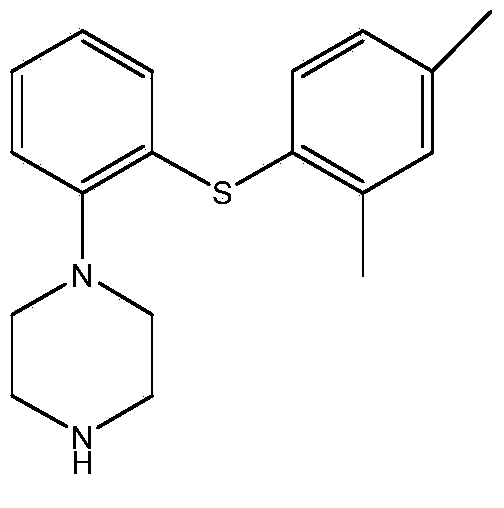
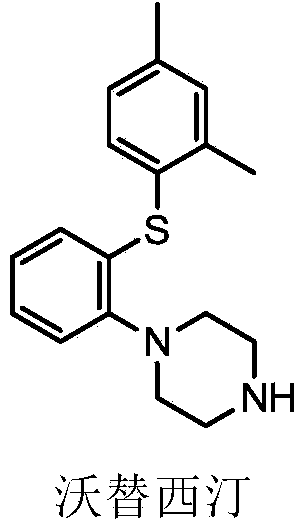
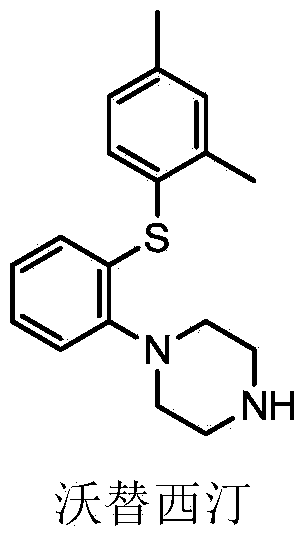
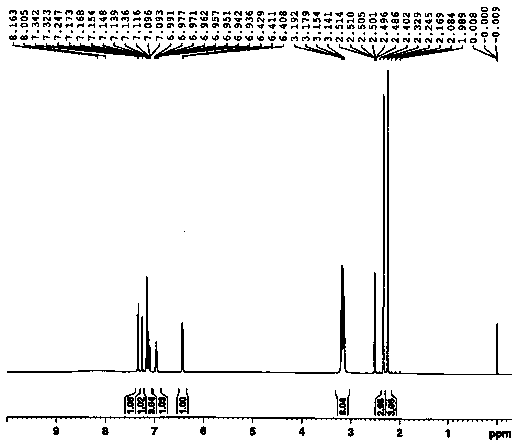
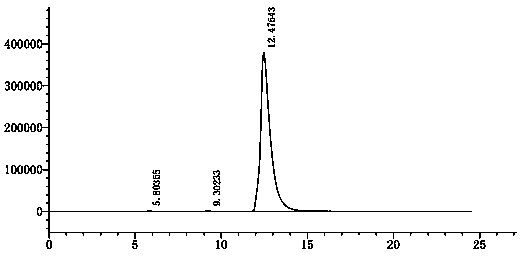
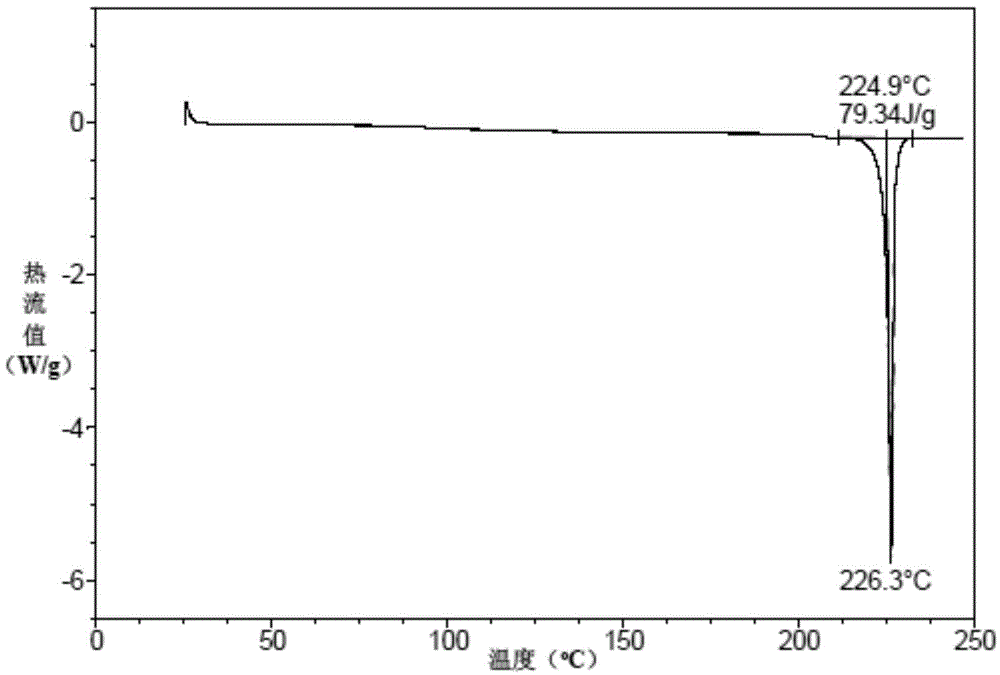
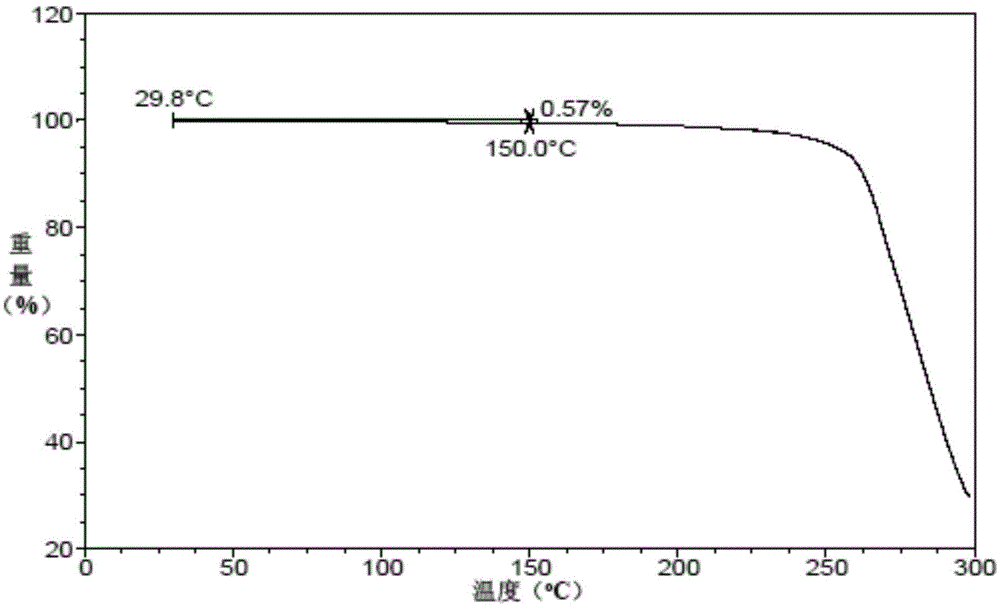
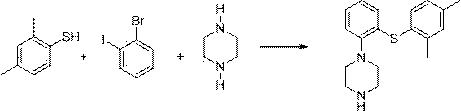Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
103 results about "Vortioxetine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
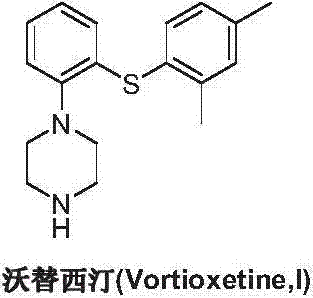
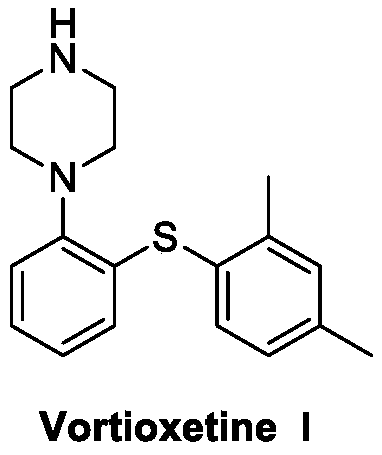
This medication is used to treat depression.
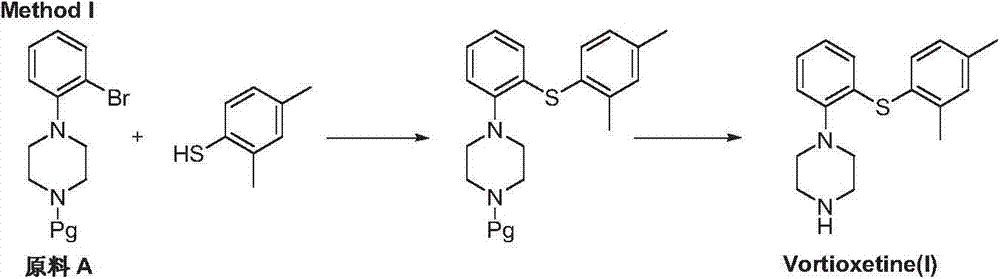
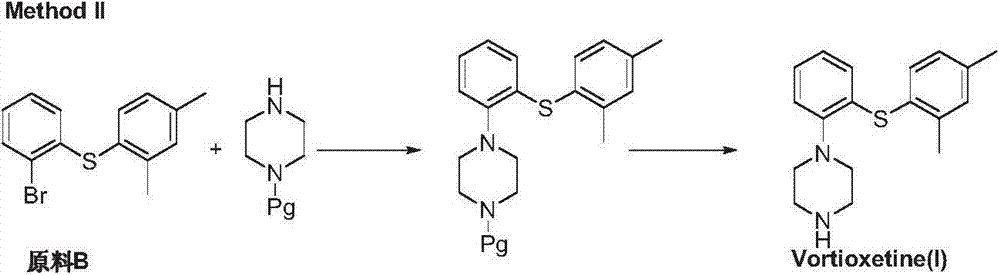
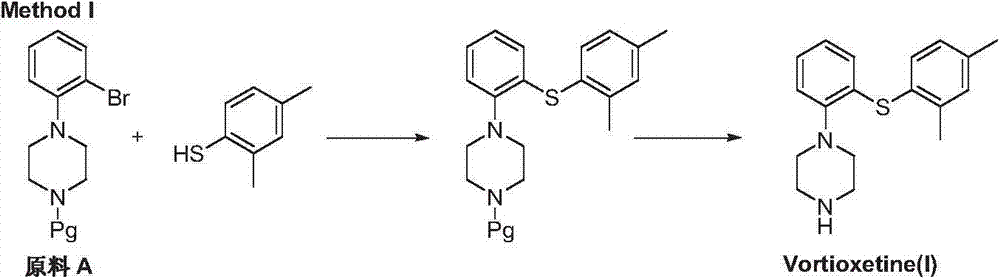
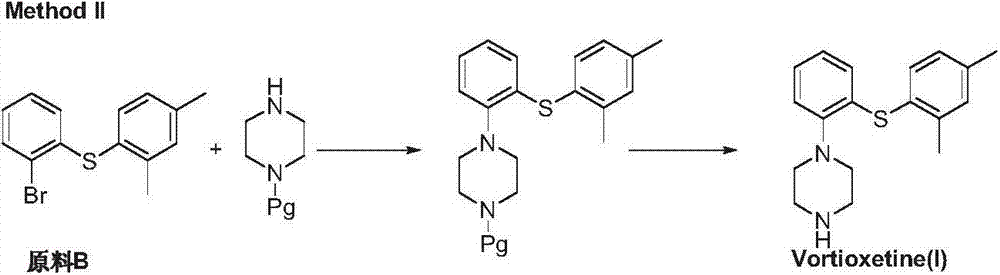
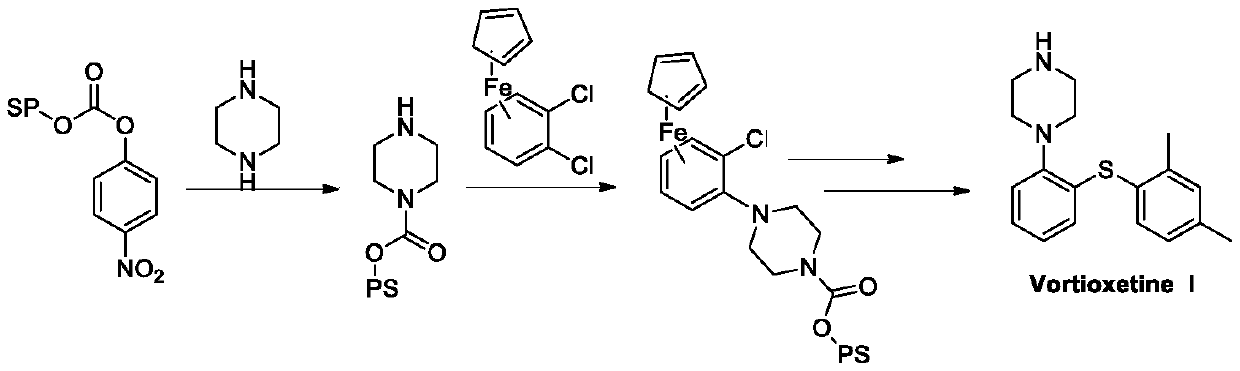
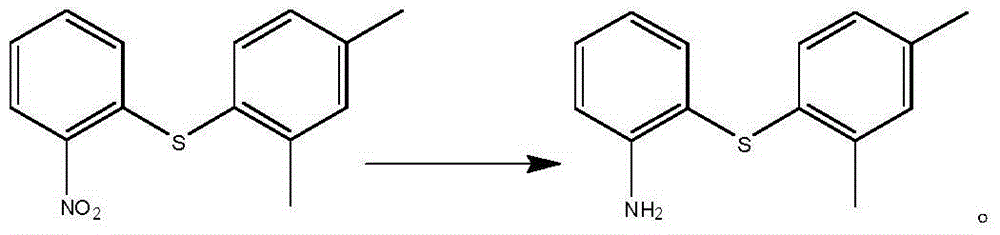
Preparation method of vortioxetine
ActiveCN103788020AEase of industrial productionEco-friendly economyOrganic chemistryNitrobenzeneAniline
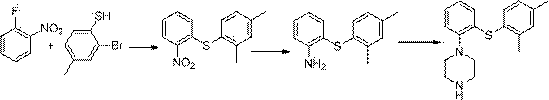
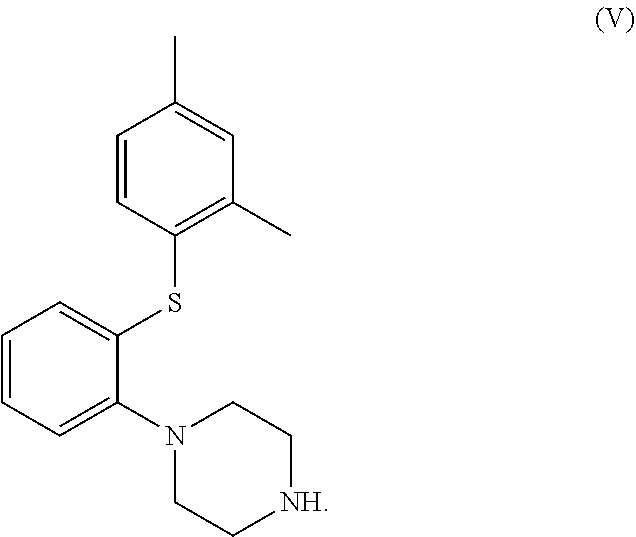
The invention discloses a preparation method of vortioxetine (I). The preparation method comprises the following steps: subjecting a compound shown in a formula (II) as a raw material and 2,4-dimethylthiophenol (III) to condensation to generate 2-(2,4-dimethylphenylthioalkyl)nitrobenzene (IV) or 2-(2,4-dimethylphenylthioalkyl)aniline (V) which is obtained by reducing the 2-(2,4-dimethylphenylthioalkyl)nitrobenzene (IV), and subjecting the 2-(2,4-dimethylphenylthioalkyl)aniline (V) and a compound shown in a formula (VI) to cyclization under alkaline conditions to obtain the vortioxetine (I). The preparation method is accessible in raw materials, is concise in process, is economical and environment-friendly and is suitable for industrial production.
Owner:优标易站(苏州)电子商务有限公司
Preparation method of vortioxetine
ActiveCN103788019AEase of industrial productionEco-friendly economyOrganic chemistryNitrobenzeneAniline
The invention discloses a preparation method of vortioxetine (I). The preparation method comprises the following steps: subjecting 2-substituted thiophenol shown in a formula (II) and 2,4-dimethylphenyl halide shown in a formula (III) to condensation to generate 2-(2,4-dimethylphenylthioalkyl)nitrobenzene (IV) or 2-(2,4-dimethylphenylthioalkyl)aniline (V) which is obtained by reducing the 2-(2,4-dimethylphenylthioalkyl)nitrobenzene (IV), and subjecting the 2-(2,4-dimethylphenylthioalkyl)aniline (V) and a compound shown in a formula (VI) to cyclization under alkaline conditions to obtain the vortioxetine (I). The preparation method is accessible in raw materials, is concise in process, is economical and environment-friendly and is suitable for industrial production.
Owner:优标易站(苏州)电子商务有限公司
Preparation method of antidepressant vortioxetine
The invention relates to the field of medicine synthesis, and particularly relates to a preparation method of antidepressant vortioxetine (I) shown in the specification. The preparation method comprises the following steps: reacting 2-(2,4-diphenylmethylthio)aniline (V) with bis(2-chloroethyl)amine hydrochloride (VI) to obtain vortioxetine hydrochloride (VII), and neutralizing VII through a base, to obtain vortioxetine (I). The total yield of vortioxetine hydrochloride (VII) prepared by the method is 59.8%, the raw materials are low-cost and easily available, and the treatment after reaction is convenient.
Owner:CHINA PHARM UNIV
Preparation method of Vortioxetine
InactiveCN104098530ARaw materials are easy to getSimple processOrganic chemistryCopper iodideCoupling reaction
The invention relates to the preparation method of Vortioxetine. The preparation method is characterized by comprising the steps of: obtaining a compound (formula 2) by carrying out coupling reaction on compound 2,4-dimethylbenzenethiol (formula 4) and compound 2-bromoiodobenzene (formula 3) in the presence of copper iodide, chiral ligand and alkali; obtaining a compound (formula 1) through the reaction of compound in formula 2 and piperazine in the presence of copper iodide, chiral ligand and alkali; or operating the twp steps in one reactor by one-pot method; the preparation method is easy to obtain material, simple in technology, high in product purity, few in by-products and beneficial to industrial production of the bulk drug.
Owner:冯修武
Orally disintegrating pharmaceutical composition and preparation method thereof
InactiveCN103989650AOrganic active ingredientsNervous disorderOrally disintegrating tabletFiller Excipient
The invention relates to an orally disintegrating pharmaceutical composition and a preparation method thereof and belongs to the field of pharmaceutical preparations. Considering the characteristic of bitter taste of hydrobromic acid vortioxetine, the invention provides a method for preparing orally disintegrating tablets which are small in particle size and can cover the bitter taste. The method is realized by mainly adopting the technical scheme, namely the composition is prepared by granulating or directly tabletting pharmaceutical microcapsules, 20-80 weight percent of filling agent, 5-10 weight percent of disintegrating agent, 0.5-2 percent of flavoring agent and 1-2 percent of lubricating agent. The orally disintegrating tablets do not have a grit feeling, sweetening agents such as saccharin sodium salt and Aspartame are not needed to be added into a subsequent tablet prescription, or the flavor of the tablets can be increased by adding a small amount of sweetening agent or essence and flavor according to market requirements, and the medicine content and encapsulation efficiency can meet the industrial large-scale production requirements.
Owner:李雪梅
Novel preparation method of hydrobromic acid Vortioxetine beta crystalline form
The invention discloses a novel preparation method of a hydrobromic acid Vortioxetine beta crystalline form. The novel preparation method comprises the steps of firstly synthesizing 2-(2,4-dimethyl-phenylsulfanyl)chlorobenzene from 2-chlorobenzenethiol and 2,4-dimethylphenol, adding bi(dibenzylidene acetone)palladium, 1,1'-binaphthyl-2,2'-bisdiphenylphosphino, sodium tert-butoxide and toluene into a reaction bottle, mixing, adding other substances to prepare Vortioxetine, dissolving prepared Vortioxetine by virtue of ethyl acetate with the weight of 14-16 times of that of Vortioxetine to obtain rough hydrobromic acid Vortioxetine, and finally purifying rough hydrobromic acid Vortioxetine to obtain finished hydrobromic acid Vortioxetine. The preparation method has the beneficial effects that the raw materials are easily available, process reaction conditions are mild, a product is high in yield and purity, and industrial production is easily realized; prepared hydrobromic acid Vortioxetine is white crystalline powder, and the purity is more than 99.5%.
Owner:郑州大明药物科技有限公司
Synthetic method of vortioxetine
The invention discloses a synthetic method of vortioxetine. The synthetic method comprises the following steps of by adopting a compound as shown in a formula (I) as a raw material, carrying out substitution reaction on the compound and 2,4-dimethyl thiophenol (II) to generate 2-(2,4-dimethyl phenyl alkyl sulfide) nitrobenzene (III); reducing 2-(2,4-dimethyl phenyl alkyl sulfide) nitrobenzene (III) to obtain 2-(2,4-dimethyl phenyl alkyl sulfide) phenylamine (IV); cyclizing 2-(2,4-dimethyl phenyl alkyl sulfide) phenylamine (IV) and N,N-bis(2-chloroethyl)-4-methyl benzsulfamide (VI) to obtain Tos-protecting vortioxetine (V); and preparing vortioxetine (VII) from Tos-protecting vortioxetine (V) under the action of a phenol additive. The synthetic method disclosed by the invention has the advantages of easily available raw material, simple process, low cost and high purity, and is suitable for industrialized production.
Owner:SUNDIA MEDITECH COMPANY LTD
Preparation method for vortioxetine
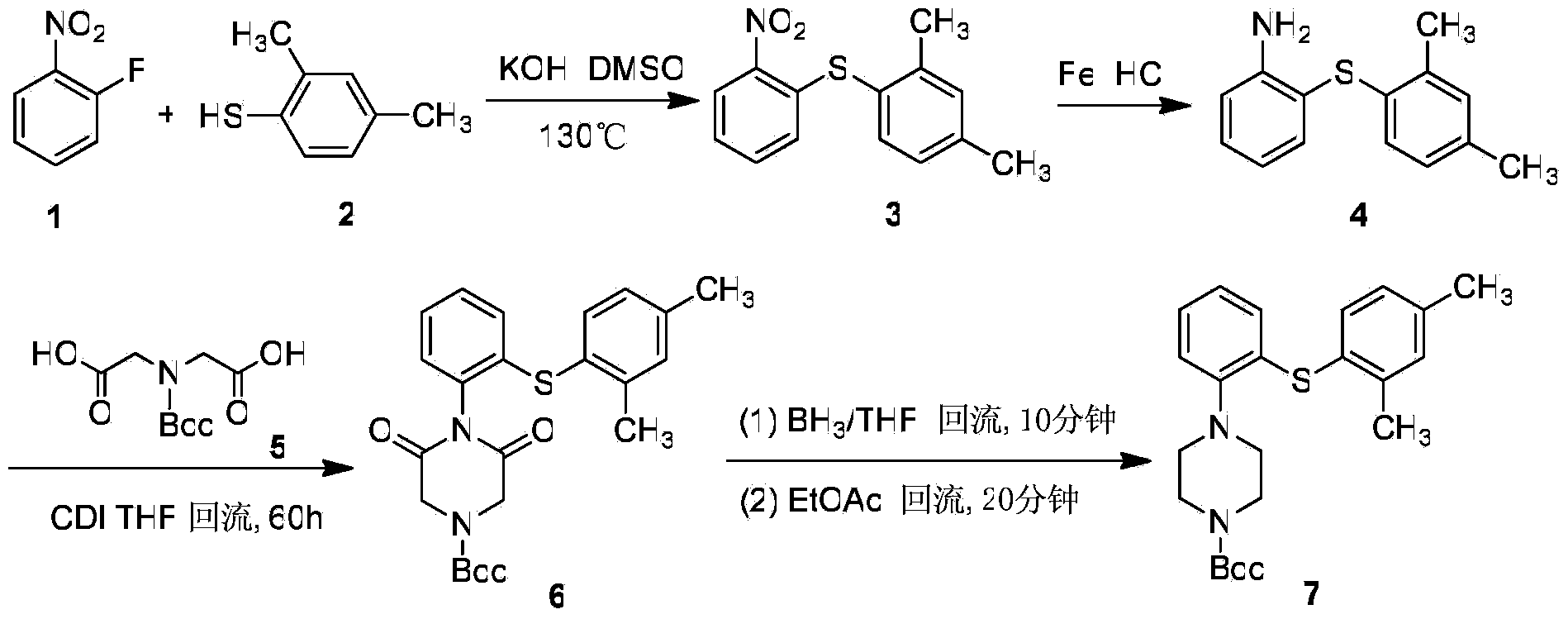
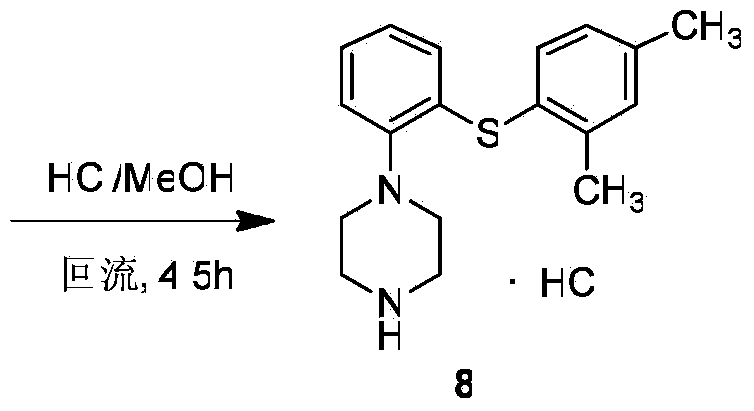
The invention discloses a preparation method for vortioxetine. The preparation method comprises the following steps of (1) performing diazotization on a compound 4 to obtain a compound 5; (2) coupling the compound 5 and a compound 6 to obtain a compound 7; (3) deacetylating the compound 7 to obtain 1-(2-(2,4-dimethyl phenylthio)phenyl)piperazine (i.e. vortioxetine) shown in formula 8. According to the method, operation is simplified, the yield is increased, the cost is greatly reduced, and the method is more suitable for industrial expansion, and has remarkable creativity and practical application value.
Owner:HANGZHOU HEZE PHARMA TECH
Preparation method for vortioxetine
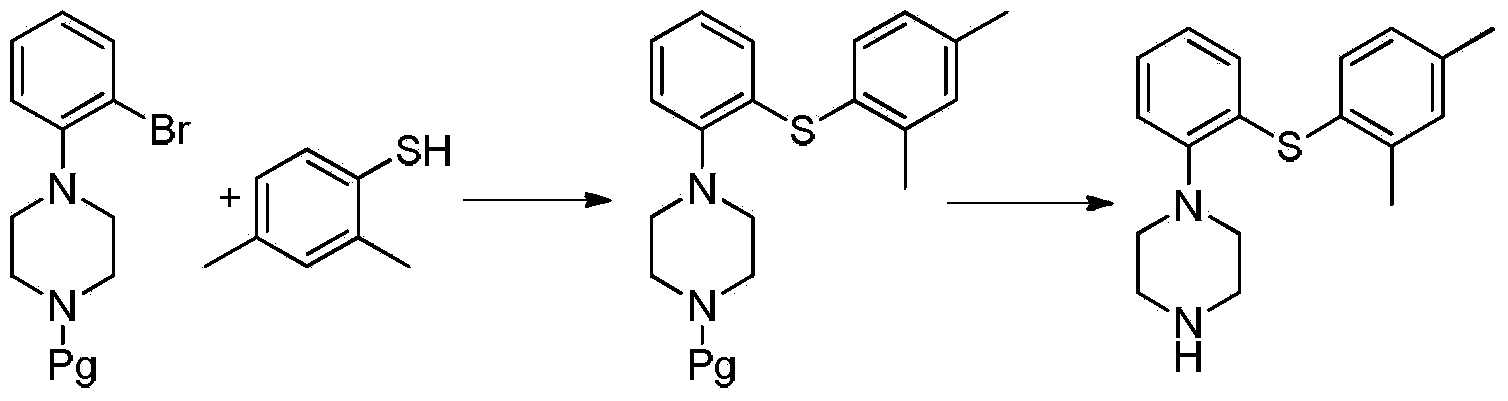
The invention relates to the field of medicament synthesis, in particular to a novel preparation method for an antidepressant medicament intermediate vortioxetine. The method comprises the following steps of reacting 2,4-dimethylthiophenol with 2-halogen chlorobenzene to obtain an intermediate 2-(2,4-dimethylthiophenyl) chlorobenzene, and reacting the intermediate with piperazine to obtain vortioxetine. According to the method, the total yield can reach 68 to 75 percent, the method has the advantages of starting materials are low in cost and easy to obtain, the process is simple, and the requirements of large-scale production are met.
Owner:合肥创新医药技术有限公司
Vortioxetine hydrobromide crystal preparation method
ActiveCN104910099AComply with medicinal requirementsHigh yieldOrganic chemistry methodsThermal insulationEthyl acetate
The invention discloses a vortioxetine hydrobromide crystal preparation method. The method comprises a, dissolving vortioxetine free alkali in ethyl acetate at a temperature of 20-30 DEG C, b, carrying out filtration, cooling the filtrate to a temperature of 0-10 DEG C, dropwisely adding an ethyl acetate solution of hydrobromic acid into the filtrate along with thermal insulation and then carrying out thermal insulation stirring for 2-8h, c, filtering the mixture subjected to thermal insulation stirring in the step b to obtain filter cake 1, leaching the filter cake 1 by ethyl acetate, and carrying out stirring washing in ethyl acetate at a temperature of 0-10 DEG C for 0.5-5h, d, filtering the mixture subjected to stirring washing in the step c to obtain filter cake 2, leaching the filter cake 2 by methyl tert-butyl ether / ethyl acetate pre-cooled at a temperature of 0-10 DEG C and carrying out stirring washing in methyl tert-butyl ether at a temperature of 10-30 DEG C for 15-24h, and e, filtering the mixture subjected to stirring washing in the step d to obtain filter cake 3, leaching the filter cake 3 by methyl tert-butyl ether and carrying out vacuum drying at a temperature of 40-50 DEG C to obtain the product. The method has the advantages of good repeatability, simple processes, a high yield and high product purity and is suitable for industrial production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Hydrobromic acid vortioxetine gastric-soluble tablet and preparation method thereof
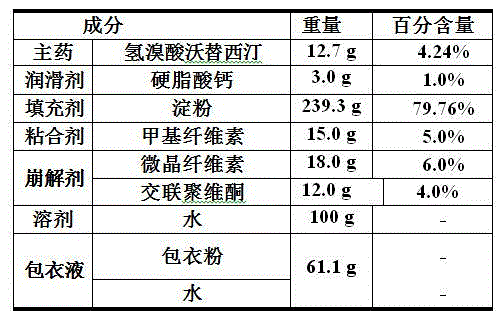
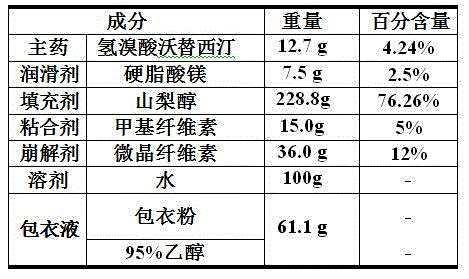
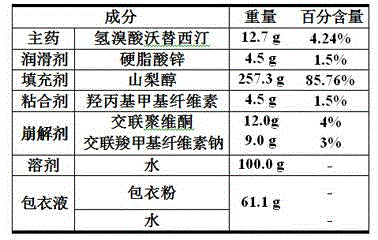
The invention relates to the technical field of medicines and in particular relates to a hydrobromic acid vortioxetine gastric-soluble tablet and a preparation method thereof. The hydrobromic acid vortioxetine gastric-soluble tablet comprises the following components in percentage by weight: 4.24% of hydrobromic acid vortioxetine, 1.0-5.0% of a lubricating agent, 81-91% of a filling agent, 2-5% of a binding agent and 2-15% of a disintegrating agent. Dissolution rate of the hydrobromic acid vortioxetine gastric-soluble tablet is similar to the foreign original standard, so that the hydrobromic acid vortioxetine gastric-soluble tablet has good application prospect and market prospect.
Owner:郑州大明药物科技有限公司
Vortioxetine semi-hydrochloride, preparation method therefor, and pharmaceutical composition thereof
InactiveCN105111167ACrystal stableHigh crystallinityOrganic active ingredientsNervous disorderSpace groupProton
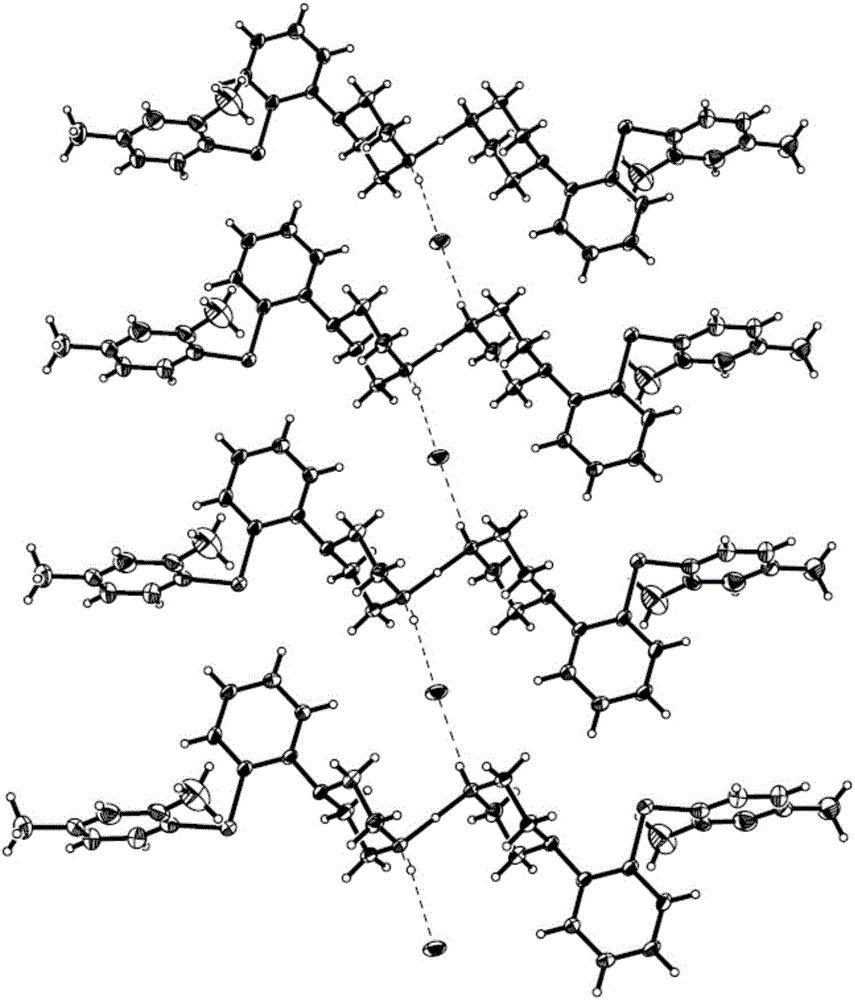
The present invention discloses a vortioxetine semi-hydrochloride, a preparation method therefor, and a pharmaceutical composition thereof. The vortioxetine semi-hydrochloride is as shown in the formula I; a space group of the salt is a monoclinic system; two vortioxetine molecules share one proton; and a hydrogen bond N-H ... Cl- is combined with vortioxetine to form a basic structural unit. The vortioxetine semi-hydrochloride provided by the invention has a stable crystal form and high crystallinity; the preparation method is simple; and the crystal does not contain a solvent.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method and intermediate of vortioxetine
ActiveCN105315184ALow costMild reaction conditionsSulfide preparationEthyl groupCombinatorial chemistry
The invention discloses a preparation method and intermediate of vortioxetine. The preparation method of vortioxetine includes the following steps that 1, a compound V is prepared according to a preparation method of the compound V; 2, the compound V and dichloro ethylamine or salt of dichloro ethylamine are subjected to a cyclization reaction in a high-boiling-point solvent to prepare vortioxetine, wherein the boiling point of the high-boiling-point solvent is above 120 DEG C at normal pressure. The preparation method is low in cost, easy to implement, safe, environmentally friendly, high in yield and suitable for industrial production, and reaction conditions are mild.
Owner:上海谷方盟医药科技有限公司
Vortioxetine manufacturing process
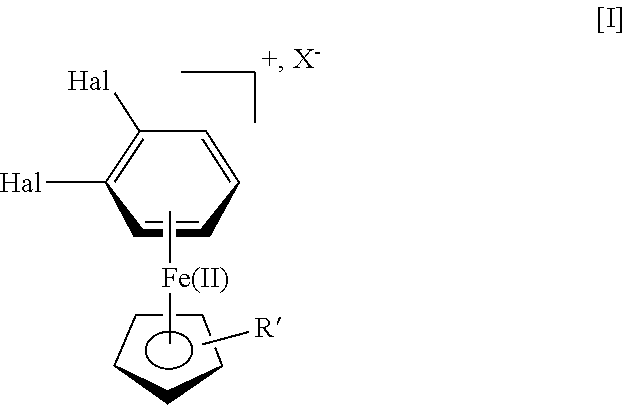
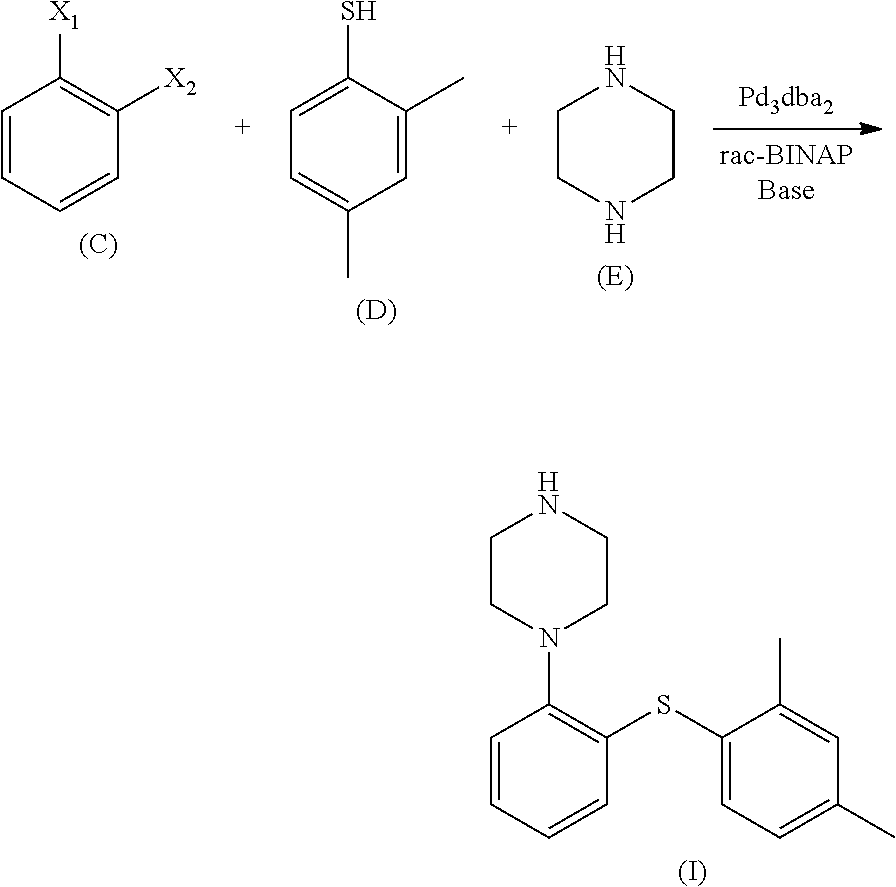
A process for the manufacture of vortioxetine is provided in which a compound of formula I, formula I is reacted with optionally substituted piperazine and 2,4-dimethylthiophenol(ate) followed by de-cmplexation.
Owner:H LUNDBECK AS
Aspartate of vortioxetine or hydrate thereof as well as preparation method and application thereof
ActiveCN104610195AEasy to synthesizeEliminate damageOrganic active ingredientsNervous disorderHydrobromideSolubility
The invention provides aspartate of vortioxetine or hydrate thereof, comprising anhydrous crystal form, semihydrate crystal form and dihydrate crystal form of aspartate of vortioxetine, as well as a preparation method and application thereof. The preparation method comprises the following steps: performing a reaction on the vortioxetine and the aspartic acid in the organic solvent or the mixed solvent of the organic solvent and the water for generating the aspartate, keeping the temperature or cooling and crystallizing. The aspartate of the vortioxetine has the function of treating major depressive disorder and also has the function of the aspartic acid, so that the defect of the inorganic acid salt to the human body is effectively compensated by the aspartate. The solubility, stability and bioavailability of the aspartate are greatly improved. The equilibrium solubility shows that the solubility of the aspartate of the vortioxetine (at 37 DEG C) is more than 7 times of that of the hydrobromide of the vortioxetine.
Owner:SHANGYU JINGXIN PHARMA +2
Preparation method of vortioxetine
PendingCN110452188AHigh purityRaw materials are easy to getOrganic chemistryBenzeneSynthesis methods
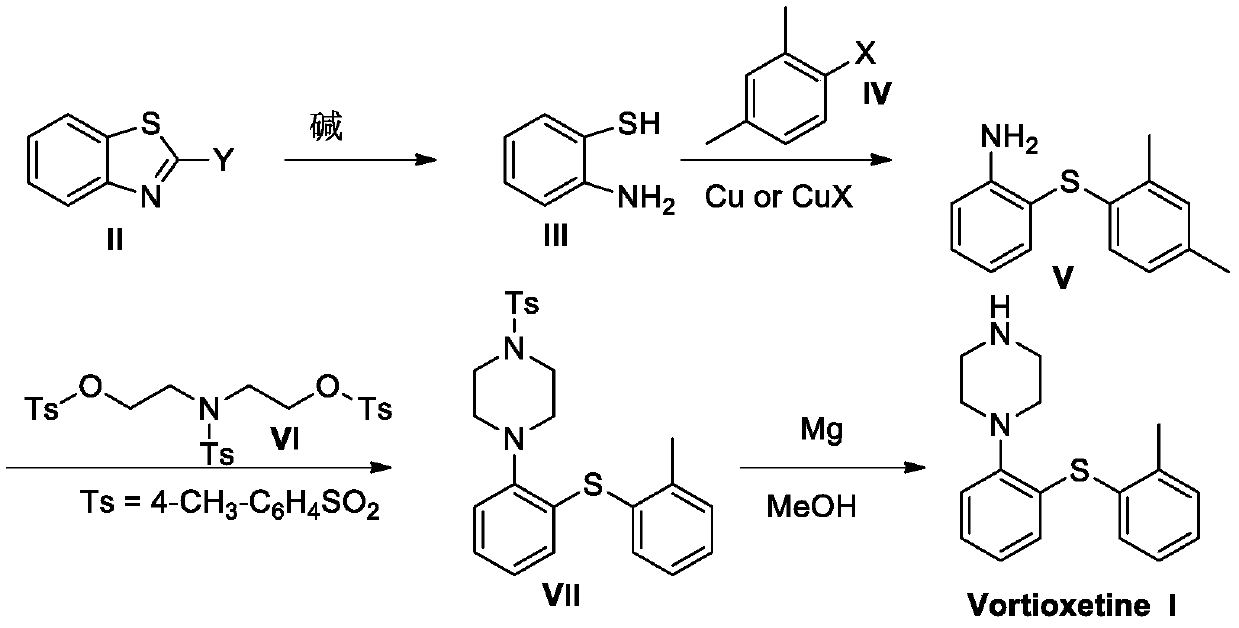
The invention discloses a preparation method of vortioxetine. A benzothiazole derivative (II) is dissociated into o-aminothiophenol (III) under the condition of alkali, and then the o-aminothiophenol(III) reacts with 2,4-dimethyl halogeno benzene under the catalysis of copper or a copper salt to generate 2-(2,4-dimethylphenylsulfanyl)aniline (V), the 2-(2,4-dimethylphenylsulfanyl)aniline (V) andbis(2-hydroxybenzenesulfonic acid)-4-methylbenzenesulfonamide (VI) are cyclized to obtain Ts-protected vortioxetine (VII), and the Ts-protected vortioxetine (VII) is converted into the vortioxetine (I) under the effect of Mg. The synthesis method has the advantages of easily available raw materials, simple process, low cost, high purity and suitability for industrial production.
Owner:BENGBU COLLEGE
Vortioxetine pharmaceutical composition and preparation method thereof
InactiveCN104644635ASolve the problem of large differences in content uniformityHigh yieldOrganic active ingredientsNervous disorderCyclodextrinPharmaceutical drug
The invention discloses a vortioxetine pharmaceutical composition and a preparation method thereof. The vortioxetine pharmaceutical composition contains hydrobromic acid vortioxetine, betadex, lactose, microcrystalline cellulose and magnesium stearate. The vortioxetine pharmaceutical composition is characterized in that the weight ratio of hydrobromic acid vortioxetine to betadex to lactose is 1 to 5 to 10. The vortioxetine pharmaceutical composition is good in stability and has obvious superiorities that the product yield is increased, the cost is lowered, the industrialization is realized, and the relatively good clinic application is achieved; the dissolution rate can be effectively increased, and the bioavailability is obviously improved. The vortioxetine pharmaceutical composition can be used for treating major depressive disorder.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Composition of amorphous vortioxetine or amorphous vortioxetine salt and pharmaceutical adjuvants, and preparation method thereof
InactiveCN106491604AGood dispersionPromote absorptionOrganic active ingredientsNervous disorderBioavailabilityPharmaceutical Adjuvants
The invention relates to a composition of vortioxetine or pharmaceutically acceptable vortioxetine salt and pharmaceutical adjuvants, and a preparation method thereof. The composition includes vortioxetine or pharmaceutically acceptable vortioxetine salt and two or more pharmaceutical adjuvants, and a weight ratio of the vortioxetine or pharmaceutically acceptable vortioxetine salt to all the pharmaceutical adjuvants is 1:(0.1-100), wherein the vortioxetine or pharmaceutically acceptable vortioxetine salt in the composition has an amorphous form, and no characteristic peak of the vortioxetine or pharmaceutically acceptable vortioxetine salt exists in an X-ray powder diffraction spectrum of the composition, subtracting the background peaks of the pharmaceutical adjuvants. The composition of vortioxetine or pharmaceutically acceptable vortioxetine salt and pharmaceutical adjuvants has good stability and dispersibility, increases the dissolvability of the vortioxetine or pharmaceutically acceptable vortioxetine salt, is in favor of improving the bioavailability of a medicinal preparation and the medicine absorption of bodies, and keeps good physical stability and chemical stability under accelerated test conditions. The preparation method of the amorphous composition has the advantages of simplicity in operation, low cost, good reappearance, easiness in realization, and suitableness for industrial production.
Owner:CHANGZHOU FANGNAN MEDICINE TECH CO LTD
Preparation method of antidepressant drug Vortioxetine
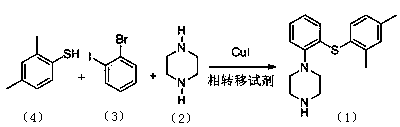
ActiveCN104292183AEase of industrial productionEasy to operateOrganic chemistryMethyl benzeneCopper iodide
The invention relates to a preparation method of an antidepressant drug Vortioxetine, which belongs to the technical field of pharmaceutical chemicals. According to the method, by taking water as a solvent, a compound 2,4-dimethylbenzenethiol shown in a formula (4), a compound 2-bromoiodobenzene shown in a formula (3) and a compound piperazine shown in a formula (2) react with copper iodide, a phase transfer reagent and alkali in an aqueous solution so as to obtain a compound shown in a formula (1). The method disclosed by the invention is easily-obtained in raw materials and simple in process; and one-pot reaction operation can be performed, and water is taken as a solvent, so that the method is green and environmental-friendly, and facilitates the industrialized production of the bulk drug.
Owner:NANTONG FINC PHARMA CHEM
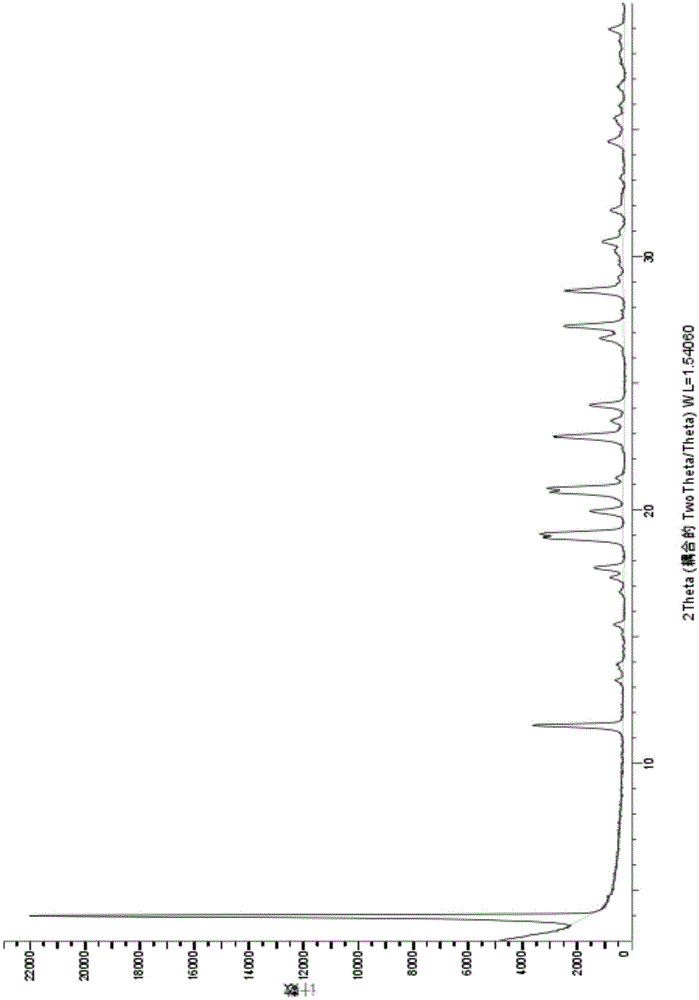
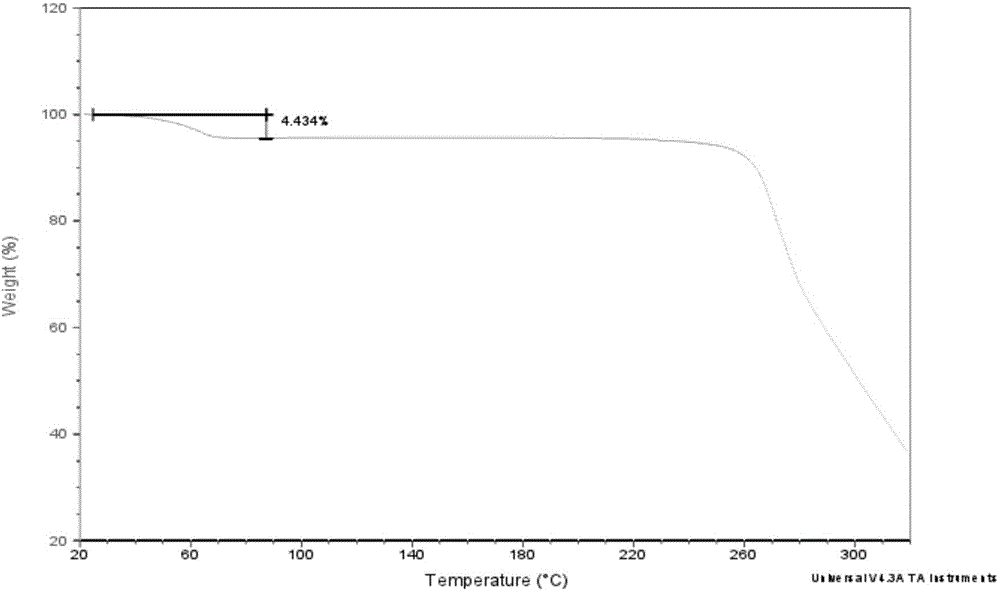
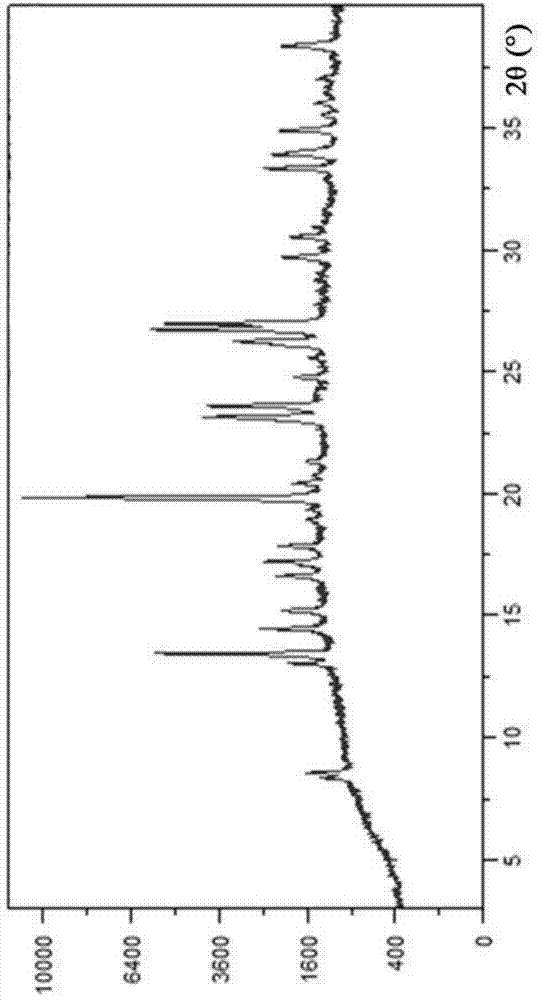
Novel crystal form of Vortioxetine hydrobromate and preparation method for novel crystal form of Vortioxetine hydrobromate
The invention relates to a novel crystal form of Vortioxetine hydrobromate and a preparation method for the novel crystal form of the Vortioxetine hydrobromate. The novel crystal form of the Vortioxetine hydrobromate is named a crystal form sigma, wherein in an X-ray powder diffraction atlas using Cu-K alpha radiation detection, the crystal form sigma has characteristic peaks when a 2[theta] angle is about 4.0 degrees, 11.5 degrees, 15.5 degrees, 17.7 degrees, 19.1 degrees, 20.8 degrees, 22.9 degrees, 27.2 degrees or 28.6 degrees. The invention also provides a method for preparing the novel crystal form sigma of the Vortioxetine hydrobromate. The method is simple and convenient and is good in reproducibility, and the obtained novel crystal form sigma of the Vortioxetine hydrobromate is high in purity and good in stability, so that the method is applicable to industrial production.
Owner:SHANGHAI AOBO PHARMTECH INC LTD
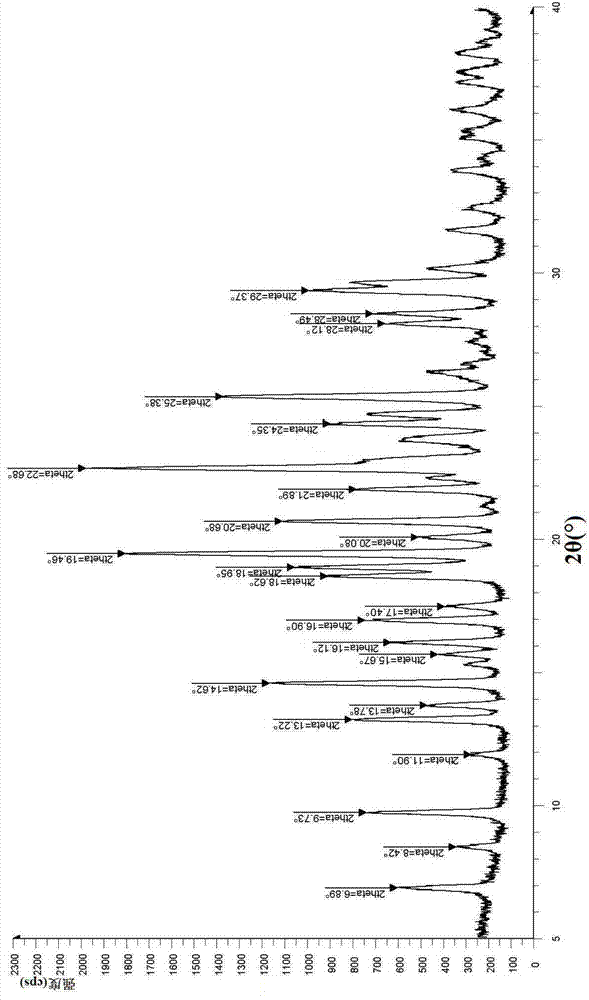
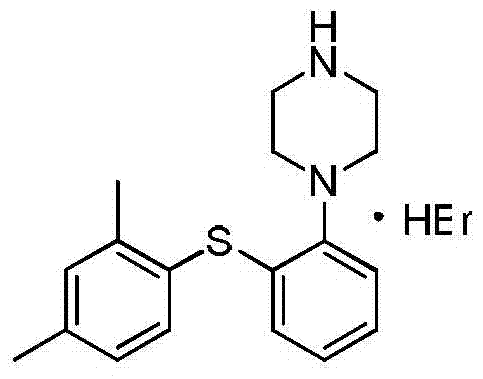
Hydrobromide of vortioxetine
The invention relates to a hydrobromide of vortioxetine and in particular relates to a crystal of a compound shown in a formula I in the specification. The crystal uses Cu-K alpha radiation. In a powder X-ray diffraction pattern shown by a 2theta angle, diffraction peaks exist at about 6.89 degrees, 9.73 degrees, 13.78 degrees and 14.62 degrees. The invention also relates to a preparation method of the crystal and a drug composition containing the compound. The compound shows the serotonin reabsorption inhibitory activity, has activities towards a serotonin receptor 1A (5-HT1A) and a serotonin receptor 3 (5-HT3), can be used for treating the CNS (central nervous system)-related diseases, and in particular can be used for treating depressive disorder, especially major depressive disorder of adults.
Owner:BEIJING LABWORLD BIO MEDICINE TECH
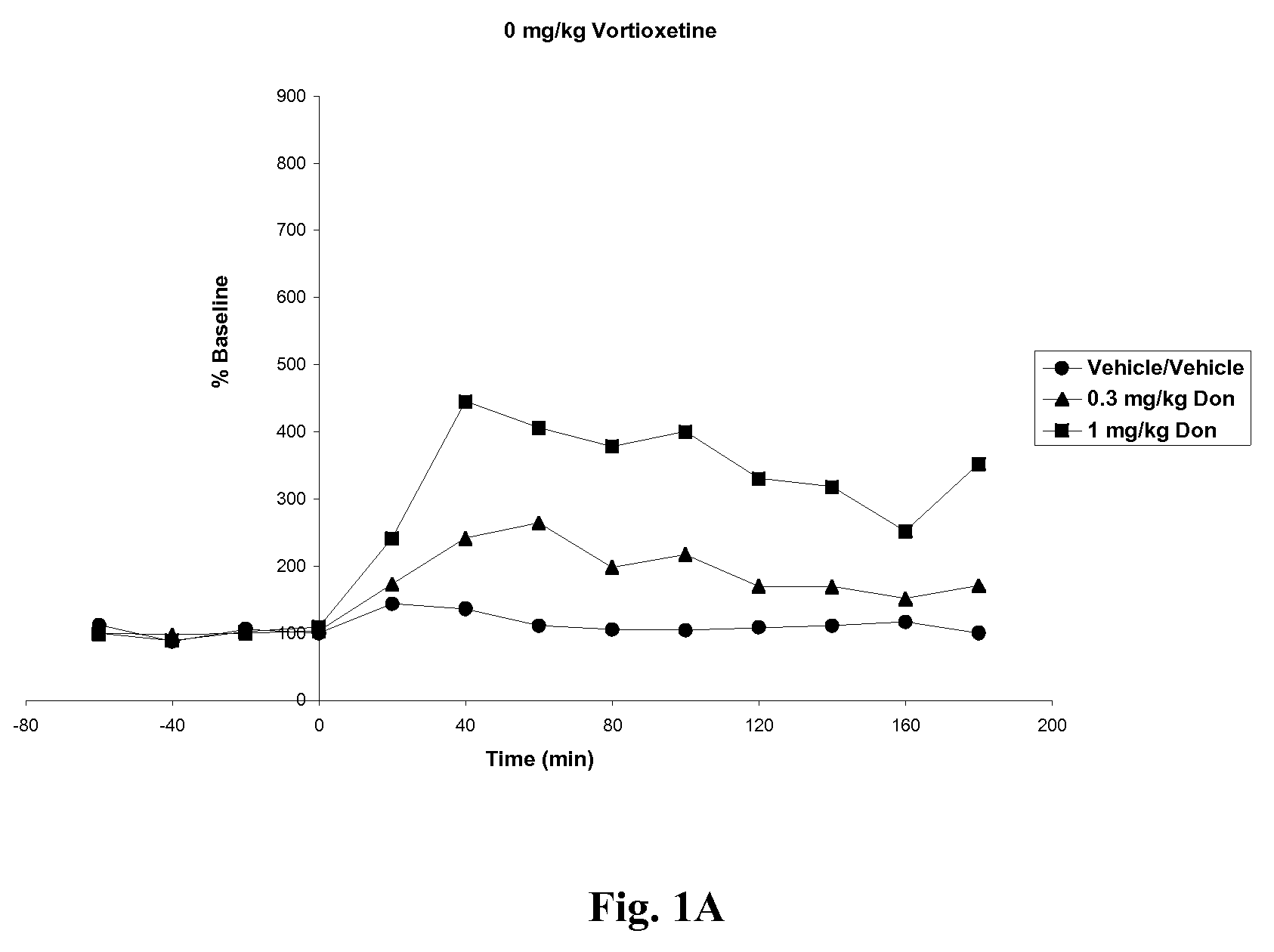
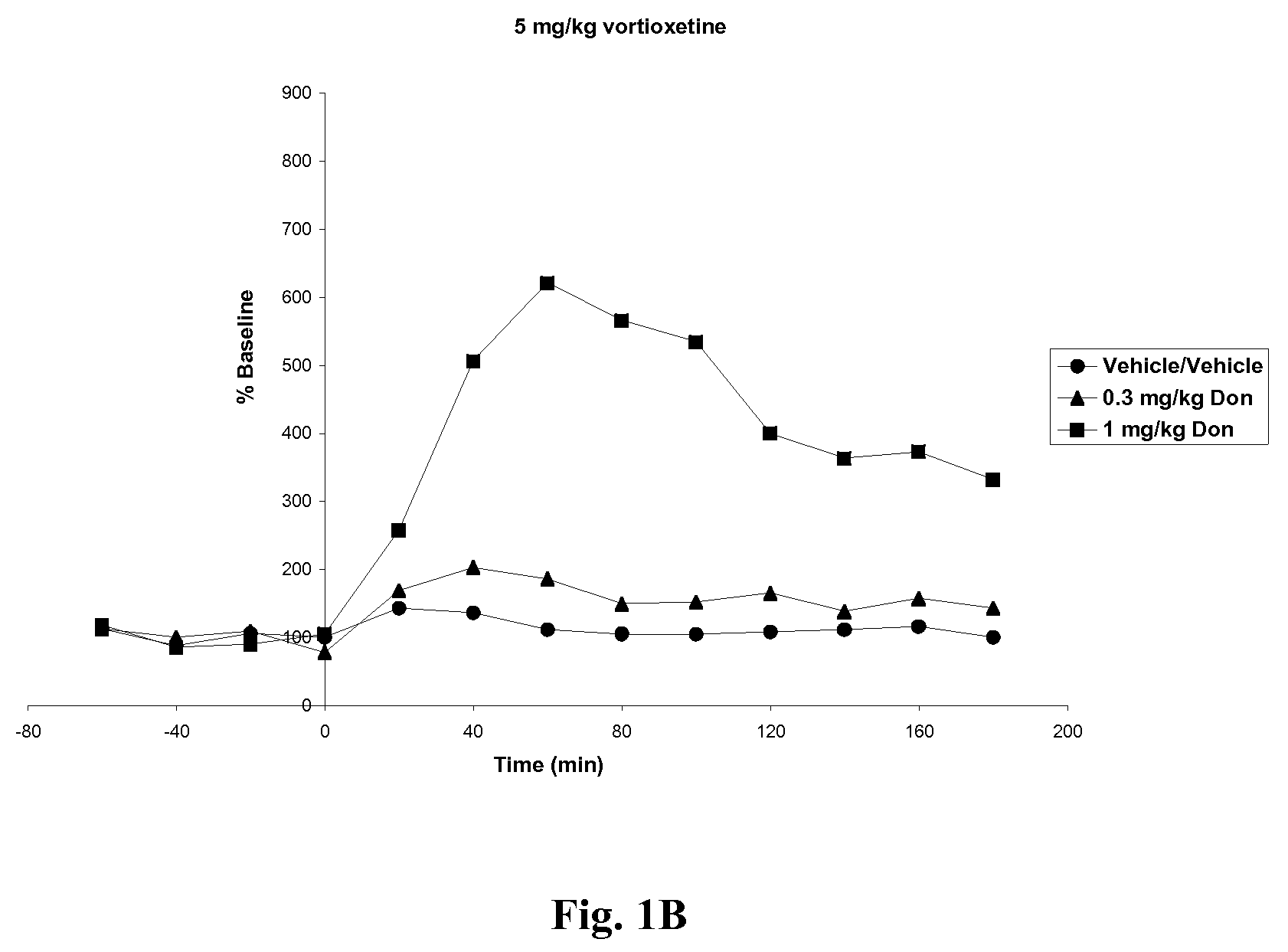
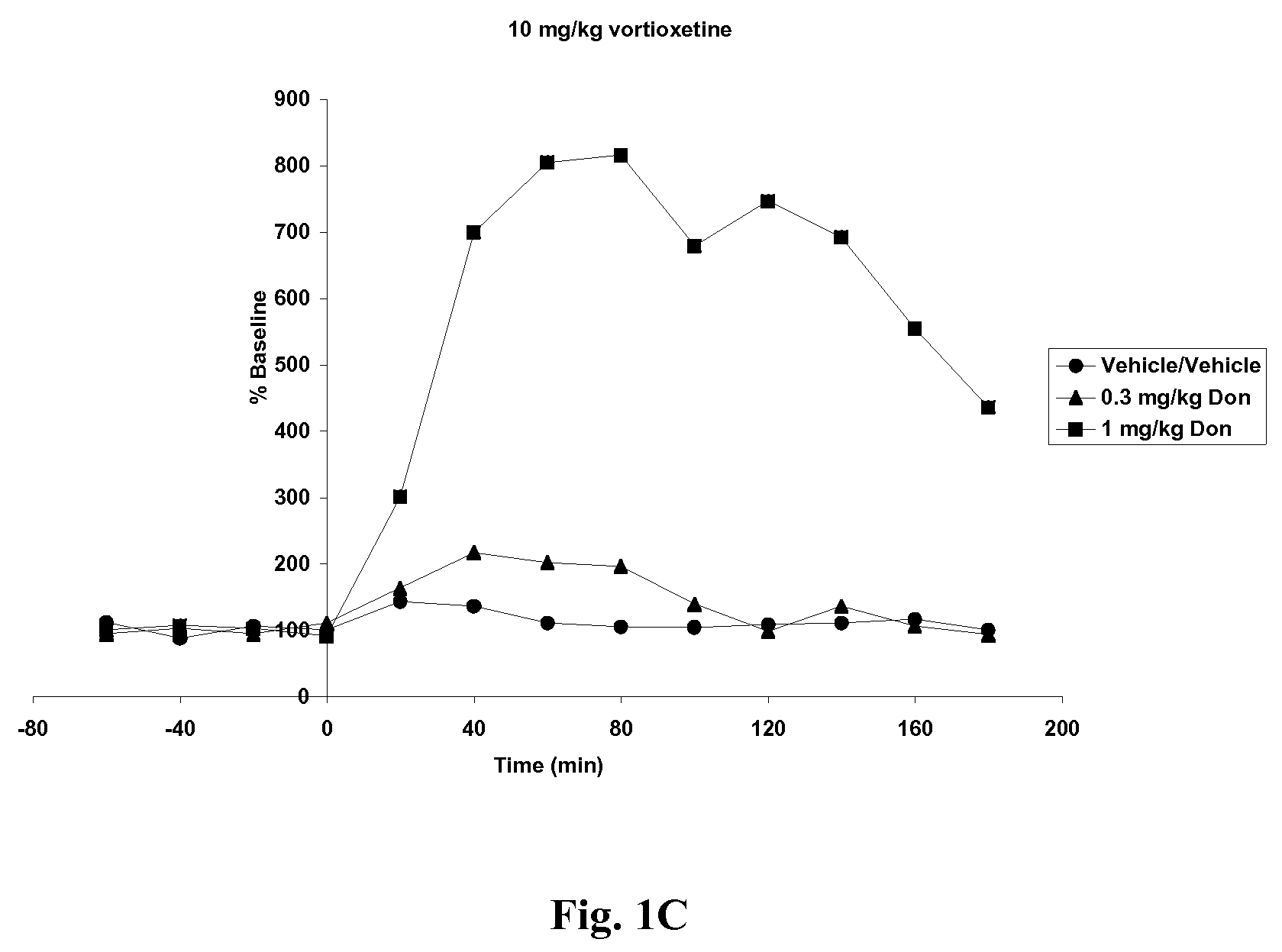
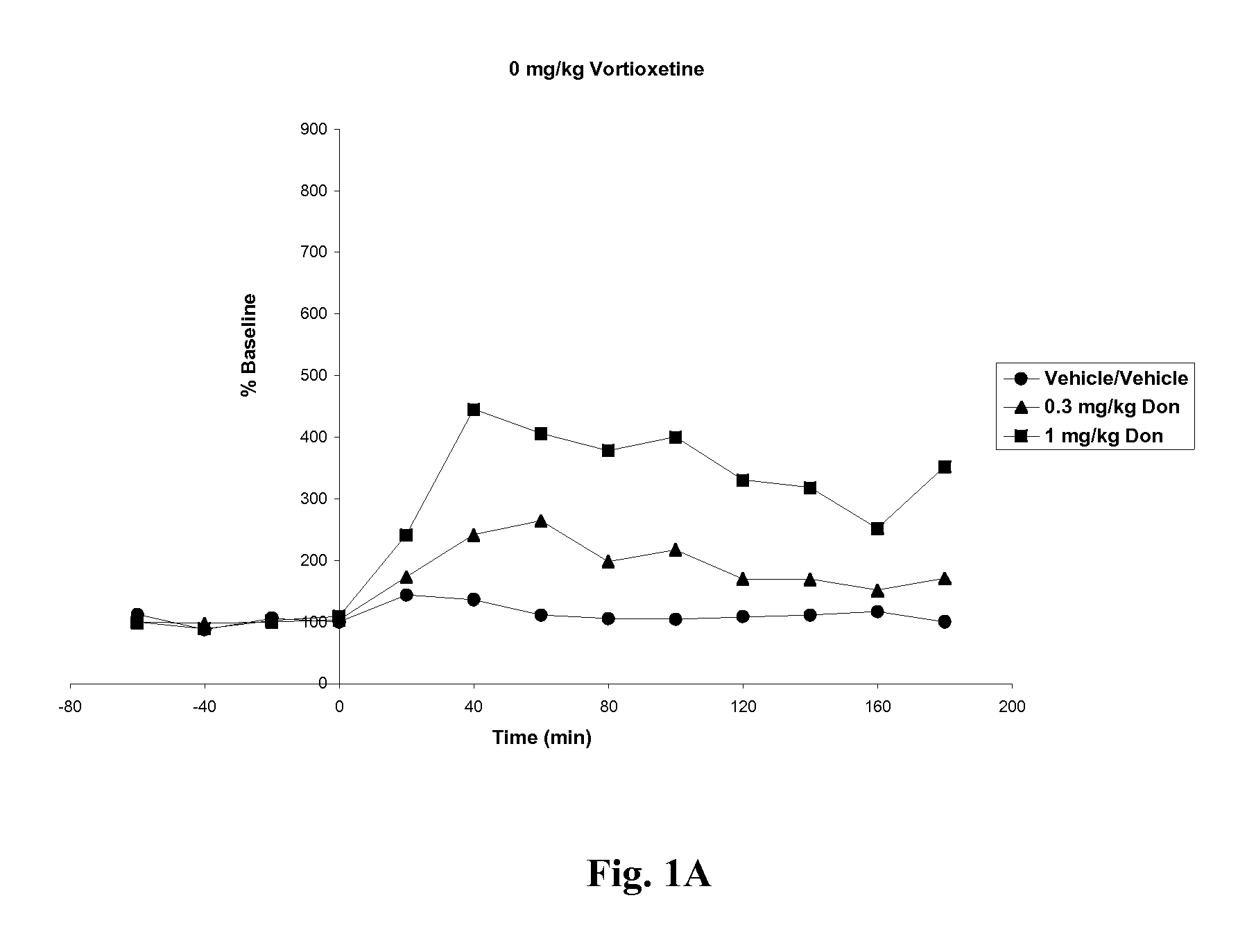
Compositions comprising vortioxetine and donepezil
ActiveUS9211288B2Improve the level ofNervous disorderIn-vivo testing preparationsDonepezilVortioxetine
Pharmaceutical compositions comprising vortioxetine and donepezil are provided and the use of such composition for the treatment of cognitive dysfunctions.
Owner:H LUNDBECK AS
Process for the preparation of an antidepressant and the intermediates thereof
The present invention relates to a process for the preparation of 1-[2-(2,4-dimethylphenylsulphanyl)phenyl]piperazine of formula (I), also known as vortioxetine, salts thereof, and intermediates useful for its synthesis.
Owner:DIPHARMA FRANCIS
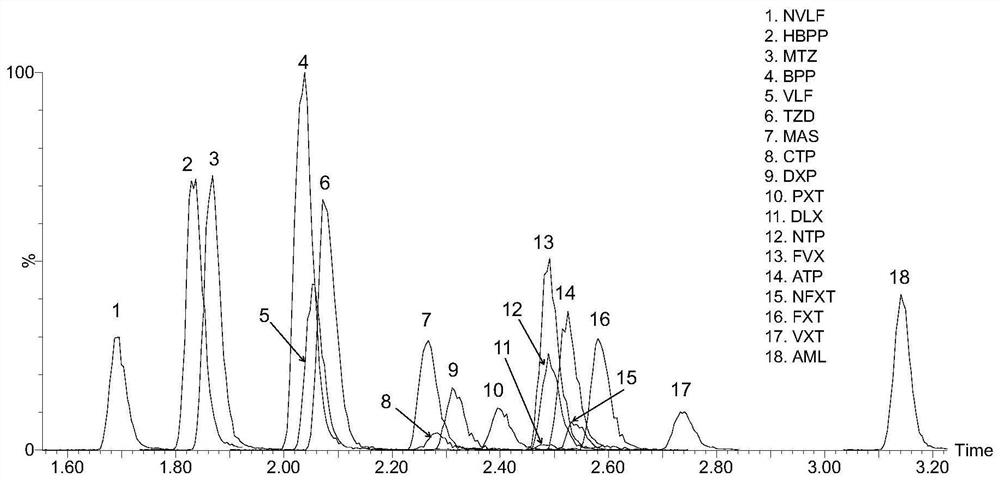
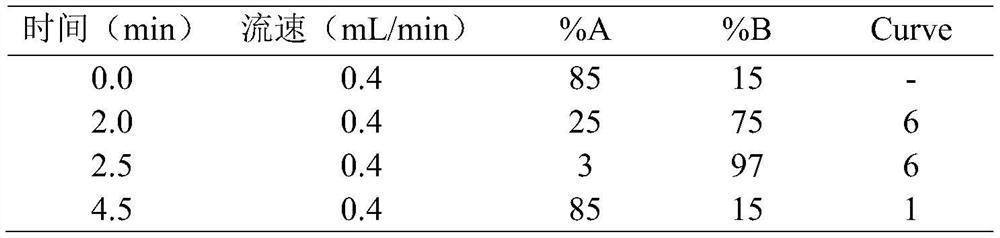
Method for detecting concentration of antidepressant drugs in serum by using ultra-high performance liquid chromatography-tandem mass spectrometry technology
The invention discloses a method for detecting the concentration of antidepressant drugs in serum by an ultra-high performance liquid chromatography-tandem mass spectrometry technology. The antidepressant drugs comprise bupropion, agomelatine, hydroxybupropion, nortriptyline, o-desmethylvenlafaxine, mianserin, mirtazapine, venlafaxine, amitriptyline, doxepin, norfluoxetine hydrochloride, duloxetine, fluoxetine, fluvoxamine, citalopram, paroxetine, trazodone and vortioxetine. After a serum sample is pretreated, a to-be-detected substance is separated from a serum matrix by utilizing ultra-highperformance liquid chromatography, a calibration curve is established by utilizing a mass spectrum isotope internal standard quantitative method and taking a concentration ratio of the standard substance to an internal standard substance as an X axis and a peak area ratio of the standard substance to the internal standard substance as a Y axis, and the content of the drugs in serum is calculated.The method is high in sensitivity, high in specificity, accurate and simple in pretreatment process, separation and detection can be completed within 4.5 min, and the accuracy degree and precision basically meet the requirements.
Owner:南京品生医学检验实验室有限公司
Preparation method of Vortioxetine
The invention relates to the technical field of Vortioxetine preparation methods. Vortioxetine is prepared through the ring-closure reactions between 2-(2,4-dimethylthiophenyl)aniline and bis(2-chloroethyl)amine hydrochloride. Compared to the prior art, the Vortioxetine can be obtained through three steps of replacement reactions, reduction reactions, and ring-closure reactions in the provided preparation method, the total yield is four times as many as that of a conventional method, and the cost is greatly reduced. Moreover, the reagents used in the preparation method are common and cheap, and do not have any side or toxic effect; the reaction conditions are mild, and thus the preparation method is suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
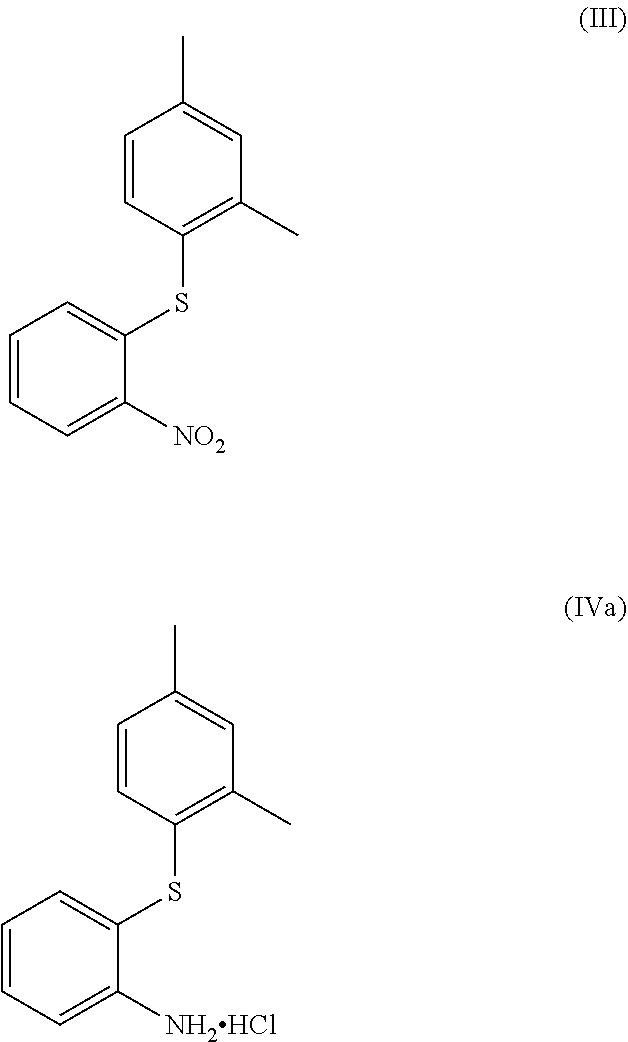
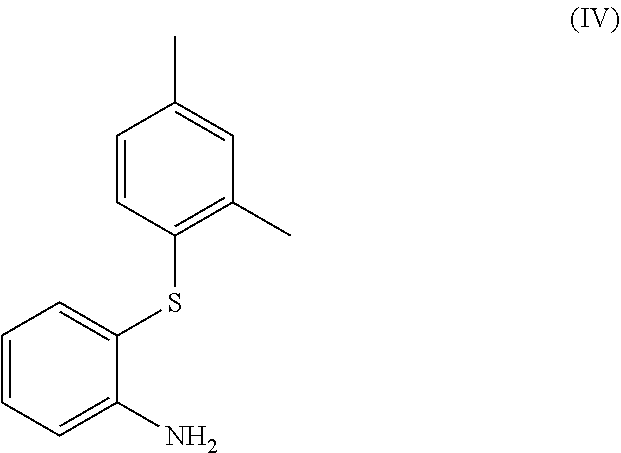
Process for the synthesis of 1-(2-((2,4-dimethylphenyl)thio)phenyl)piperazine
The present invention provides new intermediate compounds or formulae (III) and (IVa), and salts thereof, and their use in a new synthetic process for the production of 1-(2-((2,4-dimethylphenyl)thio)phenyl)piperazine (vortioxetine) an experimental drug under development for the treatment of depression and anxiety.
Owner:LEK PHARMA D D
Compositions comprising vortioxetine and donepezil
ActiveUS20150297585A1Improve the level ofNervous disorderIn-vivo testing preparationsDonepezilVortioxetine
Pharmaceutical compositions comprising vortioxetine and donepezil are provided and the use of such composition for the treatment of cognitive dysfunctions.
Owner:H LUNDBECK AS
Vortioxetine sustained-release capsules and preparation method of vortioxetine sustained-release capsules
InactiveCN106727438AGood compatibilityPrevent infiltrationOrganic active ingredientsNervous disorderSide effectSucrose
The invention discloses vortioxetine sustained-release capsules as well as a preparation method and application of the vortioxetine sustained-release capsules. The vortioxetine sustained-release capsules are prepared by loading vortioxetine sustained-release micro-pills into hollow capsules. Each vortioxetine sustained-release micro-pill is composed of a medicine-carrying pill core, an isolation type coating layer, a sustained-release coating layer and a protective coating layer from inside to outside, wherein the medicine-carrying pill core is composed of vortioxetine, an adhesive and a sucrose hollow pill core, and the specific weight percent is as follows: 0.1 percent to 10 percent of the vortioxetine, 10 percent to 30 percent of the adhesive and 10 percent to 60 percent of the sucrose hollow pill core. A sustained-release preparation can be used for prolonging the holding time of treatment concentration of the vortioxetine in a body to 12h and reducing the side effect caused by the fact that the fluctuation of plasma drug concentration is too great; meanwhile, the number of times of orally taking drug is reduced, and the vortioxetine sustained-release capsules have remarkable effect on patients with depressive disorder, who need to be treated, and are convenient to use.
Owner:佛山市弘泰药物研发有限公司
Vortioxetine orally disintegrating tablets and preparation method thereof
The invention discloses vortioxetine orally disintegrating tablets and a preparation method thereof. The orally disintegrating tablets are prepared from, by mass, 5-30 parts of vortioxetine, 10-30 parts of fatty glyceride, 5-10 parts of Tween 80, 900-2,700 parts of white sugar, 2-5 parts of polyvinylpolypyrrolidone, 10-30 parts of mannitol, 0.01-0.05 part of essence, 0.2-0.5 part of magnesium stearate and 0.2-0.5 part of superfine silica powder. Vortioxetine serves as an effective medicine ingredient, and the orally disintegrating tablets are prepared through a creative process. The prepared vortioxetine orally disintegrating tablets successfully solve the problems that the medicine dissolution rate is low, and medicine is bitter and astringent, and have a good effect on functional dyspepsia.
Owner:佛山市弘泰药物研发有限公司
A kind of vortioxetine hydrobromide crystal and preparation method thereof
ActiveCN105017176BComply with medicinal requirementsHigh yieldOrganic chemistry methodsHydrobromideX-ray
The present invention discloses a hydrobromic acid vortioxetine crystal that has diffraction peaks at 8.55+ / -0.2, 13.05+ / -0.2, 13.44+ / -0.2, 14.46+ / -0.2, 15.20+ / -0.2, 16.63+ / -0.2, 16.94+ / -0.2, 17.22+ / -0.2, 17.85+ / -0.2, 19.83+ / -0.2, 20.43+ / -0.2, 21.33+ / -0.2, 23.14+ / -0.2, 23.60+ / -0.2, 24.77+ / -0.2, 26.25+ / -0.2, 26.72+ / -0.2, 26.96+ / -0.2, 29.69+ / -0.2, 30.52+ / -0.2, 33.33+ / -0.2, 33.89+ / -0.2, 34.89+ / -0.2, 35.54+ / -0.2, 37.03+ / -0.2, and 38.33+ / -0.2 in a powder X-ray diffraction diagram represented with 2 theta. In addition, the present invention further discloses a preparation method for the crystal. The hydrobromic acid vortioxetine crystal and the preparation method therefor in the present invention have good repeatability, easy operation, good product stability, and high yield and purity, and are suitable for industrial production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com