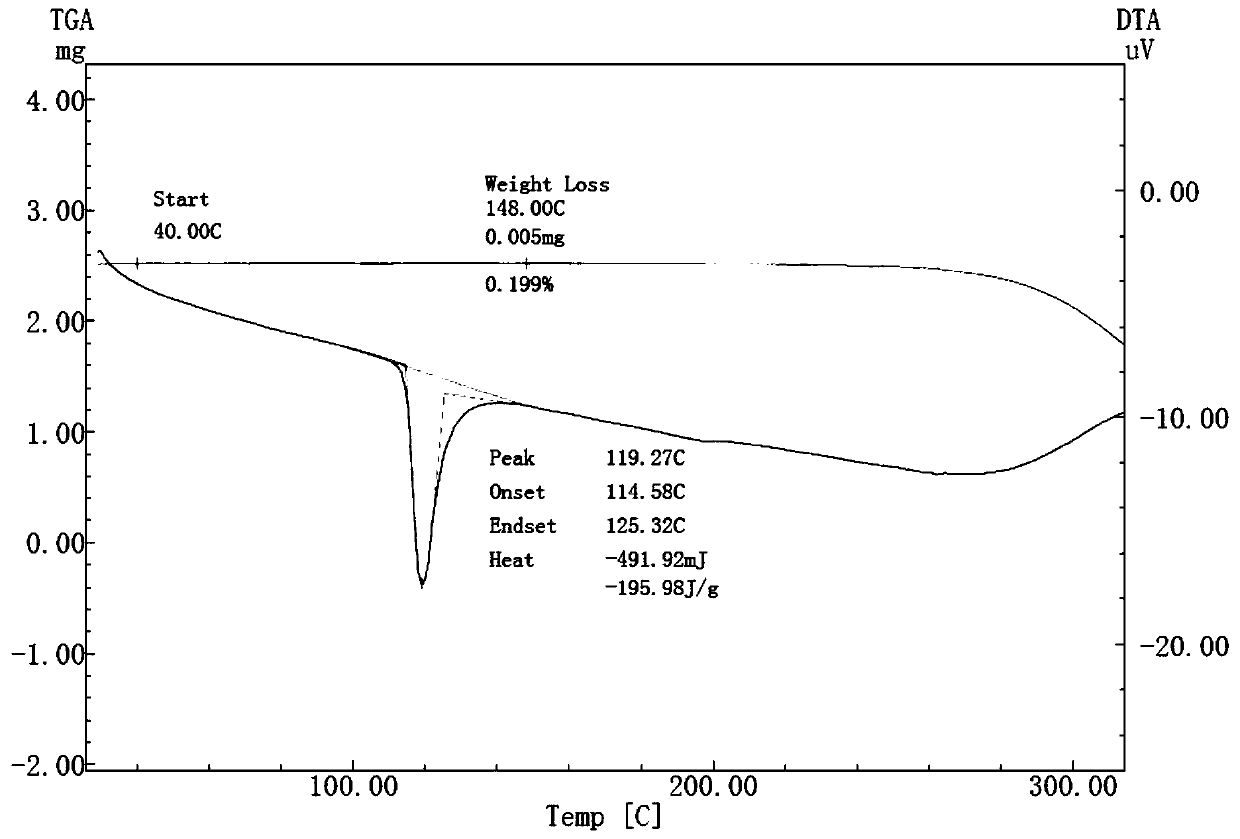
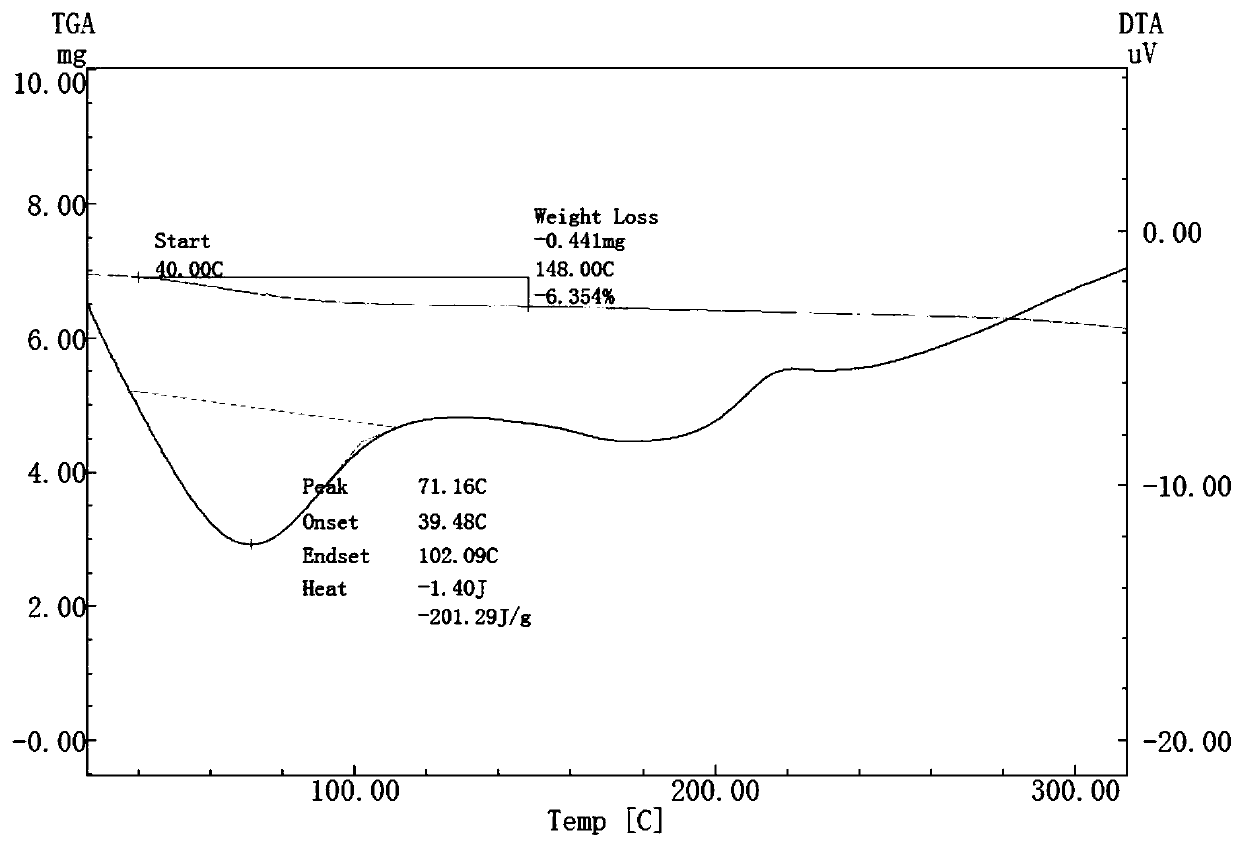
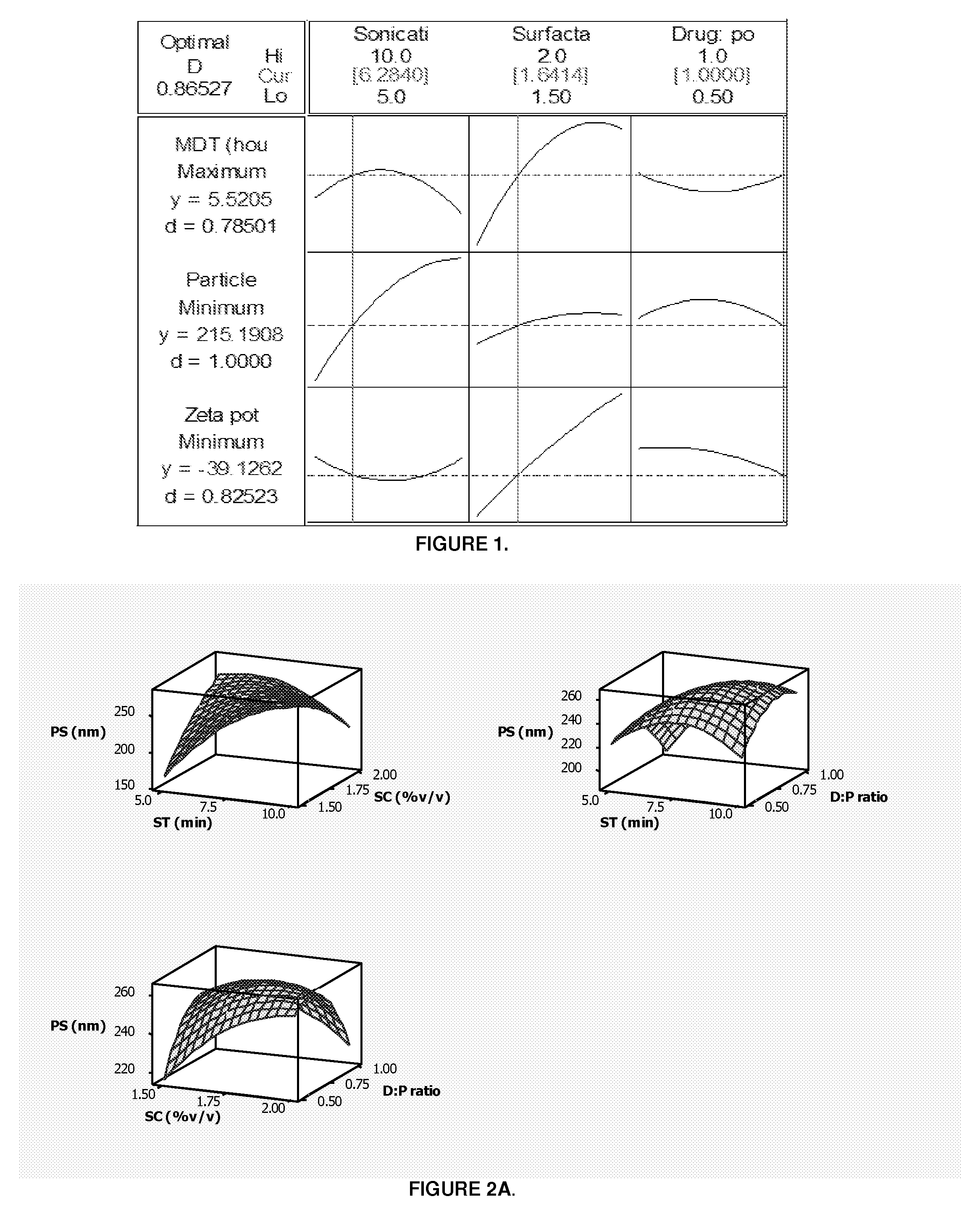
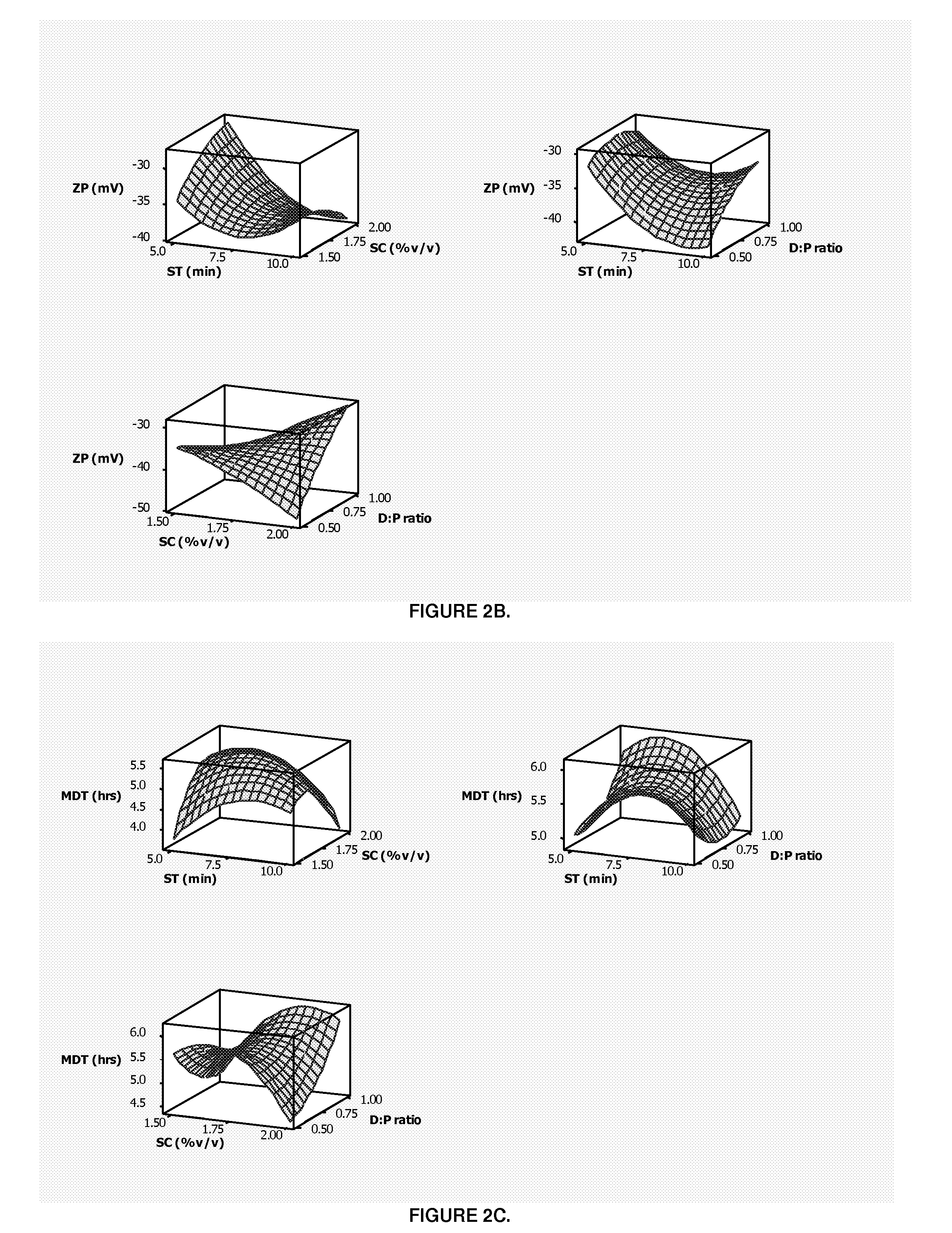
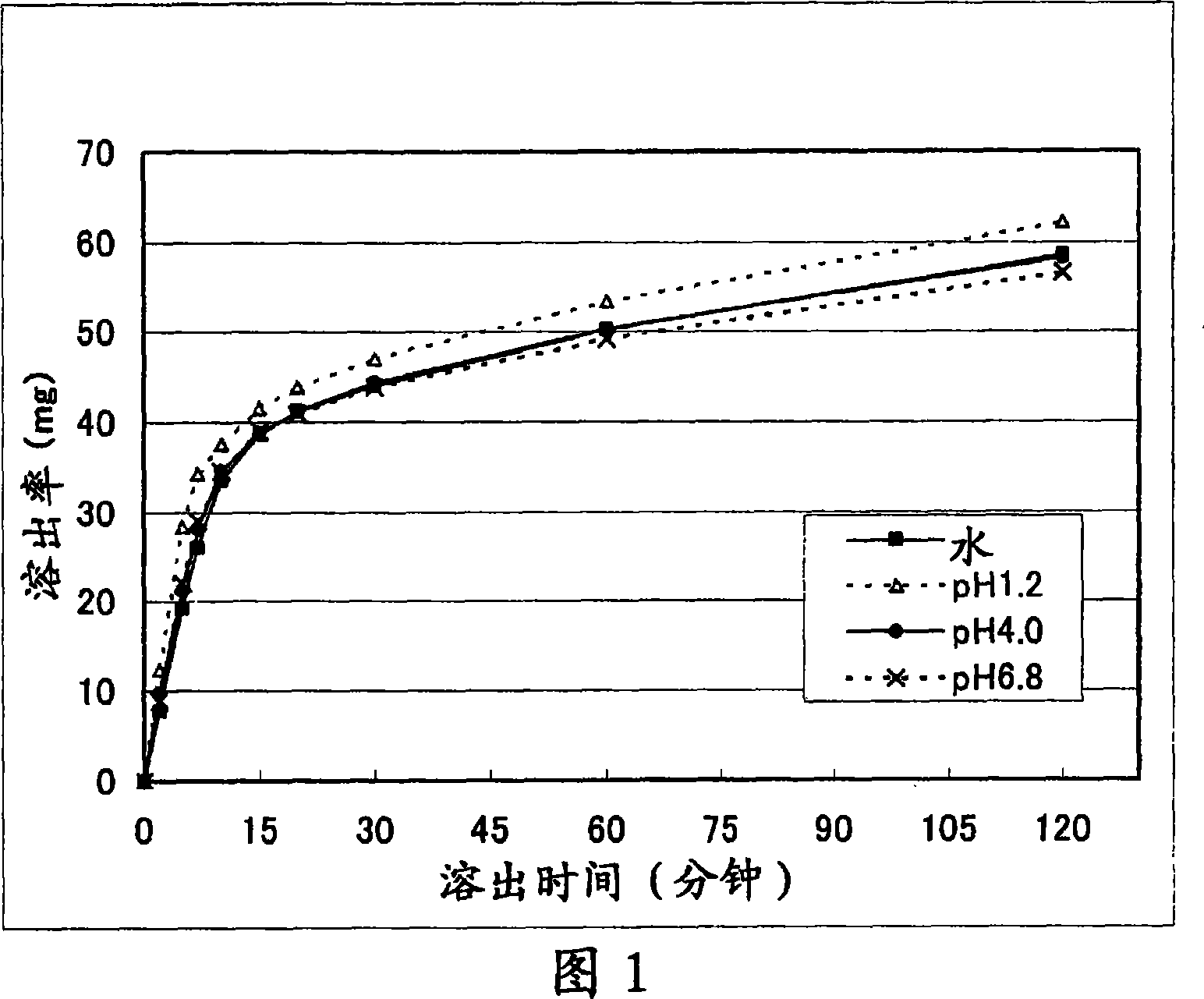
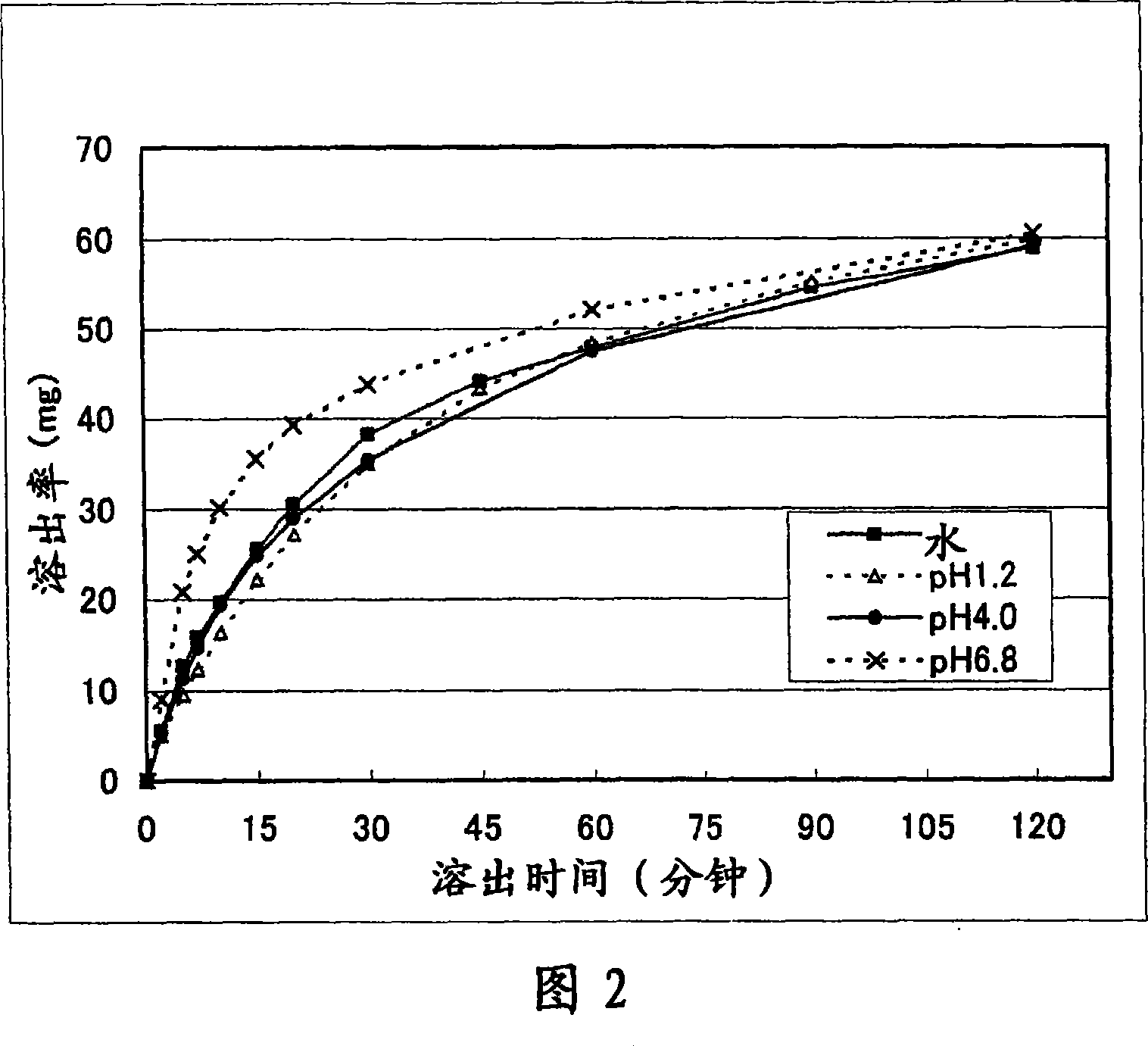
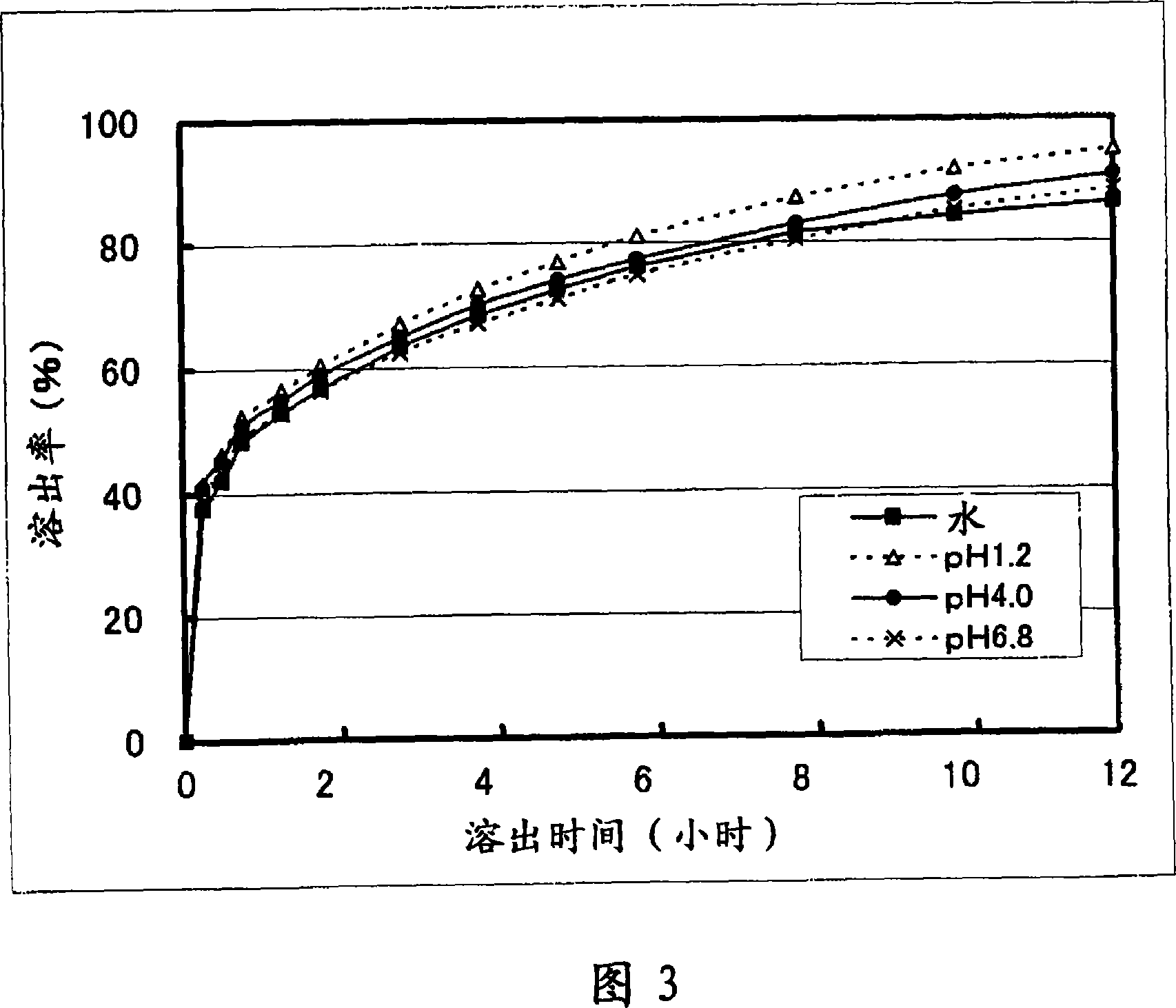
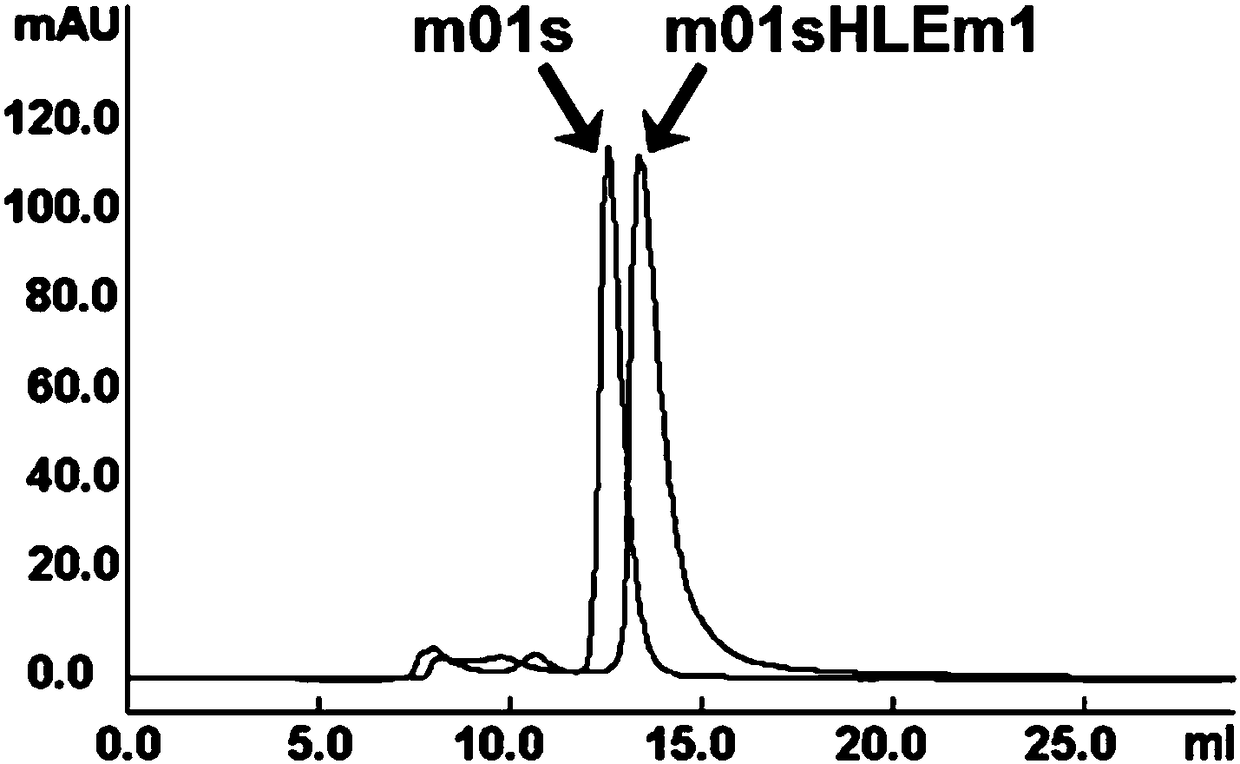
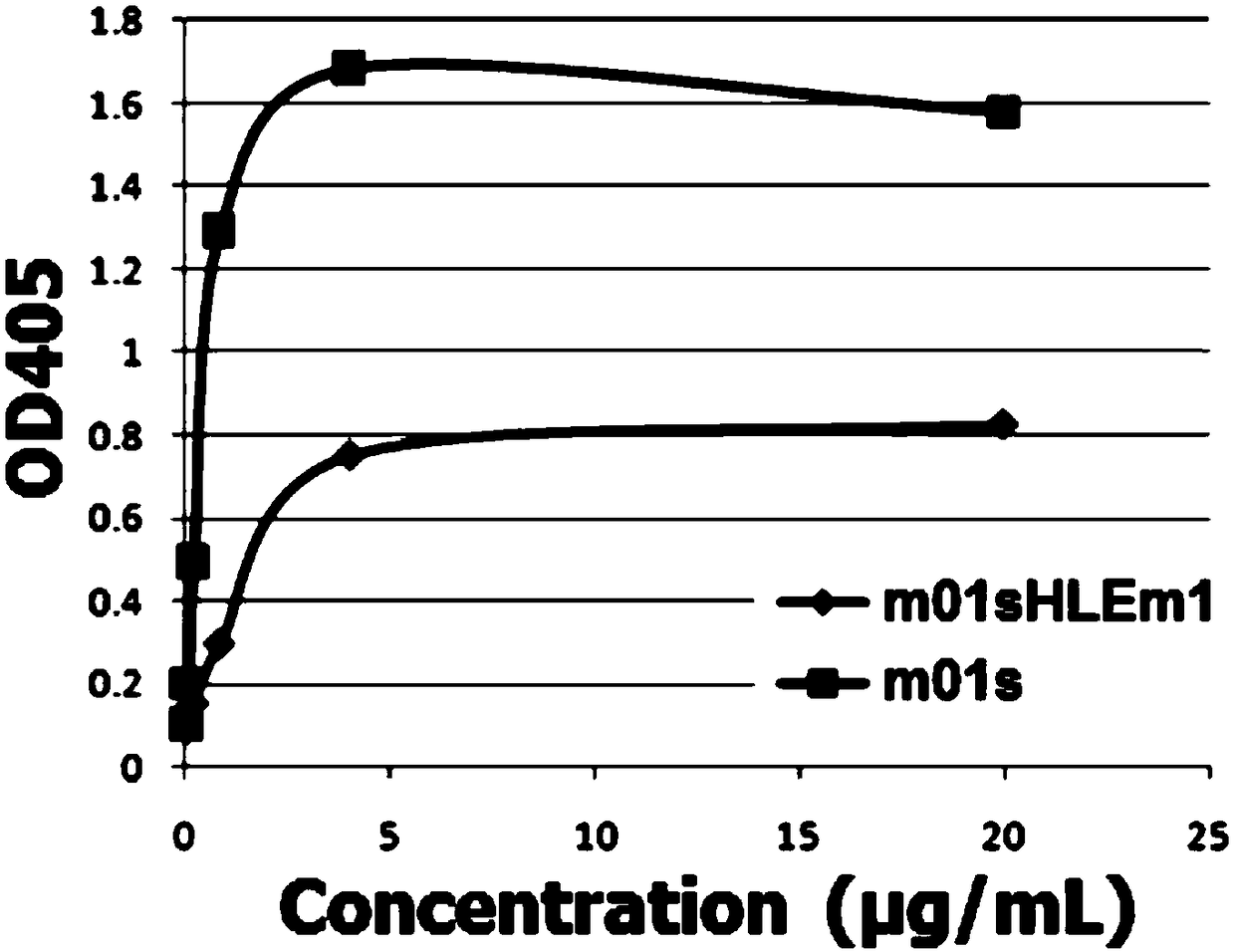
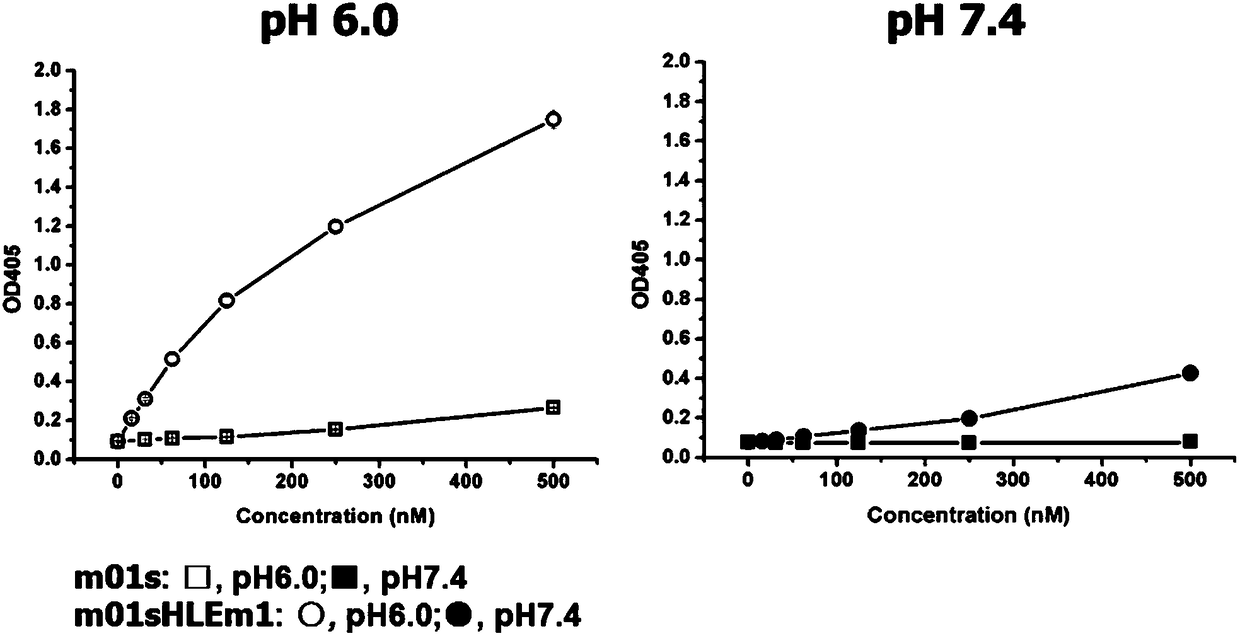
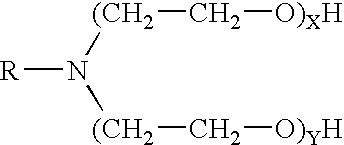Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Ph dependency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
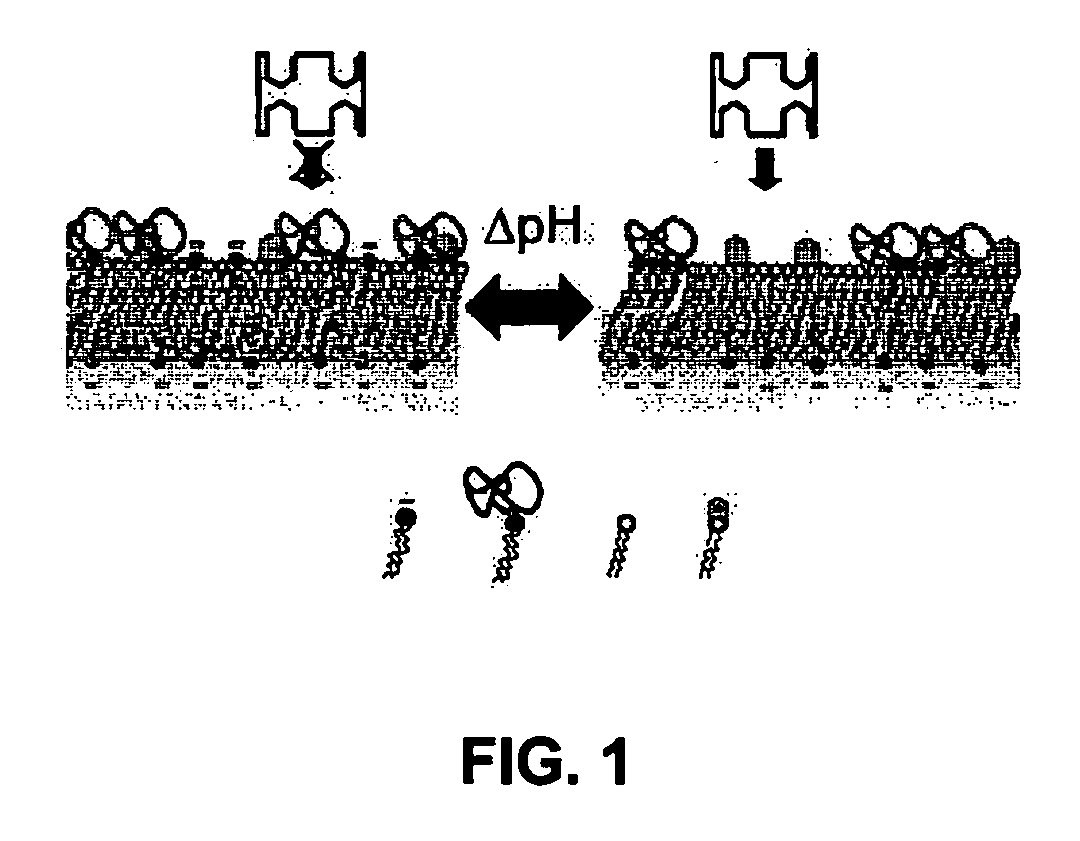
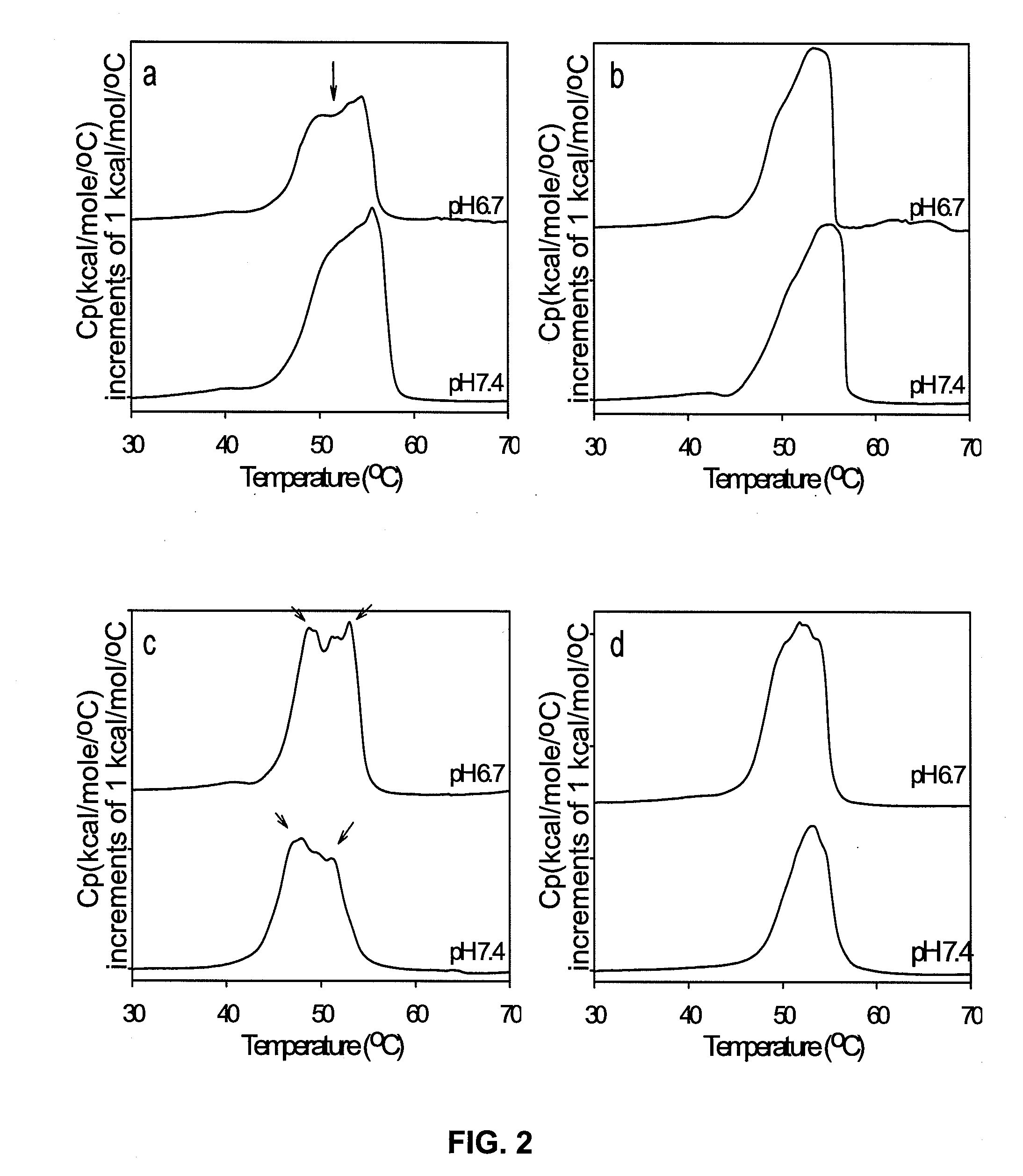
The pH dependence is usually due to the side groups of the amino acids. A change in pH changes the protonation pattern and can, in extreme cases, result in protein denaturation.
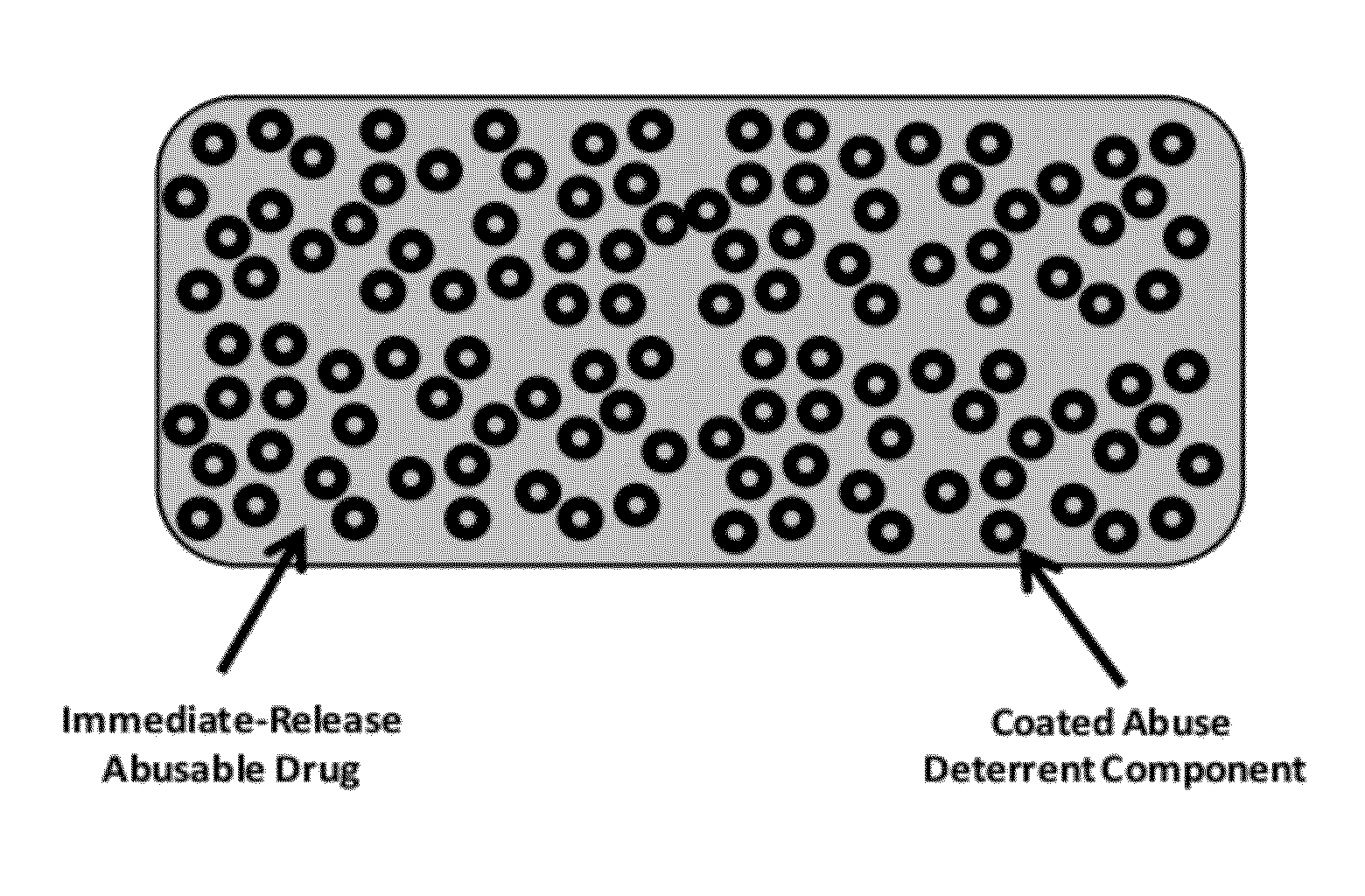
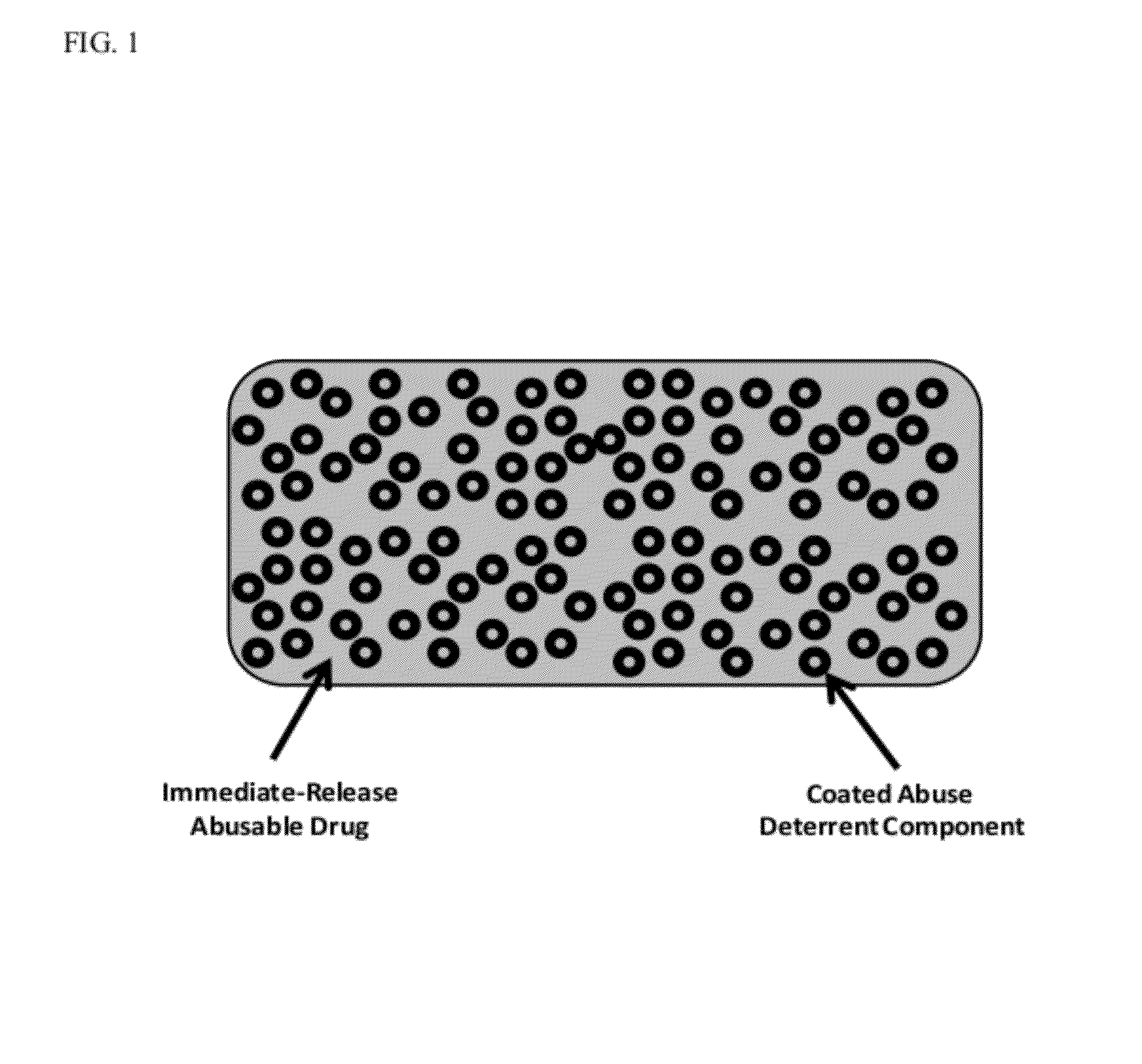
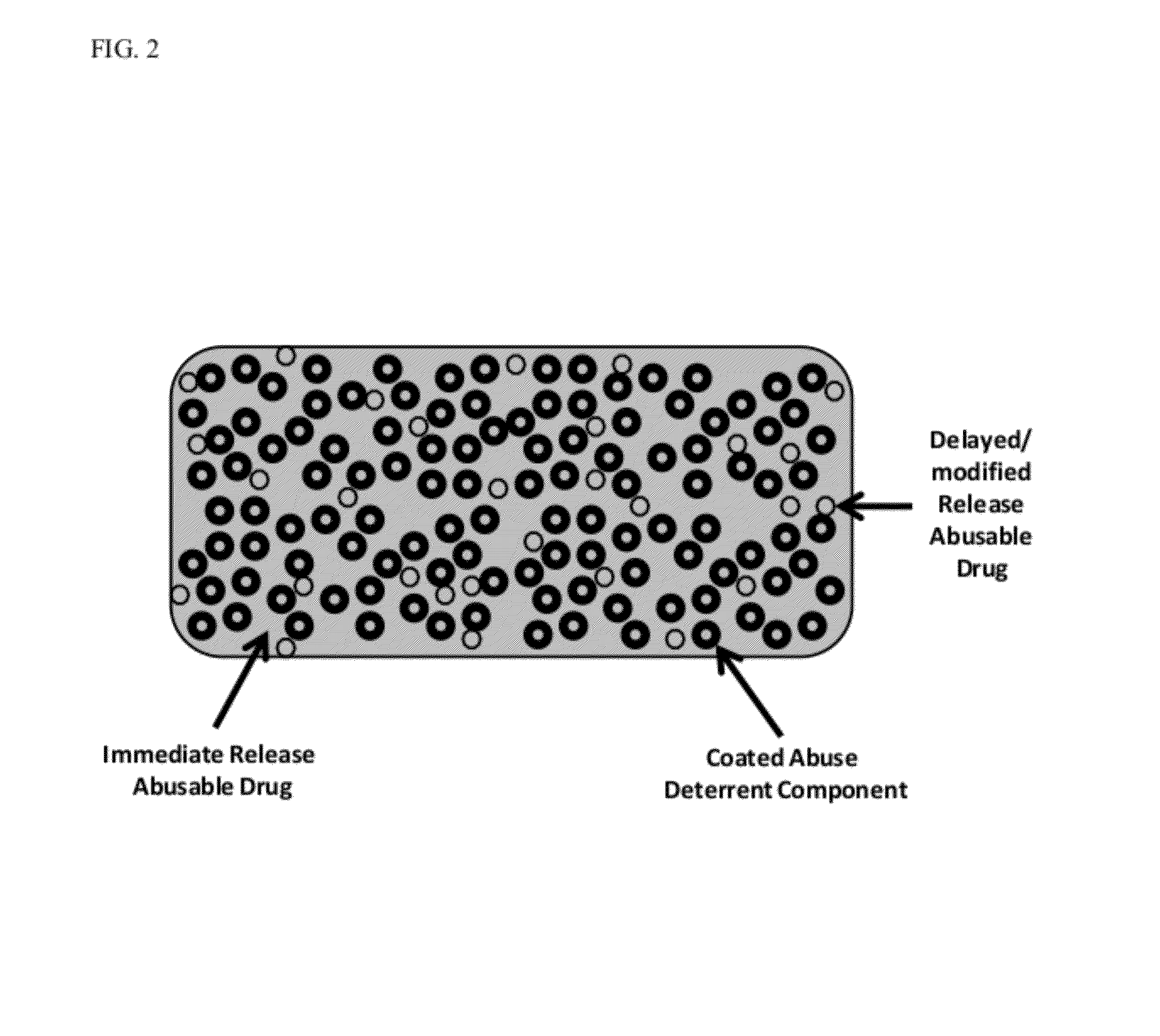
Technology for preventing abuse of solid dosage forms
InactiveUS20120321716A1Excessive amountReduce probabilityBiocidePowder deliveryAbuse deterrentPharmaceutical formulation
Abuse resistant pharmaceutical formulations are provided that contain one or more abusable drugs and one or more abuse deterrent components. The abuse deterrent component(s) prevent the abusable drug(s) from being removed / extracted to an appreciable extent and / or rate. The abuse deterrent component(s) may be in the form of pellets, beads, beadlets, granules, powders, or the like, and may comprise a core that contains a material that is both hydrophilic and hydrophobic, and optionally a pH-dependent coating.
Owner:VACHON MICHAEL +1
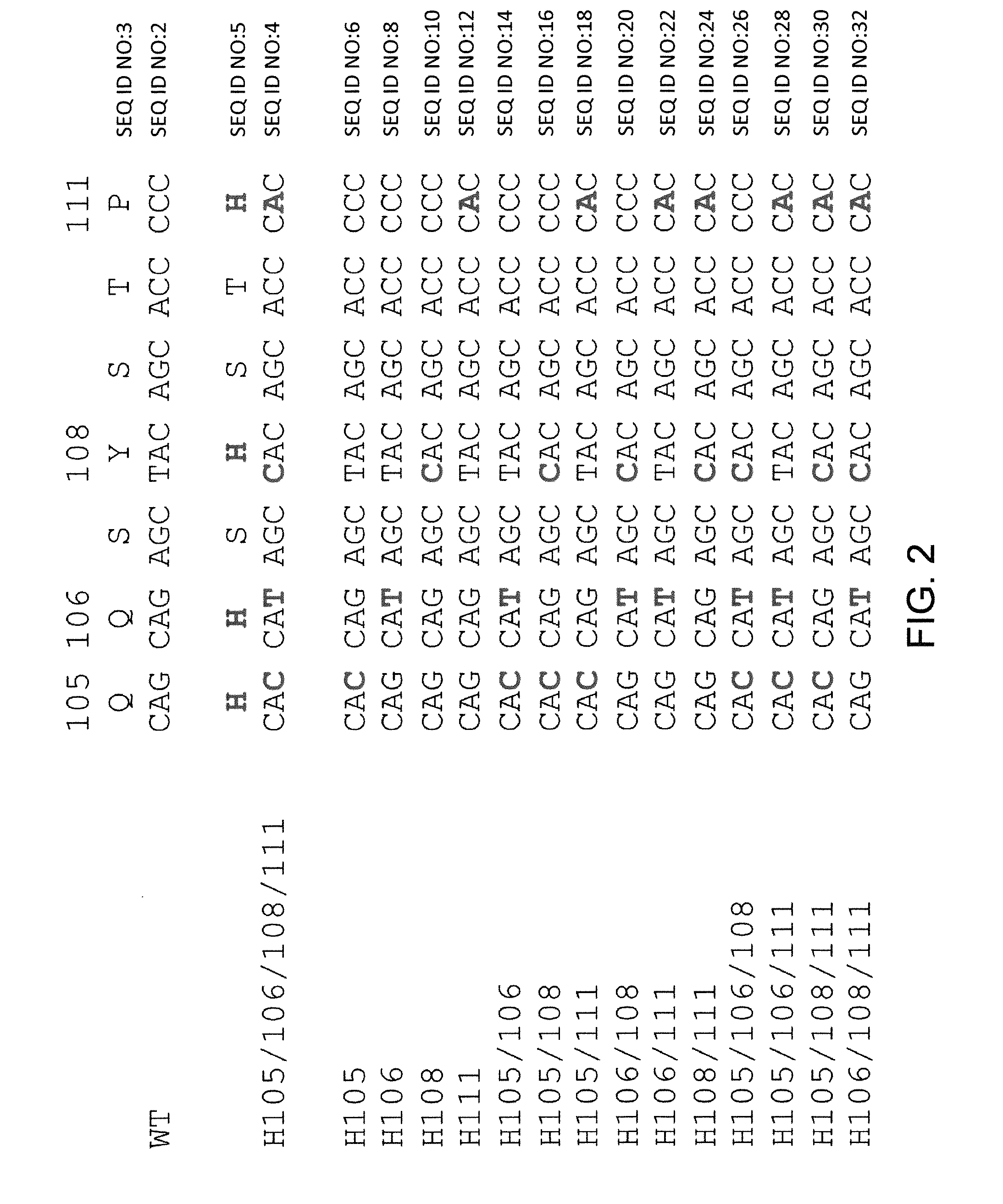
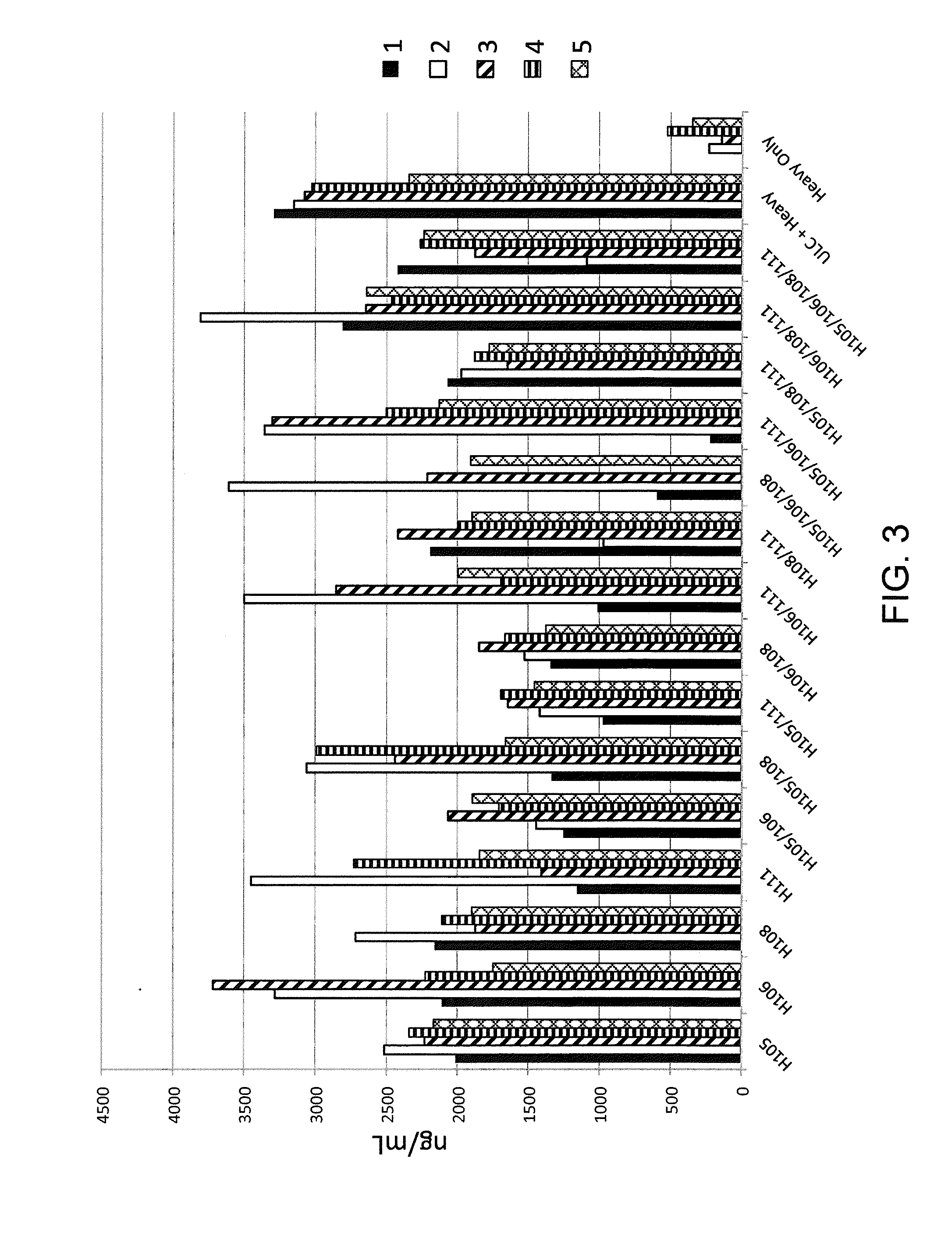
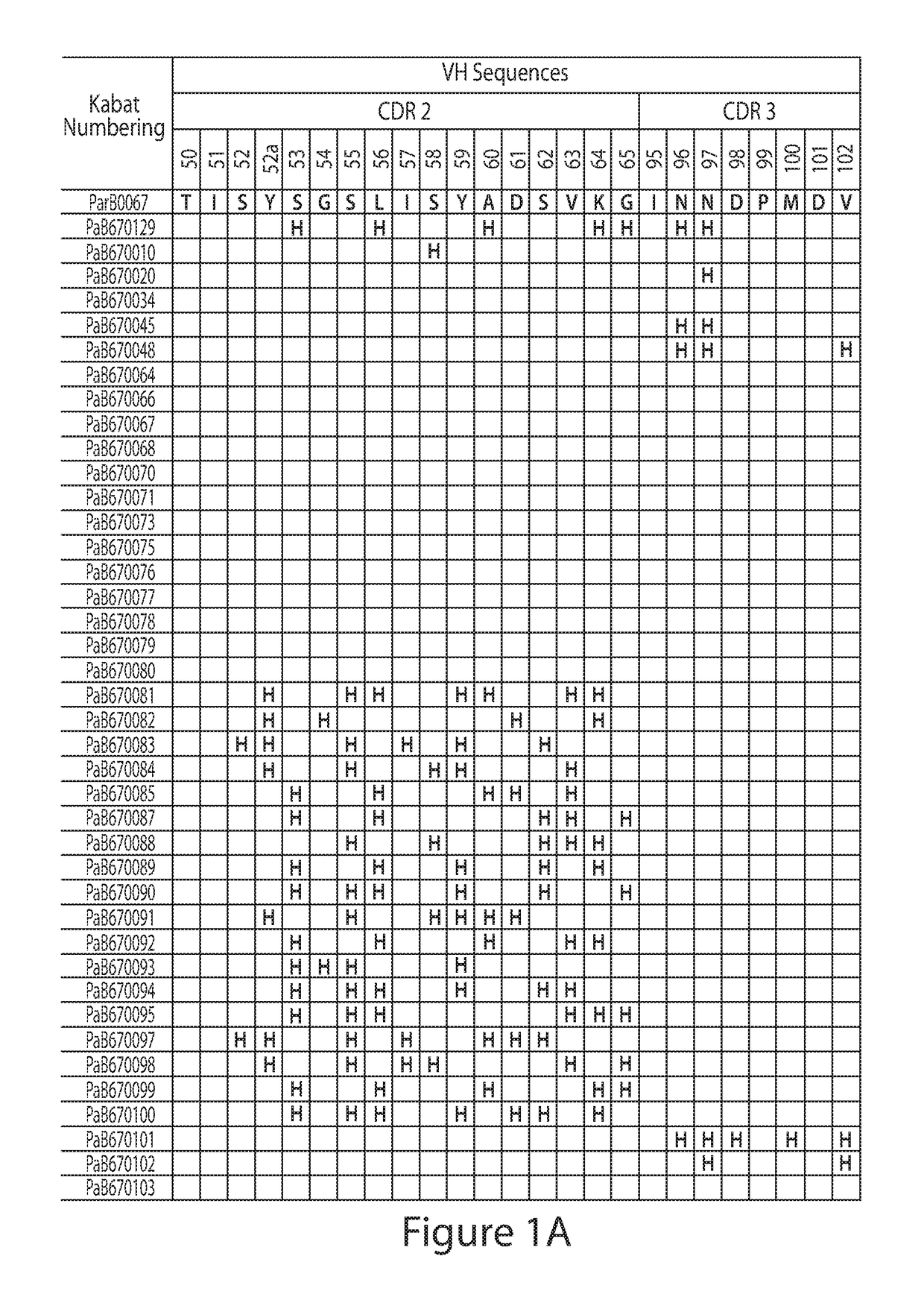
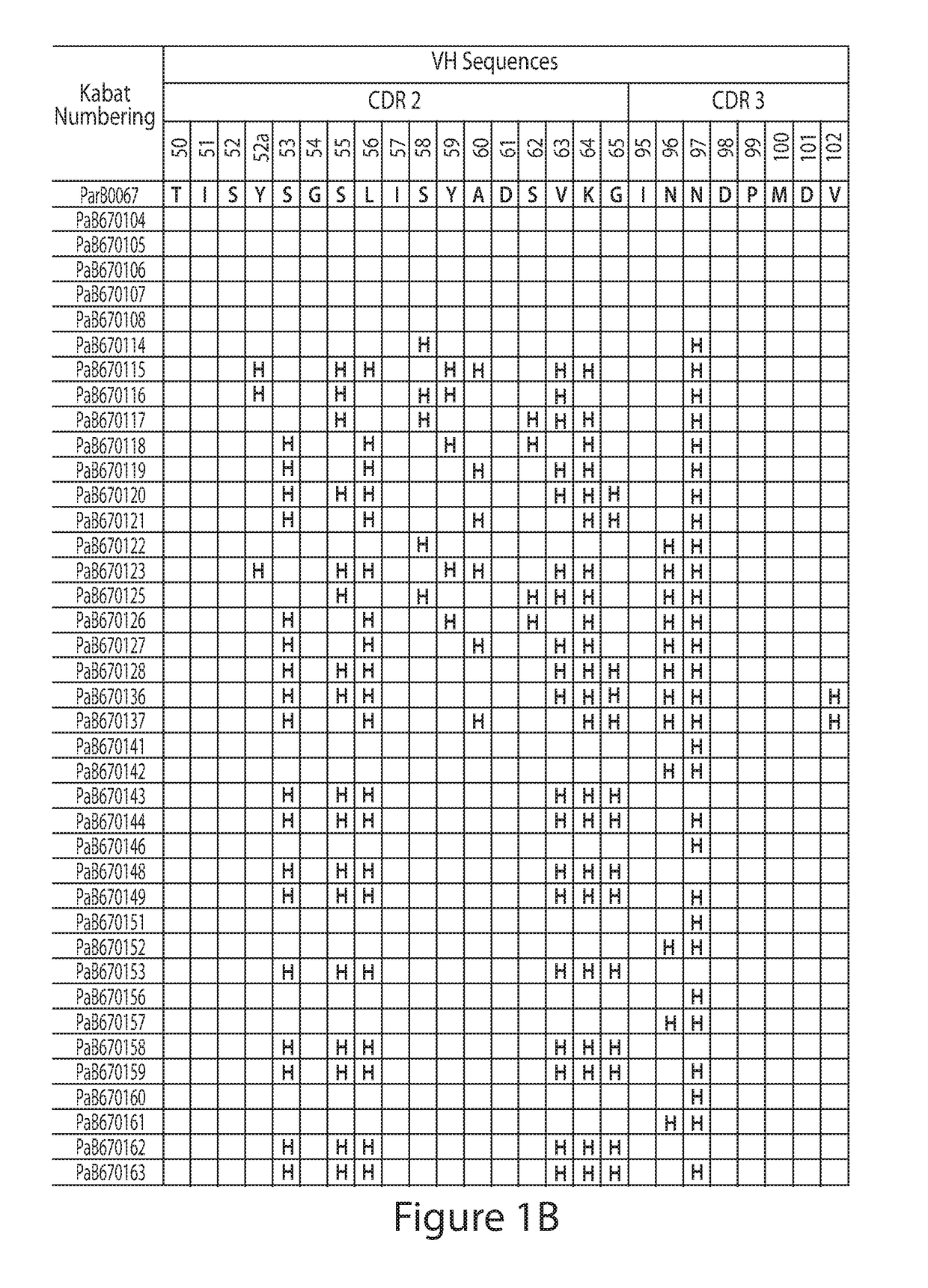
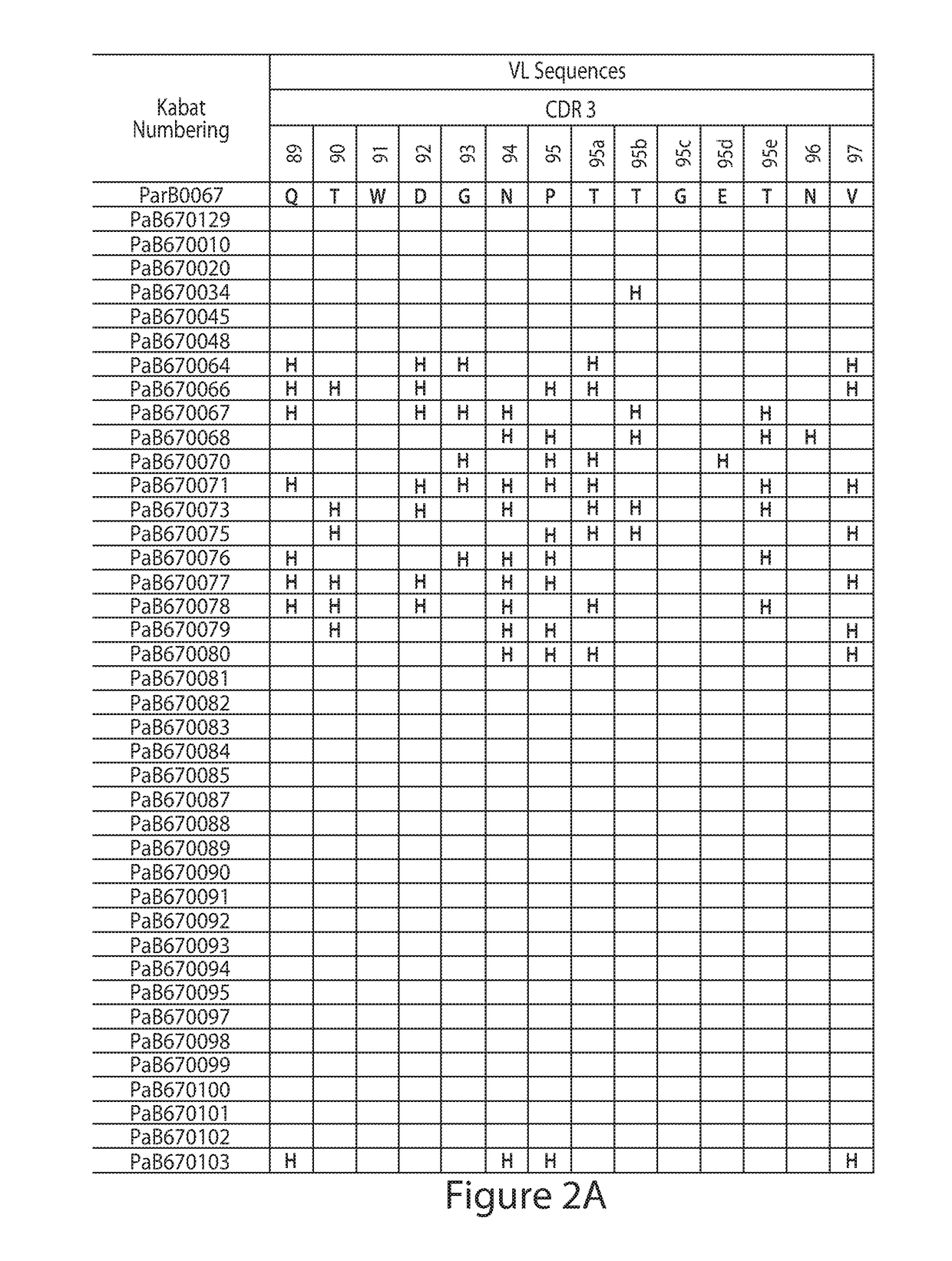
Histidine Engineered Light Chain Antibodies and Genetically Modified Non-Human Animals for Generating the Same
ActiveUS20130247234A1Reduce the binding forceImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHuman animalVariable domain
A genetically modified non-human animal is provided, wherein the non-human animal expresses an antibody repertoire capable of pH dependent binding to antigens upon immunization. A genetically modified non-human animal is provided that expresses a single light chain variable domain derived from a single rearranged light chain variable region gene in the germline of the non-human animal, wherein the single rearranged light chain variable region gene comprises a substitution of at least one non-histidine encoding codon with a histidine encoding codon. Methods of making non-human animals that express antibodies comprising a histidine-containing universal light chain are provided.
Owner:REGENERON PHARM INC
Abuse resistant opioid drug-ion exchange resin complexes having hybrid coatings
ActiveUS20120135077A1Favorable abuse resistance propertyImproved resistance characteristicsPowder deliveryNervous disorderMedicineIon-exchange resin
A sustained release formulation for opioid drugs is described. The formulation contains an opioid-ion exchange resin complex having a hybrid coating. The hybrid coating contains a cured polyvinylacetate polymer and a pH-dependent enteric coating layer mixed therein. Also provided are methods of making and using same.
Owner:TRIS PHARMA
Method for rapid detection and evaluation of cultured cell growth
Provided is a method and system for the rapid and accurate detection of growth and metabolism of a cellular microorganism in a population of microorganisms in a non-liquid, culture medium. Further provided is a gelled culture medium containing a non-toxic, water-soluble, phosphorescent compound which measures oxygen content (partial pressure) of an microorganism also contained therein, by oxygen-dependent quenching of phosphorescence; or the gel contains a fluorescent pH indicator that demonstrates growth of the microorganism by pH-dependent intensity change or wavelength shift in the emission spectrum. Further provided is a system and method for killing undesirable microorganisms or colonies in the culture medium without harming the surrounding microorganisms.
Owner:OXYGEN ENTERPRISES
Infrared shielding film
ActiveUS20150132550A1Low costGood infrared reflection performanceLayered productsThin material handlingPolyvinyl alcoholRefractive index
To provide an infrared shielding film, which has low production cost, can be prepared as a film with a large area, and has excellent reflecting property of infrared light, high visible light transmittance and excellent uniformity of optical characteristics.An infrared shielding film which has at least one unit on a substrate, the unit having a low refractive index layer that contains first metal oxide particles and a first binder and a high refractive index layer that is arranged adjacent to the low refractive index layer, contains second metal oxide particles and a second binder, and has a higher refractive index than the low refractive index layer, in which at least one layer of the low refractive index layer and the high refractive index layer contains, as a binder, at least one of the following (a) to (c):(a) a carboxyvinyl polymer that contains a monomer component, which contains a carboxylic acid, in an amount of 20 to 75% by mass of the entire composition,(b) a copolymer having pH dependency of viscosity, and(c) a modified polyvinyl alcohol.
Owner:KONICA MINOLTA INC
Sustained release tamsulosin formulations
A sustained release tamsulosin formulation contains tamsulosin, a hydrophobic polymer, a microsphere forming agent and a diluent. The hydrophobic polymers include pH-dependent and pH-independent polymers are used as the release-modulating agent to control the dissolution profile of tamsulosin formulation so that the formulation releases tamsulosin slowly and continuously as the formulation passed through the stomach and gastrointestinal tract.
Owner:STANDARD CHEM PHARM
Tagged polyfunctional reagents capble of reversibly binding target substances in a ph-dependent manner
InactiveUS20060263780A1High affinityEasy to detectBioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismVirus
Polyfunctional reagents are disclosed that are capable of reversibly binding to target substances, for example nucleic acid, proteins, polypeptides, cells, cell components, microorganisms or viruses, for use in purifying or otherwise manipulating them. The reagents comprise a tagging group for manipulating and / or detecting the target substance when bound to the polyfunctional reagent. The polyfunctional reagents work by binding the target substance at a first pH and then releasing it at a second pH, usually higher than the first. Examples of tagging groups include tagging group members of a specific binding pair which is capable of binding to a specific binding partner and / or a label.
Owner:LIFE TECH CORP
pH DEPENDENT CARRIERS FOR TARGETED RELEASE OF PHARMACEUTICALS ALONG THE GASTROINTESTINAL TRACT, COMPOSITIONS THEREFROM, AND MAKING AND USING SAME
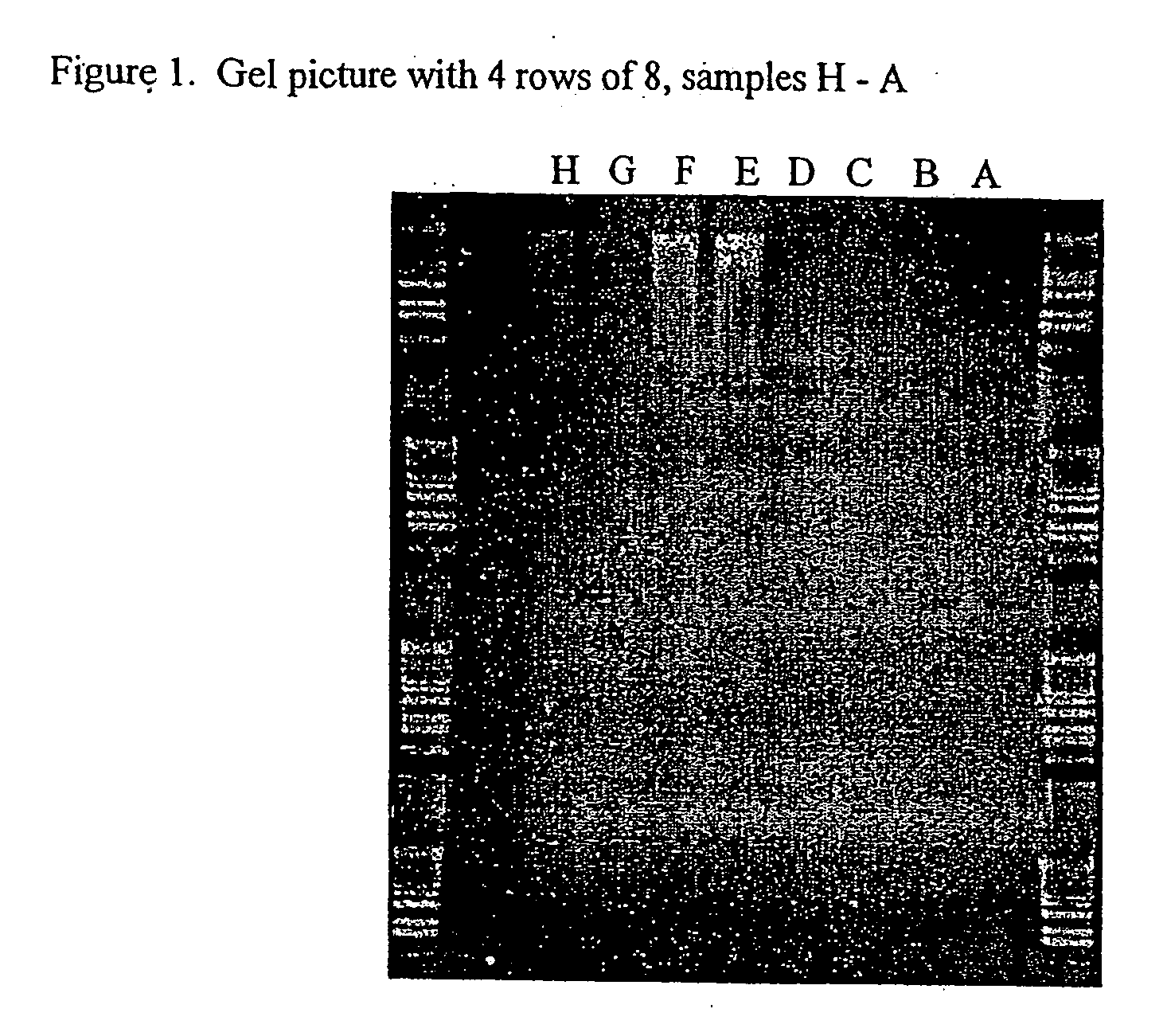
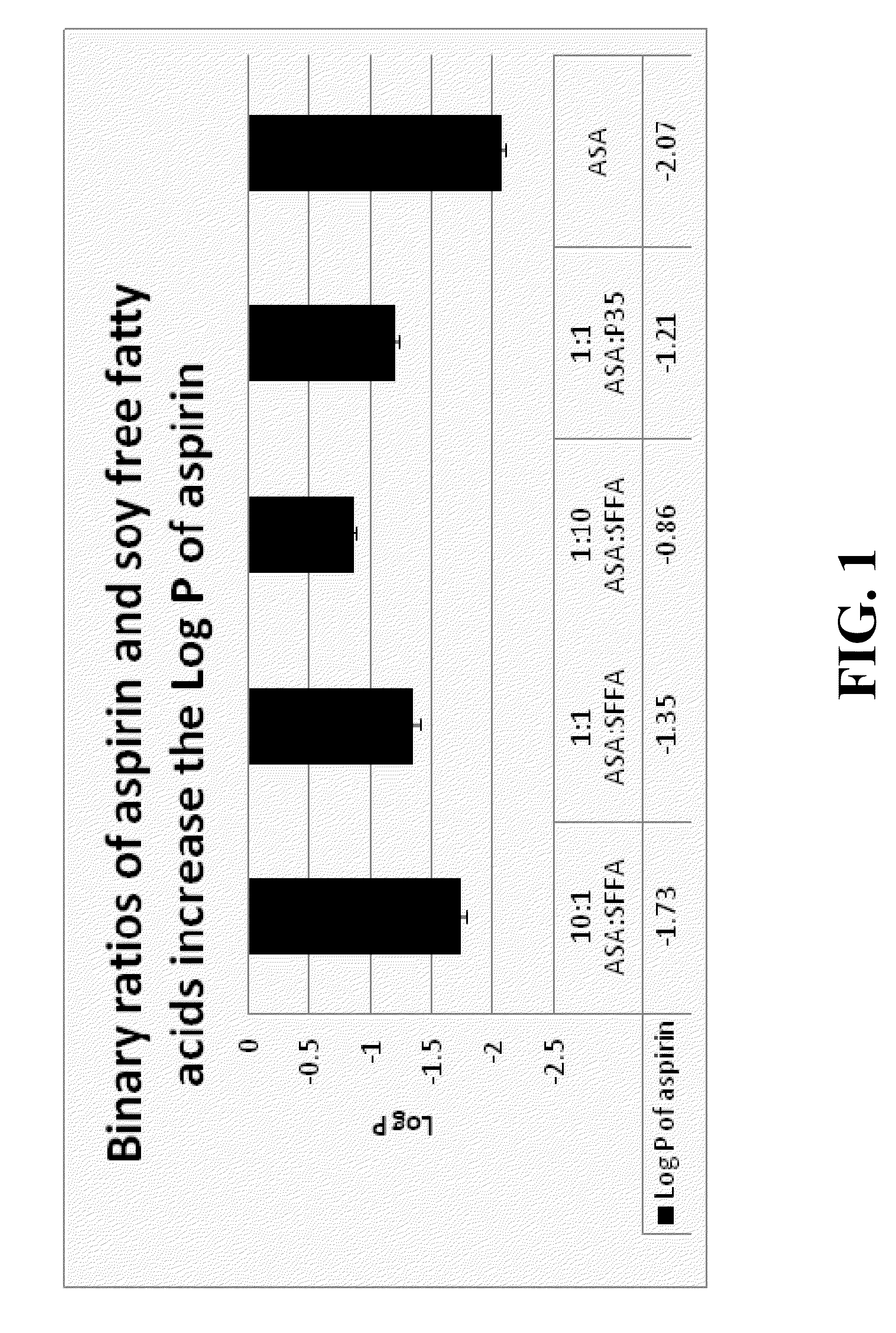
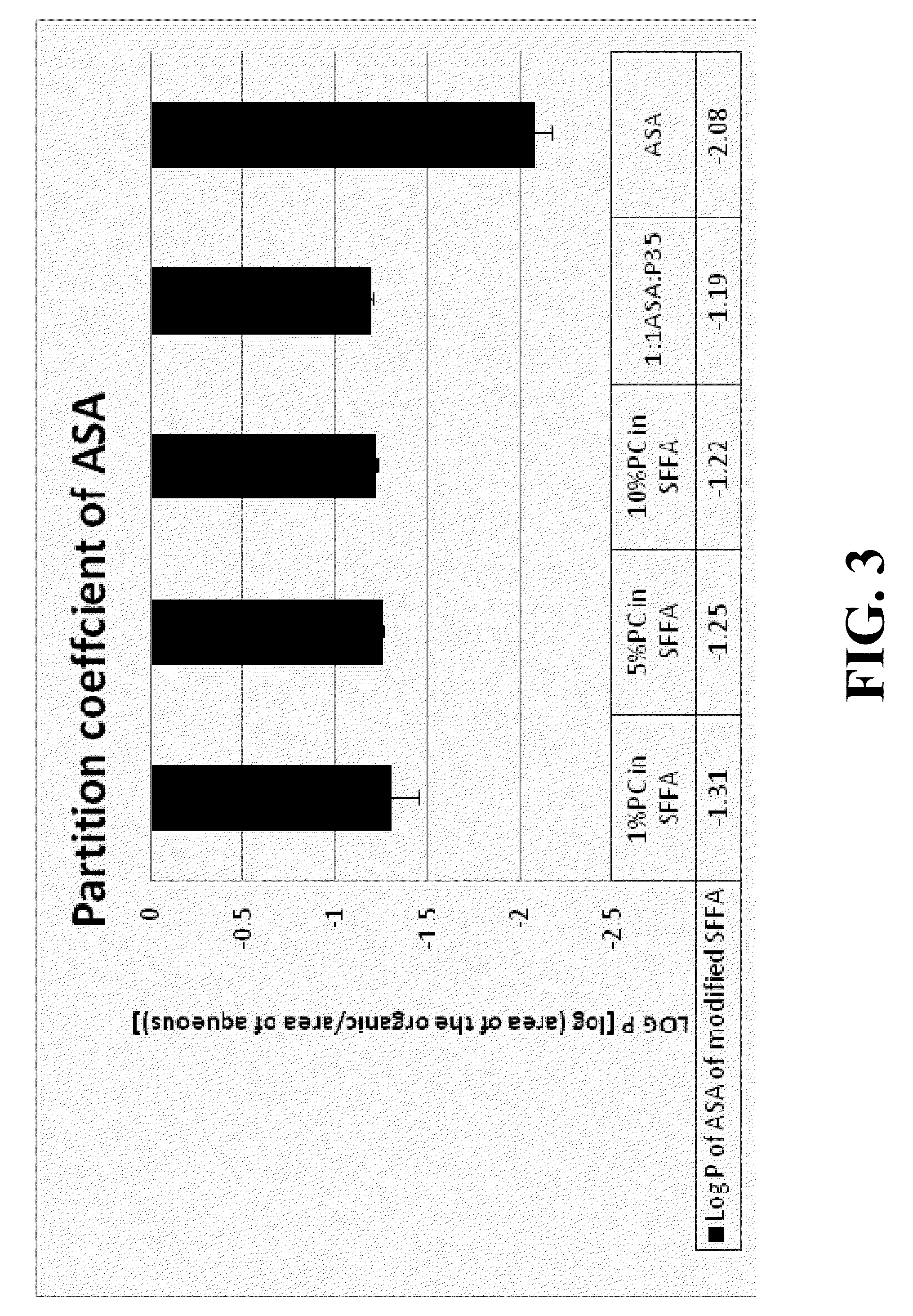
Novel drug carriers capable of targeted and / or pH dependent release of biologically active agents into selected pH environments including the gastrointestinal (GI), ophthalmic, urinary, or reproductive tracts. Unexpectedly, carriers including free fatty acids (FFA) are able to deliver biologically active agents to various pH environments. Such targeted delivery is tailorable and useful for active agents that are: (a) injurious to the upper GI tract (esophagus, stomach, and duodenum), (b) acid labile, (c) impermeable / insoluble compounds in GI fluids, (d) susceptible to first pass metabolism, and / or (e) cause stomach irritation, upset, or dyspepsia.
Owner:PLX ACQUISITION CO LLC
Methods for effecting controlled break in pH dependent foamed fracturing fluid
The invention provides a fluid for use in a subterranean formation penetrated by a wellbore, the fluid comprising: (a) water; (b) delayed release acid; (c) a surfactant comprising a tertiary alkyl amine ethoxylate generally represented by the following formula: wherein R is an alkyl group or groups, X is ethylene oxide, and Y is ethylene oxide; and (e) a gelling agent. The invention also provides a method of fracturing a subterranean formation, comprising the step of forming a foamed fracturing fluid comprising water; a surfactant comprising a tertiary alkyl amine ethoxylate generally represented by the formula above; a gelling agent; and a gas. The method also provides the step of introducing the foamed fracturing fluid into a subterranean formation at a pressure sufficient to create a fracture in the subterranean formation. The method also provides the step of introducing an acid into the subterranean formation.
Owner:HALLIBURTON ENERGY SERVICES INC
Surface renewable iridium oxide-glass or ceramic composite hydrogen ion electrode
InactiveUS8486238B2Excellent characteristicsIncreased durabilityMachining electrodesConductive materialCeramic compositeHydrogen-Ion Concentrations
Owner:KONKUK UNIV IND COOP CORP
Ph sensitive liposome compositions for controlling surface topography and binding reactivity in functionalized liposomes
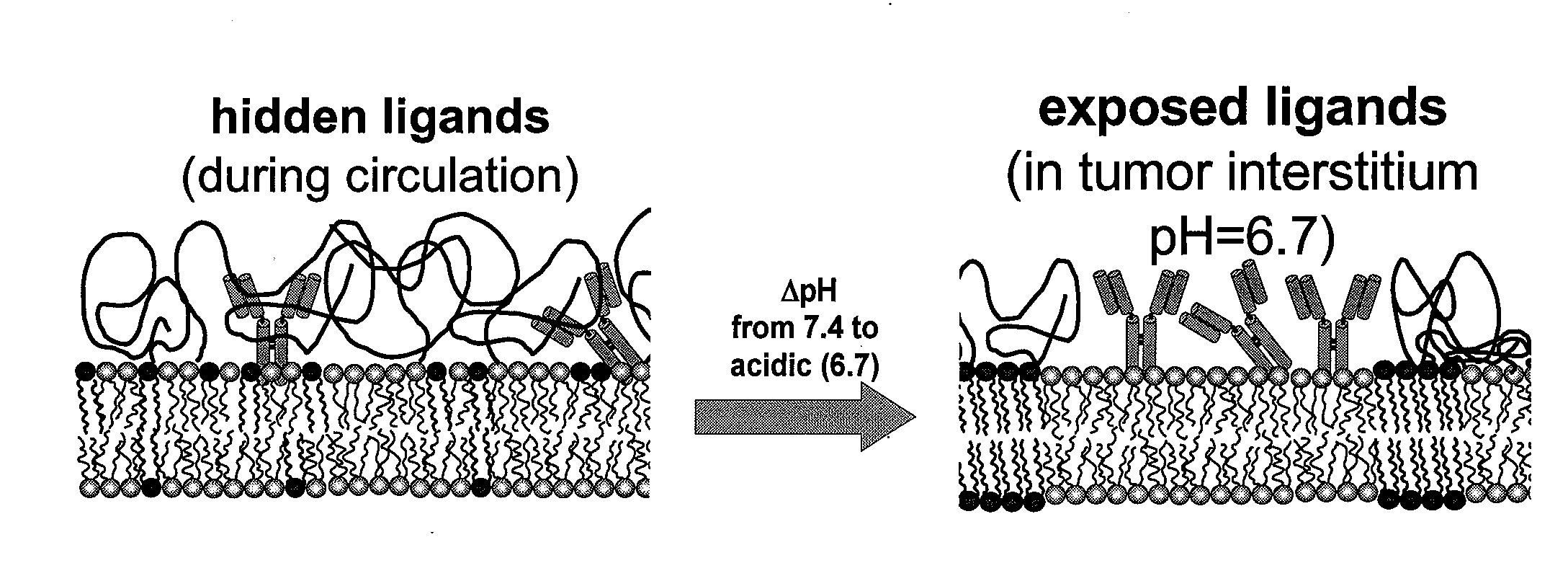
Methods for controlling surface topography and binding reactivity in functionalized lipid layers, including in the form of liposomes, using pH-dependent processes. During direct cell-to-cell communication, lipids on the extracellular side of plasma membranes reorganize, and membrane associated communication-related molecules co-localize. At co-localization sites, sometimes identified as rafts, the local cell surface topography and reactivity are altered. Integration of these processes on nanometer-sized lipid vesicles used as drug delivery carriers would precisely control their interactions with diseased cells minimizing toxicities. Included are pH-dependent processes on functionalized lipid bilayers demonstrating reversible sharp changes in binding reactivity within a narrow pH window. Cholesterol enables tuning of the membrane reorganization to occur at pH values not necessarily close to the reported pKa's of the constituent titratable lipids. One illustrative function of the invention is to use liposomes to deliver bioactive agents to cancer or tumor cells and compositions of specific lipids that form liposomes to deliver a biologically active agent.
Owner:POLYTECHNIC INSTITUTE OF NEW YORK UNIVERSITY
Vincamine sustained-release pellet preparation and preparation method thereof
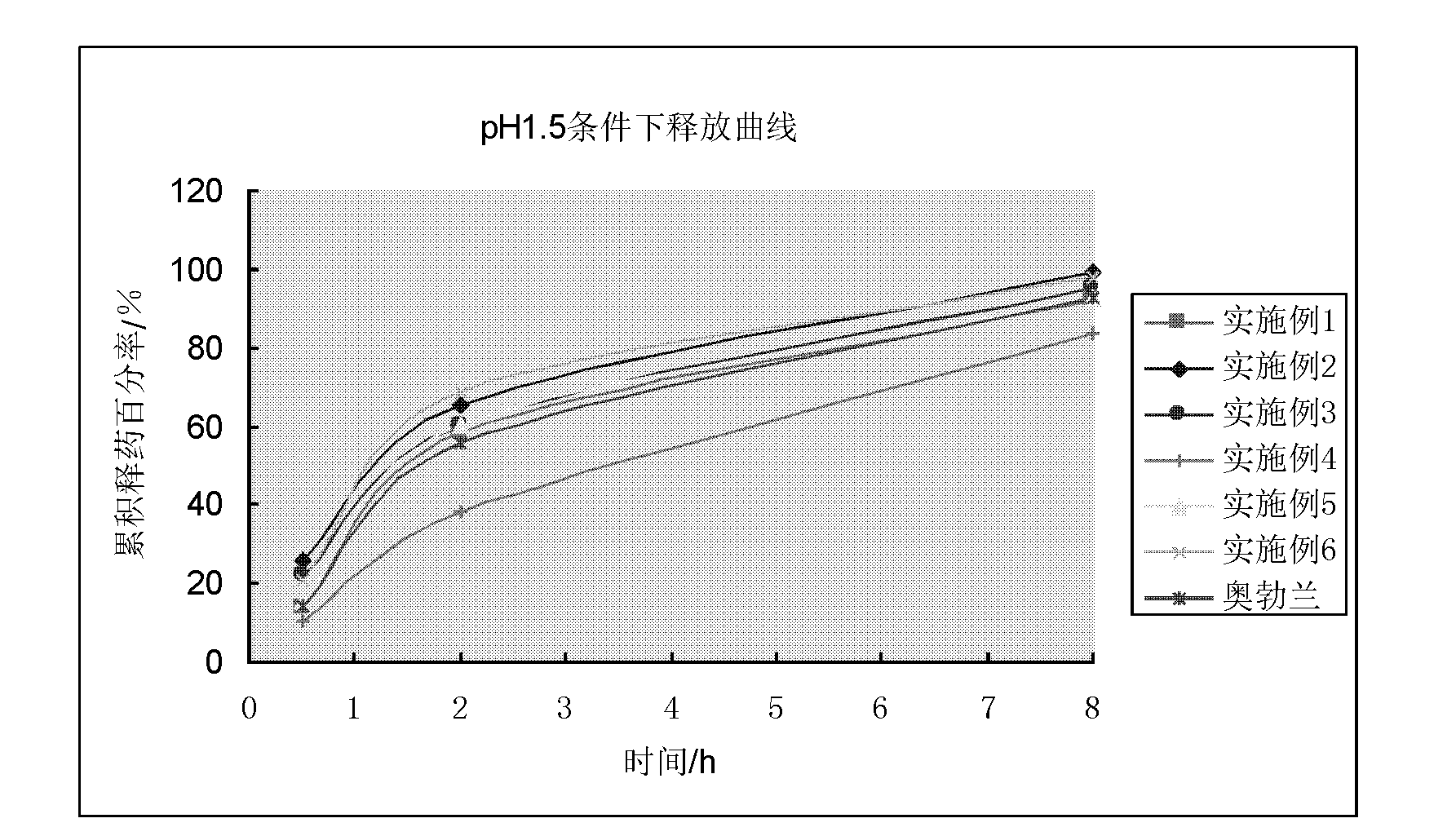
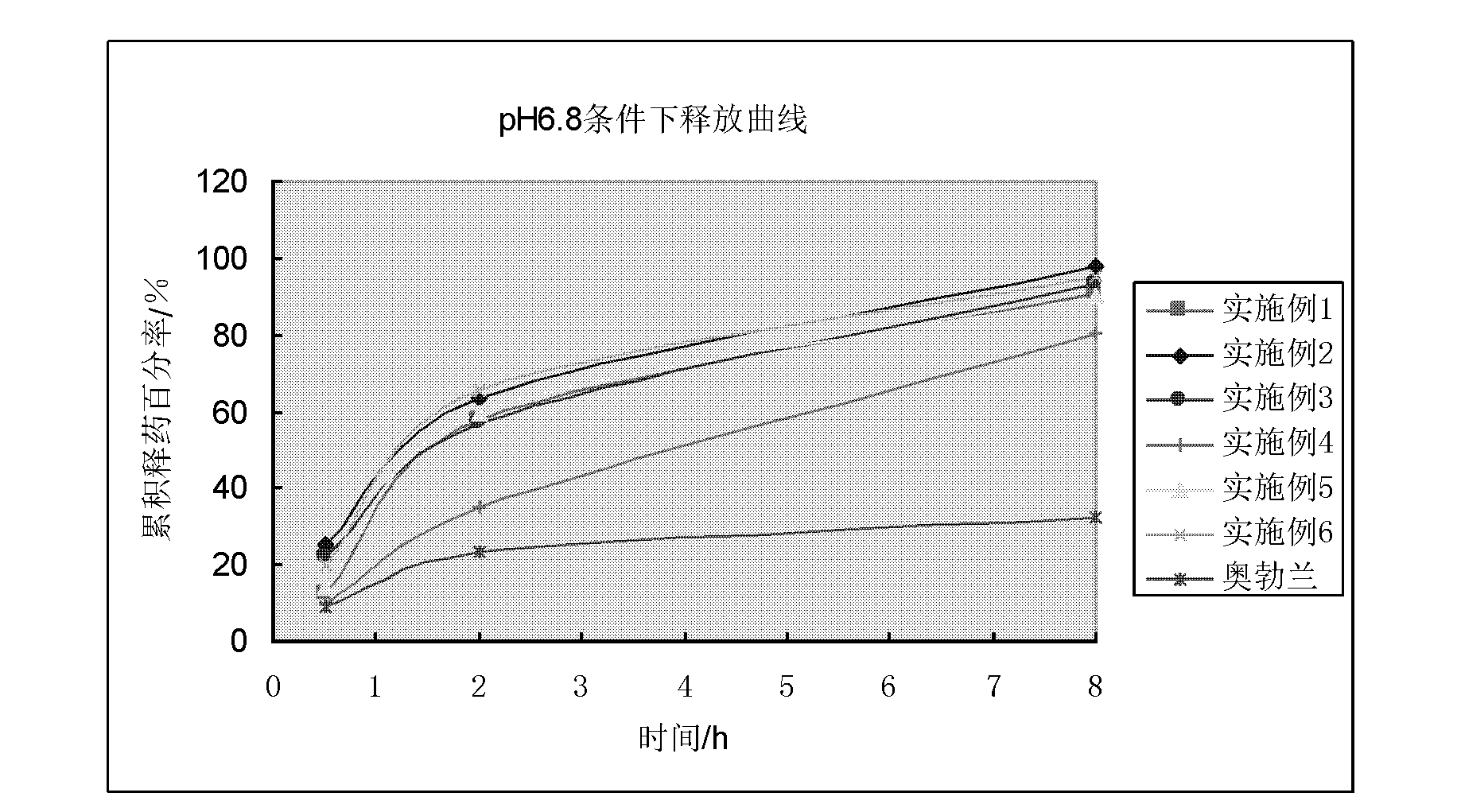
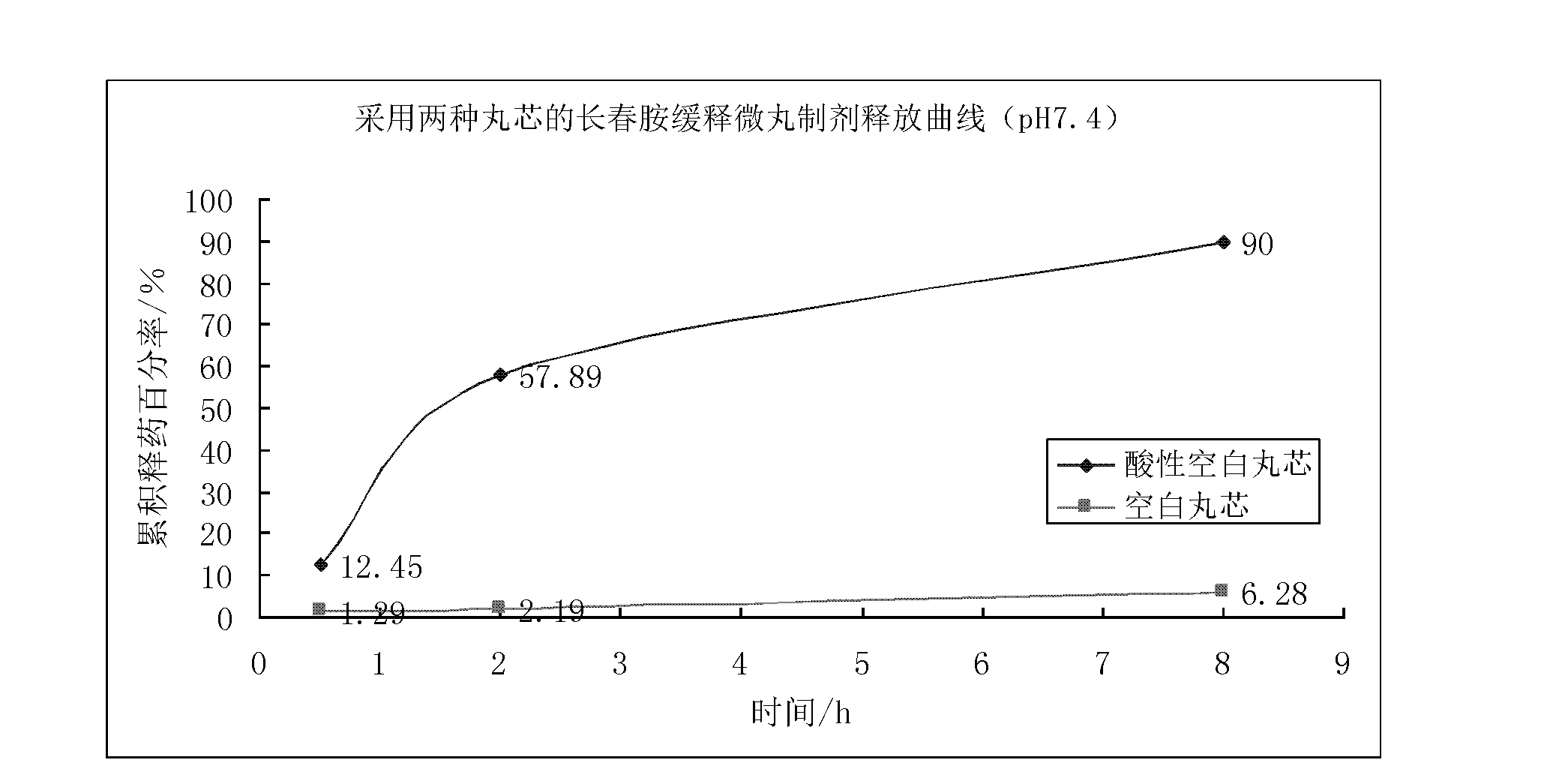
InactiveCN102657615AResolving pH DependencePromote absorptionOrganic active ingredientsNervous disorderOrganic acidSustained release pellets
The invention discloses a vincamine sustained-release pellet preparation, which comprises main medicines of vincamine, acid blank pellet cores, an adhesive and a diluent in a weight ratio of (1-10): (10-20): (1-10): (20-200), wherein the acid blank pellet cores consist of blank pellet cores and an organic acid covered on the blank pellet cores. The invention provides the vincamine preparation which can be stably released under the pH of 1.5-7.4, the release pH dependency of the conventional vincamine sustained-release preparation is solved, the vincamine preparation can be released stably and slowly in stomach and intestinal tracts, and the pharmaceutical effect is lasting. Moreover, the invention also discloses a preparation method for the vincamine sustained-release pellet preparation. The preparation method is simple, good in repeatability, high in production efficiency and suitable for industrialized mass production. The prepared medicine-carrying pellets are smooth and round, are released uniformly under the pH of 1.5-7.4, and are high in stability.
Owner:上海智同医药科技有限公司
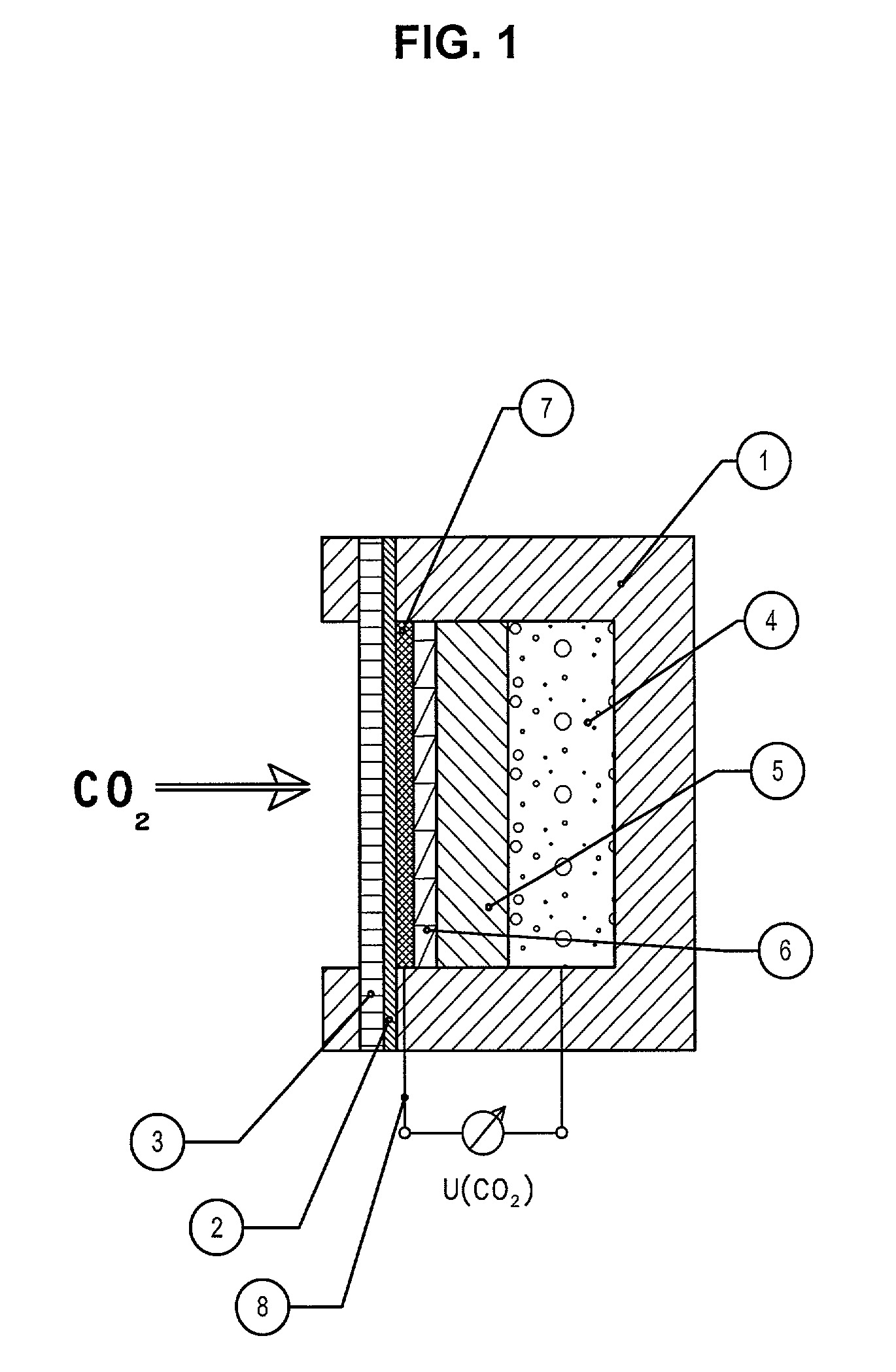
Device for the measurement of the carbon dioxide partial pressure
InactiveUS20020168296A1Small sizeLow priceMaterial thermal conductivityWithdrawing sample devicesElectrode potentialElectrode Contact
The invention describes a new device for the measurement of the partial pressure of carbon dioxide. The device is based on a simple construction and can be manufactured in a cost effective way. The pH dependency of the redox potential of organic substances is used to determine an electrical characteristic that depends on the partial pressure of carbon dioxide. The device for the potentiometric determination of the partial pressure of carbon dioxide consists of a housing with an opening for the entrance for gases, a gas permeable membrane, an electrolyte, at least one redox and one reference electrode with electrode contacts that transmit the electrode potential outwards through the housing. The gas permeable membrane has an electronically conductive layer that works as a redox electrode for a pH dependent redox system. The pH dependent redox system is adsorbed, absorbed or chemically bound onto the electronically conductive layer and interacts electrochemically with the electrode such that the pH dependent redox potential adjusts at the electrode according to the present partial pressure of carbon dioxide.
Owner:IT DR GAMBERT
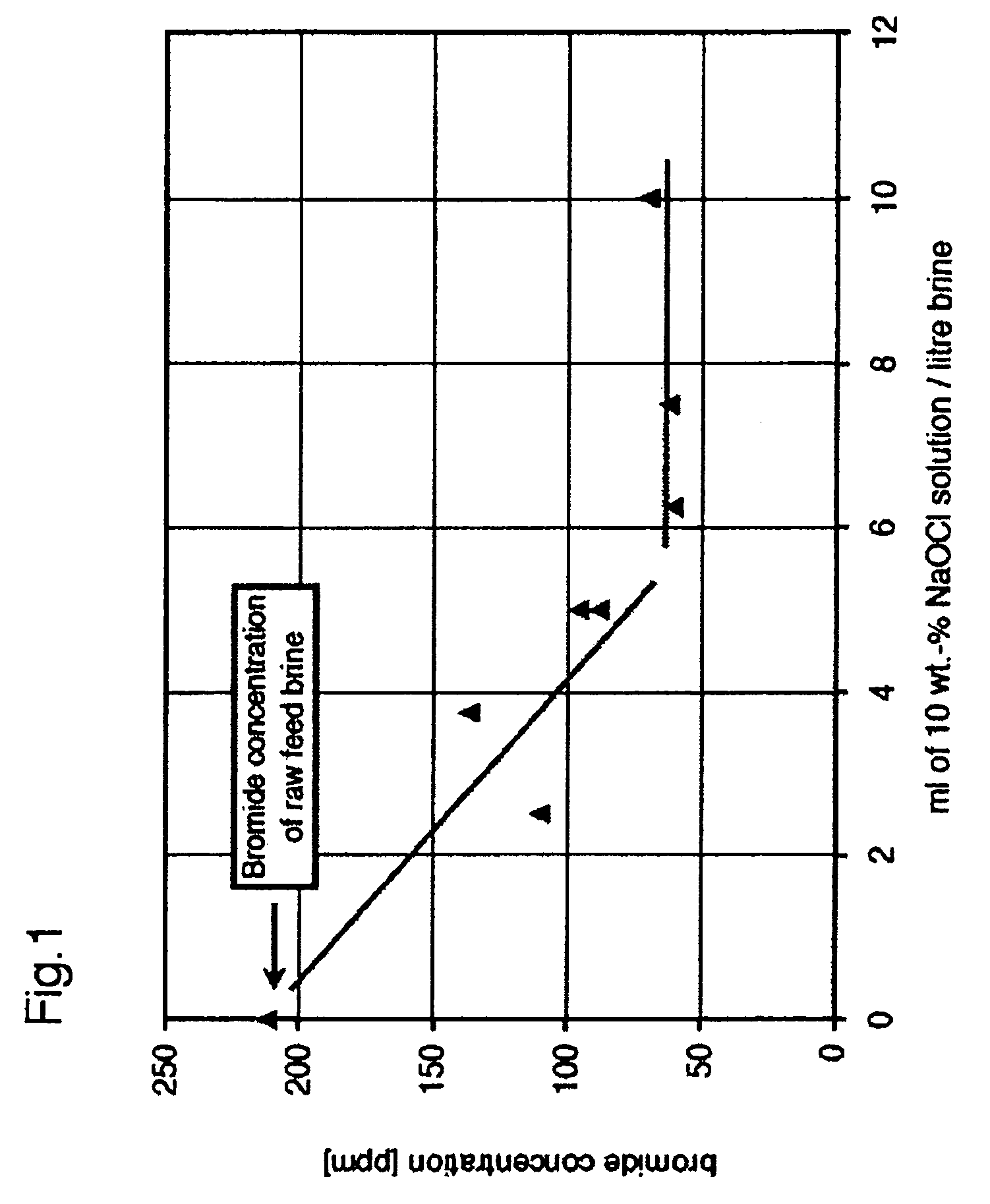
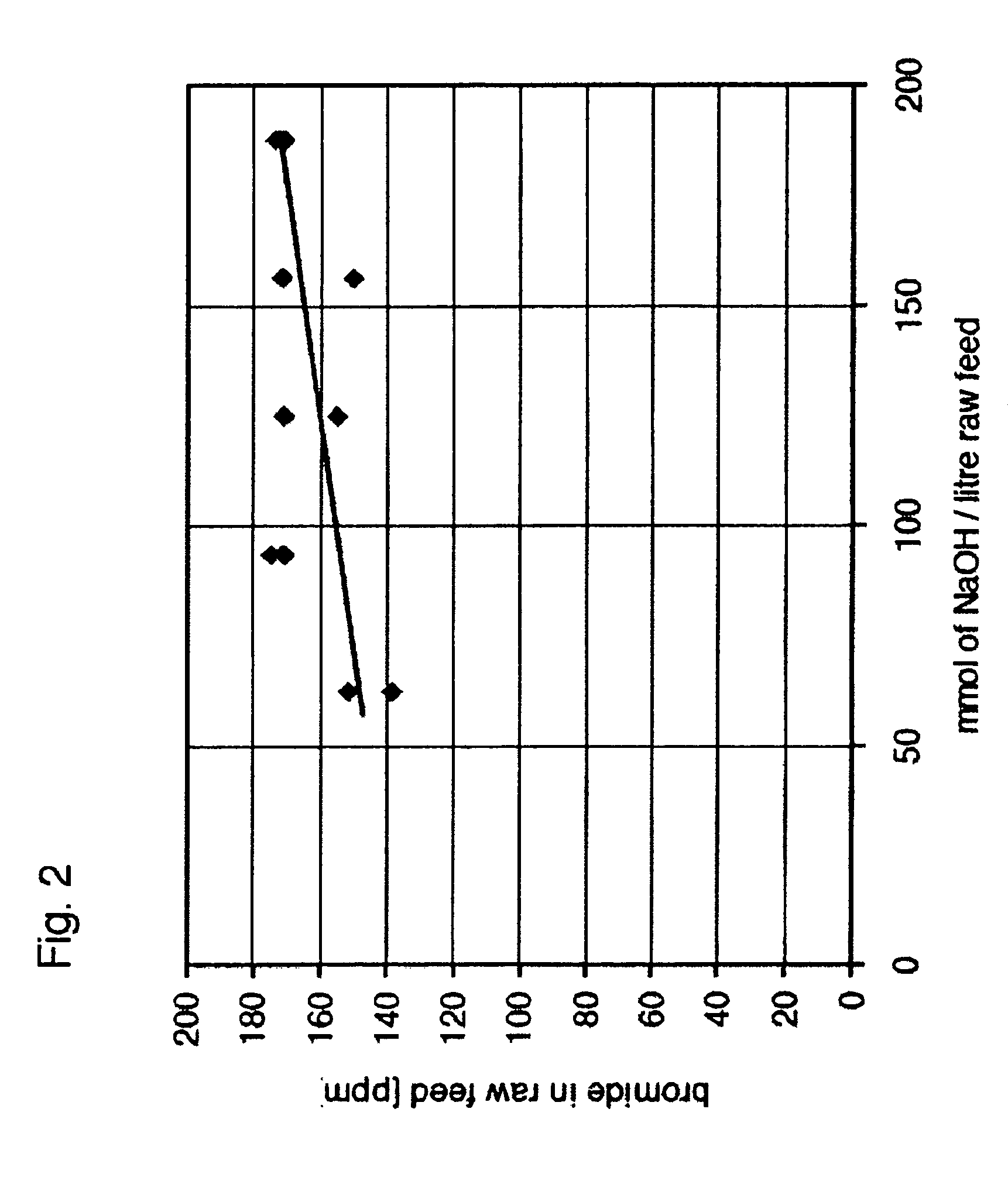
Bromide reduction process in liquid solutions
InactiveUS20060110312A1Increase supplyReduced effectivenessHypochlorous acidBromine oxygen compoundsPotassiumPh dependent
The invention provides a method for reducing bromine levels in brine solutions such as potassium chloride brine solutions. Bromide in solution is converted to hypobromite by the addition of an oxidant such as sodium hypochlorite. Hypobromite is precipitated by the addition of a metal cation such as magnesium under conditions of basic pH. The process is pH dependent such that the most efficient removal of bromine is achieved at a sodium hydroxide concentration of 90-200 mM. The pH optimum is also temperature dependent such that increased temperature lowers the optimal pH for bromide removal. The invention further provides a bromine-reduced potassium chloride product, suitable for uses in industrial applications. By the method of the invention bromine levels in a potassium chloride feed stock can be reduced by 97% or more.
Owner:IMC GLOBAL
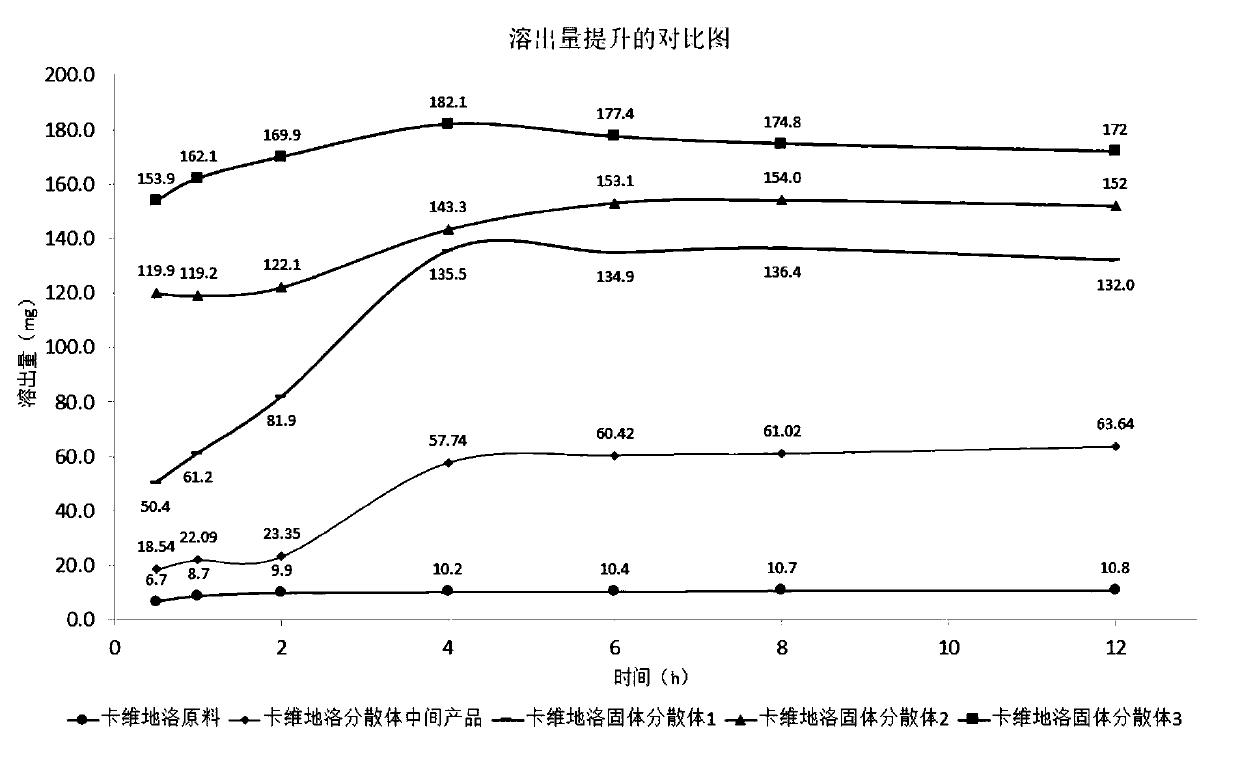
Solid dispersoid of poorly soluble drug CVD (carvedilol), preparation method and application
PendingCN110279662AHigh Gibbs EnergyImprove solubilityOrganic active ingredientsPowder deliverySolubilityProtonation
The invention discloses a solid dispersoid of poorly soluble drug CVD (carvedilol), a preparation method and an application and relates to the technical field of medicines. With the adoption of a one-step solvent coprecipitation method, CVD and a protonation reagent are dissolved in a solvent, a dispersion material is added, a suspension is obtained for coprecipitation, and finally, the solid dispersoid is prepared after the solvent is removed. The one-step solvent coprecipitation method is adopted for in-situ production of the solid dispersoid. Detection proves that CVD is transformed into an amorphous form from an original crystal form and is dispersed in the solid dispersoid, besides, CVD is salified due to addition of the protonation reagent, thus, the solubility and the dissolution rate of free alkali drugs are improved from two aspects, and the bioavailability of the BCSII type poorly soluble drug CVD is effectively improved. a drug preparation with retention floating in the stomach and time-lag trip or release in different pH parts in a body is prepared from the CVD solid dispersoid according to a polymer coating material in cooperation with the dissolubility characteristic of pH dependency.
Owner:HEFEI COSOURCE PHARMA CO LTD
Taste masking system for alprazolam
InactiveUS20060147516A1Eliminate needDisintegrates quicklyPowder deliveryOrganic active ingredientsAlprazolamSolvent
The present invention relates to taste masking system, taste masked formulations, dosage forms made from those formulations and methods of making those formulations that involve dissolving or dispersing a pH dependant polymer and alprazolam in a solvent, granulating using that material or forming layers over a solid support therewith. This can be followed with the use of an overcoating layer.
Owner:CIMA LABS
Pharmaceutical Dosage Form
InactiveUS20150004225A1Modulating releasePromote absorptionBiocidePowder deliveryControlled releaseMicrosphere
This invention relates to pharmaceutical dosage forms, particularly to pH dependent pharmaceutical dosage forms with enhanced and / or prolonged distribution of a pharmaceutical compound at a target site. More specifically, this invention relates to a controlled release intravaginal pharmaceutical dosage form and, more particularly, to a pharmaceutical dosage form which comprises microspheres encapsulated and / or embedded within a bioerodible polymeric matrix, together the microspheres and the matrix are formed into a caplet and / or tablet.
Owner:UNIVERSITY OF THE WITWATERSRAND
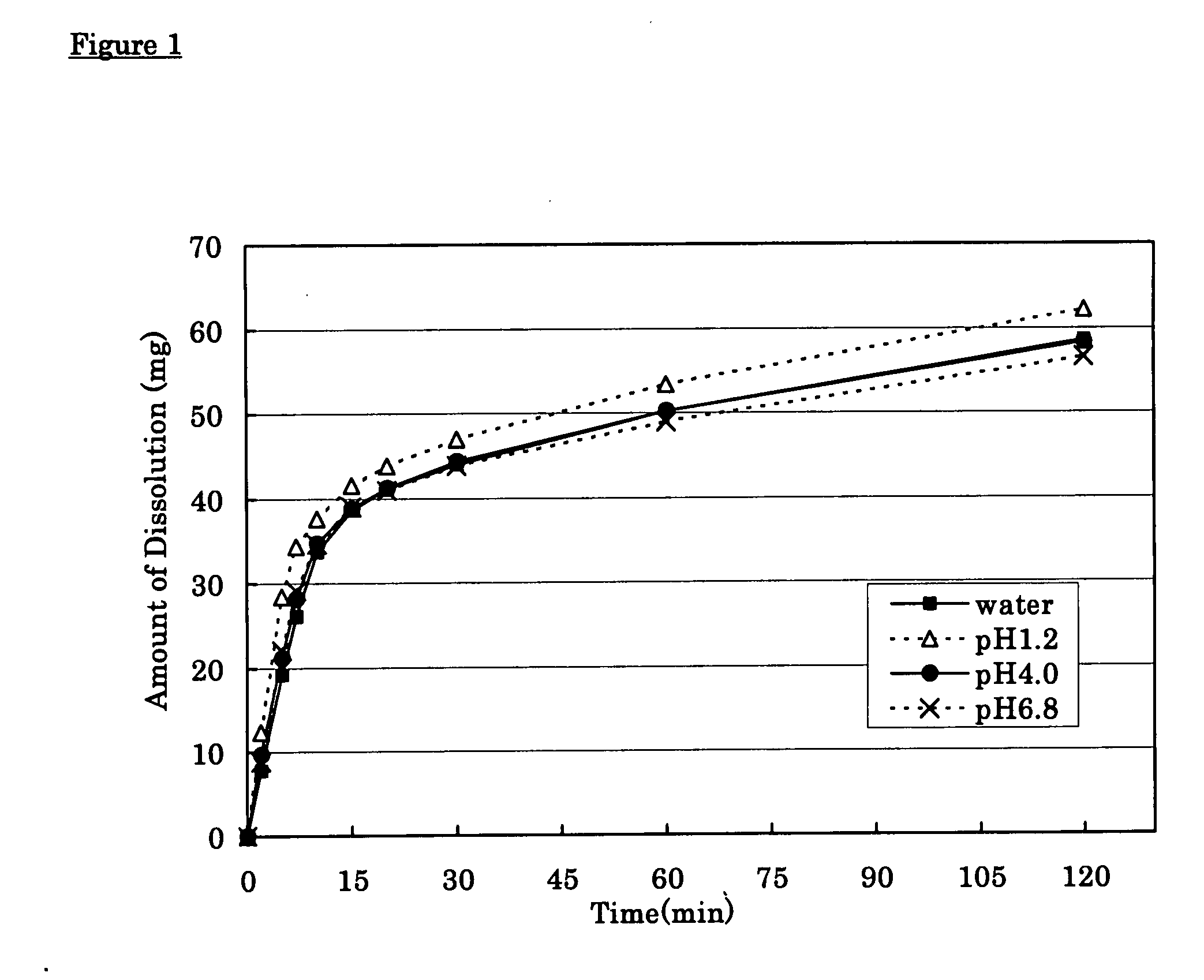
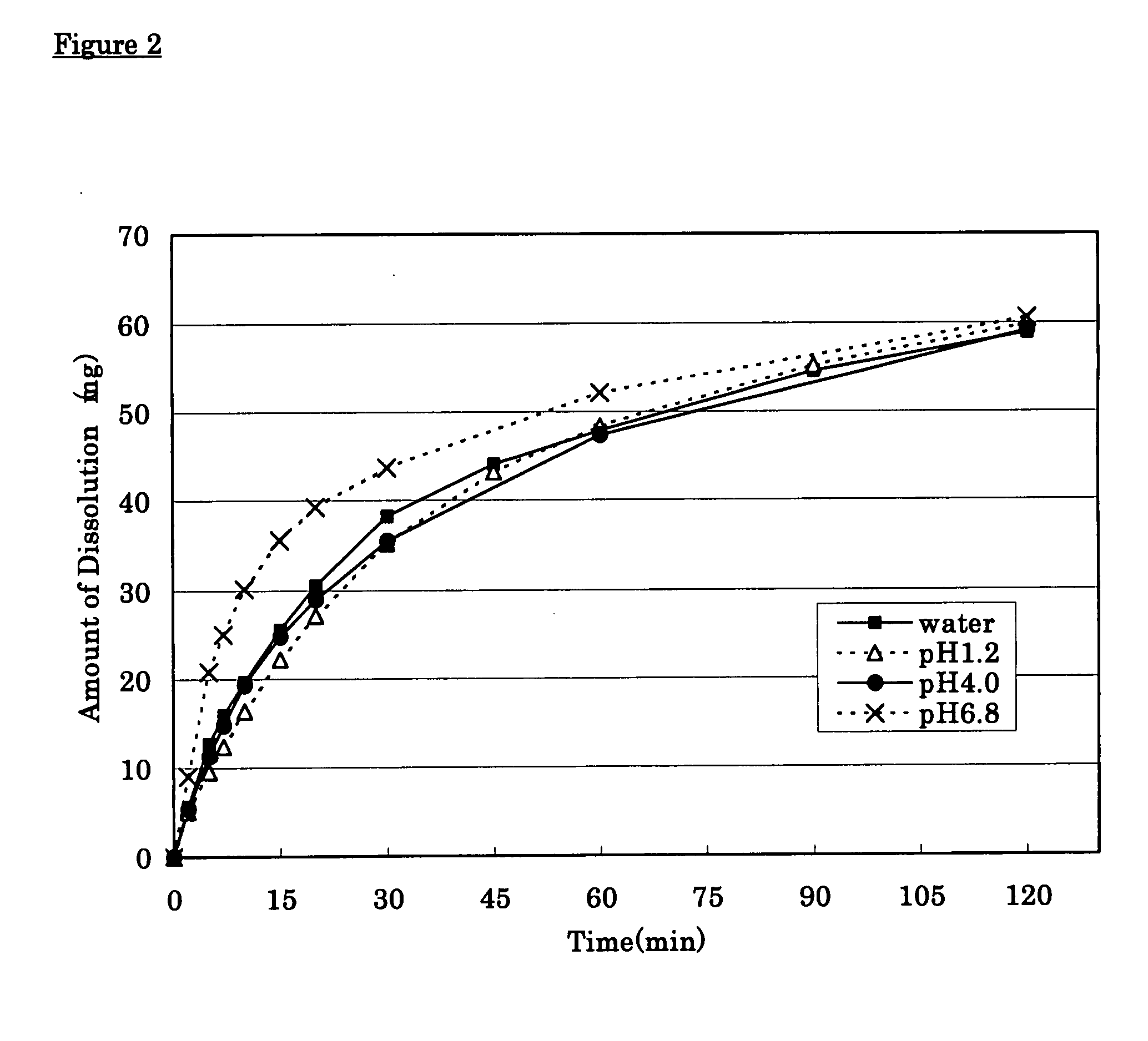
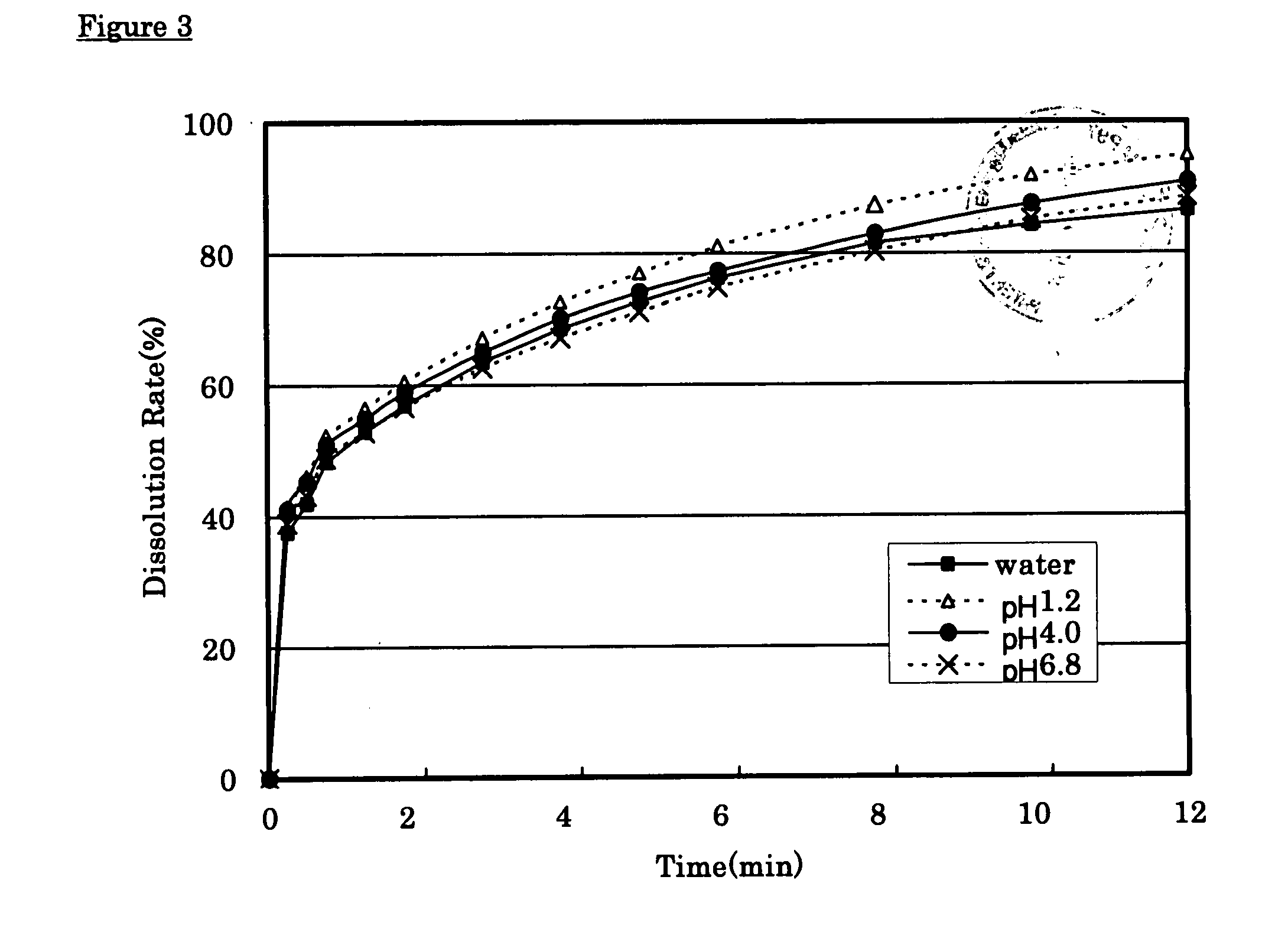
Solid pharmaceutical preparation
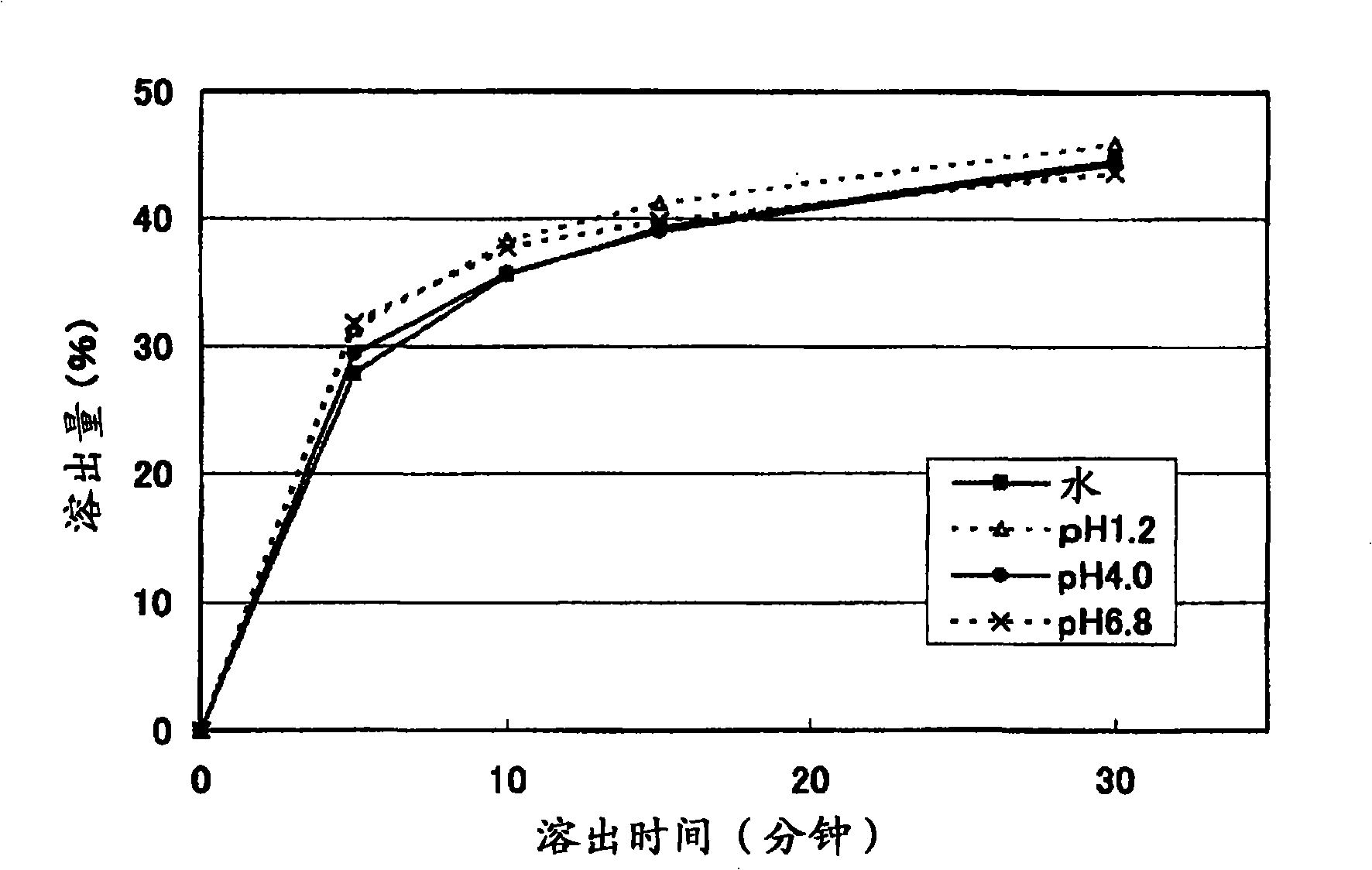
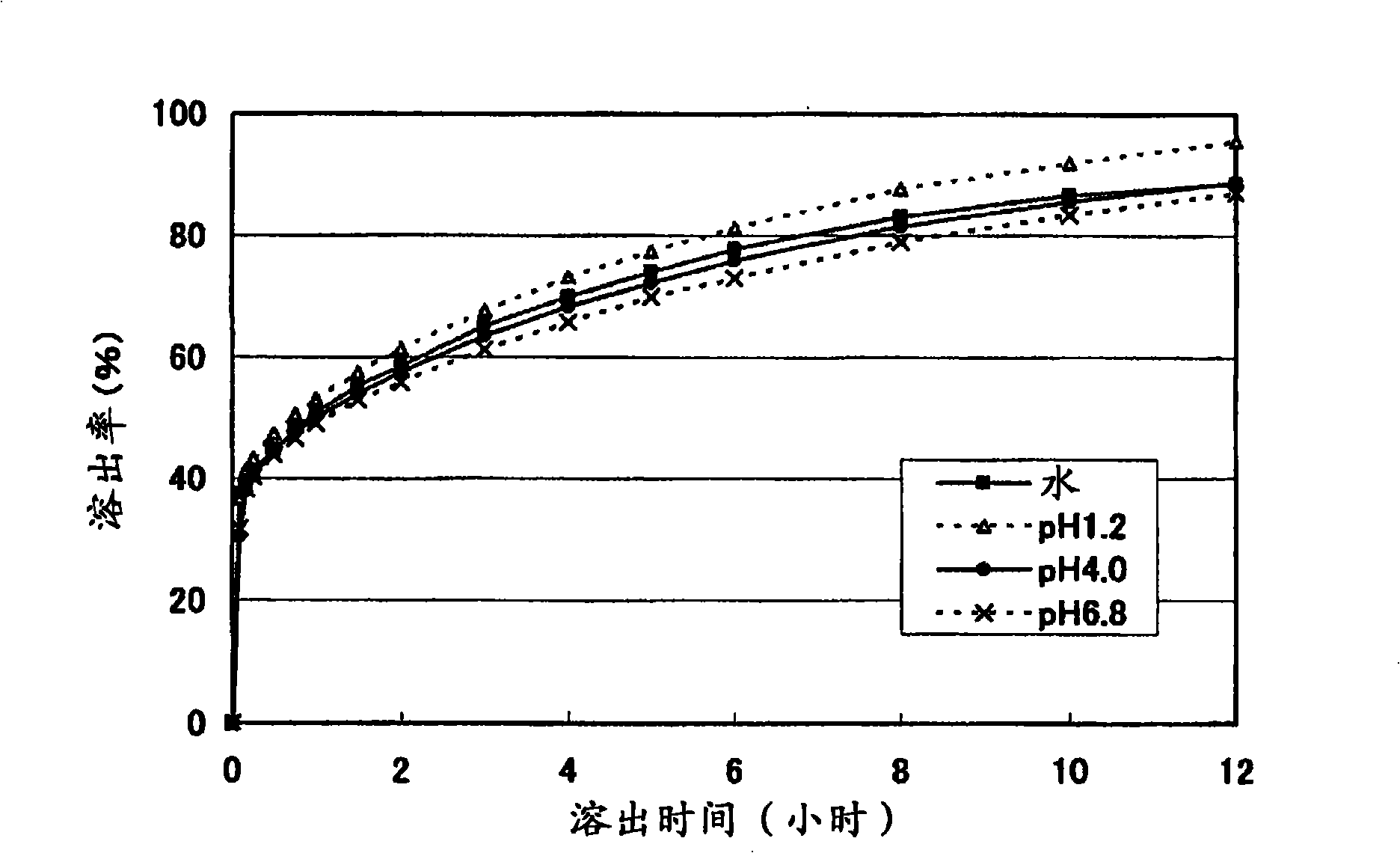
ActiveUS8367107B2Little pH dependencyStable concentrationOrganic active ingredientsNervous disorderImmediate releaseBULK ACTIVE INGREDIENT
It is intended to provide a long-acting solid pharmaceutical preparation which has an immediate release part and a sustained release part containing tramadol or a pharmaceutically acceptable salt thereof, is fast-acting and stably has an excellent release property showing little pH dependency in the initial elution. The invention relates to a long-acting solid pharmaceutical preparation characterized by having an immediate release part and a sustained release part, containing tramadol or a pharmaceutically acceptable salt thereof as an active ingredient in both parts and containing partially pregelatinized starch and an excipient as additives in the immediate release part. The preparation of the invention is a long-acting preparation in which an effective blood concentration is reached rapidly after taking it for rapid pain-relief and a drug action can be sustained for a long time thereafter and is practical as a preparation showing a stable, pH-independent and rapid initial elution behavior and, further, having a sufficient hardness enough to meet the need for avoidance of defacement, cracking, chipping, etc. during tablet coating.
Owner:NIPPON ZOKI PHARM CO LTD
Preparation and application of florfenicol slowly-releasing micropill for treating animal digestive canal infection
InactiveCN101693012AReduce absorptionSmall doseOrganic active ingredientsDigestive systemDigestive canalTreatment effect
The invention relates to preparation and application of florfenicol slowly-releasing micropills for treating animal digestive canal infection. The implementation comprises the following steps: weighing drug accessories and florfenicol raw materials according to the proportion of weight ratio 0.1-10:1, preparing micropills whose grain size dimension is between 0.5 to 3mm, wrapping a non-pH dependency high molecular polymer film outside the micropills, wherein the film contains low molecular water-soluble materials. Slowly-releasing micropills containing florfenicol is wrapped by non-pH dependency high molecular polymer materials, water-soluble materials such as mannidex, antacidin or natrii chloridum are added in the wrapping film, after people take the wrapped micropill orally, the water-soluble component in the wrapping film is dissolved to form a pore passage, and the drug is slowly released from the pore passage and exerts the long-term curative effect in the intestinal tract. The drug is different from traditional drug feeding modes in that higher drug concentration can be kept in intestinal tracts for long time, directional distribution of the drug in the intestinal tract can lower the side effects of drug to other organs and tissues, and the treatment cost can be lowered due to less drug dosage.
Owner:TIANJIN SHENGJI GRP CO LTD
Pharmaceutical composition containing acetylcholine esterase inhibitor and method for the preparation thereof
InactiveUS20110060008A1Low release rateReduce bitternessBiocideNervous disorderDonepezilOral medication
A pharmaceutical composition for oral administration comprising donepezil or pharmaceutically acceptable salt thereof, and an effective amount of pH dependent excipient as a taste masking agent, to suppress the release of donepezil in the pH environment of the oral cavity and increase the release of donepezil in acidic environment.
Owner:GENEPHARM
Solid pharmaceutical preparation
InactiveCN101031322AFull hardnessSwift independenceOrganic active ingredientsNervous disorderHas active ingredientAdditive ingredient
To provide a sustained-release solid pharmaceutical preparation having a quick-release part and a sustained-release part and stably having an excellent quick releasing characteristic with little pH dependency in an initial dissolution. The present invention relates to that, in a preparation containing a medical ingredient as an effective ingredient, particularly active analgesic ingredient, a sustained-release solid pharmaceutical preparation which is characterized in that it is a solid pharmaceutical form having a quick-release part and a sustained-release part and contains effective ingredient in both parts and the quick-release part contains a partly pregelatinized starch and a low substituted hydroxypropylcellulose as additives. In the preparation of the present invention, stable and quick initial dissolution behavior being independent upon pH is achieved even when some sustained-release part is contaminated in the quick-release part due to the difference in the tabletting method for multi-layered tablets. Furthermore, the preparation is practical as a preparation having a sufficient hardness in view of necessity that abrasion, breakage, crack, etc. are not generated when the tablet is coated.
Owner:NIPPON ZOKI PHARM CO LTD
Surface-polishable iridium oxide composite hydrogen ion electrode and method of manufacturing the same
InactiveUS20070298278A1Increased durabilityHigh surfaceMaterial analysis by electric/magnetic meansCeramic layered productsHydrogen-Ion ConcentrationsWater quality
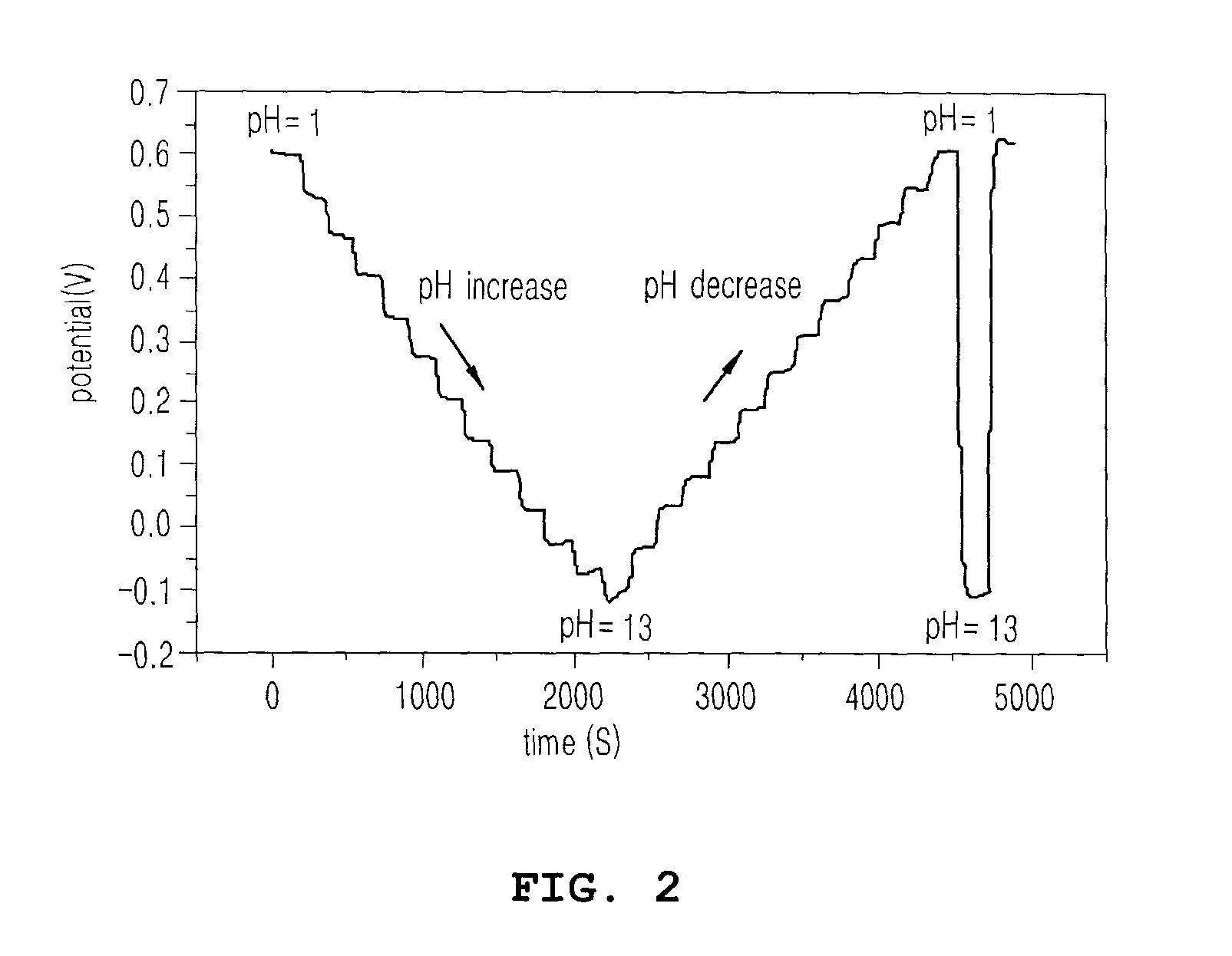
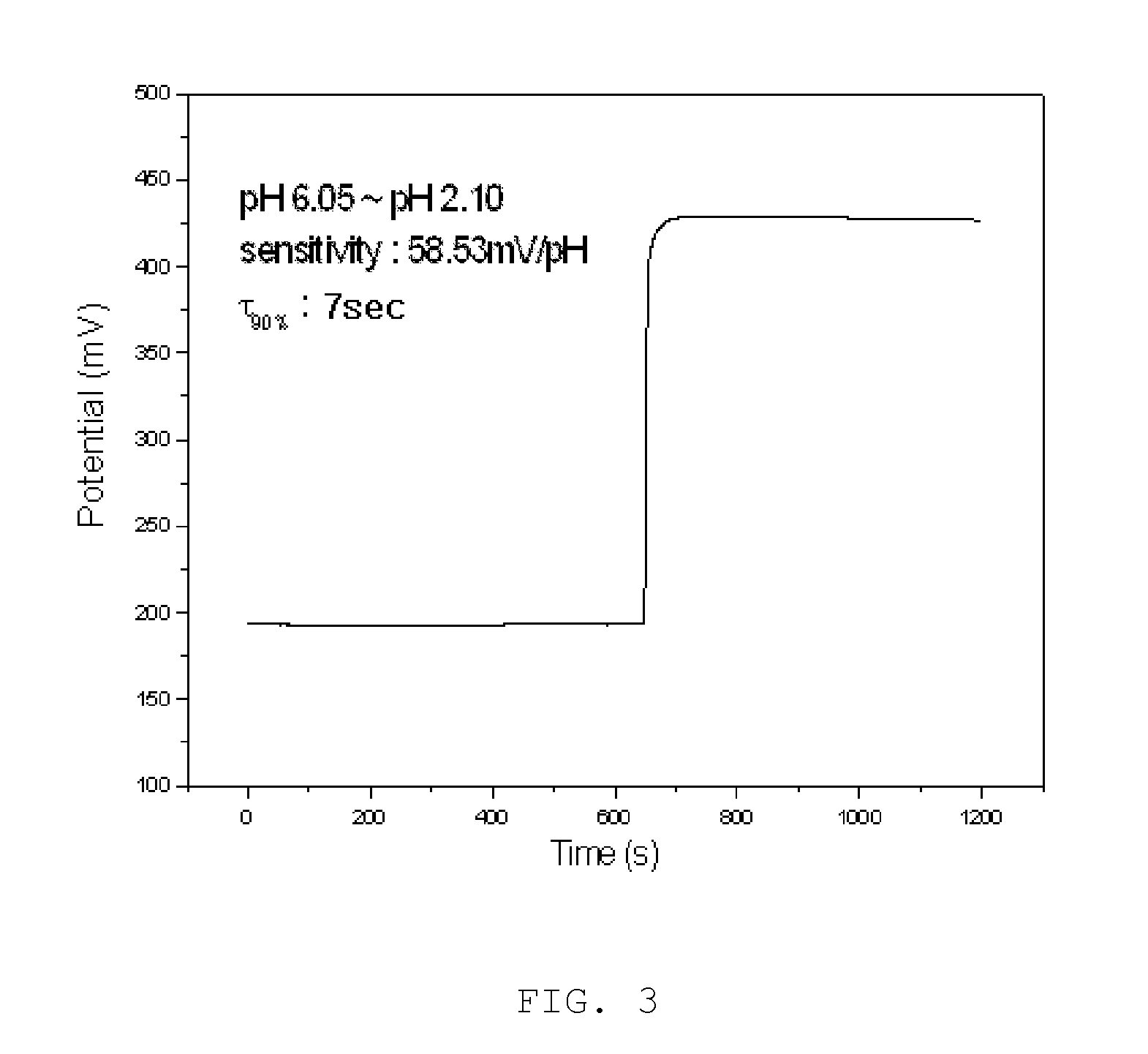
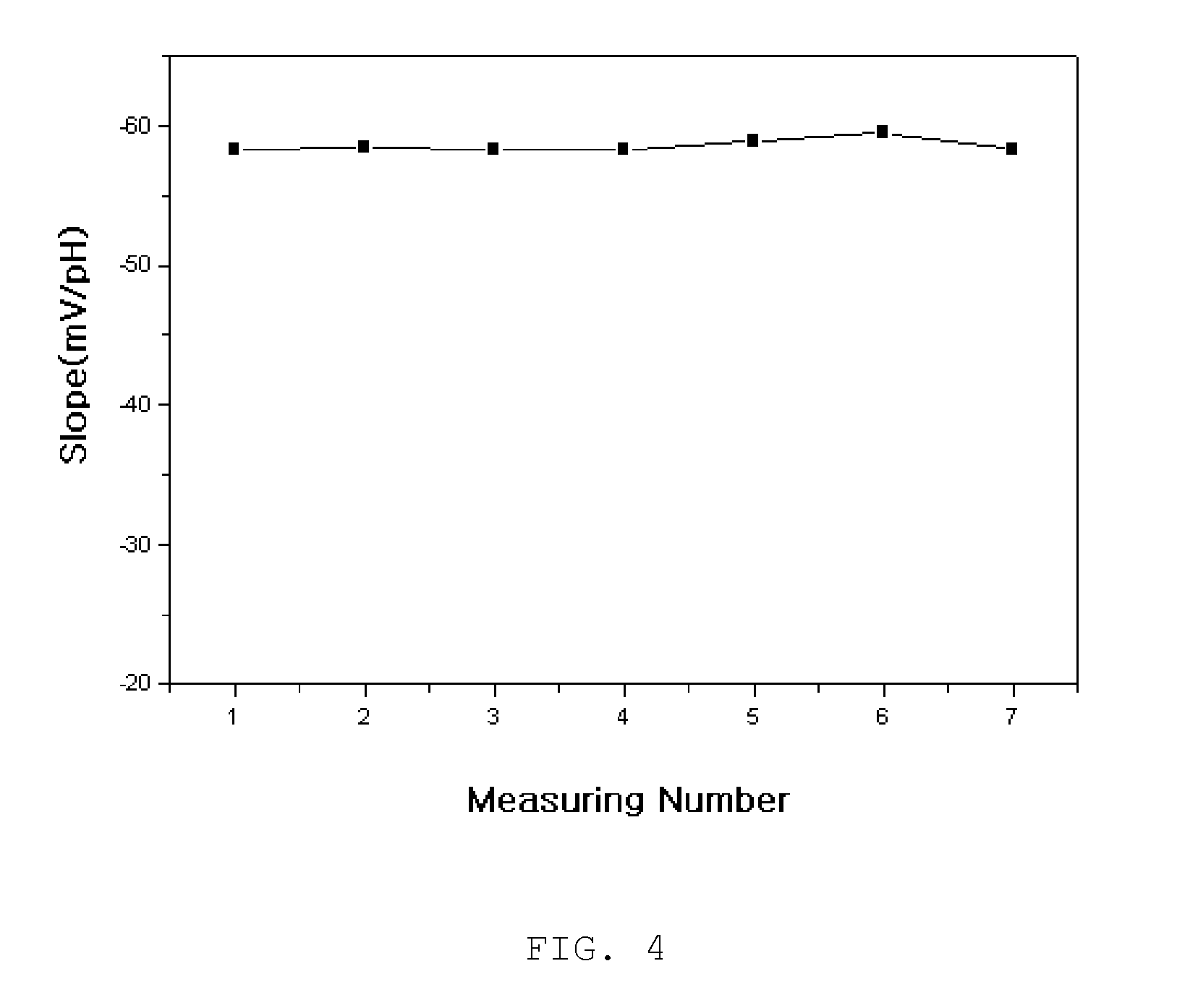
Disclosed herein is a surface-polishable iridium oxide composite hydrogen ion electrode and a method of manufacturing the same, and, more particularly, a surface-polishable iridium oxide composite hydrogen ion electrode, which has a long life due to its excellent physical strength, pH dependency approximate to a theoretical value (59 mV / pH unit), and high surface renewability, and a method of manufacturing the same. The iridium oxide composite hydrogen ion electrode according to the present invention is effective in that, when the electrode is contaminated or inactivated, the surface of the electrode can be regenerated through a simple polishing process because the electrode has high surface renewability, unlike conventional electrodes. The iridium oxide composite electrode according to the present invention can be usefully used in a water-quality monitoring system for monitoring the hydrogen ion concentration of a solution for a long period, an online pH measurement system, and pH measurement for samples, which causes serious contamination of the surface of a sensor.
Owner:KONKUK UNIV IND COOP CORP
Method for identifying whether one or more chemicals of theophylline, caffeine and theobromine are added into traditional Chinese medicines for relieving cough and asthma
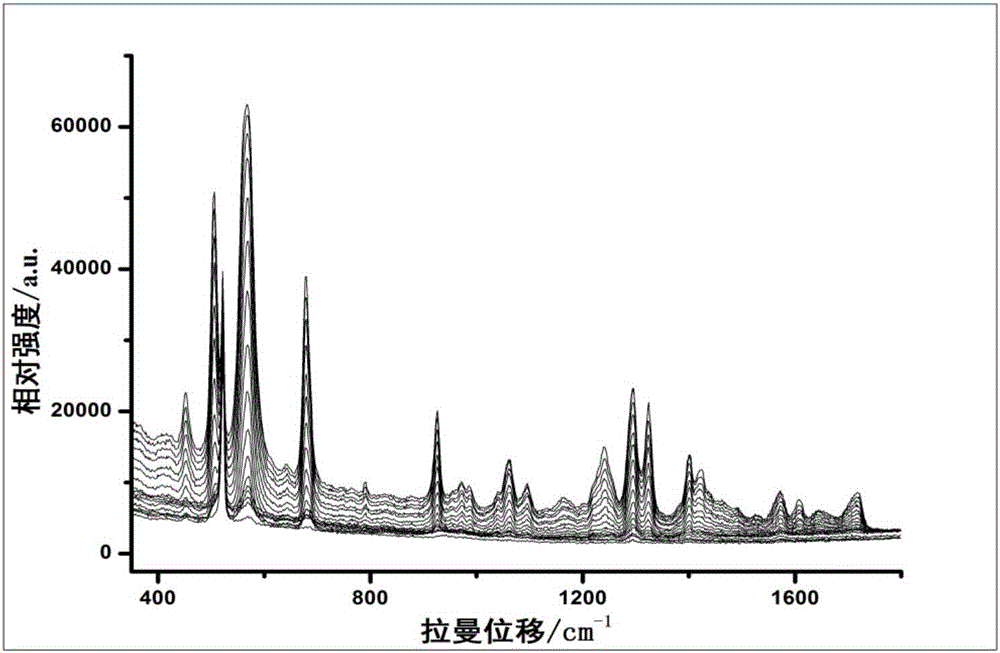
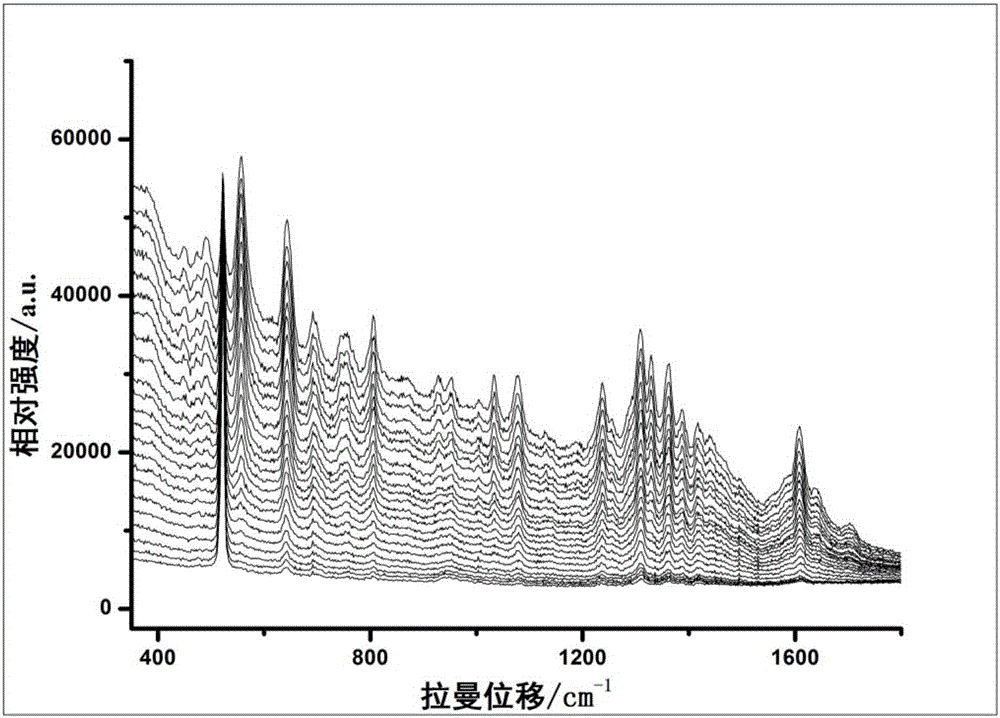
ActiveCN106525809AAccurate identificationStrong specificityRaman scatteringPre-conditionSurface-enhanced Raman spectroscopy
The invention relates to the technical field of medicine analysis and specifically relates to a method for identifying whether one or more chemicals of theophylline, caffeine and theobromine are added into traditional Chinese medicines for relieving cough and asthma. The method comprises the following steps: pre-conditioning the pH value of a to-be-detected solution; collecting a one-dimensional dynamic surface-enhanced Raman spectrum chart; drawing a pH dependency-two-dimensional correlation surface-enhanced Raman spectrum chart; and identifying whether one or more chemicals of theophylline, caffeine and theobromine are added into a to-be-detected sample. The step of pre-conditioning the pH value in the method can guarantee that the spontaneous change in pH value occurs in the most sensitive scope of substances, a more distinctive dynamic change Raman spectrum chart can be acquired, the specificity is strong, the sensitivity is high, the operation steps are simple, the detection time is short, and whether the theophylline, caffeine or theobromine is added into the traditional Chinese medicines for relieving cough and asthma can be accurately identified.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Hepatoma targeting carbon nano tube loaded with doxorubicin hydrochloride and preparation method thereof
InactiveCN104689334AGood biocompatibilitySmall side effectsOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityLiver parenchyma
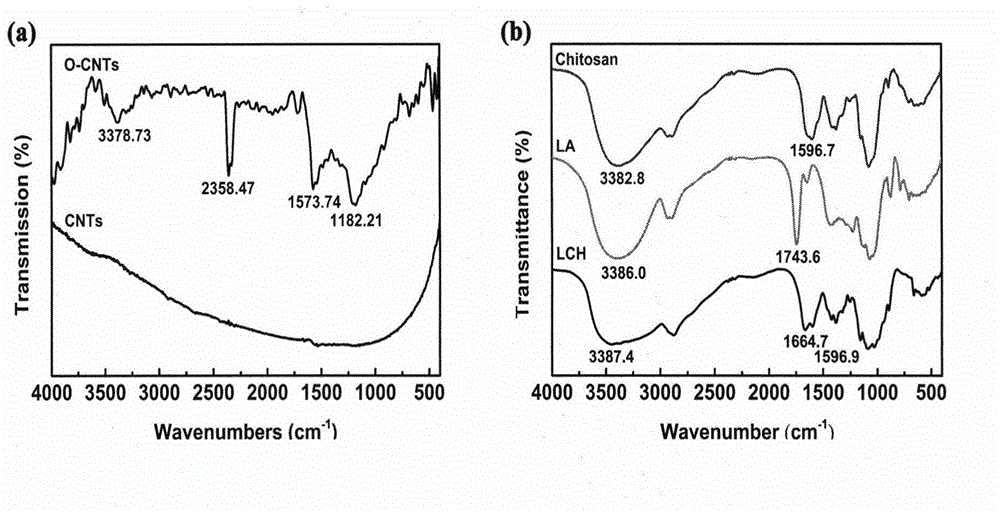
The invention discloses a hepatoma targeting carbon nano tube loaded with doxorubicin hydrochloride and a preparation method thereof. Amidation reaction of amino in a chitosan structure and carboxyl in lactobionic acid is carried out; galactosyl is coupled to chitosan so as to obtain galactosylated chitosan; the galactosylated chitosan is modified onto the carbon nano tube; therefore, the water solubility and the biocompatibility of the carbon nano tube are increased; simultaneously, the specificity of non-reductive galactose or N-acetyl-galactose is identified and taken by utilizing an asialoglycoprotein acceptor on a liver parenchyma cell membrane, so that the active hepatoma targeting effect is realized; simultaneously, doxorubicin hydrochloride is combined with a vector in a non-covalent manner through phi-phi accumulation acting force; and therefore, the hepatoma targeting carbon nano tube loaded with doxorubicin hydrochloride is prepared. The hepatoma targeting carbon nano tube disclosed by the invention is simple in preparation process, moderate in experimental condition and easy to operate; release of the medicine loaded targeting carbon nano tube has pH dependency, good biocompatibility and obvious in-vivo tumour inhibition effect; and thus, the hepatoma targeting carbon nano tube has good practical value.
Owner:CHINA PHARM UNIV
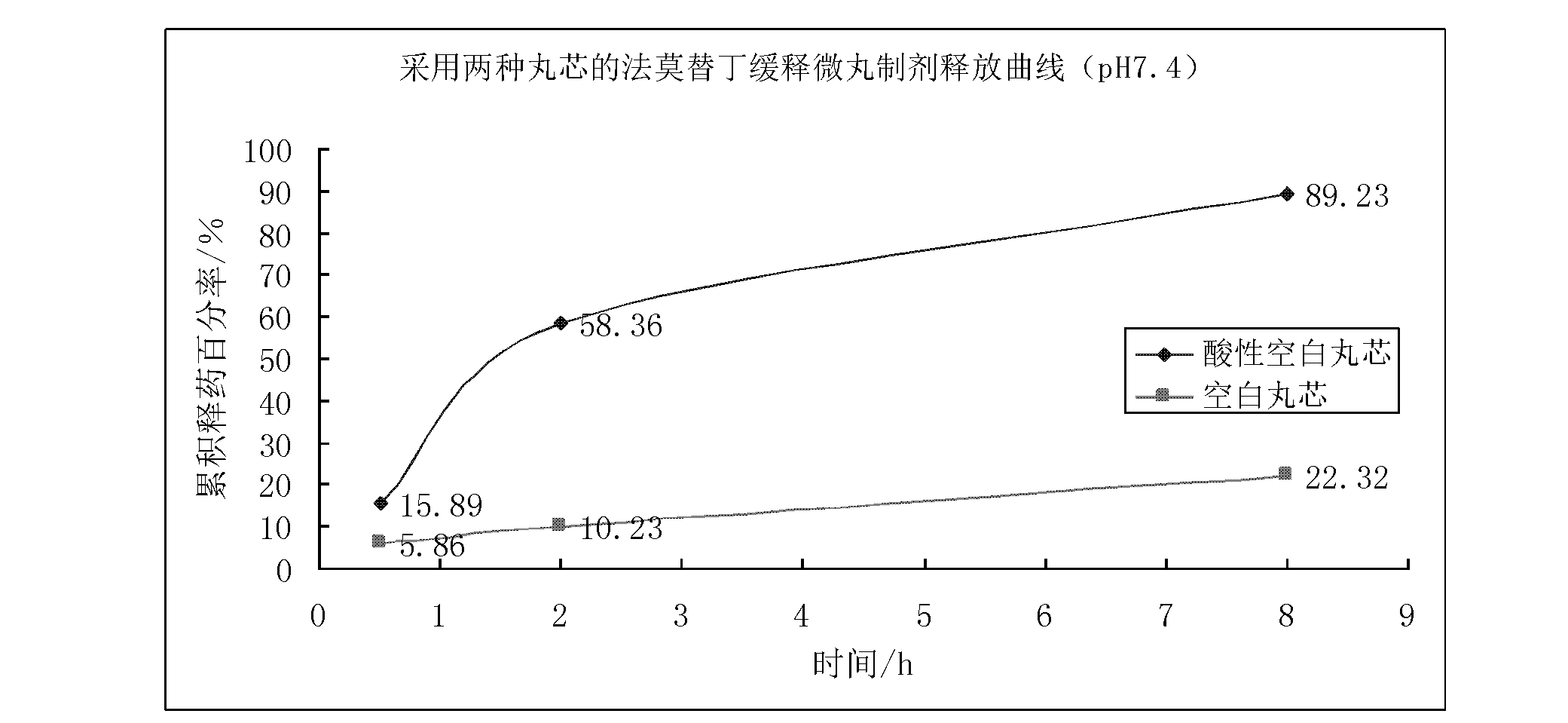
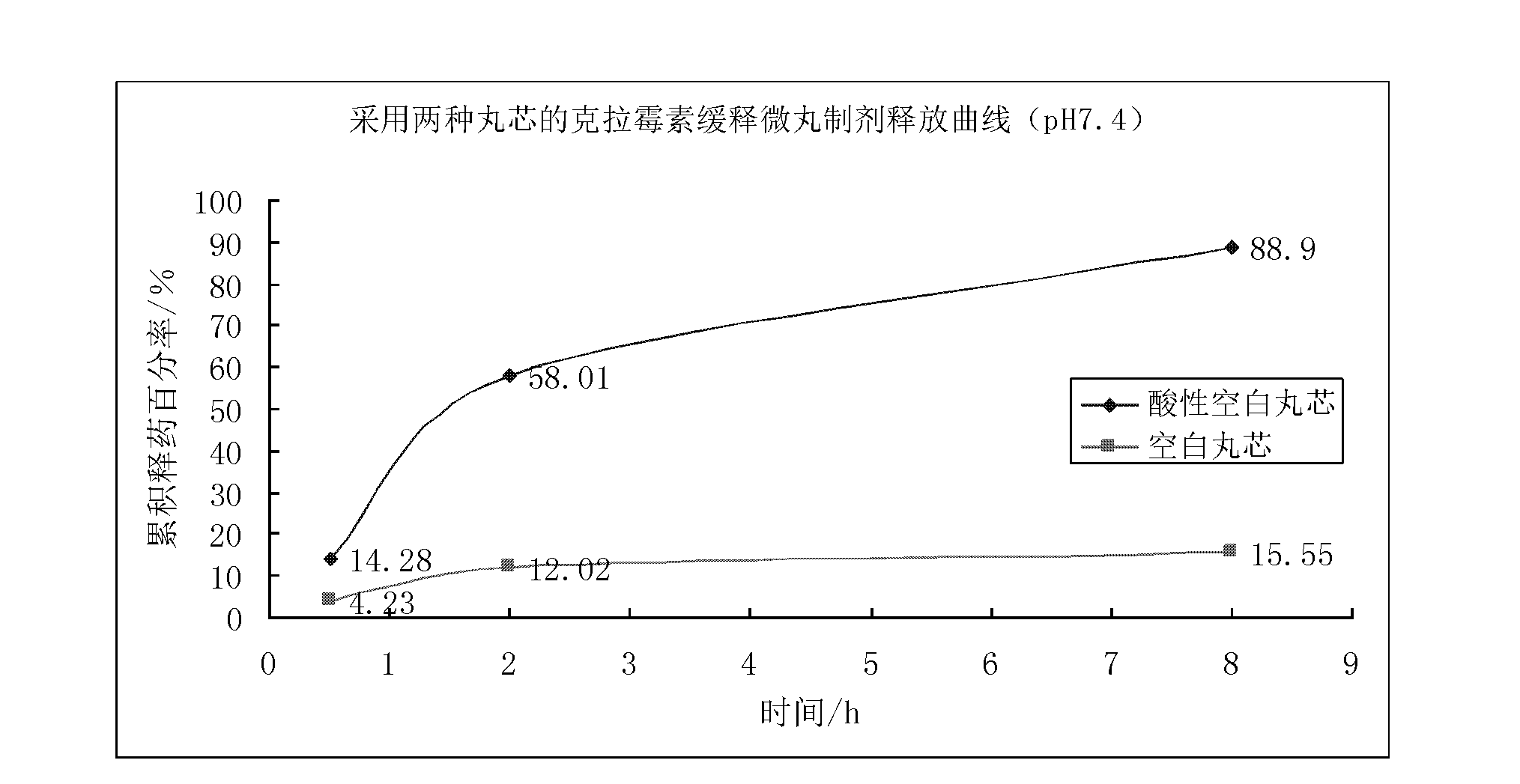
Acidic blank pellet core and preparation method thereof
InactiveCN102657625AResolves pH dependenceFacilitated releasePharmaceutical product form changePharmaceutical non-active ingredientsOrganic acidSustained release pellets
The invention discloses an acidic blank pellet core, which comprises a blank pellet core and an organic acid and is used for substituting a common blank pellet core in a sustained-release pellet preparation. Medicaments can be prepared into the sustained-release pellet preparation which can be stably released under the condition that the pH is 1.5 to 7.4, the release pH dependency of most conventional weakly alkaline medicaments is eliminated, the medicinal preparation can be released in stomach and intestinal tracts stably and slowly, and the medicament effect is lasting. Moreover, the invention also discloses a preparation method for the acidic blank pellet core. The method is simple and convenient, and facilitates industrialized production; and the acidic blank pellet core substitutes the conventional blank pellet core, is used for the sustained-release pellet preparation, can eliminate the release pH dependency of the alkaline medicaments, and has a wide application prospect.
Owner:上海智同医药科技有限公司
Solid pharmaceutical preparation
ActiveCN101410103AStable blood concentrationStable pHOrganic active ingredientsNervous disorderImmediate releaseBULK ACTIVE INGREDIENT
It is intended to provide a long-acting solid pharmaceutical preparation which has an immediate release part and a sustained release part containing tramadol or a pharmaceutically acceptable salt thereof, is fast-acting and stably has an excellent release property showing little pH dependency in the initial elution. The invention relates to a long-acting solid pharmaceutical preparation characterized by having an immediate release part and a sustained release part, containing tramadol or a pharmaceutically acceptable salt thereof as an active ingredient in both parts and containing partially pregelatinized starch and an excipient as additives in the immediate release part. The preparation of the invention is a long-acting preparation in which an effective blood concentration is reached rapidly after taking it for rapid pain-relief and a drug action can be sustained for a long time thereafter and is practical as a preparation showing a stable, pH-independent and rapid initial elution behavior and, further, having a sufficient hardness enough to meet the need for avoidance of detrition, cracking, chipping, etc. during tablet coating.
Owner:NIPPON ZOKI PHARM CO LTD
Infrared shielding film
ActiveUS9435923B2Low costGood infrared reflection performanceOptical filtersThin material handlingPolymer sciencePolyvinyl alcohol
An infrared shielding film has at least one unit on a substrate, the unit having a low refractive index layer that contains first metal oxide particles and a first binder and a high refractive index layer that is arranged adjacent to the low refractive index layer, contains second metal oxide particles and a second binder, and has a higher refractive index than the low refractive index layer, in which at least one layer of the low refractive index layer and the high refractive index layer contains, as a binder, at least one of the following (a) to (c):(a) a carboxyvinyl polymer that contains a monomer component, which contains a carboxylic acid, in an amount of 20 to 75% by mass of the entire composition,(b) a copolymer having pH dependency of viscosity, and(c) a modified polyvinyl alcohol.
Owner:KONICA MINOLTA INC
Anti-par2 antibodies and uses thereof
The present disclosure provides antibodies and antigen-binding fragments capable of binding PAR2. In some embodiments, the anti-PAR2 antibodies or antigen-binding fragments thereof bind PAR2 in a pH-dependent manner. The disclosure further provides methods for making and using the antibodies and antigen-binding fragments.
Owner:MEDIMMUNE LTD
Solid Pharmaceutical Preparation
InactiveUS20080038344A1Stable quick releasing characteristicLittle pH dependencyBiocideOrganic active ingredientsHas active ingredientAdditive ingredient
To provide a sustained-release solid pharmaceutical preparation having a quick-release part and a sustained-release part and stably having an excellent quick releasing characteristic with little pH dependency in an initial dissolution. The present invention relates to that, in a preparation containing a medical ingredient as an effective ingredient, particularly active analgesic ingredient, a sustained-release solid pharmaceutical preparation which is characterized in that it is a solid pharmaceutical form having a quick-release part and a sustained-release part and contains effective ingredient in both parts and the quick-release part contains a partly pregelatinized starch and a low substituted hydroxypropylcellulose as additives. In the preparation of the present invention, stable and quick initial dissolution behavior being independent upon pH is achieved even when some sustained-release part is contaminated in the quick-release part due to the difference in the tabletting method for multi-layered tablets. Furthermore, the preparation is practical as a preparation having a sufficient hardness in view of necessity that abrasion, breakage, crack, etc. are not generated when the tablet is coated.
Owner:NIPPON ZOKI PHARM CO LTD
Enhanced CH2 structure domain mutant combined with newborn Fc receptor as well as preparation method and application thereof
ActiveCN108101992AReduce production processCheap purificationImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationFc(alpha) receptorWild type
The invention discloses an enhanced CH2 structure domain mutant combined with a newborn Fc receptor as well as a preparation method and application thereof. According to the enhanced CH2 structure domain mutant combined with the newborn Fc receptor as well as the preparation method and application thereof disclosed by the invention, amino acid neighbouring to a helical area which interacts with FcRn in a CH2 structure domain of a constant area of a human antibody IgG is mutated to obtain the enhanced CH2 structure domain mutant. Relative to a wild type CH2 structure domain, the mutant disclosed by the invention has higher stability and anti-gathering ability, and has the advantages that the production, purification and storage costs of a monoclonal antibody or Fc fusion protein can be reduced; the CH2 structure domain mutant is combined with pH dependency of FcRn so that enhancement is achieved, and a good basis is laid for searching a novel single-domain antibody drug using the enhanced CH2 structure domain mutant as a framework.
Owner:武汉班科生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com