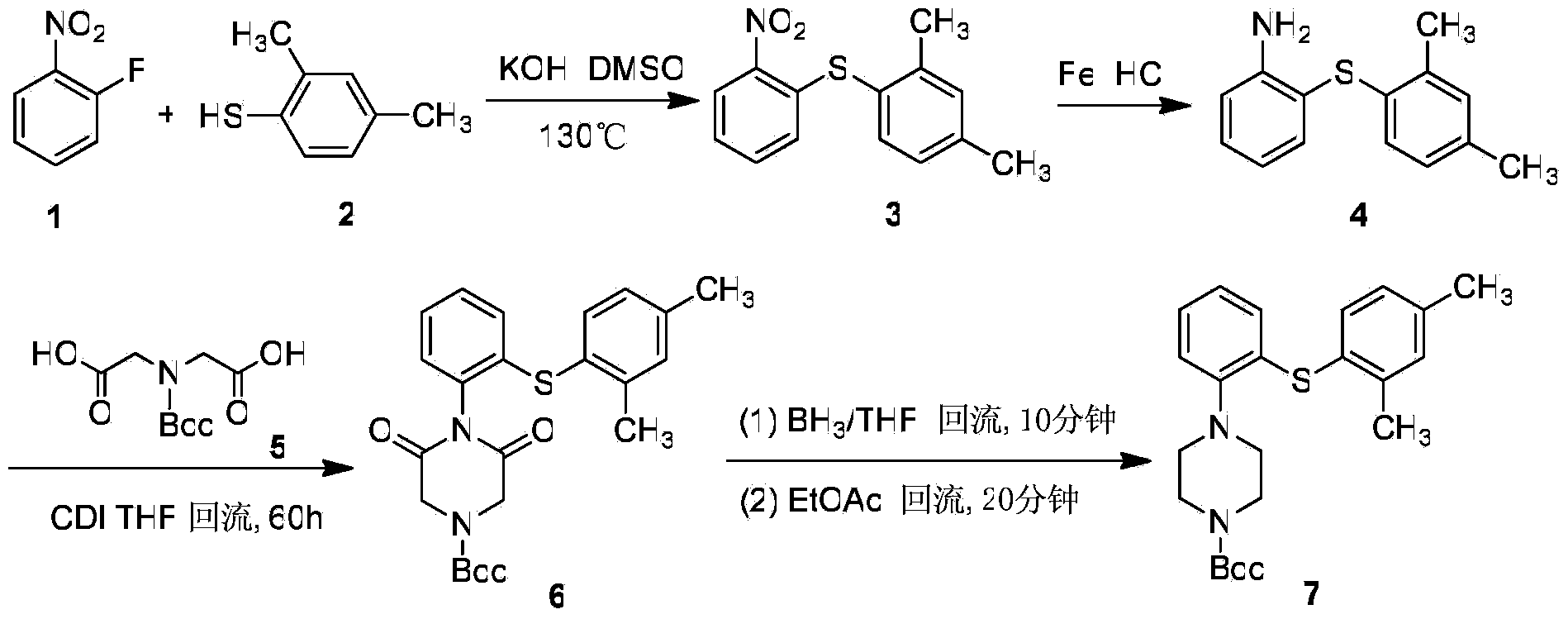
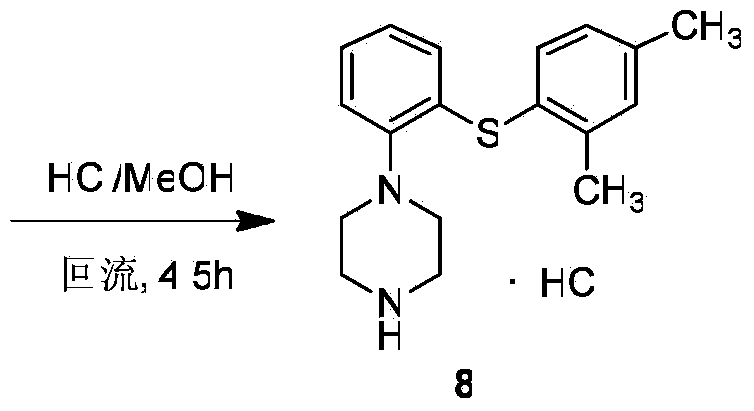
Preparation method of antidepressant vortioxetine
A vortioxetine and preparation process technology, applied in the field of drug synthesis, can solve the problems of complicated operation, high price and the like, and achieve the effects of cheap and easy-to-obtain reaction raw materials, convenient handling, and avoiding the use of palladium catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of 2-(2,4-Dimethylphenylsulfanyl)nitrobenzene(IV)
[0037] In a 500ml eggplant-shaped bottle, 2,4-dimethylthiophenol (47g, 0.341mol) was mixed with 150ml of methanol, sodium methoxide (22g, 0.409mol) was added under ice-cooling, and the reaction was stopped for 0.5 hours. Remove the solvent, add N,N-dimethylformamide (200ml) and o-chloronitrobenzene (59g, 0.375mol) to it, protect with nitrogen, react at 10°C for 24 hours, stop the reaction, pour into 600ml of water, Cool, filter with suction to obtain a tan solid, and recrystallize from absolute ethanol to obtain 79.3 g of pale yellow needle-like crystals, with a yield of 90.0%, m.p.94°C-96°C. .
[0038] 1H-NMR (300MHz, DMSO-d6), δ (ppm): 8.23 (1H, d, J=8.1Hz), 7.55~7.32 (4H, m, -ArH), 7.29 (1H, s), 7.16 (1H , d, J=7.8Hz), 6.64(1H, d, J=7.8Hz), 2.33(3H, s), 2.21(3H, s).
[0039] Synthesis of 2-(2,4-Dimethylphenylsulfanyl)aniline (V)
[0040] In a 1L three-necked flask, add compound IV (79g, 0.305mol) and...
Embodiment 2
[0049] Synthesis of 2-(2,4-Dimethylphenylsulfanyl)nitrobenzene(IV)
[0050] In a 500ml eggplant-shaped bottle, add 2,4-dimethylthiophenol (47g, 0.341mol) and potassium hydroxide (22.9g, 0.409mol), add acetonitrile (200ml) and o-fluoronitrobenzene (43g, 0.375mol), nitrogen protection, reflux reaction after 12 hours, stop the reaction, spin off the acetonitrile, extract with ethyl acetate (200ml×3), combine the organic layers, wash with saturated brine, dry over anhydrous sodium sulfate, and suction filter, The solvent was spin-dried to obtain a tan solid, which was recrystallized from absolute ethanol to obtain 63.3 g of pale yellow needle-like crystals, with a yield of 72.6%, m.p.94°C-96°C. .
[0051] 1H-NMR (300MHz, DMSO-d6), δ (ppm): 8.23 (1H, d, J=8.1Hz), 7.55~7.32 (4H, m, -ArH), 7.29 (1H, s), 7.16 (1H , d, J=7.8Hz), 6.64(1H, d, J=7.8Hz), 2.33(3H, s), 2.21(3H, s).
[0052] Synthesis of 2-(2,4-Dimethylphenylsulfanyl)aniline (V)
[0053] In a 1L three-necked flask, add ...
Embodiment 3
[0062] Synthesis of 2-(2,4-Dimethylphenylsulfanyl)nitrobenzene(IV)
[0063] In a 500ml eggplant-shaped bottle, add 2,4-dimethylthiophenol (47g, 0.341mol) and potassium hydroxide (22.9g, 0.409mol), add dimethylsulfoxide (200ml) and o-fluoronitro Benzene (43g, 0.375mol), protected by nitrogen, refluxed for 12 hours, stopped the reaction, poured into 600ml of water, extracted with ethyl acetate (200ml×3), combined the organic layers, washed with saturated brine, and dried over anhydrous sodium sulfate Suction filter overnight, and spin dry the solvent to obtain a tan solid, which was recrystallized from absolute ethanol to obtain 65.6 g of pale yellow needle-like crystals, with a yield of 75.6%, m.p.94°C-96°C. .
[0064] 1H-NMR (300MHz, DMSO-d6), δ (ppm): 8.23 (1H, d, J=8.1Hz), 7.55~7.32 (4H, m, -ArH), 7.29 (1H, s), 7.16 (1H , d, J=7.8Hz), 6.64(1H, d, J=7.8Hz), 2.33(3H, s), 2.21(3H, s).
[0065] Synthesis of 2-(2,4-Dimethylphenylsulfanyl)aniline (V)
[0066] In a 1L three-n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com