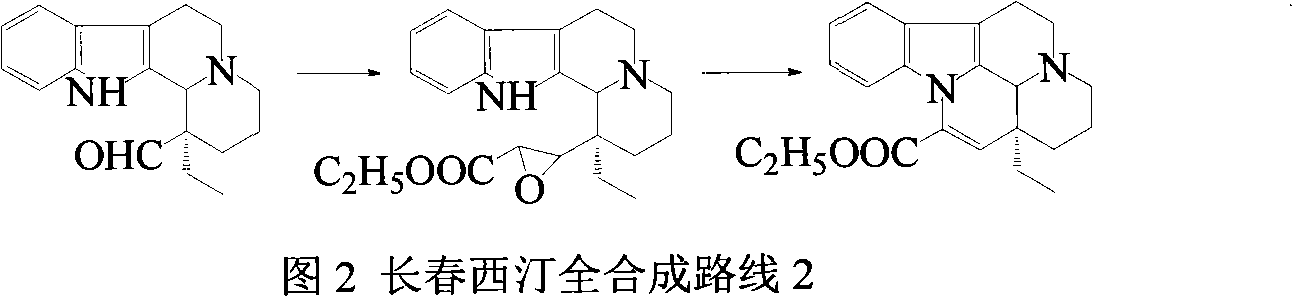
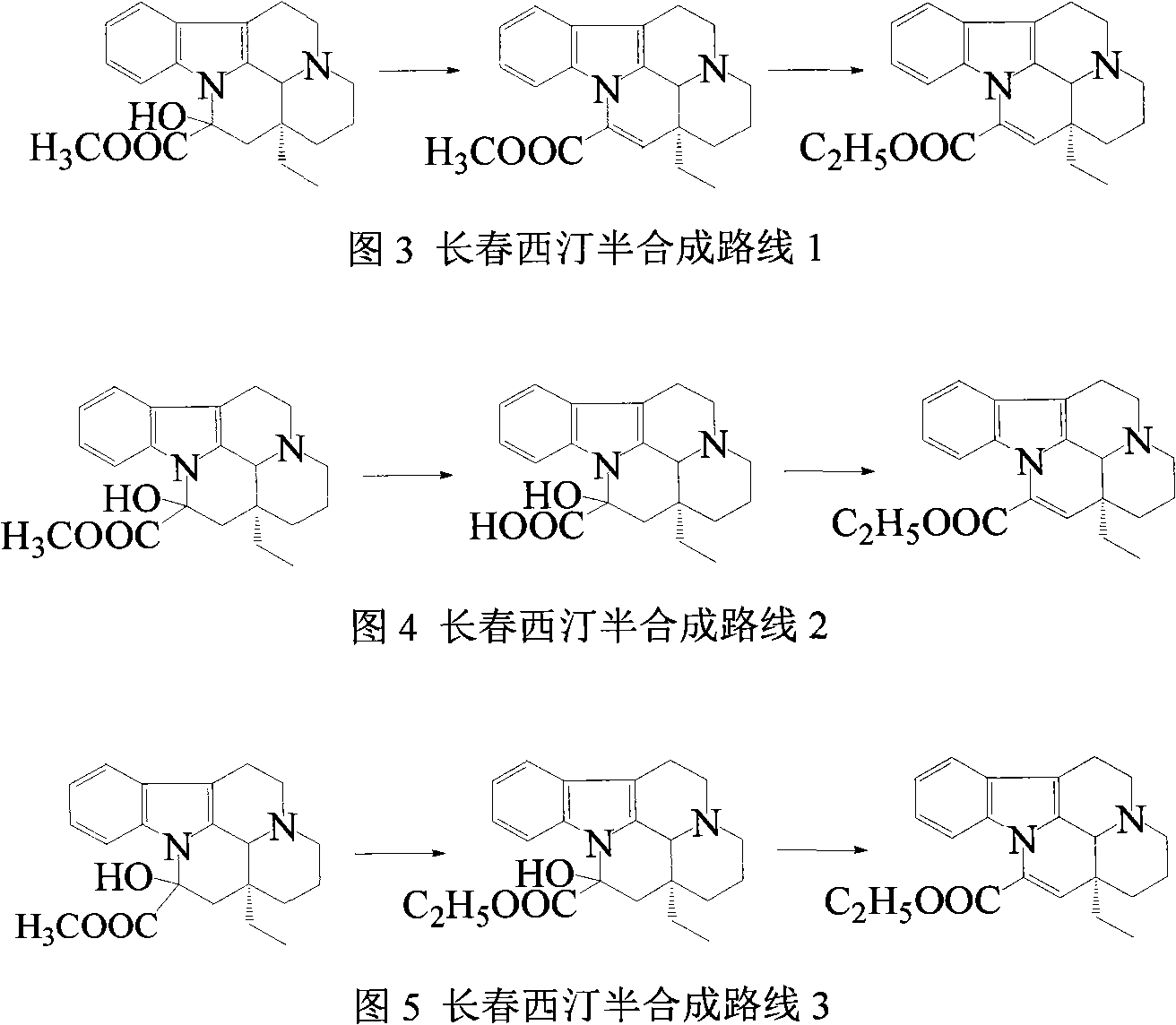
Semi-synthesis of vinpocetine through one kettle way and preparation of water-soluble vinpocetine salt
A semi-synthetic technology of vinpocetine, which is applied in the field of semi-synthesis of indole alkaloids, can solve the problems of lengthy process, low water solubility, unfavorable bioavailability, etc., and achieve simple and controllable process, high atom economy sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The synthetic technique 1 of embodiment 1 Vinpocetine
[0026] Weigh 5kg of vincamine with a purity greater than 99.0% in a 100L enamel reaction kettle, add 25L of absolute ethanol, start stirring, add 4L of concentrated phosphoric acid and 1kg of anhydrous potassium hydrogensulfate dropwise, and heat to 90°C for 1 hour. Then add 15L of glacial acetic acid (or formic acid or propionic acid), and rapidly raise the temperature to 100-105° C., and react for another 5 hours. The reaction was tracked by thin-layer chromatography until the reaction of vincamine was complete, the heating was stopped, and the temperature was lowered to room temperature. While stirring, 1% NaOH solution was added dropwise to adjust the pH value to 8, and then extracted twice with 15 L of dichloroethane, the organic phases were combined, and 1 kg of anhydrous sodium sulfate was added to dry overnight. Dichloromethane was concentrated and recovered, and the solid was crystallized from ethyl aceta...
Embodiment 2
[0027] The synthetic technique 2 of embodiment 2 Vinpocetine
[0028] Weigh 5kg of vincamine with a purity greater than 99.0% into a 100L enamel reaction kettle, add 30L of absolute ethanol, start stirring, add 4kg of p-toluenesulfonic acid and 1kg of anhydrous sodium bisulfate in batches, and heat to 90°C for 1 hour. Then 10L of trifluoroacetic acid was added, and the temperature was rapidly raised to 100-105°C, and then reacted for another 3 hours. The reaction was tracked by thin-layer chromatography until the reaction of vincamine was complete, the heating was stopped, and the temperature was lowered to room temperature. While stirring, 1% NaOH solution was added dropwise to adjust the pH value to 8, and then extracted twice with 15 L of dichloroethane, the organic phases were combined, and 1 kg of anhydrous sodium sulfate was added to dry overnight. Dichloromethane was concentrated and recovered, and the solid was crystallized from ethyl acetate to obtain coarse crystals...
Embodiment 3
[0029] Embodiment 3 Vinpocetine sulfate preparation
[0030] Weigh 10 g of Vinpocetine with a purity greater than 99.5%, disperse and dissolve it in 100 mL of absolute ethanol, stir and heat to 50°C, add 10% sulfuric acid ethanol solution dropwise, Vinpocetine gradually dissolves, add sulfuric acid ethanol solution dropwise to pH 4.5 . Concentrate under reduced pressure to remove about 60 mL of ethanol, and then cool to below 10°C to precipitate coarse crystals of vinpocetine sulfate, filter and wash with cooled absolute ethanol. Obtain 8.5kg of pure vinpocetine sulfate, the yield is 85.0%, and the purity is greater than 99.5%.
[0031] Embodiment 3 Vinpocetine maleate preparation
[0032] Take by weighing 10 g of Vinpocetine with a purity greater than 99.5%, disperse and dissolve it in 100 mL of absolute ethanol, stir and heat to 80° C., add dropwise 10% maleic acid dilute ethanol solution, Vinpocetine gradually dissolves, add dropwise sulfuric acid ethanol solution to Sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com