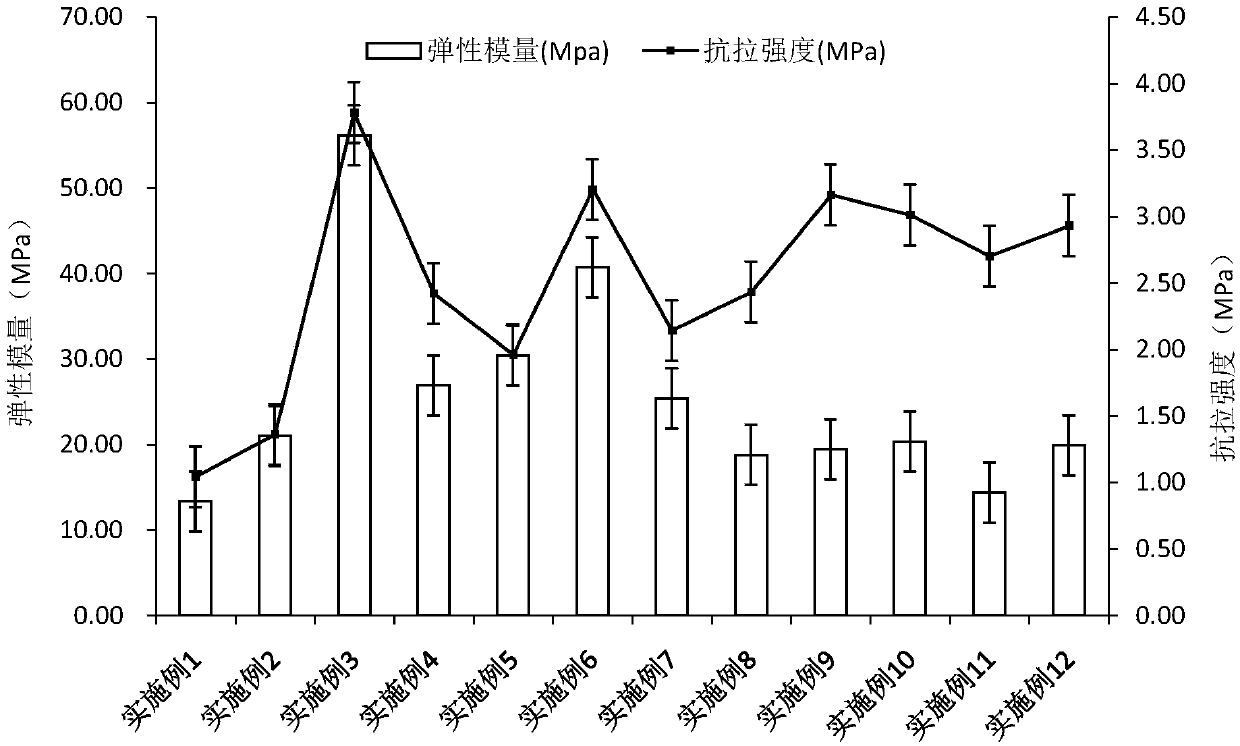
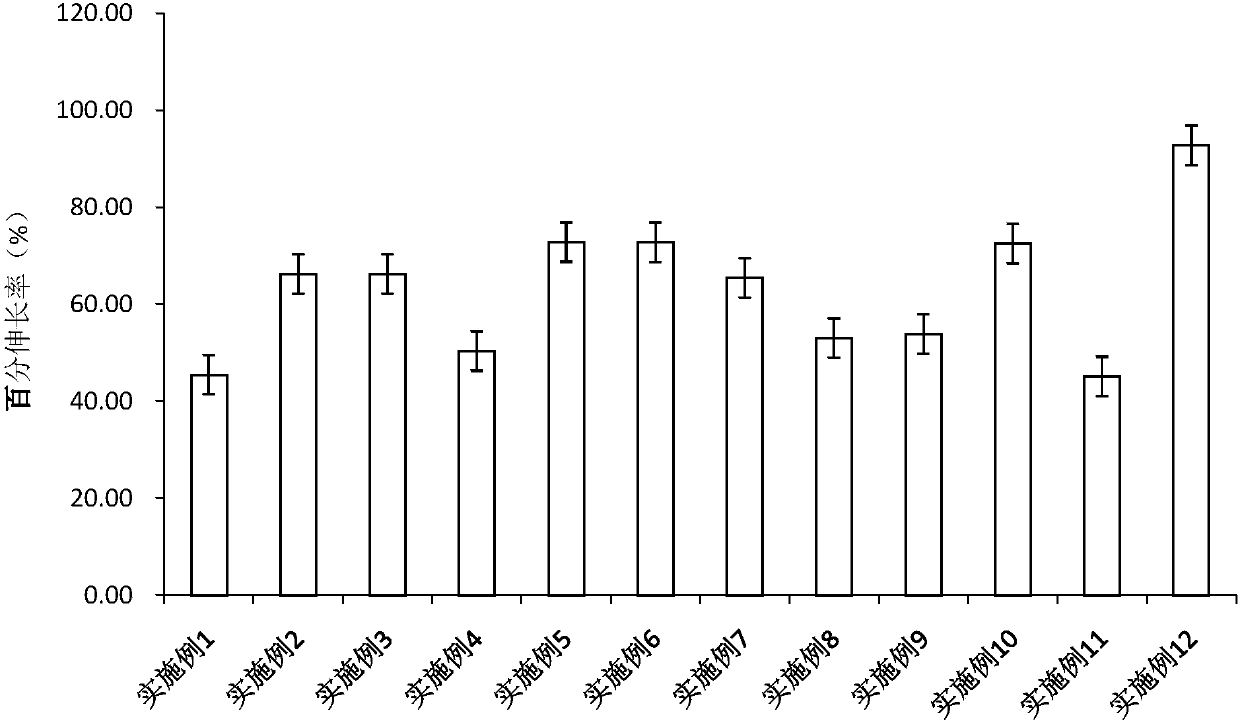
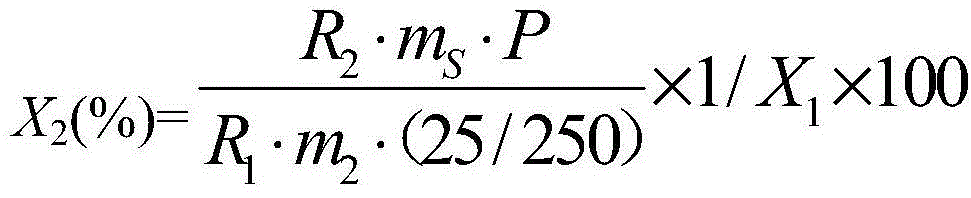Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36results about How to "Good disintegration time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Effervescent and effervescent-dispersion compositions for medicaments and methods of use thereof
InactiveUS20050074489A1Need can be addressedEnhance sensory effectPill deliveryPharmacyPharmaceutical drug
Owner:UNION SPRINGS PHARMA
Glyphosate dry suspension and preparation method and application thereof
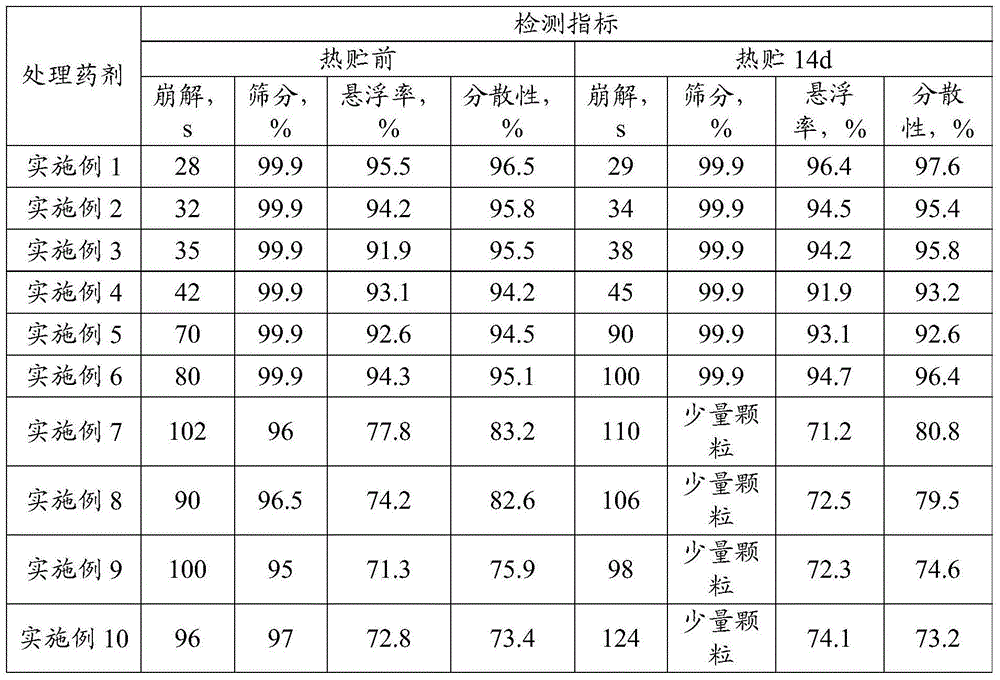
The invention provides a glyphosate dry suspension and a preparation method and application thereof. The glyphosate dry suspension is prepared by using the following components in percentage by mass: 30-85 percent of glyphosate, 2-20 percent of wetting agent, 1-20 percent of dispersing agent, 0.1-5 percent of defoaming agent, 1-5 percent of bonding agent, 1-10 percent of disintegrating agent and balance of carrier, wherein the disintegrating agent comprises one or a plurality of urea, sodium chloride, sulfate, carbonate and sodium carboxymethylcellulose. The glyphosate dry suspension provided by the invention has relatively excellent stability and disintegrating property through the proportioning of the components with the contents; the content of effective components is higher; the control efficiency is higher; according to experimental results, after 14-day heated storage, no particles occur in more than 60 percent of the glyphosate dry suspension after screening; the suspension rate highly reaches 96.4 percent; the disintegrating time is 29-124s; the control efficiency is 88-99.05 percent after 14 days after application; the control efficiency is 88-99.72 percent after 28 days after application.
Owner:SHANDONG WEIFANG RAINBOW CHEM
Hydroxyalkylcellulose microparticles
ActiveUS20120232167A1High strengthImprove suppression propertiesBiocidePill deliveryShock waveVolume average
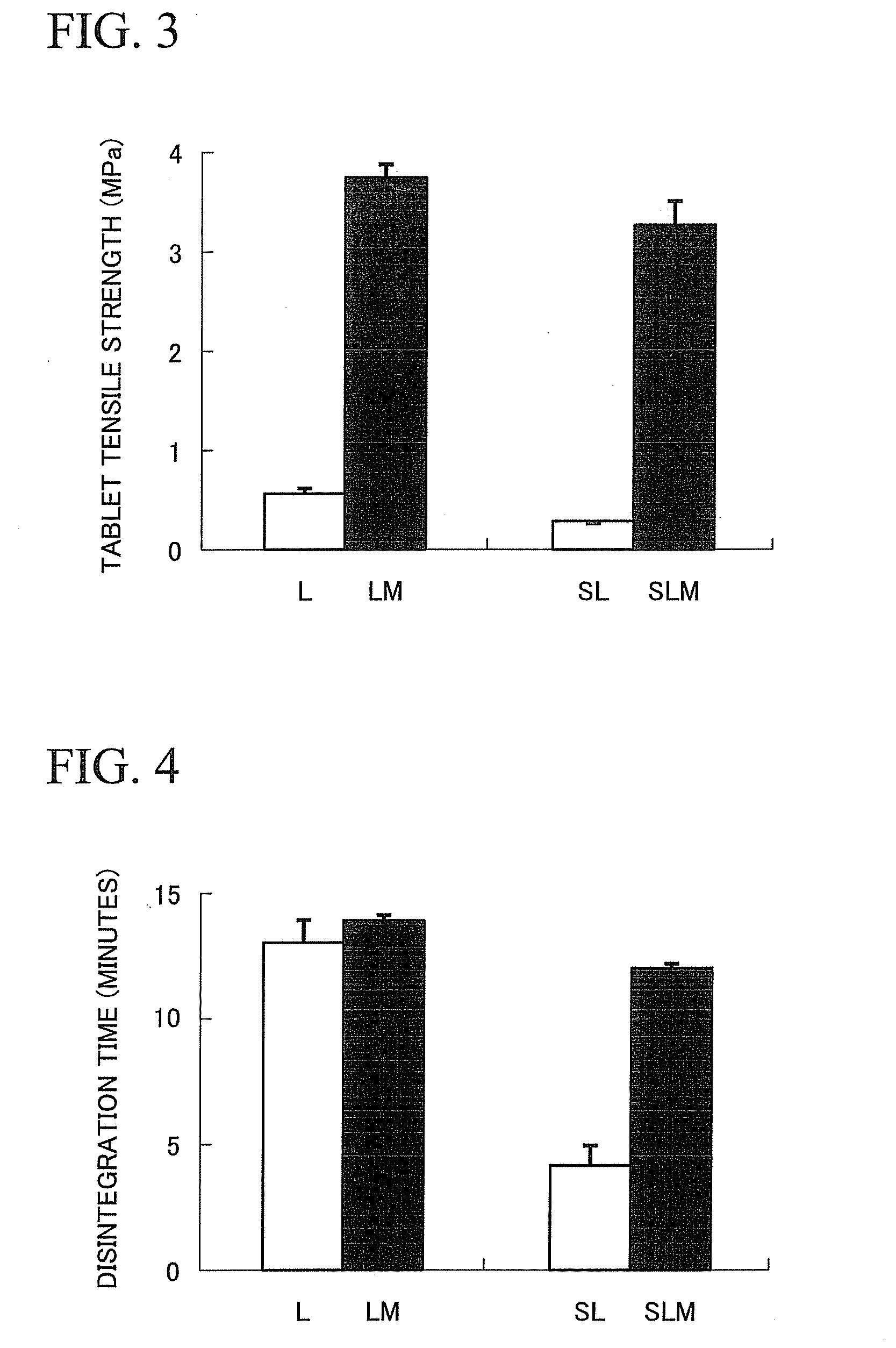
Provided is a method of producing hydroxyalkylcellulose microparticles, the method including generating a pulse shock wave, and supplying a hydroxyalkylcellulose aqueous solution to the pulse shock wave generation region, thereby crushing and drying the hydroxyalkylcellulose aqueous solution. According to the production method, hydroxyalkylcellulose microparticles having a volume-average particle size of at least 0.1 μm but less than 15 μm are obtained. By mixing the hydroxyalkylcellulose microparticles with a principal agent and subjecting the resulting mixture to a tablet compression, a solid preparation having excellent tensile strength and disintegration properties can be obtained.
Owner:NIPPON SODA CO LTD +1
Deep processing preparation and quality control method for propolis compound
ActiveCN106511396ASmooth dryingImprove the mixAntinoxious agentsUnknown materialsBiotechnologySoftgel
The invention relates to the field of propolis product processing, and particularly relates to a deep processing preparation method for a propolis powder, and a product thereof. The preparation method comprises the following steps: hardening and crushing a propolis raw material to obtain a propolis raw material powder; carrying out alcohol extraction on the propolis raw material powder to obtain a thick propolis extract paste; and mixing the thick propolis extract paste with amino acid and auxiliary materials, grinding, carrying out freeze drying, and crushing to obtain the propolis powder. According to the preparation method disclosed by the invention, the mixing condition of propolis and the auxiliary materials is improved through adding the amino acid into the thick propolis extract paste, thus the propolis can be uniformly mixed with the auxiliary materials, and then the propolis can be smoothly dried to obtain the propolis powder with good powder characteristic; and meanwhile, the propolis powder prepared by the process is suitable for propolis capsules (comprising hard capsules and soft capsules), propolis tablets and the like, and is capable of remarkably improving the problem of disintegration of propolis products.
Owner:BY HEALTH CO LTD
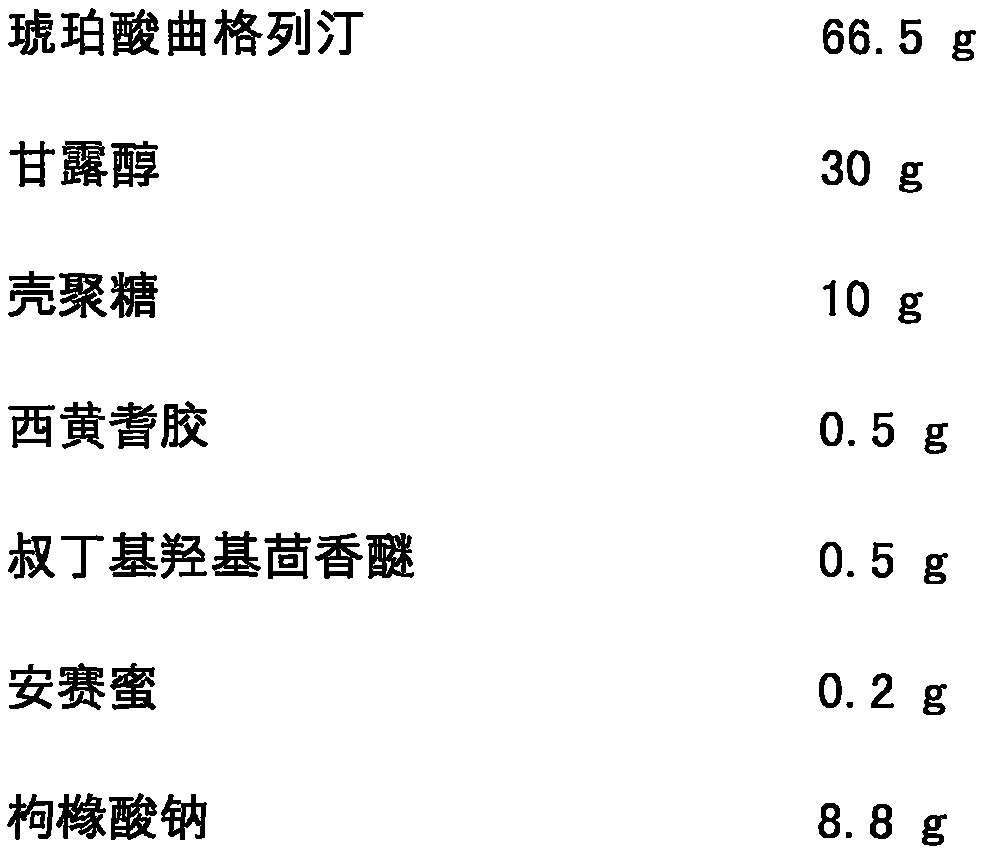
Lyophilized orally disintegrating tablets of trelagliptin succinate
InactiveCN105434382AImproved taste and disintegration timeImprove complianceOrganic active ingredientsMetabolism disorderDrugAdhesive
The invention discloses lyophilized orally disintegrating tablets of trelagliptin succinate and a preparation method thereof. Trelagliptin succinate is used as a medicament active component, and the tablets comprise the following raw materials and auxiliary materials by weight: 2-75% of trelagliptin succinate, 10-85% of a frame supporting agent, 2-55% of an adhesive, 0.5-55% of a suspending agent, 0.5-30% of an anti-oxidant, 0.2-10% of a corrigent, a proper amount of a pH regulator, and a proper amount of injection water. The lyophilized orally disintegrating tablets are prepared by a lyophilization device. The lyophilized orally disintegrating tablets has the advantages of fast disintegration speed, high dissolution rate, good stability, usage convenience, portability, and substantially enhanced medication compliance of patients.
Owner:BEIJING KANG LISHENG PHARMA TECH DEV
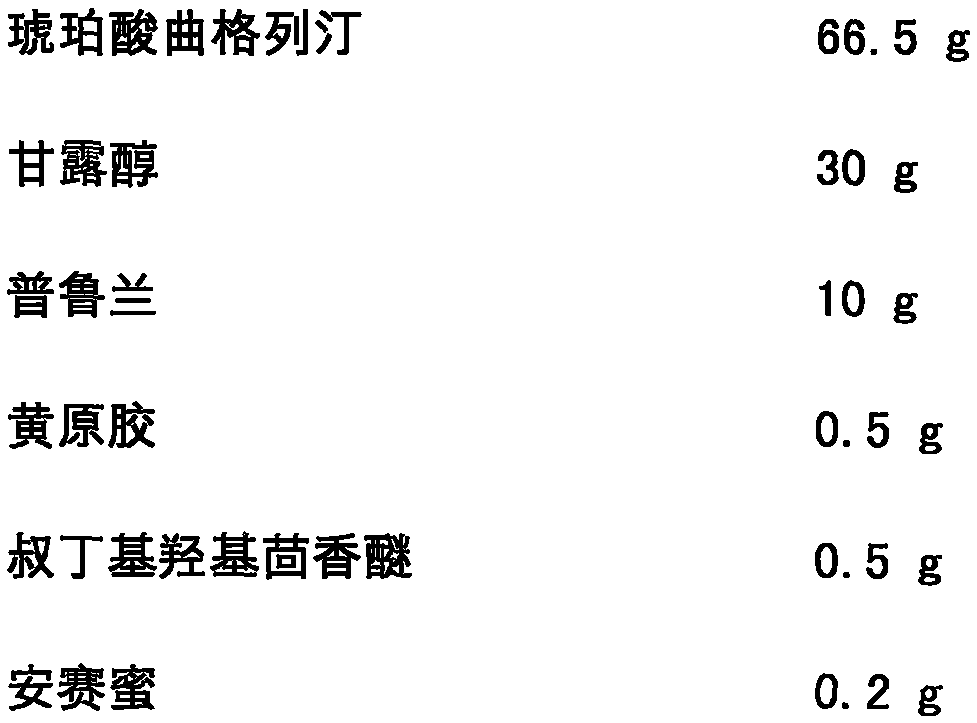
Vortioxetine freeze-dried orally disintegrating tablet and preparation method thereof
InactiveCN104873458AGreat tasteGood disintegration timeOrganic active ingredientsNervous disorderFreeze dryOrally disintegrating tablet
The invention discloses a vortioxetine freeze-dried orally disintegrating tablet and a preparation method thereof. The vortioxetine freeze-dried orally disintegrating tablet with the vortioxetine as an active pharmaceutical ingredient comprises, by weight, 2%-75% of vortioxetine, 10%-85% of a skeleton supporting agent, 2%-55% of binder, 0.5%-55% of a suspending agent, 0.5%-30% of antioxidant, 0.2%-10% of corrigent, a defined amount of pH regulator and a defined amount of injection water. The vortioxetine freeze-dried orally disintegrating tablet is prepared through a freeze-drying device and has the advantages of being high in disintegrating speed, high in dissolution rate, good in stability and convenient to take and carry, and the compliance of a patient to take medicine is greatly improved.
Owner:AVENTIS PHARMA HAINAN
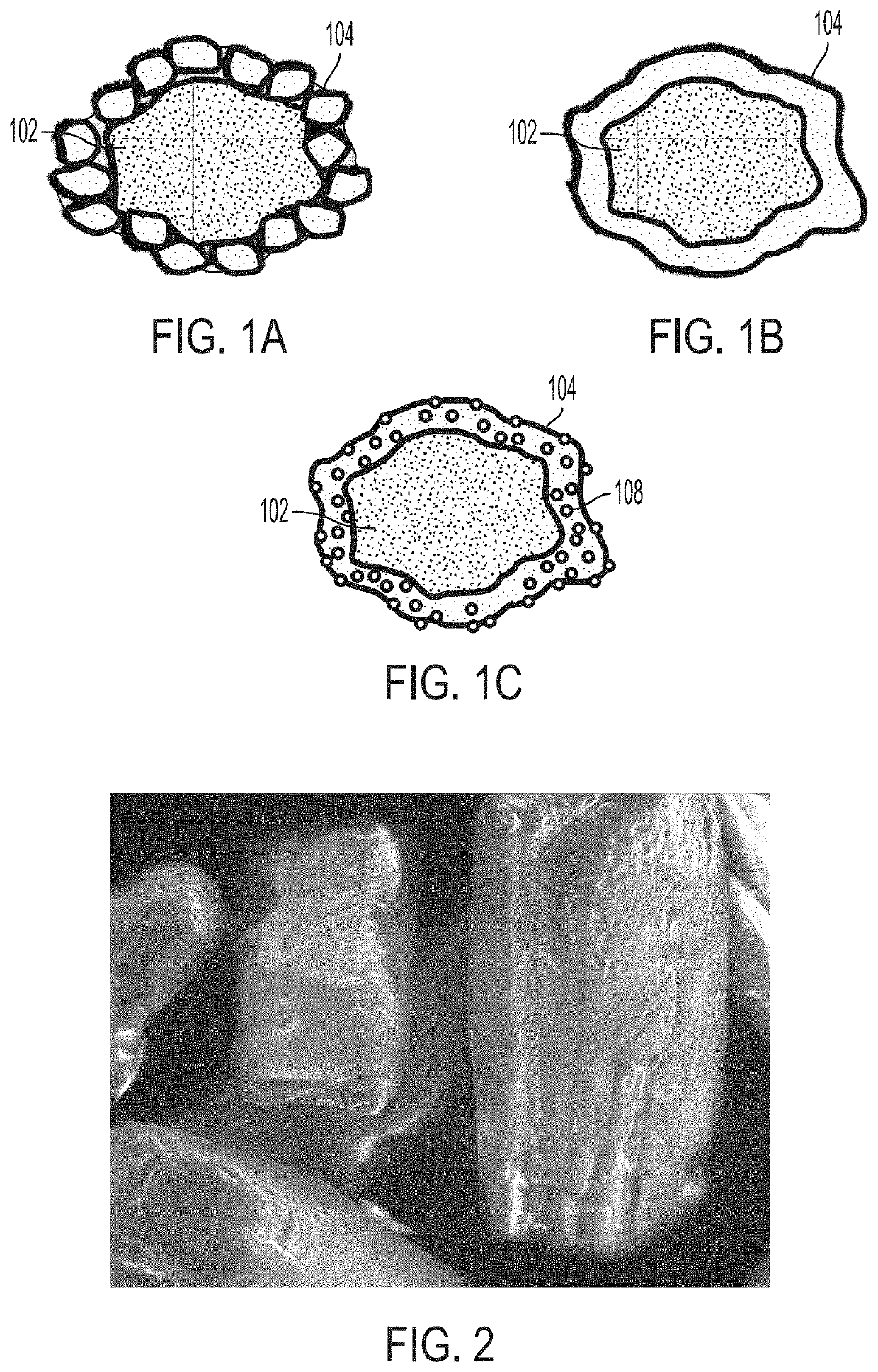
Minimizing agglomeration of drug particle coating material during storage to stabilize disintegration times of pharmaceutical products
ActiveUS20200268667A1Minimize agglomerationReduced stabilityOrganic active ingredientsAntipyreticPhysical chemistrySolvent free
Provided are pharmaceutical compositions and methods for preparing pharmaceutical compositions using solventless mixing methods. Excess coating material that is not bound to a coated API particle may be removed by a sieving process. Coating and dosing ratios can also be optimized to minimize the amount of excess unbound coating material. Specifically, a coating ratio and / or a dosing ratio can be used to minimize the residual amount of excess unbound coating material to minimize agglomeration of coating material during storage. In some embodiments, a pharmaceutical composition is provided, the pharmaceutical composition comprising: 65-85 % w / w API particles; 15-30 % w / w coating material coating the API particles; and 3-15 % w / w matrix surrounding the coated API particles, wherein the pharmaceutical composition comprises a disintegration time rate of less than 10 seconds for at least six months under storage conditions of at least 25° C. and at least 60 % relative humidity.
Owner:CATALENT U K SWINDON ZYDIS LTD
Minimizing agglomeration, aeration, and preserving the coating of pharmaceutical compositions comprising ibuprofen
ActiveUS11166919B2Minimize agglomerationReduced stabilityPowder deliveryOrganic active ingredientsSolvent freeEngineering
Owner:CATALENT U K SWINDON ZYDIS LTD
Minimizing agglomeration, aeration, and preserving the coating of pharmaceutical compositions comprising ibuprofen
ActiveUS20200268676A1Minimize agglomerationReduced stabilityOrganic active ingredientsAntipyreticSolvent freePharmaceutical Substances
Provided are pharmaceutical compositions and methods for preparing pharmaceutical compositions comprising Ibuprofen using solventless mixing methods. Excess coating material that is not bound to coated Ibuprofen may be removed by a sieving process. Coating and dosing ratios can also be optimized to minimize the amount of excess unbound coating material. Additionally, the compositions can be formulated to preserve the functional coating of coated Ibuprofen and to minimize aeration of Ibuprofen when mixed into suspension.
Owner:CATALENT U K SWINDON ZYDIS LTD
Minimizing agglomeration of drug particle coating material during storage to stabilize disintegration times of pharmaceutical products
ActiveUS11141380B2Reduced stabilityMinimize agglomerationOrganic active ingredientsPowder deliveryPhysical chemistrySolvent free
Owner:CATALENT U K SWINDON ZYDIS LTD
Preparation method of calcium iron zinc selenium vitamin tablets
InactiveCN107296282AExtended shelf lifeModerate crispnessFood shapingFood ingredient as encapsulating agentVitamin CMagnesium stearate
The invention discloses a preparation method of calcium iron zinc selenium vitamin tablets. The preparation method of the calcium iron zinc selenium vitamin tablets comprises the following steps: mixing calcium carbonate, ferrous fumarate, zinc citrate, selenium-enriched yeast, vitamin A, vitamin D3, vitamin C, magnesium stearate, maltodextrin, stevioside and starch uniformly, and performing boiling pelletization to obtain a first prefabricated material; mixing microcrystalline cellulose and a gelatin compound uniformly to obtain a second prefabricated material; mixing the second prefabricated material, the first prefabricated material and talcum powder, pelletizing for the second time, and tabletting to obtain the calcium iron zinc selenium vitamin tablets. The calcium iron zinc selenium vitamin tablets obtained by the method have extremely high stability and long shelf life of effective components, and can be disintegrated quickly after entering intestines and stomach.
Owner:安徽全康药业有限公司
Honeysuckle throat clearing tablets and preparation method thereof
ActiveCN102657778AGood disintegration timeImprove appearancePharmaceutical non-active ingredientsPill deliveryThroatHoneysuckle
The invention belongs to the field of medicinal preparations, and particularly relates to a honeysuckle throat clearing formula and a tablet preparation method thereof. The honeysuckle throat clearing tablets contain medicament extract and pharmaceutically acceptable diluting agent, lubricating agent and disintegrating agent. The honeysuckle throat clearing tablets prepared by the invention have a better treatment effect on acute pharyngitis.
Owner:GENERAL HOSPITAL OF PLA
Method for preparing soft capsules for resisting cervicitis
InactiveCN101347547ALong-term storage stabilityEasy to packCapsule deliverySexual disorderPEG 400Callicarpa kwangtungensis
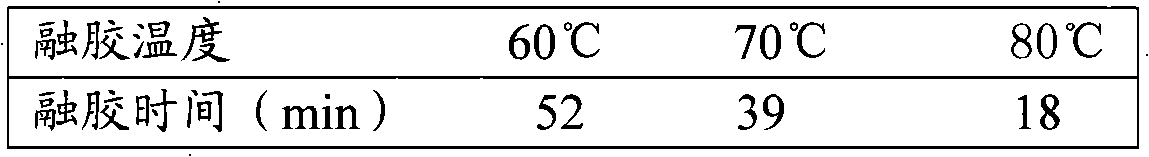
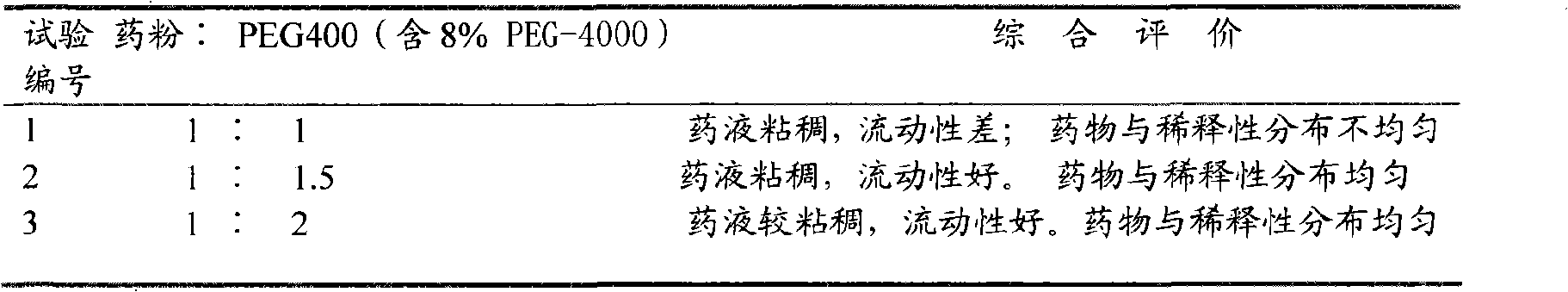
The invention discloses a preparation method of a soft capsule for treating hysteritis, including the following steps: three medicines, namely, 167 parts of callicarpa kwangtungensis dry extract, 44 parts of motherwort dry extract and 39 parts of radix linderae dry extract are ground into fine powder and evenly blended to obtain dry extract powder; the dry extract powder is added with the mixture of polyethylene glycol 400 and polyethylene glycol 4,000 which accounts for 8% of the total weight of finished products, the proportion of the dry extract to the mixture of polyethylene glycol 400 and 8% polyethylene glycol 4,000 is 1:1.5 to 2; the preparation of a capsule shell is as follows: gelatin liquid is prepared according to the proportion that gelatin: water: glycerin is 1:1:0.4, put into a gelatin dissolving tank and the temperature is set to be 70-80 DEG C; when the gelatin liquid is completely melted, the temperature is set to be 55-65 DEG C and is maintained for about 8 hours. Then pelleting, shaping, drying and packaging are carried out. The soft capsule can prevent air oxidation and moisture absorption and has the advantages of short disintegration time, no leakage of medicine, good stability, high utilization rate and good curative effect.
Owner:罗伟
Buccal tablets
InactiveCN110786505AReduce dosageImprove antioxidant capacityFood ingredient as antioxidantOrganic active ingredientsStearic acidPharmacology
The invention provides buccal tablets. The buccal tablets contain plant exosome components, antioxidant components, whitening components, stearic acid, hydroxyproline and pectin. The buccal tablets provided by the invention have significantly shortened disintegrating time; and the buccal tablets are very good in antioxidant effects, anti-wrinkle effects and whitening effects.
Owner:陕西佰瑞衡健康科技有限公司
Hydroxyalkyl cellulose
ActiveCN103228676AImprove lamination efficiencyFull strengthPharmaceutical non-active ingredientsGranular deliveryCelluloseAqueous solution
The present invention provides a hydroxyalkyl cellulose having a viscosity of 1.10 to 1.95 mPas in a 2%-concentration aqueous solution at a temperature below 20 DEG C, and a solid preparation containing the same.
Owner:NIPPON SODA CO LTD
Water-soluble tablet of fluorescent brightener 351 as well as preparation method and application of water-soluble tablet
InactiveCN103540161AConvenient and accurate measurementEasy to store and transportOrganic dyesBleaching apparatusFluorescenceMedicine
The invention provides a water-soluble tablet of a fluorescent brightener 351. The water-soluble tablet contains the fluorescent brightener 351, a binder, a wetting agent, a disintegrating agent, a dispersing agent, an effervescing agent and a filling agent. All the components are mixed and crushed and are tabletted by using a direct powder compression method. Compared with the traditional dosage form, the water-soluble tablet is better in whitening effect, safe and convenient in use and transportation, beneficial to storage and container configuration and little in environment pollution.
Owner:沈阳新纪化学有限公司
Compound uterus-warming and pain-relieving footbath effervescent tablets and preparation method thereof
ActiveCN111329972AFix stability issuesEffective isolationAntipyreticAnalgesicsEffervescent tabletTraditional medicine
The invention relates to the technical field of traditional Chinese medicine preparations and particularly discloses compound uterus-warming and pain-relieving footbath effervescent tablets and a preparation method thereof. The compound uterus-warming and pain-relieving footbath effervescent tablets are prepared from components in parts by weight as follows: 30-40 parts of extract powder, 35-50 parts of an acid source and an alkaline source, 3-8 parts of PEG, 1-3 parts of a lubricant and 15-30 parts of a filling agent. The brand-new compound uterus-warming and pain-relieving footbath effervescent tablets are provided and a brand-new compound uterus-warming and pain-relieving footbath product is provided to fill up the market vacancy of the product. Besides, the effervescent tablets have excellent quality and have better disintegrating time and effervescence effects.
Owner:JIAYING UNIV
Orally disintegrating tablets
ActiveUS20190117577A1Good disintegration timeLower Level RequirementsOrganic active ingredientsPharmaceutical containersOrally disintegrating tabletDiol
The present invention relates to rapidly disintegrating oral dosage forms, more particularly to rapidly disintegrating tablets containing (1S ,2S ,3R,5S)-3-[7-{[1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino}-5-(propylthio)-3H-[1,2,3]-triazolo[4,5-d]pyrimidin-3-yl]-55-(2-hydroxyethoxy)cyclopentane-1,2-diol and a disintegrating excipient. Blister packs suitable for use with the rapidly disintegrating oral dosage form are also disclosed.
Owner:ASTRAZENECA AB
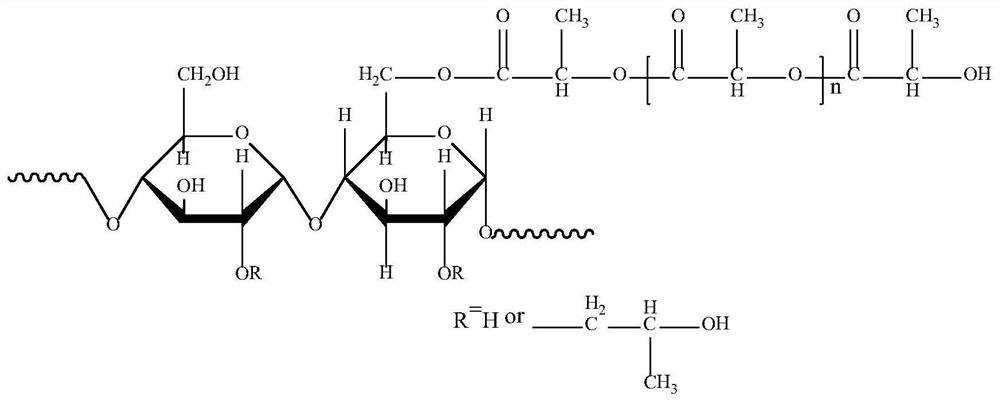
Plant-based soft capsule shell as well as preparation method and application thereof
ActiveCN111700873AImprove brittlenessHigh tensile strengthPharmaceutical non-active ingredientsCapsule deliveryCelluloseSoftgel
The invention relates to the technical field of soft capsule shell materials, in particular to a plant-based soft capsule shell and a preparation method and application thereof. The plant-based soft capsule shell comprises a hydroxypropyl starch / lactic acid graft copolymer, a cellulose / polylactic acid graft copolymer, a plasticizer, gel, edible pigment and water, wherein the substitution degree ofthe hydroxypropyl starch is not higher than 0.58, and the grafting rate of lactic acid to the hydroxypropyl starch is 14-25%. The main material of the soft capsule shell is derived from plants, and has extremely excellent disintegration time limit, and is beneficial to rapid release of the capsule in an organism. The soft capsule shell has excellent performance in the aspects of friability, disintegration time, tensile strength, elongation at break and the like, and meanwhile, and has excellent ageing resistance and remarkably-prolonged storage life.
Owner:JIANGSU FOOD & PHARMA SCI COLLEGE
A kind of pesticide water dispersible granule and preparation method thereof
ActiveCN110432265BImprove biological activityHigh suspension rateBiocideFungicidesWater dispersibleSpray dried
The invention belongs to the technical field of pesticide preparations, and provides a pesticide water-dispersible granule and a preparation method thereof. A pesticide water-dispersible granule, which is composed of the following components in parts by weight: 15-70 parts of pesticide active ingredients, 5-10 parts of dispersant, 2-5 parts of wetting agent, 5.5-9 parts of disintegrating agent, binder 1-4 parts of agent, 2-10 parts of filler, dispersant is a mixture of poloxamer, chitosan and citric acid with a mass ratio of 4:1:1.5. In the preparation method, the active ingredients of the pesticide, the dispersant, and the disintegrating agent are mixed evenly and then pulverized, then mixed with the wetting agent and binder dissolved in water to obtain a suspension, the suspension is spray-dried, mixed with the filler, squeezed Pressing granulation, drying and other steps. Through the above technical scheme, the problems of single formulation and small application range of the water-dispersible granules in the prior art are solved.
Owner:河北双吉化工有限公司
Entecavir oral instant film agent and preparation method thereof
ActiveCN107714676AEasy to carryImprove complianceAntiviralsPharmaceutical non-active ingredientsMedicinePlasticizer
The invention relates to an entecavir oral instant film agent and a preparation method thereof. By referring to the total weight, the entecavir oral instant film agent is prepared from, by mass, 0.05-15% of entecavir, 55-90% of polyving akohol / polyethylene glycol grafted copolymer, 5-15% of glycerin plasticizer, 0.5-15% of sodium alginate disintegrating agent. The invention provides a preparationmethod of the entecavir oral instant film agent, which includes steps of (1), mixing polyving akohol / polyethylene glycol grafted copolymer with water evenly to obtain polymer gel; (2), adding entecavir in the polymer gel; after mixing evenly, adding plasticizer and sodium alginate disintegrating agent, so as to obtain drug-containing solution; (3), degassing the drug-containing solution and thencoating on a substrate; drying at 40-60 DEG C and then cutting, so as to obtain the entecavir oral instant film agent. The entecavir oral instant film agent can significantly accelerate the disintegrating time limit of the film agent and solve the shortcoming that the most oral solid preparation is applied along with water at present; the film agent cannot delay the drug applying time in the absence of water resource and improves the drug compliance of a patient.
Owner:SUZHOU UNIV
Honeysuckle throat clearing tablets and preparation method thereof
ActiveCN102657778BEasy to takeImprove compliancePharmaceutical non-active ingredientsPill deliveryThroatAcute Pharyngitis
Owner:GENERAL HOSPITAL OF PLA
Hydroxyalkylcellulose microparticles
ActiveUS8568787B2High strengthImprove suppression propertiesBiocidePill deliveryShock waveVolume average
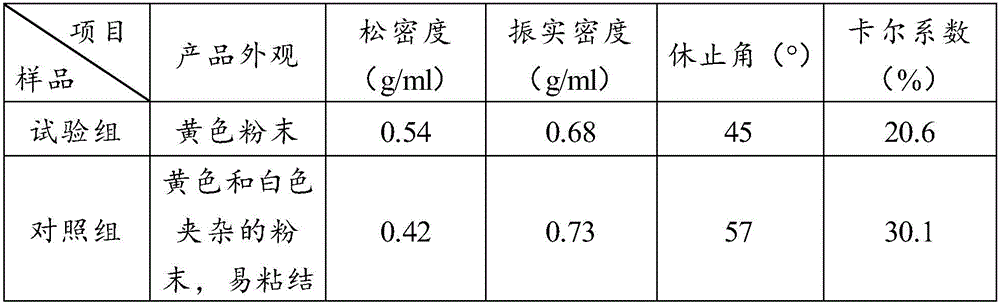
Provided is a method of producing hydroxyalkylcellulose microparticles, the method including generating a pulse shock wave, and supplying a hydroxyalkylcellulose aqueous solution to the pulse shock wave generation region, thereby crushing and drying the hydroxyalkylcellulose aqueous solution. According to the production method, hydroxyalkylcellulose microparticles having a volume-average particle size of at least 0.1 μm but less than 15 μm are obtained. By mixing the hydroxyalkylcellulose microparticles with a principal agent and subjecting the resulting mixture to a tablet compression, a solid preparation having excellent tensile strength and disintegration properties can be obtained.
Owner:NIPPON SODA CO LTD +1
Pharmaceutical compositions comprising coated api
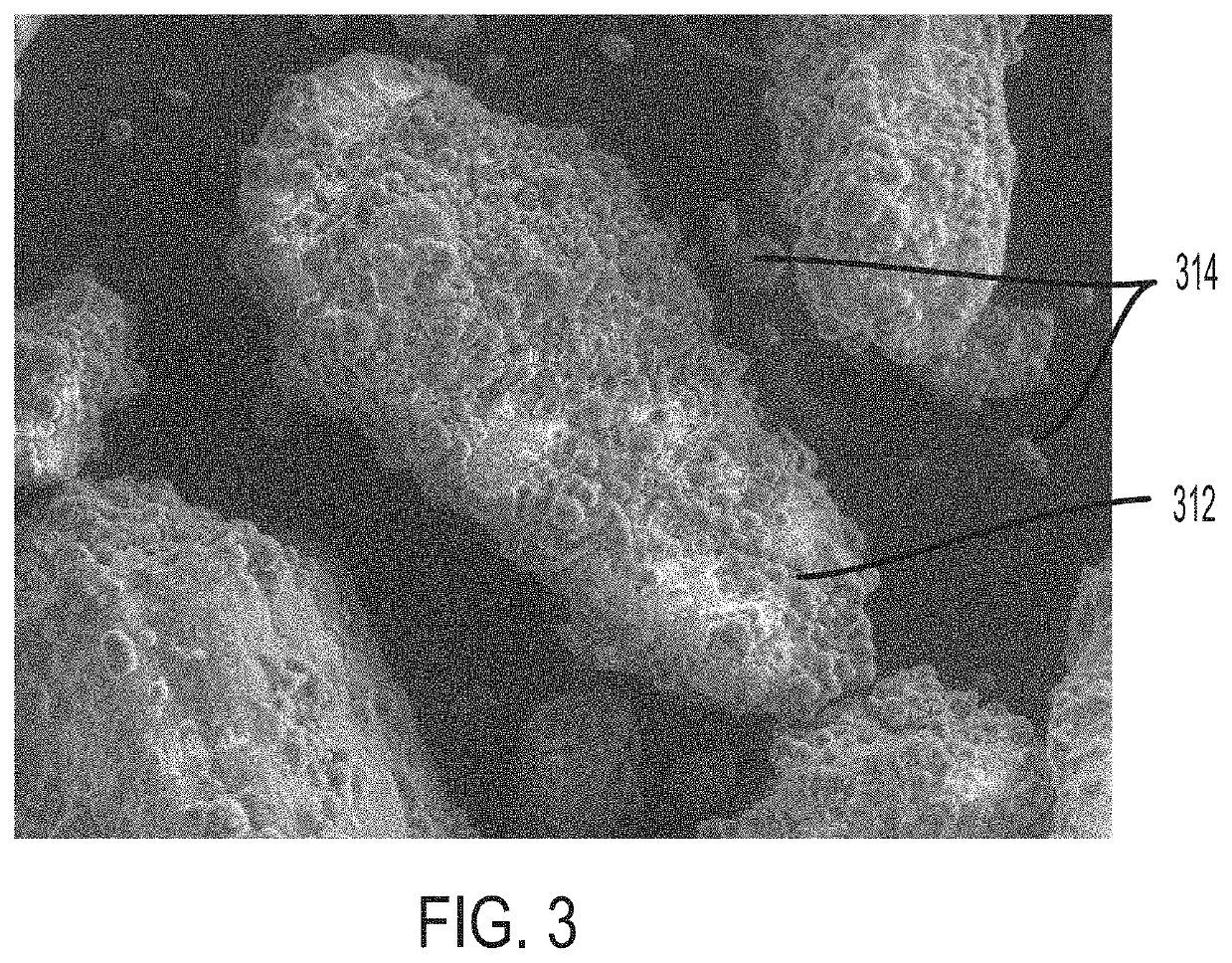
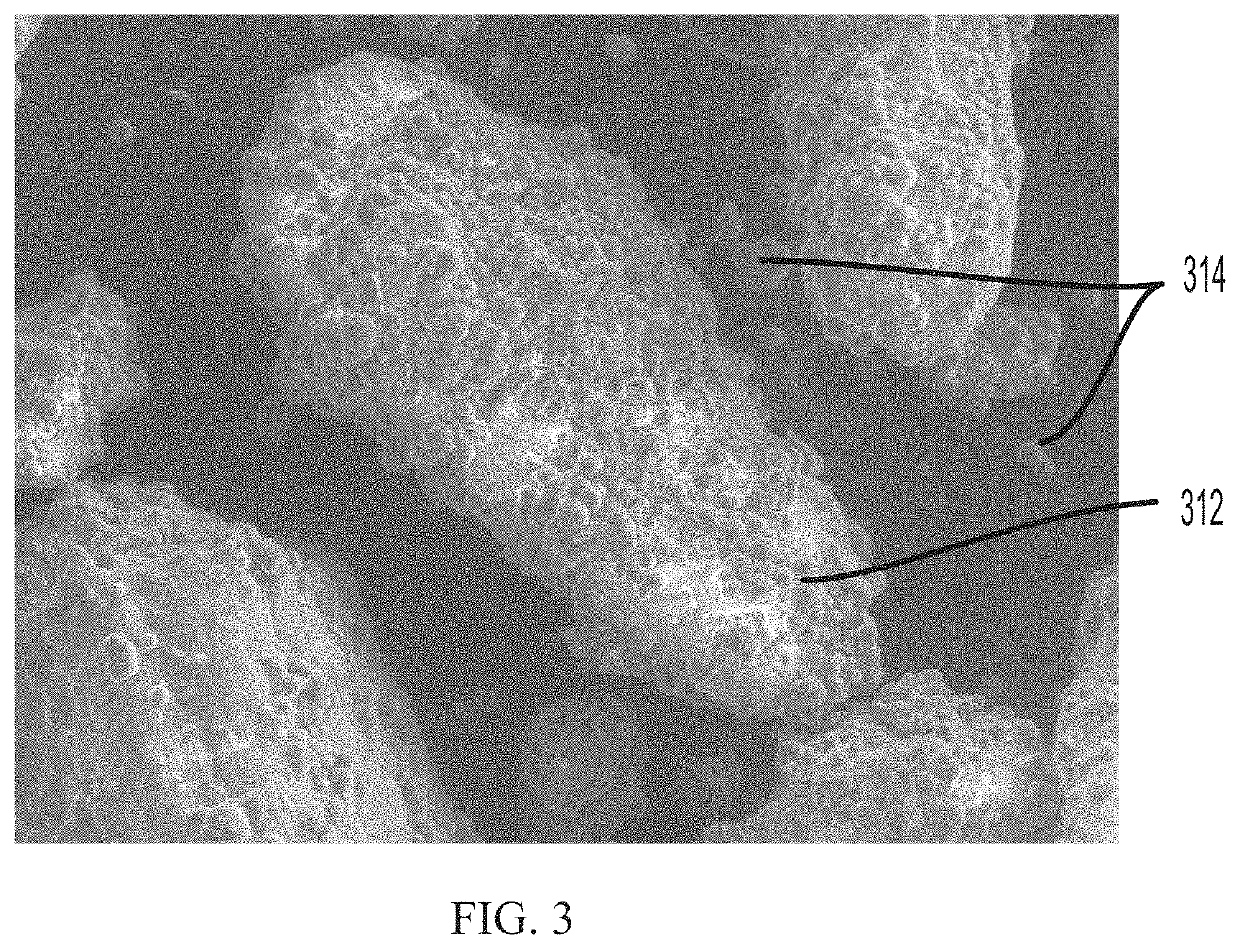
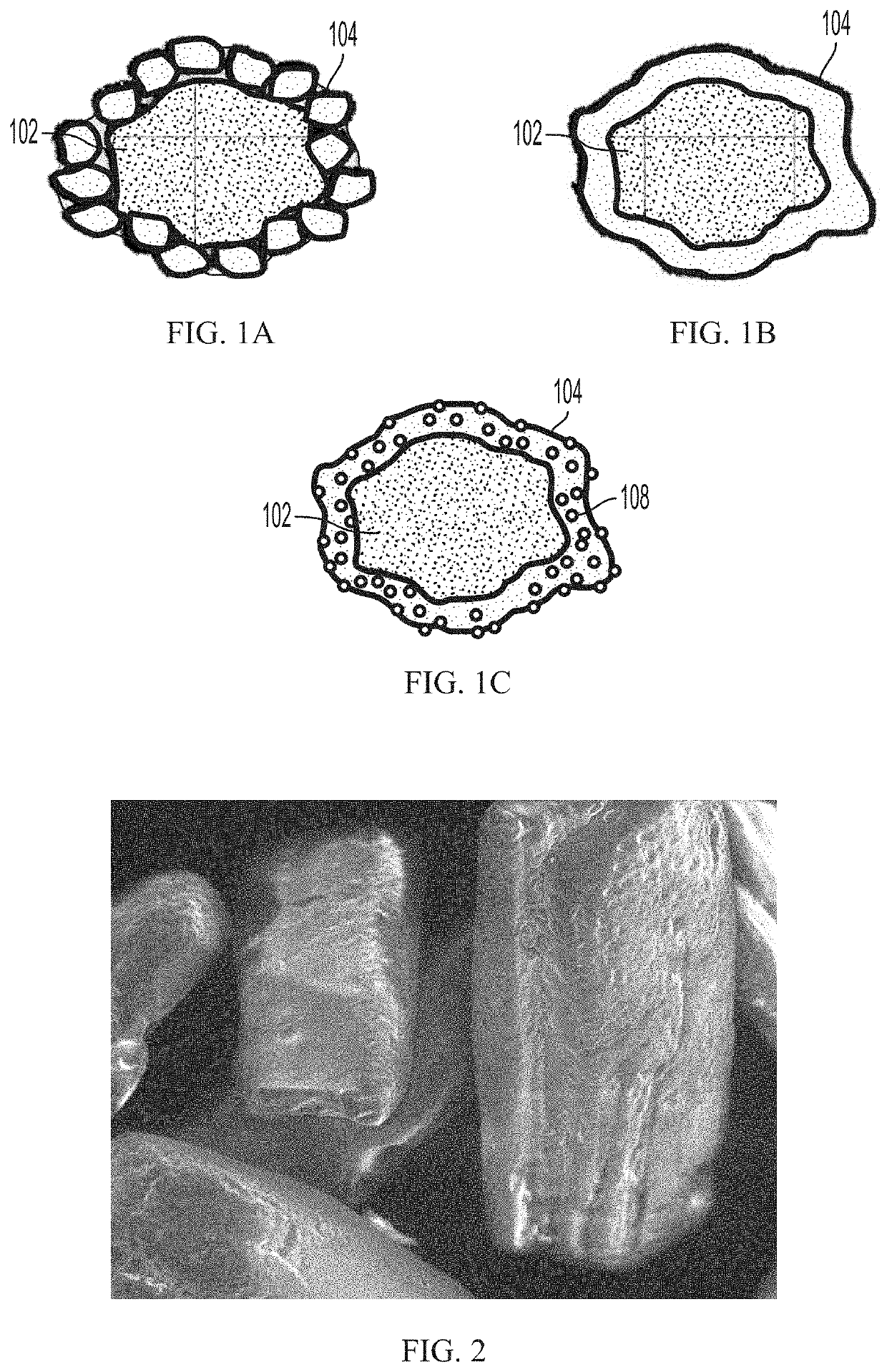
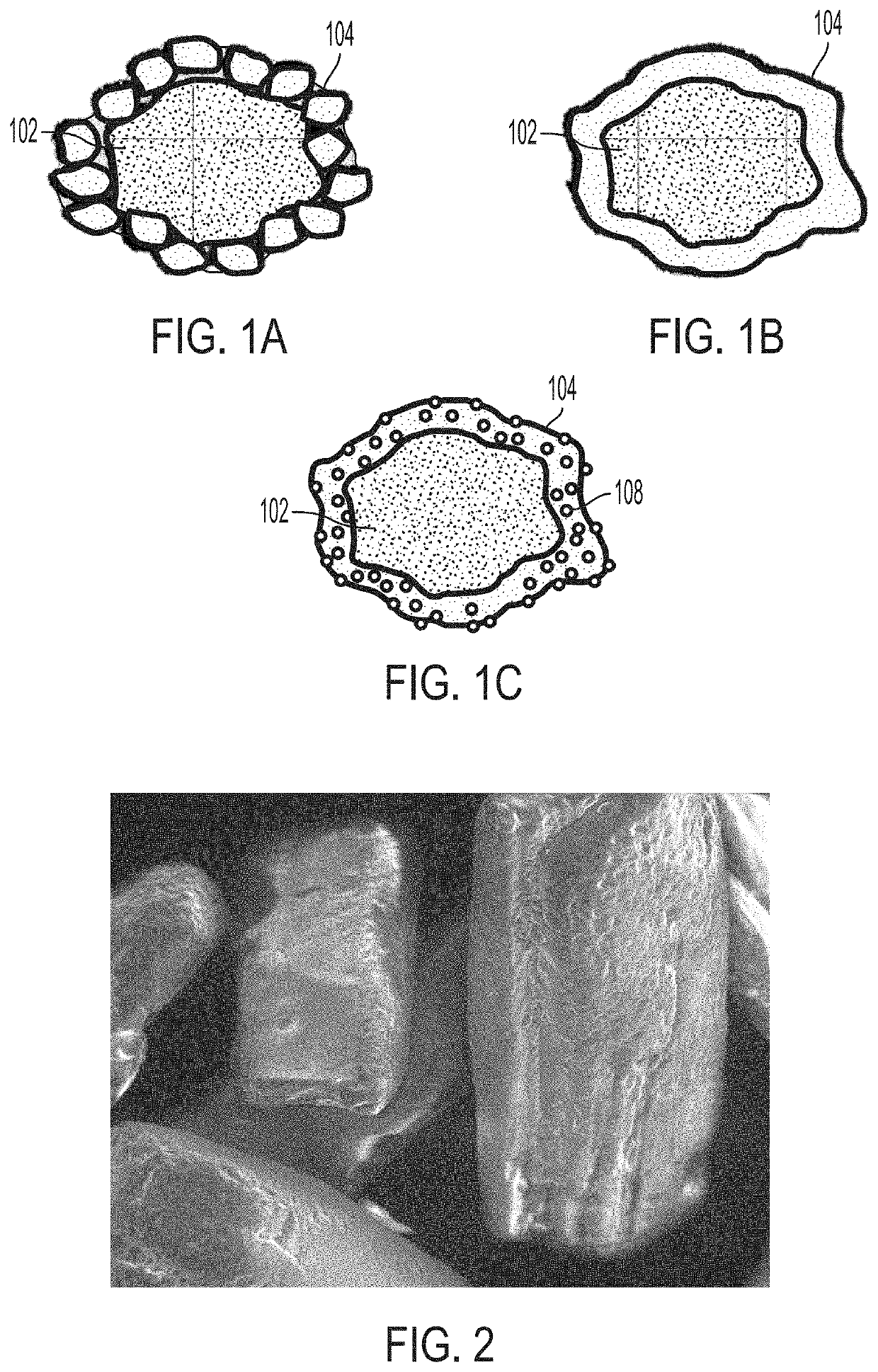
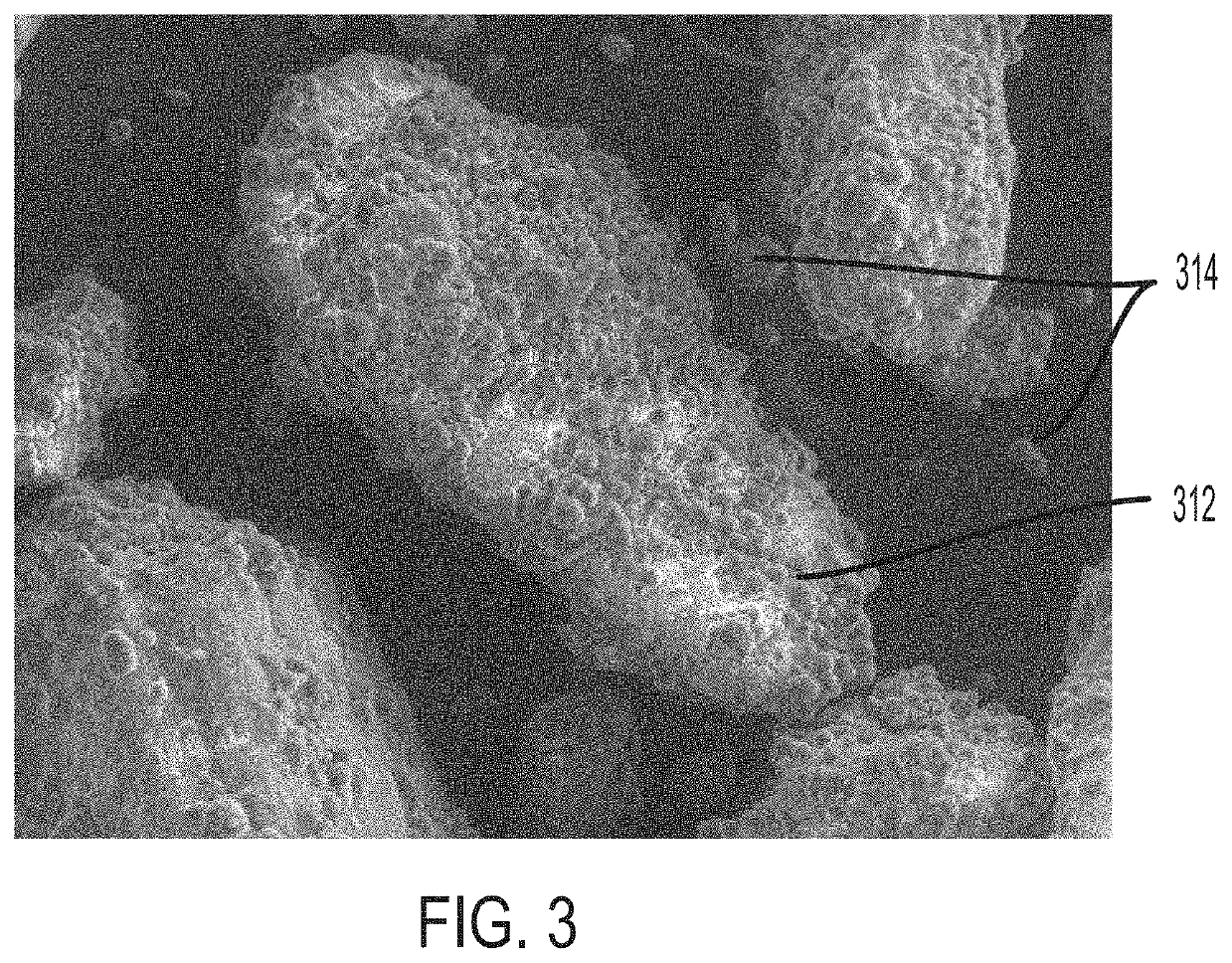
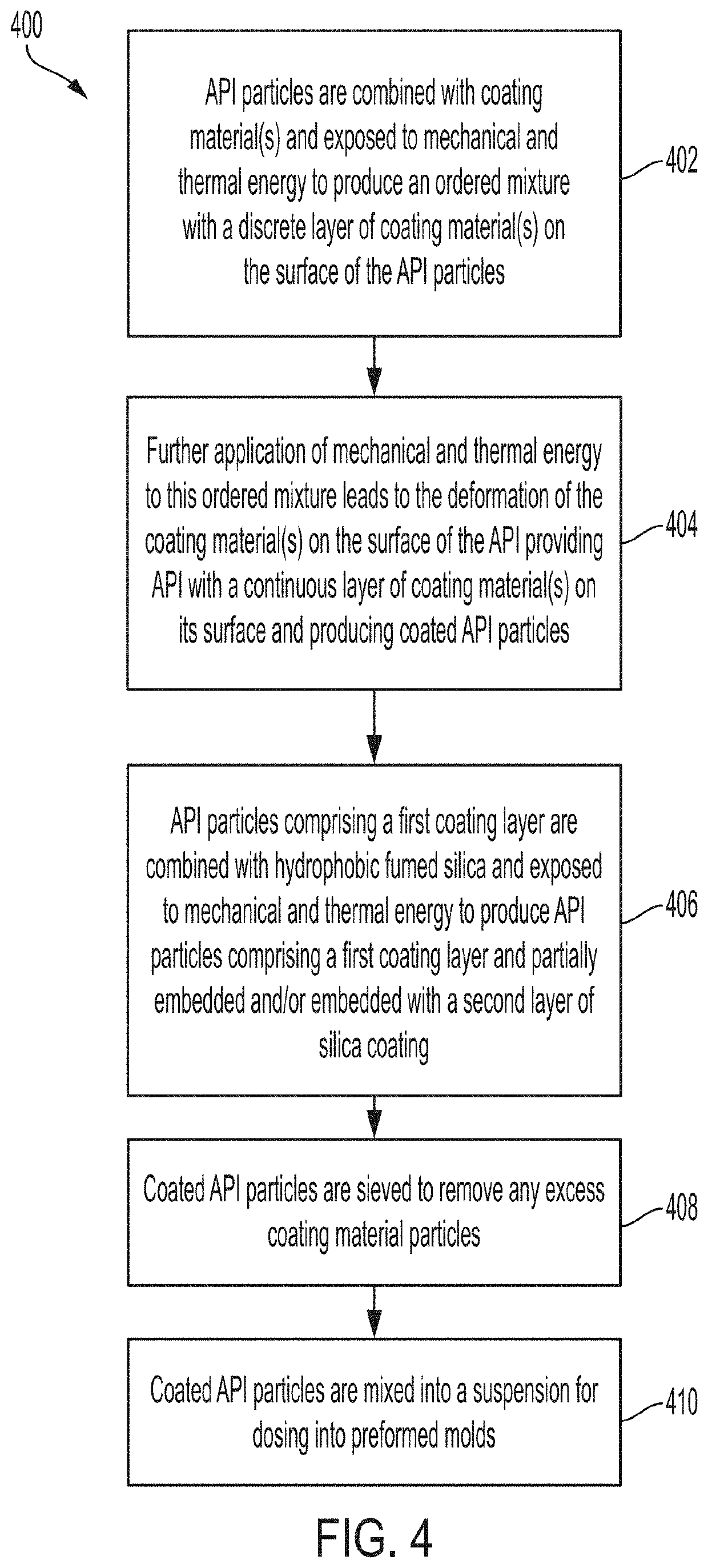
PendingUS20220031610A1Improve homogeneityIncrease dose weight accuracyOrganic active ingredientsLyophilised deliveryPharmaceutical SubstancesBiomedical engineering
Provided are pharmaceutical compositions and methods for preparing pharmaceutical compositions comprising coated API. Excess coating material that is not bound to coated API may be removed by a sieving process. Coating and dosing ratios can also be optimized to minimize the amount of excess unbound coating material. Additionally, the compositions can be formulated to preserve the functional coating of coated API and to minimize aeration of API when mixed into suspension.
Owner:CATALENT U K SWINDON ZYDIS LTD
Method of biodesintegrating metal scrap with a bacterial consortium adapted to high concentrations of ferrous sulphate and ferric sulphate
PendingUS20220325373A1Eliminate surface oxidationGood disintegration timeBacteriaMicroorganism based processesIron sulfateMicrobiology
Method of biodesintegrating metal scrap with a bacterial consortium adapted to high concentrations of ferrous sulfate and ferric sulfate, access RGM 2972 of the Chilean Collection of Genetic and Microbial Resources; intermediate solution comprising it, useful in eliminating surface oxidation in metallic structure; and oxidizing solution, useful in the hydrometallurgical extraction of copper.
Owner:RUDANAC BIOTEC SPA
Fast Disintegrating Cannabinoid Tablets
ActiveUS20210177748A1Easy to disassembleIncrease loadPowder deliveryHydroxy compound active ingredientsCannabinoidAlcohol sugars
The present invention relates in a first aspect to a fast disintegrating cannabinoid tablet, the tablet comprising a sugar alcohol composition comprising one or more sugar alcohol particles in an amount of at least 20% by weight of the tablet, a cannabinoid composition comprising one or more cannabinoids, and a disintegrant composition comprising one or more disintegrants operable to disintegrate the tablet within a period of 2 minutes or less in contact with oral saliva. In a second aspect, the invention relates to a modular tablet, wherein the tablet comprises a further tablet module that is different in composition.
Owner:NORDICCAN AS
A kind of deep processing preparation and quality control method of propolis compound
ActiveCN106420834BSmooth dryingImprove powder propertiesAntinoxious agentsUnknown materialsAlcoholPropolis
The invention relates to a processing field of a propolis product, and particularly relates to a deep processing preparation method of the propolis powder and its product. The propolis composite is prepared by hardening and crushing the propolis raw material, and obtaining the propolis raw material powder; performing alcohol extraction on the propolis raw material powder, and obtaining the thick paste of the propolis extractive; mixing the propolis extractive thick paste with amino acid; grinding, cooling and drying, crushing and obtaining the propolis powder. The preparation method adds amino acid to the propolis extractive thick paste, thus the propolis and the amino acid can form an uniform system, thus the propolis can be smoothly dried, and the propolis powder with good powder property is obtained; meanwhile, the propolis powder prepared by the process is applicable to the propolis capsule (including hard capsule and soft capsule), propolis slice and others; the method can significantly improve the collapse problem of the propolis product.
Owner:BY HEALTH CO LTD
Method for preparing spleen-tonifying stomach-harmonizing healthcare product
The invention discloses a method for preparing a spleen-tonifying stomach-harmonizing healthcare product. The method comprises a step of weighing an excipient, lalang grass rhizome, radix bupleuri, astragalus membranaceus, gynostemma pentaphylla, carthamus tinctorius, nepal dock leaves, rhizoma anemarrhenae, phyllodium elegans, geranium robertianum, alum, motherwort fruits, scindapsus officinalisschott, sage and radix scrophulariae. The product is capable of tonifying spleen and harmonizing stomach, clearing lung and brightening eyes, nourishing spleen and tonifying stomach, brightening facecolor and prolonging life as well as tonifying kidney and strengthening yang, is simple in preparation method, low in cost, good in operability, free of environment pollution, safe and environmentallyfriendly, short in disintegration time, good in decomposition state, easy to absorb, rapid in effect taking and free of conspicuous toxic or side effect; due to synergetic effects of the radix scrophulariae and the lalang grass rhizome, functions of generating body fluid and tonifying lung, refreshing mind and clearing dizziness, expelling wind and removing coldness, improving vigor, protecting liver and preventing cancer and preventing dementia can be achieved; due to the synergetic functions of the components in the formula, effects of nourishing spleen and tonifying stomach, replenishing vital essence and improving eyesight and improving vigor can be achieved, and the product can be adopted to treat mouth sore, cough and excessive phlegm, chronic diarrhea and movement disability, and can be widely produced to replace conventional materials continuously.
Owner:SUZHOU LI LIANGJI HEALTH IND LTD
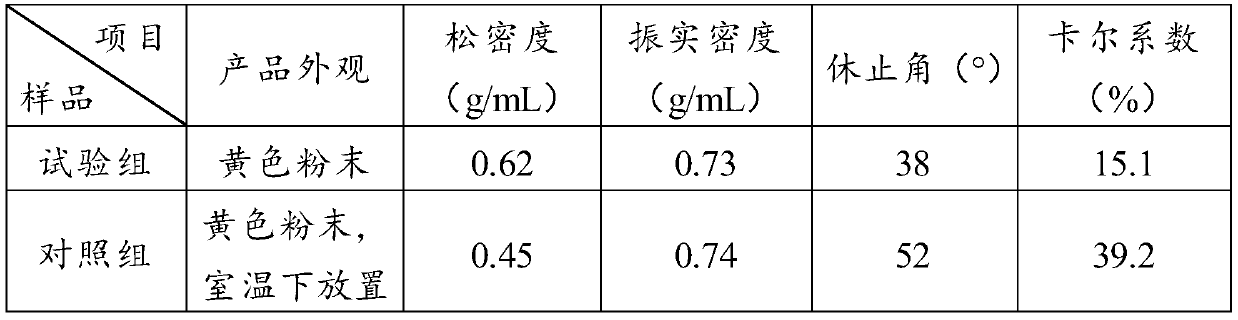
A kind of plant-based soft capsule rubber, preparation method and application thereof
ActiveCN111700873BStrong anti-agingExtended shelf lifePharmaceutical non-active ingredientsCapsule deliveryRubber materialCellulose
Owner:JIANGSU FOOD & PHARMA SCI COLLEGE
A kind of compound Nuangongzhitong foot bath effervescent tablet and preparation method thereof
ActiveCN111329972BFix stability issuesEffective isolationAntipyreticAnalgesicsEffervescent tabletTraditional medicine
The invention relates to the technical field of traditional Chinese medicine preparations, and specifically discloses a compound Nuangong analgesic foot bath effervescent tablet and a preparation method thereof. The preparation of the compound Nuangong Pain Relief Foot Bath Effervescent Tablet comprises the following components in parts by weight: 30-40 parts of extract powder; 35-50 parts of acid source and alkali source; 3-8 parts of PEG; 1 part of lubricant ~3 parts; filler 15~30 parts. The invention discloses a brand-new compound Nuan Gong pain-relieving foot bath effervescent tablet, provides a brand-new Nuan Gong pain-relieving foot bath product, and fills the market gap of such products. In addition, the effervescent tablet has excellent quality, better disintegration time and effervescent effect.
Owner:四川智谷藏御堂生物科技有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com