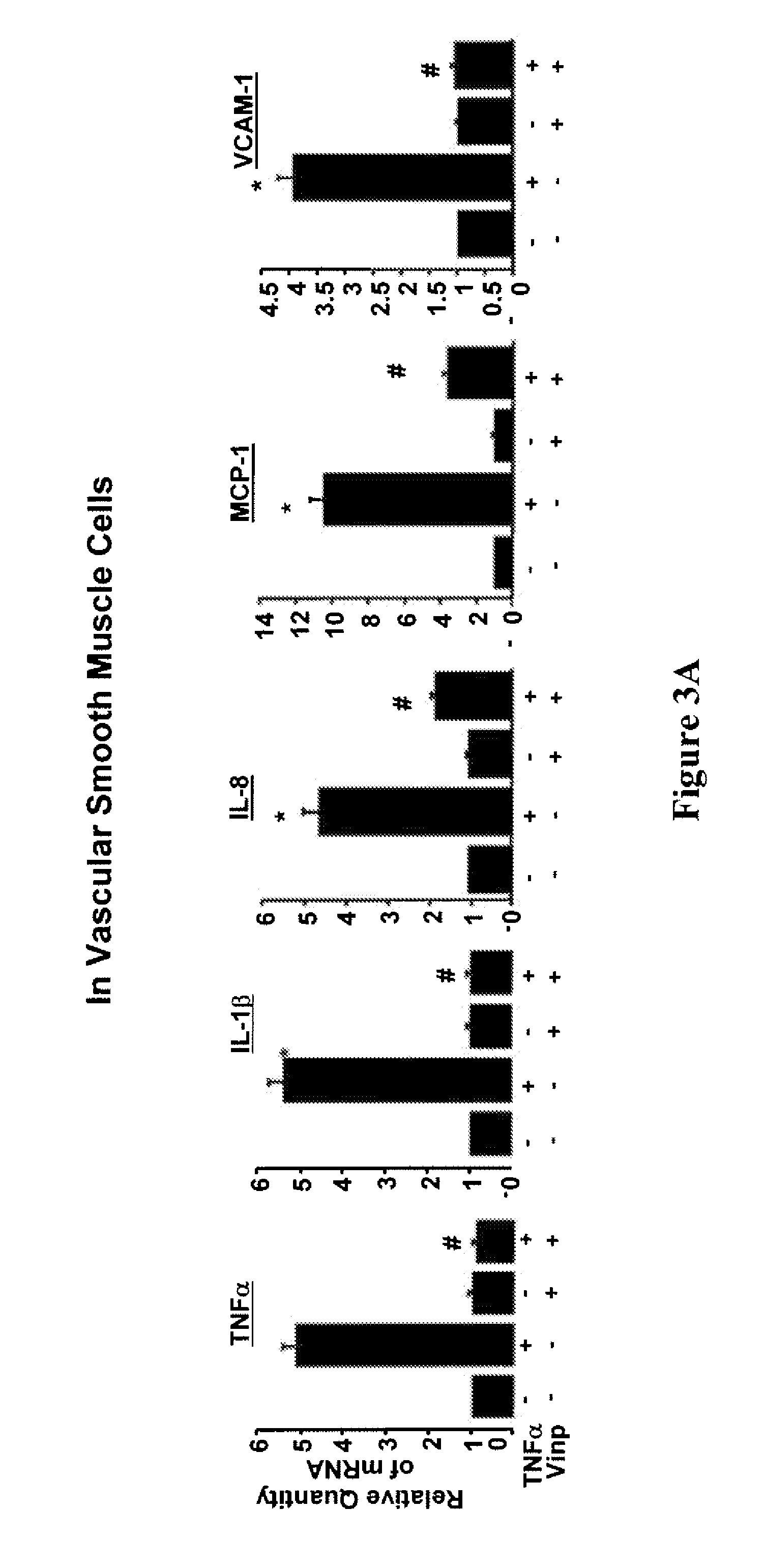
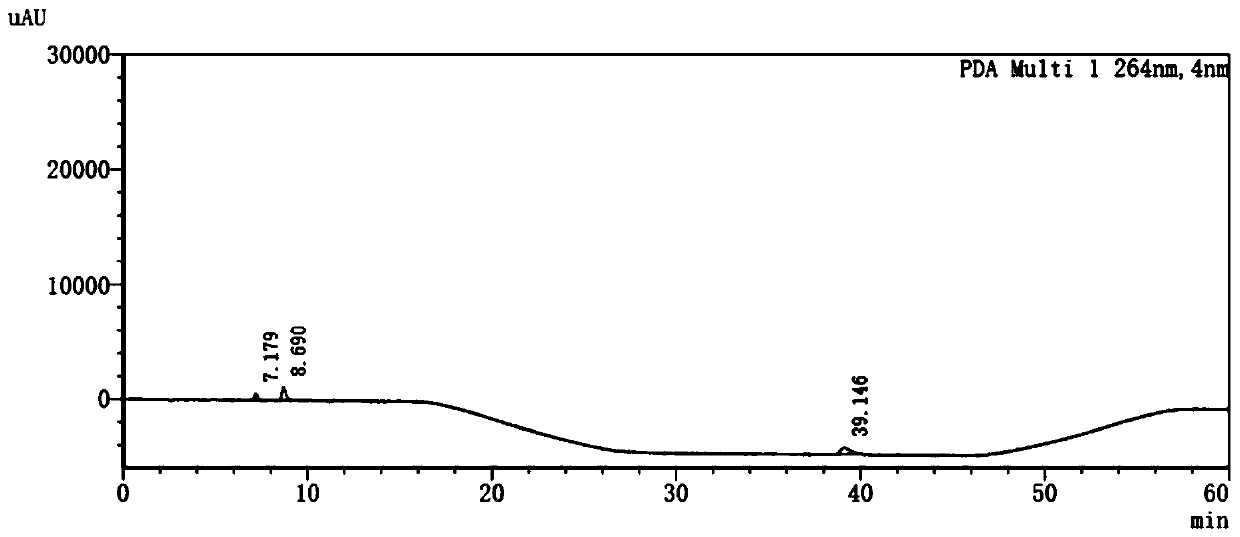
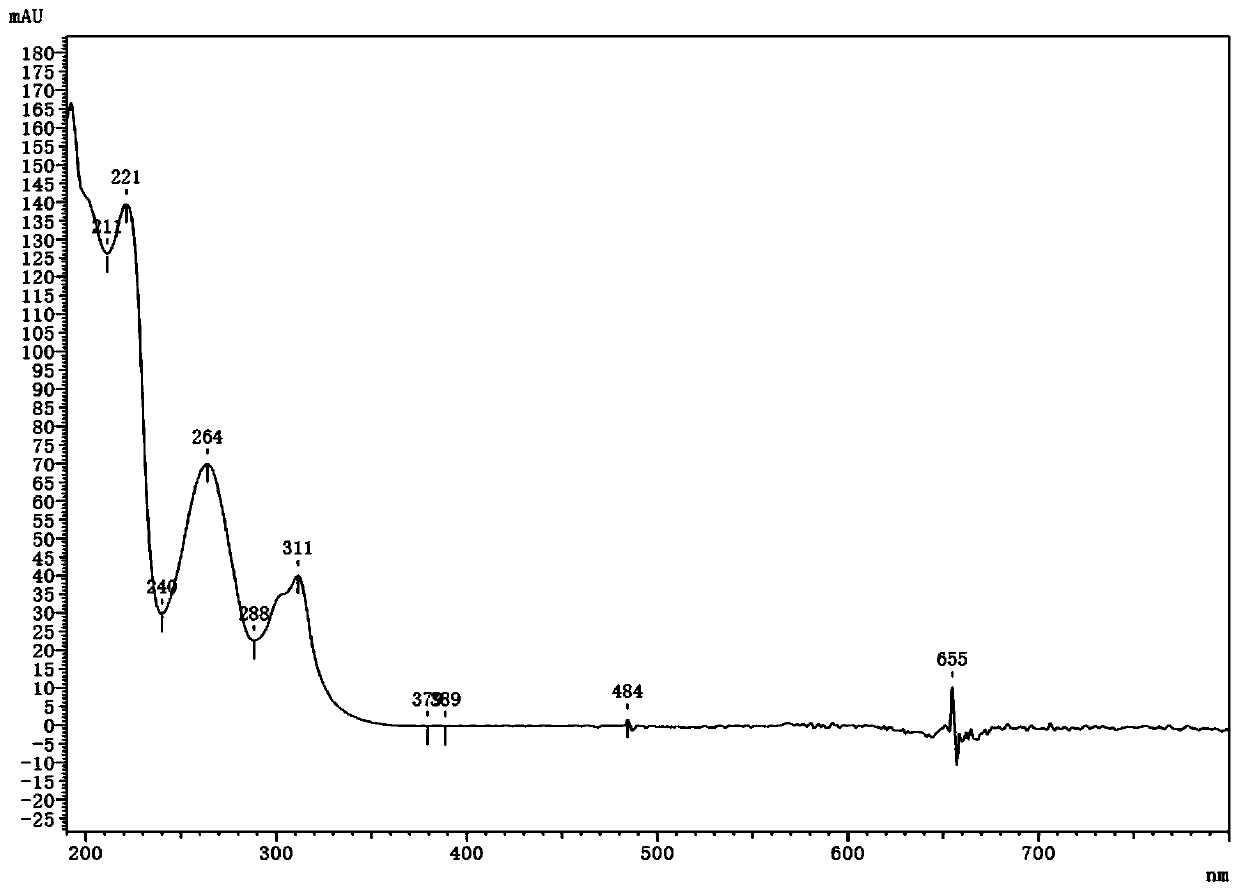
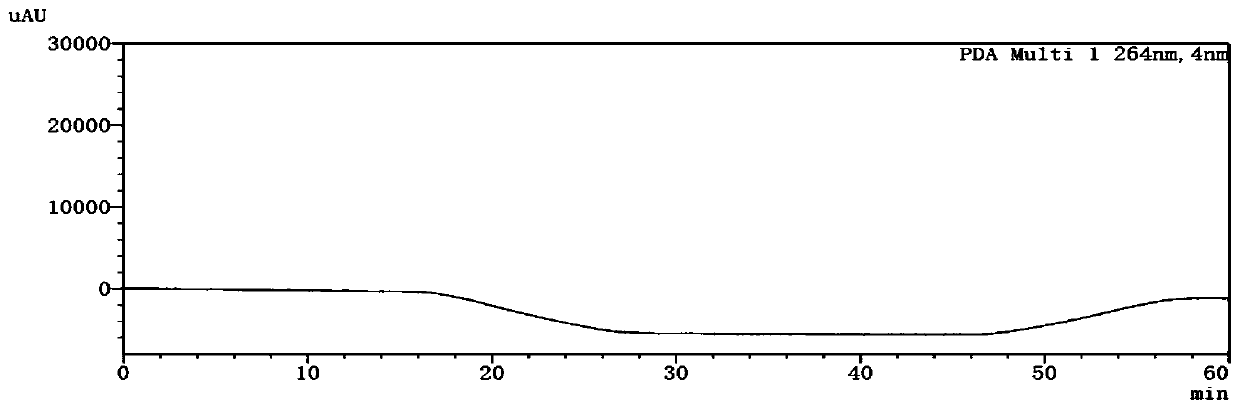
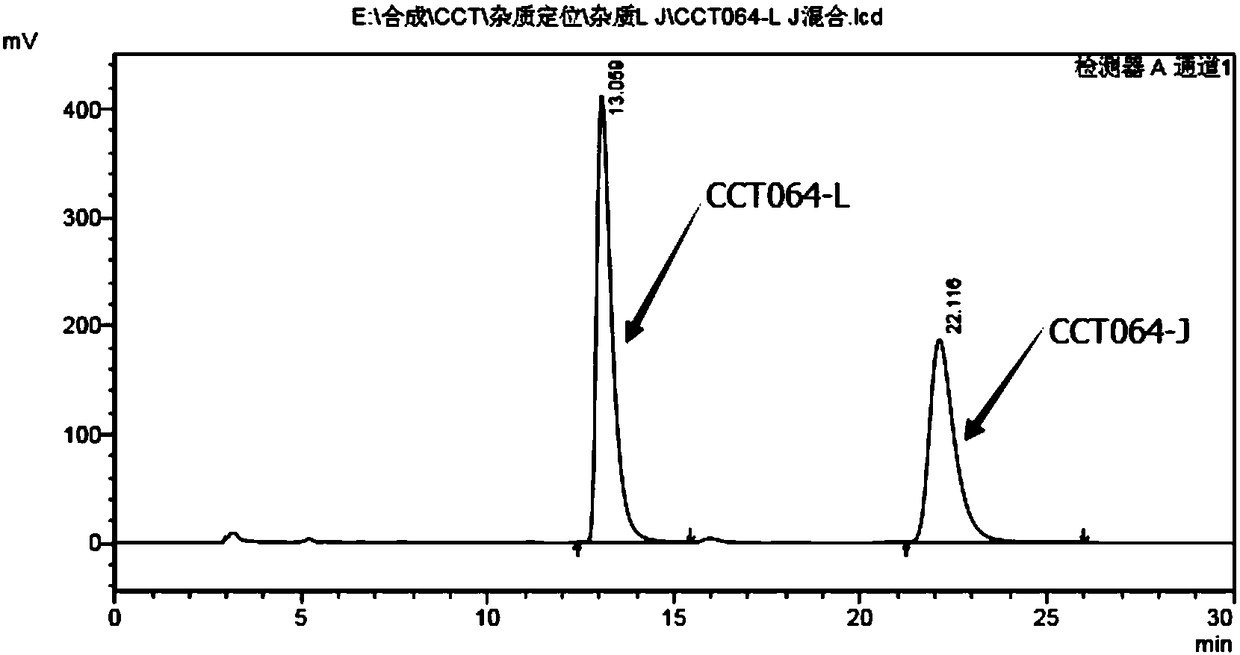
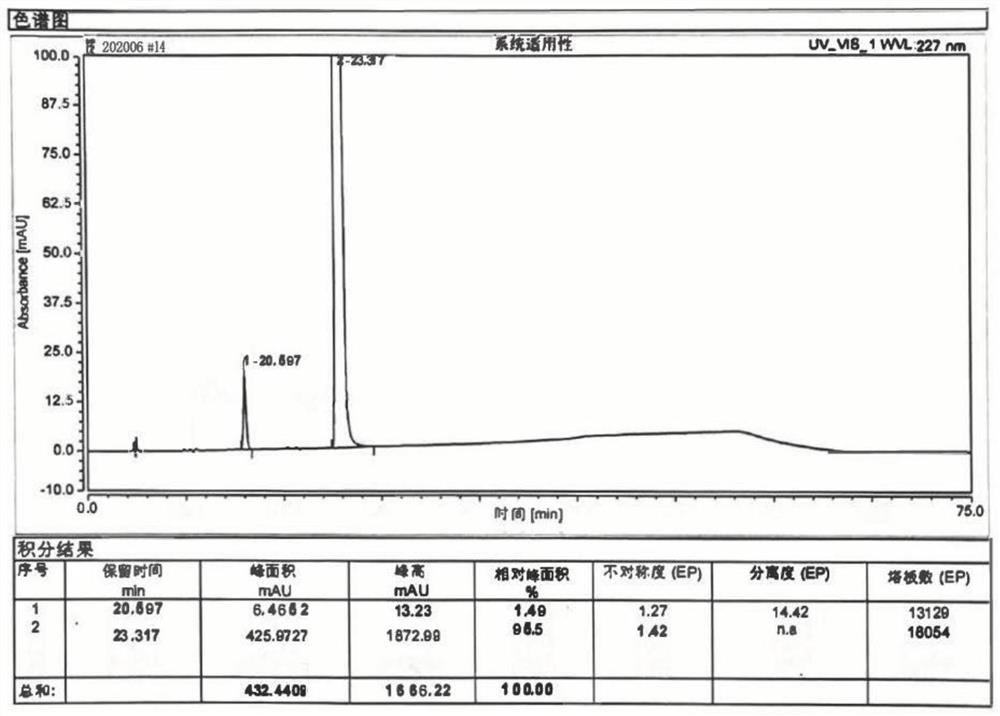
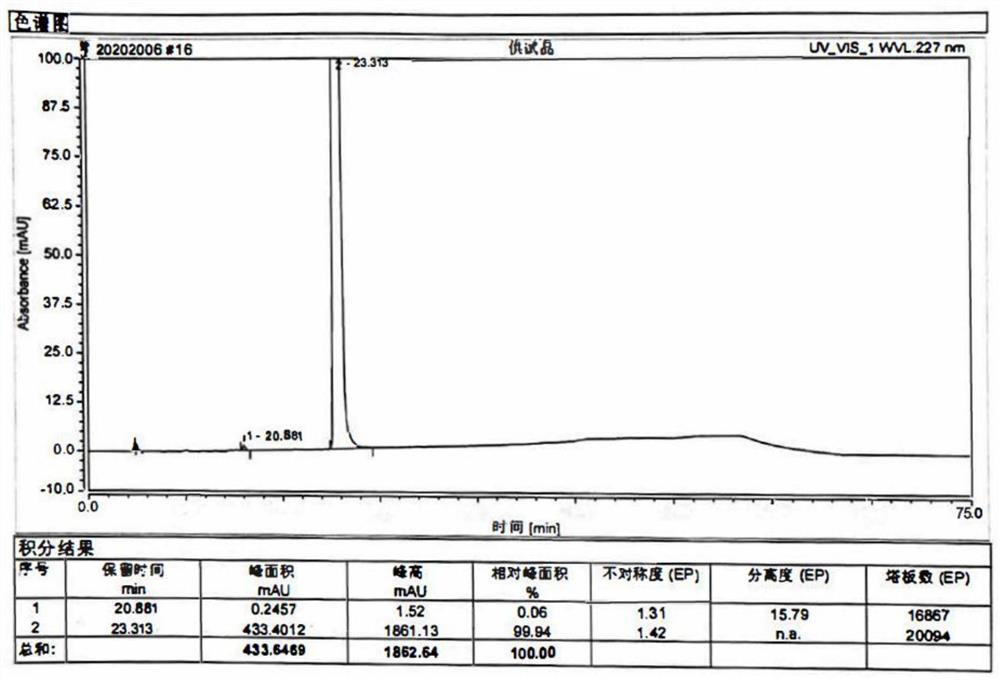
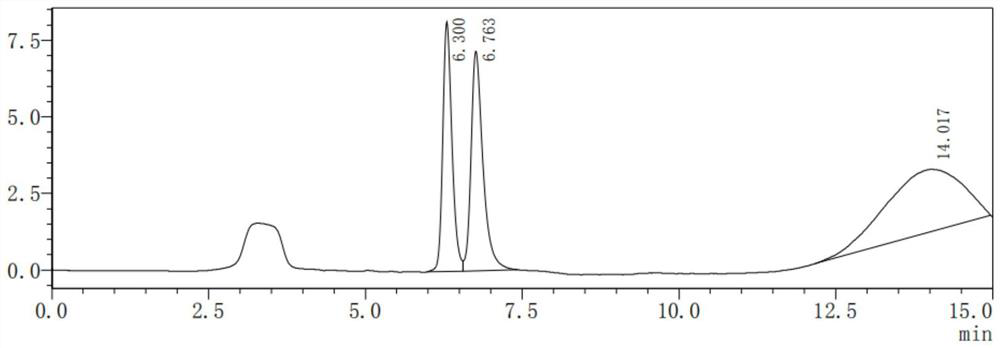
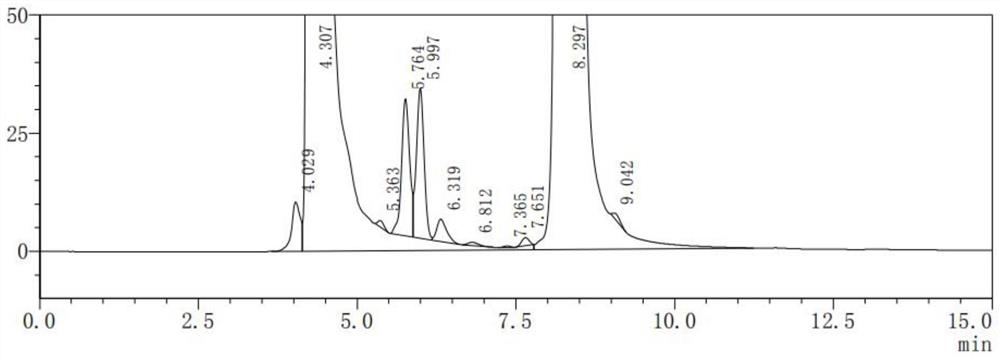
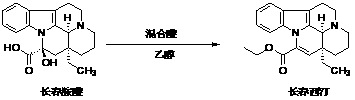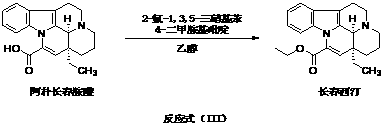Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
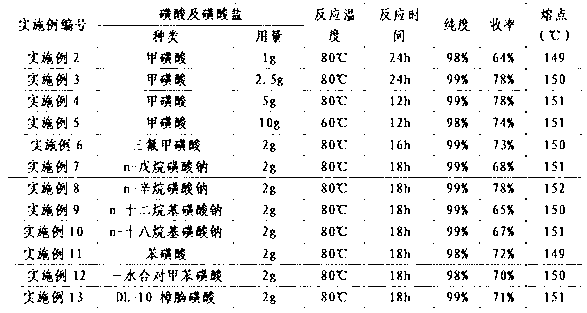
53 results about "Vincamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
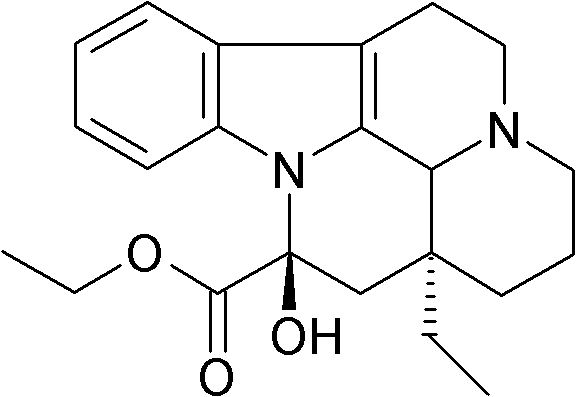
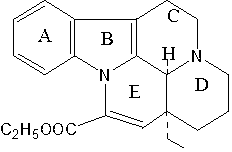
Vincamine is a monoterpenoid indole alkaloid found in the leaves of Vinca minor (lesser periwinkle), comprising about 25-65% of its indole alkaloids by weight. It can also be synthesized from related alkaloids.
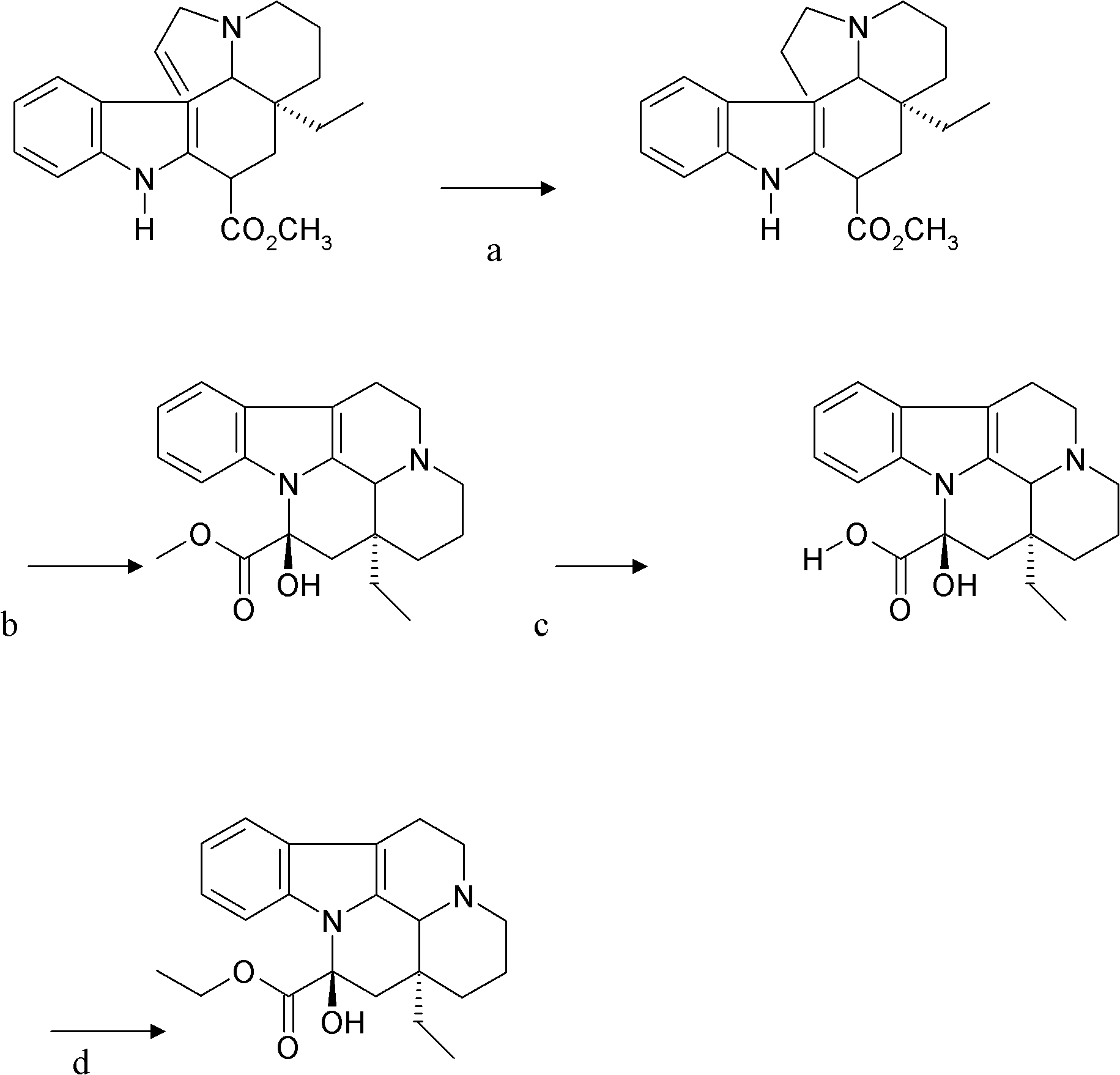
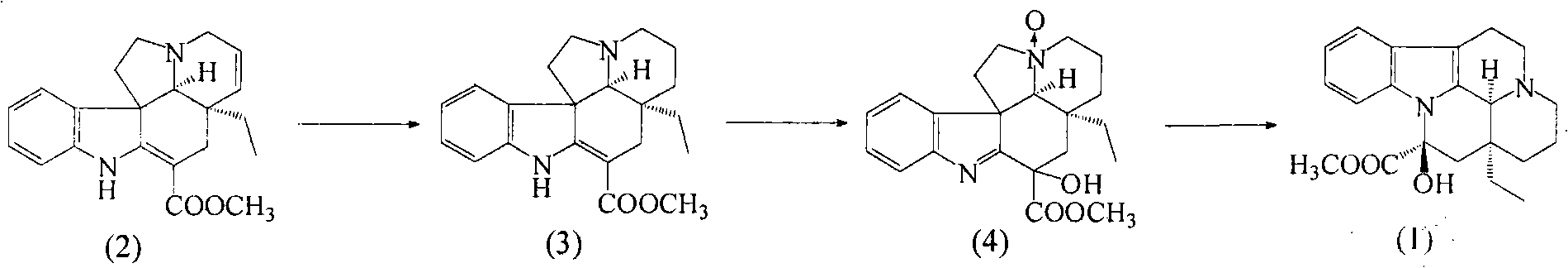
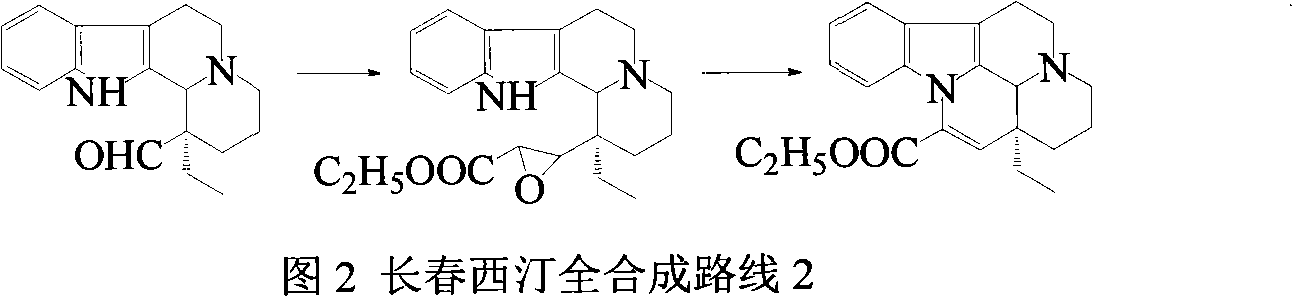
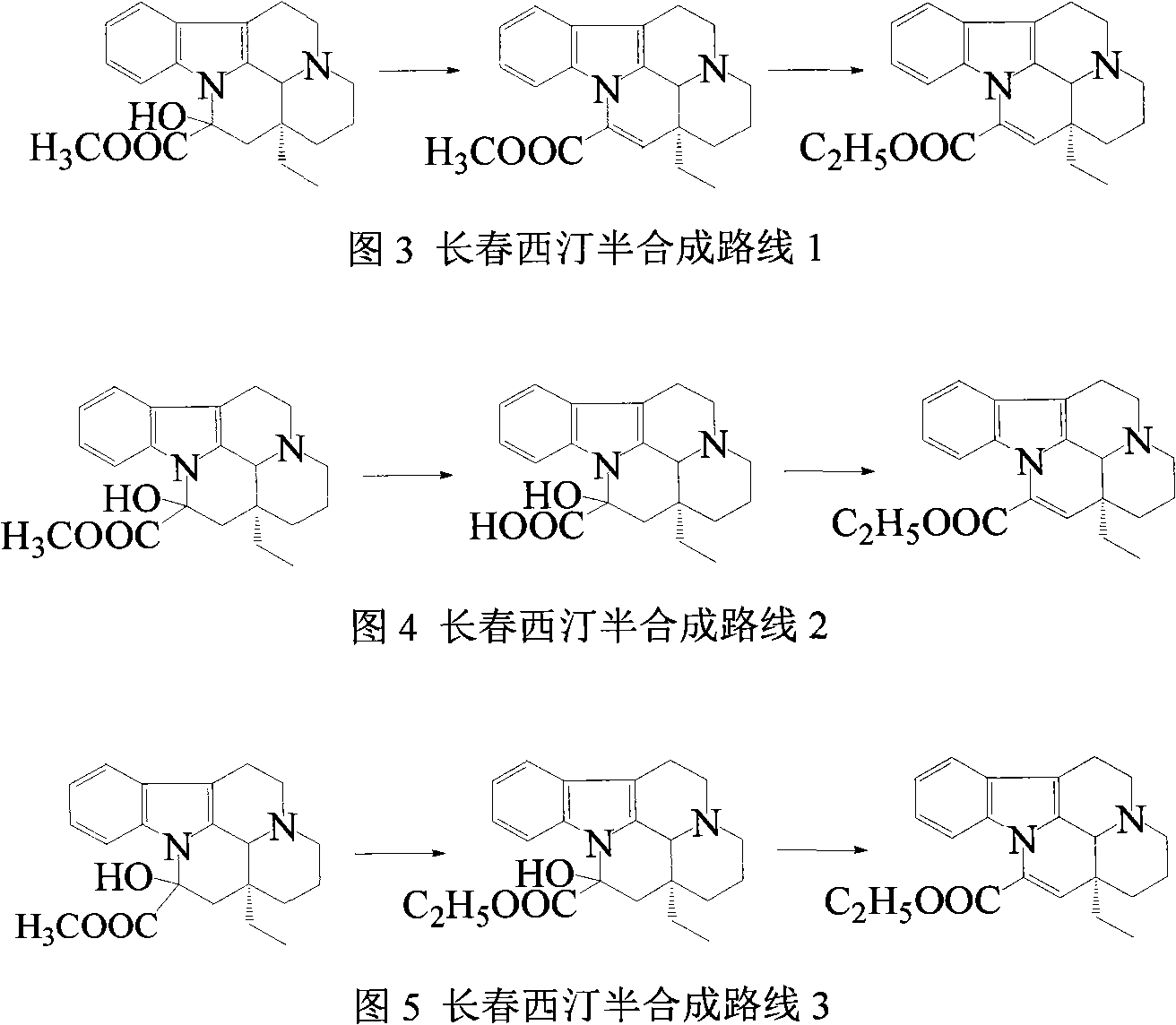
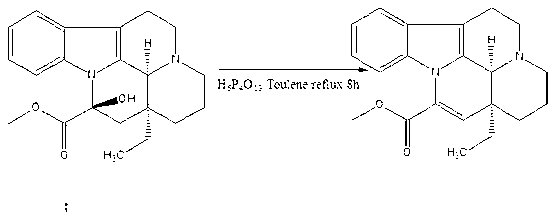
Synthetic method of vinpocetine
The invention provides a half synthetic method for industrially producing vinpocetine on the premise of ensuring product purity and yield. Voacanga seed grown in Africa is used for extracting raw material-tabersonine required by synthesis. The tabersonine is synthesized into vinpocetine by four steps: 1) preparing vincadifformine; 2) preparing vincamine; 3) preparing vincamine acid; and 4) synthesizing the vinpocetine the content of which is 99%. The invention builds an integral process from tabersonine to vinpocetine, has the advatages of simple process, high yield and lower cost, and can realize the industrial production of vinpocetine on the premise of ensuring the product purity and yield.
Owner:SHAANXI JIAHE PHYTOCHEM
Small volume vincamine injection and its prepn process
ActiveCN101066244ALong validity periodImprove stabilityOrganic active ingredientsNervous disorderMedicineBioavailability
The present invention discloses one kind of small volume vincamine injection and its preparation process. The small volume vincamine injection consists of vincamine as main medicine component 3-20 weight portions and supplementary material including co-solvent 0.1-1 weight portions, antioxidant 0.5-10 weight portions, physical stabilizer 50-100 weight portions, and blood stimulation regulator 0.5-10 weight portions. It has high stability, long validity period, high bioavailability, capacity of being used directly and other advantages.
Owner:HENAN RUNHONG PHARMA
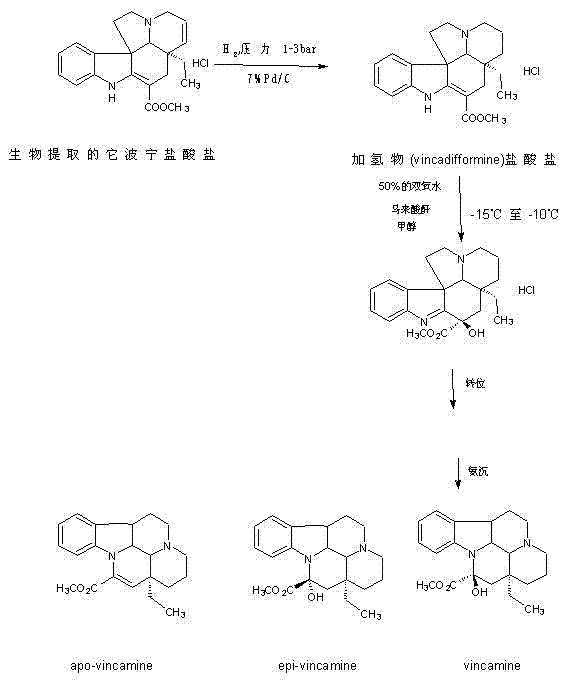
Industrialized semisynthesis of medicine-vincamine for treating cerebral ischemia
InactiveCN102108082AThe production process is green and environmentally friendlyOrganic chemistryTabersonineIschemia
The invention relates to a semisynthesis process route which takes tabersonine salt as raw material for producing vincamine. Catalytic hydrogenation, peroxidation, rearrangement, crystallization and recrystallization are sequentially performed on the tabersonine salt for getting the high-purity vincamine, and the content is greater than 99.0%. Calculated according to the tabersonine salt, the overall yield of the vincamine is 55.3%. The method has the characteristics of low cost, high yield, short time, and simplicity and safety in operation, and is completely applicable to industrialized large-scale production.
Owner:GUANGZHOU SWELLXIN SCI & TECH
Semi-synthesis of vinpocetine through one kettle way and preparation of water-soluble vinpocetine salt
InactiveCN102485723AConducive to large-scale industrial productionAtom economy is highOrganic chemistryFood additiveVinpocetine
The invention relates to a semi-synthesis of vinpocetine and a preparation of a vinpocetine salt, wherein vincamine is used as a raw material. The one kettle way for the synthesis of vinpocetine allows the production efficiency to be improved and the total yield to be above 85%; and a method of the preparation of the vinpocetine salt is simple, and the yield is above 85%. Compared with original production methods, the method of the invention, which has the advantages of high atom economy, and concise and controllable process flow, is in favor of the production of GMP (good manufacturing practice) grade bulk medicines and food additives.
Owner:江苏斯威森生物医药工程研究中心有限公司
Industrialized semi-synthesis process of vincamine
InactiveCN103232452AThe production process is green and environmentally friendlyOrganic chemistryTabersonineSafe operation
The invention relates to a semi-synthesis process for producing vincamine by taking tabersonine as a raw material. The high-purity vincamine with the content of over 99.0 percent is obtained by performing catalytic hydrogenation, peroxidation, rearrangement, crystallization and recrystallization on the tabersonine. Based on the tabersonine, the total yield of the vincamine is 55.3 percent. The semi-synthesis process has the characteristics of low cost, high yield, short time and simple and safe operation, and is completely suitable for industrialized mass production.
Owner:彭学东
Vincamine preparation method
ActiveCN106749230AReduce dosageReduce the risk of pressor reactionsOrganic chemistry methodsTabersonineBiochemical engineering
The invention relates to a vincamine preparation method in the field of compound preparation. The vincamine preparation method includes the steps of (1), tabersonine preparation, (2), vincadifformine preparation, (3), monoperoxy maleic acid preparation and (4), vincamine preparation. The vincamine preparation method has the advantages of easy availability to raw materials, simplicity and convenience in reaction process operation, high safety, low cost, high product yield, high quality and suitability for industrial production.
Owner:NORTHEAST PHARMA GRP
Method and compositions for treatment or prevention of inflammatory conditions
InactiveUS20100221340A1Inhibit the inflammatory responseAvoid stickingBiocidePowder deliveryNon steroid anti inflammatory drugPharmaceutical drug
Pharmaceutical compositions and methods for treating or preventing an inflammatory condition in a patient are disclosed. The pharmaceutical compositions and methods include the use of vincamine or a vincamine derivative, either alone or in combination with one or more additional therapeutic agents, including a steroid (preferably a corticosteroid), an angiotensin II receptor (type 1) antagonist, an angiotensin-converting enzyme (ACE) inhibitor, and a non-steroidal anti-inflammatory drug.
Owner:UNIVERSITY OF ROCHESTER
Preparation method for vinpocetine
The invention provides a preparation method for vinpocetine. The method comprises the following steps: with vincamine as a raw material and triethyl formate as an esterification reagent, subjecting vincamine and triethyl formate to a one-step reaction under the action of Lewis acid so as to obtain a crude vinpocetine product; and then carrying out washing with alkali lye, decoloring in alcohol and recrystallization so as to prepare a refined vinpocetine product. Compared with the prior art, the preparation method provided by the invention has the advantages of short process flow, simple operation and suitability for industrial production, and the prepared refined vinpocetine product has yield of more than 89%, a melting point of 148 to 151 DEG C, HPLC of greater than 99%, a whitish color, good quality and low preparation cost.
Owner:HUNAN KEYUAN BIO PRODS
Method of detecting apovincamine acid and vincamine acid in vinpocetine simultaneously
InactiveCN110455944AEasy detectionEasy to separateComponent separationChromatographic separationPhosphate
The invention provides a method of detecting apovincamine acid and vincamine acid in vinpocetine simultaneously. The method comprises steps of (1) preparing a test solution by using a vinpocetine rawmaterial or vinpocetine injection; (2) diluting the test solution by 1000 times to be used as a control solution; (3) performing chromatographic separation on the test solution and the control solution by using high-performance liquid chromatography, and recording a chromatograph, wherein chromatographic conditions are as follows: detection wavelength is 262nm-266nm, column temperature is 25DEG C-35DEG C, a mobile phase A is a phosphate buffer solution, a mobile phase B is methanol, and eluting flow rate is 0.8-1.5ml / min; and (4) calculating contents of apovincamine acid and vincamine acid inthe test solution according to a self-control method of a principle component with a calibration factor. Peaks of the vincamine acid and apovincamine acid and the peak of vinpocetine can be well separated. The method can highly adapt to a system, and has low detection limit and high precision.
Owner:武汉华龙生物制药有限公司
Vincamine sustained-release pellet preparation and preparation method thereof
InactiveCN102657615AResolving pH DependencePromote absorptionOrganic active ingredientsNervous disorderOrganic acidSustained release pellets
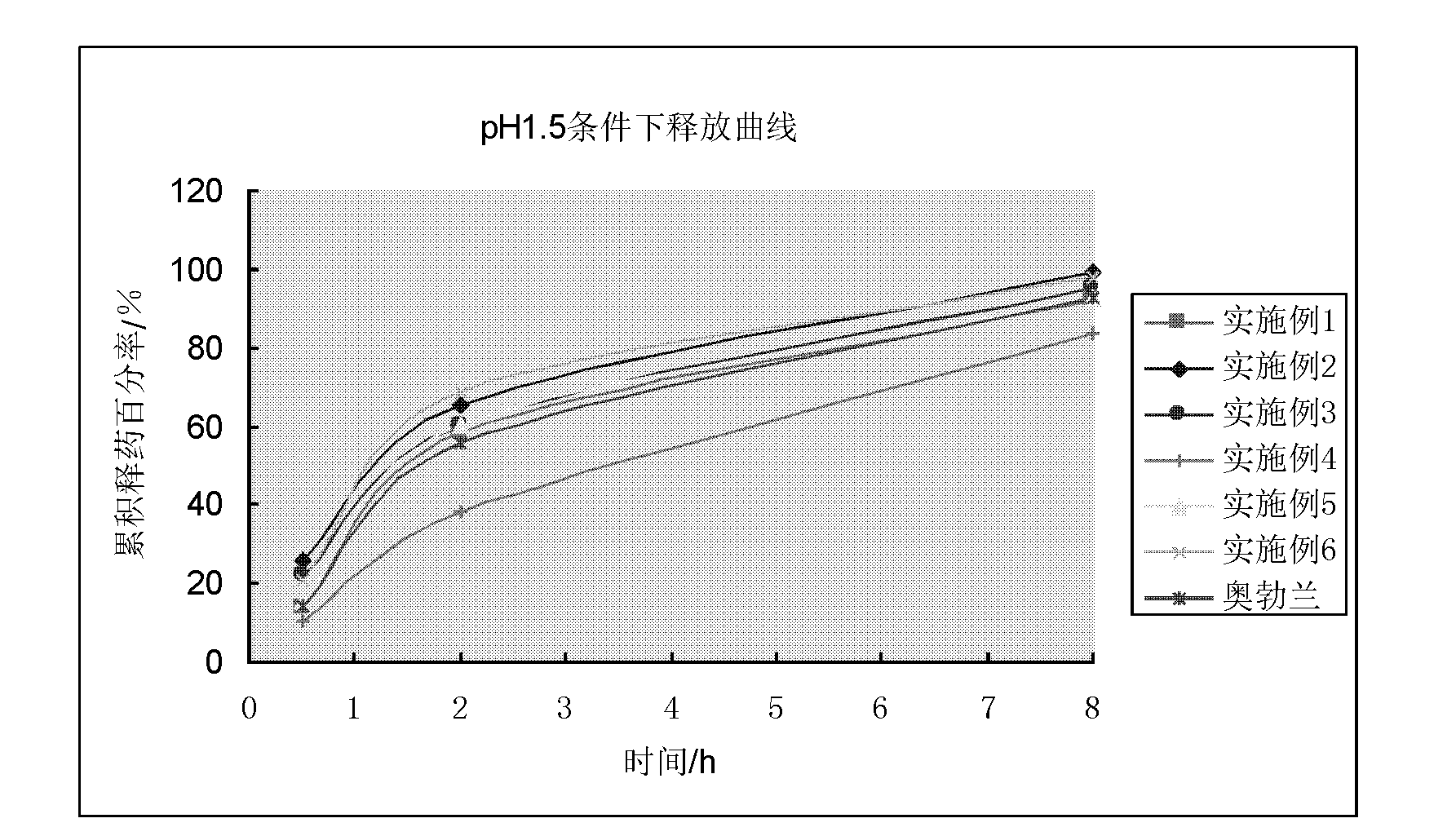
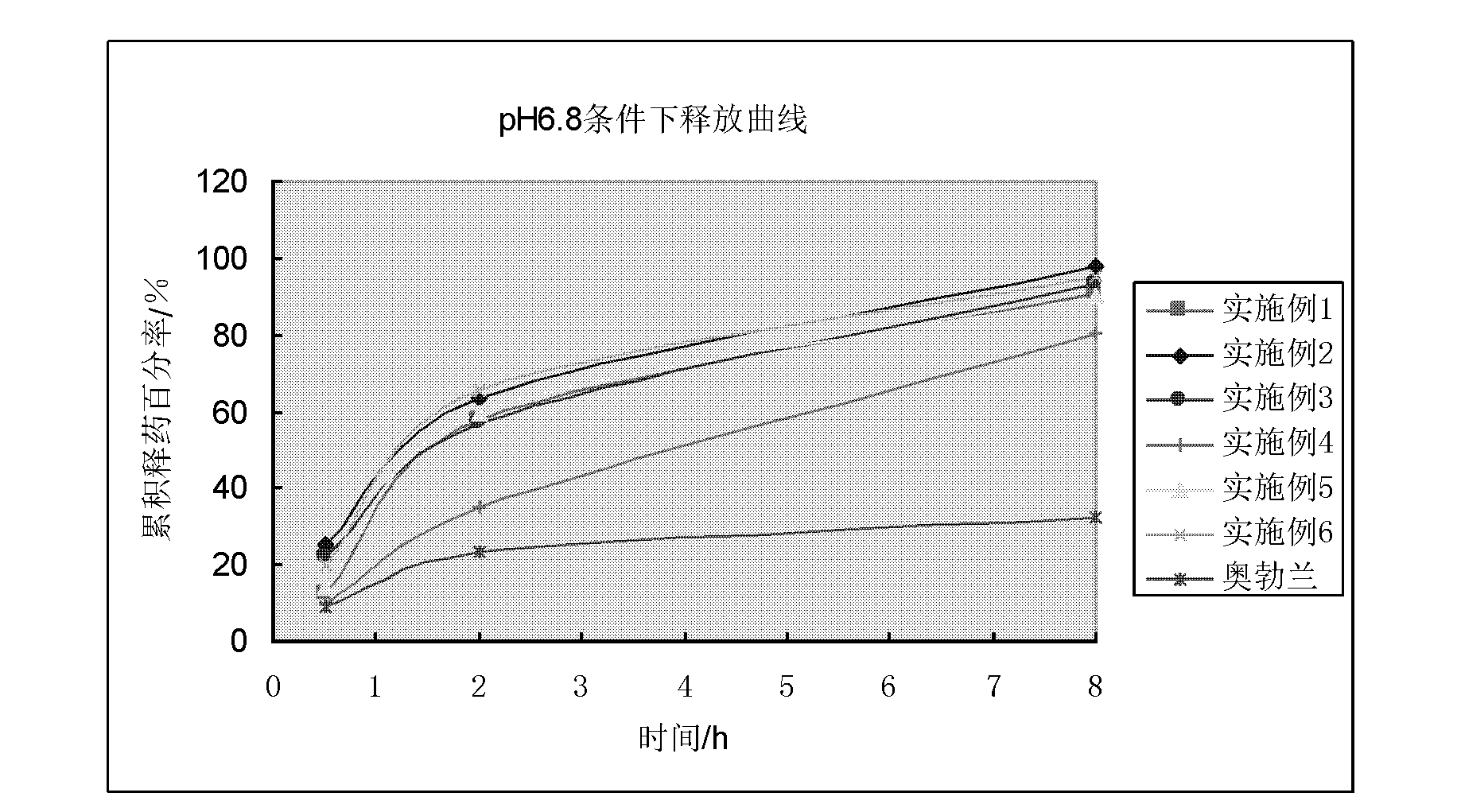
The invention discloses a vincamine sustained-release pellet preparation, which comprises main medicines of vincamine, acid blank pellet cores, an adhesive and a diluent in a weight ratio of (1-10): (10-20): (1-10): (20-200), wherein the acid blank pellet cores consist of blank pellet cores and an organic acid covered on the blank pellet cores. The invention provides the vincamine preparation which can be stably released under the pH of 1.5-7.4, the release pH dependency of the conventional vincamine sustained-release preparation is solved, the vincamine preparation can be released stably and slowly in stomach and intestinal tracts, and the pharmaceutical effect is lasting. Moreover, the invention also discloses a preparation method for the vincamine sustained-release pellet preparation. The preparation method is simple, good in repeatability, high in production efficiency and suitable for industrialized mass production. The prepared medicine-carrying pellets are smooth and round, are released uniformly under the pH of 1.5-7.4, and are high in stability.
Owner:上海智同医药科技有限公司
Semisynthetic production method of vinpocetine
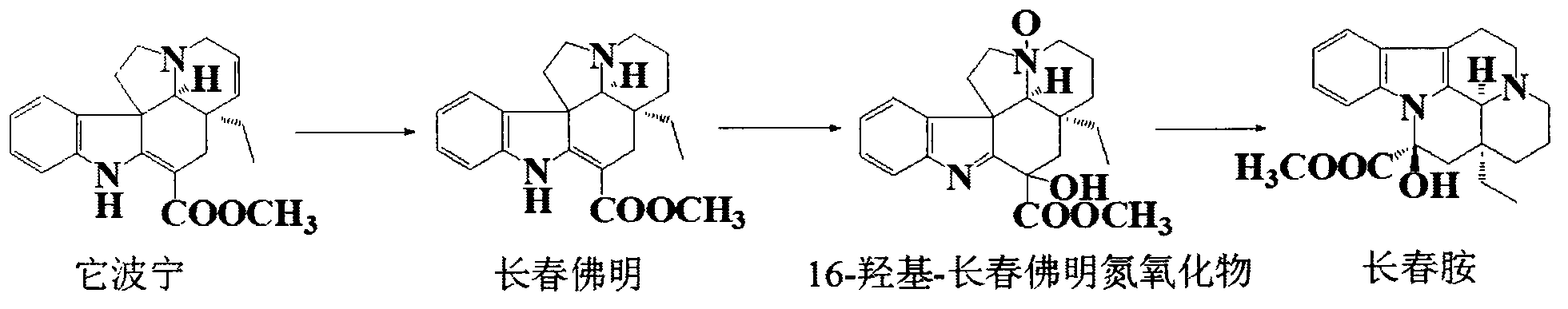
The invention discloses a semisynthetic production method of vinpocetine, a tabersonine hydrochloride is used as a raw material, Pd / C is used as a catalyst, tabersonine hydrogenation hydrochloride is obtained under normal temperature and pressure, monoperoxy maleic acid is prepared by use of hydrogen peroxide, vincamine is prepared by oxidation, reduction, transposition, settlement and washing then, and the vincamine is hydrolyzed, dehydrated and converted into vinpocetine. The semisynthetic production method of vinpocetine is simple in operation and high in safety, increases the yield, and reduces production cost, and a high purity product can be obtained without column chromatography.
Owner:广州普星药业有限公司
Slow-released vincamine capsule and its prepn process
InactiveCN1810242APromote blood circulationPromote energy metabolismOrganic active ingredientsNervous disorderSide effectSustained Release Capsule
The present invention provides one kind of slow-released vincamine capsule and its preparation process. The slow-released vincamine capsule contains slow-released vincamine micro pills comprising blank micro pill, active medicine layer and releasing coating. The slow-released vincamine capsule may have different micro pill combination for ideal slow-release speed for the patient to take 1-2 times a day. Compared with other vincamine preparations, the preparation of the present invention has the advantages of smooth blood medicine concentration, long maintenance time, less toxic side effect, high patient dependence in taking medicine, etc.
Owner:HONGGUAN BIO PHARMA CO LTD
Preparation method of vinpocetine
ActiveCN103288822AEnvironmentally friendlySimple and fast operationOrganic chemistryAlcoholPhysical chemistry
The invention relates to a preparation method of vinpocetine. The preparation method comprises the following steps of: (1) in the presence of mixed acid, reacting apovincaminic acid with absolute ethyl alcohol in a heating condition to obtain a reaction solution; (2) condensing the reaction solution at reduced pressure, diluting the concentration residue using water, adjusting the pH (Potential of Hydrogen) to 8-9, carrying out extraction by using an organic phase, washing the organic phase by using a saturated saline solution, dewatering and drying the organic phase, and carrying out filtration, reduced-pressure concentration and recrystallization, and drying to obtain the vinpocetine. The preparation method provided by the invention has the characteristics of environment protection and the like and is simple and convenient to operate, and the prepared vinpocetine has the advantages of high purity, controllable impurity, low cost and the like.
Owner:BEIJING AOHE DRUG RES INST +1
Refining method for vincamine
The invention relates to a refining method for vincamine and belongs to the field of refining of compounds. The method includes the following steps that 1, in existence of organic solvent, pulping is carried out on a vincamine rough product, then the vincamine rough product is cooled, crystallized and subjected to suction filtration, a filter cake is washed with the organic solvent, and a vincamine pulped product is obtained after drying; 2, the obtained vincamine pulped product is dissolved with the organic solvent and filtered, filtrate is subjected to vacuum concentration, and then concentrate is obtained; 3, organic solvent is added into the concentrate, the mixture is pulped, cooled, crystallized and subjected to suction filtration, a filter cake is washed with organic solvent, and a vincamine refined product is obtained after drying. The method has the advantages that the removal effect of N-oxide impurities is good, the reaction process is easy and convenient to implement, safety is high, cost is low, the yield of the obtained product is high, quality is good, and the vincamine is suitable for industrialized production.
Owner:NORTHEAST PHARMA GRP
Preparation method of high-purity vinpocetine
The invention discloses a preparation method of high-purity vinpocetine. The preparation method comprises the following steps: S1, preparing vincamine, wherein tabersonine is dissolved, a hydrogenation reaction is performed under the catalysis of palladium and carbon to obtain vincadifformine, the vincadifformine is oxidized to obtain a vincadifformine nitric oxide, and an intermediate vincamine is obtained from the vincadifformine nitric oxide reaction liquid under the catalysis of an acid; S2, dewatering the vincamine by using toluene as a solvent and adding a dewatering agent to obtain apovincamine; and S3, on the basis of the step S2, carrying out a transesterification reaction by taking ethanol as a solvent, using dichloromethane as a cosolvent and using sodium ethoxide / sodium methoxide as a catalyst to obtain a vinpocetine crude product, and re-crystallizing with ethanol to obtain vinpocetine. The process provided by the invention has the advantages of simple production operation, avoidance of high-toxicity reagents, less impurity of the obtained product, high production efficiency, simple and convent operation and environmental friendliness, wherein the purity of the productis more than 99.9%, the quality of the product is higher than the quality of the product obtained by the original research factory, and the medicinal requirements are met.
Owner:GUANGZHOU YIPINHONG PHARMA +4
Synthesis method of vinpocetine
The invention discloses a synthesis method of vinpocetine. The synthesis method comprises adding vincamine into a three-neck flask, adding toluene into the three-neck bottle, stirring the mixture in ice water bath, dropwise adding polyphosphoric acid, placing the mixture into oil bath after half an hour, installing a water separator and a condensation tube, pumping to be vacuum, replacingnitrogen for two times, and performing reaction for 8 hours at the temperature of 120 DEG C; drying solvent by distillation after reaction is finished, adding ethanol and water, simultaneously dropwise adding caustic soda solution, utilizing saturated potassium carbonate solution to regulate pH to be 12 when the pH is 9, separating out solid, filtering and performing vacuum drying to obtain apovincamine; and adding absolute ethyl alcohol into the three-neck bottle, stirring the mixture in the ice water bath for one hour, then adding caustic alcohol into the mixture, adding the apovincamine after half an hour's stirring, placing a reaction bottle in the oil bath, performing reaction for 12 hours at the temperature of 80 DEG C, then steaming out solvent, adding the solvent into hydrochloric acid, extracting the mixture through ethyl acetate, adjusting pH value of the water phase to 12, separating out solid, filtering and drying to obtain the vinpocetine. The synthesis method is few in reaction step, low in energy consumption and less in environment pollution, recrystallization is not needed, and the purity of the vinpocetine can reach 99.5%.
Owner:JIANGSU QINGJIANG PHARMA
Process for preparing vincamine by semisynthetic method
InactiveCN102276599BChoose cheapReduce the amount addedOrganic chemistryTabersonineHydrogenation reaction
The invention relates to a synthetic process for chemical medicines, in particular to a process for preparing vincamine by a semisynthetic method. At present, the process for preparing the vincamine is divided into a total synthetic method and the semisynthetic method, wherein the semisynthetic method has the problems of unstable and inflammable hydrogenation catalyst, large using amount, high hydrogenation pressure, insecurity in the preparation of monoperoxide maleic acid and the like. The process comprises the following steps of: preparing tabersonine vincadifformine hydrochloride, preparing the monoperoxide maleic acid, performing oxidizing reaction, performing reduction reaction and transposition reaction, precipitating ammonia and purifying products, wherein the low-cost and stable Pd / C hydrogenation catalyst is selected to reduce the pressure of the hydrogenation reaction; tabersonine sulfate is replaced by the tabersonine hydrochloride; the monoperoxide maleic acid is preparedby using low-concentration hydrogen peroxide; the reaction temperature is below 0 DEG C, and security coefficients are improved; the temperature of the oxidizing reaction is reduced, and the generation of isomers is reduced; purification and solvent recrystallization are performed by column chromatography; and the quality of the products reaches the pharmaceutical-grade standard.
Owner:ZHANGJIAKOU GERUI HIGH TECH
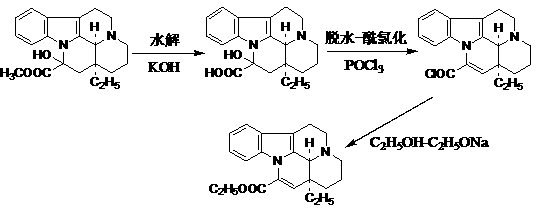
Method for efficiently synthesizing vinpocetine from vincamine
InactiveCN103304563AAccelerates the dehydration processImprove efficiencyOrganic chemistryPtru catalystVinpocetine
The invention relates to a method for efficiently synthesizing vinpocetine from vincamine. The method is used for preparing the vinpocetine by three steps of reactions including vincamine hydrolysis, dehydration-acylating chlorination and esterification. In the method, a catalyst adopted in the vincamine hydrolysis reaction is strong base KOH or NaOH, a catalyst for the vincamine acid dehydration-acylating chlorination reaction is phosphorus oxychloride, and a catalyst for the esterification reaction between the acyl chloride compound and ethanol is sodium ethoxide or triethylamine. According to the method provided by the invention, Lewis acid phosphorus oxychloride is used as a catalysis-dehydration agent, the dehydration and acylating chlorination are finished by one-step coupling, and then the vinpocetine is prepared through the esterification reaction between the acyl chloride compound and ethanol; additional dehydration treatment is not required in the reaction, the efficiency of the dehydration and esterification reaction is high, and the product yield is over 90%; and the method is simple and convenient to operate and has better prospects in industrial application and development.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
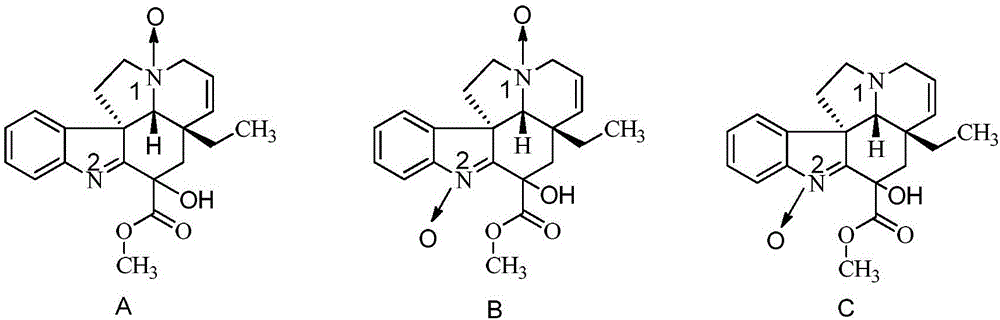
Preparation methods for chiral impurities of vincamine
The invention relates to preparation methods for chiral impurities of vincamine. Through oriented synthesis method and the later column chromatography separation, high purity product of seven chiral impurities can be obtained, and the preparation methods can be used for the qualitative and quantitative analysis of impurities in the production of vincamine, so that the separation and confirmation of the seven chiral impurities in vincamine drugs can be realized, and the drug standard of the vincamine can be enhanced, and therefore, the vincamine with high purity can be obtained. Compared with existing preparation methods for the vincamine, the preparation methods relate to the preparation of seven chiral isomers, and are more complete and simpler in operation.
Owner:北京康派森医药科技有限公司
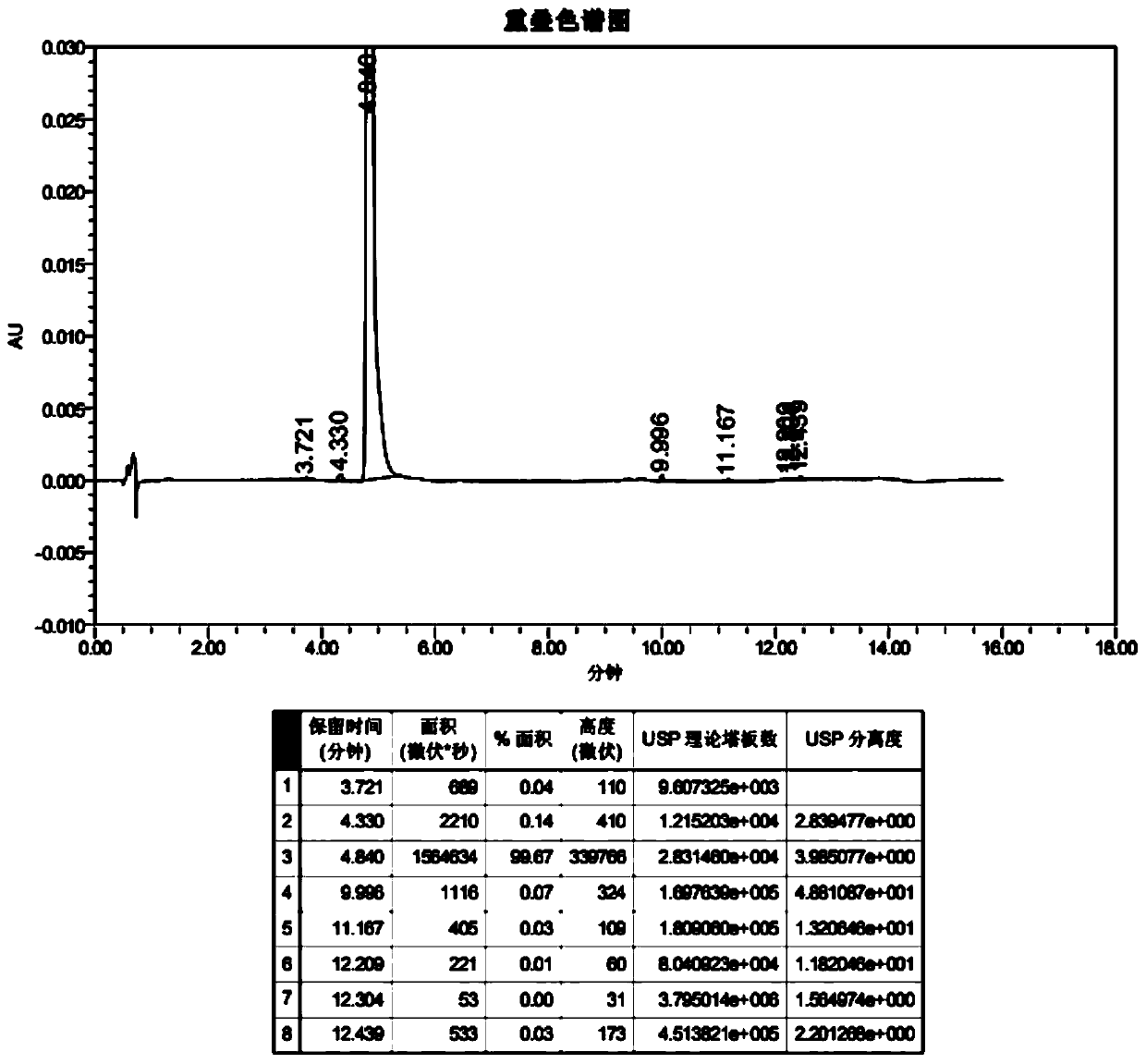
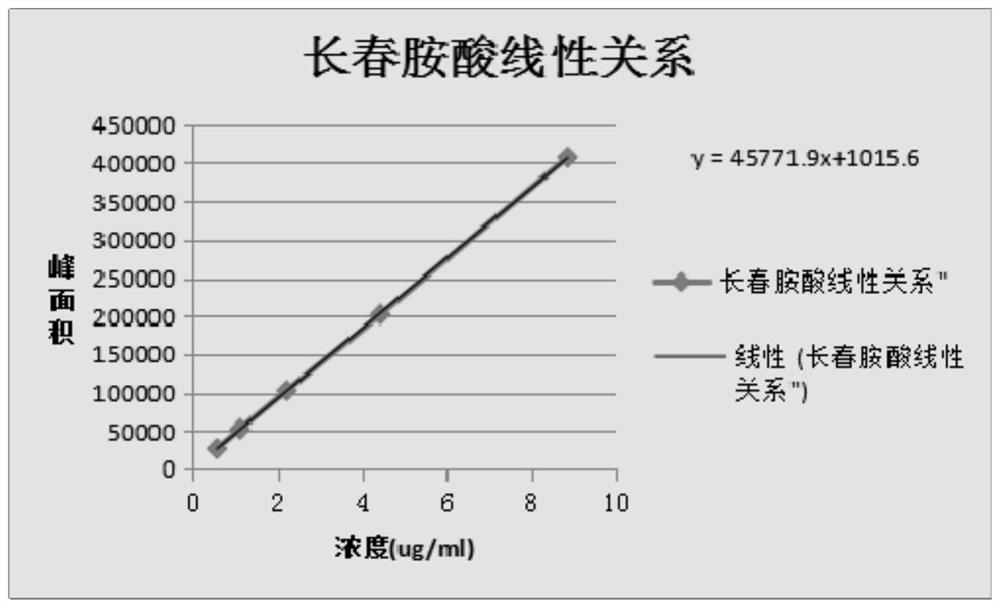
Method for measuring vincamine related substances through high performance liquid chromatography (HPLC)
The invention relates to a method for measuring vincamine related substances through high performance liquid chromatography (HPLC). The method is characterized in that an octadecyl bonded silica chromatographic column is selected, an acetonitrile-0.1M ammonium carbonate solution (7:3) serves as a mobile phase for performing isocratic elution, a detector is an ultraviolet detector, and the detection wavelength is 260nm. The method has the characteristics of high separation degree, simplicity, rapidness, high specificity, high sensitivity and the like and can be used for detection and quality control of possibly generated related substances comprising intermediates, byproducts and other organic impurities in the vincamine preparation process.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
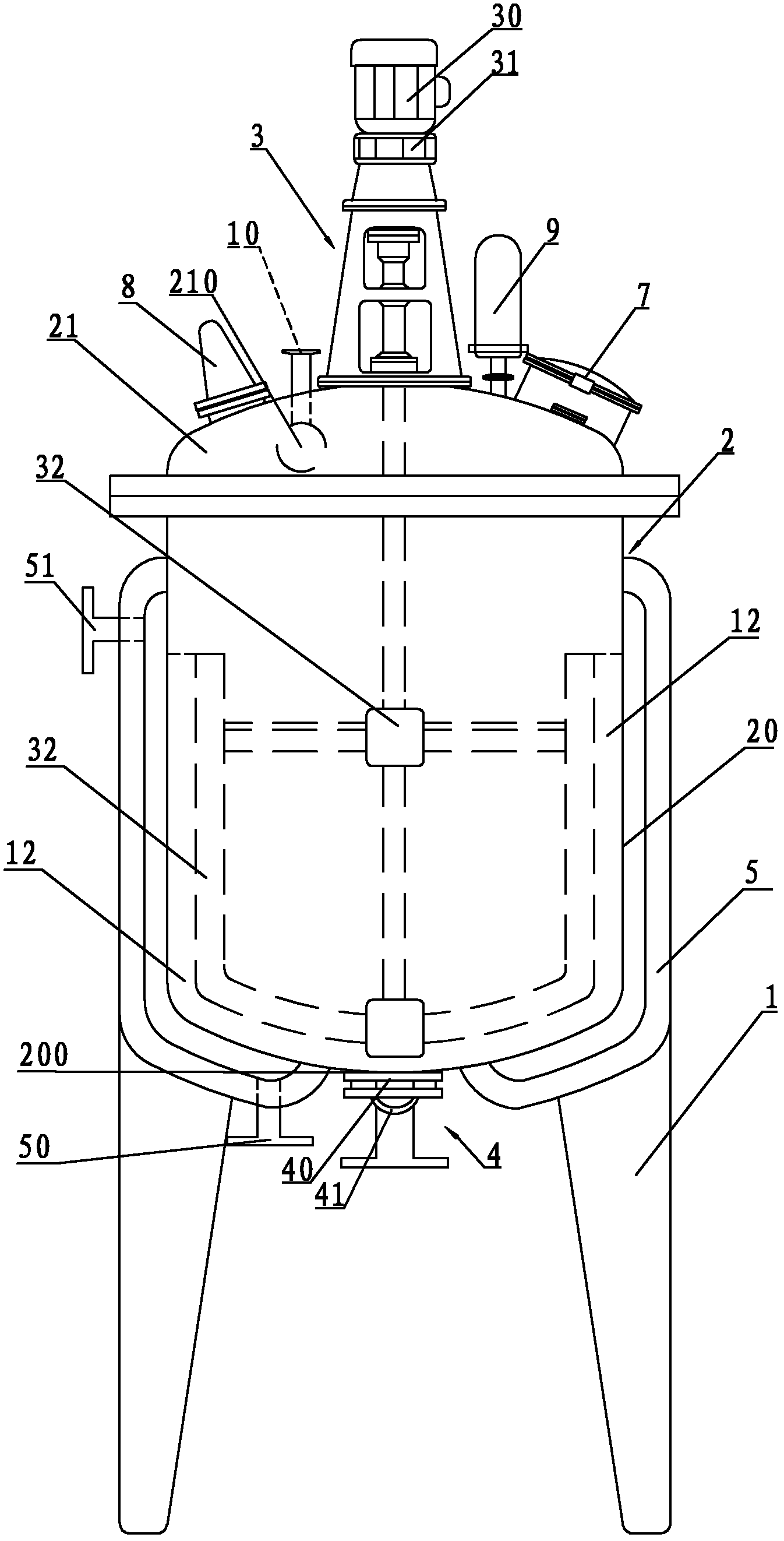
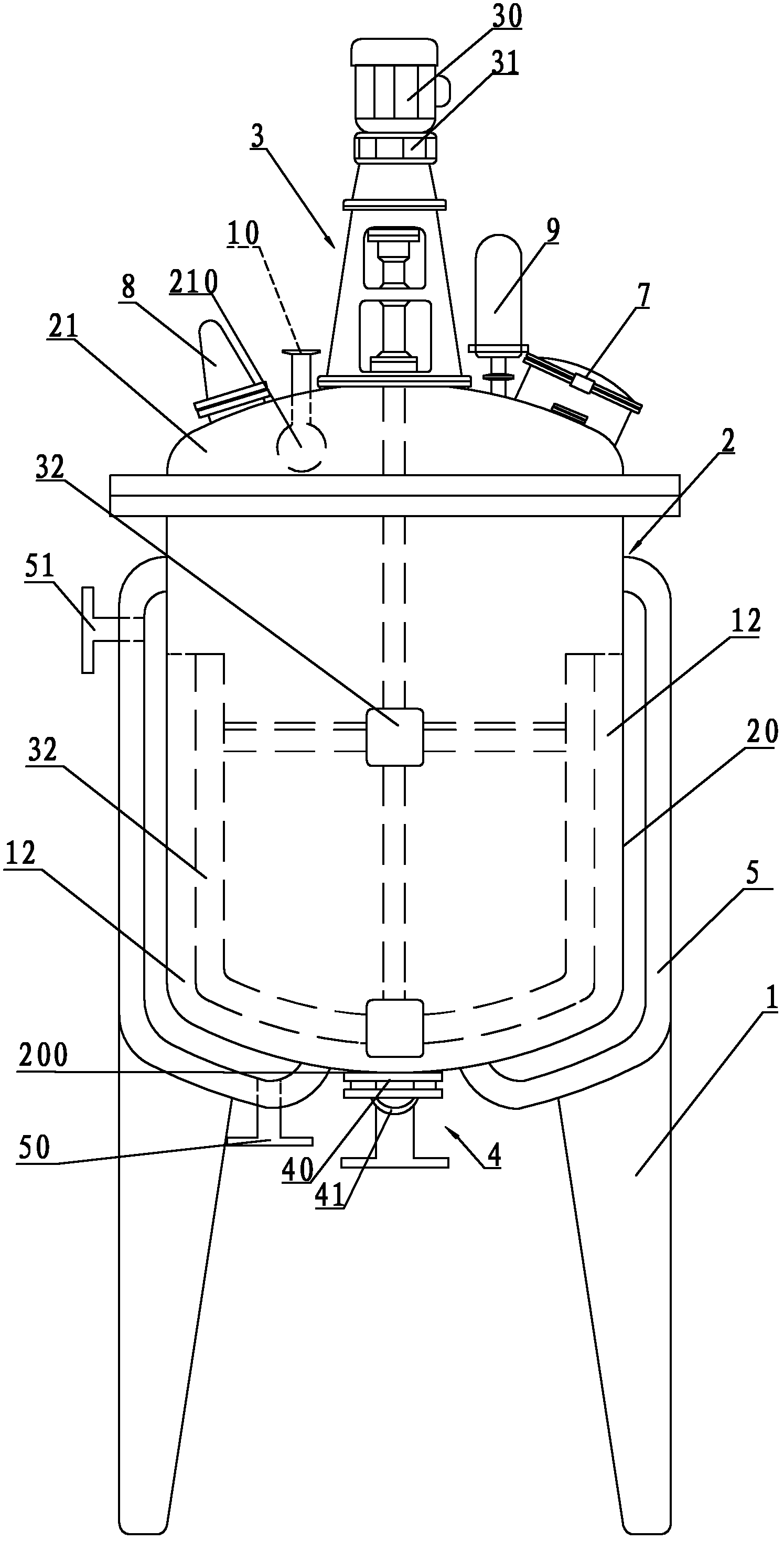
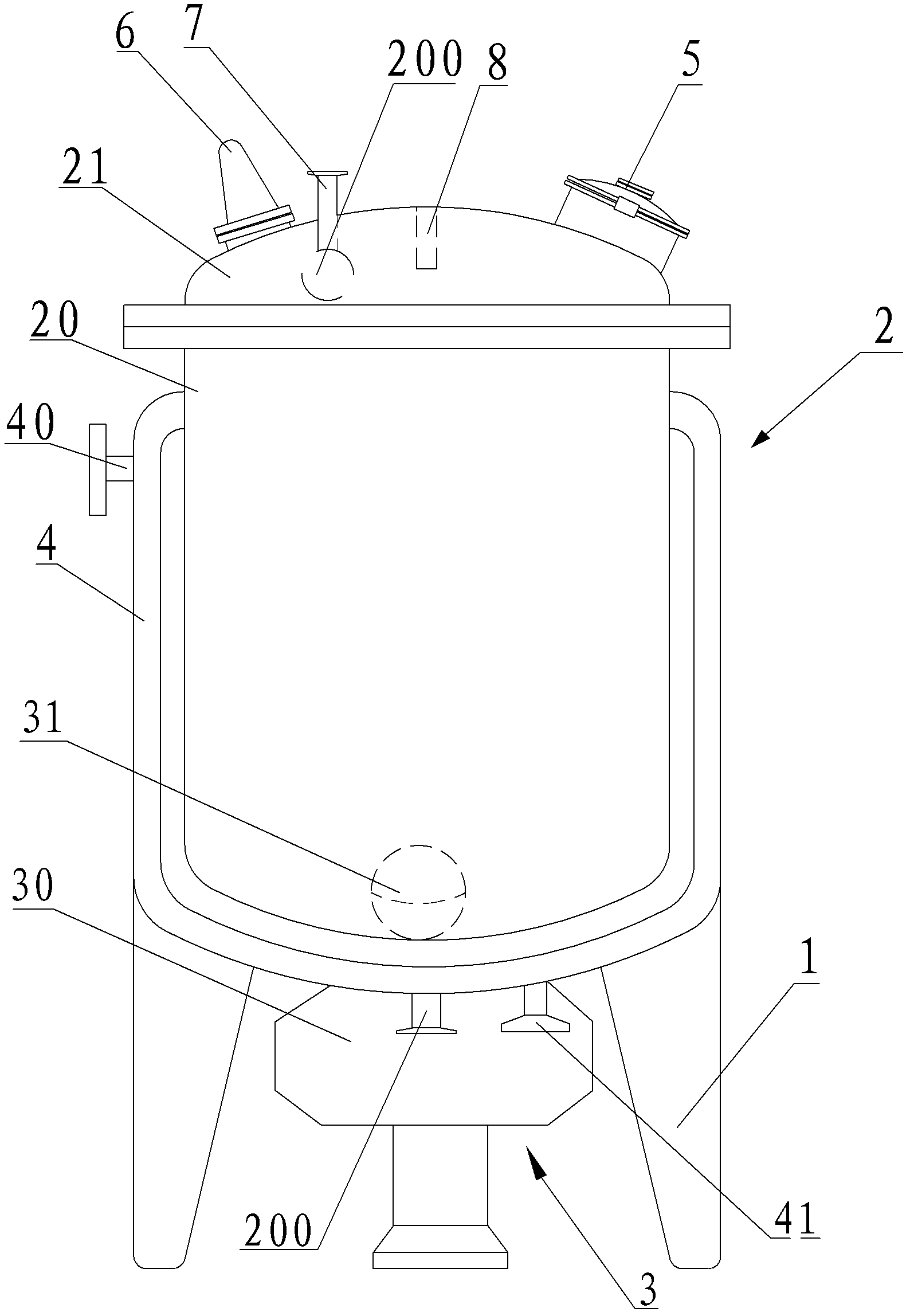
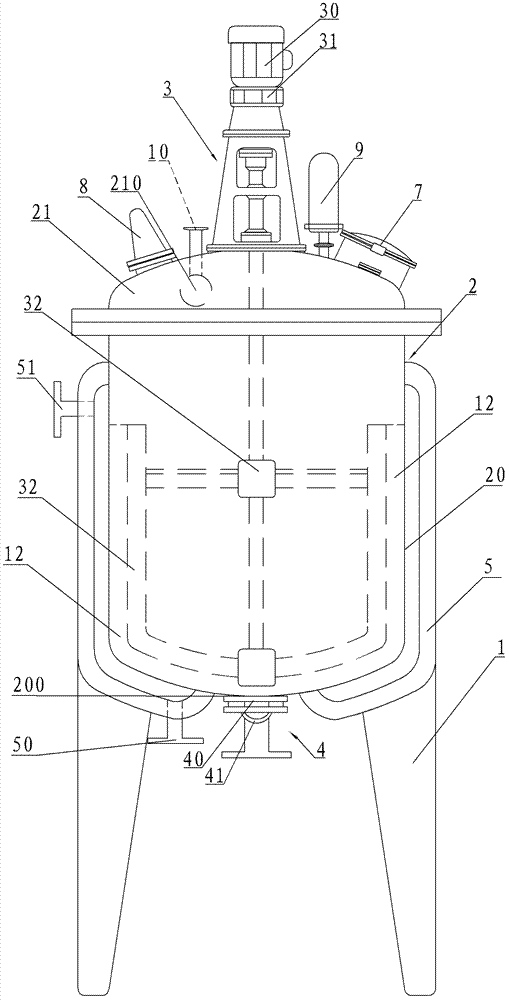
Crystallizing tank used for vincamine
ActiveCN102350083AUniform particle sizeOrganic chemistrySolution crystallizationEngineeringMechanical engineering
The invention relates to a crystallizing tank used for vincamine, comprising a tank rack, a tank and a stirring device, wherein the tank and the stirring device are arranged on the tank rack; the tank comprises a tank body and a tank cover connected with the tank body in a sealing mode; the bottom of the tank body is provided with a discharge hole; a dump valve is arranged on the discharge hole; the tank cover is provided with a feeding hole; the circumstance of the tank body is also provided with a jacket with a cooling water inlet and a cooling water outlet; and the discharge valve comprises a valve plate and a filter plate for blocking the discharge hole. Especially, the thickness of the discharge hole is same with that of the bottom of the tank body; and the valve plate is used for blocking an annular continuous arc-shaped surface formed by parts of upper surface of the discharge hole and the inner surface of the bottom of the tank. The crystallizing tank is characterized in that the valve plate of the discharge valve is used for blocking the annular continuous arc-shaped surface formed by parts of upper surface of the discharge hole and the inner surface of the bottom of the tank without any stirring dead corner, and fine-grain crystallization with even particle diameter can be obtained.
Owner:舞阳威森生物医药有限公司
Special reaction kettle for synthesizing vincamine intermediate vinblastine formin
InactiveCN102423677AImprove sealingReduce energy consumptionOrganic chemistryMixersMaterials scienceVincamine
The invention relates to a special reaction kettle for synthesizing vincamine intermediate vinblastine forming, which comprises a kettle rack, a kettle erected on the kettle rack and a stirring device, the kettle comprises a kettle body and a kettle cover which is hermetically connected with the kettle body, a material outlet is provided at the lower part of the kettle body, a material inlet is provided on the kettle cover, insulation jackets of the steam inlet and condensed water outlet are provided outside the kettle body, particularly, the stirring device is a magnetic stirring device, and comprises a magnetic device arranged at the lower part of the kettle body and a magnetic stir bar which is in the kettle body and rotated by driving of the magnetic device. The reaction kettle of the invention uses the magnetic stirring device to replace traditional mechanical stirring, the reaction kettle has good seal performance and can satisfy the sealing requirement of synthesizing the reaction kettle by catalytic hydrogenation. Simultaneously, the magnetic stirring device can provide the required stirring force for the catalytic hydrogenation reaction, compared with the mechanical stirring mode, the reaction kettle of the present invention has lower energy consumption and is in favor of reducing the production cost.
Owner:江苏斯威森生物医药工程研究中心有限公司
Preparation method of vincamine
ActiveCN112679493AFix security issuesShort reaction timeOrganic chemistryChemical/physical/physico-chemical processesSodium bicarbonateTabersonine
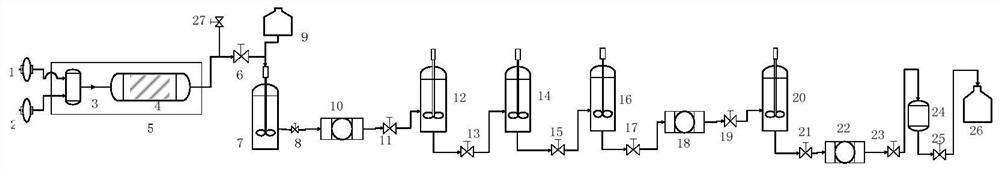
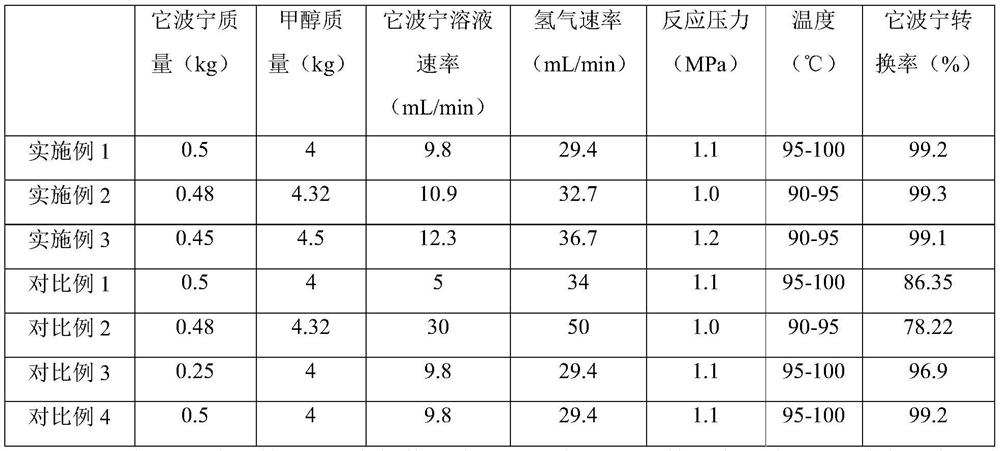
The invention provides a green preparation method of vincamine, which comprises the following steps: (1) reduction: introducing a tabersonine methanol solution and hydrogen into a micro-channel reactor through a charging pump to react, and filtering, concentrating and extracting the reaction solution twice after the reaction is finished, thereby obtaining an extracting solution; (2) oxidation: catalyzing an organic phase in the extraction liquid in the step (1) by using peroxy acid to carry out oxidation reaction, neutralizing by using a sodium bicarbonate solution after the reaction is finished, extracting and concentrating; and (3) rearrangement: dissolving the concentrated solid obtained in the step (2) with ethanol, rearranging in the presence of a catalyst, adjusting the pH value after the reaction is completed, filtering, washing and crystallizing the filter cake, and separating out the solid, namely vincamine. The preparation method is safe, environmentally friendly and capable of achieving continuous production, the product purity is 99% or above, automatic equipment can be adopted for continuous production, and the industrial production efficiency is improved.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
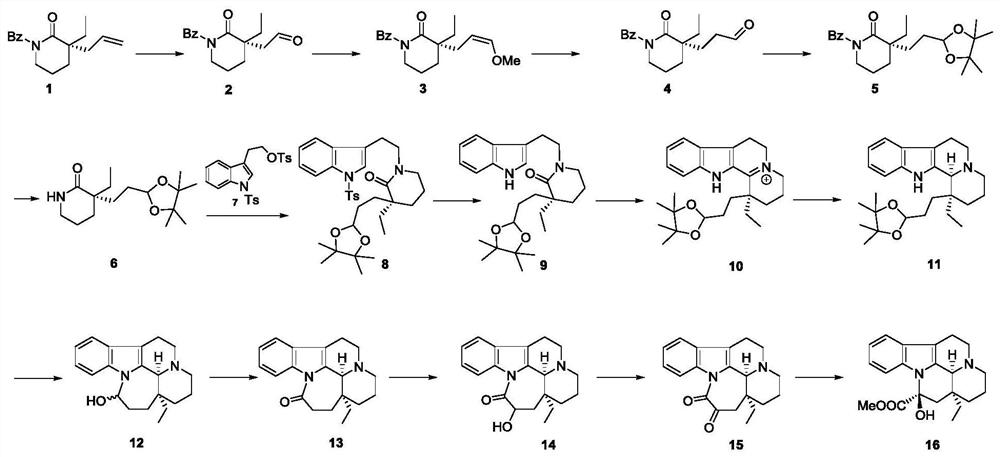
Intermediate, preparation method and application of intermediate in synthesis of vincamine
ActiveCN113321643AImprove responseHigh yieldOrganic chemistry methodsBulk chemical productionTryptopholDrugs synthesis
The invention relates to the technical field of chemical drug synthesis, and discloses an intermediate, a preparation method and application of the intermediate in synthesis of vincamine. A modular synthesis strategy is adopted, and a compound 1 with a D ring structure and a C20 quaternary carbon center and a tryptophol derivative 7 (namely the compound 7) with an indole ring are adopted as synthesis blocks for synthesis. The synthesis method is efficient; each step of the synthesis route is simple in reaction; the used reagent and solvent are cheap and easy to obtain; the operation is simple and convenient; the yield is high; and large-scale production is easy.
Owner:SICHUAN UNIV
A kind of preparation method of vinpocetine
The invention provides a preparation method for vinpocetine. The method comprises the following steps: with vincamine as a raw material and triethyl formate as an esterification reagent, subjecting vincamine and triethyl formate to a one-step reaction under the action of Lewis acid so as to obtain a crude vinpocetine product; and then carrying out washing with alkali lye, decoloring in alcohol and recrystallization so as to prepare a refined vinpocetine product. Compared with the prior art, the preparation method provided by the invention has the advantages of short process flow, simple operation and suitability for industrial production, and the prepared refined vinpocetine product has yield of more than 89%, a melting point of 148 to 151 DEG C, HPLC of greater than 99%, a whitish color, good quality and low preparation cost.
Owner:HUNAN KEYUAN BIO PRODS
Pervone vincamine injection and preparation process thereof
InactiveCN101721361BUnique dosage formEasy to useOrganic active ingredientsPharmaceutical delivery mechanismSolubilityAlkaloid
The invention provides a pervone vincamine injection and a preparation process thereof, relating to the technical field of pervone vincamine injections and preparation processes thereof. The injection is a solution type injection or frozen powder injection, and the solution type injection is divided into a water injection and a drug-carried type infusion solution, wherein the contained active constituent is the pervone vincamine, and the pervone vincamine can exit in the form of soluble salt. The invention has the advantages that compared with the prior art, the injection has the characteristics of unique formulation, convenient use, fast curative effect, safety, reliability and the like, and is applied to the clinical treatment, in particular to the intravenous injection for some patients who can not swallow drugs due to all kinds of reasons or can not carry out the oral administration due to the gastrointestinal tract dysfunction; and the preparation process is simple, convenient and economical, is easy for industrial production, has high economic and social benefits, and solves the key problem that the solubility of the pervone vincamine is lower in water because the pervone vincamine belongs to alkaloids in tryptophane system. It is especially significant to develop a pervone vincamine aqueous solution applied to the clinical treatment.
Owner:李荣立
A kind of semi-synthetic method prepares the production technology of vincamine
Owner:ZHANG JIA GANG VINSCE BIO PHARM
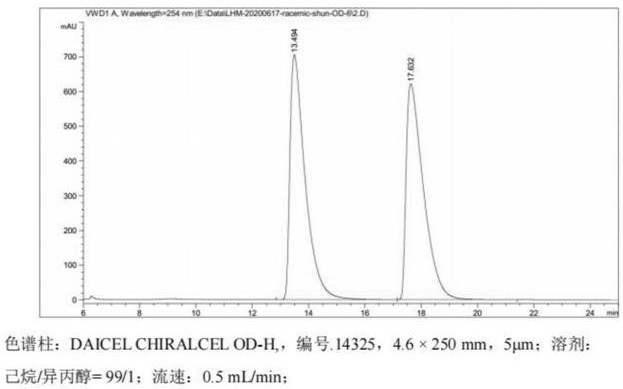
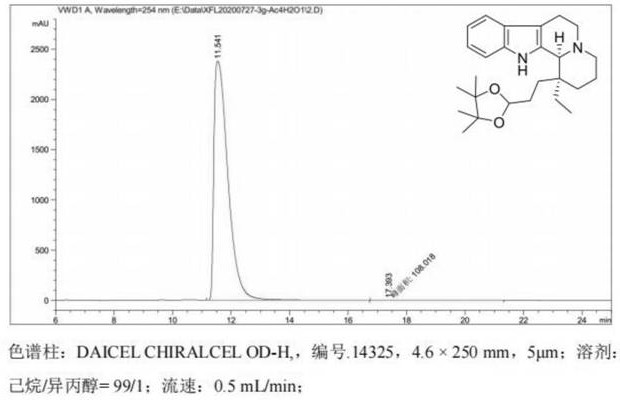
A kind of detection method of isomer impurity in vincamine
The invention discloses a method for detecting isomer impurities in vincamine, which uses high-performance liquid chromatography for detection, uses a CHIRALCEL OD-RH chiral chromatographic column as a chromatographic column, and uses a mixed solution of borax-boric acid buffer and acetonitrile Carry out isocratic elution for mobile phase, record chromatogram, calculate the content of impurity A in vincamine according to the peak area in chromatogram. The method of the invention can quantitatively determine the content of the isomer impurity A in the vincamine raw material, has the advantages of high accuracy and high sensitivity, and is beneficial to the quality control of the vincamine raw material.
Owner:开封康诺药业有限公司 +1
A kind of detection method of related substances vincristine and apo-vinblastine in injection
ActiveCN112649522BEasy to separateSensitive detection methodComponent separationFluid phaseVinpocetine
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Crystallizing tank used for vincamine
InactiveCN102350083BUniform particle sizeOrganic chemistrySolution crystallizationEngineeringMechanical engineering
The invention relates to a crystallizing tank used for vincamine, comprising a tank rack, a tank and a stirring device, wherein the tank and the stirring device are arranged on the tank rack; the tank comprises a tank body and a tank cover connected with the tank body in a sealing mode; the bottom of the tank body is provided with a discharge hole; a dump valve is arranged on the discharge hole; the tank cover is provided with a feeding hole; the circumstance of the tank body is also provided with a jacket with a cooling water inlet and a cooling water outlet; and the discharge valve comprises a valve plate and a filter plate for blocking the discharge hole. Especially, the thickness of the discharge hole is same with that of the bottom of the tank body; and the valve plate is used for blocking an annular continuous arc-shaped surface formed by parts of upper surface of the discharge hole and the inner surface of the bottom of the tank. The crystallizing tank is characterized in that the valve plate of the discharge valve is used for blocking the annular continuous arc-shaped surface formed by parts of upper surface of the discharge hole and the inner surface of the bottom of the tank without any stirring dead corner, and fine-grain crystallization with even particle diameter can be obtained.
Owner:舞阳威森生物医药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com