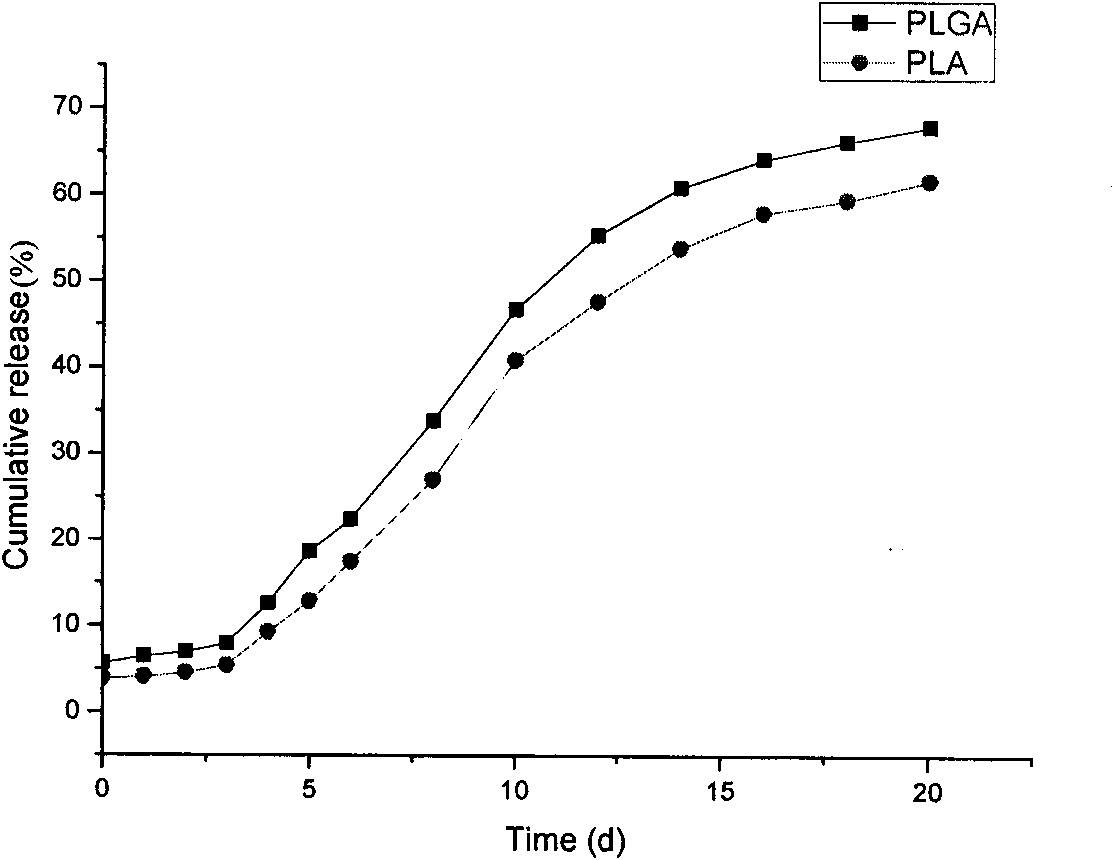
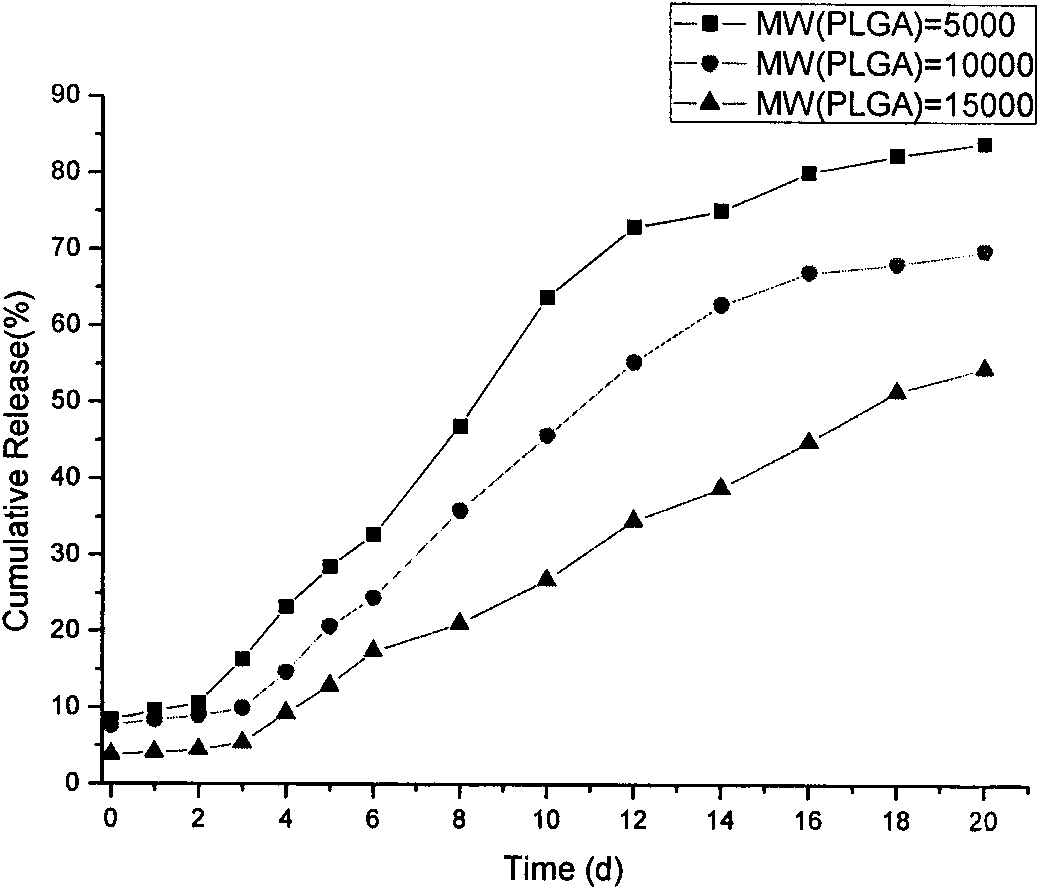
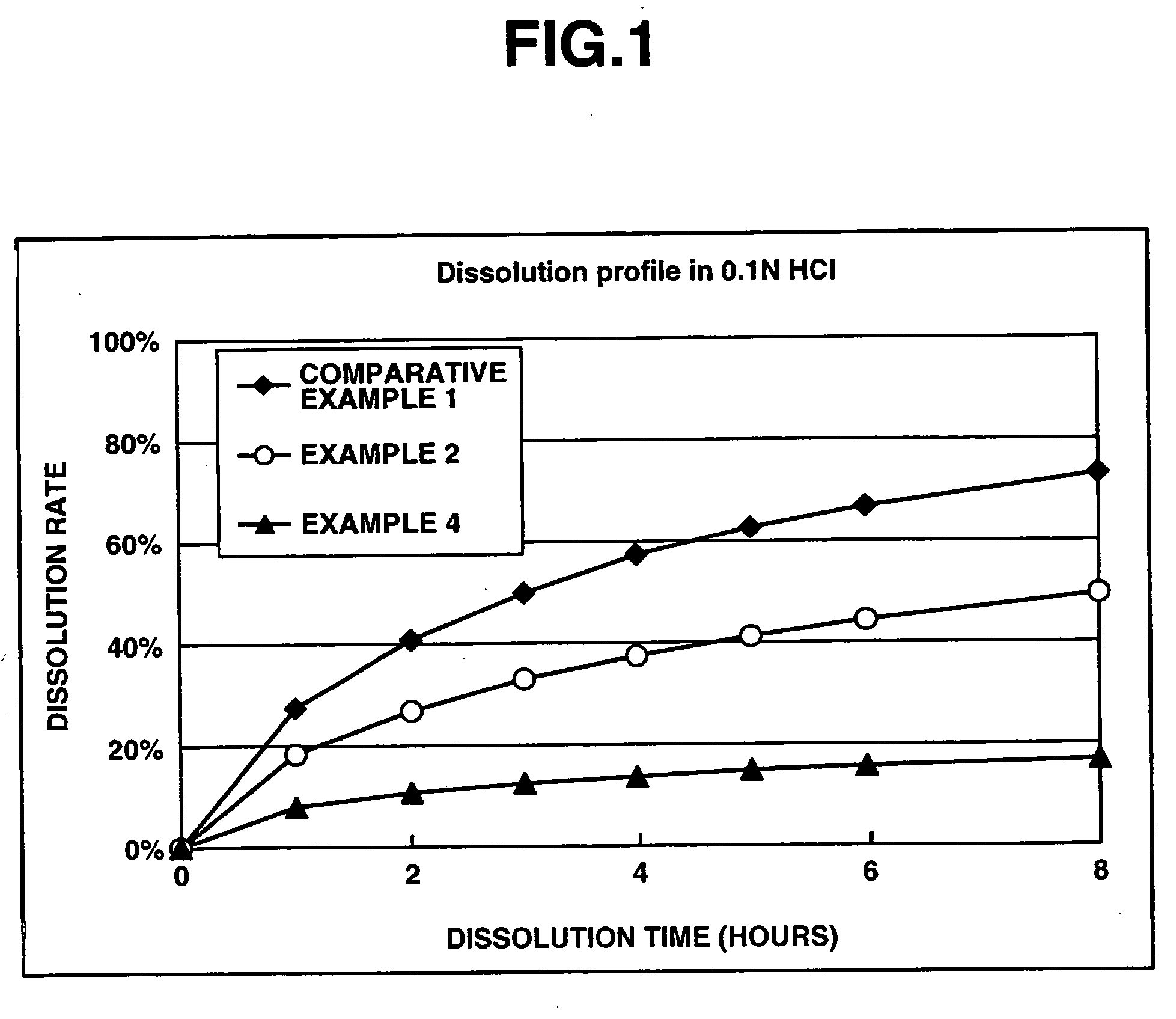
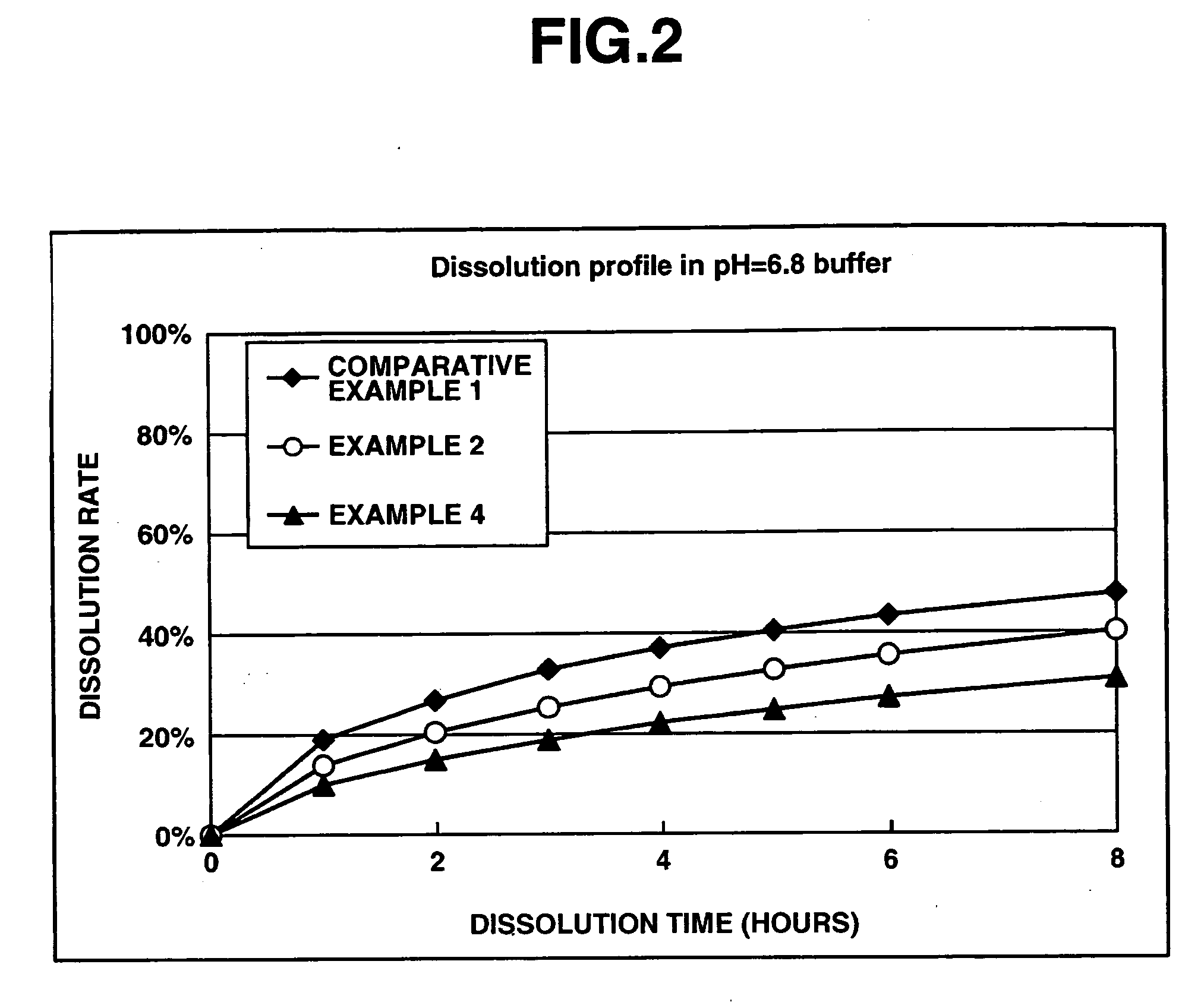
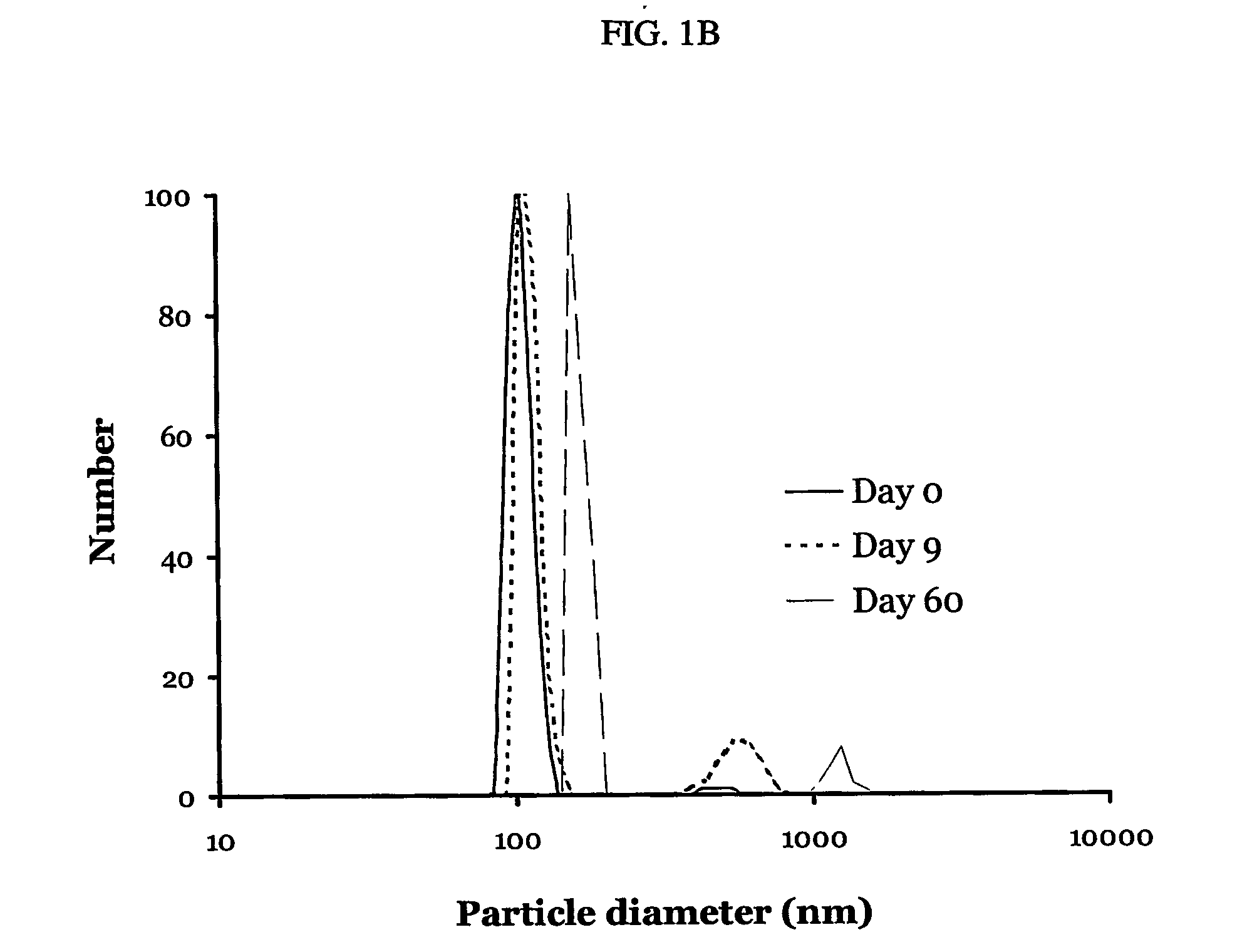
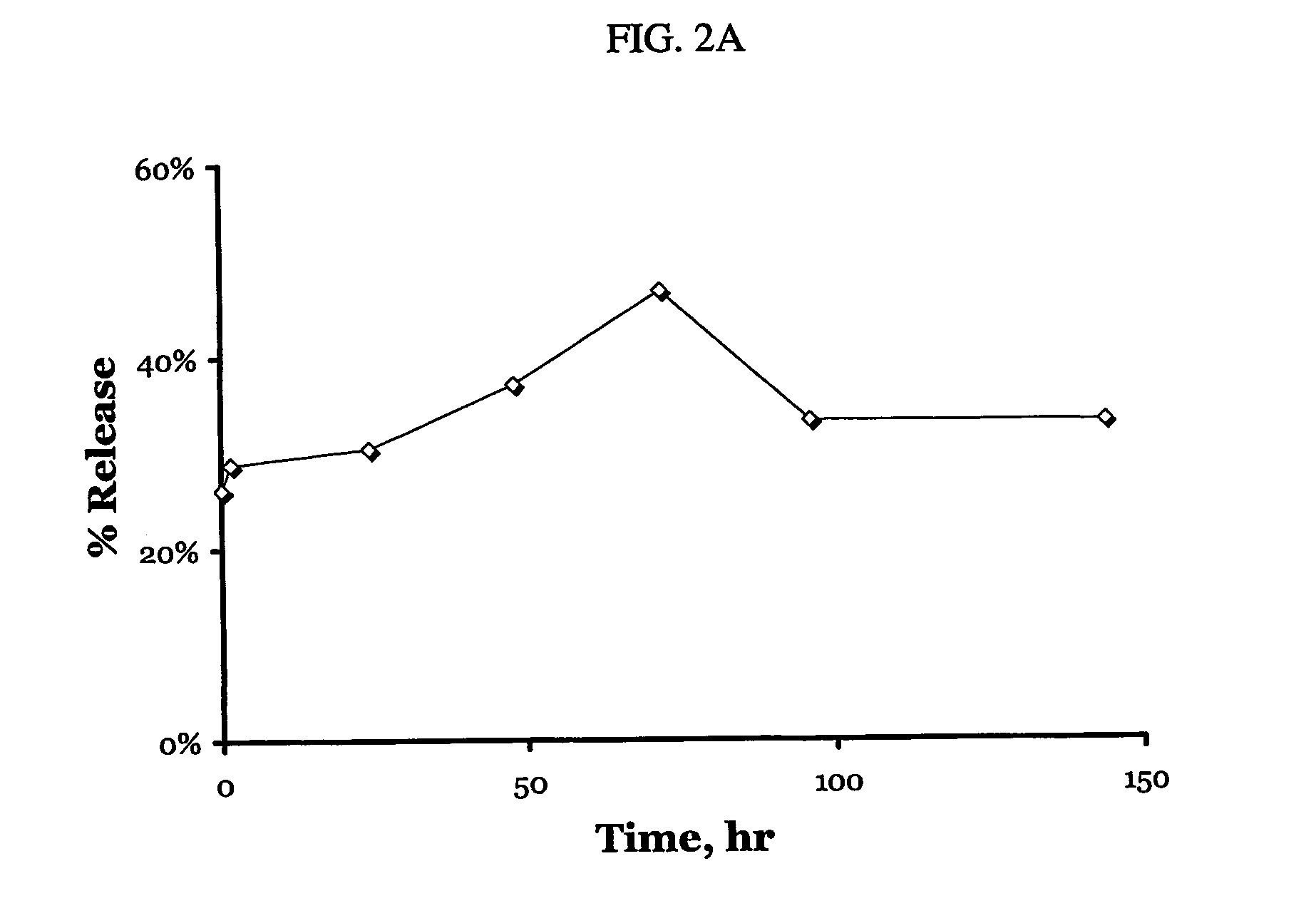
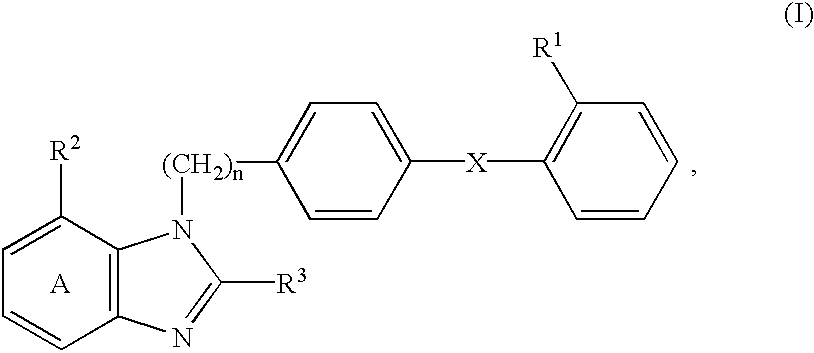
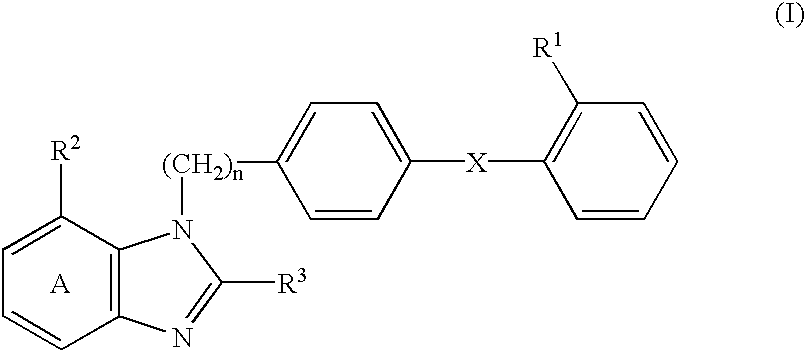
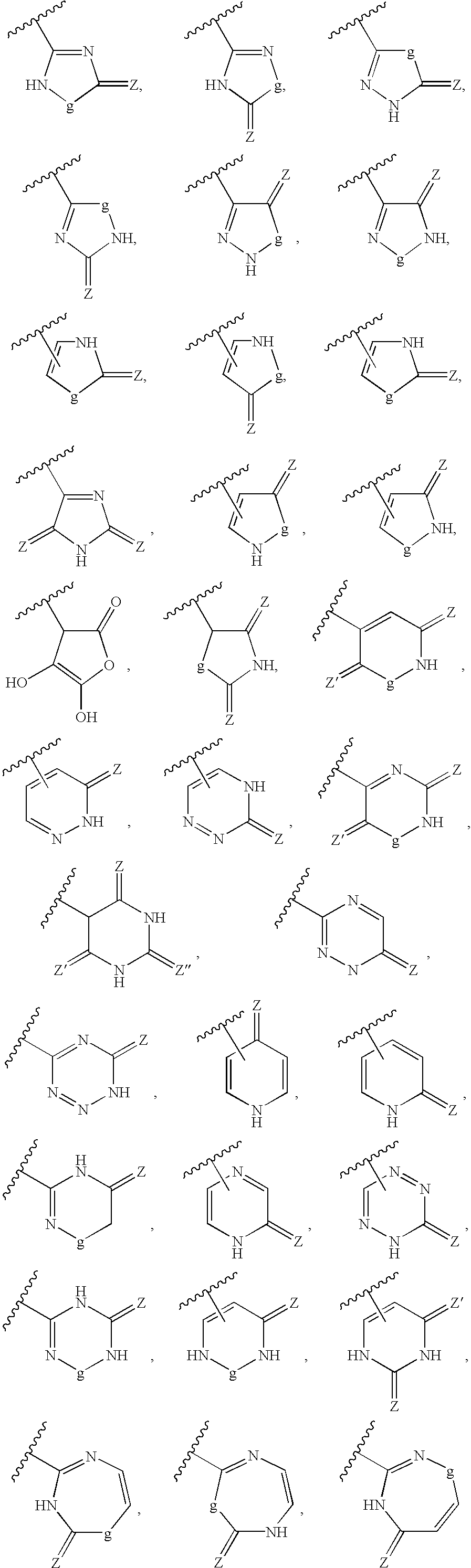
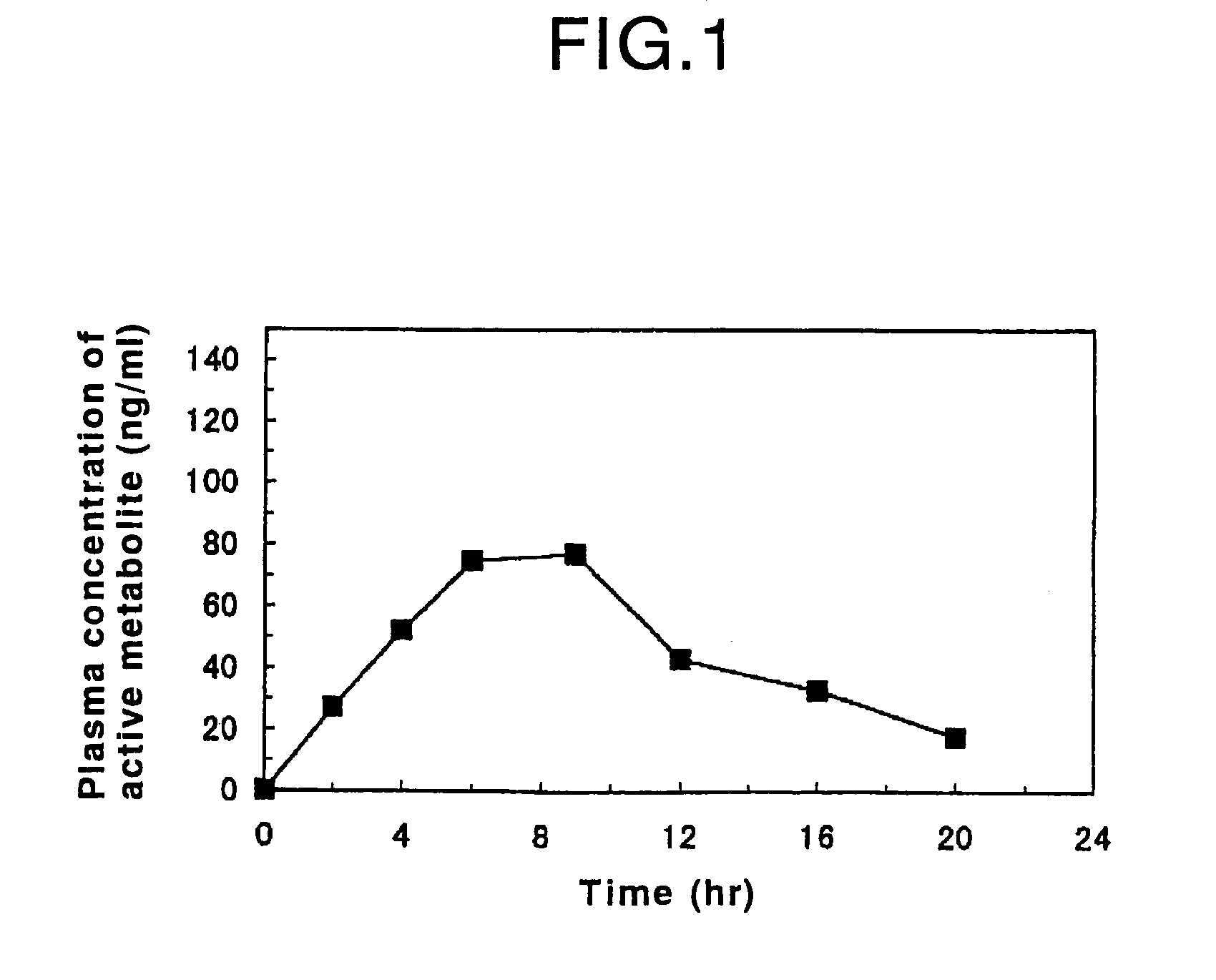
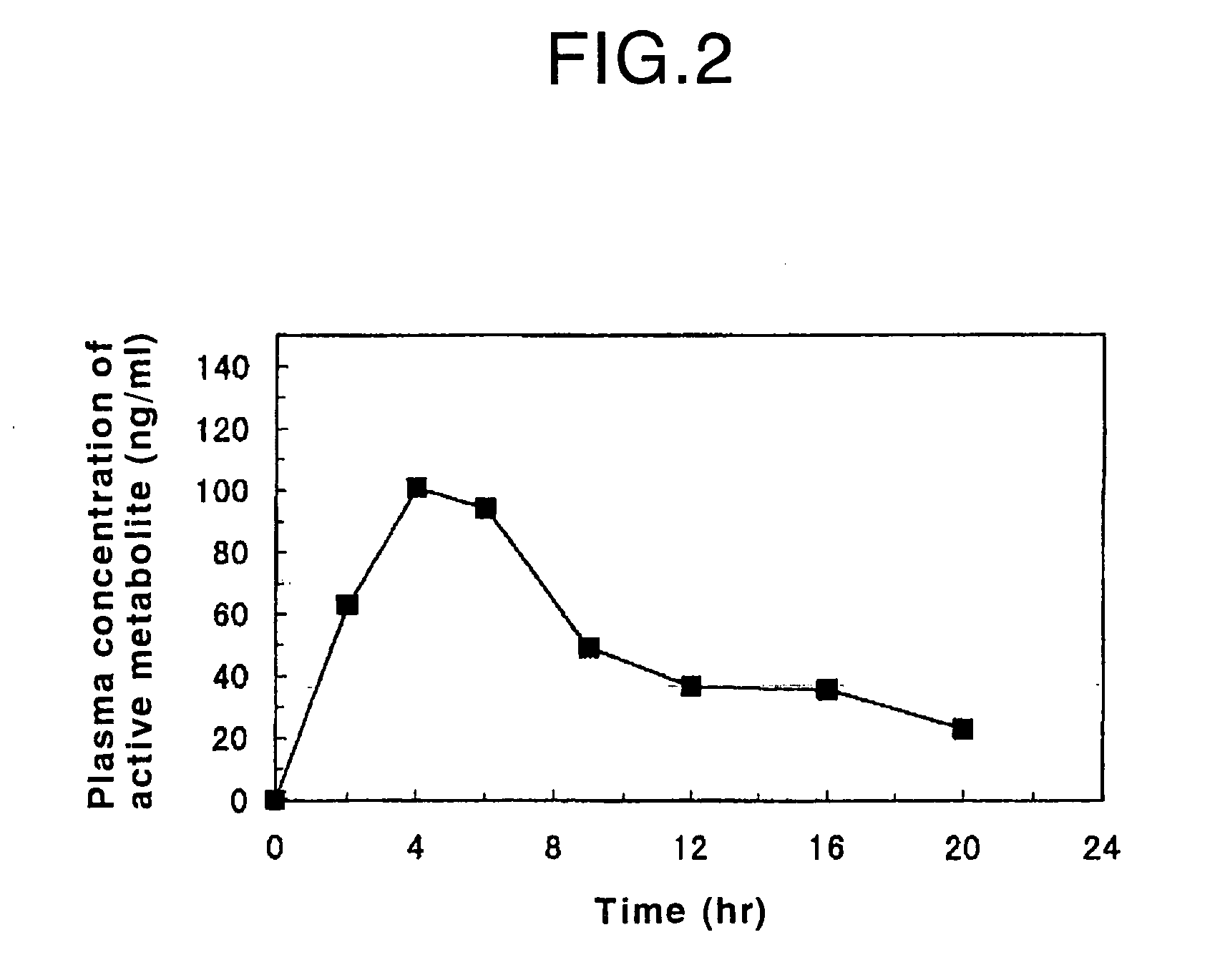
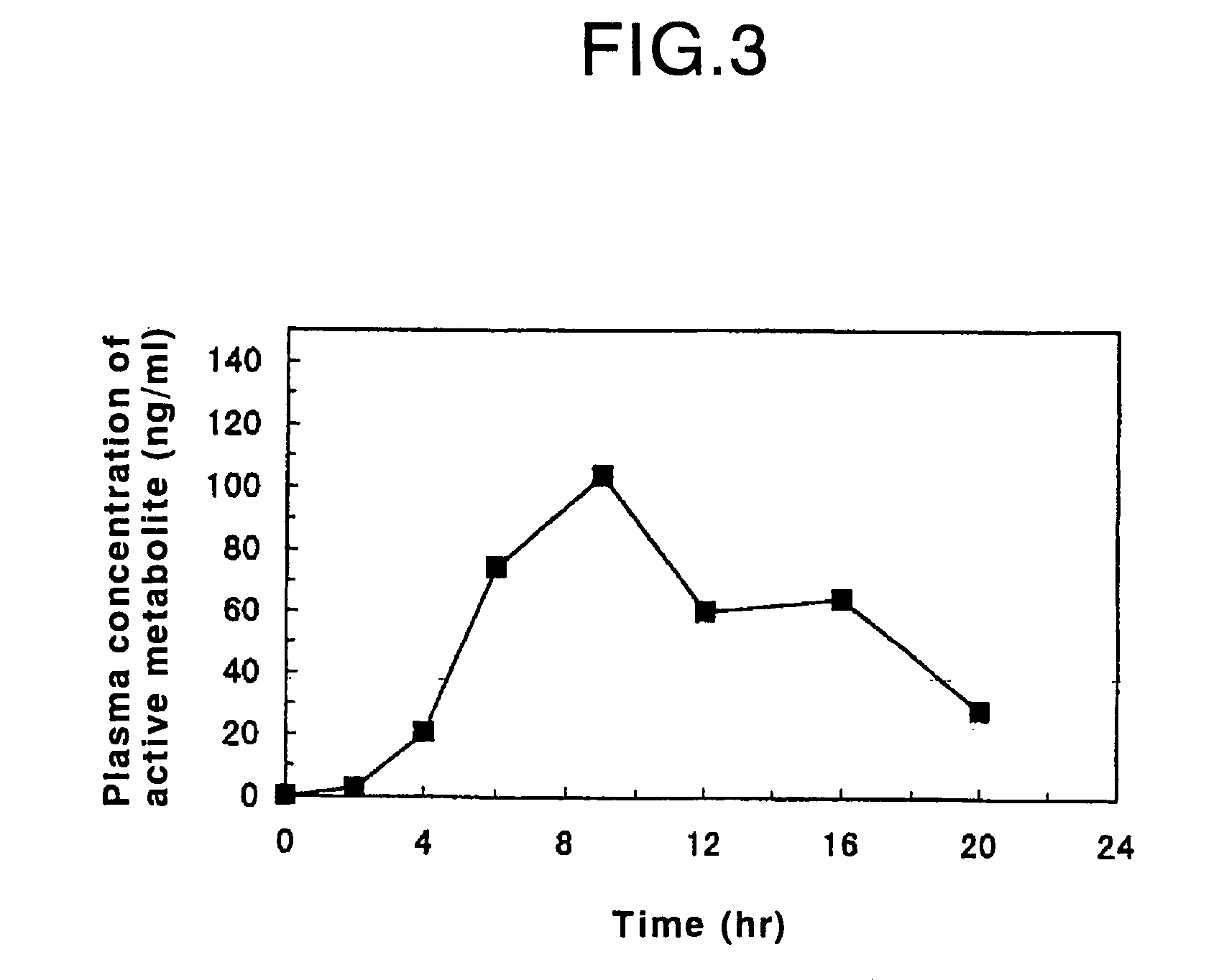
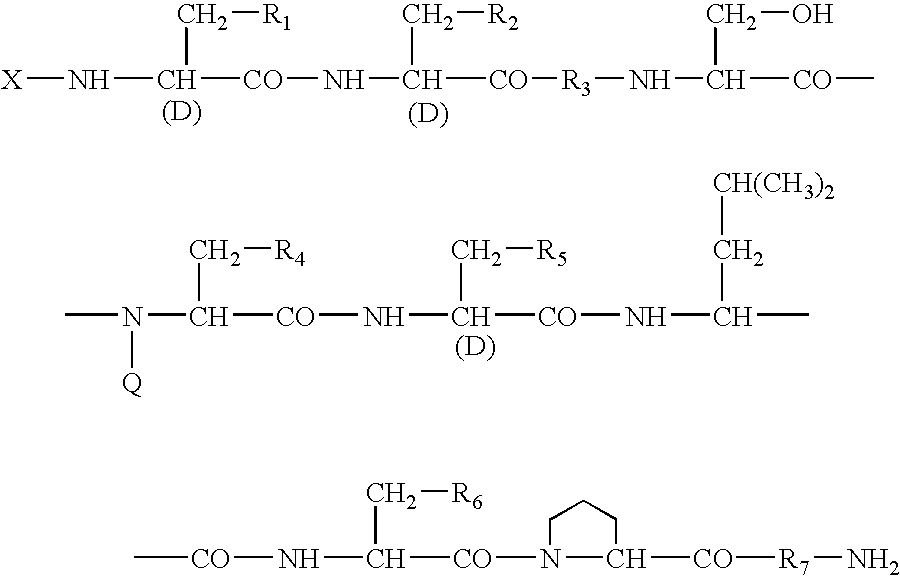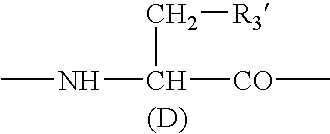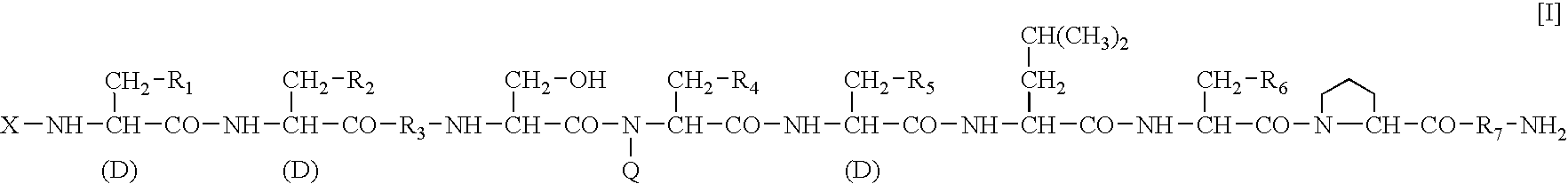Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1328 results about "Sustained-Release Preparations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sustained-release preparations formulations of medicines which limit their solubility so that they are delivered at a slow but steady rate into the blood supply. Includes oral preparations in the form of reticular bullets of metal or ceramic and slow-release granules, intramuscular injection of slowly soluble substances, e.g. procaine penicillin, or ...
Method of producing sustained-release preparation
InactiveUS6267981B1Maintain good propertiesEnhancement of entrapmentPowder deliveryPeptide/protein ingredientsEntrapmentBiodegradable polymer
This invention provides a sustained-release preparation comprising a biodegradable polymer metal salt and broactive polypeptide, with enhanced entrapment of the bioactive polypeptides, a suppression of initial burst, and a constant long-term release of the bioactive polypeptides.
Owner:TAKEDA PHARMA CO LTD
Method of producing a sustained-release preparation
InactiveUS6197350B1Reduce the number of stepsSuitable for industrializationPowder deliveryPeptide/protein ingredientsBlood concentrationOrganic solvent
A method of producing sustained-release microcapsules which comprises dispersing a physiologically active polypeptide into a solution of a biodegradable polymer and zinc oxide in an organic solvent, followed by removing the organic solvent; which provides a sustained-release preparation showing a high entrapment ratio of the physiologically active polypeptide and its constant high blood concentration levels over a long period of time.
Owner:TAKEDA PHARMA CO LTD
Hydrogel-forming sustained-release preparation
InactiveUS6436441B1Rapid drug releaseReduce the amount of solutionPowder deliveryPill deliverySmall intestinePolymer
The invention provides a hydrogel-type sustained-release preparation comprising (1) at least one drug, (2) an additive which insures a penetration of water into the core of the preparation and (3) a hydrogel-forming polymer, wherein said preparation is capable of undergoing substantially complete gelation during its stay in the upper digestive tract such as stomach and small intestine and is capable of releasing the drug in the lower digestive tract including colon.By the preparation of the invention, the drug is efficiently released and absorbed even in the colon so that a steady and sustained release effect can be achieved.
Owner:ASTELLAS PHARMA INC
Sustained release formulations
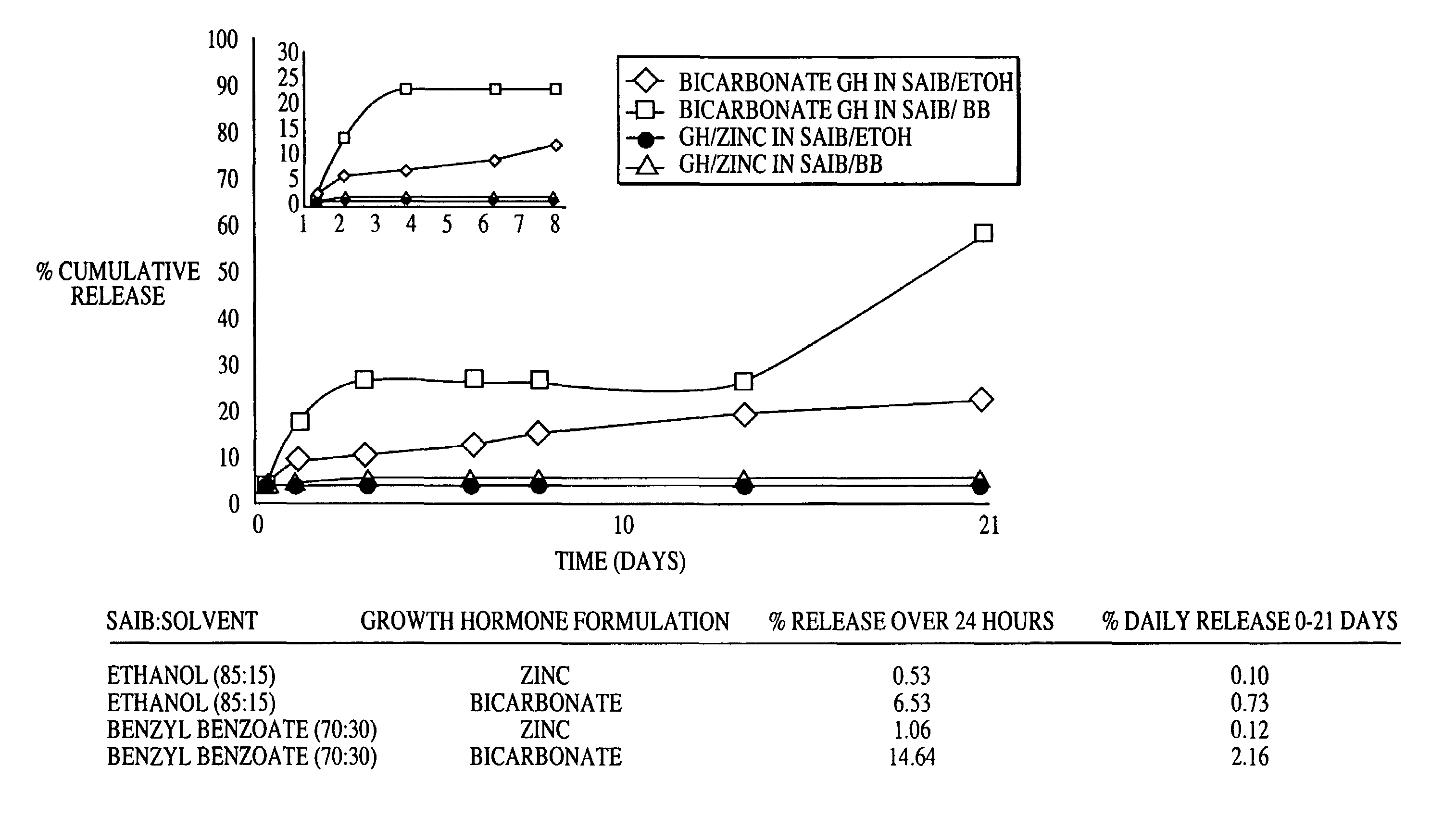
A composition for sustained release comprises a carrier material containing a non-polymeric, non-water soluble liquid material having a viscosity of at least 5,000 cP at 37° C. that does not crytallize neat under ambient physiological conditions, a multivalent metal cation, and growth hormone.
Owner:DURECT CORP
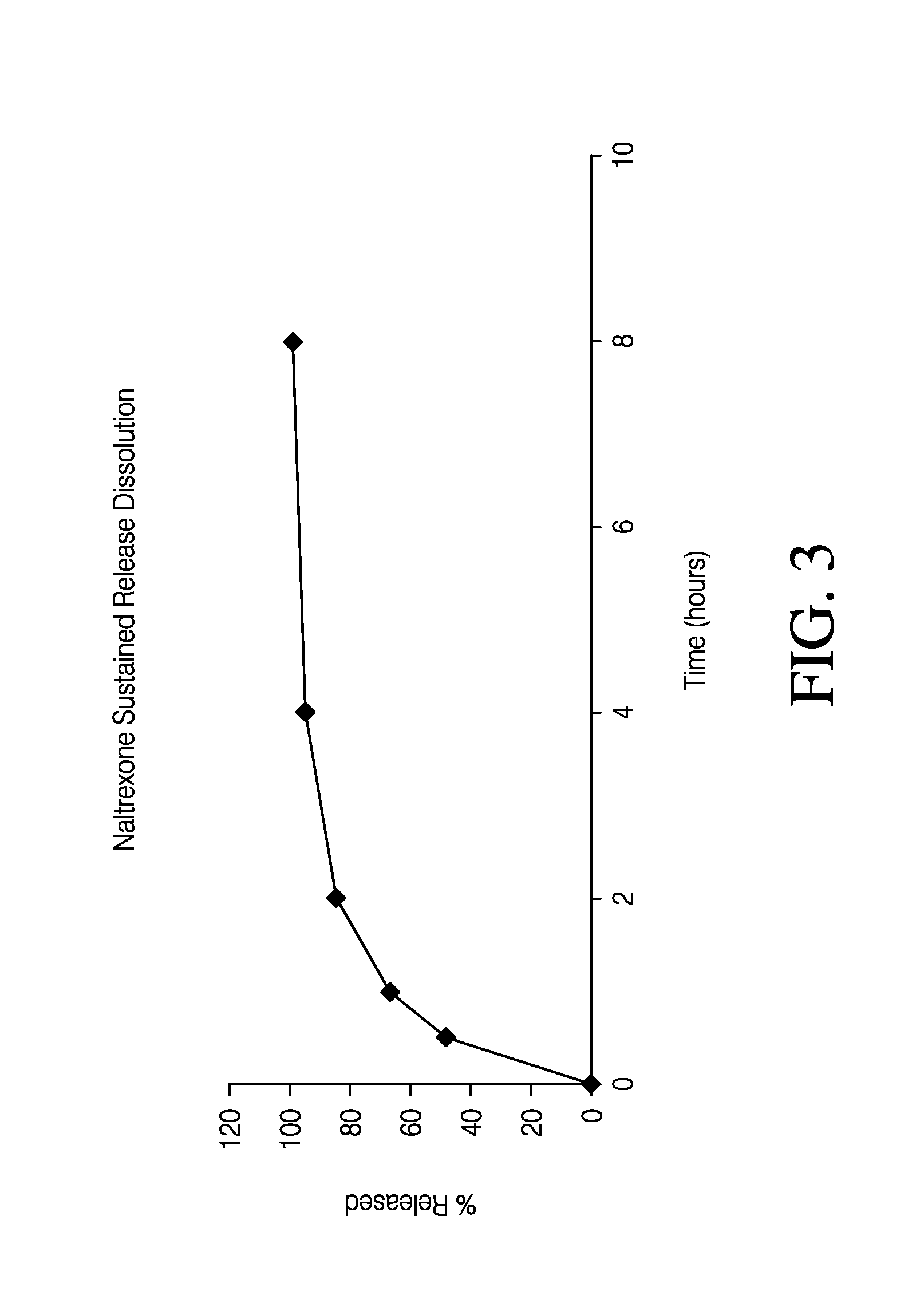
Sustained release formulation of naltrexone
ActiveUS20070281021A1Cause weight lossAvoid weight gainBiocideKetone active ingredientsSide effectCompound (substance)
A sustained-release oral dosage form of naltrexone or a pharmaceutically acceptable salt thereof is provided. The oral dosage form may be administered with another compound. Administration of the oral dosage form may reduce a side effect, which may be a side effect at least partially attributable to a weight-loss treatment. The oral dosage form may be administered to treat a weight-loss condition.
Owner:OREXIGEN THERAPEUTICS INC
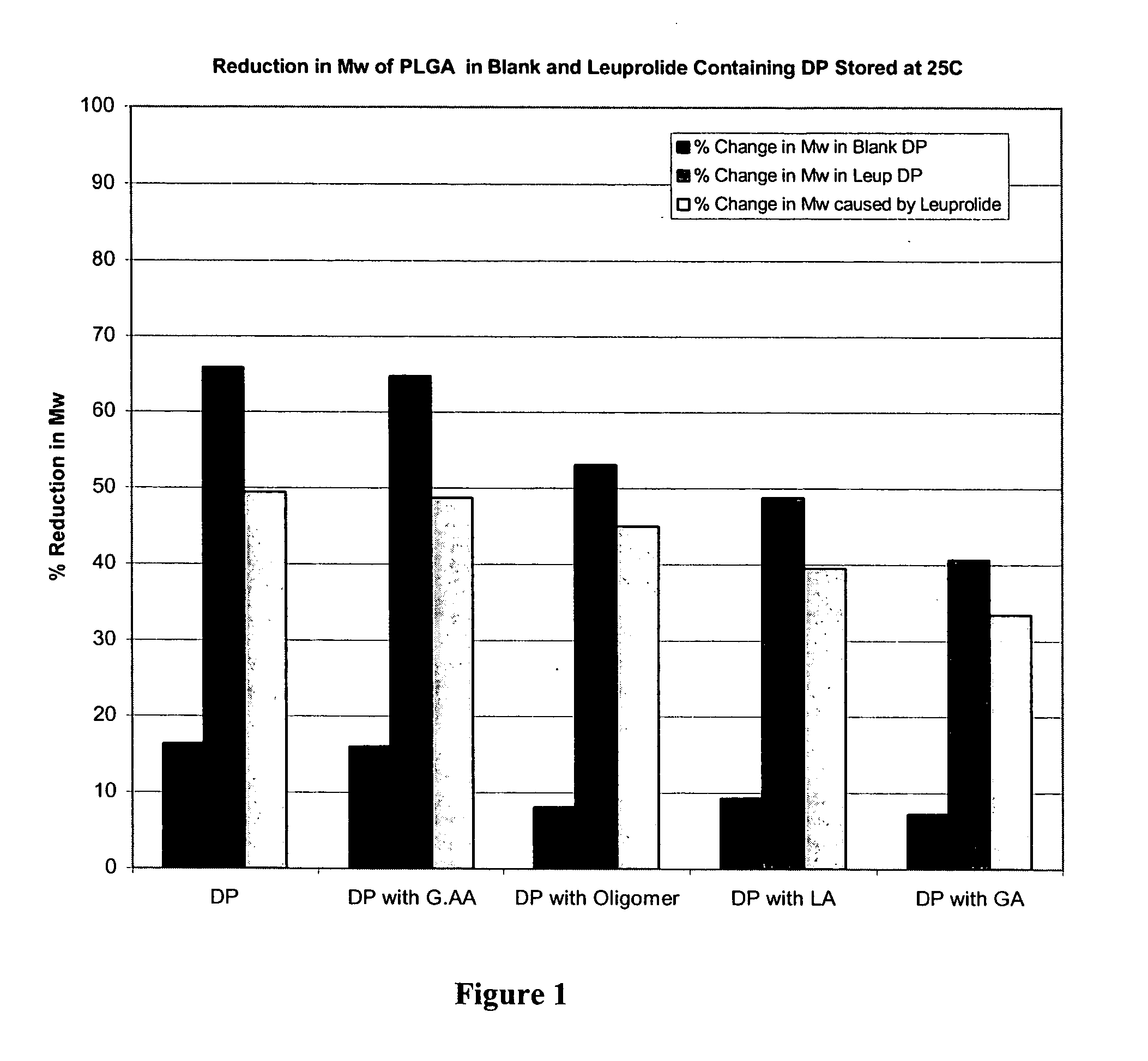
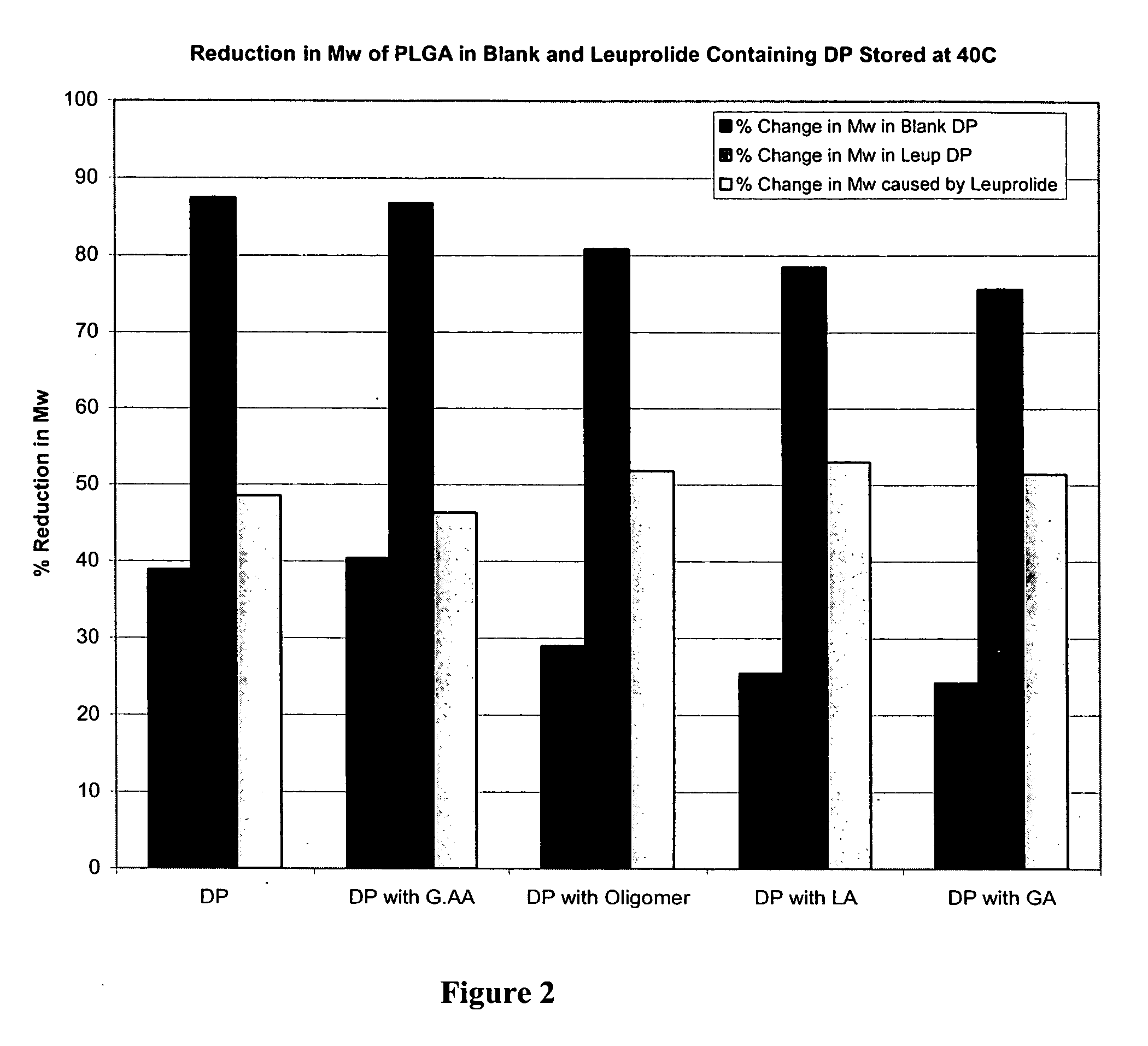
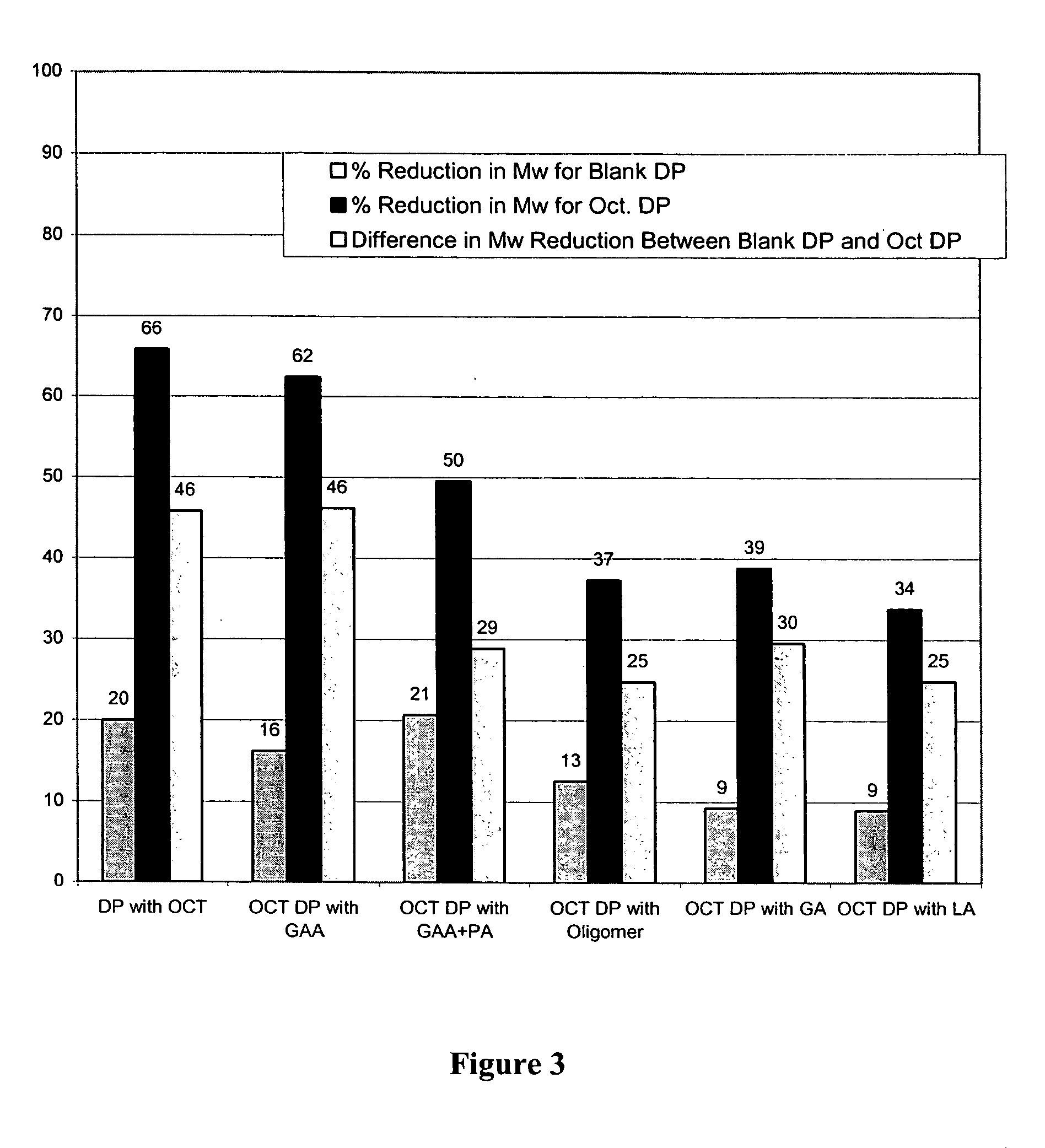
Prevention of molecular weight reduction of the polymer, impurity formation and gelling in polymer compositions
ActiveUS20050042294A1Reduce and eliminate molecular weight reductionReduce molecular weightPowder deliveryBiocideSolubilityActive agent
Polymer and drug containing compositions and method of preparing such compositions are disclosed. The dispersed phase formulation has a polymer, a pharmaceutically or biologically active agent and a small fraction of low pKa acid additive. Stable, filter sterilizable, non-gelling solutions containing GnRH analogues at least at levels typically used in sustained release formulations and a method of increasing solubility of a high level of a GnRH analogue or a freeze-dried antgonist of GnRH in a polymer containing solution are also disclosed. The amount of the acid additive in the polymer solution is such that it is sufficient to increase the solubility of the high level of the GnRH analogue in the polymer solution without affecting the release characteristics of the microspheres prepared therefrom.
Owner:OAKWOOD LAB LLC
Sustained release preparation
InactiveUS20060039974A1Reduce decreaseReduced activityOrganic active ingredientsMetabolism disorderDipeptidyl peptidaseDipeptidyl-Peptidase IV Inhibitors
The sustained-release preparation of the present invention, which contains a dipeptidyl peptidase IV inhibitor and a hydrophilic polymer, can appropriately inhibit the DPP-IV activity, and is superior in convenience or compliance.
Owner:TAKEDA PHARMA CO LTD
Methods for administering aripiprazole
InactiveUS20050032811A1Without complexityWithout expenseOrganic active ingredientsNervous disorderActive agentMicrosphere
The present invention relates, in part, to the discovery that a pharmaceutical composition comprising aripiprazole and a carrier administered in a bolus injection resulted in an extended release profile similar to that obtained by the injection of a poly lactide-co-glycolide microsphere formulation containing the active agent. This surprising result suggests that pharmacologically beneficial extended release formulations without the complexities and expense associated with the manufacture microspheres.
Owner:ALKERMES INC
Robust sustained release formulations
InactiveUS20080085304A1Avoid dose dumpingHigh drug safetyPowder deliveryPill deliverySolid Dose FormOxymorphone
Robust sustained release formulations, solid dosage forms comprising robust sustained release formulations, and methods for making and using these formulations and solid dosage forms are provided. Robustness of the sustained release formulation is related to the particle size of the hydrophilic gum. Sustained release formulations resist dose-dumping when ingested with alcohol. The formulations are useful for treating a patient suffering from a condition, e.g., pain. The formulations comprise at least one drug. In one embodiment, the drug is an opioid, e.g., oxymorphone.
Owner:ENDO PHARMA INC
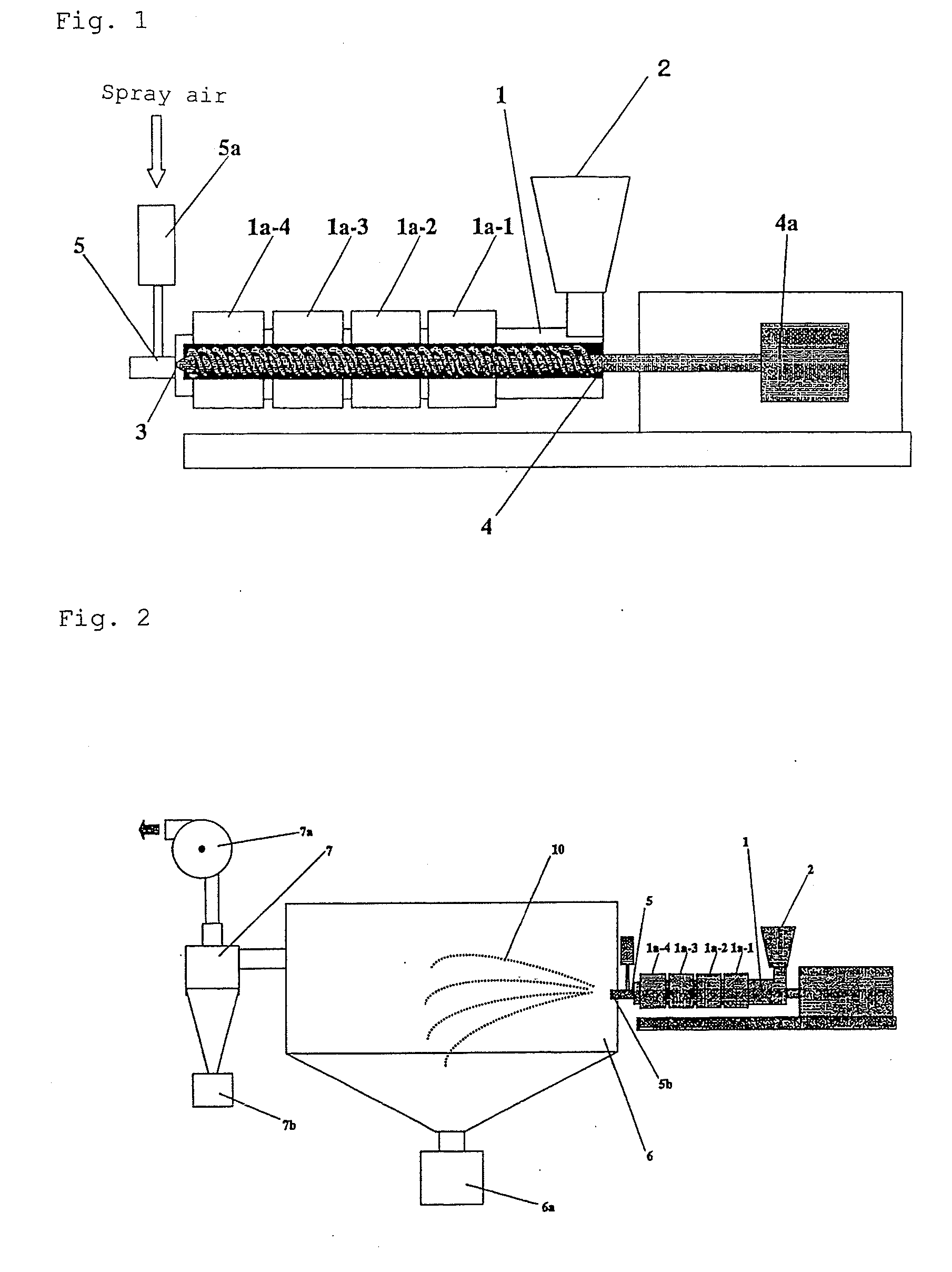
Method of producing drug-containing wax matrix particles, extruder to be used in the method and sustained-release preparation containing cilostazol
InactiveUS20090047357A1Easy to produceSolve low usagePowder deliveryBiocideSpray nozzleSustained-Release Preparations
The present invention aims to provide a method for producing, by a simple method, drug-containing wax matrix granules, particularly drug-containing wax matrix granules having an average particle diameter of 1 mm or lower, while avoiding liquid blockage due to the recrystallization of a molten drug during the period from a melting step to a spray step.Drug-containing wax matrix granules having at least one wax and at least one drug are produced by the following steps (i) and (ii): (i) supplying the at least one drug and the at least one wax to an extruder in which the temperature of a barrel and the temperature of a die are adjusted to be higher than the melting point of the at least one wax; and (ii) while melting and kneading the at least one drug and the at least one wax in the extruder to give a molten kneaded drug and wax, spraying the molten kneaded drug and wax into an atmosphere having a temperature lower than the melting point of the wax from a spray nozzle directly mounted onto a die provided at a top end of the barrel of the extruder, thereby forming the mixture into granules.
Owner:OTSUKA PHARM CO LTD
Sustained release preparations
Disclosed are sustained release drug particles suitable for forming sustained release oral pharmaceutical compositions. The sustained release drug particles comprise a drug-ion exchange resin complex and a water-permeable, diffusion barrier surrounding at least a portion of the drug-ion exchange resin complex. The diffusion barrier comprises a film-forming polymer and is free or contains no substantial traces of organic solvent. Also disclosed are oral pharmaceutical compositions, for example, oral suspensions, comprising the sustained release drug particles, a method for the controlled administration of a drug to a patient, and a method for manufacturing the sustained release drug particles. The method of manufacturing involves the use of an aqueous coating composition comprising a water-permeable film-forming polymer such as ethylcellulose.
Owner:MALLINCKRODT INC
Sustained release pharmaceutical composition
InactiveUS20050100603A1Equivalent and even more efficacyReduce the adverse eventsPowder deliveryBiocideTamsulosin hclSustained release drug
[Problem] As compared with the current oral sustained-release preparation containing tamsulosin hydrochloride which have been supplied to the medical setting at present, it is needed to provide a sustained-release pharmaceutical composition in which the efficacy is equivalent or even better, adverse events such as adverse reactions (e.g., postural hypotension) are reduced, dose can be increased and, if desired, ingestion of food is not limited in the dosage and it is also needed to provide a method for administration of tamsulosin hydrochloride in which the adverse reactions accompanied by therapy or prevention on the basis of an α1 receptor blocking action are reduced. [Means for Resolution] A sustained-release pharmaceutical composition, characterized in that, there are contained tamsulosin or a pharmaceutically acceptable salt thereof and a carrier for a sustained-release pharmaceutical composition and the ratio (Cmin / Cmax ratio) of the plasma tamsulosin concentration at 24 hours after the administration of the preparation per os (Cmin) to the maximum plasma tamsulosin concentration after the administration (Cmax) is about 0.4 or more.
Owner:ASTELLAS PHARMA INC
Sustained-release preparation utilizing thermal change and process for the production thereof
A sustained-release preparation which can release a highly water-soluble medicinally active ingredient over a long time and a process for the production thereof are provided. The preparation has a sustained-releasing layer formed by heating and melting a layer composed of both an aqueous ethylcellulose latex containing a plasticizer and a wax to miscibilize them.
Owner:SHIONOGI & CO LTD
Hydrogel-forming sustained-release preparation
InactiveUS20030203024A1Prolongs absorption periodStable levelPharmaceutical non-active ingredientsPill deliverySmall intestinePharmaceutical Substances
The invention provides a hydrogel-type sustained-release preparation comprising (1) at least one drug, (2) an additive which insures a penetration of water into the core of the preparation and (3) a hydrogel-forming polymer, wherein said preparation is capable of undergoing substantially complete gelation during its stay in the upper digestive tract such as stomach and small intestine and is capable of releasing the drug in the lower digestive tract including colon. By the preparation of the invention, the drug is efficiently released and absorbed even in the colon so that a steady and sustained release effect can be achieved.
Owner:ASTELLAS PHARMA INC
Active component of red sage root, its slowly releasing medicine and medical purpose, and its preparing process
An active component of red sage root, its slow-releasing agent, its medicinal application, and its preparing process are disclosed. In its effective region, the content of general solviolic acid is more than 80% and the content of salviolic acid A is more than 10%. Said slow-releasing agent is prepared through extracting in water or alcohol ic water, getting a supernatant, removing alcohol, dissolving in water, then washing to remove inpurities, eduting with alcohol, recovering alcohol eluent and drying. It can be used to prepare the medicine to prevent and cure cardiovascular and cerebrovascular diseases.
Owner:SHENYANG PHARMA UNIVERSITY
Polypeptide medicament sustained release microsphere or microcapsule preparation with uniform grain size and preparation method thereof
ActiveCN101559041ASmall particle sizeRegulated release ratePeptide/protein ingredientsMetabolism disorderMicrosphereMedicine
The invention discloses a polypeptide medicament sustained release microsphere or a microcapsule preparation with uniform grain size, a preparation method thereof and application. The average grain size of the microsphere or the microcapsule preparation is between 50 nanometers and 100 microns, and the grain size distribution coefficient CV value is less than 20 percent. The polypeptide medicament has a definite structure, has functions of therapy or adjuvant therapy of type-2 diabetes, and is preferably one or more of GLP-1, Exenatide, Exendin-4 and derivatives and analogs thereof. The microsphere or the microcapsule preparation uses a microsphere or a microcapsule with uniform grain size as a substrate to prepare the polypeptide medicament into a sustained release preparation through an embedding mode, and by changing the grain size of the microsphere or the microcapsule, the sustained release cycle is adjustable between one week and one month, and the microsphere or the microcapsule preparation can be applied to the therapy or the adjuvant therapy of the type-2 diabetes and body weight control. Besides, the microsphere or the microcapsule preparation has the advantages of simple preparation process and mild preparation course, and can protect the biological activity of the embedded polypeptide medicament.
Owner:辉粒药业(苏州)有限公司
Matrix type sustained-release preparation containing basic drug or salt thereof
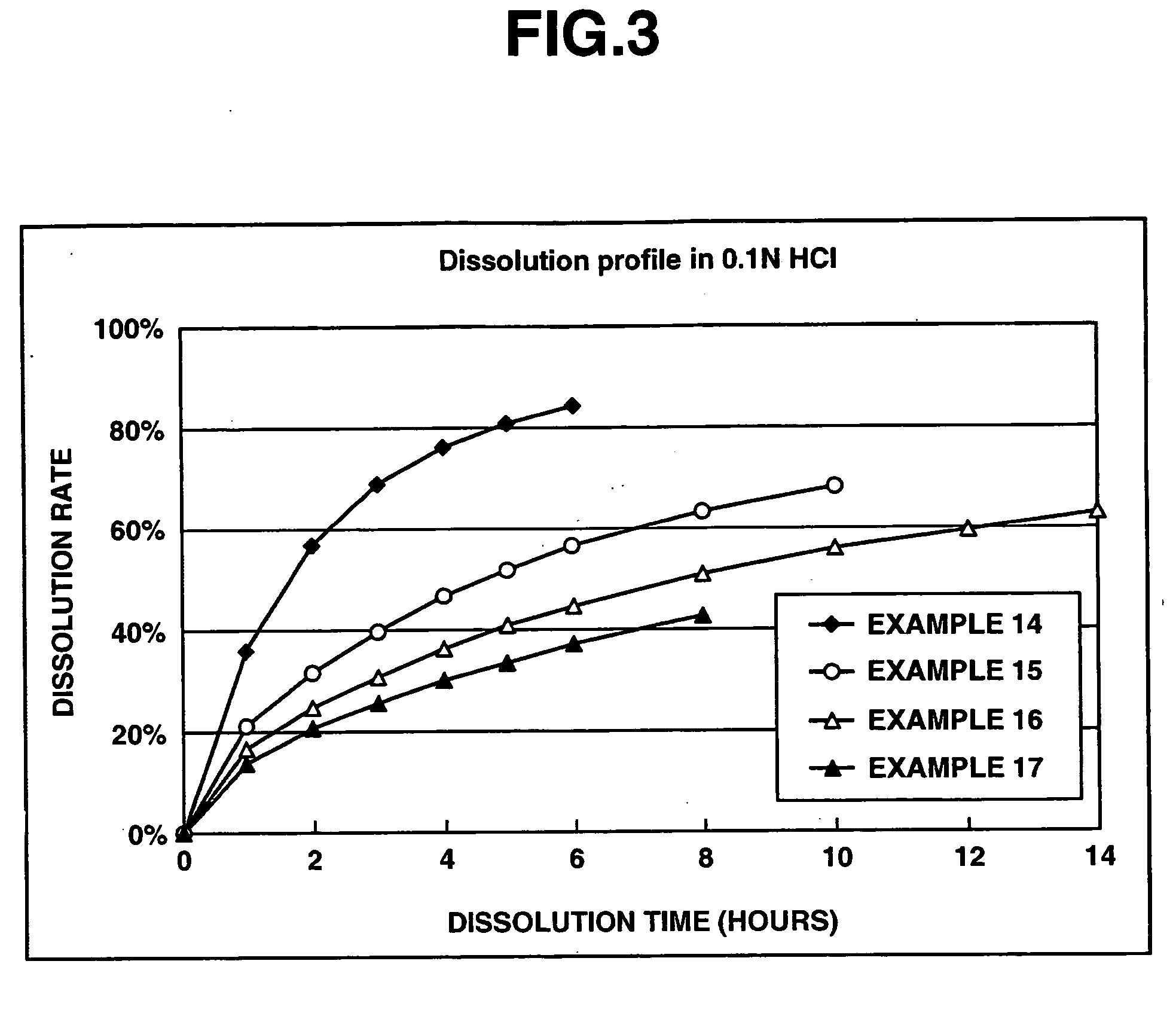
A matrix type sustained-release preparation and a manufacturing method therefor are provided wherein dissolution with low pH dependence of a basic drug or a salt thereof at the early stage of dissolution can be ensured in a dissolution test, and wherein as the dissolution test proceeds, a ratio of a dissolution rate of the basic drug or the salt thereof in an acidic test solution to a dissolution rate of the basic drug or the salt thereof in a neutral test solution (dissolution rate in the acidic test solution / dissolution rate in the neutral test solution) decreases with dissolution time at the late stage of dissolution, as compared to the early stage of dissolution. According to the present invention, the matrix type sustained-release preparation contains a basic drug or a salt thereof and at least one enteric polymer, in which solubility of the basic drug or the salt thereof in a 0.1 N hydrochloric acid solution and a neutral aqueous solution, pH 6.0 is higher than in a basic aqueous solution, pH 8.0.
Owner:EISIA R&D MANAGEMENT CO LTD
Sustained-release preparation
ActiveUS20040057996A1Superior clinical characteristicGood treatment effectPeptide/protein ingredientsSolution deliveryPolyolSustained-Release Preparations
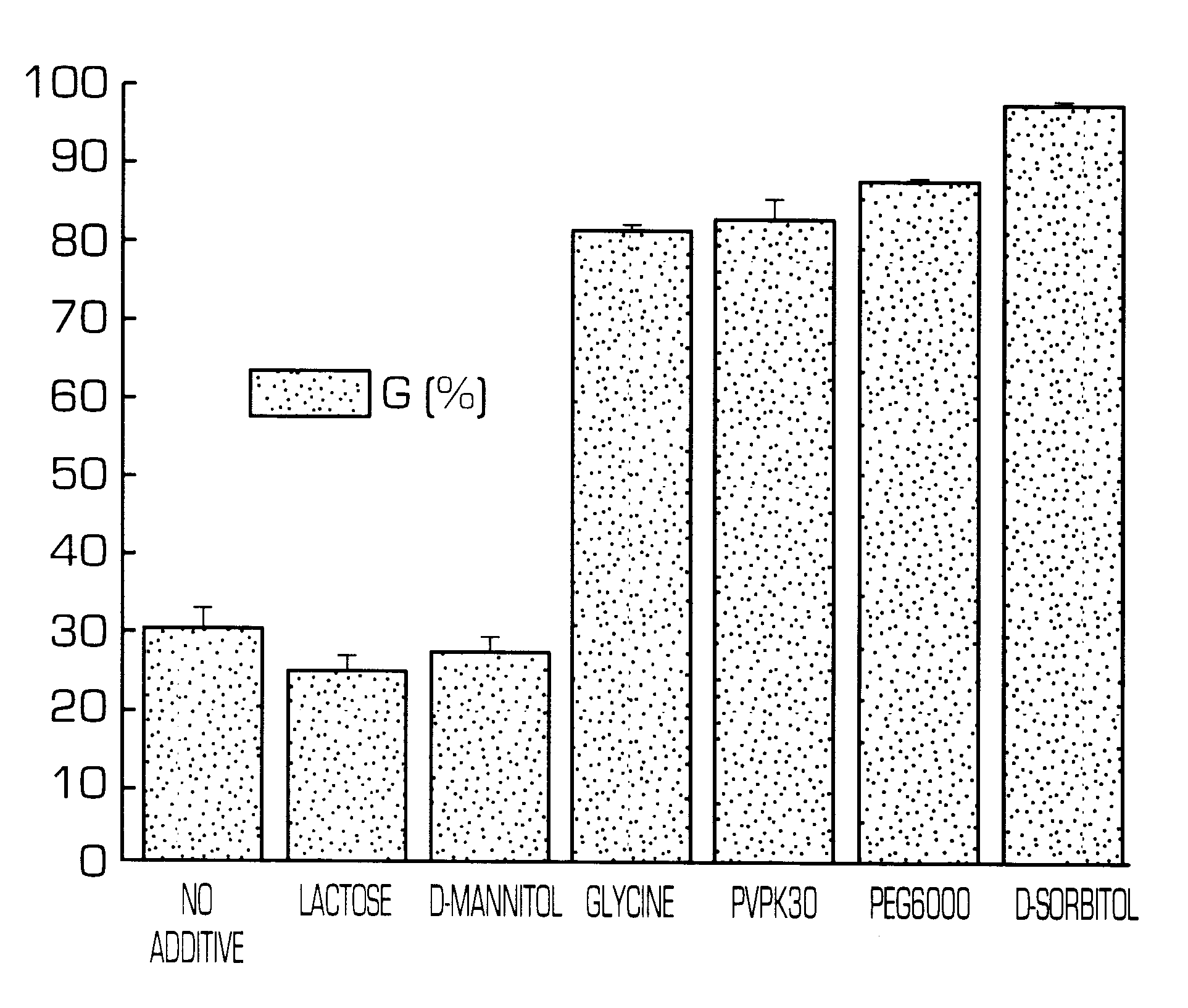
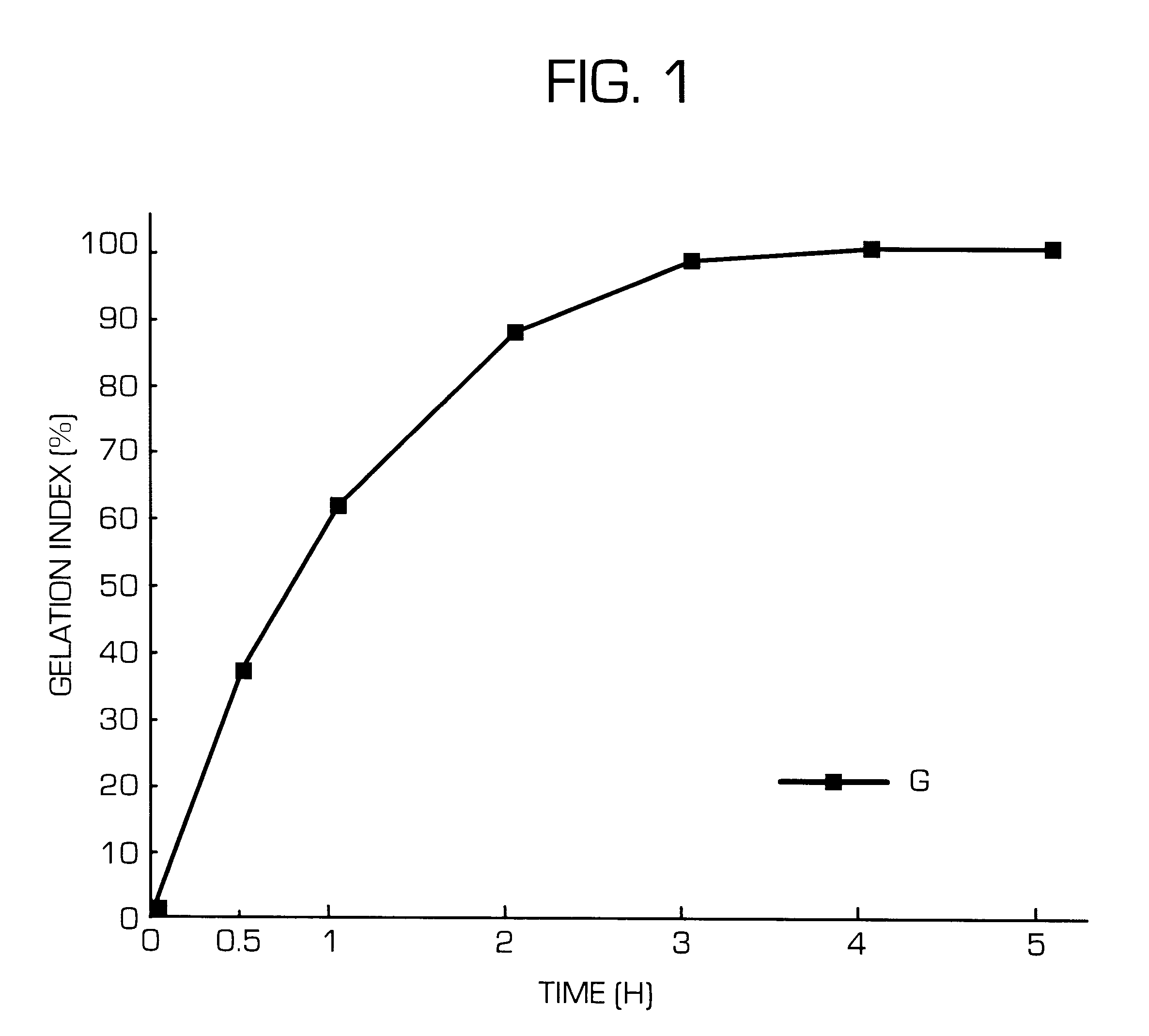
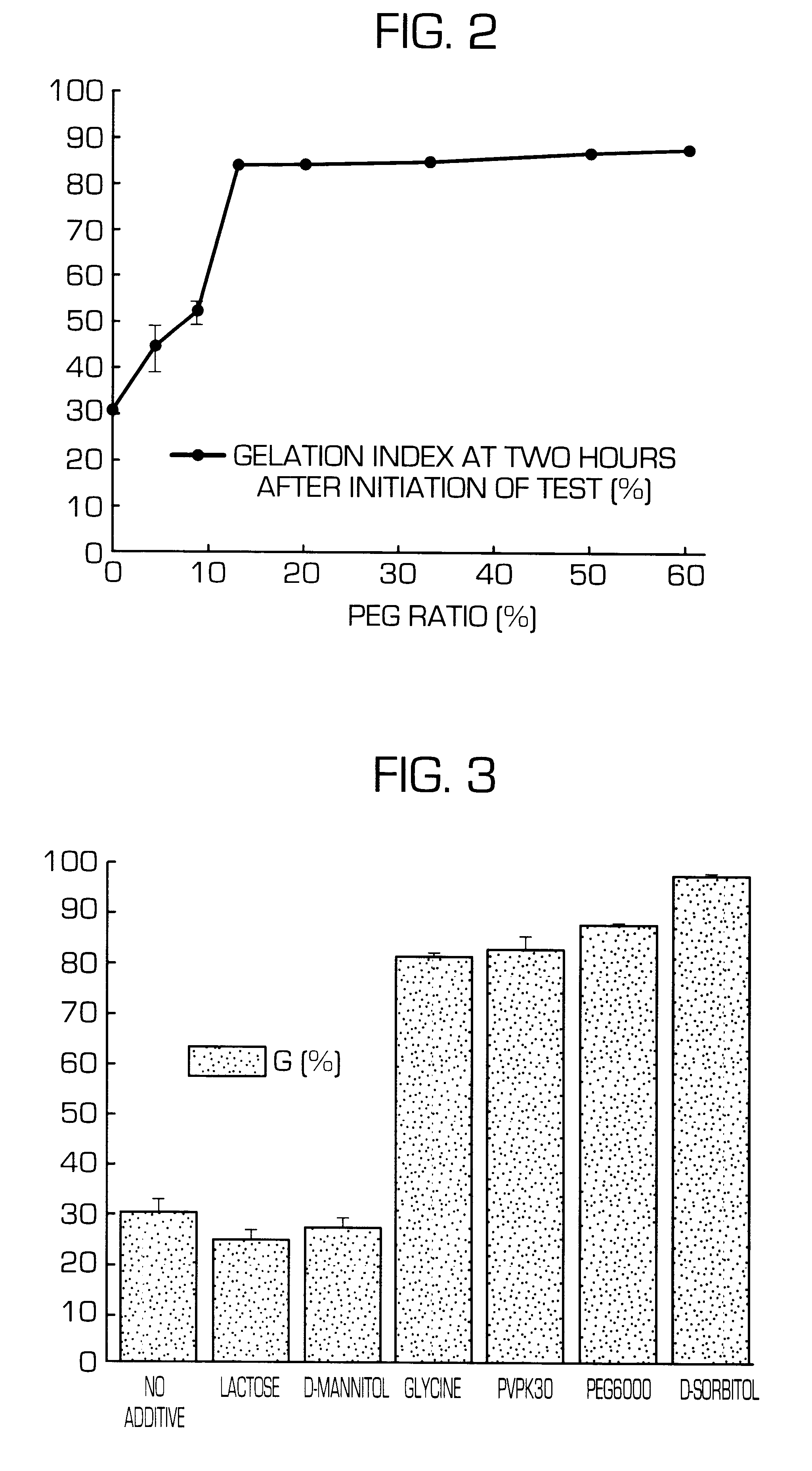
Sustained release preparations, which show suppressed initial release of a physiologically active substance and can release a constant amount of the physiologically active substance over a long period of time, and dispersion vehicle thereof can be obtained by adding a cationic substance or polyols to the outside of a matrix or dispersion vehicle thereof. Thus, sustained release preparations, which show suppressed initial release of a physiologically active substance immediately after the administration and can release a constant amount of the physiologically active substance over a long period of time and dispersion vehicle thereof can be provided.
Owner:TAKEDA PHARMA CO LTD
Sustained-release preparations and method for producing the same
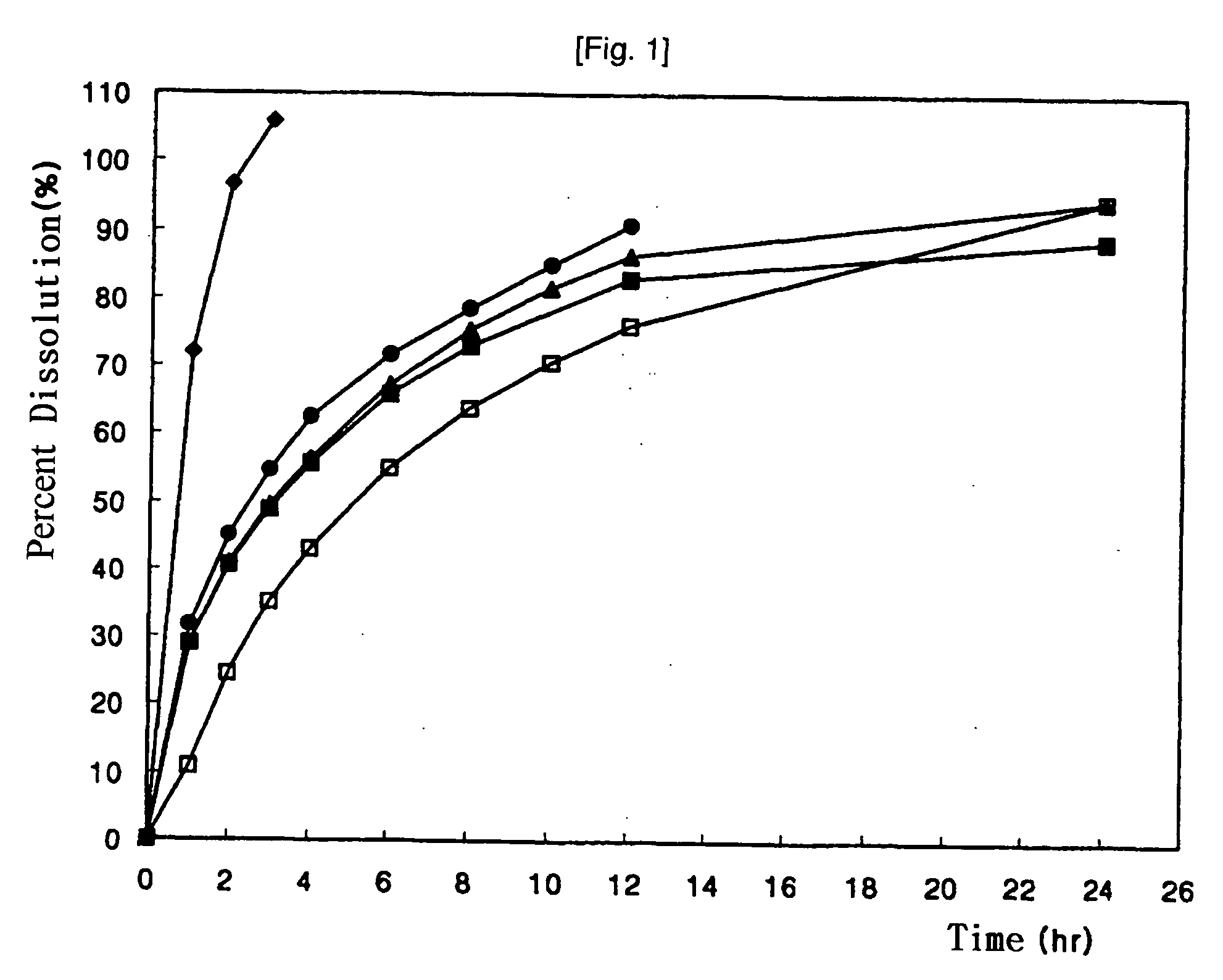
The present invention relates to sustained-release preparations prepared by double granulation and methods for producing the same. The sustained-release preparation according to the present invention enables maintenance of effective blood concentration of drug for many hours via sustained release of the drug over 12 hours or more, and further its production is easy owing to convenience of process.
Owner:AMOREPACIFIC CORP
Lactic acid polymer and process for producing the same
InactiveUS7019106B2Peptide/protein ingredientsPharmaceutical non-active ingredientsPolymerSustained-Release Preparations
A process for producing a lactic acid polymer of 15,000 to 50,000 in weight-average molecular weight, the content of polymeric materials having not more than about 5,000 in weight-average molecular weight therein being not more than about 5% by weight, characterized by hydrolyzing a high molecular weight lactic acid polymer, placing the resultant solution comprising the hydrolyzed product under a condition capable of precipitating the objective lactic acid polymer, separating the precipitated lactic acid polymer and collecting them. The lactic acid polymer is useful as a matrix for sustained-release preparations. The sustained-release microcapsule preparation encapsulating a physiologically active substance can fully prevent the initial excessive release of the physiologically active substance from the microcapsules and keep a stable release rate over a long period of time.
Owner:FUJIFILM WAKO PURE CHEM CORP +1
Slow-releasing preparation containing metformin hydrochloride and glipizide and its preparation method
InactiveCN101057849AEvenly distributedReduce local irritationOrganic active ingredientsMetabolism disorderMedicineMetformin Hydrochloride
The invention discloses a diabecron and glipizide -containing slow-release agent and the method for preparing the same. The glipizide micro-pill takes blank micro-pill as carrier, and combines glipizide and other medical findings with it. The diabecron-containing slow-release micro-pill comprises diabetosan pill, slow-release coating membrane material or other medical findings. The method for preparing diabecron-containing slow-release micro-pill takes extrusion rolling method or blank micro-pill loading method. The product is characterized by safety, high efficient, low toxicity and convenient usage. It can be used to treat non-insulin-dependent diabetes mellitus.
Owner:QIQIHAR MEDICAL UNIVERSITY
Sustained release preparations composed of biocompatible complex microparticles
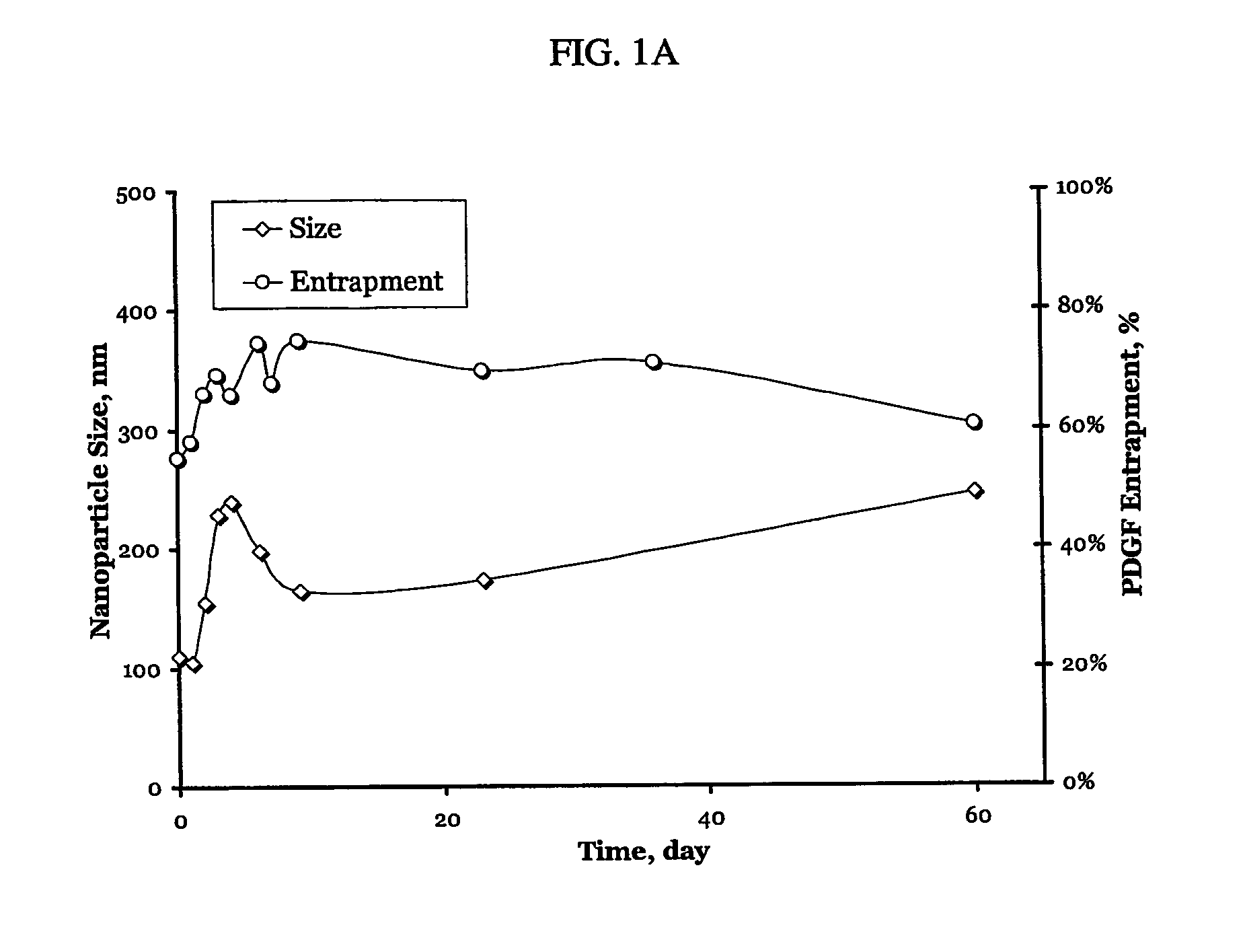
A particle includes a complex between a bioactive agent and a complexing agent, provided that the bioactive agent is other than a polynucleotide and an oligonucleotide, and wherein the particle has a bioactive function. The particle has a diameter from about 5 nm to about 100 microns. In certain embodiments, the bioactive agent is a member selected from the group consisting of a growth factor, a hormone, a peptide, a protein, and polysaccharide. In certain embodiments, the complexing agent is a member selected from the group consisting of polysaccharides, glycosaminoglycans, complex carbohydrates, polyacids, modifications and derivatives thereof. Also provided is a method of making the particle. Further provided is a method of administering the particle, including providing the particle, which is adapted to gradually release the bioactive agent; and thereby administering the particle.
Owner:CHORNY MICHAEL +1
Sustained-release preparation for AII antagonist, production and use thereof
InactiveUS6589547B1Reduce the amount of solutionControlled release ratePowder deliveryOrganic active ingredientsBlood pressureCircadian rhythm
The present invention is to provide a sustained-release preparation which comprises a compound having angiotensin II antagonistic activity, its pro-drug or their salt, and a biodegradable polymer, and if necessary, a polyvalent metal, and which is highly stable and active and shows angiotensin II antagonistic activity while maintaining circadian rhythm of blood pressure for a long time.
Owner:TAKEDA PHARMA CO LTD
Process for producing an oral sustained-release preparation of fasudil hydrochloride
InactiveUS7125567B2Good effectReduce the burden onBiocidePowder deliveryDissolutionBULK ACTIVE INGREDIENT
Disclosed is an oral sustained-release preparation which contains at least one active ingredient selected from the group consisting of fasudil hydrochloride and a hydrate thereof, the preparation comprising at least one sustained-release coated particle comprising a core having a surface and a coating formed on the surface of the core, wherein the core contains the active ingredient and the coating comprises a coating base material and a specific insoluble auxiliary material, and wherein the preparation exhibits, with respect to the active ingredient, a specific dissolution rate, as measured by the dissolution test. By using the oral sustained-release preparation of the present invention, it becomes possible to surely control the release of fasudil hydrochloride from the preparation, so that the effect of the active ingredient is maintained for a long period of time. Therefore, the burden of the patient who has to take the preparation can be decreased and the compliance with respect to the administration of the preparation can be improved. Also disclosed is a method for evaluating an oral sustained-release preparation containing the active ingredient, wherein the evaluation is conducted with respect to the sustained-release ability of the active ingredient.
Owner:ASAHI KASEI PHARMA
Sustained release pharmaceutical composition
InactiveUS20050100602A1Reduces adverse eventImprove featuresPowder deliveryBiocideTamsulosin hclJapanese Pharmacopoeia
[Problem] As compared with the current oral sustained-release preparation containing tamsulosin hydrochloride which have been supplied to the medical setting, there is a problem to provide a sustained-release pharmaceutical composition in which efficacy is equivalent or even better, adverse events such as adverse reactions (e.g., postural hypotension) are reduced, dose can be increased and, if desired, ingestion of food is not limited. [Means for Resolution] A sustained-release pharmaceutical composition, characterized in that, there are contained tamsulosin or a pharmaceutically acceptable salt thereof and a carrier for a sustained-release pharmaceutical composition and, when dissolution test is carried out according to Japanese Pharmacopoeia Dissolution Test Method 2, the tamsulosin release after 7 hours from the start of the dissolution is about 20 to about 85%.
Owner:ASTELLAS PHARMA INC
Sustained release preparation of licardipine hydrochloride and its preparing process
InactiveCN101011395AGood slow releaseQuick effectOrganic active ingredientsPharmaceutical product form changeSide effectSustained Release Capsule
The invention relates to a method for preparing Licardipine Hydrochloride slow-release agent, which can be used to treat hypertension, coronary disease or the like. The inventive slow-release agent is formed by quick-release stomach-soluble micro drop and slow-release enteric-soluble micro drop at 1:0.5-5 ratios in the hollow capsule. The inventive capsule has slow-release effect in 12 hours. The slow-release enteric-soluble micro drop comprises Licardipine Hydrochloride, medical macromolecule materials, drug release adjusting agent and some medical finding. The micro drops are prepared by extruding-rolling technique. The invention can quickly approach the blood drug density to treatment object and hold the density stably, with low side effect.
Owner:SOUTHEAST UNIV
Sustained-release preparation
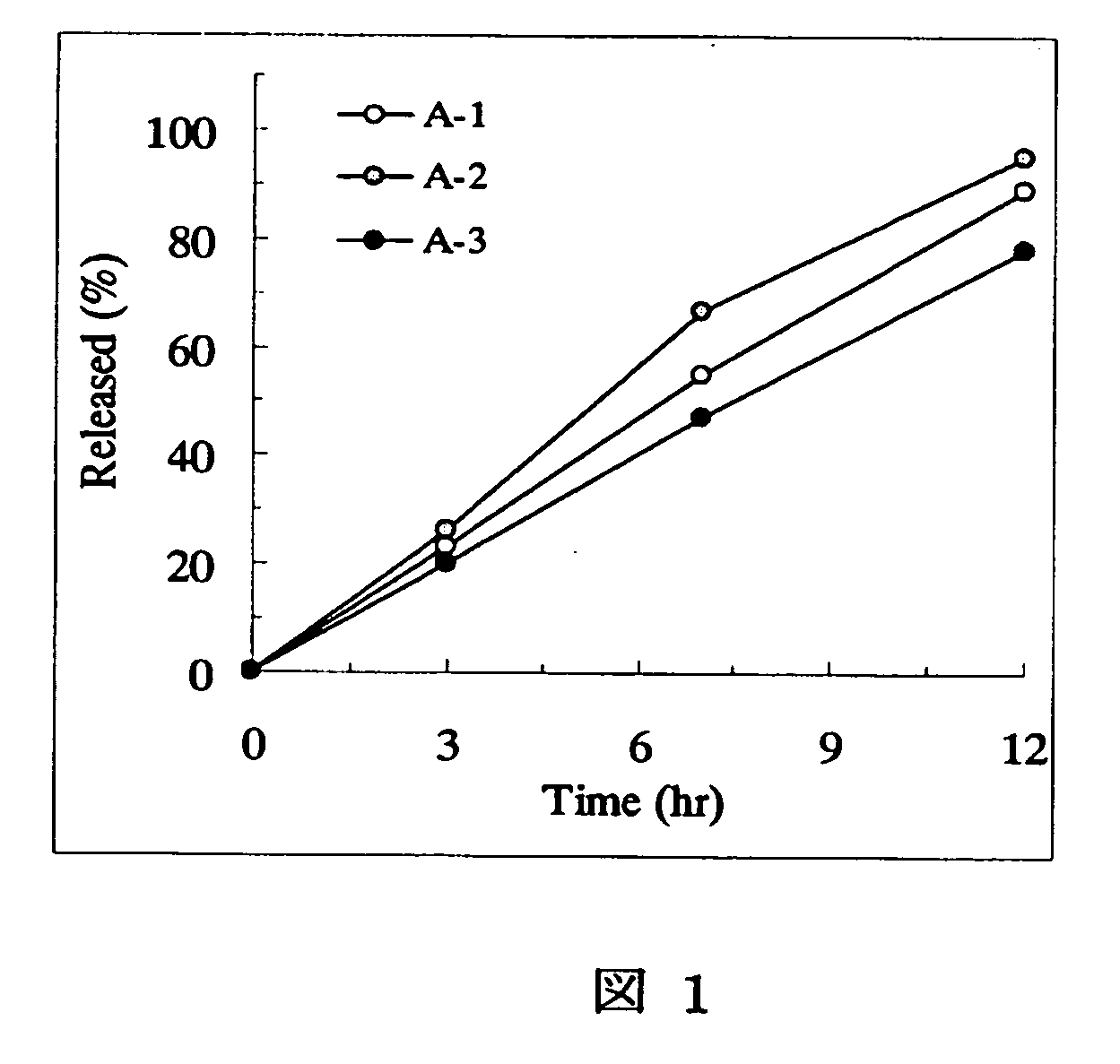
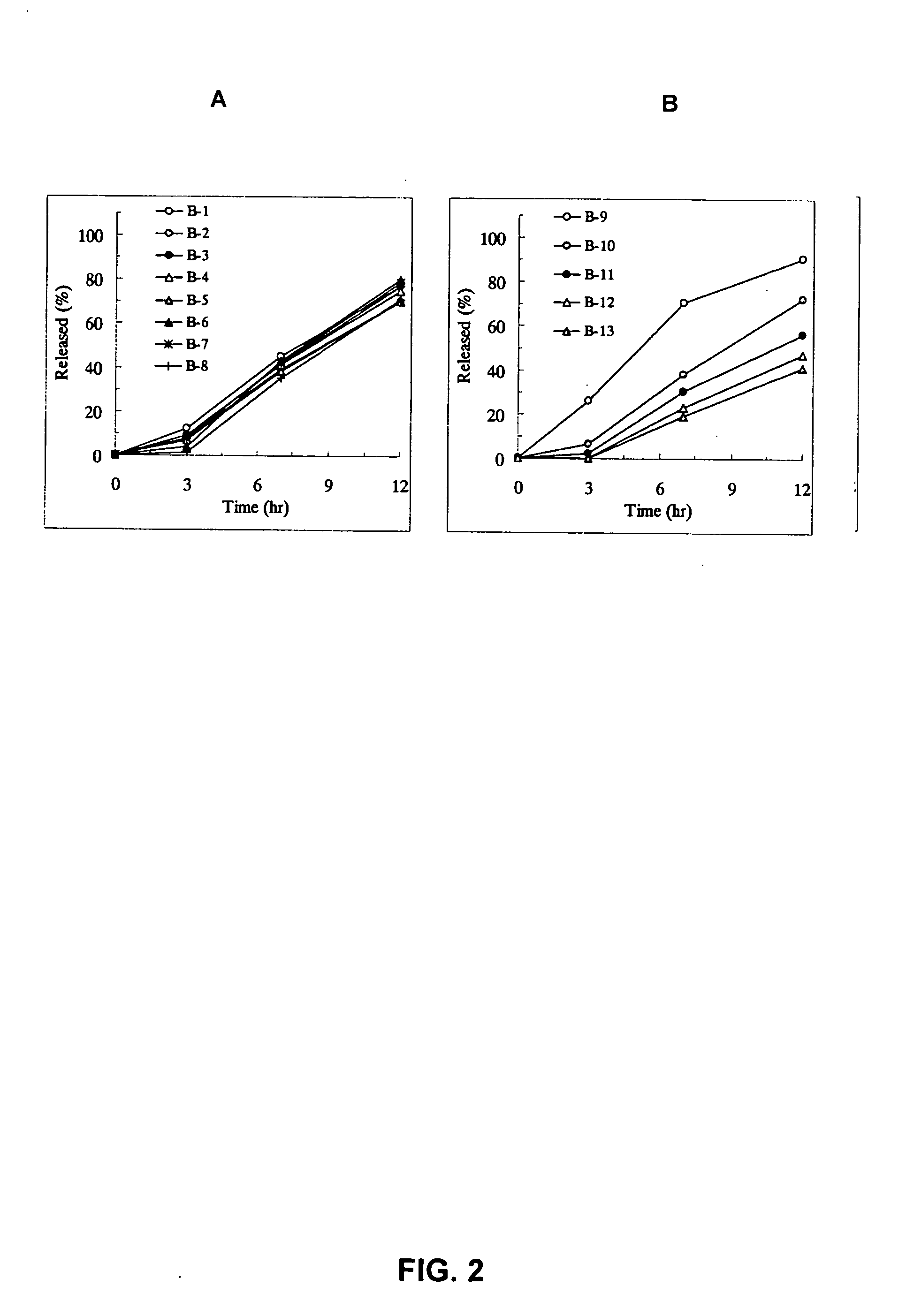
A sustained-release preparation which comprises a physiologically active peptide of general formulawherein X represents an acyl group;R1, R2 and R4 each represents an aromatic cyclic group;R3 represents a D-amino acid residue or a group of the formulawherein R3′ is a heterocyclic group;R5 represents a group of the formula —(CH2)n—R5′ wherein n is 2 or 3, and R5′ is an amino group which may optionally be substituted, an aromatic cyclic group or an O-glycosyl group;R6 represents a group of the formula —(CH2)n—R6′ wherein n is 2 or 3, and R6′ is an amino group which may optionally be substituted;R7 represents a D-amino acid residue or an azaglycyl residue; andQ represents hydrogen or a lower alkyl group, or a salt thereof and a biodegradable polymer having a terminal carboxyl group.The sustained-release preparation shows a constant release of the peptide over a long time and is substantially free from an initial burst.
Owner:TAKEDA PHARMA CO LTD
Slow/controlled release pellet composition containing ginkgo leaf extracts and preparation method thereof
InactiveCN101375869ASmall toxicityStable blood concentrationGranular deliveryGinkgophyta medical ingredientsSustained release pelletsHard Capsule
The invention belongs to the field sustained / controlled-release preparations, in particular to an oral sustained / controlled-release pellet combination containing ginkgo biloba extract and a preparation method. The oral sustained / controlled-release pellet combination is composed of (A) a core containing a pill; (B) an insulating coating layer; (C) a sustained-release coating layer; (D) and an enteric-coated coating layer. The invention is the traditional Chinese medicine multi-component sustained-release pellet combination which is taken once by 24 hours and the multi-unit sustained-release pellet combined preparation with the different drug release systems, the core containing the pill is prepared by adopting the extrusion pill rolling method, a novel sustained-release multi-layer coating technology and a fluidized bed are utilized for coating the sustained-release pellet, the rapid-release part and the sustained-release part of the coated pellet are mixedly filled into a hard capsule or pressed into a pellet tablet. The sustained-release pellet has stable coating process and good reproducibility, thereby being applicable to the industrial mass production; and the drug quality of the preparation is stable through the long-term storage. The in vitro release test shows that the multiple components of the traditional Chinese medicine can achieve the sustained-release role, the sustained-release preparation can significantly increase the transmembrane absorption and the stability of various effective active ingredients by oral drug administration, the curve of plasma drug concentration in vivo is smooth, and the design purpose of 24-hour sustained-release is achieved.
Owner:CHINA PHARM UNIV
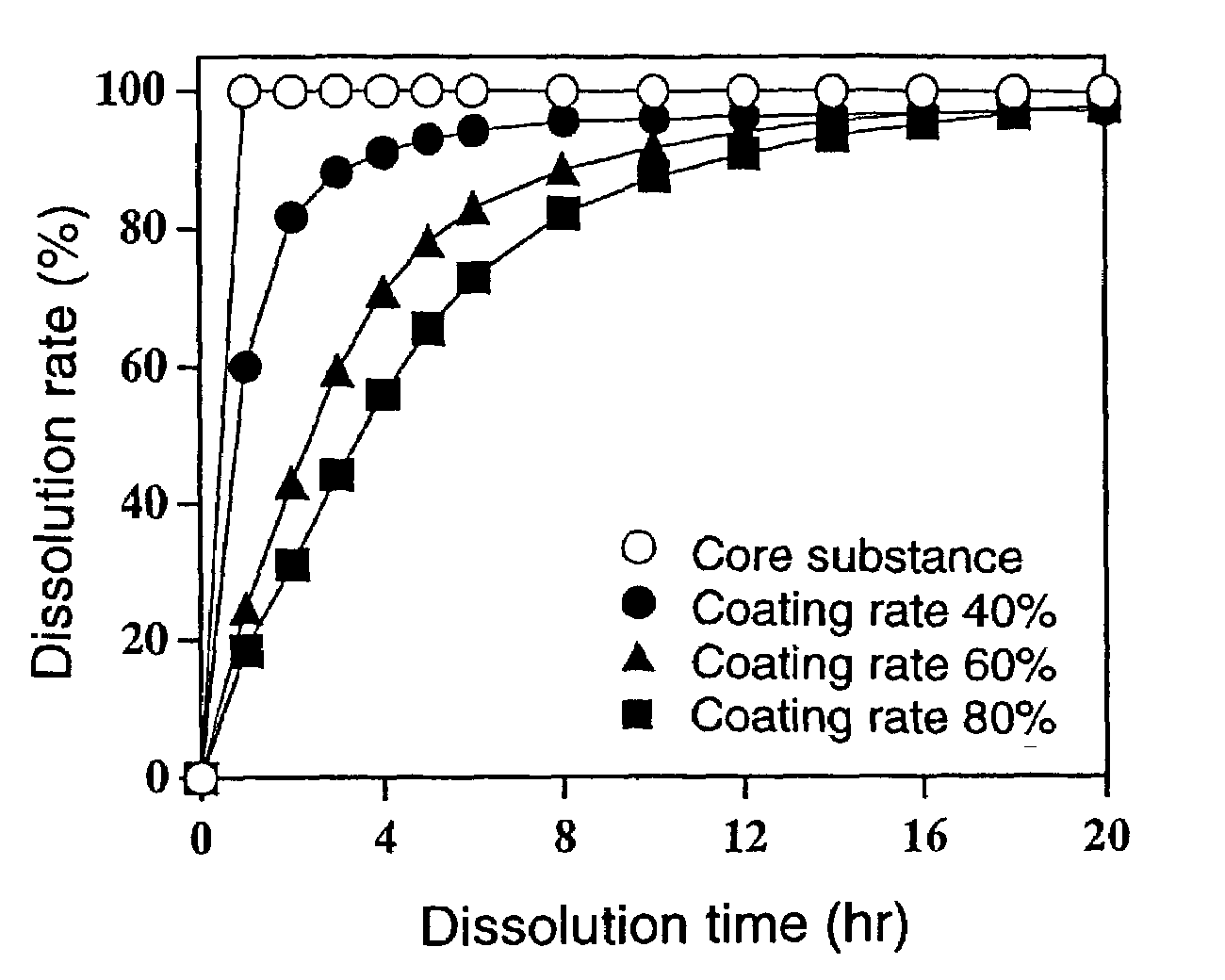
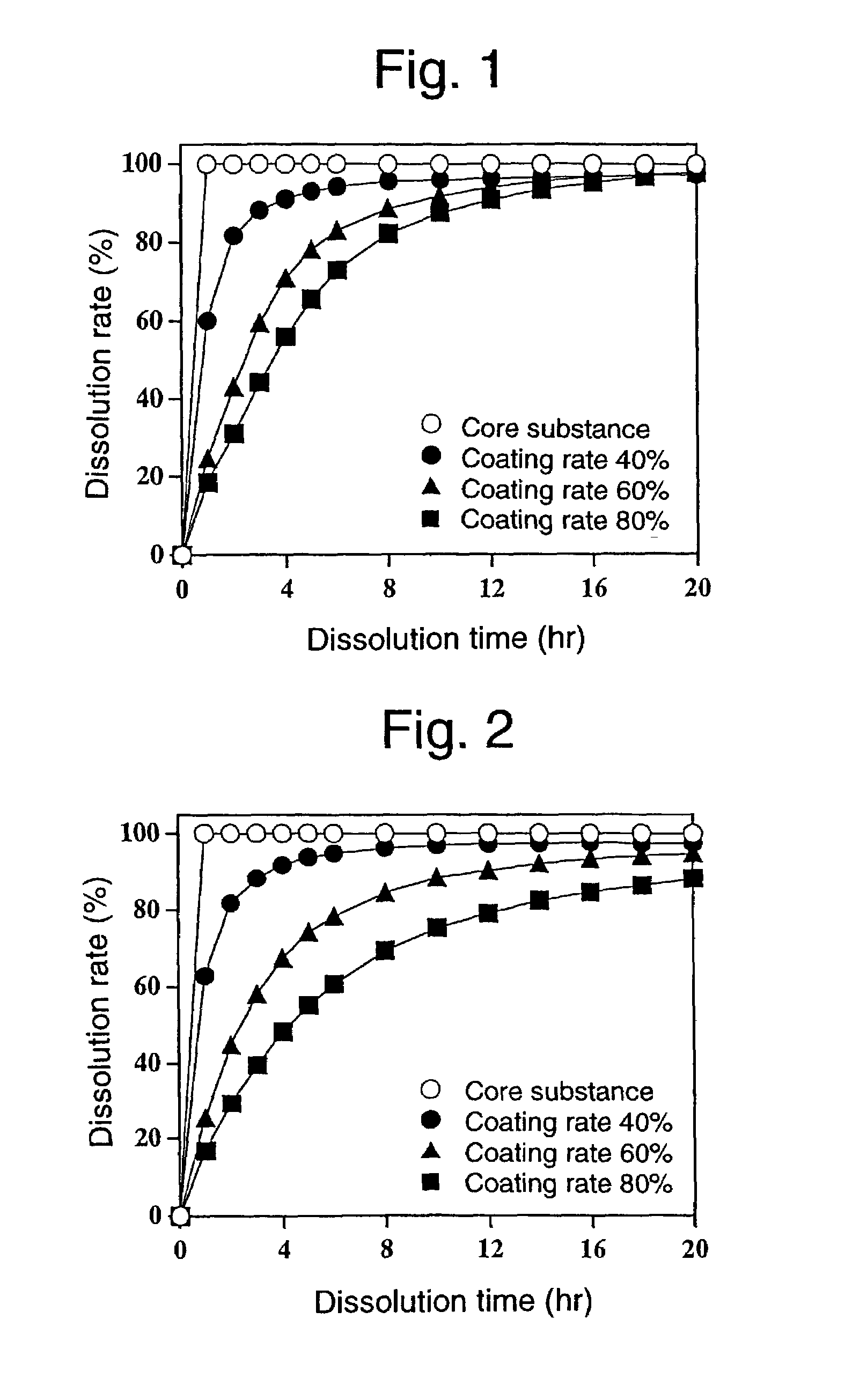
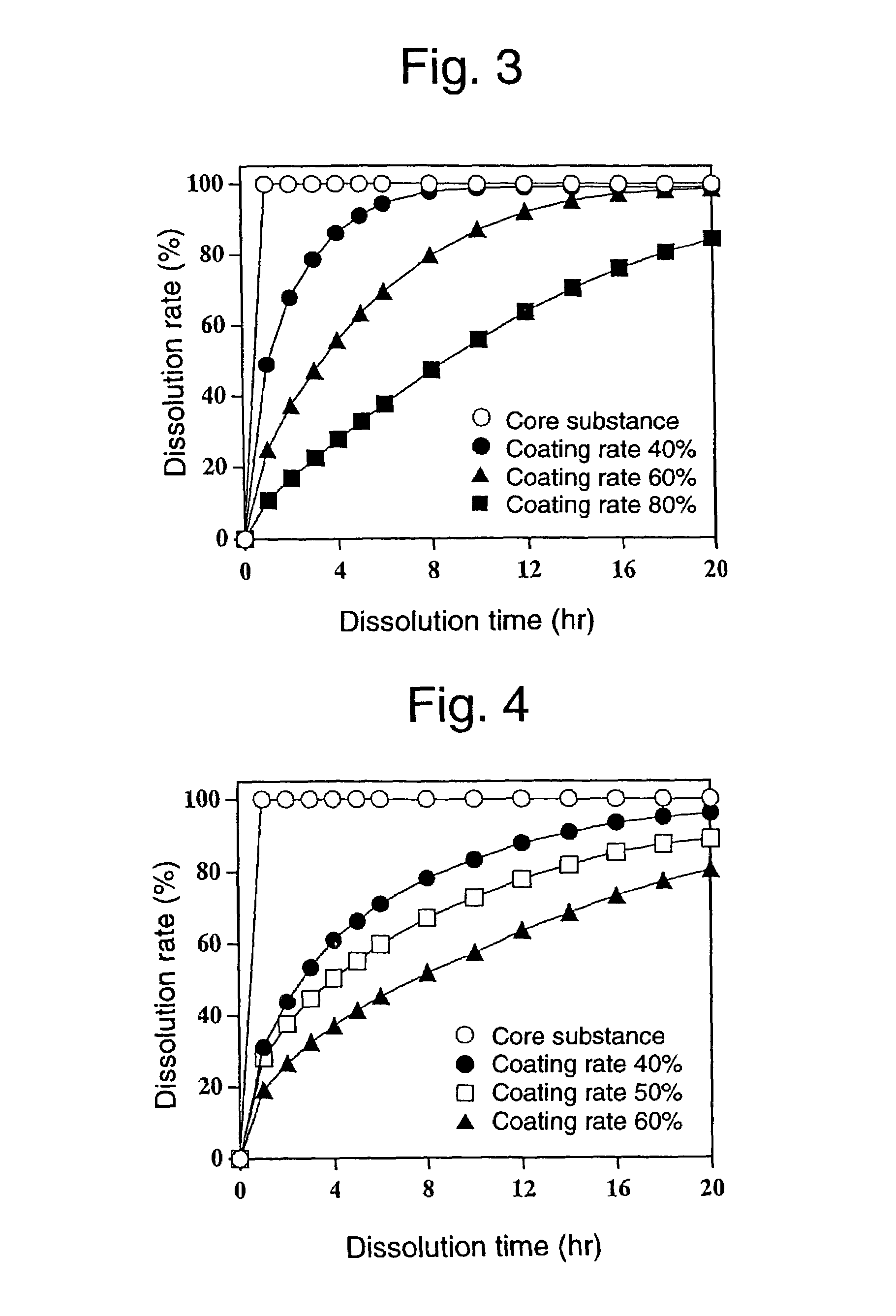
Sustained-release preparation and process for producing the same
InactiveUS7220430B2Effective controlExcellent effect of releasing drug satisfactorilyPharmaceutical non-active ingredientsCoatingsSimple Organic CompoundsSpray coating
The present invention is to provide a sustained release preparation comprising a drug-containing core substance and a multilayered coating layer covering the core substance, wherein all adjacent layers in the multilayered coating layer contain mutually different hydrophobic organic compound-water-soluble polymer mixtures; and, a method of producing a sustained release preparation, having a multilayered coating layer in which adjacent layers contain different hydrophobic organic compound-water-soluble polymer mixtures, which comprises spray-coating a solution containing a hydrophobic organic compound-water-soluble polymer mixture onto a drug-containing core substance, continuing to spray-coat a solution containing a different hydrophobic organic compound-water-soluble polymer mixture onto the resulting coating layer, and repeating this step.
Owner:MITSUBISHI TANABE PHARMA CORP
Effective part group in red sage mixture and its delayed release prepn. medicinal use and prepn process
The present invention relates to a compound salvia active component group and its slow-release preparation, medicinal application and preparation method. It features convenient administration and good stability. Said invention contains salvia active component (salvia total phenolic acid content is above 50%), notoginseng active component (notoginseng total saponin content is above 50%) and natural (or artifiical) borneol and its cyclodextrin inclusion. It can be made into various slow-release preparations, and is obtained by using water or pure water to make extraction, dissolving in water, removing impurity by using water to wash and recovering and drying. It can be used for making medicine for curing angiocardiopathy and cerebrovascular diseases.
Owner:SHENYANG PHARMA UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com