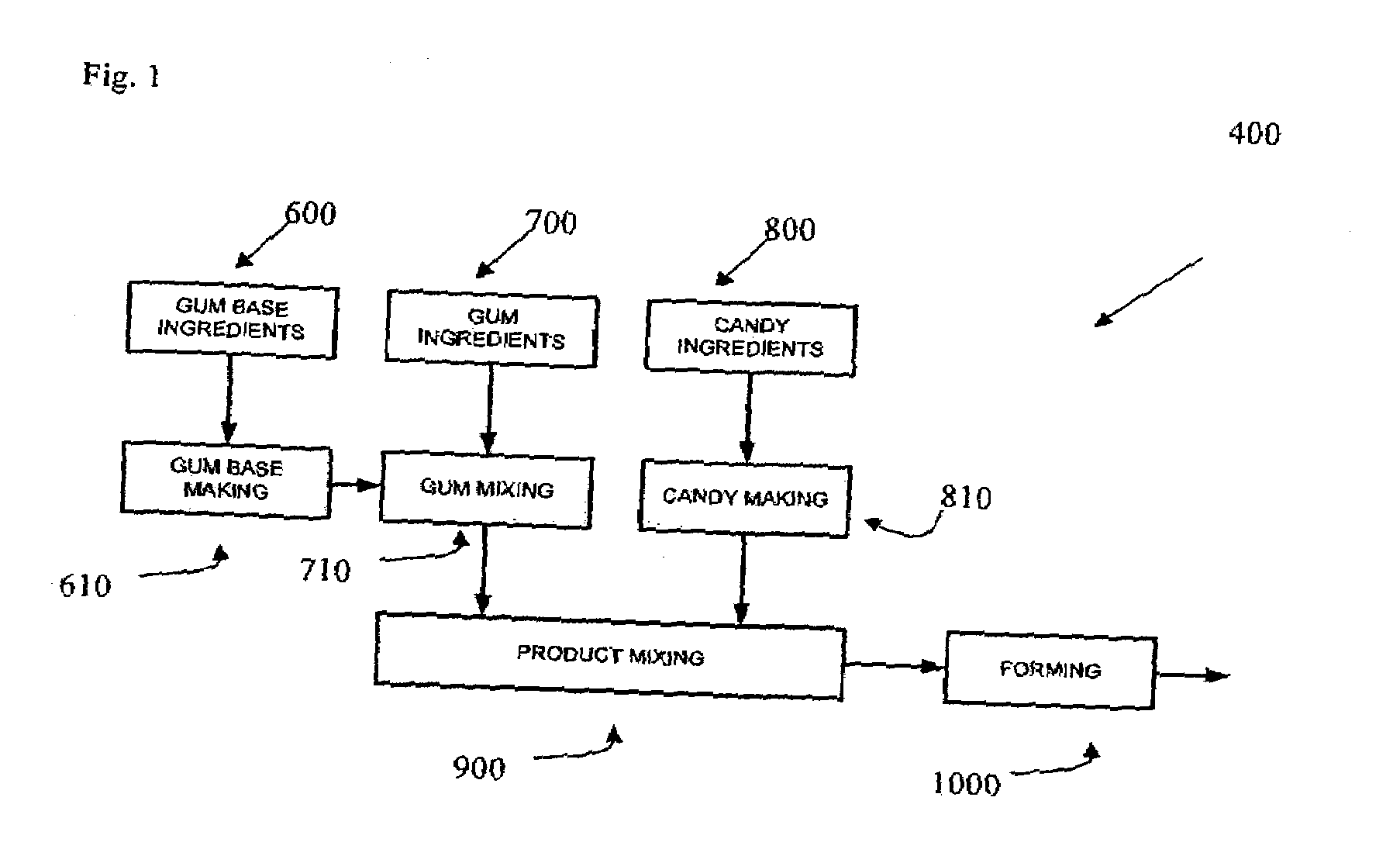
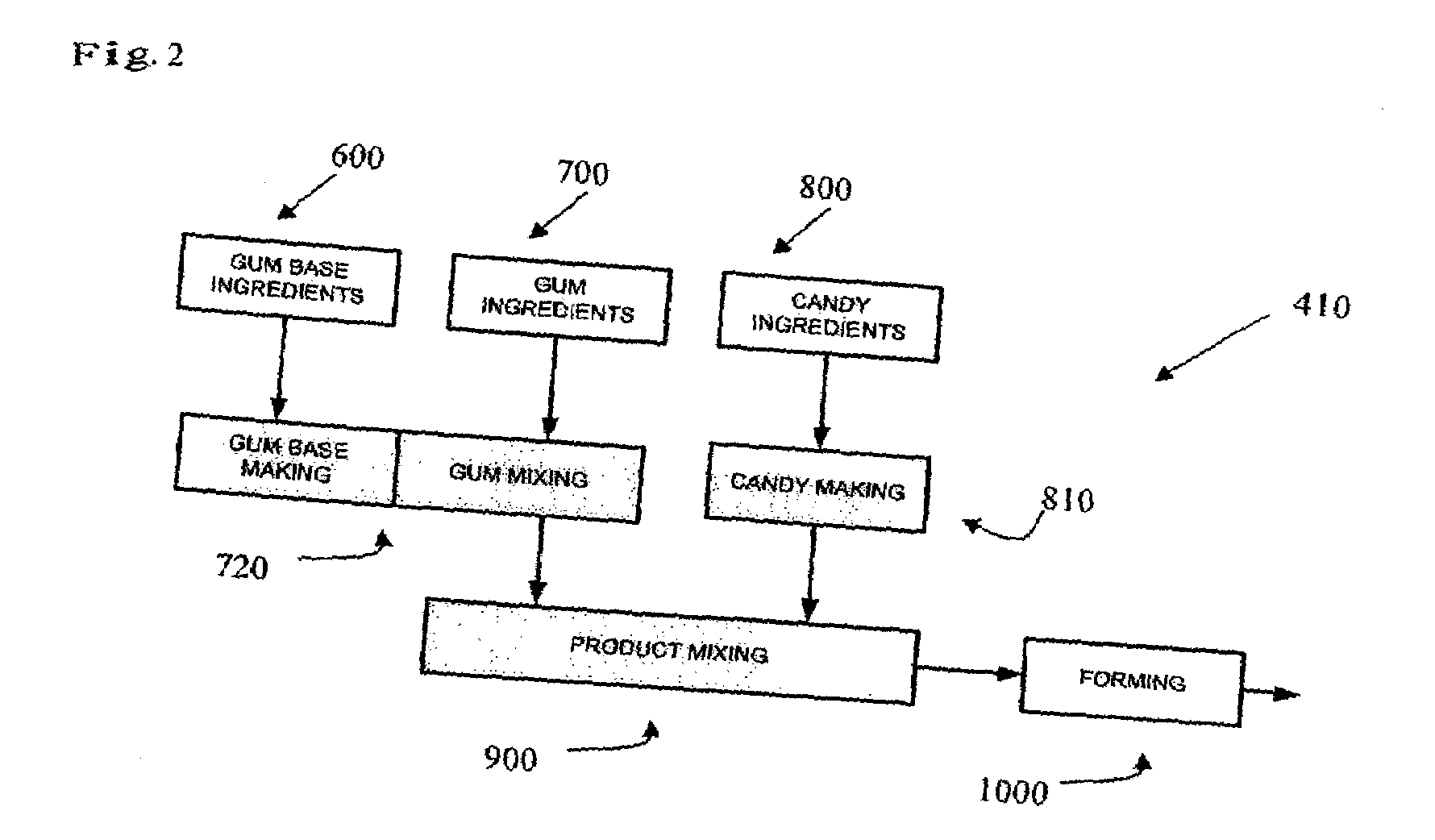
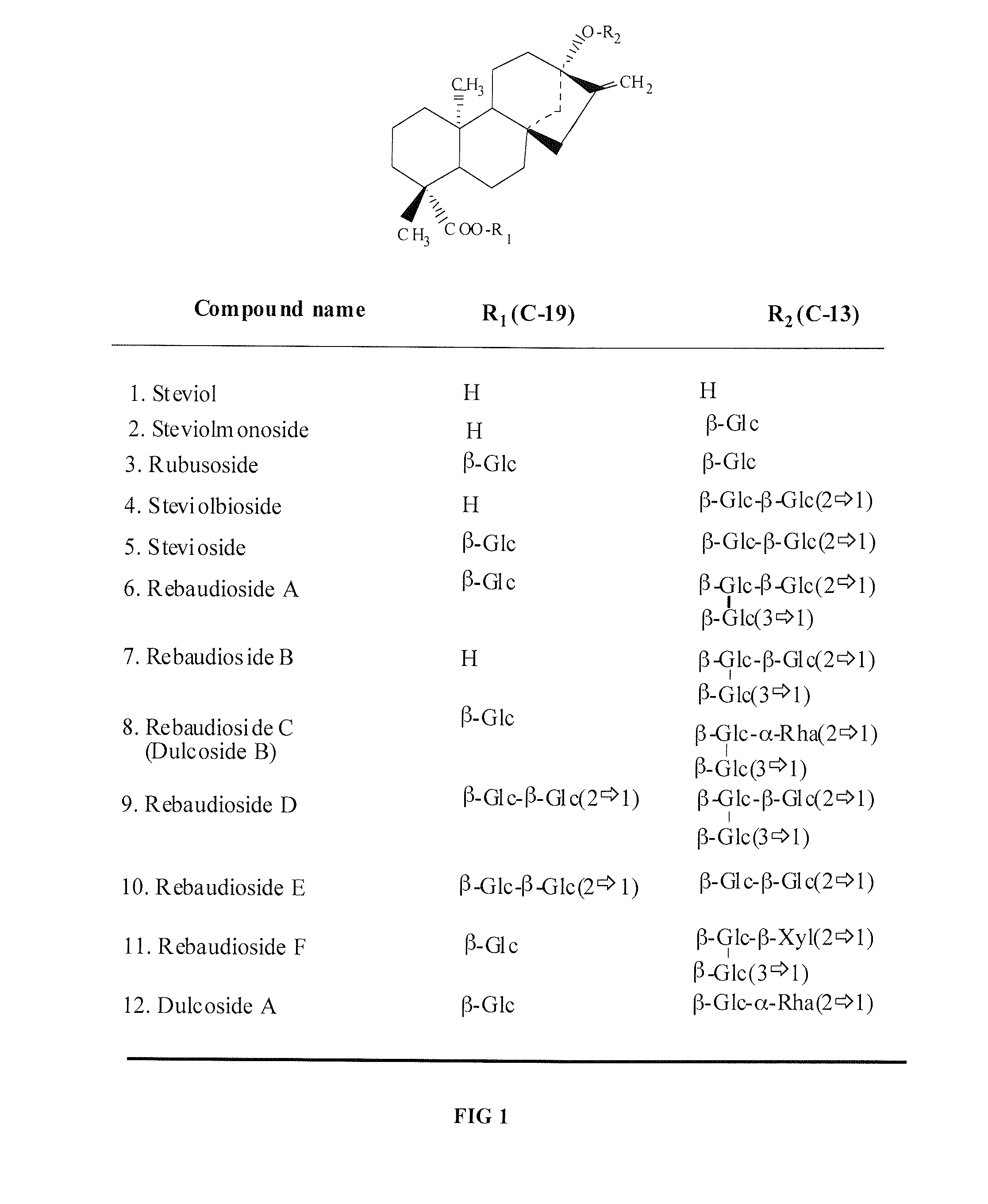
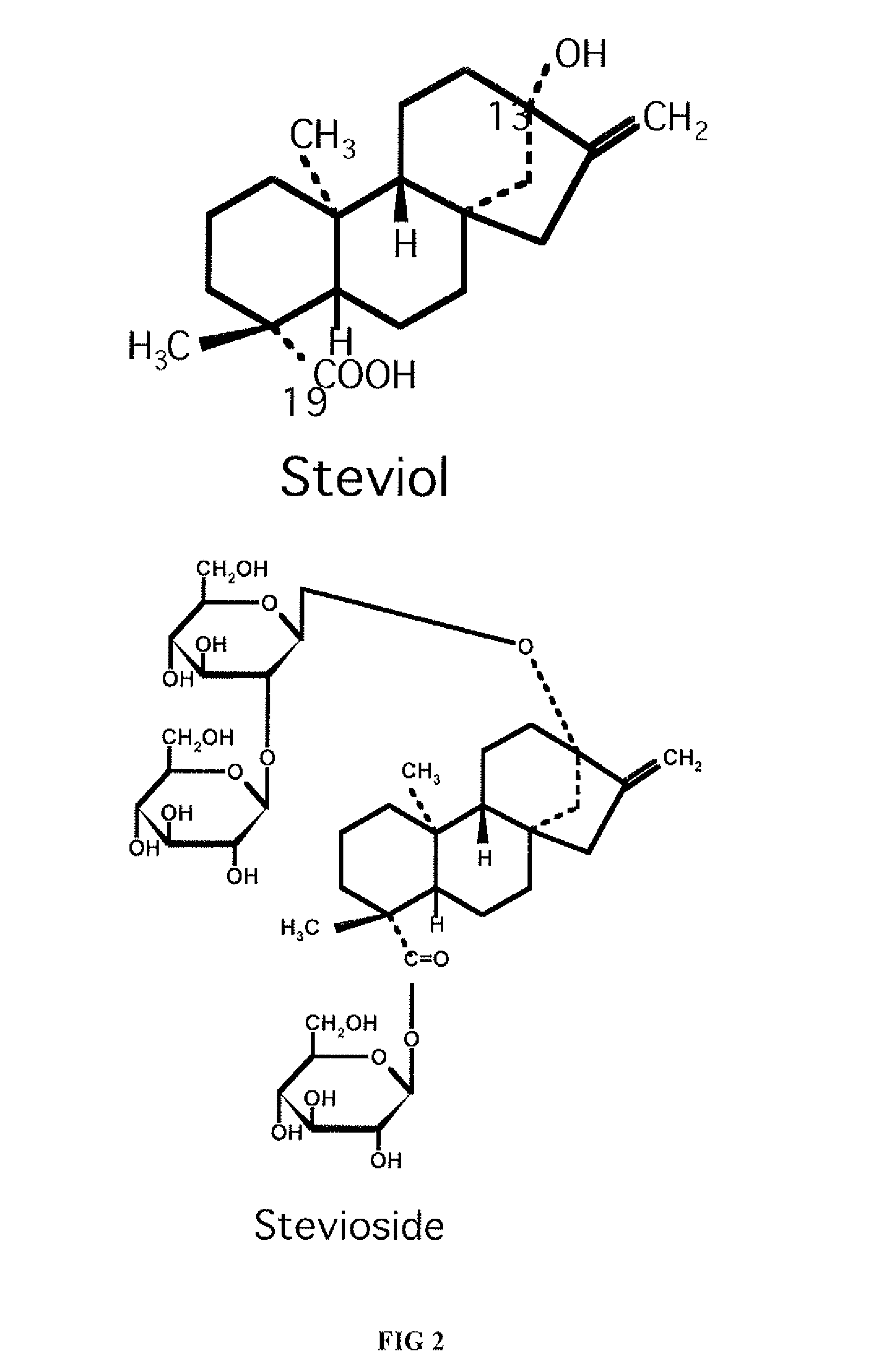
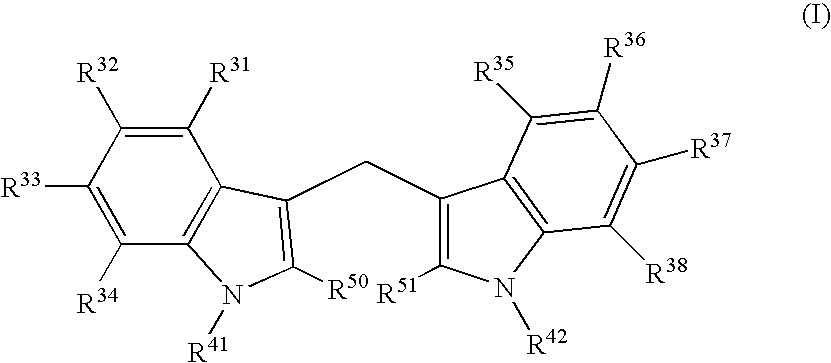
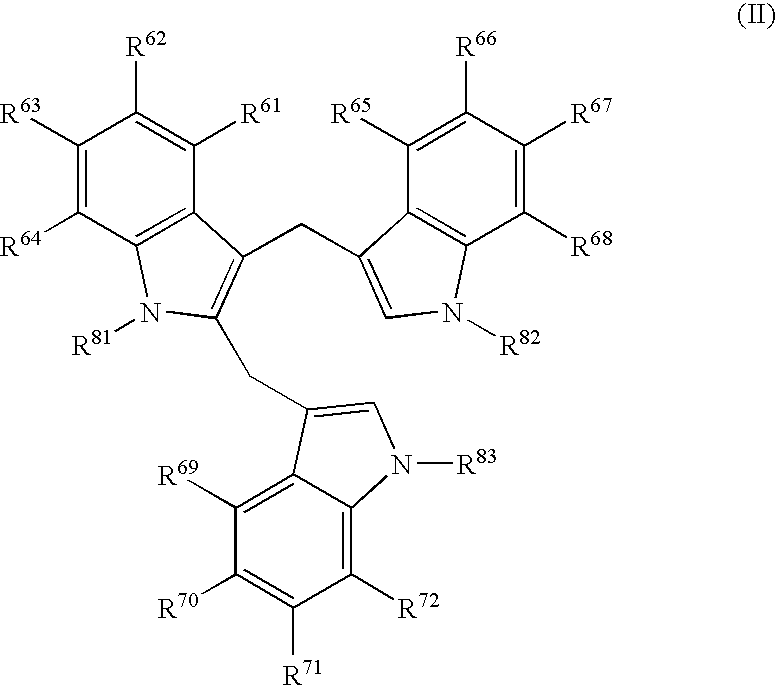
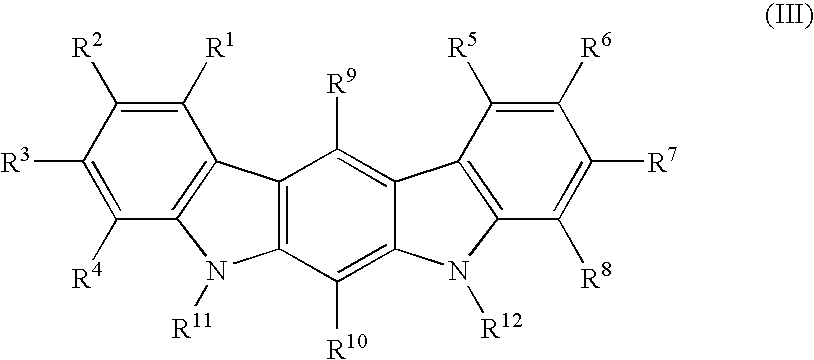
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1352 results about "Chewing gum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
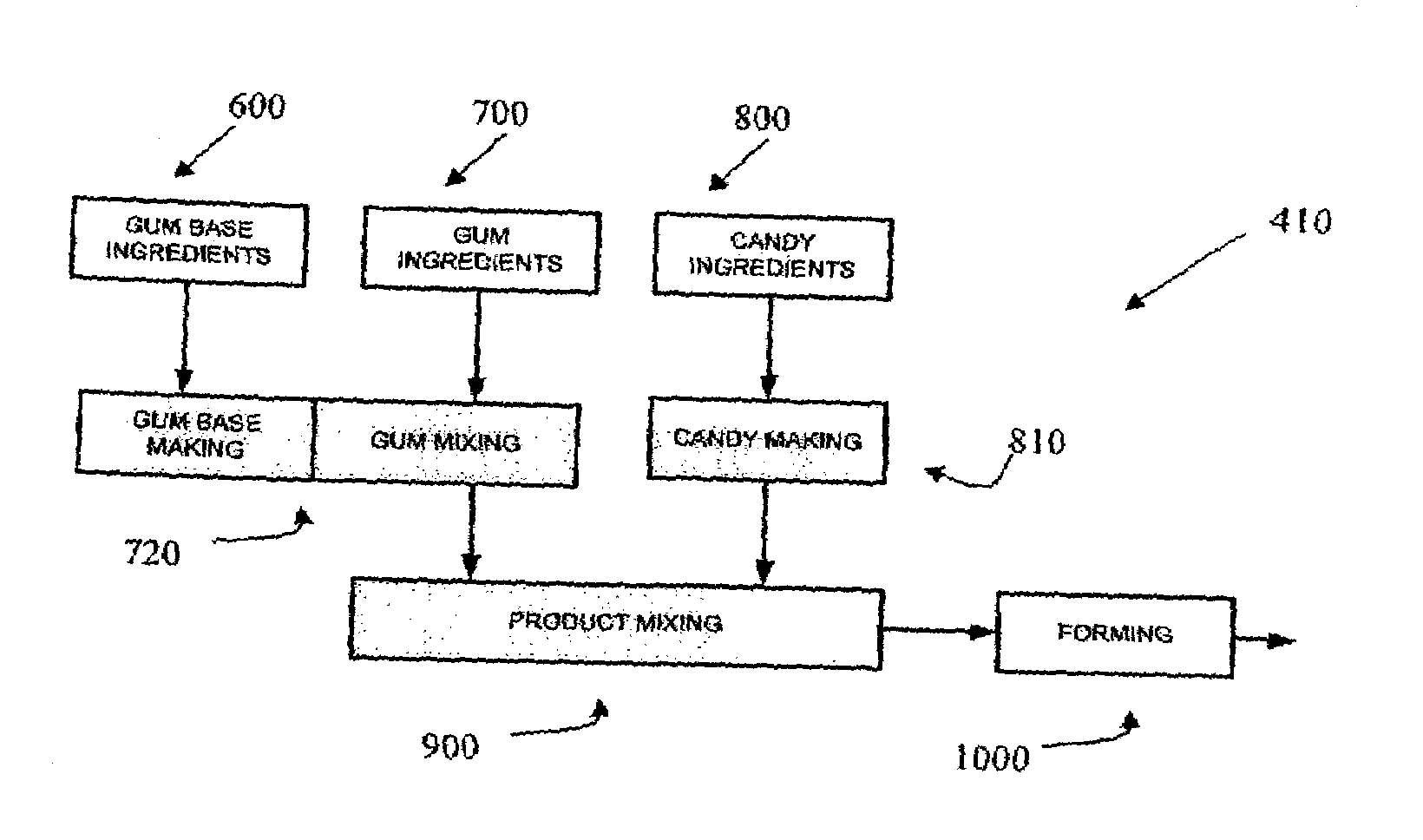
Chewing gum is a soft, cohesive substance designed to be chewed without being swallowed. Modern chewing gum is composed of gum base, sweeteners, softeners/plasticizers, flavors, colors, and, typically, a hard or powdered polyol coating. Its texture is reminiscent of rubber because of the physical-chemical properties of its polymer, plasticizer, and resin components, which contribute to its ...
Parenteral delivery systems
Hypertonic sugar compositions administered by other than ingestion and swallowing or intravascular injection, such as by intranasal spray or drops, intraocular drops or ointment, oral spray, intraotic spray or drops, lozenges, chewable tablet, chewing gum, or gargle, pulmonary inhalation, vaginal or rectal suppositories, or transdermal creams, ointments, lotions, or patches, are effective to open the blood-brain barrier to permit entry into the central nervous system of a co-administered chemical compound, such as a nutrient or a therapeutic or diagnostic agent. In this way, the compositions and methods of the invention increase the therapeutic or diagnostic efficacy of such chemical compounds.
Owner:NAITO ALBERT T
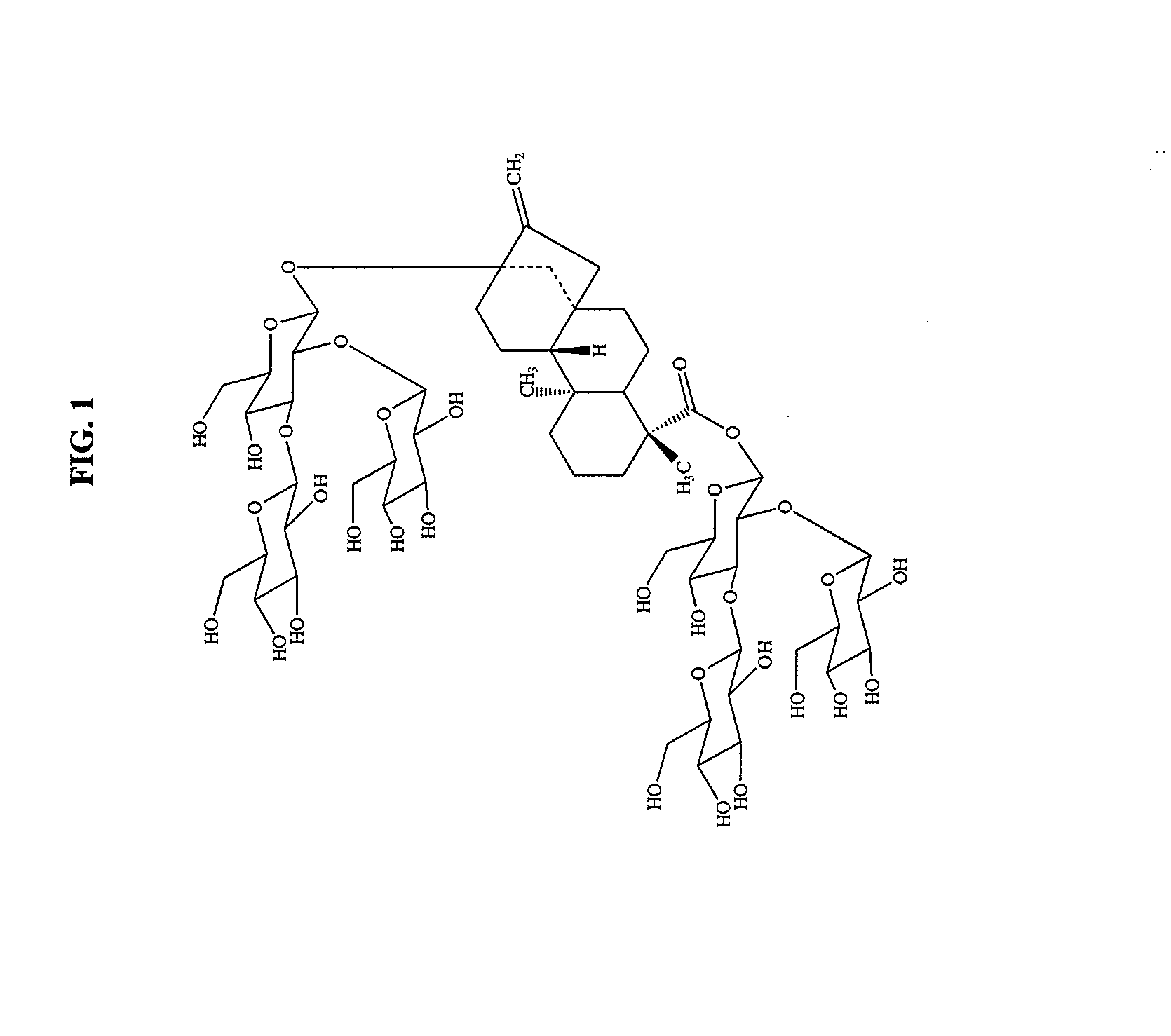
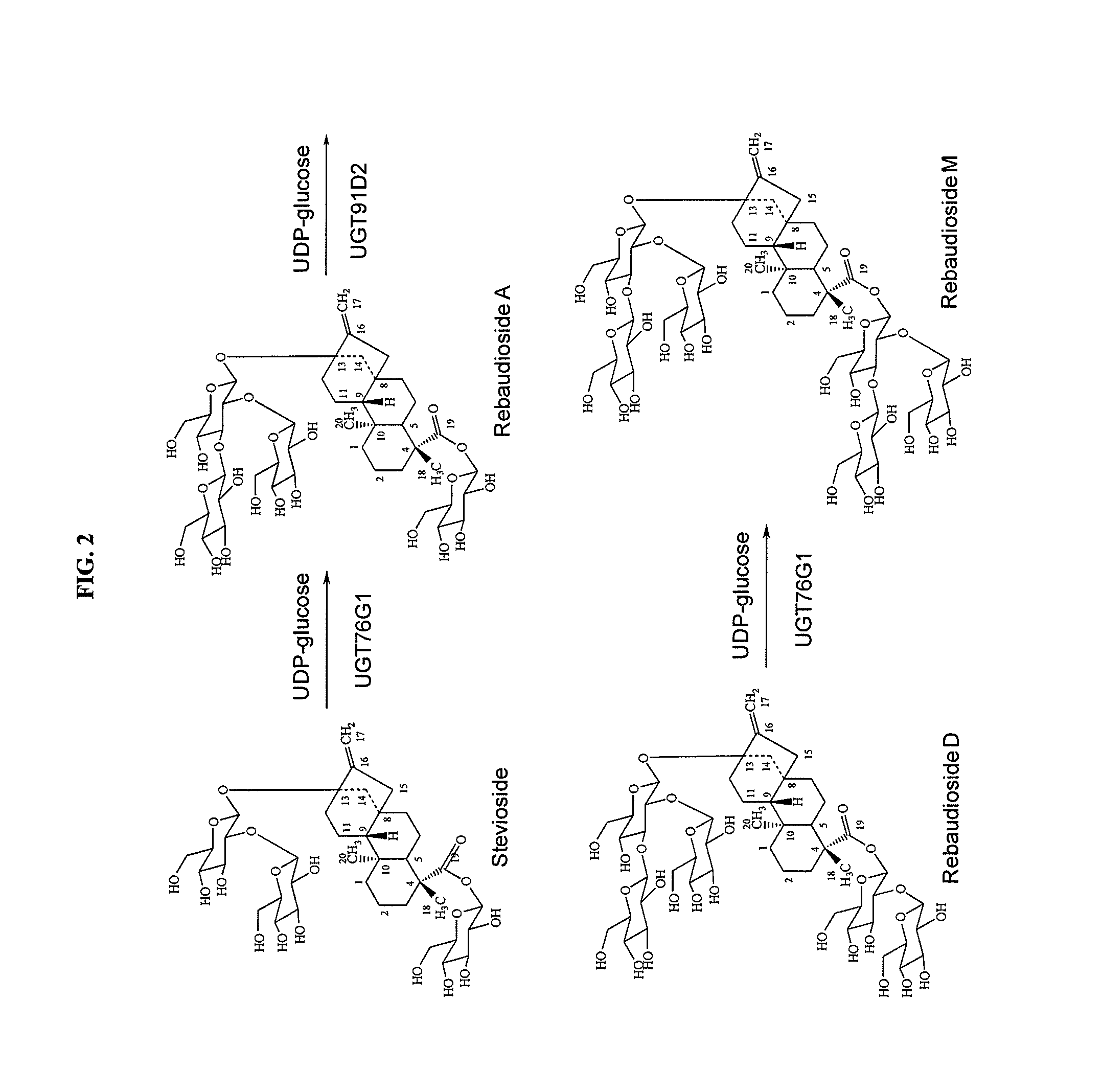
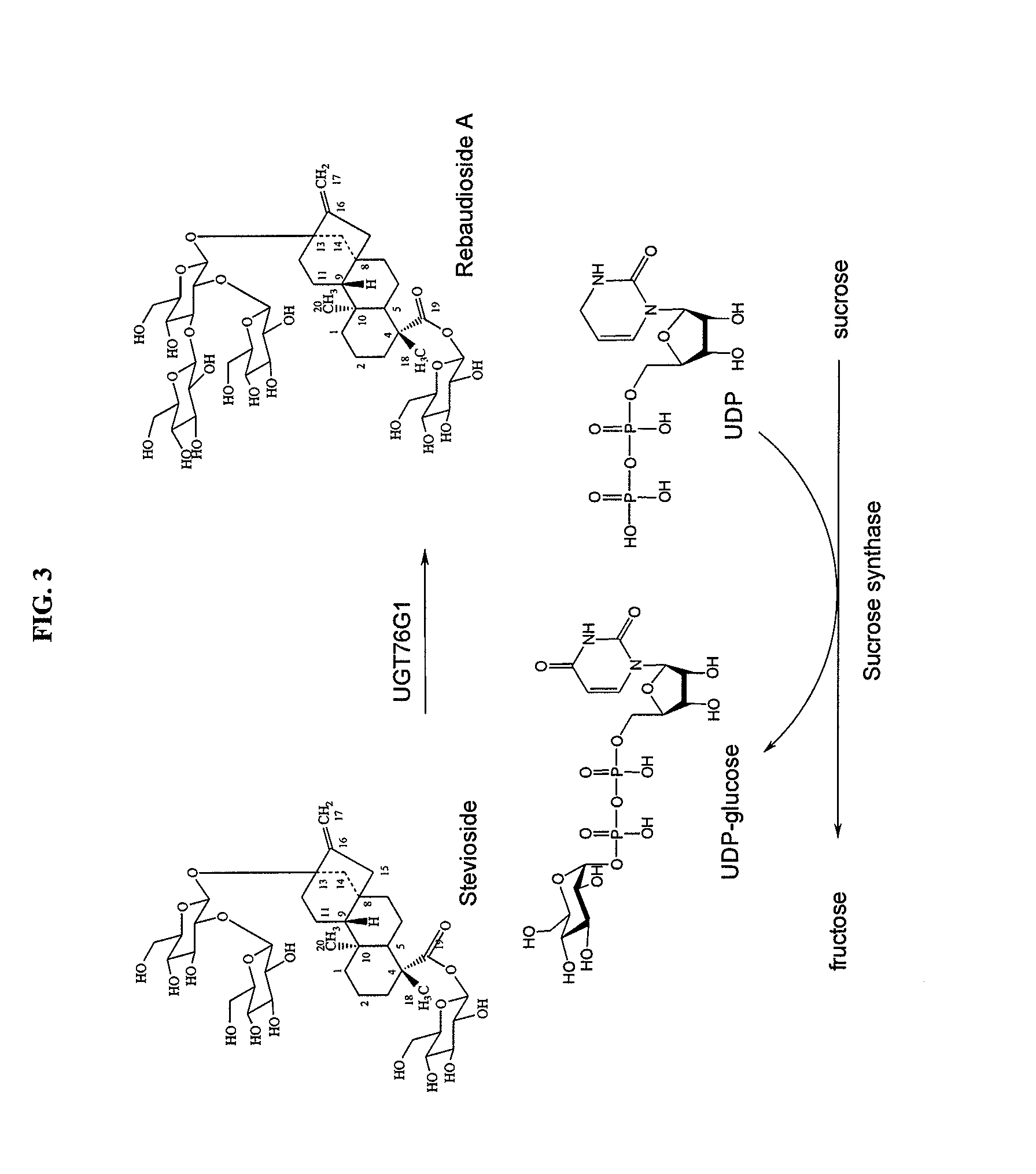
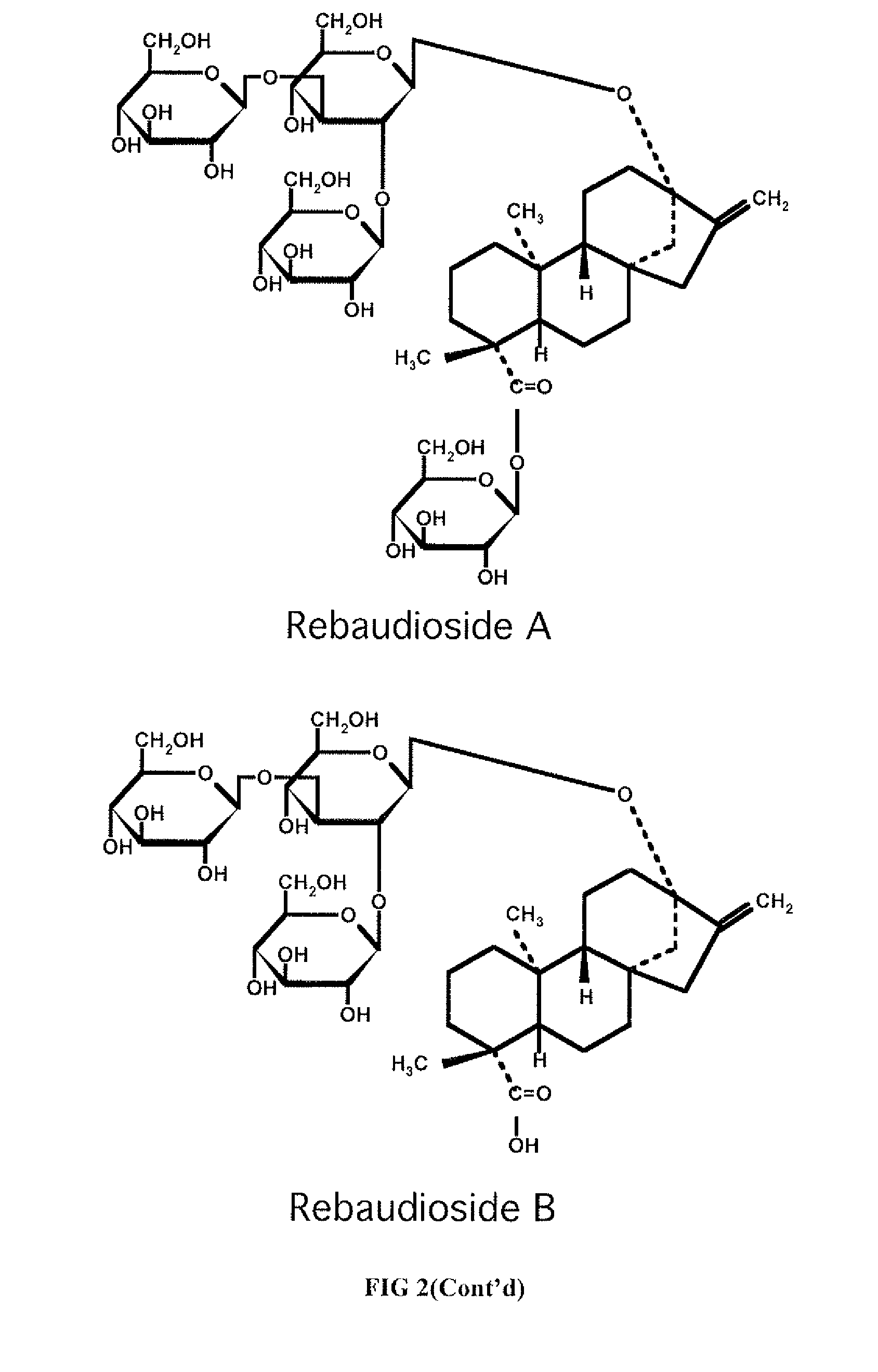
High-purity steviol glycosides
Methods of preparing highly purified steviol glycosides, particularly rebaudiosides A, D and M are described. The methods include utilizing recombinant microorganisms for converting various staring compositions to target steviol glycosides. In addition, novel steviol glycosides reb D2 and reb M2 are disclosed, as are methods of preparing the same. The highly purified rebaudiosides are useful as non-caloric sweetener in edible and chewable compositions such as any beverages, confectioneries, bakery products, cookies, and chewing gums.
Owner:PURECIRCLE SDN BHD
Natural Juncao liver-nourishing and sobering-up agent
The invention describes a health product additive and preparation for sots or hepatitis B pathogen carriers By adding medicinal fungus fermentation liquor as additive and using effective components and fine powder extracted from plants and herbal medicines by solvent as adjuvant, the health product can be made into forms of effervescent tablet, buccal tablet, chewable tablet, oral taken tablet, candy, chocolate, chewing gum, oral liquid, particle, soluble granules, capsule, aerosol, liquid beverage and solid beverage. The product can relieve alcohol effect, nourish stomach, protect liver, and help to remit hepatitis B virus and hepatitis liver cancer patient condition, achieves a quite important effect for protecting the health of the sots or hepatitis B pathogen carriers in daily or social occasions. The invention provides a good idea to the utilization of the large amount of active fermentation liquid generated with the thallus pharmacy in the medicinal fungus fermentation industries, and also provides a effective approach for increasing the utilization value of the large amount of wild plant resources such as wild jujube and haw widely distributed in the north areas.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Confectionery compositions including an elastomeric component and a saccharide component
InactiveUS20080166449A1Center edible coresCosmetic preparationsToilet preparationsElastomerChewing gum
The present invention relates to the confectionery compositions including a saccharide and a chewing gum base.
Owner:INTERCONTINENTAL GREAT BRANDS
High-Purity Rebaudioside D
The invention provides methods of purifying Rebaudioside D from the Stevia rebaudiana Bertoni plant extract along with Rebaudioside A. The methods are useful for producing high purity Rebaudioside D and Rebaudioside A. The high purity Rebaudiosides are useful as non-caloric sweeteners in edible and chewable compositions such as any beverages, confectionaries, bakeries, cookies, chewing gums, and alike.
Owner:PURECIRCLE SDN BHD
Nutraceuticals or nutritional supplements and method of making
InactiveUS6949264B1Method is newPromote absorptionContainers for annular articlesConfectioneryControlled releaseMedicine
A method for producing a chewing gum with a controlled release active agent, as well as the chewing gum so produced, is obtained by physically modifying the release properties of the active agent, such as a nutraceutical or nutritional supplement, by coating and drying. The active agent is coated by encapsulation, partially coated by agglomeration, entrapped by absorption, or treated by multiple steps of encapsulation, agglomeration, and absorption. The coated active agent is preferably then co-dried and particle sized to produce a release-modified active agent for use in chewing gum. The active agent may also be used in a coating on a chewing gum product, as part of a rolling compound applied to the chewing gum product, or as a part of the liquid in a liquid-center chewing gum product.
Owner:WM WRIGLEY JR CO
Method of controlling release of N-substituted derivatives of aspartame in chewing gum
InactiveUS6692778B2High consumer acceptanceImprove impact performanceConfectioneryChewing gumControlled releaseHigh intensity
The present invention includes a method for producing a chewing gum with a modified release sweetener selected from the group of N-substituted derivatives of aspartame, particularly neotame, as well as the chewing gum so produced. The modified release neotame or other N-substituted derivative of aspartame sweetener is obtained by physically modifying the sweetener properties by coating and drying. Neotame or another N-substituted derivative of aspartame sweetener is coated by encapsulation, partially coated by agglomeration, entrapped by absorption or extrusion, or treated by multiple steps of encapsulation, agglomeration, absorption, or extrusion. The coated sweetener is then co-dried and particle sized to produce a release-modified high-intensity sweetener. When incorporated into the chewing gum, these particles are adapted to enhance the shelf stability of the sweetener and / or produce a modified release when the gum is chewed.
Owner:WM WRIGLEY JR CO
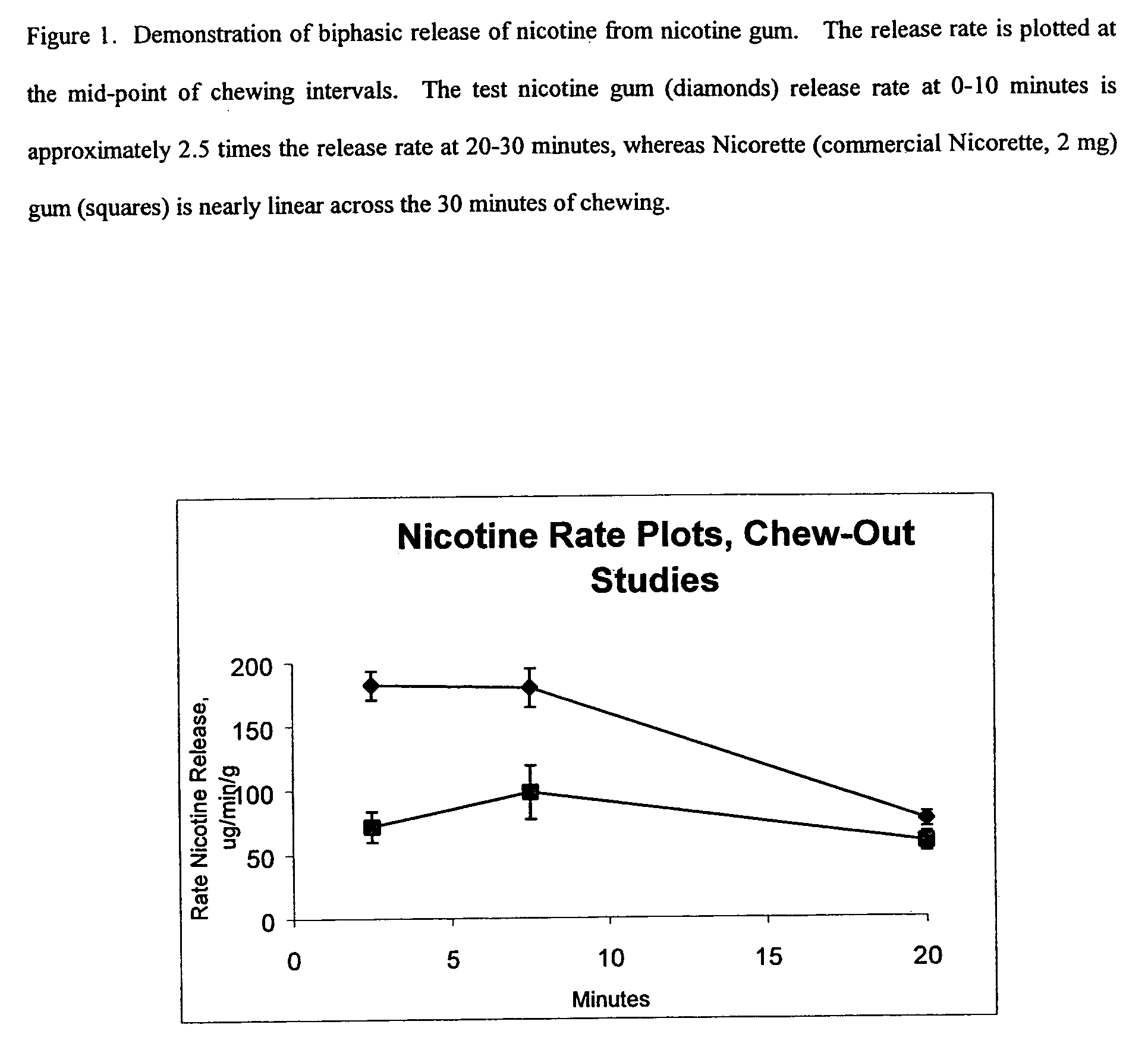
Two-stage transmucosal medicine delivery system for symptom relief
InactiveUS6893654B2Convenient and reliable and practicalReduce cravingsBiocidePowder deliveryOpiateInitial dose
A two-stage medicine delivery system provides an initial dose of medicine and a second dose of medicine. The initial and second doses are capable of achieving a rapid pharmacological effect and a prolonged pharmacological effect, respectively. The two-stage medicine delivery system preferably delivers a craving reduction substance, in which case, the rapid and prolonged pharmacological effects include a rapid and prolonged craving reduction. Preferably, the delivery system is a nicotine delivery system which is provided in chewing gum form or lozenge form and which provides the nicotine in a transmucosally absorbable form. The two-stage medicine delivery system preferably releases a buffering agent which increases a pH level in a user's mouth to facilitate absorption of the medicine when the delivery system is placed in the user's mouth. A method of making the medicine delivery system also is provided. The system and apparatus can be adapted to reduce cravings for alcohol, food, drugs (e.g., cocaine, opiates and the like) and tobacco products, especially tobacco products containing nicotine.
Owner:JSR NTI
Diindolylmethane-based compositions and methods of use thereof for promoting oral mucosal and bone health
InactiveUS20060264497A1Improve bioavailabilityEffective oral systemic useBiocideCosmetic preparationsDiseaseDental flossing
The present invention includes compositions and methods for the treatment and prevention of oral mucosal disorders and for promotion of bone health. In particular, the present invention describes new therapeutic and preventative uses for 3,3′-diindolylmethane (DIM), or a DIM-related indole, alone or in combination with anti-inflammatory agents and / or antibacterial agents, to treat oral mucosal disorders and promote bone health. The compositions of the invention are used to prevent and reverse oral mucosal disorders and bone loss (osteopenia and osteoporosis) associated with aging and chronic inflammation. Oral mucosal disorders include Periodontitis, gingivitis and related oral mucosal inflammation. Formulations of the compositions of the invention include capsules, tablets, toothpastes, oral gels, mouthwashes, mouth rinses, lozenges, chewing gum, dental floss, and dental topical formulations, and fortified foods.
Owner:BIORESPONSE
Chewing gum products containing trigeminal stimulant and method of making the same
InactiveUS20050202118A1Prolonged flavor durationImproved sensory benefitConfectioneryChewing gumFlavorStimulant
Chewing gums and methods of making same that have improved flavor duration by stimulating a trigeminal nerve of a consumer of the chewing gum are provided. The chewing gums of the present invention includes a trigeminal stimulant. The trigeminal stimulant stimulates the trigeminal nerve of the consumer to provide longer lasting flavor duration.
Owner:WM WRIGLEY JR CO
Chewing gum base and chewing gum compositions
ActiveUS6986907B2High affinityEasy to processContainers for annular articlesChewing gumPolymer scienceSpray dried
Owner:WM WRIGLEY JR CO
Flavoring of drug-containing chewing gums
ActiveUS20060275344A1Avoid problemsEnhanced release controlNervous disorderContainers for annular articlesAdditive ingredientPolymer thin films
A chewing gum comprising at least one active pharmaceutical ingredient (API) with a core onto which is applied at least one inner polymer film coating and thereafter onto which is applied at least one outer hard coating. A preferred API is nicotine. Flavoring agents may be incorporated in the core, in the at least one inner polymer film coating and / or in the at least one outer hard coating. The gums formed exhibit a long lasting effect of flavoring agent(s) and result in the domination of flavoring agents in the coating(s) over flavoring agent(s) in the core, thereby (a) avoiding problems of chemical or pharmaceutical incompatibility between an API in the core and flavoring agent(s) in the coating(s) and (b) achieving an increased control of the release of the API and of non-active excipients.
Owner:MCNEIL AB
Oral delivery of pharmaceuticals via encapsulation
An alternate drug delivery system for dissolution of pharmaceuticals in the mouth wherein a therapeutically effective amount of a drug is encapsulated using an encapsulation method. Encapsulation reduces the perceived off flavors of drugs, allowing the active components to dissolve pleasantly in the mouth. This allows more rapid absorption of the active compounds through the oral cavity compared to traditional tablets, which require breakdown and absorption in the gastrointestinal tract. The delivery system can be incorporated into a variety of applications, such as breath mint tablets or chewing gum. Benefits of this invention include portability and the ability to take pharmaceuticals without water and without the off taste of chewable tablets, thereby leading to increased patient compliance.
Owner:BATTEY ALYCE S +1
Syrups containing sorbitol, a plasticizing agent and hydrogenated starch hydrolyzate, and their use in chewing gum and other confectionaries
A chewing gum including non-crystalline sorbitol and method of making the same is provided. The chewing gum composition comprises a sugarless syrup made from aqueous sorbitol, a plasticizing agent selected from the group consisting of glycerin, propylene glycol and mixtures thereof, and a hydrogenated starch hydrolyzate syrup. Other confectionaries can be made from the same sugarless syrup.
Owner:WM WRIGLEY JR CO
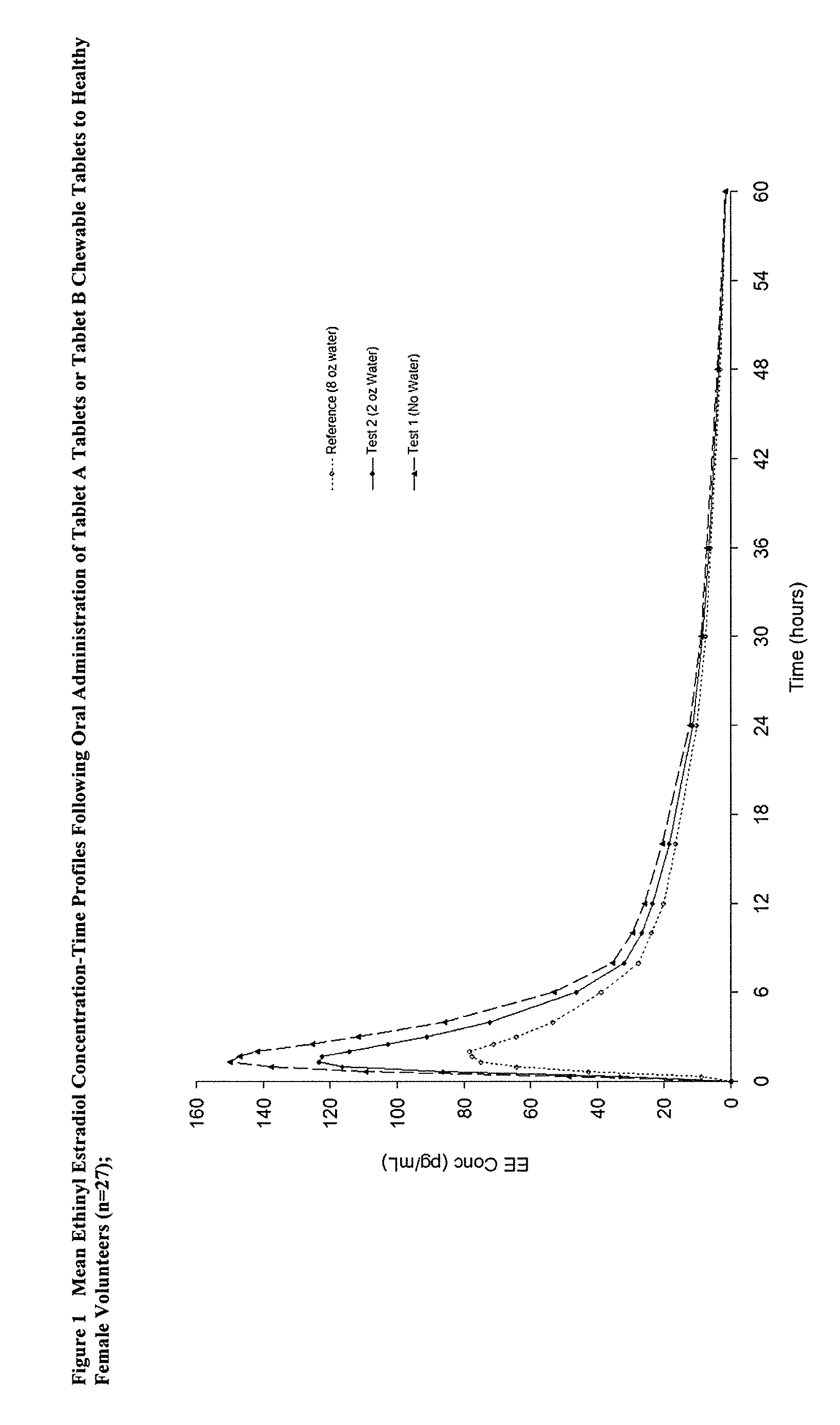
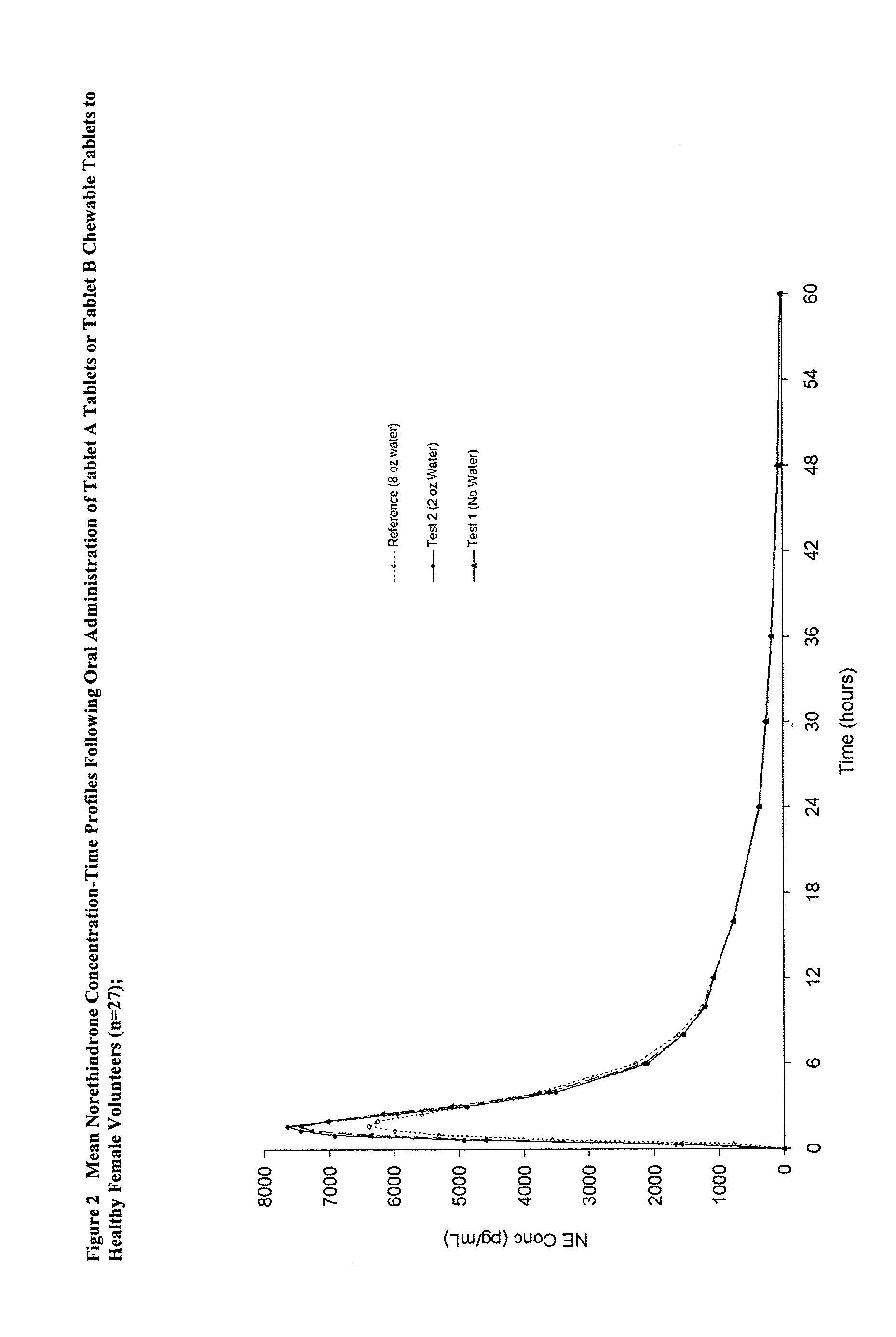
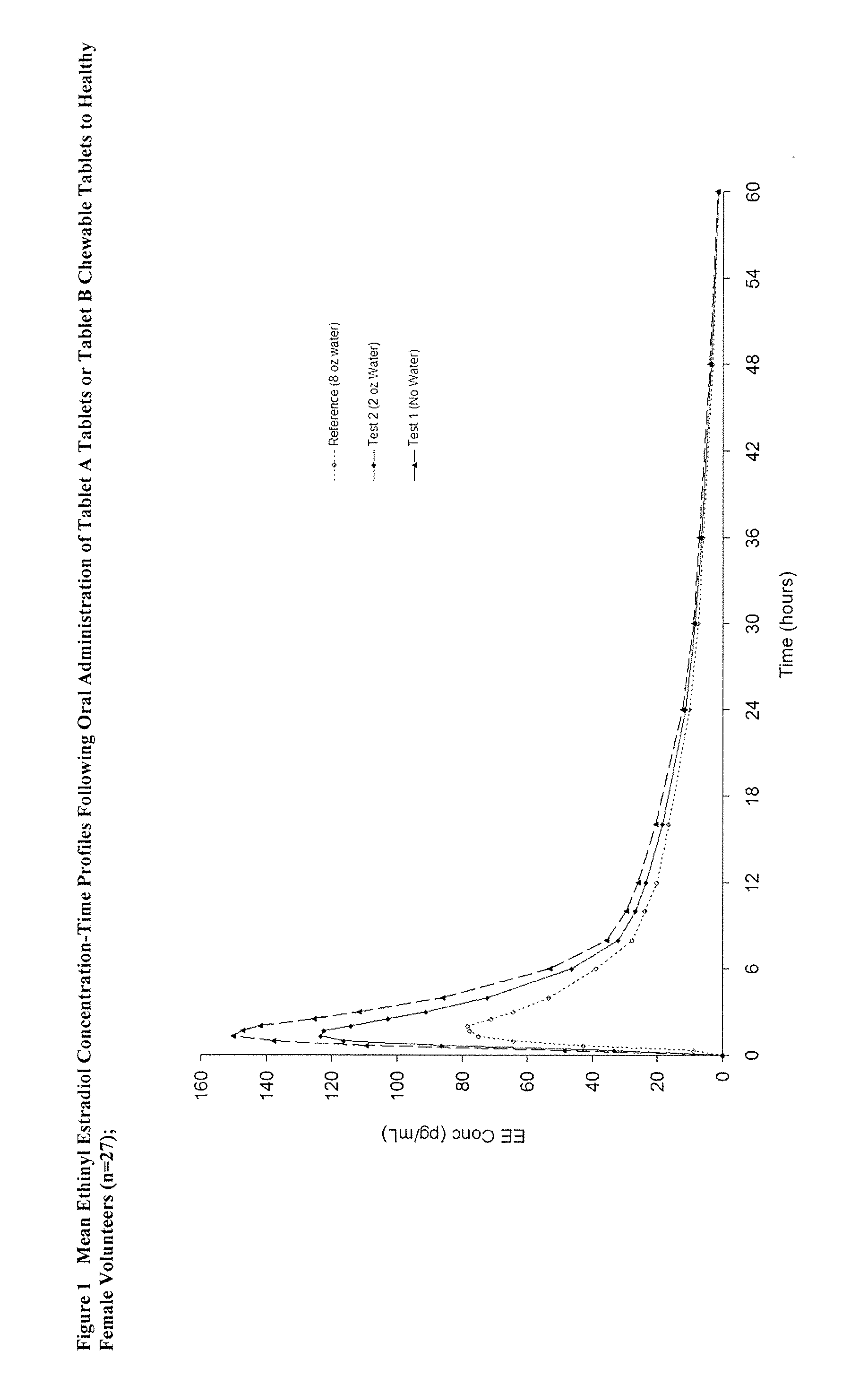
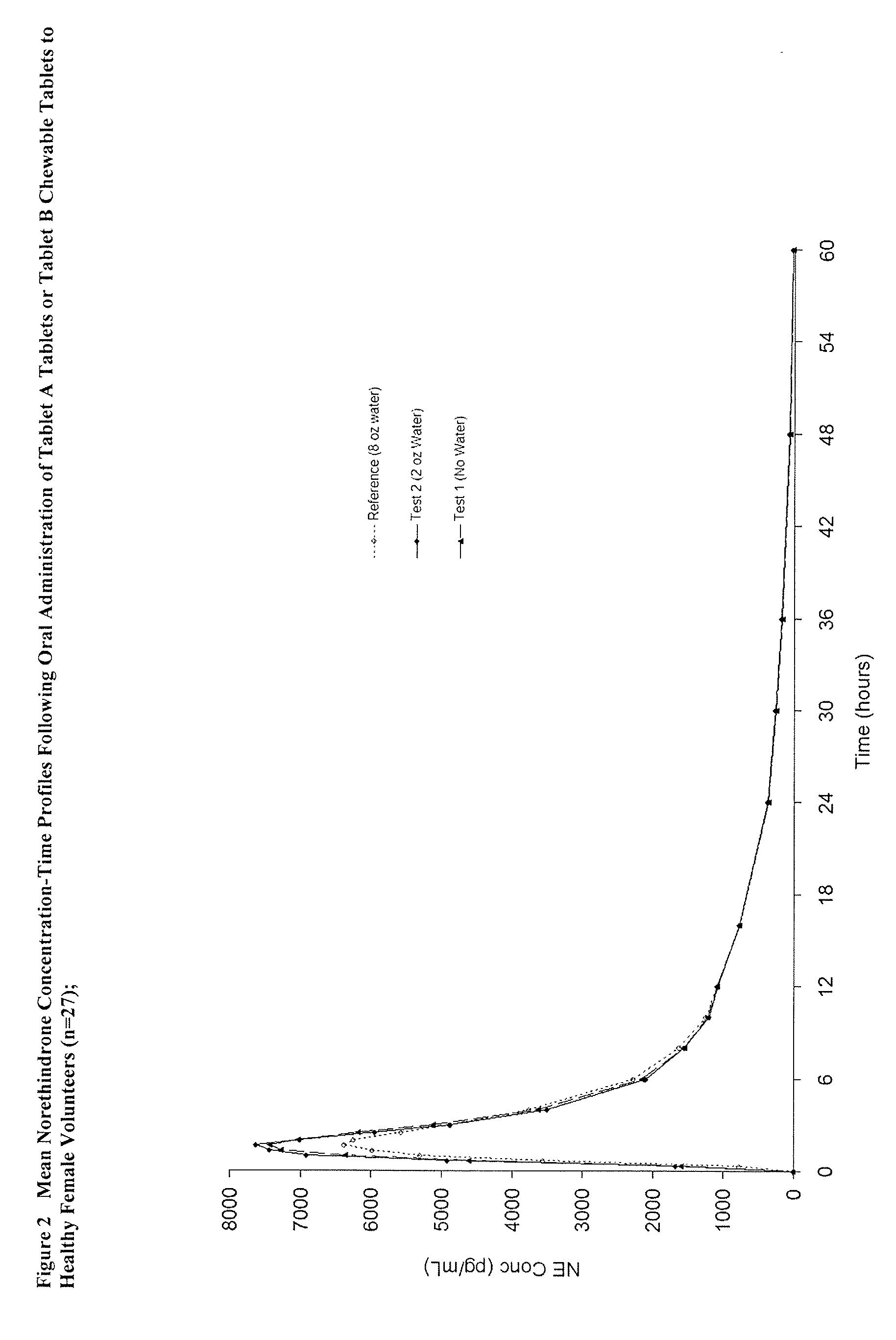
Methods to administer ethinyl estradiol and prodrugs thereof with improved bioavailability
InactiveUS20070286819A1Improve bioavailabilityReducing potential hormonal side effectOrganic active ingredientsPill deliveryHormone replacementBioavailability
Methods of improving the bioavailability of ethinyl estradiol by orally administering to a patient a solid dosage form containing ethinyl estradiol or prodrug thereof where that dosage form releases at least some of the ethinyl estradiol or prodrug thereof in the oral cavity for absorption through the oral mucosa to treat the patient for a predetermined indication such as, for example, hormone replacement therapy or contraception. The solid dosage forms may be selected from, among others, chewable tablets, fast melt tablets, films, dissolving films, mucoadhesive tablets, lozenges, and chewing gum.
Owner:WARNER CHILCOTT CO LLC
Coated chewing gum product and method of making
InactiveUS7163705B2Promote absorptionContainers for annular articlesChewing gumSodium bicarbonateWater soluble
A method for producing a coated chewing gum product with accelerated absorption of medicaments through oral mucosa, as well as the chewing gum product so produced, is obtained by using a xylitol or sorbitol coating, or by adding a water-soluble alkaline material, such as a bicarbonate salt, to the chewing gum center, a coating on the center, or both. Coatings made with sorbitol or xylitol or gum centers that include sodium bicarbonate are particularly preferred.
Owner:WM WRIGLEY JR CO
Coated degradable chewing gum with improved shelf life and process for preparing same
ActiveUS20040146599A1Reduce probabilityContainers for annular articlesConfectioneryElastomerChemical effects
A coated chewing gum element comprising about 25 to 99.9% by weight of a chewing gum centre comprising at least one environmentally degradable elastomeric or resinous polymer and about 0.1 to 75% by weight of an outer coating and processes for providing the chewing gum. The outer coating is a hard sugar or sugarless coating, a film coating or a soft coating. The application of an outer coating prevents degradation of the degradable polymers prior to chewing due to physical or chemical effects, thereby improving the shelf life of the environmentally degradable chewing gum. After chewing, the chewing gum will degrade in the environment and can be removed more readily than chewing gum based on non-degradable polymers.
Owner:FERTIN PHARMA AS
Chewing gums, lozenges, candies, tablets, liquids, and sprays for efficient delivery of medications and dietary supplements
InactiveUS20060073189A1Relieve symptomsLower of oral cavityInorganic non-active ingredientsChewing gumDietary supplementTherapeutic effect
The present invention relates to gums, lozenges, candies, tablets, liquids and spray compositions that contain orally administered medications and dietary supplements that are released in the oral cavity. The medications and dietary supplements contained therein may be delivered in a multi-phase mode. The compositions may also contain buffer systems that facilitate oral absorption. A rapid release is followed by slower release of medicant(s) and dietary supplements. The buffer system is released simultaneously with the medicant(s) and dietary supplements, thereby facilitating transmucosal and buccal absorption of active ingredient(s). The invention delivers, first, rapidly an initial pharmacologically effective dose of medicine and dietary supplements and, second, a prolonged pharmacologically sufficient dose for longer-term relief of symptoms or provision of therapeutic effect.
Owner:JSR NTI
Tooth whitening compositions and delivery systems therefor
InactiveUS20060024244A1Sufficient amountCosmetic preparationsContainers for annular articlesPolyphosphateSilicon dioxide
Stain-removing oral compositions, such as gum compositions are herein provided. The compositions include an orally acceptable carrier and a stain-removing anionic surfactant. The surfactant includes a fatty acid salt having at least one hydroxyl functionality. The fatty acid salt may be a salt of ricinoleic acid, and may be combined with a chelating agent and / or an abrasive. The chelating agent may be a polyphosphate and the abrasive may be a silica abrasive.
Owner:INTERCONTINENTAL GREAT BRANDS LLC
Chewing gum containing physiological cooling agents
A method for producing a chewing gum, as well as the chewing gum so produced, incorporates a physiological cooling agent, such as acyclic carboxamide, or combinations of physiological cooling agents. In another embodiment a combination of physiological cooling agents is made in a modified release structure. The modified release / cooling agents combination is preferably obtained by physically modifying the properties of the combination of cooling agents by coating and drying. When incorporated into gum, these particles are adapted to enhance the shelf stability of the flavor and / or produce a modified release when the gum is chewed. In another embodiment, the physiological cooling agent is present with menthol and menthone. In another embodiment, coated chewing gum has a coating that comprises a physiological cooling agent. The preferred inventive chewing gum provides a high flavor impact in which the harsh notes normally associated with such a high flavor impact have been reduced or eliminated. In addition, the preferred inventive gum provides a clean, high-quality, cooling chewing gum coating with xylitol or other polyols where xylitol has been reduced in concentration or eliminated.
Owner:WM WRIGLEY JR CO
Chewing gum compositions comprising cannabinoids
The present invention relates to a chewing gum composition comprising 0.01 to 15% by weight a cannabinoid or a derivative thereof, based on the total weight of the chewing gum composition, and to chewing gums and blistering packages comprising said chewing gums.
Owner:CANCHEW BIOTECH LLC
Oral hygiene products containing ascorbic acid and method of using the same
InactiveUS20080057007A1Efficient removalEffectively and efficiently protectCosmetic preparationsOrganic active ingredientsDiseaseAdditive ingredient
The present invention is directed to dental compositions, including dentifrices, containing ascorbic acid for removing and inhibiting dental biofilms which form plaque and tartar, and also for treating and preventing gingivitis and periodontitis. The ascorbic acid composition can contain may additional ingredients, including an enamel-strengthening component, and be used in many different forms, including breath spray, chewing gum, dental floss, dental powder, gargle, lozenge, mouth spray, mouth wash, tooth gel, tooth liquid, tooth paste and tooth strips. Also described in a method of using a dental composition containing ascorbic acid in order to treat plaque and tartar as well as gum disorders.
Owner:DENTECH
Multifunctional scrubbing cleanser
InactiveCN1524939AGood removal effectEasy to cleanSurface-active detergent compositionsInorganic saltsCleansers skin
A multifunctional scouring detergent with constituents (by weight percentage) of, mixed solvent 2-70, surface active agent 3-30, inorganic salt 0-10, water for the rest. The multifunctional cleaning agent can effectively remove the stains of chewing gum, scotch tape colloid residual and paint film coating.
Owner:李伟光
Animal chew toy containing solid food
InactiveUS20080314333A1Easy to processImprove and protect healthPeptide/protein ingredientsBoron compound active ingredientsVitamin CManganese
An animal toy containing food having a first portion with food delivery means integrated therein adapted to securely house solid food treats, including a nutritional pet supplement, to be removed by the animal during play or through chewing action, and a second side with a plurality of gum stimulation teeth integrated within and projecting therefrom that act to massage the gums of the animal. Food delivery means may be provided as a plurality of cavities integrated within the first portion (and optionally, the second portion), preferably having a grooved or threaded interior for securely holding food pieces securely therein. Alternatively, the food delivery means is provided as a food portion composing the first portion. A pet supplement may be utilized, including joint preserving and joint rebuilding compositions comprising chicken collagen type II, glucosamine hydrochloride and chondroitin sulfate, a vitamin composition comprising vitamins C, D and K, a mineral composition comprising calcium, magnesium, zinc, copper, manganese and boron, a herbal anti-oxidant cofactor blend comprising citrus bioflavonoids, red grapes anthocyanins, turmeric rhizome, boswellia resin and fennel seed.
Owner:I DID IT
Taste-masked resinate and preparation thereof
The present invention is directed to a taste-masked resinate that contains a water-insoluble active substance complexed to an ion-exchange resin in a taste-masking effective amount. The taste-masked resinate is useful in the manufacture of a dosage form such as a rapid-disintegrating tablet, a rapid-disintegrating film, an effervescent tablet, a chewable tablet, a chewing gum, a suspension, a sprinkle granule, a powder for reconstitution in a suspension and the like and a method for the preparation thereof.
Owner:JANSSEN PHARMA NV
Methods to administer solid dosage forms of ethinyl estradiol and prodrugs thereof with improved bioavailability
InactiveUS20080113953A1Improve bioavailabilityReducing potential hormonal side effectOrganic active ingredientsPharmaceutical delivery mechanismEthinyl oestradiolHormone replacement
Methods of improving the bioavailability of ethinyl estradiol by orally administering to a patient a solid dosage form containing ethinyl estradiol or prodrug thereof where that dosage form releases at least some of the ethinyl estradiol or prodrug thereof in the oral cavity for absorption through the oral mucosa to treat the patient for a predetermined indication such as, for example, hormone replacement therapy or contraception. The solid dosage forms may be selected from, among others, chewable tablets, fast melt tablets, films, dissolving films, mucoadhesive tablets, lozenges, and chewing gum.
Owner:APTALIS PHARMA
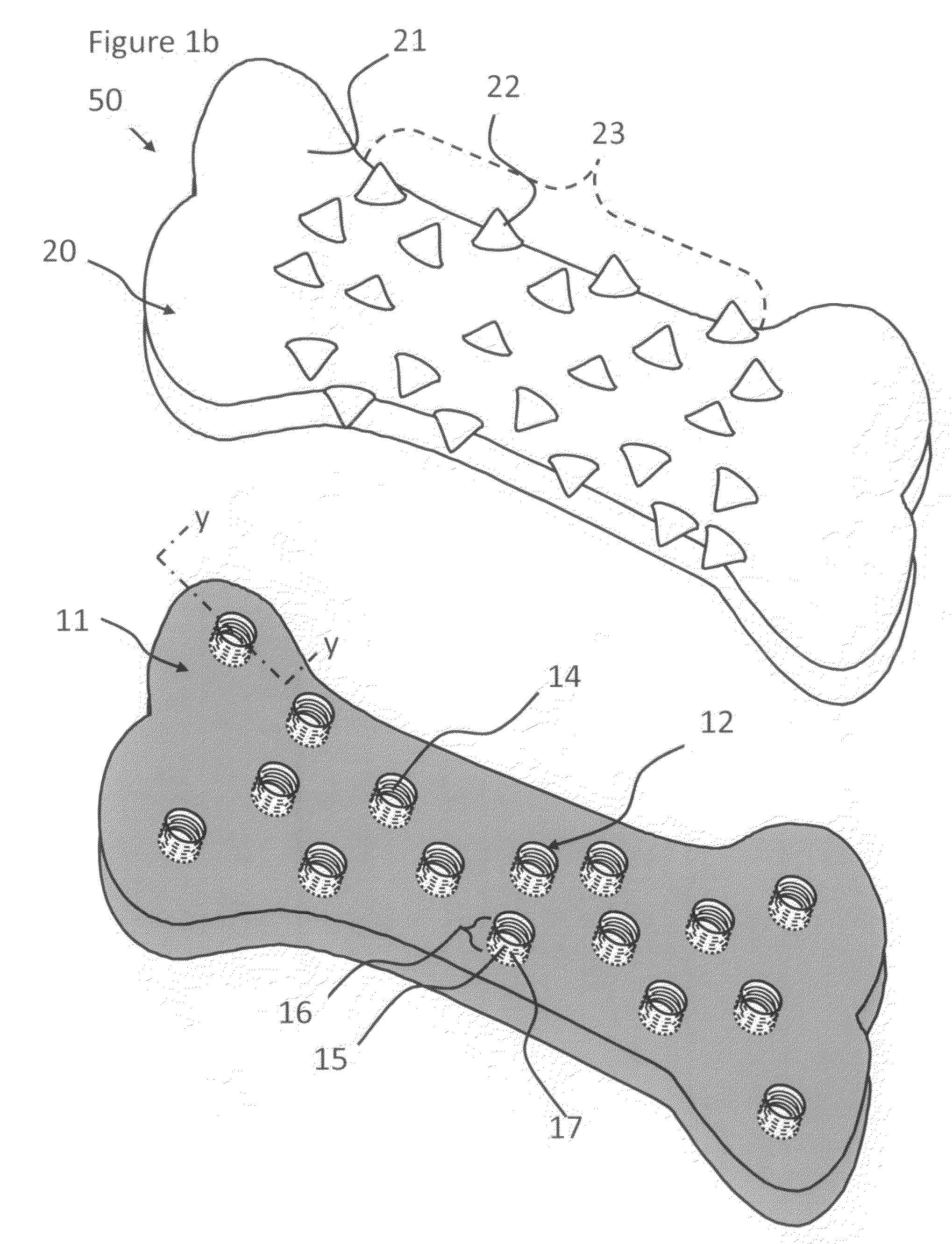
Over-coated chewing gum formulations including tableted center
Methods and products for delivering a medicament or agent to an individual are provided as well as methods for producing the product. The product includes a coating having a medicament or agent. The medicament or agent is present within the coating that surrounds a tableted gum center (the water soluble portion and a water insoluble base portion). By chewing the gum, the medicament or agent is released from the product. Continuing to chew the chewing gum creates a pressure within the buccal cavity forcing the agent or medicament directly into the systemic system of the individual through the oral mucosa contained in the buccal cavity. This greatly enhances the absorption of the drug into the systemic system as well as the bioavailability of the drug within the system.
Owner:WM WRIGLEY JR CO
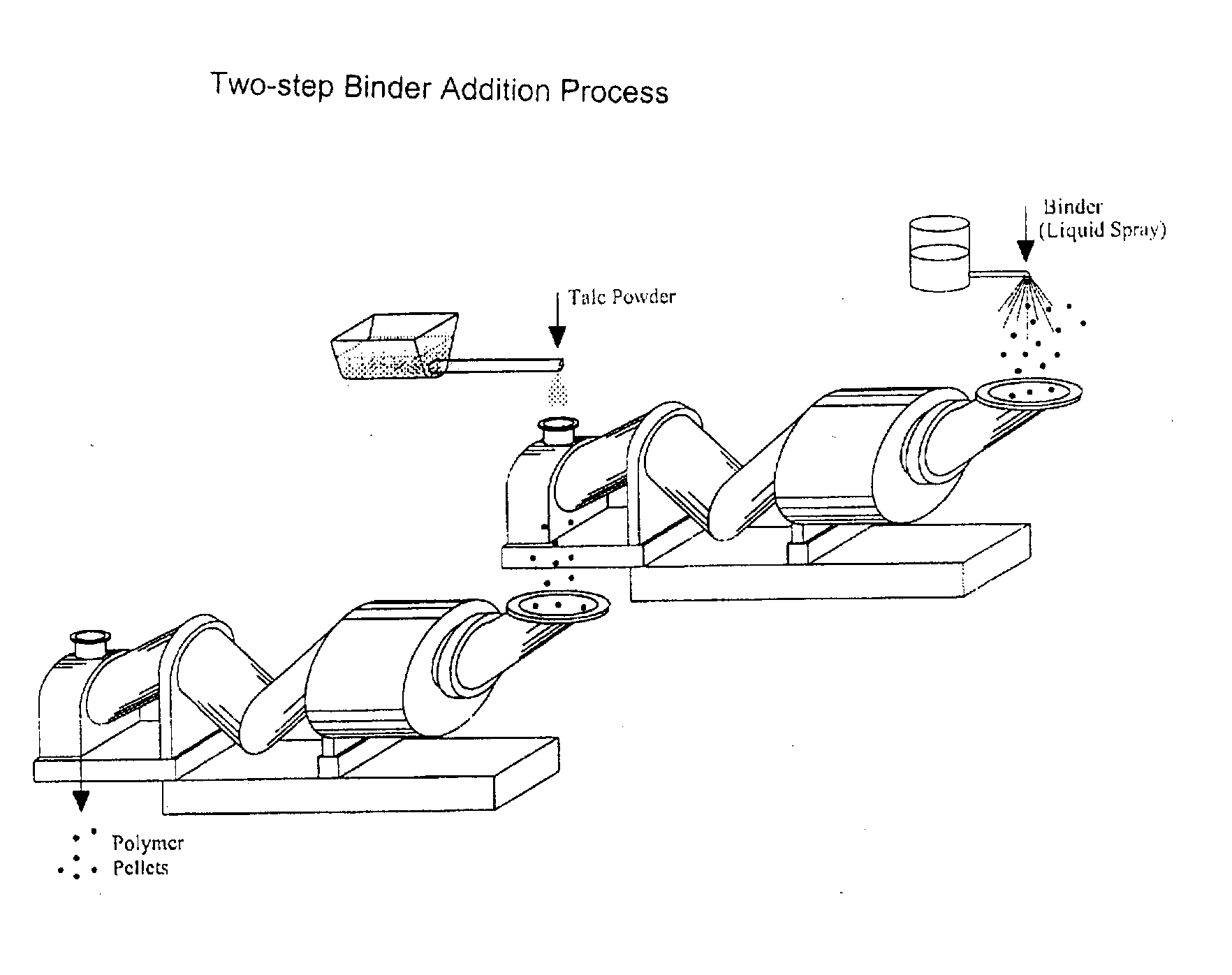
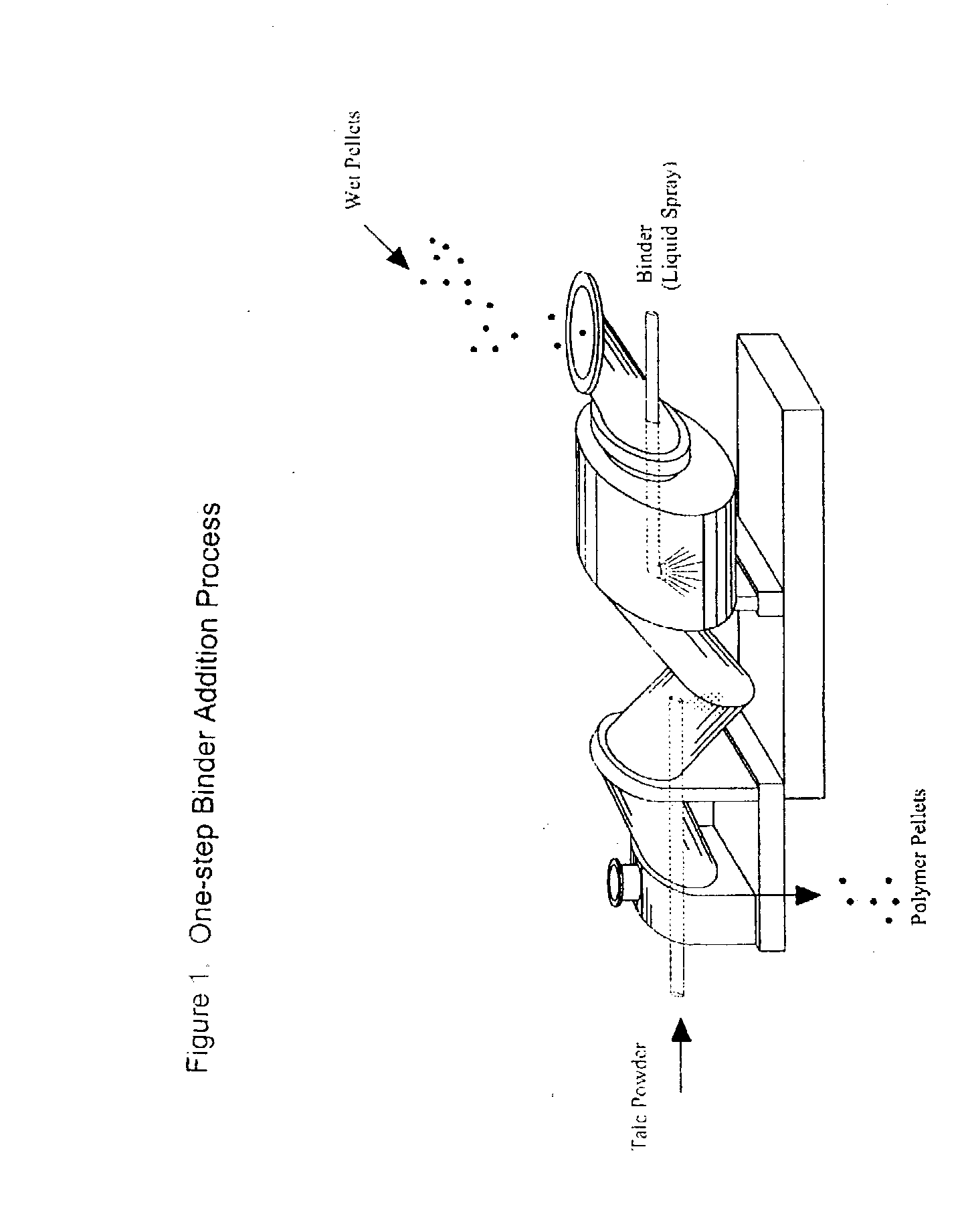
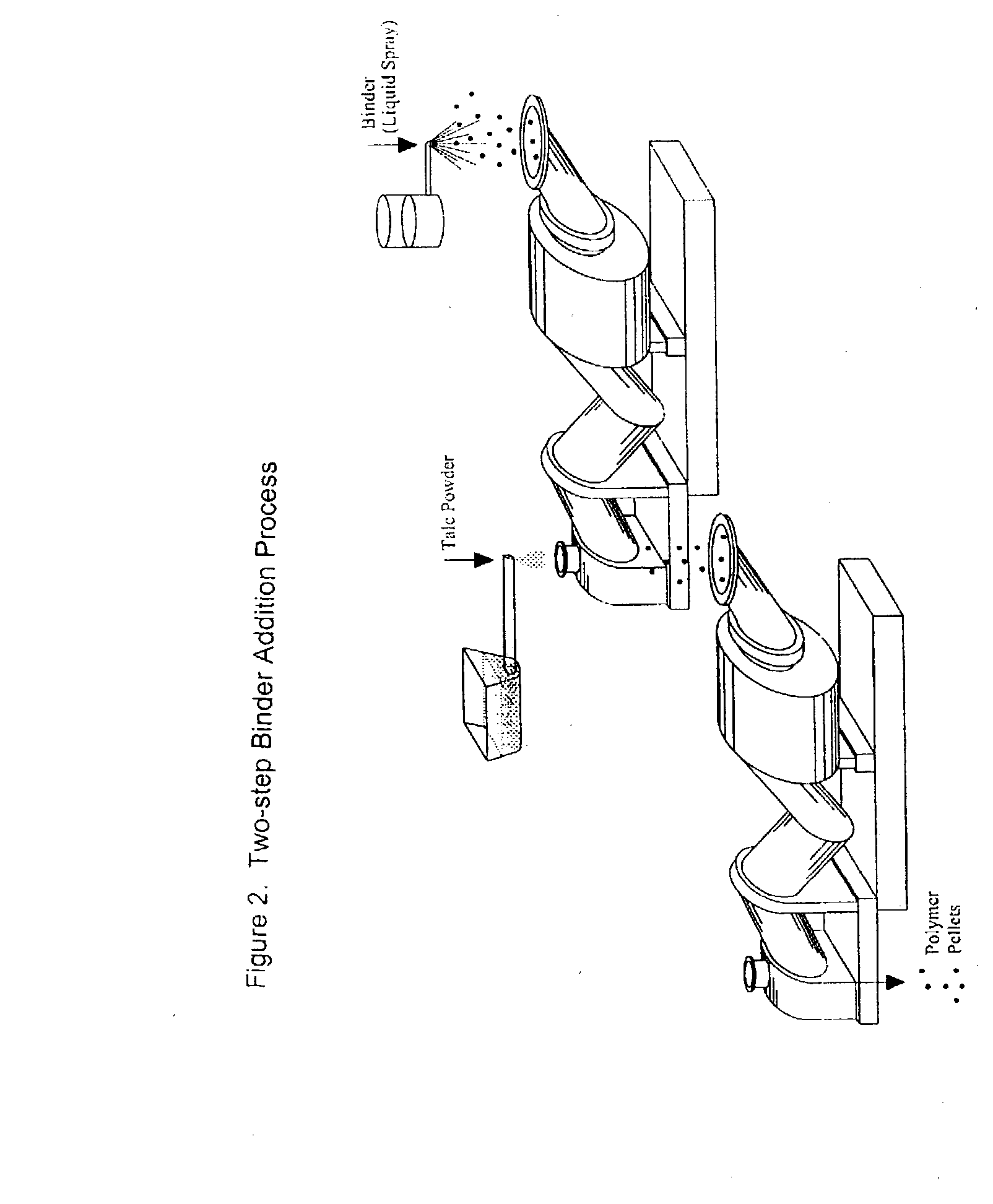
Process of Coating Tacky and Soft Polymer Pellets
InactiveUS20040209082A1Easy downstream handlingAvoid stickingConfectioneryChewing gumPolymer scienceMonoglyceride
An improved process of coating tacky or soft polymer pellets to maintain a free-flowing property, uses a liquid binder, in conjunction with an anti-tack or partitioning powder such as talc to prevent aggregation during storage. The binder is a non-volatile material such as an oil or plasticizer including triglycerides, mono- / di-glycerides, acetylated mono- / di-glycerides, fatty acids, epoxidized triglycerides, phthalates, benzoates, sebacates, lactates, citrates, mineral oils etc. Applications of this process include chewing gum bases, hot-melt adhesives, sealants, rubber masterbatches, powdered rubber, and other soft and tacky polymer materials.
Owner:WM WRIGLEY JR CO
Breath freshening and oral cleansing product with magnolia bark extract in combination with surface active agents
InactiveUS20060013779A1Growth inhibitionEnhanced antiplaque growth activityOrganic active ingredientsBiocideBiofilmSurface-active agents
An oral composition for oral cleansing, breath freshening, and anti-microbial benefits includes Magnolia Bark Extract in combination with a surface active agent. The effectiveness of Magnolia Bark Extract in inhibiting biofilm formation in the oral cavity is increased by a synergistic combination of the Magnolia Bark Extract with a surface active agent in an oral cavity delivery agent, such as chewing gum, a confectionary, a lozenge, a compressed tablet, and an edible film.
Owner:WM WRIGLEY JR CO
Snack system, including interior filing of ingredient, outer coating of ingredient, addition of ingredient, and formation of snack roll
InactiveUS20140212453A1Modest expenseQuickly replenish energy levelBiocideFrozen sweetsAdditive ingredientSnack food
A novel snack system is created in various form, variety, and with various ingredient. The snack system is created being a snack, with at least one ingredient, and at least one texture. The category of snack includes cheese, powdery candy, soft candy, creamy candy, gummy candy, hard candy, liquid candy, fudge candy, chocolate, ice cream, ice milk, sherbet, gelato, yogurt, sorbet, tofu, jelly, pudding, chewing gum, roll, pie, biscuit, cookie, donut, pastry, cake, pancake, crepe, waffle, bread, tortilla, and taco. The snack can be served at room temperature, cold from refrigerator, warm, hot, and reheated. The snack is in form selected from group consisting of interior filing of ingredient, outer coating of ingredient, and formation of snack roll. The snack is also created with addition of various ingredients. The snack is created with at least one ingredient selected from group consisting of flavoring ingredient, nutritional ingredient, health ingredient, and other ingredient.
Owner:CHANG ALICE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com