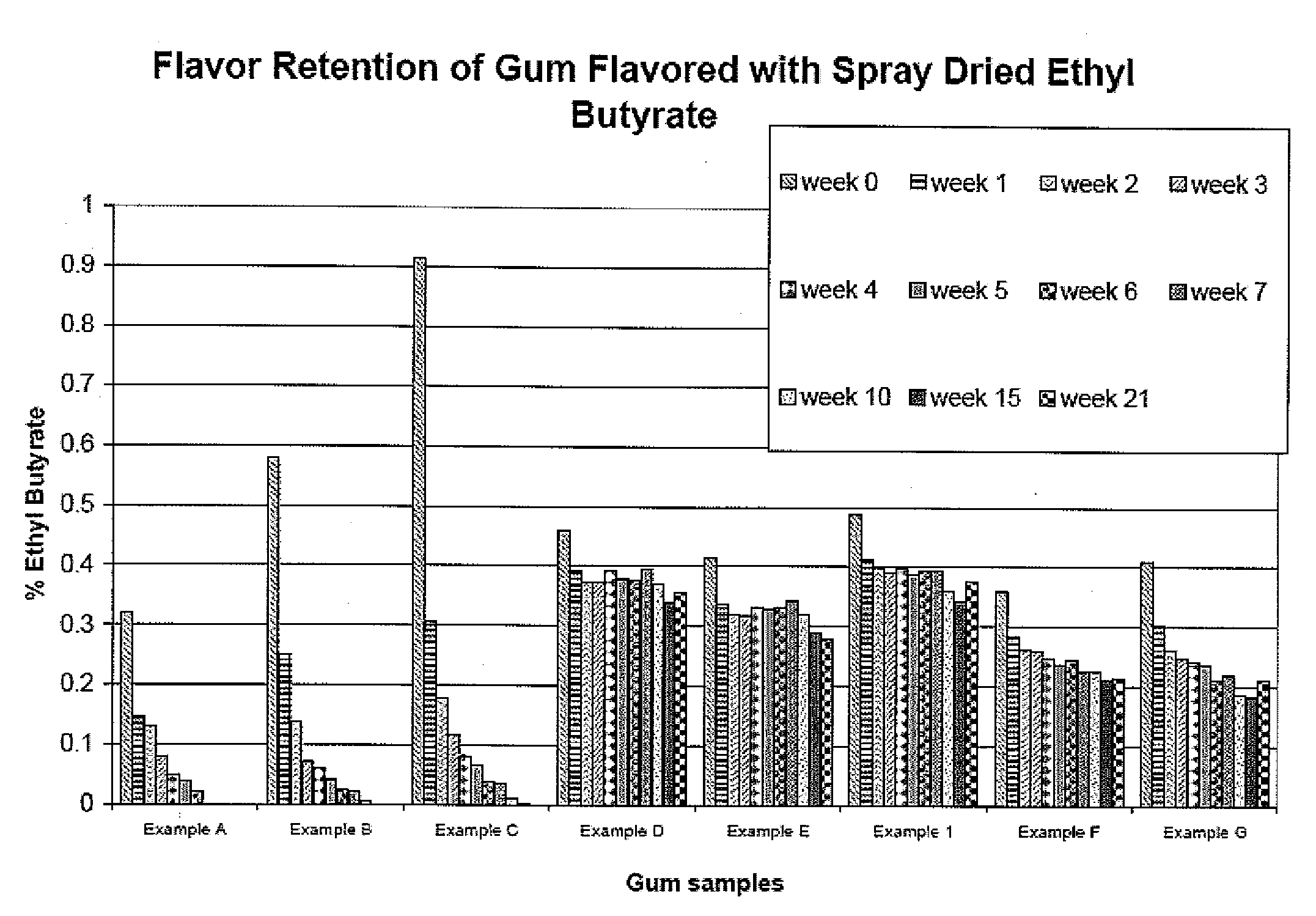
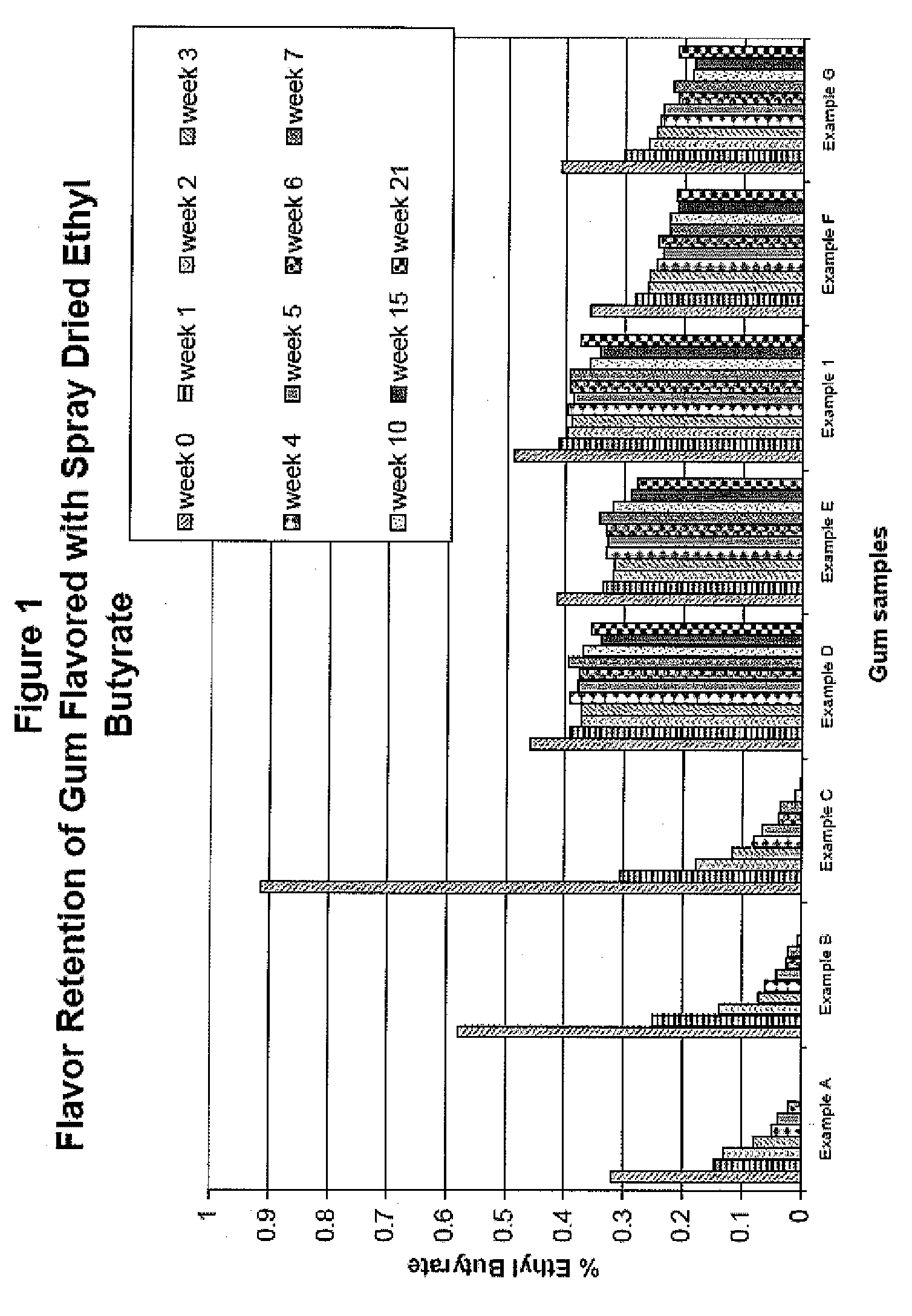
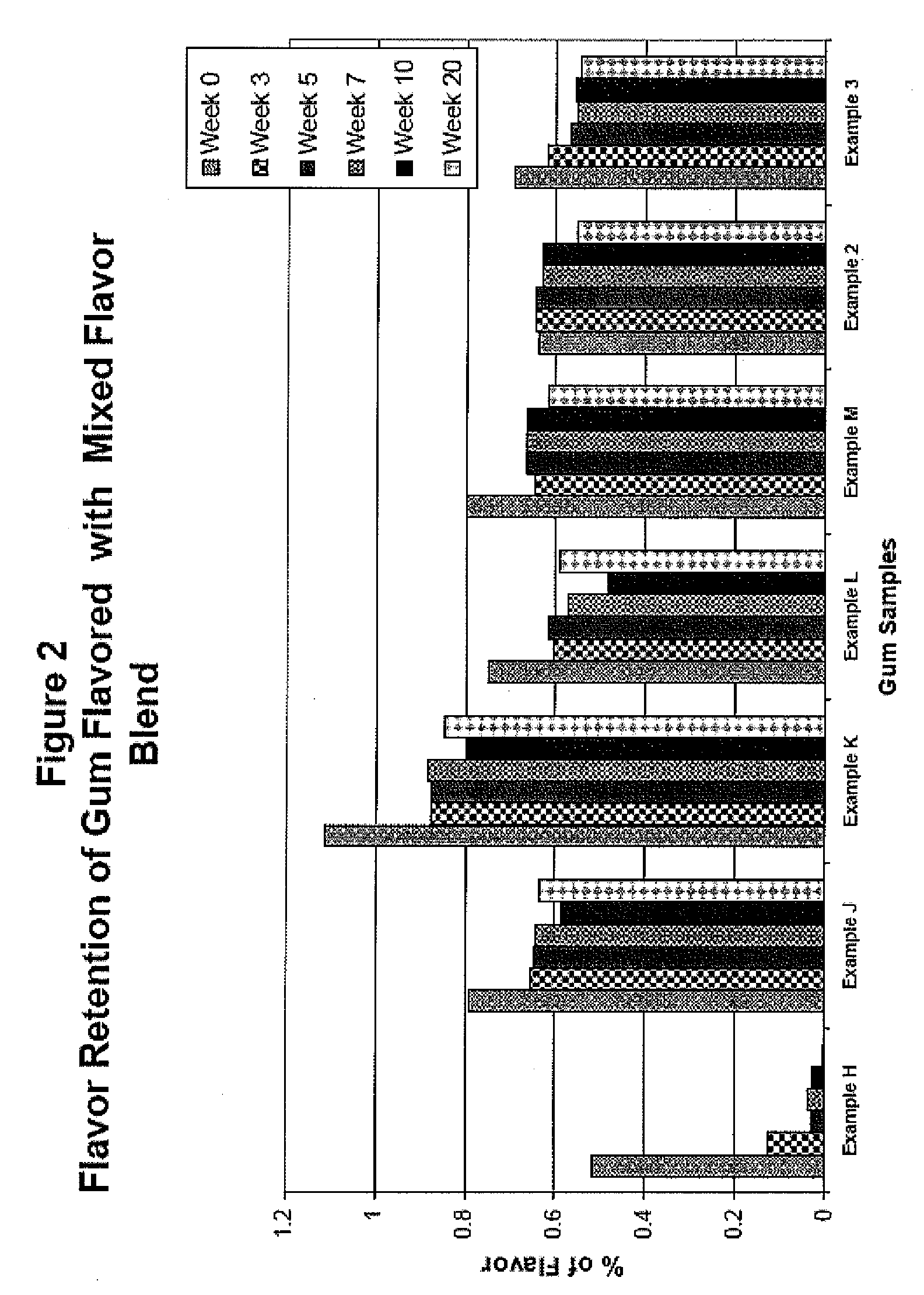
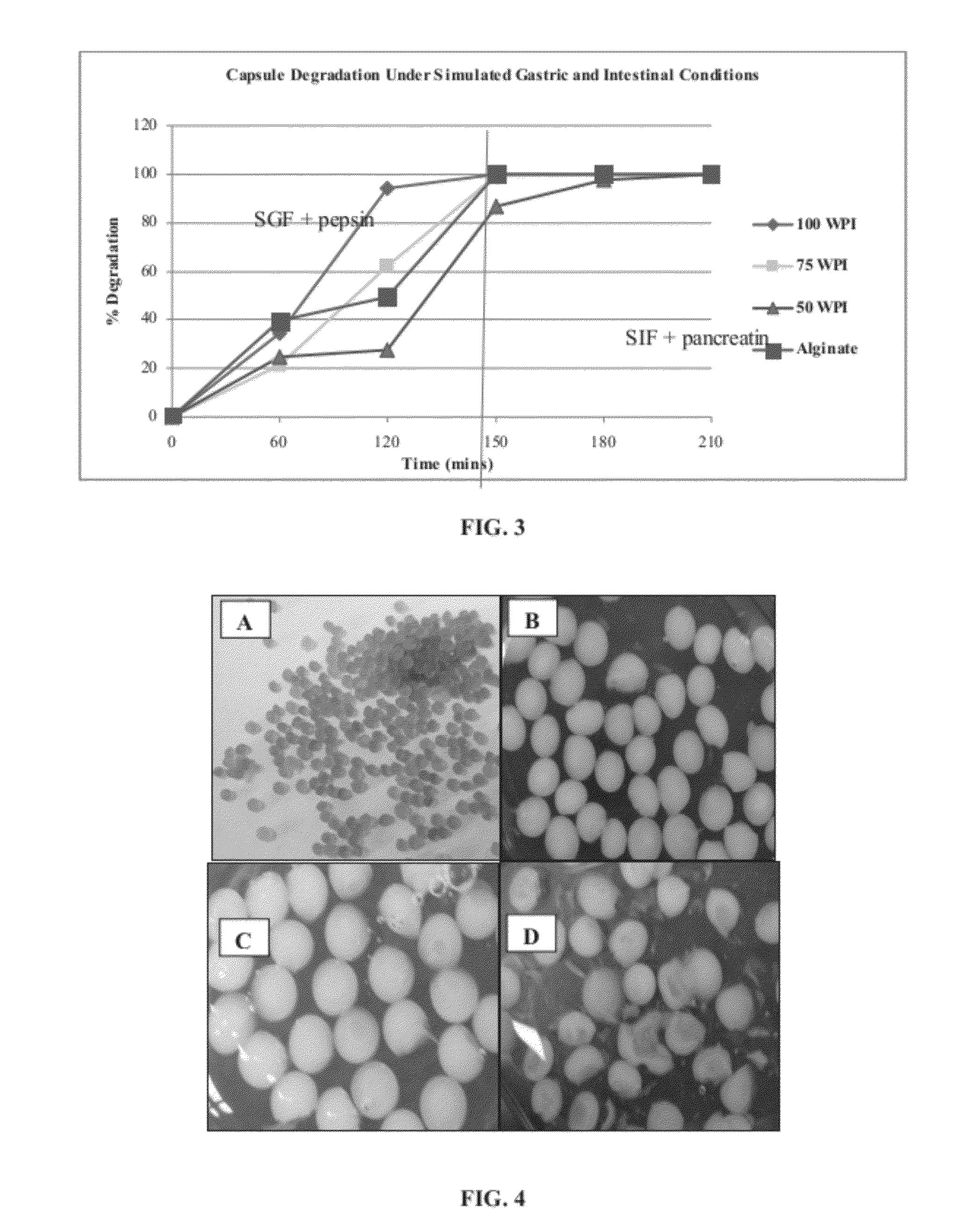
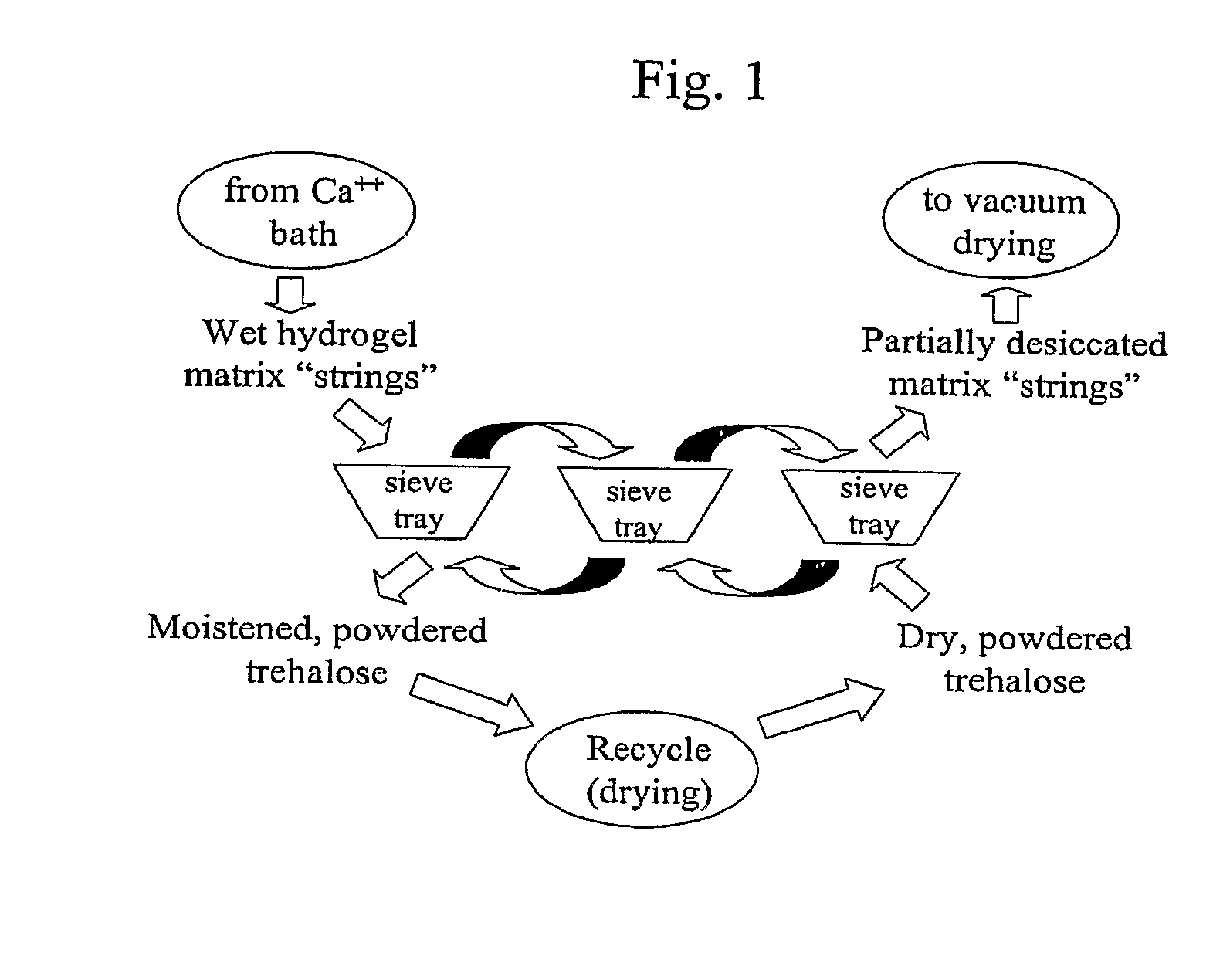
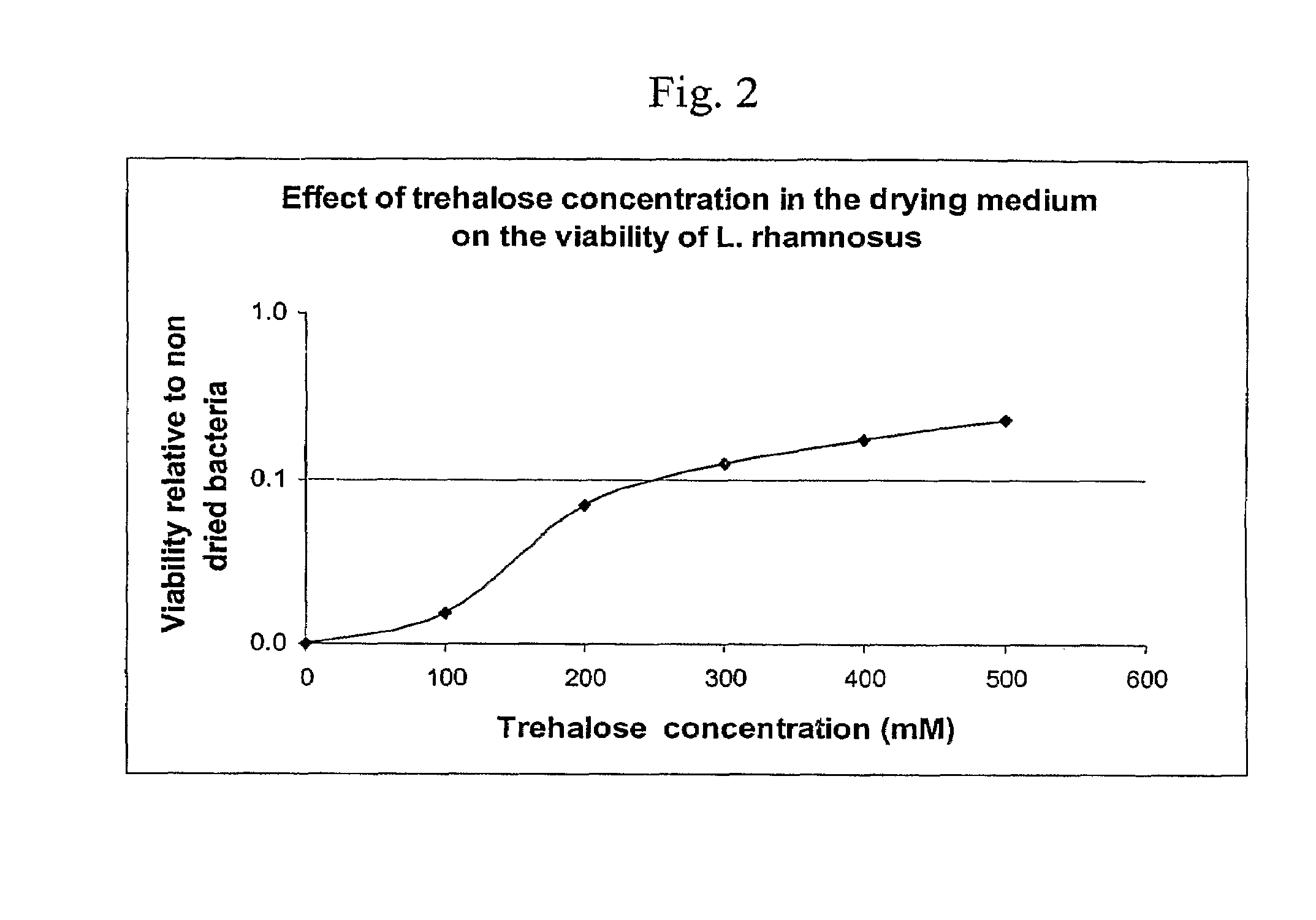
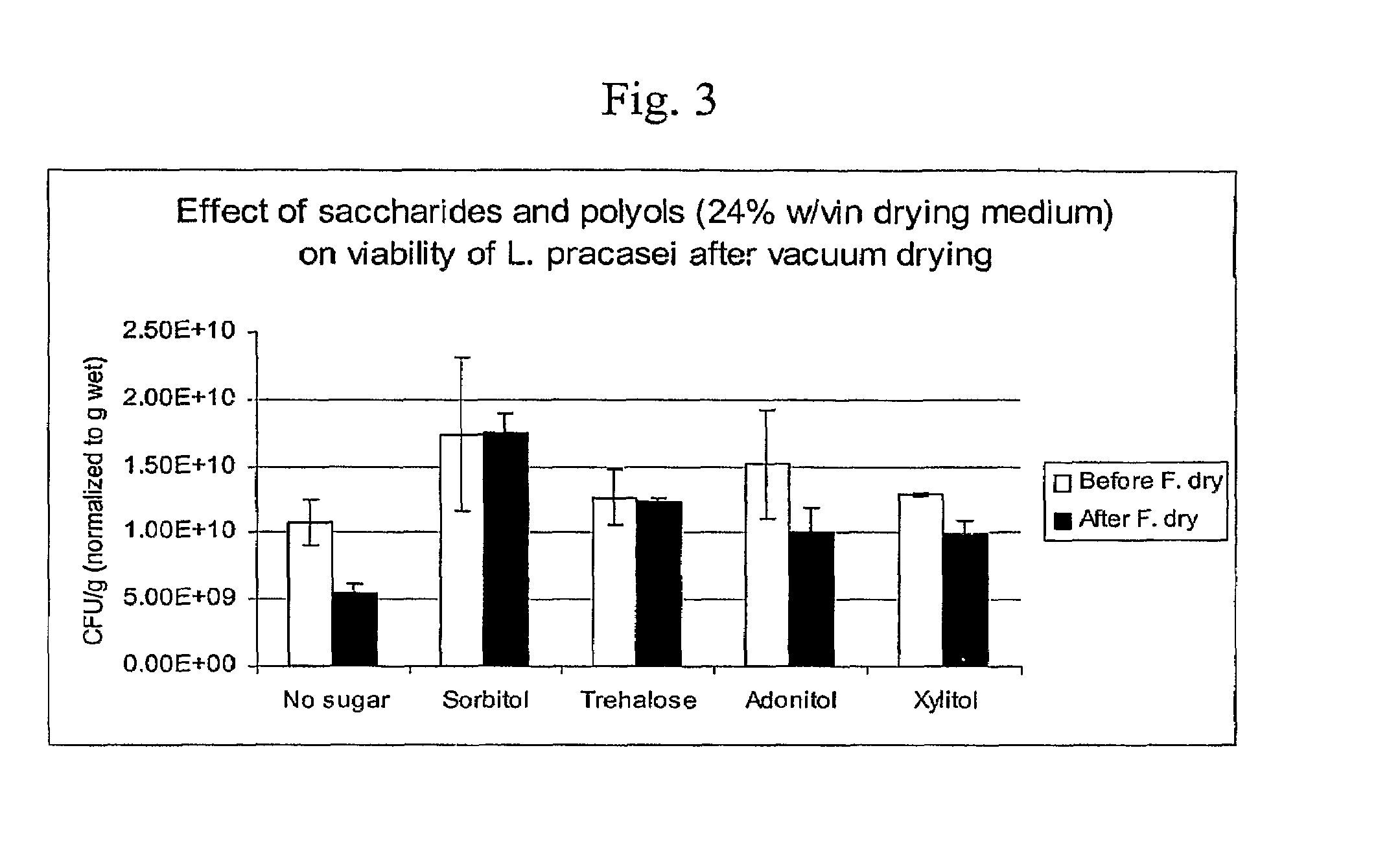
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
788results about "Food ingredient as encapsulating agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
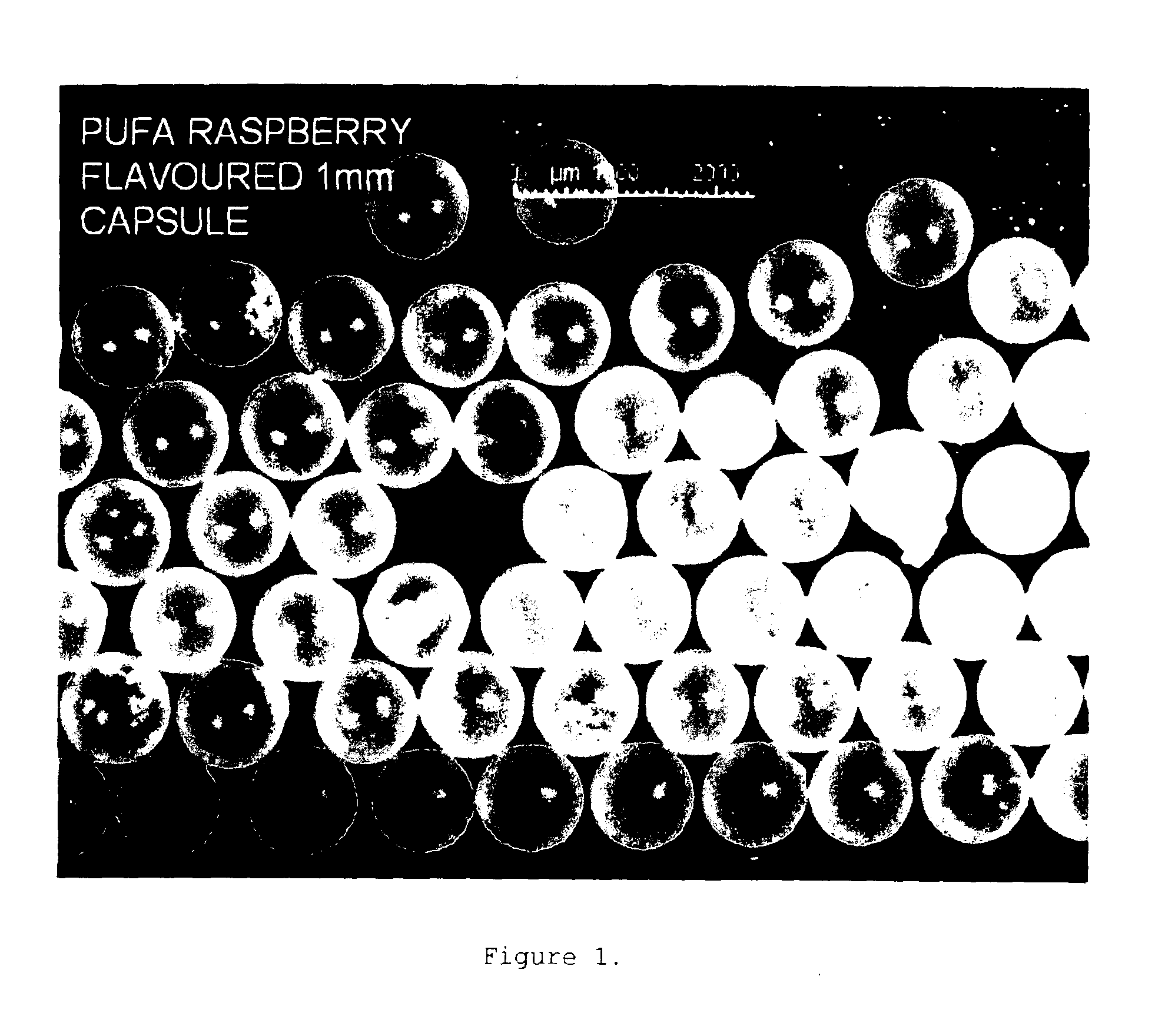
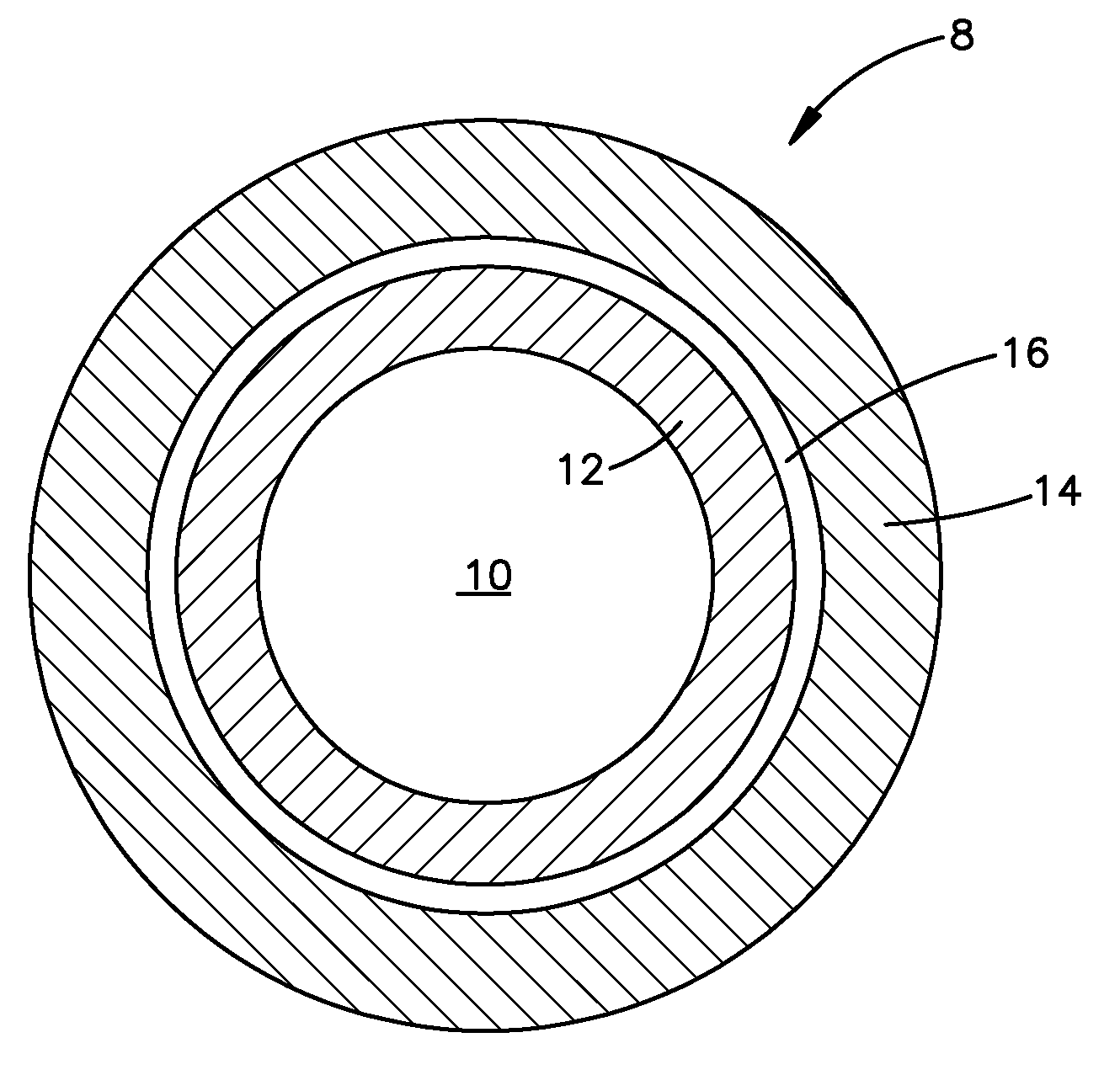
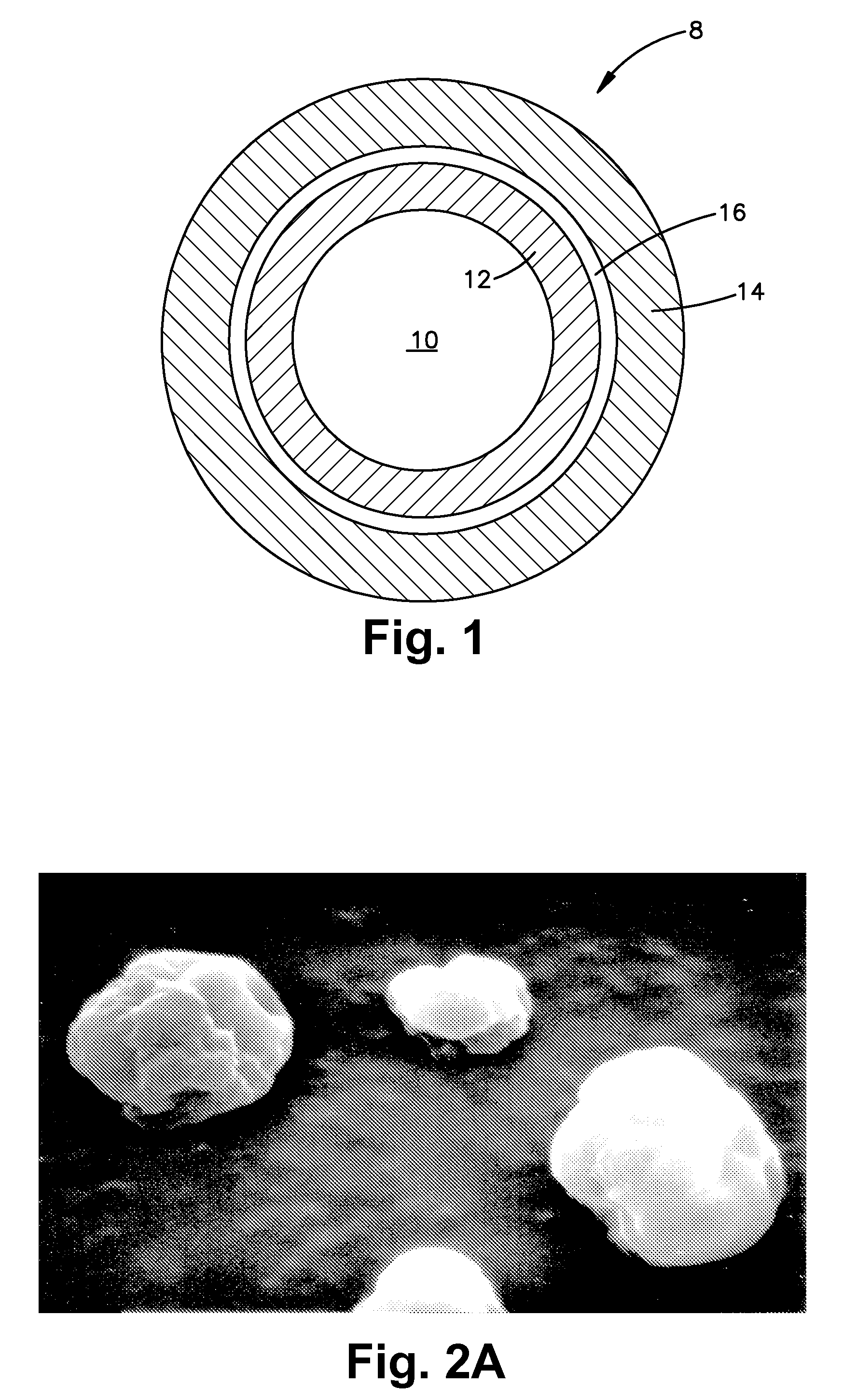
Seamless capsules containing high amounts of polyunsaturated fatty acids and a flavouring component
A seamless capsule includes a core and a shell, wherein the core includes at least one polyunsaturated fatty acid, and at least one flavouring component, the process for manufacturing the capsule and products containing the capsule are also disclosed.
Owner:V MANE FILS
Method of hydration; infusion packet system(s), support member(s), delivery system(s), and method(s); with business model(s) and Method(s)
InactiveUS20020012689A1Constant deliveryUniform deliveryBiocideOrganic active ingredientsDiagnostic Radiology ModalityDietary supplement
Liquid activated infusion packet(s) / system, promoting hydration, containing active and / or inactive ingredients and / or a support member(s). Infusion Packet(s) / System is one or more individual compartments, and / or group(s), whereby the enveloping material(s) may be totally or partially dissolvable, edible, transparent, opaque, decorated, etc. Further, including of one or more: color(s), flavor(s), aroma(s), pharmaceutical(s), nutraceutical(s), dietary supplement(s), enzyme(s), pre / pro-biotic(s), amino-acid(s), soluble-fiber(s), diagnostic agent(s) etc. regardless of form, + / - effervescence, + / - uniform / controlled-release encapsulations into liquid for humans and / or animals. Enveloping material may be in whole and / or in combination; non-synthetic / porous, and / or synthetic porous / non-porous with deliberate perforations. Infusion Packet(s) / System + / - tag, support member for assistance, consumer compliance: promotion, advertising, education, entertainment, (toy / game), etc. Manual and / or power operated parts, lights, noise, etc. Additionally incorporated; unique business modalities with test market opportunities and / or the ability to provide income and / or esteem for the health challenged.
Owner:STILLMAN SUZANNE JAFFE
Delivery system for two or more active components as part of an edible composition
InactiveUS20060034897A1Powder deliveryContainers for annular articlesActive componentPolymer chemistry
A delivery system for inclusion in an edible composition is formulated to have at least two active components with an encapsulating material for managing the release of the two actives relative to each other when used in an edible composition.
Owner:INTERCONTINENTAL GREAT BRANDS LLC
Method and composition to reduce cancer incidence
InactiveUS6090414AReduce distractionsInhibition formationBiocideTetrapeptide ingredientsHydroxybenzoate EthersS oxidation
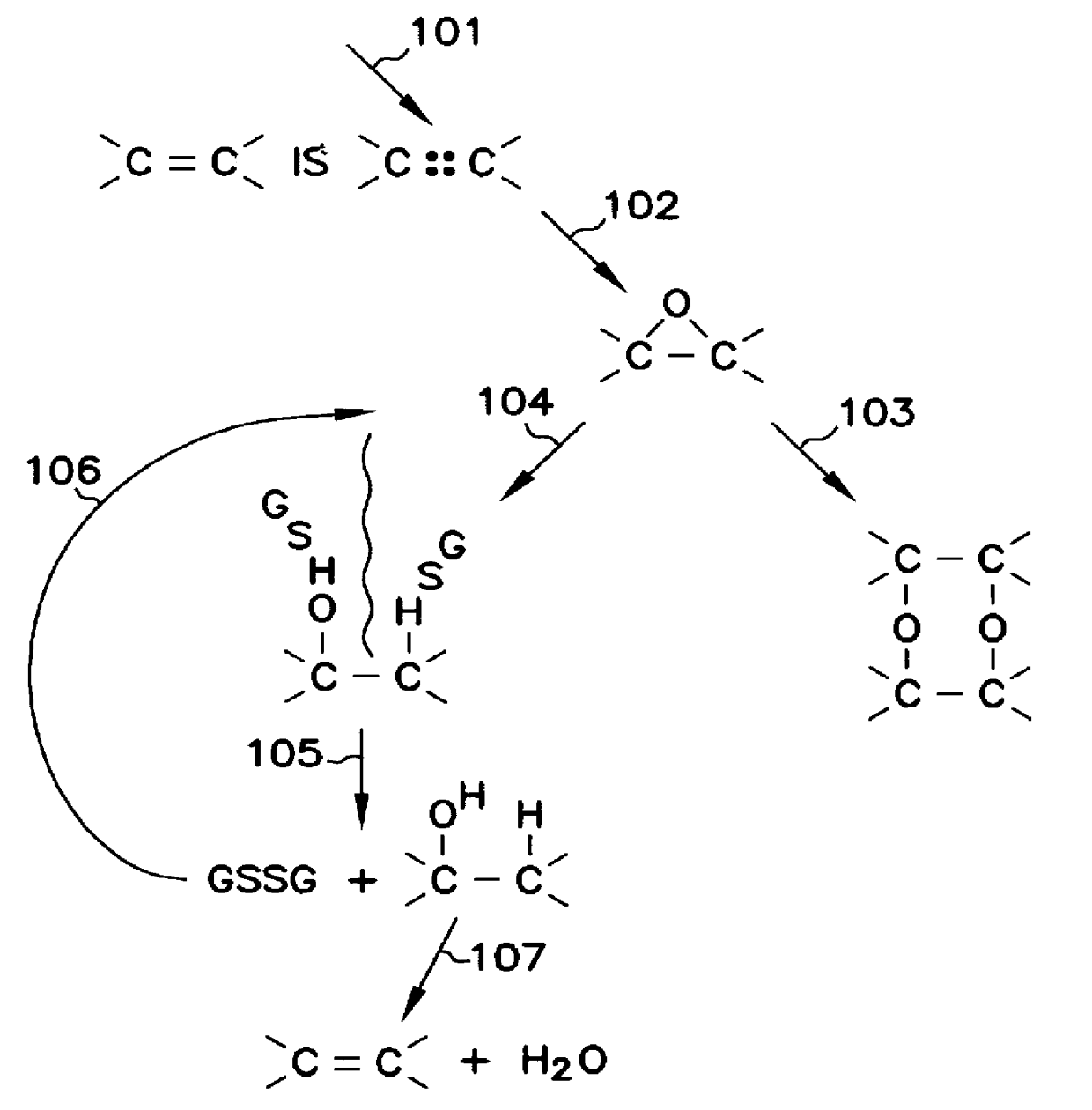
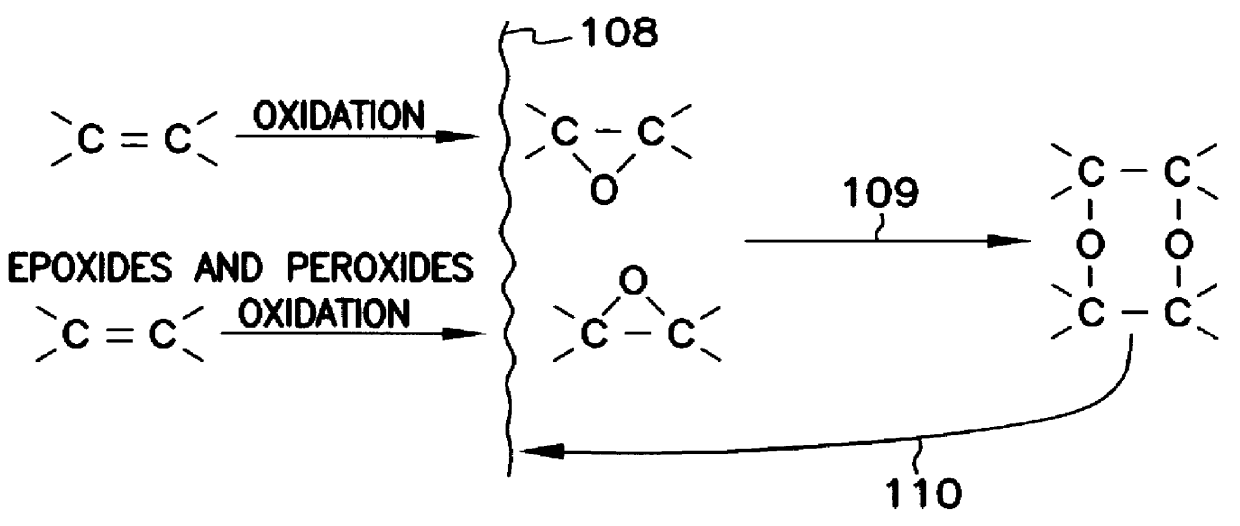
The five component composition consisting essentially of: (1) Water soluble antioxidant vitamin C or ascorbic acid, or any of its forms or derivatives, or mixtures thereof. (2) Oil soluble antioxidant vitamin E or Alpha-tocophorol, or any of its forms or derivatives, or mixtures thereof. (3) The element selenium, or a chemical (or composition) containing it, or mixtures thereof. The most preferred chemical containing selenium is dimethyl selenide and mixtures thereof. The words "dimethyl selenide" here and hereinafter mean dimethyl selenide and / or it's oxidation products, including dimethyl selenoxide. (4) A sulfur amino acid, in any form, or a sulfur peptide, or a sulfur protein, or any of their derivatives, or mixtures thereof. The mixture of methionine and cysteine, which contains as impurities some seleno-methionine and some selenocysteine, is preferred,-the tripeptide glutathione containing cysteine is also preferred. (5) Another antioxidant, other than vitamin C and other than vitamin E, which is synthetic or natural and water soluble or oil soluble, or a mixture of such antioxidants, or a combination of such forms thereof. The mixtures of butylated hydroxyanisole and ethoxyquin is preferred.
Owner:LIFE SCI LAB
Natamycin dosage form, method for preparing same and use thereof
InactiveUS20050042341A1Sustained releaseReduce releaseDough treatmentEggs preservation by coatingFood productsDosage form
The present invention relates to a novel natamycin dosage form for the food industry, and more particularly to microcapsules where natamycin is encapsulated within a physiologically acceptable shell to provide a protected natamycin product. The present invention relates also to novel processes for preparing the capsules according to the invention, as well as to the use of the capsules of the present invention. The invention further relates to food products containing natamycin in encapsulated form.
Owner:AS DE DANSKE SUKKERFABRIKKER
Method of controlling release of N-substituted derivatives of aspartame in chewing gum
InactiveUS6692778B2High consumer acceptanceImprove impact performanceConfectioneryChewing gumControlled releaseHigh intensity
The present invention includes a method for producing a chewing gum with a modified release sweetener selected from the group of N-substituted derivatives of aspartame, particularly neotame, as well as the chewing gum so produced. The modified release neotame or other N-substituted derivative of aspartame sweetener is obtained by physically modifying the sweetener properties by coating and drying. Neotame or another N-substituted derivative of aspartame sweetener is coated by encapsulation, partially coated by agglomeration, entrapped by absorption or extrusion, or treated by multiple steps of encapsulation, agglomeration, absorption, or extrusion. The coated sweetener is then co-dried and particle sized to produce a release-modified high-intensity sweetener. When incorporated into the chewing gum, these particles are adapted to enhance the shelf stability of the sweetener and / or produce a modified release when the gum is chewed.
Owner:WM WRIGLEY JR CO
Coated polyunsaturated fatty acid-containing particles and coated liquid pharmaceutical-containing particles
InactiveUS20060068019A1Powder deliveryLiquid surface applicatorsFatty acids.polyunsaturatedFatty acid
A process for coating a polyunsaturated fatty acid (PUFA)-containing carrier particle or a PUFA matrix particle, or a liquid pharmaceutical-containing carrier particle or a liquid pharmaceutical matrix particle. Also disclosed are such particles made by the process of the invention and foods, pharmaceuticals, beverages, nutritional supplements, infant formula, pet food and animal feed with incorporate such particles.
Owner:SOLAE LLC
Encapsulation compositions and process for preparing the same
InactiveUS20100289164A1Improve physical stabilityReduce lossesBiocidePowder deliveryPolymer sciencePlasticizer
Encapsulation compositions in which an encapsulate (A) is encapsulated in a matrix (B) may be prepared by:(i) mixing matrix (B) with a liquid plasticizer and encapsulate (A) in an extruder, to obtain a melted mixture of encapsulate (A) and matrix (B); and(ii) extruding the melted mixture, to obtain an extruded mixture.
Owner:MCCORMICK & CO INC
Encapsulated agglomeration of microcapsules and method for the preparation thereof
Microcapsules comprising an agglomeration of primary microcapsules, each individual primary microcapsule having a primary shell and the agglomeration being encapsulated by an outer shell, may be prepared by providing an aqueous mixture of a loading substance and a shell material, adjusting pH, temperature, concentration and / or mixing speed to form primary shells of shell material around the loading substance and cooling the aqueous mixture until the primary shells agglomerate and an outer shell of shell material forms around the agglomeration. Such microcapsules are useful for storing a substance and for delivering the substance to a desired environment.
Owner:DSM NUTRITIONAL PROD
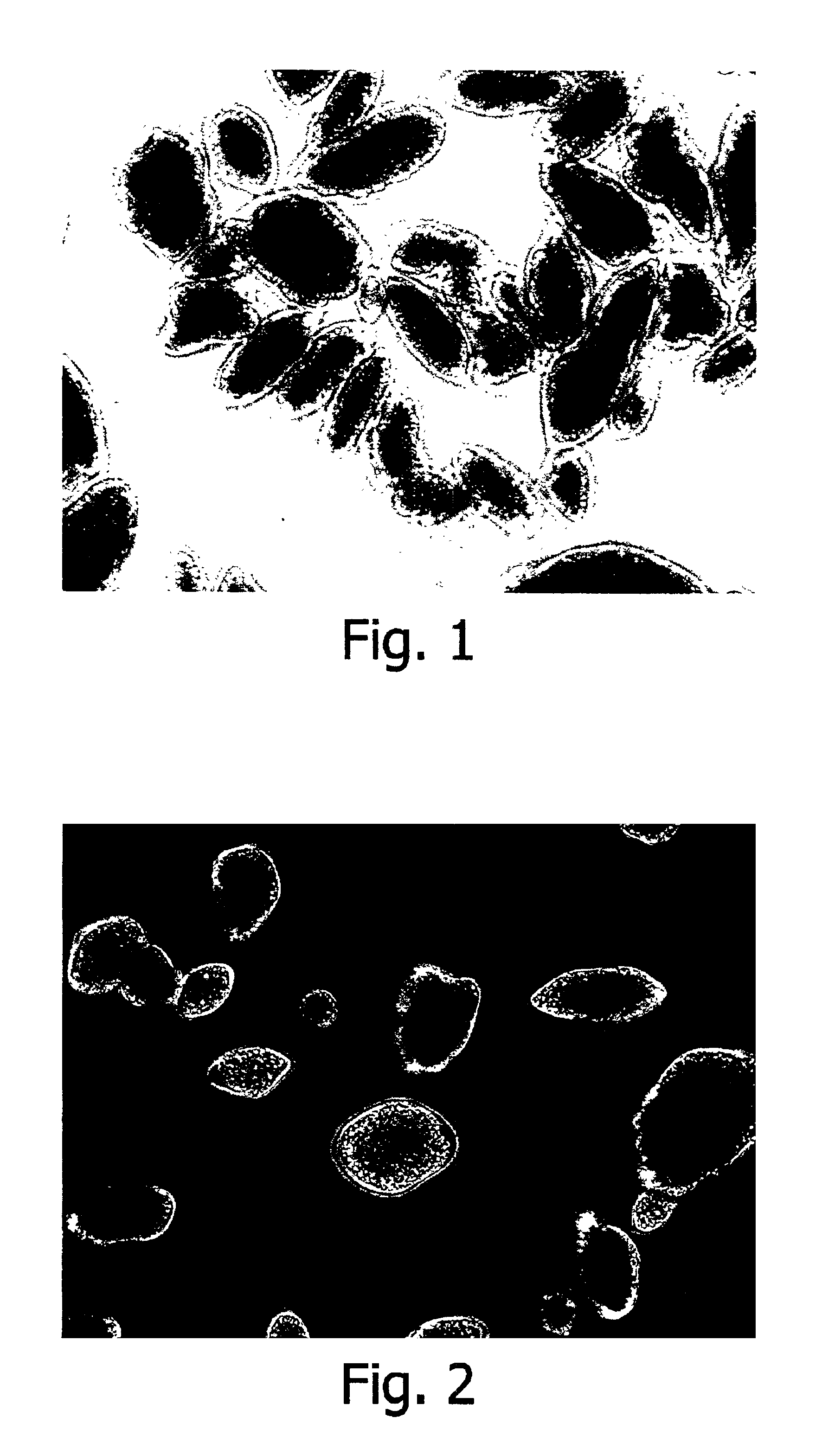
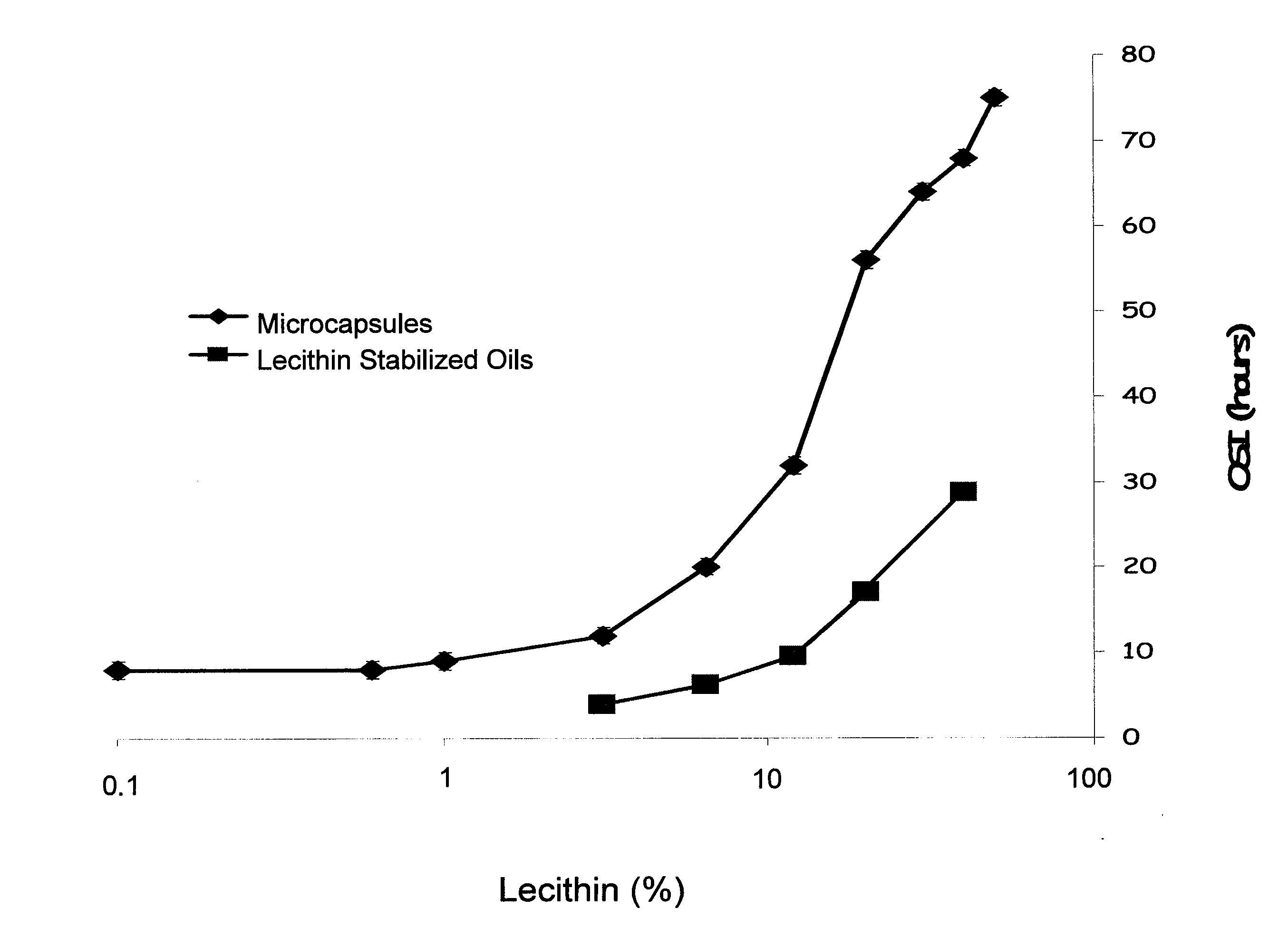
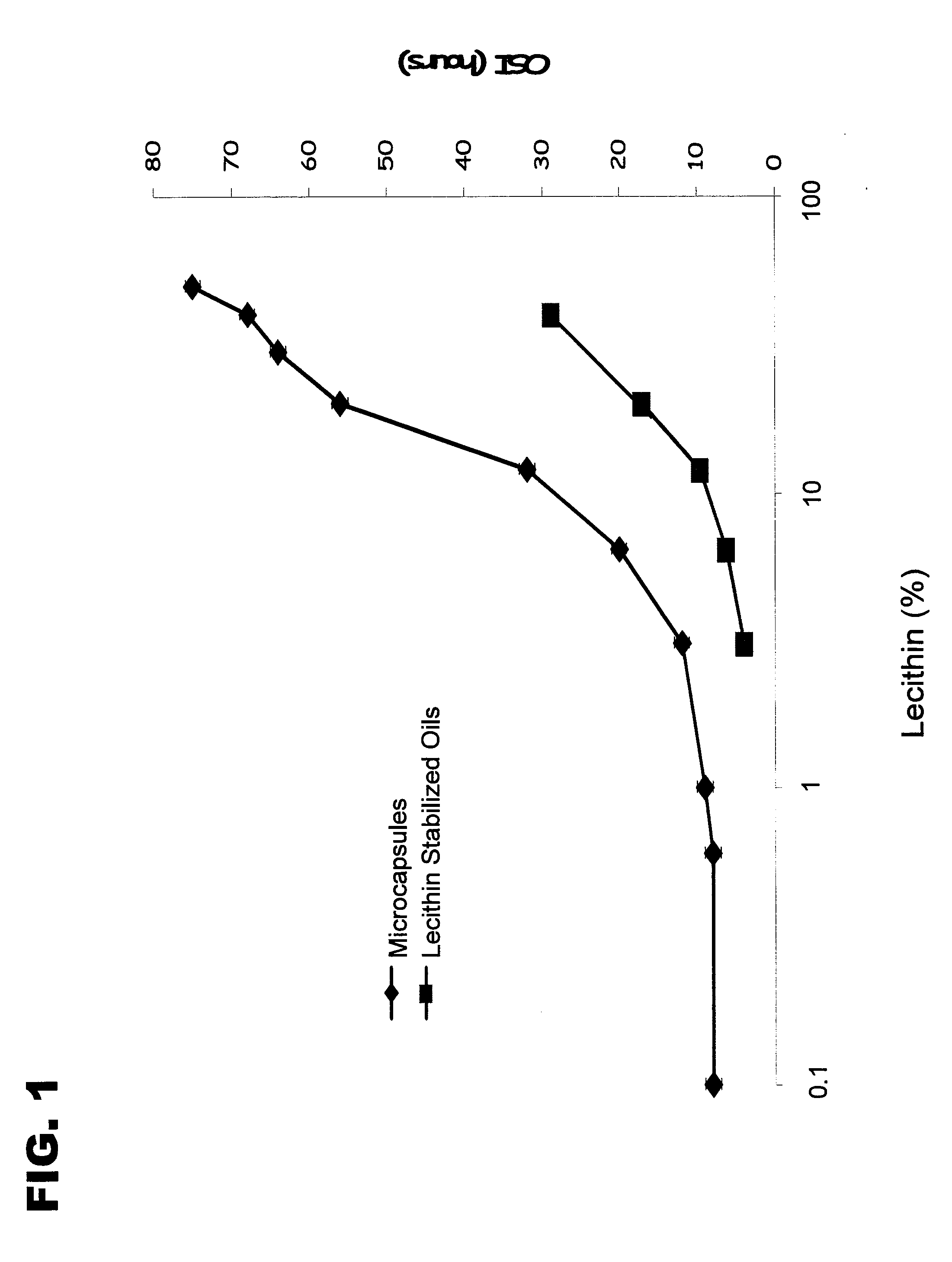
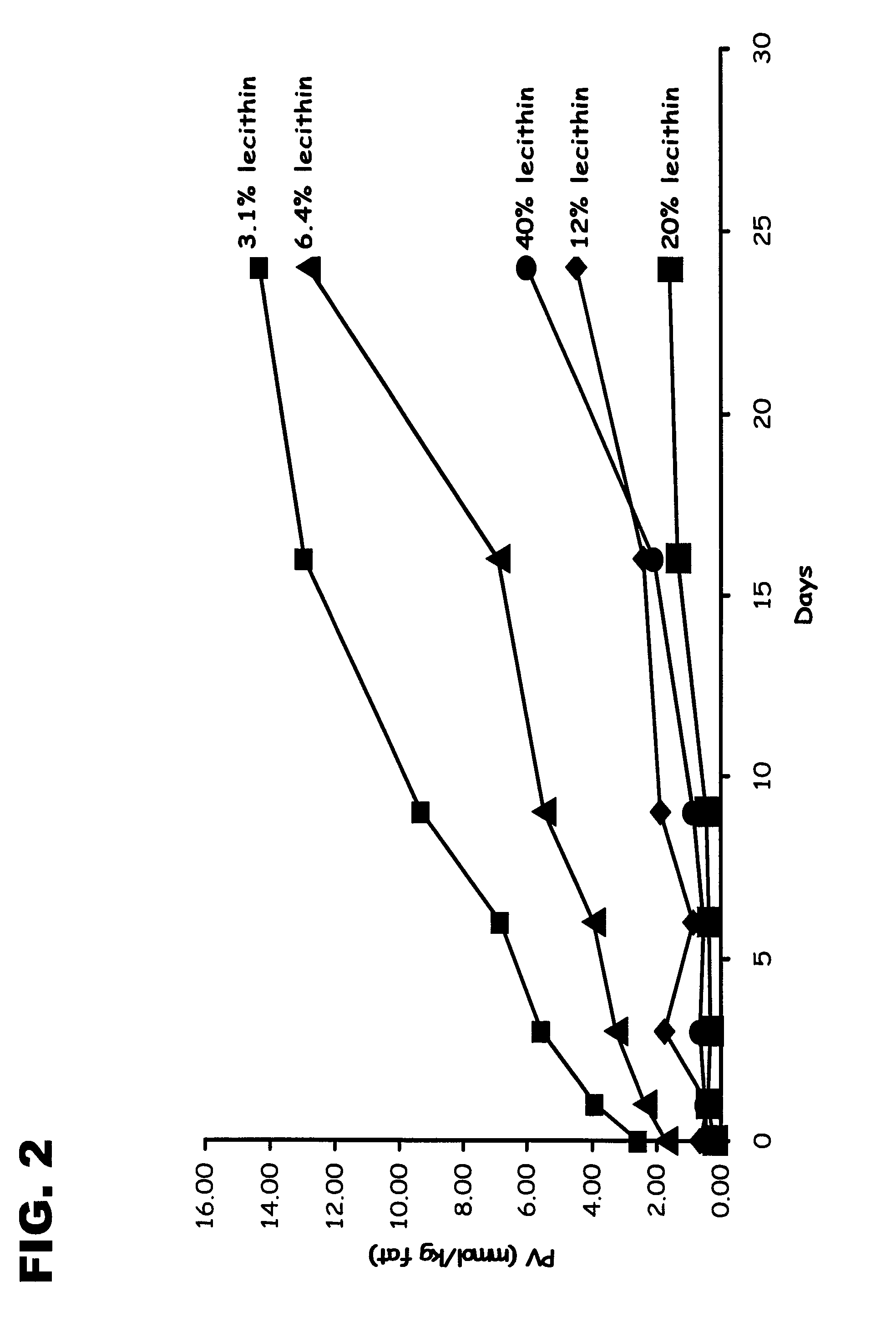
Encapsulated Phospholipid-Stabilized Oxidizable Material
InactiveUS20070141211A1Reduce oxidationLiquid surface applicatorsFatty substance preservation using additivesPhospholipidFood products
Compositions and methods to reduce the oxidation of an oxidizable material are disclosed herein. The invention provides a microcapsule comprising a core material, which is the phospholipid-stabilized oxidizable material, and a shell wall that encapsulates the core material. Food products comprising an edible material and a microcapsule of phospholipid-stabilized oxidizable material are also disclosed.
Owner:SOLAE LLC
Encapsulated flavor and/or fragrance composition
InactiveUS6932982B2Prevents release of flavorPrevents release of fragranceCosmetic preparationsPowder deliveryControlled releaseAdditive ingredient
The invention relates to a granular delivery system based on a matrix combining at least a carbohydrate material with from 1 to 7% of prehydrated agar agar. The system disclosed is particularly stable in aqueous environments and is capable of providing the controlled release of an active flavoring or perfuming ingredient there-encapsulated.
Owner:FIRMENICH SA
Acid-resistant soft gel compositions
The present disclosure describes a delivery device for administration of nutraceuticals or pharmaceuticals, which device contains a soft gel shell comprising a gelatin-based water soluble film forming polymer, an acid insoluble polymer, and at least one reducing sugar and water, including processes, gel mixtures used for device production, and coatings containing such gel mixtures.
Owner:HASSAN EMADELDIN M
Probiotic composition having acid-resistant enteric coating
InactiveUS20070059296A1Improve digestibilityImproving resistance against diseaseOrganic active ingredientsBiocideBiotechnologyTalc
A probiotic composition essentially comprises 15 to 20 wt % of milk powder, 25 to 30 wt % of corn starch, 8 to 15 wt % of modified starch (capsul), 10 to 15 wt % of ethylcellulose, 5 to 15 wt % of bacterial broth, and 10 to 15 wt % of talc. The probiotic composition is microencapsulated to form a plurality of microencapsule coated with an acid-resistant enteric coating for improving the enteric acid-resistance, the probiotic survival rate, the antimicrobial property, the stability, the moisture-proof property, and the mobility of the probiotic composition preventing from coagulation in a moist environment and for being used as an additive applied to livestock feed.
Owner:BION TECH INC
Encapsulation compositions and process for preparing the same
Encapsulation compositions in which an encapsulate (A) is encapsulated in a matrix (B) may be prepared by:(i) mixing matrix (B) with a liquid plasticizer and encapsulate (A) in an extruder, to obtain a melted mixture of encapsulate (A) and matrix (B); and(ii) extruding the melted mixture, to obtain an extruded mixture.
Owner:MCCORMICK & CO INC
Spherical single-substance particles, medicines and foodstuffs containing the particles, and method of production thereof
The present invention relates to a process for producing a spherical particle comprising an aggregate of particles containing at least 95% of a water-soluble single substance having a viscosity of 10 mPa.s or less as determined in the form of a saturated aqueous solution, the process comprising: preparing moist spherical particles of the single substance by charging, as cores, crystalline particles or granulated particles of the single substance on a rotary disc in a processing vessel of a centrifugal tumbling granulating apparatus, wherein the granulated particles are prepared by granulating a powder of the single substance, and dispersing over the cores a powder of the single substance and simultaneously spraying on the cores a liquid such as water or the like while supplying slit air to provide a fluidized condition; and then fixation treating the moist spherical particles by drying them while spraying an aqueous solution of the single substance or the like on the spherical particles in a fluidized bed apparatus; to the spherical particle produced by the process; and to a pharmaceutical preparation and a food containing the spherical particle.
Owner:FREUNT IND +1
Microcapsules
InactiveUS20070042184A1Increase release rateControl releaseBiocideEggs preservation by coatingBULK ACTIVE INGREDIENTActive ingredient
The present invention relates to microcapsules, and more particularly to microcapsules where an aqueous bead or beads comprising the active ingredient is encapsulated in or by a hydrophobic shell matrix. The present invention relates also to novel methods for preparing the microcapsules according to the invention, as well as to the use of the microcapsules of the present invention. A microcapsule of the present invention comprises a solidified hydrophobic shell matrix, an encapsulated aqueous bead or beads which is / are encapsulated in or by the solidified hydrophobic shell matrix, and an active ingredient or active ingredients dissolved or incorporated in the encapsulated aqueous bead or beads.
Owner:DUPONT NUTRITION BIOSCIENCES APS
Long-duration encapsulated flavors and chewing gum using same
InactiveUS20070231424A1Easy to keepIncreases flavor durationSugar food ingredientsChewing gumCORN SYRUP SOLIDSFood flavor
A chewing gum composition comprises about 5% to about 95% gum base, about 5% to about 96% bulking and sweetening agents, and about 0.1% to about 15% flavor, wherein at least part of the flavor is a long-duration flavor material comprising a vinyl polymer encapsulated matrix. The matrix itself includes about 30% to about 60% acacia gum, about 30% to about 60% corn syrup solids having a DE of between about 24 and about 44 or equivalent hydrogenated starch hydrolysates, and about 2% to about 20% hydrocolloid material, with the acacia gum and corn syrup solids or hydrogenated starch hydrolysates together comprising at least 80% of the matrix. The vinyl polymer comprises between about 30% and about 80% of the long-duration flavor material.
Owner:WM WRIGLEY JR CO
Stable tooth whitening gum with reactive ingredients
ActiveUS20060177383A1Reaction can be limitedExtended shelf lifeCosmetic preparationsToilet preparationsMedicineActive component
Oral compositions including an encapsulated active component(s) are provided. The oral compositions include a carrier composition including a first reactive component. The oral compositions also include an active composition. The active composition includes at least one active component encapsulated in a coating. The coating includes a hydrophilic material and at least one second reactive component, wherein an interaction between the first and second reactive components preserves the activity and / or availability of the active.
Owner:INTERCONTINENTAL GREAT BRANDS LLC
Encapsulation system for protection of probiotics during processing
Comestible products, for example beverage products, are disclosed containing encapsulated probiotic bacteria having resistance to subjection to at least thermal and acidic conditions. Beverage products include at least one aqueous liquid and capsules comprising a gelled mixture of alginate and denatured protein, and probiotic bacteria entrapped within the gelled mixture. The average particle size of the capsules is optionally less than 1000 microns (μm) in diameter, such as less than 500 μm in diameter. Methods are provided for making such encapsulated probiotics by providing a mixture comprising sodium alginate, denatured protein and active probiotic cells, and combining the mixture with a divalent cation to initiate cold gelation of the sodium alginate and denatured protein to form a second mixture. The second mixture is passed through an opening having a diameter of less than 1000 μm to form capsules. The weight ratio of protein to alginate is from 1:1 to 9:1.
Owner:PEPSI COLA TECHNICAL OPERATIONS INC +1
Carbohydrate nanoparticles for prolonged efficacy of antimicrobial peptide
A nanoparticle includes a carbohydrate carrier and a bacteriocin. A method for prolonging efficacy of a bacteriocin against a food pathogen includes providing the bacteriocin in a delivery system, and inhibiting the food pathogen by the bacteriocin. A duration of efficacy of the bacteriocin against the food pathogen when the bacteriocin is provided in the delivery system exceeds a duration of efficacy of the bacteriocin when the bacteriocin is provided without the delivery system.
Owner:PURDUE RES FOUND INC
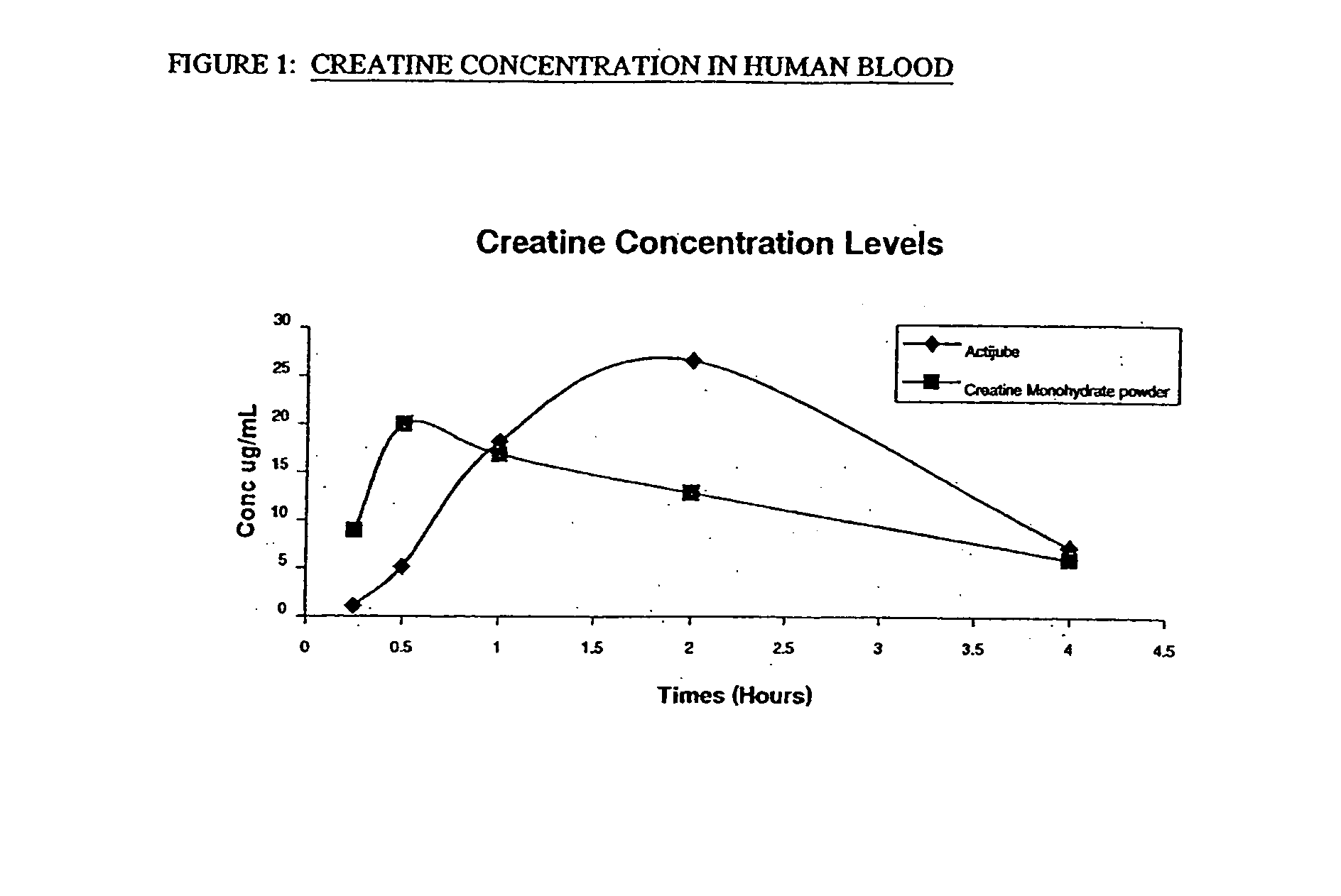
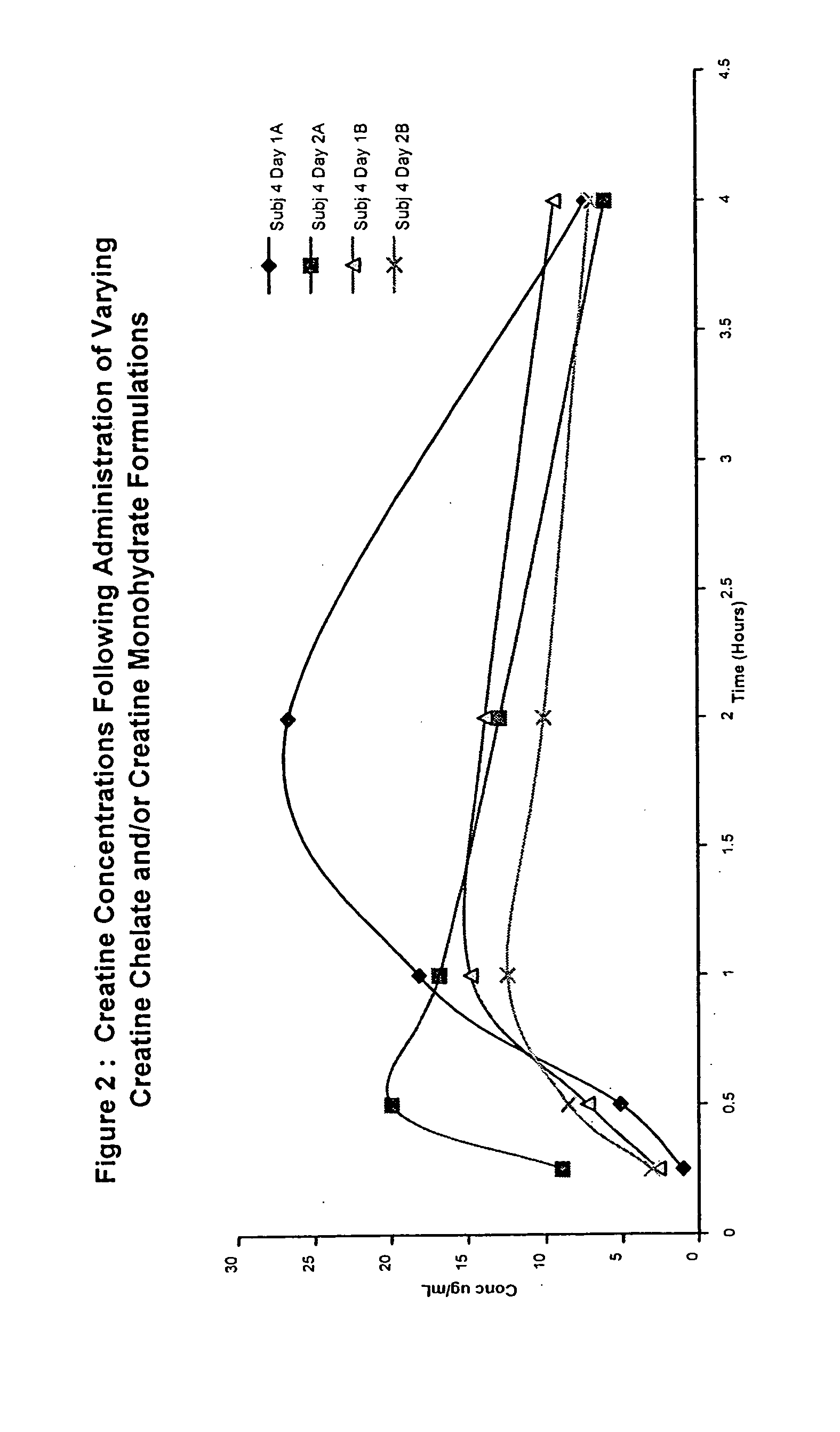
Starch-based delivery system for creatine
InactiveUS20040013732A1Promote absorptionMinimize degradationPowder deliverySugar food ingredientsAdditive ingredientVitamin
The present invention provides an oral delivery system for creatine. The creatine delivery system may additionally contain other bioactive ingredients such as nutraceuticals, botanicals, and vitamins. The delivery system comprises an ingestible matrix within which a creatine formulation and optionally one or more bioactives are substantially uniformly and completely dispersed and in which degradation of the creatine and other bioactives is minimised or eliminated. The invention also provides methods of preparing and using the delivery system.
Owner:2120812 ONTARIO
Microencapsulated Oil Product and Method of Making Same
ActiveUS20090004333A1Increase loadAvoid excessive shear and damageDough treatmentConfectionerySolid shellProtein free
A microencapsulated product comprises a core, a first shell comprising a protein and being substantially carbohydrate-free, and a second shell comprising a carbohydrate and being substantially protein-free. The double shell structure provides a strong shell that makes the microcapsule suitable for use in food products. The core can be a lipid, and in particular a structured lipid with nutritional benefits, such that the nutritional benefits can be passed on to the consumer. The microcapsules of the present invention can be used in making foods products, beverage products, and mixes for making such food and beverage products.
Owner:BUNGE OILS INC
Delivery vehicle for probiotic bacteria comprising a dry matrix of polysaccharides, saccharides and polyols in a glass form and methods of making same
ActiveUS8097245B2Promote digestionOverall firmnessBiocideSugar food ingredientsPolyolDelivery vehicle
The disclosure relates to a solid glass matrix of polysaccharide, saccharides and polyols as delivery vehicle for preservation and post gastric administration of a probiotic. The delivery vehicle is capable of releasing the probiotic at their site of action. The present invention further includes methods of making and using the solid glass matrix delivery vehicle of the invention.
Owner:ADVANCED BIONUTRITION CORP
Heat resistant probiotic compositions and healthy food comprising them
Owner:DEGAMA PROBIOTICS +1
Encapsulation of food ingredients
InactiveUS20030185960A1Small particle sizeGood emulsificationSugar food ingredientsFatty substance preservation using additivesMaillard reactionAdditive ingredient
Oxygen sensitive oils or oils containing oil soluble oxygen sensitive substances are encapsulated in proteins which have been reacted with carbohydrates that contain reducing sugar groups. An aqueous mixture of a protein preferably casein and a carbohydrate preferably a sugar is heated within the range of 60 to 160° C. so that Maillard reaction products are formed in the aqueous mixture. The oil phase, up to 50% by weight is then emulsified with the aqueous phase to form micro encapsulated oil particles. The formation of MRP may also be done after emulsification prior to drying. The emulsions can be used as food ingredients or dried to form powders
Owner:COMMONWEALTH SCI & IND RES ORG
Encapsulated agglomeration of microcapsules and method for the preparation thereof
Microcapsules comprising an agglomeration of primary microcapsules, each individual primary microcapsule having a primary shell and the agglomeration being encapsulated by an outer shell, may be prepared by providing an aqueous mixture of a loading substance and a shell material, adjusting pH, temperature, concentration and / or mixing speed to form primary shells of shell material around the loading substance and cooling the aqueous mixture until the primary shells agglomerate and an outer shell of shell material forms around the agglomeration. Such microcapsules are useful for storing a substance and for delivering the substance to a desired environment.
Owner:DSM NUTRITIONAL PROD
Compositions and Methods for Promoting Gastrointestinal and/or Cardiovascular Health
InactiveUS20110027412A1Promoting cardiovascular healthPromoting gastrointestinal healthMilk preparationDough treatmentDietary fiberMedicine
A composition for promoting gastrointestinal and / or cardiovascular health comprising a gelling dietary fiber and a non-gelling dietary fiber in a weight ratio of from about 5:1 to about 1:2.5; a coating component and a wetting component in a weight ratio of from about 20:1 to about 1:1; wherein a weight ratio of coating component plus wetting component to said gelling dietary fiber or said non-gelling dietary fiber is from about 25:1 to about 1:5 is disclosed. Also disclosed herein is a composition for promoting gastrointestinal and / or cardiovascular health comprising a gelling dietary fiber; a non-gelling dietary fiber; and an amount of a coating component sufficient to coat the particles of the gelling dietary fiber, such that the water-absorbing ability is reduced and the organoleptic properties of the composition are improved. Methods of promoting gastrointestinal and / or cardiovascular health are also included.
Owner:THE PROCTER & GAMBLE COMPANY
Nutrient clusters for food products and methods of preparation
Nutrient clusters for food products, such as for addition to Ready-To-Eat cereals, are made in the form of aggregates or clusters comprising a first particulate component; a nutrient powder blend, and sufficient binder to adhere the powder to the particulates. The nutrient clusters are in the form of pieces each weighing from about 0.3 to 5 g. and having a moisture content of about 2% to 10%. Such nutrient clusters are prepared by applying a liquid binder to the particulates to form sticky particulates, adding a powdered nutrient blend, and curing the mixtures to form hardened dried nutrient clusters. The nutrient cluster can contain 100% US recommended daily allowance of essential vitamins and minerals and can contain added macronutrients such as soy proteins, soluble fiber, and / or calcium in nutritionally dense form in as little as 5 to 15 g of nutrient clusters. The clusters find particular suitability for use in providing to-order customized cereal products in response to particular customer requirements for nutrition.
Owner:GENERAL MILLS INC
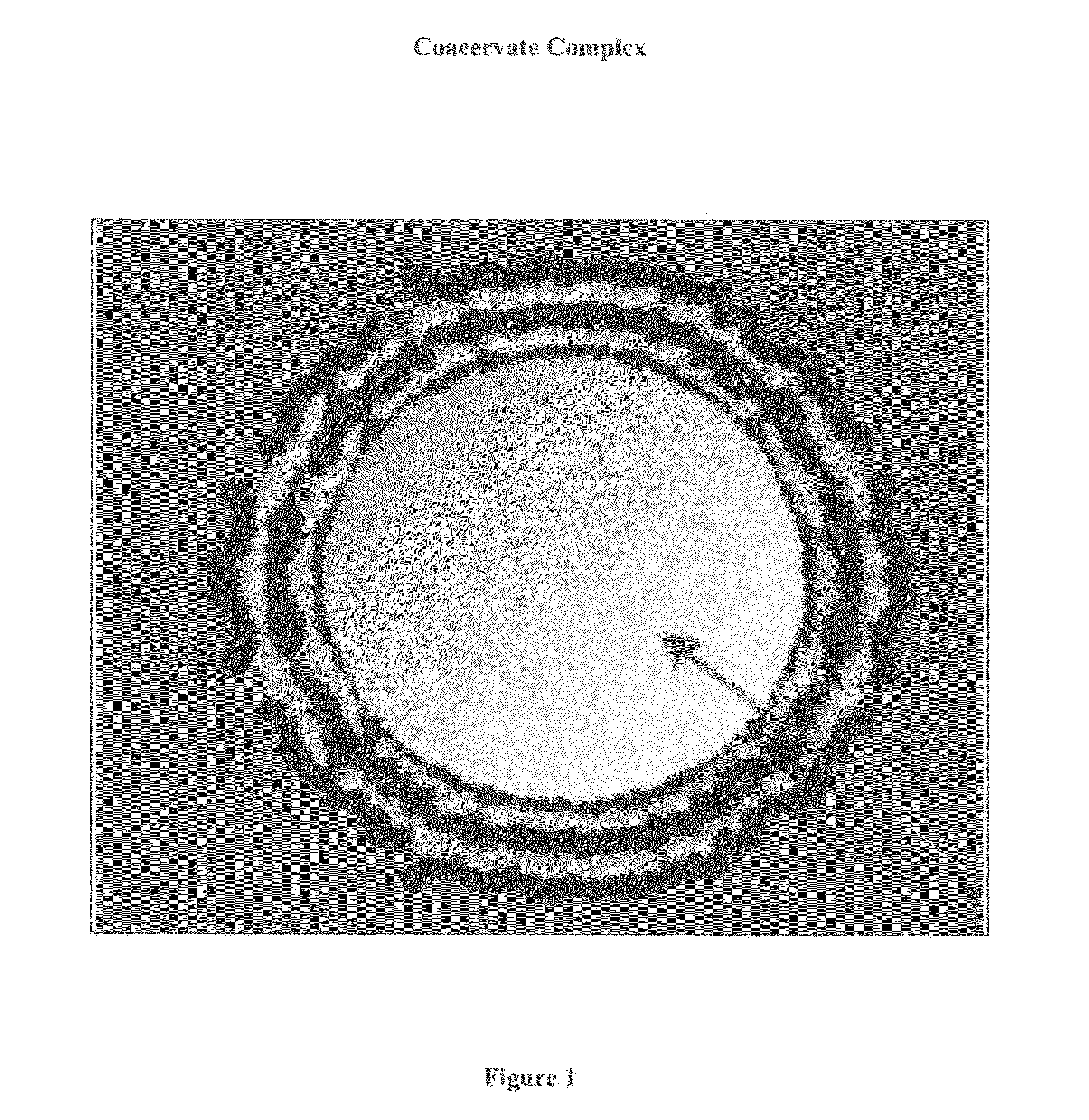
Coacervate complexes, methods and food products
InactiveUS20130004617A1Reduce and eliminate oxidationNegative effectFatty substance preservation using additivesFood shapingCoacervateFood item
Complex coacervates incorporating one or more hydrophobic substances are provided, that are stable in certain aqueous systems and food products. The coacervates may be used as an ingredient in food products, e.g., in beverages, dry foods, and semi-moist foods. Methods for producing the complex coacervates and food products are also disclosed herein.
Owner:PEPSICO INC
Omega-3 Fatty Acids Encapsulated In Zein Coatings and Food Products Incorporating the Same
Disclosed are processes for stabilizing omega-3 fatty acids for use in food products. The processes permit creation of a variety of food forms and food ingredients that contain omega-3 fatty acids like docosahexaenoic acid and eicosapentaenoic acid wherein these foods and food forms are stable for months without developing fishy aromas or tastes. This stability enables the incorporation of omega-3 fatty acids into food forms such as ready to eat cereals, trail mixes, chips, granola bars, toaster pastries, baked goods, cookies, crackers, fruit pieces and fruit leathers. The processes utilize a zein coating to protect and stabilize the omega-3 fatty acids.
Owner:KELLOGG CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com