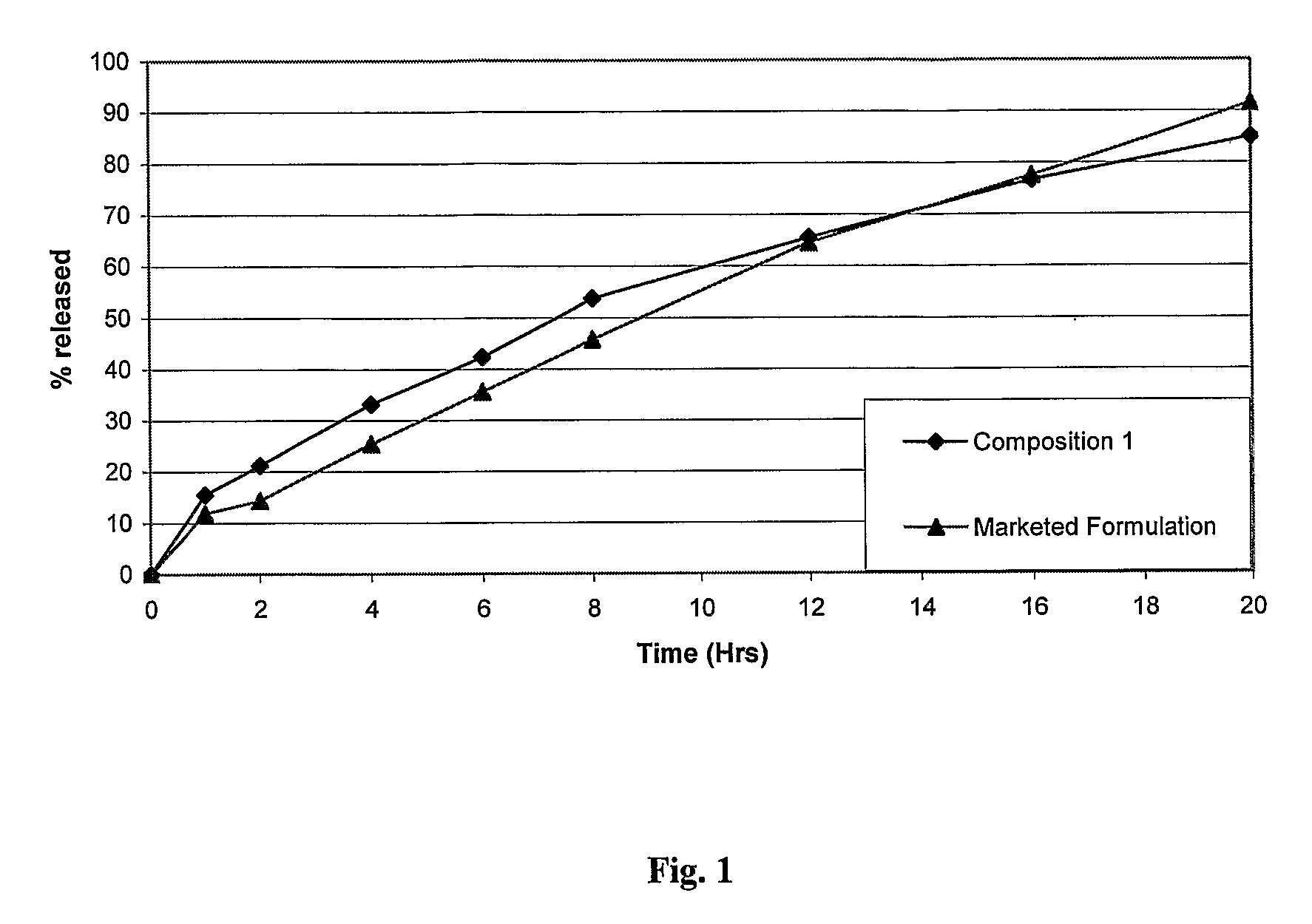
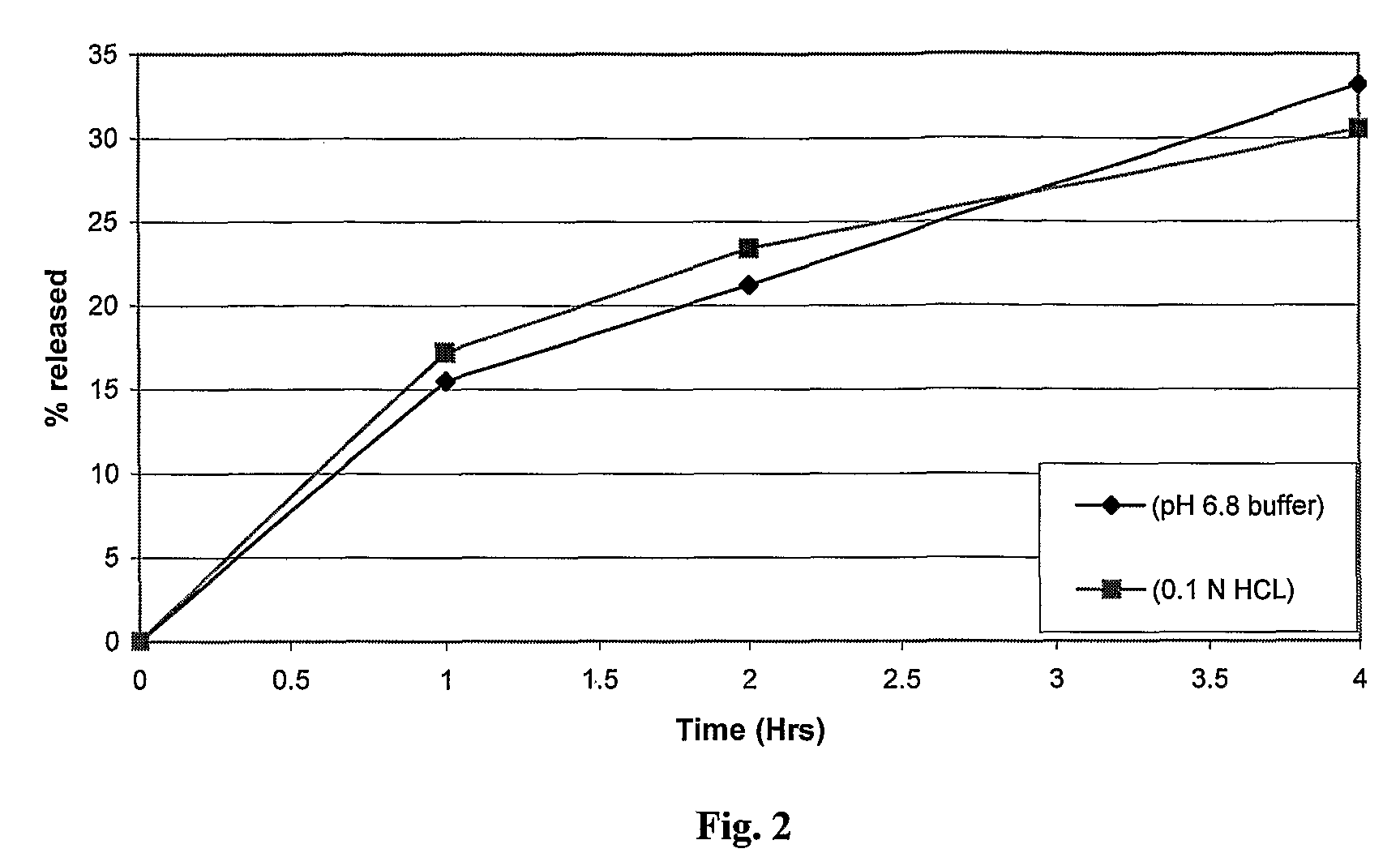
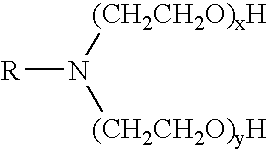
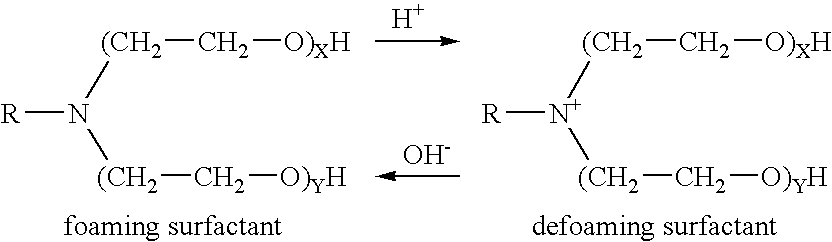
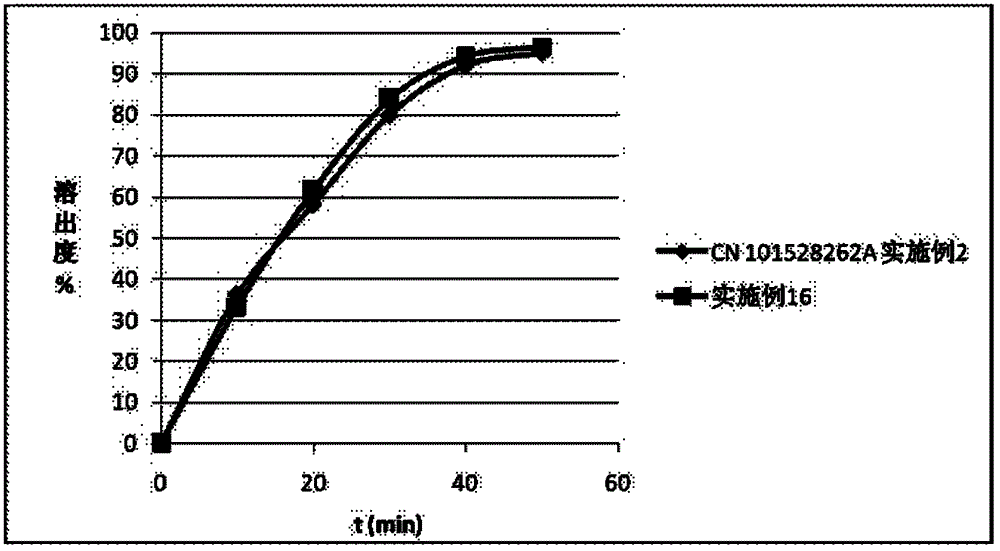
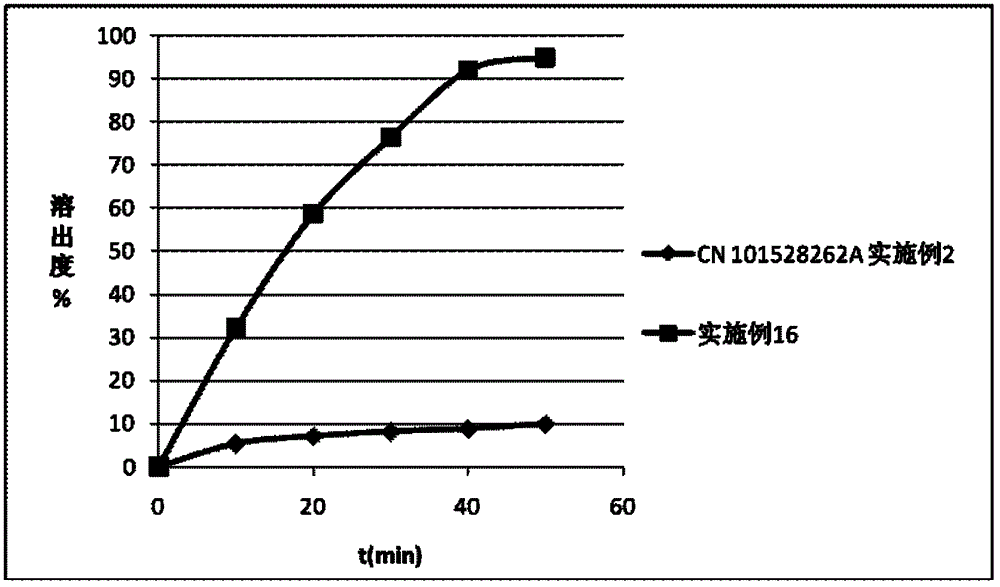
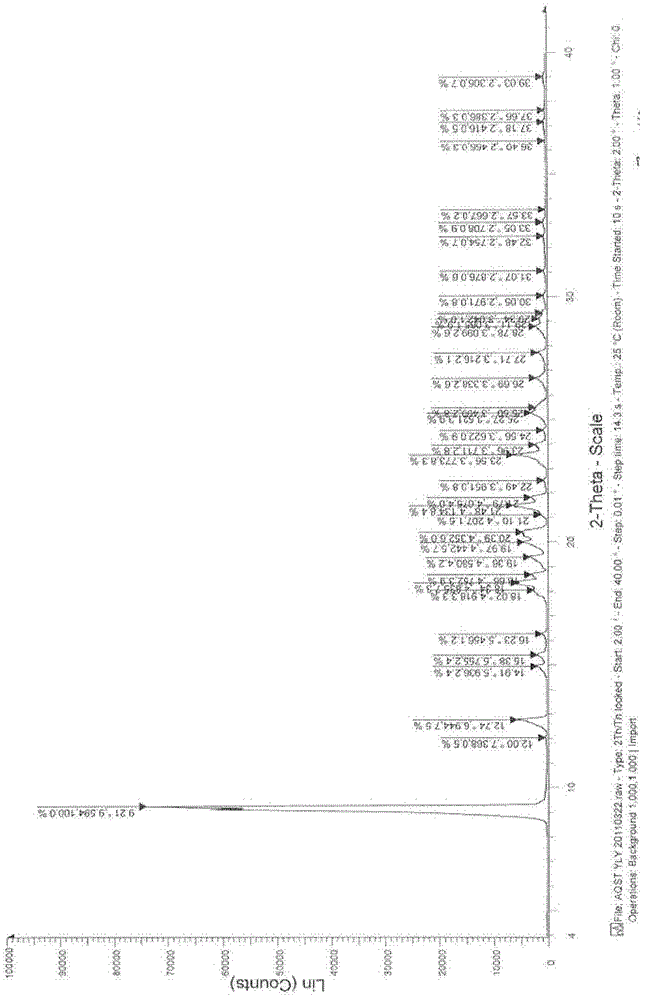
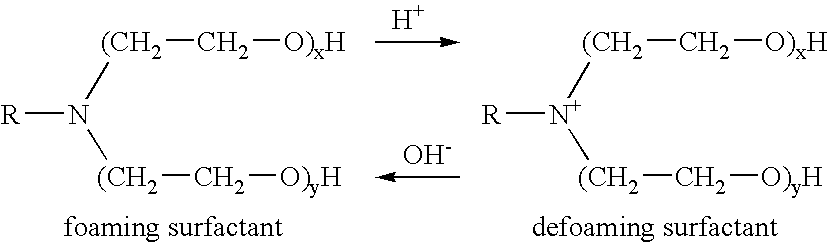
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
194 results about "Ph dependence" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
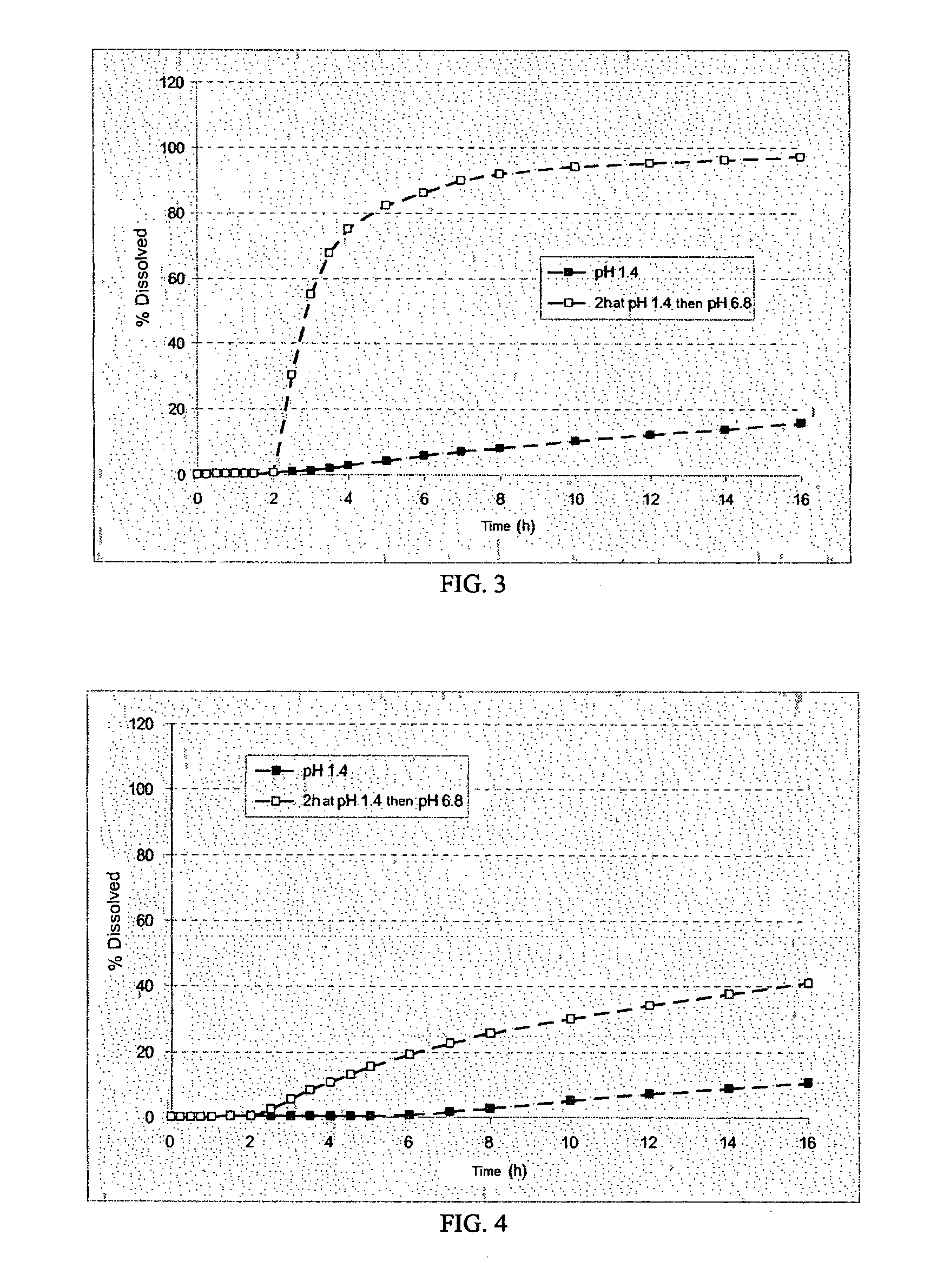
There are four ways to address pH dependence of free chlorine sensors: Restrict applications to those in which the pH varies no more than about ±0.1. Treat the sample with acid (e.g., vinegar) to lower the pH to a value where free chlorine exists only as HOCl. Measure the pH of the sample and use the pH to calculate a correction factor.
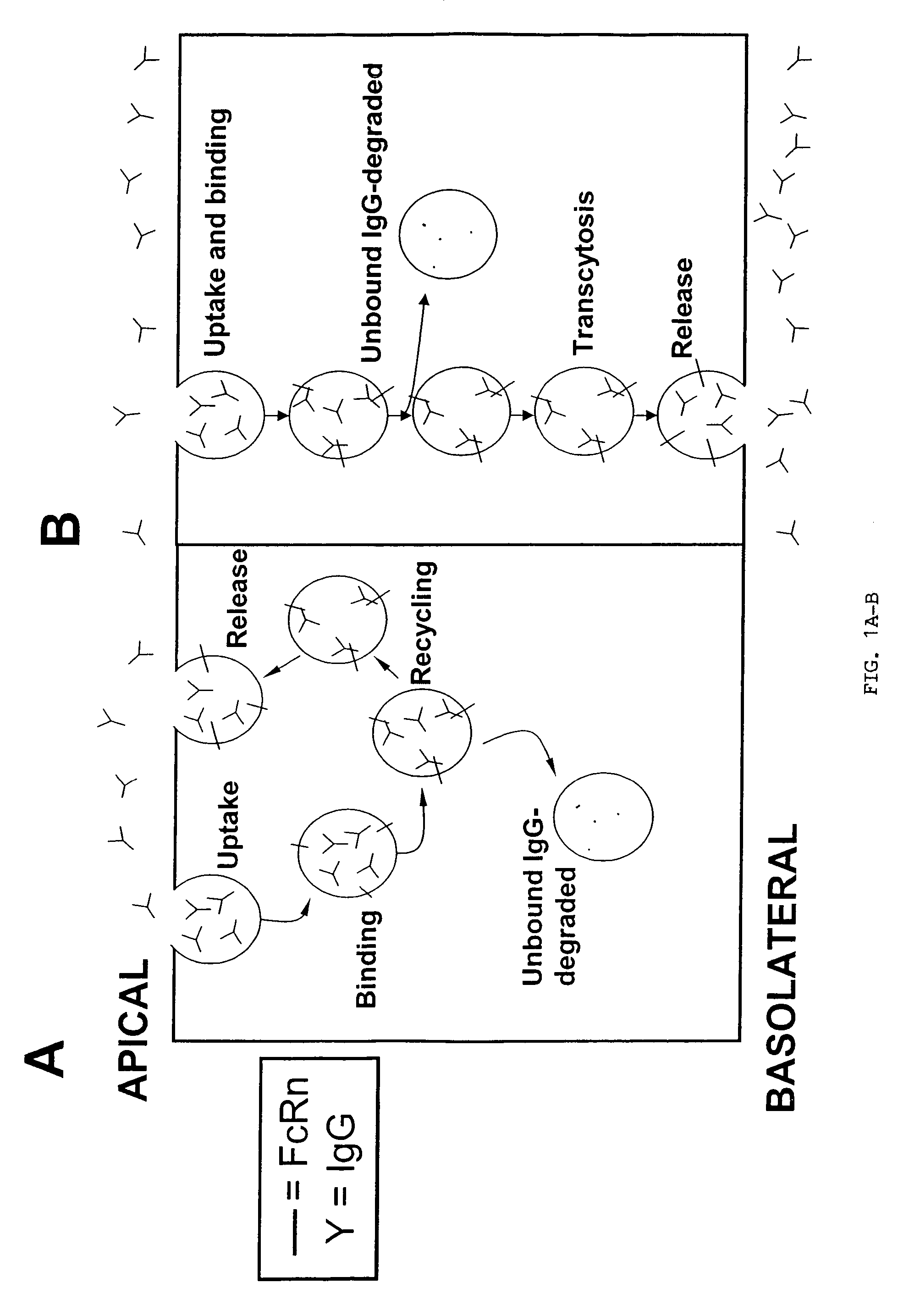
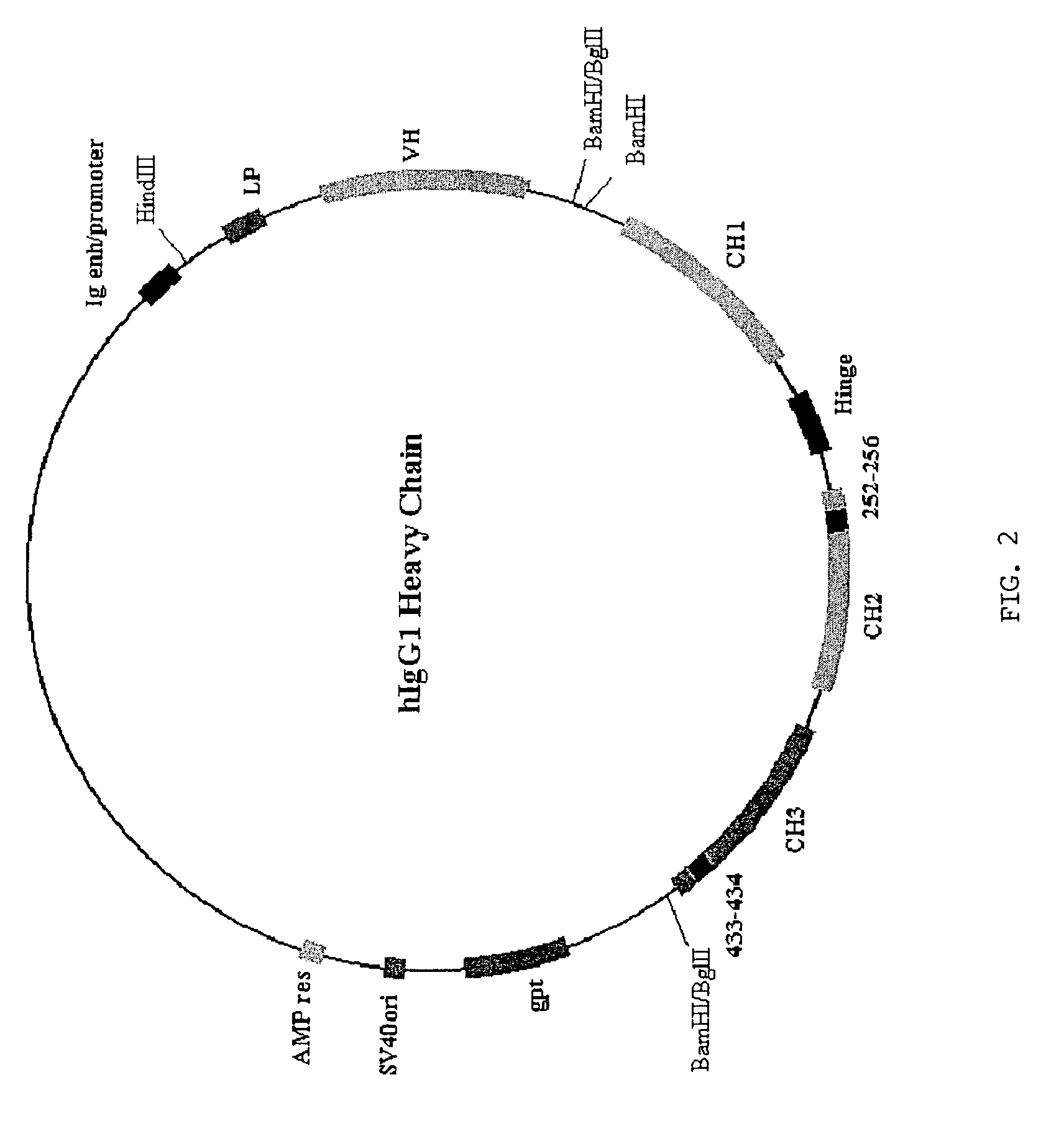
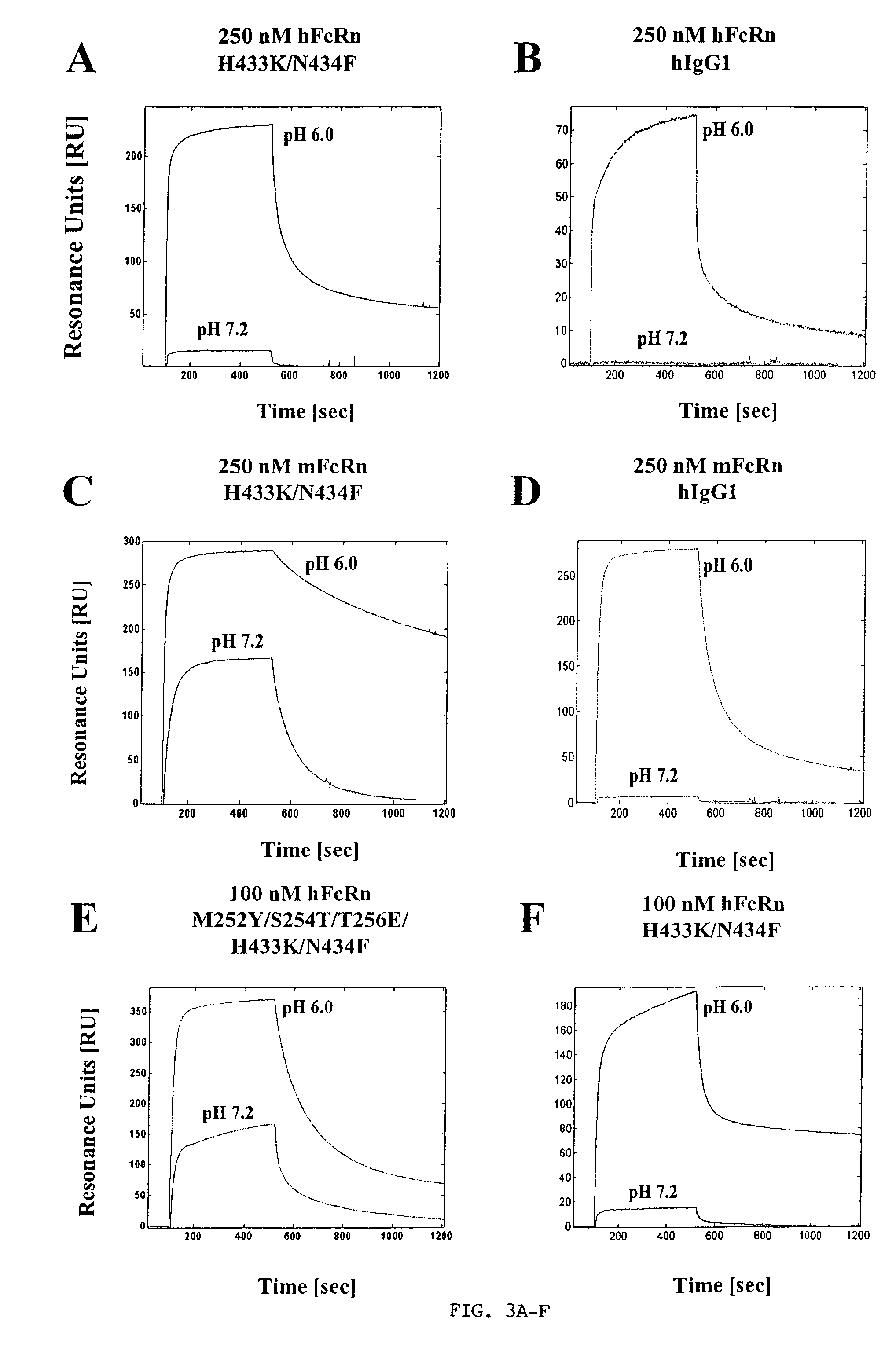
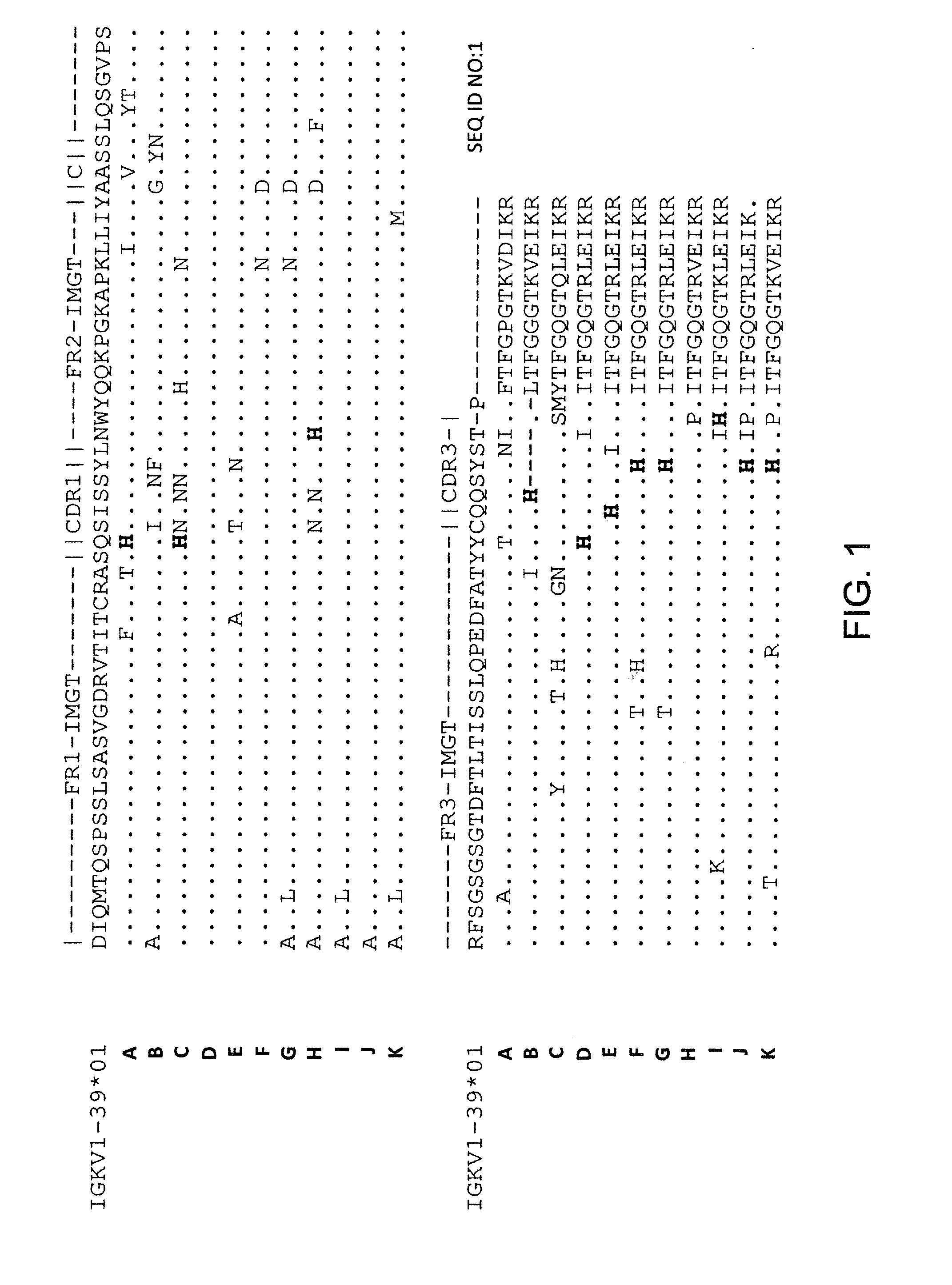
Immunoglobulin molecules with improved characteristics
ActiveUS8163881B2Increased serum half-lifeUltrasonic/sonic/infrasonic diagnosticsAntipyreticSerum igeClearance rate
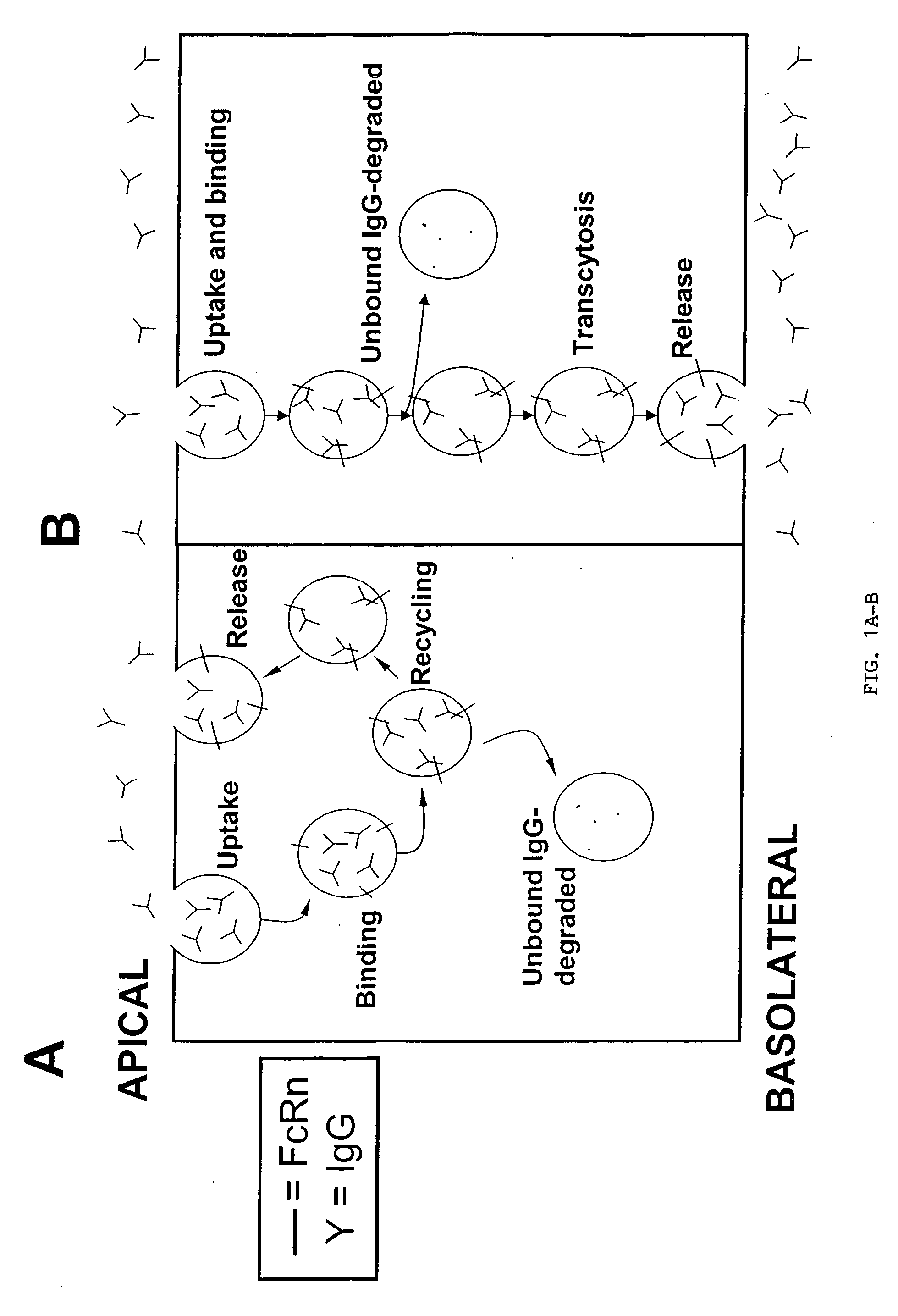
The present invention provides for IgG1 molecules with improved characteristics. In particular, substitution mutations are provided that, in combination, facilitate improved placental transfer, improved serum half-life and improved FcRn binding. Substitution mutations are also provided, that in combination, can be used to block FcRn function and thereby increase the clearance rates of other (endogenous or exogenous) IgGs, block placental transport of IgGs and have increased affinity / reduced pH dependence for FcRn binding.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Chia seed beverage and related method
InactiveUS20090181114A1Enhancing heart healthImprove regularityBiocideDigestive systemFruit juiceFlavored water
A beverage is disclosed that is effective for enhancing gastrointestinal regularity and heart health. It is formed by a liquid comprising fruit derived juices, water or naturally or artificially flavored water. A composition of matter is mixed within the liquid in a shelf stable pasteurized beverage form and formed from sterilized whole seed extracted from Salvia hispanica L. The resulting beverage exhibits a pH dependent viscosity requiring no additional thickening agents and suitable as a beverage for human consumption.
Owner:US NUTRACEUTICALS LLC
Abuse resistant opioid drug-ion exchange resin complexes having hybrid coatings
InactiveUS20120148672A1Improved resistance characteristicsPowder deliveryNervous disorderMedicineIon-exchange resin
A sustained release formulation for opioid drugs is described. The formulation contains an opioid-ion exchange resin complex having a hybrid coating. The hybrid coating contains a cured polyvinylacetate polymer and a pH-dependent enteric coating layer mixed therein. Also provided are methods of making and using same.
Owner:TRIS PHARMA
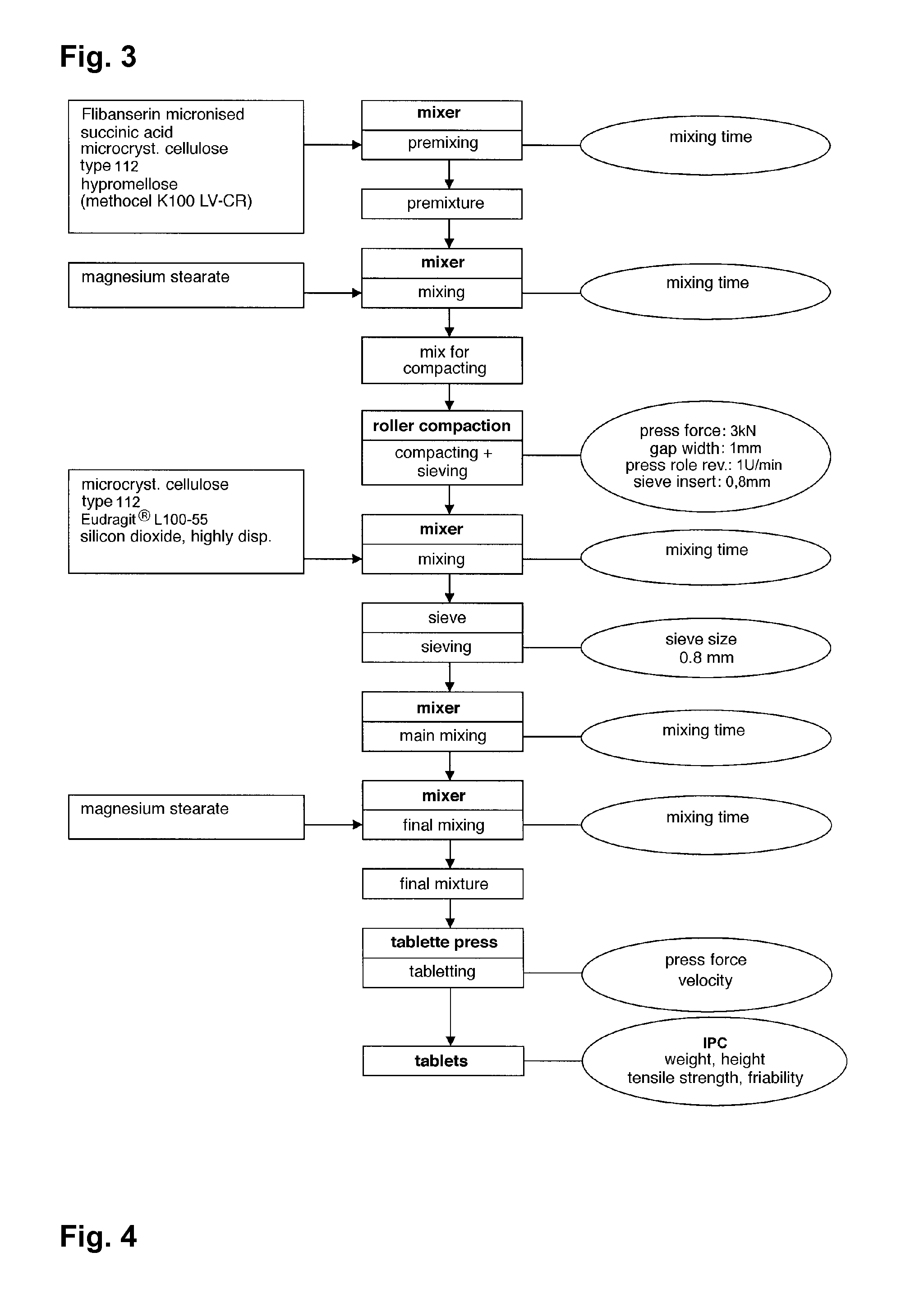
Extended release tablet formulations of flibanserin and method for manufacturing the same
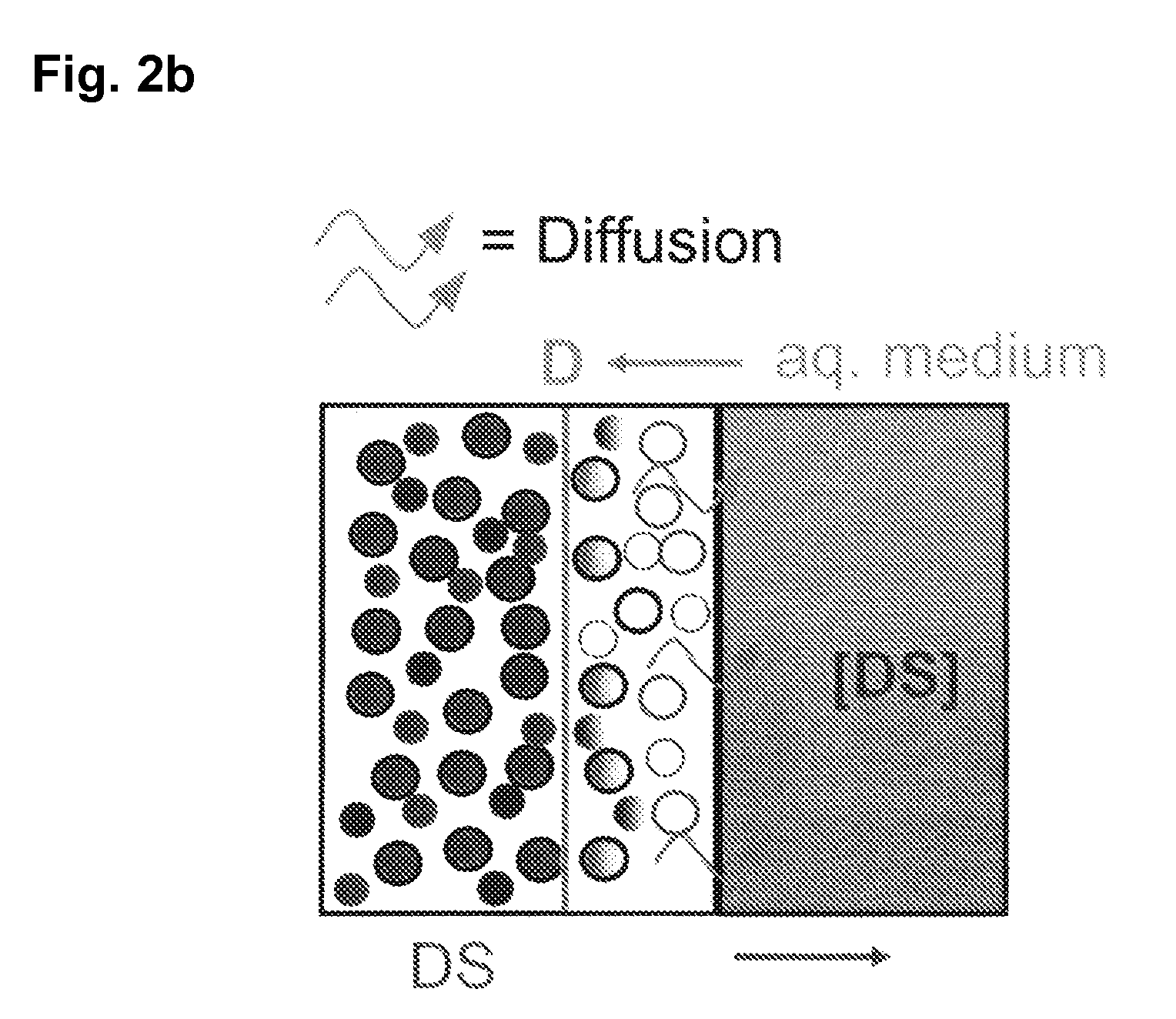
ActiveUS20080038346A1Reduce solubilitySufficiently slow releasePowder deliveryOrganic active ingredientsOral medicationExtended release tablets
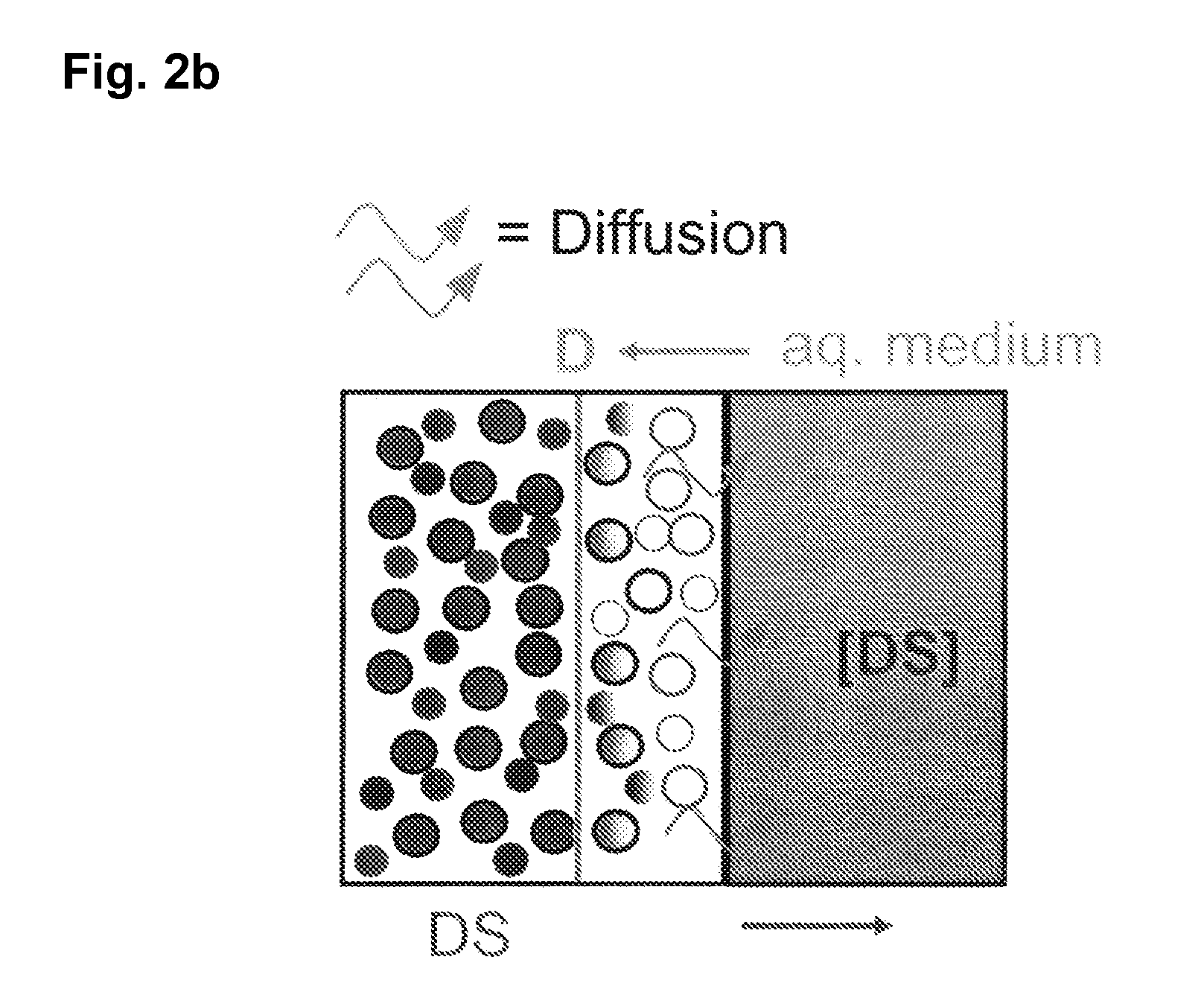
The invention is directed to a Pharmaceutical extended release system, particularly for oral administration, of a pH-dependent water-soluble active substance, comprising or essentially consisting of a) flibanserin or a pharmaceutically acceptable derivative thereof as active substance; b) one or more pharmaceutically acceptable pH-dependent polymers; c) one or more pharmaceutically acceptable pH-independent polymers; d) one or more pharmaceutically acceptable acids; and e) optionally one or more additives. The present invention provides a release profile of flibanserin which is independent on the pH in the gastrointestinal tract when administered orally resulting in a significantly improved bioavailability.
Owner:BOEHRINGER INGELHEIM INT GMBH
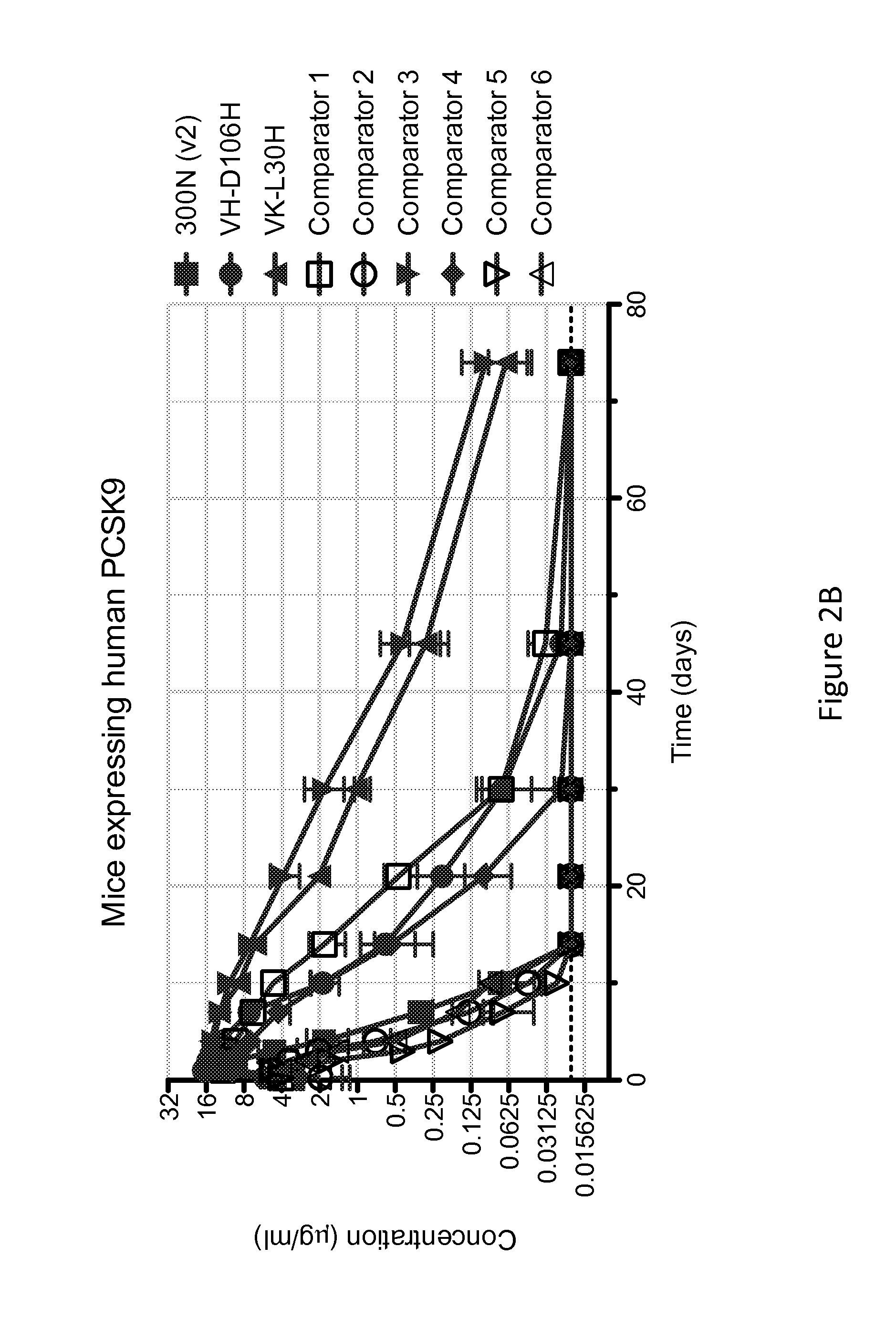
ANTI-PCSK9 ANTIBODIES WITH pH-DEPENDENT BINDING CHARACTERISTICS
ActiveUS20140044730A1High affinityReduced binding affinityMetabolism disorderAntibody ingredientsDiseaseKexin
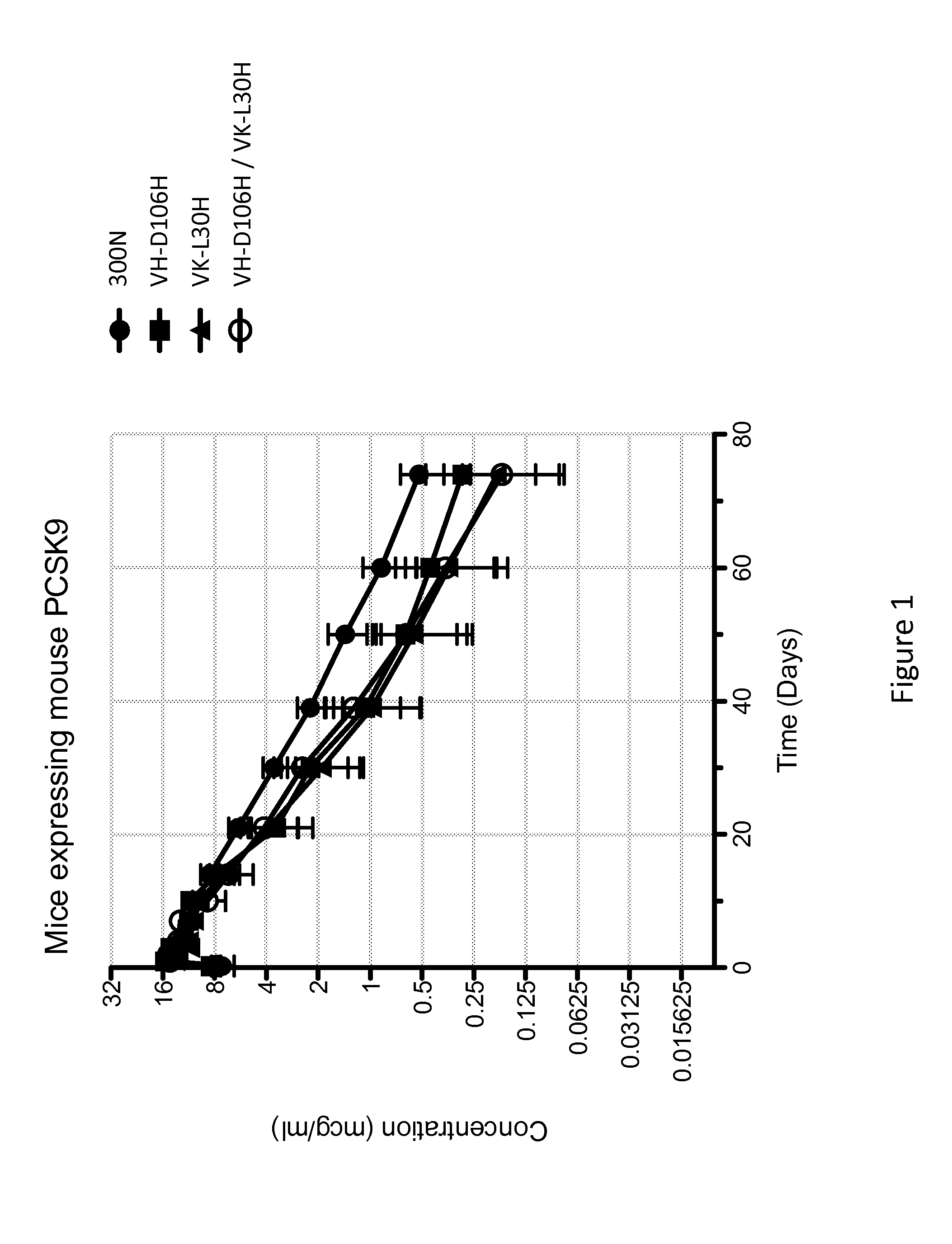
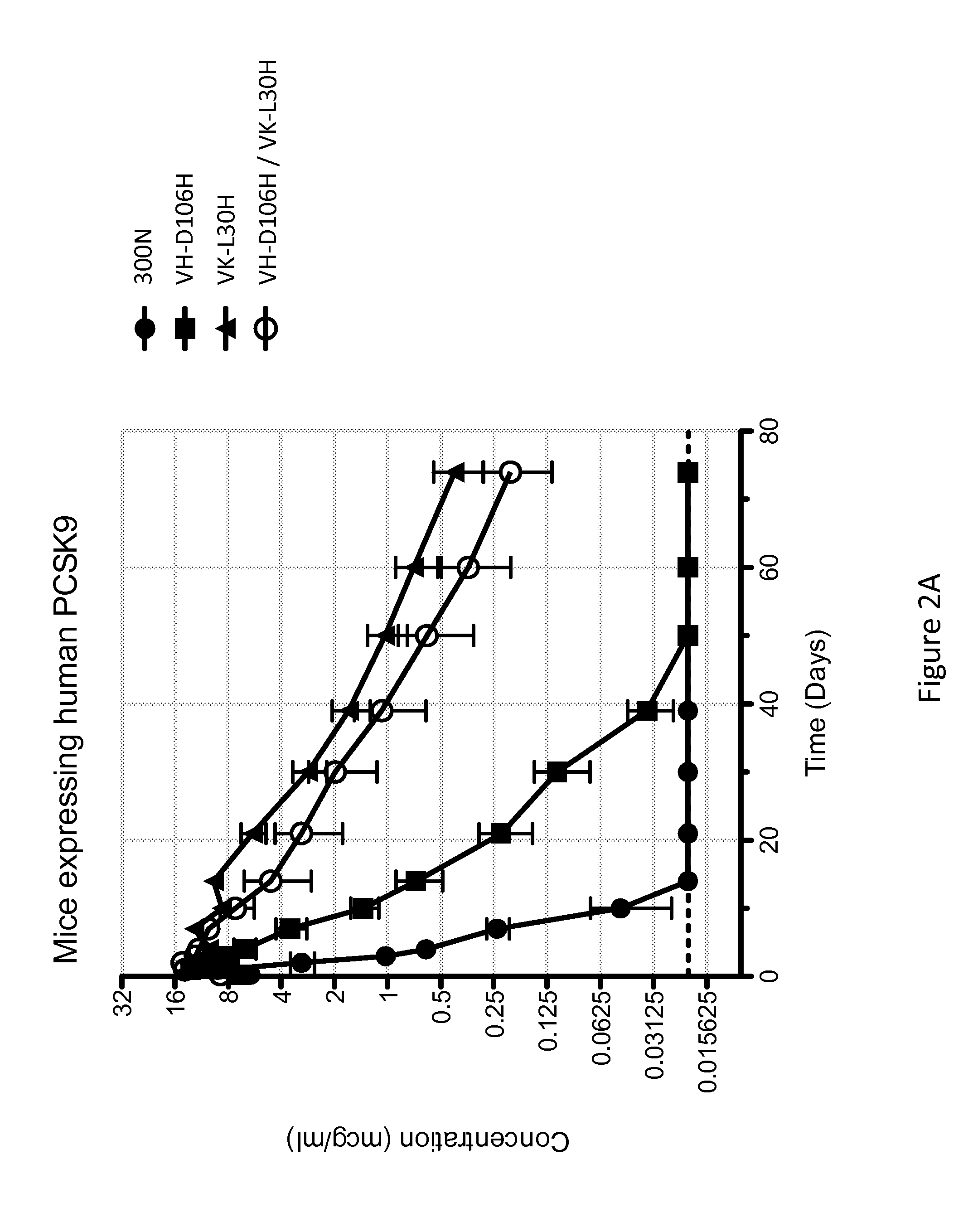
The present invention provides antibodies and antigen-binding fragments thereof that specifically bind proprotein convertase subtilisin / kexin-9 (PCSK9) with greater affinity at neutral pH than at acidic pH. The antibodies of the invention may possess one or more amino acid changes as compared to antibodies that do not exhibit pH-dependent binding properties. For example, the present invention includes anti-PCSK9 antibodies which possess one or more histidine substitutions in one or more complementarity determining regions. The antibodies of the invention, with pH-dependent binding properties, remain in circulation and exhibit cholesterol lowering activity for prolonged periods of time in animal subjects as compared to anti-PCSK9 antibodies that do not exhibit pH-dependent binding properties. The antibodies of the invention are therefore useful for treating diseases and disorders related to elevated HDL cholesterol, wherein the antibodies of the invention can be administered to a patient at a lower dose and / or with less frequent dosing as compared to antibodies that do not exhibit pH-dependent binding properties.
Owner:REGENERON PHARM INC
Sustained release ranolazine formulations
InactiveUS6852724B2Without fluctuationBiocidePharmaceutical non-active ingredientsRanolazineDissolution
A sustained release ranolazine formulation contains an intimate mixture of ranolazine and a partially neutralized pH-dependent binder to form a film that is mostly insoluble in aqueous media below pH 4.5 and soluble in aqueous media above pH 4.5. The formulation is suitable for twice daily administration of ranolazine and is useful for controlling the rate of dissolution of ranolazine, and to maintain human plasma ranolazine levels at between 850 and 4000 ng base / mL.
Owner:GILEAD SCI INC
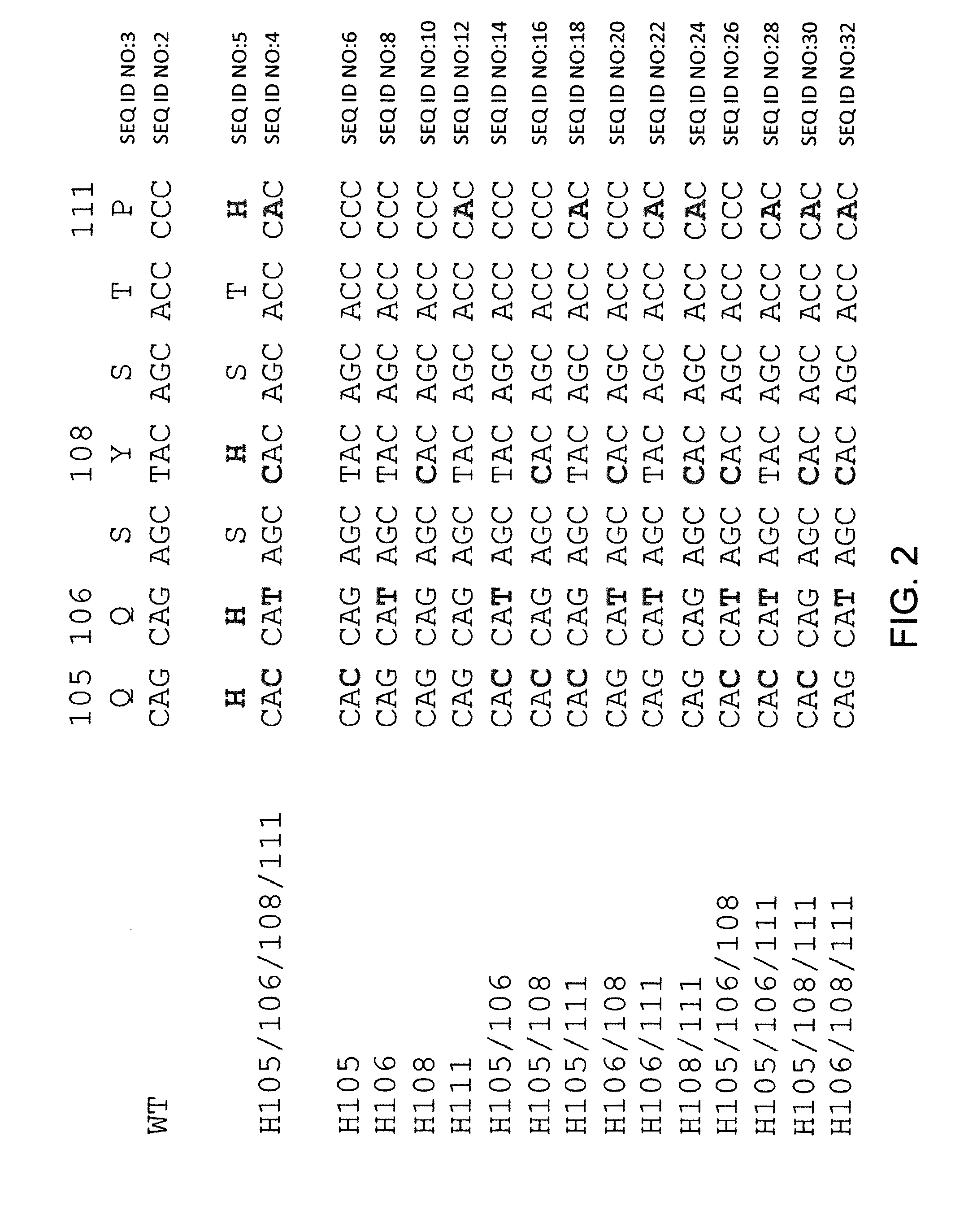
Histidine Engineered Light Chain Antibodies and Genetically Modified Non-Human Animals for Generating the Same
InactiveUS20140013456A1Reduce the binding forceNucleic acid vectorImmunoglobulinsNucleotideGenetically modified crops
A genetically modified non-human animal is provided, wherein the non-human animal expresses an antibody repertoire capable of pH dependent binding to antigens upon immunization. A genetically modified non-human animal is provided that expresses human immunoglobulin light chain variable domains derived from a limited repertoire of human immunoglobulin light chain variable gene segments that comprise histidine modifications in their germline sequence. Methods of making non-human animals that express antibodies comprising histidine residues encoded by histidine codons introduced into immunoglobulin light chain nucleotide sequences are provided.
Owner:REGENERON PHARM INC
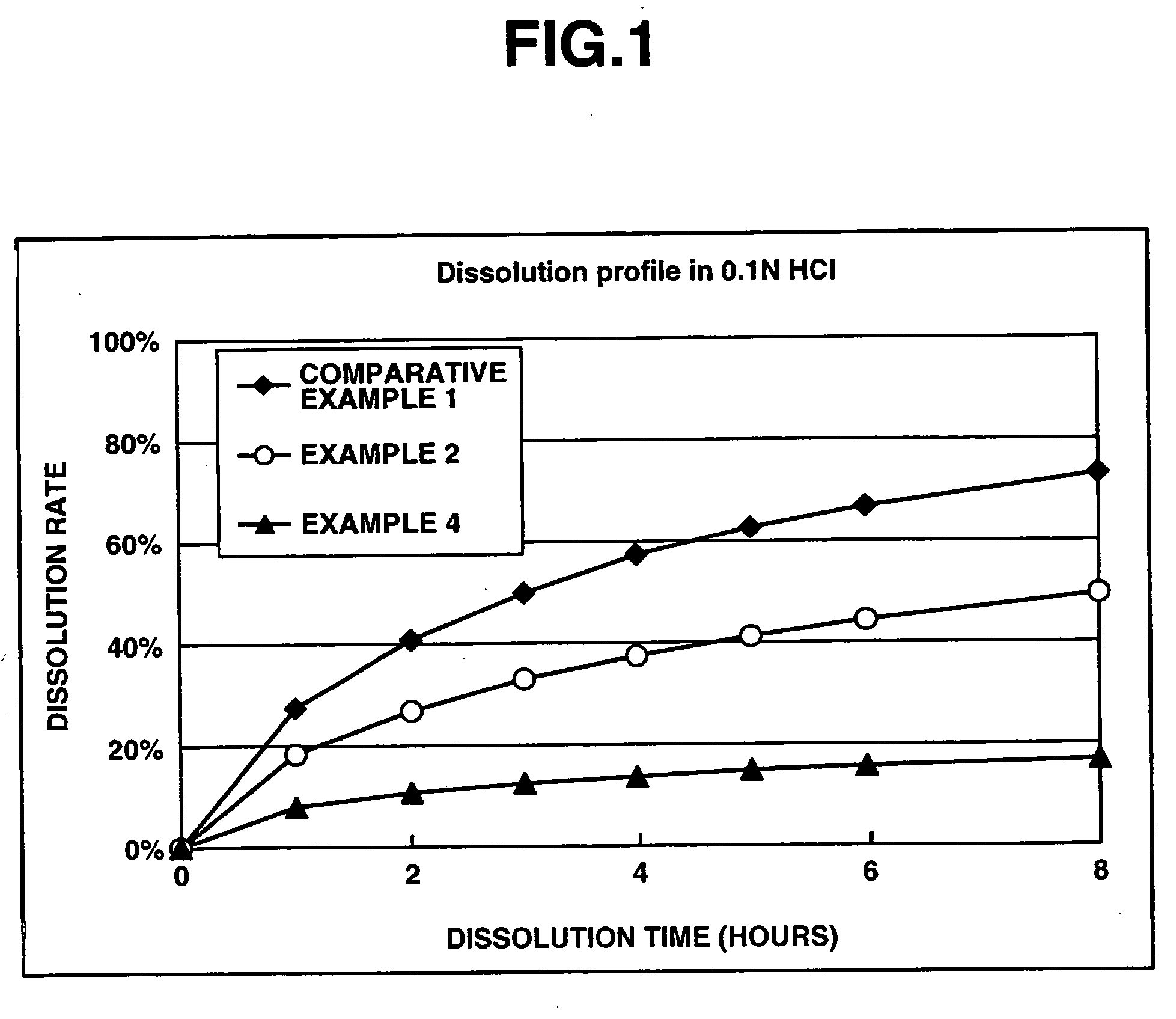
Matrix type sustained-release preparation containing basic drug or salt thereof
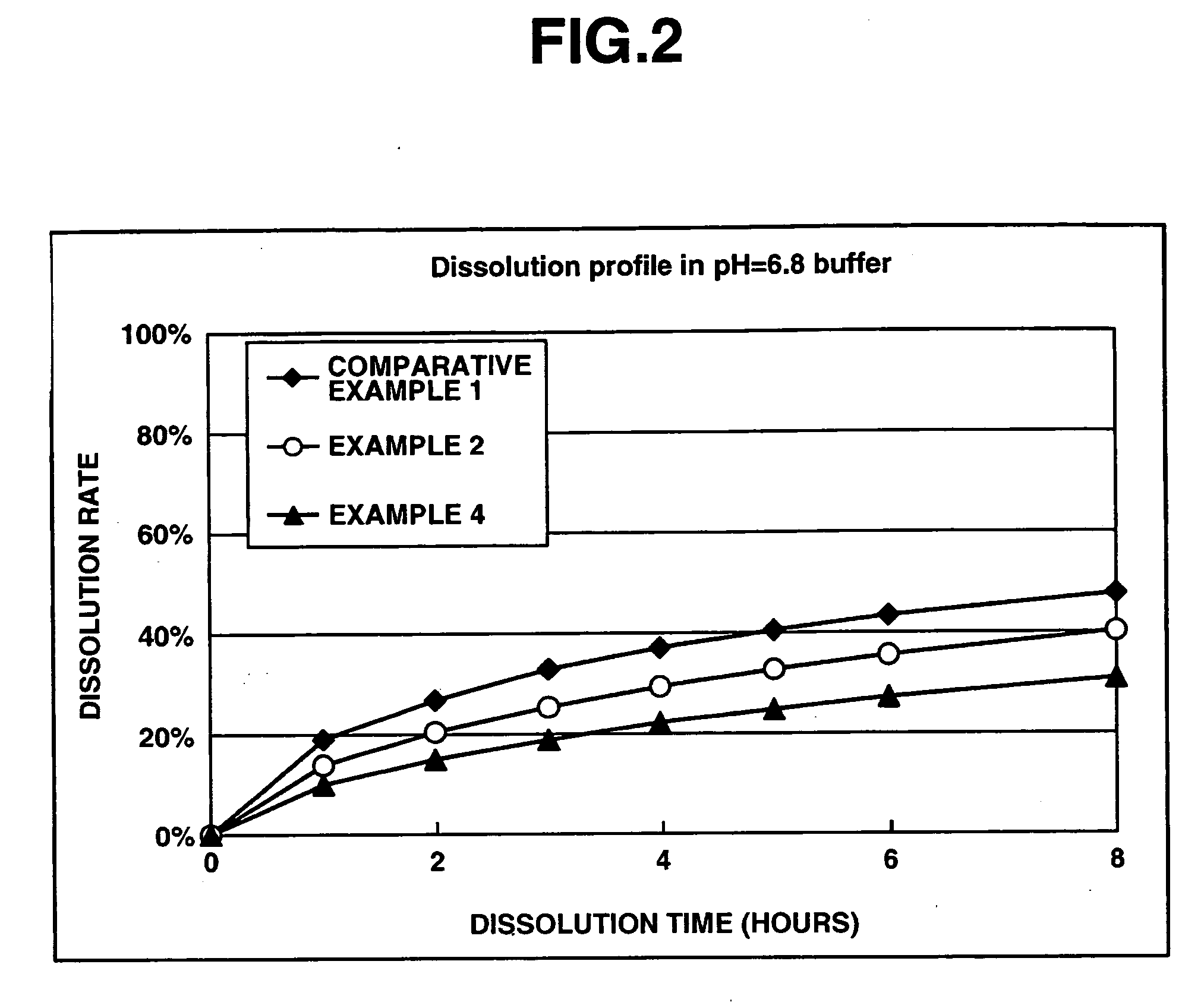
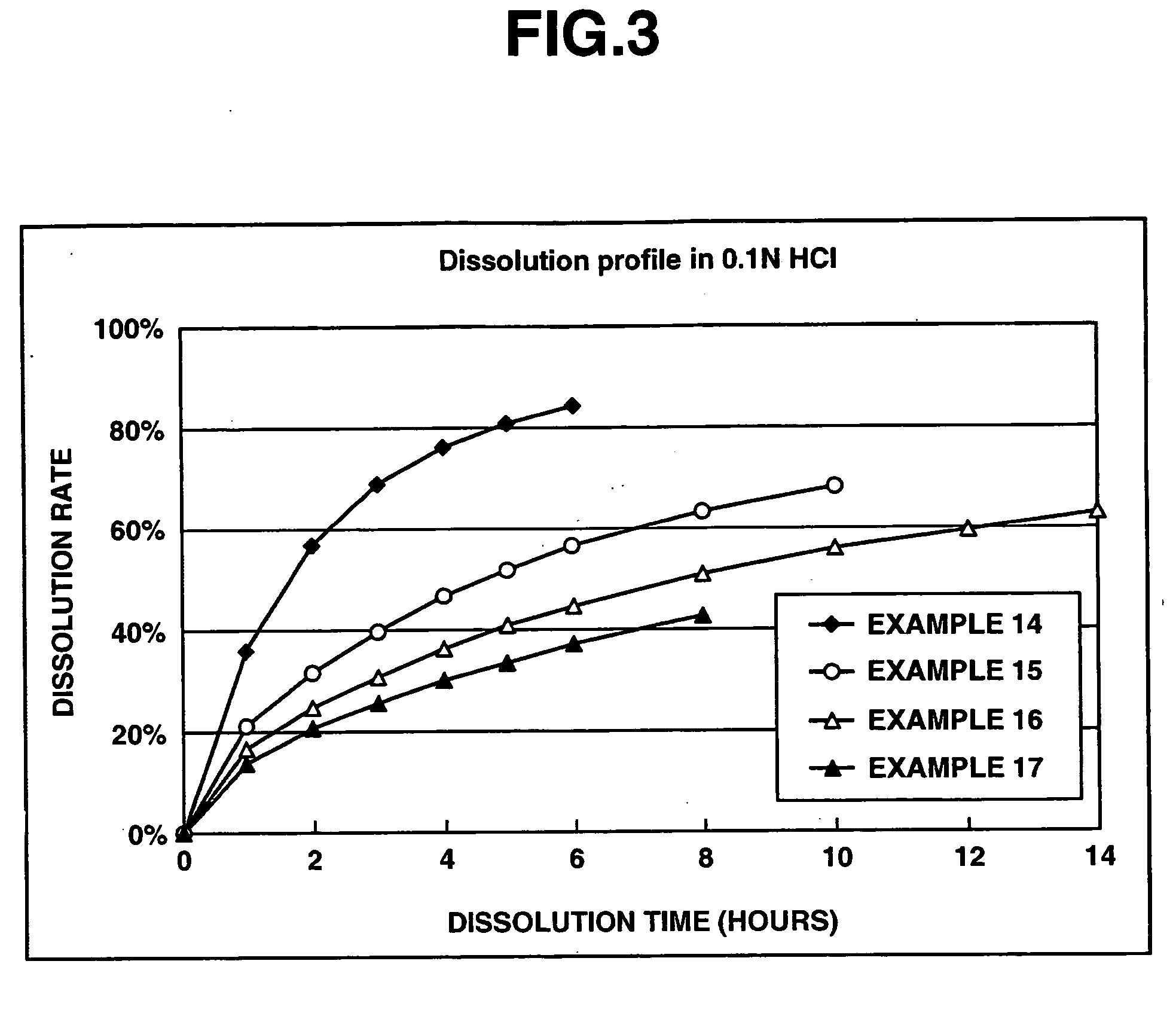
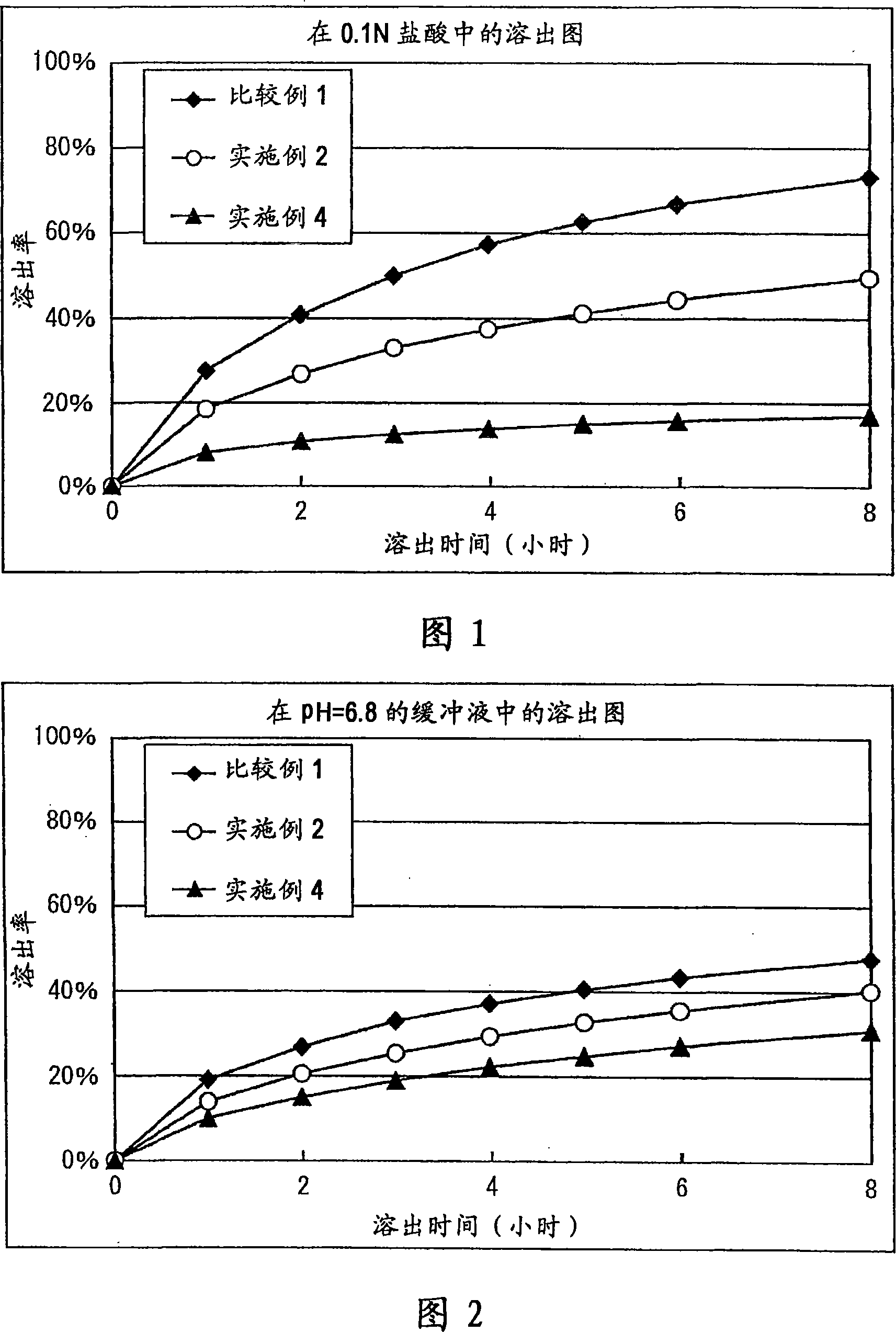
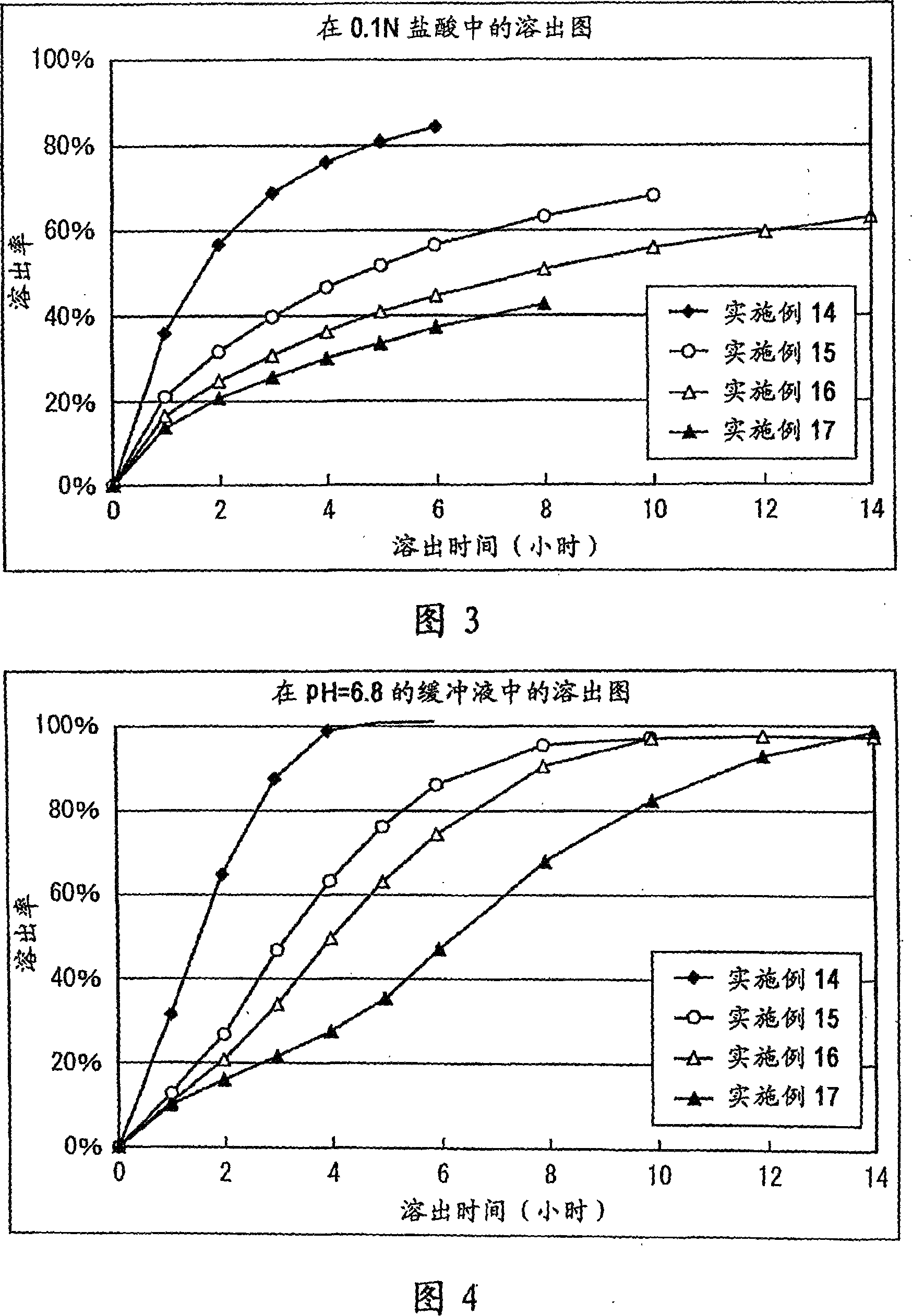
A matrix type sustained-release preparation and a manufacturing method therefor are provided wherein dissolution with low pH dependence of a basic drug or a salt thereof at the early stage of dissolution can be ensured in a dissolution test, and wherein as the dissolution test proceeds, a ratio of a dissolution rate of the basic drug or the salt thereof in an acidic test solution to a dissolution rate of the basic drug or the salt thereof in a neutral test solution (dissolution rate in the acidic test solution / dissolution rate in the neutral test solution) decreases with dissolution time at the late stage of dissolution, as compared to the early stage of dissolution. According to the present invention, the matrix type sustained-release preparation contains a basic drug or a salt thereof and at least one enteric polymer, in which solubility of the basic drug or the salt thereof in a 0.1 N hydrochloric acid solution and a neutral aqueous solution, pH 6.0 is higher than in a basic aqueous solution, pH 8.0.
Owner:EISIA R&D MANAGEMENT CO LTD
Immunoglobulin molecules with improved characteristics
ActiveUS20070041907A1Increased serum half-lifeUltrasonic/sonic/infrasonic diagnosticsAntipyreticHalf-lifeClearance rate
The present invention provides for IgG1 molecules with improved characteristics. In particular, substitution mutations are provided that, in combination, facilitate improved placental transfer, improved serum half-life and improved FcRn binding. Substitution mutations are also provided, that in combination, can be used to block FcRn function and thereby increase the clearance rates of other (endogenous or exogenous) IgGs, block placental transport of IgGs and have increased affinity / reduced pH dependence for FcRn binding.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Delayed release film coatings containing calcium silicate and substrates coated therewith
ActiveUS9233074B2Reduces caking tendencyResistant to agglomerationCoatingsDrageesCalcium silicatePh dependent
The present invention includes pH dependent, dry film coating compositions containing calcium silicate for use on orally-ingestible substrates such as tablets and the like. The film coating compositions can be applied as an aqueous suspension either directly to a substrate or after the substrate has been coated with a subcoat. In preferred aspects, the polymer is either an enteric or reverse-enteric polymer. Methods of preparing the dry film coatings, methods of preparing corresponding aqueous suspensions, methods of applying the coatings to substrates and the coated substrates themselves are also disclosed.
Owner:BPSI HLDG LLC
Self-emulsifying drug delivery system for improving bioavailability of insoluble medicine, and application thereof
ActiveCN105535979AGood water solubilityIncrease dissolution rateOrganic active ingredientsCapsule deliverySolubilityOil phase
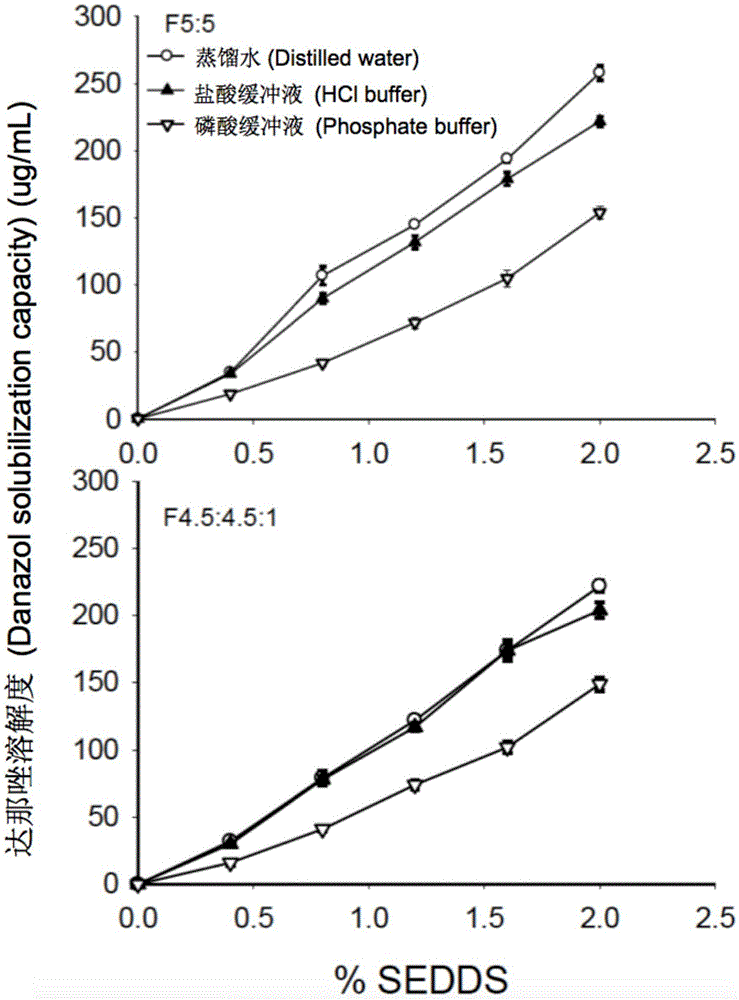
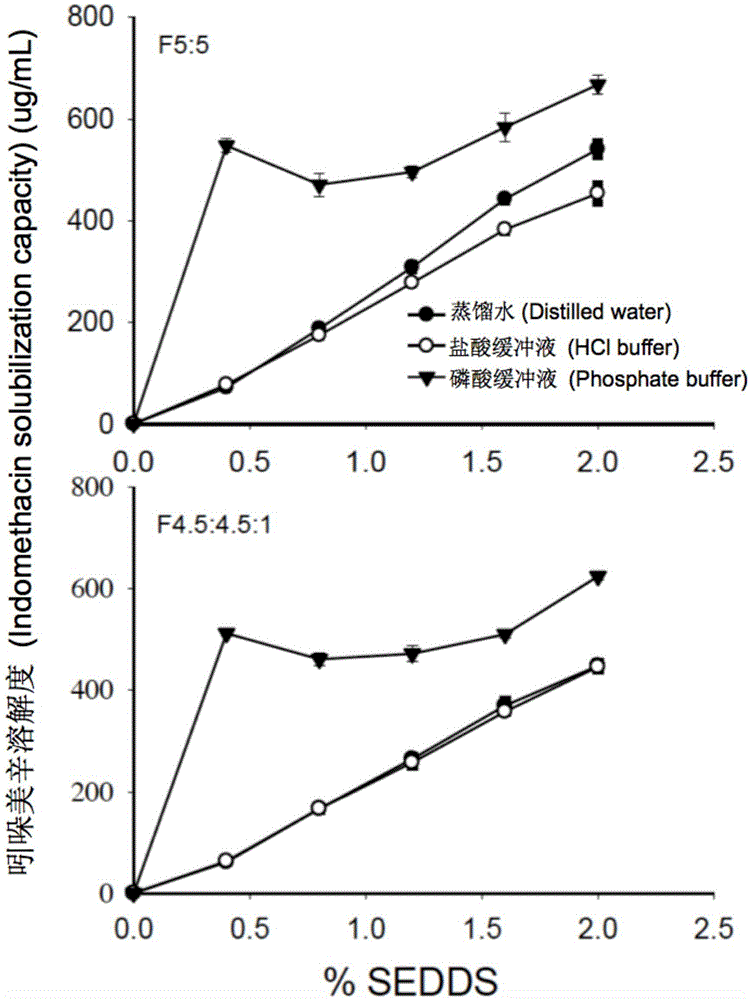
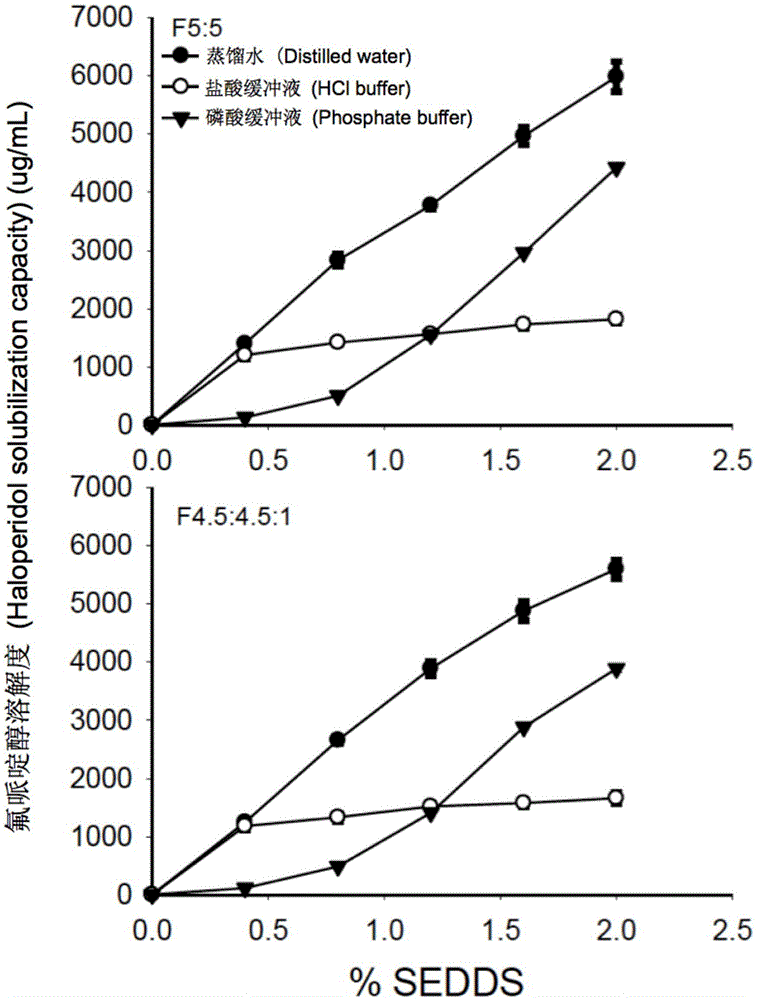
The invention provides a novel self-emulsifying drug delivery system (SEDDS). An SEDDS carrier material comprises a surfactant and an oil phase containing Capmul MCMs and medium chain fatty acids, is suitable for loading pH-dependent (weakly acidic and weakly alkaline) and a pH-independent (neutral) insoluble medicines, greatly improves the solubility of the medicines to realize optimum bioavailability, and has important application values in the development of preparations of the insoluble medicines.
Owner:李素华
Sustained release dosage form
The novel sustained release dosage form comprising an active agent and a combination of a non-swelling pH dependent release retardant and a non swelling pH independent release retardant polymer which provides pH-independent drug release for a considerable period of time after administration.
Owner:RUBICON RES PTY LTD
PROCESS FOR REMOVING FLUORINATED EMULSIFIER FROM FLUOROPOLMER DISPERSIONS USING AN ANION-EXCHANGE RESIN AND A pH-DEPENDENT SURFACTANT AND FLUOROPOLYMER DISPERSIONS CONTAINING A pH-DEPENDENT SURFACTANT
InactiveUS20080264864A1Reduce the amount requiredGood dispersionIon-exchange process apparatusWater treatment parameter controlPolymer scienceFluoropolymer
A process of reducing the amount of fluorinated emulsifiers in fluoropolymer dispersions by contacting the fluoropolymer dispersion with an anion exchange resin in the presence of a pH-dependent surfactant, and fluoropolymer dispersions containing the pH-dependent surfactant and uses thereof.
Owner:3M INNOVATIVE PROPERTIES CO
Methods of fracturing a subterranean formation using a pH dependent foamed fracturing fluid
InactiveUS6966379B2Lower Level RequirementsFine foamFluid removalFlushingFracturing fluidSURFACTANT BLEND
Methods of fracturing a subterranean formation include providing a fracturing fluid having a first pH. The fracturing fluid may be made by combining a gelling agent, a surfactant, and a proppant. The surfactant is capable of facilitating foaming of the fracturing fluid at the first pH and defoaming of the fracturing fluid when its pH is changed to a second pH. The methods of fracturing the subterranean formation further include foaming the fracturing fluid having the first pH and subsequently pumping it to the subterranean formation to fracture the formation. The pH of the fracturing fluid changes to a second pH, for example via in situ contact with an acidic material, causing the level of foam in the fracturing fluid to be reduced. As a result of the reduction of the foam, the fracturing fluid deposits the proppant into the fractures formed in the subterranean formation.
Owner:HALLIBURTON ENERGY SERVICES INC
Novel sustained release dosage form
The novel sustained release dosage form comprising an active agent and a combination of a non-swelling pH dependent release retardant and a non swelling pH independent release retardant polymer which provides pH-independent drug release for a considerable period of time after administration.
Owner:RUBICON RES PTY LTD
Azilsartan solid dispersion and preparation method and medicinal composition thereof
InactiveCN102793680AImprove solubilityReduced pH dependenceOrganic active ingredientsPill deliverySolubilityCombinatorial chemistry
The invention relates to an azilsartan solid dispersion and a preparation method and medicinal composition thereof. A solid dispersion system is prepared from azilsartan and pharmaceutically acceptable carrier materials of azilsartan. According to the invention, the problem of poor water solubility of asilsartan can be solved, the pH dependence of azilsartan dissolubility can be weakened, and azilsartan can achieve an ideal dissolving effect in different pH media.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Oral solid pharmaceutical formulations with pH-dependent multiphasic release
InactiveUS7022345B2Smooth releaseOrganic active ingredientsPowder deliveryAdditive ingredientBULK ACTIVE INGREDIENT
Oral solid pharmaceutical compositions with pH-dependent multiphasic release, containing, as active ingredient, a molecule useful in the inflammatory bowel disease therapy, are described, being such compositions suitable to the release of the active ingredient in the intestinal tract.
Owner:VALDUCCI ROBERTO
Methods of fracturing a subterranean formation using a pH dependent foamed fracturing fluid
InactiveUS20050077047A1Level of foaming is reducedFine foamFluid removalFlushingFracturing fluidSURFACTANT BLEND
Methods of fracturing a subterranean formation include providing a fracturing fluid having a first pH. The fracturing fluid may be made by combining a gelling agent, a surfactant, and a proppant. The surfactant is capable of facilitating foaming of the fracturing fluid at the first pH and defoaming of the fracturing fluid when its pH is changed to a second pH. The methods of fracturing the subterranean formation further include foaming the fracturing fluid having the first pH and subsequently pumping it to the subterranean formation to fracture the formation. The pH of the fracturing fluid changes to a second pH, for example via in situ contact with an acidic material, causing the level of foam in the fracturing fluid to be reduced. As a result of the reduction of the foam, the fracturing fluid deposits the proppant into the fractures formed in the subterranean formation.
Owner:HALLIBURTON ENERGY SERVICES INC
Method for prophylaxis and/or treatment of thromboembolism
InactiveUS6875446B2Reduce solubilityLimited food interactionPowder deliveryOrganic active ingredientsFiller ExcipientThrombus
A new oral IR formulation in solid form for a low molecular weight thrombin inhibitor having pH dependant dissolution, characterized in that the formulation comprises a filler or a combination of fillers having disintegrant properties in an amount higher than 35% w / w of the formulation.
Owner:ASTRAZENECA AB
Preparation method of taste-masking slow-release allicin composite microcapsules
InactiveCN102266308AImprove adsorption capacityHigh affinityAntibacterial agentsOrganic active ingredientsCarrying capacityIrritation
The invention discloses a method for preparing odor masking sustained-release garlicin composite micro-capsules. In the method, a garlicin / organic attapulgite complex serving as a capsule core is combined with sodium alginate to obtain a complex, and sodium alginate / chitosan micro-capsules are prepared from the obtained complex in a complex coacervation method. In the method, odor is masked and the medicine carrying capacity and envelop rate of the micro-capsules are improved by utilizing the adsorptive power and appetency of organic attapulgite on garlicin; gastric mucosae are protected against the irritation of the garlicin by utilizing the pH dependence of calcium alginate and the protective effect of attapulgite mucosae, and the micro-capsules become enteric medicine carrier materialsunder the synergistic action of the garlicin and the attapulgite on germs in intestinal tracts. In addition, the release of medicines in the micro-capsules is retarded by utilizing the detention effect of the attapulgite on the garlicin and the sustained-release performance of chitosan to improve the sustained-release performance of the micro-capsules. The preparation method is simple and environment-friendly, and can be used for sustained-release carrier materials of various medicines.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
Capsule for holding liquid-containing compositions and method for making the same
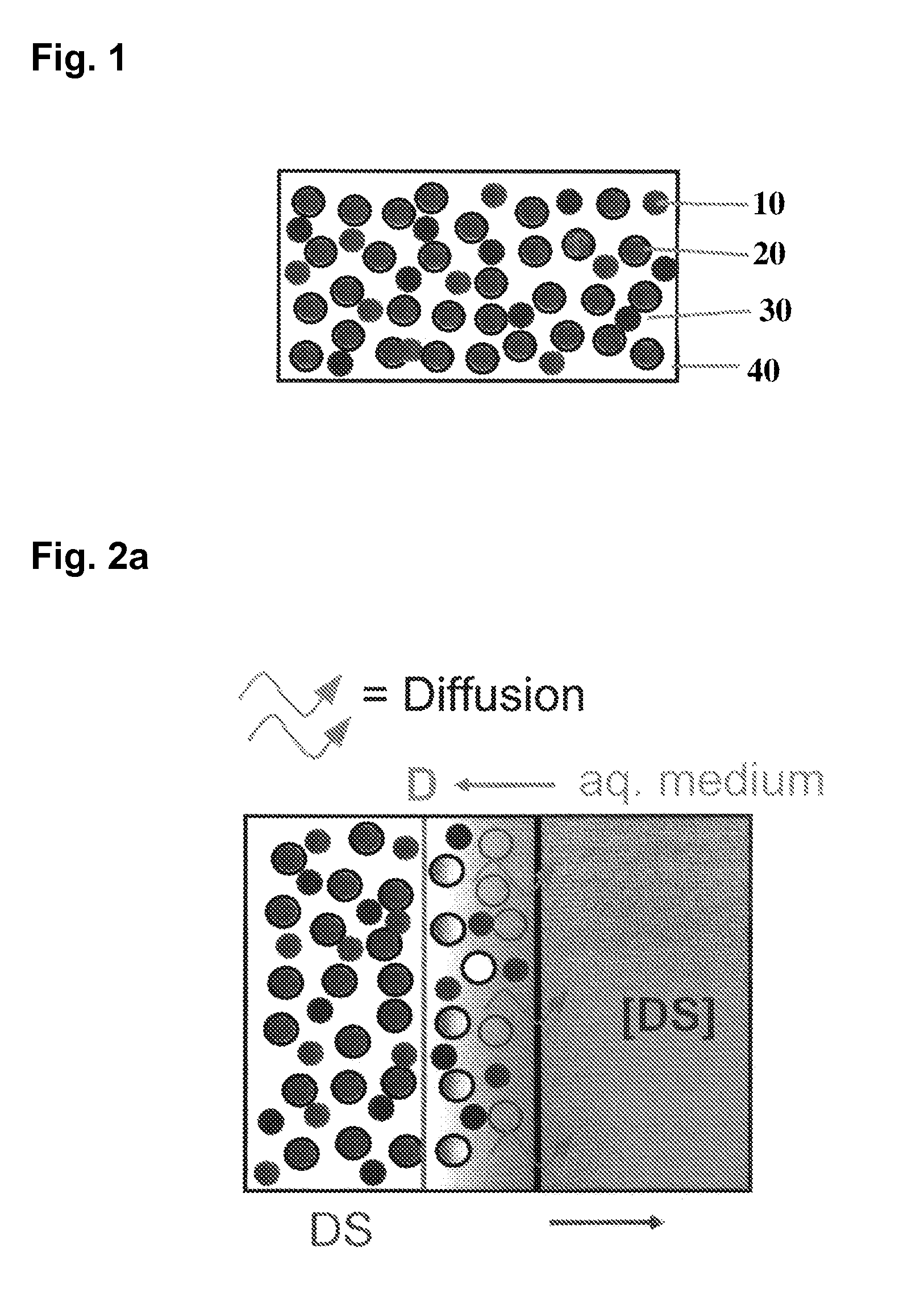
The present invention provides a capsule which comprises (1) a capsule shell, and (2) a liquid core composition. The capsule shell comprises a pH-dependent polymer and optionally a plasticizer. The liquid core composition contains liquid up to 70% by volume. The pH of the liquid core composition is adjusted to or at a pH in which the pH-dependent polymer is insoluble. The liquid core composition is preferably a decoction or condensate of the decoction containing herbal extract. The present invention further provides methods for making the capsule.
Owner:YUNG SHIN PHARMACEUTICALS INDUSTRIAL CO LTD
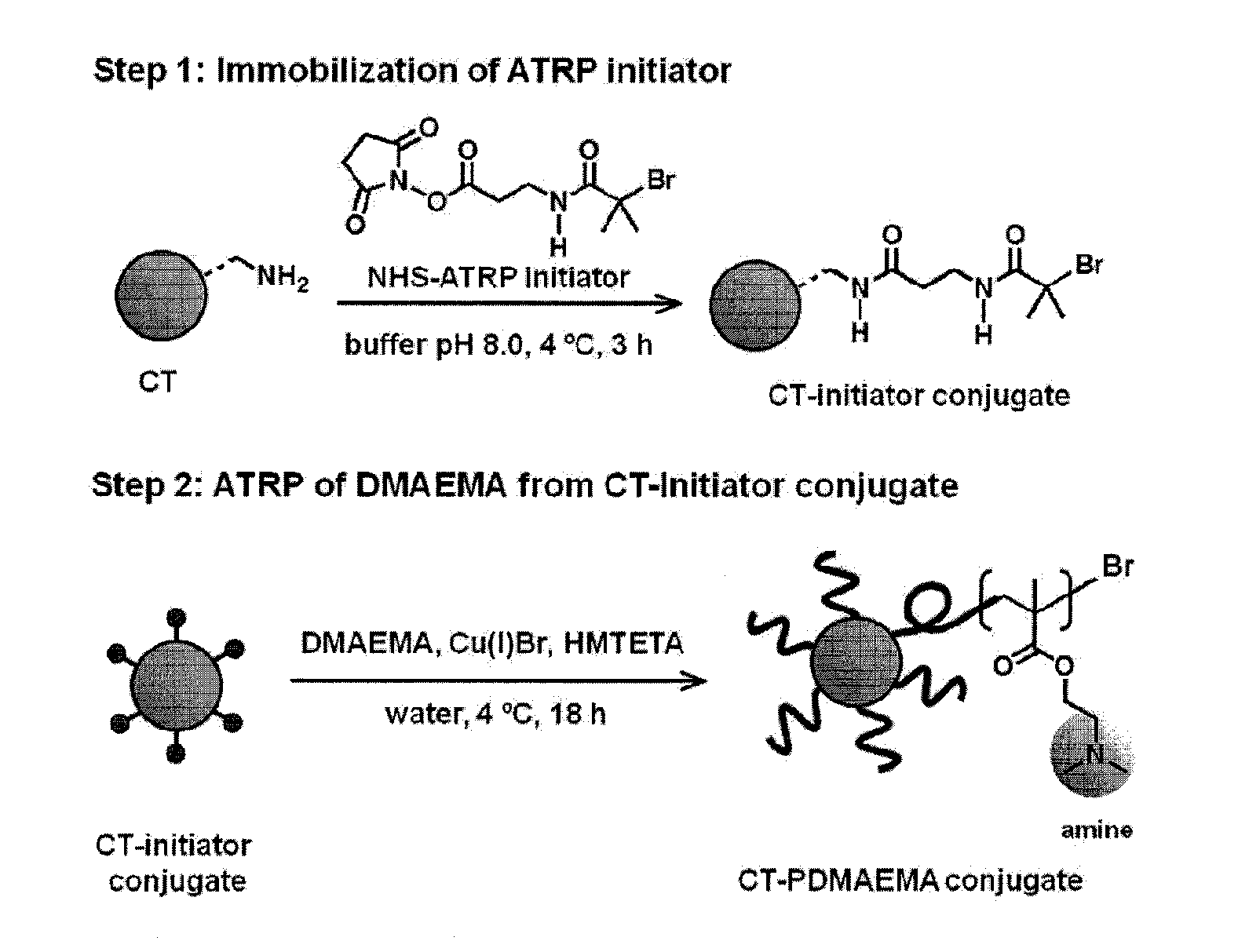
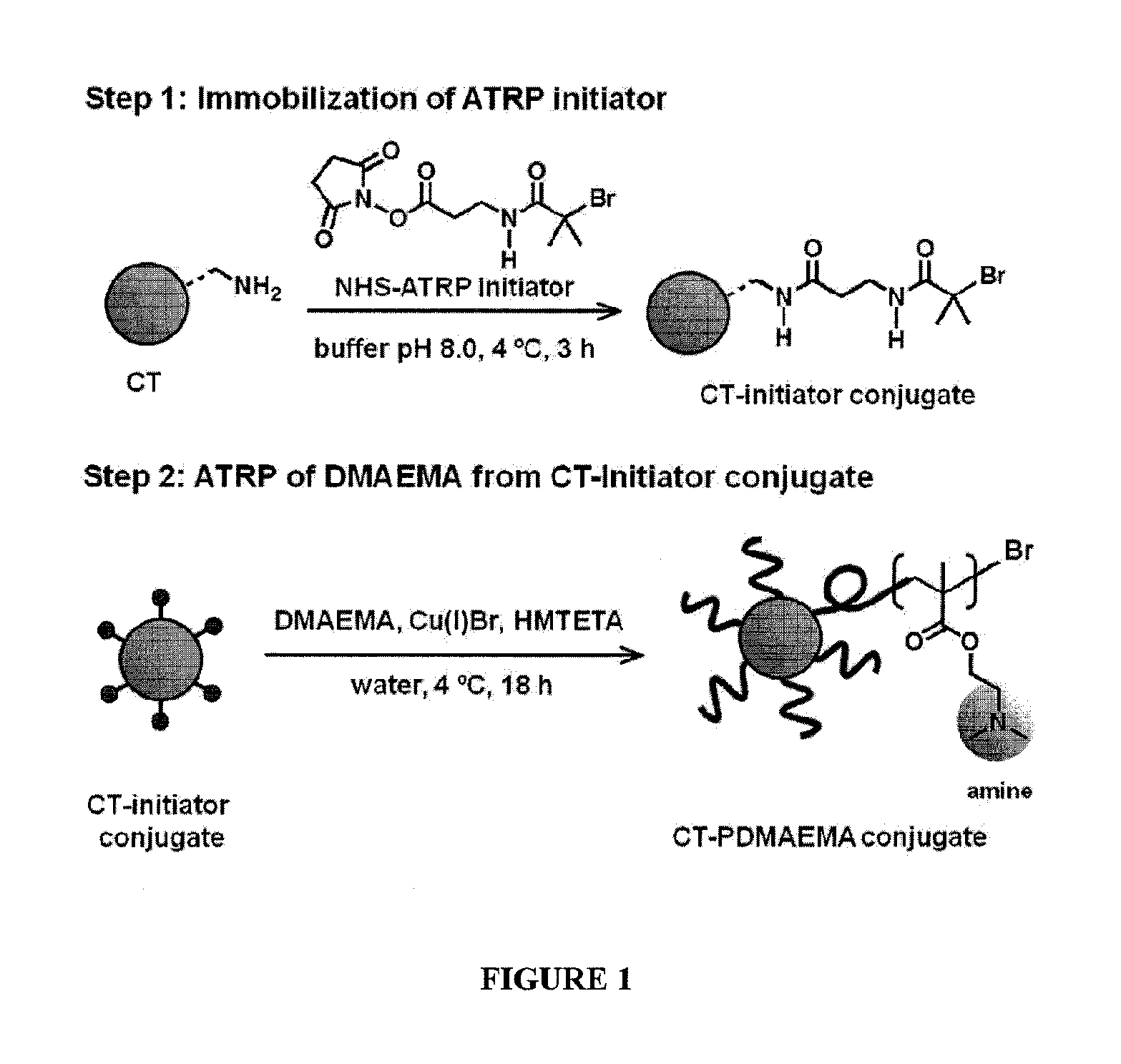
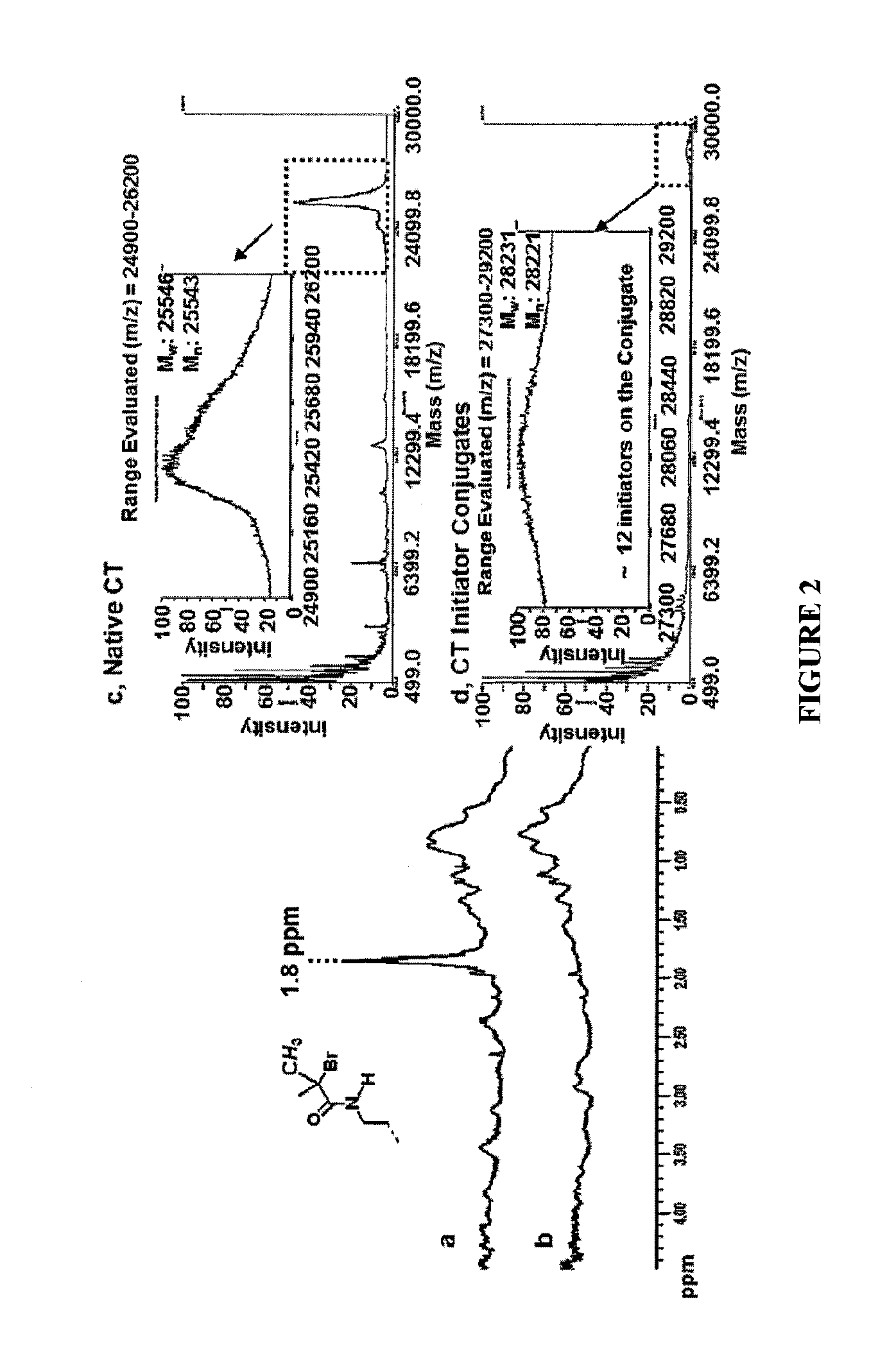
POLYMER-BASED PROTEIN ENGINEERING METHODS TO RATIONALLY TUNE ENZYME ACTIVITY, pH-DEPENDENCE AND STABILITY
ActiveUS20160101190A1Improve biological activityHigh affinityPeptide/protein ingredientsEnzyme stabilisationStimuli responsiveChymotrypsin
Using a novel water-soluble, active ester amide-containing functionalized controlled radical polymerization initiator, stimuli responsive polymers have been grown from the surface of a protein, exemplified by chymotrypsin or any protein having surface amino acids that will covalently bind to the active ester amide-containing functionalized initiator. It is shown that changes in temperature or pH can change the conformation of the polymer surrounding the enzyme, which in turn enabled the rational tailoring of enzyme activity and stability. This method has afforded an increase in the activity and stability of the enzyme by an order of magnitude at pH's where the enzyme is usually inactive or unstable. Multimodal temperature responsive protein-block copolymer conjugates are described.
Owner:CARNEGIE MELLON UNIV +1
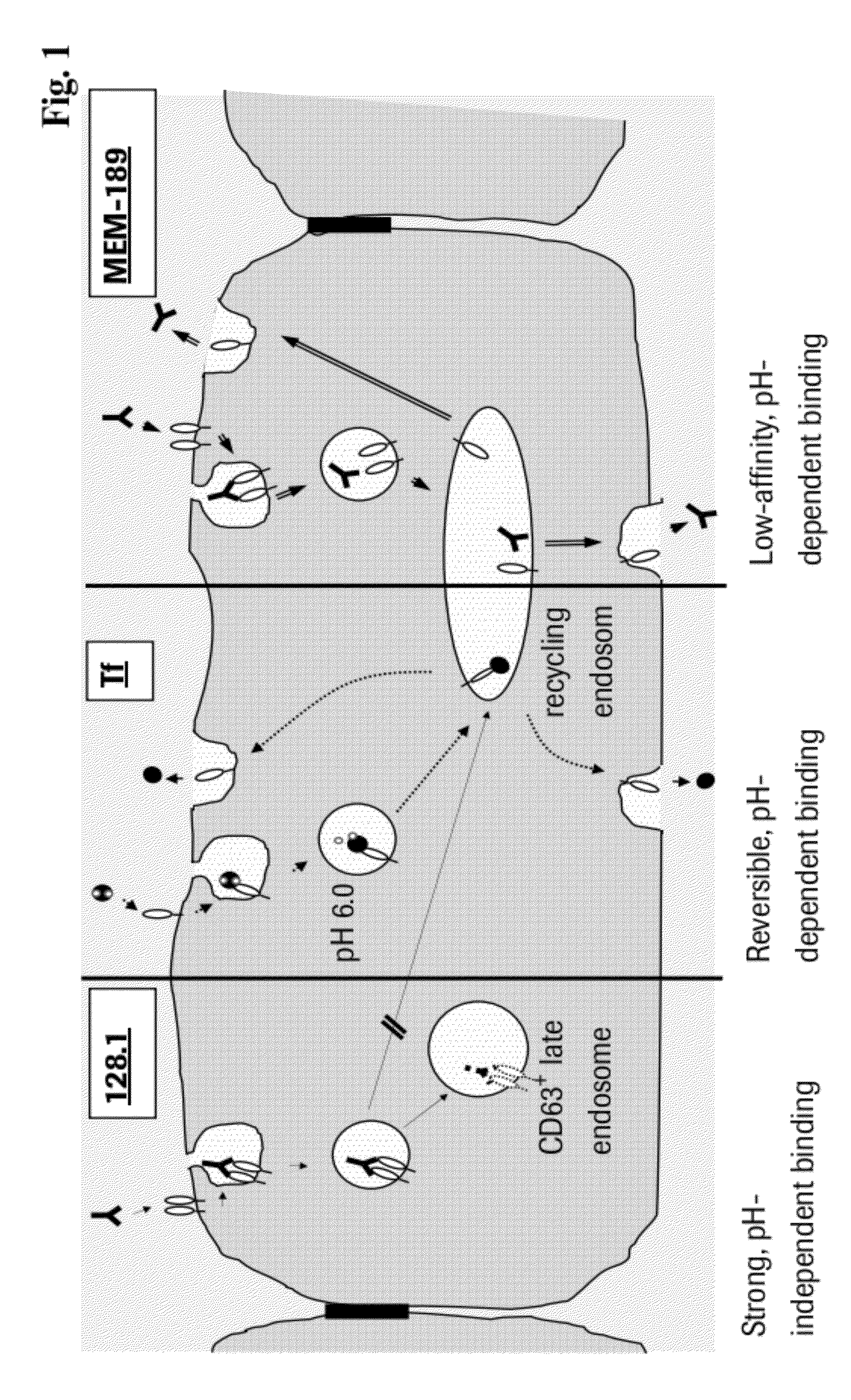
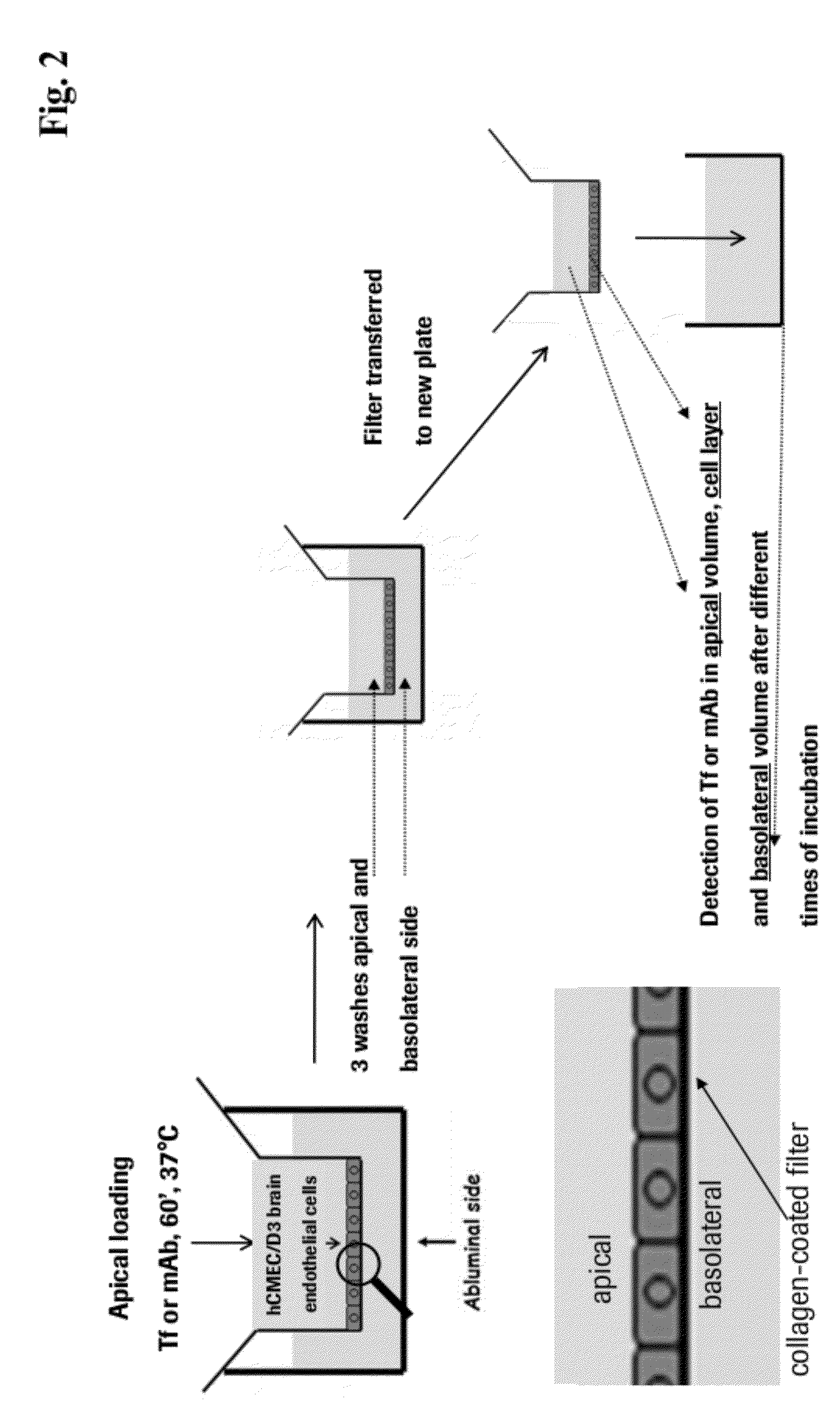
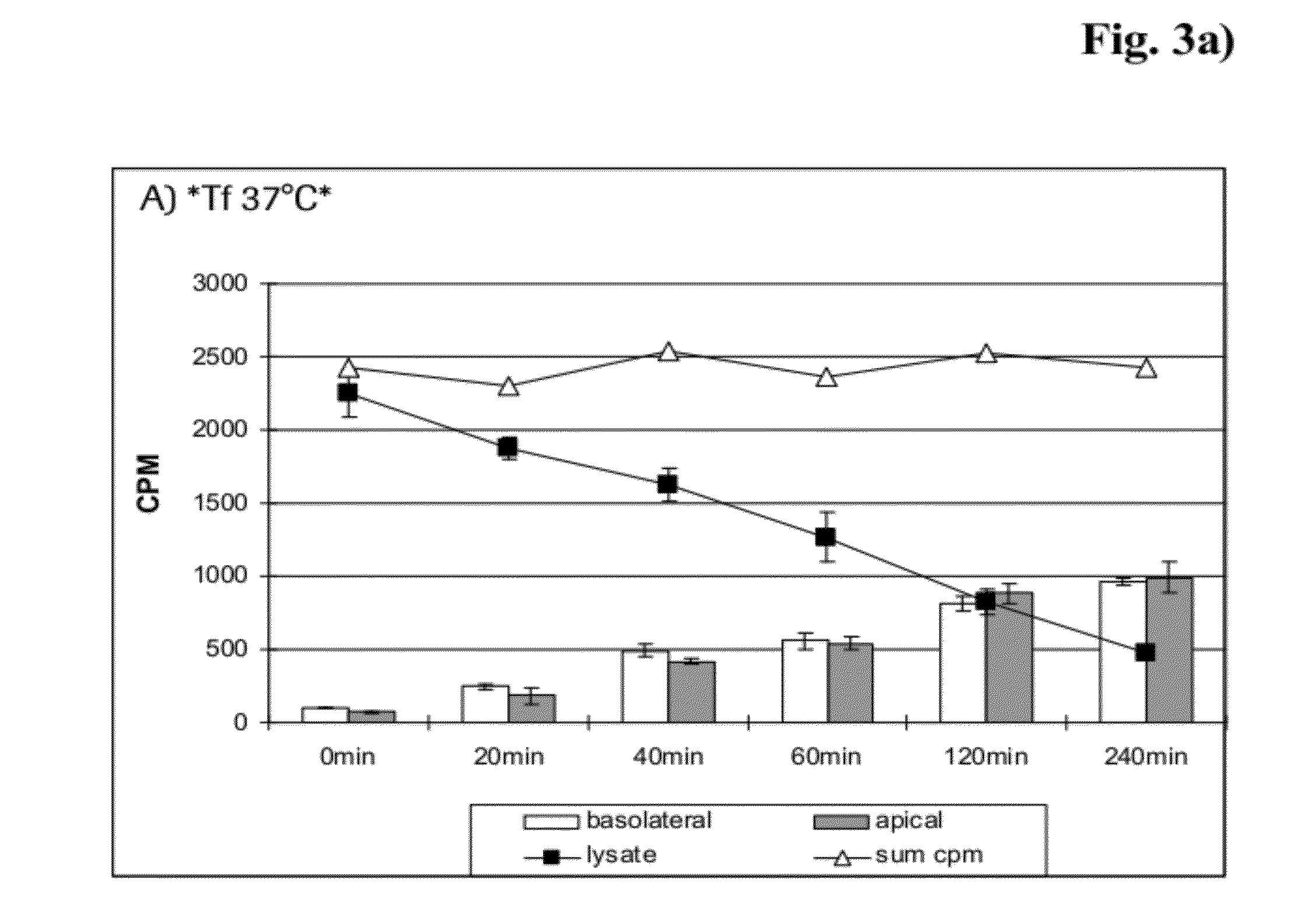
Method and Constructs for the pH Dependent Passage of the Blood-brain-barrier
InactiveUS20120282176A1Effectively crossDiminishment of any direct or indirect pathological consequencesAntibacterial agentsNervous disorderBinding siteVariable domain
Herein is reported a fusion polypeptide comprising i) at least one binding site, e.g. which comprises an antibody heavy chain variable domain and an antibody light chain variable domain, and which binds to an internalizing cell surface receptor, and ii) at least one pharmaceutically active compound, whereby the EC50-value of the binding pair that binds to an internalizing cell surface receptor determined at pH 5.5 is higher than the EC50-value of the same binding pair determined at pH 7.4 and its use for delivering a pharmaceutically active compound across the blood-brain-barrier.
Owner:ROCHE GLYCART AG
Materials and Methods For the Purification of Polyelectrolytes, Particularly Nucleic Acids
This invention describes materials and methods usable for the purification of polyelectrolytes, such as nucleic acids and proteins. The materials of the invention are separation media that possess pH-dependent groups with pKa value in the range of about 5 to about 7. Separation of the nucleic acids or proteins from a separation medium is effected at a neutral or higher pH.
Owner:LIFE TECH CORP
Oral Medicament For The Modified Release Of At Least One Active Principle, In Multimicrocapsule Form
InactiveUS20080305160A1Increase activity timeAntibacterial agentsBiocideDual releaseTherapeutic effect
The field of the invention is that of oral medicaments or pharmaceutical compositions, in particular of the type including one or more active principles. The aim of the invention is to provide an improved oral medicament to be administered in one or several daily doses and enabling the modified release of active principles (in particular of one active principle), whereby the prophylactic and therapeutic effectiveness of said medicament is improved. This aim is achieved by the oral multimicrocapsule galenic form according to the invention, in which the active principle release is controlled by a dual release trigger mechanism: “time-dependent trigger” and “pH-dependent trigger”. Said medicament includes microcapsules providing the modified release of the active principle, each comprising a core containing
Owner:FLAMEL TECHNOLOGIES
SUSTAINED RELEASE PHARMACEUTICAL COMPOSITION ON THE BASIS OF RELEASE SYSTEM COMPRISING AN ACID-SOLUBLE POLYMER AND A pH-DEPENDENT POLYMER
Sustained release pharmaceutical composition comprising at least one poorly soluble active agent(s), at least one solubilizer, a release rate controlling polymer system consisting of an acid-soluble polymer and a pH-dependent polymer, and optionally other pharmaceutically acceptable excipients. The present invention also describes a process for preparation of such compositions and method of using such compositions. The sustained release compositions are useful in providing therapeutically effective levels of active agent(s) for extended periods of time.
Owner:PANACEA BIOTEC
Medicine osmotic pump preparation
ActiveCN102871982AImprove clarityHigh resolutionMaterial analysis using wave/particle radiationSulfonylurea active ingredientsSolubilityWater insoluble
The invention provides a novel medicine osmotic pump preparation, which comprises a tablet core and a coating membrane, wherein on the basis of the total weight of the tablet core, the tablet core comprises 1 to 60 weight percent of medicine, 5 to 90 weight percent of cavity structure forming materials, 1 to 50 weight percent of cavity structure shape modifiers, 0.5 to 10 weight percent of lubricating agents and 1 to 60 weight percent of pharmaceutically acceptable auxiliary materials, and on the basis of the total weight of the coating membrane, the coating membrane comprises 40 to 90 weight percent of semipermeable membrane coating materials, 5 to 40 weight percent of pore-foaming agents and 0 to 20 weight percent of plasticizers. The cavity structure forming materials can form micro-cell-shaped, poriform or saccular cavity structures inside the tablet core after meeting water, and the control on the medicine release speed is realized through regulating and controlling the form, the size and the evolution speed of micro cavity structures. The osmotic pump preparation is applicable to water-soluble medicine and water-insoluble medicine, in particular to the control release of low-solubility medicine or pH dependency medicine.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Proton pump inhibitor formulations, and methods of preparing and using such formulations
InactiveUS20050232992A1Organic active ingredientsDigestive systemPh independentPharmaceutical formulation
Pharmaceutical formulation comprising at least one proton pump inhibitor structured and arranged to provide an initial pH independent time-based delayed-release, and a subsequent extended-release of the at least one proton pump inhibitor.
Owner:AGI THERAPEUTICS RES
Extended release tablet formulations of flibanserin and method for manufacturing the same
ActiveUS8545886B2Reduce solubilitySufficiently slow releasePowder deliveryNervous disorderExtended release tabletsOral medication
The invention is directed to a Pharmaceutical extended release system, particularly for oral administration, of a pH-dependent water-soluble active substance, comprising or essentially consisting ofa) flibanserin or a pharmaceutically acceptable derivative thereof as active substance;b) one or more pharmaceutically acceptable pH-dependent polymers;c) one or more pharmaceutically acceptable pH-independent polymers;d) one or more pharmaceutically acceptable acids; ande) optionally one or more additives.The present invention provides a release profile of flibanserin which is independent on the pH in the gastrointestinal tract when administered orally resulting in a significantly improved bioavailability.
Owner:BOEHRINGER INGELHEIM INT GMBH
Matrix type sustained-release preparation containing basic drug or salt thereof
InactiveCN101090738AReduced pH dependenceReduced risk of side effectsPowder deliveryNervous disorderSolubilityDissolution
A matrix type sustained-release preparation and a manufacturing method therefor are provided wherein dissolution with low pH dependence of a basic drug or a salt thereof at the early stage of dissolution can be ensured in a dissolution test, and wherein as the dissolution test proceeds, a ratio of a dissolution rate of the basic drug or the salt thereof in an acidic test solution to a dissolution rate of the basic drug or the salt thereof in a neutral test solution (dissolution rate in the acidic test solution / dissolution rate in the neutral test solution) decreases with dissolution time at the late stage of dissolution, as compared to the early stage of dissolution. According to the present invention, the matrix type sustained-release preparation contains a basic drug or a salt thereof and at least one enteric polymer, in which solubility of the basic drug or the salt thereof in a 0.1 N hydrochloric acid solution and a neutral aqueous solution, pH 6.0 is higher than in a basic aqueous solution, pH 8.0.
Owner:EISIA R&D MANAGEMENT CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com